User login
A high-risk medical device didn’t meet federal standards. The government paid millions for more
In 2014, when the Food and Drug Administration found serious problems with a life-sustaining heart pump, its warning letter to the manufacturer threatened to notify other federal health agencies about the inspection’s findings.
But for years, no such alert ever went out. Instead, the agency added the warning letter to an online database alongside thousands of others, following its typical procedures, an FDA spokesperson said.
Agencies such as the Centers for Medicare & Medicaid Services and the U.S. Department of Veterans Affairs went on paying to implant the HeartWare Ventricular Assist Device, or HVAD, in new patients even though federal inspectors had found problems with the device linked to patient deaths and injuries.
Taxpayer dollars continued to flow to the original device maker, HeartWare, and then to the company that acquired it in 2016, Medtronic, for 7 years while the issues raised in the warning letter remained unresolved.
If crucial safety information in FDA warning letters doesn’t make it to other arms of the government responsible for deciding which medical devices to pay for, experts said patients are the ones put at risk.
“It’s clearly a breakdown of communication,” said Dr. Rita Redberg, a cardiologist at the University of California, San Francisco, who researches medical device safety and regulation. “It’s not just the money, obviously. It’s people’s lives.”
The FDA acknowledged that it doesn’t directly notify other agencies when it issues warning letters, pointing instead to its online database, which is accessible to both government officials and the public. “The FDA’s decisions are intended to be patient-centric with the health and safety of device users as our highest priority,” the agency spokesperson said in an email.
The HeartWare letter was removed from the public database about 2 years ago, even though the problems remained unresolved and patients were still receiving implants. The database clears out letters that are more than 5 years old.
CMS, which oversees the Medicare and Medicaid programs, would not say why it continued paying for a device that didn’t meet government standards. It directed questions about the HeartWare warning letter to the FDA. “CMS does not have oversight of the manufacturing and related safety assessments of a medical device manufacturer,” a spokesperson said in an email.
The spokesperson noted that CMS requires heart pump patients to have specialized medical teams managing their care, which should monitor FDA communications regarding safety of devices.
CMS doesn’t track data on devices by manufacturer, so it’s essentially impossible to calculate its total spending on HVADs. One 2018 medical journal study found that Medicare and Medicaid paid for more than half the cost of all heart pump implants from 2009 to 2014. If that rate of spending continued, CMS may have spent more than $400 million on implanting HVADs since 2014.
A spokesperson for the VA said his agency was never notified about the HeartWare warning letter. The VA paid HeartWare and Medtronic more than $3 million after the FDA issued the letter in 2014. It offered this explanation for why: “It’s important to note that FDA Warning Letters are notifications issued to manufacturers found to be in significant violation of federal regulations. They are not product recalls.”
In the case of the HVAD, the FDA’s failure to make sure its warning reached beyond the manufacturer may have had life-and-death consequences.
In August, ProPublica reported that federal inspectors continued finding problems at the HVAD’s manufacturing plant for years. Meanwhile, the FDA received thousands of reports of suspicious deaths and injuries and more than a dozen high-risk safety alerts from the manufacturer.
The documents detailed one horrifying device failure after another. A father of four died after his device suddenly failed and his teenage daughter couldn’t resuscitate him. Another patient’s heart tissue was charred after a pump short-circuited and overheated. A teenager died after vomiting blood as his mother struggled to restart a defective pump.
In June, Medtronic ended sales and implants of the device, citing new data that showed patients with HVADs had a higher rate of deaths and strokes than those with a competing heart pump.
Medtronic declined to comment for this story. It has previously said it believed that after the 2014 warning letter the benefits of the HVAD still outweighed the risks for patients with severe heart failure.
Experts said the lack of communication between federal agencies when serious device problems are found is baffling but not surprising. It fits a broader trend of device regulators focusing more on evaluating new products than monitoring the ones already on the market.
“The priority is to get more medical devices out there, paid for and getting used,” said Dr. Joseph Ross, a professor of medicine and public health at Yale University who studies medical device regulation.
Other U.S. health care regulators move more forcefully when providers and suppliers don’t meet the government’s minimum safety requirements for an extended period, putting patients at risk.
Take hospitals. When inspectors find a facility is not meeting safety standards, CMS can issue an immediate jeopardy citation and, if problems aren’t fixed, move to withhold federal payments, which make up substantial portions of most hospitals’ revenues. In the rare cases when hospitals don’t take sufficient action, CMS follows through and revokes funding.
Redberg, the UCSF cardiologist, said the lack of similar action for medical devices offers a clear “opportunity for improvement.” At minimum, the FDA could establish processes to directly inform other agencies when it issues warning letters and finds serious problems with devices being sold in the United States.
“If the agency’s mission is to protect public health, they would want to do these things and move quickly,” she said.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
In 2014, when the Food and Drug Administration found serious problems with a life-sustaining heart pump, its warning letter to the manufacturer threatened to notify other federal health agencies about the inspection’s findings.
But for years, no such alert ever went out. Instead, the agency added the warning letter to an online database alongside thousands of others, following its typical procedures, an FDA spokesperson said.
Agencies such as the Centers for Medicare & Medicaid Services and the U.S. Department of Veterans Affairs went on paying to implant the HeartWare Ventricular Assist Device, or HVAD, in new patients even though federal inspectors had found problems with the device linked to patient deaths and injuries.
Taxpayer dollars continued to flow to the original device maker, HeartWare, and then to the company that acquired it in 2016, Medtronic, for 7 years while the issues raised in the warning letter remained unresolved.
If crucial safety information in FDA warning letters doesn’t make it to other arms of the government responsible for deciding which medical devices to pay for, experts said patients are the ones put at risk.
“It’s clearly a breakdown of communication,” said Dr. Rita Redberg, a cardiologist at the University of California, San Francisco, who researches medical device safety and regulation. “It’s not just the money, obviously. It’s people’s lives.”
The FDA acknowledged that it doesn’t directly notify other agencies when it issues warning letters, pointing instead to its online database, which is accessible to both government officials and the public. “The FDA’s decisions are intended to be patient-centric with the health and safety of device users as our highest priority,” the agency spokesperson said in an email.
The HeartWare letter was removed from the public database about 2 years ago, even though the problems remained unresolved and patients were still receiving implants. The database clears out letters that are more than 5 years old.
CMS, which oversees the Medicare and Medicaid programs, would not say why it continued paying for a device that didn’t meet government standards. It directed questions about the HeartWare warning letter to the FDA. “CMS does not have oversight of the manufacturing and related safety assessments of a medical device manufacturer,” a spokesperson said in an email.
The spokesperson noted that CMS requires heart pump patients to have specialized medical teams managing their care, which should monitor FDA communications regarding safety of devices.
CMS doesn’t track data on devices by manufacturer, so it’s essentially impossible to calculate its total spending on HVADs. One 2018 medical journal study found that Medicare and Medicaid paid for more than half the cost of all heart pump implants from 2009 to 2014. If that rate of spending continued, CMS may have spent more than $400 million on implanting HVADs since 2014.
A spokesperson for the VA said his agency was never notified about the HeartWare warning letter. The VA paid HeartWare and Medtronic more than $3 million after the FDA issued the letter in 2014. It offered this explanation for why: “It’s important to note that FDA Warning Letters are notifications issued to manufacturers found to be in significant violation of federal regulations. They are not product recalls.”
In the case of the HVAD, the FDA’s failure to make sure its warning reached beyond the manufacturer may have had life-and-death consequences.
In August, ProPublica reported that federal inspectors continued finding problems at the HVAD’s manufacturing plant for years. Meanwhile, the FDA received thousands of reports of suspicious deaths and injuries and more than a dozen high-risk safety alerts from the manufacturer.
The documents detailed one horrifying device failure after another. A father of four died after his device suddenly failed and his teenage daughter couldn’t resuscitate him. Another patient’s heart tissue was charred after a pump short-circuited and overheated. A teenager died after vomiting blood as his mother struggled to restart a defective pump.
In June, Medtronic ended sales and implants of the device, citing new data that showed patients with HVADs had a higher rate of deaths and strokes than those with a competing heart pump.
Medtronic declined to comment for this story. It has previously said it believed that after the 2014 warning letter the benefits of the HVAD still outweighed the risks for patients with severe heart failure.
Experts said the lack of communication between federal agencies when serious device problems are found is baffling but not surprising. It fits a broader trend of device regulators focusing more on evaluating new products than monitoring the ones already on the market.
“The priority is to get more medical devices out there, paid for and getting used,” said Dr. Joseph Ross, a professor of medicine and public health at Yale University who studies medical device regulation.
Other U.S. health care regulators move more forcefully when providers and suppliers don’t meet the government’s minimum safety requirements for an extended period, putting patients at risk.
Take hospitals. When inspectors find a facility is not meeting safety standards, CMS can issue an immediate jeopardy citation and, if problems aren’t fixed, move to withhold federal payments, which make up substantial portions of most hospitals’ revenues. In the rare cases when hospitals don’t take sufficient action, CMS follows through and revokes funding.
Redberg, the UCSF cardiologist, said the lack of similar action for medical devices offers a clear “opportunity for improvement.” At minimum, the FDA could establish processes to directly inform other agencies when it issues warning letters and finds serious problems with devices being sold in the United States.
“If the agency’s mission is to protect public health, they would want to do these things and move quickly,” she said.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
In 2014, when the Food and Drug Administration found serious problems with a life-sustaining heart pump, its warning letter to the manufacturer threatened to notify other federal health agencies about the inspection’s findings.
But for years, no such alert ever went out. Instead, the agency added the warning letter to an online database alongside thousands of others, following its typical procedures, an FDA spokesperson said.
Agencies such as the Centers for Medicare & Medicaid Services and the U.S. Department of Veterans Affairs went on paying to implant the HeartWare Ventricular Assist Device, or HVAD, in new patients even though federal inspectors had found problems with the device linked to patient deaths and injuries.
Taxpayer dollars continued to flow to the original device maker, HeartWare, and then to the company that acquired it in 2016, Medtronic, for 7 years while the issues raised in the warning letter remained unresolved.
If crucial safety information in FDA warning letters doesn’t make it to other arms of the government responsible for deciding which medical devices to pay for, experts said patients are the ones put at risk.
“It’s clearly a breakdown of communication,” said Dr. Rita Redberg, a cardiologist at the University of California, San Francisco, who researches medical device safety and regulation. “It’s not just the money, obviously. It’s people’s lives.”
The FDA acknowledged that it doesn’t directly notify other agencies when it issues warning letters, pointing instead to its online database, which is accessible to both government officials and the public. “The FDA’s decisions are intended to be patient-centric with the health and safety of device users as our highest priority,” the agency spokesperson said in an email.
The HeartWare letter was removed from the public database about 2 years ago, even though the problems remained unresolved and patients were still receiving implants. The database clears out letters that are more than 5 years old.
CMS, which oversees the Medicare and Medicaid programs, would not say why it continued paying for a device that didn’t meet government standards. It directed questions about the HeartWare warning letter to the FDA. “CMS does not have oversight of the manufacturing and related safety assessments of a medical device manufacturer,” a spokesperson said in an email.
The spokesperson noted that CMS requires heart pump patients to have specialized medical teams managing their care, which should monitor FDA communications regarding safety of devices.
CMS doesn’t track data on devices by manufacturer, so it’s essentially impossible to calculate its total spending on HVADs. One 2018 medical journal study found that Medicare and Medicaid paid for more than half the cost of all heart pump implants from 2009 to 2014. If that rate of spending continued, CMS may have spent more than $400 million on implanting HVADs since 2014.
A spokesperson for the VA said his agency was never notified about the HeartWare warning letter. The VA paid HeartWare and Medtronic more than $3 million after the FDA issued the letter in 2014. It offered this explanation for why: “It’s important to note that FDA Warning Letters are notifications issued to manufacturers found to be in significant violation of federal regulations. They are not product recalls.”
In the case of the HVAD, the FDA’s failure to make sure its warning reached beyond the manufacturer may have had life-and-death consequences.
In August, ProPublica reported that federal inspectors continued finding problems at the HVAD’s manufacturing plant for years. Meanwhile, the FDA received thousands of reports of suspicious deaths and injuries and more than a dozen high-risk safety alerts from the manufacturer.
The documents detailed one horrifying device failure after another. A father of four died after his device suddenly failed and his teenage daughter couldn’t resuscitate him. Another patient’s heart tissue was charred after a pump short-circuited and overheated. A teenager died after vomiting blood as his mother struggled to restart a defective pump.
In June, Medtronic ended sales and implants of the device, citing new data that showed patients with HVADs had a higher rate of deaths and strokes than those with a competing heart pump.
Medtronic declined to comment for this story. It has previously said it believed that after the 2014 warning letter the benefits of the HVAD still outweighed the risks for patients with severe heart failure.
Experts said the lack of communication between federal agencies when serious device problems are found is baffling but not surprising. It fits a broader trend of device regulators focusing more on evaluating new products than monitoring the ones already on the market.
“The priority is to get more medical devices out there, paid for and getting used,” said Dr. Joseph Ross, a professor of medicine and public health at Yale University who studies medical device regulation.
Other U.S. health care regulators move more forcefully when providers and suppliers don’t meet the government’s minimum safety requirements for an extended period, putting patients at risk.
Take hospitals. When inspectors find a facility is not meeting safety standards, CMS can issue an immediate jeopardy citation and, if problems aren’t fixed, move to withhold federal payments, which make up substantial portions of most hospitals’ revenues. In the rare cases when hospitals don’t take sufficient action, CMS follows through and revokes funding.
Redberg, the UCSF cardiologist, said the lack of similar action for medical devices offers a clear “opportunity for improvement.” At minimum, the FDA could establish processes to directly inform other agencies when it issues warning letters and finds serious problems with devices being sold in the United States.
“If the agency’s mission is to protect public health, they would want to do these things and move quickly,” she said.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Should psychiatry categorize ‘substance-induced paraphilia?’
‘substance-induced paraphilia?’
The dopamine receptors of the brain get their fair share amid the didactics we receive in residency. From discussions of antipsychotics and schizophrenia to stimulants and ADHD, dopamine plays a key role. Depending on the program and interest of faculty, methamphetamine may get its own lecture or be mixed in with other stimulants of abuse. During that discussion, a comment might be made in passing on the impact of methamphetamine on sexual desire and activity.
Experiences in the emergency department caring for patients who are intoxicated from methamphetamine then effectively make up for any gaps in trainees’ knowledge base. From patients engaging in self-pleasing pursuits in the emergency room to unfiltered reports of sexual exploits and desires, the impact of methamphetamine on sexual behavior quickly becomes apparent. Those experiences are later reinforced when residents are exposed to more long-term rehabilitation programs and have more in-depth conversations with patients about the sex-culture surrounding methamphetamine.
It is common to hear that, under the influence of methamphetamine, any available body will become an acceptable sexual partner – at times resulting in significant regrets, dangerous sexual activity, and complicated questions surrounding consent. Some early studies have found up to 72% increase in risky sexual behavior in methamphetamine users.1 This is particularly problematic as society has recently taken on the difficult and important work to re-examine the role and nature of consent in sexual activities. This falls within the larger #MeToo movement and has led to advocating for harsher sentencing of sexual offenders.
Yet simultaneously, society has also reconsidered its approach to apportioning blame on drug users.2 This shift to a more compassionate stance has resulted in a desire to treat and care for a disorder, rather than punish and condemn a poor choice. As forensic psychiatrists, we have noted this significant change. Where substance use disorders were once considered a risk factor for recidivism, they are now considered a disability that not only warrants treatment but can also diminish the share of blame one may be responsible for.
The convergence of those two societal movements often plays out in the courtroom, and in our experience when faced with those two opposing viewpoints, triers of fact (judges and juries) often favor punishing sexual offense over empathizing with an addictive disorder. While certainly not implying methamphetamine use condones sexual offense, we do posit the particular relationship between methamphetamine use and sexual activity should be explained to those entrusted with deciding guilt.
Examples of such problems are extremely common. A routine case involves IK,3 a 48-year-old male without significant history of legal problems, arrested for indecent exposure. His history of mental illness is closely intertwined with a history of substance use, leading to many psychiatric hospitalizations for methamphetamine-induced psychosis. After many hospitalizations he was placed in an assertive community treatment (ACT) team.
One day, IK is approached by an industrious drug dealer who frequents multiple board-and-cares in search for customers interested in relapsing. IK uses methamphetamine and within hours finds himself having walked miles away, naked, in the middle of an RV park. He subsequently describes the experience of unrelenting sexual desire, accompanied by ideas of reference involving billboards encouraging him to demonstrate his sexual prowess, as well as auditory hallucinations of women cheering him on. This leads to him pleasing himself publicly and his subsequent arrest.
Interviewing IK, 3 months later, he is embarrassed and apologetic. He is cognizant of the inappropriate nature of the incident and the foolishness of his actions. However, when asked whether he considers himself a sexual offender, he protests that he would never act in such a manner if not under the influence of methamphetamine. He points to his lack of significant sexual urges when sober, his lack of prior sexual offense, his lack of sexually violent offense, and his lack of unusual sexual interests.
It is unclear to us how society will or should adjudicate on such a case. It is not under the purview of forensic psychiatry to become a trier of fact. However, psychiatry should have a better working framework of how to discuss and conceptualize such situations, especially considering the dire consequences for those involved.
While any criminal conviction already has the potential to destroy a person’s life, sexual crimes bring particularly serious consequences. Entry into the national sex offender registry, in addition to carrying an unshakable stigma, comes with additional degrees of lost freedom. These individuals are prohibited from living or working in areas that have children in proximity, subjecting them to the outskirts of society and greatly restricting any chance of economic escape from poverty. Parks, libraries, and shopping malls can become off limits. Privacy for these individuals is nonexistent; from websites they visit to where they travel physically can be monitored. Even where they live and a detailed physical description are often easily accessible by members of their community.
