User login
Primer message boosts colorectal cancer screening rates
Researchers have found a simple, low-cost way to get more adults to complete a fecal immunochemical test (FIT) to screen for colorectal cancer (CRC).
In a randomized controlled trial, patients who received an electronic “primer” message through their patient portal before the test kit arrived in their mailbox were more apt to complete and return the test than peers who didn’t get the electronic message.
“We were thrilled by the magnitude of the impact,” Gregory Goshgarian, MSc, MPH, and Daniel Croymans, MD, with the department of medicine, David Geffen School of Medicine, University of California, Los Angeles, said in a joint email to this news organization.
At UCLA Health, “including a primer patient portal message is now standard practice for our FIT mailer program,” they added.
Their study was published online Feb. 4 in JAMA Network Open.
Heads-up message boosts compliance
CRC screening rates in the United States remain well below the national benchmark of 80%, and COVID-19 hasn’t helped. As a result, multiple medical and professional societies have emphasized the use of a mailed FIT outreach program.
As part of the outreach program, researchers at UCLA Health developed an electronic primer message within the electronic patient portal to alert patients due for CRC screening that they would be receiving a FIT kit in the mail.
They tested the impact of the primer messages in a randomized controlled trial involving 2,339 adults (mean age, 59 years, 57.5% women). Out of these, 1,157 received the standard mailed FIT kit (control group) and 1,182 received the standard mailed FIT kit plus a primer message sent through their personal patient portal.
Adding the primer message significantly increased the FIT completion rate at 6 months by 5.5%, with rates of 37.6% in the intervention group versus 32.1% in the control group.
After adjusting for patient demographics, the primer (versus no primer) led to significantly increased odds of completing CRC screening (adjusted odds ratio: 1.29; 95% confidence interval, 1.08-1.53; P = .004).
The primer message also shortened the time to FIT screening by 3 days (35 days with the primer vs. 38 days without).
Dr. Goshgarian and Dr. Croymans believe the priming messages worked well in their patient population because at the beginning of the intervention they identified a potential lack of awareness of the incoming FIT kit mailer as a barrier to uptake.
“We believe patients were receiving the kits with minimal advanced warning and discarding it as a mistake or hesitant to complete it because they did not understand the value to them,” they told this news organization.
“Therefore, a priming message helped to bridge that gap and allowed patients to be aware of the incoming FIT kits, know why it was important to do the FIT kit, and ultimately led to increasing our FIT kit return rates and thus CRC screening,” they said.
The researchers caution that their findings may be more relevant to patient populations who are more engaged in their health or who are more technologically savvy. In the UCLA Health system, roughly 84% of patients have an activated patient portal.
‘Good enhancement’ for health care systems
Reached for comment, Aasma Shaukat, MD, MPH, professor of medicine, NYU Langone Health, and first author of the American College of Gastroenterology (ACG) 2021 CRC screening guidelines, said the results are “interesting but not entirely surprising.”
“There’s literature supporting that a letter or notification prior to the FIT being mailed improves its uptake. Here, the authors applied it to their health care system in a quality improvement study and demonstrated it works,” Dr. Shaukat said.
“This is a good enhancement for health care systems where most of their patients are using or accessing their health chart portal,” added Dr. Shaukat.
“Caveats are that the generalizability is not known. It requires EHR [electronic health record] support tools and patients with access to a computer and enrolled and able to access their electronic chart, likely those with high literacy and English speaking.”
Funding for the study was provided by the UCLA Health Department of Medicine. Dr. Goshgarian, Dr. Croymans, and Dr. Shaukat have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have found a simple, low-cost way to get more adults to complete a fecal immunochemical test (FIT) to screen for colorectal cancer (CRC).
In a randomized controlled trial, patients who received an electronic “primer” message through their patient portal before the test kit arrived in their mailbox were more apt to complete and return the test than peers who didn’t get the electronic message.
“We were thrilled by the magnitude of the impact,” Gregory Goshgarian, MSc, MPH, and Daniel Croymans, MD, with the department of medicine, David Geffen School of Medicine, University of California, Los Angeles, said in a joint email to this news organization.
At UCLA Health, “including a primer patient portal message is now standard practice for our FIT mailer program,” they added.
Their study was published online Feb. 4 in JAMA Network Open.
Heads-up message boosts compliance
CRC screening rates in the United States remain well below the national benchmark of 80%, and COVID-19 hasn’t helped. As a result, multiple medical and professional societies have emphasized the use of a mailed FIT outreach program.
As part of the outreach program, researchers at UCLA Health developed an electronic primer message within the electronic patient portal to alert patients due for CRC screening that they would be receiving a FIT kit in the mail.
They tested the impact of the primer messages in a randomized controlled trial involving 2,339 adults (mean age, 59 years, 57.5% women). Out of these, 1,157 received the standard mailed FIT kit (control group) and 1,182 received the standard mailed FIT kit plus a primer message sent through their personal patient portal.
Adding the primer message significantly increased the FIT completion rate at 6 months by 5.5%, with rates of 37.6% in the intervention group versus 32.1% in the control group.
After adjusting for patient demographics, the primer (versus no primer) led to significantly increased odds of completing CRC screening (adjusted odds ratio: 1.29; 95% confidence interval, 1.08-1.53; P = .004).
The primer message also shortened the time to FIT screening by 3 days (35 days with the primer vs. 38 days without).
Dr. Goshgarian and Dr. Croymans believe the priming messages worked well in their patient population because at the beginning of the intervention they identified a potential lack of awareness of the incoming FIT kit mailer as a barrier to uptake.
“We believe patients were receiving the kits with minimal advanced warning and discarding it as a mistake or hesitant to complete it because they did not understand the value to them,” they told this news organization.
“Therefore, a priming message helped to bridge that gap and allowed patients to be aware of the incoming FIT kits, know why it was important to do the FIT kit, and ultimately led to increasing our FIT kit return rates and thus CRC screening,” they said.
The researchers caution that their findings may be more relevant to patient populations who are more engaged in their health or who are more technologically savvy. In the UCLA Health system, roughly 84% of patients have an activated patient portal.
‘Good enhancement’ for health care systems
Reached for comment, Aasma Shaukat, MD, MPH, professor of medicine, NYU Langone Health, and first author of the American College of Gastroenterology (ACG) 2021 CRC screening guidelines, said the results are “interesting but not entirely surprising.”
“There’s literature supporting that a letter or notification prior to the FIT being mailed improves its uptake. Here, the authors applied it to their health care system in a quality improvement study and demonstrated it works,” Dr. Shaukat said.
“This is a good enhancement for health care systems where most of their patients are using or accessing their health chart portal,” added Dr. Shaukat.
“Caveats are that the generalizability is not known. It requires EHR [electronic health record] support tools and patients with access to a computer and enrolled and able to access their electronic chart, likely those with high literacy and English speaking.”
Funding for the study was provided by the UCLA Health Department of Medicine. Dr. Goshgarian, Dr. Croymans, and Dr. Shaukat have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have found a simple, low-cost way to get more adults to complete a fecal immunochemical test (FIT) to screen for colorectal cancer (CRC).
In a randomized controlled trial, patients who received an electronic “primer” message through their patient portal before the test kit arrived in their mailbox were more apt to complete and return the test than peers who didn’t get the electronic message.
“We were thrilled by the magnitude of the impact,” Gregory Goshgarian, MSc, MPH, and Daniel Croymans, MD, with the department of medicine, David Geffen School of Medicine, University of California, Los Angeles, said in a joint email to this news organization.
At UCLA Health, “including a primer patient portal message is now standard practice for our FIT mailer program,” they added.
Their study was published online Feb. 4 in JAMA Network Open.
Heads-up message boosts compliance
CRC screening rates in the United States remain well below the national benchmark of 80%, and COVID-19 hasn’t helped. As a result, multiple medical and professional societies have emphasized the use of a mailed FIT outreach program.
As part of the outreach program, researchers at UCLA Health developed an electronic primer message within the electronic patient portal to alert patients due for CRC screening that they would be receiving a FIT kit in the mail.
They tested the impact of the primer messages in a randomized controlled trial involving 2,339 adults (mean age, 59 years, 57.5% women). Out of these, 1,157 received the standard mailed FIT kit (control group) and 1,182 received the standard mailed FIT kit plus a primer message sent through their personal patient portal.
Adding the primer message significantly increased the FIT completion rate at 6 months by 5.5%, with rates of 37.6% in the intervention group versus 32.1% in the control group.
After adjusting for patient demographics, the primer (versus no primer) led to significantly increased odds of completing CRC screening (adjusted odds ratio: 1.29; 95% confidence interval, 1.08-1.53; P = .004).
The primer message also shortened the time to FIT screening by 3 days (35 days with the primer vs. 38 days without).
Dr. Goshgarian and Dr. Croymans believe the priming messages worked well in their patient population because at the beginning of the intervention they identified a potential lack of awareness of the incoming FIT kit mailer as a barrier to uptake.
“We believe patients were receiving the kits with minimal advanced warning and discarding it as a mistake or hesitant to complete it because they did not understand the value to them,” they told this news organization.
“Therefore, a priming message helped to bridge that gap and allowed patients to be aware of the incoming FIT kits, know why it was important to do the FIT kit, and ultimately led to increasing our FIT kit return rates and thus CRC screening,” they said.
The researchers caution that their findings may be more relevant to patient populations who are more engaged in their health or who are more technologically savvy. In the UCLA Health system, roughly 84% of patients have an activated patient portal.
‘Good enhancement’ for health care systems
Reached for comment, Aasma Shaukat, MD, MPH, professor of medicine, NYU Langone Health, and first author of the American College of Gastroenterology (ACG) 2021 CRC screening guidelines, said the results are “interesting but not entirely surprising.”
“There’s literature supporting that a letter or notification prior to the FIT being mailed improves its uptake. Here, the authors applied it to their health care system in a quality improvement study and demonstrated it works,” Dr. Shaukat said.
“This is a good enhancement for health care systems where most of their patients are using or accessing their health chart portal,” added Dr. Shaukat.
“Caveats are that the generalizability is not known. It requires EHR [electronic health record] support tools and patients with access to a computer and enrolled and able to access their electronic chart, likely those with high literacy and English speaking.”
Funding for the study was provided by the UCLA Health Department of Medicine. Dr. Goshgarian, Dr. Croymans, and Dr. Shaukat have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Adding TACE to lenvatinib improves survival in liver cancer
The combination of TACE and lenvatinib “represents a potential new first-line treatment option for patients with advanced HCC,” said study author Ming Kuang, MD, PhD, professor in hepatobiliary surgery and interventional ultrasound and director of the cancer center in the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
The combination of the two approaches was “safe and effective for patients with advanced hepatocellular carcinoma and demonstrated remarkable improvements in overall survival, progression-free survival, and overall response rate, as well as acceptable toxicity,” he said.
Patients receiving combination therapy achieved a median overall survival of 17.8 months, compared with 11.5 months in the lenvatinib arm (hazard ratio, 0.45; P < .001). Similarly, median progression-free survival also favored lenvatinib plus TACE: 10.6 months vs. 6.4 months (HR, 0.43; P < .001).
The study results were presented at the Gastrointestinal Cancers Symposium.
Discussing the abstract, Anthony B. El-Khoueiry, MD, from the University of Southern California, Norris Comprehensive Cancer Center, Los Angeles, said the results are “intriguing,” and he commended the researchers on carrying out this study.
“It reinforces the feasibility of combined liver directed and systemic therapy,” he said.
“However, it does not change the standard of care in the U.S.,” he cautioned.
“Systemic therapy backbone is not the standard of care, and the design of this study was not optimal to answer the question of whether the addition of liver-directed therapy in advanced HCC improves outcomes,” he added.
Dr. EL-Khoueiry pointed out that these new results from the LAUNCH trial contrast with two studies that looked at liver-directed plus a systemic therapy. Both of those previous studies used sorafenib, one utilizing Y-90 and the other conventional TACE.
Both of those studies were negative, he said. “But there were differences between these studies and LAUNCH.”
Aside from the fact that they used sorafenib and not lenvatinib, another difference was that the patient population of LAUNCH was younger than in the other two studies. In addition, most patients in the LAUCH trial had hepatitis B, and they received a higher number of TACE treatments than in the previous studies. “One can argue that maybe treatment selection was more optimal,” Dr. El-Khoueiry commented.
He also noted that “the control arm of lenvatinib underperformed, as sorafenib median overall survival in previous trials ranges from 13 to 15 months. We would have expected lenvatinib to perform at least as well or better.” (The median overall survival was 11.5 months).
Improved outcomes with combination therapy
The LAUNCH study involved 338 treatment-naive patients with advanced HCC from 12 hospitals in China who were randomly assigned to receive either lenvatinib plus TACE (n = 170) or lenvatinib alone (n = 168).
TACE was administered on day 1 following treatment with lenvatinib, which was administered at 8 mg or 12 mg once daily, depending on the patient’s weight.
The majority of patients were 60 years of age or younger, with a median age of 54-56 years. The majority of patients were male (81.8% in the combination group vs. 78.6% in the lenvatinib-alone group), and the majority had hepatitis B (87.1% vs. 85.7%).
