User login
New agents for youth-onset type 2 diabetes ‘finally in sight’
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There are limited treatment options for children and youth with type 2 diabetes, but a few novel therapies beyond metformin are on the horizon, experts said at the annual scientific sessions of the American Diabetes Association.
“Type 2 diabetes in youth only emerged as a well-recognized pediatric medical problem in the 1990s and the first decade of the 21st century,” session chair Kenneth C. Copeland, MD, said in an interview.
“Fortunately, a number of clinical trials of antidiabetic pharmacologic agents in diabetic youth have now been completed, demonstrating both safety and efficacy, and at long last, a ... variety of agents are finally in sight,” he noted.
Type 2 diabetes in youth is profoundly different from type 2 diabetes in adults, added Dr. Copeland, pediatrics professor emeritus, University of Oklahoma, Oklahoma City. In youth, its course is typically aggressive and refractive to treatment.
Concerted efforts at lifestyle intervention are important but insufficient, and a response to metformin, even when initiated at diagnosis, is often short lived, he added.
Because of the rapid glycemic deterioration that is typical of type 2 diabetes in youth and leads to the full array of diabetic complications, early aggressive pharmacologic treatment is indicated.
“We all look forward to this next decade ushering in new treatment options, spanning the spectrum from obesity prevention to complex pharmacologic intervention,” Dr. Copeland summarized.
Increasing prevalence of T2D in youth, limited therapies
Rates of type 2 diabetes in youth continue to increase, especially among non-White groups, and most of these individuals have less than optimal diabetes control, Elvira Isganaitis, MD, MPH, a pediatric endocrinologist at the Joslin Diabetes Center and assistant professor of pediatrics at Harvard Medical School, both in Boston, told the meeting.
Although the Food and Drug Administration has approved more than 25 drugs to treat type 2 diabetes in adults, “unfortunately,” metformin is the only oral medication approved to treat the disease in a pediatric population, “and a majority of youth either do not respond to it or do not tolerate it,” she said in an interview.
Dr. Copeland observed that “the TODAY study demonstrated conclusively that, despite an often dramatic initial improvement in glycemic control upon initiation of pharmacologic and lifestyle intervention, this initial response was followed by a rapid deterioration of beta-cell function and glycemic failure, indicating that additional pharmacologic agents were sorely needed for this population.”
The RISE study also showed that, compared with adults, youth had more rapid beta-cell deterioration despite treatment.
Until the June 2019 FDA approval of the injectable glucagonlike peptide–1 receptor agonist liraglutide (Victoza, Novo Nordisk) for children 10 years or older, “except for insulin, metformin was the only antidiabetic medication available for use in youth, severely limiting treatment options,” he added.
Liraglutide ‘a huge breakthrough,’ other options on the horizon
The FDA approval of liraglutide was “a huge breakthrough” as the first noninsulin drug for pediatric type 2 diabetes since metformin was approved for pediatric use in 2000, Dr. Isganaitis said.
The ELLIPSE study, on which the approval was based, showed liraglutide was effective at lowering hemoglobin A1c and was generally well tolerated, although it was associated with a higher incidence of gastrointestinal symptoms.
In December 2020, the FDA also approved liraglutide (Saxenda) for the treatment of obesity in youth age 12 and older (at a dose of 3 mg as opposed to the 1.8-mg dose of liraglutide [Victoza]), “which is wonderful news considering that the majority of pediatric patients with type 2 diabetes also have obesity,” Dr. Isganaitis added.
“The results of studies of liraglutide on glycemia in diabetic youth are impressive, with both an additional benefit of weight loss and without unacceptable identified risks or side effects,” Dr. Copeland concurred.
Waiting in the wings
Dr. Isganaitis reported that a few phase 3 clinical trials of other therapies for pediatric patients with type 2 diabetes are in the wings.
The 24-week phase 3 T2GO clinical trial of the sodium-glucose cotransporter 2 inhibitor dapagliflozin (AstraZeneca) versus placebo in 72 patients with type 2 diabetes aged 10-24 years was completed in April 2020, and the data are being analyzed.
An AstraZeneca-sponsored phase 3 trial of the safety and efficacy of a weekly injection of the GLP-1 receptor agonist exenatide in 10- to 17-year-olds with type 2 diabetes (n = 82) has also been completed and data are being analyzed.
A Takeda-sponsored phase 3 pediatric study of the dipeptidyl peptidase–4 inhibitor alogliptin in 10- to 17-year-olds with type 2 diabetes (n = 150) is estimated to be completed by February 2022.
And the phase 3 DINAMO trial, sponsored by Boehringer Ingelheim, which is evaluating the efficacy and safety of the SGLT2 inhibitor empagliflozin (10 mg/25 mg) versus the DPP-4 inhibitor linagliptin (5 mg) versus placebo over 26 weeks in 10- to 17-year-olds with type 2 diabetes (estimated 186 participants), is expected to be completed in May 2023.
“I hope that these medications will demonstrate efficacy and allow pediatric patients with type 2 diabetes to have more treatment options,” Dr. Isganaitis concluded.
Type 2 diabetes more aggressive than type 1 diabetes in kids
According to Dr. Isganaitis, “there is a widely held misconception among the general public and even among some physicians that type 2 diabetes is somehow less worrisome or ‘milder’ than a diagnosis of type 1 diabetes.”
However, the risk of complications and severe morbidity is higher with a diagnosis of type 2 diabetes versus type 1 diabetes in a child, so “this condition needs to be managed intensively with a multidisciplinary team including pediatric endocrinology, nutrition [support], diabetes educators, and mental health support,” she emphasized.
Many people also believe that “type 2 diabetes in kids is a ‘lifestyle disease,’ ” she continued, “but in fact, there is a strong role for genetics.”
The ADA Presidents’ Select Abstract “paints a picture of youth-onset type 2 diabetes as a disease intermediate in extremity between monogenic diabetes [caused by mutations in a single gene] and type 2 diabetes [caused by multiple genes and lifestyle factors such as obesity], in which genetic variants in both insulin secretion and insulin response pathways are implicated.”
Along the same lines, Dr. Isganaitis presented an oral abstract at the meeting that showed that, among youth with newly diagnosed type 2 diabetes, those whose mothers had diabetes had faster disease progression and earlier onset of diabetes complications.
