User login
Improving Patient Experience During the COVID-19 Pandemic: One Family’s Reflections
On March 11, 2020, the novel coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization.1 On March 13, 2020, a national emergency was declared in the United States concerning the COVID-19 outbreak.2 Later that week, Mike Kueper, a 52-year-old previously healthy man and resident of the Indianapolis metropolitan area, became sick with what he would eventually learn was COVID-19. Prior to contracting the novel coronavirus, he had never had as much as an Emergency Department (ED) visit. He had never spent a night in a hospital. He and his sister, DeAnn Harvey, describe the events that followed.
DeAnn
As a 20-year veteran clinical child psychologist and mother of two teenagers, my first reaction to the governor’s call for state-wide lockdowns was that they sounded like an opportunity for time at home with my husband and children. I thought we would play games, watch movies, try new recipes, and get a much-needed reprieve from our hectic lives of sports schedules, homework, and social outings. Even a slowdown in my practice sounded good. Maybe I could finally finish those continuing education credits that were due for my upcoming license renewal. My greatest concerns about sheltering in place were about how I was going to structure my children’s online learning while at the same time getting into my office to manage my patients via telehealth. Unfortunately, this relaxed feeling was short-lived.
On March 20, 2020, a few days after the lockdown started, my brother Mike developed high fevers. During a virtual doctor visit, he was told that it could be COVID-19 and to self-quarantine. Our discussions turned to jokes about his lack of taste or smell. We had dropped off soup for him from a new recipe my daughter had tried. My son joked that Mike was lucky that he couldn’t taste it.
On the morning of March 28, my mother called to tell me that Mike needed to go to the ED. Because we needed to figure out which hospital would be the best for him and I didn’t want my children to worry too much, I jumped in my car and drove to our church parking lot. In between calls to area hospitals, I began praying for his health and for guidance and support from God. Mike, concerned about spreading the virus to the rest of the family, refused to let my parents or me drive him to the hospital.
Mike
I thought I had a regular cold, and then, once I had a temperature of 102 °F and night sweats, decided it was the flu. One night, I was so cold that I went to bed wearing winter gloves. After a virtual visit with a nurse, she said my symptoms did not sound like COVID, but recommended self-quarantine, just in case. On March 26, I noticed that my sense of taste and smell had disappeared completely, and it hurt to yawn or take deep breaths. By Saturday, March 28, I was getting sicker and was short of breath and very tired. My elderly parents wanted to drive me to the ED, but if it was COVID-19, I didn’t want them near me. After getting advice from my sister, I called a local hospital and asked if I could come into the ED. The person on the phone said if I got there within an hour, they would be able to take me. When I arrived, an aide came out to my car, put me in a protective gown and mask, and walked me in. Walking even this short distance was tiring, and from this moment, things get fuzzy. I only have glimpses of the next few days. At first, I was put into a negative pressure room. I spent the night in there. I remember talking to a doctor who asked if I had a living will. He recommended that I go on a ventilator. I asked him, “Do you expect me to die?”
I remember him saying, “That is always a possibility.”
DeAnn
Once Mike was admitted to the hospital, we didn’t hear from anyone for about 6 hours, and I started to panic. I called people I knew who worked in the hospital, and my friend who is an intensive care unit (ICU) nurse agreed to track him down. He was indeed admitted to the hospital and was receiving oxygen. When I finally got to talk to him later that night, Mike had difficulty completing sentences because he was so short of breath. I told him not to use his energy, and that if they would let me, I would be there by his side. I promised him that he was going to get through this. Around 1:30
Mike
I don’t remember much from the ICU, but I understand that it was touch and go at times. I knew I was on a ventilator, and I found out later that I was “proned’ for up to 16 hours. Being on the ventilator was horrible, but what was even worse was that, once I was off the vent and alone in my hospital room, I had no idea how I got there. I thought I had been in a plane crash. I wanted to check my phone to see where I was flying in from but couldn’t because I thought my phone had been hacked by terrorists. I had no idea what was real and what was not. It was extremely scary.
DeAnn
When I think about the doctor coming in to tell Mike they had to put him on the ventilator, my heart absolutely breaks. It hurts to think of him all alone, having to make this decision without any of his family there to support him. Neither he nor I wanted to think about it, but we knew there was the possibility that he would never come off the ventilator. We hadn’t had a chance to hug him or even see him for days before his admission. If he didn’t make it, we would never get one of his amazing “Uncle Mike” hugs again.
Our friend, the ICU nurse, made it a point to find out which nurse was assigned to Mike and made it a priority to gather information from that nurse daily, allowing our family to receive updates on Mike’s status 2-3 times a day. In addition, the ICU physician was in daily contact with my parents: however, it was still excruciating not being able to be there. I spent a lot of time pacing the house, not eating or sleeping, checking my phone for texts, fielding texts and calls from friends and family. I was unable to do even simple household tasks, and left laundry, cooking, and my kids’ online schooling to my husband.
Feeling so helpless, I turned to prayer. My close friends organized a daily prayer vigil at 7:30
Then, after 17 days, a miracle: he was taken off the ventilator and moved to the medical unit. Looking back, I think these are really the days that the presence of his family would have sped up his recovery. Mike was experiencing delirium and hallucinations as a result of illness, medications, and the time he spent in an induced coma. I wish I could have been there with him to be the one he asked if what he was experiencing was real or a hallucination. Then we could have laughed about it together; our family has always found that humor helps with healing.
Mike
I understand the purpose of the isolation, but it really did a number on my mind. I remember being in the ICU, having my catheter taken out, not knowing what was happening or how I ended up in the hospital. I was so confused and was seeing people who were not there. One morning, I woke up thinking I was in my house and I had stolen the hospital bed I was in. I was panicking and scheming about how I might get this hospital bed back to the hospital before anyone noticed. As I mentioned earlier, I thought I was on a plane that had been taken over by terrorists who were using us COVID patients as biological weapons. Then I thought agents of the Federal Bureau of Investigation were coming to interrogate me and that they were also looking for my sister. To protect DeAnn, the next time someone asked me the name of my sister, I told them, “Maria” (the name of my sister who passed away in 1991) rather than DeAnn, who is very much alive. Another time, I thought my grandmother and cousin had died in a plane crash. My cousin is a state representative in Illinois, so once I got my phone to work, I checked his Wikipedia page to see if there was a death date listed.
When I was less confused, I found it’s tough spending day after day lying in a hospital bed with no family member or friend to offer companionship, comfort, or clarity. Even though I was extremely weak and could barely walk, I was asking, daily, about when I was expected to be discharged. I had to get out.
One night, our friend the ICU nurse came into my room to sit with me and just talk. She spent about 30 minutes with me around 4:00 in the morning. It was wonderful. All I could think about was what a huge blessing it was. I don’t think she knows just how much that meant to me. More often, I would report some symptom of confusion or insomnia, and a nurse would offer me medications for sleep or pain. I did not want any more drugs in my system. Human contact would have been a far better treatment.
I was reluctant to ask for help when I needed something, like a trip to the bathroom or some ice water. When I did press the call button, I had to wait for the busy nurse or tech to put on all the protective gear, and then, when they left, watch them take all the steps to disinfect and rid themselves of the gear that they had just put on. Even so, I was excited when it came time to take my vital signs or administer medications because that meant human interaction, however brief (and even if it was 4:00 AM). I wanted to bathe or change gowns and/or socks, but I opted to wear the same gown and socks for over three-fourths of the time I was there because I did not want to burden the staff.
Video chat turned out to be one of the best tools for creating connection. I may have sobbed a few times when talking to my parents on FaceTime, but just seeing their faces made all the difference in the world.
Finally, on April 21, 25 days after I was led into the ED, I was discharged. As we reflect on this experience, my family and I have some recommendations for hospitals and health systems trying to make patient experience a priority during this pandemic:
Kueper Family Recommendations to Improve Patient Experience for Those With COVID-19
- Adopt a more systematic approach to communicating with patient families, which would greatly improve the connection between them and healthcare personnel. This is especially important for families when the patient is critically ill, and especially in times when the patient is in isolation. We were fortunate in that we received updates from nurses and physicians several times a day. This was partly due to the relationships or connections with staff members that existed previously or developed over the course of Mike’s stay. Staff members who became invested in Mike’s progress became part of his hospital “family.” Many people who have had a family member with COVID have not had this experience, nor did they have the opportunity to build relationships with the staff, which we felt were important to ensure good care and open and frequent communication with them and the patient. Therefore, we believe a more systematic approach toward communication (eg, “the team will call each day during multidisciplinary rounds at 11 AM,”) would greatly improve the connection between families and healthcare personnel.
- Allow visitation under certain conditions even while the patient is in isolation. Visitation would have been especially helpful once Mike was more awake but isolated and delirious. We know that these policies are difficult to create and navigate but believe that there should be allowance for some visitation when there is a clear clinical benefit (eg, delirium). Because Mike had little human contact the week after he was taken off the ventilator (eg, contact limited to nurses coming in to take vitals, once daily doctor visit), he had to navigate the hallucinations and delirium on his own. Even one family member by his side who could provide frequent feedback on reality would have helped to resolve the feelings of agitation and fear that can accompany delirium.
- Schedule more video chats. Even when Mike was on the ventilator, we found video chats to be an important way to understand his experience and connect with him. Although we know such chats are difficult for clinicians to schedule, it greatly improved the experience for us.
- Reassure patients that caring for them is not a burden and they should not hesitate to ask for help. Being contagious and believing you are a danger to others is a terrible feeling. No one on staff said or even implied that they were afraid to care for him, but Mike felt “dangerous” to the staff and as such hesitated to “burden” the clinicians with requests (eg, going to the bathroom, having a change of clothes). It is time-consuming and difficult to don PPE and the amount of effort it takes to enter the room is immediately obvious to the patient. Because of this, it is very important that the clinicians and staff reassure patients that it is part of the job and not a burden to come in and out of the room.
DeAnn
Having Mike alive and now home is an incredible gift. We are taking every chance we can to make up for the time that we could not see him and are so grateful for the hospital team that saved his life.
Mike
On April 21, I was discharged and sent home. Luckily, for about 2 weeks, I had a best friend, my brother, and DeAnn, separately, stay with me each night. This was a godsend as all made sure I was taking my medicine, eating, and doing my prescribed exercises. I am struggling with a long recovery. I used a walker for a while and had both a physical and occupational therapist visit me two to three times a week. I visit a neurologist for some of my symptoms that have not resolved, such as pain and atrophy in my right shoulder, hand tremors, and some numbness in my thighs. Thankfully, I was able to resume working from home, but even going up stairs causes me to become winded. I know that doctors don’t understand this disease very well, and neither do I. Sometimes I feel discouraged about how much it set me back physically. I wish things could have been different—that I could have avoided this disease altogether or had milder symptoms. But I am so grateful to be alive and so thankful for the doctors and nurses, as well as for my family, who could not be there physically during the hospitalization but did everything they could do to help me. Because of their love and support, I survived.
Disclosures
The authors have nothing to disclose.
1. World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID–11 March 2020. Published March 11, 2020. Accessed August 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
2. White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. Issued March 13, 2020. Accessed August 11, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/
On March 11, 2020, the novel coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization.1 On March 13, 2020, a national emergency was declared in the United States concerning the COVID-19 outbreak.2 Later that week, Mike Kueper, a 52-year-old previously healthy man and resident of the Indianapolis metropolitan area, became sick with what he would eventually learn was COVID-19. Prior to contracting the novel coronavirus, he had never had as much as an Emergency Department (ED) visit. He had never spent a night in a hospital. He and his sister, DeAnn Harvey, describe the events that followed.
DeAnn
As a 20-year veteran clinical child psychologist and mother of two teenagers, my first reaction to the governor’s call for state-wide lockdowns was that they sounded like an opportunity for time at home with my husband and children. I thought we would play games, watch movies, try new recipes, and get a much-needed reprieve from our hectic lives of sports schedules, homework, and social outings. Even a slowdown in my practice sounded good. Maybe I could finally finish those continuing education credits that were due for my upcoming license renewal. My greatest concerns about sheltering in place were about how I was going to structure my children’s online learning while at the same time getting into my office to manage my patients via telehealth. Unfortunately, this relaxed feeling was short-lived.
On March 20, 2020, a few days after the lockdown started, my brother Mike developed high fevers. During a virtual doctor visit, he was told that it could be COVID-19 and to self-quarantine. Our discussions turned to jokes about his lack of taste or smell. We had dropped off soup for him from a new recipe my daughter had tried. My son joked that Mike was lucky that he couldn’t taste it.
On the morning of March 28, my mother called to tell me that Mike needed to go to the ED. Because we needed to figure out which hospital would be the best for him and I didn’t want my children to worry too much, I jumped in my car and drove to our church parking lot. In between calls to area hospitals, I began praying for his health and for guidance and support from God. Mike, concerned about spreading the virus to the rest of the family, refused to let my parents or me drive him to the hospital.
Mike
I thought I had a regular cold, and then, once I had a temperature of 102 °F and night sweats, decided it was the flu. One night, I was so cold that I went to bed wearing winter gloves. After a virtual visit with a nurse, she said my symptoms did not sound like COVID, but recommended self-quarantine, just in case. On March 26, I noticed that my sense of taste and smell had disappeared completely, and it hurt to yawn or take deep breaths. By Saturday, March 28, I was getting sicker and was short of breath and very tired. My elderly parents wanted to drive me to the ED, but if it was COVID-19, I didn’t want them near me. After getting advice from my sister, I called a local hospital and asked if I could come into the ED. The person on the phone said if I got there within an hour, they would be able to take me. When I arrived, an aide came out to my car, put me in a protective gown and mask, and walked me in. Walking even this short distance was tiring, and from this moment, things get fuzzy. I only have glimpses of the next few days. At first, I was put into a negative pressure room. I spent the night in there. I remember talking to a doctor who asked if I had a living will. He recommended that I go on a ventilator. I asked him, “Do you expect me to die?”
I remember him saying, “That is always a possibility.”
DeAnn
Once Mike was admitted to the hospital, we didn’t hear from anyone for about 6 hours, and I started to panic. I called people I knew who worked in the hospital, and my friend who is an intensive care unit (ICU) nurse agreed to track him down. He was indeed admitted to the hospital and was receiving oxygen. When I finally got to talk to him later that night, Mike had difficulty completing sentences because he was so short of breath. I told him not to use his energy, and that if they would let me, I would be there by his side. I promised him that he was going to get through this. Around 1:30
Mike
I don’t remember much from the ICU, but I understand that it was touch and go at times. I knew I was on a ventilator, and I found out later that I was “proned’ for up to 16 hours. Being on the ventilator was horrible, but what was even worse was that, once I was off the vent and alone in my hospital room, I had no idea how I got there. I thought I had been in a plane crash. I wanted to check my phone to see where I was flying in from but couldn’t because I thought my phone had been hacked by terrorists. I had no idea what was real and what was not. It was extremely scary.
DeAnn
When I think about the doctor coming in to tell Mike they had to put him on the ventilator, my heart absolutely breaks. It hurts to think of him all alone, having to make this decision without any of his family there to support him. Neither he nor I wanted to think about it, but we knew there was the possibility that he would never come off the ventilator. We hadn’t had a chance to hug him or even see him for days before his admission. If he didn’t make it, we would never get one of his amazing “Uncle Mike” hugs again.
Our friend, the ICU nurse, made it a point to find out which nurse was assigned to Mike and made it a priority to gather information from that nurse daily, allowing our family to receive updates on Mike’s status 2-3 times a day. In addition, the ICU physician was in daily contact with my parents: however, it was still excruciating not being able to be there. I spent a lot of time pacing the house, not eating or sleeping, checking my phone for texts, fielding texts and calls from friends and family. I was unable to do even simple household tasks, and left laundry, cooking, and my kids’ online schooling to my husband.
Feeling so helpless, I turned to prayer. My close friends organized a daily prayer vigil at 7:30
Then, after 17 days, a miracle: he was taken off the ventilator and moved to the medical unit. Looking back, I think these are really the days that the presence of his family would have sped up his recovery. Mike was experiencing delirium and hallucinations as a result of illness, medications, and the time he spent in an induced coma. I wish I could have been there with him to be the one he asked if what he was experiencing was real or a hallucination. Then we could have laughed about it together; our family has always found that humor helps with healing.
Mike
I understand the purpose of the isolation, but it really did a number on my mind. I remember being in the ICU, having my catheter taken out, not knowing what was happening or how I ended up in the hospital. I was so confused and was seeing people who were not there. One morning, I woke up thinking I was in my house and I had stolen the hospital bed I was in. I was panicking and scheming about how I might get this hospital bed back to the hospital before anyone noticed. As I mentioned earlier, I thought I was on a plane that had been taken over by terrorists who were using us COVID patients as biological weapons. Then I thought agents of the Federal Bureau of Investigation were coming to interrogate me and that they were also looking for my sister. To protect DeAnn, the next time someone asked me the name of my sister, I told them, “Maria” (the name of my sister who passed away in 1991) rather than DeAnn, who is very much alive. Another time, I thought my grandmother and cousin had died in a plane crash. My cousin is a state representative in Illinois, so once I got my phone to work, I checked his Wikipedia page to see if there was a death date listed.
When I was less confused, I found it’s tough spending day after day lying in a hospital bed with no family member or friend to offer companionship, comfort, or clarity. Even though I was extremely weak and could barely walk, I was asking, daily, about when I was expected to be discharged. I had to get out.
One night, our friend the ICU nurse came into my room to sit with me and just talk. She spent about 30 minutes with me around 4:00 in the morning. It was wonderful. All I could think about was what a huge blessing it was. I don’t think she knows just how much that meant to me. More often, I would report some symptom of confusion or insomnia, and a nurse would offer me medications for sleep or pain. I did not want any more drugs in my system. Human contact would have been a far better treatment.
I was reluctant to ask for help when I needed something, like a trip to the bathroom or some ice water. When I did press the call button, I had to wait for the busy nurse or tech to put on all the protective gear, and then, when they left, watch them take all the steps to disinfect and rid themselves of the gear that they had just put on. Even so, I was excited when it came time to take my vital signs or administer medications because that meant human interaction, however brief (and even if it was 4:00 AM). I wanted to bathe or change gowns and/or socks, but I opted to wear the same gown and socks for over three-fourths of the time I was there because I did not want to burden the staff.
Video chat turned out to be one of the best tools for creating connection. I may have sobbed a few times when talking to my parents on FaceTime, but just seeing their faces made all the difference in the world.
Finally, on April 21, 25 days after I was led into the ED, I was discharged. As we reflect on this experience, my family and I have some recommendations for hospitals and health systems trying to make patient experience a priority during this pandemic:
Kueper Family Recommendations to Improve Patient Experience for Those With COVID-19
- Adopt a more systematic approach to communicating with patient families, which would greatly improve the connection between them and healthcare personnel. This is especially important for families when the patient is critically ill, and especially in times when the patient is in isolation. We were fortunate in that we received updates from nurses and physicians several times a day. This was partly due to the relationships or connections with staff members that existed previously or developed over the course of Mike’s stay. Staff members who became invested in Mike’s progress became part of his hospital “family.” Many people who have had a family member with COVID have not had this experience, nor did they have the opportunity to build relationships with the staff, which we felt were important to ensure good care and open and frequent communication with them and the patient. Therefore, we believe a more systematic approach toward communication (eg, “the team will call each day during multidisciplinary rounds at 11 AM,”) would greatly improve the connection between families and healthcare personnel.
- Allow visitation under certain conditions even while the patient is in isolation. Visitation would have been especially helpful once Mike was more awake but isolated and delirious. We know that these policies are difficult to create and navigate but believe that there should be allowance for some visitation when there is a clear clinical benefit (eg, delirium). Because Mike had little human contact the week after he was taken off the ventilator (eg, contact limited to nurses coming in to take vitals, once daily doctor visit), he had to navigate the hallucinations and delirium on his own. Even one family member by his side who could provide frequent feedback on reality would have helped to resolve the feelings of agitation and fear that can accompany delirium.
- Schedule more video chats. Even when Mike was on the ventilator, we found video chats to be an important way to understand his experience and connect with him. Although we know such chats are difficult for clinicians to schedule, it greatly improved the experience for us.
- Reassure patients that caring for them is not a burden and they should not hesitate to ask for help. Being contagious and believing you are a danger to others is a terrible feeling. No one on staff said or even implied that they were afraid to care for him, but Mike felt “dangerous” to the staff and as such hesitated to “burden” the clinicians with requests (eg, going to the bathroom, having a change of clothes). It is time-consuming and difficult to don PPE and the amount of effort it takes to enter the room is immediately obvious to the patient. Because of this, it is very important that the clinicians and staff reassure patients that it is part of the job and not a burden to come in and out of the room.
DeAnn
Having Mike alive and now home is an incredible gift. We are taking every chance we can to make up for the time that we could not see him and are so grateful for the hospital team that saved his life.
Mike
On April 21, I was discharged and sent home. Luckily, for about 2 weeks, I had a best friend, my brother, and DeAnn, separately, stay with me each night. This was a godsend as all made sure I was taking my medicine, eating, and doing my prescribed exercises. I am struggling with a long recovery. I used a walker for a while and had both a physical and occupational therapist visit me two to three times a week. I visit a neurologist for some of my symptoms that have not resolved, such as pain and atrophy in my right shoulder, hand tremors, and some numbness in my thighs. Thankfully, I was able to resume working from home, but even going up stairs causes me to become winded. I know that doctors don’t understand this disease very well, and neither do I. Sometimes I feel discouraged about how much it set me back physically. I wish things could have been different—that I could have avoided this disease altogether or had milder symptoms. But I am so grateful to be alive and so thankful for the doctors and nurses, as well as for my family, who could not be there physically during the hospitalization but did everything they could do to help me. Because of their love and support, I survived.
Disclosures
The authors have nothing to disclose.
On March 11, 2020, the novel coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization.1 On March 13, 2020, a national emergency was declared in the United States concerning the COVID-19 outbreak.2 Later that week, Mike Kueper, a 52-year-old previously healthy man and resident of the Indianapolis metropolitan area, became sick with what he would eventually learn was COVID-19. Prior to contracting the novel coronavirus, he had never had as much as an Emergency Department (ED) visit. He had never spent a night in a hospital. He and his sister, DeAnn Harvey, describe the events that followed.
DeAnn
As a 20-year veteran clinical child psychologist and mother of two teenagers, my first reaction to the governor’s call for state-wide lockdowns was that they sounded like an opportunity for time at home with my husband and children. I thought we would play games, watch movies, try new recipes, and get a much-needed reprieve from our hectic lives of sports schedules, homework, and social outings. Even a slowdown in my practice sounded good. Maybe I could finally finish those continuing education credits that were due for my upcoming license renewal. My greatest concerns about sheltering in place were about how I was going to structure my children’s online learning while at the same time getting into my office to manage my patients via telehealth. Unfortunately, this relaxed feeling was short-lived.
On March 20, 2020, a few days after the lockdown started, my brother Mike developed high fevers. During a virtual doctor visit, he was told that it could be COVID-19 and to self-quarantine. Our discussions turned to jokes about his lack of taste or smell. We had dropped off soup for him from a new recipe my daughter had tried. My son joked that Mike was lucky that he couldn’t taste it.
On the morning of March 28, my mother called to tell me that Mike needed to go to the ED. Because we needed to figure out which hospital would be the best for him and I didn’t want my children to worry too much, I jumped in my car and drove to our church parking lot. In between calls to area hospitals, I began praying for his health and for guidance and support from God. Mike, concerned about spreading the virus to the rest of the family, refused to let my parents or me drive him to the hospital.
