User login
FFR-Guided or Angiography-Guided Nonculprit Lesion PCI in Patients With STEMI Without Cardiogenic Shock
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Clinical Edge Journal Scan Commentary: HCC December 2021
Laparoscopic HCC resections are increasing worldwide. Ivanics et al. report on a retrospective single-institution experience in North America that involves 149 patients who were matched by propensity score. Laparoscopic liver resection was performed in 57, and open liver resection was completed in 92. The laparoscopic liver resection group experienced a lower number of serious complications (14% vs 29%; P = .01). The 1-year overall survival (OS) rate was 90.9% vs 91.3% in the laparoscopic liver resection versus open liver resection group, while 3-year OS was 79.3% vs 88.5%, and 5-year OS was 70.5% vs 83.1% (P = .26). The cumulative incidence of recurrence at 1 year was 31.1% vs 18.9% in the laparoscopic liver resection versus open liver resection group, at 3 years was 59.7% vs 40.6%, and at 5 years was 62.9% vs 49.2% (P = .06). The authors concluded that laparoscopic HCC resection had fewer short-term complications, and statistically equivalent tumor control, compared to open liver resection, and should be considered as an option for treatment of patients with resectable liver cancer.
Radioembolization is a common treatment for liver-dominant HCC. Selective internal radiation therapy (SIRT) has a high objective response rate, but has yet to demonstrate a OS benefit. This could be due to incidental damage to the healthy liver, resulting in scarring, liver decompensation and a shorter survival. Van Doom et al. retrospectively analyzed 69 patients with advanced HCC who underwent SIRT. The primary outcome was the percentage of patients who developed Child-Pugh (CP) ≥ B7 liver disease after SIRT. The secondary outcomes were OS and response. After a median follow-up of 30 months, 38/69 patients (55%) developed CP ≥ B7. A lower ALBI score at baseline was significantly associated with a better outcome. The median OS in the SIRT-treated patients was 18 months (95% CI 14–22) compared to a case-matched cohort of 300 patients treated with sorafenib between 2007 and 2016 where the median OS was 8 months (95% CI 6–12; p = 0.0027). The authors concluded that patients with intermediate- or advanced-stage HCC treated with SIRT have a substantial risk of developing liver decompensation, but improved patient selection using the ALBI score may mitigate this risk. Note is made that the sorafenib patients were treated at a time when limited systemic options were available.
Finally, Peng et al. analyzed 699 adults with newly diagnosed HCC who were initially treated with transarterial chemoembolization (TACE) between 2010 and 2013. Initial treatment with TACE resulted in a complete response (CR) in 22.3% of the patients. The patients with a CR had a better OS than those who did not achieve CR (35.8 vs 24.0 months, P < 0.001). Predictors of lower likelihood of CR included CP B cirrhosis, higher tumor load, bilobar tumor, alpha-fetoprotein (AFP) level ≥20, and platelet counts >150,000. The authors concluded that TACE is an excellent treatment for selected patients with localized HCC.
Laparoscopic HCC resections are increasing worldwide. Ivanics et al. report on a retrospective single-institution experience in North America that involves 149 patients who were matched by propensity score. Laparoscopic liver resection was performed in 57, and open liver resection was completed in 92. The laparoscopic liver resection group experienced a lower number of serious complications (14% vs 29%; P = .01). The 1-year overall survival (OS) rate was 90.9% vs 91.3% in the laparoscopic liver resection versus open liver resection group, while 3-year OS was 79.3% vs 88.5%, and 5-year OS was 70.5% vs 83.1% (P = .26). The cumulative incidence of recurrence at 1 year was 31.1% vs 18.9% in the laparoscopic liver resection versus open liver resection group, at 3 years was 59.7% vs 40.6%, and at 5 years was 62.9% vs 49.2% (P = .06). The authors concluded that laparoscopic HCC resection had fewer short-term complications, and statistically equivalent tumor control, compared to open liver resection, and should be considered as an option for treatment of patients with resectable liver cancer.
Radioembolization is a common treatment for liver-dominant HCC. Selective internal radiation therapy (SIRT) has a high objective response rate, but has yet to demonstrate a OS benefit. This could be due to incidental damage to the healthy liver, resulting in scarring, liver decompensation and a shorter survival. Van Doom et al. retrospectively analyzed 69 patients with advanced HCC who underwent SIRT. The primary outcome was the percentage of patients who developed Child-Pugh (CP) ≥ B7 liver disease after SIRT. The secondary outcomes were OS and response. After a median follow-up of 30 months, 38/69 patients (55%) developed CP ≥ B7. A lower ALBI score at baseline was significantly associated with a better outcome. The median OS in the SIRT-treated patients was 18 months (95% CI 14–22) compared to a case-matched cohort of 300 patients treated with sorafenib between 2007 and 2016 where the median OS was 8 months (95% CI 6–12; p = 0.0027). The authors concluded that patients with intermediate- or advanced-stage HCC treated with SIRT have a substantial risk of developing liver decompensation, but improved patient selection using the ALBI score may mitigate this risk. Note is made that the sorafenib patients were treated at a time when limited systemic options were available.
Finally, Peng et al. analyzed 699 adults with newly diagnosed HCC who were initially treated with transarterial chemoembolization (TACE) between 2010 and 2013. Initial treatment with TACE resulted in a complete response (CR) in 22.3% of the patients. The patients with a CR had a better OS than those who did not achieve CR (35.8 vs 24.0 months, P < 0.001). Predictors of lower likelihood of CR included CP B cirrhosis, higher tumor load, bilobar tumor, alpha-fetoprotein (AFP) level ≥20, and platelet counts >150,000. The authors concluded that TACE is an excellent treatment for selected patients with localized HCC.
Laparoscopic HCC resections are increasing worldwide. Ivanics et al. report on a retrospective single-institution experience in North America that involves 149 patients who were matched by propensity score. Laparoscopic liver resection was performed in 57, and open liver resection was completed in 92. The laparoscopic liver resection group experienced a lower number of serious complications (14% vs 29%; P = .01). The 1-year overall survival (OS) rate was 90.9% vs 91.3% in the laparoscopic liver resection versus open liver resection group, while 3-year OS was 79.3% vs 88.5%, and 5-year OS was 70.5% vs 83.1% (P = .26). The cumulative incidence of recurrence at 1 year was 31.1% vs 18.9% in the laparoscopic liver resection versus open liver resection group, at 3 years was 59.7% vs 40.6%, and at 5 years was 62.9% vs 49.2% (P = .06). The authors concluded that laparoscopic HCC resection had fewer short-term complications, and statistically equivalent tumor control, compared to open liver resection, and should be considered as an option for treatment of patients with resectable liver cancer.
Radioembolization is a common treatment for liver-dominant HCC. Selective internal radiation therapy (SIRT) has a high objective response rate, but has yet to demonstrate a OS benefit. This could be due to incidental damage to the healthy liver, resulting in scarring, liver decompensation and a shorter survival. Van Doom et al. retrospectively analyzed 69 patients with advanced HCC who underwent SIRT. The primary outcome was the percentage of patients who developed Child-Pugh (CP) ≥ B7 liver disease after SIRT. The secondary outcomes were OS and response. After a median follow-up of 30 months, 38/69 patients (55%) developed CP ≥ B7. A lower ALBI score at baseline was significantly associated with a better outcome. The median OS in the SIRT-treated patients was 18 months (95% CI 14–22) compared to a case-matched cohort of 300 patients treated with sorafenib between 2007 and 2016 where the median OS was 8 months (95% CI 6–12; p = 0.0027). The authors concluded that patients with intermediate- or advanced-stage HCC treated with SIRT have a substantial risk of developing liver decompensation, but improved patient selection using the ALBI score may mitigate this risk. Note is made that the sorafenib patients were treated at a time when limited systemic options were available.
Finally, Peng et al. analyzed 699 adults with newly diagnosed HCC who were initially treated with transarterial chemoembolization (TACE) between 2010 and 2013. Initial treatment with TACE resulted in a complete response (CR) in 22.3% of the patients. The patients with a CR had a better OS than those who did not achieve CR (35.8 vs 24.0 months, P < 0.001). Predictors of lower likelihood of CR included CP B cirrhosis, higher tumor load, bilobar tumor, alpha-fetoprotein (AFP) level ≥20, and platelet counts >150,000. The authors concluded that TACE is an excellent treatment for selected patients with localized HCC.
Clinical Edge Journal Scan Commentary: CRC December 2021
Throughout the oncology landscape we are trying to incorporate immunotherapy into the treatment paradigm, and while this has been very successful in certain types of cancer (e.g. melanoma, lung cancer, and kidney cancer), colorectal cancer has been mostly left behind by the immunotherapy revolution to date. In a phase II single-arm study out of China, Lin and coworkers added camrelizumab, an anti-PD-1 monoclonal antibody, to neoadjuvant CAPOX chemotherapy following short-course radiation for patients with locally advanced rectal cancer. Of 27 evaluable patients, 13 had a pathological complete response (pCR, 48.1%), all but one of whom were mismatch repair proficient and unlikely to respond to immunotherapy. This small study laid the groundwork for an ongoing, randomized phase III study which is designed to demonstrate a significant increase in the pCR rate compared to standard neoadjuvant long-course chemoradiation and chemotherapy.
Finally, there has been excitement in the advanced setting of adding regorafenib, an oral poly-tyrosine kinase inhibitor, to immune checkpoint inhibitors for patients with mismatch repair proficient disease. Japanese data suggested a benefit from this combination which has not been fully borne out in the American experience. Yang and coauthors retrospectively analyzed the experience of regorafenib plus immune checkpoint inhibitors in mismatch repair proficient metastatic colorectal cancer patients at 14 Chinese medical centers and determined that the objective response rate in 82 patients was only 5% with a 45% stable disease rate. However, the median duration of disease control (stable disease or better) was 6.3 months which is clinically meaningful in this population. Moreover, 65% of patients have liver metastases which have proven to be more refractory to this combination in the American data. While we await more prospective studies of this combination, we continue to hold out hope that it may be a novel therapeutic option which is so desperately needed for patients with metastatic colorectal cancer.
Throughout the oncology landscape we are trying to incorporate immunotherapy into the treatment paradigm, and while this has been very successful in certain types of cancer (e.g. melanoma, lung cancer, and kidney cancer), colorectal cancer has been mostly left behind by the immunotherapy revolution to date. In a phase II single-arm study out of China, Lin and coworkers added camrelizumab, an anti-PD-1 monoclonal antibody, to neoadjuvant CAPOX chemotherapy following short-course radiation for patients with locally advanced rectal cancer. Of 27 evaluable patients, 13 had a pathological complete response (pCR, 48.1%), all but one of whom were mismatch repair proficient and unlikely to respond to immunotherapy. This small study laid the groundwork for an ongoing, randomized phase III study which is designed to demonstrate a significant increase in the pCR rate compared to standard neoadjuvant long-course chemoradiation and chemotherapy.
Finally, there has been excitement in the advanced setting of adding regorafenib, an oral poly-tyrosine kinase inhibitor, to immune checkpoint inhibitors for patients with mismatch repair proficient disease. Japanese data suggested a benefit from this combination which has not been fully borne out in the American experience. Yang and coauthors retrospectively analyzed the experience of regorafenib plus immune checkpoint inhibitors in mismatch repair proficient metastatic colorectal cancer patients at 14 Chinese medical centers and determined that the objective response rate in 82 patients was only 5% with a 45% stable disease rate. However, the median duration of disease control (stable disease or better) was 6.3 months which is clinically meaningful in this population. Moreover, 65% of patients have liver metastases which have proven to be more refractory to this combination in the American data. While we await more prospective studies of this combination, we continue to hold out hope that it may be a novel therapeutic option which is so desperately needed for patients with metastatic colorectal cancer.
Throughout the oncology landscape we are trying to incorporate immunotherapy into the treatment paradigm, and while this has been very successful in certain types of cancer (e.g. melanoma, lung cancer, and kidney cancer), colorectal cancer has been mostly left behind by the immunotherapy revolution to date. In a phase II single-arm study out of China, Lin and coworkers added camrelizumab, an anti-PD-1 monoclonal antibody, to neoadjuvant CAPOX chemotherapy following short-course radiation for patients with locally advanced rectal cancer. Of 27 evaluable patients, 13 had a pathological complete response (pCR, 48.1%), all but one of whom were mismatch repair proficient and unlikely to respond to immunotherapy. This small study laid the groundwork for an ongoing, randomized phase III study which is designed to demonstrate a significant increase in the pCR rate compared to standard neoadjuvant long-course chemoradiation and chemotherapy.
Finally, there has been excitement in the advanced setting of adding regorafenib, an oral poly-tyrosine kinase inhibitor, to immune checkpoint inhibitors for patients with mismatch repair proficient disease. Japanese data suggested a benefit from this combination which has not been fully borne out in the American experience. Yang and coauthors retrospectively analyzed the experience of regorafenib plus immune checkpoint inhibitors in mismatch repair proficient metastatic colorectal cancer patients at 14 Chinese medical centers and determined that the objective response rate in 82 patients was only 5% with a 45% stable disease rate. However, the median duration of disease control (stable disease or better) was 6.3 months which is clinically meaningful in this population. Moreover, 65% of patients have liver metastases which have proven to be more refractory to this combination in the American data. While we await more prospective studies of this combination, we continue to hold out hope that it may be a novel therapeutic option which is so desperately needed for patients with metastatic colorectal cancer.
Platelet-rich plasma injections show no benefit in knee OA in placebo-controlled trial
A large randomized, placebo-controlled trial of platelet-rich plasma injections for knee osteoarthritis has found almost no symptomatic or structural benefit from the treatment, giving some clarity to an evidence base that has seen both positive and negative trials for the treatment modality.
Given the need for better disease-modifying treatments for osteoarthritis, there has been a lot of interest in biological therapies such as platelet-rich plasma and stem cells, the lead author of the study, Kim Bennell, PhD, told this news organization. “People have started to use it to treat osteoarthritis, but the evidence to support it was limited in terms of its quality, and there’s been very little work looking at effects on structure,” said Dr. Bennell, a research physiotherapist and chair of physiotherapy at the University of Melbourne.
Platelet-rich plasma contains a range of growth factors and cytokines that are thought to be beneficial in building cartilage and reducing inflammation. There have been several clinical trials of the treatment in knee osteoarthritis, but the current study’s authors said these were limited by factors such as a lack of blinding and were at high risk of bias. “That was the impetus to do a large, high-quality study and to look at joint structure,” Dr. Bennell said.
Study details
For the study, which was published Nov. 23 in JAMA, the researchers enrolled 288 adults older than 50 with knee osteoarthritis who had experienced knee pain on most days of the past month and had radiographic evidence of mild to moderate osteoarthritis of the tibiofemoral joint.
After having stopped all nonsteroidal anti-inflammatory and pain-relief drugs 2 weeks prior – except acetaminophen – participants were randomly assigned to receive three weekly intra-articular knee injections of either a commercially available leukocyte-poor platelet-rich plasma or saline placebo. They were then followed for 12 months.
Among the 288 participants in the study, researchers saw no statistically significant difference in the change in pain scores between the treatment and placebo groups at 12 months, although there was a nonsignificantly greater reduction in pain scores among those given platelet-rich plasma. The study also found no statistically significant difference between the two groups in the change in medial tibial cartilage volume.
The researchers also looked at a large number of secondary outcomes, including the effects of treatment on pain and function at 2 months, change in Knee Injury and Osteoarthritis Outcome (KOOS) scores, and change in quality of life scores. There were no indications of any benefits from the treatment at the 2-month follow-up, and at 12 months, the study showed no significant improvements in knee pain while walking or in pain scores, KOOS scores, or quality of life measures.
However, significantly more participants in the treatment group than in the placebo group reported overall improvement at the 2-month point – 48.2% of those in the treatment arm, compared with 36.2% of the placebo group (risk ratio, 1.37; 95% confidence interval, 1.05-1.80; P = .02). At 12 months, 42.8% of those who received platelet-rich plasma reported improved function, compared with 32.1% of those in the placebo group (risk ratio, 1.36; 95% CI, 1.00-1.86, P = .05).
The study also found that significantly more people in the platelet-rich plasma group had three or more areas of cartilage thinning at 12 months (17.1% vs. 6.8%; risk ratio, 2.71; 95% CI, 1.16-6.34; P = .02).
Even when researchers looked for treatment effects in subgroups – for example, based on disease severity, body mass index, or knee alignment – they found no significant differences from placebo.
Dr. Bennell said the results were disappointing but not surprising. “Anecdotally, people do report that they get better, but we know that there is a very large placebo effect with treatment of pain,” she said.
Results emphasize importance of placebo controls
In an accompanying editorial by Jeffrey N. Katz, MD, director of the Orthopaedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital, professor of medicine and orthopedic surgery at Harvard Medical School, and professor of epidemiology and environmental health at the Harvard T.H. Chan School of Public Health, all in Boston, draws parallels between this study and two earlier studies of platelet-rich plasma for ankle osteoarthritis and Achilles tendinopathy, both published in JAMA in 2021. None of the three studies showed any significant improvements over and above placebo.
“These findings emphasize the importance of comparing interventions with placebos in trials of injection therapies,” Dr. Katz writes. However, he notes that these studies do suggest possible benefits in secondary outcomes, such as self-reported pain and function, and that earlier studies of the treatment had had more positive outcomes.
Dr. Katz said it was premature to dismiss platelet-rich plasma as a treatment for knee osteoarthritis, but “until a new generation of trials using standardized approaches to PRP [platelet-rich plasma] therapy provides evidence of efficacy, it would be prudent to pause the use of PRP for OA and Achilles tendinitis.”
Not ready to stop using platelet-rich plasma?
When asked for comment, sports medicine physician Maarten Moen, MD, from the Bergman Clinics Naarden (the Netherlands) said the study was the largest yet of the use of platelet-rich plasma for knee osteoarthritis and that it was a well-designed, double-blind, placebo-controlled trial.
However, he also pointed out that at least six earlier randomized, placebo-controlled studies of this treatment approach have been conducted, and of those six, all but two found positive benefits for patients. “It’s a very well-performed study, but for me, it would be a bridge too far to say, ‘Now we have this study, let’s stop doing it,’ ” Dr. Moen said.
Dr. Moen said he would like to see what effect this study had on meta-analyses and systematic reviews of the treatment, as that would give the clearest indication of the overall picture of its effectiveness.
Dr. Moen’s own experience of treating patients with platelet-rich plasma also suggested that, among those who do benefit from the treatment, that benefit would most likely show between 2 and 12 months afterward. He said it would have been useful to see outcomes at 3- and 6-month intervals.
“What I tell people is that, on average, around 9 months’ effect is to be expected,” he said.
Dr. Bennell said the research group chose the 12-month follow-up because they wanted to see if there were long-term improvements in joint structure which they hoped for, given the cost of treatment.
The study was funded by the Australian National Health and Medical Research Council, and Regen Lab SA provided platelet-rich plasma kits free of charge. Two authors reported using platelet-rich plasma injections in clinical practice, one reported scientific advisory board fees from Biobone, Novartis, Tissuegene, Pfizer, and Lilly; two reported fees for contributing to UpToDate clinical guidelines, and two reported grants from the National Health and Medical Research Council outside the submitted work. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
A large randomized, placebo-controlled trial of platelet-rich plasma injections for knee osteoarthritis has found almost no symptomatic or structural benefit from the treatment, giving some clarity to an evidence base that has seen both positive and negative trials for the treatment modality.
Given the need for better disease-modifying treatments for osteoarthritis, there has been a lot of interest in biological therapies such as platelet-rich plasma and stem cells, the lead author of the study, Kim Bennell, PhD, told this news organization. “People have started to use it to treat osteoarthritis, but the evidence to support it was limited in terms of its quality, and there’s been very little work looking at effects on structure,” said Dr. Bennell, a research physiotherapist and chair of physiotherapy at the University of Melbourne.
Platelet-rich plasma contains a range of growth factors and cytokines that are thought to be beneficial in building cartilage and reducing inflammation. There have been several clinical trials of the treatment in knee osteoarthritis, but the current study’s authors said these were limited by factors such as a lack of blinding and were at high risk of bias. “That was the impetus to do a large, high-quality study and to look at joint structure,” Dr. Bennell said.
Study details
For the study, which was published Nov. 23 in JAMA, the researchers enrolled 288 adults older than 50 with knee osteoarthritis who had experienced knee pain on most days of the past month and had radiographic evidence of mild to moderate osteoarthritis of the tibiofemoral joint.
After having stopped all nonsteroidal anti-inflammatory and pain-relief drugs 2 weeks prior – except acetaminophen – participants were randomly assigned to receive three weekly intra-articular knee injections of either a commercially available leukocyte-poor platelet-rich plasma or saline placebo. They were then followed for 12 months.
Among the 288 participants in the study, researchers saw no statistically significant difference in the change in pain scores between the treatment and placebo groups at 12 months, although there was a nonsignificantly greater reduction in pain scores among those given platelet-rich plasma. The study also found no statistically significant difference between the two groups in the change in medial tibial cartilage volume.
The researchers also looked at a large number of secondary outcomes, including the effects of treatment on pain and function at 2 months, change in Knee Injury and Osteoarthritis Outcome (KOOS) scores, and change in quality of life scores. There were no indications of any benefits from the treatment at the 2-month follow-up, and at 12 months, the study showed no significant improvements in knee pain while walking or in pain scores, KOOS scores, or quality of life measures.
However, significantly more participants in the treatment group than in the placebo group reported overall improvement at the 2-month point – 48.2% of those in the treatment arm, compared with 36.2% of the placebo group (risk ratio, 1.37; 95% confidence interval, 1.05-1.80; P = .02). At 12 months, 42.8% of those who received platelet-rich plasma reported improved function, compared with 32.1% of those in the placebo group (risk ratio, 1.36; 95% CI, 1.00-1.86, P = .05).
The study also found that significantly more people in the platelet-rich plasma group had three or more areas of cartilage thinning at 12 months (17.1% vs. 6.8%; risk ratio, 2.71; 95% CI, 1.16-6.34; P = .02).
Even when researchers looked for treatment effects in subgroups – for example, based on disease severity, body mass index, or knee alignment – they found no significant differences from placebo.
Dr. Bennell said the results were disappointing but not surprising. “Anecdotally, people do report that they get better, but we know that there is a very large placebo effect with treatment of pain,” she said.
Results emphasize importance of placebo controls
In an accompanying editorial by Jeffrey N. Katz, MD, director of the Orthopaedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital, professor of medicine and orthopedic surgery at Harvard Medical School, and professor of epidemiology and environmental health at the Harvard T.H. Chan School of Public Health, all in Boston, draws parallels between this study and two earlier studies of platelet-rich plasma for ankle osteoarthritis and Achilles tendinopathy, both published in JAMA in 2021. None of the three studies showed any significant improvements over and above placebo.
“These findings emphasize the importance of comparing interventions with placebos in trials of injection therapies,” Dr. Katz writes. However, he notes that these studies do suggest possible benefits in secondary outcomes, such as self-reported pain and function, and that earlier studies of the treatment had had more positive outcomes.
Dr. Katz said it was premature to dismiss platelet-rich plasma as a treatment for knee osteoarthritis, but “until a new generation of trials using standardized approaches to PRP [platelet-rich plasma] therapy provides evidence of efficacy, it would be prudent to pause the use of PRP for OA and Achilles tendinitis.”
Not ready to stop using platelet-rich plasma?
When asked for comment, sports medicine physician Maarten Moen, MD, from the Bergman Clinics Naarden (the Netherlands) said the study was the largest yet of the use of platelet-rich plasma for knee osteoarthritis and that it was a well-designed, double-blind, placebo-controlled trial.
However, he also pointed out that at least six earlier randomized, placebo-controlled studies of this treatment approach have been conducted, and of those six, all but two found positive benefits for patients. “It’s a very well-performed study, but for me, it would be a bridge too far to say, ‘Now we have this study, let’s stop doing it,’ ” Dr. Moen said.
Dr. Moen said he would like to see what effect this study had on meta-analyses and systematic reviews of the treatment, as that would give the clearest indication of the overall picture of its effectiveness.
Dr. Moen’s own experience of treating patients with platelet-rich plasma also suggested that, among those who do benefit from the treatment, that benefit would most likely show between 2 and 12 months afterward. He said it would have been useful to see outcomes at 3- and 6-month intervals.
“What I tell people is that, on average, around 9 months’ effect is to be expected,” he said.
Dr. Bennell said the research group chose the 12-month follow-up because they wanted to see if there were long-term improvements in joint structure which they hoped for, given the cost of treatment.
The study was funded by the Australian National Health and Medical Research Council, and Regen Lab SA provided platelet-rich plasma kits free of charge. Two authors reported using platelet-rich plasma injections in clinical practice, one reported scientific advisory board fees from Biobone, Novartis, Tissuegene, Pfizer, and Lilly; two reported fees for contributing to UpToDate clinical guidelines, and two reported grants from the National Health and Medical Research Council outside the submitted work. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
A large randomized, placebo-controlled trial of platelet-rich plasma injections for knee osteoarthritis has found almost no symptomatic or structural benefit from the treatment, giving some clarity to an evidence base that has seen both positive and negative trials for the treatment modality.
