User login
Medications for opioid addiction vastly underutilized
Based on data from the National Survey on Drug Use and Health (NSDUH), investigators found only one in four individuals with OUD receive drug treatment.
In addition, receipt of medication for OUD (MOUD) was lowest among women, the uninsured, non-Hispanic Black or Hispanic people, people with low incomes, and those over age 50. Teens with OUD had the lowest rate of medication use among all demographic groups – zero.
The study is the first to estimate past-year MOUD use in a nationally representative community sample of individuals who may have needed OUD treatment.
“The overdose crisis in the U.S. is continuing unabated, unfortunately, and medication access is an important tool to target and reduce overdose deaths,” lead author Pia Mauro, PhD, assistant professor of epidemiology at Columbia University’s Mailman School of Public Health, New York, told this news organization. “Putting numbers to the distribution of people who are getting medication is important, because it shows that what we’re doing is not enough.”
The study was published online March 23 in JAMA Network Open.
Overdose deaths at an all-time high
U.S. drug overdose deaths are at a record high, with 100,306 American deaths between April 2020 and April 2021, a nearly 30% increase from the previous year. Nearly three-quarters of those deaths involved opioids.
There are currently three Food and Drug Administration–approved medications to treat OUD: methadone, buprenorphine, or naltrexone, all of which are highly effective. Buprenorphine is the only one approved for use in adolescents, and only in those 16 and older.
Before 2019, information about MOUD treatment use was not collected in the NSDUH, an annual survey conducted by the Substance Abuse and Mental Health Services Administration (SAMHSA).
Drawing on responses to the newly added MOUD-use question, Dr. Mauro and colleagues identified participants who may have needed MOUD in the previous year, including those who reported having a heroin or opioid use disorder, receiving medication for their disorder, or undergoing a non-medication OUD treatment, including cognitive behavioral therapy and self-help.
Only 27.8% of those eligible for OUD treatment received MOUD in the past year; 57.0% received no treatment; and 15.3% received non-MOUD services.
Individuals aged 18-25 were most likely to receive MOUD, with just 13.2% of those over age 50 and no one under age 18 receiving medication.
“This study points to extremely low medication use for people who may need it, and given the continued increase in drug-related overdose deaths, the majority of which involve opioids, the necessity to increase access to medication is more important than ever,” Dr. Mauro said.
MOUD use was significantly lower in non-Hispanic Black people, (adjusted relative risk ratio, 0.82; 95% confidence interval, 0.27-2.46), Hispanic people (aRRR, 0.57; 95% CI, 0.14-2.28), and in Asian, Native American or Alaska Native, Native Hawaiian, Pacific Islander, or multiracial people (aRRR, 0.28; 95% CI, 0.08-0.92) compared to White people.
Medication use was less likely in women than men (aRRR, 0.52; 95% CI, 0.29-0.95) and more likely in people who had both prescription opioid and heroin use disorder, compared with those who misused just one of the substances (aRRR, 5.07; 95% CI, 1.50-17.12).
MOUD was more common among people with public insurance than those with private or no insurance, but the overall use remained very low regardless of insurance status.
“Public insurance has consistently been positively associated with MOUD access, so our study builds on this showing the importance of public insurance to increase medication access,” Dr. Mauro said. “But even among those with public insurance, only 35% got medication. That’s only one in three.”
About 85% of participants who may have needed treatment for OUD had at least one contact with a health care provider in the past year, and more than half had contact with the criminal legal system. Only about one-third of these individuals received MOUD, which Dr. Mauro lamented as a lost opportunity for treatment.
Persistent barriers to treatment
The findings highlight persistent barriers to medication-based therapy for OUD, said Alan Leshner, PhD, chief executive officer emeritus of the American Association for the Advancement of Science and a former director of the National Institute on Drug Abuse.
“These kinds of data are critical to increase our understanding of the nature of the opioid epidemic and what to do about it,” Dr. Leshner said. “It’s particularly important to understand who does, and doesn’t, have access to lifesaving medications, but also where to focus efforts at working on the problem.”
In 2019, Dr. Leshner coauthored a report on the underutilization of medication to treat OUD. As previously reported by this news organization, that report argued that stigma, burdensome regulations, unfounded concerns about diversion of MOUDs, lack of insurance coverage, and inadequate professional training for health care providers, law enforcement, and criminal justice officials all acted as barriers that separate people with a medical disorder from desperately needed – and effective – treatment.
“The barriers are the same and have not been vigorously addressed,” Dr. Leshner said. However, recent moves by government leaders may signal a positive trend toward expanded treatment, he added.
Earlier this month, Dr. Leshner chaired a workshop on ways to improve access to methadone, one of the approved medications to treat OUD. Officials from SAMHSA, the Drug Enforcement Administration, and the FDA participated, as did Rahul Gupta, MD, director of the Office of National Drug Control Policy and the nation’s top drug policy official.
“I am optimistic that there may be a new commitment to working on this epidemic using a health-centered approach that takes into account the array of social issues that surround the problem, as well as the criminal justice issues,” Dr. Leshner said.
The study was funded by the National Institute on Drug Abuse grant. Dr. Mauro and Dr. Leshner reported no conflicts. Full disclosures are reported in the original article.
A version of this article first appeared on Medscape.com.
Based on data from the National Survey on Drug Use and Health (NSDUH), investigators found only one in four individuals with OUD receive drug treatment.
In addition, receipt of medication for OUD (MOUD) was lowest among women, the uninsured, non-Hispanic Black or Hispanic people, people with low incomes, and those over age 50. Teens with OUD had the lowest rate of medication use among all demographic groups – zero.
The study is the first to estimate past-year MOUD use in a nationally representative community sample of individuals who may have needed OUD treatment.
“The overdose crisis in the U.S. is continuing unabated, unfortunately, and medication access is an important tool to target and reduce overdose deaths,” lead author Pia Mauro, PhD, assistant professor of epidemiology at Columbia University’s Mailman School of Public Health, New York, told this news organization. “Putting numbers to the distribution of people who are getting medication is important, because it shows that what we’re doing is not enough.”
The study was published online March 23 in JAMA Network Open.
Overdose deaths at an all-time high
U.S. drug overdose deaths are at a record high, with 100,306 American deaths between April 2020 and April 2021, a nearly 30% increase from the previous year. Nearly three-quarters of those deaths involved opioids.
There are currently three Food and Drug Administration–approved medications to treat OUD: methadone, buprenorphine, or naltrexone, all of which are highly effective. Buprenorphine is the only one approved for use in adolescents, and only in those 16 and older.
Before 2019, information about MOUD treatment use was not collected in the NSDUH, an annual survey conducted by the Substance Abuse and Mental Health Services Administration (SAMHSA).
Drawing on responses to the newly added MOUD-use question, Dr. Mauro and colleagues identified participants who may have needed MOUD in the previous year, including those who reported having a heroin or opioid use disorder, receiving medication for their disorder, or undergoing a non-medication OUD treatment, including cognitive behavioral therapy and self-help.
Only 27.8% of those eligible for OUD treatment received MOUD in the past year; 57.0% received no treatment; and 15.3% received non-MOUD services.
Individuals aged 18-25 were most likely to receive MOUD, with just 13.2% of those over age 50 and no one under age 18 receiving medication.
“This study points to extremely low medication use for people who may need it, and given the continued increase in drug-related overdose deaths, the majority of which involve opioids, the necessity to increase access to medication is more important than ever,” Dr. Mauro said.
MOUD use was significantly lower in non-Hispanic Black people, (adjusted relative risk ratio, 0.82; 95% confidence interval, 0.27-2.46), Hispanic people (aRRR, 0.57; 95% CI, 0.14-2.28), and in Asian, Native American or Alaska Native, Native Hawaiian, Pacific Islander, or multiracial people (aRRR, 0.28; 95% CI, 0.08-0.92) compared to White people.
Medication use was less likely in women than men (aRRR, 0.52; 95% CI, 0.29-0.95) and more likely in people who had both prescription opioid and heroin use disorder, compared with those who misused just one of the substances (aRRR, 5.07; 95% CI, 1.50-17.12).
MOUD was more common among people with public insurance than those with private or no insurance, but the overall use remained very low regardless of insurance status.
“Public insurance has consistently been positively associated with MOUD access, so our study builds on this showing the importance of public insurance to increase medication access,” Dr. Mauro said. “But even among those with public insurance, only 35% got medication. That’s only one in three.”
About 85% of participants who may have needed treatment for OUD had at least one contact with a health care provider in the past year, and more than half had contact with the criminal legal system. Only about one-third of these individuals received MOUD, which Dr. Mauro lamented as a lost opportunity for treatment.
Persistent barriers to treatment
The findings highlight persistent barriers to medication-based therapy for OUD, said Alan Leshner, PhD, chief executive officer emeritus of the American Association for the Advancement of Science and a former director of the National Institute on Drug Abuse.
“These kinds of data are critical to increase our understanding of the nature of the opioid epidemic and what to do about it,” Dr. Leshner said. “It’s particularly important to understand who does, and doesn’t, have access to lifesaving medications, but also where to focus efforts at working on the problem.”
In 2019, Dr. Leshner coauthored a report on the underutilization of medication to treat OUD. As previously reported by this news organization, that report argued that stigma, burdensome regulations, unfounded concerns about diversion of MOUDs, lack of insurance coverage, and inadequate professional training for health care providers, law enforcement, and criminal justice officials all acted as barriers that separate people with a medical disorder from desperately needed – and effective – treatment.
“The barriers are the same and have not been vigorously addressed,” Dr. Leshner said. However, recent moves by government leaders may signal a positive trend toward expanded treatment, he added.
Earlier this month, Dr. Leshner chaired a workshop on ways to improve access to methadone, one of the approved medications to treat OUD. Officials from SAMHSA, the Drug Enforcement Administration, and the FDA participated, as did Rahul Gupta, MD, director of the Office of National Drug Control Policy and the nation’s top drug policy official.
“I am optimistic that there may be a new commitment to working on this epidemic using a health-centered approach that takes into account the array of social issues that surround the problem, as well as the criminal justice issues,” Dr. Leshner said.
The study was funded by the National Institute on Drug Abuse grant. Dr. Mauro and Dr. Leshner reported no conflicts. Full disclosures are reported in the original article.
A version of this article first appeared on Medscape.com.
Based on data from the National Survey on Drug Use and Health (NSDUH), investigators found only one in four individuals with OUD receive drug treatment.
In addition, receipt of medication for OUD (MOUD) was lowest among women, the uninsured, non-Hispanic Black or Hispanic people, people with low incomes, and those over age 50. Teens with OUD had the lowest rate of medication use among all demographic groups – zero.
The study is the first to estimate past-year MOUD use in a nationally representative community sample of individuals who may have needed OUD treatment.
“The overdose crisis in the U.S. is continuing unabated, unfortunately, and medication access is an important tool to target and reduce overdose deaths,” lead author Pia Mauro, PhD, assistant professor of epidemiology at Columbia University’s Mailman School of Public Health, New York, told this news organization. “Putting numbers to the distribution of people who are getting medication is important, because it shows that what we’re doing is not enough.”
The study was published online March 23 in JAMA Network Open.
Overdose deaths at an all-time high
U.S. drug overdose deaths are at a record high, with 100,306 American deaths between April 2020 and April 2021, a nearly 30% increase from the previous year. Nearly three-quarters of those deaths involved opioids.
There are currently three Food and Drug Administration–approved medications to treat OUD: methadone, buprenorphine, or naltrexone, all of which are highly effective. Buprenorphine is the only one approved for use in adolescents, and only in those 16 and older.
Before 2019, information about MOUD treatment use was not collected in the NSDUH, an annual survey conducted by the Substance Abuse and Mental Health Services Administration (SAMHSA).
Drawing on responses to the newly added MOUD-use question, Dr. Mauro and colleagues identified participants who may have needed MOUD in the previous year, including those who reported having a heroin or opioid use disorder, receiving medication for their disorder, or undergoing a non-medication OUD treatment, including cognitive behavioral therapy and self-help.
