User login
Short-acting opioids needed for withdrawal in U.S. hospitals, say experts
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
The commentary by Robert A. Kleinman, MD, with the Centre for Addiction and Mental Health, and department of psychiatry, University of Toronto, and Sarah E. Wakeman, MD, with the division of general internal medicine at Massachusetts General Hospital, and Harvard Medical School, Boston, was published in Annals of Internal Medicine.
Currently, short-acting opioids are not recommended in the United States for opioid withdrawal symptoms (OWS) management in the hospital, the authors wrote. Instead, withdrawal symptoms are typically treated, followed by methadone or buprenorphine or nonopioid medications, but many patients don’t get enough relief. Undertreated withdrawal can result in patients leaving the hospital against medical advice, which is linked with higher risk of death.
Addiction specialist Elisabeth Poorman, MD, of the University of Illinois Chicago, said in an interview that she agrees it’s time to start shifting the thinking on using short-acting opioids for OWS in hospitals. Use varies greatly by hospital and by clinician, she said.
“It’s time to let evidence guide us and to be flexible,” Dr. Poorman said.
The commentary authors noted that with methadone, patients must wait several hours for maximal symptom reduction, and the full benefits of methadone treatment are not realized until days after initiation.
Rapid initiation of methadone may be feasible in hospitals and has been proposed as an option, but further study is necessary before widespread use, the authors wrote.
Short-acting opioids may address limitations of other opioids
Lofexidine, an alpha-2-adrenergic agonist, is the only drug approved by the Food and Drug Administration specifically for OWS.
“However,” the authors said, “more than half of patients with OWS treated with lofexidine in phase 3 efficacy trials dropped out by day five. Clonidine, another alpha-2-agonist used off label to treat OWS, has similar effects to those of lofexidine. “
Therefore, short-acting opioids may complement methadone and buprenorphine in treating OWS in the hospital by addressing their limitations, the authors wrote.
Dr. Kleinman and Dr. Wakeman also say short-acting opioids may help with starting buprenorphine for patients exposed to fentanyl, because short-acting opioids can relieve withdrawal symptoms while fentanyl is metabolized and excreted.
Supplementation with short-acting opioids within the hospital can relieve withdrawal symptoms and help keep patients comfortable while methadone is titrated to more effective doses for long-term treatment, they wrote.
With short-acting opioids, patients may become more engaged in their care with, for example, a tamper-proof, patient-controlled analgesia pump, which would allow them to have more autonomy in administration of opioids to relieve pain and withdrawal symptoms, the authors wrote.
Dr. Kleinman and Dr. Wakeman noted that many patients who inject drugs already consume short-acting illicit drugs in the hospital, typically in washrooms and smoking areas, so supervised use of short-acting opioids helps eliminate the risk for unwitnessed overdoses.
Barriers to short-acting opioid use
Despite use of short-acting opioids internationally, barriers in the United States include limited prospective, randomized, controlled research on their benefits. There is limited institutional support for such approaches, and concerns and stigma around providing opioids to patients with OUD.
“[M]any institutions have insufficient numbers of providers who are both confident and competent with standard buprenorphine and methadone initiation approaches, a prerequisite before adopting more complex regimens,” the authors wrote.
Short-acting, full-agonist opioids, as a complement to methadone or buprenorphine, is already recommended for inpatients with OUD who are experiencing acute pain.
But the authors argue it should be an option when pain is not present, but methadone or buprenorphine have not provided enough OWS relief.
When short-acting opioids are helpful, according to outside expert
Dr. Poorman agrees and says she has found short-acting opioids simple to use in the hospital and very helpful in two situations.
One is when patients are very clear that they don’t want any medication for opioid use disorder, but they do want to be treated for their acute medical issue.
“I thought that was a fantastic tool to have to demonstrate we’re listening to them and weren’t trying to impose something on them and left the door open to come back when they did want treatment, which many of them did,” Dr. Poorman said.
The second situation is when the patient is uncertain about options but very afraid of precipitated withdrawal from buprenorphine.
She said she then found it easy to switch from those medications to buprenorphine and methadone.
Dr. Poorman described a situation she encountered previously where the patient was injecting heroin several times a day for 30-40 years. He was very clear he wasn’t going to stop injecting heroin, but he needed medical attention. He was willing to get medical attention, but he told his doctor he didn’t want to be uncomfortable while in the hospital.
It was very hard for his doctor to accept relieving his symptoms of withdrawal as part of her job, because she felt as though she was condoning his drug use, Dr. Poorman explained.
But Dr. Poorman said it’s not realistic to think that someone who clearly does not want to stop using is going to stop using because a doctor made that person go through painful withdrawal “that they’ve structured their whole life around avoiding.”
Take-home message
“We need to understand that addiction is very complex. A lot of times people come to us distressed, and it’s a great time to engage them in care but engaging them in care doesn’t mean imposing discomfort or pain on them,” Dr. Poorman noted. Instead, it means “listening to them, helping them be comfortable in a really stressful situation and then letting them know we are always there for them wherever they are on their disease process or recovery journey so that they can come back to us.”
Dr. Wakeman previously served on clinical advisory board for Celero Systems and receives textbook royalties from Springer and author payment from UpToDate. Dr. Kleinman and Dr. Poorman declared no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Inexplicably drunk: A case of an underdiagnosed condition?
A 46-year-old North Carolina man, who was pulled over on suspicion of drunk driving, vehemently denied consuming alcohol. When he refused to take a breathalyzer test, he was hospitalized and doctors confirmed what police suspected – his blood alcohol level was 0.20, two-and-a-half times the state’s legal limit – and he was charged with driving while intoxicated (DWI).
For an entire year after his arrest, the cause of his “intoxication” remained a mystery. It wasn’t until his aunt learned about a similar case that had been successfully treated at an Ohio clinic that he understood what was happening to him – he had auto brewery syndrome (ABS).
and suffer all the medical and social implications of alcoholism.
“ABS occurs when ingested carbohydrates are converted to alcohol by fungi in the gastrointestinal tract,” Fahad Malik, MD, who reported the case in BMJ Open Gastroenterology while a resident at Richmond University Medical Center in New York, told this news organization.
At the urging of his aunt, the patient attended the Ohio clinic where he underwent a complete blood count, comprehensive metabolic panel, immunology panel and urinalysis, all of which were normal.
However, stool testing revealed the presence of two strains of yeast – Saccharomyces cerevisiae, commonly used in winemaking, baking, and beer brewing, and Saccharomyces boulardii.
To confirm the ABS diagnosis, the patient received a carbohydrate meal and clinicians monitored his blood alcohol level, which, after 8 hours, reached 57 mg/dL. He was treated with antifungals for the Saccharomyces fungi in his stool and discharged on a strict carbohydrate-free diet along with special supplements, including multivitamins and probiotics, but no further antifungal therapy.
Probiotics, said Dr. Malik, competitively inhibit bad bacteria and fungi, but currently there is evidence to show they are useful for ABS.
Although the patient adhered to his prescribed treatment regimen, after a few weeks of no symptoms, intermittent “flares” returned. In one instance of inebriation, he fell and hit his head, resulting in intracranial bleeding that resulted in a transfer to a neurosurgical center. During his hospital stay, his blood alcohol levels ranged from 50 to 400 mg/dL.
Antibiotics the culprit?
Disheartened by the continuation of his symptoms, the patient sought support from an online forum. It was there he read about Dr. Malik and gastroenterologist Prasanna Wickremesinghe, MD (a colleague of Dr. Malik’s at Richmond MC), who had treated a complicated, very similar case of ABS. The patient made contact with the two physicians and they assessed him.
“We went from A to Z with the patient, because we were trying to look for similar things in the history – we wanted to know the exact point at which it started and understand when he started experiencing mental fog,” said Dr. Malik.
After speaking to the patient, Dr. Malik and Dr. Wickremesinghe traced his initial symptoms to a 2011 course of antibiotics (cephalexin 250 mg oral three times a day for 3 weeks) prescribed for a complicated traumatic thumb injury.
About a week after he finished the antibiotics, he experienced noticeable behavioral changes, including depression, brain fog, and aggressive outbursts, all of which were very uncharacteristic.
He visited his primary care physician in 2014 for treatment, which resulted in a referral to a psychiatrist, who treated him with lorazepam and fluoxetine. The patient noted that he was previously healthy, with no significant medical or psychiatric history.
Dr. Malik believes the antibiotics prescribed all those years ago is the culprit. “We were postulating that the antibiotics had changed the microbiome of his gut and allowed the fungi to develop,” he said.
Since there are no established diagnostic criteria or treatment regimen for ABS, Dr. Malik and Dr. Wickremesinghe developed their own.
Diagnosis consisted of a standardized carbohydrate challenge test vs. a carbohydrate meal, where they gave the patient 200 g of glucose by mouth after an overnight fast and drew blood at timed intervals of 0, 0.5, 1, 2, 4, 8, 16, and 24 hours to test for glucose and blood alcohol levels.
“After that we needed to isolate the fungi by examining the gut secretions through an upper and lower endoscopy,” said Dr. Wickremesinghe. Fungal cultures from the upper small gut and cecal secretions grew Candida albicans and C. parapsilosis.
Both fungi were sensitive to azoles and the physicians prescribed oral itraconazole 150 mg per day as an initial therapy. After 10 days, his symptoms did not improve so the dose was increased to 200 mg/day and the patient became “completely asymptomatic.”
“We had nothing to follow. We didn’t know how long to treat the patient, it was really just a process of trial and error,” said Dr. Malik. The physicians asked the patient to monitor his breath alcohol levels twice a day during treatment and immediately report any increases. Over time, he also received treatment with various probiotics to help normalize his gut flora.
Underdiagnosed condition?
At the time of the case study’s publication in the summer of 2019, the patient had been asymptomatic for 18 months and had been able to resume a normal diet, but still checks his breath alcohol levels from time to time.
“Before this patient’s case, I went all through the literature and found only a few cases of ABS,” said Dr. Malik.
However, he added, after this case study was published 10 other patients contacted him with a similar history of antibiotic use and the same symptoms. This, said Dr. Malik, is “significant” and suggests ABS is much more common than previously thought.
The clinicians also note that to the best of their knowledge this is the first report of antibiotic exposure initiating ABS.
“What we tried to do was set up a protocol by which to identify these patients, confirm a diagnosis, and treat them for a sufficient amount of time,” said Dr. Wickremesinghe. “We also wanted to inform other physicians that this may function as a standardized way of treating these patients, and may promote further study,” added Dr. Malik, who emphasized that the role of probiotics in ABS still needs to be studied.
Dr. Malik and Dr. Wickremesinghe note that physicians should be aware that mood changes, brain fog, and delirium in patients who deny alcohol ingestion may be the first symptoms of ABS.
Dr. Wickremesinghe said since the case study was published he and Dr. Malik have received queries from all over the world. “It’s unbelievable the amount of interest we have had in the paper, so if we have made the medical community and the general population aware of this condition and how to treat it, we have done a major thing for medicine,” he said.
A version of this article first appeared on Medscape.com.
A 46-year-old North Carolina man, who was pulled over on suspicion of drunk driving, vehemently denied consuming alcohol. When he refused to take a breathalyzer test, he was hospitalized and doctors confirmed what police suspected – his blood alcohol level was 0.20, two-and-a-half times the state’s legal limit – and he was charged with driving while intoxicated (DWI).
For an entire year after his arrest, the cause of his “intoxication” remained a mystery. It wasn’t until his aunt learned about a similar case that had been successfully treated at an Ohio clinic that he understood what was happening to him – he had auto brewery syndrome (ABS).
and suffer all the medical and social implications of alcoholism.
“ABS occurs when ingested carbohydrates are converted to alcohol by fungi in the gastrointestinal tract,” Fahad Malik, MD, who reported the case in BMJ Open Gastroenterology while a resident at Richmond University Medical Center in New York, told this news organization.
At the urging of his aunt, the patient attended the Ohio clinic where he underwent a complete blood count, comprehensive metabolic panel, immunology panel and urinalysis, all of which were normal.
However, stool testing revealed the presence of two strains of yeast – Saccharomyces cerevisiae, commonly used in winemaking, baking, and beer brewing, and Saccharomyces boulardii.
To confirm the ABS diagnosis, the patient received a carbohydrate meal and clinicians monitored his blood alcohol level, which, after 8 hours, reached 57 mg/dL. He was treated with antifungals for the Saccharomyces fungi in his stool and discharged on a strict carbohydrate-free diet along with special supplements, including multivitamins and probiotics, but no further antifungal therapy.
Probiotics, said Dr. Malik, competitively inhibit bad bacteria and fungi, but currently there is evidence to show they are useful for ABS.
Although the patient adhered to his prescribed treatment regimen, after a few weeks of no symptoms, intermittent “flares” returned. In one instance of inebriation, he fell and hit his head, resulting in intracranial bleeding that resulted in a transfer to a neurosurgical center. During his hospital stay, his blood alcohol levels ranged from 50 to 400 mg/dL.
Antibiotics the culprit?
Disheartened by the continuation of his symptoms, the patient sought support from an online forum. It was there he read about Dr. Malik and gastroenterologist Prasanna Wickremesinghe, MD (a colleague of Dr. Malik’s at Richmond MC), who had treated a complicated, very similar case of ABS. The patient made contact with the two physicians and they assessed him.
“We went from A to Z with the patient, because we were trying to look for similar things in the history – we wanted to know the exact point at which it started and understand when he started experiencing mental fog,” said Dr. Malik.
After speaking to the patient, Dr. Malik and Dr. Wickremesinghe traced his initial symptoms to a 2011 course of antibiotics (cephalexin 250 mg oral three times a day for 3 weeks) prescribed for a complicated traumatic thumb injury.
About a week after he finished the antibiotics, he experienced noticeable behavioral changes, including depression, brain fog, and aggressive outbursts, all of which were very uncharacteristic.
He visited his primary care physician in 2014 for treatment, which resulted in a referral to a psychiatrist, who treated him with lorazepam and fluoxetine. The patient noted that he was previously healthy, with no significant medical or psychiatric history.
Dr. Malik believes the antibiotics prescribed all those years ago is the culprit. “We were postulating that the antibiotics had changed the microbiome of his gut and allowed the fungi to develop,” he said.
Since there are no established diagnostic criteria or treatment regimen for ABS, Dr. Malik and Dr. Wickremesinghe developed their own.
Diagnosis consisted of a standardized carbohydrate challenge test vs. a carbohydrate meal, where they gave the patient 200 g of glucose by mouth after an overnight fast and drew blood at timed intervals of 0, 0.5, 1, 2, 4, 8, 16, and 24 hours to test for glucose and blood alcohol levels.
“After that we needed to isolate the fungi by examining the gut secretions through an upper and lower endoscopy,” said Dr. Wickremesinghe. Fungal cultures from the upper small gut and cecal secretions grew Candida albicans and C. parapsilosis.
Both fungi were sensitive to azoles and the physicians prescribed oral itraconazole 150 mg per day as an initial therapy. After 10 days, his symptoms did not improve so the dose was increased to 200 mg/day and the patient became “completely asymptomatic.”
“We had nothing to follow. We didn’t know how long to treat the patient, it was really just a process of trial and error,” said Dr. Malik. The physicians asked the patient to monitor his breath alcohol levels twice a day during treatment and immediately report any increases. Over time, he also received treatment with various probiotics to help normalize his gut flora.
Underdiagnosed condition?
At the time of the case study’s publication in the summer of 2019, the patient had been asymptomatic for 18 months and had been able to resume a normal diet, but still checks his breath alcohol levels from time to time.
“Before this patient’s case, I went all through the literature and found only a few cases of ABS,” said Dr. Malik.
However, he added, after this case study was published 10 other patients contacted him with a similar history of antibiotic use and the same symptoms. This, said Dr. Malik, is “significant” and suggests ABS is much more common than previously thought.
The clinicians also note that to the best of their knowledge this is the first report of antibiotic exposure initiating ABS.
“What we tried to do was set up a protocol by which to identify these patients, confirm a diagnosis, and treat them for a sufficient amount of time,” said Dr. Wickremesinghe. “We also wanted to inform other physicians that this may function as a standardized way of treating these patients, and may promote further study,” added Dr. Malik, who emphasized that the role of probiotics in ABS still needs to be studied.
Dr. Malik and Dr. Wickremesinghe note that physicians should be aware that mood changes, brain fog, and delirium in patients who deny alcohol ingestion may be the first symptoms of ABS.
Dr. Wickremesinghe said since the case study was published he and Dr. Malik have received queries from all over the world. “It’s unbelievable the amount of interest we have had in the paper, so if we have made the medical community and the general population aware of this condition and how to treat it, we have done a major thing for medicine,” he said.
A version of this article first appeared on Medscape.com.
