User login
First SGLT1/2 inhibitor shows ‘spectacular’ phase 3 safety and efficacy in T2D
Sotagliflozin, a novel type of sodium-glucose cotransporter inhibitor, showed the diverse benefits this drug class provides along some new twists in a pair of international pivotal trials that together enrolled nearly 12,000 patients with type 2 diabetes.
Unprecedented benefits were seen for the first time with a drug, sotagliflozin (Zynquista) that produces both sodium-glucose cotransporter 2 inhibition as well as SGLT1 inhibition.
They included a big reduction in both MIs and strokes; an ability to meaningfully reduce hyperglycemia in patients with severe renal dysfunction with an estimated glomerular filtration rate (eGFR) of 25-29 mL/min per 1.73 m2; an ability to safely and effectively start in patients still hospitalized (but stable) for an acute heart failure episode; and a striking 37% relative risk reduction in cardiovascular death, heart failure hospitalizations, or an urgent outpatient visit for heart failure in 739 of the patients enrolled in both trials who had heart failure with preserved ejection fraction (HFpEF).
These studies produced for the first time evidence from controlled, prospective, randomized trials that a drug could improve the outcome of HFpEF patients.
All these novel outcomes came on top of the usual benefits clinicians have generally seen across the SGLT2 inhibitors already on the U.S. market: reductions in cardiovascular death and heart failure hospitalizations among all patients with type 2 diabetes, preservation of renal function, and hemoglobin A1c lowering among T2D patients with eGFR levels of at least 30 mL/min per 1.73 m2.
“The data look spectacular,” summed up Deepak L. Bhatt, MD, who presented the results from the two trials, SOLOIST-WHF and SCORED, in talks at the virtual scientific sessions of the American Heart Association.
“I think sotagliflozin has the potential to be the best in class” based on the several added attributes shown in the two trials, he said in an interview. “We’ve shown that it is very safe, well tolerated, and effective.”
The primary results were a significant 33% relative risk reduction with sotagliflozin treatment, compared with placebo in the rate of total cardiovascular deaths, hospitalizations for heart failure, or urgent outpatient visits for heart failure during just over 9 months of median follow-up among patients with T2D recently hospitalized for heart failure in SOLOIST-WFH. And a significant 26% relative risk reduction with sotagliflozin for the same endpoint after a median follow-up of just over 14 months in SCORED, which enrolled patients with T2D and chronic kidney disease.
“Sotagliflozin adds to the SGLT2 inhibitor story,” and the SOLOIST-WHF results “may shift our focus to vulnerable, acute heart failure patients with an opportunity to treat during the transition phase,” when these patients leave the hospital, commented Jane E. Wilcox, MD, the study’s designated discussant and a heart failure cardiologist at Northwestern Medicine in Chicago.
A dual SGLT inhibitor
What sets sotagliflozin apart from the SGLT2 inhibitors is that it not only inhibits that protein but also SGTL1, which primarily resides in the gastrointestinal tract and is the main route for gut absorption of glucose. Dr. Bhatt said that he was unaware of any other SGLT1/2 inhibitors currently in advanced clinical testing.
The activity of sotagliflozin against the SGLT1 protein likely explains its ability to cut A1c levels in patients with severe renal dysfunction, a condition that stymies glucose lowering by SGLT2 inhibitors. In SCORED, which randomized 10,584 patients with T2D at 750 study sites in 44 countries, 813 patients (8%) had an eGFR of 25-29 mL/min per 1.73 m2 at enrollment. Sotagliflozin treatment led to an average 0.6% cut in A1c in this subgroup, and by the same average amount among the patients with GFRs of 30-60 mL/min per 1.73 m2.
“This is a huge finding for endocrinologists and primary care physicians” who treat patients with T2D who have severe renal dysfunction, said Dr. Bhatt, a professor of medicine at Harvard Medical School in Boston. “It’s a good enough reason by itself to approve this drug.”
The same mechanism may also be behind another unexpected finding in SCORED. Treatment with sotagliflozin cut the rate of total episodes of cardiovascular death, nonfatal MI, or nonfatal stroke by an absolute 1.6%, compared with placebo, and by a relative 23%. This benefit was largely driven by a 32% relative risk reduction total in MIs, and a 34% relative risk reduction in total stroke, both significant differences.
“No SGLT2 inhibitor has shown a reduction in stroke, and the MI signals have been mixed. The sizable MI and stroke effects are unique to sotagliflozin,” compared with the SGLT2 inhibitors, and likely reflect one or more mechanisms that result from blocked gut SGLT1 and a cut in GI glucose uptake, said Dr. Bhatt. “Probably some novel mechanism we don’t fully understand.”
First-ever HFpEF benefit
In contrast to these two benefits that are probably unique to drugs that inhibit the SGLT1 protein, sotagliflozin showed two other notable and unprecedented benefits that are likely generalizable to the SGLT2 inhibitors.
First is the striking benefit for HFpEF. Neither SOLOIST, which enrolled 1,222 patients with T2D and just hospitalized for worsening heart failure, nor SCORED, which enrolled patients with T2D and chronic kidney disease based exclusively on an eGFR of 25-60 mL/min per 1.73 m2, excluded patients with HFpEF, defined as heart failure patients with a left ventricular ejection fraction of at least 50%. The two studies together included a total of 739 of these patients, and they split fairly evenly between treatment with sotagliflozin or placebo.
The combined analysis showed that the incidence rate for the primary endpoint in both SOLOIST and SCORED was 59% with placebo and 39% with sotagliflozin, an absolute event reduction of 11.6 events/100 patient-years, and a significant 37% relative risk reduction, with a number needed to treat to prevent 1 event per year event of 9.
Although this observation comes from a nonprespecified combined analysis, “to me this result seems real, and I think it’s a class effect that I’m willing to extrapolate to the SGLT2 inhibitors,” Dr. Bhatt said. “It will change my practice,” he added, by spurring him to more aggressively prescribe an SGLT2 inhibitor to a patient with T2D and HFpEF.
“I think there has been some hesitation to use SGLT2 inhibitors in T2D patients with HFpEF” because of the paucity of data in this population, even though labeling and society recommendations do not rule it out. “I hope this finding will move that needle, and also generally improve SGLT2 inhibitor uptake, which has been low,” he said.
Also safe soon after acute heart failure decompensation
The other finding likely generalizable to SGLT2 inhibitors stems from the design of SOLOIST-WHF, which tested the efficacy and safety of starting sotagliflozin in patients with T2D as soon as they were stable after hospitalization for acute heart failure decompensation.
“Showing safety and efficacy when started in the hospital is pretty meaningful, because its tells patients that this drug is important and they should stay on it,” which should improve adherence, predicted Dr. Bhatt, who is also executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. “That’s the ultimate treatment path to prevent patients from falling through the cracks” and failing to receive an SGLT2 inhibitor.
SOLOIST-WHF enrolled patients hospitalized for worsening heart failure who also required intravenous diuretic treatment but had become stable enough to transition to an oral diuretic and come off oxygen. During a median follow-up of just over 9 months (both SOLOIST-WHF and SCORED ended sooner than planned because of a change in drug company sponsorship), treatment with sotagliflozin cut the primary endpoint by a relative 33%, compared with placebo, and with an absolute reduction of 25 events per 100 patient-years for a number needed to treat of 4. Sotagliflozin produced a strikingly high level of treatment efficiency driven by the high event rate in these recently decompensated patients. The benefit also appeared quickly, with a significant cut in events discernible within 28 days.
Extrapolating this finding to the SGLT2 inhibitors is “not a huge leap of faith,” Dr. Bhatt said.
“There is a role for sotagliflozin in acute heart failure. It showed benefit in these high-risk, transition-phase patients,” said Dr. Wilcox.
Simultaneously with Dr. Bhatt’s presentation, results of SOLOIST-WHF and SCORED were published online in the New England Journal of Medicine.
The trials were sponsored initially by Sanofi, and more recently by Lexicon. Dr. Bhatt has received research funding from both companies, and also from several other companies. He also is an adviser to several companies. Dr. Wilcox has been a consultant to Boehringer Ingelheim and Medtronic.
Sotagliflozin, a novel type of sodium-glucose cotransporter inhibitor, showed the diverse benefits this drug class provides along some new twists in a pair of international pivotal trials that together enrolled nearly 12,000 patients with type 2 diabetes.
Unprecedented benefits were seen for the first time with a drug, sotagliflozin (Zynquista) that produces both sodium-glucose cotransporter 2 inhibition as well as SGLT1 inhibition.
They included a big reduction in both MIs and strokes; an ability to meaningfully reduce hyperglycemia in patients with severe renal dysfunction with an estimated glomerular filtration rate (eGFR) of 25-29 mL/min per 1.73 m2; an ability to safely and effectively start in patients still hospitalized (but stable) for an acute heart failure episode; and a striking 37% relative risk reduction in cardiovascular death, heart failure hospitalizations, or an urgent outpatient visit for heart failure in 739 of the patients enrolled in both trials who had heart failure with preserved ejection fraction (HFpEF).
These studies produced for the first time evidence from controlled, prospective, randomized trials that a drug could improve the outcome of HFpEF patients.
All these novel outcomes came on top of the usual benefits clinicians have generally seen across the SGLT2 inhibitors already on the U.S. market: reductions in cardiovascular death and heart failure hospitalizations among all patients with type 2 diabetes, preservation of renal function, and hemoglobin A1c lowering among T2D patients with eGFR levels of at least 30 mL/min per 1.73 m2.
“The data look spectacular,” summed up Deepak L. Bhatt, MD, who presented the results from the two trials, SOLOIST-WHF and SCORED, in talks at the virtual scientific sessions of the American Heart Association.
“I think sotagliflozin has the potential to be the best in class” based on the several added attributes shown in the two trials, he said in an interview. “We’ve shown that it is very safe, well tolerated, and effective.”
The primary results were a significant 33% relative risk reduction with sotagliflozin treatment, compared with placebo in the rate of total cardiovascular deaths, hospitalizations for heart failure, or urgent outpatient visits for heart failure during just over 9 months of median follow-up among patients with T2D recently hospitalized for heart failure in SOLOIST-WFH. And a significant 26% relative risk reduction with sotagliflozin for the same endpoint after a median follow-up of just over 14 months in SCORED, which enrolled patients with T2D and chronic kidney disease.
“Sotagliflozin adds to the SGLT2 inhibitor story,” and the SOLOIST-WHF results “may shift our focus to vulnerable, acute heart failure patients with an opportunity to treat during the transition phase,” when these patients leave the hospital, commented Jane E. Wilcox, MD, the study’s designated discussant and a heart failure cardiologist at Northwestern Medicine in Chicago.
A dual SGLT inhibitor
What sets sotagliflozin apart from the SGLT2 inhibitors is that it not only inhibits that protein but also SGTL1, which primarily resides in the gastrointestinal tract and is the main route for gut absorption of glucose. Dr. Bhatt said that he was unaware of any other SGLT1/2 inhibitors currently in advanced clinical testing.
The activity of sotagliflozin against the SGLT1 protein likely explains its ability to cut A1c levels in patients with severe renal dysfunction, a condition that stymies glucose lowering by SGLT2 inhibitors. In SCORED, which randomized 10,584 patients with T2D at 750 study sites in 44 countries, 813 patients (8%) had an eGFR of 25-29 mL/min per 1.73 m2 at enrollment. Sotagliflozin treatment led to an average 0.6% cut in A1c in this subgroup, and by the same average amount among the patients with GFRs of 30-60 mL/min per 1.73 m2.
“This is a huge finding for endocrinologists and primary care physicians” who treat patients with T2D who have severe renal dysfunction, said Dr. Bhatt, a professor of medicine at Harvard Medical School in Boston. “It’s a good enough reason by itself to approve this drug.”
The same mechanism may also be behind another unexpected finding in SCORED. Treatment with sotagliflozin cut the rate of total episodes of cardiovascular death, nonfatal MI, or nonfatal stroke by an absolute 1.6%, compared with placebo, and by a relative 23%. This benefit was largely driven by a 32% relative risk reduction total in MIs, and a 34% relative risk reduction in total stroke, both significant differences.
“No SGLT2 inhibitor has shown a reduction in stroke, and the MI signals have been mixed. The sizable MI and stroke effects are unique to sotagliflozin,” compared with the SGLT2 inhibitors, and likely reflect one or more mechanisms that result from blocked gut SGLT1 and a cut in GI glucose uptake, said Dr. Bhatt. “Probably some novel mechanism we don’t fully understand.”
First-ever HFpEF benefit
In contrast to these two benefits that are probably unique to drugs that inhibit the SGLT1 protein, sotagliflozin showed two other notable and unprecedented benefits that are likely generalizable to the SGLT2 inhibitors.
First is the striking benefit for HFpEF. Neither SOLOIST, which enrolled 1,222 patients with T2D and just hospitalized for worsening heart failure, nor SCORED, which enrolled patients with T2D and chronic kidney disease based exclusively on an eGFR of 25-60 mL/min per 1.73 m2, excluded patients with HFpEF, defined as heart failure patients with a left ventricular ejection fraction of at least 50%. The two studies together included a total of 739 of these patients, and they split fairly evenly between treatment with sotagliflozin or placebo.
The combined analysis showed that the incidence rate for the primary endpoint in both SOLOIST and SCORED was 59% with placebo and 39% with sotagliflozin, an absolute event reduction of 11.6 events/100 patient-years, and a significant 37% relative risk reduction, with a number needed to treat to prevent 1 event per year event of 9.
Although this observation comes from a nonprespecified combined analysis, “to me this result seems real, and I think it’s a class effect that I’m willing to extrapolate to the SGLT2 inhibitors,” Dr. Bhatt said. “It will change my practice,” he added, by spurring him to more aggressively prescribe an SGLT2 inhibitor to a patient with T2D and HFpEF.
“I think there has been some hesitation to use SGLT2 inhibitors in T2D patients with HFpEF” because of the paucity of data in this population, even though labeling and society recommendations do not rule it out. “I hope this finding will move that needle, and also generally improve SGLT2 inhibitor uptake, which has been low,” he said.
Also safe soon after acute heart failure decompensation
The other finding likely generalizable to SGLT2 inhibitors stems from the design of SOLOIST-WHF, which tested the efficacy and safety of starting sotagliflozin in patients with T2D as soon as they were stable after hospitalization for acute heart failure decompensation.
“Showing safety and efficacy when started in the hospital is pretty meaningful, because its tells patients that this drug is important and they should stay on it,” which should improve adherence, predicted Dr. Bhatt, who is also executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. “That’s the ultimate treatment path to prevent patients from falling through the cracks” and failing to receive an SGLT2 inhibitor.
SOLOIST-WHF enrolled patients hospitalized for worsening heart failure who also required intravenous diuretic treatment but had become stable enough to transition to an oral diuretic and come off oxygen. During a median follow-up of just over 9 months (both SOLOIST-WHF and SCORED ended sooner than planned because of a change in drug company sponsorship), treatment with sotagliflozin cut the primary endpoint by a relative 33%, compared with placebo, and with an absolute reduction of 25 events per 100 patient-years for a number needed to treat of 4. Sotagliflozin produced a strikingly high level of treatment efficiency driven by the high event rate in these recently decompensated patients. The benefit also appeared quickly, with a significant cut in events discernible within 28 days.
Extrapolating this finding to the SGLT2 inhibitors is “not a huge leap of faith,” Dr. Bhatt said.
“There is a role for sotagliflozin in acute heart failure. It showed benefit in these high-risk, transition-phase patients,” said Dr. Wilcox.
Simultaneously with Dr. Bhatt’s presentation, results of SOLOIST-WHF and SCORED were published online in the New England Journal of Medicine.
The trials were sponsored initially by Sanofi, and more recently by Lexicon. Dr. Bhatt has received research funding from both companies, and also from several other companies. He also is an adviser to several companies. Dr. Wilcox has been a consultant to Boehringer Ingelheim and Medtronic.
Sotagliflozin, a novel type of sodium-glucose cotransporter inhibitor, showed the diverse benefits this drug class provides along some new twists in a pair of international pivotal trials that together enrolled nearly 12,000 patients with type 2 diabetes.
Unprecedented benefits were seen for the first time with a drug, sotagliflozin (Zynquista) that produces both sodium-glucose cotransporter 2 inhibition as well as SGLT1 inhibition.
They included a big reduction in both MIs and strokes; an ability to meaningfully reduce hyperglycemia in patients with severe renal dysfunction with an estimated glomerular filtration rate (eGFR) of 25-29 mL/min per 1.73 m2; an ability to safely and effectively start in patients still hospitalized (but stable) for an acute heart failure episode; and a striking 37% relative risk reduction in cardiovascular death, heart failure hospitalizations, or an urgent outpatient visit for heart failure in 739 of the patients enrolled in both trials who had heart failure with preserved ejection fraction (HFpEF).
These studies produced for the first time evidence from controlled, prospective, randomized trials that a drug could improve the outcome of HFpEF patients.
All these novel outcomes came on top of the usual benefits clinicians have generally seen across the SGLT2 inhibitors already on the U.S. market: reductions in cardiovascular death and heart failure hospitalizations among all patients with type 2 diabetes, preservation of renal function, and hemoglobin A1c lowering among T2D patients with eGFR levels of at least 30 mL/min per 1.73 m2.
“The data look spectacular,” summed up Deepak L. Bhatt, MD, who presented the results from the two trials, SOLOIST-WHF and SCORED, in talks at the virtual scientific sessions of the American Heart Association.
“I think sotagliflozin has the potential to be the best in class” based on the several added attributes shown in the two trials, he said in an interview. “We’ve shown that it is very safe, well tolerated, and effective.”
The primary results were a significant 33% relative risk reduction with sotagliflozin treatment, compared with placebo in the rate of total cardiovascular deaths, hospitalizations for heart failure, or urgent outpatient visits for heart failure during just over 9 months of median follow-up among patients with T2D recently hospitalized for heart failure in SOLOIST-WFH. And a significant 26% relative risk reduction with sotagliflozin for the same endpoint after a median follow-up of just over 14 months in SCORED, which enrolled patients with T2D and chronic kidney disease.
“Sotagliflozin adds to the SGLT2 inhibitor story,” and the SOLOIST-WHF results “may shift our focus to vulnerable, acute heart failure patients with an opportunity to treat during the transition phase,” when these patients leave the hospital, commented Jane E. Wilcox, MD, the study’s designated discussant and a heart failure cardiologist at Northwestern Medicine in Chicago.
A dual SGLT inhibitor
What sets sotagliflozin apart from the SGLT2 inhibitors is that it not only inhibits that protein but also SGTL1, which primarily resides in the gastrointestinal tract and is the main route for gut absorption of glucose. Dr. Bhatt said that he was unaware of any other SGLT1/2 inhibitors currently in advanced clinical testing.
The activity of sotagliflozin against the SGLT1 protein likely explains its ability to cut A1c levels in patients with severe renal dysfunction, a condition that stymies glucose lowering by SGLT2 inhibitors. In SCORED, which randomized 10,584 patients with T2D at 750 study sites in 44 countries, 813 patients (8%) had an eGFR of 25-29 mL/min per 1.73 m2 at enrollment. Sotagliflozin treatment led to an average 0.6% cut in A1c in this subgroup, and by the same average amount among the patients with GFRs of 30-60 mL/min per 1.73 m2.
“This is a huge finding for endocrinologists and primary care physicians” who treat patients with T2D who have severe renal dysfunction, said Dr. Bhatt, a professor of medicine at Harvard Medical School in Boston. “It’s a good enough reason by itself to approve this drug.”
The same mechanism may also be behind another unexpected finding in SCORED. Treatment with sotagliflozin cut the rate of total episodes of cardiovascular death, nonfatal MI, or nonfatal stroke by an absolute 1.6%, compared with placebo, and by a relative 23%. This benefit was largely driven by a 32% relative risk reduction total in MIs, and a 34% relative risk reduction in total stroke, both significant differences.
“No SGLT2 inhibitor has shown a reduction in stroke, and the MI signals have been mixed. The sizable MI and stroke effects are unique to sotagliflozin,” compared with the SGLT2 inhibitors, and likely reflect one or more mechanisms that result from blocked gut SGLT1 and a cut in GI glucose uptake, said Dr. Bhatt. “Probably some novel mechanism we don’t fully understand.”
First-ever HFpEF benefit
In contrast to these two benefits that are probably unique to drugs that inhibit the SGLT1 protein, sotagliflozin showed two other notable and unprecedented benefits that are likely generalizable to the SGLT2 inhibitors.
First is the striking benefit for HFpEF. Neither SOLOIST, which enrolled 1,222 patients with T2D and just hospitalized for worsening heart failure, nor SCORED, which enrolled patients with T2D and chronic kidney disease based exclusively on an eGFR of 25-60 mL/min per 1.73 m2, excluded patients with HFpEF, defined as heart failure patients with a left ventricular ejection fraction of at least 50%. The two studies together included a total of 739 of these patients, and they split fairly evenly between treatment with sotagliflozin or placebo.
The combined analysis showed that the incidence rate for the primary endpoint in both SOLOIST and SCORED was 59% with placebo and 39% with sotagliflozin, an absolute event reduction of 11.6 events/100 patient-years, and a significant 37% relative risk reduction, with a number needed to treat to prevent 1 event per year event of 9.
Although this observation comes from a nonprespecified combined analysis, “to me this result seems real, and I think it’s a class effect that I’m willing to extrapolate to the SGLT2 inhibitors,” Dr. Bhatt said. “It will change my practice,” he added, by spurring him to more aggressively prescribe an SGLT2 inhibitor to a patient with T2D and HFpEF.
“I think there has been some hesitation to use SGLT2 inhibitors in T2D patients with HFpEF” because of the paucity of data in this population, even though labeling and society recommendations do not rule it out. “I hope this finding will move that needle, and also generally improve SGLT2 inhibitor uptake, which has been low,” he said.
Also safe soon after acute heart failure decompensation
The other finding likely generalizable to SGLT2 inhibitors stems from the design of SOLOIST-WHF, which tested the efficacy and safety of starting sotagliflozin in patients with T2D as soon as they were stable after hospitalization for acute heart failure decompensation.
“Showing safety and efficacy when started in the hospital is pretty meaningful, because its tells patients that this drug is important and they should stay on it,” which should improve adherence, predicted Dr. Bhatt, who is also executive director of Interventional Cardiovascular Programs at Brigham and Women’s Hospital in Boston. “That’s the ultimate treatment path to prevent patients from falling through the cracks” and failing to receive an SGLT2 inhibitor.
SOLOIST-WHF enrolled patients hospitalized for worsening heart failure who also required intravenous diuretic treatment but had become stable enough to transition to an oral diuretic and come off oxygen. During a median follow-up of just over 9 months (both SOLOIST-WHF and SCORED ended sooner than planned because of a change in drug company sponsorship), treatment with sotagliflozin cut the primary endpoint by a relative 33%, compared with placebo, and with an absolute reduction of 25 events per 100 patient-years for a number needed to treat of 4. Sotagliflozin produced a strikingly high level of treatment efficiency driven by the high event rate in these recently decompensated patients. The benefit also appeared quickly, with a significant cut in events discernible within 28 days.
Extrapolating this finding to the SGLT2 inhibitors is “not a huge leap of faith,” Dr. Bhatt said.
“There is a role for sotagliflozin in acute heart failure. It showed benefit in these high-risk, transition-phase patients,” said Dr. Wilcox.
Simultaneously with Dr. Bhatt’s presentation, results of SOLOIST-WHF and SCORED were published online in the New England Journal of Medicine.
The trials were sponsored initially by Sanofi, and more recently by Lexicon. Dr. Bhatt has received research funding from both companies, and also from several other companies. He also is an adviser to several companies. Dr. Wilcox has been a consultant to Boehringer Ingelheim and Medtronic.
FROM AHA 2020
Factor XI inhibitor–based anticoagulation strategies gain ground
according to Jeffrey I. Weitz, MD.
These strategies could pick up where direct-acting oral anticoagulants leave off, he suggested during a presentation at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
“We all know that the direct oral anticoagulants – the DOACs – are an advance over vitamin K antagonists,” said Dr. Weitz, professor of medicine and biochemistry at McMaster University, Hamilton, Ontario.
Not only are DOACs at least as effective as vitamin K antagonists such as warfarin for stroke prevention in atrial fibrillation or for treatment of venous thromboembolism (VTE), but they also reduce intracranial bleeding and major bleeding risk in those settings, respectively, and they are more convenient to administer because they can be delivered using fixed doses without the need for coagulation monitoring, he added.
Still, new targets are needed, he said, explaining that, although DOACs moved closer to the goal of attenuating thrombosis without increasing the risk of bleeding, annual rates of major bleeding remain at 2%-3% in the atrial fibrillation population, and rates of major and clinically relevant nonmajor bleeding are about 10%.
“The fear of bleeding leads to underuse of anticoagulants for eligible patients with atrial fibrillation and inappropriate use of low-dose [non–vitamin K antagonist oral anticoagulant] regimens, which can leave patients unprotected from thrombotic complications,” he said.
Factor XI
That’s where Factor XI (FXI) may come in, Dr. Weitz said.
Current anticoagulants target enzymes, including FXa or thrombin, in the common pathway of coagulation, but the intrinsic pathway at the level of FXI and FXII has attracted attention in recent years.
The intrinsic pathway is activated when blood comes into contact with medical devices like stents, mechanical heart valves, or central venous catheters, but evidence also suggests that it plays a role in clot stabilization and growth, he explained, noting additional evidence of attenuation of thrombosis in mice deficient in FXI or FXII and in animals with FXI or FXII inhibitors.
“There is no bleeding with congenital FXII deficiency, and patients with FXI deficiency rarely have spontaneous bleeding, although they can bleed with surgery or trauma,” he noted. “Therefore, the promise of contact pathway inhibition is that we can attenuate thrombosis with little or no disruption of hemostasis.”
The initiators of the intrinsic pathway are naturally occurring polyphosphates that can activate FXI and FXII, promote platelet activation, and lead to thrombosis. A number of agents are being investigated to target these enzymes – particularly FXI, for which the strongest epidemiological and other evidence of its link with thrombosis exists. He noted that FXI deficiency appears protective against deep-vein thrombosis (DVT) and ischemic stroke, whereas high levels are linked with an increased risk of venous and arterial thrombosis.
Investigative strategies include the use of antisense oligonucleotides to reduce hepatic synthesis of FXI, aptamers to bind FXI and block its activity, antibodies to bind FXI and block its activation or activity, and small molecules to bind reversibly to the active site of FXI and block its activity “much like the DOACs block the activity of FXa or thrombin.”
“We have to remember that the DOACs have taken over from vitamin K antagonists, like warfarin, for many indications, and as they go generic their uptake will increase even further,” Dr. Weitz said. “When we compare the FXI inhibitors with existing anticoagulants, we don’t necessarily want to go up against the DOACs – we’re looking for indications where [DOACs] have yet to be tested or may be unsafe.”
Potential indications include the following:
Prevention of major adverse cardiovascular events in patients with end-stage renal disease with or without atrial fibrillation.
Provision of a safer platform for antiplatelet therapy in patients with acute coronary syndrome.
Secondary stroke prevention.
Prevention or treatment of cancer-associated VTE.
Prevention of thrombosis associated with central venous catheters, left ventricular assist devices, or mechanical heart valves.
Agents in development
Of the FXI inhibitors in development, ISIS-FXIRx, an antisense oligonucleotide against FXI, is furthest along. In a study published in Blood, ISIS-FXIRx produced a dose-dependent and sustained reduction in FXI levels in healthy volunteers, and in a later randomized study published in The New England Journal of Medicine, it significantly reduced the incidence of DVT in patients undergoing voluntary total knee arthroplasty (30.4% with enoxaparin vs. 4.2% with ISIS-FXIRx at a dose of 300 mg). Bleeding rates were 8.3% and 2.6%, respectively.
The findings showed the potential for reducing thrombosis without increasing bleeding by targeting FXI, Dr. Weitz said, adding that ISIS-FXIRx was also evaluated in a small study of patients with end-stage renal disease undergoing hemodialysis and was shown to produce a dose-dependent reduction in FXI levels and to reduce the incidence of category 3 and 4 clotting in the air trap and dialyzer, compared with placebo, when given in addition to heparin.
This suggests that FXI knockdown can attenuate device-associated clotting to a greater extent than heparin alone, Dr. Weitz said.
The FXIa-directed inhibitory antibody osocimab has also been evaluated in both healthy volunteers and in patients undergoing total knee arthroplasty. In a 2019 study of healthy volunteers, a single IV injection showed a dose-dependent pharmacokinetic profile and produced FXI inhibition for about 1 month, and in the FOXTROT trial published in January in JAMA by Dr. Weitz and colleagues, osocimab was shown to reduce the incidence of symptomatic VTE, asymptomatic DVT, and VTE-related death up to day 10-13 after total knee arthroplasty.
Osocimab at doses ranging from 0.3-1.8 mg/kg given postoperatively or preoperatively were noninferior to enoxaparin (rates of 15.7%-23.7% vs. 26.3%), and osocimab at a preoperative dose of 1.8 mg/kg was superior to both enoxaparin and apixaban (11.5% vs. 26.3% and 14.5%, respectively), he said.
Bleeding rates ranged from 0%-5% with osocimab, compared with 6% with enoxaparin and 2% with apixaban
Ongoing studies
Currently ongoing studies of FXI-directed anticoagulation strategies include a study comparing ISIS-FXIRx with placebo in 200 patients with end-stage renal disease, a study comparing osocimab with placebo in 600 patients with end-stage renal disease, and a study comparing abelacimab – an antibody that binds to FXI and prevents its activation by either FXIIa or thrombin, with enoxaparin in 700 patients undergoing total knee arthroplasty, Dr. Weitz said.
Additionally, there is “considerable activity” with small molecule inhibitors of FXIa, including a phase 2, placebo-controlled, dose-ranging study looking at the novel JNG-7003/BMS-986177 agent for secondary stroke/transient ischemic attack prevention in 2,500 patients and a phase 2 study comparing it with enoxaparin for postoperative thromboprophylaxis in 1,200 patients undergoing total knee arthroplasty.
Parallel phase 2 studies are also underway to compare the novel BAY-2433334 small molecule inhibitor with placebo for stroke/transient ischemic attack prevention, with apixaban for atrial fibrillation, and for prevention of major adverse cardiovascular events in patients with acute MI.
These ongoing trials will help determine the risk-benefit profile of FXI inhibitors he said.
Session comoderator Anne Rose, PharmD, pharmacy coordinator at the University of Wisconsin, Madison, noted that these types of agents have been discussed “for quite some time” and asked whether they will be available for use in clinical practice in the near future.
Dr. Weitz predicted it will be at least a few years. The studies are just now moving to phase 2b and will still need to be evaluated in phase 3 trials and for appropriate new indications, he said.
Dr. Weitz reported research support from Canadian Institutes of Health research, Heart and Stroke Foundation, and Canadian Fund for Innovation, and he is a consultant and/or scientific advisory board member for Anthos, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, Novartis, Pfizer, Portola, Servier , and Thetherex.
according to Jeffrey I. Weitz, MD.
These strategies could pick up where direct-acting oral anticoagulants leave off, he suggested during a presentation at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
“We all know that the direct oral anticoagulants – the DOACs – are an advance over vitamin K antagonists,” said Dr. Weitz, professor of medicine and biochemistry at McMaster University, Hamilton, Ontario.
Not only are DOACs at least as effective as vitamin K antagonists such as warfarin for stroke prevention in atrial fibrillation or for treatment of venous thromboembolism (VTE), but they also reduce intracranial bleeding and major bleeding risk in those settings, respectively, and they are more convenient to administer because they can be delivered using fixed doses without the need for coagulation monitoring, he added.
Still, new targets are needed, he said, explaining that, although DOACs moved closer to the goal of attenuating thrombosis without increasing the risk of bleeding, annual rates of major bleeding remain at 2%-3% in the atrial fibrillation population, and rates of major and clinically relevant nonmajor bleeding are about 10%.
“The fear of bleeding leads to underuse of anticoagulants for eligible patients with atrial fibrillation and inappropriate use of low-dose [non–vitamin K antagonist oral anticoagulant] regimens, which can leave patients unprotected from thrombotic complications,” he said.
Factor XI
That’s where Factor XI (FXI) may come in, Dr. Weitz said.
Current anticoagulants target enzymes, including FXa or thrombin, in the common pathway of coagulation, but the intrinsic pathway at the level of FXI and FXII has attracted attention in recent years.
The intrinsic pathway is activated when blood comes into contact with medical devices like stents, mechanical heart valves, or central venous catheters, but evidence also suggests that it plays a role in clot stabilization and growth, he explained, noting additional evidence of attenuation of thrombosis in mice deficient in FXI or FXII and in animals with FXI or FXII inhibitors.
“There is no bleeding with congenital FXII deficiency, and patients with FXI deficiency rarely have spontaneous bleeding, although they can bleed with surgery or trauma,” he noted. “Therefore, the promise of contact pathway inhibition is that we can attenuate thrombosis with little or no disruption of hemostasis.”
The initiators of the intrinsic pathway are naturally occurring polyphosphates that can activate FXI and FXII, promote platelet activation, and lead to thrombosis. A number of agents are being investigated to target these enzymes – particularly FXI, for which the strongest epidemiological and other evidence of its link with thrombosis exists. He noted that FXI deficiency appears protective against deep-vein thrombosis (DVT) and ischemic stroke, whereas high levels are linked with an increased risk of venous and arterial thrombosis.
Investigative strategies include the use of antisense oligonucleotides to reduce hepatic synthesis of FXI, aptamers to bind FXI and block its activity, antibodies to bind FXI and block its activation or activity, and small molecules to bind reversibly to the active site of FXI and block its activity “much like the DOACs block the activity of FXa or thrombin.”
“We have to remember that the DOACs have taken over from vitamin K antagonists, like warfarin, for many indications, and as they go generic their uptake will increase even further,” Dr. Weitz said. “When we compare the FXI inhibitors with existing anticoagulants, we don’t necessarily want to go up against the DOACs – we’re looking for indications where [DOACs] have yet to be tested or may be unsafe.”
Potential indications include the following:
Prevention of major adverse cardiovascular events in patients with end-stage renal disease with or without atrial fibrillation.
Provision of a safer platform for antiplatelet therapy in patients with acute coronary syndrome.
Secondary stroke prevention.
Prevention or treatment of cancer-associated VTE.
Prevention of thrombosis associated with central venous catheters, left ventricular assist devices, or mechanical heart valves.
Agents in development
Of the FXI inhibitors in development, ISIS-FXIRx, an antisense oligonucleotide against FXI, is furthest along. In a study published in Blood, ISIS-FXIRx produced a dose-dependent and sustained reduction in FXI levels in healthy volunteers, and in a later randomized study published in The New England Journal of Medicine, it significantly reduced the incidence of DVT in patients undergoing voluntary total knee arthroplasty (30.4% with enoxaparin vs. 4.2% with ISIS-FXIRx at a dose of 300 mg). Bleeding rates were 8.3% and 2.6%, respectively.
The findings showed the potential for reducing thrombosis without increasing bleeding by targeting FXI, Dr. Weitz said, adding that ISIS-FXIRx was also evaluated in a small study of patients with end-stage renal disease undergoing hemodialysis and was shown to produce a dose-dependent reduction in FXI levels and to reduce the incidence of category 3 and 4 clotting in the air trap and dialyzer, compared with placebo, when given in addition to heparin.
This suggests that FXI knockdown can attenuate device-associated clotting to a greater extent than heparin alone, Dr. Weitz said.
The FXIa-directed inhibitory antibody osocimab has also been evaluated in both healthy volunteers and in patients undergoing total knee arthroplasty. In a 2019 study of healthy volunteers, a single IV injection showed a dose-dependent pharmacokinetic profile and produced FXI inhibition for about 1 month, and in the FOXTROT trial published in January in JAMA by Dr. Weitz and colleagues, osocimab was shown to reduce the incidence of symptomatic VTE, asymptomatic DVT, and VTE-related death up to day 10-13 after total knee arthroplasty.
Osocimab at doses ranging from 0.3-1.8 mg/kg given postoperatively or preoperatively were noninferior to enoxaparin (rates of 15.7%-23.7% vs. 26.3%), and osocimab at a preoperative dose of 1.8 mg/kg was superior to both enoxaparin and apixaban (11.5% vs. 26.3% and 14.5%, respectively), he said.
Bleeding rates ranged from 0%-5% with osocimab, compared with 6% with enoxaparin and 2% with apixaban
Ongoing studies
Currently ongoing studies of FXI-directed anticoagulation strategies include a study comparing ISIS-FXIRx with placebo in 200 patients with end-stage renal disease, a study comparing osocimab with placebo in 600 patients with end-stage renal disease, and a study comparing abelacimab – an antibody that binds to FXI and prevents its activation by either FXIIa or thrombin, with enoxaparin in 700 patients undergoing total knee arthroplasty, Dr. Weitz said.
Additionally, there is “considerable activity” with small molecule inhibitors of FXIa, including a phase 2, placebo-controlled, dose-ranging study looking at the novel JNG-7003/BMS-986177 agent for secondary stroke/transient ischemic attack prevention in 2,500 patients and a phase 2 study comparing it with enoxaparin for postoperative thromboprophylaxis in 1,200 patients undergoing total knee arthroplasty.
Parallel phase 2 studies are also underway to compare the novel BAY-2433334 small molecule inhibitor with placebo for stroke/transient ischemic attack prevention, with apixaban for atrial fibrillation, and for prevention of major adverse cardiovascular events in patients with acute MI.
These ongoing trials will help determine the risk-benefit profile of FXI inhibitors he said.
Session comoderator Anne Rose, PharmD, pharmacy coordinator at the University of Wisconsin, Madison, noted that these types of agents have been discussed “for quite some time” and asked whether they will be available for use in clinical practice in the near future.
Dr. Weitz predicted it will be at least a few years. The studies are just now moving to phase 2b and will still need to be evaluated in phase 3 trials and for appropriate new indications, he said.
Dr. Weitz reported research support from Canadian Institutes of Health research, Heart and Stroke Foundation, and Canadian Fund for Innovation, and he is a consultant and/or scientific advisory board member for Anthos, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, Novartis, Pfizer, Portola, Servier , and Thetherex.
according to Jeffrey I. Weitz, MD.
These strategies could pick up where direct-acting oral anticoagulants leave off, he suggested during a presentation at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
“We all know that the direct oral anticoagulants – the DOACs – are an advance over vitamin K antagonists,” said Dr. Weitz, professor of medicine and biochemistry at McMaster University, Hamilton, Ontario.
Not only are DOACs at least as effective as vitamin K antagonists such as warfarin for stroke prevention in atrial fibrillation or for treatment of venous thromboembolism (VTE), but they also reduce intracranial bleeding and major bleeding risk in those settings, respectively, and they are more convenient to administer because they can be delivered using fixed doses without the need for coagulation monitoring, he added.
Still, new targets are needed, he said, explaining that, although DOACs moved closer to the goal of attenuating thrombosis without increasing the risk of bleeding, annual rates of major bleeding remain at 2%-3% in the atrial fibrillation population, and rates of major and clinically relevant nonmajor bleeding are about 10%.
“The fear of bleeding leads to underuse of anticoagulants for eligible patients with atrial fibrillation and inappropriate use of low-dose [non–vitamin K antagonist oral anticoagulant] regimens, which can leave patients unprotected from thrombotic complications,” he said.
Factor XI
That’s where Factor XI (FXI) may come in, Dr. Weitz said.
Current anticoagulants target enzymes, including FXa or thrombin, in the common pathway of coagulation, but the intrinsic pathway at the level of FXI and FXII has attracted attention in recent years.
The intrinsic pathway is activated when blood comes into contact with medical devices like stents, mechanical heart valves, or central venous catheters, but evidence also suggests that it plays a role in clot stabilization and growth, he explained, noting additional evidence of attenuation of thrombosis in mice deficient in FXI or FXII and in animals with FXI or FXII inhibitors.
“There is no bleeding with congenital FXII deficiency, and patients with FXI deficiency rarely have spontaneous bleeding, although they can bleed with surgery or trauma,” he noted. “Therefore, the promise of contact pathway inhibition is that we can attenuate thrombosis with little or no disruption of hemostasis.”
The initiators of the intrinsic pathway are naturally occurring polyphosphates that can activate FXI and FXII, promote platelet activation, and lead to thrombosis. A number of agents are being investigated to target these enzymes – particularly FXI, for which the strongest epidemiological and other evidence of its link with thrombosis exists. He noted that FXI deficiency appears protective against deep-vein thrombosis (DVT) and ischemic stroke, whereas high levels are linked with an increased risk of venous and arterial thrombosis.
Investigative strategies include the use of antisense oligonucleotides to reduce hepatic synthesis of FXI, aptamers to bind FXI and block its activity, antibodies to bind FXI and block its activation or activity, and small molecules to bind reversibly to the active site of FXI and block its activity “much like the DOACs block the activity of FXa or thrombin.”
“We have to remember that the DOACs have taken over from vitamin K antagonists, like warfarin, for many indications, and as they go generic their uptake will increase even further,” Dr. Weitz said. “When we compare the FXI inhibitors with existing anticoagulants, we don’t necessarily want to go up against the DOACs – we’re looking for indications where [DOACs] have yet to be tested or may be unsafe.”
Potential indications include the following:
Prevention of major adverse cardiovascular events in patients with end-stage renal disease with or without atrial fibrillation.
Provision of a safer platform for antiplatelet therapy in patients with acute coronary syndrome.
Secondary stroke prevention.
Prevention or treatment of cancer-associated VTE.
Prevention of thrombosis associated with central venous catheters, left ventricular assist devices, or mechanical heart valves.
Agents in development
Of the FXI inhibitors in development, ISIS-FXIRx, an antisense oligonucleotide against FXI, is furthest along. In a study published in Blood, ISIS-FXIRx produced a dose-dependent and sustained reduction in FXI levels in healthy volunteers, and in a later randomized study published in The New England Journal of Medicine, it significantly reduced the incidence of DVT in patients undergoing voluntary total knee arthroplasty (30.4% with enoxaparin vs. 4.2% with ISIS-FXIRx at a dose of 300 mg). Bleeding rates were 8.3% and 2.6%, respectively.
The findings showed the potential for reducing thrombosis without increasing bleeding by targeting FXI, Dr. Weitz said, adding that ISIS-FXIRx was also evaluated in a small study of patients with end-stage renal disease undergoing hemodialysis and was shown to produce a dose-dependent reduction in FXI levels and to reduce the incidence of category 3 and 4 clotting in the air trap and dialyzer, compared with placebo, when given in addition to heparin.
This suggests that FXI knockdown can attenuate device-associated clotting to a greater extent than heparin alone, Dr. Weitz said.
The FXIa-directed inhibitory antibody osocimab has also been evaluated in both healthy volunteers and in patients undergoing total knee arthroplasty. In a 2019 study of healthy volunteers, a single IV injection showed a dose-dependent pharmacokinetic profile and produced FXI inhibition for about 1 month, and in the FOXTROT trial published in January in JAMA by Dr. Weitz and colleagues, osocimab was shown to reduce the incidence of symptomatic VTE, asymptomatic DVT, and VTE-related death up to day 10-13 after total knee arthroplasty.
Osocimab at doses ranging from 0.3-1.8 mg/kg given postoperatively or preoperatively were noninferior to enoxaparin (rates of 15.7%-23.7% vs. 26.3%), and osocimab at a preoperative dose of 1.8 mg/kg was superior to both enoxaparin and apixaban (11.5% vs. 26.3% and 14.5%, respectively), he said.
Bleeding rates ranged from 0%-5% with osocimab, compared with 6% with enoxaparin and 2% with apixaban
Ongoing studies
Currently ongoing studies of FXI-directed anticoagulation strategies include a study comparing ISIS-FXIRx with placebo in 200 patients with end-stage renal disease, a study comparing osocimab with placebo in 600 patients with end-stage renal disease, and a study comparing abelacimab – an antibody that binds to FXI and prevents its activation by either FXIIa or thrombin, with enoxaparin in 700 patients undergoing total knee arthroplasty, Dr. Weitz said.
Additionally, there is “considerable activity” with small molecule inhibitors of FXIa, including a phase 2, placebo-controlled, dose-ranging study looking at the novel JNG-7003/BMS-986177 agent for secondary stroke/transient ischemic attack prevention in 2,500 patients and a phase 2 study comparing it with enoxaparin for postoperative thromboprophylaxis in 1,200 patients undergoing total knee arthroplasty.
Parallel phase 2 studies are also underway to compare the novel BAY-2433334 small molecule inhibitor with placebo for stroke/transient ischemic attack prevention, with apixaban for atrial fibrillation, and for prevention of major adverse cardiovascular events in patients with acute MI.
These ongoing trials will help determine the risk-benefit profile of FXI inhibitors he said.
Session comoderator Anne Rose, PharmD, pharmacy coordinator at the University of Wisconsin, Madison, noted that these types of agents have been discussed “for quite some time” and asked whether they will be available for use in clinical practice in the near future.
Dr. Weitz predicted it will be at least a few years. The studies are just now moving to phase 2b and will still need to be evaluated in phase 3 trials and for appropriate new indications, he said.
Dr. Weitz reported research support from Canadian Institutes of Health research, Heart and Stroke Foundation, and Canadian Fund for Innovation, and he is a consultant and/or scientific advisory board member for Anthos, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Ionis Pharmaceuticals, Janssen, Merck, Novartis, Pfizer, Portola, Servier , and Thetherex.
FROM THE THSNA BIENNIAL SUMMIT
Pediatric News welcomes Dr. Lessin to the board
Dr. Lessin has been a practicing pediatric clinician for the past 39 years at The Children’s Medical Group. In 1997, he was a founding partner of one of the first private practice “supergroups” by merging two competing pediatric practices into one and expanding it to 25 clinicians, with eight offices in three counties in New York state’s Mid-Hudson Valley. The group provides pediatric care to more than 30,000 children and has a nearly 90-year history, across its various incarnations, providing such care.
Dr. Lessin received his medical degree from Stanford (Calif.) University and trained in pediatrics at Yale-New Haven (Conn.) Medical Center. He has been active in national policy and leadership in the American Academy of Pediatrics, having served on the executive committee of the Section of Administration and Practice Management, the national Committee on Practice and Ambulatory Medicine, and his current appointment to the national Private Payer Advocacy Advisory Committee. In those roles he has authored several national policy statements and clinical guidelines, including “Increasing Immunization Coverage,” “Immunizing Parents and Other Close Family Contacts in the Pediatric Office Setting,” “Instrument-Based Pediatric Vision Screening Policy Statement,” and most recently, “Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents.” He is also the coeditor of the AAP’s ADHD toolkit for clinicians published in 2019. Dr. Lessin served as the director of clinical research for his group for 5 years and the medical director for the practice for 10 years.
He has served as a faculty member at numerous local and regional pediatric meetings. He has been a faculty member at the AAP’s annual national conference and exposition for the past decade, speaking on a variety of topics. Dr. Lessin also has been an invited speaker internationally at pediatric conferences in India and Egypt. He has participated in more than a dozen medical missions to developing countries in Latin America, the Caribbean, Africa, and Vietnam. His expertise includes practice management, the business of medicine, immunizations, ADHD, and liability topics. He has been a testifying expert witness for both defense and plaintiff in medical malpractice litigation for more than 30 years. He founded and served as president of a medical independent practice association that began with 12 physicians and grew to over 3,000 doctors. He is also a certified managed care executive. His most recent interest has been becoming a professional voice-over actor!
While performing all of the above, Dr. Lessin is a dedicated community pediatrician whose first love and primary goal has remained providing the highest quality medical care to children while helping his colleagues manage their businesses in order to be able to survive and continue to provide such care.
Dr. Lessin has been a practicing pediatric clinician for the past 39 years at The Children’s Medical Group. In 1997, he was a founding partner of one of the first private practice “supergroups” by merging two competing pediatric practices into one and expanding it to 25 clinicians, with eight offices in three counties in New York state’s Mid-Hudson Valley. The group provides pediatric care to more than 30,000 children and has a nearly 90-year history, across its various incarnations, providing such care.
Dr. Lessin received his medical degree from Stanford (Calif.) University and trained in pediatrics at Yale-New Haven (Conn.) Medical Center. He has been active in national policy and leadership in the American Academy of Pediatrics, having served on the executive committee of the Section of Administration and Practice Management, the national Committee on Practice and Ambulatory Medicine, and his current appointment to the national Private Payer Advocacy Advisory Committee. In those roles he has authored several national policy statements and clinical guidelines, including “Increasing Immunization Coverage,” “Immunizing Parents and Other Close Family Contacts in the Pediatric Office Setting,” “Instrument-Based Pediatric Vision Screening Policy Statement,” and most recently, “Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents.” He is also the coeditor of the AAP’s ADHD toolkit for clinicians published in 2019. Dr. Lessin served as the director of clinical research for his group for 5 years and the medical director for the practice for 10 years.
He has served as a faculty member at numerous local and regional pediatric meetings. He has been a faculty member at the AAP’s annual national conference and exposition for the past decade, speaking on a variety of topics. Dr. Lessin also has been an invited speaker internationally at pediatric conferences in India and Egypt. He has participated in more than a dozen medical missions to developing countries in Latin America, the Caribbean, Africa, and Vietnam. His expertise includes practice management, the business of medicine, immunizations, ADHD, and liability topics. He has been a testifying expert witness for both defense and plaintiff in medical malpractice litigation for more than 30 years. He founded and served as president of a medical independent practice association that began with 12 physicians and grew to over 3,000 doctors. He is also a certified managed care executive. His most recent interest has been becoming a professional voice-over actor!
While performing all of the above, Dr. Lessin is a dedicated community pediatrician whose first love and primary goal has remained providing the highest quality medical care to children while helping his colleagues manage their businesses in order to be able to survive and continue to provide such care.
Dr. Lessin has been a practicing pediatric clinician for the past 39 years at The Children’s Medical Group. In 1997, he was a founding partner of one of the first private practice “supergroups” by merging two competing pediatric practices into one and expanding it to 25 clinicians, with eight offices in three counties in New York state’s Mid-Hudson Valley. The group provides pediatric care to more than 30,000 children and has a nearly 90-year history, across its various incarnations, providing such care.
Dr. Lessin received his medical degree from Stanford (Calif.) University and trained in pediatrics at Yale-New Haven (Conn.) Medical Center. He has been active in national policy and leadership in the American Academy of Pediatrics, having served on the executive committee of the Section of Administration and Practice Management, the national Committee on Practice and Ambulatory Medicine, and his current appointment to the national Private Payer Advocacy Advisory Committee. In those roles he has authored several national policy statements and clinical guidelines, including “Increasing Immunization Coverage,” “Immunizing Parents and Other Close Family Contacts in the Pediatric Office Setting,” “Instrument-Based Pediatric Vision Screening Policy Statement,” and most recently, “Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents.” He is also the coeditor of the AAP’s ADHD toolkit for clinicians published in 2019. Dr. Lessin served as the director of clinical research for his group for 5 years and the medical director for the practice for 10 years.
He has served as a faculty member at numerous local and regional pediatric meetings. He has been a faculty member at the AAP’s annual national conference and exposition for the past decade, speaking on a variety of topics. Dr. Lessin also has been an invited speaker internationally at pediatric conferences in India and Egypt. He has participated in more than a dozen medical missions to developing countries in Latin America, the Caribbean, Africa, and Vietnam. His expertise includes practice management, the business of medicine, immunizations, ADHD, and liability topics. He has been a testifying expert witness for both defense and plaintiff in medical malpractice litigation for more than 30 years. He founded and served as president of a medical independent practice association that began with 12 physicians and grew to over 3,000 doctors. He is also a certified managed care executive. His most recent interest has been becoming a professional voice-over actor!
While performing all of the above, Dr. Lessin is a dedicated community pediatrician whose first love and primary goal has remained providing the highest quality medical care to children while helping his colleagues manage their businesses in order to be able to survive and continue to provide such care.
Breakthroughs in Crohn's Disease From ACG 2020 Virtual
Miguel Regueiro, MD, an expert in gastroenterology at the Cleveland Clinic, offers his choice of the most important and clinically relevant studies on Crohn's disease presented at the American College of Gastroenterology 2020 virtual annual scientific meeting.
First he looks at studies reflecting three medical approaches to treating the disease. He initially reports on the CELEST open-label extension study examining the safety and efficacy of 2 years of upadacitinib treatment.
Then he discusses the IM-UNITI long-term extension study, which took treatment with ustekinumab out to 5 years, the longest reported duration for an anti-IL-12/23 treatment.
Finally, he looks at a retrospective cohort study of the combination of vedolizumab and ustekinumab, which found that this may be an effective option for patients with refractory disease.
Switching gears, Dr Regueiro focuses on surgery-related studies, presenting an analysis of whether the type of surgical anastomosis influences long-term outcomes and opioid requirement.
Next up is a study of whether prior surgical history is the strongest predictor of postoperative Crohn's disease recurrence.
The last abstract he discusses examines whether preoperative medication exposure is associated with postoperative complications in patients undergoing ileocolic resection. The findings indicate that preoperative treatment should not be seen as a reason to delay surgery.
Miguel D. Regueiro, MD, Chairman, Professor, Department of Gastroenterology, Hepatology, and Nutrition; Vice-Chair, Digestive Disease Institute, Cleveland Clinic, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio.
Miguel D. Regueiro, MD, has disclosed the following relevant financial relationships: Serve(d) as an advisor and/or consultant for: AbbVie; Janssen; UCB; Takeda; Pfizer; Miraca Labs; Amgen; Celgene; Seres; Allergan; Genentech; Gilead; Salix; Prometheus. Received unrestricted educational grants from: AbbVie; Janssen; UCB; Pfizer; Takeda; Salix; Shire. Received research support from AbbVie; Janssen; Takeda; Pfizer.
Miguel Regueiro, MD, an expert in gastroenterology at the Cleveland Clinic, offers his choice of the most important and clinically relevant studies on Crohn's disease presented at the American College of Gastroenterology 2020 virtual annual scientific meeting.
First he looks at studies reflecting three medical approaches to treating the disease. He initially reports on the CELEST open-label extension study examining the safety and efficacy of 2 years of upadacitinib treatment.
Then he discusses the IM-UNITI long-term extension study, which took treatment with ustekinumab out to 5 years, the longest reported duration for an anti-IL-12/23 treatment.
Finally, he looks at a retrospective cohort study of the combination of vedolizumab and ustekinumab, which found that this may be an effective option for patients with refractory disease.
Switching gears, Dr Regueiro focuses on surgery-related studies, presenting an analysis of whether the type of surgical anastomosis influences long-term outcomes and opioid requirement.
Next up is a study of whether prior surgical history is the strongest predictor of postoperative Crohn's disease recurrence.
The last abstract he discusses examines whether preoperative medication exposure is associated with postoperative complications in patients undergoing ileocolic resection. The findings indicate that preoperative treatment should not be seen as a reason to delay surgery.
Miguel D. Regueiro, MD, Chairman, Professor, Department of Gastroenterology, Hepatology, and Nutrition; Vice-Chair, Digestive Disease Institute, Cleveland Clinic, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio.
Miguel D. Regueiro, MD, has disclosed the following relevant financial relationships: Serve(d) as an advisor and/or consultant for: AbbVie; Janssen; UCB; Takeda; Pfizer; Miraca Labs; Amgen; Celgene; Seres; Allergan; Genentech; Gilead; Salix; Prometheus. Received unrestricted educational grants from: AbbVie; Janssen; UCB; Pfizer; Takeda; Salix; Shire. Received research support from AbbVie; Janssen; Takeda; Pfizer.
Miguel Regueiro, MD, an expert in gastroenterology at the Cleveland Clinic, offers his choice of the most important and clinically relevant studies on Crohn's disease presented at the American College of Gastroenterology 2020 virtual annual scientific meeting.
First he looks at studies reflecting three medical approaches to treating the disease. He initially reports on the CELEST open-label extension study examining the safety and efficacy of 2 years of upadacitinib treatment.
Then he discusses the IM-UNITI long-term extension study, which took treatment with ustekinumab out to 5 years, the longest reported duration for an anti-IL-12/23 treatment.
Finally, he looks at a retrospective cohort study of the combination of vedolizumab and ustekinumab, which found that this may be an effective option for patients with refractory disease.
Switching gears, Dr Regueiro focuses on surgery-related studies, presenting an analysis of whether the type of surgical anastomosis influences long-term outcomes and opioid requirement.
Next up is a study of whether prior surgical history is the strongest predictor of postoperative Crohn's disease recurrence.
The last abstract he discusses examines whether preoperative medication exposure is associated with postoperative complications in patients undergoing ileocolic resection. The findings indicate that preoperative treatment should not be seen as a reason to delay surgery.
Miguel D. Regueiro, MD, Chairman, Professor, Department of Gastroenterology, Hepatology, and Nutrition; Vice-Chair, Digestive Disease Institute, Cleveland Clinic, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio.
Miguel D. Regueiro, MD, has disclosed the following relevant financial relationships: Serve(d) as an advisor and/or consultant for: AbbVie; Janssen; UCB; Takeda; Pfizer; Miraca Labs; Amgen; Celgene; Seres; Allergan; Genentech; Gilead; Salix; Prometheus. Received unrestricted educational grants from: AbbVie; Janssen; UCB; Pfizer; Takeda; Salix; Shire. Received research support from AbbVie; Janssen; Takeda; Pfizer.

