User login
No link between cell phones and brain tumors in large U.K. study
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Fingers take the fight to COVID-19
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Verrucous Carcinoma of the Foot: A Retrospective Study of 19 Cases and Analysis of Prognostic Factors Influencing Recurrence
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
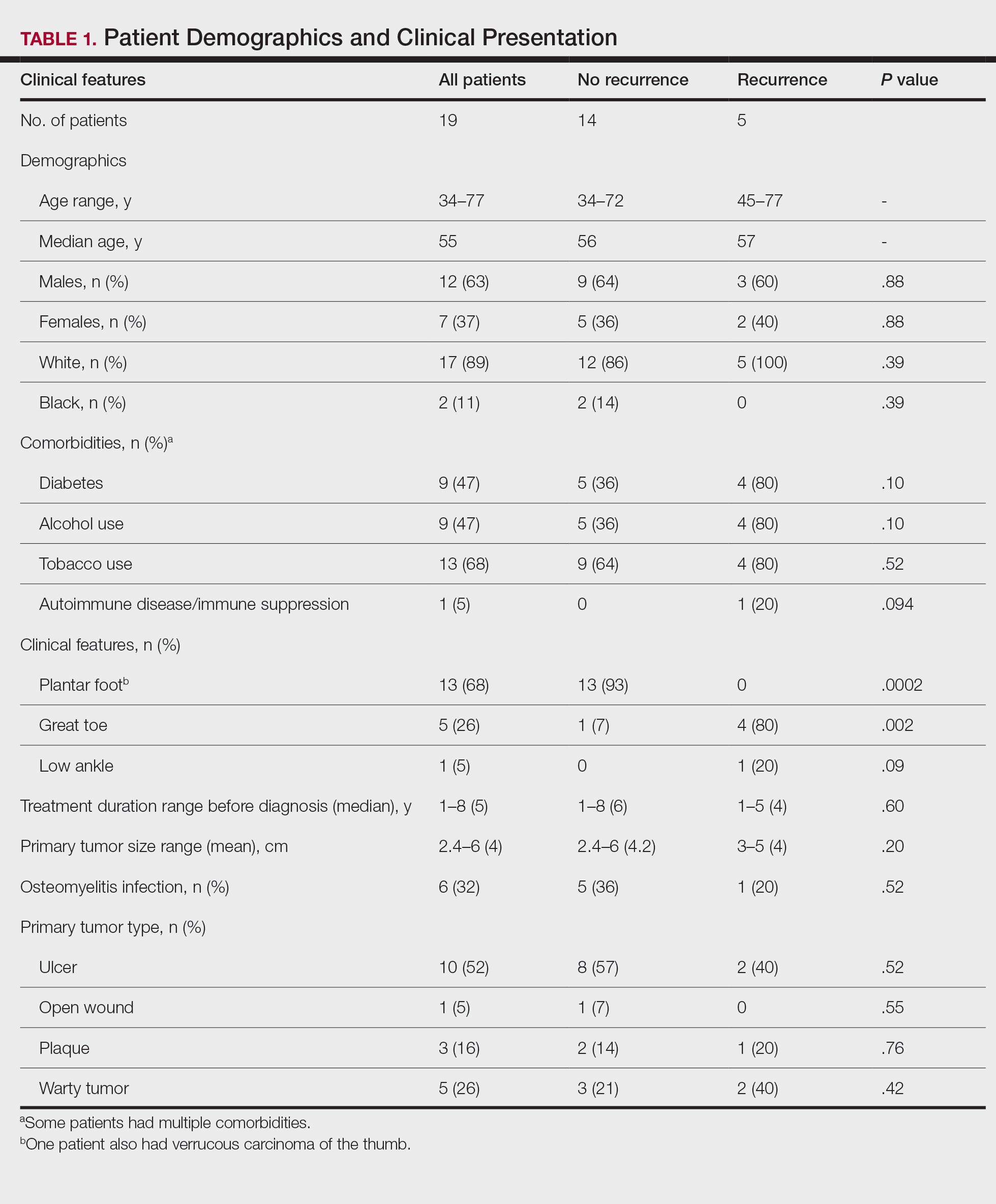