Dr. Isganaitis has reported no relevant financial relationships. Dr. Copeland has reported serving on data monitoring committees for Boehringer Ingelheim and Novo Nordisk, and on an advisory committee for a research study for Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
Contraceptive counseling for transmasculine patients
One of the most common reasons patients seek care from an ob.gyn. is for contraceptive counseling and family planning. While prescribing contraceptives for cisgender patients is often part of the daily routine for ob.gyns., many are unfamiliar with counseling and options for patients who identify as transgender. In a survey of practicing ob.gyns. in nine academic centers, 80% did not receive training on caring for transgender patients.1 Other studies have found that 5.5%-9% of transgender men have reported that a clinician informed them that testosterone was a contraceptive.2,3
Testosterone is not a reliable form of contraception and, in fact, testosterone is considered category X, as it can induce labial fusion, produce abnormal vaginal development, cause a persistent urogenital sinus, and cause clitoromegaly in the developing fetus. Given the teratogenic effects of testosterone, it is imperative that patients who do not desire pregnancy receive appropriate contraceptive options. Counseling of patients should be individualized and start by taking a comprehensive sexual history. Such strategies include using gender-inclusive language, avoiding assumptions about sexual orientation, and obtaining an anatomic inventory of both the patient and their partner(s).4 While a majority of patients achieve amenorrhea while taking testosterone, it is important to discuss the need for contraception if patients are engaging in penile-vaginal intercourse. According to a study of 41 transmasculine patients who achieved pregnancy, one-third of pregnancies were unplanned. Another study reported that 20% of transmasculine patients were taking testosterone and amenorrheic at the time of conception.2
Contraindications to certain types of contraception, such as a history of a thromboembolic event precluding a patient from using combined oral contraceptives, still apply. Transmasculine patients have additional concerns that providers should be aware of and sensitive to when prescribing contraceptives. Gender dysphoria may be exacerbated by contraceptive options that require a pelvic exam or procedure, such as an intrauterine device. For patients that desire an IUD but experience heightened distress in anticipation of the procedure, premedication with anxiolytics or topical anesthetics are reasonable options.4 Using an adequate amount of lubricant and a small speculum may also make the exam more comfortable for patients, especially if patients do not engage in receptive frontal intercourse. Of note, certain types of IUDs, such as the Paragard, may cause pelvic cramping or abnormal bleeding, which could be a trigger for dysphoria. Patients may also experience worsening dysphoria by repeatedly taking a medication that is often associated with cisgender women, such as combined oral contraceptives (COCs). Furthermore, patients may want to avoid COCs secondary to concerns about potential feminizing effects of these hormones and their counteraction of masculinizing effects of testosterone. While COCs act to lower androgen levels by increasing sex hormone–binding globulin, which subsequently binds to testosterone, the amount of estrogen in the pill does not contribute significantly to inhibiting masculinization, and patients should be counseled accordingly.4,5 Side effects such as breast tenderness, which is common among COCs and other estrogen-containing contraceptives, can increase dysphoria and make chest binding more painful. In patients who undergo gender-affirming mastectomies, these effects are less pronounced, however, there may be residual breast tissue left behind which can still produce tenderness and pain.
Sterilization is also a reasonable option in transmasculine patients desiring permanent contraception. Similar to sterilization counseling in cisgender women, a discussion about the irreversibility of the procedure and rates of regret should occur. Transmasculine patients may seek hysterectomy for contraception and to avoid further pelvic exams, cervical cancer screening, pelvic cramping, and/or uterine bleeding. Providers should be knowledgeable about the World Professional Association for Transgender Health standards of care for gender-affirming hysterectomies and counsel patients appropriately.
In summary, transmasculine and all gender-diverse patients deserve the same comprehensive care that their cisgender counterparts receive. Even if the ob.gyn. is not the prescribing physician for testosterone, we all must have a basic understanding of the effects of testosterone and provide appropriate contraceptive services and family planning options to patients when indicated.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Unger CA. J Women’s Health. 2015;24(2):114-8.
2. Abern L and Maguire K. Obstet Gynecol. 2018;131:65S.
3. Light A et al. Contraception. 2018;98:266-9.
4. Krempasky C et al. Am J Obstet Gynecol. 2020;222(2):134-43.
5. Goodman NF et al. Endocrin Pract. 2015:21(11):1291-300.
One of the most common reasons patients seek care from an ob.gyn. is for contraceptive counseling and family planning. While prescribing contraceptives for cisgender patients is often part of the daily routine for ob.gyns., many are unfamiliar with counseling and options for patients who identify as transgender. In a survey of practicing ob.gyns. in nine academic centers, 80% did not receive training on caring for transgender patients.1 Other studies have found that 5.5%-9% of transgender men have reported that a clinician informed them that testosterone was a contraceptive.2,3
Testosterone is not a reliable form of contraception and, in fact, testosterone is considered category X, as it can induce labial fusion, produce abnormal vaginal development, cause a persistent urogenital sinus, and cause clitoromegaly in the developing fetus. Given the teratogenic effects of testosterone, it is imperative that patients who do not desire pregnancy receive appropriate contraceptive options. Counseling of patients should be individualized and start by taking a comprehensive sexual history. Such strategies include using gender-inclusive language, avoiding assumptions about sexual orientation, and obtaining an anatomic inventory of both the patient and their partner(s).4 While a majority of patients achieve amenorrhea while taking testosterone, it is important to discuss the need for contraception if patients are engaging in penile-vaginal intercourse. According to a study of 41 transmasculine patients who achieved pregnancy, one-third of pregnancies were unplanned. Another study reported that 20% of transmasculine patients were taking testosterone and amenorrheic at the time of conception.2
Contraindications to certain types of contraception, such as a history of a thromboembolic event precluding a patient from using combined oral contraceptives, still apply. Transmasculine patients have additional concerns that providers should be aware of and sensitive to when prescribing contraceptives. Gender dysphoria may be exacerbated by contraceptive options that require a pelvic exam or procedure, such as an intrauterine device. For patients that desire an IUD but experience heightened distress in anticipation of the procedure, premedication with anxiolytics or topical anesthetics are reasonable options.4 Using an adequate amount of lubricant and a small speculum may also make the exam more comfortable for patients, especially if patients do not engage in receptive frontal intercourse. Of note, certain types of IUDs, such as the Paragard, may cause pelvic cramping or abnormal bleeding, which could be a trigger for dysphoria. Patients may also experience worsening dysphoria by repeatedly taking a medication that is often associated with cisgender women, such as combined oral contraceptives (COCs). Furthermore, patients may want to avoid COCs secondary to concerns about potential feminizing effects of these hormones and their counteraction of masculinizing effects of testosterone. While COCs act to lower androgen levels by increasing sex hormone–binding globulin, which subsequently binds to testosterone, the amount of estrogen in the pill does not contribute significantly to inhibiting masculinization, and patients should be counseled accordingly.4,5 Side effects such as breast tenderness, which is common among COCs and other estrogen-containing contraceptives, can increase dysphoria and make chest binding more painful. In patients who undergo gender-affirming mastectomies, these effects are less pronounced, however, there may be residual breast tissue left behind which can still produce tenderness and pain.