Mike
I thought I had a regular cold, and then, once I had a temperature of 102 °F and night sweats, decided it was the flu. One night, I was so cold that I went to bed wearing winter gloves. After a virtual visit with a nurse, she said my symptoms did not sound like COVID, but recommended self-quarantine, just in case. On March 26, I noticed that my sense of taste and smell had disappeared completely, and it hurt to yawn or take deep breaths. By Saturday, March 28, I was getting sicker and was short of breath and very tired. My elderly parents wanted to drive me to the ED, but if it was COVID-19, I didn’t want them near me. After getting advice from my sister, I called a local hospital and asked if I could come into the ED. The person on the phone said if I got there within an hour, they would be able to take me. When I arrived, an aide came out to my car, put me in a protective gown and mask, and walked me in. Walking even this short distance was tiring, and from this moment, things get fuzzy. I only have glimpses of the next few days. At first, I was put into a negative pressure room. I spent the night in there. I remember talking to a doctor who asked if I had a living will. He recommended that I go on a ventilator. I asked him, “Do you expect me to die?”
I remember him saying, “That is always a possibility.”
DeAnn
Once Mike was admitted to the hospital, we didn’t hear from anyone for about 6 hours, and I started to panic. I called people I knew who worked in the hospital, and my friend who is an intensive care unit (ICU) nurse agreed to track him down. He was indeed admitted to the hospital and was receiving oxygen. When I finally got to talk to him later that night, Mike had difficulty completing sentences because he was so short of breath. I told him not to use his energy, and that if they would let me, I would be there by his side. I promised him that he was going to get through this. Around 1:30
Mike
I don’t remember much from the ICU, but I understand that it was touch and go at times. I knew I was on a ventilator, and I found out later that I was “proned’ for up to 16 hours. Being on the ventilator was horrible, but what was even worse was that, once I was off the vent and alone in my hospital room, I had no idea how I got there. I thought I had been in a plane crash. I wanted to check my phone to see where I was flying in from but couldn’t because I thought my phone had been hacked by terrorists. I had no idea what was real and what was not. It was extremely scary.
DeAnn
When I think about the doctor coming in to tell Mike they had to put him on the ventilator, my heart absolutely breaks. It hurts to think of him all alone, having to make this decision without any of his family there to support him. Neither he nor I wanted to think about it, but we knew there was the possibility that he would never come off the ventilator. We hadn’t had a chance to hug him or even see him for days before his admission. If he didn’t make it, we would never get one of his amazing “Uncle Mike” hugs again.
Our friend, the ICU nurse, made it a point to find out which nurse was assigned to Mike and made it a priority to gather information from that nurse daily, allowing our family to receive updates on Mike’s status 2-3 times a day. In addition, the ICU physician was in daily contact with my parents: however, it was still excruciating not being able to be there. I spent a lot of time pacing the house, not eating or sleeping, checking my phone for texts, fielding texts and calls from friends and family. I was unable to do even simple household tasks, and left laundry, cooking, and my kids’ online schooling to my husband.
Feeling so helpless, I turned to prayer. My close friends organized a daily prayer vigil at 7:30
Then, after 17 days, a miracle: he was taken off the ventilator and moved to the medical unit. Looking back, I think these are really the days that the presence of his family would have sped up his recovery. Mike was experiencing delirium and hallucinations as a result of illness, medications, and the time he spent in an induced coma. I wish I could have been there with him to be the one he asked if what he was experiencing was real or a hallucination. Then we could have laughed about it together; our family has always found that humor helps with healing.
Mike
I understand the purpose of the isolation, but it really did a number on my mind. I remember being in the ICU, having my catheter taken out, not knowing what was happening or how I ended up in the hospital. I was so confused and was seeing people who were not there. One morning, I woke up thinking I was in my house and I had stolen the hospital bed I was in. I was panicking and scheming about how I might get this hospital bed back to the hospital before anyone noticed. As I mentioned earlier, I thought I was on a plane that had been taken over by terrorists who were using us COVID patients as biological weapons. Then I thought agents of the Federal Bureau of Investigation were coming to interrogate me and that they were also looking for my sister. To protect DeAnn, the next time someone asked me the name of my sister, I told them, “Maria” (the name of my sister who passed away in 1991) rather than DeAnn, who is very much alive. Another time, I thought my grandmother and cousin had died in a plane crash. My cousin is a state representative in Illinois, so once I got my phone to work, I checked his Wikipedia page to see if there was a death date listed.
When I was less confused, I found it’s tough spending day after day lying in a hospital bed with no family member or friend to offer companionship, comfort, or clarity. Even though I was extremely weak and could barely walk, I was asking, daily, about when I was expected to be discharged. I had to get out.
One night, our friend the ICU nurse came into my room to sit with me and just talk. She spent about 30 minutes with me around 4:00 in the morning. It was wonderful. All I could think about was what a huge blessing it was. I don’t think she knows just how much that meant to me. More often, I would report some symptom of confusion or insomnia, and a nurse would offer me medications for sleep or pain. I did not want any more drugs in my system. Human contact would have been a far better treatment.
I was reluctant to ask for help when I needed something, like a trip to the bathroom or some ice water. When I did press the call button, I had to wait for the busy nurse or tech to put on all the protective gear, and then, when they left, watch them take all the steps to disinfect and rid themselves of the gear that they had just put on. Even so, I was excited when it came time to take my vital signs or administer medications because that meant human interaction, however brief (and even if it was 4:00 AM). I wanted to bathe or change gowns and/or socks, but I opted to wear the same gown and socks for over three-fourths of the time I was there because I did not want to burden the staff.
Video chat turned out to be one of the best tools for creating connection. I may have sobbed a few times when talking to my parents on FaceTime, but just seeing their faces made all the difference in the world.
Finally, on April 21, 25 days after I was led into the ED, I was discharged. As we reflect on this experience, my family and I have some recommendations for hospitals and health systems trying to make patient experience a priority during this pandemic:
Kueper Family Recommendations to Improve Patient Experience for Those With COVID-19
- Adopt a more systematic approach to communicating with patient families, which would greatly improve the connection between them and healthcare personnel. This is especially important for families when the patient is critically ill, and especially in times when the patient is in isolation. We were fortunate in that we received updates from nurses and physicians several times a day. This was partly due to the relationships or connections with staff members that existed previously or developed over the course of Mike’s stay. Staff members who became invested in Mike’s progress became part of his hospital “family.” Many people who have had a family member with COVID have not had this experience, nor did they have the opportunity to build relationships with the staff, which we felt were important to ensure good care and open and frequent communication with them and the patient. Therefore, we believe a more systematic approach toward communication (eg, “the team will call each day during multidisciplinary rounds at 11 AM,”) would greatly improve the connection between families and healthcare personnel.
- Allow visitation under certain conditions even while the patient is in isolation. Visitation would have been especially helpful once Mike was more awake but isolated and delirious. We know that these policies are difficult to create and navigate but believe that there should be allowance for some visitation when there is a clear clinical benefit (eg, delirium). Because Mike had little human contact the week after he was taken off the ventilator (eg, contact limited to nurses coming in to take vitals, once daily doctor visit), he had to navigate the hallucinations and delirium on his own. Even one family member by his side who could provide frequent feedback on reality would have helped to resolve the feelings of agitation and fear that can accompany delirium.
- Schedule more video chats. Even when Mike was on the ventilator, we found video chats to be an important way to understand his experience and connect with him. Although we know such chats are difficult for clinicians to schedule, it greatly improved the experience for us.
- Reassure patients that caring for them is not a burden and they should not hesitate to ask for help. Being contagious and believing you are a danger to others is a terrible feeling. No one on staff said or even implied that they were afraid to care for him, but Mike felt “dangerous” to the staff and as such hesitated to “burden” the clinicians with requests (eg, going to the bathroom, having a change of clothes). It is time-consuming and difficult to don PPE and the amount of effort it takes to enter the room is immediately obvious to the patient. Because of this, it is very important that the clinicians and staff reassure patients that it is part of the job and not a burden to come in and out of the room.
DeAnn
Having Mike alive and now home is an incredible gift. We are taking every chance we can to make up for the time that we could not see him and are so grateful for the hospital team that saved his life.
Mike
On April 21, I was discharged and sent home. Luckily, for about 2 weeks, I had a best friend, my brother, and DeAnn, separately, stay with me each night. This was a godsend as all made sure I was taking my medicine, eating, and doing my prescribed exercises. I am struggling with a long recovery. I used a walker for a while and had both a physical and occupational therapist visit me two to three times a week. I visit a neurologist for some of my symptoms that have not resolved, such as pain and atrophy in my right shoulder, hand tremors, and some numbness in my thighs. Thankfully, I was able to resume working from home, but even going up stairs causes me to become winded. I know that doctors don’t understand this disease very well, and neither do I. Sometimes I feel discouraged about how much it set me back physically. I wish things could have been different—that I could have avoided this disease altogether or had milder symptoms. But I am so grateful to be alive and so thankful for the doctors and nurses, as well as for my family, who could not be there physically during the hospitalization but did everything they could do to help me. Because of their love and support, I survived.
Disclosures
The authors have nothing to disclose.
1. World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID–11 March 2020. Published March 11, 2020. Accessed August 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
2. White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. Issued March 13, 2020. Accessed August 11, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/
1. World Health Organization. WHO director-general’s opening remarks at the media briefing on COVID–11 March 2020. Published March 11, 2020. Accessed August 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
2. White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak. Issued March 13, 2020. Accessed August 11, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/
© 2020 Society of Hospital Medicine
Deployed: Pediatric Residents Caring for Adults During COVID-19’s First Wave in New York City
Stepping onto a busy coronavirus disease (COVID-19) unit for the first time can elicit trepidation for any medical provider. For a group of deployed pediatric residents at a New York City hospital in the spring of 2020, it was also the first time caring for adults since medical school. Imagine a pediatrician receiving this handoff: “77-year-old female with a history of diabetes, peripheral vascular disease, and COPD admitted with COVID-19 pneumonia, now intubated and proned with O2 saturations in the 80s. To do: DNR discussion.” General anxiety around COVID-19 was compounded by the discomfort of being thrust into adult medicine. But the doctoring instinct we have been honing throughout training kicked in, and we acted.
A NEW ORDER
As the COVID-19 crisis escalated in New York City, it became evident that staff from other specialties would be essential to manage the surge of patients. Hospital administrators selected a group of trainees for deployment based on their clinical experiences and willingness to volunteer. Almost overnight, a group of senior pediatric residents became adult providers, honoring the oath we each took to “remain a member of society with special obligations to all . . . fellow human beings.”¹
This health crisis brought different clinical disciplines together like never before. Entire wings of the hospital were converted into new COVID-19–dedicated wards and intensive care units (ICUs), and teams were built to optimize providers’ skills and capabilities. For example, one third-year pediatric resident was grouped with an outpatient endocrinologist—who had not practiced inpatient medicine in a decade—and a medicine intern. Hospitalists provided crucial support and guidance to these ward teams of deployed providers who were eager and willing to work but often not very knowledgeable about inpatient adult medicine.² In new ad hoc COVID-19 ICUs housed in other ICUs, where most pediatric residents were deployed, critical care attendings and neurointensivists led teams that also included anesthesiology, radiology, and neurosurgery residents, as well as nurses and advanced practice providers trained in various subspecialties of adult medicine.
PEDIATRICIANS IN AN ADULT WORLD
Although we wanted to help in any way we could, the prospect of entering this new world was incredibly daunting. We had not treated adults in several years, and during that time, our clinical experience with pediatric medicine greatly surpassed our adult training from medical school. We dug out materials on adult diseases, watched impromptu lectures on COVID-19 given by our critical care attendings, taught ourselves ventilator management in adults, and reviewed advanced cardiac life support (ACLS) protocols. But putting this all into practice was entirely different. Nothing can truly prepare you for arriving at the bedside of a hemodynamically unstable patient suffering from a virus that no one really understands.
When we arrived and introduced ourselves, we occasionally encountered surprise and curiosity from other providers. We felt that there was a perception that pediatricians do not often take care of critically ill or complex patients. Some of us were reluctant to disclose our specialty, lest it cloud perceptions of our capabilities. However, sick patients awaited us, so we got to work.
There was a steep learning curve over the first few days, from adjusting insulin for type 2 diabetes to troubleshooting renal replacement therapy issues. Accustomed to pediatric weight-based dosing, we were very anxious about ordering medications. The adult providers on our teams oriented us and helped us with many of these concerns. But the mystery of COVID-19 was a great equalizing force, leaving providers of every background with questions: Should we anticoagulate? How about steroids? Could this clinical change be another effect of the virus or a new infection?
We were pleasantly surprised that many aspects of our pediatric training proved beneficial in caring for adults. The focus on family-centered rounds and shared decision-making in pediatrics had imprinted on us the paramount importance of good communication. We were very cognizant of involving loved ones in discussions, now conducted by telephone or video call because infection-prevention guidelines precluded visitors. Family members were thankful for frequent updates, and as a result, largely embraced us as the doctors treating their loved ones. On one occasion, an internist, whose mother was a patient, was delighted to learn that the provider was a pediatric resident, saying, “I know you’ll take such good care of her.”
With the hospital inundated with sick adults, colleagues were grateful for our help. More so, they seemed appreciative of our compassion and ability to maintain a sense of humanity during the pandemonium of the pandemic despite feeling vulnerable, scared, and often powerless against COVID-19. In pediatrics, we do our best to truly engage with patients, from playing games with a 6-year-old with perforated appendicitis to holding and soothing a newborn in the neonatal ICU. We carried those skills over to the adult side. The team appreciated when a pediatric resident, with the help of an occupational therapist, used a letter board to communicate and receive assent for a tracheostomy from a nonsedated, intubated patient, directly answering the patient’s questions and addressing concerns rather than relying solely on a family member’s consent. And, though we had not previously led end-of-life discussions, we found that we were capable of doing so with the compassion instilled in us from our pediatric training. It had prepared us to face the universal challenge of communication in times of grief.
COVID-19 CHALLENGES
Besides grappling with our insecurity in treating adults, we, like all medical providers, had to balance our desire to provide care while keeping ourselves safe from COVID-19. To reduce our risk of exposure and preserve the dwindling supply of personal protective equipment (PPE), the flow of rounding, bedside care, and interventions was adapted to better cluster examinations, blood draws, and bedside tasks. Although efficient, this meant we did not enter rooms as frequently, creating an unfamiliar distance between provider and patient.³ We feared missing moments of clinical decompensation, and for pediatricians who value close patient contact, this made for a deeply uncomfortable reality.
We considered every plausible treatment for critically ill patients, sometimes unsure if they were beneficial or instead complicating the course further. Was lack of improvement a treatment failure or just the natural progression of this new illness? Unfortunately, most of the time, treatments were to no avail. Watching the respiratory, cardiovascular, renal, and neurologic devastation of COVID-19 on so many patients was horrifying. Seeing patients die without their loved ones beside them and at an alarmingly fast rate was simply crushing, as other trainees have similarly described.4 It was unlike anything we had ever experienced in pediatrics. Though we had begun to see a few pediatric COVID-19 patients in the hospital, their disease course was less severe. And, in the rare cases when pediatric patients die, they are almost invariably surrounded by family. One pediatric resident, who had never performed a single death examination before, did three in 1 week. It was emotionally trying, yet we had little time to mourn, as deathbeds were only briefly empty before the next gravely ill patients filled them.
Deployment took a toll on our bodies as well. We padded our faces to alleviate skin breakdown from 12-hour shifts spent entirely in N95 masks. We sanitized and washed our hands constantly, developing cracked skin and dermatitis, and showered meticulously after every shift. We isolated ourselves from our families and loved ones to protect them from the virus.
MOMENTS OF POSITIVITY
Despite these challenges, positive moments emerged. We worked with many wonderful colleagues from different disciplines we likely never would have met, let alone work alongside. We valued each other’s skills, talents, and knowledge. On an overnight shift in one of the ICUs, among the “ragtag team of deployees,” as one pediatric resident phrased it, each presented a topic from his or her respective specialty that might interest others. The pediatrician presented Kawasaki disease, as adult colleagues were beginning to ask questions about its cousin, the emerging multisystem inflammatory syndrome in children (MIS-C). This collegiality promoted a culture of collaboration and respect for other specialties that will hopefully continue.
A strong drive toward teamwork and shared responsibility flourished during deployment. No one was above any task. Residents and even fellows performed typical frontline tasks, such as ordering laboratory work and coordinating imaging. We all helped the proning team turn patients. Everyone shared insights, perspectives, and information gleaned from friends in different wards and hospitals and the ever-evolving literature. As we grappled with unpredictable disease courses, the traditional hierarchical roles of medicine—attending, fellow, resident—often blurred. We felt like we were all in this together.
Patient triumphs were celebrated. We danced with an 80-year-old patient admitted for almost 2 weeks when she was informed of her discharge and gave a standing ovation for a 91-year-old woman as she headed home. Music played over the hospital loudspeaker for every patient discharge. We also tried to create moments of lightheartedness. In the ICUs, we ate donated meals together and posed for pictures to express our gratitude to restaurants. Camaraderie blossomed during deployment.
ADVICE FOR THE FUTURE
Answering the call to help during the COVID-19 surge in New York City indelibly shaped our experiences as trainees and physicians. We will carry with us the lessons that we learned, both in the short term for the possible resurgence of cases and in the long term for ongoing patient care for the rest of our careers. For those residents who may be called upon next, the experience will be challenging, but rewarding. Each trainee has a foundation of knowledge, abilities, and instincts that will be useful, so trust in your training. Do not be afraid to ask questions or for help. You may be leaving your comfort zone, but you will not be alone, and families and other clinicians will be grateful to have you there. You are resilient, and you will make a difference.
Disclosures
The authors have nothing to disclose.
1. Lasagna L. Hippocratic oath—modern version. Published 1964. Accessed September 14, 2020. http://www.pbs.org/wgbh/nova/doctors/oath_modern.html
2. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314-315.https://doi.org/10.12788/jhm.3426
3. Cunningham CO, Diaz C, Slawek DE. COVID-19: the worst days of our careers. Ann Intern Med. 2020;172(11):764-765. https://doi.org/10.7326/M20-1715
4. Gallagher TH, Schleyer AM. “We signed up for this!”—student and trainee responses to the COVID-19 pandemic. N Engl J Med. 2020;382(25):e96. https://doi.org/10.1056/NEJMp2005234
Stepping onto a busy coronavirus disease (COVID-19) unit for the first time can elicit trepidation for any medical provider. For a group of deployed pediatric residents at a New York City hospital in the spring of 2020, it was also the first time caring for adults since medical school. Imagine a pediatrician receiving this handoff: “77-year-old female with a history of diabetes, peripheral vascular disease, and COPD admitted with COVID-19 pneumonia, now intubated and proned with O2 saturations in the 80s. To do: DNR discussion.” General anxiety around COVID-19 was compounded by the discomfort of being thrust into adult medicine. But the doctoring instinct we have been honing throughout training kicked in, and we acted.
A NEW ORDER
As the COVID-19 crisis escalated in New York City, it became evident that staff from other specialties would be essential to manage the surge of patients. Hospital administrators selected a group of trainees for deployment based on their clinical experiences and willingness to volunteer. Almost overnight, a group of senior pediatric residents became adult providers, honoring the oath we each took to “remain a member of society with special obligations to all . . . fellow human beings.”¹
This health crisis brought different clinical disciplines together like never before. Entire wings of the hospital were converted into new COVID-19–dedicated wards and intensive care units (ICUs), and teams were built to optimize providers’ skills and capabilities. For example, one third-year pediatric resident was grouped with an outpatient endocrinologist—who had not practiced inpatient medicine in a decade—and a medicine intern. Hospitalists provided crucial support and guidance to these ward teams of deployed providers who were eager and willing to work but often not very knowledgeable about inpatient adult medicine.² In new ad hoc COVID-19 ICUs housed in other ICUs, where most pediatric residents were deployed, critical care attendings and neurointensivists led teams that also included anesthesiology, radiology, and neurosurgery residents, as well as nurses and advanced practice providers trained in various subspecialties of adult medicine.
PEDIATRICIANS IN AN ADULT WORLD
Although we wanted to help in any way we could, the prospect of entering this new world was incredibly daunting. We had not treated adults in several years, and during that time, our clinical experience with pediatric medicine greatly surpassed our adult training from medical school. We dug out materials on adult diseases, watched impromptu lectures on COVID-19 given by our critical care attendings, taught ourselves ventilator management in adults, and reviewed advanced cardiac life support (ACLS) protocols. But putting this all into practice was entirely different. Nothing can truly prepare you for arriving at the bedside of a hemodynamically unstable patient suffering from a virus that no one really understands.
When we arrived and introduced ourselves, we occasionally encountered surprise and curiosity from other providers. We felt that there was a perception that pediatricians do not often take care of critically ill or complex patients. Some of us were reluctant to disclose our specialty, lest it cloud perceptions of our capabilities. However, sick patients awaited us, so we got to work.
There was a steep learning curve over the first few days, from adjusting insulin for type 2 diabetes to troubleshooting renal replacement therapy issues. Accustomed to pediatric weight-based dosing, we were very anxious about ordering medications. The adult providers on our teams oriented us and helped us with many of these concerns. But the mystery of COVID-19 was a great equalizing force, leaving providers of every background with questions: Should we anticoagulate? How about steroids? Could this clinical change be another effect of the virus or a new infection?
We were pleasantly surprised that many aspects of our pediatric training proved beneficial in caring for adults. The focus on family-centered rounds and shared decision-making in pediatrics had imprinted on us the paramount importance of good communication. We were very cognizant of involving loved ones in discussions, now conducted by telephone or video call because infection-prevention guidelines precluded visitors. Family members were thankful for frequent updates, and as a result, largely embraced us as the doctors treating their loved ones. On one occasion, an internist, whose mother was a patient, was delighted to learn that the provider was a pediatric resident, saying, “I know you’ll take such good care of her.”
With the hospital inundated with sick adults, colleagues were grateful for our help. More so, they seemed appreciative of our compassion and ability to maintain a sense of humanity during the pandemonium of the pandemic despite feeling vulnerable, scared, and often powerless against COVID-19. In pediatrics, we do our best to truly engage with patients, from playing games with a 6-year-old with perforated appendicitis to holding and soothing a newborn in the neonatal ICU. We carried those skills over to the adult side. The team appreciated when a pediatric resident, with the help of an occupational therapist, used a letter board to communicate and receive assent for a tracheostomy from a nonsedated, intubated patient, directly answering the patient’s questions and addressing concerns rather than relying solely on a family member’s consent. And, though we had not previously led end-of-life discussions, we found that we were capable of doing so with the compassion instilled in us from our pediatric training. It had prepared us to face the universal challenge of communication in times of grief.
COVID-19 CHALLENGES
Besides grappling with our insecurity in treating adults, we, like all medical providers, had to balance our desire to provide care while keeping ourselves safe from COVID-19. To reduce our risk of exposure and preserve the dwindling supply of personal protective equipment (PPE), the flow of rounding, bedside care, and interventions was adapted to better cluster examinations, blood draws, and bedside tasks. Although efficient, this meant we did not enter rooms as frequently, creating an unfamiliar distance between provider and patient.³ We feared missing moments of clinical decompensation, and for pediatricians who value close patient contact, this made for a deeply uncomfortable reality.
We considered every plausible treatment for critically ill patients, sometimes unsure if they were beneficial or instead complicating the course further. Was lack of improvement a treatment failure or just the natural progression of this new illness? Unfortunately, most of the time, treatments were to no avail. Watching the respiratory, cardiovascular, renal, and neurologic devastation of COVID-19 on so many patients was horrifying. Seeing patients die without their loved ones beside them and at an alarmingly fast rate was simply crushing, as other trainees have similarly described.4 It was unlike anything we had ever experienced in pediatrics. Though we had begun to see a few pediatric COVID-19 patients in the hospital, their disease course was less severe. And, in the rare cases when pediatric patients die, they are almost invariably surrounded by family. One pediatric resident, who had never performed a single death examination before, did three in 1 week. It was emotionally trying, yet we had little time to mourn, as deathbeds were only briefly empty before the next gravely ill patients filled them.
Deployment took a toll on our bodies as well. We padded our faces to alleviate skin breakdown from 12-hour shifts spent entirely in N95 masks. We sanitized and washed our hands constantly, developing cracked skin and dermatitis, and showered meticulously after every shift. We isolated ourselves from our families and loved ones to protect them from the virus.