When should it be permissible to consider sex offender status for someone on the grounds of a mental illness? A patient with obsessive-compulsive disorder might have sadistic obsessions and compulsions to commit violent sexual acts, which, along with being repugnant to society, are entirely ego-dystonic to the suffering patient. Psychosis is often characterized as involving a loss of insight and impaired reality testing. If society accepts insanity as grounds to mitigate sentencing, then why not permit it for grounds to wave the designation of sex offender to those with certain disorders, including substance use disorder? Wherever we come down on this issue,
Should IK have to register as a sex offender? Regardless of the circumstances, he did publicly masturbate. Society has determined that public sexual displays are a crime worth carrying the pariah status of sex offender – why should an exception be made for methamphetamine use? On the other hand, it is difficult to claim that IK’s behavior was entirely of his own free will. Most triers of fact will have never experienced that amount of dopamine reward. They can’t attest to the remaining free will after experiencing more pleasurable salience and positive reinforcement than ever naturally possible.
How we deal with the behavioral consequences, and other sequelae, of methamphetamine use is a growing problem. Access to and use of methamphetamine is no longer reserved for soldiers patrolling the jungles of Vietnam. Once thought to be a scourge of the West Coast, methamphetamine is now widely available throughout the United States.4 The use of methamphetamine is likely to continue to expand as society keeps pursuing the decriminalizing of drug use. Psychiatrists practicing in areas heavily affected by methamphetamine see firsthand the burden it places on community resources in the form of increased psychosis, emergency room utilization, medical resource strain, and encounters with police.5
The presence of mental illness is tied to a small but statistically significant risk of violence. However, substance use is a well-established risk factor for violence.6 What is often missed is that many sexual offenders have not committed a violent offense. However, like IK, they have been charged with indecent exposure or other nonviolent sexual offenses, such as prostitution and solicitation. Those nonviolent offenses are driven by poor judgment and impulsivity, the trademarks of substance use. The answer cannot be to incarcerate, and eventually add to the sex offender registry, the growing number of these individuals.
Yet, as psychiatrists, we seem at a loss for how to treat these patients. The prescription of allowing them to spend a night in the ED with a complementary sandwich garnished with olanzapine often feels like enabling. Substance use treatment programs are too limited, and the wait list is rarely shorter than the time it takes our patient to purchase their next hit.
There are no effective pharmacologic treatments for methamphetamine use disorder.7 The recommendations of cognitive-behavioral therapy, family and group therapy, contingency management, and a 12-step program may be sufficient for the most motivated and well-supported patients but are inadequate for the vast majority.8 As much as we want to laud the merits of community psychiatry and the ACT [assertive community treatment] model of care, it is hard to carry that banner while confronted with the reality these patients face on a day-to-day basis during any shift in the emergency room. Eventually the countless encounters with homeless, helplessly meth-addicted patients ending in discharge back to the streets begins to tarnish the bright rhetoric surrounding community care, which starts to sound more and more like abandonment of patients to suffer in futility.9
It is not up to forensic psychiatrists, or even psychiatry as a whole, to fix the myriad of inadequacies surrounding how society handles those suffering from methamphetamine addiction. However, it is essential for psychiatry to study and educate society on the interaction of methamphetamine use and sexual behavior. There has been some exploration into other risk factors for paraphilic behavior while under the influence of substances, but there is a dearth of information on this topic. Establishing a nomenclature called “substance-induced paraphilia” might be a way to bring clarity to such instances in both a forensic and general psychiatric setting.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Compton has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Psychol Addict Behav. 2016;30(2)147-57.
2. Monitor Psychol. 2019;50(6).
3. IK’s case has been modified in certain ways to maintain confidentiality.
4. J Psychoactive Drugs. 2000;(2):137-41.
5. Acad Emerg Med. 2020 Nov;27(11):1116-25.
6. Swanson JW. Mental disorder, substance abuse, and community violence: An epidemiological approach, in: Monahan J and Steadman HJ, eds. “Violence and Mental Disorder: Developments in Risk Assessment” (Chicago: University of Chicago Press, 1994, pp. 101-36).
7. Addiction. 2004 Jun;99(6)708-17.
8. Am Fam Physician. 2007 Oct 15;76(8):1169-74.
9. Perspect Biol Med. 2021;64(1)70-81.
The dopamine receptors of the brain get their fair share amid the didactics we receive in residency. From discussions of antipsychotics and schizophrenia to stimulants and ADHD, dopamine plays a key role. Depending on the program and interest of faculty, methamphetamine may get its own lecture or be mixed in with other stimulants of abuse. During that discussion, a comment might be made in passing on the impact of methamphetamine on sexual desire and activity.
Experiences in the emergency department caring for patients who are intoxicated from methamphetamine then effectively make up for any gaps in trainees’ knowledge base. From patients engaging in self-pleasing pursuits in the emergency room to unfiltered reports of sexual exploits and desires, the impact of methamphetamine on sexual behavior quickly becomes apparent. Those experiences are later reinforced when residents are exposed to more long-term rehabilitation programs and have more in-depth conversations with patients about the sex-culture surrounding methamphetamine.
It is common to hear that, under the influence of methamphetamine, any available body will become an acceptable sexual partner – at times resulting in significant regrets, dangerous sexual activity, and complicated questions surrounding consent. Some early studies have found up to 72% increase in risky sexual behavior in methamphetamine users.1 This is particularly problematic as society has recently taken on the difficult and important work to re-examine the role and nature of consent in sexual activities. This falls within the larger #MeToo movement and has led to advocating for harsher sentencing of sexual offenders.
Yet simultaneously, society has also reconsidered its approach to apportioning blame on drug users.2 This shift to a more compassionate stance has resulted in a desire to treat and care for a disorder, rather than punish and condemn a poor choice. As forensic psychiatrists, we have noted this significant change. Where substance use disorders were once considered a risk factor for recidivism, they are now considered a disability that not only warrants treatment but can also diminish the share of blame one may be responsible for.
The convergence of those two societal movements often plays out in the courtroom, and in our experience when faced with those two opposing viewpoints, triers of fact (judges and juries) often favor punishing sexual offense over empathizing with an addictive disorder. While certainly not implying methamphetamine use condones sexual offense, we do posit the particular relationship between methamphetamine use and sexual activity should be explained to those entrusted with deciding guilt.
Examples of such problems are extremely common. A routine case involves IK,3 a 48-year-old male without significant history of legal problems, arrested for indecent exposure. His history of mental illness is closely intertwined with a history of substance use, leading to many psychiatric hospitalizations for methamphetamine-induced psychosis. After many hospitalizations he was placed in an assertive community treatment (ACT) team.
One day, IK is approached by an industrious drug dealer who frequents multiple board-and-cares in search for customers interested in relapsing. IK uses methamphetamine and within hours finds himself having walked miles away, naked, in the middle of an RV park. He subsequently describes the experience of unrelenting sexual desire, accompanied by ideas of reference involving billboards encouraging him to demonstrate his sexual prowess, as well as auditory hallucinations of women cheering him on. This leads to him pleasing himself publicly and his subsequent arrest.
Interviewing IK, 3 months later, he is embarrassed and apologetic. He is cognizant of the inappropriate nature of the incident and the foolishness of his actions. However, when asked whether he considers himself a sexual offender, he protests that he would never act in such a manner if not under the influence of methamphetamine. He points to his lack of significant sexual urges when sober, his lack of prior sexual offense, his lack of sexually violent offense, and his lack of unusual sexual interests.
It is unclear to us how society will or should adjudicate on such a case. It is not under the purview of forensic psychiatry to become a trier of fact. However, psychiatry should have a better working framework of how to discuss and conceptualize such situations, especially considering the dire consequences for those involved.
While any criminal conviction already has the potential to destroy a person’s life, sexual crimes bring particularly serious consequences. Entry into the national sex offender registry, in addition to carrying an unshakable stigma, comes with additional degrees of lost freedom. These individuals are prohibited from living or working in areas that have children in proximity, subjecting them to the outskirts of society and greatly restricting any chance of economic escape from poverty. Parks, libraries, and shopping malls can become off limits. Privacy for these individuals is nonexistent; from websites they visit to where they travel physically can be monitored. Even where they live and a detailed physical description are often easily accessible by members of their community.
When should it be permissible to consider sex offender status for someone on the grounds of a mental illness? A patient with obsessive-compulsive disorder might have sadistic obsessions and compulsions to commit violent sexual acts, which, along with being repugnant to society, are entirely ego-dystonic to the suffering patient. Psychosis is often characterized as involving a loss of insight and impaired reality testing. If society accepts insanity as grounds to mitigate sentencing, then why not permit it for grounds to wave the designation of sex offender to those with certain disorders, including substance use disorder? Wherever we come down on this issue,
Should IK have to register as a sex offender? Regardless of the circumstances, he did publicly masturbate. Society has determined that public sexual displays are a crime worth carrying the pariah status of sex offender – why should an exception be made for methamphetamine use? On the other hand, it is difficult to claim that IK’s behavior was entirely of his own free will. Most triers of fact will have never experienced that amount of dopamine reward. They can’t attest to the remaining free will after experiencing more pleasurable salience and positive reinforcement than ever naturally possible.
How we deal with the behavioral consequences, and other sequelae, of methamphetamine use is a growing problem. Access to and use of methamphetamine is no longer reserved for soldiers patrolling the jungles of Vietnam. Once thought to be a scourge of the West Coast, methamphetamine is now widely available throughout the United States.4 The use of methamphetamine is likely to continue to expand as society keeps pursuing the decriminalizing of drug use. Psychiatrists practicing in areas heavily affected by methamphetamine see firsthand the burden it places on community resources in the form of increased psychosis, emergency room utilization, medical resource strain, and encounters with police.5
The presence of mental illness is tied to a small but statistically significant risk of violence. However, substance use is a well-established risk factor for violence.6 What is often missed is that many sexual offenders have not committed a violent offense. However, like IK, they have been charged with indecent exposure or other nonviolent sexual offenses, such as prostitution and solicitation. Those nonviolent offenses are driven by poor judgment and impulsivity, the trademarks of substance use. The answer cannot be to incarcerate, and eventually add to the sex offender registry, the growing number of these individuals.
Yet, as psychiatrists, we seem at a loss for how to treat these patients. The prescription of allowing them to spend a night in the ED with a complementary sandwich garnished with olanzapine often feels like enabling. Substance use treatment programs are too limited, and the wait list is rarely shorter than the time it takes our patient to purchase their next hit.
There are no effective pharmacologic treatments for methamphetamine use disorder.7 The recommendations of cognitive-behavioral therapy, family and group therapy, contingency management, and a 12-step program may be sufficient for the most motivated and well-supported patients but are inadequate for the vast majority.8 As much as we want to laud the merits of community psychiatry and the ACT [assertive community treatment] model of care, it is hard to carry that banner while confronted with the reality these patients face on a day-to-day basis during any shift in the emergency room. Eventually the countless encounters with homeless, helplessly meth-addicted patients ending in discharge back to the streets begins to tarnish the bright rhetoric surrounding community care, which starts to sound more and more like abandonment of patients to suffer in futility.9
It is not up to forensic psychiatrists, or even psychiatry as a whole, to fix the myriad of inadequacies surrounding how society handles those suffering from methamphetamine addiction. However, it is essential for psychiatry to study and educate society on the interaction of methamphetamine use and sexual behavior. There has been some exploration into other risk factors for paraphilic behavior while under the influence of substances, but there is a dearth of information on this topic. Establishing a nomenclature called “substance-induced paraphilia” might be a way to bring clarity to such instances in both a forensic and general psychiatric setting.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Compton has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Psychol Addict Behav. 2016;30(2)147-57.
2. Monitor Psychol. 2019;50(6).
3. IK’s case has been modified in certain ways to maintain confidentiality.
4. J Psychoactive Drugs. 2000;(2):137-41.
5. Acad Emerg Med. 2020 Nov;27(11):1116-25.
6. Swanson JW. Mental disorder, substance abuse, and community violence: An epidemiological approach, in: Monahan J and Steadman HJ, eds. “Violence and Mental Disorder: Developments in Risk Assessment” (Chicago: University of Chicago Press, 1994, pp. 101-36).
7. Addiction. 2004 Jun;99(6)708-17.
8. Am Fam Physician. 2007 Oct 15;76(8):1169-74.
9. Perspect Biol Med. 2021;64(1)70-81.
The dopamine receptors of the brain get their fair share amid the didactics we receive in residency. From discussions of antipsychotics and schizophrenia to stimulants and ADHD, dopamine plays a key role. Depending on the program and interest of faculty, methamphetamine may get its own lecture or be mixed in with other stimulants of abuse. During that discussion, a comment might be made in passing on the impact of methamphetamine on sexual desire and activity.
Experiences in the emergency department caring for patients who are intoxicated from methamphetamine then effectively make up for any gaps in trainees’ knowledge base. From patients engaging in self-pleasing pursuits in the emergency room to unfiltered reports of sexual exploits and desires, the impact of methamphetamine on sexual behavior quickly becomes apparent. Those experiences are later reinforced when residents are exposed to more long-term rehabilitation programs and have more in-depth conversations with patients about the sex-culture surrounding methamphetamine.
It is common to hear that, under the influence of methamphetamine, any available body will become an acceptable sexual partner – at times resulting in significant regrets, dangerous sexual activity, and complicated questions surrounding consent. Some early studies have found up to 72% increase in risky sexual behavior in methamphetamine users.1 This is particularly problematic as society has recently taken on the difficult and important work to re-examine the role and nature of consent in sexual activities. This falls within the larger #MeToo movement and has led to advocating for harsher sentencing of sexual offenders.
Yet simultaneously, society has also reconsidered its approach to apportioning blame on drug users.2 This shift to a more compassionate stance has resulted in a desire to treat and care for a disorder, rather than punish and condemn a poor choice. As forensic psychiatrists, we have noted this significant change. Where substance use disorders were once considered a risk factor for recidivism, they are now considered a disability that not only warrants treatment but can also diminish the share of blame one may be responsible for.
The convergence of those two societal movements often plays out in the courtroom, and in our experience when faced with those two opposing viewpoints, triers of fact (judges and juries) often favor punishing sexual offense over empathizing with an addictive disorder. While certainly not implying methamphetamine use condones sexual offense, we do posit the particular relationship between methamphetamine use and sexual activity should be explained to those entrusted with deciding guilt.
Examples of such problems are extremely common. A routine case involves IK,3 a 48-year-old male without significant history of legal problems, arrested for indecent exposure. His history of mental illness is closely intertwined with a history of substance use, leading to many psychiatric hospitalizations for methamphetamine-induced psychosis. After many hospitalizations he was placed in an assertive community treatment (ACT) team.
One day, IK is approached by an industrious drug dealer who frequents multiple board-and-cares in search for customers interested in relapsing. IK uses methamphetamine and within hours finds himself having walked miles away, naked, in the middle of an RV park. He subsequently describes the experience of unrelenting sexual desire, accompanied by ideas of reference involving billboards encouraging him to demonstrate his sexual prowess, as well as auditory hallucinations of women cheering him on. This leads to him pleasing himself publicly and his subsequent arrest.
Interviewing IK, 3 months later, he is embarrassed and apologetic. He is cognizant of the inappropriate nature of the incident and the foolishness of his actions. However, when asked whether he considers himself a sexual offender, he protests that he would never act in such a manner if not under the influence of methamphetamine. He points to his lack of significant sexual urges when sober, his lack of prior sexual offense, his lack of sexually violent offense, and his lack of unusual sexual interests.
It is unclear to us how society will or should adjudicate on such a case. It is not under the purview of forensic psychiatry to become a trier of fact. However, psychiatry should have a better working framework of how to discuss and conceptualize such situations, especially considering the dire consequences for those involved.
While any criminal conviction already has the potential to destroy a person’s life, sexual crimes bring particularly serious consequences. Entry into the national sex offender registry, in addition to carrying an unshakable stigma, comes with additional degrees of lost freedom. These individuals are prohibited from living or working in areas that have children in proximity, subjecting them to the outskirts of society and greatly restricting any chance of economic escape from poverty. Parks, libraries, and shopping malls can become off limits. Privacy for these individuals is nonexistent; from websites they visit to where they travel physically can be monitored. Even where they live and a detailed physical description are often easily accessible by members of their community.
When should it be permissible to consider sex offender status for someone on the grounds of a mental illness? A patient with obsessive-compulsive disorder might have sadistic obsessions and compulsions to commit violent sexual acts, which, along with being repugnant to society, are entirely ego-dystonic to the suffering patient. Psychosis is often characterized as involving a loss of insight and impaired reality testing. If society accepts insanity as grounds to mitigate sentencing, then why not permit it for grounds to wave the designation of sex offender to those with certain disorders, including substance use disorder? Wherever we come down on this issue,
Should IK have to register as a sex offender? Regardless of the circumstances, he did publicly masturbate. Society has determined that public sexual displays are a crime worth carrying the pariah status of sex offender – why should an exception be made for methamphetamine use? On the other hand, it is difficult to claim that IK’s behavior was entirely of his own free will. Most triers of fact will have never experienced that amount of dopamine reward. They can’t attest to the remaining free will after experiencing more pleasurable salience and positive reinforcement than ever naturally possible.
How we deal with the behavioral consequences, and other sequelae, of methamphetamine use is a growing problem. Access to and use of methamphetamine is no longer reserved for soldiers patrolling the jungles of Vietnam. Once thought to be a scourge of the West Coast, methamphetamine is now widely available throughout the United States.4 The use of methamphetamine is likely to continue to expand as society keeps pursuing the decriminalizing of drug use. Psychiatrists practicing in areas heavily affected by methamphetamine see firsthand the burden it places on community resources in the form of increased psychosis, emergency room utilization, medical resource strain, and encounters with police.5
The presence of mental illness is tied to a small but statistically significant risk of violence. However, substance use is a well-established risk factor for violence.6 What is often missed is that many sexual offenders have not committed a violent offense. However, like IK, they have been charged with indecent exposure or other nonviolent sexual offenses, such as prostitution and solicitation. Those nonviolent offenses are driven by poor judgment and impulsivity, the trademarks of substance use. The answer cannot be to incarcerate, and eventually add to the sex offender registry, the growing number of these individuals.