Sterilization is also a reasonable option in transmasculine patients desiring permanent contraception. Similar to sterilization counseling in cisgender women, a discussion about the irreversibility of the procedure and rates of regret should occur. Transmasculine patients may seek hysterectomy for contraception and to avoid further pelvic exams, cervical cancer screening, pelvic cramping, and/or uterine bleeding. Providers should be knowledgeable about the World Professional Association for Transgender Health standards of care for gender-affirming hysterectomies and counsel patients appropriately.
In summary, transmasculine and all gender-diverse patients deserve the same comprehensive care that their cisgender counterparts receive. Even if the ob.gyn. is not the prescribing physician for testosterone, we all must have a basic understanding of the effects of testosterone and provide appropriate contraceptive services and family planning options to patients when indicated.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Unger CA. J Women’s Health. 2015;24(2):114-8.
2. Abern L and Maguire K. Obstet Gynecol. 2018;131:65S.
3. Light A et al. Contraception. 2018;98:266-9.
4. Krempasky C et al. Am J Obstet Gynecol. 2020;222(2):134-43.
5. Goodman NF et al. Endocrin Pract. 2015:21(11):1291-300.
One of the most common reasons patients seek care from an ob.gyn. is for contraceptive counseling and family planning. While prescribing contraceptives for cisgender patients is often part of the daily routine for ob.gyns., many are unfamiliar with counseling and options for patients who identify as transgender. In a survey of practicing ob.gyns. in nine academic centers, 80% did not receive training on caring for transgender patients.1 Other studies have found that 5.5%-9% of transgender men have reported that a clinician informed them that testosterone was a contraceptive.2,3
Testosterone is not a reliable form of contraception and, in fact, testosterone is considered category X, as it can induce labial fusion, produce abnormal vaginal development, cause a persistent urogenital sinus, and cause clitoromegaly in the developing fetus. Given the teratogenic effects of testosterone, it is imperative that patients who do not desire pregnancy receive appropriate contraceptive options. Counseling of patients should be individualized and start by taking a comprehensive sexual history. Such strategies include using gender-inclusive language, avoiding assumptions about sexual orientation, and obtaining an anatomic inventory of both the patient and their partner(s).4 While a majority of patients achieve amenorrhea while taking testosterone, it is important to discuss the need for contraception if patients are engaging in penile-vaginal intercourse. According to a study of 41 transmasculine patients who achieved pregnancy, one-third of pregnancies were unplanned. Another study reported that 20% of transmasculine patients were taking testosterone and amenorrheic at the time of conception.2
Contraindications to certain types of contraception, such as a history of a thromboembolic event precluding a patient from using combined oral contraceptives, still apply. Transmasculine patients have additional concerns that providers should be aware of and sensitive to when prescribing contraceptives. Gender dysphoria may be exacerbated by contraceptive options that require a pelvic exam or procedure, such as an intrauterine device. For patients that desire an IUD but experience heightened distress in anticipation of the procedure, premedication with anxiolytics or topical anesthetics are reasonable options.4 Using an adequate amount of lubricant and a small speculum may also make the exam more comfortable for patients, especially if patients do not engage in receptive frontal intercourse. Of note, certain types of IUDs, such as the Paragard, may cause pelvic cramping or abnormal bleeding, which could be a trigger for dysphoria. Patients may also experience worsening dysphoria by repeatedly taking a medication that is often associated with cisgender women, such as combined oral contraceptives (COCs). Furthermore, patients may want to avoid COCs secondary to concerns about potential feminizing effects of these hormones and their counteraction of masculinizing effects of testosterone. While COCs act to lower androgen levels by increasing sex hormone–binding globulin, which subsequently binds to testosterone, the amount of estrogen in the pill does not contribute significantly to inhibiting masculinization, and patients should be counseled accordingly.4,5 Side effects such as breast tenderness, which is common among COCs and other estrogen-containing contraceptives, can increase dysphoria and make chest binding more painful. In patients who undergo gender-affirming mastectomies, these effects are less pronounced, however, there may be residual breast tissue left behind which can still produce tenderness and pain.
Sterilization is also a reasonable option in transmasculine patients desiring permanent contraception. Similar to sterilization counseling in cisgender women, a discussion about the irreversibility of the procedure and rates of regret should occur. Transmasculine patients may seek hysterectomy for contraception and to avoid further pelvic exams, cervical cancer screening, pelvic cramping, and/or uterine bleeding. Providers should be knowledgeable about the World Professional Association for Transgender Health standards of care for gender-affirming hysterectomies and counsel patients appropriately.
In summary, transmasculine and all gender-diverse patients deserve the same comprehensive care that their cisgender counterparts receive. Even if the ob.gyn. is not the prescribing physician for testosterone, we all must have a basic understanding of the effects of testosterone and provide appropriate contraceptive services and family planning options to patients when indicated.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Unger CA. J Women’s Health. 2015;24(2):114-8.
2. Abern L and Maguire K. Obstet Gynecol. 2018;131:65S.
3. Light A et al. Contraception. 2018;98:266-9.
4. Krempasky C et al. Am J Obstet Gynecol. 2020;222(2):134-43.
5. Goodman NF et al. Endocrin Pract. 2015:21(11):1291-300.
Cancer mortality continues to drop in females as breast cancer reversal looms
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.