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
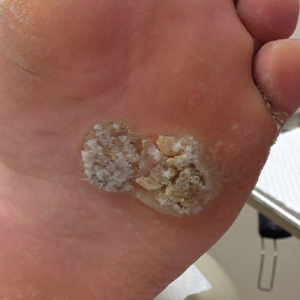
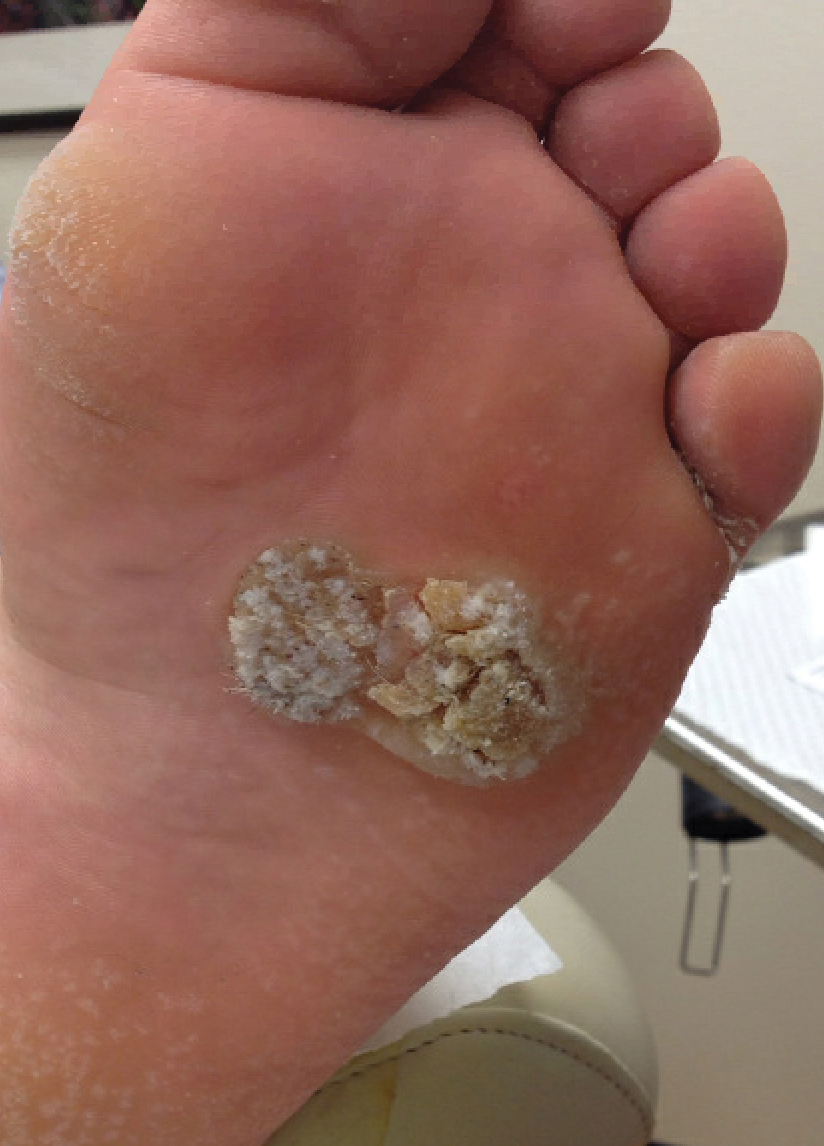
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
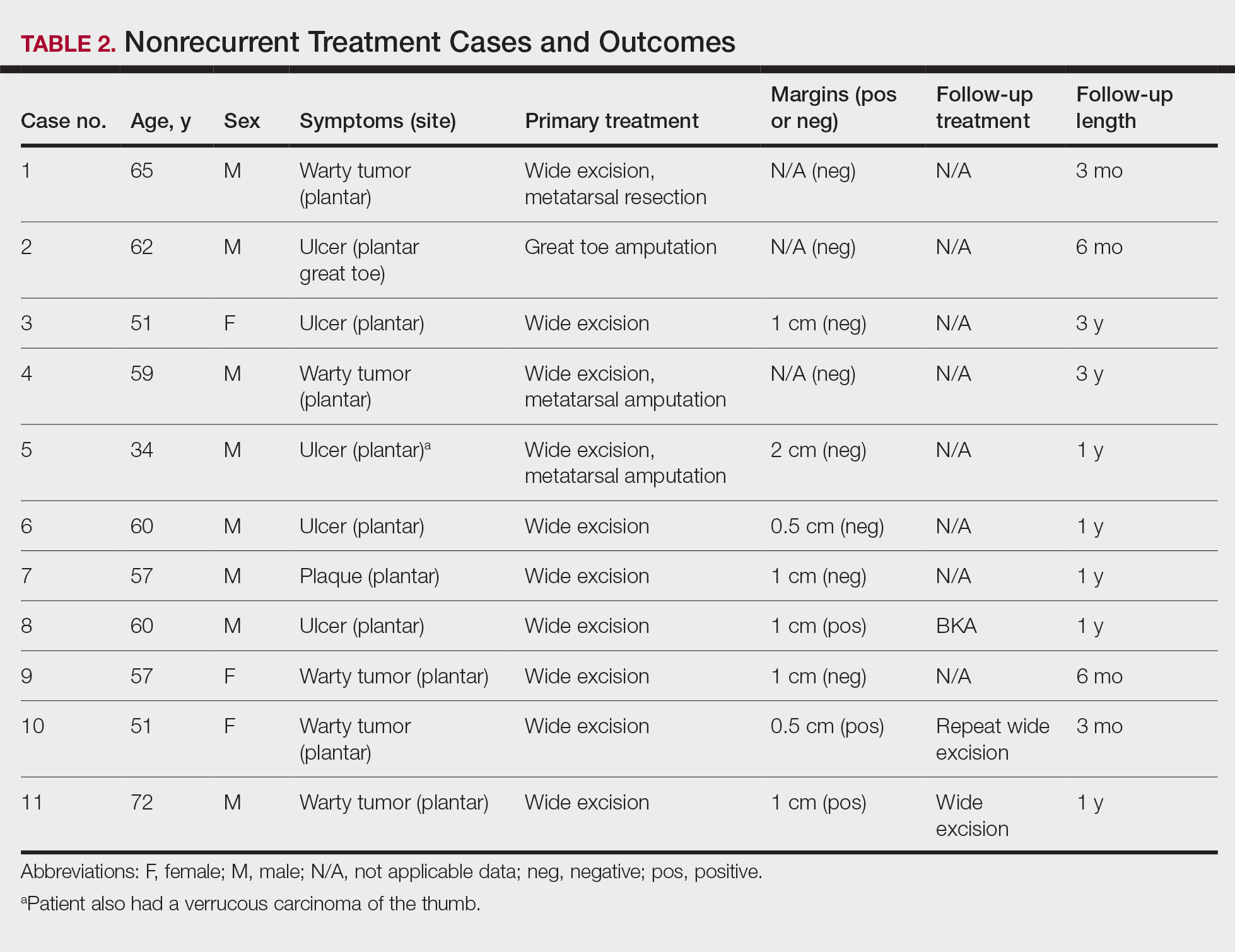
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
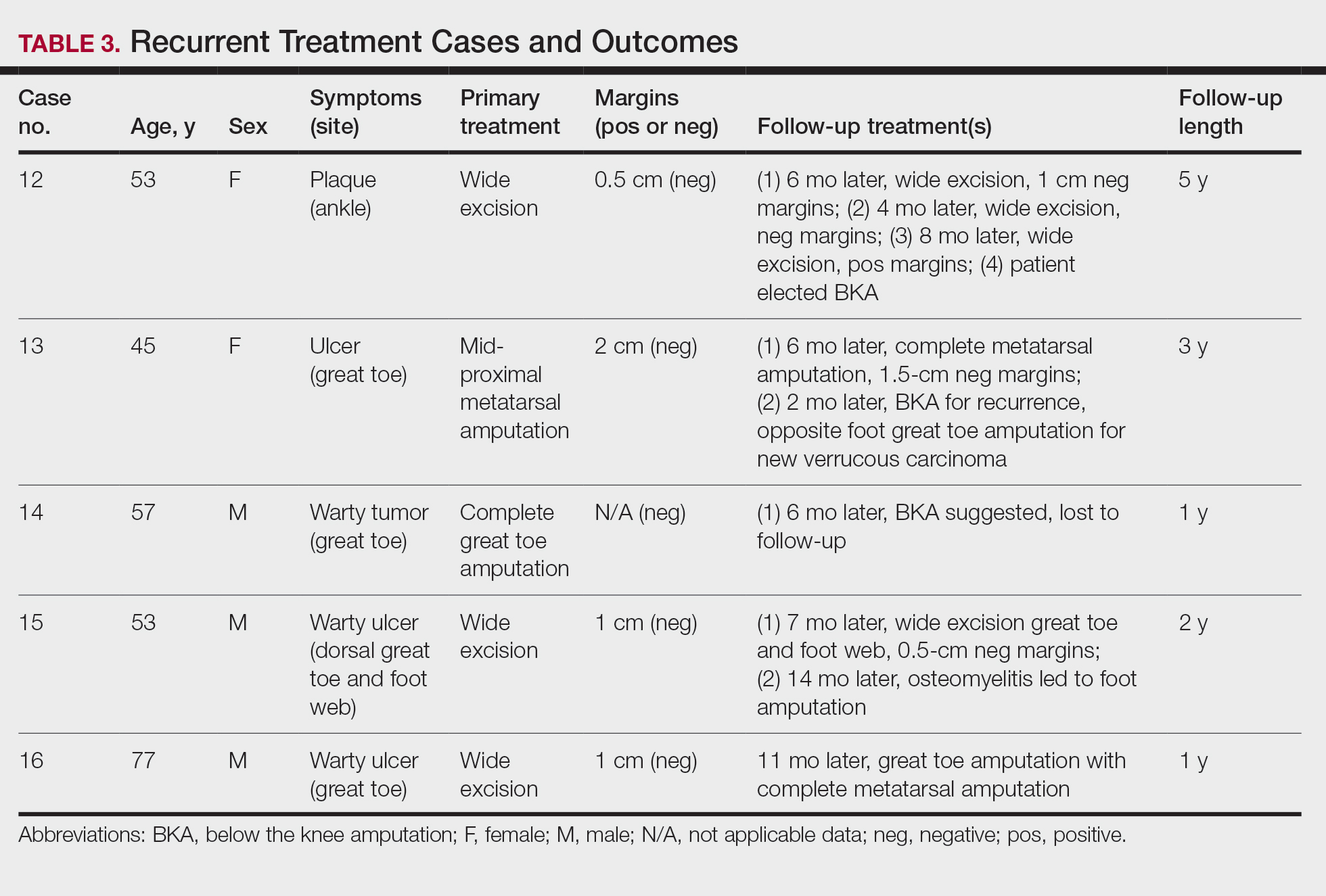
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
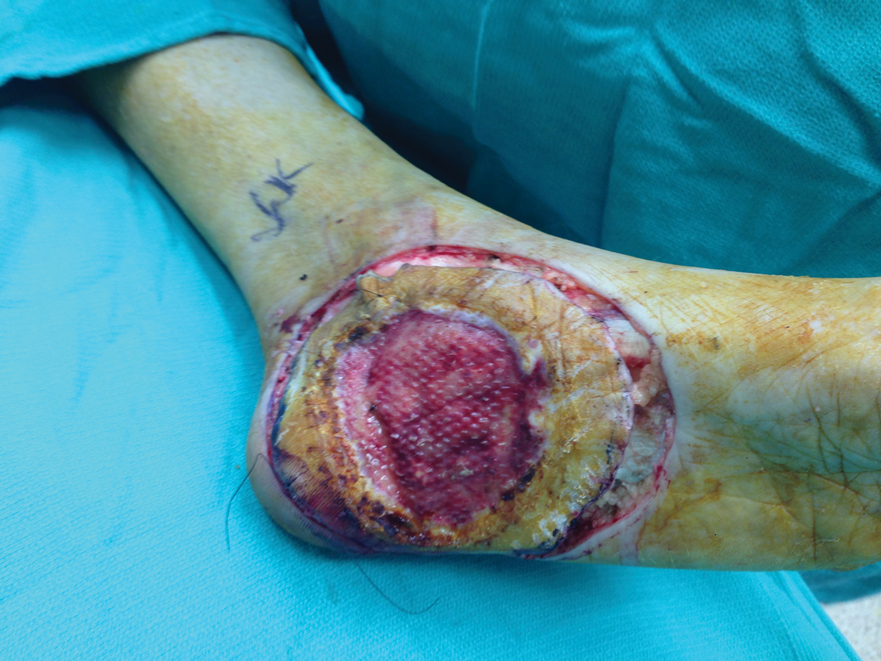