MOMENTS OF POSITIVITY
Despite these challenges, positive moments emerged. We worked with many wonderful colleagues from different disciplines we likely never would have met, let alone work alongside. We valued each other’s skills, talents, and knowledge. On an overnight shift in one of the ICUs, among the “ragtag team of deployees,” as one pediatric resident phrased it, each presented a topic from his or her respective specialty that might interest others. The pediatrician presented Kawasaki disease, as adult colleagues were beginning to ask questions about its cousin, the emerging multisystem inflammatory syndrome in children (MIS-C). This collegiality promoted a culture of collaboration and respect for other specialties that will hopefully continue.
A strong drive toward teamwork and shared responsibility flourished during deployment. No one was above any task. Residents and even fellows performed typical frontline tasks, such as ordering laboratory work and coordinating imaging. We all helped the proning team turn patients. Everyone shared insights, perspectives, and information gleaned from friends in different wards and hospitals and the ever-evolving literature. As we grappled with unpredictable disease courses, the traditional hierarchical roles of medicine—attending, fellow, resident—often blurred. We felt like we were all in this together.
Patient triumphs were celebrated. We danced with an 80-year-old patient admitted for almost 2 weeks when she was informed of her discharge and gave a standing ovation for a 91-year-old woman as she headed home. Music played over the hospital loudspeaker for every patient discharge. We also tried to create moments of lightheartedness. In the ICUs, we ate donated meals together and posed for pictures to express our gratitude to restaurants. Camaraderie blossomed during deployment.
ADVICE FOR THE FUTURE
Answering the call to help during the COVID-19 surge in New York City indelibly shaped our experiences as trainees and physicians. We will carry with us the lessons that we learned, both in the short term for the possible resurgence of cases and in the long term for ongoing patient care for the rest of our careers. For those residents who may be called upon next, the experience will be challenging, but rewarding. Each trainee has a foundation of knowledge, abilities, and instincts that will be useful, so trust in your training. Do not be afraid to ask questions or for help. You may be leaving your comfort zone, but you will not be alone, and families and other clinicians will be grateful to have you there. You are resilient, and you will make a difference.
Disclosures
The authors have nothing to disclose.
Stepping onto a busy coronavirus disease (COVID-19) unit for the first time can elicit trepidation for any medical provider. For a group of deployed pediatric residents at a New York City hospital in the spring of 2020, it was also the first time caring for adults since medical school. Imagine a pediatrician receiving this handoff: “77-year-old female with a history of diabetes, peripheral vascular disease, and COPD admitted with COVID-19 pneumonia, now intubated and proned with O2 saturations in the 80s. To do: DNR discussion.” General anxiety around COVID-19 was compounded by the discomfort of being thrust into adult medicine. But the doctoring instinct we have been honing throughout training kicked in, and we acted.
A NEW ORDER
As the COVID-19 crisis escalated in New York City, it became evident that staff from other specialties would be essential to manage the surge of patients. Hospital administrators selected a group of trainees for deployment based on their clinical experiences and willingness to volunteer. Almost overnight, a group of senior pediatric residents became adult providers, honoring the oath we each took to “remain a member of society with special obligations to all . . . fellow human beings.”¹
This health crisis brought different clinical disciplines together like never before. Entire wings of the hospital were converted into new COVID-19–dedicated wards and intensive care units (ICUs), and teams were built to optimize providers’ skills and capabilities. For example, one third-year pediatric resident was grouped with an outpatient endocrinologist—who had not practiced inpatient medicine in a decade—and a medicine intern. Hospitalists provided crucial support and guidance to these ward teams of deployed providers who were eager and willing to work but often not very knowledgeable about inpatient adult medicine.² In new ad hoc COVID-19 ICUs housed in other ICUs, where most pediatric residents were deployed, critical care attendings and neurointensivists led teams that also included anesthesiology, radiology, and neurosurgery residents, as well as nurses and advanced practice providers trained in various subspecialties of adult medicine.
PEDIATRICIANS IN AN ADULT WORLD
Although we wanted to help in any way we could, the prospect of entering this new world was incredibly daunting. We had not treated adults in several years, and during that time, our clinical experience with pediatric medicine greatly surpassed our adult training from medical school. We dug out materials on adult diseases, watched impromptu lectures on COVID-19 given by our critical care attendings, taught ourselves ventilator management in adults, and reviewed advanced cardiac life support (ACLS) protocols. But putting this all into practice was entirely different. Nothing can truly prepare you for arriving at the bedside of a hemodynamically unstable patient suffering from a virus that no one really understands.
When we arrived and introduced ourselves, we occasionally encountered surprise and curiosity from other providers. We felt that there was a perception that pediatricians do not often take care of critically ill or complex patients. Some of us were reluctant to disclose our specialty, lest it cloud perceptions of our capabilities. However, sick patients awaited us, so we got to work.
There was a steep learning curve over the first few days, from adjusting insulin for type 2 diabetes to troubleshooting renal replacement therapy issues. Accustomed to pediatric weight-based dosing, we were very anxious about ordering medications. The adult providers on our teams oriented us and helped us with many of these concerns. But the mystery of COVID-19 was a great equalizing force, leaving providers of every background with questions: Should we anticoagulate? How about steroids? Could this clinical change be another effect of the virus or a new infection?
We were pleasantly surprised that many aspects of our pediatric training proved beneficial in caring for adults. The focus on family-centered rounds and shared decision-making in pediatrics had imprinted on us the paramount importance of good communication. We were very cognizant of involving loved ones in discussions, now conducted by telephone or video call because infection-prevention guidelines precluded visitors. Family members were thankful for frequent updates, and as a result, largely embraced us as the doctors treating their loved ones. On one occasion, an internist, whose mother was a patient, was delighted to learn that the provider was a pediatric resident, saying, “I know you’ll take such good care of her.”
With the hospital inundated with sick adults, colleagues were grateful for our help. More so, they seemed appreciative of our compassion and ability to maintain a sense of humanity during the pandemonium of the pandemic despite feeling vulnerable, scared, and often powerless against COVID-19. In pediatrics, we do our best to truly engage with patients, from playing games with a 6-year-old with perforated appendicitis to holding and soothing a newborn in the neonatal ICU. We carried those skills over to the adult side. The team appreciated when a pediatric resident, with the help of an occupational therapist, used a letter board to communicate and receive assent for a tracheostomy from a nonsedated, intubated patient, directly answering the patient’s questions and addressing concerns rather than relying solely on a family member’s consent. And, though we had not previously led end-of-life discussions, we found that we were capable of doing so with the compassion instilled in us from our pediatric training. It had prepared us to face the universal challenge of communication in times of grief.
COVID-19 CHALLENGES
Besides grappling with our insecurity in treating adults, we, like all medical providers, had to balance our desire to provide care while keeping ourselves safe from COVID-19. To reduce our risk of exposure and preserve the dwindling supply of personal protective equipment (PPE), the flow of rounding, bedside care, and interventions was adapted to better cluster examinations, blood draws, and bedside tasks. Although efficient, this meant we did not enter rooms as frequently, creating an unfamiliar distance between provider and patient.³ We feared missing moments of clinical decompensation, and for pediatricians who value close patient contact, this made for a deeply uncomfortable reality.
We considered every plausible treatment for critically ill patients, sometimes unsure if they were beneficial or instead complicating the course further. Was lack of improvement a treatment failure or just the natural progression of this new illness? Unfortunately, most of the time, treatments were to no avail. Watching the respiratory, cardiovascular, renal, and neurologic devastation of COVID-19 on so many patients was horrifying. Seeing patients die without their loved ones beside them and at an alarmingly fast rate was simply crushing, as other trainees have similarly described.4 It was unlike anything we had ever experienced in pediatrics. Though we had begun to see a few pediatric COVID-19 patients in the hospital, their disease course was less severe. And, in the rare cases when pediatric patients die, they are almost invariably surrounded by family. One pediatric resident, who had never performed a single death examination before, did three in 1 week. It was emotionally trying, yet we had little time to mourn, as deathbeds were only briefly empty before the next gravely ill patients filled them.
Deployment took a toll on our bodies as well. We padded our faces to alleviate skin breakdown from 12-hour shifts spent entirely in N95 masks. We sanitized and washed our hands constantly, developing cracked skin and dermatitis, and showered meticulously after every shift. We isolated ourselves from our families and loved ones to protect them from the virus.
MOMENTS OF POSITIVITY
Despite these challenges, positive moments emerged. We worked with many wonderful colleagues from different disciplines we likely never would have met, let alone work alongside. We valued each other’s skills, talents, and knowledge. On an overnight shift in one of the ICUs, among the “ragtag team of deployees,” as one pediatric resident phrased it, each presented a topic from his or her respective specialty that might interest others. The pediatrician presented Kawasaki disease, as adult colleagues were beginning to ask questions about its cousin, the emerging multisystem inflammatory syndrome in children (MIS-C). This collegiality promoted a culture of collaboration and respect for other specialties that will hopefully continue.
A strong drive toward teamwork and shared responsibility flourished during deployment. No one was above any task. Residents and even fellows performed typical frontline tasks, such as ordering laboratory work and coordinating imaging. We all helped the proning team turn patients. Everyone shared insights, perspectives, and information gleaned from friends in different wards and hospitals and the ever-evolving literature. As we grappled with unpredictable disease courses, the traditional hierarchical roles of medicine—attending, fellow, resident—often blurred. We felt like we were all in this together.
Patient triumphs were celebrated. We danced with an 80-year-old patient admitted for almost 2 weeks when she was informed of her discharge and gave a standing ovation for a 91-year-old woman as she headed home. Music played over the hospital loudspeaker for every patient discharge. We also tried to create moments of lightheartedness. In the ICUs, we ate donated meals together and posed for pictures to express our gratitude to restaurants. Camaraderie blossomed during deployment.
ADVICE FOR THE FUTURE
Answering the call to help during the COVID-19 surge in New York City indelibly shaped our experiences as trainees and physicians. We will carry with us the lessons that we learned, both in the short term for the possible resurgence of cases and in the long term for ongoing patient care for the rest of our careers. For those residents who may be called upon next, the experience will be challenging, but rewarding. Each trainee has a foundation of knowledge, abilities, and instincts that will be useful, so trust in your training. Do not be afraid to ask questions or for help. You may be leaving your comfort zone, but you will not be alone, and families and other clinicians will be grateful to have you there. You are resilient, and you will make a difference.
Disclosures
The authors have nothing to disclose.
1. Lasagna L. Hippocratic oath—modern version. Published 1964. Accessed September 14, 2020. http://www.pbs.org/wgbh/nova/doctors/oath_modern.html
2. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314-315.https://doi.org/10.12788/jhm.3426
3. Cunningham CO, Diaz C, Slawek DE. COVID-19: the worst days of our careers. Ann Intern Med. 2020;172(11):764-765. https://doi.org/10.7326/M20-1715
4. Gallagher TH, Schleyer AM. “We signed up for this!”—student and trainee responses to the COVID-19 pandemic. N Engl J Med. 2020;382(25):e96. https://doi.org/10.1056/NEJMp2005234
1. Lasagna L. Hippocratic oath—modern version. Published 1964. Accessed September 14, 2020. http://www.pbs.org/wgbh/nova/doctors/oath_modern.html
2. Cram P, Anderson ML, Shaughnessy EE. All hands on deck: learning to “un-specialize” in the COVID-19 pandemic. J Hosp Med. 2020;15(5):314-315.https://doi.org/10.12788/jhm.3426
3. Cunningham CO, Diaz C, Slawek DE. COVID-19: the worst days of our careers. Ann Intern Med. 2020;172(11):764-765. https://doi.org/10.7326/M20-1715
4. Gallagher TH, Schleyer AM. “We signed up for this!”—student and trainee responses to the COVID-19 pandemic. N Engl J Med. 2020;382(25):e96. https://doi.org/10.1056/NEJMp2005234
© 2020 Society of Hospital Medicine
Professional Identity Formation During the COVID-19 Pandemic
In 1957, Merton wrote that the primary aim of medical education should be “to provide [learners] with a professional identity so that [they] come to think, act, and feel like a physician.”1 More than a half-century later, the Carnegie Foundation for the Advancement of Teaching echoed his sentiments in its landmark examination of the United States medical education system, which produced four key recommendations for curricular reform, including explicitly addressing professional identity formation (PIF).2 PIF is a process by which a learner transforms into a physician with the values, dispositions, and aspirations of the physician community.3 It is now recognized as crucial to developing physicians who can deliver high-quality care.2
Major changes to the learning environment can impact PIF. For example, when the Accreditation Committee for Graduate Medical Education duty-hour restrictions were implemented in 2003, several educators were concerned that the changes may negatively affect resident PIF,4 whereas others saw an opportunity to refocus curricular efforts on PIF.5 Medical education is now in the midst of another radical change with the novel coronavirus disease 2019 (COVID-19) pandemic. Over the past several months, we have begun to understand the pandemic’s effects on medical education in terms of learner welfare, educational experiences/value, innovation, and assessment.6-8 However, little has been published on the pandemic’s effect on PIF.9 We explore the impact of COVID-19 on physicians’ PIF and identify strategies to support PIF in physicians and other healthcare professionals during times of crisis.
SOCIALIZATION AND COMMUNITIES OF PRACTICE
PIF is dynamic and nonlinear, occurring at every level of the medical education hierarchy (medical student, resident, fellow, attending).10 Emphasis on PIF has grown in recent years as a response to the limitations of behavior-based educational frameworks such as competency-based medical education (CBME),3 which focuses on what the learner can “do.” PIF moves beyond “doing” to consider who the learner “is.”11 PIF occurs at the individual level as learners progress through multiple distinct identity stages during their longitudinal formation10,12-14 but also at the level of the collective. Socialization plays a crucial role; thus, PIF is heavily influenced by the environment, context, and other individuals.10
Medicine can be conceptualized as a community of practice, which is a sustaining network of individuals who share knowledge, beliefs, values, and experiences related to a common practice or purpose.15,16 In a community of practice, learning is social, includes knowledge that is tacit to the community, and is situated within the context in which it will be applied. PIF involves learners moving from “legitimate peripheral participation,” whereby they are accepted as novice community members, to “full participation,” which involves gaining competence in relevant tasks and internalizing community principles to become full partners in the community.13 Critical to this process is exposure to socializing agents (eg, attendings, nurses, peers), observation of community interactions, experiential learning in the clinical environment, and access to role models.10,16 Immersion in the clinical environment with other community members is thus crucial to PIF. This is especially important, as “medicine” is not truly a single community, but rather a “landscape of communities,” each with its own identity.17 Learners must therefore be immersed in many different clinical environments to experience the various communities within our field.
COVID-19 CHANGING THE LEARNING ENVIRONMENT
The pandemic is drastically altering the learning environment in medical education.8 Several institutions temporarily removed medical students from clinical rotations to reduce learner exposure and conserve personal protective equipment. Some residents were removed from nonessential clinical activities for similar reasons. Many attendings have been asked to work from home when not required to be present for clinical care duties. Common medical community activities, such as group meals and conferences, have been altered for physical distancing or simply canceled. Usual clinical care has rapidly evolved, with changes in rounding practices, a boon of telehealth, and cancellations of nonessential procedures. These necessary changes present constantly shifting grounds for anyone trying to integrate into a community and develop a professional identity.
Changes outside of the clinical learning environment are also affecting PIF. Social interactions, such as dinners and peer gatherings, occur via video conference or not at all. Most in-person contact happens with masks in place, physically distanced, and in smaller groups. Resident and student lounges are being modified to physically distance or reduce the number of occupants. There is often variable adherence, both intentional and unintentional, to physical distance and mask mandates, creating potential for confusion as learners try to internalize the values and norms of the medical community. Common professional rituals, such as white coat ceremonies, orientation events, and graduations, have been curtailed or canceled. Even experiences that are not commonly seen as social events but are important in the physician’s journey, such as the residency and fellowship application processes and standardized tests, are being transformed. These changes alter typical social patterns that are important in PIF and may adversely affect high-value social group interactions that serve as buffers against stressors during training.18
Finally, the pandemic has altered the timeline for many learners. Medical students at several institutions graduated early to join the workforce and help care for escalating numbers of patients during the pandemic.7 Some see the pandemic as a catalyst to move toward competency-based time-variable training, in which learners progress through training at variable rates depending on their individual performance and learning needs.19 These changes could shorten the amount of time some learners spend in a given role (eg, medical student, intern). In such situations, it is unclear whether a minimal maturational time is necessary for most learners to fully develop a professional identity.
SUPPORTING PIF DURING THE PANDEMIC
In 2019, Cruess et al published general principles for supporting PIF,17 which have been used to support PIF during the COVID-19 pandemic.20 In the Table, we describe these principles and provide examples of how to implement them in the context of the pandemic. We believe these principles are applicable for PIF in undergraduate medical education, graduate medical education, and faculty development programs. A common thread throughout the principles is that PIF is not a process that should be left to chance, but rather explicitly nurtured through systematic support and curricular initiatives.5 This may be challenging while the COVID-19 pandemic is sapping financial resources and requiring rapid changes to clinical systems, but given the central role PIF plays in physician development, it should be prioritized by educational leaders.
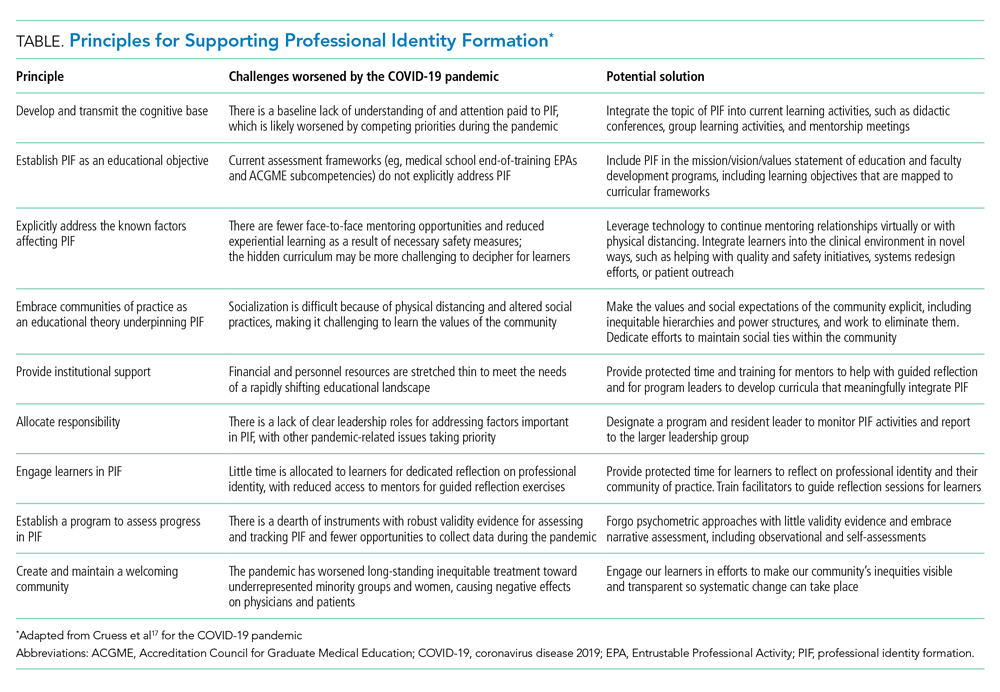
CREATING AND MAINTAINING A WELCOMING COMMUNITY: AN OPPORTUNITY
One of the final principles from Cruess et al is to create and maintain a welcoming community.17 This prompts questions such as: Is our community welcoming to everyone, where “everyone” really does mean everyone? Like other social structures, communities of practice tend to perpetuate existing power structures and inequities.17 It is no secret that medicine, like other professions, is riddled with inequities and bias based on factors such as race, gender, and socioeconomic status.21-23 The COVID-19 pandemic is likely exacerbating these inequities, such as the adverse impacts that are specifically affecting women physicians, who take on a disproportionate share of the child care at home.23 These biases impact not only the members of our professional community but also our patients, contributing to disparities in care and outcomes.
Physicians who have received inequitable treatment have laid bare the ways in which our communities of practice are failing them, and also outlined a better path on which to move forward.21,23 In addition to recruitment practices that promote diversity, meaningful programs should be developed to support inclusion, equity (in recognition, support, compensation), retention, and advancement. The disruption caused by COVID-19 can be a catalyst for this change. By taking this moment of crisis to examine the values and norms of medicine and how we systematically perpetuate harmful inequities and biases, we have an opportunity to deliberately rebuild our community of practice in a manner that helps shape the next generation’s professional identities to be better than we have been. This should always be the aim of education.
1. Merton RK. Some Preliminaries to a Sociology of Medical Education. Harvard University Press; 1957.
2. Cooke M, Irby DM, O’Brien BC. Educating Physicians: A Call for Reform of Medical School and Residency. Jossey-Bass; 2010.
3. Irby DM, Hamstra SJ. Parting the clouds: three professionalism frameworks in medical education. Acad Med. 2016;91(12):1606-1611. https://doi.org/10.1097/ACM.0000000000001190
4. Reed DA, Levine RB, Miller RG, et al. Effect of residency duty-hour limits: views of key clinical faculty. Arch Intern Med. 2007;167(14):1487-1492. https://doi.org/10.1001/archinte.167.14.1487
5. Schumacher DJ, Slovin SR, Riebschleger MP, Englander R, Hicks PJ, Carraccio C. Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883-888. https://doi.org/10.1097/ACM.0b013e318257d57d
6. Anderson ML, Turbow S, Willgerodt MA, Ruhnke GW. Education in a crisis: the opportunity of our lives. J Hosp Med. 2020;15(5):287-291. https://doi.org/10.12788/jhm.3431
7. Kinnear B, Kelleher M, Olson AP, Sall D, Schumacher DJ. Developing trust with early medical school graduates during the COVID-19 pandemic. J Hosp Med. 2020;15(6):367-369. https://doi.org/10.12788/jhm.3463
8. Woolliscroft JO. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402
9. Cullum RJ, Shaughnessy A, Mayat NY, Brown ME. Identity in lockdown: supporting primary care professional identity development in the COVID-19 generation. Educ Prim Care. 2020;31(4):200-204. https://doi.org/10.1080/14739879.2020.1779616
10. Jarvis-Selinger S, Pratt DD, Regehr G. Competency is not enough: integrating identity formation into the medical education discourse. Acad Med. 2012;87(9):1185-1190. https://doi.org/10.1097/ACM.0b013e3182604968
11. Al‐Eraky M, Marei H. A fresh look at Miller’s pyramid: assessment at the ‘Is’ and ‘Do’ levels. Med Educ. 2016;50(12):1253-1257. https://doi.org/10.1111/medu.13101
12. Forsythe GB. Identity development in professional education. Acad Med. 2005;80(10 Suppl):S112-S117. https://doi.org/10.1097/00001888-200510001-0002913.