Comparison of Resident, Advanced Practice Clinician, and Hospitalist Teams in an Academic Medical Center: Association With Clinical Outcomes and Resource Utilization
The Accreditation Council for Graduate Medical Education (ACGME) first mandated residency work hour restrictions in 2003.1 In 2011, revised work hour requirements were issued, further limiting the maximum duration of a shift and extending the duration of time off between scheduled shifts.2 Academic medical centers have been forced to adapt to work hour restrictions, and cuts in funding to research and educational missions have pressured institutions to restructure with a greater focus on high-quality, lower-cost care.3,4 In response, many academic hospitals have added hospitalist teams, or incorporated advanced practice clinicians (APCs) (nurse practitioners [NPs] and physician assistants [PAs]) to accommodate resident physician duty hour restrictions on their inpatient general medicine services.5,6 More recently, the COVID-19 pandemic has created unanticipated physician shortages forcing medical centers to rapidly expand and broaden the scope of their existing APC workforce.7
Several comparisons of clinical outcomes, cost, and patient satisfaction between different combinations of hospitalist-based, resident-based, or APC-based inpatient teams have been reported with conflicting observations.6,8-14 Roy et al reported no significant differences in mortality, length of stay (LOS), or readmissions between PA and resident teams.6 Timmermans et al reported similar cost-effectiveness, LOS, and quality of care between PA and physician teams that included a hybrid of attending only and resident teams.13,14 Alternatively, Singh et al and Iannuzzi et al reported increased LOS among PA teams,10,12 whereas Chin et al observed an increased LOS and reduced 30-day readmissions among hospitalist teams.8 While these observed differences may be attributable to heterogeneous patient populations or institution-specific team structure, the exact reasons remain unknown. Furthermore, understanding the value of alternate staffing models is essential for medical centers to prepare for potential COVID-19 related physician shortages. To our knowledge, no study to date has directly compared outcomes between resident, APC, and hospitalist team structures within an academic medical center.
We believe our institution provides a unique environment to study the differences in inpatient general medicine team structure with respect to quality and efficiency of care delivery. The objective of our study is to directly compare clinical outcomes and resource utilization among three distinct team structures: APC, resident, and solo hospitalist. We hypothesize that clinical outcomes, cost, and utilization of consult services will be similar across all team structures and hospitalist teams will discharge patients earlier than resident and APC teams.
METHODS
Study Design and Setting
We conducted a retrospective observational cohort study at the University of Utah Medical Center, a 548-bed academic medical center in Salt Lake City. An electronic database query was used to identify all patients discharged from the inpatient general internal medicine service between July 1, 2015, and July 1, 2018. Baseline patient characteristics were collected including age, gender, and Charlson comorbidity index (CCI).15 Case-mix index was determined for admissions where a Medicare Severity Diagnosis Related Group (MS-DRG) and corresponding weight was assigned.16,17 Source of admission was collected to identify patients transferred from an outside hospital, typically due to increased medical complexity or need for specialty care not available at the referring center. Time of admission was collected to classify whether a patient was admitted during the day or at night. Length of stay was calculated as the difference between discharge date/time and admission date/time. Discharge order time was collected as a measure of clinician efficiency. The number of consults per admission was determined by the number of different medical or surgical subspecialty services that wrote at least one consultation or progress note after the time of admission and were not the primary service at the time the note was written. The project was reviewed and deemed exempt by the University of Utah Institutional Review Board (IRB 00104884).
Inpatient Care Team Structure
Patients were assigned to one of three cohorts dependent on the assigned treatment team at the time of discharge. The three inpatient team structures were as follows: (1) a “resident team” composed of a senior resident (postgraduate year [PGY] 2 or PGY3) and one to two medical students or one senior resident, two interns (PGY1), and one to two medical students supervised by a hospitalist physician; (2) an “APC team” composed of one to two APCs supervised by a hospitalist physician; and (3) a “hospitalist team” composed of one attending hospitalist independently managing all patients.
Advanced Practice Clinicians
The APC service included 10 APCs (8 PAs and 2 NPs), with a combined workforce of nine APC full-time equivalents during the study period. Their experience ranged from new graduate to 11 years of clinical experience, with an average of 4.2 years. Among the 6 APCs with prior clinical experience, the majority (86%) of their years of clinical experience were within inpatient medicine, oncology, or cardiology. Recognizing the variability in clinical experience, we employed a rigorous onboarding program that entailed an average of 80 hours of didactic sessions including 1:1 teaching of the inpatient Society of Hospital Medicine core lecture series combined with initial intense clinical oversight.18 This program ranged from 2 weeks to 6 weeks depending on the individual APC’s clinical experience, progress, and comfort working independently. This onboarding program has subsequently been formalized into a 1-year APC fellowship that began after the study period concluded.
The degree of autonomy for each APC was individualized based on their clinical experience and ability to recognize limitations such as medical decision-making, clinical knowledge, and effective use of interprofessional team members (eg, peers, nursing, ancillary staff, consultants, and support personnel). Those APCs who demonstrated a sufficient level of clinical competence functioned with a high level of autonomy. During the day, APCs were expected to be the first point of contact for interprofessional team members, to respond to acute clinical changes in a patient’s condition, and to discuss active issues with the supervising attending, all with the majority of medical decision-making, direct patient communication, documentation, and care coordination performed by the APC. An experienced subset of the APC service was responsible for overnight coverage. Nocturnist APCs independently managed all cross-cover issues on patients assigned to APC and hospitalist teams and performed admissions with very little to no direct supervision of the overnight attending physician.
Patient Admission and Redistribution Process
During the study period, resident teams performed all daytime admissions (6
Study Outcomes
We divided study outcomes into two categories, clinical outcomes and resource utilization. Clinical outcomes included LOS, unplanned readmission within 30-days, and inpatient mortality and were designed to measure patient-related outcomes as a reflection of the quality of care delivered by different team structures. Resource utilization included discharge order time, discharge time, consults per admission, and total direct cost, which were designed to measure provider-related differences in efficiency and cost of care.
Statistical analysis
Baseline characteristics and unadjusted outcomes are reported as frequency and percent, normally distributed variables as mean with SD, and nonnormally distributed variables as median with interquartile range (IQR). Baseline characteristics and unadjusted outcomes were compared using the chi-square test or the t test, where appropriate. Multivariable regression analysis using generalized linear models with a log link function and gamma distribution was used for continuous outcomes. Multivariable logistic regression was used for binary outcomes.10 Covariates included in regression models were age, gender, CCI, transfer from an outside hospital, and nighttime admission. In a sensitivity analysis, we included MS-DRG weight as a covariate for 85% of hospitalizations in our cohort exclusive of observation stays, and our findings were qualitatively similar (data not reported but available on request). Adjusted continuous outcomes were estimated using marginal effects at the means.19 Due to the sensitivity of cost data and an institutional policy against disclosing cost figures, total direct costs were normalized using the unadjusted median and adjusted mean total direct cost of an admission to an APC team as the normalizing value. A P value cutoff of .05 was used to determine statistical significance. Stata/IC version 16.1 (StataCorp) was used for all analyses.
RESULTS
Study Population
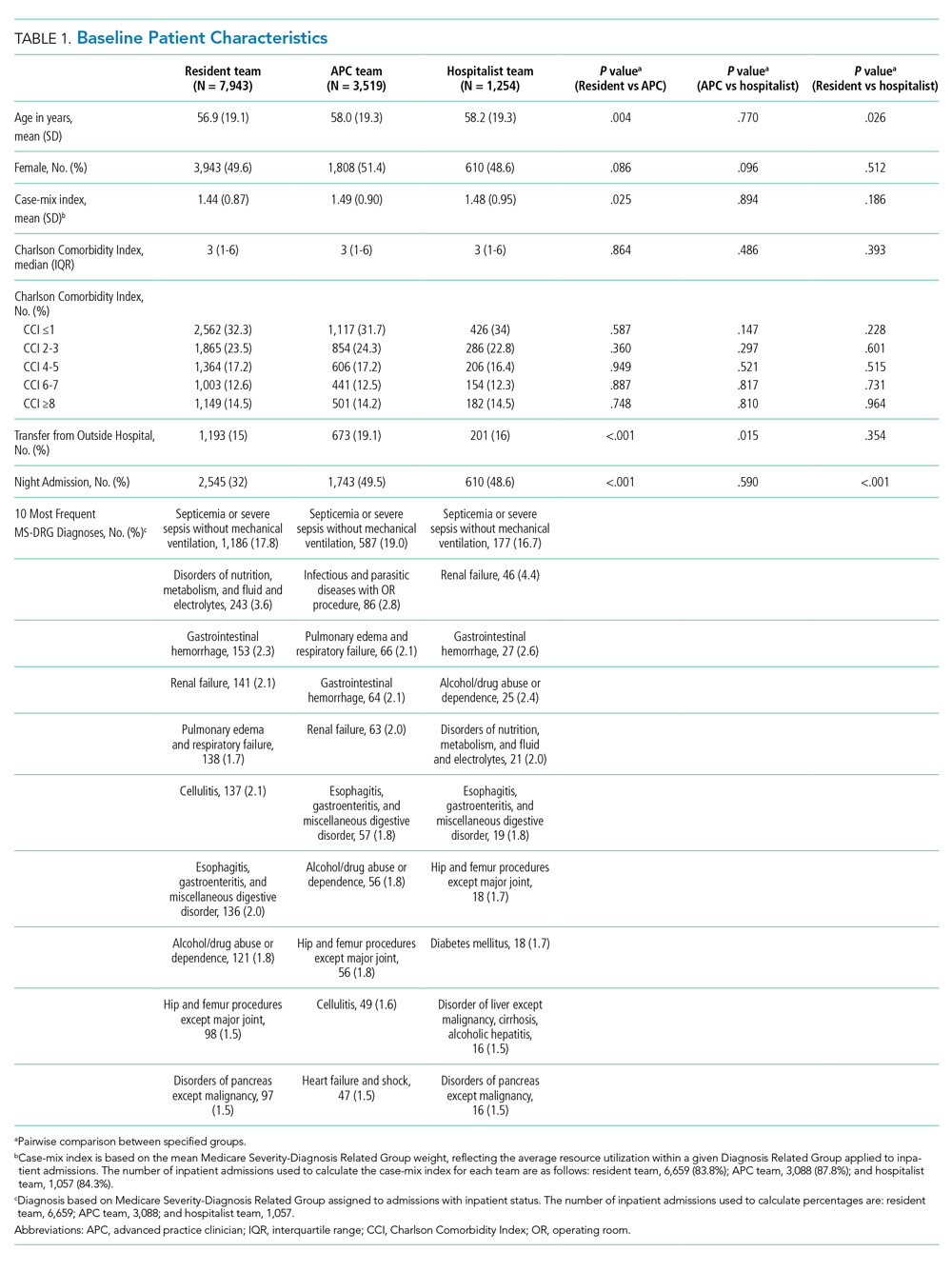
A total of 12,716 hospital admissions were identified during the study period. Of these, 7,943 (62.5%) admissions were assigned to a resident team, 3,519 (27.7%) admissions were assigned to an APC team, and the remaining 1,254 (9.9%) were assigned to a hospitalist team. Baseline patient characteristics are reported in Table 1. Patients admitted to resident teams (mean age [SD], 56.9 [19.1] years) were younger than those admitted to an APC team (58.0 [19.3] years; P = .004) or a hospitalist team (58.2 [19.3] years; P = .026). The case-mix index (mean MS-DRG weight [SD], 1.44 [0.87]) was slightly lower for resident teams than that for APC teams (1.49 [0.90]; P = .025).Resident teams had a significantly lower proportion of night admissions than did APC teams (32.0% vs 49.5%; P < .001) and hospitalist teams (48.6%; P < .001). APC teams were assigned more patients transferred from an outside hospital (19.1%), compared with resident teams (15.0%; P < .001) and hospitalist teams (16.0%; P = .015). No other significant differences were observed in baseline characteristics between cohorts.
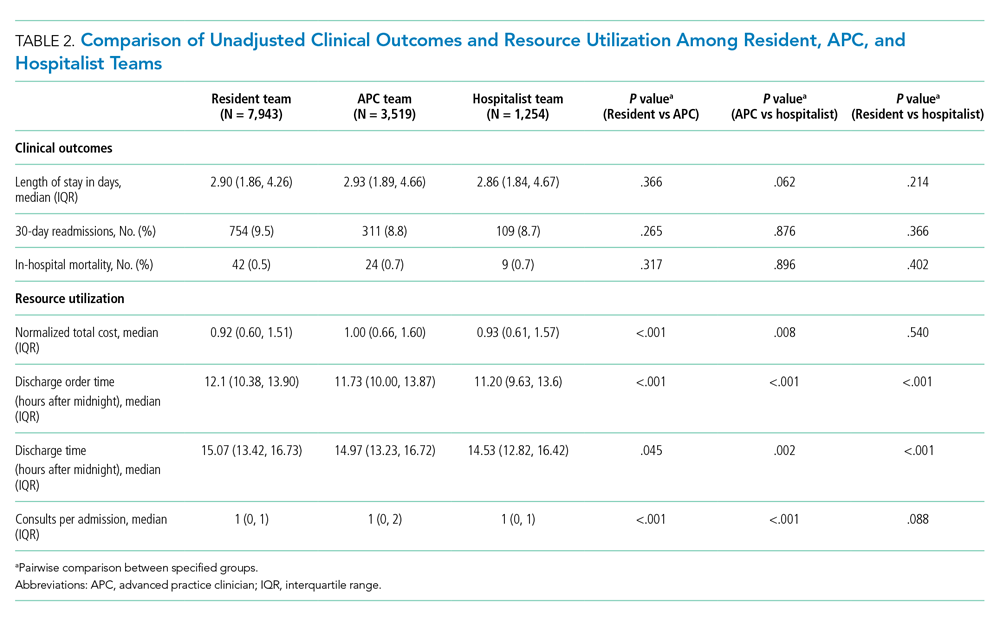
Clinical Outcomes
Unadjusted analysis demonstrated the LOS was similar among resident, APC, and hospitalist teams with a median (IQR) LOS of 2.90 (1.86, 4.26) days, 2.93 (1.89, 4.66) days, and 2.86 (1.84, 4.67) days, respectively. No significant differences were observed in unadjusted 30-day readmissions or inpatient mortality among the team structures (Table 2). Following multivariable adjustment for differences in baseline characteristics, no significant differences were observed in LOS, 30-day readmission, or inpatient mortality among teams (Table 3).
Resource Utilization
In unadjusted comparisons, hospitalist teams were observed to place discharge orders more than 30 minutes earlier than APC teams (median hours after midnight [IQR], 11.20 [9.63, 13.60] vs 11.73 [10.00, 13.87]; P < .001) and 54 minutes earlier than resident teams (12.10 [10.38, 13.90]; P < .001) (Table 2). Consistent with the earlier placement of discharge orders, hospitalist patients were also discharged from the hospital 26 and 32 minutes earlier than APC and resident patients, respectively. APC teams also discharged patients slightly earlier (6 minutes) than resident teams (median hours after midnight [IQR], 14.97 [13.23, 16.72] vs 15.07 [13.42, 16.73]; P = .045). Median consultation use among teams was similar, although statistically significant differences were present. Normalized total direct cost was 8% higher (P < .001) for admissions to APC teams than that for resident teams and 7% higher (P = .008) than that for hospitalist teams in unadjusted analysis (Table 2).
Following multivariable adjustment, the mean differences in discharge order time and discharge time remained significant with hospitalist teams discharging patients an average of 20 to 30 minutes earlier than APC and resident teams (Table 3). Consultant utilization remained significantly different between teams, with APC teams utilizing consultants on average 15% more than hospitalist teams (P < .001) and 7% more than resident teams (P = .001). The differences in total direct costs were not significant after adjusted analysis.

