User login
Global dementia cases may triple by 2050 unless risk factors are reduced
new research suggests.
Results from a study of 195 countries and territories estimates that by 2050, 153 million people are expected to have dementia worldwide – up from 57 million in 2019. In the United States, the number is expected to increase 100%, from an estimated 5.3 million in 2019 to 10.5 million in 2050.
The increase is largely driven by population growth and population aging, but researchers noted that expanding access to education and addressing risk factors such as obesity, high blood sugar, and smoking could blunt the rise in cases.
The study predicts increases in dementia in every country included in the analysis. The sharpest rise is expected in north Africa and the Middle East (367%) and sub-Saharan Africa (357%). The smallest increases will be in high-income countries in Asia Pacific (53%) and western Europe (74%).
Although the United States had the 37th lowest percentage increase across all countries considered, “this expected increase is still large and requires attention from policy and decision-makers,” said coinvestigator Emma Nichols, MPH, a researcher with the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The findings were published online Jan. 6, 2022, in The Lancet Public Health (doi: 10.1016/S2468-2667[21]00249-8).
Dementia prevalence
For the study, researchers used country-specific estimates of dementia prevalence from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 study to project dementia prevalence globally, by world region, and at the country level.
They also used information on projected trends in four important dementia risk factors (high body mass index, high fasting plasma glucose, smoking, and education) to estimate how changes in these risk factors might impact dementia prevalence between 2019 and 2050.
Despite large increases in the projected number of people living with dementia, age-standardized both-sex prevalence remained stable between 2019 and 2050, with a global percentage change of 0.1% (95% uncertainty interval, –7.5 to 10.8).
Dementia prevalence was higher in women than in men and increased with age, doubling about every 5 years until 85 years of age in both 2019 and 2050 (female-to-male ratio, 1.67; 95% UI, 1.52-1.85).
Projected increases in cases could largely be attributed to population growth and population aging, although their relative importance varied by world region. Population growth contributed most to the increases in sub-Saharan Africa and population aging contributed most to the increases in east Asia.
The countries with the highest expected percentage change in total number of dementia cases between 2019 and 2050 were: Qatar (1,926%), United Arab Emirates (1,795%), Bahrain (1,084%), Oman (943%), Saudi Arabia (898%), Kuwait (850%), Iraq (559%), Maldives (554%), Jordan (522%), and Equatorial Guinea (498%).
The countries with the lowest expected percentage change in total number of dementia cases between 2019 and 2050 were Japan (27%), Bulgaria (37%), Serbia (38%), Lithuania (44%), Greece (45%), Latvia (47%), Croatia (55%), Ukraine (55%), Italy (56%), and Finland (58%).
Modifiable risk factors
Researchers also calculated how changes in risk factors might affect dementia prevalence. They found that improvements in global education access would reduce dementia prevalence by an estimated 6.2 million cases worldwide by 2050. However, that decrease would be offset by expected increases in obesity, high blood sugar, and smoking, which investigators estimate will result in an additional 6.8 million dementia cases.
The projections are based on expected trends in population aging, population growth, and risk factor trajectories, but “projections could change if effective interventions for modifiable risk factors are developed and deployed,” Ms. Nichols said.
In 2020, the Lancet Commission on Dementia Prevention, Intervention, and Care issued an update of its 2017 report, identifying 12 modifiable risk factors that could delay or prevent 40% of dementia cases. The risk factors were low education, hypertension, hearing impairment, smoking, midlife obesity, depression, physical inactivity, diabetes, social isolation, excessive alcohol consumption, head injury, and air pollution.
“Countries, including the U.S., should look to develop effective interventions for modifiable risk factors, but also should invest in the resources needed to support those with dementia and their caregivers,” Ms. Nichols said. She added that additional support for research and resources to develop therapeutic interventions is also warranted.
Oversimplifying mechanisms?
In an accompanying commentary, Michaël Schwarzinger, MD, and Carole Dufouil, PhD, of Bordeaux (France) University Hospital, noted that the authors’ efforts to build on GBD 2019 oversimplify the underlying mechanisms that cause dementia. The authors “provide somehow apocalyptic projections that do not factor in advisable changes in lifestyle over the lifetime,” they wrote.
“There is a considerable and urgent need to reinforce a public health approach towards dementia to better inform the people and decision-makers about the appropriate means to delay or avoid these dire projections,” the editorialists added.
The study was funded by the Bill and Melinda Gates Foundation and Gates Ventures. Ms. Nichols and the editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a study of 195 countries and territories estimates that by 2050, 153 million people are expected to have dementia worldwide – up from 57 million in 2019. In the United States, the number is expected to increase 100%, from an estimated 5.3 million in 2019 to 10.5 million in 2050.
The increase is largely driven by population growth and population aging, but researchers noted that expanding access to education and addressing risk factors such as obesity, high blood sugar, and smoking could blunt the rise in cases.
The study predicts increases in dementia in every country included in the analysis. The sharpest rise is expected in north Africa and the Middle East (367%) and sub-Saharan Africa (357%). The smallest increases will be in high-income countries in Asia Pacific (53%) and western Europe (74%).
Although the United States had the 37th lowest percentage increase across all countries considered, “this expected increase is still large and requires attention from policy and decision-makers,” said coinvestigator Emma Nichols, MPH, a researcher with the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The findings were published online Jan. 6, 2022, in The Lancet Public Health (doi: 10.1016/S2468-2667[21]00249-8).
Dementia prevalence
For the study, researchers used country-specific estimates of dementia prevalence from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 study to project dementia prevalence globally, by world region, and at the country level.
They also used information on projected trends in four important dementia risk factors (high body mass index, high fasting plasma glucose, smoking, and education) to estimate how changes in these risk factors might impact dementia prevalence between 2019 and 2050.
Despite large increases in the projected number of people living with dementia, age-standardized both-sex prevalence remained stable between 2019 and 2050, with a global percentage change of 0.1% (95% uncertainty interval, –7.5 to 10.8).
Dementia prevalence was higher in women than in men and increased with age, doubling about every 5 years until 85 years of age in both 2019 and 2050 (female-to-male ratio, 1.67; 95% UI, 1.52-1.85).
Projected increases in cases could largely be attributed to population growth and population aging, although their relative importance varied by world region. Population growth contributed most to the increases in sub-Saharan Africa and population aging contributed most to the increases in east Asia.
The countries with the highest expected percentage change in total number of dementia cases between 2019 and 2050 were: Qatar (1,926%), United Arab Emirates (1,795%), Bahrain (1,084%), Oman (943%), Saudi Arabia (898%), Kuwait (850%), Iraq (559%), Maldives (554%), Jordan (522%), and Equatorial Guinea (498%).