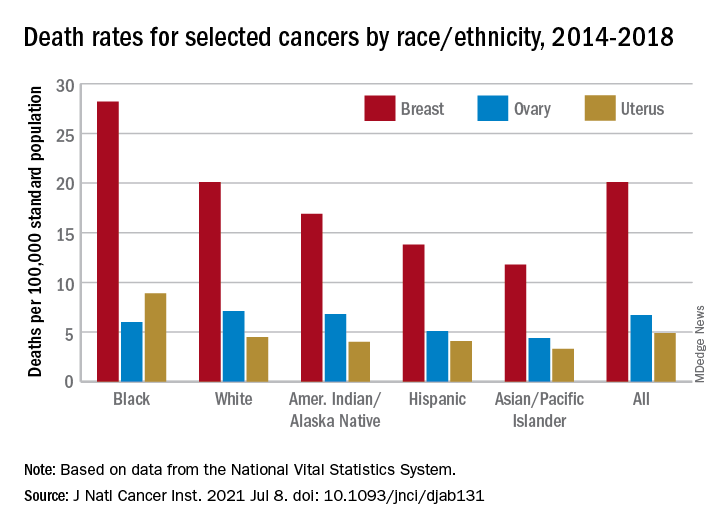
The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.

The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
Overall cancer mortality in females continues to decrease in the United States, but “previous declining trends in death rates slowed” for breast cancer in recent years, according to an annual report by several national organizations.

The analysis of long-term trends in cancer death rates shows that a decline of 1.4% per year from 2001 to 2016 accelerated to 2.1% per year in 2016-2018, the American Cancer Society, Centers for Disease Control and Prevention, National Cancer Institute, and the North American Association of Central Cancer Registries said.
Decreases in overall cancer mortality were seen in females of all races and ethnic groups over the most recent 5-year period included in the report, 2014-2018, varying from –1.6% per year in both non-Hispanic Blacks and Whites to –0.9% for non-Hispanic American Indians/Alaska Natives (AI/ANs), Farhad Islami, MD, PhD, of the American Cancer Society, Atlanta, and associates said in the Journal of the National Cancer Institute.
Over those 5 years, death rates fell for 14 of the 20 most common cancers in females; increased for liver, uterus, brain, pancreas, and soft tissue including heart; and remained stable for cancers of the oral cavity/pharynx, they reported.
Breast cancer was among those that declined, but the rate of that decline has been slowing. Mortality declined by an average of 2.3% per year in 2003-2007, by 1.6% a year in 2007-2014, and by just 1.0% annually during 2014-2018, based on data from the National Center for Health Statistics’ National Vital Statistics System.
Mortality from all cancers in 2014-2018 was 133.5 deaths per 100,000 standard population, with the racial/ethnic gap ranging from 85.4 per 100,000 (non-Hispanic Asian/Pacific Islander) to 154.9 (non-Hispanic Black), Dr. Islami and associates said.
Melanoma had the largest decline in mortality over that period among the 20 most common cancers in females, falling by an average of 4.4% per year, with lung cancer next at 4.3%. Among those with increased death rates, uterine cancer saw the largest rise at 2.0% a year, the research team said.
The deaths caused by cancer of the uterus were most common in non-Hispanic Black females, 8.9 per 100,000 population, followed by non-Hispanic White (4.5), Hispanic (4.1), non-Hispanic AI/AN (4.0), and non-Hispanic Asian/Pacific Islander (3.3), they reported.
“Long-term increasing trends in uterine cancer death rates parallel trends in incidence, although death rates are increasing at a somewhat faster rate. Increasing uterine cancer incidence has been attributed to increasing obesity prevalence and decreased use of combined hormone replacement therapy,” Dr. Islami and associates pointed out.
Breast cancer deaths also were most common among Blacks in 2014-2018, occurring at a rate of 28.2 per 100,000, as were deaths from cancer of the cervix (3.4 per 100,000), while ovarian cancers deaths were highest in White females (7.1 per 100,000), the researchers noted.
The continuing racial and ethnic disparity “largely reflects a combination of multiple intertwined factors” of tumor biology, diagnosis, treatment, and systemic discrimination, they wrote, adding that Black persons “are more likely to have a higher exposure to some cancer risk factors and limited access to healthy food, safe places for physical activity, and evidence-based cancer preventive services.”
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Rising rates of T1D in children: Is COVID to blame?
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
In early 2020, the COVID-19 pandemic changed everything about life as we know it, with widespread shutdowns across the globe. The U.S. health care system quickly adapted, pivoting to telehealth visits when able and proactively managing outpatient conditions to prevent overwhelming hospital resources and utilization. Meanwhile, at my practice, the typical rate of about one new-onset pediatric type 1 diabetes (T1D) case per week increased to about two per week.
However, the new diabetes cases continued to accumulate, and I saw more patients being diagnosed who did not have a known family history of autoimmunity. I began to ask friends at other centers whether they were noticing the same trend.
One colleague documented a 36% increase in her large center compared with the previous year. Another noted a 40% rise at his children’s hospital. We observed that there was often a respiratory illness reported several weeks before presenting with T1D. Sometimes the child was known to be COVID-positive. Sometimes the child had not been tested. Sometimes we suspected that COVID had been a preceding illness and then found negative SARS-CoV-2 antibodies – but we were not certain whether the result was meaningful given the time lapsed since infection.
Soon, reports emerged of large increases in severe diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state at initial presentation, a trend reported in other countries.
Is COVID-19 a trigger for T1D?
There is known precedent for increased risk for T1D after viral infections in patients who are already genetically susceptible. Mechanisms of immune-mediated islet cell failure would make sense following SARS-CoV-2 infection; direct islet toxicity was noted with SARS-CoV-1 and has been suspected with SARS-CoV-2 but not proven. Some have suggested that hypercoagulability with COVID-19 may lead to ischemic damage to the pancreas.
With multiple potential pathways for islet damage, increases in insulin-dependent diabetes would logically follow. Still, whether this is the case remains unclear. There is not yet definitive evidence that there is uptake of SARS-CoV-2 via receptors in the pancreatic beta cells.
Our current understanding of T1D pathogenesis is that susceptible individuals develop autoimmunity in response to an environmental trigger, with beta-cell failure developing over months to years. Perhaps vulnerable patients with genetic risk for pancreatic autoimmunity were stressed by SARS-CoV-2 infection and were diagnosed earlier than they might have been, showing some lead-time bias. Adult patients with COVID-19 demonstrated hyperglycemia that has been reversible in some cases, like the stress hyperglycemia seen with other infections and surgery in response to proinflammatory states.