Only 27.8% of those eligible for OUD treatment received MOUD in the past year; 57.0% received no treatment; and 15.3% received non-MOUD services.
Individuals aged 18-25 were most likely to receive MOUD, with just 13.2% of those over age 50 and no one under age 18 receiving medication.
“This study points to extremely low medication use for people who may need it, and given the continued increase in drug-related overdose deaths, the majority of which involve opioids, the necessity to increase access to medication is more important than ever,” Dr. Mauro said.
MOUD use was significantly lower in non-Hispanic Black people, (adjusted relative risk ratio, 0.82; 95% confidence interval, 0.27-2.46), Hispanic people (aRRR, 0.57; 95% CI, 0.14-2.28), and in Asian, Native American or Alaska Native, Native Hawaiian, Pacific Islander, or multiracial people (aRRR, 0.28; 95% CI, 0.08-0.92) compared to White people.
Medication use was less likely in women than men (aRRR, 0.52; 95% CI, 0.29-0.95) and more likely in people who had both prescription opioid and heroin use disorder, compared with those who misused just one of the substances (aRRR, 5.07; 95% CI, 1.50-17.12).
MOUD was more common among people with public insurance than those with private or no insurance, but the overall use remained very low regardless of insurance status.
“Public insurance has consistently been positively associated with MOUD access, so our study builds on this showing the importance of public insurance to increase medication access,” Dr. Mauro said. “But even among those with public insurance, only 35% got medication. That’s only one in three.”
About 85% of participants who may have needed treatment for OUD had at least one contact with a health care provider in the past year, and more than half had contact with the criminal legal system. Only about one-third of these individuals received MOUD, which Dr. Mauro lamented as a lost opportunity for treatment.
Persistent barriers to treatment
The findings highlight persistent barriers to medication-based therapy for OUD, said Alan Leshner, PhD, chief executive officer emeritus of the American Association for the Advancement of Science and a former director of the National Institute on Drug Abuse.
“These kinds of data are critical to increase our understanding of the nature of the opioid epidemic and what to do about it,” Dr. Leshner said. “It’s particularly important to understand who does, and doesn’t, have access to lifesaving medications, but also where to focus efforts at working on the problem.”
In 2019, Dr. Leshner coauthored a report on the underutilization of medication to treat OUD. As previously reported by this news organization, that report argued that stigma, burdensome regulations, unfounded concerns about diversion of MOUDs, lack of insurance coverage, and inadequate professional training for health care providers, law enforcement, and criminal justice officials all acted as barriers that separate people with a medical disorder from desperately needed – and effective – treatment.
“The barriers are the same and have not been vigorously addressed,” Dr. Leshner said. However, recent moves by government leaders may signal a positive trend toward expanded treatment, he added.
Earlier this month, Dr. Leshner chaired a workshop on ways to improve access to methadone, one of the approved medications to treat OUD. Officials from SAMHSA, the Drug Enforcement Administration, and the FDA participated, as did Rahul Gupta, MD, director of the Office of National Drug Control Policy and the nation’s top drug policy official.
“I am optimistic that there may be a new commitment to working on this epidemic using a health-centered approach that takes into account the array of social issues that surround the problem, as well as the criminal justice issues,” Dr. Leshner said.
The study was funded by the National Institute on Drug Abuse grant. Dr. Mauro and Dr. Leshner reported no conflicts. Full disclosures are reported in the original article.
A version of this article first appeared on Medscape.com.
Calcium scores predict sudden-death risk in preclinical CAD
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
The risk for sudden cardiac death (SCD) climbs steadily in tandem with coronary artery calcium (CAC) burden, independent of more conventional risk factors, in primary-prevention patients considered low- to intermediate-risk, researchers say.
The findings, based on a large cohort study, strengthen the case for initial CAC imaging as a gatekeeper to further testing in such patients who have mostly subclinical atherosclerotic cardiovascular disease (ASCVD), they conclude.
The CAC scan is “evolving into a primary-prevention screening test, not only for initiating statin therapy, but now as a screening modality for risk stratifying someone for sudden cardiac arrest,” Alexander C. Razavi, MD, MPH, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“Our data reinforce this and give some quantitative measures of when we should start to consider that.”
A CAC score of 100 to 399 in this “primarily asymptomatic,” predominantly White and male cohort elevated the risk for SCD over an average of 10.6 years by a factor of 2.8, compared with a score of 0. The risk went up four times with CAC scores of 400-999, and almost five times with scores above 1,000.
The risk association was independent of age and sex but also diabetes, smoking, hypertension, dyslipidemia, and family history of heart disease.
That and other findings, Dr. Razavi said, suggest CAC scores in low- to intermediate-risk patients like those studied may sharpen SCD risk-stratification beyond what is possible using traditional risk factors.
Dr. Razavi is lead author on the study’s March 21 publication in JACC Cardiovascular Imaging, and is slated to present the results April 2 during the American College of Cardiology (ACC) 2022 Scientific Session, to be held virtually and in-person in Washington, D.C.
The study’s 66,636 primary-prevention patients, part of the Coronary Artery Calcium Consortium observational cohort, were without known coronary disease at enrollment, from 1991-2010, at four major American centers. They had been referred to CAC imaging because of the presence of at least one ASCVD risk factor, such as dyslipidemia, family history of premature heart disease, hypertension, or diabetes, the researchers note.
They observed 211 SCD events, for a rate of about 0.3%, over a median of 10.6 years. The adjusted stepwise higher risk (SHR) for an SCD event went up continuously with CAC scores (P for trend < .001). The SHR values, compared with a CAC score of 0, were:
- 1.3 (95% CI, 0.7-2.4) for a CAC score score of 1 to 99
- 2.8 (95% CI, 1.6-5.0) for a CAC score of 100 to 399
- 4.0 (95% CI, 2.2-7.3) for a CAC score of 400 to 999
- 4.9 (95% CI, 2.6-9.9) for a CAC score above 1,000
The magnitude of the CAC score’s association with SCD risk in the study was “surprising,” Dr. Razavi said. The CAC score, starting at about 100, seems “more strongly associated with a sudden cardiac arrest” than more familiar SCD risk predictors, such as prolonged heart-rate-corrected QT interval or QRS duration.
Dr. Razavi reported no conflicts. Disclosures for the other authors are in the report.
A version of this article first appeared on Medscape.com.
Cancer Data Trends 2022: Cancer in Women
Zullig LL, Goldstein KM, Sims KJ, et al. Cancer among women treated in the Veterans Affairs Healthcare System. J Womens Health (Larchmt). 2019;28(2):268-275. doi:10.1089/jwh.2018.6936
Kondo K, Low A, Everson T, et al. Prevalence of and interventions to reduce health disparities in vulnerable veteran populations: a map of the evidence. VA Evidence-based Synthesis Program (ESP) project #05-225. Published May 2017. Accessed December 22, 2021. https://www.hsrd.research.va.gov/publications/esp/DisparitiesInterventions-REPORT.pdf
Aggarwal A, Liu ML, Krasnow SH. Breast cancer in male veteran population: an analysis from VA cancer registry. J Community Support Oncol. 2014;12(8):293-297. doi:10.12788/jcso.0066
Department of Defense Breast Cancer Research Program. The breast cancer landscape. Published October 2020. Accessed December 23, 2021. https://cdmrp.army.mil/bcrp/pdfs/Breast%20Cancer%20Landscape2020.pdf
Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
Kennedy K. “The enemy is lurking in our bodies”— Women veterans say toxic exposure caused breast cancer. The War Horse. Published October 14, 2021. Accessed December 29, 2021. https://thewarhorse.org/military-women-face-higher-breast-cancer-rates-from-exposure
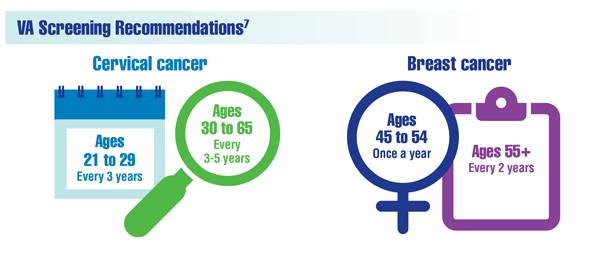
US Department of Veteran Affairs, National Center for Health Promotion and Disease Prevention. Get recommended screening tests and immunizations for women. Updated November 24, 2021. Accessed December 29, 2021. https://www.prevention.va.gov/Healthy_Living/Get_Recommended_Screening_Tests_and_Immunizations_for_Women.asp
Zullig LL, Goldstein KM, Sims KJ, et al. Cancer among women treated in the Veterans Affairs Healthcare System. J Womens Health (Larchmt). 2019;28(2):268-275. doi:10.1089/jwh.2018.6936
Kondo K, Low A, Everson T, et al. Prevalence of and interventions to reduce health disparities in vulnerable veteran populations: a map of the evidence. VA Evidence-based Synthesis Program (ESP) project #05-225. Published May 2017. Accessed December 22, 2021. https://www.hsrd.research.va.gov/publications/esp/DisparitiesInterventions-REPORT.pdf
Aggarwal A, Liu ML, Krasnow SH. Breast cancer in male veteran population: an analysis from VA cancer registry. J Community Support Oncol. 2014;12(8):293-297. doi:10.12788/jcso.0066
Department of Defense Breast Cancer Research Program. The breast cancer landscape. Published October 2020. Accessed December 23, 2021. https://cdmrp.army.mil/bcrp/pdfs/Breast%20Cancer%20Landscape2020.pdf
Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
Kennedy K. “The enemy is lurking in our bodies”— Women veterans say toxic exposure caused breast cancer. The War Horse. Published October 14, 2021. Accessed December 29, 2021. https://thewarhorse.org/military-women-face-higher-breast-cancer-rates-from-exposure
US Department of Veteran Affairs, National Center for Health Promotion and Disease Prevention. Get recommended screening tests and immunizations for women. Updated November 24, 2021. Accessed December 29, 2021. https://www.prevention.va.gov/Healthy_Living/Get_Recommended_Screening_Tests_and_Immunizations_for_Women.asp
Zullig LL, Goldstein KM, Sims KJ, et al. Cancer among women treated in the Veterans Affairs Healthcare System. J Womens Health (Larchmt). 2019;28(2):268-275. doi:10.1089/jwh.2018.6936
Kondo K, Low A, Everson T, et al. Prevalence of and interventions to reduce health disparities in vulnerable veteran populations: a map of the evidence. VA Evidence-based Synthesis Program (ESP) project #05-225. Published May 2017. Accessed December 22, 2021. https://www.hsrd.research.va.gov/publications/esp/DisparitiesInterventions-REPORT.pdf
Aggarwal A, Liu ML, Krasnow SH. Breast cancer in male veteran population: an analysis from VA cancer registry. J Community Support Oncol. 2014;12(8):293-297. doi:10.12788/jcso.0066
Department of Defense Breast Cancer Research Program. The breast cancer landscape. Published October 2020. Accessed December 23, 2021. https://cdmrp.army.mil/bcrp/pdfs/Breast%20Cancer%20Landscape2020.pdf
Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
Kennedy K. “The enemy is lurking in our bodies”— Women veterans say toxic exposure caused breast cancer. The War Horse. Published October 14, 2021. Accessed December 29, 2021. https://thewarhorse.org/military-women-face-higher-breast-cancer-rates-from-exposure
US Department of Veteran Affairs, National Center for Health Promotion and Disease Prevention. Get recommended screening tests and immunizations for women. Updated November 24, 2021. Accessed December 29, 2021. https://www.prevention.va.gov/Healthy_Living/Get_Recommended_Screening_Tests_and_Immunizations_for_Women.asp
You’re not on a ‘best doctor’ list – does it matter?
Thousands of doctors get a shout out every year when they make the “Top Doctor” lists in various magazines. Some may be your colleagues or competitors. Should you be concerned if you’re not on the list?