The countries with the lowest expected percentage change in total number of dementia cases between 2019 and 2050 were Japan (27%), Bulgaria (37%), Serbia (38%), Lithuania (44%), Greece (45%), Latvia (47%), Croatia (55%), Ukraine (55%), Italy (56%), and Finland (58%).
Modifiable risk factors
Researchers also calculated how changes in risk factors might affect dementia prevalence. They found that improvements in global education access would reduce dementia prevalence by an estimated 6.2 million cases worldwide by 2050. However, that decrease would be offset by expected increases in obesity, high blood sugar, and smoking, which investigators estimate will result in an additional 6.8 million dementia cases.
The projections are based on expected trends in population aging, population growth, and risk factor trajectories, but “projections could change if effective interventions for modifiable risk factors are developed and deployed,” Ms. Nichols said.
In 2020, the Lancet Commission on Dementia Prevention, Intervention, and Care issued an update of its 2017 report, identifying 12 modifiable risk factors that could delay or prevent 40% of dementia cases. The risk factors were low education, hypertension, hearing impairment, smoking, midlife obesity, depression, physical inactivity, diabetes, social isolation, excessive alcohol consumption, head injury, and air pollution.
“Countries, including the U.S., should look to develop effective interventions for modifiable risk factors, but also should invest in the resources needed to support those with dementia and their caregivers,” Ms. Nichols said. She added that additional support for research and resources to develop therapeutic interventions is also warranted.
Oversimplifying mechanisms?
In an accompanying commentary, Michaël Schwarzinger, MD, and Carole Dufouil, PhD, of Bordeaux (France) University Hospital, noted that the authors’ efforts to build on GBD 2019 oversimplify the underlying mechanisms that cause dementia. The authors “provide somehow apocalyptic projections that do not factor in advisable changes in lifestyle over the lifetime,” they wrote.
“There is a considerable and urgent need to reinforce a public health approach towards dementia to better inform the people and decision-makers about the appropriate means to delay or avoid these dire projections,” the editorialists added.
The study was funded by the Bill and Melinda Gates Foundation and Gates Ventures. Ms. Nichols and the editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a study of 195 countries and territories estimates that by 2050, 153 million people are expected to have dementia worldwide – up from 57 million in 2019. In the United States, the number is expected to increase 100%, from an estimated 5.3 million in 2019 to 10.5 million in 2050.
The increase is largely driven by population growth and population aging, but researchers noted that expanding access to education and addressing risk factors such as obesity, high blood sugar, and smoking could blunt the rise in cases.
The study predicts increases in dementia in every country included in the analysis. The sharpest rise is expected in north Africa and the Middle East (367%) and sub-Saharan Africa (357%). The smallest increases will be in high-income countries in Asia Pacific (53%) and western Europe (74%).
Although the United States had the 37th lowest percentage increase across all countries considered, “this expected increase is still large and requires attention from policy and decision-makers,” said coinvestigator Emma Nichols, MPH, a researcher with the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
The findings were published online Jan. 6, 2022, in The Lancet Public Health (doi: 10.1016/S2468-2667[21]00249-8).
Dementia prevalence
For the study, researchers used country-specific estimates of dementia prevalence from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 study to project dementia prevalence globally, by world region, and at the country level.
They also used information on projected trends in four important dementia risk factors (high body mass index, high fasting plasma glucose, smoking, and education) to estimate how changes in these risk factors might impact dementia prevalence between 2019 and 2050.
Despite large increases in the projected number of people living with dementia, age-standardized both-sex prevalence remained stable between 2019 and 2050, with a global percentage change of 0.1% (95% uncertainty interval, –7.5 to 10.8).
Dementia prevalence was higher in women than in men and increased with age, doubling about every 5 years until 85 years of age in both 2019 and 2050 (female-to-male ratio, 1.67; 95% UI, 1.52-1.85).
Projected increases in cases could largely be attributed to population growth and population aging, although their relative importance varied by world region. Population growth contributed most to the increases in sub-Saharan Africa and population aging contributed most to the increases in east Asia.
The countries with the highest expected percentage change in total number of dementia cases between 2019 and 2050 were: Qatar (1,926%), United Arab Emirates (1,795%), Bahrain (1,084%), Oman (943%), Saudi Arabia (898%), Kuwait (850%), Iraq (559%), Maldives (554%), Jordan (522%), and Equatorial Guinea (498%).
The countries with the lowest expected percentage change in total number of dementia cases between 2019 and 2050 were Japan (27%), Bulgaria (37%), Serbia (38%), Lithuania (44%), Greece (45%), Latvia (47%), Croatia (55%), Ukraine (55%), Italy (56%), and Finland (58%).
Modifiable risk factors
Researchers also calculated how changes in risk factors might affect dementia prevalence. They found that improvements in global education access would reduce dementia prevalence by an estimated 6.2 million cases worldwide by 2050. However, that decrease would be offset by expected increases in obesity, high blood sugar, and smoking, which investigators estimate will result in an additional 6.8 million dementia cases.
The projections are based on expected trends in population aging, population growth, and risk factor trajectories, but “projections could change if effective interventions for modifiable risk factors are developed and deployed,” Ms. Nichols said.
In 2020, the Lancet Commission on Dementia Prevention, Intervention, and Care issued an update of its 2017 report, identifying 12 modifiable risk factors that could delay or prevent 40% of dementia cases. The risk factors were low education, hypertension, hearing impairment, smoking, midlife obesity, depression, physical inactivity, diabetes, social isolation, excessive alcohol consumption, head injury, and air pollution.
“Countries, including the U.S., should look to develop effective interventions for modifiable risk factors, but also should invest in the resources needed to support those with dementia and their caregivers,” Ms. Nichols said. She added that additional support for research and resources to develop therapeutic interventions is also warranted.
Oversimplifying mechanisms?
In an accompanying commentary, Michaël Schwarzinger, MD, and Carole Dufouil, PhD, of Bordeaux (France) University Hospital, noted that the authors’ efforts to build on GBD 2019 oversimplify the underlying mechanisms that cause dementia. The authors “provide somehow apocalyptic projections that do not factor in advisable changes in lifestyle over the lifetime,” they wrote.
“There is a considerable and urgent need to reinforce a public health approach towards dementia to better inform the people and decision-makers about the appropriate means to delay or avoid these dire projections,” the editorialists added.