At a median follow-up of 18.4 months for the lenvatinib-TACE group and 17.0 months for the lenvatinib group, the results showed a significantly improved overall survival of 17.8 months with the combination vs. 11.5 months for monotherapy (HR, 0.45; P < .001). Median progression-free survival (PFS) was also significantly longer, at 10.6 months vs. 6.4 months, respectively (HR, 0.43; P < .001).
The overall response rate was 54.1% vs. 25.0% (P < .001), and one complete response was observed in each study arm. The complete response rate was 2.9% vs. 0.6%; partial response rate, 51.2% vs. 24.4%; stable disease rate, 40.0% vs. 48.2%; and rate of disease progression, 5.9% vs. 26.8% for the lenvatinib-TACE group and lenvatinib monotherapy groups. The disease control rate was 94.1% vs. 73.2%.
Grade 3-4 adverse events that occurred more frequently in the lenvatinib-TACE group than in the lenvatinib group included increased liver enzymes, with increased ALT in 17.6% vs. 1.2%; increased AST in 22.9% vs. 1.8%; and hyperbilirubinemia in 9.4% vs. 3.0%.
“Subgroup analysis shows that the combination group had better overall survival and progression-free survival in most of the analyzed subgroups,” said Dr. Kuang. “Multivariate analysis also found that portal vein tumor thrombus and treatment allocation were independent risk factors of overall survival, and that age, portal vein tumor thrombus, and treatment allocation were independent risk factors of progression-free survival.”
Study limitations
In his discussion of the abstract, Dr. El-Khoueiry noted that the LAUNCH trial had several limitations, one being the heterogeneity of the patient population and potential imbalance. “There is limited information regarding extrahepatic disease burden and distribution,” he explained. “Another limitation is that the younger population – with the majority having hepatitis B – limits the broad applicability of the result and has a potential impact on the low rate of treatment discontinuation.”
This study received no industry funding. Dr. Kuang has disclosed no relevant financial relationships. Dr. El-Khoueiry reported relationships with ABL bio, Agenus, Astex, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, CytomX Therapeutics, Eisai, EMD Serono, Exelixis, Fulgent Genetics, Gilead Sciences, Merck, Pieris Pharmaceuticals, QED Therapeutics, and Roche/Genentech.
A version of this article first appeared on Medscape.com.
The combination of TACE and lenvatinib “represents a potential new first-line treatment option for patients with advanced HCC,” said study author Ming Kuang, MD, PhD, professor in hepatobiliary surgery and interventional ultrasound and director of the cancer center in the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
The combination of the two approaches was “safe and effective for patients with advanced hepatocellular carcinoma and demonstrated remarkable improvements in overall survival, progression-free survival, and overall response rate, as well as acceptable toxicity,” he said.
Patients receiving combination therapy achieved a median overall survival of 17.8 months, compared with 11.5 months in the lenvatinib arm (hazard ratio, 0.45; P < .001). Similarly, median progression-free survival also favored lenvatinib plus TACE: 10.6 months vs. 6.4 months (HR, 0.43; P < .001).
The study results were presented at the Gastrointestinal Cancers Symposium.
Discussing the abstract, Anthony B. El-Khoueiry, MD, from the University of Southern California, Norris Comprehensive Cancer Center, Los Angeles, said the results are “intriguing,” and he commended the researchers on carrying out this study.
“It reinforces the feasibility of combined liver directed and systemic therapy,” he said.
“However, it does not change the standard of care in the U.S.,” he cautioned.
“Systemic therapy backbone is not the standard of care, and the design of this study was not optimal to answer the question of whether the addition of liver-directed therapy in advanced HCC improves outcomes,” he added.
Dr. EL-Khoueiry pointed out that these new results from the LAUNCH trial contrast with two studies that looked at liver-directed plus a systemic therapy. Both of those previous studies used sorafenib, one utilizing Y-90 and the other conventional TACE.
Both of those studies were negative, he said. “But there were differences between these studies and LAUNCH.”
Aside from the fact that they used sorafenib and not lenvatinib, another difference was that the patient population of LAUNCH was younger than in the other two studies. In addition, most patients in the LAUCH trial had hepatitis B, and they received a higher number of TACE treatments than in the previous studies. “One can argue that maybe treatment selection was more optimal,” Dr. El-Khoueiry commented.
He also noted that “the control arm of lenvatinib underperformed, as sorafenib median overall survival in previous trials ranges from 13 to 15 months. We would have expected lenvatinib to perform at least as well or better.” (The median overall survival was 11.5 months).
Improved outcomes with combination therapy
The LAUNCH study involved 338 treatment-naive patients with advanced HCC from 12 hospitals in China who were randomly assigned to receive either lenvatinib plus TACE (n = 170) or lenvatinib alone (n = 168).
TACE was administered on day 1 following treatment with lenvatinib, which was administered at 8 mg or 12 mg once daily, depending on the patient’s weight.
The majority of patients were 60 years of age or younger, with a median age of 54-56 years. The majority of patients were male (81.8% in the combination group vs. 78.6% in the lenvatinib-alone group), and the majority had hepatitis B (87.1% vs. 85.7%).
At a median follow-up of 18.4 months for the lenvatinib-TACE group and 17.0 months for the lenvatinib group, the results showed a significantly improved overall survival of 17.8 months with the combination vs. 11.5 months for monotherapy (HR, 0.45; P < .001). Median progression-free survival (PFS) was also significantly longer, at 10.6 months vs. 6.4 months, respectively (HR, 0.43; P < .001).
The overall response rate was 54.1% vs. 25.0% (P < .001), and one complete response was observed in each study arm. The complete response rate was 2.9% vs. 0.6%; partial response rate, 51.2% vs. 24.4%; stable disease rate, 40.0% vs. 48.2%; and rate of disease progression, 5.9% vs. 26.8% for the lenvatinib-TACE group and lenvatinib monotherapy groups. The disease control rate was 94.1% vs. 73.2%.
Grade 3-4 adverse events that occurred more frequently in the lenvatinib-TACE group than in the lenvatinib group included increased liver enzymes, with increased ALT in 17.6% vs. 1.2%; increased AST in 22.9% vs. 1.8%; and hyperbilirubinemia in 9.4% vs. 3.0%.
“Subgroup analysis shows that the combination group had better overall survival and progression-free survival in most of the analyzed subgroups,” said Dr. Kuang. “Multivariate analysis also found that portal vein tumor thrombus and treatment allocation were independent risk factors of overall survival, and that age, portal vein tumor thrombus, and treatment allocation were independent risk factors of progression-free survival.”
Study limitations
In his discussion of the abstract, Dr. El-Khoueiry noted that the LAUNCH trial had several limitations, one being the heterogeneity of the patient population and potential imbalance. “There is limited information regarding extrahepatic disease burden and distribution,” he explained. “Another limitation is that the younger population – with the majority having hepatitis B – limits the broad applicability of the result and has a potential impact on the low rate of treatment discontinuation.”
This study received no industry funding. Dr. Kuang has disclosed no relevant financial relationships. Dr. El-Khoueiry reported relationships with ABL bio, Agenus, Astex, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, CytomX Therapeutics, Eisai, EMD Serono, Exelixis, Fulgent Genetics, Gilead Sciences, Merck, Pieris Pharmaceuticals, QED Therapeutics, and Roche/Genentech.
A version of this article first appeared on Medscape.com.
The combination of TACE and lenvatinib “represents a potential new first-line treatment option for patients with advanced HCC,” said study author Ming Kuang, MD, PhD, professor in hepatobiliary surgery and interventional ultrasound and director of the cancer center in the First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
The combination of the two approaches was “safe and effective for patients with advanced hepatocellular carcinoma and demonstrated remarkable improvements in overall survival, progression-free survival, and overall response rate, as well as acceptable toxicity,” he said.
Patients receiving combination therapy achieved a median overall survival of 17.8 months, compared with 11.5 months in the lenvatinib arm (hazard ratio, 0.45; P < .001). Similarly, median progression-free survival also favored lenvatinib plus TACE: 10.6 months vs. 6.4 months (HR, 0.43; P < .001).
The study results were presented at the Gastrointestinal Cancers Symposium.
Discussing the abstract, Anthony B. El-Khoueiry, MD, from the University of Southern California, Norris Comprehensive Cancer Center, Los Angeles, said the results are “intriguing,” and he commended the researchers on carrying out this study.
“It reinforces the feasibility of combined liver directed and systemic therapy,” he said.
“However, it does not change the standard of care in the U.S.,” he cautioned.
“Systemic therapy backbone is not the standard of care, and the design of this study was not optimal to answer the question of whether the addition of liver-directed therapy in advanced HCC improves outcomes,” he added.
Dr. EL-Khoueiry pointed out that these new results from the LAUNCH trial contrast with two studies that looked at liver-directed plus a systemic therapy. Both of those previous studies used sorafenib, one utilizing Y-90 and the other conventional TACE.
Both of those studies were negative, he said. “But there were differences between these studies and LAUNCH.”
Aside from the fact that they used sorafenib and not lenvatinib, another difference was that the patient population of LAUNCH was younger than in the other two studies. In addition, most patients in the LAUCH trial had hepatitis B, and they received a higher number of TACE treatments than in the previous studies. “One can argue that maybe treatment selection was more optimal,” Dr. El-Khoueiry commented.
He also noted that “the control arm of lenvatinib underperformed, as sorafenib median overall survival in previous trials ranges from 13 to 15 months. We would have expected lenvatinib to perform at least as well or better.” (The median overall survival was 11.5 months).
Improved outcomes with combination therapy
The LAUNCH study involved 338 treatment-naive patients with advanced HCC from 12 hospitals in China who were randomly assigned to receive either lenvatinib plus TACE (n = 170) or lenvatinib alone (n = 168).
TACE was administered on day 1 following treatment with lenvatinib, which was administered at 8 mg or 12 mg once daily, depending on the patient’s weight.
The majority of patients were 60 years of age or younger, with a median age of 54-56 years. The majority of patients were male (81.8% in the combination group vs. 78.6% in the lenvatinib-alone group), and the majority had hepatitis B (87.1% vs. 85.7%).
At a median follow-up of 18.4 months for the lenvatinib-TACE group and 17.0 months for the lenvatinib group, the results showed a significantly improved overall survival of 17.8 months with the combination vs. 11.5 months for monotherapy (HR, 0.45; P < .001). Median progression-free survival (PFS) was also significantly longer, at 10.6 months vs. 6.4 months, respectively (HR, 0.43; P < .001).
The overall response rate was 54.1% vs. 25.0% (P < .001), and one complete response was observed in each study arm. The complete response rate was 2.9% vs. 0.6%; partial response rate, 51.2% vs. 24.4%; stable disease rate, 40.0% vs. 48.2%; and rate of disease progression, 5.9% vs. 26.8% for the lenvatinib-TACE group and lenvatinib monotherapy groups. The disease control rate was 94.1% vs. 73.2%.
Grade 3-4 adverse events that occurred more frequently in the lenvatinib-TACE group than in the lenvatinib group included increased liver enzymes, with increased ALT in 17.6% vs. 1.2%; increased AST in 22.9% vs. 1.8%; and hyperbilirubinemia in 9.4% vs. 3.0%.
“Subgroup analysis shows that the combination group had better overall survival and progression-free survival in most of the analyzed subgroups,” said Dr. Kuang. “Multivariate analysis also found that portal vein tumor thrombus and treatment allocation were independent risk factors of overall survival, and that age, portal vein tumor thrombus, and treatment allocation were independent risk factors of progression-free survival.”
Study limitations
In his discussion of the abstract, Dr. El-Khoueiry noted that the LAUNCH trial had several limitations, one being the heterogeneity of the patient population and potential imbalance. “There is limited information regarding extrahepatic disease burden and distribution,” he explained. “Another limitation is that the younger population – with the majority having hepatitis B – limits the broad applicability of the result and has a potential impact on the low rate of treatment discontinuation.”
This study received no industry funding. Dr. Kuang has disclosed no relevant financial relationships. Dr. El-Khoueiry reported relationships with ABL bio, Agenus, Astex, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, CytomX Therapeutics, Eisai, EMD Serono, Exelixis, Fulgent Genetics, Gilead Sciences, Merck, Pieris Pharmaceuticals, QED Therapeutics, and Roche/Genentech.
A version of this article first appeared on Medscape.com.
FROM GI CANCERS SYMPOSIUM 2022
USDA announces stricter standards for school nutrition
The U.S. Department of Agriculture has announced new changes to school nutrition standards for the next 2 school years, which will reinstate health goals that were rolled back during the Trump administration.
The Biden administration is also tightening rules for fat and salt content in foods after restrictions were eased during the pandemic, according to the Washington Post.
“Nutritious school meals give America’s children the foundation for successful, healthy lives,” Tom Vilsack, the U.S. agriculture secretary, said in a statement on Feb. 4.
“We applaud schools’ heroic efforts throughout the challenges of this pandemic to continue serving kids the most nutritious meals possible,” he said. “The standards we’re putting in place of the next 2 school years will help schools transition to a future that builds on the tremendous strides they’ve made improving school meal nutrition over the past decade.”
For the 2022-2023 school year, schools and childcare providers will be required to offer low-fat or nonfat unflavored milks and limit the fat in sweet flavored milks. In addition, at least 80% of the grains served during school breakfasts and lunches each week must be considered rich in whole grains.