Best Doctor lists are clearly popular with readers and make money for the magazines. They can also bring in patient revenue for doctors and their employers who promote them in news releases and on their websites.
For doctors on some of the top lists, the recognition can bring not only patients, but national or international visibility.
While the dollar value is hard to come by, some doctors say that these lists have attracted new patients to their practice.
Sarah St. Louis, MD, a physician manager of Associates in Urogynecology, is one of Orlando Style magazine’s Doctors of the Year and Orlando Family Magazine’s Top Doctors.
Several new patients have told her that they read about her in the magazines’ Top Doctor lists. “Urogynecology is not a well-known specialty – it’s a helpful way to get the word out about the women’s health specialty and what I do,” said Dr. St. Louis, an early career physician who started her practice in 2017.
The additional patient revenue has been worth the cost of displaying her profile in Orlando Style, which was about $800 for a half-page spread with her photo.
Top Doctor lists also work well for specialty practices whose patients can self-refer, such as plastic surgery, dermatology, orthopedics, gastroenterology, and geriatric medicine, said Andrea Eliscu, RN, founder and president of Medical Marketing in Orlando.
Being in a competitive market also matters. If a practice is the only one in town, those doctors may not need the publicity as much as doctors in an urban practice that faces stiff competition.
How do doctors get on these lists?
In most cases, doctors have to be nominated by their peers, a process that some say is flawed because it may shut out doctors who are less popular or well-connected.
Forty-eight regional magazines, including Chicago magazine and Philadelphia Magazine , partner with Castle Connolly to use their online Top Doctor database of more than 61,000 physicians in every major metropolitan area, said Steve Leibforth, managing director of Castle Connolly’s Top Doctors.
The company says it sends annual surveys to tens of thousands of practicing doctors asking them to nominate colleagues in their specialty. The nominated doctors are vetted by Castle Connolly’s physician-led research team on several criteria including professional qualifications, education, hospital and faculty appointments, research leadership, professional reputation and disciplinary history, and outcomes data when available, said Mr. Leibforth.
Washingtonian magazine says it sends annual online surveys to 13,500 physicians in the DC metro area asking them to nominate one colleague in their specialty. The top vote-getters in each of 39 categories are designated Top Doctors.
Orlando Family Magazine says its annual Top Doctor selections are based on reader polls and doctor nominations.
Consumers’ Research Council of America uses a point system based on each year the doctor has been in practice, education and continuing education, board certification, and membership in professional medical societies.
Doctors have many ways to promote that they’re listed as a “top” doctor. Dr. St. Louis takes advantage of the magazine’s free reprints, which she puts in her waiting room.
Others buy plaques to hang up in their waiting rooms or offices and announce the distinction on their websites, blogs, or social media. “They have to maximize the magazine distinction or it’s worthless,” said Ms. Eliscu.
Employers also like to spread the word when their doctors make it on “Top Doctor” lists.
“With Emory physicians making up nearly 50 percent of the list, that’s more than any other health system in Atlanta,” said an Emory University press release after nearly half of the university’s doctors made the Top Doctors list in Atlanta magazine.
Patients may be impressed: What about your peers?
Dr. St. Louis said that making some of these lists is less impressive than having a peer-reviewed journal article or receiving professional awards.
“Just because a physician is listed in a magazine as a ‘top doctor’ does not mean they are the best. There are far more medical, clinical, and scientific points to consider than just a pretty picture in a style magazine,” she said.
Wanda Filer, MD, MBA, who practiced family medicine until last year when she became chief medical officer for VaxCare in Orlando, said she ignores the many congratulatory letters in the mail announcing that she’s made one list or another.
“I don’t put much credence in the lists. I get notifications fairly often, and to me it always looks like they’re trying to sell a plaque. I’d rather let my work speak for itself.”
Arlen Meyers, MD, MBA, president and CEO of the Society of Physician Entrepreneurs and a paid strategic adviser to RYTE, a data-driven site for “best doctors” and “best hospitals,” said he received several of these “top doctor” awards when he was a professor of otolaryngology at the University of Colorado.
He has been critical of these awards for some time. “These doctor beauty pageants may be good for business but have little value for patients.”
He would like to see a new approach that is driven by data and what patients value. “If I have a lump in my thyroid, I want to know the best doctor to treat me based on outcomes data.”
He said a good rating system would include a data-driven approach based on treatment outcomes, publicly available data, price transparency, and patient values.
Whether a physician feels honored to be named a top physician or sees little value in it, most doctors are aware of the list’s marketing value for their practices and many choose to make use of it.
A version of this article first appeared on Medscape.com.
Thousands of doctors get a shout out every year when they make the “Top Doctor” lists in various magazines. Some may be your colleagues or competitors. Should you be concerned if you’re not on the list?
Best Doctor lists are clearly popular with readers and make money for the magazines. They can also bring in patient revenue for doctors and their employers who promote them in news releases and on their websites.
For doctors on some of the top lists, the recognition can bring not only patients, but national or international visibility.
While the dollar value is hard to come by, some doctors say that these lists have attracted new patients to their practice.
Sarah St. Louis, MD, a physician manager of Associates in Urogynecology, is one of Orlando Style magazine’s Doctors of the Year and Orlando Family Magazine’s Top Doctors.
Several new patients have told her that they read about her in the magazines’ Top Doctor lists. “Urogynecology is not a well-known specialty – it’s a helpful way to get the word out about the women’s health specialty and what I do,” said Dr. St. Louis, an early career physician who started her practice in 2017.
The additional patient revenue has been worth the cost of displaying her profile in Orlando Style, which was about $800 for a half-page spread with her photo.
Top Doctor lists also work well for specialty practices whose patients can self-refer, such as plastic surgery, dermatology, orthopedics, gastroenterology, and geriatric medicine, said Andrea Eliscu, RN, founder and president of Medical Marketing in Orlando.
Being in a competitive market also matters. If a practice is the only one in town, those doctors may not need the publicity as much as doctors in an urban practice that faces stiff competition.
How do doctors get on these lists?
In most cases, doctors have to be nominated by their peers, a process that some say is flawed because it may shut out doctors who are less popular or well-connected.
Forty-eight regional magazines, including Chicago magazine and Philadelphia Magazine , partner with Castle Connolly to use their online Top Doctor database of more than 61,000 physicians in every major metropolitan area, said Steve Leibforth, managing director of Castle Connolly’s Top Doctors.
The company says it sends annual surveys to tens of thousands of practicing doctors asking them to nominate colleagues in their specialty. The nominated doctors are vetted by Castle Connolly’s physician-led research team on several criteria including professional qualifications, education, hospital and faculty appointments, research leadership, professional reputation and disciplinary history, and outcomes data when available, said Mr. Leibforth.
Washingtonian magazine says it sends annual online surveys to 13,500 physicians in the DC metro area asking them to nominate one colleague in their specialty. The top vote-getters in each of 39 categories are designated Top Doctors.
Orlando Family Magazine says its annual Top Doctor selections are based on reader polls and doctor nominations.
Consumers’ Research Council of America uses a point system based on each year the doctor has been in practice, education and continuing education, board certification, and membership in professional medical societies.
Doctors have many ways to promote that they’re listed as a “top” doctor. Dr. St. Louis takes advantage of the magazine’s free reprints, which she puts in her waiting room.
Others buy plaques to hang up in their waiting rooms or offices and announce the distinction on their websites, blogs, or social media. “They have to maximize the magazine distinction or it’s worthless,” said Ms. Eliscu.
Employers also like to spread the word when their doctors make it on “Top Doctor” lists.
“With Emory physicians making up nearly 50 percent of the list, that’s more than any other health system in Atlanta,” said an Emory University press release after nearly half of the university’s doctors made the Top Doctors list in Atlanta magazine.
Patients may be impressed: What about your peers?
Dr. St. Louis said that making some of these lists is less impressive than having a peer-reviewed journal article or receiving professional awards.
“Just because a physician is listed in a magazine as a ‘top doctor’ does not mean they are the best. There are far more medical, clinical, and scientific points to consider than just a pretty picture in a style magazine,” she said.
Wanda Filer, MD, MBA, who practiced family medicine until last year when she became chief medical officer for VaxCare in Orlando, said she ignores the many congratulatory letters in the mail announcing that she’s made one list or another.
“I don’t put much credence in the lists. I get notifications fairly often, and to me it always looks like they’re trying to sell a plaque. I’d rather let my work speak for itself.”
Arlen Meyers, MD, MBA, president and CEO of the Society of Physician Entrepreneurs and a paid strategic adviser to RYTE, a data-driven site for “best doctors” and “best hospitals,” said he received several of these “top doctor” awards when he was a professor of otolaryngology at the University of Colorado.
He has been critical of these awards for some time. “These doctor beauty pageants may be good for business but have little value for patients.”
He would like to see a new approach that is driven by data and what patients value. “If I have a lump in my thyroid, I want to know the best doctor to treat me based on outcomes data.”
He said a good rating system would include a data-driven approach based on treatment outcomes, publicly available data, price transparency, and patient values.
Whether a physician feels honored to be named a top physician or sees little value in it, most doctors are aware of the list’s marketing value for their practices and many choose to make use of it.
A version of this article first appeared on Medscape.com.
Thousands of doctors get a shout out every year when they make the “Top Doctor” lists in various magazines. Some may be your colleagues or competitors. Should you be concerned if you’re not on the list?
Best Doctor lists are clearly popular with readers and make money for the magazines. They can also bring in patient revenue for doctors and their employers who promote them in news releases and on their websites.
For doctors on some of the top lists, the recognition can bring not only patients, but national or international visibility.
While the dollar value is hard to come by, some doctors say that these lists have attracted new patients to their practice.
Sarah St. Louis, MD, a physician manager of Associates in Urogynecology, is one of Orlando Style magazine’s Doctors of the Year and Orlando Family Magazine’s Top Doctors.
Several new patients have told her that they read about her in the magazines’ Top Doctor lists. “Urogynecology is not a well-known specialty – it’s a helpful way to get the word out about the women’s health specialty and what I do,” said Dr. St. Louis, an early career physician who started her practice in 2017.
The additional patient revenue has been worth the cost of displaying her profile in Orlando Style, which was about $800 for a half-page spread with her photo.
Top Doctor lists also work well for specialty practices whose patients can self-refer, such as plastic surgery, dermatology, orthopedics, gastroenterology, and geriatric medicine, said Andrea Eliscu, RN, founder and president of Medical Marketing in Orlando.
Being in a competitive market also matters. If a practice is the only one in town, those doctors may not need the publicity as much as doctors in an urban practice that faces stiff competition.
How do doctors get on these lists?
In most cases, doctors have to be nominated by their peers, a process that some say is flawed because it may shut out doctors who are less popular or well-connected.
Forty-eight regional magazines, including Chicago magazine and Philadelphia Magazine , partner with Castle Connolly to use their online Top Doctor database of more than 61,000 physicians in every major metropolitan area, said Steve Leibforth, managing director of Castle Connolly’s Top Doctors.
The company says it sends annual surveys to tens of thousands of practicing doctors asking them to nominate colleagues in their specialty. The nominated doctors are vetted by Castle Connolly’s physician-led research team on several criteria including professional qualifications, education, hospital and faculty appointments, research leadership, professional reputation and disciplinary history, and outcomes data when available, said Mr. Leibforth.
Washingtonian magazine says it sends annual online surveys to 13,500 physicians in the DC metro area asking them to nominate one colleague in their specialty. The top vote-getters in each of 39 categories are designated Top Doctors.
Orlando Family Magazine says its annual Top Doctor selections are based on reader polls and doctor nominations.
Consumers’ Research Council of America uses a point system based on each year the doctor has been in practice, education and continuing education, board certification, and membership in professional medical societies.
Doctors have many ways to promote that they’re listed as a “top” doctor. Dr. St. Louis takes advantage of the magazine’s free reprints, which she puts in her waiting room.
Others buy plaques to hang up in their waiting rooms or offices and announce the distinction on their websites, blogs, or social media. “They have to maximize the magazine distinction or it’s worthless,” said Ms. Eliscu.
