User login
Docs fight back after losing hospital privileges, patients, and income
In April, a group of more than a dozen cardiologists at St. Louis Heart and Vascular (SLHV) lost their privileges at SSM Health, an eight-hospital system in St. Louis.
The physicians did not lose their privileges because of a clinical failure. Rather, it was because of SSM’s decision to enter into an exclusive contract with another set of cardiologists.
“The current situation is economically untenable for us,” said Harvey Serota, MD, founder and medical director of SLHV. “This is an existential threat to the practice.”
Because of the exclusive contract, many of SLHV’s patients are now being redirected to SSM-contracted cardiologists. Volume for the group’s new $15 million catheterization lab has plummeted. SLHV is suing SSM to restore its privileges, claiming lack of due process, restraint of trade, interference with its business, and breach of contract.
Losing privileges because a hospital seeks to increase their profits is becoming all too familiar for many independent specialists in fields such as cardiology, orthopedic surgery, and urology, as the hospitals that hosted them become their competitors and forge exclusive contracts with opposing groups.
What can these doctors do if they’re shut out? File a lawsuit, as SLHV has done? Demand a hearing before the medical staff and try to resolve the problem? Or simply give up their privileges and move on?
Unfortunately, none of these approaches offer a quick or certain solution, and each comes with risks.
Generally, courts have upheld hospitals’ use of exclusive contracts, which is also known as economic credentialing, says Barry F. Rosen, a health law attorney at Gordon Feinblatt, in Baltimore.
“Courts have long recognized exclusive contracts, and challenges by excluded doctors usually fail,” he says.
However, Mr. Rosen can cite several examples in which excluded doctors launched legal challenges that prevailed, owing to nuances in the law. The legal field in this area is tangled, and it varies by state.
Can hospitals make exclusive deals?
Hospitals have long used exclusive contracts for hospital-based specialists – anesthesiologists, radiologists, pathologists, emergency physicians, and hospitalists. They say that restricting patients to one group of anesthesiologists or radiologists enhances operational efficiency and that these contracts do not disrupt patients, because patients have no ties to hospital-based physicians. Such contracts are often more profitable for the hospital because of the negotiated rates.
Exclusive contracts in other specialties, however, are less accepted because they involve markedly different strategies and have different effects. In such cases, the hospital is no longer simply enhancing operational efficiency but is competing with physicians on staff, and the arrangement can disrupt the care of patients of the excluded doctors.
In the courts, these concerns might form the basis of an antitrust action or a claim of tortious interference with physicians’ ability to provide care for their patients, but neither claim is easy to win, Mr. Rosen says.
In antitrust cases, “the issue is not whether the excluded doctor was injured but whether the action harmed competition,” Mr. Rosen says. “Will the exclusion lead to higher prices?”
In the case of interference with patient care, “you will always find interference by one entity in the affairs of another,” he says, “but tortious interference applies to situations where something nefarious is going on, such as the other side was out to destroy your business and create a monopoly.”
Hospitals may try to restrict the privileges of physicians who invest in competing facilities such as cath labs and ambulatory surgery centers (ASCs), says Gregory Mertz, managing director of Physician Strategies Group, a consultancy in Virginia Beach.
“However, any revenge that a hospital might take against the doctors who started an ASC would usually not be publicly admitted,” Mr. Mertz says. “Revenge would be exacted in subtle ways.”
In the St. Louis situation, SSM did not cite SLHV’s cath lab as a reason for its exclusive contract. SSM stated in court documents that the decision was based on the recommendations of an expert panel. Furthermore, SSM said the board created the panel in response to a state report that cited the limited experience of some SLHV cardiologists in treating a rare type of heart attack.
Mr. Mertz says the board’s interest in the state’s concern and then its forming the special panel lent a great deal of legitimacy to SSM’s decision to start an exclusive contract. “SSM can show evidence that the board’s decision was based on a clinical matter and not on trying to squeeze out the cardiologists,” he says.
In SLHV’s defense, Dr. Serota says the practice offered to stop taking calls for the type of heart attack that was cited, but the hospital did not respond to its offer. He says SSM should have consulted the hospital’s medical staff to address the state’s concern and to create the exclusive contract, because these decisions involved clinical issues that the medical staff understands better than the board.
The law, however, does not require a hospital board to consult with its medical staff, says Alice G. Gosfield, a health care attorney in Philadelphia. “The board has ultimate legal control of everything in the hospital,” she says. However, the board often delegates certain functions to the medical staff in the hospital bylaws, and depending on the wording of the bylaws, it is still possible that the board violated the bylaws, Ms. Gosfield adds.
Can excluded physicians get peer review?
Can the hospital medical staff help restore the privileges of excluded physicians? Don’t these physicians have the right to peer review – a hearing before the medical staff?
Indeed, the Joint Commission, which accredits hospitals, states that the hospital must have “mechanisms, including a fair hearing and appeal process, for addressing adverse decisions for existing medical staff members and other individuals holding clinical privileges for renewal, revocation, or revision of clinical privileges.”
However, excluded physicians may not have a right to a hearing if they have not been fully stripped of privileges. SSM discontinued adult cardiology privileges for SLHV doctors but retained some doctors’ internal medicine privileges. Dr. Serota says internal medicine privileges are useless to cardiologists, but because the doctors’ privileges had not been fully removed, they cannot ask for a hearing.
More fundamentally, exclusive contracts are not a good fit for peer review. Mr. Rosen says the hearings were designed to review the physicians’ clinical competence or behavior, but excluded physicians do not have these problems. About all the hearing could focus on is the hospital’s policy, which the board would not want to allow. To avoid this, “the hospital might rule out a hearing as contrary to the intent of the bylaws,” Mr. Rosen says.
Furthermore, even if peer review goes forward, “what the medical staff decides is only advisory, and the hospital board makes the final decision,” Mr. Rosen says. He notes that the doctor could challenge the decision in court, but the hospital might still prevail.
Excluded physicians sometimes prevail
Although it is rare for excluded physicians to win a lawsuit against their hospital, it does happen, says Michael R. Callahan, health lawyer at Katten Muchin Rosenman, in Chicago.
Mr. Callahan cites a 2010 decision by the Arkansas Supreme Court that stopped the state’s largest health system from denying physicians’ privileges. Among other things, the hospital was found to have tortiously interfered with the physicians’ contracts with patients.
In a 2007 decision, a West Virginia court ruled that hospitals that have a mission to serve the public cannot exclude physicians for nonquality issues. In addition, some states, such as Texas, limit the economic factors that can be considered when credentialing decisions are made. Other states, such as Ohio, give hospitals a great deal of leeway to alter credentialing.
Dr. Serota is optimistic about his Missouri lawsuit. Although the judge in the case did not immediately grant SLHV’s request for restoration of privileges while the case proceeds, she did grant expedited discovery – allowing SLHV to obtain documents from SSM that could strengthen the doctors’ case – and she agreed to a hearing on SLHV’s request for a temporary restoration of privileges.
Ms. Gosfield says Dr. Serota’s optimism seems justified, but she adds that such cases cost a lot of money and that they may still not be winnable.
Often plaintiffs can settle lawsuits before they go to trial, but Mr. Callahan says hospitals are loath to restore privileges in a settlement because they don’t want to undermine an exclusivity deal. “The exclusive group expects a certain volume, which can’t be reached if the competing doctors are allowed back in,” he says.
Many physicians don’t challenge the exclusion
Quite often, excluded doctors decide not to challenge the decision. For example, Dr. Serota says groups of orthopedic surgeons and urologists have decided not to challenge similar decisions by SSM. “They wanted to move on,” he says.
Mr. Callahan says many excluded doctors also don’t even ask for a hearing. “They expect that the hospital’s decision will be upheld,” he says.
This was the case for Devendra K. Amin, MD, an independent cardiologist in Easton, Pa. Dr. Amin has not had any hospital privileges since July 2020. Even though he is board certified in interventional cardiology, which involves catheterization, Dr. Amin says he cannot perform these procedures because they can only be performed in a hospital in the area.
In the 1990s, Dr. Amin says, he had invasive cardiology privileges at five hospitals, but then those hospitals consolidated, and the remaining ones started constricting his privileges. First he could no longer work in the emergency department, then he could no longer read echocardiograms and interpret stress test results, because that work was assigned exclusively to employed doctors, he says.
Then the one remaining hospital announced that privileges would only be available to physicians by invitation, and he was not invited. Dr. Amin says he could have regained general cardiology privileges if he had accepted employment at the hospital, but he did not want to do this. A recruiter and the head of the cardiology section at the hospital even took him out to dinner 2 years ago to discuss employment, but there was a stipulation that the hospital would not agree to.
“I wanted to get back my interventional privileges back,” Dr. Amin says, “but they told me that would not be possible because they had an exclusive contract with a group.”
Dr. Amin says that now, he can only work as a general cardiologist with reduced volume. He says primary care physicians in the local hospital systems only refer to cardiologists within their systems. “When these patients do come to me, it is only because they specifically requested to see me,” Dr. Amin says.
He does not want to challenge the decisions regarding privileging. “Look, I am 68 years old,” Dr. Amin says. “I’m not retiring yet, but I don’t want to get into a battle with a hospital that has very deep pockets. I’m not a confrontational person to begin with, and I don’t want to spend the next 10 years of my life in litigation.”
Diverging expectations
The law on exclusive contracts does not provide easy answers for excluded doctors, and often it defies physicians’ conception of their own role in the hospital.
Many physicians expect the hospital to be a haven where they can do their work without being cut out by a competitor. This view is reinforced by organizations such as the American Medical Association.
The AMA Council on Medical Service states that privileges “can only be abridged upon recommendation of the medical staff and only for reason related to professional competence, adherence to standards of care, and other parameters agreed to by the medical staff.”
But the courts don’t tend to agree with that position. “Hospitals have a fiduciary duty to protect their own financial interests,” Mr. Callahan says. “This may involve anything that furthers the hospital’s mission to provide high-quality health care services to its patient community.”
At the same time, however, there are plenty of instances in which courts have ruled that exclusive contracts had gone too far. But usually it takes a lawyer experienced in these cases to know what those exceptions are.
A version of this article first appeared on Medscape.com.
In April, a group of more than a dozen cardiologists at St. Louis Heart and Vascular (SLHV) lost their privileges at SSM Health, an eight-hospital system in St. Louis.
The physicians did not lose their privileges because of a clinical failure. Rather, it was because of SSM’s decision to enter into an exclusive contract with another set of cardiologists.
“The current situation is economically untenable for us,” said Harvey Serota, MD, founder and medical director of SLHV. “This is an existential threat to the practice.”
Because of the exclusive contract, many of SLHV’s patients are now being redirected to SSM-contracted cardiologists. Volume for the group’s new $15 million catheterization lab has plummeted. SLHV is suing SSM to restore its privileges, claiming lack of due process, restraint of trade, interference with its business, and breach of contract.
Losing privileges because a hospital seeks to increase their profits is becoming all too familiar for many independent specialists in fields such as cardiology, orthopedic surgery, and urology, as the hospitals that hosted them become their competitors and forge exclusive contracts with opposing groups.
What can these doctors do if they’re shut out? File a lawsuit, as SLHV has done? Demand a hearing before the medical staff and try to resolve the problem? Or simply give up their privileges and move on?
Unfortunately, none of these approaches offer a quick or certain solution, and each comes with risks.
Generally, courts have upheld hospitals’ use of exclusive contracts, which is also known as economic credentialing, says Barry F. Rosen, a health law attorney at Gordon Feinblatt, in Baltimore.
“Courts have long recognized exclusive contracts, and challenges by excluded doctors usually fail,” he says.
However, Mr. Rosen can cite several examples in which excluded doctors launched legal challenges that prevailed, owing to nuances in the law. The legal field in this area is tangled, and it varies by state.
Can hospitals make exclusive deals?
Hospitals have long used exclusive contracts for hospital-based specialists – anesthesiologists, radiologists, pathologists, emergency physicians, and hospitalists. They say that restricting patients to one group of anesthesiologists or radiologists enhances operational efficiency and that these contracts do not disrupt patients, because patients have no ties to hospital-based physicians. Such contracts are often more profitable for the hospital because of the negotiated rates.
Exclusive contracts in other specialties, however, are less accepted because they involve markedly different strategies and have different effects. In such cases, the hospital is no longer simply enhancing operational efficiency but is competing with physicians on staff, and the arrangement can disrupt the care of patients of the excluded doctors.
In the courts, these concerns might form the basis of an antitrust action or a claim of tortious interference with physicians’ ability to provide care for their patients, but neither claim is easy to win, Mr. Rosen says.
In antitrust cases, “the issue is not whether the excluded doctor was injured but whether the action harmed competition,” Mr. Rosen says. “Will the exclusion lead to higher prices?”
In the case of interference with patient care, “you will always find interference by one entity in the affairs of another,” he says, “but tortious interference applies to situations where something nefarious is going on, such as the other side was out to destroy your business and create a monopoly.”
Hospitals may try to restrict the privileges of physicians who invest in competing facilities such as cath labs and ambulatory surgery centers (ASCs), says Gregory Mertz, managing director of Physician Strategies Group, a consultancy in Virginia Beach.
“However, any revenge that a hospital might take against the doctors who started an ASC would usually not be publicly admitted,” Mr. Mertz says. “Revenge would be exacted in subtle ways.”
In the St. Louis situation, SSM did not cite SLHV’s cath lab as a reason for its exclusive contract. SSM stated in court documents that the decision was based on the recommendations of an expert panel. Furthermore, SSM said the board created the panel in response to a state report that cited the limited experience of some SLHV cardiologists in treating a rare type of heart attack.
Mr. Mertz says the board’s interest in the state’s concern and then its forming the special panel lent a great deal of legitimacy to SSM’s decision to start an exclusive contract. “SSM can show evidence that the board’s decision was based on a clinical matter and not on trying to squeeze out the cardiologists,” he says.
In SLHV’s defense, Dr. Serota says the practice offered to stop taking calls for the type of heart attack that was cited, but the hospital did not respond to its offer. He says SSM should have consulted the hospital’s medical staff to address the state’s concern and to create the exclusive contract, because these decisions involved clinical issues that the medical staff understands better than the board.
The law, however, does not require a hospital board to consult with its medical staff, says Alice G. Gosfield, a health care attorney in Philadelphia. “The board has ultimate legal control of everything in the hospital,” she says. However, the board often delegates certain functions to the medical staff in the hospital bylaws, and depending on the wording of the bylaws, it is still possible that the board violated the bylaws, Ms. Gosfield adds.
Can excluded physicians get peer review?
Can the hospital medical staff help restore the privileges of excluded physicians? Don’t these physicians have the right to peer review – a hearing before the medical staff?
Indeed, the Joint Commission, which accredits hospitals, states that the hospital must have “mechanisms, including a fair hearing and appeal process, for addressing adverse decisions for existing medical staff members and other individuals holding clinical privileges for renewal, revocation, or revision of clinical privileges.”
However, excluded physicians may not have a right to a hearing if they have not been fully stripped of privileges. SSM discontinued adult cardiology privileges for SLHV doctors but retained some doctors’ internal medicine privileges. Dr. Serota says internal medicine privileges are useless to cardiologists, but because the doctors’ privileges had not been fully removed, they cannot ask for a hearing.
More fundamentally, exclusive contracts are not a good fit for peer review. Mr. Rosen says the hearings were designed to review the physicians’ clinical competence or behavior, but excluded physicians do not have these problems. About all the hearing could focus on is the hospital’s policy, which the board would not want to allow. To avoid this, “the hospital might rule out a hearing as contrary to the intent of the bylaws,” Mr. Rosen says.
Furthermore, even if peer review goes forward, “what the medical staff decides is only advisory, and the hospital board makes the final decision,” Mr. Rosen says. He notes that the doctor could challenge the decision in court, but the hospital might still prevail.
Excluded physicians sometimes prevail
Although it is rare for excluded physicians to win a lawsuit against their hospital, it does happen, says Michael R. Callahan, health lawyer at Katten Muchin Rosenman, in Chicago.
Mr. Callahan cites a 2010 decision by the Arkansas Supreme Court that stopped the state’s largest health system from denying physicians’ privileges. Among other things, the hospital was found to have tortiously interfered with the physicians’ contracts with patients.
In a 2007 decision, a West Virginia court ruled that hospitals that have a mission to serve the public cannot exclude physicians for nonquality issues. In addition, some states, such as Texas, limit the economic factors that can be considered when credentialing decisions are made. Other states, such as Ohio, give hospitals a great deal of leeway to alter credentialing.
Dr. Serota is optimistic about his Missouri lawsuit. Although the judge in the case did not immediately grant SLHV’s request for restoration of privileges while the case proceeds, she did grant expedited discovery – allowing SLHV to obtain documents from SSM that could strengthen the doctors’ case – and she agreed to a hearing on SLHV’s request for a temporary restoration of privileges.
Ms. Gosfield says Dr. Serota’s optimism seems justified, but she adds that such cases cost a lot of money and that they may still not be winnable.
