User login
Replace routine preoperative testing with individualized risk assessment and indicated testing
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
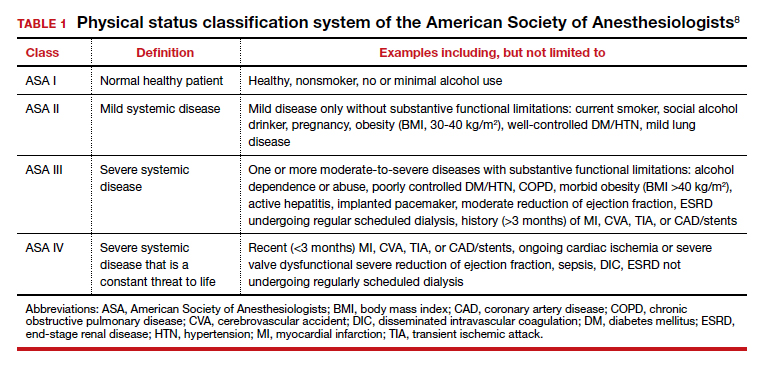
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8
In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11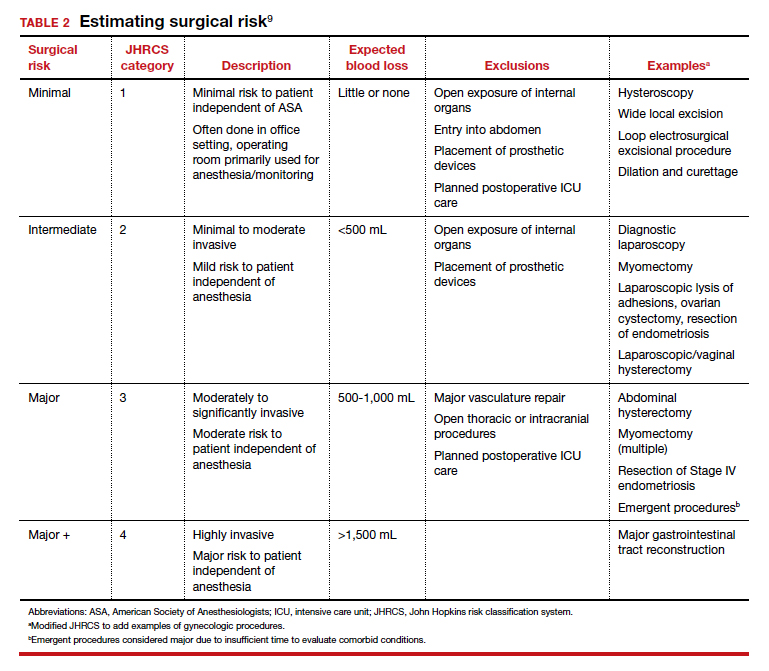
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
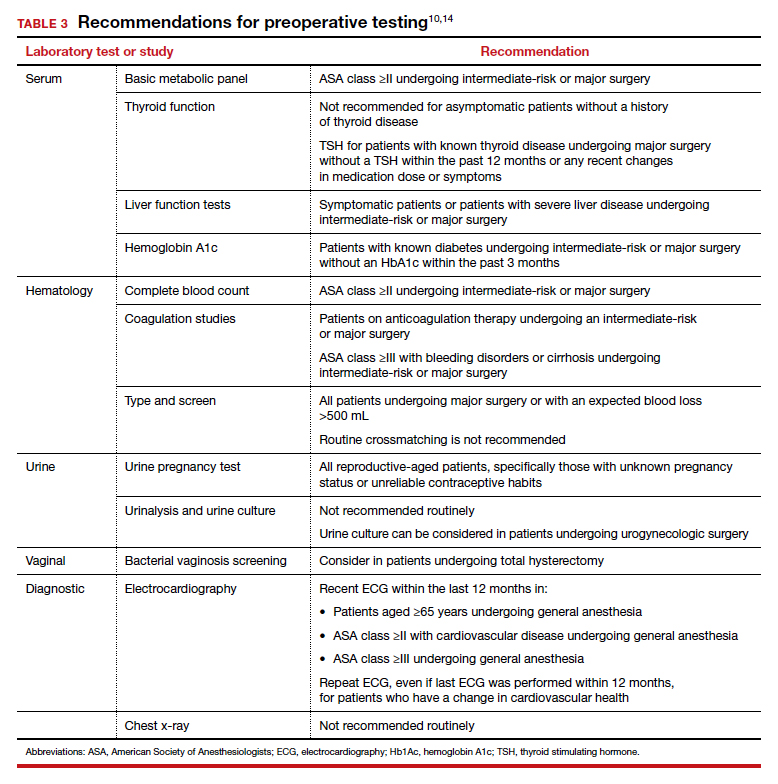
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14
The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8

In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14

The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8

In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14

The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
The pill toolbox: How to choose a combined oral contraceptive
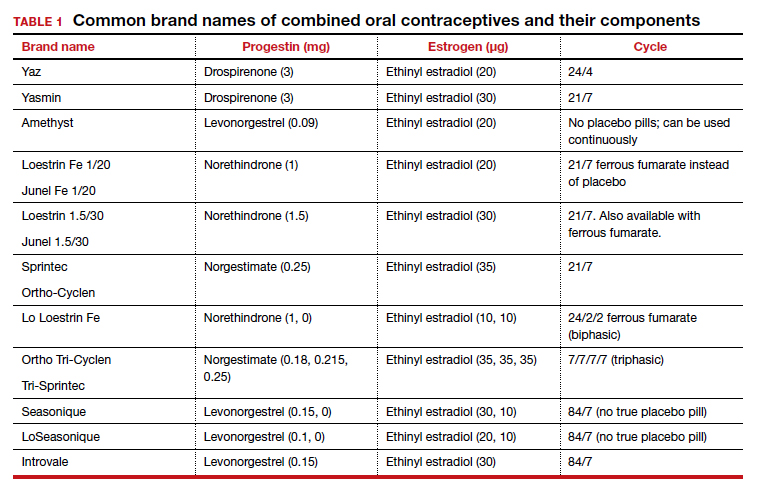
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.
Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).
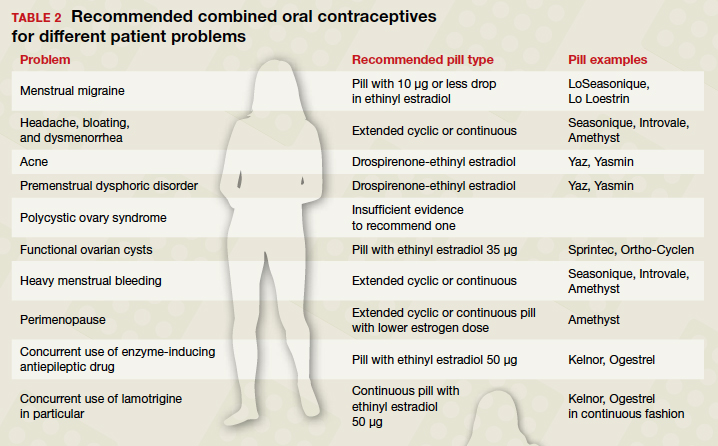
When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
For obese postmenopausal women, what options may decrease endometrial cancer risk?
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
Burnt Out ? The Phenomenon of Type 2 Diabetes Mellitus in End-Stage Renal Disease
In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice with a hemoglobin A1c target of 6 to 8%, using fructosamine levels or other measures for better assessment of glycemic control.
More than 34 million adults in the US have type 2 diabetes mellitus (T2DM), a chronic progressive disease identified by worsening hyperglycemia and micro- and macrovascular complications.1 Consequently, 12.2% of the US adult population is currently at risk for macrovascular diseases, such as stroke and coronary artery disease (CAD) and microvascular diseases, such as neuropathy and diabetic nephropathy.1
T2DM is the most common comorbid risk factor for chronic kidney disease (CKD) and the leading cause of end-stage renal disease (ESRD). As of 2017, about 750,000 Americans have CKD stage 5 requiring dialysis, and 50% of these patients have preexisting diabetic nephropathy.2 Rates of mortality and morbidity are observed to be higher in patients with both CKD and T2DM compared with patients with CKD without T2DM.2 Previous clinical trials, including the United Kingdom Prospective Diabetes Study of 1998, have proven that optimal glycemic control decreases the risk of complications of T2DM (ie, nephropathy) in the general population.3 Conversely, tight glycemic control that targets hemoglobin A1c (HbA1c) < 7%, in patients with T2DM with ESRD has not shown the same benefits and may lead to worse outcomes. It is postulated that this may be due to the increased incidence of hypoglycemia in this patient population.4
Dialysis has varying effects on patients both with and without T2DM. While patients with ESRD without T2DM have the potential to develop impaired glucose tolerance and T2DM, about 33% of patients with T2DM on dialysis actually have HbA1c < 6%.5 In these patients, glycemic control improves spontaneously as their disease progresses, leading to a decrease or cessation of insulin or other antidiabetic medications. This phenomenon, known as burnt-out diabetes, is characterized by (1) alterations in glucose homeostasis and normoglycemia without antidiabetic treatment; (2) HbA1c levels < 6% despite having established T2DM; (3) decline in insulin requirements or cessation of insulin altogether; and (4) spontaneous hypoglycemia.
There is a misconception that burnt-out diabetes is a favorable condition due to the alteration of the natural course of T2DM. Although this may be true, patients with this condition are prone to develop hypoglycemic episodes and may be linked to poor survival outcomes due to low HbA1c.6,7
Since Kalantar-Zadeh and colleagues presented a 2009 case study, there has been a lack of research regarding this unique condition.8 The purpose of this case study is to shed further light on burnt-out diabetes and present a patient case pertaining to the challenges of glycemic control in ESRD.
Case Presentation
Mr. A is a 49-year-old Hispanic male veteran with a history of ESRD on hemodialysis (HD) for 6 years, anemia of CKD, and T2DM for 22 years. The patient also has an extensive cardiovascular disease history, including hypertension, hyperlipidemia, and CAD status post-4-vessel coronary artery bypass graft in December 2014. The patient receives in-home HD Monday, Wednesday, and Friday and is on the wait list for kidney transplantation. The patient’s T2DM is managed by a primary care clinical pharmacy specialist (CPS) at the
Mr. A’s antidiabetic regimen is 45 units of subcutaneous insulin glargine every morning; insulin aspart sliding scale (about 15-27 units) subcutaneous 3 times daily with meals; and saxagliptin 2.5 mg by mouth once daily.
At a follow-up visit with the CPS, Mr. A stated, “I feel fine except for the occasional low blood sugar episode.” The patient’s most recent HbA1c was 6.1%, and he reported medication adherence and no signs or symptoms of hyperglycemia (ie, polydipsia, polyphagia, nocturia, visual disturbances). Mr. A reported no use of alcohol, tobacco, or illicit drugs. He walks 1 mile every other day and participates in self-monitoring blood glucose (SMBG) about 2 to 3 times daily (Table 1).
Although Mr. A’s most recent HbA1c was well controlled, his estimated fasting blood glucose at the same laboratory draw was 224 mg/dL. His SMBG readings in the past month also were elevated with higher readings in the evening. Mr. A attributed the elevated readings to dietary excursions and a high carbohydrate intake. At this visit, the CPS increased his insulin glargine dose to 50 units subcutaneous every morning and educated him on lifestyle modifications. Follow-up with the CPS was scheduled for 2 months from the day of the visit.
Analysis
Few articles on potential contributors to burnt-out diabetes have been published.6,7 These articles discuss decreased renal and hepatic clearance of insulin (which increases its half-life) hypoglycemia during HD, and low HbA1c due to preexisting anemia. Inappropriately low HbA1c levels may be secondary to, but not limited to, hemolysis, recent blood transfusion, acute blood loss, and medications, such as erythropoietin-stimulating agents (ESAs).9 The conditions that affect red blood cell turnover are common in patients with advanced CKD and may result in discrepancies in HbA1c levels.
Glycated hemoglobin is a series of minor hemoglobin components formed by the adduction of various carbohydrate molecules to hemoglobin. HbA1c is the largest fraction formed and the most consistent index of the concentration of glucose in the blood.10 Hence, HbA1c is the traditional indicator of overall glycemic control. The current HbA1c goals recommended by the American Diabetes Association are derived from landmark trials conducted with patients in the general adult diabetic non-CKD population. However, hemoglobin measurements can be confounded by conditions present in ESRD and tend to underestimate glucose measurements in patients with T2DM on HD. Despite this, HbA1c is still regarded as a reasonable measure of glycemic control even in patients with ESRD; however, alternative markers of glycemia may be preferable.11
Although HbA1c is the gold standard, there are other laboratory measures of average glycemic control available. Fructosamine is a ketoamine formed when glucose binds to serum proteins. When these proteins are exposed to high concentrations of glucose, they experience increased glycation. Fructosamine assays measure the total glycated serum proteins, of which albumin accounts for about 90%.11 Because the half-life of serum proteins is about 20 days, fructosamine levels can reflect glycemic control over a 2- to 3-week period. This is advantageous in conditions that affect the average age of red blood cells, in pregnancy where frequent monitoring and measures of short-term glucose control are especially important, and in the evaluation of a medication adjustment in the management of T2DM. However, this test is not without its limitations. It is less reliable in settings of decreased protein levels (eg, liver disease), there is a lack of availability in routine practice, and reference levels have not been established.11
Fructosamine has been shown to be strongly associated with mean blood glucose and HbA1c (Table 2). In 2010, Mittman and colleagues published a study that compared HbA1c with fructosamine and their correlation to glycemic control and morbidity, defined as rates of hospitalization and infection.12 The study included 100 patients with T2DM on HD with a mean age of 63 years, 54% were women, mean HbA1c of 7.2%, and mean dialysis duration of 3 years. Average follow-up was 3 years. At the end of follow-up, Mittman and colleagues found that HbA1c and fructosamine were highly correlated and associated with serum glucose (P < .01). However, fructosamine was found to be more highly correlated with mean glucose levels when those levels were below 150 mg/dL (P = .01). A higher fructosamine level, not HbA1c was a more significant predictor of hospitalization (P = .007) and infection (P = .001). Mittman and colleagues presented evidence for the use of fructosamine over HbA1c in patients with T2DM on HD.12
Hypoglycemic Episodes
At the 2-month follow-up visit with the CPS, Mr. A reported having 5 hypoglycemic episodes in the past 30 days. He also stated he would forget to take his insulin aspart dose before dinner about 3 to 4 times a week but would take it 30 to 60 minutes after the meal. Mr. A did not bring his glucometer or SMBG readings to the visit, but he indicated that his blood glucose levels continued to fluctuate and were elevated when consuming carbohydrates.
