User login
Light Brown and Pink Macule on the Upper Arm
The Diagnosis: Desmoplastic Spitz Nevus
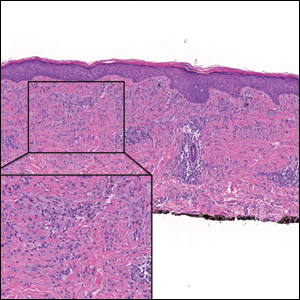
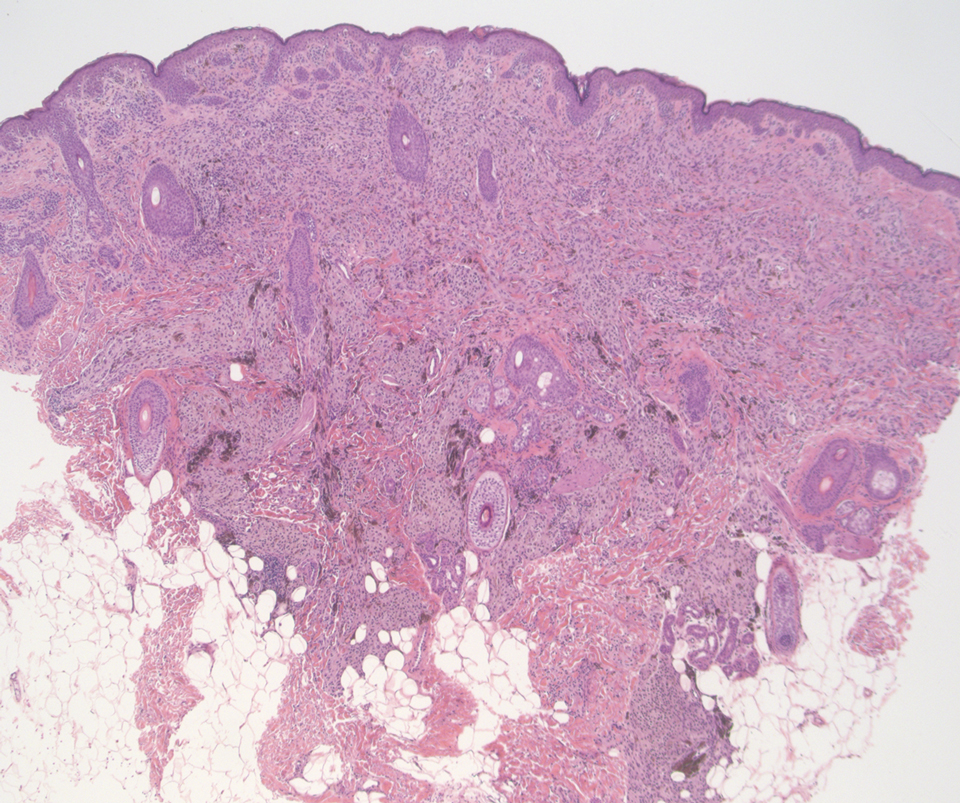
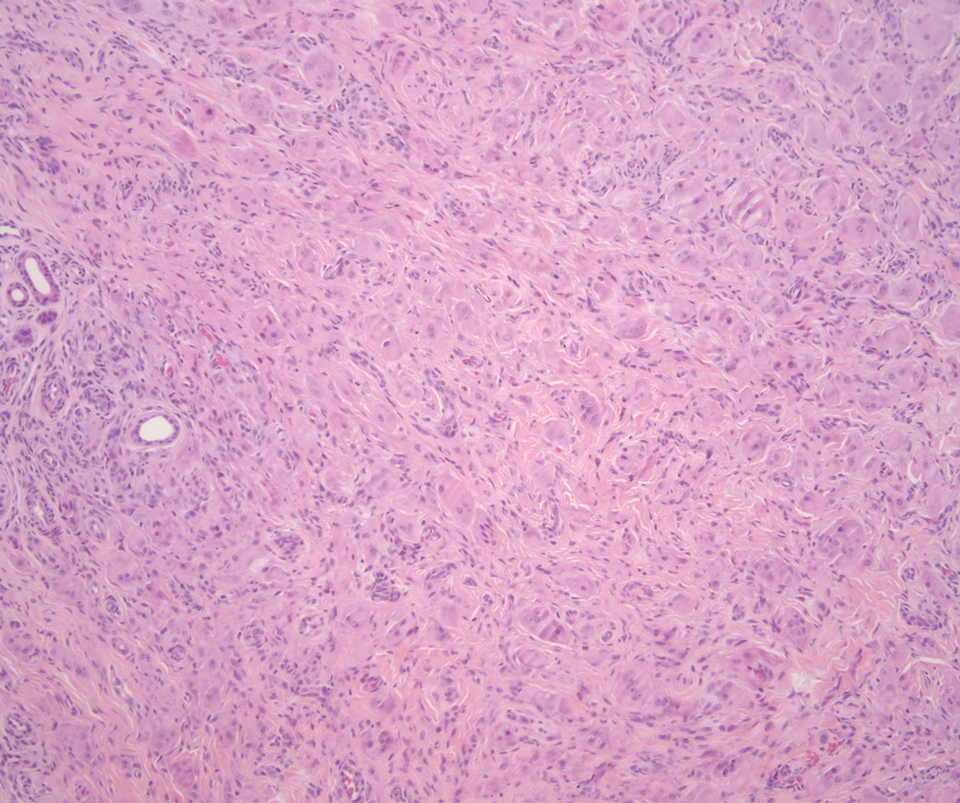
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).
Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
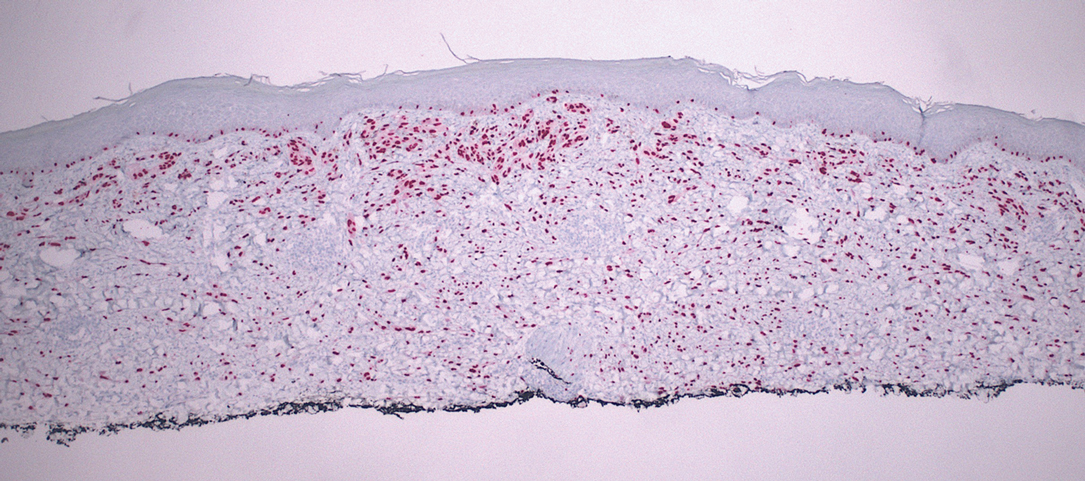
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15
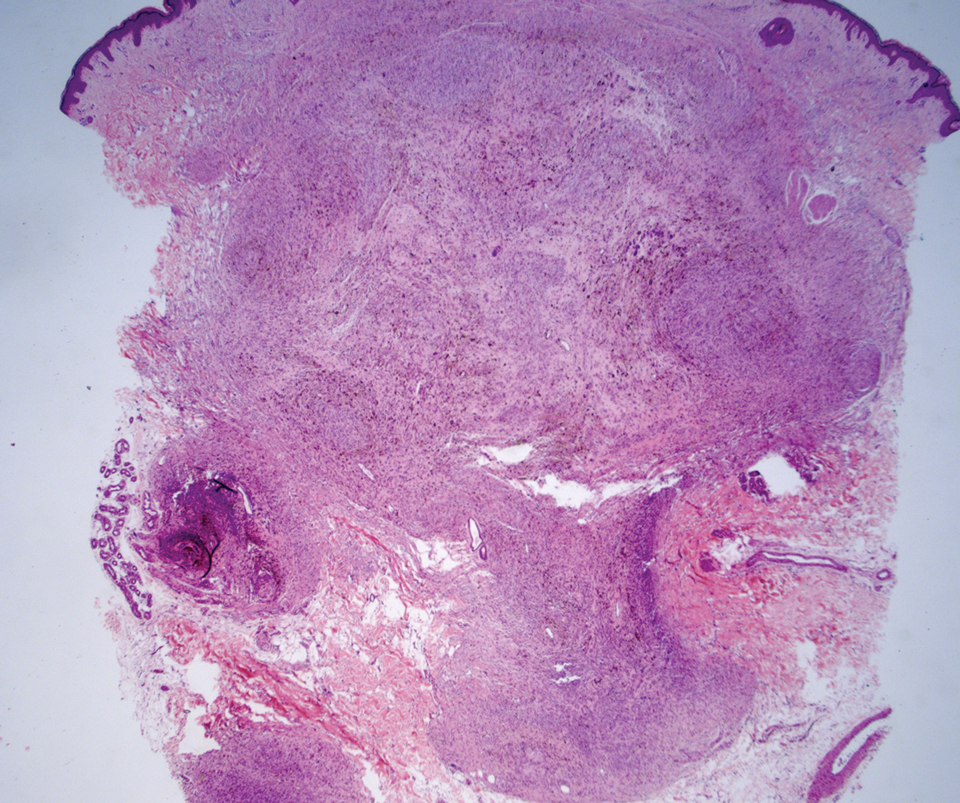
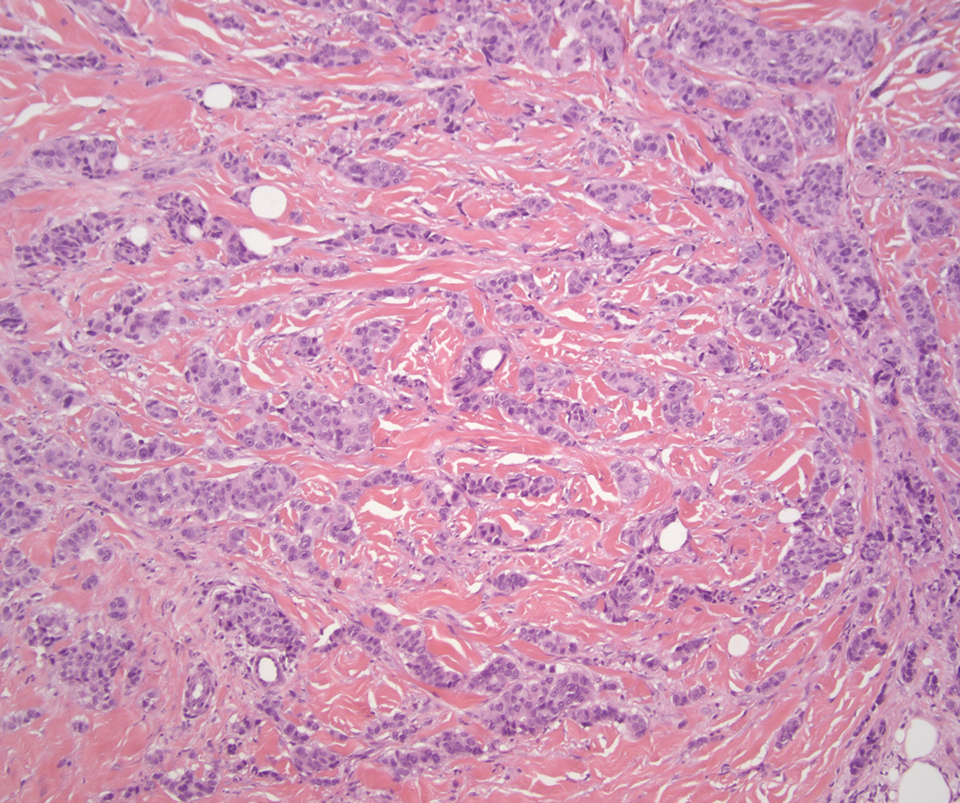
The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.
Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.
- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).

Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15

The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.

Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.


The Diagnosis: Desmoplastic Spitz Nevus
Desmoplastic Spitz nevus is a rare variant of Spitz nevus that commonly presents as a red to brown papule on the head, neck, or extremities. It is pertinent to review the histologic features of this neoplasm, as it can be confused with other more sinister entities such as spitzoid melanoma. Histologically, there is a dermal infiltrate of melanocytes containing eosinophilic cytoplasm and vesicular nuclei. Junctional involvement is rare, and there should be no pagetoid spread.1 This entity features abundant stromal fibrosis formed by dense collagen bundles, low cellular density, and polygonal-shaped melanocytes, which helps to differentiate it from melanoma.2,3 In a retrospective study comparing the characteristics of desmoplastic Spitz nevi with desmoplastic melanoma, desmoplastic Spitz nevi histologically were more symmetric and circumscribed with greater melanocytic maturation and adnexal structure involvement.3 Although this entity demonstrates maturation from the superficial to the deep dermis, it also may feature deep dermal vascular proliferation.4 S-100 and SRY-related HMG box 10, SOX-10, are noted to be positive in desmoplastic Spitz nevi, which can help to differentiate it from nonmelanocytic entities (Figure 1).

Although spitzoid lesions can be ambiguous and difficult even for experts to classify, spitzoid melanoma tends to have a high Breslow thickness, high cell density, marked atypia, and an increased nucleus to cytoplasm ratio.5 Additionally, desmoplastic melanoma was found to more often display “melanocytic junctional nests associated with discohesive cells, variations in size and shape of the nests, lentiginous melanocytic proliferation, actinic elastosis, pagetoid spread, dermal mitosis, perineural involvement and brisk inflammatory infiltrate.”3 Given the challenge of histologically separating desmoplastic Spitz nevi from melanoma, immunostaining can be useful. For example, Hilliard et al6 used a p16 antibody to differentiate desmoplastic Spitz nevi from desmoplastic melanoma, finding that most desmoplastic melanomas (81.8%; n=11) were negative for p16, whereas all desmoplastic Spitz nevi were at least moderately positive. However, another study re-evaluated the utility of p16 in desmoplastic melanoma and found that 72.7% (16/22) were at least focally reactive for the immunostain.7 Thus, caution must be exercised when using p16.
PReferentially expressed Antigen in MElanoma (PRAME) is a newer nuclear immunohistochemical marker that tends to be positive in melanomas and negative in nevi. Desmoplastic Spitz nevi would be expected to be negative for PRAME, while desmoplastic melanoma may be positive; however, this marker seems to be less effective in desmoplastic melanoma than in most other subtypes of the malignancy. In one study, only 35% (n=20) of desmoplastic melanomas were positive for PRAME.8 Likewise, another study showed that some benign Spitz nevi may diffusely express PRAME.9 As such, PRAME should be used prudently.
For cases in which immunohistochemistry is equivocal, molecular testing may aid in differentiating Spitz nevi from melanoma. For example, comparative genomic hybridization has revealed an increased copy number of chromosome 11p in approximately 20% of Spitz nevi cases10; this finding is not seen in melanoma. Mutation analyses of HRas proto-oncogene, GTPase, HRAS; B-Raf proto-oncogene, serine/threonine kinase, BRAF; and NRAS proto-oncogene, GTPase, NRAS, also have shown some promise in distinguishing spitzoid lesions from melanoma, but these analyses may be oversimplified.11 Fluorescence in situ hybridization (FISH) is another diagnostic modality that has been studied to differentiate benign nevi from melanoma. One study challenged the utility of FISH, reporting 7 of 15 desmoplastic melanomas tested positive compared to 0 of 15 sclerotic melanocytic nevi.12 Thus, negative FISH cannot reliably rule out melanoma. Ultimately, a combination of immunostains along with FISH or another genetic study would prove to be most effective in ruling out melanoma in difficult cases. Even then, a dermatopathologist may be faced with a degree of uncertainty.
Cellular blue nevi predominantly affect adults younger than 40 years and commonly are seen on the buttocks.13 This benign neoplasm demonstrates areas that are distinctly sclerotic as well as those that are cellular in nature.14 This entity demonstrates a well-circumscribed dermal growth pattern with 2 main populations of cells. The sclerotic portion of the cellular blue nevus mimics that of the blue nevus in that it is noted superficially with irregular margins. The cellular aspect of the nevus features spindle cells contained within well-circumscribed nodules (Figure 2). Stromal melanophages are not uncommon, and some can be observed adjacent to nerve fibers. Although this blue nevus variant displays features of the common blue nevus, its melanocytes track along adnexal and neurovascular structures similar to the deep penetrating nevus and the desmoplastic Spitz nevus. However, these melanocytes are variable in morphology and can appear on a spectrum spanning from pale and lightly pigmented to clear.15

The breast is the most common site of origin of tumor metastasis to the skin. These cutaneous metastases can vary in both their clinical and histological presentations. For example, cutaneous metastatic breast adenocarcinoma often can present clinically as pink-violaceous papules and plaques on the breast or on other parts of the body. Histologically, it can demonstrate a varying degree of patterns such as collagen infiltration by single cells, cords, tubules, and sheets of atypical cells (Figure 3) that can be observed together in areas of mucin or can form glandular structures.16 Metastatic breast carcinoma is noted to be positive for gross cystic disease fluid protein-15, estrogen receptor, and cytokeratin 7, which can help differentiate this entity from other tumors of glandular origin.16 Although rare, primary melanoma of the breast has been reported in the literature.17,18 These malignant melanocytic lesions easily could be differentiated from other breast tumors such as adenocarcinoma using immunohistochemical staining patterns.