Often plaintiffs can settle lawsuits before they go to trial, but Mr. Callahan says hospitals are loath to restore privileges in a settlement because they don’t want to undermine an exclusivity deal. “The exclusive group expects a certain volume, which can’t be reached if the competing doctors are allowed back in,” he says.
Many physicians don’t challenge the exclusion
Quite often, excluded doctors decide not to challenge the decision. For example, Dr. Serota says groups of orthopedic surgeons and urologists have decided not to challenge similar decisions by SSM. “They wanted to move on,” he says.
Mr. Callahan says many excluded doctors also don’t even ask for a hearing. “They expect that the hospital’s decision will be upheld,” he says.
This was the case for Devendra K. Amin, MD, an independent cardiologist in Easton, Pa. Dr. Amin has not had any hospital privileges since July 2020. Even though he is board certified in interventional cardiology, which involves catheterization, Dr. Amin says he cannot perform these procedures because they can only be performed in a hospital in the area.
In the 1990s, Dr. Amin says, he had invasive cardiology privileges at five hospitals, but then those hospitals consolidated, and the remaining ones started constricting his privileges. First he could no longer work in the emergency department, then he could no longer read echocardiograms and interpret stress test results, because that work was assigned exclusively to employed doctors, he says.
Then the one remaining hospital announced that privileges would only be available to physicians by invitation, and he was not invited. Dr. Amin says he could have regained general cardiology privileges if he had accepted employment at the hospital, but he did not want to do this. A recruiter and the head of the cardiology section at the hospital even took him out to dinner 2 years ago to discuss employment, but there was a stipulation that the hospital would not agree to.
“I wanted to get back my interventional privileges back,” Dr. Amin says, “but they told me that would not be possible because they had an exclusive contract with a group.”
Dr. Amin says that now, he can only work as a general cardiologist with reduced volume. He says primary care physicians in the local hospital systems only refer to cardiologists within their systems. “When these patients do come to me, it is only because they specifically requested to see me,” Dr. Amin says.
He does not want to challenge the decisions regarding privileging. “Look, I am 68 years old,” Dr. Amin says. “I’m not retiring yet, but I don’t want to get into a battle with a hospital that has very deep pockets. I’m not a confrontational person to begin with, and I don’t want to spend the next 10 years of my life in litigation.”
Diverging expectations
The law on exclusive contracts does not provide easy answers for excluded doctors, and often it defies physicians’ conception of their own role in the hospital.
Many physicians expect the hospital to be a haven where they can do their work without being cut out by a competitor. This view is reinforced by organizations such as the American Medical Association.
The AMA Council on Medical Service states that privileges “can only be abridged upon recommendation of the medical staff and only for reason related to professional competence, adherence to standards of care, and other parameters agreed to by the medical staff.”
But the courts don’t tend to agree with that position. “Hospitals have a fiduciary duty to protect their own financial interests,” Mr. Callahan says. “This may involve anything that furthers the hospital’s mission to provide high-quality health care services to its patient community.”
At the same time, however, there are plenty of instances in which courts have ruled that exclusive contracts had gone too far. But usually it takes a lawyer experienced in these cases to know what those exceptions are.
A version of this article first appeared on Medscape.com.
In April, a group of more than a dozen cardiologists at St. Louis Heart and Vascular (SLHV) lost their privileges at SSM Health, an eight-hospital system in St. Louis.
The physicians did not lose their privileges because of a clinical failure. Rather, it was because of SSM’s decision to enter into an exclusive contract with another set of cardiologists.
“The current situation is economically untenable for us,” said Harvey Serota, MD, founder and medical director of SLHV. “This is an existential threat to the practice.”
Because of the exclusive contract, many of SLHV’s patients are now being redirected to SSM-contracted cardiologists. Volume for the group’s new $15 million catheterization lab has plummeted. SLHV is suing SSM to restore its privileges, claiming lack of due process, restraint of trade, interference with its business, and breach of contract.
Losing privileges because a hospital seeks to increase their profits is becoming all too familiar for many independent specialists in fields such as cardiology, orthopedic surgery, and urology, as the hospitals that hosted them become their competitors and forge exclusive contracts with opposing groups.
What can these doctors do if they’re shut out? File a lawsuit, as SLHV has done? Demand a hearing before the medical staff and try to resolve the problem? Or simply give up their privileges and move on?
Unfortunately, none of these approaches offer a quick or certain solution, and each comes with risks.
Generally, courts have upheld hospitals’ use of exclusive contracts, which is also known as economic credentialing, says Barry F. Rosen, a health law attorney at Gordon Feinblatt, in Baltimore.
“Courts have long recognized exclusive contracts, and challenges by excluded doctors usually fail,” he says.
However, Mr. Rosen can cite several examples in which excluded doctors launched legal challenges that prevailed, owing to nuances in the law. The legal field in this area is tangled, and it varies by state.
Can hospitals make exclusive deals?
Hospitals have long used exclusive contracts for hospital-based specialists – anesthesiologists, radiologists, pathologists, emergency physicians, and hospitalists. They say that restricting patients to one group of anesthesiologists or radiologists enhances operational efficiency and that these contracts do not disrupt patients, because patients have no ties to hospital-based physicians. Such contracts are often more profitable for the hospital because of the negotiated rates.
Exclusive contracts in other specialties, however, are less accepted because they involve markedly different strategies and have different effects. In such cases, the hospital is no longer simply enhancing operational efficiency but is competing with physicians on staff, and the arrangement can disrupt the care of patients of the excluded doctors.
In the courts, these concerns might form the basis of an antitrust action or a claim of tortious interference with physicians’ ability to provide care for their patients, but neither claim is easy to win, Mr. Rosen says.
In antitrust cases, “the issue is not whether the excluded doctor was injured but whether the action harmed competition,” Mr. Rosen says. “Will the exclusion lead to higher prices?”
In the case of interference with patient care, “you will always find interference by one entity in the affairs of another,” he says, “but tortious interference applies to situations where something nefarious is going on, such as the other side was out to destroy your business and create a monopoly.”
Hospitals may try to restrict the privileges of physicians who invest in competing facilities such as cath labs and ambulatory surgery centers (ASCs), says Gregory Mertz, managing director of Physician Strategies Group, a consultancy in Virginia Beach.
“However, any revenge that a hospital might take against the doctors who started an ASC would usually not be publicly admitted,” Mr. Mertz says. “Revenge would be exacted in subtle ways.”
In the St. Louis situation, SSM did not cite SLHV’s cath lab as a reason for its exclusive contract. SSM stated in court documents that the decision was based on the recommendations of an expert panel. Furthermore, SSM said the board created the panel in response to a state report that cited the limited experience of some SLHV cardiologists in treating a rare type of heart attack.
Mr. Mertz says the board’s interest in the state’s concern and then its forming the special panel lent a great deal of legitimacy to SSM’s decision to start an exclusive contract. “SSM can show evidence that the board’s decision was based on a clinical matter and not on trying to squeeze out the cardiologists,” he says.
In SLHV’s defense, Dr. Serota says the practice offered to stop taking calls for the type of heart attack that was cited, but the hospital did not respond to its offer. He says SSM should have consulted the hospital’s medical staff to address the state’s concern and to create the exclusive contract, because these decisions involved clinical issues that the medical staff understands better than the board.
The law, however, does not require a hospital board to consult with its medical staff, says Alice G. Gosfield, a health care attorney in Philadelphia. “The board has ultimate legal control of everything in the hospital,” she says. However, the board often delegates certain functions to the medical staff in the hospital bylaws, and depending on the wording of the bylaws, it is still possible that the board violated the bylaws, Ms. Gosfield adds.
Can excluded physicians get peer review?
Can the hospital medical staff help restore the privileges of excluded physicians? Don’t these physicians have the right to peer review – a hearing before the medical staff?
Indeed, the Joint Commission, which accredits hospitals, states that the hospital must have “mechanisms, including a fair hearing and appeal process, for addressing adverse decisions for existing medical staff members and other individuals holding clinical privileges for renewal, revocation, or revision of clinical privileges.”
However, excluded physicians may not have a right to a hearing if they have not been fully stripped of privileges. SSM discontinued adult cardiology privileges for SLHV doctors but retained some doctors’ internal medicine privileges. Dr. Serota says internal medicine privileges are useless to cardiologists, but because the doctors’ privileges had not been fully removed, they cannot ask for a hearing.
More fundamentally, exclusive contracts are not a good fit for peer review. Mr. Rosen says the hearings were designed to review the physicians’ clinical competence or behavior, but excluded physicians do not have these problems. About all the hearing could focus on is the hospital’s policy, which the board would not want to allow. To avoid this, “the hospital might rule out a hearing as contrary to the intent of the bylaws,” Mr. Rosen says.
Furthermore, even if peer review goes forward, “what the medical staff decides is only advisory, and the hospital board makes the final decision,” Mr. Rosen says. He notes that the doctor could challenge the decision in court, but the hospital might still prevail.
Excluded physicians sometimes prevail
Although it is rare for excluded physicians to win a lawsuit against their hospital, it does happen, says Michael R. Callahan, health lawyer at Katten Muchin Rosenman, in Chicago.
Mr. Callahan cites a 2010 decision by the Arkansas Supreme Court that stopped the state’s largest health system from denying physicians’ privileges. Among other things, the hospital was found to have tortiously interfered with the physicians’ contracts with patients.
In a 2007 decision, a West Virginia court ruled that hospitals that have a mission to serve the public cannot exclude physicians for nonquality issues. In addition, some states, such as Texas, limit the economic factors that can be considered when credentialing decisions are made. Other states, such as Ohio, give hospitals a great deal of leeway to alter credentialing.
Dr. Serota is optimistic about his Missouri lawsuit. Although the judge in the case did not immediately grant SLHV’s request for restoration of privileges while the case proceeds, she did grant expedited discovery – allowing SLHV to obtain documents from SSM that could strengthen the doctors’ case – and she agreed to a hearing on SLHV’s request for a temporary restoration of privileges.
Ms. Gosfield says Dr. Serota’s optimism seems justified, but she adds that such cases cost a lot of money and that they may still not be winnable.
Often plaintiffs can settle lawsuits before they go to trial, but Mr. Callahan says hospitals are loath to restore privileges in a settlement because they don’t want to undermine an exclusivity deal. “The exclusive group expects a certain volume, which can’t be reached if the competing doctors are allowed back in,” he says.
Many physicians don’t challenge the exclusion
Quite often, excluded doctors decide not to challenge the decision. For example, Dr. Serota says groups of orthopedic surgeons and urologists have decided not to challenge similar decisions by SSM. “They wanted to move on,” he says.
Mr. Callahan says many excluded doctors also don’t even ask for a hearing. “They expect that the hospital’s decision will be upheld,” he says.
This was the case for Devendra K. Amin, MD, an independent cardiologist in Easton, Pa. Dr. Amin has not had any hospital privileges since July 2020. Even though he is board certified in interventional cardiology, which involves catheterization, Dr. Amin says he cannot perform these procedures because they can only be performed in a hospital in the area.
In the 1990s, Dr. Amin says, he had invasive cardiology privileges at five hospitals, but then those hospitals consolidated, and the remaining ones started constricting his privileges. First he could no longer work in the emergency department, then he could no longer read echocardiograms and interpret stress test results, because that work was assigned exclusively to employed doctors, he says.
Then the one remaining hospital announced that privileges would only be available to physicians by invitation, and he was not invited. Dr. Amin says he could have regained general cardiology privileges if he had accepted employment at the hospital, but he did not want to do this. A recruiter and the head of the cardiology section at the hospital even took him out to dinner 2 years ago to discuss employment, but there was a stipulation that the hospital would not agree to.
“I wanted to get back my interventional privileges back,” Dr. Amin says, “but they told me that would not be possible because they had an exclusive contract with a group.”
Dr. Amin says that now, he can only work as a general cardiologist with reduced volume. He says primary care physicians in the local hospital systems only refer to cardiologists within their systems. “When these patients do come to me, it is only because they specifically requested to see me,” Dr. Amin says.
He does not want to challenge the decisions regarding privileging. “Look, I am 68 years old,” Dr. Amin says. “I’m not retiring yet, but I don’t want to get into a battle with a hospital that has very deep pockets. I’m not a confrontational person to begin with, and I don’t want to spend the next 10 years of my life in litigation.”
Diverging expectations
The law on exclusive contracts does not provide easy answers for excluded doctors, and often it defies physicians’ conception of their own role in the hospital.
Many physicians expect the hospital to be a haven where they can do their work without being cut out by a competitor. This view is reinforced by organizations such as the American Medical Association.
The AMA Council on Medical Service states that privileges “can only be abridged upon recommendation of the medical staff and only for reason related to professional competence, adherence to standards of care, and other parameters agreed to by the medical staff.”
But the courts don’t tend to agree with that position. “Hospitals have a fiduciary duty to protect their own financial interests,” Mr. Callahan says. “This may involve anything that furthers the hospital’s mission to provide high-quality health care services to its patient community.”
At the same time, however, there are plenty of instances in which courts have ruled that exclusive contracts had gone too far. But usually it takes a lawyer experienced in these cases to know what those exceptions are.
A version of this article first appeared on Medscape.com.
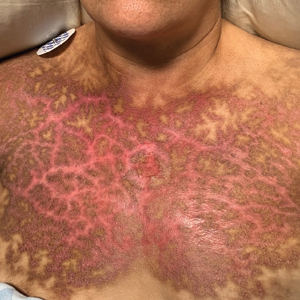
Reticular Rash on the Chest
The Diagnosis: Erythema Ab Igne
Based on the clinical findings and history, a diagnosis of erythema ab igne (EAI), a skin reaction to chronic infrared radiation exposure, was made. The name of this condition translates from Latin as “redness from fire”; other names include toasted skin syndrome and fire stains. The most common presentation is reticulated hyperpigmentation, erythema, and cutaneous atrophy, as well as possible crusting, scaling, or telangiectasia. The rash also typically presents in areas of heat exposure—from heated blankets, heating pads, or the use of infrared heaters or lamps.1,2 The patient usually will have pain and pruritus over the affected areas. The diagnosis of EAI largely is clinical and based on the patient’s history of exposure; it rarely requires biopsy and histologic analysis. However, some of the common histopathologic findings include hyperkeratosis, a hyperpigmented basal layer, hemosiderin deposits, prominent melanophages, basal cell degeneration, course collagen, and elastosis.2,3 These changes are common with UV radiation exposure and thermal damage. The primary treatment in all cases is to remove or reduce the source of infrared radiation. However, EAI has been reported to be successfully treated with removal of the insult as well as topical agents such as imiquimod and 5-fluorouracil.4 Possible complications include increased risk for malignancies such as squamous cell carcinoma in the affected area.1
The possible differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectatica congenita. All of these conditions are related to dysfunction of the cutaneous vasculature that creates a reticular, mottled, reddish purple rash. When the livedo is reversible and idiopathic, it is referred to as livedo reticularis, but when it is generalized and permanent it is referred to as livedo racemosa. Livedo racemosa can be caused by a variety of conditions, including systemic lupus erythematosus and antiphospholipid syndrome.1 Physiologic livedo reticularis that is more transient and can be reversed by warming is referred to as cutis marmorata. Finally, cutis marmorata telangiectatica congenita primarily is found in neonates, and although persistent, it usually improves with age. Erythema ab igne also is a type of livedo with a known heat exposure and localized distribution.
Our patient was educated on the etiology of the rash, specifically related to heating pad usage for multiple years, and the risk for cutaneous malignancy after longstanding EAI. It was recommended that she discontinue use of a heating pad on the affected areas to allow them to properly heal. If she found that heating pad usage was necessary, she was advised to limit use to 5 to 10 minutes with 2 to 3 hours in between applications. In addition, she was advised to apply petroleum jelly daily for assistance with wound healing as well as anti-itch sensitive lotion twice daily on the arms and back to alleviate some of the tingling pain. We explained that areas of hyperpigmentation may improve with time; however, areas of erythema/ atrophy may be long-lasting.
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635.
- Dellavalle RP, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Finlayson GR, Sams WM Jr, Smith JG Jr. Erythema ab igne: a histopathological study. J Invest Dermatol. 1966;46:104-108.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
The Diagnosis: Erythema Ab Igne
Based on the clinical findings and history, a diagnosis of erythema ab igne (EAI), a skin reaction to chronic infrared radiation exposure, was made. The name of this condition translates from Latin as “redness from fire”; other names include toasted skin syndrome and fire stains. The most common presentation is reticulated hyperpigmentation, erythema, and cutaneous atrophy, as well as possible crusting, scaling, or telangiectasia. The rash also typically presents in areas of heat exposure—from heated blankets, heating pads, or the use of infrared heaters or lamps.1,2 The patient usually will have pain and pruritus over the affected areas. The diagnosis of EAI largely is clinical and based on the patient’s history of exposure; it rarely requires biopsy and histologic analysis. However, some of the common histopathologic findings include hyperkeratosis, a hyperpigmented basal layer, hemosiderin deposits, prominent melanophages, basal cell degeneration, course collagen, and elastosis.2,3 These changes are common with UV radiation exposure and thermal damage. The primary treatment in all cases is to remove or reduce the source of infrared radiation. However, EAI has been reported to be successfully treated with removal of the insult as well as topical agents such as imiquimod and 5-fluorouracil.4 Possible complications include increased risk for malignancies such as squamous cell carcinoma in the affected area.1
The possible differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectatica congenita. All of these conditions are related to dysfunction of the cutaneous vasculature that creates a reticular, mottled, reddish purple rash. When the livedo is reversible and idiopathic, it is referred to as livedo reticularis, but when it is generalized and permanent it is referred to as livedo racemosa. Livedo racemosa can be caused by a variety of conditions, including systemic lupus erythematosus and antiphospholipid syndrome.1 Physiologic livedo reticularis that is more transient and can be reversed by warming is referred to as cutis marmorata. Finally, cutis marmorata telangiectatica congenita primarily is found in neonates, and although persistent, it usually improves with age. Erythema ab igne also is a type of livedo with a known heat exposure and localized distribution.