Yet, as psychiatrists, we seem at a loss for how to treat these patients. The prescription of allowing them to spend a night in the ED with a complementary sandwich garnished with olanzapine often feels like enabling. Substance use treatment programs are too limited, and the wait list is rarely shorter than the time it takes our patient to purchase their next hit.
There are no effective pharmacologic treatments for methamphetamine use disorder.7 The recommendations of cognitive-behavioral therapy, family and group therapy, contingency management, and a 12-step program may be sufficient for the most motivated and well-supported patients but are inadequate for the vast majority.8 As much as we want to laud the merits of community psychiatry and the ACT [assertive community treatment] model of care, it is hard to carry that banner while confronted with the reality these patients face on a day-to-day basis during any shift in the emergency room. Eventually the countless encounters with homeless, helplessly meth-addicted patients ending in discharge back to the streets begins to tarnish the bright rhetoric surrounding community care, which starts to sound more and more like abandonment of patients to suffer in futility.9
It is not up to forensic psychiatrists, or even psychiatry as a whole, to fix the myriad of inadequacies surrounding how society handles those suffering from methamphetamine addiction. However, it is essential for psychiatry to study and educate society on the interaction of methamphetamine use and sexual behavior. There has been some exploration into other risk factors for paraphilic behavior while under the influence of substances, but there is a dearth of information on this topic. Establishing a nomenclature called “substance-induced paraphilia” might be a way to bring clarity to such instances in both a forensic and general psychiatric setting.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Compton has no conflicts of interest. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Psychol Addict Behav. 2016;30(2)147-57.
2. Monitor Psychol. 2019;50(6).
3. IK’s case has been modified in certain ways to maintain confidentiality.
4. J Psychoactive Drugs. 2000;(2):137-41.
5. Acad Emerg Med. 2020 Nov;27(11):1116-25.
6. Swanson JW. Mental disorder, substance abuse, and community violence: An epidemiological approach, in: Monahan J and Steadman HJ, eds. “Violence and Mental Disorder: Developments in Risk Assessment” (Chicago: University of Chicago Press, 1994, pp. 101-36).
7. Addiction. 2004 Jun;99(6)708-17.
8. Am Fam Physician. 2007 Oct 15;76(8):1169-74.
9. Perspect Biol Med. 2021;64(1)70-81.
‘substance-induced paraphilia?’
‘substance-induced paraphilia?’
Flexible sigmoidoscopy ADR linked to long-term survival
Gastroenterology centers with higher adenoma detection rates (ADR) with the use of flexible sigmoidoscopy (FS) had a lower long-term colorectal cancer incidence and lower CRC mortality among its patients, according to a new study.
Detection and removal of polyps during colonoscopy screening is vital to the prevention of CRC, and previous research has shown that centers with higher detection rates are associated with lower rates of CRC diagnosis within 3-5 years after a negative screen.
In Clinical Gastroenterology and Hepatology, researchers led by Amanda J. Cross, PhD, a professor of cancer epidemiology at Imperial College London, published an analysis of the UK Flexible Sigmoidoscopy Screening Trial, which found that FS screening between the ages 55 and 64 led to a 35% reduction of CRC incidence and a 41% reduction in CRC over a mean follow-up 17.1 years. The screening program had no apparent effect on incidence and mortality of proximal cancers. The researchers speculated that this was because few patients underwent proximal examination during follow-up colonoscopy.
“Considering only 5% of participants were referred for follow-up colonoscopy and 4% were referred for surveillance, we conclude that the improved detection of adenomas at FS has a measurable impact on long-term distal CRC outcomes, even when there is infrequent colonoscopy use. It is possible that high detectors also were more adept at polypectomy than intermediate or low detectors, and achieved more complete resection of detected lesions,” the authors wrote.
The researchers analyzed data from 38,550 patients who underwent screening at 14 U.K. hospitals, between 1994 and 1999. A single endoscopist was responsible for nearly all FS screens performed at each participating hospital.
The mean patient age was 60 years, and 49% were male. The researchers calculated ADRs for each center using the percentage of patients who had at least one adenoma detected during screening, which included any distal adenomas discovered during follow-up colonoscopy.
The ADR overall was 12%. The researchers used multivariate logistic regression to rank individual centers as having high (15%; five centers), intermediate (12%; four centers), or low (9%; four centers) detection rates.
There was a strong association between detection rates of small adenomas and a center’s ADR (P < .001), but not for large or advanced adenomas. In the high-detector group, 6.2% of patients screened were referred to colonoscopy versus 4.5% in the intermediate group and 4.5% in the low group. About half of colonoscopies were conducted by the same endoscopist who performed FS.
During follow-up, the distal CRC incidence was 1.5% in the high ADR group, 1.4% in the intermediate group, and 1.7% in the low group, and mortality rates were 0.4%, 0.4%, and 0.5%, respectively.
Compared with unscreened controls, risk of distal CRC was lowest among individuals who underwent screening in the high ADR group (hazard ratio, 0.34; 95% confidence interval, 0.27-0.42), followed by the intermediate group (HR, 0.46; 95% CI, 0.36-0.59), and the low ADR group (HR, 0.55; 95% CI, 0.44-0.68; P < .05 for all).
Compared with unscreened controls, CRC mortality was lower among individuals who underwent screening in the high ADR group (HR, 0.22; 95% CI, 0.13-0.37), followed by the intermediate group (HR, 0.30; 95% CI, 0.17-0.55), and the low ADR group (HR, 0.54; 95% CI, 0.34-0.86; P < .05 for between group differences).
All-site CRC incidence followed similar trends, with the lowest risks in the high ADR group (HR, 0.58; 95% CI, 0.50-0.67), followed by intermediate ADR (HR, 0.65; 95% CI, 0.55-0.77) and low ADR groups (HR, 0.72; 95% CI, 0.61-0.85; between group differences not statistically significant).
All-site CRC mortality was lowest in the high ADR group (HR, 0.52; 95% CI, 0.39-0.69), followed by the intermediate group (HR, 0.53; 95% CI, 0.38-0.73), and the low ADR group (HR, 0.68; 95% CI, 0.51-0.92; between-group differences not statistically significant).
The number needed to screen (NNS) to prevent one CRC diagnosis was 78 in the high ADR group (95% CI, 61-106), 103 in the intermediate group (95% CI, 74-171), and 125 in the low ADR group (95% CI, 82-256). The NNS to prevent one CRC death was 226 (95% CI, 159-387), 247 (95% CI, 165-490), and 349 respectively (95% CI, 192-1,904).
However, the researchers also pointed out that efforts to increase ADR could result in more complications, such as perforations or gastrointestinal bleeding, as well as more frequent diagnosis and recommended surveillance for diminutive adenomas.
The study is limited by the fact that endoscopists were either gastroenterologists or surgeons and the study population was made up of individuals who desired screening.
The UK Flexible Sigmoidoscopy Screening Trial was funded by the UK Medical Research Council and the National Institute for Health Research. The authors disclosed no conflicts of interest.
Adenoma detection rate (ADR) is an important quality indicator for colonoscopy. A higher ADR is associated with a lower risk of postcolonoscopy colorectal cancer (CRC). Flexible sigmoidoscopy (FS) is an evidence-based CRC screening modality, supported by multiple randomized trials reporting long-term reduction in CRC incidence and mortality. However, the impact of ADR of endoscopist performing FS on long-term outcomes is not known.
In this post hoc analysis from the UK Flexible Sigmoidoscopy Screening Trial the authors stratified the 13 endoscopy centers performing screening FS on 40,085 average-risk individuals aged between 55 and 64 years by their ADR into high, intermediate, and low with ADRs of 15%, 12%, and 9% respectively, and compared the relative reduction in CRC incidence and mortality with 113,195 controls over a median of 17 years. The authors reported greater reduction in both CRC incidence and mortality for CRC between high and low detectors (relative reduction of 42% versus 28% for CRC incidence and 48% versus 32% for CRC mortality respectively). Differences by ADR for distal CRC were more pronounced between high and low ADR centers (66% versus 45% for CRC incidence and 78% versus 46% for CRC mortality respectively); however, the test for interaction was not statistically significant, suggesting the three ADR groups cannot be differentiated from each other for the outcomes.
While FS is rarely used for screening in the United States, and U.K. guidelines also recently moved away from FS, the study illustrates that quality of FS is important, and that ADR can be a valid quality indicator for flexible sigmoidoscopy.
Aasma Shaukat, MD MPH AGAF, is Robert M. and Mary H. Glickman Professor of Medicine and Population Health and director of GI outcomes research at New York University. She reported having no relevant conflicts of interest.
Adenoma detection rate (ADR) is an important quality indicator for colonoscopy. A higher ADR is associated with a lower risk of postcolonoscopy colorectal cancer (CRC). Flexible sigmoidoscopy (FS) is an evidence-based CRC screening modality, supported by multiple randomized trials reporting long-term reduction in CRC incidence and mortality. However, the impact of ADR of endoscopist performing FS on long-term outcomes is not known.
In this post hoc analysis from the UK Flexible Sigmoidoscopy Screening Trial the authors stratified the 13 endoscopy centers performing screening FS on 40,085 average-risk individuals aged between 55 and 64 years by their ADR into high, intermediate, and low with ADRs of 15%, 12%, and 9% respectively, and compared the relative reduction in CRC incidence and mortality with 113,195 controls over a median of 17 years. The authors reported greater reduction in both CRC incidence and mortality for CRC between high and low detectors (relative reduction of 42% versus 28% for CRC incidence and 48% versus 32% for CRC mortality respectively). Differences by ADR for distal CRC were more pronounced between high and low ADR centers (66% versus 45% for CRC incidence and 78% versus 46% for CRC mortality respectively); however, the test for interaction was not statistically significant, suggesting the three ADR groups cannot be differentiated from each other for the outcomes.
While FS is rarely used for screening in the United States, and U.K. guidelines also recently moved away from FS, the study illustrates that quality of FS is important, and that ADR can be a valid quality indicator for flexible sigmoidoscopy.
Aasma Shaukat, MD MPH AGAF, is Robert M. and Mary H. Glickman Professor of Medicine and Population Health and director of GI outcomes research at New York University. She reported having no relevant conflicts of interest.
Adenoma detection rate (ADR) is an important quality indicator for colonoscopy. A higher ADR is associated with a lower risk of postcolonoscopy colorectal cancer (CRC). Flexible sigmoidoscopy (FS) is an evidence-based CRC screening modality, supported by multiple randomized trials reporting long-term reduction in CRC incidence and mortality. However, the impact of ADR of endoscopist performing FS on long-term outcomes is not known.
In this post hoc analysis from the UK Flexible Sigmoidoscopy Screening Trial the authors stratified the 13 endoscopy centers performing screening FS on 40,085 average-risk individuals aged between 55 and 64 years by their ADR into high, intermediate, and low with ADRs of 15%, 12%, and 9% respectively, and compared the relative reduction in CRC incidence and mortality with 113,195 controls over a median of 17 years. The authors reported greater reduction in both CRC incidence and mortality for CRC between high and low detectors (relative reduction of 42% versus 28% for CRC incidence and 48% versus 32% for CRC mortality respectively). Differences by ADR for distal CRC were more pronounced between high and low ADR centers (66% versus 45% for CRC incidence and 78% versus 46% for CRC mortality respectively); however, the test for interaction was not statistically significant, suggesting the three ADR groups cannot be differentiated from each other for the outcomes.
While FS is rarely used for screening in the United States, and U.K. guidelines also recently moved away from FS, the study illustrates that quality of FS is important, and that ADR can be a valid quality indicator for flexible sigmoidoscopy.
Aasma Shaukat, MD MPH AGAF, is Robert M. and Mary H. Glickman Professor of Medicine and Population Health and director of GI outcomes research at New York University. She reported having no relevant conflicts of interest.
Gastroenterology centers with higher adenoma detection rates (ADR) with the use of flexible sigmoidoscopy (FS) had a lower long-term colorectal cancer incidence and lower CRC mortality among its patients, according to a new study.
Detection and removal of polyps during colonoscopy screening is vital to the prevention of CRC, and previous research has shown that centers with higher detection rates are associated with lower rates of CRC diagnosis within 3-5 years after a negative screen.
In Clinical Gastroenterology and Hepatology, researchers led by Amanda J. Cross, PhD, a professor of cancer epidemiology at Imperial College London, published an analysis of the UK Flexible Sigmoidoscopy Screening Trial, which found that FS screening between the ages 55 and 64 led to a 35% reduction of CRC incidence and a 41% reduction in CRC over a mean follow-up 17.1 years. The screening program had no apparent effect on incidence and mortality of proximal cancers. The researchers speculated that this was because few patients underwent proximal examination during follow-up colonoscopy.
“Considering only 5% of participants were referred for follow-up colonoscopy and 4% were referred for surveillance, we conclude that the improved detection of adenomas at FS has a measurable impact on long-term distal CRC outcomes, even when there is infrequent colonoscopy use. It is possible that high detectors also were more adept at polypectomy than intermediate or low detectors, and achieved more complete resection of detected lesions,” the authors wrote.
The researchers analyzed data from 38,550 patients who underwent screening at 14 U.K. hospitals, between 1994 and 1999. A single endoscopist was responsible for nearly all FS screens performed at each participating hospital.
The mean patient age was 60 years, and 49% were male. The researchers calculated ADRs for each center using the percentage of patients who had at least one adenoma detected during screening, which included any distal adenomas discovered during follow-up colonoscopy.
The ADR overall was 12%. The researchers used multivariate logistic regression to rank individual centers as having high (15%; five centers), intermediate (12%; four centers), or low (9%; four centers) detection rates.
There was a strong association between detection rates of small adenomas and a center’s ADR (P < .001), but not for large or advanced adenomas. In the high-detector group, 6.2% of patients screened were referred to colonoscopy versus 4.5% in the intermediate group and 4.5% in the low group. About half of colonoscopies were conducted by the same endoscopist who performed FS.
During follow-up, the distal CRC incidence was 1.5% in the high ADR group, 1.4% in the intermediate group, and 1.7% in the low group, and mortality rates were 0.4%, 0.4%, and 0.5%, respectively.
Compared with unscreened controls, risk of distal CRC was lowest among individuals who underwent screening in the high ADR group (hazard ratio, 0.34; 95% confidence interval, 0.27-0.42), followed by the intermediate group (HR, 0.46; 95% CI, 0.36-0.59), and the low ADR group (HR, 0.55; 95% CI, 0.44-0.68; P < .05 for all).
Compared with unscreened controls, CRC mortality was lower among individuals who underwent screening in the high ADR group (HR, 0.22; 95% CI, 0.13-0.37), followed by the intermediate group (HR, 0.30; 95% CI, 0.17-0.55), and the low ADR group (HR, 0.54; 95% CI, 0.34-0.86; P < .05 for between group differences).
All-site CRC incidence followed similar trends, with the lowest risks in the high ADR group (HR, 0.58; 95% CI, 0.50-0.67), followed by intermediate ADR (HR, 0.65; 95% CI, 0.55-0.77) and low ADR groups (HR, 0.72; 95% CI, 0.61-0.85; between group differences not statistically significant).
All-site CRC mortality was lowest in the high ADR group (HR, 0.52; 95% CI, 0.39-0.69), followed by the intermediate group (HR, 0.53; 95% CI, 0.38-0.73), and the low ADR group (HR, 0.68; 95% CI, 0.51-0.92; between-group differences not statistically significant).
The number needed to screen (NNS) to prevent one CRC diagnosis was 78 in the high ADR group (95% CI, 61-106), 103 in the intermediate group (95% CI, 74-171), and 125 in the low ADR group (95% CI, 82-256). The NNS to prevent one CRC death was 226 (95% CI, 159-387), 247 (95% CI, 165-490), and 349 respectively (95% CI, 192-1,904).
However, the researchers also pointed out that efforts to increase ADR could result in more complications, such as perforations or gastrointestinal bleeding, as well as more frequent diagnosis and recommended surveillance for diminutive adenomas.
The study is limited by the fact that endoscopists were either gastroenterologists or surgeons and the study population was made up of individuals who desired screening.
The UK Flexible Sigmoidoscopy Screening Trial was funded by the UK Medical Research Council and the National Institute for Health Research. The authors disclosed no conflicts of interest.
Gastroenterology centers with higher adenoma detection rates (ADR) with the use of flexible sigmoidoscopy (FS) had a lower long-term colorectal cancer incidence and lower CRC mortality among its patients, according to a new study.
Detection and removal of polyps during colonoscopy screening is vital to the prevention of CRC, and previous research has shown that centers with higher detection rates are associated with lower rates of CRC diagnosis within 3-5 years after a negative screen.
In Clinical Gastroenterology and Hepatology, researchers led by Amanda J. Cross, PhD, a professor of cancer epidemiology at Imperial College London, published an analysis of the UK Flexible Sigmoidoscopy Screening Trial, which found that FS screening between the ages 55 and 64 led to a 35% reduction of CRC incidence and a 41% reduction in CRC over a mean follow-up 17.1 years. The screening program had no apparent effect on incidence and mortality of proximal cancers. The researchers speculated that this was because few patients underwent proximal examination during follow-up colonoscopy.
“Considering only 5% of participants were referred for follow-up colonoscopy and 4% were referred for surveillance, we conclude that the improved detection of adenomas at FS has a measurable impact on long-term distal CRC outcomes, even when there is infrequent colonoscopy use. It is possible that high detectors also were more adept at polypectomy than intermediate or low detectors, and achieved more complete resection of detected lesions,” the authors wrote.
The researchers analyzed data from 38,550 patients who underwent screening at 14 U.K. hospitals, between 1994 and 1999. A single endoscopist was responsible for nearly all FS screens performed at each participating hospital.
The mean patient age was 60 years, and 49% were male. The researchers calculated ADRs for each center using the percentage of patients who had at least one adenoma detected during screening, which included any distal adenomas discovered during follow-up colonoscopy.
The ADR overall was 12%. The researchers used multivariate logistic regression to rank individual centers as having high (15%; five centers), intermediate (12%; four centers), or low (9%; four centers) detection rates.
There was a strong association between detection rates of small adenomas and a center’s ADR (P < .001), but not for large or advanced adenomas. In the high-detector group, 6.2% of patients screened were referred to colonoscopy versus 4.5% in the intermediate group and 4.5% in the low group. About half of colonoscopies were conducted by the same endoscopist who performed FS.