The true question seems to be whether there is a unique type of diabetes related to direct viral toxicity. Do newly diagnosed patients have measurable traditional antibodies, like anti-glutamic acid decarboxylase or anti-islet cell antibodies? Is there proof of preceding SARS-CoV-2 infection? In the new cases that I thought were unusual at first glance, I found typical pancreatic autoimmunity and negative SARS-CoV-2 antibodies. The small cohorts reported thus far have had similar findings.
A stronger case can be made for the risk of developing diabetes (types 1 and 2) with rapid weight gain. Another marked pattern that pediatric endocrinologists have observed has been increased weight gain in children with closed schools, decreased activity, and more social isolation. I have seen weight change as great as 100 lb in a teen over the past year; 30- to 50-lb weight increases over the course of the pandemic have been common. Considering the “accelerator hypothesis” of faster onset of type 2 diabetes with rapid weight gain, implications for hastening of T1D with weight gain have also been considered. The full impact of these dramatic weight changes will take time to understand.
The true story may not emerge for years
Anecdotes and theoretical concerns may give us pause, but they are far from scientific truth. Efforts are underway to explore this perceived trend with international registries, including the CoviDIAB Registry as well as T1D Exchange. The true story may not emerge until years have passed to see the cumulative fallout of COVID-19. Regardless, these troubling observations should be considered as pandemic safeguards continue to loosen.
While pediatric mortality from COVID-19 has been relatively low (though sadly not zero), some have placed too little focus on possible morbidity. Long-term effects like long COVID and neuropsychiatric sequelae are becoming evident in all populations, including children. If a lifelong illness like diabetes can be directly linked to COVID-19, protecting children from infection with measures like masks becomes all the more crucial until vaccines are more readily available. Despite our rapid progress with understanding COVID-19 disease, there is still much left to learn.
A version of this article first appeared on Medscape.com.
Latest FDA pembrolizumab approval expands label to cutaneous SCCs
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
Children and COVID: New vaccinations drop as the case count rises
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.
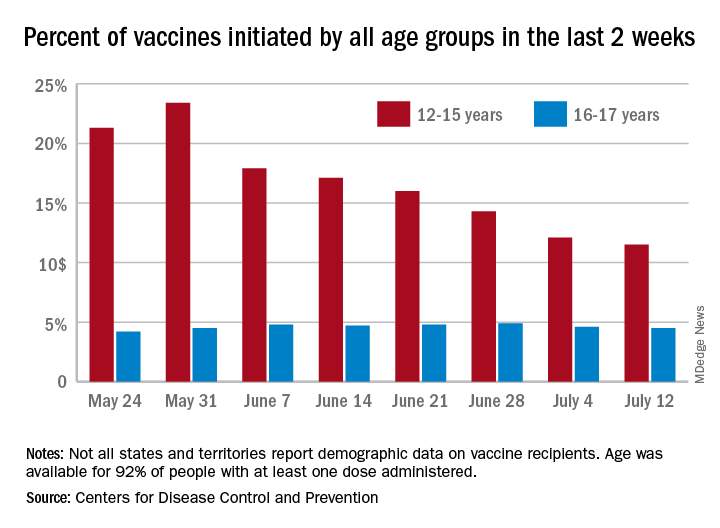
Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
Patients on methotrexate show T-cell response to Pfizer vaccine
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Optimizing Severe Asthma Treatment: Challenges and Approaches to Care
Asthma is a complex, heterogeneous disease with unmet treatment needs. Patients with severe asthma generally continue to have severe disease despite being on controller therapies.
Uncontrolled asthma can result in unnecessary suffering and interfere with daily activities. It also increases the risk for exacerbations and places substantial burden on the healthcare system.
In this ReCAP, Drs Sandhya Khurana and Steve N. Georas, from the Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care in Rochester, New York, discuss current challenges in the management of severe asthma, and how advances in the understanding of phenotypes and therapeutic options guide their approaches to asthma management.
They review key indicators of severe asthma, tools to assess asthma control, approaches to phenotyping patients, treatment options for type 2 and non–type 2 asthma, and emerging agents.
--
Sandhya Khurana, MD is a Professor, Department of Medicine, University of Rochester, Director, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Sandhya Khurana, MD, has disclosed the following relevant financial relationships: Received research grant from: GlaxoSmithKline.
Steve N. Georas, MD is a Professor, Department of Medicine, University of Rochester; Walter & Carmina Mary Parkes Family Endowed Professor; Director, Pulmonary Function Labs, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Steve N. Georas, MD, has disclosed no relevant financial relationships.
Asthma is a complex, heterogeneous disease with unmet treatment needs. Patients with severe asthma generally continue to have severe disease despite being on controller therapies.
Uncontrolled asthma can result in unnecessary suffering and interfere with daily activities. It also increases the risk for exacerbations and places substantial burden on the healthcare system.
In this ReCAP, Drs Sandhya Khurana and Steve N. Georas, from the Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care in Rochester, New York, discuss current challenges in the management of severe asthma, and how advances in the understanding of phenotypes and therapeutic options guide their approaches to asthma management.
They review key indicators of severe asthma, tools to assess asthma control, approaches to phenotyping patients, treatment options for type 2 and non–type 2 asthma, and emerging agents.
--
Sandhya Khurana, MD is a Professor, Department of Medicine, University of Rochester, Director, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Sandhya Khurana, MD, has disclosed the following relevant financial relationships: Received research grant from: GlaxoSmithKline.
Steve N. Georas, MD is a Professor, Department of Medicine, University of Rochester; Walter & Carmina Mary Parkes Family Endowed Professor; Director, Pulmonary Function Labs, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Steve N. Georas, MD, has disclosed no relevant financial relationships.
Asthma is a complex, heterogeneous disease with unmet treatment needs. Patients with severe asthma generally continue to have severe disease despite being on controller therapies.
Uncontrolled asthma can result in unnecessary suffering and interfere with daily activities. It also increases the risk for exacerbations and places substantial burden on the healthcare system.
In this ReCAP, Drs Sandhya Khurana and Steve N. Georas, from the Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care in Rochester, New York, discuss current challenges in the management of severe asthma, and how advances in the understanding of phenotypes and therapeutic options guide their approaches to asthma management.
They review key indicators of severe asthma, tools to assess asthma control, approaches to phenotyping patients, treatment options for type 2 and non–type 2 asthma, and emerging agents.