13. Cruess RL, Cruess SR, Boudreau JD, Snell L, Steinert Y. A schematic representation of the professional identity formation and socialization of medical students and residents: a guide for medical educators. Acad Med. 2015;90(6):718-725. https://doi.org/10.1097/ACM.0000000000000700
14. Kegan R. The Evolving Self: Problem and Process in Human Development. Harvard University Press; 1982.
15. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
16. Lave J, Wenger E. Situated Learning: Legitimate Peripheral Participation. Cambridge University Press; 1991.
17. Cruess SR, Cruess RL, Steinert Y. Supporting the development of a professional identity: general principles. Med Teach. 2019;41(6):641-649. https://doi.org/10.1080/0142159X.2018.1536260
18. Mavor KI, McNeill KG, Anderson K, Kerr A, O’Reilly E, Platow MJ. Beyond prevalence to process: the role of self and identity in medical student well‐being. Med Educ. 2014;48(4):351-360. https://doi.org/10.1111/medu.12375
19. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
20. Stetson GV, Kryzhanovskaya IV, Lomen‐Hoerth C, Hauer KE. Professional identity formation in disorienting times. Med Educ. 2020;54(8):765-766. https://doi.org/10.1111/medu.14202
21. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(9):572-573. https://doi.org/10.12788/jhm.3492
22. Beagan BL. Everyday classism in medical school: experiencing marginality and resistance. Med Educ. 2005;39(8):777-784. https://doi.org/10.1111/j.1365-2929.2005.02225.x
23. Jones Y, Durand V, Morton K, et al. Collateral damage: how COVID-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
In 1957, Merton wrote that the primary aim of medical education should be “to provide [learners] with a professional identity so that [they] come to think, act, and feel like a physician.”1 More than a half-century later, the Carnegie Foundation for the Advancement of Teaching echoed his sentiments in its landmark examination of the United States medical education system, which produced four key recommendations for curricular reform, including explicitly addressing professional identity formation (PIF).2 PIF is a process by which a learner transforms into a physician with the values, dispositions, and aspirations of the physician community.3 It is now recognized as crucial to developing physicians who can deliver high-quality care.2
Major changes to the learning environment can impact PIF. For example, when the Accreditation Committee for Graduate Medical Education duty-hour restrictions were implemented in 2003, several educators were concerned that the changes may negatively affect resident PIF,4 whereas others saw an opportunity to refocus curricular efforts on PIF.5 Medical education is now in the midst of another radical change with the novel coronavirus disease 2019 (COVID-19) pandemic. Over the past several months, we have begun to understand the pandemic’s effects on medical education in terms of learner welfare, educational experiences/value, innovation, and assessment.6-8 However, little has been published on the pandemic’s effect on PIF.9 We explore the impact of COVID-19 on physicians’ PIF and identify strategies to support PIF in physicians and other healthcare professionals during times of crisis.
SOCIALIZATION AND COMMUNITIES OF PRACTICE
PIF is dynamic and nonlinear, occurring at every level of the medical education hierarchy (medical student, resident, fellow, attending).10 Emphasis on PIF has grown in recent years as a response to the limitations of behavior-based educational frameworks such as competency-based medical education (CBME),3 which focuses on what the learner can “do.” PIF moves beyond “doing” to consider who the learner “is.”11 PIF occurs at the individual level as learners progress through multiple distinct identity stages during their longitudinal formation10,12-14 but also at the level of the collective. Socialization plays a crucial role; thus, PIF is heavily influenced by the environment, context, and other individuals.10
Medicine can be conceptualized as a community of practice, which is a sustaining network of individuals who share knowledge, beliefs, values, and experiences related to a common practice or purpose.15,16 In a community of practice, learning is social, includes knowledge that is tacit to the community, and is situated within the context in which it will be applied. PIF involves learners moving from “legitimate peripheral participation,” whereby they are accepted as novice community members, to “full participation,” which involves gaining competence in relevant tasks and internalizing community principles to become full partners in the community.13 Critical to this process is exposure to socializing agents (eg, attendings, nurses, peers), observation of community interactions, experiential learning in the clinical environment, and access to role models.10,16 Immersion in the clinical environment with other community members is thus crucial to PIF. This is especially important, as “medicine” is not truly a single community, but rather a “landscape of communities,” each with its own identity.17 Learners must therefore be immersed in many different clinical environments to experience the various communities within our field.
COVID-19 CHANGING THE LEARNING ENVIRONMENT
The pandemic is drastically altering the learning environment in medical education.8 Several institutions temporarily removed medical students from clinical rotations to reduce learner exposure and conserve personal protective equipment. Some residents were removed from nonessential clinical activities for similar reasons. Many attendings have been asked to work from home when not required to be present for clinical care duties. Common medical community activities, such as group meals and conferences, have been altered for physical distancing or simply canceled. Usual clinical care has rapidly evolved, with changes in rounding practices, a boon of telehealth, and cancellations of nonessential procedures. These necessary changes present constantly shifting grounds for anyone trying to integrate into a community and develop a professional identity.
Changes outside of the clinical learning environment are also affecting PIF. Social interactions, such as dinners and peer gatherings, occur via video conference or not at all. Most in-person contact happens with masks in place, physically distanced, and in smaller groups. Resident and student lounges are being modified to physically distance or reduce the number of occupants. There is often variable adherence, both intentional and unintentional, to physical distance and mask mandates, creating potential for confusion as learners try to internalize the values and norms of the medical community. Common professional rituals, such as white coat ceremonies, orientation events, and graduations, have been curtailed or canceled. Even experiences that are not commonly seen as social events but are important in the physician’s journey, such as the residency and fellowship application processes and standardized tests, are being transformed. These changes alter typical social patterns that are important in PIF and may adversely affect high-value social group interactions that serve as buffers against stressors during training.18
Finally, the pandemic has altered the timeline for many learners. Medical students at several institutions graduated early to join the workforce and help care for escalating numbers of patients during the pandemic.7 Some see the pandemic as a catalyst to move toward competency-based time-variable training, in which learners progress through training at variable rates depending on their individual performance and learning needs.19 These changes could shorten the amount of time some learners spend in a given role (eg, medical student, intern). In such situations, it is unclear whether a minimal maturational time is necessary for most learners to fully develop a professional identity.
SUPPORTING PIF DURING THE PANDEMIC
In 2019, Cruess et al published general principles for supporting PIF,17 which have been used to support PIF during the COVID-19 pandemic.20 In the Table, we describe these principles and provide examples of how to implement them in the context of the pandemic. We believe these principles are applicable for PIF in undergraduate medical education, graduate medical education, and faculty development programs. A common thread throughout the principles is that PIF is not a process that should be left to chance, but rather explicitly nurtured through systematic support and curricular initiatives.5 This may be challenging while the COVID-19 pandemic is sapping financial resources and requiring rapid changes to clinical systems, but given the central role PIF plays in physician development, it should be prioritized by educational leaders.

CREATING AND MAINTAINING A WELCOMING COMMUNITY: AN OPPORTUNITY
One of the final principles from Cruess et al is to create and maintain a welcoming community.17 This prompts questions such as: Is our community welcoming to everyone, where “everyone” really does mean everyone? Like other social structures, communities of practice tend to perpetuate existing power structures and inequities.17 It is no secret that medicine, like other professions, is riddled with inequities and bias based on factors such as race, gender, and socioeconomic status.21-23 The COVID-19 pandemic is likely exacerbating these inequities, such as the adverse impacts that are specifically affecting women physicians, who take on a disproportionate share of the child care at home.23 These biases impact not only the members of our professional community but also our patients, contributing to disparities in care and outcomes.
Physicians who have received inequitable treatment have laid bare the ways in which our communities of practice are failing them, and also outlined a better path on which to move forward.21,23 In addition to recruitment practices that promote diversity, meaningful programs should be developed to support inclusion, equity (in recognition, support, compensation), retention, and advancement. The disruption caused by COVID-19 can be a catalyst for this change. By taking this moment of crisis to examine the values and norms of medicine and how we systematically perpetuate harmful inequities and biases, we have an opportunity to deliberately rebuild our community of practice in a manner that helps shape the next generation’s professional identities to be better than we have been. This should always be the aim of education.
In 1957, Merton wrote that the primary aim of medical education should be “to provide [learners] with a professional identity so that [they] come to think, act, and feel like a physician.”1 More than a half-century later, the Carnegie Foundation for the Advancement of Teaching echoed his sentiments in its landmark examination of the United States medical education system, which produced four key recommendations for curricular reform, including explicitly addressing professional identity formation (PIF).2 PIF is a process by which a learner transforms into a physician with the values, dispositions, and aspirations of the physician community.3 It is now recognized as crucial to developing physicians who can deliver high-quality care.2
Major changes to the learning environment can impact PIF. For example, when the Accreditation Committee for Graduate Medical Education duty-hour restrictions were implemented in 2003, several educators were concerned that the changes may negatively affect resident PIF,4 whereas others saw an opportunity to refocus curricular efforts on PIF.5 Medical education is now in the midst of another radical change with the novel coronavirus disease 2019 (COVID-19) pandemic. Over the past several months, we have begun to understand the pandemic’s effects on medical education in terms of learner welfare, educational experiences/value, innovation, and assessment.6-8 However, little has been published on the pandemic’s effect on PIF.9 We explore the impact of COVID-19 on physicians’ PIF and identify strategies to support PIF in physicians and other healthcare professionals during times of crisis.
SOCIALIZATION AND COMMUNITIES OF PRACTICE
PIF is dynamic and nonlinear, occurring at every level of the medical education hierarchy (medical student, resident, fellow, attending).10 Emphasis on PIF has grown in recent years as a response to the limitations of behavior-based educational frameworks such as competency-based medical education (CBME),3 which focuses on what the learner can “do.” PIF moves beyond “doing” to consider who the learner “is.”11 PIF occurs at the individual level as learners progress through multiple distinct identity stages during their longitudinal formation10,12-14 but also at the level of the collective. Socialization plays a crucial role; thus, PIF is heavily influenced by the environment, context, and other individuals.10
Medicine can be conceptualized as a community of practice, which is a sustaining network of individuals who share knowledge, beliefs, values, and experiences related to a common practice or purpose.15,16 In a community of practice, learning is social, includes knowledge that is tacit to the community, and is situated within the context in which it will be applied. PIF involves learners moving from “legitimate peripheral participation,” whereby they are accepted as novice community members, to “full participation,” which involves gaining competence in relevant tasks and internalizing community principles to become full partners in the community.13 Critical to this process is exposure to socializing agents (eg, attendings, nurses, peers), observation of community interactions, experiential learning in the clinical environment, and access to role models.10,16 Immersion in the clinical environment with other community members is thus crucial to PIF. This is especially important, as “medicine” is not truly a single community, but rather a “landscape of communities,” each with its own identity.17 Learners must therefore be immersed in many different clinical environments to experience the various communities within our field.
COVID-19 CHANGING THE LEARNING ENVIRONMENT
The pandemic is drastically altering the learning environment in medical education.8 Several institutions temporarily removed medical students from clinical rotations to reduce learner exposure and conserve personal protective equipment. Some residents were removed from nonessential clinical activities for similar reasons. Many attendings have been asked to work from home when not required to be present for clinical care duties. Common medical community activities, such as group meals and conferences, have been altered for physical distancing or simply canceled. Usual clinical care has rapidly evolved, with changes in rounding practices, a boon of telehealth, and cancellations of nonessential procedures. These necessary changes present constantly shifting grounds for anyone trying to integrate into a community and develop a professional identity.
Changes outside of the clinical learning environment are also affecting PIF. Social interactions, such as dinners and peer gatherings, occur via video conference or not at all. Most in-person contact happens with masks in place, physically distanced, and in smaller groups. Resident and student lounges are being modified to physically distance or reduce the number of occupants. There is often variable adherence, both intentional and unintentional, to physical distance and mask mandates, creating potential for confusion as learners try to internalize the values and norms of the medical community. Common professional rituals, such as white coat ceremonies, orientation events, and graduations, have been curtailed or canceled. Even experiences that are not commonly seen as social events but are important in the physician’s journey, such as the residency and fellowship application processes and standardized tests, are being transformed. These changes alter typical social patterns that are important in PIF and may adversely affect high-value social group interactions that serve as buffers against stressors during training.18
Finally, the pandemic has altered the timeline for many learners. Medical students at several institutions graduated early to join the workforce and help care for escalating numbers of patients during the pandemic.7 Some see the pandemic as a catalyst to move toward competency-based time-variable training, in which learners progress through training at variable rates depending on their individual performance and learning needs.19 These changes could shorten the amount of time some learners spend in a given role (eg, medical student, intern). In such situations, it is unclear whether a minimal maturational time is necessary for most learners to fully develop a professional identity.
SUPPORTING PIF DURING THE PANDEMIC
In 2019, Cruess et al published general principles for supporting PIF,17 which have been used to support PIF during the COVID-19 pandemic.20 In the Table, we describe these principles and provide examples of how to implement them in the context of the pandemic. We believe these principles are applicable for PIF in undergraduate medical education, graduate medical education, and faculty development programs. A common thread throughout the principles is that PIF is not a process that should be left to chance, but rather explicitly nurtured through systematic support and curricular initiatives.5 This may be challenging while the COVID-19 pandemic is sapping financial resources and requiring rapid changes to clinical systems, but given the central role PIF plays in physician development, it should be prioritized by educational leaders.

CREATING AND MAINTAINING A WELCOMING COMMUNITY: AN OPPORTUNITY
One of the final principles from Cruess et al is to create and maintain a welcoming community.17 This prompts questions such as: Is our community welcoming to everyone, where “everyone” really does mean everyone? Like other social structures, communities of practice tend to perpetuate existing power structures and inequities.17 It is no secret that medicine, like other professions, is riddled with inequities and bias based on factors such as race, gender, and socioeconomic status.21-23 The COVID-19 pandemic is likely exacerbating these inequities, such as the adverse impacts that are specifically affecting women physicians, who take on a disproportionate share of the child care at home.23 These biases impact not only the members of our professional community but also our patients, contributing to disparities in care and outcomes.
Physicians who have received inequitable treatment have laid bare the ways in which our communities of practice are failing them, and also outlined a better path on which to move forward.21,23 In addition to recruitment practices that promote diversity, meaningful programs should be developed to support inclusion, equity (in recognition, support, compensation), retention, and advancement. The disruption caused by COVID-19 can be a catalyst for this change. By taking this moment of crisis to examine the values and norms of medicine and how we systematically perpetuate harmful inequities and biases, we have an opportunity to deliberately rebuild our community of practice in a manner that helps shape the next generation’s professional identities to be better than we have been. This should always be the aim of education.
1. Merton RK. Some Preliminaries to a Sociology of Medical Education. Harvard University Press; 1957.
2. Cooke M, Irby DM, O’Brien BC. Educating Physicians: A Call for Reform of Medical School and Residency. Jossey-Bass; 2010.
3. Irby DM, Hamstra SJ. Parting the clouds: three professionalism frameworks in medical education. Acad Med. 2016;91(12):1606-1611. https://doi.org/10.1097/ACM.0000000000001190
4. Reed DA, Levine RB, Miller RG, et al. Effect of residency duty-hour limits: views of key clinical faculty. Arch Intern Med. 2007;167(14):1487-1492. https://doi.org/10.1001/archinte.167.14.1487
5. Schumacher DJ, Slovin SR, Riebschleger MP, Englander R, Hicks PJ, Carraccio C. Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883-888. https://doi.org/10.1097/ACM.0b013e318257d57d
6. Anderson ML, Turbow S, Willgerodt MA, Ruhnke GW. Education in a crisis: the opportunity of our lives. J Hosp Med. 2020;15(5):287-291. https://doi.org/10.12788/jhm.3431
7. Kinnear B, Kelleher M, Olson AP, Sall D, Schumacher DJ. Developing trust with early medical school graduates during the COVID-19 pandemic. J Hosp Med. 2020;15(6):367-369. https://doi.org/10.12788/jhm.3463
8. Woolliscroft JO. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402
9. Cullum RJ, Shaughnessy A, Mayat NY, Brown ME. Identity in lockdown: supporting primary care professional identity development in the COVID-19 generation. Educ Prim Care. 2020;31(4):200-204. https://doi.org/10.1080/14739879.2020.1779616
10. Jarvis-Selinger S, Pratt DD, Regehr G. Competency is not enough: integrating identity formation into the medical education discourse. Acad Med. 2012;87(9):1185-1190. https://doi.org/10.1097/ACM.0b013e3182604968
11. Al‐Eraky M, Marei H. A fresh look at Miller’s pyramid: assessment at the ‘Is’ and ‘Do’ levels. Med Educ. 2016;50(12):1253-1257. https://doi.org/10.1111/medu.13101
12. Forsythe GB. Identity development in professional education. Acad Med. 2005;80(10 Suppl):S112-S117. https://doi.org/10.1097/00001888-200510001-0002913.
13. Cruess RL, Cruess SR, Boudreau JD, Snell L, Steinert Y. A schematic representation of the professional identity formation and socialization of medical students and residents: a guide for medical educators. Acad Med. 2015;90(6):718-725. https://doi.org/10.1097/ACM.0000000000000700
14. Kegan R. The Evolving Self: Problem and Process in Human Development. Harvard University Press; 1982.
15. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
16. Lave J, Wenger E. Situated Learning: Legitimate Peripheral Participation. Cambridge University Press; 1991.
17. Cruess SR, Cruess RL, Steinert Y. Supporting the development of a professional identity: general principles. Med Teach. 2019;41(6):641-649. https://doi.org/10.1080/0142159X.2018.1536260
18. Mavor KI, McNeill KG, Anderson K, Kerr A, O’Reilly E, Platow MJ. Beyond prevalence to process: the role of self and identity in medical student well‐being. Med Educ. 2014;48(4):351-360. https://doi.org/10.1111/medu.12375
19. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
20. Stetson GV, Kryzhanovskaya IV, Lomen‐Hoerth C, Hauer KE. Professional identity formation in disorienting times. Med Educ. 2020;54(8):765-766. https://doi.org/10.1111/medu.14202
21. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(9):572-573. https://doi.org/10.12788/jhm.3492
22. Beagan BL. Everyday classism in medical school: experiencing marginality and resistance. Med Educ. 2005;39(8):777-784. https://doi.org/10.1111/j.1365-2929.2005.02225.x
23. Jones Y, Durand V, Morton K, et al. Collateral damage: how COVID-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
1. Merton RK. Some Preliminaries to a Sociology of Medical Education. Harvard University Press; 1957.
2. Cooke M, Irby DM, O’Brien BC. Educating Physicians: A Call for Reform of Medical School and Residency. Jossey-Bass; 2010.
3. Irby DM, Hamstra SJ. Parting the clouds: three professionalism frameworks in medical education. Acad Med. 2016;91(12):1606-1611. https://doi.org/10.1097/ACM.0000000000001190
4. Reed DA, Levine RB, Miller RG, et al. Effect of residency duty-hour limits: views of key clinical faculty. Arch Intern Med. 2007;167(14):1487-1492. https://doi.org/10.1001/archinte.167.14.1487
5. Schumacher DJ, Slovin SR, Riebschleger MP, Englander R, Hicks PJ, Carraccio C. Perspective: beyond counting hours: the importance of supervision, professionalism, transitions of care, and workload in residency training. Acad Med. 2012;87(7):883-888. https://doi.org/10.1097/ACM.0b013e318257d57d
6. Anderson ML, Turbow S, Willgerodt MA, Ruhnke GW. Education in a crisis: the opportunity of our lives. J Hosp Med. 2020;15(5):287-291. https://doi.org/10.12788/jhm.3431
7. Kinnear B, Kelleher M, Olson AP, Sall D, Schumacher DJ. Developing trust with early medical school graduates during the COVID-19 pandemic. J Hosp Med. 2020;15(6):367-369. https://doi.org/10.12788/jhm.3463
8. Woolliscroft JO. Innovation in response to the COVID-19 pandemic crisis. Acad Med. 2020;95(8):1140-1142. https://doi.org/10.1097/ACM.0000000000003402
9. Cullum RJ, Shaughnessy A, Mayat NY, Brown ME. Identity in lockdown: supporting primary care professional identity development in the COVID-19 generation. Educ Prim Care. 2020;31(4):200-204. https://doi.org/10.1080/14739879.2020.1779616
10. Jarvis-Selinger S, Pratt DD, Regehr G. Competency is not enough: integrating identity formation into the medical education discourse. Acad Med. 2012;87(9):1185-1190. https://doi.org/10.1097/ACM.0b013e3182604968
11. Al‐Eraky M, Marei H. A fresh look at Miller’s pyramid: assessment at the ‘Is’ and ‘Do’ levels. Med Educ. 2016;50(12):1253-1257. https://doi.org/10.1111/medu.13101
12. Forsythe GB. Identity development in professional education. Acad Med. 2005;80(10 Suppl):S112-S117. https://doi.org/10.1097/00001888-200510001-0002913.
13. Cruess RL, Cruess SR, Boudreau JD, Snell L, Steinert Y. A schematic representation of the professional identity formation and socialization of medical students and residents: a guide for medical educators. Acad Med. 2015;90(6):718-725. https://doi.org/10.1097/ACM.0000000000000700
14. Kegan R. The Evolving Self: Problem and Process in Human Development. Harvard University Press; 1982.
15. Cruess RL, Cruess SR, Steinert Y. Medicine as a community of practice: implications for medical education. Acad Med. 2018;93(2):185-191. https://doi.org/10.1097/ACM.0000000000001826
16. Lave J, Wenger E. Situated Learning: Legitimate Peripheral Participation. Cambridge University Press; 1991.
17. Cruess SR, Cruess RL, Steinert Y. Supporting the development of a professional identity: general principles. Med Teach. 2019;41(6):641-649. https://doi.org/10.1080/0142159X.2018.1536260
18. Mavor KI, McNeill KG, Anderson K, Kerr A, O’Reilly E, Platow MJ. Beyond prevalence to process: the role of self and identity in medical student well‐being. Med Educ. 2014;48(4):351-360. https://doi.org/10.1111/medu.12375
19. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
20. Stetson GV, Kryzhanovskaya IV, Lomen‐Hoerth C, Hauer KE. Professional identity formation in disorienting times. Med Educ. 2020;54(8):765-766. https://doi.org/10.1111/medu.14202
21. Unaka NI, Reynolds KL. Truth in tension: reflections on racism in medicine. J Hosp Med. 2020;15(9):572-573. https://doi.org/10.12788/jhm.3492
22. Beagan BL. Everyday classism in medical school: experiencing marginality and resistance. Med Educ. 2005;39(8):777-784. https://doi.org/10.1111/j.1365-2929.2005.02225.x
23. Jones Y, Durand V, Morton K, et al. Collateral damage: how COVID-19 is adversely impacting women physicians. J Hosp Med. 2020;15(8):507-509. https://doi.org/10.12788/jhm.3470
© 2021 Society of Hospital Medicine
Creating Psychological Safety on Medical Teams in Times of Crisis
Hospitalized patients receive care via a team-based approach. Because of frequent turnover and constant changes in team members, medical teams require rapid establishment of psychological safety. Psychological safety, or “being able to show and employ one’s self without fear of negative consequences of self-image, status or career,”1 is at the core of successful team functioning. Google studied successful teams and found diverse personalities and skillsets work together most productively if they incorporate certain team dynamics; chief among these are focusing on shared values and psychological safety.2 Times of acute crisis, especially those in which clinicians are working in unfamiliar settings and with new teams, increase the need for psychological safety. During the first wave of the coronavirus disease 2019 (COVID-19) pandemic, many hospitals responded by forming ad hoc teams of non-hospitalist clinicians, including redeployed outpatient physicians and subspecialists.3 Because this situation was an acute crisis in which strangers (some new to the field) were suddenly working side by side, it was an excellent example of a moment that required rapid establishment of psychological safety. As subsequent waves of COVID-19 arrive, this will likely occur again. In this perspective, we identify strategies that help to establish psychological safety on medical teams, aiming to increase the effectiveness of teams caring for hospitalized patients, enhance leaders’ abilities to improve team function, and allow for delivery of high-quality patient care.
WHY IS PSYCHOLOGICAL SAFETY IMPORTANT?
Psychological safety creates a nonthreatening team environment in which clinicians can ask questions and seek help with unfamiliar clinical scenarios. When psychological safety is present, the team dynamic encourages interpersonal risk-taking, improves learning, and increases the likelihood that team members will suggest new ideas.4 A culture of openness where people feel accepted and respected plays a vital role in helping people thrive in challenging and high-stakes work environments.5 In healthcare, team members who do not fear punishment for mistakes are more likely to disclose errors.6 Psychological safety has been associated with decreased anxiety in stressful situations, thereby freeing learners’ mental capacity to explore, innovate, and absorb new information.6
STRATEGIES TO IMPLEMENT PSYCHOLOGICAL SAFETY
Through a thorough literature review, we identified strategies that can increase psychological safety on clinical teams. We focused on strategies applicable to acute crises, like COVID-19, when dynamic teams and uncertainty are rife. These strategies primarily focus on “team leaders,” generally the attendings or senior residents, who influence the team’s culture.