During follow-up, the distal CRC incidence was 1.5% in the high ADR group, 1.4% in the intermediate group, and 1.7% in the low group, and mortality rates were 0.4%, 0.4%, and 0.5%, respectively.
Compared with unscreened controls, risk of distal CRC was lowest among individuals who underwent screening in the high ADR group (hazard ratio, 0.34; 95% confidence interval, 0.27-0.42), followed by the intermediate group (HR, 0.46; 95% CI, 0.36-0.59), and the low ADR group (HR, 0.55; 95% CI, 0.44-0.68; P < .05 for all).
Compared with unscreened controls, CRC mortality was lower among individuals who underwent screening in the high ADR group (HR, 0.22; 95% CI, 0.13-0.37), followed by the intermediate group (HR, 0.30; 95% CI, 0.17-0.55), and the low ADR group (HR, 0.54; 95% CI, 0.34-0.86; P < .05 for between group differences).
All-site CRC incidence followed similar trends, with the lowest risks in the high ADR group (HR, 0.58; 95% CI, 0.50-0.67), followed by intermediate ADR (HR, 0.65; 95% CI, 0.55-0.77) and low ADR groups (HR, 0.72; 95% CI, 0.61-0.85; between group differences not statistically significant).
All-site CRC mortality was lowest in the high ADR group (HR, 0.52; 95% CI, 0.39-0.69), followed by the intermediate group (HR, 0.53; 95% CI, 0.38-0.73), and the low ADR group (HR, 0.68; 95% CI, 0.51-0.92; between-group differences not statistically significant).
The number needed to screen (NNS) to prevent one CRC diagnosis was 78 in the high ADR group (95% CI, 61-106), 103 in the intermediate group (95% CI, 74-171), and 125 in the low ADR group (95% CI, 82-256). The NNS to prevent one CRC death was 226 (95% CI, 159-387), 247 (95% CI, 165-490), and 349 respectively (95% CI, 192-1,904).
However, the researchers also pointed out that efforts to increase ADR could result in more complications, such as perforations or gastrointestinal bleeding, as well as more frequent diagnosis and recommended surveillance for diminutive adenomas.
The study is limited by the fact that endoscopists were either gastroenterologists or surgeons and the study population was made up of individuals who desired screening.
The UK Flexible Sigmoidoscopy Screening Trial was funded by the UK Medical Research Council and the National Institute for Health Research. The authors disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Bleeding after reperfusion contributes to cardiac injury in MI
The damage to the heart caused by a myocardial infarction is not just a result of ischemia caused by the blocked artery but is also brought about by bleeding in the myocardium after the artery has been opened, a new study suggests.
This observation is leading to new approaches to limiting infarct size and treating MI.
“In MI treatment, we have always focused on opening up the artery as quickly as possible to limit the myocardial damage caused by ischemia,” the study’s senior author, Rohan Dharmakumar, PhD, Indiana University, Indianapolis, told this news organization.
“We are pursuing a completely new approach focusing on limiting the damage after revascularization,” he said. “We are totally rethinking what a myocardial infarction is – what causes the injury and the time course of the injury – our results suggest that it’s not just ischemic damage and a lot of the harm is caused by hemorrhage after reperfusion.”
It has been known for many years that hemorrhage is often seen in the myocardium in large MIs, but it has not been established before now whether it contributes to the injury or not, Dr. Dharmakumar explained.
“This study was done to look at that – and we found that the hemorrhage drives a second layer of injury on top of the ischemia.”
Dr. Dharmakumar said this hemorrhage is part of the phenomenon known as reperfusion injury. “This has been known to exist for many years, but we haven’t fully understood all the factors contributing to it. Our results suggest that hemorrhage is a major component of reperfusion injury – probably the dominant factor,” he said.
The researchers are now working on therapeutic approaches to try to prevent this hemorrhage and/or to minimize its effect.
“We are studying how hemorrhage drives damage and how to block these biological processes,” Dr. Dharmakumar said. “Our studies suggest that hemorrhage could account for up to half of the damage caused by a myocardial infarction. If we can limit that, we should be able to reduce the size of the infarct and this should translate into better long-term outcomes.
“I’m very excited about these results,” he added. “We are already seeing a remarkable improvement in animal models with some of the potential therapeutic approaches we are working on.”
The current study is published in the January 2022 issue of the Journal of the American College of Cardiology (JACC).
The authors explain that it is now recognized that reperfusion injury can contribute to increasing infarct size, which they refer to as “infarct surge.” Previous studies have also shown that reperfusion injury can contribute to as much as 50% of the final infarct size, but the factors contributing to the observed variability are not known, and previous attempts to limit infarct surge from reperfusion injury have failed.
They noted that after reperfusion, microvessels can remain obstructed, resulting in intramyocardial hemorrhage. They conducted the current study to investigate whether such hemorrhage causes expansion of the infarct.
They studied 70 patients with ST-segment elevation MI who were categorized with cardiovascular MRI to have intramyocardial hemorrhage or not following primary PCI, and for whom serial cardiac troponin measures were used to assess infarct size.
Results showed that while troponin levels were not different before reperfusion, patients with intramyocardial hemorrhage had significantly higher cardiac troponin levels after reperfusion and these levels peaked earlier than in patients without hemorrhage.
In animal models, those with intramyocardial hemorrhage had a more rapid expansion of myocardial necrosis than did those without hemorrhage, and within 72 hours of reperfusion, a fourfold greater loss in salvageable myocardium was evident in hemorrhagic MIs.
“We have shown that damage to the heart continues after revascularization as measured by rapidly increasing troponin levels in the hearts that have had a hemorrhage,” Dr. Dharmakumar said.
“Hemorrhage in the myocardium was associated with larger infarctions, and in infarcts causing the same area of myocardium to be at risk, those with hemorrhage after revascularization lost a lot more of the salvageable myocardium than those without hemorrhage,” he added.
Dr. Dharmakumar estimates that such hemorrhage occurs in about half of MIs after revascularization, with risk factors including male gender, anterior wall MIs, and smoking.
He pointed out that previous attempts to treat or prevent reperfusion injury have not been successful, probably because they have not been addressing the key mechanism. “We have not been looking at hemorrhage in this regard until now. This is because it is only recently that we have had the tools to be able to identify hemorrhage in the heart with the use of cardiac MRI.”
Final frontier
In an accompanying editorial, Colin Berry, MBChB, University of Glasgow, and Borja Ibáñez, MD, Jiménez Díaz Foundation University Hospital, Madrid, said they applaud the investigators for providing new, mechanistic insights into a difficult clinical problem that has an unmet therapeutic need.
But they pointed out that it is difficult to completely dissect the impact of hemorrhage versus MI size on adverse remodeling, noting that it might be the case that more severe ischemia/reperfusion events are associated with large MI sizes and higher degree of hemorrhage.
However, they concluded that: “Intramyocardial hemorrhage represents the final frontier for preventing heart failure post-MI. It is readily detected using CMR, and clinical research of novel therapeutic approaches merits prioritization.”
This work was supported by grants from National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Dharmakumar and coauthor Robert Finney, PhD, have ownership interest in Cardiotheranostics. Dr. Berry is employed by the University of Glasgow, which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Causeway Therapeutics, Coroventis, Genentech, GlaxoSmithKline, HeartFlow, Menarini, Neovasc, Siemens Healthcare, and Valo Health.
A version of this article first appeared on Medscape.com.
The damage to the heart caused by a myocardial infarction is not just a result of ischemia caused by the blocked artery but is also brought about by bleeding in the myocardium after the artery has been opened, a new study suggests.
This observation is leading to new approaches to limiting infarct size and treating MI.
“In MI treatment, we have always focused on opening up the artery as quickly as possible to limit the myocardial damage caused by ischemia,” the study’s senior author, Rohan Dharmakumar, PhD, Indiana University, Indianapolis, told this news organization.
“We are pursuing a completely new approach focusing on limiting the damage after revascularization,” he said. “We are totally rethinking what a myocardial infarction is – what causes the injury and the time course of the injury – our results suggest that it’s not just ischemic damage and a lot of the harm is caused by hemorrhage after reperfusion.”
It has been known for many years that hemorrhage is often seen in the myocardium in large MIs, but it has not been established before now whether it contributes to the injury or not, Dr. Dharmakumar explained.
“This study was done to look at that – and we found that the hemorrhage drives a second layer of injury on top of the ischemia.”
Dr. Dharmakumar said this hemorrhage is part of the phenomenon known as reperfusion injury. “This has been known to exist for many years, but we haven’t fully understood all the factors contributing to it. Our results suggest that hemorrhage is a major component of reperfusion injury – probably the dominant factor,” he said.
The researchers are now working on therapeutic approaches to try to prevent this hemorrhage and/or to minimize its effect.
“We are studying how hemorrhage drives damage and how to block these biological processes,” Dr. Dharmakumar said. “Our studies suggest that hemorrhage could account for up to half of the damage caused by a myocardial infarction. If we can limit that, we should be able to reduce the size of the infarct and this should translate into better long-term outcomes.
“I’m very excited about these results,” he added. “We are already seeing a remarkable improvement in animal models with some of the potential therapeutic approaches we are working on.”
The current study is published in the January 2022 issue of the Journal of the American College of Cardiology (JACC).
The authors explain that it is now recognized that reperfusion injury can contribute to increasing infarct size, which they refer to as “infarct surge.” Previous studies have also shown that reperfusion injury can contribute to as much as 50% of the final infarct size, but the factors contributing to the observed variability are not known, and previous attempts to limit infarct surge from reperfusion injury have failed.
They noted that after reperfusion, microvessels can remain obstructed, resulting in intramyocardial hemorrhage. They conducted the current study to investigate whether such hemorrhage causes expansion of the infarct.
They studied 70 patients with ST-segment elevation MI who were categorized with cardiovascular MRI to have intramyocardial hemorrhage or not following primary PCI, and for whom serial cardiac troponin measures were used to assess infarct size.
Results showed that while troponin levels were not different before reperfusion, patients with intramyocardial hemorrhage had significantly higher cardiac troponin levels after reperfusion and these levels peaked earlier than in patients without hemorrhage.
In animal models, those with intramyocardial hemorrhage had a more rapid expansion of myocardial necrosis than did those without hemorrhage, and within 72 hours of reperfusion, a fourfold greater loss in salvageable myocardium was evident in hemorrhagic MIs.
“We have shown that damage to the heart continues after revascularization as measured by rapidly increasing troponin levels in the hearts that have had a hemorrhage,” Dr. Dharmakumar said.
“Hemorrhage in the myocardium was associated with larger infarctions, and in infarcts causing the same area of myocardium to be at risk, those with hemorrhage after revascularization lost a lot more of the salvageable myocardium than those without hemorrhage,” he added.
Dr. Dharmakumar estimates that such hemorrhage occurs in about half of MIs after revascularization, with risk factors including male gender, anterior wall MIs, and smoking.
He pointed out that previous attempts to treat or prevent reperfusion injury have not been successful, probably because they have not been addressing the key mechanism. “We have not been looking at hemorrhage in this regard until now. This is because it is only recently that we have had the tools to be able to identify hemorrhage in the heart with the use of cardiac MRI.”
Final frontier
In an accompanying editorial, Colin Berry, MBChB, University of Glasgow, and Borja Ibáñez, MD, Jiménez Díaz Foundation University Hospital, Madrid, said they applaud the investigators for providing new, mechanistic insights into a difficult clinical problem that has an unmet therapeutic need.
But they pointed out that it is difficult to completely dissect the impact of hemorrhage versus MI size on adverse remodeling, noting that it might be the case that more severe ischemia/reperfusion events are associated with large MI sizes and higher degree of hemorrhage.
However, they concluded that: “Intramyocardial hemorrhage represents the final frontier for preventing heart failure post-MI. It is readily detected using CMR, and clinical research of novel therapeutic approaches merits prioritization.”
This work was supported by grants from National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Dharmakumar and coauthor Robert Finney, PhD, have ownership interest in Cardiotheranostics. Dr. Berry is employed by the University of Glasgow, which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Causeway Therapeutics, Coroventis, Genentech, GlaxoSmithKline, HeartFlow, Menarini, Neovasc, Siemens Healthcare, and Valo Health.
A version of this article first appeared on Medscape.com.
The damage to the heart caused by a myocardial infarction is not just a result of ischemia caused by the blocked artery but is also brought about by bleeding in the myocardium after the artery has been opened, a new study suggests.
This observation is leading to new approaches to limiting infarct size and treating MI.
“In MI treatment, we have always focused on opening up the artery as quickly as possible to limit the myocardial damage caused by ischemia,” the study’s senior author, Rohan Dharmakumar, PhD, Indiana University, Indianapolis, told this news organization.
“We are pursuing a completely new approach focusing on limiting the damage after revascularization,” he said. “We are totally rethinking what a myocardial infarction is – what causes the injury and the time course of the injury – our results suggest that it’s not just ischemic damage and a lot of the harm is caused by hemorrhage after reperfusion.”
It has been known for many years that hemorrhage is often seen in the myocardium in large MIs, but it has not been established before now whether it contributes to the injury or not, Dr. Dharmakumar explained.
“This study was done to look at that – and we found that the hemorrhage drives a second layer of injury on top of the ischemia.”
Dr. Dharmakumar said this hemorrhage is part of the phenomenon known as reperfusion injury. “This has been known to exist for many years, but we haven’t fully understood all the factors contributing to it. Our results suggest that hemorrhage is a major component of reperfusion injury – probably the dominant factor,” he said.
The researchers are now working on therapeutic approaches to try to prevent this hemorrhage and/or to minimize its effect.
“We are studying how hemorrhage drives damage and how to block these biological processes,” Dr. Dharmakumar said. “Our studies suggest that hemorrhage could account for up to half of the damage caused by a myocardial infarction. If we can limit that, we should be able to reduce the size of the infarct and this should translate into better long-term outcomes.
“I’m very excited about these results,” he added. “We are already seeing a remarkable improvement in animal models with some of the potential therapeutic approaches we are working on.”
The current study is published in the January 2022 issue of the Journal of the American College of Cardiology (JACC).
The authors explain that it is now recognized that reperfusion injury can contribute to increasing infarct size, which they refer to as “infarct surge.” Previous studies have also shown that reperfusion injury can contribute to as much as 50% of the final infarct size, but the factors contributing to the observed variability are not known, and previous attempts to limit infarct surge from reperfusion injury have failed.
They noted that after reperfusion, microvessels can remain obstructed, resulting in intramyocardial hemorrhage. They conducted the current study to investigate whether such hemorrhage causes expansion of the infarct.
They studied 70 patients with ST-segment elevation MI who were categorized with cardiovascular MRI to have intramyocardial hemorrhage or not following primary PCI, and for whom serial cardiac troponin measures were used to assess infarct size.
Results showed that while troponin levels were not different before reperfusion, patients with intramyocardial hemorrhage had significantly higher cardiac troponin levels after reperfusion and these levels peaked earlier than in patients without hemorrhage.
In animal models, those with intramyocardial hemorrhage had a more rapid expansion of myocardial necrosis than did those without hemorrhage, and within 72 hours of reperfusion, a fourfold greater loss in salvageable myocardium was evident in hemorrhagic MIs.
“We have shown that damage to the heart continues after revascularization as measured by rapidly increasing troponin levels in the hearts that have had a hemorrhage,” Dr. Dharmakumar said.
“Hemorrhage in the myocardium was associated with larger infarctions, and in infarcts causing the same area of myocardium to be at risk, those with hemorrhage after revascularization lost a lot more of the salvageable myocardium than those without hemorrhage,” he added.
Dr. Dharmakumar estimates that such hemorrhage occurs in about half of MIs after revascularization, with risk factors including male gender, anterior wall MIs, and smoking.
He pointed out that previous attempts to treat or prevent reperfusion injury have not been successful, probably because they have not been addressing the key mechanism. “We have not been looking at hemorrhage in this regard until now. This is because it is only recently that we have had the tools to be able to identify hemorrhage in the heart with the use of cardiac MRI.”
Final frontier
In an accompanying editorial, Colin Berry, MBChB, University of Glasgow, and Borja Ibáñez, MD, Jiménez Díaz Foundation University Hospital, Madrid, said they applaud the investigators for providing new, mechanistic insights into a difficult clinical problem that has an unmet therapeutic need.
But they pointed out that it is difficult to completely dissect the impact of hemorrhage versus MI size on adverse remodeling, noting that it might be the case that more severe ischemia/reperfusion events are associated with large MI sizes and higher degree of hemorrhage.
However, they concluded that: “Intramyocardial hemorrhage represents the final frontier for preventing heart failure post-MI. It is readily detected using CMR, and clinical research of novel therapeutic approaches merits prioritization.”
This work was supported by grants from National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Dharmakumar and coauthor Robert Finney, PhD, have ownership interest in Cardiotheranostics. Dr. Berry is employed by the University of Glasgow, which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Causeway Therapeutics, Coroventis, Genentech, GlaxoSmithKline, HeartFlow, Menarini, Neovasc, Siemens Healthcare, and Valo Health.
A version of this article first appeared on Medscape.com.
Similar 10-year survival after CABG, PCI in heavy calcification
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Changing terminology in LGBTQ+ spaces: How to keep up with the lingo
For those of us who see adolescent patients on a regular basis, it seems that they use new vocabulary almost every day. While you may not need to know what “lit” means, you probably do need to understand terms used to describe your patients’ identities. At times it feels like we, as providers, have to be on TikTok to keep up with our patients, and while this may be an amusing way to educate ourselves, a judicious Google search can be much more helpful. The interesting part about LGBTQ+ terminology is that it stems from the community and thus is frequently updated to reflect our evolving understanding of gender, sexuality, and identity. That being said, it can make it difficult for those who are not plugged in to the community to keep up to date. While we have learned in medicine to use accurate terminology and appropriate three-letter acronyms (or “TLAs” as one of my residents referenced them when I was a medical student) to describe medical conditions, the LGBTQ+ community has its own set of terms and acronyms. These new words may seem daunting, but they are often based in Latin roots or prefixes such as a-, demi-, poly-, and pan-, which may be familiar to those of us who use plenty of other Latin-based terms in medicine and our everyday lives. By paying attention to how people define and use terminology, we can better recognize their true identities and become better providers.
