User login
Dermatology Battles COVID-19 With Comfort
We are in unprecedented times. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is attacking our communities and, as with any battle, we face unexpected challenges from the global pandemic. What can dermatologists, as highly skilled health care experts, do to support the fight against coronavirus disease 2019 (COVID-19)?
In early 2020, I became involved in a fulfilling and stimulating opportunity to contribute as a US Navy reservist, having just returned from a 3-month deployment. I served in the Medical Operations Center aboard the hospital ship USNS Comfort, which was docked in New York Harbor, as liaison to surrounding New York City hospitals. I also served as sole dermatologist on the ship, caring for the dermatologic needs of our team and consulting on numerous COVID-19 inpatients.
In May 2020, upon return to Virginia from New York City, I served as senior medical officer to medically clear other Navy Reserve health care workers returning from the field hospital at the Jacob K. Javits Convention Center of New York and from serving as embedded caregivers in existing New York City hospitals. I share 2 very important observations from my work there: First, COVID-19 is devastatingly real; second, we dermatologists can be valuable team members in the fight against this disease.
It is normal for us to feel scared, confused, and helpless; as 1% of the physician population, dermatologists represent a small focused fraction of the health care force. Nevertheless, we are all well-trained medical professionals who have taken the same Hippocratic Oath as other physicians. As members of the global health care team, we can each play a role in defeating COVID-19: We can be a trusted voice of reason, set an example, implement safe and effective distancing and hygiene precautions, and assist our local overburdened medical teams.
The magnitude and severity of COVID-19 can create a mass casualty–type phenomenon, overwhelming health care systems if the disease curve is not flattened. We can help flatten that curve by lengthening the pulse duration (to use dermatology jargon): that is, slowing the abrupt impact of cases to allow health care systems to triage, treat, and discharge in a more controlled manner.
How We Can Make a Difference
Despite representing a fraction of the health care team, we see a larger percentage of the population. On the Comfort, for example, dermatology visits accounted for approximately 20% of outpatient crew visits. We have an opportunity and a voice to reach a large percentage of the population directly. Whether we are now seeing patients face-to-face or virtually, we can spread the public health message and set an example. Wearing masks and social distancing do help to slow and markedly decrease the spread of SARS-CoV-2.
When you see patients in your office, consider the following:
• Have patients wait outside the office in their car and call the receptionist upon arrival.
• Have the receptionist call back the patient when the office is ready.
• Prescreen the patient before having him/her enter the clinic.
• Do not allow handshaking.
• Require everyone to wear a mask.
• Wear gloves.
• Have ample hand sanitizer openly available for all.
• Thoroughly clean or disinfect surfaces between patients.
Recalling the Difficult Experience of a Colleague-Patient
I think back to a crew member of Comfort who presented with new-onset pruritus and erythematous papules on the arms, legs, and torso. She was an intensive care unit nurse working 13-hour days, every day, for weeks on a COVID-positive unit—double-masked, gowned, wearing eye protection, in a warmer than usual intensive care unit, managing the most critically ill patients she’s ever cared for. Outside work, her life consisted of a commute on a government-chartered bus between Comfort and a contracted hotel while eating boxed meals. For 6 hours daily, she would—unsuccessfully—attempt to sleep with raging pruritus. Treating this routine case of eczema had a domino effect, improving her quality of life and thus allowing her to provide better care for the critically ill.
Let Us All Join in the Fight
As well-educated medical experts, we have the ability and the opportunity to reach outside our comfort zone and assist our medical colleagues. As I saw in New York City, the spectrum of specialists bravely worked together to meet overwhelming demand on the health care system and care for thousands of critically ill and dying patients. Dermatologists treated extensive eczema, ulcers, and other dermatoses on caretakers; triaged patients for appropriate allocation of care; and delivered care outside their comfort zone as physician extenders on inpatient and critical care units.
We are all in this together. I encourage all dermatologists who are in an area of need to ask your health care system how you can join the fight against SARS-CoV-2. Let’s step forward to help, in recognition of the oath we took to “prevent disease whenever we can.”
We are in unprecedented times. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is attacking our communities and, as with any battle, we face unexpected challenges from the global pandemic. What can dermatologists, as highly skilled health care experts, do to support the fight against coronavirus disease 2019 (COVID-19)?
In early 2020, I became involved in a fulfilling and stimulating opportunity to contribute as a US Navy reservist, having just returned from a 3-month deployment. I served in the Medical Operations Center aboard the hospital ship USNS Comfort, which was docked in New York Harbor, as liaison to surrounding New York City hospitals. I also served as sole dermatologist on the ship, caring for the dermatologic needs of our team and consulting on numerous COVID-19 inpatients.
In May 2020, upon return to Virginia from New York City, I served as senior medical officer to medically clear other Navy Reserve health care workers returning from the field hospital at the Jacob K. Javits Convention Center of New York and from serving as embedded caregivers in existing New York City hospitals. I share 2 very important observations from my work there: First, COVID-19 is devastatingly real; second, we dermatologists can be valuable team members in the fight against this disease.
It is normal for us to feel scared, confused, and helpless; as 1% of the physician population, dermatologists represent a small focused fraction of the health care force. Nevertheless, we are all well-trained medical professionals who have taken the same Hippocratic Oath as other physicians. As members of the global health care team, we can each play a role in defeating COVID-19: We can be a trusted voice of reason, set an example, implement safe and effective distancing and hygiene precautions, and assist our local overburdened medical teams.
The magnitude and severity of COVID-19 can create a mass casualty–type phenomenon, overwhelming health care systems if the disease curve is not flattened. We can help flatten that curve by lengthening the pulse duration (to use dermatology jargon): that is, slowing the abrupt impact of cases to allow health care systems to triage, treat, and discharge in a more controlled manner.
How We Can Make a Difference
Despite representing a fraction of the health care team, we see a larger percentage of the population. On the Comfort, for example, dermatology visits accounted for approximately 20% of outpatient crew visits. We have an opportunity and a voice to reach a large percentage of the population directly. Whether we are now seeing patients face-to-face or virtually, we can spread the public health message and set an example. Wearing masks and social distancing do help to slow and markedly decrease the spread of SARS-CoV-2.
When you see patients in your office, consider the following:
• Have patients wait outside the office in their car and call the receptionist upon arrival.
• Have the receptionist call back the patient when the office is ready.
• Prescreen the patient before having him/her enter the clinic.
• Do not allow handshaking.
• Require everyone to wear a mask.
• Wear gloves.
• Have ample hand sanitizer openly available for all.
• Thoroughly clean or disinfect surfaces between patients.
Recalling the Difficult Experience of a Colleague-Patient
I think back to a crew member of Comfort who presented with new-onset pruritus and erythematous papules on the arms, legs, and torso. She was an intensive care unit nurse working 13-hour days, every day, for weeks on a COVID-positive unit—double-masked, gowned, wearing eye protection, in a warmer than usual intensive care unit, managing the most critically ill patients she’s ever cared for. Outside work, her life consisted of a commute on a government-chartered bus between Comfort and a contracted hotel while eating boxed meals. For 6 hours daily, she would—unsuccessfully—attempt to sleep with raging pruritus. Treating this routine case of eczema had a domino effect, improving her quality of life and thus allowing her to provide better care for the critically ill.
Let Us All Join in the Fight
As well-educated medical experts, we have the ability and the opportunity to reach outside our comfort zone and assist our medical colleagues. As I saw in New York City, the spectrum of specialists bravely worked together to meet overwhelming demand on the health care system and care for thousands of critically ill and dying patients. Dermatologists treated extensive eczema, ulcers, and other dermatoses on caretakers; triaged patients for appropriate allocation of care; and delivered care outside their comfort zone as physician extenders on inpatient and critical care units.
We are all in this together. I encourage all dermatologists who are in an area of need to ask your health care system how you can join the fight against SARS-CoV-2. Let’s step forward to help, in recognition of the oath we took to “prevent disease whenever we can.”
We are in unprecedented times. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is attacking our communities and, as with any battle, we face unexpected challenges from the global pandemic. What can dermatologists, as highly skilled health care experts, do to support the fight against coronavirus disease 2019 (COVID-19)?
In early 2020, I became involved in a fulfilling and stimulating opportunity to contribute as a US Navy reservist, having just returned from a 3-month deployment. I served in the Medical Operations Center aboard the hospital ship USNS Comfort, which was docked in New York Harbor, as liaison to surrounding New York City hospitals. I also served as sole dermatologist on the ship, caring for the dermatologic needs of our team and consulting on numerous COVID-19 inpatients.
In May 2020, upon return to Virginia from New York City, I served as senior medical officer to medically clear other Navy Reserve health care workers returning from the field hospital at the Jacob K. Javits Convention Center of New York and from serving as embedded caregivers in existing New York City hospitals. I share 2 very important observations from my work there: First, COVID-19 is devastatingly real; second, we dermatologists can be valuable team members in the fight against this disease.
It is normal for us to feel scared, confused, and helpless; as 1% of the physician population, dermatologists represent a small focused fraction of the health care force. Nevertheless, we are all well-trained medical professionals who have taken the same Hippocratic Oath as other physicians. As members of the global health care team, we can each play a role in defeating COVID-19: We can be a trusted voice of reason, set an example, implement safe and effective distancing and hygiene precautions, and assist our local overburdened medical teams.
The magnitude and severity of COVID-19 can create a mass casualty–type phenomenon, overwhelming health care systems if the disease curve is not flattened. We can help flatten that curve by lengthening the pulse duration (to use dermatology jargon): that is, slowing the abrupt impact of cases to allow health care systems to triage, treat, and discharge in a more controlled manner.
How We Can Make a Difference
Despite representing a fraction of the health care team, we see a larger percentage of the population. On the Comfort, for example, dermatology visits accounted for approximately 20% of outpatient crew visits. We have an opportunity and a voice to reach a large percentage of the population directly. Whether we are now seeing patients face-to-face or virtually, we can spread the public health message and set an example. Wearing masks and social distancing do help to slow and markedly decrease the spread of SARS-CoV-2.
When you see patients in your office, consider the following:
• Have patients wait outside the office in their car and call the receptionist upon arrival.
• Have the receptionist call back the patient when the office is ready.
• Prescreen the patient before having him/her enter the clinic.
• Do not allow handshaking.
• Require everyone to wear a mask.
• Wear gloves.
• Have ample hand sanitizer openly available for all.
• Thoroughly clean or disinfect surfaces between patients.
Recalling the Difficult Experience of a Colleague-Patient
I think back to a crew member of Comfort who presented with new-onset pruritus and erythematous papules on the arms, legs, and torso. She was an intensive care unit nurse working 13-hour days, every day, for weeks on a COVID-positive unit—double-masked, gowned, wearing eye protection, in a warmer than usual intensive care unit, managing the most critically ill patients she’s ever cared for. Outside work, her life consisted of a commute on a government-chartered bus between Comfort and a contracted hotel while eating boxed meals. For 6 hours daily, she would—unsuccessfully—attempt to sleep with raging pruritus. Treating this routine case of eczema had a domino effect, improving her quality of life and thus allowing her to provide better care for the critically ill.
Let Us All Join in the Fight
As well-educated medical experts, we have the ability and the opportunity to reach outside our comfort zone and assist our medical colleagues. As I saw in New York City, the spectrum of specialists bravely worked together to meet overwhelming demand on the health care system and care for thousands of critically ill and dying patients. Dermatologists treated extensive eczema, ulcers, and other dermatoses on caretakers; triaged patients for appropriate allocation of care; and delivered care outside their comfort zone as physician extenders on inpatient and critical care units.
We are all in this together. I encourage all dermatologists who are in an area of need to ask your health care system how you can join the fight against SARS-CoV-2. Let’s step forward to help, in recognition of the oath we took to “prevent disease whenever we can.”
Practice Points
- Be aware of and promote coronavirus disease 2019 guidelines and recommendations from the Centers for Disease Control and Prevention and your local health department.
- Be prepared to push the limits of your comfort zone in an effort to assist the health care community.
Skin Eruption and Gastrointestinal Symptoms as Presentation of COVID-19
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
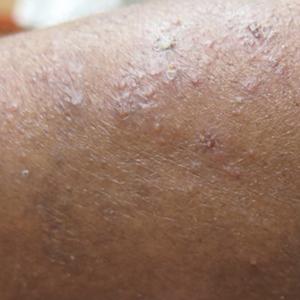
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.
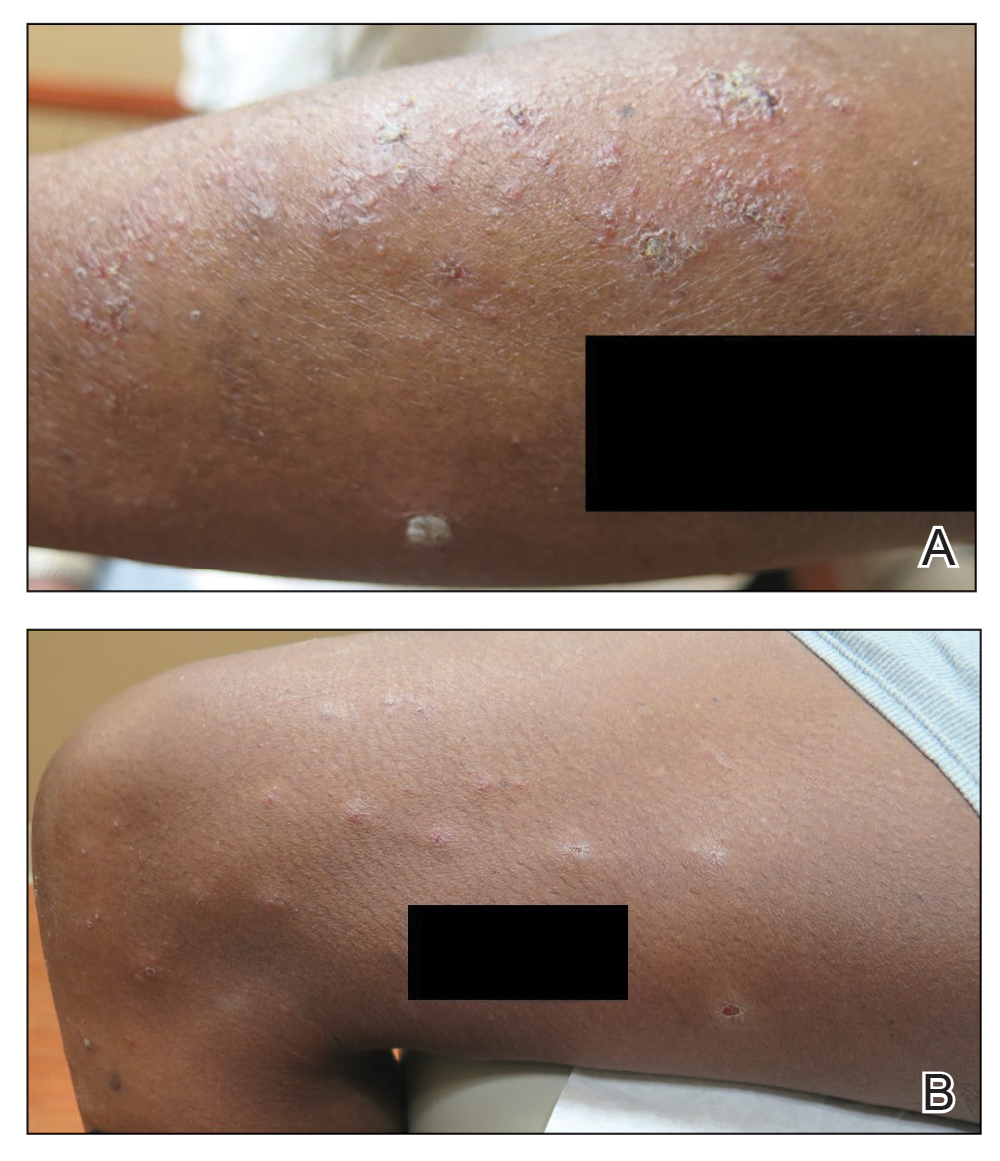
The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.