DISCUSSION
Many academic medical centers have expanded their workforce with APC or nonteaching hospitalist teams to accommodate the increasing volume of hospital admissions, resident work hour restrictions,1,2 and medical complexity of an aging population. Several hospitals have reported comparative outcomes between different care delivery models, with conflicting results.6,8,10-12 In our study, we directly evaluated three inpatient care delivery models and found that hospitalist teams discharged patients more efficiently and utilized fewer consultants, compared with APC and resident teams. In spite of this improved efficiency, no significant differences were observed in cost or other clinical outcomes.
Our findings are important and further strengthen the evidence supporting the use of APCs on inpatient general medicine services and are of particular interest to academic centers struggling to expand staffing in order to offset the growth in patient volume and reduction in resident workforce. We believe several findings from our study warrant further discussion.
First, although hospitalist teams were able to discharge patients more efficiently, this observation may be influenced by factors of workflow rather than caused by significant disparities in efficiency between provider types (ie, APC vs hospitalist vs resident physician). As with most academic centers, patients assigned to resident teams are presented by house staff to an attending physician who is ultimately responsible for patient care decisions. Therefore, it is conceivable that delays in the discharge process are in part related to the convention of bedside rounding and discussing the care plan prior to discharge.20 In fact, we recognized this as a bottleneck and changed our discharge process for resident teams in June 2017, with a measurable improvement in discharge times. In the absence of this intervention, our observed differences in discharge times among teams may have been even greater.
Second, no significant differences in clinical outcomes were observed in our adjusted analyses, which suggests that a similar quality of care is delivered to patients regardless of team structure, an important observation when considering different staffing models.
Third, we observed a significant increase in consultation use among resident and APC teams, compared with hospitalists. While we are not able to precisely identify the basis for this variation, we believe it could reflect differences in clinical experience, comfort with diagnostic uncertainty, or the unequal distribution of patients transferred from outside hospitals for tertiary care. Interestingly, the greater consultation use did not correlate with higher healthcare costs, a finding recently reported by Stevens et al.21
Fourth, we believe the lack of differences in cost and clinical outcomes among team structures may be of particular interest to academic centers when considering physician burnout, salaries, and clinical education. The relationship between clerical burden, such as completing clinical documentation and computerized physician order entry, has been implicated as a risk factor for physician burnout.22 Incorporating APCs into roles similar to those performed by resident physicians may reduce the clerical burden on hospitalists, thereby reducing the risk of physician burnout. The addition of APCs may also represent opportunities for cost savings for healthcare centers when comparing the median salary of an APC to that of an internal medicine hospitalist.23,24 Moreover, academic hospitalists have been shown to be excellent medical educators and report increased job satisfaction with a variety of duties beyond direct patient care.24,25 Unforeseen benefits of adding APC teams within our institution has been the added teaching opportunities for APCs and APC students, increased collegiality with the APCs, and the creation of an APC fellowship program with a focus on inpatient medicine. Similar postgraduate training programs have been reported and serve as effective models to train APCs for hospital-based practice.26
Lastly, although this project was conceived and completed prior to the COVID-19 pandemic, our observations may be informative for medical centers experiencing a workforce shortage caused by a surge of COVID-19 patients. During a physician shortage we believe our APC team model could be rapidly expanded to accommodate a large influx of patients. This expansion could be accomplished through a single attending physician overseeing multiple APC teams. In this model, the supervising physician would only evaluate the most complex patients with most patients being managed solely by an APC from admission to discharge. Such changes may require temporary suspension of state laws restricting APC independent practice.27,28
Our findings contrast those of previous reports in that we did not observe significant differences in clinical outcomes (ie, LOS, inpatient mortality, and 30-day readmissions) or total direct cost.8,10,21 Other institutions have noted an increased LOS among APC teams and hospitalist teams, compared with resident teams.8,10 Furthermore, Chin et al and Iannuzzi et al reported reductions in healthcare cost for resident teams, whereas our study did not identify significant cost differences among team structures. Although we cannot pinpoint the exact reason(s) for these dissimilarities, it is plausible that unmeasured factors such as institutional differences in APC training, direct physician supervision, admission processes, or inpatient team census may play a role.
Several study limitations should be recognized. First, the retrospective, nonrandomized design is one of the largest limitations of our study. Administrative data was obtained via an electronic query of our data warehouse, and although we aimed to identify as many patient characteristics as possible to adjust for cofounding effects, undetected differences among cohorts may exist. Second, our inpatient admission process may have placed undue burden on resident teams to perform all daytime admissions, inadvertently affecting study outcomes. It is possible the observed benefits of a solo hospitalist team are attributable to the lack of admitting duties rather than inherent advantages of the team structure. If this were the case, we would expect similar benefits among APC teams, which we did not note. Third, the study was performed at a single academic center, which may limit the generalizability of our results. Fourth, it is possible the outcomes are similar among teams because our hospitalist faculty rotate proportionately between the different teams. Lastly, the study was underpowered to detect a significant difference in mortality between hospitalist and APC teams. A post hoc power calculation based on our observed sample and effect sizes estimated 75% power to detect a mortality difference between hospitalists and APCs; other mortality comparisons were adequately powered.
CONCLUSION
We observed similar total direct costs, LOS, 30-day readmission, and inpatient mortality between hospitalist, APC, and resident teams. APC and resident teams utilized more consultants and discharged patient later than hospitalists. Our analysis suggests clinical outcomes are not significantly affected by inpatient team structure, and the addition of general medicine inpatient APC or hospitalist teams represent safe and efficient alternatives to traditional resident teams within an academic medical center.
Disclosures
All authors declare they have no conflicts of interest.
1. Report of the Work Group on Resident Duty Hours and the Learning Environment, June 11, 2002. Accreditation Council for Graduate Medical Education; 2003.
2. ACGME Task Force on Quality Care and Professionalism. Philibert I, Amis Steve, eds. The ACGME 2011 Duty Hour Standards: Enhancing Quality of Care, Supervision, and Resident Professional Development. Accreditation Council for Graduate Medical Education; 2011. https://www.acgme.org/Portals/0/PDFs/jgme-monograph[1].pdf
3. Konstam MA, Hill JA, Kovacs RJ, et al. The academic medical system: reinvention to survive the revolution in health care. J Am Coll Cardiol. 2017;69(10):1305-1312. https://doi.org/10.1016/j.jacc.2016.12.024
4. The future of the academic medical center: strategies to avoid a margin meltdown. Health Research Institute. February 2012. https://uofuhealth.utah.edu/hcr/2012/resources/the-future-of-academic-medical-centers.pdf
5. Moote M, Krsek C, Kleinpell R, Todd B. Physician assistant and nurse practitioner utilization in academic medical centers. Am J Med Qual. 2019;34(5):465-472. https://doi.org/ 10.1177/1062860619873216
6. Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368. https://doi.org/10.1002/jhm.352
7. Denne E. Behind the scenes at Northwell Health as PAs respond to COVID-19. American Academy of Physician Assistants. May 11, 2020. Accessed May 15, 2020. https://www.aapa.org/news-central/2020/05/behind-the-scenes-at-northwell-heath-as-pas-respond-to-covid-19/
8. Chin DL, Wilson MH, Bang H, Romano PS. Comparing patient outcomes of academician-preceptors, hospitalist-preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672-1678. https://doi.org/10.1007/s11606-014-2982-y
9. Cowan MJ, Shapiro M, Hays RD, et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79-85. https://doi.org/10.1097/00005110-200602000-00006
10. Iannuzzi MC, Iannuzzi JC, Holtsbery A, Wright SM, Knohl SJ. Comparing hospitalist-resident to hospitalist-midlevel practitioner team performance on length of stay and direct patient care cost. J Grad Med Educ. 2015;7(1):65-69. https://doi.org/10.4300/jgme-d-14-00234.1
11. Kapu AN, Kleinpell R, Pilon B. Quality and financial impact of adding nurse practitioners to inpatient care teams. J Nurs Adm. 2014;44(2):87-96. https://doi.org/10.1097/nna.0000000000000031
12. Singh S, Fletcher KE, Schapira MM, et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist-physician assistant model vs a traditional resident-based model. J Hosp Med. 2011;6(3):122-130. https://doi.org/10.1002/jhm.826
13. Timmermans MJC, van Vught A, Peters YAS, et al. The impact of the implementation of physician assistants in inpatient care: a multicenter matched-controlled study. PLoS One. 2017;12(8):e0178212. https://doi.org/10.1371/journal.pone.0178212
14. Timmermans MJC, van den Brink GT, van Vught A, et al. The involvement of physician assistants in inpatient care in hospitals in the Netherlands: a cost-effectiveness analysis. BMJ Open. 2017;7(7):e016405. https://doi.org/10.1136/bmjopen-2017-016405
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8
16. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. 2020. Updated April 28, 2020. Accessed May 5, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
17. Fetter RB, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis-related groups. Med Care. 1980;18(2 Suppl):iii, 1-53.
18. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
19. Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308-331. https://doi.org/10.1177%2F1536867X1201200209
20. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
21. Stevens JP, Hatfield LA, Nyweide DJ, Landon B. Association of variation in consultant use among hospitalist physicians with outcomes among Medicare beneficiaries. JAMA Netw Open. 2020;3(2):e1921750. https://doi.org/10.1001/jamanetworkopen.2019.21750
22. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91(7):836-848. https://doi.org/10.1016/j.mayocp.2016.05.007
23. 2019 AAPA Salary Report. American Academy of PAs. 2019. https://www.aapa.org/shop/salary-report-2019/
24. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
25. Dalen JE, Ryan KJ, Waterbrook AL, Alpert JS. Hospitalists, medical education, and US health care costs. Am J Med. 2018;131(11):1267-1269. https://doi.org/10.1016/j.amjmed.2018.05.016
26. Will KK, Budavari AI, Wilkens JA, Mishark K, Hartsell ZC. A hospitalist postgraduate training program for physician assistants. J Hosp Med. 2010;5(2):94-98. https://doi.org/10.1002/jhm.619
27. Utah Physician Assistant Act. Utah Code. Published 2019. Accessed May 8, 2020. https://le.utah.gov/xcode/Title58/Chapter70A/C58-70a_2019051420190514.pdf
28. Nurse Practice Act. Utah Code. Published 2019. Accessed May 8, 2020. https://le.utah.gov/xcode/Title58/Chapter31B/C58-31b_1800010118000101.pdf
The Accreditation Council for Graduate Medical Education (ACGME) first mandated residency work hour restrictions in 2003.1 In 2011, revised work hour requirements were issued, further limiting the maximum duration of a shift and extending the duration of time off between scheduled shifts.2 Academic medical centers have been forced to adapt to work hour restrictions, and cuts in funding to research and educational missions have pressured institutions to restructure with a greater focus on high-quality, lower-cost care.3,4 In response, many academic hospitals have added hospitalist teams, or incorporated advanced practice clinicians (APCs) (nurse practitioners [NPs] and physician assistants [PAs]) to accommodate resident physician duty hour restrictions on their inpatient general medicine services.5,6 More recently, the COVID-19 pandemic has created unanticipated physician shortages forcing medical centers to rapidly expand and broaden the scope of their existing APC workforce.7
Several comparisons of clinical outcomes, cost, and patient satisfaction between different combinations of hospitalist-based, resident-based, or APC-based inpatient teams have been reported with conflicting observations.6,8-14 Roy et al reported no significant differences in mortality, length of stay (LOS), or readmissions between PA and resident teams.6 Timmermans et al reported similar cost-effectiveness, LOS, and quality of care between PA and physician teams that included a hybrid of attending only and resident teams.13,14 Alternatively, Singh et al and Iannuzzi et al reported increased LOS among PA teams,10,12 whereas Chin et al observed an increased LOS and reduced 30-day readmissions among hospitalist teams.8 While these observed differences may be attributable to heterogeneous patient populations or institution-specific team structure, the exact reasons remain unknown. Furthermore, understanding the value of alternate staffing models is essential for medical centers to prepare for potential COVID-19 related physician shortages. To our knowledge, no study to date has directly compared outcomes between resident, APC, and hospitalist team structures within an academic medical center.
We believe our institution provides a unique environment to study the differences in inpatient general medicine team structure with respect to quality and efficiency of care delivery. The objective of our study is to directly compare clinical outcomes and resource utilization among three distinct team structures: APC, resident, and solo hospitalist. We hypothesize that clinical outcomes, cost, and utilization of consult services will be similar across all team structures and hospitalist teams will discharge patients earlier than resident and APC teams.
METHODS
Study Design and Setting
We conducted a retrospective observational cohort study at the University of Utah Medical Center, a 548-bed academic medical center in Salt Lake City. An electronic database query was used to identify all patients discharged from the inpatient general internal medicine service between July 1, 2015, and July 1, 2018. Baseline patient characteristics were collected including age, gender, and Charlson comorbidity index (CCI).15 Case-mix index was determined for admissions where a Medicare Severity Diagnosis Related Group (MS-DRG) and corresponding weight was assigned.16,17 Source of admission was collected to identify patients transferred from an outside hospital, typically due to increased medical complexity or need for specialty care not available at the referring center. Time of admission was collected to classify whether a patient was admitted during the day or at night. Length of stay was calculated as the difference between discharge date/time and admission date/time. Discharge order time was collected as a measure of clinician efficiency. The number of consults per admission was determined by the number of different medical or surgical subspecialty services that wrote at least one consultation or progress note after the time of admission and were not the primary service at the time the note was written. The project was reviewed and deemed exempt by the University of Utah Institutional Review Board (IRB 00104884).
Inpatient Care Team Structure
Patients were assigned to one of three cohorts dependent on the assigned treatment team at the time of discharge. The three inpatient team structures were as follows: (1) a “resident team” composed of a senior resident (postgraduate year [PGY] 2 or PGY3) and one to two medical students or one senior resident, two interns (PGY1), and one to two medical students supervised by a hospitalist physician; (2) an “APC team” composed of one to two APCs supervised by a hospitalist physician; and (3) a “hospitalist team” composed of one attending hospitalist independently managing all patients.
Advanced Practice Clinicians
The APC service included 10 APCs (8 PAs and 2 NPs), with a combined workforce of nine APC full-time equivalents during the study period. Their experience ranged from new graduate to 11 years of clinical experience, with an average of 4.2 years. Among the 6 APCs with prior clinical experience, the majority (86%) of their years of clinical experience were within inpatient medicine, oncology, or cardiology. Recognizing the variability in clinical experience, we employed a rigorous onboarding program that entailed an average of 80 hours of didactic sessions including 1:1 teaching of the inpatient Society of Hospital Medicine core lecture series combined with initial intense clinical oversight.18 This program ranged from 2 weeks to 6 weeks depending on the individual APC’s clinical experience, progress, and comfort working independently. This onboarding program has subsequently been formalized into a 1-year APC fellowship that began after the study period concluded.
The degree of autonomy for each APC was individualized based on their clinical experience and ability to recognize limitations such as medical decision-making, clinical knowledge, and effective use of interprofessional team members (eg, peers, nursing, ancillary staff, consultants, and support personnel). Those APCs who demonstrated a sufficient level of clinical competence functioned with a high level of autonomy. During the day, APCs were expected to be the first point of contact for interprofessional team members, to respond to acute clinical changes in a patient’s condition, and to discuss active issues with the supervising attending, all with the majority of medical decision-making, direct patient communication, documentation, and care coordination performed by the APC. An experienced subset of the APC service was responsible for overnight coverage. Nocturnist APCs independently managed all cross-cover issues on patients assigned to APC and hospitalist teams and performed admissions with very little to no direct supervision of the overnight attending physician.
Patient Admission and Redistribution Process
During the study period, resident teams performed all daytime admissions (6
Study Outcomes
We divided study outcomes into two categories, clinical outcomes and resource utilization. Clinical outcomes included LOS, unplanned readmission within 30-days, and inpatient mortality and were designed to measure patient-related outcomes as a reflection of the quality of care delivered by different team structures. Resource utilization included discharge order time, discharge time, consults per admission, and total direct cost, which were designed to measure provider-related differences in efficiency and cost of care.
Statistical analysis
Baseline characteristics and unadjusted outcomes are reported as frequency and percent, normally distributed variables as mean with SD, and nonnormally distributed variables as median with interquartile range (IQR). Baseline characteristics and unadjusted outcomes were compared using the chi-square test or the t test, where appropriate. Multivariable regression analysis using generalized linear models with a log link function and gamma distribution was used for continuous outcomes. Multivariable logistic regression was used for binary outcomes.10 Covariates included in regression models were age, gender, CCI, transfer from an outside hospital, and nighttime admission. In a sensitivity analysis, we included MS-DRG weight as a covariate for 85% of hospitalizations in our cohort exclusive of observation stays, and our findings were qualitatively similar (data not reported but available on request). Adjusted continuous outcomes were estimated using marginal effects at the means.19 Due to the sensitivity of cost data and an institutional policy against disclosing cost figures, total direct costs were normalized using the unadjusted median and adjusted mean total direct cost of an admission to an APC team as the normalizing value. A P value cutoff of .05 was used to determine statistical significance. Stata/IC version 16.1 (StataCorp) was used for all analyses.
RESULTS
Study Population
A total of 12,716 hospital admissions were identified during the study period. Of these, 7,943 (62.5%) admissions were assigned to a resident team, 3,519 (27.7%) admissions were assigned to an APC team, and the remaining 1,254 (9.9%) were assigned to a hospitalist team. Baseline patient characteristics are reported in Table 1. Patients admitted to resident teams (mean age [SD], 56.9 [19.1] years) were younger than those admitted to an APC team (58.0 [19.3] years; P = .004) or a hospitalist team (58.2 [19.3] years; P = .026). The case-mix index (mean MS-DRG weight [SD], 1.44 [0.87]) was slightly lower for resident teams than that for APC teams (1.49 [0.90]; P = .025).Resident teams had a significantly lower proportion of night admissions than did APC teams (32.0% vs 49.5%; P < .001) and hospitalist teams (48.6%; P < .001). APC teams were assigned more patients transferred from an outside hospital (19.1%), compared with resident teams (15.0%; P < .001) and hospitalist teams (16.0%; P = .015). No other significant differences were observed in baseline characteristics between cohorts.

Clinical Outcomes
Unadjusted analysis demonstrated the LOS was similar among resident, APC, and hospitalist teams with a median (IQR) LOS of 2.90 (1.86, 4.26) days, 2.93 (1.89, 4.66) days, and 2.86 (1.84, 4.67) days, respectively. No significant differences were observed in unadjusted 30-day readmissions or inpatient mortality among the team structures (Table 2). Following multivariable adjustment for differences in baseline characteristics, no significant differences were observed in LOS, 30-day readmission, or inpatient mortality among teams (Table 3).

Resource Utilization
In unadjusted comparisons, hospitalist teams were observed to place discharge orders more than 30 minutes earlier than APC teams (median hours after midnight [IQR], 11.20 [9.63, 13.60] vs 11.73 [10.00, 13.87]; P < .001) and 54 minutes earlier than resident teams (12.10 [10.38, 13.90]; P < .001) (Table 2). Consistent with the earlier placement of discharge orders, hospitalist patients were also discharged from the hospital 26 and 32 minutes earlier than APC and resident patients, respectively. APC teams also discharged patients slightly earlier (6 minutes) than resident teams (median hours after midnight [IQR], 14.97 [13.23, 16.72] vs 15.07 [13.42, 16.73]; P = .045). Median consultation use among teams was similar, although statistically significant differences were present. Normalized total direct cost was 8% higher (P < .001) for admissions to APC teams than that for resident teams and 7% higher (P = .008) than that for hospitalist teams in unadjusted analysis (Table 2).
Following multivariable adjustment, the mean differences in discharge order time and discharge time remained significant with hospitalist teams discharging patients an average of 20 to 30 minutes earlier than APC and resident teams (Table 3). Consultant utilization remained significantly different between teams, with APC teams utilizing consultants on average 15% more than hospitalist teams (P < .001) and 7% more than resident teams (P = .001). The differences in total direct costs were not significant after adjusted analysis.

