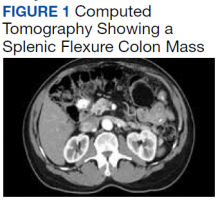
User login
Not so crazy: Pancreas transplants in type 2 diabetes rising
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
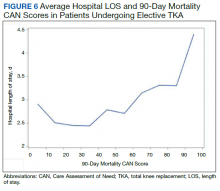
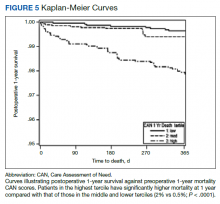
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Simultaneous
Traditionally, recipients of pancreas transplants have been people with type 1 diabetes who also have either chronic kidney disease (CKD) or hypoglycemic unawareness. The former group could receive either a simultaneous pancreas-kidney or a pancreas after kidney transplant, while the latter – if they have normal kidney function – would be eligible for a pancreas transplant alone.
But increasingly in recent years, patients with type 2 diabetes and CKD have been receiving simultaneous pancreas-kidney transplants, with similar success rates to those of people with type 1 diabetes.
Such candidates are typically sufficiently fit, not morbidly obese, and taking insulin regardless of their C-peptide status, said Jon S. Odorico, MD, professor of surgery and director of pancreas and islet transplantation at the University of Wisconsin–Madison Transplant Program.
“One might ask: Is it a crazy idea to do a pancreas transplant for patients with type 2 diabetes? Based on the known mechanisms of hyperglycemia in these patients, it might seem so,” he said, noting that while individuals with type 2 diabetes usually have insulin resistance, many also have relative or absolute deficiency of insulin production.
“So by replacing beta-cell mass, pancreas transplantation addresses this beta-cell defect mechanism,” he explained when discussing the topic during a symposium held June 26 at the virtual American Diabetes Association (ADA) 81st Scientific Sessions.
Arguments in favor of simultaneous pancreas-kidney transplant in people with type 2 diabetes and CKD include the fact that type 2 diabetes is the leading cause of kidney disease in the United States – roughly 50-60% of candidates on the kidney transplant waiting list also have type 2 diabetes – and that kidney transplant alone tends to worsen diabetes control due to the required immunosuppression.
Moreover, due to a 2014 allocation policy change that separates simultaneous pancreas-kidney from kidney transplant–alone donor organs, waiting times are shorter for the former, and kidney quality is generally better than for kidney transplant alone, unless a living kidney donor is available.
And, Dr. Odorico added, “adding a pancreas to a kidney transplant does not appear to jeopardize patient survival or kidney graft survival in appropriately selected patients with diabetes.” However, he also noted that because type 2 diabetes is so heterogeneous, ideal candidates for simultaneous pancreas-kidney transplant are not yet clear.
Currently, people with type 2 diabetes account for about 20% of those receiving simultaneous pancreas-kidney transplants and about 50% of pancreas after kidney transplants. Few pancreas transplants alone are performed in type 2 diabetes because those individuals rarely experience severe life-threatening hypoglycemia, Dr. Odorico explained.
Criteria have shifted over time, C-peptide removed in 2019
In an interview, symposium moderator Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program at the University of California, San Francisco, said he agreed that “it’s a surprising trend. It doesn’t make intuitive sense. In type 1 diabetes, it makes sense to replace the beta cells. But type 2 is due to a whole cluster of etiologies ... The view in the public domain is that it’s not due to the lack of insulin but problems with insulin resistance and obesity. So it doesn’t make a whole lot of sense to give you more insulin if it’s a receptor problem.”
But Dr. Stock noted that because in the past diabetes type wasn’t always rigorously assessed using C-peptide and antibody testing, which most centers measure today, “a number of transplants were done in people who turned out to have type 2. Our perception is that everybody who has type 2 is obese, but that’s not true anymore.”
Once it became apparent that some patients with type 2 diabetes who received pancreas transplants seemed to be doing well, the pancreas transplantation committee of the United Network for Organ Sharing (UNOS) established general criteria for the procedure in people with diabetes. They had to be taking insulin and have a C-peptide value of 2 ng/mL or below or taking insulin with a C-peptide greater than 2 ng/mL and a body mass index less than or equal to the maximum allowable BMI (28 kg/m2 at the time).
Dr. Stock, who chaired that committee from 2005 to 2007, said: “We thought it was risky to offer a scarce pool of donor pancreases to people with type 2 when we had people with type 1 who we know will benefit from it. So initially, the committee decided to limit pancreas transplantation to those with type 2 who have fairly low insulin requirements and BMIs that are more in the range of people with type 1. And lo and behold the results were comparable.”
Subsequent to Dr. Stock’s tenure as chair, the UNOS committee decided that the BMI and C-peptide criteria for simultaneous pancreas-kidney were no longer scientifically justifiable and were potentially discriminatory both to minority populations with type 2 diabetes and people with type 1 diabetes who have a high BMI, so in 2019, they removed them.
Individual transplant centers must follow UNOS rules, but they can also add their own criteria. Some don’t perform simultaneous pancreas-kidney transplants in people with type 2 diabetes at all.
At Dr. Odorico’s center, which began doing so in 2012, patients with type 2 diabetes account for nearly 40% of all simultaneous pancreas-kidney transplants. Indications there include age 20-60 years, insulin dependent with requirements less than 1 unit/kg/day, CKD stage 3-5, predialysis or on dialysis, and BMI <33 kg/m2.
“They are highly selected and a fairly fit group of patients,” Dr. Odorico noted.
Those who don’t meet all the requirements for simultaneous pancreas-kidney transplants may still be eligible for kidney transplant alone, from either a living or deceased donor, he said.
Dr. Stock’s criteria at UCSF are even more stringent for both BMI and insulin requirements.
SPK outcomes similar for type 1 and type 2 diabetes: Emerging data
Data to guide this area are accumulating slowly. Thus far, all studies have been retrospective and have used variable definitions for diabetes type and for graft failure. However, they’re fairly consistent in showing similar outcomes by diabetes type and little impact of C-peptide level on patient survival or survival of either kidney or pancreas graft, particularly after adjustment for confounding factors between the two types.
In a study from Dr. Odorico’s center of 284 type 1 and 39 type 2 diabetes patients undergoing simultaneous pancreas-kidney transplant between 2006 and 2017, pretransplant BMI and insulin requirements did not affect patient or graft survival in either type. There was a suggestion of greater risk for post-transplant diabetes with very high pretransplant insulin requirements (>75 units/day) but the numbers were too small to be definitive.
“It’s clear we will be doing more pancreas transplants in the future in this group of patients, and it’s ripe for further investigation,” Dr. Odorico concluded.
Beta cells for all?
Dr. Stock added one more aspect. While of course whole-organ transplantation is limited by the shortage of human donors, stem cell–derived beta cells could potentially produce an unlimited supply. Both Dr. Stock and Dr. Odorico are working on different approaches to this.
“We’re really close,” he said, noting, “the data we get for people with type 2 diabetes undergoing solid organ pancreas transplant could also be applied to cellular therapy ... We need to get a better understanding of which patients will benefit. The data we have so far are very promising.”
Dr. Odorico is scientific founder, stock equity holder, scientific advisory board chair, and a prior grant support recipient from Regenerative Medical Solutions. He has reported receiving clinical trial support from Veloxis Pharmaceuticals, CareDx, Natera, and Vertex Pharmaceuticals. Dr. Stock has reported being on the scientific advisory board of Encellin and receives funding from the California Institute of Regenerative Medicine and National Institutes of Health.
A version of this article first appeared on Medscape.com.
Most U.S. adults age 50+ report good health: Survey
a nonprofit hospice/advanced illness care organization based in Virginia.
Among the respondents, 41% said their health was very good or excellent.
However, the ratings differed largely by race, employment status, and income.
Employment status was also associated with a significant difference in the way people viewed their health at the top tier and bottom tier.
The middle tier (“good” health) was reported similarly (from 33% to 37%) whether a person was employed, retired, or not employed. However, employed respondents were much more likely to report they had “excellent” or “very good” health (51% vs. 44% for retirees and 21% for the not employed).
Conversely, those who were not employed were far more likely to report “fair” or “poor” health (45%) than those who were employed (13%) or retired (20%).
Similarly, respondents with incomes of less than $50,000 were three times more likely to report their health as “fair” or “poor” than were those with incomes of more than $100,000 (36% vs. 12%).
WebMD/CCH surveyed 3,464 U.S. residents ages 50 and older between Aug. 13 and Nov. 9, 2020. WebMD.com readers were randomly invited to take a 10-minute online survey.
Aging at home a priority
The survey also highlighted a strong preference for aging in place, says Steve Cone, chief of communications and philanthropy at CCH.
“More now than ever before, thanks to the COVID experience, baby boomers and their children really believe that’s the holy grail,” he says.
Mr. Cone notes that the quick spread of COVID-19 through some nursing homes early in the pandemic likely has strengthened people’s resolve to live out their lives in their own homes.
The survey indicated that 85% of people aged 50+ who are living in their own home, a family member’s home, or a loved one’s home responded that it is “very important” or “important” to stay in their home as they age.
When asked what services they would need to continue their living situation, the most common responses were housekeeping, home repair services, and transportation (listed as needs by 35% to 45% of respondents). Regarding changes they would have to make to feel safe in their home as they age, installing grab bars and/or safety rails in the bath/shower was the most popular answer (50%).
Use of telemedicine
Respondents were also asked about their acceptance of telemedicine, and 62% said they would be likely or very likely to engage in virtual visits with a doctor it in the future.
However, the likelihood varied by income level. Specifically, respondents with incomes over $100,000 were significantly more likely to say they would use telemedicine in the future than were those with incomes below $50,000 (74% vs. 60%). They were also more likely to already have used telemedicine.
Although respondents generally embraced telemedicine, they are less confident about some types of monitoring, according to Mr. Cone.
Emergency response (64%) was the leading type of remote monitoring respondents ages 50 and older would allow. Only a minority of respondents would allow the other types of monitoring asked about in the survey.
Close to one-quarter of respondents would not allow any type of monitoring.
Fewer than one-third would allow tracking of medication compliance, refrigerator use, sleep habits, or bathroom use.
People see monitoring of some movements as “Orwellian,” Mr. Cone says.
Knowledge of hospice
The survey findings support the need for more widespread use of hospice so people can stay in their homes as they age, Mr. Cone says.
When illness gets severe, “There’s no reason you have to get rushed to the emergency room or wind up in a hospital,” Mr. Cone says.
He notes that hospice and palliative care can come to patients wherever they reside – in their home, an assisted living center, a nursing home, or even a hospital room.
“That doesn’t mean the physician isn’t involved,” he says. “But working as a team, we can keep them in their homes and their lifestyle intact.”
Patients whose doctors attest that they are likely to live a maximum 6 months are eligible for hospice. But most families wait too long to long to start hospice or palliative care for a patient, Mr. Cone says, and may not be aware of what these services typically cover, including meal preparation and pet care.
In the survey, nearly one-third of respondents said they did not know that palliative care is something that “can be given at any stage of a serious illness” or “provides non-medical services (e.g., patient/family communication, help with insurance issues, scheduling appointments, arranging transportation).”
He notes palliative care and hospice are covered by Medicare and Medicaid and also by most private insurance plans or by individual companies providing the service.
However, health care providers may have to overcome a general reluctance to discuss hospice when sharing options for those severely ill.
The survey showed that while 51% of those 50 and older are at least “slightly interested” in learning more about hospice, a nearly equal number say they are “not at all interested” (49%).
Most using hospice are White
More than 90% of those surveyed reported that aspects of hospice care, including “comfort and relief from pain at the end of patients’ lives,” providing a dedicated care team, and an alternative to other care settings, are “very important” or “important.”
However, national hospice use rates are extremely low for minorities and the LGBTQ community, according to Mr. Cone. Among Medicare hospice recipients, 82% were white, 8.2% Black, 6.7% Hispanic, and 1.8% Asian or Pacific Islander, according to the National Hospice and Palliative Care Organization.
Those numbers signal a need for outreach to those communities with information on what services are available and how to access them, he says.
Health costs top concern
The survey also asked about level of concern regarding matters including family, health, financials, and end-of-life directives and found adults aged 50 and older expressed the greatest amount of concern for health care costs that are not covered by insurance.
More than half (56%) said they were concerned or very concerned about those costs, which was higher than the percentage concerned about losing a spouse (49%).
Respondents were less concerned (“slightly concerned” or “not at all concerned”) about their children living far away, planning end-of life-directives, and falling or having reduced mobility.
A version of this article first appeared on WebMD.com.
a nonprofit hospice/advanced illness care organization based in Virginia.
Among the respondents, 41% said their health was very good or excellent.
However, the ratings differed largely by race, employment status, and income.
Employment status was also associated with a significant difference in the way people viewed their health at the top tier and bottom tier.
The middle tier (“good” health) was reported similarly (from 33% to 37%) whether a person was employed, retired, or not employed. However, employed respondents were much more likely to report they had “excellent” or “very good” health (51% vs. 44% for retirees and 21% for the not employed).
Conversely, those who were not employed were far more likely to report “fair” or “poor” health (45%) than those who were employed (13%) or retired (20%).
Similarly, respondents with incomes of less than $50,000 were three times more likely to report their health as “fair” or “poor” than were those with incomes of more than $100,000 (36% vs. 12%).
WebMD/CCH surveyed 3,464 U.S. residents ages 50 and older between Aug. 13 and Nov. 9, 2020. WebMD.com readers were randomly invited to take a 10-minute online survey.
Aging at home a priority
The survey also highlighted a strong preference for aging in place, says Steve Cone, chief of communications and philanthropy at CCH.
“More now than ever before, thanks to the COVID experience, baby boomers and their children really believe that’s the holy grail,” he says.
Mr. Cone notes that the quick spread of COVID-19 through some nursing homes early in the pandemic likely has strengthened people’s resolve to live out their lives in their own homes.
The survey indicated that 85% of people aged 50+ who are living in their own home, a family member’s home, or a loved one’s home responded that it is “very important” or “important” to stay in their home as they age.
When asked what services they would need to continue their living situation, the most common responses were housekeeping, home repair services, and transportation (listed as needs by 35% to 45% of respondents). Regarding changes they would have to make to feel safe in their home as they age, installing grab bars and/or safety rails in the bath/shower was the most popular answer (50%).
Use of telemedicine
Respondents were also asked about their acceptance of telemedicine, and 62% said they would be likely or very likely to engage in virtual visits with a doctor it in the future.
However, the likelihood varied by income level. Specifically, respondents with incomes over $100,000 were significantly more likely to say they would use telemedicine in the future than were those with incomes below $50,000 (74% vs. 60%). They were also more likely to already have used telemedicine.
Although respondents generally embraced telemedicine, they are less confident about some types of monitoring, according to Mr. Cone.
Emergency response (64%) was the leading type of remote monitoring respondents ages 50 and older would allow. Only a minority of respondents would allow the other types of monitoring asked about in the survey.
Close to one-quarter of respondents would not allow any type of monitoring.
Fewer than one-third would allow tracking of medication compliance, refrigerator use, sleep habits, or bathroom use.
People see monitoring of some movements as “Orwellian,” Mr. Cone says.
Knowledge of hospice
The survey findings support the need for more widespread use of hospice so people can stay in their homes as they age, Mr. Cone says.
When illness gets severe, “There’s no reason you have to get rushed to the emergency room or wind up in a hospital,” Mr. Cone says.
He notes that hospice and palliative care can come to patients wherever they reside – in their home, an assisted living center, a nursing home, or even a hospital room.
“That doesn’t mean the physician isn’t involved,” he says. “But working as a team, we can keep them in their homes and their lifestyle intact.”
Patients whose doctors attest that they are likely to live a maximum 6 months are eligible for hospice. But most families wait too long to long to start hospice or palliative care for a patient, Mr. Cone says, and may not be aware of what these services typically cover, including meal preparation and pet care.
In the survey, nearly one-third of respondents said they did not know that palliative care is something that “can be given at any stage of a serious illness” or “provides non-medical services (e.g., patient/family communication, help with insurance issues, scheduling appointments, arranging transportation).”
He notes palliative care and hospice are covered by Medicare and Medicaid and also by most private insurance plans or by individual companies providing the service.
However, health care providers may have to overcome a general reluctance to discuss hospice when sharing options for those severely ill.
The survey showed that while 51% of those 50 and older are at least “slightly interested” in learning more about hospice, a nearly equal number say they are “not at all interested” (49%).
Most using hospice are White
More than 90% of those surveyed reported that aspects of hospice care, including “comfort and relief from pain at the end of patients’ lives,” providing a dedicated care team, and an alternative to other care settings, are “very important” or “important.”
However, national hospice use rates are extremely low for minorities and the LGBTQ community, according to Mr. Cone. Among Medicare hospice recipients, 82% were white, 8.2% Black, 6.7% Hispanic, and 1.8% Asian or Pacific Islander, according to the National Hospice and Palliative Care Organization.
Those numbers signal a need for outreach to those communities with information on what services are available and how to access them, he says.
Health costs top concern
The survey also asked about level of concern regarding matters including family, health, financials, and end-of-life directives and found adults aged 50 and older expressed the greatest amount of concern for health care costs that are not covered by insurance.
More than half (56%) said they were concerned or very concerned about those costs, which was higher than the percentage concerned about losing a spouse (49%).
Respondents were less concerned (“slightly concerned” or “not at all concerned”) about their children living far away, planning end-of life-directives, and falling or having reduced mobility.
A version of this article first appeared on WebMD.com.
a nonprofit hospice/advanced illness care organization based in Virginia.
Among the respondents, 41% said their health was very good or excellent.
However, the ratings differed largely by race, employment status, and income.
Employment status was also associated with a significant difference in the way people viewed their health at the top tier and bottom tier.
The middle tier (“good” health) was reported similarly (from 33% to 37%) whether a person was employed, retired, or not employed. However, employed respondents were much more likely to report they had “excellent” or “very good” health (51% vs. 44% for retirees and 21% for the not employed).
Conversely, those who were not employed were far more likely to report “fair” or “poor” health (45%) than those who were employed (13%) or retired (20%).
Similarly, respondents with incomes of less than $50,000 were three times more likely to report their health as “fair” or “poor” than were those with incomes of more than $100,000 (36% vs. 12%).
WebMD/CCH surveyed 3,464 U.S. residents ages 50 and older between Aug. 13 and Nov. 9, 2020. WebMD.com readers were randomly invited to take a 10-minute online survey.
Aging at home a priority
The survey also highlighted a strong preference for aging in place, says Steve Cone, chief of communications and philanthropy at CCH.
“More now than ever before, thanks to the COVID experience, baby boomers and their children really believe that’s the holy grail,” he says.
Mr. Cone notes that the quick spread of COVID-19 through some nursing homes early in the pandemic likely has strengthened people’s resolve to live out their lives in their own homes.
The survey indicated that 85% of people aged 50+ who are living in their own home, a family member’s home, or a loved one’s home responded that it is “very important” or “important” to stay in their home as they age.
When asked what services they would need to continue their living situation, the most common responses were housekeeping, home repair services, and transportation (listed as needs by 35% to 45% of respondents). Regarding changes they would have to make to feel safe in their home as they age, installing grab bars and/or safety rails in the bath/shower was the most popular answer (50%).
Use of telemedicine
Respondents were also asked about their acceptance of telemedicine, and 62% said they would be likely or very likely to engage in virtual visits with a doctor it in the future.
However, the likelihood varied by income level. Specifically, respondents with incomes over $100,000 were significantly more likely to say they would use telemedicine in the future than were those with incomes below $50,000 (74% vs. 60%). They were also more likely to already have used telemedicine.
Although respondents generally embraced telemedicine, they are less confident about some types of monitoring, according to Mr. Cone.
Emergency response (64%) was the leading type of remote monitoring respondents ages 50 and older would allow. Only a minority of respondents would allow the other types of monitoring asked about in the survey.
Close to one-quarter of respondents would not allow any type of monitoring.
Fewer than one-third would allow tracking of medication compliance, refrigerator use, sleep habits, or bathroom use.
People see monitoring of some movements as “Orwellian,” Mr. Cone says.
Knowledge of hospice
The survey findings support the need for more widespread use of hospice so people can stay in their homes as they age, Mr. Cone says.
When illness gets severe, “There’s no reason you have to get rushed to the emergency room or wind up in a hospital,” Mr. Cone says.
He notes that hospice and palliative care can come to patients wherever they reside – in their home, an assisted living center, a nursing home, or even a hospital room.
“That doesn’t mean the physician isn’t involved,” he says. “But working as a team, we can keep them in their homes and their lifestyle intact.”
Patients whose doctors attest that they are likely to live a maximum 6 months are eligible for hospice. But most families wait too long to long to start hospice or palliative care for a patient, Mr. Cone says, and may not be aware of what these services typically cover, including meal preparation and pet care.
In the survey, nearly one-third of respondents said they did not know that palliative care is something that “can be given at any stage of a serious illness” or “provides non-medical services (e.g., patient/family communication, help with insurance issues, scheduling appointments, arranging transportation).”
He notes palliative care and hospice are covered by Medicare and Medicaid and also by most private insurance plans or by individual companies providing the service.
However, health care providers may have to overcome a general reluctance to discuss hospice when sharing options for those severely ill.
The survey showed that while 51% of those 50 and older are at least “slightly interested” in learning more about hospice, a nearly equal number say they are “not at all interested” (49%).
Most using hospice are White
More than 90% of those surveyed reported that aspects of hospice care, including “comfort and relief from pain at the end of patients’ lives,” providing a dedicated care team, and an alternative to other care settings, are “very important” or “important.”
However, national hospice use rates are extremely low for minorities and the LGBTQ community, according to Mr. Cone. Among Medicare hospice recipients, 82% were white, 8.2% Black, 6.7% Hispanic, and 1.8% Asian or Pacific Islander, according to the National Hospice and Palliative Care Organization.
Those numbers signal a need for outreach to those communities with information on what services are available and how to access them, he says.
Health costs top concern
The survey also asked about level of concern regarding matters including family, health, financials, and end-of-life directives and found adults aged 50 and older expressed the greatest amount of concern for health care costs that are not covered by insurance.
More than half (56%) said they were concerned or very concerned about those costs, which was higher than the percentage concerned about losing a spouse (49%).
Respondents were less concerned (“slightly concerned” or “not at all concerned”) about their children living far away, planning end-of life-directives, and falling or having reduced mobility.
A version of this article first appeared on WebMD.com.
St. Jude to pay $27 million to end DOJ suit over faulty ICDs
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
St. Jude Medical, now part of Abbott Laboratories, will pay the American government $27 million to settle allegations that it knowingly sold defective implantable cardiac defibrillators to health care facilities, which were implanted into patients, causing injuries and two deaths, the U.S. Department of Justice (DOJ) has announced.
“Medical device manufacturers have an obligation to be truthful with the Food and Drug Administration, and the U.S. government will not pay for devices that are unsafe and risk injury or death,” Jonathan F. Lenzner, Acting U.S. Attorney for the District of Maryland, said in a July 8 statement.
“The government contends that St. Jude knowingly caused the submission of false claims and failed to inform the FDA with critical information about prior injuries and a death which, had the FDA been made aware, would have led to a recall,” Mr. Lenzner added.
Those claims were submitted to the Medicare, TRICARE, and Federal Employees Health Benefits programs, according to the settlement agreement.
“The U.S. Attorney’s Office is committed to protecting Medicare and other federal health care programs from fraud, and in doing so, strengthen[ing] patient safety,” Mr. Lenzner said.
Premature battery depletion
The government alleges that St. Jude failed to disclose “serious adverse health events” related to premature battery depletion of certain models of its Fortify, Fortify Assura, Quadra, and Unify implantable defibrillators.
The government further alleges that, by 2013, St. Jude knew that lithium clusters could form on the batteries, causing them to short and run out of power. But it took until late 2014 for St. Jude to ask the FDA to approve a change to prevent lithium clusters from draining the battery.
And at this point, St. Jude told the FDA that “no serious injury, permanent harm, or deaths have been reported associated with this” issue, the government alleges.
However, according to the government’s allegations, St. Jude was aware at that time of two reported serious injuries and one death associated with the faulty batteries and continued to distribute devices that had been manufactured without the new design.
Not until August 2016 did St. Jude inform the FDA that the number of premature battery depletion events had increased to 729, including two deaths and 29 events associated with loss of pacing, the government alleges.
In October 2016, St. Jude issued a medical advisory regarding the battery problem, which the FDA classified as a Class I recall, the most serious type.
After the recall, St. Jude no longer sold the older devices, but thousands of them had been implanted into patients between November 2014 and October 2016.
In September 2017, as reported by this news organization, a nationwide class-action lawsuit was filed against St. Jude Medical and parent company Abbott Laboratories alleging that, despite knowing about a battery-depletion defect in some of its cardiac defibrillators as early as 2011, St. Jude failed to adequately report the risk and waited nearly 5 years before issuing a recall.
“To ensure the health and safety of patients, manufacturers of implantable cardiac devices must be transparent when communicating with the government about safety issues and incidents,” Acting Assistant Attorney General Brian Boynton, from the DOJ’s Civil Division, said in the DOJ statement announcing the settlement.
“We will hold accountable those companies whose conduct violates the law and puts patients’ health at risk,” Mr. Boynton said.
The civil settlement includes the resolution of claims brought under the qui tam, or whistleblower, provisions of the False Claims Act by Debbie Burke, a patient who received one of the devices that was subject to recall.
The claims resolved by the settlement are allegations only; there has been no determination of liability, the DOJ noted. St. Jude denies the allegations raised in the lawsuit.
A version of this article first appeared on Medscape.com.
Standard medical mask can protect wearer from aerosols
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PPIs could be bad news for oral cancer therapies
A substantial proportion of patients with cancer use proton pump inhibitors (PPIs), and up to one-third of these patients are also using oral cancer treatments that could be adversely affected by concomitant PPI use, according to a cross-sectional analysis.
Amit Patel, MD, a gastroenterologist with Duke University, Durham, N.C., was not involved in the study but commented on it in an interview. The “sobering” study findings highlight the need for “clinicians to carefully and regularly assess the indications and need for PPI, which are often overutilized, and consider ‘deprescribing’ based on clinical guidance,” he explained.
Previous research indicates the use of PPIs can lower the bioavailability and efficacy of oral cancer treatments, such as tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors. In the current study, published in JAMA Network Open, researchers sought to identify how many patients with cancer were taking treatments at risk for altered efficacy from PPI use and what factors were associated with use of PPIs.
The study findings
Jean-Luc Raoul, MD, and colleagues, analyzed physician-reported medical data of 566 women and 306 men with cancer from four comprehensive cancer centers in France, with a median age of 63 years. A total of 229 patients in the study (26.3%) were taking PPIs.
Most patients (71.1%) were using PPIs on a regular basis; reasons included epigastric pain (50.0%), retrosternal pain (14.0%), proven esophageal or gastric ulcer (8.0%), or gastroprotection (15.0%).
Factors associated with PPI use in this cohort included older age (odds ratio, 1.02; P <.001), Eastern Cooperative Oncology Group performance status (PS) (PS 1: OR, 1.92; PS 2: OR, 2.51; PS 3: OR, 2.33; P <.001), receipt of hormone therapy (OR, 0.59; P =.01), metastatic stage (P =.03), and tumor site (P =.045).
Older age and PS are particularly important characteristics, explained Dr. Patel. “Unfortunately, older patients with cancer and/or poor PS are more likely to have medical interactions that may result in their being prescribed PPI medications, often for indications that may not justify their use, and/or for indefinite durations.”
He noted that clinicians who are considering prescribing PPI medications should carefully address the indications for PPIs in the clinical scenario, the evidence supporting PPI use for the indication, ratio of benefits and risks, and potential alternatives to PPI use to mitigate potential issues with other therapies.
Approximately 29% of patients who took drugs whose efficacy might be affected by PPI use were also taking other medications, including capecitabine (n = 5), sunitinib (n = 5), cabozantinib (n = 2), pazopanib (n = 1), gefitinib (n = 1), erlotinib (n = 1), and sorafenib (n = 1). Another 39 out of 90 patients (25.6%) taking PPIs were also receiving checkpoint inhibitors. Of the 20 patients who took TKIs and PPIs, a total of 16 reported long-term PPI use. The most common reason for long-term use of PPIs was related to epigastric pain (n = 11).
Since this study was based on physician-reported data, the analysis was limited by the lack of data for all patients seen by each participating physician. In spite of this limitation, the investigators reported no sources of major bias and suggested the study’s prospective nature and relatively large-sized cohorts strengthened the analysis.
PPI use and cancer care
Although issues exist with PPIs in respect to cancer therapies, there are some strategies which may help reduce possible negative effects, Dr. Patel said. “When PPI medications are prescribed, they should be used at the lowest effective dose for the shortest necessary duration, and their use should be regularly reevaluated for dose reduction and/or potential discontinuation.”
Dr. Patel noted that, based on the indication for PPIs, alternatives to PPIs should be considered in the setting of potential drug-drug interactions that may affect the efficacy of oral cancer therapies. “For example, for intermittent typical reflux symptoms such as heartburn, over-the-counter antacids may be considered, along with reflux lifestyle medications,” he explained.
Likewise, the study authors stated in their research letter that “PPIs should be actively identified and substituted” in certain cases. The authors added that antacids are also the best option for patients taking checkpoint inhibitors.