Laboratory tests 1 month prior to the 2-month follow-up visit showed HbA1c of 7.3%, which had increased from his previous level of 6.1%. He was counseled on the proper administration of insulin aspart and lifestyle modifications. A fructosamine level was ordered at this visit to further assess his glycemic control. A follow-up appointment and laboratory workup (fructosamine and HbA1c) were scheduled for 2 months from the visit (Table 3).
Mr. A was educated on the unreliability of his HbA1c levels secondary to his condition of ESRD on HD. He was counseled on the purpose of fructosamine and how it may be a better predictor of his glycemic control and morbidity. Mr. A continued to be followed closely by the primary care CPS for T2DM management.
Discussion
Management of T2DM in patients with ESRD presents challenges for clinicians in determining HbA1c goals and selecting appropriate medication options. The 2012 Kidney Disease Outcomes Quality Initiative (KDOQI) diabetes guideline does not recommend treatment for patients with substantially reduced kidney function to a target HbA1c < 7% due to risk of hypoglycemia.13 Although a target HbA1c > 7% is suggested for these patients, little is known about appropriate glycemic control in these patients as there is a paucity of prospective, randomized clinical trials that include patients with advanced CKD.13
Moreover, many oral antidiabetic medications and their metabolites are cleared by the kidneys and, therefore, pose with potential harm for patients with CKD. Because of this, insulin is the medication of choice for patients with ESRD.7 Although insulin requirements may diminish with worsening kidney function, insulin provides the safest method of glycemic control. Insulin dosing can be individualized according to a patient’s renal status as there is no uniformity in renal dose adjustments. There are some noninsulin antidiabetic agents that can be used in ESRD, but use of these agents requires close monitoring and evaluation of the medication’s pharmacokinetics (Table 4). Overall, medication management can be a difficult task for patients with T2DM and ESRD, but antidiabetic regimens may be reduced or discontinued altogether in burnt-out diabetes.
One of 3 patients with T2DM and ESRD on dialysis has burnt-out diabetes, defined as a phenomenon in which glucose homeostasis is altered to cause normoglycemia, spontaneous hypoglycemia, and decreased insulin requirements in established patients with T2DM.5 Although Mr. A had a normal-to-low HbA1c, he did not meet these criteria. Due to his elevated SMBG readings, he did not have normoglycemia and did require an increase in his basal insulin dose. Therefore, our patient did not have burnt-out diabetes.
Mr. A represents the relevant issue of inappropriately and unreliably low HbA1c levels due to various factors in ESRD. Our patient did not receive a blood transfusion in the past 2 years and was not on ESA therapy; nevertheless, Mr. A was a patient with ESRD on HD with a diagnosis of anemia. These diagnoses are confounders for low HbA1c values. When fructosamine levels were drawn for Mr. A on September 11, 2018 and November 6, 2018, they correlated well with his serum glucose and SMBG readings. This indicated to the CPS that the patient’s glycemic control was poor despite a promising HbA1c level.
This patient’s case and supporting evidence suggests that other measures of glycemic control (eg, fructosamine) can be used to supplement HbA1c, serum glucose, and glucometer readings to provide an accurate assessment of glycemic control in T2DM. Fructosamine also can assist HbA1c with predicting morbidity and potentially mortality, which are of great importance in this patient population.
Kalantar-Zadeh and colleagues conducted a study of 23,618 patients with T2DM on dialysis to observe mortality in association with HbA1c.5 This analysis showed that patients with HbA1c levels < 5% or > 8% had a higher risk of mortality; higher values of HbA1c (> 10%) were associated with increased death risk vs all other values. In the unadjusted analysis, HbA1c levels between 6 and 8% had the lowest death risk (hazard ratios [HR] 0.8 - 0.9, 95% CI) compared with those of higher and lower HbA1c ranges.5 In nonanemic patients, HbA1c > 6% was associated with increased death risk, whereas anemic patients did not show this trend.
Other studies made similar observations. In 2001, Morioka and colleagues published an observational study of 150 patients with DM on intermittent hemodialysis. The study analyzed survival and HbA1c levels at 1, 3, and 5 years. The study found that at 1, 3, and 5 years, patients with HbA1c < 7.5% had better survival than did patients with HbA1c > 7.5% (3.6 years vs 2.0 years, P = .008). Morioka and colleagues also found that there was a 13% increase in death per 1% increase in HbA1c.14 Oomichi and colleagues conducted an observational study of 114 patients with T2DM and ESRD on intermittent hemodialysis. Patients with fair control (HbA1c 6.5 - 8%) and good control (HbA1c < 6.5%) were compared with patients with poor control (HbA1c > 8%); it was found that the poor control group had nearly triple the mortality when compared with the good and fair control groups (HR = 2.89, P = .01).15 Park and colleagues also saw a similar observation in a study of 1,239 patients with ESRD and DM; 70% of these patients were on intermittent hemodialysis. Patients with poor control (HbA1c ≥ 8%) had worse survival outcomes than those with HbA1c < 8% (HR 2.2, P < .001).16
Our patient case forced us to ask the question, “What should our patient’s HbA1c goals be?” In the study by Oomichi and colleagues, a HbA1c level of 8% has usefulness as a “signpost for management of glycemic control.”15 All patients’ goals should be individualized based on various factors (eg, age, comorbidities), but based on the survival studies above, a HbA1c goal range of 6 to 8% may be optimal.
Conclusions
Patients with T2DM and ESRD on dialysis may have higher morbidity and mortality rates than the rates of those without T2DM. It has been shown in various studies that very low HbA1c (< 5%) and high HbA1c (> 8%) are associated with poor survival. Some patients with T2DM on dialysis may experience burnt-out diabetes in which they may have normoglycemia and a HbA1c below goal; despite these facts, this condition is not positive and can be linked to bad outcomes. In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice, and we recommend a HbA1c target of 6 to 8%. In this patient population, consider using fructosamine levels or other measures of glycemic control to supplement HbA1c and glucose values to provide a better assessment of glycemic control, morbidity, and mortality. Larger clinical trials are needed to assist in answering questions regarding mortality and optimal HbA1c targets in burnt-out diabetes.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Updated August 28, 2020. Accessed November 17, 2020.
2. Saran R, Robinson B, et al. US renal data system 2019 annual data report: epidemiology of klidney disease in the United States. Am J Kidney Dis. 2020 Jan;75(1 suppl 1):A6-A7. doi:10.1053/j.ajkd.2019.09.003. Epub 2019 Nov 5.
3. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
4. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. doi:10.1056/NEJMoa0802743
5. Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1c and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049-10.55. doi:10.2337/dc06-2127
6. Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep. 2012;12(4):432-439. doi:10.1007/s11892-012-0286-3
7. Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27(2):135-145. doi:10.1111/sdi.12198
8. Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19(1):33-37. doi:10.1053/j.jrn.2008.11.012
9. Unnikrishnan R, Anjana RM, Mohan V. Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528-531. doi:10.4103/2230-8210.98004
10. Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. 2011;5(6):1572-1583. doi:10.1177/193229681100500634
11. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr. 2012;25(3):141-148. doi:10.2337/diaspect.25.3.141
12. Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int. 2010;78 (suppl 117):S41-S45. doi:10.1038/ki.2010.193
13. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886. doi:10.1053/j.ajkd.2012.07.005
14. Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909-913. doi.10.2337/diacare.24.5.909

15. Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496-1500. doi:10.2337/dc05-1887
16. Park JI, Bae E, Kim YL, et al. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: a prospective cohort study in Korea. PLoS ONE. 2015;10(8):e0136085. doi:10.1371/journal.pone.0136085
17. Glucotrol tablets [
18. Amaryl [package insert]. Bridgewater, NJ: Sanofi-Aventis; December 2018.
19. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb; May 2018.
20. Actos [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; December 2017.
21. Precose [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; March 2015.
22. Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; June 2019.
23. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; June 2019.
24. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; October 2018.
In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice with a hemoglobin A1c target of 6 to 8%, using fructosamine levels or other measures for better assessment of glycemic control.
In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice with a hemoglobin A1c target of 6 to 8%, using fructosamine levels or other measures for better assessment of glycemic control.
More than 34 million adults in the US have type 2 diabetes mellitus (T2DM), a chronic progressive disease identified by worsening hyperglycemia and micro- and macrovascular complications.1 Consequently, 12.2% of the US adult population is currently at risk for macrovascular diseases, such as stroke and coronary artery disease (CAD) and microvascular diseases, such as neuropathy and diabetic nephropathy.1
T2DM is the most common comorbid risk factor for chronic kidney disease (CKD) and the leading cause of end-stage renal disease (ESRD). As of 2017, about 750,000 Americans have CKD stage 5 requiring dialysis, and 50% of these patients have preexisting diabetic nephropathy.2 Rates of mortality and morbidity are observed to be higher in patients with both CKD and T2DM compared with patients with CKD without T2DM.2 Previous clinical trials, including the United Kingdom Prospective Diabetes Study of 1998, have proven that optimal glycemic control decreases the risk of complications of T2DM (ie, nephropathy) in the general population.3 Conversely, tight glycemic control that targets hemoglobin A1c (HbA1c) < 7%, in patients with T2DM with ESRD has not shown the same benefits and may lead to worse outcomes. It is postulated that this may be due to the increased incidence of hypoglycemia in this patient population.4
Dialysis has varying effects on patients both with and without T2DM. While patients with ESRD without T2DM have the potential to develop impaired glucose tolerance and T2DM, about 33% of patients with T2DM on dialysis actually have HbA1c < 6%.5 In these patients, glycemic control improves spontaneously as their disease progresses, leading to a decrease or cessation of insulin or other antidiabetic medications. This phenomenon, known as burnt-out diabetes, is characterized by (1) alterations in glucose homeostasis and normoglycemia without antidiabetic treatment; (2) HbA1c levels < 6% despite having established T2DM; (3) decline in insulin requirements or cessation of insulin altogether; and (4) spontaneous hypoglycemia.
There is a misconception that burnt-out diabetes is a favorable condition due to the alteration of the natural course of T2DM. Although this may be true, patients with this condition are prone to develop hypoglycemic episodes and may be linked to poor survival outcomes due to low HbA1c.6,7
Since Kalantar-Zadeh and colleagues presented a 2009 case study, there has been a lack of research regarding this unique condition.8 The purpose of this case study is to shed further light on burnt-out diabetes and present a patient case pertaining to the challenges of glycemic control in ESRD.
Case Presentation
Mr. A is a 49-year-old Hispanic male veteran with a history of ESRD on hemodialysis (HD) for 6 years, anemia of CKD, and T2DM for 22 years. The patient also has an extensive cardiovascular disease history, including hypertension, hyperlipidemia, and CAD status post-4-vessel coronary artery bypass graft in December 2014. The patient receives in-home HD Monday, Wednesday, and Friday and is on the wait list for kidney transplantation. The patient’s T2DM is managed by a primary care clinical pharmacy specialist (CPS) at the
Mr. A’s antidiabetic regimen is 45 units of subcutaneous insulin glargine every morning; insulin aspart sliding scale (about 15-27 units) subcutaneous 3 times daily with meals; and saxagliptin 2.5 mg by mouth once daily.
At a follow-up visit with the CPS, Mr. A stated, “I feel fine except for the occasional low blood sugar episode.” The patient’s most recent HbA1c was 6.1%, and he reported medication adherence and no signs or symptoms of hyperglycemia (ie, polydipsia, polyphagia, nocturia, visual disturbances). Mr. A reported no use of alcohol, tobacco, or illicit drugs. He walks 1 mile every other day and participates in self-monitoring blood glucose (SMBG) about 2 to 3 times daily (Table 1).
Although Mr. A’s most recent HbA1c was well controlled, his estimated fasting blood glucose at the same laboratory draw was 224 mg/dL. His SMBG readings in the past month also were elevated with higher readings in the evening. Mr. A attributed the elevated readings to dietary excursions and a high carbohydrate intake. At this visit, the CPS increased his insulin glargine dose to 50 units subcutaneous every morning and educated him on lifestyle modifications. Follow-up with the CPS was scheduled for 2 months from the day of the visit.