Employers also like to spread the word when their doctors make it on “Top Doctor” lists.
“With Emory physicians making up nearly 50 percent of the list, that’s more than any other health system in Atlanta,” said an Emory University press release after nearly half of the university’s doctors made the Top Doctors list in Atlanta magazine.
Patients may be impressed: What about your peers?
Dr. St. Louis said that making some of these lists is less impressive than having a peer-reviewed journal article or receiving professional awards.
“Just because a physician is listed in a magazine as a ‘top doctor’ does not mean they are the best. There are far more medical, clinical, and scientific points to consider than just a pretty picture in a style magazine,” she said.
Wanda Filer, MD, MBA, who practiced family medicine until last year when she became chief medical officer for VaxCare in Orlando, said she ignores the many congratulatory letters in the mail announcing that she’s made one list or another.
“I don’t put much credence in the lists. I get notifications fairly often, and to me it always looks like they’re trying to sell a plaque. I’d rather let my work speak for itself.”
Arlen Meyers, MD, MBA, president and CEO of the Society of Physician Entrepreneurs and a paid strategic adviser to RYTE, a data-driven site for “best doctors” and “best hospitals,” said he received several of these “top doctor” awards when he was a professor of otolaryngology at the University of Colorado.
He has been critical of these awards for some time. “These doctor beauty pageants may be good for business but have little value for patients.”
He would like to see a new approach that is driven by data and what patients value. “If I have a lump in my thyroid, I want to know the best doctor to treat me based on outcomes data.”
He said a good rating system would include a data-driven approach based on treatment outcomes, publicly available data, price transparency, and patient values.
Whether a physician feels honored to be named a top physician or sees little value in it, most doctors are aware of the list’s marketing value for their practices and many choose to make use of it.
A version of this article first appeared on Medscape.com.
Black men at higher risk for mortality from sleep apnea
There has been a flattening of sleep apnea–related mortality rates in the United States over the past 10 years. The exception is among Black men, for whom mortality from sleep apnea has continuously increased over the past 21 years, new research shows.
“OSA (obstructive sleep apnea) has been recognized as an important cause of medical morbidity and mortality and contributes to the development of systemic hypertension, cardiovascular disease, and abnormalities in glucose metabolism,” noted Yu-Che Lee, MD, University at Buffalo–Catholic Health System, Buffalo, N.Y., and colleagues.
“This study provides the first systematic assessment and demonstrates remarkable demographic disparities of age-adjusted sleep apnea–related mortality in the U.S., with higher rates in males than females and Blacks than Whites,” they concluded.
The study was published online in Sleep Medicine.
Twenty-one year interval
Data on sleep apnea–related mortality were obtained from the National Center for Health Statistics and were provided by the Centers for Disease Control and Prevention for the years 1999-2019. Over that 21-year interval, sleep apnea was documented as the underlying cause of death in 17,053 decedents, including 2,593 Black patients and 14,127 White patients.
The age-adjusted mortality rate attributed to sleep apnea was 2.5 per 1,000,000 population. The mortality rate was higher for men, at 3.1 per 1,000,000, than among women, 1.9 per 1,000,000 (P < .001). For both sexes, “unadjusted mortality rates were higher in groups aged ≥ 35 years, and the highest mortality rates were observed in groups aged 75-84,” the authors noted. The rate was 11.3 per 1,000,000 for those aged 75-84 and 13.3 per 1,000,000 for those older than 85.
This was also true among Black and White patients, the authors added, although the age-adjusted mortality rate was higher among Black patients than among other racial groups, at 3.5 per 1,000,000 (P < .001). “Over the 21-year study period, the overall age-adjusted mortality rate rose from 1.2 per 1,000,000 population in 1999 to 2.8 per 1,000,000 in 2019,” Dr. Lee and colleagues noted. While the annual percentage change in sleep apnea–related mortality rose by 10.2% (95% confidence interval [CI], 8.4%-12.0%) between 1999 and 2018, no significant change was observed between 2008 and 2019.
On the other hand, when examined by race and sex, age-adjusted mortality rates increased significantly by an annual percentage change of 7.5% (95% CI, 3.3%-11.9%) among Black women and by 8.2% (95% CI, 6.8%-9.6%) between 1999 and 2009 in White men and by 11.5% (95% CI, 8.9%-14.1%) in White women. “Again, these uptrends were no longer observed after that time interval,” the authors stressed.
Only among Black men was there no turning point in age-adjusted mortality rates; they experienced a steady, significant, 2.7% (95% CI, 1.2%-4.2%) annual percent increase in age-adjusted mortality rate between 1999 and 2019. The highest age-adjusted mortality rate for Black persons was recorded in Indiana, at 6.5 per 1,000,000 population; Utah recorded the highest mortality rate for White persons, at 5.7 per 1,000,000.
For both Black persons and White persons, the lowest mortality rates were in New York, at 1.2 per 1,000,000 and 1.5 per 1,000,000, respectively. Among four geographic regions analyzed, the highest age-adjusted mortality rates were in the Midwest for both sexes; Black men in the West and those in three other regional groups in the Northwest had the lowest mortality rates.
Multiple causes of death
Black women were more likely to have multiple causes of death, including cardiac arrest, heart failure, and hypertension. White women were more likely to die of arrhythmia, respiratory failure, pneumonia, and depression. Black men were also more likely to die of cardiac arrest, hypertension, and obesity; arrhythmias, ischemic heart disease, and chronic obstructive pulmonary disease were more common in White men.
The authors pointed out that continuous positive airway pressure (CPAP) is the mainstay of therapy for adults with OSA, but many studies have demonstrated decreased CPAP adherence among Black persons. For example, one report indicated that Black persons use CPAP on average 92 minutes less a day after 1 month of therapy than do White persons, for reasons that are not well understood. Asked by this news organization why Black men are so adversely affected by sleep apnea, Dr. Lee pointed out that studies have shown that sleep apnea is more severe in Black men when first diagnosed.
“We know that the severity of sleep apnea is a risk factor for mortality and cardiovascular outcomes,” he said, “so maybe delayed diagnosis, delayed treatment, and noncompliance with CPAP among Black men may help explain why mortality from sleep apnea among Black men has continued to increase.” Why nonadherence to CPAP is higher among Black men is also not clear. Even when access to CPAP is equal for Black patients and White patients, studies have found that rates of noncompliance to CPAP are higher among Black persons than among White patients.
“This is again a hypothesis,” Dr. Lee emphasized, “but perhaps health literacy among Blacks is lower than it is among White patients, and they may not realize that CPAP can improve health outcomes from sleep apnea,” he suggested. The use of CPAP requires a high level of self-advocacy, which might explain part of their noncompliance.
Other health behaviors and environmental factors may contribute to the tendency among Black patients to be noncompliant with CPAP. “I think this is the first study to show that there is a significant racial disparity in mortality from sleep apnea among Black males, and it should give physicians some insight into the problem; they can develop strategies or interventions to try and reduce racial disparities in outcomes from sleep apnea,” Dr. Lee said.
“So, this study is only the beginning, and we need to have more insight and strategies to improve outcomes among Black males,” he affirmed.
Asked to comment on the findings, Diego Mazzotti, PhD, said the study helps bring attention to existing health disparities related to sleep disorders. “Some of the trends observed by the authors seem to explain the increased recognition that sleep apnea may be a risk factor for cardiovascular morbidity and mortality,” said Dr. Mazzotti, assistant professor in the division of medical informatics at the University of Kansas Medical Center in Kansas City.
“Trends in certain minority groups and certain regions in the U.S. suggest that physicians need to recognize the impact of untreated sleep apnea on the cardiovascular health of these patients,” he said.
Dr. Lee and Dr. Mazzotti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There has been a flattening of sleep apnea–related mortality rates in the United States over the past 10 years. The exception is among Black men, for whom mortality from sleep apnea has continuously increased over the past 21 years, new research shows.
“OSA (obstructive sleep apnea) has been recognized as an important cause of medical morbidity and mortality and contributes to the development of systemic hypertension, cardiovascular disease, and abnormalities in glucose metabolism,” noted Yu-Che Lee, MD, University at Buffalo–Catholic Health System, Buffalo, N.Y., and colleagues.
“This study provides the first systematic assessment and demonstrates remarkable demographic disparities of age-adjusted sleep apnea–related mortality in the U.S., with higher rates in males than females and Blacks than Whites,” they concluded.
The study was published online in Sleep Medicine.
Twenty-one year interval
Data on sleep apnea–related mortality were obtained from the National Center for Health Statistics and were provided by the Centers for Disease Control and Prevention for the years 1999-2019. Over that 21-year interval, sleep apnea was documented as the underlying cause of death in 17,053 decedents, including 2,593 Black patients and 14,127 White patients.
The age-adjusted mortality rate attributed to sleep apnea was 2.5 per 1,000,000 population. The mortality rate was higher for men, at 3.1 per 1,000,000, than among women, 1.9 per 1,000,000 (P < .001). For both sexes, “unadjusted mortality rates were higher in groups aged ≥ 35 years, and the highest mortality rates were observed in groups aged 75-84,” the authors noted. The rate was 11.3 per 1,000,000 for those aged 75-84 and 13.3 per 1,000,000 for those older than 85.
This was also true among Black and White patients, the authors added, although the age-adjusted mortality rate was higher among Black patients than among other racial groups, at 3.5 per 1,000,000 (P < .001). “Over the 21-year study period, the overall age-adjusted mortality rate rose from 1.2 per 1,000,000 population in 1999 to 2.8 per 1,000,000 in 2019,” Dr. Lee and colleagues noted. While the annual percentage change in sleep apnea–related mortality rose by 10.2% (95% confidence interval [CI], 8.4%-12.0%) between 1999 and 2018, no significant change was observed between 2008 and 2019.
On the other hand, when examined by race and sex, age-adjusted mortality rates increased significantly by an annual percentage change of 7.5% (95% CI, 3.3%-11.9%) among Black women and by 8.2% (95% CI, 6.8%-9.6%) between 1999 and 2009 in White men and by 11.5% (95% CI, 8.9%-14.1%) in White women. “Again, these uptrends were no longer observed after that time interval,” the authors stressed.
Only among Black men was there no turning point in age-adjusted mortality rates; they experienced a steady, significant, 2.7% (95% CI, 1.2%-4.2%) annual percent increase in age-adjusted mortality rate between 1999 and 2019. The highest age-adjusted mortality rate for Black persons was recorded in Indiana, at 6.5 per 1,000,000 population; Utah recorded the highest mortality rate for White persons, at 5.7 per 1,000,000.
For both Black persons and White persons, the lowest mortality rates were in New York, at 1.2 per 1,000,000 and 1.5 per 1,000,000, respectively. Among four geographic regions analyzed, the highest age-adjusted mortality rates were in the Midwest for both sexes; Black men in the West and those in three other regional groups in the Northwest had the lowest mortality rates.
Multiple causes of death
Black women were more likely to have multiple causes of death, including cardiac arrest, heart failure, and hypertension. White women were more likely to die of arrhythmia, respiratory failure, pneumonia, and depression. Black men were also more likely to die of cardiac arrest, hypertension, and obesity; arrhythmias, ischemic heart disease, and chronic obstructive pulmonary disease were more common in White men.
The authors pointed out that continuous positive airway pressure (CPAP) is the mainstay of therapy for adults with OSA, but many studies have demonstrated decreased CPAP adherence among Black persons. For example, one report indicated that Black persons use CPAP on average 92 minutes less a day after 1 month of therapy than do White persons, for reasons that are not well understood. Asked by this news organization why Black men are so adversely affected by sleep apnea, Dr. Lee pointed out that studies have shown that sleep apnea is more severe in Black men when first diagnosed.