For the 2023-2024 school year, the weekly sodium limit for school lunches will be decreased by 10%.
The changes mark a shift from the Trump administration, which eased policies on whole grains, nonfat milk, and sodium, the newspaper reported. Then the pandemic forced additional changes as school districts scrambled to package meals for students. The USDA granted extra flexibility and eased some guidelines to ensure that children could be fed while schools were closed or focused on remote learning.
Now the USDA is updating the nutrition standards to “give schools clear expectations for gradual transition from current pandemic operations to more nutritious meals,” Stacy Dean, the USDA’s deputy undersecretary for food, nutrition, and consumer services, told reporters.
The Biden administration’s changes represent a shift back to Obama-era nutrition standards from 2012, according to the Post. But some nutrition advocates have said the new changes don’t address enough issues, such as added sugars. Fruit and vegetable requirements, for instance, will remain the same as the 2012 standards.
That said, some advocates have said the transition could be tough as schools move out of pandemic-era protocols. The School Nutrition Association, which represents school food service manufacturers and professionals, has urged Congress to provide additional support and waiver extensions for the next school year.
“School nutrition professionals are frantic just trying to get enough food on the tray for our students amid relentless supply chain disruptions and labor shortages,” Beth Wallace, the association’s president, told the Washington Post.
The shift will likely require a balancing act and slow transition. The USDA has been consulting with stakeholders for months to determine how to move toward stricter school nutrition standards while also acknowledging the pandemic, supply chain disruptions, and labor shortages.
“This approach is really going to help move forward the nutrition of the meals and allows the schools to continue to function effectively,” Geri Henchy, director of nutrition policy at the Food Research and Action Center, told the Post.
“Schools can’t make big changes at this point because of the supply chain and staffing,” she said. “They have a lot of waivers at this point that are helping them, and this balances the needs of all the different sectors.”
The USDA plans to issue a proposed rule in fall 2022 to update nutrition standards for the future, the department said in its announcement, which would be finalized for the 2024-2025 school year.
A version of this article first appeared on WebMD.com.
The U.S. Department of Agriculture has announced new changes to school nutrition standards for the next 2 school years, which will reinstate health goals that were rolled back during the Trump administration.
The Biden administration is also tightening rules for fat and salt content in foods after restrictions were eased during the pandemic, according to the Washington Post.
“Nutritious school meals give America’s children the foundation for successful, healthy lives,” Tom Vilsack, the U.S. agriculture secretary, said in a statement on Feb. 4.
“We applaud schools’ heroic efforts throughout the challenges of this pandemic to continue serving kids the most nutritious meals possible,” he said. “The standards we’re putting in place of the next 2 school years will help schools transition to a future that builds on the tremendous strides they’ve made improving school meal nutrition over the past decade.”
For the 2022-2023 school year, schools and childcare providers will be required to offer low-fat or nonfat unflavored milks and limit the fat in sweet flavored milks. In addition, at least 80% of the grains served during school breakfasts and lunches each week must be considered rich in whole grains.
For the 2023-2024 school year, the weekly sodium limit for school lunches will be decreased by 10%.
The changes mark a shift from the Trump administration, which eased policies on whole grains, nonfat milk, and sodium, the newspaper reported. Then the pandemic forced additional changes as school districts scrambled to package meals for students. The USDA granted extra flexibility and eased some guidelines to ensure that children could be fed while schools were closed or focused on remote learning.
Now the USDA is updating the nutrition standards to “give schools clear expectations for gradual transition from current pandemic operations to more nutritious meals,” Stacy Dean, the USDA’s deputy undersecretary for food, nutrition, and consumer services, told reporters.
The Biden administration’s changes represent a shift back to Obama-era nutrition standards from 2012, according to the Post. But some nutrition advocates have said the new changes don’t address enough issues, such as added sugars. Fruit and vegetable requirements, for instance, will remain the same as the 2012 standards.
That said, some advocates have said the transition could be tough as schools move out of pandemic-era protocols. The School Nutrition Association, which represents school food service manufacturers and professionals, has urged Congress to provide additional support and waiver extensions for the next school year.
“School nutrition professionals are frantic just trying to get enough food on the tray for our students amid relentless supply chain disruptions and labor shortages,” Beth Wallace, the association’s president, told the Washington Post.
The shift will likely require a balancing act and slow transition. The USDA has been consulting with stakeholders for months to determine how to move toward stricter school nutrition standards while also acknowledging the pandemic, supply chain disruptions, and labor shortages.
“This approach is really going to help move forward the nutrition of the meals and allows the schools to continue to function effectively,” Geri Henchy, director of nutrition policy at the Food Research and Action Center, told the Post.
“Schools can’t make big changes at this point because of the supply chain and staffing,” she said. “They have a lot of waivers at this point that are helping them, and this balances the needs of all the different sectors.”
The USDA plans to issue a proposed rule in fall 2022 to update nutrition standards for the future, the department said in its announcement, which would be finalized for the 2024-2025 school year.
A version of this article first appeared on WebMD.com.
The U.S. Department of Agriculture has announced new changes to school nutrition standards for the next 2 school years, which will reinstate health goals that were rolled back during the Trump administration.
The Biden administration is also tightening rules for fat and salt content in foods after restrictions were eased during the pandemic, according to the Washington Post.
“Nutritious school meals give America’s children the foundation for successful, healthy lives,” Tom Vilsack, the U.S. agriculture secretary, said in a statement on Feb. 4.
“We applaud schools’ heroic efforts throughout the challenges of this pandemic to continue serving kids the most nutritious meals possible,” he said. “The standards we’re putting in place of the next 2 school years will help schools transition to a future that builds on the tremendous strides they’ve made improving school meal nutrition over the past decade.”
For the 2022-2023 school year, schools and childcare providers will be required to offer low-fat or nonfat unflavored milks and limit the fat in sweet flavored milks. In addition, at least 80% of the grains served during school breakfasts and lunches each week must be considered rich in whole grains.
For the 2023-2024 school year, the weekly sodium limit for school lunches will be decreased by 10%.
The changes mark a shift from the Trump administration, which eased policies on whole grains, nonfat milk, and sodium, the newspaper reported. Then the pandemic forced additional changes as school districts scrambled to package meals for students. The USDA granted extra flexibility and eased some guidelines to ensure that children could be fed while schools were closed or focused on remote learning.
Now the USDA is updating the nutrition standards to “give schools clear expectations for gradual transition from current pandemic operations to more nutritious meals,” Stacy Dean, the USDA’s deputy undersecretary for food, nutrition, and consumer services, told reporters.
The Biden administration’s changes represent a shift back to Obama-era nutrition standards from 2012, according to the Post. But some nutrition advocates have said the new changes don’t address enough issues, such as added sugars. Fruit and vegetable requirements, for instance, will remain the same as the 2012 standards.
That said, some advocates have said the transition could be tough as schools move out of pandemic-era protocols. The School Nutrition Association, which represents school food service manufacturers and professionals, has urged Congress to provide additional support and waiver extensions for the next school year.
“School nutrition professionals are frantic just trying to get enough food on the tray for our students amid relentless supply chain disruptions and labor shortages,” Beth Wallace, the association’s president, told the Washington Post.
The shift will likely require a balancing act and slow transition. The USDA has been consulting with stakeholders for months to determine how to move toward stricter school nutrition standards while also acknowledging the pandemic, supply chain disruptions, and labor shortages.
“This approach is really going to help move forward the nutrition of the meals and allows the schools to continue to function effectively,” Geri Henchy, director of nutrition policy at the Food Research and Action Center, told the Post.
“Schools can’t make big changes at this point because of the supply chain and staffing,” she said. “They have a lot of waivers at this point that are helping them, and this balances the needs of all the different sectors.”
The USDA plans to issue a proposed rule in fall 2022 to update nutrition standards for the future, the department said in its announcement, which would be finalized for the 2024-2025 school year.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases down again, but still ‘extremely high’
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.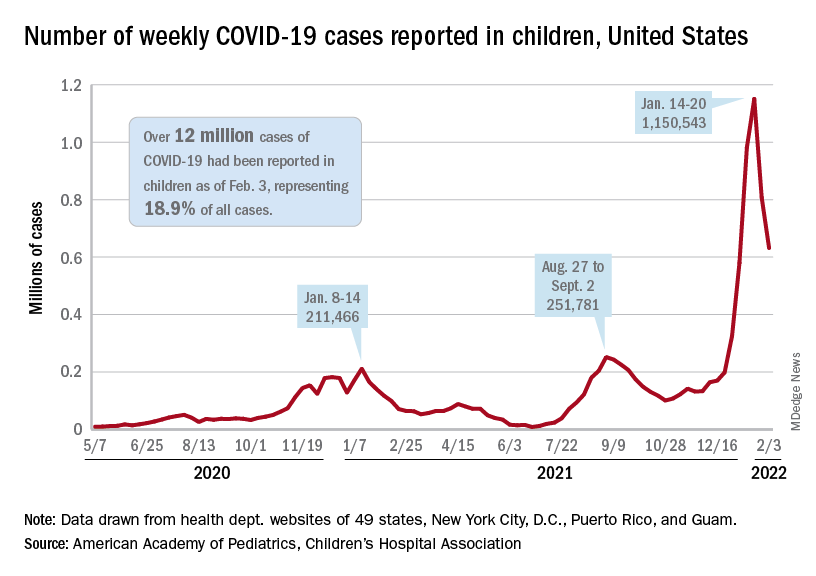
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
Eating dinner late ups diabetes risk; melatonin involved
which increase the risk of type 2 diabetes.
And people who are carriers of the G allele of the MTNR1B gene have greater impairment in glucose tolerance after eating a late dinner.
“In natural late eaters [in Spain], we simulated early and late dinner timing by administering a glucose drink and compared effects on blood sugar control over 2 hours,” said senior author Richa Saxena, PhD, a principal investigator at the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
The study also compared outcomes in carriers and noncarriers of the G allele variant of the melatonin receptor gene, Dr. Saxena pointed out in a press release from the hospital.
“We found that late eating disturbed blood sugar control in the whole group,” added lead author Marta Garaulet, PhD.
“This impaired glucose control was predominantly seen in genetic risk variant carriers, representing about half of the cohort,” said Dr. Garaulet, professor of physiology and nutrition, University of Murcia (Spain).
The study results “may be important in the effort toward prevention of type 2 diabetes,” according to co–senior author Frank A.J.L. Scheer, PhD.
“Our findings are applicable to about a third of the population in the industrialized world who consume food close to bedtime, as well as other populations who eat at night, including shift workers, or those experiencing jet lag or night-eating disorders, as well as those who routinely use melatonin supplements close to food intake,” said Dr. Scheer, director of the medical chronobiology program at Brigham and Women’s Hospital, Boston.
The results suggest people should not eat within 2 hours of bedtime, said the researchers.
“Notably, our study does not include patients with diabetes, so additional studies are needed to examine the impact of food timing and its link with melatonin and receptor variation in patients with diabetes,” Dr. Scheer said.
The findings, from the MTNR1B SNP*Food Timing Interaction on Glucose Control (ONTIME-MT) randomized crossover study, were recently published in Diabetes Care.
Melatonin plays a key role in glucose metabolism
Melatonin, a hormone primarily released at night that helps control the sleep-wake cycle, typically rises around 2 hours before bedtime, the researchers explained.
The discovery of MTNR1B as a type 2 diabetes–associated gene “suggests that, beyond sleep and circadian regulation, melatonin plays a key role in glucose metabolism,” they noted. However, whether melatonin improves or impairs glucose control is controversial, and the effect of MTNR1B genotypes on glucose control is not clear.
“We decided to test if late eating that usually occurs with elevated melatonin levels results in disturbed blood sugar control,” Dr. Saxena explained.
To investigate this, researchers enrolled 845 adults in Spain who were 18-70 years old and did not have diabetes. Participants were a mean age of 38 years and 71% were women. They had a mean body mass index of 25.7 kg/m2 and 18% had obesity.
On average, they typically ate dinner at 21:38 (9:38 p.m.) and went to bed at 24:32 (12:32 a.m.).
DNA analysis from participants’ blood samples determined that 50% had the CC genotype of the MTNR1B gene, 40% had the CG genotype, and 10% had the GG genotype.
Each participant underwent two oral glucose tolerance tests. They fasted for 8 hours and then had a 2-hour 75-g oral glucose tolerance test either 1 hour before bedtime (simulating a late dinner) or 4 hours before bedtime (simulating an early dinner). Then they repeated the test at the opposite dinner time on another night.
The average serum melatonin values were 3.5-fold higher after the late dinner than after the early dinner, resulting in 6.7% lower insulin area under the curve and 8.3% higher glucose AUC.
Genotype differences in glucose tolerance were attributed to reductions in beta-cell function.
“Our results confirm that late eating acutely impairs glucose tolerance through a defect in insulin secretion,” the researchers reiterated.
ONTIME-MT was funded by the National Institutes of Health; the Spanish Government of Investigation, Development, and Innovation; and the Seneca Foundation. The researchers reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
which increase the risk of type 2 diabetes.
And people who are carriers of the G allele of the MTNR1B gene have greater impairment in glucose tolerance after eating a late dinner.