The first, and perhaps most important, piece of advice is to maintain cultural humility. Know when to admit you don’t recognize a term and politely ask the definition. For example, the first time I heard the term “demiboy” I said “I’m not familiar with that word. Can you explain what it means to you?” Phrasing the question as such is also helpful in that it gives the individuals a chance to really define their identity. In addition, some words may be used differently by various individuals and by asking what the word means to them, you can have a better understanding of how they are using the terminology. In this particular instance, the patient felt more masculine, but not 100%, partway between agender (meaning having no gender identity) and being “all male.” By embracing cultural humility, we place the patients in the role of expert on their own identity and orientation. According to Maria Ruud, DNP, of the University of Minnesota, Minneapolis, cultural humility is the “ongoing self-reflection and education …[seeking] to gain an awareness of their own assumptions and biases that may contribute to health disparities.”1
Another reason it is important to keep up on the language is that some adolescents, particularly younger adolescents, may not be using the terminology correctly. It can be very helpful to know the difference between polyamorous and pansexual when a 12-year-old describes themselves as polyamorous (having consenting, nonmonogamous relationships) but provides the definition for pansexual (being attracted to all gender identities). Yes, this has happened to me, and yes, my resident was appropriately confused. Correcting someone else’s vocabulary can be tricky and even inappropriate or condescending; therefore, tread cautiously. It may be appropriate, however, to correct colleagues’ or even patients’ family members’ language if they are using terms that may be hurtful to your patients. I do not allow slurs in my clinic, and when parents are using incorrect pronouns on purpose, I will often let them know that it is my job to respect their child’s identity where it is in the moment and that they have asked me to use specific pronouns, so I will continue to refer to their child with those pronouns. Reflecting the language of the patient can be a powerful statement providing them with the autonomy that they deserve as burgeoning adults navigating the complicated journey of identity.
As providers who often have to defend ourselves against “Dr. Google,” we may be leery of just searching randomly for the definition of a new word and hoping a site is credible. One site that I have used repeatedly is www.itspronouncedmetrosexual.com by Sam Killermann,2 a gender and sexuality educator.
Mr. Killermann has also produced an E-book that is regularly updated to reflect changing terminology, which can be obtained for a small donation. As Mr. Killermann explains, “New language can be intimidating, and the language of gender and sexuality is often that.”3 In reality, the definitions aren’t scary and often the words can describe something you already know exists but didn’t recognize had a specific term. Not everyone can know every term and its definition; in fact, many members of the LGBTQ+ community don’t know or even understand every term. Below is a shortened list with some of the more common terms you may encounter; however, individuals may use them differently so it is never out of place to clarify your understanding of the term’s definition.
With these resources, along with cultural humility and reflection of others’ language, we can all start to have more meaningful conversations with our patients around their identity and relationships with others.
Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at [email protected].
References
1. Ruud M. Nursing for women’s health. 2018;22(3):255-63.
2. Killermann S. It’s Pronounced Metrosexual. 2020.
3. Killermann S. Defining LGBTQ+: A guide to gender and sexuality terminology. 2019, Feb 25.
4. The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered care for the lesbian, gay, bisexual, and transgender (LGBT) community: A field guide. Oak Brook, Ill. 2011.
5. LGBT health disparities. American Psychiatric Association Public Interest Government Relations Office. 2013 May.
6. Lawlis S et al. Health services for LGBTQ+ patients. Psychiatr Ann. 2019;49(10):426-35.
7. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
8. Center of Excellence for Transgender Health, department of family and community medicine, UCSF. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2016 Jun 17.
For those of us who see adolescent patients on a regular basis, it seems that they use new vocabulary almost every day. While you may not need to know what “lit” means, you probably do need to understand terms used to describe your patients’ identities. At times it feels like we, as providers, have to be on TikTok to keep up with our patients, and while this may be an amusing way to educate ourselves, a judicious Google search can be much more helpful. The interesting part about LGBTQ+ terminology is that it stems from the community and thus is frequently updated to reflect our evolving understanding of gender, sexuality, and identity. That being said, it can make it difficult for those who are not plugged in to the community to keep up to date. While we have learned in medicine to use accurate terminology and appropriate three-letter acronyms (or “TLAs” as one of my residents referenced them when I was a medical student) to describe medical conditions, the LGBTQ+ community has its own set of terms and acronyms. These new words may seem daunting, but they are often based in Latin roots or prefixes such as a-, demi-, poly-, and pan-, which may be familiar to those of us who use plenty of other Latin-based terms in medicine and our everyday lives. By paying attention to how people define and use terminology, we can better recognize their true identities and become better providers.
The first, and perhaps most important, piece of advice is to maintain cultural humility. Know when to admit you don’t recognize a term and politely ask the definition. For example, the first time I heard the term “demiboy” I said “I’m not familiar with that word. Can you explain what it means to you?” Phrasing the question as such is also helpful in that it gives the individuals a chance to really define their identity. In addition, some words may be used differently by various individuals and by asking what the word means to them, you can have a better understanding of how they are using the terminology. In this particular instance, the patient felt more masculine, but not 100%, partway between agender (meaning having no gender identity) and being “all male.” By embracing cultural humility, we place the patients in the role of expert on their own identity and orientation. According to Maria Ruud, DNP, of the University of Minnesota, Minneapolis, cultural humility is the “ongoing self-reflection and education …[seeking] to gain an awareness of their own assumptions and biases that may contribute to health disparities.”1
Another reason it is important to keep up on the language is that some adolescents, particularly younger adolescents, may not be using the terminology correctly. It can be very helpful to know the difference between polyamorous and pansexual when a 12-year-old describes themselves as polyamorous (having consenting, nonmonogamous relationships) but provides the definition for pansexual (being attracted to all gender identities). Yes, this has happened to me, and yes, my resident was appropriately confused. Correcting someone else’s vocabulary can be tricky and even inappropriate or condescending; therefore, tread cautiously. It may be appropriate, however, to correct colleagues’ or even patients’ family members’ language if they are using terms that may be hurtful to your patients. I do not allow slurs in my clinic, and when parents are using incorrect pronouns on purpose, I will often let them know that it is my job to respect their child’s identity where it is in the moment and that they have asked me to use specific pronouns, so I will continue to refer to their child with those pronouns. Reflecting the language of the patient can be a powerful statement providing them with the autonomy that they deserve as burgeoning adults navigating the complicated journey of identity.
As providers who often have to defend ourselves against “Dr. Google,” we may be leery of just searching randomly for the definition of a new word and hoping a site is credible. One site that I have used repeatedly is www.itspronouncedmetrosexual.com by Sam Killermann,2 a gender and sexuality educator.
Mr. Killermann has also produced an E-book that is regularly updated to reflect changing terminology, which can be obtained for a small donation. As Mr. Killermann explains, “New language can be intimidating, and the language of gender and sexuality is often that.”3 In reality, the definitions aren’t scary and often the words can describe something you already know exists but didn’t recognize had a specific term. Not everyone can know every term and its definition; in fact, many members of the LGBTQ+ community don’t know or even understand every term. Below is a shortened list with some of the more common terms you may encounter; however, individuals may use them differently so it is never out of place to clarify your understanding of the term’s definition.
With these resources, along with cultural humility and reflection of others’ language, we can all start to have more meaningful conversations with our patients around their identity and relationships with others.

Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at [email protected].
References
1. Ruud M. Nursing for women’s health. 2018;22(3):255-63.
2. Killermann S. It’s Pronounced Metrosexual. 2020.
3. Killermann S. Defining LGBTQ+: A guide to gender and sexuality terminology. 2019, Feb 25.
4. The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered care for the lesbian, gay, bisexual, and transgender (LGBT) community: A field guide. Oak Brook, Ill. 2011.
5. LGBT health disparities. American Psychiatric Association Public Interest Government Relations Office. 2013 May.
6. Lawlis S et al. Health services for LGBTQ+ patients. Psychiatr Ann. 2019;49(10):426-35.
7. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
8. Center of Excellence for Transgender Health, department of family and community medicine, UCSF. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2016 Jun 17.
For those of us who see adolescent patients on a regular basis, it seems that they use new vocabulary almost every day. While you may not need to know what “lit” means, you probably do need to understand terms used to describe your patients’ identities. At times it feels like we, as providers, have to be on TikTok to keep up with our patients, and while this may be an amusing way to educate ourselves, a judicious Google search can be much more helpful. The interesting part about LGBTQ+ terminology is that it stems from the community and thus is frequently updated to reflect our evolving understanding of gender, sexuality, and identity. That being said, it can make it difficult for those who are not plugged in to the community to keep up to date. While we have learned in medicine to use accurate terminology and appropriate three-letter acronyms (or “TLAs” as one of my residents referenced them when I was a medical student) to describe medical conditions, the LGBTQ+ community has its own set of terms and acronyms. These new words may seem daunting, but they are often based in Latin roots or prefixes such as a-, demi-, poly-, and pan-, which may be familiar to those of us who use plenty of other Latin-based terms in medicine and our everyday lives. By paying attention to how people define and use terminology, we can better recognize their true identities and become better providers.
The first, and perhaps most important, piece of advice is to maintain cultural humility. Know when to admit you don’t recognize a term and politely ask the definition. For example, the first time I heard the term “demiboy” I said “I’m not familiar with that word. Can you explain what it means to you?” Phrasing the question as such is also helpful in that it gives the individuals a chance to really define their identity. In addition, some words may be used differently by various individuals and by asking what the word means to them, you can have a better understanding of how they are using the terminology. In this particular instance, the patient felt more masculine, but not 100%, partway between agender (meaning having no gender identity) and being “all male.” By embracing cultural humility, we place the patients in the role of expert on their own identity and orientation. According to Maria Ruud, DNP, of the University of Minnesota, Minneapolis, cultural humility is the “ongoing self-reflection and education …[seeking] to gain an awareness of their own assumptions and biases that may contribute to health disparities.”1
Another reason it is important to keep up on the language is that some adolescents, particularly younger adolescents, may not be using the terminology correctly. It can be very helpful to know the difference between polyamorous and pansexual when a 12-year-old describes themselves as polyamorous (having consenting, nonmonogamous relationships) but provides the definition for pansexual (being attracted to all gender identities). Yes, this has happened to me, and yes, my resident was appropriately confused. Correcting someone else’s vocabulary can be tricky and even inappropriate or condescending; therefore, tread cautiously. It may be appropriate, however, to correct colleagues’ or even patients’ family members’ language if they are using terms that may be hurtful to your patients. I do not allow slurs in my clinic, and when parents are using incorrect pronouns on purpose, I will often let them know that it is my job to respect their child’s identity where it is in the moment and that they have asked me to use specific pronouns, so I will continue to refer to their child with those pronouns. Reflecting the language of the patient can be a powerful statement providing them with the autonomy that they deserve as burgeoning adults navigating the complicated journey of identity.
As providers who often have to defend ourselves against “Dr. Google,” we may be leery of just searching randomly for the definition of a new word and hoping a site is credible. One site that I have used repeatedly is www.itspronouncedmetrosexual.com by Sam Killermann,2 a gender and sexuality educator.
Mr. Killermann has also produced an E-book that is regularly updated to reflect changing terminology, which can be obtained for a small donation. As Mr. Killermann explains, “New language can be intimidating, and the language of gender and sexuality is often that.”3 In reality, the definitions aren’t scary and often the words can describe something you already know exists but didn’t recognize had a specific term. Not everyone can know every term and its definition; in fact, many members of the LGBTQ+ community don’t know or even understand every term. Below is a shortened list with some of the more common terms you may encounter; however, individuals may use them differently so it is never out of place to clarify your understanding of the term’s definition.
With these resources, along with cultural humility and reflection of others’ language, we can all start to have more meaningful conversations with our patients around their identity and relationships with others.

Dr. Lawlis is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, and an adolescent medicine specialist at OU Children’s. She has no relevant financial disclosures. Email her at [email protected].
References
1. Ruud M. Nursing for women’s health. 2018;22(3):255-63.
2. Killermann S. It’s Pronounced Metrosexual. 2020.
3. Killermann S. Defining LGBTQ+: A guide to gender and sexuality terminology. 2019, Feb 25.
4. The Joint Commission. Advancing effective communication, cultural competence, and patient- and family-centered care for the lesbian, gay, bisexual, and transgender (LGBT) community: A field guide. Oak Brook, Ill. 2011.
5. LGBT health disparities. American Psychiatric Association Public Interest Government Relations Office. 2013 May.
6. Lawlis S et al. Health services for LGBTQ+ patients. Psychiatr Ann. 2019;49(10):426-35.
7. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
8. Center of Excellence for Transgender Health, department of family and community medicine, UCSF. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2016 Jun 17.
Aquatic Antagonists: Jellyfish Stings
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6
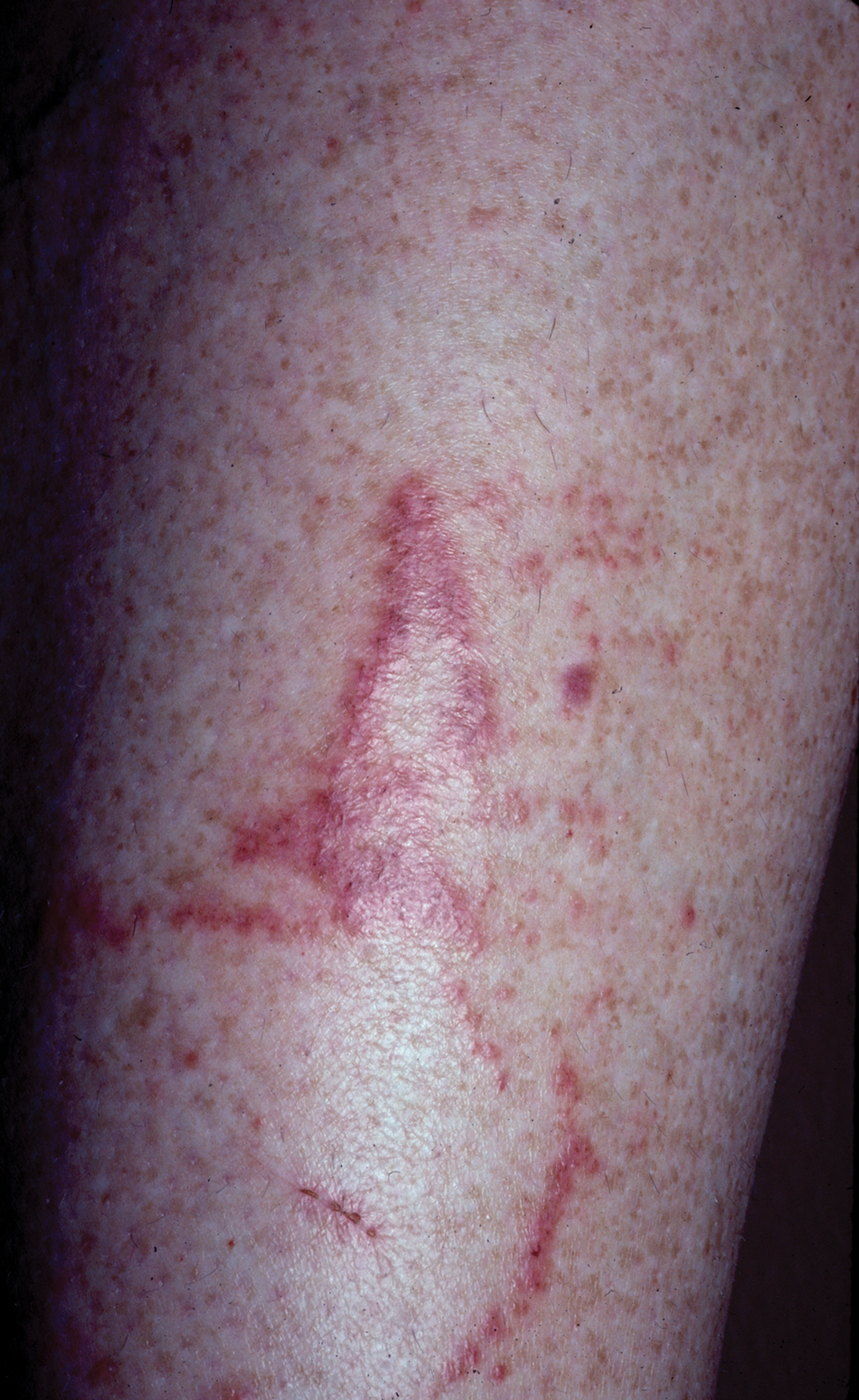
Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
Jellyfish stings are one of the most common marine injuries, with an estimated 150 million stings occurring annually worldwide.1 Most jellyfish stings result in painful localized skin reactions that are self-limited and can be treated with conservative measures including hot water immersion and topical anesthetics. Life-threatening systemic reactions (eg, anaphylaxis, Irukandji syndrome) can occur with some species.2-4 Mainstream media reports do not reflect the true incidence and variability of jellyfish-related injuries that are commonly encountered in the clinic.3
Characteristics of Jellyfish
There are roughly 10,000 known species of jellyfish, with approximately 100 of them posing danger to humans.5 Jellyfish belong to the phylum Cnidaria, which is comprised of 5 classes of both free-floating and sessile animals: Staurozoa (stauromedusae), Hydrozoa (hydroids, fire corals, and Portuguese man-of-war), Scyphozoa (true jellyfish), Anthozoa (corals and sea anemones), and Cubozoa (box jellyfish and Irukandji jellyfish).1,2,6 Jellyfish typically have several tentacles suspended from a free-floating gelatinous body or bell; these tentacles are covered with thousands of cells unique to Cnidaria called nematocytes or cnidocytes containing specialized stinging organelles known as nematocysts. When triggered by physical (eg, human or foreign-body contact) or chemical stimuli, each nematocyst ejects a hollow filament or barb externally, releasing venom into the victim.7,8

The scyphozoan, hydrozoan, and cubozoan life cycles generally consist of a bottom-dwelling, sessile polyp form that produces multiple free-swimming ephyrae through an asexual reproductive process called strobilation. These ephyrae grow into the fully mature medusae, recognizable as jellyfish (Figure 1).5 Additionally, jellyfish populations experience cycles of temporal and spatial population abundance and crashes known as jellyfish blooms. In 2017, Kaffenberger et al9 reviewed the shifting landscape of skin diseases in North America attributable to major changes in climate and weather patterns, including the rise in jellyfish blooms and envenomation outbreaks worldwide (eg, Physalia physalis [Portuguese man-of-war][Figure 2] along the southeastern US coastline, Porpita pacifica off Japanese beaches). Some research suggests jellyfish surges relate to climate change and human interactions with jellyfish habitats by way of eutrophication and fishing (removing predators of jellyfish).9,10

Clinical Presentation
Jellyfish injuries can vary greatly in clinical symptoms, but they do follow some basic patterns. The severity of pain and symptoms is related to the jellyfish species, the number of stinging cells (nematocysts) that are triggered, and the potency of the venom that is absorbed by the victim.11-13 Most stings are minor, and patients experience immediate localized pain with serpiginous raised erythematous or urticarial lesions following the distribution of tentacle contact; these lesions have been described as tentaclelike and resembling a string of beads (Figure 3).12 Pain usually lasts a couple hours, while the skin lesions can last hours to days and can even recur years later. This pattern fits that of the well-known hydrozoans P physalis and Physalia utriculus (bluebottle), which are endemic to the Atlantic and Indo-Pacific Oceans, respectively. The scyphozoan jellyfish causing similar presentations include Pelagia noctiluca (Mauve stinger), Aurelia aurita (Moon jellyfish), and Cyanea species. The cubozoan Chironex fleckeri (Australian box jellyfish or sea wasp) also causes tentaclelike stings but is widely considered the most dangerous jellyfish, as its venom is known to cause cardiac or respiratory arrest.4,11 More than 100 fatalities have been reported following severe envenomations from C fleckeri in Australian and Indo-Pacific waters.6

Stings from another box jellyfish species, Carukia barnesi, cause a unique presentation known as Irukandji syndrome. Carukia barnesi is a small box jellyfish with a bell measuring roughly 2 cm in diameter. It has nematocysts on both its bell and tentacles. It inhabits deeper waters and typically stings divers but also can wash ashore and injure beach tourists. Although Irukandji syndrome usually is associated with C barnesi, which is endemic to Northern Australian beaches, other jellyfish species including P physalis rarely have been linked to this potentially fatal syndrome.6,11 Unlike the immediate cutaneous and systemic findings described in C fleckeri encounters, symptoms of Irukandji-like stings can be delayed by up to 30 minutes. Patients may present with severe generalized pain (lower back, chest, headache), signs of excess catecholamine release (tachycardia, hypertension, anxiety, diaphoresis, agitation), or cardiopulmonary decompensation (arrhythmia, cardiac arrest, pulmonary edema).6,11,14.15 Anaphylactic reactions also have been reported in those sensitized by prior stings.16
Management
Prevention of drowning is key in all marine injuries. Rescuers should remove the individual from the water, establish the ABCs—airway, breathing, and circulation—and seek acute medical attention. If immediate resuscitation is not required, douse the wound as soon as possible with a solution that halts further nematocyst discharge, which may contain alcohol, vinegar, or bicarbonate, depending on the prevalent species. General guidance is available to providers through evidence-based, point-of-care databases including UpToDate and DynaMed, as well as through the American Heart Association (AHA) or a country’s equivalent council on emergency care if residing outside the United States. Pressure immobilization bandages as a means of decreasing venom redistribution is no longer recommended by the AHA because animal studies have shown increased nematocyst discharge after pressure application.17 As such, touching or applying pressure to the affected area is not recommended until after a proper rinse solution has been applied. Tentacles may be removed mechanically with gloved hands or sand and seawater with minimal compression or agitation.