A 46-year-old North Carolina man, who was pulled over on suspicion of drunk driving, vehemently denied consuming alcohol. When he refused to take a breathalyzer test, he was hospitalized and doctors confirmed what police suspected – his blood alcohol level was 0.20, two-and-a-half times the state’s legal limit – and he was charged with driving while intoxicated (DWI).
For an entire year after his arrest, the cause of his “intoxication” remained a mystery. It wasn’t until his aunt learned about a similar case that had been successfully treated at an Ohio clinic that he understood what was happening to him – he had auto brewery syndrome (ABS).
and suffer all the medical and social implications of alcoholism.
“ABS occurs when ingested carbohydrates are converted to alcohol by fungi in the gastrointestinal tract,” Fahad Malik, MD, who reported the case in BMJ Open Gastroenterology while a resident at Richmond University Medical Center in New York, told this news organization.
At the urging of his aunt, the patient attended the Ohio clinic where he underwent a complete blood count, comprehensive metabolic panel, immunology panel and urinalysis, all of which were normal.
However, stool testing revealed the presence of two strains of yeast – Saccharomyces cerevisiae, commonly used in winemaking, baking, and beer brewing, and Saccharomyces boulardii.
To confirm the ABS diagnosis, the patient received a carbohydrate meal and clinicians monitored his blood alcohol level, which, after 8 hours, reached 57 mg/dL. He was treated with antifungals for the Saccharomyces fungi in his stool and discharged on a strict carbohydrate-free diet along with special supplements, including multivitamins and probiotics, but no further antifungal therapy.
Probiotics, said Dr. Malik, competitively inhibit bad bacteria and fungi, but currently there is evidence to show they are useful for ABS.
Although the patient adhered to his prescribed treatment regimen, after a few weeks of no symptoms, intermittent “flares” returned. In one instance of inebriation, he fell and hit his head, resulting in intracranial bleeding that resulted in a transfer to a neurosurgical center. During his hospital stay, his blood alcohol levels ranged from 50 to 400 mg/dL.
Antibiotics the culprit?
Disheartened by the continuation of his symptoms, the patient sought support from an online forum. It was there he read about Dr. Malik and gastroenterologist Prasanna Wickremesinghe, MD (a colleague of Dr. Malik’s at Richmond MC), who had treated a complicated, very similar case of ABS. The patient made contact with the two physicians and they assessed him.
“We went from A to Z with the patient, because we were trying to look for similar things in the history – we wanted to know the exact point at which it started and understand when he started experiencing mental fog,” said Dr. Malik.
After speaking to the patient, Dr. Malik and Dr. Wickremesinghe traced his initial symptoms to a 2011 course of antibiotics (cephalexin 250 mg oral three times a day for 3 weeks) prescribed for a complicated traumatic thumb injury.
About a week after he finished the antibiotics, he experienced noticeable behavioral changes, including depression, brain fog, and aggressive outbursts, all of which were very uncharacteristic.
He visited his primary care physician in 2014 for treatment, which resulted in a referral to a psychiatrist, who treated him with lorazepam and fluoxetine. The patient noted that he was previously healthy, with no significant medical or psychiatric history.
Dr. Malik believes the antibiotics prescribed all those years ago is the culprit. “We were postulating that the antibiotics had changed the microbiome of his gut and allowed the fungi to develop,” he said.
Since there are no established diagnostic criteria or treatment regimen for ABS, Dr. Malik and Dr. Wickremesinghe developed their own.
Diagnosis consisted of a standardized carbohydrate challenge test vs. a carbohydrate meal, where they gave the patient 200 g of glucose by mouth after an overnight fast and drew blood at timed intervals of 0, 0.5, 1, 2, 4, 8, 16, and 24 hours to test for glucose and blood alcohol levels.
“After that we needed to isolate the fungi by examining the gut secretions through an upper and lower endoscopy,” said Dr. Wickremesinghe. Fungal cultures from the upper small gut and cecal secretions grew Candida albicans and C. parapsilosis.
Both fungi were sensitive to azoles and the physicians prescribed oral itraconazole 150 mg per day as an initial therapy. After 10 days, his symptoms did not improve so the dose was increased to 200 mg/day and the patient became “completely asymptomatic.”
“We had nothing to follow. We didn’t know how long to treat the patient, it was really just a process of trial and error,” said Dr. Malik. The physicians asked the patient to monitor his breath alcohol levels twice a day during treatment and immediately report any increases. Over time, he also received treatment with various probiotics to help normalize his gut flora.
Underdiagnosed condition?
At the time of the case study’s publication in the summer of 2019, the patient had been asymptomatic for 18 months and had been able to resume a normal diet, but still checks his breath alcohol levels from time to time.
“Before this patient’s case, I went all through the literature and found only a few cases of ABS,” said Dr. Malik.
However, he added, after this case study was published 10 other patients contacted him with a similar history of antibiotic use and the same symptoms. This, said Dr. Malik, is “significant” and suggests ABS is much more common than previously thought.
The clinicians also note that to the best of their knowledge this is the first report of antibiotic exposure initiating ABS.
“What we tried to do was set up a protocol by which to identify these patients, confirm a diagnosis, and treat them for a sufficient amount of time,” said Dr. Wickremesinghe. “We also wanted to inform other physicians that this may function as a standardized way of treating these patients, and may promote further study,” added Dr. Malik, who emphasized that the role of probiotics in ABS still needs to be studied.
Dr. Malik and Dr. Wickremesinghe note that physicians should be aware that mood changes, brain fog, and delirium in patients who deny alcohol ingestion may be the first symptoms of ABS.
Dr. Wickremesinghe said since the case study was published he and Dr. Malik have received queries from all over the world. “It’s unbelievable the amount of interest we have had in the paper, so if we have made the medical community and the general population aware of this condition and how to treat it, we have done a major thing for medicine,” he said.
A version of this article first appeared on Medscape.com.
TikTok trends: Scalp popping, EpiPen tutorial, and plant juice
With the holidays just around the corner (how did that happen?), it’s a good time to remind yourself of the things you’re grateful for.
Perhaps you’re grateful for spending chilly evenings under a warm blanket binge-watching your favorite shows or being able to safely gather with loved ones. If you’re William Shatner, maybe you’re grateful for that quick trip to space (because apparently, that’s a thing now) and the poetic tweets it induced. Down here on earth, TikTok has surpassed 1 billion users, and while we’re not grateful, necessarily, we are entertained.
Here are the latest ugly, good, and bad TikToks that have been trending lately.
The Ugly: Scalp popping
Warning: Don’t watch this if you’re easily freaked out by weird body sounds. It’s like cracking your knuckles but way, way worse.
This TikTok from @asmr.barber has 1.7 million likes, and lots of people are trying it out for themselves. The viral video features the (disturbed) art of scalp popping, also known as hair cracking. It features what is assumed to be some sort of barber or professional (here’s hoping) twisting a client’s hair around his fingers and then yanking, creating an audible popping sound. Many are posting their own hair-cracking attempts on the platform. It’s unclear if this is supposed to feel good or just be grossly satisfying, though some users claim it helps with migraines.
But it turns out this might be more than kind of gross; it can be dangerous, too.
Anthony Youn, MD, a board-certified plastic surgeon, comments on the trend with concern: “What the hell is going on here?” Not something you want to hear from a doctor. Dr. Youn explained that the popping sound comes from the galea aponeurotica, a fibrous sheet of connective tissue under your scalp, being pulled off the skull.
In a comment, Dr. Youn continued to warn people of replicating this trend: “It can tear the inside of the scalp, which can bleed a ton on the inside. Think boxer or MMA fighter with scalp hematoma.”
Let’s keep our scalps attached to our skulls, people. If I never have to hear that sound again, I’ll be eternally grateful.
The Good: Doctor demonstrates correct EpiPen use
This reaction TikTok from medical student Mutahir Farhan (aka @madmedicine) has over 252,000 likes and hundreds of comments. In it, Ms. Farhan watches a video of a young woman attempting to administer an EpiPen to her friend, with the caption “How NOT to use an EpiPen” over it (in bright red, of course).
The woman in the video is using the wrong end of the EpiPen against her friend’s leg, so it isn’t working. When she uses her thumb to press down and help, her thumb is actually pressed against the needle end and the EpiPen sticks her instead of her friend. Ouch!
Ms. Farhan goes on to explain the anatomy of the EpiPen and shows his audience of 1.1 million followers where to inject it.
“You gotta remember that the orange tip is where the needle comes out. Otherwise, you’re going to end up stabbing yourself with epinephrine, like that girl in the video,” Ms. Farhan says. He goes on to instruct the important, but often overlooked, follow-up: “After you stab someone with epinephrine, call 911 or go to the ER, so that we can make sure they’re actually okay and good to go.”
The Bad: Liquid chlorophyll
Here is another one of those tricky trends that are so widespread and popular that it’s hard to find exactly where it originated from. A video from @lenamaiah has over 5 million views and 800,000 likes, which even by TikTok standards, is a lot. TikTok is rife with similar videos, which feature drops of liquid chlorophyll being added to water and smoothies.
The pretty emerald hue is mesmerizing and it’s hard to resist trying it out when it’s being peddled by seemingly every pretty, smooth-skinned pseudo-model on the platform. In this video, Lena says drinking a glass of water with a few drops of chlorophyll can reduce inflammation, get rid of eye bags, boost your vitamin levels, reduce free radical damage, detoxify your system, and file your taxes. Okay, I made that last one up, but it follows, doesn’t it? This stuff sounds pretty good. Maybe too good.
Chlorophyll, if you skipped biology class (somehow, I doubt you did), is what makes plants green. Medscape has a detailed explanation of chlorophyll, but all you really need to know is that it’s the secret to that cool thing plants do: photosynthesis, or turning sunlight into energy. Scientists have been trying to find uses for it in people since the 1940s. Unfortunately, studies never found much that it can do for us, aside from being kind of deodorizing. So, while it’s been historically marketed as toothpaste and deodorant, the new TikTok claims of it being a cure-all or the next big skincare supplement are not widely substantiated by scientific studies. The only real evidence of it being effective is word of mouth from those who claim to like the way they look or feel since taking it, which isn’t enough for doctors to recommend it.
TikTok’s resident dermatologist, Muneeb Shah, DO, stitched a TikTok from another user, with his captions explaining, “[There’s] no scientific evidence for liquid chlorophyll [helping] rosacea or acne.”
His advice: “Chlorophyll is great, but just eat more veggies.”
A version of this article first appeared on Medscape.com.
With the holidays just around the corner (how did that happen?), it’s a good time to remind yourself of the things you’re grateful for.
Perhaps you’re grateful for spending chilly evenings under a warm blanket binge-watching your favorite shows or being able to safely gather with loved ones. If you’re William Shatner, maybe you’re grateful for that quick trip to space (because apparently, that’s a thing now) and the poetic tweets it induced. Down here on earth, TikTok has surpassed 1 billion users, and while we’re not grateful, necessarily, we are entertained.
Here are the latest ugly, good, and bad TikToks that have been trending lately.
The Ugly: Scalp popping
Warning: Don’t watch this if you’re easily freaked out by weird body sounds. It’s like cracking your knuckles but way, way worse.
This TikTok from @asmr.barber has 1.7 million likes, and lots of people are trying it out for themselves. The viral video features the (disturbed) art of scalp popping, also known as hair cracking. It features what is assumed to be some sort of barber or professional (here’s hoping) twisting a client’s hair around his fingers and then yanking, creating an audible popping sound. Many are posting their own hair-cracking attempts on the platform. It’s unclear if this is supposed to feel good or just be grossly satisfying, though some users claim it helps with migraines.
But it turns out this might be more than kind of gross; it can be dangerous, too.
Anthony Youn, MD, a board-certified plastic surgeon, comments on the trend with concern: “What the hell is going on here?” Not something you want to hear from a doctor. Dr. Youn explained that the popping sound comes from the galea aponeurotica, a fibrous sheet of connective tissue under your scalp, being pulled off the skull.
In a comment, Dr. Youn continued to warn people of replicating this trend: “It can tear the inside of the scalp, which can bleed a ton on the inside. Think boxer or MMA fighter with scalp hematoma.”
Let’s keep our scalps attached to our skulls, people. If I never have to hear that sound again, I’ll be eternally grateful.
The Good: Doctor demonstrates correct EpiPen use
This reaction TikTok from medical student Mutahir Farhan (aka @madmedicine) has over 252,000 likes and hundreds of comments. In it, Ms. Farhan watches a video of a young woman attempting to administer an EpiPen to her friend, with the caption “How NOT to use an EpiPen” over it (in bright red, of course).
The woman in the video is using the wrong end of the EpiPen against her friend’s leg, so it isn’t working. When she uses her thumb to press down and help, her thumb is actually pressed against the needle end and the EpiPen sticks her instead of her friend. Ouch!
Ms. Farhan goes on to explain the anatomy of the EpiPen and shows his audience of 1.1 million followers where to inject it.
“You gotta remember that the orange tip is where the needle comes out. Otherwise, you’re going to end up stabbing yourself with epinephrine, like that girl in the video,” Ms. Farhan says. He goes on to instruct the important, but often overlooked, follow-up: “After you stab someone with epinephrine, call 911 or go to the ER, so that we can make sure they’re actually okay and good to go.”
The Bad: Liquid chlorophyll
Here is another one of those tricky trends that are so widespread and popular that it’s hard to find exactly where it originated from. A video from @lenamaiah has over 5 million views and 800,000 likes, which even by TikTok standards, is a lot. TikTok is rife with similar videos, which feature drops of liquid chlorophyll being added to water and smoothies.
The pretty emerald hue is mesmerizing and it’s hard to resist trying it out when it’s being peddled by seemingly every pretty, smooth-skinned pseudo-model on the platform. In this video, Lena says drinking a glass of water with a few drops of chlorophyll can reduce inflammation, get rid of eye bags, boost your vitamin levels, reduce free radical damage, detoxify your system, and file your taxes. Okay, I made that last one up, but it follows, doesn’t it? This stuff sounds pretty good. Maybe too good.
Chlorophyll, if you skipped biology class (somehow, I doubt you did), is what makes plants green. Medscape has a detailed explanation of chlorophyll, but all you really need to know is that it’s the secret to that cool thing plants do: photosynthesis, or turning sunlight into energy. Scientists have been trying to find uses for it in people since the 1940s. Unfortunately, studies never found much that it can do for us, aside from being kind of deodorizing. So, while it’s been historically marketed as toothpaste and deodorant, the new TikTok claims of it being a cure-all or the next big skincare supplement are not widely substantiated by scientific studies. The only real evidence of it being effective is word of mouth from those who claim to like the way they look or feel since taking it, which isn’t enough for doctors to recommend it.
TikTok’s resident dermatologist, Muneeb Shah, DO, stitched a TikTok from another user, with his captions explaining, “[There’s] no scientific evidence for liquid chlorophyll [helping] rosacea or acne.”
His advice: “Chlorophyll is great, but just eat more veggies.”
A version of this article first appeared on Medscape.com.
With the holidays just around the corner (how did that happen?), it’s a good time to remind yourself of the things you’re grateful for.
Perhaps you’re grateful for spending chilly evenings under a warm blanket binge-watching your favorite shows or being able to safely gather with loved ones. If you’re William Shatner, maybe you’re grateful for that quick trip to space (because apparently, that’s a thing now) and the poetic tweets it induced. Down here on earth, TikTok has surpassed 1 billion users, and while we’re not grateful, necessarily, we are entertained.
Here are the latest ugly, good, and bad TikToks that have been trending lately.
The Ugly: Scalp popping
Warning: Don’t watch this if you’re easily freaked out by weird body sounds. It’s like cracking your knuckles but way, way worse.
This TikTok from @asmr.barber has 1.7 million likes, and lots of people are trying it out for themselves. The viral video features the (disturbed) art of scalp popping, also known as hair cracking. It features what is assumed to be some sort of barber or professional (here’s hoping) twisting a client’s hair around his fingers and then yanking, creating an audible popping sound. Many are posting their own hair-cracking attempts on the platform. It’s unclear if this is supposed to feel good or just be grossly satisfying, though some users claim it helps with migraines.
But it turns out this might be more than kind of gross; it can be dangerous, too.
Anthony Youn, MD, a board-certified plastic surgeon, comments on the trend with concern: “What the hell is going on here?” Not something you want to hear from a doctor. Dr. Youn explained that the popping sound comes from the galea aponeurotica, a fibrous sheet of connective tissue under your scalp, being pulled off the skull.
In a comment, Dr. Youn continued to warn people of replicating this trend: “It can tear the inside of the scalp, which can bleed a ton on the inside. Think boxer or MMA fighter with scalp hematoma.”
Let’s keep our scalps attached to our skulls, people. If I never have to hear that sound again, I’ll be eternally grateful.
The Good: Doctor demonstrates correct EpiPen use
This reaction TikTok from medical student Mutahir Farhan (aka @madmedicine) has over 252,000 likes and hundreds of comments. In it, Ms. Farhan watches a video of a young woman attempting to administer an EpiPen to her friend, with the caption “How NOT to use an EpiPen” over it (in bright red, of course).
The woman in the video is using the wrong end of the EpiPen against her friend’s leg, so it isn’t working. When she uses her thumb to press down and help, her thumb is actually pressed against the needle end and the EpiPen sticks her instead of her friend. Ouch!
