User login
Rap music mention of mental health topics more than doubles
Mental health distress is rising but often is undertreated among children and young adults in the United States, wrote Alex Kresovich, MA, of the University of North Carolina, Chapel Hill, and colleagues.
“Mental health risk especially is increasing among young Black/ African American male individuals (YBAAM), who are often disproportionately exposed to environmental, economic, and family stressors linked with depression and anxiety,” they said. Adolescents and young adults, especially YBAAM, make up a large part of the audience for rap music.
In recent years, more rap artists have disclosed mental health issues, and they have included mental health topics such as depression and suicidal thoughts into their music, the researchers said.
In a study published in JAMA Pediatrics, the researchers identified 125 songs from the period between 1998 and 2018, then assessed them for references to mental health. The song selections included the top 25 rap songs in 1998, 2003, 2008, 2013, and 2018, based on the Billboard music charts.
The majority of the songs (123) featured lead artists from North America, and 97 of them were Black/African American males. The average age of the artists was 28 years. “Prominent artists captured in the sample included 50 Cent, Drake, Eminem, Kanye West, Jay-Z, and Lil’Wayne, among others,” they said. The researchers divided mental health issues into four categories: anxiety or anxious thinking; depression or depressive thinking; metaphors (such as struggling with mental stability); and suicide or suicidal ideation.
Mental health references rise
Across the study period, 35 songs (28%) mentioned anxiety, 28 (22%) mentioned depression, 8 (6%) mentioned suicide, and 26 (21%) mentioned a mental health metaphor. The proportion of songs with a mental health reference increased in a significant linear trend across the study period for suicide (0%-12%), depression (16%-32%), and mental health metaphors (8%-44%).
All references to suicide or suicidal ideation were found in songs that were popular between 2013 and 2018, the researchers noted.
“This increase is important, given that rap artists serve as role models to their audience, which extends beyond YBAAM to include U.S. young people across strata, constituting a large group with increased risk of mental health issues and underuse of mental health services,” Mr. Kresovich and associates said.
In addition, the researchers found that stressors related to environmental conditions and love were significantly more likely to co-occur with mental health references (adjusted odds ratios 8.1 and 4.8, respectively).
The study findings were limited by several factors including the selection of songs only from the Billboard hot rap songs year-end charts, which “does not fully represent the population of rap music between 1998 and 2018,” the researchers said. In addition, they could not address causation or motivations for the increased mental health references over the study period. “We are also unable to ascertain how U.S. youth interact with this music or are positively or negatively affected by its messages.”
“For example, positively framed references to mental health awareness, treatment, or support may lead to reduced stigma and increased willingness to seek treatment,” Mr. Kresovich and associates wrote. “However, negatively framed references to mental health struggles might lead to negative outcomes, including copycat behavior in which listeners model harmful behavior, such as suicide attempts, if those behaviors are described in lyrics (i.e., the Werther effect),” they added.
Despite these limitations, the results support the need for more research on the impact of rap music as a way to reduce stigma and potentially reduce mental health risk in adolescents and young adults, Mr. Kresovich and associates concluded.
Music may help raise tough topics
The study is important because children and adolescents have more control than ever over the media they consume, Sarah Vinson, MD, founder of the Lorio Psych Group in Atlanta, said in an interview.
“With more and more children with access to their own devices, they spend a great amount of time consuming content, including music,” Dr. Vinson said. “The norms reflected in the lyrics they hear have an impact on their emerging view of themselves, others, and the world.”
The increased recognition of mental health issues by rap musicians as a topic “certainly has the potential to have a positive impact; however, the way that it is discussed can influence [the] nature of that impact,” she explained.
“It is important for people who are dealing with the normal range of human emotions to know that they are not alone. It is even more important for people dealing with suicidality or mental illness to know that,” Dr. Vinson said.
“Validation and sense of connection are human needs, and stigma related to mental illness can be isolating,” she emphasized. “Rappers have a platform and are often people that children and adolescents look up to, for better or for worse.” Through their music, “the rappers are signaling that these topics are worthy of our attention and okay to talk about.”
Unfortunately, many barriers persist for adolescents in need of mental health treatment, said Dr. Vinson. “The children’s mental health workforce, quantitatively, is not enough to meet the current needs,” she said. “Mental health is not reimbursed at the same rate as other kinds of health care, which contributes to healthy systems not prioritizing these services. Additionally, the racial, ethnic, and socioeconomic background of those who are mental health providers is not reflective of the larger population, and mental health training insufficiently incorporates the cultural and structural humility needed to help professionals navigate those differences,” she explained.
“Children at increased risk are those who face many of those environmental barriers that the rappers reference in those lyrics. They are likely to have even poorer access because they are disproportionately impacted by residential segregation, transportation challenges, financial barriers, and structural racism in mental health care,” Dr. Vinson added. A take-home message for clinicians is to find out what their patients are listening to. “One way to understand what is on the hearts and minds of children is to ask them what’s in their playlist,” she said.
Additional research is needed to examine “moderating factors for the impact, good or bad, of increased mental health content in hip hop for young listeners’ mental health awareness, symptoms and/or interest in seeking treatment,” Dr. Vinson concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Dr. Vinson served as chair for a workshop on mental health and hip-hop at the American Psychiatric Association annual meeting. She had no financial conflicts to disclose.
SOURCE: Kresovich A et al. JAMA Pediatr. 2020 Dec 7. doi: 10.1001/jamapediatrics.2020.5155.
This article was updated on December 21, 2020.
Mental health distress is rising but often is undertreated among children and young adults in the United States, wrote Alex Kresovich, MA, of the University of North Carolina, Chapel Hill, and colleagues.
“Mental health risk especially is increasing among young Black/ African American male individuals (YBAAM), who are often disproportionately exposed to environmental, economic, and family stressors linked with depression and anxiety,” they said. Adolescents and young adults, especially YBAAM, make up a large part of the audience for rap music.
In recent years, more rap artists have disclosed mental health issues, and they have included mental health topics such as depression and suicidal thoughts into their music, the researchers said.
In a study published in JAMA Pediatrics, the researchers identified 125 songs from the period between 1998 and 2018, then assessed them for references to mental health. The song selections included the top 25 rap songs in 1998, 2003, 2008, 2013, and 2018, based on the Billboard music charts.
The majority of the songs (123) featured lead artists from North America, and 97 of them were Black/African American males. The average age of the artists was 28 years. “Prominent artists captured in the sample included 50 Cent, Drake, Eminem, Kanye West, Jay-Z, and Lil’Wayne, among others,” they said. The researchers divided mental health issues into four categories: anxiety or anxious thinking; depression or depressive thinking; metaphors (such as struggling with mental stability); and suicide or suicidal ideation.
Mental health references rise
Across the study period, 35 songs (28%) mentioned anxiety, 28 (22%) mentioned depression, 8 (6%) mentioned suicide, and 26 (21%) mentioned a mental health metaphor. The proportion of songs with a mental health reference increased in a significant linear trend across the study period for suicide (0%-12%), depression (16%-32%), and mental health metaphors (8%-44%).
All references to suicide or suicidal ideation were found in songs that were popular between 2013 and 2018, the researchers noted.
“This increase is important, given that rap artists serve as role models to their audience, which extends beyond YBAAM to include U.S. young people across strata, constituting a large group with increased risk of mental health issues and underuse of mental health services,” Mr. Kresovich and associates said.
In addition, the researchers found that stressors related to environmental conditions and love were significantly more likely to co-occur with mental health references (adjusted odds ratios 8.1 and 4.8, respectively).
The study findings were limited by several factors including the selection of songs only from the Billboard hot rap songs year-end charts, which “does not fully represent the population of rap music between 1998 and 2018,” the researchers said. In addition, they could not address causation or motivations for the increased mental health references over the study period. “We are also unable to ascertain how U.S. youth interact with this music or are positively or negatively affected by its messages.”
“For example, positively framed references to mental health awareness, treatment, or support may lead to reduced stigma and increased willingness to seek treatment,” Mr. Kresovich and associates wrote. “However, negatively framed references to mental health struggles might lead to negative outcomes, including copycat behavior in which listeners model harmful behavior, such as suicide attempts, if those behaviors are described in lyrics (i.e., the Werther effect),” they added.
Despite these limitations, the results support the need for more research on the impact of rap music as a way to reduce stigma and potentially reduce mental health risk in adolescents and young adults, Mr. Kresovich and associates concluded.
Music may help raise tough topics
The study is important because children and adolescents have more control than ever over the media they consume, Sarah Vinson, MD, founder of the Lorio Psych Group in Atlanta, said in an interview.
“With more and more children with access to their own devices, they spend a great amount of time consuming content, including music,” Dr. Vinson said. “The norms reflected in the lyrics they hear have an impact on their emerging view of themselves, others, and the world.”
The increased recognition of mental health issues by rap musicians as a topic “certainly has the potential to have a positive impact; however, the way that it is discussed can influence [the] nature of that impact,” she explained.
“It is important for people who are dealing with the normal range of human emotions to know that they are not alone. It is even more important for people dealing with suicidality or mental illness to know that,” Dr. Vinson said.
“Validation and sense of connection are human needs, and stigma related to mental illness can be isolating,” she emphasized. “Rappers have a platform and are often people that children and adolescents look up to, for better or for worse.” Through their music, “the rappers are signaling that these topics are worthy of our attention and okay to talk about.”
Unfortunately, many barriers persist for adolescents in need of mental health treatment, said Dr. Vinson. “The children’s mental health workforce, quantitatively, is not enough to meet the current needs,” she said. “Mental health is not reimbursed at the same rate as other kinds of health care, which contributes to healthy systems not prioritizing these services. Additionally, the racial, ethnic, and socioeconomic background of those who are mental health providers is not reflective of the larger population, and mental health training insufficiently incorporates the cultural and structural humility needed to help professionals navigate those differences,” she explained.
“Children at increased risk are those who face many of those environmental barriers that the rappers reference in those lyrics. They are likely to have even poorer access because they are disproportionately impacted by residential segregation, transportation challenges, financial barriers, and structural racism in mental health care,” Dr. Vinson added. A take-home message for clinicians is to find out what their patients are listening to. “One way to understand what is on the hearts and minds of children is to ask them what’s in their playlist,” she said.
Additional research is needed to examine “moderating factors for the impact, good or bad, of increased mental health content in hip hop for young listeners’ mental health awareness, symptoms and/or interest in seeking treatment,” Dr. Vinson concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Dr. Vinson served as chair for a workshop on mental health and hip-hop at the American Psychiatric Association annual meeting. She had no financial conflicts to disclose.
SOURCE: Kresovich A et al. JAMA Pediatr. 2020 Dec 7. doi: 10.1001/jamapediatrics.2020.5155.
This article was updated on December 21, 2020.
Mental health distress is rising but often is undertreated among children and young adults in the United States, wrote Alex Kresovich, MA, of the University of North Carolina, Chapel Hill, and colleagues.
“Mental health risk especially is increasing among young Black/ African American male individuals (YBAAM), who are often disproportionately exposed to environmental, economic, and family stressors linked with depression and anxiety,” they said. Adolescents and young adults, especially YBAAM, make up a large part of the audience for rap music.
In recent years, more rap artists have disclosed mental health issues, and they have included mental health topics such as depression and suicidal thoughts into their music, the researchers said.
In a study published in JAMA Pediatrics, the researchers identified 125 songs from the period between 1998 and 2018, then assessed them for references to mental health. The song selections included the top 25 rap songs in 1998, 2003, 2008, 2013, and 2018, based on the Billboard music charts.
The majority of the songs (123) featured lead artists from North America, and 97 of them were Black/African American males. The average age of the artists was 28 years. “Prominent artists captured in the sample included 50 Cent, Drake, Eminem, Kanye West, Jay-Z, and Lil’Wayne, among others,” they said. The researchers divided mental health issues into four categories: anxiety or anxious thinking; depression or depressive thinking; metaphors (such as struggling with mental stability); and suicide or suicidal ideation.
Mental health references rise
Across the study period, 35 songs (28%) mentioned anxiety, 28 (22%) mentioned depression, 8 (6%) mentioned suicide, and 26 (21%) mentioned a mental health metaphor. The proportion of songs with a mental health reference increased in a significant linear trend across the study period for suicide (0%-12%), depression (16%-32%), and mental health metaphors (8%-44%).
All references to suicide or suicidal ideation were found in songs that were popular between 2013 and 2018, the researchers noted.
