User login
Using telehealth to deliver palliative care to cancer patients
Traditional delivery of palliative care to outpatients with cancer is associated with many challenges.
Telehealth can eliminate some of these challenges but comes with issues of its own, according to results of the REACH PC trial.
Jennifer S. Temel, MD, of Massachusetts General Hospital in Boston, discussed the use of telemedicine in palliative care, including results from REACH PC, during an educational session at the ASCO Virtual Quality Care Symposium 2020.
Dr. Temel noted that, for cancer patients, an in-person visit with a palliative care specialist can cost time, induce fatigue, and increase financial burden from transportation and parking expenses.
For caregivers and family, an in-person visit may necessitate absence from family and/or work, require complex scheduling to coordinate with other office visits, and result in additional transportation and/or parking expenses.
For health care systems, to have a dedicated palliative care clinic requires precious space and financial expenditures for office personnel and other resources.
These issues make it attractive to consider whether telehealth could be used for palliative care services.
Scarcity of palliative care specialists
In the United States, there is roughly 1 palliative care physician for every 20,000 older adults with a life-limiting illness, according to research published in Annual Review of Public Health in 2014.
In its 2019 state-by-state report card, the Center to Advance Palliative Care noted that only 72% of U.S. hospitals with 50 or more beds have a palliative care team.
For patients with serious illnesses and those who are socioeconomically or geographically disadvantaged, palliative care is often inaccessible.
Inefficiencies in the current system are an additional impediment. Palliative care specialists frequently see patients during a portion of the patient’s routine visit to subspecialty or primary care clinics. This limits the palliative care specialist’s ability to perform comprehensive assessments and provide patient-centered care efficiently.
Special considerations regarding telehealth for palliative care
As a specialty, palliative care involves interactions that could make the use of telehealth problematic. For example, conveyance of interest, warmth, and touch are challenging or impossible in a video format.
Palliative care specialists engage with patients regarding relatively serious topics such as prognosis and end-of-life preferences. There is uncertainty about how those discussions would be received by patients and their caregivers via video.
Furthermore, there are logistical impediments such as prescribing opioids with video or across state lines.
Despite these concerns, the ENABLE study showed that supplementing usual oncology care with weekly (transitioning to monthly) telephone-based educational palliative care produced higher quality of life and mood than did usual oncology care alone. These results were published in JAMA in 2009.
REACH PC study demonstrates feasibility of telehealth model
Dr. Temel described the ongoing REACH PC trial in which palliative care is delivered via video visits and compared with in-person palliative care for patients with advanced non–small cell lung cancer.
The primary aim of REACH PC is to determine whether telehealth palliative care is equivalent to traditional palliative care in improving quality of life as a supplement to routine oncology care.
Currently, REACH PC has enrolled 581 patients at its 20 sites, spanning a geographically diverse area. Just over half of patients approached about REACH PC agreed to enroll in it. Ultimately, 1,250 enrollees are sought.
Among patients who declined to participate, 7.6% indicated “discomfort with technology” as the reason. Most refusals were due to lack of interest in research (35.1%) and/or palliative care (22.9%).
Older adults were prominent among enrollees. More than 60% were older than 60 years of age, and more than one-third were older than 70 years.
Among patients who began the trial, there were slightly more withdrawals in the telehealth participants, in comparison with in-person participants (13.6% versus 9.1%).
When palliative care clinicians were queried about video visits, 64.3% said there were no challenges. This is comparable to the 65.5% of clinicians who had no challenges with in-person visits.
When problems occurred with video visits, they were most frequently technical (19.1%). Only 1.4% of clinicians reported difficulty addressing topics that felt uncomfortable over video, and 1.5% reported difficulty establishing rapport.
The success rates of video and in-person visits were similar. About 80% of visits accomplished planned goals.
‘Webside’ manner
Strategies such as reflective listening and summarizing what patients say (to verify an accurate understanding of the patient’s perspective) are key to successful palliative care visits, regardless of the setting.
For telehealth visits, Dr. Temel described techniques she defined as “webside manner,” to compensate for the inability of the clinician to touch a patient. These techniques include leaning in toward the camera, nodding, and pausing to be certain the patient has finished speaking before the clinician speaks again.
Is telehealth the future of palliative care?
I include myself among those oncologists who have voiced concern about moving from face-to-face to remote visits for complicated consultations such as those required for palliative care. Nonetheless, from the preliminary results of the REACH PC trial, it appears that telehealth could be a valuable tool.
To minimize differences between in-person and remote delivery of palliative care, practical strategies for ensuring rapport and facilitating a trusting relationship should be defined further and disseminated.
In addition, we need to be vigilant for widening inequities of care from rapid movement to the use of technology (i.e., an equity gap). In their telehealth experience during the COVID-19 pandemic, investigators at Houston Methodist Cancer Center found that patients declining virtual visits tended to be older, lower-income, and less likely to have commercial insurance. These results were recently published in JCO Oncology Practice.
For the foregoing reasons, hybrid systems for palliative care services will probably always be needed.
Going forward, we should heed the advice of Alvin Toffler in his book Future Shock. Mr. Toffler said, “The illiterate of the 21st century will not be those who cannot read and write, but those who cannot learn, unlearn, and relearn.”
The traditional model for delivering palliative care will almost certainly need to be reimagined and relearned.
Dr. Temel disclosed institutional research funding from Pfizer.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Traditional delivery of palliative care to outpatients with cancer is associated with many challenges.
Telehealth can eliminate some of these challenges but comes with issues of its own, according to results of the REACH PC trial.
Jennifer S. Temel, MD, of Massachusetts General Hospital in Boston, discussed the use of telemedicine in palliative care, including results from REACH PC, during an educational session at the ASCO Virtual Quality Care Symposium 2020.
Dr. Temel noted that, for cancer patients, an in-person visit with a palliative care specialist can cost time, induce fatigue, and increase financial burden from transportation and parking expenses.
For caregivers and family, an in-person visit may necessitate absence from family and/or work, require complex scheduling to coordinate with other office visits, and result in additional transportation and/or parking expenses.
For health care systems, to have a dedicated palliative care clinic requires precious space and financial expenditures for office personnel and other resources.
These issues make it attractive to consider whether telehealth could be used for palliative care services.
Scarcity of palliative care specialists
In the United States, there is roughly 1 palliative care physician for every 20,000 older adults with a life-limiting illness, according to research published in Annual Review of Public Health in 2014.
In its 2019 state-by-state report card, the Center to Advance Palliative Care noted that only 72% of U.S. hospitals with 50 or more beds have a palliative care team.
For patients with serious illnesses and those who are socioeconomically or geographically disadvantaged, palliative care is often inaccessible.
Inefficiencies in the current system are an additional impediment. Palliative care specialists frequently see patients during a portion of the patient’s routine visit to subspecialty or primary care clinics. This limits the palliative care specialist’s ability to perform comprehensive assessments and provide patient-centered care efficiently.
Special considerations regarding telehealth for palliative care
As a specialty, palliative care involves interactions that could make the use of telehealth problematic. For example, conveyance of interest, warmth, and touch are challenging or impossible in a video format.
Palliative care specialists engage with patients regarding relatively serious topics such as prognosis and end-of-life preferences. There is uncertainty about how those discussions would be received by patients and their caregivers via video.
Furthermore, there are logistical impediments such as prescribing opioids with video or across state lines.
Despite these concerns, the ENABLE study showed that supplementing usual oncology care with weekly (transitioning to monthly) telephone-based educational palliative care produced higher quality of life and mood than did usual oncology care alone. These results were published in JAMA in 2009.
REACH PC study demonstrates feasibility of telehealth model
Dr. Temel described the ongoing REACH PC trial in which palliative care is delivered via video visits and compared with in-person palliative care for patients with advanced non–small cell lung cancer.
The primary aim of REACH PC is to determine whether telehealth palliative care is equivalent to traditional palliative care in improving quality of life as a supplement to routine oncology care.
Currently, REACH PC has enrolled 581 patients at its 20 sites, spanning a geographically diverse area. Just over half of patients approached about REACH PC agreed to enroll in it. Ultimately, 1,250 enrollees are sought.
Among patients who declined to participate, 7.6% indicated “discomfort with technology” as the reason. Most refusals were due to lack of interest in research (35.1%) and/or palliative care (22.9%).
Older adults were prominent among enrollees. More than 60% were older than 60 years of age, and more than one-third were older than 70 years.
Among patients who began the trial, there were slightly more withdrawals in the telehealth participants, in comparison with in-person participants (13.6% versus 9.1%).
When palliative care clinicians were queried about video visits, 64.3% said there were no challenges. This is comparable to the 65.5% of clinicians who had no challenges with in-person visits.
When problems occurred with video visits, they were most frequently technical (19.1%). Only 1.4% of clinicians reported difficulty addressing topics that felt uncomfortable over video, and 1.5% reported difficulty establishing rapport.
The success rates of video and in-person visits were similar. About 80% of visits accomplished planned goals.
‘Webside’ manner
Strategies such as reflective listening and summarizing what patients say (to verify an accurate understanding of the patient’s perspective) are key to successful palliative care visits, regardless of the setting.
For telehealth visits, Dr. Temel described techniques she defined as “webside manner,” to compensate for the inability of the clinician to touch a patient. These techniques include leaning in toward the camera, nodding, and pausing to be certain the patient has finished speaking before the clinician speaks again.
Is telehealth the future of palliative care?
I include myself among those oncologists who have voiced concern about moving from face-to-face to remote visits for complicated consultations such as those required for palliative care. Nonetheless, from the preliminary results of the REACH PC trial, it appears that telehealth could be a valuable tool.
To minimize differences between in-person and remote delivery of palliative care, practical strategies for ensuring rapport and facilitating a trusting relationship should be defined further and disseminated.
In addition, we need to be vigilant for widening inequities of care from rapid movement to the use of technology (i.e., an equity gap). In their telehealth experience during the COVID-19 pandemic, investigators at Houston Methodist Cancer Center found that patients declining virtual visits tended to be older, lower-income, and less likely to have commercial insurance. These results were recently published in JCO Oncology Practice.
For the foregoing reasons, hybrid systems for palliative care services will probably always be needed.
Going forward, we should heed the advice of Alvin Toffler in his book Future Shock. Mr. Toffler said, “The illiterate of the 21st century will not be those who cannot read and write, but those who cannot learn, unlearn, and relearn.”
The traditional model for delivering palliative care will almost certainly need to be reimagined and relearned.
Dr. Temel disclosed institutional research funding from Pfizer.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Traditional delivery of palliative care to outpatients with cancer is associated with many challenges.
Telehealth can eliminate some of these challenges but comes with issues of its own, according to results of the REACH PC trial.
Jennifer S. Temel, MD, of Massachusetts General Hospital in Boston, discussed the use of telemedicine in palliative care, including results from REACH PC, during an educational session at the ASCO Virtual Quality Care Symposium 2020.
Dr. Temel noted that, for cancer patients, an in-person visit with a palliative care specialist can cost time, induce fatigue, and increase financial burden from transportation and parking expenses.
For caregivers and family, an in-person visit may necessitate absence from family and/or work, require complex scheduling to coordinate with other office visits, and result in additional transportation and/or parking expenses.
For health care systems, to have a dedicated palliative care clinic requires precious space and financial expenditures for office personnel and other resources.
These issues make it attractive to consider whether telehealth could be used for palliative care services.
Scarcity of palliative care specialists
In the United States, there is roughly 1 palliative care physician for every 20,000 older adults with a life-limiting illness, according to research published in Annual Review of Public Health in 2014.
In its 2019 state-by-state report card, the Center to Advance Palliative Care noted that only 72% of U.S. hospitals with 50 or more beds have a palliative care team.
For patients with serious illnesses and those who are socioeconomically or geographically disadvantaged, palliative care is often inaccessible.
Inefficiencies in the current system are an additional impediment. Palliative care specialists frequently see patients during a portion of the patient’s routine visit to subspecialty or primary care clinics. This limits the palliative care specialist’s ability to perform comprehensive assessments and provide patient-centered care efficiently.
Special considerations regarding telehealth for palliative care
As a specialty, palliative care involves interactions that could make the use of telehealth problematic. For example, conveyance of interest, warmth, and touch are challenging or impossible in a video format.
Palliative care specialists engage with patients regarding relatively serious topics such as prognosis and end-of-life preferences. There is uncertainty about how those discussions would be received by patients and their caregivers via video.
Furthermore, there are logistical impediments such as prescribing opioids with video or across state lines.
Despite these concerns, the ENABLE study showed that supplementing usual oncology care with weekly (transitioning to monthly) telephone-based educational palliative care produced higher quality of life and mood than did usual oncology care alone. These results were published in JAMA in 2009.
REACH PC study demonstrates feasibility of telehealth model
Dr. Temel described the ongoing REACH PC trial in which palliative care is delivered via video visits and compared with in-person palliative care for patients with advanced non–small cell lung cancer.
The primary aim of REACH PC is to determine whether telehealth palliative care is equivalent to traditional palliative care in improving quality of life as a supplement to routine oncology care.
Currently, REACH PC has enrolled 581 patients at its 20 sites, spanning a geographically diverse area. Just over half of patients approached about REACH PC agreed to enroll in it. Ultimately, 1,250 enrollees are sought.
Among patients who declined to participate, 7.6% indicated “discomfort with technology” as the reason. Most refusals were due to lack of interest in research (35.1%) and/or palliative care (22.9%).
Older adults were prominent among enrollees. More than 60% were older than 60 years of age, and more than one-third were older than 70 years.
Among patients who began the trial, there were slightly more withdrawals in the telehealth participants, in comparison with in-person participants (13.6% versus 9.1%).
When palliative care clinicians were queried about video visits, 64.3% said there were no challenges. This is comparable to the 65.5% of clinicians who had no challenges with in-person visits.
When problems occurred with video visits, they were most frequently technical (19.1%). Only 1.4% of clinicians reported difficulty addressing topics that felt uncomfortable over video, and 1.5% reported difficulty establishing rapport.
The success rates of video and in-person visits were similar. About 80% of visits accomplished planned goals.
‘Webside’ manner
Strategies such as reflective listening and summarizing what patients say (to verify an accurate understanding of the patient’s perspective) are key to successful palliative care visits, regardless of the setting.
For telehealth visits, Dr. Temel described techniques she defined as “webside manner,” to compensate for the inability of the clinician to touch a patient. These techniques include leaning in toward the camera, nodding, and pausing to be certain the patient has finished speaking before the clinician speaks again.