Ms. Farhan goes on to explain the anatomy of the EpiPen and shows his audience of 1.1 million followers where to inject it.
“You gotta remember that the orange tip is where the needle comes out. Otherwise, you’re going to end up stabbing yourself with epinephrine, like that girl in the video,” Ms. Farhan says. He goes on to instruct the important, but often overlooked, follow-up: “After you stab someone with epinephrine, call 911 or go to the ER, so that we can make sure they’re actually okay and good to go.”
The Bad: Liquid chlorophyll
Here is another one of those tricky trends that are so widespread and popular that it’s hard to find exactly where it originated from. A video from @lenamaiah has over 5 million views and 800,000 likes, which even by TikTok standards, is a lot. TikTok is rife with similar videos, which feature drops of liquid chlorophyll being added to water and smoothies.
The pretty emerald hue is mesmerizing and it’s hard to resist trying it out when it’s being peddled by seemingly every pretty, smooth-skinned pseudo-model on the platform. In this video, Lena says drinking a glass of water with a few drops of chlorophyll can reduce inflammation, get rid of eye bags, boost your vitamin levels, reduce free radical damage, detoxify your system, and file your taxes. Okay, I made that last one up, but it follows, doesn’t it? This stuff sounds pretty good. Maybe too good.
Chlorophyll, if you skipped biology class (somehow, I doubt you did), is what makes plants green. Medscape has a detailed explanation of chlorophyll, but all you really need to know is that it’s the secret to that cool thing plants do: photosynthesis, or turning sunlight into energy. Scientists have been trying to find uses for it in people since the 1940s. Unfortunately, studies never found much that it can do for us, aside from being kind of deodorizing. So, while it’s been historically marketed as toothpaste and deodorant, the new TikTok claims of it being a cure-all or the next big skincare supplement are not widely substantiated by scientific studies. The only real evidence of it being effective is word of mouth from those who claim to like the way they look or feel since taking it, which isn’t enough for doctors to recommend it.
TikTok’s resident dermatologist, Muneeb Shah, DO, stitched a TikTok from another user, with his captions explaining, “[There’s] no scientific evidence for liquid chlorophyll [helping] rosacea or acne.”
His advice: “Chlorophyll is great, but just eat more veggies.”
A version of this article first appeared on Medscape.com.
‘Misleading’ results in colchicine COVID-19 trials meta-analysis
A new meta-analysis appears to show that colchicine has no benefit as a treatment for COVID-19, but its inclusion of trials studying differing patient populations and testing different outcomes led to “misleading” results, says a researcher involved in one of the trials.
The meta-analysis, which includes data from the recent Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, was published Nov. 22 in RMD Open.
Kedar Gautambhai Mehta, MBBS, MD, of the GMERS Medical College Gotri in Vadodara, India, and colleagues included outcomes from six studies of 16,148 patients with COVID-19 who received colchicine or supportive care. They evaluated the efficacy outcomes of mortality, need for ventilation, intensive care unit admission, and length of stay in hospital, as well as safety outcomes of adverse events, serious adverse events, and diarrhea.
The studies in the meta-analysis included a randomized, controlled trial (RCT) of 105 patients hospitalized with COVID-19 in Greece, the international, open-label RECOVERY RCT of 11,340 patients hospitalized with COVID-19, an RCT of 72 hospitalized patients with moderate or severe COVID-19 in Brazil, an RCT of 100 patients hospitalized with COVID-19 in Iran, the international COLCORONA trial of 4,488 patients with COVID-19 who were treated with colchicine or placebo on an outpatient basis, and the randomized COLORIT trial of 43 patients hospitalized with COVID-19 in Russia.
Studies “asked very different questions” about colchicine
Commenting on the meta-analysis, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology with New York University, said the authors combined studies “that are not comparable and that asked very different questions.” Two of the studies in the meta-analysis are very large, and four are very small, which skews the results, he explained.
“The larger studies therefore drive the outcome, and while the small studies are potentially insight providing, the large studies are the only ones worth giving our attention to in the context of the meta-analysis,” he said. The two largest studies – RECOVERY and COLCORONA – taken together show no benefit for colchicine as a treatment, even though the former demonstrated no benefit and the latter did show a benefit, explained Dr. Pillinger, a co–principal investigator for the COLCORONA trial in the United States.
The studies were designed differently and should not have been included in the same analysis, Dr. Pillinger argued. In the case of COLCORONA, early treatment with colchicine was the intervention, whereas RECOVERY focused on hospitalized patients.
“In designing [COLCORONA], the author group (of whom I was a member) expressly rejected the idea that colchicine might be useful for the sicker hospitalized patients, based on the long experience with colchicine of some of us as rheumatologists,” Dr. Pillinger said.
“In short, COLCORONA proved a benefit of colchicine in outpatient COVID-19, and its authors presumed there would be no inpatient benefit; RECOVERY went ahead and proved a lack of inpatient benefit, at least when high-dose steroids were also given,” he said. “While there is no conflict between these results, the combination of the two studies in this meta-analysis suggests there might be no benefit for colchicine overall, which is misleading and can lead physicians to reject the potential of outpatient colchicine, even for future studies.”
Dr. Pillinger said he still believes colchicine has potential value as a COVID-19 treatment option for patients with mild disease, “especially for low–vaccine rate, resource-starved countries.
“It would be unfortunate if meta-analyses such as this one would put a stop to colchicine’s use, or at least its further investigation,” he said.
Study details
The authors of the study assessed heterogeneity of the trials’ data across the outcomes using an I2 test. They evaluated the quality of the evidence for the outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).
The results of their meta-analysis showed that colchicine offered no significant improvement in mortality in six studies (risk difference, –0.0; 95% confidence interval, –0.01 to 0.01; I2 = 15%). It showed no benefit with respect to requiring ventilatory support in five studies of 15,519 patients (risk ratio, 0.67; 95% CI, 0.38-1.21; I2 = 47%); being admitted to the ICU in three studies with 220 patients (RR, 0.49; 95% CI, 0.19-1.25; I2 = 34%); and length of stay while in the hospital in four studies of 11,560 patients (mean difference, –1.17; 95% CI, –3.02 to 0.67; I2 = 77%).
There was no difference in serious adverse events in three studies with 4,665 patients (RD, –0.01; 95% CI, –0.02 to 0.00; I2 = 28%) for patients who received colchicine, compared with supportive care alone. Patients who received colchicine were more likely to have a higher rate of adverse events (RR, 1.58; 95% CI, 1.07-2.33; I2 = 81%) and to experience diarrhea (RR, 1.93; 95% CI, 1.62-2.29; I2 = 0%) than were patients who received supportive care alone. The researchers note that for most outcomes, the GRADE quality of evidence was moderate.
“Our findings on colchicine should be interpreted cautiously due to the inclusion of open-labeled, randomized clinical trials,” Dr. Mehta and colleagues write. “The analysis of efficacy and safety outcomes are based on a small number of RCTs in control interventions.”
The authors reported no relevant financial relationships. Dr. Pillinger is co–principal investigator of the U.S. component of the COLCORONA trial; he reported no other relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis appears to show that colchicine has no benefit as a treatment for COVID-19, but its inclusion of trials studying differing patient populations and testing different outcomes led to “misleading” results, says a researcher involved in one of the trials.
The meta-analysis, which includes data from the recent Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, was published Nov. 22 in RMD Open.
Kedar Gautambhai Mehta, MBBS, MD, of the GMERS Medical College Gotri in Vadodara, India, and colleagues included outcomes from six studies of 16,148 patients with COVID-19 who received colchicine or supportive care. They evaluated the efficacy outcomes of mortality, need for ventilation, intensive care unit admission, and length of stay in hospital, as well as safety outcomes of adverse events, serious adverse events, and diarrhea.
The studies in the meta-analysis included a randomized, controlled trial (RCT) of 105 patients hospitalized with COVID-19 in Greece, the international, open-label RECOVERY RCT of 11,340 patients hospitalized with COVID-19, an RCT of 72 hospitalized patients with moderate or severe COVID-19 in Brazil, an RCT of 100 patients hospitalized with COVID-19 in Iran, the international COLCORONA trial of 4,488 patients with COVID-19 who were treated with colchicine or placebo on an outpatient basis, and the randomized COLORIT trial of 43 patients hospitalized with COVID-19 in Russia.
Studies “asked very different questions” about colchicine
Commenting on the meta-analysis, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology with New York University, said the authors combined studies “that are not comparable and that asked very different questions.” Two of the studies in the meta-analysis are very large, and four are very small, which skews the results, he explained.
“The larger studies therefore drive the outcome, and while the small studies are potentially insight providing, the large studies are the only ones worth giving our attention to in the context of the meta-analysis,” he said. The two largest studies – RECOVERY and COLCORONA – taken together show no benefit for colchicine as a treatment, even though the former demonstrated no benefit and the latter did show a benefit, explained Dr. Pillinger, a co–principal investigator for the COLCORONA trial in the United States.
The studies were designed differently and should not have been included in the same analysis, Dr. Pillinger argued. In the case of COLCORONA, early treatment with colchicine was the intervention, whereas RECOVERY focused on hospitalized patients.
“In designing [COLCORONA], the author group (of whom I was a member) expressly rejected the idea that colchicine might be useful for the sicker hospitalized patients, based on the long experience with colchicine of some of us as rheumatologists,” Dr. Pillinger said.
“In short, COLCORONA proved a benefit of colchicine in outpatient COVID-19, and its authors presumed there would be no inpatient benefit; RECOVERY went ahead and proved a lack of inpatient benefit, at least when high-dose steroids were also given,” he said. “While there is no conflict between these results, the combination of the two studies in this meta-analysis suggests there might be no benefit for colchicine overall, which is misleading and can lead physicians to reject the potential of outpatient colchicine, even for future studies.”
Dr. Pillinger said he still believes colchicine has potential value as a COVID-19 treatment option for patients with mild disease, “especially for low–vaccine rate, resource-starved countries.
“It would be unfortunate if meta-analyses such as this one would put a stop to colchicine’s use, or at least its further investigation,” he said.
Study details
The authors of the study assessed heterogeneity of the trials’ data across the outcomes using an I2 test. They evaluated the quality of the evidence for the outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).
The results of their meta-analysis showed that colchicine offered no significant improvement in mortality in six studies (risk difference, –0.0; 95% confidence interval, –0.01 to 0.01; I2 = 15%). It showed no benefit with respect to requiring ventilatory support in five studies of 15,519 patients (risk ratio, 0.67; 95% CI, 0.38-1.21; I2 = 47%); being admitted to the ICU in three studies with 220 patients (RR, 0.49; 95% CI, 0.19-1.25; I2 = 34%); and length of stay while in the hospital in four studies of 11,560 patients (mean difference, –1.17; 95% CI, –3.02 to 0.67; I2 = 77%).
There was no difference in serious adverse events in three studies with 4,665 patients (RD, –0.01; 95% CI, –0.02 to 0.00; I2 = 28%) for patients who received colchicine, compared with supportive care alone. Patients who received colchicine were more likely to have a higher rate of adverse events (RR, 1.58; 95% CI, 1.07-2.33; I2 = 81%) and to experience diarrhea (RR, 1.93; 95% CI, 1.62-2.29; I2 = 0%) than were patients who received supportive care alone. The researchers note that for most outcomes, the GRADE quality of evidence was moderate.
“Our findings on colchicine should be interpreted cautiously due to the inclusion of open-labeled, randomized clinical trials,” Dr. Mehta and colleagues write. “The analysis of efficacy and safety outcomes are based on a small number of RCTs in control interventions.”
The authors reported no relevant financial relationships. Dr. Pillinger is co–principal investigator of the U.S. component of the COLCORONA trial; he reported no other relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis appears to show that colchicine has no benefit as a treatment for COVID-19, but its inclusion of trials studying differing patient populations and testing different outcomes led to “misleading” results, says a researcher involved in one of the trials.
The meta-analysis, which includes data from the recent Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, was published Nov. 22 in RMD Open.
Kedar Gautambhai Mehta, MBBS, MD, of the GMERS Medical College Gotri in Vadodara, India, and colleagues included outcomes from six studies of 16,148 patients with COVID-19 who received colchicine or supportive care. They evaluated the efficacy outcomes of mortality, need for ventilation, intensive care unit admission, and length of stay in hospital, as well as safety outcomes of adverse events, serious adverse events, and diarrhea.
The studies in the meta-analysis included a randomized, controlled trial (RCT) of 105 patients hospitalized with COVID-19 in Greece, the international, open-label RECOVERY RCT of 11,340 patients hospitalized with COVID-19, an RCT of 72 hospitalized patients with moderate or severe COVID-19 in Brazil, an RCT of 100 patients hospitalized with COVID-19 in Iran, the international COLCORONA trial of 4,488 patients with COVID-19 who were treated with colchicine or placebo on an outpatient basis, and the randomized COLORIT trial of 43 patients hospitalized with COVID-19 in Russia.
Studies “asked very different questions” about colchicine
Commenting on the meta-analysis, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology with New York University, said the authors combined studies “that are not comparable and that asked very different questions.” Two of the studies in the meta-analysis are very large, and four are very small, which skews the results, he explained.
“The larger studies therefore drive the outcome, and while the small studies are potentially insight providing, the large studies are the only ones worth giving our attention to in the context of the meta-analysis,” he said. The two largest studies – RECOVERY and COLCORONA – taken together show no benefit for colchicine as a treatment, even though the former demonstrated no benefit and the latter did show a benefit, explained Dr. Pillinger, a co–principal investigator for the COLCORONA trial in the United States.
The studies were designed differently and should not have been included in the same analysis, Dr. Pillinger argued. In the case of COLCORONA, early treatment with colchicine was the intervention, whereas RECOVERY focused on hospitalized patients.
“In designing [COLCORONA], the author group (of whom I was a member) expressly rejected the idea that colchicine might be useful for the sicker hospitalized patients, based on the long experience with colchicine of some of us as rheumatologists,” Dr. Pillinger said.
“In short, COLCORONA proved a benefit of colchicine in outpatient COVID-19, and its authors presumed there would be no inpatient benefit; RECOVERY went ahead and proved a lack of inpatient benefit, at least when high-dose steroids were also given,” he said. “While there is no conflict between these results, the combination of the two studies in this meta-analysis suggests there might be no benefit for colchicine overall, which is misleading and can lead physicians to reject the potential of outpatient colchicine, even for future studies.”
Dr. Pillinger said he still believes colchicine has potential value as a COVID-19 treatment option for patients with mild disease, “especially for low–vaccine rate, resource-starved countries.
“It would be unfortunate if meta-analyses such as this one would put a stop to colchicine’s use, or at least its further investigation,” he said.
Study details
The authors of the study assessed heterogeneity of the trials’ data across the outcomes using an I2 test. They evaluated the quality of the evidence for the outcomes using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).
The results of their meta-analysis showed that colchicine offered no significant improvement in mortality in six studies (risk difference, –0.0; 95% confidence interval, –0.01 to 0.01; I2 = 15%). It showed no benefit with respect to requiring ventilatory support in five studies of 15,519 patients (risk ratio, 0.67; 95% CI, 0.38-1.21; I2 = 47%); being admitted to the ICU in three studies with 220 patients (RR, 0.49; 95% CI, 0.19-1.25; I2 = 34%); and length of stay while in the hospital in four studies of 11,560 patients (mean difference, –1.17; 95% CI, –3.02 to 0.67; I2 = 77%).
There was no difference in serious adverse events in three studies with 4,665 patients (RD, –0.01; 95% CI, –0.02 to 0.00; I2 = 28%) for patients who received colchicine, compared with supportive care alone. Patients who received colchicine were more likely to have a higher rate of adverse events (RR, 1.58; 95% CI, 1.07-2.33; I2 = 81%) and to experience diarrhea (RR, 1.93; 95% CI, 1.62-2.29; I2 = 0%) than were patients who received supportive care alone. The researchers note that for most outcomes, the GRADE quality of evidence was moderate.
“Our findings on colchicine should be interpreted cautiously due to the inclusion of open-labeled, randomized clinical trials,” Dr. Mehta and colleagues write. “The analysis of efficacy and safety outcomes are based on a small number of RCTs in control interventions.”
The authors reported no relevant financial relationships. Dr. Pillinger is co–principal investigator of the U.S. component of the COLCORONA trial; he reported no other relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Positive Outcomes Following a Multidisciplinary Approach in the Diagnosis and Prevention of Hospital Delirium
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens
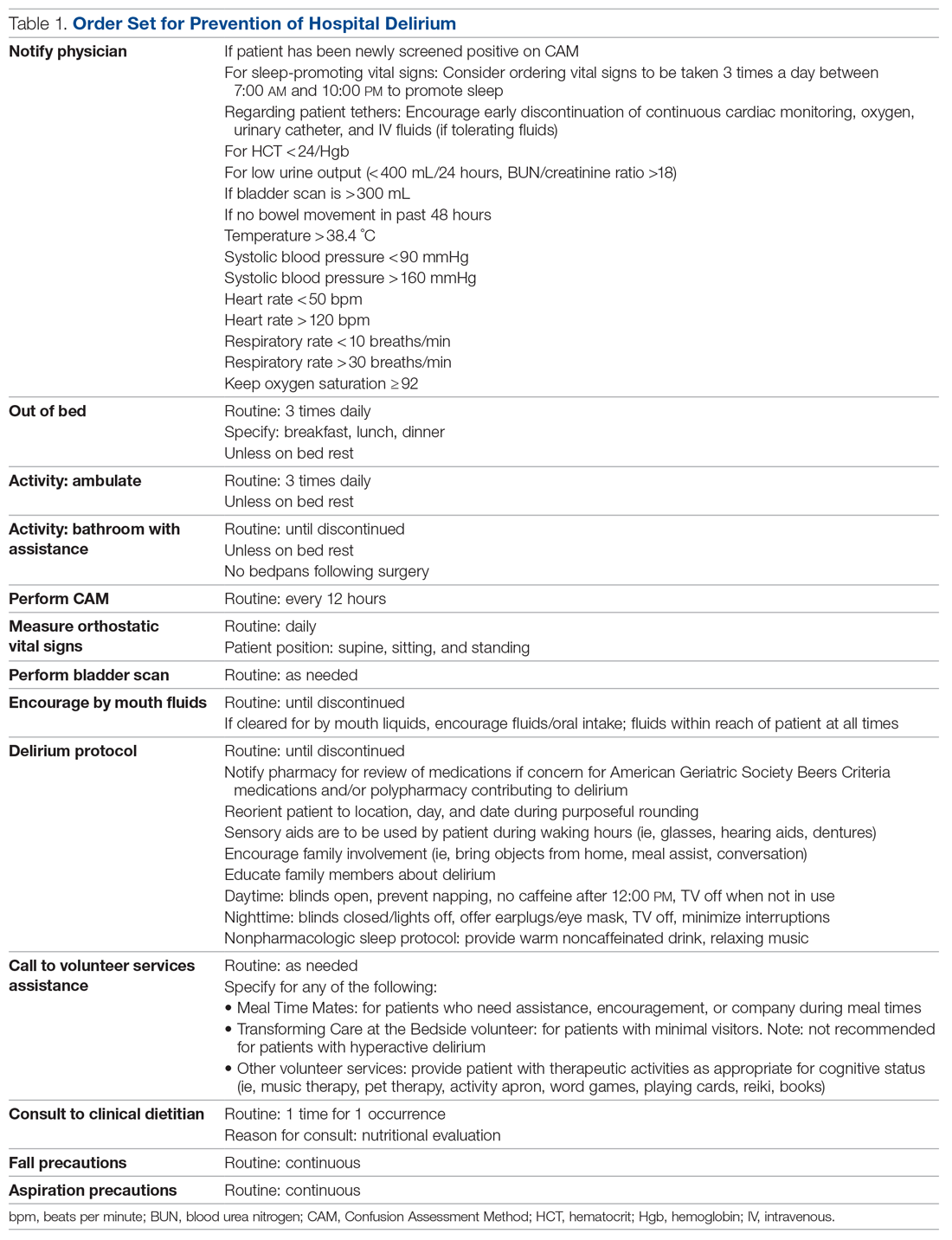
Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.
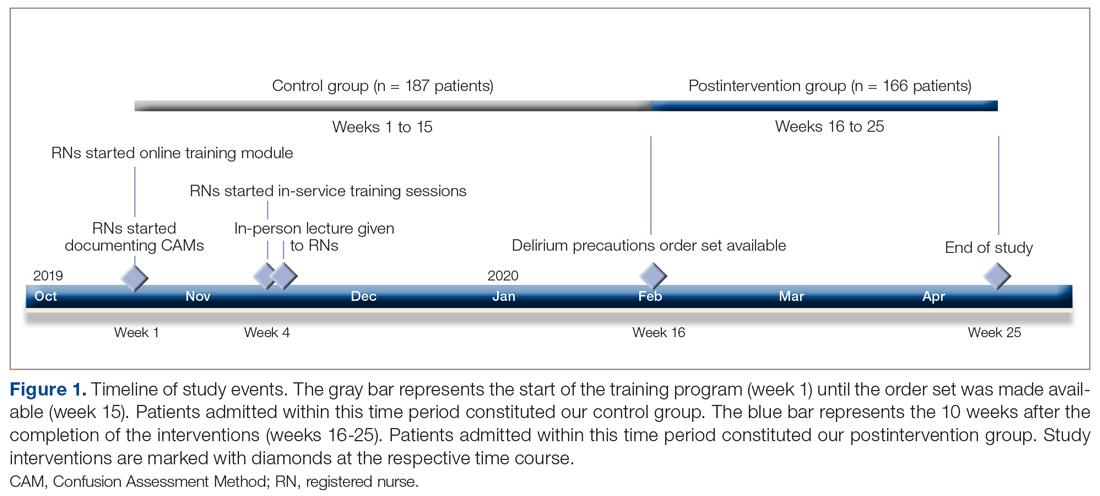
Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.
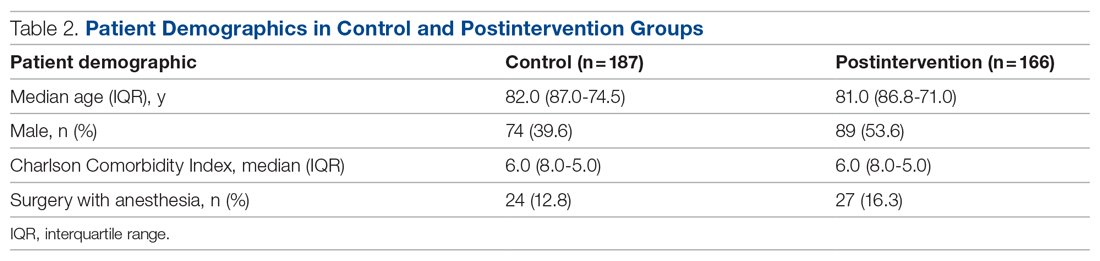
In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.
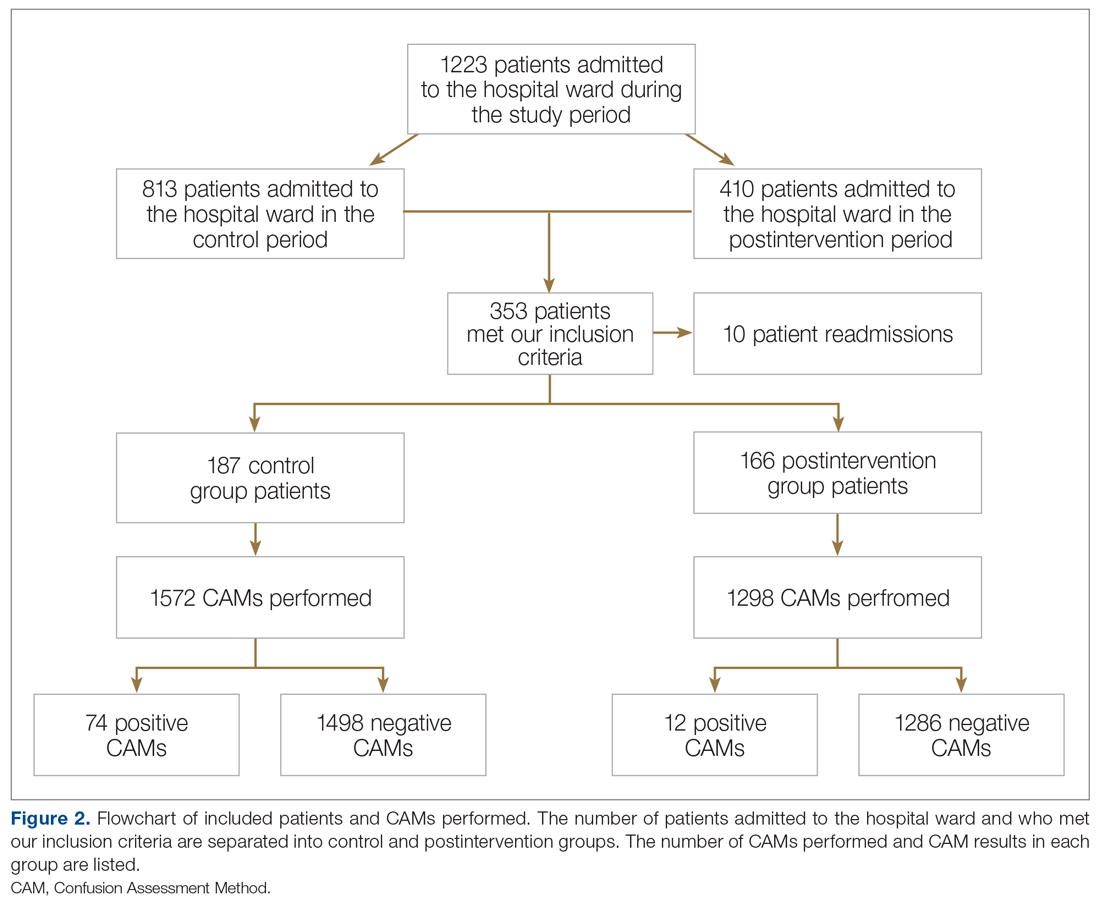
Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.
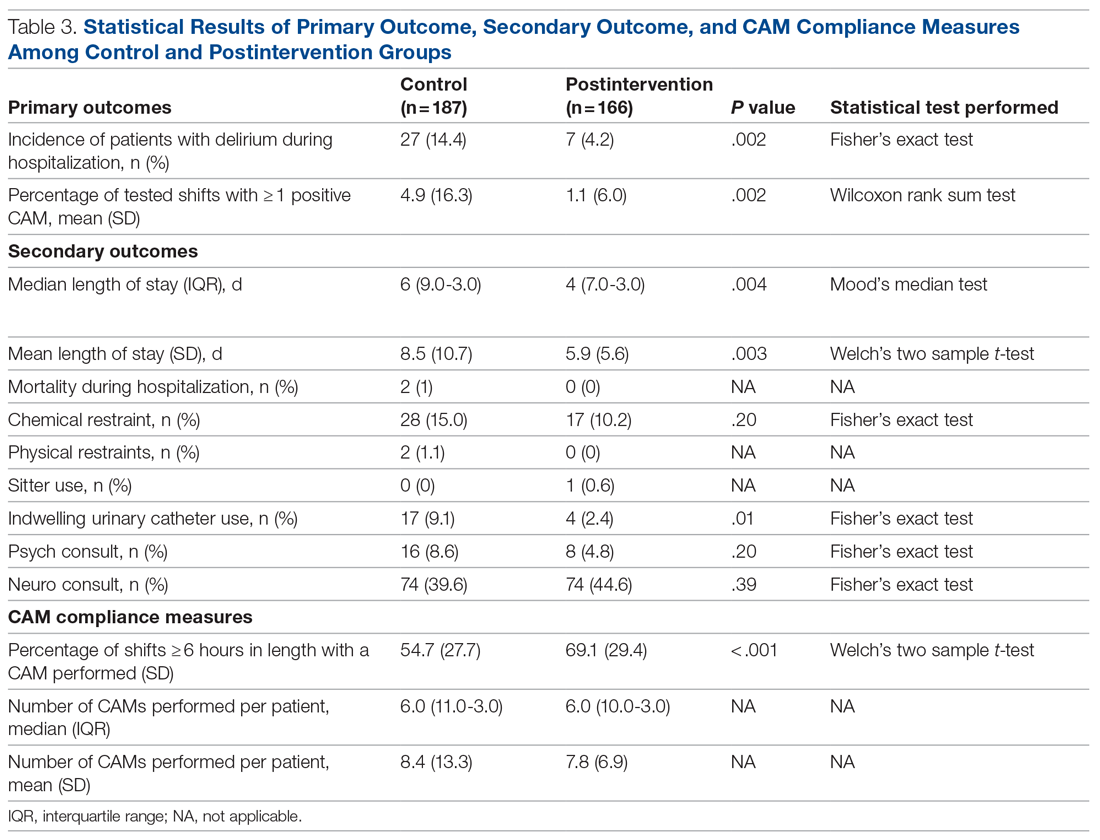
Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.
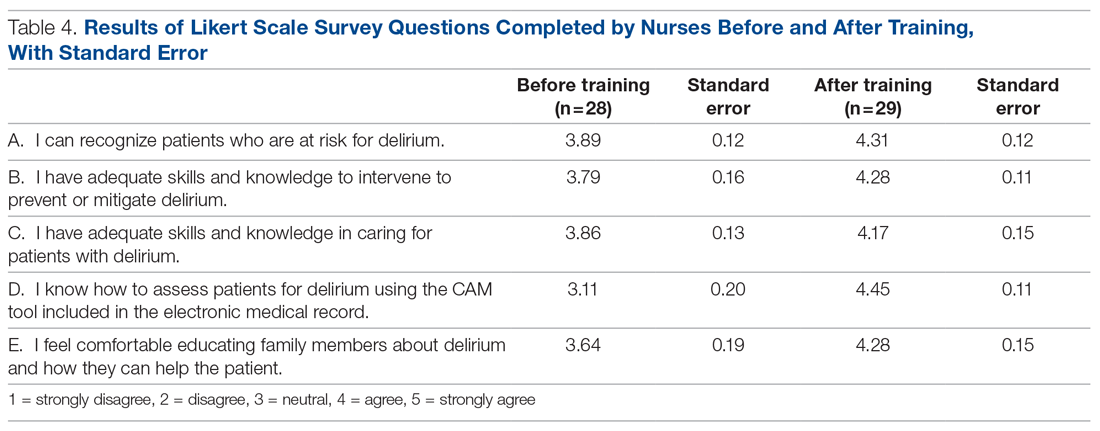
Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; [email protected].
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens

Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.

Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.

In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.

Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.

Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.

Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; [email protected].
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
From the Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, CA (Drs. Ching, Darwish, Li, Wong, Simpson, and Funk), the Department of Anesthesia, Cedars-Sinai Medical Center, Los Angeles, CA (Keith Siegel), and the Department of Psychiatry, Cedars-Sinai Medical Center, Los Angeles, CA (Dr. Bamgbose).
Objectives: To reduce the incidence and duration of delirium among patients in a hospital ward through standardized delirium screening tools and nonpharmacologic interventions. To advance nursing-focused education on delirium-prevention strategies. To measure the efficacy of the interventions with the aim of reproducing best practices.
Background: Delirium is associated with poor patient outcomes but may be preventable in a significant percentage of hospitalized patients.
Methods: Following nursing-focused education to prevent delirium, we prospectively evaluated patient care outcomes in a consecutive series of patients who were admitted to a hospital medical-surgical ward within a 25-week period. All patients who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria (N = 353). Standards for Quality Improvement Reporting Excellence guidelines were adhered to.
Results: There were 187 patients in the control group, and 166 in the postintervention group. Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days), mean length of stay (8.5 days vs 5.9 days), and use of an indwelling urinary catheter (9.1% vs 2.4%).
Conclusion: A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs.
Delirium is a disorder characterized by inattention and acute changes in cognition. It is defined by the American Psychiatric Association’s fifth edition of the Diagnostic and Statistical Manual of Mental Disorders as a disturbance in attention, awareness, and cognition over hours to a few days that is not better explained by a preexisting, established, or other evolving neurocognitive disorder.1 Delirium is common yet often under-recognized among hospitalized patients, particularly in the elderly. The incidence of delirium in elderly patients on admission is estimated to be 11% to 25%, and an additional 29% to 31% of elderly patients will develop delirium during the hospitalization.2 Delirium costs the health care system an estimated $38 billion to $152 billion per year.3 It is associated with negative outcomes, such as increased new placements to nursing homes, increased mortality, increased risk of dementia, and further cognitive deterioration among patients with dementia.4-6
Despite its prevalence, delirium may be preventable in a significant percentage of hospitalized patients. Targeted intervention strategies, such as frequent reorientation, maximizing sleep, early mobilization, restricting use of psychoactive medications, and addressing hearing or vision impairment, have been demonstrated to significantly reduce the incidence of hospital delirium.7,8 To achieve these goals, we explored the use of a multimodal strategy centered on nursing education. We integrated consistent, standardized delirium screening and nonpharmacologic interventions as part of a preventative protocol to reduce the incidence of delirium in the hospital ward.
Methods
We evaluated a consecutive series of patients who were admitted to a designated hospital medical-surgical ward within a 25-week period between October 2019 and April 2020. All patients during this period who had at least 1 Confusion Assessment Method (CAM) documented by a nurse during hospitalization met our inclusion criteria. Patients who did not have a CAM documented were excluded from the analysis. Delirium was defined according to the CAM diagnostic algorithm.9
Core nursing staff regularly assigned to the ward completed a multimodal training program designed to improve recognition, documentation, and prevention of hospital delirium. Prior to the training, the nurses completed a 5-point Likert scale survey assessing their level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium. Nurses completed the same survey after the study period ended.
The training curriculum for nurses began with an online module reviewing the epidemiology and risk factors for delirium. Nurses then participated in a series of in-service training sessions led by a team of physicians, during which the CAM and nonpharmacologic delirium prevention measures were reviewed then practiced first-hand. Nursing staff attended an in-person lecture reviewing the current body of literature on delirium risk factors and effective nursing interventions. After formal training was completed, nurses were instructed to document CAM screens

Patients admitted to the hospital unit from the start of the training program (week 1) until the order set was made available (week 15) constituted our control group. The postintervention study group consisted of patients admitted for 10 weeks after the completion of the interventions (weeks 16-25). A timeline of the study events is shown in Figure 1.

Patient demographics and hospital-stay metrics determined a priori were attained via the Cedars-Sinai Enterprise Information Services core. Age, sex, medical history, and incidence of surgery with anesthesia during hospitalization were recorded. The Charlson Comorbidity Index was calculated from patients’ listed diagnoses following discharge. Primary outcomes included incidence of patients with delirium during hospitalization, percentage of tested shifts with positive CAM screens, length of hospital stay, and survival. Secondary outcomes included measures associated with delirium, including the use of chemical restraints, physical restraints, sitters, indwelling urinary catheters, and new psychiatry and neurology consults. Chemical restraints were defined as administration of a new antipsychotic medication or benzodiazepine for the specific indication of hyperactive delirium or agitation.
Statistical analysis was conducted by a statistician, using R version 3.6.3.10P values of < .05 were considered significant. Categorical variables were analyzed using Fisher’s exact test. Continuous variables were analyzed with Welch’s t-test or, for highly skewed continuous variables, with Wilcoxon rank-sum test or Mood’s median test. All patient data were anonymized and stored securely in accordance with institutional guidelines.
Our project was deemed to represent nonhuman subject research and therefore did not require Institutional Review Board (IRB) approval upon review by our institution’s IRB committee and Office of Research Compliance and Quality Improvement. Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were adhered to (Supplementary File can be found at mdedge.com/jcomjournal).
Results
We evaluated 353 patients who met our inclusion criteria: 187 in the control group, and 166 in the postintervention group. Ten patients were readmitted to the ward after their initial discharge; only the initial admission encounters were included in our analysis. Median age, sex, median Charlson Comorbidity Index, and incidence of surgery with anesthesia during hospitalization were comparable between the control and postintervention groups and are summarized in Table 2.

In the control group, 1572 CAMs were performed, with 74 positive CAMs recorded among 27 patients with delirium. In the postintervention group, 1298 CAMs were performed, with 12 positive CAMs recorded among 7 patients with delirium (Figure 2). Primary and secondary outcomes, as well as CAM compliance measures, are summarized in Table 3.

Compared to the control group, the postintervention group had a significant decrease in the incidence of delirium during hospitalization (14.4% vs 4.2%, P = .002) and a significant decrease in the mean percentage of tested nursing shifts with 1 or more positive CAM (4.9% vs 1.1%, P = .002). Significant differences in secondary outcomes between the control and postintervention groups included median length of stay (6 days vs 4 days, P = .004), mean length of stay (8.5 days vs 5.9 days, P = .003), and use of an indwelling urinary catheter (9.1% vs 2.4%, P = .012). There was a trend towards decreased incidence of chemical restraints and psychiatry consults, which did not reach statistical significance. Differences in mortality during hospitalization, physical restraint use, and sitter use could not be assessed due to low incidence.

Compliance with nursing CAM assessments was evaluated. Compared to the control group, the postintervention group saw a significant increase in the percentage of shifts with a CAM performed (54.7% vs 69.1%, P < .001). The median and mean number of CAMs performed per patient were similar between the control and postintervention groups.
Results of nursing surveys completed before and after the training program are listed in Table 4. After training, nurses had a greater level of confidence with recognizing delirium risk factors, preventing delirium, addressing delirium, utilizing the CAM tool, and educating others about delirium.