Deep penetrating nevi most often are observed clinically as blue, brown, or black papules or nodules on the head or neck.19 Histologically, this lesion features a wedge-shaped infiltrate of deep dermal melanocytes with oval nuclei. It commonly extends to the reticular dermis or further into the subcutis (Figure 4).20,21 This neoplasm frequently tracks along adnexal and neurovascular structures, resulting in a plexiform appearance.22 The adnexal involvement of deep penetrating nevi is a shared feature with desmoplastic Spitz nevi. The presence of any number of melanophages is characteristic of this lesion.23 Lastly, there is a well-documented association between β-catenin mutations and deep penetrating nevi.24 Multicentric reticulohistiocytosis (MRH) is a rare form of non-Langerhans cell histiocytosis that has the pathognomonic clinical finding of pink-red papules (coral beading) with a predilection for acral surfaces. Histology of affected skin reveals a dermal infiltrate of ground glass as well as eosinophilic histiocytes that most often stain positive for CD68 and human alveolar macrophage 56 but negative for S-100 and CD1a (Figure 5).25 Although MRH is rare, negative staining for S-100 could serve as a useful diagnostic clue to differentiate it from other entities that are positive for S-100, such as the desmoplastic Spitz nevus. Arthritis mutilans is a potential complication of MRH, but a reported association with an underlying malignancy is seen in approximately 25% of cases.26 Thus, the cutaneous, rheumatologic, and oncologic implications of this disease help to distinguish it from other differential diagnoses that may be considered.


- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
- Luzar B, Bastian BC, North JP, et al. Melanocytic nevi. In: Calonje E, Brenn T, Lazar AJ, et al, eds. McKee’s Pathology of the Skin. 5th ed. Elsevier; 2020:1275-1280.
- Busam KJ, Gerami P. Spitz nevi. In: Busam KJ, Gerami P, Scolyer RA, eds. Pathology of Melanocytic Tumors. Elsevier; 2019:37-60.
- Nojavan H, Cribier B, Mehregan DR. Desmoplastic Spitz nevus: a histopathological review and comparison with desmoplastic melanoma [in French]. Ann Dermatol Venereol. 2009;136:689-695.
- Tomizawa K. Desmoplastic Spitz nevus showing vascular proliferation more prominently in the deep portion. Am J Dermatopathol. 2002;24:184-185.
- Requena C, Botella R, Nagore E, et al. Characteristics of spitzoid melanoma and clues for differential diagnosis with Spitz nevus. Am J Dermatopathol. 2012;34:478-486.
- Hilliard NJ, Krahl D, Sellheyer K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J Cutan Pathol. 2009;36:753-759.
- Blokhin E, Pulitzer M, Busam KJ. Immunohistochemical expression of p16 in desmoplastic melanoma. J Cutan Pathol. 2013;40:796-800.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131.
- Bauer J, Bastian BC. DNA copy number changes in the diagnosis of melanocytic tumors [in German]. Pathologe. 2007;28:464-473.
- Luo S, Sepehr A, Tsao H. Spitz nevi and other spitzoid lesions part I. background and diagnoses. J Am Acad Dermatol. 2011;65:1073-1084.
- Gerami P, Beilfuss B, Haghighat Z, et al. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329-334.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017; 37:401-415.
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405.
- Phadke PA, Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2011;31:345-358.
- Ko CJ. Metastatic tumors and simulators. In: Elston DM, Ferringer T, eds. Dermatopathology. 3rd ed. Elsevier Limited; 2019:496-504.
- Drueppel D, Schultheis B, Solass W, et al. Primary malignant melanoma of the breast: case report and review of the literature. Anticancer Res. 2015;35:1709-1713.
- Kurul S, Tas¸ F, Büyükbabani N, et al. Different manifestations of malignant melanoma in the breast: a report of 12 cases and a review of the literature. Jpn J Clin Oncol. 2005;35:202-206.
- Strazzula L, Senna MM, Yasuda M, et al. The deep penetrating nevus. J Am Acad Dermatol. 2014;71:1234-1240.
- Mehregan DA, Mehregan AH. Deep penetrating nevus. Arch Dermatol. 1993;129:328-331.
- Robson A, Morley-Quante M, Hempel H, et al. Deep penetrating naevus: clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology. 2003;43:529-537.
- Luzar B, Calonje E. Deep penetrating nevus: a review. Arch Pathol Lab Med. 2011;135:321-326.
- Cooper PH. Deep penetrating (plexiform spindle cell) nevus. a frequent participant in combined nevus. J Cutan Pathol. 1992;19:172-180.
- de la Fouchardière A, Caillot C, Jacquemus J, et al. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019;474:539-550.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Selmi C, Greenspan A, Huntley A, et al. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511.
A 37-year-old woman with a history of fibrocystic breast disease and a family history of breast cancer presented with a light brown macule on the right upper arm of 10 years’ duration. The patient first noticed this macule 10 years prior; however, within the last 4 months she noticed a small amount of homogenous darkening and occasional pruritus. Physical examination revealed a 4.0-mm, light brown and pink macule on the right upper arm. Dermoscopy showed a homogenous pigment network with reticular lines and branched streaks centrally. No crystalline structures, milky red globules, or pseudopods were appreciated. A tangential shave biopsy was obtained and submitted for hematoxylin and eosin staining.
Motor function restored in three men after complete paralysis from spinal cord injury
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
The Final Rule for 2022: What’s New and How Changes in the Medicare Physician Fee Schedule and Quality Payment Program Affect Dermatologists
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
On November 2, 2021, the Centers for Medicare & Medicaid Services (CMS) released its final rule for the 2022 Medicare Physician Fee Schedule (PFS) and the Quality Payment Program (QPP).1,2 These guidelines contain updates that will remarkably impact the field of medicine—and dermatology in particular—in 2022. This article will walk you through some of the updates most relevant to dermatology and how they may affect your practice.
Process for the Final Rule
The CMS releases an annual rule for the PFS and QPP. The interim rule generally is released over the summer with preliminary guidelines for the upcoming payment year. There is then a period of open comment where those affected by these changes, including physicians and medical associations, can submit comments to support what has been proposed or advocate for any changes. This input is then reviewed, and a final rule generally is published in the fall.
For this calendar year, the interim 2022 rule was released on July 13, 2021,3 and included many of guidelines that will be discussed in more detail in this article. Many associations that represent medicine overall and specifically dermatology, including the American Medical Association and the American Academy of Dermatology, submitted comments in response to these proposals.4,5
PFS Conversion Factor
The PFS conversion factor is updated annually to ensure budget neutrality in the setting of changes in relative value units. For 2022, the PFS conversion factor is $34.6062, representing a reduction of approximately $0.29 from the 2021 PFS conversion factor of $34.8931.6 This reduction does not take into account other payment adjustments due to legislative changes.
In combination, these changes previously were estimated to represent an overall payment cut of 10% or higher for dermatology, with those practitioners doing more procedural work or dermatopathology likely being impacted more heavily. However, with the passing of the Protecting Medicare and American Farmers from Sequester Cuts Act, it is estimated that the reductions in payment to dermatology will begin at 0.75% and reach 2.75% in the second half of the year with the phased-in reinstatement of the Medicare sequester.4,5,7
Clinical Labor Pricing Updates
Starting in 2022, the CMS will utilize updated wage rates from the US Bureau of Labor Statistics to revise clinical labor costs over a 4-year period. Clinical labor rates are important, as they are used to calculate practice expense within the PFS. These clinical labor rates were last updated in 2002.8 Median wage data, as opposed to mean data, from the US Bureau of Labor Statistics will be utilized to calculate the updated clinical labor rates.
A multiyear implementation plan was put into place by CMS due to multiple concerns, including that current wage rates are inadequate and may not reflect current labor rate information. Additionally, comments on this proposal voiced concern that updating the supply and equipment pricing without updating the clinical labor pricing could create distortions in the allocation of direct practice expense, which also factored into the implementation of a multiyear plan.8
It is anticipated that specialties that rely primarily on clinical labor will receive the largest increases in these rates and that specialties that rely primarily on supply or equipment items are anticipated to receive the largest reductions relative to other specialties. Dermatology is estimated to have a 0% change during the year 1 transition period; however, it will have an estimated 1% reduction in clinical labor pricing overall once the updates are completed.1 Pathology also is estimated to have a similar overall decrease during this transition period.
Evaluation and Management Visits
The biggest update in this area primarily is related to refining policies for split (shared) evaluation and management (E/M) visits and teaching physician activities. Split E/M visits are defined by the CMS as visits provided in the facility setting by a physician and nonphysician practitioner in the same group, with the visit billed by whomever provides the substantive portion of the visit. For 2022, the term substantive portion will be defined by the CMS as history, physical examination, medical decision-making, or more than half of the total time; for 2023, it will be defined as more than half of the total time spent.3 A split visit also can apply to an E/M visit provided in part by both a teaching physician and resident. Split visits can be reported for new or established patients. For proper reimbursement, the 2 practitioners who performed the services must be documented in the medical record, and the practitioner who provided the substantive portion must sign and date the encounter in the medical record. Additionally, the CMS has indicated the modifier FS must be included on the claim to indicate the split visit.9
For dermatologists who act as teaching physicians, it is important to note that many of the existing CMS policies for billing E/M services are still in place, specifically that if a resident participates in a service in a teaching setting, the teaching physician can bill for the service only if they are present for the key or critical portion of the service. A primary care exception does exist, in which teaching physicians at certain teaching hospital primary care centers can bill for some services performed independently by a resident without the physical presence of the teaching physician; however, this often is not applicable within dermatology.
With updated outpatient E/M guidelines, if time is being selected to bill, only the time that the teaching physician was present can be included to determine the overall E/M level.
Billing for Physician Assistant Services
Currently Medicare can only make payments to the employer or independent contractor of a physician assistant (PA); however, starting January 1, 2022, the CMS has authorized Medicare to make direct payments to PAs for qualifying professional services, in the same manner that nurse practitioners can currently bill. This also will allow PAs to incorporate as a group and bill Medicare for PA services. This stems from a congressional mandate within the Consolidated Appropriations Act of 2021.8 As a result, in states where PAs can practice independently, they can opt out of physician-led care teams and furnish services independently, including dermatologic services.
QPP Updates
Several changes were made to the Merit-Based Incentive Payment System (MIPS). Some of these changes include:
- Increase the MIPS performance threshold to 75 points from 60 points.
- Set the performance threshold at 89 points.
- Reduce the quality performance category weight from 40% to 30% of the final MIPS score.
- Increase the cost performance category weight from 20% to 30% of the final MIPS score.
- The extreme and uncontrollable circumstances application also has been extended to the end of 2022, allowing those remarkably impacted by the COVID-19 public health emergency to request for reweighting on any or all MIPS performance categories.
Cost Measures and MIPS Value Pathways
The melanoma resection cost measure will be implemented in 2022, representing the first dermatology cost measure, which will include the cost to Medicare over a 1-year period for all patient care for the excision of a melanoma. Although cost measures will be part of the MIPS value pathways (MVPs) reporting, dermatology currently is not part of the MVP; however, with the CMS moving forward with an initial set of MVPs that physicians can voluntarily report on in 2023, there is a possibility that dermatology will be asked to be part of the program in the future.10
Final Thoughts
There are many upcoming changes as part of the 2022 final rule, including to the conversion factor, E/M split visits, PA billing, and the QPP. Advocacy in these areas to the CMS and lawmakers, either directly or through dermatologic and other medical societies, is critical to help influence eventual recommendations.
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
- Medicare Program; CY 2022 payment policies under the Physician Fee Schedule and other changes to part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86:64996-66031. To be codified at 42 CFR §403, §405, §410, §411, §414, §415, §423, §424, and §425. https://www.federalregister.gov/documents/2021/11/19/2021-23972/medicare-program-cy-2022-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Centers for Medicare & Medicaid Services. CMS physician payment rule promotes greater access to telehealth services, diabetes prevention programs. Published November 2, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/press-releases/cms-physician-payment-rule-promotes-greater-access-telehealth-services-diabetes-prevention-programs
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2022 Medicare Physician Fee Schedule proposed rule. Published July 13, 2021. Accessed January 10, 2022. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- American Academy of Dermatology. Dermatology World Weekly. October 27, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- O’Reilly KB. 2022 Medicare pay schedule confirms Congress needs to act. American Medical Association website. Published November 10, 2021. Accessed January 10, 2021. https://www.ama-assn.org/practice-management/medicare-medicaid/2022-medicare-pay-schedule-confirms-congress-needs-act
- History of Medicare conversion factors. American Medical Association website. Accessed January 19, 2022. https://www.ama-assn.org/system/files/2021-01/cf-history.pdf
- American Academy of Dermatology. Dermatology World Weekly. December 15, 2021. Accessed January 20, 2022. https://www.aad.org/dw/weekly
- American Medical Association. CY 2022 Medicare Physician Fee Schedule (PFS) and Quality Payment Program (QPP) final rule summary. Accessed January 10, 2021. https://www.ama-assn.org/system/files/2022-pfs-qpp-final-rule.pdf
- Centers for Medicare & Medicaid Services. January 2022 alpha-numeric HCPCS file. Updated December 20, 2021. Accessed January 20, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/HCPCS-Quarterly-Update
- CMS finalizes Medicare payments for 2022. American Academy of Dermatology website. NEED PUB DATE. Accessed January 20, 2022. https://www.aad.org/member/practice/mips/fee-schedule/2022-fee-schedule-final
Practice Points
- The Centers for Medicare & Medicaid Services (CMS) 2022 final rule contains multiple updates affecting the practice of dermatology.
- Adjustments to the conversion factor and legislative-level actions have led to changes in reimbursement for many procedures within dermatology and beyond.
- Other notable updates include refining the definition of split evaluation and management visits, clinical labor pricing, and billing for physician assistant services.
- Changes in the Merit-Based Incentive Payment System (MIPS), cost measures, and MIPS value pathways also will impact many dermatology practices.
RECAM vs. RUCAM: Finding a better way to diagnose DILI
Researchers looking for a better way to diagnose drug-induced liver injury (DILI) have found evidence to support the use of the Revised Electronic Causality Assessment Method (RECAM).
The broadly used Roussel Uclaf Causality Assessment Method (RUCAM), introduced in 1993, “has been a valuable clinical framework for DILI diagnosis,” but it has been clouded by subjectivity and poor reliability, wrote the authors, led by Paul H. Hayashi, MD, MPH, with the Food and Drug Administration, in Hepatology. Citing a review from the Journal of Hepatology, Dr. Hayashi and colleagues noted three major problems: “(1) unclear operating instructions and subjectivity leading to poor reliability and usability, (2) unclear validity due to lack of an accepted gold standard and (3) domain criteria that are not evidence-based.”
Currently, a diagnosis of DILI is primarily based on clinicians’ judgment and ruling out alternative diagnoses, the authors of this study wrote. The lack of an evidence-based and reliable diagnostic tool is a significant obstacle in clinical care and research .
Reaching a new method
The researchers used classification tree analysis to set diagnostic cut-offs for RECAM and then compared RECAM with RUCAM for correlation with expert opinion diagnostic categories in 194 DILI cases (98 from the Drug-Induced Liver Injury Network, 96 from the Spanish DILI Registry).
The area under receiver operator curves for identifying at least probable DILI were the same at 0.89 for both RECAM and RUCAM.
The authors wrote, “However, RECAM diagnostic categories have better observed overall agreement with expert opinion (0.62 vs. 0.56 weighted kappa, P = .14), and had better sensitivity to detect extreme diagnostic categories (73 vs. 54 for highly likely or high probable, P = .02; 65 vs. 48 for unlikely/excluded, P = .08) than RUCAM diagnostic categories.”
They concluded that RECAM “is at least as capable as RUCAM in diagnosing DILI compared to expert opinion but is better than RUCAM at the diagnostic extremes.”
RECAM appears to add objectivity and clarity that can improve precision and reliability when diagnosing DILI and improve diagnostic standardization, according to authors. It has automated scoring, which reduces subjective input and should lead to better reliability among raters, something that has limited RUCAM’s adaptation in clinical practice and research.
RECAM has automatic warnings for data inconsistencies, which DILI and RUCAM do not. In RUCAM, a different diagnosis or other data could rule out DILI, but the case would still gain points in other criteria.
The authors explained, “Even when data clearly diagnose acute viral hepatitis or autoimmune hepatitis by simplified autoimmune hepatitis score, points are still given for latency, dechallenge, or underlying hepatotoxicity risk of the drug. In these situations of highly implausible DILI, RECAM gives warnings to stop with an imputed total score of –6. One can over-ride these warnings, if one believes DILI may be concurrent with the non-DILI diagnosis. However, –6 points are still assessed.”
Diagnosis of exclusion
Paul Martin, MD, chief, division of digestive health and liver diseases, Mandel Chair in Gastroenterology and professor of medicine at the University of Miami, said in an interview that he hopes RECAM will become widely used and better address a condition that sometimes doesn’t get enough attention. DILI remains underappreciated, he said, despite it being a major cause of morbidity and mortality in some patients.