Is telehealth the future of palliative care?
I include myself among those oncologists who have voiced concern about moving from face-to-face to remote visits for complicated consultations such as those required for palliative care. Nonetheless, from the preliminary results of the REACH PC trial, it appears that telehealth could be a valuable tool.
To minimize differences between in-person and remote delivery of palliative care, practical strategies for ensuring rapport and facilitating a trusting relationship should be defined further and disseminated.
In addition, we need to be vigilant for widening inequities of care from rapid movement to the use of technology (i.e., an equity gap). In their telehealth experience during the COVID-19 pandemic, investigators at Houston Methodist Cancer Center found that patients declining virtual visits tended to be older, lower-income, and less likely to have commercial insurance. These results were recently published in JCO Oncology Practice.
For the foregoing reasons, hybrid systems for palliative care services will probably always be needed.
Going forward, we should heed the advice of Alvin Toffler in his book Future Shock. Mr. Toffler said, “The illiterate of the 21st century will not be those who cannot read and write, but those who cannot learn, unlearn, and relearn.”
The traditional model for delivering palliative care will almost certainly need to be reimagined and relearned.
Dr. Temel disclosed institutional research funding from Pfizer.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM ASCO QUALITY CARE SYMPOSIUM 2020
Key Studies in Ulcerative Colitis From ACG 2020 Virtual Conference
Miguel Regueiro, MD, an expert in gastroenterology at the Cleveland Clinic, reflects on the most important and clinically relevant studies on ulcerative colitis presented at the American College of Gastroenterology 2020 virtual annual scientific meeting. He starts with four studies from the OCTAVE clinical trials program. These studies examined the efficacy and safety of tofacitinib after treatment interruption and in pregnant women, presenting almost 7 years of follow-up. Long-term follow-up remains the theme as he turns to the VISIBLE open-label extension of treatment with vedolizumab SC, where the long-term safety of the drug was confirmed and clinical remission rates were maintained out to 2 years. He reports that a post-hoc analysis of the VARSITY trial appeared to show that vedolizumab achieves greater early control vs adalimumab. Dr Regueiro next discusses the late-breaking, phase 3 True North study of ozanimod for moderate to severe ulcerative colitis before finishing up with an analysis of the long-term trends for colectomy since the turn of the century.
Miguel D. Regueiro, MD, Chairman, Professor, Department of Gastroenterology, Hepatology, and Nutrition; Vice-Chair, Digestive Disease Institute, Cleveland Clinic, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio.
Miguel D. Regueiro, MD, has disclosed the following relevant financial relationships: Serve(d) as an advisor and/or consultant for: AbbVie; Janssen; UCB; Takeda; Pfizer; Miraca Labs; Amgen; Celgene; Seres; Allergan; Genentech; Gilead; Salix; Prometheus. Received unrestricted educational grants from: AbbVie; Janssen; UCB; Pfizer; Takeda; Salix; Shire. Received research support from AbbVie; Janssen; Takeda; Pfizer.
Miguel Regueiro, MD, an expert in gastroenterology at the Cleveland Clinic, reflects on the most important and clinically relevant studies on ulcerative colitis presented at the American College of Gastroenterology 2020 virtual annual scientific meeting. He starts with four studies from the OCTAVE clinical trials program. These studies examined the efficacy and safety of tofacitinib after treatment interruption and in pregnant women, presenting almost 7 years of follow-up. Long-term follow-up remains the theme as he turns to the VISIBLE open-label extension of treatment with vedolizumab SC, where the long-term safety of the drug was confirmed and clinical remission rates were maintained out to 2 years. He reports that a post-hoc analysis of the VARSITY trial appeared to show that vedolizumab achieves greater early control vs adalimumab. Dr Regueiro next discusses the late-breaking, phase 3 True North study of ozanimod for moderate to severe ulcerative colitis before finishing up with an analysis of the long-term trends for colectomy since the turn of the century.
Miguel D. Regueiro, MD, Chairman, Professor, Department of Gastroenterology, Hepatology, and Nutrition; Vice-Chair, Digestive Disease Institute, Cleveland Clinic, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio.
Miguel D. Regueiro, MD, has disclosed the following relevant financial relationships: Serve(d) as an advisor and/or consultant for: AbbVie; Janssen; UCB; Takeda; Pfizer; Miraca Labs; Amgen; Celgene; Seres; Allergan; Genentech; Gilead; Salix; Prometheus. Received unrestricted educational grants from: AbbVie; Janssen; UCB; Pfizer; Takeda; Salix; Shire. Received research support from AbbVie; Janssen; Takeda; Pfizer.
Miguel Regueiro, MD, an expert in gastroenterology at the Cleveland Clinic, reflects on the most important and clinically relevant studies on ulcerative colitis presented at the American College of Gastroenterology 2020 virtual annual scientific meeting. He starts with four studies from the OCTAVE clinical trials program. These studies examined the efficacy and safety of tofacitinib after treatment interruption and in pregnant women, presenting almost 7 years of follow-up. Long-term follow-up remains the theme as he turns to the VISIBLE open-label extension of treatment with vedolizumab SC, where the long-term safety of the drug was confirmed and clinical remission rates were maintained out to 2 years. He reports that a post-hoc analysis of the VARSITY trial appeared to show that vedolizumab achieves greater early control vs adalimumab. Dr Regueiro next discusses the late-breaking, phase 3 True North study of ozanimod for moderate to severe ulcerative colitis before finishing up with an analysis of the long-term trends for colectomy since the turn of the century.
Miguel D. Regueiro, MD, Chairman, Professor, Department of Gastroenterology, Hepatology, and Nutrition; Vice-Chair, Digestive Disease Institute, Cleveland Clinic, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio.
Miguel D. Regueiro, MD, has disclosed the following relevant financial relationships: Serve(d) as an advisor and/or consultant for: AbbVie; Janssen; UCB; Takeda; Pfizer; Miraca Labs; Amgen; Celgene; Seres; Allergan; Genentech; Gilead; Salix; Prometheus. Received unrestricted educational grants from: AbbVie; Janssen; UCB; Pfizer; Takeda; Salix; Shire. Received research support from AbbVie; Janssen; Takeda; Pfizer.

Siblings of patients with bipolar disorder at increased risk
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
The siblings of patients with bipolar disorder not only face a significantly increased lifetime risk of that affective disorder, but a whole panoply of other psychiatric disorders, according to a new Danish longitudinal national registry study.
“Our data show the healthy siblings of patients with bipolar disorder are themselves at increased risk of developing any kind of psychiatric disorder. Mainly bipolar disorder, but all other kinds as well,” Lars Vedel Kessing, MD, DMSc, said in presenting the results of the soon-to-be-published Danish study at the virtual congress of the European College of Neuropsychopharmacology.
Moreover, the long-term Danish study also demonstrated that several major psychiatric disorders follow a previously unappreciated bimodal distribution of age of onset in the siblings of patients with bipolar disorder. For example, the incidence of new-onset bipolar disorder and unipolar depression in the siblings was markedly increased during youth and early adulthood, compared with controls drawn from the general Danish population. Then, incidence rates dropped off and plateaued at a lower level in midlife before surging after age 60 years. The same was true for somatoform disorders as well as alcohol and substance use disorders.
“Strategies to prevent onset of psychiatric illness in individuals with a first-generation family history of bipolar disorder should not be limited to adolescence and early adulthood but should be lifelong, likely with differentiated age-specific approaches. And this is not now the case.
“Generally, most researchers and clinicians are focusing more on the early part of life and not the later part of life from age 60 and up, even though this is indeed also a risk period for any kind of psychiatric illness as well as bipolar disorder,” according to Dr. Kessing, professor of psychiatry at the University of Copenhagen.
Dr. Kessing, a past recipient of the Brain and Behavior Research Foundation’s Outstanding Achievement in Mood Disorders Research Award, also described his research group’s successful innovative efforts to prevent first recurrences after a single manic episode or bipolar disorder.
Danish national sibling study
The longitudinal registry study included all 19,995 Danish patients with a primary diagnosis of bipolar disorder during 1995-2017, along with 13,923 of their siblings and 278,460 age- and gender-matched controls drawn from the general population.
The cumulative incidence of any psychiatric disorder was 66% greater in siblings than controls. Leading the way was a 374% increased risk of bipolar disorder.
Strategies to prevent a first relapse of bipolar disorder
Dr. Kessing and coinvestigators demonstrated in a meta-analysis that, with current standard therapies, the risk of recurrence among patients after a single manic or mixed episode is high in both adult and pediatric patients. In three studies of adults, the risk of recurrence was 35% during the first year after recovery from the index episode and 59% at 2 years. In three studies of children and adolescents, the risk of recurrence within 1 year after recovery was 40% in children and 52% in adolescents. This makes a compelling case for starting maintenance therapy following onset of a single manic or mixed episode, according to the investigators.
More than half a decade ago, Dr. Kessing and colleagues demonstrated in a study of 4,714 Danish patients with bipolar disorder who were prescribed lithium while in a psychiatric hospital that those who started the drug for prophylaxis early – that is, following their first psychiatric contact – had a significantly higher response to lithium monotherapy than those who started it only after repeated contacts. Indeed, their risk of nonresponse to lithium prophylaxis as evidenced by repeat hospital admission after a 6-month lithium stabilization period was 13% lower than in those starting the drug later.
Early intervention aiming to stop clinical progression of bipolar disorder intuitively seems appealing, so Dr. Kessing and colleagues created a specialized outpatient mood disorders clinic combining optimized pharmacotherapy and evidence-based group psychoeducation. They then put it to the test in a clinical trial in which 158 patients discharged from an initial psychiatric hospital admission for bipolar disorder were randomized to the specialized outpatient mood disorders clinic or standard care.
The rate of psychiatric hospital readmission within the next 6 years was 40% lower in the group assigned to the specialized early intervention clinic. Their rate of adherence to medication – mostly lithium and antipsychotics – was significantly higher. So were their treatment satisfaction scores. And the clincher: The total net direct cost of treatment in the specialized mood disorders clinic averaged 3,194 euro less per patient, an 11% reduction relative to the cost of standard care, a striking economic benefit achieved mainly through avoided hospitalizations.
In a subsequent subgroup analysis of the randomized trial data, Dr. Kessing and coinvestigators demonstrated that young adults with bipolar disorder not only benefited from participation in the specialized outpatient clinic, but they appeared to have derived greater benefit than the older patients. The rehospitalization rate was 67% lower in 18- to 25-year-old patients randomized to the specialized outpatient mood disorder clinic than in standard-care controls, compared with a 32% relative risk reduction in outpatient clinic patients aged 26 years or older).
“There are now several centers around the world which also use this model involving early intervention,” Dr. Kessing said. “It is so important that, when the diagnosis is made for the first time, the patient gets sufficient evidence-based treatment comprised of mood maintenance medication as well as group-based psychoeducation, which is the psychotherapeutic intervention for which there is the strongest evidence of an effect.”
The sibling study was funded free of commercial support. Dr. Kessing reported serving as a consultant to Lundbeck.
SOURCE: Kessing LV. ECNP 2020, Session S.25.
FROM ECNP 2020
Opt-out policy at a syringe service program increased HIV/HCV testing
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
Bundled opt-out HIV/hepatitis C virus (HCV) testing increased the percentage of syringe service program (SSP) clients who received HIV and HCV rapid tests at enrollment into the program. Researchers conducted a retrospective comparative analysis of patient testing patterns before and after opt-out policy implementation in a single SSP program, according to a report published online in the International Journal of Drug Policy.
Because HCV is the most common infectious disease among people who inject drugs (PWID), engaging PWID in harm reduction services, such as SSPs, is critical to reduce HCV and HIV transmission, according to Tyler S. Bartholomew of the University of Miami, and colleagues. They added that testing for HIV and HCV among PWID is important for improvement of diagnosis and linkage to care.
Their study, conducted in the 37 months between December 2016 and January 2020 assessed 512 SSP participants 15 months prior to and 547 SSP participants 22 months after implementation of bundled HIV/HCV opt-out testing.
Opt-out optimal
There was a significant increase in uptake of HIV/HCV testing by 42.4% (95% confidence interval, 26.2%-58.5%; P < 0.001) immediately after the policy changed to opt-out testing, according to the researchers. In addition, they found that the significant predictors of accepting both HIV/HCV tests were cocaine injection (adjusted odds ratio, 2.36), self-reported HIV-positive status (aOR, 0.39), and self-reported HCV-positive status (aOR, 0.27).
The authors explained that participants who injected cocaine in the previous 30 days, compared with other drugs, might have had higher odds of accepting HIV/HCV testing because of their known added risk factors. Previous studies have shown that people who use stimulants describe higher rates of condomless sex, sex work, and sex in exchange for money or drugs, compared with people who use nonstimulant drugs.
“Our paper is the first of which we are aware to suggest that implementation of routine opt-out HIV/HCV testing among PWID at SSPs could enhance HIV/HCV testing among this high incidence population,” the researchers concluded.
The authors reported funding from the National Cancer Institute and the Frontlines of Communities in the United States, a program of Gilead Sciences. They provided no other disclosures.
SOURCE: Bartholomew TS et al. Int J Drug Policy. 2020; doi: 10.1016/j.drugpo.2020.102875.
FROM INTERNATIONAL JOURNAL OF DRUG POLICY
Open enrollment 2021: A big start for HealthCare.gov
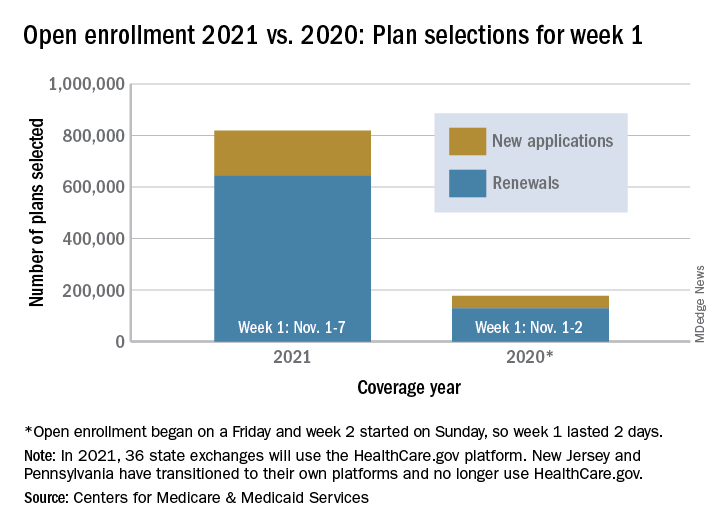
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.