Analysis
Few articles on potential contributors to burnt-out diabetes have been published.6,7 These articles discuss decreased renal and hepatic clearance of insulin (which increases its half-life) hypoglycemia during HD, and low HbA1c due to preexisting anemia. Inappropriately low HbA1c levels may be secondary to, but not limited to, hemolysis, recent blood transfusion, acute blood loss, and medications, such as erythropoietin-stimulating agents (ESAs).9 The conditions that affect red blood cell turnover are common in patients with advanced CKD and may result in discrepancies in HbA1c levels.
Glycated hemoglobin is a series of minor hemoglobin components formed by the adduction of various carbohydrate molecules to hemoglobin. HbA1c is the largest fraction formed and the most consistent index of the concentration of glucose in the blood.10 Hence, HbA1c is the traditional indicator of overall glycemic control. The current HbA1c goals recommended by the American Diabetes Association are derived from landmark trials conducted with patients in the general adult diabetic non-CKD population. However, hemoglobin measurements can be confounded by conditions present in ESRD and tend to underestimate glucose measurements in patients with T2DM on HD. Despite this, HbA1c is still regarded as a reasonable measure of glycemic control even in patients with ESRD; however, alternative markers of glycemia may be preferable.11
Although HbA1c is the gold standard, there are other laboratory measures of average glycemic control available. Fructosamine is a ketoamine formed when glucose binds to serum proteins. When these proteins are exposed to high concentrations of glucose, they experience increased glycation. Fructosamine assays measure the total glycated serum proteins, of which albumin accounts for about 90%.11 Because the half-life of serum proteins is about 20 days, fructosamine levels can reflect glycemic control over a 2- to 3-week period. This is advantageous in conditions that affect the average age of red blood cells, in pregnancy where frequent monitoring and measures of short-term glucose control are especially important, and in the evaluation of a medication adjustment in the management of T2DM. However, this test is not without its limitations. It is less reliable in settings of decreased protein levels (eg, liver disease), there is a lack of availability in routine practice, and reference levels have not been established.11
Fructosamine has been shown to be strongly associated with mean blood glucose and HbA1c (Table 2). In 2010, Mittman and colleagues published a study that compared HbA1c with fructosamine and their correlation to glycemic control and morbidity, defined as rates of hospitalization and infection.12 The study included 100 patients with T2DM on HD with a mean age of 63 years, 54% were women, mean HbA1c of 7.2%, and mean dialysis duration of 3 years. Average follow-up was 3 years. At the end of follow-up, Mittman and colleagues found that HbA1c and fructosamine were highly correlated and associated with serum glucose (P < .01). However, fructosamine was found to be more highly correlated with mean glucose levels when those levels were below 150 mg/dL (P = .01). A higher fructosamine level, not HbA1c was a more significant predictor of hospitalization (P = .007) and infection (P = .001). Mittman and colleagues presented evidence for the use of fructosamine over HbA1c in patients with T2DM on HD.12
Hypoglycemic Episodes
At the 2-month follow-up visit with the CPS, Mr. A reported having 5 hypoglycemic episodes in the past 30 days. He also stated he would forget to take his insulin aspart dose before dinner about 3 to 4 times a week but would take it 30 to 60 minutes after the meal. Mr. A did not bring his glucometer or SMBG readings to the visit, but he indicated that his blood glucose levels continued to fluctuate and were elevated when consuming carbohydrates.
Laboratory tests 1 month prior to the 2-month follow-up visit showed HbA1c of 7.3%, which had increased from his previous level of 6.1%. He was counseled on the proper administration of insulin aspart and lifestyle modifications. A fructosamine level was ordered at this visit to further assess his glycemic control. A follow-up appointment and laboratory workup (fructosamine and HbA1c) were scheduled for 2 months from the visit (Table 3).
Mr. A was educated on the unreliability of his HbA1c levels secondary to his condition of ESRD on HD. He was counseled on the purpose of fructosamine and how it may be a better predictor of his glycemic control and morbidity. Mr. A continued to be followed closely by the primary care CPS for T2DM management.
Discussion
Management of T2DM in patients with ESRD presents challenges for clinicians in determining HbA1c goals and selecting appropriate medication options. The 2012 Kidney Disease Outcomes Quality Initiative (KDOQI) diabetes guideline does not recommend treatment for patients with substantially reduced kidney function to a target HbA1c < 7% due to risk of hypoglycemia.13 Although a target HbA1c > 7% is suggested for these patients, little is known about appropriate glycemic control in these patients as there is a paucity of prospective, randomized clinical trials that include patients with advanced CKD.13
Moreover, many oral antidiabetic medications and their metabolites are cleared by the kidneys and, therefore, pose with potential harm for patients with CKD. Because of this, insulin is the medication of choice for patients with ESRD.7 Although insulin requirements may diminish with worsening kidney function, insulin provides the safest method of glycemic control. Insulin dosing can be individualized according to a patient’s renal status as there is no uniformity in renal dose adjustments. There are some noninsulin antidiabetic agents that can be used in ESRD, but use of these agents requires close monitoring and evaluation of the medication’s pharmacokinetics (Table 4). Overall, medication management can be a difficult task for patients with T2DM and ESRD, but antidiabetic regimens may be reduced or discontinued altogether in burnt-out diabetes.
One of 3 patients with T2DM and ESRD on dialysis has burnt-out diabetes, defined as a phenomenon in which glucose homeostasis is altered to cause normoglycemia, spontaneous hypoglycemia, and decreased insulin requirements in established patients with T2DM.5 Although Mr. A had a normal-to-low HbA1c, he did not meet these criteria. Due to his elevated SMBG readings, he did not have normoglycemia and did require an increase in his basal insulin dose. Therefore, our patient did not have burnt-out diabetes.
Mr. A represents the relevant issue of inappropriately and unreliably low HbA1c levels due to various factors in ESRD. Our patient did not receive a blood transfusion in the past 2 years and was not on ESA therapy; nevertheless, Mr. A was a patient with ESRD on HD with a diagnosis of anemia. These diagnoses are confounders for low HbA1c values. When fructosamine levels were drawn for Mr. A on September 11, 2018 and November 6, 2018, they correlated well with his serum glucose and SMBG readings. This indicated to the CPS that the patient’s glycemic control was poor despite a promising HbA1c level.
This patient’s case and supporting evidence suggests that other measures of glycemic control (eg, fructosamine) can be used to supplement HbA1c, serum glucose, and glucometer readings to provide an accurate assessment of glycemic control in T2DM. Fructosamine also can assist HbA1c with predicting morbidity and potentially mortality, which are of great importance in this patient population.
Kalantar-Zadeh and colleagues conducted a study of 23,618 patients with T2DM on dialysis to observe mortality in association with HbA1c.5 This analysis showed that patients with HbA1c levels < 5% or > 8% had a higher risk of mortality; higher values of HbA1c (> 10%) were associated with increased death risk vs all other values. In the unadjusted analysis, HbA1c levels between 6 and 8% had the lowest death risk (hazard ratios [HR] 0.8 - 0.9, 95% CI) compared with those of higher and lower HbA1c ranges.5 In nonanemic patients, HbA1c > 6% was associated with increased death risk, whereas anemic patients did not show this trend.
Other studies made similar observations. In 2001, Morioka and colleagues published an observational study of 150 patients with DM on intermittent hemodialysis. The study analyzed survival and HbA1c levels at 1, 3, and 5 years. The study found that at 1, 3, and 5 years, patients with HbA1c < 7.5% had better survival than did patients with HbA1c > 7.5% (3.6 years vs 2.0 years, P = .008). Morioka and colleagues also found that there was a 13% increase in death per 1% increase in HbA1c.14 Oomichi and colleagues conducted an observational study of 114 patients with T2DM and ESRD on intermittent hemodialysis. Patients with fair control (HbA1c 6.5 - 8%) and good control (HbA1c < 6.5%) were compared with patients with poor control (HbA1c > 8%); it was found that the poor control group had nearly triple the mortality when compared with the good and fair control groups (HR = 2.89, P = .01).15 Park and colleagues also saw a similar observation in a study of 1,239 patients with ESRD and DM; 70% of these patients were on intermittent hemodialysis. Patients with poor control (HbA1c ≥ 8%) had worse survival outcomes than those with HbA1c < 8% (HR 2.2, P < .001).16
Our patient case forced us to ask the question, “What should our patient’s HbA1c goals be?” In the study by Oomichi and colleagues, a HbA1c level of 8% has usefulness as a “signpost for management of glycemic control.”15 All patients’ goals should be individualized based on various factors (eg, age, comorbidities), but based on the survival studies above, a HbA1c goal range of 6 to 8% may be optimal.
Conclusions
Patients with T2DM and ESRD on dialysis may have higher morbidity and mortality rates than the rates of those without T2DM. It has been shown in various studies that very low HbA1c (< 5%) and high HbA1c (> 8%) are associated with poor survival. Some patients with T2DM on dialysis may experience burnt-out diabetes in which they may have normoglycemia and a HbA1c below goal; despite these facts, this condition is not positive and can be linked to bad outcomes. In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice, and we recommend a HbA1c target of 6 to 8%. In this patient population, consider using fructosamine levels or other measures of glycemic control to supplement HbA1c and glucose values to provide a better assessment of glycemic control, morbidity, and mortality. Larger clinical trials are needed to assist in answering questions regarding mortality and optimal HbA1c targets in burnt-out diabetes.
More than 34 million adults in the US have type 2 diabetes mellitus (T2DM), a chronic progressive disease identified by worsening hyperglycemia and micro- and macrovascular complications.1 Consequently, 12.2% of the US adult population is currently at risk for macrovascular diseases, such as stroke and coronary artery disease (CAD) and microvascular diseases, such as neuropathy and diabetic nephropathy.1
T2DM is the most common comorbid risk factor for chronic kidney disease (CKD) and the leading cause of end-stage renal disease (ESRD). As of 2017, about 750,000 Americans have CKD stage 5 requiring dialysis, and 50% of these patients have preexisting diabetic nephropathy.2 Rates of mortality and morbidity are observed to be higher in patients with both CKD and T2DM compared with patients with CKD without T2DM.2 Previous clinical trials, including the United Kingdom Prospective Diabetes Study of 1998, have proven that optimal glycemic control decreases the risk of complications of T2DM (ie, nephropathy) in the general population.3 Conversely, tight glycemic control that targets hemoglobin A1c (HbA1c) < 7%, in patients with T2DM with ESRD has not shown the same benefits and may lead to worse outcomes. It is postulated that this may be due to the increased incidence of hypoglycemia in this patient population.4
Dialysis has varying effects on patients both with and without T2DM. While patients with ESRD without T2DM have the potential to develop impaired glucose tolerance and T2DM, about 33% of patients with T2DM on dialysis actually have HbA1c < 6%.5 In these patients, glycemic control improves spontaneously as their disease progresses, leading to a decrease or cessation of insulin or other antidiabetic medications. This phenomenon, known as burnt-out diabetes, is characterized by (1) alterations in glucose homeostasis and normoglycemia without antidiabetic treatment; (2) HbA1c levels < 6% despite having established T2DM; (3) decline in insulin requirements or cessation of insulin altogether; and (4) spontaneous hypoglycemia.
There is a misconception that burnt-out diabetes is a favorable condition due to the alteration of the natural course of T2DM. Although this may be true, patients with this condition are prone to develop hypoglycemic episodes and may be linked to poor survival outcomes due to low HbA1c.6,7
Since Kalantar-Zadeh and colleagues presented a 2009 case study, there has been a lack of research regarding this unique condition.8 The purpose of this case study is to shed further light on burnt-out diabetes and present a patient case pertaining to the challenges of glycemic control in ESRD.
Case Presentation
Mr. A is a 49-year-old Hispanic male veteran with a history of ESRD on hemodialysis (HD) for 6 years, anemia of CKD, and T2DM for 22 years. The patient also has an extensive cardiovascular disease history, including hypertension, hyperlipidemia, and CAD status post-4-vessel coronary artery bypass graft in December 2014. The patient receives in-home HD Monday, Wednesday, and Friday and is on the wait list for kidney transplantation. The patient’s T2DM is managed by a primary care clinical pharmacy specialist (CPS) at the
Mr. A’s antidiabetic regimen is 45 units of subcutaneous insulin glargine every morning; insulin aspart sliding scale (about 15-27 units) subcutaneous 3 times daily with meals; and saxagliptin 2.5 mg by mouth once daily.
At a follow-up visit with the CPS, Mr. A stated, “I feel fine except for the occasional low blood sugar episode.” The patient’s most recent HbA1c was 6.1%, and he reported medication adherence and no signs or symptoms of hyperglycemia (ie, polydipsia, polyphagia, nocturia, visual disturbances). Mr. A reported no use of alcohol, tobacco, or illicit drugs. He walks 1 mile every other day and participates in self-monitoring blood glucose (SMBG) about 2 to 3 times daily (Table 1).
Although Mr. A’s most recent HbA1c was well controlled, his estimated fasting blood glucose at the same laboratory draw was 224 mg/dL. His SMBG readings in the past month also were elevated with higher readings in the evening. Mr. A attributed the elevated readings to dietary excursions and a high carbohydrate intake. At this visit, the CPS increased his insulin glargine dose to 50 units subcutaneous every morning and educated him on lifestyle modifications. Follow-up with the CPS was scheduled for 2 months from the day of the visit.