Our patient was educated on the etiology of the rash, specifically related to heating pad usage for multiple years, and the risk for cutaneous malignancy after longstanding EAI. It was recommended that she discontinue use of a heating pad on the affected areas to allow them to properly heal. If she found that heating pad usage was necessary, she was advised to limit use to 5 to 10 minutes with 2 to 3 hours in between applications. In addition, she was advised to apply petroleum jelly daily for assistance with wound healing as well as anti-itch sensitive lotion twice daily on the arms and back to alleviate some of the tingling pain. We explained that areas of hyperpigmentation may improve with time; however, areas of erythema/ atrophy may be long-lasting.
The Diagnosis: Erythema Ab Igne
Based on the clinical findings and history, a diagnosis of erythema ab igne (EAI), a skin reaction to chronic infrared radiation exposure, was made. The name of this condition translates from Latin as “redness from fire”; other names include toasted skin syndrome and fire stains. The most common presentation is reticulated hyperpigmentation, erythema, and cutaneous atrophy, as well as possible crusting, scaling, or telangiectasia. The rash also typically presents in areas of heat exposure—from heated blankets, heating pads, or the use of infrared heaters or lamps.1,2 The patient usually will have pain and pruritus over the affected areas. The diagnosis of EAI largely is clinical and based on the patient’s history of exposure; it rarely requires biopsy and histologic analysis. However, some of the common histopathologic findings include hyperkeratosis, a hyperpigmented basal layer, hemosiderin deposits, prominent melanophages, basal cell degeneration, course collagen, and elastosis.2,3 These changes are common with UV radiation exposure and thermal damage. The primary treatment in all cases is to remove or reduce the source of infrared radiation. However, EAI has been reported to be successfully treated with removal of the insult as well as topical agents such as imiquimod and 5-fluorouracil.4 Possible complications include increased risk for malignancies such as squamous cell carcinoma in the affected area.1
The possible differential for EAI includes livedo reticularis, livedo racemosa, cutis marmorata, and cutis marmorata telangiectatica congenita. All of these conditions are related to dysfunction of the cutaneous vasculature that creates a reticular, mottled, reddish purple rash. When the livedo is reversible and idiopathic, it is referred to as livedo reticularis, but when it is generalized and permanent it is referred to as livedo racemosa. Livedo racemosa can be caused by a variety of conditions, including systemic lupus erythematosus and antiphospholipid syndrome.1 Physiologic livedo reticularis that is more transient and can be reversed by warming is referred to as cutis marmorata. Finally, cutis marmorata telangiectatica congenita primarily is found in neonates, and although persistent, it usually improves with age. Erythema ab igne also is a type of livedo with a known heat exposure and localized distribution.
Our patient was educated on the etiology of the rash, specifically related to heating pad usage for multiple years, and the risk for cutaneous malignancy after longstanding EAI. It was recommended that she discontinue use of a heating pad on the affected areas to allow them to properly heal. If she found that heating pad usage was necessary, she was advised to limit use to 5 to 10 minutes with 2 to 3 hours in between applications. In addition, she was advised to apply petroleum jelly daily for assistance with wound healing as well as anti-itch sensitive lotion twice daily on the arms and back to alleviate some of the tingling pain. We explained that areas of hyperpigmentation may improve with time; however, areas of erythema/ atrophy may be long-lasting.
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635.
- Dellavalle RP, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Finlayson GR, Sams WM Jr, Smith JG Jr. Erythema ab igne: a histopathological study. J Invest Dermatol. 1966;46:104-108.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:E2635.
- Dellavalle RP, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Finlayson GR, Sams WM Jr, Smith JG Jr. Erythema ab igne: a histopathological study. J Invest Dermatol. 1966;46:104-108.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
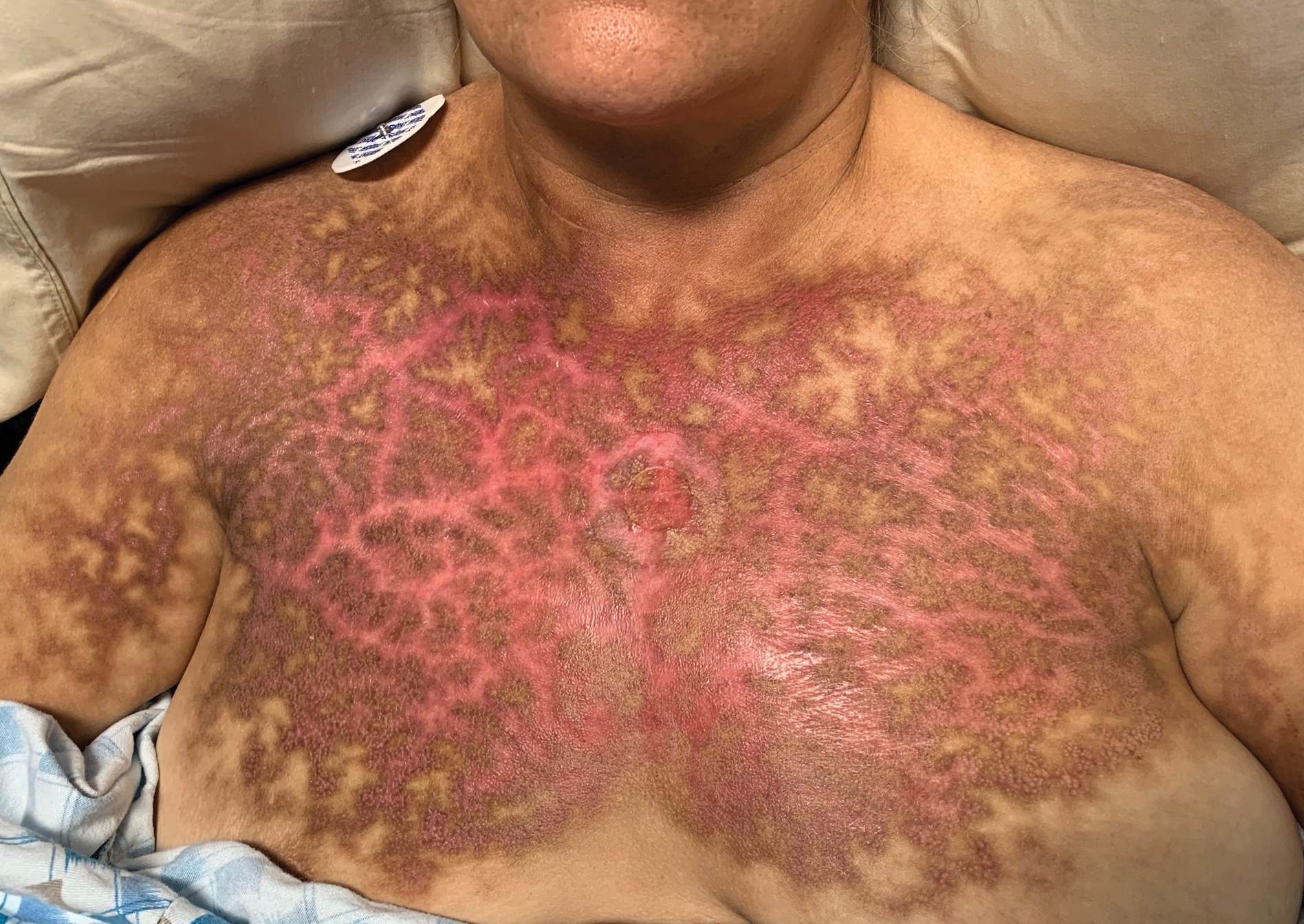
A 53-year-old woman with a history of diabetes mellitus, hypertension, chronic complex regional pain syndrome type 1, and chronic prescription opiate use presented to the hospital with a pruritic rash on the chest of 15 years’ duration that started a few weeks after a left shoulder repair. The patient was using fentanyl patches and acetaminophen with oxycodone as well as a heating pad for 20 to 22 hours per day for many years to help with her chronic pain. She also described similar lesions on the abdomen and back when she used the heating pad on those areas for weeks at a time. Vital signs were within normal limits. Physical examination revealed a lacy, reticular, eroded, well-demarcated rash on the chest along with areas of cracking. Laboratory evaluation did not reveal any abnormalities.
Task force affirms routine gestational diabetes testing
Asymptomatic pregnant women with no previous diagnosis of type 1 or 2 diabetes should be screened for gestational diabetes at 24 weeks’ gestation or later, according to an updated recommendation from the U.S. Preventive Services Task Force.
Pregnant individuals who develop gestational diabetes are at increased risk for complications including preeclampsia, fetal macrosomia, and neonatal hypoglycemia, as well as negative long-term outcomes for themselves and their children, wrote lead author Karina W. Davidson, PhD, of Feinstein Institute for Medical Research, Manhasset, N.Y., and colleagues. The statement was published online in JAMA.
The B recommendation and I statement reflect “moderate certainty” that current evidence supports the recommendation in terms of harms versus benefits, and is consistent with the 2014 USPSTF recommendation.
The statement calls for a one-time screening using a glucose tolerance test at or after 24 weeks’ gestation. Although most screening in the United States takes place prior to 28 weeks’ gestation, it can be performed later in patients who begin prenatal care after 28 weeks’ gestation, according to the statement. Data on the harms and benefits of gestational diabetes screening prior to 24 weeks’ gestation are limited, the authors noted. Gestational diabetes was defined as diabetes that develops during pregnancy that is not clearly overt diabetes.
To update the 2014 recommendation, the USPSTF commissioned a systematic review. In 45 prospective studies on the accuracy of gestational diabetes screening, several tests, included oral glucose challenge test, oral glucose tolerance test, and fasting plasma glucose using either a one- or two-step approach were accurate detectors of gestational diabetes; therefore, the USPSTF does not recommend a specific test.
In 13 trials on the impact of treating gestational diabetes on intermediate and health outcomes, treatment was associated with a reduced risk of outcomes, including primary cesarean delivery (but not total cesarean delivery) and preterm delivery, but not with a reduced risk of outcomes including preeclampsia, emergency cesarean delivery, induction of labor, or maternal birth trauma.
The task force also reviewed seven studies of harms associated with screening for gestational diabetes, including three on psychosocial harms, three on hospital experiences, and one of the odds of cesarean delivery after a diagnosis of gestational diabetes. No increase in anxiety or depression occurred following a positive diagnosis or false-positive test result, but data suggested that a gestational diabetes diagnosis may be associated with higher rates of cesarean delivery.
A total of 13 trials evaluated the harms associated with treatment of gestational diabetes, and found no association between treatment and increased risk of several outcomes including severe maternal hypoglycemia, low birth weight, and small for gestational age, and no effect was noted on the number of cesarean deliveries.
Evidence gaps that require additional research include randomized, controlled trials on the effects of gestational diabetes screening on health outcomes, as well as benefits versus harms of screening for pregnant individuals prior to 24 weeks, and studies on the effects of screening in subpopulations of race/ethnicity, age, and socioeconomic factors, according to the task force. Additional research also is needed in areas of maternal health outcomes, long-term outcomes, and the effect on outcomes of one-step versus two-step screening, the USPSTF said.
However, “screening for and detecting gestational diabetes provides a potential opportunity to control blood glucose levels (through lifestyle changes, pharmacological interventions, or both) and reduce the risk of macrosomia and LGA [large for gestational age] infants,” the task force wrote. “In turn, this can prevent associated complications such as primary cesarean delivery, shoulder dystocia, and [neonatal] ICU admissions.”
Support screening with counseling on risk reduction
The USPSTF recommendation is important at this time because “the prevalence of gestational diabetes is increasing secondary to rising rates of obesity,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
“In 2014, based on a systematic review of literature, the USPSTF recommended screening all asymptomatic pregnant women for gestational diabetes mellitus [GDM] starting at 24 weeks’ gestation. The recommended gestational age for screening coincides with increasing insulin resistance during pregnancy with advancing gestational age,” Dr. Krishna said.
“An updated systematic review by the USPSTF concluded that existing literature continues to affirm current recommendations of universal screening for GDM at 24 weeks gestation or later. There continues, however, to be no consensus on the optimal approach to screening,” she noted.
“Screening can be performed as a two-step or one-step approach,” said Dr. Krishna. “The two-step approach is commonly used in the United States, and all pregnant women are first screened with a 50-gram oral glucose solution followed by a diagnostic test if they have a positive initial screening.
“Women with risk factors for diabetes, such as prior GDM, obesity, strong family history of diabetes, or history of fetal macrosomia, should be screened early in pregnancy for GDM and have the GDM screen repeated at 24 weeks’ gestation or later if normal in early pregnancy,” Dr. Krishna said. “Pregnant women should be counseled on the importance of diet and exercise and appropriate weight gain in pregnancy to reduce the risk of GDM. Overall, timely diagnosis of gestational diabetes is crucial to improving maternal and fetal pregnancy outcomes.”
The full recommendation statement is also available on the USPSTF website. The research was supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Krishna had no disclosures, but serves on the editorial advisory board of Ob.Gyn News.
Asymptomatic pregnant women with no previous diagnosis of type 1 or 2 diabetes should be screened for gestational diabetes at 24 weeks’ gestation or later, according to an updated recommendation from the U.S. Preventive Services Task Force.
Pregnant individuals who develop gestational diabetes are at increased risk for complications including preeclampsia, fetal macrosomia, and neonatal hypoglycemia, as well as negative long-term outcomes for themselves and their children, wrote lead author Karina W. Davidson, PhD, of Feinstein Institute for Medical Research, Manhasset, N.Y., and colleagues. The statement was published online in JAMA.
The B recommendation and I statement reflect “moderate certainty” that current evidence supports the recommendation in terms of harms versus benefits, and is consistent with the 2014 USPSTF recommendation.
The statement calls for a one-time screening using a glucose tolerance test at or after 24 weeks’ gestation. Although most screening in the United States takes place prior to 28 weeks’ gestation, it can be performed later in patients who begin prenatal care after 28 weeks’ gestation, according to the statement. Data on the harms and benefits of gestational diabetes screening prior to 24 weeks’ gestation are limited, the authors noted. Gestational diabetes was defined as diabetes that develops during pregnancy that is not clearly overt diabetes.
To update the 2014 recommendation, the USPSTF commissioned a systematic review. In 45 prospective studies on the accuracy of gestational diabetes screening, several tests, included oral glucose challenge test, oral glucose tolerance test, and fasting plasma glucose using either a one- or two-step approach were accurate detectors of gestational diabetes; therefore, the USPSTF does not recommend a specific test.
In 13 trials on the impact of treating gestational diabetes on intermediate and health outcomes, treatment was associated with a reduced risk of outcomes, including primary cesarean delivery (but not total cesarean delivery) and preterm delivery, but not with a reduced risk of outcomes including preeclampsia, emergency cesarean delivery, induction of labor, or maternal birth trauma.
The task force also reviewed seven studies of harms associated with screening for gestational diabetes, including three on psychosocial harms, three on hospital experiences, and one of the odds of cesarean delivery after a diagnosis of gestational diabetes. No increase in anxiety or depression occurred following a positive diagnosis or false-positive test result, but data suggested that a gestational diabetes diagnosis may be associated with higher rates of cesarean delivery.
A total of 13 trials evaluated the harms associated with treatment of gestational diabetes, and found no association between treatment and increased risk of several outcomes including severe maternal hypoglycemia, low birth weight, and small for gestational age, and no effect was noted on the number of cesarean deliveries.
Evidence gaps that require additional research include randomized, controlled trials on the effects of gestational diabetes screening on health outcomes, as well as benefits versus harms of screening for pregnant individuals prior to 24 weeks, and studies on the effects of screening in subpopulations of race/ethnicity, age, and socioeconomic factors, according to the task force. Additional research also is needed in areas of maternal health outcomes, long-term outcomes, and the effect on outcomes of one-step versus two-step screening, the USPSTF said.
However, “screening for and detecting gestational diabetes provides a potential opportunity to control blood glucose levels (through lifestyle changes, pharmacological interventions, or both) and reduce the risk of macrosomia and LGA [large for gestational age] infants,” the task force wrote. “In turn, this can prevent associated complications such as primary cesarean delivery, shoulder dystocia, and [neonatal] ICU admissions.”