The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.

The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
Practice Points
- Patients with coronavirus disease 2019 (COVID-19) typically present with fever, dry cough, dyspnea, and fatigue, but cutaneous manifestations also have been reported.
- Awareness of atypical presentations of COVID-19, including uncommon cutaneous manifestations, may identify more cases and help slow the expansion of this pandemic.
Toxoplasma gondii infection may protect against MS
Key clinical point: Toxoplasma gondii (T. gondii) is negatively associated with multiple sclerosis (MS), suggesting a possible protective role of the parasite in MS.
Major finding: Anti-T. gondii antibodies were detected in 38 MS patients (29.5%) and 130 healthy controls (45.4%). After adjustment, T. gondii seropositivity was significantly associated with a reduced risk of MS (adjusted odds ratio, 0.56; P = .02).
Study details: The data come from an Italian population‑based case-control study of 129 patients with MS and 287 age- and sex-matched controls.
Disclosures: This research was funded by the Department of Medical and Surgical Sciences and Advanced Technologies “G.F. Ingrassia,” University of Catania, Italy. The authors declared no conflicts of interest.
Source: Nicoletti A et al. Sci Rep. 2020 Nov 2. doi: 10.1038/s41598-020-75830-y.
Key clinical point: Toxoplasma gondii (T. gondii) is negatively associated with multiple sclerosis (MS), suggesting a possible protective role of the parasite in MS.
Major finding: Anti-T. gondii antibodies were detected in 38 MS patients (29.5%) and 130 healthy controls (45.4%). After adjustment, T. gondii seropositivity was significantly associated with a reduced risk of MS (adjusted odds ratio, 0.56; P = .02).
Study details: The data come from an Italian population‑based case-control study of 129 patients with MS and 287 age- and sex-matched controls.
Disclosures: This research was funded by the Department of Medical and Surgical Sciences and Advanced Technologies “G.F. Ingrassia,” University of Catania, Italy. The authors declared no conflicts of interest.
Source: Nicoletti A et al. Sci Rep. 2020 Nov 2. doi: 10.1038/s41598-020-75830-y.
Key clinical point: Toxoplasma gondii (T. gondii) is negatively associated with multiple sclerosis (MS), suggesting a possible protective role of the parasite in MS.
Major finding: Anti-T. gondii antibodies were detected in 38 MS patients (29.5%) and 130 healthy controls (45.4%). After adjustment, T. gondii seropositivity was significantly associated with a reduced risk of MS (adjusted odds ratio, 0.56; P = .02).
Study details: The data come from an Italian population‑based case-control study of 129 patients with MS and 287 age- and sex-matched controls.
Disclosures: This research was funded by the Department of Medical and Surgical Sciences and Advanced Technologies “G.F. Ingrassia,” University of Catania, Italy. The authors declared no conflicts of interest.
Source: Nicoletti A et al. Sci Rep. 2020 Nov 2. doi: 10.1038/s41598-020-75830-y.
Combined Treatment of Disfiguring Facial Angiofibromas in Tuberous Sclerosis Complex With Surgical Debulking and Topical Sirolimus
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
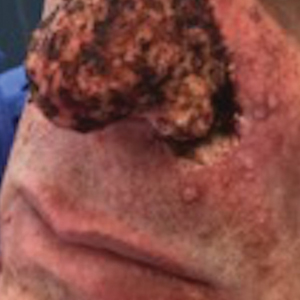
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
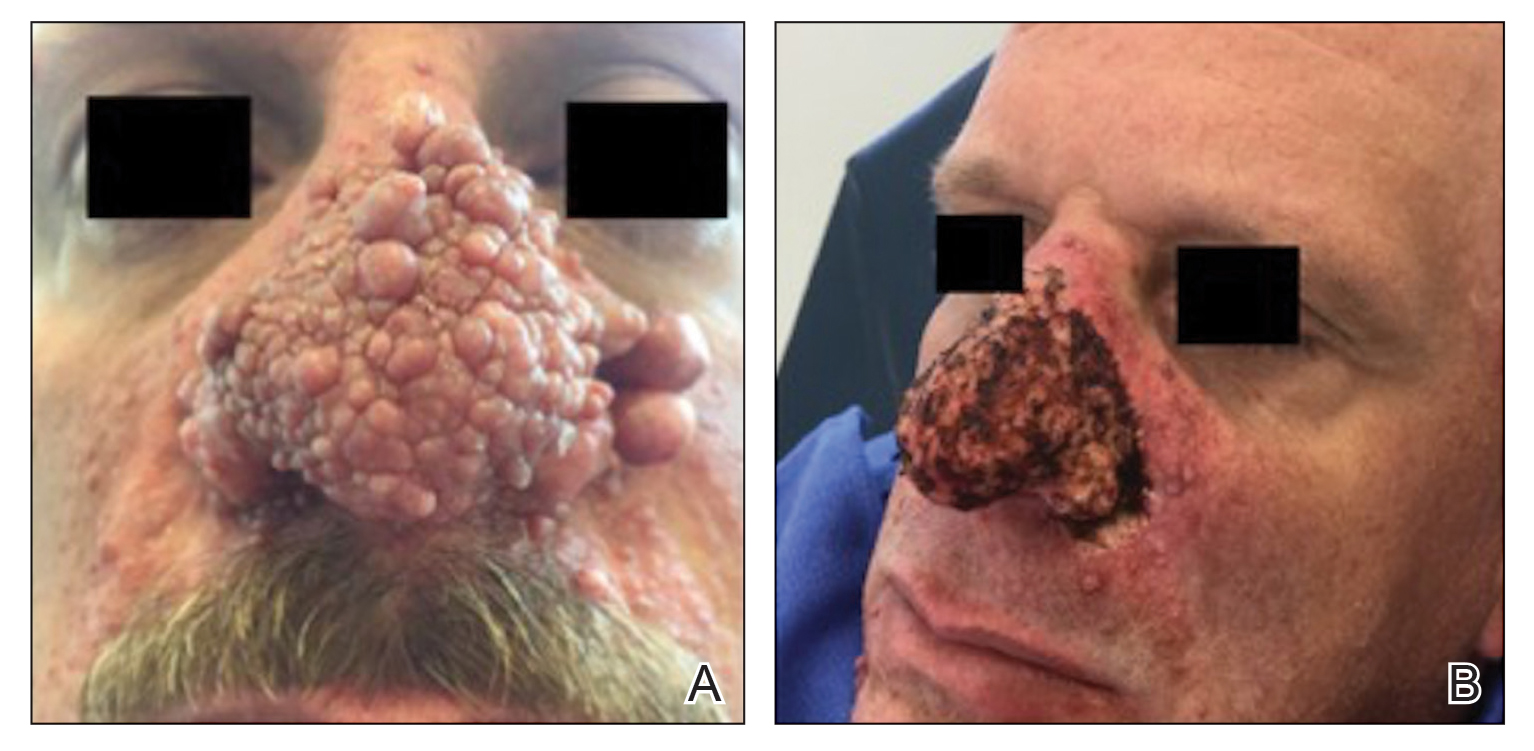
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
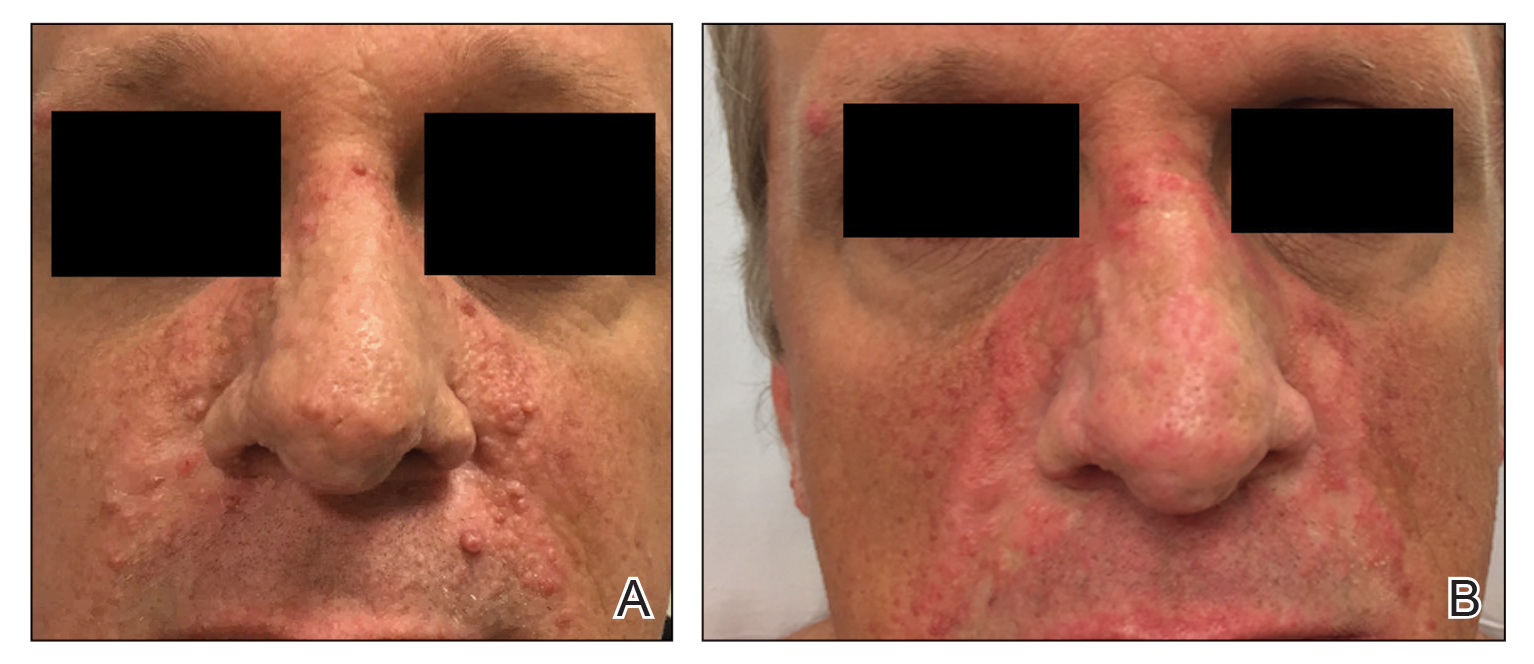
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.

Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).

Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.

Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).

Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Doctor in a Bottle: Examining the Increase in Essential Oil Use
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
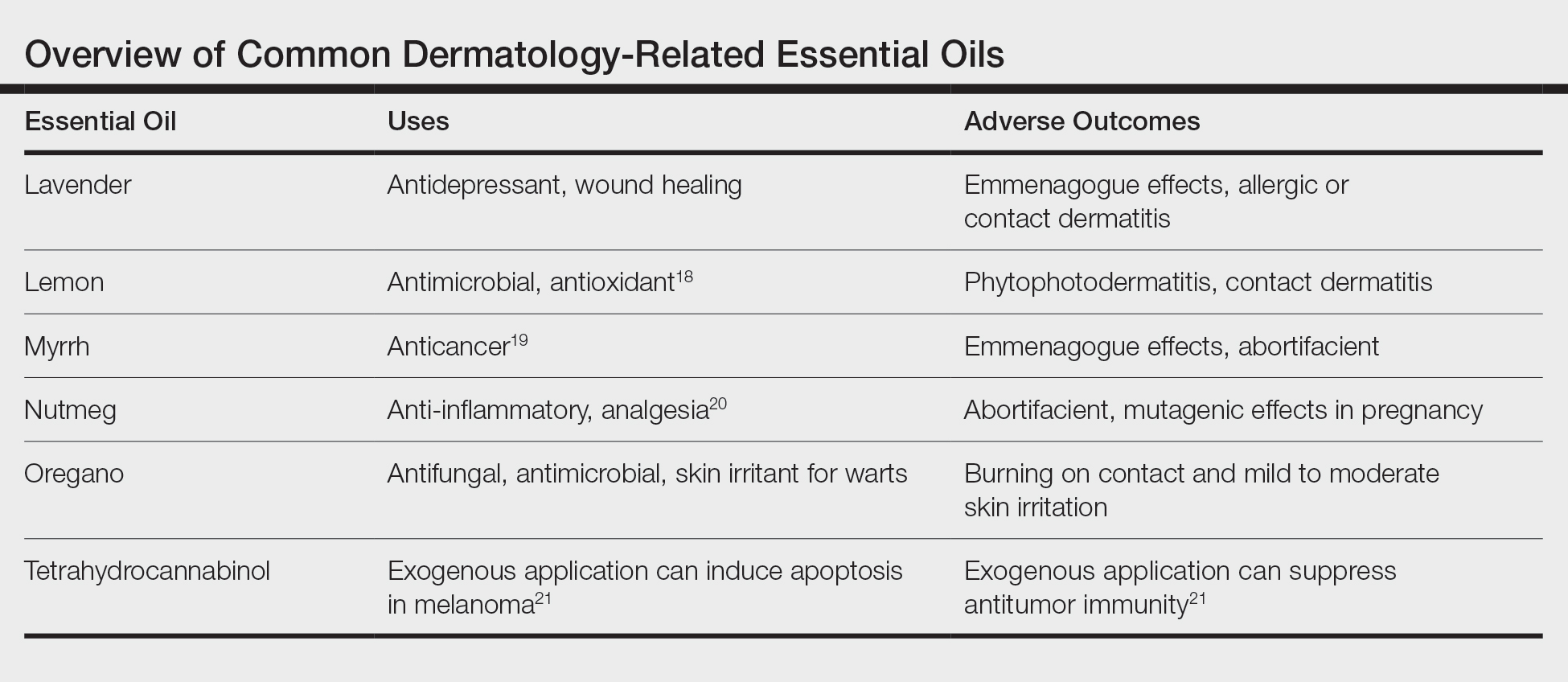
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.

What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.

- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
Practice Points
- Essential oils are a rising trend of nonprescribed topical supplements used by patients to self-treat.
- Research into historically medicinal essential oils may unlock treatment opportunities in the near future.
- Keeping an open-minded line of communication is critical for divulgence of potential home remedies that could be causing patients harm.
- Understanding the mindset of the essential oil–using community is key to building trust and treating these patients who are often distrusting of Western medicine.
Mobile Apps for Professional Dermatology Education: An Objective Review
With today’s technology, it is easier than ever to access web-based tools that enrich traditional dermatology education. The literature supports the use of these innovative platforms to enhance learning at the student and trainee levels. A controlled study of pediatric residents showed that online modules effectively supplemented clinical experience with atopic dermatitis.1 In a randomized diagnostic study of medical students, practice with an image-based web application (app) that teaches rapid recognition of melanoma proved more effective than learning a rule-based algorithm.2 Given the visual nature of dermatology, pattern recognition is an essential skill that is fostered through experience and is only made more accessible with technology.
With the added benefit of convenience and accessibility, mobile apps can supplement experiential learning. Mirroring the overall growth of mobile apps, the number of available dermatology apps has increased.3 Dermatology mobile apps serve purposes ranging from quick reference tools to comprehensive modules, journals, and question banks. At an academic hospital in Taiwan, both nondermatology and dermatology trainees’ examination performance improved after 3 weeks of using a smartphone-based wallpaper learning module displaying morphologic characteristics of fungi.4 With the expansion of virtual microscopy, mobile apps also have been created as a learning tool for dermatopathology, giving trainees the flexibility and autonomy to view slides on their own time.5 Nevertheless, the literature on dermatology mobile apps designed for the education of medical students and trainees is limited, demonstrating a need for further investigation.
Prior studies have reviewed dermatology apps for patients and practicing dermatologists.6-8 Herein, we focus on mobile apps targeting students and residents learning dermatology. General dermatology reference apps and educational aid apps have grown by 33% and 32%, respectively, from 2014 to 2017.3 As with any resource meant to educate future and current medical providers, there must be an objective review process in place to ensure accurate, unbiased, evidence-based teaching.
Well-organized, comprehensive information and a user-friendly interface are additional factors of importance when selecting an educational mobile app. When discussing supplemental resources, accessibility and affordability also are priorities given the high cost of a medical education at baseline. Overall, there is a need for a standardized method to evaluate the key factors of an educational mobile app that make it appropriate for this demographic. We conducted a search of mobile apps relating to dermatology education for students and residents.
Methods
We searched for publicly available mobile apps relating to dermatology education in the App Store (Apple Inc) from September to November 2019 using the search terms dermatology education, dermoscopy education, melanoma education, skin cancer education, psoriasis education, rosacea education, acne education, eczema education, dermal fillers education, and Mohs surgery education. We excluded apps that were not in English, were created for a conference, cost more than $5 to download, or did not include a specific dermatology education section. In this way, we hoped to evaluate apps that were relevant, accessible, and affordable.
We modeled our study after a review of patient education apps performed by Masud et al6 and utilized their quantified grading rubric (scale of 1 to 4). We found their established criteria—educational objectives, content, accuracy, design, and conflict of interest—to be equally applicable for evaluating apps designed for professional education.6 Each app earned a minimum of 1 point and a maximum of 4 points per criterion. One point was given if the app did not fulfill the criterion, 2 points for minimally fulfilling the criterion, 3 points for mostly fulfilling the criterion, and 4 points if the criterion was completely fulfilled. Two medical students (E.H. and N.C.)—one at the preclinical stage and the other at the clinical stage of medical education—reviewed the apps using the given rubric, then discussed and resolved any discrepancies in points assigned. A dermatology resident (M.A.) independently reviewed the apps using the given rubric.
The mean of the student score and the resident score was calculated for each category. The sum of the averages for each category was considered the final score for an app, determining its overall quality. Apps with a total score of 5 to 10 were considered poor and inadequate for education. A total score of 10.5 to 15 indicated that an app was somewhat adequate (ie, useful for education in some aspects but falling short in others). Apps that were considered adequate for education, across all or most criteria, received a total score ranging from 15.5 to 20.
Results
Our search generated 130 apps. After applying exclusion criteria, 42 apps were eligible for review. At the time of publication, 36 of these apps were still available. The possible range of scores based on the rubric was 5 to 20. The actual range of scores was 7 to 20. Of the 36 apps, 2 (5.6%) were poor, 16 (44.4%) were somewhat adequate, and 18 (50%) were adequate. Formats included primary resources, such as clinical decision support tools, journals, references, and a podcast (Table 1). Additionally, interactive learning tools included games, learning modules, and apps for self-evaluation (Table 2). Thirty apps covered general dermatology; others focused on skin cancer (n=5) and cosmetic dermatology (n=1). Regarding cost, 29 apps were free to download, whereas 7 charged a fee (mean price, $2.56).