Analysis
Few articles on potential contributors to burnt-out diabetes have been published.6,7 These articles discuss decreased renal and hepatic clearance of insulin (which increases its half-life) hypoglycemia during HD, and low HbA1c due to preexisting anemia. Inappropriately low HbA1c levels may be secondary to, but not limited to, hemolysis, recent blood transfusion, acute blood loss, and medications, such as erythropoietin-stimulating agents (ESAs).9 The conditions that affect red blood cell turnover are common in patients with advanced CKD and may result in discrepancies in HbA1c levels.
Glycated hemoglobin is a series of minor hemoglobin components formed by the adduction of various carbohydrate molecules to hemoglobin. HbA1c is the largest fraction formed and the most consistent index of the concentration of glucose in the blood.10 Hence, HbA1c is the traditional indicator of overall glycemic control. The current HbA1c goals recommended by the American Diabetes Association are derived from landmark trials conducted with patients in the general adult diabetic non-CKD population. However, hemoglobin measurements can be confounded by conditions present in ESRD and tend to underestimate glucose measurements in patients with T2DM on HD. Despite this, HbA1c is still regarded as a reasonable measure of glycemic control even in patients with ESRD; however, alternative markers of glycemia may be preferable.11
Although HbA1c is the gold standard, there are other laboratory measures of average glycemic control available. Fructosamine is a ketoamine formed when glucose binds to serum proteins. When these proteins are exposed to high concentrations of glucose, they experience increased glycation. Fructosamine assays measure the total glycated serum proteins, of which albumin accounts for about 90%.11 Because the half-life of serum proteins is about 20 days, fructosamine levels can reflect glycemic control over a 2- to 3-week period. This is advantageous in conditions that affect the average age of red blood cells, in pregnancy where frequent monitoring and measures of short-term glucose control are especially important, and in the evaluation of a medication adjustment in the management of T2DM. However, this test is not without its limitations. It is less reliable in settings of decreased protein levels (eg, liver disease), there is a lack of availability in routine practice, and reference levels have not been established.11
Fructosamine has been shown to be strongly associated with mean blood glucose and HbA1c (Table 2). In 2010, Mittman and colleagues published a study that compared HbA1c with fructosamine and their correlation to glycemic control and morbidity, defined as rates of hospitalization and infection.12 The study included 100 patients with T2DM on HD with a mean age of 63 years, 54% were women, mean HbA1c of 7.2%, and mean dialysis duration of 3 years. Average follow-up was 3 years. At the end of follow-up, Mittman and colleagues found that HbA1c and fructosamine were highly correlated and associated with serum glucose (P < .01). However, fructosamine was found to be more highly correlated with mean glucose levels when those levels were below 150 mg/dL (P = .01). A higher fructosamine level, not HbA1c was a more significant predictor of hospitalization (P = .007) and infection (P = .001). Mittman and colleagues presented evidence for the use of fructosamine over HbA1c in patients with T2DM on HD.12
Hypoglycemic Episodes
At the 2-month follow-up visit with the CPS, Mr. A reported having 5 hypoglycemic episodes in the past 30 days. He also stated he would forget to take his insulin aspart dose before dinner about 3 to 4 times a week but would take it 30 to 60 minutes after the meal. Mr. A did not bring his glucometer or SMBG readings to the visit, but he indicated that his blood glucose levels continued to fluctuate and were elevated when consuming carbohydrates.
Laboratory tests 1 month prior to the 2-month follow-up visit showed HbA1c of 7.3%, which had increased from his previous level of 6.1%. He was counseled on the proper administration of insulin aspart and lifestyle modifications. A fructosamine level was ordered at this visit to further assess his glycemic control. A follow-up appointment and laboratory workup (fructosamine and HbA1c) were scheduled for 2 months from the visit (Table 3).
Mr. A was educated on the unreliability of his HbA1c levels secondary to his condition of ESRD on HD. He was counseled on the purpose of fructosamine and how it may be a better predictor of his glycemic control and morbidity. Mr. A continued to be followed closely by the primary care CPS for T2DM management.
Discussion
Management of T2DM in patients with ESRD presents challenges for clinicians in determining HbA1c goals and selecting appropriate medication options. The 2012 Kidney Disease Outcomes Quality Initiative (KDOQI) diabetes guideline does not recommend treatment for patients with substantially reduced kidney function to a target HbA1c < 7% due to risk of hypoglycemia.13 Although a target HbA1c > 7% is suggested for these patients, little is known about appropriate glycemic control in these patients as there is a paucity of prospective, randomized clinical trials that include patients with advanced CKD.13
Moreover, many oral antidiabetic medications and their metabolites are cleared by the kidneys and, therefore, pose with potential harm for patients with CKD. Because of this, insulin is the medication of choice for patients with ESRD.7 Although insulin requirements may diminish with worsening kidney function, insulin provides the safest method of glycemic control. Insulin dosing can be individualized according to a patient’s renal status as there is no uniformity in renal dose adjustments. There are some noninsulin antidiabetic agents that can be used in ESRD, but use of these agents requires close monitoring and evaluation of the medication’s pharmacokinetics (Table 4). Overall, medication management can be a difficult task for patients with T2DM and ESRD, but antidiabetic regimens may be reduced or discontinued altogether in burnt-out diabetes.
One of 3 patients with T2DM and ESRD on dialysis has burnt-out diabetes, defined as a phenomenon in which glucose homeostasis is altered to cause normoglycemia, spontaneous hypoglycemia, and decreased insulin requirements in established patients with T2DM.5 Although Mr. A had a normal-to-low HbA1c, he did not meet these criteria. Due to his elevated SMBG readings, he did not have normoglycemia and did require an increase in his basal insulin dose. Therefore, our patient did not have burnt-out diabetes.
Mr. A represents the relevant issue of inappropriately and unreliably low HbA1c levels due to various factors in ESRD. Our patient did not receive a blood transfusion in the past 2 years and was not on ESA therapy; nevertheless, Mr. A was a patient with ESRD on HD with a diagnosis of anemia. These diagnoses are confounders for low HbA1c values. When fructosamine levels were drawn for Mr. A on September 11, 2018 and November 6, 2018, they correlated well with his serum glucose and SMBG readings. This indicated to the CPS that the patient’s glycemic control was poor despite a promising HbA1c level.
This patient’s case and supporting evidence suggests that other measures of glycemic control (eg, fructosamine) can be used to supplement HbA1c, serum glucose, and glucometer readings to provide an accurate assessment of glycemic control in T2DM. Fructosamine also can assist HbA1c with predicting morbidity and potentially mortality, which are of great importance in this patient population.
Kalantar-Zadeh and colleagues conducted a study of 23,618 patients with T2DM on dialysis to observe mortality in association with HbA1c.5 This analysis showed that patients with HbA1c levels < 5% or > 8% had a higher risk of mortality; higher values of HbA1c (> 10%) were associated with increased death risk vs all other values. In the unadjusted analysis, HbA1c levels between 6 and 8% had the lowest death risk (hazard ratios [HR] 0.8 - 0.9, 95% CI) compared with those of higher and lower HbA1c ranges.5 In nonanemic patients, HbA1c > 6% was associated with increased death risk, whereas anemic patients did not show this trend.
Other studies made similar observations. In 2001, Morioka and colleagues published an observational study of 150 patients with DM on intermittent hemodialysis. The study analyzed survival and HbA1c levels at 1, 3, and 5 years. The study found that at 1, 3, and 5 years, patients with HbA1c < 7.5% had better survival than did patients with HbA1c > 7.5% (3.6 years vs 2.0 years, P = .008). Morioka and colleagues also found that there was a 13% increase in death per 1% increase in HbA1c.14 Oomichi and colleagues conducted an observational study of 114 patients with T2DM and ESRD on intermittent hemodialysis. Patients with fair control (HbA1c 6.5 - 8%) and good control (HbA1c < 6.5%) were compared with patients with poor control (HbA1c > 8%); it was found that the poor control group had nearly triple the mortality when compared with the good and fair control groups (HR = 2.89, P = .01).15 Park and colleagues also saw a similar observation in a study of 1,239 patients with ESRD and DM; 70% of these patients were on intermittent hemodialysis. Patients with poor control (HbA1c ≥ 8%) had worse survival outcomes than those with HbA1c < 8% (HR 2.2, P < .001).16
Our patient case forced us to ask the question, “What should our patient’s HbA1c goals be?” In the study by Oomichi and colleagues, a HbA1c level of 8% has usefulness as a “signpost for management of glycemic control.”15 All patients’ goals should be individualized based on various factors (eg, age, comorbidities), but based on the survival studies above, a HbA1c goal range of 6 to 8% may be optimal.
Conclusions
Patients with T2DM and ESRD on dialysis may have higher morbidity and mortality rates than the rates of those without T2DM. It has been shown in various studies that very low HbA1c (< 5%) and high HbA1c (> 8%) are associated with poor survival. Some patients with T2DM on dialysis may experience burnt-out diabetes in which they may have normoglycemia and a HbA1c below goal; despite these facts, this condition is not positive and can be linked to bad outcomes. In patients with T2DM and ESRD, insulin is the antidiabetic medication of choice, and we recommend a HbA1c target of 6 to 8%. In this patient population, consider using fructosamine levels or other measures of glycemic control to supplement HbA1c and glucose values to provide a better assessment of glycemic control, morbidity, and mortality. Larger clinical trials are needed to assist in answering questions regarding mortality and optimal HbA1c targets in burnt-out diabetes.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Updated August 28, 2020. Accessed November 17, 2020.
2. Saran R, Robinson B, et al. US renal data system 2019 annual data report: epidemiology of klidney disease in the United States. Am J Kidney Dis. 2020 Jan;75(1 suppl 1):A6-A7. doi:10.1053/j.ajkd.2019.09.003. Epub 2019 Nov 5.
3. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
4. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. doi:10.1056/NEJMoa0802743
5. Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1c and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049-10.55. doi:10.2337/dc06-2127
6. Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep. 2012;12(4):432-439. doi:10.1007/s11892-012-0286-3
7. Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27(2):135-145. doi:10.1111/sdi.12198
8. Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19(1):33-37. doi:10.1053/j.jrn.2008.11.012
9. Unnikrishnan R, Anjana RM, Mohan V. Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528-531. doi:10.4103/2230-8210.98004
10. Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. 2011;5(6):1572-1583. doi:10.1177/193229681100500634
11. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr. 2012;25(3):141-148. doi:10.2337/diaspect.25.3.141
12. Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int. 2010;78 (suppl 117):S41-S45. doi:10.1038/ki.2010.193
13. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886. doi:10.1053/j.ajkd.2012.07.005
14. Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909-913. doi.10.2337/diacare.24.5.909

15. Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496-1500. doi:10.2337/dc05-1887
16. Park JI, Bae E, Kim YL, et al. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: a prospective cohort study in Korea. PLoS ONE. 2015;10(8):e0136085. doi:10.1371/journal.pone.0136085
17. Glucotrol tablets [
18. Amaryl [package insert]. Bridgewater, NJ: Sanofi-Aventis; December 2018.
19. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb; May 2018.
20. Actos [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; December 2017.
21. Precose [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; March 2015.
22. Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; June 2019.
23. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; June 2019.
24. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; October 2018.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Updated August 28, 2020. Accessed November 17, 2020.
2. Saran R, Robinson B, et al. US renal data system 2019 annual data report: epidemiology of klidney disease in the United States. Am J Kidney Dis. 2020 Jan;75(1 suppl 1):A6-A7. doi:10.1053/j.ajkd.2019.09.003. Epub 2019 Nov 5.
3. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865.
4. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. doi:10.1056/NEJMoa0802743
5. Kalantar-Zadeh K, Kopple JD, Regidor DL, et al. A1c and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30(5):1049-10.55. doi:10.2337/dc06-2127
6. Park J, Lertdumrongluk P, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K. Glycemic control in diabetic dialysis patients and the burnt-out diabetes phenomenon. Curr Diab Rep. 2012;12(4):432-439. doi:10.1007/s11892-012-0286-3
7. Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27(2):135-145. doi:10.1111/sdi.12198
8. Kalantar-Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt-out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19(1):33-37. doi:10.1053/j.jrn.2008.11.012
9. Unnikrishnan R, Anjana RM, Mohan V. Drugs affecting HbA1c levels. Indian J Endocrinol Metab. 2012;16(4):528-531. doi:10.4103/2230-8210.98004
10. Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? J Diabetes Sci Technol. 2011;5(6):1572-1583. doi:10.1177/193229681100500634
11. Wright LAC, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes: reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectr. 2012;25(3):141-148. doi:10.2337/diaspect.25.3.141
12. Mittman N, Desiraju B, Fazil I, et al. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int. 2010;78 (suppl 117):S41-S45. doi:10.1038/ki.2010.193
13. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60(5):850-886. doi:10.1053/j.ajkd.2012.07.005
14. Morioka T, Emoto M, Tabata T, et al. Glycemic control is a predictor of survival for diabetic patients on hemodialysis. Diabetes Care. 2001;24(5):909-913. doi.10.2337/diacare.24.5.909

15. Oomichi T, Emoto M, Tabata T, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diabetes Care. 2006;29(7):1496-1500. doi:10.2337/dc05-1887
16. Park JI, Bae E, Kim YL, et al. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: a prospective cohort study in Korea. PLoS ONE. 2015;10(8):e0136085. doi:10.1371/journal.pone.0136085
17. Glucotrol tablets [
18. Amaryl [package insert]. Bridgewater, NJ: Sanofi-Aventis; December 2018.
19. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb; May 2018.