Given the need for better disease-modifying treatments for osteoarthritis, there has been a lot of interest in biological therapies such as platelet-rich plasma and stem cells, the lead author of the study, Kim Bennell, PhD, told this news organization. “People have started to use it to treat osteoarthritis, but the evidence to support it was limited in terms of its quality, and there’s been very little work looking at effects on structure,” said Dr. Bennell, a research physiotherapist and chair of physiotherapy at the University of Melbourne.
Platelet-rich plasma contains a range of growth factors and cytokines that are thought to be beneficial in building cartilage and reducing inflammation. There have been several clinical trials of the treatment in knee osteoarthritis, but the current study’s authors said these were limited by factors such as a lack of blinding and were at high risk of bias. “That was the impetus to do a large, high-quality study and to look at joint structure,” Dr. Bennell said.
Study details
For the study, which was published Nov. 23 in JAMA, the researchers enrolled 288 adults older than 50 with knee osteoarthritis who had experienced knee pain on most days of the past month and had radiographic evidence of mild to moderate osteoarthritis of the tibiofemoral joint.
After having stopped all nonsteroidal anti-inflammatory and pain-relief drugs 2 weeks prior – except acetaminophen – participants were randomly assigned to receive three weekly intra-articular knee injections of either a commercially available leukocyte-poor platelet-rich plasma or saline placebo. They were then followed for 12 months.
Among the 288 participants in the study, researchers saw no statistically significant difference in the change in pain scores between the treatment and placebo groups at 12 months, although there was a nonsignificantly greater reduction in pain scores among those given platelet-rich plasma. The study also found no statistically significant difference between the two groups in the change in medial tibial cartilage volume.
The researchers also looked at a large number of secondary outcomes, including the effects of treatment on pain and function at 2 months, change in Knee Injury and Osteoarthritis Outcome (KOOS) scores, and change in quality of life scores. There were no indications of any benefits from the treatment at the 2-month follow-up, and at 12 months, the study showed no significant improvements in knee pain while walking or in pain scores, KOOS scores, or quality of life measures.
However, significantly more participants in the treatment group than in the placebo group reported overall improvement at the 2-month point – 48.2% of those in the treatment arm, compared with 36.2% of the placebo group (risk ratio, 1.37; 95% confidence interval, 1.05-1.80; P = .02). At 12 months, 42.8% of those who received platelet-rich plasma reported improved function, compared with 32.1% of those in the placebo group (risk ratio, 1.36; 95% CI, 1.00-1.86, P = .05).
The study also found that significantly more people in the platelet-rich plasma group had three or more areas of cartilage thinning at 12 months (17.1% vs. 6.8%; risk ratio, 2.71; 95% CI, 1.16-6.34; P = .02).
Even when researchers looked for treatment effects in subgroups – for example, based on disease severity, body mass index, or knee alignment – they found no significant differences from placebo.
Dr. Bennell said the results were disappointing but not surprising. “Anecdotally, people do report that they get better, but we know that there is a very large placebo effect with treatment of pain,” she said.
Results emphasize importance of placebo controls
In an accompanying editorial by Jeffrey N. Katz, MD, director of the Orthopaedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital, professor of medicine and orthopedic surgery at Harvard Medical School, and professor of epidemiology and environmental health at the Harvard T.H. Chan School of Public Health, all in Boston, draws parallels between this study and two earlier studies of platelet-rich plasma for ankle osteoarthritis and Achilles tendinopathy, both published in JAMA in 2021. None of the three studies showed any significant improvements over and above placebo.
“These findings emphasize the importance of comparing interventions with placebos in trials of injection therapies,” Dr. Katz writes. However, he notes that these studies do suggest possible benefits in secondary outcomes, such as self-reported pain and function, and that earlier studies of the treatment had had more positive outcomes.
Dr. Katz said it was premature to dismiss platelet-rich plasma as a treatment for knee osteoarthritis, but “until a new generation of trials using standardized approaches to PRP [platelet-rich plasma] therapy provides evidence of efficacy, it would be prudent to pause the use of PRP for OA and Achilles tendinitis.”
Not ready to stop using platelet-rich plasma?
When asked for comment, sports medicine physician Maarten Moen, MD, from the Bergman Clinics Naarden (the Netherlands) said the study was the largest yet of the use of platelet-rich plasma for knee osteoarthritis and that it was a well-designed, double-blind, placebo-controlled trial.
However, he also pointed out that at least six earlier randomized, placebo-controlled studies of this treatment approach have been conducted, and of those six, all but two found positive benefits for patients. “It’s a very well-performed study, but for me, it would be a bridge too far to say, ‘Now we have this study, let’s stop doing it,’ ” Dr. Moen said.
Dr. Moen said he would like to see what effect this study had on meta-analyses and systematic reviews of the treatment, as that would give the clearest indication of the overall picture of its effectiveness.
Dr. Moen’s own experience of treating patients with platelet-rich plasma also suggested that, among those who do benefit from the treatment, that benefit would most likely show between 2 and 12 months afterward. He said it would have been useful to see outcomes at 3- and 6-month intervals.
“What I tell people is that, on average, around 9 months’ effect is to be expected,” he said.
Dr. Bennell said the research group chose the 12-month follow-up because they wanted to see if there were long-term improvements in joint structure which they hoped for, given the cost of treatment.
The study was funded by the Australian National Health and Medical Research Council, and Regen Lab SA provided platelet-rich plasma kits free of charge. Two authors reported using platelet-rich plasma injections in clinical practice, one reported scientific advisory board fees from Biobone, Novartis, Tissuegene, Pfizer, and Lilly; two reported fees for contributing to UpToDate clinical guidelines, and two reported grants from the National Health and Medical Research Council outside the submitted work. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
FROM JAMA
Free Clinic Diagnosis Data Improvement Project Using International Classification of Diseases and Electronic Health Record
From Pacific Lutheran School of Nursing, Tacoma, WA.
Objective: This quality improvement project aimed to enhance The Olympia Free Clinic’s (TOFC) data availability using
Methods: A new system was implemented for inputting ICD codes into Practice Fusion, the clinic’s EHR. During the initial phase, TOFC’s 21 volunteer providers entered the codes associated with the appropriate diagnosis for each of 157 encounters using a simplified map of options, including a map of the 20 most common diagnoses and a more comprehensive 60-code map.
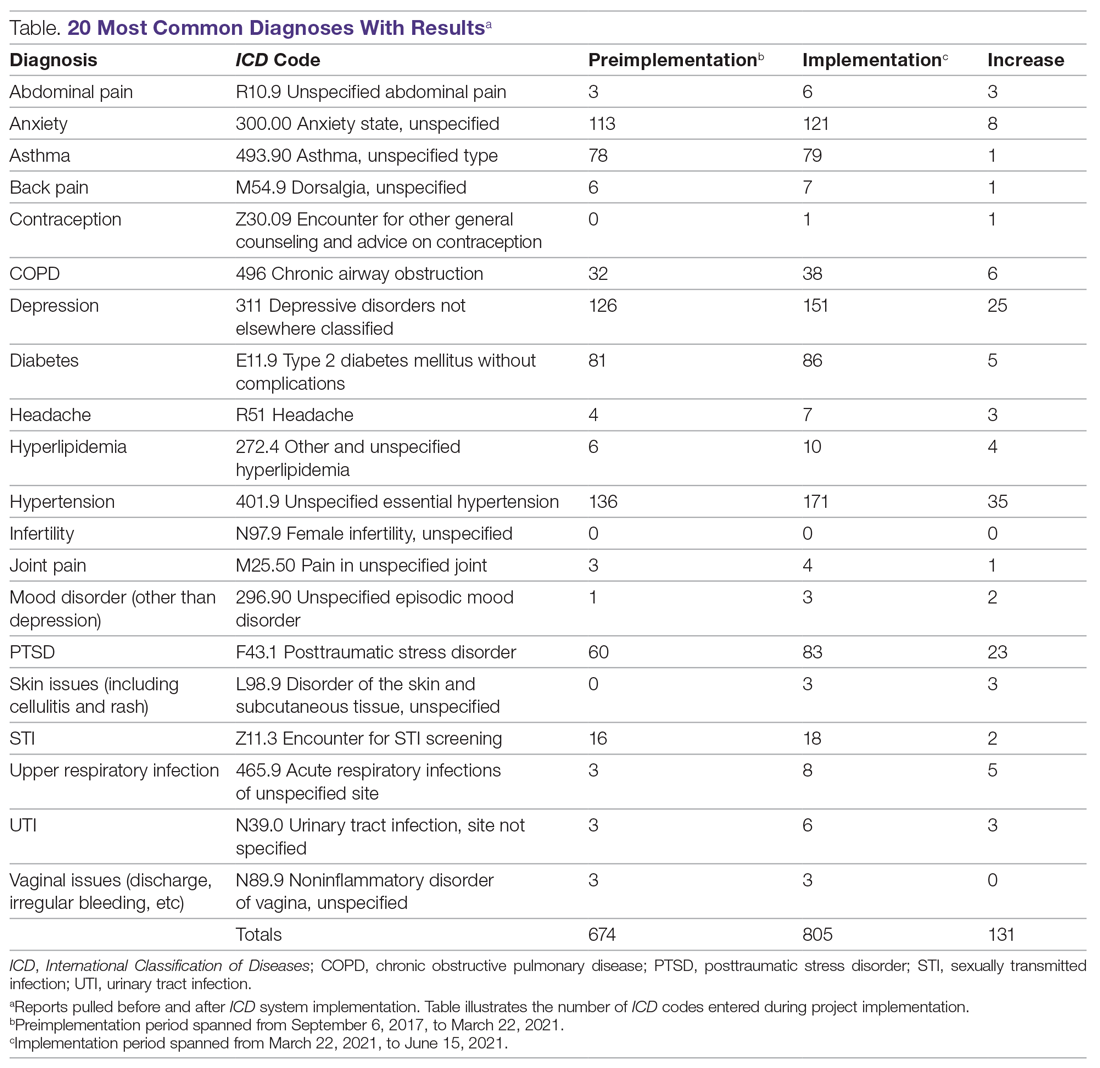
Results: An EHR report found that 128 new diagnoses were entered during project implementation, hypertension being the most common diagnosis, followed by depression, then posttraumatic stress disorder.
Conclusion: The knowledge of patient diagnoses enabled the clinic to make more-informed decisions.
Keywords: free clinic, data, quality improvement, electronic health record, International Classification of Diseases
Data creates a starting point, a goal, background, understanding of needs and context, and allows for tracking and improvement over time. This quality improvement (QI) project for The Olympia Free Clinic (TOFC) implemented a new system for tracking patient diagnoses. The 21 primary TOFC providers were encouraged to input mapped International Statistical Classification of Diseases and Related Health Problems (ICD) codes into the electronic health record (EHR). The clinic’s providers consisted of mostly retired, but some actively practicing, medical doctors, doctors of osteopathy, nurse practitioners, physician assistants, and psychiatrists.
Previous to this project, the clinic lacked any concrete data on patient demographics or diagnoses. For example, the clinic was unable to accurately answer the National Association of Free and Charitable Clinics’ questions about how many patients TOFC providers saw with diabetes, hypertension, asthma, and hyperlipidemia.1 Additionally, the needs of the clinic and its population were based on educated guesses.
As a free clinic staffed by volunteers and open 2 days a week, TOFC focused solely on giving care to those who needed it, operating pragmatically and addressing any issues as they arose. However, this strategy left the clinic unable to answer questions like “How many TOFC patients have diabetes?” By answering these questions, the clinic can better assess their resource and staffing needs.
Purpose
The project enlisted 21 volunteer providers to record diagnoses through ICD codes on the approximately 2000 active patients between March 22, 2021, and June 15, 2021. Tracking patient diagnoses improves clinic data, outcomes, and decision-making. By working on data improvement, the clinic can better understand its patient population and their needs, enhance clinical care, create better outcomes, make informed decisions, and raise eligibility for grants. The clinic was at a turning point as they reevaluated their mission statement and decided whether they would continue to focus on acute ailments or expand to formally manage chronic diseases as well. This decision needed to be made with knowledge, understanding, and context, which diagnosis data can provide. For example, the knowledge that the clinic’s 3 most common diagnoses are chronic conditions demonstrated that an official shift in their mission may have been warranted.
Literature Review
QI projects are effective and common in the free clinic setting.2-4 To the author’s knowledge, no literature to date shows the implementation of a system to better track diagnoses using a free clinic’s EHR with ICD codes.
Data bring value to clinics in many ways. It can also lead to more informed and better distribution of resources, such as preventative health and social services, patient education, and medical inventory.4
The focus of the US health care system is shifting to a value-based system under the Patient Protection and Affordable Care Act.5 Outcome measurements and improvement play a key role in this.6 Without knowing diagnoses, we cannot effectively track outcomes and have no data on which to base improvements. Insurance and reimbursement requirements typically hold health care facilities accountable for making these outcomes and improvements a reality.5,6 Free clinics, however, lack these motivations, which explains why a free clinic may be deficient in data and tracking methods. Tracking diagnosis codes will, going forward, allow TOFC to see outcomes and trends over time, track the effectiveness of the treatments, and change course if need be.6
TOFC fully implemented the EHR in 2018, giving the clinic better capabilities for pulling reports and tracking data. Although there were growing pains, many TOFC providers were already familiar with ICD codes, which, along with an EHR, provide a system to easily retrieve, store, and analyze diagnoses for evidence-based and informed decision-making.7 This made using ICD codes and the EHR an obvious choice to track patient diagnoses. However, most of the providers were not putting them in ICD codes before this project was implemented. Instead, diagnoses were typed in the notes and, therefore, not easy to generate in a report without having to open each chart for each individual encounter and combing through the notes. To make matters worse, providers were never trained on how to enter the codes in the EHR, and most providers saw no reason to, because the clinic does not bill for services.
Methods
A needs assessment determined that TOFC lacked data. This QI project used a combination of primary and secondary continuous quality improvement data.8 The primary data came from pulling the reports on Practice Fusion to see how many times each diagnosis code was put in during the implementation phase of this project. Secondary data came from interviewing the providers and asking whether they put in the diagnosis codes.
ICD diagnosis entry
Practice Fusion is the EHR TOFC uses and was therefore the platform for this QI project. Two ICD maps were created, which incorporated both International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) codes. There are tens of thousands of ICD codes in existence, but because TOFC is a free clinic that does not bill or receive reimbursement, the codes did not need to be as specific as they do in a paid clinic. Therefore, the maps put all the variations of each disease into a single category. For example, every patient with diabetes would receive the same ICD code regardless of whether their diabetes was controlled, uncontrolled, or any other variation. The goal of simplifying the codes was to improve compliance with ICD code entry and make reports easier to generate. The maps allowed the options to be simplified and, therefore, more user friendly for both the providers and the data collectors pulling reports. As some ICD-9 codes were already being used, these codes were incorporated so providers could keep using what they were already familiar with. To create the map, generic ICD codes were selected to represent each disease.
An initial survey was conducted prior to implementation with 10 providers, 2 nurses, and 2 staff members, asking which diagnoses they thought were seen most often in the clinic. Based off those answers, a map was created with the 20 most commonly used ICD codes, which can be seen in the Table. A more comprehensive map was also created, with 61 encompassing diagnoses.
To start the implementation process, providers were emailed an explanation of the project, the ICD code maps, and step-by-step instructions on how to enter a diagnosis into the EHR. Additionally, the 20 most common diagnoses forms were posted on the walls at the provider stations along with pictures illustrating how to input the codes in the EHR. The more comprehensive map was attached to the nurse clipboards that accompanied each encounter. The first night the providers volunteered after receiving the email, the researcher would review with them how to input the diagnosis code and have them test the method on a practice patient, either in person or over the phone.
A starting report was pulled March 22, 2021, covering encounters between September 6, 2017, and March 22, 2021, for the 20 most common diagnoses. Another report was pulled at the completion of the implementation phase, on June 15, 2021, covering March 22, 2021, to June 15, 2021. Willing providers and staff members were surveyed after implementation completion. The providers were asked whether they use the ICD codes, whether they would do so in the future, and whether they found it helpful when other providers had entered diagnoses. If they answered no to any of the questions, there were asked why, and whether they had any suggestions for improvements. The 4 staff members were asked whether they thought the data were helpful for their role and, if so, how they would use it.
Surveys
Surveys were conducted after the project was completed with willing and available providers and staff members in order to assess the utility of the project as well as to ensure future improvements and sustainability of the system.
Provider surveys
Do you currently input mapped ICD-10 codes when you chart for each encounter?
Yes No
If yes, do you intend to continue inputting the ICD codes in your encounters in the future?
Yes No
If no to either question above, please explain:
Do you have any recommendations for making it easier to input ICD codes or another way to track patients’ diagnoses?
Staff surveys
Is this data helpful for your role?
Yes No
If yes, how will you use this data?
Results
During the implementation phase, hypertension was the most common diagnosis seen at TOFC, accounting for 35 of 131 (27%) top 20 diagnoses entered. Depression was second, accounting for about 20% of diagnoses. Posttraumatic stress disorder was the third most common, making up 18% of diagnoses. There were 157 encounters during the implementation phase and 128 ICD diagnoses entered into the chart during this time period, suggesting that most encounters had a corresponding diagnosis code entered. See the Table for more details.
Survey results
Provider surveys
Six providers answered the survey questions. Four answered “yes” to both questions and 2 answered “no” to both questions. Reasons cited for why they did not input the ICD codes included not remembering to enter the codes or not remembering how to enter the codes. Recommendations for making it easier included incorporating the diagnosis in the assessment section of the EHR instead of standing alone as its own section, replacing ICD-9 codes with ICD-10 codes on the maps, making more specific codes for options, like typing more mental health diagnoses, and implementing more training on how to enter the codes.
Staff surveys
Three of 4 staff members responded to the survey. All 3 indicated that the data collected from this project assisted in their role. Stated uses for this data included grant applications and funding; community education, such as presentations and outreach; program development and monitoring; quality improvement; supply purchasing (eg, medications in stock to treat most commonly seen conditions), scheduling clinics and providers; allocating resources and supplies; and accepting or rejecting medical supply donations.
Discussion
Before this project, 668 of the top 20 most common diagnosis codes were entered from when TOFC introduced use of the EHR in the clinic in 2017, until the beginning of the implementation phase of this project in March 2021. During the 3 months of the implementation phase, 131 diagnoses were entered, representing almost 20% of the amount that were entered in 3 and a half years. Pulling the reports for these 20 diagnoses took less than 1 hour. During the needs assessment phase of this project, diagnoses for 3 months were extracted from the EHR by combing through provider notes and extracting the data from the notes—a process that took 11 hours.
Knowledge of diagnoses and the reasons for clinic attendance help the clinic make decisions about staffing, resources, and services. The TOFC board of directors used this data to assist with the decision of whether or not to change the clinic’s mission to include primary care as an official clinic function. The original purpose of the clinic was to address acute issues for people who lacked the resources for medical care. For example, a homeless person with an abscess could come to the clinic and have the abscess drained and treated. The results of this project illustrate that, in reality, most of the diagnoses actually seen in the clinic are more chronic in nature and require consistent, ongoing care. For instance, the project identified 52 clinic patients receiving consistent diabetic care. This type of data can help the clinic determine whether it should accept diabetes-associated donations and whether it needs to recruit a volunteer diabetes educator. Generally, this data can help guide other decisions as well, like what medications should be kept in the pharmacy, whether there are certain specialists the clinic should seek to partner with, and whether the clinic should embark on any particular education campaigns. By inputting ICD codes, diagnosis data are easily obtained to assist with future decisions.
A limitation of this project was that the reports could only be pulled within a certain time frame if the start date of the diagnosis was specified. As most providers did not indicate a start date with their entered diagnosis code, the only way to compare the before and after was to count the total before and the total after the implementation time frame. In other words, comparison reports could not be pulled retroactively, so some data on the less common diagnosis codes are missing from this paper, as reports for the comprehensive map were not pulled ahead of time. Providers may have omitted the start date when entering the diagnosis codes because many of these patients had their diagnoses for years—seeing different providers each time—so starting the diagnosis at that particular encounter did not make sense. Additionally, during training, although how to enter the start date was demonstrated, the emphasis and priority was placed on actually entering the ICD code, in an effort to keep the process simple and increase participation.
Conclusion
Evidence-based care and informed decision-making require data. In a free clinic, this can be difficult to obtain due to limited staffing and the absence of billing and insurance requirements. ICD codes and EHRs are powerful tools to collect data and information about clinic needs. This project improved TOFC’s knowledge about what kind of patients and diagnoses they see.
Corresponding author: Sarah M. Shanahan, MSN, RN, Pacific Lutheran University School of Nursing, Ramstad, Room 214, Tacoma, WA 98447; [email protected].
Financial disclosures: None.
1. National Association of Free and Charitable Clinics. 2021 NAFC Member Data & Standards Report. https://www.nafcclinics.org/sites/default/files/NAFC%202021%20Data%20Report%20Final.pdf
2. Lee JS, Combs K, Pasarica M; KNIGHTS Research Group. Improving efficiency while improving patient care in a student-run free clinic. J Am Board Fam Med. 2017;30(4):513-519. doi:10.3122/jabfm.2017.04.170044
3. Lu KB, Thiel B, Atkins CA, et al. Satisfaction with healthcare received at an interprofessional student-run free clinic: invested in training the next generation of healthcare professionals. Cureus. 2018;10(3):e2282. doi:10.7759/cureus.2282
4. Tran T, Briones C, Gillet AS, et al. “Knowing” your population: who are we caring for at Tulane University School of Medicine’s student-run free clinics? J Public Health (Oxf). 2020:1-7. doi:10.1007/s10389-020-01389-7
5. Sennett C. Healthcare reform: quality outcomes measurement and reporting. Am Health Drug Benefits. 2010;3(5):350-352.
6. Mazzali C, Duca P. Use of administrative data in healthcare research. Intern Emerg Med. 2015;10(4):517-524. doi:10.1007/s11739-015-1213-9
7. Moons E, Khanna A, Akkasi A, Moens MF. A comparison of deep learning methods for ICD coding of clinical records. Appl Sci. 2020;10(15):5262. doi:10.3390/app10155262
8. Finkelman A. Quality Improvement: A Guide for Integration in Nursing. Jones & Bartlett Learning; 2018.
From Pacific Lutheran School of Nursing, Tacoma, WA.
Objective: This quality improvement project aimed to enhance The Olympia Free Clinic’s (TOFC) data availability using
Methods: A new system was implemented for inputting ICD codes into Practice Fusion, the clinic’s EHR. During the initial phase, TOFC’s 21 volunteer providers entered the codes associated with the appropriate diagnosis for each of 157 encounters using a simplified map of options, including a map of the 20 most common diagnoses and a more comprehensive 60-code map.
Results: An EHR report found that 128 new diagnoses were entered during project implementation, hypertension being the most common diagnosis, followed by depression, then posttraumatic stress disorder.
Conclusion: The knowledge of patient diagnoses enabled the clinic to make more-informed decisions.
Keywords: free clinic, data, quality improvement, electronic health record, International Classification of Diseases
Data creates a starting point, a goal, background, understanding of needs and context, and allows for tracking and improvement over time. This quality improvement (QI) project for The Olympia Free Clinic (TOFC) implemented a new system for tracking patient diagnoses. The 21 primary TOFC providers were encouraged to input mapped International Statistical Classification of Diseases and Related Health Problems (ICD) codes into the electronic health record (EHR). The clinic’s providers consisted of mostly retired, but some actively practicing, medical doctors, doctors of osteopathy, nurse practitioners, physician assistants, and psychiatrists.
Previous to this project, the clinic lacked any concrete data on patient demographics or diagnoses. For example, the clinic was unable to accurately answer the National Association of Free and Charitable Clinics’ questions about how many patients TOFC providers saw with diabetes, hypertension, asthma, and hyperlipidemia.1 Additionally, the needs of the clinic and its population were based on educated guesses.
As a free clinic staffed by volunteers and open 2 days a week, TOFC focused solely on giving care to those who needed it, operating pragmatically and addressing any issues as they arose. However, this strategy left the clinic unable to answer questions like “How many TOFC patients have diabetes?” By answering these questions, the clinic can better assess their resource and staffing needs.
Purpose
The project enlisted 21 volunteer providers to record diagnoses through ICD codes on the approximately 2000 active patients between March 22, 2021, and June 15, 2021. Tracking patient diagnoses improves clinic data, outcomes, and decision-making. By working on data improvement, the clinic can better understand its patient population and their needs, enhance clinical care, create better outcomes, make informed decisions, and raise eligibility for grants. The clinic was at a turning point as they reevaluated their mission statement and decided whether they would continue to focus on acute ailments or expand to formally manage chronic diseases as well. This decision needed to be made with knowledge, understanding, and context, which diagnosis data can provide. For example, the knowledge that the clinic’s 3 most common diagnoses are chronic conditions demonstrated that an official shift in their mission may have been warranted.
Literature Review
QI projects are effective and common in the free clinic setting.2-4 To the author’s knowledge, no literature to date shows the implementation of a system to better track diagnoses using a free clinic’s EHR with ICD codes.
Data bring value to clinics in many ways. It can also lead to more informed and better distribution of resources, such as preventative health and social services, patient education, and medical inventory.4
The focus of the US health care system is shifting to a value-based system under the Patient Protection and Affordable Care Act.5 Outcome measurements and improvement play a key role in this.6 Without knowing diagnoses, we cannot effectively track outcomes and have no data on which to base improvements. Insurance and reimbursement requirements typically hold health care facilities accountable for making these outcomes and improvements a reality.5,6 Free clinics, however, lack these motivations, which explains why a free clinic may be deficient in data and tracking methods. Tracking diagnosis codes will, going forward, allow TOFC to see outcomes and trends over time, track the effectiveness of the treatments, and change course if need be.6
TOFC fully implemented the EHR in 2018, giving the clinic better capabilities for pulling reports and tracking data. Although there were growing pains, many TOFC providers were already familiar with ICD codes, which, along with an EHR, provide a system to easily retrieve, store, and analyze diagnoses for evidence-based and informed decision-making.7 This made using ICD codes and the EHR an obvious choice to track patient diagnoses. However, most of the providers were not putting them in ICD codes before this project was implemented. Instead, diagnoses were typed in the notes and, therefore, not easy to generate in a report without having to open each chart for each individual encounter and combing through the notes. To make matters worse, providers were never trained on how to enter the codes in the EHR, and most providers saw no reason to, because the clinic does not bill for services.
Methods
A needs assessment determined that TOFC lacked data. This QI project used a combination of primary and secondary continuous quality improvement data.8 The primary data came from pulling the reports on Practice Fusion to see how many times each diagnosis code was put in during the implementation phase of this project. Secondary data came from interviewing the providers and asking whether they put in the diagnosis codes.
ICD diagnosis entry
Practice Fusion is the EHR TOFC uses and was therefore the platform for this QI project. Two ICD maps were created, which incorporated both International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) codes. There are tens of thousands of ICD codes in existence, but because TOFC is a free clinic that does not bill or receive reimbursement, the codes did not need to be as specific as they do in a paid clinic. Therefore, the maps put all the variations of each disease into a single category. For example, every patient with diabetes would receive the same ICD code regardless of whether their diabetes was controlled, uncontrolled, or any other variation. The goal of simplifying the codes was to improve compliance with ICD code entry and make reports easier to generate. The maps allowed the options to be simplified and, therefore, more user friendly for both the providers and the data collectors pulling reports. As some ICD-9 codes were already being used, these codes were incorporated so providers could keep using what they were already familiar with. To create the map, generic ICD codes were selected to represent each disease.
An initial survey was conducted prior to implementation with 10 providers, 2 nurses, and 2 staff members, asking which diagnoses they thought were seen most often in the clinic. Based off those answers, a map was created with the 20 most commonly used ICD codes, which can be seen in the Table. A more comprehensive map was also created, with 61 encompassing diagnoses.