“This increase is important, given that rap artists serve as role models to their audience, which extends beyond YBAAM to include U.S. young people across strata, constituting a large group with increased risk of mental health issues and underuse of mental health services,” Mr. Kresovich and associates said.
In addition, the researchers found that stressors related to environmental conditions and love were significantly more likely to co-occur with mental health references (adjusted odds ratios 8.1 and 4.8, respectively).
The study findings were limited by several factors including the selection of songs only from the Billboard hot rap songs year-end charts, which “does not fully represent the population of rap music between 1998 and 2018,” the researchers said. In addition, they could not address causation or motivations for the increased mental health references over the study period. “We are also unable to ascertain how U.S. youth interact with this music or are positively or negatively affected by its messages.”
“For example, positively framed references to mental health awareness, treatment, or support may lead to reduced stigma and increased willingness to seek treatment,” Mr. Kresovich and associates wrote. “However, negatively framed references to mental health struggles might lead to negative outcomes, including copycat behavior in which listeners model harmful behavior, such as suicide attempts, if those behaviors are described in lyrics (i.e., the Werther effect),” they added.
Despite these limitations, the results support the need for more research on the impact of rap music as a way to reduce stigma and potentially reduce mental health risk in adolescents and young adults, Mr. Kresovich and associates concluded.
Music may help raise tough topics
The study is important because children and adolescents have more control than ever over the media they consume, Sarah Vinson, MD, founder of the Lorio Psych Group in Atlanta, said in an interview.
“With more and more children with access to their own devices, they spend a great amount of time consuming content, including music,” Dr. Vinson said. “The norms reflected in the lyrics they hear have an impact on their emerging view of themselves, others, and the world.”
The increased recognition of mental health issues by rap musicians as a topic “certainly has the potential to have a positive impact; however, the way that it is discussed can influence [the] nature of that impact,” she explained.
“It is important for people who are dealing with the normal range of human emotions to know that they are not alone. It is even more important for people dealing with suicidality or mental illness to know that,” Dr. Vinson said.
“Validation and sense of connection are human needs, and stigma related to mental illness can be isolating,” she emphasized. “Rappers have a platform and are often people that children and adolescents look up to, for better or for worse.” Through their music, “the rappers are signaling that these topics are worthy of our attention and okay to talk about.”
Unfortunately, many barriers persist for adolescents in need of mental health treatment, said Dr. Vinson. “The children’s mental health workforce, quantitatively, is not enough to meet the current needs,” she said. “Mental health is not reimbursed at the same rate as other kinds of health care, which contributes to healthy systems not prioritizing these services. Additionally, the racial, ethnic, and socioeconomic background of those who are mental health providers is not reflective of the larger population, and mental health training insufficiently incorporates the cultural and structural humility needed to help professionals navigate those differences,” she explained.
“Children at increased risk are those who face many of those environmental barriers that the rappers reference in those lyrics. They are likely to have even poorer access because they are disproportionately impacted by residential segregation, transportation challenges, financial barriers, and structural racism in mental health care,” Dr. Vinson added. A take-home message for clinicians is to find out what their patients are listening to. “One way to understand what is on the hearts and minds of children is to ask them what’s in their playlist,” she said.
Additional research is needed to examine “moderating factors for the impact, good or bad, of increased mental health content in hip hop for young listeners’ mental health awareness, symptoms and/or interest in seeking treatment,” Dr. Vinson concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Dr. Vinson served as chair for a workshop on mental health and hip-hop at the American Psychiatric Association annual meeting. She had no financial conflicts to disclose.
SOURCE: Kresovich A et al. JAMA Pediatr. 2020 Dec 7. doi: 10.1001/jamapediatrics.2020.5155.
This article was updated on December 21, 2020.
FROM JAMA PEDIATRICS
Cryoballoon, cryospray found equivalent for eradicating Barret’s esophagus
Cryoballoon and cryospray ablation were equivalent for eradicating dysplastic Barrett’s esophagus, according to the findings of a single-center retrospective study of 71 ablation-naive patients.
At 18 months, rates of complete eradication of dysplasia were 95.6% in patients who received cryoballoon therapy and 96% in recipients of cryospray, reported Mohammed Alshelleh, MD, of Northwell Health System, a tertiary care system in New Hyde Park, N.Y. Rates of complete eradication of intestinal metaplasia were 84.75% and 80%, respectively. However, selection bias was likely, and a post hoc power calculation suggested that the cryospray group was underpowered by four patients. “Prospective studies are needed to confirm [these] data,” Dr. Alshelleh and associates wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
Cryotherapy is common for treating dysplastic Barrett’s esophagus when patients do not achieve remission with radiofrequency ablation. For treatment-naive individuals, prospective studies suggest that cryotherapy may be less painful and as effective as radiofrequency ablation, but no studies have directly compared the two commercially available systems: a cryogenic balloon catheter (C2 Cryoballoon, Pentax Medical, Montvale, N.J.) that delivers cryogenic nitrous oxide (–85° C) into an inflated balloon in direct contact with the esophageal mucosa, and a spray cryotherapy system (truFreeze, Steris Endoscopy, Mentor, Ohio), which flash-freezes the mucosa to –196° C by delivering liquid nitrogen through a low-pressure catheter that is not directly in contact with the esophagus.
For the study, the investigators retrospectively compared rates of complete eradication of dysplasia, and complete eradication of intestinal metaplasia, among ablation-naive patients at their institution who had received one of these two cryogenic modalities between 2015 and 2019. All patients were treated at least twice, at 3-month intervals, and were followed for least 12 months, or until complete eradication of intestinal metaplasia was confirmed by at least one endoscopic biopsy. In all, 46 patients received cryoballoon therapy and 25 received cryospray. Outcomes between the two modalities showed no significant differences in subgroups stratified by baseline histology, nor were there significant differences in rates of postprocedural stricture (8.7% in the cryoballoon group vs. 12% in the cryospray group). However, the investigators acknowledged that the study was underpowered. Overall, clinicians tended to prefer cryoballoon because it uses prefilled nitrous oxide cartridges, making it unnecessary to fill up a large nitrogen tank or use a “cumbersome decompression tube,” the investigators wrote. “However, in patients with a very large hiatal hernia or if there was a need to treat in a retroflexed position, spray cryotherapy was used given its ease of use over cryoballoon in these scenarios. Finally, cryospray is more amenable to treat larger surface areas of Barrett’s versus the focal cryoballoon that treats focal areas, and thus was the cryotherapy choice for a long segment of Barrett’s.”
The investigators reported receiving no grant support. One investigator disclosed ties to Olympus America, Pentax Medical Research, and Ninepoint Medical.
SOURCE: Alshelleh M et al. Tech Innov Gastrointest Endosc. 2020 Jul 26. doi: 10.1016/j.tige.2020.07.004.
The role of cryotherapy in Barrett’s esophagus eradication continues to evolve. Early data on liquid nitrogen (LN) cryospray included patients who failed radiofrequency ablation or had long segment or nodular disease, resulting in eradication rates lower than those for RFA. More recent studies, with cohorts similar to RFA studies, show comparable results with LN cryospray and the newer nitrous oxide cryoballoon. Cryotherapy tends to produce less postprocedure pain compared with RFA, especially when treating longer segments, and this is a common reason for choosing cryotherapy. This study by Alshelleh et al. compared complete eradication rates of dysplasia and intestinal metaplasia between cryospray and cryoballoon in a retrospective single-center study. Complete eradication rate of dysplasia was 95%-96% and that of intestinal metaplasia was 80%-85%, comparable with reported results for RFA.
Bruce D. Greenwald, MD, is a professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore. He receives research funding from and serves as a consultant for Steris.
The role of cryotherapy in Barrett’s esophagus eradication continues to evolve. Early data on liquid nitrogen (LN) cryospray included patients who failed radiofrequency ablation or had long segment or nodular disease, resulting in eradication rates lower than those for RFA. More recent studies, with cohorts similar to RFA studies, show comparable results with LN cryospray and the newer nitrous oxide cryoballoon. Cryotherapy tends to produce less postprocedure pain compared with RFA, especially when treating longer segments, and this is a common reason for choosing cryotherapy. This study by Alshelleh et al. compared complete eradication rates of dysplasia and intestinal metaplasia between cryospray and cryoballoon in a retrospective single-center study. Complete eradication rate of dysplasia was 95%-96% and that of intestinal metaplasia was 80%-85%, comparable with reported results for RFA.
Bruce D. Greenwald, MD, is a professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore. He receives research funding from and serves as a consultant for Steris.
The role of cryotherapy in Barrett’s esophagus eradication continues to evolve. Early data on liquid nitrogen (LN) cryospray included patients who failed radiofrequency ablation or had long segment or nodular disease, resulting in eradication rates lower than those for RFA. More recent studies, with cohorts similar to RFA studies, show comparable results with LN cryospray and the newer nitrous oxide cryoballoon. Cryotherapy tends to produce less postprocedure pain compared with RFA, especially when treating longer segments, and this is a common reason for choosing cryotherapy. This study by Alshelleh et al. compared complete eradication rates of dysplasia and intestinal metaplasia between cryospray and cryoballoon in a retrospective single-center study. Complete eradication rate of dysplasia was 95%-96% and that of intestinal metaplasia was 80%-85%, comparable with reported results for RFA.
Bruce D. Greenwald, MD, is a professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore. He receives research funding from and serves as a consultant for Steris.
Cryoballoon and cryospray ablation were equivalent for eradicating dysplastic Barrett’s esophagus, according to the findings of a single-center retrospective study of 71 ablation-naive patients.
At 18 months, rates of complete eradication of dysplasia were 95.6% in patients who received cryoballoon therapy and 96% in recipients of cryospray, reported Mohammed Alshelleh, MD, of Northwell Health System, a tertiary care system in New Hyde Park, N.Y. Rates of complete eradication of intestinal metaplasia were 84.75% and 80%, respectively. However, selection bias was likely, and a post hoc power calculation suggested that the cryospray group was underpowered by four patients. “Prospective studies are needed to confirm [these] data,” Dr. Alshelleh and associates wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
Cryotherapy is common for treating dysplastic Barrett’s esophagus when patients do not achieve remission with radiofrequency ablation. For treatment-naive individuals, prospective studies suggest that cryotherapy may be less painful and as effective as radiofrequency ablation, but no studies have directly compared the two commercially available systems: a cryogenic balloon catheter (C2 Cryoballoon, Pentax Medical, Montvale, N.J.) that delivers cryogenic nitrous oxide (–85° C) into an inflated balloon in direct contact with the esophageal mucosa, and a spray cryotherapy system (truFreeze, Steris Endoscopy, Mentor, Ohio), which flash-freezes the mucosa to –196° C by delivering liquid nitrogen through a low-pressure catheter that is not directly in contact with the esophagus.
For the study, the investigators retrospectively compared rates of complete eradication of dysplasia, and complete eradication of intestinal metaplasia, among ablation-naive patients at their institution who had received one of these two cryogenic modalities between 2015 and 2019. All patients were treated at least twice, at 3-month intervals, and were followed for least 12 months, or until complete eradication of intestinal metaplasia was confirmed by at least one endoscopic biopsy. In all, 46 patients received cryoballoon therapy and 25 received cryospray. Outcomes between the two modalities showed no significant differences in subgroups stratified by baseline histology, nor were there significant differences in rates of postprocedural stricture (8.7% in the cryoballoon group vs. 12% in the cryospray group). However, the investigators acknowledged that the study was underpowered. Overall, clinicians tended to prefer cryoballoon because it uses prefilled nitrous oxide cartridges, making it unnecessary to fill up a large nitrogen tank or use a “cumbersome decompression tube,” the investigators wrote. “However, in patients with a very large hiatal hernia or if there was a need to treat in a retroflexed position, spray cryotherapy was used given its ease of use over cryoballoon in these scenarios. Finally, cryospray is more amenable to treat larger surface areas of Barrett’s versus the focal cryoballoon that treats focal areas, and thus was the cryotherapy choice for a long segment of Barrett’s.”
The investigators reported receiving no grant support. One investigator disclosed ties to Olympus America, Pentax Medical Research, and Ninepoint Medical.
SOURCE: Alshelleh M et al. Tech Innov Gastrointest Endosc. 2020 Jul 26. doi: 10.1016/j.tige.2020.07.004.
Cryoballoon and cryospray ablation were equivalent for eradicating dysplastic Barrett’s esophagus, according to the findings of a single-center retrospective study of 71 ablation-naive patients.