Discuss Mistakes
Creating a culture in which openly discussing mistakes is normalized and learning is fostered is especially important for healthcare providers redeployed to COVID-19 wards. Acknowledging errors can be challenging, especially in medicine, because success is often celebrated.7 Creating an environment where discussing mistakes in a nonjudgmental manner is the norm helps people disclose and learn from errors. By modeling fallibility, team leaders can create an environment where learning from mistakes seems less threatening.8 Leaders can say, “I may miss something. I encourage all members of the team to share what they know.”9
Provide Frequent Updates and Seek Feedback
Information and guidelines are changing frequently as we learn more about the novel coronavirus. The barrage of new information and periodic policy changes can be disconcerting. Leaders can dispel some of the team’s anxiety by providing a unified message that distills new information into clear and essential updates.9 They can reassure the team that updates will be provided frequently, be honest about what is known, and offer some predictability by providing updates at set times via consistent forms of communication during times of crisis. They can show empathy by inquiring about individual worries and responding to concerns about changes that are being made in response to COVID-19.10 Leaders should routinely seek feedback. They can ask, “How are things going for you? What can we improve? What should we do differently? How can I make you feel more comfortable or help you learn more effectively?” Inviting input communicates that everyone’s opinion is respected and creates a climate where everyone feels comfortable asking questions or respectfully expressing diverging opinions.9
Foster Creativity and Seek New Ideas
Curiosity and creativity are associated with better group outcomes.11 Curiosity, or the motivation to learn and seek new ideas, improves individual and group dynamics by stimulating better job performance, inspiring leaders to discover more creative solutions, and encouraging employees to develop more trusting relationships, which makes them less likely to stereotype coworkers and patients as they ask questions and learn about others.12 People who approach situations with a more creative perspective are less likely to react defensively. Curious people tend to try to learn about and understand different points of view.13 For example, if an order is not placed for a patient, a curious team leader might think about why—was there disagreement on the order, confusion on how to place it, or was it inadvertently forgotten?—and be able help avoid similar scenarios in the future. This ability to see things from another person’s point of view helps individuals with diverse clinical, social, and ethnic backgrounds function as a harmonious team. An attitude of curiosity and interest in learning about what each person can contribute based on their unique training and personality will help well-functioning teams form in response to COVID-19. Openness to new ideas allows for more innovative solutions, which are important in times of crisis. To promote curiosity, team members should discuss differences and varied opinions openly. This open dialogue provides individuals opportunities to learn from each other.5
Build Connection and Trust
A culture of trust—the belief that others will act for the good of the team—helps create psychological safety.10 Leaders can build trust by making expectations clear, being consistent, being inclusive, and modeling behaviors they wish to encourage.6 Predictability reduces anxiety and promotes psychological safety.9 Defining goals and expectations helps people relax, ask questions, and focus on learning.14 In times of crisis, leaders can tell teams what changes to expect, spell out new priorities, and assign specific tasks to give people a way to contribute.15
Activities that create connection also build trust, enhancing the team’s sense of psychological safety. Shared experiences foster connections.2 Leaders can encourage team bonding by setting aside time to share stories and coping strategies.10 Chief residents in the early days of the pandemic found defining social distancing only as physical separation and focusing on emotional bonds helped maintain a sense of community. Debriefing about emotional patient encounters and discussing interesting clinical cases during video calls were ways to implement this strategy.10 Team members feel connected to each other and dedicated to their work if they focus on the meaning of the work to them, as well as its impact on society.2 This shared belief that what they are doing matters to their community helps bond them.2 Currently, the shared experience of treating a novel illness during the COVID-19 pandemic and the common goal of patient well-being unites healthcare providers across the globe. Leaders can create solidarity by emphasizing the shared identity of fighting COVID-19 and reminding teams of the impact of their work. Leaders can say, “Remember, we are here to improve patients’ health and form emotional bonds with people and their families.” These reminders have been shown to promote psychological safety and connection.16
Make Team Members Feel Valued
As many healthcare providers work harder or in unfamiliar environments during this pandemic, recognition of their efforts by leaders can be especially motivating and meaningful. When individuals on a team feel their work is valued, it helps create a sense of psychological safety.17 Employees feel valued when they believe their leaders care, they are in socially supportive environments, and are given resources for professional growth.17 Diversity and inclusion are also associated with feeling valued.17 During the height of the initial COVID-19 surge at our hospital, the chief of the Department of Medicine regularly sent messages and photographs of trainees and faculty, showing teams coming together during these unprecedented times. This boosted morale and created comradery and is an excellent example of a leader modeling inclusion.
Gratitude strengthens relationships and motivates people, especially when its expression is thoughtful and unique to individuals.18 Sincere compliments, acknowledgement of hard work, inclusiveness, and gratitude all contribute to team members feeling at ease and are key to leading, especially in times of crisis.17 Leaders can provide motivation by affirming the team’s ability to work together. Leaders can say, “I believe in each and every one of your capabilities—and I believe even more so in our joint capabilities. We can do this together.”19
CONCLUSION
Psychological safety is a powerful predictor of team performance, increased engagement, and satisfaction. It is critical for creating teams that can deal with uncertainty in high-risk situations, promoting a culture that is safe to acknowledge mistakes and take chances, which is important for optimal team functioning. Crises like the COVID-19 pandemic emphasize the need for psychologically safe team climates to promote learning, safe patient care, and team support. Hospitalists often care for patients when they are at their most vulnerable. Respecting and connecting with patients, through good and efficient teamwork, is important to providing effective care. The strategies suggested in this article strive to help hospitalists create a respectful culture to strengthen relationships with patients and colleagues in order to create an inclusive environment in times of crisis.
1. Kahn WA. Psychological conditions of personal engagement and disengagement at work. Acad Manage J. 1990;33(4):692-724. https://doi.org/10.5465/256287
2. Rozovsky J. The five keys to a successful Google team. re:Work. Posted November 17, 2015. Accessed October 11, 2020. https://rework.withgoogle.com/blog/five-keys-to-a-successful-google-team/
3. Hettle D, Sutherland K, Miles E, et al. Cross-skilling training to support medical redeployment in the COVID-19 pandemic. Future Healthc J. 2020:fhj.2020-0049. https://doi.org/10.7861/fhj.2020-0049
4. Edmondson AC, Lei Z. Psychological safety: the history, renaissance, and future of an interpersonal construct. Annu Rev Organ Psychol Organ Behav. 2014;1(1):23-43. https://doi.org/10.1146/annurev-orgpsych-031413-091305
5. Edmondson A. Psychological safety and learning behavior in work teams. Admin Sci Quart.1999;44(2):350-383. https://doi.org/10.2307/2666999
6. Turner S, Harder N. Psychological safe environment: a concept analysis. Clin Simul Nurs. 2018;18:47-55. https://doi.org/10.1016/j.ecns.2018.02.004
7. Jug R, Jiang XS, Bean SM. Giving and receiving effective feedback: a review article and how-to guide. Arch Pathol Lab Med. 2019;143(2):244-250. https://doi.org/10.5858/arpa.2018-0058-RA
8. Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781. https://doi.org/10.1001/jama.1983.03340060055026
9. Edmondson AC, Woolley AW. Understanding outcomes of organizational learning interventions. In: Easterby-Smith M, Lyles M, eds. Blackwell Handbook of Organizational Learning and Knowledge Management. Blackwell Publishing; 2003.
10. Rakowsky S, Flashner BM, Doolin J, et al. Five questions for residency leadership in the time of COVID-19: reflections of chief medical residents from an internal medicine program. Acad Med. 2020;95(8):1152-1154. https://doi.org/10.1097/ACM.0000000000003419
11. Armstrong K. If you can’t beat it, join it: uncertainty and trust in medicine. Ann Intern Med. 2018;168(11):818-819. https://doi.org/10.7326/M18-0445
12. Gino F. The business case for curiosity. Harvard Bus Rev. 2018;96(5):48-57.
13. Kashdan TB, DeWall CN, Pond RS, et al. Curiosity protects against interpersonal aggression: cross-sectional, daily process, and behavioral evidence. J Pers. 2013;81(1):87-102. https://doi.org/10.1111/j.1467-6494.2012.00783.x
14. Epstein RM, Krasner MS. Physician resilience: what it means, why it matters, and how to promote it. Acad Med. 2013;88(3):301-303. https://doi.org/10.1097/ACM.0b013e318280cff0
15. Petriglieri G. The psychology behind effective crisis leadership. Harvard Bus Rev. Published April 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/the-psychology-behind-effective-crisis-leadership
16. Edmondson A. Building a psychologically safe workplace: TEDx Talk. May 4, 2014. Accessed October 11, 2020. https://youtube.com/watch?v=LhoLuui9gX8
17. Simpkin AL, Chang Y, Yu L, Campbell EG, Armstrong K, Walensky RP. Assessment of job satisfaction and feeling valued in academic medicine. JAMA Intern Med. 2019;179(7):992-994. https://doi.org/10.1001/jamainternmed.2019.0377
18. Nawaz S. In times of crisis, a little thanks goes a long way. Harvard Bus Rev. Published May 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/05/in-times-of-crisis-a-little-thanks-goes-a-long-way
19. Knight R. How to talk to your team when the future is uncertain. Harvard Bus Rev. Published April 20, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/how-to-talk-to-your-team-when-the-future-is-uncertain
Hospitalized patients receive care via a team-based approach. Because of frequent turnover and constant changes in team members, medical teams require rapid establishment of psychological safety. Psychological safety, or “being able to show and employ one’s self without fear of negative consequences of self-image, status or career,”1 is at the core of successful team functioning. Google studied successful teams and found diverse personalities and skillsets work together most productively if they incorporate certain team dynamics; chief among these are focusing on shared values and psychological safety.2 Times of acute crisis, especially those in which clinicians are working in unfamiliar settings and with new teams, increase the need for psychological safety. During the first wave of the coronavirus disease 2019 (COVID-19) pandemic, many hospitals responded by forming ad hoc teams of non-hospitalist clinicians, including redeployed outpatient physicians and subspecialists.3 Because this situation was an acute crisis in which strangers (some new to the field) were suddenly working side by side, it was an excellent example of a moment that required rapid establishment of psychological safety. As subsequent waves of COVID-19 arrive, this will likely occur again. In this perspective, we identify strategies that help to establish psychological safety on medical teams, aiming to increase the effectiveness of teams caring for hospitalized patients, enhance leaders’ abilities to improve team function, and allow for delivery of high-quality patient care.
WHY IS PSYCHOLOGICAL SAFETY IMPORTANT?
Psychological safety creates a nonthreatening team environment in which clinicians can ask questions and seek help with unfamiliar clinical scenarios. When psychological safety is present, the team dynamic encourages interpersonal risk-taking, improves learning, and increases the likelihood that team members will suggest new ideas.4 A culture of openness where people feel accepted and respected plays a vital role in helping people thrive in challenging and high-stakes work environments.5 In healthcare, team members who do not fear punishment for mistakes are more likely to disclose errors.6 Psychological safety has been associated with decreased anxiety in stressful situations, thereby freeing learners’ mental capacity to explore, innovate, and absorb new information.6
STRATEGIES TO IMPLEMENT PSYCHOLOGICAL SAFETY
Through a thorough literature review, we identified strategies that can increase psychological safety on clinical teams. We focused on strategies applicable to acute crises, like COVID-19, when dynamic teams and uncertainty are rife. These strategies primarily focus on “team leaders,” generally the attendings or senior residents, who influence the team’s culture.
Discuss Mistakes
Creating a culture in which openly discussing mistakes is normalized and learning is fostered is especially important for healthcare providers redeployed to COVID-19 wards. Acknowledging errors can be challenging, especially in medicine, because success is often celebrated.7 Creating an environment where discussing mistakes in a nonjudgmental manner is the norm helps people disclose and learn from errors. By modeling fallibility, team leaders can create an environment where learning from mistakes seems less threatening.8 Leaders can say, “I may miss something. I encourage all members of the team to share what they know.”9
Provide Frequent Updates and Seek Feedback
Information and guidelines are changing frequently as we learn more about the novel coronavirus. The barrage of new information and periodic policy changes can be disconcerting. Leaders can dispel some of the team’s anxiety by providing a unified message that distills new information into clear and essential updates.9 They can reassure the team that updates will be provided frequently, be honest about what is known, and offer some predictability by providing updates at set times via consistent forms of communication during times of crisis. They can show empathy by inquiring about individual worries and responding to concerns about changes that are being made in response to COVID-19.10 Leaders should routinely seek feedback. They can ask, “How are things going for you? What can we improve? What should we do differently? How can I make you feel more comfortable or help you learn more effectively?” Inviting input communicates that everyone’s opinion is respected and creates a climate where everyone feels comfortable asking questions or respectfully expressing diverging opinions.9
Foster Creativity and Seek New Ideas
Curiosity and creativity are associated with better group outcomes.11 Curiosity, or the motivation to learn and seek new ideas, improves individual and group dynamics by stimulating better job performance, inspiring leaders to discover more creative solutions, and encouraging employees to develop more trusting relationships, which makes them less likely to stereotype coworkers and patients as they ask questions and learn about others.12 People who approach situations with a more creative perspective are less likely to react defensively. Curious people tend to try to learn about and understand different points of view.13 For example, if an order is not placed for a patient, a curious team leader might think about why—was there disagreement on the order, confusion on how to place it, or was it inadvertently forgotten?—and be able help avoid similar scenarios in the future. This ability to see things from another person’s point of view helps individuals with diverse clinical, social, and ethnic backgrounds function as a harmonious team. An attitude of curiosity and interest in learning about what each person can contribute based on their unique training and personality will help well-functioning teams form in response to COVID-19. Openness to new ideas allows for more innovative solutions, which are important in times of crisis. To promote curiosity, team members should discuss differences and varied opinions openly. This open dialogue provides individuals opportunities to learn from each other.5
Build Connection and Trust
A culture of trust—the belief that others will act for the good of the team—helps create psychological safety.10 Leaders can build trust by making expectations clear, being consistent, being inclusive, and modeling behaviors they wish to encourage.6 Predictability reduces anxiety and promotes psychological safety.9 Defining goals and expectations helps people relax, ask questions, and focus on learning.14 In times of crisis, leaders can tell teams what changes to expect, spell out new priorities, and assign specific tasks to give people a way to contribute.15
Activities that create connection also build trust, enhancing the team’s sense of psychological safety. Shared experiences foster connections.2 Leaders can encourage team bonding by setting aside time to share stories and coping strategies.10 Chief residents in the early days of the pandemic found defining social distancing only as physical separation and focusing on emotional bonds helped maintain a sense of community. Debriefing about emotional patient encounters and discussing interesting clinical cases during video calls were ways to implement this strategy.10 Team members feel connected to each other and dedicated to their work if they focus on the meaning of the work to them, as well as its impact on society.2 This shared belief that what they are doing matters to their community helps bond them.2 Currently, the shared experience of treating a novel illness during the COVID-19 pandemic and the common goal of patient well-being unites healthcare providers across the globe. Leaders can create solidarity by emphasizing the shared identity of fighting COVID-19 and reminding teams of the impact of their work. Leaders can say, “Remember, we are here to improve patients’ health and form emotional bonds with people and their families.” These reminders have been shown to promote psychological safety and connection.16
Make Team Members Feel Valued
As many healthcare providers work harder or in unfamiliar environments during this pandemic, recognition of their efforts by leaders can be especially motivating and meaningful. When individuals on a team feel their work is valued, it helps create a sense of psychological safety.17 Employees feel valued when they believe their leaders care, they are in socially supportive environments, and are given resources for professional growth.17 Diversity and inclusion are also associated with feeling valued.17 During the height of the initial COVID-19 surge at our hospital, the chief of the Department of Medicine regularly sent messages and photographs of trainees and faculty, showing teams coming together during these unprecedented times. This boosted morale and created comradery and is an excellent example of a leader modeling inclusion.
Gratitude strengthens relationships and motivates people, especially when its expression is thoughtful and unique to individuals.18 Sincere compliments, acknowledgement of hard work, inclusiveness, and gratitude all contribute to team members feeling at ease and are key to leading, especially in times of crisis.17 Leaders can provide motivation by affirming the team’s ability to work together. Leaders can say, “I believe in each and every one of your capabilities—and I believe even more so in our joint capabilities. We can do this together.”19
CONCLUSION
Psychological safety is a powerful predictor of team performance, increased engagement, and satisfaction. It is critical for creating teams that can deal with uncertainty in high-risk situations, promoting a culture that is safe to acknowledge mistakes and take chances, which is important for optimal team functioning. Crises like the COVID-19 pandemic emphasize the need for psychologically safe team climates to promote learning, safe patient care, and team support. Hospitalists often care for patients when they are at their most vulnerable. Respecting and connecting with patients, through good and efficient teamwork, is important to providing effective care. The strategies suggested in this article strive to help hospitalists create a respectful culture to strengthen relationships with patients and colleagues in order to create an inclusive environment in times of crisis.
Hospitalized patients receive care via a team-based approach. Because of frequent turnover and constant changes in team members, medical teams require rapid establishment of psychological safety. Psychological safety, or “being able to show and employ one’s self without fear of negative consequences of self-image, status or career,”1 is at the core of successful team functioning. Google studied successful teams and found diverse personalities and skillsets work together most productively if they incorporate certain team dynamics; chief among these are focusing on shared values and psychological safety.2 Times of acute crisis, especially those in which clinicians are working in unfamiliar settings and with new teams, increase the need for psychological safety. During the first wave of the coronavirus disease 2019 (COVID-19) pandemic, many hospitals responded by forming ad hoc teams of non-hospitalist clinicians, including redeployed outpatient physicians and subspecialists.3 Because this situation was an acute crisis in which strangers (some new to the field) were suddenly working side by side, it was an excellent example of a moment that required rapid establishment of psychological safety. As subsequent waves of COVID-19 arrive, this will likely occur again. In this perspective, we identify strategies that help to establish psychological safety on medical teams, aiming to increase the effectiveness of teams caring for hospitalized patients, enhance leaders’ abilities to improve team function, and allow for delivery of high-quality patient care.
WHY IS PSYCHOLOGICAL SAFETY IMPORTANT?
Psychological safety creates a nonthreatening team environment in which clinicians can ask questions and seek help with unfamiliar clinical scenarios. When psychological safety is present, the team dynamic encourages interpersonal risk-taking, improves learning, and increases the likelihood that team members will suggest new ideas.4 A culture of openness where people feel accepted and respected plays a vital role in helping people thrive in challenging and high-stakes work environments.5 In healthcare, team members who do not fear punishment for mistakes are more likely to disclose errors.6 Psychological safety has been associated with decreased anxiety in stressful situations, thereby freeing learners’ mental capacity to explore, innovate, and absorb new information.6
STRATEGIES TO IMPLEMENT PSYCHOLOGICAL SAFETY
Through a thorough literature review, we identified strategies that can increase psychological safety on clinical teams. We focused on strategies applicable to acute crises, like COVID-19, when dynamic teams and uncertainty are rife. These strategies primarily focus on “team leaders,” generally the attendings or senior residents, who influence the team’s culture.
Discuss Mistakes
Creating a culture in which openly discussing mistakes is normalized and learning is fostered is especially important for healthcare providers redeployed to COVID-19 wards. Acknowledging errors can be challenging, especially in medicine, because success is often celebrated.7 Creating an environment where discussing mistakes in a nonjudgmental manner is the norm helps people disclose and learn from errors. By modeling fallibility, team leaders can create an environment where learning from mistakes seems less threatening.8 Leaders can say, “I may miss something. I encourage all members of the team to share what they know.”9
Provide Frequent Updates and Seek Feedback
Information and guidelines are changing frequently as we learn more about the novel coronavirus. The barrage of new information and periodic policy changes can be disconcerting. Leaders can dispel some of the team’s anxiety by providing a unified message that distills new information into clear and essential updates.9 They can reassure the team that updates will be provided frequently, be honest about what is known, and offer some predictability by providing updates at set times via consistent forms of communication during times of crisis. They can show empathy by inquiring about individual worries and responding to concerns about changes that are being made in response to COVID-19.10 Leaders should routinely seek feedback. They can ask, “How are things going for you? What can we improve? What should we do differently? How can I make you feel more comfortable or help you learn more effectively?” Inviting input communicates that everyone’s opinion is respected and creates a climate where everyone feels comfortable asking questions or respectfully expressing diverging opinions.9
Foster Creativity and Seek New Ideas
Curiosity and creativity are associated with better group outcomes.11 Curiosity, or the motivation to learn and seek new ideas, improves individual and group dynamics by stimulating better job performance, inspiring leaders to discover more creative solutions, and encouraging employees to develop more trusting relationships, which makes them less likely to stereotype coworkers and patients as they ask questions and learn about others.12 People who approach situations with a more creative perspective are less likely to react defensively. Curious people tend to try to learn about and understand different points of view.13 For example, if an order is not placed for a patient, a curious team leader might think about why—was there disagreement on the order, confusion on how to place it, or was it inadvertently forgotten?—and be able help avoid similar scenarios in the future. This ability to see things from another person’s point of view helps individuals with diverse clinical, social, and ethnic backgrounds function as a harmonious team. An attitude of curiosity and interest in learning about what each person can contribute based on their unique training and personality will help well-functioning teams form in response to COVID-19. Openness to new ideas allows for more innovative solutions, which are important in times of crisis. To promote curiosity, team members should discuss differences and varied opinions openly. This open dialogue provides individuals opportunities to learn from each other.5
Build Connection and Trust
A culture of trust—the belief that others will act for the good of the team—helps create psychological safety.10 Leaders can build trust by making expectations clear, being consistent, being inclusive, and modeling behaviors they wish to encourage.6 Predictability reduces anxiety and promotes psychological safety.9 Defining goals and expectations helps people relax, ask questions, and focus on learning.14 In times of crisis, leaders can tell teams what changes to expect, spell out new priorities, and assign specific tasks to give people a way to contribute.15
Activities that create connection also build trust, enhancing the team’s sense of psychological safety. Shared experiences foster connections.2 Leaders can encourage team bonding by setting aside time to share stories and coping strategies.10 Chief residents in the early days of the pandemic found defining social distancing only as physical separation and focusing on emotional bonds helped maintain a sense of community. Debriefing about emotional patient encounters and discussing interesting clinical cases during video calls were ways to implement this strategy.10 Team members feel connected to each other and dedicated to their work if they focus on the meaning of the work to them, as well as its impact on society.2 This shared belief that what they are doing matters to their community helps bond them.2 Currently, the shared experience of treating a novel illness during the COVID-19 pandemic and the common goal of patient well-being unites healthcare providers across the globe. Leaders can create solidarity by emphasizing the shared identity of fighting COVID-19 and reminding teams of the impact of their work. Leaders can say, “Remember, we are here to improve patients’ health and form emotional bonds with people and their families.” These reminders have been shown to promote psychological safety and connection.16
Make Team Members Feel Valued
As many healthcare providers work harder or in unfamiliar environments during this pandemic, recognition of their efforts by leaders can be especially motivating and meaningful. When individuals on a team feel their work is valued, it helps create a sense of psychological safety.17 Employees feel valued when they believe their leaders care, they are in socially supportive environments, and are given resources for professional growth.17 Diversity and inclusion are also associated with feeling valued.17 During the height of the initial COVID-19 surge at our hospital, the chief of the Department of Medicine regularly sent messages and photographs of trainees and faculty, showing teams coming together during these unprecedented times. This boosted morale and created comradery and is an excellent example of a leader modeling inclusion.