“Any algorithm or criteria that can improve diagnostic accuracy is useful because typically it is a diagnosis of exclusion,” Dr. Martin said. “This new system seems to be as good as any other prior algorithms to diagnose drug-induced liver injury.”
He added, “This should help clinicians with individual patients with unexplained liver disease.”
The authors noted some limitations. RECAM was developed in U.S. and Spanish cohorts, so its performance in other regions is unclear. Both registries have minimum enrollment requirements for liver enzyme and bilirubin elevation, so it is not known how effective RECAM is in less severe cases. It also needs to be tested by other clinicians, including nonhepatologists.
The authors also added, “It is currently limited to single-agent medication cases leaving the user to score each medication individually in multidrug cases. However, any competing medication causing loss of points in the RUCAM, probably deserves its own RECAM score.”
The DILIN is structured as a cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Hayashi is employed by the FDA, but the conclusions of this paper do not reflect any opinion of the FDA. One coauthor has advised Pfizer, GSK, and NuCANA through Nottingham University Consultants, and another has received support from Gilead and AbbVie and consulted for Sanofi. The remaining authors have no conflicts. Dr. Martin reports no relevant financial relationships.
Researchers looking for a better way to diagnose drug-induced liver injury (DILI) have found evidence to support the use of the Revised Electronic Causality Assessment Method (RECAM).
The broadly used Roussel Uclaf Causality Assessment Method (RUCAM), introduced in 1993, “has been a valuable clinical framework for DILI diagnosis,” but it has been clouded by subjectivity and poor reliability, wrote the authors, led by Paul H. Hayashi, MD, MPH, with the Food and Drug Administration, in Hepatology. Citing a review from the Journal of Hepatology, Dr. Hayashi and colleagues noted three major problems: “(1) unclear operating instructions and subjectivity leading to poor reliability and usability, (2) unclear validity due to lack of an accepted gold standard and (3) domain criteria that are not evidence-based.”
Currently, a diagnosis of DILI is primarily based on clinicians’ judgment and ruling out alternative diagnoses, the authors of this study wrote. The lack of an evidence-based and reliable diagnostic tool is a significant obstacle in clinical care and research .
Reaching a new method
The researchers used classification tree analysis to set diagnostic cut-offs for RECAM and then compared RECAM with RUCAM for correlation with expert opinion diagnostic categories in 194 DILI cases (98 from the Drug-Induced Liver Injury Network, 96 from the Spanish DILI Registry).
The area under receiver operator curves for identifying at least probable DILI were the same at 0.89 for both RECAM and RUCAM.
The authors wrote, “However, RECAM diagnostic categories have better observed overall agreement with expert opinion (0.62 vs. 0.56 weighted kappa, P = .14), and had better sensitivity to detect extreme diagnostic categories (73 vs. 54 for highly likely or high probable, P = .02; 65 vs. 48 for unlikely/excluded, P = .08) than RUCAM diagnostic categories.”
They concluded that RECAM “is at least as capable as RUCAM in diagnosing DILI compared to expert opinion but is better than RUCAM at the diagnostic extremes.”
RECAM appears to add objectivity and clarity that can improve precision and reliability when diagnosing DILI and improve diagnostic standardization, according to authors. It has automated scoring, which reduces subjective input and should lead to better reliability among raters, something that has limited RUCAM’s adaptation in clinical practice and research.
RECAM has automatic warnings for data inconsistencies, which DILI and RUCAM do not. In RUCAM, a different diagnosis or other data could rule out DILI, but the case would still gain points in other criteria.
The authors explained, “Even when data clearly diagnose acute viral hepatitis or autoimmune hepatitis by simplified autoimmune hepatitis score, points are still given for latency, dechallenge, or underlying hepatotoxicity risk of the drug. In these situations of highly implausible DILI, RECAM gives warnings to stop with an imputed total score of –6. One can over-ride these warnings, if one believes DILI may be concurrent with the non-DILI diagnosis. However, –6 points are still assessed.”
Diagnosis of exclusion
Paul Martin, MD, chief, division of digestive health and liver diseases, Mandel Chair in Gastroenterology and professor of medicine at the University of Miami, said in an interview that he hopes RECAM will become widely used and better address a condition that sometimes doesn’t get enough attention. DILI remains underappreciated, he said, despite it being a major cause of morbidity and mortality in some patients.
“Any algorithm or criteria that can improve diagnostic accuracy is useful because typically it is a diagnosis of exclusion,” Dr. Martin said. “This new system seems to be as good as any other prior algorithms to diagnose drug-induced liver injury.”
He added, “This should help clinicians with individual patients with unexplained liver disease.”
The authors noted some limitations. RECAM was developed in U.S. and Spanish cohorts, so its performance in other regions is unclear. Both registries have minimum enrollment requirements for liver enzyme and bilirubin elevation, so it is not known how effective RECAM is in less severe cases. It also needs to be tested by other clinicians, including nonhepatologists.
The authors also added, “It is currently limited to single-agent medication cases leaving the user to score each medication individually in multidrug cases. However, any competing medication causing loss of points in the RUCAM, probably deserves its own RECAM score.”
The DILIN is structured as a cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Hayashi is employed by the FDA, but the conclusions of this paper do not reflect any opinion of the FDA. One coauthor has advised Pfizer, GSK, and NuCANA through Nottingham University Consultants, and another has received support from Gilead and AbbVie and consulted for Sanofi. The remaining authors have no conflicts. Dr. Martin reports no relevant financial relationships.
Researchers looking for a better way to diagnose drug-induced liver injury (DILI) have found evidence to support the use of the Revised Electronic Causality Assessment Method (RECAM).
The broadly used Roussel Uclaf Causality Assessment Method (RUCAM), introduced in 1993, “has been a valuable clinical framework for DILI diagnosis,” but it has been clouded by subjectivity and poor reliability, wrote the authors, led by Paul H. Hayashi, MD, MPH, with the Food and Drug Administration, in Hepatology. Citing a review from the Journal of Hepatology, Dr. Hayashi and colleagues noted three major problems: “(1) unclear operating instructions and subjectivity leading to poor reliability and usability, (2) unclear validity due to lack of an accepted gold standard and (3) domain criteria that are not evidence-based.”
Currently, a diagnosis of DILI is primarily based on clinicians’ judgment and ruling out alternative diagnoses, the authors of this study wrote. The lack of an evidence-based and reliable diagnostic tool is a significant obstacle in clinical care and research .
Reaching a new method
The researchers used classification tree analysis to set diagnostic cut-offs for RECAM and then compared RECAM with RUCAM for correlation with expert opinion diagnostic categories in 194 DILI cases (98 from the Drug-Induced Liver Injury Network, 96 from the Spanish DILI Registry).
The area under receiver operator curves for identifying at least probable DILI were the same at 0.89 for both RECAM and RUCAM.
The authors wrote, “However, RECAM diagnostic categories have better observed overall agreement with expert opinion (0.62 vs. 0.56 weighted kappa, P = .14), and had better sensitivity to detect extreme diagnostic categories (73 vs. 54 for highly likely or high probable, P = .02; 65 vs. 48 for unlikely/excluded, P = .08) than RUCAM diagnostic categories.”
They concluded that RECAM “is at least as capable as RUCAM in diagnosing DILI compared to expert opinion but is better than RUCAM at the diagnostic extremes.”
RECAM appears to add objectivity and clarity that can improve precision and reliability when diagnosing DILI and improve diagnostic standardization, according to authors. It has automated scoring, which reduces subjective input and should lead to better reliability among raters, something that has limited RUCAM’s adaptation in clinical practice and research.
RECAM has automatic warnings for data inconsistencies, which DILI and RUCAM do not. In RUCAM, a different diagnosis or other data could rule out DILI, but the case would still gain points in other criteria.
The authors explained, “Even when data clearly diagnose acute viral hepatitis or autoimmune hepatitis by simplified autoimmune hepatitis score, points are still given for latency, dechallenge, or underlying hepatotoxicity risk of the drug. In these situations of highly implausible DILI, RECAM gives warnings to stop with an imputed total score of –6. One can over-ride these warnings, if one believes DILI may be concurrent with the non-DILI diagnosis. However, –6 points are still assessed.”
Diagnosis of exclusion
Paul Martin, MD, chief, division of digestive health and liver diseases, Mandel Chair in Gastroenterology and professor of medicine at the University of Miami, said in an interview that he hopes RECAM will become widely used and better address a condition that sometimes doesn’t get enough attention. DILI remains underappreciated, he said, despite it being a major cause of morbidity and mortality in some patients.
“Any algorithm or criteria that can improve diagnostic accuracy is useful because typically it is a diagnosis of exclusion,” Dr. Martin said. “This new system seems to be as good as any other prior algorithms to diagnose drug-induced liver injury.”
He added, “This should help clinicians with individual patients with unexplained liver disease.”
The authors noted some limitations. RECAM was developed in U.S. and Spanish cohorts, so its performance in other regions is unclear. Both registries have minimum enrollment requirements for liver enzyme and bilirubin elevation, so it is not known how effective RECAM is in less severe cases. It also needs to be tested by other clinicians, including nonhepatologists.
The authors also added, “It is currently limited to single-agent medication cases leaving the user to score each medication individually in multidrug cases. However, any competing medication causing loss of points in the RUCAM, probably deserves its own RECAM score.”
The DILIN is structured as a cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases.
Dr. Hayashi is employed by the FDA, but the conclusions of this paper do not reflect any opinion of the FDA. One coauthor has advised Pfizer, GSK, and NuCANA through Nottingham University Consultants, and another has received support from Gilead and AbbVie and consulted for Sanofi. The remaining authors have no conflicts. Dr. Martin reports no relevant financial relationships.
FROM HEPATOLOGY
Does using A1c to diagnose diabetes miss some patients?
The introduction of hemoglobin A1c as an option for diagnosing type 2 diabetes over a decade ago may have resulted in underdiagnosis, new research indicates.
In 2011, the World Health Organization advised that A1c measurement, with a cutoff value of 6.5%, could be used to diagnose diabetes. The American Diabetes Association had issued similar guidance in 2010.
Prior to that time, the less-convenient 2-hour oral glucose tolerance test (OGTT) and fasting blood glucose (FBG) were the only recommended tests. While WHO made no recommendations for interpreting values below 6.5%, the ADA designated 5.7%-6.4% as prediabetes.
The new study, published online in The Lancet Regional Health–Europe, showed that the incidence of type 2 diabetes in Denmark had been increasing prior to the 2012 adoption of A1c as a diagnostic option but declined thereafter. And all-cause mortality among people with type 2 diabetes, which had been dropping, began to increase after that time.
“Our findings suggest that fewer patients have been diagnosed with [type 2 diabetes] since A1c testing was introduced as a convenient diagnostic option. We may thus be missing a group with borderline increased A1c values that is still at high metabolic and cardiovascular risk,” Jakob S. Knudsen, MD, of the department of clinical epidemiology, Aarhus (Denmark) University Hospital, and colleagues wrote.
Therefore, Dr. Knudsen said in an interview, clinicians should “consider testing with FBG or OGTT when presented with borderline A1c values.”
The reason for the increase in mortality after incident type 2 diabetes diagnosis, he said, “is that the patients who would have reduced the average mortality are no longer diagnosed...This does not reflect that we are treating already diagnosed patients any worse, rather some patients are not diagnosed.”
But M. Sue Kirkman, MD, emeritus professor of medicine at the University of North Carolina at Chapel Hill, who was part of the writing group for the 2010 ADA guidelines, isn’t convinced.
“This is an interesting paper, but it is a bit hard to believe that a change in WHO recommendations would have such a large and almost immediate impact on incidence and mortality. It seems likely that ... factors [other] than just the changes in recommendations for the diagnostic test account for these findings,” she said.
Dr. Kirkman pointed to new data just out from the Centers for Disease Control and Prevention on Jan. 26 that don›t show evidence of a higher proportion of people in the United States who have undiagnosed diabetes, “which would be expected if more cases were being ‘missed’ by A1c.”
She added that the CDC incidence data “show a continuing steady rate of decline in incidence that began in 2008, before any organizations recommended using A1c to screen for or diagnose diabetes.” Moreover, “there is evidence that type 2 diabetes incidence has fallen or plateaued in many countries since 2006, well before the WHO recommendation, with most of the studies from developed countries.”
But Dr. Knudsen also cited other data, including a study that showed a drop or stabilization in diagnosed diabetes incidence in high-income countries since 2010.
“That study concluded that the reasons for the declines in the incidence of diagnosed diabetes warrant further investigation with appropriate data sources, which was a main objective of our study,” wrote Dr. Knudsen and coauthors.
Dr. Knudsen said in an interview: “We are not the first to make the point that this sudden change is related to A1c introduction...but we are the first to have the data to clearly show that is the case.”
Diabetes incidence dropped but mortality rose after 2010
The population-based longitudinal study used four Danish medical databases and included 415,553 patients treated for type 2 diabetes for the first time from 1995-2018 and 2,060,279 matched comparators not treated for diabetes.
From 1995 until the 2012 introduction of A1c as a diagnostic option, the annual standardized incidence rates of type 2 diabetes more than doubled, from 193 per 100,000 population to 396 per 100,000 population, at a rate of 4.1% per year.
But from 2011 to 2018, the annual standardized incidence rate declined by 36%, to 253 per 100,000 population, a 5.7% annualized decrease.
The increase prior to 2011 occurred in both men and women and in all age groups, while the subsequent decline was seen primarily in the older age groups. The all-cause mortality risk within the first year after diabetes diagnosis was higher than subsequent 1-year mortality risks and not different between men and women.
From the periods 1995-1997 to 2010-2012, the adjusted mortality rate among those with type 2 diabetes decreased by 44%, from 72 deaths per 1000 person-years to 40 deaths per 1000 person-years (adjusted mortality rate ratio, 0.55). After that low level in 2010-2012, mortality increased by 27% to 48 per 1000 person-years (adjusted mortality rate ratio 0.69, compared with 1995-1997).
The reversed mortality trend after 2010-2012 was caused almost entirely by the increase in the first year after diabetes diagnosis, Dr. Knudsen and colleagues noted.
According to Dr. Kirkman, “A1c is strongly predictive of complications and mortality. That plus its ease of use and the fact that more people may be screened mean it’s still a good option. But for any of these tests, people who are slightly below the cut-point should not be considered normal or low risk.”
Indeed, Dr. Knudsen and colleagues said, “these findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.”
Dr. Knudsen and Dr. Kirkman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The introduction of hemoglobin A1c as an option for diagnosing type 2 diabetes over a decade ago may have resulted in underdiagnosis, new research indicates.
In 2011, the World Health Organization advised that A1c measurement, with a cutoff value of 6.5%, could be used to diagnose diabetes. The American Diabetes Association had issued similar guidance in 2010.
Prior to that time, the less-convenient 2-hour oral glucose tolerance test (OGTT) and fasting blood glucose (FBG) were the only recommended tests. While WHO made no recommendations for interpreting values below 6.5%, the ADA designated 5.7%-6.4% as prediabetes.
The new study, published online in The Lancet Regional Health–Europe, showed that the incidence of type 2 diabetes in Denmark had been increasing prior to the 2012 adoption of A1c as a diagnostic option but declined thereafter. And all-cause mortality among people with type 2 diabetes, which had been dropping, began to increase after that time.
“Our findings suggest that fewer patients have been diagnosed with [type 2 diabetes] since A1c testing was introduced as a convenient diagnostic option. We may thus be missing a group with borderline increased A1c values that is still at high metabolic and cardiovascular risk,” Jakob S. Knudsen, MD, of the department of clinical epidemiology, Aarhus (Denmark) University Hospital, and colleagues wrote.
Therefore, Dr. Knudsen said in an interview, clinicians should “consider testing with FBG or OGTT when presented with borderline A1c values.”
The reason for the increase in mortality after incident type 2 diabetes diagnosis, he said, “is that the patients who would have reduced the average mortality are no longer diagnosed...This does not reflect that we are treating already diagnosed patients any worse, rather some patients are not diagnosed.”
But M. Sue Kirkman, MD, emeritus professor of medicine at the University of North Carolina at Chapel Hill, who was part of the writing group for the 2010 ADA guidelines, isn’t convinced.
“This is an interesting paper, but it is a bit hard to believe that a change in WHO recommendations would have such a large and almost immediate impact on incidence and mortality. It seems likely that ... factors [other] than just the changes in recommendations for the diagnostic test account for these findings,” she said.
Dr. Kirkman pointed to new data just out from the Centers for Disease Control and Prevention on Jan. 26 that don›t show evidence of a higher proportion of people in the United States who have undiagnosed diabetes, “which would be expected if more cases were being ‘missed’ by A1c.”
She added that the CDC incidence data “show a continuing steady rate of decline in incidence that began in 2008, before any organizations recommended using A1c to screen for or diagnose diabetes.” Moreover, “there is evidence that type 2 diabetes incidence has fallen or plateaued in many countries since 2006, well before the WHO recommendation, with most of the studies from developed countries.”
But Dr. Knudsen also cited other data, including a study that showed a drop or stabilization in diagnosed diabetes incidence in high-income countries since 2010.
“That study concluded that the reasons for the declines in the incidence of diagnosed diabetes warrant further investigation with appropriate data sources, which was a main objective of our study,” wrote Dr. Knudsen and coauthors.
Dr. Knudsen said in an interview: “We are not the first to make the point that this sudden change is related to A1c introduction...but we are the first to have the data to clearly show that is the case.”