Support screening with counseling on risk reduction
The USPSTF recommendation is important at this time because “the prevalence of gestational diabetes is increasing secondary to rising rates of obesity,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
“In 2014, based on a systematic review of literature, the USPSTF recommended screening all asymptomatic pregnant women for gestational diabetes mellitus [GDM] starting at 24 weeks’ gestation. The recommended gestational age for screening coincides with increasing insulin resistance during pregnancy with advancing gestational age,” Dr. Krishna said.
“An updated systematic review by the USPSTF concluded that existing literature continues to affirm current recommendations of universal screening for GDM at 24 weeks gestation or later. There continues, however, to be no consensus on the optimal approach to screening,” she noted.
“Screening can be performed as a two-step or one-step approach,” said Dr. Krishna. “The two-step approach is commonly used in the United States, and all pregnant women are first screened with a 50-gram oral glucose solution followed by a diagnostic test if they have a positive initial screening.
“Women with risk factors for diabetes, such as prior GDM, obesity, strong family history of diabetes, or history of fetal macrosomia, should be screened early in pregnancy for GDM and have the GDM screen repeated at 24 weeks’ gestation or later if normal in early pregnancy,” Dr. Krishna said. “Pregnant women should be counseled on the importance of diet and exercise and appropriate weight gain in pregnancy to reduce the risk of GDM. Overall, timely diagnosis of gestational diabetes is crucial to improving maternal and fetal pregnancy outcomes.”
The full recommendation statement is also available on the USPSTF website. The research was supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Krishna had no disclosures, but serves on the editorial advisory board of Ob.Gyn News.
Asymptomatic pregnant women with no previous diagnosis of type 1 or 2 diabetes should be screened for gestational diabetes at 24 weeks’ gestation or later, according to an updated recommendation from the U.S. Preventive Services Task Force.
Pregnant individuals who develop gestational diabetes are at increased risk for complications including preeclampsia, fetal macrosomia, and neonatal hypoglycemia, as well as negative long-term outcomes for themselves and their children, wrote lead author Karina W. Davidson, PhD, of Feinstein Institute for Medical Research, Manhasset, N.Y., and colleagues. The statement was published online in JAMA.
The B recommendation and I statement reflect “moderate certainty” that current evidence supports the recommendation in terms of harms versus benefits, and is consistent with the 2014 USPSTF recommendation.
The statement calls for a one-time screening using a glucose tolerance test at or after 24 weeks’ gestation. Although most screening in the United States takes place prior to 28 weeks’ gestation, it can be performed later in patients who begin prenatal care after 28 weeks’ gestation, according to the statement. Data on the harms and benefits of gestational diabetes screening prior to 24 weeks’ gestation are limited, the authors noted. Gestational diabetes was defined as diabetes that develops during pregnancy that is not clearly overt diabetes.
To update the 2014 recommendation, the USPSTF commissioned a systematic review. In 45 prospective studies on the accuracy of gestational diabetes screening, several tests, included oral glucose challenge test, oral glucose tolerance test, and fasting plasma glucose using either a one- or two-step approach were accurate detectors of gestational diabetes; therefore, the USPSTF does not recommend a specific test.
In 13 trials on the impact of treating gestational diabetes on intermediate and health outcomes, treatment was associated with a reduced risk of outcomes, including primary cesarean delivery (but not total cesarean delivery) and preterm delivery, but not with a reduced risk of outcomes including preeclampsia, emergency cesarean delivery, induction of labor, or maternal birth trauma.
The task force also reviewed seven studies of harms associated with screening for gestational diabetes, including three on psychosocial harms, three on hospital experiences, and one of the odds of cesarean delivery after a diagnosis of gestational diabetes. No increase in anxiety or depression occurred following a positive diagnosis or false-positive test result, but data suggested that a gestational diabetes diagnosis may be associated with higher rates of cesarean delivery.
A total of 13 trials evaluated the harms associated with treatment of gestational diabetes, and found no association between treatment and increased risk of several outcomes including severe maternal hypoglycemia, low birth weight, and small for gestational age, and no effect was noted on the number of cesarean deliveries.
Evidence gaps that require additional research include randomized, controlled trials on the effects of gestational diabetes screening on health outcomes, as well as benefits versus harms of screening for pregnant individuals prior to 24 weeks, and studies on the effects of screening in subpopulations of race/ethnicity, age, and socioeconomic factors, according to the task force. Additional research also is needed in areas of maternal health outcomes, long-term outcomes, and the effect on outcomes of one-step versus two-step screening, the USPSTF said.
However, “screening for and detecting gestational diabetes provides a potential opportunity to control blood glucose levels (through lifestyle changes, pharmacological interventions, or both) and reduce the risk of macrosomia and LGA [large for gestational age] infants,” the task force wrote. “In turn, this can prevent associated complications such as primary cesarean delivery, shoulder dystocia, and [neonatal] ICU admissions.”
Support screening with counseling on risk reduction
The USPSTF recommendation is important at this time because “the prevalence of gestational diabetes is increasing secondary to rising rates of obesity,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview.
“In 2014, based on a systematic review of literature, the USPSTF recommended screening all asymptomatic pregnant women for gestational diabetes mellitus [GDM] starting at 24 weeks’ gestation. The recommended gestational age for screening coincides with increasing insulin resistance during pregnancy with advancing gestational age,” Dr. Krishna said.
“An updated systematic review by the USPSTF concluded that existing literature continues to affirm current recommendations of universal screening for GDM at 24 weeks gestation or later. There continues, however, to be no consensus on the optimal approach to screening,” she noted.
“Screening can be performed as a two-step or one-step approach,” said Dr. Krishna. “The two-step approach is commonly used in the United States, and all pregnant women are first screened with a 50-gram oral glucose solution followed by a diagnostic test if they have a positive initial screening.
“Women with risk factors for diabetes, such as prior GDM, obesity, strong family history of diabetes, or history of fetal macrosomia, should be screened early in pregnancy for GDM and have the GDM screen repeated at 24 weeks’ gestation or later if normal in early pregnancy,” Dr. Krishna said. “Pregnant women should be counseled on the importance of diet and exercise and appropriate weight gain in pregnancy to reduce the risk of GDM. Overall, timely diagnosis of gestational diabetes is crucial to improving maternal and fetal pregnancy outcomes.”
The full recommendation statement is also available on the USPSTF website. The research was supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose. Dr. Krishna had no disclosures, but serves on the editorial advisory board of Ob.Gyn News.
FROM JAMA
Heart doc offering ‘fountain of youth’ jailed for 6 1/2 years
Cardiologist Samirkumar J. Shah, MD, was sentenced to 78 months in prison after his conviction on two counts of federal health care fraud involving more than $13 million.
As part of his sentence, Dr. Shah, 58, of Fox Chapel, Pa., must pay $1.7 million in restitution and other penalties and undergo 3 years of supervised release after prison.
“Dr. Shah risked the health of his patients so he could make millions of dollars through unnecessary procedures, and lied and fabricated records for years to perpetuate his fraud scheme,” acting U.S. Attorney Stephen R. Kaufman said in an Aug. 5 statement from the Department of Justice.
As previously reported, Dr. Shah was convicted June 14, 2019, of submitting fraudulent claims to private and federal insurance programs between 2008 and 2013 for external counterpulsation (ECP) therapy, a lower limb compression treatment approved for patients with coronary artery disease and refractory angina.
Dr. Shah, however, advertised ECP as the “fountain of youth,” claimed it made patients “younger and smarter,” and offered the treatment for conditions such as obesity, hypertension, hypotension, diabetes, and erectile dysfunction.
Patients were required to undergo diagnostic ultrasounds as a precautionary measure prior to starting ECP, but witness testimony established that Dr. Shah did not review any of the imaging before approving new patients for ECP, placing his patients at risk for serious injury or even death, the DOJ stated.
The evidence also showed that Dr. Shah double-billed insurers, routinely submitted fabricated patient files, and made false statements concerning his practice, patient population, recording keeping, and compliance with coverage guidelines, the government said.
During the scheme, Dr. Shah submitted ECP-related claims for Medicare Part B, UPMC Health Plan, Highmark Blue Cross Blue Shield, and Gateway Health Plan beneficiaries totalling more than $13 million and received reimbursement payments in excess of $3.5 million.
“Rather than upholding the oath he swore and providing care for patients who trusted him, this defendant misled patients and drained critical Medicaid funds from families who needed it,” said Attorney General Josh Shapiro. “We will not let anyone put their patients’ lives at risk for a profit.”
“Today’s sentence holds Mr. Shah accountable for his appalling actions,” said FBI Pittsburgh Special Agent in Charge Mike Nordwall. “Mr. Shah used his position as a doctor to illegally profit from a health care program paid for by taxpayers. Fraud of this magnitude will not be tolerated.”
Dr. Shah has been in custody since July 15, 2021, after skipping out on his original July 14 sentencing date. The Tribune-Review reported that Dr. Shah filed a last-minute request for a continuance, claiming he had an adverse reaction to the Pfizer COVID-19 vaccination and was advised by his doctor that he needed “strict bedrest for at least 6 weeks.”
Dr. Shah reportedly turned himself after presiding U.S. District Judge David S. Cercone denied the motion and issued an arrest warrant.
A version of this article first appeared on Medscape.com.
Cardiologist Samirkumar J. Shah, MD, was sentenced to 78 months in prison after his conviction on two counts of federal health care fraud involving more than $13 million.
As part of his sentence, Dr. Shah, 58, of Fox Chapel, Pa., must pay $1.7 million in restitution and other penalties and undergo 3 years of supervised release after prison.
“Dr. Shah risked the health of his patients so he could make millions of dollars through unnecessary procedures, and lied and fabricated records for years to perpetuate his fraud scheme,” acting U.S. Attorney Stephen R. Kaufman said in an Aug. 5 statement from the Department of Justice.
As previously reported, Dr. Shah was convicted June 14, 2019, of submitting fraudulent claims to private and federal insurance programs between 2008 and 2013 for external counterpulsation (ECP) therapy, a lower limb compression treatment approved for patients with coronary artery disease and refractory angina.
Dr. Shah, however, advertised ECP as the “fountain of youth,” claimed it made patients “younger and smarter,” and offered the treatment for conditions such as obesity, hypertension, hypotension, diabetes, and erectile dysfunction.
Patients were required to undergo diagnostic ultrasounds as a precautionary measure prior to starting ECP, but witness testimony established that Dr. Shah did not review any of the imaging before approving new patients for ECP, placing his patients at risk for serious injury or even death, the DOJ stated.
The evidence also showed that Dr. Shah double-billed insurers, routinely submitted fabricated patient files, and made false statements concerning his practice, patient population, recording keeping, and compliance with coverage guidelines, the government said.
During the scheme, Dr. Shah submitted ECP-related claims for Medicare Part B, UPMC Health Plan, Highmark Blue Cross Blue Shield, and Gateway Health Plan beneficiaries totalling more than $13 million and received reimbursement payments in excess of $3.5 million.
“Rather than upholding the oath he swore and providing care for patients who trusted him, this defendant misled patients and drained critical Medicaid funds from families who needed it,” said Attorney General Josh Shapiro. “We will not let anyone put their patients’ lives at risk for a profit.”
“Today’s sentence holds Mr. Shah accountable for his appalling actions,” said FBI Pittsburgh Special Agent in Charge Mike Nordwall. “Mr. Shah used his position as a doctor to illegally profit from a health care program paid for by taxpayers. Fraud of this magnitude will not be tolerated.”
Dr. Shah has been in custody since July 15, 2021, after skipping out on his original July 14 sentencing date. The Tribune-Review reported that Dr. Shah filed a last-minute request for a continuance, claiming he had an adverse reaction to the Pfizer COVID-19 vaccination and was advised by his doctor that he needed “strict bedrest for at least 6 weeks.”
Dr. Shah reportedly turned himself after presiding U.S. District Judge David S. Cercone denied the motion and issued an arrest warrant.
A version of this article first appeared on Medscape.com.
Cardiologist Samirkumar J. Shah, MD, was sentenced to 78 months in prison after his conviction on two counts of federal health care fraud involving more than $13 million.
As part of his sentence, Dr. Shah, 58, of Fox Chapel, Pa., must pay $1.7 million in restitution and other penalties and undergo 3 years of supervised release after prison.
“Dr. Shah risked the health of his patients so he could make millions of dollars through unnecessary procedures, and lied and fabricated records for years to perpetuate his fraud scheme,” acting U.S. Attorney Stephen R. Kaufman said in an Aug. 5 statement from the Department of Justice.
As previously reported, Dr. Shah was convicted June 14, 2019, of submitting fraudulent claims to private and federal insurance programs between 2008 and 2013 for external counterpulsation (ECP) therapy, a lower limb compression treatment approved for patients with coronary artery disease and refractory angina.
Dr. Shah, however, advertised ECP as the “fountain of youth,” claimed it made patients “younger and smarter,” and offered the treatment for conditions such as obesity, hypertension, hypotension, diabetes, and erectile dysfunction.
Patients were required to undergo diagnostic ultrasounds as a precautionary measure prior to starting ECP, but witness testimony established that Dr. Shah did not review any of the imaging before approving new patients for ECP, placing his patients at risk for serious injury or even death, the DOJ stated.
The evidence also showed that Dr. Shah double-billed insurers, routinely submitted fabricated patient files, and made false statements concerning his practice, patient population, recording keeping, and compliance with coverage guidelines, the government said.
During the scheme, Dr. Shah submitted ECP-related claims for Medicare Part B, UPMC Health Plan, Highmark Blue Cross Blue Shield, and Gateway Health Plan beneficiaries totalling more than $13 million and received reimbursement payments in excess of $3.5 million.
“Rather than upholding the oath he swore and providing care for patients who trusted him, this defendant misled patients and drained critical Medicaid funds from families who needed it,” said Attorney General Josh Shapiro. “We will not let anyone put their patients’ lives at risk for a profit.”
“Today’s sentence holds Mr. Shah accountable for his appalling actions,” said FBI Pittsburgh Special Agent in Charge Mike Nordwall. “Mr. Shah used his position as a doctor to illegally profit from a health care program paid for by taxpayers. Fraud of this magnitude will not be tolerated.”
Dr. Shah has been in custody since July 15, 2021, after skipping out on his original July 14 sentencing date. The Tribune-Review reported that Dr. Shah filed a last-minute request for a continuance, claiming he had an adverse reaction to the Pfizer COVID-19 vaccination and was advised by his doctor that he needed “strict bedrest for at least 6 weeks.”
Dr. Shah reportedly turned himself after presiding U.S. District Judge David S. Cercone denied the motion and issued an arrest warrant.
A version of this article first appeared on Medscape.com.
Tackle obesity to drop risk for secondary cardiac event
Patients who had been hospitalized for heart attack or cardiovascular revascularization procedures commonly were overweight (46%) or had obesity (35%), but at a follow-up visit, few had lost weight or planned to do so, according to researchers who conduced a large European study.
The findings emphasize that obesity needs to be recognized as a disease that has to be optimally managed to lessen the risk for a secondary cardiovascular event, the authors stressed.
The study, by Dirk De Bacquer, PhD, professor, department of public health, Ghent (Belgium) University, and colleagues, was published recently in the European Heart Journal – Quality of Care and Clinical Outcomes.
The researchers analyzed data from more than 10,000 patients in the EUROASPIRE IV and V studies who were hospitalized for acute myocardial infarction (MI), coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI) and answered a survey 16 months later on average.
Although 20% of the patients with obesity had lost 5% or more of their initial weight, 16% had gained 5% or more of their initial weight.
Notably, “the discharge letter did not record the weight status in a quarter of [the patients with obesity] and a substantial proportion reported to have never been told by a healthcare professional [that they were] overweight,” the investigators wrote.
“It seems,” Dr. De Bacquer and colleagues noted, “that obesity is not considered by physicians as a serious medical problem, which requires attention, recommendations, and obvious advice on personal weight targets.”
However, “the benefits for patients who lost weight in our study, resulting in a healthier cardiovascular risk profile, are really worthwhile,” they pointed out.
Cardiovascular rehabilitation should include weight loss intervention
“The safest and most effective approach for managing body weight” in patients with coronary artery disease and obesity “is adopting a healthy eating pattern and increasing levels of physical activity,” they wrote.
Their findings that “patients who reported reducing their fat and sugar intake, consuming more fruit, vegetables, and fish and doing more regular physical activity, had significant weight loss,” support this.
Dr. De Bacquer and colleagues recommend that cardiovascular prevention and rehabilitation programs “should include weight loss intervention, including different forms of self-support, as a specific component of a comprehensive intervention to reduce total cardiovascular risk, extend life expectancy, and improve quality of life.”
Clinicians should “consider the incremental value of telehealth intervention as well as recently described pharmacological interventions,” they added, noting that the study did not look at these options or at metabolic surgery.
Invited to comment, one expert pointed out that two new observational studies of metabolic surgery in patients with obesity and coronary artery disease reported positive outcomes.
Another expert took issue with the “patient blaming” tone of the article and the lack of actionable ways to help patients lose weight.