At 18 months, rates of complete eradication of dysplasia were 95.6% in patients who received cryoballoon therapy and 96% in recipients of cryospray, reported Mohammed Alshelleh, MD, of Northwell Health System, a tertiary care system in New Hyde Park, N.Y. Rates of complete eradication of intestinal metaplasia were 84.75% and 80%, respectively. However, selection bias was likely, and a post hoc power calculation suggested that the cryospray group was underpowered by four patients. “Prospective studies are needed to confirm [these] data,” Dr. Alshelleh and associates wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
Cryotherapy is common for treating dysplastic Barrett’s esophagus when patients do not achieve remission with radiofrequency ablation. For treatment-naive individuals, prospective studies suggest that cryotherapy may be less painful and as effective as radiofrequency ablation, but no studies have directly compared the two commercially available systems: a cryogenic balloon catheter (C2 Cryoballoon, Pentax Medical, Montvale, N.J.) that delivers cryogenic nitrous oxide (–85° C) into an inflated balloon in direct contact with the esophageal mucosa, and a spray cryotherapy system (truFreeze, Steris Endoscopy, Mentor, Ohio), which flash-freezes the mucosa to –196° C by delivering liquid nitrogen through a low-pressure catheter that is not directly in contact with the esophagus.
For the study, the investigators retrospectively compared rates of complete eradication of dysplasia, and complete eradication of intestinal metaplasia, among ablation-naive patients at their institution who had received one of these two cryogenic modalities between 2015 and 2019. All patients were treated at least twice, at 3-month intervals, and were followed for least 12 months, or until complete eradication of intestinal metaplasia was confirmed by at least one endoscopic biopsy. In all, 46 patients received cryoballoon therapy and 25 received cryospray. Outcomes between the two modalities showed no significant differences in subgroups stratified by baseline histology, nor were there significant differences in rates of postprocedural stricture (8.7% in the cryoballoon group vs. 12% in the cryospray group). However, the investigators acknowledged that the study was underpowered. Overall, clinicians tended to prefer cryoballoon because it uses prefilled nitrous oxide cartridges, making it unnecessary to fill up a large nitrogen tank or use a “cumbersome decompression tube,” the investigators wrote. “However, in patients with a very large hiatal hernia or if there was a need to treat in a retroflexed position, spray cryotherapy was used given its ease of use over cryoballoon in these scenarios. Finally, cryospray is more amenable to treat larger surface areas of Barrett’s versus the focal cryoballoon that treats focal areas, and thus was the cryotherapy choice for a long segment of Barrett’s.”
The investigators reported receiving no grant support. One investigator disclosed ties to Olympus America, Pentax Medical Research, and Ninepoint Medical.
SOURCE: Alshelleh M et al. Tech Innov Gastrointest Endosc. 2020 Jul 26. doi: 10.1016/j.tige.2020.07.004.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Phase 1 study: Beta-blocker may improve melanoma treatment response
Response rates were high without dose-limiting toxicities in a small phase 1 study that evaluated the addition of propranolol to pembrolizumab in treatment-naive patients with metastatic melanoma.
“To our knowledge, this effort is the,” wrote the two co-first authors, Shipra Gandhi, MD, and Manu Pandey, MBBS, from the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and coauthors.
The need for combinations built on anti-PD1 checkpoint inhibitor therapy strategies in metastatic melanoma that safely improve outcomes is underscored by the high (59%) grade 3 or 4 treatment-related adverse event (TRAE) rates when an anti-CTLA4 agent (ipilimumab) was added to an anti-PD-1 agent (nivolumab), they noted. In contrast, a TRAE rate of only 17% has been reported with pembrolizumab monotherapy.
The phase 1b study was stimulated by preclinical, retrospective observations of improved overall survival (OS) in cancer patients treated with beta-blockers. These were preceded by murine melanoma studies showing decreased tumor growth and metastasis with the nonselective beta-blocker propranolol. “Propranolol exerts an antitumor effect,” the authors stated, “by favorably modulating the tumor microenvironment (TME) by decreasing myeloid-derived suppressor cells and increasing CD8+ T-cell and natural killer cells in the TME.” Other research in a melanoma model in chronically-stressed mice has demonstrated synergy between an anti-PD1 antibody and propranolol.
“We know that stress can have a significant negative effect on health, but the extent to which stress may impact the outcome of cancer therapy is not well understood at all,” Dr. Ghandi said in a statement provided by Roswell Park. “We set out to better understand this relationship and to explore its implications for cancer treatment.”
The investigators recruited nine White adults (median age 65 years) with treatment-naive, histologically confirmed unresectable stage III or IV melanoma and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 to the open-label, single arm, nonrandomized, single-center, dose-finding study. Patients received standard of care intravenous pembrolizumab 200 mg every 3 weeks and, in three groups, propranolol doses of 10 mg, 20 mg, or 30 mg twice a day until 2 years on study or disease progression or the development of dose-limiting toxicities (DLTs). Assessing the safety and efficacy (overall response rate [ORR] within 6 months of starting therapy) of pembrolizumab with the increasing doses of propranolol and selecting the recommended phase 2 dose were the study’s primary objectives.
Objective responses (complete or partial responses) were reported in seven of the nine patients, with partial tumor responses in two patients in the propranolol 10-mg group, two partial responses in the 20-mg group, and three partial responses in the 30-mg group.
While all patients experienced TRAEs, only one was above grade 2. The most commonly reported TRAEs were fatigue, rash and vitiligo, reported in four of the nine patients. Two patients in the 20-mg twice-a-day group discontinued therapy because of TRAEs (hemophagocytic lymphohistiocytosis and labyrinthitis). No DLTs were observed at any of the three dose levels, and no deaths occurred on study treatment.
The authors said that propranolol 30 mg twice a day was chosen as the recommended phase 2 dose, because in combination with pembrolizumab, there were no DLTs, and preliminary antitumor efficacy was observed in all three patients. Also, in all three patients, the investigators observed a trend toward higher CD8+T-cell percentage, higher ratios of CD8+T-cell/ Treg and CD8+T-cell/ polymorphonuclear myeloid-derived suppressor cells. They underscored, however, that the small size and significant heterogeneity in biomarkers made a statistically sound and meaningful interpretation of biomarkers for deciding the phase 2 dose difficult.
“In repurposing propranolol,” Dr. Pandey said in the Roswell statement, “we’ve gained important insights on how to manage stress in people with cancer – who can face dangerously elevated levels of mental and physical stress related to their diagnosis and treatment.”
In an interview, one of the two senior authors, Elizabeth Repasky, PhD, professor of oncology and immunology at Roswell Park, said, “it’s exciting that an extremely inexpensive drug like propranolol that could be used in every country around the world could have an impact on cancer by blocking stress, especially chronic stress.” Her murine research showing that adding propranolol to immunotherapy or radiotherapy or chemotherapy improved tumor growth control provided rationale for the current study.
“The breakthrough in this study is that it reveals the immune system as the best target to look at, and shows that what stress reduction is doing is improving a patient’s immune response to his or her own tumor,” Dr. Repasky said. “The mind/body connection is so important, but we have not had a handle on how to study it,” she added.
Further research funded by Herd of Hope grants at Roswell will look at tumor effects of propranolol and nonpharmacological reducers of chronic stress such as exercise, meditation, yoga, and Tai Chi, with first studies in breast cancer.
The study was funded by Roswell Park, private, and NIH grants. The authors had no disclosures.
SOURCE: Gandhi S et al. Clin Cancer Res. 2020 Oct 30. doi: 10.1158/1078-0432.CCR-20-2381
Response rates were high without dose-limiting toxicities in a small phase 1 study that evaluated the addition of propranolol to pembrolizumab in treatment-naive patients with metastatic melanoma.
“To our knowledge, this effort is the,” wrote the two co-first authors, Shipra Gandhi, MD, and Manu Pandey, MBBS, from the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and coauthors.
The need for combinations built on anti-PD1 checkpoint inhibitor therapy strategies in metastatic melanoma that safely improve outcomes is underscored by the high (59%) grade 3 or 4 treatment-related adverse event (TRAE) rates when an anti-CTLA4 agent (ipilimumab) was added to an anti-PD-1 agent (nivolumab), they noted. In contrast, a TRAE rate of only 17% has been reported with pembrolizumab monotherapy.
The phase 1b study was stimulated by preclinical, retrospective observations of improved overall survival (OS) in cancer patients treated with beta-blockers. These were preceded by murine melanoma studies showing decreased tumor growth and metastasis with the nonselective beta-blocker propranolol. “Propranolol exerts an antitumor effect,” the authors stated, “by favorably modulating the tumor microenvironment (TME) by decreasing myeloid-derived suppressor cells and increasing CD8+ T-cell and natural killer cells in the TME.” Other research in a melanoma model in chronically-stressed mice has demonstrated synergy between an anti-PD1 antibody and propranolol.
“We know that stress can have a significant negative effect on health, but the extent to which stress may impact the outcome of cancer therapy is not well understood at all,” Dr. Ghandi said in a statement provided by Roswell Park. “We set out to better understand this relationship and to explore its implications for cancer treatment.”
The investigators recruited nine White adults (median age 65 years) with treatment-naive, histologically confirmed unresectable stage III or IV melanoma and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 to the open-label, single arm, nonrandomized, single-center, dose-finding study. Patients received standard of care intravenous pembrolizumab 200 mg every 3 weeks and, in three groups, propranolol doses of 10 mg, 20 mg, or 30 mg twice a day until 2 years on study or disease progression or the development of dose-limiting toxicities (DLTs). Assessing the safety and efficacy (overall response rate [ORR] within 6 months of starting therapy) of pembrolizumab with the increasing doses of propranolol and selecting the recommended phase 2 dose were the study’s primary objectives.
Objective responses (complete or partial responses) were reported in seven of the nine patients, with partial tumor responses in two patients in the propranolol 10-mg group, two partial responses in the 20-mg group, and three partial responses in the 30-mg group.
While all patients experienced TRAEs, only one was above grade 2. The most commonly reported TRAEs were fatigue, rash and vitiligo, reported in four of the nine patients. Two patients in the 20-mg twice-a-day group discontinued therapy because of TRAEs (hemophagocytic lymphohistiocytosis and labyrinthitis). No DLTs were observed at any of the three dose levels, and no deaths occurred on study treatment.
The authors said that propranolol 30 mg twice a day was chosen as the recommended phase 2 dose, because in combination with pembrolizumab, there were no DLTs, and preliminary antitumor efficacy was observed in all three patients. Also, in all three patients, the investigators observed a trend toward higher CD8+T-cell percentage, higher ratios of CD8+T-cell/ Treg and CD8+T-cell/ polymorphonuclear myeloid-derived suppressor cells. They underscored, however, that the small size and significant heterogeneity in biomarkers made a statistically sound and meaningful interpretation of biomarkers for deciding the phase 2 dose difficult.
“In repurposing propranolol,” Dr. Pandey said in the Roswell statement, “we’ve gained important insights on how to manage stress in people with cancer – who can face dangerously elevated levels of mental and physical stress related to their diagnosis and treatment.”
In an interview, one of the two senior authors, Elizabeth Repasky, PhD, professor of oncology and immunology at Roswell Park, said, “it’s exciting that an extremely inexpensive drug like propranolol that could be used in every country around the world could have an impact on cancer by blocking stress, especially chronic stress.” Her murine research showing that adding propranolol to immunotherapy or radiotherapy or chemotherapy improved tumor growth control provided rationale for the current study.
“The breakthrough in this study is that it reveals the immune system as the best target to look at, and shows that what stress reduction is doing is improving a patient’s immune response to his or her own tumor,” Dr. Repasky said. “The mind/body connection is so important, but we have not had a handle on how to study it,” she added.
Further research funded by Herd of Hope grants at Roswell will look at tumor effects of propranolol and nonpharmacological reducers of chronic stress such as exercise, meditation, yoga, and Tai Chi, with first studies in breast cancer.
The study was funded by Roswell Park, private, and NIH grants. The authors had no disclosures.
SOURCE: Gandhi S et al. Clin Cancer Res. 2020 Oct 30. doi: 10.1158/1078-0432.CCR-20-2381
Response rates were high without dose-limiting toxicities in a small phase 1 study that evaluated the addition of propranolol to pembrolizumab in treatment-naive patients with metastatic melanoma.