When acetic acid is appropriate, such as for cubozoan stings, commercially available vinegar (5% acetic acid in the United States) is preferred.16,17 Tap water can cause discharge of nematocysts, and seawater is preferred when no other solution is available.18 Most marine venoms are heat labile. Immersion in hot water can produce pain relief, but ice can be just as efficacious and is preferred by some patients. Prior reports of patients stung by Physalia species demonstrated greater pain relief with hot water immersion compared to ice pack application.18,19
In the setting of anaphylaxis, patients should receive epinephrine and be transported to a hospital with appropriate hemodynamic monitoring and supportive care. If the species of jellyfish has been identified, species-specific antivenin also may be available in certain regions (eg, C fleckeri antivenin manufactured in Australia), but it is unclear if it improves outcomes when compared with supportive care alone.6,16
Conclusion
Following jellyfish stings, most skin lesions will spontaneously resolve. Patients likely will present days to weeks following the inciting event with mild cutaneous symptoms that are amenable to topical corticosteroids. Recurrent dermatitis following a jellyfish sting is uncommon and is thought to be due to an immunologic mechanism consistent with type IV hypersensitivity reactions. Patients may require multiple courses of treatment before complete resolution.20
Patient education regarding marine envenomation and mechanical barriers such as wetsuits or stinger suits can reduce the risk for injury from jellyfish stings. Sting-inhibiting lotions also are commercially available, though more research is needed.21 Many beaches that are known to harbor the dangerous box jellyfish provide stinger nets to direct travelers to safer waters. Complete avoidance during jellyfish season is recommended in highly endemic areas.1
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
- Cegolon L, Heymann WC, Lange JH, et al. Jellyfish stings and their management: a review. Mar Drugs. 2013;11:523-550.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Ward NT, Darracq MA, Tomaszewski C, et al. Evidence-based treatment of jellyfish stings in North America and Hawaii. Ann Emerg Med. 2012;60:399-414.
- Burnett JW, Calton GJ, Burnett HW. Jellyfish envenomation syndromes. J Am Acad Dermatol. 1986;14:100-106.
- Brotz L, Cheung WWL, Kleisner K, et al. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia. 2012;690:3-20.
- Ottuso PT. Aquatic antagonists: Cubozoan jellyfish (Chironex fleckeri and Carukia barnesi). Cutis. 2010;85:133-136.
- Lakkis NA, Maalouf GJ, Mahmassani DM. Jellyfish stings: a practical approach. Wilderness Environ Med. 2015;26:422-429.
- Li L, McGee RG, Isbister G, et al. Interventions for the symptoms and signs resulting from jellyfish stings. Cochrane Database Syst Rev. 2013;12:CD009688.
- Kaffenberger BH, Shetlar D, Norton SA, et al. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76:140-147.
- Purcell JE, Uye S, Lo W. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series. 2007;350:153-174.
- Berling I, Isbister G. Marine envenomations. Aust Fam Physician. 2015;44:28-32.
- Tibballs J, Yanagihara AA, Turner HC, et al. Immunological and toxinological responses to jellyfish stings. Inflamm Allergy Drug Targets. 2011;10:438-446.
- Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006;48:830-859.
- Stein MR, Marracini JV, Rothschild NE, et al. Fatal Portuguese man-o’-war (Physalia physalis) envenomation. Ann Emerg Med. 1989;18:312-315.
- Burnett JW, Gable WD. A fatal jellyfish envenomation by the Portuguese man-o’war. Toxicon. 1989;27:823-824.
- Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am. 2019;33:17-38.
- Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 suppl 2):S315-S367.
- Wilcox CL, Headlam JL, Doyle TK, et al. Assessing the efficacy of first-aid measures in Physalia sp. envenomation, using solution- and blood agarose-based models. Toxins (Basel). 2017;9:149.
- Wilcox CL, Yanagihara AA. Heated debates: hot-water immersion or ice packs as first aid for cnidarian envenomations? Toxins (Basel). 2016;8:97.
- Loredana Asztalos M, Rubin AI, Elenitsas R, et al. Recurrent dermatitis and dermal hypersensitivity following a jellyfish sting: a case report and review of literature. Pediatr Dermatol. 2014;31:217-219.
- Boulware DR. A randomized, controlled field trial for the prevention of jellyfish stings with a topical sting inhibitor. J Travel Med. 2006;13:166-171.
Practice Points
- Jellyfish stings occur an estimated 150 million times annually worldwide, with numbers expected to rise due to climate change.
- Most stings result in painful self-limited cutaneous symptoms that resolve spontaneously. Box jellyfish (Cubozoa) stings carry a greater risk for causing severe systemic reactions.
- Treatment of skin reactions includes removal of tentacles and hot water immersion. Vinegar dousing for at least 30 seconds is recommended for box jellyfish stings. Supportive care and monitoring for cardiovascular collapse are key. The role of antivenin is uncertain.
The child with hypertension: Diagnosis and management
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
Justin L. Berk, MD, MPH, MBA: Welcome back to The Cribsiders, our video recap of our pediatric medicine podcast. We interview leading experts in the field to bring clinical pearls and practice-changing knowledge, and answer lingering questions about core topics in pediatric medicine. Chris, what is our topic today?
Christopher J. Chiu, MD: I was really happy to be able to talk about our recent episode with Dr. Carissa Baker-Smith, a pediatric cardiologist and director of the Nemours preventive cardiology program. She helped us review the pediatric screening guidelines for blood pressure, including initial workup and treatment.
Dr. Berk: This was a really great episode that a lot of people found really helpful. What were some of the key takeaway pearls that you think listeners would be interested in?
Dr. Chiu: We talked about when and how we should be checking blood pressures in children. Blood pressure should be checked at every well-child visit starting at age 3. But if they have other risk factors like kidney disease or a condition such as coarctation of the aorta, then blood pressure should be checked at every visit.
Dr. Berk: One thing she spoke about was how blood pressures should be measured. How should we be checking blood pressures in the clinic?
Dr. Chiu: Clinic blood pressures are usually checked with oscillometric devices. They can differ by manufacturer, but basically they find a mean arterial pressure and then each device has a method of calculating systolic and diastolic pressures. Now after that, if the child’s blood pressure is maybe abnormal, you want to double-check a manual blood pressure using Korotkoff sounds to confirm the blood pressure.
She reminded us that blood pressure should be measured with the child sitting with their back supported, feet flat on the floor, and arm at the level of the heart. Make sure you use the right size cuff. The bladder of the cuff should be 40% of the width of the arm, and about 80%-100% of the arm circumference. She recommends sizing up if you have to.
Dr. Berk: Accuracy of blood pressure management was a really important point, especially for diagnosis at this stage. Can you walk us through what we learned about diagnosis of hypertension?
Dr. Chiu: The definitions of hypertension come from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Up until the age of 13, they define prehypertension as systolic and diastolic blood pressures between the 90th and 95th percentile, or if the blood pressure exceeds 120/80 mm Hg. Hypertension is defined when blood pressure reaches the 95th percentile. Now age 13 is when it gets a little hazy. Many changes in the guidelines happen at age 13, when hypertension starts being defined by adult guidelines. The 2017 adult hypertension guidelines define stage 1 hypertension as 130/89 to 139/89, and stage 2 hypertension as greater than 140/90.
Dr. Berk: How about workup of hypertension? The work of pediatric hypertension is always a little bit complex. What are some of the pearls you took away?
Dr. Chui: She talked about tailoring the workup to the child. So when we’re doing our workup, obviously physical exam should be the first thing we do. You have to assess and compare pulses, which is one of the most important parts of the initial evaluation. Obviously, looking at coarctation of the aorta, but also looking for things like a cushingoid appearance. If the child is less than 6 years of age, she recommends a referral to nephrology for more comprehensive renovascular workup, which probably will include renal ultrasound, urinalysis, metabolic panel, and thyroid studies.
We have to be cognizant of secondary causes of hypertension, such as endocrine tumors, hyperthyroidism, aortic disease, or even medication-induced hypertension. She told us that in the majority of these cases, especially with our obese older children, primary hypertension or essential hypertension is the most likely cause.
Dr. Berk: That was my big takeaway. If they’re really young, they need a big workup, but otherwise it is likely primary hypertension. What did we learn about treatment?
Dr. Chui: Just as we tailor our assessment to the child, we also have to tailor treatment. We know that lifestyle modification is usually the first line of treatment, especially for primary hypertension, and Dr. Baker-Smith tells us that we really need to perform counseling that meets the patient where they are. So if they like dancing to the newest TikTok trends or music videos, maybe we can encourage them to move more that way. Using our motivational interviewing skills is really key here.
If you want to start medication, Dr. Baker-Smith uses things like low-dose ACE inhibitors or calcium channel blockers, but obviously it’ll be tailored to the patient and any underlying conditions.
Dr. Berk: That’s great – a lot of wonderful pearls on the diagnosis and management of pediatric hypertension. Thank you for joining us for another video recap of The Cribsiders pediatric podcast. You can download the full podcast, Off the Cuff: Managing Pediatric Hypertension in Your Primary Care Clinic, on any podcast player, or check out our website at www.theCribsiders.com.
Christopher J. Chiu, MD, is assistant professor, department of internal medicine, division of general internal medicine, Ohio State University, Columbus; lead physician, general internal medicine, OSU Outpatient Care East; department of internal medicine, division of general internal medicine, Ohio State University Wexner Medical Center. Dr. Chiu has disclosed no relevant financial relationships. Justin L. Berk, MD, MPH, MBA, is assistant professor, department of medicine; assistant professor, department of pediatrics, Brown University, Providence, R.I.
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
Justin L. Berk, MD, MPH, MBA: Welcome back to The Cribsiders, our video recap of our pediatric medicine podcast. We interview leading experts in the field to bring clinical pearls and practice-changing knowledge, and answer lingering questions about core topics in pediatric medicine. Chris, what is our topic today?
Christopher J. Chiu, MD: I was really happy to be able to talk about our recent episode with Dr. Carissa Baker-Smith, a pediatric cardiologist and director of the Nemours preventive cardiology program. She helped us review the pediatric screening guidelines for blood pressure, including initial workup and treatment.
Dr. Berk: This was a really great episode that a lot of people found really helpful. What were some of the key takeaway pearls that you think listeners would be interested in?
Dr. Chiu: We talked about when and how we should be checking blood pressures in children. Blood pressure should be checked at every well-child visit starting at age 3. But if they have other risk factors like kidney disease or a condition such as coarctation of the aorta, then blood pressure should be checked at every visit.
Dr. Berk: One thing she spoke about was how blood pressures should be measured. How should we be checking blood pressures in the clinic?
Dr. Chiu: Clinic blood pressures are usually checked with oscillometric devices. They can differ by manufacturer, but basically they find a mean arterial pressure and then each device has a method of calculating systolic and diastolic pressures. Now after that, if the child’s blood pressure is maybe abnormal, you want to double-check a manual blood pressure using Korotkoff sounds to confirm the blood pressure.
She reminded us that blood pressure should be measured with the child sitting with their back supported, feet flat on the floor, and arm at the level of the heart. Make sure you use the right size cuff. The bladder of the cuff should be 40% of the width of the arm, and about 80%-100% of the arm circumference. She recommends sizing up if you have to.
Dr. Berk: Accuracy of blood pressure management was a really important point, especially for diagnosis at this stage. Can you walk us through what we learned about diagnosis of hypertension?
Dr. Chiu: The definitions of hypertension come from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Up until the age of 13, they define prehypertension as systolic and diastolic blood pressures between the 90th and 95th percentile, or if the blood pressure exceeds 120/80 mm Hg. Hypertension is defined when blood pressure reaches the 95th percentile. Now age 13 is when it gets a little hazy. Many changes in the guidelines happen at age 13, when hypertension starts being defined by adult guidelines. The 2017 adult hypertension guidelines define stage 1 hypertension as 130/89 to 139/89, and stage 2 hypertension as greater than 140/90.
Dr. Berk: How about workup of hypertension? The work of pediatric hypertension is always a little bit complex. What are some of the pearls you took away?
Dr. Chui: She talked about tailoring the workup to the child. So when we’re doing our workup, obviously physical exam should be the first thing we do. You have to assess and compare pulses, which is one of the most important parts of the initial evaluation. Obviously, looking at coarctation of the aorta, but also looking for things like a cushingoid appearance. If the child is less than 6 years of age, she recommends a referral to nephrology for more comprehensive renovascular workup, which probably will include renal ultrasound, urinalysis, metabolic panel, and thyroid studies.
We have to be cognizant of secondary causes of hypertension, such as endocrine tumors, hyperthyroidism, aortic disease, or even medication-induced hypertension. She told us that in the majority of these cases, especially with our obese older children, primary hypertension or essential hypertension is the most likely cause.
Dr. Berk: That was my big takeaway. If they’re really young, they need a big workup, but otherwise it is likely primary hypertension. What did we learn about treatment?
Dr. Chui: Just as we tailor our assessment to the child, we also have to tailor treatment. We know that lifestyle modification is usually the first line of treatment, especially for primary hypertension, and Dr. Baker-Smith tells us that we really need to perform counseling that meets the patient where they are. So if they like dancing to the newest TikTok trends or music videos, maybe we can encourage them to move more that way. Using our motivational interviewing skills is really key here.
If you want to start medication, Dr. Baker-Smith uses things like low-dose ACE inhibitors or calcium channel blockers, but obviously it’ll be tailored to the patient and any underlying conditions.
Dr. Berk: That’s great – a lot of wonderful pearls on the diagnosis and management of pediatric hypertension. Thank you for joining us for another video recap of The Cribsiders pediatric podcast. You can download the full podcast, Off the Cuff: Managing Pediatric Hypertension in Your Primary Care Clinic, on any podcast player, or check out our website at www.theCribsiders.com.
Christopher J. Chiu, MD, is assistant professor, department of internal medicine, division of general internal medicine, Ohio State University, Columbus; lead physician, general internal medicine, OSU Outpatient Care East; department of internal medicine, division of general internal medicine, Ohio State University Wexner Medical Center. Dr. Chiu has disclosed no relevant financial relationships. Justin L. Berk, MD, MPH, MBA, is assistant professor, department of medicine; assistant professor, department of pediatrics, Brown University, Providence, R.I.