Discussion
Our study utilized a standardized delirium assessment tool to compare patient cohorts before and after nurse-targeted training interventions on delirium recognition and prevention. Our interventions emphasized nonpharmacologic intervention strategies, which are recommended as first-line in the management of patients with delirium.11 Patients were not excluded from the analysis based on preexisting medical conditions or recent surgery with anesthesia, to allow for conditions that are representative of community hospitals. We also did not use an inclusion criterion based on age; however, the majority of our patients were greater than 70 years old, representing those at highest risk for delirium.2 Significant outcomes among patients in the postintervention group include decreased incidence of delirium, lower average length of stay, decreased indwelling urinary catheter use, and increased compliance with delirium screening by nursing staff.
While the study’s focus was primarily on delirium prevention rather than treatment, these strategies may also have conferred the benefit of reversing delirium symptoms. In addition to measuring incidence of delirium, our primary outcome of percentage of tested shifts with 1 or more positive CAM was intended to assess the overall duration in which patients had delirium during their hospitalization. The reduction in shifts with positive CAMs observed in the postintervention group is notable, given that a significant percentage of patients with hospital delirium have the potential for symptom reversibility.12
Multiple studies have shown that admitted patients who develop delirium experience prolonged hospital stays, often up to 5 to 10 days longer.12-14 The decreased incidence and duration of delirium in our postintervention group is a reasonable explanation for the observed decrease in average length of stay. Our study is in line with previously documented initiatives that show that nonpharmacologic interventions can effectively address downstream health and fiscal sequelae of hospital delirium. For example, a volunteer-based initiative named the Hospital Elder Life Program, from which elements in our order set were modeled after, demonstrated significant reductions in delirium incidence, length of stay, and health care costs.14-16 Other initiatives that focused on educational training for nurses to assess and prevent delirium have also demonstrated similar positive results.17-19 Our study provides a model for effective nursing-focused education that can be reproduced in the hospital setting.
Unlike some other studies, which identified delirium based only on physician assessments, our initiative utilized the CAM performed by floor nurses to identify delirium. While this method
Our study demonstrated an increase in the overall compliance with the CAM screening during the postintervention period, which is significant given the under-recognition of delirium by health care professionals.20 We attribute this increase to greater realized importance and a higher level of confidence from nursing staff in recognizing and addressing delirium, as supported by survey data. While the increased screening of patients should be considered a positive outcome, it also poses the possibility that the observed decrease in delirium incidence in the postintervention group was in fact due to more CAMs performed on patients without delirium. Likewise, nurses may have become more adept at recognizing true delirium, as opposed to delirium mimics, in the latter period of the study.
Perhaps the greatest limitation of our study is the variability in performing and recording CAMs, as some patients had multiple CAMs recorded while others did not have any CAMs recorded. This may have been affected in part by the increase in COVID-19 cases in our hospital towards the latter half of the study, which resulted in changes in nursing assignments as well as patient comorbidities in ways that cannot be easily quantified. Given the limited size of our patient cohorts, certain outcomes, such as the use of sitters, physical restraints, and in-hospital mortality, were unable to be assessed for changes statistically. Causative relationships between our interventions and associated outcome measures are necessarily limited in a binary comparison between control and postintervention groups.
Within these limitations, our study demonstrates promising results in core dimensions of patient care. We anticipate further quality improvement initiatives involving greater numbers of nursing staff and patients to better quantify the impact of nonpharmacologic nursing-centered interventions for preventing hospital delirium.
Conclusion
A multimodal strategy involving nursing-focused training and nonpharmacologic interventions to address hospital delirium is associated with improved patient care outcomes and nursing confidence. Nurses play an integral role in the early recognition and prevention of hospital delirium, which directly translates to reducing burdens in both patient functionality and health care costs. Education and tools to equip nurses to perform standardized delirium screening and interventions should be prioritized.
Acknowledgment: The authors thanks Olena Svetlov, NP, Oscar Abarca, Jose Chavez, and Jenita Gutierrez.
Corresponding author: Jason Ching, MD, Department of Neurology, Cedars-Sinai Medical Center, 8700 Beverly Blvd, Los Angeles, CA 90048; [email protected].
Financial disclosures: None.
Funding: This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
2. Vasilevskis EE, Han JH, Hughes CG, et al. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277-287. doi:10.1016/j.bpa.2012.07003
3. Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27-32. doi:10.1001/archinternmed.2007.4
4. McCusker J, Cole M, Abrahamowicz M, et al. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463. doi:10.1001/archinte.162.4.457
5. Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. doi:10.1001/jama.2010.1013
6. Gross AL, Jones RN, Habtemariam DA, et al. Delirium and long-term cognitive trajectory among persons with dementia. Arch Intern Med. 2012;172(17):1324-1331. doi:10.1001/archinternmed.2012.3203
7. Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32(4):257-263. doi:10.3109/07853890009011770
8. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi:10.1002/14651858.CD005563.pub3
9. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi:10.7326/0003-4819-113-12-941
10. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2017.
11. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. doi:10.1038/nrneurol.2009.24
12. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364. doi:10.1093/ageing/afl005
13. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi:10.1001/jama.291.14.1753
14. Chen CC, Lin MT, Tien YW, et al. Modified Hospital Elder Life Program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213(2):245-252. doi:10.1016/j.jamcollsurg.2011.05.004
15. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the Hospital Elder Life Program in a community hospital. Psychosomatics. 2013;54(3):219-226. doi:10.1016/j.psym.2013.01.010
16. Rubin FH, Neal K, Fenlon K, et al. Sustainability and scalability of the Hospital Elder Life Program at a community hospital. J Am Geriatr Soc. 2011;59(2):359-365. doi:10.1111/j.1532-5415.2010.03243.x
17. Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49(5):523-532. doi:10.1046/j.1532-5415.2001.49109.x
18. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. doi:10.1111/j.1532-5415.2005.53210.x
19. Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34(2):152-156. doi:10.1093/ageing/afi0320. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200. doi:10.1111/j.1553-2712.2008.00339.x
Social media use associated with depression in adults
Use of social media has been linked to increased anxiety and depression, as well as reduced well-being in adolescents and young adults, but similar associations in older adults have not been well studied, and longitudinal data are lacking, Ron H. Perlis, MD, of Massachusetts General Hospital, Boston, and colleagues wrote in their paper, which was published in JAMA Network Open.
To examine the association between social media use and depressive symptoms in older adults, the researchers reviewed data from 13 waves of an internet survey conducted each month between May 2020 and May 2021. The survey respondents included individuals aged 18 years and older, with a mean age of 56 years.
In the study the researchers analyzed responses from 5,395 individuals aged 18 years and older, with a mean age of 56 years. The study participants had minimal or no depressive symptoms at baseline, according to scores on the nine-item Patient Health Questionnaire (PHQ-9).
Overall, 8.9% of the respondents reported a worsening of 5 points or more on the PHQ-9 score on a follow-up survey, which was the primary outcome. Participants who reported using social media platforms Snapchat, Facebook, or TikTok were significantly more likely to report increased depressive symptoms, compared with those who did not report use of social media. The fully adjusted odds ratio was largest for Snapchat (aOR, 1.53), followed by Facebook (aOR, 1.42), and TikTok (aOR, 1.39).
Incorporating recent television and internet news terms, such as COVID-19, changed the association for Snapchat, for which the aOR decreased from 1.53 to 1.12 when news source terms were included in the survey. TikTok and Facebook associations remained similar.
When the results were further stratified by age, use of TikTok and Snapchat was associated with depressive symptoms in those aged 35 years and older, but not in those younger than 35 years. However, the opposite pattern emerged for Facebook; use was associated with depressive symptoms for individuals younger than 35 years, but not in those aged 35 years and older (aOR, 2.60 vs. aOR, 1.12).
The association between increased self-reported depressive symptoms and use of certain social media platforms was not impacted by baseline social support or face-to-face interactions, the researchers noted.
Family physician was surprised results weren’t more significant
In the current study, “I was honestly surprised the results weren’t more significant,” Mary Ann Dakkak, MD, of Boston University said in an interview. “That said, social media uses during the COVID pandemic may have been a necessary social outlet and form of connection for many people who were otherwise isolated.”
To still see a significant increase in depression when social media could have been a positive force may suggest a heavier impact during “normal” times, she added.
“It is not surprising that what we see in youth is shown among adults,” noted Dr. Dakkak, who was not involved with this study. “I always tell my patients that what is good for their children is good for the adults too, and vice versa.
“We expect to see outcomes of this on youth and adults who have been more isolated, who have used more screen time for learning, work, connection and boredom, in the near future,” she said. “The complex nature of why social media may have been used more heavily for connection during a time when in-person meetings were not possible may be a heavy confounder as the typical profile of heavy social media users may have differed during the COVID shutdowns.”
Psychiatrist: Balance benefits of social media with mental health risks
The current study was likely conducted before the recent news on “hidden” Facebook data and the implications that Facebook knew it was contributing to worsened mental health in teens, particularly around self-esteem, Jessica “Jessi” Gold, MD, a psychiatrist at Washington University, St. Louis, said in an interview.
“If you look more specifically at other studies, however, the data around social media and mental health is constantly varied, with some showing benefits and some showing negatives, and none conclusively suggesting either way,” said Dr. Gold, who also was not involved with the new research. “More data are needed, especially longitudinally and on a broader age group, to understand social media’s impact on mental health over time.
“It is also even more important in the wake of COVID-19, as so many people have turned to social media as a primary source of social support and connection, and are using it even more than before,” she emphasized.
In the current study, “I think the most interesting information is that, for TikTok and Snapchat, the effects seemed to be more pronounced in those older than 35 years who used social media,” said Dr. Gold.
What this study leaves unanswered is “whether people who might develop depression are simply more prone to use social media in the first place, such as to seek out social support,” Dr. Gold said. “Also, we don’t know anything about how long they are using social media or what they are using it for, which to me is important for understanding more about the nuance of the relationship with mental health and social media.”
Experts advise clinicians to discuss social media with patients
This new research suggests that clinicians should be talking to their patients about how social media impacts their emotional reactions, as well as their sleep, Dr. Gold said.
“Patients should be asking themselves how they are feeling when they are on social media and not using it before sleep. They should also be considering time limits and how to effectively use social media while taking care of their mental health,” she said. This conversation between clinician and patient should be had with any patient of any age, who uses social media, not only with teenagers.
“This is also a conversation about moderation, and knowing that individuals may feel they benefit from social media, that they should balance these benefits with potential mental health risks,” she said.
“Studies such as this one shed light onto why social media consumption should be at least a point of discussion with our patients,” said Dr. Dakkak.
She advised clinicians to ask and listen to patients and their families when it comes to screen time habits. “Whenever I see a patient with mood symptoms, I ask about their habits – eating, sleeping, socializing, screen time – including phone time. I ask about the family dynamics around screen time.
“I’ve added screen time to my adolescent assessment. Discussing safe use of cell phones and social media can have a significant impact on adolescent behavior and wellbeing, and parents are very thankful for the help,” she said. “This study encourages us to add screen time to the assessments we do at all adult ages, especially if mood symptoms exist,” Dr. Dakkak emphasized.
Suggestions for future research
Dr. Dakkak added that more areas for research include the differences in the impact of social media use on content creators versus content consumers. Also, “I would like to see research using the real data of use, the times of use, interruptions in sleep and use, possible confounding variables to include exercise, presence of intimate relationship and school/job performance.”
Given the many confounding variables, more controlled studies are needed to examine mental health outcomes in use, how long people use social media, and the impact of interventions such as time limits, Dr. Gold said.
“We can’t ignore the benefits of social media, such as helping those with social anxiety, finding peer support, and normalizing mental health, and those factors need to be studied and measured more effectively as well, she said.
Take-home message
It is important to recognize that the current study represents a correlation, not causality, said Dr. Gold. In addressing the issues of how social media impact mental health, “as always, the hardest thing is that many people get their news from social media, and often get social support from social media, so there has to be a balance of not removing social media completely, but of helping people see how it affects their mental health and how to find balance.”
The study findings were limited by several factors, including the inability to control for all potential confounders, the inability to assess the nature of social media use, and the lack of dose-response data, the researchers noted. Although the surveys in the current study were not specific to COVID-19, the effects of social media on depression may be specific to the content, and the findings may not generalize beyond the COVID-19 pandemic period.
Approximately two-thirds (66%) of the study participants identified as female, and 76% as White; 11% as Black; 6% as Asian; 5% as Hispanic; and 2% as American Indian or Alaska Native, Pacific Islander or Native Hawaiian, or other.
The National Institute of Mental Health provided a grant for the study to Dr. Pelis, who disclosed consulting fees from various companies and equity in Psy Therapeutics. The study’s lead author also serves as associate editor for JAMA Network Open, but was not involved in the decision process for publication of this study. Dr. Gold disclosed conducting a conference for Johnson & Johnson about social media and health care workers, and was on the advisory council.
Use of social media has been linked to increased anxiety and depression, as well as reduced well-being in adolescents and young adults, but similar associations in older adults have not been well studied, and longitudinal data are lacking, Ron H. Perlis, MD, of Massachusetts General Hospital, Boston, and colleagues wrote in their paper, which was published in JAMA Network Open.
To examine the association between social media use and depressive symptoms in older adults, the researchers reviewed data from 13 waves of an internet survey conducted each month between May 2020 and May 2021. The survey respondents included individuals aged 18 years and older, with a mean age of 56 years.
In the study the researchers analyzed responses from 5,395 individuals aged 18 years and older, with a mean age of 56 years. The study participants had minimal or no depressive symptoms at baseline, according to scores on the nine-item Patient Health Questionnaire (PHQ-9).
Overall, 8.9% of the respondents reported a worsening of 5 points or more on the PHQ-9 score on a follow-up survey, which was the primary outcome. Participants who reported using social media platforms Snapchat, Facebook, or TikTok were significantly more likely to report increased depressive symptoms, compared with those who did not report use of social media. The fully adjusted odds ratio was largest for Snapchat (aOR, 1.53), followed by Facebook (aOR, 1.42), and TikTok (aOR, 1.39).
Incorporating recent television and internet news terms, such as COVID-19, changed the association for Snapchat, for which the aOR decreased from 1.53 to 1.12 when news source terms were included in the survey. TikTok and Facebook associations remained similar.
When the results were further stratified by age, use of TikTok and Snapchat was associated with depressive symptoms in those aged 35 years and older, but not in those younger than 35 years. However, the opposite pattern emerged for Facebook; use was associated with depressive symptoms for individuals younger than 35 years, but not in those aged 35 years and older (aOR, 2.60 vs. aOR, 1.12).
The association between increased self-reported depressive symptoms and use of certain social media platforms was not impacted by baseline social support or face-to-face interactions, the researchers noted.
Family physician was surprised results weren’t more significant
In the current study, “I was honestly surprised the results weren’t more significant,” Mary Ann Dakkak, MD, of Boston University said in an interview. “That said, social media uses during the COVID pandemic may have been a necessary social outlet and form of connection for many people who were otherwise isolated.”
To still see a significant increase in depression when social media could have been a positive force may suggest a heavier impact during “normal” times, she added.
“It is not surprising that what we see in youth is shown among adults,” noted Dr. Dakkak, who was not involved with this study. “I always tell my patients that what is good for their children is good for the adults too, and vice versa.
“We expect to see outcomes of this on youth and adults who have been more isolated, who have used more screen time for learning, work, connection and boredom, in the near future,” she said. “The complex nature of why social media may have been used more heavily for connection during a time when in-person meetings were not possible may be a heavy confounder as the typical profile of heavy social media users may have differed during the COVID shutdowns.”
Psychiatrist: Balance benefits of social media with mental health risks
The current study was likely conducted before the recent news on “hidden” Facebook data and the implications that Facebook knew it was contributing to worsened mental health in teens, particularly around self-esteem, Jessica “Jessi” Gold, MD, a psychiatrist at Washington University, St. Louis, said in an interview.
“If you look more specifically at other studies, however, the data around social media and mental health is constantly varied, with some showing benefits and some showing negatives, and none conclusively suggesting either way,” said Dr. Gold, who also was not involved with the new research. “More data are needed, especially longitudinally and on a broader age group, to understand social media’s impact on mental health over time.
“It is also even more important in the wake of COVID-19, as so many people have turned to social media as a primary source of social support and connection, and are using it even more than before,” she emphasized.
In the current study, “I think the most interesting information is that, for TikTok and Snapchat, the effects seemed to be more pronounced in those older than 35 years who used social media,” said Dr. Gold.