DISCUSSION
Many academic medical centers have expanded their workforce with APC or nonteaching hospitalist teams to accommodate the increasing volume of hospital admissions, resident work hour restrictions,1,2 and medical complexity of an aging population. Several hospitals have reported comparative outcomes between different care delivery models, with conflicting results.6,8,10-12 In our study, we directly evaluated three inpatient care delivery models and found that hospitalist teams discharged patients more efficiently and utilized fewer consultants, compared with APC and resident teams. In spite of this improved efficiency, no significant differences were observed in cost or other clinical outcomes.
Our findings are important and further strengthen the evidence supporting the use of APCs on inpatient general medicine services and are of particular interest to academic centers struggling to expand staffing in order to offset the growth in patient volume and reduction in resident workforce. We believe several findings from our study warrant further discussion.
First, although hospitalist teams were able to discharge patients more efficiently, this observation may be influenced by factors of workflow rather than caused by significant disparities in efficiency between provider types (ie, APC vs hospitalist vs resident physician). As with most academic centers, patients assigned to resident teams are presented by house staff to an attending physician who is ultimately responsible for patient care decisions. Therefore, it is conceivable that delays in the discharge process are in part related to the convention of bedside rounding and discussing the care plan prior to discharge.20 In fact, we recognized this as a bottleneck and changed our discharge process for resident teams in June 2017, with a measurable improvement in discharge times. In the absence of this intervention, our observed differences in discharge times among teams may have been even greater.
Second, no significant differences in clinical outcomes were observed in our adjusted analyses, which suggests that a similar quality of care is delivered to patients regardless of team structure, an important observation when considering different staffing models.
Third, we observed a significant increase in consultation use among resident and APC teams, compared with hospitalists. While we are not able to precisely identify the basis for this variation, we believe it could reflect differences in clinical experience, comfort with diagnostic uncertainty, or the unequal distribution of patients transferred from outside hospitals for tertiary care. Interestingly, the greater consultation use did not correlate with higher healthcare costs, a finding recently reported by Stevens et al.21
Fourth, we believe the lack of differences in cost and clinical outcomes among team structures may be of particular interest to academic centers when considering physician burnout, salaries, and clinical education. The relationship between clerical burden, such as completing clinical documentation and computerized physician order entry, has been implicated as a risk factor for physician burnout.22 Incorporating APCs into roles similar to those performed by resident physicians may reduce the clerical burden on hospitalists, thereby reducing the risk of physician burnout. The addition of APCs may also represent opportunities for cost savings for healthcare centers when comparing the median salary of an APC to that of an internal medicine hospitalist.23,24 Moreover, academic hospitalists have been shown to be excellent medical educators and report increased job satisfaction with a variety of duties beyond direct patient care.24,25 Unforeseen benefits of adding APC teams within our institution has been the added teaching opportunities for APCs and APC students, increased collegiality with the APCs, and the creation of an APC fellowship program with a focus on inpatient medicine. Similar postgraduate training programs have been reported and serve as effective models to train APCs for hospital-based practice.26
Lastly, although this project was conceived and completed prior to the COVID-19 pandemic, our observations may be informative for medical centers experiencing a workforce shortage caused by a surge of COVID-19 patients. During a physician shortage we believe our APC team model could be rapidly expanded to accommodate a large influx of patients. This expansion could be accomplished through a single attending physician overseeing multiple APC teams. In this model, the supervising physician would only evaluate the most complex patients with most patients being managed solely by an APC from admission to discharge. Such changes may require temporary suspension of state laws restricting APC independent practice.27,28
Our findings contrast those of previous reports in that we did not observe significant differences in clinical outcomes (ie, LOS, inpatient mortality, and 30-day readmissions) or total direct cost.8,10,21 Other institutions have noted an increased LOS among APC teams and hospitalist teams, compared with resident teams.8,10 Furthermore, Chin et al and Iannuzzi et al reported reductions in healthcare cost for resident teams, whereas our study did not identify significant cost differences among team structures. Although we cannot pinpoint the exact reason(s) for these dissimilarities, it is plausible that unmeasured factors such as institutional differences in APC training, direct physician supervision, admission processes, or inpatient team census may play a role.
Several study limitations should be recognized. First, the retrospective, nonrandomized design is one of the largest limitations of our study. Administrative data was obtained via an electronic query of our data warehouse, and although we aimed to identify as many patient characteristics as possible to adjust for cofounding effects, undetected differences among cohorts may exist. Second, our inpatient admission process may have placed undue burden on resident teams to perform all daytime admissions, inadvertently affecting study outcomes. It is possible the observed benefits of a solo hospitalist team are attributable to the lack of admitting duties rather than inherent advantages of the team structure. If this were the case, we would expect similar benefits among APC teams, which we did not note. Third, the study was performed at a single academic center, which may limit the generalizability of our results. Fourth, it is possible the outcomes are similar among teams because our hospitalist faculty rotate proportionately between the different teams. Lastly, the study was underpowered to detect a significant difference in mortality between hospitalist and APC teams. A post hoc power calculation based on our observed sample and effect sizes estimated 75% power to detect a mortality difference between hospitalists and APCs; other mortality comparisons were adequately powered.
CONCLUSION
We observed similar total direct costs, LOS, 30-day readmission, and inpatient mortality between hospitalist, APC, and resident teams. APC and resident teams utilized more consultants and discharged patient later than hospitalists. Our analysis suggests clinical outcomes are not significantly affected by inpatient team structure, and the addition of general medicine inpatient APC or hospitalist teams represent safe and efficient alternatives to traditional resident teams within an academic medical center.
Disclosures
All authors declare they have no conflicts of interest.
The Accreditation Council for Graduate Medical Education (ACGME) first mandated residency work hour restrictions in 2003.1 In 2011, revised work hour requirements were issued, further limiting the maximum duration of a shift and extending the duration of time off between scheduled shifts.2 Academic medical centers have been forced to adapt to work hour restrictions, and cuts in funding to research and educational missions have pressured institutions to restructure with a greater focus on high-quality, lower-cost care.3,4 In response, many academic hospitals have added hospitalist teams, or incorporated advanced practice clinicians (APCs) (nurse practitioners [NPs] and physician assistants [PAs]) to accommodate resident physician duty hour restrictions on their inpatient general medicine services.5,6 More recently, the COVID-19 pandemic has created unanticipated physician shortages forcing medical centers to rapidly expand and broaden the scope of their existing APC workforce.7
Several comparisons of clinical outcomes, cost, and patient satisfaction between different combinations of hospitalist-based, resident-based, or APC-based inpatient teams have been reported with conflicting observations.6,8-14 Roy et al reported no significant differences in mortality, length of stay (LOS), or readmissions between PA and resident teams.6 Timmermans et al reported similar cost-effectiveness, LOS, and quality of care between PA and physician teams that included a hybrid of attending only and resident teams.13,14 Alternatively, Singh et al and Iannuzzi et al reported increased LOS among PA teams,10,12 whereas Chin et al observed an increased LOS and reduced 30-day readmissions among hospitalist teams.8 While these observed differences may be attributable to heterogeneous patient populations or institution-specific team structure, the exact reasons remain unknown. Furthermore, understanding the value of alternate staffing models is essential for medical centers to prepare for potential COVID-19 related physician shortages. To our knowledge, no study to date has directly compared outcomes between resident, APC, and hospitalist team structures within an academic medical center.
We believe our institution provides a unique environment to study the differences in inpatient general medicine team structure with respect to quality and efficiency of care delivery. The objective of our study is to directly compare clinical outcomes and resource utilization among three distinct team structures: APC, resident, and solo hospitalist. We hypothesize that clinical outcomes, cost, and utilization of consult services will be similar across all team structures and hospitalist teams will discharge patients earlier than resident and APC teams.
METHODS
Study Design and Setting
We conducted a retrospective observational cohort study at the University of Utah Medical Center, a 548-bed academic medical center in Salt Lake City. An electronic database query was used to identify all patients discharged from the inpatient general internal medicine service between July 1, 2015, and July 1, 2018. Baseline patient characteristics were collected including age, gender, and Charlson comorbidity index (CCI).15 Case-mix index was determined for admissions where a Medicare Severity Diagnosis Related Group (MS-DRG) and corresponding weight was assigned.16,17 Source of admission was collected to identify patients transferred from an outside hospital, typically due to increased medical complexity or need for specialty care not available at the referring center. Time of admission was collected to classify whether a patient was admitted during the day or at night. Length of stay was calculated as the difference between discharge date/time and admission date/time. Discharge order time was collected as a measure of clinician efficiency. The number of consults per admission was determined by the number of different medical or surgical subspecialty services that wrote at least one consultation or progress note after the time of admission and were not the primary service at the time the note was written. The project was reviewed and deemed exempt by the University of Utah Institutional Review Board (IRB 00104884).
Inpatient Care Team Structure
Patients were assigned to one of three cohorts dependent on the assigned treatment team at the time of discharge. The three inpatient team structures were as follows: (1) a “resident team” composed of a senior resident (postgraduate year [PGY] 2 or PGY3) and one to two medical students or one senior resident, two interns (PGY1), and one to two medical students supervised by a hospitalist physician; (2) an “APC team” composed of one to two APCs supervised by a hospitalist physician; and (3) a “hospitalist team” composed of one attending hospitalist independently managing all patients.
Advanced Practice Clinicians
The APC service included 10 APCs (8 PAs and 2 NPs), with a combined workforce of nine APC full-time equivalents during the study period. Their experience ranged from new graduate to 11 years of clinical experience, with an average of 4.2 years. Among the 6 APCs with prior clinical experience, the majority (86%) of their years of clinical experience were within inpatient medicine, oncology, or cardiology. Recognizing the variability in clinical experience, we employed a rigorous onboarding program that entailed an average of 80 hours of didactic sessions including 1:1 teaching of the inpatient Society of Hospital Medicine core lecture series combined with initial intense clinical oversight.18 This program ranged from 2 weeks to 6 weeks depending on the individual APC’s clinical experience, progress, and comfort working independently. This onboarding program has subsequently been formalized into a 1-year APC fellowship that began after the study period concluded.
The degree of autonomy for each APC was individualized based on their clinical experience and ability to recognize limitations such as medical decision-making, clinical knowledge, and effective use of interprofessional team members (eg, peers, nursing, ancillary staff, consultants, and support personnel). Those APCs who demonstrated a sufficient level of clinical competence functioned with a high level of autonomy. During the day, APCs were expected to be the first point of contact for interprofessional team members, to respond to acute clinical changes in a patient’s condition, and to discuss active issues with the supervising attending, all with the majority of medical decision-making, direct patient communication, documentation, and care coordination performed by the APC. An experienced subset of the APC service was responsible for overnight coverage. Nocturnist APCs independently managed all cross-cover issues on patients assigned to APC and hospitalist teams and performed admissions with very little to no direct supervision of the overnight attending physician.
Patient Admission and Redistribution Process
During the study period, resident teams performed all daytime admissions (6
Study Outcomes
We divided study outcomes into two categories, clinical outcomes and resource utilization. Clinical outcomes included LOS, unplanned readmission within 30-days, and inpatient mortality and were designed to measure patient-related outcomes as a reflection of the quality of care delivered by different team structures. Resource utilization included discharge order time, discharge time, consults per admission, and total direct cost, which were designed to measure provider-related differences in efficiency and cost of care.
Statistical analysis
Baseline characteristics and unadjusted outcomes are reported as frequency and percent, normally distributed variables as mean with SD, and nonnormally distributed variables as median with interquartile range (IQR). Baseline characteristics and unadjusted outcomes were compared using the chi-square test or the t test, where appropriate. Multivariable regression analysis using generalized linear models with a log link function and gamma distribution was used for continuous outcomes. Multivariable logistic regression was used for binary outcomes.10 Covariates included in regression models were age, gender, CCI, transfer from an outside hospital, and nighttime admission. In a sensitivity analysis, we included MS-DRG weight as a covariate for 85% of hospitalizations in our cohort exclusive of observation stays, and our findings were qualitatively similar (data not reported but available on request). Adjusted continuous outcomes were estimated using marginal effects at the means.19 Due to the sensitivity of cost data and an institutional policy against disclosing cost figures, total direct costs were normalized using the unadjusted median and adjusted mean total direct cost of an admission to an APC team as the normalizing value. A P value cutoff of .05 was used to determine statistical significance. Stata/IC version 16.1 (StataCorp) was used for all analyses.
RESULTS
Study Population
A total of 12,716 hospital admissions were identified during the study period. Of these, 7,943 (62.5%) admissions were assigned to a resident team, 3,519 (27.7%) admissions were assigned to an APC team, and the remaining 1,254 (9.9%) were assigned to a hospitalist team. Baseline patient characteristics are reported in Table 1. Patients admitted to resident teams (mean age [SD], 56.9 [19.1] years) were younger than those admitted to an APC team (58.0 [19.3] years; P = .004) or a hospitalist team (58.2 [19.3] years; P = .026). The case-mix index (mean MS-DRG weight [SD], 1.44 [0.87]) was slightly lower for resident teams than that for APC teams (1.49 [0.90]; P = .025).Resident teams had a significantly lower proportion of night admissions than did APC teams (32.0% vs 49.5%; P < .001) and hospitalist teams (48.6%; P < .001). APC teams were assigned more patients transferred from an outside hospital (19.1%), compared with resident teams (15.0%; P < .001) and hospitalist teams (16.0%; P = .015). No other significant differences were observed in baseline characteristics between cohorts.

Clinical Outcomes
Unadjusted analysis demonstrated the LOS was similar among resident, APC, and hospitalist teams with a median (IQR) LOS of 2.90 (1.86, 4.26) days, 2.93 (1.89, 4.66) days, and 2.86 (1.84, 4.67) days, respectively. No significant differences were observed in unadjusted 30-day readmissions or inpatient mortality among the team structures (Table 2). Following multivariable adjustment for differences in baseline characteristics, no significant differences were observed in LOS, 30-day readmission, or inpatient mortality among teams (Table 3).

Resource Utilization
In unadjusted comparisons, hospitalist teams were observed to place discharge orders more than 30 minutes earlier than APC teams (median hours after midnight [IQR], 11.20 [9.63, 13.60] vs 11.73 [10.00, 13.87]; P < .001) and 54 minutes earlier than resident teams (12.10 [10.38, 13.90]; P < .001) (Table 2). Consistent with the earlier placement of discharge orders, hospitalist patients were also discharged from the hospital 26 and 32 minutes earlier than APC and resident patients, respectively. APC teams also discharged patients slightly earlier (6 minutes) than resident teams (median hours after midnight [IQR], 14.97 [13.23, 16.72] vs 15.07 [13.42, 16.73]; P = .045). Median consultation use among teams was similar, although statistically significant differences were present. Normalized total direct cost was 8% higher (P < .001) for admissions to APC teams than that for resident teams and 7% higher (P = .008) than that for hospitalist teams in unadjusted analysis (Table 2).
Following multivariable adjustment, the mean differences in discharge order time and discharge time remained significant with hospitalist teams discharging patients an average of 20 to 30 minutes earlier than APC and resident teams (Table 3). Consultant utilization remained significantly different between teams, with APC teams utilizing consultants on average 15% more than hospitalist teams (P < .001) and 7% more than resident teams (P = .001). The differences in total direct costs were not significant after adjusted analysis.

DISCUSSION
Many academic medical centers have expanded their workforce with APC or nonteaching hospitalist teams to accommodate the increasing volume of hospital admissions, resident work hour restrictions,1,2 and medical complexity of an aging population. Several hospitals have reported comparative outcomes between different care delivery models, with conflicting results.6,8,10-12 In our study, we directly evaluated three inpatient care delivery models and found that hospitalist teams discharged patients more efficiently and utilized fewer consultants, compared with APC and resident teams. In spite of this improved efficiency, no significant differences were observed in cost or other clinical outcomes.
Our findings are important and further strengthen the evidence supporting the use of APCs on inpatient general medicine services and are of particular interest to academic centers struggling to expand staffing in order to offset the growth in patient volume and reduction in resident workforce. We believe several findings from our study warrant further discussion.
First, although hospitalist teams were able to discharge patients more efficiently, this observation may be influenced by factors of workflow rather than caused by significant disparities in efficiency between provider types (ie, APC vs hospitalist vs resident physician). As with most academic centers, patients assigned to resident teams are presented by house staff to an attending physician who is ultimately responsible for patient care decisions. Therefore, it is conceivable that delays in the discharge process are in part related to the convention of bedside rounding and discussing the care plan prior to discharge.20 In fact, we recognized this as a bottleneck and changed our discharge process for resident teams in June 2017, with a measurable improvement in discharge times. In the absence of this intervention, our observed differences in discharge times among teams may have been even greater.
Second, no significant differences in clinical outcomes were observed in our adjusted analyses, which suggests that a similar quality of care is delivered to patients regardless of team structure, an important observation when considering different staffing models.
Third, we observed a significant increase in consultation use among resident and APC teams, compared with hospitalists. While we are not able to precisely identify the basis for this variation, we believe it could reflect differences in clinical experience, comfort with diagnostic uncertainty, or the unequal distribution of patients transferred from outside hospitals for tertiary care. Interestingly, the greater consultation use did not correlate with higher healthcare costs, a finding recently reported by Stevens et al.21
Fourth, we believe the lack of differences in cost and clinical outcomes among team structures may be of particular interest to academic centers when considering physician burnout, salaries, and clinical education. The relationship between clerical burden, such as completing clinical documentation and computerized physician order entry, has been implicated as a risk factor for physician burnout.22 Incorporating APCs into roles similar to those performed by resident physicians may reduce the clerical burden on hospitalists, thereby reducing the risk of physician burnout. The addition of APCs may also represent opportunities for cost savings for healthcare centers when comparing the median salary of an APC to that of an internal medicine hospitalist.23,24 Moreover, academic hospitalists have been shown to be excellent medical educators and report increased job satisfaction with a variety of duties beyond direct patient care.24,25 Unforeseen benefits of adding APC teams within our institution has been the added teaching opportunities for APCs and APC students, increased collegiality with the APCs, and the creation of an APC fellowship program with a focus on inpatient medicine. Similar postgraduate training programs have been reported and serve as effective models to train APCs for hospital-based practice.26
Lastly, although this project was conceived and completed prior to the COVID-19 pandemic, our observations may be informative for medical centers experiencing a workforce shortage caused by a surge of COVID-19 patients. During a physician shortage we believe our APC team model could be rapidly expanded to accommodate a large influx of patients. This expansion could be accomplished through a single attending physician overseeing multiple APC teams. In this model, the supervising physician would only evaluate the most complex patients with most patients being managed solely by an APC from admission to discharge. Such changes may require temporary suspension of state laws restricting APC independent practice.27,28
Our findings contrast those of previous reports in that we did not observe significant differences in clinical outcomes (ie, LOS, inpatient mortality, and 30-day readmissions) or total direct cost.8,10,21 Other institutions have noted an increased LOS among APC teams and hospitalist teams, compared with resident teams.8,10 Furthermore, Chin et al and Iannuzzi et al reported reductions in healthcare cost for resident teams, whereas our study did not identify significant cost differences among team structures. Although we cannot pinpoint the exact reason(s) for these dissimilarities, it is plausible that unmeasured factors such as institutional differences in APC training, direct physician supervision, admission processes, or inpatient team census may play a role.
Several study limitations should be recognized. First, the retrospective, nonrandomized design is one of the largest limitations of our study. Administrative data was obtained via an electronic query of our data warehouse, and although we aimed to identify as many patient characteristics as possible to adjust for cofounding effects, undetected differences among cohorts may exist. Second, our inpatient admission process may have placed undue burden on resident teams to perform all daytime admissions, inadvertently affecting study outcomes. It is possible the observed benefits of a solo hospitalist team are attributable to the lack of admitting duties rather than inherent advantages of the team structure. If this were the case, we would expect similar benefits among APC teams, which we did not note. Third, the study was performed at a single academic center, which may limit the generalizability of our results. Fourth, it is possible the outcomes are similar among teams because our hospitalist faculty rotate proportionately between the different teams. Lastly, the study was underpowered to detect a significant difference in mortality between hospitalist and APC teams. A post hoc power calculation based on our observed sample and effect sizes estimated 75% power to detect a mortality difference between hospitalists and APCs; other mortality comparisons were adequately powered.
CONCLUSION
We observed similar total direct costs, LOS, 30-day readmission, and inpatient mortality between hospitalist, APC, and resident teams. APC and resident teams utilized more consultants and discharged patient later than hospitalists. Our analysis suggests clinical outcomes are not significantly affected by inpatient team structure, and the addition of general medicine inpatient APC or hospitalist teams represent safe and efficient alternatives to traditional resident teams within an academic medical center.
Disclosures
All authors declare they have no conflicts of interest.
1. Report of the Work Group on Resident Duty Hours and the Learning Environment, June 11, 2002. Accreditation Council for Graduate Medical Education; 2003.
2. ACGME Task Force on Quality Care and Professionalism. Philibert I, Amis Steve, eds. The ACGME 2011 Duty Hour Standards: Enhancing Quality of Care, Supervision, and Resident Professional Development. Accreditation Council for Graduate Medical Education; 2011. https://www.acgme.org/Portals/0/PDFs/jgme-monograph[1].pdf
3. Konstam MA, Hill JA, Kovacs RJ, et al. The academic medical system: reinvention to survive the revolution in health care. J Am Coll Cardiol. 2017;69(10):1305-1312. https://doi.org/10.1016/j.jacc.2016.12.024
4. The future of the academic medical center: strategies to avoid a margin meltdown. Health Research Institute. February 2012. https://uofuhealth.utah.edu/hcr/2012/resources/the-future-of-academic-medical-centers.pdf
5. Moote M, Krsek C, Kleinpell R, Todd B. Physician assistant and nurse practitioner utilization in academic medical centers. Am J Med Qual. 2019;34(5):465-472. https://doi.org/ 10.1177/1062860619873216
6. Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368. https://doi.org/10.1002/jhm.352
7. Denne E. Behind the scenes at Northwell Health as PAs respond to COVID-19. American Academy of Physician Assistants. May 11, 2020. Accessed May 15, 2020. https://www.aapa.org/news-central/2020/05/behind-the-scenes-at-northwell-heath-as-pas-respond-to-covid-19/
8. Chin DL, Wilson MH, Bang H, Romano PS. Comparing patient outcomes of academician-preceptors, hospitalist-preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672-1678. https://doi.org/10.1007/s11606-014-2982-y
9. Cowan MJ, Shapiro M, Hays RD, et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79-85. https://doi.org/10.1097/00005110-200602000-00006
10. Iannuzzi MC, Iannuzzi JC, Holtsbery A, Wright SM, Knohl SJ. Comparing hospitalist-resident to hospitalist-midlevel practitioner team performance on length of stay and direct patient care cost. J Grad Med Educ. 2015;7(1):65-69. https://doi.org/10.4300/jgme-d-14-00234.1
11. Kapu AN, Kleinpell R, Pilon B. Quality and financial impact of adding nurse practitioners to inpatient care teams. J Nurs Adm. 2014;44(2):87-96. https://doi.org/10.1097/nna.0000000000000031
12. Singh S, Fletcher KE, Schapira MM, et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist-physician assistant model vs a traditional resident-based model. J Hosp Med. 2011;6(3):122-130. https://doi.org/10.1002/jhm.826
13. Timmermans MJC, van Vught A, Peters YAS, et al. The impact of the implementation of physician assistants in inpatient care: a multicenter matched-controlled study. PLoS One. 2017;12(8):e0178212. https://doi.org/10.1371/journal.pone.0178212
14. Timmermans MJC, van den Brink GT, van Vught A, et al. The involvement of physician assistants in inpatient care in hospitals in the Netherlands: a cost-effectiveness analysis. BMJ Open. 2017;7(7):e016405. https://doi.org/10.1136/bmjopen-2017-016405
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8
16. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. 2020. Updated April 28, 2020. Accessed May 5, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
17. Fetter RB, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis-related groups. Med Care. 1980;18(2 Suppl):iii, 1-53.
18. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
19. Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308-331. https://doi.org/10.1177%2F1536867X1201200209
20. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
21. Stevens JP, Hatfield LA, Nyweide DJ, Landon B. Association of variation in consultant use among hospitalist physicians with outcomes among Medicare beneficiaries. JAMA Netw Open. 2020;3(2):e1921750. https://doi.org/10.1001/jamanetworkopen.2019.21750
22. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91(7):836-848. https://doi.org/10.1016/j.mayocp.2016.05.007
23. 2019 AAPA Salary Report. American Academy of PAs. 2019. https://www.aapa.org/shop/salary-report-2019/
24. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
25. Dalen JE, Ryan KJ, Waterbrook AL, Alpert JS. Hospitalists, medical education, and US health care costs. Am J Med. 2018;131(11):1267-1269. https://doi.org/10.1016/j.amjmed.2018.05.016
26. Will KK, Budavari AI, Wilkens JA, Mishark K, Hartsell ZC. A hospitalist postgraduate training program for physician assistants. J Hosp Med. 2010;5(2):94-98. https://doi.org/10.1002/jhm.619
27. Utah Physician Assistant Act. Utah Code. Published 2019. Accessed May 8, 2020. https://le.utah.gov/xcode/Title58/Chapter70A/C58-70a_2019051420190514.pdf
28. Nurse Practice Act. Utah Code. Published 2019. Accessed May 8, 2020. https://le.utah.gov/xcode/Title58/Chapter31B/C58-31b_1800010118000101.pdf
1. Report of the Work Group on Resident Duty Hours and the Learning Environment, June 11, 2002. Accreditation Council for Graduate Medical Education; 2003.
2. ACGME Task Force on Quality Care and Professionalism. Philibert I, Amis Steve, eds. The ACGME 2011 Duty Hour Standards: Enhancing Quality of Care, Supervision, and Resident Professional Development. Accreditation Council for Graduate Medical Education; 2011. https://www.acgme.org/Portals/0/PDFs/jgme-monograph[1].pdf
3. Konstam MA, Hill JA, Kovacs RJ, et al. The academic medical system: reinvention to survive the revolution in health care. J Am Coll Cardiol. 2017;69(10):1305-1312. https://doi.org/10.1016/j.jacc.2016.12.024
4. The future of the academic medical center: strategies to avoid a margin meltdown. Health Research Institute. February 2012. https://uofuhealth.utah.edu/hcr/2012/resources/the-future-of-academic-medical-centers.pdf
5. Moote M, Krsek C, Kleinpell R, Todd B. Physician assistant and nurse practitioner utilization in academic medical centers. Am J Med Qual. 2019;34(5):465-472. https://doi.org/ 10.1177/1062860619873216
6. Roy CL, Liang CL, Lund M, et al. Implementation of a physician assistant/hospitalist service in an academic medical center: impact on efficiency and patient outcomes. J Hosp Med. 2008;3(5):361-368. https://doi.org/10.1002/jhm.352
7. Denne E. Behind the scenes at Northwell Health as PAs respond to COVID-19. American Academy of Physician Assistants. May 11, 2020. Accessed May 15, 2020. https://www.aapa.org/news-central/2020/05/behind-the-scenes-at-northwell-heath-as-pas-respond-to-covid-19/
8. Chin DL, Wilson MH, Bang H, Romano PS. Comparing patient outcomes of academician-preceptors, hospitalist-preceptors, and hospitalists on internal medicine services in an academic medical center. J Gen Intern Med. 2014;29(12):1672-1678. https://doi.org/10.1007/s11606-014-2982-y
9. Cowan MJ, Shapiro M, Hays RD, et al. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nurs Adm. 2006;36(2):79-85. https://doi.org/10.1097/00005110-200602000-00006
10. Iannuzzi MC, Iannuzzi JC, Holtsbery A, Wright SM, Knohl SJ. Comparing hospitalist-resident to hospitalist-midlevel practitioner team performance on length of stay and direct patient care cost. J Grad Med Educ. 2015;7(1):65-69. https://doi.org/10.4300/jgme-d-14-00234.1
11. Kapu AN, Kleinpell R, Pilon B. Quality and financial impact of adding nurse practitioners to inpatient care teams. J Nurs Adm. 2014;44(2):87-96. https://doi.org/10.1097/nna.0000000000000031
12. Singh S, Fletcher KE, Schapira MM, et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist-physician assistant model vs a traditional resident-based model. J Hosp Med. 2011;6(3):122-130. https://doi.org/10.1002/jhm.826
13. Timmermans MJC, van Vught A, Peters YAS, et al. The impact of the implementation of physician assistants in inpatient care: a multicenter matched-controlled study. PLoS One. 2017;12(8):e0178212. https://doi.org/10.1371/journal.pone.0178212
14. Timmermans MJC, van den Brink GT, van Vught A, et al. The involvement of physician assistants in inpatient care in hospitals in the Netherlands: a cost-effectiveness analysis. BMJ Open. 2017;7(7):e016405. https://doi.org/10.1136/bmjopen-2017-016405
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8
16. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. 2020. Updated April 28, 2020. Accessed May 5, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
17. Fetter RB, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis-related groups. Med Care. 1980;18(2 Suppl):iii, 1-53.
18. Nichani S, Crocker J, Fitterman N, Lukela M. Updating the core competencies in hospital medicine--2017 revision: introduction and methodology. J Hosp Med. 2017;12(4):283-287. https://doi.org/10.12788/jhm.2715
19. Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308-331. https://doi.org/10.1177%2F1536867X1201200209
20. Goolsarran N, Olowo G, Ling Y, Abbasi S, Taub E, Teressa G. Outcomes of a resident-led early hospital discharge intervention. J Gen Intern Med. 2020;35(2):437-443. https://doi.org/10.1007/s11606-019-05563-w
21. Stevens JP, Hatfield LA, Nyweide DJ, Landon B. Association of variation in consultant use among hospitalist physicians with outcomes among Medicare beneficiaries. JAMA Netw Open. 2020;3(2):e1921750. https://doi.org/10.1001/jamanetworkopen.2019.21750
22. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91(7):836-848. https://doi.org/10.1016/j.mayocp.2016.05.007
23. 2019 AAPA Salary Report. American Academy of PAs. 2019. https://www.aapa.org/shop/salary-report-2019/
24. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
25. Dalen JE, Ryan KJ, Waterbrook AL, Alpert JS. Hospitalists, medical education, and US health care costs. Am J Med. 2018;131(11):1267-1269. https://doi.org/10.1016/j.amjmed.2018.05.016
26. Will KK, Budavari AI, Wilkens JA, Mishark K, Hartsell ZC. A hospitalist postgraduate training program for physician assistants. J Hosp Med. 2010;5(2):94-98. https://doi.org/10.1002/jhm.619
27. Utah Physician Assistant Act. Utah Code. Published 2019. Accessed May 8, 2020. https://le.utah.gov/xcode/Title58/Chapter70A/C58-70a_2019051420190514.pdf
28. Nurse Practice Act. Utah Code. Published 2019. Accessed May 8, 2020. https://le.utah.gov/xcode/Title58/Chapter31B/C58-31b_1800010118000101.pdf
© 2020 Society of Hospital Medicine
Performance of Pediatric Readmission Measures
Readmission rates are frequently used as a hospital quality metric, with use including payment incentive at the hospital level,1 specific condition quality measurement,2 balancing measures for quality improvement projects,3-5 transition success,6,7 and use in public hospital rankings.8 Currently, four methods are commonly used to evaluate pediatric readmissions, each with strengths and limitations, including the following (Appendix Table 1):
1. All-cause readmissions: A measure of any readmission within a given time period regardless of the reason for readmission.9
2. Unplanned readmission/time flag: A measure intended to identify unplanned readmissions. This measure relies on time designations within the electronic health record. The time between hospital registration and admission is calculated, and if the readmission is registered more than 24 hours prior to admission, the readmission is considered planned.10 Hereafter, this measure will be referred to as the time flag measure.
3. Pediatric all-condition readmission (PACR): A measure intended to identify unplanned readmission through the exclusion of certain procedures and diagnoses.11
4. Potentially preventable readmission (PPR): A method to identify preventable readmissions based on a proprietary algorithm developed by
While all four of these measures are used to assess quality, there is little known about these measures’ ability to exclude planned readmissions and identify only preventable pediatric readmission, which conceptually is most relevant to the quality of care. However, many of these measures were not intended to capture preventability, but instead capture the related issue of whether the readmission was planned. Therefore, we sought to evaluate the four readmission measures as they relate to both preventability and unplanned status as determined through medical record review with multidisciplinary care provider input.
METHODS
As part of a hospital-wide readmission reduction quality improvement collaborative at a free-standing tertiary care children’s hospital, clinicians from hospital medicine, cardiology, neonatology, and neurology teams reviewed 30-day readmissions using a standardized abstraction tool. All readmission events (observation or inpatient encounter) after any discharge (observation or inpatient encounter) from eligible units were reviewed; therefore, each hospitalization was a potential index hospitalization. We classified the preventability of each readmission with use of a previously described Likert scale with high interrater reliability.14 For these analyses, readmissions were considered preventable if the reviewing team rated them as either “more likely preventable” or “preventable in most circumstances.” Each readmission was also evaluated as planned or unplanned. Methods for readmission review and classification are in the Appendix.
We included all readmissions between July 2014 and June 2016. We compared the medical record review classifications with the assessments from each of the four measures of pediatric readmission. We calculated sensitivity and specificity for both outcomes (planned/unplanned and preventable/not preventable) for all four measures. For standardization of discussion, we categorized description of measure performance as “very poor” as less than 50%, “poor” between 50%-75%, “fair” as 75%-85%, “good” as 85%-90%, “very good” as 90%-95% and excellent as greater than 95%. We also calculated positive and negative predictive value (PPV and NPV) over plausible ranges of prevalence using the sensitivity and specificity of each comparison (Appendix).
Of note, certain exclusions are outlined by the PACR and PPR algorithms. The PACR evaluates only readmission events that occur in children younger than 18 years. The PPR algorithm does not assign preventability if either the index or readmission event is classified as an observation stay or if it is part of a larger chain of readmissions.
RESULTS
Among 30-day readmissions considered, 1,643 were eligible for medical record review; 1,125 reviews were completed by the clinical teams (68.5%). The median time to readmission was 7 days (interquartile range [IQR], 4-18). Most children were non-Hispanic White (71%) or Black (20%). The median age at hospitalization was 2.3 years (IQR 0.4-12.1). Most children had Medicaid (56%) or private (41%) insurance. Most of the reviews were performed in cardiology (43%) and hospital medicine (37%) with patients in neurology (13%) and neonatology (7%) constituting the remaining reviews. Uncontrolled advancement of chronic disease was the most common readmission category on medical record review (25.1%), followed by unrelated readmission (20.7%), scheduled readmission (20.4%), and progression of acute disease (16.6%) (Appendix Table 2).
Assessment of Preventable and Unplanned Readmissions
On multidisciplinary medical record review, most readmissions were classified as not preventable (84.5%). Specifically, 64% were not preventable and unplanned; 20% were deemed not preventable and planned. Only 15% were classified as unplanned and preventable and 1% as planned and preventable (Appendix Figure: Population A/B).
Matching Chart Review to the Four Algorithms
All 1,125 readmissions were assessed by the all-cause and time flag readmission measures (Appendix Figure: Population A/B). After applying algorithm exclusions (details in Appendix), only 804 of the 1,125 (71.5%) reviewed readmissions matched for PACR readmission comparison (Appendix Figure: Population C); 487 of the 1,125 (43.3%) of the reviewed readmissions matched for PPR comparison (Appendix Figure: Population D).
All-Cause
Because all-cause determines only if a readmission occurs, the measure is by definition 100% sensitive and 0% specific in both assessment of preventability and unplanned readmission (Table: Section A).
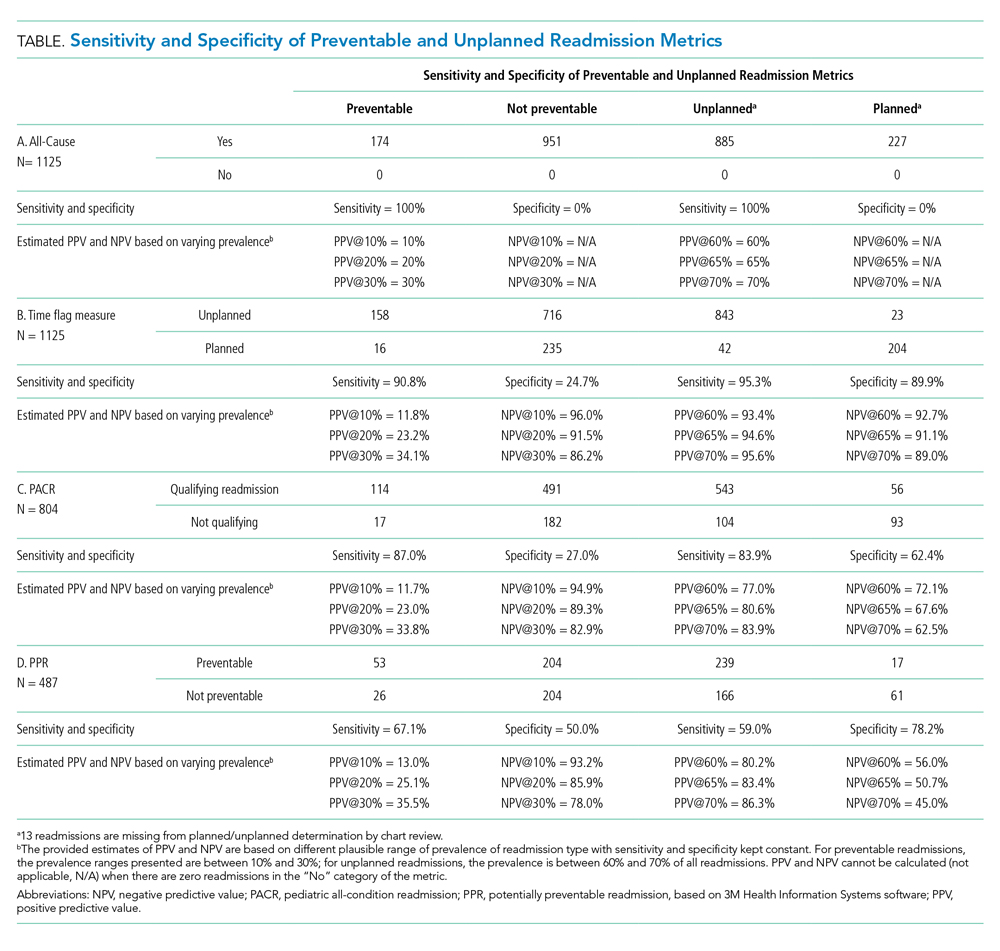
Time Flag
The time flag measure identified 80% (866/1,112) of the readmissions as unplanned. This measure had very good sensitivity but very poor specificity in identifying preventable readmissions, which corresponded to very poor PPV and good to excellent NPV. In terms of identifying unplanned readmissions, the time flag measure had excellent sensitivity and very good specificity, which corresponded to very good to excellent PPV and good to very good NPV (Table: Section B).
PACR
The PACR algorithm identified 75% (599/796) of readmissions as unplanned. The PACR has good sensitivity but very poor specificity in identifying preventable readmissions, which corresponded to very poor PPV and fair to very good NPV. In terms of identifying unplanned readmissions, the PACR had fair sensitivity but poor specificity, which corresponded to fair PPV and poor NPV (Table: Section C).
PPR
The PPR algorithm identified 53% (257/487) of admissions as potentially preventable. The PPR algorithm had poor sensitivity and specificity in identifying preventable readmissions, which corresponded to very poor PPV and fair to very good NPV. In terms of identifying unplanned readmissions, the PPR algorithm had poor sensitivity and fair specificity in identifying unplanned readmissions, which corresponded to fair to good PPV and very poor to poor NPV (Table: Section D).
Evaluation of Excluded Readmission Events
Because both the PACR and PPR had large numbers of algorithm exclusions, we describe the preventability and unplanned assessment of the excluded readmission events. Both algorithms excluded preventable events. Of the 321 readmissions excluded by the PACR algorithm, 13.4% were classified as preventable by chart review. Likewise, 14.9% of 638 readmissions excluded by PPR were classified as preventable by chart review.
DISCUSSION
The ability to accurately capture preventable pediatric readmission is a goal for hospital quality experts and health policymakers alike. Of the four commonly used readmission measures to assess readmission, only PPR is designed to focus on preventability. Unfortunately, none of these four measures is adequately sensitive or specific to identify preventable readmissions; all measures had very poor PPV for preventability. Of the four measures, the time flag measure had the best sensitivity, specificity, PPV, and NPV for identifying unplanned readmissions.
The overall percentage of unplanned readmissions identified by both the time flag and by PACR measures match the overall percentage of unplanned readmissions identified in chart review: The time flag measure identified 80% of admissions as unplanned versus 79% identified by chart review (Appendix Figure: Population A/B); PACR classified 75% as unplanned versus 81% identified by chart review for PACR-eligible readmissions (Appendix Figure: Population C). In contrast, the PPR algorithm classified many more readmissions as potentially preventable (53%) than were identified by chart review at only 16% (Appendix Figure: Population D). The PACR and PPR algorithms also exclude a significant number of readmissions that are unplanned and a smaller, but not trivial, number of readmissions that are preventable; these exclusions limit their accuracy.
The ability to apply these four measures in real time during a hospitalization varies by metric. Two of the measures, the all-cause and time flag, can be applied during a readmission event, which is appealing for quality improvement initiatives. These measures allow for notification of providers that a current hospitalization is a readmission event, which allows providers the opportunity to learn from these events as they occur (Appendix Table 1). While “unplanned” is not the same as “potentially preventable,” almost all potentially preventable readmissions are unplanned; therefore, accurately identifying unplanned readmissions is more beneficial than all-cause. Additionally, a low all-cause readmission rate can be indicative of poor access to scheduled procedures. Nevertheless, all-cause readmission is sometimes used to measure quality.1,8 While the time flag measure may be more useful for quality improvement initiatives and hospital providers, it relies on hospital registration time, which is not widely available in administrative data sources and, therefore, has limited usefulness to policymakers.
Both PACR and PPR require administrative claims analysis, which is appealing from a policy standpoint. However, the reliance on claims data means the inclusion/exclusion of events can occur only retrospectively, which limits the usefulness of these measures in learning and intervening in real time. When the two measures are compared, PACR offers better sensitivity and PPR offers better specificity with regard to identifying unplanned readmission. The PPR software overcalls preventable readmissions, identifying more readmissions as preventable than there actually are. Nevertheless, Medicaid in several states uses PPR for payment incentive.1,15-17 Given the poor performance of PPR in assessing both preventable and unplanned pediatric readmission, the use of this measure as a quality metric should be limited.
This study should be considered in the context of several limitations. Because the assessment of preventability was determined as part of a learning quality improvement collaborative and not as a planned research endeavor, not all readmission reviews were completed nor were other existent tools18 that allow for preventability assessment via more structured medical record review used. Second, we reviewed cases only from certain clinical services, which would limit generalizability of these findings to all pediatric admissions. However, given the low sensitivity and specificity of some of the metrics, we would not anticipate that the addition of other types of admissions would improve the sensitivity and specificity enough to ensure reliability. Third, while we relied on an established method to determine preventability, prior work has demonstrated that additional information gathered from families may change preventability.19 Finally, due to the exclusions required by the PPR and PACR algorithms, not all readmission events were reviewed. However, these exclusions reflect the actual specifications of use for both measures.
CONCLUSION
The PPR software has poor fidelity in identifying preventable and unplanned pediatric readmission; this finding has broad policy implications given how widely it is used by state Medicaid offices to assess financial penalties. Among the four pediatric readmission measures used, the time flag metric best identifies unplanned readmissions.
Disclosures
The authors have no conflicts of interest or financial relationships relevant to this article to disclose.
Funding
Dr Auger’s research is supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1). The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
1. State Medicaid Payment Policies for Inpatient Hospital Services. Medicaid and CHIP Payment and Access Commission; December 2018. Accessed June 1, 2019. https://www.macpac.gov/publication/macpac-inpatient-hospital-payment-landscapes/
2. Mangione-Smith R, Zhou C, Williams DJ, et al. Pediatric Respiratory Illness Measurement System (PRIMES) scores and outcomes. Pediatrics. 2019;144(2):e20190242. https://doi.org/10.1542/peds.2019-0242
3. Biondi EA, McCulloh R, Staggs VS, et al. Reducing Variability in the Infant Sepsis Evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3):e20182201. https://doi.org/10.1542/peds.2018-2201
4. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving discharge efficiency in medically complex pediatric patients. Pediatrics. 2016;138(2):e20153832. https://doi.org/10.1542/peds.2015-3832
5. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
6. Auger KA, Simmons JM, Tubbs-Cooley HL, et al; H20 Trial Study Group. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2017-3919
7. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482
8. Olmsted MG, Powell R, Murphy J, Bell Denise, Stanley M, Sanchz R. Methodology: U.S. News & World Report Best Children’s Hospitals 2019-20. U.S. News & World Report; June 17, 2019. Accessed June 16, 2020. https://www.usnews.com/static/documents/health/best-hospitals/BCH_Methodology_2019-20.pdf
9. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
10. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e102. https://doi.org/10.1016/j.jpeds.2015.11.051
11. Readmissions-Content. Boston Children’s Hospital. Accessed April 8, 2019. http://www.childrenshospital.org/research-and-innovation/research/centers/center-of-excellence-for-pediatric-quality-measurement-cepqm/cepqm-measures/pediatric-readmissions/content
12. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-9.e5. https://doi.org/10.1016/j.jpeds.2014.10.052
13. Auger KA, Teufel RJ, Harris JM, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2):e20161720. https://doi.org/10.1542/peds.2016-1720
14. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
15. Potentially Preventable Events. Texas Health and Human Services. Accessed May 19, 2019. https://hhs.texas.gov/about-hhs/process-improvement/medicaid-chip-quality-efficiency-improvement/potentially-preventable-events
16. Potentially Preventable Readmissions. New York State Department of Health. Accessed May 28, 2019. https://regs.health.ny.gov/sites/default/files/pdf/recently_adopted_regulations/2011-02-23_potentially_preventable_readmissions.pdf
17. Potentially Preventable Readmissions Policy. Illinois Department of Healthcare and Family Services. Accessed May 28, 2019. https://www.illinois.gov/hfs/SiteCollectionDocuments/PPR_Overview.pdf
18. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
19. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
Readmission rates are frequently used as a hospital quality metric, with use including payment incentive at the hospital level,1 specific condition quality measurement,2 balancing measures for quality improvement projects,3-5 transition success,6,7 and use in public hospital rankings.8 Currently, four methods are commonly used to evaluate pediatric readmissions, each with strengths and limitations, including the following (Appendix Table 1):
1. All-cause readmissions: A measure of any readmission within a given time period regardless of the reason for readmission.9
2. Unplanned readmission/time flag: A measure intended to identify unplanned readmissions. This measure relies on time designations within the electronic health record. The time between hospital registration and admission is calculated, and if the readmission is registered more than 24 hours prior to admission, the readmission is considered planned.10 Hereafter, this measure will be referred to as the time flag measure.
3. Pediatric all-condition readmission (PACR): A measure intended to identify unplanned readmission through the exclusion of certain procedures and diagnoses.11
4. Potentially preventable readmission (PPR): A method to identify preventable readmissions based on a proprietary algorithm developed by
While all four of these measures are used to assess quality, there is little known about these measures’ ability to exclude planned readmissions and identify only preventable pediatric readmission, which conceptually is most relevant to the quality of care. However, many of these measures were not intended to capture preventability, but instead capture the related issue of whether the readmission was planned. Therefore, we sought to evaluate the four readmission measures as they relate to both preventability and unplanned status as determined through medical record review with multidisciplinary care provider input.
METHODS
As part of a hospital-wide readmission reduction quality improvement collaborative at a free-standing tertiary care children’s hospital, clinicians from hospital medicine, cardiology, neonatology, and neurology teams reviewed 30-day readmissions using a standardized abstraction tool. All readmission events (observation or inpatient encounter) after any discharge (observation or inpatient encounter) from eligible units were reviewed; therefore, each hospitalization was a potential index hospitalization. We classified the preventability of each readmission with use of a previously described Likert scale with high interrater reliability.14 For these analyses, readmissions were considered preventable if the reviewing team rated them as either “more likely preventable” or “preventable in most circumstances.” Each readmission was also evaluated as planned or unplanned. Methods for readmission review and classification are in the Appendix.
We included all readmissions between July 2014 and June 2016. We compared the medical record review classifications with the assessments from each of the four measures of pediatric readmission. We calculated sensitivity and specificity for both outcomes (planned/unplanned and preventable/not preventable) for all four measures. For standardization of discussion, we categorized description of measure performance as “very poor” as less than 50%, “poor” between 50%-75%, “fair” as 75%-85%, “good” as 85%-90%, “very good” as 90%-95% and excellent as greater than 95%. We also calculated positive and negative predictive value (PPV and NPV) over plausible ranges of prevalence using the sensitivity and specificity of each comparison (Appendix).
Of note, certain exclusions are outlined by the PACR and PPR algorithms. The PACR evaluates only readmission events that occur in children younger than 18 years. The PPR algorithm does not assign preventability if either the index or readmission event is classified as an observation stay or if it is part of a larger chain of readmissions.
RESULTS
Among 30-day readmissions considered, 1,643 were eligible for medical record review; 1,125 reviews were completed by the clinical teams (68.5%). The median time to readmission was 7 days (interquartile range [IQR], 4-18). Most children were non-Hispanic White (71%) or Black (20%). The median age at hospitalization was 2.3 years (IQR 0.4-12.1). Most children had Medicaid (56%) or private (41%) insurance. Most of the reviews were performed in cardiology (43%) and hospital medicine (37%) with patients in neurology (13%) and neonatology (7%) constituting the remaining reviews. Uncontrolled advancement of chronic disease was the most common readmission category on medical record review (25.1%), followed by unrelated readmission (20.7%), scheduled readmission (20.4%), and progression of acute disease (16.6%) (Appendix Table 2).
Assessment of Preventable and Unplanned Readmissions
On multidisciplinary medical record review, most readmissions were classified as not preventable (84.5%). Specifically, 64% were not preventable and unplanned; 20% were deemed not preventable and planned. Only 15% were classified as unplanned and preventable and 1% as planned and preventable (Appendix Figure: Population A/B).
Matching Chart Review to the Four Algorithms
All 1,125 readmissions were assessed by the all-cause and time flag readmission measures (Appendix Figure: Population A/B). After applying algorithm exclusions (details in Appendix), only 804 of the 1,125 (71.5%) reviewed readmissions matched for PACR readmission comparison (Appendix Figure: Population C); 487 of the 1,125 (43.3%) of the reviewed readmissions matched for PPR comparison (Appendix Figure: Population D).
All-Cause
Because all-cause determines only if a readmission occurs, the measure is by definition 100% sensitive and 0% specific in both assessment of preventability and unplanned readmission (Table: Section A).