The study was funded by the Bill and Melinda Gates Foundation and Gates Ventures. Ms. Nichols and the editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PUBLIC HEALTH
Pig heart successfully transplanted to man
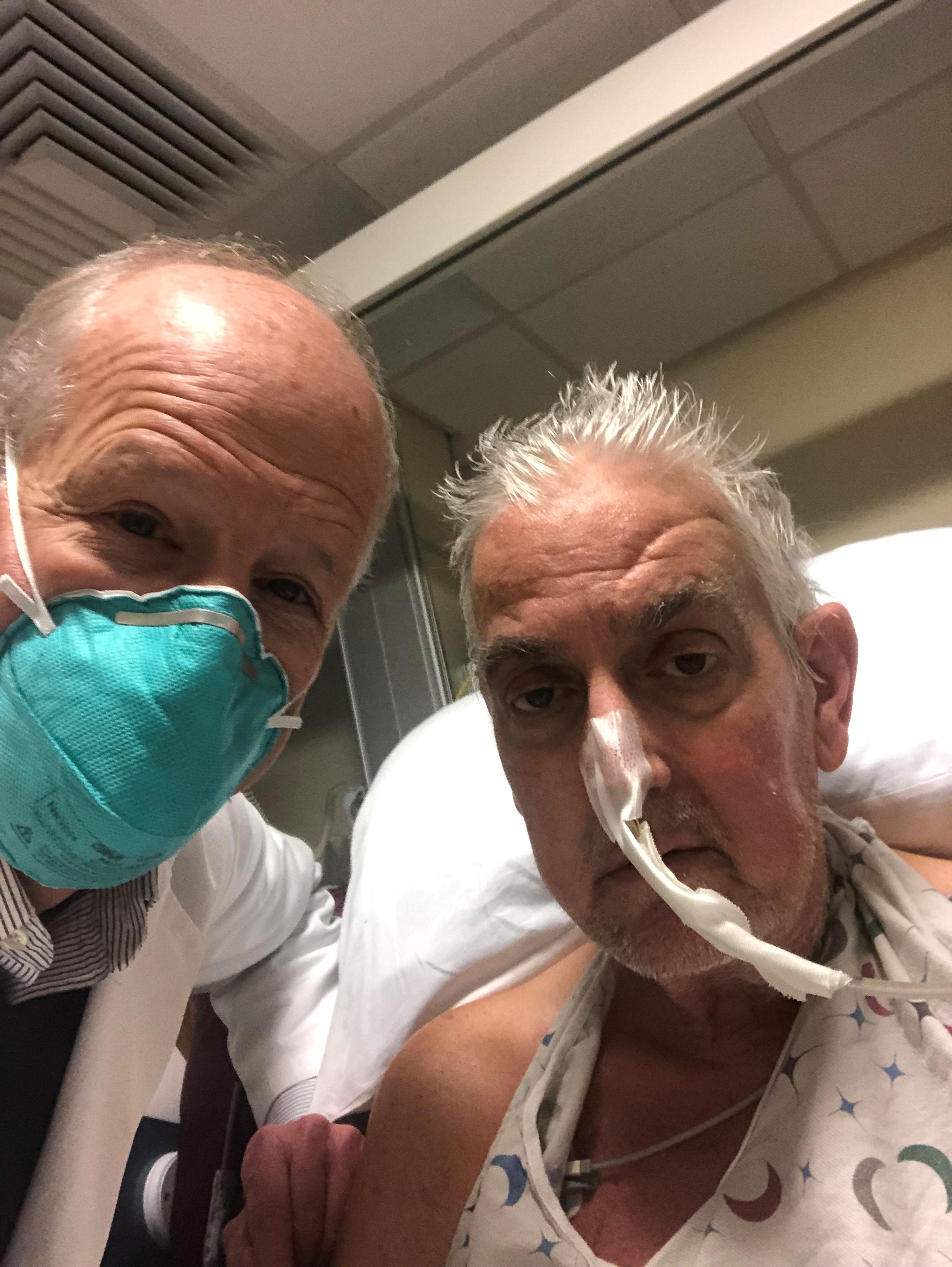
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
GERD: Upper endoscopy may reduce GI cancer mortality
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
This study from Holmberg and colleagues has the potential to revolutionize future clinical guidelines determining endoscopic investigations for GERD patients.
The cohort for analysis is staggering in magnitude: The authors analyzed real-world data from over 1 million participants with GERD in four Scandinavian databases. The results show strong and precise reductions in both risk and mortality from upper gastrointestinal cancer in the whole cohort. This reduction was consistent across all subgroup and sensitivity analyses.
These findings are important as GERD alone does not necessarily warrant an upper endoscopy investigation in current practice. This study provides strong evidence that a one-off endoscopic investigation in patients with GERD could bring meaningful opportunities for early detection of esophageal and gastric cancers – and in turn lead to fewer patients dying from these tumors. The immediacy of the return for investment is also impressive; with the risk reduction being strongest in the first few years of follow-up.
The elusive next step, as highlighted by the authors, is to ensure implementation of endoscopic screening can be done in a cost-effective manner. This is even more important because many health care systems across the world struggle with endoscopy capacity during the COVID-19 pandemic.
Helen Coleman, PhD, BSc(Hons), is a professor of cancer epidemiology at Queen’s University Belfast (Northern Ireland); joint deputy director of the Northern Ireland Cancer Registry; a Cancer Research UK Fellow; and a visiting scientist with the Fitzgerald Lab at the University of Cambridge (England). She has no conflicts.
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
Among individuals with gastroesophageal reflux disease (GERD), a negative upper endoscopy is associated with decreased risk in incidence and mortality from gastrointestinal cancer. The benefit persisted through 5-10 years following the procedure.
The finding is similar to the survival benefit seen with colonoscopies and colorectal cancer, and may be attributable to endoscopic treatment of premalignant lesions.
“The relatively high incidence rate of upper gastrointestinal cancer in patients with GERD indicates that a one-time upper endoscopy may be beneficial,” wrote the authors, who were led by Dag Holmberg, MD, PhD, of the department of molecular medicine and surgery at the Karolinska Institutet and Karolinska University Hospital, both in Stockholm. The study was published in Gastroenterology.
GERD is the most frequent reason patients undergo an upper endoscopy, but the results are often negative. It is generally a benign condition, but can lead to Barrett’s esophagus, as well as esophageal and gastric cardia adenocarcinoma. Upper endoscopy can identify other esophageal cancers like gastric noncardia cancer and duodenal cancer, which may cause dyspepsia or GERD-like symptoms.
To determine the potential benefit of upper endoscopy, the researchers conducted a population-based, four-nation cohort study that included 1,062,740 individuals with GERD in Denmark, Finland, Norway, and Sweden. The data were gathered from national patient registries, cancer registries, and cause of death registries. The study encompassed data from 1979 through the end of 2018.
The median age was 58 years, and 52% of participants were women.
The researchers defined a negative endoscopy as no diagnosis of gastrointestinal cancer within 6 months of the procedure; 69.3% of procedures were negative.
During the follow-up period, 0.34% of participants developed and 0.27% died of upper gastrointestinal cancer. Among those with negative endoscopies, 0.23% developed and 0.22% died from upper gastrointestinal cancer.