“To our knowledge, this effort is the,” wrote the two co-first authors, Shipra Gandhi, MD, and Manu Pandey, MBBS, from the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and coauthors.
The need for combinations built on anti-PD1 checkpoint inhibitor therapy strategies in metastatic melanoma that safely improve outcomes is underscored by the high (59%) grade 3 or 4 treatment-related adverse event (TRAE) rates when an anti-CTLA4 agent (ipilimumab) was added to an anti-PD-1 agent (nivolumab), they noted. In contrast, a TRAE rate of only 17% has been reported with pembrolizumab monotherapy.
The phase 1b study was stimulated by preclinical, retrospective observations of improved overall survival (OS) in cancer patients treated with beta-blockers. These were preceded by murine melanoma studies showing decreased tumor growth and metastasis with the nonselective beta-blocker propranolol. “Propranolol exerts an antitumor effect,” the authors stated, “by favorably modulating the tumor microenvironment (TME) by decreasing myeloid-derived suppressor cells and increasing CD8+ T-cell and natural killer cells in the TME.” Other research in a melanoma model in chronically-stressed mice has demonstrated synergy between an anti-PD1 antibody and propranolol.
“We know that stress can have a significant negative effect on health, but the extent to which stress may impact the outcome of cancer therapy is not well understood at all,” Dr. Ghandi said in a statement provided by Roswell Park. “We set out to better understand this relationship and to explore its implications for cancer treatment.”
The investigators recruited nine White adults (median age 65 years) with treatment-naive, histologically confirmed unresectable stage III or IV melanoma and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 to the open-label, single arm, nonrandomized, single-center, dose-finding study. Patients received standard of care intravenous pembrolizumab 200 mg every 3 weeks and, in three groups, propranolol doses of 10 mg, 20 mg, or 30 mg twice a day until 2 years on study or disease progression or the development of dose-limiting toxicities (DLTs). Assessing the safety and efficacy (overall response rate [ORR] within 6 months of starting therapy) of pembrolizumab with the increasing doses of propranolol and selecting the recommended phase 2 dose were the study’s primary objectives.
Objective responses (complete or partial responses) were reported in seven of the nine patients, with partial tumor responses in two patients in the propranolol 10-mg group, two partial responses in the 20-mg group, and three partial responses in the 30-mg group.
While all patients experienced TRAEs, only one was above grade 2. The most commonly reported TRAEs were fatigue, rash and vitiligo, reported in four of the nine patients. Two patients in the 20-mg twice-a-day group discontinued therapy because of TRAEs (hemophagocytic lymphohistiocytosis and labyrinthitis). No DLTs were observed at any of the three dose levels, and no deaths occurred on study treatment.
The authors said that propranolol 30 mg twice a day was chosen as the recommended phase 2 dose, because in combination with pembrolizumab, there were no DLTs, and preliminary antitumor efficacy was observed in all three patients. Also, in all three patients, the investigators observed a trend toward higher CD8+T-cell percentage, higher ratios of CD8+T-cell/ Treg and CD8+T-cell/ polymorphonuclear myeloid-derived suppressor cells. They underscored, however, that the small size and significant heterogeneity in biomarkers made a statistically sound and meaningful interpretation of biomarkers for deciding the phase 2 dose difficult.
“In repurposing propranolol,” Dr. Pandey said in the Roswell statement, “we’ve gained important insights on how to manage stress in people with cancer – who can face dangerously elevated levels of mental and physical stress related to their diagnosis and treatment.”
In an interview, one of the two senior authors, Elizabeth Repasky, PhD, professor of oncology and immunology at Roswell Park, said, “it’s exciting that an extremely inexpensive drug like propranolol that could be used in every country around the world could have an impact on cancer by blocking stress, especially chronic stress.” Her murine research showing that adding propranolol to immunotherapy or radiotherapy or chemotherapy improved tumor growth control provided rationale for the current study.
“The breakthrough in this study is that it reveals the immune system as the best target to look at, and shows that what stress reduction is doing is improving a patient’s immune response to his or her own tumor,” Dr. Repasky said. “The mind/body connection is so important, but we have not had a handle on how to study it,” she added.
Further research funded by Herd of Hope grants at Roswell will look at tumor effects of propranolol and nonpharmacological reducers of chronic stress such as exercise, meditation, yoga, and Tai Chi, with first studies in breast cancer.
The study was funded by Roswell Park, private, and NIH grants. The authors had no disclosures.
SOURCE: Gandhi S et al. Clin Cancer Res. 2020 Oct 30. doi: 10.1158/1078-0432.CCR-20-2381
FROM CLINICAL CANCER RESEARCH
New child COVID-19 cases down in last weekly count
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 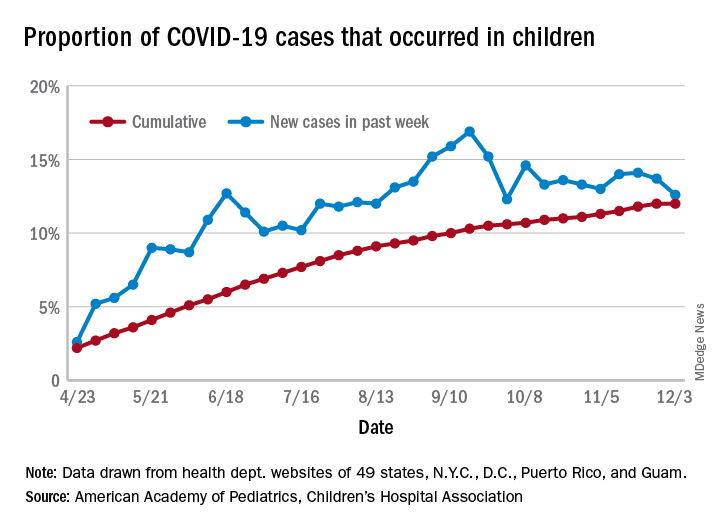
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
‘Practice changing’: Ruxolitinib as second-line in chronic GVHD
When chronic graft-versus-host disease (cGVHD) develops as a complication of allogeneic hematopoietic stem cell transplant (alloHSCT), treatment options are limited. New findings show that ruxolitinib (Jakafi) was superior to standard therapy in reducing symptoms of cGVHD in the second-line setting, and the results are potentially practice changing.
The new data, from the REACH3 trial, were presented at the annual meeting of the American Society of Hematology, held virtually this year.
This trial is “almost certainly a practice changer,” Robert Brodsky, MD, ASH secretary, said during a press preview webinar.
Chronic GVHD occurs in approximately 30%-70% of patients who undergo alloSCT, and “has been really hard to treat,” said Dr. Brodsky, of Johns Hopkins University, Baltimore. “Steroids are the first-line treatment, but after that, nothing else has shown any improvement and even steroids don’t work that well.”
Of the patients assessed, 50% of those who received ruxolitinib responded to therapy compared with only 25% who received standard therapies.
“This is the first multicenter randomized controlled trial for chronic GVHD that is positive,” said senior study author Robert Zeiser, PhD, of University Medical Center, Freiburg, Germany. “It shows a significant advantage for ruxolitinib. It is likely that this trial will lead to approval for this indication and change the guidelines for the treatment of this disease.”
Ruxolitinib, a JAK inhibitor first marketed for use in myelofibrosis, is already approved for acute GVHD. The Food and Drug Administration approved that indication last year on the basis of data from two previous trials, REACH 1 and REACH 2. The trials found that ruxolitinib was superior to best available therapy for treating patients with acute GVHD.
Superior to best available therapy
In the current REACH 3 study, Dr. Zeiser and colleagues compared ruxolitinib with best available therapy in 329 patients with moderate-to-severe cGVHD (both steroid dependent and steroid resistant).
All patients had undergone alloSCT and were randomly assigned to ruxolitinib (10 mg twice daily) for six 28-day cycles or investigator-selected best available therapy (BAT), of which there were 10 options. Patients continued receiving their regimen of corticosteroids, and viral prophylaxis and antibiotics were allowed as needed for infection prevention and treatment.
The study permitted crossover: Patients on BAT were allowed to start on ruxolitinib on or after cycle 7 day 1 for those who did not achieve or maintain a response, developed toxicity to BAT, or had a cGVHD flare.
The study met its primary endpoint of overall response rate (ORR), with a clear and substantial improvement among patients taking ruxolitinib (50% vs 26%; odds ratio, 2.99; P < .0001a), Dr. Zeiser noted. The complete response rate was also higher (7% vs. 3%).
Both key secondary endpoints also showed that ruxolitinib was superior to BAT. Failure-free survival was significantly longer in the ruxolitinib group (median not reached vs 5.7 months; hazard ratio, 0.370; P < .0001). There was also an improvement in symptoms based on changes in the modified Lee symptom score (mLSS; 0 [no symptoms] to 100 [worst symptoms]) at cycle 7 day 1; the results show that the mLSS responder rate was higher in patients on ruxolitinib (24% vs. 11%; odds ratio, 2.62; P = .0011).
A total of 31 patients in the ruxolitinib group died (19%) along with 27 in the BAT group (16%), with the cGVHD as the main cause of death.
Adverse events were comparable in both groups (ruxolitinib 98% [grade ≥ 3, 57%]; BAT, 92% [grade ≥ 3, 58%], with the most common being anemia (29% vs. 13%), hypertension (16% vs. 13%), pyrexia (16% vs. 9%), and ALT increase (15% vs 4%).
More options for patients
“The addition of ruxolitinib is definitely practice changing for this very difficult to treat population,” said James Essell, MD, medical director of the Blood Cancer Center at Mercy Health, Cincinnati, who was not involved in the study.
However, he added, “more options are still required, as evidenced by the continued deaths of patients despite this new option.”
Dr. Essell pointed out that ibrutinib (Imbruvica) is already approved for the treatment of cGVHD. “Ruxolitinib offers another option for treating this group of patients,” he said, and predicted that “it will be used frequently and has a different toxicity profile, ultimately improving the care for patients with cGVHD.”
It is likely that ruxolitinib will be considered earlier in the treatment of cGVHD to avoid the toxicity of chronic steroid use, he added, but price is a consideration. “The cost of ruxolitinib is over 200 times more than prednisone, limiting the adoption front line without a clinical trial.”
Another expert approached for comment was enthusiastic. “The abstract gave good evidence and efficacy with chronic GVHD,” said Ryotaro Nakamura, MD, associate professor of hematology & hematopoietic cell transplantation at City of Hope, Duarte, Calif. He noted that there have been two previous REACH trials which showed a benefit for ruxolitinib in acute GVHD.
What this means is that there is now global evidence that ruxolitinib is better than anything else so far, he said, and this latest trial is just part of the “practice-changing data,” from the three studies. “It is practice changing in that it is providing options now for these patients,” he said.
Dr. Zeiser has disclosed relationships with Incyte, Novartis and Mallinckrodt; other authors disclosed relationships with industry as noted in the abstract. Dr. Essell and Dr. Nakamura have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When chronic graft-versus-host disease (cGVHD) develops as a complication of allogeneic hematopoietic stem cell transplant (alloHSCT), treatment options are limited. New findings show that ruxolitinib (Jakafi) was superior to standard therapy in reducing symptoms of cGVHD in the second-line setting, and the results are potentially practice changing.
The new data, from the REACH3 trial, were presented at the annual meeting of the American Society of Hematology, held virtually this year.
This trial is “almost certainly a practice changer,” Robert Brodsky, MD, ASH secretary, said during a press preview webinar.
Chronic GVHD occurs in approximately 30%-70% of patients who undergo alloSCT, and “has been really hard to treat,” said Dr. Brodsky, of Johns Hopkins University, Baltimore. “Steroids are the first-line treatment, but after that, nothing else has shown any improvement and even steroids don’t work that well.”
Of the patients assessed, 50% of those who received ruxolitinib responded to therapy compared with only 25% who received standard therapies.
“This is the first multicenter randomized controlled trial for chronic GVHD that is positive,” said senior study author Robert Zeiser, PhD, of University Medical Center, Freiburg, Germany. “It shows a significant advantage for ruxolitinib. It is likely that this trial will lead to approval for this indication and change the guidelines for the treatment of this disease.”
Ruxolitinib, a JAK inhibitor first marketed for use in myelofibrosis, is already approved for acute GVHD. The Food and Drug Administration approved that indication last year on the basis of data from two previous trials, REACH 1 and REACH 2. The trials found that ruxolitinib was superior to best available therapy for treating patients with acute GVHD.