What this study leaves unanswered is “whether people who might develop depression are simply more prone to use social media in the first place, such as to seek out social support,” Dr. Gold said. “Also, we don’t know anything about how long they are using social media or what they are using it for, which to me is important for understanding more about the nuance of the relationship with mental health and social media.”
Experts advise clinicians to discuss social media with patients
This new research suggests that clinicians should be talking to their patients about how social media impacts their emotional reactions, as well as their sleep, Dr. Gold said.
“Patients should be asking themselves how they are feeling when they are on social media and not using it before sleep. They should also be considering time limits and how to effectively use social media while taking care of their mental health,” she said. This conversation between clinician and patient should be had with any patient of any age, who uses social media, not only with teenagers.
“This is also a conversation about moderation, and knowing that individuals may feel they benefit from social media, that they should balance these benefits with potential mental health risks,” she said.
“Studies such as this one shed light onto why social media consumption should be at least a point of discussion with our patients,” said Dr. Dakkak.
She advised clinicians to ask and listen to patients and their families when it comes to screen time habits. “Whenever I see a patient with mood symptoms, I ask about their habits – eating, sleeping, socializing, screen time – including phone time. I ask about the family dynamics around screen time.
“I’ve added screen time to my adolescent assessment. Discussing safe use of cell phones and social media can have a significant impact on adolescent behavior and wellbeing, and parents are very thankful for the help,” she said. “This study encourages us to add screen time to the assessments we do at all adult ages, especially if mood symptoms exist,” Dr. Dakkak emphasized.
Suggestions for future research
Dr. Dakkak added that more areas for research include the differences in the impact of social media use on content creators versus content consumers. Also, “I would like to see research using the real data of use, the times of use, interruptions in sleep and use, possible confounding variables to include exercise, presence of intimate relationship and school/job performance.”
Given the many confounding variables, more controlled studies are needed to examine mental health outcomes in use, how long people use social media, and the impact of interventions such as time limits, Dr. Gold said.
“We can’t ignore the benefits of social media, such as helping those with social anxiety, finding peer support, and normalizing mental health, and those factors need to be studied and measured more effectively as well, she said.
Take-home message
It is important to recognize that the current study represents a correlation, not causality, said Dr. Gold. In addressing the issues of how social media impact mental health, “as always, the hardest thing is that many people get their news from social media, and often get social support from social media, so there has to be a balance of not removing social media completely, but of helping people see how it affects their mental health and how to find balance.”
The study findings were limited by several factors, including the inability to control for all potential confounders, the inability to assess the nature of social media use, and the lack of dose-response data, the researchers noted. Although the surveys in the current study were not specific to COVID-19, the effects of social media on depression may be specific to the content, and the findings may not generalize beyond the COVID-19 pandemic period.
Approximately two-thirds (66%) of the study participants identified as female, and 76% as White; 11% as Black; 6% as Asian; 5% as Hispanic; and 2% as American Indian or Alaska Native, Pacific Islander or Native Hawaiian, or other.
The National Institute of Mental Health provided a grant for the study to Dr. Pelis, who disclosed consulting fees from various companies and equity in Psy Therapeutics. The study’s lead author also serves as associate editor for JAMA Network Open, but was not involved in the decision process for publication of this study. Dr. Gold disclosed conducting a conference for Johnson & Johnson about social media and health care workers, and was on the advisory council.
Use of social media has been linked to increased anxiety and depression, as well as reduced well-being in adolescents and young adults, but similar associations in older adults have not been well studied, and longitudinal data are lacking, Ron H. Perlis, MD, of Massachusetts General Hospital, Boston, and colleagues wrote in their paper, which was published in JAMA Network Open.
To examine the association between social media use and depressive symptoms in older adults, the researchers reviewed data from 13 waves of an internet survey conducted each month between May 2020 and May 2021. The survey respondents included individuals aged 18 years and older, with a mean age of 56 years.
In the study the researchers analyzed responses from 5,395 individuals aged 18 years and older, with a mean age of 56 years. The study participants had minimal or no depressive symptoms at baseline, according to scores on the nine-item Patient Health Questionnaire (PHQ-9).
Overall, 8.9% of the respondents reported a worsening of 5 points or more on the PHQ-9 score on a follow-up survey, which was the primary outcome. Participants who reported using social media platforms Snapchat, Facebook, or TikTok were significantly more likely to report increased depressive symptoms, compared with those who did not report use of social media. The fully adjusted odds ratio was largest for Snapchat (aOR, 1.53), followed by Facebook (aOR, 1.42), and TikTok (aOR, 1.39).
Incorporating recent television and internet news terms, such as COVID-19, changed the association for Snapchat, for which the aOR decreased from 1.53 to 1.12 when news source terms were included in the survey. TikTok and Facebook associations remained similar.
When the results were further stratified by age, use of TikTok and Snapchat was associated with depressive symptoms in those aged 35 years and older, but not in those younger than 35 years. However, the opposite pattern emerged for Facebook; use was associated with depressive symptoms for individuals younger than 35 years, but not in those aged 35 years and older (aOR, 2.60 vs. aOR, 1.12).
The association between increased self-reported depressive symptoms and use of certain social media platforms was not impacted by baseline social support or face-to-face interactions, the researchers noted.
Family physician was surprised results weren’t more significant
In the current study, “I was honestly surprised the results weren’t more significant,” Mary Ann Dakkak, MD, of Boston University said in an interview. “That said, social media uses during the COVID pandemic may have been a necessary social outlet and form of connection for many people who were otherwise isolated.”
To still see a significant increase in depression when social media could have been a positive force may suggest a heavier impact during “normal” times, she added.
“It is not surprising that what we see in youth is shown among adults,” noted Dr. Dakkak, who was not involved with this study. “I always tell my patients that what is good for their children is good for the adults too, and vice versa.
“We expect to see outcomes of this on youth and adults who have been more isolated, who have used more screen time for learning, work, connection and boredom, in the near future,” she said. “The complex nature of why social media may have been used more heavily for connection during a time when in-person meetings were not possible may be a heavy confounder as the typical profile of heavy social media users may have differed during the COVID shutdowns.”
Psychiatrist: Balance benefits of social media with mental health risks
The current study was likely conducted before the recent news on “hidden” Facebook data and the implications that Facebook knew it was contributing to worsened mental health in teens, particularly around self-esteem, Jessica “Jessi” Gold, MD, a psychiatrist at Washington University, St. Louis, said in an interview.
“If you look more specifically at other studies, however, the data around social media and mental health is constantly varied, with some showing benefits and some showing negatives, and none conclusively suggesting either way,” said Dr. Gold, who also was not involved with the new research. “More data are needed, especially longitudinally and on a broader age group, to understand social media’s impact on mental health over time.
“It is also even more important in the wake of COVID-19, as so many people have turned to social media as a primary source of social support and connection, and are using it even more than before,” she emphasized.
In the current study, “I think the most interesting information is that, for TikTok and Snapchat, the effects seemed to be more pronounced in those older than 35 years who used social media,” said Dr. Gold.
What this study leaves unanswered is “whether people who might develop depression are simply more prone to use social media in the first place, such as to seek out social support,” Dr. Gold said. “Also, we don’t know anything about how long they are using social media or what they are using it for, which to me is important for understanding more about the nuance of the relationship with mental health and social media.”
Experts advise clinicians to discuss social media with patients
This new research suggests that clinicians should be talking to their patients about how social media impacts their emotional reactions, as well as their sleep, Dr. Gold said.
“Patients should be asking themselves how they are feeling when they are on social media and not using it before sleep. They should also be considering time limits and how to effectively use social media while taking care of their mental health,” she said. This conversation between clinician and patient should be had with any patient of any age, who uses social media, not only with teenagers.
“This is also a conversation about moderation, and knowing that individuals may feel they benefit from social media, that they should balance these benefits with potential mental health risks,” she said.
“Studies such as this one shed light onto why social media consumption should be at least a point of discussion with our patients,” said Dr. Dakkak.
She advised clinicians to ask and listen to patients and their families when it comes to screen time habits. “Whenever I see a patient with mood symptoms, I ask about their habits – eating, sleeping, socializing, screen time – including phone time. I ask about the family dynamics around screen time.
“I’ve added screen time to my adolescent assessment. Discussing safe use of cell phones and social media can have a significant impact on adolescent behavior and wellbeing, and parents are very thankful for the help,” she said. “This study encourages us to add screen time to the assessments we do at all adult ages, especially if mood symptoms exist,” Dr. Dakkak emphasized.
Suggestions for future research
Dr. Dakkak added that more areas for research include the differences in the impact of social media use on content creators versus content consumers. Also, “I would like to see research using the real data of use, the times of use, interruptions in sleep and use, possible confounding variables to include exercise, presence of intimate relationship and school/job performance.”
Given the many confounding variables, more controlled studies are needed to examine mental health outcomes in use, how long people use social media, and the impact of interventions such as time limits, Dr. Gold said.
“We can’t ignore the benefits of social media, such as helping those with social anxiety, finding peer support, and normalizing mental health, and those factors need to be studied and measured more effectively as well, she said.
Take-home message
It is important to recognize that the current study represents a correlation, not causality, said Dr. Gold. In addressing the issues of how social media impact mental health, “as always, the hardest thing is that many people get their news from social media, and often get social support from social media, so there has to be a balance of not removing social media completely, but of helping people see how it affects their mental health and how to find balance.”
The study findings were limited by several factors, including the inability to control for all potential confounders, the inability to assess the nature of social media use, and the lack of dose-response data, the researchers noted. Although the surveys in the current study were not specific to COVID-19, the effects of social media on depression may be specific to the content, and the findings may not generalize beyond the COVID-19 pandemic period.
Approximately two-thirds (66%) of the study participants identified as female, and 76% as White; 11% as Black; 6% as Asian; 5% as Hispanic; and 2% as American Indian or Alaska Native, Pacific Islander or Native Hawaiian, or other.
The National Institute of Mental Health provided a grant for the study to Dr. Pelis, who disclosed consulting fees from various companies and equity in Psy Therapeutics. The study’s lead author also serves as associate editor for JAMA Network Open, but was not involved in the decision process for publication of this study. Dr. Gold disclosed conducting a conference for Johnson & Johnson about social media and health care workers, and was on the advisory council.
FROM JAMA NETWORK OPEN
Oakland score identifies patients with lower GI bleed at low risk for adverse events
Background: The Oakland score was initially designed to be used in patients presenting with LGIB in the urgent, emergent, or primary care setting to help predict risk of readmission and determine if outpatient management is feasible. National guidelines in the United Kingdom have recommended use of the Oakland score despite limited external validation for the triage of patients with acute LGIB. This study aimed to externally validate the Oakland score in a large population in the United States and compare the performance at two thresholds.
Study design: Retrospective observational study.
Setting: 140 hospitals across the United States.
Synopsis: In this prognostic study, 38,067 patients were identified retrospectively using ICD-10 codes that were consistent with a diagnosis of LGIB and were admitted to the hospital. The Oakland score consisted of seven variables, including age, sex, prior hospitalization with LGIB, digital rectal exam results, heart rate, systolic blood pressure, and hemoglobin concentration. The primary outcome was safe discharge from the hospital, defined as absence of in-hospital rebleeding, RBC transfusion, therapeutic colonoscopy, mesenteric embolization or laparotomy for bleeding, in-hospital death, or readmission with subsequent LGIB in 28 days. In total, 47.9% of the identified patients experienced no adverse outcomes and were classified as meeting criteria for safe discharge. In addition, 8.7% of patients scored 8 points or fewer with a sensitivity of 98.4% and specificity of 16.0% for safe discharge. A sensitivity of 96% was maintained after increasing the threshold to 10 points or fewer with a specificity of 31.9%, suggesting the threshold can be increased while still maintaining adequate sensitivity. The study suggests that, by using the Oakland score threshold of 8, hospital admission may be avoided in low-risk patients leading to a savings of at least $44.5 million and even more if the threshold is increased to 10. Low specificity does present limitation of the score as some patients considered to be at risk for adverse events may have been safely discharged and managed as an outpatient, avoiding hospitalization.
Bottom line: The Oakland score was externally validated for use in assessing risk of adverse outcomes in patients with LGIB and had a high sensitivity but low specificity for identifying low-risk patients.
Citation: Oakland K et al. External validation of the Oakland score to assess safe hospital discharge among adult patients with acute lower gastrointestinal bleeding in the US. JAMA Netw Open. 2020 Jul 1;3:e209630. doi:
Dr. Steker is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: The Oakland score was initially designed to be used in patients presenting with LGIB in the urgent, emergent, or primary care setting to help predict risk of readmission and determine if outpatient management is feasible. National guidelines in the United Kingdom have recommended use of the Oakland score despite limited external validation for the triage of patients with acute LGIB. This study aimed to externally validate the Oakland score in a large population in the United States and compare the performance at two thresholds.
Study design: Retrospective observational study.
Setting: 140 hospitals across the United States.
Synopsis: In this prognostic study, 38,067 patients were identified retrospectively using ICD-10 codes that were consistent with a diagnosis of LGIB and were admitted to the hospital. The Oakland score consisted of seven variables, including age, sex, prior hospitalization with LGIB, digital rectal exam results, heart rate, systolic blood pressure, and hemoglobin concentration. The primary outcome was safe discharge from the hospital, defined as absence of in-hospital rebleeding, RBC transfusion, therapeutic colonoscopy, mesenteric embolization or laparotomy for bleeding, in-hospital death, or readmission with subsequent LGIB in 28 days. In total, 47.9% of the identified patients experienced no adverse outcomes and were classified as meeting criteria for safe discharge. In addition, 8.7% of patients scored 8 points or fewer with a sensitivity of 98.4% and specificity of 16.0% for safe discharge. A sensitivity of 96% was maintained after increasing the threshold to 10 points or fewer with a specificity of 31.9%, suggesting the threshold can be increased while still maintaining adequate sensitivity. The study suggests that, by using the Oakland score threshold of 8, hospital admission may be avoided in low-risk patients leading to a savings of at least $44.5 million and even more if the threshold is increased to 10. Low specificity does present limitation of the score as some patients considered to be at risk for adverse events may have been safely discharged and managed as an outpatient, avoiding hospitalization.
Bottom line: The Oakland score was externally validated for use in assessing risk of adverse outcomes in patients with LGIB and had a high sensitivity but low specificity for identifying low-risk patients.
Citation: Oakland K et al. External validation of the Oakland score to assess safe hospital discharge among adult patients with acute lower gastrointestinal bleeding in the US. JAMA Netw Open. 2020 Jul 1;3:e209630. doi:
Dr. Steker is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Background: The Oakland score was initially designed to be used in patients presenting with LGIB in the urgent, emergent, or primary care setting to help predict risk of readmission and determine if outpatient management is feasible. National guidelines in the United Kingdom have recommended use of the Oakland score despite limited external validation for the triage of patients with acute LGIB. This study aimed to externally validate the Oakland score in a large population in the United States and compare the performance at two thresholds.
Study design: Retrospective observational study.
Setting: 140 hospitals across the United States.
Synopsis: In this prognostic study, 38,067 patients were identified retrospectively using ICD-10 codes that were consistent with a diagnosis of LGIB and were admitted to the hospital. The Oakland score consisted of seven variables, including age, sex, prior hospitalization with LGIB, digital rectal exam results, heart rate, systolic blood pressure, and hemoglobin concentration. The primary outcome was safe discharge from the hospital, defined as absence of in-hospital rebleeding, RBC transfusion, therapeutic colonoscopy, mesenteric embolization or laparotomy for bleeding, in-hospital death, or readmission with subsequent LGIB in 28 days. In total, 47.9% of the identified patients experienced no adverse outcomes and were classified as meeting criteria for safe discharge. In addition, 8.7% of patients scored 8 points or fewer with a sensitivity of 98.4% and specificity of 16.0% for safe discharge. A sensitivity of 96% was maintained after increasing the threshold to 10 points or fewer with a specificity of 31.9%, suggesting the threshold can be increased while still maintaining adequate sensitivity. The study suggests that, by using the Oakland score threshold of 8, hospital admission may be avoided in low-risk patients leading to a savings of at least $44.5 million and even more if the threshold is increased to 10. Low specificity does present limitation of the score as some patients considered to be at risk for adverse events may have been safely discharged and managed as an outpatient, avoiding hospitalization.
Bottom line: The Oakland score was externally validated for use in assessing risk of adverse outcomes in patients with LGIB and had a high sensitivity but low specificity for identifying low-risk patients.
Citation: Oakland K et al. External validation of the Oakland score to assess safe hospital discharge among adult patients with acute lower gastrointestinal bleeding in the US. JAMA Netw Open. 2020 Jul 1;3:e209630. doi:
Dr. Steker is a hospitalist at Northwestern Memorial Hospital and instructor of medicine, Feinberg School of Medicine, both in Chicago.
Prevalence of undiagnosed vitiligo is ‘remarkably high’
A new