Participants with a negative endoscopy had a lower risk of being diagnosed with upper gastrointestinal cancer during the follow-up period (adjusted hazard ratio, 0.45; 95% confidence interval, 0.43-0.48). The reduction in risk was similar across age sexes and age groups, but among procedures performed after 2008, the risk reduction was even higher (aHR, 0.34; P < .001).
The effect was strongest in the first year after the procedure, but it persisted out to 5 years before returning to baseline risk levels.
A negative endoscopy was also associated with decreased mortality risk from upper gastrointestinal cancer versus those who hadn’t had an endoscopy (aHR, 0.39; 95% CI, 0.37-0.42). The protective value continued for at least 10 years.
Esophageal adenocarcinoma developed in 0.12% of participants, and 0.10% died of the disease. Among those with a negative endoscopy, 0.09% developed adenocarcinoma, and 0.07% died (aHR vs. no upper endoscopy, 0.33; 95% CI, 0.30-0.37).
The rapid return to baseline risk was notable, and different from what occurs after negative colonoscopies. However, new tumors can readily form within one year, and the risk may reflect early malignant or premalignant lesions that were missed during the procedure.
In fact, a meta-analysis found that 11.3% of upper gastrointestinal cancers had escaped detection during an endoscopy in the previous 3 years before diagnosis, and case reviews of patients diagnosed with gastrointestinal cancer soon after an upper endoscopy usually reveal suspicious or indeterminate results that the endoscopist or pathologist missed.
Quality indicators for upper endoscopy include procedure time, rate of targeted biopsies, and computer-aided detection, but it isn’t clear what impact these measures have on outcomes. However, the greater risk reduction found with endoscopies performed more recently suggests that newer quality indicators and technological improvements may be improving outcomes.
The relatively low incidence of esophageal and gastric cancer in Western countries has discouraged widespread adoption of endoscopic screening, but the researchers point out that the risk of gastrointestinal cancer among individuals with GERD is similar to the risk of colorectal cancer in the 60-69 age group in the United States, for whom colonoscopy is recommended.
“The present study indicates that upper endoscopy may be beneficial for patients with GERD, but to make upper endoscopy screening more cost beneficial at its initiation, the target group may be limited to include patients at highest risk of cancer. Such previous cost-effectiveness studies have indicated that endoscopy is cost effective in men at aged 50 years or older with chronic GERD,” the authors wrote.
The study was funded by Swedish Research Council and Swedish Cancer Society. The authors disclosed no relevant conflicts of interest.
FROM GASTROENTEROLOGY
Increased electronic media use and youth suicidality: What can clinicians do?
Pediatric suicide was an emerging public health crisis prior to COVID-19, and recent data indicate that pediatric suicide attempts continued to increase during the pandemic.1 In October 2021, the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and the Children’s Hospital Association declared a national state of emergency for pediatric mental health because of a surge in youth suicide attempts.2 Isolation mediated by the degradation of community and exacerbated by the pandemic, has been identified as a contributor to increasing pediatric suicidality.
It is impossible to understand this current public health crisis and to seek solutions without recognizing the ways in which the degradation of community and consequent social isolation play a central role. While the degradation of community and the isolation epidemic that preceded COVID-19 have been mediated by multiple factors, one factor associated with mental health problems in youth is electronic media use.3 During COVID-19, when physical distancing and virtual learning have been necessary to curb the spread, electronic media use has increased exponentially in the pediatric demographic. Some of this increase in screen time has been attributable to virtual schooling, but electronic devices also have become the only means by which kids can stay in contact with one another. While electronic communication has been viewed as an antidote to isolation, disturbing consequences associated with electronic media use have also been noted in our pediatric population.
In the health care system where I (P.L.L.) work as a pediatrician and a child and adolescent psychiatrist, electronic media use has been implicated in more than 90% of our inpatient admissions for suicidal ideation. Use of electronic devices has contributed to suicidal thoughts and attempts in most patients admitted to our acute crisis stabilization unit over the past year. During the pandemic, and in the absence of meaningful interpersonal interactions, many in our pediatric population have become even more dependent on electronic devices to cope with isolation. This has created an often-devastating irony, where the very devices already associated with mental health problems in youth are now being endorsed as “necessary” by mental health professionals.
So how does electronic media use relate to isolation and the continued degradation of community, and why might electronic media use be exacerbating pediatric suicide? One way we have coped with the deterioration of our communities has been the creation of the synthetic community-substitutes found on electronic devices. Unfortunately, our electronic devices create only an illusion of community, where interpersonal interaction occurs by way of inanimate objects, and by electronic text and ideograms. These become substitutes for genuine intimacy, personal contact, and reciprocity. Instead of engaging with one another, our youth are spending hours daily in isolation engaging with a piece of plastic. The mirage generated by pixels on a plastic screen creates an illusion of connectivity, but in reality, this only increases the isolation of our youth.
Human evolution and connection
Intimate social connectivity, woven together in our communities, was a fundamental mechanism for human survival. Historically, for our hunter-gatherer ancestors, the community provided access to our fundamental needs, such as safety from predators and access to substantive nutrition.4 Community allowed our ancestors to survive and procreate, and facilitated their triumph over predation and disease.5 Our distinction as the dominant species on Earth has been afforded by our social connectivity. Unfortunately, in the virtual worlds of our electronic devices the intimate social connectivity of community is absent. Our children wander in isolation, left to navigate age-old evolutionary pressures in the absence of the fundamental advantage for our survival as a species.
Unlike the living, breathing bears and wolves that threatened our ancestors, in the virtual world of the electronic device children are stalked by invisible predators seeking sexual or monetary exploitation. Children are being consumed by digital advertising and social media platforms that perpetually reinforce the requirement of perfection, and they fall prey to cyberbullies who mercilessly disparage their imperfections. In their virtual worlds, where their value is predicated upon anonymous others’ opinions, they succumb to the idea that they will never be enough.6 Their fundamental needs of competence and relatedness go unmet, and they lose their sense of purpose, belonging, and often their will to live. More importantly, absent from their children’s virtual worlds, and preoccupied within their own, parents cannot protect their children from online predators, deflect the vicious attacks of cyberbullies, or reframe their children’s imperfections as distinctive or empowering. They are unable to provide their children with the substantive interpersonal contact necessary for resilience and that bolsters their self-worth.
Human beings are inherently social creatures, who regardless of era require community to meet their fundamental needs. As the duration of daily screen time steadily increases, our youth are spending more and more of their waking hours living in isolation in an electronic world. Without the protective social connectivity of community, they are hunted by online predators, and they are consumed by the predatory culture of perfectionism that is contradictory to the reciprocal caretaking necessary to support their healthy development. Evolutionary biology informs us that, when children are isolated, they are susceptible to predation and disease. And in the socialized isolation of their electronic worlds, they are succumbing to predation and to the depressive diseases that are exacerbating the pediatric mental health crisis.