Medical therapy or bariatric surgery as other options?
“The study demonstrated how prevalent obesity is in patients with heart disease“ and “confirmed how difficult it is to achieve weight loss, in particular, in patients with heart disease, where weight loss would be beneficial,” Erik Näslund, MD, PhD, said in an interview.
Even though “current guidelines stress weight-loss counseling, some patients actually gained weight,” observed Dr. Näslund, of Danderyd Hospital and Karolinska Institutet, Stockholm.
On the other hand, patients who lost 5% or more of their initial weight had reduced comorbidities that are associated with cardiovascular disease.
“The best way to achieve long-term weight loss in patients with severe obesity is metabolic (bariatric) surgery,” noted Dr. Näslund, who was not involved in the study. “There are now two recent papers in the journal Circulation that demonstrate that metabolic surgery has a role in the secondary prevention of cardiovascular disease in patients with severe obesity” – one study from Dr. Näslund’s group (Circulation. 2021;143:1458-67), as previously reported, and one study from researchers in Ontario, Canada (Circulation. 2021;143:1468-80).
However, those were observational studies, and the findings would need to be confirmed in a randomized clinical trial before they could be used as recommended practice of care, he cautioned. In addition, most patients in the current study would not fulfill the minimum body weight criteria for metabolic surgery.
“Therefore, there is a need for intensified medical therapy for these patients,” as another treatment option, said Dr. Näslund.
“It would be interesting,” he speculated, “to study how the new glucagon-like peptide-1 (GLP-1) receptor agonist therapies could work in this setting as a weight loss agent and perhaps have a positive independent cardiovascular benefit.”
Obesity is a disease; clinicians need to be respectful
Meanwhile, Obesity Society fellow and spokesperson Fatima Cody Stanford, MD, said in an interview that she didn’t think the language and tone of the article was respectful for patients with obesity, and the researchers “talked about the old narrative of how we support patients with obesity.”
Lifestyle modification can be at the core of treatment, but medication or bariatric surgery may be other options to “help patients get to their best selves.
“Patients with obesity deserve to be cared for and treated with respect,” said Dr. Stanford, an obesity medicine physician scientist at Massachusetts General Hospital and Harvard Medical School, Boston.
Treatment needs to be individualized and clinicians need to listen to patient concerns. For example, a patient with obesity may not be able to follow advice to walk more. “I can barely stand up,” one patient with obesity and osteoarthritis told Dr. Stanford.
And patients’ insurance may not cover cardiac rehabilitation – especially patients from racial minorities or those with lower socioeconomic status, she noted.
“My feeling has always been that it is important to be respectful to all patients,” Dr. Näslund agreed. “I do agree that we need to recognize obesity as a chronic disease, and the paper in EHJ demonstrates this, as obesity was not registered in many of the discharge notes.
“If we as healthcare workers measured a weight of our patients the same way that we take a blood pressure,” he said, “perhaps the [stigma] of obesity would be reduced.”
Study findings
The researchers examined pooled data from EUROASPIRE IV (2012-13) and EUROASPIRE V (2016-17) surveys of patients who were overweight or had obesity who had been discharged from hospital after MI, CABG, or PCI to determine if they had received lifestyle advice for weight loss, if they had acted on this advice, and if losing weight altered their cardiovascular disease risk factors.
They identified 10,507 adult patients in 29 mainly European countries who had complete survey data.
The mean age of the patients was 63 at the time of their hospitalization; 25% were women. Many had hypertension (66%-88%), dyslipidemia (69%-80%), or diabetes (16%-37%).
The prevalence of obesity varied from 8% to 46% in men and from 18% to 57% in women, in different countries. Patients with obesity had a mean body weight of 97 kg (213 pounds).
One of the most “striking” findings was the “apparent lack of motivation” to lose weight, Dr. De Bacquer and colleagues wrote. Half of the patients with obesity had not attempted to lose weight in the month before the follow-up visit and most did not plan to do so in the following month.
Goal setting is an important aspect of behavior modification techniques, they wrote, yet 7% of the patients did not know their body weight and 21% did not have an optimal weight target.
Half of the patients had been advised to follow a cardiac rehabilitation program and two-thirds had been advised to follow dietary recommendations and move more.
Those who made positive dietary changes and were more physically active were more likely to lose at least 5% of their weight.
And patients who lost at least 5% of their initial weight were less likely to have hypertension, dyslipidemia, or diabetes compared with patients who had gained this much weight, which “is likely to translate into improved prognosis on the long term,” the authors wrote.
EUROASPIRE IV and V were supported through research grants to the European Society of Cardiology from Amgen, AstraZeneca, Bristol-Myers Squibb/Emea Sarl, GlaxoSmithKline, Hoffmann-La Roche, and Merck, Sharp & Dohme (EUROASPIRE IV) and Amarin, Amgen, Daiichi Sankyo, Eli Lilly, Pfizer, Sanofi, Ferrer, and Novo Nordisk (EUROASPIRE V). Dr. De Bacquer, Dr. Näslund, and Dr. Stanford have no disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who had been hospitalized for heart attack or cardiovascular revascularization procedures commonly were overweight (46%) or had obesity (35%), but at a follow-up visit, few had lost weight or planned to do so, according to researchers who conduced a large European study.
The findings emphasize that obesity needs to be recognized as a disease that has to be optimally managed to lessen the risk for a secondary cardiovascular event, the authors stressed.
The study, by Dirk De Bacquer, PhD, professor, department of public health, Ghent (Belgium) University, and colleagues, was published recently in the European Heart Journal – Quality of Care and Clinical Outcomes.
The researchers analyzed data from more than 10,000 patients in the EUROASPIRE IV and V studies who were hospitalized for acute myocardial infarction (MI), coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI) and answered a survey 16 months later on average.
Although 20% of the patients with obesity had lost 5% or more of their initial weight, 16% had gained 5% or more of their initial weight.
Notably, “the discharge letter did not record the weight status in a quarter of [the patients with obesity] and a substantial proportion reported to have never been told by a healthcare professional [that they were] overweight,” the investigators wrote.
“It seems,” Dr. De Bacquer and colleagues noted, “that obesity is not considered by physicians as a serious medical problem, which requires attention, recommendations, and obvious advice on personal weight targets.”
However, “the benefits for patients who lost weight in our study, resulting in a healthier cardiovascular risk profile, are really worthwhile,” they pointed out.
Cardiovascular rehabilitation should include weight loss intervention
“The safest and most effective approach for managing body weight” in patients with coronary artery disease and obesity “is adopting a healthy eating pattern and increasing levels of physical activity,” they wrote.
Their findings that “patients who reported reducing their fat and sugar intake, consuming more fruit, vegetables, and fish and doing more regular physical activity, had significant weight loss,” support this.
Dr. De Bacquer and colleagues recommend that cardiovascular prevention and rehabilitation programs “should include weight loss intervention, including different forms of self-support, as a specific component of a comprehensive intervention to reduce total cardiovascular risk, extend life expectancy, and improve quality of life.”
Clinicians should “consider the incremental value of telehealth intervention as well as recently described pharmacological interventions,” they added, noting that the study did not look at these options or at metabolic surgery.
Invited to comment, one expert pointed out that two new observational studies of metabolic surgery in patients with obesity and coronary artery disease reported positive outcomes.
Another expert took issue with the “patient blaming” tone of the article and the lack of actionable ways to help patients lose weight.
Medical therapy or bariatric surgery as other options?
“The study demonstrated how prevalent obesity is in patients with heart disease“ and “confirmed how difficult it is to achieve weight loss, in particular, in patients with heart disease, where weight loss would be beneficial,” Erik Näslund, MD, PhD, said in an interview.
Even though “current guidelines stress weight-loss counseling, some patients actually gained weight,” observed Dr. Näslund, of Danderyd Hospital and Karolinska Institutet, Stockholm.
On the other hand, patients who lost 5% or more of their initial weight had reduced comorbidities that are associated with cardiovascular disease.
“The best way to achieve long-term weight loss in patients with severe obesity is metabolic (bariatric) surgery,” noted Dr. Näslund, who was not involved in the study. “There are now two recent papers in the journal Circulation that demonstrate that metabolic surgery has a role in the secondary prevention of cardiovascular disease in patients with severe obesity” – one study from Dr. Näslund’s group (Circulation. 2021;143:1458-67), as previously reported, and one study from researchers in Ontario, Canada (Circulation. 2021;143:1468-80).
However, those were observational studies, and the findings would need to be confirmed in a randomized clinical trial before they could be used as recommended practice of care, he cautioned. In addition, most patients in the current study would not fulfill the minimum body weight criteria for metabolic surgery.
“Therefore, there is a need for intensified medical therapy for these patients,” as another treatment option, said Dr. Näslund.
“It would be interesting,” he speculated, “to study how the new glucagon-like peptide-1 (GLP-1) receptor agonist therapies could work in this setting as a weight loss agent and perhaps have a positive independent cardiovascular benefit.”
Obesity is a disease; clinicians need to be respectful
Meanwhile, Obesity Society fellow and spokesperson Fatima Cody Stanford, MD, said in an interview that she didn’t think the language and tone of the article was respectful for patients with obesity, and the researchers “talked about the old narrative of how we support patients with obesity.”
Lifestyle modification can be at the core of treatment, but medication or bariatric surgery may be other options to “help patients get to their best selves.
“Patients with obesity deserve to be cared for and treated with respect,” said Dr. Stanford, an obesity medicine physician scientist at Massachusetts General Hospital and Harvard Medical School, Boston.
Treatment needs to be individualized and clinicians need to listen to patient concerns. For example, a patient with obesity may not be able to follow advice to walk more. “I can barely stand up,” one patient with obesity and osteoarthritis told Dr. Stanford.
And patients’ insurance may not cover cardiac rehabilitation – especially patients from racial minorities or those with lower socioeconomic status, she noted.
“My feeling has always been that it is important to be respectful to all patients,” Dr. Näslund agreed. “I do agree that we need to recognize obesity as a chronic disease, and the paper in EHJ demonstrates this, as obesity was not registered in many of the discharge notes.
“If we as healthcare workers measured a weight of our patients the same way that we take a blood pressure,” he said, “perhaps the [stigma] of obesity would be reduced.”
Study findings
The researchers examined pooled data from EUROASPIRE IV (2012-13) and EUROASPIRE V (2016-17) surveys of patients who were overweight or had obesity who had been discharged from hospital after MI, CABG, or PCI to determine if they had received lifestyle advice for weight loss, if they had acted on this advice, and if losing weight altered their cardiovascular disease risk factors.
They identified 10,507 adult patients in 29 mainly European countries who had complete survey data.
The mean age of the patients was 63 at the time of their hospitalization; 25% were women. Many had hypertension (66%-88%), dyslipidemia (69%-80%), or diabetes (16%-37%).
The prevalence of obesity varied from 8% to 46% in men and from 18% to 57% in women, in different countries. Patients with obesity had a mean body weight of 97 kg (213 pounds).
One of the most “striking” findings was the “apparent lack of motivation” to lose weight, Dr. De Bacquer and colleagues wrote. Half of the patients with obesity had not attempted to lose weight in the month before the follow-up visit and most did not plan to do so in the following month.
Goal setting is an important aspect of behavior modification techniques, they wrote, yet 7% of the patients did not know their body weight and 21% did not have an optimal weight target.
Half of the patients had been advised to follow a cardiac rehabilitation program and two-thirds had been advised to follow dietary recommendations and move more.
Those who made positive dietary changes and were more physically active were more likely to lose at least 5% of their weight.
And patients who lost at least 5% of their initial weight were less likely to have hypertension, dyslipidemia, or diabetes compared with patients who had gained this much weight, which “is likely to translate into improved prognosis on the long term,” the authors wrote.
EUROASPIRE IV and V were supported through research grants to the European Society of Cardiology from Amgen, AstraZeneca, Bristol-Myers Squibb/Emea Sarl, GlaxoSmithKline, Hoffmann-La Roche, and Merck, Sharp & Dohme (EUROASPIRE IV) and Amarin, Amgen, Daiichi Sankyo, Eli Lilly, Pfizer, Sanofi, Ferrer, and Novo Nordisk (EUROASPIRE V). Dr. De Bacquer, Dr. Näslund, and Dr. Stanford have no disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who had been hospitalized for heart attack or cardiovascular revascularization procedures commonly were overweight (46%) or had obesity (35%), but at a follow-up visit, few had lost weight or planned to do so, according to researchers who conduced a large European study.
The findings emphasize that obesity needs to be recognized as a disease that has to be optimally managed to lessen the risk for a secondary cardiovascular event, the authors stressed.
The study, by Dirk De Bacquer, PhD, professor, department of public health, Ghent (Belgium) University, and colleagues, was published recently in the European Heart Journal – Quality of Care and Clinical Outcomes.
The researchers analyzed data from more than 10,000 patients in the EUROASPIRE IV and V studies who were hospitalized for acute myocardial infarction (MI), coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI) and answered a survey 16 months later on average.
Although 20% of the patients with obesity had lost 5% or more of their initial weight, 16% had gained 5% or more of their initial weight.
Notably, “the discharge letter did not record the weight status in a quarter of [the patients with obesity] and a substantial proportion reported to have never been told by a healthcare professional [that they were] overweight,” the investigators wrote.
“It seems,” Dr. De Bacquer and colleagues noted, “that obesity is not considered by physicians as a serious medical problem, which requires attention, recommendations, and obvious advice on personal weight targets.”
However, “the benefits for patients who lost weight in our study, resulting in a healthier cardiovascular risk profile, are really worthwhile,” they pointed out.
Cardiovascular rehabilitation should include weight loss intervention
“The safest and most effective approach for managing body weight” in patients with coronary artery disease and obesity “is adopting a healthy eating pattern and increasing levels of physical activity,” they wrote.
Their findings that “patients who reported reducing their fat and sugar intake, consuming more fruit, vegetables, and fish and doing more regular physical activity, had significant weight loss,” support this.
Dr. De Bacquer and colleagues recommend that cardiovascular prevention and rehabilitation programs “should include weight loss intervention, including different forms of self-support, as a specific component of a comprehensive intervention to reduce total cardiovascular risk, extend life expectancy, and improve quality of life.”
Clinicians should “consider the incremental value of telehealth intervention as well as recently described pharmacological interventions,” they added, noting that the study did not look at these options or at metabolic surgery.
Invited to comment, one expert pointed out that two new observational studies of metabolic surgery in patients with obesity and coronary artery disease reported positive outcomes.
Another expert took issue with the “patient blaming” tone of the article and the lack of actionable ways to help patients lose weight.
Medical therapy or bariatric surgery as other options?
“The study demonstrated how prevalent obesity is in patients with heart disease“ and “confirmed how difficult it is to achieve weight loss, in particular, in patients with heart disease, where weight loss would be beneficial,” Erik Näslund, MD, PhD, said in an interview.
Even though “current guidelines stress weight-loss counseling, some patients actually gained weight,” observed Dr. Näslund, of Danderyd Hospital and Karolinska Institutet, Stockholm.
On the other hand, patients who lost 5% or more of their initial weight had reduced comorbidities that are associated with cardiovascular disease.
“The best way to achieve long-term weight loss in patients with severe obesity is metabolic (bariatric) surgery,” noted Dr. Näslund, who was not involved in the study. “There are now two recent papers in the journal Circulation that demonstrate that metabolic surgery has a role in the secondary prevention of cardiovascular disease in patients with severe obesity” – one study from Dr. Näslund’s group (Circulation. 2021;143:1458-67), as previously reported, and one study from researchers in Ontario, Canada (Circulation. 2021;143:1468-80).
However, those were observational studies, and the findings would need to be confirmed in a randomized clinical trial before they could be used as recommended practice of care, he cautioned. In addition, most patients in the current study would not fulfill the minimum body weight criteria for metabolic surgery.
“Therefore, there is a need for intensified medical therapy for these patients,” as another treatment option, said Dr. Näslund.
“It would be interesting,” he speculated, “to study how the new glucagon-like peptide-1 (GLP-1) receptor agonist therapies could work in this setting as a weight loss agent and perhaps have a positive independent cardiovascular benefit.”
Obesity is a disease; clinicians need to be respectful
Meanwhile, Obesity Society fellow and spokesperson Fatima Cody Stanford, MD, said in an interview that she didn’t think the language and tone of the article was respectful for patients with obesity, and the researchers “talked about the old narrative of how we support patients with obesity.”
Lifestyle modification can be at the core of treatment, but medication or bariatric surgery may be other options to “help patients get to their best selves.
“Patients with obesity deserve to be cared for and treated with respect,” said Dr. Stanford, an obesity medicine physician scientist at Massachusetts General Hospital and Harvard Medical School, Boston.
Treatment needs to be individualized and clinicians need to listen to patient concerns. For example, a patient with obesity may not be able to follow advice to walk more. “I can barely stand up,” one patient with obesity and osteoarthritis told Dr. Stanford.
And patients’ insurance may not cover cardiac rehabilitation – especially patients from racial minorities or those with lower socioeconomic status, she noted.