To start the implementation process, providers were emailed an explanation of the project, the ICD code maps, and step-by-step instructions on how to enter a diagnosis into the EHR. Additionally, the 20 most common diagnoses forms were posted on the walls at the provider stations along with pictures illustrating how to input the codes in the EHR. The more comprehensive map was attached to the nurse clipboards that accompanied each encounter. The first night the providers volunteered after receiving the email, the researcher would review with them how to input the diagnosis code and have them test the method on a practice patient, either in person or over the phone.
A starting report was pulled March 22, 2021, covering encounters between September 6, 2017, and March 22, 2021, for the 20 most common diagnoses. Another report was pulled at the completion of the implementation phase, on June 15, 2021, covering March 22, 2021, to June 15, 2021. Willing providers and staff members were surveyed after implementation completion. The providers were asked whether they use the ICD codes, whether they would do so in the future, and whether they found it helpful when other providers had entered diagnoses. If they answered no to any of the questions, there were asked why, and whether they had any suggestions for improvements. The 4 staff members were asked whether they thought the data were helpful for their role and, if so, how they would use it.
Surveys
Surveys were conducted after the project was completed with willing and available providers and staff members in order to assess the utility of the project as well as to ensure future improvements and sustainability of the system.
Provider surveys
Do you currently input mapped ICD-10 codes when you chart for each encounter?
Yes No
If yes, do you intend to continue inputting the ICD codes in your encounters in the future?
Yes No
If no to either question above, please explain:
Do you have any recommendations for making it easier to input ICD codes or another way to track patients’ diagnoses?
Staff surveys
Is this data helpful for your role?
Yes No
If yes, how will you use this data?
Results
During the implementation phase, hypertension was the most common diagnosis seen at TOFC, accounting for 35 of 131 (27%) top 20 diagnoses entered. Depression was second, accounting for about 20% of diagnoses. Posttraumatic stress disorder was the third most common, making up 18% of diagnoses. There were 157 encounters during the implementation phase and 128 ICD diagnoses entered into the chart during this time period, suggesting that most encounters had a corresponding diagnosis code entered. See the Table for more details.
Survey results
Provider surveys
Six providers answered the survey questions. Four answered “yes” to both questions and 2 answered “no” to both questions. Reasons cited for why they did not input the ICD codes included not remembering to enter the codes or not remembering how to enter the codes. Recommendations for making it easier included incorporating the diagnosis in the assessment section of the EHR instead of standing alone as its own section, replacing ICD-9 codes with ICD-10 codes on the maps, making more specific codes for options, like typing more mental health diagnoses, and implementing more training on how to enter the codes.
Staff surveys
Three of 4 staff members responded to the survey. All 3 indicated that the data collected from this project assisted in their role. Stated uses for this data included grant applications and funding; community education, such as presentations and outreach; program development and monitoring; quality improvement; supply purchasing (eg, medications in stock to treat most commonly seen conditions), scheduling clinics and providers; allocating resources and supplies; and accepting or rejecting medical supply donations.
Discussion
Before this project, 668 of the top 20 most common diagnosis codes were entered from when TOFC introduced use of the EHR in the clinic in 2017, until the beginning of the implementation phase of this project in March 2021. During the 3 months of the implementation phase, 131 diagnoses were entered, representing almost 20% of the amount that were entered in 3 and a half years. Pulling the reports for these 20 diagnoses took less than 1 hour. During the needs assessment phase of this project, diagnoses for 3 months were extracted from the EHR by combing through provider notes and extracting the data from the notes—a process that took 11 hours.
Knowledge of diagnoses and the reasons for clinic attendance help the clinic make decisions about staffing, resources, and services. The TOFC board of directors used this data to assist with the decision of whether or not to change the clinic’s mission to include primary care as an official clinic function. The original purpose of the clinic was to address acute issues for people who lacked the resources for medical care. For example, a homeless person with an abscess could come to the clinic and have the abscess drained and treated. The results of this project illustrate that, in reality, most of the diagnoses actually seen in the clinic are more chronic in nature and require consistent, ongoing care. For instance, the project identified 52 clinic patients receiving consistent diabetic care. This type of data can help the clinic determine whether it should accept diabetes-associated donations and whether it needs to recruit a volunteer diabetes educator. Generally, this data can help guide other decisions as well, like what medications should be kept in the pharmacy, whether there are certain specialists the clinic should seek to partner with, and whether the clinic should embark on any particular education campaigns. By inputting ICD codes, diagnosis data are easily obtained to assist with future decisions.
A limitation of this project was that the reports could only be pulled within a certain time frame if the start date of the diagnosis was specified. As most providers did not indicate a start date with their entered diagnosis code, the only way to compare the before and after was to count the total before and the total after the implementation time frame. In other words, comparison reports could not be pulled retroactively, so some data on the less common diagnosis codes are missing from this paper, as reports for the comprehensive map were not pulled ahead of time. Providers may have omitted the start date when entering the diagnosis codes because many of these patients had their diagnoses for years—seeing different providers each time—so starting the diagnosis at that particular encounter did not make sense. Additionally, during training, although how to enter the start date was demonstrated, the emphasis and priority was placed on actually entering the ICD code, in an effort to keep the process simple and increase participation.
Conclusion
Evidence-based care and informed decision-making require data. In a free clinic, this can be difficult to obtain due to limited staffing and the absence of billing and insurance requirements. ICD codes and EHRs are powerful tools to collect data and information about clinic needs. This project improved TOFC’s knowledge about what kind of patients and diagnoses they see.
Corresponding author: Sarah M. Shanahan, MSN, RN, Pacific Lutheran University School of Nursing, Ramstad, Room 214, Tacoma, WA 98447; [email protected].
Financial disclosures: None.
From Pacific Lutheran School of Nursing, Tacoma, WA.
Objective: This quality improvement project aimed to enhance The Olympia Free Clinic’s (TOFC) data availability using
Methods: A new system was implemented for inputting ICD codes into Practice Fusion, the clinic’s EHR. During the initial phase, TOFC’s 21 volunteer providers entered the codes associated with the appropriate diagnosis for each of 157 encounters using a simplified map of options, including a map of the 20 most common diagnoses and a more comprehensive 60-code map.
Results: An EHR report found that 128 new diagnoses were entered during project implementation, hypertension being the most common diagnosis, followed by depression, then posttraumatic stress disorder.
Conclusion: The knowledge of patient diagnoses enabled the clinic to make more-informed decisions.
Keywords: free clinic, data, quality improvement, electronic health record, International Classification of Diseases
Data creates a starting point, a goal, background, understanding of needs and context, and allows for tracking and improvement over time. This quality improvement (QI) project for The Olympia Free Clinic (TOFC) implemented a new system for tracking patient diagnoses. The 21 primary TOFC providers were encouraged to input mapped International Statistical Classification of Diseases and Related Health Problems (ICD) codes into the electronic health record (EHR). The clinic’s providers consisted of mostly retired, but some actively practicing, medical doctors, doctors of osteopathy, nurse practitioners, physician assistants, and psychiatrists.
Previous to this project, the clinic lacked any concrete data on patient demographics or diagnoses. For example, the clinic was unable to accurately answer the National Association of Free and Charitable Clinics’ questions about how many patients TOFC providers saw with diabetes, hypertension, asthma, and hyperlipidemia.1 Additionally, the needs of the clinic and its population were based on educated guesses.
As a free clinic staffed by volunteers and open 2 days a week, TOFC focused solely on giving care to those who needed it, operating pragmatically and addressing any issues as they arose. However, this strategy left the clinic unable to answer questions like “How many TOFC patients have diabetes?” By answering these questions, the clinic can better assess their resource and staffing needs.
Purpose
The project enlisted 21 volunteer providers to record diagnoses through ICD codes on the approximately 2000 active patients between March 22, 2021, and June 15, 2021. Tracking patient diagnoses improves clinic data, outcomes, and decision-making. By working on data improvement, the clinic can better understand its patient population and their needs, enhance clinical care, create better outcomes, make informed decisions, and raise eligibility for grants. The clinic was at a turning point as they reevaluated their mission statement and decided whether they would continue to focus on acute ailments or expand to formally manage chronic diseases as well. This decision needed to be made with knowledge, understanding, and context, which diagnosis data can provide. For example, the knowledge that the clinic’s 3 most common diagnoses are chronic conditions demonstrated that an official shift in their mission may have been warranted.
Literature Review
QI projects are effective and common in the free clinic setting.2-4 To the author’s knowledge, no literature to date shows the implementation of a system to better track diagnoses using a free clinic’s EHR with ICD codes.
Data bring value to clinics in many ways. It can also lead to more informed and better distribution of resources, such as preventative health and social services, patient education, and medical inventory.4
The focus of the US health care system is shifting to a value-based system under the Patient Protection and Affordable Care Act.5 Outcome measurements and improvement play a key role in this.6 Without knowing diagnoses, we cannot effectively track outcomes and have no data on which to base improvements. Insurance and reimbursement requirements typically hold health care facilities accountable for making these outcomes and improvements a reality.5,6 Free clinics, however, lack these motivations, which explains why a free clinic may be deficient in data and tracking methods. Tracking diagnosis codes will, going forward, allow TOFC to see outcomes and trends over time, track the effectiveness of the treatments, and change course if need be.6
TOFC fully implemented the EHR in 2018, giving the clinic better capabilities for pulling reports and tracking data. Although there were growing pains, many TOFC providers were already familiar with ICD codes, which, along with an EHR, provide a system to easily retrieve, store, and analyze diagnoses for evidence-based and informed decision-making.7 This made using ICD codes and the EHR an obvious choice to track patient diagnoses. However, most of the providers were not putting them in ICD codes before this project was implemented. Instead, diagnoses were typed in the notes and, therefore, not easy to generate in a report without having to open each chart for each individual encounter and combing through the notes. To make matters worse, providers were never trained on how to enter the codes in the EHR, and most providers saw no reason to, because the clinic does not bill for services.
Methods
A needs assessment determined that TOFC lacked data. This QI project used a combination of primary and secondary continuous quality improvement data.8 The primary data came from pulling the reports on Practice Fusion to see how many times each diagnosis code was put in during the implementation phase of this project. Secondary data came from interviewing the providers and asking whether they put in the diagnosis codes.
ICD diagnosis entry
Practice Fusion is the EHR TOFC uses and was therefore the platform for this QI project. Two ICD maps were created, which incorporated both International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) codes. There are tens of thousands of ICD codes in existence, but because TOFC is a free clinic that does not bill or receive reimbursement, the codes did not need to be as specific as they do in a paid clinic. Therefore, the maps put all the variations of each disease into a single category. For example, every patient with diabetes would receive the same ICD code regardless of whether their diabetes was controlled, uncontrolled, or any other variation. The goal of simplifying the codes was to improve compliance with ICD code entry and make reports easier to generate. The maps allowed the options to be simplified and, therefore, more user friendly for both the providers and the data collectors pulling reports. As some ICD-9 codes were already being used, these codes were incorporated so providers could keep using what they were already familiar with. To create the map, generic ICD codes were selected to represent each disease.
An initial survey was conducted prior to implementation with 10 providers, 2 nurses, and 2 staff members, asking which diagnoses they thought were seen most often in the clinic. Based off those answers, a map was created with the 20 most commonly used ICD codes, which can be seen in the Table. A more comprehensive map was also created, with 61 encompassing diagnoses.

