User login
Reappraisal as a way to cope with pandemic news
Our emotional health and that of our patients has taken a terrible beating at the hands of the COVID-19 pandemic. Suicides, substance abuse, levels of depression, and anxiety have risen dramatically. It is tempting to believe that it is the unfortunate events alone we hear about and experience that are causing us to feel the way we do. However, James Gross, PhD, professor of psychology and director of the Stanford (Calif.) University psychophysiology laboratory said: “It is actually the thoughts that we have about the situation that are leading us to feel negative emotions or fail to feel positive emotions.” (YouTube video – https://www.youtube.com/watch?v=Ay4_L1RfkIs).
With this premise as a jumping off point, a large group of psychophysiologists at a variety of centers around the world began a study of more than 20,000 subjects in more than 87 countries and regions. Half of the subjects were exposed to a brief (about 5 min) emotional regulation strategy called “reappraisal.” All the subjects were then shown images of the COVID-19 crisis culled from news sources and were then surveyed about their emotions. The researchers discovered that those subjects exposed to the reappraisal intervention demonstrated significantly increased positive responses and significantly decreased negative responses compared to the two control groups.
Reappraisal is an intervention that encourages individuals to think differently about their current situation in hopes of improving their emotional responses. The researchers tested two different types of reappraisal: “Reconstruing,” which aims to change the way the situation is represented mentally – for example, viewing it as controllable – and “repurposing,” in which the subject is encouraged to focus on the potentially positive outcomes of the situation. In other words, reappraisal basically tries to instill a glass-half-full, silver-lining mindset. The investigators report that both reappraisal strategies were equally effective at influencing the subjects’ responses.
The authors claimed that their findings suggest that reappraisal interventions might be of value for health care and other essential workers who have demonstrated a vulnerability to emotion upheaval during the pandemic. The authors also envisioned opportunities for political and business leaders to implement national and global reappraisal–based initiatives to generate resilience on a national and even global scale.
I will admit that, although I am usually skeptical of studies aimed at quantifying emotions, I found this study interesting. After watching a half hour of television news or reading the online edition of the New York Times I think we could all use a pep talk from someone who might be able to help us look on the bright side of things. However, I doubt that a single 5-minute reappraisal intervention is going to have much lasting benefit in the face of the shear magnitude of bad news we are fed every day. Catastrophic news sells newspapers and it is unlikely that dynamic is ever going to change.
I guess we could try mandating that every half hour of network news be followed by a 5-minute session of reconstruing or repurposing. That is, if we could find someone who could consistently put a positive spin on the news of the day. Even if we could locate that one-in-a-million individual with an absolutely unshakably sunny disposition and a knack for finding silver linings, I suspect after a few weeks he or she would be labeled the arch Pollyanna and be drummed off the air.
That is not to say that we should write off the findings of this international study as a statistical quirk. It may be, but clearly these last 2 years have taken a toll on our emotions and even those of us who are congenital optimists need a pep talk from time to time. Although my forte is denial, I think I already know how to reconstrue and repurpose, but I’m ready to listen to anyone who can help me learn to do it better.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Our emotional health and that of our patients has taken a terrible beating at the hands of the COVID-19 pandemic. Suicides, substance abuse, levels of depression, and anxiety have risen dramatically. It is tempting to believe that it is the unfortunate events alone we hear about and experience that are causing us to feel the way we do. However, James Gross, PhD, professor of psychology and director of the Stanford (Calif.) University psychophysiology laboratory said: “It is actually the thoughts that we have about the situation that are leading us to feel negative emotions or fail to feel positive emotions.” (YouTube video – https://www.youtube.com/watch?v=Ay4_L1RfkIs).
With this premise as a jumping off point, a large group of psychophysiologists at a variety of centers around the world began a study of more than 20,000 subjects in more than 87 countries and regions. Half of the subjects were exposed to a brief (about 5 min) emotional regulation strategy called “reappraisal.” All the subjects were then shown images of the COVID-19 crisis culled from news sources and were then surveyed about their emotions. The researchers discovered that those subjects exposed to the reappraisal intervention demonstrated significantly increased positive responses and significantly decreased negative responses compared to the two control groups.
Reappraisal is an intervention that encourages individuals to think differently about their current situation in hopes of improving their emotional responses. The researchers tested two different types of reappraisal: “Reconstruing,” which aims to change the way the situation is represented mentally – for example, viewing it as controllable – and “repurposing,” in which the subject is encouraged to focus on the potentially positive outcomes of the situation. In other words, reappraisal basically tries to instill a glass-half-full, silver-lining mindset. The investigators report that both reappraisal strategies were equally effective at influencing the subjects’ responses.
The authors claimed that their findings suggest that reappraisal interventions might be of value for health care and other essential workers who have demonstrated a vulnerability to emotion upheaval during the pandemic. The authors also envisioned opportunities for political and business leaders to implement national and global reappraisal–based initiatives to generate resilience on a national and even global scale.
I will admit that, although I am usually skeptical of studies aimed at quantifying emotions, I found this study interesting. After watching a half hour of television news or reading the online edition of the New York Times I think we could all use a pep talk from someone who might be able to help us look on the bright side of things. However, I doubt that a single 5-minute reappraisal intervention is going to have much lasting benefit in the face of the shear magnitude of bad news we are fed every day. Catastrophic news sells newspapers and it is unlikely that dynamic is ever going to change.
I guess we could try mandating that every half hour of network news be followed by a 5-minute session of reconstruing or repurposing. That is, if we could find someone who could consistently put a positive spin on the news of the day. Even if we could locate that one-in-a-million individual with an absolutely unshakably sunny disposition and a knack for finding silver linings, I suspect after a few weeks he or she would be labeled the arch Pollyanna and be drummed off the air.
That is not to say that we should write off the findings of this international study as a statistical quirk. It may be, but clearly these last 2 years have taken a toll on our emotions and even those of us who are congenital optimists need a pep talk from time to time. Although my forte is denial, I think I already know how to reconstrue and repurpose, but I’m ready to listen to anyone who can help me learn to do it better.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Our emotional health and that of our patients has taken a terrible beating at the hands of the COVID-19 pandemic. Suicides, substance abuse, levels of depression, and anxiety have risen dramatically. It is tempting to believe that it is the unfortunate events alone we hear about and experience that are causing us to feel the way we do. However, James Gross, PhD, professor of psychology and director of the Stanford (Calif.) University psychophysiology laboratory said: “It is actually the thoughts that we have about the situation that are leading us to feel negative emotions or fail to feel positive emotions.” (YouTube video – https://www.youtube.com/watch?v=Ay4_L1RfkIs).
With this premise as a jumping off point, a large group of psychophysiologists at a variety of centers around the world began a study of more than 20,000 subjects in more than 87 countries and regions. Half of the subjects were exposed to a brief (about 5 min) emotional regulation strategy called “reappraisal.” All the subjects were then shown images of the COVID-19 crisis culled from news sources and were then surveyed about their emotions. The researchers discovered that those subjects exposed to the reappraisal intervention demonstrated significantly increased positive responses and significantly decreased negative responses compared to the two control groups.
Reappraisal is an intervention that encourages individuals to think differently about their current situation in hopes of improving their emotional responses. The researchers tested two different types of reappraisal: “Reconstruing,” which aims to change the way the situation is represented mentally – for example, viewing it as controllable – and “repurposing,” in which the subject is encouraged to focus on the potentially positive outcomes of the situation. In other words, reappraisal basically tries to instill a glass-half-full, silver-lining mindset. The investigators report that both reappraisal strategies were equally effective at influencing the subjects’ responses.
The authors claimed that their findings suggest that reappraisal interventions might be of value for health care and other essential workers who have demonstrated a vulnerability to emotion upheaval during the pandemic. The authors also envisioned opportunities for political and business leaders to implement national and global reappraisal–based initiatives to generate resilience on a national and even global scale.
I will admit that, although I am usually skeptical of studies aimed at quantifying emotions, I found this study interesting. After watching a half hour of television news or reading the online edition of the New York Times I think we could all use a pep talk from someone who might be able to help us look on the bright side of things. However, I doubt that a single 5-minute reappraisal intervention is going to have much lasting benefit in the face of the shear magnitude of bad news we are fed every day. Catastrophic news sells newspapers and it is unlikely that dynamic is ever going to change.
I guess we could try mandating that every half hour of network news be followed by a 5-minute session of reconstruing or repurposing. That is, if we could find someone who could consistently put a positive spin on the news of the day. Even if we could locate that one-in-a-million individual with an absolutely unshakably sunny disposition and a knack for finding silver linings, I suspect after a few weeks he or she would be labeled the arch Pollyanna and be drummed off the air.
That is not to say that we should write off the findings of this international study as a statistical quirk. It may be, but clearly these last 2 years have taken a toll on our emotions and even those of us who are congenital optimists need a pep talk from time to time. Although my forte is denial, I think I already know how to reconstrue and repurpose, but I’m ready to listen to anyone who can help me learn to do it better.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As COVID resurges, vaccinated Americans rage against holdouts
Outraged at vaccine-hesitant people, some are even calling for mandates requiring all Americans to get inoculated, arguing the holdouts are allowing the Delta coronavirus variant to gain traction and reverse the progress the United States was making against the virus.
“I am angry, I am resentful, and I think it’s a fair and appropriate response,” said Jonathan Hyman, a Berea, Ohio, attorney who blames the unvaccinated for the backslide in pandemic progress.
Mr. Hyman has been following the difficult guidelines health experts have been urging from the beginning. He has been masking up, avoiding large gatherings, postponing travel, and he signed up to receive the vaccine as soon as it was available.
“We have been responsible, I did everything I was supposed to do,” said Mr. Hyman, 48, who didn’t visit his parents for 18 months to keep them safe. “Yet here we are, 16, 17 months later, and it feels like we’re in the exact same place we were last summer, and it’s all because some people refuse to do the responsible things they were told to do.”
James Simmons, a retired South Florida high school finance teacher, is also angered by the vaccine holdouts, citing new spikes in COVID-19 infections, hospitalization rates, and deaths across the country – nearly all of which are among unvaccinated people.
“I can’t fathom the fact that people have seen over 600,000 Americans die from COVID, yet are resistant to a vaccine that provides direct protection for themselves and others,” said Mr. Simmons, 63, who received the shot early. “Their irresponsible decision is an affront to those of us who are vaccinated and still wear masks for the benefit of our society.”
Melissa Martin, an Atlanta resident who contracted a serious case of COVID-19 in September 2020, says it is “perplexing and frustrating” that so many Americans are refusing the vaccine. She believes the anger so many vaccinated people feel is tied to fear.
“I believe at the core of this anger is a fear of losing the ones we love,” said Ms. Martin, 55, who has been vaccinated, as has her fiancé, Shane McGeehin. “I was very angry last year after contracting COVID. The experience of having COVID was negative physically, emotionally, and socially.”
She recalled arguing with friends and relatives who downplayed how severe the virus was and who still refuse vaccination, despite seeing how COVID affected her.
“I am trying to understand why they feel the way they do,” she said, “but I would describe the emotions I have now towards those who do not get the vaccine as frustration, confusion, and disbelief.”
Leana Wen, MD, an emergency medicine doctor and public health policy professor at George Washington University, said such sentiments are common and justified.
“I understand that feeling of frustration and anger, because it is the unvaccinated who are setting back the progress that we’ve made [because of] the many sacrifices that many people have undergone,” said Dr. Wen, author of the newly published book “Lifelines: A Doctor’s Journey in the Fight for Public Health.”
“I think it is appropriate for the vaccinated to feel like they’re being punished right now,” she said. “We as a country had the opportunity to beat this virus – to return to prepandemic normal [life] and have our kids go back to school without worrying about coronavirus and our economy fully recovering. We came so close to achieving this, but we didn’t, and now COVID-19 is surging again. The vaccinated are having to pay the price for the choices that some have made to not end this pandemic.”
COVID rising, driving anger
The rising anger among vaccinated Americans comes as health officials are reporting huge spikes in new cases, hospitalizations, and deaths. Meanwhile, only about half of all Americans fully vaccinated, according to the Centers for Disease Control and Prevention.
Per Aug. 6 estimates from the CDC, the nation is averaging more than 100,000 new cases every day – the highest levels seen since February.