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
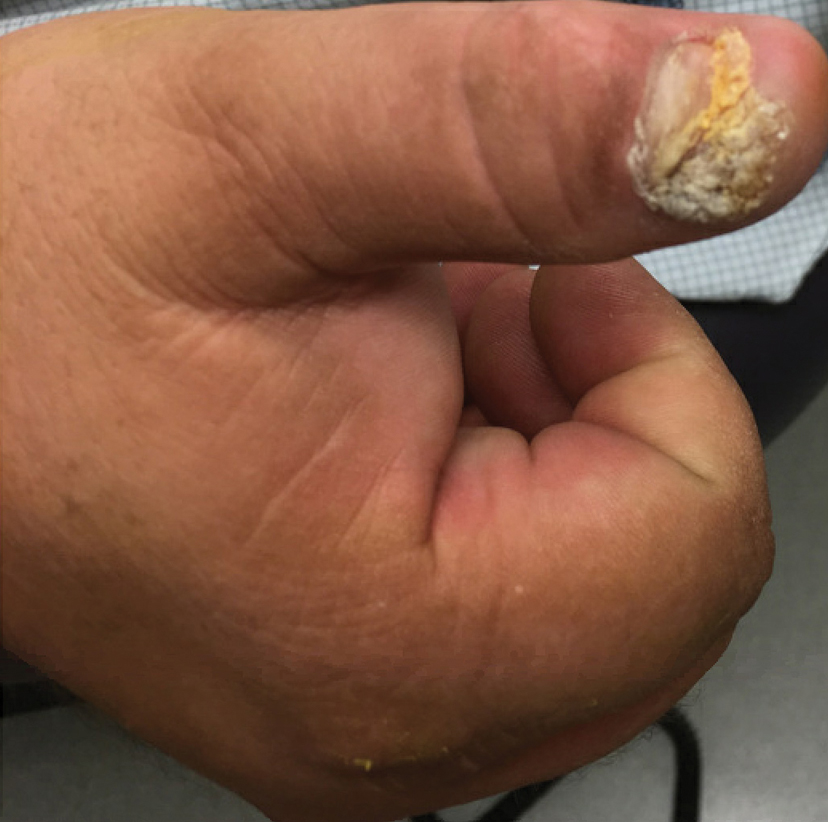
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.

Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).

Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.

Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation

Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).

The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.

Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.

Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.

Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).

Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.

Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation

Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).

The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.

Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.

Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Practice Points
- Clinicians should have a high suspicion for verrucous carcinoma in the setting of a chronic ulceration or warty lesion that is resistant to traditional treatment. Early biopsy with tissue collection of the raised ulcer borders and the deep dermis layer of warty lesions is imperative for diagnosis.
- Verrucous carcinoma originating on the nonglabrous surface of the foot may have a higher rate of recurrence often occurring within months of previous treatment. Patients presenting with nonhealing surgical sites in this area should be treated with a high level of suspicion for recurrence.
Obesity increasing the risk for cancer: It’s complicated
The link between obesity and cancer has increasingly been emphasized in public health messages, but is the current message correct?
“Being overweight or having obesity increases your risk of getting cancer,” warns the U.S. Centers for Disease Control and Prevention. It warns that overweight/obesity is “linked with a higher risk of getting 13 types of cancer ... [which] make up 40% of all cancers diagnosed in the United States each year.”
But that message, which is also promulgated by many cancer organizations, is based on data from observational studies, which have many limitations.
In addition, it found an inverse relationship for breast cancer, in which early-life obesity was associated with a reduced risk of breast cancer, and the relationship with obesity was “complicated” for lung and prostate cancer.
The study, headed by Zhe Fang, MBBS, Harvard T. H. Chan School of Public Health, Boston, Mass., was published in the Journal of the National Cancer Institute
“For a seemingly straightforward question of whether excessive body fatness causes cancer, the answer may not be straightforward after all,” writes Song Yao, PhD, professor of oncology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., in an accompanying editorial
“How to craft a simple public health message to convey the complexity and nuances of the relationships may be a challenge to be grappled with going forward,” he added.
In an interview, Dr. Yao said that it “really depends on what kind of message you want to get out.”
“If you want to talk about cancer overall, as one disease, we all know that a clear association with obesity does not exist,” he said. “It’s not that simple.”
“You really cannot say that obesity increases cancer risk overall,” he said.
For some cancers included in the study, Dr. Yao continued, it was “very clear that obesity increased the risk ... but for some other cancer types, we either don’t have enough data yet or the association is not as consistent.”
This, he said, is especially the case for prostate and lung cancer.
All of this indicates that there is a complex relationship between obesity and cancer risk, he maintains.
“We always think obesity is bad, not only for cancer but also for more common conditions, like hypertension, diabetes, and cardiovascular disease,” Dr. Yao noted. This points to the link between obesity and chronic inflammation, he added.
However, there are also other hypotheses, including synthesis of estrogen in adipose tissue, which may explain the link between obesity and breast cancer risk in older women.
However, in younger women, obesity protects against breast cancer, and “we really don’t know why,” Dr. Yao said.
The new study used Mendelian randomization to examine these relationships. This is a “new tool that we have developed over the past 20 years or so, largely because there is so much data coming from genome-wide association studies,” Dr. Yao explained.
It has “advantages” over other methods, including observational studies. One of its strengths is that it is “not impacted by reverse causality,” because genetic risk does not change over time.
However, he said, it is “quite straightforward to think that the genetics do not change, but at the same time, the environment we live in throughout our life course changes,” and the impact of genetic variants may be “washed out.”
How genetics influences cancer risk may therefore change over time, and it is a “dynamic process,” Dr. Yao commented.
In addition, this approach has its own limitations, he said, because it depends on how much of the variation in a given measure can be attributed to genetic factors.
New conclusions
In their study, Dr. Fang and colleagues reviewed 204 meta-analyses of 2,179 individual estimates from 507 cohort or case-control studies. They found “strong evidence” that supports the association between obesity and 11 cancers.
These are esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney.
They note, however, that the associations “may be causal for some malignancies” but that the co-occurrence of obesity with various cancer risk factors means that others may be “susceptible to potential confounding bias.”
To overcome some of these limitations, the team looked to Mendelian randomization studies that examined the association between genetic variants linked to body mass index (BMI), indicating lifetime risk of high BMI, and cancer risk for a range of cancer types.
These Mendelian randomization studies were then compared with the results of large-scale conventional observational studies, as well as with evidence in reports from the International Agency for Research on Cancer and the World Cancer Research Fund–American Institute of Cancer Research, which also include experimental studies.
The researchers say that, overall, the Mendelian randomization studies “further establish the causality of obesity” with six cancer types: colorectal, endometrial, ovarian, kidney, and pancreatic cancer, and esophageal adenocarcinoma.
In addition, these studies further establish the inverse relationship of early-life obesity with breast cancer.
However, the approach could not confirm a positive association between obesity and gallbladder and gastric cardia cancer, as well as multiple myeloma.
“This could be due to low power,” the team suggests, “and larger studies are required.”
With respect to lung cancer, the Mendelian randomization identified a positive association with obesity that supports the inverse association identified in observational studies, that is, that obesity may reduce the risk for lung cancer.