Diabetes incidence dropped but mortality rose after 2010
The population-based longitudinal study used four Danish medical databases and included 415,553 patients treated for type 2 diabetes for the first time from 1995-2018 and 2,060,279 matched comparators not treated for diabetes.
From 1995 until the 2012 introduction of A1c as a diagnostic option, the annual standardized incidence rates of type 2 diabetes more than doubled, from 193 per 100,000 population to 396 per 100,000 population, at a rate of 4.1% per year.
But from 2011 to 2018, the annual standardized incidence rate declined by 36%, to 253 per 100,000 population, a 5.7% annualized decrease.
The increase prior to 2011 occurred in both men and women and in all age groups, while the subsequent decline was seen primarily in the older age groups. The all-cause mortality risk within the first year after diabetes diagnosis was higher than subsequent 1-year mortality risks and not different between men and women.
From the periods 1995-1997 to 2010-2012, the adjusted mortality rate among those with type 2 diabetes decreased by 44%, from 72 deaths per 1000 person-years to 40 deaths per 1000 person-years (adjusted mortality rate ratio, 0.55). After that low level in 2010-2012, mortality increased by 27% to 48 per 1000 person-years (adjusted mortality rate ratio 0.69, compared with 1995-1997).
The reversed mortality trend after 2010-2012 was caused almost entirely by the increase in the first year after diabetes diagnosis, Dr. Knudsen and colleagues noted.
According to Dr. Kirkman, “A1c is strongly predictive of complications and mortality. That plus its ease of use and the fact that more people may be screened mean it’s still a good option. But for any of these tests, people who are slightly below the cut-point should not be considered normal or low risk.”
Indeed, Dr. Knudsen and colleagues said, “these findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.”
Dr. Knudsen and Dr. Kirkman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The introduction of hemoglobin A1c as an option for diagnosing type 2 diabetes over a decade ago may have resulted in underdiagnosis, new research indicates.
In 2011, the World Health Organization advised that A1c measurement, with a cutoff value of 6.5%, could be used to diagnose diabetes. The American Diabetes Association had issued similar guidance in 2010.
Prior to that time, the less-convenient 2-hour oral glucose tolerance test (OGTT) and fasting blood glucose (FBG) were the only recommended tests. While WHO made no recommendations for interpreting values below 6.5%, the ADA designated 5.7%-6.4% as prediabetes.
The new study, published online in The Lancet Regional Health–Europe, showed that the incidence of type 2 diabetes in Denmark had been increasing prior to the 2012 adoption of A1c as a diagnostic option but declined thereafter. And all-cause mortality among people with type 2 diabetes, which had been dropping, began to increase after that time.
“Our findings suggest that fewer patients have been diagnosed with [type 2 diabetes] since A1c testing was introduced as a convenient diagnostic option. We may thus be missing a group with borderline increased A1c values that is still at high metabolic and cardiovascular risk,” Jakob S. Knudsen, MD, of the department of clinical epidemiology, Aarhus (Denmark) University Hospital, and colleagues wrote.
Therefore, Dr. Knudsen said in an interview, clinicians should “consider testing with FBG or OGTT when presented with borderline A1c values.”
The reason for the increase in mortality after incident type 2 diabetes diagnosis, he said, “is that the patients who would have reduced the average mortality are no longer diagnosed...This does not reflect that we are treating already diagnosed patients any worse, rather some patients are not diagnosed.”
But M. Sue Kirkman, MD, emeritus professor of medicine at the University of North Carolina at Chapel Hill, who was part of the writing group for the 2010 ADA guidelines, isn’t convinced.
“This is an interesting paper, but it is a bit hard to believe that a change in WHO recommendations would have such a large and almost immediate impact on incidence and mortality. It seems likely that ... factors [other] than just the changes in recommendations for the diagnostic test account for these findings,” she said.
Dr. Kirkman pointed to new data just out from the Centers for Disease Control and Prevention on Jan. 26 that don›t show evidence of a higher proportion of people in the United States who have undiagnosed diabetes, “which would be expected if more cases were being ‘missed’ by A1c.”
She added that the CDC incidence data “show a continuing steady rate of decline in incidence that began in 2008, before any organizations recommended using A1c to screen for or diagnose diabetes.” Moreover, “there is evidence that type 2 diabetes incidence has fallen or plateaued in many countries since 2006, well before the WHO recommendation, with most of the studies from developed countries.”
But Dr. Knudsen also cited other data, including a study that showed a drop or stabilization in diagnosed diabetes incidence in high-income countries since 2010.
“That study concluded that the reasons for the declines in the incidence of diagnosed diabetes warrant further investigation with appropriate data sources, which was a main objective of our study,” wrote Dr. Knudsen and coauthors.
Dr. Knudsen said in an interview: “We are not the first to make the point that this sudden change is related to A1c introduction...but we are the first to have the data to clearly show that is the case.”
Diabetes incidence dropped but mortality rose after 2010
The population-based longitudinal study used four Danish medical databases and included 415,553 patients treated for type 2 diabetes for the first time from 1995-2018 and 2,060,279 matched comparators not treated for diabetes.
From 1995 until the 2012 introduction of A1c as a diagnostic option, the annual standardized incidence rates of type 2 diabetes more than doubled, from 193 per 100,000 population to 396 per 100,000 population, at a rate of 4.1% per year.
But from 2011 to 2018, the annual standardized incidence rate declined by 36%, to 253 per 100,000 population, a 5.7% annualized decrease.
The increase prior to 2011 occurred in both men and women and in all age groups, while the subsequent decline was seen primarily in the older age groups. The all-cause mortality risk within the first year after diabetes diagnosis was higher than subsequent 1-year mortality risks and not different between men and women.
From the periods 1995-1997 to 2010-2012, the adjusted mortality rate among those with type 2 diabetes decreased by 44%, from 72 deaths per 1000 person-years to 40 deaths per 1000 person-years (adjusted mortality rate ratio, 0.55). After that low level in 2010-2012, mortality increased by 27% to 48 per 1000 person-years (adjusted mortality rate ratio 0.69, compared with 1995-1997).
The reversed mortality trend after 2010-2012 was caused almost entirely by the increase in the first year after diabetes diagnosis, Dr. Knudsen and colleagues noted.
According to Dr. Kirkman, “A1c is strongly predictive of complications and mortality. That plus its ease of use and the fact that more people may be screened mean it’s still a good option. But for any of these tests, people who are slightly below the cut-point should not be considered normal or low risk.”
Indeed, Dr. Knudsen and colleagues said, “these findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.”
Dr. Knudsen and Dr. Kirkman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET REGIONAL HEALTH–EUROPE
Oral Isotretinoin for Acne in the US Military: How Accelerated Courses and Teledermatology Can Minimize the Duty-Limiting Impacts of Treatment
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
Acne vulgaris is an extremely common dermatologic disease affecting 40 to 50 million individuals in the United States each year, with a prevalence of 85% in adolescents and young adults aged 12 to 24 years. For some patients, the disease may persist well into adulthood, affecting 8% of adults aged 25 and 34 years.1 Acne negatively impacts patients’ quality of life and productivity, with an estimated direct and indirect cost of over $3 billion per year.2
Oral isotretinoin, a vitamin A derivative, is approved by the US Food and Drug Administration for the treatment of severe nodulocystic acne. Isotretinoin reduces the size and secretions of sebaceous glands, inhibits growth and resulting inflammation of Cutibacterium acnes, and normalizes the differentiation of follicular keratinocytes, resulting in permanent changes in the pathogenesis of acne that may lead to remission.3 The use of oral isotretinoin in the active-duty US Military population may cause service members to be nondeployable or limit their ability to function in special roles (eg, pilot, submariner).4 Treatment regimens that minimize the course duration of isotretinoin and reduce the risk for relapse that requires a retrial of isotretinoin may, in turn, increase a service member’s readiness, deployment availability, and ability to perform unique occupational roles.
Additionally, teledermatology has been increasingly utilized to maintain treatment continuity for patients on isotretinoin during the COVID-19 pandemic.5 Application of this technology in the military also may be used to facilitate timely isotretinoin treatment regimens in active-duty service members to minimize course duration and increase readiness.
In this article, we discuss an accelerated course of oral isotretinoin as a safe and effective option for military service members bound by duty restrictions and operational timelines and explore the role of teledermatology for the treatment of acne in military service members.
Isotretinoin for Acne
Isotretinoin typically is initiated at a dosage of 0.5 mg/kg daily, increasing to 1 mg/kg daily with a goal cumulative dose between 120 and 150 mg/kg. Relapse may occur after completing a treatment course and is associated with cumulative dosing less than 120 mg/kg.6 The average duration of acne treatment with oral isotretinoin is approximately 6 months.7 At therapeutic doses, nearly all patients experience side effects, most commonly dryness and desquamation of the skin and mucous membranes, as well as possible involvement of the lips, eyes, and nose. Notable extracutaneous side effects include headache, visual disturbances at night, idiopathic intracranial hypertension, and myalgia. Serum cholesterol, triglycerides, and transaminases may be increased in patients taking isotretinoin, which requires routine monitoring using serum lipid profiles and liver function studies. A potential association between isotretinoin and inflammatory bowel disease and changes in mood have been reported, but current data do not suggest an evidence-based link.6,8 Isotretinoin is a potent teratogen, and in the United States, all patients are required to enroll in iPLEDGE, a US Food and Drug Administration–approved pregnancy prevention program that monitors prescribing and dispensing of the medication. For patients who can become pregnant, iPLEDGE requires use of 2 forms of contraception as well as monthly pregnancy tests prior to dispensing the medication.
Acne in Military Service Members
Acne is exceedingly common in the active-duty military population. In 2018, more than 40% of soldiers, sailors, airmen, and marines were 25 years or younger, and 75% of all US service members were 35 years or younger, corresponding to acne peak incidences.1,9 Management of acne in this population requires unique treatment considerations due to distinctive occupational requirements of and hazards faced by military personnel. Use of personal protective equipment, including gas masks, safety restraints, parachute rigging, and flak jackets, may be limiting in individuals with moderate to severe acne.10 For example, severe nodulocystic acne on the chin and jawline can interfere with proper wear of the chin strap on a Kevlar helmet. The severity of acne often necessitates the use of oral isotretinoin therapy, which is considered disqualifying for many special military assignments, including submarine duty, nuclear field duty, and diving duty.11 In military aviation communities, oral isotretinoin requires grounding for the duration of therapy plus 3 months after cessation. Slit-lamp examination, triglycerides, and transaminase levels must be normal prior to returning to unrestricted duty.12 Furthermore, use of oral isotretinoin may limit overseas assignments or deployment eligibility.4
The high prevalence of acne and the operationally limiting consequences of isotretinoin therapy present a unique challenge for dermatologists treating military personnel. The average duration of isotretinoin treatment is approximately 6 months,7 which represents a considerable amount of time during an average 4-year enlistment contract. Therapeutic treatment strategies that (1) reduce the duration of oral isotretinoin therapy, (2) reduce the risk for relapse, and (3) increase medication compliance can reduce the operational impact of this acne treatment. Such treatment strategies are discussed below.
High-Dose Isotretinoin
An optimal isotretinoin dosing regimen would achieve swift resolution of acne lesions and reduce the overall relapse rate requiring retrial of isotretinoin, thereby minimizing the operational- and duty-limiting impacts of the medication. Cyrulnik et al13 studied treatment outcomes of high-dose isotretinoin for acne vulgaris using a mean dosage of 1.6 mg/kg daily with an average cumulative dosage of 290 mg/kg. They demonstrated 100% clearance of lesions over 6 months, with a 12.5% relapse rate at 3 years. Aside from an increased rate of elevated transaminases, incidence of adverse effects and laboratory abnormalities were not significantly increased compared to conventional dosing regimens.13 The goal cumulative dosing of 120 to 150 mg/kg can be achieved 1 to 2 months earlier using a dosage of 1.6 mg/kg daily vs a conventional dosage of 1 mg/kg daily.
It has been hypothesized that higher cumulative doses of oral isotretinoin reduce the risk for relapse of acne and retrial of oral isotretinoin.14 Blasiak et al15 studied relapse and retrial of oral isotretinoin in acne patients who received cumulative dosing higher or lower than 220 mg/kg. A clinically but not statistically significant reduced relapse rate was observed in the cohort that received cumulative dosing higher than 220 mg/kg. No statistically significant difference in rates of adverse advents was observed aside from an increase in retinoid dermatitis in the cohort that received cumulative dosing higher than 220 mg/kg. Higher but not statistically significant rates of adverse events were seen in the group that received dosing higher than 220 mg/kg.15 Cumulative doses of oral isotretinoin higher than the 120 to 150 mg/kg range may decrease the risk for acne relapse and the need for an additional course of oral isotretinoin, which would reduce a service member’s total time away from deployment and full duty.
Relapse requiring a retrial of oral isotretinoin not only increases the operational cost of acne treatment but also considerably increases the monetary cost to the health care system. In a cost-analysis model, cumulative doses of oral isotretinoin higher than 230 mg/kg have a decreased overall cost compared to traditional cumulative dosing of less than 150 mg/kg due to the cost of relapse.16
Limitations of high daily and cumulative dosing regimens of oral isotretinoin are chiefly the dose-dependent rate of adverse effects. Low-dose regimens are associated with a reduced risk of isotretinoin-related side effects.6,17 Acute acne flares may be seen following initial administration of oral isotretinoin and are aggravated by increases in dosage.18 Isotretinoin-induced acne fulminans is a rare but devastating complication observed with high initial doses of oral isotretinoin in patients with severe acne.19 The risks and benefits of high daily and cumulatively dosed isotretinoin must be carefully considered in patients with severe acne.
Teledermatology: A Force for Readiness
The COVID-19 pandemic drastically changed the dermatology practice landscape with recommendations to cancel all elective outpatient visits in favor of teledermatology encounters.20 This decreased access to care, which resulted in an increase in drug interruption for dermatology patients, including patients on oral isotretinoin.21 Teledermatology has been increasingly utilized to maintain continuity of care for the management of patients taking isotretinoin.5 Routine utilization of teledermatology evaluation in military practices could expedite care, decrease patient travel time, and allow for in-clinic visits to be utilized for higher-acuity concerns.22
The use of teledermatology for uncomplicated oral isotretinoin management has the potential to increase medication compliance and decrease the amount of travel time for active-duty service members; for example, consider a military dermatology practice based in San Diego, California, that accepts referrals from military bases 3 hours away by car. After an initial consultation for consideration and initiation of oral isotretinoin, teledermatology appointments can save the active-duty service member 3 hours of travel time for each follow-up visit per month. This ultimately increases operational productivity, reduces barriers to accessing care, and improves patient satisfaction.23
Although military personnel usually are located at duty stations for 2 to 4 years, training exercises and military vocational schools often temporarily take personnel away from their home station. These temporary-duty assignments have the potential to interrupt medical follow-up appointments and may cause delays in treatment for individuals who miss monthly isotretinoin visits. When deemed appropriate by the prescribing dermatologist, teledermatology allows for increased continuity of care for active-duty service members and maintenance of a therapeutic isotretinoin course despite temporary geographic displacement.
By facilitating regular follow-up appointments, teledermatology can minimize the amount of time an active-duty service member is on a course of oral isotretinoin, thereby reducing the operational and duty-limiting implications of the medication.
Final Thoughts
Acne is a common dermatologic concern within the active-duty military population. Oral isotretinoin is indicated for treatment-resistant moderate or severe acne; however, it limits the ability of service members to deploy and is disqualifying for special military assignments. High daily- and cumulative-dose isotretinoin treatment strategies can reduce the duration of therapy and may be associated with a decrease in acne relapse and the need for retrial. Teledermatology can increase access to care and facilitate the completion of oral isotretinoin courses in a timely manner. These treatment strategies may help mitigate the duty-limiting impact of oral isotretinoin therapy in military service members.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34-S37. doi:10.1016/s0190-9622(98)70442-6
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. doi:10.1016/j.jaad.2006.05.048
- James WD. Clinical practice. acne. N Engl J Med. 2005;352:1463-1472. doi:10.1056/NEJMcp033487
- Burke KR, Larrymore DC, Cho SH. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Rosamilia LL. Isotretinoin meets COVID-19: revisiting a fragmented paradigm. Cutis. 2021;108:8-12. doi:10.12788/cutis.0299
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Huang KE, Carstensen SE, Feldman SR. The duration of acne treatment. J Drugs Dermatol. 2014;13:655-656.