Gratitude strengthens relationships and motivates people, especially when its expression is thoughtful and unique to individuals.18 Sincere compliments, acknowledgement of hard work, inclusiveness, and gratitude all contribute to team members feeling at ease and are key to leading, especially in times of crisis.17 Leaders can provide motivation by affirming the team’s ability to work together. Leaders can say, “I believe in each and every one of your capabilities—and I believe even more so in our joint capabilities. We can do this together.”19
CONCLUSION
Psychological safety is a powerful predictor of team performance, increased engagement, and satisfaction. It is critical for creating teams that can deal with uncertainty in high-risk situations, promoting a culture that is safe to acknowledge mistakes and take chances, which is important for optimal team functioning. Crises like the COVID-19 pandemic emphasize the need for psychologically safe team climates to promote learning, safe patient care, and team support. Hospitalists often care for patients when they are at their most vulnerable. Respecting and connecting with patients, through good and efficient teamwork, is important to providing effective care. The strategies suggested in this article strive to help hospitalists create a respectful culture to strengthen relationships with patients and colleagues in order to create an inclusive environment in times of crisis.
1. Kahn WA. Psychological conditions of personal engagement and disengagement at work. Acad Manage J. 1990;33(4):692-724. https://doi.org/10.5465/256287
2. Rozovsky J. The five keys to a successful Google team. re:Work. Posted November 17, 2015. Accessed October 11, 2020. https://rework.withgoogle.com/blog/five-keys-to-a-successful-google-team/
3. Hettle D, Sutherland K, Miles E, et al. Cross-skilling training to support medical redeployment in the COVID-19 pandemic. Future Healthc J. 2020:fhj.2020-0049. https://doi.org/10.7861/fhj.2020-0049
4. Edmondson AC, Lei Z. Psychological safety: the history, renaissance, and future of an interpersonal construct. Annu Rev Organ Psychol Organ Behav. 2014;1(1):23-43. https://doi.org/10.1146/annurev-orgpsych-031413-091305
5. Edmondson A. Psychological safety and learning behavior in work teams. Admin Sci Quart.1999;44(2):350-383. https://doi.org/10.2307/2666999
6. Turner S, Harder N. Psychological safe environment: a concept analysis. Clin Simul Nurs. 2018;18:47-55. https://doi.org/10.1016/j.ecns.2018.02.004
7. Jug R, Jiang XS, Bean SM. Giving and receiving effective feedback: a review article and how-to guide. Arch Pathol Lab Med. 2019;143(2):244-250. https://doi.org/10.5858/arpa.2018-0058-RA
8. Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781. https://doi.org/10.1001/jama.1983.03340060055026
9. Edmondson AC, Woolley AW. Understanding outcomes of organizational learning interventions. In: Easterby-Smith M, Lyles M, eds. Blackwell Handbook of Organizational Learning and Knowledge Management. Blackwell Publishing; 2003.
10. Rakowsky S, Flashner BM, Doolin J, et al. Five questions for residency leadership in the time of COVID-19: reflections of chief medical residents from an internal medicine program. Acad Med. 2020;95(8):1152-1154. https://doi.org/10.1097/ACM.0000000000003419
11. Armstrong K. If you can’t beat it, join it: uncertainty and trust in medicine. Ann Intern Med. 2018;168(11):818-819. https://doi.org/10.7326/M18-0445
12. Gino F. The business case for curiosity. Harvard Bus Rev. 2018;96(5):48-57.
13. Kashdan TB, DeWall CN, Pond RS, et al. Curiosity protects against interpersonal aggression: cross-sectional, daily process, and behavioral evidence. J Pers. 2013;81(1):87-102. https://doi.org/10.1111/j.1467-6494.2012.00783.x
14. Epstein RM, Krasner MS. Physician resilience: what it means, why it matters, and how to promote it. Acad Med. 2013;88(3):301-303. https://doi.org/10.1097/ACM.0b013e318280cff0
15. Petriglieri G. The psychology behind effective crisis leadership. Harvard Bus Rev. Published April 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/the-psychology-behind-effective-crisis-leadership
16. Edmondson A. Building a psychologically safe workplace: TEDx Talk. May 4, 2014. Accessed October 11, 2020. https://youtube.com/watch?v=LhoLuui9gX8
17. Simpkin AL, Chang Y, Yu L, Campbell EG, Armstrong K, Walensky RP. Assessment of job satisfaction and feeling valued in academic medicine. JAMA Intern Med. 2019;179(7):992-994. https://doi.org/10.1001/jamainternmed.2019.0377
18. Nawaz S. In times of crisis, a little thanks goes a long way. Harvard Bus Rev. Published May 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/05/in-times-of-crisis-a-little-thanks-goes-a-long-way
19. Knight R. How to talk to your team when the future is uncertain. Harvard Bus Rev. Published April 20, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/how-to-talk-to-your-team-when-the-future-is-uncertain
1. Kahn WA. Psychological conditions of personal engagement and disengagement at work. Acad Manage J. 1990;33(4):692-724. https://doi.org/10.5465/256287
2. Rozovsky J. The five keys to a successful Google team. re:Work. Posted November 17, 2015. Accessed October 11, 2020. https://rework.withgoogle.com/blog/five-keys-to-a-successful-google-team/
3. Hettle D, Sutherland K, Miles E, et al. Cross-skilling training to support medical redeployment in the COVID-19 pandemic. Future Healthc J. 2020:fhj.2020-0049. https://doi.org/10.7861/fhj.2020-0049
4. Edmondson AC, Lei Z. Psychological safety: the history, renaissance, and future of an interpersonal construct. Annu Rev Organ Psychol Organ Behav. 2014;1(1):23-43. https://doi.org/10.1146/annurev-orgpsych-031413-091305
5. Edmondson A. Psychological safety and learning behavior in work teams. Admin Sci Quart.1999;44(2):350-383. https://doi.org/10.2307/2666999
6. Turner S, Harder N. Psychological safe environment: a concept analysis. Clin Simul Nurs. 2018;18:47-55. https://doi.org/10.1016/j.ecns.2018.02.004
7. Jug R, Jiang XS, Bean SM. Giving and receiving effective feedback: a review article and how-to guide. Arch Pathol Lab Med. 2019;143(2):244-250. https://doi.org/10.5858/arpa.2018-0058-RA
8. Ende J. Feedback in clinical medical education. JAMA. 1983;250(6):777-781. https://doi.org/10.1001/jama.1983.03340060055026
9. Edmondson AC, Woolley AW. Understanding outcomes of organizational learning interventions. In: Easterby-Smith M, Lyles M, eds. Blackwell Handbook of Organizational Learning and Knowledge Management. Blackwell Publishing; 2003.
10. Rakowsky S, Flashner BM, Doolin J, et al. Five questions for residency leadership in the time of COVID-19: reflections of chief medical residents from an internal medicine program. Acad Med. 2020;95(8):1152-1154. https://doi.org/10.1097/ACM.0000000000003419
11. Armstrong K. If you can’t beat it, join it: uncertainty and trust in medicine. Ann Intern Med. 2018;168(11):818-819. https://doi.org/10.7326/M18-0445
12. Gino F. The business case for curiosity. Harvard Bus Rev. 2018;96(5):48-57.
13. Kashdan TB, DeWall CN, Pond RS, et al. Curiosity protects against interpersonal aggression: cross-sectional, daily process, and behavioral evidence. J Pers. 2013;81(1):87-102. https://doi.org/10.1111/j.1467-6494.2012.00783.x
14. Epstein RM, Krasner MS. Physician resilience: what it means, why it matters, and how to promote it. Acad Med. 2013;88(3):301-303. https://doi.org/10.1097/ACM.0b013e318280cff0
15. Petriglieri G. The psychology behind effective crisis leadership. Harvard Bus Rev. Published April 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/the-psychology-behind-effective-crisis-leadership
16. Edmondson A. Building a psychologically safe workplace: TEDx Talk. May 4, 2014. Accessed October 11, 2020. https://youtube.com/watch?v=LhoLuui9gX8
17. Simpkin AL, Chang Y, Yu L, Campbell EG, Armstrong K, Walensky RP. Assessment of job satisfaction and feeling valued in academic medicine. JAMA Intern Med. 2019;179(7):992-994. https://doi.org/10.1001/jamainternmed.2019.0377
18. Nawaz S. In times of crisis, a little thanks goes a long way. Harvard Bus Rev. Published May 22, 2020. Accessed October 11, 2020. https://hbr.org/2020/05/in-times-of-crisis-a-little-thanks-goes-a-long-way
19. Knight R. How to talk to your team when the future is uncertain. Harvard Bus Rev. Published April 20, 2020. Accessed October 11, 2020. https://hbr.org/2020/04/how-to-talk-to-your-team-when-the-future-is-uncertain
© 2021 Society of Hospital Medicine
Metapneumovirus infections clinically indistinguishable from flu, RSV
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
FROM IDWEEK 2020
Osteoporosis drugs don’t worsen COVID-19 risk, may help
New observational data are the first to support recommendations to continue osteoporosis medications during the COVID-19 pandemic, and even suggest that some agents may protect against the virus.
Findings from the cross-sectional study of 2,102 patients with osteoporosis, osteoarthritis, and/or fibromyalgia – so-called noninflammatory rheumatic conditions – during March 1 to May 3, 2020, were recently published in Aging by Josep Blanch-Rubió, MD, scientific clinical director of the Rheumatology Service, Hospital del Mar, Barcelona, and colleagues.
Patients taking denosumab, zoledronate, and calcium showed trends toward lower incidence of developing symptomatic presumed COVID-19 (polymerase chain reaction tests weren’t widely available at the time), as did those taking the antidepressant serotonin/norepinephrine inhibitor duloxetine.
Some analgesics, particularly pregabalin and most other antidepressants, were associated with higher incidences of COVID-19, while oral bisphosphonates, vitamin D, thiazide diuretics, antihypertensive drugs, and chronic nonsteroidal anti-inflammatory drugs had no effect on COVID-19 incidence.
These data are the first to support guidance issued in May 2020 by the American Society for Bone and Mineral Research and four other professional societies advising continuation of osteoporosis medications during the pandemic. That statement’s authors acknowledged that, lacking data, their recommendations were based primarily on expert opinion.
“There were guidelines without any scientific base. ... This is the first scientific evidence showing that indeed you should continue your osteoporosis treatment if you have COVID-19. This is the first study to provide scientific support for the guidelines,” study coauthor Rafael Maldonado, MD, PhD, of the Laboratory of Neuropharmacology, Universitat Pompeu Fabra, Barcelona, said in an interview.
And while the data don’t offer proof of benefit for any drug – all of the 95% confidence intervals crossed 1.0 – they do show trends that deserve further study, Dr. Maldonado said.
“What we observed is that there is no harm. Treatments should be continued.”
“But we obtained very interesting results with denosumab, zoledronate, calcium, and duloxetine. ... There is a clear tendency, and the message is we should promote studies to see if these four treatments provide benefit.”
Different mechanisms for each?
Asked to comment on the findings, Matthew T. Drake, MD, PhD, said in an interview, “I would agree that there’s no reason any of these medications should be stopped or discontinued since there’s no evidence that they make the risk for infection worse.”
“But how [some of them may] improve or reduce the infection risk in my mind is somewhat unclear. ... It’s hard to come up with a unifying explanation” because those mentioned as potentially beneficial “are fairly different,” he noted.
Dr. Drake, associate professor of medicine in the department of endocrinology at the Mayo Clinic, Rochester, Minn., said he agreed with the study authors that denosumab’s targeting of the RANK/RANKL system is a possible anti-COVID-19 mechanism for that drug because that system is involved in immune response.
Regarding zoledronate/zoledronic acid, both the Spanish authors and Dr. Drake pointed to a landmark study linking the intravenous drug to longer survival in patients with hip fracture. The study authors note that there could be several mechanisms for an overall survival benefit, but additionally, “zoledronate may make dendritic cells and their precursors less susceptible to SARS-CoV-2 infection, which could explain the beneficial effects here ... on COVID-19 incidence.”
And, the authors hypothesized, the reason for the lack of benefit with oral bisphosphonates might relate to the higher potency of the intravenous zoledronate. Dr. Drake added that its higher bioavailability may also play a role.
As for calcium, the authors suggest that the beneficial effect against COVID-19 could relate to its action in generating two immune cell types – T follicular helper cells and T follicular regulatory cells – which promote an appropriate immune response against infectious agents, including viruses.
Data supporting the guidelines
Of the 2,102 patients in the study by Blanch-Rubió and colleagues, 80.5% were women, and their mean age was 66.4 years. Overall, 63.7% had osteoarthritis, 43.5% had osteoporosis, and 27.2% had fibromyalgia. Treatments included vitamin D in 62%, calcium in 23.3%, denosumab in 12.6%, and intravenous zoledronate in 8.5%. Over half were taking analgesics and nearly a third antidepressants, with 9.9% taking duloxetine.
During the study period, 5.2%, or 109 individuals, were diagnosed with COVID-19 based on presenting for medical care with hallmark symptoms.
After adjustments for sex, age, diabetes, pulmonary disease, cardiovascular disease, chronic kidney disease, and active cancer or treatment, the relative risks for COVID-19 were 0.58 for denosumab, 0.62 for intravenous zoledronate, and 0.64 for calcium, all nonsignificant trends. No associations were found between COVID-19 and oral bisphosphonates, vitamin D, or thiazide diuretics. Increased but nonsignificant relative risks for COVID-19 were seen with analgesics, particularly pregabalin (1.55), gabapentin (1.39), and opioids (1.25).
Among antidepressants, there was a relative risk of 1.54 for selective serotonin reuptake inhibitors, 1.38 for amitriptyline, and 1.22 for all dual-action antidepressants together. In contrast, there was a negative association with the dual-action antidepressant duloxetine, with an adjusted relative risk of 0.68.
“The good news,” Dr. Drake said, “is that none of it appears bad.”
Dr. Blanch-Rubió has received grants or consulting fees from Amgen, Laboratorio Stada, Gedeon-Rhicter Ibérica, Lilly España, Pfizer, Gebro Pharma, and UCB Pharma. Dr. Maldonado has received research grants or consulting fees from Aelis, Almirall, Boehringer Ingelheim, BrainCo, Esteve, Ferrer, GlaxoSmithKline, Grünenthal, GW Pharmaceuticals, Janus, Lundbeck, Pharmaleads, Phytoplant, Rhodes, Sanofi, Spherium, Union de Pharmacologie Scientifique Appliquée, Upjohn, and Uriach. Dr. Drake has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New observational data are the first to support recommendations to continue osteoporosis medications during the COVID-19 pandemic, and even suggest that some agents may protect against the virus.
Findings from the cross-sectional study of 2,102 patients with osteoporosis, osteoarthritis, and/or fibromyalgia – so-called noninflammatory rheumatic conditions – during March 1 to May 3, 2020, were recently published in Aging by Josep Blanch-Rubió, MD, scientific clinical director of the Rheumatology Service, Hospital del Mar, Barcelona, and colleagues.
Patients taking denosumab, zoledronate, and calcium showed trends toward lower incidence of developing symptomatic presumed COVID-19 (polymerase chain reaction tests weren’t widely available at the time), as did those taking the antidepressant serotonin/norepinephrine inhibitor duloxetine.
Some analgesics, particularly pregabalin and most other antidepressants, were associated with higher incidences of COVID-19, while oral bisphosphonates, vitamin D, thiazide diuretics, antihypertensive drugs, and chronic nonsteroidal anti-inflammatory drugs had no effect on COVID-19 incidence.
These data are the first to support guidance issued in May 2020 by the American Society for Bone and Mineral Research and four other professional societies advising continuation of osteoporosis medications during the pandemic. That statement’s authors acknowledged that, lacking data, their recommendations were based primarily on expert opinion.
“There were guidelines without any scientific base. ... This is the first scientific evidence showing that indeed you should continue your osteoporosis treatment if you have COVID-19. This is the first study to provide scientific support for the guidelines,” study coauthor Rafael Maldonado, MD, PhD, of the Laboratory of Neuropharmacology, Universitat Pompeu Fabra, Barcelona, said in an interview.
And while the data don’t offer proof of benefit for any drug – all of the 95% confidence intervals crossed 1.0 – they do show trends that deserve further study, Dr. Maldonado said.
“What we observed is that there is no harm. Treatments should be continued.”
“But we obtained very interesting results with denosumab, zoledronate, calcium, and duloxetine. ... There is a clear tendency, and the message is we should promote studies to see if these four treatments provide benefit.”
Different mechanisms for each?
Asked to comment on the findings, Matthew T. Drake, MD, PhD, said in an interview, “I would agree that there’s no reason any of these medications should be stopped or discontinued since there’s no evidence that they make the risk for infection worse.”
“But how [some of them may] improve or reduce the infection risk in my mind is somewhat unclear. ... It’s hard to come up with a unifying explanation” because those mentioned as potentially beneficial “are fairly different,” he noted.
Dr. Drake, associate professor of medicine in the department of endocrinology at the Mayo Clinic, Rochester, Minn., said he agreed with the study authors that denosumab’s targeting of the RANK/RANKL system is a possible anti-COVID-19 mechanism for that drug because that system is involved in immune response.
Regarding zoledronate/zoledronic acid, both the Spanish authors and Dr. Drake pointed to a landmark study linking the intravenous drug to longer survival in patients with hip fracture. The study authors note that there could be several mechanisms for an overall survival benefit, but additionally, “zoledronate may make dendritic cells and their precursors less susceptible to SARS-CoV-2 infection, which could explain the beneficial effects here ... on COVID-19 incidence.”
And, the authors hypothesized, the reason for the lack of benefit with oral bisphosphonates might relate to the higher potency of the intravenous zoledronate. Dr. Drake added that its higher bioavailability may also play a role.
As for calcium, the authors suggest that the beneficial effect against COVID-19 could relate to its action in generating two immune cell types – T follicular helper cells and T follicular regulatory cells – which promote an appropriate immune response against infectious agents, including viruses.
Data supporting the guidelines
Of the 2,102 patients in the study by Blanch-Rubió and colleagues, 80.5% were women, and their mean age was 66.4 years. Overall, 63.7% had osteoarthritis, 43.5% had osteoporosis, and 27.2% had fibromyalgia. Treatments included vitamin D in 62%, calcium in 23.3%, denosumab in 12.6%, and intravenous zoledronate in 8.5%. Over half were taking analgesics and nearly a third antidepressants, with 9.9% taking duloxetine.
During the study period, 5.2%, or 109 individuals, were diagnosed with COVID-19 based on presenting for medical care with hallmark symptoms.
After adjustments for sex, age, diabetes, pulmonary disease, cardiovascular disease, chronic kidney disease, and active cancer or treatment, the relative risks for COVID-19 were 0.58 for denosumab, 0.62 for intravenous zoledronate, and 0.64 for calcium, all nonsignificant trends. No associations were found between COVID-19 and oral bisphosphonates, vitamin D, or thiazide diuretics. Increased but nonsignificant relative risks for COVID-19 were seen with analgesics, particularly pregabalin (1.55), gabapentin (1.39), and opioids (1.25).
Among antidepressants, there was a relative risk of 1.54 for selective serotonin reuptake inhibitors, 1.38 for amitriptyline, and 1.22 for all dual-action antidepressants together. In contrast, there was a negative association with the dual-action antidepressant duloxetine, with an adjusted relative risk of 0.68.
“The good news,” Dr. Drake said, “is that none of it appears bad.”
Dr. Blanch-Rubió has received grants or consulting fees from Amgen, Laboratorio Stada, Gedeon-Rhicter Ibérica, Lilly España, Pfizer, Gebro Pharma, and UCB Pharma. Dr. Maldonado has received research grants or consulting fees from Aelis, Almirall, Boehringer Ingelheim, BrainCo, Esteve, Ferrer, GlaxoSmithKline, Grünenthal, GW Pharmaceuticals, Janus, Lundbeck, Pharmaleads, Phytoplant, Rhodes, Sanofi, Spherium, Union de Pharmacologie Scientifique Appliquée, Upjohn, and Uriach. Dr. Drake has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New observational data are the first to support recommendations to continue osteoporosis medications during the COVID-19 pandemic, and even suggest that some agents may protect against the virus.
Findings from the cross-sectional study of 2,102 patients with osteoporosis, osteoarthritis, and/or fibromyalgia – so-called noninflammatory rheumatic conditions – during March 1 to May 3, 2020, were recently published in Aging by Josep Blanch-Rubió, MD, scientific clinical director of the Rheumatology Service, Hospital del Mar, Barcelona, and colleagues.
Patients taking denosumab, zoledronate, and calcium showed trends toward lower incidence of developing symptomatic presumed COVID-19 (polymerase chain reaction tests weren’t widely available at the time), as did those taking the antidepressant serotonin/norepinephrine inhibitor duloxetine.
Some analgesics, particularly pregabalin and most other antidepressants, were associated with higher incidences of COVID-19, while oral bisphosphonates, vitamin D, thiazide diuretics, antihypertensive drugs, and chronic nonsteroidal anti-inflammatory drugs had no effect on COVID-19 incidence.
These data are the first to support guidance issued in May 2020 by the American Society for Bone and Mineral Research and four other professional societies advising continuation of osteoporosis medications during the pandemic. That statement’s authors acknowledged that, lacking data, their recommendations were based primarily on expert opinion.
“There were guidelines without any scientific base. ... This is the first scientific evidence showing that indeed you should continue your osteoporosis treatment if you have COVID-19. This is the first study to provide scientific support for the guidelines,” study coauthor Rafael Maldonado, MD, PhD, of the Laboratory of Neuropharmacology, Universitat Pompeu Fabra, Barcelona, said in an interview.
And while the data don’t offer proof of benefit for any drug – all of the 95% confidence intervals crossed 1.0 – they do show trends that deserve further study, Dr. Maldonado said.
“What we observed is that there is no harm. Treatments should be continued.”
“But we obtained very interesting results with denosumab, zoledronate, calcium, and duloxetine. ... There is a clear tendency, and the message is we should promote studies to see if these four treatments provide benefit.”
Different mechanisms for each?
Asked to comment on the findings, Matthew T. Drake, MD, PhD, said in an interview, “I would agree that there’s no reason any of these medications should be stopped or discontinued since there’s no evidence that they make the risk for infection worse.”
“But how [some of them may] improve or reduce the infection risk in my mind is somewhat unclear. ... It’s hard to come up with a unifying explanation” because those mentioned as potentially beneficial “are fairly different,” he noted.
Dr. Drake, associate professor of medicine in the department of endocrinology at the Mayo Clinic, Rochester, Minn., said he agreed with the study authors that denosumab’s targeting of the RANK/RANKL system is a possible anti-COVID-19 mechanism for that drug because that system is involved in immune response.
Regarding zoledronate/zoledronic acid, both the Spanish authors and Dr. Drake pointed to a landmark study linking the intravenous drug to longer survival in patients with hip fracture. The study authors note that there could be several mechanisms for an overall survival benefit, but additionally, “zoledronate may make dendritic cells and their precursors less susceptible to SARS-CoV-2 infection, which could explain the beneficial effects here ... on COVID-19 incidence.”
And, the authors hypothesized, the reason for the lack of benefit with oral bisphosphonates might relate to the higher potency of the intravenous zoledronate. Dr. Drake added that its higher bioavailability may also play a role.
As for calcium, the authors suggest that the beneficial effect against COVID-19 could relate to its action in generating two immune cell types – T follicular helper cells and T follicular regulatory cells – which promote an appropriate immune response against infectious agents, including viruses.
Data supporting the guidelines
Of the 2,102 patients in the study by Blanch-Rubió and colleagues, 80.5% were women, and their mean age was 66.4 years. Overall, 63.7% had osteoarthritis, 43.5% had osteoporosis, and 27.2% had fibromyalgia. Treatments included vitamin D in 62%, calcium in 23.3%, denosumab in 12.6%, and intravenous zoledronate in 8.5%. Over half were taking analgesics and nearly a third antidepressants, with 9.9% taking duloxetine.
During the study period, 5.2%, or 109 individuals, were diagnosed with COVID-19 based on presenting for medical care with hallmark symptoms.
After adjustments for sex, age, diabetes, pulmonary disease, cardiovascular disease, chronic kidney disease, and active cancer or treatment, the relative risks for COVID-19 were 0.58 for denosumab, 0.62 for intravenous zoledronate, and 0.64 for calcium, all nonsignificant trends. No associations were found between COVID-19 and oral bisphosphonates, vitamin D, or thiazide diuretics. Increased but nonsignificant relative risks for COVID-19 were seen with analgesics, particularly pregabalin (1.55), gabapentin (1.39), and opioids (1.25).