“We know that the severity of sleep apnea is a risk factor for mortality and cardiovascular outcomes,” he said, “so maybe delayed diagnosis, delayed treatment, and noncompliance with CPAP among Black men may help explain why mortality from sleep apnea among Black men has continued to increase.” Why nonadherence to CPAP is higher among Black men is also not clear. Even when access to CPAP is equal for Black patients and White patients, studies have found that rates of noncompliance to CPAP are higher among Black persons than among White patients.
“This is again a hypothesis,” Dr. Lee emphasized, “but perhaps health literacy among Blacks is lower than it is among White patients, and they may not realize that CPAP can improve health outcomes from sleep apnea,” he suggested. The use of CPAP requires a high level of self-advocacy, which might explain part of their noncompliance.
Other health behaviors and environmental factors may contribute to the tendency among Black patients to be noncompliant with CPAP. “I think this is the first study to show that there is a significant racial disparity in mortality from sleep apnea among Black males, and it should give physicians some insight into the problem; they can develop strategies or interventions to try and reduce racial disparities in outcomes from sleep apnea,” Dr. Lee said.
“So, this study is only the beginning, and we need to have more insight and strategies to improve outcomes among Black males,” he affirmed.
Asked to comment on the findings, Diego Mazzotti, PhD, said the study helps bring attention to existing health disparities related to sleep disorders. “Some of the trends observed by the authors seem to explain the increased recognition that sleep apnea may be a risk factor for cardiovascular morbidity and mortality,” said Dr. Mazzotti, assistant professor in the division of medical informatics at the University of Kansas Medical Center in Kansas City.
“Trends in certain minority groups and certain regions in the U.S. suggest that physicians need to recognize the impact of untreated sleep apnea on the cardiovascular health of these patients,” he said.
Dr. Lee and Dr. Mazzotti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There has been a flattening of sleep apnea–related mortality rates in the United States over the past 10 years. The exception is among Black men, for whom mortality from sleep apnea has continuously increased over the past 21 years, new research shows.
“OSA (obstructive sleep apnea) has been recognized as an important cause of medical morbidity and mortality and contributes to the development of systemic hypertension, cardiovascular disease, and abnormalities in glucose metabolism,” noted Yu-Che Lee, MD, University at Buffalo–Catholic Health System, Buffalo, N.Y., and colleagues.
“This study provides the first systematic assessment and demonstrates remarkable demographic disparities of age-adjusted sleep apnea–related mortality in the U.S., with higher rates in males than females and Blacks than Whites,” they concluded.
The study was published online in Sleep Medicine.
Twenty-one year interval
Data on sleep apnea–related mortality were obtained from the National Center for Health Statistics and were provided by the Centers for Disease Control and Prevention for the years 1999-2019. Over that 21-year interval, sleep apnea was documented as the underlying cause of death in 17,053 decedents, including 2,593 Black patients and 14,127 White patients.
The age-adjusted mortality rate attributed to sleep apnea was 2.5 per 1,000,000 population. The mortality rate was higher for men, at 3.1 per 1,000,000, than among women, 1.9 per 1,000,000 (P < .001). For both sexes, “unadjusted mortality rates were higher in groups aged ≥ 35 years, and the highest mortality rates were observed in groups aged 75-84,” the authors noted. The rate was 11.3 per 1,000,000 for those aged 75-84 and 13.3 per 1,000,000 for those older than 85.
This was also true among Black and White patients, the authors added, although the age-adjusted mortality rate was higher among Black patients than among other racial groups, at 3.5 per 1,000,000 (P < .001). “Over the 21-year study period, the overall age-adjusted mortality rate rose from 1.2 per 1,000,000 population in 1999 to 2.8 per 1,000,000 in 2019,” Dr. Lee and colleagues noted. While the annual percentage change in sleep apnea–related mortality rose by 10.2% (95% confidence interval [CI], 8.4%-12.0%) between 1999 and 2018, no significant change was observed between 2008 and 2019.
On the other hand, when examined by race and sex, age-adjusted mortality rates increased significantly by an annual percentage change of 7.5% (95% CI, 3.3%-11.9%) among Black women and by 8.2% (95% CI, 6.8%-9.6%) between 1999 and 2009 in White men and by 11.5% (95% CI, 8.9%-14.1%) in White women. “Again, these uptrends were no longer observed after that time interval,” the authors stressed.
Only among Black men was there no turning point in age-adjusted mortality rates; they experienced a steady, significant, 2.7% (95% CI, 1.2%-4.2%) annual percent increase in age-adjusted mortality rate between 1999 and 2019. The highest age-adjusted mortality rate for Black persons was recorded in Indiana, at 6.5 per 1,000,000 population; Utah recorded the highest mortality rate for White persons, at 5.7 per 1,000,000.
For both Black persons and White persons, the lowest mortality rates were in New York, at 1.2 per 1,000,000 and 1.5 per 1,000,000, respectively. Among four geographic regions analyzed, the highest age-adjusted mortality rates were in the Midwest for both sexes; Black men in the West and those in three other regional groups in the Northwest had the lowest mortality rates.
Multiple causes of death
Black women were more likely to have multiple causes of death, including cardiac arrest, heart failure, and hypertension. White women were more likely to die of arrhythmia, respiratory failure, pneumonia, and depression. Black men were also more likely to die of cardiac arrest, hypertension, and obesity; arrhythmias, ischemic heart disease, and chronic obstructive pulmonary disease were more common in White men.
The authors pointed out that continuous positive airway pressure (CPAP) is the mainstay of therapy for adults with OSA, but many studies have demonstrated decreased CPAP adherence among Black persons. For example, one report indicated that Black persons use CPAP on average 92 minutes less a day after 1 month of therapy than do White persons, for reasons that are not well understood. Asked by this news organization why Black men are so adversely affected by sleep apnea, Dr. Lee pointed out that studies have shown that sleep apnea is more severe in Black men when first diagnosed.
“We know that the severity of sleep apnea is a risk factor for mortality and cardiovascular outcomes,” he said, “so maybe delayed diagnosis, delayed treatment, and noncompliance with CPAP among Black men may help explain why mortality from sleep apnea among Black men has continued to increase.” Why nonadherence to CPAP is higher among Black men is also not clear. Even when access to CPAP is equal for Black patients and White patients, studies have found that rates of noncompliance to CPAP are higher among Black persons than among White patients.
“This is again a hypothesis,” Dr. Lee emphasized, “but perhaps health literacy among Blacks is lower than it is among White patients, and they may not realize that CPAP can improve health outcomes from sleep apnea,” he suggested. The use of CPAP requires a high level of self-advocacy, which might explain part of their noncompliance.
Other health behaviors and environmental factors may contribute to the tendency among Black patients to be noncompliant with CPAP. “I think this is the first study to show that there is a significant racial disparity in mortality from sleep apnea among Black males, and it should give physicians some insight into the problem; they can develop strategies or interventions to try and reduce racial disparities in outcomes from sleep apnea,” Dr. Lee said.
“So, this study is only the beginning, and we need to have more insight and strategies to improve outcomes among Black males,” he affirmed.
Asked to comment on the findings, Diego Mazzotti, PhD, said the study helps bring attention to existing health disparities related to sleep disorders. “Some of the trends observed by the authors seem to explain the increased recognition that sleep apnea may be a risk factor for cardiovascular morbidity and mortality,” said Dr. Mazzotti, assistant professor in the division of medical informatics at the University of Kansas Medical Center in Kansas City.
“Trends in certain minority groups and certain regions in the U.S. suggest that physicians need to recognize the impact of untreated sleep apnea on the cardiovascular health of these patients,” he said.
Dr. Lee and Dr. Mazzotti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even light drinking ups CV risk; harm rises along with intake
Even very light alcohol intake is associated with an increased risk for cardiovascular disease, compared with not drinking at all, and the risk increases exponentially as alcohol intake rises, even at moderate levels, a new study shows.
“Our findings suggest that the observed benefit in individuals with light to moderate alcohol intake, which is consistently shown in epidemiological studies, is likely due to other positive lifestyle factors that are common in these individuals who drink lightly,” senior author Krishna Aragam, MD, Massachusetts General Hospital, Boston, told this news organization.
“Our results also showed that while all levels of alcohol were linked to increased risk of cardiovascular disease, the association was not linear. Rather, light alcohol intake was associated with rather modest risk increases, but there were exponential increases in cardiovascular risk with increasing amounts of alcohol consumption,” he said.
As the risk gradient appeared to increase quite sharply even between 1 and 2 drinks per day, Dr. Aragam suggested that what might be regarded as safe levels of drinking may trend downward in the future.
The study was published online March 25 in JAMA Network Open.
The cohort study used data from the UK Biobank, collected between 2006 and 2010 with follow-up until 2016, to assess the relationship between various levels of alcohol consumption and risk for cardiovascular disease.
Data were analyzed from 371,463 participants (mean age, 57 years; 46% men) who consumed an average of 9.2 standard drinks per week. Of these participants, 33% had hypertension and 7.5% had coronary artery disease.
“Use of the UK biobank database gives the advantage of a large, well-phenotyped population with a lot of information on various lifestyle factors that could be potential confounders,” Dr. Aragam noted.
Results showed that well-established J- or U-shaped curves were seen for the association between alcohol consumption and both the prevalence and hazards of hypertension, coronary artery disease, myocardial infarction, stroke, heart failure, and atrial fibrillation.
However, individuals in the light and moderate consumption group had healthier lifestyle behaviors than abstainers, self-reporting better overall health and exhibiting lower rates of smoking, lower body mass index, higher physical activity, and higher vegetable intake.
Adjustment for these lifestyle factors attenuated the cardioprotective associations with modest alcohol intake. For example, in baseline models, moderate intake was associated with significantly lower risk of hypertension and coronary artery disease, but adjustment for just six lifestyle factors rendered these results insignificant.
“Adjustments for yet unmeasured or unknown factors may further attenuate, if not eliminate, the residual, cardioprotective associations observed among light drinkers,” the researchers suggest.
They also conducted genetic analyses to examine the effect of alcohol and cardiovascular disease.
Dr. Aragam explained that previous work has shown good evidence, in individuals who choose to drink, that several relevant genetic variants predict levels of alcohol consumption quite accurately.
“Mendelian randomization using these gene variants allows for stronger inferences about potential causality than do observational studies, as they are less affected by confounding factors,” he noted.
Newer techniques in Mendelian randomization in which data on several gene variants linked to alcohol consumption are combined into a score allow for a greater understanding of the risk linked to different amount of alcohol intake, he added.
In these Mendelian randomization analyses, a 1-standard deviation increase in genetically predicted alcohol consumption was associated with 1.3-fold higher risk of hypertension (P < .001) and 1.4-fold higher risk of coronary artery disease (P = .006).
Further analyses suggested nonlinear associations between alcohol consumption and both hypertension and coronary artery disease; light alcohol intake was associated with minimal increases in cardiovascular risk, whereas heavier consumption was associated with exponential increases in risk of both clinical and subclinical cardiovascular disease.
These results were replicated in a second database of 30,716 individuals from the Mass General Brigham Biobank.
“The findings of this study suggest that the observed cardioprotective effects of light to moderate alcohol intake may be largely mediated by confounding lifestyle factors,” the researchers conclude. “Genetic analyses suggest causal associations between alcohol intake and cardiovascular disease but with unequal and exponential increases in risk at greater levels of intake, which should be accounted for in health recommendations around the habitual consumption of alcohol.”
What is an acceptable level?
“Specifically, our results suggest that consuming as many as 7 drinks per week is associated with relatively modest increases in cardiovascular risk,” they write.
But they point out that there are unequal increases in cardiovascular risk when progressing from 0 to 7 versus 7 to 14 drinks per week in both men and women.
“Although risk thresholds are inherently somewhat subjective, these findings again bring into question whether an average consumption of 2 drinks per day (14 drinks per week) should be designated a low-risk behavior,” they say.
“Furthermore, as several-fold increases in risk were observed for those consuming 21 or more drinks per week, our results emphasize the importance of aggressive efforts to reduce alcohol intake among heavy drinkers,” they add.