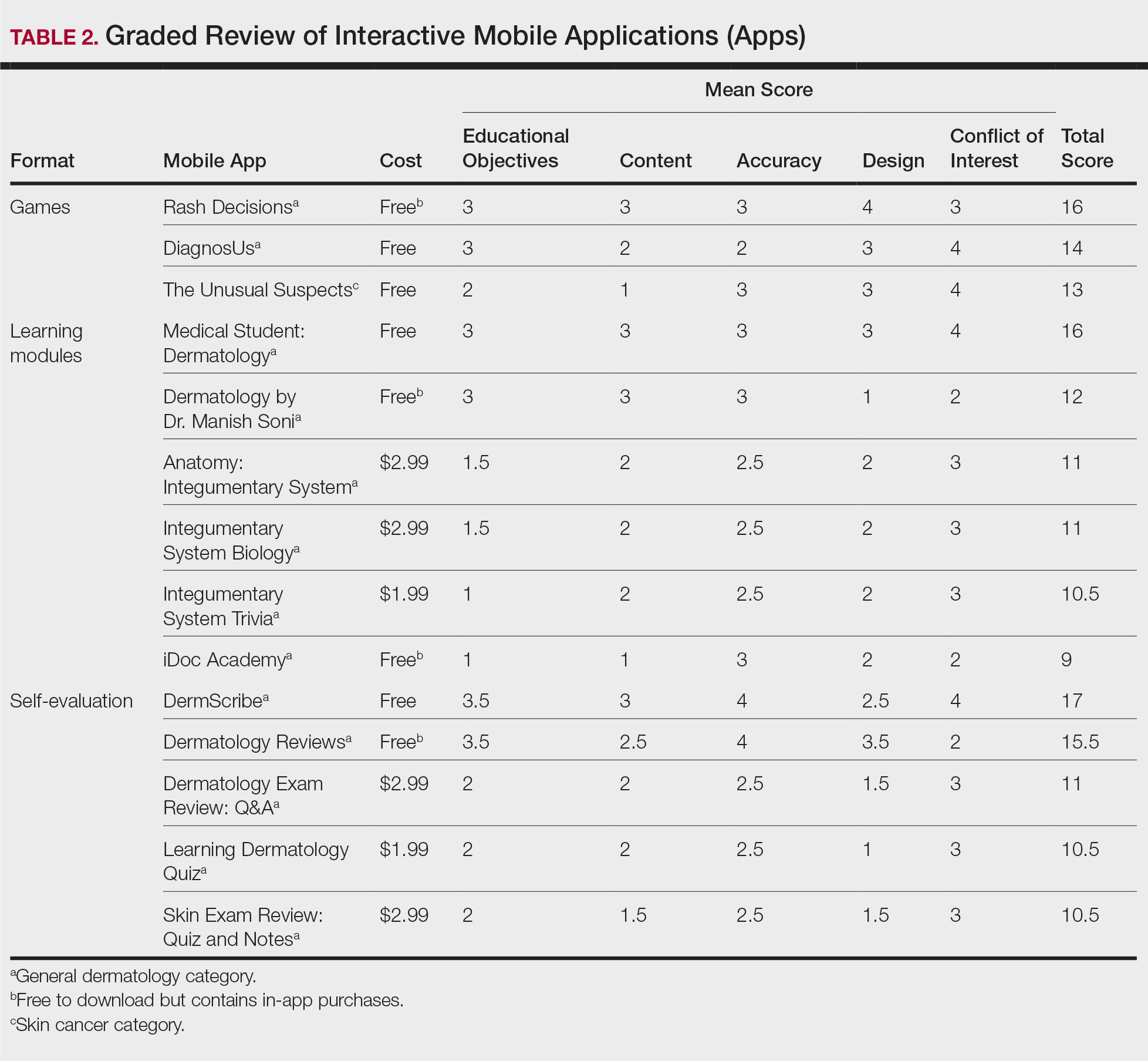
Comment
In addition to the convenience of having an educational tool in their white-coat pocket, learners of dermatology have been shown to benefit from supplementing their curriculum with mobile apps, which sets the stage for formal integration of mobile apps into dermatology teaching in the future.8 Prior to widespread adoption, mobile apps must be evaluated for content and utility, starting with an objective rubric.
Without official scientific standards in place, it was unsurprising that only half of the dermatology education applications were classified as adequate in this study. Among the types of apps offered—clinical decision support tools, journals, references, podcast, games, learning modules, and self-evaluation—certain categories scored higher than others. App formats with the highest average score (16.5 out of 20) were journals and podcast.
One barrier to utilization of these apps was that a subscription to the journals and podcast was required to obtain access to all available content. Students and trainees can seek out library resources at their academic institutions to take advantage of journal subscriptions available to them at no additional cost. Dermatology residents can take advantage of their complimentary membership in the American Academy of Dermatology for a free subscription to AAD Dialogues in Dermatology (otherwise $179 annually for nonresident members and $320 annually for nonmembers).
On the other hand, learning module was the lowest-rated format (average score, 11.3 out of 20), with only Medical Student: Dermatology qualifying as adequate (total score, 16). This finding is worrisome given that students and residents might look to learning modules for quick targeted lessons on specific topics.
The lowest-scoring app, a clinical decision support tool called Naturelize, received a total score of 7. Although it listed the indications and contraindications for dermal filler types to be used in different locations on the face, there was a clear conflict of interest, oversimplified design, and little evidence-based education, mirroring the current state of cosmetic dermatology training in residency, in which trainees think they are inadequately prepared for aesthetic procedures and comparative effectiveness research is lacking.9-11
At the opposite end of the spectrum, MyDermPath+ was a reference app with a total score of 20. The app cited credible authors with a medical degree (MD) and had an easy-to-use, well-designed interface, including a reference guide, differential builder, and quiz for a range of topics within dermatology. As a free download without in-app purchases or advertisements, there was no evidence of conflict of interest. The position of a dermatopathology app as the top dermatology education mobile app might reflect an increased emphasis on dermatopathology education in residency as well as a transition to digitization of slides.5
The second-highest scoring apps (total score of 19 points) were Dermatology Database and VisualDx. Both were references covering a wide range of dermatology topics. Dermatology Database was a comprehensive search tool for diseases, drugs, procedures, and terms that was simple and entirely free to use but did not cite references. VisualDx, as its name suggests, offered quality clinical images, complete guides with references, and a unique differential builder. An annual subscription is $399.99, but the process to gain free access through a participating academic institution was simple.
Games were a unique mobile app format; however, 2 of 3 games scored in the somewhat adequate range. The game DiagnosUs, which tested users’ ability to differentiate skin cancer and psoriasis from dermatitis on clinical images, would benefit from more comprehensive content as well as professional verification of true diagnoses, which earned the app 2 points in both the content and accuracy categories. The Unusual Suspects tested the ABCDE algorithm in a short learning module, followed by a simple game that involved identification of melanoma in a timed setting. Although the design was novel and interactive, the game was limited to the same 5 melanoma tumors overlaid on pictures of normal skin. The narrow scope earned 1 point for content, the redundancy in the game earned 3 points for design, and the lack of real clinical images earned 2 points for educational objectives. Although game-format mobile apps have the capability to challenge the user’s knowledge with a built-in feedback or reward system, improvements should be made to ensure that apps are equally educational as they are engaging.
AAD Dialogues in Dermatology was the only app in the form of a podcast and provided expert interviews along with disclosures, transcripts, commentary, and references. More than half the content in the app could not be accessed without a subscription, earning 2.5 points in the conflict of interest category. Additionally, several flaws resulted in a design score of 2.5, including inconsistent availability of transcripts, poor quality of sound on some episodes, difficulty distinguishing new episodes from those already played, and a glitch that removed the episode duration. Still, the app was a valuable and comprehensive resource, with clear objectives and cited references. With improvements in content, affordability, and user experience, apps in unique formats such as games and podcasts might appeal to kinesthetic and auditory learners.
An important factor to consider when discussing mobile apps for students and residents is cost. With rising prices of board examinations and preparation materials, supplementary study tools should not come with an exorbitant price tag. Therefore, we limited our evaluation to apps that were free or cost less than $5 to download. Even so, subscriptions and other in-app purchases were an obstacle in one-third of apps, ranging from $4.99 to unlock additional content in Rash Decisions to $69.99 to access most topics in Fitzpatrick’s Color Atlas. The highest-rated app in our study, MyDermPath+, historically cost $19.99 to download but became free with a grant from the Sulzberger Foundation.12 An initial investment to develop quality apps for the purpose of dermatology education might pay off in the end.
To evaluate the apps from the perspective of the target demographic of this study, 2 medical students—one in the preclinical stage and the other in the clinical stage of medical education—and a dermatology resident graded the apps. Certain limitations exist in this type of study, including differing learning styles, which might influence the types of apps that evaluators found most impactful to their education. Interestingly, some apps earned a higher resident score than student score. In particular, RightSite (a reference that helps with anatomically correct labeling) and Mohs Surgery Appropriate Use Criteria (a clinical decision support tool to determine whether to perform Mohs surgery) each had a 3-point discrepancy (data not shown). A resident might benefit from these practical apps in day-to-day practice, but a student would be less likely to find them useful as a learning tool.
Still, by defining adequate teaching value using specific categories of educational objectives, content, accuracy, design, and conflict of interest, we attempted to minimize the effect of personal preference on the grading process. Although we acknowledge a degree of subjectivity, we found that utilizing a previously published rubric with defined criteria was crucial in remaining unbiased.
Conclusion
Further studies should evaluate additional apps available on Apple’s iPad (tablet), as well as those on other operating systems, including Google’s Android. To ensure the existence of mobile apps as adequate education tools, they should be peer reviewed prior to publication or before widespread use by future and current providers at the minimum. To maximize free access to highly valuable resources available in the palm of their hand, students and trainees should contact the library at their academic institution.
- Craddock MF, Blondin HM, Youssef MJ, et al. Online education improves pediatric residents' understanding of atopic dermatitis. Pediatr Dermatol. 2018;35:64-69.
- Lacy FA, Coman GC, Holliday AC, et al. Assessment of smartphone application for teaching intuitive visual diagnosis of melanoma. JAMA Dermatol. 2018;154:730-731.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology--2017 update. Dermatol Online J. 2018;24:13.
- Liu R-F, Wang F-Y, Yen H, et al. A new mobile learning module using smartphone wallpapers in identification of medical fungi for medical students and residents. Int J Dermatol. 2018;57:458-462.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Mercer JM. An array of mobile apps for dermatologists. J Cutan Med Surg. 2014;18:295-297.
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol. 2013;68:e23-e28.
- Waldman A, Sobanko JF, Alam M. Practice and educational gaps in cosmetic dermatologic surgery. Dermatol Clin. 2016;34:341-346.
- Nielson CB, Harb JN, Motaparthi K. Education in cosmetic procedural dermatology: resident experiences and perceptions. J Clin Aesthet Dermatol. 2019;12:E70-E72.
- Hanna MG, Parwani AV, Pantanowitz L, et al. Smartphone applications: a contemporary resource for dermatopathology. J Pathol Inform. 2015;6:44.
With today’s technology, it is easier than ever to access web-based tools that enrich traditional dermatology education. The literature supports the use of these innovative platforms to enhance learning at the student and trainee levels. A controlled study of pediatric residents showed that online modules effectively supplemented clinical experience with atopic dermatitis.1 In a randomized diagnostic study of medical students, practice with an image-based web application (app) that teaches rapid recognition of melanoma proved more effective than learning a rule-based algorithm.2 Given the visual nature of dermatology, pattern recognition is an essential skill that is fostered through experience and is only made more accessible with technology.
With the added benefit of convenience and accessibility, mobile apps can supplement experiential learning. Mirroring the overall growth of mobile apps, the number of available dermatology apps has increased.3 Dermatology mobile apps serve purposes ranging from quick reference tools to comprehensive modules, journals, and question banks. At an academic hospital in Taiwan, both nondermatology and dermatology trainees’ examination performance improved after 3 weeks of using a smartphone-based wallpaper learning module displaying morphologic characteristics of fungi.4 With the expansion of virtual microscopy, mobile apps also have been created as a learning tool for dermatopathology, giving trainees the flexibility and autonomy to view slides on their own time.5 Nevertheless, the literature on dermatology mobile apps designed for the education of medical students and trainees is limited, demonstrating a need for further investigation.
Prior studies have reviewed dermatology apps for patients and practicing dermatologists.6-8 Herein, we focus on mobile apps targeting students and residents learning dermatology. General dermatology reference apps and educational aid apps have grown by 33% and 32%, respectively, from 2014 to 2017.3 As with any resource meant to educate future and current medical providers, there must be an objective review process in place to ensure accurate, unbiased, evidence-based teaching.
Well-organized, comprehensive information and a user-friendly interface are additional factors of importance when selecting an educational mobile app. When discussing supplemental resources, accessibility and affordability also are priorities given the high cost of a medical education at baseline. Overall, there is a need for a standardized method to evaluate the key factors of an educational mobile app that make it appropriate for this demographic. We conducted a search of mobile apps relating to dermatology education for students and residents.
Methods
We searched for publicly available mobile apps relating to dermatology education in the App Store (Apple Inc) from September to November 2019 using the search terms dermatology education, dermoscopy education, melanoma education, skin cancer education, psoriasis education, rosacea education, acne education, eczema education, dermal fillers education, and Mohs surgery education. We excluded apps that were not in English, were created for a conference, cost more than $5 to download, or did not include a specific dermatology education section. In this way, we hoped to evaluate apps that were relevant, accessible, and affordable.
We modeled our study after a review of patient education apps performed by Masud et al6 and utilized their quantified grading rubric (scale of 1 to 4). We found their established criteria—educational objectives, content, accuracy, design, and conflict of interest—to be equally applicable for evaluating apps designed for professional education.6 Each app earned a minimum of 1 point and a maximum of 4 points per criterion. One point was given if the app did not fulfill the criterion, 2 points for minimally fulfilling the criterion, 3 points for mostly fulfilling the criterion, and 4 points if the criterion was completely fulfilled. Two medical students (E.H. and N.C.)—one at the preclinical stage and the other at the clinical stage of medical education—reviewed the apps using the given rubric, then discussed and resolved any discrepancies in points assigned. A dermatology resident (M.A.) independently reviewed the apps using the given rubric.
The mean of the student score and the resident score was calculated for each category. The sum of the averages for each category was considered the final score for an app, determining its overall quality. Apps with a total score of 5 to 10 were considered poor and inadequate for education. A total score of 10.5 to 15 indicated that an app was somewhat adequate (ie, useful for education in some aspects but falling short in others). Apps that were considered adequate for education, across all or most criteria, received a total score ranging from 15.5 to 20.
Results
Our search generated 130 apps. After applying exclusion criteria, 42 apps were eligible for review. At the time of publication, 36 of these apps were still available. The possible range of scores based on the rubric was 5 to 20. The actual range of scores was 7 to 20. Of the 36 apps, 2 (5.6%) were poor, 16 (44.4%) were somewhat adequate, and 18 (50%) were adequate. Formats included primary resources, such as clinical decision support tools, journals, references, and a podcast (Table 1). Additionally, interactive learning tools included games, learning modules, and apps for self-evaluation (Table 2). Thirty apps covered general dermatology; others focused on skin cancer (n=5) and cosmetic dermatology (n=1). Regarding cost, 29 apps were free to download, whereas 7 charged a fee (mean price, $2.56).