Creating and building community amid a pandemic has been challenging at best. However, now that we have better tools to fight COVID, it is important to encourage our young patients to reduce their nonacademic screen time, and to get outside and engage with others. Their mental health depends on it.
Dr. Loper is a pediatrician and child and adolescent psychiatrist at Prisma Health–Midlands in Columbia, S.C. He is an assistant professor in the department of neuropsychiatry and behavioral science at the University of South Carolina, Columbia. Dr. Loper has no conflicts of interest. Dr. Kaminstein is an adjunct assistant professor at the graduate school of education and affiliated faculty in the organizational dynamics program, School of Arts and Sciences, at the University of Pennsylvania, Philadelphia. He is a social psychologist who has been studying groups and organizations for more than 40 years. He has no conflicts of interest.
References
1. MMWR. 2021 Jun 18;70(24):888-94.
2. Ray G. “Pediatricians, Child and Adolescent Psychiatrists and Children’s Hospitals Declare National Emergency in Children’s Mental Health.” Childrenshospitals.org. 2021 Oct 19.
3. JAMA Netw Open. 2020(8):e2011381.
4. Am J Phys Anthropol. 2018 April:165(4):777-800.
5. The influence of predation on primate and early human evolution: Impetus for cooperation, in “Origins of Altruism and Cooperation. Developments in Primatology: Progress and Prospects.” (Basingstoke, England: Springer Nature, 2011, pp. 19-40).
6. Media Psychology. 2020;23(1):52-78.
Pediatric suicide was an emerging public health crisis prior to COVID-19, and recent data indicate that pediatric suicide attempts continued to increase during the pandemic.1 In October 2021, the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and the Children’s Hospital Association declared a national state of emergency for pediatric mental health because of a surge in youth suicide attempts.2 Isolation mediated by the degradation of community and exacerbated by the pandemic, has been identified as a contributor to increasing pediatric suicidality.
It is impossible to understand this current public health crisis and to seek solutions without recognizing the ways in which the degradation of community and consequent social isolation play a central role. While the degradation of community and the isolation epidemic that preceded COVID-19 have been mediated by multiple factors, one factor associated with mental health problems in youth is electronic media use.3 During COVID-19, when physical distancing and virtual learning have been necessary to curb the spread, electronic media use has increased exponentially in the pediatric demographic. Some of this increase in screen time has been attributable to virtual schooling, but electronic devices also have become the only means by which kids can stay in contact with one another. While electronic communication has been viewed as an antidote to isolation, disturbing consequences associated with electronic media use have also been noted in our pediatric population.
In the health care system where I (P.L.L.) work as a pediatrician and a child and adolescent psychiatrist, electronic media use has been implicated in more than 90% of our inpatient admissions for suicidal ideation. Use of electronic devices has contributed to suicidal thoughts and attempts in most patients admitted to our acute crisis stabilization unit over the past year. During the pandemic, and in the absence of meaningful interpersonal interactions, many in our pediatric population have become even more dependent on electronic devices to cope with isolation. This has created an often-devastating irony, where the very devices already associated with mental health problems in youth are now being endorsed as “necessary” by mental health professionals.
So how does electronic media use relate to isolation and the continued degradation of community, and why might electronic media use be exacerbating pediatric suicide? One way we have coped with the deterioration of our communities has been the creation of the synthetic community-substitutes found on electronic devices. Unfortunately, our electronic devices create only an illusion of community, where interpersonal interaction occurs by way of inanimate objects, and by electronic text and ideograms. These become substitutes for genuine intimacy, personal contact, and reciprocity. Instead of engaging with one another, our youth are spending hours daily in isolation engaging with a piece of plastic. The mirage generated by pixels on a plastic screen creates an illusion of connectivity, but in reality, this only increases the isolation of our youth.
Human evolution and connection
Intimate social connectivity, woven together in our communities, was a fundamental mechanism for human survival. Historically, for our hunter-gatherer ancestors, the community provided access to our fundamental needs, such as safety from predators and access to substantive nutrition.4 Community allowed our ancestors to survive and procreate, and facilitated their triumph over predation and disease.5 Our distinction as the dominant species on Earth has been afforded by our social connectivity. Unfortunately, in the virtual worlds of our electronic devices the intimate social connectivity of community is absent. Our children wander in isolation, left to navigate age-old evolutionary pressures in the absence of the fundamental advantage for our survival as a species.
Unlike the living, breathing bears and wolves that threatened our ancestors, in the virtual world of the electronic device children are stalked by invisible predators seeking sexual or monetary exploitation. Children are being consumed by digital advertising and social media platforms that perpetually reinforce the requirement of perfection, and they fall prey to cyberbullies who mercilessly disparage their imperfections. In their virtual worlds, where their value is predicated upon anonymous others’ opinions, they succumb to the idea that they will never be enough.6 Their fundamental needs of competence and relatedness go unmet, and they lose their sense of purpose, belonging, and often their will to live. More importantly, absent from their children’s virtual worlds, and preoccupied within their own, parents cannot protect their children from online predators, deflect the vicious attacks of cyberbullies, or reframe their children’s imperfections as distinctive or empowering. They are unable to provide their children with the substantive interpersonal contact necessary for resilience and that bolsters their self-worth.
Human beings are inherently social creatures, who regardless of era require community to meet their fundamental needs. As the duration of daily screen time steadily increases, our youth are spending more and more of their waking hours living in isolation in an electronic world. Without the protective social connectivity of community, they are hunted by online predators, and they are consumed by the predatory culture of perfectionism that is contradictory to the reciprocal caretaking necessary to support their healthy development. Evolutionary biology informs us that, when children are isolated, they are susceptible to predation and disease. And in the socialized isolation of their electronic worlds, they are succumbing to predation and to the depressive diseases that are exacerbating the pediatric mental health crisis.
Creating and building community amid a pandemic has been challenging at best. However, now that we have better tools to fight COVID, it is important to encourage our young patients to reduce their nonacademic screen time, and to get outside and engage with others. Their mental health depends on it.
Dr. Loper is a pediatrician and child and adolescent psychiatrist at Prisma Health–Midlands in Columbia, S.C. He is an assistant professor in the department of neuropsychiatry and behavioral science at the University of South Carolina, Columbia. Dr. Loper has no conflicts of interest. Dr. Kaminstein is an adjunct assistant professor at the graduate school of education and affiliated faculty in the organizational dynamics program, School of Arts and Sciences, at the University of Pennsylvania, Philadelphia. He is a social psychologist who has been studying groups and organizations for more than 40 years. He has no conflicts of interest.