“In natural late eaters [in Spain], we simulated early and late dinner timing by administering a glucose drink and compared effects on blood sugar control over 2 hours,” said senior author Richa Saxena, PhD, a principal investigator at the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
The study also compared outcomes in carriers and noncarriers of the G allele variant of the melatonin receptor gene, Dr. Saxena pointed out in a press release from the hospital.
“We found that late eating disturbed blood sugar control in the whole group,” added lead author Marta Garaulet, PhD.
“This impaired glucose control was predominantly seen in genetic risk variant carriers, representing about half of the cohort,” said Dr. Garaulet, professor of physiology and nutrition, University of Murcia (Spain).
The study results “may be important in the effort toward prevention of type 2 diabetes,” according to co–senior author Frank A.J.L. Scheer, PhD.
“Our findings are applicable to about a third of the population in the industrialized world who consume food close to bedtime, as well as other populations who eat at night, including shift workers, or those experiencing jet lag or night-eating disorders, as well as those who routinely use melatonin supplements close to food intake,” said Dr. Scheer, director of the medical chronobiology program at Brigham and Women’s Hospital, Boston.
The results suggest people should not eat within 2 hours of bedtime, said the researchers.
“Notably, our study does not include patients with diabetes, so additional studies are needed to examine the impact of food timing and its link with melatonin and receptor variation in patients with diabetes,” Dr. Scheer said.
The findings, from the MTNR1B SNP*Food Timing Interaction on Glucose Control (ONTIME-MT) randomized crossover study, were recently published in Diabetes Care.
Melatonin plays a key role in glucose metabolism
Melatonin, a hormone primarily released at night that helps control the sleep-wake cycle, typically rises around 2 hours before bedtime, the researchers explained.
The discovery of MTNR1B as a type 2 diabetes–associated gene “suggests that, beyond sleep and circadian regulation, melatonin plays a key role in glucose metabolism,” they noted. However, whether melatonin improves or impairs glucose control is controversial, and the effect of MTNR1B genotypes on glucose control is not clear.
“We decided to test if late eating that usually occurs with elevated melatonin levels results in disturbed blood sugar control,” Dr. Saxena explained.
To investigate this, researchers enrolled 845 adults in Spain who were 18-70 years old and did not have diabetes. Participants were a mean age of 38 years and 71% were women. They had a mean body mass index of 25.7 kg/m2 and 18% had obesity.
On average, they typically ate dinner at 21:38 (9:38 p.m.) and went to bed at 24:32 (12:32 a.m.).
DNA analysis from participants’ blood samples determined that 50% had the CC genotype of the MTNR1B gene, 40% had the CG genotype, and 10% had the GG genotype.
Each participant underwent two oral glucose tolerance tests. They fasted for 8 hours and then had a 2-hour 75-g oral glucose tolerance test either 1 hour before bedtime (simulating a late dinner) or 4 hours before bedtime (simulating an early dinner). Then they repeated the test at the opposite dinner time on another night.
The average serum melatonin values were 3.5-fold higher after the late dinner than after the early dinner, resulting in 6.7% lower insulin area under the curve and 8.3% higher glucose AUC.
Genotype differences in glucose tolerance were attributed to reductions in beta-cell function.
“Our results confirm that late eating acutely impairs glucose tolerance through a defect in insulin secretion,” the researchers reiterated.
ONTIME-MT was funded by the National Institutes of Health; the Spanish Government of Investigation, Development, and Innovation; and the Seneca Foundation. The researchers reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
which increase the risk of type 2 diabetes.
And people who are carriers of the G allele of the MTNR1B gene have greater impairment in glucose tolerance after eating a late dinner.
“In natural late eaters [in Spain], we simulated early and late dinner timing by administering a glucose drink and compared effects on blood sugar control over 2 hours,” said senior author Richa Saxena, PhD, a principal investigator at the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
The study also compared outcomes in carriers and noncarriers of the G allele variant of the melatonin receptor gene, Dr. Saxena pointed out in a press release from the hospital.
“We found that late eating disturbed blood sugar control in the whole group,” added lead author Marta Garaulet, PhD.
“This impaired glucose control was predominantly seen in genetic risk variant carriers, representing about half of the cohort,” said Dr. Garaulet, professor of physiology and nutrition, University of Murcia (Spain).
The study results “may be important in the effort toward prevention of type 2 diabetes,” according to co–senior author Frank A.J.L. Scheer, PhD.
“Our findings are applicable to about a third of the population in the industrialized world who consume food close to bedtime, as well as other populations who eat at night, including shift workers, or those experiencing jet lag or night-eating disorders, as well as those who routinely use melatonin supplements close to food intake,” said Dr. Scheer, director of the medical chronobiology program at Brigham and Women’s Hospital, Boston.
The results suggest people should not eat within 2 hours of bedtime, said the researchers.
“Notably, our study does not include patients with diabetes, so additional studies are needed to examine the impact of food timing and its link with melatonin and receptor variation in patients with diabetes,” Dr. Scheer said.
The findings, from the MTNR1B SNP*Food Timing Interaction on Glucose Control (ONTIME-MT) randomized crossover study, were recently published in Diabetes Care.
Melatonin plays a key role in glucose metabolism
Melatonin, a hormone primarily released at night that helps control the sleep-wake cycle, typically rises around 2 hours before bedtime, the researchers explained.
The discovery of MTNR1B as a type 2 diabetes–associated gene “suggests that, beyond sleep and circadian regulation, melatonin plays a key role in glucose metabolism,” they noted. However, whether melatonin improves or impairs glucose control is controversial, and the effect of MTNR1B genotypes on glucose control is not clear.
“We decided to test if late eating that usually occurs with elevated melatonin levels results in disturbed blood sugar control,” Dr. Saxena explained.
To investigate this, researchers enrolled 845 adults in Spain who were 18-70 years old and did not have diabetes. Participants were a mean age of 38 years and 71% were women. They had a mean body mass index of 25.7 kg/m2 and 18% had obesity.
On average, they typically ate dinner at 21:38 (9:38 p.m.) and went to bed at 24:32 (12:32 a.m.).
DNA analysis from participants’ blood samples determined that 50% had the CC genotype of the MTNR1B gene, 40% had the CG genotype, and 10% had the GG genotype.
Each participant underwent two oral glucose tolerance tests. They fasted for 8 hours and then had a 2-hour 75-g oral glucose tolerance test either 1 hour before bedtime (simulating a late dinner) or 4 hours before bedtime (simulating an early dinner). Then they repeated the test at the opposite dinner time on another night.
The average serum melatonin values were 3.5-fold higher after the late dinner than after the early dinner, resulting in 6.7% lower insulin area under the curve and 8.3% higher glucose AUC.
Genotype differences in glucose tolerance were attributed to reductions in beta-cell function.
“Our results confirm that late eating acutely impairs glucose tolerance through a defect in insulin secretion,” the researchers reiterated.
ONTIME-MT was funded by the National Institutes of Health; the Spanish Government of Investigation, Development, and Innovation; and the Seneca Foundation. The researchers reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Updated endometriosis guidelines emphasize less laparoscopy, more hormone therapy
Updated guidelines for the management and treatment of endometriosis reflect changes in clinical practice to guide clinician and patient decision-making, according to a statement from the European Society of Human Reproduction and Embryology, which issued the guidelines in February 2022.
Although the exact prevalence of endometriosis remains unclear, estimates suggest that approximately 190 million women and adolescent girls are affected by endometriosis during their reproductive years, and women continue to suffer beyond menopause, according to the authors. Endometriosis has a significant impact on society through both direct and indirect health care costs comparable to those of type 2 diabetes, rheumatoid arthritis, and Crohn’s disease, they noted.
The guidelines are the first update on the topic of endometriosis since 2014, and include more than 100 recommendations, according to the European Society of Human Reproduction and Embryology (ESHRE). The target audience, according to the authors, is secondary and tertiary health care providers who treat women with endometriosis. The recommendations were based on research papers published up to Dec. 1, 2020.
Although most of the recent studies confirm previous ESHRE recommendations, several topics reflect significant changes in clinical practice.
Notably, laparoscopy is no longer recommended as the diagnostic gold standard, and should be used only in patients with negative imaging for whom empirical treatment was unsuccessful.
For pain management, studies support the use of GnRH antagonists as a second-line treatment, while laparoscopic uterosacral nerve ablation and presacral neurectomy are no longer included in the recommendations.
The guidelines include new information on pregnancy and fertility preservation for women with endometriosis. The Endometriosis Fertility Index (EFI) was added to support joint decision-making for women seeking pregnancy after surgery. However, the extended use of GnRH antagonist prior to assisted reproductive technology treatments to improve live birth rate is not recommended.
Endometriosis in adolescent patients is included in the guidelines for the first time, and strong recommendations include taking a careful history and using ultrasound if appropriate, but the use of serum biomarkers is not recommended for diagnosis. Strong recommendations for treatment strategies for adolescents include hormonal contraceptives or progestins as a first-line therapy.
Recommendations for managing endometriosis in menopause are more extensive than in previous guidelines and the strongest update is against the use of estrogen-only treatment in these patients. However, the guidelines continue to recommend treating women with a history of endometriosis after surgical menopause with combined estrogen-progestogen therapy “at least up to the age of natural menopause.”
Expanded recommendations related to endometriosis and cancer begin with a strong recommendation for clinicians to advise women that endometriosis is not associated with a significantly higher risk of cancer overall. “Although endometriosis is associated with a higher risk of ovarian, breast, and thyroid cancers in particular, the increase in absolute risk compared with women in the general population is low,” the authors wrote. Other strong recommendations include reassuring women with endometriosis of the low risk of malignancy associated with hormonal contraceptive use, and performing cancer screening according to the existing population-based guidelines without additional screening. Epidemiologic data show that complete excision of visible endometriosis may reduce the risk of ovarian cancer, but the potential benefits must be weighed against the risks of surgery, including morbidity, pain, and ovarian reserve, the authors said.
The guidelines include recommendations related to asymptomatic endometriosis, extrapelvic endometriosis, and primary prevention of endometriosis, but without major changes to the 2014 guidelines.
Guidelines expand strategies, but research gaps remain
In 2021, an international working group of the American Association of Gynecologic Laparoscopists, the European Society for Gynecologic Endoscopy, ESHRE, and the World Endometriosis Society defined endometriosis as “a disease characterized by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process,” Mark P. Trolice, MD, director of The IVF Center, Orlando, Fla., and professor of obstetrics and gynecology at the University of Central Florida, said in an interview.
Although the current guidelines represent the second update since 2005, many unanswered questions remain, Dr. Trolice said. “There is a large diagnostic void between the onset of symptoms and the time to a reliable diagnosis averaging between 8 and 12 years,” he emphasized.
Dr. Trolice noted the change of the addition of an oral GnRH antagonist, “now FDA approved for the treatment of pain associated with endometriosis,” he said. However, “Extended GnRH agonist prior to ART is not recommended due to the lack of any clear benefit,” he noted.
Dr. Trolice noted the inclusion of the Endometriosis Fertility Index (EFI), published in 2010, “as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and IUI [intrauterine insemination]) based on patient characteristics, revised ASRM staging, and ‘least function score of the adnexa.’ ” He agreed with the need for expanded information on the topics of endometriosis and adolescence and endometriosis and cancer.
The most important changes for clinical practice include reducing unnecessary laparoscopy and procedures without benefit, such as laparoscopic uterosacral nerve ablation and presacral neurectomy, and GnRH suppression using an oral antagonist, said Dr. Trolice. Other especially practical guidance includes the recommendation to discontinue advising patients that pregnancy will reduce symptoms of endometriosis, and to avoid prescribing estrogen-only treatment in menopause given the risk of malignant transformation of endometriosis, he said.
Another clinically useful recommendation, though not a significant update, is the need to identify extrapelvic endometriosis symptoms, such as cyclical shoulder pain, cyclical spontaneous pneumothorax, cyclical cough, or nodules that enlarge during menses, Dr. Trolice added.
Barriers to implementing the updated guidelines include lack of education of clinicians, including primary care providers, and the lack of definitive evidence for many areas, he noted.
As for additional research, more data are needed to explore the genetic, mutational, and epigenetic profile of endometriosis, and to identify biomarkers to noninvasively detect and provide a prognosis for endometriosis, and optimal methods for prevention and management, said Dr. Trolice. Other research gaps include “definitive medical and surgical treatment of endometriosis for improvement of fertility, quality of life, and reduction of pain,” he noted. From a fertility standpoint, more studies are needed on “the use of ovarian tissue or oocytes cryopreservation in adolescents and adults who undergo ovarian surgery for endometriomas, and the role of the EFI as a presurgical triage tool and to predict IUI outcomes,” said Dr. Trolice.
Overall, society recommendations such as these from ESHRE “serve as guides for physicians by providing evidence-based medicine and dispelling prior unproven practices so patients may receive the most effective care of endometriosis, throughout a woman’s life,” Dr. Trolice emphasized.
The current guideline will be considered for revision in 2025, and the full version is available on the ESHRE website.
Members of the ESHRE guideline development group received no payment for participating in the development process, although they were reimbursed for travel expenses related to guideline meetings.