Comment
In addition to the convenience of having an educational tool in their white-coat pocket, learners of dermatology have been shown to benefit from supplementing their curriculum with mobile apps, which sets the stage for formal integration of mobile apps into dermatology teaching in the future.8 Prior to widespread adoption, mobile apps must be evaluated for content and utility, starting with an objective rubric.
Without official scientific standards in place, it was unsurprising that only half of the dermatology education applications were classified as adequate in this study. Among the types of apps offered—clinical decision support tools, journals, references, podcast, games, learning modules, and self-evaluation—certain categories scored higher than others. App formats with the highest average score (16.5 out of 20) were journals and podcast.
One barrier to utilization of these apps was that a subscription to the journals and podcast was required to obtain access to all available content. Students and trainees can seek out library resources at their academic institutions to take advantage of journal subscriptions available to them at no additional cost. Dermatology residents can take advantage of their complimentary membership in the American Academy of Dermatology for a free subscription to AAD Dialogues in Dermatology (otherwise $179 annually for nonresident members and $320 annually for nonmembers).
On the other hand, learning module was the lowest-rated format (average score, 11.3 out of 20), with only Medical Student: Dermatology qualifying as adequate (total score, 16). This finding is worrisome given that students and residents might look to learning modules for quick targeted lessons on specific topics.
The lowest-scoring app, a clinical decision support tool called Naturelize, received a total score of 7. Although it listed the indications and contraindications for dermal filler types to be used in different locations on the face, there was a clear conflict of interest, oversimplified design, and little evidence-based education, mirroring the current state of cosmetic dermatology training in residency, in which trainees think they are inadequately prepared for aesthetic procedures and comparative effectiveness research is lacking.9-11
At the opposite end of the spectrum, MyDermPath+ was a reference app with a total score of 20. The app cited credible authors with a medical degree (MD) and had an easy-to-use, well-designed interface, including a reference guide, differential builder, and quiz for a range of topics within dermatology. As a free download without in-app purchases or advertisements, there was no evidence of conflict of interest. The position of a dermatopathology app as the top dermatology education mobile app might reflect an increased emphasis on dermatopathology education in residency as well as a transition to digitization of slides.5
The second-highest scoring apps (total score of 19 points) were Dermatology Database and VisualDx. Both were references covering a wide range of dermatology topics. Dermatology Database was a comprehensive search tool for diseases, drugs, procedures, and terms that was simple and entirely free to use but did not cite references. VisualDx, as its name suggests, offered quality clinical images, complete guides with references, and a unique differential builder. An annual subscription is $399.99, but the process to gain free access through a participating academic institution was simple.
Games were a unique mobile app format; however, 2 of 3 games scored in the somewhat adequate range. The game DiagnosUs, which tested users’ ability to differentiate skin cancer and psoriasis from dermatitis on clinical images, would benefit from more comprehensive content as well as professional verification of true diagnoses, which earned the app 2 points in both the content and accuracy categories. The Unusual Suspects tested the ABCDE algorithm in a short learning module, followed by a simple game that involved identification of melanoma in a timed setting. Although the design was novel and interactive, the game was limited to the same 5 melanoma tumors overlaid on pictures of normal skin. The narrow scope earned 1 point for content, the redundancy in the game earned 3 points for design, and the lack of real clinical images earned 2 points for educational objectives. Although game-format mobile apps have the capability to challenge the user’s knowledge with a built-in feedback or reward system, improvements should be made to ensure that apps are equally educational as they are engaging.
AAD Dialogues in Dermatology was the only app in the form of a podcast and provided expert interviews along with disclosures, transcripts, commentary, and references. More than half the content in the app could not be accessed without a subscription, earning 2.5 points in the conflict of interest category. Additionally, several flaws resulted in a design score of 2.5, including inconsistent availability of transcripts, poor quality of sound on some episodes, difficulty distinguishing new episodes from those already played, and a glitch that removed the episode duration. Still, the app was a valuable and comprehensive resource, with clear objectives and cited references. With improvements in content, affordability, and user experience, apps in unique formats such as games and podcasts might appeal to kinesthetic and auditory learners.
An important factor to consider when discussing mobile apps for students and residents is cost. With rising prices of board examinations and preparation materials, supplementary study tools should not come with an exorbitant price tag. Therefore, we limited our evaluation to apps that were free or cost less than $5 to download. Even so, subscriptions and other in-app purchases were an obstacle in one-third of apps, ranging from $4.99 to unlock additional content in Rash Decisions to $69.99 to access most topics in Fitzpatrick’s Color Atlas. The highest-rated app in our study, MyDermPath+, historically cost $19.99 to download but became free with a grant from the Sulzberger Foundation.12 An initial investment to develop quality apps for the purpose of dermatology education might pay off in the end.
To evaluate the apps from the perspective of the target demographic of this study, 2 medical students—one in the preclinical stage and the other in the clinical stage of medical education—and a dermatology resident graded the apps. Certain limitations exist in this type of study, including differing learning styles, which might influence the types of apps that evaluators found most impactful to their education. Interestingly, some apps earned a higher resident score than student score. In particular, RightSite (a reference that helps with anatomically correct labeling) and Mohs Surgery Appropriate Use Criteria (a clinical decision support tool to determine whether to perform Mohs surgery) each had a 3-point discrepancy (data not shown). A resident might benefit from these practical apps in day-to-day practice, but a student would be less likely to find them useful as a learning tool.
Still, by defining adequate teaching value using specific categories of educational objectives, content, accuracy, design, and conflict of interest, we attempted to minimize the effect of personal preference on the grading process. Although we acknowledge a degree of subjectivity, we found that utilizing a previously published rubric with defined criteria was crucial in remaining unbiased.
Conclusion
Further studies should evaluate additional apps available on Apple’s iPad (tablet), as well as those on other operating systems, including Google’s Android. To ensure the existence of mobile apps as adequate education tools, they should be peer reviewed prior to publication or before widespread use by future and current providers at the minimum. To maximize free access to highly valuable resources available in the palm of their hand, students and trainees should contact the library at their academic institution.
With today’s technology, it is easier than ever to access web-based tools that enrich traditional dermatology education. The literature supports the use of these innovative platforms to enhance learning at the student and trainee levels. A controlled study of pediatric residents showed that online modules effectively supplemented clinical experience with atopic dermatitis.1 In a randomized diagnostic study of medical students, practice with an image-based web application (app) that teaches rapid recognition of melanoma proved more effective than learning a rule-based algorithm.2 Given the visual nature of dermatology, pattern recognition is an essential skill that is fostered through experience and is only made more accessible with technology.
With the added benefit of convenience and accessibility, mobile apps can supplement experiential learning. Mirroring the overall growth of mobile apps, the number of available dermatology apps has increased.3 Dermatology mobile apps serve purposes ranging from quick reference tools to comprehensive modules, journals, and question banks. At an academic hospital in Taiwan, both nondermatology and dermatology trainees’ examination performance improved after 3 weeks of using a smartphone-based wallpaper learning module displaying morphologic characteristics of fungi.4 With the expansion of virtual microscopy, mobile apps also have been created as a learning tool for dermatopathology, giving trainees the flexibility and autonomy to view slides on their own time.5 Nevertheless, the literature on dermatology mobile apps designed for the education of medical students and trainees is limited, demonstrating a need for further investigation.
Prior studies have reviewed dermatology apps for patients and practicing dermatologists.6-8 Herein, we focus on mobile apps targeting students and residents learning dermatology. General dermatology reference apps and educational aid apps have grown by 33% and 32%, respectively, from 2014 to 2017.3 As with any resource meant to educate future and current medical providers, there must be an objective review process in place to ensure accurate, unbiased, evidence-based teaching.
Well-organized, comprehensive information and a user-friendly interface are additional factors of importance when selecting an educational mobile app. When discussing supplemental resources, accessibility and affordability also are priorities given the high cost of a medical education at baseline. Overall, there is a need for a standardized method to evaluate the key factors of an educational mobile app that make it appropriate for this demographic. We conducted a search of mobile apps relating to dermatology education for students and residents.
Methods
We searched for publicly available mobile apps relating to dermatology education in the App Store (Apple Inc) from September to November 2019 using the search terms dermatology education, dermoscopy education, melanoma education, skin cancer education, psoriasis education, rosacea education, acne education, eczema education, dermal fillers education, and Mohs surgery education. We excluded apps that were not in English, were created for a conference, cost more than $5 to download, or did not include a specific dermatology education section. In this way, we hoped to evaluate apps that were relevant, accessible, and affordable.
We modeled our study after a review of patient education apps performed by Masud et al6 and utilized their quantified grading rubric (scale of 1 to 4). We found their established criteria—educational objectives, content, accuracy, design, and conflict of interest—to be equally applicable for evaluating apps designed for professional education.6 Each app earned a minimum of 1 point and a maximum of 4 points per criterion. One point was given if the app did not fulfill the criterion, 2 points for minimally fulfilling the criterion, 3 points for mostly fulfilling the criterion, and 4 points if the criterion was completely fulfilled. Two medical students (E.H. and N.C.)—one at the preclinical stage and the other at the clinical stage of medical education—reviewed the apps using the given rubric, then discussed and resolved any discrepancies in points assigned. A dermatology resident (M.A.) independently reviewed the apps using the given rubric.
The mean of the student score and the resident score was calculated for each category. The sum of the averages for each category was considered the final score for an app, determining its overall quality. Apps with a total score of 5 to 10 were considered poor and inadequate for education. A total score of 10.5 to 15 indicated that an app was somewhat adequate (ie, useful for education in some aspects but falling short in others). Apps that were considered adequate for education, across all or most criteria, received a total score ranging from 15.5 to 20.
Results
Our search generated 130 apps. After applying exclusion criteria, 42 apps were eligible for review. At the time of publication, 36 of these apps were still available. The possible range of scores based on the rubric was 5 to 20. The actual range of scores was 7 to 20. Of the 36 apps, 2 (5.6%) were poor, 16 (44.4%) were somewhat adequate, and 18 (50%) were adequate. Formats included primary resources, such as clinical decision support tools, journals, references, and a podcast (Table 1). Additionally, interactive learning tools included games, learning modules, and apps for self-evaluation (Table 2). Thirty apps covered general dermatology; others focused on skin cancer (n=5) and cosmetic dermatology (n=1). Regarding cost, 29 apps were free to download, whereas 7 charged a fee (mean price, $2.56).