“My feeling has always been that it is important to be respectful to all patients,” Dr. Näslund agreed. “I do agree that we need to recognize obesity as a chronic disease, and the paper in EHJ demonstrates this, as obesity was not registered in many of the discharge notes.
“If we as healthcare workers measured a weight of our patients the same way that we take a blood pressure,” he said, “perhaps the [stigma] of obesity would be reduced.”
Study findings
The researchers examined pooled data from EUROASPIRE IV (2012-13) and EUROASPIRE V (2016-17) surveys of patients who were overweight or had obesity who had been discharged from hospital after MI, CABG, or PCI to determine if they had received lifestyle advice for weight loss, if they had acted on this advice, and if losing weight altered their cardiovascular disease risk factors.
They identified 10,507 adult patients in 29 mainly European countries who had complete survey data.
The mean age of the patients was 63 at the time of their hospitalization; 25% were women. Many had hypertension (66%-88%), dyslipidemia (69%-80%), or diabetes (16%-37%).
The prevalence of obesity varied from 8% to 46% in men and from 18% to 57% in women, in different countries. Patients with obesity had a mean body weight of 97 kg (213 pounds).
One of the most “striking” findings was the “apparent lack of motivation” to lose weight, Dr. De Bacquer and colleagues wrote. Half of the patients with obesity had not attempted to lose weight in the month before the follow-up visit and most did not plan to do so in the following month.
Goal setting is an important aspect of behavior modification techniques, they wrote, yet 7% of the patients did not know their body weight and 21% did not have an optimal weight target.
Half of the patients had been advised to follow a cardiac rehabilitation program and two-thirds had been advised to follow dietary recommendations and move more.
Those who made positive dietary changes and were more physically active were more likely to lose at least 5% of their weight.
And patients who lost at least 5% of their initial weight were less likely to have hypertension, dyslipidemia, or diabetes compared with patients who had gained this much weight, which “is likely to translate into improved prognosis on the long term,” the authors wrote.
EUROASPIRE IV and V were supported through research grants to the European Society of Cardiology from Amgen, AstraZeneca, Bristol-Myers Squibb/Emea Sarl, GlaxoSmithKline, Hoffmann-La Roche, and Merck, Sharp & Dohme (EUROASPIRE IV) and Amarin, Amgen, Daiichi Sankyo, Eli Lilly, Pfizer, Sanofi, Ferrer, and Novo Nordisk (EUROASPIRE V). Dr. De Bacquer, Dr. Näslund, and Dr. Stanford have no disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Plant-based lignan intake linked to lower CHD risk
Consumption of a plant-based diet rich in lignans is associated with a lower risk of coronary heart disease (CHD), new research suggests.
In a prospective cohort study that followed almost 214,108 men and women who were free of CHD and cancer at baseline, increased long-term intake of lignans, polyphenolic substances produced by plants, was associated with significantly lower risk of total CHD in both men and women.
The benefit was increased in participants with a greater intake of fiber, suggesting that synergistic effects between the two might exist in relation to CHD reduction.
The results were published online in the Journal of the American College of Cardiology.
“Lignan is an estrogen-like molecule, so it exerts some estrogenic effects which are cardioprotective. It also has anti-inflammatory properties,” first author Yang Hu, ScD, a research fellow at the Harvard School of Public Health, Boston, said in an interview.
“Our results that showed an inverse association between lignan consumption and heart disease risk were expected, because it is known that lignans, which are predominantly from plant-based foods, like whole grains, fruit, vegetables, red wine, and coffee, are all associated with lower CHD risk,” Dr. Hu said.
What is novel about the current study is that it established a threshold for lignan consumption, above which there is no CHD benefit, he said.
“It is not a matter of the more you consume, the lower your risk. There is a certain amount of lignan you have to reach, after which there is no more benefit,” Dr. Hu said.
Dr. Hu and associates prospectively followed 214,108 men and women in three cohorts who did not have cardiovascular disease or cancer at baseline. The cohorts were the Health Professionals Follow-Up Study, Nurses’ Health Study, and Nurses’ Health Study II.
Diets were assessed using the Food Frequency Questionnaire every 2-4 years at follow-up visits.
During 5.5 million person-years of follow-up, Dr. Hu and associates documented 10,244 CHD cases, including 6,283 nonfatal myocardial infarctions and 3,961 fatal CHD cases.
The results showed that higher total lignan intake, and all individual lignan intake as well, were associated with significantly lower risk of total CHD.
Participants with higher total lignan intake were older and had more favorable health and lifestyle profiles including lower body mass index, lower prevalence of hypertension and hypercholesterolemia, high levels of physical activity, and better diet quality.
Overall, the pooled hazard ratios of CHD were 0.85 (95% confidence interval, 0.79-0.92) for total lignans, 0.76 (95% CI, 0.71-0.82) for matairesinol, 0.87 (95% CI, 0.81-0.93) for secoisolariciresinol, 0.89 (95% CI, 0.83-0.95) for pinoresinol, and 0.89 (95% CI: 0.83- 0.95) for lariciresinol (all P values for trend ≤ .003).
In addition, nonlinear relationships were found for total lignan, matairesinol, and secoisolariciresinol: The risk reduction plateaued at intakes above approximately 300 mcg/d for total lignan; 10 mcg/d for matairesinol, and 100 mcg/d for secoisolariciresinol.
The inverse associations for total lignan intake were more apparent among participants with higher total fiber intake.
In addition, lignan intake was more strongly associated with plasma concentrations of enterolactone when fiber intake was higher.
Dr. Hu said a next avenue of research will explore the synergistic association between lignans and fiber in further lowering CHD risk.
Lignans are exclusively metabolized by gut microbiota, Dr. Hu noted. “This opens another avenue of research because we can take further steps to see how the gut microbiota compositions and fiber interact with the production of lignans and how these might affect disease risk for other conditions, such as diabetes.”
An important study
“The evidence is building that there is an association between polyphenol intake and chronic disease, especially for CVD [cardiovascular disease],” David J.A. Jenkins, MD, PhD, and colleagues wrote in an accompanying editorial.
“Plant polyphenols may be important components of healthy plant-based diets that contribute to freedom from chronic diseases such as CVD, diabetes, and possibly cancer and so are associated with a reduction in all-cause mortality,” they wrote.
“I think this is an important study even though the results are not unexpected,” Dr. Jenkins, professor in the departments of medicine and nutritional sciences at the University of Toronto, said in an interview.
“We do know that plant polyphenols are important sources of antioxidants and may have many protective roles in preventing destruction of proteins and DNA destruction, so the results here reinforce very strongly the concept of plant foods and their importance in the diet,” he said.
The data reaffirmed the value of eating a variety of plant foods and eating them in a less processed form, because they have higher amounts of their phenolic compounds, Dr. Jenkins said.
“Things like wheat, oats, barley, [and] whole grain foods will have more phenolic components with them, as do fruits and vegetables,” he said.
Dr. Hu and Dr. Jenkins disclosed no relevant financial relationships.
Aversion of this article first appeared on Medscape.com.
Consumption of a plant-based diet rich in lignans is associated with a lower risk of coronary heart disease (CHD), new research suggests.
In a prospective cohort study that followed almost 214,108 men and women who were free of CHD and cancer at baseline, increased long-term intake of lignans, polyphenolic substances produced by plants, was associated with significantly lower risk of total CHD in both men and women.
The benefit was increased in participants with a greater intake of fiber, suggesting that synergistic effects between the two might exist in relation to CHD reduction.
The results were published online in the Journal of the American College of Cardiology.
“Lignan is an estrogen-like molecule, so it exerts some estrogenic effects which are cardioprotective. It also has anti-inflammatory properties,” first author Yang Hu, ScD, a research fellow at the Harvard School of Public Health, Boston, said in an interview.
“Our results that showed an inverse association between lignan consumption and heart disease risk were expected, because it is known that lignans, which are predominantly from plant-based foods, like whole grains, fruit, vegetables, red wine, and coffee, are all associated with lower CHD risk,” Dr. Hu said.
What is novel about the current study is that it established a threshold for lignan consumption, above which there is no CHD benefit, he said.
“It is not a matter of the more you consume, the lower your risk. There is a certain amount of lignan you have to reach, after which there is no more benefit,” Dr. Hu said.
Dr. Hu and associates prospectively followed 214,108 men and women in three cohorts who did not have cardiovascular disease or cancer at baseline. The cohorts were the Health Professionals Follow-Up Study, Nurses’ Health Study, and Nurses’ Health Study II.
Diets were assessed using the Food Frequency Questionnaire every 2-4 years at follow-up visits.
During 5.5 million person-years of follow-up, Dr. Hu and associates documented 10,244 CHD cases, including 6,283 nonfatal myocardial infarctions and 3,961 fatal CHD cases.
The results showed that higher total lignan intake, and all individual lignan intake as well, were associated with significantly lower risk of total CHD.
Participants with higher total lignan intake were older and had more favorable health and lifestyle profiles including lower body mass index, lower prevalence of hypertension and hypercholesterolemia, high levels of physical activity, and better diet quality.
Overall, the pooled hazard ratios of CHD were 0.85 (95% confidence interval, 0.79-0.92) for total lignans, 0.76 (95% CI, 0.71-0.82) for matairesinol, 0.87 (95% CI, 0.81-0.93) for secoisolariciresinol, 0.89 (95% CI, 0.83-0.95) for pinoresinol, and 0.89 (95% CI: 0.83- 0.95) for lariciresinol (all P values for trend ≤ .003).
In addition, nonlinear relationships were found for total lignan, matairesinol, and secoisolariciresinol: The risk reduction plateaued at intakes above approximately 300 mcg/d for total lignan; 10 mcg/d for matairesinol, and 100 mcg/d for secoisolariciresinol.
The inverse associations for total lignan intake were more apparent among participants with higher total fiber intake.
In addition, lignan intake was more strongly associated with plasma concentrations of enterolactone when fiber intake was higher.
Dr. Hu said a next avenue of research will explore the synergistic association between lignans and fiber in further lowering CHD risk.
Lignans are exclusively metabolized by gut microbiota, Dr. Hu noted. “This opens another avenue of research because we can take further steps to see how the gut microbiota compositions and fiber interact with the production of lignans and how these might affect disease risk for other conditions, such as diabetes.”
An important study
“The evidence is building that there is an association between polyphenol intake and chronic disease, especially for CVD [cardiovascular disease],” David J.A. Jenkins, MD, PhD, and colleagues wrote in an accompanying editorial.
“Plant polyphenols may be important components of healthy plant-based diets that contribute to freedom from chronic diseases such as CVD, diabetes, and possibly cancer and so are associated with a reduction in all-cause mortality,” they wrote.
“I think this is an important study even though the results are not unexpected,” Dr. Jenkins, professor in the departments of medicine and nutritional sciences at the University of Toronto, said in an interview.
“We do know that plant polyphenols are important sources of antioxidants and may have many protective roles in preventing destruction of proteins and DNA destruction, so the results here reinforce very strongly the concept of plant foods and their importance in the diet,” he said.
The data reaffirmed the value of eating a variety of plant foods and eating them in a less processed form, because they have higher amounts of their phenolic compounds, Dr. Jenkins said.
“Things like wheat, oats, barley, [and] whole grain foods will have more phenolic components with them, as do fruits and vegetables,” he said.
Dr. Hu and Dr. Jenkins disclosed no relevant financial relationships.
Aversion of this article first appeared on Medscape.com.
Consumption of a plant-based diet rich in lignans is associated with a lower risk of coronary heart disease (CHD), new research suggests.
In a prospective cohort study that followed almost 214,108 men and women who were free of CHD and cancer at baseline, increased long-term intake of lignans, polyphenolic substances produced by plants, was associated with significantly lower risk of total CHD in both men and women.
The benefit was increased in participants with a greater intake of fiber, suggesting that synergistic effects between the two might exist in relation to CHD reduction.
The results were published online in the Journal of the American College of Cardiology.
“Lignan is an estrogen-like molecule, so it exerts some estrogenic effects which are cardioprotective. It also has anti-inflammatory properties,” first author Yang Hu, ScD, a research fellow at the Harvard School of Public Health, Boston, said in an interview.
“Our results that showed an inverse association between lignan consumption and heart disease risk were expected, because it is known that lignans, which are predominantly from plant-based foods, like whole grains, fruit, vegetables, red wine, and coffee, are all associated with lower CHD risk,” Dr. Hu said.
What is novel about the current study is that it established a threshold for lignan consumption, above which there is no CHD benefit, he said.
“It is not a matter of the more you consume, the lower your risk. There is a certain amount of lignan you have to reach, after which there is no more benefit,” Dr. Hu said.
Dr. Hu and associates prospectively followed 214,108 men and women in three cohorts who did not have cardiovascular disease or cancer at baseline. The cohorts were the Health Professionals Follow-Up Study, Nurses’ Health Study, and Nurses’ Health Study II.
Diets were assessed using the Food Frequency Questionnaire every 2-4 years at follow-up visits.
During 5.5 million person-years of follow-up, Dr. Hu and associates documented 10,244 CHD cases, including 6,283 nonfatal myocardial infarctions and 3,961 fatal CHD cases.
The results showed that higher total lignan intake, and all individual lignan intake as well, were associated with significantly lower risk of total CHD.
Participants with higher total lignan intake were older and had more favorable health and lifestyle profiles including lower body mass index, lower prevalence of hypertension and hypercholesterolemia, high levels of physical activity, and better diet quality.
Overall, the pooled hazard ratios of CHD were 0.85 (95% confidence interval, 0.79-0.92) for total lignans, 0.76 (95% CI, 0.71-0.82) for matairesinol, 0.87 (95% CI, 0.81-0.93) for secoisolariciresinol, 0.89 (95% CI, 0.83-0.95) for pinoresinol, and 0.89 (95% CI: 0.83- 0.95) for lariciresinol (all P values for trend ≤ .003).
In addition, nonlinear relationships were found for total lignan, matairesinol, and secoisolariciresinol: The risk reduction plateaued at intakes above approximately 300 mcg/d for total lignan; 10 mcg/d for matairesinol, and 100 mcg/d for secoisolariciresinol.
The inverse associations for total lignan intake were more apparent among participants with higher total fiber intake.
In addition, lignan intake was more strongly associated with plasma concentrations of enterolactone when fiber intake was higher.
Dr. Hu said a next avenue of research will explore the synergistic association between lignans and fiber in further lowering CHD risk.
Lignans are exclusively metabolized by gut microbiota, Dr. Hu noted. “This opens another avenue of research because we can take further steps to see how the gut microbiota compositions and fiber interact with the production of lignans and how these might affect disease risk for other conditions, such as diabetes.”
An important study
“The evidence is building that there is an association between polyphenol intake and chronic disease, especially for CVD [cardiovascular disease],” David J.A. Jenkins, MD, PhD, and colleagues wrote in an accompanying editorial.
“Plant polyphenols may be important components of healthy plant-based diets that contribute to freedom from chronic diseases such as CVD, diabetes, and possibly cancer and so are associated with a reduction in all-cause mortality,” they wrote.
“I think this is an important study even though the results are not unexpected,” Dr. Jenkins, professor in the departments of medicine and nutritional sciences at the University of Toronto, said in an interview.
“We do know that plant polyphenols are important sources of antioxidants and may have many protective roles in preventing destruction of proteins and DNA destruction, so the results here reinforce very strongly the concept of plant foods and their importance in the diet,” he said.
The data reaffirmed the value of eating a variety of plant foods and eating them in a less processed form, because they have higher amounts of their phenolic compounds, Dr. Jenkins said.
“Things like wheat, oats, barley, [and] whole grain foods will have more phenolic components with them, as do fruits and vegetables,” he said.
Dr. Hu and Dr. Jenkins disclosed no relevant financial relationships.
Aversion of this article first appeared on Medscape.com.
Infusion shown effective for acquired von Willebrand disease
Acquired von Willebrand disease (aVWD) is a rare and serious condition associated with lymphoproliferative disorders, malignancy, autoimmune disorders, and cardiovascular disease. It is most commonly caused by monoclonal gammopathy of undetermined significance (MGUS), which acts to clear von Willebrand factor from the patient’s bloodstream. However, a continuous-infusion of plasma-derived von Willebrand factor (VWF) concentrate provided adequate hemostasis in aVWD resulting from MGUS, according to Kathryn E. Dane, PharmD, of Johns Hopkins University, Baltimore, and colleagues.
The infusion rapidly achieved target ristocetin cofactor activity with or without intravenous immunoglobulin in three patients, as detailed in the report published online in Blood Advances.
The three consecutive patients with aVWD were treated with plasma-derived VWF concentrate administered for periprocedural optimization (patient 1, an 85-year old woman) or to treat bleeding episodes (patient 2, an 88-year-old man; and patient 3, a 53-year-old woman). Factor VIII activity was measured via a 1-stage clotting test and von Willebrand factor activity was measured with a ristocetin cofactor assay.
Promising results
All three patients demonstrated increased VWF ristocetin cofactor and factor VIII activities within hours of initiation of the continuous infusion concentrate, according to the report.