Dr. Trolice had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Updated guidelines for the management and treatment of endometriosis reflect changes in clinical practice to guide clinician and patient decision-making, according to a statement from the European Society of Human Reproduction and Embryology, which issued the guidelines in February 2022.
Although the exact prevalence of endometriosis remains unclear, estimates suggest that approximately 190 million women and adolescent girls are affected by endometriosis during their reproductive years, and women continue to suffer beyond menopause, according to the authors. Endometriosis has a significant impact on society through both direct and indirect health care costs comparable to those of type 2 diabetes, rheumatoid arthritis, and Crohn’s disease, they noted.
The guidelines are the first update on the topic of endometriosis since 2014, and include more than 100 recommendations, according to the European Society of Human Reproduction and Embryology (ESHRE). The target audience, according to the authors, is secondary and tertiary health care providers who treat women with endometriosis. The recommendations were based on research papers published up to Dec. 1, 2020.
Although most of the recent studies confirm previous ESHRE recommendations, several topics reflect significant changes in clinical practice.
Notably, laparoscopy is no longer recommended as the diagnostic gold standard, and should be used only in patients with negative imaging for whom empirical treatment was unsuccessful.
For pain management, studies support the use of GnRH antagonists as a second-line treatment, while laparoscopic uterosacral nerve ablation and presacral neurectomy are no longer included in the recommendations.
The guidelines include new information on pregnancy and fertility preservation for women with endometriosis. The Endometriosis Fertility Index (EFI) was added to support joint decision-making for women seeking pregnancy after surgery. However, the extended use of GnRH antagonist prior to assisted reproductive technology treatments to improve live birth rate is not recommended.
Endometriosis in adolescent patients is included in the guidelines for the first time, and strong recommendations include taking a careful history and using ultrasound if appropriate, but the use of serum biomarkers is not recommended for diagnosis. Strong recommendations for treatment strategies for adolescents include hormonal contraceptives or progestins as a first-line therapy.
Recommendations for managing endometriosis in menopause are more extensive than in previous guidelines and the strongest update is against the use of estrogen-only treatment in these patients. However, the guidelines continue to recommend treating women with a history of endometriosis after surgical menopause with combined estrogen-progestogen therapy “at least up to the age of natural menopause.”
Expanded recommendations related to endometriosis and cancer begin with a strong recommendation for clinicians to advise women that endometriosis is not associated with a significantly higher risk of cancer overall. “Although endometriosis is associated with a higher risk of ovarian, breast, and thyroid cancers in particular, the increase in absolute risk compared with women in the general population is low,” the authors wrote. Other strong recommendations include reassuring women with endometriosis of the low risk of malignancy associated with hormonal contraceptive use, and performing cancer screening according to the existing population-based guidelines without additional screening. Epidemiologic data show that complete excision of visible endometriosis may reduce the risk of ovarian cancer, but the potential benefits must be weighed against the risks of surgery, including morbidity, pain, and ovarian reserve, the authors said.
The guidelines include recommendations related to asymptomatic endometriosis, extrapelvic endometriosis, and primary prevention of endometriosis, but without major changes to the 2014 guidelines.
Guidelines expand strategies, but research gaps remain
In 2021, an international working group of the American Association of Gynecologic Laparoscopists, the European Society for Gynecologic Endoscopy, ESHRE, and the World Endometriosis Society defined endometriosis as “a disease characterized by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process,” Mark P. Trolice, MD, director of The IVF Center, Orlando, Fla., and professor of obstetrics and gynecology at the University of Central Florida, said in an interview.
Although the current guidelines represent the second update since 2005, many unanswered questions remain, Dr. Trolice said. “There is a large diagnostic void between the onset of symptoms and the time to a reliable diagnosis averaging between 8 and 12 years,” he emphasized.
Dr. Trolice noted the change of the addition of an oral GnRH antagonist, “now FDA approved for the treatment of pain associated with endometriosis,” he said. However, “Extended GnRH agonist prior to ART is not recommended due to the lack of any clear benefit,” he noted.
Dr. Trolice noted the inclusion of the Endometriosis Fertility Index (EFI), published in 2010, “as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and IUI [intrauterine insemination]) based on patient characteristics, revised ASRM staging, and ‘least function score of the adnexa.’ ” He agreed with the need for expanded information on the topics of endometriosis and adolescence and endometriosis and cancer.
The most important changes for clinical practice include reducing unnecessary laparoscopy and procedures without benefit, such as laparoscopic uterosacral nerve ablation and presacral neurectomy, and GnRH suppression using an oral antagonist, said Dr. Trolice. Other especially practical guidance includes the recommendation to discontinue advising patients that pregnancy will reduce symptoms of endometriosis, and to avoid prescribing estrogen-only treatment in menopause given the risk of malignant transformation of endometriosis, he said.
Another clinically useful recommendation, though not a significant update, is the need to identify extrapelvic endometriosis symptoms, such as cyclical shoulder pain, cyclical spontaneous pneumothorax, cyclical cough, or nodules that enlarge during menses, Dr. Trolice added.
Barriers to implementing the updated guidelines include lack of education of clinicians, including primary care providers, and the lack of definitive evidence for many areas, he noted.
As for additional research, more data are needed to explore the genetic, mutational, and epigenetic profile of endometriosis, and to identify biomarkers to noninvasively detect and provide a prognosis for endometriosis, and optimal methods for prevention and management, said Dr. Trolice. Other research gaps include “definitive medical and surgical treatment of endometriosis for improvement of fertility, quality of life, and reduction of pain,” he noted. From a fertility standpoint, more studies are needed on “the use of ovarian tissue or oocytes cryopreservation in adolescents and adults who undergo ovarian surgery for endometriomas, and the role of the EFI as a presurgical triage tool and to predict IUI outcomes,” said Dr. Trolice.
Overall, society recommendations such as these from ESHRE “serve as guides for physicians by providing evidence-based medicine and dispelling prior unproven practices so patients may receive the most effective care of endometriosis, throughout a woman’s life,” Dr. Trolice emphasized.
The current guideline will be considered for revision in 2025, and the full version is available on the ESHRE website.
Members of the ESHRE guideline development group received no payment for participating in the development process, although they were reimbursed for travel expenses related to guideline meetings.
Dr. Trolice had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Updated guidelines for the management and treatment of endometriosis reflect changes in clinical practice to guide clinician and patient decision-making, according to a statement from the European Society of Human Reproduction and Embryology, which issued the guidelines in February 2022.
Although the exact prevalence of endometriosis remains unclear, estimates suggest that approximately 190 million women and adolescent girls are affected by endometriosis during their reproductive years, and women continue to suffer beyond menopause, according to the authors. Endometriosis has a significant impact on society through both direct and indirect health care costs comparable to those of type 2 diabetes, rheumatoid arthritis, and Crohn’s disease, they noted.
The guidelines are the first update on the topic of endometriosis since 2014, and include more than 100 recommendations, according to the European Society of Human Reproduction and Embryology (ESHRE). The target audience, according to the authors, is secondary and tertiary health care providers who treat women with endometriosis. The recommendations were based on research papers published up to Dec. 1, 2020.
Although most of the recent studies confirm previous ESHRE recommendations, several topics reflect significant changes in clinical practice.
Notably, laparoscopy is no longer recommended as the diagnostic gold standard, and should be used only in patients with negative imaging for whom empirical treatment was unsuccessful.
For pain management, studies support the use of GnRH antagonists as a second-line treatment, while laparoscopic uterosacral nerve ablation and presacral neurectomy are no longer included in the recommendations.
The guidelines include new information on pregnancy and fertility preservation for women with endometriosis. The Endometriosis Fertility Index (EFI) was added to support joint decision-making for women seeking pregnancy after surgery. However, the extended use of GnRH antagonist prior to assisted reproductive technology treatments to improve live birth rate is not recommended.
Endometriosis in adolescent patients is included in the guidelines for the first time, and strong recommendations include taking a careful history and using ultrasound if appropriate, but the use of serum biomarkers is not recommended for diagnosis. Strong recommendations for treatment strategies for adolescents include hormonal contraceptives or progestins as a first-line therapy.
Recommendations for managing endometriosis in menopause are more extensive than in previous guidelines and the strongest update is against the use of estrogen-only treatment in these patients. However, the guidelines continue to recommend treating women with a history of endometriosis after surgical menopause with combined estrogen-progestogen therapy “at least up to the age of natural menopause.”
Expanded recommendations related to endometriosis and cancer begin with a strong recommendation for clinicians to advise women that endometriosis is not associated with a significantly higher risk of cancer overall. “Although endometriosis is associated with a higher risk of ovarian, breast, and thyroid cancers in particular, the increase in absolute risk compared with women in the general population is low,” the authors wrote. Other strong recommendations include reassuring women with endometriosis of the low risk of malignancy associated with hormonal contraceptive use, and performing cancer screening according to the existing population-based guidelines without additional screening. Epidemiologic data show that complete excision of visible endometriosis may reduce the risk of ovarian cancer, but the potential benefits must be weighed against the risks of surgery, including morbidity, pain, and ovarian reserve, the authors said.
The guidelines include recommendations related to asymptomatic endometriosis, extrapelvic endometriosis, and primary prevention of endometriosis, but without major changes to the 2014 guidelines.
Guidelines expand strategies, but research gaps remain
In 2021, an international working group of the American Association of Gynecologic Laparoscopists, the European Society for Gynecologic Endoscopy, ESHRE, and the World Endometriosis Society defined endometriosis as “a disease characterized by the presence of endometrium-like epithelium and/or stroma outside the endometrium and myometrium, usually with an associated inflammatory process,” Mark P. Trolice, MD, director of The IVF Center, Orlando, Fla., and professor of obstetrics and gynecology at the University of Central Florida, said in an interview.
Although the current guidelines represent the second update since 2005, many unanswered questions remain, Dr. Trolice said. “There is a large diagnostic void between the onset of symptoms and the time to a reliable diagnosis averaging between 8 and 12 years,” he emphasized.
Dr. Trolice noted the change of the addition of an oral GnRH antagonist, “now FDA approved for the treatment of pain associated with endometriosis,” he said. However, “Extended GnRH agonist prior to ART is not recommended due to the lack of any clear benefit,” he noted.
Dr. Trolice noted the inclusion of the Endometriosis Fertility Index (EFI), published in 2010, “as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and IUI [intrauterine insemination]) based on patient characteristics, revised ASRM staging, and ‘least function score of the adnexa.’ ” He agreed with the need for expanded information on the topics of endometriosis and adolescence and endometriosis and cancer.
The most important changes for clinical practice include reducing unnecessary laparoscopy and procedures without benefit, such as laparoscopic uterosacral nerve ablation and presacral neurectomy, and GnRH suppression using an oral antagonist, said Dr. Trolice. Other especially practical guidance includes the recommendation to discontinue advising patients that pregnancy will reduce symptoms of endometriosis, and to avoid prescribing estrogen-only treatment in menopause given the risk of malignant transformation of endometriosis, he said.
Another clinically useful recommendation, though not a significant update, is the need to identify extrapelvic endometriosis symptoms, such as cyclical shoulder pain, cyclical spontaneous pneumothorax, cyclical cough, or nodules that enlarge during menses, Dr. Trolice added.
Barriers to implementing the updated guidelines include lack of education of clinicians, including primary care providers, and the lack of definitive evidence for many areas, he noted.
As for additional research, more data are needed to explore the genetic, mutational, and epigenetic profile of endometriosis, and to identify biomarkers to noninvasively detect and provide a prognosis for endometriosis, and optimal methods for prevention and management, said Dr. Trolice. Other research gaps include “definitive medical and surgical treatment of endometriosis for improvement of fertility, quality of life, and reduction of pain,” he noted. From a fertility standpoint, more studies are needed on “the use of ovarian tissue or oocytes cryopreservation in adolescents and adults who undergo ovarian surgery for endometriomas, and the role of the EFI as a presurgical triage tool and to predict IUI outcomes,” said Dr. Trolice.
Overall, society recommendations such as these from ESHRE “serve as guides for physicians by providing evidence-based medicine and dispelling prior unproven practices so patients may receive the most effective care of endometriosis, throughout a woman’s life,” Dr. Trolice emphasized.
The current guideline will be considered for revision in 2025, and the full version is available on the ESHRE website.
Members of the ESHRE guideline development group received no payment for participating in the development process, although they were reimbursed for travel expenses related to guideline meetings.
Dr. Trolice had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Native American Tribes Settle ‘Epic’ Opioid Deal
Hundreds of Native American tribes have tentatively settled in what one of the lead attorneys describes as “an epic deal”: The top 3 pharmaceutical distributors in the US and Johnson & Johnson have agreed to pay $665 million for deceptive marketing practices and overdistribution of opioids. Native Americans were among those hardest hit by the opioid epidemic. Between 2006 and 2014, Native Americans were nearly 50% more likely than non-Natives to die of an opioid overdose. In 2014, they ranked number 1 for death by opioid overdose.