Time Flag
The time flag measure identified 80% (866/1,112) of the readmissions as unplanned. This measure had very good sensitivity but very poor specificity in identifying preventable readmissions, which corresponded to very poor PPV and good to excellent NPV. In terms of identifying unplanned readmissions, the time flag measure had excellent sensitivity and very good specificity, which corresponded to very good to excellent PPV and good to very good NPV (Table: Section B).
PACR
The PACR algorithm identified 75% (599/796) of readmissions as unplanned. The PACR has good sensitivity but very poor specificity in identifying preventable readmissions, which corresponded to very poor PPV and fair to very good NPV. In terms of identifying unplanned readmissions, the PACR had fair sensitivity but poor specificity, which corresponded to fair PPV and poor NPV (Table: Section C).
PPR
The PPR algorithm identified 53% (257/487) of admissions as potentially preventable. The PPR algorithm had poor sensitivity and specificity in identifying preventable readmissions, which corresponded to very poor PPV and fair to very good NPV. In terms of identifying unplanned readmissions, the PPR algorithm had poor sensitivity and fair specificity in identifying unplanned readmissions, which corresponded to fair to good PPV and very poor to poor NPV (Table: Section D).
Evaluation of Excluded Readmission Events
Because both the PACR and PPR had large numbers of algorithm exclusions, we describe the preventability and unplanned assessment of the excluded readmission events. Both algorithms excluded preventable events. Of the 321 readmissions excluded by the PACR algorithm, 13.4% were classified as preventable by chart review. Likewise, 14.9% of 638 readmissions excluded by PPR were classified as preventable by chart review.
DISCUSSION
The ability to accurately capture preventable pediatric readmission is a goal for hospital quality experts and health policymakers alike. Of the four commonly used readmission measures to assess readmission, only PPR is designed to focus on preventability. Unfortunately, none of these four measures is adequately sensitive or specific to identify preventable readmissions; all measures had very poor PPV for preventability. Of the four measures, the time flag measure had the best sensitivity, specificity, PPV, and NPV for identifying unplanned readmissions.
The overall percentage of unplanned readmissions identified by both the time flag and by PACR measures match the overall percentage of unplanned readmissions identified in chart review: The time flag measure identified 80% of admissions as unplanned versus 79% identified by chart review (Appendix Figure: Population A/B); PACR classified 75% as unplanned versus 81% identified by chart review for PACR-eligible readmissions (Appendix Figure: Population C). In contrast, the PPR algorithm classified many more readmissions as potentially preventable (53%) than were identified by chart review at only 16% (Appendix Figure: Population D). The PACR and PPR algorithms also exclude a significant number of readmissions that are unplanned and a smaller, but not trivial, number of readmissions that are preventable; these exclusions limit their accuracy.
The ability to apply these four measures in real time during a hospitalization varies by metric. Two of the measures, the all-cause and time flag, can be applied during a readmission event, which is appealing for quality improvement initiatives. These measures allow for notification of providers that a current hospitalization is a readmission event, which allows providers the opportunity to learn from these events as they occur (Appendix Table 1). While “unplanned” is not the same as “potentially preventable,” almost all potentially preventable readmissions are unplanned; therefore, accurately identifying unplanned readmissions is more beneficial than all-cause. Additionally, a low all-cause readmission rate can be indicative of poor access to scheduled procedures. Nevertheless, all-cause readmission is sometimes used to measure quality.1,8 While the time flag measure may be more useful for quality improvement initiatives and hospital providers, it relies on hospital registration time, which is not widely available in administrative data sources and, therefore, has limited usefulness to policymakers.
Both PACR and PPR require administrative claims analysis, which is appealing from a policy standpoint. However, the reliance on claims data means the inclusion/exclusion of events can occur only retrospectively, which limits the usefulness of these measures in learning and intervening in real time. When the two measures are compared, PACR offers better sensitivity and PPR offers better specificity with regard to identifying unplanned readmission. The PPR software overcalls preventable readmissions, identifying more readmissions as preventable than there actually are. Nevertheless, Medicaid in several states uses PPR for payment incentive.1,15-17 Given the poor performance of PPR in assessing both preventable and unplanned pediatric readmission, the use of this measure as a quality metric should be limited.
This study should be considered in the context of several limitations. Because the assessment of preventability was determined as part of a learning quality improvement collaborative and not as a planned research endeavor, not all readmission reviews were completed nor were other existent tools18 that allow for preventability assessment via more structured medical record review used. Second, we reviewed cases only from certain clinical services, which would limit generalizability of these findings to all pediatric admissions. However, given the low sensitivity and specificity of some of the metrics, we would not anticipate that the addition of other types of admissions would improve the sensitivity and specificity enough to ensure reliability. Third, while we relied on an established method to determine preventability, prior work has demonstrated that additional information gathered from families may change preventability.19 Finally, due to the exclusions required by the PPR and PACR algorithms, not all readmission events were reviewed. However, these exclusions reflect the actual specifications of use for both measures.
CONCLUSION
The PPR software has poor fidelity in identifying preventable and unplanned pediatric readmission; this finding has broad policy implications given how widely it is used by state Medicaid offices to assess financial penalties. Among the four pediatric readmission measures used, the time flag metric best identifies unplanned readmissions.
Disclosures
The authors have no conflicts of interest or financial relationships relevant to this article to disclose.
Funding
Dr Auger’s research is supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1). The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Readmission rates are frequently used as a hospital quality metric, with use including payment incentive at the hospital level,1 specific condition quality measurement,2 balancing measures for quality improvement projects,3-5 transition success,6,7 and use in public hospital rankings.8 Currently, four methods are commonly used to evaluate pediatric readmissions, each with strengths and limitations, including the following (Appendix Table 1):
1. All-cause readmissions: A measure of any readmission within a given time period regardless of the reason for readmission.9
2. Unplanned readmission/time flag: A measure intended to identify unplanned readmissions. This measure relies on time designations within the electronic health record. The time between hospital registration and admission is calculated, and if the readmission is registered more than 24 hours prior to admission, the readmission is considered planned.10 Hereafter, this measure will be referred to as the time flag measure.
3. Pediatric all-condition readmission (PACR): A measure intended to identify unplanned readmission through the exclusion of certain procedures and diagnoses.11
4. Potentially preventable readmission (PPR): A method to identify preventable readmissions based on a proprietary algorithm developed by
While all four of these measures are used to assess quality, there is little known about these measures’ ability to exclude planned readmissions and identify only preventable pediatric readmission, which conceptually is most relevant to the quality of care. However, many of these measures were not intended to capture preventability, but instead capture the related issue of whether the readmission was planned. Therefore, we sought to evaluate the four readmission measures as they relate to both preventability and unplanned status as determined through medical record review with multidisciplinary care provider input.
METHODS
As part of a hospital-wide readmission reduction quality improvement collaborative at a free-standing tertiary care children’s hospital, clinicians from hospital medicine, cardiology, neonatology, and neurology teams reviewed 30-day readmissions using a standardized abstraction tool. All readmission events (observation or inpatient encounter) after any discharge (observation or inpatient encounter) from eligible units were reviewed; therefore, each hospitalization was a potential index hospitalization. We classified the preventability of each readmission with use of a previously described Likert scale with high interrater reliability.14 For these analyses, readmissions were considered preventable if the reviewing team rated them as either “more likely preventable” or “preventable in most circumstances.” Each readmission was also evaluated as planned or unplanned. Methods for readmission review and classification are in the Appendix.
We included all readmissions between July 2014 and June 2016. We compared the medical record review classifications with the assessments from each of the four measures of pediatric readmission. We calculated sensitivity and specificity for both outcomes (planned/unplanned and preventable/not preventable) for all four measures. For standardization of discussion, we categorized description of measure performance as “very poor” as less than 50%, “poor” between 50%-75%, “fair” as 75%-85%, “good” as 85%-90%, “very good” as 90%-95% and excellent as greater than 95%. We also calculated positive and negative predictive value (PPV and NPV) over plausible ranges of prevalence using the sensitivity and specificity of each comparison (Appendix).
Of note, certain exclusions are outlined by the PACR and PPR algorithms. The PACR evaluates only readmission events that occur in children younger than 18 years. The PPR algorithm does not assign preventability if either the index or readmission event is classified as an observation stay or if it is part of a larger chain of readmissions.
RESULTS
Among 30-day readmissions considered, 1,643 were eligible for medical record review; 1,125 reviews were completed by the clinical teams (68.5%). The median time to readmission was 7 days (interquartile range [IQR], 4-18). Most children were non-Hispanic White (71%) or Black (20%). The median age at hospitalization was 2.3 years (IQR 0.4-12.1). Most children had Medicaid (56%) or private (41%) insurance. Most of the reviews were performed in cardiology (43%) and hospital medicine (37%) with patients in neurology (13%) and neonatology (7%) constituting the remaining reviews. Uncontrolled advancement of chronic disease was the most common readmission category on medical record review (25.1%), followed by unrelated readmission (20.7%), scheduled readmission (20.4%), and progression of acute disease (16.6%) (Appendix Table 2).
Assessment of Preventable and Unplanned Readmissions
On multidisciplinary medical record review, most readmissions were classified as not preventable (84.5%). Specifically, 64% were not preventable and unplanned; 20% were deemed not preventable and planned. Only 15% were classified as unplanned and preventable and 1% as planned and preventable (Appendix Figure: Population A/B).
Matching Chart Review to the Four Algorithms
All 1,125 readmissions were assessed by the all-cause and time flag readmission measures (Appendix Figure: Population A/B). After applying algorithm exclusions (details in Appendix), only 804 of the 1,125 (71.5%) reviewed readmissions matched for PACR readmission comparison (Appendix Figure: Population C); 487 of the 1,125 (43.3%) of the reviewed readmissions matched for PPR comparison (Appendix Figure: Population D).
All-Cause
Because all-cause determines only if a readmission occurs, the measure is by definition 100% sensitive and 0% specific in both assessment of preventability and unplanned readmission (Table: Section A).