Southern states, with the lowest vaccination rates in the country, have been particularly hard-hit. Florida and Louisiana recently set 7-day records for new cases and hospitalizations, beating previous peaks last summer. Those two states, along with Mississippi, North Carolina, South Carolina, Tennessee, Kentucky, and Georgia, account for 41% of all new COVID-19 hospitalizations in the country, according to the CDC.
“It’s time to start blaming the unvaccinated folks, not the regular folks,” an angry Gov. Kay Ivey (R) of Alabama, told reporters. “It’s the unvaccinated folks that are letting us down.”
In response to the resurgence in cases, President Joe Biden has ordered new vaccine mandates for millions of federal workers.
California started requiring health care professionals to be vaccinated in August 2021, removing the option for unvaccinated employees to submit to regular testing.
New York City became the first in the country to require proof of vaccination for all workers and customers to enter restaurants, gyms, concert halls, movie theaters, and Broadway venues.
Nearly 60 major medical organizations, including the American Medical Association and the American Nurses Association, have called for mandatory vaccination of all health care workers.
Meanwhile, many businesses are requiring workers to be vaccinated before returning to offices and other workplaces. Colleges across the country are mandating the shots for students and staff. And some states and cities are also returning to mask mandates, including Hawaii; Louisiana; Washington, D.C.; San Francisco; and Los Angeles.
Experts say the 90 million unvaccinated Americans are most at risk from COVID and have helped the new Delta variant gain a foothold and spread, posing a risk of “breakthrough” cases even in vaccinated people.
Delta is more contagious and causes more severe disease than other known variants of the virus, according to the CDC. It is also more contagious than the viruses that cause Middle East respiratory syndrome, severe acute respiratory syndrome, Ebola, the common cold, flu, and smallpox
Calls for mandates grow
With Delta helping to drive new spikes in COVID cases, some vaccinated Americans argue that the federal government should be taking a harder line with holdouts. Others have even advocated withholding government stimulus checks or tax credits from vaccine refusers and cutting federal funding to states that don’t meet vaccine targets.
Eric Jaffe, a creative writer and producer from Florida who is vaccinated, said he would like to see government agencies and private businesses do more to put pressure on unvaccinated Americans to get the shot.
“In the interest of public safety, I believe the government and private businesses need to [make] life difficult for the unvaccinated,” said Mr. Jaffe, 29, whose parents both contracted the virus but recovered. “They should not be allowed to dine at restaurants, ride public transportation, attend concerts, or broadly be in spaces with large concentrations of people without passing a COVID test at the door.
“They’ll stand in long lines and be inconvenienced at every turn, while vaccinated people get to fly through security, TSA PreCheck-style. The holdouts at [this] point are beyond convincing. The vaccinated should be able to return to a level of normalcy, and the unvaccinated should face restrictions. Any other dynamic puts the stress on citizens who did the right thing.”
Elif Akcali, 49, who teaches engineering at the University of Florida, Gainesville, worries that the rights of people who refuse the vaccine are being put ahead of those of vaccinated people. She’s also concerned for people who face greater COVID risks, including health care workers and children too young to be inoculated.
“Each infection is an opportunity for the virus to evolve into a stronger version in itself,” said Ms. Akcali, who felt such a sense of relief when she received her vaccination that she teared up. “Each hospitalization is an unnecessary burden to health care workers and the system. Each death brings heartbreak to someone in their circle.”
Ed Berliner, an Emmy Award–winning broadcast journalist and Florida-based media specialist, blames social media for spreading misinformation that has taken root with unvaccinated Americans.
“When America rallied together to combat polio, there were two things we didn’t have. One was a lack of the sewer-dwelling, troll-infested social media, which has become the main source of news for the less intelligent and arrogant,” said Mr. Berliner, CEO of Entourage Media and host of The Man in the Arena, a talk show. “Second, children were dying across the country, and that made people sit up and take notice.”
Mr. Berliner, who knows two people who’ve died from COVID and who received the vaccine early, also believes too many political leaders are still fueling falsehoods that are giving unvaccinated Americans a license to refuse the shot.
“We are also here because governments and officials spend too little time being brutally honest, choosing instead to dance around issues with soft words,” he said. “The first words out of their mouths should have been: ‘What we are doing is trying to save lives. Help us save your life and that of everyone else.’ Would it have made a difference? We will never know.”
Shon Neyland, senior pastor at the Highland Christian Center church in Portland, Ore., said vaccine tensions have divided his congregation, with about half refusing the shot by his estimation. But he said it’s important to understand why some are making that choice, rather than rage at them and hammer home the benefits of the shot.
Many vaccine holdouts don’t trust the government or medical establishment or have bought into political arguments against the shot, he says. Some conservative evangelicals are also swayed by spiritual beliefs that COVID-19 is a sign of “biblical end-times prophesies” and the vaccine is “the mark of the beast.”
But he has tried to counter those beliefs and biases, arguing they are false and unfounded, urging members of his church to get the vaccine, and partnering with local health officials to run clinics to deliver it.
“I gently try to show them that the vaccine is for our own good and, in fact, is a blessing from God, and it’s up to us to accept the blessing [so] we can get back to somewhat of normalcy,” said Mr. Neyland, author of “The Courage to Stand: A New America.”
“I also believe that to get a vaccine this quick, this was nothing short of a miracle to turn the tide so quickly. Now, for us to resist, it would cause us to continue to suffer and lose lives. And you can’t turn away from the lives that have already been lost.”
Mr. Hyman fears we may not have seen the worst of the pandemic and that the Delta variant won’t be the last or most virulent mutation to emerge.
“The number of unvaccinated people is allowing this virus to continue circulating in the community,” he noted. “And while I have a tremendous amount of confidence that the vaccine protects me now from Delta, I have less confidence that it’s going to protect me from whatever [variant] comes next.
“So, I have a tremendous amount of concern for my own health and safety and welfare, and that of the people that I love. But I’m also concerned about what’s it going to do to businesses [and] the economy. Are we going to have more shutdowns if cases continue trending up? I’m very concerned as to what this could do [to] the country.”
A version of this article first appeared on WebMD.com.
Outraged at vaccine-hesitant people, some are even calling for mandates requiring all Americans to get inoculated, arguing the holdouts are allowing the Delta coronavirus variant to gain traction and reverse the progress the United States was making against the virus.
“I am angry, I am resentful, and I think it’s a fair and appropriate response,” said Jonathan Hyman, a Berea, Ohio, attorney who blames the unvaccinated for the backslide in pandemic progress.
Mr. Hyman has been following the difficult guidelines health experts have been urging from the beginning. He has been masking up, avoiding large gatherings, postponing travel, and he signed up to receive the vaccine as soon as it was available.
“We have been responsible, I did everything I was supposed to do,” said Mr. Hyman, 48, who didn’t visit his parents for 18 months to keep them safe. “Yet here we are, 16, 17 months later, and it feels like we’re in the exact same place we were last summer, and it’s all because some people refuse to do the responsible things they were told to do.”
James Simmons, a retired South Florida high school finance teacher, is also angered by the vaccine holdouts, citing new spikes in COVID-19 infections, hospitalization rates, and deaths across the country – nearly all of which are among unvaccinated people.
“I can’t fathom the fact that people have seen over 600,000 Americans die from COVID, yet are resistant to a vaccine that provides direct protection for themselves and others,” said Mr. Simmons, 63, who received the shot early. “Their irresponsible decision is an affront to those of us who are vaccinated and still wear masks for the benefit of our society.”
Melissa Martin, an Atlanta resident who contracted a serious case of COVID-19 in September 2020, says it is “perplexing and frustrating” that so many Americans are refusing the vaccine. She believes the anger so many vaccinated people feel is tied to fear.
“I believe at the core of this anger is a fear of losing the ones we love,” said Ms. Martin, 55, who has been vaccinated, as has her fiancé, Shane McGeehin. “I was very angry last year after contracting COVID. The experience of having COVID was negative physically, emotionally, and socially.”
She recalled arguing with friends and relatives who downplayed how severe the virus was and who still refuse vaccination, despite seeing how COVID affected her.
“I am trying to understand why they feel the way they do,” she said, “but I would describe the emotions I have now towards those who do not get the vaccine as frustration, confusion, and disbelief.”
Leana Wen, MD, an emergency medicine doctor and public health policy professor at George Washington University, said such sentiments are common and justified.
“I understand that feeling of frustration and anger, because it is the unvaccinated who are setting back the progress that we’ve made [because of] the many sacrifices that many people have undergone,” said Dr. Wen, author of the newly published book “Lifelines: A Doctor’s Journey in the Fight for Public Health.”
“I think it is appropriate for the vaccinated to feel like they’re being punished right now,” she said. “We as a country had the opportunity to beat this virus – to return to prepandemic normal [life] and have our kids go back to school without worrying about coronavirus and our economy fully recovering. We came so close to achieving this, but we didn’t, and now COVID-19 is surging again. The vaccinated are having to pay the price for the choices that some have made to not end this pandemic.”
COVID rising, driving anger
The rising anger among vaccinated Americans comes as health officials are reporting huge spikes in new cases, hospitalizations, and deaths. Meanwhile, only about half of all Americans fully vaccinated, according to the Centers for Disease Control and Prevention.
Per Aug. 6 estimates from the CDC, the nation is averaging more than 100,000 new cases every day – the highest levels seen since February.
Southern states, with the lowest vaccination rates in the country, have been particularly hard-hit. Florida and Louisiana recently set 7-day records for new cases and hospitalizations, beating previous peaks last summer. Those two states, along with Mississippi, North Carolina, South Carolina, Tennessee, Kentucky, and Georgia, account for 41% of all new COVID-19 hospitalizations in the country, according to the CDC.
“It’s time to start blaming the unvaccinated folks, not the regular folks,” an angry Gov. Kay Ivey (R) of Alabama, told reporters. “It’s the unvaccinated folks that are letting us down.”
In response to the resurgence in cases, President Joe Biden has ordered new vaccine mandates for millions of federal workers.
California started requiring health care professionals to be vaccinated in August 2021, removing the option for unvaccinated employees to submit to regular testing.
New York City became the first in the country to require proof of vaccination for all workers and customers to enter restaurants, gyms, concert halls, movie theaters, and Broadway venues.
Nearly 60 major medical organizations, including the American Medical Association and the American Nurses Association, have called for mandatory vaccination of all health care workers.
Meanwhile, many businesses are requiring workers to be vaccinated before returning to offices and other workplaces. Colleges across the country are mandating the shots for students and staff. And some states and cities are also returning to mask mandates, including Hawaii; Louisiana; Washington, D.C.; San Francisco; and Los Angeles.
Experts say the 90 million unvaccinated Americans are most at risk from COVID and have helped the new Delta variant gain a foothold and spread, posing a risk of “breakthrough” cases even in vaccinated people.
Delta is more contagious and causes more severe disease than other known variants of the virus, according to the CDC. It is also more contagious than the viruses that cause Middle East respiratory syndrome, severe acute respiratory syndrome, Ebola, the common cold, flu, and smallpox
Calls for mandates grow
With Delta helping to drive new spikes in COVID cases, some vaccinated Americans argue that the federal government should be taking a harder line with holdouts. Others have even advocated withholding government stimulus checks or tax credits from vaccine refusers and cutting federal funding to states that don’t meet vaccine targets.
Eric Jaffe, a creative writer and producer from Florida who is vaccinated, said he would like to see government agencies and private businesses do more to put pressure on unvaccinated Americans to get the shot.
“In the interest of public safety, I believe the government and private businesses need to [make] life difficult for the unvaccinated,” said Mr. Jaffe, 29, whose parents both contracted the virus but recovered. “They should not be allowed to dine at restaurants, ride public transportation, attend concerts, or broadly be in spaces with large concentrations of people without passing a COVID test at the door.
“They’ll stand in long lines and be inconvenienced at every turn, while vaccinated people get to fly through security, TSA PreCheck-style. The holdouts at [this] point are beyond convincing. The vaccinated should be able to return to a level of normalcy, and the unvaccinated should face restrictions. Any other dynamic puts the stress on citizens who did the right thing.”
Elif Akcali, 49, who teaches engineering at the University of Florida, Gainesville, worries that the rights of people who refuse the vaccine are being put ahead of those of vaccinated people. She’s also concerned for people who face greater COVID risks, including health care workers and children too young to be inoculated.