Overprescribing was rampant. In some areas, such as southwestern Virginia, eastern Kentucky, and Alabama, prescriptions were 5 to 6 times higher than the national average. The overprescribing was largely due to massive and aggressive billion-dollar marketing campaigns, which misrepresented the safety of opioid medications. Purdue Pharma, for instance, trained sales representatives to claim that the risk of addiction was “less than 1 percent.” In an interview with Smithsonian Magazine, Caleb Alexander, MD, codirector of Johns Hopkins’ Center for Drug Safety and Effectiveness, said, “When I was in residency training, we were taught that one needn’t worry about the addictive potential of opioids if a patient had true pain.” He said it was no accident that physicians were cultivated to overestimate the effectiveness for chronic, noncancer pain while underestimating the risks.
Native Americans were not only in the target group for prescriptions, but also apparently singularly targeted. “We were preyed upon,” said Chickasaw Nation Governor Bill Anoatubby in the Washington Post. “It was unconscionable.” A Washington Post analysis found that, between 2006 and 2014, opioid distributors shipped an average of 36 pills per person in the US. States in the so-called opioid belt (mostly Southern states), received an average of 60 to 66 pills per person. The distributors shipped 57 pills per person to Oklahoma, home to nearly 322,000 Native Americans. (The opioid death rate for Native Americans in Oklahoma from 2006 to 2014 was more than triple the nationwide rate for non-Natives.) In South Dakota as recently as 2015, enough opioids were prescribed to medicate every adult around-the-clock for 19 consecutive days. Native Americans comprise 9% of South Dakota’s population; however, almost 30% of the patients are being treated for opioid use disorder.
In the settlement, which is a first for tribes, McKesson, Cardinal Health, and AmerisourceBergen would pay $515 million over 7 years. Johnson & Johnson would contribute $150 million in 2 years to the federally recognized tribes. “This settlement is a real turning point in history,” said Lloyd Miller, one of the attorneys representing one-third of the litigating tribes.
But the money is still small compensation for ravaging millions of lives. “Flooding the Native community with Western medicine—sedating a population rather than seeking to understand its needs and challenges—is not an acceptable means of handling its trauma,” the Lakota People’s Law Project says in an article on its website. Thus, the money dispersal will be overseen by a panel of tribal health experts, to go toward programs that aid drug users and their communities.
The funds will be managed in a way that will consider the long-term damage, Native American leaders vow. Children, for instance, have not been exempt from the sequelae of the overprescribing. Foster care systems are “overrun” with children of addicted parents, the Law Project says, and the children are placed in homes outside the tribe. “In the long run, this has the potential to curtail tribal membership, break down familial lines, and degrade cultural values.”
Dealing with the problem has drained tribal resources—doubly strained by the COVID-19 epidemic. Chairman Douglas Yankton, of the Spirit Lake Nation in North Dakota, said in a statement, “The dollars that will flow to Tribes under this initial settlement will help fund crucial, on-reservation, culturally appropriate opioid treatment services.”
However, Chairman Kristopher Peters, of the Squaxin Island Tribe in Washington State, told the Washington Post, “There is no amount of money that’s going to solve the generational issues that have been created from this. Our hope is that we can use these funds to help revitalize our culture and help heal our people.”
Johnson & Johnson says it no longer sells prescription opioids in the US
Hundreds of Native American tribes have tentatively settled in what one of the lead attorneys describes as “an epic deal”: The top 3 pharmaceutical distributors in the US and Johnson & Johnson have agreed to pay $665 million for deceptive marketing practices and overdistribution of opioids. Native Americans were among those hardest hit by the opioid epidemic. Between 2006 and 2014, Native Americans were nearly 50% more likely than non-Natives to die of an opioid overdose. In 2014, they ranked number 1 for death by opioid overdose.
Overprescribing was rampant. In some areas, such as southwestern Virginia, eastern Kentucky, and Alabama, prescriptions were 5 to 6 times higher than the national average. The overprescribing was largely due to massive and aggressive billion-dollar marketing campaigns, which misrepresented the safety of opioid medications. Purdue Pharma, for instance, trained sales representatives to claim that the risk of addiction was “less than 1 percent.” In an interview with Smithsonian Magazine, Caleb Alexander, MD, codirector of Johns Hopkins’ Center for Drug Safety and Effectiveness, said, “When I was in residency training, we were taught that one needn’t worry about the addictive potential of opioids if a patient had true pain.” He said it was no accident that physicians were cultivated to overestimate the effectiveness for chronic, noncancer pain while underestimating the risks.
Native Americans were not only in the target group for prescriptions, but also apparently singularly targeted. “We were preyed upon,” said Chickasaw Nation Governor Bill Anoatubby in the Washington Post. “It was unconscionable.” A Washington Post analysis found that, between 2006 and 2014, opioid distributors shipped an average of 36 pills per person in the US. States in the so-called opioid belt (mostly Southern states), received an average of 60 to 66 pills per person. The distributors shipped 57 pills per person to Oklahoma, home to nearly 322,000 Native Americans. (The opioid death rate for Native Americans in Oklahoma from 2006 to 2014 was more than triple the nationwide rate for non-Natives.) In South Dakota as recently as 2015, enough opioids were prescribed to medicate every adult around-the-clock for 19 consecutive days. Native Americans comprise 9% of South Dakota’s population; however, almost 30% of the patients are being treated for opioid use disorder.
In the settlement, which is a first for tribes, McKesson, Cardinal Health, and AmerisourceBergen would pay $515 million over 7 years. Johnson & Johnson would contribute $150 million in 2 years to the federally recognized tribes. “This settlement is a real turning point in history,” said Lloyd Miller, one of the attorneys representing one-third of the litigating tribes.
But the money is still small compensation for ravaging millions of lives. “Flooding the Native community with Western medicine—sedating a population rather than seeking to understand its needs and challenges—is not an acceptable means of handling its trauma,” the Lakota People’s Law Project says in an article on its website. Thus, the money dispersal will be overseen by a panel of tribal health experts, to go toward programs that aid drug users and their communities.
The funds will be managed in a way that will consider the long-term damage, Native American leaders vow. Children, for instance, have not been exempt from the sequelae of the overprescribing. Foster care systems are “overrun” with children of addicted parents, the Law Project says, and the children are placed in homes outside the tribe. “In the long run, this has the potential to curtail tribal membership, break down familial lines, and degrade cultural values.”
Dealing with the problem has drained tribal resources—doubly strained by the COVID-19 epidemic. Chairman Douglas Yankton, of the Spirit Lake Nation in North Dakota, said in a statement, “The dollars that will flow to Tribes under this initial settlement will help fund crucial, on-reservation, culturally appropriate opioid treatment services.”
However, Chairman Kristopher Peters, of the Squaxin Island Tribe in Washington State, told the Washington Post, “There is no amount of money that’s going to solve the generational issues that have been created from this. Our hope is that we can use these funds to help revitalize our culture and help heal our people.”
Johnson & Johnson says it no longer sells prescription opioids in the US
Hundreds of Native American tribes have tentatively settled in what one of the lead attorneys describes as “an epic deal”: The top 3 pharmaceutical distributors in the US and Johnson & Johnson have agreed to pay $665 million for deceptive marketing practices and overdistribution of opioids. Native Americans were among those hardest hit by the opioid epidemic. Between 2006 and 2014, Native Americans were nearly 50% more likely than non-Natives to die of an opioid overdose. In 2014, they ranked number 1 for death by opioid overdose.
Overprescribing was rampant. In some areas, such as southwestern Virginia, eastern Kentucky, and Alabama, prescriptions were 5 to 6 times higher than the national average. The overprescribing was largely due to massive and aggressive billion-dollar marketing campaigns, which misrepresented the safety of opioid medications. Purdue Pharma, for instance, trained sales representatives to claim that the risk of addiction was “less than 1 percent.” In an interview with Smithsonian Magazine, Caleb Alexander, MD, codirector of Johns Hopkins’ Center for Drug Safety and Effectiveness, said, “When I was in residency training, we were taught that one needn’t worry about the addictive potential of opioids if a patient had true pain.” He said it was no accident that physicians were cultivated to overestimate the effectiveness for chronic, noncancer pain while underestimating the risks.
Native Americans were not only in the target group for prescriptions, but also apparently singularly targeted. “We were preyed upon,” said Chickasaw Nation Governor Bill Anoatubby in the Washington Post. “It was unconscionable.” A Washington Post analysis found that, between 2006 and 2014, opioid distributors shipped an average of 36 pills per person in the US. States in the so-called opioid belt (mostly Southern states), received an average of 60 to 66 pills per person. The distributors shipped 57 pills per person to Oklahoma, home to nearly 322,000 Native Americans. (The opioid death rate for Native Americans in Oklahoma from 2006 to 2014 was more than triple the nationwide rate for non-Natives.) In South Dakota as recently as 2015, enough opioids were prescribed to medicate every adult around-the-clock for 19 consecutive days. Native Americans comprise 9% of South Dakota’s population; however, almost 30% of the patients are being treated for opioid use disorder.
In the settlement, which is a first for tribes, McKesson, Cardinal Health, and AmerisourceBergen would pay $515 million over 7 years. Johnson & Johnson would contribute $150 million in 2 years to the federally recognized tribes. “This settlement is a real turning point in history,” said Lloyd Miller, one of the attorneys representing one-third of the litigating tribes.
But the money is still small compensation for ravaging millions of lives. “Flooding the Native community with Western medicine—sedating a population rather than seeking to understand its needs and challenges—is not an acceptable means of handling its trauma,” the Lakota People’s Law Project says in an article on its website. Thus, the money dispersal will be overseen by a panel of tribal health experts, to go toward programs that aid drug users and their communities.
The funds will be managed in a way that will consider the long-term damage, Native American leaders vow. Children, for instance, have not been exempt from the sequelae of the overprescribing. Foster care systems are “overrun” with children of addicted parents, the Law Project says, and the children are placed in homes outside the tribe. “In the long run, this has the potential to curtail tribal membership, break down familial lines, and degrade cultural values.”
Dealing with the problem has drained tribal resources—doubly strained by the COVID-19 epidemic. Chairman Douglas Yankton, of the Spirit Lake Nation in North Dakota, said in a statement, “The dollars that will flow to Tribes under this initial settlement will help fund crucial, on-reservation, culturally appropriate opioid treatment services.”
However, Chairman Kristopher Peters, of the Squaxin Island Tribe in Washington State, told the Washington Post, “There is no amount of money that’s going to solve the generational issues that have been created from this. Our hope is that we can use these funds to help revitalize our culture and help heal our people.”
Johnson & Johnson says it no longer sells prescription opioids in the US
Enough is enough: the pandemic and loss of female oncologists
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Nuances in Training During the Age of Teledermatology
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
The COVID-19 pandemic largely altered the practice of medicine, including a rapid expansion of telemedicine following the March 2020 World Health Organization guidelines for social distancing, which recommended suspension of all nonurgent in-person visits.1 Expectedly, COVID-related urgent care visits initially comprised the bulk of the new telemedicine wave: NYU Langone Health (New York, New York), for example, saw a 683% increase in virtual visits between March and April 2020, most (55.3%) of which were for respiratory concerns. In-person visits, on the other hand, concurrently fell by more than 80%. Interestingly, nonurgent ambulatory care specialties also saw a considerable uptick in virtual encounters, from less than 50 visits in a typical day to an average of 7000 in a 10-day stretch.2
As a largely ambulatory specialty that relies on visual examination, dermatology was no exception to the swing toward telemedicine, or teledermatology (TD). Before the COVID-19 pandemic, 14.1% (82 of 582 respondents) of practicing US dermatologists reported having used teledermatology, compared to 96.9% (572/591) during the pandemic.3 Even at my home institution (Massachusetts General Hospital [Boston, Massachusetts] and its 12 affiliated dermatology clinics), the number of in-person visits in April 2020 (n=67) was less than 1% of that in April 2019 (n=7919), whereas there was a total of 1564 virtual visits in April 2020 compared to zero the year prior. Virtual provider-to-provider consults (e-consultations) also saw an increase of more than 20%, suggesting that dermatology’s avid adoption of TD also had improved the perceived accessibility of our specialty.4
The adoption and adaptation of TD are projected to continue to grow rapidly across the globe, as digitalization has enhanced access without increasing costs, shortened wait times, and even created opportunities for primary care providers based in rural or overseas locations to learn the diagnosis and treatment of skin disease.5 Residents and fellows should be privy to the nuances of training and practicing in this digital era, as our careers inevitably will involve some facet of TD.
The Art of Medicine
Touch, a sense that perhaps ranks second to sight in dermatology, is absent in TD. In either synchronous (live-interactive, face video visits) or asynchronous (store-and-forward, where digital photographs and clinical information sent by patients or referring physicians are assessed at a later time) TD, the skin cannot be rubbed for texture, pinched for thickness, or pushed for blanching. Instead, all we have is vision. Irwin Braverman, MD, Professor Emeritus of Dermatology at Yale University (New Haven, Connecticut), alongside Jacqueline Dolev, MD, dermatologist and Yale graduate, and Linda Friedlaender, curator at the Yale Center for British Art, founded an observational skills workshop in which trainees learn to observe and describe the paintings housed in the museum, noting all memorable details: the color of the sky, the actions of the animals, and the facial expressions of the people. A study of 90 participants over a 2-year period found that following the workshop, the ability to identify key diagnostic details from clinical photography improved by more than 10%.6 Other studies also utilizing fine art as a medical training tool to improve “visual literacy” saw similarly increased sophistication in the description of clinical imagery, which translated to better diagnostic acumen.7 Confined to video and photographs, TD necessitates trainees and practicing dermatologists to be excellent visual diagnosticians. Although surveyed dermatologists believe TD is presently appropriate for acne, benign lesions, or follow-up appointments,3 conditions for which patients have been examined via TD have included drug eruptions, premalignant or malignant neoplasms, infections, and papulosquamous or inflammatory dermatoses.8 At the very least, clinicians should be versed in identifying those conditions that require in-person evaluation, as patients cannot be held responsible to distinguish which situations can and cannot be addressed virtually.