To start the implementation process, providers were emailed an explanation of the project, the ICD code maps, and step-by-step instructions on how to enter a diagnosis into the EHR. Additionally, the 20 most common diagnoses forms were posted on the walls at the provider stations along with pictures illustrating how to input the codes in the EHR. The more comprehensive map was attached to the nurse clipboards that accompanied each encounter. The first night the providers volunteered after receiving the email, the researcher would review with them how to input the diagnosis code and have them test the method on a practice patient, either in person or over the phone.
A starting report was pulled March 22, 2021, covering encounters between September 6, 2017, and March 22, 2021, for the 20 most common diagnoses. Another report was pulled at the completion of the implementation phase, on June 15, 2021, covering March 22, 2021, to June 15, 2021. Willing providers and staff members were surveyed after implementation completion. The providers were asked whether they use the ICD codes, whether they would do so in the future, and whether they found it helpful when other providers had entered diagnoses. If they answered no to any of the questions, there were asked why, and whether they had any suggestions for improvements. The 4 staff members were asked whether they thought the data were helpful for their role and, if so, how they would use it.
Surveys
Surveys were conducted after the project was completed with willing and available providers and staff members in order to assess the utility of the project as well as to ensure future improvements and sustainability of the system.
Provider surveys
Do you currently input mapped ICD-10 codes when you chart for each encounter?
Yes No
If yes, do you intend to continue inputting the ICD codes in your encounters in the future?
Yes No
If no to either question above, please explain:
Do you have any recommendations for making it easier to input ICD codes or another way to track patients’ diagnoses?
Staff surveys
Is this data helpful for your role?
Yes No
If yes, how will you use this data?
Results
During the implementation phase, hypertension was the most common diagnosis seen at TOFC, accounting for 35 of 131 (27%) top 20 diagnoses entered. Depression was second, accounting for about 20% of diagnoses. Posttraumatic stress disorder was the third most common, making up 18% of diagnoses. There were 157 encounters during the implementation phase and 128 ICD diagnoses entered into the chart during this time period, suggesting that most encounters had a corresponding diagnosis code entered. See the Table for more details.
Survey results
Provider surveys
Six providers answered the survey questions. Four answered “yes” to both questions and 2 answered “no” to both questions. Reasons cited for why they did not input the ICD codes included not remembering to enter the codes or not remembering how to enter the codes. Recommendations for making it easier included incorporating the diagnosis in the assessment section of the EHR instead of standing alone as its own section, replacing ICD-9 codes with ICD-10 codes on the maps, making more specific codes for options, like typing more mental health diagnoses, and implementing more training on how to enter the codes.
Staff surveys
Three of 4 staff members responded to the survey. All 3 indicated that the data collected from this project assisted in their role. Stated uses for this data included grant applications and funding; community education, such as presentations and outreach; program development and monitoring; quality improvement; supply purchasing (eg, medications in stock to treat most commonly seen conditions), scheduling clinics and providers; allocating resources and supplies; and accepting or rejecting medical supply donations.
Discussion
Before this project, 668 of the top 20 most common diagnosis codes were entered from when TOFC introduced use of the EHR in the clinic in 2017, until the beginning of the implementation phase of this project in March 2021. During the 3 months of the implementation phase, 131 diagnoses were entered, representing almost 20% of the amount that were entered in 3 and a half years. Pulling the reports for these 20 diagnoses took less than 1 hour. During the needs assessment phase of this project, diagnoses for 3 months were extracted from the EHR by combing through provider notes and extracting the data from the notes—a process that took 11 hours.
Knowledge of diagnoses and the reasons for clinic attendance help the clinic make decisions about staffing, resources, and services. The TOFC board of directors used this data to assist with the decision of whether or not to change the clinic’s mission to include primary care as an official clinic function. The original purpose of the clinic was to address acute issues for people who lacked the resources for medical care. For example, a homeless person with an abscess could come to the clinic and have the abscess drained and treated. The results of this project illustrate that, in reality, most of the diagnoses actually seen in the clinic are more chronic in nature and require consistent, ongoing care. For instance, the project identified 52 clinic patients receiving consistent diabetic care. This type of data can help the clinic determine whether it should accept diabetes-associated donations and whether it needs to recruit a volunteer diabetes educator. Generally, this data can help guide other decisions as well, like what medications should be kept in the pharmacy, whether there are certain specialists the clinic should seek to partner with, and whether the clinic should embark on any particular education campaigns. By inputting ICD codes, diagnosis data are easily obtained to assist with future decisions.
A limitation of this project was that the reports could only be pulled within a certain time frame if the start date of the diagnosis was specified. As most providers did not indicate a start date with their entered diagnosis code, the only way to compare the before and after was to count the total before and the total after the implementation time frame. In other words, comparison reports could not be pulled retroactively, so some data on the less common diagnosis codes are missing from this paper, as reports for the comprehensive map were not pulled ahead of time. Providers may have omitted the start date when entering the diagnosis codes because many of these patients had their diagnoses for years—seeing different providers each time—so starting the diagnosis at that particular encounter did not make sense. Additionally, during training, although how to enter the start date was demonstrated, the emphasis and priority was placed on actually entering the ICD code, in an effort to keep the process simple and increase participation.
Conclusion
Evidence-based care and informed decision-making require data. In a free clinic, this can be difficult to obtain due to limited staffing and the absence of billing and insurance requirements. ICD codes and EHRs are powerful tools to collect data and information about clinic needs. This project improved TOFC’s knowledge about what kind of patients and diagnoses they see.
Corresponding author: Sarah M. Shanahan, MSN, RN, Pacific Lutheran University School of Nursing, Ramstad, Room 214, Tacoma, WA 98447; [email protected].
Financial disclosures: None.
1. National Association of Free and Charitable Clinics. 2021 NAFC Member Data & Standards Report. https://www.nafcclinics.org/sites/default/files/NAFC%202021%20Data%20Report%20Final.pdf
2. Lee JS, Combs K, Pasarica M; KNIGHTS Research Group. Improving efficiency while improving patient care in a student-run free clinic. J Am Board Fam Med. 2017;30(4):513-519. doi:10.3122/jabfm.2017.04.170044
3. Lu KB, Thiel B, Atkins CA, et al. Satisfaction with healthcare received at an interprofessional student-run free clinic: invested in training the next generation of healthcare professionals. Cureus. 2018;10(3):e2282. doi:10.7759/cureus.2282
4. Tran T, Briones C, Gillet AS, et al. “Knowing” your population: who are we caring for at Tulane University School of Medicine’s student-run free clinics? J Public Health (Oxf). 2020:1-7. doi:10.1007/s10389-020-01389-7
5. Sennett C. Healthcare reform: quality outcomes measurement and reporting. Am Health Drug Benefits. 2010;3(5):350-352.
6. Mazzali C, Duca P. Use of administrative data in healthcare research. Intern Emerg Med. 2015;10(4):517-524. doi:10.1007/s11739-015-1213-9
7. Moons E, Khanna A, Akkasi A, Moens MF. A comparison of deep learning methods for ICD coding of clinical records. Appl Sci. 2020;10(15):5262. doi:10.3390/app10155262
8. Finkelman A. Quality Improvement: A Guide for Integration in Nursing. Jones & Bartlett Learning; 2018.
1. National Association of Free and Charitable Clinics. 2021 NAFC Member Data & Standards Report. https://www.nafcclinics.org/sites/default/files/NAFC%202021%20Data%20Report%20Final.pdf
2. Lee JS, Combs K, Pasarica M; KNIGHTS Research Group. Improving efficiency while improving patient care in a student-run free clinic. J Am Board Fam Med. 2017;30(4):513-519. doi:10.3122/jabfm.2017.04.170044
3. Lu KB, Thiel B, Atkins CA, et al. Satisfaction with healthcare received at an interprofessional student-run free clinic: invested in training the next generation of healthcare professionals. Cureus. 2018;10(3):e2282. doi:10.7759/cureus.2282
4. Tran T, Briones C, Gillet AS, et al. “Knowing” your population: who are we caring for at Tulane University School of Medicine’s student-run free clinics? J Public Health (Oxf). 2020:1-7. doi:10.1007/s10389-020-01389-7
5. Sennett C. Healthcare reform: quality outcomes measurement and reporting. Am Health Drug Benefits. 2010;3(5):350-352.
6. Mazzali C, Duca P. Use of administrative data in healthcare research. Intern Emerg Med. 2015;10(4):517-524. doi:10.1007/s11739-015-1213-9
7. Moons E, Khanna A, Akkasi A, Moens MF. A comparison of deep learning methods for ICD coding of clinical records. Appl Sci. 2020;10(15):5262. doi:10.3390/app10155262
8. Finkelman A. Quality Improvement: A Guide for Integration in Nursing. Jones & Bartlett Learning; 2018.
The Use of Nasogastric Tube Bridle Kits in COVID-19 Intensive Care Unit Patients
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).
The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).