Superior to best available therapy
In the current REACH 3 study, Dr. Zeiser and colleagues compared ruxolitinib with best available therapy in 329 patients with moderate-to-severe cGVHD (both steroid dependent and steroid resistant).
All patients had undergone alloSCT and were randomly assigned to ruxolitinib (10 mg twice daily) for six 28-day cycles or investigator-selected best available therapy (BAT), of which there were 10 options. Patients continued receiving their regimen of corticosteroids, and viral prophylaxis and antibiotics were allowed as needed for infection prevention and treatment.
The study permitted crossover: Patients on BAT were allowed to start on ruxolitinib on or after cycle 7 day 1 for those who did not achieve or maintain a response, developed toxicity to BAT, or had a cGVHD flare.
The study met its primary endpoint of overall response rate (ORR), with a clear and substantial improvement among patients taking ruxolitinib (50% vs 26%; odds ratio, 2.99; P < .0001a), Dr. Zeiser noted. The complete response rate was also higher (7% vs. 3%).
Both key secondary endpoints also showed that ruxolitinib was superior to BAT. Failure-free survival was significantly longer in the ruxolitinib group (median not reached vs 5.7 months; hazard ratio, 0.370; P < .0001). There was also an improvement in symptoms based on changes in the modified Lee symptom score (mLSS; 0 [no symptoms] to 100 [worst symptoms]) at cycle 7 day 1; the results show that the mLSS responder rate was higher in patients on ruxolitinib (24% vs. 11%; odds ratio, 2.62; P = .0011).
A total of 31 patients in the ruxolitinib group died (19%) along with 27 in the BAT group (16%), with the cGVHD as the main cause of death.
Adverse events were comparable in both groups (ruxolitinib 98% [grade ≥ 3, 57%]; BAT, 92% [grade ≥ 3, 58%], with the most common being anemia (29% vs. 13%), hypertension (16% vs. 13%), pyrexia (16% vs. 9%), and ALT increase (15% vs 4%).
More options for patients
“The addition of ruxolitinib is definitely practice changing for this very difficult to treat population,” said James Essell, MD, medical director of the Blood Cancer Center at Mercy Health, Cincinnati, who was not involved in the study.
However, he added, “more options are still required, as evidenced by the continued deaths of patients despite this new option.”
Dr. Essell pointed out that ibrutinib (Imbruvica) is already approved for the treatment of cGVHD. “Ruxolitinib offers another option for treating this group of patients,” he said, and predicted that “it will be used frequently and has a different toxicity profile, ultimately improving the care for patients with cGVHD.”
It is likely that ruxolitinib will be considered earlier in the treatment of cGVHD to avoid the toxicity of chronic steroid use, he added, but price is a consideration. “The cost of ruxolitinib is over 200 times more than prednisone, limiting the adoption front line without a clinical trial.”
Another expert approached for comment was enthusiastic. “The abstract gave good evidence and efficacy with chronic GVHD,” said Ryotaro Nakamura, MD, associate professor of hematology & hematopoietic cell transplantation at City of Hope, Duarte, Calif. He noted that there have been two previous REACH trials which showed a benefit for ruxolitinib in acute GVHD.
What this means is that there is now global evidence that ruxolitinib is better than anything else so far, he said, and this latest trial is just part of the “practice-changing data,” from the three studies. “It is practice changing in that it is providing options now for these patients,” he said.
Dr. Zeiser has disclosed relationships with Incyte, Novartis and Mallinckrodt; other authors disclosed relationships with industry as noted in the abstract. Dr. Essell and Dr. Nakamura have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
When chronic graft-versus-host disease (cGVHD) develops as a complication of allogeneic hematopoietic stem cell transplant (alloHSCT), treatment options are limited. New findings show that ruxolitinib (Jakafi) was superior to standard therapy in reducing symptoms of cGVHD in the second-line setting, and the results are potentially practice changing.
The new data, from the REACH3 trial, were presented at the annual meeting of the American Society of Hematology, held virtually this year.
This trial is “almost certainly a practice changer,” Robert Brodsky, MD, ASH secretary, said during a press preview webinar.
Chronic GVHD occurs in approximately 30%-70% of patients who undergo alloSCT, and “has been really hard to treat,” said Dr. Brodsky, of Johns Hopkins University, Baltimore. “Steroids are the first-line treatment, but after that, nothing else has shown any improvement and even steroids don’t work that well.”
Of the patients assessed, 50% of those who received ruxolitinib responded to therapy compared with only 25% who received standard therapies.
“This is the first multicenter randomized controlled trial for chronic GVHD that is positive,” said senior study author Robert Zeiser, PhD, of University Medical Center, Freiburg, Germany. “It shows a significant advantage for ruxolitinib. It is likely that this trial will lead to approval for this indication and change the guidelines for the treatment of this disease.”
Ruxolitinib, a JAK inhibitor first marketed for use in myelofibrosis, is already approved for acute GVHD. The Food and Drug Administration approved that indication last year on the basis of data from two previous trials, REACH 1 and REACH 2. The trials found that ruxolitinib was superior to best available therapy for treating patients with acute GVHD.
Superior to best available therapy
In the current REACH 3 study, Dr. Zeiser and colleagues compared ruxolitinib with best available therapy in 329 patients with moderate-to-severe cGVHD (both steroid dependent and steroid resistant).
All patients had undergone alloSCT and were randomly assigned to ruxolitinib (10 mg twice daily) for six 28-day cycles or investigator-selected best available therapy (BAT), of which there were 10 options. Patients continued receiving their regimen of corticosteroids, and viral prophylaxis and antibiotics were allowed as needed for infection prevention and treatment.
The study permitted crossover: Patients on BAT were allowed to start on ruxolitinib on or after cycle 7 day 1 for those who did not achieve or maintain a response, developed toxicity to BAT, or had a cGVHD flare.
The study met its primary endpoint of overall response rate (ORR), with a clear and substantial improvement among patients taking ruxolitinib (50% vs 26%; odds ratio, 2.99; P < .0001a), Dr. Zeiser noted. The complete response rate was also higher (7% vs. 3%).
Both key secondary endpoints also showed that ruxolitinib was superior to BAT. Failure-free survival was significantly longer in the ruxolitinib group (median not reached vs 5.7 months; hazard ratio, 0.370; P < .0001). There was also an improvement in symptoms based on changes in the modified Lee symptom score (mLSS; 0 [no symptoms] to 100 [worst symptoms]) at cycle 7 day 1; the results show that the mLSS responder rate was higher in patients on ruxolitinib (24% vs. 11%; odds ratio, 2.62; P = .0011).
A total of 31 patients in the ruxolitinib group died (19%) along with 27 in the BAT group (16%), with the cGVHD as the main cause of death.
Adverse events were comparable in both groups (ruxolitinib 98% [grade ≥ 3, 57%]; BAT, 92% [grade ≥ 3, 58%], with the most common being anemia (29% vs. 13%), hypertension (16% vs. 13%), pyrexia (16% vs. 9%), and ALT increase (15% vs 4%).
More options for patients
“The addition of ruxolitinib is definitely practice changing for this very difficult to treat population,” said James Essell, MD, medical director of the Blood Cancer Center at Mercy Health, Cincinnati, who was not involved in the study.
However, he added, “more options are still required, as evidenced by the continued deaths of patients despite this new option.”
Dr. Essell pointed out that ibrutinib (Imbruvica) is already approved for the treatment of cGVHD. “Ruxolitinib offers another option for treating this group of patients,” he said, and predicted that “it will be used frequently and has a different toxicity profile, ultimately improving the care for patients with cGVHD.”
It is likely that ruxolitinib will be considered earlier in the treatment of cGVHD to avoid the toxicity of chronic steroid use, he added, but price is a consideration. “The cost of ruxolitinib is over 200 times more than prednisone, limiting the adoption front line without a clinical trial.”
Another expert approached for comment was enthusiastic. “The abstract gave good evidence and efficacy with chronic GVHD,” said Ryotaro Nakamura, MD, associate professor of hematology & hematopoietic cell transplantation at City of Hope, Duarte, Calif. He noted that there have been two previous REACH trials which showed a benefit for ruxolitinib in acute GVHD.
What this means is that there is now global evidence that ruxolitinib is better than anything else so far, he said, and this latest trial is just part of the “practice-changing data,” from the three studies. “It is practice changing in that it is providing options now for these patients,” he said.
Dr. Zeiser has disclosed relationships with Incyte, Novartis and Mallinckrodt; other authors disclosed relationships with industry as noted in the abstract. Dr. Essell and Dr. Nakamura have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Air pollution linked to brain amyloid pathology
Higher levels of air pollution were associated with an increased risk for amyloid-beta pathology in a new study of older adults with cognitive impairment. “Many studies have now found a link between air pollution and clinical outcomes of dementia or cognitive decline,” said lead author Leonardo Iaccarino, PhD, Weill Institute for Neurosciences, University of California, San Francisco. “But this study is now showing a clear link between air pollution and a biomarker of Alzheimer’s disease: It shows a relationship between bad air quality and pathology in the brain.
“We believe that exposure to air pollution should be considered as one factor in the lifetime risk of developing Alzheimer’s disease,” he added. “We believe it is a significant determinant. Our results suggest that, if we can reduce occupational and residential exposure to air pollution, then this could help reduce the risk of Alzheimer’s disease.”
The study was published online Nov. 30 in JAMA Neurology.
A modifiable risk factor
Dr. Iaccarino explained that it is well known that air pollution is linked to poor health outcomes. “As well as cardiovascular and respiratory disease, there is also growing interest in the relationship between air pollution and brain health,” he said. “The link is becoming more and more convincing, with evidence from laboratory, animal, and human studies suggesting that individuals exposed to poor air quality have an increased risk of cognitive decline and dementia.”
In addition, this year, the Lancet Commission included air pollution in its updated list of modifiable risk factors for dementia.
For the current study, the researchers analyzed data from the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) Study, which included more than 18,000 U.S. participants with cognitive impairment who received an amyloid positron-emission tomography scan between 2016 and 2018.
The investigators used data from the IDEAS study to assess the relationship between the air quality at the place of residence of each patient and the likelihood of a positive amyloid PET result. Public records from the U.S. Environmental Protection Agency were used to estimate air quality in individual ZIP-code areas during two periods – 2002-2003 (approximately 14 years before the amyloid PET scan) and 2015-2016 (approximately 1 year before the amyloid PET scan).
Results showed that those living in an area with increased air pollution, as determined using concentrations of predicted fine particulate matter (PM2.5), had a higher probability of a positive amyloid PET scan. This association was dose dependent and statistically significant after adjusting for demographic, lifestyle, and socioeconomic factors as well as medical comorbidities. The association was seen in both periods; the adjusted odds ratio was 1.10 in 2002-2003 and 1.15 in 2015-2016.
“This shows about a 10% increased probability of a positive amyloid test for individuals living in the worst polluted areas, compared with those in the least polluted areas,” Dr. Iaccarino explained.
Every unit increase in PM2.5 in 2002-2003 was associated with an increased probability of positive amyloid findings on PET of 0.5%. Every unit increase in PM2.5 in for the 2015-2016 period was associated with an increased probability of positive amyloid findings on PET of 0.8%.
“This was a very large cohort study, and we adjusted for multiple other factors, so these are pretty robust findings,” Dr. Iaccarino said.
Exposure to higher ozone concentrations was not associated with amyloid positivity on PET scans in either time window.
“These findings suggest that brain amyloid-beta accumulation could be one of the biological pathways in the increased incidence of dementia and cognitive decline associated with exposure to air pollution,” the researchers stated.
A public health concern
“Adverse effects of airborne toxic pollutants associated with amyloid-beta pathology should be considered in public health policy decisions and should inform individual lifetime risk of developing Alzheimer’s disease and dementia,” they concluded.
Dr. Iaccarino noted that, although governments need to take primary action in reducing air pollution, individuals can make some changes to reduce their exposure to poor-quality air.
“Such changes could include not going out or using masks when pollution levels are very high (as happened recently in California with the wildfires) and avoiding areas where the air quality is known to be bad. In addition, there are activities which increase indoor air pollution which can be changed, such as certain types of cooking, cigarette smoking, use of coal fires,” he commented.
“Based on our findings, it would be reasonable to take action on these things, especially for individuals at higher risk of cardiovascular and respiratory disease or Alzheimer’s,” he added.
On a more optimistic note, Dr. Iaccarino pointed out that air quality in the United States has improved significantly in recent years. Meaningful improvements were found between the two periods in this analysis study (2002-2016), “so we are going in the right direction.”