20. Actos [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; December 2017.
21. Precose [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; March 2015.
22. Nesina [package insert]. Deerfield, IL: Takeda Pharmaceuticals America Inc; June 2019.
23. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; June 2019.
24. Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; October 2018.
Recalled to Life: The Best and Worst of 2020 Is the Year 2020
Some who read Federal Practitioner regularly may recall that since 2017, I have been dedicating the December and January editorials to a more substantive version of the popular best and worst awards that appear in the media this time of year. Everything from the most comfortable slippers to the weirdest lawsuits is scored annually. In an effort to elevate the ranking routine, this column has reviewed and evaluated ethical and unethical events and decisions in the 3 federal health care systems Federal Practitioner primarily serves. In previous years it was a challenge requiring research and deliberation to select the most inspiring and troubling occurrences in the world of federal health care. This year neither great effort or prolonged study was required as the choice was immediate and obvious—the year itself. A year in which our individual identities as health care professionals serving in the US Department of Defense, US Department of Veterans Affairs (VA), and US Public Health Service is subsumed in our realities as citizens of a nation in crisis.
The opening lines of A Tale of Two Cities have become such a literary platitude taken out of the context of the novel that the terror and fascination with which Dickens wrote these oft-quoted lines has been diluted and dulled.1 In citing the entire paragraph as the epigraph, I hope to recapture the moral seriousness of its message, which is so relevant in 2020. While protesting the widespread injustice that fueled the progress of London’s industrial revolution, Dickens also feared such discontent would ignite a bloody uprising as it had done in Paris.1 This passage is a classic example of the literary device of parallelism that so perfectly expressed Dickens’ reflections on the trajectory of the unprecedented historical impact of the French Revolution. A parallelism that also aptly captures the contemporary contrasts and comparisons of the best and worst of 2020.
It is estimated that at least 66% of those eligible to vote did so on November 3, 2020, the highest turnout in more than a century, demonstrating the strength of the United States as a representative democracy.2 It is not about partisan politics, it is that more than 150 million citizens braved the winter, the virus, and potential intimidation to cast a ballot for their values.3 Still, America has never been more divided, and Dickens’ fear of political upheaval has never been more real in our country, or at least since the Civil War.
As I write this editorial, manufacturers for 2 vaccines have submitted phase 3 trial data to the US Food and Drug Administration for Emergency Use Authorizations and a third consortium may follow suit soon. Scientists report that the 2 vaccines, which were developed in less than a year, have high efficacy rates (> 90%) with only modest adverse effects.4 It is an unparalleled, really unimaginable, scientific feat. Americans’ characteristic gift for logistical efficiency and scientific innovation faces daunting administrative and technical barriers to achieve a similar viral victory, yet we may have faced even more formidable odds in World War II.
As of December 4, 2020, Johns Hopkins University reports that more than 275,000 Americans have died of coronavirus.5 The United States is on track to reach 200,000 cases a day with the signature holiday season of family festivities brutally morphed into gatherings of contagion.6 Hospitals across the country are running out of intensive care beds and nurses and doctors to staff them. Unlike the Spring surge in the Northeast, cases are rising in 49 states, and there is nowhere in the land from which respite and reinforcements can come.7
Thousands of health care professionals are exhausted, many with COVID-19 or recovering from it, morally distressed, and emotionally spent. Masks and social distancing are no longer public health essentials but elements of a culture war. Those same nurses, doctors, and public health officers still show up day after night for what is much closer to war than work. They struggle to prevent patients from going on ventilators they may never come off and use the few available therapies to keep as many patients alive as possible—whether those patients believe in COVID-19, wore a mask, no matter who they voted for—because that is what it means to practice health care according to a code of ethics.
In March 2020, I pledged to devote every editorial to COVID-19 for as long as the pandemic lasted, as one small candle for all those who have died of COVID-19, who are suffering as survivors of it, and who take risks and labor to deliver essential services from groceries to intensive care. Prudent public health officials wisely advise that the vaccine(s) are not a miracle cure to revive a depleted country, in part because it may undermine life-saving public health measures.8 And so the columns will continue in 2021 to illuminate the ethical issues of the pandemic as they affect all of us as federal health care professionals and Americans.
The Tale of Two Cities chapter that begins with the “best of times, and the worst of times” is entitled “Recalled to Life.” Let that be our hope and prayer for the coming year.
1. Dickens C. A Tale of Two Cities. Douglas-Fairhust ed. New York: Norton; 2020.
2. Schaul K, Rabinowitz K, Mellnik T. 2020 turnout is the highest in over a century. Washington Post, November 5, 2020. https://www.washingtonpost.com/graphics/2020/elections/voter-turnout. Accessed November 23, 2020.
3. Desilver D. In past elections, U.S. trailed most developed countries in voter turnout. https://www.pewresearch.org/fact-tank/2020/11/03/in-past-elections-u-s-trailed-most-developed-countries-in-voter-turnout. Published November 3, 2020. Accessed November 23, 2020.
4. Herper M, Garde D. Moderna to submit Covid-19 vaccine to FDA as full results show 94% efficacy.https://www.statnews.com/2020/11/30/moderna-covid-19-vaccine-full-results. Published November 30, 2020. Accessed November 30, 2020.
5. Johns Hopkins University and Medicine. Coronavirus research center. https://coronavirus.jhu.edu. Updated November 23, 2020. Accessed December 4, 2020.
6. Hawkins D, Knowles H. As U.S. coronavirus cases soar toward 200,000 a day holiday travel is surging. Washington Post, November 21, 2020. https://www.washingtonpost.com/health/2020/11/21/coronavirus-thanksgiving-travel. Accessed November 23, 2020.
7. Goldhill O. ‘People are going to die’: Hospitals in half the states are facing massive staffing shortages as COVID-19 surges. November 19, 2020. https://www.statnews.com/2020/11/19/covid19-hospitals-in-half-the-states-facing-massive-staffing-shortage. Published November 19, 2020. Accessed November 23, 2020.
8. Lazar K. Is Pfizer’s vaccine a ‘magic bullet?’ Scientists warn masks, distancing may last well into 2021. Boston Globe . November 9, 2020. https://www.bostonglobe.com/2020/11/09/metro/is-pfizer-vaccine-magic-bullet-scientists-warn-public-should-be-prepared-live-with-masks-social-distancing-months. Accessed November 23, 2020.
Some who read Federal Practitioner regularly may recall that since 2017, I have been dedicating the December and January editorials to a more substantive version of the popular best and worst awards that appear in the media this time of year. Everything from the most comfortable slippers to the weirdest lawsuits is scored annually. In an effort to elevate the ranking routine, this column has reviewed and evaluated ethical and unethical events and decisions in the 3 federal health care systems Federal Practitioner primarily serves. In previous years it was a challenge requiring research and deliberation to select the most inspiring and troubling occurrences in the world of federal health care. This year neither great effort or prolonged study was required as the choice was immediate and obvious—the year itself. A year in which our individual identities as health care professionals serving in the US Department of Defense, US Department of Veterans Affairs (VA), and US Public Health Service is subsumed in our realities as citizens of a nation in crisis.
The opening lines of A Tale of Two Cities have become such a literary platitude taken out of the context of the novel that the terror and fascination with which Dickens wrote these oft-quoted lines has been diluted and dulled.1 In citing the entire paragraph as the epigraph, I hope to recapture the moral seriousness of its message, which is so relevant in 2020. While protesting the widespread injustice that fueled the progress of London’s industrial revolution, Dickens also feared such discontent would ignite a bloody uprising as it had done in Paris.1 This passage is a classic example of the literary device of parallelism that so perfectly expressed Dickens’ reflections on the trajectory of the unprecedented historical impact of the French Revolution. A parallelism that also aptly captures the contemporary contrasts and comparisons of the best and worst of 2020.
It is estimated that at least 66% of those eligible to vote did so on November 3, 2020, the highest turnout in more than a century, demonstrating the strength of the United States as a representative democracy.2 It is not about partisan politics, it is that more than 150 million citizens braved the winter, the virus, and potential intimidation to cast a ballot for their values.3 Still, America has never been more divided, and Dickens’ fear of political upheaval has never been more real in our country, or at least since the Civil War.
As I write this editorial, manufacturers for 2 vaccines have submitted phase 3 trial data to the US Food and Drug Administration for Emergency Use Authorizations and a third consortium may follow suit soon. Scientists report that the 2 vaccines, which were developed in less than a year, have high efficacy rates (> 90%) with only modest adverse effects.4 It is an unparalleled, really unimaginable, scientific feat. Americans’ characteristic gift for logistical efficiency and scientific innovation faces daunting administrative and technical barriers to achieve a similar viral victory, yet we may have faced even more formidable odds in World War II.
As of December 4, 2020, Johns Hopkins University reports that more than 275,000 Americans have died of coronavirus.5 The United States is on track to reach 200,000 cases a day with the signature holiday season of family festivities brutally morphed into gatherings of contagion.6 Hospitals across the country are running out of intensive care beds and nurses and doctors to staff them. Unlike the Spring surge in the Northeast, cases are rising in 49 states, and there is nowhere in the land from which respite and reinforcements can come.7
Thousands of health care professionals are exhausted, many with COVID-19 or recovering from it, morally distressed, and emotionally spent. Masks and social distancing are no longer public health essentials but elements of a culture war. Those same nurses, doctors, and public health officers still show up day after night for what is much closer to war than work. They struggle to prevent patients from going on ventilators they may never come off and use the few available therapies to keep as many patients alive as possible—whether those patients believe in COVID-19, wore a mask, no matter who they voted for—because that is what it means to practice health care according to a code of ethics.
In March 2020, I pledged to devote every editorial to COVID-19 for as long as the pandemic lasted, as one small candle for all those who have died of COVID-19, who are suffering as survivors of it, and who take risks and labor to deliver essential services from groceries to intensive care. Prudent public health officials wisely advise that the vaccine(s) are not a miracle cure to revive a depleted country, in part because it may undermine life-saving public health measures.8 And so the columns will continue in 2021 to illuminate the ethical issues of the pandemic as they affect all of us as federal health care professionals and Americans.
The Tale of Two Cities chapter that begins with the “best of times, and the worst of times” is entitled “Recalled to Life.” Let that be our hope and prayer for the coming year.
Some who read Federal Practitioner regularly may recall that since 2017, I have been dedicating the December and January editorials to a more substantive version of the popular best and worst awards that appear in the media this time of year. Everything from the most comfortable slippers to the weirdest lawsuits is scored annually. In an effort to elevate the ranking routine, this column has reviewed and evaluated ethical and unethical events and decisions in the 3 federal health care systems Federal Practitioner primarily serves. In previous years it was a challenge requiring research and deliberation to select the most inspiring and troubling occurrences in the world of federal health care. This year neither great effort or prolonged study was required as the choice was immediate and obvious—the year itself. A year in which our individual identities as health care professionals serving in the US Department of Defense, US Department of Veterans Affairs (VA), and US Public Health Service is subsumed in our realities as citizens of a nation in crisis.
The opening lines of A Tale of Two Cities have become such a literary platitude taken out of the context of the novel that the terror and fascination with which Dickens wrote these oft-quoted lines has been diluted and dulled.1 In citing the entire paragraph as the epigraph, I hope to recapture the moral seriousness of its message, which is so relevant in 2020. While protesting the widespread injustice that fueled the progress of London’s industrial revolution, Dickens also feared such discontent would ignite a bloody uprising as it had done in Paris.1 This passage is a classic example of the literary device of parallelism that so perfectly expressed Dickens’ reflections on the trajectory of the unprecedented historical impact of the French Revolution. A parallelism that also aptly captures the contemporary contrasts and comparisons of the best and worst of 2020.
It is estimated that at least 66% of those eligible to vote did so on November 3, 2020, the highest turnout in more than a century, demonstrating the strength of the United States as a representative democracy.2 It is not about partisan politics, it is that more than 150 million citizens braved the winter, the virus, and potential intimidation to cast a ballot for their values.3 Still, America has never been more divided, and Dickens’ fear of political upheaval has never been more real in our country, or at least since the Civil War.
As I write this editorial, manufacturers for 2 vaccines have submitted phase 3 trial data to the US Food and Drug Administration for Emergency Use Authorizations and a third consortium may follow suit soon. Scientists report that the 2 vaccines, which were developed in less than a year, have high efficacy rates (> 90%) with only modest adverse effects.4 It is an unparalleled, really unimaginable, scientific feat. Americans’ characteristic gift for logistical efficiency and scientific innovation faces daunting administrative and technical barriers to achieve a similar viral victory, yet we may have faced even more formidable odds in World War II.