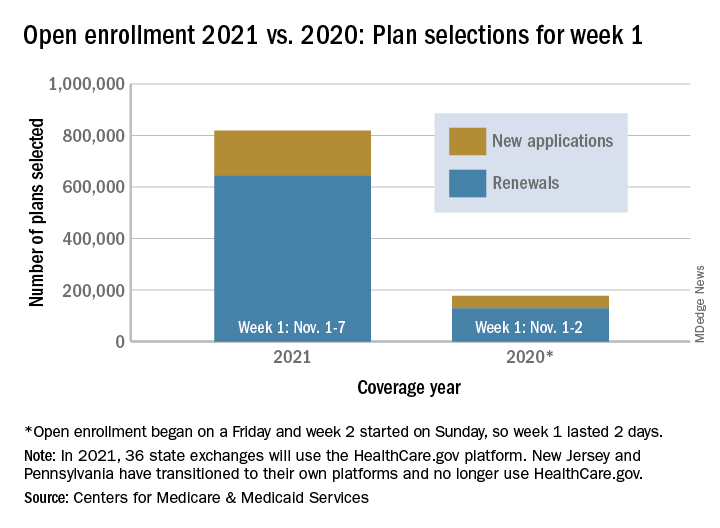
The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.

The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
Over 818,000 plans were selected for the 2021 coverage year during the first week, Nov.1-7, of this year’s open enrollment on the federal health insurance exchange, according to the Centers for Medicare & Medicaid Services.

The bulk of those plans, nearly 79%, were renewals by consumers who had coverage through the federal exchange this year. The balance covers new plans selected by individuals who were not covered through HealthCare.gov this year, the CMS noted in a written statement.
The total enrollment for week 1 marks a considerable increase over last year’s first week of open enrollment, which saw approximately 177,000 plans selected, but Nov. 1 fell on a Friday in 2019, so that total represents only 2 days since weeks are tracked as running from Sunday to Saturday, the CMS explained.
For the 2021 benefit year, the HealthCare.gov platform covers 36 states, down from 38 for the 2020 benefit year, because New Jersey and Pennsylvania have “transitioned to their own state-based exchange platforms,” the CMS noted, adding that the two accounted for 7% of all plans selected last year.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS said, and its weekly snapshot “does not report the number of consumers who have paid premiums to effectuate their enrollment.”
This year’s open-enrollment period on HealthCare.gov is scheduled to conclude Dec. 15.
Nail Unit Squamous Cell Carcinoma: Updates on Diagnosis, Surgical Approach, and the Use of Mohs Micrographic Surgery
Nail unit squamous cell carcinoma (NSCC) is a malignant neoplasm that can arise from any part of the nail unit. Diagnosis often is delayed due to its clinical presentation mimicking benign conditions such as onychomycosis, warts, and paronychia. Nail unit SCC has a low rate of metastasis; however, a delayed diagnosis often can result in local destruction and bone invasion. It is imperative for dermatologists who are early in their training to recognize this entity and refer for treatment. Many approaches have been used to treat NSCC, including wide local excision, digital amputation, cryotherapy, topical modalities, and recently Mohs micrographic surgery (MMS). This article provides an overview of the clinical presentation and diagnosis of NSCC, the role of human papillomavirus (HPV) in NSCC pathogenesis, and the evidence supporting surgical management.
NSCC Clinical Presentation and Diagnosis
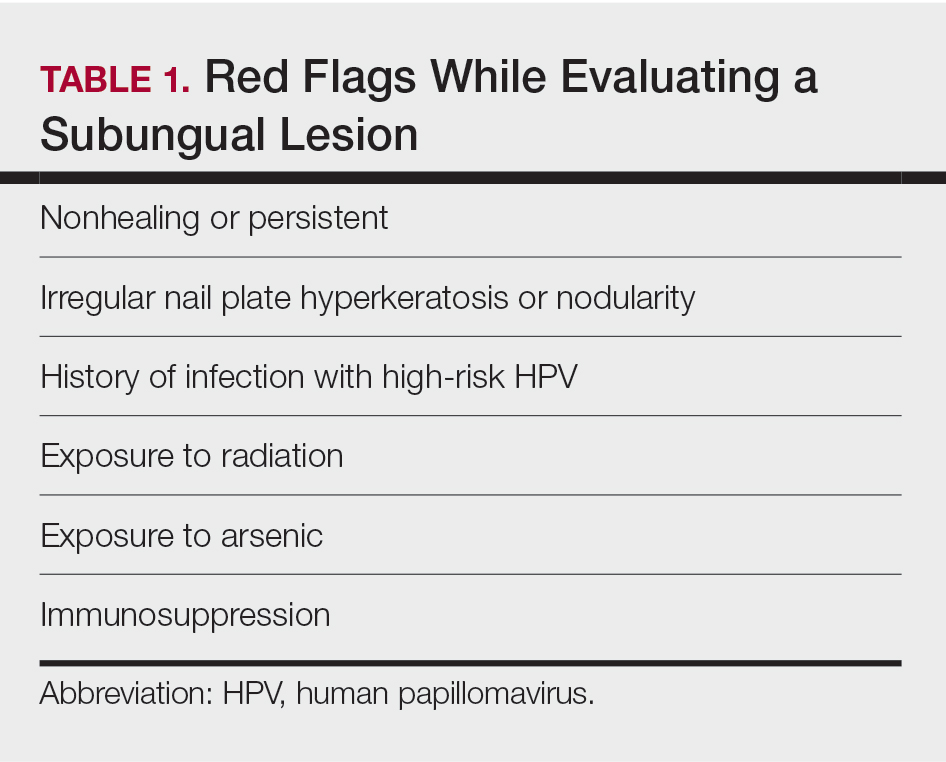
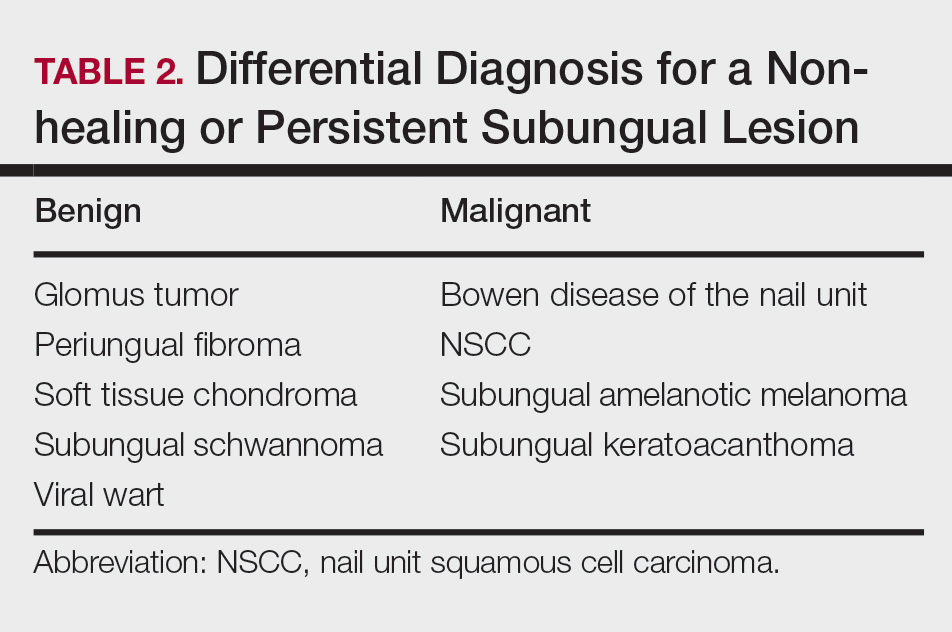
Nail unit squamous cell carcinoma is a malignant neoplasm that can arise from any part of the nail unit including the nail bed, matrix, groove, and nail fold.1 Although NSCC is the most common malignant nail neoplasm, its diagnosis often is delayed partly due to the clinical presentation of NSCC mimicking benign conditions such as onychomycosis, warts, and paronychia.2,3 Nail unit SCC most commonly is mistaken for verruca vulgaris, and thus it is important to exclude malignancy in nonresolving verrucae of the fingernails or toenails. Another reason for a delay in the diagnosis is the painless and often asymptomatic presentation of this tumor, which keeps patients from seeking care.4 While evaluating a subungual lesion, dermatologists should keep in mind red flags that would prompt a biopsy to rule out NSCC (Table 1), including chronic nonhealing lesions, nail plate nodularity, known history of infection with HPV types 16 and 18, history of radiation or arsenic exposure, and immunosuppression. Table 2 lists the differential diagnosis of a persisting or nonhealing subungual tumor.
Nail unit SCC has a low rate of metastasis; however, a delayed diagnosis often can result in local destruction and bone invasion.5 Based on several reports, NSCC more commonly is found in middle-aged and older individuals, has a male predilection, and more often is seen on fingernails than toenails.1,2,6 Figure A shows an example of the clinical presentation of NSCC affecting the right thumb.
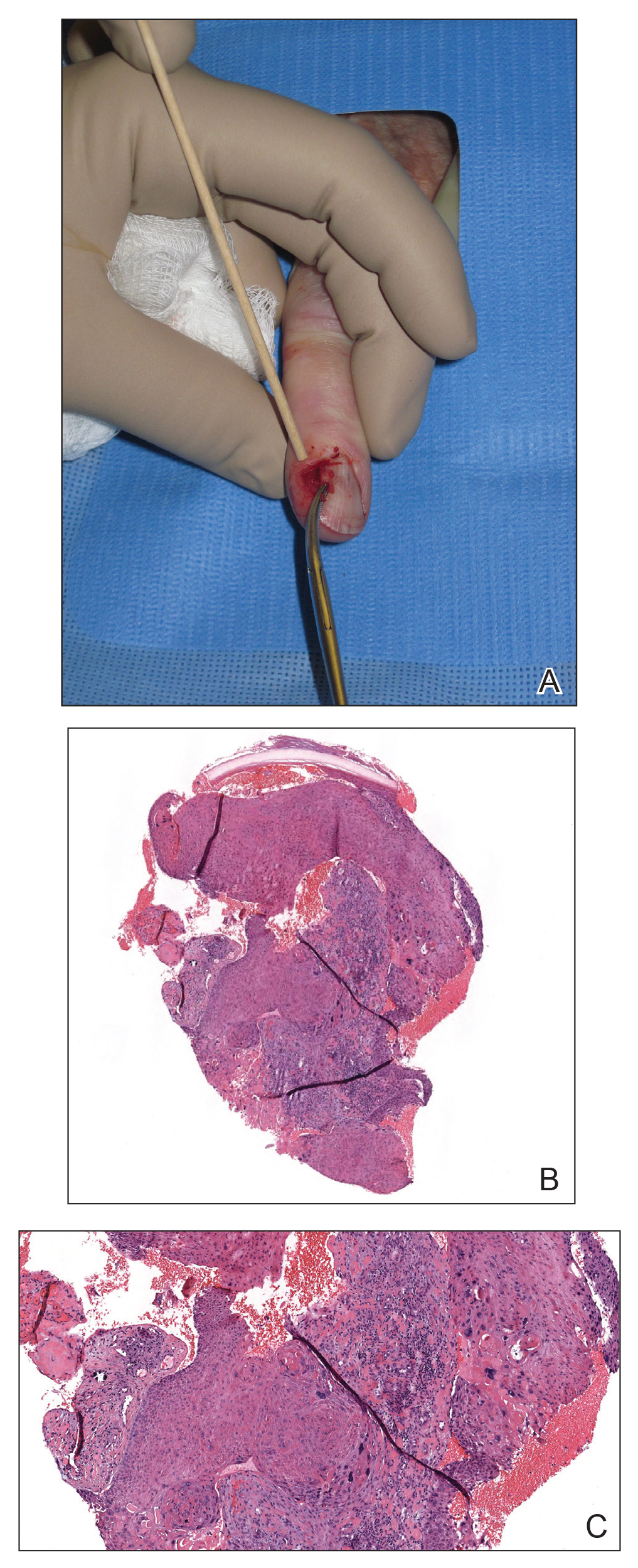
Although there often is a delay in the presentation and biopsy of NSCC, no correlation has been observed between time to biopsy and rate of disease invasion and recurrence.7 Nevertheless, Starace et al7 noted that a low threshold for biopsy of nail unit lesions is necessary. It is recommended to perform a deep shave or a nail matrix biopsy, especially if matrical involvement is suspected.8 Patients should be closely followed after a diagnosis of NSCC is made, especially if they are immunocompromised or have genetic skin cancer syndromes, as multiple NSCCs can occur in the same individual.9 For instance, one report discussed a patient with xeroderma pigmentosum who developed 3 separate NSCCs. Interestingly, in this patient, the authors suspected HPV as a cause for the field cancerization, as 2 of 3 NSCCs were noted on initial histopathology to have arisen from verrucae.10
Histologic Features
A biopsy from an NSCC tumor shows features similar to cutaneous SCC in the affected areas (ie, nail bed, nail matrix, nail groove, nail fold). Characteristic histologic findings include tongues or whorls of atypical squamous epithelium that invade deeply into the dermis.11 The cells appear as atypical keratinocytes, exhibit distinct intracellular bridges, and possess hyperchromatic and pleomorphic nuclei with dyskeratosis and keratin pearls within the dermis.12 Immunoperoxidase staining for cytokeratin AE1/AE3 can be helpful to confirm the diagnosis and assess whether the depth of invasion involves the bone.13 Figures B and C demonstrate the histopathology of NSCC biopsied from the tumor shown in Figure A.
Role of HPV in NSCC Pathogenesis
There is no clear pathogenic etiology for NSCC; however, there have been some reports of HPV as a risk factor. Shimizu et al14 reviewed 136 cases of HPV-associated NSCC and found that half of the cases were associated with high-risk HPV. They also found that 24% of the patients with NSCC had a history of other HPV-associated diseases. As such, the authors hypothesized that there is a possibility for genitodigital HPV transmission and that NSCC could be a reservoir for sexually transmitted high-risk HPV.14 Other risk factors are radiation exposure, chemical insult, and chronic trauma.15 The higher propensity for fingernails likely is reflective of the role of UV light exposure and infection with HPV in the development of these tumors.14,15
Several nonsurgical approaches have been suggested to treat NSCC, including topical agents, cryotherapy, CO2 laser, and photodynamic therapy.3,16 Unfortunately, there are no large case series to demonstrate the cure rate or effectiveness of these methods.17 In one study, the authors did not recommend use of photodynamic therapy or topical modalities such as imiquimod cream 5% or fluorouracil cream 5% as first-line treatments of NSCC due to the difficulty in ensuring complete treatment of the sulci of the lateral and proximal nail folds.18
More evidence in the literature supports surgical approaches, including wide local excision, MMS, and digital amputation. Clinicians should consider relapse rates and the impact on digital functioning when choosing a surgical approach.