References
1. MMWR. 2021 Jun 18;70(24):888-94.
2. Ray G. “Pediatricians, Child and Adolescent Psychiatrists and Children’s Hospitals Declare National Emergency in Children’s Mental Health.” Childrenshospitals.org. 2021 Oct 19.
3. JAMA Netw Open. 2020(8):e2011381.
4. Am J Phys Anthropol. 2018 April:165(4):777-800.
5. The influence of predation on primate and early human evolution: Impetus for cooperation, in “Origins of Altruism and Cooperation. Developments in Primatology: Progress and Prospects.” (Basingstoke, England: Springer Nature, 2011, pp. 19-40).
6. Media Psychology. 2020;23(1):52-78.
Pediatric suicide was an emerging public health crisis prior to COVID-19, and recent data indicate that pediatric suicide attempts continued to increase during the pandemic.1 In October 2021, the American Academy of Pediatrics, the American Academy of Child and Adolescent Psychiatry, and the Children’s Hospital Association declared a national state of emergency for pediatric mental health because of a surge in youth suicide attempts.2 Isolation mediated by the degradation of community and exacerbated by the pandemic, has been identified as a contributor to increasing pediatric suicidality.
It is impossible to understand this current public health crisis and to seek solutions without recognizing the ways in which the degradation of community and consequent social isolation play a central role. While the degradation of community and the isolation epidemic that preceded COVID-19 have been mediated by multiple factors, one factor associated with mental health problems in youth is electronic media use.3 During COVID-19, when physical distancing and virtual learning have been necessary to curb the spread, electronic media use has increased exponentially in the pediatric demographic. Some of this increase in screen time has been attributable to virtual schooling, but electronic devices also have become the only means by which kids can stay in contact with one another. While electronic communication has been viewed as an antidote to isolation, disturbing consequences associated with electronic media use have also been noted in our pediatric population.
In the health care system where I (P.L.L.) work as a pediatrician and a child and adolescent psychiatrist, electronic media use has been implicated in more than 90% of our inpatient admissions for suicidal ideation. Use of electronic devices has contributed to suicidal thoughts and attempts in most patients admitted to our acute crisis stabilization unit over the past year. During the pandemic, and in the absence of meaningful interpersonal interactions, many in our pediatric population have become even more dependent on electronic devices to cope with isolation. This has created an often-devastating irony, where the very devices already associated with mental health problems in youth are now being endorsed as “necessary” by mental health professionals.
So how does electronic media use relate to isolation and the continued degradation of community, and why might electronic media use be exacerbating pediatric suicide? One way we have coped with the deterioration of our communities has been the creation of the synthetic community-substitutes found on electronic devices. Unfortunately, our electronic devices create only an illusion of community, where interpersonal interaction occurs by way of inanimate objects, and by electronic text and ideograms. These become substitutes for genuine intimacy, personal contact, and reciprocity. Instead of engaging with one another, our youth are spending hours daily in isolation engaging with a piece of plastic. The mirage generated by pixels on a plastic screen creates an illusion of connectivity, but in reality, this only increases the isolation of our youth.
Human evolution and connection
Intimate social connectivity, woven together in our communities, was a fundamental mechanism for human survival. Historically, for our hunter-gatherer ancestors, the community provided access to our fundamental needs, such as safety from predators and access to substantive nutrition.4 Community allowed our ancestors to survive and procreate, and facilitated their triumph over predation and disease.5 Our distinction as the dominant species on Earth has been afforded by our social connectivity. Unfortunately, in the virtual worlds of our electronic devices the intimate social connectivity of community is absent. Our children wander in isolation, left to navigate age-old evolutionary pressures in the absence of the fundamental advantage for our survival as a species.
Unlike the living, breathing bears and wolves that threatened our ancestors, in the virtual world of the electronic device children are stalked by invisible predators seeking sexual or monetary exploitation. Children are being consumed by digital advertising and social media platforms that perpetually reinforce the requirement of perfection, and they fall prey to cyberbullies who mercilessly disparage their imperfections. In their virtual worlds, where their value is predicated upon anonymous others’ opinions, they succumb to the idea that they will never be enough.6 Their fundamental needs of competence and relatedness go unmet, and they lose their sense of purpose, belonging, and often their will to live. More importantly, absent from their children’s virtual worlds, and preoccupied within their own, parents cannot protect their children from online predators, deflect the vicious attacks of cyberbullies, or reframe their children’s imperfections as distinctive or empowering. They are unable to provide their children with the substantive interpersonal contact necessary for resilience and that bolsters their self-worth.
Human beings are inherently social creatures, who regardless of era require community to meet their fundamental needs. As the duration of daily screen time steadily increases, our youth are spending more and more of their waking hours living in isolation in an electronic world. Without the protective social connectivity of community, they are hunted by online predators, and they are consumed by the predatory culture of perfectionism that is contradictory to the reciprocal caretaking necessary to support their healthy development. Evolutionary biology informs us that, when children are isolated, they are susceptible to predation and disease. And in the socialized isolation of their electronic worlds, they are succumbing to predation and to the depressive diseases that are exacerbating the pediatric mental health crisis.
Creating and building community amid a pandemic has been challenging at best. However, now that we have better tools to fight COVID, it is important to encourage our young patients to reduce their nonacademic screen time, and to get outside and engage with others. Their mental health depends on it.
Dr. Loper is a pediatrician and child and adolescent psychiatrist at Prisma Health–Midlands in Columbia, S.C. He is an assistant professor in the department of neuropsychiatry and behavioral science at the University of South Carolina, Columbia. Dr. Loper has no conflicts of interest. Dr. Kaminstein is an adjunct assistant professor at the graduate school of education and affiliated faculty in the organizational dynamics program, School of Arts and Sciences, at the University of Pennsylvania, Philadelphia. He is a social psychologist who has been studying groups and organizations for more than 40 years. He has no conflicts of interest.
References
1. MMWR. 2021 Jun 18;70(24):888-94.
2. Ray G. “Pediatricians, Child and Adolescent Psychiatrists and Children’s Hospitals Declare National Emergency in Children’s Mental Health.” Childrenshospitals.org. 2021 Oct 19.
3. JAMA Netw Open. 2020(8):e2011381.
4. Am J Phys Anthropol. 2018 April:165(4):777-800.
5. The influence of predation on primate and early human evolution: Impetus for cooperation, in “Origins of Altruism and Cooperation. Developments in Primatology: Progress and Prospects.” (Basingstoke, England: Springer Nature, 2011, pp. 19-40).
6. Media Psychology. 2020;23(1):52-78.
Pediatric antibiotic prescriptions plummeted in pandemic
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
FROM PEDIATRICS
Ubrogepant beneficial in migraine regardless of prior preventive medication use
Key clinical point: Ubrogepant was effective and safe in patients with migraine, regardless of prior or concomitant preventive medication use.