“For those patients who absolutely must take TKI and PPI, clinicians can also consider staggering the dosing schedule, such as taking the TKI in the morning at least 2 hours before PPI and/or with an acidic beverage,” added Dr. Patel.
Although the findings from this study raise potential concerns, Dr. Patel stated further clinical investigations are needed to help the medical community better understand the specific effects of PPIs on the efficacy of various chemotherapeutic agents and to also help develop better management options for patients in these settings.
The authors reported relationships with Bayer, Merck, Transgene, and others. Dr. Patel has no relevant conflicts of interest to report.
A substantial proportion of patients with cancer use proton pump inhibitors (PPIs), and up to one-third of these patients are also using oral cancer treatments that could be adversely affected by concomitant PPI use, according to a cross-sectional analysis.
Amit Patel, MD, a gastroenterologist with Duke University, Durham, N.C., was not involved in the study but commented on it in an interview. The “sobering” study findings highlight the need for “clinicians to carefully and regularly assess the indications and need for PPI, which are often overutilized, and consider ‘deprescribing’ based on clinical guidance,” he explained.
Previous research indicates the use of PPIs can lower the bioavailability and efficacy of oral cancer treatments, such as tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors. In the current study, published in JAMA Network Open, researchers sought to identify how many patients with cancer were taking treatments at risk for altered efficacy from PPI use and what factors were associated with use of PPIs.
The study findings
Jean-Luc Raoul, MD, and colleagues, analyzed physician-reported medical data of 566 women and 306 men with cancer from four comprehensive cancer centers in France, with a median age of 63 years. A total of 229 patients in the study (26.3%) were taking PPIs.
Most patients (71.1%) were using PPIs on a regular basis; reasons included epigastric pain (50.0%), retrosternal pain (14.0%), proven esophageal or gastric ulcer (8.0%), or gastroprotection (15.0%).
Factors associated with PPI use in this cohort included older age (odds ratio, 1.02; P <.001), Eastern Cooperative Oncology Group performance status (PS) (PS 1: OR, 1.92; PS 2: OR, 2.51; PS 3: OR, 2.33; P <.001), receipt of hormone therapy (OR, 0.59; P =.01), metastatic stage (P =.03), and tumor site (P =.045).
Older age and PS are particularly important characteristics, explained Dr. Patel. “Unfortunately, older patients with cancer and/or poor PS are more likely to have medical interactions that may result in their being prescribed PPI medications, often for indications that may not justify their use, and/or for indefinite durations.”
He noted that clinicians who are considering prescribing PPI medications should carefully address the indications for PPIs in the clinical scenario, the evidence supporting PPI use for the indication, ratio of benefits and risks, and potential alternatives to PPI use to mitigate potential issues with other therapies.
Approximately 29% of patients who took drugs whose efficacy might be affected by PPI use were also taking other medications, including capecitabine (n = 5), sunitinib (n = 5), cabozantinib (n = 2), pazopanib (n = 1), gefitinib (n = 1), erlotinib (n = 1), and sorafenib (n = 1). Another 39 out of 90 patients (25.6%) taking PPIs were also receiving checkpoint inhibitors. Of the 20 patients who took TKIs and PPIs, a total of 16 reported long-term PPI use. The most common reason for long-term use of PPIs was related to epigastric pain (n = 11).
Since this study was based on physician-reported data, the analysis was limited by the lack of data for all patients seen by each participating physician. In spite of this limitation, the investigators reported no sources of major bias and suggested the study’s prospective nature and relatively large-sized cohorts strengthened the analysis.
PPI use and cancer care
Although issues exist with PPIs in respect to cancer therapies, there are some strategies which may help reduce possible negative effects, Dr. Patel said. “When PPI medications are prescribed, they should be used at the lowest effective dose for the shortest necessary duration, and their use should be regularly reevaluated for dose reduction and/or potential discontinuation.”
Dr. Patel noted that, based on the indication for PPIs, alternatives to PPIs should be considered in the setting of potential drug-drug interactions that may affect the efficacy of oral cancer therapies. “For example, for intermittent typical reflux symptoms such as heartburn, over-the-counter antacids may be considered, along with reflux lifestyle medications,” he explained.
Likewise, the study authors stated in their research letter that “PPIs should be actively identified and substituted” in certain cases. The authors added that antacids are also the best option for patients taking checkpoint inhibitors.
“For those patients who absolutely must take TKI and PPI, clinicians can also consider staggering the dosing schedule, such as taking the TKI in the morning at least 2 hours before PPI and/or with an acidic beverage,” added Dr. Patel.
Although the findings from this study raise potential concerns, Dr. Patel stated further clinical investigations are needed to help the medical community better understand the specific effects of PPIs on the efficacy of various chemotherapeutic agents and to also help develop better management options for patients in these settings.
The authors reported relationships with Bayer, Merck, Transgene, and others. Dr. Patel has no relevant conflicts of interest to report.
A substantial proportion of patients with cancer use proton pump inhibitors (PPIs), and up to one-third of these patients are also using oral cancer treatments that could be adversely affected by concomitant PPI use, according to a cross-sectional analysis.
Amit Patel, MD, a gastroenterologist with Duke University, Durham, N.C., was not involved in the study but commented on it in an interview. The “sobering” study findings highlight the need for “clinicians to carefully and regularly assess the indications and need for PPI, which are often overutilized, and consider ‘deprescribing’ based on clinical guidance,” he explained.
Previous research indicates the use of PPIs can lower the bioavailability and efficacy of oral cancer treatments, such as tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors. In the current study, published in JAMA Network Open, researchers sought to identify how many patients with cancer were taking treatments at risk for altered efficacy from PPI use and what factors were associated with use of PPIs.
The study findings
Jean-Luc Raoul, MD, and colleagues, analyzed physician-reported medical data of 566 women and 306 men with cancer from four comprehensive cancer centers in France, with a median age of 63 years. A total of 229 patients in the study (26.3%) were taking PPIs.
Most patients (71.1%) were using PPIs on a regular basis; reasons included epigastric pain (50.0%), retrosternal pain (14.0%), proven esophageal or gastric ulcer (8.0%), or gastroprotection (15.0%).
Factors associated with PPI use in this cohort included older age (odds ratio, 1.02; P <.001), Eastern Cooperative Oncology Group performance status (PS) (PS 1: OR, 1.92; PS 2: OR, 2.51; PS 3: OR, 2.33; P <.001), receipt of hormone therapy (OR, 0.59; P =.01), metastatic stage (P =.03), and tumor site (P =.045).
Older age and PS are particularly important characteristics, explained Dr. Patel. “Unfortunately, older patients with cancer and/or poor PS are more likely to have medical interactions that may result in their being prescribed PPI medications, often for indications that may not justify their use, and/or for indefinite durations.”
He noted that clinicians who are considering prescribing PPI medications should carefully address the indications for PPIs in the clinical scenario, the evidence supporting PPI use for the indication, ratio of benefits and risks, and potential alternatives to PPI use to mitigate potential issues with other therapies.
Approximately 29% of patients who took drugs whose efficacy might be affected by PPI use were also taking other medications, including capecitabine (n = 5), sunitinib (n = 5), cabozantinib (n = 2), pazopanib (n = 1), gefitinib (n = 1), erlotinib (n = 1), and sorafenib (n = 1). Another 39 out of 90 patients (25.6%) taking PPIs were also receiving checkpoint inhibitors. Of the 20 patients who took TKIs and PPIs, a total of 16 reported long-term PPI use. The most common reason for long-term use of PPIs was related to epigastric pain (n = 11).
Since this study was based on physician-reported data, the analysis was limited by the lack of data for all patients seen by each participating physician. In spite of this limitation, the investigators reported no sources of major bias and suggested the study’s prospective nature and relatively large-sized cohorts strengthened the analysis.
PPI use and cancer care
Although issues exist with PPIs in respect to cancer therapies, there are some strategies which may help reduce possible negative effects, Dr. Patel said. “When PPI medications are prescribed, they should be used at the lowest effective dose for the shortest necessary duration, and their use should be regularly reevaluated for dose reduction and/or potential discontinuation.”
Dr. Patel noted that, based on the indication for PPIs, alternatives to PPIs should be considered in the setting of potential drug-drug interactions that may affect the efficacy of oral cancer therapies. “For example, for intermittent typical reflux symptoms such as heartburn, over-the-counter antacids may be considered, along with reflux lifestyle medications,” he explained.
Likewise, the study authors stated in their research letter that “PPIs should be actively identified and substituted” in certain cases. The authors added that antacids are also the best option for patients taking checkpoint inhibitors.
“For those patients who absolutely must take TKI and PPI, clinicians can also consider staggering the dosing schedule, such as taking the TKI in the morning at least 2 hours before PPI and/or with an acidic beverage,” added Dr. Patel.
Although the findings from this study raise potential concerns, Dr. Patel stated further clinical investigations are needed to help the medical community better understand the specific effects of PPIs on the efficacy of various chemotherapeutic agents and to also help develop better management options for patients in these settings.
The authors reported relationships with Bayer, Merck, Transgene, and others. Dr. Patel has no relevant conflicts of interest to report.
FROM JAMA NETWORK OPEN
Drawing Down From Crisis: More Lessons From a Soldier
Last year, I wrote an article for the Journal of Hospital Medicine offering tips to healthcare providers in what was then an expanding COVID-19 environment.1 These lessons were drawn from my experiences during the “tough fights” and crisis situations of my military career, situations similar to what healthcare providers experienced during the pandemic.
Now, as vaccination rates rise and hospitalization rates fall, the nation and healthcare profession begin the transition to “normalcy.” What should healthcare professionals expect as they transition from a year of operating in a crisis to resumption of the habitual? What memories and lessons will linger from a long, tough fight against COVID-19, and how might physicians best approach the many post-crisis challenges they will surely face?
My military experiences inform the tips I offer to those in the medical profession. Both professions depend on adeptly leading and building a functional and effective organizational culture under trying circumstances. It may seem strange, but the challenges healthcare workers (HCWs) faced in fighting COVID-19 are comparable to what soldiers experience on a battlefield. And now, as citizens return to “normal” (however normal is defined), only naïve HCWs will believe they can simply resume their previous habits and practices. This part of the journey will present new challenges and unique opportunities.
Healthcare has changed…and so have you! Just like soldiers coming home from the battlefield face a necessarily new and different world, HCWs will also face changing circumstances, environments, and organizational requirements. Given this new landscape, I offer some of my lessons learned coming out of combat to help you adapt.
REFLECTIONS
Heading home from my last combat tour in Iraq, I found myself gazing out the aircraft window and pondering my personal experiences during a very long combat tour commanding a multinational task force. Pulling out my green soldier’s notebook, I rapidly scratched out some reflections on where I was, what I had learned, and what I needed to address personally and professionally. In talking with physicians in the healthcare organization where I now work, this emotional checklist seems to mirror some of the same thoughts they face coming out of the COVID-19 crisis.
Expect exhaustion. There’s a military axiom that “fatigue can make cowards of us all,” and while I don’t think I had succumbed to cowardice in battle, after 15 months in combat I was exhausted. Commanders in combat—or HCWs fighting a pandemic—face unrelenting demands from a variety of audiences. Leaders are asked to solve unsolvable problems, be at the right place at the right time with the right answers, have more energy than others, be upbeat, and exhibit behaviors that will motivate the “troops.” That’s true even if they’re exhausted and weary to the bone, serving on multiple teams, and attending endless meetings. There is also the common and unfortunate expectation that leaders should not take any time for themselves.
During the pandemic, most HCWs reported sleeping less, having little time to interact casually with others, and having less time for personal reflection, exercise, personal growth, or even prayer. My solution for addressing exhaustion was to develop a personal plan to address each one of these areas—mental, emotional, physical, spiritual—with a detailed rest and recovery strategy. I wrote my plan down, knowing that I would need to discuss this blueprint with both my employer and my spouse, who I suspected would have different ideas on what my schedule should look like after returning “home.” Healthcare providers have been through the same kinds of stresses and need to ask themselves: What recovery plan have I designed to help me overcome the fatigue I feel, and have I talked about this plan with the people who will be affected by it?
Take pride in what your teams accomplished. I was proud of how my teams had accomplished the impossible and how they had adapted to continually changing situations. Whenever military organizations know they’ll face the enemy in combat, they feel heightened anxiety, increased fear, and concern about the preparedness of their team. The Army, like any successful team, attempts to mitigate those emotions through training. During my reflections, I remembered the teams that came together to accomplish very tough missions. Some of those teams were those I had concerns about prior to deployment, but fortunately they often surprised me with their adaptability and successes in combat.
Leaders in healthcare can likely relate. Even in normal situations, organizational fault lines exist between physicians, nurses, and administrators. These fault lines may manifest as communication disconnects and distrust between different members who may not completely trust one another due to differences in training, culture, or role within the organization. But during a crisis, rifts dissipate and trust evolves as different cultures are forced to work together. Many healthcare organizations report that, during the COVID crisis, most personality conflicts, communication disconnects, and organizational dysfunctions receded, and organizations saw more and greater coordination and collaboration. Extensive research on leadership demonstrates that crises drive teams to communicate better and become more effective and efficient in accomplishing stated goals, resulting in team members who relish “being there” for one another like never before. These positive changes must be reinforced to ensure these newly formed high-performing teams do not revert back to work silos, which usually occurs due to distrust.
Just as important as pride in teams is the pride in the accomplishment of specific individuals during times of crisis. Diverse members of any organization deliver some of the best solutions to the toughest problems when they are included in the discussion, allowed to bring their ideas to the table, and rewarded for their actions (and their courage)! Just one example is given by Dr Sasha Shillcut as she describes the innovations and adaptations of the women physicians she observed in her organization during the COVID-19 crisis,2 and there are many examples of other organizations citing similar transformation in areas like telemedicine, emergency department procedures, and equipment design and use.3,4
Anticipate “survivor’s guilt.” During my three combat tours, 253 soldiers under my command or in my organization sacrificed their lives for the mission, and many more were wounded in action. There are times when bad dreams remind me of some of the circumstances surrounding the incidents that took the lives of those who died, and I often wake with a start and in a sweat. The first question I always ask myself in the middle of the night when this happens is, “Why did they die, and why did I survive?” That question is always followed by, “What might I have done differently to prevent those deaths?”
As we draw down from treating patients during the COVID-19 crisis, healthcare providers must also be wary of “survivor’s guilt.” Survivor’s guilt is a strong emotion for anyone who has survived a crisis, especially when their friends or loved ones have not. Healthcare providers have lost many patients, but they have also lost colleagues, friends, and family members. Because you are in the healing profession, many of you will question what more you could have done to prevent the loss of life. You likely won’t ever be completely satisfied with the answer, but I have a recommendation that may assuage your emotions.
In combat, we continually memorialized our fallen comrades in ceremonies that are attended by the entire unit. One of my commanders had an idea to keep pictures of those who had made the ultimate sacrifice, and on my desk is a box with the 253 pictures of those dedicated individuals who were killed in action under my command or in my unit. On the top of the box are the words “Make It Matter.” I look at those pictures often to remember them and their selfless service to the nation, and I often ask myself whether I am “making it matter” in my daily activities. Does your healthcare facility have plans for a memorial service for all those who died while in your care? Is there a special tribute in your hospital to those healthcare providers who paid the ultimate sacrifice in caring for patients? Most importantly, have you rededicated yourself to your profession, knowing that what you learned during the pandemic will help you be a better physician in the future, and do you have the knowledge that you are making a meaningful difference every day you serve in healthcare?
Relish being home. On that flight back to family, my excitement was palpable. But there were challenges too, as I knew I had to continue to focus on my team, my organization, and my profession. While images on the internet often show soldiers returning from war rushing into the arms of their loved ones, soldiers never leave the demands associated with wearing the cloth of the country. As a result, many marriages and families are damaged when one member who has been so singularly focused returns home and is still caught up in the demands of the job. They find it is difficult to pick up where they’ve left off, forgetting their family has also been under a different kind of intense stress.
These same challenges will face HCWs. Many of you voluntarily distanced yourself from family and friends due to a fear of transmitting the disease. Spouses and children underwent traumatic challenges in their jobs, holding together the household and piloting kids through schooling. My biggest recommendation is this: strive for a return to a healthy balance, be wary of any sharp edges that appear in your personality or in your relationships, and be open in communicating with those you love. Relying on friends, counselors, and mentors who can provide trusted advice—as well as therapy, if necessary—is not a sign of weakness, but a sign of strength and courage. The pandemic has affected our lives more than we can imagine, and “coming out” of the crisis will continue to test our humanity and civility like never before. Trust me on this one. I’ve been there.
RECOMMENDATIONS FOR POST-CRISIS ACTIONS
These reflections open us to issues physicians must address in the months after your “redeployment” from dealing with the pandemic. When soldiers redeploy from combat, every unit develops a plan to address personal and professional growth for individual members of the team. Additionally, leaders develop a plan to sustain performance and improve teams and organizational approaches. The objective? Polish the diamond from what we learned during the crisis, while preparing for those things that might detract from effectiveness in future crises. It’s an SOP (standard operating procedure) for military units to do these things. Is this approach also advisable for healthcare professionals and teams in responding to crises?
Crises increase stress on individuals and disrupt the functioning of organizations, but crises also provide phenomenal opportunities for growth.5 Adaptive organizations, be they military or healthcare, must take time to understand how the crises affected people and the organizational framework, while also preparing for potential future disruptions. While HCWs and their respective organizations are usually adept at learning from short-term emergencies (eg, limited disease outbreaks, natural disasters, mass-casualty events), they are less practiced in addressing crises that affect the profession for months. It has been a century since the medical profession has been faced with a global pandemic, but experts suggest other pandemics may be on the short-term horizon.6 We ought to use this past year of experiences to prepare for them.
Pay attention to your personal needs and the conditions of others on your team. After returning from combat, I was exhausted and stressed intellectually, physically, emotionally, and spiritually. From what I’ve seen, healthcare providers fit that same description, and the fatigue is palpable. Many of you have experienced extreme stress. I have experienced extremepost-traumatic stress, and it is important to understand that this will affect some on your team.7 In addition to addressing stress—and this is advice I give to all the physicians I know—find the time to get a physical examination. While the Army requires yearly physicals for all soldiers (especially generals!), most healthcare providers I know are shockingly deficient in taking the time to get a checkup from one of their colleagues. Commit to fixing that.
Reflect on what you have learned during this period. Take an afternoon with an adult beverage (if that’s your style) and reflect on what you learned and what others might learn from your unique experiences. Then, take some notes and shape your ideas. What did you experience? What adaptations did you or your team make during the pandemic? What worked and what didn’t? What things do you want to sustain in your practice and what things do you want to eliminate? What did you learn about the medical arts…or even about your Hippocratic Oath? If you have a mentor, share these thoughts with them; if you don’t have a mentor, find one and then share your thoughts with them. Get some outside feedback.
Assess team strengths and weaknesses. If you’re a formal physician leader (someone with a title and a position on your team), it’s your responsibility to provide feedback on both people and processes. If you’re an informal leader (someone who is a member of the team but doesn’t have specific leadership responsibilities outside your clinical role) and you don’t see this happening, volunteer to run the session for your formal leader and your organization. This session should last several hours and be held in a comfortable setting. You should prepare your team so they aren’t defensive about the points that may arise. Determine strengths and opportunities by asking for feedback on communication, behaviors, medical knowledge, emotional intelligence, and execution of tasks. Determine which processes and systems either worked or didn’t work, and either polish the approaches or drive change to improve systems as you get back to normal. Crises provide an opportunity to fix what’s broken while also reinforcing the things that worked in the crisis that might not be normal procedure. Don’t go back to old ways if those weren’t the things or the approaches you were using under critical conditions.
Encourage completion of an organization-wide after-action review (AAR). As I started writing this article, I watched CNN’s Dr Sanjay Gupta conduct a review of actions with the key physicians who contributed to the last administration’s response to the pandemic. In watching that session—and having conducted hundreds of AARs in my military career—there was discussion of obvious good and bad leadership and management procedures, process issues that needed to be addressed, and decision-making that might be applauded or questioned. Every healthcare organization ought to conduct a similar AAR, with a review of the most important aspects of actions and teamwork, the hospital’s operations, logistical preparation, and leader and organization procedures that demand to be addressed.
The successful conduct of any AAR requires asking (and getting answers to) four questions: What happened?; Why did it happen the way it did?; What needs to be fixed or “polished” in the processes, systems, or leadership approach?; and Who is responsible for ensuring the fixes or adjustments occur? The facilitator (and the key leaders of the organization) must ask the right questions, must be deeply involved in getting the right people to comment on the issues, and must “pin the rose” on someone who will be responsible for carrying through on the fixes. At the end of the AAR, after the key topics are discussed, with a plan for addressing each, the person in charge of the organization must publish an action plan with details for ensuring the fixes.
Like all citizens across our nation, my family is grateful for the skill and professionalism exhibited by clinicians and healthcare providers during this devastating pandemic. While we are all breathing a sigh of relief as we see the end in sight, true professionals must take the opportunity to learn and grow from this crisis and adapt. Hopefully, the reflections and recommendations in this article—things I learned from a different profession—will provide ideas to my new colleagues in healthcare.
1. Hertling M. Ten tips for a crisis: lessons from a soldier. J Hosp Med. 2020;15(5): 275-276. https://doi.org/10.12788/jhm.3424
2. Shillcut S. The inspiring women physicians of the COVID-19 pandemic. MedPage Today. April 9, 2020. Accessed July 7, 2021. https://www.kevinmd.com/blog/2020/04/the-insiring-women-physicians-of-the-covid-19-pandemic.html
3. Daley B. Three medical innovations fueled by COVID-19 that will outlast the pandemic. The Conversation. March 9, 2021. Accessed July 7, 2021. https://theconversation.com/3-medical-innovations-fueled-by-covid-19-that-will-outlast-the-pandemic-156464
4. Drees J, Dyrda L, Adams K. Ten big advancements in healthcare tech during the pandemic. Becker’s Health IT. July 6, 2020. Accessed July 7, 2021. https://www.beckershospitalreview.com/digital-transformation/10-big-advancements-in-healthcare-tech-during-the-pandemic.html
5. Wang J. Developing organizational learning capacity in crisis management. Adv Developing Hum Resources. 10(3):425-445. https://doi.org/10.1177/1523422308316464
6. Morens DM, Fauci AS. Emerging pandemic diseases: how we got COVID-19. Cell. 2020;182(5):1077-1092. https://doi.org/10.1016/j.cell.2020.08.021
7. What is posttraumatic stress disorder? American Psychiatric Association. Reviewed August 2020. Accessed July 7, 2021. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd
Last year, I wrote an article for the Journal of Hospital Medicine offering tips to healthcare providers in what was then an expanding COVID-19 environment.1 These lessons were drawn from my experiences during the “tough fights” and crisis situations of my military career, situations similar to what healthcare providers experienced during the pandemic.
Now, as vaccination rates rise and hospitalization rates fall, the nation and healthcare profession begin the transition to “normalcy.” What should healthcare professionals expect as they transition from a year of operating in a crisis to resumption of the habitual? What memories and lessons will linger from a long, tough fight against COVID-19, and how might physicians best approach the many post-crisis challenges they will surely face?
My military experiences inform the tips I offer to those in the medical profession. Both professions depend on adeptly leading and building a functional and effective organizational culture under trying circumstances. It may seem strange, but the challenges healthcare workers (HCWs) faced in fighting COVID-19 are comparable to what soldiers experience on a battlefield. And now, as citizens return to “normal” (however normal is defined), only naïve HCWs will believe they can simply resume their previous habits and practices. This part of the journey will present new challenges and unique opportunities.
Healthcare has changed…and so have you! Just like soldiers coming home from the battlefield face a necessarily new and different world, HCWs will also face changing circumstances, environments, and organizational requirements. Given this new landscape, I offer some of my lessons learned coming out of combat to help you adapt.
REFLECTIONS
Heading home from my last combat tour in Iraq, I found myself gazing out the aircraft window and pondering my personal experiences during a very long combat tour commanding a multinational task force. Pulling out my green soldier’s notebook, I rapidly scratched out some reflections on where I was, what I had learned, and what I needed to address personally and professionally. In talking with physicians in the healthcare organization where I now work, this emotional checklist seems to mirror some of the same thoughts they face coming out of the COVID-19 crisis.
Expect exhaustion. There’s a military axiom that “fatigue can make cowards of us all,” and while I don’t think I had succumbed to cowardice in battle, after 15 months in combat I was exhausted. Commanders in combat—or HCWs fighting a pandemic—face unrelenting demands from a variety of audiences. Leaders are asked to solve unsolvable problems, be at the right place at the right time with the right answers, have more energy than others, be upbeat, and exhibit behaviors that will motivate the “troops.” That’s true even if they’re exhausted and weary to the bone, serving on multiple teams, and attending endless meetings. There is also the common and unfortunate expectation that leaders should not take any time for themselves.
During the pandemic, most HCWs reported sleeping less, having little time to interact casually with others, and having less time for personal reflection, exercise, personal growth, or even prayer. My solution for addressing exhaustion was to develop a personal plan to address each one of these areas—mental, emotional, physical, spiritual—with a detailed rest and recovery strategy. I wrote my plan down, knowing that I would need to discuss this blueprint with both my employer and my spouse, who I suspected would have different ideas on what my schedule should look like after returning “home.” Healthcare providers have been through the same kinds of stresses and need to ask themselves: What recovery plan have I designed to help me overcome the fatigue I feel, and have I talked about this plan with the people who will be affected by it?
Take pride in what your teams accomplished. I was proud of how my teams had accomplished the impossible and how they had adapted to continually changing situations. Whenever military organizations know they’ll face the enemy in combat, they feel heightened anxiety, increased fear, and concern about the preparedness of their team. The Army, like any successful team, attempts to mitigate those emotions through training. During my reflections, I remembered the teams that came together to accomplish very tough missions. Some of those teams were those I had concerns about prior to deployment, but fortunately they often surprised me with their adaptability and successes in combat.
Leaders in healthcare can likely relate. Even in normal situations, organizational fault lines exist between physicians, nurses, and administrators. These fault lines may manifest as communication disconnects and distrust between different members who may not completely trust one another due to differences in training, culture, or role within the organization. But during a crisis, rifts dissipate and trust evolves as different cultures are forced to work together. Many healthcare organizations report that, during the COVID crisis, most personality conflicts, communication disconnects, and organizational dysfunctions receded, and organizations saw more and greater coordination and collaboration. Extensive research on leadership demonstrates that crises drive teams to communicate better and become more effective and efficient in accomplishing stated goals, resulting in team members who relish “being there” for one another like never before. These positive changes must be reinforced to ensure these newly formed high-performing teams do not revert back to work silos, which usually occurs due to distrust.
Just as important as pride in teams is the pride in the accomplishment of specific individuals during times of crisis. Diverse members of any organization deliver some of the best solutions to the toughest problems when they are included in the discussion, allowed to bring their ideas to the table, and rewarded for their actions (and their courage)! Just one example is given by Dr Sasha Shillcut as she describes the innovations and adaptations of the women physicians she observed in her organization during the COVID-19 crisis,2 and there are many examples of other organizations citing similar transformation in areas like telemedicine, emergency department procedures, and equipment design and use.3,4
Anticipate “survivor’s guilt.” During my three combat tours, 253 soldiers under my command or in my organization sacrificed their lives for the mission, and many more were wounded in action. There are times when bad dreams remind me of some of the circumstances surrounding the incidents that took the lives of those who died, and I often wake with a start and in a sweat. The first question I always ask myself in the middle of the night when this happens is, “Why did they die, and why did I survive?” That question is always followed by, “What might I have done differently to prevent those deaths?”
As we draw down from treating patients during the COVID-19 crisis, healthcare providers must also be wary of “survivor’s guilt.” Survivor’s guilt is a strong emotion for anyone who has survived a crisis, especially when their friends or loved ones have not. Healthcare providers have lost many patients, but they have also lost colleagues, friends, and family members. Because you are in the healing profession, many of you will question what more you could have done to prevent the loss of life. You likely won’t ever be completely satisfied with the answer, but I have a recommendation that may assuage your emotions.
In combat, we continually memorialized our fallen comrades in ceremonies that are attended by the entire unit. One of my commanders had an idea to keep pictures of those who had made the ultimate sacrifice, and on my desk is a box with the 253 pictures of those dedicated individuals who were killed in action under my command or in my unit. On the top of the box are the words “Make It Matter.” I look at those pictures often to remember them and their selfless service to the nation, and I often ask myself whether I am “making it matter” in my daily activities. Does your healthcare facility have plans for a memorial service for all those who died while in your care? Is there a special tribute in your hospital to those healthcare providers who paid the ultimate sacrifice in caring for patients? Most importantly, have you rededicated yourself to your profession, knowing that what you learned during the pandemic will help you be a better physician in the future, and do you have the knowledge that you are making a meaningful difference every day you serve in healthcare?
Relish being home. On that flight back to family, my excitement was palpable. But there were challenges too, as I knew I had to continue to focus on my team, my organization, and my profession. While images on the internet often show soldiers returning from war rushing into the arms of their loved ones, soldiers never leave the demands associated with wearing the cloth of the country. As a result, many marriages and families are damaged when one member who has been so singularly focused returns home and is still caught up in the demands of the job. They find it is difficult to pick up where they’ve left off, forgetting their family has also been under a different kind of intense stress.