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
Justin L. Berk, MD, MPH, MBA: Welcome back to The Cribsiders, our video recap of our pediatric medicine podcast. We interview leading experts in the field to bring clinical pearls and practice-changing knowledge, and answer lingering questions about core topics in pediatric medicine. Chris, what is our topic today?
Christopher J. Chiu, MD: I was really happy to be able to talk about our recent episode with Dr. Carissa Baker-Smith, a pediatric cardiologist and director of the Nemours preventive cardiology program. She helped us review the pediatric screening guidelines for blood pressure, including initial workup and treatment.
Dr. Berk: This was a really great episode that a lot of people found really helpful. What were some of the key takeaway pearls that you think listeners would be interested in?
Dr. Chiu: We talked about when and how we should be checking blood pressures in children. Blood pressure should be checked at every well-child visit starting at age 3. But if they have other risk factors like kidney disease or a condition such as coarctation of the aorta, then blood pressure should be checked at every visit.
Dr. Berk: One thing she spoke about was how blood pressures should be measured. How should we be checking blood pressures in the clinic?
Dr. Chiu: Clinic blood pressures are usually checked with oscillometric devices. They can differ by manufacturer, but basically they find a mean arterial pressure and then each device has a method of calculating systolic and diastolic pressures. Now after that, if the child’s blood pressure is maybe abnormal, you want to double-check a manual blood pressure using Korotkoff sounds to confirm the blood pressure.
She reminded us that blood pressure should be measured with the child sitting with their back supported, feet flat on the floor, and arm at the level of the heart. Make sure you use the right size cuff. The bladder of the cuff should be 40% of the width of the arm, and about 80%-100% of the arm circumference. She recommends sizing up if you have to.
Dr. Berk: Accuracy of blood pressure management was a really important point, especially for diagnosis at this stage. Can you walk us through what we learned about diagnosis of hypertension?
Dr. Chiu: The definitions of hypertension come from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Up until the age of 13, they define prehypertension as systolic and diastolic blood pressures between the 90th and 95th percentile, or if the blood pressure exceeds 120/80 mm Hg. Hypertension is defined when blood pressure reaches the 95th percentile. Now age 13 is when it gets a little hazy. Many changes in the guidelines happen at age 13, when hypertension starts being defined by adult guidelines. The 2017 adult hypertension guidelines define stage 1 hypertension as 130/89 to 139/89, and stage 2 hypertension as greater than 140/90.
Dr. Berk: How about workup of hypertension? The work of pediatric hypertension is always a little bit complex. What are some of the pearls you took away?
Dr. Chui: She talked about tailoring the workup to the child. So when we’re doing our workup, obviously physical exam should be the first thing we do. You have to assess and compare pulses, which is one of the most important parts of the initial evaluation. Obviously, looking at coarctation of the aorta, but also looking for things like a cushingoid appearance. If the child is less than 6 years of age, she recommends a referral to nephrology for more comprehensive renovascular workup, which probably will include renal ultrasound, urinalysis, metabolic panel, and thyroid studies.
We have to be cognizant of secondary causes of hypertension, such as endocrine tumors, hyperthyroidism, aortic disease, or even medication-induced hypertension. She told us that in the majority of these cases, especially with our obese older children, primary hypertension or essential hypertension is the most likely cause.
Dr. Berk: That was my big takeaway. If they’re really young, they need a big workup, but otherwise it is likely primary hypertension. What did we learn about treatment?
Dr. Chui: Just as we tailor our assessment to the child, we also have to tailor treatment. We know that lifestyle modification is usually the first line of treatment, especially for primary hypertension, and Dr. Baker-Smith tells us that we really need to perform counseling that meets the patient where they are. So if they like dancing to the newest TikTok trends or music videos, maybe we can encourage them to move more that way. Using our motivational interviewing skills is really key here.
If you want to start medication, Dr. Baker-Smith uses things like low-dose ACE inhibitors or calcium channel blockers, but obviously it’ll be tailored to the patient and any underlying conditions.
Dr. Berk: That’s great – a lot of wonderful pearls on the diagnosis and management of pediatric hypertension. Thank you for joining us for another video recap of The Cribsiders pediatric podcast. You can download the full podcast, Off the Cuff: Managing Pediatric Hypertension in Your Primary Care Clinic, on any podcast player, or check out our website at www.theCribsiders.com.
Christopher J. Chiu, MD, is assistant professor, department of internal medicine, division of general internal medicine, Ohio State University, Columbus; lead physician, general internal medicine, OSU Outpatient Care East; department of internal medicine, division of general internal medicine, Ohio State University Wexner Medical Center. Dr. Chiu has disclosed no relevant financial relationships. Justin L. Berk, MD, MPH, MBA, is assistant professor, department of medicine; assistant professor, department of pediatrics, Brown University, Providence, R.I.
Scheduled Acetaminophen to Minimize Neuropsychiatric Symptoms in Wernicke-Korsakoff Syndrome
To manage the physical, cognitive, and emotional symptoms of a veteran hospitalized for Wernicke-Korsakoff syndrome secondary to chronic alcohol overuse, acetaminophen was administered in place of psychoactive medications.
Alcohol is the most common substance misused by veterans. 1 Veterans may m isuse alcohol as a result of mental illness or posttraumatic stress disorder (PTSD), having difficulties adjusting to civilian life, or because of heavy drinking habits acquired before leaving active duty. 2 One potential long-term effect of chronic alcohol misuse is Wernicke-Korsakoff syndrome (WKS), a neuropsychiatric condition secondary to a deficiency of thiamine. 3 The disease is characterized by altered mental status, oculomotor findings, and ataxia. 3 Patients with WKS may exhibit challenging behaviors, including aggression, disinhibition, and lack of awareness of their illness. 4 Due to long-standing cognitive and physical deficits, many patients require lifelong care with a focus on a palliative approach. 3
The mainstay of pharmacologic management for the neuropsychiatric symptoms of WKS continues to be psychoactive medications, such as antipsychotics, benzodiazepines, antidepressants, and anticonvulsant medications.4-6 Though atypical antipsychotic medications remain the most widely used, they have a high adverse effect (AE) profile.5,6 Among the potential AEs are metabolic syndrome, anticholinergic effects, QTc prolongation, orthostatic hypotension, extrapyramidal effects, sedation, and falls. There also is a US Food and Drug Administration boxed warning for increased risk of mortality.7 With the goal of improving and maintaining patient safety, pharmacologic interventions with lower AEs may be beneficial in the management of the neuropsychiatric symptoms of WKS.
This case describes a veteran who was initially hospitalized due to confusion, ataxia, and nystagmus secondary to chronic alcohol overuse. The aim of the case was to consider the use of acetaminophen in place of psychoactive medications as a way to manage neuropsychiatric symptoms of WKS even when pain was not present.
Case Presentation
A veteran presented to the local US Department of Veterans Affairs (VA) emergency department (ED) due to their spouse’s concern of acute onset confusion and ambulatory difficulties. The veteran’s medical history included extensive alcohol misuse, mild asthma, and diet-controlled hyperlipidemia. On initial evaluation, the veteran displayed symptoms of ataxia and confusion. When asked why the veteran was at the ED, the response was, “I just came to the hospital to find my sister.” Based on their medical history, clinical evaluation, and altered mental status, the veteran was admitted to the acute care medical service with a presumptive diagnosis of WKS.
On admission, the laboratory evaluation revealed normal alanine transaminase (ALT) and aspartate transaminase (AST) levels but markedly elevated γ-glutamyl transferase (GGT) consistent with alcohol toxicity. COVID-19 testing was negative. Magnetic resonance imaging (MRI) of the brain revealed evidence of alterations in the mammillary bodies and moderately severe cortical and cerebellar volume loss suggestive of long-standing alcohol use.
The veteran was hospitalized for 12 days and treated with high-dose IV thiamine, which resulted in improvement of their ophthalmic disorder (nystagmus) and ataxia. However, they continued to exhibit poor recall, confusion, and occasional agitation characterized by verbal outbursts and aggression toward the staff.
The veteran’s spouse worked full time and did not feel capable of providing the necessary follow-up care at home. The safest discharge plan found was to transfer the veteran to the local VA community living center (CLC) for physical therapy and further support of their marked cognitive decline and agitation.
Following admission to the CLC, the veteran was placed in a secured memory unit with staff trained specifically on management of veterans with cognitive impairment and behavioral concerns. As the veteran did not have decisional capacity on admission, the staff arranged a meeting with the spouse. Based on that conversation, the goals of care were to focus on a palliative approach and the hope that the veteran would one day be able to return home to their spouse.
At the CLC, the veteran was initially treated with thiamine 200 mg orally once daily and albuterol inhaler as needed. A clinical psychologist performed a comprehensive psychological evaluation on admission, which confirmed evidence of WKS with symptoms, including confusion, disorientation, and confabulation. There was no evidence of cultural diversity factors regarding the veteran’s delusional beliefs.
After the first full day in the CLC, the nursing staff observed anger and agitation that seemed to start midafternoon and continued until around dinnertime. The veteran displayed verbal outbursts, refusal to cooperate with the staff, and multiple attempts to leave the CLC. With the guidance of a geriatric psychiatrist, risperidone 1 mg once daily as needed was initiated, and staff continued with verbal redirection, both with limited efficacy. After 3 days, due to safety concerns for the veteran, other CLC patients, and CLC staff, risperidone dosing was increased to 1 mg twice daily, which had limited efficacy. Lorazepam 1 mg once daily also was added. A careful medication review was performed to minimize any potential AEs or interactions that might have contributed to the veteran’s behavior, but no pharmacologic interventions were found to fully abate their behavioral issues.
After 5 weeks of ongoing intermittent behavioral issues, the medical team again met to discuss new treatment options.A case reported by Husebo and colleagues used scheduled acetaminophen to help relieve neuropsychiatric symptoms of dementia in a patient who exhibited similar behavioral issues and did not respond well to antipsychotics or benzodiazepines.8 Although our veteran did not express or exhibit obvious pain, the medical team chose to trial this intervention, and the veteran was started on acetaminophen 650 mg orally 3 times daily. A comprehensive metabolic panel, including GGT and thyroid-stimulating hormone, was performed before starting acetaminophen; no abnormalities were noted. The clinical examination did not reveal physical abnormalities other than ataxia.
After 5 days of therapy with the scheduled acetaminophen, the veteran’s clinical behavior dramatically improved. The veteran exhibited infrequent agitated behavior and became cooperative with staff. Three days later, the scheduled lorazepam was discontinued, and eventually they were tapered off risperidone. One month after starting scheduled acetaminophen, the veteran had improved to a point where the staff determined a safe discharge plan could be initiated. The veteran’s nystagmus resolved and behavioral issues improved, although cognitive impairment persisted.
Due to COVID-19, a teleconference was scheduled with the veteran’s spouse to discuss a discharge plan. The spouse was pleased that the veteran had progressed adequately both functionally and behaviorally to make a safe discharge home possible. The spouse arranged to take family leave from their job to help support the veteran after discharge. The veteran was able to return home with a safe discharge plan 1 week later. The acetaminophen was continued with twice-daily dosing and was continued because there were no new behavioral issues. This was done to enhance postfacility adherence and minimize the risk of drug-drug interactions. Attempts to follow up with the veteran postdischarge were unfortunately unsuccessful as the family lived out of the local area.
Discussion
Alcohol misuse is a common finding in many US veterans, as well as in the general population.1,3 As a result, it is not uncommon to see patients with physical and psychological symptoms related to this abuse. Many of these patients will become verbally and physically abusive, thus having appropriate pharmacologic and nonpharmacologic interventions is important.
In this case study, the veteran was diagnosed with WKS and exhibited physical, cognitive, and emotional symptoms consistent with this disease. Although the physical symptoms improved with thiamine and abstinence from alcohol, their cognitive impairment, verbal outbursts, and aggressive demeanor persisted.
After using antipsychotic and anxiolytic medications with minimal clinical improvement, a trial of acetaminophen 650 mg 3 times daily was instituted. The patient’s behavior improved; demeanor became calmer, and they were easily redirected by the nursing staff. Psychological support was again employed, which enhanced and supported the veteran’s calmer demeanor. Although there is limited medical literature on the use of acetaminophen in clinical situations not related to pain, there has been research documenting its effect on social interaction.9,10
Acetaminophen is an analgesic medication that acts through central neural mechanisms. It has been hypothesized that social and physical pain rely on shared neurochemical underpinnings, and some of the regions of the brain involved in affective experience of physical pain also have been found to be involved in the experience of social pain.11 Acetaminophen may impact an individual’s social well-being as social pain processes.11 It has been shown to blunt reactivity to both physical pain as well as negative stimuli.11
Conclusions
A 2019 survey on alcohol and drug use found 5.6% of adults aged ≥ 18 have an alcohol use disorder.12 In severe cases, this can result in WKS. Although replacement of thiamine is critical for physical improvement, psychological deficits may persist. Small studies have advanced the concept of using scheduled acetaminophen even when the patient is not verbalizing or displaying pain.13 Although more research needs to be done on this topic, this palliative approach may be worth considering, especially if the risks of antipsychotics and anxiolytics outweigh the benefits.
1. National Institute on Drug Abuse. Substance use and military life drug facts. Published October 2019. Accessed November 10, 2021. https://www.drugabuse.gov/publications/drugfacts/substance-use-military-life
2. National Veterans Foundation. What statistics show about veteran substance abuse and why proper treatment is important. Published March 30, 2016. Accessed November 10, 2021. https://nvf.org/veteran-substance-abuse-statistics
3. National Center for Biotechnology Information. Korsakoff syndrome. Updated July 10, 2020. Accessed November 10, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539854
4. Gerridzen IJ, Goossensen MA. Patients with Korsakoff syndrome in nursing homes: characteristics, comorbidity, and use of psychotropic drugs. Int Psychogeriatr. 2014;26(1):115-121. doi:10.1017/S1041610213001543
5. Press D, Alexander M. Management of neuropsychiatric symptoms of dementia. Updated October 2021. Accessed November 10, 2021. https://www.uptodate.com/contents/management-of-neuropsychiatric-symptoms-of-dementia
6. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: Managing safety concerns. Am J Psychiatry. 2012;169(9):900-906. doi:10.1176/appi.ajp.2012.12030342
7. Jibson MD. Second-generation antipsychotic medications: pharmacology, administration, and side effects. https://www.uptodate.com/contents/second-generation-antipsychotic-medications-pharmacology-administration-and-side-effects
8. Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi:10.1136/bmj.d4065
9. Fung K, Alden LE. Once hurt, twice shy: social pain contributes to social anxiety. Emotion. 2017;(2):231-239. doi:10.1037/emo0000223
10. Roberts ID, Krajbich I, Cheavens JS, Campo JV, Way BM. Acetaminophen Reduces Distrust in Individuals with Borderline Personality Disorder Features. Clin Psychol Sci. 2018;6(1):145-154. doi:10.1177/2167702617731374
11. Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-937. doi:10.1177/0956797610374741
12. National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. Updated June 2021. Accessed November 2, 202November 10, 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
13. Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatrics Soc. 2005;53(11):1921-9. doi:10.1111/j.1532-5415.2005.53572.x
To manage the physical, cognitive, and emotional symptoms of a veteran hospitalized for Wernicke-Korsakoff syndrome secondary to chronic alcohol overuse, acetaminophen was administered in place of psychoactive medications.
To manage the physical, cognitive, and emotional symptoms of a veteran hospitalized for Wernicke-Korsakoff syndrome secondary to chronic alcohol overuse, acetaminophen was administered in place of psychoactive medications.
Alcohol is the most common substance misused by veterans. 1 Veterans may m isuse alcohol as a result of mental illness or posttraumatic stress disorder (PTSD), having difficulties adjusting to civilian life, or because of heavy drinking habits acquired before leaving active duty. 2 One potential long-term effect of chronic alcohol misuse is Wernicke-Korsakoff syndrome (WKS), a neuropsychiatric condition secondary to a deficiency of thiamine. 3 The disease is characterized by altered mental status, oculomotor findings, and ataxia. 3 Patients with WKS may exhibit challenging behaviors, including aggression, disinhibition, and lack of awareness of their illness. 4 Due to long-standing cognitive and physical deficits, many patients require lifelong care with a focus on a palliative approach. 3
The mainstay of pharmacologic management for the neuropsychiatric symptoms of WKS continues to be psychoactive medications, such as antipsychotics, benzodiazepines, antidepressants, and anticonvulsant medications.4-6 Though atypical antipsychotic medications remain the most widely used, they have a high adverse effect (AE) profile.5,6 Among the potential AEs are metabolic syndrome, anticholinergic effects, QTc prolongation, orthostatic hypotension, extrapyramidal effects, sedation, and falls. There also is a US Food and Drug Administration boxed warning for increased risk of mortality.7 With the goal of improving and maintaining patient safety, pharmacologic interventions with lower AEs may be beneficial in the management of the neuropsychiatric symptoms of WKS.
This case describes a veteran who was initially hospitalized due to confusion, ataxia, and nystagmus secondary to chronic alcohol overuse. The aim of the case was to consider the use of acetaminophen in place of psychoactive medications as a way to manage neuropsychiatric symptoms of WKS even when pain was not present.
Case Presentation
A veteran presented to the local US Department of Veterans Affairs (VA) emergency department (ED) due to their spouse’s concern of acute onset confusion and ambulatory difficulties. The veteran’s medical history included extensive alcohol misuse, mild asthma, and diet-controlled hyperlipidemia. On initial evaluation, the veteran displayed symptoms of ataxia and confusion. When asked why the veteran was at the ED, the response was, “I just came to the hospital to find my sister.” Based on their medical history, clinical evaluation, and altered mental status, the veteran was admitted to the acute care medical service with a presumptive diagnosis of WKS.
On admission, the laboratory evaluation revealed normal alanine transaminase (ALT) and aspartate transaminase (AST) levels but markedly elevated γ-glutamyl transferase (GGT) consistent with alcohol toxicity. COVID-19 testing was negative. Magnetic resonance imaging (MRI) of the brain revealed evidence of alterations in the mammillary bodies and moderately severe cortical and cerebellar volume loss suggestive of long-standing alcohol use.