Issues of Patient-Physician Confidentiality
Teledermatology is not without its shortcomings; critics have noted diagnostic challenges with poor quality photographs or videos, inability to perform total-body skin examinations, and socioeconomic limitations due to broadband availability and speed.5,9 Although most of these shortcomings are outside of our control, a key challenge within the purview of the provider is the protection of patient privacy.
Much of the salient concerns regarding patient-physician confidentiality involve asynchronous TD, where store-and-forward data sharing allows physicians to download patient photographs or information onto their personal email or smartphones.10 Although some hospital systems provide encryption software or hospital-sponsored devices to ensure security, physicians may opt to use their personal phones or laptops out of convenience or to save time.10,11 One study found that less than 30% of smartphone users choose to activate user authentication on their devices, even ones as simple as a passphrase.11 The digital exchange of information thus poses an immense risk for compromising protected health information (PHI), as personal devices can be easily lost, stolen, or hacked. Indeed, in 2015, more than 113 million individuals were affected by a breach of PHI, the majority over hacked network servers.12 With the growing diversity of mediums through which PHI is exchanged, such as videoconferencing and instant messaging, the potential medicolegal risks of information breach continue to climb. The US Department of Health & Human Services urges health care providers to uphold best practices for security, including encrypting data, updating all software including antivirus software, using multifactor authentication, and following local cybersecurity regulations or recommendations.13 For synchronous TD, suggested best practices include utilizing headphones during live appointments, avoiding public wireless networks, and ensuring the provider and patient both scan the room with their device’s camera before the start of the visit.14
On the Horizon of Teledermatology
What can we expect in the coming years? Increased utilization of telemedicine will translate into data that will help address questions surrounding safety, diagnostic accuracy, privacy, and accessibility. One aspect of TD in need of clarity is a guideline on payment and reimbursement, and whether TD can continue to be financially attractive to providers. Starting in 2020, the Centers for Medicare & Medicaid Services removed geographic restrictions for reimbursement of telemedicine visits, enabling even urban-residing patients to enjoy the convenience of TD. This followed a prior relaxation of restrictions, where even prerecorded patient information became eligible for Medicare reimbursement.9 However, as virtual visits tend to be shorter with fewer diagnostic services compared to in-person visits, the reimbursement structure of TD must be nuanced, which is the subject of ongoing study and modification in the wake of the COVID-19 pandemic.15
Another point to consider is the explosion of direct-to-consumer TD, which allows patients to receive virtual dermatologic care or prescription medication without a pre-established relationship with any physician. In 2017, there were 22 direct-to-consumer TD services available to US patients in 45 states, 16 (73%) of which provided dermatologic care for any concern while 6 (27%) were limited to acne or antiaging and were largely prescription oriented. Orchestrated mostly by the for-profit private sector, direct-to-consumer companies are poorly regulated and have raised concerns over questionable practices, such as the use of non–US board-certified physicians, exorbitant fees, and failure to disclose medication side effects.16 A study of 16 direct-to-consumer telemedicine sites found substantial discordance in the suggested management of the same patient, and many of the services relied heavily on patient-provided self-diagnoses, such as a case where psoriasis medication was dispensed for a psoriasis patient who submitted a photograph of his syphilitic rash.17 Despite these problems, consumers show a willingness to pay out of pocket to access these services for their shorter waiting times and convenience.18 Hence, we must learn to ask about direct-to-consumer service use when obtaining a thorough history and be open to counseling our patients on the proper use and potential risks of direct-to-consumer TD.
Final Thoughts
The telemedicine industry is expected to reach more than $130 billion by 2025, with more than 90% of surveyed health care executives planning for the adoption and incorporation of telemedicine into their business models.19 The COVID-19 pandemic was an impetus for an exponential adoption of TD, and it would behoove current residents to realize that the practice of dermatology will continue to be increasingly digitalized within the coming years. Whether through formal training or self-assessment, we must strive to grow as proficient virtual dermatologists while upholding professionalism, patient safety, and health information privacy.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
- Yeboah CB, Harvey N, Krishnan R, et al. The impact of COVID-19 on teledermatology: a review. Dermatol Clin. 2021;39:599-608.
- Mann DM, Chen J, Chunara R, et al. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132-1135.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Su MY, Das S. Expansion of asynchronous teledermatology during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E471-E472.
- Maddukuri S, Patel J, Lipoff JB. Teledermatology addressing disparities in health care access: a review [published online March 12, 2021]. Curr Dermatol Rep. doi:10.1007/s13671-021-00329-2
- Dolev JC, Friedlaender LK, Braverman IM. Use of fine art to enhance visual diagnostic skills. JAMA. 2001;286:1020-1021.
- Naghshineh S, Hafler JP, Miller AR, et al. Formal art observation training improves medical students’ visual diagnostic skills. J Gen Intern Med. 2008;23:991-997.
- Lee KJ, Finnane A, Soyer HP. Recent trends in teledermatology and teledermoscopy. Dermatol Pract Concept. 2018;8:214-223.
- Wang RH, Barbieri JS, Nguyen HP, et al. Clinical effectiveness and cost-effectiveness of teledermatology: where are we now, and what are the barriers to adoption? J Am Acad Dermatol. 2020;83:299-307.
- Stevenson P, Finnane AR, Soyer HP. Teledermatology and clinical photography: safeguarding patient privacy and mitigating medico-legal risk. Med J Aust. 2016;204:198-200e1.
- Smith KA, Zhou L, Watzlaf VJM. User authentication in smartphones for telehealth. Int J Telerehabil. 2017;9:3-12.
- Breaches of unsecured protected health information. Health IT website. Updated July 22, 2021. Accessed January 16, 2022. https://www.healthit.gov/data/quickstats/breaches-unsecured-protected-health-information
- Jalali MS, Landman A, Gordon WJ. Telemedicine, privacy, and information security in the age of COVID-19. J Am Med Inform Assoc. 2021;28:671-672.
- Telehealth for behavioral health care: protecting patients’ privacy. United States Department of Health and Human Services website. Updated July 2, 2021. Accessed January 16, 2022. https://telehealth.hhs.gov/providers/telehealth-for-behavioral-health/preparing-patients-for-telebehavioral-health/protecting-patients-privacy/
- Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323:2375-2376.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Snoswell CL, Whitty JA, Caffery LJ, et al. Consumer preference and willingness to pay for direct-to-consumer mobile teledermoscopy services in Australia [published online August 13, 2021]. Dermatology. doi:10.1159/000517257
- Elliott T, Yopes MC. Direct-to-consumer telemedicine. J Allergy Clin Immunol Pract. 2019;7:2546-2552.
Resident Pearl
- The COVID-19 pandemic has accelerated the adoption of teledermatology, enhancing patient access to dermatologic care while also facilitating multidisciplinary discourse and providing opportunities for education and training. However, these virtual interactions require a vigilance for patient privacy and security with an added emphasis on visual diagnostics to deliver high-quality care.
New PET tracer detects more metastases in cancer patients
leading to predictions of a “paradigm shift” in this field.
The new tracer, 68Ga-FAPI (fibroblast activation protein inhibitor), detected more metastases in patients with lung cancer than the standard tracer, 18F-FDG (fluorodeoxyglucose), which has been in use for years.
The study by Chinese researchers was published in Radiology.
The team imaged 34 lung cancer patients with both 68Ga-FAPI and 18F-FDG. Performance was similar for primary tumors and for lung, liver, and adrenal gland metastases. However, FAPI imaging detected more metastases in the lymph nodes (356 vs. 320), brain (23 vs. 10), bone (109 vs. 91), and pleura (66 vs. 35). However, neither modality outperformed MRI for brain metastases, the researchers note.
An accompanying editorial concluded that 68Ga-FAPI PET/CT scanning marks “an important paradigm shift to more specific identification and characterization of a variety of cancers.”
“This may also mark the arrival of a new era in nuclear medicine where molecular imaging helps visualize and characterize the entire tumor burden in one setting,” write editorialists Francine Jacobson, MD, and Annick Van den Abbeele, MD, from Harvard University and Brigham and Women’s Hospital and the Dana Farber Cancer Center, in Boston.
This study was the one of the latest in a fast-growing body of literature reporting that tracers targeting FAP with a small-molecule inhibitor (FAPI) outperform FDG tracers, not just in lung cancer but across a broad range of cancers, including breast, hepatic, gastrointestinal, head-neck, gynecologic, and many other tumor types.
The possibilities aren’t limited to imaging, either. Several companies are planning trials to target FAP with radiopharmaceuticals.
FAP is associated with wound repair and is highly expressed by the fibroblasts tightly packed in with cancer cells, particularly in stroma-dense tumors. FAP is rarely expressed by healthy tissue.
The underlying idea is to deliver a radionuclide to cancer-associated fibroblasts, using either a positron emitter, such as gallium-68 (68Ga), for PET imaging or a beta particle or other short-radiation emitter to kill nearby cancer cells as part of treatment.
Targeting FAP holds the promise of PET imaging that is more selective for cancer than FDG. FDG resolution depends on glucose uptake, which is high in active tumors but is also high in inflamed tissues as well as in the brain, gastrointestinal tract, and other areas. Uptake by background tissue can make it difficult to distinguish tumors from their surroundings. FDG uptake can also be lower in small and indolent tumors.
On the therapy side, there’s hope that FAP targeting will lead to radiopharmaceuticals that work across tumor types, not just in specific cancers.
High interest in FAP
Overall, FAP “is a target of high interest for the whole medical oncology community. The preliminary data are good, but this will take a while” to get to market, said Jeremie Calais, MD, a nuclear medicine specialist and FAP researcher at the University of California, Los Angeles.
Interest in FAP as a radiopharmaceutical target is being driven by the success of two agents that have served as a kind of proof of concept, Dr. Calais said.
The first is Novartis’s 177Lu-PSMA-617, which was granted priority review by the U.S. Food and Drug Administration in September 2021 following phase 3 results that showed a progression-free survival benefit of about 5 months when added to standard of care for metastatic castration-resistant prostate cancer, as well as an overall survival benefit of 4 months.
PSMA-617 binds prostate cancer cells that express prostate-specific membrane antigen. The lutetium-177 (177Lu) bombards them with beta particles and gamma radiation.
FAP researchers are also encouraged by the success of 177Lu dotatate (Lutathera), from Advanced Accelerator Applications, which delivers the radionucleotide to gastroenteropancreatic neuroendocrine tumors that express somatostatin receptors.
The FDA approved this agent in 2018 in part on the basis of phase 3 results that found a 20-month progression-free survival of 65.2% when Lutathera was added to octreotide for metastatic disease vs. 10.8% when it wasn’t.
Novartis is now looking into developing FAP-targeted radiopharmaceuticals, along with Clovis and Point Biopharma, among others.
“That’s the key goal” of industry research, “more so than FAP as a diagnostic tool,” Dr. Calais commented to this news organization. There’s “huge potential” if it works out, he said, in part because it won’t be limited to one tumor type.
Clovis recently launched a phase 1/2 trial of its candidate, 177Lu-FAP-2286, for advanced/metastatic solid tumors.
In the company’s “luMIERE” trial, subjects will be infused with 68Ga-FAP-2286 to image the tumor. Once uptake is confirmed, they’ll be infused with 177Lu-FAP-2286 for treatment.
The two-step process – uptake confirmation, then treatment – is dubbed “theranostics” and is the standard approach for radiopharmaceutical therapy, Dr. Calais said.
His own team is working to confirm that imaging accurately reflects FAP expression in tumors by comparing preoperative imaging results with FAP expression on surgical specimens. So far, his team has found that they are strongly correlated.
FAPI PET imaging research is much farther along than therapeutic applications, with almost 200 research articles listed on PubMed in 2021, up from just 3 in 2018. One 2019 paper reported “remarkably high uptake and image contrast” across 28 cancers in 80 patients, including breast, esophagus, lung, pancreatic, head-neck, and colorectal tumors.
Imaging studies so far have tended to be small, with many currently focused on identifying the optimal molecule for targeting FAP and the best positron emitter to combine with it.
FAPI tracers are not available yet commercially, so researchers are creating them themselves. One team recently reported it’s recipe for automated synthesis using commercially available synthesis modules.
Sofie, a maker of FDG and other tracers, hopes to change that and is working to bring FAP tracers to market. The company announced in November 2021 a phase 2 study of 68Ga FAPI-46 to image pancreatic ductal adenocarcinoma. It’s the first step in a broader development program for oncologic and nononcologic indications, Sofie said in a press release.
Dr. Calais sees potential for indications where FAPI has already outperformed FDG in the literature, particularly for gastrointestinal cancers. He doesn’t think it will ever replace FDG for indications such as lymphoma, where it “works perfectly well.”