Among antidepressants, there was a relative risk of 1.54 for selective serotonin reuptake inhibitors, 1.38 for amitriptyline, and 1.22 for all dual-action antidepressants together. In contrast, there was a negative association with the dual-action antidepressant duloxetine, with an adjusted relative risk of 0.68.
“The good news,” Dr. Drake said, “is that none of it appears bad.”
Dr. Blanch-Rubió has received grants or consulting fees from Amgen, Laboratorio Stada, Gedeon-Rhicter Ibérica, Lilly España, Pfizer, Gebro Pharma, and UCB Pharma. Dr. Maldonado has received research grants or consulting fees from Aelis, Almirall, Boehringer Ingelheim, BrainCo, Esteve, Ferrer, GlaxoSmithKline, Grünenthal, GW Pharmaceuticals, Janus, Lundbeck, Pharmaleads, Phytoplant, Rhodes, Sanofi, Spherium, Union de Pharmacologie Scientifique Appliquée, Upjohn, and Uriach. Dr. Drake has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Cardiac arrest in COVID-19 pandemic: ‘Survival is possible’
In the early weeks of the COVID-19 pandemic in the United States, rates of sustained return of spontaneous circulation after out-of-hospital cardiac arrest were lower throughout the country, compared with a year earlier, in one study.
A second study of that period showed that patients with COVID-19 had rates that were better than previously reported of surviving in-hospital cardiac arrest.
Paul S. Chan, MD, presented the out-of-hospital cardiac arrest research, and Oscar J. Mitchell, MD, presented the in-hospital cardiac arrest findings in a late-breaking resuscitation science session at the American Heart Association scientific sessions. The former study was also simultaneously published online Nov. 14 in JAMA Cardiology.
Importantly, “the survival rates were not zero in either setting,” said Dr. Chan, commenting on the implications of both studies taken together.
“The survival rates – either return of circulation or survival to discharge – were not futile,” Dr. Chan, from Saint Luke’s Mid America Heart Institute, Kansas City, Missouri, said in an interview.
“And I think that’s an overall important message – that we can’t write off patients who have a cardiac arrest at this point,” he stressed. “They deserve a response. Although the outcomes might not be as good as we had seen in years prior, we are seeing patients making it out of the hospital and surviving.”
Dr. Mitchell, from the University of Pennsylvania in Philadelphia, echoed this message in an interview.
“I think that the key finding here is that survival is possible after patients with COVID-19 suffer an in-hospital cardiac arrest,” Dr. Mitchell said. “We hope that the information from our study will be of use to frontline providers who are treating patients with COVID-19.”
“In coming weeks, there will likely be increased hospital strain and enormous challenges to providing COVID-19 care,” added Benjamin S. Abella, MD, the senior author of the in-hospital study. Dr. Abella is also from the University of Pennsylvania and was cochair of the Resuscitation Science symposium during the AHA meeting.
“It is crucial that hospital leaders prepare now for how they will manage COVID-19 resuscitation efforts,” Dr. Abella said. “Emergency medicine and critical care leaders must be mindful that many COVID-19 patients with arrest could survive to return to their families.”
“It is important to note both studies demonstrated variations in outcome and that those differences were associated with the differential COVID prevalence and mortality,” session comoderator Cindy H. Hsu, MD, PhD, University of Michigan, said in an interview.
“Future studies,” she said, “should address knowledge gaps including associated comorbidities and affected resuscitation process variables during the COVID-19 pandemic.”
Out-of-hospital cardiac arrest, March 2019 vs. March 2020
Compared with 2019, in 2020, the reported rates of return of spontaneous circulation after out-of-hospital cardiac arrest fell from 25% to 10.6% in New York and from 13.5% to 5.0% in northern Italy – two areas that were severely affected, Dr. Chan noted.
In this study, the researchers aimed to examine whether out-of-hospital cardiac arrest outcomes would be similar throughout the United States, including areas that were less severely affected, in the first weeks of the pandemic.
They linked data from the Cardiac Arrest Registry to Enhance Survival (CARES), which covers an area with about 152 million U.S. residents, with COVID-19 disease mortality data.
There were 9,863 out-of-hospital arrests from March 16 to April 30, 2020, compared with 9,440 cases during this time in 2019.
The patients in both years had a similar age (mean, 62 years) and sex (62% male), but there were more Black patients in 2020 (28% vs. 23%).
Overall, in communities with low to high rates of death from COVID-19, the rate of return of spontaneous circulation was 18% lower in that early pandemic period than in the same time in the previous year (23% vs. 29.8%; adjusted rate ratio, 0.82).
The rates of return of spontaneous circulation were also lower in communities with a low rate of COVID-19 mortality, but to a lesser extent (11%-15% lower in 2020 vs. 2019).
In the subset of emergency medical agencies with complete data on hospital survival, overall rates of survival to discharge were 17% lower during the studied pandemic period versus the same time a year earlier (6.6% vs. 9.8%; adjusted RR, 0.83).
This drop in survival was greater in communities with moderate to high COVID-19 mortality.
These outcomes were not explained by differences in emergency medical services arrival or treatment times, rates of bystander CPR, or initial out-of-hospital cardiac arrest rhythm.
Dr. Chan was a coauthor of an interim guidance issued April 9, 2020, by the AHA and several other medical societies for ways to protect frontline workers from contracting COVID-19 while they were performing CPR.
Communities that were not heavily affected by COVID-19 could have also been following the recommendations, which might have affected outcomes, he speculated.
For example, “when we pause chest compressions it can potentially worsen survival even if it’s for a short period of time. That might explain the lower rates of return of circulation.”
“That guidance was really meant for heavily affected communities,” Dr. Chan added. “Of course, as we speak, the pandemic is pretty much everywhere in the United States. It’s not just in the northeast; it’s not just in Arizona, Florida, California, Texas like it was in the summer. You are seeing surges in 46 of the 50 states.
“If your community is heavily affected by COVID-19 in terms of deaths at this time, paramedics will need to take caution to also help protect themselves, and the guidance may apply at that point,” he said.
In-hospital cardiac arrest, March Through May 2020
The early studies of in-hospital cardiac arrest in patients with COVID-19 showed “concerningly low rates” of return of spontaneous circulation and survival, said Dr. Mitchell.
“The first was a study from Wuhan, which demonstrated a 2.9% 30-day survival and the second was a small cohort from NYC with 0% survival to hospital discharge,” he said. “This raised concerns that offering CPR to patients who had a cardiac arrest from COVID-19 might only hold a low probability of success.”
To investigate this, the researchers formed a COVID study group comprising two hospitals in New York and nine hospitals in the Northeast and West Coast.
They identified 260 hospitalized adult patients with COVID-19 who had in-hospital cardiac arrest between March 1 and May 31, 2020. The patients had a median age of 69 years, and 72% were male. Most had preexisting comorbidities. Most of the cardiac arrests were in the ICU (64%), and almost all were witnessed (91%).
Return of spontaneous circulation occurred in 22% of the patients, and 12% had survived 30 days later. Of the 260 cardiac arrests, most (204) occurred in the New York hospitals.
There was a huge variation in outcomes. The rate of sustained return of spontaneous circulation was much lower in the two hospitals in New York compared with elsewhere (11% vs. 64%), as was 30-day survival (6% vs. 36%).
“Variation in outcomes from [in-hospital cardiac arrest] has been well described prior to the COVID-19 pandemic,” said Dr. Mitchell, “and is felt to be due to a range of factors, including variation in detection and prevention of cardiac arrest, management of patients during the cardiac arrest, and differences in postarrest care – including targeted temperature management and neuroprognostication.”
“We hypothesize that the strains of the COVID-19 pandemic may have amplified these variations (although we were unable to compare hospital performance before and after the pandemic),” he said.
Nevertheless, “in contrast to [earlier] studies, we have found that survival with a good neurological status is possible after in-hospital cardiac arrest in patients with COVID-19, which is certainly reassuring for those of us on the front line.”
Dr. Chan has received research support from the American Heart Association (which helps fund CARES); the National Heart, Lung, and Blood Institute; and Optum Rx. Dr. Abella has received honoraria from NeuroproteXeon, Becton Dickinson, and Physio-Control, and research grants from Medtronic, PCORI, Physio-Control, Stryker, and TerSera. Dr. Mitchell has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the early weeks of the COVID-19 pandemic in the United States, rates of sustained return of spontaneous circulation after out-of-hospital cardiac arrest were lower throughout the country, compared with a year earlier, in one study.
A second study of that period showed that patients with COVID-19 had rates that were better than previously reported of surviving in-hospital cardiac arrest.
Paul S. Chan, MD, presented the out-of-hospital cardiac arrest research, and Oscar J. Mitchell, MD, presented the in-hospital cardiac arrest findings in a late-breaking resuscitation science session at the American Heart Association scientific sessions. The former study was also simultaneously published online Nov. 14 in JAMA Cardiology.
Importantly, “the survival rates were not zero in either setting,” said Dr. Chan, commenting on the implications of both studies taken together.
“The survival rates – either return of circulation or survival to discharge – were not futile,” Dr. Chan, from Saint Luke’s Mid America Heart Institute, Kansas City, Missouri, said in an interview.
“And I think that’s an overall important message – that we can’t write off patients who have a cardiac arrest at this point,” he stressed. “They deserve a response. Although the outcomes might not be as good as we had seen in years prior, we are seeing patients making it out of the hospital and surviving.”
Dr. Mitchell, from the University of Pennsylvania in Philadelphia, echoed this message in an interview.
“I think that the key finding here is that survival is possible after patients with COVID-19 suffer an in-hospital cardiac arrest,” Dr. Mitchell said. “We hope that the information from our study will be of use to frontline providers who are treating patients with COVID-19.”
“In coming weeks, there will likely be increased hospital strain and enormous challenges to providing COVID-19 care,” added Benjamin S. Abella, MD, the senior author of the in-hospital study. Dr. Abella is also from the University of Pennsylvania and was cochair of the Resuscitation Science symposium during the AHA meeting.
“It is crucial that hospital leaders prepare now for how they will manage COVID-19 resuscitation efforts,” Dr. Abella said. “Emergency medicine and critical care leaders must be mindful that many COVID-19 patients with arrest could survive to return to their families.”
“It is important to note both studies demonstrated variations in outcome and that those differences were associated with the differential COVID prevalence and mortality,” session comoderator Cindy H. Hsu, MD, PhD, University of Michigan, said in an interview.
“Future studies,” she said, “should address knowledge gaps including associated comorbidities and affected resuscitation process variables during the COVID-19 pandemic.”
Out-of-hospital cardiac arrest, March 2019 vs. March 2020
Compared with 2019, in 2020, the reported rates of return of spontaneous circulation after out-of-hospital cardiac arrest fell from 25% to 10.6% in New York and from 13.5% to 5.0% in northern Italy – two areas that were severely affected, Dr. Chan noted.
In this study, the researchers aimed to examine whether out-of-hospital cardiac arrest outcomes would be similar throughout the United States, including areas that were less severely affected, in the first weeks of the pandemic.
They linked data from the Cardiac Arrest Registry to Enhance Survival (CARES), which covers an area with about 152 million U.S. residents, with COVID-19 disease mortality data.
There were 9,863 out-of-hospital arrests from March 16 to April 30, 2020, compared with 9,440 cases during this time in 2019.
The patients in both years had a similar age (mean, 62 years) and sex (62% male), but there were more Black patients in 2020 (28% vs. 23%).
Overall, in communities with low to high rates of death from COVID-19, the rate of return of spontaneous circulation was 18% lower in that early pandemic period than in the same time in the previous year (23% vs. 29.8%; adjusted rate ratio, 0.82).
The rates of return of spontaneous circulation were also lower in communities with a low rate of COVID-19 mortality, but to a lesser extent (11%-15% lower in 2020 vs. 2019).
In the subset of emergency medical agencies with complete data on hospital survival, overall rates of survival to discharge were 17% lower during the studied pandemic period versus the same time a year earlier (6.6% vs. 9.8%; adjusted RR, 0.83).
This drop in survival was greater in communities with moderate to high COVID-19 mortality.
These outcomes were not explained by differences in emergency medical services arrival or treatment times, rates of bystander CPR, or initial out-of-hospital cardiac arrest rhythm.
Dr. Chan was a coauthor of an interim guidance issued April 9, 2020, by the AHA and several other medical societies for ways to protect frontline workers from contracting COVID-19 while they were performing CPR.
Communities that were not heavily affected by COVID-19 could have also been following the recommendations, which might have affected outcomes, he speculated.
For example, “when we pause chest compressions it can potentially worsen survival even if it’s for a short period of time. That might explain the lower rates of return of circulation.”
“That guidance was really meant for heavily affected communities,” Dr. Chan added. “Of course, as we speak, the pandemic is pretty much everywhere in the United States. It’s not just in the northeast; it’s not just in Arizona, Florida, California, Texas like it was in the summer. You are seeing surges in 46 of the 50 states.
“If your community is heavily affected by COVID-19 in terms of deaths at this time, paramedics will need to take caution to also help protect themselves, and the guidance may apply at that point,” he said.
In-hospital cardiac arrest, March Through May 2020
The early studies of in-hospital cardiac arrest in patients with COVID-19 showed “concerningly low rates” of return of spontaneous circulation and survival, said Dr. Mitchell.
“The first was a study from Wuhan, which demonstrated a 2.9% 30-day survival and the second was a small cohort from NYC with 0% survival to hospital discharge,” he said. “This raised concerns that offering CPR to patients who had a cardiac arrest from COVID-19 might only hold a low probability of success.”
To investigate this, the researchers formed a COVID study group comprising two hospitals in New York and nine hospitals in the Northeast and West Coast.
They identified 260 hospitalized adult patients with COVID-19 who had in-hospital cardiac arrest between March 1 and May 31, 2020. The patients had a median age of 69 years, and 72% were male. Most had preexisting comorbidities. Most of the cardiac arrests were in the ICU (64%), and almost all were witnessed (91%).
Return of spontaneous circulation occurred in 22% of the patients, and 12% had survived 30 days later. Of the 260 cardiac arrests, most (204) occurred in the New York hospitals.
There was a huge variation in outcomes. The rate of sustained return of spontaneous circulation was much lower in the two hospitals in New York compared with elsewhere (11% vs. 64%), as was 30-day survival (6% vs. 36%).
“Variation in outcomes from [in-hospital cardiac arrest] has been well described prior to the COVID-19 pandemic,” said Dr. Mitchell, “and is felt to be due to a range of factors, including variation in detection and prevention of cardiac arrest, management of patients during the cardiac arrest, and differences in postarrest care – including targeted temperature management and neuroprognostication.”
“We hypothesize that the strains of the COVID-19 pandemic may have amplified these variations (although we were unable to compare hospital performance before and after the pandemic),” he said.
Nevertheless, “in contrast to [earlier] studies, we have found that survival with a good neurological status is possible after in-hospital cardiac arrest in patients with COVID-19, which is certainly reassuring for those of us on the front line.”
Dr. Chan has received research support from the American Heart Association (which helps fund CARES); the National Heart, Lung, and Blood Institute; and Optum Rx. Dr. Abella has received honoraria from NeuroproteXeon, Becton Dickinson, and Physio-Control, and research grants from Medtronic, PCORI, Physio-Control, Stryker, and TerSera. Dr. Mitchell has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the early weeks of the COVID-19 pandemic in the United States, rates of sustained return of spontaneous circulation after out-of-hospital cardiac arrest were lower throughout the country, compared with a year earlier, in one study.
A second study of that period showed that patients with COVID-19 had rates that were better than previously reported of surviving in-hospital cardiac arrest.
Paul S. Chan, MD, presented the out-of-hospital cardiac arrest research, and Oscar J. Mitchell, MD, presented the in-hospital cardiac arrest findings in a late-breaking resuscitation science session at the American Heart Association scientific sessions. The former study was also simultaneously published online Nov. 14 in JAMA Cardiology.
Importantly, “the survival rates were not zero in either setting,” said Dr. Chan, commenting on the implications of both studies taken together.
“The survival rates – either return of circulation or survival to discharge – were not futile,” Dr. Chan, from Saint Luke’s Mid America Heart Institute, Kansas City, Missouri, said in an interview.
“And I think that’s an overall important message – that we can’t write off patients who have a cardiac arrest at this point,” he stressed. “They deserve a response. Although the outcomes might not be as good as we had seen in years prior, we are seeing patients making it out of the hospital and surviving.”
Dr. Mitchell, from the University of Pennsylvania in Philadelphia, echoed this message in an interview.
“I think that the key finding here is that survival is possible after patients with COVID-19 suffer an in-hospital cardiac arrest,” Dr. Mitchell said. “We hope that the information from our study will be of use to frontline providers who are treating patients with COVID-19.”
“In coming weeks, there will likely be increased hospital strain and enormous challenges to providing COVID-19 care,” added Benjamin S. Abella, MD, the senior author of the in-hospital study. Dr. Abella is also from the University of Pennsylvania and was cochair of the Resuscitation Science symposium during the AHA meeting.
“It is crucial that hospital leaders prepare now for how they will manage COVID-19 resuscitation efforts,” Dr. Abella said. “Emergency medicine and critical care leaders must be mindful that many COVID-19 patients with arrest could survive to return to their families.”
“It is important to note both studies demonstrated variations in outcome and that those differences were associated with the differential COVID prevalence and mortality,” session comoderator Cindy H. Hsu, MD, PhD, University of Michigan, said in an interview.
“Future studies,” she said, “should address knowledge gaps including associated comorbidities and affected resuscitation process variables during the COVID-19 pandemic.”
Out-of-hospital cardiac arrest, March 2019 vs. March 2020
Compared with 2019, in 2020, the reported rates of return of spontaneous circulation after out-of-hospital cardiac arrest fell from 25% to 10.6% in New York and from 13.5% to 5.0% in northern Italy – two areas that were severely affected, Dr. Chan noted.
In this study, the researchers aimed to examine whether out-of-hospital cardiac arrest outcomes would be similar throughout the United States, including areas that were less severely affected, in the first weeks of the pandemic.
They linked data from the Cardiac Arrest Registry to Enhance Survival (CARES), which covers an area with about 152 million U.S. residents, with COVID-19 disease mortality data.
There were 9,863 out-of-hospital arrests from March 16 to April 30, 2020, compared with 9,440 cases during this time in 2019.
The patients in both years had a similar age (mean, 62 years) and sex (62% male), but there were more Black patients in 2020 (28% vs. 23%).
Overall, in communities with low to high rates of death from COVID-19, the rate of return of spontaneous circulation was 18% lower in that early pandemic period than in the same time in the previous year (23% vs. 29.8%; adjusted rate ratio, 0.82).
The rates of return of spontaneous circulation were also lower in communities with a low rate of COVID-19 mortality, but to a lesser extent (11%-15% lower in 2020 vs. 2019).
In the subset of emergency medical agencies with complete data on hospital survival, overall rates of survival to discharge were 17% lower during the studied pandemic period versus the same time a year earlier (6.6% vs. 9.8%; adjusted RR, 0.83).
This drop in survival was greater in communities with moderate to high COVID-19 mortality.
These outcomes were not explained by differences in emergency medical services arrival or treatment times, rates of bystander CPR, or initial out-of-hospital cardiac arrest rhythm.
Dr. Chan was a coauthor of an interim guidance issued April 9, 2020, by the AHA and several other medical societies for ways to protect frontline workers from contracting COVID-19 while they were performing CPR.
Communities that were not heavily affected by COVID-19 could have also been following the recommendations, which might have affected outcomes, he speculated.
For example, “when we pause chest compressions it can potentially worsen survival even if it’s for a short period of time. That might explain the lower rates of return of circulation.”
“That guidance was really meant for heavily affected communities,” Dr. Chan added. “Of course, as we speak, the pandemic is pretty much everywhere in the United States. It’s not just in the northeast; it’s not just in Arizona, Florida, California, Texas like it was in the summer. You are seeing surges in 46 of the 50 states.
“If your community is heavily affected by COVID-19 in terms of deaths at this time, paramedics will need to take caution to also help protect themselves, and the guidance may apply at that point,” he said.
In-hospital cardiac arrest, March Through May 2020
The early studies of in-hospital cardiac arrest in patients with COVID-19 showed “concerningly low rates” of return of spontaneous circulation and survival, said Dr. Mitchell.
“The first was a study from Wuhan, which demonstrated a 2.9% 30-day survival and the second was a small cohort from NYC with 0% survival to hospital discharge,” he said. “This raised concerns that offering CPR to patients who had a cardiac arrest from COVID-19 might only hold a low probability of success.”
To investigate this, the researchers formed a COVID study group comprising two hospitals in New York and nine hospitals in the Northeast and West Coast.
They identified 260 hospitalized adult patients with COVID-19 who had in-hospital cardiac arrest between March 1 and May 31, 2020. The patients had a median age of 69 years, and 72% were male. Most had preexisting comorbidities. Most of the cardiac arrests were in the ICU (64%), and almost all were witnessed (91%).
Return of spontaneous circulation occurred in 22% of the patients, and 12% had survived 30 days later. Of the 260 cardiac arrests, most (204) occurred in the New York hospitals.
There was a huge variation in outcomes. The rate of sustained return of spontaneous circulation was much lower in the two hospitals in New York compared with elsewhere (11% vs. 64%), as was 30-day survival (6% vs. 36%).
“Variation in outcomes from [in-hospital cardiac arrest] has been well described prior to the COVID-19 pandemic,” said Dr. Mitchell, “and is felt to be due to a range of factors, including variation in detection and prevention of cardiac arrest, management of patients during the cardiac arrest, and differences in postarrest care – including targeted temperature management and neuroprognostication.”
“We hypothesize that the strains of the COVID-19 pandemic may have amplified these variations (although we were unable to compare hospital performance before and after the pandemic),” he said.
Nevertheless, “in contrast to [earlier] studies, we have found that survival with a good neurological status is possible after in-hospital cardiac arrest in patients with COVID-19, which is certainly reassuring for those of us on the front line.”
Dr. Chan has received research support from the American Heart Association (which helps fund CARES); the National Heart, Lung, and Blood Institute; and Optum Rx. Dr. Abella has received honoraria from NeuroproteXeon, Becton Dickinson, and Physio-Control, and research grants from Medtronic, PCORI, Physio-Control, Stryker, and TerSera. Dr. Mitchell has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AHA 2020
Escaping the daily grind
Few films have universal appeal these days, but one that comes close is the 1993 classic Groundhog Day, in which the protagonist is trapped in a time loop, doomed to living the same day over and over for many years.
One reason that this story resonates with so many, I think, is that we are all living a similar life. Not as a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. . And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of one a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lessons; or a long weekend away with my wife.
And until COVID-19 put a temporary stop to them earlier this year, we have embarked on at least one longer adventure each year, some of which have been shared in these pages. Our 2019 expedition to Easter Island remains among the most memorable, and fulfilled a dream I’ve had since I read Thor Heyerdahl’s Aku Aku in grade school. As we explored the giant stone moai – which are found nowhere else in the world – I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out. When our trip was over, I returned ready to take on the world, and my practice, anew.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money; this is breaking the routine, clearing the cobwebs, living your life. Trust me, your practice will still be there when you return. And while COVID-19 will not last forever, there are plenty of other “sharpeners” while we wait.
More than once I’ve recounted the story of Alex Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few films have universal appeal these days, but one that comes close is the 1993 classic Groundhog Day, in which the protagonist is trapped in a time loop, doomed to living the same day over and over for many years.
One reason that this story resonates with so many, I think, is that we are all living a similar life. Not as a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. . And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of one a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lessons; or a long weekend away with my wife.