--
Sandhya Khurana, MD is a Professor, Department of Medicine, University of Rochester, Director, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Sandhya Khurana, MD, has disclosed the following relevant financial relationships: Received research grant from: GlaxoSmithKline.
Steve N. Georas, MD is a Professor, Department of Medicine, University of Rochester; Walter & Carmina Mary Parkes Family Endowed Professor; Director, Pulmonary Function Labs, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Steve N. Georas, MD, has disclosed no relevant financial relationships.

Gender pay gap most pronounced in procedural specialties
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Good survival, outcomes with TARE for HCC in practice
Patients with hepatocellular carcinoma (HCC) can be offered transarterial radioembolization (TARE) as a safe and effective first-line treatment or adjunct to other locoregional therapies, authors of a large multicenter study reported.
Among 422 patients with HCC treated with TARE in eight European countries, the median overall survival was 16.5 months, with fewer than 10% of patients experiencing grade 3 or greater adverse events, reported Frank Kolligs, MD, from Helios Hospital Berlin-Buch.
“This exploratory study evaluated factors that can influence the application and outcome of transarterial radioembolization in clinical practice. TARE is generally applied according to guideline recommendations, and randomized, controlled trials are needed to confirm the effect of personalized dosimetry on the effectiveness of TARE,” he said in an oral abstract presented at the meeting sponsored by the European Association for the Study of the Liver.
Intriguingly, the investigators found evidence suggesting that patients whose treatments were planned using a partition model had better survival outcomes than those patients who treatments were based on calculated body surface area or measured BSA (mBSA), but this finding will need to be explored in more detail, Dr. Kolligs said.
The partition model incorporates variables such as tumor volume and liver volume, shunt fractions, the ratio of radiation uptake between tumor and normal tissues, vascular anatomy and other factors to estimate the optimal dose.
Study design
Dr. Kolligs and colleagues looked at prospective data from the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Registry for SIR-Spheres Therapy to evaluate the real-world clinical application of TARE with yttrium Y-90 resin microspheres in Europe, clinical outcomes, safety, and quality of life.
They selected data from centers with a minimum of 10 cases performed in the previous 12 months and at least 40 total cases overall.
The patients included adults 18 years and older scheduled for treatment with Y-90 resin microspheres for primary or metastatic liver tumors, with no specific exclusion criteria. The patients were followed for at least 24 months at recommended intervals of every 3 months. The first patient was enrolled in January 2015, and the last follow-up visit was in December 2019. A total of 422 registry patients had a diagnosis of HCC and were included in the study.
The median age was 68 years (range, 60-74), 80.8% were male, 70.9% had cirrhosis, 14.5% had ascites, and 8.5% had extrahepatic disease. About 32% of patients had one tumor nodule, 33% had two to five nodules, and the remainder had either more than five or an uncountable number.
In all, 14% of patients had Barcelona Clinic Liver Cancer stage A disease, 51.4% had stage B, 33.6% had stage C, and 0.9% stage D.
About one-third of patients had portal vein occlusion. Tumors were in both left and right lobes in 35.5%, the left lobe alone in 12.1%, and the right lobe alone in 52.4%.
Half of all patients (50.2%) received TARE as first-line therapy, 44.8% had it following surgery (17.1%), ablation (14.7%), and/or transarterial chemoembolization (; 23%). In addition, 9.7% of patients received systemic therapy prior to TARE, primarily with sorafenib (Nexavar).
Treatment intent was palliative for 57.3% of patients, and tumor downsizing/downstaging in 32.5% (remainder unspecified).
Survival and prognostic factors
As noted before, median overall survival was 16.5 months. Median progression-free survival was 6.1 months, and median hepatic PFS was 6.7 months.
Factors prognostic for better overall survival included hepatitis B or C virus as the cirrhosis cause versus alcohol (hazard ratio for death, 0.51 for each; P = .0060 for HBV and P = .0007 for HCV); unilobar versus bilobar tumors (HR, 0.67; P = .0422 for left-lobe; HR 0.55; P < .0001 for right); prior surgery (HR, 0.67; P = .0258); prior ablation (HR, 0.65; P = .0394); and curative versus palliative intent (HR, 0.53; P < .0001).
Factors associated with worse overall survival were presence of ascites (HR 1.75, P = .001); presence of extrahepatic disease before TARE (HR, 1.81, P = .0037); tumor burden greater than 5 nodules (HR, 1.67; P = .0073); main portal vein occlusion (HR, 2.14; P = .0064); lobar portal vein occlusion (HR, 1.77; P = .0083); total bilirubin greater than 1.5 mg/dL (HR, 1.69; P = .0094); albumin-bilirubin grade A2 (HR, 1.66; P = .0005); ALB1 grade A3 (HR, 3.92; P < .0001); and BSA/mBSA versus partition-model dosimetry (HR, 1.89; P < .0001).
The safety analysis showed that 36.7% of patients had at least one adverse event, but only 7.1% had at least one grade 3 or greater event.
Grade 3 or greater events were abdominal pain (nine patients), fatigue (six), nausea (three), radioembolization-induced liver disease (three), vomiting (two), and GI ulceration (one). Fifteen additional patients had other unspecified events.
The investigators acknowledged broad inclusion criteria, relatively high rates of loss to follow-up, and differences in national guidelines and local standards of practice as potential limitations to their findings.
In the question-and-answer following the presentation, session comoderator María Varela, MD, PhD, a pathologist in the liver unit at the Hospital Universitario Central de Asturia, Oviedo, Spain, questioned why about one-third of patients received TARE for downstaging, but only 13 underwent subsequent surgical resection.
“We don’t have a detailed analysis of this subgroup of patients who received curative intent as yet, ” Dr. Kolligs said.
Pierre Nahon, MD, from the University of Paris and Hôpital Jean Verdier in Bondy, France, commented that, among this heterogenous population, one of the best indications for TARE is probably localized HCC with adjacent portal vein thrombosis.
He asked whether the investigators had examined overall survival among patients with localized unilobar HCC with adjacent small portal vein thrombosis.
“We find that patients with portal vein occlusion have a worse prognosis in the total group,” Dr. Kolligs replied. “To look into the question whether partial thrombosis with a small tumor might benefit is an interesting question, and we should look into that, but I don’t have any data on that yet.”
Another audience member asked: “According to your data, which patients are the best candidates for radioembolization?”
“According to these data, the best candidates are of course patients with good liver function, ascites should ideally not be present, and what is probably is important is that we identify or include patients without extrahepatic disease,” he said.