For wide local excisions, the most common approach is en bloc excision of the nail unit including the lateral nail folds, the proximal nail fold, and the distal nail fold. The excision starts with a transverse incision on the base of the distal phalanx, which is then prolonged laterally and distally to the distal nail fold down to the bone. After the incision is made to the depth of the bone, the matrical horns are destroyed by electrocoagulation, and the defect is closed either by a full-thickness skin graft or secondary intent.19
Topin-Ruiz et al19 followed patients with biopsy-proven NSCC without bone invasion who underwent en bloc excision followed by full-thickness skin graft. In their consecutive series of 55 patients with 5 years of follow-up, the rate of recurrence was only 4%. There was a low rate of complications including graft infection, delayed wound healing, and severe pain in a small percentage of patients. They also reported a high patient satisfaction rate.19 Due to the low recurrence rate, this study suggested that total excision of the nail unit followed by a full-thickness skin graft is a safe and efficient treatment of NSCC without bone involvement. Similarly, in another case series, wide local excision of the entire nail apparatus had a relapse rate of only 5%, in contrast to partial excision of the nail unit with a relapse of 56%.20 These studies suggest that wide nail unit excision is an acceptable and effective approach; however, in cases in which invasion cannot be ruled out, histologic clearance would be a reasonable approach.21 As such, several case series demonstrated the merits of MMS for NSCC. de Berker et al22 reported 8 patients with NSCC treated using slow MMS and showed tumor clearance after a mean of 3 stages over a mean period of 6.9 days. In all cases, the wounds were allowed to heal by secondary intention, and the distal phalanx was preserved. During a mean follow-up period of 3.1 years, no recurrence was seen, and involved digits remained functional.22
Other studies tested the efficacy of MMS for NSCC. Young et al23 reported the outcomes of 14 NSCC cases treated with MMS. In their case series, they found that the mean number of MMS surgical stages required to achieve histologic clearance was 2, while the mean number of tissue sections was 4.23 All cases were allowed to heal by secondary intent with excellent outcomes, except for 1 patient who received primary closure of a small defect. They reported a 78% cure rate with an average time to recurrence of 47 months.23 In a series of 42 cases of NSCC treated with MMS, Gou et al17 noted a cure rate close to 93%. In their study, recurrences were observed in only 3 patients (7.1%). These recurrent cases were then successfully treated with another round of MMS.17 This study’s cure rate was comparable to the cure rate of MMS for SCC in other cutaneous areas. Goldminz and Bennett24 demonstrated a cure rate of 92% in their case series of 25 patients. Two patients developed recurrent disease and were treated again with MMS resulting in no subsequent recurrence. In this study, the authors allowed all defects to heal by secondary intention and found that there were excellent cosmetic and functional outcomes.24 Dika et al25 evaluated the long-term effectiveness of MMS in the treatment of NSCC, in particular its ability to reduce the number of digital amputations. Fifteen patients diagnosed with NSCC were treated with MMS as the first-line surgical approach and were followed for 2 to 5 years. They found that in utilizing MMS, they were able to avoid amputations in 13 of 15 cases with no recurrence in any of these tumors. Two cases, however, still required amputation of the distal phalanx.25
Although these studies suggest that MMS achieves a high cure rate ranging from 78% to 93%, it is not yet clear in the literature whether MMS is superior to wide local excision. More studies and clinical trials comparing these 2 surgical approaches should be performed to identify which surgical approach would be the gold standard for NSCC and which select cases would benefit from MMS as first-line treatment.
Final Thoughts
Nail unit SCC is one of the most common nail unit malignancies and can mimic several benign entities. Dermatologists who are early in their training should consider biopsy of subungual lesions with certain red flags (Table 1). It is important to diagnose NSCC for early intervention. Referral for wide local excision or MMS would be ideal. There are data in the literature supporting both surgical approaches as being effective; however, there are no trials comparing both approaches. Distal amputation should be considered as a last resort when wide local excision is not reasonable or when MMS fails to achieve clear margins, thereby reducing unnecessary amputations and patient morbidity.17
- Dika E, Starace M, Patrizi A, et al. Squamous cell carcinoma of the nail unit: a clinical histopathologic study and a proposal for classification. Dermatol Surg. 2019;45:365-370.
- Lee TM, Jo G, Kim M, et al. Squamous cell carcinoma of the nail unit: a retrospective review of 19 cases in Asia and comparative review of Western literature. Int J Dermatol. 2019;58:428-432.
- Tambe SA, Patil PD, Saple DG, et al. Squamous cell carcinoma of the nail bed: the great mimicker. J Cutan Aesthet Surg. 2017;10:59-60.
- Perrin C. Tumors of the nail unit. a review. part II: acquired localized longitudinal pachyonychia and masked nail tumors. Am J Dermatopathol. 2013;35:693-712.
- Li PF, Zhu N, Lu H. Squamous cell carcinoma of the nail bed: a case report. World J Clin Cases. 2019;7:3590-3594.
- Kaul S, Singal A, Grover C, et al. Clinical and histological spectrum of nail psoriasis: a cross-sectional study. J Cutan Pathol. 2018;45:824-830.
- Starace M, Alessandrini A, Dika E, et al. Squamous cell carcinoma of the nail unit. Dermatol Pract Concept. 2018;8:238-244.
- Kelly KJ, Kalani AD, Storrs S, et al. Subungual squamous cell carcinoma of the toe: working toward a standardized therapeutic approach. J Surg Educ. 2008;65:297-301.
- Ormerod E, De Berker D. Nail unit squamous cell carcinoma in people with immunosuppression. Br J Dermatol. 2015;173:701-712.
- Ventéjou S, Bagny K, Waldmeyer J, et al. Skin cancers in patients of skin phototype V or VI with xeroderma pigmentosum type C (XP-C): a retrospective study. Ann Dermatol Venereol. 2019;146:192-203.
- Mikhail GR. Subungual epidermoid carcinoma. J Am Acad Dermatol. 1984;11:291-298.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Kurokawa I, Senba Y, Kakeda M, et al. Cytokeratin expression in subungual squamous cell carcinoma. J Int Med Res. 2006;34:441-443.
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370.
- Tang N, Maloney ME, Clark AH, et al. A retrospective study of nail squamous cell carcinoma at 2 institutions. Dermatol Surg. 2016;42(suppl 1):S8-S17.
- An Q, Zheng S, Zhang L, et al. Subungual squamous cell carcinoma treated by topical photodynamic therapy. Chin Med J (Engl). 2020;133:881-882.
- Gou D, Nijhawan RI, Srivastava D. Mohs micrographic surgery as the standard of care for nail unit squamous cell carcinoma. Dermatol Surg. 2020;46:725-732.
- Dika E, Fanti PA, Patrizi A, et al. Mohs surgery for squamous cell carcinoma of the nail unit: 10 years of experience. Dermatol Surg. 2015;41:1015-1019.
- Topin-Ruiz S, Surinach C, Dalle S, et al. Surgical treatment of subungual squamous cell carcinoma by wide excision of the nail unit and skin graft reconstruction: an evaluation of treatment efficiency and outcomes. JAMA Dermatol. 2017;153:442-448.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Zaiac MN, Weiss E. Mohs micrographic surgery of the nail unit and squamous cell carcinoma. Dermatol Surg. 2001;27:246-251.
- de Berker DA, Dahl MG, Malcolm AJ, et al. Micrographic surgery for subungual squamous cell carcinoma. Br J Plast Surg. 1996;49:414-419.
- Young LC, Tuxen AJ, Goodman G. Mohs’ micrographic surgery as treatment for squamous dysplasia of the nail unit. Australas J Dermatol. 2012;53:123-127.
- Goldminz D, Bennett RG. Mohs micrographic surgery of the nail unit. J Dermatol Surg Oncol. 1992;18:721-726.
- Dika E, Piraccini BM, Balestri R, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
Nail unit squamous cell carcinoma (NSCC) is a malignant neoplasm that can arise from any part of the nail unit. Diagnosis often is delayed due to its clinical presentation mimicking benign conditions such as onychomycosis, warts, and paronychia. Nail unit SCC has a low rate of metastasis; however, a delayed diagnosis often can result in local destruction and bone invasion. It is imperative for dermatologists who are early in their training to recognize this entity and refer for treatment. Many approaches have been used to treat NSCC, including wide local excision, digital amputation, cryotherapy, topical modalities, and recently Mohs micrographic surgery (MMS). This article provides an overview of the clinical presentation and diagnosis of NSCC, the role of human papillomavirus (HPV) in NSCC pathogenesis, and the evidence supporting surgical management.
NSCC Clinical Presentation and Diagnosis
Nail unit squamous cell carcinoma is a malignant neoplasm that can arise from any part of the nail unit including the nail bed, matrix, groove, and nail fold.1 Although NSCC is the most common malignant nail neoplasm, its diagnosis often is delayed partly due to the clinical presentation of NSCC mimicking benign conditions such as onychomycosis, warts, and paronychia.2,3 Nail unit SCC most commonly is mistaken for verruca vulgaris, and thus it is important to exclude malignancy in nonresolving verrucae of the fingernails or toenails. Another reason for a delay in the diagnosis is the painless and often asymptomatic presentation of this tumor, which keeps patients from seeking care.4 While evaluating a subungual lesion, dermatologists should keep in mind red flags that would prompt a biopsy to rule out NSCC (Table 1), including chronic nonhealing lesions, nail plate nodularity, known history of infection with HPV types 16 and 18, history of radiation or arsenic exposure, and immunosuppression. Table 2 lists the differential diagnosis of a persisting or nonhealing subungual tumor.


Nail unit SCC has a low rate of metastasis; however, a delayed diagnosis often can result in local destruction and bone invasion.5 Based on several reports, NSCC more commonly is found in middle-aged and older individuals, has a male predilection, and more often is seen on fingernails than toenails.1,2,6 Figure A shows an example of the clinical presentation of NSCC affecting the right thumb.

Although there often is a delay in the presentation and biopsy of NSCC, no correlation has been observed between time to biopsy and rate of disease invasion and recurrence.7 Nevertheless, Starace et al7 noted that a low threshold for biopsy of nail unit lesions is necessary. It is recommended to perform a deep shave or a nail matrix biopsy, especially if matrical involvement is suspected.8 Patients should be closely followed after a diagnosis of NSCC is made, especially if they are immunocompromised or have genetic skin cancer syndromes, as multiple NSCCs can occur in the same individual.9 For instance, one report discussed a patient with xeroderma pigmentosum who developed 3 separate NSCCs. Interestingly, in this patient, the authors suspected HPV as a cause for the field cancerization, as 2 of 3 NSCCs were noted on initial histopathology to have arisen from verrucae.10
Histologic Features
A biopsy from an NSCC tumor shows features similar to cutaneous SCC in the affected areas (ie, nail bed, nail matrix, nail groove, nail fold). Characteristic histologic findings include tongues or whorls of atypical squamous epithelium that invade deeply into the dermis.11 The cells appear as atypical keratinocytes, exhibit distinct intracellular bridges, and possess hyperchromatic and pleomorphic nuclei with dyskeratosis and keratin pearls within the dermis.12 Immunoperoxidase staining for cytokeratin AE1/AE3 can be helpful to confirm the diagnosis and assess whether the depth of invasion involves the bone.13 Figures B and C demonstrate the histopathology of NSCC biopsied from the tumor shown in Figure A.
Role of HPV in NSCC Pathogenesis
There is no clear pathogenic etiology for NSCC; however, there have been some reports of HPV as a risk factor. Shimizu et al14 reviewed 136 cases of HPV-associated NSCC and found that half of the cases were associated with high-risk HPV. They also found that 24% of the patients with NSCC had a history of other HPV-associated diseases. As such, the authors hypothesized that there is a possibility for genitodigital HPV transmission and that NSCC could be a reservoir for sexually transmitted high-risk HPV.14 Other risk factors are radiation exposure, chemical insult, and chronic trauma.15 The higher propensity for fingernails likely is reflective of the role of UV light exposure and infection with HPV in the development of these tumors.14,15
Several nonsurgical approaches have been suggested to treat NSCC, including topical agents, cryotherapy, CO2 laser, and photodynamic therapy.3,16 Unfortunately, there are no large case series to demonstrate the cure rate or effectiveness of these methods.17 In one study, the authors did not recommend use of photodynamic therapy or topical modalities such as imiquimod cream 5% or fluorouracil cream 5% as first-line treatments of NSCC due to the difficulty in ensuring complete treatment of the sulci of the lateral and proximal nail folds.18
More evidence in the literature supports surgical approaches, including wide local excision, MMS, and digital amputation. Clinicians should consider relapse rates and the impact on digital functioning when choosing a surgical approach.