These same challenges will face HCWs. Many of you voluntarily distanced yourself from family and friends due to a fear of transmitting the disease. Spouses and children underwent traumatic challenges in their jobs, holding together the household and piloting kids through schooling. My biggest recommendation is this: strive for a return to a healthy balance, be wary of any sharp edges that appear in your personality or in your relationships, and be open in communicating with those you love. Relying on friends, counselors, and mentors who can provide trusted advice—as well as therapy, if necessary—is not a sign of weakness, but a sign of strength and courage. The pandemic has affected our lives more than we can imagine, and “coming out” of the crisis will continue to test our humanity and civility like never before. Trust me on this one. I’ve been there.
RECOMMENDATIONS FOR POST-CRISIS ACTIONS
These reflections open us to issues physicians must address in the months after your “redeployment” from dealing with the pandemic. When soldiers redeploy from combat, every unit develops a plan to address personal and professional growth for individual members of the team. Additionally, leaders develop a plan to sustain performance and improve teams and organizational approaches. The objective? Polish the diamond from what we learned during the crisis, while preparing for those things that might detract from effectiveness in future crises. It’s an SOP (standard operating procedure) for military units to do these things. Is this approach also advisable for healthcare professionals and teams in responding to crises?
Crises increase stress on individuals and disrupt the functioning of organizations, but crises also provide phenomenal opportunities for growth.5 Adaptive organizations, be they military or healthcare, must take time to understand how the crises affected people and the organizational framework, while also preparing for potential future disruptions. While HCWs and their respective organizations are usually adept at learning from short-term emergencies (eg, limited disease outbreaks, natural disasters, mass-casualty events), they are less practiced in addressing crises that affect the profession for months. It has been a century since the medical profession has been faced with a global pandemic, but experts suggest other pandemics may be on the short-term horizon.6 We ought to use this past year of experiences to prepare for them.
Pay attention to your personal needs and the conditions of others on your team. After returning from combat, I was exhausted and stressed intellectually, physically, emotionally, and spiritually. From what I’ve seen, healthcare providers fit that same description, and the fatigue is palpable. Many of you have experienced extreme stress. I have experienced extremepost-traumatic stress, and it is important to understand that this will affect some on your team.7 In addition to addressing stress—and this is advice I give to all the physicians I know—find the time to get a physical examination. While the Army requires yearly physicals for all soldiers (especially generals!), most healthcare providers I know are shockingly deficient in taking the time to get a checkup from one of their colleagues. Commit to fixing that.
Reflect on what you have learned during this period. Take an afternoon with an adult beverage (if that’s your style) and reflect on what you learned and what others might learn from your unique experiences. Then, take some notes and shape your ideas. What did you experience? What adaptations did you or your team make during the pandemic? What worked and what didn’t? What things do you want to sustain in your practice and what things do you want to eliminate? What did you learn about the medical arts…or even about your Hippocratic Oath? If you have a mentor, share these thoughts with them; if you don’t have a mentor, find one and then share your thoughts with them. Get some outside feedback.
Assess team strengths and weaknesses. If you’re a formal physician leader (someone with a title and a position on your team), it’s your responsibility to provide feedback on both people and processes. If you’re an informal leader (someone who is a member of the team but doesn’t have specific leadership responsibilities outside your clinical role) and you don’t see this happening, volunteer to run the session for your formal leader and your organization. This session should last several hours and be held in a comfortable setting. You should prepare your team so they aren’t defensive about the points that may arise. Determine strengths and opportunities by asking for feedback on communication, behaviors, medical knowledge, emotional intelligence, and execution of tasks. Determine which processes and systems either worked or didn’t work, and either polish the approaches or drive change to improve systems as you get back to normal. Crises provide an opportunity to fix what’s broken while also reinforcing the things that worked in the crisis that might not be normal procedure. Don’t go back to old ways if those weren’t the things or the approaches you were using under critical conditions.
Encourage completion of an organization-wide after-action review (AAR). As I started writing this article, I watched CNN’s Dr Sanjay Gupta conduct a review of actions with the key physicians who contributed to the last administration’s response to the pandemic. In watching that session—and having conducted hundreds of AARs in my military career—there was discussion of obvious good and bad leadership and management procedures, process issues that needed to be addressed, and decision-making that might be applauded or questioned. Every healthcare organization ought to conduct a similar AAR, with a review of the most important aspects of actions and teamwork, the hospital’s operations, logistical preparation, and leader and organization procedures that demand to be addressed.
The successful conduct of any AAR requires asking (and getting answers to) four questions: What happened?; Why did it happen the way it did?; What needs to be fixed or “polished” in the processes, systems, or leadership approach?; and Who is responsible for ensuring the fixes or adjustments occur? The facilitator (and the key leaders of the organization) must ask the right questions, must be deeply involved in getting the right people to comment on the issues, and must “pin the rose” on someone who will be responsible for carrying through on the fixes. At the end of the AAR, after the key topics are discussed, with a plan for addressing each, the person in charge of the organization must publish an action plan with details for ensuring the fixes.
Like all citizens across our nation, my family is grateful for the skill and professionalism exhibited by clinicians and healthcare providers during this devastating pandemic. While we are all breathing a sigh of relief as we see the end in sight, true professionals must take the opportunity to learn and grow from this crisis and adapt. Hopefully, the reflections and recommendations in this article—things I learned from a different profession—will provide ideas to my new colleagues in healthcare.
Last year, I wrote an article for the Journal of Hospital Medicine offering tips to healthcare providers in what was then an expanding COVID-19 environment.1 These lessons were drawn from my experiences during the “tough fights” and crisis situations of my military career, situations similar to what healthcare providers experienced during the pandemic.
Now, as vaccination rates rise and hospitalization rates fall, the nation and healthcare profession begin the transition to “normalcy.” What should healthcare professionals expect as they transition from a year of operating in a crisis to resumption of the habitual? What memories and lessons will linger from a long, tough fight against COVID-19, and how might physicians best approach the many post-crisis challenges they will surely face?
My military experiences inform the tips I offer to those in the medical profession. Both professions depend on adeptly leading and building a functional and effective organizational culture under trying circumstances. It may seem strange, but the challenges healthcare workers (HCWs) faced in fighting COVID-19 are comparable to what soldiers experience on a battlefield. And now, as citizens return to “normal” (however normal is defined), only naïve HCWs will believe they can simply resume their previous habits and practices. This part of the journey will present new challenges and unique opportunities.
Healthcare has changed…and so have you! Just like soldiers coming home from the battlefield face a necessarily new and different world, HCWs will also face changing circumstances, environments, and organizational requirements. Given this new landscape, I offer some of my lessons learned coming out of combat to help you adapt.
REFLECTIONS
Heading home from my last combat tour in Iraq, I found myself gazing out the aircraft window and pondering my personal experiences during a very long combat tour commanding a multinational task force. Pulling out my green soldier’s notebook, I rapidly scratched out some reflections on where I was, what I had learned, and what I needed to address personally and professionally. In talking with physicians in the healthcare organization where I now work, this emotional checklist seems to mirror some of the same thoughts they face coming out of the COVID-19 crisis.
Expect exhaustion. There’s a military axiom that “fatigue can make cowards of us all,” and while I don’t think I had succumbed to cowardice in battle, after 15 months in combat I was exhausted. Commanders in combat—or HCWs fighting a pandemic—face unrelenting demands from a variety of audiences. Leaders are asked to solve unsolvable problems, be at the right place at the right time with the right answers, have more energy than others, be upbeat, and exhibit behaviors that will motivate the “troops.” That’s true even if they’re exhausted and weary to the bone, serving on multiple teams, and attending endless meetings. There is also the common and unfortunate expectation that leaders should not take any time for themselves.
During the pandemic, most HCWs reported sleeping less, having little time to interact casually with others, and having less time for personal reflection, exercise, personal growth, or even prayer. My solution for addressing exhaustion was to develop a personal plan to address each one of these areas—mental, emotional, physical, spiritual—with a detailed rest and recovery strategy. I wrote my plan down, knowing that I would need to discuss this blueprint with both my employer and my spouse, who I suspected would have different ideas on what my schedule should look like after returning “home.” Healthcare providers have been through the same kinds of stresses and need to ask themselves: What recovery plan have I designed to help me overcome the fatigue I feel, and have I talked about this plan with the people who will be affected by it?
Take pride in what your teams accomplished. I was proud of how my teams had accomplished the impossible and how they had adapted to continually changing situations. Whenever military organizations know they’ll face the enemy in combat, they feel heightened anxiety, increased fear, and concern about the preparedness of their team. The Army, like any successful team, attempts to mitigate those emotions through training. During my reflections, I remembered the teams that came together to accomplish very tough missions. Some of those teams were those I had concerns about prior to deployment, but fortunately they often surprised me with their adaptability and successes in combat.
Leaders in healthcare can likely relate. Even in normal situations, organizational fault lines exist between physicians, nurses, and administrators. These fault lines may manifest as communication disconnects and distrust between different members who may not completely trust one another due to differences in training, culture, or role within the organization. But during a crisis, rifts dissipate and trust evolves as different cultures are forced to work together. Many healthcare organizations report that, during the COVID crisis, most personality conflicts, communication disconnects, and organizational dysfunctions receded, and organizations saw more and greater coordination and collaboration. Extensive research on leadership demonstrates that crises drive teams to communicate better and become more effective and efficient in accomplishing stated goals, resulting in team members who relish “being there” for one another like never before. These positive changes must be reinforced to ensure these newly formed high-performing teams do not revert back to work silos, which usually occurs due to distrust.
Just as important as pride in teams is the pride in the accomplishment of specific individuals during times of crisis. Diverse members of any organization deliver some of the best solutions to the toughest problems when they are included in the discussion, allowed to bring their ideas to the table, and rewarded for their actions (and their courage)! Just one example is given by Dr Sasha Shillcut as she describes the innovations and adaptations of the women physicians she observed in her organization during the COVID-19 crisis,2 and there are many examples of other organizations citing similar transformation in areas like telemedicine, emergency department procedures, and equipment design and use.3,4
Anticipate “survivor’s guilt.” During my three combat tours, 253 soldiers under my command or in my organization sacrificed their lives for the mission, and many more were wounded in action. There are times when bad dreams remind me of some of the circumstances surrounding the incidents that took the lives of those who died, and I often wake with a start and in a sweat. The first question I always ask myself in the middle of the night when this happens is, “Why did they die, and why did I survive?” That question is always followed by, “What might I have done differently to prevent those deaths?”
As we draw down from treating patients during the COVID-19 crisis, healthcare providers must also be wary of “survivor’s guilt.” Survivor’s guilt is a strong emotion for anyone who has survived a crisis, especially when their friends or loved ones have not. Healthcare providers have lost many patients, but they have also lost colleagues, friends, and family members. Because you are in the healing profession, many of you will question what more you could have done to prevent the loss of life. You likely won’t ever be completely satisfied with the answer, but I have a recommendation that may assuage your emotions.
In combat, we continually memorialized our fallen comrades in ceremonies that are attended by the entire unit. One of my commanders had an idea to keep pictures of those who had made the ultimate sacrifice, and on my desk is a box with the 253 pictures of those dedicated individuals who were killed in action under my command or in my unit. On the top of the box are the words “Make It Matter.” I look at those pictures often to remember them and their selfless service to the nation, and I often ask myself whether I am “making it matter” in my daily activities. Does your healthcare facility have plans for a memorial service for all those who died while in your care? Is there a special tribute in your hospital to those healthcare providers who paid the ultimate sacrifice in caring for patients? Most importantly, have you rededicated yourself to your profession, knowing that what you learned during the pandemic will help you be a better physician in the future, and do you have the knowledge that you are making a meaningful difference every day you serve in healthcare?
Relish being home. On that flight back to family, my excitement was palpable. But there were challenges too, as I knew I had to continue to focus on my team, my organization, and my profession. While images on the internet often show soldiers returning from war rushing into the arms of their loved ones, soldiers never leave the demands associated with wearing the cloth of the country. As a result, many marriages and families are damaged when one member who has been so singularly focused returns home and is still caught up in the demands of the job. They find it is difficult to pick up where they’ve left off, forgetting their family has also been under a different kind of intense stress.
These same challenges will face HCWs. Many of you voluntarily distanced yourself from family and friends due to a fear of transmitting the disease. Spouses and children underwent traumatic challenges in their jobs, holding together the household and piloting kids through schooling. My biggest recommendation is this: strive for a return to a healthy balance, be wary of any sharp edges that appear in your personality or in your relationships, and be open in communicating with those you love. Relying on friends, counselors, and mentors who can provide trusted advice—as well as therapy, if necessary—is not a sign of weakness, but a sign of strength and courage. The pandemic has affected our lives more than we can imagine, and “coming out” of the crisis will continue to test our humanity and civility like never before. Trust me on this one. I’ve been there.
RECOMMENDATIONS FOR POST-CRISIS ACTIONS
These reflections open us to issues physicians must address in the months after your “redeployment” from dealing with the pandemic. When soldiers redeploy from combat, every unit develops a plan to address personal and professional growth for individual members of the team. Additionally, leaders develop a plan to sustain performance and improve teams and organizational approaches. The objective? Polish the diamond from what we learned during the crisis, while preparing for those things that might detract from effectiveness in future crises. It’s an SOP (standard operating procedure) for military units to do these things. Is this approach also advisable for healthcare professionals and teams in responding to crises?
Crises increase stress on individuals and disrupt the functioning of organizations, but crises also provide phenomenal opportunities for growth.5 Adaptive organizations, be they military or healthcare, must take time to understand how the crises affected people and the organizational framework, while also preparing for potential future disruptions. While HCWs and their respective organizations are usually adept at learning from short-term emergencies (eg, limited disease outbreaks, natural disasters, mass-casualty events), they are less practiced in addressing crises that affect the profession for months. It has been a century since the medical profession has been faced with a global pandemic, but experts suggest other pandemics may be on the short-term horizon.6 We ought to use this past year of experiences to prepare for them.
Pay attention to your personal needs and the conditions of others on your team. After returning from combat, I was exhausted and stressed intellectually, physically, emotionally, and spiritually. From what I’ve seen, healthcare providers fit that same description, and the fatigue is palpable. Many of you have experienced extreme stress. I have experienced extremepost-traumatic stress, and it is important to understand that this will affect some on your team.7 In addition to addressing stress—and this is advice I give to all the physicians I know—find the time to get a physical examination. While the Army requires yearly physicals for all soldiers (especially generals!), most healthcare providers I know are shockingly deficient in taking the time to get a checkup from one of their colleagues. Commit to fixing that.
Reflect on what you have learned during this period. Take an afternoon with an adult beverage (if that’s your style) and reflect on what you learned and what others might learn from your unique experiences. Then, take some notes and shape your ideas. What did you experience? What adaptations did you or your team make during the pandemic? What worked and what didn’t? What things do you want to sustain in your practice and what things do you want to eliminate? What did you learn about the medical arts…or even about your Hippocratic Oath? If you have a mentor, share these thoughts with them; if you don’t have a mentor, find one and then share your thoughts with them. Get some outside feedback.
Assess team strengths and weaknesses. If you’re a formal physician leader (someone with a title and a position on your team), it’s your responsibility to provide feedback on both people and processes. If you’re an informal leader (someone who is a member of the team but doesn’t have specific leadership responsibilities outside your clinical role) and you don’t see this happening, volunteer to run the session for your formal leader and your organization. This session should last several hours and be held in a comfortable setting. You should prepare your team so they aren’t defensive about the points that may arise. Determine strengths and opportunities by asking for feedback on communication, behaviors, medical knowledge, emotional intelligence, and execution of tasks. Determine which processes and systems either worked or didn’t work, and either polish the approaches or drive change to improve systems as you get back to normal. Crises provide an opportunity to fix what’s broken while also reinforcing the things that worked in the crisis that might not be normal procedure. Don’t go back to old ways if those weren’t the things or the approaches you were using under critical conditions.
Encourage completion of an organization-wide after-action review (AAR). As I started writing this article, I watched CNN’s Dr Sanjay Gupta conduct a review of actions with the key physicians who contributed to the last administration’s response to the pandemic. In watching that session—and having conducted hundreds of AARs in my military career—there was discussion of obvious good and bad leadership and management procedures, process issues that needed to be addressed, and decision-making that might be applauded or questioned. Every healthcare organization ought to conduct a similar AAR, with a review of the most important aspects of actions and teamwork, the hospital’s operations, logistical preparation, and leader and organization procedures that demand to be addressed.
The successful conduct of any AAR requires asking (and getting answers to) four questions: What happened?; Why did it happen the way it did?; What needs to be fixed or “polished” in the processes, systems, or leadership approach?; and Who is responsible for ensuring the fixes or adjustments occur? The facilitator (and the key leaders of the organization) must ask the right questions, must be deeply involved in getting the right people to comment on the issues, and must “pin the rose” on someone who will be responsible for carrying through on the fixes. At the end of the AAR, after the key topics are discussed, with a plan for addressing each, the person in charge of the organization must publish an action plan with details for ensuring the fixes.
Like all citizens across our nation, my family is grateful for the skill and professionalism exhibited by clinicians and healthcare providers during this devastating pandemic. While we are all breathing a sigh of relief as we see the end in sight, true professionals must take the opportunity to learn and grow from this crisis and adapt. Hopefully, the reflections and recommendations in this article—things I learned from a different profession—will provide ideas to my new colleagues in healthcare.
1. Hertling M. Ten tips for a crisis: lessons from a soldier. J Hosp Med. 2020;15(5): 275-276. https://doi.org/10.12788/jhm.3424
2. Shillcut S. The inspiring women physicians of the COVID-19 pandemic. MedPage Today. April 9, 2020. Accessed July 7, 2021. https://www.kevinmd.com/blog/2020/04/the-insiring-women-physicians-of-the-covid-19-pandemic.html
3. Daley B. Three medical innovations fueled by COVID-19 that will outlast the pandemic. The Conversation. March 9, 2021. Accessed July 7, 2021. https://theconversation.com/3-medical-innovations-fueled-by-covid-19-that-will-outlast-the-pandemic-156464
4. Drees J, Dyrda L, Adams K. Ten big advancements in healthcare tech during the pandemic. Becker’s Health IT. July 6, 2020. Accessed July 7, 2021. https://www.beckershospitalreview.com/digital-transformation/10-big-advancements-in-healthcare-tech-during-the-pandemic.html
5. Wang J. Developing organizational learning capacity in crisis management. Adv Developing Hum Resources. 10(3):425-445. https://doi.org/10.1177/1523422308316464
6. Morens DM, Fauci AS. Emerging pandemic diseases: how we got COVID-19. Cell. 2020;182(5):1077-1092. https://doi.org/10.1016/j.cell.2020.08.021
7. What is posttraumatic stress disorder? American Psychiatric Association. Reviewed August 2020. Accessed July 7, 2021. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd
1. Hertling M. Ten tips for a crisis: lessons from a soldier. J Hosp Med. 2020;15(5): 275-276. https://doi.org/10.12788/jhm.3424
2. Shillcut S. The inspiring women physicians of the COVID-19 pandemic. MedPage Today. April 9, 2020. Accessed July 7, 2021. https://www.kevinmd.com/blog/2020/04/the-insiring-women-physicians-of-the-covid-19-pandemic.html
3. Daley B. Three medical innovations fueled by COVID-19 that will outlast the pandemic. The Conversation. March 9, 2021. Accessed July 7, 2021. https://theconversation.com/3-medical-innovations-fueled-by-covid-19-that-will-outlast-the-pandemic-156464
4. Drees J, Dyrda L, Adams K. Ten big advancements in healthcare tech during the pandemic. Becker’s Health IT. July 6, 2020. Accessed July 7, 2021. https://www.beckershospitalreview.com/digital-transformation/10-big-advancements-in-healthcare-tech-during-the-pandemic.html
5. Wang J. Developing organizational learning capacity in crisis management. Adv Developing Hum Resources. 10(3):425-445. https://doi.org/10.1177/1523422308316464
6. Morens DM, Fauci AS. Emerging pandemic diseases: how we got COVID-19. Cell. 2020;182(5):1077-1092. https://doi.org/10.1016/j.cell.2020.08.021
7. What is posttraumatic stress disorder? American Psychiatric Association. Reviewed August 2020. Accessed July 7, 2021. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd
© 2021 Society of Hospital Medicine
The Peer Review Process During the COVID-19 Pandemic
The COVID-19 pandemic put unparalleled strain on US health care systems and individual health care providers (HCPs), which has been well documented. Like all other medical peer reviewed journals, Federal Practitioner relies heavily on the generosity and dedication of federal HCPs. As the pandemic unfolded, we questioned whether HCPs would have the time and energy to write new articles, complete research projects, and review the work of their peers. To assess the impact of COVID-19 on the journal, we compared data from a full year during the COVID-19 pandemic with that of the previous year to determine whether and how the pandemic reshaped the peer review and publication process.
For the purposes of this review, we will compare a full year of COVID-19 journal performance with the prior year. Since COVID-19 infections spiked at different times in different places, there is no clear starting point for the pandemic. Similarly, states varied widely in their vaccination rates and opening procedures. Nevertheless, the period from May 1, 2020 to April 30, 2021, most of the country experienced COVID-19 restrictions, and the number of cases rose dramatically.
From May 1, 2020 to April 30, 2021, Federal Practitioner received 208 submissions, 110% increase over the previous year (189 submissions from May 1, 2019 to April 30, 2020) and a 28% increase over a 2-year period. After submission, it took an average of 9.0 days to the first reviewer invitation compared with 10.3 days in the previous year and 4.7 days 2 years prior. Time from the initial submission to the first decision (ie, accept, reject, or revise) took 72.8 days in the COVID-19 year compared with 91.1 days in the previous year and 69.6 days 2 years prior. In both periods it took reviewers a mean 9.5 days to complete a review from the date invited, and the rate of late reviews was unchanged as well.
During the COVID-19 pandemic year, 1481 reviewer invitations were sent to potential reviewers and 498 reviews were completed (33.6%) by 195 individual reviewers: an average of 2.4 reviews per manuscript. Most reviewers recommended to accept the manuscript, and just 14.7% of reviewers recommended to reject the manuscript (Table). The previous year 1295 invitations were sent to potential reviewers and 460 reviews were completed (38.1%) by 181 individual reviewers for an average of 2.4 reviews per manuscript.
For the original submissions, the journal accepted just 26 (12.7%) articles, recommended revisions for 105 (51.2%) submissions, and rejected 74 (36.1%) submissions from May 1, 2020 to April 30, 3021. One hundred seven manuscripts were revised once, and 75.7% were accepted, and 2.8% were rejected. Twenty-two articles had a second revision and 1 had a third revision and all were published. In the year before the pandemic, just 16 (9.5%) manuscripts were accepted in their original form and 59 (39.1%) were rejected.
Federal Practitioner published 113 articles from May 2020 to April 2021. These articles included 44 (38.9%) original studies, 25 (22.1%) case studies, 20 (17.7%) program profiles, 16 (14.2%) commentaries/editorials, and 8 (7.1%) review articles; 19 (16.8%) articles were focused on COVID-19. The prior year saw Federal Practitioner publish 106 articles in 18 issues. Of these articles 36.8% were original studies, 22.6% were program profiles, 18.9% were case studies, 13.2% were commentaries/editorials, and 8.5% were review articles.
Despite the impact of COVID-19, federal HCPs continued to contribute to this journal without significant interruption. The journal saw a 10% increase in submissions during the pandemic year compared with the previous year but that was in keeping with prior increases in submissions. Similarly, the journal saw more individual reviewers submit more total reviews from May 2020 to April 2021 compared with the previous year. The broad spectrum of reviewers involved in the process and the growing volume of both reviews and submissions suggest that our reviewers remained available and committed to the peer review process despite the impact of a pandemic.
Reducing the time to first decision remains an important priority for the journal. Although the time was shortened during the pandemic, it still took longer to inform authors of the first decision compared with 2 years before. There is no indication that COVID-19 had an impact on the speed of decision making. Reviewers were as timely during the pandemic as they were the year before.
Similarly, there was little difference in the types of articles that were published, other than the obvious increase in COVID-19 submissions. Most of the articles on COVID-19 were editorials and columns, though the journal also published case studies, program profiles, and review articles on treatment. During the pandemic, a higher percentage of articles were original studies and case reports, and fewer were program profiles compared with the types the year before. It is unclear if these differences resulted from random fluctuations in unsolicited manuscripts or are part of a larger trend. The journal managed to publish slightly more articles from May 2020 to April 2021 compared with May 2019 to April 2020 despite fewer issues. This is likely due to increased submissions and articles published online.
For the original submissions, the journal accepted just 26 (12.7%) articles, recommended revisions for 105 (51.2%) submissions and rejected 74 (36.1%) submissions from May 2020 to April 3021. One hundred seven manuscripts were revised once and 75.7% were accepted and 2.8% were rejected. Twenty-two articles had a second revision and 1 had a third revision and all were published. In the year prior to the pandemic, just 16 (9.5%) manuscripts were accepted in their original form, and 59 (39.1%) were rejected.
Although Federal Practitioner improved the efficiency of its decision making, there is still significant room for improvement. We are committed to providing our authors with more rapid decisions and reducing the time to the first decision. Seventy-two days is still too long for authors to wait to hear about the initial decision on their article. Future reviews of the publication process should focus not only on the types of articles that are included, but their subjects as well. Given the great diversity of clinical care practiced across the US Department of Veterans Affairs, US Department of Defense, and the US Public Health Service, the journal must ensure that its articles reflect its diverse audience. We would like to see articles come from authors associated with all 3 major branches of our audience, as well as small portions of the readership (eg, Federal Bureau of Prisons, National Institutes of Health) and ask our readers to help us promote the journal to potential authors in all Federal Health Care organizations. We are especially interested in submissions on or from underserved populations.
Despite the significant burdens on HCPs and federal health care systems, Federal Practitioner managed to increase the speed of publication and the number of articles between May 2020 and April 2021 thanks to the work of all the authors and reviewers who contributed their time and energy to the publication during this challenging period. Their efforts are impressive and greatly appreciated. We pledge to continue to improve our process to reduce the time to publication and to continue to provide regular updates on our process and performance.
The COVID-19 pandemic put unparalleled strain on US health care systems and individual health care providers (HCPs), which has been well documented. Like all other medical peer reviewed journals, Federal Practitioner relies heavily on the generosity and dedication of federal HCPs. As the pandemic unfolded, we questioned whether HCPs would have the time and energy to write new articles, complete research projects, and review the work of their peers. To assess the impact of COVID-19 on the journal, we compared data from a full year during the COVID-19 pandemic with that of the previous year to determine whether and how the pandemic reshaped the peer review and publication process.
For the purposes of this review, we will compare a full year of COVID-19 journal performance with the prior year. Since COVID-19 infections spiked at different times in different places, there is no clear starting point for the pandemic. Similarly, states varied widely in their vaccination rates and opening procedures. Nevertheless, the period from May 1, 2020 to April 30, 2021, most of the country experienced COVID-19 restrictions, and the number of cases rose dramatically.
From May 1, 2020 to April 30, 2021, Federal Practitioner received 208 submissions, 110% increase over the previous year (189 submissions from May 1, 2019 to April 30, 2020) and a 28% increase over a 2-year period. After submission, it took an average of 9.0 days to the first reviewer invitation compared with 10.3 days in the previous year and 4.7 days 2 years prior. Time from the initial submission to the first decision (ie, accept, reject, or revise) took 72.8 days in the COVID-19 year compared with 91.1 days in the previous year and 69.6 days 2 years prior. In both periods it took reviewers a mean 9.5 days to complete a review from the date invited, and the rate of late reviews was unchanged as well.
During the COVID-19 pandemic year, 1481 reviewer invitations were sent to potential reviewers and 498 reviews were completed (33.6%) by 195 individual reviewers: an average of 2.4 reviews per manuscript. Most reviewers recommended to accept the manuscript, and just 14.7% of reviewers recommended to reject the manuscript (Table). The previous year 1295 invitations were sent to potential reviewers and 460 reviews were completed (38.1%) by 181 individual reviewers for an average of 2.4 reviews per manuscript.
For the original submissions, the journal accepted just 26 (12.7%) articles, recommended revisions for 105 (51.2%) submissions, and rejected 74 (36.1%) submissions from May 1, 2020 to April 30, 3021. One hundred seven manuscripts were revised once, and 75.7% were accepted, and 2.8% were rejected. Twenty-two articles had a second revision and 1 had a third revision and all were published. In the year before the pandemic, just 16 (9.5%) manuscripts were accepted in their original form and 59 (39.1%) were rejected.
Federal Practitioner published 113 articles from May 2020 to April 2021. These articles included 44 (38.9%) original studies, 25 (22.1%) case studies, 20 (17.7%) program profiles, 16 (14.2%) commentaries/editorials, and 8 (7.1%) review articles; 19 (16.8%) articles were focused on COVID-19. The prior year saw Federal Practitioner publish 106 articles in 18 issues. Of these articles 36.8% were original studies, 22.6% were program profiles, 18.9% were case studies, 13.2% were commentaries/editorials, and 8.5% were review articles.
Despite the impact of COVID-19, federal HCPs continued to contribute to this journal without significant interruption. The journal saw a 10% increase in submissions during the pandemic year compared with the previous year but that was in keeping with prior increases in submissions. Similarly, the journal saw more individual reviewers submit more total reviews from May 2020 to April 2021 compared with the previous year. The broad spectrum of reviewers involved in the process and the growing volume of both reviews and submissions suggest that our reviewers remained available and committed to the peer review process despite the impact of a pandemic.