- Bettoli V, Guerra-Tapia A, Herane MI, et al. Challenges and solutions in oral isotretinoin in acne: reflections on 35 years of experience. Clin Cosmet Investig Dermatol. 2019;12:943-951. doi:10.2147/CCID.S234231
- US Department of Defense. 2018 demographics report: profile of the military community. Accessed January 18, 2022. https://download.militaryonesource.mil/12038/MOS/Reports/2018-demographics-report.pdf
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- US Department of the Navy. Change 167. manual of the medical department. Published February 15, 2019. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- US Department of the Navy. US Navy aeromedical reference and waiver guide. Published August 11, 2021. Accessed January 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3d%3d
- Cyrulnik AA, Viola KV, Gewirtzman AJ, et al. High-dose isotretinoin in acne vulgaris: improved treatment outcomes and quality of life. Int J Dermatol. 2012;51:1123-1130. doi:10.1111/j.1365-4632.2011.05409.x
- Coloe J, Du H, Morrell DS. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65:422-423. doi:10.1016/j.jaad.2010.06.025
- Blasiak RC, Stamey CR, Burkhart CN, et al. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149:1392-1398. doi:10.1001/jamadermatol.2013.6746
- Zeitany AE, Bowers EV, Morrell DS. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74:174-176. doi:10.1016/j.jaad.2015.08.012
- Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644-666. doi:10.1016/j.jaad.2005.11.1061
- Borghi A, Mantovani L, Minghetti S, et al. Acute acne flare following isotretinoin administration: potential protective role of low starting dose. Dermatology. 2009;218:178-180. doi:10.1159/000182270
- Greywal T, Zaenglein AL, Baldwin HE, et al. Evidence-based recommendations for the management of acne fulminans and its variants. J Am Acad Dermatol. 2017;77:109-117. doi:10.1016/j.jaad.2016.11.028
- Kwatra SG, Sweren RJ, Grossberg AL. Dermatology practices as vectors for COVID-19 transmission: a call for immediate cessation of nonemergent dermatology visits. J Am Acad Dermatol. 2020;82:E179-E180. doi:10.1016/j.jaad.2020.03.037
- Alshiyab DM, Al-Qarqaz FA, Muhaidat JM. Impact of COVID-19 pandemic on the continuity of care for dermatologic patients on systemic therapy during the period of strict lockdown. Ann Med Surg (Lond). 2020;60:571-574. doi:10.1016/j.amsu.2020.11.056
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335,337,345.
- Ruggiero A, Megna M, Annunziata MC, et al. Teledermatology for acne during COVID-19: high patients’ satisfaction in spite of the emergency. J Eur Acad Dermatol Venereol. 2020;34:E662-E663. doi:10.1111/jdv.16746
Practice Points
- Acne is a common skin disease with a high prevalence in the active-duty US Military population.
- Oral isotretinoin is a commonly utilized acne medication that can limit the ability for military service members to deploy and is considered disqualifying for some special duty assignments.
- High daily- and cumulative-dose oral isotretinoin therapy as well as teledermatology can minimize the duty-limiting impact of isotretinoin therapy for military service members.
Vaginal progesterone for preterm birth has mixed results
The potential effectiveness of using vaginal progesterone to prevent preterm birth in two different populations was the focus of a pair of studies with mixed results at the annual meeting sponsored by the Society for Maternal-Fetal Medicine on Feb. 3. One study found no benefit from vaginal progesterone in those with first trimester bleeding, while the other, in a head-to-head comparison with 17-alpha-hydroxyprogesterone caproate (17-OHPC), found vaginal progesterone performs similarly to 17-OHPC in singleton pregnancies with a history of preterm birth.
While the first study does not suggest any changes in clinical practice, the second one suggests that vaginal progesterone is an alternative to 17-OHPC, as the American College of Obstetricians and Gynecologists currently recommends. SMFM currently only includes 17-OHPC in its guidelines.
“In otherwise low-risk pregnancies with first trimester bleeding, progesterone should not be prescribed for the prevention of miscarriage or prematurity,” Haim A. Abenhaim, MD, MPH, of the Jewish General Hospital at McGill University, Montreal, told attendees in his presentation.
”Publishing the negative result is so important because this helps the overall body of literature reduce the amount of publication bias that exists in the literature,” Michael Richley, MD, an ob.gyn. and maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview. Dr. Richley was not involved in the research but attended the presentation.
Vaginal progesterone for first trimester bleeding
Most preterm birth occurs in pregnancies with no identifiable risk factors, but first-trimester bleeding may indicate subchorionic hemorrhage from placental detachment, which can increase the risk of preterm birth. Other risk factors where progesterone has previously shown effectiveness in reducing preterm birth risk include short cervix and a history of prior preterm birth.
The first study (PREEMPT) was a double-blind, randomized controlled trial conducted at six Canadian hospitals with 533 women. The participants all experienced bleeding within the first 14 weeks of pregnancy and a documented subchorionic hemorrhage. The trial excluded those who already required progesterone, had contraindications to progesterone or had an alternate cause of bleeding.
The intervention group included 264 women randomly assigned to use 200 mg of micronized progesterone administered with a vaginal suppository at bedtime, while the placebo group included 269 women who used a vaginal suppository with no medication, both administered until 34 weeks of pregnancy. The groups were not significantly different in age, race, or former pregnancies, live births, and miscarriages. They were also similar in clinical characteristics of bleeding and subchorionic hemorrhage.
The proportion of term births was similar between the progesterone (74.6%) and placebo (70.6%) groups (P =.3), as was the proportion of preterm births (10.2% progesterone vs. 12.3% placebo, P =.46). There were also no significant differences in the secondary outcomes of cramping, hospital admission, bed rest, or preventive measures, including pessary, cerclage, antibiotics, magnesium, and nifedipine. Newborns across both groups were statistically similar in average birth weight and distribution of birth weights and in incidence of neonatal ICU admission or respiratory distress syndrome. Adverse events were similar across both groups.
”The results are not surprising as several studies in the past have shown similar lack of efficacy,” Dr. said. “The pathophysiology of subchorionic hematoma is different from the multifactorial etiologies of spontaneous preterm birth, and given our lack of clear understanding of the actions of progesterone, the lack of efficacy in this subgroup with subchorionic hematoma is not surprising.”
Dr. Richley did note that having a low-risk population to start with may have affected the findings, which might be different in a high-risk population.
“I don’t believe this will change anything within clinical practice. At this time, progesterone is not used in any form in the setting of first trimester threatened abortion by maternal-fetal medicine specialists,” Dr. Richley said. “There may be other subgroups of clinicians who do prescribe progesterone in this setting, and these data should further encourage them to move away from this practice.”
Dr. Abenhaim noted a couple unexpected issues that occurred during the course of the study, such as underreporting of subchorionic hemorrhage with radiologic confirmation that resulted in a smaller population and a change in protocol to include patients with no identifiable secondary source of bleeding. The pandemic also halted enrollment, and the investigators halted the trial when recruitment could have continued since interim analysis showed no likely benefit.
Though first trimester bleeding is associated with a 25%-30% increased risk of miscarriage or preterm birth, the findings showed that progesterone did not prevent miscarriage or prematurity, or increase the live birth rate, in low-risk patients with first trimester bleeding.
Vaginal progesterone vs. 17-OHPC
Although a 2017 meta-analysis had found vaginal progesterone to be superior to 17-OHPC in preventing preterm birth, few studies were available, and they had wide confidence intervals. This open-label randomized controlled trial took place at five U.S. sites and included participants who had a singleton pregnancy less than 24 weeks along and a history of singleton preterm birth between 16 and 37 weeks. The trials excluded those with placenta previa or accreta, preterm labor, preterm premature rupture of the membranes, clinical chorioamnionitis, or a major fetal anomaly or chromosomal disorder.
Among 205 women initially randomized, 94 in each group completed the trial, either inserting 200 mg of micronized progesterone daily with a vaginal suppository or receiving 250 mg of weekly intramuscular injections of 17-OHPC from 16 to 36 weeks’ gestation. The only significant difference between the groups in demographics or clinical features was that the vaginal progesterone group had a higher proportion of multiple past preterm births (33%) compared with the 17-OHPC group (17%). Cervical length and use of cerclage were also similar between the groups.
Though 30.9% of the vaginal progesterone group delivered preterm before 37 weeks, compared with 38.3% in the 17-OHPC group, the difference was not significant (P =.28). There was a borderline statistical difference between gestational age at delivery: 37.4 weeks in the vaginal progesterone group versus 36.3 weeks in the 17-OHPC group (P =.047). Neonatal outcomes were clinically similar between the two groups. Therapy initiation did slightly differ between the groups, with an average start 1 gestational week earlier in the vaginal progesterone group (16.9 vs. 17.8, P =.001) and a higher proportion of patients in the 17-OHPC group initiating therapy after 20 weeks (16.5% vs. 2.2%, P =.001). Adherence was otherwise similar between the groups, and the groups reported similar rates and types of side effects.
The trial did not meet the primary endpoint of vaginal progesterone reducing risk of recurrent preterm birth by 50%, compared with 17-OHPC, but it may increase latency to delivery, Rupsa C. Boelig, MD, of Thomas Jefferson University, Philadelphia, told attendees. Though this was the largest trial to compare vaginal progesterone with 17-OHPC for preventing preterm birth, it was underpowered to detect a difference in efficacy, to conduct subgroup analyses, and to assess secondary outcomes, Dr. Boelig noted. “Baseline difference in preterm birth risk may affect apparent relative efficacy of vaginal progesterone.”
Nevertheless, the “totality of evidence appears to be greater for vaginal progesterone,” Dr. Boelig said, making vaginal progesterone an acceptable alternative to 17-OHPC. ACOG recommendations currently include offering either, but SMFM recommendations only mention 17-OHPC.
It’s worth noting, however, that the future of 17-OHPC, a synthetic compound, compared with naturally occurring micronized progesterone, continues to be uncertain following a 2020 study that found no evidence of its efficacy, leading the Food and Drug Administration to withdraw its approval for prevention of preterm birth. ”These findings are important especially in light of the controversy surrounding 17-OHP,” Jenny Mei, MD, a maternal-fetal medicine fellow at UCLA, said in an interview after attending the presentation. “It is also sometimes difficult for patients to commit to weekly 17-OHPC injections, which requires time and many doctors visits, as compared to vaginal progesterone, which patients can administer at home.” Since this study does not have a placebo group, “it does not address the question of the overall efficacy of either medication compared to a control,” Dr. Mei said. ”It is also a somewhat small patient population so the results may change with a larger population. The authors conclude it is worth readdressing the use of vaginal progesterone for these patients.”
Herman L. Hedriana, MD, professor and director of the division of maternal-fetal medicine and the maternal-fetal medicine fellowship program at the University of California, Davis, also pointed out notable differences between the two compounds.
“One has to remember that the formulation and mechanism of action are very different between 17-OHPC and the vaginal application of micronized progesterone. We do not have enough data to say one is superior versus the other,” said Dr. Hedriana, who was not involved in the research. “With 17-OHPC, the mechanism of action appears to be influenced by how the drug is metabolized based on race and ethnicity makeup, and may be influence by epigenetics,” while the mechanism for vaginal progesterone is probably local “given it is applied directly next to the cervix; hence, the results are it is effective in short cervices.” But those differing mechanisms don’t change the clinical significance of the findings. “One can use vaginal progesterone or 17-OHPC based on patient preference and availability,” Dr. Hedriana said.
The researchers of both studies reported no personal financial or industry disclosures, though Dr. Boelig disclosed that she had taken 17-OHPC and had cerclages during both her pregnancies, which resulted in healthy children today. The PREEMPT trial was funded by the Canadian Institutes of Health Research. The head-to-head trial was funded by the National Institute of Child Health and Development, the March of Dimes, the EW Thrasher Foundation, the PhRMA Foundation, and Covis Pharma, who manufactures the 17-OHPC drug Makena.
This story was updated on 2/8/2022.
The potential effectiveness of using vaginal progesterone to prevent preterm birth in two different populations was the focus of a pair of studies with mixed results at the annual meeting sponsored by the Society for Maternal-Fetal Medicine on Feb. 3. One study found no benefit from vaginal progesterone in those with first trimester bleeding, while the other, in a head-to-head comparison with 17-alpha-hydroxyprogesterone caproate (17-OHPC), found vaginal progesterone performs similarly to 17-OHPC in singleton pregnancies with a history of preterm birth.
While the first study does not suggest any changes in clinical practice, the second one suggests that vaginal progesterone is an alternative to 17-OHPC, as the American College of Obstetricians and Gynecologists currently recommends. SMFM currently only includes 17-OHPC in its guidelines.
“In otherwise low-risk pregnancies with first trimester bleeding, progesterone should not be prescribed for the prevention of miscarriage or prematurity,” Haim A. Abenhaim, MD, MPH, of the Jewish General Hospital at McGill University, Montreal, told attendees in his presentation.
”Publishing the negative result is so important because this helps the overall body of literature reduce the amount of publication bias that exists in the literature,” Michael Richley, MD, an ob.gyn. and maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview. Dr. Richley was not involved in the research but attended the presentation.
Vaginal progesterone for first trimester bleeding
Most preterm birth occurs in pregnancies with no identifiable risk factors, but first-trimester bleeding may indicate subchorionic hemorrhage from placental detachment, which can increase the risk of preterm birth. Other risk factors where progesterone has previously shown effectiveness in reducing preterm birth risk include short cervix and a history of prior preterm birth.
The first study (PREEMPT) was a double-blind, randomized controlled trial conducted at six Canadian hospitals with 533 women. The participants all experienced bleeding within the first 14 weeks of pregnancy and a documented subchorionic hemorrhage. The trial excluded those who already required progesterone, had contraindications to progesterone or had an alternate cause of bleeding.
The intervention group included 264 women randomly assigned to use 200 mg of micronized progesterone administered with a vaginal suppository at bedtime, while the placebo group included 269 women who used a vaginal suppository with no medication, both administered until 34 weeks of pregnancy. The groups were not significantly different in age, race, or former pregnancies, live births, and miscarriages. They were also similar in clinical characteristics of bleeding and subchorionic hemorrhage.
The proportion of term births was similar between the progesterone (74.6%) and placebo (70.6%) groups (P =.3), as was the proportion of preterm births (10.2% progesterone vs. 12.3% placebo, P =.46). There were also no significant differences in the secondary outcomes of cramping, hospital admission, bed rest, or preventive measures, including pessary, cerclage, antibiotics, magnesium, and nifedipine. Newborns across both groups were statistically similar in average birth weight and distribution of birth weights and in incidence of neonatal ICU admission or respiratory distress syndrome. Adverse events were similar across both groups.
”The results are not surprising as several studies in the past have shown similar lack of efficacy,” Dr. said. “The pathophysiology of subchorionic hematoma is different from the multifactorial etiologies of spontaneous preterm birth, and given our lack of clear understanding of the actions of progesterone, the lack of efficacy in this subgroup with subchorionic hematoma is not surprising.”
Dr. Richley did note that having a low-risk population to start with may have affected the findings, which might be different in a high-risk population.
“I don’t believe this will change anything within clinical practice. At this time, progesterone is not used in any form in the setting of first trimester threatened abortion by maternal-fetal medicine specialists,” Dr. Richley said. “There may be other subgroups of clinicians who do prescribe progesterone in this setting, and these data should further encourage them to move away from this practice.”
Dr. Abenhaim noted a couple unexpected issues that occurred during the course of the study, such as underreporting of subchorionic hemorrhage with radiologic confirmation that resulted in a smaller population and a change in protocol to include patients with no identifiable secondary source of bleeding. The pandemic also halted enrollment, and the investigators halted the trial when recruitment could have continued since interim analysis showed no likely benefit.
Though first trimester bleeding is associated with a 25%-30% increased risk of miscarriage or preterm birth, the findings showed that progesterone did not prevent miscarriage or prematurity, or increase the live birth rate, in low-risk patients with first trimester bleeding.
Vaginal progesterone vs. 17-OHPC
Although a 2017 meta-analysis had found vaginal progesterone to be superior to 17-OHPC in preventing preterm birth, few studies were available, and they had wide confidence intervals. This open-label randomized controlled trial took place at five U.S. sites and included participants who had a singleton pregnancy less than 24 weeks along and a history of singleton preterm birth between 16 and 37 weeks. The trials excluded those with placenta previa or accreta, preterm labor, preterm premature rupture of the membranes, clinical chorioamnionitis, or a major fetal anomaly or chromosomal disorder.
Among 205 women initially randomized, 94 in each group completed the trial, either inserting 200 mg of micronized progesterone daily with a vaginal suppository or receiving 250 mg of weekly intramuscular injections of 17-OHPC from 16 to 36 weeks’ gestation. The only significant difference between the groups in demographics or clinical features was that the vaginal progesterone group had a higher proportion of multiple past preterm births (33%) compared with the 17-OHPC group (17%). Cervical length and use of cerclage were also similar between the groups.
Though 30.9% of the vaginal progesterone group delivered preterm before 37 weeks, compared with 38.3% in the 17-OHPC group, the difference was not significant (P =.28). There was a borderline statistical difference between gestational age at delivery: 37.4 weeks in the vaginal progesterone group versus 36.3 weeks in the 17-OHPC group (P =.047). Neonatal outcomes were clinically similar between the two groups. Therapy initiation did slightly differ between the groups, with an average start 1 gestational week earlier in the vaginal progesterone group (16.9 vs. 17.8, P =.001) and a higher proportion of patients in the 17-OHPC group initiating therapy after 20 weeks (16.5% vs. 2.2%, P =.001). Adherence was otherwise similar between the groups, and the groups reported similar rates and types of side effects.
The trial did not meet the primary endpoint of vaginal progesterone reducing risk of recurrent preterm birth by 50%, compared with 17-OHPC, but it may increase latency to delivery, Rupsa C. Boelig, MD, of Thomas Jefferson University, Philadelphia, told attendees. Though this was the largest trial to compare vaginal progesterone with 17-OHPC for preventing preterm birth, it was underpowered to detect a difference in efficacy, to conduct subgroup analyses, and to assess secondary outcomes, Dr. Boelig noted. “Baseline difference in preterm birth risk may affect apparent relative efficacy of vaginal progesterone.”