“The remarkably high number of participants with undiagnosed vitiligo” indicates a need for “the development and validation of teledermatology apps that allow for potential diagnosis,” Kavita Gandhi, MS, of the patient and health impact group at Pfizer in Collegeville, Pa., and associates said in JAMA Dermatology.
The estimated range of 0.76%-1.11% prevalence represents 1.9 million to 2.8 million adults with vitiligo in the general population, based on responses from 40,888 participants surveyed between Dec. 30, 2019, and March 11, 2020, and further physician evaluation of photos uploaded by 113 respondents, they explained. The investigators used a representative sample of the U.S. population, of people ages 18-85 years.
A prior vitiligo diagnosis was reported by 314 participants, and another 249 screened positive through the survey, for a self-reported overall prevalence of 1.38% in the adult population and a previously undiagnosed prevalence of 0.61%. The physician adjudication brought the overall prevalence down to 0.76% and the undiagnosed prevalence to 0.29%. “These findings suggest that up to 40% of adults with vitiligo in the U.S. may be undiagnosed,” the investigators wrote.
Survey questions covering the laterality of lesions broke the 1.38% overall prevalence down to 0.77% nonsegmental vitiligo (self-reported as bilateral) and 0.61% segmental (unilateral). The 0.76% overall prevalence provided by the three dermatologist reviewers worked out to 0.58% classified as nonsegmental and 0.18% as segmental, Ms. Gandhi and associates said.
“The distinction between segmental and nonsegmental vitiligo is of prime importance [since] patients are usually concerned by the spreading of the disease and its unpredictable course, which is the hallmark of nonsegmental vitiligo,” the researchers noted.
The analysis was the first, to the authors’ knowledge, to identify several trends among the undiagnosed population. The proportion of nonwhite adults was higher in the undiagnosed group (40.2%) than among those with a diagnosis (31.5%), as was Hispanic, Latino, or Spanish origin (21.3% vs. 15.3%). Unilateral presentation was seen in 54.2% of the undiagnosed adults and 37.3% of those with diagnosed vitiligo, they reported.
The study was sponsored by Pfizer, which employs several of the investigators. Two of the investigators disclosed multiple conflicts of interest involving other companies.
A new

“The remarkably high number of participants with undiagnosed vitiligo” indicates a need for “the development and validation of teledermatology apps that allow for potential diagnosis,” Kavita Gandhi, MS, of the patient and health impact group at Pfizer in Collegeville, Pa., and associates said in JAMA Dermatology.
The estimated range of 0.76%-1.11% prevalence represents 1.9 million to 2.8 million adults with vitiligo in the general population, based on responses from 40,888 participants surveyed between Dec. 30, 2019, and March 11, 2020, and further physician evaluation of photos uploaded by 113 respondents, they explained. The investigators used a representative sample of the U.S. population, of people ages 18-85 years.
A prior vitiligo diagnosis was reported by 314 participants, and another 249 screened positive through the survey, for a self-reported overall prevalence of 1.38% in the adult population and a previously undiagnosed prevalence of 0.61%. The physician adjudication brought the overall prevalence down to 0.76% and the undiagnosed prevalence to 0.29%. “These findings suggest that up to 40% of adults with vitiligo in the U.S. may be undiagnosed,” the investigators wrote.
Survey questions covering the laterality of lesions broke the 1.38% overall prevalence down to 0.77% nonsegmental vitiligo (self-reported as bilateral) and 0.61% segmental (unilateral). The 0.76% overall prevalence provided by the three dermatologist reviewers worked out to 0.58% classified as nonsegmental and 0.18% as segmental, Ms. Gandhi and associates said.
“The distinction between segmental and nonsegmental vitiligo is of prime importance [since] patients are usually concerned by the spreading of the disease and its unpredictable course, which is the hallmark of nonsegmental vitiligo,” the researchers noted.
The analysis was the first, to the authors’ knowledge, to identify several trends among the undiagnosed population. The proportion of nonwhite adults was higher in the undiagnosed group (40.2%) than among those with a diagnosis (31.5%), as was Hispanic, Latino, or Spanish origin (21.3% vs. 15.3%). Unilateral presentation was seen in 54.2% of the undiagnosed adults and 37.3% of those with diagnosed vitiligo, they reported.
The study was sponsored by Pfizer, which employs several of the investigators. Two of the investigators disclosed multiple conflicts of interest involving other companies.
A new