Time Flag
The time flag measure identified 80% (866/1,112) of the readmissions as unplanned. This measure had very good sensitivity but very poor specificity in identifying preventable readmissions, which corresponded to very poor PPV and good to excellent NPV. In terms of identifying unplanned readmissions, the time flag measure had excellent sensitivity and very good specificity, which corresponded to very good to excellent PPV and good to very good NPV (Table: Section B).
PACR
The PACR algorithm identified 75% (599/796) of readmissions as unplanned. The PACR has good sensitivity but very poor specificity in identifying preventable readmissions, which corresponded to very poor PPV and fair to very good NPV. In terms of identifying unplanned readmissions, the PACR had fair sensitivity but poor specificity, which corresponded to fair PPV and poor NPV (Table: Section C).
PPR
The PPR algorithm identified 53% (257/487) of admissions as potentially preventable. The PPR algorithm had poor sensitivity and specificity in identifying preventable readmissions, which corresponded to very poor PPV and fair to very good NPV. In terms of identifying unplanned readmissions, the PPR algorithm had poor sensitivity and fair specificity in identifying unplanned readmissions, which corresponded to fair to good PPV and very poor to poor NPV (Table: Section D).
Evaluation of Excluded Readmission Events
Because both the PACR and PPR had large numbers of algorithm exclusions, we describe the preventability and unplanned assessment of the excluded readmission events. Both algorithms excluded preventable events. Of the 321 readmissions excluded by the PACR algorithm, 13.4% were classified as preventable by chart review. Likewise, 14.9% of 638 readmissions excluded by PPR were classified as preventable by chart review.
DISCUSSION
The ability to accurately capture preventable pediatric readmission is a goal for hospital quality experts and health policymakers alike. Of the four commonly used readmission measures to assess readmission, only PPR is designed to focus on preventability. Unfortunately, none of these four measures is adequately sensitive or specific to identify preventable readmissions; all measures had very poor PPV for preventability. Of the four measures, the time flag measure had the best sensitivity, specificity, PPV, and NPV for identifying unplanned readmissions.
The overall percentage of unplanned readmissions identified by both the time flag and by PACR measures match the overall percentage of unplanned readmissions identified in chart review: The time flag measure identified 80% of admissions as unplanned versus 79% identified by chart review (Appendix Figure: Population A/B); PACR classified 75% as unplanned versus 81% identified by chart review for PACR-eligible readmissions (Appendix Figure: Population C). In contrast, the PPR algorithm classified many more readmissions as potentially preventable (53%) than were identified by chart review at only 16% (Appendix Figure: Population D). The PACR and PPR algorithms also exclude a significant number of readmissions that are unplanned and a smaller, but not trivial, number of readmissions that are preventable; these exclusions limit their accuracy.
The ability to apply these four measures in real time during a hospitalization varies by metric. Two of the measures, the all-cause and time flag, can be applied during a readmission event, which is appealing for quality improvement initiatives. These measures allow for notification of providers that a current hospitalization is a readmission event, which allows providers the opportunity to learn from these events as they occur (Appendix Table 1). While “unplanned” is not the same as “potentially preventable,” almost all potentially preventable readmissions are unplanned; therefore, accurately identifying unplanned readmissions is more beneficial than all-cause. Additionally, a low all-cause readmission rate can be indicative of poor access to scheduled procedures. Nevertheless, all-cause readmission is sometimes used to measure quality.1,8 While the time flag measure may be more useful for quality improvement initiatives and hospital providers, it relies on hospital registration time, which is not widely available in administrative data sources and, therefore, has limited usefulness to policymakers.
Both PACR and PPR require administrative claims analysis, which is appealing from a policy standpoint. However, the reliance on claims data means the inclusion/exclusion of events can occur only retrospectively, which limits the usefulness of these measures in learning and intervening in real time. When the two measures are compared, PACR offers better sensitivity and PPR offers better specificity with regard to identifying unplanned readmission. The PPR software overcalls preventable readmissions, identifying more readmissions as preventable than there actually are. Nevertheless, Medicaid in several states uses PPR for payment incentive.1,15-17 Given the poor performance of PPR in assessing both preventable and unplanned pediatric readmission, the use of this measure as a quality metric should be limited.
This study should be considered in the context of several limitations. Because the assessment of preventability was determined as part of a learning quality improvement collaborative and not as a planned research endeavor, not all readmission reviews were completed nor were other existent tools18 that allow for preventability assessment via more structured medical record review used. Second, we reviewed cases only from certain clinical services, which would limit generalizability of these findings to all pediatric admissions. However, given the low sensitivity and specificity of some of the metrics, we would not anticipate that the addition of other types of admissions would improve the sensitivity and specificity enough to ensure reliability. Third, while we relied on an established method to determine preventability, prior work has demonstrated that additional information gathered from families may change preventability.19 Finally, due to the exclusions required by the PPR and PACR algorithms, not all readmission events were reviewed. However, these exclusions reflect the actual specifications of use for both measures.
CONCLUSION
The PPR software has poor fidelity in identifying preventable and unplanned pediatric readmission; this finding has broad policy implications given how widely it is used by state Medicaid offices to assess financial penalties. Among the four pediatric readmission measures used, the time flag metric best identifies unplanned readmissions.
Disclosures
The authors have no conflicts of interest or financial relationships relevant to this article to disclose.
Funding
Dr Auger’s research is supported by a grant from the Agency for Healthcare Research and Quality (1K08HS204735-01A1). The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 5UL1TR001425-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
1. State Medicaid Payment Policies for Inpatient Hospital Services. Medicaid and CHIP Payment and Access Commission; December 2018. Accessed June 1, 2019. https://www.macpac.gov/publication/macpac-inpatient-hospital-payment-landscapes/
2. Mangione-Smith R, Zhou C, Williams DJ, et al. Pediatric Respiratory Illness Measurement System (PRIMES) scores and outcomes. Pediatrics. 2019;144(2):e20190242. https://doi.org/10.1542/peds.2019-0242
3. Biondi EA, McCulloh R, Staggs VS, et al. Reducing Variability in the Infant Sepsis Evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3):e20182201. https://doi.org/10.1542/peds.2018-2201
4. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving discharge efficiency in medically complex pediatric patients. Pediatrics. 2016;138(2):e20153832. https://doi.org/10.1542/peds.2015-3832
5. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
6. Auger KA, Simmons JM, Tubbs-Cooley HL, et al; H20 Trial Study Group. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2017-3919
7. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482
8. Olmsted MG, Powell R, Murphy J, Bell Denise, Stanley M, Sanchz R. Methodology: U.S. News & World Report Best Children’s Hospitals 2019-20. U.S. News & World Report; June 17, 2019. Accessed June 16, 2020. https://www.usnews.com/static/documents/health/best-hospitals/BCH_Methodology_2019-20.pdf
9. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
10. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e102. https://doi.org/10.1016/j.jpeds.2015.11.051
11. Readmissions-Content. Boston Children’s Hospital. Accessed April 8, 2019. http://www.childrenshospital.org/research-and-innovation/research/centers/center-of-excellence-for-pediatric-quality-measurement-cepqm/cepqm-measures/pediatric-readmissions/content
12. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-9.e5. https://doi.org/10.1016/j.jpeds.2014.10.052
13. Auger KA, Teufel RJ, Harris JM, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2):e20161720. https://doi.org/10.1542/peds.2016-1720
14. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
15. Potentially Preventable Events. Texas Health and Human Services. Accessed May 19, 2019. https://hhs.texas.gov/about-hhs/process-improvement/medicaid-chip-quality-efficiency-improvement/potentially-preventable-events
16. Potentially Preventable Readmissions. New York State Department of Health. Accessed May 28, 2019. https://regs.health.ny.gov/sites/default/files/pdf/recently_adopted_regulations/2011-02-23_potentially_preventable_readmissions.pdf
17. Potentially Preventable Readmissions Policy. Illinois Department of Healthcare and Family Services. Accessed May 28, 2019. https://www.illinois.gov/hfs/SiteCollectionDocuments/PPR_Overview.pdf
18. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
19. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
1. State Medicaid Payment Policies for Inpatient Hospital Services. Medicaid and CHIP Payment and Access Commission; December 2018. Accessed June 1, 2019. https://www.macpac.gov/publication/macpac-inpatient-hospital-payment-landscapes/
2. Mangione-Smith R, Zhou C, Williams DJ, et al. Pediatric Respiratory Illness Measurement System (PRIMES) scores and outcomes. Pediatrics. 2019;144(2):e20190242. https://doi.org/10.1542/peds.2019-0242
3. Biondi EA, McCulloh R, Staggs VS, et al. Reducing Variability in the Infant Sepsis Evaluation (REVISE): a national quality initiative. Pediatrics. 2019;144(3):e20182201. https://doi.org/10.1542/peds.2018-2201
4. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving discharge efficiency in medically complex pediatric patients. Pediatrics. 2016;138(2):e20153832. https://doi.org/10.1542/peds.2015-3832
5. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. https://doi.org/10.1136/bmjqs-2013-002556
6. Auger KA, Simmons JM, Tubbs-Cooley HL, et al; H20 Trial Study Group. Postdischarge nurse home visits and reuse: the Hospital to Home Outcomes (H2O) trial. Pediatrics. 2018;142(1):e20173919. https://doi.org/10.1542/peds.2017-3919
7. Auger KA, Shah SS, Tubbs-Cooley HL, et al. Effects of a 1-time nurse-led telephone call after pediatric discharge: the H2O II randomized clinical trial. JAMA Pediatr. 2018;172(9):e181482. https://doi.org/10.1001/jamapediatrics.2018.1482
8. Olmsted MG, Powell R, Murphy J, Bell Denise, Stanley M, Sanchz R. Methodology: U.S. News & World Report Best Children’s Hospitals 2019-20. U.S. News & World Report; June 17, 2019. Accessed June 16, 2020. https://www.usnews.com/static/documents/health/best-hospitals/BCH_Methodology_2019-20.pdf
9. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527
10. Auger KA, Mueller EL, Weinberg SH, et al. A validated method for identifying unplanned pediatric readmission. J Pediatr. 2016;170:105-12.e102. https://doi.org/10.1016/j.jpeds.2015.11.051
11. Readmissions-Content. Boston Children’s Hospital. Accessed April 8, 2019. http://www.childrenshospital.org/research-and-innovation/research/centers/center-of-excellence-for-pediatric-quality-measurement-cepqm/cepqm-measures/pediatric-readmissions/content
12. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-9.e5. https://doi.org/10.1016/j.jpeds.2014.10.052
13. Auger KA, Teufel RJ, Harris JM, et al. Children’s hospital characteristics and readmission metrics. Pediatrics. 2017;139(2):e20161720. https://doi.org/10.1542/peds.2016-1720
14. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of early readmissions at a children’s hospital. Pediatrics. 2013;131(1):e171-e181. https://doi.org/10.1542/peds.2012-0820
15. Potentially Preventable Events. Texas Health and Human Services. Accessed May 19, 2019. https://hhs.texas.gov/about-hhs/process-improvement/medicaid-chip-quality-efficiency-improvement/potentially-preventable-events
16. Potentially Preventable Readmissions. New York State Department of Health. Accessed May 28, 2019. https://regs.health.ny.gov/sites/default/files/pdf/recently_adopted_regulations/2011-02-23_potentially_preventable_readmissions.pdf
17. Potentially Preventable Readmissions Policy. Illinois Department of Healthcare and Family Services. Accessed May 28, 2019. https://www.illinois.gov/hfs/SiteCollectionDocuments/PPR_Overview.pdf
18. Jonas JA, Devon EP, Ronan JC, et al. Determining preventability of pediatric readmissions using fault tree analysis. J Hosp Med. 2016;11(5):329-335. https://doi.org/10.1002/jhm.2555
19. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182. https://doi.org/10.1542/peds.2015-4182
© 2020 Society of Hospital Medicine
Healthcare Resource Utilization Following a Discharge Against Medical Advice: An Analysis of Commercially Insured Adults
Discharges against medical advice (DAMAs), in which a patient leaves the hospital prior to a physician-recommended endpoint, represent approximately 1% to 2% of inpatient discharges in the United States.1 When compared with routine discharges, a DAMA is associated with adverse clinical consequences, including an increased risk of all-cause mortality.2,3 Additionally, due to incomplete care, a DAMA may result in increased healthcare resource utilization (HcRU), including the use of inpatient, emergency department (ED), and outpatient services in the postdischarge period. Quantifying these relationships can provide important information regarding an individual’s healthcare-seeking behavior following a DAMA.
Prior literature has focused on the association between a DAMA and the risk of inpatient readmission. Relative to routine discharges, a DAMA is associated with a 1.5 to 2 times increased risk of a 30-day readmission.3-9 However, these estimates are based on mixed-payer populations primarily composed (65%-80%) of individuals with public (Medicaid, Medicare) or no insurance. Further, they do not differentiate this association by payer type. It is unclear if prior results apply to commercially insured adults. These individuals represent a small but nonnegligible proportion (19%) of all DAMAs in the United States.10 Quantifying relationships among commercially insured adults can help advance our understanding of readmission patterns in the DAMA population.
There is limited evidence regarding the relationship between a DAMA and outpatient HcRU in the postdischarge period. Use of ED services after a DAMA has been explored only in specific disease populations such as asthma.4 Additionally, prior studies have reported a reduced frequency in the receipt of medication prescriptions and outpatient follow-up plans among individuals with a DAMA at the time of discharge.11,12 Whether these practices translate to altered patterns of postdischarge prescription drug fills or use of outpatient services is not known.
To address these substantive gaps in the literature, the present study evaluates the association between a DAMA and all-cause HcRU in the postdischarge period among commercially insured adults. We examined HcRU across all points of service including inpatient readmissions, ED visits, physician office visits, nonphysician outpatient encounters, and prescription drug fills. These results can serve as a benchmark for comparison to future studies on DAMAs among publicly insured or uninsured individuals. Furthermore, such knowledge can help providers, payers, and policy planners make evidence-based decisions regarding postdischarge healthcare delivery.
METHODS
Data Source
This retrospective study used a 10% random sample of enrollees in the IQVIA PharMetrics® Plus database (purchased by University of Maryland, Baltimore, under license from IQVIA). The database is composed of fully adjudicated claims and enrollment information from over 70 contributing US health plans and self-insured employer groups for over 140 million unique enrollees from 2006 onward. The enrollee population is generally representative of the commercially insured population that is younger than 65 years of age (with a subset of commercial Medicare and Medicaid) with respect to age and gender.
The database allows longitudinal follow-up for individuals using three files: medical claims, pharmacy claims, and insurance eligibility. The average length of enrollment is 39 months. The claims data represent payments to providers for services rendered to individuals covered by health plans. The medical claims file contains information on diagnostic and therapeutic services rendered in the inpatient and outpatient settings. The pharmacy claims file captures data on prescription drugs dispensed in retail and mail-order settings. The eligibility file contains demographic and insurance eligibility information for individuals.
Study Population
We identified all individuals aged 18 to 64 years with an inpatient admission record between January 1, 2007, and December 31, 2015. All individuals with continuous medical and prescription drug coverage from 6 months prior to the hospital admission date (baseline period) through 30 days following the discharge date (follow-up period) were included. Inpatient admissions with a missing discharge disposition or those that resulted in in-hospital death, discharge to a short-term hospital, skilled nursing facility, intermediate care facility, or any other type of facility were not considered for analysis. Only the first eligible inpatient admission was considered for analysis.
Main Predictor Variable
Individuals with a DAMA were analyzed as the case group. A DAMA was identified using the “Patient Status Code” variable, which represents the discharge disposition of each individual. Individuals who were discharged to home/self-care or discharged to a home health organization formed the control group (hereafter referred to as routine discharge).
Demographic, Clinical, and Hospitalization Characteristics
An individual’s age, sex, and region of residence were determined at the date of hospital admission. The Elixhauser algorithm was used to categorize comorbid conditions (as scores of 0, 1-2, ≥3 depending on number of comorbidities) based on International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis codes during the baseline period.13,14 The following characteristics of each individual’s eligible inpatient admission were captured: year, timing (weekday or weekend), length of stay (LOS, measured in days), and receipt of a surgical procedure.
Outcomes
All-cause HcRU was identified during the 30-day postdischarge period. Specifically, we identified inpatient readmissions, ED visits, physician office visits, nonphysician outpatient encounters (for example, pathology, radiology, outpatient surgical services), and prescription drug fills. Binary variables (yes or no) were created for inpatient readmissions and ED visits while the remaining HcRU categories (ie, physician office visits, nonphysician outpatient encounters, and prescription drug fills) were analyzed as count variables. In the sensitivity analyses, we provide results for HcRU outcomes among a subgroup of individuals who had at least 90 days of continuous medical and prescription drug benefits following the hospital discharge.
Statistical Analysis
Descriptive Analysis
Measures of interest were reported using summary statistics depending on the nature of the variable. Continuous variables were described using t tests, and categorical variables were described using chi-square tests.
Propensity Score Matching
Cases and controls were matched using a 1:1 greedy matching algorithm based on propensity scores.15 We developed propensity scores based on confounders that we hypothesized would be associated with a DAMA and postdischarge HcRU. The propensity score model included the following variables: age, sex, region of residence, Elixhauser comorbidity index score, year of admission, timing of admission, LOS, and presence of any surgical procedure during the inpatient admission. The best match between cases and controls was determined based on the absolute difference in their propensity scores, which allowed for a maximal caliper width of 0.2 of the standard deviation of the logit of the propensity score.16 A standardized difference value of less than 0.1 was used to assess balance in baseline patient and hospital characteristics between cases and controls consistent with prior literature.17,18 Proportions and balance, as measured by standardized differences between baseline covariates across cases and controls in the matched sample, are displayed in tabular format (Appendix Table 1).
Healthcare Resource Utilization
We estimated the adjusted odds ratio (AOR) using a logistic regression model. The AOR quantified the association between a DAMA and the prevalence of all-cause inpatient readmissions and ED visits during the 30-day postdischarge period. We estimated incident rate ratios (IRR) for count outcomes. Given the large number of individuals with no physician office visits, nonphysician outpatient encounters, or prescription drug fills, we estimated model parameters for IRRs using a finite mixture negative binomial hurdle model.19 We considered the data to represent a mixture of a constant distribution (which always generates zero counts) and a zero-truncated distribution (which always generates nonzero counts). The finite mixture count models include two outcomes: the mixing probabilities and the count distribution. The mixing probabilities quantify the probability that an observation for the HcRU category will be drawn from either the constant distribution (with mass at zero) or the count distribution. Conditional on having positive values, a zero-truncated generalized linear model (GLM) governs the count variable. Compared with other GLM specifications (eg, Poisson, negative binomial, zero-inflated), the negative binomial hurdle model presented the best-fitting model across several information criteria statistics (Appendix Figures 1-3 and Appendix Tables 2-4).
The GLM results provided IRR for the counts of HcRU. Ratios were interpreted as evidence of increased HcRU (IRR ≥ 1.0) or decreased HcRU (IRR < 1.0) among individuals with a DAMA compared with those discharged routinely. For all HcRU analyses, we reported results for the matched sample. All analyses were conducted using SAS version 9.4 (SAS Institute), and statistical significance was determined at α= .05. The study received the University of Maryland, Baltimore, Institutional Review Board approval (HP-00081497).
RESULTS
The unmatched sample included 457,530 individuals, of whom 0.5% had a DAMA. A consort diagram illustrating cohort inclusion and exclusion criteria is presented in Appendix Figure 4. Demographic, clinical, and inpatient admission characteristics of the unmatched sample and for subgroups defined by discharge status are displayed in Table 1. In the unmatched sample, the median age at admission was higher for individuals with a DAMA than it was for those discharged routinely (43 vs 42 years, respectively), and the proportion of males was higher among those with a DAMA (58.4% vs 33.1%). There were statistically significant differences based on the geographic region of residence and the comorbidity burden across both groups. The median LOS was shorter (1 day vs 2 days), the proportion of weekend admissions was higher (22.2% vs 16.3%), and the proportion of inpatient surgical procedures was lower (12.9% vs 59.2%) among those with a DAMA compared with that among those with routine discharges. The propensity score-matched sample included 2,245 cases and 2,245 controls (Appendix Table 1). Standardized differences for all baseline factors were less than 0.1, indicating that cases and controls were matched on the included baseline factors.
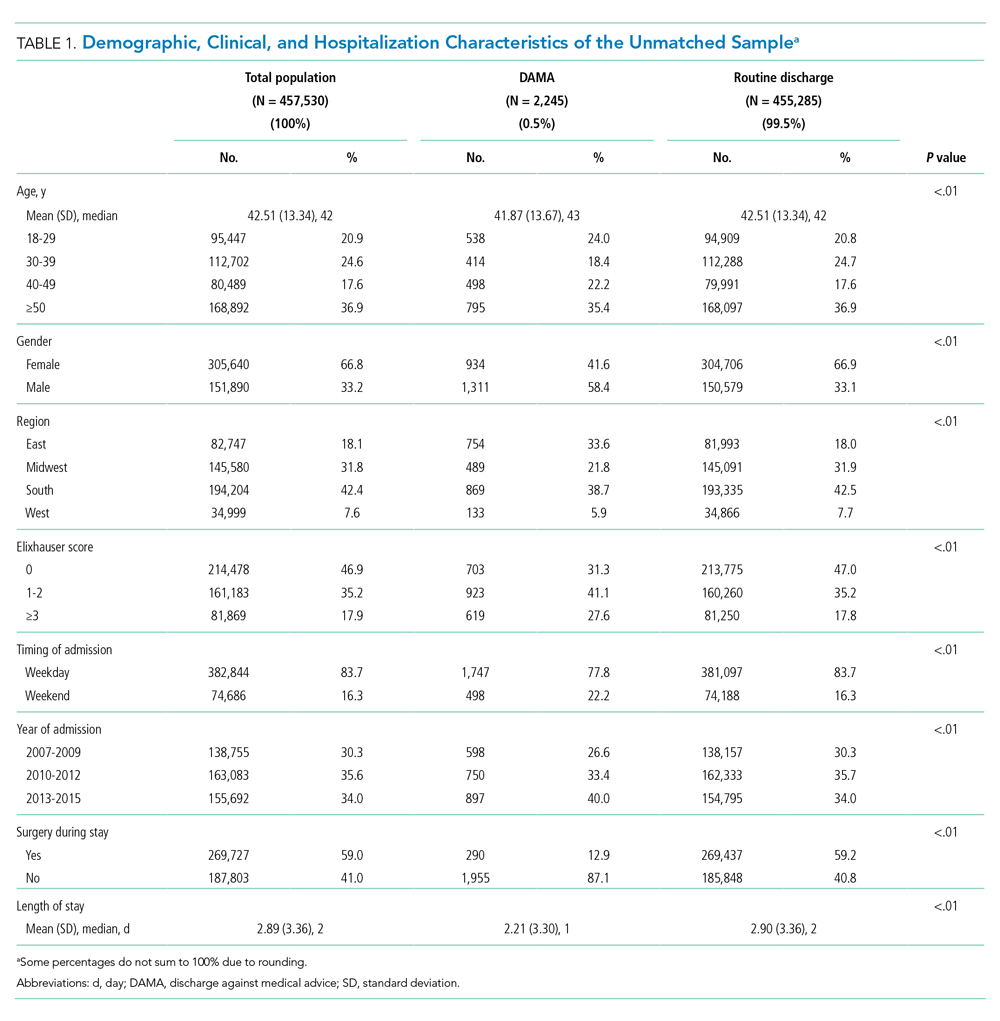
Summary Statistics: Proportions and Counts
Across the DAMA and routine discharge groups, the proportion of individuals with a 30-day inpatient readmission was similar (19.5% vs 18.7%; P = .47), whereas the proportion with an ED visit was higher (18.6% vs 9.1%; P < .01). There were no differences in the median number of inpatient readmissions (median, 0) and ED visits (median, 0) across both groups. Individuals with a DAMA and those discharged routinely displayed similar median counts of 30-day physician office (median, 1) and nonphysician outpatient encounters (median, 1) (Table 2). Individuals with a DAMA displayed a lower median number of prescription drug fills (median, 2 vs 3) than that among those with a routine discharge (Table 2).
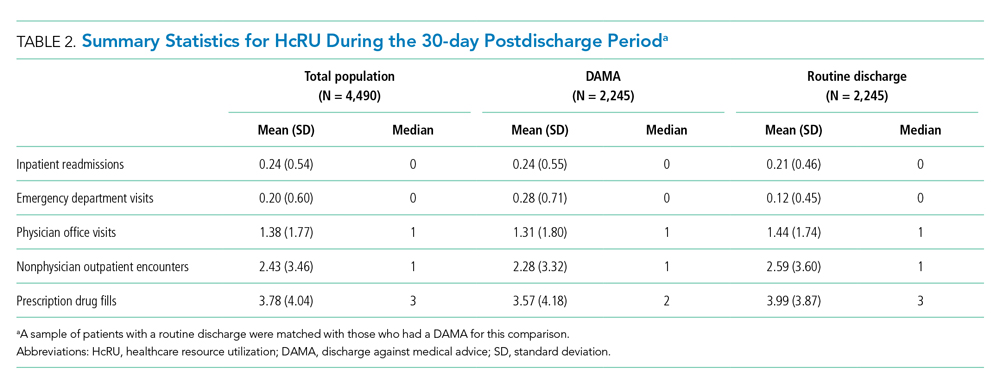
Main Analysis: Thirty-Day Healthcare Resource Utilization
The associations between a DAMA and 30-day inpatient readmissions and ED visits based on the matched sample are presented in Table 3. Individuals with a DAMA had increased odds for an ED visit (AOR, 2.28; 95% CI, 1.90-2.72) but no significant difference in the odds of a 30-day inpatient readmission (AOR, 1.06; 95% CI, 0.91-1.23) compared with those discharged routinely.

The association between a DAMA and count HcRU outcomes is presented in Table 4. Compared with those discharged routinely, individuals with a DAMA displayed no significant difference in rates for physician office visits (IRR, 1.01; 95% CI, 0.91-1.11), nonphysician outpatient encounters (IRR, 0.89; 95% CI, 0.78-1.00), and prescription drug fills (IRR, 1.03; 95% CI, 0.97-1.09) during the 30-day postdischarge period.
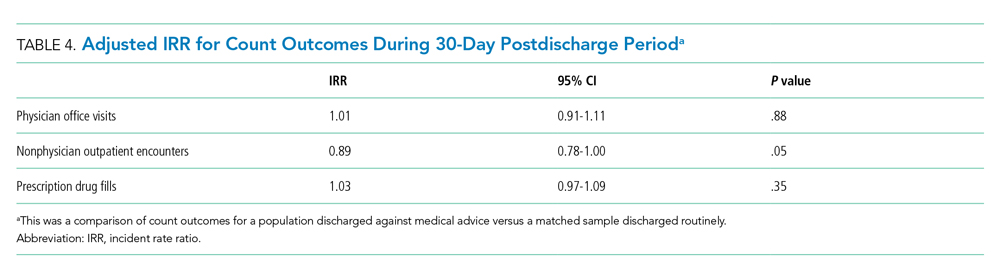
Sensitivity Analysis: Ninety-Day Healthcare Resource Utilization
Relative to those discharged routinely, individuals with a DAMA had statistically significant increased odds of 90-day inpatient readmissions (AOR, 1.18; 95% CI, 1.02-1.36), odds of ED visits (AOR, 2.16; 95% CI, 1.85-2.51), and rates of prescription drug fills (IRR, 1.32; 95% CI, 1.29-1.35). No statistically significant differences were observed in the rates of physician office visits and nonphysician outpatient encounters across both groups.
DISCUSSION
In this commercially insured sample of working age individuals, we identified an association between a DAMA and the likelihood and intensity of postdischarge HcRU. The direction of the association varied across categories of HcRU and the duration of follow-up. A DAMA was associated with increased odds of 30-day ED visits but not 30-day readmissions compared with routine discharges. No significant differences were observed in the rates of 30-day physician office visits, nonphysician outpatient encounters, and prescription drug fills across both groups. To our knowledge, this is the first study on DAMAs that examines postdischarge HcRU outside the inpatient setting.
The 0.5% prevalence of DAMAs in our study was lower than the approximate 1% to 2% value that is typically reported in the literature. Prior studies have typically reported results based on mixed-payer populations.3-10 These mixed-payer populations include publicly insured (Medicare or Medicaid) or uninsured stays, which account for a disproportionate share of all DAMAs. In contrast, commercially insured stays account for the lowest proportion of all DAMAs.10 Similar to prior literature,5 the DAMA group in our study was younger, had a higher proportion of males, had a higher comorbidity burden, and had a shorter LOS than the routinely discharged group.
We observed a greater likelihood of ED utilization after a DAMA. Similar findings have been reported, which may indicate that patients with a DAMA receive inadequate treatment at the time of discharge and may require further acute treatment. For example, a prior study reported that, after a DAMA, individuals with asthma were four times more likely to have an ED visit within 14 days compared with those discharged routinely.4
Contrary to prior findings,3-9 we found no significant difference in the odds of a 30-day inpatient readmission across the DAMA and routine discharge groups, which may be attributable to differences in the populations studied. Those previous studies used mixed payer populations and did not differentiate results by payer type. The mixed payer populations in these studies were older (mean ages, 55 years and above) and had an increased comorbidity burden compared with our commercially insured population. Furthermore, some of these studies were either limited to single sites,8 single state hospital systems,3,4,9 or focused on specific medical populations.3,4,6-9 Our national sample of commercially insured adults is considerably younger, with a mean age of 43 years. Thirty days may be too brief to observe enough inpatient readmissions for the purpose of comparative analyses. This is suggested by our results, which indicated that there is an association between DAMA and 90-day inpatient readmission. Additionally, nonsignificant findings for 30-day inpatient readmissions may also be due to the small sample size of the DAMA group in our study, which may have limited robust statistical inference. Future studies in a larger population of commercially insured individuals with a DAMA are required to confirm these findings.
Nonsignificant differences in the rates of 30-day physician office visits, nonphysician outpatient encounters, and prescription drug fills across both groups may explain the null association with 30-day inpatient readmissions. Prior literature on specific medical populations or individuals with general hospital admissions report that early outpatient follow-up can help prevent 30-day readmissions.20-25 In our sample, we observed similar rates of outpatient follow-up across the DAMA and routinely discharged groups. Prior studies based on single hospital sites have reported that, at the time of discharge, a lower proportion of individuals with a DAMA received medication prescriptions and outpatient follow-up plans compared with those discharged routinely.11,12 In contrast, we evaluated prescription drug fills and outpatient visits during the postdischarge period, which may explain the difference in findings.
The present study has several strengths. To the best of our knowledge, our study represents the first and largest retrospective analysis of DAMAs in a national sample of commercially insured adults. In addition to a large generalizable sample, we examine HcRU after a DAMA across major points of service over a longitudinal postdischarge period. Our results provide a comprehensive understanding of utilization outcomes in this population including those outside the inpatient setting, which has been the focus of prior literature. These findings can help guide the implementation of appropriate patient- and system-level interventions to optimize DAMA prevention and mitigate the associated utilization burden on the healthcare system in the postdischarge period.26,27
Our findings should be interpreted with certain limitations in mind. First, this study used data based on a commercially insured sample of patients and may not be generalizable to publicly insured or uninsured samples. Second, like prior DAMA studies that used the Nationwide Readmissions Database instead,5-7 our study was unable to account for individual-level factors such as race, marital status, family social support, income, health literacy, and activation in self-care. Further, given the limitations of our data, we were unable to control for hospital characteristics such as bed size, urban-rural designation, teaching status, and control (eg, private or government ownership). Despite the use of propensity score methods to balance both comparison groups on observable sources of confounding, we cannot rule out the possibility of residual confounding. Lastly, due to a lack of data on postdischarge mortality outcomes, we could not control for competing risk of death in our analysis. However, in a population with an average age of 43 years, we did not expect high or differential 30- or 90-day postdischarge mortality rates across both groups.
Our findings suggest several important directions for future research. First, it will be useful to examine these associations among publicly insured and uninsured samples in which a DAMA is more prevalent and in which the associations with HcRU may be more pronounced than they are in the commercially insured population. Secondly, future research should identify subgroups of DAMA patients with an increased propensity for postdischarge HcRU. This can help in the design of individualized outpatient follow-up plans that address patient-specific medical and social needs. Finally, our findings highlight the need for education, practice guidelines, and suitable interventions to help providers in the prevention and management of a DAMA.
CONCLUSION
Using data from a commercially insured population, we identified associations between a DAMA and postdischarge HcRU. The associations differed by category of HcRU. We identified a positive association with the likelihood of ED utilization but no association with the likelihood of 30-day inpatient readmission or general outpatient utilization. Our results indicate that the examination of inpatient readmissions after a DAMA should not be considered in isolation. The identification of the full range of outpatient and inpatient HcRU after a DAMA in a broad population of patients can improve our understanding of outcomes following a DAMA and support appropriate system-level interventions designed to reduce their prevalence.
Acknowledgments
The statements, findings, conclusions, views, and opinions contained and expressed in this manuscript are based in part on data obtained under license from IQVIA. Source: IQVIA PharMetrics® Plus January 2006 – December 2015, IQVIA. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA or any of its affiliated or subsidiary entities.
Disclosures
Dr Onukwugha reports grants from Bayer Healthcare Pharmaceuticals, grants from Pfizer, Inc, and personal fees from Novo Nordisk outside the submitted work. The other authors have nothing to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs, the U.S. Government, or the VA National Center for Ethics in Health Care.
Funding
The authors acknowledge the support of the University of Maryland, Baltimore Institute for Clinical & Translational Research (ICTR) through the ICTR Voucher Program.
1. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. https://doi.org/10.4065/84.3.255
2. Garland A, Ramsey CD, Fransoo R, et al. Rates of readmission and death associated with leaving hospital against medical advice: a population-based study. CMAJ. 2013;185(14):1207-1214. https://doi.org/10.1503/cmaj.130029
3. Fiscella K, Meldrum S, Barnett S. Hospital discharge against advice after myocardial infarction: deaths and readmissions. Am J Med. 2007;120(12):1047-1053. https://doi.org/10.1016/j.amjmed.2007.08.024
4. Baptist AP, Warrier I, Arora R, Ager J, Massanari RM. Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes. J Allergy Clin Immunol. 2007;119(4):924-929. https://doi.org/10.1016/j.jaci.2006.11.695
5. Kumar N. Burden of 30-day readmissions associated with discharge against medical advice among inpatients in the United States. Am J Med. 2019;132(6):708-717.e4. https://doi.org/10.1016/j.amjmed.2019.01.023
6. Kwok CS, Walsh MN, Volgman A, et al. Discharge against medical advice after hospitalisation for acute myocardial infarction. Heart. 2019;105(4):315-321. https://doi.org/10.1136/heartjnl-2018-313671
7. Patel B, Prousi G, Shah M, et al. Thirty-day readmission rate in acute heart failure patients discharged against medical advice in a matched cohort study. Mayo Clin Proc. 2018;93(10):1397-1403. https://doi.org/10.1016/j.mayocp.2018.04.023
8. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594-602. https://doi.org/10.1016/j.amjmed.2011.12.017
9. Onukwugha E, Mullins D, Loh FE, Saunders E, Shaya FT, Weir MR. Readmissions after unauthorized discharges in the cardiovascular setting. Med Care. 2011;49(2):215-224. https://doi.org/10.1097/mlr.0b013e31820192a5
10. Stranges E, Wier L, Merrill CT, Steiner C. Hospitalizations in which Patients Leave the Hospital against Medical Advice (AMA), 2007. HCUP Statistical Brief #78. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; August 2009. Accessed 04/07 2020.http://www.hcup-us.ahrq.gov/reports/statbriefs/sb78.pdf
11. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. https://doi.org/10.1002/jhm.2087
12. Stearns CR, Bakamjian A, Sattar S, Weintraub MR. Discharges against medical advice at a county hospital: provider perceptions and practice. J Hosp Med. 2017;12(1):11-17. https://doi.org/10.1002/jhm.2672
13. Garland A, Fransoo R, Olafson K, et al. The Epidemiology and Outcomes of Critical Illness in Manitoba. Manitoba Centre for Health Policy; April 2012. Accessed April 7, 2020. http://mchp-appserv.cpe.umanitoba.ca/reference/MCHP_ICU_Report_WEB_(20120403).pdf
14. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
15. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-1069. https://doi.org/10.1002/sim.6004
16. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. https://doi.org/10.1002/pst.433
17. Austin PC, Mamdani MM. A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use. Stat Med. 2006;25(12):2084-2106. https://doi.org/10.1002/sim.2328
18. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. https://doi.org/10.1016/s0895-4356(00)00321-8
19. Mullahy J. Specification and testing of some modified count data models. J Econometrics. 1986;33(3):341-365. https://doi.org/10.1016/0304-4076(86)90002-3
20. Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354-360. https://doi.org/10.1002/jhm.129
21. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
22. Leschke J, Panepinto JA, Nimmer M, Hoffmann RG, Yan K, Brousseau DC. Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406-409. https://doi.org/10.1002/pbc.23140
23. Misky GJ, Wald HL, Coleman EA. Post‐hospitalization transitions: Examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
24. Muus K, Knudson A, Klug MG, Gokun J, Sarrazin M, Kaboli P. Effect of post-discharge follow-up care on re-admissions among US veterans with congestive heart failure: a rural-urban comparison. Rural Remote Health. 2010;10(2):1447.https://doi.org/10.22605/RRH1447
25. Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow-up visits as part of a heart failure readmission quality improvement initiative. Am J Med. 2013;126(11):989-994.e1. https://doi.org/10.1016/j.amjmed.2013.06.027
26. Alfandre D. Improving quality in against medical advice discharges—more empirical evidence, enhanced professional education, and directed systems changes. J Hosp Med. 2017;12(1):59-60. https://doi.org/10.1002/jhm.2678
27. Nagarajan M, Offurum AI, Gulati M, Onukwugha E. Discharges Against Medical Advice: Prevalence, Predictors, and Populations. In: Alfandre D, ed. Against‐Medical‐Advice Discharges from the Hospital. Springer; 2018:11-29.
Discharges against medical advice (DAMAs), in which a patient leaves the hospital prior to a physician-recommended endpoint, represent approximately 1% to 2% of inpatient discharges in the United States.1 When compared with routine discharges, a DAMA is associated with adverse clinical consequences, including an increased risk of all-cause mortality.2,3 Additionally, due to incomplete care, a DAMA may result in increased healthcare resource utilization (HcRU), including the use of inpatient, emergency department (ED), and outpatient services in the postdischarge period. Quantifying these relationships can provide important information regarding an individual’s healthcare-seeking behavior following a DAMA.
Prior literature has focused on the association between a DAMA and the risk of inpatient readmission. Relative to routine discharges, a DAMA is associated with a 1.5 to 2 times increased risk of a 30-day readmission.3-9 However, these estimates are based on mixed-payer populations primarily composed (65%-80%) of individuals with public (Medicaid, Medicare) or no insurance. Further, they do not differentiate this association by payer type. It is unclear if prior results apply to commercially insured adults. These individuals represent a small but nonnegligible proportion (19%) of all DAMAs in the United States.10 Quantifying relationships among commercially insured adults can help advance our understanding of readmission patterns in the DAMA population.
There is limited evidence regarding the relationship between a DAMA and outpatient HcRU in the postdischarge period. Use of ED services after a DAMA has been explored only in specific disease populations such as asthma.4 Additionally, prior studies have reported a reduced frequency in the receipt of medication prescriptions and outpatient follow-up plans among individuals with a DAMA at the time of discharge.11,12 Whether these practices translate to altered patterns of postdischarge prescription drug fills or use of outpatient services is not known.
To address these substantive gaps in the literature, the present study evaluates the association between a DAMA and all-cause HcRU in the postdischarge period among commercially insured adults. We examined HcRU across all points of service including inpatient readmissions, ED visits, physician office visits, nonphysician outpatient encounters, and prescription drug fills. These results can serve as a benchmark for comparison to future studies on DAMAs among publicly insured or uninsured individuals. Furthermore, such knowledge can help providers, payers, and policy planners make evidence-based decisions regarding postdischarge healthcare delivery.
METHODS
Data Source
This retrospective study used a 10% random sample of enrollees in the IQVIA PharMetrics® Plus database (purchased by University of Maryland, Baltimore, under license from IQVIA). The database is composed of fully adjudicated claims and enrollment information from over 70 contributing US health plans and self-insured employer groups for over 140 million unique enrollees from 2006 onward. The enrollee population is generally representative of the commercially insured population that is younger than 65 years of age (with a subset of commercial Medicare and Medicaid) with respect to age and gender.
The database allows longitudinal follow-up for individuals using three files: medical claims, pharmacy claims, and insurance eligibility. The average length of enrollment is 39 months. The claims data represent payments to providers for services rendered to individuals covered by health plans. The medical claims file contains information on diagnostic and therapeutic services rendered in the inpatient and outpatient settings. The pharmacy claims file captures data on prescription drugs dispensed in retail and mail-order settings. The eligibility file contains demographic and insurance eligibility information for individuals.
Study Population
We identified all individuals aged 18 to 64 years with an inpatient admission record between January 1, 2007, and December 31, 2015. All individuals with continuous medical and prescription drug coverage from 6 months prior to the hospital admission date (baseline period) through 30 days following the discharge date (follow-up period) were included. Inpatient admissions with a missing discharge disposition or those that resulted in in-hospital death, discharge to a short-term hospital, skilled nursing facility, intermediate care facility, or any other type of facility were not considered for analysis. Only the first eligible inpatient admission was considered for analysis.
Main Predictor Variable
Individuals with a DAMA were analyzed as the case group. A DAMA was identified using the “Patient Status Code” variable, which represents the discharge disposition of each individual. Individuals who were discharged to home/self-care or discharged to a home health organization formed the control group (hereafter referred to as routine discharge).
Demographic, Clinical, and Hospitalization Characteristics
An individual’s age, sex, and region of residence were determined at the date of hospital admission. The Elixhauser algorithm was used to categorize comorbid conditions (as scores of 0, 1-2, ≥3 depending on number of comorbidities) based on International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis codes during the baseline period.13,14 The following characteristics of each individual’s eligible inpatient admission were captured: year, timing (weekday or weekend), length of stay (LOS, measured in days), and receipt of a surgical procedure.
Outcomes
All-cause HcRU was identified during the 30-day postdischarge period. Specifically, we identified inpatient readmissions, ED visits, physician office visits, nonphysician outpatient encounters (for example, pathology, radiology, outpatient surgical services), and prescription drug fills. Binary variables (yes or no) were created for inpatient readmissions and ED visits while the remaining HcRU categories (ie, physician office visits, nonphysician outpatient encounters, and prescription drug fills) were analyzed as count variables. In the sensitivity analyses, we provide results for HcRU outcomes among a subgroup of individuals who had at least 90 days of continuous medical and prescription drug benefits following the hospital discharge.
Statistical Analysis
Descriptive Analysis
Measures of interest were reported using summary statistics depending on the nature of the variable. Continuous variables were described using t tests, and categorical variables were described using chi-square tests.
Propensity Score Matching
Cases and controls were matched using a 1:1 greedy matching algorithm based on propensity scores.15 We developed propensity scores based on confounders that we hypothesized would be associated with a DAMA and postdischarge HcRU. The propensity score model included the following variables: age, sex, region of residence, Elixhauser comorbidity index score, year of admission, timing of admission, LOS, and presence of any surgical procedure during the inpatient admission. The best match between cases and controls was determined based on the absolute difference in their propensity scores, which allowed for a maximal caliper width of 0.2 of the standard deviation of the logit of the propensity score.16 A standardized difference value of less than 0.1 was used to assess balance in baseline patient and hospital characteristics between cases and controls consistent with prior literature.17,18 Proportions and balance, as measured by standardized differences between baseline covariates across cases and controls in the matched sample, are displayed in tabular format (Appendix Table 1).
Healthcare Resource Utilization
We estimated the adjusted odds ratio (AOR) using a logistic regression model. The AOR quantified the association between a DAMA and the prevalence of all-cause inpatient readmissions and ED visits during the 30-day postdischarge period. We estimated incident rate ratios (IRR) for count outcomes. Given the large number of individuals with no physician office visits, nonphysician outpatient encounters, or prescription drug fills, we estimated model parameters for IRRs using a finite mixture negative binomial hurdle model.19 We considered the data to represent a mixture of a constant distribution (which always generates zero counts) and a zero-truncated distribution (which always generates nonzero counts). The finite mixture count models include two outcomes: the mixing probabilities and the count distribution. The mixing probabilities quantify the probability that an observation for the HcRU category will be drawn from either the constant distribution (with mass at zero) or the count distribution. Conditional on having positive values, a zero-truncated generalized linear model (GLM) governs the count variable. Compared with other GLM specifications (eg, Poisson, negative binomial, zero-inflated), the negative binomial hurdle model presented the best-fitting model across several information criteria statistics (Appendix Figures 1-3 and Appendix Tables 2-4).
The GLM results provided IRR for the counts of HcRU. Ratios were interpreted as evidence of increased HcRU (IRR ≥ 1.0) or decreased HcRU (IRR < 1.0) among individuals with a DAMA compared with those discharged routinely. For all HcRU analyses, we reported results for the matched sample. All analyses were conducted using SAS version 9.4 (SAS Institute), and statistical significance was determined at α= .05. The study received the University of Maryland, Baltimore, Institutional Review Board approval (HP-00081497).
RESULTS
The unmatched sample included 457,530 individuals, of whom 0.5% had a DAMA. A consort diagram illustrating cohort inclusion and exclusion criteria is presented in Appendix Figure 4. Demographic, clinical, and inpatient admission characteristics of the unmatched sample and for subgroups defined by discharge status are displayed in Table 1. In the unmatched sample, the median age at admission was higher for individuals with a DAMA than it was for those discharged routinely (43 vs 42 years, respectively), and the proportion of males was higher among those with a DAMA (58.4% vs 33.1%). There were statistically significant differences based on the geographic region of residence and the comorbidity burden across both groups. The median LOS was shorter (1 day vs 2 days), the proportion of weekend admissions was higher (22.2% vs 16.3%), and the proportion of inpatient surgical procedures was lower (12.9% vs 59.2%) among those with a DAMA compared with that among those with routine discharges. The propensity score-matched sample included 2,245 cases and 2,245 controls (Appendix Table 1). Standardized differences for all baseline factors were less than 0.1, indicating that cases and controls were matched on the included baseline factors.