Reducing the time to first decision remains an important priority for the journal. Although the time was shortened during the pandemic, it still took longer to inform authors of the first decision compared with 2 years before. There is no indication that COVID-19 had an impact on the speed of decision making. Reviewers were as timely during the pandemic as they were the year before.
Similarly, there was little difference in the types of articles that were published, other than the obvious increase in COVID-19 submissions. Most of the articles on COVID-19 were editorials and columns, though the journal also published case studies, program profiles, and review articles on treatment. During the pandemic, a higher percentage of articles were original studies and case reports, and fewer were program profiles compared with the types the year before. It is unclear if these differences resulted from random fluctuations in unsolicited manuscripts or are part of a larger trend. The journal managed to publish slightly more articles from May 2020 to April 2021 compared with May 2019 to April 2020 despite fewer issues. This is likely due to increased submissions and articles published online.
For the original submissions, the journal accepted just 26 (12.7%) articles, recommended revisions for 105 (51.2%) submissions and rejected 74 (36.1%) submissions from May 2020 to April 3021. One hundred seven manuscripts were revised once and 75.7% were accepted and 2.8% were rejected. Twenty-two articles had a second revision and 1 had a third revision and all were published. In the year prior to the pandemic, just 16 (9.5%) manuscripts were accepted in their original form, and 59 (39.1%) were rejected.
Although Federal Practitioner improved the efficiency of its decision making, there is still significant room for improvement. We are committed to providing our authors with more rapid decisions and reducing the time to the first decision. Seventy-two days is still too long for authors to wait to hear about the initial decision on their article. Future reviews of the publication process should focus not only on the types of articles that are included, but their subjects as well. Given the great diversity of clinical care practiced across the US Department of Veterans Affairs, US Department of Defense, and the US Public Health Service, the journal must ensure that its articles reflect its diverse audience. We would like to see articles come from authors associated with all 3 major branches of our audience, as well as small portions of the readership (eg, Federal Bureau of Prisons, National Institutes of Health) and ask our readers to help us promote the journal to potential authors in all Federal Health Care organizations. We are especially interested in submissions on or from underserved populations.
Despite the significant burdens on HCPs and federal health care systems, Federal Practitioner managed to increase the speed of publication and the number of articles between May 2020 and April 2021 thanks to the work of all the authors and reviewers who contributed their time and energy to the publication during this challenging period. Their efforts are impressive and greatly appreciated. We pledge to continue to improve our process to reduce the time to publication and to continue to provide regular updates on our process and performance.
The COVID-19 pandemic put unparalleled strain on US health care systems and individual health care providers (HCPs), which has been well documented. Like all other medical peer reviewed journals, Federal Practitioner relies heavily on the generosity and dedication of federal HCPs. As the pandemic unfolded, we questioned whether HCPs would have the time and energy to write new articles, complete research projects, and review the work of their peers. To assess the impact of COVID-19 on the journal, we compared data from a full year during the COVID-19 pandemic with that of the previous year to determine whether and how the pandemic reshaped the peer review and publication process.
For the purposes of this review, we will compare a full year of COVID-19 journal performance with the prior year. Since COVID-19 infections spiked at different times in different places, there is no clear starting point for the pandemic. Similarly, states varied widely in their vaccination rates and opening procedures. Nevertheless, the period from May 1, 2020 to April 30, 2021, most of the country experienced COVID-19 restrictions, and the number of cases rose dramatically.
From May 1, 2020 to April 30, 2021, Federal Practitioner received 208 submissions, 110% increase over the previous year (189 submissions from May 1, 2019 to April 30, 2020) and a 28% increase over a 2-year period. After submission, it took an average of 9.0 days to the first reviewer invitation compared with 10.3 days in the previous year and 4.7 days 2 years prior. Time from the initial submission to the first decision (ie, accept, reject, or revise) took 72.8 days in the COVID-19 year compared with 91.1 days in the previous year and 69.6 days 2 years prior. In both periods it took reviewers a mean 9.5 days to complete a review from the date invited, and the rate of late reviews was unchanged as well.
During the COVID-19 pandemic year, 1481 reviewer invitations were sent to potential reviewers and 498 reviews were completed (33.6%) by 195 individual reviewers: an average of 2.4 reviews per manuscript. Most reviewers recommended to accept the manuscript, and just 14.7% of reviewers recommended to reject the manuscript (Table). The previous year 1295 invitations were sent to potential reviewers and 460 reviews were completed (38.1%) by 181 individual reviewers for an average of 2.4 reviews per manuscript.
For the original submissions, the journal accepted just 26 (12.7%) articles, recommended revisions for 105 (51.2%) submissions, and rejected 74 (36.1%) submissions from May 1, 2020 to April 30, 3021. One hundred seven manuscripts were revised once, and 75.7% were accepted, and 2.8% were rejected. Twenty-two articles had a second revision and 1 had a third revision and all were published. In the year before the pandemic, just 16 (9.5%) manuscripts were accepted in their original form and 59 (39.1%) were rejected.
Federal Practitioner published 113 articles from May 2020 to April 2021. These articles included 44 (38.9%) original studies, 25 (22.1%) case studies, 20 (17.7%) program profiles, 16 (14.2%) commentaries/editorials, and 8 (7.1%) review articles; 19 (16.8%) articles were focused on COVID-19. The prior year saw Federal Practitioner publish 106 articles in 18 issues. Of these articles 36.8% were original studies, 22.6% were program profiles, 18.9% were case studies, 13.2% were commentaries/editorials, and 8.5% were review articles.
Despite the impact of COVID-19, federal HCPs continued to contribute to this journal without significant interruption. The journal saw a 10% increase in submissions during the pandemic year compared with the previous year but that was in keeping with prior increases in submissions. Similarly, the journal saw more individual reviewers submit more total reviews from May 2020 to April 2021 compared with the previous year. The broad spectrum of reviewers involved in the process and the growing volume of both reviews and submissions suggest that our reviewers remained available and committed to the peer review process despite the impact of a pandemic.
Reducing the time to first decision remains an important priority for the journal. Although the time was shortened during the pandemic, it still took longer to inform authors of the first decision compared with 2 years before. There is no indication that COVID-19 had an impact on the speed of decision making. Reviewers were as timely during the pandemic as they were the year before.
Similarly, there was little difference in the types of articles that were published, other than the obvious increase in COVID-19 submissions. Most of the articles on COVID-19 were editorials and columns, though the journal also published case studies, program profiles, and review articles on treatment. During the pandemic, a higher percentage of articles were original studies and case reports, and fewer were program profiles compared with the types the year before. It is unclear if these differences resulted from random fluctuations in unsolicited manuscripts or are part of a larger trend. The journal managed to publish slightly more articles from May 2020 to April 2021 compared with May 2019 to April 2020 despite fewer issues. This is likely due to increased submissions and articles published online.
For the original submissions, the journal accepted just 26 (12.7%) articles, recommended revisions for 105 (51.2%) submissions and rejected 74 (36.1%) submissions from May 2020 to April 3021. One hundred seven manuscripts were revised once and 75.7% were accepted and 2.8% were rejected. Twenty-two articles had a second revision and 1 had a third revision and all were published. In the year prior to the pandemic, just 16 (9.5%) manuscripts were accepted in their original form, and 59 (39.1%) were rejected.
Although Federal Practitioner improved the efficiency of its decision making, there is still significant room for improvement. We are committed to providing our authors with more rapid decisions and reducing the time to the first decision. Seventy-two days is still too long for authors to wait to hear about the initial decision on their article. Future reviews of the publication process should focus not only on the types of articles that are included, but their subjects as well. Given the great diversity of clinical care practiced across the US Department of Veterans Affairs, US Department of Defense, and the US Public Health Service, the journal must ensure that its articles reflect its diverse audience. We would like to see articles come from authors associated with all 3 major branches of our audience, as well as small portions of the readership (eg, Federal Bureau of Prisons, National Institutes of Health) and ask our readers to help us promote the journal to potential authors in all Federal Health Care organizations. We are especially interested in submissions on or from underserved populations.
Despite the significant burdens on HCPs and federal health care systems, Federal Practitioner managed to increase the speed of publication and the number of articles between May 2020 and April 2021 thanks to the work of all the authors and reviewers who contributed their time and energy to the publication during this challenging period. Their efforts are impressive and greatly appreciated. We pledge to continue to improve our process to reduce the time to publication and to continue to provide regular updates on our process and performance.
Constipation and Postprandial Pain in a Patient With Shortness of Breath
A 62-year-old male veteran with a history of pulmonary embolism (PE) and prostate cancer status after brachytherapy presented to the emergency department with new onset shortness of breath and left-sided chest pain after prolonged car travel. He underwent a chest computed tomography (CT) angiogram that showed no PE recurrence; however, the scan revealed an incidental transverse colon mass that appeared well circumscribed, homogeneous, and radiolucent with no enhancement, septations, or hypervascularity but no evidence of colonic distension or obstruction (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
The patient reported having chronic constipation and a dull, left-sided abdominal discomfort for the past year. He noted that his abdominal pain worsened after eating and mildly improved after taking castor oil. He had no surgical history and no family history of cancer. The patient reported no fever, fatigue, weight loss, chills, nausea, vomiting, diarrhea, hematochezia, dysuria, hematuria, or melena. Vital signs, physical examination, and initial routine laboratory work were all within appropriate ranges, and a fecal occult blood test was negative.
A colonoscopy was performed, revealing a near-obstructing submucosal mass in the transverse colon near the splenic flexure with a smooth surface and a positive Cushion (Pillow) sign (Figure 2). The patient underwent surgical exploration that resulted in finding a soft, 11-cm lesion arising from the mesenteric side of the transverse colon (Figure 3). Hematoxylin and eosin (H&E) stains were used on a sample from the mass (Figure 4).
The tumor was enucleated via a colotomy over the mass, and the colotomy repaired primarily. Gross examination revealed homogenous yellow fatty tissue, and the H&E stains showed mature, well-differentiated adipocytes with uniform nuclei surrounded by a fibrous capsule. Based on this pathologic examination, this patient was diagnosed with a lipoma of the transverse colon. The resected tissue showed negative margins, indicating full removal of the lipoma.
The patient stabilized well after surgery and remained under inpatient care for observation; due to lack of appetite following the surgery, the patient did not start eating solids again until 2 days after the lipoma removal. By postoperative day 4, the patient had return of bowel function and was tolerating a regular diet with no recurrence of his prandial pain, shortness of breath, or left-sided chest pain. While the precise cause of the patient’s initial presentation of shortness of breath and left-sided chest pain was not ascertained, it is likely that the lipoma, near completely obstructed his bowel, caused abdominal contents and distended intestines to push against his diaphragm, leading to pain and dyspnea. This was likely exacerbated by sensitization to these symptoms from his prior PE. He was discharged home on postoperative day 4 with outpatient follow-up with general surgery.
Discussion
Lipomas are common benign tumors arising from aberrantly multiplying adipocytes. Although lipomas are most commonly found subcutaneously, the lesions can occur anywhere along the gastrointestinal (GI) tract, most often in the colon.1 The incidence rate of colon lipomas ranges from 0.2 to 4.4% among patients in their fifth to sixth decades of life, more commonly found in females.2 These lesions are the most common submucosal mesenchymal lesions of the colon, with a predilection for the right ascending colon.1 The etiology of colon lipomas is largely unknown; one known cause is trauma, thought to induce cytokine release or HMGA2-LPP fusion gene arrangements leading to adipocyte proliferation.3
Most colon lipomas are asymptomatic and discovered incidentally; symptoms typically arise when the lesions are > 2 cm in diameter and include abdominal pain, changes in bowel habits, rectal bleeding, and in extreme cases, obstruction and perforation.4 On CT imaging, colon lipomas will appear radiolucent, homogenous, and well circumscribed. The lesions usually do not warrant intervention unless they are symptomatic. If symptomatic, resection of the lesion is the first-line treatment and usually results in complete resolution of symptoms with no recurrence.2
While either a surgical or endoscopic approach may be used for resection, an increased risk of perforation of the colon with larger lipomas has been shown with endoscopic excision.5 With surgical resection, an open or minimally invasive approach may be offered, based on surgeon comfort with minimally invasive colon procedures. Minimally invasive colonic surgeries may be associated with a shorter length of stay, decreased postoperative pain, and faster return of bowel function. In this case, the surgeon chose an open approach due to the large size of the mass (11 cm) as well as location of the mass in the transverse colon, which made it easy to access directly through a small laparotomy incision made in the superior midline over the transverse colon.
When a colonic mesenchymal mass is seen on colonoscopy, it is important to consider other, nonbenign lesions that present this way. The most common malignant mesenchymal tumor of the GI tract is a gastrointestinal stromal tumor (GIST), a soft-tissue sarcoma that occurs predominantly in the stomach and small intestine.6 These tumors arise from the interstitial cells of Cajal (ICC) and are associated with mutations of KIT and PDGFR-α genes.7 The incidence in the United States is approximately 0.70 per 100,000 people per year, predominantly found in adults in their fifth or sixth decade of life.8 While this tumor typically occurs in the upper GI tract, very rarely, GISTs can be found in the colon.6 Common constitutional symptoms of colon GIST are similar to those of colon lipomas and include abdominal pain, changes in bowel habits, nausea, vomiting, and in some cases, weight loss.
CT imaging is often enough to differentiate a colon lipoma from a colon GIST. On CT, large GIST tumors tend to show irregular, lobulated margins, mucosal ulceration, central necrosis, cavitation, hemorrhage, and hypervascularity—vastly different from the CT findings of colon lipomas. If imaging is equivocal, an ultrasound-guided fine needle aspiration biopsy may be performed, differentiating GIST through the presence of ICC tumor cells as well as KIT and PDGFR-α proteins.
In our patient, colonoscopy showed a positive Cushion sign (tumor indented on depression with biopsy forceps), pathognomonic for a colon lipoma, and CT imaging showed a radiolucent, well-circumscribed lesion.9 This was more consistent with a colon lipoma than a GIST. Because the patient was symptomatic with a near obstructing lesion, the appropriate next step was removal of the lesion. Had this instead been a GIST tumor, a more extensive oncologic surgical resection would have been warranted, with adequate mesentery and lymph nodes collected.
This case is notable because colon lipomas exceeding 2 cm are rare and are usually an incidental finding on CT. However, larger lipomas can lead to symptoms, including obstruction if not removed in a timely manner.
1. Nallamothu G, Adler DG. Large colonic lipomas. Gastroenterol Hepatol (NY). 2011;7(7):490-492.
2. Crocetti D, Sapienza P, Sterpetti AV, et al. Surgery for symptomatic colon lipoma: a systematic review of the literature. Anticancer Res. 2014;34(11):6271-6276.
3. Italiano A, Ebran N, Attias R, et al. NFIB rearrangement in superficial, retroperitoneal, and colonic lipomas with aberrations involving chromosome band 9p22. Genes Chromosomes Cancer. 2008;47(11):971-977. doi:10.1002/gcc.20602
4. Agrawal A, Singh KJ. Symptomatic intestinal lipomas: our experience. Med J Armed Forces India. 2011;67(4):374-376. doi:10.1016/S0377-1237(11)60090-7
5. Kim GW, Kwon CI, Song SH, et al. Endoscopic resection of giant colonic lipoma: case series with partial resection. Clin Endosc. 2013;46(5):586-590. doi:10.5946/ce.2013.46.5.586
6. Reddy RM, Fleshman JW. Colorectal gastrointestinal stromal tumors: a brief review. Clin Colon Rectal Surg. 2006;19(2):69-77. doi:10.1055/s-2006-942347
7. Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol. 2005;40(8):775-780. doi:10.1007/s00535-005-1674-0
8. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11(2):e4120. Published 2019 Feb 22. doi:10.7759/cureus.4120
9. Kyawzaw K, Emmanuel O, Sandar L,2 Febin J,Naing LA, Madhavi R. Pillow sign in colonoscopy. MOJ Clin Med Case Rep. 2018;8(2):57-58. doi:10.15406/mojcr.2018.08.00240
A 62-year-old male veteran with a history of pulmonary embolism (PE) and prostate cancer status after brachytherapy presented to the emergency department with new onset shortness of breath and left-sided chest pain after prolonged car travel. He underwent a chest computed tomography (CT) angiogram that showed no PE recurrence; however, the scan revealed an incidental transverse colon mass that appeared well circumscribed, homogeneous, and radiolucent with no enhancement, septations, or hypervascularity but no evidence of colonic distension or obstruction (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
The patient reported having chronic constipation and a dull, left-sided abdominal discomfort for the past year. He noted that his abdominal pain worsened after eating and mildly improved after taking castor oil. He had no surgical history and no family history of cancer. The patient reported no fever, fatigue, weight loss, chills, nausea, vomiting, diarrhea, hematochezia, dysuria, hematuria, or melena. Vital signs, physical examination, and initial routine laboratory work were all within appropriate ranges, and a fecal occult blood test was negative.
A colonoscopy was performed, revealing a near-obstructing submucosal mass in the transverse colon near the splenic flexure with a smooth surface and a positive Cushion (Pillow) sign (Figure 2). The patient underwent surgical exploration that resulted in finding a soft, 11-cm lesion arising from the mesenteric side of the transverse colon (Figure 3). Hematoxylin and eosin (H&E) stains were used on a sample from the mass (Figure 4).
The tumor was enucleated via a colotomy over the mass, and the colotomy repaired primarily. Gross examination revealed homogenous yellow fatty tissue, and the H&E stains showed mature, well-differentiated adipocytes with uniform nuclei surrounded by a fibrous capsule. Based on this pathologic examination, this patient was diagnosed with a lipoma of the transverse colon. The resected tissue showed negative margins, indicating full removal of the lipoma.
The patient stabilized well after surgery and remained under inpatient care for observation; due to lack of appetite following the surgery, the patient did not start eating solids again until 2 days after the lipoma removal. By postoperative day 4, the patient had return of bowel function and was tolerating a regular diet with no recurrence of his prandial pain, shortness of breath, or left-sided chest pain. While the precise cause of the patient’s initial presentation of shortness of breath and left-sided chest pain was not ascertained, it is likely that the lipoma, near completely obstructed his bowel, caused abdominal contents and distended intestines to push against his diaphragm, leading to pain and dyspnea. This was likely exacerbated by sensitization to these symptoms from his prior PE. He was discharged home on postoperative day 4 with outpatient follow-up with general surgery.
Discussion
Lipomas are common benign tumors arising from aberrantly multiplying adipocytes. Although lipomas are most commonly found subcutaneously, the lesions can occur anywhere along the gastrointestinal (GI) tract, most often in the colon.1 The incidence rate of colon lipomas ranges from 0.2 to 4.4% among patients in their fifth to sixth decades of life, more commonly found in females.2 These lesions are the most common submucosal mesenchymal lesions of the colon, with a predilection for the right ascending colon.1 The etiology of colon lipomas is largely unknown; one known cause is trauma, thought to induce cytokine release or HMGA2-LPP fusion gene arrangements leading to adipocyte proliferation.3
Most colon lipomas are asymptomatic and discovered incidentally; symptoms typically arise when the lesions are > 2 cm in diameter and include abdominal pain, changes in bowel habits, rectal bleeding, and in extreme cases, obstruction and perforation.4 On CT imaging, colon lipomas will appear radiolucent, homogenous, and well circumscribed. The lesions usually do not warrant intervention unless they are symptomatic. If symptomatic, resection of the lesion is the first-line treatment and usually results in complete resolution of symptoms with no recurrence.2
While either a surgical or endoscopic approach may be used for resection, an increased risk of perforation of the colon with larger lipomas has been shown with endoscopic excision.5 With surgical resection, an open or minimally invasive approach may be offered, based on surgeon comfort with minimally invasive colon procedures. Minimally invasive colonic surgeries may be associated with a shorter length of stay, decreased postoperative pain, and faster return of bowel function. In this case, the surgeon chose an open approach due to the large size of the mass (11 cm) as well as location of the mass in the transverse colon, which made it easy to access directly through a small laparotomy incision made in the superior midline over the transverse colon.
When a colonic mesenchymal mass is seen on colonoscopy, it is important to consider other, nonbenign lesions that present this way. The most common malignant mesenchymal tumor of the GI tract is a gastrointestinal stromal tumor (GIST), a soft-tissue sarcoma that occurs predominantly in the stomach and small intestine.6 These tumors arise from the interstitial cells of Cajal (ICC) and are associated with mutations of KIT and PDGFR-α genes.7 The incidence in the United States is approximately 0.70 per 100,000 people per year, predominantly found in adults in their fifth or sixth decade of life.8 While this tumor typically occurs in the upper GI tract, very rarely, GISTs can be found in the colon.6 Common constitutional symptoms of colon GIST are similar to those of colon lipomas and include abdominal pain, changes in bowel habits, nausea, vomiting, and in some cases, weight loss.
CT imaging is often enough to differentiate a colon lipoma from a colon GIST. On CT, large GIST tumors tend to show irregular, lobulated margins, mucosal ulceration, central necrosis, cavitation, hemorrhage, and hypervascularity—vastly different from the CT findings of colon lipomas. If imaging is equivocal, an ultrasound-guided fine needle aspiration biopsy may be performed, differentiating GIST through the presence of ICC tumor cells as well as KIT and PDGFR-α proteins.
In our patient, colonoscopy showed a positive Cushion sign (tumor indented on depression with biopsy forceps), pathognomonic for a colon lipoma, and CT imaging showed a radiolucent, well-circumscribed lesion.9 This was more consistent with a colon lipoma than a GIST. Because the patient was symptomatic with a near obstructing lesion, the appropriate next step was removal of the lesion. Had this instead been a GIST tumor, a more extensive oncologic surgical resection would have been warranted, with adequate mesentery and lymph nodes collected.
This case is notable because colon lipomas exceeding 2 cm are rare and are usually an incidental finding on CT. However, larger lipomas can lead to symptoms, including obstruction if not removed in a timely manner.
A 62-year-old male veteran with a history of pulmonary embolism (PE) and prostate cancer status after brachytherapy presented to the emergency department with new onset shortness of breath and left-sided chest pain after prolonged car travel. He underwent a chest computed tomography (CT) angiogram that showed no PE recurrence; however, the scan revealed an incidental transverse colon mass that appeared well circumscribed, homogeneous, and radiolucent with no enhancement, septations, or hypervascularity but no evidence of colonic distension or obstruction (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
The patient reported having chronic constipation and a dull, left-sided abdominal discomfort for the past year. He noted that his abdominal pain worsened after eating and mildly improved after taking castor oil. He had no surgical history and no family history of cancer. The patient reported no fever, fatigue, weight loss, chills, nausea, vomiting, diarrhea, hematochezia, dysuria, hematuria, or melena. Vital signs, physical examination, and initial routine laboratory work were all within appropriate ranges, and a fecal occult blood test was negative.
A colonoscopy was performed, revealing a near-obstructing submucosal mass in the transverse colon near the splenic flexure with a smooth surface and a positive Cushion (Pillow) sign (Figure 2). The patient underwent surgical exploration that resulted in finding a soft, 11-cm lesion arising from the mesenteric side of the transverse colon (Figure 3). Hematoxylin and eosin (H&E) stains were used on a sample from the mass (Figure 4).
The tumor was enucleated via a colotomy over the mass, and the colotomy repaired primarily. Gross examination revealed homogenous yellow fatty tissue, and the H&E stains showed mature, well-differentiated adipocytes with uniform nuclei surrounded by a fibrous capsule. Based on this pathologic examination, this patient was diagnosed with a lipoma of the transverse colon. The resected tissue showed negative margins, indicating full removal of the lipoma.
The patient stabilized well after surgery and remained under inpatient care for observation; due to lack of appetite following the surgery, the patient did not start eating solids again until 2 days after the lipoma removal. By postoperative day 4, the patient had return of bowel function and was tolerating a regular diet with no recurrence of his prandial pain, shortness of breath, or left-sided chest pain. While the precise cause of the patient’s initial presentation of shortness of breath and left-sided chest pain was not ascertained, it is likely that the lipoma, near completely obstructed his bowel, caused abdominal contents and distended intestines to push against his diaphragm, leading to pain and dyspnea. This was likely exacerbated by sensitization to these symptoms from his prior PE. He was discharged home on postoperative day 4 with outpatient follow-up with general surgery.
Discussion
Lipomas are common benign tumors arising from aberrantly multiplying adipocytes. Although lipomas are most commonly found subcutaneously, the lesions can occur anywhere along the gastrointestinal (GI) tract, most often in the colon.1 The incidence rate of colon lipomas ranges from 0.2 to 4.4% among patients in their fifth to sixth decades of life, more commonly found in females.2 These lesions are the most common submucosal mesenchymal lesions of the colon, with a predilection for the right ascending colon.1 The etiology of colon lipomas is largely unknown; one known cause is trauma, thought to induce cytokine release or HMGA2-LPP fusion gene arrangements leading to adipocyte proliferation.3
Most colon lipomas are asymptomatic and discovered incidentally; symptoms typically arise when the lesions are > 2 cm in diameter and include abdominal pain, changes in bowel habits, rectal bleeding, and in extreme cases, obstruction and perforation.4 On CT imaging, colon lipomas will appear radiolucent, homogenous, and well circumscribed. The lesions usually do not warrant intervention unless they are symptomatic. If symptomatic, resection of the lesion is the first-line treatment and usually results in complete resolution of symptoms with no recurrence.2
While either a surgical or endoscopic approach may be used for resection, an increased risk of perforation of the colon with larger lipomas has been shown with endoscopic excision.5 With surgical resection, an open or minimally invasive approach may be offered, based on surgeon comfort with minimally invasive colon procedures. Minimally invasive colonic surgeries may be associated with a shorter length of stay, decreased postoperative pain, and faster return of bowel function. In this case, the surgeon chose an open approach due to the large size of the mass (11 cm) as well as location of the mass in the transverse colon, which made it easy to access directly through a small laparotomy incision made in the superior midline over the transverse colon.
When a colonic mesenchymal mass is seen on colonoscopy, it is important to consider other, nonbenign lesions that present this way. The most common malignant mesenchymal tumor of the GI tract is a gastrointestinal stromal tumor (GIST), a soft-tissue sarcoma that occurs predominantly in the stomach and small intestine.6 These tumors arise from the interstitial cells of Cajal (ICC) and are associated with mutations of KIT and PDGFR-α genes.7 The incidence in the United States is approximately 0.70 per 100,000 people per year, predominantly found in adults in their fifth or sixth decade of life.8 While this tumor typically occurs in the upper GI tract, very rarely, GISTs can be found in the colon.6 Common constitutional symptoms of colon GIST are similar to those of colon lipomas and include abdominal pain, changes in bowel habits, nausea, vomiting, and in some cases, weight loss.
CT imaging is often enough to differentiate a colon lipoma from a colon GIST. On CT, large GIST tumors tend to show irregular, lobulated margins, mucosal ulceration, central necrosis, cavitation, hemorrhage, and hypervascularity—vastly different from the CT findings of colon lipomas. If imaging is equivocal, an ultrasound-guided fine needle aspiration biopsy may be performed, differentiating GIST through the presence of ICC tumor cells as well as KIT and PDGFR-α proteins.
In our patient, colonoscopy showed a positive Cushion sign (tumor indented on depression with biopsy forceps), pathognomonic for a colon lipoma, and CT imaging showed a radiolucent, well-circumscribed lesion.9 This was more consistent with a colon lipoma than a GIST. Because the patient was symptomatic with a near obstructing lesion, the appropriate next step was removal of the lesion. Had this instead been a GIST tumor, a more extensive oncologic surgical resection would have been warranted, with adequate mesentery and lymph nodes collected.
This case is notable because colon lipomas exceeding 2 cm are rare and are usually an incidental finding on CT. However, larger lipomas can lead to symptoms, including obstruction if not removed in a timely manner.
1. Nallamothu G, Adler DG. Large colonic lipomas. Gastroenterol Hepatol (NY). 2011;7(7):490-492.
2. Crocetti D, Sapienza P, Sterpetti AV, et al. Surgery for symptomatic colon lipoma: a systematic review of the literature. Anticancer Res. 2014;34(11):6271-6276.
3. Italiano A, Ebran N, Attias R, et al. NFIB rearrangement in superficial, retroperitoneal, and colonic lipomas with aberrations involving chromosome band 9p22. Genes Chromosomes Cancer. 2008;47(11):971-977. doi:10.1002/gcc.20602
4. Agrawal A, Singh KJ. Symptomatic intestinal lipomas: our experience. Med J Armed Forces India. 2011;67(4):374-376. doi:10.1016/S0377-1237(11)60090-7
5. Kim GW, Kwon CI, Song SH, et al. Endoscopic resection of giant colonic lipoma: case series with partial resection. Clin Endosc. 2013;46(5):586-590. doi:10.5946/ce.2013.46.5.586
6. Reddy RM, Fleshman JW. Colorectal gastrointestinal stromal tumors: a brief review. Clin Colon Rectal Surg. 2006;19(2):69-77. doi:10.1055/s-2006-942347
7. Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol. 2005;40(8):775-780. doi:10.1007/s00535-005-1674-0
8. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11(2):e4120. Published 2019 Feb 22. doi:10.7759/cureus.4120
9. Kyawzaw K, Emmanuel O, Sandar L,2 Febin J,Naing LA, Madhavi R. Pillow sign in colonoscopy. MOJ Clin Med Case Rep. 2018;8(2):57-58. doi:10.15406/mojcr.2018.08.00240
1. Nallamothu G, Adler DG. Large colonic lipomas. Gastroenterol Hepatol (NY). 2011;7(7):490-492.
2. Crocetti D, Sapienza P, Sterpetti AV, et al. Surgery for symptomatic colon lipoma: a systematic review of the literature. Anticancer Res. 2014;34(11):6271-6276.