Dr. Aragam elaborated: “Our data suggest that reducing alcohol intake will reduce cardiovascular risk in all individuals, but the extent of the relative risk reduction is quite different depending on the current levels of consumption. For the same absolute reduction in alcohol intake, the gains in terms of reduction in cardiovascular risk will be more pronounced in those who drink heavily and will be more modest in those who drink at a light level.”
The results also suggest that while all levels of alcohol intake increase cardiovascular risk, there are low levels of alcohol consumption that do not carry major elevations in risk, but these are probably lower than those currently recommended, Dr. Aragam pointed out.
“This doesn’t mean that everyone has to give up drinking alcohol completely, just that you shouldn’t consume with the goal of improving cardiovascular health. In fact, our analyses suggest that in an otherwise healthy person, up to 1 drink per day may not pose outsized risks,” he said. “And, even in a less healthy person who might be smoking, eating poorly, and drinking up to 1 drink per day, it may be a higher priority to focus on smoking cessation and diet than cutting back further on alcohol.”
“Beyond that amount, though, the jury is still out. Our models suggested marked increases in risk even between 1 and 2 drinks per day, and of course even greater risk increases beyond that. So, it’s probably worth revisiting what one might consider a ‘safe’ amount within the moderate drinking categories. The conservative move for now might be to advise a limit of 1 drink per day,” he said.
Dr. Aragam is supported by grants from the National Institutes of Health and the American Heart Association. He reports receiving speaking fees from the Novartis Institute for Biomedical Research.
A version of this article first appeared on Medscape.com.
Even very light alcohol intake is associated with an increased risk for cardiovascular disease, compared with not drinking at all, and the risk increases exponentially as alcohol intake rises, even at moderate levels, a new study shows.
“Our findings suggest that the observed benefit in individuals with light to moderate alcohol intake, which is consistently shown in epidemiological studies, is likely due to other positive lifestyle factors that are common in these individuals who drink lightly,” senior author Krishna Aragam, MD, Massachusetts General Hospital, Boston, told this news organization.
“Our results also showed that while all levels of alcohol were linked to increased risk of cardiovascular disease, the association was not linear. Rather, light alcohol intake was associated with rather modest risk increases, but there were exponential increases in cardiovascular risk with increasing amounts of alcohol consumption,” he said.
As the risk gradient appeared to increase quite sharply even between 1 and 2 drinks per day, Dr. Aragam suggested that what might be regarded as safe levels of drinking may trend downward in the future.
The study was published online March 25 in JAMA Network Open.
The cohort study used data from the UK Biobank, collected between 2006 and 2010 with follow-up until 2016, to assess the relationship between various levels of alcohol consumption and risk for cardiovascular disease.
Data were analyzed from 371,463 participants (mean age, 57 years; 46% men) who consumed an average of 9.2 standard drinks per week. Of these participants, 33% had hypertension and 7.5% had coronary artery disease.
“Use of the UK biobank database gives the advantage of a large, well-phenotyped population with a lot of information on various lifestyle factors that could be potential confounders,” Dr. Aragam noted.
Results showed that well-established J- or U-shaped curves were seen for the association between alcohol consumption and both the prevalence and hazards of hypertension, coronary artery disease, myocardial infarction, stroke, heart failure, and atrial fibrillation.
However, individuals in the light and moderate consumption group had healthier lifestyle behaviors than abstainers, self-reporting better overall health and exhibiting lower rates of smoking, lower body mass index, higher physical activity, and higher vegetable intake.
Adjustment for these lifestyle factors attenuated the cardioprotective associations with modest alcohol intake. For example, in baseline models, moderate intake was associated with significantly lower risk of hypertension and coronary artery disease, but adjustment for just six lifestyle factors rendered these results insignificant.
“Adjustments for yet unmeasured or unknown factors may further attenuate, if not eliminate, the residual, cardioprotective associations observed among light drinkers,” the researchers suggest.
They also conducted genetic analyses to examine the effect of alcohol and cardiovascular disease.
Dr. Aragam explained that previous work has shown good evidence, in individuals who choose to drink, that several relevant genetic variants predict levels of alcohol consumption quite accurately.
“Mendelian randomization using these gene variants allows for stronger inferences about potential causality than do observational studies, as they are less affected by confounding factors,” he noted.
Newer techniques in Mendelian randomization in which data on several gene variants linked to alcohol consumption are combined into a score allow for a greater understanding of the risk linked to different amount of alcohol intake, he added.
In these Mendelian randomization analyses, a 1-standard deviation increase in genetically predicted alcohol consumption was associated with 1.3-fold higher risk of hypertension (P < .001) and 1.4-fold higher risk of coronary artery disease (P = .006).
Further analyses suggested nonlinear associations between alcohol consumption and both hypertension and coronary artery disease; light alcohol intake was associated with minimal increases in cardiovascular risk, whereas heavier consumption was associated with exponential increases in risk of both clinical and subclinical cardiovascular disease.
These results were replicated in a second database of 30,716 individuals from the Mass General Brigham Biobank.
“The findings of this study suggest that the observed cardioprotective effects of light to moderate alcohol intake may be largely mediated by confounding lifestyle factors,” the researchers conclude. “Genetic analyses suggest causal associations between alcohol intake and cardiovascular disease but with unequal and exponential increases in risk at greater levels of intake, which should be accounted for in health recommendations around the habitual consumption of alcohol.”
What is an acceptable level?
“Specifically, our results suggest that consuming as many as 7 drinks per week is associated with relatively modest increases in cardiovascular risk,” they write.
But they point out that there are unequal increases in cardiovascular risk when progressing from 0 to 7 versus 7 to 14 drinks per week in both men and women.
“Although risk thresholds are inherently somewhat subjective, these findings again bring into question whether an average consumption of 2 drinks per day (14 drinks per week) should be designated a low-risk behavior,” they say.
“Furthermore, as several-fold increases in risk were observed for those consuming 21 or more drinks per week, our results emphasize the importance of aggressive efforts to reduce alcohol intake among heavy drinkers,” they add.
Dr. Aragam elaborated: “Our data suggest that reducing alcohol intake will reduce cardiovascular risk in all individuals, but the extent of the relative risk reduction is quite different depending on the current levels of consumption. For the same absolute reduction in alcohol intake, the gains in terms of reduction in cardiovascular risk will be more pronounced in those who drink heavily and will be more modest in those who drink at a light level.”
The results also suggest that while all levels of alcohol intake increase cardiovascular risk, there are low levels of alcohol consumption that do not carry major elevations in risk, but these are probably lower than those currently recommended, Dr. Aragam pointed out.
“This doesn’t mean that everyone has to give up drinking alcohol completely, just that you shouldn’t consume with the goal of improving cardiovascular health. In fact, our analyses suggest that in an otherwise healthy person, up to 1 drink per day may not pose outsized risks,” he said. “And, even in a less healthy person who might be smoking, eating poorly, and drinking up to 1 drink per day, it may be a higher priority to focus on smoking cessation and diet than cutting back further on alcohol.”
“Beyond that amount, though, the jury is still out. Our models suggested marked increases in risk even between 1 and 2 drinks per day, and of course even greater risk increases beyond that. So, it’s probably worth revisiting what one might consider a ‘safe’ amount within the moderate drinking categories. The conservative move for now might be to advise a limit of 1 drink per day,” he said.
Dr. Aragam is supported by grants from the National Institutes of Health and the American Heart Association. He reports receiving speaking fees from the Novartis Institute for Biomedical Research.
A version of this article first appeared on Medscape.com.
Even very light alcohol intake is associated with an increased risk for cardiovascular disease, compared with not drinking at all, and the risk increases exponentially as alcohol intake rises, even at moderate levels, a new study shows.
“Our findings suggest that the observed benefit in individuals with light to moderate alcohol intake, which is consistently shown in epidemiological studies, is likely due to other positive lifestyle factors that are common in these individuals who drink lightly,” senior author Krishna Aragam, MD, Massachusetts General Hospital, Boston, told this news organization.
“Our results also showed that while all levels of alcohol were linked to increased risk of cardiovascular disease, the association was not linear. Rather, light alcohol intake was associated with rather modest risk increases, but there were exponential increases in cardiovascular risk with increasing amounts of alcohol consumption,” he said.
As the risk gradient appeared to increase quite sharply even between 1 and 2 drinks per day, Dr. Aragam suggested that what might be regarded as safe levels of drinking may trend downward in the future.
The study was published online March 25 in JAMA Network Open.
The cohort study used data from the UK Biobank, collected between 2006 and 2010 with follow-up until 2016, to assess the relationship between various levels of alcohol consumption and risk for cardiovascular disease.
Data were analyzed from 371,463 participants (mean age, 57 years; 46% men) who consumed an average of 9.2 standard drinks per week. Of these participants, 33% had hypertension and 7.5% had coronary artery disease.
“Use of the UK biobank database gives the advantage of a large, well-phenotyped population with a lot of information on various lifestyle factors that could be potential confounders,” Dr. Aragam noted.
Results showed that well-established J- or U-shaped curves were seen for the association between alcohol consumption and both the prevalence and hazards of hypertension, coronary artery disease, myocardial infarction, stroke, heart failure, and atrial fibrillation.
However, individuals in the light and moderate consumption group had healthier lifestyle behaviors than abstainers, self-reporting better overall health and exhibiting lower rates of smoking, lower body mass index, higher physical activity, and higher vegetable intake.
Adjustment for these lifestyle factors attenuated the cardioprotective associations with modest alcohol intake. For example, in baseline models, moderate intake was associated with significantly lower risk of hypertension and coronary artery disease, but adjustment for just six lifestyle factors rendered these results insignificant.
“Adjustments for yet unmeasured or unknown factors may further attenuate, if not eliminate, the residual, cardioprotective associations observed among light drinkers,” the researchers suggest.
They also conducted genetic analyses to examine the effect of alcohol and cardiovascular disease.
Dr. Aragam explained that previous work has shown good evidence, in individuals who choose to drink, that several relevant genetic variants predict levels of alcohol consumption quite accurately.
“Mendelian randomization using these gene variants allows for stronger inferences about potential causality than do observational studies, as they are less affected by confounding factors,” he noted.
Newer techniques in Mendelian randomization in which data on several gene variants linked to alcohol consumption are combined into a score allow for a greater understanding of the risk linked to different amount of alcohol intake, he added.
In these Mendelian randomization analyses, a 1-standard deviation increase in genetically predicted alcohol consumption was associated with 1.3-fold higher risk of hypertension (P < .001) and 1.4-fold higher risk of coronary artery disease (P = .006).
Further analyses suggested nonlinear associations between alcohol consumption and both hypertension and coronary artery disease; light alcohol intake was associated with minimal increases in cardiovascular risk, whereas heavier consumption was associated with exponential increases in risk of both clinical and subclinical cardiovascular disease.
These results were replicated in a second database of 30,716 individuals from the Mass General Brigham Biobank.
“The findings of this study suggest that the observed cardioprotective effects of light to moderate alcohol intake may be largely mediated by confounding lifestyle factors,” the researchers conclude. “Genetic analyses suggest causal associations between alcohol intake and cardiovascular disease but with unequal and exponential increases in risk at greater levels of intake, which should be accounted for in health recommendations around the habitual consumption of alcohol.”
What is an acceptable level?
“Specifically, our results suggest that consuming as many as 7 drinks per week is associated with relatively modest increases in cardiovascular risk,” they write.
But they point out that there are unequal increases in cardiovascular risk when progressing from 0 to 7 versus 7 to 14 drinks per week in both men and women.
“Although risk thresholds are inherently somewhat subjective, these findings again bring into question whether an average consumption of 2 drinks per day (14 drinks per week) should be designated a low-risk behavior,” they say.
“Furthermore, as several-fold increases in risk were observed for those consuming 21 or more drinks per week, our results emphasize the importance of aggressive efforts to reduce alcohol intake among heavy drinkers,” they add.