For wide local excisions, the most common approach is en bloc excision of the nail unit including the lateral nail folds, the proximal nail fold, and the distal nail fold. The excision starts with a transverse incision on the base of the distal phalanx, which is then prolonged laterally and distally to the distal nail fold down to the bone. After the incision is made to the depth of the bone, the matrical horns are destroyed by electrocoagulation, and the defect is closed either by a full-thickness skin graft or secondary intent.19
Topin-Ruiz et al19 followed patients with biopsy-proven NSCC without bone invasion who underwent en bloc excision followed by full-thickness skin graft. In their consecutive series of 55 patients with 5 years of follow-up, the rate of recurrence was only 4%. There was a low rate of complications including graft infection, delayed wound healing, and severe pain in a small percentage of patients. They also reported a high patient satisfaction rate.19 Due to the low recurrence rate, this study suggested that total excision of the nail unit followed by a full-thickness skin graft is a safe and efficient treatment of NSCC without bone involvement. Similarly, in another case series, wide local excision of the entire nail apparatus had a relapse rate of only 5%, in contrast to partial excision of the nail unit with a relapse of 56%.20 These studies suggest that wide nail unit excision is an acceptable and effective approach; however, in cases in which invasion cannot be ruled out, histologic clearance would be a reasonable approach.21 As such, several case series demonstrated the merits of MMS for NSCC. de Berker et al22 reported 8 patients with NSCC treated using slow MMS and showed tumor clearance after a mean of 3 stages over a mean period of 6.9 days. In all cases, the wounds were allowed to heal by secondary intention, and the distal phalanx was preserved. During a mean follow-up period of 3.1 years, no recurrence was seen, and involved digits remained functional.22
Other studies tested the efficacy of MMS for NSCC. Young et al23 reported the outcomes of 14 NSCC cases treated with MMS. In their case series, they found that the mean number of MMS surgical stages required to achieve histologic clearance was 2, while the mean number of tissue sections was 4.23 All cases were allowed to heal by secondary intent with excellent outcomes, except for 1 patient who received primary closure of a small defect. They reported a 78% cure rate with an average time to recurrence of 47 months.23 In a series of 42 cases of NSCC treated with MMS, Gou et al17 noted a cure rate close to 93%. In their study, recurrences were observed in only 3 patients (7.1%). These recurrent cases were then successfully treated with another round of MMS.17 This study’s cure rate was comparable to the cure rate of MMS for SCC in other cutaneous areas. Goldminz and Bennett24 demonstrated a cure rate of 92% in their case series of 25 patients. Two patients developed recurrent disease and were treated again with MMS resulting in no subsequent recurrence. In this study, the authors allowed all defects to heal by secondary intention and found that there were excellent cosmetic and functional outcomes.24 Dika et al25 evaluated the long-term effectiveness of MMS in the treatment of NSCC, in particular its ability to reduce the number of digital amputations. Fifteen patients diagnosed with NSCC were treated with MMS as the first-line surgical approach and were followed for 2 to 5 years. They found that in utilizing MMS, they were able to avoid amputations in 13 of 15 cases with no recurrence in any of these tumors. Two cases, however, still required amputation of the distal phalanx.25
Although these studies suggest that MMS achieves a high cure rate ranging from 78% to 93%, it is not yet clear in the literature whether MMS is superior to wide local excision. More studies and clinical trials comparing these 2 surgical approaches should be performed to identify which surgical approach would be the gold standard for NSCC and which select cases would benefit from MMS as first-line treatment.
Final Thoughts
Nail unit SCC is one of the most common nail unit malignancies and can mimic several benign entities. Dermatologists who are early in their training should consider biopsy of subungual lesions with certain red flags (Table 1). It is important to diagnose NSCC for early intervention. Referral for wide local excision or MMS would be ideal. There are data in the literature supporting both surgical approaches as being effective; however, there are no trials comparing both approaches. Distal amputation should be considered as a last resort when wide local excision is not reasonable or when MMS fails to achieve clear margins, thereby reducing unnecessary amputations and patient morbidity.17
Nail unit squamous cell carcinoma (NSCC) is a malignant neoplasm that can arise from any part of the nail unit. Diagnosis often is delayed due to its clinical presentation mimicking benign conditions such as onychomycosis, warts, and paronychia. Nail unit SCC has a low rate of metastasis; however, a delayed diagnosis often can result in local destruction and bone invasion. It is imperative for dermatologists who are early in their training to recognize this entity and refer for treatment. Many approaches have been used to treat NSCC, including wide local excision, digital amputation, cryotherapy, topical modalities, and recently Mohs micrographic surgery (MMS). This article provides an overview of the clinical presentation and diagnosis of NSCC, the role of human papillomavirus (HPV) in NSCC pathogenesis, and the evidence supporting surgical management.
NSCC Clinical Presentation and Diagnosis
Nail unit squamous cell carcinoma is a malignant neoplasm that can arise from any part of the nail unit including the nail bed, matrix, groove, and nail fold.1 Although NSCC is the most common malignant nail neoplasm, its diagnosis often is delayed partly due to the clinical presentation of NSCC mimicking benign conditions such as onychomycosis, warts, and paronychia.2,3 Nail unit SCC most commonly is mistaken for verruca vulgaris, and thus it is important to exclude malignancy in nonresolving verrucae of the fingernails or toenails. Another reason for a delay in the diagnosis is the painless and often asymptomatic presentation of this tumor, which keeps patients from seeking care.4 While evaluating a subungual lesion, dermatologists should keep in mind red flags that would prompt a biopsy to rule out NSCC (Table 1), including chronic nonhealing lesions, nail plate nodularity, known history of infection with HPV types 16 and 18, history of radiation or arsenic exposure, and immunosuppression. Table 2 lists the differential diagnosis of a persisting or nonhealing subungual tumor.


Nail unit SCC has a low rate of metastasis; however, a delayed diagnosis often can result in local destruction and bone invasion.5 Based on several reports, NSCC more commonly is found in middle-aged and older individuals, has a male predilection, and more often is seen on fingernails than toenails.1,2,6 Figure A shows an example of the clinical presentation of NSCC affecting the right thumb.

Although there often is a delay in the presentation and biopsy of NSCC, no correlation has been observed between time to biopsy and rate of disease invasion and recurrence.7 Nevertheless, Starace et al7 noted that a low threshold for biopsy of nail unit lesions is necessary. It is recommended to perform a deep shave or a nail matrix biopsy, especially if matrical involvement is suspected.8 Patients should be closely followed after a diagnosis of NSCC is made, especially if they are immunocompromised or have genetic skin cancer syndromes, as multiple NSCCs can occur in the same individual.9 For instance, one report discussed a patient with xeroderma pigmentosum who developed 3 separate NSCCs. Interestingly, in this patient, the authors suspected HPV as a cause for the field cancerization, as 2 of 3 NSCCs were noted on initial histopathology to have arisen from verrucae.10
Histologic Features
A biopsy from an NSCC tumor shows features similar to cutaneous SCC in the affected areas (ie, nail bed, nail matrix, nail groove, nail fold). Characteristic histologic findings include tongues or whorls of atypical squamous epithelium that invade deeply into the dermis.11 The cells appear as atypical keratinocytes, exhibit distinct intracellular bridges, and possess hyperchromatic and pleomorphic nuclei with dyskeratosis and keratin pearls within the dermis.12 Immunoperoxidase staining for cytokeratin AE1/AE3 can be helpful to confirm the diagnosis and assess whether the depth of invasion involves the bone.13 Figures B and C demonstrate the histopathology of NSCC biopsied from the tumor shown in Figure A.
Role of HPV in NSCC Pathogenesis
There is no clear pathogenic etiology for NSCC; however, there have been some reports of HPV as a risk factor. Shimizu et al14 reviewed 136 cases of HPV-associated NSCC and found that half of the cases were associated with high-risk HPV. They also found that 24% of the patients with NSCC had a history of other HPV-associated diseases. As such, the authors hypothesized that there is a possibility for genitodigital HPV transmission and that NSCC could be a reservoir for sexually transmitted high-risk HPV.14 Other risk factors are radiation exposure, chemical insult, and chronic trauma.15 The higher propensity for fingernails likely is reflective of the role of UV light exposure and infection with HPV in the development of these tumors.14,15
Several nonsurgical approaches have been suggested to treat NSCC, including topical agents, cryotherapy, CO2 laser, and photodynamic therapy.3,16 Unfortunately, there are no large case series to demonstrate the cure rate or effectiveness of these methods.17 In one study, the authors did not recommend use of photodynamic therapy or topical modalities such as imiquimod cream 5% or fluorouracil cream 5% as first-line treatments of NSCC due to the difficulty in ensuring complete treatment of the sulci of the lateral and proximal nail folds.18
More evidence in the literature supports surgical approaches, including wide local excision, MMS, and digital amputation. Clinicians should consider relapse rates and the impact on digital functioning when choosing a surgical approach.
For wide local excisions, the most common approach is en bloc excision of the nail unit including the lateral nail folds, the proximal nail fold, and the distal nail fold. The excision starts with a transverse incision on the base of the distal phalanx, which is then prolonged laterally and distally to the distal nail fold down to the bone. After the incision is made to the depth of the bone, the matrical horns are destroyed by electrocoagulation, and the defect is closed either by a full-thickness skin graft or secondary intent.19
Topin-Ruiz et al19 followed patients with biopsy-proven NSCC without bone invasion who underwent en bloc excision followed by full-thickness skin graft. In their consecutive series of 55 patients with 5 years of follow-up, the rate of recurrence was only 4%. There was a low rate of complications including graft infection, delayed wound healing, and severe pain in a small percentage of patients. They also reported a high patient satisfaction rate.19 Due to the low recurrence rate, this study suggested that total excision of the nail unit followed by a full-thickness skin graft is a safe and efficient treatment of NSCC without bone involvement. Similarly, in another case series, wide local excision of the entire nail apparatus had a relapse rate of only 5%, in contrast to partial excision of the nail unit with a relapse of 56%.20 These studies suggest that wide nail unit excision is an acceptable and effective approach; however, in cases in which invasion cannot be ruled out, histologic clearance would be a reasonable approach.21 As such, several case series demonstrated the merits of MMS for NSCC. de Berker et al22 reported 8 patients with NSCC treated using slow MMS and showed tumor clearance after a mean of 3 stages over a mean period of 6.9 days. In all cases, the wounds were allowed to heal by secondary intention, and the distal phalanx was preserved. During a mean follow-up period of 3.1 years, no recurrence was seen, and involved digits remained functional.22
Other studies tested the efficacy of MMS for NSCC. Young et al23 reported the outcomes of 14 NSCC cases treated with MMS. In their case series, they found that the mean number of MMS surgical stages required to achieve histologic clearance was 2, while the mean number of tissue sections was 4.23 All cases were allowed to heal by secondary intent with excellent outcomes, except for 1 patient who received primary closure of a small defect. They reported a 78% cure rate with an average time to recurrence of 47 months.23 In a series of 42 cases of NSCC treated with MMS, Gou et al17 noted a cure rate close to 93%. In their study, recurrences were observed in only 3 patients (7.1%). These recurrent cases were then successfully treated with another round of MMS.17 This study’s cure rate was comparable to the cure rate of MMS for SCC in other cutaneous areas. Goldminz and Bennett24 demonstrated a cure rate of 92% in their case series of 25 patients. Two patients developed recurrent disease and were treated again with MMS resulting in no subsequent recurrence. In this study, the authors allowed all defects to heal by secondary intention and found that there were excellent cosmetic and functional outcomes.24 Dika et al25 evaluated the long-term effectiveness of MMS in the treatment of NSCC, in particular its ability to reduce the number of digital amputations. Fifteen patients diagnosed with NSCC were treated with MMS as the first-line surgical approach and were followed for 2 to 5 years. They found that in utilizing MMS, they were able to avoid amputations in 13 of 15 cases with no recurrence in any of these tumors. Two cases, however, still required amputation of the distal phalanx.25
Although these studies suggest that MMS achieves a high cure rate ranging from 78% to 93%, it is not yet clear in the literature whether MMS is superior to wide local excision. More studies and clinical trials comparing these 2 surgical approaches should be performed to identify which surgical approach would be the gold standard for NSCC and which select cases would benefit from MMS as first-line treatment.
Final Thoughts
Nail unit SCC is one of the most common nail unit malignancies and can mimic several benign entities. Dermatologists who are early in their training should consider biopsy of subungual lesions with certain red flags (Table 1). It is important to diagnose NSCC for early intervention. Referral for wide local excision or MMS would be ideal. There are data in the literature supporting both surgical approaches as being effective; however, there are no trials comparing both approaches. Distal amputation should be considered as a last resort when wide local excision is not reasonable or when MMS fails to achieve clear margins, thereby reducing unnecessary amputations and patient morbidity.17
- Dika E, Starace M, Patrizi A, et al. Squamous cell carcinoma of the nail unit: a clinical histopathologic study and a proposal for classification. Dermatol Surg. 2019;45:365-370.
- Lee TM, Jo G, Kim M, et al. Squamous cell carcinoma of the nail unit: a retrospective review of 19 cases in Asia and comparative review of Western literature. Int J Dermatol. 2019;58:428-432.
- Tambe SA, Patil PD, Saple DG, et al. Squamous cell carcinoma of the nail bed: the great mimicker. J Cutan Aesthet Surg. 2017;10:59-60.
- Perrin C. Tumors of the nail unit. a review. part II: acquired localized longitudinal pachyonychia and masked nail tumors. Am J Dermatopathol. 2013;35:693-712.
- Li PF, Zhu N, Lu H. Squamous cell carcinoma of the nail bed: a case report. World J Clin Cases. 2019;7:3590-3594.
- Kaul S, Singal A, Grover C, et al. Clinical and histological spectrum of nail psoriasis: a cross-sectional study. J Cutan Pathol. 2018;45:824-830.
- Starace M, Alessandrini A, Dika E, et al. Squamous cell carcinoma of the nail unit. Dermatol Pract Concept. 2018;8:238-244.
- Kelly KJ, Kalani AD, Storrs S, et al. Subungual squamous cell carcinoma of the toe: working toward a standardized therapeutic approach. J Surg Educ. 2008;65:297-301.
- Ormerod E, De Berker D. Nail unit squamous cell carcinoma in people with immunosuppression. Br J Dermatol. 2015;173:701-712.
- Ventéjou S, Bagny K, Waldmeyer J, et al. Skin cancers in patients of skin phototype V or VI with xeroderma pigmentosum type C (XP-C): a retrospective study. Ann Dermatol Venereol. 2019;146:192-203.
- Mikhail GR. Subungual epidermoid carcinoma. J Am Acad Dermatol. 1984;11:291-298.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Kurokawa I, Senba Y, Kakeda M, et al. Cytokeratin expression in subungual squamous cell carcinoma. J Int Med Res. 2006;34:441-443.
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370.
- Tang N, Maloney ME, Clark AH, et al. A retrospective study of nail squamous cell carcinoma at 2 institutions. Dermatol Surg. 2016;42(suppl 1):S8-S17.
- An Q, Zheng S, Zhang L, et al. Subungual squamous cell carcinoma treated by topical photodynamic therapy. Chin Med J (Engl). 2020;133:881-882.
- Gou D, Nijhawan RI, Srivastava D. Mohs micrographic surgery as the standard of care for nail unit squamous cell carcinoma. Dermatol Surg. 2020;46:725-732.
- Dika E, Fanti PA, Patrizi A, et al. Mohs surgery for squamous cell carcinoma of the nail unit: 10 years of experience. Dermatol Surg. 2015;41:1015-1019.
- Topin-Ruiz S, Surinach C, Dalle S, et al. Surgical treatment of subungual squamous cell carcinoma by wide excision of the nail unit and skin graft reconstruction: an evaluation of treatment efficiency and outcomes. JAMA Dermatol. 2017;153:442-448.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Zaiac MN, Weiss E. Mohs micrographic surgery of the nail unit and squamous cell carcinoma. Dermatol Surg. 2001;27:246-251.