3. Italiano A, Ebran N, Attias R, et al. NFIB rearrangement in superficial, retroperitoneal, and colonic lipomas with aberrations involving chromosome band 9p22. Genes Chromosomes Cancer. 2008;47(11):971-977. doi:10.1002/gcc.20602
4. Agrawal A, Singh KJ. Symptomatic intestinal lipomas: our experience. Med J Armed Forces India. 2011;67(4):374-376. doi:10.1016/S0377-1237(11)60090-7
5. Kim GW, Kwon CI, Song SH, et al. Endoscopic resection of giant colonic lipoma: case series with partial resection. Clin Endosc. 2013;46(5):586-590. doi:10.5946/ce.2013.46.5.586
6. Reddy RM, Fleshman JW. Colorectal gastrointestinal stromal tumors: a brief review. Clin Colon Rectal Surg. 2006;19(2):69-77. doi:10.1055/s-2006-942347
7. Shinomura Y, Kinoshita K, Tsutsui S, Hirota S. Pathophysiology, diagnosis, and treatment of gastrointestinal stromal tumors. J Gastroenterol. 2005;40(8):775-780. doi:10.1007/s00535-005-1674-0
8. Patel N, Benipal B. Incidence of gastrointestinal stromal tumors in the United States from 2001-2015: a United States cancer statistics analysis of 50 states. Cureus. 2019;11(2):e4120. Published 2019 Feb 22. doi:10.7759/cureus.4120
9. Kyawzaw K, Emmanuel O, Sandar L,2 Febin J,Naing LA, Madhavi R. Pillow sign in colonoscopy. MOJ Clin Med Case Rep. 2018;8(2):57-58. doi:10.15406/mojcr.2018.08.00240
Home Modifications for Rural Veterans With Disabilities
The US Department of Veterans Affairs (VA) created the Home Improvements and Structural Alterations (HISA) program to help provide necessary home modifications (HMs) to veterans with disabilities (VWDs) that will facilitate the provision of medical services at home and improve home accessibility and functional independence. The Veterans Health Administration (VHA) has more than 9 million veteran enrollees; of those, 2.7 million are classified as rural or highly rural.1 Rural veterans (RVs) possess higher rate of disability compared with that of urban veterans.2-5 RVs have unequal access to screening of ambulatory care sensitive conditions (eg, hypertension, diabetes mellitus).6 Furthermore, RVs are at risk of poor medical outcomes due to distance from health care facilities and specialist care, which can be a barrier to emergency care when issues arise. These barriers, among others, are associated with compromised health quality of life and health outcomes for RVs.3,6 The HISA program may be key to decreasing falls and other serious mishaps in the home. Therefore, understanding use of the HISA program by RVs is important. However, to date little information has been available regarding use of HISA benefits by RVs or characteristics of RVs who receive HISA benefits.
HISA Alterations Program
HISA was initially developed by VA to improve veterans’ transition from acute medical care to home.7,8 However, to obtain HISA grants currently, there is an average 3 to 6 months application process.7 Through the HISA program, VWDs can be prescribed the following HMs, including (but not limited to): flooring replacement, permanent ramps, roll-in showers, installation of central air-conditioning systems, improved lighting, kitchen/bathroom modifications, and home inspections. The HMs prescribed depend on an assessment of medical need by health care providers (HCPs).8
As time passed and the veteran population aged, the program now primarily helps ensure the ability to enter into essential areas and safety in the home.5 The amount of a HISA payment is based on whether a veteran’s health condition is related to military service as defined by the VHA service connection medical evaluation process. Barriers to obtaining a HISA HM can include difficulty in navigating the evaluation process and difficulty in finding a qualified contractor or builder to do the HM.7
This article aims to: (1) Detail the sociodemographic and clinical characteristics of rural HISA users (RHUs); (2) report on HISA usage patterns in number, types, and cost of HMs; (3) compare use amid the diverse VA medical centers (VAMCs) and related complexity levels and Veterans Integrated Service Networks (VISNs); and (4) examine the relationship between travel time/distance and HISA utilization. The long-term goal is to provide accurate information to researchers, HM administrators, health care providers and policy makers on HISA program utilization by rural VWDs, which may help improve its use and bring awareness of its users. This study was approved by the affiliate University of Florida Institutional Review Board and VA research and development committee at the North Florida/South Georgia Veterans Health System.
Methods
Data were obtained from 3 VA sources: the National Prosthetics Patient Database (NPPD), the VHA Medical Inpatient Dataset, and the VHA Outpatient Dataset.7 The NPPD is a national administrative database that contains information on prosthetic-associated products ordered by HCPs for patients, such as portable ramps, handrails, home oxygen equipment, and orthotic and prosthetic apparatus. Data obtained from the NPPD included cost of HMs, clinical characteristics, VISN, and VAMC. VA facilities are categorized into complexity levels 1a, 1b, 1c, 2, and 3. Complexity level 1a to 1c VAMCs address medical cases that entail “heightening involvedness,” meaning a larger number of patients presented with medical concerns needing medical specialists. Complexity levels 2 and 3 have fewer resources, lower patient numbers, and less medically complex patients. Finally, the VHA Medical Inpatient and Outpatient Datasets administrated by VA Informatics and Computing Infrastructure, consist of in-depth health services national data on inpatient and outpatient encounters and procedures.
The study cohort was divided into those with service-connected conditions (Class 1) or those with conditions not related to military service (Class 2). If veterans were identified in both classes, they were assigned to Class 1. The cost variable is determined by using the veterans’ classification. Class 1 veterans receive a lifetime limit of $6800, and Class 2 veterans receive a lifetime limit of $2000. A Class 2 veteran with ≥ 50% disability rating is eligible for a HISA lifetime limit of $6800. Whenever a value exceeds allowed limit of $6800 or $2000, due to data entry error or other reasons, the study team reassigned the cost value to the maximum allowed value.
Travel distance and time were derived by loading patient zip codes and HISA facility locations into the geographical information system program and using the nearest facility and find-route tools. These tools used a road network that simulates real-world driving conditions to calculate distance.
Study Variables
VWDs of any age, gender, and race/ethnicity who qualified for HISA and received HMs from fiscal year ( FY) 2015 through FY 2018 were identified (N = 30,823). Most VWDs were nonrural subjects (n = 19,970), and 43 had no Federal Information Processing System data. The final study cohort consisted of 10,810 HISA recipients. The NPPD, inpatient and outpatient data were merged by scrambled social security numbers to retrieve the following data: age, gender, race, ethnicity, marital status, Class (1 or 2), mean and total number of inpatient days, and type of HMs prescribed.
We also recorded rurality using the VA Rural-Urban Commuting Areas (RUCA) system, but we combined the rural and highly rural designation.1 Census tracts with a RUCA score of 10.0 are deemed highly rural, the remainder are considered rural except those with a RUCA score of 1.0 or 1.1. Travel time and distance from a veteran’s home to the VA facility that provided the HISA prescription were determined from zip codes. The current study focuses on VAMCs prescribing stations (affiliated sites of administrative parent medical facilities) where the HISA users obtained the HM, not the parent station (administrative parent medical facilities).
HISA Utilization
To characterize HISA utilization geographically and over time, the number of users were mapped by county. Areas where users were increasing (hot spots) or decreasing (cold spots) also were mapped. The maps were created using Environmental Systems Research Institute ArcGIS Pro 2.2.1 software. We chose to use natural breaks (Jenks) data classification method in a choropleth to symbolize the change over time map. We then used the Getis Ord GI* optimized hot spot analysis tool in the ArcGIS Pro spatial statistics tool set to generate the hot/cold spot maps. This tool identifies clusters of high values (hot spots) and low values (cold spots) creating a new output layer, RHUs by county, with a Z score, P value, and CI for each county. The Gi Bin field classifies statistically significant hot and cold spots. Counties sorted into the ± 3 category (bin) have a clustering characteristic (eg, with neighboring counties) that is statistically significant with a 99% CI; the ± 2 bin indicates a 95% CI for those county clustering sorted therein; ± 1 reflects a 90% CI; and 0 bin contains county features that have no statistical significant clustering with neighboring counties.
Data Analysis
Data were cleaned and analyzed using SAS 9.4 and R 3.5.3. Descriptive statistics are provided for sociodemographic characteristics, clinical characteristics, and class. ANOVA and t tests were used to compare continuous variables between groups, while χ2 and Fisher exact tests were used for dichotomous and categorical outcome variables. The threshold for statistical significance for these tests was set at α = .001.
Results
There were 10,810 RHUs from FY 2015 through FY 2018 and HISA utilization increased each year (Figure 1). Although some years may show usage decreases relative to previous fiscal years, the cumulative trends showed an increase relative to FY 2015 for both Classes of RVs (Figure 2). There was a 45.4% increase from FY 2015 to FY 2018 with a mean 13.6% yearly increase. Class 1 increased 21.0% and Class 2 increased 39.5% from FY 2015 to FY 2016 (Figure 3).
Most RHUs were male, White, and married. Class 1 and Class 2 RHUs differed significantly by age, race, marital status, and disability conditions: Class 1 RHUs were aged 6.6 years younger with a mean age of 69.1 years compared with 75.7 years for Class 2 users. For Class 1 RHUs, a plurality (29.4%) were aged 65 to 69 years; while a plurality (41.4%) of Class 2 users were aged ≥ 80 years. Musculoskeletal was the most common identified type of condition for all RHUs (Table 1).
To better understand HISA utilization patterns and net RHUs per county, we used a map to detail RHUs by county and change over time (Figure 4). Additionally, we compared US counties by RHUs from FY 2015 to FY 2018 and determined how clusters of high numbers of RHUs (hot spots) and low numbers of RHUs (cold spots) shifted over this period (Figure 5). While HISA utilization grew over the study period, the net count of RHUs per county varied by 9 to 20 persons/county. The population of RHUs increased over time in the Southwest, Southeast, and over much of the East/Northeast, while in the Central and Midwest regions, number of RHUs seems to decrease in population and/or use of the system. The cold spots in the Midwest and South Central US seem to increase with a significant relationship to neighboring counties having a low number of RHUs.
There were 11,166 HM prescribed to RHUs (Table 2). Bathroom HMs also were the dominant HM type for all facilities regardless of complexity levels (Table 3). The San Antonio, Texas, VAMC demonstrated the highest Class 1 vs Class 2 difference in HISA use (Class 1: 87.7% and Class 2: 12.3%). Except for the Des Moines VAMC, all other VAMCs showed HISA use > 60% by Class 1.
Cost Data
Air-conditioning installation ($5007) was the costliest HM overall (Table 4), closely followed by bathroom ($4978) and kitchen modifications ($4305). Bathroom renovations were the costliest HM type for both Class 1 and Class 2, closely followed by electrical repair and air-conditioning installation for Class 1 and driveway reconstruction and wooden ramp construction for Class 2.
The mean award received for HM was $4687 (Table 5). While the number of RHUs increased from FY 2015 to FY 2016, the average cost decreased, both overall ($280) and for Class 1 ($195) and Class 2 ($153). Except for a small decline in the number of Class 2 HISA recipients from FY 2017 to FY 2018, overall, the number of RHUs continuously grew from FY 2015 to FY 2018: 977 for the overall cohort, 678 for Class 1 and 299 for Class 2. Despite the obvious gain in the number of RHUs, the average costs did not notably change over time. VISN 21 had the highest mean cost, followed by VISNs 17, 6, 22, and 20.
Travel
Travel time and distance to the HISA prescribing facility differed significantly between Class 1 and Class 2 HISA users. RHUs had to travel about 95 minutes from their place of residence to access the HISA benefits program. There were no statistically significant differences between Class 1 and 2 users with respect to travel time and distance traveled (Table 6).
The majority of Class 1 and Class 2 veterans accessed the HISA from their nearest facility. However, nearly one-quarter of both Class 1 and 2 RHUs (24% each) did not. Among the 2598 who accessed the nonnearest facility, 97 (3.7%) accessed a facility that is ≤ 40 miles. Many (44%) users traveled 40 to 100 miles, and another 43.2% traveled 100 to 200 miles from their residence to access a HM prescription. Some 2598 users (1.1%) traveled > 500 miles to access a facility.
Discussion
Although utilization of the HISA program has steadily increased, overall participation by subpopulations such as RHUs can still be improved significantly. Veterans aged ≤ 46 years who have a disability that is common to those receiving HISA benefits have low HISA utilization. Similarly, veterans with sensory disabilities also have low use. These subpopulations are among those in great need of attention and services.
A study by Lucas and Zelaya, using the 2016 National Health Interview Survey data with an aim to measure degree of vision problems, dual sensory impairment, and hearing trouble in male veterans aged ≥ 18 years, found that veterans were more likely to report dual sensory impairment and balance difficulties when compared with nonveterans.9 The number of female veterans is growing but had very low representation in this study.10 This emerging VHA population requires information and education on their HM benefits.
Home Modifications
The most common HM prescribed for RHUs was for the bathroom. Further investigation is warranted as to why, given the diversity of HM types that the grant covers, low prescription rates exist across most of the HM types. There may be a lack of knowledge by providers and VWD as to the range of HMs that can be awarded under the grant. It is important that HCPs and veterans receive education on HISA HM options.
Semeah and colleagues pointed out the need for an assessment of the HISA HM ordering system to ensure that multiple HMs items (eg, kitchen, air conditioning, fees, driveway, and plumbing) are listed among the forced choices shown to clinicians to select from.7 Poor housing in rural America is widespread: 63% of rural dwellings need renovations and/or repairs to be accessible to individuals with disabilities, with > 6.7 million rural homes having no or faulty plumbing or kitchens; yet in this study, prescriptions for these HMs accounted for < 1%.11,12
VISN 6 had the most HISA awards with 1364, while VISN 21 had the fewest (245). Across all VISNs, Class 1 RHUs received more prescriptions than did Class 2 RHUs. Future research may seek to examine whether prescribers are fully aware of the eligibility of HM prescription to Class 2 veterans. VISN 21 ($5354); VISN 17 ($5302); and VISN 6 ($5301) had the highest mean HM expenditures. The national mean cost for HISA HMs were $4978 for bathrooms and $4305 for kitchens; for non-HISA HMs in FY 2017, the mean costs were $6362 and $12,255, respectively. A noteworthy concern is whether the maximum grant limit awards are sufficient to perform more expensive and complex HMs, such as the kitchen or major bathroom alternations.13
Facilities categorized as 1a, 1b, or 1c provided
North Florida/Sough Georgia was the highest-prescribing VAMC with 39% more HM prescriptions than the second highest prescribing facility (Durham, NC). Unfortunately, the data presented here cannot establish causality for the large variance difference between the top facilities, and the skewed distribution of total RHUs across VAMCs.
Travel-Related Variables
HISA beneficiaries face significant travel-related challenges. Just 3.6% of RHUs could access a facility within 40 miles of their home and 43.2% traveled 100 to 200 miles from their home to access a HM prescription. Further exploration is warranted to understand how travel patterns impact access to or the uptake of HISA.
RVs already have problems with accessing care because of long travel time.14,15 The choice or necessity to travel to a farther facility for HISA prescription is problematic for RVs, especially when transportation is often reported in the literature as a barrier to resources for people living in rural communities.15-17 When patients have travel barriers, they wait longer to obtain medical services and often wait for their conditions to worsen before seeking services.15,18 Once HM is completed, telerehabilitation is an effective delivery method used for delivering health care services to people in remote places.18,19 Considering that HISA use has the potential to improve quality of life, afford comfort, facilitate the accomplishment of activities of daily living for RVs, it is important that future studies examine how existing telehealth technologies can be used to improve HISA access.
Future Directions
County-level analyses is warranted in future studies exploring potential variables associated with HISA use; for example, county-level rates of primary care physicians and other HCPs. Future research should explore how long distance travel impacts the HISA application process and HM implementation. Further research also should focus on the HISA application structure and process to identify causes of delays. The HISA application process takes a mean 6 months to complete, yet the duration of hospital stays is 1 to 3 weeks, thus it is impossible to connect HISA to hospital discharge, which was the original intent of the program. Future research can examine how telehealth services can expedite HISA obtainment and coordination of the application process. Future research also may study the possible causes of the wide variations in HM prescriptions per facility. It is also important that educational programs provide information on the array of HM items that veterans can obtain.
Conclusions
In our previous study of the HISA cohort (2011-2017), we documented that an increase in utilization of the HISA program was warranted based on the low national budgetary appropriation and identification of significant low participation by vulnerable subpopulations, including veterans residing in rural areas or having returned from recent conflicts.7 The present study documents national utilization patterns, demographic profiles, and clinical characteristics of RHUs from FY 2015 through FY 2018, data that may be useful to policy makers and HISA administrators in predicting future use and users. It is important to note that the data and information presented in this article identify trends. The work in no way establishes a gold standard or any targeted goal of utilization. Future research could focus on conceptualizing or theorizing what steps are necessary to set such a gold standard of utilization rate and steps toward achievement.
Acknowledgments
This research was supported by grant 15521 from the US Department of Veterans Affairs, Office of Rural Health . Furthermore, the research was supported in part by grant K12 HD055929 from the National Institutes of Health.
1. US Department of Veterans Affairs, Veteran Health Administration, Office of Rural Health. Rural veteran health care challenges. Updated February 9, 2021. Accessed June 11, 2021. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
2. Holder, K.A. Veterans in rural America, 2011–2015. Published January 2017. Accessed June 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2017/acs/acs-36.pdf
3. Pezzin LE, Bogner HR, Kurichi JE, et al. Preventable hospitalizations, barriers to care, and disability. Medicine (Baltimore). 2018;97(19):e0691. doi:10.1097/MD.0000000000010691
4. Rosenbach ML. Access and satisfaction within the disabled Medicare population. Health Care Financ Rev. 1995;17(2):147-167.
5. Semeah LM, Ganesh SP, Wang X, et al. Home modification and health services utilization in rural and urban veterans with disabilities. Housing Policy Debate. 2021. Published online: March 4, 2021. doi:10.1080/10511482.2020.1858923
6. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, and Wilt TJ. Rural vs. urban ambulatory health care: A Systematic Review. Published May 2011. Accessed June 11, 2021. https://www.hsrd.research.va.gov/publications/esp/ambulatory.pdf
7. Semeah LM, Wang X, Cowper Ripley DC, et al. Improving health through a home modification service for veterans. In: Fiedler BA, ed. Three Facets of Public Health and Paths to Improvements. Academic Press; 2020:381-416.
8. Semeah LM, Ahrentzen S, Jia H, Cowper-Ripley DC, Levy CE, Mann WC. The home improvements and structural alterations benefits program: veterans with disabilities and home accessibility. J Disability Policy Studies. 2017;28(1):43-51. doi:10.1177/1044207317696275
9. Lucas, JW, Zelaya, CE. Hearing difficulty, vision trouble, and balance problems among male veterans and nonveterans. Published June 12, 2020. Accessed June 11, 2021. https://www.cdc.gov/nchs/data/nhsr/nhsr142-508.pdf
10. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed June 11, 2021. https://www.va.gov/vetdata/docs/SpecialReports/Women_Veterans_2015_Final.pdf
11. US Department of Housing and Urban Development, Office of Policy Development and Research. Housing challenges of rural seniors. Published 2017. Accessed June 11, 2021. https://www.huduser.gov/portal/periodicals/em/summer17/highlight1.html
12. Pendall R, Goodman L, Zhu J, Gold A. The future of rural housing. Published October 2016. Accessed June 11, 202.1 https://www.urban.org/sites/default/files/publication/85101/2000972-the-future-of-rural-housing_6.pdf
13. Joint Center for Housing Studies at Harvard University. Improving America’s housing 2019. Published 2019. Accessed June 11, 2021. https://www.jchs.harvard.edu/sites/default/files/reports/files/Harvard_JCHS_Improving_Americas_Housing_2019.pdf
14. Schooley BL, Horan TA, Lee PW, West PA. Rural veteran access to healthcare services: investigating the role of information and communication technologies in overcoming spatial barriers. Perspect Health Inf Manag. 2010;7(Spring):1f. Published 2010 Apr 1.
15. Ripley DC, Kwong PL, Vogel WB, Kurichi JE, Bates BE, Davenport C. How does geographic access affect in-hospital mortality for veterans with acute ischemic stroke?. Med Care. 2015;53(6):501-509. doi:10.1097/MLR.0000000000000366
16. Cowper-Ripley DC, Reker DM, Hayes J, et al. Geographic access to VHA rehabilitation services for traumatically injured veterans. Fed Pract. 2009;26(10):28-39.
17. Smith M, Towne S, Herrera-Venson A, Cameron K, Horel S, Ory M, et al. Delivery of fall prevention interventions for at-risk older adults in rural areas: Findings from a national dissemination. International journal of environmental research and public health. 2018;15:2798. doi: 10.3390/ijerph15122798
18. Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for Rural Veterans: A Qualitative Assessment of Barriers and Facilitators to Implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
19. Sarfo FS, Akassi J, Kyem G, et al. Long-Term Outcomes of Stroke in a Ghanaian Outpatient Clinic. J Stroke Cerebrovasc Dis. 2018;27(4):1090-1099. doi:10.1016/j.jstrokecerebrovasdis.2017.11.017
The US Department of Veterans Affairs (VA) created the Home Improvements and Structural Alterations (HISA) program to help provide necessary home modifications (HMs) to veterans with disabilities (VWDs) that will facilitate the provision of medical services at home and improve home accessibility and functional independence. The Veterans Health Administration (VHA) has more than 9 million veteran enrollees; of those, 2.7 million are classified as rural or highly rural.1 Rural veterans (RVs) possess higher rate of disability compared with that of urban veterans.2-5 RVs have unequal access to screening of ambulatory care sensitive conditions (eg, hypertension, diabetes mellitus).6 Furthermore, RVs are at risk of poor medical outcomes due to distance from health care facilities and specialist care, which can be a barrier to emergency care when issues arise. These barriers, among others, are associated with compromised health quality of life and health outcomes for RVs.3,6 The HISA program may be key to decreasing falls and other serious mishaps in the home. Therefore, understanding use of the HISA program by RVs is important. However, to date little information has been available regarding use of HISA benefits by RVs or characteristics of RVs who receive HISA benefits.
HISA Alterations Program
HISA was initially developed by VA to improve veterans’ transition from acute medical care to home.7,8 However, to obtain HISA grants currently, there is an average 3 to 6 months application process.7 Through the HISA program, VWDs can be prescribed the following HMs, including (but not limited to): flooring replacement, permanent ramps, roll-in showers, installation of central air-conditioning systems, improved lighting, kitchen/bathroom modifications, and home inspections. The HMs prescribed depend on an assessment of medical need by health care providers (HCPs).8
As time passed and the veteran population aged, the program now primarily helps ensure the ability to enter into essential areas and safety in the home.5 The amount of a HISA payment is based on whether a veteran’s health condition is related to military service as defined by the VHA service connection medical evaluation process. Barriers to obtaining a HISA HM can include difficulty in navigating the evaluation process and difficulty in finding a qualified contractor or builder to do the HM.7
This article aims to: (1) Detail the sociodemographic and clinical characteristics of rural HISA users (RHUs); (2) report on HISA usage patterns in number, types, and cost of HMs; (3) compare use amid the diverse VA medical centers (VAMCs) and related complexity levels and Veterans Integrated Service Networks (VISNs); and (4) examine the relationship between travel time/distance and HISA utilization. The long-term goal is to provide accurate information to researchers, HM administrators, health care providers and policy makers on HISA program utilization by rural VWDs, which may help improve its use and bring awareness of its users. This study was approved by the affiliate University of Florida Institutional Review Board and VA research and development committee at the North Florida/South Georgia Veterans Health System.
Methods
Data were obtained from 3 VA sources: the National Prosthetics Patient Database (NPPD), the VHA Medical Inpatient Dataset, and the VHA Outpatient Dataset.7 The NPPD is a national administrative database that contains information on prosthetic-associated products ordered by HCPs for patients, such as portable ramps, handrails, home oxygen equipment, and orthotic and prosthetic apparatus. Data obtained from the NPPD included cost of HMs, clinical characteristics, VISN, and VAMC. VA facilities are categorized into complexity levels 1a, 1b, 1c, 2, and 3. Complexity level 1a to 1c VAMCs address medical cases that entail “heightening involvedness,” meaning a larger number of patients presented with medical concerns needing medical specialists. Complexity levels 2 and 3 have fewer resources, lower patient numbers, and less medically complex patients. Finally, the VHA Medical Inpatient and Outpatient Datasets administrated by VA Informatics and Computing Infrastructure, consist of in-depth health services national data on inpatient and outpatient encounters and procedures.
The study cohort was divided into those with service-connected conditions (Class 1) or those with conditions not related to military service (Class 2). If veterans were identified in both classes, they were assigned to Class 1. The cost variable is determined by using the veterans’ classification. Class 1 veterans receive a lifetime limit of $6800, and Class 2 veterans receive a lifetime limit of $2000. A Class 2 veteran with ≥ 50% disability rating is eligible for a HISA lifetime limit of $6800. Whenever a value exceeds allowed limit of $6800 or $2000, due to data entry error or other reasons, the study team reassigned the cost value to the maximum allowed value.
Travel distance and time were derived by loading patient zip codes and HISA facility locations into the geographical information system program and using the nearest facility and find-route tools. These tools used a road network that simulates real-world driving conditions to calculate distance.
Study Variables
VWDs of any age, gender, and race/ethnicity who qualified for HISA and received HMs from fiscal year ( FY) 2015 through FY 2018 were identified (N = 30,823). Most VWDs were nonrural subjects (n = 19,970), and 43 had no Federal Information Processing System data. The final study cohort consisted of 10,810 HISA recipients. The NPPD, inpatient and outpatient data were merged by scrambled social security numbers to retrieve the following data: age, gender, race, ethnicity, marital status, Class (1 or 2), mean and total number of inpatient days, and type of HMs prescribed.
We also recorded rurality using the VA Rural-Urban Commuting Areas (RUCA) system, but we combined the rural and highly rural designation.1 Census tracts with a RUCA score of 10.0 are deemed highly rural, the remainder are considered rural except those with a RUCA score of 1.0 or 1.1. Travel time and distance from a veteran’s home to the VA facility that provided the HISA prescription were determined from zip codes. The current study focuses on VAMCs prescribing stations (affiliated sites of administrative parent medical facilities) where the HISA users obtained the HM, not the parent station (administrative parent medical facilities).
HISA Utilization
To characterize HISA utilization geographically and over time, the number of users were mapped by county. Areas where users were increasing (hot spots) or decreasing (cold spots) also were mapped. The maps were created using Environmental Systems Research Institute ArcGIS Pro 2.2.1 software. We chose to use natural breaks (Jenks) data classification method in a choropleth to symbolize the change over time map. We then used the Getis Ord GI* optimized hot spot analysis tool in the ArcGIS Pro spatial statistics tool set to generate the hot/cold spot maps. This tool identifies clusters of high values (hot spots) and low values (cold spots) creating a new output layer, RHUs by county, with a Z score, P value, and CI for each county. The Gi Bin field classifies statistically significant hot and cold spots. Counties sorted into the ± 3 category (bin) have a clustering characteristic (eg, with neighboring counties) that is statistically significant with a 99% CI; the ± 2 bin indicates a 95% CI for those county clustering sorted therein; ± 1 reflects a 90% CI; and 0 bin contains county features that have no statistical significant clustering with neighboring counties.
Data Analysis
Data were cleaned and analyzed using SAS 9.4 and R 3.5.3. Descriptive statistics are provided for sociodemographic characteristics, clinical characteristics, and class. ANOVA and t tests were used to compare continuous variables between groups, while χ2 and Fisher exact tests were used for dichotomous and categorical outcome variables. The threshold for statistical significance for these tests was set at α = .001.
Results
There were 10,810 RHUs from FY 2015 through FY 2018 and HISA utilization increased each year (Figure 1). Although some years may show usage decreases relative to previous fiscal years, the cumulative trends showed an increase relative to FY 2015 for both Classes of RVs (Figure 2). There was a 45.4% increase from FY 2015 to FY 2018 with a mean 13.6% yearly increase. Class 1 increased 21.0% and Class 2 increased 39.5% from FY 2015 to FY 2016 (Figure 3).
Most RHUs were male, White, and married. Class 1 and Class 2 RHUs differed significantly by age, race, marital status, and disability conditions: Class 1 RHUs were aged 6.6 years younger with a mean age of 69.1 years compared with 75.7 years for Class 2 users. For Class 1 RHUs, a plurality (29.4%) were aged 65 to 69 years; while a plurality (41.4%) of Class 2 users were aged ≥ 80 years. Musculoskeletal was the most common identified type of condition for all RHUs (Table 1).