The study was sponsored by CIRSE. Dr. Kolligs disclosed speaking activities and consulting for several companies. Dr. Varela disclosed speaking for several companies and advisory board activity for Bayer. Dr. Nahon disclosed honoraria and consulting fees from several companies.
Patients with hepatocellular carcinoma (HCC) can be offered transarterial radioembolization (TARE) as a safe and effective first-line treatment or adjunct to other locoregional therapies, authors of a large multicenter study reported.
Among 422 patients with HCC treated with TARE in eight European countries, the median overall survival was 16.5 months, with fewer than 10% of patients experiencing grade 3 or greater adverse events, reported Frank Kolligs, MD, from Helios Hospital Berlin-Buch.
“This exploratory study evaluated factors that can influence the application and outcome of transarterial radioembolization in clinical practice. TARE is generally applied according to guideline recommendations, and randomized, controlled trials are needed to confirm the effect of personalized dosimetry on the effectiveness of TARE,” he said in an oral abstract presented at the meeting sponsored by the European Association for the Study of the Liver.
Intriguingly, the investigators found evidence suggesting that patients whose treatments were planned using a partition model had better survival outcomes than those patients who treatments were based on calculated body surface area or measured BSA (mBSA), but this finding will need to be explored in more detail, Dr. Kolligs said.
The partition model incorporates variables such as tumor volume and liver volume, shunt fractions, the ratio of radiation uptake between tumor and normal tissues, vascular anatomy and other factors to estimate the optimal dose.
Study design
Dr. Kolligs and colleagues looked at prospective data from the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Registry for SIR-Spheres Therapy to evaluate the real-world clinical application of TARE with yttrium Y-90 resin microspheres in Europe, clinical outcomes, safety, and quality of life.
They selected data from centers with a minimum of 10 cases performed in the previous 12 months and at least 40 total cases overall.
The patients included adults 18 years and older scheduled for treatment with Y-90 resin microspheres for primary or metastatic liver tumors, with no specific exclusion criteria. The patients were followed for at least 24 months at recommended intervals of every 3 months. The first patient was enrolled in January 2015, and the last follow-up visit was in December 2019. A total of 422 registry patients had a diagnosis of HCC and were included in the study.
The median age was 68 years (range, 60-74), 80.8% were male, 70.9% had cirrhosis, 14.5% had ascites, and 8.5% had extrahepatic disease. About 32% of patients had one tumor nodule, 33% had two to five nodules, and the remainder had either more than five or an uncountable number.
In all, 14% of patients had Barcelona Clinic Liver Cancer stage A disease, 51.4% had stage B, 33.6% had stage C, and 0.9% stage D.
About one-third of patients had portal vein occlusion. Tumors were in both left and right lobes in 35.5%, the left lobe alone in 12.1%, and the right lobe alone in 52.4%.
Half of all patients (50.2%) received TARE as first-line therapy, 44.8% had it following surgery (17.1%), ablation (14.7%), and/or transarterial chemoembolization (; 23%). In addition, 9.7% of patients received systemic therapy prior to TARE, primarily with sorafenib (Nexavar).
Treatment intent was palliative for 57.3% of patients, and tumor downsizing/downstaging in 32.5% (remainder unspecified).
Survival and prognostic factors
As noted before, median overall survival was 16.5 months. Median progression-free survival was 6.1 months, and median hepatic PFS was 6.7 months.
Factors prognostic for better overall survival included hepatitis B or C virus as the cirrhosis cause versus alcohol (hazard ratio for death, 0.51 for each; P = .0060 for HBV and P = .0007 for HCV); unilobar versus bilobar tumors (HR, 0.67; P = .0422 for left-lobe; HR 0.55; P < .0001 for right); prior surgery (HR, 0.67; P = .0258); prior ablation (HR, 0.65; P = .0394); and curative versus palliative intent (HR, 0.53; P < .0001).
Factors associated with worse overall survival were presence of ascites (HR 1.75, P = .001); presence of extrahepatic disease before TARE (HR, 1.81, P = .0037); tumor burden greater than 5 nodules (HR, 1.67; P = .0073); main portal vein occlusion (HR, 2.14; P = .0064); lobar portal vein occlusion (HR, 1.77; P = .0083); total bilirubin greater than 1.5 mg/dL (HR, 1.69; P = .0094); albumin-bilirubin grade A2 (HR, 1.66; P = .0005); ALB1 grade A3 (HR, 3.92; P < .0001); and BSA/mBSA versus partition-model dosimetry (HR, 1.89; P < .0001).
The safety analysis showed that 36.7% of patients had at least one adverse event, but only 7.1% had at least one grade 3 or greater event.
Grade 3 or greater events were abdominal pain (nine patients), fatigue (six), nausea (three), radioembolization-induced liver disease (three), vomiting (two), and GI ulceration (one). Fifteen additional patients had other unspecified events.
The investigators acknowledged broad inclusion criteria, relatively high rates of loss to follow-up, and differences in national guidelines and local standards of practice as potential limitations to their findings.
In the question-and-answer following the presentation, session comoderator María Varela, MD, PhD, a pathologist in the liver unit at the Hospital Universitario Central de Asturia, Oviedo, Spain, questioned why about one-third of patients received TARE for downstaging, but only 13 underwent subsequent surgical resection.
“We don’t have a detailed analysis of this subgroup of patients who received curative intent as yet, ” Dr. Kolligs said.
Pierre Nahon, MD, from the University of Paris and Hôpital Jean Verdier in Bondy, France, commented that, among this heterogenous population, one of the best indications for TARE is probably localized HCC with adjacent portal vein thrombosis.
He asked whether the investigators had examined overall survival among patients with localized unilobar HCC with adjacent small portal vein thrombosis.
“We find that patients with portal vein occlusion have a worse prognosis in the total group,” Dr. Kolligs replied. “To look into the question whether partial thrombosis with a small tumor might benefit is an interesting question, and we should look into that, but I don’t have any data on that yet.”
Another audience member asked: “According to your data, which patients are the best candidates for radioembolization?”
“According to these data, the best candidates are of course patients with good liver function, ascites should ideally not be present, and what is probably is important is that we identify or include patients without extrahepatic disease,” he said.