- de Berker DA, Dahl MG, Malcolm AJ, et al. Micrographic surgery for subungual squamous cell carcinoma. Br J Plast Surg. 1996;49:414-419.
- Young LC, Tuxen AJ, Goodman G. Mohs’ micrographic surgery as treatment for squamous dysplasia of the nail unit. Australas J Dermatol. 2012;53:123-127.
- Goldminz D, Bennett RG. Mohs micrographic surgery of the nail unit. J Dermatol Surg Oncol. 1992;18:721-726.
- Dika E, Piraccini BM, Balestri R, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Dika E, Starace M, Patrizi A, et al. Squamous cell carcinoma of the nail unit: a clinical histopathologic study and a proposal for classification. Dermatol Surg. 2019;45:365-370.
- Lee TM, Jo G, Kim M, et al. Squamous cell carcinoma of the nail unit: a retrospective review of 19 cases in Asia and comparative review of Western literature. Int J Dermatol. 2019;58:428-432.
- Tambe SA, Patil PD, Saple DG, et al. Squamous cell carcinoma of the nail bed: the great mimicker. J Cutan Aesthet Surg. 2017;10:59-60.
- Perrin C. Tumors of the nail unit. a review. part II: acquired localized longitudinal pachyonychia and masked nail tumors. Am J Dermatopathol. 2013;35:693-712.
- Li PF, Zhu N, Lu H. Squamous cell carcinoma of the nail bed: a case report. World J Clin Cases. 2019;7:3590-3594.
- Kaul S, Singal A, Grover C, et al. Clinical and histological spectrum of nail psoriasis: a cross-sectional study. J Cutan Pathol. 2018;45:824-830.
- Starace M, Alessandrini A, Dika E, et al. Squamous cell carcinoma of the nail unit. Dermatol Pract Concept. 2018;8:238-244.
- Kelly KJ, Kalani AD, Storrs S, et al. Subungual squamous cell carcinoma of the toe: working toward a standardized therapeutic approach. J Surg Educ. 2008;65:297-301.
- Ormerod E, De Berker D. Nail unit squamous cell carcinoma in people with immunosuppression. Br J Dermatol. 2015;173:701-712.
- Ventéjou S, Bagny K, Waldmeyer J, et al. Skin cancers in patients of skin phototype V or VI with xeroderma pigmentosum type C (XP-C): a retrospective study. Ann Dermatol Venereol. 2019;146:192-203.
- Mikhail GR. Subungual epidermoid carcinoma. J Am Acad Dermatol. 1984;11:291-298.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Kurokawa I, Senba Y, Kakeda M, et al. Cytokeratin expression in subungual squamous cell carcinoma. J Int Med Res. 2006;34:441-443.
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370.
- Tang N, Maloney ME, Clark AH, et al. A retrospective study of nail squamous cell carcinoma at 2 institutions. Dermatol Surg. 2016;42(suppl 1):S8-S17.
- An Q, Zheng S, Zhang L, et al. Subungual squamous cell carcinoma treated by topical photodynamic therapy. Chin Med J (Engl). 2020;133:881-882.
- Gou D, Nijhawan RI, Srivastava D. Mohs micrographic surgery as the standard of care for nail unit squamous cell carcinoma. Dermatol Surg. 2020;46:725-732.
- Dika E, Fanti PA, Patrizi A, et al. Mohs surgery for squamous cell carcinoma of the nail unit: 10 years of experience. Dermatol Surg. 2015;41:1015-1019.
- Topin-Ruiz S, Surinach C, Dalle S, et al. Surgical treatment of subungual squamous cell carcinoma by wide excision of the nail unit and skin graft reconstruction: an evaluation of treatment efficiency and outcomes. JAMA Dermatol. 2017;153:442-448.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Zaiac MN, Weiss E. Mohs micrographic surgery of the nail unit and squamous cell carcinoma. Dermatol Surg. 2001;27:246-251.
- de Berker DA, Dahl MG, Malcolm AJ, et al. Micrographic surgery for subungual squamous cell carcinoma. Br J Plast Surg. 1996;49:414-419.
- Young LC, Tuxen AJ, Goodman G. Mohs’ micrographic surgery as treatment for squamous dysplasia of the nail unit. Australas J Dermatol. 2012;53:123-127.
- Goldminz D, Bennett RG. Mohs micrographic surgery of the nail unit. J Dermatol Surg Oncol. 1992;18:721-726.
- Dika E, Piraccini BM, Balestri R, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
Resident Pearls
- The diagnosis of nail unit squamous cell carcinoma often is delayed due to its clinical presentation, which frequently mimics benign nail conditions.
- Treatment includes wide local excision, Mohs micrographic surgery, digital amputation, cryotherapy, and topical modalities.
FDA-approved peanut immunotherapy protocol comes with a cost
Peanut allergy immunotherapy now comes with approval from the US Food and Drug Administration (FDA), but it also comes with protocols, standards, and paperwork. Whether it will be widely adopted has yet to be determined.
A few dozen allergists around the world have been offering food allergy immunotherapy for many years, having developed their own measuring techniques using store-bought food.
But the vast majority of allergists are not interested in developing home-grown treatments, not only because it involves research and development, but also because it comes with legal risks.
“Finally we have another treatment option,” said Edwin Kim, MD, from the UNC Allergy and Immunology Clinic in Chapel Hill, N.C. “This is what we were waiting for. It’s not cowboy stuff; this works.”
In January, the FDA approved peanut allergen powder (Palforzia) for patients 4-17 years of age, as reported by Medscape Medical News.
The pill contains measured doses of peanut flour and comes with a protocol that will allow allergists to bring patients to a peanut tolerance of 300 mg (about one peanut) and a black-box warning about anaphylaxis risk.
And before allergists can prescribe it, they must take a Risk Evaluation and Mitigation Strategy course to learn about dosing and the allergic reaction protocol.
“That may scare some away,” said Dr. Kim, who discussed the FDA-approved option during his presentation at the American College of Allergy, Asthma & Immunology 2020 Annual Scientific Meeting.
Allergic reaction, including the potential for anaphylaxis, has always been an issue with immunotherapy.
“People make the argument that there is a difference” between an expected allergic reaction – such as one that occurs after the administration of immunotherapy – and an unexpected reaction, he said. Because an expected reaction can be treated quickly, “some feel these expected reactions don’t matter so much.”
“Others say a reaction is a reaction” and argue that if, a treatment causes reaction, then it doesn’t make sense, he explained.
It comes down to patients – they must be willing to take a risk to develop tolerance and improve their quality of life – and the allergists willing to treat them.
The peanut powder involves paperwork, preauthorization forms, denials of care, a higher price tag, regimented procedures, and a prerequisite number of visits with patients. “Not everyone will want to do this,” said Dr. Kim.
The regimen involves three phases. During initial dose escalation, five doses are administered in the office on day 1. Then, over the next 6 months, updoses are administered during 11 in-office sessions and a 300-mg tolerance is achieved. Finally, to maintain tolerance to one peanut, daily doses are administered at home.
The drug cost alone is about $4,200 a year, according to Institute for Clinical and Economic Review. Peanut flour from the grocery store is cheaper, but comes with the risk of bacteria or other contamination.
“This product offers some reassurance, and that matters,” Dr. Kim said.
It’s good to have more options for food allergy treatment. “We need a more proactive way to treat food allergy; avoidance is not good enough,” he explained. “And presumably, at some point, the patient will be able to eat a grocery-store peanut instead of buying the pills.”
The art of medicine
But not all allergists will be able to make the protocol work. And it’s not clear whether there is room to alter treatment and offer patients with a higher tolerance a higher starting dose. What we do know, though, is that “the product leaves little room for ‘the art of medicine,’ ” Kim said.
That art is practiced by Arnon Elizur, MD, from the Shamir Medical Center in Tzrifin, Israel, but it’s backed by a rigid home-grown protocol.
Since 2010, he has treated 1,800 patients for peanut allergy, updosing slowly to a tolerance of 3,000 mg of peanut, the equivalent of 10 peanuts. He keeps the maintenance dose at four peanuts (1,200 mg). His center takes a personalized approach, starting patients on the highest dose they can tolerate and working up, with daily patient check-ins from home and a staff available around the clock to answer questions and deal with reactions.
“We aim for full sensitization,” Dr. Elizur said in an interview.
The peanut pill is “a big step forward” for immunotherapy, he said. It is “a standardized product, checked for bacteria and allergen content, which is available to a wide community of physicians.”
But, he pointed out, “it’s expensive.” And it’s only for peanut. “There are millions of food-allergic patients around the world dying from adverse reactions to many different kinds of food. We don’t want to wait for years for a product for all of them. We can use the actual food.”
He questions the lifelong maintenance protocol with a daily 300-mg pill. “If you can’t eat a peanut, why would you buy a drug that’s a peanut?” he asked.
He also said he’s disappointed that the product is not indicated for adults.
At the Shamir clinic, reactions are closely monitored. “Some are mild, others we treat with autoinjectors, epinephrine,” he reported. “Those are the most undesirable.”
Data from his center show that reactions occur in about 15% of patients. But his treatment success rates are good. In an average of 8 months, he is able to get 80% of his adult patients to full sensitization.
But it’s not for all patients or for all clinics, he acknowledged. “We continue to look at this balance in quality of life throughout the process. Our goal is to improve the quality of life threshold.”
Treatment that involves “native food” is “a lot of work” and requires “a lot of investment,” Dr. Elizur said. His center uses a web reporting system to maintain a 24/7 dialogue with patients, “and we look at the reports every day.” They also have a physician on call at all times. “Not everyone can commit to providing care throughout the day and night.”
His center charges the equivalent of $US3,000 per food allergy treated. “That’s whether it takes 6 months or 2 years,” he said.
There are more than 1,000 people on his 3-year waiting list.
“This is the first year that the American College of Allergy, Asthma, and Immunology is not hosting a pro–con debate on oral immunotherapy,” Dr. Kim pointed out. “We have a therapy now.”
However, the pandemic has slowed treatment uptake. “Immunotherapy is not easy to do, whether it’s FDA approved or not,” he explained. With at least 11 doctor visits in the first 6 months – each visit is between 30 minutes and 2-3 hours long – it hasn’t been possible to set up this year. “It’s not ideal.”
It will be interesting to see “how this will roll out and how it will be adopted,” Dr. Kim said. “From a food allergy point of view, the next 12 months are going to be very interesting.”
Dr. Kim reports receiving consulting honorarium from Aimmune, the maker of Palforzia; being on the clinical medical advisory board for DBV Technologies; and consulting for Aimmune, Allakos, Allergenis, DBV, Duke Clinical Research Institute, Ukko Incorporated, Vibrant America, and Kenota Health. Dr. Elizur has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Peanut allergy immunotherapy now comes with approval from the US Food and Drug Administration (FDA), but it also comes with protocols, standards, and paperwork. Whether it will be widely adopted has yet to be determined.
A few dozen allergists around the world have been offering food allergy immunotherapy for many years, having developed their own measuring techniques using store-bought food.
But the vast majority of allergists are not interested in developing home-grown treatments, not only because it involves research and development, but also because it comes with legal risks.
“Finally we have another treatment option,” said Edwin Kim, MD, from the UNC Allergy and Immunology Clinic in Chapel Hill, N.C. “This is what we were waiting for. It’s not cowboy stuff; this works.”
In January, the FDA approved peanut allergen powder (Palforzia) for patients 4-17 years of age, as reported by Medscape Medical News.
The pill contains measured doses of peanut flour and comes with a protocol that will allow allergists to bring patients to a peanut tolerance of 300 mg (about one peanut) and a black-box warning about anaphylaxis risk.
And before allergists can prescribe it, they must take a Risk Evaluation and Mitigation Strategy course to learn about dosing and the allergic reaction protocol.
“That may scare some away,” said Dr. Kim, who discussed the FDA-approved option during his presentation at the American College of Allergy, Asthma & Immunology 2020 Annual Scientific Meeting.
Allergic reaction, including the potential for anaphylaxis, has always been an issue with immunotherapy.
“People make the argument that there is a difference” between an expected allergic reaction – such as one that occurs after the administration of immunotherapy – and an unexpected reaction, he said. Because an expected reaction can be treated quickly, “some feel these expected reactions don’t matter so much.”
“Others say a reaction is a reaction” and argue that if, a treatment causes reaction, then it doesn’t make sense, he explained.
It comes down to patients – they must be willing to take a risk to develop tolerance and improve their quality of life – and the allergists willing to treat them.
The peanut powder involves paperwork, preauthorization forms, denials of care, a higher price tag, regimented procedures, and a prerequisite number of visits with patients. “Not everyone will want to do this,” said Dr. Kim.
The regimen involves three phases. During initial dose escalation, five doses are administered in the office on day 1. Then, over the next 6 months, updoses are administered during 11 in-office sessions and a 300-mg tolerance is achieved. Finally, to maintain tolerance to one peanut, daily doses are administered at home.
The drug cost alone is about $4,200 a year, according to Institute for Clinical and Economic Review. Peanut flour from the grocery store is cheaper, but comes with the risk of bacteria or other contamination.
“This product offers some reassurance, and that matters,” Dr. Kim said.
It’s good to have more options for food allergy treatment. “We need a more proactive way to treat food allergy; avoidance is not good enough,” he explained. “And presumably, at some point, the patient will be able to eat a grocery-store peanut instead of buying the pills.”
The art of medicine
But not all allergists will be able to make the protocol work. And it’s not clear whether there is room to alter treatment and offer patients with a higher tolerance a higher starting dose. What we do know, though, is that “the product leaves little room for ‘the art of medicine,’ ” Kim said.
That art is practiced by Arnon Elizur, MD, from the Shamir Medical Center in Tzrifin, Israel, but it’s backed by a rigid home-grown protocol.
Since 2010, he has treated 1,800 patients for peanut allergy, updosing slowly to a tolerance of 3,000 mg of peanut, the equivalent of 10 peanuts. He keeps the maintenance dose at four peanuts (1,200 mg). His center takes a personalized approach, starting patients on the highest dose they can tolerate and working up, with daily patient check-ins from home and a staff available around the clock to answer questions and deal with reactions.
“We aim for full sensitization,” Dr. Elizur said in an interview.
The peanut pill is “a big step forward” for immunotherapy, he said. It is “a standardized product, checked for bacteria and allergen content, which is available to a wide community of physicians.”