To better understand HISA utilization patterns and net RHUs per county, we used a map to detail RHUs by county and change over time (Figure 4). Additionally, we compared US counties by RHUs from FY 2015 to FY 2018 and determined how clusters of high numbers of RHUs (hot spots) and low numbers of RHUs (cold spots) shifted over this period (Figure 5). While HISA utilization grew over the study period, the net count of RHUs per county varied by 9 to 20 persons/county. The population of RHUs increased over time in the Southwest, Southeast, and over much of the East/Northeast, while in the Central and Midwest regions, number of RHUs seems to decrease in population and/or use of the system. The cold spots in the Midwest and South Central US seem to increase with a significant relationship to neighboring counties having a low number of RHUs.
There were 11,166 HM prescribed to RHUs (Table 2). Bathroom HMs also were the dominant HM type for all facilities regardless of complexity levels (Table 3). The San Antonio, Texas, VAMC demonstrated the highest Class 1 vs Class 2 difference in HISA use (Class 1: 87.7% and Class 2: 12.3%). Except for the Des Moines VAMC, all other VAMCs showed HISA use > 60% by Class 1.
Cost Data
Air-conditioning installation ($5007) was the costliest HM overall (Table 4), closely followed by bathroom ($4978) and kitchen modifications ($4305). Bathroom renovations were the costliest HM type for both Class 1 and Class 2, closely followed by electrical repair and air-conditioning installation for Class 1 and driveway reconstruction and wooden ramp construction for Class 2.
The mean award received for HM was $4687 (Table 5). While the number of RHUs increased from FY 2015 to FY 2016, the average cost decreased, both overall ($280) and for Class 1 ($195) and Class 2 ($153). Except for a small decline in the number of Class 2 HISA recipients from FY 2017 to FY 2018, overall, the number of RHUs continuously grew from FY 2015 to FY 2018: 977 for the overall cohort, 678 for Class 1 and 299 for Class 2. Despite the obvious gain in the number of RHUs, the average costs did not notably change over time. VISN 21 had the highest mean cost, followed by VISNs 17, 6, 22, and 20.
Travel
Travel time and distance to the HISA prescribing facility differed significantly between Class 1 and Class 2 HISA users. RHUs had to travel about 95 minutes from their place of residence to access the HISA benefits program. There were no statistically significant differences between Class 1 and 2 users with respect to travel time and distance traveled (Table 6).
The majority of Class 1 and Class 2 veterans accessed the HISA from their nearest facility. However, nearly one-quarter of both Class 1 and 2 RHUs (24% each) did not. Among the 2598 who accessed the nonnearest facility, 97 (3.7%) accessed a facility that is ≤ 40 miles. Many (44%) users traveled 40 to 100 miles, and another 43.2% traveled 100 to 200 miles from their residence to access a HM prescription. Some 2598 users (1.1%) traveled > 500 miles to access a facility.
Discussion
Although utilization of the HISA program has steadily increased, overall participation by subpopulations such as RHUs can still be improved significantly. Veterans aged ≤ 46 years who have a disability that is common to those receiving HISA benefits have low HISA utilization. Similarly, veterans with sensory disabilities also have low use. These subpopulations are among those in great need of attention and services.
A study by Lucas and Zelaya, using the 2016 National Health Interview Survey data with an aim to measure degree of vision problems, dual sensory impairment, and hearing trouble in male veterans aged ≥ 18 years, found that veterans were more likely to report dual sensory impairment and balance difficulties when compared with nonveterans.9 The number of female veterans is growing but had very low representation in this study.10 This emerging VHA population requires information and education on their HM benefits.
Home Modifications
The most common HM prescribed for RHUs was for the bathroom. Further investigation is warranted as to why, given the diversity of HM types that the grant covers, low prescription rates exist across most of the HM types. There may be a lack of knowledge by providers and VWD as to the range of HMs that can be awarded under the grant. It is important that HCPs and veterans receive education on HISA HM options.
Semeah and colleagues pointed out the need for an assessment of the HISA HM ordering system to ensure that multiple HMs items (eg, kitchen, air conditioning, fees, driveway, and plumbing) are listed among the forced choices shown to clinicians to select from.7 Poor housing in rural America is widespread: 63% of rural dwellings need renovations and/or repairs to be accessible to individuals with disabilities, with > 6.7 million rural homes having no or faulty plumbing or kitchens; yet in this study, prescriptions for these HMs accounted for < 1%.11,12
VISN 6 had the most HISA awards with 1364, while VISN 21 had the fewest (245). Across all VISNs, Class 1 RHUs received more prescriptions than did Class 2 RHUs. Future research may seek to examine whether prescribers are fully aware of the eligibility of HM prescription to Class 2 veterans. VISN 21 ($5354); VISN 17 ($5302); and VISN 6 ($5301) had the highest mean HM expenditures. The national mean cost for HISA HMs were $4978 for bathrooms and $4305 for kitchens; for non-HISA HMs in FY 2017, the mean costs were $6362 and $12,255, respectively. A noteworthy concern is whether the maximum grant limit awards are sufficient to perform more expensive and complex HMs, such as the kitchen or major bathroom alternations.13
Facilities categorized as 1a, 1b, or 1c provided
North Florida/Sough Georgia was the highest-prescribing VAMC with 39% more HM prescriptions than the second highest prescribing facility (Durham, NC). Unfortunately, the data presented here cannot establish causality for the large variance difference between the top facilities, and the skewed distribution of total RHUs across VAMCs.
Travel-Related Variables
HISA beneficiaries face significant travel-related challenges. Just 3.6% of RHUs could access a facility within 40 miles of their home and 43.2% traveled 100 to 200 miles from their home to access a HM prescription. Further exploration is warranted to understand how travel patterns impact access to or the uptake of HISA.
RVs already have problems with accessing care because of long travel time.14,15 The choice or necessity to travel to a farther facility for HISA prescription is problematic for RVs, especially when transportation is often reported in the literature as a barrier to resources for people living in rural communities.15-17 When patients have travel barriers, they wait longer to obtain medical services and often wait for their conditions to worsen before seeking services.15,18 Once HM is completed, telerehabilitation is an effective delivery method used for delivering health care services to people in remote places.18,19 Considering that HISA use has the potential to improve quality of life, afford comfort, facilitate the accomplishment of activities of daily living for RVs, it is important that future studies examine how existing telehealth technologies can be used to improve HISA access.
Future Directions
County-level analyses is warranted in future studies exploring potential variables associated with HISA use; for example, county-level rates of primary care physicians and other HCPs. Future research should explore how long distance travel impacts the HISA application process and HM implementation. Further research also should focus on the HISA application structure and process to identify causes of delays. The HISA application process takes a mean 6 months to complete, yet the duration of hospital stays is 1 to 3 weeks, thus it is impossible to connect HISA to hospital discharge, which was the original intent of the program. Future research can examine how telehealth services can expedite HISA obtainment and coordination of the application process. Future research also may study the possible causes of the wide variations in HM prescriptions per facility. It is also important that educational programs provide information on the array of HM items that veterans can obtain.
Conclusions
In our previous study of the HISA cohort (2011-2017), we documented that an increase in utilization of the HISA program was warranted based on the low national budgetary appropriation and identification of significant low participation by vulnerable subpopulations, including veterans residing in rural areas or having returned from recent conflicts.7 The present study documents national utilization patterns, demographic profiles, and clinical characteristics of RHUs from FY 2015 through FY 2018, data that may be useful to policy makers and HISA administrators in predicting future use and users. It is important to note that the data and information presented in this article identify trends. The work in no way establishes a gold standard or any targeted goal of utilization. Future research could focus on conceptualizing or theorizing what steps are necessary to set such a gold standard of utilization rate and steps toward achievement.
Acknowledgments
This research was supported by grant 15521 from the US Department of Veterans Affairs, Office of Rural Health . Furthermore, the research was supported in part by grant K12 HD055929 from the National Institutes of Health.
The US Department of Veterans Affairs (VA) created the Home Improvements and Structural Alterations (HISA) program to help provide necessary home modifications (HMs) to veterans with disabilities (VWDs) that will facilitate the provision of medical services at home and improve home accessibility and functional independence. The Veterans Health Administration (VHA) has more than 9 million veteran enrollees; of those, 2.7 million are classified as rural or highly rural.1 Rural veterans (RVs) possess higher rate of disability compared with that of urban veterans.2-5 RVs have unequal access to screening of ambulatory care sensitive conditions (eg, hypertension, diabetes mellitus).6 Furthermore, RVs are at risk of poor medical outcomes due to distance from health care facilities and specialist care, which can be a barrier to emergency care when issues arise. These barriers, among others, are associated with compromised health quality of life and health outcomes for RVs.3,6 The HISA program may be key to decreasing falls and other serious mishaps in the home. Therefore, understanding use of the HISA program by RVs is important. However, to date little information has been available regarding use of HISA benefits by RVs or characteristics of RVs who receive HISA benefits.
HISA Alterations Program
HISA was initially developed by VA to improve veterans’ transition from acute medical care to home.7,8 However, to obtain HISA grants currently, there is an average 3 to 6 months application process.7 Through the HISA program, VWDs can be prescribed the following HMs, including (but not limited to): flooring replacement, permanent ramps, roll-in showers, installation of central air-conditioning systems, improved lighting, kitchen/bathroom modifications, and home inspections. The HMs prescribed depend on an assessment of medical need by health care providers (HCPs).8
As time passed and the veteran population aged, the program now primarily helps ensure the ability to enter into essential areas and safety in the home.5 The amount of a HISA payment is based on whether a veteran’s health condition is related to military service as defined by the VHA service connection medical evaluation process. Barriers to obtaining a HISA HM can include difficulty in navigating the evaluation process and difficulty in finding a qualified contractor or builder to do the HM.7
This article aims to: (1) Detail the sociodemographic and clinical characteristics of rural HISA users (RHUs); (2) report on HISA usage patterns in number, types, and cost of HMs; (3) compare use amid the diverse VA medical centers (VAMCs) and related complexity levels and Veterans Integrated Service Networks (VISNs); and (4) examine the relationship between travel time/distance and HISA utilization. The long-term goal is to provide accurate information to researchers, HM administrators, health care providers and policy makers on HISA program utilization by rural VWDs, which may help improve its use and bring awareness of its users. This study was approved by the affiliate University of Florida Institutional Review Board and VA research and development committee at the North Florida/South Georgia Veterans Health System.
Methods
Data were obtained from 3 VA sources: the National Prosthetics Patient Database (NPPD), the VHA Medical Inpatient Dataset, and the VHA Outpatient Dataset.7 The NPPD is a national administrative database that contains information on prosthetic-associated products ordered by HCPs for patients, such as portable ramps, handrails, home oxygen equipment, and orthotic and prosthetic apparatus. Data obtained from the NPPD included cost of HMs, clinical characteristics, VISN, and VAMC. VA facilities are categorized into complexity levels 1a, 1b, 1c, 2, and 3. Complexity level 1a to 1c VAMCs address medical cases that entail “heightening involvedness,” meaning a larger number of patients presented with medical concerns needing medical specialists. Complexity levels 2 and 3 have fewer resources, lower patient numbers, and less medically complex patients. Finally, the VHA Medical Inpatient and Outpatient Datasets administrated by VA Informatics and Computing Infrastructure, consist of in-depth health services national data on inpatient and outpatient encounters and procedures.
The study cohort was divided into those with service-connected conditions (Class 1) or those with conditions not related to military service (Class 2). If veterans were identified in both classes, they were assigned to Class 1. The cost variable is determined by using the veterans’ classification. Class 1 veterans receive a lifetime limit of $6800, and Class 2 veterans receive a lifetime limit of $2000. A Class 2 veteran with ≥ 50% disability rating is eligible for a HISA lifetime limit of $6800. Whenever a value exceeds allowed limit of $6800 or $2000, due to data entry error or other reasons, the study team reassigned the cost value to the maximum allowed value.
Travel distance and time were derived by loading patient zip codes and HISA facility locations into the geographical information system program and using the nearest facility and find-route tools. These tools used a road network that simulates real-world driving conditions to calculate distance.
Study Variables
VWDs of any age, gender, and race/ethnicity who qualified for HISA and received HMs from fiscal year ( FY) 2015 through FY 2018 were identified (N = 30,823). Most VWDs were nonrural subjects (n = 19,970), and 43 had no Federal Information Processing System data. The final study cohort consisted of 10,810 HISA recipients. The NPPD, inpatient and outpatient data were merged by scrambled social security numbers to retrieve the following data: age, gender, race, ethnicity, marital status, Class (1 or 2), mean and total number of inpatient days, and type of HMs prescribed.
We also recorded rurality using the VA Rural-Urban Commuting Areas (RUCA) system, but we combined the rural and highly rural designation.1 Census tracts with a RUCA score of 10.0 are deemed highly rural, the remainder are considered rural except those with a RUCA score of 1.0 or 1.1. Travel time and distance from a veteran’s home to the VA facility that provided the HISA prescription were determined from zip codes. The current study focuses on VAMCs prescribing stations (affiliated sites of administrative parent medical facilities) where the HISA users obtained the HM, not the parent station (administrative parent medical facilities).
HISA Utilization
To characterize HISA utilization geographically and over time, the number of users were mapped by county. Areas where users were increasing (hot spots) or decreasing (cold spots) also were mapped. The maps were created using Environmental Systems Research Institute ArcGIS Pro 2.2.1 software. We chose to use natural breaks (Jenks) data classification method in a choropleth to symbolize the change over time map. We then used the Getis Ord GI* optimized hot spot analysis tool in the ArcGIS Pro spatial statistics tool set to generate the hot/cold spot maps. This tool identifies clusters of high values (hot spots) and low values (cold spots) creating a new output layer, RHUs by county, with a Z score, P value, and CI for each county. The Gi Bin field classifies statistically significant hot and cold spots. Counties sorted into the ± 3 category (bin) have a clustering characteristic (eg, with neighboring counties) that is statistically significant with a 99% CI; the ± 2 bin indicates a 95% CI for those county clustering sorted therein; ± 1 reflects a 90% CI; and 0 bin contains county features that have no statistical significant clustering with neighboring counties.
Data Analysis
Data were cleaned and analyzed using SAS 9.4 and R 3.5.3. Descriptive statistics are provided for sociodemographic characteristics, clinical characteristics, and class. ANOVA and t tests were used to compare continuous variables between groups, while χ2 and Fisher exact tests were used for dichotomous and categorical outcome variables. The threshold for statistical significance for these tests was set at α = .001.
Results
There were 10,810 RHUs from FY 2015 through FY 2018 and HISA utilization increased each year (Figure 1). Although some years may show usage decreases relative to previous fiscal years, the cumulative trends showed an increase relative to FY 2015 for both Classes of RVs (Figure 2). There was a 45.4% increase from FY 2015 to FY 2018 with a mean 13.6% yearly increase. Class 1 increased 21.0% and Class 2 increased 39.5% from FY 2015 to FY 2016 (Figure 3).
Most RHUs were male, White, and married. Class 1 and Class 2 RHUs differed significantly by age, race, marital status, and disability conditions: Class 1 RHUs were aged 6.6 years younger with a mean age of 69.1 years compared with 75.7 years for Class 2 users. For Class 1 RHUs, a plurality (29.4%) were aged 65 to 69 years; while a plurality (41.4%) of Class 2 users were aged ≥ 80 years. Musculoskeletal was the most common identified type of condition for all RHUs (Table 1).
To better understand HISA utilization patterns and net RHUs per county, we used a map to detail RHUs by county and change over time (Figure 4). Additionally, we compared US counties by RHUs from FY 2015 to FY 2018 and determined how clusters of high numbers of RHUs (hot spots) and low numbers of RHUs (cold spots) shifted over this period (Figure 5). While HISA utilization grew over the study period, the net count of RHUs per county varied by 9 to 20 persons/county. The population of RHUs increased over time in the Southwest, Southeast, and over much of the East/Northeast, while in the Central and Midwest regions, number of RHUs seems to decrease in population and/or use of the system. The cold spots in the Midwest and South Central US seem to increase with a significant relationship to neighboring counties having a low number of RHUs.
There were 11,166 HM prescribed to RHUs (Table 2). Bathroom HMs also were the dominant HM type for all facilities regardless of complexity levels (Table 3). The San Antonio, Texas, VAMC demonstrated the highest Class 1 vs Class 2 difference in HISA use (Class 1: 87.7% and Class 2: 12.3%). Except for the Des Moines VAMC, all other VAMCs showed HISA use > 60% by Class 1.
Cost Data
Air-conditioning installation ($5007) was the costliest HM overall (Table 4), closely followed by bathroom ($4978) and kitchen modifications ($4305). Bathroom renovations were the costliest HM type for both Class 1 and Class 2, closely followed by electrical repair and air-conditioning installation for Class 1 and driveway reconstruction and wooden ramp construction for Class 2.
The mean award received for HM was $4687 (Table 5). While the number of RHUs increased from FY 2015 to FY 2016, the average cost decreased, both overall ($280) and for Class 1 ($195) and Class 2 ($153). Except for a small decline in the number of Class 2 HISA recipients from FY 2017 to FY 2018, overall, the number of RHUs continuously grew from FY 2015 to FY 2018: 977 for the overall cohort, 678 for Class 1 and 299 for Class 2. Despite the obvious gain in the number of RHUs, the average costs did not notably change over time. VISN 21 had the highest mean cost, followed by VISNs 17, 6, 22, and 20.
Travel
Travel time and distance to the HISA prescribing facility differed significantly between Class 1 and Class 2 HISA users. RHUs had to travel about 95 minutes from their place of residence to access the HISA benefits program. There were no statistically significant differences between Class 1 and 2 users with respect to travel time and distance traveled (Table 6).
The majority of Class 1 and Class 2 veterans accessed the HISA from their nearest facility. However, nearly one-quarter of both Class 1 and 2 RHUs (24% each) did not. Among the 2598 who accessed the nonnearest facility, 97 (3.7%) accessed a facility that is ≤ 40 miles. Many (44%) users traveled 40 to 100 miles, and another 43.2% traveled 100 to 200 miles from their residence to access a HM prescription. Some 2598 users (1.1%) traveled > 500 miles to access a facility.
Discussion
Although utilization of the HISA program has steadily increased, overall participation by subpopulations such as RHUs can still be improved significantly. Veterans aged ≤ 46 years who have a disability that is common to those receiving HISA benefits have low HISA utilization. Similarly, veterans with sensory disabilities also have low use. These subpopulations are among those in great need of attention and services.
A study by Lucas and Zelaya, using the 2016 National Health Interview Survey data with an aim to measure degree of vision problems, dual sensory impairment, and hearing trouble in male veterans aged ≥ 18 years, found that veterans were more likely to report dual sensory impairment and balance difficulties when compared with nonveterans.9 The number of female veterans is growing but had very low representation in this study.10 This emerging VHA population requires information and education on their HM benefits.
Home Modifications
The most common HM prescribed for RHUs was for the bathroom. Further investigation is warranted as to why, given the diversity of HM types that the grant covers, low prescription rates exist across most of the HM types. There may be a lack of knowledge by providers and VWD as to the range of HMs that can be awarded under the grant. It is important that HCPs and veterans receive education on HISA HM options.
Semeah and colleagues pointed out the need for an assessment of the HISA HM ordering system to ensure that multiple HMs items (eg, kitchen, air conditioning, fees, driveway, and plumbing) are listed among the forced choices shown to clinicians to select from.7 Poor housing in rural America is widespread: 63% of rural dwellings need renovations and/or repairs to be accessible to individuals with disabilities, with > 6.7 million rural homes having no or faulty plumbing or kitchens; yet in this study, prescriptions for these HMs accounted for < 1%.11,12
VISN 6 had the most HISA awards with 1364, while VISN 21 had the fewest (245). Across all VISNs, Class 1 RHUs received more prescriptions than did Class 2 RHUs. Future research may seek to examine whether prescribers are fully aware of the eligibility of HM prescription to Class 2 veterans. VISN 21 ($5354); VISN 17 ($5302); and VISN 6 ($5301) had the highest mean HM expenditures. The national mean cost for HISA HMs were $4978 for bathrooms and $4305 for kitchens; for non-HISA HMs in FY 2017, the mean costs were $6362 and $12,255, respectively. A noteworthy concern is whether the maximum grant limit awards are sufficient to perform more expensive and complex HMs, such as the kitchen or major bathroom alternations.13
Facilities categorized as 1a, 1b, or 1c provided
North Florida/Sough Georgia was the highest-prescribing VAMC with 39% more HM prescriptions than the second highest prescribing facility (Durham, NC). Unfortunately, the data presented here cannot establish causality for the large variance difference between the top facilities, and the skewed distribution of total RHUs across VAMCs.
Travel-Related Variables
HISA beneficiaries face significant travel-related challenges. Just 3.6% of RHUs could access a facility within 40 miles of their home and 43.2% traveled 100 to 200 miles from their home to access a HM prescription. Further exploration is warranted to understand how travel patterns impact access to or the uptake of HISA.
RVs already have problems with accessing care because of long travel time.14,15 The choice or necessity to travel to a farther facility for HISA prescription is problematic for RVs, especially when transportation is often reported in the literature as a barrier to resources for people living in rural communities.15-17 When patients have travel barriers, they wait longer to obtain medical services and often wait for their conditions to worsen before seeking services.15,18 Once HM is completed, telerehabilitation is an effective delivery method used for delivering health care services to people in remote places.18,19 Considering that HISA use has the potential to improve quality of life, afford comfort, facilitate the accomplishment of activities of daily living for RVs, it is important that future studies examine how existing telehealth technologies can be used to improve HISA access.
Future Directions
County-level analyses is warranted in future studies exploring potential variables associated with HISA use; for example, county-level rates of primary care physicians and other HCPs. Future research should explore how long distance travel impacts the HISA application process and HM implementation. Further research also should focus on the HISA application structure and process to identify causes of delays. The HISA application process takes a mean 6 months to complete, yet the duration of hospital stays is 1 to 3 weeks, thus it is impossible to connect HISA to hospital discharge, which was the original intent of the program. Future research can examine how telehealth services can expedite HISA obtainment and coordination of the application process. Future research also may study the possible causes of the wide variations in HM prescriptions per facility. It is also important that educational programs provide information on the array of HM items that veterans can obtain.
Conclusions
In our previous study of the HISA cohort (2011-2017), we documented that an increase in utilization of the HISA program was warranted based on the low national budgetary appropriation and identification of significant low participation by vulnerable subpopulations, including veterans residing in rural areas or having returned from recent conflicts.7 The present study documents national utilization patterns, demographic profiles, and clinical characteristics of RHUs from FY 2015 through FY 2018, data that may be useful to policy makers and HISA administrators in predicting future use and users. It is important to note that the data and information presented in this article identify trends. The work in no way establishes a gold standard or any targeted goal of utilization. Future research could focus on conceptualizing or theorizing what steps are necessary to set such a gold standard of utilization rate and steps toward achievement.
Acknowledgments
This research was supported by grant 15521 from the US Department of Veterans Affairs, Office of Rural Health . Furthermore, the research was supported in part by grant K12 HD055929 from the National Institutes of Health.
1. US Department of Veterans Affairs, Veteran Health Administration, Office of Rural Health. Rural veteran health care challenges. Updated February 9, 2021. Accessed June 11, 2021. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
2. Holder, K.A. Veterans in rural America, 2011–2015. Published January 2017. Accessed June 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2017/acs/acs-36.pdf
3. Pezzin LE, Bogner HR, Kurichi JE, et al. Preventable hospitalizations, barriers to care, and disability. Medicine (Baltimore). 2018;97(19):e0691. doi:10.1097/MD.0000000000010691
4. Rosenbach ML. Access and satisfaction within the disabled Medicare population. Health Care Financ Rev. 1995;17(2):147-167.
5. Semeah LM, Ganesh SP, Wang X, et al. Home modification and health services utilization in rural and urban veterans with disabilities. Housing Policy Debate. 2021. Published online: March 4, 2021. doi:10.1080/10511482.2020.1858923
6. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, and Wilt TJ. Rural vs. urban ambulatory health care: A Systematic Review. Published May 2011. Accessed June 11, 2021. https://www.hsrd.research.va.gov/publications/esp/ambulatory.pdf
7. Semeah LM, Wang X, Cowper Ripley DC, et al. Improving health through a home modification service for veterans. In: Fiedler BA, ed. Three Facets of Public Health and Paths to Improvements. Academic Press; 2020:381-416.
8. Semeah LM, Ahrentzen S, Jia H, Cowper-Ripley DC, Levy CE, Mann WC. The home improvements and structural alterations benefits program: veterans with disabilities and home accessibility. J Disability Policy Studies. 2017;28(1):43-51. doi:10.1177/1044207317696275
9. Lucas, JW, Zelaya, CE. Hearing difficulty, vision trouble, and balance problems among male veterans and nonveterans. Published June 12, 2020. Accessed June 11, 2021. https://www.cdc.gov/nchs/data/nhsr/nhsr142-508.pdf
10. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed June 11, 2021. https://www.va.gov/vetdata/docs/SpecialReports/Women_Veterans_2015_Final.pdf
11. US Department of Housing and Urban Development, Office of Policy Development and Research. Housing challenges of rural seniors. Published 2017. Accessed June 11, 2021. https://www.huduser.gov/portal/periodicals/em/summer17/highlight1.html
12. Pendall R, Goodman L, Zhu J, Gold A. The future of rural housing. Published October 2016. Accessed June 11, 202.1 https://www.urban.org/sites/default/files/publication/85101/2000972-the-future-of-rural-housing_6.pdf
13. Joint Center for Housing Studies at Harvard University. Improving America’s housing 2019. Published 2019. Accessed June 11, 2021. https://www.jchs.harvard.edu/sites/default/files/reports/files/Harvard_JCHS_Improving_Americas_Housing_2019.pdf
14. Schooley BL, Horan TA, Lee PW, West PA. Rural veteran access to healthcare services: investigating the role of information and communication technologies in overcoming spatial barriers. Perspect Health Inf Manag. 2010;7(Spring):1f. Published 2010 Apr 1.
15. Ripley DC, Kwong PL, Vogel WB, Kurichi JE, Bates BE, Davenport C. How does geographic access affect in-hospital mortality for veterans with acute ischemic stroke?. Med Care. 2015;53(6):501-509. doi:10.1097/MLR.0000000000000366
16. Cowper-Ripley DC, Reker DM, Hayes J, et al. Geographic access to VHA rehabilitation services for traumatically injured veterans. Fed Pract. 2009;26(10):28-39.
17. Smith M, Towne S, Herrera-Venson A, Cameron K, Horel S, Ory M, et al. Delivery of fall prevention interventions for at-risk older adults in rural areas: Findings from a national dissemination. International journal of environmental research and public health. 2018;15:2798. doi: 10.3390/ijerph15122798
18. Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for Rural Veterans: A Qualitative Assessment of Barriers and Facilitators to Implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
19. Sarfo FS, Akassi J, Kyem G, et al. Long-Term Outcomes of Stroke in a Ghanaian Outpatient Clinic. J Stroke Cerebrovasc Dis. 2018;27(4):1090-1099. doi:10.1016/j.jstrokecerebrovasdis.2017.11.017
1. US Department of Veterans Affairs, Veteran Health Administration, Office of Rural Health. Rural veteran health care challenges. Updated February 9, 2021. Accessed June 11, 2021. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
2. Holder, K.A. Veterans in rural America, 2011–2015. Published January 2017. Accessed June 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2017/acs/acs-36.pdf
3. Pezzin LE, Bogner HR, Kurichi JE, et al. Preventable hospitalizations, barriers to care, and disability. Medicine (Baltimore). 2018;97(19):e0691. doi:10.1097/MD.0000000000010691
4. Rosenbach ML. Access and satisfaction within the disabled Medicare population. Health Care Financ Rev. 1995;17(2):147-167.
5. Semeah LM, Ganesh SP, Wang X, et al. Home modification and health services utilization in rural and urban veterans with disabilities. Housing Policy Debate. 2021. Published online: March 4, 2021. doi:10.1080/10511482.2020.1858923
6. Spoont M, Greer N, Su J, Fitzgerald P, Rutks I, and Wilt TJ. Rural vs. urban ambulatory health care: A Systematic Review. Published May 2011. Accessed June 11, 2021. https://www.hsrd.research.va.gov/publications/esp/ambulatory.pdf
7. Semeah LM, Wang X, Cowper Ripley DC, et al. Improving health through a home modification service for veterans. In: Fiedler BA, ed. Three Facets of Public Health and Paths to Improvements. Academic Press; 2020:381-416.
8. Semeah LM, Ahrentzen S, Jia H, Cowper-Ripley DC, Levy CE, Mann WC. The home improvements and structural alterations benefits program: veterans with disabilities and home accessibility. J Disability Policy Studies. 2017;28(1):43-51. doi:10.1177/1044207317696275
9. Lucas, JW, Zelaya, CE. Hearing difficulty, vision trouble, and balance problems among male veterans and nonveterans. Published June 12, 2020. Accessed June 11, 2021. https://www.cdc.gov/nchs/data/nhsr/nhsr142-508.pdf
10. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Published February 2017. Accessed June 11, 2021. https://www.va.gov/vetdata/docs/SpecialReports/Women_Veterans_2015_Final.pdf
11. US Department of Housing and Urban Development, Office of Policy Development and Research. Housing challenges of rural seniors. Published 2017. Accessed June 11, 2021. https://www.huduser.gov/portal/periodicals/em/summer17/highlight1.html
12. Pendall R, Goodman L, Zhu J, Gold A. The future of rural housing. Published October 2016. Accessed June 11, 202.1 https://www.urban.org/sites/default/files/publication/85101/2000972-the-future-of-rural-housing_6.pdf
13. Joint Center for Housing Studies at Harvard University. Improving America’s housing 2019. Published 2019. Accessed June 11, 2021. https://www.jchs.harvard.edu/sites/default/files/reports/files/Harvard_JCHS_Improving_Americas_Housing_2019.pdf
14. Schooley BL, Horan TA, Lee PW, West PA. Rural veteran access to healthcare services: investigating the role of information and communication technologies in overcoming spatial barriers. Perspect Health Inf Manag. 2010;7(Spring):1f. Published 2010 Apr 1.