The IDEAS Study was funded by the Alzheimer’s Association, the American College of Radiology, Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging. Dr. Iaccarino has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Higher levels of air pollution were associated with an increased risk for amyloid-beta pathology in a new study of older adults with cognitive impairment. “Many studies have now found a link between air pollution and clinical outcomes of dementia or cognitive decline,” said lead author Leonardo Iaccarino, PhD, Weill Institute for Neurosciences, University of California, San Francisco. “But this study is now showing a clear link between air pollution and a biomarker of Alzheimer’s disease: It shows a relationship between bad air quality and pathology in the brain.
“We believe that exposure to air pollution should be considered as one factor in the lifetime risk of developing Alzheimer’s disease,” he added. “We believe it is a significant determinant. Our results suggest that, if we can reduce occupational and residential exposure to air pollution, then this could help reduce the risk of Alzheimer’s disease.”
The study was published online Nov. 30 in JAMA Neurology.
A modifiable risk factor
Dr. Iaccarino explained that it is well known that air pollution is linked to poor health outcomes. “As well as cardiovascular and respiratory disease, there is also growing interest in the relationship between air pollution and brain health,” he said. “The link is becoming more and more convincing, with evidence from laboratory, animal, and human studies suggesting that individuals exposed to poor air quality have an increased risk of cognitive decline and dementia.”
In addition, this year, the Lancet Commission included air pollution in its updated list of modifiable risk factors for dementia.
For the current study, the researchers analyzed data from the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) Study, which included more than 18,000 U.S. participants with cognitive impairment who received an amyloid positron-emission tomography scan between 2016 and 2018.
The investigators used data from the IDEAS study to assess the relationship between the air quality at the place of residence of each patient and the likelihood of a positive amyloid PET result. Public records from the U.S. Environmental Protection Agency were used to estimate air quality in individual ZIP-code areas during two periods – 2002-2003 (approximately 14 years before the amyloid PET scan) and 2015-2016 (approximately 1 year before the amyloid PET scan).
Results showed that those living in an area with increased air pollution, as determined using concentrations of predicted fine particulate matter (PM2.5), had a higher probability of a positive amyloid PET scan. This association was dose dependent and statistically significant after adjusting for demographic, lifestyle, and socioeconomic factors as well as medical comorbidities. The association was seen in both periods; the adjusted odds ratio was 1.10 in 2002-2003 and 1.15 in 2015-2016.
“This shows about a 10% increased probability of a positive amyloid test for individuals living in the worst polluted areas, compared with those in the least polluted areas,” Dr. Iaccarino explained.
Every unit increase in PM2.5 in 2002-2003 was associated with an increased probability of positive amyloid findings on PET of 0.5%. Every unit increase in PM2.5 in for the 2015-2016 period was associated with an increased probability of positive amyloid findings on PET of 0.8%.
“This was a very large cohort study, and we adjusted for multiple other factors, so these are pretty robust findings,” Dr. Iaccarino said.
Exposure to higher ozone concentrations was not associated with amyloid positivity on PET scans in either time window.
“These findings suggest that brain amyloid-beta accumulation could be one of the biological pathways in the increased incidence of dementia and cognitive decline associated with exposure to air pollution,” the researchers stated.
A public health concern
“Adverse effects of airborne toxic pollutants associated with amyloid-beta pathology should be considered in public health policy decisions and should inform individual lifetime risk of developing Alzheimer’s disease and dementia,” they concluded.
Dr. Iaccarino noted that, although governments need to take primary action in reducing air pollution, individuals can make some changes to reduce their exposure to poor-quality air.
“Such changes could include not going out or using masks when pollution levels are very high (as happened recently in California with the wildfires) and avoiding areas where the air quality is known to be bad. In addition, there are activities which increase indoor air pollution which can be changed, such as certain types of cooking, cigarette smoking, use of coal fires,” he commented.
“Based on our findings, it would be reasonable to take action on these things, especially for individuals at higher risk of cardiovascular and respiratory disease or Alzheimer’s,” he added.
On a more optimistic note, Dr. Iaccarino pointed out that air quality in the United States has improved significantly in recent years. Meaningful improvements were found between the two periods in this analysis study (2002-2016), “so we are going in the right direction.”
The IDEAS Study was funded by the Alzheimer’s Association, the American College of Radiology, Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging. Dr. Iaccarino has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Higher levels of air pollution were associated with an increased risk for amyloid-beta pathology in a new study of older adults with cognitive impairment. “Many studies have now found a link between air pollution and clinical outcomes of dementia or cognitive decline,” said lead author Leonardo Iaccarino, PhD, Weill Institute for Neurosciences, University of California, San Francisco. “But this study is now showing a clear link between air pollution and a biomarker of Alzheimer’s disease: It shows a relationship between bad air quality and pathology in the brain.
“We believe that exposure to air pollution should be considered as one factor in the lifetime risk of developing Alzheimer’s disease,” he added. “We believe it is a significant determinant. Our results suggest that, if we can reduce occupational and residential exposure to air pollution, then this could help reduce the risk of Alzheimer’s disease.”
The study was published online Nov. 30 in JAMA Neurology.
A modifiable risk factor
Dr. Iaccarino explained that it is well known that air pollution is linked to poor health outcomes. “As well as cardiovascular and respiratory disease, there is also growing interest in the relationship between air pollution and brain health,” he said. “The link is becoming more and more convincing, with evidence from laboratory, animal, and human studies suggesting that individuals exposed to poor air quality have an increased risk of cognitive decline and dementia.”
In addition, this year, the Lancet Commission included air pollution in its updated list of modifiable risk factors for dementia.
For the current study, the researchers analyzed data from the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) Study, which included more than 18,000 U.S. participants with cognitive impairment who received an amyloid positron-emission tomography scan between 2016 and 2018.
The investigators used data from the IDEAS study to assess the relationship between the air quality at the place of residence of each patient and the likelihood of a positive amyloid PET result. Public records from the U.S. Environmental Protection Agency were used to estimate air quality in individual ZIP-code areas during two periods – 2002-2003 (approximately 14 years before the amyloid PET scan) and 2015-2016 (approximately 1 year before the amyloid PET scan).
Results showed that those living in an area with increased air pollution, as determined using concentrations of predicted fine particulate matter (PM2.5), had a higher probability of a positive amyloid PET scan. This association was dose dependent and statistically significant after adjusting for demographic, lifestyle, and socioeconomic factors as well as medical comorbidities. The association was seen in both periods; the adjusted odds ratio was 1.10 in 2002-2003 and 1.15 in 2015-2016.
“This shows about a 10% increased probability of a positive amyloid test for individuals living in the worst polluted areas, compared with those in the least polluted areas,” Dr. Iaccarino explained.
Every unit increase in PM2.5 in 2002-2003 was associated with an increased probability of positive amyloid findings on PET of 0.5%. Every unit increase in PM2.5 in for the 2015-2016 period was associated with an increased probability of positive amyloid findings on PET of 0.8%.
“This was a very large cohort study, and we adjusted for multiple other factors, so these are pretty robust findings,” Dr. Iaccarino said.
Exposure to higher ozone concentrations was not associated with amyloid positivity on PET scans in either time window.
“These findings suggest that brain amyloid-beta accumulation could be one of the biological pathways in the increased incidence of dementia and cognitive decline associated with exposure to air pollution,” the researchers stated.
A public health concern
“Adverse effects of airborne toxic pollutants associated with amyloid-beta pathology should be considered in public health policy decisions and should inform individual lifetime risk of developing Alzheimer’s disease and dementia,” they concluded.
Dr. Iaccarino noted that, although governments need to take primary action in reducing air pollution, individuals can make some changes to reduce their exposure to poor-quality air.
“Such changes could include not going out or using masks when pollution levels are very high (as happened recently in California with the wildfires) and avoiding areas where the air quality is known to be bad. In addition, there are activities which increase indoor air pollution which can be changed, such as certain types of cooking, cigarette smoking, use of coal fires,” he commented.
“Based on our findings, it would be reasonable to take action on these things, especially for individuals at higher risk of cardiovascular and respiratory disease or Alzheimer’s,” he added.
On a more optimistic note, Dr. Iaccarino pointed out that air quality in the United States has improved significantly in recent years. Meaningful improvements were found between the two periods in this analysis study (2002-2016), “so we are going in the right direction.”
The IDEAS Study was funded by the Alzheimer’s Association, the American College of Radiology, Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging. Dr. Iaccarino has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NEUROLOGY
Joint guidelines favor antibody testing for certain Lyme disease manifestations
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
New clinical practice guidelines on Lyme disease place a strong emphasis on antibody testing to assess for rheumatologic and neurologic syndromes. “Diagnostically, we recommend testing via antibodies, and an index of antibodies in cerebrospinal fluid [CSF] versus serum. Importantly, we recommend against using polymerase chain reaction [PCR] in CSF,” Jeffrey A. Rumbaugh, MD, PhD, a coauthor of the guidelines and a member of the American Academy of Neurology, said in an interview.
The Infectious Diseases Society of America, AAN, and the American College of Rheumatology convened a multidisciplinary panel to develop the 43 recommendations, seeking input from 12 additional medical specialties, and patients. The panel conducted a systematic review of available evidence on preventing, diagnosing, and treating Lyme disease, using the Grading of Recommendations Assessment, Development and Evaluation model to evaluate clinical evidence and strength of recommendations. The guidelines were simultaneous published in Clinical Infectious Diseases, Neurology, Arthritis & Rheumatology, and Arthritis Care & Research.
This is the first time these organizations have collaborated on joint Lyme disease guidelines, which focus mainly on neurologic, cardiac, and rheumatologic manifestations.
“We are very excited to provide these updated guidelines to assist clinicians working in numerous medical specialties around the country, and even the world, as they care for patients suffering from Lyme disease,” Dr. Rumbaugh said.
When to use and not to use PCR
Guideline authors called for specific testing regimens depending on presentation of symptoms. Generally, they advised that individuals with a skin rash suggestive of early disease seek a clinical diagnosis instead of laboratory testing.
Recommendations on Lyme arthritis support previous IDSA guidelines published in 2006, Linda K. Bockenstedt, MD, professor of medicine at Yale University, New Haven, Conn., and a coauthor of the guidelines, said in an interview.
To evaluate for potential Lyme arthritis, clinicians should choose serum antibody testing over PCR or culture of blood or synovial fluid/tissue. However, if a doctor is assessing a seropositive patient for Lyme arthritis diagnosis but needs more information for treatment decisions, the authors recommended PCR applied to synovial fluid or tissue over Borrelia culture.
“Synovial fluid can be analyzed by PCR, but sensitivity is generally lower than serology,” Dr. Bockenstedt explained. Additionally, culture of joint fluid or synovial tissue for Lyme spirochetes has 0% sensitivity in multiple studies. “For these reasons, we recommend serum antibody testing over PCR of joint fluid or other methods for an initial diagnosis.”
Serum antibody testing over PCR or culture is also recommended for identifying Lyme neuroborreliosis in the peripheral nervous system (PNS) or CNS.
Despite the recent popularity of Lyme PCR testing in hospitals and labs, “with Lyme at least, antibodies are better in the CSF,” Dr. Rumbaugh said. Studies have shown that “most patients with even early neurologic Lyme disease are seropositive by conventional antibody testing at time of initial clinical presentation, and that intrathecal antibody production, as demonstrated by an elevated CSF:serum index, is highly specific for CNS involvement.”
If done correctly, antibody testing is both sensitive and specific for neurologic Lyme disease. “On the other hand, sensitivity of Lyme PCR performed on CSF has been only in the 5%-17% range in studies. Incidentally, Lyme PCR on blood is also not sensitive and therefore not recommended,” Dr. Rumbaugh said.
Guideline authors recommended testing in patients with the following conditions: acute neurologic disorders such as meningitis, painful radiculoneuritis, mononeuropathy multiplex; evidence of spinal cord or brain inflammation; and acute myocarditis/pericarditis of unknown cause in an appropriate epidemiologic setting.
They did not recommend testing in patients with typical amyotrophic lateral sclerosis; relapsing remitting multiple sclerosis; Parkinson’s disease, dementia, or cognitive decline; new-onset seizures; other neurologic syndromes or those lacking clinical or epidemiologic history that would support a diagnosis of Lyme disease; and patients with chronic cardiomyopathy of unknown cause.