The study was sponsored by CIRSE. Dr. Kolligs disclosed speaking activities and consulting for several companies. Dr. Varela disclosed speaking for several companies and advisory board activity for Bayer. Dr. Nahon disclosed honoraria and consulting fees from several companies.
Patients with hepatocellular carcinoma (HCC) can be offered transarterial radioembolization (TARE) as a safe and effective first-line treatment or adjunct to other locoregional therapies, authors of a large multicenter study reported.
Among 422 patients with HCC treated with TARE in eight European countries, the median overall survival was 16.5 months, with fewer than 10% of patients experiencing grade 3 or greater adverse events, reported Frank Kolligs, MD, from Helios Hospital Berlin-Buch.
“This exploratory study evaluated factors that can influence the application and outcome of transarterial radioembolization in clinical practice. TARE is generally applied according to guideline recommendations, and randomized, controlled trials are needed to confirm the effect of personalized dosimetry on the effectiveness of TARE,” he said in an oral abstract presented at the meeting sponsored by the European Association for the Study of the Liver.
Intriguingly, the investigators found evidence suggesting that patients whose treatments were planned using a partition model had better survival outcomes than those patients who treatments were based on calculated body surface area or measured BSA (mBSA), but this finding will need to be explored in more detail, Dr. Kolligs said.
The partition model incorporates variables such as tumor volume and liver volume, shunt fractions, the ratio of radiation uptake between tumor and normal tissues, vascular anatomy and other factors to estimate the optimal dose.
Study design
Dr. Kolligs and colleagues looked at prospective data from the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Registry for SIR-Spheres Therapy to evaluate the real-world clinical application of TARE with yttrium Y-90 resin microspheres in Europe, clinical outcomes, safety, and quality of life.
They selected data from centers with a minimum of 10 cases performed in the previous 12 months and at least 40 total cases overall.
The patients included adults 18 years and older scheduled for treatment with Y-90 resin microspheres for primary or metastatic liver tumors, with no specific exclusion criteria. The patients were followed for at least 24 months at recommended intervals of every 3 months. The first patient was enrolled in January 2015, and the last follow-up visit was in December 2019. A total of 422 registry patients had a diagnosis of HCC and were included in the study.
The median age was 68 years (range, 60-74), 80.8% were male, 70.9% had cirrhosis, 14.5% had ascites, and 8.5% had extrahepatic disease. About 32% of patients had one tumor nodule, 33% had two to five nodules, and the remainder had either more than five or an uncountable number.
In all, 14% of patients had Barcelona Clinic Liver Cancer stage A disease, 51.4% had stage B, 33.6% had stage C, and 0.9% stage D.
About one-third of patients had portal vein occlusion. Tumors were in both left and right lobes in 35.5%, the left lobe alone in 12.1%, and the right lobe alone in 52.4%.
Half of all patients (50.2%) received TARE as first-line therapy, 44.8% had it following surgery (17.1%), ablation (14.7%), and/or transarterial chemoembolization (; 23%). In addition, 9.7% of patients received systemic therapy prior to TARE, primarily with sorafenib (Nexavar).
Treatment intent was palliative for 57.3% of patients, and tumor downsizing/downstaging in 32.5% (remainder unspecified).
Survival and prognostic factors
As noted before, median overall survival was 16.5 months. Median progression-free survival was 6.1 months, and median hepatic PFS was 6.7 months.
Factors prognostic for better overall survival included hepatitis B or C virus as the cirrhosis cause versus alcohol (hazard ratio for death, 0.51 for each; P = .0060 for HBV and P = .0007 for HCV); unilobar versus bilobar tumors (HR, 0.67; P = .0422 for left-lobe; HR 0.55; P < .0001 for right); prior surgery (HR, 0.67; P = .0258); prior ablation (HR, 0.65; P = .0394); and curative versus palliative intent (HR, 0.53; P < .0001).
Factors associated with worse overall survival were presence of ascites (HR 1.75, P = .001); presence of extrahepatic disease before TARE (HR, 1.81, P = .0037); tumor burden greater than 5 nodules (HR, 1.67; P = .0073); main portal vein occlusion (HR, 2.14; P = .0064); lobar portal vein occlusion (HR, 1.77; P = .0083); total bilirubin greater than 1.5 mg/dL (HR, 1.69; P = .0094); albumin-bilirubin grade A2 (HR, 1.66; P = .0005); ALB1 grade A3 (HR, 3.92; P < .0001); and BSA/mBSA versus partition-model dosimetry (HR, 1.89; P < .0001).
The safety analysis showed that 36.7% of patients had at least one adverse event, but only 7.1% had at least one grade 3 or greater event.
Grade 3 or greater events were abdominal pain (nine patients), fatigue (six), nausea (three), radioembolization-induced liver disease (three), vomiting (two), and GI ulceration (one). Fifteen additional patients had other unspecified events.
The investigators acknowledged broad inclusion criteria, relatively high rates of loss to follow-up, and differences in national guidelines and local standards of practice as potential limitations to their findings.
In the question-and-answer following the presentation, session comoderator María Varela, MD, PhD, a pathologist in the liver unit at the Hospital Universitario Central de Asturia, Oviedo, Spain, questioned why about one-third of patients received TARE for downstaging, but only 13 underwent subsequent surgical resection.
“We don’t have a detailed analysis of this subgroup of patients who received curative intent as yet, ” Dr. Kolligs said.
Pierre Nahon, MD, from the University of Paris and Hôpital Jean Verdier in Bondy, France, commented that, among this heterogenous population, one of the best indications for TARE is probably localized HCC with adjacent portal vein thrombosis.
He asked whether the investigators had examined overall survival among patients with localized unilobar HCC with adjacent small portal vein thrombosis.
“We find that patients with portal vein occlusion have a worse prognosis in the total group,” Dr. Kolligs replied. “To look into the question whether partial thrombosis with a small tumor might benefit is an interesting question, and we should look into that, but I don’t have any data on that yet.”
Another audience member asked: “According to your data, which patients are the best candidates for radioembolization?”
“According to these data, the best candidates are of course patients with good liver function, ascites should ideally not be present, and what is probably is important is that we identify or include patients without extrahepatic disease,” he said.
The study was sponsored by CIRSE. Dr. Kolligs disclosed speaking activities and consulting for several companies. Dr. Varela disclosed speaking for several companies and advisory board activity for Bayer. Dr. Nahon disclosed honoraria and consulting fees from several companies.
FROM ILC 2021