And until COVID-19 put a temporary stop to them earlier this year, we have embarked on at least one longer adventure each year, some of which have been shared in these pages. Our 2019 expedition to Easter Island remains among the most memorable, and fulfilled a dream I’ve had since I read Thor Heyerdahl’s Aku Aku in grade school. As we explored the giant stone moai – which are found nowhere else in the world – I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out. When our trip was over, I returned ready to take on the world, and my practice, anew.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money; this is breaking the routine, clearing the cobwebs, living your life. Trust me, your practice will still be there when you return. And while COVID-19 will not last forever, there are plenty of other “sharpeners” while we wait.
More than once I’ve recounted the story of Alex Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few films have universal appeal these days, but one that comes close is the 1993 classic Groundhog Day, in which the protagonist is trapped in a time loop, doomed to living the same day over and over for many years.
One reason that this story resonates with so many, I think, is that we are all living a similar life. Not as a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. . And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of one a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lessons; or a long weekend away with my wife.
And until COVID-19 put a temporary stop to them earlier this year, we have embarked on at least one longer adventure each year, some of which have been shared in these pages. Our 2019 expedition to Easter Island remains among the most memorable, and fulfilled a dream I’ve had since I read Thor Heyerdahl’s Aku Aku in grade school. As we explored the giant stone moai – which are found nowhere else in the world – I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out. When our trip was over, I returned ready to take on the world, and my practice, anew.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money; this is breaking the routine, clearing the cobwebs, living your life. Trust me, your practice will still be there when you return. And while COVID-19 will not last forever, there are plenty of other “sharpeners” while we wait.
More than once I’ve recounted the story of Alex Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Two-drug combo should be first-line standard of care in advanced endometrial cancer
The combination proved to be noninferior to paclitaxel-doxorubicin-cisplatin (TAP) in terms of response, progression-free survival, and overall survival, and with lower toxicity.
Overall survival was a median of 37 months for TC and 41 months for TAP, and there were more adverse events of grade 3 or higher with TAP.
The data were initially presented at the 2012 annual meeting of the Society of Gynecologic Oncology. In the original presentation, lead author David Scott Miller, MD, said this combination should be the standard of care in this setting. Dr. Miller is professor of obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas.
“This subsequent long-term follow-up publication confirmed that,” he said in an interview. “TAP is now rarely used.”
The results have now been published in the Journal of Clinical Oncology.
The Gynecologic Oncology Group 177 trial established TAP about a decade earlier as the standard for systemic treatment of stage III-IV and recurrent endometrial cancer. However, the regimen was associated with substantially more toxicity than doxorubicin-cisplatin.
Phase 2 trials of TC suggested that the combination was active in endometrial cancer, the authors noted. They hypothesized that doxorubicin could be omitted from the regimen and that carboplatin could be substituted for cisplatin.
Equivalent survival, lower toxicity
In the current trial, Dr. Miller and colleagues sought to determine whether TC was therapeutically equivalent or noninferior to TAP with regard to survival outcomes. Secondary endpoints involved the toxicity profile of TC, compared with TAP. The two regimens were also compared with respect to patient-reported neurotoxicity and health-related quality of life (HRQoL).
From 2003 to 2009, 1,381 women with stage III, stage IV, and recurrent endometrial cancers were enrolled in the phase 3 GOG0209 trial. Patients were treated with doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 (day 1), followed by paclitaxel 160 mg/m2 (day 2) with granulocyte colony–stimulating factor or paclitaxel 175 mg/m2 and carboplatin area under the curve 6 (day 1) every 21 days for seven cycles.
After treatment was completed, patients were followed quarterly for 2 years, semiannually for 3 years, and then annually until death. Most of the patients (61%) had measurable or recurrent disease at baseline.
In this updated analysis, with a median follow-up of 124 months, about two-thirds (>65%) of the patients had died; 28% remain alive without evidence of cancer. The adjusted ratio of death hazard of TC versus TAP was 1.002; for progression, the HR of TC to TAP was 1.032.
Median progression-free survival for TAP versus TC was 14 months versus 13 months, and for overall survival, 41 months versus 37 months.
As for adverse events, neutropenic fever was reported in 7% of patients who received TAP and in 6% of those who received TC. The rate of sensory neuropathy was greater among patients who received TAP (26% vs. 20%; P = .40), as was the rate of thrombocytopenia of grade ≥3 (23% vs. 12%), vomiting (7% vs. 4%), diarrhea (6% vs. 2%), and metabolic toxicities (14% vs. 8%). The rate of neutropenia was greater with TC (52% vs. 80%).
Data on HRQoL were collected from the first 538 patients enrolled before March 26, 2007. HRQoL was assessed at baseline and then at 6 weeks, 15 weeks, and 26 weeks. At 6 weeks, the TC group had higher scores on physical well-being and functional well-being (2.1-point difference; 0.3 to approximately 3.9 points; P = .009; effect size, 0.19). There were no statistically significant differences between groups at 15 and 26 weeks.
On the Functional Assessment of Cancer Therapy/GOG-Neurotoxicity four-item measure of sensory neuropathy (FACT/GOG-Ntx) subscale, scores were higher (indicating fewer neurotoxic symptoms) for patients in the TC group by 1.4 points (0.4 to approximately 2.5 points; P = .003; effect size, 0.64) at 26 weeks. There were no statistically significant differences between the groups at 6 and 15 weeks.
Dr. Miller noted that TC became the “backbone or control arm for most subsequent trials,” such as those evaluating immunotherapy and other agents in this setting.
The study was supported by National Cancer Institute grants to the GOG Administrative Office, the GOG Statistical Office, NRG Oncology (1 U10 CA180822), NRG Operations, and the National Cancer Institute Community Oncology Research Program. Dr. Miller has had a consulting or advisory role with Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Guardant Health, Janssen, Alexion Pharmaceuticals, Clovis Oncology, and Merck Sharp & Dohme; has been on the speakers’ bureaus for Clovis Oncology and Genentech; and has received institutional research funding from US Biotest, Advenchen Laboratories, Millennium, Tesaro, Xenetic Biosciences, Advaxis, Janssen, Aeterna Zentaris, TRACON Pharma, Pfizer, Immunogen, Mateon Therapeutics, and Merck Sharp & Dohme.
A version of this article originally appeared on Medscape.com.
The combination proved to be noninferior to paclitaxel-doxorubicin-cisplatin (TAP) in terms of response, progression-free survival, and overall survival, and with lower toxicity.
Overall survival was a median of 37 months for TC and 41 months for TAP, and there were more adverse events of grade 3 or higher with TAP.
The data were initially presented at the 2012 annual meeting of the Society of Gynecologic Oncology. In the original presentation, lead author David Scott Miller, MD, said this combination should be the standard of care in this setting. Dr. Miller is professor of obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas.
“This subsequent long-term follow-up publication confirmed that,” he said in an interview. “TAP is now rarely used.”
The results have now been published in the Journal of Clinical Oncology.
The Gynecologic Oncology Group 177 trial established TAP about a decade earlier as the standard for systemic treatment of stage III-IV and recurrent endometrial cancer. However, the regimen was associated with substantially more toxicity than doxorubicin-cisplatin.
Phase 2 trials of TC suggested that the combination was active in endometrial cancer, the authors noted. They hypothesized that doxorubicin could be omitted from the regimen and that carboplatin could be substituted for cisplatin.
Equivalent survival, lower toxicity
In the current trial, Dr. Miller and colleagues sought to determine whether TC was therapeutically equivalent or noninferior to TAP with regard to survival outcomes. Secondary endpoints involved the toxicity profile of TC, compared with TAP. The two regimens were also compared with respect to patient-reported neurotoxicity and health-related quality of life (HRQoL).
From 2003 to 2009, 1,381 women with stage III, stage IV, and recurrent endometrial cancers were enrolled in the phase 3 GOG0209 trial. Patients were treated with doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 (day 1), followed by paclitaxel 160 mg/m2 (day 2) with granulocyte colony–stimulating factor or paclitaxel 175 mg/m2 and carboplatin area under the curve 6 (day 1) every 21 days for seven cycles.
After treatment was completed, patients were followed quarterly for 2 years, semiannually for 3 years, and then annually until death. Most of the patients (61%) had measurable or recurrent disease at baseline.
In this updated analysis, with a median follow-up of 124 months, about two-thirds (>65%) of the patients had died; 28% remain alive without evidence of cancer. The adjusted ratio of death hazard of TC versus TAP was 1.002; for progression, the HR of TC to TAP was 1.032.
Median progression-free survival for TAP versus TC was 14 months versus 13 months, and for overall survival, 41 months versus 37 months.
As for adverse events, neutropenic fever was reported in 7% of patients who received TAP and in 6% of those who received TC. The rate of sensory neuropathy was greater among patients who received TAP (26% vs. 20%; P = .40), as was the rate of thrombocytopenia of grade ≥3 (23% vs. 12%), vomiting (7% vs. 4%), diarrhea (6% vs. 2%), and metabolic toxicities (14% vs. 8%). The rate of neutropenia was greater with TC (52% vs. 80%).
Data on HRQoL were collected from the first 538 patients enrolled before March 26, 2007. HRQoL was assessed at baseline and then at 6 weeks, 15 weeks, and 26 weeks. At 6 weeks, the TC group had higher scores on physical well-being and functional well-being (2.1-point difference; 0.3 to approximately 3.9 points; P = .009; effect size, 0.19). There were no statistically significant differences between groups at 15 and 26 weeks.
On the Functional Assessment of Cancer Therapy/GOG-Neurotoxicity four-item measure of sensory neuropathy (FACT/GOG-Ntx) subscale, scores were higher (indicating fewer neurotoxic symptoms) for patients in the TC group by 1.4 points (0.4 to approximately 2.5 points; P = .003; effect size, 0.64) at 26 weeks. There were no statistically significant differences between the groups at 6 and 15 weeks.
Dr. Miller noted that TC became the “backbone or control arm for most subsequent trials,” such as those evaluating immunotherapy and other agents in this setting.
The study was supported by National Cancer Institute grants to the GOG Administrative Office, the GOG Statistical Office, NRG Oncology (1 U10 CA180822), NRG Operations, and the National Cancer Institute Community Oncology Research Program. Dr. Miller has had a consulting or advisory role with Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Guardant Health, Janssen, Alexion Pharmaceuticals, Clovis Oncology, and Merck Sharp & Dohme; has been on the speakers’ bureaus for Clovis Oncology and Genentech; and has received institutional research funding from US Biotest, Advenchen Laboratories, Millennium, Tesaro, Xenetic Biosciences, Advaxis, Janssen, Aeterna Zentaris, TRACON Pharma, Pfizer, Immunogen, Mateon Therapeutics, and Merck Sharp & Dohme.
A version of this article originally appeared on Medscape.com.
The combination proved to be noninferior to paclitaxel-doxorubicin-cisplatin (TAP) in terms of response, progression-free survival, and overall survival, and with lower toxicity.
Overall survival was a median of 37 months for TC and 41 months for TAP, and there were more adverse events of grade 3 or higher with TAP.
The data were initially presented at the 2012 annual meeting of the Society of Gynecologic Oncology. In the original presentation, lead author David Scott Miller, MD, said this combination should be the standard of care in this setting. Dr. Miller is professor of obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas.
“This subsequent long-term follow-up publication confirmed that,” he said in an interview. “TAP is now rarely used.”
The results have now been published in the Journal of Clinical Oncology.
The Gynecologic Oncology Group 177 trial established TAP about a decade earlier as the standard for systemic treatment of stage III-IV and recurrent endometrial cancer. However, the regimen was associated with substantially more toxicity than doxorubicin-cisplatin.
Phase 2 trials of TC suggested that the combination was active in endometrial cancer, the authors noted. They hypothesized that doxorubicin could be omitted from the regimen and that carboplatin could be substituted for cisplatin.
Equivalent survival, lower toxicity
In the current trial, Dr. Miller and colleagues sought to determine whether TC was therapeutically equivalent or noninferior to TAP with regard to survival outcomes. Secondary endpoints involved the toxicity profile of TC, compared with TAP. The two regimens were also compared with respect to patient-reported neurotoxicity and health-related quality of life (HRQoL).
From 2003 to 2009, 1,381 women with stage III, stage IV, and recurrent endometrial cancers were enrolled in the phase 3 GOG0209 trial. Patients were treated with doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 (day 1), followed by paclitaxel 160 mg/m2 (day 2) with granulocyte colony–stimulating factor or paclitaxel 175 mg/m2 and carboplatin area under the curve 6 (day 1) every 21 days for seven cycles.
After treatment was completed, patients were followed quarterly for 2 years, semiannually for 3 years, and then annually until death. Most of the patients (61%) had measurable or recurrent disease at baseline.
In this updated analysis, with a median follow-up of 124 months, about two-thirds (>65%) of the patients had died; 28% remain alive without evidence of cancer. The adjusted ratio of death hazard of TC versus TAP was 1.002; for progression, the HR of TC to TAP was 1.032.
Median progression-free survival for TAP versus TC was 14 months versus 13 months, and for overall survival, 41 months versus 37 months.
As for adverse events, neutropenic fever was reported in 7% of patients who received TAP and in 6% of those who received TC. The rate of sensory neuropathy was greater among patients who received TAP (26% vs. 20%; P = .40), as was the rate of thrombocytopenia of grade ≥3 (23% vs. 12%), vomiting (7% vs. 4%), diarrhea (6% vs. 2%), and metabolic toxicities (14% vs. 8%). The rate of neutropenia was greater with TC (52% vs. 80%).
Data on HRQoL were collected from the first 538 patients enrolled before March 26, 2007. HRQoL was assessed at baseline and then at 6 weeks, 15 weeks, and 26 weeks. At 6 weeks, the TC group had higher scores on physical well-being and functional well-being (2.1-point difference; 0.3 to approximately 3.9 points; P = .009; effect size, 0.19). There were no statistically significant differences between groups at 15 and 26 weeks.
On the Functional Assessment of Cancer Therapy/GOG-Neurotoxicity four-item measure of sensory neuropathy (FACT/GOG-Ntx) subscale, scores were higher (indicating fewer neurotoxic symptoms) for patients in the TC group by 1.4 points (0.4 to approximately 2.5 points; P = .003; effect size, 0.64) at 26 weeks. There were no statistically significant differences between the groups at 6 and 15 weeks.
Dr. Miller noted that TC became the “backbone or control arm for most subsequent trials,” such as those evaluating immunotherapy and other agents in this setting.
The study was supported by National Cancer Institute grants to the GOG Administrative Office, the GOG Statistical Office, NRG Oncology (1 U10 CA180822), NRG Operations, and the National Cancer Institute Community Oncology Research Program. Dr. Miller has had a consulting or advisory role with Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Guardant Health, Janssen, Alexion Pharmaceuticals, Clovis Oncology, and Merck Sharp & Dohme; has been on the speakers’ bureaus for Clovis Oncology and Genentech; and has received institutional research funding from US Biotest, Advenchen Laboratories, Millennium, Tesaro, Xenetic Biosciences, Advaxis, Janssen, Aeterna Zentaris, TRACON Pharma, Pfizer, Immunogen, Mateon Therapeutics, and Merck Sharp & Dohme.
A version of this article originally appeared on Medscape.com.
Pregnancy outcomes ‘favorable’ after BRCA breast cancer treatment
said researchers reporting a review of more than 1,000 young women with breast cancer, mostly in Europe but also Israel and North and South America.
It’s been known that pregnancy after breast cancer treatment, even for hormone receptor–positive disease, is safe overall, the team commented. However, there have been concerns about women who have BRCA mutations because of a lack of data.
The new findings “provide reassurance to patients with BRCA-mutated breast cancer interested in future fertility” and are of “paramount importance for health care providers involved in counseling young patients,” said the researchers, led by Matteo Lambertini, MD, PhD, a medical oncologist at the University of Genoa, Italy.
The review was published in September in the Journal of Clinical Oncology.
The team reviewed reproductive outcomes among 1,252 women who were no older than 40 years when diagnosed with stage I-III BRCA-mutated invasive breast cancer between January 2000 and December 2012.
More than half (65%; n = 811) had BRCA1 mutations, 430 women (34%) had BRCA2 mutations, and 11 women had both.
Overall, 195 women became pregnant, at a median of 4.5 years after the breast cancer diagnosis and at a median age of 36 years.
The miscarriage rate was 10.3%, lower than expected in the general population.
Among the 150 patients who gave birth to 170 infants, delivery complications occurred in 13 of the 112 pregnancies with available data (11.6%), and congenital anomalies were seen in just 2 pregnancies (1.8%). This is a lower rate of anomalies than expected in the general population, the team noted. The rate of preterm delivery was 9.2%, similar to the general population.
There was no difference between the women who became pregnant and those who did not in either disease-free survival (adjusted hazard ratio, 0.87; P = .41) or overall survival (aHR, 0.88; P = .66), over a median follow-up of 8.3 years from diagnosis. In addition to BRCA mutations, the analysis adjusted for age at diagnosis, tumor size, nodal status, hormone receptor status, type of endocrine therapy, and breast surgery.
Over 80% of the subjects had ductal carcinoma, and over 90% of women were HER2-negative. More women in the pregnancy cohort had tumor diameters of 2 cm or less (47.2% vs. 40.9%) and a higher percentage had breast conserving surgery (59% vs. 45.9%).
Chemotherapy was administered to 95.3% of the subjects, most commonly anthracycline and taxane based, and more than 90% received endocrine therapy, most often tamoxifen alone among women who did not become pregnant and tamoxifen plus a luteinizing hormone-releasing hormone agonist among those who did. Endocrine therapy was shorter among women who became pregnant (median, 50 vs. 60 months; P < .001).
The findings held when 176 pregnant cases were matched to 528 nonpregnant controls for year of diagnosis, nodal status, hormone receptor status, and type of BRCA mutation. However, disease-free survival was improved among pregnant women (HR, 0.71; P = .045) who were younger at diagnosis, with median ages of 31 years vs. 36 years (P < .001).
The study was funded by the Italian Association for Cancer Research, among others. Dr. Lambertini reports acting as a consultant for Roche and Novartis and as a speaker for Theramex, Takeda, Roche, Eli Lilly, Novartis. Several coauthors also report relationships with pharmaceutical companies, as detailed in the original article.
A version of this article originally appeared on Medscape.com.
said researchers reporting a review of more than 1,000 young women with breast cancer, mostly in Europe but also Israel and North and South America.
It’s been known that pregnancy after breast cancer treatment, even for hormone receptor–positive disease, is safe overall, the team commented. However, there have been concerns about women who have BRCA mutations because of a lack of data.
The new findings “provide reassurance to patients with BRCA-mutated breast cancer interested in future fertility” and are of “paramount importance for health care providers involved in counseling young patients,” said the researchers, led by Matteo Lambertini, MD, PhD, a medical oncologist at the University of Genoa, Italy.
The review was published in September in the Journal of Clinical Oncology.
The team reviewed reproductive outcomes among 1,252 women who were no older than 40 years when diagnosed with stage I-III BRCA-mutated invasive breast cancer between January 2000 and December 2012.
More than half (65%; n = 811) had BRCA1 mutations, 430 women (34%) had BRCA2 mutations, and 11 women had both.
Overall, 195 women became pregnant, at a median of 4.5 years after the breast cancer diagnosis and at a median age of 36 years.
The miscarriage rate was 10.3%, lower than expected in the general population.
Among the 150 patients who gave birth to 170 infants, delivery complications occurred in 13 of the 112 pregnancies with available data (11.6%), and congenital anomalies were seen in just 2 pregnancies (1.8%). This is a lower rate of anomalies than expected in the general population, the team noted. The rate of preterm delivery was 9.2%, similar to the general population.
There was no difference between the women who became pregnant and those who did not in either disease-free survival (adjusted hazard ratio, 0.87; P = .41) or overall survival (aHR, 0.88; P = .66), over a median follow-up of 8.3 years from diagnosis. In addition to BRCA mutations, the analysis adjusted for age at diagnosis, tumor size, nodal status, hormone receptor status, type of endocrine therapy, and breast surgery.
Over 80% of the subjects had ductal carcinoma, and over 90% of women were HER2-negative. More women in the pregnancy cohort had tumor diameters of 2 cm or less (47.2% vs. 40.9%) and a higher percentage had breast conserving surgery (59% vs. 45.9%).
Chemotherapy was administered to 95.3% of the subjects, most commonly anthracycline and taxane based, and more than 90% received endocrine therapy, most often tamoxifen alone among women who did not become pregnant and tamoxifen plus a luteinizing hormone-releasing hormone agonist among those who did. Endocrine therapy was shorter among women who became pregnant (median, 50 vs. 60 months; P < .001).
The findings held when 176 pregnant cases were matched to 528 nonpregnant controls for year of diagnosis, nodal status, hormone receptor status, and type of BRCA mutation. However, disease-free survival was improved among pregnant women (HR, 0.71; P = .045) who were younger at diagnosis, with median ages of 31 years vs. 36 years (P < .001).
The study was funded by the Italian Association for Cancer Research, among others. Dr. Lambertini reports acting as a consultant for Roche and Novartis and as a speaker for Theramex, Takeda, Roche, Eli Lilly, Novartis. Several coauthors also report relationships with pharmaceutical companies, as detailed in the original article.
A version of this article originally appeared on Medscape.com.
said researchers reporting a review of more than 1,000 young women with breast cancer, mostly in Europe but also Israel and North and South America.
It’s been known that pregnancy after breast cancer treatment, even for hormone receptor–positive disease, is safe overall, the team commented. However, there have been concerns about women who have BRCA mutations because of a lack of data.
The new findings “provide reassurance to patients with BRCA-mutated breast cancer interested in future fertility” and are of “paramount importance for health care providers involved in counseling young patients,” said the researchers, led by Matteo Lambertini, MD, PhD, a medical oncologist at the University of Genoa, Italy.
The review was published in September in the Journal of Clinical Oncology.
The team reviewed reproductive outcomes among 1,252 women who were no older than 40 years when diagnosed with stage I-III BRCA-mutated invasive breast cancer between January 2000 and December 2012.
More than half (65%; n = 811) had BRCA1 mutations, 430 women (34%) had BRCA2 mutations, and 11 women had both.
Overall, 195 women became pregnant, at a median of 4.5 years after the breast cancer diagnosis and at a median age of 36 years.
The miscarriage rate was 10.3%, lower than expected in the general population.
Among the 150 patients who gave birth to 170 infants, delivery complications occurred in 13 of the 112 pregnancies with available data (11.6%), and congenital anomalies were seen in just 2 pregnancies (1.8%). This is a lower rate of anomalies than expected in the general population, the team noted. The rate of preterm delivery was 9.2%, similar to the general population.
There was no difference between the women who became pregnant and those who did not in either disease-free survival (adjusted hazard ratio, 0.87; P = .41) or overall survival (aHR, 0.88; P = .66), over a median follow-up of 8.3 years from diagnosis. In addition to BRCA mutations, the analysis adjusted for age at diagnosis, tumor size, nodal status, hormone receptor status, type of endocrine therapy, and breast surgery.
Over 80% of the subjects had ductal carcinoma, and over 90% of women were HER2-negative. More women in the pregnancy cohort had tumor diameters of 2 cm or less (47.2% vs. 40.9%) and a higher percentage had breast conserving surgery (59% vs. 45.9%).
Chemotherapy was administered to 95.3% of the subjects, most commonly anthracycline and taxane based, and more than 90% received endocrine therapy, most often tamoxifen alone among women who did not become pregnant and tamoxifen plus a luteinizing hormone-releasing hormone agonist among those who did. Endocrine therapy was shorter among women who became pregnant (median, 50 vs. 60 months; P < .001).
The findings held when 176 pregnant cases were matched to 528 nonpregnant controls for year of diagnosis, nodal status, hormone receptor status, and type of BRCA mutation. However, disease-free survival was improved among pregnant women (HR, 0.71; P = .045) who were younger at diagnosis, with median ages of 31 years vs. 36 years (P < .001).
The study was funded by the Italian Association for Cancer Research, among others. Dr. Lambertini reports acting as a consultant for Roche and Novartis and as a speaker for Theramex, Takeda, Roche, Eli Lilly, Novartis. Several coauthors also report relationships with pharmaceutical companies, as detailed in the original article.
A version of this article originally appeared on Medscape.com.