As of December 4, 2020, Johns Hopkins University reports that more than 275,000 Americans have died of coronavirus.5 The United States is on track to reach 200,000 cases a day with the signature holiday season of family festivities brutally morphed into gatherings of contagion.6 Hospitals across the country are running out of intensive care beds and nurses and doctors to staff them. Unlike the Spring surge in the Northeast, cases are rising in 49 states, and there is nowhere in the land from which respite and reinforcements can come.7
Thousands of health care professionals are exhausted, many with COVID-19 or recovering from it, morally distressed, and emotionally spent. Masks and social distancing are no longer public health essentials but elements of a culture war. Those same nurses, doctors, and public health officers still show up day after night for what is much closer to war than work. They struggle to prevent patients from going on ventilators they may never come off and use the few available therapies to keep as many patients alive as possible—whether those patients believe in COVID-19, wore a mask, no matter who they voted for—because that is what it means to practice health care according to a code of ethics.
In March 2020, I pledged to devote every editorial to COVID-19 for as long as the pandemic lasted, as one small candle for all those who have died of COVID-19, who are suffering as survivors of it, and who take risks and labor to deliver essential services from groceries to intensive care. Prudent public health officials wisely advise that the vaccine(s) are not a miracle cure to revive a depleted country, in part because it may undermine life-saving public health measures.8 And so the columns will continue in 2021 to illuminate the ethical issues of the pandemic as they affect all of us as federal health care professionals and Americans.
The Tale of Two Cities chapter that begins with the “best of times, and the worst of times” is entitled “Recalled to Life.” Let that be our hope and prayer for the coming year.
1. Dickens C. A Tale of Two Cities. Douglas-Fairhust ed. New York: Norton; 2020.
2. Schaul K, Rabinowitz K, Mellnik T. 2020 turnout is the highest in over a century. Washington Post, November 5, 2020. https://www.washingtonpost.com/graphics/2020/elections/voter-turnout. Accessed November 23, 2020.
3. Desilver D. In past elections, U.S. trailed most developed countries in voter turnout. https://www.pewresearch.org/fact-tank/2020/11/03/in-past-elections-u-s-trailed-most-developed-countries-in-voter-turnout. Published November 3, 2020. Accessed November 23, 2020.
4. Herper M, Garde D. Moderna to submit Covid-19 vaccine to FDA as full results show 94% efficacy.https://www.statnews.com/2020/11/30/moderna-covid-19-vaccine-full-results. Published November 30, 2020. Accessed November 30, 2020.
5. Johns Hopkins University and Medicine. Coronavirus research center. https://coronavirus.jhu.edu. Updated November 23, 2020. Accessed December 4, 2020.
6. Hawkins D, Knowles H. As U.S. coronavirus cases soar toward 200,000 a day holiday travel is surging. Washington Post, November 21, 2020. https://www.washingtonpost.com/health/2020/11/21/coronavirus-thanksgiving-travel. Accessed November 23, 2020.
7. Goldhill O. ‘People are going to die’: Hospitals in half the states are facing massive staffing shortages as COVID-19 surges. November 19, 2020. https://www.statnews.com/2020/11/19/covid19-hospitals-in-half-the-states-facing-massive-staffing-shortage. Published November 19, 2020. Accessed November 23, 2020.
8. Lazar K. Is Pfizer’s vaccine a ‘magic bullet?’ Scientists warn masks, distancing may last well into 2021. Boston Globe . November 9, 2020. https://www.bostonglobe.com/2020/11/09/metro/is-pfizer-vaccine-magic-bullet-scientists-warn-public-should-be-prepared-live-with-masks-social-distancing-months. Accessed November 23, 2020.
1. Dickens C. A Tale of Two Cities. Douglas-Fairhust ed. New York: Norton; 2020.
2. Schaul K, Rabinowitz K, Mellnik T. 2020 turnout is the highest in over a century. Washington Post, November 5, 2020. https://www.washingtonpost.com/graphics/2020/elections/voter-turnout. Accessed November 23, 2020.
3. Desilver D. In past elections, U.S. trailed most developed countries in voter turnout. https://www.pewresearch.org/fact-tank/2020/11/03/in-past-elections-u-s-trailed-most-developed-countries-in-voter-turnout. Published November 3, 2020. Accessed November 23, 2020.
4. Herper M, Garde D. Moderna to submit Covid-19 vaccine to FDA as full results show 94% efficacy.https://www.statnews.com/2020/11/30/moderna-covid-19-vaccine-full-results. Published November 30, 2020. Accessed November 30, 2020.
5. Johns Hopkins University and Medicine. Coronavirus research center. https://coronavirus.jhu.edu. Updated November 23, 2020. Accessed December 4, 2020.
6. Hawkins D, Knowles H. As U.S. coronavirus cases soar toward 200,000 a day holiday travel is surging. Washington Post, November 21, 2020. https://www.washingtonpost.com/health/2020/11/21/coronavirus-thanksgiving-travel. Accessed November 23, 2020.
7. Goldhill O. ‘People are going to die’: Hospitals in half the states are facing massive staffing shortages as COVID-19 surges. November 19, 2020. https://www.statnews.com/2020/11/19/covid19-hospitals-in-half-the-states-facing-massive-staffing-shortage. Published November 19, 2020. Accessed November 23, 2020.
8. Lazar K. Is Pfizer’s vaccine a ‘magic bullet?’ Scientists warn masks, distancing may last well into 2021. Boston Globe . November 9, 2020. https://www.bostonglobe.com/2020/11/09/metro/is-pfizer-vaccine-magic-bullet-scientists-warn-public-should-be-prepared-live-with-masks-social-distancing-months. Accessed November 23, 2020.
Prophylactic antibiotics for myomectomy?
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
FDA clears first drug for rare genetic causes of severe obesity
The Food and Drug Administration has approved setmelanotide (Imcivree, Rhythm Pharmaceuticals) for weight management in adults and children as young as 6 years with obesity because of proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing.
Individuals with these rare genetic causes of severe obesity have a normal weight at birth but develop persistent severe obesity within months because of insatiable hunger (hyperphagia).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for these disorders.
“Many patients and families who live with these diseases face an often-burdensome stigma associated with severe obesity. To manage this obesity and control disruptive food-seeking behavior, caregivers often lock cabinets and refrigerators and significantly limit social activities,” said Jennifer Miller, MD, a pediatric endocrinologist at University of Florida Health, Gainesville, in a press release issued by the company.
“This FDA approval marks an important turning point, providing a much needed therapy and supporting the use of genetic testing to identify and properly diagnose patients with these rare genetic diseases of obesity,” she noted.
David Meeker, MD, chair, president, and CEO of Rhythm Pharmaceuticals, added: “We are advancing a first-in-class, precision medicine that is designed to directly address the underlying cause of obesities driven by genetic deficits in the MC4R pathway.”
Setmelanotide was evaluated in two phase 3 clinical trials. In one trial, 80% of patients with obesity caused by POMC or PCSK1 deficiency achieved greater than 10% weight loss after 1 year of treatment.
In the other trial, 45.5% of patients with obesity caused by LEPR deficiency achieved greater than 10% weight loss with 1 year of treatment.
Results for the two trials were recently published in The Lancet Diabetes & Endocrinology and discussed at the ObesityWeek Interactive 2020 meeting.
Setmelanotide was generally well tolerated in both trials. The most common adverse events were injection-site reactions, skin hyperpigmentation, and nausea.
The drug label notes that disturbances in sexual arousal, depression, and suicidal ideation; skin pigmentation; and darkening of preexisting nevi may occur with setmelanotide treatment.
The drug label also notes a risk for serious adverse reactions because of benzyl alcohol preservative in neonates and low-birth-weight infants. Setmelanotide is not approved for use in neonates or infants.
The company expects the drug to be commercially available in the United States in the first quarter of 2021.
Setmelanotide for the treatment of obesity associated with rare genetic defects had FDA breakthrough therapy designation as well as orphan drug designation.
The company is also evaluating setmelanotide for reduction in hunger and body weight in a pivotal phase 3 trial in people living with Bardet-Biedl or Alström syndrome, and top-line data are due soon.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved setmelanotide (Imcivree, Rhythm Pharmaceuticals) for weight management in adults and children as young as 6 years with obesity because of proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing.
Individuals with these rare genetic causes of severe obesity have a normal weight at birth but develop persistent severe obesity within months because of insatiable hunger (hyperphagia).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for these disorders.
“Many patients and families who live with these diseases face an often-burdensome stigma associated with severe obesity. To manage this obesity and control disruptive food-seeking behavior, caregivers often lock cabinets and refrigerators and significantly limit social activities,” said Jennifer Miller, MD, a pediatric endocrinologist at University of Florida Health, Gainesville, in a press release issued by the company.
“This FDA approval marks an important turning point, providing a much needed therapy and supporting the use of genetic testing to identify and properly diagnose patients with these rare genetic diseases of obesity,” she noted.
David Meeker, MD, chair, president, and CEO of Rhythm Pharmaceuticals, added: “We are advancing a first-in-class, precision medicine that is designed to directly address the underlying cause of obesities driven by genetic deficits in the MC4R pathway.”
Setmelanotide was evaluated in two phase 3 clinical trials. In one trial, 80% of patients with obesity caused by POMC or PCSK1 deficiency achieved greater than 10% weight loss after 1 year of treatment.
In the other trial, 45.5% of patients with obesity caused by LEPR deficiency achieved greater than 10% weight loss with 1 year of treatment.
Results for the two trials were recently published in The Lancet Diabetes & Endocrinology and discussed at the ObesityWeek Interactive 2020 meeting.
Setmelanotide was generally well tolerated in both trials. The most common adverse events were injection-site reactions, skin hyperpigmentation, and nausea.
The drug label notes that disturbances in sexual arousal, depression, and suicidal ideation; skin pigmentation; and darkening of preexisting nevi may occur with setmelanotide treatment.
The drug label also notes a risk for serious adverse reactions because of benzyl alcohol preservative in neonates and low-birth-weight infants. Setmelanotide is not approved for use in neonates or infants.
The company expects the drug to be commercially available in the United States in the first quarter of 2021.
Setmelanotide for the treatment of obesity associated with rare genetic defects had FDA breakthrough therapy designation as well as orphan drug designation.
The company is also evaluating setmelanotide for reduction in hunger and body weight in a pivotal phase 3 trial in people living with Bardet-Biedl or Alström syndrome, and top-line data are due soon.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved setmelanotide (Imcivree, Rhythm Pharmaceuticals) for weight management in adults and children as young as 6 years with obesity because of proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing.
Individuals with these rare genetic causes of severe obesity have a normal weight at birth but develop persistent severe obesity within months because of insatiable hunger (hyperphagia).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for these disorders.
“Many patients and families who live with these diseases face an often-burdensome stigma associated with severe obesity. To manage this obesity and control disruptive food-seeking behavior, caregivers often lock cabinets and refrigerators and significantly limit social activities,” said Jennifer Miller, MD, a pediatric endocrinologist at University of Florida Health, Gainesville, in a press release issued by the company.
“This FDA approval marks an important turning point, providing a much needed therapy and supporting the use of genetic testing to identify and properly diagnose patients with these rare genetic diseases of obesity,” she noted.
David Meeker, MD, chair, president, and CEO of Rhythm Pharmaceuticals, added: “We are advancing a first-in-class, precision medicine that is designed to directly address the underlying cause of obesities driven by genetic deficits in the MC4R pathway.”
Setmelanotide was evaluated in two phase 3 clinical trials. In one trial, 80% of patients with obesity caused by POMC or PCSK1 deficiency achieved greater than 10% weight loss after 1 year of treatment.
In the other trial, 45.5% of patients with obesity caused by LEPR deficiency achieved greater than 10% weight loss with 1 year of treatment.
Results for the two trials were recently published in The Lancet Diabetes & Endocrinology and discussed at the ObesityWeek Interactive 2020 meeting.
Setmelanotide was generally well tolerated in both trials. The most common adverse events were injection-site reactions, skin hyperpigmentation, and nausea.
The drug label notes that disturbances in sexual arousal, depression, and suicidal ideation; skin pigmentation; and darkening of preexisting nevi may occur with setmelanotide treatment.
The drug label also notes a risk for serious adverse reactions because of benzyl alcohol preservative in neonates and low-birth-weight infants. Setmelanotide is not approved for use in neonates or infants.
The company expects the drug to be commercially available in the United States in the first quarter of 2021.
Setmelanotide for the treatment of obesity associated with rare genetic defects had FDA breakthrough therapy designation as well as orphan drug designation.
The company is also evaluating setmelanotide for reduction in hunger and body weight in a pivotal phase 3 trial in people living with Bardet-Biedl or Alström syndrome, and top-line data are due soon.
A version of this article originally appeared on Medscape.com.
VTE prophylaxis is feasible, effective in some high-risk cancer patients
Primary thromboprophylaxis is feasible and worth considering for high-risk ambulatory patients with cancer who are initiating systemic chemotherapy, according to Marc Carrier, MD.
Risk scores can identify patients at high risk for venous thromboembolism (VTE), and treatments that are effective and associated with low bleeding risk are available, Dr. Carrier explained at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
However, caution is advised in patients with certain types of cancer, including some gastrointestinal and genitourinary cancers, because of the possibility of increased major and clinically relevant nonmajor bleeding risk, he said.
VTE and cancer
VTE is relatively rare in the general population, occurring in about 1 or 2 per 1,000 people annually. The risk increases 4.1-fold in patients with cancer, and 6.5-fold in patients with cancer receiving chemotherapy.
“So just putting these numbers together, we’re no longer talking about 1 in 1,000, but 1 in 200, so [this is] something that is very common among cancer patients,” said Dr. Carrier, a professor at the University of Ottawa and chief of the division of hematology at The Ottawa Hospital.