The veteran was hospitalized for 12 days and treated with high-dose IV thiamine, which resulted in improvement of their ophthalmic disorder (nystagmus) and ataxia. However, they continued to exhibit poor recall, confusion, and occasional agitation characterized by verbal outbursts and aggression toward the staff.
The veteran’s spouse worked full time and did not feel capable of providing the necessary follow-up care at home. The safest discharge plan found was to transfer the veteran to the local VA community living center (CLC) for physical therapy and further support of their marked cognitive decline and agitation.
Following admission to the CLC, the veteran was placed in a secured memory unit with staff trained specifically on management of veterans with cognitive impairment and behavioral concerns. As the veteran did not have decisional capacity on admission, the staff arranged a meeting with the spouse. Based on that conversation, the goals of care were to focus on a palliative approach and the hope that the veteran would one day be able to return home to their spouse.
At the CLC, the veteran was initially treated with thiamine 200 mg orally once daily and albuterol inhaler as needed. A clinical psychologist performed a comprehensive psychological evaluation on admission, which confirmed evidence of WKS with symptoms, including confusion, disorientation, and confabulation. There was no evidence of cultural diversity factors regarding the veteran’s delusional beliefs.
After the first full day in the CLC, the nursing staff observed anger and agitation that seemed to start midafternoon and continued until around dinnertime. The veteran displayed verbal outbursts, refusal to cooperate with the staff, and multiple attempts to leave the CLC. With the guidance of a geriatric psychiatrist, risperidone 1 mg once daily as needed was initiated, and staff continued with verbal redirection, both with limited efficacy. After 3 days, due to safety concerns for the veteran, other CLC patients, and CLC staff, risperidone dosing was increased to 1 mg twice daily, which had limited efficacy. Lorazepam 1 mg once daily also was added. A careful medication review was performed to minimize any potential AEs or interactions that might have contributed to the veteran’s behavior, but no pharmacologic interventions were found to fully abate their behavioral issues.
After 5 weeks of ongoing intermittent behavioral issues, the medical team again met to discuss new treatment options.A case reported by Husebo and colleagues used scheduled acetaminophen to help relieve neuropsychiatric symptoms of dementia in a patient who exhibited similar behavioral issues and did not respond well to antipsychotics or benzodiazepines.8 Although our veteran did not express or exhibit obvious pain, the medical team chose to trial this intervention, and the veteran was started on acetaminophen 650 mg orally 3 times daily. A comprehensive metabolic panel, including GGT and thyroid-stimulating hormone, was performed before starting acetaminophen; no abnormalities were noted. The clinical examination did not reveal physical abnormalities other than ataxia.
After 5 days of therapy with the scheduled acetaminophen, the veteran’s clinical behavior dramatically improved. The veteran exhibited infrequent agitated behavior and became cooperative with staff. Three days later, the scheduled lorazepam was discontinued, and eventually they were tapered off risperidone. One month after starting scheduled acetaminophen, the veteran had improved to a point where the staff determined a safe discharge plan could be initiated. The veteran’s nystagmus resolved and behavioral issues improved, although cognitive impairment persisted.
Due to COVID-19, a teleconference was scheduled with the veteran’s spouse to discuss a discharge plan. The spouse was pleased that the veteran had progressed adequately both functionally and behaviorally to make a safe discharge home possible. The spouse arranged to take family leave from their job to help support the veteran after discharge. The veteran was able to return home with a safe discharge plan 1 week later. The acetaminophen was continued with twice-daily dosing and was continued because there were no new behavioral issues. This was done to enhance postfacility adherence and minimize the risk of drug-drug interactions. Attempts to follow up with the veteran postdischarge were unfortunately unsuccessful as the family lived out of the local area.
Discussion
Alcohol misuse is a common finding in many US veterans, as well as in the general population.1,3 As a result, it is not uncommon to see patients with physical and psychological symptoms related to this abuse. Many of these patients will become verbally and physically abusive, thus having appropriate pharmacologic and nonpharmacologic interventions is important.
In this case study, the veteran was diagnosed with WKS and exhibited physical, cognitive, and emotional symptoms consistent with this disease. Although the physical symptoms improved with thiamine and abstinence from alcohol, their cognitive impairment, verbal outbursts, and aggressive demeanor persisted.
After using antipsychotic and anxiolytic medications with minimal clinical improvement, a trial of acetaminophen 650 mg 3 times daily was instituted. The patient’s behavior improved; demeanor became calmer, and they were easily redirected by the nursing staff. Psychological support was again employed, which enhanced and supported the veteran’s calmer demeanor. Although there is limited medical literature on the use of acetaminophen in clinical situations not related to pain, there has been research documenting its effect on social interaction.9,10
Acetaminophen is an analgesic medication that acts through central neural mechanisms. It has been hypothesized that social and physical pain rely on shared neurochemical underpinnings, and some of the regions of the brain involved in affective experience of physical pain also have been found to be involved in the experience of social pain.11 Acetaminophen may impact an individual’s social well-being as social pain processes.11 It has been shown to blunt reactivity to both physical pain as well as negative stimuli.11
Conclusions
A 2019 survey on alcohol and drug use found 5.6% of adults aged ≥ 18 have an alcohol use disorder.12 In severe cases, this can result in WKS. Although replacement of thiamine is critical for physical improvement, psychological deficits may persist. Small studies have advanced the concept of using scheduled acetaminophen even when the patient is not verbalizing or displaying pain.13 Although more research needs to be done on this topic, this palliative approach may be worth considering, especially if the risks of antipsychotics and anxiolytics outweigh the benefits.
Alcohol is the most common substance misused by veterans. 1 Veterans may m isuse alcohol as a result of mental illness or posttraumatic stress disorder (PTSD), having difficulties adjusting to civilian life, or because of heavy drinking habits acquired before leaving active duty. 2 One potential long-term effect of chronic alcohol misuse is Wernicke-Korsakoff syndrome (WKS), a neuropsychiatric condition secondary to a deficiency of thiamine. 3 The disease is characterized by altered mental status, oculomotor findings, and ataxia. 3 Patients with WKS may exhibit challenging behaviors, including aggression, disinhibition, and lack of awareness of their illness. 4 Due to long-standing cognitive and physical deficits, many patients require lifelong care with a focus on a palliative approach. 3
The mainstay of pharmacologic management for the neuropsychiatric symptoms of WKS continues to be psychoactive medications, such as antipsychotics, benzodiazepines, antidepressants, and anticonvulsant medications.4-6 Though atypical antipsychotic medications remain the most widely used, they have a high adverse effect (AE) profile.5,6 Among the potential AEs are metabolic syndrome, anticholinergic effects, QTc prolongation, orthostatic hypotension, extrapyramidal effects, sedation, and falls. There also is a US Food and Drug Administration boxed warning for increased risk of mortality.7 With the goal of improving and maintaining patient safety, pharmacologic interventions with lower AEs may be beneficial in the management of the neuropsychiatric symptoms of WKS.
This case describes a veteran who was initially hospitalized due to confusion, ataxia, and nystagmus secondary to chronic alcohol overuse. The aim of the case was to consider the use of acetaminophen in place of psychoactive medications as a way to manage neuropsychiatric symptoms of WKS even when pain was not present.
Case Presentation
A veteran presented to the local US Department of Veterans Affairs (VA) emergency department (ED) due to their spouse’s concern of acute onset confusion and ambulatory difficulties. The veteran’s medical history included extensive alcohol misuse, mild asthma, and diet-controlled hyperlipidemia. On initial evaluation, the veteran displayed symptoms of ataxia and confusion. When asked why the veteran was at the ED, the response was, “I just came to the hospital to find my sister.” Based on their medical history, clinical evaluation, and altered mental status, the veteran was admitted to the acute care medical service with a presumptive diagnosis of WKS.
On admission, the laboratory evaluation revealed normal alanine transaminase (ALT) and aspartate transaminase (AST) levels but markedly elevated γ-glutamyl transferase (GGT) consistent with alcohol toxicity. COVID-19 testing was negative. Magnetic resonance imaging (MRI) of the brain revealed evidence of alterations in the mammillary bodies and moderately severe cortical and cerebellar volume loss suggestive of long-standing alcohol use.
The veteran was hospitalized for 12 days and treated with high-dose IV thiamine, which resulted in improvement of their ophthalmic disorder (nystagmus) and ataxia. However, they continued to exhibit poor recall, confusion, and occasional agitation characterized by verbal outbursts and aggression toward the staff.
The veteran’s spouse worked full time and did not feel capable of providing the necessary follow-up care at home. The safest discharge plan found was to transfer the veteran to the local VA community living center (CLC) for physical therapy and further support of their marked cognitive decline and agitation.
Following admission to the CLC, the veteran was placed in a secured memory unit with staff trained specifically on management of veterans with cognitive impairment and behavioral concerns. As the veteran did not have decisional capacity on admission, the staff arranged a meeting with the spouse. Based on that conversation, the goals of care were to focus on a palliative approach and the hope that the veteran would one day be able to return home to their spouse.
At the CLC, the veteran was initially treated with thiamine 200 mg orally once daily and albuterol inhaler as needed. A clinical psychologist performed a comprehensive psychological evaluation on admission, which confirmed evidence of WKS with symptoms, including confusion, disorientation, and confabulation. There was no evidence of cultural diversity factors regarding the veteran’s delusional beliefs.
After the first full day in the CLC, the nursing staff observed anger and agitation that seemed to start midafternoon and continued until around dinnertime. The veteran displayed verbal outbursts, refusal to cooperate with the staff, and multiple attempts to leave the CLC. With the guidance of a geriatric psychiatrist, risperidone 1 mg once daily as needed was initiated, and staff continued with verbal redirection, both with limited efficacy. After 3 days, due to safety concerns for the veteran, other CLC patients, and CLC staff, risperidone dosing was increased to 1 mg twice daily, which had limited efficacy. Lorazepam 1 mg once daily also was added. A careful medication review was performed to minimize any potential AEs or interactions that might have contributed to the veteran’s behavior, but no pharmacologic interventions were found to fully abate their behavioral issues.
After 5 weeks of ongoing intermittent behavioral issues, the medical team again met to discuss new treatment options.A case reported by Husebo and colleagues used scheduled acetaminophen to help relieve neuropsychiatric symptoms of dementia in a patient who exhibited similar behavioral issues and did not respond well to antipsychotics or benzodiazepines.8 Although our veteran did not express or exhibit obvious pain, the medical team chose to trial this intervention, and the veteran was started on acetaminophen 650 mg orally 3 times daily. A comprehensive metabolic panel, including GGT and thyroid-stimulating hormone, was performed before starting acetaminophen; no abnormalities were noted. The clinical examination did not reveal physical abnormalities other than ataxia.
After 5 days of therapy with the scheduled acetaminophen, the veteran’s clinical behavior dramatically improved. The veteran exhibited infrequent agitated behavior and became cooperative with staff. Three days later, the scheduled lorazepam was discontinued, and eventually they were tapered off risperidone. One month after starting scheduled acetaminophen, the veteran had improved to a point where the staff determined a safe discharge plan could be initiated. The veteran’s nystagmus resolved and behavioral issues improved, although cognitive impairment persisted.
Due to COVID-19, a teleconference was scheduled with the veteran’s spouse to discuss a discharge plan. The spouse was pleased that the veteran had progressed adequately both functionally and behaviorally to make a safe discharge home possible. The spouse arranged to take family leave from their job to help support the veteran after discharge. The veteran was able to return home with a safe discharge plan 1 week later. The acetaminophen was continued with twice-daily dosing and was continued because there were no new behavioral issues. This was done to enhance postfacility adherence and minimize the risk of drug-drug interactions. Attempts to follow up with the veteran postdischarge were unfortunately unsuccessful as the family lived out of the local area.
Discussion
Alcohol misuse is a common finding in many US veterans, as well as in the general population.1,3 As a result, it is not uncommon to see patients with physical and psychological symptoms related to this abuse. Many of these patients will become verbally and physically abusive, thus having appropriate pharmacologic and nonpharmacologic interventions is important.
In this case study, the veteran was diagnosed with WKS and exhibited physical, cognitive, and emotional symptoms consistent with this disease. Although the physical symptoms improved with thiamine and abstinence from alcohol, their cognitive impairment, verbal outbursts, and aggressive demeanor persisted.
After using antipsychotic and anxiolytic medications with minimal clinical improvement, a trial of acetaminophen 650 mg 3 times daily was instituted. The patient’s behavior improved; demeanor became calmer, and they were easily redirected by the nursing staff. Psychological support was again employed, which enhanced and supported the veteran’s calmer demeanor. Although there is limited medical literature on the use of acetaminophen in clinical situations not related to pain, there has been research documenting its effect on social interaction.9,10
Acetaminophen is an analgesic medication that acts through central neural mechanisms. It has been hypothesized that social and physical pain rely on shared neurochemical underpinnings, and some of the regions of the brain involved in affective experience of physical pain also have been found to be involved in the experience of social pain.11 Acetaminophen may impact an individual’s social well-being as social pain processes.11 It has been shown to blunt reactivity to both physical pain as well as negative stimuli.11
Conclusions
A 2019 survey on alcohol and drug use found 5.6% of adults aged ≥ 18 have an alcohol use disorder.12 In severe cases, this can result in WKS. Although replacement of thiamine is critical for physical improvement, psychological deficits may persist. Small studies have advanced the concept of using scheduled acetaminophen even when the patient is not verbalizing or displaying pain.13 Although more research needs to be done on this topic, this palliative approach may be worth considering, especially if the risks of antipsychotics and anxiolytics outweigh the benefits.
1. National Institute on Drug Abuse. Substance use and military life drug facts. Published October 2019. Accessed November 10, 2021. https://www.drugabuse.gov/publications/drugfacts/substance-use-military-life
2. National Veterans Foundation. What statistics show about veteran substance abuse and why proper treatment is important. Published March 30, 2016. Accessed November 10, 2021. https://nvf.org/veteran-substance-abuse-statistics
3. National Center for Biotechnology Information. Korsakoff syndrome. Updated July 10, 2020. Accessed November 10, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539854
4. Gerridzen IJ, Goossensen MA. Patients with Korsakoff syndrome in nursing homes: characteristics, comorbidity, and use of psychotropic drugs. Int Psychogeriatr. 2014;26(1):115-121. doi:10.1017/S1041610213001543
5. Press D, Alexander M. Management of neuropsychiatric symptoms of dementia. Updated October 2021. Accessed November 10, 2021. https://www.uptodate.com/contents/management-of-neuropsychiatric-symptoms-of-dementia
6. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: Managing safety concerns. Am J Psychiatry. 2012;169(9):900-906. doi:10.1176/appi.ajp.2012.12030342
7. Jibson MD. Second-generation antipsychotic medications: pharmacology, administration, and side effects. https://www.uptodate.com/contents/second-generation-antipsychotic-medications-pharmacology-administration-and-side-effects
8. Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi:10.1136/bmj.d4065
9. Fung K, Alden LE. Once hurt, twice shy: social pain contributes to social anxiety. Emotion. 2017;(2):231-239. doi:10.1037/emo0000223
10. Roberts ID, Krajbich I, Cheavens JS, Campo JV, Way BM. Acetaminophen Reduces Distrust in Individuals with Borderline Personality Disorder Features. Clin Psychol Sci. 2018;6(1):145-154. doi:10.1177/2167702617731374
11. Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-937. doi:10.1177/0956797610374741
12. National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. Updated June 2021. Accessed November 2, 202November 10, 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
13. Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatrics Soc. 2005;53(11):1921-9. doi:10.1111/j.1532-5415.2005.53572.x
1. National Institute on Drug Abuse. Substance use and military life drug facts. Published October 2019. Accessed November 10, 2021. https://www.drugabuse.gov/publications/drugfacts/substance-use-military-life
2. National Veterans Foundation. What statistics show about veteran substance abuse and why proper treatment is important. Published March 30, 2016. Accessed November 10, 2021. https://nvf.org/veteran-substance-abuse-statistics
3. National Center for Biotechnology Information. Korsakoff syndrome. Updated July 10, 2020. Accessed November 10, 2021. https://www.ncbi.nlm.nih.gov/books/NBK539854
4. Gerridzen IJ, Goossensen MA. Patients with Korsakoff syndrome in nursing homes: characteristics, comorbidity, and use of psychotropic drugs. Int Psychogeriatr. 2014;26(1):115-121. doi:10.1017/S1041610213001543
5. Press D, Alexander M. Management of neuropsychiatric symptoms of dementia. Updated October 2021. Accessed November 10, 2021. https://www.uptodate.com/contents/management-of-neuropsychiatric-symptoms-of-dementia
6. Steinberg M, Lyketsos CG. Atypical antipsychotic use in patients with dementia: Managing safety concerns. Am J Psychiatry. 2012;169(9):900-906. doi:10.1176/appi.ajp.2012.12030342
7. Jibson MD. Second-generation antipsychotic medications: pharmacology, administration, and side effects. https://www.uptodate.com/contents/second-generation-antipsychotic-medications-pharmacology-administration-and-side-effects
8. Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. doi:10.1136/bmj.d4065
9. Fung K, Alden LE. Once hurt, twice shy: social pain contributes to social anxiety. Emotion. 2017;(2):231-239. doi:10.1037/emo0000223
10. Roberts ID, Krajbich I, Cheavens JS, Campo JV, Way BM. Acetaminophen Reduces Distrust in Individuals with Borderline Personality Disorder Features. Clin Psychol Sci. 2018;6(1):145-154. doi:10.1177/2167702617731374
11. Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-937. doi:10.1177/0956797610374741
12. National Institute on Alcohol Abuse and Alcoholism. Alcohol facts and statistics. Updated June 2021. Accessed November 2, 202November 10, 2021. https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics
13. Chibnall JT, Tait RC, Harman B, Luebbert RA. Effect of acetaminophen on behavior, well-being, and psychotropic medication use in nursing home residents with moderate-to-severe dementia. J Am Geriatrics Soc. 2005;53(11):1921-9. doi:10.1111/j.1532-5415.2005.53572.x
With sexually transmitted infections off the charts, California pushes at-home tests
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.