The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).

The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
Assessment of Same-Day Naloxone Availability in New Mexico Pharmacies
From the Department of Medicine, University of California San Diego (Dr. Haponyuk), Department of Emergency Medicine, University of Tennessee (Dr. Dejong), the Department of Family Medicine, University of New Mexico (Dr. Gutfrucht), and the Department of Internal Medicine, University of New Mexico (Dr. Barrett)
Objective: Naloxone availability can reduce the risk of death from opioid overdoses, although prescriber, legislative, and payment barriers to accessing this life-saving medication exist. A previously underreported barrier involves same-day availability, the lack of which may force patients to travel to multiple pharmacies and having delays in access or risking not filling their prescription. This study sought to determine same-day availability of naloxone in pharmacies in the state of New Mexico.
Methods: Same-day availability of naloxone was assessed via an audit survey.
Results: Of the 183 pharamacies screened, only 84.7% had same-day availability, including only 72% in Albuquerque, the state’s most populous city/municipality.
Conclusion: These results highlight the extent of a previously underexplored challenge to patient care and barrier to patient safety, and future directions for more patient-centered care.
Keywords: naloxone; barriers to care; opioid overdose prevention.
The US is enduring an ongoing epidemic of deaths due to opioid use, which have increased in frequency since the onset of the COVID-19 pandemic.1 One strategy to reduce the risk of mortality from opioid use is to ensure the widespread availability of naloxone. Individual states have implemented harm reduction strategies to increase access to naloxone, including improving availability via a statewide standing order that it may be dispensed without a prescription.2,3 Such naloxone access laws are being widely adopted and are believed to reduce overdose deaths.4
There are many barriers to patients receiving naloxone despite their clinicians providing a prescription for it, including stigmatization, financial cost, and local availability.5-9 However, the stigma associated with naloxone extends to both patients and pharmacists. Pharmacists in West Virginia, for example, showed widespread concerns about having naloxone available for patients to purchase over the counter, for fear that increasing naloxone access may increase overdoses.6 A study in Tennessee also found pharmacists hesitant to recommend naloxone.7 Another study of rural pharmacies in Georgia found that just over half carried naloxone despite a state law that naloxone be available without a prescription.8 Challenges are not limited to rural areas, however; a study in Philadelphia found that more than one-third of pharmacies required a prescription to dispense naloxone, contrary to state law.9 Thus, in a rapidly changing regulatory environment, there are many evolving barriers to patients receiving naloxone.
New Mexico has an opioid overdose rate higher than the national average, coming in 15th out of 50 states when last ranked in 2018, with overdose rates that vary across demographic variables.10 Consequently, New Mexico state law added language requiring clinicians prescribing opioids for 5 days or longer to co-prescribe naloxone along with written information on how to administer the opioid antagonist.11 New Mexico is also a geographically large state with a relatively low overall population characterized by striking health disparities, particularly as related to access to care.
The purpose of this study is to describe the same-day availability of naloxone throughout the state of New Mexico after a change in state law requiring co-prescription was enacted, to help identify challenges to patients receiving it. Comprehensive examination of barriers to patients accessing this life-saving medication can advise strategies to both improve patient-centered care and potentially reduce deaths.
Methods
To better understand barriers to patients obtaining naloxone, in July and August of 2019 we performed an audit (“secret shopper”) study of all pharmacies in the state, posing as patients wishing to obtain naloxone. A publicly available list of every pharmacy in New Mexico was used to identify 89 pharmacies in Albuquerque (the most populous city in New Mexico) and 106 pharmacies throughout the rest of the state.12
Every pharmacy was called via a publicly available phone number during business hours (confirmed via an internet search), at least 2 hours prior to closing. One of 3 researchers telephoned pharmacies posing as a patient and inquired whether naloxone would be available for pick up the same day. If the pharmacy confirmed it was available that day, the call concluded. If naloxone was unavailable for same day pick up, researchers asked when it would be next available. Each pharmacy was called once, and neither insurance information nor cost was offered or requested. All questions were asked in English by native English speakers.
All responses were recorded in a secure spreadsheet. Once all responses were received and reviewed, they were characterized in discrete response categories: same day, within 1 to 2 days, within 3 to 4 days, within a week, or unsure/unknown. Naloxone availability was also tracked by city/municipality, and this was compared to the state’s population distribution.
No personally identifiable information was obtained. This study was Institutional Review Board exempt.
Results
Responses were recorded from 183 pharmacies. Seventeen locations were eliminated from our analysis because their phone system was inoperable or the pharmacy was permanently closed. Of the pharmacies reached, 84.7% (155/183) reported they have naloxone available for pick up on the same day (Figure 1). Of the 15.3% (28) pharmacies that did not have same-day availability, 60.7% (17 pharmacies) reported availability in 1 to 2 days, 3.6% had availability in 3 to 4 days, 3.6% had availability in 1 week, and 32.1% were unsure of next availability (Figure 2). More than one-third of the state’s patients reside in municipalities where naloxone is immediately available in at least 72% of pharmacies (Table).13
Discussion
Increased access to naloxone at the state and community level is associated with reduced risk for death from overdose, and, consequently, widespread availability is recommended.14-17 Statewide real-time pharmacy availability of naloxone—as patients would experience availability—has not been previously reported. These findings suggest unpredictable same-day availability that may affect experience and care outcomes. That other studies have found similar challenges in naloxone availability in other municipalities and regions suggests this barrier to access is widespread,6-9 and likely affects patients throughout the country.
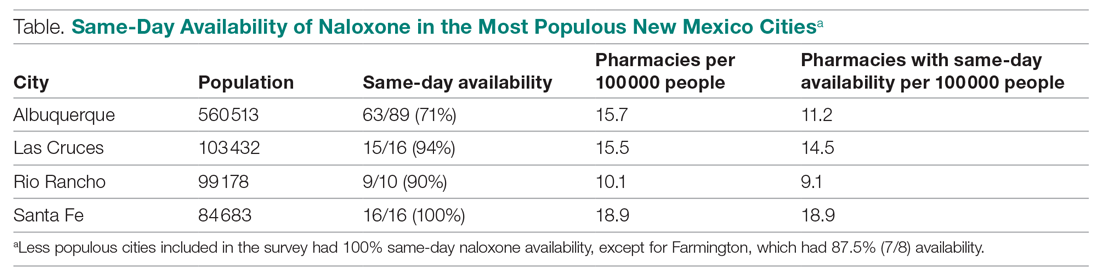
Many patients have misgivings about naloxone, and it places an undue burden on them to travel to multiple pharmacies or take repeated trips to fill prescriptions. Additionally, patients without reliable transportation may be unable to return at a later date. Although we found most pharmacies in New Mexico without immediate availability of naloxone reported they could have it within several days, such a delay may reduce the likelihood that patients will fill their prescription at all. It is also concerning that many pharmacies are unsure of when naloxone will be available, particularly when some of these may be the only pharmacy easily accessible to patients or the one where they regularly fill their prescriptions.
Barriers to naloxone availability requires further study due to possible negative consequences for patient safety and risks for exacerbating health disparities among vulnerable populations. Further research may focus on examining the effects on patients when naloxone dispensing is delayed or impossible, why there is variability in naloxone availability between different pharmacies and municipalities, the reasons for uncertainty when naloxone will be available, and effective solutions. Expanded naloxone distribution in community locations and in clinics offers one potential patient-centered solution that should be explored, but it is likely that more widespread and systemic solutions will require policy and regulatory changes at the state and national levels.
Limitations of this study include that the findings may be relevant for solely 1 state, such as in the case of state-specific barriers to keeping naloxone in stock that we are unaware of. However, it is unclear why that would be the case, and it is more likely that similar barriers are pervasive. Additionally, repeat phone calls, which we did not follow up with, may have yielded more pharmacies with naloxone availability. However, due to the stigma associated with obtaining naloxone, it may be that patients will not make multiple calls either—highlighting how important real-time availability is.
Conclusion
Urgent solutions are needed to address the epidemic of deaths from opioid overdoses. Naloxone availability is an important tool for reducing these deaths, resulting in numerous state laws attempting to increase access. Despite this, there are persistent barriers to patients receiving naloxone, including a lack of same-day availability at pharmacies. Our results suggest that this underexplored barrier is widespread. Improving both availability and accessibility of naloxone may include legislative policy solutions as well as patient-oriented solutions, such as distribution in clinics and hospitals when opioid prescriptions are first written. Further research should be conducted to determine patient-centered, effective solutions that can improve outcomes.
Corresponding author: Eileen Barrett, MD, MPH, Department of Internal Medicine, University of New Mexico; [email protected].
Financial disclosures: None.
1. Mason M, Welch SB, Arunkumar P, et al. Notes from the field: opioid overdose deaths before, during, and after an 11-week COVID-19 stay-at-home order—Cook County, Illinois, January 1, 2018–October 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):362-363. doi:10.15585/mmwr.mm7010a3
2. Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). Accessed October 6, 2021. https://www.kff.org/other/state-indicator/opioid-overdose-death
3. Sohn M, Talbert JC, Huang Z, et al. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215. doi:10.1001/jamanetworkopen.2019.6215
4. Smart R, Pardo B, Davis CS. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction. 2021;116(1):6-17. doi:10.1111/add.15163
5. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017;32(3):277-283. doi:10.1007/s11606-016-3895-8
6. Thornton JD, Lyvers E, Scott VGG, Dwibedi N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J Am Pharm Assoc (2003). 2017;57(2S):S12-S18.e4. doi:10.1016/j.japh.2016.12.070
7. Spivey C, Wilder A, Chisholm-Burns MA, et al. Evaluation of naloxone access, pricing, and barriers to dispensing in Tennessee retail community pharmacies. J Am Pharm Assoc (2003). 2020;60(5):694-701.e1. doi:10.1016/j.japh.2020.01.030
8. Nguyen JL, Gilbert LR, Beasley L, et al. Availability of naloxone at rural Georgia pharmacies, 2019. JAMA Netw Open. 2020;3(2):e1921227. doi:10.1001/jamanetworkopen.2019.21227
9. Guadamuz JS, Alexander GC, Chaudhri T, et al. Availability and cost of naloxone nasal spray at pharmacies in Philadelphia, Pennsylvania. JAMA Netw Open. 2019;2(6):e195388. doi:10.1001/jamanetworkopen.2019.5388
10. Edge K. Changes in drug overdose mortality in New Mexico. New Mexico Epidemiology. July 2020 (3). https://www.nmhealth.org/data/view/report/2402/
11. Senate Bill 221. 54th Legislature, State of New Mexico, First Session, 2019 (introduced by William P. Soules). Accessed October 6, 2021. https://nmlegis.gov/Sessions/19%20Regular/bills/senate/SB0221.pdf
12. GoodRx. Find pharmacies in New Mexico. Accessed October 6, 2021. https://www.goodrx.com/pharmacy-near-me/all/nm
13. U.S. Census Bureau. QuickFacts: New Mexico. Accessed October 6, 2021. https://www.census.gov/quickfacts/NM
14. Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4(2):e2037259. doi:10.1001/jamanetworkopen.2020.37259
15. You HS, Ha J, Kang CY, et al. Regional variation in states’ naloxone accessibility laws in association with opioid overdose death rates—observational study (STROBE compliant). Medicine (Baltimore). 2020;99(22):e20033. doi:10.1097/MD.0000000000020033
16. Pew Charitable Trusts. Expanded access to naloxone can curb opioid overdose deaths. October 20, 2020. Accessed October 6, 2021. https://www.
17. Centers for Disease Control and Prevention. Still not enough naloxone where it’s most needed. August 6, 2019. Accessed October 6, 2021. https://www.cdc.gov/media/releases/2019/p0806-naloxone.html
From the Department of Medicine, University of California San Diego (Dr. Haponyuk), Department of Emergency Medicine, University of Tennessee (Dr. Dejong), the Department of Family Medicine, University of New Mexico (Dr. Gutfrucht), and the Department of Internal Medicine, University of New Mexico (Dr. Barrett)
Objective: Naloxone availability can reduce the risk of death from opioid overdoses, although prescriber, legislative, and payment barriers to accessing this life-saving medication exist. A previously underreported barrier involves same-day availability, the lack of which may force patients to travel to multiple pharmacies and having delays in access or risking not filling their prescription. This study sought to determine same-day availability of naloxone in pharmacies in the state of New Mexico.
Methods: Same-day availability of naloxone was assessed via an audit survey.
Results: Of the 183 pharamacies screened, only 84.7% had same-day availability, including only 72% in Albuquerque, the state’s most populous city/municipality.
Conclusion: These results highlight the extent of a previously underexplored challenge to patient care and barrier to patient safety, and future directions for more patient-centered care.
Keywords: naloxone; barriers to care; opioid overdose prevention.
The US is enduring an ongoing epidemic of deaths due to opioid use, which have increased in frequency since the onset of the COVID-19 pandemic.1 One strategy to reduce the risk of mortality from opioid use is to ensure the widespread availability of naloxone. Individual states have implemented harm reduction strategies to increase access to naloxone, including improving availability via a statewide standing order that it may be dispensed without a prescription.2,3 Such naloxone access laws are being widely adopted and are believed to reduce overdose deaths.4
There are many barriers to patients receiving naloxone despite their clinicians providing a prescription for it, including stigmatization, financial cost, and local availability.5-9 However, the stigma associated with naloxone extends to both patients and pharmacists. Pharmacists in West Virginia, for example, showed widespread concerns about having naloxone available for patients to purchase over the counter, for fear that increasing naloxone access may increase overdoses.6 A study in Tennessee also found pharmacists hesitant to recommend naloxone.7 Another study of rural pharmacies in Georgia found that just over half carried naloxone despite a state law that naloxone be available without a prescription.8 Challenges are not limited to rural areas, however; a study in Philadelphia found that more than one-third of pharmacies required a prescription to dispense naloxone, contrary to state law.9 Thus, in a rapidly changing regulatory environment, there are many evolving barriers to patients receiving naloxone.
New Mexico has an opioid overdose rate higher than the national average, coming in 15th out of 50 states when last ranked in 2018, with overdose rates that vary across demographic variables.10 Consequently, New Mexico state law added language requiring clinicians prescribing opioids for 5 days or longer to co-prescribe naloxone along with written information on how to administer the opioid antagonist.11 New Mexico is also a geographically large state with a relatively low overall population characterized by striking health disparities, particularly as related to access to care.
The purpose of this study is to describe the same-day availability of naloxone throughout the state of New Mexico after a change in state law requiring co-prescription was enacted, to help identify challenges to patients receiving it. Comprehensive examination of barriers to patients accessing this life-saving medication can advise strategies to both improve patient-centered care and potentially reduce deaths.
Methods
To better understand barriers to patients obtaining naloxone, in July and August of 2019 we performed an audit (“secret shopper”) study of all pharmacies in the state, posing as patients wishing to obtain naloxone. A publicly available list of every pharmacy in New Mexico was used to identify 89 pharmacies in Albuquerque (the most populous city in New Mexico) and 106 pharmacies throughout the rest of the state.12
Every pharmacy was called via a publicly available phone number during business hours (confirmed via an internet search), at least 2 hours prior to closing. One of 3 researchers telephoned pharmacies posing as a patient and inquired whether naloxone would be available for pick up the same day. If the pharmacy confirmed it was available that day, the call concluded. If naloxone was unavailable for same day pick up, researchers asked when it would be next available. Each pharmacy was called once, and neither insurance information nor cost was offered or requested. All questions were asked in English by native English speakers.
All responses were recorded in a secure spreadsheet. Once all responses were received and reviewed, they were characterized in discrete response categories: same day, within 1 to 2 days, within 3 to 4 days, within a week, or unsure/unknown. Naloxone availability was also tracked by city/municipality, and this was compared to the state’s population distribution.
No personally identifiable information was obtained. This study was Institutional Review Board exempt.