Dr. Aragam elaborated: “Our data suggest that reducing alcohol intake will reduce cardiovascular risk in all individuals, but the extent of the relative risk reduction is quite different depending on the current levels of consumption. For the same absolute reduction in alcohol intake, the gains in terms of reduction in cardiovascular risk will be more pronounced in those who drink heavily and will be more modest in those who drink at a light level.”
The results also suggest that while all levels of alcohol intake increase cardiovascular risk, there are low levels of alcohol consumption that do not carry major elevations in risk, but these are probably lower than those currently recommended, Dr. Aragam pointed out.
“This doesn’t mean that everyone has to give up drinking alcohol completely, just that you shouldn’t consume with the goal of improving cardiovascular health. In fact, our analyses suggest that in an otherwise healthy person, up to 1 drink per day may not pose outsized risks,” he said. “And, even in a less healthy person who might be smoking, eating poorly, and drinking up to 1 drink per day, it may be a higher priority to focus on smoking cessation and diet than cutting back further on alcohol.”
“Beyond that amount, though, the jury is still out. Our models suggested marked increases in risk even between 1 and 2 drinks per day, and of course even greater risk increases beyond that. So, it’s probably worth revisiting what one might consider a ‘safe’ amount within the moderate drinking categories. The conservative move for now might be to advise a limit of 1 drink per day,” he said.
Dr. Aragam is supported by grants from the National Institutes of Health and the American Heart Association. He reports receiving speaking fees from the Novartis Institute for Biomedical Research.
A version of this article first appeared on Medscape.com.
Be aware of gallbladder, biliary disease with newer obesity drugs
Treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with a 37% increase in the relative risk of gallbladder or biliary disease, compared with controls – especially when used at high doses, for a longer time, and for weight loss rather than type 2 diabetes – a new meta-analysis has found.
The results “indicate that physicians and patients should be concerned about the risks of gallbladder or biliary diseases with using GLP-1 agonists,” study authors Liyun He and colleagues from Peking Union Medical College, Beijing, summarize.
However, “the overall absolute risk increase for gallbladder and biliary disease with use of GLP-1 receptor agonists was small (an additional 27 cases per 10,000 persons treated per year),” they note.
“This absolute risk increase should be weighed against the benefits of treatment with GLP-1 agonists,” which include glucose control, decreased cardiovascular risk, and weight loss, they add.
The findings are from a meta-analysis of 76 randomized controlled trials of GLP-1 agonists published online March 28 in JAMA Internal Medicine.
In an accompanying editorial, Shanzay Haider, MD, and Kasia J. Lipska, MD, also characterize the absolute risk of these complications as “modest.”
“The highest risk for these complications,” they add, “occurred among individuals in the weight loss, compared with the type 2 diabetes studies (119 vs. 13 more events per 10,000 persons per year).”
“Ultimately, the decision to start, continue, or change the dose of a GLP-1 agonist should be reached through a collaborative and individualized discussion between a clinician and a patient,” Dr. Haider and Dr. Lipska, from Yale School of Medicine, New Haven, Conn., summarize.
The study authors also note that few of the trials reported biliary-related events.
“Future trials [of drugs in this class] should prespecify gallbladder and biliary diseases as potential adverse events, and fully test for and report on these outcomes,” they urge.
Certain drugs in this class are now approved by the U.S. Food and Drug Administration for weight loss at higher doses than for type 2 diabetes – subcutaneous liraglutide (3.0 mg) and subcutaneous semaglutide (2.4 mg) – “suggesting that GLP-1 agonist drugs will increasingly be used at high doses for weight control,” the authors note.
Controversial link
The association between GLP-1 agonists and gallbladder or biliary disease is controversial, the authors write.
Several randomized controlled trials reported higher rates of gallbladder disorders in patients who received a GLP-1 agonist versus placebo, but it is not clear if this is a class effect.
Liraglutide “has drawn the most attention” about this risk, and a post-hoc analysis of the LEADER trial found a significantly increased risk of acute biliary obstruction with liraglutide versus placebo.
To investigate this, the researchers identified 76 randomized controlled trials of GLP-1 agonists in 103,371 patients that had data for the following safety outcomes: cholelithiasis (gallstones, 61 trials), cholecystitis (inflamed gallbladder, 53 trials), biliary disease (21 trials), cholecystectomy (surgical removal of the gallbladder, seven trials), and biliary cancer (12 trials).
Sixty trials were for type 2 diabetes, 13 were for weight loss, and three were for nonalcoholic steatohepatitis, polycystic ovary syndrome, and schizophrenia. They were classed as short or long (≤ 26 weeks or > 26 weeks).
The GLP-1 agonists were liraglutide (21 trials), subcutaneous semaglutide (14), dulaglutide (11), exenatide (9), albiglutide (8), oral semaglutide (8), and lixisenatide (6).
Participants were a mean age of 58 years and 41% were women. They had a mean BMI of 31.6 kg/m2 and 36.9 kg/m2 in trials of GLP-1 agonists for type 2 diabetes and weight loss, respectively.
Patients who received a GLP-1 agonist versus controls had significantly increased rates of cholelithiasis (RR, 1.27; P = .001), cholecystitis (RR, 1.36; P < .001), biliary disease (RR, 1.55; P = .02), and cholecystectomy (RR, 1.70; P < .001) but a nonsignificant increased rate of biliary cancer (RR, 1.43; P = .22).
Use of GLP-1 agonists was associated with a greater increased risk of gallbladder or biliary diseases in trials for weight loss (RR, 2.29) than in trials for type 2 diabetes or other diseases (RR, 1.27; P < .001 for interaction).
Use of these drugs was also associated with higher risks of these complications at higher doses and when given for a longer duration.
Limitations of the meta-analysis include that the individual studies were not designed to evaluate the risk of gallbladder or biliary diseases associated with GLP-1 agonists.
Also, biliary-related events may have been under-reported, because this was not a predefined safety outcome in most of the trials. The meta-analysis lacked patient-level data, and it may have been underpowered for subgroup analyses.
The work was supported by grants from the National Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Sciences, the CAMS Innovation Fund for Medical Sciences, and the Training Program for Excellent Talents in Dongcheng District. The researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with a 37% increase in the relative risk of gallbladder or biliary disease, compared with controls – especially when used at high doses, for a longer time, and for weight loss rather than type 2 diabetes – a new meta-analysis has found.
The results “indicate that physicians and patients should be concerned about the risks of gallbladder or biliary diseases with using GLP-1 agonists,” study authors Liyun He and colleagues from Peking Union Medical College, Beijing, summarize.
However, “the overall absolute risk increase for gallbladder and biliary disease with use of GLP-1 receptor agonists was small (an additional 27 cases per 10,000 persons treated per year),” they note.
“This absolute risk increase should be weighed against the benefits of treatment with GLP-1 agonists,” which include glucose control, decreased cardiovascular risk, and weight loss, they add.
The findings are from a meta-analysis of 76 randomized controlled trials of GLP-1 agonists published online March 28 in JAMA Internal Medicine.
In an accompanying editorial, Shanzay Haider, MD, and Kasia J. Lipska, MD, also characterize the absolute risk of these complications as “modest.”
“The highest risk for these complications,” they add, “occurred among individuals in the weight loss, compared with the type 2 diabetes studies (119 vs. 13 more events per 10,000 persons per year).”
“Ultimately, the decision to start, continue, or change the dose of a GLP-1 agonist should be reached through a collaborative and individualized discussion between a clinician and a patient,” Dr. Haider and Dr. Lipska, from Yale School of Medicine, New Haven, Conn., summarize.
The study authors also note that few of the trials reported biliary-related events.
“Future trials [of drugs in this class] should prespecify gallbladder and biliary diseases as potential adverse events, and fully test for and report on these outcomes,” they urge.
Certain drugs in this class are now approved by the U.S. Food and Drug Administration for weight loss at higher doses than for type 2 diabetes – subcutaneous liraglutide (3.0 mg) and subcutaneous semaglutide (2.4 mg) – “suggesting that GLP-1 agonist drugs will increasingly be used at high doses for weight control,” the authors note.
Controversial link
The association between GLP-1 agonists and gallbladder or biliary disease is controversial, the authors write.
Several randomized controlled trials reported higher rates of gallbladder disorders in patients who received a GLP-1 agonist versus placebo, but it is not clear if this is a class effect.
Liraglutide “has drawn the most attention” about this risk, and a post-hoc analysis of the LEADER trial found a significantly increased risk of acute biliary obstruction with liraglutide versus placebo.
To investigate this, the researchers identified 76 randomized controlled trials of GLP-1 agonists in 103,371 patients that had data for the following safety outcomes: cholelithiasis (gallstones, 61 trials), cholecystitis (inflamed gallbladder, 53 trials), biliary disease (21 trials), cholecystectomy (surgical removal of the gallbladder, seven trials), and biliary cancer (12 trials).
Sixty trials were for type 2 diabetes, 13 were for weight loss, and three were for nonalcoholic steatohepatitis, polycystic ovary syndrome, and schizophrenia. They were classed as short or long (≤ 26 weeks or > 26 weeks).
The GLP-1 agonists were liraglutide (21 trials), subcutaneous semaglutide (14), dulaglutide (11), exenatide (9), albiglutide (8), oral semaglutide (8), and lixisenatide (6).
Participants were a mean age of 58 years and 41% were women. They had a mean BMI of 31.6 kg/m2 and 36.9 kg/m2 in trials of GLP-1 agonists for type 2 diabetes and weight loss, respectively.
Patients who received a GLP-1 agonist versus controls had significantly increased rates of cholelithiasis (RR, 1.27; P = .001), cholecystitis (RR, 1.36; P < .001), biliary disease (RR, 1.55; P = .02), and cholecystectomy (RR, 1.70; P < .001) but a nonsignificant increased rate of biliary cancer (RR, 1.43; P = .22).
Use of GLP-1 agonists was associated with a greater increased risk of gallbladder or biliary diseases in trials for weight loss (RR, 2.29) than in trials for type 2 diabetes or other diseases (RR, 1.27; P < .001 for interaction).
Use of these drugs was also associated with higher risks of these complications at higher doses and when given for a longer duration.
Limitations of the meta-analysis include that the individual studies were not designed to evaluate the risk of gallbladder or biliary diseases associated with GLP-1 agonists.
Also, biliary-related events may have been under-reported, because this was not a predefined safety outcome in most of the trials. The meta-analysis lacked patient-level data, and it may have been underpowered for subgroup analyses.
The work was supported by grants from the National Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Sciences, the CAMS Innovation Fund for Medical Sciences, and the Training Program for Excellent Talents in Dongcheng District. The researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a glucagon-like peptide-1 (GLP-1) receptor agonist was associated with a 37% increase in the relative risk of gallbladder or biliary disease, compared with controls – especially when used at high doses, for a longer time, and for weight loss rather than type 2 diabetes – a new meta-analysis has found.
The results “indicate that physicians and patients should be concerned about the risks of gallbladder or biliary diseases with using GLP-1 agonists,” study authors Liyun He and colleagues from Peking Union Medical College, Beijing, summarize.
However, “the overall absolute risk increase for gallbladder and biliary disease with use of GLP-1 receptor agonists was small (an additional 27 cases per 10,000 persons treated per year),” they note.
“This absolute risk increase should be weighed against the benefits of treatment with GLP-1 agonists,” which include glucose control, decreased cardiovascular risk, and weight loss, they add.
The findings are from a meta-analysis of 76 randomized controlled trials of GLP-1 agonists published online March 28 in JAMA Internal Medicine.
In an accompanying editorial, Shanzay Haider, MD, and Kasia J. Lipska, MD, also characterize the absolute risk of these complications as “modest.”
“The highest risk for these complications,” they add, “occurred among individuals in the weight loss, compared with the type 2 diabetes studies (119 vs. 13 more events per 10,000 persons per year).”
“Ultimately, the decision to start, continue, or change the dose of a GLP-1 agonist should be reached through a collaborative and individualized discussion between a clinician and a patient,” Dr. Haider and Dr. Lipska, from Yale School of Medicine, New Haven, Conn., summarize.