“Each infection is an opportunity for the virus to evolve into a stronger version in itself,” said Ms. Akcali, who felt such a sense of relief when she received her vaccination that she teared up. “Each hospitalization is an unnecessary burden to health care workers and the system. Each death brings heartbreak to someone in their circle.”
Ed Berliner, an Emmy Award–winning broadcast journalist and Florida-based media specialist, blames social media for spreading misinformation that has taken root with unvaccinated Americans.
“When America rallied together to combat polio, there were two things we didn’t have. One was a lack of the sewer-dwelling, troll-infested social media, which has become the main source of news for the less intelligent and arrogant,” said Mr. Berliner, CEO of Entourage Media and host of The Man in the Arena, a talk show. “Second, children were dying across the country, and that made people sit up and take notice.”
Mr. Berliner, who knows two people who’ve died from COVID and who received the vaccine early, also believes too many political leaders are still fueling falsehoods that are giving unvaccinated Americans a license to refuse the shot.
“We are also here because governments and officials spend too little time being brutally honest, choosing instead to dance around issues with soft words,” he said. “The first words out of their mouths should have been: ‘What we are doing is trying to save lives. Help us save your life and that of everyone else.’ Would it have made a difference? We will never know.”
Shon Neyland, senior pastor at the Highland Christian Center church in Portland, Ore., said vaccine tensions have divided his congregation, with about half refusing the shot by his estimation. But he said it’s important to understand why some are making that choice, rather than rage at them and hammer home the benefits of the shot.
Many vaccine holdouts don’t trust the government or medical establishment or have bought into political arguments against the shot, he says. Some conservative evangelicals are also swayed by spiritual beliefs that COVID-19 is a sign of “biblical end-times prophesies” and the vaccine is “the mark of the beast.”
But he has tried to counter those beliefs and biases, arguing they are false and unfounded, urging members of his church to get the vaccine, and partnering with local health officials to run clinics to deliver it.
“I gently try to show them that the vaccine is for our own good and, in fact, is a blessing from God, and it’s up to us to accept the blessing [so] we can get back to somewhat of normalcy,” said Mr. Neyland, author of “The Courage to Stand: A New America.”
“I also believe that to get a vaccine this quick, this was nothing short of a miracle to turn the tide so quickly. Now, for us to resist, it would cause us to continue to suffer and lose lives. And you can’t turn away from the lives that have already been lost.”
Mr. Hyman fears we may not have seen the worst of the pandemic and that the Delta variant won’t be the last or most virulent mutation to emerge.
“The number of unvaccinated people is allowing this virus to continue circulating in the community,” he noted. “And while I have a tremendous amount of confidence that the vaccine protects me now from Delta, I have less confidence that it’s going to protect me from whatever [variant] comes next.
“So, I have a tremendous amount of concern for my own health and safety and welfare, and that of the people that I love. But I’m also concerned about what’s it going to do to businesses [and] the economy. Are we going to have more shutdowns if cases continue trending up? I’m very concerned as to what this could do [to] the country.”
A version of this article first appeared on WebMD.com.
Outraged at vaccine-hesitant people, some are even calling for mandates requiring all Americans to get inoculated, arguing the holdouts are allowing the Delta coronavirus variant to gain traction and reverse the progress the United States was making against the virus.
“I am angry, I am resentful, and I think it’s a fair and appropriate response,” said Jonathan Hyman, a Berea, Ohio, attorney who blames the unvaccinated for the backslide in pandemic progress.
Mr. Hyman has been following the difficult guidelines health experts have been urging from the beginning. He has been masking up, avoiding large gatherings, postponing travel, and he signed up to receive the vaccine as soon as it was available.
“We have been responsible, I did everything I was supposed to do,” said Mr. Hyman, 48, who didn’t visit his parents for 18 months to keep them safe. “Yet here we are, 16, 17 months later, and it feels like we’re in the exact same place we were last summer, and it’s all because some people refuse to do the responsible things they were told to do.”
James Simmons, a retired South Florida high school finance teacher, is also angered by the vaccine holdouts, citing new spikes in COVID-19 infections, hospitalization rates, and deaths across the country – nearly all of which are among unvaccinated people.
“I can’t fathom the fact that people have seen over 600,000 Americans die from COVID, yet are resistant to a vaccine that provides direct protection for themselves and others,” said Mr. Simmons, 63, who received the shot early. “Their irresponsible decision is an affront to those of us who are vaccinated and still wear masks for the benefit of our society.”
Melissa Martin, an Atlanta resident who contracted a serious case of COVID-19 in September 2020, says it is “perplexing and frustrating” that so many Americans are refusing the vaccine. She believes the anger so many vaccinated people feel is tied to fear.
“I believe at the core of this anger is a fear of losing the ones we love,” said Ms. Martin, 55, who has been vaccinated, as has her fiancé, Shane McGeehin. “I was very angry last year after contracting COVID. The experience of having COVID was negative physically, emotionally, and socially.”
She recalled arguing with friends and relatives who downplayed how severe the virus was and who still refuse vaccination, despite seeing how COVID affected her.
“I am trying to understand why they feel the way they do,” she said, “but I would describe the emotions I have now towards those who do not get the vaccine as frustration, confusion, and disbelief.”
Leana Wen, MD, an emergency medicine doctor and public health policy professor at George Washington University, said such sentiments are common and justified.
“I understand that feeling of frustration and anger, because it is the unvaccinated who are setting back the progress that we’ve made [because of] the many sacrifices that many people have undergone,” said Dr. Wen, author of the newly published book “Lifelines: A Doctor’s Journey in the Fight for Public Health.”
“I think it is appropriate for the vaccinated to feel like they’re being punished right now,” she said. “We as a country had the opportunity to beat this virus – to return to prepandemic normal [life] and have our kids go back to school without worrying about coronavirus and our economy fully recovering. We came so close to achieving this, but we didn’t, and now COVID-19 is surging again. The vaccinated are having to pay the price for the choices that some have made to not end this pandemic.”
COVID rising, driving anger
The rising anger among vaccinated Americans comes as health officials are reporting huge spikes in new cases, hospitalizations, and deaths. Meanwhile, only about half of all Americans fully vaccinated, according to the Centers for Disease Control and Prevention.
Per Aug. 6 estimates from the CDC, the nation is averaging more than 100,000 new cases every day – the highest levels seen since February.
Southern states, with the lowest vaccination rates in the country, have been particularly hard-hit. Florida and Louisiana recently set 7-day records for new cases and hospitalizations, beating previous peaks last summer. Those two states, along with Mississippi, North Carolina, South Carolina, Tennessee, Kentucky, and Georgia, account for 41% of all new COVID-19 hospitalizations in the country, according to the CDC.
“It’s time to start blaming the unvaccinated folks, not the regular folks,” an angry Gov. Kay Ivey (R) of Alabama, told reporters. “It’s the unvaccinated folks that are letting us down.”
In response to the resurgence in cases, President Joe Biden has ordered new vaccine mandates for millions of federal workers.
California started requiring health care professionals to be vaccinated in August 2021, removing the option for unvaccinated employees to submit to regular testing.
New York City became the first in the country to require proof of vaccination for all workers and customers to enter restaurants, gyms, concert halls, movie theaters, and Broadway venues.
Nearly 60 major medical organizations, including the American Medical Association and the American Nurses Association, have called for mandatory vaccination of all health care workers.
Meanwhile, many businesses are requiring workers to be vaccinated before returning to offices and other workplaces. Colleges across the country are mandating the shots for students and staff. And some states and cities are also returning to mask mandates, including Hawaii; Louisiana; Washington, D.C.; San Francisco; and Los Angeles.
Experts say the 90 million unvaccinated Americans are most at risk from COVID and have helped the new Delta variant gain a foothold and spread, posing a risk of “breakthrough” cases even in vaccinated people.
Delta is more contagious and causes more severe disease than other known variants of the virus, according to the CDC. It is also more contagious than the viruses that cause Middle East respiratory syndrome, severe acute respiratory syndrome, Ebola, the common cold, flu, and smallpox
Calls for mandates grow
With Delta helping to drive new spikes in COVID cases, some vaccinated Americans argue that the federal government should be taking a harder line with holdouts. Others have even advocated withholding government stimulus checks or tax credits from vaccine refusers and cutting federal funding to states that don’t meet vaccine targets.
Eric Jaffe, a creative writer and producer from Florida who is vaccinated, said he would like to see government agencies and private businesses do more to put pressure on unvaccinated Americans to get the shot.
“In the interest of public safety, I believe the government and private businesses need to [make] life difficult for the unvaccinated,” said Mr. Jaffe, 29, whose parents both contracted the virus but recovered. “They should not be allowed to dine at restaurants, ride public transportation, attend concerts, or broadly be in spaces with large concentrations of people without passing a COVID test at the door.
“They’ll stand in long lines and be inconvenienced at every turn, while vaccinated people get to fly through security, TSA PreCheck-style. The holdouts at [this] point are beyond convincing. The vaccinated should be able to return to a level of normalcy, and the unvaccinated should face restrictions. Any other dynamic puts the stress on citizens who did the right thing.”
Elif Akcali, 49, who teaches engineering at the University of Florida, Gainesville, worries that the rights of people who refuse the vaccine are being put ahead of those of vaccinated people. She’s also concerned for people who face greater COVID risks, including health care workers and children too young to be inoculated.
“Each infection is an opportunity for the virus to evolve into a stronger version in itself,” said Ms. Akcali, who felt such a sense of relief when she received her vaccination that she teared up. “Each hospitalization is an unnecessary burden to health care workers and the system. Each death brings heartbreak to someone in their circle.”
Ed Berliner, an Emmy Award–winning broadcast journalist and Florida-based media specialist, blames social media for spreading misinformation that has taken root with unvaccinated Americans.
“When America rallied together to combat polio, there were two things we didn’t have. One was a lack of the sewer-dwelling, troll-infested social media, which has become the main source of news for the less intelligent and arrogant,” said Mr. Berliner, CEO of Entourage Media and host of The Man in the Arena, a talk show. “Second, children were dying across the country, and that made people sit up and take notice.”
Mr. Berliner, who knows two people who’ve died from COVID and who received the vaccine early, also believes too many political leaders are still fueling falsehoods that are giving unvaccinated Americans a license to refuse the shot.
“We are also here because governments and officials spend too little time being brutally honest, choosing instead to dance around issues with soft words,” he said. “The first words out of their mouths should have been: ‘What we are doing is trying to save lives. Help us save your life and that of everyone else.’ Would it have made a difference? We will never know.”
Shon Neyland, senior pastor at the Highland Christian Center church in Portland, Ore., said vaccine tensions have divided his congregation, with about half refusing the shot by his estimation. But he said it’s important to understand why some are making that choice, rather than rage at them and hammer home the benefits of the shot.
Many vaccine holdouts don’t trust the government or medical establishment or have bought into political arguments against the shot, he says. Some conservative evangelicals are also swayed by spiritual beliefs that COVID-19 is a sign of “biblical end-times prophesies” and the vaccine is “the mark of the beast.”
But he has tried to counter those beliefs and biases, arguing they are false and unfounded, urging members of his church to get the vaccine, and partnering with local health officials to run clinics to deliver it.
“I gently try to show them that the vaccine is for our own good and, in fact, is a blessing from God, and it’s up to us to accept the blessing [so] we can get back to somewhat of normalcy,” said Mr. Neyland, author of “The Courage to Stand: A New America.”
“I also believe that to get a vaccine this quick, this was nothing short of a miracle to turn the tide so quickly. Now, for us to resist, it would cause us to continue to suffer and lose lives. And you can’t turn away from the lives that have already been lost.”
Mr. Hyman fears we may not have seen the worst of the pandemic and that the Delta variant won’t be the last or most virulent mutation to emerge.
“The number of unvaccinated people is allowing this virus to continue circulating in the community,” he noted. “And while I have a tremendous amount of confidence that the vaccine protects me now from Delta, I have less confidence that it’s going to protect me from whatever [variant] comes next.
“So, I have a tremendous amount of concern for my own health and safety and welfare, and that of the people that I love. But I’m also concerned about what’s it going to do to businesses [and] the economy. Are we going to have more shutdowns if cases continue trending up? I’m very concerned as to what this could do [to] the country.”
A version of this article first appeared on WebMD.com.
Anaplasmosis quadruples in New York state
Anaplasmosis prevalence in New York state nearly quadrupled statewide from 2010 to 2018, new research suggests, increasing by more than eightfold in the region surrounding Albany, the state capital. The proportion of ticks carrying Anaplasma phagocytophilum, the bacterium that causes the tick-borne disease, also increased during the study period.