Results
Responses were recorded from 183 pharmacies. Seventeen locations were eliminated from our analysis because their phone system was inoperable or the pharmacy was permanently closed. Of the pharmacies reached, 84.7% (155/183) reported they have naloxone available for pick up on the same day (Figure 1). Of the 15.3% (28) pharmacies that did not have same-day availability, 60.7% (17 pharmacies) reported availability in 1 to 2 days, 3.6% had availability in 3 to 4 days, 3.6% had availability in 1 week, and 32.1% were unsure of next availability (Figure 2). More than one-third of the state’s patients reside in municipalities where naloxone is immediately available in at least 72% of pharmacies (Table).13

Discussion
Increased access to naloxone at the state and community level is associated with reduced risk for death from overdose, and, consequently, widespread availability is recommended.14-17 Statewide real-time pharmacy availability of naloxone—as patients would experience availability—has not been previously reported. These findings suggest unpredictable same-day availability that may affect experience and care outcomes. That other studies have found similar challenges in naloxone availability in other municipalities and regions suggests this barrier to access is widespread,6-9 and likely affects patients throughout the country.

Many patients have misgivings about naloxone, and it places an undue burden on them to travel to multiple pharmacies or take repeated trips to fill prescriptions. Additionally, patients without reliable transportation may be unable to return at a later date. Although we found most pharmacies in New Mexico without immediate availability of naloxone reported they could have it within several days, such a delay may reduce the likelihood that patients will fill their prescription at all. It is also concerning that many pharmacies are unsure of when naloxone will be available, particularly when some of these may be the only pharmacy easily accessible to patients or the one where they regularly fill their prescriptions.
Barriers to naloxone availability requires further study due to possible negative consequences for patient safety and risks for exacerbating health disparities among vulnerable populations. Further research may focus on examining the effects on patients when naloxone dispensing is delayed or impossible, why there is variability in naloxone availability between different pharmacies and municipalities, the reasons for uncertainty when naloxone will be available, and effective solutions. Expanded naloxone distribution in community locations and in clinics offers one potential patient-centered solution that should be explored, but it is likely that more widespread and systemic solutions will require policy and regulatory changes at the state and national levels.
Limitations of this study include that the findings may be relevant for solely 1 state, such as in the case of state-specific barriers to keeping naloxone in stock that we are unaware of. However, it is unclear why that would be the case, and it is more likely that similar barriers are pervasive. Additionally, repeat phone calls, which we did not follow up with, may have yielded more pharmacies with naloxone availability. However, due to the stigma associated with obtaining naloxone, it may be that patients will not make multiple calls either—highlighting how important real-time availability is.
Conclusion
Urgent solutions are needed to address the epidemic of deaths from opioid overdoses. Naloxone availability is an important tool for reducing these deaths, resulting in numerous state laws attempting to increase access. Despite this, there are persistent barriers to patients receiving naloxone, including a lack of same-day availability at pharmacies. Our results suggest that this underexplored barrier is widespread. Improving both availability and accessibility of naloxone may include legislative policy solutions as well as patient-oriented solutions, such as distribution in clinics and hospitals when opioid prescriptions are first written. Further research should be conducted to determine patient-centered, effective solutions that can improve outcomes.
Corresponding author: Eileen Barrett, MD, MPH, Department of Internal Medicine, University of New Mexico; [email protected].
Financial disclosures: None.
From the Department of Medicine, University of California San Diego (Dr. Haponyuk), Department of Emergency Medicine, University of Tennessee (Dr. Dejong), the Department of Family Medicine, University of New Mexico (Dr. Gutfrucht), and the Department of Internal Medicine, University of New Mexico (Dr. Barrett)
Objective: Naloxone availability can reduce the risk of death from opioid overdoses, although prescriber, legislative, and payment barriers to accessing this life-saving medication exist. A previously underreported barrier involves same-day availability, the lack of which may force patients to travel to multiple pharmacies and having delays in access or risking not filling their prescription. This study sought to determine same-day availability of naloxone in pharmacies in the state of New Mexico.
Methods: Same-day availability of naloxone was assessed via an audit survey.
Results: Of the 183 pharamacies screened, only 84.7% had same-day availability, including only 72% in Albuquerque, the state’s most populous city/municipality.
Conclusion: These results highlight the extent of a previously underexplored challenge to patient care and barrier to patient safety, and future directions for more patient-centered care.
Keywords: naloxone; barriers to care; opioid overdose prevention.
The US is enduring an ongoing epidemic of deaths due to opioid use, which have increased in frequency since the onset of the COVID-19 pandemic.1 One strategy to reduce the risk of mortality from opioid use is to ensure the widespread availability of naloxone. Individual states have implemented harm reduction strategies to increase access to naloxone, including improving availability via a statewide standing order that it may be dispensed without a prescription.2,3 Such naloxone access laws are being widely adopted and are believed to reduce overdose deaths.4
There are many barriers to patients receiving naloxone despite their clinicians providing a prescription for it, including stigmatization, financial cost, and local availability.5-9 However, the stigma associated with naloxone extends to both patients and pharmacists. Pharmacists in West Virginia, for example, showed widespread concerns about having naloxone available for patients to purchase over the counter, for fear that increasing naloxone access may increase overdoses.6 A study in Tennessee also found pharmacists hesitant to recommend naloxone.7 Another study of rural pharmacies in Georgia found that just over half carried naloxone despite a state law that naloxone be available without a prescription.8 Challenges are not limited to rural areas, however; a study in Philadelphia found that more than one-third of pharmacies required a prescription to dispense naloxone, contrary to state law.9 Thus, in a rapidly changing regulatory environment, there are many evolving barriers to patients receiving naloxone.
New Mexico has an opioid overdose rate higher than the national average, coming in 15th out of 50 states when last ranked in 2018, with overdose rates that vary across demographic variables.10 Consequently, New Mexico state law added language requiring clinicians prescribing opioids for 5 days or longer to co-prescribe naloxone along with written information on how to administer the opioid antagonist.11 New Mexico is also a geographically large state with a relatively low overall population characterized by striking health disparities, particularly as related to access to care.
The purpose of this study is to describe the same-day availability of naloxone throughout the state of New Mexico after a change in state law requiring co-prescription was enacted, to help identify challenges to patients receiving it. Comprehensive examination of barriers to patients accessing this life-saving medication can advise strategies to both improve patient-centered care and potentially reduce deaths.
Methods
To better understand barriers to patients obtaining naloxone, in July and August of 2019 we performed an audit (“secret shopper”) study of all pharmacies in the state, posing as patients wishing to obtain naloxone. A publicly available list of every pharmacy in New Mexico was used to identify 89 pharmacies in Albuquerque (the most populous city in New Mexico) and 106 pharmacies throughout the rest of the state.12
Every pharmacy was called via a publicly available phone number during business hours (confirmed via an internet search), at least 2 hours prior to closing. One of 3 researchers telephoned pharmacies posing as a patient and inquired whether naloxone would be available for pick up the same day. If the pharmacy confirmed it was available that day, the call concluded. If naloxone was unavailable for same day pick up, researchers asked when it would be next available. Each pharmacy was called once, and neither insurance information nor cost was offered or requested. All questions were asked in English by native English speakers.
All responses were recorded in a secure spreadsheet. Once all responses were received and reviewed, they were characterized in discrete response categories: same day, within 1 to 2 days, within 3 to 4 days, within a week, or unsure/unknown. Naloxone availability was also tracked by city/municipality, and this was compared to the state’s population distribution.
No personally identifiable information was obtained. This study was Institutional Review Board exempt.

Results
Responses were recorded from 183 pharmacies. Seventeen locations were eliminated from our analysis because their phone system was inoperable or the pharmacy was permanently closed. Of the pharmacies reached, 84.7% (155/183) reported they have naloxone available for pick up on the same day (Figure 1). Of the 15.3% (28) pharmacies that did not have same-day availability, 60.7% (17 pharmacies) reported availability in 1 to 2 days, 3.6% had availability in 3 to 4 days, 3.6% had availability in 1 week, and 32.1% were unsure of next availability (Figure 2). More than one-third of the state’s patients reside in municipalities where naloxone is immediately available in at least 72% of pharmacies (Table).13

Discussion
Increased access to naloxone at the state and community level is associated with reduced risk for death from overdose, and, consequently, widespread availability is recommended.14-17 Statewide real-time pharmacy availability of naloxone—as patients would experience availability—has not been previously reported. These findings suggest unpredictable same-day availability that may affect experience and care outcomes. That other studies have found similar challenges in naloxone availability in other municipalities and regions suggests this barrier to access is widespread,6-9 and likely affects patients throughout the country.