The study authors also note that few of the trials reported biliary-related events.
“Future trials [of drugs in this class] should prespecify gallbladder and biliary diseases as potential adverse events, and fully test for and report on these outcomes,” they urge.
Certain drugs in this class are now approved by the U.S. Food and Drug Administration for weight loss at higher doses than for type 2 diabetes – subcutaneous liraglutide (3.0 mg) and subcutaneous semaglutide (2.4 mg) – “suggesting that GLP-1 agonist drugs will increasingly be used at high doses for weight control,” the authors note.
Controversial link
The association between GLP-1 agonists and gallbladder or biliary disease is controversial, the authors write.
Several randomized controlled trials reported higher rates of gallbladder disorders in patients who received a GLP-1 agonist versus placebo, but it is not clear if this is a class effect.
Liraglutide “has drawn the most attention” about this risk, and a post-hoc analysis of the LEADER trial found a significantly increased risk of acute biliary obstruction with liraglutide versus placebo.
To investigate this, the researchers identified 76 randomized controlled trials of GLP-1 agonists in 103,371 patients that had data for the following safety outcomes: cholelithiasis (gallstones, 61 trials), cholecystitis (inflamed gallbladder, 53 trials), biliary disease (21 trials), cholecystectomy (surgical removal of the gallbladder, seven trials), and biliary cancer (12 trials).
Sixty trials were for type 2 diabetes, 13 were for weight loss, and three were for nonalcoholic steatohepatitis, polycystic ovary syndrome, and schizophrenia. They were classed as short or long (≤ 26 weeks or > 26 weeks).
The GLP-1 agonists were liraglutide (21 trials), subcutaneous semaglutide (14), dulaglutide (11), exenatide (9), albiglutide (8), oral semaglutide (8), and lixisenatide (6).
Participants were a mean age of 58 years and 41% were women. They had a mean BMI of 31.6 kg/m2 and 36.9 kg/m2 in trials of GLP-1 agonists for type 2 diabetes and weight loss, respectively.
Patients who received a GLP-1 agonist versus controls had significantly increased rates of cholelithiasis (RR, 1.27; P = .001), cholecystitis (RR, 1.36; P < .001), biliary disease (RR, 1.55; P = .02), and cholecystectomy (RR, 1.70; P < .001) but a nonsignificant increased rate of biliary cancer (RR, 1.43; P = .22).
Use of GLP-1 agonists was associated with a greater increased risk of gallbladder or biliary diseases in trials for weight loss (RR, 2.29) than in trials for type 2 diabetes or other diseases (RR, 1.27; P < .001 for interaction).
Use of these drugs was also associated with higher risks of these complications at higher doses and when given for a longer duration.
Limitations of the meta-analysis include that the individual studies were not designed to evaluate the risk of gallbladder or biliary diseases associated with GLP-1 agonists.
Also, biliary-related events may have been under-reported, because this was not a predefined safety outcome in most of the trials. The meta-analysis lacked patient-level data, and it may have been underpowered for subgroup analyses.
The work was supported by grants from the National Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Sciences, the CAMS Innovation Fund for Medical Sciences, and the Training Program for Excellent Talents in Dongcheng District. The researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Removal of Isotretinoin Gender-Based Guidelines: Inclusivity Takes Precedence
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Resident Pearls
- Major changes in the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) system recently took place, including simplifying registration categories while making the process more inclusive for patients.
- It is important to practice culturally sensitive language when discussing subjects regarding gender identification and sexual practices. Sample questions have been provided to help familiarize practitioners with optimal ways to approach these patient encounters.
- There likely will be more changes with iPLEDGE REMS in the future as the American Academy of Dermatology Association continues to work on solutions regarding decreasing monthly qualifications for patients who cannot get pregnant and possible removal of patient attestation requirements.
Polio: Five African countries vaccinating 23 million children
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When polio paralyzed a 3-year-old girl in Lilongwe, Malawi, in November 2021, public health experts in Malawi’s Ministry of Health responded quickly. The ministry partnered with the Global Polio Eradication Initiative, the World Health Organization, and the United Nations International Children’s Emergency Fund to mobilize a surge team of personnel and resources to vaccinate all 2.9 million Malawian children aged under 5 years, WHO reported in a news release.
The first of four sequential campaigns began on March 20 and expanded on March 24 to neighboring Mozambique, Tanzania, and Zambia. The multinational, multiagency effort aims to include Zimbabwean children as well and deliver over 80 million supplemental doses of bivalent oral polio vaccines to over 23 million children in these five countries by July.
Because it takes multiple polio vaccine doses to become fully immunized, the children are expected to receive four rounds of vaccine regardless of their vaccination history.
“It is important to conduct the campaigns now to boost the immunity of our children,” Annie Chauma-Mwale, MBBS, MPH, the chief medical officer of epidemiology and surveillance in Malawi’s Ministry of Health in Lilongwe, said in an interview. “Polio is not only a medical issue. Polio is also a socioeconomic issue with long-term impacts on the child, the country, and the globe.
“In Malawi, we are using our community health and health care facility structures to ensure we do not miss any eligible child,” explained Dr. Chauma-Mwale, who is also the deputy incident manager of the poliovirus outbreak response. “We aim to play our role in the global eradication of polio by protecting the vulnerable and curtailing any potential transmission as early as possible.”
Of the three variants of wild, naturally occurring poliovirus, types 2 and 3 have been eradicated, but wild poliovirus type 1 (WPV1) remains endemic in Afghanistan and Pakistan.
As reported recently by this news organization, the girl in Malawi was infected with a WPV1 strain that had been circulating for years in Pakistan’s Sindh Province.
Malawi’s most recent clinically confirmed WPV1 case was reported in 1992, and this is the first WPV1 case detected in Africa since 2016. The continent was declared free of indigenous wild polio in 2020 and is still considered free of wild poliovirus because the child’s illness was imported from elsewhere.
The 3-year-old girl developed acute flaccid paralysis in November 2021. In February 2022, virus from her stool was sequenced by the National Institute of Communicable Disease in South Africa and the U.S. Centers for Disease Control and Prevention. On Feb. 16, Malawi was notified of the case, which was genetically linked to a sequence detected in Sindh Province around 2 years earlier.
‘Do not ignore polio’
Within 24 hours, the Government of Malawi declared a public health emergency and activated the national Emergency Operations Centre. Within 72 hours, the GPEI rapid response team arrived in the country. The Ministry of Health partnered with GPEI, WHO, and UNICEF to mobilize the campaign and begin vaccinating children on March 20.
‘’We rely on clinicians to support the surveillance of polio through case searches, both active and passive,” Mike Nenani Chisema, MBBS, MPH, the program manager of the expanded program on immunization and the polio response operations manager in Malawi’s Ministry of Health, said in an interview.
He noted that the young girl was diagnosed correctly and millions of children are now being protected against the disease, thanks to the acumen of one hospital clinician.
“Remember, we still have polio in some countries, and every country is at risk,” he cautioned. “Don’t forget to look for the obvious and do not ignore polio, regardless of economic status.’’
According to GPEI, all countries – especially those with weak immunization and other public health programs whose residents trade or travel to and from endemic countries – are at risk for imported polio.
Anita Gupta, DO, MPP, PharmD, an adjunct assistant professor of anesthesiology and critical care medicine and pain medicine at the Johns Hopkins University, Baltimore, said that she welcomes this effort.
“Given the decades of published evidence and understanding on the vaccine’s safety and efficacy, this program in Malawi is the right step to take,” Gupta, who is not involved in the campaigns, said in an interview. “Polio is preventable, and acting now will prevent spread later.”
Dr. Chauma-Mwale and Dr. Chisema are employees of Malawi’s Ministry of Health. Dr. Gupta disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychotropic med use tied to ‘striking’ post-COVID dementia risk
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large study of more than 1,700 patients who had been hospitalized with COVID showed a greater than twofold increased risk for post-COVID dementia in those taking antipsychotics and mood stabilizers/anticonvulsants – medications often used to treat schizophrenia, psychosis, bipolar disorder, and seizures.
“We know that pre-existing psychiatric illness is associated with poor COVID-19 outcomes, but our study is the first to show an association with certain psychiatric medications and dementia,” co-investigator Liron Sinvani, MD, the Feinstein Institutes for Medical Research, Manhasset, New York, said in an interview.
“Our study highlights the potential interaction between baseline neuropsychiatric disease, psychotropic medications, COVID-19, and dementia,” Dr. Sinvani added.
The findings were published online March 18 in Frontiers in Medicine.
‘Striking’ dementia rate
Using electronic health records, the researchers evaluated pre-COVID psychotropic medication use and post-COVID dementia onset in 1,755 adults aged 65 and older. All were hospitalized with COVID-19 at Northwell Health between March 1 and April 20, 2020.
A “striking” 13% of the participants (n = 223) developed dementia within 1-year of follow-up, the investigators report.
Among the 438 patients (25%) exposed to at least one psychotropic medication before COVID-19, 105 (24%) developed dementia in the year following COVID versus 118 of 1,317 (9%) patients with no pre-COVID exposure to psychotropic medication (odds ratio, 3.2; 95% confidence interval, 2.37-4.32).
Both pre-COVID psychotropic medication use (OR, 2.7; 95% CI, 1.8-4.0, P < .001) and delirium (OR, 3.0; 95% CI, 1.9-4.6, P < .001) were significantly associated with post-COVID dementia at 1 year.
In a sensitivity analysis in the subset of 423 patients with at least one documented neurologic or psychiatric diagnosis at the time of COVID admission, and after adjusting for confounding factors, pre-COVID psychotropic medication use remained significantly linked to post-COVID dementia onset (OR, 3.09; 95% CI, 1.5-6.6, P = .002).
Drug classes most strongly associated with 1-year post-COVID dementia onset were antipsychotics (OR, 2.8, 95% CI, 1.7-4.4, P < .001) and mood stabilizers/anticonvulsants (OR, 2.4, 95% CI, 1.39-4.02, P = .001).
In a further exploratory analysis, the psychotropics valproic acid (multiple brands) and haloperidol (Haldol) had the largest association with post-COVID dementia.
Antidepressants as a class were not associated with post-COVID dementia, but the potential effects of two commonly prescribed antidepressants in older adults, mirtazapine (Remeron) and escitalopram (Lexapro), “warrant further investigation,” the researchers note.
Predictive risk marker?
“This research shows that psychotropic medications can be considered a predictive risk marker for post-COVID dementia. In patients taking psychotropic medications, COVID-19 could have accelerated progression of dementia after hospitalization,” lead author Yun Freudenberg-Hua, MD, the Feinstein Institutes, said in a news release.
It is unclear why psychotropic medications may raise the risk for dementia onset after COVID, the investigators note.
“It is intuitive that psychotropic medications indicate pre-existing neuropsychiatric conditions in which COVID-19 occurs. It is possible that psychotropic medications may potentiate the neurostructural changes that have been found in the brain of those who have recovered from COVID-19,” they write.
The sensitivity analysis in patients with documented neurologic and psychiatric diagnoses supports this interpretation.
COVID-19 may also accelerate the underlying brain disorders for which psychotropic medications were prescribed, leading to the greater incidence of post-COVID dementia, the researchers write.
“It is important to note that this study is in no way recommending people should stop taking antipsychotics but simply that clinicians need to factor in a patient’s medication history while considering post-COVID aftereffects,” Dr. Freudenberg-Hua said.
“Given that the number of patients with dementia is projected to triple in the next 30 years, these findings have significant public health implications,” Dr. Sinvani added.
She noted that “care partners and health care professionals” should look for early signs of dementia, such as forgetfulness and depressive symptoms, in their patients.
“Future studies must continue to evaluate these associations, which are key for potential future interventions to prevent dementia,” Dr. Sinvani said.
The study was funded by the National Institutes of Health. Dr. Freudenberg-Hua co-owns stock and stock options from Regeneron Pharmaceuticals. Dr. Sinvani has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN MEDICINE