Although not as well-recognized as Lyme disease, anaplasmosis is one of the most common tickborne diseases in the United States. The bacterial disease is primarily transmitted to humans by the bites of blacklegged ticks infected with A. phagocytophilum, and often causes fever, headache, muscle aches, and chills. If treatment is delayed – or if a patient has underlying medical conditions – anaplasmosis can lead to difficulty breathing, bleeding problems, organ failure, and even death.
Since anaplasmosis become a nationally notifiable disease in 1999, cases have increased 16-fold in the United States, from 351 cases in 2000 to a high of 5,762 cases in 2017, according to data from the Centers for Disease Control and Prevention. Just eight states – Vermont, Maine, Rhode Island, Minnesota, Massachusetts, Wisconsin, New Hampshire, and New York – make up 90% of reported cases.
“While Lyme disease remains the most common tick-borne illness reported in New York state, anaplasmosis continues to account for a growing proportion of our tick-borne disease cases each year,” Melissa Prusinski, a research scientist at the New York State Department of Health and author of the study, told this news organization in an email. “It is critically important to investigate the environmental and epidemiological drivers facilitating this increase to better understand why and how risk for this serious illness is increasing.” The results were published in Emerging Infectious Diseases.
For the study, investigators analyzed human anaplasmosis cases reported to the New York State Department of Health from 2010-2018. They also included data from tick collection and pathogen testing in order to determine whether the prevalence of A. phagocytophilum in ticks increased along with cases. All New York State counties were included in the study, apart from the five boroughs of New York City: Manhattan, Brooklyn, the Bronx, Queens, and Staten Island.
There were 5,146 reported anaplasmosis cases in New York, with annual case numbers peaking at 1,112 in 2017. Researchers reported a dip in cases in 2018, a trend that was also seen nationally. Anaplasmosis incidence surged in the area surrounding Albany, increasing 8.4-fold from 4.3 cases per 100,000 people in 2010 to 36.3 cases per 100,000 persons in 2018.
Ms. Prusinski noted that the rapid increase in and around this inland hot spot is unlike the gradual spread of Lyme disease and other tick-borne illnesses like babesiosis, which spreads from coastal areas both northward and westward across New York. The research team also found that the incidence of ticks infected with A. phagocytophilum nearly doubled statewide and increased fourfold – from 2.9% to 12% – between 2010 and 2018 in the Albany area.
This increase in cases could be the result, at least in part, of more robust testing efforts over time, said Susan Elias, PhD, of the Vector-Borne Disease Laboratory at the Maine Medical Center Research Institute in Scarborough. She was not involved with the recently published study. “The more you look for something, the more you find,” she said. For example, she added, a 602% surge in anaplasmosis cases in Maine from 2013-2017 occurred alongside a 10-fold increase in use of tick-borne disease panels that test for multiple pathogens.
Ms. Prusinski agreed that increased testing at least partially explains the surge of cases in New York, but she did not have data on how many tick-borne disease panels were used to diagnose cases in the state.
Proliferation of A. phagocytophilum in tick populations could also partially explain this dramatic increase in cases. With the suburbanization of America, “we have basically laid out a buffet” for ticks, Dr. Elias said. Patches of forest and yards create edge habitats where ticks, and the small mammals they feed on, thrive. “Then, once you have a large expanding blacklegged tick population, it makes it easier for the pathogens and carriers to amplify,” she added.
While the study did not differentiate between a variant of A. phagocytophilum associated with small mammals that causes illness and another found in white-tailed deer that is nonpathogenic, Ms. Prusinski suspects that the infectious variant is likely more prevalent and is circulating in animals and ticks in and around Albany. Research is ongoing to see if this could help explain the spread of disease in this anaplasmosis hotspot.
“The unique geographic pattern of anaplasmosis spread in New York state and elsewhere leads to many further questions about the vector ecology and epidemiology of this emerging tick-borne illness,” Ms. Prusinski added. “Learning all we can about this dynamic disease system will help us better identify at-risk populations and may lead to novel ways to prevent anaplasmosis.”
Dr. Elias and Ms. Prusinski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anaplasmosis prevalence in New York state nearly quadrupled statewide from 2010 to 2018, new research suggests, increasing by more than eightfold in the region surrounding Albany, the state capital. The proportion of ticks carrying Anaplasma phagocytophilum, the bacterium that causes the tick-borne disease, also increased during the study period.
Although not as well-recognized as Lyme disease, anaplasmosis is one of the most common tickborne diseases in the United States. The bacterial disease is primarily transmitted to humans by the bites of blacklegged ticks infected with A. phagocytophilum, and often causes fever, headache, muscle aches, and chills. If treatment is delayed – or if a patient has underlying medical conditions – anaplasmosis can lead to difficulty breathing, bleeding problems, organ failure, and even death.
Since anaplasmosis become a nationally notifiable disease in 1999, cases have increased 16-fold in the United States, from 351 cases in 2000 to a high of 5,762 cases in 2017, according to data from the Centers for Disease Control and Prevention. Just eight states – Vermont, Maine, Rhode Island, Minnesota, Massachusetts, Wisconsin, New Hampshire, and New York – make up 90% of reported cases.
“While Lyme disease remains the most common tick-borne illness reported in New York state, anaplasmosis continues to account for a growing proportion of our tick-borne disease cases each year,” Melissa Prusinski, a research scientist at the New York State Department of Health and author of the study, told this news organization in an email. “It is critically important to investigate the environmental and epidemiological drivers facilitating this increase to better understand why and how risk for this serious illness is increasing.” The results were published in Emerging Infectious Diseases.
For the study, investigators analyzed human anaplasmosis cases reported to the New York State Department of Health from 2010-2018. They also included data from tick collection and pathogen testing in order to determine whether the prevalence of A. phagocytophilum in ticks increased along with cases. All New York State counties were included in the study, apart from the five boroughs of New York City: Manhattan, Brooklyn, the Bronx, Queens, and Staten Island.
There were 5,146 reported anaplasmosis cases in New York, with annual case numbers peaking at 1,112 in 2017. Researchers reported a dip in cases in 2018, a trend that was also seen nationally. Anaplasmosis incidence surged in the area surrounding Albany, increasing 8.4-fold from 4.3 cases per 100,000 people in 2010 to 36.3 cases per 100,000 persons in 2018.
Ms. Prusinski noted that the rapid increase in and around this inland hot spot is unlike the gradual spread of Lyme disease and other tick-borne illnesses like babesiosis, which spreads from coastal areas both northward and westward across New York. The research team also found that the incidence of ticks infected with A. phagocytophilum nearly doubled statewide and increased fourfold – from 2.9% to 12% – between 2010 and 2018 in the Albany area.
This increase in cases could be the result, at least in part, of more robust testing efforts over time, said Susan Elias, PhD, of the Vector-Borne Disease Laboratory at the Maine Medical Center Research Institute in Scarborough. She was not involved with the recently published study. “The more you look for something, the more you find,” she said. For example, she added, a 602% surge in anaplasmosis cases in Maine from 2013-2017 occurred alongside a 10-fold increase in use of tick-borne disease panels that test for multiple pathogens.
Ms. Prusinski agreed that increased testing at least partially explains the surge of cases in New York, but she did not have data on how many tick-borne disease panels were used to diagnose cases in the state.
Proliferation of A. phagocytophilum in tick populations could also partially explain this dramatic increase in cases. With the suburbanization of America, “we have basically laid out a buffet” for ticks, Dr. Elias said. Patches of forest and yards create edge habitats where ticks, and the small mammals they feed on, thrive. “Then, once you have a large expanding blacklegged tick population, it makes it easier for the pathogens and carriers to amplify,” she added.
While the study did not differentiate between a variant of A. phagocytophilum associated with small mammals that causes illness and another found in white-tailed deer that is nonpathogenic, Ms. Prusinski suspects that the infectious variant is likely more prevalent and is circulating in animals and ticks in and around Albany. Research is ongoing to see if this could help explain the spread of disease in this anaplasmosis hotspot.
“The unique geographic pattern of anaplasmosis spread in New York state and elsewhere leads to many further questions about the vector ecology and epidemiology of this emerging tick-borne illness,” Ms. Prusinski added. “Learning all we can about this dynamic disease system will help us better identify at-risk populations and may lead to novel ways to prevent anaplasmosis.”
Dr. Elias and Ms. Prusinski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anaplasmosis prevalence in New York state nearly quadrupled statewide from 2010 to 2018, new research suggests, increasing by more than eightfold in the region surrounding Albany, the state capital. The proportion of ticks carrying Anaplasma phagocytophilum, the bacterium that causes the tick-borne disease, also increased during the study period.
Although not as well-recognized as Lyme disease, anaplasmosis is one of the most common tickborne diseases in the United States. The bacterial disease is primarily transmitted to humans by the bites of blacklegged ticks infected with A. phagocytophilum, and often causes fever, headache, muscle aches, and chills. If treatment is delayed – or if a patient has underlying medical conditions – anaplasmosis can lead to difficulty breathing, bleeding problems, organ failure, and even death.
Since anaplasmosis become a nationally notifiable disease in 1999, cases have increased 16-fold in the United States, from 351 cases in 2000 to a high of 5,762 cases in 2017, according to data from the Centers for Disease Control and Prevention. Just eight states – Vermont, Maine, Rhode Island, Minnesota, Massachusetts, Wisconsin, New Hampshire, and New York – make up 90% of reported cases.
“While Lyme disease remains the most common tick-borne illness reported in New York state, anaplasmosis continues to account for a growing proportion of our tick-borne disease cases each year,” Melissa Prusinski, a research scientist at the New York State Department of Health and author of the study, told this news organization in an email. “It is critically important to investigate the environmental and epidemiological drivers facilitating this increase to better understand why and how risk for this serious illness is increasing.” The results were published in Emerging Infectious Diseases.
For the study, investigators analyzed human anaplasmosis cases reported to the New York State Department of Health from 2010-2018. They also included data from tick collection and pathogen testing in order to determine whether the prevalence of A. phagocytophilum in ticks increased along with cases. All New York State counties were included in the study, apart from the five boroughs of New York City: Manhattan, Brooklyn, the Bronx, Queens, and Staten Island.
There were 5,146 reported anaplasmosis cases in New York, with annual case numbers peaking at 1,112 in 2017. Researchers reported a dip in cases in 2018, a trend that was also seen nationally. Anaplasmosis incidence surged in the area surrounding Albany, increasing 8.4-fold from 4.3 cases per 100,000 people in 2010 to 36.3 cases per 100,000 persons in 2018.
Ms. Prusinski noted that the rapid increase in and around this inland hot spot is unlike the gradual spread of Lyme disease and other tick-borne illnesses like babesiosis, which spreads from coastal areas both northward and westward across New York. The research team also found that the incidence of ticks infected with A. phagocytophilum nearly doubled statewide and increased fourfold – from 2.9% to 12% – between 2010 and 2018 in the Albany area.
This increase in cases could be the result, at least in part, of more robust testing efforts over time, said Susan Elias, PhD, of the Vector-Borne Disease Laboratory at the Maine Medical Center Research Institute in Scarborough. She was not involved with the recently published study. “The more you look for something, the more you find,” she said. For example, she added, a 602% surge in anaplasmosis cases in Maine from 2013-2017 occurred alongside a 10-fold increase in use of tick-borne disease panels that test for multiple pathogens.
Ms. Prusinski agreed that increased testing at least partially explains the surge of cases in New York, but she did not have data on how many tick-borne disease panels were used to diagnose cases in the state.
Proliferation of A. phagocytophilum in tick populations could also partially explain this dramatic increase in cases. With the suburbanization of America, “we have basically laid out a buffet” for ticks, Dr. Elias said. Patches of forest and yards create edge habitats where ticks, and the small mammals they feed on, thrive. “Then, once you have a large expanding blacklegged tick population, it makes it easier for the pathogens and carriers to amplify,” she added.
While the study did not differentiate between a variant of A. phagocytophilum associated with small mammals that causes illness and another found in white-tailed deer that is nonpathogenic, Ms. Prusinski suspects that the infectious variant is likely more prevalent and is circulating in animals and ticks in and around Albany. Research is ongoing to see if this could help explain the spread of disease in this anaplasmosis hotspot.