The mortality rate associated with cancer-associated thrombosis is about 9%, comparable to that associated with infection in the cancer outpatient setting, which underscores the importance of educating patients about the signs and symptoms of VTE so they can seek medical treatment quickly if necessary, he added.
It may also be useful to discuss prophylaxis or other ways to prevent venous thromboembolic complications with certain patients, he said, noting that in an observational cohort study of nearly 600 patients at the University of Ottawa, 25% of those initiating chemotherapy were identified as intermediate or high risk using the validated Khorana risk score, and thus would likely benefit from thromboprophylaxis.
Risk assessment
The Khorana risk score assesses VTE risk based on cancer site, blood counts, and body mass index. It is simple to use and has been validated in more than 20,000 people in multiple countries, Dr. Carrier said.
In a well-known validation study, Ay et al. showed a VTE complication rate of 10% in patients with a Khorana risk score of 2 or higher who were followed up to 6 months.
“This is huge,” Dr. Carrier stressed. “This is much higher than what we tolerate for all sorts of different populations for which we would recommend anticoagulation or thromboprophylaxis.”
The question is whether the risk score can be helpful in a real-world clinic setting, he said, adding: “I’d like to think the answer to that is yes.”
In the University of Ottawa cohort study, 11% of high-risk patients experienced a VTE complication, compared with 4% of those with lower risk, suggesting that the validation data for the Khorana risk score is not only accurate, it is “actually applicable in real-world practice, and you can use it in your own center,” he said.
Further, recent studies have demonstrated that treatment based on Khorana risk score assessment reduces VTE complications.
Prophylaxis options
Low-molecular-weight heparin (LMWH) has been shown in several studies to be associated with a significant relative VTE risk reduction in patients with cancer initiating chemotherapy – with only a slight, nonsignificant increase in the risk of major bleeding.
However, the absolute benefit was small, and LMWH is “parenteral, relatively costly, and, based on that, although we showed relatively good risk-benefit ratio, it never really got translated to clinical practice,” Dr. Carrier said.
In fact, a 2015 American Society of Clinical Oncology guidelines update recommended against routine thromboprophylaxis in this setting, but stated that it could be considered in select high-risk patients identified using a validated risk-assessment tool.
The guidelines noted that “individual risk factors such as biomarkers and cancer site don’t reliably identify high-risk patients.”
More recent data provide additional support for risk assessment and treatment based on Khorana risk score of 2 or higher.
The AVERT trial, for which Dr. Carrier was the first author, showed that the direct-acting oral anticoagulant (DOAC) apixaban reduced VTE incidence, compared with placebo, in patients with Khorana score of 2 or higher (4.2% vs. 10.2%; hazard ratio, 0.41 overall, and 1.0 vs. 7.3; HR, 0.14 on treatment), and the CASSINI trial showed that another DOAC, rivaroxaban, reduced VTE incidence, compared with placebo, in those with Khorana score of 2 or higher (5.9 vs. 6.7; HR, 0.6 overall, and 2.6 vs. 6.4; HR, 0.40 on treatment). The differences in the on-treatment populations were statistically significant.
The two trials, which included a variety of tumor types, showed similar rates of major bleeding, with an absolute difference of about 1% between treatment and placebo, which was not statistically significant in the on-treatment analyses (HR, 1.89 in AVERT and HR, 1.96 in CASSINI).
A systematic review of these trials showed an overall significant decrease in VTE complication risk with treatment in high-risk patients, and a nonstatistically significant major bleeding risk increase.
Based on these findings, ASCO guidelines were updated in 2020 to state that “routine thromboprophylaxis should not be offered to all patients with cancer. ... However, high-risk outpatients with cancer may be offered thromboprophylaxis with apixaban, rivaroxaban or LMWH, providing there are no significant risk factors for bleeding or drug-drug interactions, and after having a full discussion with patients ... to make sure they understand the risk-benefit ratio and the rationale for that particular recommendation,” he said.
Real-world implementation
Implementing this approach in the clinic setting requires a practical model, such as the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC) program, a prospective quality improvement research initiative developed in collaboration with the Jeffords Institute for Quality at the University of Vermont Medical Center and described in a recent report, Dr. Carrier said.
The “Vermont model” is “really a comprehensive model that includes identifying patients with the electronic medical records, gathering the formal education and insight from other health care providers like pharmacists and nurses in order to really come up with personalized care for your patients,” he explained.
In 918 outpatients with cancer who were included in the program, VTE awareness increased from less than 5% before VTEPACC to nearly 82% during the implementation phase and 94.7% after 2 years, with nearly 94% of high-risk patients receiving VTE prophylaxis at that time.
“So we can certainly do that in our own center.” he said. “It’s a matter of coming up with the model and making sure that the patients are seen at the right time.”
Given the high frequency of VTE in patients with cancer initiating chemotherapy, the usefulness of risk scores such as the Khorana risk score for identifying those at high risk, and the availability of safe and effective interventions for reducing risk, “we should probably use the data and incorporate them into clinical practice by implementation of programs for primary prevention,” he said.
A word of caution
Caution is warranted, however, when it comes to using DOACs in patients with higher-risk or potentially higher-risk tumor types, he added.
“It’s an important question we are facing as clinicians on a daily basis,” he said, responding to an attendee’s query, as shared by session moderator James Douketis, MD, professor of medicine at McMaster University, Hamilton, Ont., regarding possible bleeding risks in certain genitourinary cancers.
A recent meta-analysis published in Nature, for example, noted that, in the SELECT-D trial, rivaroxaban was associated with significantly higher incidence of clinically relevant nonmajor bleeding, most often in bladder and colorectal cancers, and most often at genitourinary and gastrointestinal sites.
Both Dr. Carrier and fellow panelist Michael Streiff, MD, professor of medicine at Johns Hopkins University and medical director at the Johns Hopkins Hospital Special Coagulation Laboratory, Baltimore, said they approach DOAC use cautiously, but don’t rule it out entirely, in patients with unresected genitourinary tumors that could pose a risk of bleeding.
“It’s worth mentioning and being cautious. In my own personal practice, I’m very careful with unresected urothelial-type tumors or, for example, bladder cancer, for the same reason as [with] unresected luminal GI tumors,” Dr. Carrier said, adding that he’s also mindful that patients with nephropathy were excluded from U.S. DOAC trials because of bleeding risk.
He said he sometimes tries a LMWH challenge first in higher-risk patients, and then might try a DOAC if no bleeding occurs.
“But it certainly is controversial,” he noted.
Dr. Streiff added that he also worries less with genitourinary cancers than with upper GI lesions because “the signals weren’t as big as in GI” cancers, but he noted that “the drugs are going out through the kidneys ... so I’m cautious in those populations.”
“So caution, but not complete exclusion, is the operative management,” Dr. Douketis said, summarizing the panelists’ consensus.
Dr. Carrier reported clinical trial or advisory board participation for Bayer, Pfizer, Servier, Leo Pharma, and/or BMS.
Primary thromboprophylaxis is feasible and worth considering for high-risk ambulatory patients with cancer who are initiating systemic chemotherapy, according to Marc Carrier, MD.
Risk scores can identify patients at high risk for venous thromboembolism (VTE), and treatments that are effective and associated with low bleeding risk are available, Dr. Carrier explained at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
However, caution is advised in patients with certain types of cancer, including some gastrointestinal and genitourinary cancers, because of the possibility of increased major and clinically relevant nonmajor bleeding risk, he said.
VTE and cancer
VTE is relatively rare in the general population, occurring in about 1 or 2 per 1,000 people annually. The risk increases 4.1-fold in patients with cancer, and 6.5-fold in patients with cancer receiving chemotherapy.
“So just putting these numbers together, we’re no longer talking about 1 in 1,000, but 1 in 200, so [this is] something that is very common among cancer patients,” said Dr. Carrier, a professor at the University of Ottawa and chief of the division of hematology at The Ottawa Hospital.
The mortality rate associated with cancer-associated thrombosis is about 9%, comparable to that associated with infection in the cancer outpatient setting, which underscores the importance of educating patients about the signs and symptoms of VTE so they can seek medical treatment quickly if necessary, he added.
It may also be useful to discuss prophylaxis or other ways to prevent venous thromboembolic complications with certain patients, he said, noting that in an observational cohort study of nearly 600 patients at the University of Ottawa, 25% of those initiating chemotherapy were identified as intermediate or high risk using the validated Khorana risk score, and thus would likely benefit from thromboprophylaxis.
Risk assessment
The Khorana risk score assesses VTE risk based on cancer site, blood counts, and body mass index. It is simple to use and has been validated in more than 20,000 people in multiple countries, Dr. Carrier said.
In a well-known validation study, Ay et al. showed a VTE complication rate of 10% in patients with a Khorana risk score of 2 or higher who were followed up to 6 months.
“This is huge,” Dr. Carrier stressed. “This is much higher than what we tolerate for all sorts of different populations for which we would recommend anticoagulation or thromboprophylaxis.”
The question is whether the risk score can be helpful in a real-world clinic setting, he said, adding: “I’d like to think the answer to that is yes.”
In the University of Ottawa cohort study, 11% of high-risk patients experienced a VTE complication, compared with 4% of those with lower risk, suggesting that the validation data for the Khorana risk score is not only accurate, it is “actually applicable in real-world practice, and you can use it in your own center,” he said.
Further, recent studies have demonstrated that treatment based on Khorana risk score assessment reduces VTE complications.
Prophylaxis options
Low-molecular-weight heparin (LMWH) has been shown in several studies to be associated with a significant relative VTE risk reduction in patients with cancer initiating chemotherapy – with only a slight, nonsignificant increase in the risk of major bleeding.
However, the absolute benefit was small, and LMWH is “parenteral, relatively costly, and, based on that, although we showed relatively good risk-benefit ratio, it never really got translated to clinical practice,” Dr. Carrier said.
In fact, a 2015 American Society of Clinical Oncology guidelines update recommended against routine thromboprophylaxis in this setting, but stated that it could be considered in select high-risk patients identified using a validated risk-assessment tool.
The guidelines noted that “individual risk factors such as biomarkers and cancer site don’t reliably identify high-risk patients.”
More recent data provide additional support for risk assessment and treatment based on Khorana risk score of 2 or higher.
The AVERT trial, for which Dr. Carrier was the first author, showed that the direct-acting oral anticoagulant (DOAC) apixaban reduced VTE incidence, compared with placebo, in patients with Khorana score of 2 or higher (4.2% vs. 10.2%; hazard ratio, 0.41 overall, and 1.0 vs. 7.3; HR, 0.14 on treatment), and the CASSINI trial showed that another DOAC, rivaroxaban, reduced VTE incidence, compared with placebo, in those with Khorana score of 2 or higher (5.9 vs. 6.7; HR, 0.6 overall, and 2.6 vs. 6.4; HR, 0.40 on treatment). The differences in the on-treatment populations were statistically significant.
The two trials, which included a variety of tumor types, showed similar rates of major bleeding, with an absolute difference of about 1% between treatment and placebo, which was not statistically significant in the on-treatment analyses (HR, 1.89 in AVERT and HR, 1.96 in CASSINI).
A systematic review of these trials showed an overall significant decrease in VTE complication risk with treatment in high-risk patients, and a nonstatistically significant major bleeding risk increase.
Based on these findings, ASCO guidelines were updated in 2020 to state that “routine thromboprophylaxis should not be offered to all patients with cancer. ... However, high-risk outpatients with cancer may be offered thromboprophylaxis with apixaban, rivaroxaban or LMWH, providing there are no significant risk factors for bleeding or drug-drug interactions, and after having a full discussion with patients ... to make sure they understand the risk-benefit ratio and the rationale for that particular recommendation,” he said.
Real-world implementation
Implementing this approach in the clinic setting requires a practical model, such as the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC) program, a prospective quality improvement research initiative developed in collaboration with the Jeffords Institute for Quality at the University of Vermont Medical Center and described in a recent report, Dr. Carrier said.
The “Vermont model” is “really a comprehensive model that includes identifying patients with the electronic medical records, gathering the formal education and insight from other health care providers like pharmacists and nurses in order to really come up with personalized care for your patients,” he explained.
In 918 outpatients with cancer who were included in the program, VTE awareness increased from less than 5% before VTEPACC to nearly 82% during the implementation phase and 94.7% after 2 years, with nearly 94% of high-risk patients receiving VTE prophylaxis at that time.
“So we can certainly do that in our own center.” he said. “It’s a matter of coming up with the model and making sure that the patients are seen at the right time.”
Given the high frequency of VTE in patients with cancer initiating chemotherapy, the usefulness of risk scores such as the Khorana risk score for identifying those at high risk, and the availability of safe and effective interventions for reducing risk, “we should probably use the data and incorporate them into clinical practice by implementation of programs for primary prevention,” he said.
A word of caution
Caution is warranted, however, when it comes to using DOACs in patients with higher-risk or potentially higher-risk tumor types, he added.
“It’s an important question we are facing as clinicians on a daily basis,” he said, responding to an attendee’s query, as shared by session moderator James Douketis, MD, professor of medicine at McMaster University, Hamilton, Ont., regarding possible bleeding risks in certain genitourinary cancers.