Major finding: Ubrogepant vs. placebo was associated with significantly higher responder rates for pain freedom (P £ .005), absence of most bothersome symptom (50 mg ubrogepant vs. placebo; P £ .001), and pain relief (P £ .011) at 2 hours, with the responder rates not being significantly different among patients with vs. without preventive medication use (all P > .05). No serious or treatment-related adverse events were reported.
Study details: Findings are from a pooled analysis of ACHIEVE I and ACHIEVE II phase 3 trials (n = 2,247) and the long-term safety extension trial (n = 813) including patients with migraine with or without a history of preventive medication use.
Disclosures: This study was funded by Allergan (before its acquisition by AbbVie). Some investigators, including the lead author, reported advising or consulting for; being a speaker, on the board of directors, or a contributing author for; receiving grants, research support, and honoraria from; holding stocks or patents with; or employment with various sources including Allergan and AbbVie.
Source: Blumenfeld AM et al. Add Ther. 2021 (Dec 7). Doi: 10.1007/s12325-021-01923-3.
Key clinical point: Ubrogepant was effective and safe in patients with migraine, regardless of prior or concomitant preventive medication use.
Major finding: Ubrogepant vs. placebo was associated with significantly higher responder rates for pain freedom (P £ .005), absence of most bothersome symptom (50 mg ubrogepant vs. placebo; P £ .001), and pain relief (P £ .011) at 2 hours, with the responder rates not being significantly different among patients with vs. without preventive medication use (all P > .05). No serious or treatment-related adverse events were reported.
Study details: Findings are from a pooled analysis of ACHIEVE I and ACHIEVE II phase 3 trials (n = 2,247) and the long-term safety extension trial (n = 813) including patients with migraine with or without a history of preventive medication use.
Disclosures: This study was funded by Allergan (before its acquisition by AbbVie). Some investigators, including the lead author, reported advising or consulting for; being a speaker, on the board of directors, or a contributing author for; receiving grants, research support, and honoraria from; holding stocks or patents with; or employment with various sources including Allergan and AbbVie.
Source: Blumenfeld AM et al. Add Ther. 2021 (Dec 7). Doi: 10.1007/s12325-021-01923-3.
Key clinical point: Ubrogepant was effective and safe in patients with migraine, regardless of prior or concomitant preventive medication use.
Major finding: Ubrogepant vs. placebo was associated with significantly higher responder rates for pain freedom (P £ .005), absence of most bothersome symptom (50 mg ubrogepant vs. placebo; P £ .001), and pain relief (P £ .011) at 2 hours, with the responder rates not being significantly different among patients with vs. without preventive medication use (all P > .05). No serious or treatment-related adverse events were reported.
Study details: Findings are from a pooled analysis of ACHIEVE I and ACHIEVE II phase 3 trials (n = 2,247) and the long-term safety extension trial (n = 813) including patients with migraine with or without a history of preventive medication use.
Disclosures: This study was funded by Allergan (before its acquisition by AbbVie). Some investigators, including the lead author, reported advising or consulting for; being a speaker, on the board of directors, or a contributing author for; receiving grants, research support, and honoraria from; holding stocks or patents with; or employment with various sources including Allergan and AbbVie.
Source: Blumenfeld AM et al. Add Ther. 2021 (Dec 7). Doi: 10.1007/s12325-021-01923-3.
Fremanezumab effective and safe in older patients with chronic or episodic migraine
Key clinical point: Fremanezumab was effective and well tolerated over 12 weeks in older patients with chronic or episodic migraine.
Major finding: Quarterly and monthly fremanezumab vs. placebo showed greater reduction in monthly average migraine days (least-squares mean change from baseline [D] −4.3 and −4.6 vs. −2.3), headache days of at least moderate severity (D −3.9 and −4.2 vs. −2.1), and acute medication use (D −3.7 and −4.0 vs. −1.3) over 12 weeks (all P < .05). Adverse events were similar across groups.
Study details: Findings are pooled subgroup analysis of 3 phase 3 studies (HALO CM, HALO EM, and FOCUS) including 246 participants aged ≥60 years with chronic or episodic migraine with an inadequate response to 2-4 prior migraine preventives. They were randomly assigned to quarterly fremanezumab, monthly fremanezumab, or matched monthly placebo.
Disclosures: This study was funded by Teva Pharmaceuticals Ltd. Some investigators, including the lead author, reported participating in clinical trials and receiving honoraria, research support, and legal or personal fees from and being former or current employees of various sources, including Teva Pharmaceuticals.
Source: Nahas SJ et al. J Headache Pain. 2021;22:141 (Nov 24). Doi: 10.1186/s10194-021-01351-2.
Key clinical point: Fremanezumab was effective and well tolerated over 12 weeks in older patients with chronic or episodic migraine.
Major finding: Quarterly and monthly fremanezumab vs. placebo showed greater reduction in monthly average migraine days (least-squares mean change from baseline [D] −4.3 and −4.6 vs. −2.3), headache days of at least moderate severity (D −3.9 and −4.2 vs. −2.1), and acute medication use (D −3.7 and −4.0 vs. −1.3) over 12 weeks (all P < .05). Adverse events were similar across groups.
Study details: Findings are pooled subgroup analysis of 3 phase 3 studies (HALO CM, HALO EM, and FOCUS) including 246 participants aged ≥60 years with chronic or episodic migraine with an inadequate response to 2-4 prior migraine preventives. They were randomly assigned to quarterly fremanezumab, monthly fremanezumab, or matched monthly placebo.
Disclosures: This study was funded by Teva Pharmaceuticals Ltd. Some investigators, including the lead author, reported participating in clinical trials and receiving honoraria, research support, and legal or personal fees from and being former or current employees of various sources, including Teva Pharmaceuticals.
Source: Nahas SJ et al. J Headache Pain. 2021;22:141 (Nov 24). Doi: 10.1186/s10194-021-01351-2.
Key clinical point: Fremanezumab was effective and well tolerated over 12 weeks in older patients with chronic or episodic migraine.
Major finding: Quarterly and monthly fremanezumab vs. placebo showed greater reduction in monthly average migraine days (least-squares mean change from baseline [D] −4.3 and −4.6 vs. −2.3), headache days of at least moderate severity (D −3.9 and −4.2 vs. −2.1), and acute medication use (D −3.7 and −4.0 vs. −1.3) over 12 weeks (all P < .05). Adverse events were similar across groups.
Study details: Findings are pooled subgroup analysis of 3 phase 3 studies (HALO CM, HALO EM, and FOCUS) including 246 participants aged ≥60 years with chronic or episodic migraine with an inadequate response to 2-4 prior migraine preventives. They were randomly assigned to quarterly fremanezumab, monthly fremanezumab, or matched monthly placebo.