“The unique geographic pattern of anaplasmosis spread in New York state and elsewhere leads to many further questions about the vector ecology and epidemiology of this emerging tick-borne illness,” Ms. Prusinski added. “Learning all we can about this dynamic disease system will help us better identify at-risk populations and may lead to novel ways to prevent anaplasmosis.”
Dr. Elias and Ms. Prusinski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Flavonoids dietary ‘powerhouses’ for cognitive decline prevention
, new research shows.
Among the different types of flavonoids, flavones (found in some spices and yellow or orange fruits and vegetables) and anthocyanins (found in blueberries, blackberries, and cherries) seem to have most protective effect, the researchers report.
“There is mounting evidence suggesting flavonoids are powerhouses when it comes to preventing your thinking skills from declining as you get older,” study investigator Walter Willett, MD, DrPH, Harvard University, Boston, said in a statement.
“Our results are exciting because they show that making simple changes to your diet could help prevent cognitive decline,” said Dr. Willett.
The study was published online July 28 in the journal Neurology.
Antioxidant punch
Flavonoids, naturally occurring phytochemicals found in plants, are strong antioxidants. Considering the likely role of oxidative stress in age-related cognitive decline, flavonoids have been proposed as a potentially important preventive.
For the study, Dr. Willett and colleagues prospectively examined associations between long-term dietary flavonoids (flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, polymeric flavonoids, and proanthocyanidins) and subjective cognitive decline in 49,493 women from the Nurses’ Health Study (1984-2006) and 27,842 men from the Health Professionals Follow-up Study (1986-2002).
Those in the highest quintile of flavonoid consumption consumed about 600 mg daily on average while those in the lowest quintile got only about 150 mg daily.
After adjusting for age, total energy intake, major nondietary factors, and specific dietary factors, a higher intake of total flavonoids was associated with lower likelihood of self-reported subjective cognitive decline during follow up.
Individuals in the highest quintile of daily consumption had about a 20% lower risk of subjective cognitive decline relative to peers in the lowest quintile (pooled multivariable-adjusted odds ratio: 0.81; 95% confidence interval, 0.76-0.89).
The strongest protective associations were found for flavones (OR, 0.62; 95% confidence interval, 0.57-0.68), flavanones (OR, 0.64; 95% CI, 0.58-0.68), and anthocyanins (OR, 0.76; 95% CI, 0.72-0.84) (P trend < .0001 for all groups).
“The people in our study who did the best over time ate an average of at least half a serving per day of foods like orange juice, oranges, peppers, celery, grapefruits, grapefruit juice, apples, and pears,” Dr. Willett said.
“While it is possible other phytochemicals are at work here, a colorful diet rich in flavonoids – and specifically flavones and anthocyanins – seems to be a good bet for promoting long-term brain health,” he added.
A limitation of the study is that participants reported on their diets and may not recall perfectly what they ate or how much.
Healthy diet best bet for brain health
Reached for comment, Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association, said this study “adds to our understanding of which elements of a healthy diet may be important in reducing dementia risk; flavonols may be one of those elements.”
“However, at this point, people should not put too much stock in specific nutrients – including subsets of flavonols – for reducing dementia risk until more research is done. Rather, they should focus on eating an overall healthy diet,” he said.
“It would be wonderful if a particular food or supplement could delay or prevent Alzheimer’s disease, but we do not have scientific evidence to support such claims. Randomized controlled clinical trials are necessary to evaluate whether any food or supplement has a scientifically proven beneficial effect,” Dr. Weber added.
For now, the Alzheimer’s Association “encourages everyone to eat a healthy and balanced diet as a way to help reduce the risk of cognitive decline,” Dr. Weber said.
“With more than 6 million Americans living with Alzheimer’s disease and other dementia today, there is a pressing need to test the effectiveness of a healthy lifestyle regimen to reduce risk of cognitive decline in a large and diverse population,” he added.
The Alzheimer’s Association has launched a 2-year clinical trial, called the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), to do just that.
“While we research that definitive lifestyle ‘recipe,’ there are things we can do today that may decrease our risk of cognitive decline as we age. Eating a heart-healthy diet, exercising regularly, and staying cognitively engaged are just a few,” Dr. Weber added.
Also weighing in, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said the study results are not surprising.
“Scientific data on the ability of flavonoids to prevent age-related chronic diseases, including cognitive decline, has accumulated immensely over the last decade. This epidemiological study reinforces findings from smaller shorter-duration clinical trials and mechanistic studies,” said Dr. Wallace, who was not involved in the study.
“Flavonoids show great potential in reducing inflammation and oxidative stress in the body. They are also vasodilators that help improve blood flow, which is important for the cardiovascular and cerebrovascular systems,” he noted.
“Typically, foods rich in flavonoids are also nutrient-dense in vitamins, minerals, and dietary fiber (eg, fruits and vegetables). Anthocyanins in blueberries have long been known to prevent cognitive decline with age,” Dr. Wallace said.
Dr. Wallace was part of a 14-member panel of nutrition scientists who recently reviewed available evidence around fruit and vegetable intake and health.
“Our findings are consistent with this study in regard to cognitive decline and other disease states. Cruciferous vegetables, dark-green leafy vegetables, citrus fruits, and dark-colored berries seem to have superior effects on health promotion and disease prevention in general,” said Dr. Wallace.
This work was supported by grants from the National Institutes of Health. The authors have disclosed no relevant financial relationships. Dr. Weber has no relevant disclosures. Dr. Wallace is principal and chief executive officer of the Think Healthy Group; editor of the Journal of Dietary Supplements; and deputy editor-in-chief of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
, new research shows.
Among the different types of flavonoids, flavones (found in some spices and yellow or orange fruits and vegetables) and anthocyanins (found in blueberries, blackberries, and cherries) seem to have most protective effect, the researchers report.
“There is mounting evidence suggesting flavonoids are powerhouses when it comes to preventing your thinking skills from declining as you get older,” study investigator Walter Willett, MD, DrPH, Harvard University, Boston, said in a statement.
“Our results are exciting because they show that making simple changes to your diet could help prevent cognitive decline,” said Dr. Willett.
The study was published online July 28 in the journal Neurology.
Antioxidant punch
Flavonoids, naturally occurring phytochemicals found in plants, are strong antioxidants. Considering the likely role of oxidative stress in age-related cognitive decline, flavonoids have been proposed as a potentially important preventive.
For the study, Dr. Willett and colleagues prospectively examined associations between long-term dietary flavonoids (flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, polymeric flavonoids, and proanthocyanidins) and subjective cognitive decline in 49,493 women from the Nurses’ Health Study (1984-2006) and 27,842 men from the Health Professionals Follow-up Study (1986-2002).
Those in the highest quintile of flavonoid consumption consumed about 600 mg daily on average while those in the lowest quintile got only about 150 mg daily.
After adjusting for age, total energy intake, major nondietary factors, and specific dietary factors, a higher intake of total flavonoids was associated with lower likelihood of self-reported subjective cognitive decline during follow up.
Individuals in the highest quintile of daily consumption had about a 20% lower risk of subjective cognitive decline relative to peers in the lowest quintile (pooled multivariable-adjusted odds ratio: 0.81; 95% confidence interval, 0.76-0.89).
The strongest protective associations were found for flavones (OR, 0.62; 95% confidence interval, 0.57-0.68), flavanones (OR, 0.64; 95% CI, 0.58-0.68), and anthocyanins (OR, 0.76; 95% CI, 0.72-0.84) (P trend < .0001 for all groups).
“The people in our study who did the best over time ate an average of at least half a serving per day of foods like orange juice, oranges, peppers, celery, grapefruits, grapefruit juice, apples, and pears,” Dr. Willett said.
“While it is possible other phytochemicals are at work here, a colorful diet rich in flavonoids – and specifically flavones and anthocyanins – seems to be a good bet for promoting long-term brain health,” he added.
A limitation of the study is that participants reported on their diets and may not recall perfectly what they ate or how much.
Healthy diet best bet for brain health
Reached for comment, Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association, said this study “adds to our understanding of which elements of a healthy diet may be important in reducing dementia risk; flavonols may be one of those elements.”
“However, at this point, people should not put too much stock in specific nutrients – including subsets of flavonols – for reducing dementia risk until more research is done. Rather, they should focus on eating an overall healthy diet,” he said.
“It would be wonderful if a particular food or supplement could delay or prevent Alzheimer’s disease, but we do not have scientific evidence to support such claims. Randomized controlled clinical trials are necessary to evaluate whether any food or supplement has a scientifically proven beneficial effect,” Dr. Weber added.
For now, the Alzheimer’s Association “encourages everyone to eat a healthy and balanced diet as a way to help reduce the risk of cognitive decline,” Dr. Weber said.
“With more than 6 million Americans living with Alzheimer’s disease and other dementia today, there is a pressing need to test the effectiveness of a healthy lifestyle regimen to reduce risk of cognitive decline in a large and diverse population,” he added.
The Alzheimer’s Association has launched a 2-year clinical trial, called the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), to do just that.
“While we research that definitive lifestyle ‘recipe,’ there are things we can do today that may decrease our risk of cognitive decline as we age. Eating a heart-healthy diet, exercising regularly, and staying cognitively engaged are just a few,” Dr. Weber added.
Also weighing in, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said the study results are not surprising.
“Scientific data on the ability of flavonoids to prevent age-related chronic diseases, including cognitive decline, has accumulated immensely over the last decade. This epidemiological study reinforces findings from smaller shorter-duration clinical trials and mechanistic studies,” said Dr. Wallace, who was not involved in the study.
“Flavonoids show great potential in reducing inflammation and oxidative stress in the body. They are also vasodilators that help improve blood flow, which is important for the cardiovascular and cerebrovascular systems,” he noted.
“Typically, foods rich in flavonoids are also nutrient-dense in vitamins, minerals, and dietary fiber (eg, fruits and vegetables). Anthocyanins in blueberries have long been known to prevent cognitive decline with age,” Dr. Wallace said.
Dr. Wallace was part of a 14-member panel of nutrition scientists who recently reviewed available evidence around fruit and vegetable intake and health.
“Our findings are consistent with this study in regard to cognitive decline and other disease states. Cruciferous vegetables, dark-green leafy vegetables, citrus fruits, and dark-colored berries seem to have superior effects on health promotion and disease prevention in general,” said Dr. Wallace.
This work was supported by grants from the National Institutes of Health. The authors have disclosed no relevant financial relationships. Dr. Weber has no relevant disclosures. Dr. Wallace is principal and chief executive officer of the Think Healthy Group; editor of the Journal of Dietary Supplements; and deputy editor-in-chief of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
, new research shows.
Among the different types of flavonoids, flavones (found in some spices and yellow or orange fruits and vegetables) and anthocyanins (found in blueberries, blackberries, and cherries) seem to have most protective effect, the researchers report.
“There is mounting evidence suggesting flavonoids are powerhouses when it comes to preventing your thinking skills from declining as you get older,” study investigator Walter Willett, MD, DrPH, Harvard University, Boston, said in a statement.
“Our results are exciting because they show that making simple changes to your diet could help prevent cognitive decline,” said Dr. Willett.
The study was published online July 28 in the journal Neurology.
Antioxidant punch
Flavonoids, naturally occurring phytochemicals found in plants, are strong antioxidants. Considering the likely role of oxidative stress in age-related cognitive decline, flavonoids have been proposed as a potentially important preventive.
For the study, Dr. Willett and colleagues prospectively examined associations between long-term dietary flavonoids (flavonols, flavones, flavanones, flavan-3-ols, anthocyanins, polymeric flavonoids, and proanthocyanidins) and subjective cognitive decline in 49,493 women from the Nurses’ Health Study (1984-2006) and 27,842 men from the Health Professionals Follow-up Study (1986-2002).
Those in the highest quintile of flavonoid consumption consumed about 600 mg daily on average while those in the lowest quintile got only about 150 mg daily.
After adjusting for age, total energy intake, major nondietary factors, and specific dietary factors, a higher intake of total flavonoids was associated with lower likelihood of self-reported subjective cognitive decline during follow up.
Individuals in the highest quintile of daily consumption had about a 20% lower risk of subjective cognitive decline relative to peers in the lowest quintile (pooled multivariable-adjusted odds ratio: 0.81; 95% confidence interval, 0.76-0.89).