“We hypothesize that the efficacy of CI VWF concentrate in aVWD may be related to continuous provision of VWF, allowing binding and neutralization of anti-VWF IgG antibodies, and providing adequate circulating unbound VWF for appropriate hemostatic efficacy,” the researchers concluded.
The authors reported that they had no competing financial interests.
Acquired von Willebrand disease (aVWD) is a rare and serious condition associated with lymphoproliferative disorders, malignancy, autoimmune disorders, and cardiovascular disease. It is most commonly caused by monoclonal gammopathy of undetermined significance (MGUS), which acts to clear von Willebrand factor from the patient’s bloodstream. However, a continuous-infusion of plasma-derived von Willebrand factor (VWF) concentrate provided adequate hemostasis in aVWD resulting from MGUS, according to Kathryn E. Dane, PharmD, of Johns Hopkins University, Baltimore, and colleagues.
The infusion rapidly achieved target ristocetin cofactor activity with or without intravenous immunoglobulin in three patients, as detailed in the report published online in Blood Advances.
The three consecutive patients with aVWD were treated with plasma-derived VWF concentrate administered for periprocedural optimization (patient 1, an 85-year old woman) or to treat bleeding episodes (patient 2, an 88-year-old man; and patient 3, a 53-year-old woman). Factor VIII activity was measured via a 1-stage clotting test and von Willebrand factor activity was measured with a ristocetin cofactor assay.
Promising results
All three patients demonstrated increased VWF ristocetin cofactor and factor VIII activities within hours of initiation of the continuous infusion concentrate, according to the report.
“We hypothesize that the efficacy of CI VWF concentrate in aVWD may be related to continuous provision of VWF, allowing binding and neutralization of anti-VWF IgG antibodies, and providing adequate circulating unbound VWF for appropriate hemostatic efficacy,” the researchers concluded.
The authors reported that they had no competing financial interests.
Acquired von Willebrand disease (aVWD) is a rare and serious condition associated with lymphoproliferative disorders, malignancy, autoimmune disorders, and cardiovascular disease. It is most commonly caused by monoclonal gammopathy of undetermined significance (MGUS), which acts to clear von Willebrand factor from the patient’s bloodstream. However, a continuous-infusion of plasma-derived von Willebrand factor (VWF) concentrate provided adequate hemostasis in aVWD resulting from MGUS, according to Kathryn E. Dane, PharmD, of Johns Hopkins University, Baltimore, and colleagues.
The infusion rapidly achieved target ristocetin cofactor activity with or without intravenous immunoglobulin in three patients, as detailed in the report published online in Blood Advances.
The three consecutive patients with aVWD were treated with plasma-derived VWF concentrate administered for periprocedural optimization (patient 1, an 85-year old woman) or to treat bleeding episodes (patient 2, an 88-year-old man; and patient 3, a 53-year-old woman). Factor VIII activity was measured via a 1-stage clotting test and von Willebrand factor activity was measured with a ristocetin cofactor assay.
Promising results
All three patients demonstrated increased VWF ristocetin cofactor and factor VIII activities within hours of initiation of the continuous infusion concentrate, according to the report.
“We hypothesize that the efficacy of CI VWF concentrate in aVWD may be related to continuous provision of VWF, allowing binding and neutralization of anti-VWF IgG antibodies, and providing adequate circulating unbound VWF for appropriate hemostatic efficacy,” the researchers concluded.
The authors reported that they had no competing financial interests.
FROM BLOOD ADVANCES
He Needs More Than Lip Service for This Lesion
ANSWER
The correct answer is all of the above (choice “g”).
DISCUSSION
Squamous cell carcinoma (SCC) of the lip—almost always the lower lip—is quite common and appears to be caused by several factors. These can include exposure to ultraviolet light, ionizing radiation, arsenic (through contaminated groundwater or certain medications), tobacco, and human papillomavirus.
Early on in its manifestation, this patient’s SCC could have been excised with margins, producing an excellent prognosis. But with the extended delay in diagnosis and apparent related adenopathy, the patient’s future looked much less certain. At the very least, he would face extensive surgery, possible lymph node dissection, and maybe chemo and radiation therapies. Metastasis to the brain and lung(s) were very real possibilities.
The history associated with this case is far from uncommon, since these cancers are often mistaken for infection, which further delays correct diagnosis and treatment. Compounding this problem—and for reasons unclear to this author—affected patients are often referred to the wrong specialist. The critical missing piece of information was a diagnosis, which could only have been obtained through an incisional biopsy. Only then could the patient be referred appropriately.
Fortunately, in this case, the patient ended up under the care of a head and neck surgeon who planned to address this tumor after imaging of the head, neck, and lungs was completed.
ANSWER
The correct answer is all of the above (choice “g”).
DISCUSSION
Squamous cell carcinoma (SCC) of the lip—almost always the lower lip—is quite common and appears to be caused by several factors. These can include exposure to ultraviolet light, ionizing radiation, arsenic (through contaminated groundwater or certain medications), tobacco, and human papillomavirus.
Early on in its manifestation, this patient’s SCC could have been excised with margins, producing an excellent prognosis. But with the extended delay in diagnosis and apparent related adenopathy, the patient’s future looked much less certain. At the very least, he would face extensive surgery, possible lymph node dissection, and maybe chemo and radiation therapies. Metastasis to the brain and lung(s) were very real possibilities.
The history associated with this case is far from uncommon, since these cancers are often mistaken for infection, which further delays correct diagnosis and treatment. Compounding this problem—and for reasons unclear to this author—affected patients are often referred to the wrong specialist. The critical missing piece of information was a diagnosis, which could only have been obtained through an incisional biopsy. Only then could the patient be referred appropriately.
Fortunately, in this case, the patient ended up under the care of a head and neck surgeon who planned to address this tumor after imaging of the head, neck, and lungs was completed.
ANSWER
The correct answer is all of the above (choice “g”).
DISCUSSION
Squamous cell carcinoma (SCC) of the lip—almost always the lower lip—is quite common and appears to be caused by several factors. These can include exposure to ultraviolet light, ionizing radiation, arsenic (through contaminated groundwater or certain medications), tobacco, and human papillomavirus.
Early on in its manifestation, this patient’s SCC could have been excised with margins, producing an excellent prognosis. But with the extended delay in diagnosis and apparent related adenopathy, the patient’s future looked much less certain. At the very least, he would face extensive surgery, possible lymph node dissection, and maybe chemo and radiation therapies. Metastasis to the brain and lung(s) were very real possibilities.
The history associated with this case is far from uncommon, since these cancers are often mistaken for infection, which further delays correct diagnosis and treatment. Compounding this problem—and for reasons unclear to this author—affected patients are often referred to the wrong specialist. The critical missing piece of information was a diagnosis, which could only have been obtained through an incisional biopsy. Only then could the patient be referred appropriately.
Fortunately, in this case, the patient ended up under the care of a head and neck surgeon who planned to address this tumor after imaging of the head, neck, and lungs was completed.
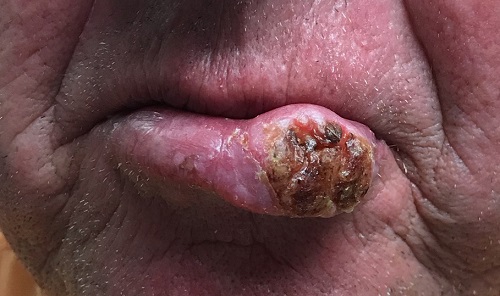
Several years ago, the patient developed what he thought was a cold sore, which waxed and waned but never quite healed. It grew considerably over time but caused no pain, so the patient and his wife assumed it was benign. A few times over the years, his primary care provider (PCP) prescribed oral antibiotics, then later acyclovir, to treat the lesion. When it became large enough to interfere with normal speech, the patient’s PCP referred him to a dentist, who in turn arranged an urgent consultation with dermatology.
The patient was retired but had spent more than 50 years working and recreating outdoors without any protection. For 40 of those adult years, he had smoked. His health was good in other respects.
The 3-cm warty mass on the vermillion surface of the lower lip, just to the left of center, was focally eroded and quite firm to touch. Palpation of local nodal locations revealed several fixed nontender nodes, most notably in the submental area. His sun-exposed skin was quite sun damaged, with stellate scarring, abundant dyschromia, telangiectasias, and marked solar atrophy.
A 5-mm punch biopsy was performed, showing poorly differentiated squamous cell carcinoma.
Colorectal polyps often recur after incomplete resection
When gastroenterologists resect polyps during colonoscopy, they may inadvertently leave some neoplastic tissue behind. Biopsies from resection margins sometimes contain polyp remnants, even after a lesion has been visibly removed, research has shown. But the significance of incomplete resection has been unclear.
A new study indicates that incomplete resection, compared with complete resection, substantially increases the likelihood that a polyp will be found during follow-up colonoscopy.
The evidence “strongly supports the hypothesis” that residual polyp tissue likely contributes to neoplastic polyp recurrence and, by extension, interval colorectal cancer, said study author Heiko Pohl, MD, and colleagues. Dr. Pohl is affiliated with the White River Junction Veterans Affairs Medical Center in Vermont and Dartmouth-Hitchcock Medical Center and Dartmouth Geisel School of Medicine in Hanover, N.H.
Among 166 participants in the Complete Adenoma Resection (CARE) study who went on to have a surveillance colonoscopy in the following years, metachronous neoplasia was detected more often in colon segments where polyps had been incompletely resected, compared with colon segments with complete resections (52% vs. 23%), according to a study in Annals of Internal Medicine.
In addition, colon segments that had had incomplete resections in the CARE study were more likely to have polyps that were 10 mm or greater (18% vs. 3%). The 32 participants who had at least one incomplete resection during the original CARE study were three times more likely to have a polyp at follow-up, Dr. Pohl and coauthors found.
There were no instances of cancer or high-grade dysplasia during follow-up, however.
Key questions remain, including “Why do people get cancer after colonoscopy?” said Shai Friedland, MD, MS, who was not involved in the study. “Maybe it is because of these polyps that were not removed completely.”
On the other hand, lesions that are more difficult to see in the first place and more dangerous might be responsible when colorectal cancer does occur after colonoscopy, said Dr. Friedland, professor of gastroenterology and hepatology at Stanford (Calif.) University and a gastroenterologist at Veterans Affairs Palo Alto (Calif.) Health Care System.
Although it is not surprising that polyp remnants may appear as polyps during follow-up examinations, the difference in polyp recurrence between groups “must be quite striking” for it to clearly be seen in this small study, Dr. Friedland said. The results suggest that there is a need to “redouble our efforts to make sure that we remove polyps completely and not just hope that they disappear after an incomplete job,” he added.
In the CARE study, which was conducted between 2009 and 2012 at two academic medical centers, researchers found that about 10% of neoplastic polyps with visibly complete removal may nonetheless be incompletely resected, based on the presence of neoplastic tissue in biopsies taken from resection margins.
The investigators focused on 5- to 20-mm nonpedunculated colorectal polyps that were removed during routine polypectomy using electrocautery snare resection.
Participants with incomplete polyp resections were encouraged to have a surveillance examination within 1 year, and those with complete resections received guideline-based surveillance recommendations. In the follow-up analysis, those with an incomplete resection had a median time to first examination of 17 months, and those with complete resections had a median time to first examination of 45 months.
“These results highlight the importance of achieving complete resection because the risk for subsequent advanced neoplasia detection statistically significantly increases without it,” Dr. Pohl said. “The large polyp size after incomplete resection within a generally short follow-up time may imply fast growth, even if none of the presumed recurrent polyps contained advanced histologic characteristics.”
Between-group differences in the participants and polyps could confound the observations, the authors noted. It is possible that endoscopists who did the follow-up colonoscopies looked harder for polyps in colon segments that had prior incomplete resections, on account of the initial study findings, they said. “It is also plausible that endoscopists with a greater incomplete resection rate would be more likely to miss polyps, which could then be detected at follow-up,” though detection and resection skills do not necessarily correlate, the investigators said.
With resection, the goal is to remove the polyp in one piece with clear margins, and researchers have known that the polyp removal techniques used in the study “are far from perfect,” Dr. Friedland said. One question is whether the field should focus on improving standard techniques, or incorporating newer, more advanced techniques such as endoscopic submucosal dissection, which is effective but may entail significant perforation risk when performed by someone who is not proficient, he said.
“One of the major focuses we had over the years is finding the polyps to prevent future cancer,” Dr. Pohl said in an interview. But the CARE study showed that “we do not do a good job of taking polyps off completely,” he added. The present analysis underscores that clinicians should do their best to remove polyps completely, even if they lack a way to confirm complete resection in routine practice, as is currently the case, Dr. Pohl said. To that end, clinicians should aim to refine their skills and techniques. Video evaluations with guidance from experts may prove helpful, he suggested. “We need to think hard about quality assessment,” Dr. Pohl said.
Dr. Pohl disclosed grants from Boston Scientific, Cosmo Pharmaceuticals, and Steris outside of the study. Dr. Friedland had no disclosures.
When gastroenterologists resect polyps during colonoscopy, they may inadvertently leave some neoplastic tissue behind. Biopsies from resection margins sometimes contain polyp remnants, even after a lesion has been visibly removed, research has shown. But the significance of incomplete resection has been unclear.
A new study indicates that incomplete resection, compared with complete resection, substantially increases the likelihood that a polyp will be found during follow-up colonoscopy.
The evidence “strongly supports the hypothesis” that residual polyp tissue likely contributes to neoplastic polyp recurrence and, by extension, interval colorectal cancer, said study author Heiko Pohl, MD, and colleagues. Dr. Pohl is affiliated with the White River Junction Veterans Affairs Medical Center in Vermont and Dartmouth-Hitchcock Medical Center and Dartmouth Geisel School of Medicine in Hanover, N.H.
Among 166 participants in the Complete Adenoma Resection (CARE) study who went on to have a surveillance colonoscopy in the following years, metachronous neoplasia was detected more often in colon segments where polyps had been incompletely resected, compared with colon segments with complete resections (52% vs. 23%), according to a study in Annals of Internal Medicine.
In addition, colon segments that had had incomplete resections in the CARE study were more likely to have polyps that were 10 mm or greater (18% vs. 3%). The 32 participants who had at least one incomplete resection during the original CARE study were three times more likely to have a polyp at follow-up, Dr. Pohl and coauthors found.
There were no instances of cancer or high-grade dysplasia during follow-up, however.
Key questions remain, including “Why do people get cancer after colonoscopy?” said Shai Friedland, MD, MS, who was not involved in the study. “Maybe it is because of these polyps that were not removed completely.”
On the other hand, lesions that are more difficult to see in the first place and more dangerous might be responsible when colorectal cancer does occur after colonoscopy, said Dr. Friedland, professor of gastroenterology and hepatology at Stanford (Calif.) University and a gastroenterologist at Veterans Affairs Palo Alto (Calif.) Health Care System.
Although it is not surprising that polyp remnants may appear as polyps during follow-up examinations, the difference in polyp recurrence between groups “must be quite striking” for it to clearly be seen in this small study, Dr. Friedland said. The results suggest that there is a need to “redouble our efforts to make sure that we remove polyps completely and not just hope that they disappear after an incomplete job,” he added.
In the CARE study, which was conducted between 2009 and 2012 at two academic medical centers, researchers found that about 10% of neoplastic polyps with visibly complete removal may nonetheless be incompletely resected, based on the presence of neoplastic tissue in biopsies taken from resection margins.
The investigators focused on 5- to 20-mm nonpedunculated colorectal polyps that were removed during routine polypectomy using electrocautery snare resection.
Participants with incomplete polyp resections were encouraged to have a surveillance examination within 1 year, and those with complete resections received guideline-based surveillance recommendations. In the follow-up analysis, those with an incomplete resection had a median time to first examination of 17 months, and those with complete resections had a median time to first examination of 45 months.
“These results highlight the importance of achieving complete resection because the risk for subsequent advanced neoplasia detection statistically significantly increases without it,” Dr. Pohl said. “The large polyp size after incomplete resection within a generally short follow-up time may imply fast growth, even if none of the presumed recurrent polyps contained advanced histologic characteristics.”
Between-group differences in the participants and polyps could confound the observations, the authors noted. It is possible that endoscopists who did the follow-up colonoscopies looked harder for polyps in colon segments that had prior incomplete resections, on account of the initial study findings, they said. “It is also plausible that endoscopists with a greater incomplete resection rate would be more likely to miss polyps, which could then be detected at follow-up,” though detection and resection skills do not necessarily correlate, the investigators said.
With resection, the goal is to remove the polyp in one piece with clear margins, and researchers have known that the polyp removal techniques used in the study “are far from perfect,” Dr. Friedland said. One question is whether the field should focus on improving standard techniques, or incorporating newer, more advanced techniques such as endoscopic submucosal dissection, which is effective but may entail significant perforation risk when performed by someone who is not proficient, he said.