Nevertheless, the “totality of evidence appears to be greater for vaginal progesterone,” Dr. Boelig said, making vaginal progesterone an acceptable alternative to 17-OHPC. ACOG recommendations currently include offering either, but SMFM recommendations only mention 17-OHPC.
It’s worth noting, however, that the future of 17-OHPC, a synthetic compound, compared with naturally occurring micronized progesterone, continues to be uncertain following a 2020 study that found no evidence of its efficacy, leading the Food and Drug Administration to withdraw its approval for prevention of preterm birth. ”These findings are important especially in light of the controversy surrounding 17-OHP,” Jenny Mei, MD, a maternal-fetal medicine fellow at UCLA, said in an interview after attending the presentation. “It is also sometimes difficult for patients to commit to weekly 17-OHPC injections, which requires time and many doctors visits, as compared to vaginal progesterone, which patients can administer at home.” Since this study does not have a placebo group, “it does not address the question of the overall efficacy of either medication compared to a control,” Dr. Mei said. ”It is also a somewhat small patient population so the results may change with a larger population. The authors conclude it is worth readdressing the use of vaginal progesterone for these patients.”
Herman L. Hedriana, MD, professor and director of the division of maternal-fetal medicine and the maternal-fetal medicine fellowship program at the University of California, Davis, also pointed out notable differences between the two compounds.
“One has to remember that the formulation and mechanism of action are very different between 17-OHPC and the vaginal application of micronized progesterone. We do not have enough data to say one is superior versus the other,” said Dr. Hedriana, who was not involved in the research. “With 17-OHPC, the mechanism of action appears to be influenced by how the drug is metabolized based on race and ethnicity makeup, and may be influence by epigenetics,” while the mechanism for vaginal progesterone is probably local “given it is applied directly next to the cervix; hence, the results are it is effective in short cervices.” But those differing mechanisms don’t change the clinical significance of the findings. “One can use vaginal progesterone or 17-OHPC based on patient preference and availability,” Dr. Hedriana said.
The researchers of both studies reported no personal financial or industry disclosures, though Dr. Boelig disclosed that she had taken 17-OHPC and had cerclages during both her pregnancies, which resulted in healthy children today. The PREEMPT trial was funded by the Canadian Institutes of Health Research. The head-to-head trial was funded by the National Institute of Child Health and Development, the March of Dimes, the EW Thrasher Foundation, the PhRMA Foundation, and Covis Pharma, who manufactures the 17-OHPC drug Makena.
This story was updated on 2/8/2022.
The potential effectiveness of using vaginal progesterone to prevent preterm birth in two different populations was the focus of a pair of studies with mixed results at the annual meeting sponsored by the Society for Maternal-Fetal Medicine on Feb. 3. One study found no benefit from vaginal progesterone in those with first trimester bleeding, while the other, in a head-to-head comparison with 17-alpha-hydroxyprogesterone caproate (17-OHPC), found vaginal progesterone performs similarly to 17-OHPC in singleton pregnancies with a history of preterm birth.
While the first study does not suggest any changes in clinical practice, the second one suggests that vaginal progesterone is an alternative to 17-OHPC, as the American College of Obstetricians and Gynecologists currently recommends. SMFM currently only includes 17-OHPC in its guidelines.
“In otherwise low-risk pregnancies with first trimester bleeding, progesterone should not be prescribed for the prevention of miscarriage or prematurity,” Haim A. Abenhaim, MD, MPH, of the Jewish General Hospital at McGill University, Montreal, told attendees in his presentation.
”Publishing the negative result is so important because this helps the overall body of literature reduce the amount of publication bias that exists in the literature,” Michael Richley, MD, an ob.gyn. and maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview. Dr. Richley was not involved in the research but attended the presentation.
Vaginal progesterone for first trimester bleeding
Most preterm birth occurs in pregnancies with no identifiable risk factors, but first-trimester bleeding may indicate subchorionic hemorrhage from placental detachment, which can increase the risk of preterm birth. Other risk factors where progesterone has previously shown effectiveness in reducing preterm birth risk include short cervix and a history of prior preterm birth.
The first study (PREEMPT) was a double-blind, randomized controlled trial conducted at six Canadian hospitals with 533 women. The participants all experienced bleeding within the first 14 weeks of pregnancy and a documented subchorionic hemorrhage. The trial excluded those who already required progesterone, had contraindications to progesterone or had an alternate cause of bleeding.
The intervention group included 264 women randomly assigned to use 200 mg of micronized progesterone administered with a vaginal suppository at bedtime, while the placebo group included 269 women who used a vaginal suppository with no medication, both administered until 34 weeks of pregnancy. The groups were not significantly different in age, race, or former pregnancies, live births, and miscarriages. They were also similar in clinical characteristics of bleeding and subchorionic hemorrhage.
The proportion of term births was similar between the progesterone (74.6%) and placebo (70.6%) groups (P =.3), as was the proportion of preterm births (10.2% progesterone vs. 12.3% placebo, P =.46). There were also no significant differences in the secondary outcomes of cramping, hospital admission, bed rest, or preventive measures, including pessary, cerclage, antibiotics, magnesium, and nifedipine. Newborns across both groups were statistically similar in average birth weight and distribution of birth weights and in incidence of neonatal ICU admission or respiratory distress syndrome. Adverse events were similar across both groups.
”The results are not surprising as several studies in the past have shown similar lack of efficacy,” Dr. said. “The pathophysiology of subchorionic hematoma is different from the multifactorial etiologies of spontaneous preterm birth, and given our lack of clear understanding of the actions of progesterone, the lack of efficacy in this subgroup with subchorionic hematoma is not surprising.”
Dr. Richley did note that having a low-risk population to start with may have affected the findings, which might be different in a high-risk population.
“I don’t believe this will change anything within clinical practice. At this time, progesterone is not used in any form in the setting of first trimester threatened abortion by maternal-fetal medicine specialists,” Dr. Richley said. “There may be other subgroups of clinicians who do prescribe progesterone in this setting, and these data should further encourage them to move away from this practice.”
Dr. Abenhaim noted a couple unexpected issues that occurred during the course of the study, such as underreporting of subchorionic hemorrhage with radiologic confirmation that resulted in a smaller population and a change in protocol to include patients with no identifiable secondary source of bleeding. The pandemic also halted enrollment, and the investigators halted the trial when recruitment could have continued since interim analysis showed no likely benefit.
Though first trimester bleeding is associated with a 25%-30% increased risk of miscarriage or preterm birth, the findings showed that progesterone did not prevent miscarriage or prematurity, or increase the live birth rate, in low-risk patients with first trimester bleeding.
Vaginal progesterone vs. 17-OHPC
Although a 2017 meta-analysis had found vaginal progesterone to be superior to 17-OHPC in preventing preterm birth, few studies were available, and they had wide confidence intervals. This open-label randomized controlled trial took place at five U.S. sites and included participants who had a singleton pregnancy less than 24 weeks along and a history of singleton preterm birth between 16 and 37 weeks. The trials excluded those with placenta previa or accreta, preterm labor, preterm premature rupture of the membranes, clinical chorioamnionitis, or a major fetal anomaly or chromosomal disorder.
Among 205 women initially randomized, 94 in each group completed the trial, either inserting 200 mg of micronized progesterone daily with a vaginal suppository or receiving 250 mg of weekly intramuscular injections of 17-OHPC from 16 to 36 weeks’ gestation. The only significant difference between the groups in demographics or clinical features was that the vaginal progesterone group had a higher proportion of multiple past preterm births (33%) compared with the 17-OHPC group (17%). Cervical length and use of cerclage were also similar between the groups.
Though 30.9% of the vaginal progesterone group delivered preterm before 37 weeks, compared with 38.3% in the 17-OHPC group, the difference was not significant (P =.28). There was a borderline statistical difference between gestational age at delivery: 37.4 weeks in the vaginal progesterone group versus 36.3 weeks in the 17-OHPC group (P =.047). Neonatal outcomes were clinically similar between the two groups. Therapy initiation did slightly differ between the groups, with an average start 1 gestational week earlier in the vaginal progesterone group (16.9 vs. 17.8, P =.001) and a higher proportion of patients in the 17-OHPC group initiating therapy after 20 weeks (16.5% vs. 2.2%, P =.001). Adherence was otherwise similar between the groups, and the groups reported similar rates and types of side effects.
The trial did not meet the primary endpoint of vaginal progesterone reducing risk of recurrent preterm birth by 50%, compared with 17-OHPC, but it may increase latency to delivery, Rupsa C. Boelig, MD, of Thomas Jefferson University, Philadelphia, told attendees. Though this was the largest trial to compare vaginal progesterone with 17-OHPC for preventing preterm birth, it was underpowered to detect a difference in efficacy, to conduct subgroup analyses, and to assess secondary outcomes, Dr. Boelig noted. “Baseline difference in preterm birth risk may affect apparent relative efficacy of vaginal progesterone.”
Nevertheless, the “totality of evidence appears to be greater for vaginal progesterone,” Dr. Boelig said, making vaginal progesterone an acceptable alternative to 17-OHPC. ACOG recommendations currently include offering either, but SMFM recommendations only mention 17-OHPC.
It’s worth noting, however, that the future of 17-OHPC, a synthetic compound, compared with naturally occurring micronized progesterone, continues to be uncertain following a 2020 study that found no evidence of its efficacy, leading the Food and Drug Administration to withdraw its approval for prevention of preterm birth. ”These findings are important especially in light of the controversy surrounding 17-OHP,” Jenny Mei, MD, a maternal-fetal medicine fellow at UCLA, said in an interview after attending the presentation. “It is also sometimes difficult for patients to commit to weekly 17-OHPC injections, which requires time and many doctors visits, as compared to vaginal progesterone, which patients can administer at home.” Since this study does not have a placebo group, “it does not address the question of the overall efficacy of either medication compared to a control,” Dr. Mei said. ”It is also a somewhat small patient population so the results may change with a larger population. The authors conclude it is worth readdressing the use of vaginal progesterone for these patients.”
Herman L. Hedriana, MD, professor and director of the division of maternal-fetal medicine and the maternal-fetal medicine fellowship program at the University of California, Davis, also pointed out notable differences between the two compounds.
“One has to remember that the formulation and mechanism of action are very different between 17-OHPC and the vaginal application of micronized progesterone. We do not have enough data to say one is superior versus the other,” said Dr. Hedriana, who was not involved in the research. “With 17-OHPC, the mechanism of action appears to be influenced by how the drug is metabolized based on race and ethnicity makeup, and may be influence by epigenetics,” while the mechanism for vaginal progesterone is probably local “given it is applied directly next to the cervix; hence, the results are it is effective in short cervices.” But those differing mechanisms don’t change the clinical significance of the findings. “One can use vaginal progesterone or 17-OHPC based on patient preference and availability,” Dr. Hedriana said.
The researchers of both studies reported no personal financial or industry disclosures, though Dr. Boelig disclosed that she had taken 17-OHPC and had cerclages during both her pregnancies, which resulted in healthy children today. The PREEMPT trial was funded by the Canadian Institutes of Health Research. The head-to-head trial was funded by the National Institute of Child Health and Development, the March of Dimes, the EW Thrasher Foundation, the PhRMA Foundation, and Covis Pharma, who manufactures the 17-OHPC drug Makena.
This story was updated on 2/8/2022.
FROM THE PREGNANCY MEETING
Aspirin use risk for postpartum bleeding unclear
Low-dose aspirin may increase risk of postpartum bleeding if patients don’t discontinue its use at least 7 days before delivery, but it’s otherwise unclear whether its use increases bleeding risk, according to research presented Feb. 5 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“These findings were a little surprising to me because we have generally been taught that aspirin is safe to continue up until delivery with minimal risk,” Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview after attending the presentation. “Theoretically it makes sense that it may increase bleeding risk, but multiple studies in the past analyzing its use with gynecological surgery show minimal risk, which was conferred to the obstetrical population as well.”
She noted, however, that patients prescribed low-dose aspirin already have risk factors that may increase their risk of postpartum bleeding, and the study’s finding of possible increased risk was not statistically significant after accounting for those confounders. “I wouldn’t change my practice management over it, but it does raise awareness that all interventions likely come with some risk,” Dr. Mei said.
Hypertensive disorders of pregnancy are responsible for 6.6% of U.S. pregnancy-related deaths. The SMFM currently recommends low-dose aspirin starting at 12 weeks’ gestation in patients at high risk for preeclampsia, which includes people with multifetal gestation, chronic hypertension, pregestational diabetes, renal disease, autoimmune disease, or a history of preeclampsia. However, previous research has shown mixed results on the safety of low-dose aspirin in terms of bleeding risk, Kelsey White, MD, a second-year maternal-fetal medicine fellow of the Yale University, New Haven, Conn., told attendees.
This retrospective study compared a bleeding composite endpoint among those who did and did not take low-dose aspirin between January 2018 and April 2021. The composite included an estimated blood loss of greater than 1,000 mL, postpartum hemorrhage based on ICD-9/10 code diagnosis, and red blood cell transfusion. The researchers also compared bleeding risk within the aspirin group based on discontinuation at greater or less than 7 days before delivery.
Among 16,980 patients, 11.3% were prescribed low-dose aspirin. The patients prescribed low-dose aspirin significantly differed from those not prescribed it in all demographic and clinical characteristics except placenta accreta spectrum. The average age of the aspirin group was 39 years, compared with 24 years in the nonaspirin group (P < .01). More of the aspirin group patients were Hispanic and Black, and 52.3% of patients taking aspirin had a body mass index greater than 30 kg/m2, compared with 22.9% of the nonaspirin group. Rates of diabetes, lupus, fibroids, nonaspirin anticoagulation use, cesarean delivery, and preterm delivery were all greater in the aspirin group.
In addition, 43.9% of the patients in the aspirin group had a hypertensive disorder, including 20.2% with preeclampsia, compared with 17.1% with hypertensive disorders, including 6.2% with preeclampsia, in the group not taking aspirin (P < .0001). “This shows that a high-risk population was prescribed aspirin, which correlates to the recommended prescription guidelines,” Dr. White said.
The postpartum bleeding composite outcome occurred in 14.7% of patients in the low-dose aspirin group, compared with 9.2% of patients in the nonaspirin group, for an unadjusted 1.7 times greater risk of bleeding (95% confidence interval, 1.49-1.96). After adjustment for confounders, the risk declined and was no longer statistically significant (aOR = 1.15; 95% CI, 0.98-1.34).
Meanwhile, 15% of those who discontinued aspirin within 7 days of delivery had postpartum bleeding, compared with 9% of those who discontinued aspirin at 7 or more days before delivery (P = .03).
Therefore, while the study found only a possible, nonsignificant association between low-dose aspirin and postpartum bleeding, risk of bleeding was significantly greater among those who discontinued aspirin only in the last week before delivery.
”Our study is timely and supports a recent Swedish study [that] found an increased risk of intrapartum bleeding, postpartum hemorrhage, and postpartum hematoma,” Dr. White said. She also noted that the United States Preventive Services Task Force changed their recommendation in 2021 for low-dose aspirin prophylaxis for cardiovascular disease.
“They now recommend against the use of low-dose aspirin for prevention in adults without a history of cardiovascular disease,” Dr. White said. “The change in recommendations came after recent randomized control trials showed that low-dose aspirin had very little benefit and may increase the risk of bleeding.”
However, Dr. White added that they “do not believe this study should be used to make any clinical decisions.” While the study had a large sample size, it was limited by its retrospective reliance on EMR data, including the EMR medication list, and the researchers couldn’t assess patient compliance or patient use of over-the-counter aspirin not recorded in the EMR.
Deirdre Lyell, MD, a professor of maternal-fetal medicine at Stanford (Calif.) University, agreed that the findings should not impact clinical practice given its limitations.
“The investigators could not entirely identify who stopped low-dose aspirin and when. When they estimated timing of stoppage of low-dose aspirin, their data suggested a small benefit among those who discontinued it at least 7 days before delivery, though this should be interpreted with caution, given the potential inaccuracy in these data,” Dr. Lyell, who was not involved in the study, said in an interview. “Their study did not examine factors that should be used to confirm if there are real differences in blood loss, such as changes in blood counts before and after delivery, or more use of medications that we use to stop heavy bleeding.”
In fact, Dr. Lyell noted, other research at the SMFM meeting found ”that low-dose aspirin is not used frequently enough in patients who might benefit, such as those at high risk for preeclampsia,” she said. ”Low-dose aspirin among those at increased risk has been shown to reduce rates of preeclampsia, reducing the likelihood of risky situations for moms and babies.”
The authors had no disclosures. Dr. Lyell has consulted for Bloomlife, a uterine contraction and fetal monitor. Dr. White and Dr. Mei had no disclosures.
Low-dose aspirin may increase risk of postpartum bleeding if patients don’t discontinue its use at least 7 days before delivery, but it’s otherwise unclear whether its use increases bleeding risk, according to research presented Feb. 5 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“These findings were a little surprising to me because we have generally been taught that aspirin is safe to continue up until delivery with minimal risk,” Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview after attending the presentation. “Theoretically it makes sense that it may increase bleeding risk, but multiple studies in the past analyzing its use with gynecological surgery show minimal risk, which was conferred to the obstetrical population as well.”
She noted, however, that patients prescribed low-dose aspirin already have risk factors that may increase their risk of postpartum bleeding, and the study’s finding of possible increased risk was not statistically significant after accounting for those confounders. “I wouldn’t change my practice management over it, but it does raise awareness that all interventions likely come with some risk,” Dr. Mei said.