Summary Statistics: Proportions and Counts
Across the DAMA and routine discharge groups, the proportion of individuals with a 30-day inpatient readmission was similar (19.5% vs 18.7%; P = .47), whereas the proportion with an ED visit was higher (18.6% vs 9.1%; P < .01). There were no differences in the median number of inpatient readmissions (median, 0) and ED visits (median, 0) across both groups. Individuals with a DAMA and those discharged routinely displayed similar median counts of 30-day physician office (median, 1) and nonphysician outpatient encounters (median, 1) (Table 2). Individuals with a DAMA displayed a lower median number of prescription drug fills (median, 2 vs 3) than that among those with a routine discharge (Table 2).

Main Analysis: Thirty-Day Healthcare Resource Utilization
The associations between a DAMA and 30-day inpatient readmissions and ED visits based on the matched sample are presented in Table 3. Individuals with a DAMA had increased odds for an ED visit (AOR, 2.28; 95% CI, 1.90-2.72) but no significant difference in the odds of a 30-day inpatient readmission (AOR, 1.06; 95% CI, 0.91-1.23) compared with those discharged routinely.

The association between a DAMA and count HcRU outcomes is presented in Table 4. Compared with those discharged routinely, individuals with a DAMA displayed no significant difference in rates for physician office visits (IRR, 1.01; 95% CI, 0.91-1.11), nonphysician outpatient encounters (IRR, 0.89; 95% CI, 0.78-1.00), and prescription drug fills (IRR, 1.03; 95% CI, 0.97-1.09) during the 30-day postdischarge period.

Sensitivity Analysis: Ninety-Day Healthcare Resource Utilization
Relative to those discharged routinely, individuals with a DAMA had statistically significant increased odds of 90-day inpatient readmissions (AOR, 1.18; 95% CI, 1.02-1.36), odds of ED visits (AOR, 2.16; 95% CI, 1.85-2.51), and rates of prescription drug fills (IRR, 1.32; 95% CI, 1.29-1.35). No statistically significant differences were observed in the rates of physician office visits and nonphysician outpatient encounters across both groups.
DISCUSSION
In this commercially insured sample of working age individuals, we identified an association between a DAMA and the likelihood and intensity of postdischarge HcRU. The direction of the association varied across categories of HcRU and the duration of follow-up. A DAMA was associated with increased odds of 30-day ED visits but not 30-day readmissions compared with routine discharges. No significant differences were observed in the rates of 30-day physician office visits, nonphysician outpatient encounters, and prescription drug fills across both groups. To our knowledge, this is the first study on DAMAs that examines postdischarge HcRU outside the inpatient setting.
The 0.5% prevalence of DAMAs in our study was lower than the approximate 1% to 2% value that is typically reported in the literature. Prior studies have typically reported results based on mixed-payer populations.3-10 These mixed-payer populations include publicly insured (Medicare or Medicaid) or uninsured stays, which account for a disproportionate share of all DAMAs. In contrast, commercially insured stays account for the lowest proportion of all DAMAs.10 Similar to prior literature,5 the DAMA group in our study was younger, had a higher proportion of males, had a higher comorbidity burden, and had a shorter LOS than the routinely discharged group.
We observed a greater likelihood of ED utilization after a DAMA. Similar findings have been reported, which may indicate that patients with a DAMA receive inadequate treatment at the time of discharge and may require further acute treatment. For example, a prior study reported that, after a DAMA, individuals with asthma were four times more likely to have an ED visit within 14 days compared with those discharged routinely.4
Contrary to prior findings,3-9 we found no significant difference in the odds of a 30-day inpatient readmission across the DAMA and routine discharge groups, which may be attributable to differences in the populations studied. Those previous studies used mixed payer populations and did not differentiate results by payer type. The mixed payer populations in these studies were older (mean ages, 55 years and above) and had an increased comorbidity burden compared with our commercially insured population. Furthermore, some of these studies were either limited to single sites,8 single state hospital systems,3,4,9 or focused on specific medical populations.3,4,6-9 Our national sample of commercially insured adults is considerably younger, with a mean age of 43 years. Thirty days may be too brief to observe enough inpatient readmissions for the purpose of comparative analyses. This is suggested by our results, which indicated that there is an association between DAMA and 90-day inpatient readmission. Additionally, nonsignificant findings for 30-day inpatient readmissions may also be due to the small sample size of the DAMA group in our study, which may have limited robust statistical inference. Future studies in a larger population of commercially insured individuals with a DAMA are required to confirm these findings.
Nonsignificant differences in the rates of 30-day physician office visits, nonphysician outpatient encounters, and prescription drug fills across both groups may explain the null association with 30-day inpatient readmissions. Prior literature on specific medical populations or individuals with general hospital admissions report that early outpatient follow-up can help prevent 30-day readmissions.20-25 In our sample, we observed similar rates of outpatient follow-up across the DAMA and routinely discharged groups. Prior studies based on single hospital sites have reported that, at the time of discharge, a lower proportion of individuals with a DAMA received medication prescriptions and outpatient follow-up plans compared with those discharged routinely.11,12 In contrast, we evaluated prescription drug fills and outpatient visits during the postdischarge period, which may explain the difference in findings.
The present study has several strengths. To the best of our knowledge, our study represents the first and largest retrospective analysis of DAMAs in a national sample of commercially insured adults. In addition to a large generalizable sample, we examine HcRU after a DAMA across major points of service over a longitudinal postdischarge period. Our results provide a comprehensive understanding of utilization outcomes in this population including those outside the inpatient setting, which has been the focus of prior literature. These findings can help guide the implementation of appropriate patient- and system-level interventions to optimize DAMA prevention and mitigate the associated utilization burden on the healthcare system in the postdischarge period.26,27
Our findings should be interpreted with certain limitations in mind. First, this study used data based on a commercially insured sample of patients and may not be generalizable to publicly insured or uninsured samples. Second, like prior DAMA studies that used the Nationwide Readmissions Database instead,5-7 our study was unable to account for individual-level factors such as race, marital status, family social support, income, health literacy, and activation in self-care. Further, given the limitations of our data, we were unable to control for hospital characteristics such as bed size, urban-rural designation, teaching status, and control (eg, private or government ownership). Despite the use of propensity score methods to balance both comparison groups on observable sources of confounding, we cannot rule out the possibility of residual confounding. Lastly, due to a lack of data on postdischarge mortality outcomes, we could not control for competing risk of death in our analysis. However, in a population with an average age of 43 years, we did not expect high or differential 30- or 90-day postdischarge mortality rates across both groups.
Our findings suggest several important directions for future research. First, it will be useful to examine these associations among publicly insured and uninsured samples in which a DAMA is more prevalent and in which the associations with HcRU may be more pronounced than they are in the commercially insured population. Secondly, future research should identify subgroups of DAMA patients with an increased propensity for postdischarge HcRU. This can help in the design of individualized outpatient follow-up plans that address patient-specific medical and social needs. Finally, our findings highlight the need for education, practice guidelines, and suitable interventions to help providers in the prevention and management of a DAMA.
CONCLUSION
Using data from a commercially insured population, we identified associations between a DAMA and postdischarge HcRU. The associations differed by category of HcRU. We identified a positive association with the likelihood of ED utilization but no association with the likelihood of 30-day inpatient readmission or general outpatient utilization. Our results indicate that the examination of inpatient readmissions after a DAMA should not be considered in isolation. The identification of the full range of outpatient and inpatient HcRU after a DAMA in a broad population of patients can improve our understanding of outcomes following a DAMA and support appropriate system-level interventions designed to reduce their prevalence.
Acknowledgments
The statements, findings, conclusions, views, and opinions contained and expressed in this manuscript are based in part on data obtained under license from IQVIA. Source: IQVIA PharMetrics® Plus January 2006 – December 2015, IQVIA. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA or any of its affiliated or subsidiary entities.
Disclosures
Dr Onukwugha reports grants from Bayer Healthcare Pharmaceuticals, grants from Pfizer, Inc, and personal fees from Novo Nordisk outside the submitted work. The other authors have nothing to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs, the U.S. Government, or the VA National Center for Ethics in Health Care.
Funding
The authors acknowledge the support of the University of Maryland, Baltimore Institute for Clinical & Translational Research (ICTR) through the ICTR Voucher Program.
Discharges against medical advice (DAMAs), in which a patient leaves the hospital prior to a physician-recommended endpoint, represent approximately 1% to 2% of inpatient discharges in the United States.1 When compared with routine discharges, a DAMA is associated with adverse clinical consequences, including an increased risk of all-cause mortality.2,3 Additionally, due to incomplete care, a DAMA may result in increased healthcare resource utilization (HcRU), including the use of inpatient, emergency department (ED), and outpatient services in the postdischarge period. Quantifying these relationships can provide important information regarding an individual’s healthcare-seeking behavior following a DAMA.
Prior literature has focused on the association between a DAMA and the risk of inpatient readmission. Relative to routine discharges, a DAMA is associated with a 1.5 to 2 times increased risk of a 30-day readmission.3-9 However, these estimates are based on mixed-payer populations primarily composed (65%-80%) of individuals with public (Medicaid, Medicare) or no insurance. Further, they do not differentiate this association by payer type. It is unclear if prior results apply to commercially insured adults. These individuals represent a small but nonnegligible proportion (19%) of all DAMAs in the United States.10 Quantifying relationships among commercially insured adults can help advance our understanding of readmission patterns in the DAMA population.
There is limited evidence regarding the relationship between a DAMA and outpatient HcRU in the postdischarge period. Use of ED services after a DAMA has been explored only in specific disease populations such as asthma.4 Additionally, prior studies have reported a reduced frequency in the receipt of medication prescriptions and outpatient follow-up plans among individuals with a DAMA at the time of discharge.11,12 Whether these practices translate to altered patterns of postdischarge prescription drug fills or use of outpatient services is not known.
To address these substantive gaps in the literature, the present study evaluates the association between a DAMA and all-cause HcRU in the postdischarge period among commercially insured adults. We examined HcRU across all points of service including inpatient readmissions, ED visits, physician office visits, nonphysician outpatient encounters, and prescription drug fills. These results can serve as a benchmark for comparison to future studies on DAMAs among publicly insured or uninsured individuals. Furthermore, such knowledge can help providers, payers, and policy planners make evidence-based decisions regarding postdischarge healthcare delivery.
METHODS
Data Source
This retrospective study used a 10% random sample of enrollees in the IQVIA PharMetrics® Plus database (purchased by University of Maryland, Baltimore, under license from IQVIA). The database is composed of fully adjudicated claims and enrollment information from over 70 contributing US health plans and self-insured employer groups for over 140 million unique enrollees from 2006 onward. The enrollee population is generally representative of the commercially insured population that is younger than 65 years of age (with a subset of commercial Medicare and Medicaid) with respect to age and gender.
The database allows longitudinal follow-up for individuals using three files: medical claims, pharmacy claims, and insurance eligibility. The average length of enrollment is 39 months. The claims data represent payments to providers for services rendered to individuals covered by health plans. The medical claims file contains information on diagnostic and therapeutic services rendered in the inpatient and outpatient settings. The pharmacy claims file captures data on prescription drugs dispensed in retail and mail-order settings. The eligibility file contains demographic and insurance eligibility information for individuals.
Study Population
We identified all individuals aged 18 to 64 years with an inpatient admission record between January 1, 2007, and December 31, 2015. All individuals with continuous medical and prescription drug coverage from 6 months prior to the hospital admission date (baseline period) through 30 days following the discharge date (follow-up period) were included. Inpatient admissions with a missing discharge disposition or those that resulted in in-hospital death, discharge to a short-term hospital, skilled nursing facility, intermediate care facility, or any other type of facility were not considered for analysis. Only the first eligible inpatient admission was considered for analysis.
Main Predictor Variable
Individuals with a DAMA were analyzed as the case group. A DAMA was identified using the “Patient Status Code” variable, which represents the discharge disposition of each individual. Individuals who were discharged to home/self-care or discharged to a home health organization formed the control group (hereafter referred to as routine discharge).
Demographic, Clinical, and Hospitalization Characteristics
An individual’s age, sex, and region of residence were determined at the date of hospital admission. The Elixhauser algorithm was used to categorize comorbid conditions (as scores of 0, 1-2, ≥3 depending on number of comorbidities) based on International Classification of Diseases, Ninth Revision, Clinical Modification, diagnosis codes during the baseline period.13,14 The following characteristics of each individual’s eligible inpatient admission were captured: year, timing (weekday or weekend), length of stay (LOS, measured in days), and receipt of a surgical procedure.
Outcomes
All-cause HcRU was identified during the 30-day postdischarge period. Specifically, we identified inpatient readmissions, ED visits, physician office visits, nonphysician outpatient encounters (for example, pathology, radiology, outpatient surgical services), and prescription drug fills. Binary variables (yes or no) were created for inpatient readmissions and ED visits while the remaining HcRU categories (ie, physician office visits, nonphysician outpatient encounters, and prescription drug fills) were analyzed as count variables. In the sensitivity analyses, we provide results for HcRU outcomes among a subgroup of individuals who had at least 90 days of continuous medical and prescription drug benefits following the hospital discharge.
Statistical Analysis
Descriptive Analysis
Measures of interest were reported using summary statistics depending on the nature of the variable. Continuous variables were described using t tests, and categorical variables were described using chi-square tests.
Propensity Score Matching
Cases and controls were matched using a 1:1 greedy matching algorithm based on propensity scores.15 We developed propensity scores based on confounders that we hypothesized would be associated with a DAMA and postdischarge HcRU. The propensity score model included the following variables: age, sex, region of residence, Elixhauser comorbidity index score, year of admission, timing of admission, LOS, and presence of any surgical procedure during the inpatient admission. The best match between cases and controls was determined based on the absolute difference in their propensity scores, which allowed for a maximal caliper width of 0.2 of the standard deviation of the logit of the propensity score.16 A standardized difference value of less than 0.1 was used to assess balance in baseline patient and hospital characteristics between cases and controls consistent with prior literature.17,18 Proportions and balance, as measured by standardized differences between baseline covariates across cases and controls in the matched sample, are displayed in tabular format (Appendix Table 1).
Healthcare Resource Utilization
We estimated the adjusted odds ratio (AOR) using a logistic regression model. The AOR quantified the association between a DAMA and the prevalence of all-cause inpatient readmissions and ED visits during the 30-day postdischarge period. We estimated incident rate ratios (IRR) for count outcomes. Given the large number of individuals with no physician office visits, nonphysician outpatient encounters, or prescription drug fills, we estimated model parameters for IRRs using a finite mixture negative binomial hurdle model.19 We considered the data to represent a mixture of a constant distribution (which always generates zero counts) and a zero-truncated distribution (which always generates nonzero counts). The finite mixture count models include two outcomes: the mixing probabilities and the count distribution. The mixing probabilities quantify the probability that an observation for the HcRU category will be drawn from either the constant distribution (with mass at zero) or the count distribution. Conditional on having positive values, a zero-truncated generalized linear model (GLM) governs the count variable. Compared with other GLM specifications (eg, Poisson, negative binomial, zero-inflated), the negative binomial hurdle model presented the best-fitting model across several information criteria statistics (Appendix Figures 1-3 and Appendix Tables 2-4).
The GLM results provided IRR for the counts of HcRU. Ratios were interpreted as evidence of increased HcRU (IRR ≥ 1.0) or decreased HcRU (IRR < 1.0) among individuals with a DAMA compared with those discharged routinely. For all HcRU analyses, we reported results for the matched sample. All analyses were conducted using SAS version 9.4 (SAS Institute), and statistical significance was determined at α= .05. The study received the University of Maryland, Baltimore, Institutional Review Board approval (HP-00081497).
RESULTS
The unmatched sample included 457,530 individuals, of whom 0.5% had a DAMA. A consort diagram illustrating cohort inclusion and exclusion criteria is presented in Appendix Figure 4. Demographic, clinical, and inpatient admission characteristics of the unmatched sample and for subgroups defined by discharge status are displayed in Table 1. In the unmatched sample, the median age at admission was higher for individuals with a DAMA than it was for those discharged routinely (43 vs 42 years, respectively), and the proportion of males was higher among those with a DAMA (58.4% vs 33.1%). There were statistically significant differences based on the geographic region of residence and the comorbidity burden across both groups. The median LOS was shorter (1 day vs 2 days), the proportion of weekend admissions was higher (22.2% vs 16.3%), and the proportion of inpatient surgical procedures was lower (12.9% vs 59.2%) among those with a DAMA compared with that among those with routine discharges. The propensity score-matched sample included 2,245 cases and 2,245 controls (Appendix Table 1). Standardized differences for all baseline factors were less than 0.1, indicating that cases and controls were matched on the included baseline factors.

Summary Statistics: Proportions and Counts
Across the DAMA and routine discharge groups, the proportion of individuals with a 30-day inpatient readmission was similar (19.5% vs 18.7%; P = .47), whereas the proportion with an ED visit was higher (18.6% vs 9.1%; P < .01). There were no differences in the median number of inpatient readmissions (median, 0) and ED visits (median, 0) across both groups. Individuals with a DAMA and those discharged routinely displayed similar median counts of 30-day physician office (median, 1) and nonphysician outpatient encounters (median, 1) (Table 2). Individuals with a DAMA displayed a lower median number of prescription drug fills (median, 2 vs 3) than that among those with a routine discharge (Table 2).

Main Analysis: Thirty-Day Healthcare Resource Utilization
The associations between a DAMA and 30-day inpatient readmissions and ED visits based on the matched sample are presented in Table 3. Individuals with a DAMA had increased odds for an ED visit (AOR, 2.28; 95% CI, 1.90-2.72) but no significant difference in the odds of a 30-day inpatient readmission (AOR, 1.06; 95% CI, 0.91-1.23) compared with those discharged routinely.

The association between a DAMA and count HcRU outcomes is presented in Table 4. Compared with those discharged routinely, individuals with a DAMA displayed no significant difference in rates for physician office visits (IRR, 1.01; 95% CI, 0.91-1.11), nonphysician outpatient encounters (IRR, 0.89; 95% CI, 0.78-1.00), and prescription drug fills (IRR, 1.03; 95% CI, 0.97-1.09) during the 30-day postdischarge period.

Sensitivity Analysis: Ninety-Day Healthcare Resource Utilization
Relative to those discharged routinely, individuals with a DAMA had statistically significant increased odds of 90-day inpatient readmissions (AOR, 1.18; 95% CI, 1.02-1.36), odds of ED visits (AOR, 2.16; 95% CI, 1.85-2.51), and rates of prescription drug fills (IRR, 1.32; 95% CI, 1.29-1.35). No statistically significant differences were observed in the rates of physician office visits and nonphysician outpatient encounters across both groups.
DISCUSSION
In this commercially insured sample of working age individuals, we identified an association between a DAMA and the likelihood and intensity of postdischarge HcRU. The direction of the association varied across categories of HcRU and the duration of follow-up. A DAMA was associated with increased odds of 30-day ED visits but not 30-day readmissions compared with routine discharges. No significant differences were observed in the rates of 30-day physician office visits, nonphysician outpatient encounters, and prescription drug fills across both groups. To our knowledge, this is the first study on DAMAs that examines postdischarge HcRU outside the inpatient setting.
The 0.5% prevalence of DAMAs in our study was lower than the approximate 1% to 2% value that is typically reported in the literature. Prior studies have typically reported results based on mixed-payer populations.3-10 These mixed-payer populations include publicly insured (Medicare or Medicaid) or uninsured stays, which account for a disproportionate share of all DAMAs. In contrast, commercially insured stays account for the lowest proportion of all DAMAs.10 Similar to prior literature,5 the DAMA group in our study was younger, had a higher proportion of males, had a higher comorbidity burden, and had a shorter LOS than the routinely discharged group.
We observed a greater likelihood of ED utilization after a DAMA. Similar findings have been reported, which may indicate that patients with a DAMA receive inadequate treatment at the time of discharge and may require further acute treatment. For example, a prior study reported that, after a DAMA, individuals with asthma were four times more likely to have an ED visit within 14 days compared with those discharged routinely.4
Contrary to prior findings,3-9 we found no significant difference in the odds of a 30-day inpatient readmission across the DAMA and routine discharge groups, which may be attributable to differences in the populations studied. Those previous studies used mixed payer populations and did not differentiate results by payer type. The mixed payer populations in these studies were older (mean ages, 55 years and above) and had an increased comorbidity burden compared with our commercially insured population. Furthermore, some of these studies were either limited to single sites,8 single state hospital systems,3,4,9 or focused on specific medical populations.3,4,6-9 Our national sample of commercially insured adults is considerably younger, with a mean age of 43 years. Thirty days may be too brief to observe enough inpatient readmissions for the purpose of comparative analyses. This is suggested by our results, which indicated that there is an association between DAMA and 90-day inpatient readmission. Additionally, nonsignificant findings for 30-day inpatient readmissions may also be due to the small sample size of the DAMA group in our study, which may have limited robust statistical inference. Future studies in a larger population of commercially insured individuals with a DAMA are required to confirm these findings.
Nonsignificant differences in the rates of 30-day physician office visits, nonphysician outpatient encounters, and prescription drug fills across both groups may explain the null association with 30-day inpatient readmissions. Prior literature on specific medical populations or individuals with general hospital admissions report that early outpatient follow-up can help prevent 30-day readmissions.20-25 In our sample, we observed similar rates of outpatient follow-up across the DAMA and routinely discharged groups. Prior studies based on single hospital sites have reported that, at the time of discharge, a lower proportion of individuals with a DAMA received medication prescriptions and outpatient follow-up plans compared with those discharged routinely.11,12 In contrast, we evaluated prescription drug fills and outpatient visits during the postdischarge period, which may explain the difference in findings.
The present study has several strengths. To the best of our knowledge, our study represents the first and largest retrospective analysis of DAMAs in a national sample of commercially insured adults. In addition to a large generalizable sample, we examine HcRU after a DAMA across major points of service over a longitudinal postdischarge period. Our results provide a comprehensive understanding of utilization outcomes in this population including those outside the inpatient setting, which has been the focus of prior literature. These findings can help guide the implementation of appropriate patient- and system-level interventions to optimize DAMA prevention and mitigate the associated utilization burden on the healthcare system in the postdischarge period.26,27
Our findings should be interpreted with certain limitations in mind. First, this study used data based on a commercially insured sample of patients and may not be generalizable to publicly insured or uninsured samples. Second, like prior DAMA studies that used the Nationwide Readmissions Database instead,5-7 our study was unable to account for individual-level factors such as race, marital status, family social support, income, health literacy, and activation in self-care. Further, given the limitations of our data, we were unable to control for hospital characteristics such as bed size, urban-rural designation, teaching status, and control (eg, private or government ownership). Despite the use of propensity score methods to balance both comparison groups on observable sources of confounding, we cannot rule out the possibility of residual confounding. Lastly, due to a lack of data on postdischarge mortality outcomes, we could not control for competing risk of death in our analysis. However, in a population with an average age of 43 years, we did not expect high or differential 30- or 90-day postdischarge mortality rates across both groups.
Our findings suggest several important directions for future research. First, it will be useful to examine these associations among publicly insured and uninsured samples in which a DAMA is more prevalent and in which the associations with HcRU may be more pronounced than they are in the commercially insured population. Secondly, future research should identify subgroups of DAMA patients with an increased propensity for postdischarge HcRU. This can help in the design of individualized outpatient follow-up plans that address patient-specific medical and social needs. Finally, our findings highlight the need for education, practice guidelines, and suitable interventions to help providers in the prevention and management of a DAMA.
CONCLUSION
Using data from a commercially insured population, we identified associations between a DAMA and postdischarge HcRU. The associations differed by category of HcRU. We identified a positive association with the likelihood of ED utilization but no association with the likelihood of 30-day inpatient readmission or general outpatient utilization. Our results indicate that the examination of inpatient readmissions after a DAMA should not be considered in isolation. The identification of the full range of outpatient and inpatient HcRU after a DAMA in a broad population of patients can improve our understanding of outcomes following a DAMA and support appropriate system-level interventions designed to reduce their prevalence.
Acknowledgments
The statements, findings, conclusions, views, and opinions contained and expressed in this manuscript are based in part on data obtained under license from IQVIA. Source: IQVIA PharMetrics® Plus January 2006 – December 2015, IQVIA. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IQVIA or any of its affiliated or subsidiary entities.
Disclosures
Dr Onukwugha reports grants from Bayer Healthcare Pharmaceuticals, grants from Pfizer, Inc, and personal fees from Novo Nordisk outside the submitted work. The other authors have nothing to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs, the U.S. Government, or the VA National Center for Ethics in Health Care.
Funding
The authors acknowledge the support of the University of Maryland, Baltimore Institute for Clinical & Translational Research (ICTR) through the ICTR Voucher Program.
1. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. https://doi.org/10.4065/84.3.255
2. Garland A, Ramsey CD, Fransoo R, et al. Rates of readmission and death associated with leaving hospital against medical advice: a population-based study. CMAJ. 2013;185(14):1207-1214. https://doi.org/10.1503/cmaj.130029
3. Fiscella K, Meldrum S, Barnett S. Hospital discharge against advice after myocardial infarction: deaths and readmissions. Am J Med. 2007;120(12):1047-1053. https://doi.org/10.1016/j.amjmed.2007.08.024
4. Baptist AP, Warrier I, Arora R, Ager J, Massanari RM. Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes. J Allergy Clin Immunol. 2007;119(4):924-929. https://doi.org/10.1016/j.jaci.2006.11.695
5. Kumar N. Burden of 30-day readmissions associated with discharge against medical advice among inpatients in the United States. Am J Med. 2019;132(6):708-717.e4. https://doi.org/10.1016/j.amjmed.2019.01.023
6. Kwok CS, Walsh MN, Volgman A, et al. Discharge against medical advice after hospitalisation for acute myocardial infarction. Heart. 2019;105(4):315-321. https://doi.org/10.1136/heartjnl-2018-313671
7. Patel B, Prousi G, Shah M, et al. Thirty-day readmission rate in acute heart failure patients discharged against medical advice in a matched cohort study. Mayo Clin Proc. 2018;93(10):1397-1403. https://doi.org/10.1016/j.mayocp.2018.04.023
8. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594-602. https://doi.org/10.1016/j.amjmed.2011.12.017
9. Onukwugha E, Mullins D, Loh FE, Saunders E, Shaya FT, Weir MR. Readmissions after unauthorized discharges in the cardiovascular setting. Med Care. 2011;49(2):215-224. https://doi.org/10.1097/mlr.0b013e31820192a5
10. Stranges E, Wier L, Merrill CT, Steiner C. Hospitalizations in which Patients Leave the Hospital against Medical Advice (AMA), 2007. HCUP Statistical Brief #78. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; August 2009. Accessed 04/07 2020.http://www.hcup-us.ahrq.gov/reports/statbriefs/sb78.pdf
11. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. https://doi.org/10.1002/jhm.2087
12. Stearns CR, Bakamjian A, Sattar S, Weintraub MR. Discharges against medical advice at a county hospital: provider perceptions and practice. J Hosp Med. 2017;12(1):11-17. https://doi.org/10.1002/jhm.2672
13. Garland A, Fransoo R, Olafson K, et al. The Epidemiology and Outcomes of Critical Illness in Manitoba. Manitoba Centre for Health Policy; April 2012. Accessed April 7, 2020. http://mchp-appserv.cpe.umanitoba.ca/reference/MCHP_ICU_Report_WEB_(20120403).pdf
14. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
15. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-1069. https://doi.org/10.1002/sim.6004
16. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. https://doi.org/10.1002/pst.433
17. Austin PC, Mamdani MM. A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use. Stat Med. 2006;25(12):2084-2106. https://doi.org/10.1002/sim.2328
18. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. https://doi.org/10.1016/s0895-4356(00)00321-8
19. Mullahy J. Specification and testing of some modified count data models. J Econometrics. 1986;33(3):341-365. https://doi.org/10.1016/0304-4076(86)90002-3
20. Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354-360. https://doi.org/10.1002/jhm.129
21. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
22. Leschke J, Panepinto JA, Nimmer M, Hoffmann RG, Yan K, Brousseau DC. Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406-409. https://doi.org/10.1002/pbc.23140
23. Misky GJ, Wald HL, Coleman EA. Post‐hospitalization transitions: Examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
24. Muus K, Knudson A, Klug MG, Gokun J, Sarrazin M, Kaboli P. Effect of post-discharge follow-up care on re-admissions among US veterans with congestive heart failure: a rural-urban comparison. Rural Remote Health. 2010;10(2):1447.https://doi.org/10.22605/RRH1447
25. Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow-up visits as part of a heart failure readmission quality improvement initiative. Am J Med. 2013;126(11):989-994.e1. https://doi.org/10.1016/j.amjmed.2013.06.027
26. Alfandre D. Improving quality in against medical advice discharges—more empirical evidence, enhanced professional education, and directed systems changes. J Hosp Med. 2017;12(1):59-60. https://doi.org/10.1002/jhm.2678
27. Nagarajan M, Offurum AI, Gulati M, Onukwugha E. Discharges Against Medical Advice: Prevalence, Predictors, and Populations. In: Alfandre D, ed. Against‐Medical‐Advice Discharges from the Hospital. Springer; 2018:11-29.
1. Alfandre DJ. “I’m going home”: discharges against medical advice. Mayo Clin Proc. 2009;84(3):255-260. https://doi.org/10.4065/84.3.255
2. Garland A, Ramsey CD, Fransoo R, et al. Rates of readmission and death associated with leaving hospital against medical advice: a population-based study. CMAJ. 2013;185(14):1207-1214. https://doi.org/10.1503/cmaj.130029
3. Fiscella K, Meldrum S, Barnett S. Hospital discharge against advice after myocardial infarction: deaths and readmissions. Am J Med. 2007;120(12):1047-1053. https://doi.org/10.1016/j.amjmed.2007.08.024
4. Baptist AP, Warrier I, Arora R, Ager J, Massanari RM. Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes. J Allergy Clin Immunol. 2007;119(4):924-929. https://doi.org/10.1016/j.jaci.2006.11.695
5. Kumar N. Burden of 30-day readmissions associated with discharge against medical advice among inpatients in the United States. Am J Med. 2019;132(6):708-717.e4. https://doi.org/10.1016/j.amjmed.2019.01.023
6. Kwok CS, Walsh MN, Volgman A, et al. Discharge against medical advice after hospitalisation for acute myocardial infarction. Heart. 2019;105(4):315-321. https://doi.org/10.1136/heartjnl-2018-313671
7. Patel B, Prousi G, Shah M, et al. Thirty-day readmission rate in acute heart failure patients discharged against medical advice in a matched cohort study. Mayo Clin Proc. 2018;93(10):1397-1403. https://doi.org/10.1016/j.mayocp.2018.04.023
8. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125(6):594-602. https://doi.org/10.1016/j.amjmed.2011.12.017
9. Onukwugha E, Mullins D, Loh FE, Saunders E, Shaya FT, Weir MR. Readmissions after unauthorized discharges in the cardiovascular setting. Med Care. 2011;49(2):215-224. https://doi.org/10.1097/mlr.0b013e31820192a5
10. Stranges E, Wier L, Merrill CT, Steiner C. Hospitalizations in which Patients Leave the Hospital against Medical Advice (AMA), 2007. HCUP Statistical Brief #78. Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality; August 2009. Accessed 04/07 2020.http://www.hcup-us.ahrq.gov/reports/statbriefs/sb78.pdf
11. Edwards J, Markert R, Bricker D. Discharge against medical advice: how often do we intervene? J Hosp Med. 2013;8(10):574-577. https://doi.org/10.1002/jhm.2087
12. Stearns CR, Bakamjian A, Sattar S, Weintraub MR. Discharges against medical advice at a county hospital: provider perceptions and practice. J Hosp Med. 2017;12(1):11-17. https://doi.org/10.1002/jhm.2672
13. Garland A, Fransoo R, Olafson K, et al. The Epidemiology and Outcomes of Critical Illness in Manitoba. Manitoba Centre for Health Policy; April 2012. Accessed April 7, 2020. http://mchp-appserv.cpe.umanitoba.ca/reference/MCHP_ICU_Report_WEB_(20120403).pdf
14. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
15. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-1069. https://doi.org/10.1002/sim.6004
16. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. https://doi.org/10.1002/pst.433
17. Austin PC, Mamdani MM. A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use. Stat Med. 2006;25(12):2084-2106. https://doi.org/10.1002/sim.2328
18. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. https://doi.org/10.1016/s0895-4356(00)00321-8
19. Mullahy J. Specification and testing of some modified count data models. J Econometrics. 1986;33(3):341-365. https://doi.org/10.1016/0304-4076(86)90002-3
20. Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354-360. https://doi.org/10.1002/jhm.129
21. Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716-1722. https://doi.org/10.1001/jama.2010.533
22. Leschke J, Panepinto JA, Nimmer M, Hoffmann RG, Yan K, Brousseau DC. Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406-409. https://doi.org/10.1002/pbc.23140
23. Misky GJ, Wald HL, Coleman EA. Post‐hospitalization transitions: Examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392-397. https://doi.org/10.1002/jhm.666
24. Muus K, Knudson A, Klug MG, Gokun J, Sarrazin M, Kaboli P. Effect of post-discharge follow-up care on re-admissions among US veterans with congestive heart failure: a rural-urban comparison. Rural Remote Health. 2010;10(2):1447.https://doi.org/10.22605/RRH1447
25. Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow-up visits as part of a heart failure readmission quality improvement initiative. Am J Med. 2013;126(11):989-994.e1. https://doi.org/10.1016/j.amjmed.2013.06.027
26. Alfandre D. Improving quality in against medical advice discharges—more empirical evidence, enhanced professional education, and directed systems changes. J Hosp Med. 2017;12(1):59-60. https://doi.org/10.1002/jhm.2678
27. Nagarajan M, Offurum AI, Gulati M, Onukwugha E. Discharges Against Medical Advice: Prevalence, Predictors, and Populations. In: Alfandre D, ed. Against‐Medical‐Advice Discharges from the Hospital. Springer; 2018:11-29.
© 2020 Society of Hospital Medicine
Safety Assessment of a Noninvasive Respiratory Protocol for Adults With COVID-19
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).
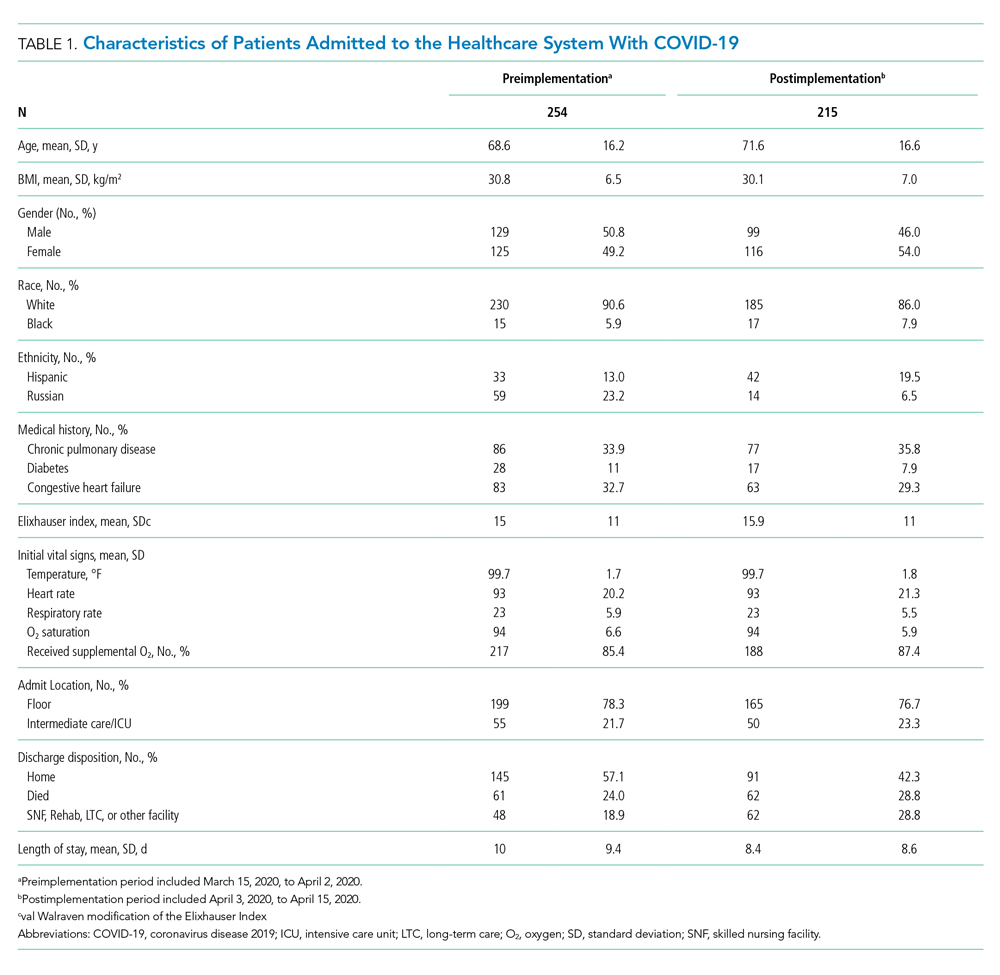
Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).
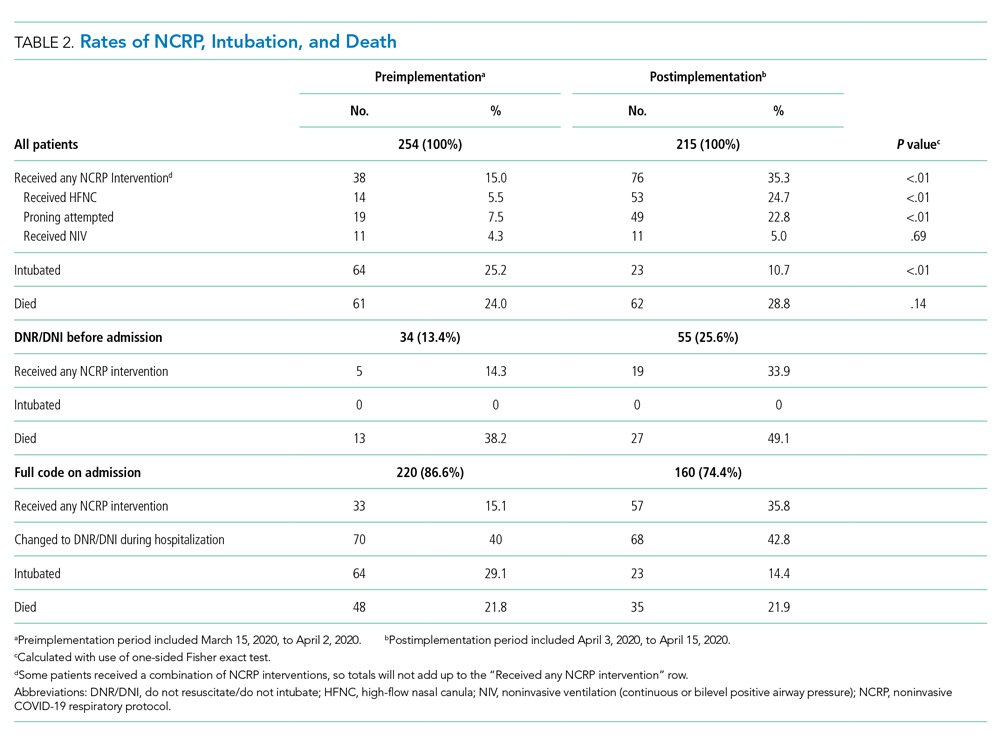
Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).
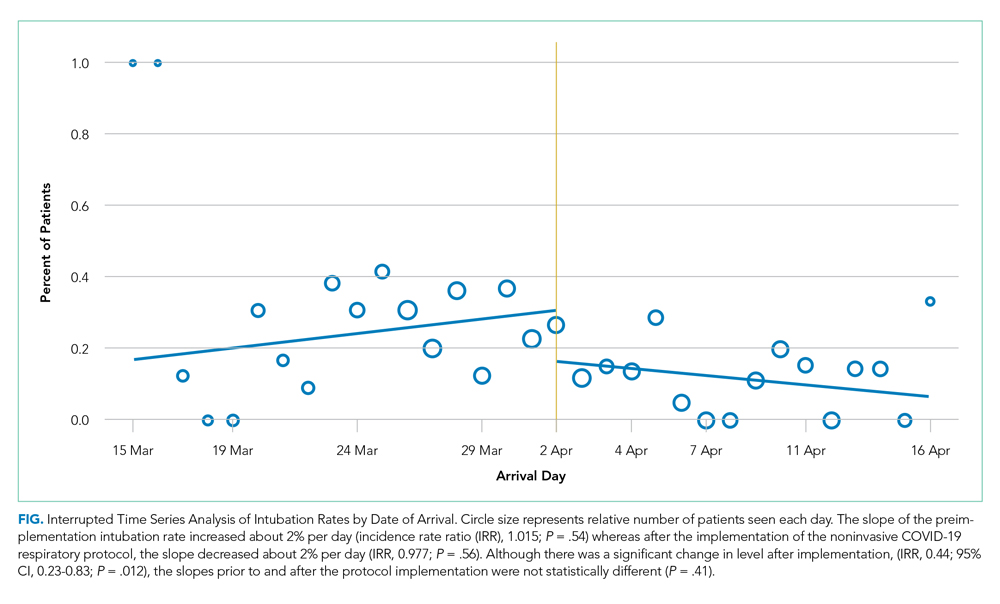
Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).

Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).

Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).

Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
Hypoxemic respiratory failure is a hallmark of severe coronavirus disease 2019 (COVID-19). Initial guidelines favored early mechanical ventilation (MV) over traditional noninvasive strategies, such as high-flow nasal cannula (HFNC) and noninvasive positive pressure ventilation (NIV), based on perceived ineffectiveness and dangers extrapolated from severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) patients.1,2 As COVID-19 progressed, early MV became associated with prolonged ventilator courses and high mortality.3-6 Simultaneously, data emerged that HFNC/NIV and self-proning, could successfully stabilize some COVID-19 patients.7-10 Based on evolving evidence, we implemented a noninvasive COVID-19 respiratory protocol (NCRP) that promoted the early use of HFNC, NIV, and self-proning for hypoxemia in patients with COVID-19, with the intention of avoiding MV in some patients. The protocol was implemented throughout our hospital system, from the Emergency Departments (EDs) to the medical floors and critical care units.
Although preliminary evidence supported the use of HFNC, NIV, and self-proning, the impact of a system-wide noninvasive COVID-19 respiratory protocol on safety has not been well described. The objective of this study was to evaluate patient safety outcomes after implementation of the NCRP, including intubation rate and mortality.
METHODS
Study Design and Setting
We performed a retrospective chart review, adhering to SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, to assess safety outcomes after implementation of the NCRP.11 Baystate Health is a not-for-profit, integrated healthcare system in western Massachusetts composed of four hospitals and one free-standing ED with 980 beds serving over 800,000 people. The Baystate Health IRB determined that this project did not meet criteria for Human Subjects Research.
Selection of Participants
A consecutive sample of adults (≥18 years old) admitted to the hospital with a positive nucleic acid test for SARS-CoV-2 (reverse transcriptase–polymerase chain reaction [RT-PCR]) test via nasopharyngeal swab (Cepheid or Roche Cobas 6800) between March 15, 2020, and April 15, 2020, were included. Participants were identified by either an order for the COVID-19 test with a positive result or a discharge diagnosis of COVID-19. Daily rapid response team (RRT), intensive care unit (ICU), and COVID-19 unit logs were reviewed to ensure all COVID-19 patients were included. Patients with positive tests admitted for reasons unrelated to COVID-19 infections, such as patients in labor, were excluded.
Interventions
At the start of the COVID-19 pandemic, the Baystate Health system adopted a conservative approach to the respiratory management of patients with COVID-19. This approach started with nasal cannula up to 6 L/min or nonrebreather up to 15 L/min. If the patient remained in respiratory distress, intubation was recommended.
Based on emerging evidence, the NCRP was created. The details of the NCRP implementation have been previously described.12 Briefly, over a 4-day period (April 3, 2020, to April 7, 2020), a multidisciplinary team developed, refined, and rapidly implemented a COVID-19 respiratory protocol that encouraged the early use of HFNC, NIV, and self-proning in clinically appropriate patients with hypoxemia and respiratory distress due to COVID-19 prior to intubation across all departments of the Baystate Health system (Appendix 1).
Measurements
A chart review was performed using a structured data collection form (Appendix 2). The data collection form was piloted by three physician-researchers. Data abstraction was performed by 16 clinicians. Abstractors were practicing emergency providers and hospitalists and were blinded to the study outcomes. Abstractors received a 1-hour training and abstracted data from at least five charts in parallel with investigators. An additional 10% of charts were double abstracted to calculate interrater reliability for five variables determined a priori.
To validate the capture of outcomes of interest, we triangulated data sources by cross-referencing the monthly RRT log, the ICU list, all orders for HFNC, and RRT activations. Data abstraction occurred from April 21, 2020, to April 30, 2020. Patients who were still hospitalized after April 30,2020, were followed until hospital discharge, ending July 1, 2020.
Outcomes and Analysis
The primary outcome was mortality, defined as the proportion of deaths by admissions during the post–NCRP implementation period (April 3, 2020, to April 15, 2020), compared with the preimplementation period (March 15, 2020, to April 2, 2020). Deaths were stratified by patient code status (do not resuscitate/do not intubate [DNR/DNI] established prior to admission vs Full Code or presumed Full Code). Mortality outcomes were evaluated using one-sided Fisher exact tests.
To assess whether the protocol led to an increase in the use of the interventions and a decrease in intubations, we compared the use of proning, HFNC, NIV, and intubation before the protocol was implemented and with use after. Intubation rates were analyzed using interrupted time series (piecemeal regression), without adjustments, using a cut point of April 2, 2020.
Secondary outcomes included unexpected cardiac arrests, ICU transfers and consultations, and RRT activations during the postimplementation period, compared with the preimplementation period. Secondary outcomes were evaluated using standard chi-square tests (χ2). Additional descriptive outcomes included use of the NCRP, overall and by components, and in-hospital rates of MV.
RESULTS
From March 15, 2020, through April 15, 2020, there were 469 patients with COVID-19 admitted to the four hospitals of the Baystate Health system. Patients had an average age of 70 years (SD, 16.4), 241 (52%) were female, and 336 (72%) spoke English as their primary language. Most patients, 405 (86.4%), required supplemental oxygen upon being admitted to the hospital (Table 1).

Postimplementation Mortality
Overall, 123 (26.2%) patients died during the study period. In the preimplementation cohort, 24% (61 of 254) of patients died, compared with 28.8% (62 of 215) in the postimplementation cohort (one-sided Fisher exact, P = .14). Excluding patients with an established DNR/DNI prior to admission, 21.8% (48 of 220) patients died in the preimplementation period vs 21.9% (35 of 160) patients after implementation of the NCRP (Table 2).

Secondary Safety Outcomes
There was no increase in RRT activations (preimplementation, 16.5% [42 of 254], vs postimplementation, 11.6% [25 of 215]; χ2P = 0.17) or ICU consultations (preimplementation, 18.1% [47 of 254], vs postimplementation, 16.3% [35 of 215]; χ2P = 0.52). ICU transfers decreased in the postimplementation period (preimplementation, 26.8% [68 of 254], vs postimplementation, 13.5% [29 of 215], χ2P < .001). There was one unexpected cardiac arrest documented in the postimplementation period, compared to none before implementation.
NCRP Protocol Implementation
After implementation, the proportion of patients using HFNC increased from 5.5% (14 of 254) to 24.7% (53 of 215), and self-proning increased from 7.5% (19 of 254) to 22.8% (49 of 215). The proportion of patients who were intubated (MV) decreased from 25.2% (64 of 254) to 10.7% (23 of 215) (χ2P < .01). Interrupted time series analysis demonstrated an immediate reduction in the proportion of patients intubated after the intervention (incident rate ratio, 0.44; 95% CI, 0.23-0.83; P = .012) (Figure). The median time from admission to MV was longer in the postimplementation period patients (postimplementation, 1.4 days; interquartile range, 0.21-2.9; vs preimplementation, 0.66 days; IQR 0.23-1.69).

Interrater Reliability
Interrater reliability for variables chosen a priori was k = 1.0 for self-proning, k = 1.0 for intubation, k = 0.95 for discharge disposition, k = 0.94 for nasal cannula, and k = 0.74 for HFNC.
DISCUSSION
The rapid spread of SARS-CoV-2 led to early recommendations based on minimal data. As evidence emerged, hospitals were forced to adapt to protect patients and medical providers. As a healthcare system, we incorporated emerging evidence to rapidly implement a noninvasive respiratory treatment protocol. Aware of the methodological problems in evaluating the NCRP itself, we integrated best practices of quality improvement to examine multiple patient safety outcomes after NCRP implementation. We found the rate of intubation decreased with no significant increase in mortality, ICU transfers, RRT activations, or unexpected deaths after the implementation of the NCRP.
Although we were unable to measure all confounders and changes that co-occurred during the study period, initial vital signs, age, BMI, past medical history, and use of oxygen were similar between the pre- and postimplementation cohorts. Further, there were many constants worth noting. First, COVID-19 respiratory protocols were highly regulated to ensure patient safety and minimize COVID-19 transmission. Second, there were no new nonrespiratory treatments or medications during the study. Third, although the COVID-19 hospital census rose during the study, it never overwhelmed resources; there was no rationing of clinical care.
The nonsignificant increase in mortality in the postimplementation period was limited to patients with an established DNR/DNI prior to admission. Established DNR/DNI patients were largely from skilled nursing facilities that were disproportionally impacted in the postimplementation period through clustered outbreaks of COVID-19 in our region, which likely contributed to the increased mortality.13
Additionally, despite decreased MV rates in the postimplementation period, we did not find a concurrent decrease in mortality. We do not believe this is a failure of noninvasive treatments. Rather, the increased proportion of DNR/DNI patients, combined with increased nursing home outbreaks in the postimplementation period likely influenced mortality. The postimplementation decreases in ICU transfers and RRT activations supports this hypothesis.
Finally, it is worth nothing that, although the goal of decreasing intubations was to improve patient care and decrease mortality, a decrease in intubations alone, without a change in mortality, may be important because mechanical ventilation has been associated with increased morbidity, such as posttraumatic stress disorder.14
Taken together, the post–NCRP implementation period appears to have been safe for patients, compared to the preimplementation period’s protocol. Future research may help understand the impact of specific noninvasive interventions on COVID-19–related MV and mortality.
Limitations
Given the urgency of COVID-19 treatment, the NCRP was designed as a quality improvement initiative rather than a prospective trial. Issues of selection bias and confounding limit our ability to evaluate the effect of the NCRP itself. Additionally, unmeasured patient and provider factors may have influenced outcomes. For example, increased provider knowledge and experience treating COVID-19 may have improved outcomes over time, and unmeasured patient characteristics may have been different in the pre- and postimplementation groups. Finally, our study was limited to a single healthcare system, which may limit generalizability
That said, the objective of our study was to evaluate patient safety outcomes of the NCRP, an important first step while other hospital systems continue to confront increasing rates of COVID-19 and must decide on appropriate respiratory management. To that end, our enrollment captured 469 COVID-19 admissions across four diverse hospitals without obvious differences in initial measured covariates. Further, the strict protocolization of respiratory treatments, the evaluation of multiple safety outcomes, and the complete patient follow-up all support the conclusion that NCRP in the postimplementation period did not increase adverse patient outcomes. Further studies are needed to determine the efficacy of the NCRP protocol itself.
CONCLUSION
In our health system, patients with COVID-19 did not experience a significant increase in mortality, RRT activations, or ICU admissions despite decreased rates of MV after implementation of a respiratory protocol that encouraged early noninvasive management of COVID-19 respiratory distress.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Elizabeth Coray, Joseph Lahey, Richard Gabor, Cheryl Greenstein, Sarah Badach, Marie Boutin, Adrienne Wurl, Anthony Kitchen, Michelle Holton, Matthew Shapiro, Eleanor Ragone, Nageshwar Jonnalagadda, Ryan Flynn, Raghuveer Rakasi, and Jasmine Paadam.
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
1. Brown CA 3rd, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):80-84. https://doi.org/10.1002/emp2.12063
2. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with middle east respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160(6):389-397. https://doi.org/10.7326/m13-2486
3. Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564. https://doi.org/10.1164/rccm.202004-1163le
4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
5. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/s0140-6736(20)31189-2
6. Farfel JM, Franca SA, Sitta Mdo C, Filho WJ, Carvalho CR. Age, invasive ventilatory support and outcomes in elderly patients admitted to intensive care units. Age Ageing. 2009;38(5):515-520. https://doi.org/10.1093/ageing/afp119
7. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. https://doi.org/10.1111/acem.13994
8. Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33. https://doi.org/10.1186/s13613-020-00650-2
9. Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. https://doi.org/10.1186/s13613-020-00653-z
10. Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887 https://doi.org/10.1007/s00134-020-06022-5
11. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for quality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
12. Westafer LM, Elia T, Medarametla V, Lagu T. A transdiciplinary COVID-19 early respiratory intervention protocol: an implementation story. J Hosp Med. 2020;15(6):372-374. https://doi.org/10.12788/jhm.3456
13. COVID-19 Response Reporting. Mass.gov. Accessed July 20, 2020. https://www.mass.gov/info-details/covid-19-response-reporting#covid-19-daily-dashboard-
14. Shaw RJ, Harvey JE, Bernard R, Gunary R, Tiley M, Steiner H. Comparison of short-term psychological outcomes of respiratory failure treated by either invasive or non-invasive ventilation. Psychosomatics. 2009;50(6):586-591. https://doi.org/10.1176/appi.psy.50.6.586
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Therapeutic Monitoring of Vancomycin
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
© 2020 Society of Hospital Medicine
Rethinking Hospital-Associated Disability for Patients With COVID-19
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
Between February 1 and July 1, 2020, SARS-CoV-2 killed over 120,000 people in the United States alone. Nearly 80% of deaths occurred in those 65 years and older; by contrast, this age group constituted only 65% of deaths from influenza during the same time period.1 Though the reasons for these differences have not been completely elucidated, one thing is abundantly clear: Our nation’s oldest and most frail have been among the most likely to die of COVID-19. With an estimated mortality rate of 4.7% in the United States, we are fortunate that most infected patients survive2,3; however, many survivors require an exceptionally long hospital stay in isolation. Hospitalizations for patients with COVID-19 are distinct and confer a high risk for hospital-associated disability (HAD). HAD, defined as a new loss of ability to complete one or more activities of daily living (ADLs) without assistance after hospital discharge, occurs in approximately one-third of all hospitalized patients.4 In this perspective, we explore why HAD might be worse in patients with COVID-19 and offer new models for delivery of physical and occupational therapy to help them with functional recovery during and after hospitalization.
HOSPITAL-ASSOCIATED DISABILITY BEFORE COVID-19
Functional decline, a life-altering condition that patients experience as part of posthospital syndrome,5 is characterized by loss of mobility, cognitive decline, and HAD. The effects of functional decline can lead to a cascade of readmissions, institutionalization, and even death. During hospitalization, patients spend 87% to 100% of their time in bed. This immobilization is a major contributor to the development of HAD.6,7 The $58.5 billion dollars in yearly Medicare spending that is attributed to post–acute care also highlights the financial toll arising from such disability.8 Early mobilization with physical and occupational therapy is important to prevent HAD. However, even under normal conditions, care teams face innumerable barriers to mobilizing patients: symptomatic patients can be resistant to mobilizing during illness, providers have fears of worsening symptoms or falls, and some providers are unaware of the importance of mobilization. In patients with COVID-19, the barriers are only magnified.
HOSPITAL-ASSOCIATED DISABILITY DURING COVID-19
Given the increasing numbers of COVID-19 survivors discharged from the hospital, it is critical to consider why HAD could be an even larger problem in these patients. Consider their age, symptom burden, and illness severity: Among 5,700 patients who were admitted for COVID-19 in the New York City area, most were elderly (median age, 63 years), many were tachypneic (17%), and many required supplemental oxygen (28%).9 Fourteen percent of these patients required care in the intensive care unit (ICU), most of whom required mechanical ventilation (86%), which independently places them at higher risk of HAD. Given these severe respiratory issues in COVID-19, mobilization may cause significant discomfort. Being symptomatic is, by far, the most common reason hospitalized patients refuse to ambulate.10 As a result, this could make early mobilization for these COVID-19 patients exceptionally difficult.
Patients with COVID-19 also experience prolonged hospitalization. The median hospital length of stay (LOS) is 9.3 days for survivors of SARS-CoV-2 infection compared with the 7-day average LOS for patients with pneumonia requiring ICU admission and 5-day average LOS for patients with influenza.11-13 Complications of COVID, such as cardiac injury, critical illness polyneuropathy or myopathy, or cognitive impairment, also contribute to the significant need for rehabilitation long after recovery from the acute illness.14
Physical and occupational therapy involve prolonged close contact with patients, a known risk factor for contracting SARS-CoV-2.15 For staff, mobilizing a patient with COVID-19 takes longer due to intricate PPE donning and doffing procedures and patients requiring rest breaks because of weakness and respiratory-related recovery time. For patients who are mobilized, their activity is constrained by isolation restrictions that prohibit patients from leaving the confines of their hospital rooms. On March 23, 2020, the World Confederation for Physical Therapy (WCPT) endorsed guidelines created by the Australian Physiotherapy Association (APA) on caring for patients with COVID-19 acknowledging this risk16. The guidelines suggested that personal protective equipment (PPE) required for reducing risk of droplet transmission is appropriate for some scenarios, but they noted that exercising may induce coughing or expectoration, which could make physical therapy an aerosol-generating procedure. Therefore, the guidelines recommended that therapists wear N95 masks and recommend that direct face-to-face physical therapy should be limited to patients with certain functional limitations, including frailty, multiple comorbidities, and advanced age.
Patients with COVID-19 face additional barriers to accessing therapy services following hospital discharge. Post–acute care placement may be difficult due to limited availability of isolation rooms for patients with COVID-19 and the requirement of negative results for recovering patients. For those who manage to secure a bed, PPE shortages in nursing facilities could lead to lower prioritization of therapy interventions among staff and more bedridden days for the patients. Given social distancing restrictions, home health and outpatient therapy may not be possible for similar reasons.
The confluence of often highly symptomatic and even fragile patients, time-consuming visits with high concern for contagion, limited space to freely mobilize, and barriers to post–acute care illustrates why it is likely that COVID-19 admissions will be associated with a higher degree of HAD than admissions for other illnesses.
COVID-19: INNOVATION IN THERAPY SERVICES
The entire healthcare system has had to evolve and innovate rapidly to combat the morbidity and mortality of COVID-19. In the case of HAD, nursing staff, new billing guidelines, hospital redesign, and telemedicine are all facilitating novel ways to mobilize patients during and after hospitalization.
To limit the numbers of staff exposed to patients with COVID-19, the APA recommends engaging nursing staff in initial therapy evaluations and simple exercises that can be performed in a hospital room. Meaningful in-room exercise for some patients may include getting out of bed and walking to the bathroom to brush their teeth or complete other ADLs. Assessment of cognition should be carefully considered for discharge planning given its effects on the patient’s ability to independently participate in exercises and ADLs. For this reason, treatment and prevention of delirium or cognitive changes with interventions targeting environmental modifications, maintenance of healthy sleep-wake cycles, and orientation strategies are vital.
Therapy evaluations can also be administered remotely via phone call or video. To help facilitate telehealth visits, the Centers for Medicaid & Medicare Services has released new guidelines under the Coronavirus Aid, Relief, and Economic Security (CARES) Act. Physical and occupational therapists have been historically excluded from the list of providers able to bill for telehealth services, but the CARES Act allows physical and occupational therapists who accept Medicare part B to bill for telehealth services and e-visits. The new rule applies to patients in healthcare facilities or patients at home.17 Transitioning some physical and occupational therapy to telehealth could prove to be a critical resource for patients with COVID-19 trying to regain strength and independence during and after hospitalization.
Other solutions include converting areas of a hospital into rehabilitation units solely for patients recovering from COVID-19. Alternatively, rural hospitals, which usually run below capacity, or certain post–acute care facilities that are already prepared to manage infectious patients could serve as dedicated COVID-19 rehabilitation facilities, which can offer novel ways to continue therapy services after discharge while decreasing new exposures to COVID-19.18
Given the social isolation patients with COVID-19 experience during hospitalization, virtual group exercise classes may help for overall recovery. Most therapy companies already offer this service, and several include an app that allows therapists to monitor the patient’s exercises and progress. However, when transitioning to telemedicine, it is also important to consider the needs of those who may not be able to navigate technology effectively. For example, some elderly patients can be limited by a range of issues from poor computer skills and “technophobia” to visual and cognitive impairments. Having a friend or family member available to assist with technology should be considered. Additionally, being elderly, having lower income, or having a lower level of education makes it less likely that a patient will have access to internet or smartphones. Therefore, patients with these limitations may be poor candidates for telehealth and require post–acute care for their therapy services.19,20
CONCLUSION
With all the devastation that COVID-19 has created, it might be easy to forget the importance of physical and occupational therapy. But without this focus, the disability resulting from COVID-19 hospitalizations could inflict considerable long-lasting effects on our patients at great cost to an already strained healthcare system. Immediate changes in how we adapt and innovate these services for patients with COVID-19 are critical. It may prove to have enormous impact on patients and the healthcare system long after the worst of the virus is forgotten.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Arora is funded by National Heart, Lung and Blood Institute (NHLBI Grant K24HL136859).
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
1. Provisional COVID-19 Death Counts by Sex, Age, and State. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
2. Rajgor DD, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776-777. https://dx.doi.org/10.1016/S1473-3099(20)30244-9
3. Coronavirus Resource Center: Maps & Trends: Mortality Analyses. Johns Hopkins University & Medicine. Accessed April 26, 2020. https://coronavirus.jhu.edu/data/mortality
4. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461.e5. https://doi.org/10.1016/j.jamda.2019.09.015
5. Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. https://doi.org/10.1056/nejmp1212324
6. Summary Health Statistics: National Health Interview Survey, 2017. Tables P10a-P10c; p. 1-9. Centers for Disease Control and Prevention. Accessed April 26,2020. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_P-10.pdf
7. Fazio S, Stocking J, Kuhn B, et al. How much do hospitalized adults move? a systematic review and meta-analysis. Appl Nurs Res. 2020;51:151189. https://doi.org/10.1016/j.apnr.2019.151189
8. Fact Sheet: Post-Acute Care. American Hospital Association. July 2019. Accessed April 26, 2020. https://www.aha.org/system/files/media/file/2019/07/fact-sheet-post-acute-care-0719.pdf
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
10. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305-313. https://doi.org/10.1002/jhm.209
11. Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ 2020;369:m1923. https://doi.org/10.1136/bmj.m1923
12. Williams S, Gousen S, DeFrances C. National Hospital Care Survey Demonstration Projects: pneumonia inpatient hospitalizations and emergency department visits. Natl Health Stat Report. 2018;(116):1-11.
13. Milenkovic M, Russo CA, Elixhauser A. Hospital Stays for Influenza, 2004: Statistical Brief #16. 2006 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Accessed April 26, 2020 https://www.ncbi.nlm.nih.gov/books/NBK63484/
14. Simpson R, Robinson L. Rehabilitation after critical illness in people with COVID-19 infection. Am J Phys Med Rehabil. 2020;99(6):470-474. https://doi.org/10.1097/phm.0000000000001443
15. Coronavirus Disease 2019 (COVID-19): Social Distancing. Centers for Disease Control and Prevention. Accessed April 26, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html
16. Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73-82. https://doi.org/10.1016/j.jphys.2020.03.011
17. COVID1-9 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services. Accessed April 23, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
18. Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19: thinking ahead. JAMA. 2020;323(20):2007-2008. https://doi.org/10.1001/jama.2020.4686
19. Eung-Hun K, Stolvar A, Lober WB, et al. Challenges to using an electronic health record by a low-income elderly population. J Med Internet Res. 2009;11(4):e44. https://doi.org/10.2196/jmir.1256
20. Rajasekaran K. Access to telemedicine-are we doing all that we can during the COVID-19 pandemic? Otolaryngol Head Neck Surg. 2020;163(1):104-106. https://doi.org/10.1177/0194599820925049
© 2020 Society of Hospital Medicine