The authors also called for judicious use of electrocardiogram to screen for Lyme carditis, recommending it only in patients signs or symptoms of this condition. However, patients at risk for or showing signs of severe cardiac complications of Lyme disease should be hospitalized and monitored via ECG.
Timelines for antibiotics
Most patients with Lyme disease should receive oral antibiotics, although duration times vary depending on the disease state. “We recommend that prophylactic antibiotic therapy be given to adults and children only within 72 hours of removal of an identified high-risk tick bite, but not for bites that are equivocal risk or low risk,” according to the guideline authors.
Specific antibiotic treatment regimens by condition are as follows: 10-14 days for early-stage disease, 14 days for Lyme carditis, 14-21 days for neurologic Lyme disease, and 28 days for late Lyme arthritis.
“Despite arthritis occurring late in the course of infection, treatment with a 28-day course of oral antibiotic is effective, although the rates of complete resolution of joint swelling can vary,” Dr. Bockenstedt said. Clinicians may consider a second 28-day course of oral antibiotics or a 2- to 4-week course of ceftriaxone in patients with persistent swelling, after an initial course of oral antibiotics.
Citing knowledge gaps, the authors made no recommendation on secondary antibiotic treatment for unresolved Lyme arthritis. Rheumatologists can play an important role in the care of this small subset of patients, Dr. Bockenstedt noted. “Studies of patients with ‘postantibiotic Lyme arthritis’ show that they can be treated successfully with intra-articular steroids, nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, biologic response modifiers, and even synovectomy with successful outcomes.” Some of these therapies also work in cases where first courses of oral and intravenous antibiotics are unsuccessful.
“Antibiotic therapy for longer than 8 weeks is not expected to provide additional benefit to patients with persistent arthritis if that treatment has included one course of IV therapy,” the authors clarified.
For patients with Lyme disease–associated meningitis, cranial neuropathy, radiculoneuropathy, or other PNS manifestations, the authors recommended intravenous ceftriaxone, cefotaxime, penicillin G, or oral doxycycline over other antimicrobials.
“For most neurologic presentations, oral doxycycline is just as effective as appropriate IV antibiotics,” Dr. Rumbaugh said. “The exception is the relatively rare situation where the patient is felt to have parenchymal involvement of brain or spinal cord, in which case the guidelines recommend IV antibiotics over oral antibiotics.” In the studies, there was no statistically significant difference between oral or intravenous regimens in response rate or risk of adverse effects.
Patients with nonspecific symptoms such as fatigue, pain, or cognitive impairment following treatment should not receive additional antibiotic therapy if there’s no evidence of treatment failure or infection. These two markers “would include objective signs of disease activity, such as arthritis, meningitis, or neuropathy,” the guideline authors wrote in comments accompanying the recommendation.
Clinicians caring for patients with symptomatic bradycardia caused by Lyme carditis should consider temporary pacing measures instead of a permanent pacemaker. For patients hospitalized with Lyme carditis, “we suggest initially using IV ceftriaxone over oral antibiotics until there is evidence of clinical improvement, then switching to oral antibiotics to complete treatment,” they advised. Outpatients with this condition should receive oral antibiotics instead of intravenous antibiotics.
Advice on antibodies testing ‘particularly cogent’
For individuals without expertise in these areas, the recommendations are clear and useful, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and research professor at the University of Florence (Italy), said in an interview.
“As a rheumatologist, I would have appreciated literature references for some of the recommendations but, nevertheless, find these useful. I applaud the care with which the evidence was gathered and the general formatting, which tried to review multiple possible scenarios surrounding Lyme arthritis,” said Dr. Furst, offering a third-party perspective.
The advice on using antibodies tests to make a diagnosis of Lyme arthritis “is particularly cogent and more useful than trying to culture these fastidious organisms,” he added.
The IDSA, AAN, and ACR provided support for the guideline. Dr. Bockenstedt reported receiving research funding from the National Institutes of Health and the Gordon and the Llura Gund Foundation and remuneration from L2 Diagnostics for investigator-initiated NIH-sponsored research. Dr. Rumbaugh had no conflicts of interest to disclose. Dr. Furst reported no conflicts of interest in commenting on these guidelines.
SOURCE: Rumbaugh JA et al. Clin Infect Dis. 2020 Nov 30. doi: 10.1093/cid/ciaa1215.
FROM CLINICAL INFECTIOUS DISEASES
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
Oral steroids benefit patients with cluster headache
Adjunctive oral prednisone appears to significantly reduce cluster headache attacks, new research shows. Results of the multicenter, randomized, double-blind trial show that patients who received the steroid had 25% fewer attacks in the first week of therapy, compared with their counterparts who received placebo.
In addition, more than a third of patients in the prednisone group were pain free, and for almost half, headache frequency was reduced by at least 50% at day 7 of treatment.
These findings provide clear evidence that prednisone, in conjunction with the use of verapamil, is effective in cluster headache, said lead author Mark Obermann, MD, director, Center for Neurology, Asklepios Hospitals Seesen (Germany), and associate professor, University of Duisburg-Essen (Germany).
The key message, he added, is that all patients with cluster headache should receive prednisone at the start of an episode.
The study was published online Nov. 24 in the Lancet Neurology.
‘Suicide headaches’
Cluster headaches are intense unilateral attacks of facial and head pain. They last 15-180 minutes and predominantly affect men. They are accompanied by trigeminal autonomic symptoms and are extremely painful. “They’re referred to as ‘suicide headaches’ because the pain is so severe that patients often report they think about killing themselves to get rid of the pain,” said Dr. Obermann.
The cause is unclear, although there is some evidence that the hypothalamus is involved. The headaches sometimes follow a “strict circadian pattern,” said Dr. Obermann. He noted that the attacks might occur over a few weeks or months and then not return for months or even years.
An estimated 1 in 1,000 people experience cluster headache, but the condition is underrecognized, and research is scarce and poorly funded. Previous research does show that the calcium channel blocker verapamil, which is used to treat high blood pressure, is effective in cluster headache. However, it takes about 14 days to work and has to be slowly titrated because of cardiac side effects, said Dr. Obermann. For these reasons, international guidelines recommend initiating short-term preventive treatment with corticosteroids to suppress, or at least lessen, cluster headache attacks until long-term prevention is effective.
Although some clinicians treat cluster headaches with corticosteroids, others don’t because of a lack of evidence that shows they are effective. “There’s no evidence whatsoever on what the correct dose is or whether it helps at all. This is the gap we wanted to close,” said Dr. Obermann.
The study included 116 adult patients with cluster headache from 10 centers who were experiencing a cluster headache episode and were not taking prophylactic medication.
The trial only included patients who had an attack within 30 days of their current episode. The investigators included this restriction to reduce the possibility of spontaneous remission, which is “a big problem” in cluster headache trials, he said. To confirm that episodes were cluster headache attacks, patients were also required to have moderate to severe pain, indicated by a score of at least 5 on a numerical rating scale in which 0 indicates no pain and 10 indicates the worse imaginable pain.
Participants were allowed to use treatments for acute attack, but these therapies were limited to triptans, high-flow oxygen, intranasal lidocaine, ergotamine, and oral analgesics.
Debilitating pain
Patients were randomly assigned to receive oral prednisone (n = 53) or placebo (n = 56). The study groups were matched with respect to demographic and clinical characteristics. Prednisone was initiated at 100 mg/d for 5 days and was then tapered by 20 mg every 3 days in the active-treatment group. All patients also received oral verapamil at a starting dose of 40 mg three times per day. The dose was increased every 3 days by 40 mg to a maximum of 360 mg/d.
All participants received pantoprazole 20 mg to prevent the gastric side effects of prednisone. An attack was defined as a unilateral headache of moderate to severe intensity. The study lasted 28 days.
The study’s primary outcome was the mean number of cluster headache attacks during the first week of treatment with prednisone versus placebo.
The mean number of attacks during the first week of treatment was 7.1 in the prednisone group and 9.5 in the placebo group, for a difference of –2.4 attacks (95% confidence interval, –4.8 to –0.03; P = .002). “This might not sound like much,” but reducing the number of daily attacks from, say, eight to six “really makes a difference because the attacks are so painful,” said Dr. Obermann.
The prednisone group also came out on top for a number secondary outcomes. After the first 7 days, attacks ceased in 35% of the prednisone group versus 7% in the placebo group.
‘Clear evidence’ of efficacy
About 49% of patients who took prednisone reported a reduction of at least 50% in attack frequency at day 7. By comparison, 15% of patients who received placebo reported such a reduction. The number of cluster attacks at day 28 was less in the prednisone group than in the patients who received placebo.
With respect to treatment effect, the difference between prednisone and placebo gradually lessened over time “in parallel to the verapamil dose reaching its therapeutic effect,” the investigators noted. “Therefore, attack frequency reduction slowly converged between groups,” they added.
The study results provide “clear evidence” and should reassure clinicians that short-term prednisone early in a cluster headache attack is effective, said Dr. Obermann.
Adverse events, which included headache, palpitations, dizziness, and nausea, were as expected and were similar in the two groups. There were only two severe adverse events, both of which occurred in participants in the placebo group.
Dr. Obermann said the investigators were surprised that so many patients in the study were taking analgesics. “Analgesics don’t work in cluster headache; they just don’t work in this kind of pain.”
He noted that prednisone exposure of study patients spanned only 19 days and amounted to only 1,100 mg, which he believes is safe.
The prednisone dose used in the study is “what most clinicians use in clinical practice,” although there have been reports of success using 500 mg of IV prednisone over 5 days, said Dr. Obermann. He added that it would be “interesting to see if 50 mg would be just as good” as a starting dose.
Potential limitations of the study include the fact that the majority of participants were White, so the findings may not be generalizable to other populations.
Long-awaited results
In an accompanying editorial, Anne Ducros, MD, PhD, professor of neurology and director of the Headache Center, Montpellier (France) University Hospital, said the study provides “strong and long-awaited evidence supporting the use of oral steroids as a transitional treatment option.”
The trial “raises many topics for future research,” one of which is the long-term safety of prednisone for patients with cluster headache, said Dr. Ducros. She noted that use of high-dose steroids once or twice a year for 15 years or more “has the potential for severe systemic toxic effects,” such as corticosteroid-induced osteonecrosis of the femoral head.
Other questions about corticosteroid use for patients with cluster headache remain. These include understanding whether these agents provide better efficacy than occipital nerve injections and determining the optimal verapamil regimen, she noted.
In addition, the risk for oral steroid misuse needs to be studied, she said. She noted that drug misuse is common among patients with cluster headache.
Despite these questions, the results of this new study “provide an important step forward for patients with cluster headache, for whom safe and effective transitional therapies are much needed,” Dr. Ducros wrote.
Dr. Obermann has received fees from Sanofi, Biogen, Novartis, Teva Pharmaceuticals, and Eli Lilly and grants from Allergan and Heel Pharmaceuticals outside of this work. Dr. Ducros has received fees from Amgen, Novartis, Teva, and Eli Lilly; grants from the Programme Hospitalier de Recherche Clinique and from the Appel d’Offre Interne of Montpellier University Hospital; and nonfinancial support from SOS Oxygene.
A version of this article originally appeared on Medscape.com.
Adjunctive oral prednisone appears to significantly reduce cluster headache attacks, new research shows. Results of the multicenter, randomized, double-blind trial show that patients who received the steroid had 25% fewer attacks in the first week of therapy, compared with their counterparts who received placebo.
In addition, more than a third of patients in the prednisone group were pain free, and for almost half, headache frequency was reduced by at least 50% at day 7 of treatment.
These findings provide clear evidence that prednisone, in conjunction with the use of verapamil, is effective in cluster headache, said lead author Mark Obermann, MD, director, Center for Neurology, Asklepios Hospitals Seesen (Germany), and associate professor, University of Duisburg-Essen (Germany).
The key message, he added, is that all patients with cluster headache should receive prednisone at the start of an episode.
The study was published online Nov. 24 in the Lancet Neurology.
‘Suicide headaches’
Cluster headaches are intense unilateral attacks of facial and head pain. They last 15-180 minutes and predominantly affect men. They are accompanied by trigeminal autonomic symptoms and are extremely painful. “They’re referred to as ‘suicide headaches’ because the pain is so severe that patients often report they think about killing themselves to get rid of the pain,” said Dr. Obermann.
The cause is unclear, although there is some evidence that the hypothalamus is involved. The headaches sometimes follow a “strict circadian pattern,” said Dr. Obermann. He noted that the attacks might occur over a few weeks or months and then not return for months or even years.