Hypertensive disorders of pregnancy are responsible for 6.6% of U.S. pregnancy-related deaths. The SMFM currently recommends low-dose aspirin starting at 12 weeks’ gestation in patients at high risk for preeclampsia, which includes people with multifetal gestation, chronic hypertension, pregestational diabetes, renal disease, autoimmune disease, or a history of preeclampsia. However, previous research has shown mixed results on the safety of low-dose aspirin in terms of bleeding risk, Kelsey White, MD, a second-year maternal-fetal medicine fellow of the Yale University, New Haven, Conn., told attendees.
This retrospective study compared a bleeding composite endpoint among those who did and did not take low-dose aspirin between January 2018 and April 2021. The composite included an estimated blood loss of greater than 1,000 mL, postpartum hemorrhage based on ICD-9/10 code diagnosis, and red blood cell transfusion. The researchers also compared bleeding risk within the aspirin group based on discontinuation at greater or less than 7 days before delivery.
Among 16,980 patients, 11.3% were prescribed low-dose aspirin. The patients prescribed low-dose aspirin significantly differed from those not prescribed it in all demographic and clinical characteristics except placenta accreta spectrum. The average age of the aspirin group was 39 years, compared with 24 years in the nonaspirin group (P < .01). More of the aspirin group patients were Hispanic and Black, and 52.3% of patients taking aspirin had a body mass index greater than 30 kg/m2, compared with 22.9% of the nonaspirin group. Rates of diabetes, lupus, fibroids, nonaspirin anticoagulation use, cesarean delivery, and preterm delivery were all greater in the aspirin group.
In addition, 43.9% of the patients in the aspirin group had a hypertensive disorder, including 20.2% with preeclampsia, compared with 17.1% with hypertensive disorders, including 6.2% with preeclampsia, in the group not taking aspirin (P < .0001). “This shows that a high-risk population was prescribed aspirin, which correlates to the recommended prescription guidelines,” Dr. White said.
The postpartum bleeding composite outcome occurred in 14.7% of patients in the low-dose aspirin group, compared with 9.2% of patients in the nonaspirin group, for an unadjusted 1.7 times greater risk of bleeding (95% confidence interval, 1.49-1.96). After adjustment for confounders, the risk declined and was no longer statistically significant (aOR = 1.15; 95% CI, 0.98-1.34).
Meanwhile, 15% of those who discontinued aspirin within 7 days of delivery had postpartum bleeding, compared with 9% of those who discontinued aspirin at 7 or more days before delivery (P = .03).
Therefore, while the study found only a possible, nonsignificant association between low-dose aspirin and postpartum bleeding, risk of bleeding was significantly greater among those who discontinued aspirin only in the last week before delivery.
”Our study is timely and supports a recent Swedish study [that] found an increased risk of intrapartum bleeding, postpartum hemorrhage, and postpartum hematoma,” Dr. White said. She also noted that the United States Preventive Services Task Force changed their recommendation in 2021 for low-dose aspirin prophylaxis for cardiovascular disease.
“They now recommend against the use of low-dose aspirin for prevention in adults without a history of cardiovascular disease,” Dr. White said. “The change in recommendations came after recent randomized control trials showed that low-dose aspirin had very little benefit and may increase the risk of bleeding.”
However, Dr. White added that they “do not believe this study should be used to make any clinical decisions.” While the study had a large sample size, it was limited by its retrospective reliance on EMR data, including the EMR medication list, and the researchers couldn’t assess patient compliance or patient use of over-the-counter aspirin not recorded in the EMR.
Deirdre Lyell, MD, a professor of maternal-fetal medicine at Stanford (Calif.) University, agreed that the findings should not impact clinical practice given its limitations.
“The investigators could not entirely identify who stopped low-dose aspirin and when. When they estimated timing of stoppage of low-dose aspirin, their data suggested a small benefit among those who discontinued it at least 7 days before delivery, though this should be interpreted with caution, given the potential inaccuracy in these data,” Dr. Lyell, who was not involved in the study, said in an interview. “Their study did not examine factors that should be used to confirm if there are real differences in blood loss, such as changes in blood counts before and after delivery, or more use of medications that we use to stop heavy bleeding.”
In fact, Dr. Lyell noted, other research at the SMFM meeting found ”that low-dose aspirin is not used frequently enough in patients who might benefit, such as those at high risk for preeclampsia,” she said. ”Low-dose aspirin among those at increased risk has been shown to reduce rates of preeclampsia, reducing the likelihood of risky situations for moms and babies.”
The authors had no disclosures. Dr. Lyell has consulted for Bloomlife, a uterine contraction and fetal monitor. Dr. White and Dr. Mei had no disclosures.
Low-dose aspirin may increase risk of postpartum bleeding if patients don’t discontinue its use at least 7 days before delivery, but it’s otherwise unclear whether its use increases bleeding risk, according to research presented Feb. 5 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“These findings were a little surprising to me because we have generally been taught that aspirin is safe to continue up until delivery with minimal risk,” Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview after attending the presentation. “Theoretically it makes sense that it may increase bleeding risk, but multiple studies in the past analyzing its use with gynecological surgery show minimal risk, which was conferred to the obstetrical population as well.”
She noted, however, that patients prescribed low-dose aspirin already have risk factors that may increase their risk of postpartum bleeding, and the study’s finding of possible increased risk was not statistically significant after accounting for those confounders. “I wouldn’t change my practice management over it, but it does raise awareness that all interventions likely come with some risk,” Dr. Mei said.
Hypertensive disorders of pregnancy are responsible for 6.6% of U.S. pregnancy-related deaths. The SMFM currently recommends low-dose aspirin starting at 12 weeks’ gestation in patients at high risk for preeclampsia, which includes people with multifetal gestation, chronic hypertension, pregestational diabetes, renal disease, autoimmune disease, or a history of preeclampsia. However, previous research has shown mixed results on the safety of low-dose aspirin in terms of bleeding risk, Kelsey White, MD, a second-year maternal-fetal medicine fellow of the Yale University, New Haven, Conn., told attendees.
This retrospective study compared a bleeding composite endpoint among those who did and did not take low-dose aspirin between January 2018 and April 2021. The composite included an estimated blood loss of greater than 1,000 mL, postpartum hemorrhage based on ICD-9/10 code diagnosis, and red blood cell transfusion. The researchers also compared bleeding risk within the aspirin group based on discontinuation at greater or less than 7 days before delivery.
Among 16,980 patients, 11.3% were prescribed low-dose aspirin. The patients prescribed low-dose aspirin significantly differed from those not prescribed it in all demographic and clinical characteristics except placenta accreta spectrum. The average age of the aspirin group was 39 years, compared with 24 years in the nonaspirin group (P < .01). More of the aspirin group patients were Hispanic and Black, and 52.3% of patients taking aspirin had a body mass index greater than 30 kg/m2, compared with 22.9% of the nonaspirin group. Rates of diabetes, lupus, fibroids, nonaspirin anticoagulation use, cesarean delivery, and preterm delivery were all greater in the aspirin group.
In addition, 43.9% of the patients in the aspirin group had a hypertensive disorder, including 20.2% with preeclampsia, compared with 17.1% with hypertensive disorders, including 6.2% with preeclampsia, in the group not taking aspirin (P < .0001). “This shows that a high-risk population was prescribed aspirin, which correlates to the recommended prescription guidelines,” Dr. White said.
The postpartum bleeding composite outcome occurred in 14.7% of patients in the low-dose aspirin group, compared with 9.2% of patients in the nonaspirin group, for an unadjusted 1.7 times greater risk of bleeding (95% confidence interval, 1.49-1.96). After adjustment for confounders, the risk declined and was no longer statistically significant (aOR = 1.15; 95% CI, 0.98-1.34).
Meanwhile, 15% of those who discontinued aspirin within 7 days of delivery had postpartum bleeding, compared with 9% of those who discontinued aspirin at 7 or more days before delivery (P = .03).
Therefore, while the study found only a possible, nonsignificant association between low-dose aspirin and postpartum bleeding, risk of bleeding was significantly greater among those who discontinued aspirin only in the last week before delivery.
”Our study is timely and supports a recent Swedish study [that] found an increased risk of intrapartum bleeding, postpartum hemorrhage, and postpartum hematoma,” Dr. White said. She also noted that the United States Preventive Services Task Force changed their recommendation in 2021 for low-dose aspirin prophylaxis for cardiovascular disease.
“They now recommend against the use of low-dose aspirin for prevention in adults without a history of cardiovascular disease,” Dr. White said. “The change in recommendations came after recent randomized control trials showed that low-dose aspirin had very little benefit and may increase the risk of bleeding.”
However, Dr. White added that they “do not believe this study should be used to make any clinical decisions.” While the study had a large sample size, it was limited by its retrospective reliance on EMR data, including the EMR medication list, and the researchers couldn’t assess patient compliance or patient use of over-the-counter aspirin not recorded in the EMR.
Deirdre Lyell, MD, a professor of maternal-fetal medicine at Stanford (Calif.) University, agreed that the findings should not impact clinical practice given its limitations.
“The investigators could not entirely identify who stopped low-dose aspirin and when. When they estimated timing of stoppage of low-dose aspirin, their data suggested a small benefit among those who discontinued it at least 7 days before delivery, though this should be interpreted with caution, given the potential inaccuracy in these data,” Dr. Lyell, who was not involved in the study, said in an interview. “Their study did not examine factors that should be used to confirm if there are real differences in blood loss, such as changes in blood counts before and after delivery, or more use of medications that we use to stop heavy bleeding.”
In fact, Dr. Lyell noted, other research at the SMFM meeting found ”that low-dose aspirin is not used frequently enough in patients who might benefit, such as those at high risk for preeclampsia,” she said. ”Low-dose aspirin among those at increased risk has been shown to reduce rates of preeclampsia, reducing the likelihood of risky situations for moms and babies.”
The authors had no disclosures. Dr. Lyell has consulted for Bloomlife, a uterine contraction and fetal monitor. Dr. White and Dr. Mei had no disclosures.
AT THE PREGNANCY MEETING
Highly virulent HIV variant discovered in the Netherlands
led by researchers at the University of Oxford’s Big Data Institute.
In a study published in the journal Science, researchers identified a VB variant (virulent subtype B) of HIV-1 linked to higher viral loads, increased transmissibility, and a faster decline in CD4 cell levels, leading to increased immune deficiency.
In light of the ongoing pandemic and current focus on SARS-CoV-19 virus variants such as Delta or Omicron, the discovery provides a salutary reminder that other viral pathogens, including those responsible for many long-standing endemic diseases, undergo a similar process of mutation.
Lead author Dr. Chris Wymant said: “Before this study, the genetics of the HIV virus were known to be relevant for virulence, implying that the evolution of a new variant could change its impact on health. Discovery of the VB variant demonstrated this, providing a rare example of the risk posed by viral virulence evolution.”
Global disease, local variants
Human immunodeficiency virus (HIV) infections affect around 38 million people worldwide, with more than half a million people dying from AIDS-related illnesses each year. The disease-causing retroviruses, of which the HIV-1 virus is most common, destroy CD4+ T cells, causing immune deficiency and leading eventually to AIDS.
RNA viruses such as HIV-1 have long been a particular concern to scientists because their error-prone replication, lacking the error-correcting mechanisms of DNA, results in more spontaneous mutations and so a higher potential for acquiring new characteristics.
The VB variant of HIV-1 was first detected in samples from 2,461 HIV-positive people whose viral genomes were sequenced as part of the ongoing BEEHIVE project. Within this cohort, researchers identified 17 people with very highly elevated viral loads.
As 15 of these individuals came from the Netherlands, the researchers next examined virus gene data from 6,706 HIV-positive patients in a Dutch HIV cohort study (ATHENA), identifying a further 92 people carrying the same VB variant.
By analysing patterns of genetic variation in the samples, researchers estimated that the VB variant first emerged in the Netherlands in the late 1990s, occurring through de novo mutations rather than recombination. It spread more quickly than other HIV variants initially, but cases have been declining since around 2010, most likely due to the availability of more effective combination anti-retroviral treatments.
Increased virulence
The researchers found a number of differences in people infected with the VB variant compared with those infected by other HIV variants. Prior to starting anti-retroviral treatment, individuals with the VB variant were found to have:
Around a 3.5- to 5.5-fold increase in viral load (a marker for viral virulence)
Double the rate of CD4 cell decline compared with individuals with other subtype-B strains, even after adjusting for viral load
Increased risk of transmitting the virus (the study used the virus ‘local branching index’ as a proxy for transmissibility).
Reassuringly, after starting anti-retroviral treatment, individuals with the VB variant showed similar CD4 cell recovery and survival to individuals with other HIV variants. However, the authors emphasise that due of the more rapid decline in immune function with the VB variant, it is critical to identify VB-positive individuals early and start treatment promptly.
Senior author Professor Christophe Fraser explained: “Our findings emphasise the importance of World Health Organization guidance that individuals at risk of acquiring HIV have access to regular testing to allow early diagnosis, followed by immediate treatment.
“This limits the amount of time HIV can damage an individual’s immune system and jeopardise their health. It also ensures that HIV is suppressed as quickly as possible, which prevents transmission to other individuals.”
A version of this article first appeared on Medscape.com.
led by researchers at the University of Oxford’s Big Data Institute.
In a study published in the journal Science, researchers identified a VB variant (virulent subtype B) of HIV-1 linked to higher viral loads, increased transmissibility, and a faster decline in CD4 cell levels, leading to increased immune deficiency.
In light of the ongoing pandemic and current focus on SARS-CoV-19 virus variants such as Delta or Omicron, the discovery provides a salutary reminder that other viral pathogens, including those responsible for many long-standing endemic diseases, undergo a similar process of mutation.
Lead author Dr. Chris Wymant said: “Before this study, the genetics of the HIV virus were known to be relevant for virulence, implying that the evolution of a new variant could change its impact on health. Discovery of the VB variant demonstrated this, providing a rare example of the risk posed by viral virulence evolution.”
Global disease, local variants
Human immunodeficiency virus (HIV) infections affect around 38 million people worldwide, with more than half a million people dying from AIDS-related illnesses each year. The disease-causing retroviruses, of which the HIV-1 virus is most common, destroy CD4+ T cells, causing immune deficiency and leading eventually to AIDS.
RNA viruses such as HIV-1 have long been a particular concern to scientists because their error-prone replication, lacking the error-correcting mechanisms of DNA, results in more spontaneous mutations and so a higher potential for acquiring new characteristics.
The VB variant of HIV-1 was first detected in samples from 2,461 HIV-positive people whose viral genomes were sequenced as part of the ongoing BEEHIVE project. Within this cohort, researchers identified 17 people with very highly elevated viral loads.
As 15 of these individuals came from the Netherlands, the researchers next examined virus gene data from 6,706 HIV-positive patients in a Dutch HIV cohort study (ATHENA), identifying a further 92 people carrying the same VB variant.
By analysing patterns of genetic variation in the samples, researchers estimated that the VB variant first emerged in the Netherlands in the late 1990s, occurring through de novo mutations rather than recombination. It spread more quickly than other HIV variants initially, but cases have been declining since around 2010, most likely due to the availability of more effective combination anti-retroviral treatments.
Increased virulence
The researchers found a number of differences in people infected with the VB variant compared with those infected by other HIV variants. Prior to starting anti-retroviral treatment, individuals with the VB variant were found to have:
Around a 3.5- to 5.5-fold increase in viral load (a marker for viral virulence)
Double the rate of CD4 cell decline compared with individuals with other subtype-B strains, even after adjusting for viral load
Increased risk of transmitting the virus (the study used the virus ‘local branching index’ as a proxy for transmissibility).
Reassuringly, after starting anti-retroviral treatment, individuals with the VB variant showed similar CD4 cell recovery and survival to individuals with other HIV variants. However, the authors emphasise that due of the more rapid decline in immune function with the VB variant, it is critical to identify VB-positive individuals early and start treatment promptly.
Senior author Professor Christophe Fraser explained: “Our findings emphasise the importance of World Health Organization guidance that individuals at risk of acquiring HIV have access to regular testing to allow early diagnosis, followed by immediate treatment.
“This limits the amount of time HIV can damage an individual’s immune system and jeopardise their health. It also ensures that HIV is suppressed as quickly as possible, which prevents transmission to other individuals.”
A version of this article first appeared on Medscape.com.
led by researchers at the University of Oxford’s Big Data Institute.
In a study published in the journal Science, researchers identified a VB variant (virulent subtype B) of HIV-1 linked to higher viral loads, increased transmissibility, and a faster decline in CD4 cell levels, leading to increased immune deficiency.
In light of the ongoing pandemic and current focus on SARS-CoV-19 virus variants such as Delta or Omicron, the discovery provides a salutary reminder that other viral pathogens, including those responsible for many long-standing endemic diseases, undergo a similar process of mutation.
Lead author Dr. Chris Wymant said: “Before this study, the genetics of the HIV virus were known to be relevant for virulence, implying that the evolution of a new variant could change its impact on health. Discovery of the VB variant demonstrated this, providing a rare example of the risk posed by viral virulence evolution.”
Global disease, local variants
Human immunodeficiency virus (HIV) infections affect around 38 million people worldwide, with more than half a million people dying from AIDS-related illnesses each year. The disease-causing retroviruses, of which the HIV-1 virus is most common, destroy CD4+ T cells, causing immune deficiency and leading eventually to AIDS.