The researchers suggest this may reflect reverse causality related to the loss of lean body mass before diagnosis, as well as confounding by smoking.
For prostate cancer, the evidence was “conflicting” and “implies a complicated role of obesity,” Dr. Zhang and colleagues comment.
The link between obesity and lower prostate-specific antigen levels, they suggest, may result in a detection bias by masking the presence of prostate cancer, or it “could be biological” in origin, owing to reduced androgen levels.
For six cancer types for which a causal relationship with obesity could be established, the effect estimates from the Mendelian randomization studies were stronger than those seen in conventional studies, with the magnitude of risk ranging from 1.14-fold for early-life obesity and breast cancer to 1.37-fold for adult obesity and esophageal adenocarcinoma.
In another editorial accompanying the new study, Graham A. Colditz, MD, DrPH, from Washington University School of Medicine, St. Louis, underlined that childhood and adolescent obesity and their contribution to cancer risk need further attention.
“To reap the reward from past research, we must act to implement effective strategies to reduce childhood and adolescent adiposity, reduce excess weight gain in adult years, and maintain a healthy weight,” he writes.
“This will require us to change the way we live, but COVID-19 has shown we can make changes to how we live and work. Let us keep the changes we have already made, or take on new ones, that will cut our collective cancer toll,” he implores.
No funding for the study was described. Dr. Colditz is supported by the Breast Cancer Research Foundation. No other relevant financial relationships were described.
A version of this article first appeared on Medscape.com.
The link between obesity and cancer has increasingly been emphasized in public health messages, but is the current message correct?
“Being overweight or having obesity increases your risk of getting cancer,” warns the U.S. Centers for Disease Control and Prevention. It warns that overweight/obesity is “linked with a higher risk of getting 13 types of cancer ... [which] make up 40% of all cancers diagnosed in the United States each year.”
But that message, which is also promulgated by many cancer organizations, is based on data from observational studies, which have many limitations.
In addition, it found an inverse relationship for breast cancer, in which early-life obesity was associated with a reduced risk of breast cancer, and the relationship with obesity was “complicated” for lung and prostate cancer.
The study, headed by Zhe Fang, MBBS, Harvard T. H. Chan School of Public Health, Boston, Mass., was published in the Journal of the National Cancer Institute
“For a seemingly straightforward question of whether excessive body fatness causes cancer, the answer may not be straightforward after all,” writes Song Yao, PhD, professor of oncology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., in an accompanying editorial
“How to craft a simple public health message to convey the complexity and nuances of the relationships may be a challenge to be grappled with going forward,” he added.
In an interview, Dr. Yao said that it “really depends on what kind of message you want to get out.”
“If you want to talk about cancer overall, as one disease, we all know that a clear association with obesity does not exist,” he said. “It’s not that simple.”
“You really cannot say that obesity increases cancer risk overall,” he said.
For some cancers included in the study, Dr. Yao continued, it was “very clear that obesity increased the risk ... but for some other cancer types, we either don’t have enough data yet or the association is not as consistent.”
This, he said, is especially the case for prostate and lung cancer.
All of this indicates that there is a complex relationship between obesity and cancer risk, he maintains.
“We always think obesity is bad, not only for cancer but also for more common conditions, like hypertension, diabetes, and cardiovascular disease,” Dr. Yao noted. This points to the link between obesity and chronic inflammation, he added.
However, there are also other hypotheses, including synthesis of estrogen in adipose tissue, which may explain the link between obesity and breast cancer risk in older women.
However, in younger women, obesity protects against breast cancer, and “we really don’t know why,” Dr. Yao said.
The new study used Mendelian randomization to examine these relationships. This is a “new tool that we have developed over the past 20 years or so, largely because there is so much data coming from genome-wide association studies,” Dr. Yao explained.
It has “advantages” over other methods, including observational studies. One of its strengths is that it is “not impacted by reverse causality,” because genetic risk does not change over time.
However, he said, it is “quite straightforward to think that the genetics do not change, but at the same time, the environment we live in throughout our life course changes,” and the impact of genetic variants may be “washed out.”
How genetics influences cancer risk may therefore change over time, and it is a “dynamic process,” Dr. Yao commented.
In addition, this approach has its own limitations, he said, because it depends on how much of the variation in a given measure can be attributed to genetic factors.
New conclusions
In their study, Dr. Fang and colleagues reviewed 204 meta-analyses of 2,179 individual estimates from 507 cohort or case-control studies. They found “strong evidence” that supports the association between obesity and 11 cancers.
These are esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney.
They note, however, that the associations “may be causal for some malignancies” but that the co-occurrence of obesity with various cancer risk factors means that others may be “susceptible to potential confounding bias.”
To overcome some of these limitations, the team looked to Mendelian randomization studies that examined the association between genetic variants linked to body mass index (BMI), indicating lifetime risk of high BMI, and cancer risk for a range of cancer types.