An estimated 1 in 1,000 people experience cluster headache, but the condition is underrecognized, and research is scarce and poorly funded. Previous research does show that the calcium channel blocker verapamil, which is used to treat high blood pressure, is effective in cluster headache. However, it takes about 14 days to work and has to be slowly titrated because of cardiac side effects, said Dr. Obermann. For these reasons, international guidelines recommend initiating short-term preventive treatment with corticosteroids to suppress, or at least lessen, cluster headache attacks until long-term prevention is effective.
Although some clinicians treat cluster headaches with corticosteroids, others don’t because of a lack of evidence that shows they are effective. “There’s no evidence whatsoever on what the correct dose is or whether it helps at all. This is the gap we wanted to close,” said Dr. Obermann.
The study included 116 adult patients with cluster headache from 10 centers who were experiencing a cluster headache episode and were not taking prophylactic medication.
The trial only included patients who had an attack within 30 days of their current episode. The investigators included this restriction to reduce the possibility of spontaneous remission, which is “a big problem” in cluster headache trials, he said. To confirm that episodes were cluster headache attacks, patients were also required to have moderate to severe pain, indicated by a score of at least 5 on a numerical rating scale in which 0 indicates no pain and 10 indicates the worse imaginable pain.
Participants were allowed to use treatments for acute attack, but these therapies were limited to triptans, high-flow oxygen, intranasal lidocaine, ergotamine, and oral analgesics.
Debilitating pain
Patients were randomly assigned to receive oral prednisone (n = 53) or placebo (n = 56). The study groups were matched with respect to demographic and clinical characteristics. Prednisone was initiated at 100 mg/d for 5 days and was then tapered by 20 mg every 3 days in the active-treatment group. All patients also received oral verapamil at a starting dose of 40 mg three times per day. The dose was increased every 3 days by 40 mg to a maximum of 360 mg/d.
All participants received pantoprazole 20 mg to prevent the gastric side effects of prednisone. An attack was defined as a unilateral headache of moderate to severe intensity. The study lasted 28 days.
The study’s primary outcome was the mean number of cluster headache attacks during the first week of treatment with prednisone versus placebo.
The mean number of attacks during the first week of treatment was 7.1 in the prednisone group and 9.5 in the placebo group, for a difference of –2.4 attacks (95% confidence interval, –4.8 to –0.03; P = .002). “This might not sound like much,” but reducing the number of daily attacks from, say, eight to six “really makes a difference because the attacks are so painful,” said Dr. Obermann.
The prednisone group also came out on top for a number secondary outcomes. After the first 7 days, attacks ceased in 35% of the prednisone group versus 7% in the placebo group.
‘Clear evidence’ of efficacy
About 49% of patients who took prednisone reported a reduction of at least 50% in attack frequency at day 7. By comparison, 15% of patients who received placebo reported such a reduction. The number of cluster attacks at day 28 was less in the prednisone group than in the patients who received placebo.
With respect to treatment effect, the difference between prednisone and placebo gradually lessened over time “in parallel to the verapamil dose reaching its therapeutic effect,” the investigators noted. “Therefore, attack frequency reduction slowly converged between groups,” they added.
The study results provide “clear evidence” and should reassure clinicians that short-term prednisone early in a cluster headache attack is effective, said Dr. Obermann.
Adverse events, which included headache, palpitations, dizziness, and nausea, were as expected and were similar in the two groups. There were only two severe adverse events, both of which occurred in participants in the placebo group.
Dr. Obermann said the investigators were surprised that so many patients in the study were taking analgesics. “Analgesics don’t work in cluster headache; they just don’t work in this kind of pain.”
He noted that prednisone exposure of study patients spanned only 19 days and amounted to only 1,100 mg, which he believes is safe.
The prednisone dose used in the study is “what most clinicians use in clinical practice,” although there have been reports of success using 500 mg of IV prednisone over 5 days, said Dr. Obermann. He added that it would be “interesting to see if 50 mg would be just as good” as a starting dose.
Potential limitations of the study include the fact that the majority of participants were White, so the findings may not be generalizable to other populations.
Long-awaited results
In an accompanying editorial, Anne Ducros, MD, PhD, professor of neurology and director of the Headache Center, Montpellier (France) University Hospital, said the study provides “strong and long-awaited evidence supporting the use of oral steroids as a transitional treatment option.”
The trial “raises many topics for future research,” one of which is the long-term safety of prednisone for patients with cluster headache, said Dr. Ducros. She noted that use of high-dose steroids once or twice a year for 15 years or more “has the potential for severe systemic toxic effects,” such as corticosteroid-induced osteonecrosis of the femoral head.
Other questions about corticosteroid use for patients with cluster headache remain. These include understanding whether these agents provide better efficacy than occipital nerve injections and determining the optimal verapamil regimen, she noted.
In addition, the risk for oral steroid misuse needs to be studied, she said. She noted that drug misuse is common among patients with cluster headache.
Despite these questions, the results of this new study “provide an important step forward for patients with cluster headache, for whom safe and effective transitional therapies are much needed,” Dr. Ducros wrote.
Dr. Obermann has received fees from Sanofi, Biogen, Novartis, Teva Pharmaceuticals, and Eli Lilly and grants from Allergan and Heel Pharmaceuticals outside of this work. Dr. Ducros has received fees from Amgen, Novartis, Teva, and Eli Lilly; grants from the Programme Hospitalier de Recherche Clinique and from the Appel d’Offre Interne of Montpellier University Hospital; and nonfinancial support from SOS Oxygene.
A version of this article originally appeared on Medscape.com.
Adjunctive oral prednisone appears to significantly reduce cluster headache attacks, new research shows. Results of the multicenter, randomized, double-blind trial show that patients who received the steroid had 25% fewer attacks in the first week of therapy, compared with their counterparts who received placebo.
In addition, more than a third of patients in the prednisone group were pain free, and for almost half, headache frequency was reduced by at least 50% at day 7 of treatment.
These findings provide clear evidence that prednisone, in conjunction with the use of verapamil, is effective in cluster headache, said lead author Mark Obermann, MD, director, Center for Neurology, Asklepios Hospitals Seesen (Germany), and associate professor, University of Duisburg-Essen (Germany).
The key message, he added, is that all patients with cluster headache should receive prednisone at the start of an episode.
The study was published online Nov. 24 in the Lancet Neurology.
‘Suicide headaches’
Cluster headaches are intense unilateral attacks of facial and head pain. They last 15-180 minutes and predominantly affect men. They are accompanied by trigeminal autonomic symptoms and are extremely painful. “They’re referred to as ‘suicide headaches’ because the pain is so severe that patients often report they think about killing themselves to get rid of the pain,” said Dr. Obermann.
The cause is unclear, although there is some evidence that the hypothalamus is involved. The headaches sometimes follow a “strict circadian pattern,” said Dr. Obermann. He noted that the attacks might occur over a few weeks or months and then not return for months or even years.
An estimated 1 in 1,000 people experience cluster headache, but the condition is underrecognized, and research is scarce and poorly funded. Previous research does show that the calcium channel blocker verapamil, which is used to treat high blood pressure, is effective in cluster headache. However, it takes about 14 days to work and has to be slowly titrated because of cardiac side effects, said Dr. Obermann. For these reasons, international guidelines recommend initiating short-term preventive treatment with corticosteroids to suppress, or at least lessen, cluster headache attacks until long-term prevention is effective.
Although some clinicians treat cluster headaches with corticosteroids, others don’t because of a lack of evidence that shows they are effective. “There’s no evidence whatsoever on what the correct dose is or whether it helps at all. This is the gap we wanted to close,” said Dr. Obermann.
The study included 116 adult patients with cluster headache from 10 centers who were experiencing a cluster headache episode and were not taking prophylactic medication.
The trial only included patients who had an attack within 30 days of their current episode. The investigators included this restriction to reduce the possibility of spontaneous remission, which is “a big problem” in cluster headache trials, he said. To confirm that episodes were cluster headache attacks, patients were also required to have moderate to severe pain, indicated by a score of at least 5 on a numerical rating scale in which 0 indicates no pain and 10 indicates the worse imaginable pain.
Participants were allowed to use treatments for acute attack, but these therapies were limited to triptans, high-flow oxygen, intranasal lidocaine, ergotamine, and oral analgesics.
Debilitating pain
Patients were randomly assigned to receive oral prednisone (n = 53) or placebo (n = 56). The study groups were matched with respect to demographic and clinical characteristics. Prednisone was initiated at 100 mg/d for 5 days and was then tapered by 20 mg every 3 days in the active-treatment group. All patients also received oral verapamil at a starting dose of 40 mg three times per day. The dose was increased every 3 days by 40 mg to a maximum of 360 mg/d.
All participants received pantoprazole 20 mg to prevent the gastric side effects of prednisone. An attack was defined as a unilateral headache of moderate to severe intensity. The study lasted 28 days.
The study’s primary outcome was the mean number of cluster headache attacks during the first week of treatment with prednisone versus placebo.
The mean number of attacks during the first week of treatment was 7.1 in the prednisone group and 9.5 in the placebo group, for a difference of –2.4 attacks (95% confidence interval, –4.8 to –0.03; P = .002). “This might not sound like much,” but reducing the number of daily attacks from, say, eight to six “really makes a difference because the attacks are so painful,” said Dr. Obermann.
The prednisone group also came out on top for a number secondary outcomes. After the first 7 days, attacks ceased in 35% of the prednisone group versus 7% in the placebo group.
‘Clear evidence’ of efficacy
About 49% of patients who took prednisone reported a reduction of at least 50% in attack frequency at day 7. By comparison, 15% of patients who received placebo reported such a reduction. The number of cluster attacks at day 28 was less in the prednisone group than in the patients who received placebo.
With respect to treatment effect, the difference between prednisone and placebo gradually lessened over time “in parallel to the verapamil dose reaching its therapeutic effect,” the investigators noted. “Therefore, attack frequency reduction slowly converged between groups,” they added.
The study results provide “clear evidence” and should reassure clinicians that short-term prednisone early in a cluster headache attack is effective, said Dr. Obermann.
Adverse events, which included headache, palpitations, dizziness, and nausea, were as expected and were similar in the two groups. There were only two severe adverse events, both of which occurred in participants in the placebo group.
Dr. Obermann said the investigators were surprised that so many patients in the study were taking analgesics. “Analgesics don’t work in cluster headache; they just don’t work in this kind of pain.”
He noted that prednisone exposure of study patients spanned only 19 days and amounted to only 1,100 mg, which he believes is safe.
The prednisone dose used in the study is “what most clinicians use in clinical practice,” although there have been reports of success using 500 mg of IV prednisone over 5 days, said Dr. Obermann. He added that it would be “interesting to see if 50 mg would be just as good” as a starting dose.
Potential limitations of the study include the fact that the majority of participants were White, so the findings may not be generalizable to other populations.
Long-awaited results
In an accompanying editorial, Anne Ducros, MD, PhD, professor of neurology and director of the Headache Center, Montpellier (France) University Hospital, said the study provides “strong and long-awaited evidence supporting the use of oral steroids as a transitional treatment option.”
The trial “raises many topics for future research,” one of which is the long-term safety of prednisone for patients with cluster headache, said Dr. Ducros. She noted that use of high-dose steroids once or twice a year for 15 years or more “has the potential for severe systemic toxic effects,” such as corticosteroid-induced osteonecrosis of the femoral head.
Other questions about corticosteroid use for patients with cluster headache remain. These include understanding whether these agents provide better efficacy than occipital nerve injections and determining the optimal verapamil regimen, she noted.
In addition, the risk for oral steroid misuse needs to be studied, she said. She noted that drug misuse is common among patients with cluster headache.
Despite these questions, the results of this new study “provide an important step forward for patients with cluster headache, for whom safe and effective transitional therapies are much needed,” Dr. Ducros wrote.
Dr. Obermann has received fees from Sanofi, Biogen, Novartis, Teva Pharmaceuticals, and Eli Lilly and grants from Allergan and Heel Pharmaceuticals outside of this work. Dr. Ducros has received fees from Amgen, Novartis, Teva, and Eli Lilly; grants from the Programme Hospitalier de Recherche Clinique and from the Appel d’Offre Interne of Montpellier University Hospital; and nonfinancial support from SOS Oxygene.
A version of this article originally appeared on Medscape.com.
Excess antibiotics and adverse events in patients with pneumonia
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.