Comment
In addition to the convenience of having an educational tool in their white-coat pocket, learners of dermatology have been shown to benefit from supplementing their curriculum with mobile apps, which sets the stage for formal integration of mobile apps into dermatology teaching in the future.8 Prior to widespread adoption, mobile apps must be evaluated for content and utility, starting with an objective rubric.
Without official scientific standards in place, it was unsurprising that only half of the dermatology education applications were classified as adequate in this study. Among the types of apps offered—clinical decision support tools, journals, references, podcast, games, learning modules, and self-evaluation—certain categories scored higher than others. App formats with the highest average score (16.5 out of 20) were journals and podcast.
One barrier to utilization of these apps was that a subscription to the journals and podcast was required to obtain access to all available content. Students and trainees can seek out library resources at their academic institutions to take advantage of journal subscriptions available to them at no additional cost. Dermatology residents can take advantage of their complimentary membership in the American Academy of Dermatology for a free subscription to AAD Dialogues in Dermatology (otherwise $179 annually for nonresident members and $320 annually for nonmembers).
On the other hand, learning module was the lowest-rated format (average score, 11.3 out of 20), with only Medical Student: Dermatology qualifying as adequate (total score, 16). This finding is worrisome given that students and residents might look to learning modules for quick targeted lessons on specific topics.
The lowest-scoring app, a clinical decision support tool called Naturelize, received a total score of 7. Although it listed the indications and contraindications for dermal filler types to be used in different locations on the face, there was a clear conflict of interest, oversimplified design, and little evidence-based education, mirroring the current state of cosmetic dermatology training in residency, in which trainees think they are inadequately prepared for aesthetic procedures and comparative effectiveness research is lacking.9-11
At the opposite end of the spectrum, MyDermPath+ was a reference app with a total score of 20. The app cited credible authors with a medical degree (MD) and had an easy-to-use, well-designed interface, including a reference guide, differential builder, and quiz for a range of topics within dermatology. As a free download without in-app purchases or advertisements, there was no evidence of conflict of interest. The position of a dermatopathology app as the top dermatology education mobile app might reflect an increased emphasis on dermatopathology education in residency as well as a transition to digitization of slides.5
The second-highest scoring apps (total score of 19 points) were Dermatology Database and VisualDx. Both were references covering a wide range of dermatology topics. Dermatology Database was a comprehensive search tool for diseases, drugs, procedures, and terms that was simple and entirely free to use but did not cite references. VisualDx, as its name suggests, offered quality clinical images, complete guides with references, and a unique differential builder. An annual subscription is $399.99, but the process to gain free access through a participating academic institution was simple.
Games were a unique mobile app format; however, 2 of 3 games scored in the somewhat adequate range. The game DiagnosUs, which tested users’ ability to differentiate skin cancer and psoriasis from dermatitis on clinical images, would benefit from more comprehensive content as well as professional verification of true diagnoses, which earned the app 2 points in both the content and accuracy categories. The Unusual Suspects tested the ABCDE algorithm in a short learning module, followed by a simple game that involved identification of melanoma in a timed setting. Although the design was novel and interactive, the game was limited to the same 5 melanoma tumors overlaid on pictures of normal skin. The narrow scope earned 1 point for content, the redundancy in the game earned 3 points for design, and the lack of real clinical images earned 2 points for educational objectives. Although game-format mobile apps have the capability to challenge the user’s knowledge with a built-in feedback or reward system, improvements should be made to ensure that apps are equally educational as they are engaging.
AAD Dialogues in Dermatology was the only app in the form of a podcast and provided expert interviews along with disclosures, transcripts, commentary, and references. More than half the content in the app could not be accessed without a subscription, earning 2.5 points in the conflict of interest category. Additionally, several flaws resulted in a design score of 2.5, including inconsistent availability of transcripts, poor quality of sound on some episodes, difficulty distinguishing new episodes from those already played, and a glitch that removed the episode duration. Still, the app was a valuable and comprehensive resource, with clear objectives and cited references. With improvements in content, affordability, and user experience, apps in unique formats such as games and podcasts might appeal to kinesthetic and auditory learners.
An important factor to consider when discussing mobile apps for students and residents is cost. With rising prices of board examinations and preparation materials, supplementary study tools should not come with an exorbitant price tag. Therefore, we limited our evaluation to apps that were free or cost less than $5 to download. Even so, subscriptions and other in-app purchases were an obstacle in one-third of apps, ranging from $4.99 to unlock additional content in Rash Decisions to $69.99 to access most topics in Fitzpatrick’s Color Atlas. The highest-rated app in our study, MyDermPath+, historically cost $19.99 to download but became free with a grant from the Sulzberger Foundation.12 An initial investment to develop quality apps for the purpose of dermatology education might pay off in the end.
To evaluate the apps from the perspective of the target demographic of this study, 2 medical students—one in the preclinical stage and the other in the clinical stage of medical education—and a dermatology resident graded the apps. Certain limitations exist in this type of study, including differing learning styles, which might influence the types of apps that evaluators found most impactful to their education. Interestingly, some apps earned a higher resident score than student score. In particular, RightSite (a reference that helps with anatomically correct labeling) and Mohs Surgery Appropriate Use Criteria (a clinical decision support tool to determine whether to perform Mohs surgery) each had a 3-point discrepancy (data not shown). A resident might benefit from these practical apps in day-to-day practice, but a student would be less likely to find them useful as a learning tool.
Still, by defining adequate teaching value using specific categories of educational objectives, content, accuracy, design, and conflict of interest, we attempted to minimize the effect of personal preference on the grading process. Although we acknowledge a degree of subjectivity, we found that utilizing a previously published rubric with defined criteria was crucial in remaining unbiased.
Conclusion
Further studies should evaluate additional apps available on Apple’s iPad (tablet), as well as those on other operating systems, including Google’s Android. To ensure the existence of mobile apps as adequate education tools, they should be peer reviewed prior to publication or before widespread use by future and current providers at the minimum. To maximize free access to highly valuable resources available in the palm of their hand, students and trainees should contact the library at their academic institution.
- Craddock MF, Blondin HM, Youssef MJ, et al. Online education improves pediatric residents' understanding of atopic dermatitis. Pediatr Dermatol. 2018;35:64-69.
- Lacy FA, Coman GC, Holliday AC, et al. Assessment of smartphone application for teaching intuitive visual diagnosis of melanoma. JAMA Dermatol. 2018;154:730-731.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology--2017 update. Dermatol Online J. 2018;24:13.
- Liu R-F, Wang F-Y, Yen H, et al. A new mobile learning module using smartphone wallpapers in identification of medical fungi for medical students and residents. Int J Dermatol. 2018;57:458-462.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Mercer JM. An array of mobile apps for dermatologists. J Cutan Med Surg. 2014;18:295-297.
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol. 2013;68:e23-e28.
- Waldman A, Sobanko JF, Alam M. Practice and educational gaps in cosmetic dermatologic surgery. Dermatol Clin. 2016;34:341-346.
- Nielson CB, Harb JN, Motaparthi K. Education in cosmetic procedural dermatology: resident experiences and perceptions. J Clin Aesthet Dermatol. 2019;12:E70-E72.
- Hanna MG, Parwani AV, Pantanowitz L, et al. Smartphone applications: a contemporary resource for dermatopathology. J Pathol Inform. 2015;6:44.
- Craddock MF, Blondin HM, Youssef MJ, et al. Online education improves pediatric residents' understanding of atopic dermatitis. Pediatr Dermatol. 2018;35:64-69.
- Lacy FA, Coman GC, Holliday AC, et al. Assessment of smartphone application for teaching intuitive visual diagnosis of melanoma. JAMA Dermatol. 2018;154:730-731.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology--2017 update. Dermatol Online J. 2018;24:13.
- Liu R-F, Wang F-Y, Yen H, et al. A new mobile learning module using smartphone wallpapers in identification of medical fungi for medical students and residents. Int J Dermatol. 2018;57:458-462.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Mercer JM. An array of mobile apps for dermatologists. J Cutan Med Surg. 2014;18:295-297.
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol. 2013;68:e23-e28.
- Waldman A, Sobanko JF, Alam M. Practice and educational gaps in cosmetic dermatologic surgery. Dermatol Clin. 2016;34:341-346.
- Nielson CB, Harb JN, Motaparthi K. Education in cosmetic procedural dermatology: resident experiences and perceptions. J Clin Aesthet Dermatol. 2019;12:E70-E72.
- Hanna MG, Parwani AV, Pantanowitz L, et al. Smartphone applications: a contemporary resource for dermatopathology. J Pathol Inform. 2015;6:44.
Practice Points
- Mobile applications (apps) are a convenient way to learn dermatology, but there is no objective method to assess their quality.
- To determine which apps are most useful for education, we performed a graded review of dermatology apps targeted to students and residents.
- By applying a rubric to 36 affordable apps, we identified 18 (50%) with adequate teaching value.
How to identify, evaluate, and treat patients with ‘Percocet use disorder’
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
In recent years, Percocet (oxycodone/paracetamol) has experienced a meteoric rise to prominence because of the presence of conspicuous references in pop culture and the ever-evolving hip-hop scene,1 so much so that even propafenone is being mislabeled as the agent.2 It is of utmost importance for clinicians to be made aware of the adverse effects and the treatment protocols associated with Percocet as well as propafenone.
Propafenone is identified as a class 1C antiarrhythmic with adverse effects associated with that particular class of drugs (e.g., generalized tonic-clonic seizures coupled with widened QRS complex), however, Percocet’s toxidrome is the product of the opioid/nonopioid (in the form of oxycodone/acetaminophen) components found within the formulation. Percocet is often recreationally used with MDMA (“molly”) or ecstasy as popularized by the lyrics of “Mask Off” by Future (“Percocets, Molly, Percocets”).3,4
Addressing the challenge of imitation Percocet pills
Differentiating the untoward effects of Percocet and propafenone isn’t too challenging because the agents belong to separate classes – the problem is the use of deceitful labels on propafenone with both medications sporting the “512 imprint” on their respective pills. Initial symptoms of propafenone ingestion may include weakness and dizziness followed by seizures.5As an emergent situation, the patient should be immediately treated with a sodium bicarbonate infusion to effectively reverse the sodium channel blockade associated with the widened QRS.
However, a more likely scenario is that of Percocet counterfeit pills designed to illicitly emulate the properties of officially marketed Percocet. As expected, Percocet overdose management will require that the clinician be familiar with treating general opioid toxicity (in this case, derived from the oxycodone component), in particular respiratory or CNS depression. Symptoms of opioid overdose also include the loss of consciousness with pupillary miosis. Therapy entails the use of naloxone and/or mechanical ventilation for respiratory support. The patient can also exhibit cardiovascular compromise. If further information is elicited during a patient interview, it may reveal a history of drug procurement from the streets.
Epidemiologists from Georgia collaborated with the state’s department of public health’s office of emergency services, forensic experts, and drug enforcement professionals to evaluate almost 40 cases of counterfeit Percocet overdoses during the period spanning the second week of June 2017. Of these cases, a cluster triad was identified consisting of general opioid toxicity symptoms (for example, CNS or respiratory depression with concomitant pupillary constriction, a history of drug procurement, and a history of ingesting only one or two pills with rapid deterioration.6 Unfortunately, the screening process is often hindered by the fact that synthetic opioids such as Percocet are not readily identified on urine drug screens (UDS).
Despite shortcomings in assessment procedures, a UDS will yield positive results for multiple drugs, a feature that is common to seasoned opioid users and serves as an instrumental diagnostic clue in the investigative process. To address the crisis and prevent further spread, numerous Georgia agencies (e.g., drug trafficking and legal authorities) worked with the health care community to expediently identify cases of interest and bring forth public awareness concerning the ongoing perils of counterfeit drug intake. Future investigations might benefit from the implementation of DNA-verified UDS, because those screens are versatile enough to detect the presence of synthetic urine substitutes within the context of opioid use.7,8 Moreover, an expanded panel could be tailored to provide coverage for semisynthetics, including hydrocodone, oxycodone, hydromorphone, and oxymorphone.9
As a well-received painkiller from the opioid family, Percocet derives its analgesic properties from the fast-acting oxycodone; hepatic failure is also possible from Percocet (because of the acetaminophen component) or counterfeit Percocet overdose but is less common unless the Tylenol content approaches 4 grams. By binding to the brain’s opiate receptors, Percocet modulates pain pathways leading to a dulling of pain sensation along with euphoria, which is particularly attractive to drug seekers. Chronic Percocet use corresponds with a myriad of psychological and physical consequences, and the Drug Enforcement Administration recognizes oxycodone as a Schedule II drug.
A chronic Percocet user may try to disrupt the cycle of symptoms by abruptly ceasing use of the offending agent. This can precipitate the development of classical opioid-based withdrawal symptoms, including but not limited to nausea, vomiting, irritability, tachycardia, body aches, and episodes of cold sweats. Physicians have noted that misuse (i.e., deviations from intended prescribed) might include crushing and snorting as well as “doctor-shopping” behaviors for a continuous supply of Percocet.
Treatment recommendations
According to Sarah Wakeman, MD, medical director of the substance use disorders initiative at Massachusetts General Hospital in Boston, there are apparently two clinical manifestations of Percocet use. The primary consequence is derived from the oxycodone component of Percocet; as an opioid, oxycodone toxicity leads to disrupted breathing and oxygenation, negatively impacting vital organs such as the brain or the heart. Patients experiencing a lack of oxygen will often display cyanosis and may not respond appropriately to stimuli. For individuals suspected of succumbing to overdose, Dr. Wakeman reportedly advised that the clinician or trained professional rub his or her knuckles along the breastbone of the potential user – a drug overdose patient will fail to wake up. On the other hand, a Percocet user may exhibit the symptoms of liver failure depending on the overall level of acetaminophen in the formulation. To prevent relapses, Percocet use disorder is best managed in a professional setting under the direction of trained clinicians; users are provided medications to address ongoing cravings and symptoms associated with the withdrawal process. A detoxification center can tailor the treatment with opioid-based medications such as methadone, buprenorphine, and naltrexone to help patients be weaned off Percocet.
Clinicians may further improve the efficacy of a therapeutic regimen by incorporating a personalized plan with a comprehensive substance UDS panel for monitoring and treatment purposes. This may prove to be beneficial in the event of suspected polysubstance use, as is the case with patients who dabble with Percocet and “molly.” Preparations can also be instituted at the outset of therapy with genetic testing implemented in high-risk patients who exhibit an inclination for opioid use disorder.10 Genetic polymorphisms provide robust clinical assets for evaluating patients most at risk for relapse. For individuals with biological susceptibility, arrangements can be made to incorporate nonopioid treatment alternatives.
References
1. Thomas BB. The death of Lil Peep: How the U.S. prescription drug epidemic is changing hip-hop. The Guardian. 2017 Nov 16.
2. D’Orazio JL and Curtis JA. J Emer Med. 2011 Aug 1;41(2):172-5.
3. Levy L. These are the drugs influencing pop culture now. Vulture. 2018 Feb 6.
4. Kounang N and Bender M. “What is Percocet? Drug facts, side effects, abuse and more.” CNN. 2018 Jul 12.
5. The dangers of Percocet use and overdose. American Addiction Centers. Last updated 2020 Feb 3. https://americanaddictioncenters.org/percocet-treatment/dangers-of-use-and-overdose.
6. Edison L et al. MMWR. 2017 Oct 20;66(41):1119-20.
7. Choudhry Z et al. J Psychiatry. 2015. doi: 10.4172/2378-5756.10000319.
8. Islam F and Choudhry Z. Current Psychiatry. 2018 Dec;17(12):43-4.
9. Jupe N. Ask the Experts: DOT 5-panel drug test regimen. Quest Diagnostics. 2018 Mar 21. https://blog.employersolutions.com/ask-experts-dot-5-panel-drug-test-regimen/.
10. Ahmed S et al. Pharmacogenomics. 2019 Jun 28;20(9):685-703.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam reported no relevant disclosures. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF. He reported no relevant disclosures.
Reliability of Biopsy Margin Status for Basal Cell Carcinoma: A Retrospective Study
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).
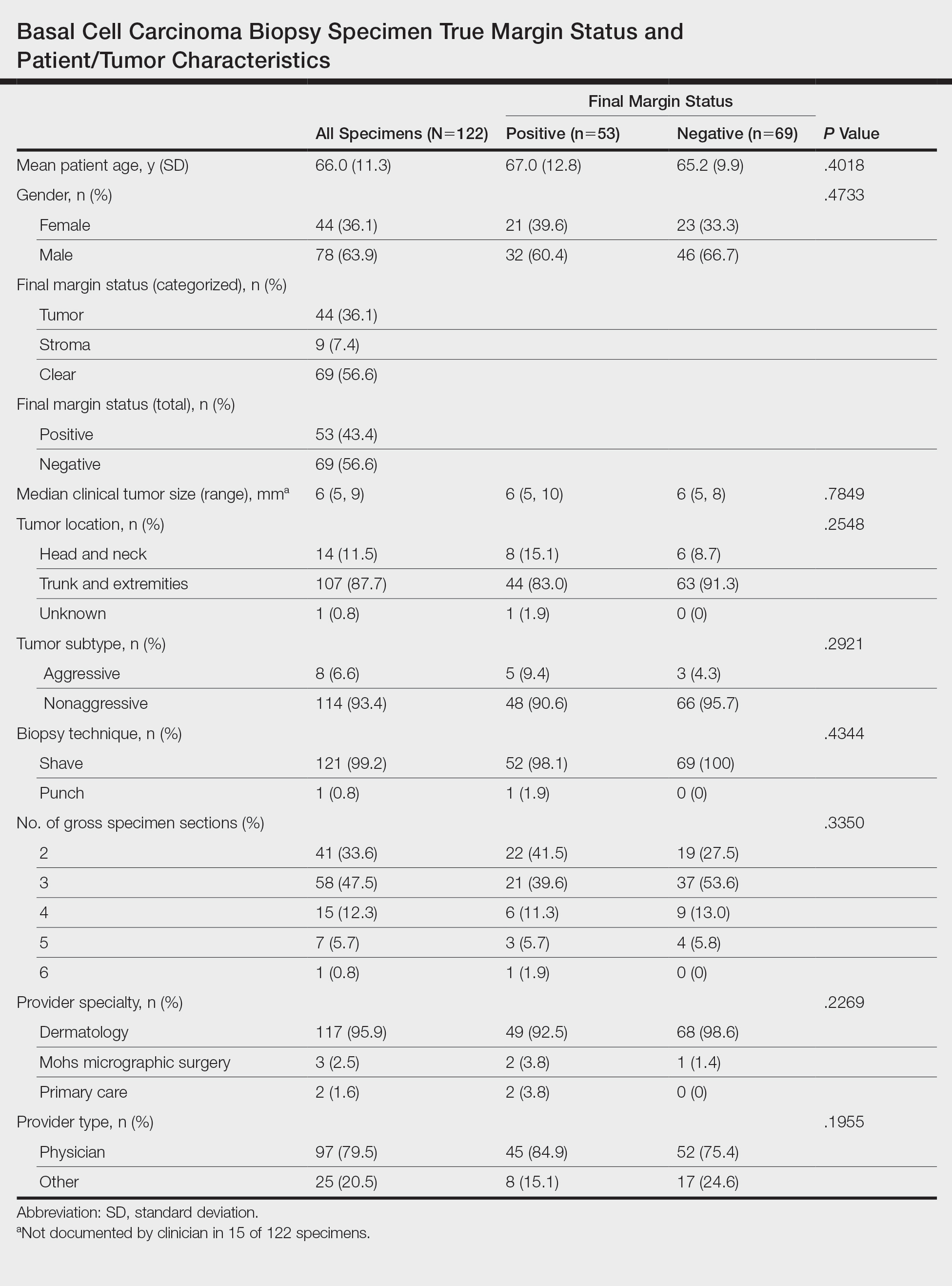
We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).