Many patients have misgivings about naloxone, and it places an undue burden on them to travel to multiple pharmacies or take repeated trips to fill prescriptions. Additionally, patients without reliable transportation may be unable to return at a later date. Although we found most pharmacies in New Mexico without immediate availability of naloxone reported they could have it within several days, such a delay may reduce the likelihood that patients will fill their prescription at all. It is also concerning that many pharmacies are unsure of when naloxone will be available, particularly when some of these may be the only pharmacy easily accessible to patients or the one where they regularly fill their prescriptions.
Barriers to naloxone availability requires further study due to possible negative consequences for patient safety and risks for exacerbating health disparities among vulnerable populations. Further research may focus on examining the effects on patients when naloxone dispensing is delayed or impossible, why there is variability in naloxone availability between different pharmacies and municipalities, the reasons for uncertainty when naloxone will be available, and effective solutions. Expanded naloxone distribution in community locations and in clinics offers one potential patient-centered solution that should be explored, but it is likely that more widespread and systemic solutions will require policy and regulatory changes at the state and national levels.
Limitations of this study include that the findings may be relevant for solely 1 state, such as in the case of state-specific barriers to keeping naloxone in stock that we are unaware of. However, it is unclear why that would be the case, and it is more likely that similar barriers are pervasive. Additionally, repeat phone calls, which we did not follow up with, may have yielded more pharmacies with naloxone availability. However, due to the stigma associated with obtaining naloxone, it may be that patients will not make multiple calls either—highlighting how important real-time availability is.
Conclusion
Urgent solutions are needed to address the epidemic of deaths from opioid overdoses. Naloxone availability is an important tool for reducing these deaths, resulting in numerous state laws attempting to increase access. Despite this, there are persistent barriers to patients receiving naloxone, including a lack of same-day availability at pharmacies. Our results suggest that this underexplored barrier is widespread. Improving both availability and accessibility of naloxone may include legislative policy solutions as well as patient-oriented solutions, such as distribution in clinics and hospitals when opioid prescriptions are first written. Further research should be conducted to determine patient-centered, effective solutions that can improve outcomes.
Corresponding author: Eileen Barrett, MD, MPH, Department of Internal Medicine, University of New Mexico; [email protected].
Financial disclosures: None.
1. Mason M, Welch SB, Arunkumar P, et al. Notes from the field: opioid overdose deaths before, during, and after an 11-week COVID-19 stay-at-home order—Cook County, Illinois, January 1, 2018–October 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):362-363. doi:10.15585/mmwr.mm7010a3
2. Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). Accessed October 6, 2021. https://www.kff.org/other/state-indicator/opioid-overdose-death
3. Sohn M, Talbert JC, Huang Z, et al. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215. doi:10.1001/jamanetworkopen.2019.6215
4. Smart R, Pardo B, Davis CS. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction. 2021;116(1):6-17. doi:10.1111/add.15163
5. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017;32(3):277-283. doi:10.1007/s11606-016-3895-8
6. Thornton JD, Lyvers E, Scott VGG, Dwibedi N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J Am Pharm Assoc (2003). 2017;57(2S):S12-S18.e4. doi:10.1016/j.japh.2016.12.070
7. Spivey C, Wilder A, Chisholm-Burns MA, et al. Evaluation of naloxone access, pricing, and barriers to dispensing in Tennessee retail community pharmacies. J Am Pharm Assoc (2003). 2020;60(5):694-701.e1. doi:10.1016/j.japh.2020.01.030
8. Nguyen JL, Gilbert LR, Beasley L, et al. Availability of naloxone at rural Georgia pharmacies, 2019. JAMA Netw Open. 2020;3(2):e1921227. doi:10.1001/jamanetworkopen.2019.21227
9. Guadamuz JS, Alexander GC, Chaudhri T, et al. Availability and cost of naloxone nasal spray at pharmacies in Philadelphia, Pennsylvania. JAMA Netw Open. 2019;2(6):e195388. doi:10.1001/jamanetworkopen.2019.5388
10. Edge K. Changes in drug overdose mortality in New Mexico. New Mexico Epidemiology. July 2020 (3). https://www.nmhealth.org/data/view/report/2402/
11. Senate Bill 221. 54th Legislature, State of New Mexico, First Session, 2019 (introduced by William P. Soules). Accessed October 6, 2021. https://nmlegis.gov/Sessions/19%20Regular/bills/senate/SB0221.pdf
12. GoodRx. Find pharmacies in New Mexico. Accessed October 6, 2021. https://www.goodrx.com/pharmacy-near-me/all/nm
13. U.S. Census Bureau. QuickFacts: New Mexico. Accessed October 6, 2021. https://www.census.gov/quickfacts/NM
14. Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4(2):e2037259. doi:10.1001/jamanetworkopen.2020.37259
15. You HS, Ha J, Kang CY, et al. Regional variation in states’ naloxone accessibility laws in association with opioid overdose death rates—observational study (STROBE compliant). Medicine (Baltimore). 2020;99(22):e20033. doi:10.1097/MD.0000000000020033
16. Pew Charitable Trusts. Expanded access to naloxone can curb opioid overdose deaths. October 20, 2020. Accessed October 6, 2021. https://www.
17. Centers for Disease Control and Prevention. Still not enough naloxone where it’s most needed. August 6, 2019. Accessed October 6, 2021. https://www.cdc.gov/media/releases/2019/p0806-naloxone.html
1. Mason M, Welch SB, Arunkumar P, et al. Notes from the field: opioid overdose deaths before, during, and after an 11-week COVID-19 stay-at-home order—Cook County, Illinois, January 1, 2018–October 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):362-363. doi:10.15585/mmwr.mm7010a3
2. Kaiser Family Foundation. Opioid overdose death rates and all drug overdose death rates per 100,000 population (age-adjusted). Accessed October 6, 2021. https://www.kff.org/other/state-indicator/opioid-overdose-death
3. Sohn M, Talbert JC, Huang Z, et al. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw Open. 2019;2(6):e196215. doi:10.1001/jamanetworkopen.2019.6215
4. Smart R, Pardo B, Davis CS. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction. 2021;116(1):6-17. doi:10.1111/add.15163
5. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: a qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017;32(3):277-283. doi:10.1007/s11606-016-3895-8
6. Thornton JD, Lyvers E, Scott VGG, Dwibedi N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J Am Pharm Assoc (2003). 2017;57(2S):S12-S18.e4. doi:10.1016/j.japh.2016.12.070
7. Spivey C, Wilder A, Chisholm-Burns MA, et al. Evaluation of naloxone access, pricing, and barriers to dispensing in Tennessee retail community pharmacies. J Am Pharm Assoc (2003). 2020;60(5):694-701.e1. doi:10.1016/j.japh.2020.01.030
8. Nguyen JL, Gilbert LR, Beasley L, et al. Availability of naloxone at rural Georgia pharmacies, 2019. JAMA Netw Open. 2020;3(2):e1921227. doi:10.1001/jamanetworkopen.2019.21227
9. Guadamuz JS, Alexander GC, Chaudhri T, et al. Availability and cost of naloxone nasal spray at pharmacies in Philadelphia, Pennsylvania. JAMA Netw Open. 2019;2(6):e195388. doi:10.1001/jamanetworkopen.2019.5388
10. Edge K. Changes in drug overdose mortality in New Mexico. New Mexico Epidemiology. July 2020 (3). https://www.nmhealth.org/data/view/report/2402/
11. Senate Bill 221. 54th Legislature, State of New Mexico, First Session, 2019 (introduced by William P. Soules). Accessed October 6, 2021. https://nmlegis.gov/Sessions/19%20Regular/bills/senate/SB0221.pdf
12. GoodRx. Find pharmacies in New Mexico. Accessed October 6, 2021. https://www.goodrx.com/pharmacy-near-me/all/nm
13. U.S. Census Bureau. QuickFacts: New Mexico. Accessed October 6, 2021. https://www.census.gov/quickfacts/NM
14. Linas BP, Savinkina A, Madushani RWMA, et al. Projected estimates of opioid mortality after community-level interventions. JAMA Netw Open. 2021;4(2):e2037259. doi:10.1001/jamanetworkopen.2020.37259
15. You HS, Ha J, Kang CY, et al. Regional variation in states’ naloxone accessibility laws in association with opioid overdose death rates—observational study (STROBE compliant). Medicine (Baltimore). 2020;99(22):e20033. doi:10.1097/MD.0000000000020033
16. Pew Charitable Trusts. Expanded access to naloxone can curb opioid overdose deaths. October 20, 2020. Accessed October 6, 2021. https://www.
17. Centers for Disease Control and Prevention. Still not enough naloxone where it’s most needed. August 6, 2019. Accessed October 6, 2021. https://www.cdc.gov/media/releases/2019/p0806-naloxone.html
Association Between Physiotherapy Outcome Measures and the Functional Independence Measure: A Retrospective Analysis
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)
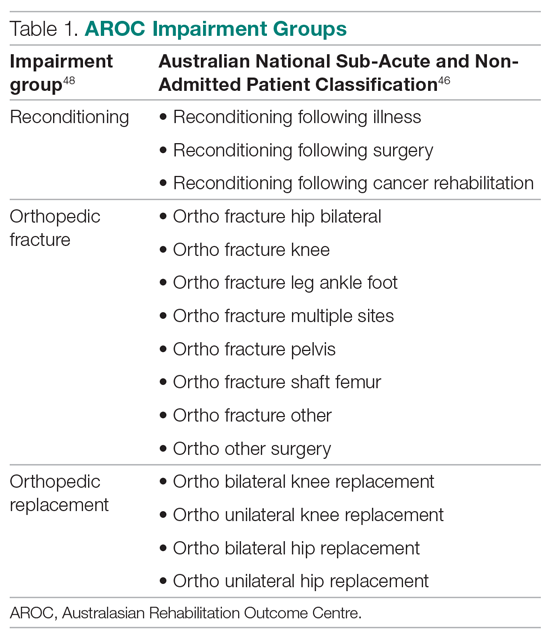
Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.
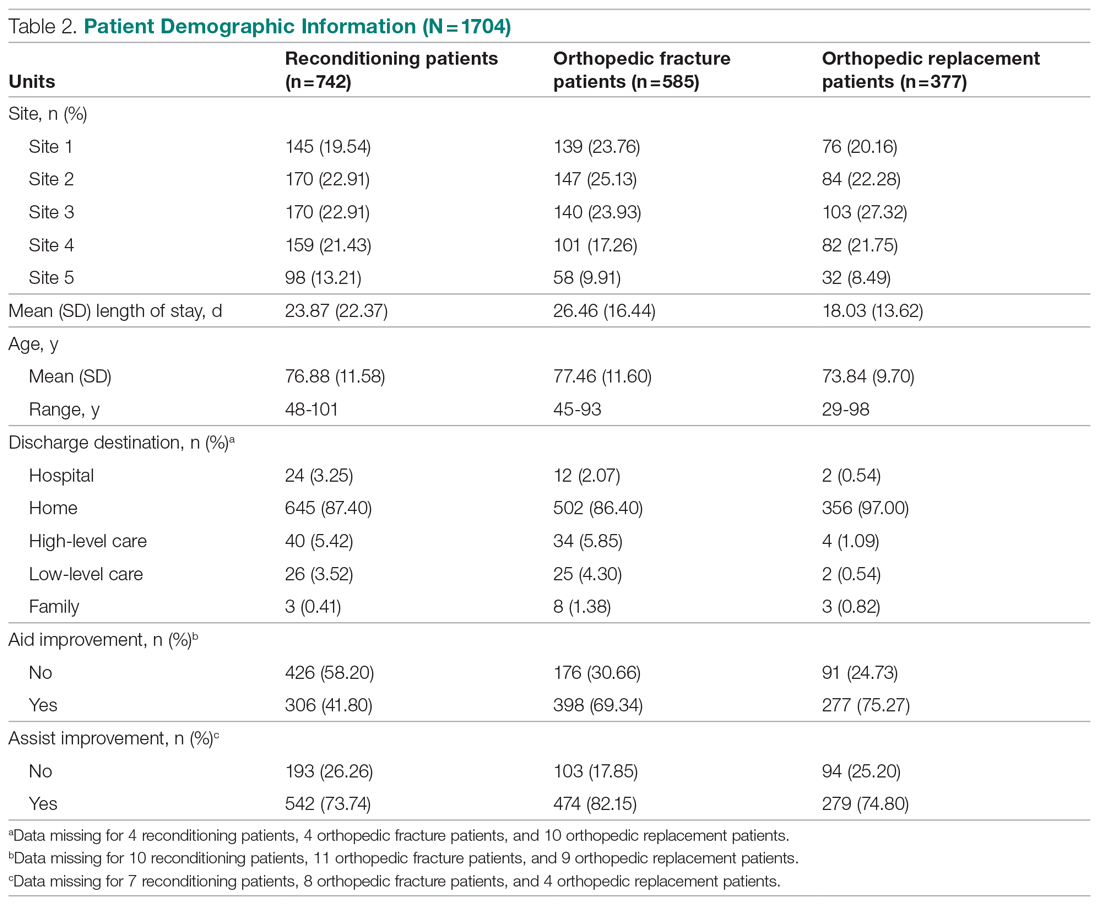
Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).
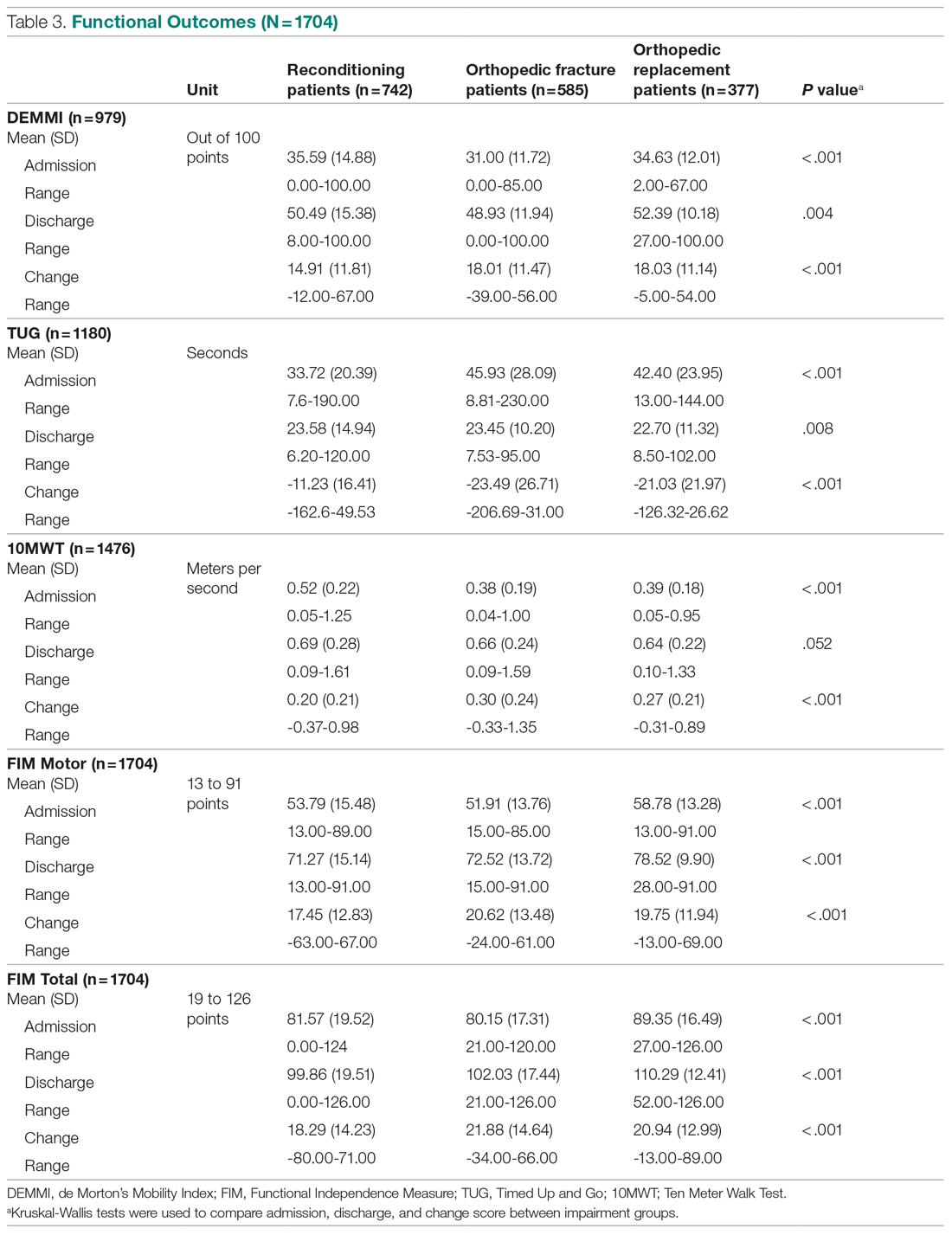
Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).
Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)

Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.

Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).

Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).

Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)

Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.

Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).

Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).

Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
High-poverty areas host more firearm-related youth deaths
Higher poverty concentration at the county level significantly increased the risk of firearm-related deaths in children and youth aged 5-24 years in the United States, based on a review of approximately 67,000 fatalities.
Firearms are the second-leading cause of death in children and young adults in the United States, according to data from the Centers for Disease Control and Prevention, wrote Jefferson T. Barrett, MD, of The Children’s Hospital at Montefiore, New York, and colleagues. County-level poverty has been associated with increased injury mortality in children, but the association between county-level poverty and firearm-related mortality in particular has not been well studied.
In a cross-sectional study published in JAMA Pediatrics, 67,905 firearm-related deaths in children and youth aged 5-24 years that occurred between Jan. 1, 2007, and Dec. 31, 2016 were analyzed. The deaths included 42,512 homicides (62.6%), 23,034 suicides (33.9%), and 1,627 unintentional deaths (2.4%).
County poverty data were acquired from the U.S. Census Bureau. County-level poverty was divided into five categories based on percentage of the population living below the federal poverty level: 0%-4.9%, 5%-9.9%, 10%-14.9%, 15%-19.9%, and 20% or more.
Overall, 88.6% of the total deaths were in males. Notably, 44.8% of total firearm-related deaths and 63.9% of homicides occurred in non-Hispanic Blacks, who make up only 14% of the youth population in the United States, the researchers wrote.
The total number of firearm-related deaths was 248 in the lowest quintile of poverty concentration, followed by 6,841, 18,551, 27,305, and 14,960 in the remaining quintiles.
In a multivariate regression model that included demographics, urban versus rural, and statewide firearm prevalence, youth in counties with the highest quintile of poverty concentration had an increased rate of total firearm-related deaths (adjusted incidence rate ratio, 2.29), as well as increased rates of homicides, suicides, and unintentional deaths (aIRR, 3.55, 1.45, and 9.32, respectively), compared with those living in the lowest quintile of poverty concentration. Individuals in the highest poverty quintile accounted for 22.0% of total firearm-related deaths, 25.5% of homicides, 15.3% of suicides, and 25.1% of unintentional deaths.
The researchers also calculated the population-attributable fraction (PAF) and years of potential life lost. “The PAF represents the proportion of deaths associated with a particular exposure, which was concentrated county poverty in this study,” they explained. The PAF for all firearm-related deaths was 0.51, PAFs for homicides, suicides, and unintentional deaths were 0.66, 0.30, and 0.86, respectively. The PAF calculation translated to 34,292 firearm-related deaths that may not have occurred if youth in all counties had the same risk as those in counties with the lowest poverty concentration.
“Over the 10-year study period, we observed 3,833,105 years of potential life lost in youth aged 5-24 years from firearm-related deaths,” the researchers wrote.
The study findings were limited by several factors including the potential bias of a cross-section design, and inability to account for all the ways that county-level poverty might increase the risk of firearm-related death in children and teens, the researchers noted. Other potential limitations include possible misclassification of death, lack of data on individual family incomes, shifts in counties in the poverty categories over time, and the use of statewide, rather than countywide, estimates of firearm ownership.
However, the results are consistent with those of previous studies, and add that “mortality rates were consistent even after controlling for demographic variables, county urbanicity, and statewide firearm prevalence,” the researchers concluded.
Address structural racism to reduce disparities
“Firearm-related homicides among youth aged 5-24 years are among the causes of death with the greatest disparities,” based on CDC fatal injury reports, wrote Alice M. Ellyson, PhD, Frederick P. Rivara, MD, and Ali Rowhani-Rahbar, MD, all of the University of Washington, Seattle, in an accompanying editorial.
The current study builds on previous research, including studies showing an association between income inequality and firearm-related homicide, they said. More research is needed to determine how to intervene in the pathways between poverty and firearm-related death. For example, if access to high-quality health care is a factor, programs to increase access to health insurance, such as the Affordable Care Act and Children’s Health Insurance Program, or to increase access to high-quality trauma care may help reduce firearm-related death in youth.
“The study of where, how, and why racism operates as a factor in both poverty and firearm-related death must continue, especially considering the disparities consistently documented in Alaska Native or American Indian, Black, and Hispanic communities,” the editorialists wrote.
“Key potential mechanisms for reducing the consequences of poverty for firearm-related death are often denied to racial and ethnic minority groups through a variety of structures, policies, and systems in health care, employment, housing, transportation, and education,” they emphasized, and the impact of racism, not only on the pathways to poverty, but also on mediators between poverty and firearm-related death, must be explored.
Findings spotlight need to for poverty programs
The study was an interesting look at the specific relationship between poverty and firearm-related deaths in people aged younger than 25 years in the United States, Tim Joos, MD, of Seattle said in an interview.
“Although America is not a poor country, the combination of poverty within America and its unique gun culture seems to prove deadly for its youth,” Dr. Joos said. “The strongest relationship is between firearm-related homicide and poverty, but unintentional firearm deaths and poverty also are clearly linked, whereas the link between firearm-related suicide and poverty appears to be present, but small.”.
In the current study, “the authors note that firearm deaths are the second-leading cause of death among all people ages 15-24 years,” said Dr. Joos. “Many of us have followed children from infancy just to have them meet this untimely end as adolescents, wishing we had a vaccine or other remedy in our toolbelt for this particular scourge.
“As our country currently debates the size of the social safety net, this study is one of many that suggests government programs aimed at poverty alleviation would substantially contribute to the health of American youth,” Dr. Joos added.
The study received no outside funding. Lead author Dr. Barrett had no financial conflicts to disclose. Dr. Ellyson disclosed funds from the CDC, the state of Washington, and the Grandmothers Against Gun Violence Foundation for research outside the submitted work. Dr. Rivara disclosed funds from the National Institutes of Health, the State of Washington, and the National Collaborative on Gun Violence Research for research outside the submitted work. Dr. Rowhani-Rahbar disclosed funds from the CDC, National Institutes of Health, National Collaborative on Gun Violence Research, Fund for a Safer Future, and state of Washington for research outside the submitted work. Dr. Joos had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Higher poverty concentration at the county level significantly increased the risk of firearm-related deaths in children and youth aged 5-24 years in the United States, based on a review of approximately 67,000 fatalities.
Firearms are the second-leading cause of death in children and young adults in the United States, according to data from the Centers for Disease Control and Prevention, wrote Jefferson T. Barrett, MD, of The Children’s Hospital at Montefiore, New York, and colleagues. County-level poverty has been associated with increased injury mortality in children, but the association between county-level poverty and firearm-related mortality in particular has not been well studied.
In a cross-sectional study published in JAMA Pediatrics, 67,905 firearm-related deaths in children and youth aged 5-24 years that occurred between Jan. 1, 2007, and Dec. 31, 2016 were analyzed. The deaths included 42,512 homicides (62.6%), 23,034 suicides (33.9%), and 1,627 unintentional deaths (2.4%).
County poverty data were acquired from the U.S. Census Bureau. County-level poverty was divided into five categories based on percentage of the population living below the federal poverty level: 0%-4.9%, 5%-9.9%, 10%-14.9%, 15%-19.9%, and 20% or more.
Overall, 88.6% of the total deaths were in males. Notably, 44.8% of total firearm-related deaths and 63.9% of homicides occurred in non-Hispanic Blacks, who make up only 14% of the youth population in the United States, the researchers wrote.
The total number of firearm-related deaths was 248 in the lowest quintile of poverty concentration, followed by 6,841, 18,551, 27,305, and 14,960 in the remaining quintiles.
In a multivariate regression model that included demographics, urban versus rural, and statewide firearm prevalence, youth in counties with the highest quintile of poverty concentration had an increased rate of total firearm-related deaths (adjusted incidence rate ratio, 2.29), as well as increased rates of homicides, suicides, and unintentional deaths (aIRR, 3.55, 1.45, and 9.32, respectively), compared with those living in the lowest quintile of poverty concentration. Individuals in the highest poverty quintile accounted for 22.0% of total firearm-related deaths, 25.5% of homicides, 15.3% of suicides, and 25.1% of unintentional deaths.
The researchers also calculated the population-attributable fraction (PAF) and years of potential life lost. “The PAF represents the proportion of deaths associated with a particular exposure, which was concentrated county poverty in this study,” they explained. The PAF for all firearm-related deaths was 0.51, PAFs for homicides, suicides, and unintentional deaths were 0.66, 0.30, and 0.86, respectively. The PAF calculation translated to 34,292 firearm-related deaths that may not have occurred if youth in all counties had the same risk as those in counties with the lowest poverty concentration.
“Over the 10-year study period, we observed 3,833,105 years of potential life lost in youth aged 5-24 years from firearm-related deaths,” the researchers wrote.
The study findings were limited by several factors including the potential bias of a cross-section design, and inability to account for all the ways that county-level poverty might increase the risk of firearm-related death in children and teens, the researchers noted. Other potential limitations include possible misclassification of death, lack of data on individual family incomes, shifts in counties in the poverty categories over time, and the use of statewide, rather than countywide, estimates of firearm ownership.
However, the results are consistent with those of previous studies, and add that “mortality rates were consistent even after controlling for demographic variables, county urbanicity, and statewide firearm prevalence,” the researchers concluded.
Address structural racism to reduce disparities
“Firearm-related homicides among youth aged 5-24 years are among the causes of death with the greatest disparities,” based on CDC fatal injury reports, wrote Alice M. Ellyson, PhD, Frederick P. Rivara, MD, and Ali Rowhani-Rahbar, MD, all of the University of Washington, Seattle, in an accompanying editorial.
The current study builds on previous research, including studies showing an association between income inequality and firearm-related homicide, they said. More research is needed to determine how to intervene in the pathways between poverty and firearm-related death. For example, if access to high-quality health care is a factor, programs to increase access to health insurance, such as the Affordable Care Act and Children’s Health Insurance Program, or to increase access to high-quality trauma care may help reduce firearm-related death in youth.
“The study of where, how, and why racism operates as a factor in both poverty and firearm-related death must continue, especially considering the disparities consistently documented in Alaska Native or American Indian, Black, and Hispanic communities,” the editorialists wrote.
“Key potential mechanisms for reducing the consequences of poverty for firearm-related death are often denied to racial and ethnic minority groups through a variety of structures, policies, and systems in health care, employment, housing, transportation, and education,” they emphasized, and the impact of racism, not only on the pathways to poverty, but also on mediators between poverty and firearm-related death, must be explored.
Findings spotlight need to for poverty programs
The study was an interesting look at the specific relationship between poverty and firearm-related deaths in people aged younger than 25 years in the United States, Tim Joos, MD, of Seattle said in an interview.
“Although America is not a poor country, the combination of poverty within America and its unique gun culture seems to prove deadly for its youth,” Dr. Joos said. “The strongest relationship is between firearm-related homicide and poverty, but unintentional firearm deaths and poverty also are clearly linked, whereas the link between firearm-related suicide and poverty appears to be present, but small.”.
In the current study, “the authors note that firearm deaths are the second-leading cause of death among all people ages 15-24 years,” said Dr. Joos. “Many of us have followed children from infancy just to have them meet this untimely end as adolescents, wishing we had a vaccine or other remedy in our toolbelt for this particular scourge.
“As our country currently debates the size of the social safety net, this study is one of many that suggests government programs aimed at poverty alleviation would substantially contribute to the health of American youth,” Dr. Joos added.
The study received no outside funding. Lead author Dr. Barrett had no financial conflicts to disclose. Dr. Ellyson disclosed funds from the CDC, the state of Washington, and the Grandmothers Against Gun Violence Foundation for research outside the submitted work. Dr. Rivara disclosed funds from the National Institutes of Health, the State of Washington, and the National Collaborative on Gun Violence Research for research outside the submitted work. Dr. Rowhani-Rahbar disclosed funds from the CDC, National Institutes of Health, National Collaborative on Gun Violence Research, Fund for a Safer Future, and state of Washington for research outside the submitted work. Dr. Joos had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Higher poverty concentration at the county level significantly increased the risk of firearm-related deaths in children and youth aged 5-24 years in the United States, based on a review of approximately 67,000 fatalities.
Firearms are the second-leading cause of death in children and young adults in the United States, according to data from the Centers for Disease Control and Prevention, wrote Jefferson T. Barrett, MD, of The Children’s Hospital at Montefiore, New York, and colleagues. County-level poverty has been associated with increased injury mortality in children, but the association between county-level poverty and firearm-related mortality in particular has not been well studied.
In a cross-sectional study published in JAMA Pediatrics, 67,905 firearm-related deaths in children and youth aged 5-24 years that occurred between Jan. 1, 2007, and Dec. 31, 2016 were analyzed. The deaths included 42,512 homicides (62.6%), 23,034 suicides (33.9%), and 1,627 unintentional deaths (2.4%).
County poverty data were acquired from the U.S. Census Bureau. County-level poverty was divided into five categories based on percentage of the population living below the federal poverty level: 0%-4.9%, 5%-9.9%, 10%-14.9%, 15%-19.9%, and 20% or more.
Overall, 88.6% of the total deaths were in males. Notably, 44.8% of total firearm-related deaths and 63.9% of homicides occurred in non-Hispanic Blacks, who make up only 14% of the youth population in the United States, the researchers wrote.
The total number of firearm-related deaths was 248 in the lowest quintile of poverty concentration, followed by 6,841, 18,551, 27,305, and 14,960 in the remaining quintiles.
In a multivariate regression model that included demographics, urban versus rural, and statewide firearm prevalence, youth in counties with the highest quintile of poverty concentration had an increased rate of total firearm-related deaths (adjusted incidence rate ratio, 2.29), as well as increased rates of homicides, suicides, and unintentional deaths (aIRR, 3.55, 1.45, and 9.32, respectively), compared with those living in the lowest quintile of poverty concentration. Individuals in the highest poverty quintile accounted for 22.0% of total firearm-related deaths, 25.5% of homicides, 15.3% of suicides, and 25.1% of unintentional deaths.
The researchers also calculated the population-attributable fraction (PAF) and years of potential life lost. “The PAF represents the proportion of deaths associated with a particular exposure, which was concentrated county poverty in this study,” they explained. The PAF for all firearm-related deaths was 0.51, PAFs for homicides, suicides, and unintentional deaths were 0.66, 0.30, and 0.86, respectively. The PAF calculation translated to 34,292 firearm-related deaths that may not have occurred if youth in all counties had the same risk as those in counties with the lowest poverty concentration.
“Over the 10-year study period, we observed 3,833,105 years of potential life lost in youth aged 5-24 years from firearm-related deaths,” the researchers wrote.
The study findings were limited by several factors including the potential bias of a cross-section design, and inability to account for all the ways that county-level poverty might increase the risk of firearm-related death in children and teens, the researchers noted. Other potential limitations include possible misclassification of death, lack of data on individual family incomes, shifts in counties in the poverty categories over time, and the use of statewide, rather than countywide, estimates of firearm ownership.
However, the results are consistent with those of previous studies, and add that “mortality rates were consistent even after controlling for demographic variables, county urbanicity, and statewide firearm prevalence,” the researchers concluded.
Address structural racism to reduce disparities
“Firearm-related homicides among youth aged 5-24 years are among the causes of death with the greatest disparities,” based on CDC fatal injury reports, wrote Alice M. Ellyson, PhD, Frederick P. Rivara, MD, and Ali Rowhani-Rahbar, MD, all of the University of Washington, Seattle, in an accompanying editorial.
The current study builds on previous research, including studies showing an association between income inequality and firearm-related homicide, they said. More research is needed to determine how to intervene in the pathways between poverty and firearm-related death. For example, if access to high-quality health care is a factor, programs to increase access to health insurance, such as the Affordable Care Act and Children’s Health Insurance Program, or to increase access to high-quality trauma care may help reduce firearm-related death in youth.
“The study of where, how, and why racism operates as a factor in both poverty and firearm-related death must continue, especially considering the disparities consistently documented in Alaska Native or American Indian, Black, and Hispanic communities,” the editorialists wrote.
“Key potential mechanisms for reducing the consequences of poverty for firearm-related death are often denied to racial and ethnic minority groups through a variety of structures, policies, and systems in health care, employment, housing, transportation, and education,” they emphasized, and the impact of racism, not only on the pathways to poverty, but also on mediators between poverty and firearm-related death, must be explored.
Findings spotlight need to for poverty programs
The study was an interesting look at the specific relationship between poverty and firearm-related deaths in people aged younger than 25 years in the United States, Tim Joos, MD, of Seattle said in an interview.
“Although America is not a poor country, the combination of poverty within America and its unique gun culture seems to prove deadly for its youth,” Dr. Joos said. “The strongest relationship is between firearm-related homicide and poverty, but unintentional firearm deaths and poverty also are clearly linked, whereas the link between firearm-related suicide and poverty appears to be present, but small.”.
In the current study, “the authors note that firearm deaths are the second-leading cause of death among all people ages 15-24 years,” said Dr. Joos. “Many of us have followed children from infancy just to have them meet this untimely end as adolescents, wishing we had a vaccine or other remedy in our toolbelt for this particular scourge.
“As our country currently debates the size of the social safety net, this study is one of many that suggests government programs aimed at poverty alleviation would substantially contribute to the health of American youth,” Dr. Joos added.
The study received no outside funding. Lead author Dr. Barrett had no financial conflicts to disclose. Dr. Ellyson disclosed funds from the CDC, the state of Washington, and the Grandmothers Against Gun Violence Foundation for research outside the submitted work. Dr. Rivara disclosed funds from the National Institutes of Health, the State of Washington, and the National Collaborative on Gun Violence Research for research outside the submitted work. Dr. Rowhani-Rahbar disclosed funds from the CDC, National Institutes of Health, National Collaborative on Gun Violence Research, Fund for a Safer Future, and state of Washington for research outside the submitted work. Dr. Joos had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Children and COVID: New cases increase for third straight week
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.