These Mendelian randomization studies were then compared with the results of large-scale conventional observational studies, as well as with evidence in reports from the International Agency for Research on Cancer and the World Cancer Research Fund–American Institute of Cancer Research, which also include experimental studies.
The researchers say that, overall, the Mendelian randomization studies “further establish the causality of obesity” with six cancer types: colorectal, endometrial, ovarian, kidney, and pancreatic cancer, and esophageal adenocarcinoma.
In addition, these studies further establish the inverse relationship of early-life obesity with breast cancer.
However, the approach could not confirm a positive association between obesity and gallbladder and gastric cardia cancer, as well as multiple myeloma.
“This could be due to low power,” the team suggests, “and larger studies are required.”
With respect to lung cancer, the Mendelian randomization identified a positive association with obesity that supports the inverse association identified in observational studies, that is, that obesity may reduce the risk for lung cancer.
The researchers suggest this may reflect reverse causality related to the loss of lean body mass before diagnosis, as well as confounding by smoking.
For prostate cancer, the evidence was “conflicting” and “implies a complicated role of obesity,” Dr. Zhang and colleagues comment.
The link between obesity and lower prostate-specific antigen levels, they suggest, may result in a detection bias by masking the presence of prostate cancer, or it “could be biological” in origin, owing to reduced androgen levels.
For six cancer types for which a causal relationship with obesity could be established, the effect estimates from the Mendelian randomization studies were stronger than those seen in conventional studies, with the magnitude of risk ranging from 1.14-fold for early-life obesity and breast cancer to 1.37-fold for adult obesity and esophageal adenocarcinoma.
In another editorial accompanying the new study, Graham A. Colditz, MD, DrPH, from Washington University School of Medicine, St. Louis, underlined that childhood and adolescent obesity and their contribution to cancer risk need further attention.
“To reap the reward from past research, we must act to implement effective strategies to reduce childhood and adolescent adiposity, reduce excess weight gain in adult years, and maintain a healthy weight,” he writes.
“This will require us to change the way we live, but COVID-19 has shown we can make changes to how we live and work. Let us keep the changes we have already made, or take on new ones, that will cut our collective cancer toll,” he implores.
No funding for the study was described. Dr. Colditz is supported by the Breast Cancer Research Foundation. No other relevant financial relationships were described.
A version of this article first appeared on Medscape.com.
The link between obesity and cancer has increasingly been emphasized in public health messages, but is the current message correct?
“Being overweight or having obesity increases your risk of getting cancer,” warns the U.S. Centers for Disease Control and Prevention. It warns that overweight/obesity is “linked with a higher risk of getting 13 types of cancer ... [which] make up 40% of all cancers diagnosed in the United States each year.”
But that message, which is also promulgated by many cancer organizations, is based on data from observational studies, which have many limitations.
In addition, it found an inverse relationship for breast cancer, in which early-life obesity was associated with a reduced risk of breast cancer, and the relationship with obesity was “complicated” for lung and prostate cancer.
The study, headed by Zhe Fang, MBBS, Harvard T. H. Chan School of Public Health, Boston, Mass., was published in the Journal of the National Cancer Institute
“For a seemingly straightforward question of whether excessive body fatness causes cancer, the answer may not be straightforward after all,” writes Song Yao, PhD, professor of oncology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., in an accompanying editorial
“How to craft a simple public health message to convey the complexity and nuances of the relationships may be a challenge to be grappled with going forward,” he added.
In an interview, Dr. Yao said that it “really depends on what kind of message you want to get out.”
“If you want to talk about cancer overall, as one disease, we all know that a clear association with obesity does not exist,” he said. “It’s not that simple.”
“You really cannot say that obesity increases cancer risk overall,” he said.
For some cancers included in the study, Dr. Yao continued, it was “very clear that obesity increased the risk ... but for some other cancer types, we either don’t have enough data yet or the association is not as consistent.”
This, he said, is especially the case for prostate and lung cancer.
All of this indicates that there is a complex relationship between obesity and cancer risk, he maintains.
“We always think obesity is bad, not only for cancer but also for more common conditions, like hypertension, diabetes, and cardiovascular disease,” Dr. Yao noted. This points to the link between obesity and chronic inflammation, he added.
However, there are also other hypotheses, including synthesis of estrogen in adipose tissue, which may explain the link between obesity and breast cancer risk in older women.
However, in younger women, obesity protects against breast cancer, and “we really don’t know why,” Dr. Yao said.
The new study used Mendelian randomization to examine these relationships. This is a “new tool that we have developed over the past 20 years or so, largely because there is so much data coming from genome-wide association studies,” Dr. Yao explained.
It has “advantages” over other methods, including observational studies. One of its strengths is that it is “not impacted by reverse causality,” because genetic risk does not change over time.
However, he said, it is “quite straightforward to think that the genetics do not change, but at the same time, the environment we live in throughout our life course changes,” and the impact of genetic variants may be “washed out.”