We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
Basal cell carcinoma (BCC) is the most common type of skin cancer frequently encountered in both dermatology and primary care settings.1 When biopsies of these neoplasms are performed to confirm the diagnosis, pathology reports may indicate positive or negative margin status. No guidelines exist for reporting biopsy margin status for BCC, resulting in varied reporting practices among dermatopathologists. Furthermore, the terminology used to describe margin status can be ambiguous and differs among pathologists; language such as “approaches the margin” or “margins appear free” may be used, with nonuniform interpretation between pathologists and providers, leading to variability in patient management.2
When interpreting a negative margin status on a pathology report, one must question if the BCC extends beyond the margin in unexamined sections of the specimen, which could be the result of an irregular tumor growth pattern or tissue processing. It has been estimated that less than 2% of the peripheral surgical margin is ultimately examined when serial cross-sections are prepared histologically (the bread loaf technique). However, this estimation would depend on several variables, including the number and thickness of sections and the amount of tissue discarded during processing.3 Importantly, reports of a false-negative margin could lead both the clinician and patient to believe that the neoplasm has been completely removed, which could have serious consequences.
Our study sought to determine the reliability of negative biopsy margin status for BCC. We examined BCC biopsy specimens initially determined to have uninvolved margins on routine tissue processing and determined the proportion with truly negative margins after complete tissue block sectioning of the initial biopsy specimen. We felt this technique was a more accurate measurement of true margin status than examination of a re-excision specimen. We also identified any factors that were predictive of positive true margins.
Methods
We conducted a retrospective study evaluating tissue samples collected at Geisinger Health System (Danville, Pennsylvania) from January to December 2016. Specimens were queried via the electronic database system at our institution (CoPath). We included BCC biopsy specimens with negative histologic margins on initial assessment that subsequently had block exhaust levels routinely ordered. These levels are cut every 100 to 150 µm, generating approximately 8 glass slides. We excluded all tumors that did not fit these criteria as well as those in patients younger than 18 years. Data collection was performed utilizing specimen pathology reports in addition to the note from the corresponding clinician office visit from the institution’s electronic medical record (Epic). Appropriate statistical calculations were performed. This study was approved by an institutional review board at our institution, which is required for all research involving human participants. This served to ensure the proper review and storage of patients’ protected health information.
Results
The search yielded a total of 122 specimens from 104 patients after appropriate exclusions. We examined a total of 122 BCC biopsy specimens with negative initial margins: 121 (99.2%) shave biopsies and 1 (0.8%) punch biopsy. Of 122 specimens with negative initial margins, 53 (43.4%) were found to have a truly positive margin based on the presence of either tumor or stroma at the lateral or deep tissue edge after complete tissue block sectioning. Sixty-nine (56.6%) specimens had clear margins and were categorized as truly negative after complete tissue block sectioning. Specimens with positive and negative final margin status did not differ significantly with respect to patient age; gender; biopsy technique; number of gross specimen sections; or tumor characteristics, including location, size, and subtype (Table)(P>.05).

We also examined the type of treatment performed, which varied and included curettage, electrodesiccation and curettage, excision, and Mohs micrographic surgery. Clinicians, who were not made aware of the exhaust level protocol, chose not to pursue further treatment in 6 (4.9%) of the cases because of negative biopsy margins. Four (66.7%) of the 6 providers were physicians, and 2 (33.3%) were advanced practitioners. All of the providers practiced within the Department of Dermatology.
Comment
Our findings support prior smaller studies investigating this topic. A prospective study by Schnebelen et al4 examined 27 BCC biopsy specimens and found that 8 (30%) were erroneously classified as negative on routine examination. This study similarly determined true margin status by assessing the margins at complete tissue block exhaustion.4 Willardson et al5 also demonstrated the poor predictive value of margin status based on the presence of residual BCC in subsequent excisions. They found that 34 (24%) of 143 cases with negative biopsy margins contained residual tumor in the corresponding excision.5
Our study revealed that almost half of BCC biopsy specimens that had negative histologic margins with routine sectioning had truly positive margins on complete block exhaustion. This finding was independent of multiple factors, including tumor subtype, indicating that even nonaggressive tumors are prone to false-negative margin reports. We also found that reports of negative margins persuaded some clinicians to forgo definitive treatment. This study serves to remind clinicians of the limitations of margin assessment and provides impetus for dermatopathologists to consider modifying how margin status is reported.
Limitations of this study include a small number of cases and limited generalizability. Institutions that routinely examine more levels of each biopsy specimen may be less likely to erroneously categorize a positive margin as negative. Furthermore, despite exhausting the tissue block, we still may have underestimated the number of cases with truly positive margins, as this method inherently does not allow for complete margin examination.
Acknowledgments
We thank the Geisinger Department of Dermatopathology and the Geisinger Biostatistics & Research Data Core (Danville, Pennsylvania) for their assistance with our project.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1149-1276.
- Abide JM, Nahai F, Bennett RG. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492-497.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-473.
- Schnebelen AM, Gardner JM, Shalin SC. Margin status in shave biopsies of nonmelanoma skin cancers: is it worth reporting? Arch Pathol Lab Med. 2016;140:678-681.
- Willardson HB, Lombardo J, Raines M, et al. Predictive value of basal cell carcinoma biopsies with negative margins: a retrospective cohort study. J Am Acad Dermatol. 2018;79:42-46.
Practice Points
- Clinicians must recognize the limitations of margin assessment of biopsy specimens and not rely on margin status to dictate treatment.
- Dermatopathologists should consider modifying how margin status is reported, either by omitting it or clarifying its limitations on the pathology report.
Patch Testing 101, Part 2: After the Patch Test
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.
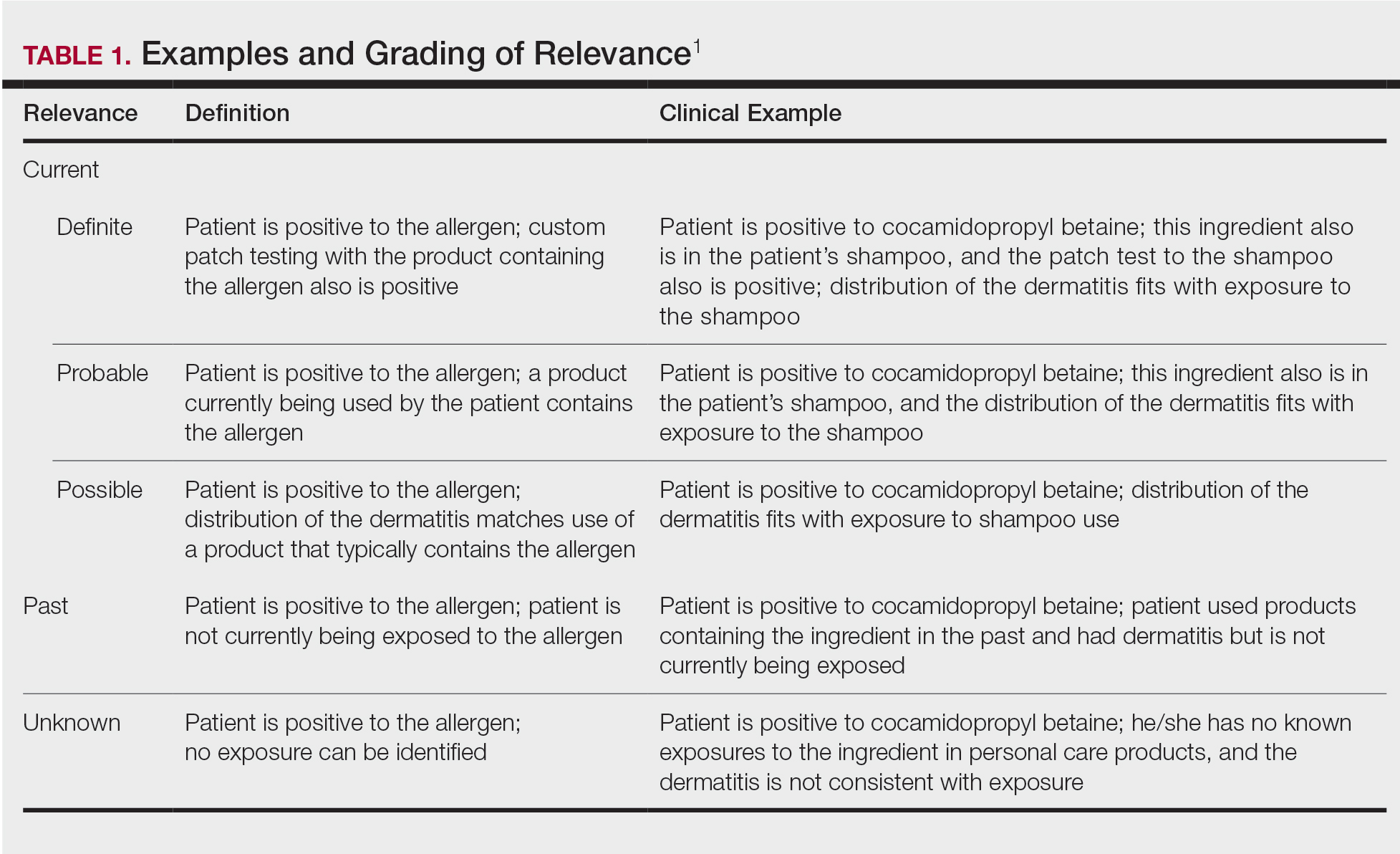
True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.

Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.

True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.

Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
The first part of this 2-part series addressed the basics of patch testing, including patch test systems, allergens, and patch test readings. In the second part of this series, we examine the incredibly important and absolutely vital steps that come after the patch test: determining relevance, patient counseling, and identifying allergen-free products for patient use. Let’s dive in!
Determining Relevance
The purpose of determining relevance is to assess whether the positive patch test explains the patient’s dermatitis. It is important to consider all of the patient’s exposures, including at home, at work, and during recreational activities. Several relevance grading scales exist. The North American Contact Dermatitis Group grades relevance as current, past, or unknown. Current relevance is further divided into definite, probable, and possible.1 Table 1 includes explanations and clinical examples of each relevance type.

True relevance is only known weeks or months after patch testing is complete. If the patient avoids allergens and is subsequently free of dermatitis, the allergens identified through patch testing were relevant. However, if the patient avoids allergens and sees no improvement in dermatitis, the allergens were not relevant. Gipson et al2 analyzed relevance as documented by the physician at final patch test reading vs patient opinion of relevance 30 days to 3 years after the final reading and found that there was variable agreement between the 2 groups; percentage agreement for formaldehyde-releasing preservatives was 88%, neomycin was 78%, nickel was 71%, fragrances was 65%, and gold was 56%. These differences underscore the need for ongoing research on patch test methods, determination of relevance, and standards for patient follow-up.2
Patient Counseling
Patient counseling is one of the most important and complex parts of patch testing. We have consulted with patients who had already completed patch testing with other providers but did not receive comprehensive allergen counseling and therefore did not improve. It is up to you to explain positive allergens to your patients in a way that they understand, can retain long-term, and can use to their advantage to keep their skin free of dermatitis, which is an incredibly difficult feat to accomplish. The resources that we describe next are the very basic requirements for proficient patch testing.
There are several tools that can be utilized to develop patch test counseling skills (Table 2). Membership with the American Contact Dermatitis Society (ACDS) includes opportunities for virtual and in-person (post–coronavirus disease 2019) lectures and conferences, videos, patch test support information, and patient resources. The European Society of Contact Dermatitis is similar, with a focus on European-based patch testers. Both societies are affiliated with academic journals—Dermatitis and Contact Dermatitis, respectively—which are phenomenal educational resources. Dermatitis Academy (https://www.dermatitisacademy.com) and Contact Dermatitis Institute (https://www.contactdermatitisinstitute.com) are websites that are privately designed and managed by US-based patch test experts.

Allergen Information Handouts
Allergen information should be presented in both verbal and written formats as well as in the patient’s preferred language and education level. Patch test counseling is detailed and complex. Patients rarely remember everything that is discussed; written information allows them to review again when necessary. Allergen information sheets typically include the name of the allergen, alternative names, types of products that might contain the allergen, and other pertinent facts. They also can be helpful for the physician who does not patch test full time; in this case, they can be used as a quick reference to guide patient counseling. It is helpful to highlight or underline important points and make notes when relevant. Importantly, reviewing information sheets with the patient allows time for questions.
Allergen information sheets are provided by manufacturers of patch test materials, including SmartPractice (allergEAZE, T.R.U.E. Test) and Chemotechnique (Dormer)(Table 2). The ACDS also provides a selection of allergen information sheets for members to share with their patients. The ACDS allergen handouts are designed for patient use, are vetted by practicing patch test dermatologists, and contain up-to-date information for patients. We recommend that you choose the handout(s) that are most appropriate for your patient; this decision can be made based on patient education or reading level, the region of the world where you are patch testing or where the patient lives, the patient’s primary language, and the specific allergen. Information on rare or new allergens may not be available on every website resource.
Identification of Allergen-Free Products
We ask patients to bring their personal care products to their patch test reading visit, and once positive allergens are known, we search for the presence of that allergen in their products. It is helpful for patients if products that are “safe” and “not safe” are sorted for them. We frequently emphasize that just one exposure to an allergen in a personal care product can be the source of the dermatitis. If a product label does not include ingredients, they often can be identified with a quick web search (use your favorite search engine or see Table 2 for websites); however, caution is advised, as lists found online may not match those found on in-store products.3 Reviewing the patient’s own products in the clinic is preferred over searching for ingredient lists online. If the product’s ingredients cannot be found (eg, ingredients that are found on external packaging), the patient has several choices: do not use, complete repeat open application testing if it is a leave-on product, or check to see if it is on a product database safe list.
We explain to patients that once they have confirmed that they are using only “safe” allergen-free products, it can take up to 6 to 8 weeks for dermatitis to improve, and at that point, the skin may only be about 75% to 80% clear. A clear description of what to expect and when is needed for a strong patient-physician partnership. For example, if the patient expects to be clear in 2 days but is not and stops avoiding their allergens because they think the process has failed, their dermatitis will not improve.
Product Databases
Because allergens sometimes have multiple different chemical names and cross-reactivity is abundant, avoidance of both the allergen and cross-reactors can be daunting for many patients (and dermatologists!). The use of a product database to aid in product selection is an invaluable resource. Product databases help patients avoid not only their allergens but also common cross-reactors by relying on complex cross-reactor programming. The ACDS owns and maintains the Contact Allergy Management Program (CAMP). Another resource is SkinSafe, which is powered by HER Inc and developed with the Mayo Clinic. Both CAMP and SkinSafe have mobile apps and update product lists frequently; they allow for much easier shopping and identification of safe products.
We typically use CAMP for generation of patient safe lists. We enter the patient’s allergens into the database, and a safe list is generated and shared with the patient. Next, we educate the patient on how to use the safe list. It is vital that the concept of exact product matching be explained to patients, as not all products from one brand or type of product is necessarily safe for a given individual. We also share information on how to download the CAMP app onto mobile devices and tablets.
Product safe lists are important resources for patients to be successful in avoiding allergens but are not a substitute for reading labels. Both CAMP and SkinSafe can potentially contain ingredient list errors due to companies frequently changing their product formulations.3 Although safe lists are an important part in selecting safe skin care products, they are not a substitute for label reading.
Counseling Pitfalls and Pearls
Language
Chemotechnique handouts are available in English, Swedish, French, and Spanish, and ACDS handouts are available in English and Spanish. If language interpretation is needed, inform the interpreter before the visit begins that you will be discussing patch test information and products so they can carefully interpret the details of the discussion.
Barriers to Allergen Avoidance
There are several barriers to long-term avoidance of contact allergy. In a European-based study of methylisothiazolinone (MI) contact allergy 2 to 5 years after patch testing, challenges described by patients included label reading, verifying products, difficulty obtaining ingredients of industrial products, the need to have their “safe” products always available for use, remembering allergen name, avoiding workplace allergens, finding acceptable MI-free products, and navigating the cost of MI-free products.4
Patient allergen recall is a well-documented long-term concern. In the previously mentioned European study (N=139), 11% of patients identified remembering the allergen name as a contributor to difficulty with avoidance.4 A Swedish study evaluated patient allergen recall at 1, 5, and 10 years after patch testing was completed; 96% of 252 patients remembered that they had completed patch testing, 79% (111/141) remembered that they had positive results, and only 29% (41/141) correctly recalled their allergens.5 Patients who had completed patch testing 10 years prior were less likely to correctly recall their allergens (P=.0045). Recall also was less likely if there was more than 1 allergen as well as in males.5 Korkmaz and Boyvat6 analyzed outcomes 6 months after patch testing in Turkey and found that 38 of 51 (74.5%) correctly recalled their allergens. Patients with more than 1 positive allergen were less likely to recall their allergens (P=.046), and patients with higher baseline investigator global assessment (P=.036) and dermatology life quality index (P=.041) scores were more likely to recall their allergens.6 A US-based study (N=757) noted that 34.1% of patients correctly recalled all of their allergens.7 Patients were less likely to remember if they had 3 or more positives but were more likely to remember if they were aged 50 to 59 years (compared to other age groups) or female as well as if their occupation was nursing (as compared to other occupations).
Additional barriers include hidden sources of allergens, as has been reported in the cases of undeclared MI8 and formaldehyde9 in personal care products. Although this phenomenon is thought to be the exception and not the rule, possible reasons for the presence of these undeclared allergens include their use as preservatives in raw materials,8,9 or in the case of formaldehyde, theorized release from product packaging or auto-oxidation and degradation of other chemicals present within the product.9
Readers may recall that we mentioned the option of identifying product ingredients with online search engines or databases, but it is not a perfect system. Comstock and Reeder3 reviewed and compared online ingredient lists from Amazon and several product databases to products taken off shelves at Target and Walgreens and found that 27.7% of online ingredient lists did not match the in-store labels.3 These differences likely are due to changes in product formulations, ingredient variability based on production site, outdated product on store shelves, or data entry error and may not be entirely avoidable. Regardless, patch test experts should be aware of this possibility. When in doubt, always check the product’s original packaging.
Finally, the elephant in the room: We challenge you, as dermatologists and patch test enthusiasts, to name all of the formaldehyde releasers or perhaps declare whether linalool and hydroxycitronellol are fragrances, preservatives, or surfactants. How about naming the relationship between cocamidopropyl betaine, amidoamine, and dimethylaminopropylamine? Difficult stuff, right? And we are medical specialists. It is downright impossible for many of our patients to memorize the names of these chemicals, let alone know their cross-reactors or other important chemical relationships. We mention that providing a safe list is part of patient counseling, but we bring up this knowledge gap to illustrate that patch testing without providing resources to select safe care products is almost as bad as not patch testing at all because in many cases patients may be left without the tools they need to be successful. Do not let this be your downfall!
Final Interpretation
The most challenging and nuanced part of patch testing happens after the actual patch test: assessment of relevance, allergen counseling, and identification of appropriate products for patient use. You now have the tools to successfully counsel your patients after patch testing; get to it!
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Gipson KA, Carlson SW, Nedorost ST. Physician-patient agreement in the assessment of allergen relevance. Dermatitis. 2010;21:275-279.
- Comstock JR, Reeder MJ. Accuracy of product ingredient labeling: comparing drugstore products with online databases and online retailers. Dermatitis. 2020;31:106-111.
- Bouschon P, Waton J, Pereira B, et al. Methylisothiazolinone allergic contact dermatitis: assessment of relapses in 139 patients after avoidance advice. Contact Dermatitis. 2019;80:304-310.
- Jamil WN, Erikssohn I, Lindberg M. How well is the outcome of patch testing remembered by the patients? a 10-year follow-up of testing with the Swedish baseline series at the department of dermatology in Örebro, Sweden. Contact Dermatitis. 2012;66:215-220.
- Korkmaz P, Boyvat A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis. 2019;30:135-141.
- Scalf LA, Genebriera J, Davis MD, et al. Patients’ perceptions of the usefulness and outcome of patch testing. J Am Acad Dermatol. 2007;56:928-932.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Nikle A, Ericson M, Warshaw E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis. 2019;30:67-73.
Practice Points
- Positive patch test reactions must be interpreted in the context of the patient’s exposures, both current and past.
- Allergen information sheets and product database safe lists are invaluable tools to help patients select safe skin care products.
Dermatology and Vaccines: We Must Do Better
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.