15. Ripley DC, Kwong PL, Vogel WB, Kurichi JE, Bates BE, Davenport C. How does geographic access affect in-hospital mortality for veterans with acute ischemic stroke?. Med Care. 2015;53(6):501-509. doi:10.1097/MLR.0000000000000366
16. Cowper-Ripley DC, Reker DM, Hayes J, et al. Geographic access to VHA rehabilitation services for traumatically injured veterans. Fed Pract. 2009;26(10):28-39.
17. Smith M, Towne S, Herrera-Venson A, Cameron K, Horel S, Ory M, et al. Delivery of fall prevention interventions for at-risk older adults in rural areas: Findings from a national dissemination. International journal of environmental research and public health. 2018;15:2798. doi: 10.3390/ijerph15122798
18. Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for Rural Veterans: A Qualitative Assessment of Barriers and Facilitators to Implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
19. Sarfo FS, Akassi J, Kyem G, et al. Long-Term Outcomes of Stroke in a Ghanaian Outpatient Clinic. J Stroke Cerebrovasc Dis. 2018;27(4):1090-1099. doi:10.1016/j.jstrokecerebrovasdis.2017.11.017
Preoperative Care Assessment of Need Scores Are Associated With Postoperative Mortality and Length of Stay in Veterans Undergoing Knee Replacement
Risk calculators can be of great value in guiding clinical decision making, patient-centered precision medicine, and resource allocation.1 Several perioperative risk prediction models have emerged in recent decades that estimate specific hazards (eg, cardiovascular complications after noncardiac surgery) with varying accuracy and utility. In the perioperative sphere, the time windows are often limited to an index hospitalization or 30 days following surgery or discharge.2-9 Although longer periods are of interest to patients, families, and health systems, few widely used or validated models are designed to look beyond this very narrow window.10,11 In addition, perioperative risk prediction models do not routinely incorporate parameters of a wide variety of health or demographic domains, such as patterns of health care, health care utilization, or medication use.
In 2013, in response to the need for near real-time information to guide delivery of enhanced care management services, the Veterans Health Administration (VHA) Office of Informatics and Analytics developed automated risk prediction models that used detailed electronic health record (EHR) data. These models were used to report Care Assessment Need (CAN) scores each week for all VHA enrollees and include data from a wide array of health domains. These CAN scores predict the risk for hospitalization, death, or either event within 90 days and 1 year.12,13 Each score is reported as both a predicted probability (0-1) and as a percentile in relation to all other VHA enrollees (a value between 1 and 99).13 The data used to calculate CAN scores are listed in Table 1.12
Surgical procedures or admissions would not be differentiated from nonsurgical admissions or other procedural clinic visits, and as such, it is not possible to isolate the effect of undergoing a surgical procedure from another health-related event on the CAN score. At the same time though, a short-term increase in system utilization caused by an elective surgical procedure such as a total knee replacement (TKR) would presumably be reflected in a change in CAN score, but this has not been studied.
Since their introduction, CAN scores have been routinely accessed by primary care teams and used to facilitate care coordination for thousands of VHA patients. However, these CAN scores are currently not available to VHA surgeons, anesthesiologists, or other perioperative clinicians. In this study, we examine the distributions of preoperative CAN scores and explore the relationships of preoperative CAN 1-year mortality scores with 1-year survival following discharge and length of stay (LOS) during index hospitalization in a cohort of US veterans who underwent TKR, the most common elective operation performed within the VHA system.
Methods
Following approval of the Durham Veterans Affairs Medical Center Institutional Review Board, all necessary data were extracted from the VHA Corporate Data Warehouse (CDW) repository.14 Informed consent was waived due to the minimal risk nature of the study.
We used Current Procedural Terminology codes (27438, 27446, 27447, 27486, 27487, 27488) and International Classification of Diseases, 9th edition clinical modification procedure codes (81.54, 81.55, 81.59, 00.80-00.84) to identify all veterans who had undergone primary or revision TKR between July 2014 and December 2015 in VHA Veterans Integrated Service Network 1 (Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode Island, New York, Pennsylvania, West Virginia, Virginia, North Carolina). Because we focused on outcomes following hospital discharge, patients who died before discharge were excluded from the analysis. Preoperative CAN 1-year mortality score was chosen as the measure under the assumption that long-term survival may be the most meaningful of the 4 possible CAN score measures.
Our primary objective was to determine distribution of preoperative CAN scores in the study population. Our secondary was to study relationships among the preoperative CAN 1-year mortality scores and 1-year mortality and hospital LOS.
Study Variables
For each patient, we extracted the date of index surgery. The primary exposure or independent variable was the CAN score in the week prior to this date. Because prior study has shown that CAN scores trajectories do not significantly change over time, the date-stamped CAN scores in the week before surgery represent what would have been available to clinicians in a preoperative setting.15 Since CAN scores are refreshed and overwritten every week, we extracted archived scores from the CDW.
For the 1-year survival outcome, the primary dependent variable, we queried the vital status files in the CDW for the date of death if applicable. We confirmed survival beyond 1 year by examining vital signs in the CDW for a minimum of 2 independent encounters beyond 1 year after the date of discharge. To compute the index LOS, the secondary outcome, we computed the difference between the date of admission and date of hospital discharge.
Statistical Methods
The parameters and performance of the multivariable logistic regression models developed to compute the various CAN mortality and hospitalization risk scores have been previously described.12 Briefly, Wang and colleagues created parsimonious regression models using backward selection. Model discrimination was evaluated using C (concordance)-statistic. Model calibration was assessed by comparing predicted vs observed event rates by risk deciles and performing Cox proportional hazards regression.
We plotted histograms to display preoperative CAN scores as a simple measure of distribution (Figure 1). We also examined the cumulative proportion of patients at each preoperative CAN 1-year mortality score.
Using a conventional t test, we compared means of preoperative CAN 1-year mortality scores in patients who survived vs those who died within 1 year. We also constructed a plot of the proportion of patients who had died within 1 year vs preoperative CAN 1-year mortality scores. Kaplan-Meier curves were then constructed examining 1-year survival by CAN 1-year mortality score by terciles.
Finally, we examined the relationship between preoperative CAN 1-year mortality scores and index LOS in 2 ways: We plotted LOS across CAN scores, and we constructed a
Results
We identified 8206 patients who had undergone a TKR over the 18-month study period. The overall mean (SD) for age was 65 (8.41) years; 93% were male, and 78% were White veterans. Patient demographics are well described in a previous publication.16,17
In terms of model parameters for the CAN score models, C-statistics for the 90-day outcome models were as follows: 0.833 for the model predicting hospitalization (95% CI, 0.832-0.834); 0.865 for the model predicting death (95% CI, 0.863-0.876); and 0.811 for the model predicting either event (95% CI, 0.810-0.812). C-statistics for the 1-year outcome models were 0.809 for the model predicting hospitalization (95% CI, 0.808-0.810); 0.851 for the model predicting death (95% CI, 0.849-0.852); and 0.787 for the model predicting either event (95% CI, 0.786-0.787). Models were well calibrated with α = 0 and β = 1, demonstrating strong agreement between observed and predicted event rates.
The distribution of preoperative CAN 1-year mortality scores was close to normal (median, 50; interquartile range, 40; mean [SD] 48 [25.6]) (eTable). The original CAN score models were developed having an equal number of patients in each strata and as such, are normally distributed.12 Our cohort was similar in pattern of distribution. Distributions of the remaining preoperative CAN scores (90-day mortality, 1-year hospitalization, 90-day hospitalization) are shown in Figures 2, 3, and 4. Not surprisingly, histograms for both 90-day and 1-year hospitalization were skewed toward higher scores, indicating that these patients were expected to be hospitalized in the near future.
Overall, 1.4% (110/8096) of patients died within 1 year of surgery. Comparing 1-year mortality CAN scores in survivors vs nonsurvivors, we found statistically significant differences in means (47 vs 66 respectively, P < .001) and medians (45 vs 75 respectively, P < .001) (Table 2). In the plot examining the relationship between preoperative 1-year mortality CAN scores and 1-year mortality, the percentage who died within 1 year increased initially for patients with CAN scores > 60 and again exponentially for patients with CAN scores > 80. Examining Kaplan-Meier curves, we found that survivors and nonsurvivors separated early after surgery, and the differences between the top tercile and the middle/lower terciles were statistically significant (P < .001). Mortality rates were about 0.5% in the lower and middle terciles but about 2% in the upper tercile (Figure 5).
In the plot examining the relationship between CAN scores and index LOS, the LOS rose significantly beyond a CAN score of 60 and dramatically beyond a CAN score of 80 (Figure 6). LOESS curves also showed 2 inflection points suggesting an incremental and sequential rise in the LOS with increasing CAN scores (Figure 7). Mean (SD) LOS in days for the lowest to highest terciles was 2.6 (1.7), 2.8 (2.1), and 3.6 (2.2), respectively.
Discussion
CAN scores are automatically generated each week by EHR-based multivariable risk models. These scores have excellent predictive accuracy for 90-day and 1-year mortality and hospitalization and are routinely used by VHA primary care teams to assist with clinical operations.13 We studied the distribution of CAN 1-year mortality scores in a preoperative context and examined relationships of the preoperative CAN 1-year mortality scores with postoperative mortality and LOS in 8206 veterans who underwent TKR.
There are several noteworthy findings. First, the overall 1-year mortality rate observed following TKR (1.4%) was similar to other published reports.18,19 Not surprisingly, preoperative CAN 1-year mortality scores were significantly higher in veterans who died compared with those of survivors. The majority of patients who died had a preoperative CAN 1-year mortality score > 75 while most who survived had a preoperative CAN 1-year mortality score < 45 (P < .001). Interestingly, the same scores showed a nonlinear correlation with LOS. Index LOS was about 4 days in patients in the highest tercile of CAN scores vs 2.5 days in the lowest tercile, but the initial increase in LOS was detected at a CAN score of about 55 to 60.
In addition, mortality rate varied widely in different segments of the population when grouped according to preoperative CAN scores. One-year mortality rates in the highest tercile reached 2%, about 4-fold higher than that of lower terciles (0.5%). Examination of the Kaplan-Meier curves showed that this difference in mortality between the highest tercile and the lower 2 groups appears soon after discharge and continues to increase over time, suggesting that the factors contributing to the increased mortality are present at the time of discharge and persist beyond the postoperative period. In summary, although CAN scores were not designed for use in the perioperative context, we found that preoperative CAN 1-year mortality scores are broadly predictive of mortality, but especially for increases in LOS following elective TKA, both increases in hospital LOS following elective TKA and mortality over the year after TKA.
Our findings raise several important questions. The decision to undergo elective surgery is complex. Arguably, individuals who undergo elective knee replacement should be healthy enough to undergo, recover, and reap the benefits from a procedure that does not extend life. The distribution of preoperative CAN 1-year mortality scores for our study population was similar to that of the general VHA enrollee population with similar measured mortality rates (≤ 0.5% vs ≥ 1.7% in the low and high terciles, respectively).1 Further study comparing outcomes in matched cohorts who did and did not undergo joint replacement would be of interest. In lieu of this, though, the association of high but not extreme CAN scores with increased hospital LOS may potentially be used to guide allocation of resources to this group, obviating the increased cost and risk to which this group is exposed. And the additional insight afforded by CAN scores may enhance shared decision-making models by identifying patients at the very highest risk (eg, 1-year mortality CAN score ≥ 90), patients who conceivably might not survive long enough to recover from and enjoy their reconstructed knee, who might in the long run be harmed by undergoing the procedure.
Many total joint arthroplasties are performed in older patients, a population in which frailty is increasingly recognized as a significant risk factor for poor outcomes.20,21 CAN scores reliably identify high-risk patients and have been shown to correlate with frailty in this group.22 Multiple authors have reported improved outcomes with cost reductions after implementation of programs targeting modifiable risk factors in high-risk surgical candidates.23-25 A preoperative assessment that includes the CAN score may be valuable in identifying patients who would benefit most from prehabilitation programs or other interventions designed to blunt the impact of frailty. It is true that many elements used to calculate the CAN score would not be considered modifiable, especially in the short term. However, specific contributors to frailty, such as nutritional status and polypharmacy might be potential candidates. As with all multivariable risk prediction models, there are multiple paths to a high CAN score, and further research to identify clinically relevant subgroups may help inform efforts to improve perioperative care within this population.
Hospital LOS is of intense interest for many reasons, not least its utility as a surrogate for cost and increased risk for immediate perioperative adverse events, such as multidrug-resistant hospital acquired infections, need for postacute facility-based rehabilitation, and deconditioning that increase risks of falls and fractures in the older population.26-29 In addition, its importance is magnified due to the COVID-19 pandemic context in which restarting elective surgery programs has changed traditional criteria by which patients are scheduled for surgery.
We have shown that elevated CAN scores are able to identify patients at risk for extended hospital stays and, as such, may be useful additional data in allocating scarce operating room time and other resources for optimal patient and health care provider safety.30,31 Individual surgeons and hospital systems would, of course, decide which patients should be triaged to go first, based on local priorities; however, choosing lower risk patients with minimal risk of morbidity and mortality while pursuing prehabilitation for higher risk patients is a reasonable approach.
Limitations
Our study has several limitations. Only a single surgical procedure was included, albeit the most common one performed in the VHA. In addition, no information was available concerning the precise clinical course for these patients, such as the duration of surgery, anesthetic technique, and management of acute, perioperative course. Although the assumption was made that patients received standard care in a manner such that these factors would not significantly affect either their mortality or their LOS out of proportion to their preoperative clinical status, confounding cannot be excluded. Therefore, further study is necessary to determine whether CAN scores can accurately predict mortality and/or LOS for patients undergoing other procedures. Further, a clinical trial is required to assess whether systematic provision of the CAN score at the point of surgery would impact care and, more important, impact outcomes. In addition, multivariable analyses were not performed, including and excluding various components of the CAN score models. Currently, CAN scores could be made available to the surgical/anesthesia communities at minimal or no cost and are updated automatically. Model calibration and discrimination in this particular setting were not validated.
Because our interest is in leveraging an existing resource to a current clinical and operational problem rather than in creating or validating a new tool, we chose to test the simple bivariate relationship between preoperative CAN scores and outcomes. We chose the preoperative 1-year mortality CAN score from among the 4 options under the assumption that long-term survival is the most meaningful of the 4 candidate outcomes. Finally, while the CAN scores are currently only calculated and generated for patients cared for within the VHA, few data elements are unavailable to civilian health systems. The most problematic would be documentation of actual prescription filling, but this is a topic of increasing interest to the medical and academic communities and access to such information we hope will improve.32-34
Conclusions
Although designed for use by VHA primary care teams, CAN scores also may have value for perioperative clinicians, predicting mortality and prolonged hospital LOS in those with elevated 1-year mortality scores. Advantages of CAN scores relative to other perioperative risk calculators lies in their ability to predict long-term rather than 30-day survival and that they are automatically generated on a near-real-time basis for all patients who receive care in VHA ambulatory clinics. Further study is needed to determine practical utility in shared decision making, preoperative evaluation and optimization, and perioperative resource allocation.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) National Center for Patient Safety, Field Office 10A4E, through the Patient Safety Center of Inquiry at the Durham VA Medical Center in North Carolina. The study also received support from the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham VA Health Care System.
1. McNair AGK, MacKichan F, Donovan JL, et al. What surgeons tell patients and what patients want to know before major cancer surgery: a qualitative study. BMC Cancer. 2016;16:258. doi:10.1186/s12885-016-2292-3
2. Grover FL, Hammermeister KE, Burchfiel C. Initial report of the Veterans Administration Preoperative Risk Assessment Study for Cardiac Surgery. Ann Thorac Surg. 1990;50(1):12-26; discussion 27-18. doi:10.1016/0003-4975(90)90073-f
3. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
4. Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702. doi:10.1097/SLA.0b013e31824b45af
5. Keller DS, Kroll D, Papaconstantinou HT, Ellis CN. Development and validation of a methodology to reduce mortality using the veterans affairs surgical quality improvement program risk calculator. J Am Coll Surg. 2017;224(4):602-607. doi:10.1016/j.jamcollsurg.2016.12.033
6. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842.e831-833. doi:10.1016/j.jamcollsurg.2013.07.385
7. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26-35. doi:10.7326/0003-4819-152-1-201001050-00007
8. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi:10.1161/CIRCULATIONAHA.110.015701
9. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi:10.1161/01.cir.100.10.1043
10. Smith T, Li X, Nylander W, Gunnar W. Thirty-day postoperative mortality risk estimates and 1-year survival in Veterans Health Administration surgery patients. JAMA Surg. 2016;151(5):417-422. doi:10.1001/jamasurg.2015.4882
11. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90- day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149-1154. doi:10.1002/bjs.8813
12. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1016/j.amjcard.2012.06.038
13. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
14. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
15. Wong ES, Yoon J, Piegari RI, Rosland AM, Fihn SD, Chang ET. Identifying latent subgroups of high-risk patients using risk score trajectories. J Gen Intern Med. 2018;33(12):2120-2126. doi:10.1007/s11606-018-4653-x
16. Chen Q, Hsia HL, Overman R, et al. Impact of an opioid safety initiative on patients undergoing total knee arthroplasty: a time series analysis. Anesthesiology. 2019;131(2):369-380. doi:10.1097/ALN.0000000000002771
17. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
18. Inacio MCS, Dillon MT, Miric A, Navarro RA, Paxton EW. Mortality after total knee and total hip arthroplasty in a large integrated health care system. Perm J. 2017;21:16-171. doi:10.7812/TPP/16-171
19. Lee QJ, Mak WP, Wong YC. Mortality following primary total knee replacement in public hospitals in Hong Kong. Hong Kong Med J. 2016;22(3):237-241. doi:10.12809/hkmj154712
20. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi:10.1186/s12877-016-0329-8
21. Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2019;155(1):e194620. doi:10.1001/jamasurg.2019.4620
22. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
23. Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33(12):3642- 3648. doi:10.1016/j.arth.2018.08.018
24. Sodhi N, Anis HK, Coste M, et al. A nationwide analysis of preoperative planning on operative times and postoperative complications in total knee arthroplasty. J Knee Surg. 2019;32(11):1040-1045. doi:10.1055/s-0039-1677790
25. Krause A, Sayeed Z, El-Othmani M, Pallekonda V, Mihalko W, Saleh KJ. Outpatient total knee arthroplasty: are we there yet? (part 1). Orthop Clin North Am. 2018;49(1):1-6. doi:10.1016/j.ocl.2017.08.002
26. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca- Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644-652. doi:10.1093/cid/cix411
27. Nikkel LE, Kates SL, Schreck M, Maceroli M, Mahmood B, Elfar JC. Length of hospital stay after hip fracture and risk of early mortality after discharge in New York state: retrospective cohort study. BMJ. 2015;351:h6246. doi:10.1136/bmj.h6246
28. Marfil-Garza BA, Belaunzarán-Zamudio PF, Gulias-Herrero A, et al. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS One. 2018;13(11):e0207203. doi:10.1371/journal.pone.0207203
29. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi:10.1111/j.1532-5415.1990.tb03451.x
30. Iyengar KP, Jain VK, Vaish A, Vaishya R, Maini L, Lal H. Post COVID-19: planning strategies to resume orthopaedic surgery -challenges and considerations. J Clin Orthop Trauma. 2020;11(suppl 3):S291-S295. doi:10.1016/j.jcot.2020.04.028
31. O’Connor CM, Anoushiravani AA, DiCaprio MR, Healy WL, Iorio R. Economic recovery after the COVID-19 pandemic: resuming elective orthopedic surgery and total joint arthroplasty. J Arthroplasty. 2020;35(suppl 7):S32-S36. doi:10.1016/j.arth.2020.04.038.
32. Mauseth SA, Skurtveit S, Skovlund E, Langhammer A, Spigset O. Medication use and association with urinary incontinence in women: data from the Norwegian Prescription Database and the HUNT study. Neurourol Urodyn. 2018;37(4):1448-1457. doi:10.1002/nau.23473
33. Sultan RS, Correll CU, Schoenbaum M, King M, Walkup JT, Olfson M. National patterns of commonly prescribed psychotropic medications to young people. J Child Adolesc Psychopharmacol. 2018;28(3):158-165. doi:10.1089/cap.2017.0077
34. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S.-the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21(12):702-712. doi:10.1089/dia.2019.0213
Risk calculators can be of great value in guiding clinical decision making, patient-centered precision medicine, and resource allocation.1 Several perioperative risk prediction models have emerged in recent decades that estimate specific hazards (eg, cardiovascular complications after noncardiac surgery) with varying accuracy and utility. In the perioperative sphere, the time windows are often limited to an index hospitalization or 30 days following surgery or discharge.2-9 Although longer periods are of interest to patients, families, and health systems, few widely used or validated models are designed to look beyond this very narrow window.10,11 In addition, perioperative risk prediction models do not routinely incorporate parameters of a wide variety of health or demographic domains, such as patterns of health care, health care utilization, or medication use.
In 2013, in response to the need for near real-time information to guide delivery of enhanced care management services, the Veterans Health Administration (VHA) Office of Informatics and Analytics developed automated risk prediction models that used detailed electronic health record (EHR) data. These models were used to report Care Assessment Need (CAN) scores each week for all VHA enrollees and include data from a wide array of health domains. These CAN scores predict the risk for hospitalization, death, or either event within 90 days and 1 year.12,13 Each score is reported as both a predicted probability (0-1) and as a percentile in relation to all other VHA enrollees (a value between 1 and 99).13 The data used to calculate CAN scores are listed in Table 1.12
Surgical procedures or admissions would not be differentiated from nonsurgical admissions or other procedural clinic visits, and as such, it is not possible to isolate the effect of undergoing a surgical procedure from another health-related event on the CAN score. At the same time though, a short-term increase in system utilization caused by an elective surgical procedure such as a total knee replacement (TKR) would presumably be reflected in a change in CAN score, but this has not been studied.
Since their introduction, CAN scores have been routinely accessed by primary care teams and used to facilitate care coordination for thousands of VHA patients. However, these CAN scores are currently not available to VHA surgeons, anesthesiologists, or other perioperative clinicians. In this study, we examine the distributions of preoperative CAN scores and explore the relationships of preoperative CAN 1-year mortality scores with 1-year survival following discharge and length of stay (LOS) during index hospitalization in a cohort of US veterans who underwent TKR, the most common elective operation performed within the VHA system.
Methods
Following approval of the Durham Veterans Affairs Medical Center Institutional Review Board, all necessary data were extracted from the VHA Corporate Data Warehouse (CDW) repository.14 Informed consent was waived due to the minimal risk nature of the study.
We used Current Procedural Terminology codes (27438, 27446, 27447, 27486, 27487, 27488) and International Classification of Diseases, 9th edition clinical modification procedure codes (81.54, 81.55, 81.59, 00.80-00.84) to identify all veterans who had undergone primary or revision TKR between July 2014 and December 2015 in VHA Veterans Integrated Service Network 1 (Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode Island, New York, Pennsylvania, West Virginia, Virginia, North Carolina). Because we focused on outcomes following hospital discharge, patients who died before discharge were excluded from the analysis. Preoperative CAN 1-year mortality score was chosen as the measure under the assumption that long-term survival may be the most meaningful of the 4 possible CAN score measures.
Our primary objective was to determine distribution of preoperative CAN scores in the study population. Our secondary was to study relationships among the preoperative CAN 1-year mortality scores and 1-year mortality and hospital LOS.
Study Variables
For each patient, we extracted the date of index surgery. The primary exposure or independent variable was the CAN score in the week prior to this date. Because prior study has shown that CAN scores trajectories do not significantly change over time, the date-stamped CAN scores in the week before surgery represent what would have been available to clinicians in a preoperative setting.15 Since CAN scores are refreshed and overwritten every week, we extracted archived scores from the CDW.
For the 1-year survival outcome, the primary dependent variable, we queried the vital status files in the CDW for the date of death if applicable. We confirmed survival beyond 1 year by examining vital signs in the CDW for a minimum of 2 independent encounters beyond 1 year after the date of discharge. To compute the index LOS, the secondary outcome, we computed the difference between the date of admission and date of hospital discharge.
Statistical Methods
The parameters and performance of the multivariable logistic regression models developed to compute the various CAN mortality and hospitalization risk scores have been previously described.12 Briefly, Wang and colleagues created parsimonious regression models using backward selection. Model discrimination was evaluated using C (concordance)-statistic. Model calibration was assessed by comparing predicted vs observed event rates by risk deciles and performing Cox proportional hazards regression.
We plotted histograms to display preoperative CAN scores as a simple measure of distribution (Figure 1). We also examined the cumulative proportion of patients at each preoperative CAN 1-year mortality score.
Using a conventional t test, we compared means of preoperative CAN 1-year mortality scores in patients who survived vs those who died within 1 year. We also constructed a plot of the proportion of patients who had died within 1 year vs preoperative CAN 1-year mortality scores. Kaplan-Meier curves were then constructed examining 1-year survival by CAN 1-year mortality score by terciles.
Finally, we examined the relationship between preoperative CAN 1-year mortality scores and index LOS in 2 ways: We plotted LOS across CAN scores, and we constructed a
Results
We identified 8206 patients who had undergone a TKR over the 18-month study period. The overall mean (SD) for age was 65 (8.41) years; 93% were male, and 78% were White veterans. Patient demographics are well described in a previous publication.16,17
In terms of model parameters for the CAN score models, C-statistics for the 90-day outcome models were as follows: 0.833 for the model predicting hospitalization (95% CI, 0.832-0.834); 0.865 for the model predicting death (95% CI, 0.863-0.876); and 0.811 for the model predicting either event (95% CI, 0.810-0.812). C-statistics for the 1-year outcome models were 0.809 for the model predicting hospitalization (95% CI, 0.808-0.810); 0.851 for the model predicting death (95% CI, 0.849-0.852); and 0.787 for the model predicting either event (95% CI, 0.786-0.787). Models were well calibrated with α = 0 and β = 1, demonstrating strong agreement between observed and predicted event rates.
The distribution of preoperative CAN 1-year mortality scores was close to normal (median, 50; interquartile range, 40; mean [SD] 48 [25.6]) (eTable). The original CAN score models were developed having an equal number of patients in each strata and as such, are normally distributed.12 Our cohort was similar in pattern of distribution. Distributions of the remaining preoperative CAN scores (90-day mortality, 1-year hospitalization, 90-day hospitalization) are shown in Figures 2, 3, and 4. Not surprisingly, histograms for both 90-day and 1-year hospitalization were skewed toward higher scores, indicating that these patients were expected to be hospitalized in the near future.
Overall, 1.4% (110/8096) of patients died within 1 year of surgery. Comparing 1-year mortality CAN scores in survivors vs nonsurvivors, we found statistically significant differences in means (47 vs 66 respectively, P < .001) and medians (45 vs 75 respectively, P < .001) (Table 2). In the plot examining the relationship between preoperative 1-year mortality CAN scores and 1-year mortality, the percentage who died within 1 year increased initially for patients with CAN scores > 60 and again exponentially for patients with CAN scores > 80. Examining Kaplan-Meier curves, we found that survivors and nonsurvivors separated early after surgery, and the differences between the top tercile and the middle/lower terciles were statistically significant (P < .001). Mortality rates were about 0.5% in the lower and middle terciles but about 2% in the upper tercile (Figure 5).
In the plot examining the relationship between CAN scores and index LOS, the LOS rose significantly beyond a CAN score of 60 and dramatically beyond a CAN score of 80 (Figure 6). LOESS curves also showed 2 inflection points suggesting an incremental and sequential rise in the LOS with increasing CAN scores (Figure 7). Mean (SD) LOS in days for the lowest to highest terciles was 2.6 (1.7), 2.8 (2.1), and 3.6 (2.2), respectively.
Discussion
CAN scores are automatically generated each week by EHR-based multivariable risk models. These scores have excellent predictive accuracy for 90-day and 1-year mortality and hospitalization and are routinely used by VHA primary care teams to assist with clinical operations.13 We studied the distribution of CAN 1-year mortality scores in a preoperative context and examined relationships of the preoperative CAN 1-year mortality scores with postoperative mortality and LOS in 8206 veterans who underwent TKR.
There are several noteworthy findings. First, the overall 1-year mortality rate observed following TKR (1.4%) was similar to other published reports.18,19 Not surprisingly, preoperative CAN 1-year mortality scores were significantly higher in veterans who died compared with those of survivors. The majority of patients who died had a preoperative CAN 1-year mortality score > 75 while most who survived had a preoperative CAN 1-year mortality score < 45 (P < .001). Interestingly, the same scores showed a nonlinear correlation with LOS. Index LOS was about 4 days in patients in the highest tercile of CAN scores vs 2.5 days in the lowest tercile, but the initial increase in LOS was detected at a CAN score of about 55 to 60.