But, he pointed out, “it’s expensive.” And it’s only for peanut. “There are millions of food-allergic patients around the world dying from adverse reactions to many different kinds of food. We don’t want to wait for years for a product for all of them. We can use the actual food.”
He questions the lifelong maintenance protocol with a daily 300-mg pill. “If you can’t eat a peanut, why would you buy a drug that’s a peanut?” he asked.
He also said he’s disappointed that the product is not indicated for adults.
At the Shamir clinic, reactions are closely monitored. “Some are mild, others we treat with autoinjectors, epinephrine,” he reported. “Those are the most undesirable.”
Data from his center show that reactions occur in about 15% of patients. But his treatment success rates are good. In an average of 8 months, he is able to get 80% of his adult patients to full sensitization.
But it’s not for all patients or for all clinics, he acknowledged. “We continue to look at this balance in quality of life throughout the process. Our goal is to improve the quality of life threshold.”
Treatment that involves “native food” is “a lot of work” and requires “a lot of investment,” Dr. Elizur said. His center uses a web reporting system to maintain a 24/7 dialogue with patients, “and we look at the reports every day.” They also have a physician on call at all times. “Not everyone can commit to providing care throughout the day and night.”
His center charges the equivalent of $US3,000 per food allergy treated. “That’s whether it takes 6 months or 2 years,” he said.
There are more than 1,000 people on his 3-year waiting list.
“This is the first year that the American College of Allergy, Asthma, and Immunology is not hosting a pro–con debate on oral immunotherapy,” Dr. Kim pointed out. “We have a therapy now.”
However, the pandemic has slowed treatment uptake. “Immunotherapy is not easy to do, whether it’s FDA approved or not,” he explained. With at least 11 doctor visits in the first 6 months – each visit is between 30 minutes and 2-3 hours long – it hasn’t been possible to set up this year. “It’s not ideal.”
It will be interesting to see “how this will roll out and how it will be adopted,” Dr. Kim said. “From a food allergy point of view, the next 12 months are going to be very interesting.”
Dr. Kim reports receiving consulting honorarium from Aimmune, the maker of Palforzia; being on the clinical medical advisory board for DBV Technologies; and consulting for Aimmune, Allakos, Allergenis, DBV, Duke Clinical Research Institute, Ukko Incorporated, Vibrant America, and Kenota Health. Dr. Elizur has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Peanut allergy immunotherapy now comes with approval from the US Food and Drug Administration (FDA), but it also comes with protocols, standards, and paperwork. Whether it will be widely adopted has yet to be determined.
A few dozen allergists around the world have been offering food allergy immunotherapy for many years, having developed their own measuring techniques using store-bought food.
But the vast majority of allergists are not interested in developing home-grown treatments, not only because it involves research and development, but also because it comes with legal risks.
“Finally we have another treatment option,” said Edwin Kim, MD, from the UNC Allergy and Immunology Clinic in Chapel Hill, N.C. “This is what we were waiting for. It’s not cowboy stuff; this works.”
In January, the FDA approved peanut allergen powder (Palforzia) for patients 4-17 years of age, as reported by Medscape Medical News.
The pill contains measured doses of peanut flour and comes with a protocol that will allow allergists to bring patients to a peanut tolerance of 300 mg (about one peanut) and a black-box warning about anaphylaxis risk.
And before allergists can prescribe it, they must take a Risk Evaluation and Mitigation Strategy course to learn about dosing and the allergic reaction protocol.
“That may scare some away,” said Dr. Kim, who discussed the FDA-approved option during his presentation at the American College of Allergy, Asthma & Immunology 2020 Annual Scientific Meeting.
Allergic reaction, including the potential for anaphylaxis, has always been an issue with immunotherapy.
“People make the argument that there is a difference” between an expected allergic reaction – such as one that occurs after the administration of immunotherapy – and an unexpected reaction, he said. Because an expected reaction can be treated quickly, “some feel these expected reactions don’t matter so much.”
“Others say a reaction is a reaction” and argue that if, a treatment causes reaction, then it doesn’t make sense, he explained.
It comes down to patients – they must be willing to take a risk to develop tolerance and improve their quality of life – and the allergists willing to treat them.
The peanut powder involves paperwork, preauthorization forms, denials of care, a higher price tag, regimented procedures, and a prerequisite number of visits with patients. “Not everyone will want to do this,” said Dr. Kim.
The regimen involves three phases. During initial dose escalation, five doses are administered in the office on day 1. Then, over the next 6 months, updoses are administered during 11 in-office sessions and a 300-mg tolerance is achieved. Finally, to maintain tolerance to one peanut, daily doses are administered at home.
The drug cost alone is about $4,200 a year, according to Institute for Clinical and Economic Review. Peanut flour from the grocery store is cheaper, but comes with the risk of bacteria or other contamination.
“This product offers some reassurance, and that matters,” Dr. Kim said.
It’s good to have more options for food allergy treatment. “We need a more proactive way to treat food allergy; avoidance is not good enough,” he explained. “And presumably, at some point, the patient will be able to eat a grocery-store peanut instead of buying the pills.”
The art of medicine
But not all allergists will be able to make the protocol work. And it’s not clear whether there is room to alter treatment and offer patients with a higher tolerance a higher starting dose. What we do know, though, is that “the product leaves little room for ‘the art of medicine,’ ” Kim said.
That art is practiced by Arnon Elizur, MD, from the Shamir Medical Center in Tzrifin, Israel, but it’s backed by a rigid home-grown protocol.
Since 2010, he has treated 1,800 patients for peanut allergy, updosing slowly to a tolerance of 3,000 mg of peanut, the equivalent of 10 peanuts. He keeps the maintenance dose at four peanuts (1,200 mg). His center takes a personalized approach, starting patients on the highest dose they can tolerate and working up, with daily patient check-ins from home and a staff available around the clock to answer questions and deal with reactions.
“We aim for full sensitization,” Dr. Elizur said in an interview.
The peanut pill is “a big step forward” for immunotherapy, he said. It is “a standardized product, checked for bacteria and allergen content, which is available to a wide community of physicians.”
But, he pointed out, “it’s expensive.” And it’s only for peanut. “There are millions of food-allergic patients around the world dying from adverse reactions to many different kinds of food. We don’t want to wait for years for a product for all of them. We can use the actual food.”
He questions the lifelong maintenance protocol with a daily 300-mg pill. “If you can’t eat a peanut, why would you buy a drug that’s a peanut?” he asked.
He also said he’s disappointed that the product is not indicated for adults.
At the Shamir clinic, reactions are closely monitored. “Some are mild, others we treat with autoinjectors, epinephrine,” he reported. “Those are the most undesirable.”
Data from his center show that reactions occur in about 15% of patients. But his treatment success rates are good. In an average of 8 months, he is able to get 80% of his adult patients to full sensitization.
But it’s not for all patients or for all clinics, he acknowledged. “We continue to look at this balance in quality of life throughout the process. Our goal is to improve the quality of life threshold.”
Treatment that involves “native food” is “a lot of work” and requires “a lot of investment,” Dr. Elizur said. His center uses a web reporting system to maintain a 24/7 dialogue with patients, “and we look at the reports every day.” They also have a physician on call at all times. “Not everyone can commit to providing care throughout the day and night.”
His center charges the equivalent of $US3,000 per food allergy treated. “That’s whether it takes 6 months or 2 years,” he said.
There are more than 1,000 people on his 3-year waiting list.
“This is the first year that the American College of Allergy, Asthma, and Immunology is not hosting a pro–con debate on oral immunotherapy,” Dr. Kim pointed out. “We have a therapy now.”
However, the pandemic has slowed treatment uptake. “Immunotherapy is not easy to do, whether it’s FDA approved or not,” he explained. With at least 11 doctor visits in the first 6 months – each visit is between 30 minutes and 2-3 hours long – it hasn’t been possible to set up this year. “It’s not ideal.”
It will be interesting to see “how this will roll out and how it will be adopted,” Dr. Kim said. “From a food allergy point of view, the next 12 months are going to be very interesting.”
Dr. Kim reports receiving consulting honorarium from Aimmune, the maker of Palforzia; being on the clinical medical advisory board for DBV Technologies; and consulting for Aimmune, Allakos, Allergenis, DBV, Duke Clinical Research Institute, Ukko Incorporated, Vibrant America, and Kenota Health. Dr. Elizur has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Osteoporosis Journal Scans: November 2020
Although the effects of vitamin K on blood coagulation are well-established, it is now clear that many extracellular proteins are carboxylated in a vitamin K-dependent manner, including bone matrix proteins such as osteocalcin and matrix gla protein. Previous studies have reported a relationship between vitamin K levels and bone density and fracture risk. However, optimal circulating vitamin K levels for skeletal health remain unknown. In this cross-sectional study of 374 women with post-menopausal osteoporosis, the authors assessed the relationship between vitamin K levels, vitamin K dependent bone-relevant circulating proteins, bone density, and fractures. In doing so, it was noted that women with prevalent fractures showed lower vitamin K levels than those without fractures. No relationship between vitamin K levels and bone density was noted. Interestingly, different serum levels of vitamin K were associated with optimal carboxylation of different vitamin K-dependent proteins: lower vitamin K levels are needed to support clotting factors than bone matrix proteins. Overall, this study suggests that higher intake is needed to obtain the full skeletal benefit of vitamin K versus its effects on coagulation. Future prospective studies are needed to test this intriguing hypothesis, and to further explore the relationship between vitamin K and bone quality.
For over 20 years, bisphosphonates have been first line therapy to increase bone density and reduce fracture risk in patients with osteoporosis. At present, multiple oral and intravenous bisphosphonates are approved for this indication by regulatory agencies worldwide. Several ‘next-generation’ bisphosphonates with optimized anti-resorptive and pharmacokinetic properties have been developed. Of these agents, minodronate is a particularly potent, third generation azaryl bisphosphonate that is currently approved for osteoporosis treatment in Japan. In this meta-analysis of 13 randomized controlled trials, the effects of minodronate were assessed versus other commonly-used osteoporosis medications. Compared with other drugs (alendronate, risedronate, raloxifene, or eldecalcitol), minodronate more potently suppressed serum bone resorption markers (NTX and TRAcP-5b) and, because bone formation and resorption are coupled, more potently suppressed the bone formation marker bone-specific alkaline phosphatase. In addition to these effects on serum markers, minodronate reduced vertebral fractures more than other medications. Across studies, no differences were noted between minodronate and comparators at the level of bone mineral density. Minodronate treatment is associated with a high incidence of gastrointestinal adverse effects than comparator medications. Taken together, these findings suggest that minodronate represents a potent, orally-available bisphosphonate for vertebral fracture reduction in patients with osteoporosis in Japan.
Chronic obstructive pulmonary disease (COPD) is well-known to be a risk factor for osteoporosis and fragility fractures. However, the interplay between inhaled corticosteroid use in COPD and skeletal outcomes remains unclear. While systemic glucocorticoid therapy clearly impairs bone mass and increases fracture risk, whether inhaled steroids have similar effects remain unknown. Furthermore, since inhaled corticosteroids can reduce lung inflammation and COPD flares, it is possible that, by controlling pulmonary disease, these agents may actually promote bone health. In this real-world retrospective Swedish cohort study, 9,651 COPD patients and 59,454 reference controls were identified. Matching using propensity scoring was performed to identify two populations (COPD and control) with similar characteristics other than the presence of COPD. As expected, COPD patients showed an increased rate of osteoporosis-related events versus controls over approximately 5 years of subsequent follow-up. Amongst COPD patients, high-dose inhaled corticosteroid treatment also increased risk of osteoporosis-related events compared to COPD patients on no or low-dose inhaled steroids. These findings confirm the known relationship between COPD and fracture risk, and suggest that extra attention should be paid to fracture risk in COPD patients receiving high-dose inhaled corticosteroids.
Wrist fractures are common in patients with osteoporosis. In addition to causing pain and triggering functional decline, the presence of a wrist fracture indicates an increased risk of additional fragility fractures in the near future. Most wrist fractures occur in the ultradistal radius, a skeletal site rich in trabecular bone. In contrast, wrist bone density by DXA is most commonly reported in the distal 1/3 radius, a region of the radius with more cortical bone. Abaloparatide is a PTHrP analog that increases bone density and reduces fracture risk. In this sub-analysis of the ACTIVE and ACTIVExtend randomized clinical trial, the effects of abaloparatide on wrist fractures and BMD at various regions of the wrist were assessed. Compared to placebo, abaloparatide treatment significantly increased ultradistal wrist BMD after 18 months of therapy. These gains were preserved during the subsequent extension study when patients were maintained on alendronate. Very few wrist fractures were noted during this study thus precluding robust statistical analysis of the effects of abaloparatide on wrist fracture risk. However, numerically fewer wrist fractures were noted in abaloparatide-treated patients versus controls. Taken together, these results highlight the potential importance of measuring ultradistal radius BMD for patients undergoing therapy with bone anabolic agents. Future studies are needed to better standardize methods for obtaining ultradistal radius BMD measurements and to define least significant change thresholds at this potentially-important skeletal site.
Marc Wein, M.D., Ph.D
Assistant Professor of Medicine
Massachusetts General Hospital Endocrine Unit, Harvard Medical School
Although the effects of vitamin K on blood coagulation are well-established, it is now clear that many extracellular proteins are carboxylated in a vitamin K-dependent manner, including bone matrix proteins such as osteocalcin and matrix gla protein. Previous studies have reported a relationship between vitamin K levels and bone density and fracture risk. However, optimal circulating vitamin K levels for skeletal health remain unknown. In this cross-sectional study of 374 women with post-menopausal osteoporosis, the authors assessed the relationship between vitamin K levels, vitamin K dependent bone-relevant circulating proteins, bone density, and fractures. In doing so, it was noted that women with prevalent fractures showed lower vitamin K levels than those without fractures. No relationship between vitamin K levels and bone density was noted. Interestingly, different serum levels of vitamin K were associated with optimal carboxylation of different vitamin K-dependent proteins: lower vitamin K levels are needed to support clotting factors than bone matrix proteins. Overall, this study suggests that higher intake is needed to obtain the full skeletal benefit of vitamin K versus its effects on coagulation. Future prospective studies are needed to test this intriguing hypothesis, and to further explore the relationship between vitamin K and bone quality.