“On the other hand, you have lesions located in a tissue that has some background level” of FDG uptake. “These things are okay with FDG, but I think maybe FAP can help” because of the improved signal-to-noise ratio, Dr. Calais commented. Unlike FDG, “you mostly never see background uptake with FAP-imaging agents,” he said.
Other pluses include quicker distribution throughout the body than FDG, so scan times are shorter, and also patients do not need to fast beforehand.
Dr. Calais predicts that FAPI tracers will reach the market within 5 years.
A version of this article first appeared on Medscape.com.
leading to predictions of a “paradigm shift” in this field.
The new tracer, 68Ga-FAPI (fibroblast activation protein inhibitor), detected more metastases in patients with lung cancer than the standard tracer, 18F-FDG (fluorodeoxyglucose), which has been in use for years.
The study by Chinese researchers was published in Radiology.
The team imaged 34 lung cancer patients with both 68Ga-FAPI and 18F-FDG. Performance was similar for primary tumors and for lung, liver, and adrenal gland metastases. However, FAPI imaging detected more metastases in the lymph nodes (356 vs. 320), brain (23 vs. 10), bone (109 vs. 91), and pleura (66 vs. 35). However, neither modality outperformed MRI for brain metastases, the researchers note.
An accompanying editorial concluded that 68Ga-FAPI PET/CT scanning marks “an important paradigm shift to more specific identification and characterization of a variety of cancers.”
“This may also mark the arrival of a new era in nuclear medicine where molecular imaging helps visualize and characterize the entire tumor burden in one setting,” write editorialists Francine Jacobson, MD, and Annick Van den Abbeele, MD, from Harvard University and Brigham and Women’s Hospital and the Dana Farber Cancer Center, in Boston.
This study was the one of the latest in a fast-growing body of literature reporting that tracers targeting FAP with a small-molecule inhibitor (FAPI) outperform FDG tracers, not just in lung cancer but across a broad range of cancers, including breast, hepatic, gastrointestinal, head-neck, gynecologic, and many other tumor types.
The possibilities aren’t limited to imaging, either. Several companies are planning trials to target FAP with radiopharmaceuticals.
FAP is associated with wound repair and is highly expressed by the fibroblasts tightly packed in with cancer cells, particularly in stroma-dense tumors. FAP is rarely expressed by healthy tissue.
The underlying idea is to deliver a radionuclide to cancer-associated fibroblasts, using either a positron emitter, such as gallium-68 (68Ga), for PET imaging or a beta particle or other short-radiation emitter to kill nearby cancer cells as part of treatment.
Targeting FAP holds the promise of PET imaging that is more selective for cancer than FDG. FDG resolution depends on glucose uptake, which is high in active tumors but is also high in inflamed tissues as well as in the brain, gastrointestinal tract, and other areas. Uptake by background tissue can make it difficult to distinguish tumors from their surroundings. FDG uptake can also be lower in small and indolent tumors.
On the therapy side, there’s hope that FAP targeting will lead to radiopharmaceuticals that work across tumor types, not just in specific cancers.
High interest in FAP
Overall, FAP “is a target of high interest for the whole medical oncology community. The preliminary data are good, but this will take a while” to get to market, said Jeremie Calais, MD, a nuclear medicine specialist and FAP researcher at the University of California, Los Angeles.
Interest in FAP as a radiopharmaceutical target is being driven by the success of two agents that have served as a kind of proof of concept, Dr. Calais said.
The first is Novartis’s 177Lu-PSMA-617, which was granted priority review by the U.S. Food and Drug Administration in September 2021 following phase 3 results that showed a progression-free survival benefit of about 5 months when added to standard of care for metastatic castration-resistant prostate cancer, as well as an overall survival benefit of 4 months.
PSMA-617 binds prostate cancer cells that express prostate-specific membrane antigen. The lutetium-177 (177Lu) bombards them with beta particles and gamma radiation.
FAP researchers are also encouraged by the success of 177Lu dotatate (Lutathera), from Advanced Accelerator Applications, which delivers the radionucleotide to gastroenteropancreatic neuroendocrine tumors that express somatostatin receptors.
The FDA approved this agent in 2018 in part on the basis of phase 3 results that found a 20-month progression-free survival of 65.2% when Lutathera was added to octreotide for metastatic disease vs. 10.8% when it wasn’t.
Novartis is now looking into developing FAP-targeted radiopharmaceuticals, along with Clovis and Point Biopharma, among others.
“That’s the key goal” of industry research, “more so than FAP as a diagnostic tool,” Dr. Calais commented to this news organization. There’s “huge potential” if it works out, he said, in part because it won’t be limited to one tumor type.
Clovis recently launched a phase 1/2 trial of its candidate, 177Lu-FAP-2286, for advanced/metastatic solid tumors.
In the company’s “luMIERE” trial, subjects will be infused with 68Ga-FAP-2286 to image the tumor. Once uptake is confirmed, they’ll be infused with 177Lu-FAP-2286 for treatment.
The two-step process – uptake confirmation, then treatment – is dubbed “theranostics” and is the standard approach for radiopharmaceutical therapy, Dr. Calais said.
His own team is working to confirm that imaging accurately reflects FAP expression in tumors by comparing preoperative imaging results with FAP expression on surgical specimens. So far, his team has found that they are strongly correlated.
FAPI PET imaging research is much farther along than therapeutic applications, with almost 200 research articles listed on PubMed in 2021, up from just 3 in 2018. One 2019 paper reported “remarkably high uptake and image contrast” across 28 cancers in 80 patients, including breast, esophagus, lung, pancreatic, head-neck, and colorectal tumors.
Imaging studies so far have tended to be small, with many currently focused on identifying the optimal molecule for targeting FAP and the best positron emitter to combine with it.
FAPI tracers are not available yet commercially, so researchers are creating them themselves. One team recently reported it’s recipe for automated synthesis using commercially available synthesis modules.
Sofie, a maker of FDG and other tracers, hopes to change that and is working to bring FAP tracers to market. The company announced in November 2021 a phase 2 study of 68Ga FAPI-46 to image pancreatic ductal adenocarcinoma. It’s the first step in a broader development program for oncologic and nononcologic indications, Sofie said in a press release.
Dr. Calais sees potential for indications where FAPI has already outperformed FDG in the literature, particularly for gastrointestinal cancers. He doesn’t think it will ever replace FDG for indications such as lymphoma, where it “works perfectly well.”
“On the other hand, you have lesions located in a tissue that has some background level” of FDG uptake. “These things are okay with FDG, but I think maybe FAP can help” because of the improved signal-to-noise ratio, Dr. Calais commented. Unlike FDG, “you mostly never see background uptake with FAP-imaging agents,” he said.
Other pluses include quicker distribution throughout the body than FDG, so scan times are shorter, and also patients do not need to fast beforehand.
Dr. Calais predicts that FAPI tracers will reach the market within 5 years.
A version of this article first appeared on Medscape.com.
leading to predictions of a “paradigm shift” in this field.
The new tracer, 68Ga-FAPI (fibroblast activation protein inhibitor), detected more metastases in patients with lung cancer than the standard tracer, 18F-FDG (fluorodeoxyglucose), which has been in use for years.
The study by Chinese researchers was published in Radiology.
The team imaged 34 lung cancer patients with both 68Ga-FAPI and 18F-FDG. Performance was similar for primary tumors and for lung, liver, and adrenal gland metastases. However, FAPI imaging detected more metastases in the lymph nodes (356 vs. 320), brain (23 vs. 10), bone (109 vs. 91), and pleura (66 vs. 35). However, neither modality outperformed MRI for brain metastases, the researchers note.
An accompanying editorial concluded that 68Ga-FAPI PET/CT scanning marks “an important paradigm shift to more specific identification and characterization of a variety of cancers.”
“This may also mark the arrival of a new era in nuclear medicine where molecular imaging helps visualize and characterize the entire tumor burden in one setting,” write editorialists Francine Jacobson, MD, and Annick Van den Abbeele, MD, from Harvard University and Brigham and Women’s Hospital and the Dana Farber Cancer Center, in Boston.
This study was the one of the latest in a fast-growing body of literature reporting that tracers targeting FAP with a small-molecule inhibitor (FAPI) outperform FDG tracers, not just in lung cancer but across a broad range of cancers, including breast, hepatic, gastrointestinal, head-neck, gynecologic, and many other tumor types.
The possibilities aren’t limited to imaging, either. Several companies are planning trials to target FAP with radiopharmaceuticals.
FAP is associated with wound repair and is highly expressed by the fibroblasts tightly packed in with cancer cells, particularly in stroma-dense tumors. FAP is rarely expressed by healthy tissue.
The underlying idea is to deliver a radionuclide to cancer-associated fibroblasts, using either a positron emitter, such as gallium-68 (68Ga), for PET imaging or a beta particle or other short-radiation emitter to kill nearby cancer cells as part of treatment.
Targeting FAP holds the promise of PET imaging that is more selective for cancer than FDG. FDG resolution depends on glucose uptake, which is high in active tumors but is also high in inflamed tissues as well as in the brain, gastrointestinal tract, and other areas. Uptake by background tissue can make it difficult to distinguish tumors from their surroundings. FDG uptake can also be lower in small and indolent tumors.
On the therapy side, there’s hope that FAP targeting will lead to radiopharmaceuticals that work across tumor types, not just in specific cancers.
High interest in FAP
Overall, FAP “is a target of high interest for the whole medical oncology community. The preliminary data are good, but this will take a while” to get to market, said Jeremie Calais, MD, a nuclear medicine specialist and FAP researcher at the University of California, Los Angeles.
Interest in FAP as a radiopharmaceutical target is being driven by the success of two agents that have served as a kind of proof of concept, Dr. Calais said.
The first is Novartis’s 177Lu-PSMA-617, which was granted priority review by the U.S. Food and Drug Administration in September 2021 following phase 3 results that showed a progression-free survival benefit of about 5 months when added to standard of care for metastatic castration-resistant prostate cancer, as well as an overall survival benefit of 4 months.
PSMA-617 binds prostate cancer cells that express prostate-specific membrane antigen. The lutetium-177 (177Lu) bombards them with beta particles and gamma radiation.
FAP researchers are also encouraged by the success of 177Lu dotatate (Lutathera), from Advanced Accelerator Applications, which delivers the radionucleotide to gastroenteropancreatic neuroendocrine tumors that express somatostatin receptors.
The FDA approved this agent in 2018 in part on the basis of phase 3 results that found a 20-month progression-free survival of 65.2% when Lutathera was added to octreotide for metastatic disease vs. 10.8% when it wasn’t.
Novartis is now looking into developing FAP-targeted radiopharmaceuticals, along with Clovis and Point Biopharma, among others.
“That’s the key goal” of industry research, “more so than FAP as a diagnostic tool,” Dr. Calais commented to this news organization. There’s “huge potential” if it works out, he said, in part because it won’t be limited to one tumor type.
Clovis recently launched a phase 1/2 trial of its candidate, 177Lu-FAP-2286, for advanced/metastatic solid tumors.
In the company’s “luMIERE” trial, subjects will be infused with 68Ga-FAP-2286 to image the tumor. Once uptake is confirmed, they’ll be infused with 177Lu-FAP-2286 for treatment.
The two-step process – uptake confirmation, then treatment – is dubbed “theranostics” and is the standard approach for radiopharmaceutical therapy, Dr. Calais said.
His own team is working to confirm that imaging accurately reflects FAP expression in tumors by comparing preoperative imaging results with FAP expression on surgical specimens. So far, his team has found that they are strongly correlated.
FAPI PET imaging research is much farther along than therapeutic applications, with almost 200 research articles listed on PubMed in 2021, up from just 3 in 2018. One 2019 paper reported “remarkably high uptake and image contrast” across 28 cancers in 80 patients, including breast, esophagus, lung, pancreatic, head-neck, and colorectal tumors.
Imaging studies so far have tended to be small, with many currently focused on identifying the optimal molecule for targeting FAP and the best positron emitter to combine with it.
FAPI tracers are not available yet commercially, so researchers are creating them themselves. One team recently reported it’s recipe for automated synthesis using commercially available synthesis modules.
Sofie, a maker of FDG and other tracers, hopes to change that and is working to bring FAP tracers to market. The company announced in November 2021 a phase 2 study of 68Ga FAPI-46 to image pancreatic ductal adenocarcinoma. It’s the first step in a broader development program for oncologic and nononcologic indications, Sofie said in a press release.
Dr. Calais sees potential for indications where FAPI has already outperformed FDG in the literature, particularly for gastrointestinal cancers. He doesn’t think it will ever replace FDG for indications such as lymphoma, where it “works perfectly well.”
“On the other hand, you have lesions located in a tissue that has some background level” of FDG uptake. “These things are okay with FDG, but I think maybe FAP can help” because of the improved signal-to-noise ratio, Dr. Calais commented. Unlike FDG, “you mostly never see background uptake with FAP-imaging agents,” he said.
Other pluses include quicker distribution throughout the body than FDG, so scan times are shorter, and also patients do not need to fast beforehand.
Dr. Calais predicts that FAPI tracers will reach the market within 5 years.
A version of this article first appeared on Medscape.com.
FROM RADIOLOGY