In addition, mortality rate varied widely in different segments of the population when grouped according to preoperative CAN scores. One-year mortality rates in the highest tercile reached 2%, about 4-fold higher than that of lower terciles (0.5%). Examination of the Kaplan-Meier curves showed that this difference in mortality between the highest tercile and the lower 2 groups appears soon after discharge and continues to increase over time, suggesting that the factors contributing to the increased mortality are present at the time of discharge and persist beyond the postoperative period. In summary, although CAN scores were not designed for use in the perioperative context, we found that preoperative CAN 1-year mortality scores are broadly predictive of mortality, but especially for increases in LOS following elective TKA, both increases in hospital LOS following elective TKA and mortality over the year after TKA.
Our findings raise several important questions. The decision to undergo elective surgery is complex. Arguably, individuals who undergo elective knee replacement should be healthy enough to undergo, recover, and reap the benefits from a procedure that does not extend life. The distribution of preoperative CAN 1-year mortality scores for our study population was similar to that of the general VHA enrollee population with similar measured mortality rates (≤ 0.5% vs ≥ 1.7% in the low and high terciles, respectively).1 Further study comparing outcomes in matched cohorts who did and did not undergo joint replacement would be of interest. In lieu of this, though, the association of high but not extreme CAN scores with increased hospital LOS may potentially be used to guide allocation of resources to this group, obviating the increased cost and risk to which this group is exposed. And the additional insight afforded by CAN scores may enhance shared decision-making models by identifying patients at the very highest risk (eg, 1-year mortality CAN score ≥ 90), patients who conceivably might not survive long enough to recover from and enjoy their reconstructed knee, who might in the long run be harmed by undergoing the procedure.
Many total joint arthroplasties are performed in older patients, a population in which frailty is increasingly recognized as a significant risk factor for poor outcomes.20,21 CAN scores reliably identify high-risk patients and have been shown to correlate with frailty in this group.22 Multiple authors have reported improved outcomes with cost reductions after implementation of programs targeting modifiable risk factors in high-risk surgical candidates.23-25 A preoperative assessment that includes the CAN score may be valuable in identifying patients who would benefit most from prehabilitation programs or other interventions designed to blunt the impact of frailty. It is true that many elements used to calculate the CAN score would not be considered modifiable, especially in the short term. However, specific contributors to frailty, such as nutritional status and polypharmacy might be potential candidates. As with all multivariable risk prediction models, there are multiple paths to a high CAN score, and further research to identify clinically relevant subgroups may help inform efforts to improve perioperative care within this population.
Hospital LOS is of intense interest for many reasons, not least its utility as a surrogate for cost and increased risk for immediate perioperative adverse events, such as multidrug-resistant hospital acquired infections, need for postacute facility-based rehabilitation, and deconditioning that increase risks of falls and fractures in the older population.26-29 In addition, its importance is magnified due to the COVID-19 pandemic context in which restarting elective surgery programs has changed traditional criteria by which patients are scheduled for surgery.
We have shown that elevated CAN scores are able to identify patients at risk for extended hospital stays and, as such, may be useful additional data in allocating scarce operating room time and other resources for optimal patient and health care provider safety.30,31 Individual surgeons and hospital systems would, of course, decide which patients should be triaged to go first, based on local priorities; however, choosing lower risk patients with minimal risk of morbidity and mortality while pursuing prehabilitation for higher risk patients is a reasonable approach.
Limitations
Our study has several limitations. Only a single surgical procedure was included, albeit the most common one performed in the VHA. In addition, no information was available concerning the precise clinical course for these patients, such as the duration of surgery, anesthetic technique, and management of acute, perioperative course. Although the assumption was made that patients received standard care in a manner such that these factors would not significantly affect either their mortality or their LOS out of proportion to their preoperative clinical status, confounding cannot be excluded. Therefore, further study is necessary to determine whether CAN scores can accurately predict mortality and/or LOS for patients undergoing other procedures. Further, a clinical trial is required to assess whether systematic provision of the CAN score at the point of surgery would impact care and, more important, impact outcomes. In addition, multivariable analyses were not performed, including and excluding various components of the CAN score models. Currently, CAN scores could be made available to the surgical/anesthesia communities at minimal or no cost and are updated automatically. Model calibration and discrimination in this particular setting were not validated.
Because our interest is in leveraging an existing resource to a current clinical and operational problem rather than in creating or validating a new tool, we chose to test the simple bivariate relationship between preoperative CAN scores and outcomes. We chose the preoperative 1-year mortality CAN score from among the 4 options under the assumption that long-term survival is the most meaningful of the 4 candidate outcomes. Finally, while the CAN scores are currently only calculated and generated for patients cared for within the VHA, few data elements are unavailable to civilian health systems. The most problematic would be documentation of actual prescription filling, but this is a topic of increasing interest to the medical and academic communities and access to such information we hope will improve.32-34
Conclusions
Although designed for use by VHA primary care teams, CAN scores also may have value for perioperative clinicians, predicting mortality and prolonged hospital LOS in those with elevated 1-year mortality scores. Advantages of CAN scores relative to other perioperative risk calculators lies in their ability to predict long-term rather than 30-day survival and that they are automatically generated on a near-real-time basis for all patients who receive care in VHA ambulatory clinics. Further study is needed to determine practical utility in shared decision making, preoperative evaluation and optimization, and perioperative resource allocation.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) National Center for Patient Safety, Field Office 10A4E, through the Patient Safety Center of Inquiry at the Durham VA Medical Center in North Carolina. The study also received support from the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham VA Health Care System.
Risk calculators can be of great value in guiding clinical decision making, patient-centered precision medicine, and resource allocation.1 Several perioperative risk prediction models have emerged in recent decades that estimate specific hazards (eg, cardiovascular complications after noncardiac surgery) with varying accuracy and utility. In the perioperative sphere, the time windows are often limited to an index hospitalization or 30 days following surgery or discharge.2-9 Although longer periods are of interest to patients, families, and health systems, few widely used or validated models are designed to look beyond this very narrow window.10,11 In addition, perioperative risk prediction models do not routinely incorporate parameters of a wide variety of health or demographic domains, such as patterns of health care, health care utilization, or medication use.
In 2013, in response to the need for near real-time information to guide delivery of enhanced care management services, the Veterans Health Administration (VHA) Office of Informatics and Analytics developed automated risk prediction models that used detailed electronic health record (EHR) data. These models were used to report Care Assessment Need (CAN) scores each week for all VHA enrollees and include data from a wide array of health domains. These CAN scores predict the risk for hospitalization, death, or either event within 90 days and 1 year.12,13 Each score is reported as both a predicted probability (0-1) and as a percentile in relation to all other VHA enrollees (a value between 1 and 99).13 The data used to calculate CAN scores are listed in Table 1.12
Surgical procedures or admissions would not be differentiated from nonsurgical admissions or other procedural clinic visits, and as such, it is not possible to isolate the effect of undergoing a surgical procedure from another health-related event on the CAN score. At the same time though, a short-term increase in system utilization caused by an elective surgical procedure such as a total knee replacement (TKR) would presumably be reflected in a change in CAN score, but this has not been studied.
Since their introduction, CAN scores have been routinely accessed by primary care teams and used to facilitate care coordination for thousands of VHA patients. However, these CAN scores are currently not available to VHA surgeons, anesthesiologists, or other perioperative clinicians. In this study, we examine the distributions of preoperative CAN scores and explore the relationships of preoperative CAN 1-year mortality scores with 1-year survival following discharge and length of stay (LOS) during index hospitalization in a cohort of US veterans who underwent TKR, the most common elective operation performed within the VHA system.
Methods
Following approval of the Durham Veterans Affairs Medical Center Institutional Review Board, all necessary data were extracted from the VHA Corporate Data Warehouse (CDW) repository.14 Informed consent was waived due to the minimal risk nature of the study.
We used Current Procedural Terminology codes (27438, 27446, 27447, 27486, 27487, 27488) and International Classification of Diseases, 9th edition clinical modification procedure codes (81.54, 81.55, 81.59, 00.80-00.84) to identify all veterans who had undergone primary or revision TKR between July 2014 and December 2015 in VHA Veterans Integrated Service Network 1 (Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode Island, New York, Pennsylvania, West Virginia, Virginia, North Carolina). Because we focused on outcomes following hospital discharge, patients who died before discharge were excluded from the analysis. Preoperative CAN 1-year mortality score was chosen as the measure under the assumption that long-term survival may be the most meaningful of the 4 possible CAN score measures.
Our primary objective was to determine distribution of preoperative CAN scores in the study population. Our secondary was to study relationships among the preoperative CAN 1-year mortality scores and 1-year mortality and hospital LOS.
Study Variables
For each patient, we extracted the date of index surgery. The primary exposure or independent variable was the CAN score in the week prior to this date. Because prior study has shown that CAN scores trajectories do not significantly change over time, the date-stamped CAN scores in the week before surgery represent what would have been available to clinicians in a preoperative setting.15 Since CAN scores are refreshed and overwritten every week, we extracted archived scores from the CDW.
For the 1-year survival outcome, the primary dependent variable, we queried the vital status files in the CDW for the date of death if applicable. We confirmed survival beyond 1 year by examining vital signs in the CDW for a minimum of 2 independent encounters beyond 1 year after the date of discharge. To compute the index LOS, the secondary outcome, we computed the difference between the date of admission and date of hospital discharge.
Statistical Methods
The parameters and performance of the multivariable logistic regression models developed to compute the various CAN mortality and hospitalization risk scores have been previously described.12 Briefly, Wang and colleagues created parsimonious regression models using backward selection. Model discrimination was evaluated using C (concordance)-statistic. Model calibration was assessed by comparing predicted vs observed event rates by risk deciles and performing Cox proportional hazards regression.
We plotted histograms to display preoperative CAN scores as a simple measure of distribution (Figure 1). We also examined the cumulative proportion of patients at each preoperative CAN 1-year mortality score.
Using a conventional t test, we compared means of preoperative CAN 1-year mortality scores in patients who survived vs those who died within 1 year. We also constructed a plot of the proportion of patients who had died within 1 year vs preoperative CAN 1-year mortality scores. Kaplan-Meier curves were then constructed examining 1-year survival by CAN 1-year mortality score by terciles.
Finally, we examined the relationship between preoperative CAN 1-year mortality scores and index LOS in 2 ways: We plotted LOS across CAN scores, and we constructed a
Results
We identified 8206 patients who had undergone a TKR over the 18-month study period. The overall mean (SD) for age was 65 (8.41) years; 93% were male, and 78% were White veterans. Patient demographics are well described in a previous publication.16,17
In terms of model parameters for the CAN score models, C-statistics for the 90-day outcome models were as follows: 0.833 for the model predicting hospitalization (95% CI, 0.832-0.834); 0.865 for the model predicting death (95% CI, 0.863-0.876); and 0.811 for the model predicting either event (95% CI, 0.810-0.812). C-statistics for the 1-year outcome models were 0.809 for the model predicting hospitalization (95% CI, 0.808-0.810); 0.851 for the model predicting death (95% CI, 0.849-0.852); and 0.787 for the model predicting either event (95% CI, 0.786-0.787). Models were well calibrated with α = 0 and β = 1, demonstrating strong agreement between observed and predicted event rates.
The distribution of preoperative CAN 1-year mortality scores was close to normal (median, 50; interquartile range, 40; mean [SD] 48 [25.6]) (eTable). The original CAN score models were developed having an equal number of patients in each strata and as such, are normally distributed.12 Our cohort was similar in pattern of distribution. Distributions of the remaining preoperative CAN scores (90-day mortality, 1-year hospitalization, 90-day hospitalization) are shown in Figures 2, 3, and 4. Not surprisingly, histograms for both 90-day and 1-year hospitalization were skewed toward higher scores, indicating that these patients were expected to be hospitalized in the near future.
Overall, 1.4% (110/8096) of patients died within 1 year of surgery. Comparing 1-year mortality CAN scores in survivors vs nonsurvivors, we found statistically significant differences in means (47 vs 66 respectively, P < .001) and medians (45 vs 75 respectively, P < .001) (Table 2). In the plot examining the relationship between preoperative 1-year mortality CAN scores and 1-year mortality, the percentage who died within 1 year increased initially for patients with CAN scores > 60 and again exponentially for patients with CAN scores > 80. Examining Kaplan-Meier curves, we found that survivors and nonsurvivors separated early after surgery, and the differences between the top tercile and the middle/lower terciles were statistically significant (P < .001). Mortality rates were about 0.5% in the lower and middle terciles but about 2% in the upper tercile (Figure 5).
In the plot examining the relationship between CAN scores and index LOS, the LOS rose significantly beyond a CAN score of 60 and dramatically beyond a CAN score of 80 (Figure 6). LOESS curves also showed 2 inflection points suggesting an incremental and sequential rise in the LOS with increasing CAN scores (Figure 7). Mean (SD) LOS in days for the lowest to highest terciles was 2.6 (1.7), 2.8 (2.1), and 3.6 (2.2), respectively.
Discussion
CAN scores are automatically generated each week by EHR-based multivariable risk models. These scores have excellent predictive accuracy for 90-day and 1-year mortality and hospitalization and are routinely used by VHA primary care teams to assist with clinical operations.13 We studied the distribution of CAN 1-year mortality scores in a preoperative context and examined relationships of the preoperative CAN 1-year mortality scores with postoperative mortality and LOS in 8206 veterans who underwent TKR.
There are several noteworthy findings. First, the overall 1-year mortality rate observed following TKR (1.4%) was similar to other published reports.18,19 Not surprisingly, preoperative CAN 1-year mortality scores were significantly higher in veterans who died compared with those of survivors. The majority of patients who died had a preoperative CAN 1-year mortality score > 75 while most who survived had a preoperative CAN 1-year mortality score < 45 (P < .001). Interestingly, the same scores showed a nonlinear correlation with LOS. Index LOS was about 4 days in patients in the highest tercile of CAN scores vs 2.5 days in the lowest tercile, but the initial increase in LOS was detected at a CAN score of about 55 to 60.
In addition, mortality rate varied widely in different segments of the population when grouped according to preoperative CAN scores. One-year mortality rates in the highest tercile reached 2%, about 4-fold higher than that of lower terciles (0.5%). Examination of the Kaplan-Meier curves showed that this difference in mortality between the highest tercile and the lower 2 groups appears soon after discharge and continues to increase over time, suggesting that the factors contributing to the increased mortality are present at the time of discharge and persist beyond the postoperative period. In summary, although CAN scores were not designed for use in the perioperative context, we found that preoperative CAN 1-year mortality scores are broadly predictive of mortality, but especially for increases in LOS following elective TKA, both increases in hospital LOS following elective TKA and mortality over the year after TKA.
Our findings raise several important questions. The decision to undergo elective surgery is complex. Arguably, individuals who undergo elective knee replacement should be healthy enough to undergo, recover, and reap the benefits from a procedure that does not extend life. The distribution of preoperative CAN 1-year mortality scores for our study population was similar to that of the general VHA enrollee population with similar measured mortality rates (≤ 0.5% vs ≥ 1.7% in the low and high terciles, respectively).1 Further study comparing outcomes in matched cohorts who did and did not undergo joint replacement would be of interest. In lieu of this, though, the association of high but not extreme CAN scores with increased hospital LOS may potentially be used to guide allocation of resources to this group, obviating the increased cost and risk to which this group is exposed. And the additional insight afforded by CAN scores may enhance shared decision-making models by identifying patients at the very highest risk (eg, 1-year mortality CAN score ≥ 90), patients who conceivably might not survive long enough to recover from and enjoy their reconstructed knee, who might in the long run be harmed by undergoing the procedure.
Many total joint arthroplasties are performed in older patients, a population in which frailty is increasingly recognized as a significant risk factor for poor outcomes.20,21 CAN scores reliably identify high-risk patients and have been shown to correlate with frailty in this group.22 Multiple authors have reported improved outcomes with cost reductions after implementation of programs targeting modifiable risk factors in high-risk surgical candidates.23-25 A preoperative assessment that includes the CAN score may be valuable in identifying patients who would benefit most from prehabilitation programs or other interventions designed to blunt the impact of frailty. It is true that many elements used to calculate the CAN score would not be considered modifiable, especially in the short term. However, specific contributors to frailty, such as nutritional status and polypharmacy might be potential candidates. As with all multivariable risk prediction models, there are multiple paths to a high CAN score, and further research to identify clinically relevant subgroups may help inform efforts to improve perioperative care within this population.
Hospital LOS is of intense interest for many reasons, not least its utility as a surrogate for cost and increased risk for immediate perioperative adverse events, such as multidrug-resistant hospital acquired infections, need for postacute facility-based rehabilitation, and deconditioning that increase risks of falls and fractures in the older population.26-29 In addition, its importance is magnified due to the COVID-19 pandemic context in which restarting elective surgery programs has changed traditional criteria by which patients are scheduled for surgery.
We have shown that elevated CAN scores are able to identify patients at risk for extended hospital stays and, as such, may be useful additional data in allocating scarce operating room time and other resources for optimal patient and health care provider safety.30,31 Individual surgeons and hospital systems would, of course, decide which patients should be triaged to go first, based on local priorities; however, choosing lower risk patients with minimal risk of morbidity and mortality while pursuing prehabilitation for higher risk patients is a reasonable approach.
Limitations
Our study has several limitations. Only a single surgical procedure was included, albeit the most common one performed in the VHA. In addition, no information was available concerning the precise clinical course for these patients, such as the duration of surgery, anesthetic technique, and management of acute, perioperative course. Although the assumption was made that patients received standard care in a manner such that these factors would not significantly affect either their mortality or their LOS out of proportion to their preoperative clinical status, confounding cannot be excluded. Therefore, further study is necessary to determine whether CAN scores can accurately predict mortality and/or LOS for patients undergoing other procedures. Further, a clinical trial is required to assess whether systematic provision of the CAN score at the point of surgery would impact care and, more important, impact outcomes. In addition, multivariable analyses were not performed, including and excluding various components of the CAN score models. Currently, CAN scores could be made available to the surgical/anesthesia communities at minimal or no cost and are updated automatically. Model calibration and discrimination in this particular setting were not validated.
Because our interest is in leveraging an existing resource to a current clinical and operational problem rather than in creating or validating a new tool, we chose to test the simple bivariate relationship between preoperative CAN scores and outcomes. We chose the preoperative 1-year mortality CAN score from among the 4 options under the assumption that long-term survival is the most meaningful of the 4 candidate outcomes. Finally, while the CAN scores are currently only calculated and generated for patients cared for within the VHA, few data elements are unavailable to civilian health systems. The most problematic would be documentation of actual prescription filling, but this is a topic of increasing interest to the medical and academic communities and access to such information we hope will improve.32-34
Conclusions
Although designed for use by VHA primary care teams, CAN scores also may have value for perioperative clinicians, predicting mortality and prolonged hospital LOS in those with elevated 1-year mortality scores. Advantages of CAN scores relative to other perioperative risk calculators lies in their ability to predict long-term rather than 30-day survival and that they are automatically generated on a near-real-time basis for all patients who receive care in VHA ambulatory clinics. Further study is needed to determine practical utility in shared decision making, preoperative evaluation and optimization, and perioperative resource allocation.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) National Center for Patient Safety, Field Office 10A4E, through the Patient Safety Center of Inquiry at the Durham VA Medical Center in North Carolina. The study also received support from the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham VA Health Care System.
1. McNair AGK, MacKichan F, Donovan JL, et al. What surgeons tell patients and what patients want to know before major cancer surgery: a qualitative study. BMC Cancer. 2016;16:258. doi:10.1186/s12885-016-2292-3
2. Grover FL, Hammermeister KE, Burchfiel C. Initial report of the Veterans Administration Preoperative Risk Assessment Study for Cardiac Surgery. Ann Thorac Surg. 1990;50(1):12-26; discussion 27-18. doi:10.1016/0003-4975(90)90073-f
3. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
4. Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702. doi:10.1097/SLA.0b013e31824b45af
5. Keller DS, Kroll D, Papaconstantinou HT, Ellis CN. Development and validation of a methodology to reduce mortality using the veterans affairs surgical quality improvement program risk calculator. J Am Coll Surg. 2017;224(4):602-607. doi:10.1016/j.jamcollsurg.2016.12.033
6. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842.e831-833. doi:10.1016/j.jamcollsurg.2013.07.385
7. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26-35. doi:10.7326/0003-4819-152-1-201001050-00007
8. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi:10.1161/CIRCULATIONAHA.110.015701
9. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi:10.1161/01.cir.100.10.1043
10. Smith T, Li X, Nylander W, Gunnar W. Thirty-day postoperative mortality risk estimates and 1-year survival in Veterans Health Administration surgery patients. JAMA Surg. 2016;151(5):417-422. doi:10.1001/jamasurg.2015.4882
11. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90- day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149-1154. doi:10.1002/bjs.8813
12. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1016/j.amjcard.2012.06.038
13. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
14. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
15. Wong ES, Yoon J, Piegari RI, Rosland AM, Fihn SD, Chang ET. Identifying latent subgroups of high-risk patients using risk score trajectories. J Gen Intern Med. 2018;33(12):2120-2126. doi:10.1007/s11606-018-4653-x
16. Chen Q, Hsia HL, Overman R, et al. Impact of an opioid safety initiative on patients undergoing total knee arthroplasty: a time series analysis. Anesthesiology. 2019;131(2):369-380. doi:10.1097/ALN.0000000000002771
17. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
18. Inacio MCS, Dillon MT, Miric A, Navarro RA, Paxton EW. Mortality after total knee and total hip arthroplasty in a large integrated health care system. Perm J. 2017;21:16-171. doi:10.7812/TPP/16-171
19. Lee QJ, Mak WP, Wong YC. Mortality following primary total knee replacement in public hospitals in Hong Kong. Hong Kong Med J. 2016;22(3):237-241. doi:10.12809/hkmj154712
20. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi:10.1186/s12877-016-0329-8
21. Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2019;155(1):e194620. doi:10.1001/jamasurg.2019.4620
22. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
23. Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33(12):3642- 3648. doi:10.1016/j.arth.2018.08.018
24. Sodhi N, Anis HK, Coste M, et al. A nationwide analysis of preoperative planning on operative times and postoperative complications in total knee arthroplasty. J Knee Surg. 2019;32(11):1040-1045. doi:10.1055/s-0039-1677790
25. Krause A, Sayeed Z, El-Othmani M, Pallekonda V, Mihalko W, Saleh KJ. Outpatient total knee arthroplasty: are we there yet? (part 1). Orthop Clin North Am. 2018;49(1):1-6. doi:10.1016/j.ocl.2017.08.002
26. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca- Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644-652. doi:10.1093/cid/cix411
27. Nikkel LE, Kates SL, Schreck M, Maceroli M, Mahmood B, Elfar JC. Length of hospital stay after hip fracture and risk of early mortality after discharge in New York state: retrospective cohort study. BMJ. 2015;351:h6246. doi:10.1136/bmj.h6246
28. Marfil-Garza BA, Belaunzarán-Zamudio PF, Gulias-Herrero A, et al. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS One. 2018;13(11):e0207203. doi:10.1371/journal.pone.0207203
29. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi:10.1111/j.1532-5415.1990.tb03451.x
30. Iyengar KP, Jain VK, Vaish A, Vaishya R, Maini L, Lal H. Post COVID-19: planning strategies to resume orthopaedic surgery -challenges and considerations. J Clin Orthop Trauma. 2020;11(suppl 3):S291-S295. doi:10.1016/j.jcot.2020.04.028
31. O’Connor CM, Anoushiravani AA, DiCaprio MR, Healy WL, Iorio R. Economic recovery after the COVID-19 pandemic: resuming elective orthopedic surgery and total joint arthroplasty. J Arthroplasty. 2020;35(suppl 7):S32-S36. doi:10.1016/j.arth.2020.04.038.
32. Mauseth SA, Skurtveit S, Skovlund E, Langhammer A, Spigset O. Medication use and association with urinary incontinence in women: data from the Norwegian Prescription Database and the HUNT study. Neurourol Urodyn. 2018;37(4):1448-1457. doi:10.1002/nau.23473
33. Sultan RS, Correll CU, Schoenbaum M, King M, Walkup JT, Olfson M. National patterns of commonly prescribed psychotropic medications to young people. J Child Adolesc Psychopharmacol. 2018;28(3):158-165. doi:10.1089/cap.2017.0077
34. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S.-the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21(12):702-712. doi:10.1089/dia.2019.0213
1. McNair AGK, MacKichan F, Donovan JL, et al. What surgeons tell patients and what patients want to know before major cancer surgery: a qualitative study. BMC Cancer. 2016;16:258. doi:10.1186/s12885-016-2292-3
2. Grover FL, Hammermeister KE, Burchfiel C. Initial report of the Veterans Administration Preoperative Risk Assessment Study for Cardiac Surgery. Ann Thorac Surg. 1990;50(1):12-26; discussion 27-18. doi:10.1016/0003-4975(90)90073-f
3. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
4. Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702. doi:10.1097/SLA.0b013e31824b45af
5. Keller DS, Kroll D, Papaconstantinou HT, Ellis CN. Development and validation of a methodology to reduce mortality using the veterans affairs surgical quality improvement program risk calculator. J Am Coll Surg. 2017;224(4):602-607. doi:10.1016/j.jamcollsurg.2016.12.033
6. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842.e831-833. doi:10.1016/j.jamcollsurg.2013.07.385
7. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26-35. doi:10.7326/0003-4819-152-1-201001050-00007
8. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi:10.1161/CIRCULATIONAHA.110.015701
9. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi:10.1161/01.cir.100.10.1043
10. Smith T, Li X, Nylander W, Gunnar W. Thirty-day postoperative mortality risk estimates and 1-year survival in Veterans Health Administration surgery patients. JAMA Surg. 2016;151(5):417-422. doi:10.1001/jamasurg.2015.4882
11. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90- day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149-1154. doi:10.1002/bjs.8813
12. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1016/j.amjcard.2012.06.038
13. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
14. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
15. Wong ES, Yoon J, Piegari RI, Rosland AM, Fihn SD, Chang ET. Identifying latent subgroups of high-risk patients using risk score trajectories. J Gen Intern Med. 2018;33(12):2120-2126. doi:10.1007/s11606-018-4653-x
16. Chen Q, Hsia HL, Overman R, et al. Impact of an opioid safety initiative on patients undergoing total knee arthroplasty: a time series analysis. Anesthesiology. 2019;131(2):369-380. doi:10.1097/ALN.0000000000002771
17. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
18. Inacio MCS, Dillon MT, Miric A, Navarro RA, Paxton EW. Mortality after total knee and total hip arthroplasty in a large integrated health care system. Perm J. 2017;21:16-171. doi:10.7812/TPP/16-171
19. Lee QJ, Mak WP, Wong YC. Mortality following primary total knee replacement in public hospitals in Hong Kong. Hong Kong Med J. 2016;22(3):237-241. doi:10.12809/hkmj154712
20. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi:10.1186/s12877-016-0329-8
21. Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2019;155(1):e194620. doi:10.1001/jamasurg.2019.4620
22. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
23. Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33(12):3642- 3648. doi:10.1016/j.arth.2018.08.018
24. Sodhi N, Anis HK, Coste M, et al. A nationwide analysis of preoperative planning on operative times and postoperative complications in total knee arthroplasty. J Knee Surg. 2019;32(11):1040-1045. doi:10.1055/s-0039-1677790
25. Krause A, Sayeed Z, El-Othmani M, Pallekonda V, Mihalko W, Saleh KJ. Outpatient total knee arthroplasty: are we there yet? (part 1). Orthop Clin North Am. 2018;49(1):1-6. doi:10.1016/j.ocl.2017.08.002
26. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca- Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644-652. doi:10.1093/cid/cix411
27. Nikkel LE, Kates SL, Schreck M, Maceroli M, Mahmood B, Elfar JC. Length of hospital stay after hip fracture and risk of early mortality after discharge in New York state: retrospective cohort study. BMJ. 2015;351:h6246. doi:10.1136/bmj.h6246
28. Marfil-Garza BA, Belaunzarán-Zamudio PF, Gulias-Herrero A, et al. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS One. 2018;13(11):e0207203. doi:10.1371/journal.pone.0207203
29. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi:10.1111/j.1532-5415.1990.tb03451.x
30. Iyengar KP, Jain VK, Vaish A, Vaishya R, Maini L, Lal H. Post COVID-19: planning strategies to resume orthopaedic surgery -challenges and considerations. J Clin Orthop Trauma. 2020;11(suppl 3):S291-S295. doi:10.1016/j.jcot.2020.04.028
31. O’Connor CM, Anoushiravani AA, DiCaprio MR, Healy WL, Iorio R. Economic recovery after the COVID-19 pandemic: resuming elective orthopedic surgery and total joint arthroplasty. J Arthroplasty. 2020;35(suppl 7):S32-S36. doi:10.1016/j.arth.2020.04.038.
32. Mauseth SA, Skurtveit S, Skovlund E, Langhammer A, Spigset O. Medication use and association with urinary incontinence in women: data from the Norwegian Prescription Database and the HUNT study. Neurourol Urodyn. 2018;37(4):1448-1457. doi:10.1002/nau.23473
33. Sultan RS, Correll CU, Schoenbaum M, King M, Walkup JT, Olfson M. National patterns of commonly prescribed psychotropic medications to young people. J Child Adolesc Psychopharmacol. 2018;28(3):158-165. doi:10.1089/cap.2017.0077
34. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S.-the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21(12):702-712. doi:10.1089/dia.2019.0213