RNA viruses such as HIV-1 have long been a particular concern to scientists because their error-prone replication, lacking the error-correcting mechanisms of DNA, results in more spontaneous mutations and so a higher potential for acquiring new characteristics.
The VB variant of HIV-1 was first detected in samples from 2,461 HIV-positive people whose viral genomes were sequenced as part of the ongoing BEEHIVE project. Within this cohort, researchers identified 17 people with very highly elevated viral loads.
As 15 of these individuals came from the Netherlands, the researchers next examined virus gene data from 6,706 HIV-positive patients in a Dutch HIV cohort study (ATHENA), identifying a further 92 people carrying the same VB variant.
By analysing patterns of genetic variation in the samples, researchers estimated that the VB variant first emerged in the Netherlands in the late 1990s, occurring through de novo mutations rather than recombination. It spread more quickly than other HIV variants initially, but cases have been declining since around 2010, most likely due to the availability of more effective combination anti-retroviral treatments.
Increased virulence
The researchers found a number of differences in people infected with the VB variant compared with those infected by other HIV variants. Prior to starting anti-retroviral treatment, individuals with the VB variant were found to have:
Around a 3.5- to 5.5-fold increase in viral load (a marker for viral virulence)
Double the rate of CD4 cell decline compared with individuals with other subtype-B strains, even after adjusting for viral load
Increased risk of transmitting the virus (the study used the virus ‘local branching index’ as a proxy for transmissibility).
Reassuringly, after starting anti-retroviral treatment, individuals with the VB variant showed similar CD4 cell recovery and survival to individuals with other HIV variants. However, the authors emphasise that due of the more rapid decline in immune function with the VB variant, it is critical to identify VB-positive individuals early and start treatment promptly.
Senior author Professor Christophe Fraser explained: “Our findings emphasise the importance of World Health Organization guidance that individuals at risk of acquiring HIV have access to regular testing to allow early diagnosis, followed by immediate treatment.
“This limits the amount of time HIV can damage an individual’s immune system and jeopardise their health. It also ensures that HIV is suppressed as quickly as possible, which prevents transmission to other individuals.”
A version of this article first appeared on Medscape.com.
FROM SCIENCE
C. difficile: New vancomycin-resistant strains raise concerns
Samples from patients in the United States and Kenya show an increasing emergence of previously undetected vancomycin-resistant strains of Clostridioides difficile, sparking concern as recurrences in the treatment of C. difficile infection (CDI) continue to rise.
“Our results may help explain a decreasing effectiveness of antibiotic-based therapy in C. difficile infection, since a significant proportion of patients harboring strains with reduced susceptibility to vancomycin may not respond to treatment,” reported the authors in research published recently in Clinical Infectious Diseases.
The spread of the resistant strains “has serious public health implications, underscoring an urgent need for a comprehensive analysis of the circulating strains to help inform clinical decisions,” they added.
Commenting on the findings, Cornelius J. Clancy, MD, professor of medicine at the University of Pittsburgh, and chief of infectious diseases at the Veterans Affairs Pittsburgh Healthcare System, echoed the concern.
“The casual belief has been that [C. difficile] strains at most centers can be assumed to be vancomycin susceptible,” he told this news organization. “This study shows that this assumption can no longer be taken as a given.”
Dr. Clancy, who was not involved with this research, noted that “based on this study, there might be need for the Infectious Diseases Society of America and other organizations to offer guidance on generating good, quality surveillance data for C. difficile resistance.”
With C. difficile showing the ability to resist multiple antibiotics, drugs in the armamentarium to treat the infection have declined in recent years, and recurrences with the infection are reported in up to 25% of cases.
Oral vancomycin is recommended as the antibiotic of choice by the IDSA and the Society for Healthcare Epidemiology of America for severe as well as nonsevere cases of CDI, and although there are reports of nine vancomycin-resistant gene clusters, most involve Enterococcus.
To take a closer look at the prevalence of vancomycin-resistant C. difficile strains, first author Charles Darkoh, PhD, with the Center for Infectious Diseases at the University of Texas Health Science Center, Houston, and colleagues analyzed stool samples from patients with CDI, including 438 patients in Houston, taken between 2012 and 2017, and 98 in Nairobi, Kenya, taken in 2017.
They found that, among samples from patients in Houston, over the time period, 26% showed vancomycin nonsusceptible C. difficile isolates and 29% had isolates that were metronidazole resistant.
And among samples from the Nairobi patients, 67% harbored vancomycin-resistant isolates and 85% had isolates resistant to metronidazole.
Of note, the proportion of samples containing vancomycin-resistant C. difficile in the Houston patients showed a marked increase over time, from «complete absence» in 2012 to approximately 35% in 2017, the authors reported.
“These nonsusceptibility rates significantly exceeded prior reports from other studies conducted in the United States and Europe from 2011 to 2014, suggesting a lower percentage of resistance to both metronidazole and vancomycin,” the authors wrote.
Further experiments on mouse models infected with one of the vancomycin-resistant isolates showed that treatment with vancomycin failed to eradicate the infection, and 5-day survival was significantly lower after vancomycin treatment in those mice (25%) versus those infected with strains known to be vancomycin sensitive (50%).
Unrecognized genetic strains
Whole-genome sequencing of 10 of the resistant isolates showed no matches with gene clusters that have been previously recognized as being vancomycin resistant, suggesting the emergence of new clusters.
“Together, these results suggest unknown genetic elements associated with vancomycin nonsusceptibility in isolates circulating in the patient population,” the authors wrote.
Dr. Darkoh told this news organization that the research team is currently working to further investigate the patterns and mechanisms.
“We are currently working on a follow-up study for the next 5 years to find out how widespread this is,” he said. “We want to make sure it’s not necessarily just occurring in the settings we studied, and we also need to establish the mechanism of resistance.”
Further commenting on the results, Dr. Clancy noted that “the extent of resistance caught many in the field a bit off guard, as they are higher than previously reported.”
“The data are also concerning because most centers do not routinely test C. difficile for drug susceptibility.”
Dr. Clancy noted that “another immediately pressing need is to understand mechanisms of resistance. It was quite striking that vancomycin-resistant strains in this study did not carry vanA genes, pointing to previously unrecognized mechanisms of resistance.”
“As is often the case, antibiotic overuse was likely a factor in the resistances, with overtesting often leading to overtreatment of C. difficile,” Dr. Clancy said. “The situation may have been compounded by failure to appreciate how entrenched C. difficile resistance may be at certain hospitals, since widespread susceptibility testing is generally not routinely performed.”
As alternative treatments, Dr. Clancy pointed to the recent IDSA update, which included a stronger endorsement of fidaxomicin.
“Of course, there is also the need to assure that data on resistance to agents like fidaxomicin are generated going forward,” he noted.
The study was supported by was supported by National Institutes of Health, the National Institute of Allergy and Infectious Diseases, the Texas Medical Center Digestive Diseases Center, and the University of Texas Health Science Center. Dr. Darkoh has disclosed no relevant financial relationships. One coauthor received grant support from Merck, Entasis Pharmaceuticals, and MeMed Diagnostics. Dr. Clancy disclosed advisory board, consulting and/or research relationships with Merck, Qpex Biopharma, Shionogi, Astellas, Cidara, Scynexis, and Needham & Associates.
A version of this article first appeared on Medscape.com.
Samples from patients in the United States and Kenya show an increasing emergence of previously undetected vancomycin-resistant strains of Clostridioides difficile, sparking concern as recurrences in the treatment of C. difficile infection (CDI) continue to rise.
“Our results may help explain a decreasing effectiveness of antibiotic-based therapy in C. difficile infection, since a significant proportion of patients harboring strains with reduced susceptibility to vancomycin may not respond to treatment,” reported the authors in research published recently in Clinical Infectious Diseases.
The spread of the resistant strains “has serious public health implications, underscoring an urgent need for a comprehensive analysis of the circulating strains to help inform clinical decisions,” they added.
Commenting on the findings, Cornelius J. Clancy, MD, professor of medicine at the University of Pittsburgh, and chief of infectious diseases at the Veterans Affairs Pittsburgh Healthcare System, echoed the concern.
“The casual belief has been that [C. difficile] strains at most centers can be assumed to be vancomycin susceptible,” he told this news organization. “This study shows that this assumption can no longer be taken as a given.”
Dr. Clancy, who was not involved with this research, noted that “based on this study, there might be need for the Infectious Diseases Society of America and other organizations to offer guidance on generating good, quality surveillance data for C. difficile resistance.”
With C. difficile showing the ability to resist multiple antibiotics, drugs in the armamentarium to treat the infection have declined in recent years, and recurrences with the infection are reported in up to 25% of cases.
Oral vancomycin is recommended as the antibiotic of choice by the IDSA and the Society for Healthcare Epidemiology of America for severe as well as nonsevere cases of CDI, and although there are reports of nine vancomycin-resistant gene clusters, most involve Enterococcus.
To take a closer look at the prevalence of vancomycin-resistant C. difficile strains, first author Charles Darkoh, PhD, with the Center for Infectious Diseases at the University of Texas Health Science Center, Houston, and colleagues analyzed stool samples from patients with CDI, including 438 patients in Houston, taken between 2012 and 2017, and 98 in Nairobi, Kenya, taken in 2017.
They found that, among samples from patients in Houston, over the time period, 26% showed vancomycin nonsusceptible C. difficile isolates and 29% had isolates that were metronidazole resistant.
And among samples from the Nairobi patients, 67% harbored vancomycin-resistant isolates and 85% had isolates resistant to metronidazole.
Of note, the proportion of samples containing vancomycin-resistant C. difficile in the Houston patients showed a marked increase over time, from «complete absence» in 2012 to approximately 35% in 2017, the authors reported.
“These nonsusceptibility rates significantly exceeded prior reports from other studies conducted in the United States and Europe from 2011 to 2014, suggesting a lower percentage of resistance to both metronidazole and vancomycin,” the authors wrote.
Further experiments on mouse models infected with one of the vancomycin-resistant isolates showed that treatment with vancomycin failed to eradicate the infection, and 5-day survival was significantly lower after vancomycin treatment in those mice (25%) versus those infected with strains known to be vancomycin sensitive (50%).
Unrecognized genetic strains
Whole-genome sequencing of 10 of the resistant isolates showed no matches with gene clusters that have been previously recognized as being vancomycin resistant, suggesting the emergence of new clusters.
“Together, these results suggest unknown genetic elements associated with vancomycin nonsusceptibility in isolates circulating in the patient population,” the authors wrote.
Dr. Darkoh told this news organization that the research team is currently working to further investigate the patterns and mechanisms.
“We are currently working on a follow-up study for the next 5 years to find out how widespread this is,” he said. “We want to make sure it’s not necessarily just occurring in the settings we studied, and we also need to establish the mechanism of resistance.”
Further commenting on the results, Dr. Clancy noted that “the extent of resistance caught many in the field a bit off guard, as they are higher than previously reported.”
“The data are also concerning because most centers do not routinely test C. difficile for drug susceptibility.”
Dr. Clancy noted that “another immediately pressing need is to understand mechanisms of resistance. It was quite striking that vancomycin-resistant strains in this study did not carry vanA genes, pointing to previously unrecognized mechanisms of resistance.”
“As is often the case, antibiotic overuse was likely a factor in the resistances, with overtesting often leading to overtreatment of C. difficile,” Dr. Clancy said. “The situation may have been compounded by failure to appreciate how entrenched C. difficile resistance may be at certain hospitals, since widespread susceptibility testing is generally not routinely performed.”
As alternative treatments, Dr. Clancy pointed to the recent IDSA update, which included a stronger endorsement of fidaxomicin.
“Of course, there is also the need to assure that data on resistance to agents like fidaxomicin are generated going forward,” he noted.
The study was supported by was supported by National Institutes of Health, the National Institute of Allergy and Infectious Diseases, the Texas Medical Center Digestive Diseases Center, and the University of Texas Health Science Center. Dr. Darkoh has disclosed no relevant financial relationships. One coauthor received grant support from Merck, Entasis Pharmaceuticals, and MeMed Diagnostics. Dr. Clancy disclosed advisory board, consulting and/or research relationships with Merck, Qpex Biopharma, Shionogi, Astellas, Cidara, Scynexis, and Needham & Associates.
A version of this article first appeared on Medscape.com.
Samples from patients in the United States and Kenya show an increasing emergence of previously undetected vancomycin-resistant strains of Clostridioides difficile, sparking concern as recurrences in the treatment of C. difficile infection (CDI) continue to rise.
“Our results may help explain a decreasing effectiveness of antibiotic-based therapy in C. difficile infection, since a significant proportion of patients harboring strains with reduced susceptibility to vancomycin may not respond to treatment,” reported the authors in research published recently in Clinical Infectious Diseases.
The spread of the resistant strains “has serious public health implications, underscoring an urgent need for a comprehensive analysis of the circulating strains to help inform clinical decisions,” they added.
Commenting on the findings, Cornelius J. Clancy, MD, professor of medicine at the University of Pittsburgh, and chief of infectious diseases at the Veterans Affairs Pittsburgh Healthcare System, echoed the concern.
“The casual belief has been that [C. difficile] strains at most centers can be assumed to be vancomycin susceptible,” he told this news organization. “This study shows that this assumption can no longer be taken as a given.”
Dr. Clancy, who was not involved with this research, noted that “based on this study, there might be need for the Infectious Diseases Society of America and other organizations to offer guidance on generating good, quality surveillance data for C. difficile resistance.”
With C. difficile showing the ability to resist multiple antibiotics, drugs in the armamentarium to treat the infection have declined in recent years, and recurrences with the infection are reported in up to 25% of cases.
Oral vancomycin is recommended as the antibiotic of choice by the IDSA and the Society for Healthcare Epidemiology of America for severe as well as nonsevere cases of CDI, and although there are reports of nine vancomycin-resistant gene clusters, most involve Enterococcus.
To take a closer look at the prevalence of vancomycin-resistant C. difficile strains, first author Charles Darkoh, PhD, with the Center for Infectious Diseases at the University of Texas Health Science Center, Houston, and colleagues analyzed stool samples from patients with CDI, including 438 patients in Houston, taken between 2012 and 2017, and 98 in Nairobi, Kenya, taken in 2017.
They found that, among samples from patients in Houston, over the time period, 26% showed vancomycin nonsusceptible C. difficile isolates and 29% had isolates that were metronidazole resistant.
And among samples from the Nairobi patients, 67% harbored vancomycin-resistant isolates and 85% had isolates resistant to metronidazole.
Of note, the proportion of samples containing vancomycin-resistant C. difficile in the Houston patients showed a marked increase over time, from «complete absence» in 2012 to approximately 35% in 2017, the authors reported.
“These nonsusceptibility rates significantly exceeded prior reports from other studies conducted in the United States and Europe from 2011 to 2014, suggesting a lower percentage of resistance to both metronidazole and vancomycin,” the authors wrote.
Further experiments on mouse models infected with one of the vancomycin-resistant isolates showed that treatment with vancomycin failed to eradicate the infection, and 5-day survival was significantly lower after vancomycin treatment in those mice (25%) versus those infected with strains known to be vancomycin sensitive (50%).
Unrecognized genetic strains
Whole-genome sequencing of 10 of the resistant isolates showed no matches with gene clusters that have been previously recognized as being vancomycin resistant, suggesting the emergence of new clusters.
“Together, these results suggest unknown genetic elements associated with vancomycin nonsusceptibility in isolates circulating in the patient population,” the authors wrote.
Dr. Darkoh told this news organization that the research team is currently working to further investigate the patterns and mechanisms.
“We are currently working on a follow-up study for the next 5 years to find out how widespread this is,” he said. “We want to make sure it’s not necessarily just occurring in the settings we studied, and we also need to establish the mechanism of resistance.”
Further commenting on the results, Dr. Clancy noted that “the extent of resistance caught many in the field a bit off guard, as they are higher than previously reported.”
“The data are also concerning because most centers do not routinely test C. difficile for drug susceptibility.”
Dr. Clancy noted that “another immediately pressing need is to understand mechanisms of resistance. It was quite striking that vancomycin-resistant strains in this study did not carry vanA genes, pointing to previously unrecognized mechanisms of resistance.”
“As is often the case, antibiotic overuse was likely a factor in the resistances, with overtesting often leading to overtreatment of C. difficile,” Dr. Clancy said. “The situation may have been compounded by failure to appreciate how entrenched C. difficile resistance may be at certain hospitals, since widespread susceptibility testing is generally not routinely performed.”
As alternative treatments, Dr. Clancy pointed to the recent IDSA update, which included a stronger endorsement of fidaxomicin.
“Of course, there is also the need to assure that data on resistance to agents like fidaxomicin are generated going forward,” he noted.
The study was supported by was supported by National Institutes of Health, the National Institute of Allergy and Infectious Diseases, the Texas Medical Center Digestive Diseases Center, and the University of Texas Health Science Center. Dr. Darkoh has disclosed no relevant financial relationships. One coauthor received grant support from Merck, Entasis Pharmaceuticals, and MeMed Diagnostics. Dr. Clancy disclosed advisory board, consulting and/or research relationships with Merck, Qpex Biopharma, Shionogi, Astellas, Cidara, Scynexis, and Needham & Associates.
A version of this article first appeared on Medscape.com.
FROM CLINICAL INFECTIOUS DISEASES