“The remarkably high number of participants with undiagnosed vitiligo” indicates a need for “the development and validation of teledermatology apps that allow for potential diagnosis,” Kavita Gandhi, MS, of the patient and health impact group at Pfizer in Collegeville, Pa., and associates said in JAMA Dermatology.
The estimated range of 0.76%-1.11% prevalence represents 1.9 million to 2.8 million adults with vitiligo in the general population, based on responses from 40,888 participants surveyed between Dec. 30, 2019, and March 11, 2020, and further physician evaluation of photos uploaded by 113 respondents, they explained. The investigators used a representative sample of the U.S. population, of people ages 18-85 years.
A prior vitiligo diagnosis was reported by 314 participants, and another 249 screened positive through the survey, for a self-reported overall prevalence of 1.38% in the adult population and a previously undiagnosed prevalence of 0.61%. The physician adjudication brought the overall prevalence down to 0.76% and the undiagnosed prevalence to 0.29%. “These findings suggest that up to 40% of adults with vitiligo in the U.S. may be undiagnosed,” the investigators wrote.
Survey questions covering the laterality of lesions broke the 1.38% overall prevalence down to 0.77% nonsegmental vitiligo (self-reported as bilateral) and 0.61% segmental (unilateral). The 0.76% overall prevalence provided by the three dermatologist reviewers worked out to 0.58% classified as nonsegmental and 0.18% as segmental, Ms. Gandhi and associates said.
“The distinction between segmental and nonsegmental vitiligo is of prime importance [since] patients are usually concerned by the spreading of the disease and its unpredictable course, which is the hallmark of nonsegmental vitiligo,” the researchers noted.
The analysis was the first, to the authors’ knowledge, to identify several trends among the undiagnosed population. The proportion of nonwhite adults was higher in the undiagnosed group (40.2%) than among those with a diagnosis (31.5%), as was Hispanic, Latino, or Spanish origin (21.3% vs. 15.3%). Unilateral presentation was seen in 54.2% of the undiagnosed adults and 37.3% of those with diagnosed vitiligo, they reported.
The study was sponsored by Pfizer, which employs several of the investigators. Two of the investigators disclosed multiple conflicts of interest involving other companies.
FROM JAMA DERMATOLOGY
Predicting cardiac shock mortality in the ICU
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Addition of echocardiogram measurement of biventricular dysfunction improved the accuracy of prognosis among patients with cardiac shock (CS) in the cardiac intensive care unit.
In patients in the cardiac ICU with CS, biventricular dysfunction (BVD), as assessed using transthoracic echocardiography, improves clinical risk stratification when combined with the Society for Cardiovascular Angiography and Interventions shock stage.
No improvements in risk stratification was seen with patients with left or right ventricular systolic dysfunction (LVSD or RVSD) alone, according to an article published in the journal Chest.
Ventricular systolic dysfunction is commonly seen in patients who have suffered cardiac shock, most often on the left side. Although echocardiography is often performed on these patients during diagnosis, previous studies looking at ventricular dysfunction used invasive hemodynamic parameters, which made it challenging to incorporate their findings into general cardiac ICU practice.
Pinning down cardiac shock
Although treatment of acute MI and heart failure has improved greatly, particularly with the implementation of percutaneous coronary intervention (primary PCI) for ST-segment elevation MI. This has reduced the rate of future heart failure, but cardiac shock can occur before or after the procedure, with a 30-day mortality of 30%-40%. This outcome hasn’t improved in the last 20 years.
Efforts to improve cardiac shock outcomes through percutaneous mechanical circulatory support devices have been hindered by the fact that CS patients are heterogeneous, and prognosis may depend on a range of factors.
SCAI was developed as a five-stage classification system for CS to improve communication of patient status, as well as to improve differentiation among patients participation in clinical trials. It does not include measures of ventricular dysfunction.
Simple measure boosts prognosis accuracy
The new work adds an additional layer to the SCAI shock stage. “Adding echocardiography allows discrimination between levels of risk for each SCAI stage,” said David Baran, MD, who was asked for comment. Dr. Baran was the lead author on the original SCAI study and is system director of advanced heart failure at Sentara Heart Hospital, as well as a professor of medicine at Eastern Virginia Medical School, both in Norfolk.
The work also underscores the value of repeated measures of prognosis during a patient’s stay in the ICU. “If a patient is not improving, it may prompt a consideration of whether transfer or consultation with a tertiary center may be of value. Conversely, if a patient doesn’t have high-risk features and is responding to therapy, it is reassuring to have data supporting low mortality with that care plan,” said Dr. Baran.
The study may be biased, since not every patient undergoes an echocardiogram. Still, “the authors make a convincing case that biventricular dysfunction is a powerful negative marker across the spectrum of SCAI stages,” said Dr. Baran.
Echocardiography is simple and generally available, and some are even portable and used with a smartphone. But patient body size interferes with echocardiography, as can the presence of a ventilator or multiple surgical dressings. “The key advantage of echo is that it is completely noninvasive and can be brought to the patient in the ICU, unlike other testing which involves moving the patient to the testing environment,” said Dr. Baran.
The researchers analyzed data from 3,158 patients admitted to the cardiac ICU at the Mayo Clinic Hospital St. Mary’s Campus in Rochester, Minn., 51.8% of whom had acute coronary syndromes. They defined LVSD as a left ventricular ejection fraction less than 40%, and RVSD as at least moderate systolic dysfunction determined by semiquantitative measurement. BVD constituted the presence of both LVSD and RVSD. They examined the association of in-hospital mortality with these parameters combined with SCAI stage.
BVD a risk factor
Overall in-hospital mortality was 10%. A total of 22.3% of patients had LVSD and 11.8% had RVSD; 16.4% had moderate or greater BVD. There was no association between LVSD or RVSD and in-hospital mortality after adjustment for SCAI stage, but there was a significant association for BVD (adjusted hazard ratio, 1.815; P = .0023). When combined with SCAI, BVC led to an improved ability to predict hospital mortality (area under the curve, 0.784 vs. 0.766; P < .001). Adding semiquantitative RVSD and LVSD led to more improvement (AUC, 0.794; P < .01 vs. both).
RVSD was associated with higher in-hospital mortality (adjusted odds ratio, 1.421; P = .02), and there was a trend toward greater mortality with LVSD (aOR, 1.336; P = .06). There was little change when SCAI shock stage A patients were excluded (aOR, 1.840; P < .001).
Patients with BVD had greater in-hospital mortality than those without ventricular dysfunction (aOR, 1.815; P = .0023), but other between-group comparisons were not significant.
The researchers performed a classification and regression tree analysis using left ventricular ejection fraction (LVEF) and semiquantitative RVSD. It found that RVSD was a better predictor of in-hospital mortality than LVSD, and the best cutoff for LVSD was different among patients with RVSD and patients without RVSD.
Patients with mild or greater RVD and LVEF greater than 24% were considered high risk; those with borderline or low RVSD and LVEF less than 33%, or mild or greater RVSD with LVEF of at least 24%, were considered intermediate risk. Patients with borderline or no RVSD and LVEF of at least 33% were considered low risk. Hospital mortality was 22% in the high-risk group, 12.2% in the intermediate group, and 3.3% in the low-risk group (aOR vs. intermediate, 0.493; P = .0006; aOR vs. high risk, 0.357; P < .0001).
The study authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rhinosinusitis without nasal polyps lowers QoL in COPD
Concomitant rhinosinusitis without nasal polyps (RSsNP) in patients with chronic obstructive pulmonary disease (COPD) is associated with a poorer, disease-specific, health-related quality of life (HRQoL), a Norwegian study is showing.
“Chronic rhinosinusitis has an impact on patients’ HRQoL,” lead author Marte Rystad Øie, Trondheim (Norway) University Hospital, said in an interview.
“We found that RSsNP in COPD was associated with more psychological issues, higher COPD symptom burden, and overall COPD-related HRQoL after adjusting for lung function, so RSsNP does have clinical relevance and [our findings] support previous studies that have suggested that rhinosinusitis should be recognized as a comorbidity in COPD,” she emphasized.
The study was published in the Nov. 1 issue of Respiratory Medicine.
Study sample
The study sample consisted of 90 patients with COPD and 93 control subjects, all age 40-80 years. “Generic HRQoL was measured with the Norwegian version of the SF-36v2 Health Survey Standard questionnaire,” the authors wrote, and responses were compared between patients with COPD and controls as well as between subgroups of patients who had COPD both with and without RSsNP.
Disease-specific HRQoL was assessed by the Sinonasal Outcome Test-22 (SNOT-22); the St. Georges Respiratory Questionnaire (SGRQ), and the COPD Assessment Test (CAT), and responses were again compared between patients who had COPD with and without RSsNP. In the COPD group, “severe” and “very severe” airflow obstruction was present in 56.5% of patients with RSsNP compared with 38.6% of patients without RSsNP, as Ms. Øie reported.
Furthermore, total SNOT-22 along with psychological subscale scores were both significantly higher in patients who had COPD with RSsNP than those without RSsNP. Among those with RSsNP, the mean value of the total SNOT-22 score was 36.8 whereas the mean value of the psychological subscale score was 22.6. Comparable mean values among patients who had COPD without RSsNP were 9.5 and 6.5, respectively (P < .05).
Total scores on the SGRQ were again significantly greater in patients who had COPD with RSsNP at a mean of 43.3 compared with a mean of 34 in those without RSsNP, investigators observe. Similarly, scores for the symptom and activity domains again on the SGRQ were significantly greater for patients who had COPD with RSsNP than those without nasal polyps. As for the total CAT score, once again it was significantly higher in patients who had COPD with RSsNP at a mean of 18.8 compared with a mean of 13.5 in those without RSsNP (P < .05).
Indeed, patients with RSsNP were four times more likely to have CAT scores indicating the condition was having a high or very high impact on their HRQoL compared with patients without RSsNP (P < .001). As the authors pointed out, having a high impact on HRQoL translates into patients having to stop their desired activities and having no good days in the week.
“This suggests that having RSsNP substantially adds to the activity limitation experienced by patients with COPD,” they emphasized. The authors also found that RSsNP was significantly associated with poorer physical functioning after adjusting for COPD as reflected by SF-36v2 findings, again suggesting that patients who had COPD with concomitant RSsNP have an additional limitation in activity and a heavier symptom burden.
As Ms. Øie explained, rhinosinusitis has two clinical phenotypes: that with nasal polyps and that without nasal polyps, the latter being twice as prevalent. In fact, rhinosinusitis with nasal polyps is associated with asthma, as she pointed out. Given, however, that rhinosinusitis without polyps is amenable to treatment with daily use of nasal steroids, it is possible to reduce the burden of symptoms and psychological stress associated with RSsNP in COPD.
Limitations of the study include the fact that investigators did not assess patients for the presence of any comorbidities that could contribute to poorer HRQoL in this patient population.
The study was funded by Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Concomitant rhinosinusitis without nasal polyps (RSsNP) in patients with chronic obstructive pulmonary disease (COPD) is associated with a poorer, disease-specific, health-related quality of life (HRQoL), a Norwegian study is showing.
“Chronic rhinosinusitis has an impact on patients’ HRQoL,” lead author Marte Rystad Øie, Trondheim (Norway) University Hospital, said in an interview.
“We found that RSsNP in COPD was associated with more psychological issues, higher COPD symptom burden, and overall COPD-related HRQoL after adjusting for lung function, so RSsNP does have clinical relevance and [our findings] support previous studies that have suggested that rhinosinusitis should be recognized as a comorbidity in COPD,” she emphasized.
The study was published in the Nov. 1 issue of Respiratory Medicine.
Study sample
The study sample consisted of 90 patients with COPD and 93 control subjects, all age 40-80 years. “Generic HRQoL was measured with the Norwegian version of the SF-36v2 Health Survey Standard questionnaire,” the authors wrote, and responses were compared between patients with COPD and controls as well as between subgroups of patients who had COPD both with and without RSsNP.
Disease-specific HRQoL was assessed by the Sinonasal Outcome Test-22 (SNOT-22); the St. Georges Respiratory Questionnaire (SGRQ), and the COPD Assessment Test (CAT), and responses were again compared between patients who had COPD with and without RSsNP. In the COPD group, “severe” and “very severe” airflow obstruction was present in 56.5% of patients with RSsNP compared with 38.6% of patients without RSsNP, as Ms. Øie reported.
Furthermore, total SNOT-22 along with psychological subscale scores were both significantly higher in patients who had COPD with RSsNP than those without RSsNP. Among those with RSsNP, the mean value of the total SNOT-22 score was 36.8 whereas the mean value of the psychological subscale score was 22.6. Comparable mean values among patients who had COPD without RSsNP were 9.5 and 6.5, respectively (P < .05).
Total scores on the SGRQ were again significantly greater in patients who had COPD with RSsNP at a mean of 43.3 compared with a mean of 34 in those without RSsNP, investigators observe. Similarly, scores for the symptom and activity domains again on the SGRQ were significantly greater for patients who had COPD with RSsNP than those without nasal polyps. As for the total CAT score, once again it was significantly higher in patients who had COPD with RSsNP at a mean of 18.8 compared with a mean of 13.5 in those without RSsNP (P < .05).
Indeed, patients with RSsNP were four times more likely to have CAT scores indicating the condition was having a high or very high impact on their HRQoL compared with patients without RSsNP (P < .001). As the authors pointed out, having a high impact on HRQoL translates into patients having to stop their desired activities and having no good days in the week.
“This suggests that having RSsNP substantially adds to the activity limitation experienced by patients with COPD,” they emphasized. The authors also found that RSsNP was significantly associated with poorer physical functioning after adjusting for COPD as reflected by SF-36v2 findings, again suggesting that patients who had COPD with concomitant RSsNP have an additional limitation in activity and a heavier symptom burden.
As Ms. Øie explained, rhinosinusitis has two clinical phenotypes: that with nasal polyps and that without nasal polyps, the latter being twice as prevalent. In fact, rhinosinusitis with nasal polyps is associated with asthma, as she pointed out. Given, however, that rhinosinusitis without polyps is amenable to treatment with daily use of nasal steroids, it is possible to reduce the burden of symptoms and psychological stress associated with RSsNP in COPD.
Limitations of the study include the fact that investigators did not assess patients for the presence of any comorbidities that could contribute to poorer HRQoL in this patient population.
The study was funded by Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Concomitant rhinosinusitis without nasal polyps (RSsNP) in patients with chronic obstructive pulmonary disease (COPD) is associated with a poorer, disease-specific, health-related quality of life (HRQoL), a Norwegian study is showing.
“Chronic rhinosinusitis has an impact on patients’ HRQoL,” lead author Marte Rystad Øie, Trondheim (Norway) University Hospital, said in an interview.
“We found that RSsNP in COPD was associated with more psychological issues, higher COPD symptom burden, and overall COPD-related HRQoL after adjusting for lung function, so RSsNP does have clinical relevance and [our findings] support previous studies that have suggested that rhinosinusitis should be recognized as a comorbidity in COPD,” she emphasized.
The study was published in the Nov. 1 issue of Respiratory Medicine.
Study sample
The study sample consisted of 90 patients with COPD and 93 control subjects, all age 40-80 years. “Generic HRQoL was measured with the Norwegian version of the SF-36v2 Health Survey Standard questionnaire,” the authors wrote, and responses were compared between patients with COPD and controls as well as between subgroups of patients who had COPD both with and without RSsNP.
Disease-specific HRQoL was assessed by the Sinonasal Outcome Test-22 (SNOT-22); the St. Georges Respiratory Questionnaire (SGRQ), and the COPD Assessment Test (CAT), and responses were again compared between patients who had COPD with and without RSsNP. In the COPD group, “severe” and “very severe” airflow obstruction was present in 56.5% of patients with RSsNP compared with 38.6% of patients without RSsNP, as Ms. Øie reported.
Furthermore, total SNOT-22 along with psychological subscale scores were both significantly higher in patients who had COPD with RSsNP than those without RSsNP. Among those with RSsNP, the mean value of the total SNOT-22 score was 36.8 whereas the mean value of the psychological subscale score was 22.6. Comparable mean values among patients who had COPD without RSsNP were 9.5 and 6.5, respectively (P < .05).
Total scores on the SGRQ were again significantly greater in patients who had COPD with RSsNP at a mean of 43.3 compared with a mean of 34 in those without RSsNP, investigators observe. Similarly, scores for the symptom and activity domains again on the SGRQ were significantly greater for patients who had COPD with RSsNP than those without nasal polyps. As for the total CAT score, once again it was significantly higher in patients who had COPD with RSsNP at a mean of 18.8 compared with a mean of 13.5 in those without RSsNP (P < .05).
Indeed, patients with RSsNP were four times more likely to have CAT scores indicating the condition was having a high or very high impact on their HRQoL compared with patients without RSsNP (P < .001). As the authors pointed out, having a high impact on HRQoL translates into patients having to stop their desired activities and having no good days in the week.
“This suggests that having RSsNP substantially adds to the activity limitation experienced by patients with COPD,” they emphasized. The authors also found that RSsNP was significantly associated with poorer physical functioning after adjusting for COPD as reflected by SF-36v2 findings, again suggesting that patients who had COPD with concomitant RSsNP have an additional limitation in activity and a heavier symptom burden.
As Ms. Øie explained, rhinosinusitis has two clinical phenotypes: that with nasal polyps and that without nasal polyps, the latter being twice as prevalent. In fact, rhinosinusitis with nasal polyps is associated with asthma, as she pointed out. Given, however, that rhinosinusitis without polyps is amenable to treatment with daily use of nasal steroids, it is possible to reduce the burden of symptoms and psychological stress associated with RSsNP in COPD.
Limitations of the study include the fact that investigators did not assess patients for the presence of any comorbidities that could contribute to poorer HRQoL in this patient population.
The study was funded by Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.