The strongest protective associations were found for flavones (OR, 0.62; 95% confidence interval, 0.57-0.68), flavanones (OR, 0.64; 95% CI, 0.58-0.68), and anthocyanins (OR, 0.76; 95% CI, 0.72-0.84) (P trend < .0001 for all groups).
“The people in our study who did the best over time ate an average of at least half a serving per day of foods like orange juice, oranges, peppers, celery, grapefruits, grapefruit juice, apples, and pears,” Dr. Willett said.
“While it is possible other phytochemicals are at work here, a colorful diet rich in flavonoids – and specifically flavones and anthocyanins – seems to be a good bet for promoting long-term brain health,” he added.
A limitation of the study is that participants reported on their diets and may not recall perfectly what they ate or how much.
Healthy diet best bet for brain health
Reached for comment, Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association, said this study “adds to our understanding of which elements of a healthy diet may be important in reducing dementia risk; flavonols may be one of those elements.”
“However, at this point, people should not put too much stock in specific nutrients – including subsets of flavonols – for reducing dementia risk until more research is done. Rather, they should focus on eating an overall healthy diet,” he said.
“It would be wonderful if a particular food or supplement could delay or prevent Alzheimer’s disease, but we do not have scientific evidence to support such claims. Randomized controlled clinical trials are necessary to evaluate whether any food or supplement has a scientifically proven beneficial effect,” Dr. Weber added.
For now, the Alzheimer’s Association “encourages everyone to eat a healthy and balanced diet as a way to help reduce the risk of cognitive decline,” Dr. Weber said.
“With more than 6 million Americans living with Alzheimer’s disease and other dementia today, there is a pressing need to test the effectiveness of a healthy lifestyle regimen to reduce risk of cognitive decline in a large and diverse population,” he added.
The Alzheimer’s Association has launched a 2-year clinical trial, called the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), to do just that.
“While we research that definitive lifestyle ‘recipe,’ there are things we can do today that may decrease our risk of cognitive decline as we age. Eating a heart-healthy diet, exercising regularly, and staying cognitively engaged are just a few,” Dr. Weber added.
Also weighing in, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said the study results are not surprising.
“Scientific data on the ability of flavonoids to prevent age-related chronic diseases, including cognitive decline, has accumulated immensely over the last decade. This epidemiological study reinforces findings from smaller shorter-duration clinical trials and mechanistic studies,” said Dr. Wallace, who was not involved in the study.
“Flavonoids show great potential in reducing inflammation and oxidative stress in the body. They are also vasodilators that help improve blood flow, which is important for the cardiovascular and cerebrovascular systems,” he noted.
“Typically, foods rich in flavonoids are also nutrient-dense in vitamins, minerals, and dietary fiber (eg, fruits and vegetables). Anthocyanins in blueberries have long been known to prevent cognitive decline with age,” Dr. Wallace said.
Dr. Wallace was part of a 14-member panel of nutrition scientists who recently reviewed available evidence around fruit and vegetable intake and health.
“Our findings are consistent with this study in regard to cognitive decline and other disease states. Cruciferous vegetables, dark-green leafy vegetables, citrus fruits, and dark-colored berries seem to have superior effects on health promotion and disease prevention in general,” said Dr. Wallace.
This work was supported by grants from the National Institutes of Health. The authors have disclosed no relevant financial relationships. Dr. Weber has no relevant disclosures. Dr. Wallace is principal and chief executive officer of the Think Healthy Group; editor of the Journal of Dietary Supplements; and deputy editor-in-chief of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Exploring the Utility of Artificial Intelligence During COVID-19 in Dermatology Practice
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
With the need to adapt to the given challenges associated with COVID-19, artificial intelligence (AI) serves as a potential tool in providing access to medical-based diagnosis in a novel way. Artificial intelligence is defined as intelligence harnessed by machines that have the ability to perform what is called cognitive thinking and to mimic the problem-solving abilities of the human mind. Virtual AI in dermatology entails neural network–based guidance that includes developing algorithms to detect skin pathology through photographs.1 To use AI in dermatology, recognition of visual patterns must be established to give diagnoses. These neural networks have been used to classify skin diseases, including cancer, actinic keratosis, and warts.2
AI for Skin Cancer
The use of AI to classify melanoma and nonmelanoma skin cancer has been studied extensively, including the following 2 research projects.
Convolutional Neural Network
In 2017, Stanford University published a study in which a deep-learning algorithm known as a convolutional neural network was used to classify skin lesions.3 The network was trained using a dataset of 129,450 clinical images of 2032 diseases. Its performance was compared to that of 21 board-certified dermatologists on biopsy-proven clinical images with 2 classifications of cases: (1) keratinocyte carcinoma as opposed to benign seborrheic keratosis and (2) malignant melanoma as opposed to benign nevi—the first representing the most common skin cancers, and the second, the deadliest skin cancers. The study showed that the machine could accurately identify and classify skin cancers compared to the work of board-certified dermatologists. The study did not include demographic information, which limits its external validity.3
Dermoscopic Image Classification
A 2019 study by Brinker and colleagues4 showed the superiority of automated dermoscopic melanoma image classifications compared to the work of board-certified dermatologists. For the study, 804 biopsy-proven images of melanoma and nevi (1:1 ratio) were randomly presented to dermatologists for their evaluation and recommended treatment (yielding 19,296 recommendations). The dermatologists classified the lesions with a sensitivity of 67.2% and specificity of 62.2%; the trained convolutional neural network attained both higher sensitivity (82.3%) and higher specificity (77.9%).4
Smartphone Diagnosis of Melanoma
An application of AI has been to use smartphone apps for the diagnosis of melanoma. The most utilized and novel algorithm-based smartphone app that assesses skin lesions for malignancy characteristics is SkinVision. With a simple download from Apple’s App Store, this technology allows a person to check their skin spots by taking a photograph and receiving algorithmic risk-assessment feedback. This inexpensive software ($51.78 a year) also allows a patient’s physician to assess the photograph and then validate their assessment by comparing it with the algorithmic analysis that the program provides.5
A review of SkinVision conducted by Thissen and colleagues6 found that, in a hypothetical population of 1000 adults of whom 3% actually had melanoma, 4 of those 30 people would not have been flagged as at “high risk” by SkinVision. There also was a high false-positive rate with the app, with more than 200 people flagged as at high risk. The analysis pegged SkinVision as having a sensitivity of 88% and specificity of 79%.6
In summary, systematic review of diagnostic accuracy has shown that, although there is accuracy in AI analyses, it should be used only as a guide for health care advice due to variability in algorithm performance.7
Utility of AI in Telehealth
Artificial intelligence algorithms could be created to ensure telehealth image accuracy, stratify risk, and track patient progress. With teledermatology visits on the rise during the COVID-19 pandemic, AI algorithms could ensure that photographs of appropriate quality are taken. Also, patients could be organized by risk factors with such algorithms, allowing physicians to save time on triage and stratification. Algorithms also could be used to track a telehealth patient’s treatment and progress.8
Furthermore, there is a need for an algorithm that has the ability to detect, quantify, and monitor changes in dermatologic conditions using images that patients have uploaded. This capability will lead to creation of a standardized quantification scale that will allow physicians to virtually track the progression of visible skin pathologies.
Hazards of Racial Bias in AI
Artificial intelligence is limited by racial disparity bias seen in computerized medicine. For years, the majority of dermatology research, especially in skin cancer, has been conducted on fairer-skinned populations. This bias has existed at the expense of darker-skinned patients, whose skin conditions and symptoms present differently,9 and reflects directly in available data sets that can be used to develop AI algorithms. Because these data are inadequate to the task, AI might misdiagnose skin cancer in people of color or miss an existing condition entirely.10 Consequently, the higher rate of skin cancer mortality that is reported in people of color is likely to persist with the rise of AI in dermatology.11 A more representative database of imaged skin lesions needs to be utilized to create a diversely representative and applicable data set for AI algorithms.12
Benefits of Conversational Agents
Another method by which AI could be incorporated into dermatology is through what is known as a conversational agent (CA)—AI software that engages in a dialogue with users by interpreting their voice and replying to them through text, image, or voice.13 Conversational agents facilitate remote patient management, allow clinicians to focus on other functions, and aid in data collection.14 A 2014 study showed that patients were significantly more likely to disclose history and emotions when informed they were interacting with a CA than with a human clinician (P=.007).15 Such benefits could be invaluable in dermatology, where emotions and patient perceptions of skin conditions play into the treatment process.
However, some evidence showed that CAs cannot respond to patients’ statements in all circumstances.16 It also is unclear how well CAs recognize nuanced statements that might signal potential harm. This fits into the greater theme of a major problem with AI: the lack of a reliable response in all circumstances.13
Final Thoughts
The practical implementations of AI in dermatology are still being explored. Given the uncertainty surrounding the COVID-19 pandemic and the future of patient care, AI might serve as an important asset in assisting with the diagnosis and treatment of dermatologic conditions, physician productivity, and patient monitoring.
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
- Amisha, Malik P, Pathania M, et al. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328-2331. doi:10.4103/jfmpc.jfmpc_440_19
- Han SS, Kim MS, Lim W, et al. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-1538. doi:10.1016/j.jid.2018.01.028
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. doi:10.1038/nature21056
- Brinker TJ, Hekler A, Enk AH, et al. Deep neural networks are superior to dermatologists in melanoma image classification. Eur J Cancer. 2019;119:11-17. doi:10.1016/j.ejca.2019.05.023
- Regulated medical device for detecting skin cancer. SkinVision website. Accessed July 23, 2021. https://www.skinvision.com/hcp/
- Thissen M, Udrea A, Hacking M, et al. mHealth app for risk assessment of pigmented and nonpigmented skin lesions—a study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-954. doi:10.1089/tmj.2016.0259
- Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi:10.1136/bmj.m127
- Puri P, Comfere N, Pittelkow MR, et al. COVID-19: an opportunity to build dermatology’s digital future. Dermatol Ther. 2020;33:e14149. doi:10.1111/dth.14149
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-1248. doi:10.1001/jamadermatol.2018.2348
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:S0190-9622(13)01296-6
- Alabdulkareem A. Artificial intelligence and dermatologists: friends or foes? J Dermatol Dermatolog Surg. 2019;23:57-60. doi:10.4103/jdds.jdds_19_19
- McGreevey JD 3rd, Hanson CW 3rd, Koppel R. Clinical, legal, and ethical aspects of artificial intelligence-assisted conversational agents in health care. JAMA. 2020;324:552-553. doi:10.1001/jama.2020.2724
- Piau A, Crissey R, Brechemier D, et al. A smartphone chatbot application to optimize monitoring of older patients with cancer. Int J Med Inform. 2019;128:18-23. doi:10.1016/j.ijmedinf.2019.05.013
- Lucas GM, Gratch J, King A, et al. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94-100. https://doi.org/10.1016/j.chb.2014.04.043
- Miner AS, Milstein A, Schueller S, et al. Smartphone-based conversational agents and responses to questions about mental health, interpersonal violence, and physical health. JAMA Intern Med. 2016;176:619-625. doi:10.1001/jamainternmed.2016.0400
Practice Points
- Dermatologists should amass pictures of dermatologic conditions in skin of color to contribute to growing awareness and knowledge of presentation of disease in this population.
- Dermatologists should use artificial intelligence as a tool for delivering more efficient and beneficial patient care.