A recent meta-analysis published in Nature, for example, noted that, in the SELECT-D trial, rivaroxaban was associated with significantly higher incidence of clinically relevant nonmajor bleeding, most often in bladder and colorectal cancers, and most often at genitourinary and gastrointestinal sites.
Both Dr. Carrier and fellow panelist Michael Streiff, MD, professor of medicine at Johns Hopkins University and medical director at the Johns Hopkins Hospital Special Coagulation Laboratory, Baltimore, said they approach DOAC use cautiously, but don’t rule it out entirely, in patients with unresected genitourinary tumors that could pose a risk of bleeding.
“It’s worth mentioning and being cautious. In my own personal practice, I’m very careful with unresected urothelial-type tumors or, for example, bladder cancer, for the same reason as [with] unresected luminal GI tumors,” Dr. Carrier said, adding that he’s also mindful that patients with nephropathy were excluded from U.S. DOAC trials because of bleeding risk.
He said he sometimes tries a LMWH challenge first in higher-risk patients, and then might try a DOAC if no bleeding occurs.
“But it certainly is controversial,” he noted.
Dr. Streiff added that he also worries less with genitourinary cancers than with upper GI lesions because “the signals weren’t as big as in GI” cancers, but he noted that “the drugs are going out through the kidneys ... so I’m cautious in those populations.”
“So caution, but not complete exclusion, is the operative management,” Dr. Douketis said, summarizing the panelists’ consensus.
Dr. Carrier reported clinical trial or advisory board participation for Bayer, Pfizer, Servier, Leo Pharma, and/or BMS.
Primary thromboprophylaxis is feasible and worth considering for high-risk ambulatory patients with cancer who are initiating systemic chemotherapy, according to Marc Carrier, MD.
Risk scores can identify patients at high risk for venous thromboembolism (VTE), and treatments that are effective and associated with low bleeding risk are available, Dr. Carrier explained at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
However, caution is advised in patients with certain types of cancer, including some gastrointestinal and genitourinary cancers, because of the possibility of increased major and clinically relevant nonmajor bleeding risk, he said.
VTE and cancer
VTE is relatively rare in the general population, occurring in about 1 or 2 per 1,000 people annually. The risk increases 4.1-fold in patients with cancer, and 6.5-fold in patients with cancer receiving chemotherapy.
“So just putting these numbers together, we’re no longer talking about 1 in 1,000, but 1 in 200, so [this is] something that is very common among cancer patients,” said Dr. Carrier, a professor at the University of Ottawa and chief of the division of hematology at The Ottawa Hospital.
The mortality rate associated with cancer-associated thrombosis is about 9%, comparable to that associated with infection in the cancer outpatient setting, which underscores the importance of educating patients about the signs and symptoms of VTE so they can seek medical treatment quickly if necessary, he added.
It may also be useful to discuss prophylaxis or other ways to prevent venous thromboembolic complications with certain patients, he said, noting that in an observational cohort study of nearly 600 patients at the University of Ottawa, 25% of those initiating chemotherapy were identified as intermediate or high risk using the validated Khorana risk score, and thus would likely benefit from thromboprophylaxis.
Risk assessment
The Khorana risk score assesses VTE risk based on cancer site, blood counts, and body mass index. It is simple to use and has been validated in more than 20,000 people in multiple countries, Dr. Carrier said.
In a well-known validation study, Ay et al. showed a VTE complication rate of 10% in patients with a Khorana risk score of 2 or higher who were followed up to 6 months.
“This is huge,” Dr. Carrier stressed. “This is much higher than what we tolerate for all sorts of different populations for which we would recommend anticoagulation or thromboprophylaxis.”
The question is whether the risk score can be helpful in a real-world clinic setting, he said, adding: “I’d like to think the answer to that is yes.”
In the University of Ottawa cohort study, 11% of high-risk patients experienced a VTE complication, compared with 4% of those with lower risk, suggesting that the validation data for the Khorana risk score is not only accurate, it is “actually applicable in real-world practice, and you can use it in your own center,” he said.
Further, recent studies have demonstrated that treatment based on Khorana risk score assessment reduces VTE complications.
Prophylaxis options
Low-molecular-weight heparin (LMWH) has been shown in several studies to be associated with a significant relative VTE risk reduction in patients with cancer initiating chemotherapy – with only a slight, nonsignificant increase in the risk of major bleeding.
However, the absolute benefit was small, and LMWH is “parenteral, relatively costly, and, based on that, although we showed relatively good risk-benefit ratio, it never really got translated to clinical practice,” Dr. Carrier said.
In fact, a 2015 American Society of Clinical Oncology guidelines update recommended against routine thromboprophylaxis in this setting, but stated that it could be considered in select high-risk patients identified using a validated risk-assessment tool.
The guidelines noted that “individual risk factors such as biomarkers and cancer site don’t reliably identify high-risk patients.”
More recent data provide additional support for risk assessment and treatment based on Khorana risk score of 2 or higher.
The AVERT trial, for which Dr. Carrier was the first author, showed that the direct-acting oral anticoagulant (DOAC) apixaban reduced VTE incidence, compared with placebo, in patients with Khorana score of 2 or higher (4.2% vs. 10.2%; hazard ratio, 0.41 overall, and 1.0 vs. 7.3; HR, 0.14 on treatment), and the CASSINI trial showed that another DOAC, rivaroxaban, reduced VTE incidence, compared with placebo, in those with Khorana score of 2 or higher (5.9 vs. 6.7; HR, 0.6 overall, and 2.6 vs. 6.4; HR, 0.40 on treatment). The differences in the on-treatment populations were statistically significant.
The two trials, which included a variety of tumor types, showed similar rates of major bleeding, with an absolute difference of about 1% between treatment and placebo, which was not statistically significant in the on-treatment analyses (HR, 1.89 in AVERT and HR, 1.96 in CASSINI).
A systematic review of these trials showed an overall significant decrease in VTE complication risk with treatment in high-risk patients, and a nonstatistically significant major bleeding risk increase.
Based on these findings, ASCO guidelines were updated in 2020 to state that “routine thromboprophylaxis should not be offered to all patients with cancer. ... However, high-risk outpatients with cancer may be offered thromboprophylaxis with apixaban, rivaroxaban or LMWH, providing there are no significant risk factors for bleeding or drug-drug interactions, and after having a full discussion with patients ... to make sure they understand the risk-benefit ratio and the rationale for that particular recommendation,” he said.
Real-world implementation
Implementing this approach in the clinic setting requires a practical model, such as the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC) program, a prospective quality improvement research initiative developed in collaboration with the Jeffords Institute for Quality at the University of Vermont Medical Center and described in a recent report, Dr. Carrier said.
The “Vermont model” is “really a comprehensive model that includes identifying patients with the electronic medical records, gathering the formal education and insight from other health care providers like pharmacists and nurses in order to really come up with personalized care for your patients,” he explained.
In 918 outpatients with cancer who were included in the program, VTE awareness increased from less than 5% before VTEPACC to nearly 82% during the implementation phase and 94.7% after 2 years, with nearly 94% of high-risk patients receiving VTE prophylaxis at that time.
“So we can certainly do that in our own center.” he said. “It’s a matter of coming up with the model and making sure that the patients are seen at the right time.”
Given the high frequency of VTE in patients with cancer initiating chemotherapy, the usefulness of risk scores such as the Khorana risk score for identifying those at high risk, and the availability of safe and effective interventions for reducing risk, “we should probably use the data and incorporate them into clinical practice by implementation of programs for primary prevention,” he said.
A word of caution
Caution is warranted, however, when it comes to using DOACs in patients with higher-risk or potentially higher-risk tumor types, he added.
“It’s an important question we are facing as clinicians on a daily basis,” he said, responding to an attendee’s query, as shared by session moderator James Douketis, MD, professor of medicine at McMaster University, Hamilton, Ont., regarding possible bleeding risks in certain genitourinary cancers.
A recent meta-analysis published in Nature, for example, noted that, in the SELECT-D trial, rivaroxaban was associated with significantly higher incidence of clinically relevant nonmajor bleeding, most often in bladder and colorectal cancers, and most often at genitourinary and gastrointestinal sites.
Both Dr. Carrier and fellow panelist Michael Streiff, MD, professor of medicine at Johns Hopkins University and medical director at the Johns Hopkins Hospital Special Coagulation Laboratory, Baltimore, said they approach DOAC use cautiously, but don’t rule it out entirely, in patients with unresected genitourinary tumors that could pose a risk of bleeding.
“It’s worth mentioning and being cautious. In my own personal practice, I’m very careful with unresected urothelial-type tumors or, for example, bladder cancer, for the same reason as [with] unresected luminal GI tumors,” Dr. Carrier said, adding that he’s also mindful that patients with nephropathy were excluded from U.S. DOAC trials because of bleeding risk.
He said he sometimes tries a LMWH challenge first in higher-risk patients, and then might try a DOAC if no bleeding occurs.
“But it certainly is controversial,” he noted.
Dr. Streiff added that he also worries less with genitourinary cancers than with upper GI lesions because “the signals weren’t as big as in GI” cancers, but he noted that “the drugs are going out through the kidneys ... so I’m cautious in those populations.”
“So caution, but not complete exclusion, is the operative management,” Dr. Douketis said, summarizing the panelists’ consensus.
Dr. Carrier reported clinical trial or advisory board participation for Bayer, Pfizer, Servier, Leo Pharma, and/or BMS.
FROM THE THSNA BIENNIAL SUMMIT
Vaginal cleansing protocol curbs deep SSIs after cesarean
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
FROM ACOG 2020
Patient with CKD: Contrast or no contrast?
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.
A 67-year-old man with stage 3 chronic kidney disease (CKD) develops abdominal pain over 24 hours. He has had low grade fevers and nausea. He has a history of colon cancer and had a resection four years ago. Abdominal exam reveals tenderness to palpation, including rebound tenderness in his right lower quadrant. Labs: hemoglobin: 13; hematocrit: 39; white blood cells: 18,000; platelets: 333; blood urea nitrogen: 28; creatinine: 1.8 (estimated glomerular filtration rate: 37); sodium: 136; potassium: 3.9; bicarbonate: 24; chlorine: 105; and lipase: 10.
What testing would you recommend?
A) Ultrasound
B) Non contrast computed tomography (CT)
C) Contrast CT
D) MRI without gadolinium
The correct answer here is to get a contrast CT scan, as it will give you the most appropriate diagnostic information.
For years, we have hesitated to order contrast studies in our patients with CKD, for fear of causing contrast-induced nephrotoxicity. We might choose less helpful studies that avoid contrast, or might not obtain imaging that is needed. Over the years I have especially seen this in the case of avoiding computed tomography angiography (CTA) for evaluation of pulmonary embolus and choosing the much less useful ventilation/perfusion scan. The problem arises with the fact that patients with CKD are more likely to develop worsening renal function when they get sick.
Lee and colleagues performed an analysis of six retrospective studies involving a total of 55,963 participants. They found that patients with CKD receiving contrast material did not have an increased risk of deteriorating renal function compared with those without CKD (odds ratio, 1.07; 95% confidence interval, 0.98-1.17).1
The early studies reporting contrast-induced renal disease were in patients who received high osmolality contrast agents.2 Most patients now receive low osmolality agents, with less nephrotoxicity.3
Key points of guidelines
This year, the American College of Radiology and the National Kidney Foundation put out joint guidelines that helped clarify why there is a diminished concern for contrast-induced kidney disease in the modern era.4 Below are some of the key points of these guidelines:
- The risk of contrast-induced acute kidney injury (AKI) from intravenous iodinated contrast media is lower than previously thought.
- Necessary contrast material–enhanced CT without a suitable alternative should not be avoided solely on the basis of contrast-induced chronic kidney insufficiency risk.
- Contrast-induced AKI risk should be determined primarily by using CKD stage and AKI.
- Patients at high risk for contrast-induced kidney injury include those with recent AKI and those with estimated glomerular filtration rate (eGFR) less than 30 mL/min per 1.73 m2.
Data supporting guidelines
The data from several studies used to support these recommendations were impressive, showing just how low the risk for contrast-induced AKI is in most patients. In these studies, the risk of contrast-induced AKI has been estimated to be near 0% for patients with an eGFR greater than or equal to 45 and 0%-2% for patients with an eGFR of 30-44.5-7 This information and recommendations make imaging much easier. In most of our patients, we can get contrast studies when we need them. The group to be concerned about are patients with eGFRs less than 30. The guidelines single out this group as the patients where risk/benefit needs to be calculated before proceeding with the study, and to use prophylactic saline hydration in patients not undergoing dialysis.
Myth: Contrast-induced renal disease is common.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lee YC et al. Contrast-induced acute kidney injury among patients with chronic kidney disease undergoing imaging studies: A meta-analysis. Am J Roentgenol. 2019 Oct;213(4):728-35.
2. Luk L et al. Intravenous contrast-induced nephropathy: The rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017 May;24(3):169-75.
3. Goldfarb S et al. Low-osmolality contrast media and the risk of contrast-associated nephrotoxicity. Invest Radiol. 1993;28(Suppl 5):7-10.
4. Davenport MS, et al. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020 Jan 22;2(1):85-93.
5. Davenport MS et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94-105.
6. McDonald RJ et al. Intravenous contrast material–induced nephropathy: Causal or coincident phenomenon? Radiology. 2013;267(1):106-18.
7. McDonald JS et al. Risk of intravenous contrast material–mediated acute kidney injury: A propensity scorematched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73.