Disclosures: This study was funded by Teva Pharmaceuticals Ltd. Some investigators, including the lead author, reported participating in clinical trials and receiving honoraria, research support, and legal or personal fees from and being former or current employees of various sources, including Teva Pharmaceuticals.
Source: Nahas SJ et al. J Headache Pain. 2021;22:141 (Nov 24). Doi: 10.1186/s10194-021-01351-2.
Important to screen for dry eye in patients with migraine
Key clinical point: Patients with migraine are more likely to suffer from dry eye than patients without migraine.
Major finding: The prevalence of dry eye was significantly higher in patients with vs. without migraine (odds ratio, 1.55; P < .001).
Study details: Findings are from a meta-analysis of 7 case-control or cross-sectional studies including a total population of 1,033,288 individuals with or without migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China and Fundamental Research Funds of the State Key Laboratory of Ophthalmology. The authors declared no conflict of interests.
Source: Chen H et al. Cornea. 2021 (Nov 6). Doi: 10.1097/ICO.0000000000002851.
Key clinical point: Patients with migraine are more likely to suffer from dry eye than patients without migraine.
Major finding: The prevalence of dry eye was significantly higher in patients with vs. without migraine (odds ratio, 1.55; P < .001).
Study details: Findings are from a meta-analysis of 7 case-control or cross-sectional studies including a total population of 1,033,288 individuals with or without migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China and Fundamental Research Funds of the State Key Laboratory of Ophthalmology. The authors declared no conflict of interests.
Source: Chen H et al. Cornea. 2021 (Nov 6). Doi: 10.1097/ICO.0000000000002851.
Key clinical point: Patients with migraine are more likely to suffer from dry eye than patients without migraine.
Major finding: The prevalence of dry eye was significantly higher in patients with vs. without migraine (odds ratio, 1.55; P < .001).
Study details: Findings are from a meta-analysis of 7 case-control or cross-sectional studies including a total population of 1,033,288 individuals with or without migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China and Fundamental Research Funds of the State Key Laboratory of Ophthalmology. The authors declared no conflict of interests.
Source: Chen H et al. Cornea. 2021 (Nov 6). Doi: 10.1097/ICO.0000000000002851.
Migraine patients should be evaluated for celiac disease and associated gastrointestinal symptoms
Key clinical point: Prevalence of migraine was higher in patients with celiac disease (CD) vs. healthy controls, with patients with CD and migraine showing worse gastrointestinal symptoms compared with those without migraine.
Major finding: The prevalence of migraine was higher in patients with vs. without CD (20.7% vs. 11.9%; P < .001). Patients with CD with vs. without migraine headache had a higher prevalence of abdominal pain (80.1% vs. 71.8%; P = .025), constipation (47.8% vs. 35.5%; P = .011), and diarrhea (60.8% vs. 45.9%; P = .002).
Study details: This case-control cross-sectional study included 1,000 adult patients with CD and 1,000 healthy controls.
Disclosures: No funding was received for this study. The authors declared no conflict of interests.
Source: Fanaeian MM et al. PLoS One. 2021;16(11):e0259502 (Nov 17). Doi: 10.1371/journal.pone.0259502.
Key clinical point: Prevalence of migraine was higher in patients with celiac disease (CD) vs. healthy controls, with patients with CD and migraine showing worse gastrointestinal symptoms compared with those without migraine.
Major finding: The prevalence of migraine was higher in patients with vs. without CD (20.7% vs. 11.9%; P < .001). Patients with CD with vs. without migraine headache had a higher prevalence of abdominal pain (80.1% vs. 71.8%; P = .025), constipation (47.8% vs. 35.5%; P = .011), and diarrhea (60.8% vs. 45.9%; P = .002).
Study details: This case-control cross-sectional study included 1,000 adult patients with CD and 1,000 healthy controls.
Disclosures: No funding was received for this study. The authors declared no conflict of interests.
Source: Fanaeian MM et al. PLoS One. 2021;16(11):e0259502 (Nov 17). Doi: 10.1371/journal.pone.0259502.
Key clinical point: Prevalence of migraine was higher in patients with celiac disease (CD) vs. healthy controls, with patients with CD and migraine showing worse gastrointestinal symptoms compared with those without migraine.
Major finding: The prevalence of migraine was higher in patients with vs. without CD (20.7% vs. 11.9%; P < .001). Patients with CD with vs. without migraine headache had a higher prevalence of abdominal pain (80.1% vs. 71.8%; P = .025), constipation (47.8% vs. 35.5%; P = .011), and diarrhea (60.8% vs. 45.9%; P = .002).
Study details: This case-control cross-sectional study included 1,000 adult patients with CD and 1,000 healthy controls.
Disclosures: No funding was received for this study. The authors declared no conflict of interests.
Source: Fanaeian MM et al. PLoS One. 2021;16(11):e0259502 (Nov 17). Doi: 10.1371/journal.pone.0259502.
Migraine: Occipital bending higher in patients with visual aura
Key clinical point: The frequency of occipital bending (OB) was 2-fold higher in patients with migraine with visual aura than those without visual aura.
Major finding: Overall, the prevalence of OB was 33.3% and was significantly higher in patients with migraine with vs. without visual aura (57.1% vs. 25.4%; odds ratio 3.9; P = .015).
Study details: Findings are from a retrospective analysis of 84 patients with migraine with (n = 21) or without visual aura (n = 63).
Disclosures: No source of funding was identified. The authors declared no conflict of interests.
Source: Özkan E et al. Headache. 2021 (Nov 28). Doi: 10.1111/head.14240.
Key clinical point: The frequency of occipital bending (OB) was 2-fold higher in patients with migraine with visual aura than those without visual aura.
Major finding: Overall, the prevalence of OB was 33.3% and was significantly higher in patients with migraine with vs. without visual aura (57.1% vs. 25.4%; odds ratio 3.9; P = .015).
Study details: Findings are from a retrospective analysis of 84 patients with migraine with (n = 21) or without visual aura (n = 63).
Disclosures: No source of funding was identified. The authors declared no conflict of interests.
Source: Özkan E et al. Headache. 2021 (Nov 28). Doi: 10.1111/head.14240.
Key clinical point: The frequency of occipital bending (OB) was 2-fold higher in patients with migraine with visual aura than those without visual aura.
Major finding: Overall, the prevalence of OB was 33.3% and was significantly higher in patients with migraine with vs. without visual aura (57.1% vs. 25.4%; odds ratio 3.9; P = .015).
Study details: Findings are from a retrospective analysis of 84 patients with migraine with (n = 21) or without visual aura (n = 63).
Disclosures: No source of funding was identified. The authors declared no conflict of interests.
Source: Özkan E et al. Headache. 2021 (Nov 28). Doi: 10.1111/head.14240.