Mobile App Usage Among Dermatology Residents in America
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
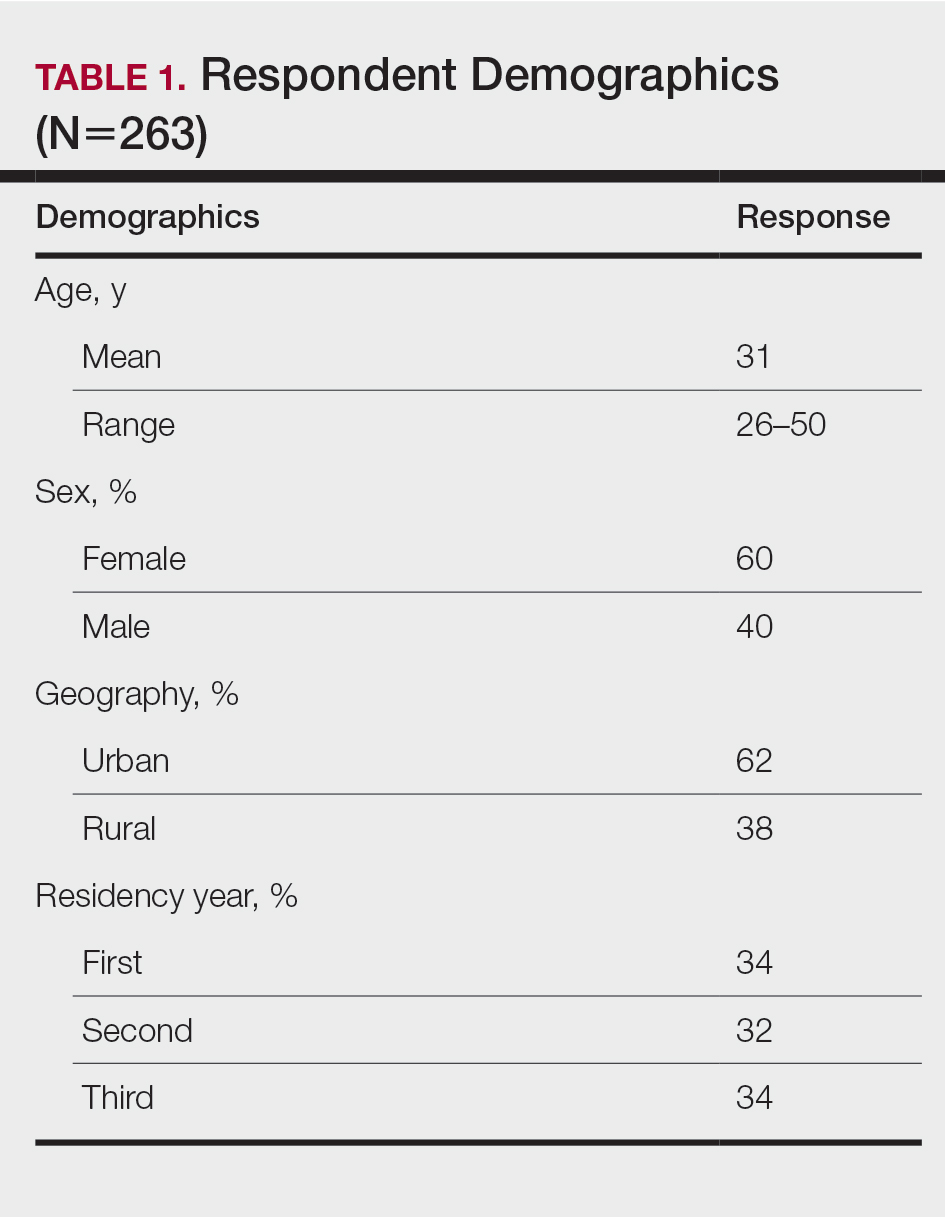
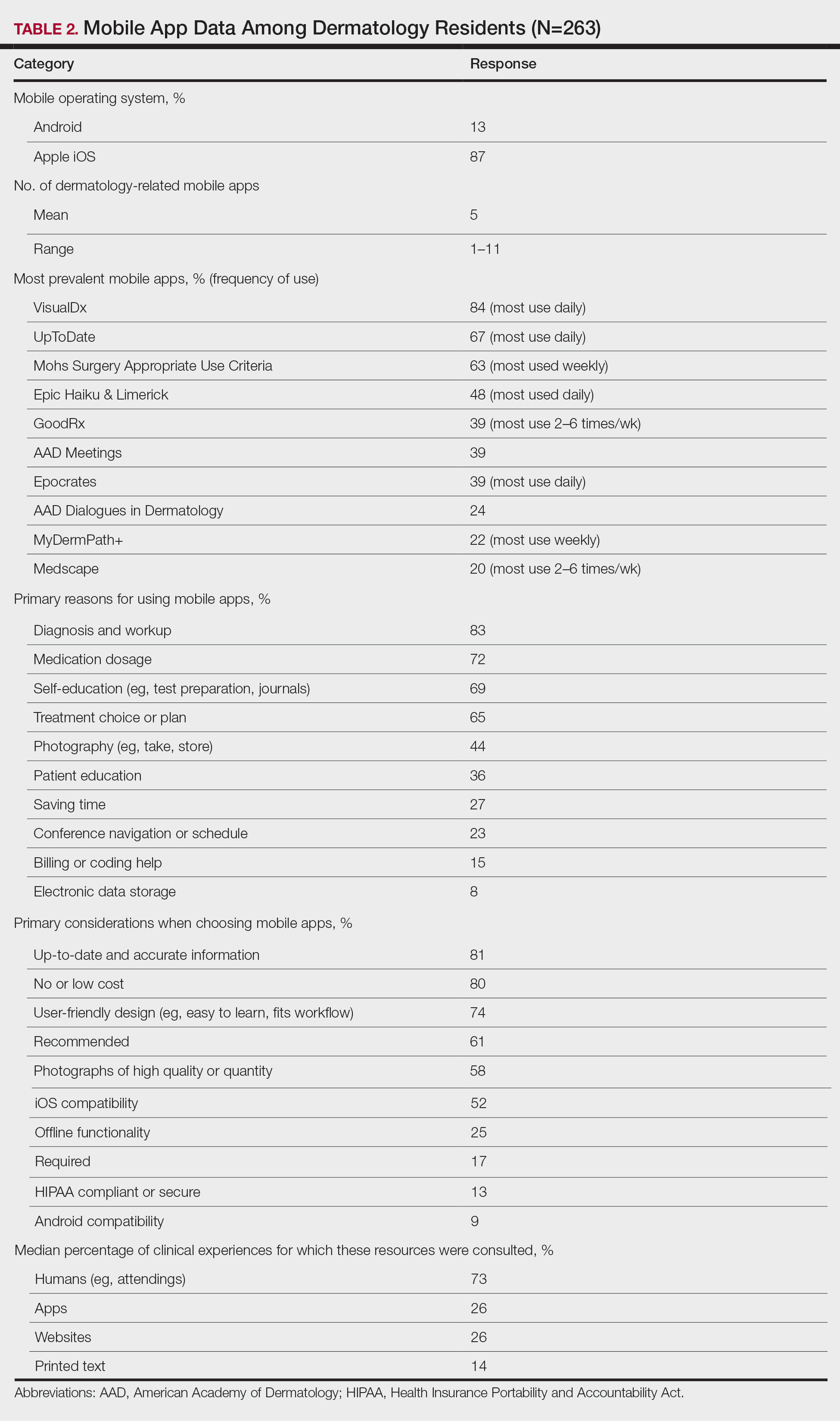
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).


Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).


Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Practice Points
- Virtual resources, including mobile apps, have become critical tools for learning and patient care during dermatology resident training for reasons that should be elucidated.
- Dermatology residents of different years and sexes utilize mobile apps in different amounts and for different purposes.
Aquatic Antagonists: Sea Cucumbers (Holothuroidea)
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9

In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.

Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9

In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Practice Points
- Sea cucumbers produce a toxin known as holothurin, which is contained in specialized structures called Cuvierian tubules and secreted onto the outer surface of the body wall. Some species also eject portions of their toxic inner organs through the anus as a defensive mechanism.
- In humans, the holothurin toxins cause an acute irritant dermatitis upon contact with the skin and a painful chemical conjunctivitis upon contact with the eyes.
- In addition to their own toxin, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through their integument, causing additional effects on human skin.
- The dermatologic effects of sea cucumbers can be prevented with the use of gloves and swim masks or goggles.
Tirzepatide questions persist despite serial phase 3 success in type 2 diabetes
The streak of positive phase 3 trial results for the novel “twincretin” tirzepatide when treating patients with type 2 diabetes continued in a report in The Lancet on results from the SURPASS-3 trial, which compared weekly subcutaneous injections of tirzepatide against daily treatment with insulin degludec in patients inadequately controlled on metformin alone or on metformin plus a sodium-glucose cotransporter 2 inhibitor.
Despite positive results in SURPASS-3, as well as in four other pivotal trials that are in the process of releasing full results, the safety and efficacy picture of tirzepatide still includes several as-yet unresolved issues, including the true incidence rate of gastrointestinal adverse effects, the role these effects play in weight loss during tirzepatide treatment, and the drug’s effect on important endpoints beyond weight loss and glycemic control such as cardiovascular outcomes and renal function, said two Australian experts who coauthored a comment on the new SURPASS-3 report.
Tirzepatide is called a “twincretin” because the molecule acts as both a glucagonlike peptide–1 receptor agonist, the drug class that includes semaglutide (Ozempic, Rybelsus, Wegovy) and liraglutide (Saxenda, Victoza), and also as a glucose-dependent insulinotropic polypeptide (GIP). Trial results reported to date suggest that tirzepatide “might be more potent than available GLP-1 receptor agonists,” based on evidence of superior glycemic control it produced relative to semaglutide in results from the SURPASS-2 phase 3 trial reported in August 2021, wrote Christopher K. Rayner, MD, and Michael Horowitz, MD, in their comment.
Uncertainty about gastrointestinal adverse effects
“Limitations of SURPASS-3 include the relatively small number of Asian and Black” patients enrolled, “and an open-label design that carries a risk for bias” when tallying the incidence of gastrointestinal adverse effects, which the trial recorded based on self-reports by enrolled patients.
A better design would use validated questionnaires geared to discerning gastrointestinal symptoms like the ones used in trials involving patients with functional gastrointestinal disorders, wrote Dr. Rayner, a professor of gastroenterology at the University of Adelaide, and Dr. Horowitz, a professor at the same institution and also director of the endocrine and metabolic unit at Royal Adelaide Hospital.
This approach would “allow for more robust evaluation of whether gastrointestinal symptoms are associated with increased weight loss,” they proposed, a possible partial explanation for the weight loss of some patients treated with a GLP-1 receptor agonist.
Additional outstanding questions about tirzepatide include the contribution resulting from the drug’s stimulation of the GIP receptor, as well as the role of GLP-1 receptor stimulation by tirzepatide in slowing gastric emptying. And they also cite the still-unreported effects of tirzepatide on cardiovascular events, fatty liver disease, and kidney function, and its longer-term effects with chronic treatment beyond a year.
All five of the recently completed SURPASS trials ran for 40-52 weeks.
Tirzepatide surpasses insulin degludec’s glycemic control
SURPASS-3 enrolled 1,444 patients with type 2 diabetes at 122 sites in 13 countries during 2019. The study’s primary endpoint was mean change in hemoglobin A1c from baseline after 52 weeks on treatment. The results showed that the A1c reduction with tirzepatide treatment significantly exceeded the drop produced by insulin degludec by 0.59%, 0.86%, and 1.04%, respectively, across the three tirzepatide dosages tested in a dose-response fashion, according to the recent publication.
The most common treatment-emergent adverse effects were gastrointestinal, which decreased with continued treatment, and tirzepatide produced fewer episodes of hypoglycemia, compared with insulin degludec (Tresiba).
In addition to full reports now out from SURPASS-2 and SURPASS-3, researchers also recently published full primary results from SURPASS-1. Results from SURPASS-5 appeared in a poster presented at the American Diabetes Association scientific sessions in June 2021 but have not yet been published in a full report, and the primary results from SURPASS-4are expected in a report during the European Association for the Study of Diabetes in September 2021.
SURPASS-3 and the other trials of tirzepatide were funded by Lilly, the company developing the drug. Dr. Rayner has been an adviser to Allergen and Glyscend, and has received research funding from Sanofi and Novartis. Dr. Horowitz has received symposia fees from Lilly, as well as from AstraZeneca and Boehringer Ingelheim.
The streak of positive phase 3 trial results for the novel “twincretin” tirzepatide when treating patients with type 2 diabetes continued in a report in The Lancet on results from the SURPASS-3 trial, which compared weekly subcutaneous injections of tirzepatide against daily treatment with insulin degludec in patients inadequately controlled on metformin alone or on metformin plus a sodium-glucose cotransporter 2 inhibitor.
Despite positive results in SURPASS-3, as well as in four other pivotal trials that are in the process of releasing full results, the safety and efficacy picture of tirzepatide still includes several as-yet unresolved issues, including the true incidence rate of gastrointestinal adverse effects, the role these effects play in weight loss during tirzepatide treatment, and the drug’s effect on important endpoints beyond weight loss and glycemic control such as cardiovascular outcomes and renal function, said two Australian experts who coauthored a comment on the new SURPASS-3 report.