For over 20 years, bisphosphonates have been first line therapy to increase bone density and reduce fracture risk in patients with osteoporosis. At present, multiple oral and intravenous bisphosphonates are approved for this indication by regulatory agencies worldwide. Several ‘next-generation’ bisphosphonates with optimized anti-resorptive and pharmacokinetic properties have been developed. Of these agents, minodronate is a particularly potent, third generation azaryl bisphosphonate that is currently approved for osteoporosis treatment in Japan. In this meta-analysis of 13 randomized controlled trials, the effects of minodronate were assessed versus other commonly-used osteoporosis medications. Compared with other drugs (alendronate, risedronate, raloxifene, or eldecalcitol), minodronate more potently suppressed serum bone resorption markers (NTX and TRAcP-5b) and, because bone formation and resorption are coupled, more potently suppressed the bone formation marker bone-specific alkaline phosphatase. In addition to these effects on serum markers, minodronate reduced vertebral fractures more than other medications. Across studies, no differences were noted between minodronate and comparators at the level of bone mineral density. Minodronate treatment is associated with a high incidence of gastrointestinal adverse effects than comparator medications. Taken together, these findings suggest that minodronate represents a potent, orally-available bisphosphonate for vertebral fracture reduction in patients with osteoporosis in Japan.
Chronic obstructive pulmonary disease (COPD) is well-known to be a risk factor for osteoporosis and fragility fractures. However, the interplay between inhaled corticosteroid use in COPD and skeletal outcomes remains unclear. While systemic glucocorticoid therapy clearly impairs bone mass and increases fracture risk, whether inhaled steroids have similar effects remain unknown. Furthermore, since inhaled corticosteroids can reduce lung inflammation and COPD flares, it is possible that, by controlling pulmonary disease, these agents may actually promote bone health. In this real-world retrospective Swedish cohort study, 9,651 COPD patients and 59,454 reference controls were identified. Matching using propensity scoring was performed to identify two populations (COPD and control) with similar characteristics other than the presence of COPD. As expected, COPD patients showed an increased rate of osteoporosis-related events versus controls over approximately 5 years of subsequent follow-up. Amongst COPD patients, high-dose inhaled corticosteroid treatment also increased risk of osteoporosis-related events compared to COPD patients on no or low-dose inhaled steroids. These findings confirm the known relationship between COPD and fracture risk, and suggest that extra attention should be paid to fracture risk in COPD patients receiving high-dose inhaled corticosteroids.
Wrist fractures are common in patients with osteoporosis. In addition to causing pain and triggering functional decline, the presence of a wrist fracture indicates an increased risk of additional fragility fractures in the near future. Most wrist fractures occur in the ultradistal radius, a skeletal site rich in trabecular bone. In contrast, wrist bone density by DXA is most commonly reported in the distal 1/3 radius, a region of the radius with more cortical bone. Abaloparatide is a PTHrP analog that increases bone density and reduces fracture risk. In this sub-analysis of the ACTIVE and ACTIVExtend randomized clinical trial, the effects of abaloparatide on wrist fractures and BMD at various regions of the wrist were assessed. Compared to placebo, abaloparatide treatment significantly increased ultradistal wrist BMD after 18 months of therapy. These gains were preserved during the subsequent extension study when patients were maintained on alendronate. Very few wrist fractures were noted during this study thus precluding robust statistical analysis of the effects of abaloparatide on wrist fracture risk. However, numerically fewer wrist fractures were noted in abaloparatide-treated patients versus controls. Taken together, these results highlight the potential importance of measuring ultradistal radius BMD for patients undergoing therapy with bone anabolic agents. Future studies are needed to better standardize methods for obtaining ultradistal radius BMD measurements and to define least significant change thresholds at this potentially-important skeletal site.
Marc Wein, M.D., Ph.D
Assistant Professor of Medicine
Massachusetts General Hospital Endocrine Unit, Harvard Medical School
Although the effects of vitamin K on blood coagulation are well-established, it is now clear that many extracellular proteins are carboxylated in a vitamin K-dependent manner, including bone matrix proteins such as osteocalcin and matrix gla protein. Previous studies have reported a relationship between vitamin K levels and bone density and fracture risk. However, optimal circulating vitamin K levels for skeletal health remain unknown. In this cross-sectional study of 374 women with post-menopausal osteoporosis, the authors assessed the relationship between vitamin K levels, vitamin K dependent bone-relevant circulating proteins, bone density, and fractures. In doing so, it was noted that women with prevalent fractures showed lower vitamin K levels than those without fractures. No relationship between vitamin K levels and bone density was noted. Interestingly, different serum levels of vitamin K were associated with optimal carboxylation of different vitamin K-dependent proteins: lower vitamin K levels are needed to support clotting factors than bone matrix proteins. Overall, this study suggests that higher intake is needed to obtain the full skeletal benefit of vitamin K versus its effects on coagulation. Future prospective studies are needed to test this intriguing hypothesis, and to further explore the relationship between vitamin K and bone quality.
For over 20 years, bisphosphonates have been first line therapy to increase bone density and reduce fracture risk in patients with osteoporosis. At present, multiple oral and intravenous bisphosphonates are approved for this indication by regulatory agencies worldwide. Several ‘next-generation’ bisphosphonates with optimized anti-resorptive and pharmacokinetic properties have been developed. Of these agents, minodronate is a particularly potent, third generation azaryl bisphosphonate that is currently approved for osteoporosis treatment in Japan. In this meta-analysis of 13 randomized controlled trials, the effects of minodronate were assessed versus other commonly-used osteoporosis medications. Compared with other drugs (alendronate, risedronate, raloxifene, or eldecalcitol), minodronate more potently suppressed serum bone resorption markers (NTX and TRAcP-5b) and, because bone formation and resorption are coupled, more potently suppressed the bone formation marker bone-specific alkaline phosphatase. In addition to these effects on serum markers, minodronate reduced vertebral fractures more than other medications. Across studies, no differences were noted between minodronate and comparators at the level of bone mineral density. Minodronate treatment is associated with a high incidence of gastrointestinal adverse effects than comparator medications. Taken together, these findings suggest that minodronate represents a potent, orally-available bisphosphonate for vertebral fracture reduction in patients with osteoporosis in Japan.
Chronic obstructive pulmonary disease (COPD) is well-known to be a risk factor for osteoporosis and fragility fractures. However, the interplay between inhaled corticosteroid use in COPD and skeletal outcomes remains unclear. While systemic glucocorticoid therapy clearly impairs bone mass and increases fracture risk, whether inhaled steroids have similar effects remain unknown. Furthermore, since inhaled corticosteroids can reduce lung inflammation and COPD flares, it is possible that, by controlling pulmonary disease, these agents may actually promote bone health. In this real-world retrospective Swedish cohort study, 9,651 COPD patients and 59,454 reference controls were identified. Matching using propensity scoring was performed to identify two populations (COPD and control) with similar characteristics other than the presence of COPD. As expected, COPD patients showed an increased rate of osteoporosis-related events versus controls over approximately 5 years of subsequent follow-up. Amongst COPD patients, high-dose inhaled corticosteroid treatment also increased risk of osteoporosis-related events compared to COPD patients on no or low-dose inhaled steroids. These findings confirm the known relationship between COPD and fracture risk, and suggest that extra attention should be paid to fracture risk in COPD patients receiving high-dose inhaled corticosteroids.
Wrist fractures are common in patients with osteoporosis. In addition to causing pain and triggering functional decline, the presence of a wrist fracture indicates an increased risk of additional fragility fractures in the near future. Most wrist fractures occur in the ultradistal radius, a skeletal site rich in trabecular bone. In contrast, wrist bone density by DXA is most commonly reported in the distal 1/3 radius, a region of the radius with more cortical bone. Abaloparatide is a PTHrP analog that increases bone density and reduces fracture risk. In this sub-analysis of the ACTIVE and ACTIVExtend randomized clinical trial, the effects of abaloparatide on wrist fractures and BMD at various regions of the wrist were assessed. Compared to placebo, abaloparatide treatment significantly increased ultradistal wrist BMD after 18 months of therapy. These gains were preserved during the subsequent extension study when patients were maintained on alendronate. Very few wrist fractures were noted during this study thus precluding robust statistical analysis of the effects of abaloparatide on wrist fracture risk. However, numerically fewer wrist fractures were noted in abaloparatide-treated patients versus controls. Taken together, these results highlight the potential importance of measuring ultradistal radius BMD for patients undergoing therapy with bone anabolic agents. Future studies are needed to better standardize methods for obtaining ultradistal radius BMD measurements and to define least significant change thresholds at this potentially-important skeletal site.
Marc Wein, M.D., Ph.D
Assistant Professor of Medicine
Massachusetts General Hospital Endocrine Unit, Harvard Medical School
Hypogonadism is a key risk factor for fractures in glucocorticoid-induced osteoporosis
Key clinical point: Hypogonadism is a major risk factor for the development of fractures in men and women treated with glucocorticoid (GC).
Major finding: Major risk factors for vertebral fracture were hypogonadism (odds ratio [OR], 12.38; P = .01) and receiving GC boluses (OR 3.45; P = .01) and that for friability fracture were hypogonadism (OR, 7.03; P = .01) and a FRAX index greater than 20 (OR, 7.08; P = .02).
Study details: A cross-sectional study of 127 adults receiving chronic GC treatment for a rheumatological autoimmune disease.
Disclosures: This study was funded in part by the Societat Catalana de Reumatologia. The authors declared no conflicts of interest.
Citation: Florez H et al. RMD Open. 2020 Sep 10. doi: 10.1136/rmdopen-2020-001355.
Key clinical point: Hypogonadism is a major risk factor for the development of fractures in men and women treated with glucocorticoid (GC).
Major finding: Major risk factors for vertebral fracture were hypogonadism (odds ratio [OR], 12.38; P = .01) and receiving GC boluses (OR 3.45; P = .01) and that for friability fracture were hypogonadism (OR, 7.03; P = .01) and a FRAX index greater than 20 (OR, 7.08; P = .02).
Study details: A cross-sectional study of 127 adults receiving chronic GC treatment for a rheumatological autoimmune disease.
Disclosures: This study was funded in part by the Societat Catalana de Reumatologia. The authors declared no conflicts of interest.
Citation: Florez H et al. RMD Open. 2020 Sep 10. doi: 10.1136/rmdopen-2020-001355.
Key clinical point: Hypogonadism is a major risk factor for the development of fractures in men and women treated with glucocorticoid (GC).
Major finding: Major risk factors for vertebral fracture were hypogonadism (odds ratio [OR], 12.38; P = .01) and receiving GC boluses (OR 3.45; P = .01) and that for friability fracture were hypogonadism (OR, 7.03; P = .01) and a FRAX index greater than 20 (OR, 7.08; P = .02).
Study details: A cross-sectional study of 127 adults receiving chronic GC treatment for a rheumatological autoimmune disease.
Disclosures: This study was funded in part by the Societat Catalana de Reumatologia. The authors declared no conflicts of interest.
Citation: Florez H et al. RMD Open. 2020 Sep 10. doi: 10.1136/rmdopen-2020-001355.
Forearm BMD and fracture incidence in postmenopausal women with osteoporosis
Key clinical point: Abaloparatide/alendronate treatment shows numerically lower risks for wrist fractures compared with placebo/alendronate, suggesting a potential correlation between ultradistal (UD) radius bone mineral density (BMD) and wrist fracture.
Major finding: BMD gains at the UD radius following treatment with abaloparatide in ACTIVE were maintained over the subsequent 24 months of treatment with alendronate in ACTIVExtend (cumulative month 43 treatment difference, 0.89%; P = .20). Conversely, UD radius BMD in the placebo group during ACTIVE decreased to below ACTIVE baseline.
Study details: The data come from a subanalysis of the phase 3 ACTIVExtend trial.
Disclosures: Funding for this study was provided by Radius Health, Inc. NB Watts, RK Dore, and MS LeBoff reported ties with various pharmaceutical companies. B Mitlak and Y Wang were employees of and own company stock in Radius Health, Inc. G Hattersley was a former employee of and a consultant to Radius Health, Inc. TD Rozental is the Editor in Chief of the Journal of Hand Surgery Global Online. S Baim reported no conflicts of interest.
Citation: Watts NB et al. Osteoporos Int. 2020 Sep 15. doi: 10.1007/s00198-020-05555-1.
Key clinical point: Abaloparatide/alendronate treatment shows numerically lower risks for wrist fractures compared with placebo/alendronate, suggesting a potential correlation between ultradistal (UD) radius bone mineral density (BMD) and wrist fracture.
Major finding: BMD gains at the UD radius following treatment with abaloparatide in ACTIVE were maintained over the subsequent 24 months of treatment with alendronate in ACTIVExtend (cumulative month 43 treatment difference, 0.89%; P = .20). Conversely, UD radius BMD in the placebo group during ACTIVE decreased to below ACTIVE baseline.
Study details: The data come from a subanalysis of the phase 3 ACTIVExtend trial.
Disclosures: Funding for this study was provided by Radius Health, Inc. NB Watts, RK Dore, and MS LeBoff reported ties with various pharmaceutical companies. B Mitlak and Y Wang were employees of and own company stock in Radius Health, Inc. G Hattersley was a former employee of and a consultant to Radius Health, Inc. TD Rozental is the Editor in Chief of the Journal of Hand Surgery Global Online. S Baim reported no conflicts of interest.
Citation: Watts NB et al. Osteoporos Int. 2020 Sep 15. doi: 10.1007/s00198-020-05555-1.
Key clinical point: Abaloparatide/alendronate treatment shows numerically lower risks for wrist fractures compared with placebo/alendronate, suggesting a potential correlation between ultradistal (UD) radius bone mineral density (BMD) and wrist fracture.
Major finding: BMD gains at the UD radius following treatment with abaloparatide in ACTIVE were maintained over the subsequent 24 months of treatment with alendronate in ACTIVExtend (cumulative month 43 treatment difference, 0.89%; P = .20). Conversely, UD radius BMD in the placebo group during ACTIVE decreased to below ACTIVE baseline.
Study details: The data come from a subanalysis of the phase 3 ACTIVExtend trial.
Disclosures: Funding for this study was provided by Radius Health, Inc. NB Watts, RK Dore, and MS LeBoff reported ties with various pharmaceutical companies. B Mitlak and Y Wang were employees of and own company stock in Radius Health, Inc. G Hattersley was a former employee of and a consultant to Radius Health, Inc. TD Rozental is the Editor in Chief of the Journal of Hand Surgery Global Online. S Baim reported no conflicts of interest.
Citation: Watts NB et al. Osteoporos Int. 2020 Sep 15. doi: 10.1007/s00198-020-05555-1.