“One of the major focuses we had over the years is finding the polyps to prevent future cancer,” Dr. Pohl said in an interview. But the CARE study showed that “we do not do a good job of taking polyps off completely,” he added. The present analysis underscores that clinicians should do their best to remove polyps completely, even if they lack a way to confirm complete resection in routine practice, as is currently the case, Dr. Pohl said. To that end, clinicians should aim to refine their skills and techniques. Video evaluations with guidance from experts may prove helpful, he suggested. “We need to think hard about quality assessment,” Dr. Pohl said.
Dr. Pohl disclosed grants from Boston Scientific, Cosmo Pharmaceuticals, and Steris outside of the study. Dr. Friedland had no disclosures.
When gastroenterologists resect polyps during colonoscopy, they may inadvertently leave some neoplastic tissue behind. Biopsies from resection margins sometimes contain polyp remnants, even after a lesion has been visibly removed, research has shown. But the significance of incomplete resection has been unclear.
A new study indicates that incomplete resection, compared with complete resection, substantially increases the likelihood that a polyp will be found during follow-up colonoscopy.
The evidence “strongly supports the hypothesis” that residual polyp tissue likely contributes to neoplastic polyp recurrence and, by extension, interval colorectal cancer, said study author Heiko Pohl, MD, and colleagues. Dr. Pohl is affiliated with the White River Junction Veterans Affairs Medical Center in Vermont and Dartmouth-Hitchcock Medical Center and Dartmouth Geisel School of Medicine in Hanover, N.H.
Among 166 participants in the Complete Adenoma Resection (CARE) study who went on to have a surveillance colonoscopy in the following years, metachronous neoplasia was detected more often in colon segments where polyps had been incompletely resected, compared with colon segments with complete resections (52% vs. 23%), according to a study in Annals of Internal Medicine.
In addition, colon segments that had had incomplete resections in the CARE study were more likely to have polyps that were 10 mm or greater (18% vs. 3%). The 32 participants who had at least one incomplete resection during the original CARE study were three times more likely to have a polyp at follow-up, Dr. Pohl and coauthors found.
There were no instances of cancer or high-grade dysplasia during follow-up, however.
Key questions remain, including “Why do people get cancer after colonoscopy?” said Shai Friedland, MD, MS, who was not involved in the study. “Maybe it is because of these polyps that were not removed completely.”
On the other hand, lesions that are more difficult to see in the first place and more dangerous might be responsible when colorectal cancer does occur after colonoscopy, said Dr. Friedland, professor of gastroenterology and hepatology at Stanford (Calif.) University and a gastroenterologist at Veterans Affairs Palo Alto (Calif.) Health Care System.
Although it is not surprising that polyp remnants may appear as polyps during follow-up examinations, the difference in polyp recurrence between groups “must be quite striking” for it to clearly be seen in this small study, Dr. Friedland said. The results suggest that there is a need to “redouble our efforts to make sure that we remove polyps completely and not just hope that they disappear after an incomplete job,” he added.
In the CARE study, which was conducted between 2009 and 2012 at two academic medical centers, researchers found that about 10% of neoplastic polyps with visibly complete removal may nonetheless be incompletely resected, based on the presence of neoplastic tissue in biopsies taken from resection margins.
The investigators focused on 5- to 20-mm nonpedunculated colorectal polyps that were removed during routine polypectomy using electrocautery snare resection.
Participants with incomplete polyp resections were encouraged to have a surveillance examination within 1 year, and those with complete resections received guideline-based surveillance recommendations. In the follow-up analysis, those with an incomplete resection had a median time to first examination of 17 months, and those with complete resections had a median time to first examination of 45 months.
“These results highlight the importance of achieving complete resection because the risk for subsequent advanced neoplasia detection statistically significantly increases without it,” Dr. Pohl said. “The large polyp size after incomplete resection within a generally short follow-up time may imply fast growth, even if none of the presumed recurrent polyps contained advanced histologic characteristics.”
Between-group differences in the participants and polyps could confound the observations, the authors noted. It is possible that endoscopists who did the follow-up colonoscopies looked harder for polyps in colon segments that had prior incomplete resections, on account of the initial study findings, they said. “It is also plausible that endoscopists with a greater incomplete resection rate would be more likely to miss polyps, which could then be detected at follow-up,” though detection and resection skills do not necessarily correlate, the investigators said.
With resection, the goal is to remove the polyp in one piece with clear margins, and researchers have known that the polyp removal techniques used in the study “are far from perfect,” Dr. Friedland said. One question is whether the field should focus on improving standard techniques, or incorporating newer, more advanced techniques such as endoscopic submucosal dissection, which is effective but may entail significant perforation risk when performed by someone who is not proficient, he said.
“One of the major focuses we had over the years is finding the polyps to prevent future cancer,” Dr. Pohl said in an interview. But the CARE study showed that “we do not do a good job of taking polyps off completely,” he added. The present analysis underscores that clinicians should do their best to remove polyps completely, even if they lack a way to confirm complete resection in routine practice, as is currently the case, Dr. Pohl said. To that end, clinicians should aim to refine their skills and techniques. Video evaluations with guidance from experts may prove helpful, he suggested. “We need to think hard about quality assessment,” Dr. Pohl said.
Dr. Pohl disclosed grants from Boston Scientific, Cosmo Pharmaceuticals, and Steris outside of the study. Dr. Friedland had no disclosures.
Medical residents need breastfeeding support too
As working mothers with babies in tow when the COVID-19 crisis struck, countless uncertainties threatened our already precarious work-life balance. We suddenly had many questions:
“If my daycare closes, what will I do for childcare?”
“How do I navigate diaper changes, feedings, and naps with my hectic remote work schedule?”
“If I’m constantly interrupted during the day, should I skip sleep to catch up on work and not let my colleagues down?”
As professionals who work closely with medical trainees, we knew our parenting dilemmas were being experienced even more acutely by our frontline worker colleagues.
Medical training is an increasingly common time to start a family. In a recent study, 34% of trainees in Harvard-affiliated residency programs became parents during training, and another 52% planned to do so. Trainees have higher breastfeeding initiation rates but lower continuation rates than the general population. Early nursing cessation among trainees is well documented nationally and is most often attributed to work-related barriers. These barriers range from insufficient time and limited access to facilities to a lack of support and discrimination by supervisors and peers.
This trend does not discriminate by specialty. Even among training programs known to be “family friendly,” the average duration of nursing is just 4.5 months. Residents of color are disproportionately affected by inadequate support. Studies show that Black parents breastfeed at lower rates than White parents. This has been largely attributed to structural racism and implicit bias, such as Black parents receiving less assistance initiating nursing after delivery. Adequate lactation support and inclusivity are also lacking for transgender parents who choose to breastfeed or chestfeed.
The very nature of residency training, which includes shifts that can span more than 24 hours, conflicts with many health-promoting behaviors like sleeping and eating well. However, its interference with lactation is correlated with gender. Women are disproportionately affected by the negative outcomes of unmet lactation goals. These include work-life imbalance, career dissatisfaction, and negative emotions. In a study of pediatric residents, one in four did not achieve their breastfeeding goals. Respondents reported feeling “sad, devastated, defeated, disappointed, guilty, embarrassed, frustrated, angry, like a failure, and inadequate.” Among physician mothers more broadly, discrimination related to pregnancy, parental leave, and nursing is associated with higher self-reported burnout.
Navigating nursing during residency training has more than just emotional and psychological consequences – it also has professional ones. Pursuing personal lactation goals can delay residency program completion and board certification, influence specialty selection, negatively impact research productivity, impede career advancement, and lead to misgivings about career choice.
Trainees and their families are not the only ones harmed by inadequate support in residency programs. Patients and their families are affected, too. Research suggests that physicians’ personal breastfeeding practices affect the advice they give to patients. Those who receive lactation support are more likely to help patients meet their own goals. In the previously mentioned study of pediatric residents, more than 90% of the 400 respondents said their own or their partner’s nursing experience affected their interaction with lactating patients in their clinic or hospital.
Increased lactation support is a straightforward, low-cost, high-impact intervention. It benefits trainee well-being, satisfaction, workflow, and future patient care. The Accreditation Council for Graduate Medical Education mandated in July 2019 that all residency programs provide adequate lactation facilities – including refrigeration capabilities and proximity for safe patient care. However, to our knowledge, rates of compliance with this new policy and citation for noncompliance have yet to be seen. Regardless, facilities alone are not enough. Residency programs should develop and enforce formal lactation policies.
Several institutions have successfully piloted such policies in recent years. One in particular from the University of Michigan’s surgery residency program inspired the development of a lactation policy within the internal medicine residency at our institution. These policies designate appropriate spaces at each clinical rotation site, clarify that residents are encouraged to take pumping breaks as needed – in coordination with clinical teams so as not to compromise patient care – and communicate support from supervisors.
Our program also established an informal peer mentoring program. Residents with experience pumping at work pair up with newer trainees. The policy benefits residents who wish to chestfeed or breastfeed, normalizes lactation, and empowers trainees by diminishing the need to ask for individual accommodations. It also costs the program nothing.
As more women enter medicine and more trainees become parents during residency, the need for support in this area will only continue to grow. The widespread lack of such resources, and the fact that clean and private facilities are only now being mandated, is symbolic. If even this basic need is rarely acknowledged or met, what other resident needs are being neglected?
A version of this article first appeared on Medscape.com.
As working mothers with babies in tow when the COVID-19 crisis struck, countless uncertainties threatened our already precarious work-life balance. We suddenly had many questions:
“If my daycare closes, what will I do for childcare?”
“How do I navigate diaper changes, feedings, and naps with my hectic remote work schedule?”
“If I’m constantly interrupted during the day, should I skip sleep to catch up on work and not let my colleagues down?”
As professionals who work closely with medical trainees, we knew our parenting dilemmas were being experienced even more acutely by our frontline worker colleagues.
Medical training is an increasingly common time to start a family. In a recent study, 34% of trainees in Harvard-affiliated residency programs became parents during training, and another 52% planned to do so. Trainees have higher breastfeeding initiation rates but lower continuation rates than the general population. Early nursing cessation among trainees is well documented nationally and is most often attributed to work-related barriers. These barriers range from insufficient time and limited access to facilities to a lack of support and discrimination by supervisors and peers.
This trend does not discriminate by specialty. Even among training programs known to be “family friendly,” the average duration of nursing is just 4.5 months. Residents of color are disproportionately affected by inadequate support. Studies show that Black parents breastfeed at lower rates than White parents. This has been largely attributed to structural racism and implicit bias, such as Black parents receiving less assistance initiating nursing after delivery. Adequate lactation support and inclusivity are also lacking for transgender parents who choose to breastfeed or chestfeed.
The very nature of residency training, which includes shifts that can span more than 24 hours, conflicts with many health-promoting behaviors like sleeping and eating well. However, its interference with lactation is correlated with gender. Women are disproportionately affected by the negative outcomes of unmet lactation goals. These include work-life imbalance, career dissatisfaction, and negative emotions. In a study of pediatric residents, one in four did not achieve their breastfeeding goals. Respondents reported feeling “sad, devastated, defeated, disappointed, guilty, embarrassed, frustrated, angry, like a failure, and inadequate.” Among physician mothers more broadly, discrimination related to pregnancy, parental leave, and nursing is associated with higher self-reported burnout.
Navigating nursing during residency training has more than just emotional and psychological consequences – it also has professional ones. Pursuing personal lactation goals can delay residency program completion and board certification, influence specialty selection, negatively impact research productivity, impede career advancement, and lead to misgivings about career choice.
Trainees and their families are not the only ones harmed by inadequate support in residency programs. Patients and their families are affected, too. Research suggests that physicians’ personal breastfeeding practices affect the advice they give to patients. Those who receive lactation support are more likely to help patients meet their own goals. In the previously mentioned study of pediatric residents, more than 90% of the 400 respondents said their own or their partner’s nursing experience affected their interaction with lactating patients in their clinic or hospital.
Increased lactation support is a straightforward, low-cost, high-impact intervention. It benefits trainee well-being, satisfaction, workflow, and future patient care. The Accreditation Council for Graduate Medical Education mandated in July 2019 that all residency programs provide adequate lactation facilities – including refrigeration capabilities and proximity for safe patient care. However, to our knowledge, rates of compliance with this new policy and citation for noncompliance have yet to be seen. Regardless, facilities alone are not enough. Residency programs should develop and enforce formal lactation policies.
Several institutions have successfully piloted such policies in recent years. One in particular from the University of Michigan’s surgery residency program inspired the development of a lactation policy within the internal medicine residency at our institution. These policies designate appropriate spaces at each clinical rotation site, clarify that residents are encouraged to take pumping breaks as needed – in coordination with clinical teams so as not to compromise patient care – and communicate support from supervisors.
Our program also established an informal peer mentoring program. Residents with experience pumping at work pair up with newer trainees. The policy benefits residents who wish to chestfeed or breastfeed, normalizes lactation, and empowers trainees by diminishing the need to ask for individual accommodations. It also costs the program nothing.
As more women enter medicine and more trainees become parents during residency, the need for support in this area will only continue to grow. The widespread lack of such resources, and the fact that clean and private facilities are only now being mandated, is symbolic. If even this basic need is rarely acknowledged or met, what other resident needs are being neglected?
A version of this article first appeared on Medscape.com.
As working mothers with babies in tow when the COVID-19 crisis struck, countless uncertainties threatened our already precarious work-life balance. We suddenly had many questions:
“If my daycare closes, what will I do for childcare?”
“How do I navigate diaper changes, feedings, and naps with my hectic remote work schedule?”
“If I’m constantly interrupted during the day, should I skip sleep to catch up on work and not let my colleagues down?”
As professionals who work closely with medical trainees, we knew our parenting dilemmas were being experienced even more acutely by our frontline worker colleagues.
Medical training is an increasingly common time to start a family. In a recent study, 34% of trainees in Harvard-affiliated residency programs became parents during training, and another 52% planned to do so. Trainees have higher breastfeeding initiation rates but lower continuation rates than the general population. Early nursing cessation among trainees is well documented nationally and is most often attributed to work-related barriers. These barriers range from insufficient time and limited access to facilities to a lack of support and discrimination by supervisors and peers.
This trend does not discriminate by specialty. Even among training programs known to be “family friendly,” the average duration of nursing is just 4.5 months. Residents of color are disproportionately affected by inadequate support. Studies show that Black parents breastfeed at lower rates than White parents. This has been largely attributed to structural racism and implicit bias, such as Black parents receiving less assistance initiating nursing after delivery. Adequate lactation support and inclusivity are also lacking for transgender parents who choose to breastfeed or chestfeed.
The very nature of residency training, which includes shifts that can span more than 24 hours, conflicts with many health-promoting behaviors like sleeping and eating well. However, its interference with lactation is correlated with gender. Women are disproportionately affected by the negative outcomes of unmet lactation goals. These include work-life imbalance, career dissatisfaction, and negative emotions. In a study of pediatric residents, one in four did not achieve their breastfeeding goals. Respondents reported feeling “sad, devastated, defeated, disappointed, guilty, embarrassed, frustrated, angry, like a failure, and inadequate.” Among physician mothers more broadly, discrimination related to pregnancy, parental leave, and nursing is associated with higher self-reported burnout.
Navigating nursing during residency training has more than just emotional and psychological consequences – it also has professional ones. Pursuing personal lactation goals can delay residency program completion and board certification, influence specialty selection, negatively impact research productivity, impede career advancement, and lead to misgivings about career choice.
Trainees and their families are not the only ones harmed by inadequate support in residency programs. Patients and their families are affected, too. Research suggests that physicians’ personal breastfeeding practices affect the advice they give to patients. Those who receive lactation support are more likely to help patients meet their own goals. In the previously mentioned study of pediatric residents, more than 90% of the 400 respondents said their own or their partner’s nursing experience affected their interaction with lactating patients in their clinic or hospital.
Increased lactation support is a straightforward, low-cost, high-impact intervention. It benefits trainee well-being, satisfaction, workflow, and future patient care. The Accreditation Council for Graduate Medical Education mandated in July 2019 that all residency programs provide adequate lactation facilities – including refrigeration capabilities and proximity for safe patient care. However, to our knowledge, rates of compliance with this new policy and citation for noncompliance have yet to be seen. Regardless, facilities alone are not enough. Residency programs should develop and enforce formal lactation policies.
Several institutions have successfully piloted such policies in recent years. One in particular from the University of Michigan’s surgery residency program inspired the development of a lactation policy within the internal medicine residency at our institution. These policies designate appropriate spaces at each clinical rotation site, clarify that residents are encouraged to take pumping breaks as needed – in coordination with clinical teams so as not to compromise patient care – and communicate support from supervisors.
Our program also established an informal peer mentoring program. Residents with experience pumping at work pair up with newer trainees. The policy benefits residents who wish to chestfeed or breastfeed, normalizes lactation, and empowers trainees by diminishing the need to ask for individual accommodations. It also costs the program nothing.
As more women enter medicine and more trainees become parents during residency, the need for support in this area will only continue to grow. The widespread lack of such resources, and the fact that clean and private facilities are only now being mandated, is symbolic. If even this basic need is rarely acknowledged or met, what other resident needs are being neglected?
A version of this article first appeared on Medscape.com.