Tirzepatide is called a “twincretin” because the molecule acts as both a glucagonlike peptide–1 receptor agonist, the drug class that includes semaglutide (Ozempic, Rybelsus, Wegovy) and liraglutide (Saxenda, Victoza), and also as a glucose-dependent insulinotropic polypeptide (GIP). Trial results reported to date suggest that tirzepatide “might be more potent than available GLP-1 receptor agonists,” based on evidence of superior glycemic control it produced relative to semaglutide in results from the SURPASS-2 phase 3 trial reported in August 2021, wrote Christopher K. Rayner, MD, and Michael Horowitz, MD, in their comment.
Uncertainty about gastrointestinal adverse effects
“Limitations of SURPASS-3 include the relatively small number of Asian and Black” patients enrolled, “and an open-label design that carries a risk for bias” when tallying the incidence of gastrointestinal adverse effects, which the trial recorded based on self-reports by enrolled patients.
A better design would use validated questionnaires geared to discerning gastrointestinal symptoms like the ones used in trials involving patients with functional gastrointestinal disorders, wrote Dr. Rayner, a professor of gastroenterology at the University of Adelaide, and Dr. Horowitz, a professor at the same institution and also director of the endocrine and metabolic unit at Royal Adelaide Hospital.
This approach would “allow for more robust evaluation of whether gastrointestinal symptoms are associated with increased weight loss,” they proposed, a possible partial explanation for the weight loss of some patients treated with a GLP-1 receptor agonist.
Additional outstanding questions about tirzepatide include the contribution resulting from the drug’s stimulation of the GIP receptor, as well as the role of GLP-1 receptor stimulation by tirzepatide in slowing gastric emptying. And they also cite the still-unreported effects of tirzepatide on cardiovascular events, fatty liver disease, and kidney function, and its longer-term effects with chronic treatment beyond a year.
All five of the recently completed SURPASS trials ran for 40-52 weeks.
Tirzepatide surpasses insulin degludec’s glycemic control
SURPASS-3 enrolled 1,444 patients with type 2 diabetes at 122 sites in 13 countries during 2019. The study’s primary endpoint was mean change in hemoglobin A1c from baseline after 52 weeks on treatment. The results showed that the A1c reduction with tirzepatide treatment significantly exceeded the drop produced by insulin degludec by 0.59%, 0.86%, and 1.04%, respectively, across the three tirzepatide dosages tested in a dose-response fashion, according to the recent publication.
The most common treatment-emergent adverse effects were gastrointestinal, which decreased with continued treatment, and tirzepatide produced fewer episodes of hypoglycemia, compared with insulin degludec (Tresiba).
In addition to full reports now out from SURPASS-2 and SURPASS-3, researchers also recently published full primary results from SURPASS-1. Results from SURPASS-5 appeared in a poster presented at the American Diabetes Association scientific sessions in June 2021 but have not yet been published in a full report, and the primary results from SURPASS-4are expected in a report during the European Association for the Study of Diabetes in September 2021.
SURPASS-3 and the other trials of tirzepatide were funded by Lilly, the company developing the drug. Dr. Rayner has been an adviser to Allergen and Glyscend, and has received research funding from Sanofi and Novartis. Dr. Horowitz has received symposia fees from Lilly, as well as from AstraZeneca and Boehringer Ingelheim.
The streak of positive phase 3 trial results for the novel “twincretin” tirzepatide when treating patients with type 2 diabetes continued in a report in The Lancet on results from the SURPASS-3 trial, which compared weekly subcutaneous injections of tirzepatide against daily treatment with insulin degludec in patients inadequately controlled on metformin alone or on metformin plus a sodium-glucose cotransporter 2 inhibitor.
Despite positive results in SURPASS-3, as well as in four other pivotal trials that are in the process of releasing full results, the safety and efficacy picture of tirzepatide still includes several as-yet unresolved issues, including the true incidence rate of gastrointestinal adverse effects, the role these effects play in weight loss during tirzepatide treatment, and the drug’s effect on important endpoints beyond weight loss and glycemic control such as cardiovascular outcomes and renal function, said two Australian experts who coauthored a comment on the new SURPASS-3 report.
Tirzepatide is called a “twincretin” because the molecule acts as both a glucagonlike peptide–1 receptor agonist, the drug class that includes semaglutide (Ozempic, Rybelsus, Wegovy) and liraglutide (Saxenda, Victoza), and also as a glucose-dependent insulinotropic polypeptide (GIP). Trial results reported to date suggest that tirzepatide “might be more potent than available GLP-1 receptor agonists,” based on evidence of superior glycemic control it produced relative to semaglutide in results from the SURPASS-2 phase 3 trial reported in August 2021, wrote Christopher K. Rayner, MD, and Michael Horowitz, MD, in their comment.
Uncertainty about gastrointestinal adverse effects
“Limitations of SURPASS-3 include the relatively small number of Asian and Black” patients enrolled, “and an open-label design that carries a risk for bias” when tallying the incidence of gastrointestinal adverse effects, which the trial recorded based on self-reports by enrolled patients.
A better design would use validated questionnaires geared to discerning gastrointestinal symptoms like the ones used in trials involving patients with functional gastrointestinal disorders, wrote Dr. Rayner, a professor of gastroenterology at the University of Adelaide, and Dr. Horowitz, a professor at the same institution and also director of the endocrine and metabolic unit at Royal Adelaide Hospital.
This approach would “allow for more robust evaluation of whether gastrointestinal symptoms are associated with increased weight loss,” they proposed, a possible partial explanation for the weight loss of some patients treated with a GLP-1 receptor agonist.
Additional outstanding questions about tirzepatide include the contribution resulting from the drug’s stimulation of the GIP receptor, as well as the role of GLP-1 receptor stimulation by tirzepatide in slowing gastric emptying. And they also cite the still-unreported effects of tirzepatide on cardiovascular events, fatty liver disease, and kidney function, and its longer-term effects with chronic treatment beyond a year.
All five of the recently completed SURPASS trials ran for 40-52 weeks.
Tirzepatide surpasses insulin degludec’s glycemic control
SURPASS-3 enrolled 1,444 patients with type 2 diabetes at 122 sites in 13 countries during 2019. The study’s primary endpoint was mean change in hemoglobin A1c from baseline after 52 weeks on treatment. The results showed that the A1c reduction with tirzepatide treatment significantly exceeded the drop produced by insulin degludec by 0.59%, 0.86%, and 1.04%, respectively, across the three tirzepatide dosages tested in a dose-response fashion, according to the recent publication.
The most common treatment-emergent adverse effects were gastrointestinal, which decreased with continued treatment, and tirzepatide produced fewer episodes of hypoglycemia, compared with insulin degludec (Tresiba).
In addition to full reports now out from SURPASS-2 and SURPASS-3, researchers also recently published full primary results from SURPASS-1. Results from SURPASS-5 appeared in a poster presented at the American Diabetes Association scientific sessions in June 2021 but have not yet been published in a full report, and the primary results from SURPASS-4are expected in a report during the European Association for the Study of Diabetes in September 2021.
SURPASS-3 and the other trials of tirzepatide were funded by Lilly, the company developing the drug. Dr. Rayner has been an adviser to Allergen and Glyscend, and has received research funding from Sanofi and Novartis. Dr. Horowitz has received symposia fees from Lilly, as well as from AstraZeneca and Boehringer Ingelheim.
FROM THE LANCET
The Top 100 Most-Cited Articles on Nail Psoriasis: A Bibliometric Analysis
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
FDA approves new enzyme replacement therapy for Pompe disease
Pompe disease is a rare genetic disease that occurs in an estimated 1 in 40,000 births. It is caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA), which leads to a buildup of glycogen in skeletal and cardiac muscle cells, causing muscle weakness and premature death from respiratory failure or heart failure.
Nexviazyme, administered by intravenous infusion every 2 weeks, supplements GAA and helps reduce glycogen accumulation.
The approval of this product “brings patients with Pompe disease another enzyme replacement therapy option for this rare disease,” said Janet Maynard, MD, deputy director, Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine, in the FDA’s Center for Drug Evaluation and Research, in a news release.
In 2010, the FDA approved alglucosidase alfa (Lumizyme) for the treatment of late-onset Pompe disease.
“The FDA will continue to work with stakeholders to advance the development of additional new, effective, and safe therapies for rare diseases, including Pompe disease,” said Dr. Maynard.
The approval is based on positive phase 3 data that demonstrated improvements in key disease burden measures, including respiratory function and walking disease, and that established the drug’s safety profile, Genzyme said in a news release.
The most common side effects were headache, fatigue, diarrhea, nausea, joint pain, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia, and urticaria.
Serious reactions included hypersensitivity reactions, such as anaphylaxis, and infusion-associated reactions, including respiratory distress, chills, and pyrexia.
Patients susceptible to fluid volume overload or those with compromised cardiac or respiratory function may be at risk for serious acute cardiorespiratory failure.
The FDA granted Nexviazyme orphan drug designation, priority review, and breakthrough status.
Genzyme expects the new therapy to be available in the United States in the coming weeks and said it will be priced on par with Lumizyme.
A version of this article first appeared on Medscape.com.
Pompe disease is a rare genetic disease that occurs in an estimated 1 in 40,000 births. It is caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA), which leads to a buildup of glycogen in skeletal and cardiac muscle cells, causing muscle weakness and premature death from respiratory failure or heart failure.
Nexviazyme, administered by intravenous infusion every 2 weeks, supplements GAA and helps reduce glycogen accumulation.
The approval of this product “brings patients with Pompe disease another enzyme replacement therapy option for this rare disease,” said Janet Maynard, MD, deputy director, Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine, in the FDA’s Center for Drug Evaluation and Research, in a news release.
In 2010, the FDA approved alglucosidase alfa (Lumizyme) for the treatment of late-onset Pompe disease.
“The FDA will continue to work with stakeholders to advance the development of additional new, effective, and safe therapies for rare diseases, including Pompe disease,” said Dr. Maynard.
The approval is based on positive phase 3 data that demonstrated improvements in key disease burden measures, including respiratory function and walking disease, and that established the drug’s safety profile, Genzyme said in a news release.
The most common side effects were headache, fatigue, diarrhea, nausea, joint pain, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia, and urticaria.
Serious reactions included hypersensitivity reactions, such as anaphylaxis, and infusion-associated reactions, including respiratory distress, chills, and pyrexia.
Patients susceptible to fluid volume overload or those with compromised cardiac or respiratory function may be at risk for serious acute cardiorespiratory failure.
The FDA granted Nexviazyme orphan drug designation, priority review, and breakthrough status.
Genzyme expects the new therapy to be available in the United States in the coming weeks and said it will be priced on par with Lumizyme.
A version of this article first appeared on Medscape.com.
Pompe disease is a rare genetic disease that occurs in an estimated 1 in 40,000 births. It is caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA), which leads to a buildup of glycogen in skeletal and cardiac muscle cells, causing muscle weakness and premature death from respiratory failure or heart failure.
Nexviazyme, administered by intravenous infusion every 2 weeks, supplements GAA and helps reduce glycogen accumulation.
The approval of this product “brings patients with Pompe disease another enzyme replacement therapy option for this rare disease,” said Janet Maynard, MD, deputy director, Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine, in the FDA’s Center for Drug Evaluation and Research, in a news release.
In 2010, the FDA approved alglucosidase alfa (Lumizyme) for the treatment of late-onset Pompe disease.
“The FDA will continue to work with stakeholders to advance the development of additional new, effective, and safe therapies for rare diseases, including Pompe disease,” said Dr. Maynard.
The approval is based on positive phase 3 data that demonstrated improvements in key disease burden measures, including respiratory function and walking disease, and that established the drug’s safety profile, Genzyme said in a news release.
The most common side effects were headache, fatigue, diarrhea, nausea, joint pain, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia, and urticaria.
Serious reactions included hypersensitivity reactions, such as anaphylaxis, and infusion-associated reactions, including respiratory distress, chills, and pyrexia.
Patients susceptible to fluid volume overload or those with compromised cardiac or respiratory function may be at risk for serious acute cardiorespiratory failure.
The FDA granted Nexviazyme orphan drug designation, priority review, and breakthrough status.
Genzyme expects the new therapy to be available in the United States in the coming weeks and said it will be priced on par with Lumizyme.
A version of this article first appeared on Medscape.com.