How genetics influences cancer risk may therefore change over time, and it is a “dynamic process,” Dr. Yao commented.
In addition, this approach has its own limitations, he said, because it depends on how much of the variation in a given measure can be attributed to genetic factors.
New conclusions
In their study, Dr. Fang and colleagues reviewed 204 meta-analyses of 2,179 individual estimates from 507 cohort or case-control studies. They found “strong evidence” that supports the association between obesity and 11 cancers.
These are esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney.
They note, however, that the associations “may be causal for some malignancies” but that the co-occurrence of obesity with various cancer risk factors means that others may be “susceptible to potential confounding bias.”
To overcome some of these limitations, the team looked to Mendelian randomization studies that examined the association between genetic variants linked to body mass index (BMI), indicating lifetime risk of high BMI, and cancer risk for a range of cancer types.
These Mendelian randomization studies were then compared with the results of large-scale conventional observational studies, as well as with evidence in reports from the International Agency for Research on Cancer and the World Cancer Research Fund–American Institute of Cancer Research, which also include experimental studies.
The researchers say that, overall, the Mendelian randomization studies “further establish the causality of obesity” with six cancer types: colorectal, endometrial, ovarian, kidney, and pancreatic cancer, and esophageal adenocarcinoma.
In addition, these studies further establish the inverse relationship of early-life obesity with breast cancer.
However, the approach could not confirm a positive association between obesity and gallbladder and gastric cardia cancer, as well as multiple myeloma.
“This could be due to low power,” the team suggests, “and larger studies are required.”
With respect to lung cancer, the Mendelian randomization identified a positive association with obesity that supports the inverse association identified in observational studies, that is, that obesity may reduce the risk for lung cancer.
The researchers suggest this may reflect reverse causality related to the loss of lean body mass before diagnosis, as well as confounding by smoking.
For prostate cancer, the evidence was “conflicting” and “implies a complicated role of obesity,” Dr. Zhang and colleagues comment.
The link between obesity and lower prostate-specific antigen levels, they suggest, may result in a detection bias by masking the presence of prostate cancer, or it “could be biological” in origin, owing to reduced androgen levels.
For six cancer types for which a causal relationship with obesity could be established, the effect estimates from the Mendelian randomization studies were stronger than those seen in conventional studies, with the magnitude of risk ranging from 1.14-fold for early-life obesity and breast cancer to 1.37-fold for adult obesity and esophageal adenocarcinoma.
In another editorial accompanying the new study, Graham A. Colditz, MD, DrPH, from Washington University School of Medicine, St. Louis, underlined that childhood and adolescent obesity and their contribution to cancer risk need further attention.
“To reap the reward from past research, we must act to implement effective strategies to reduce childhood and adolescent adiposity, reduce excess weight gain in adult years, and maintain a healthy weight,” he writes.
“This will require us to change the way we live, but COVID-19 has shown we can make changes to how we live and work. Let us keep the changes we have already made, or take on new ones, that will cut our collective cancer toll,” he implores.
No funding for the study was described. Dr. Colditz is supported by the Breast Cancer Research Foundation. No other relevant financial relationships were described.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Diet’s impact on the microbiome: It’s real, and broad
WASHINGTON – – including amino acid metabolites – that may modify health, said Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia.
During the 2022 Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility, Dr. Wu led a plenary session in which the impact of diet on the microbiome was characterized as important, rapid, personalized, likely modest relative to other contributing ecological factors, influenced by the process of cooking, and exceedingly difficult to tease apart and characterize in human studies.
In a human study published in 2021, Dr. Wu and coinvestigators performed a controlled feeding experiment with 30 healthy volunteers, randomizing them to several weeks of a vegan diet, an omnivore diet (a typical American diet), and an exclusive enteral nutrition diet (EEN) devoid of dietary fiber.
They compared the composition and metabolic function of the gut microbiome during three phases: an initial dietary phase (days 1-5), a purge phase in which antibiotics and polyethylene glycol were administered to transiently reduce bacterial load in the gut (days 6-8), and a recovery phase (days 9-15).
Diversity of the gut microbiota recovered from the purge phase in both vegans and omnivores, but not in those receiving EEN. “The EEN diet was having a profound effect on the [short-term] recovery of microbiota,” said Dr. Wu, the Ferdinand G. Weisbrod Professor in Gastroenterology, in describing the Food And Resulting Microbial Metabolites study.
Using genetic sequencing, microbial culturing, and bioinformatics processing, the researchers also determined that EEN subsequently led to metabolites that were distinct from omnivores and vegans. Unexpectedly, bacterial metabolites of amino acid origin – not only carbohydrate origin – were altered in the EEN group, suggesting a broad impact of dietary fiber on the bacterial metabolome. EEN-induced alterations in the microbiome and metabolome resolved after the study period, he noted.
In other words, “depriving or supplying the gut microbiome with one dietary component (i.e., fiber) can directly impact metabolites of an unrelated portion of the diet (i.e., amino acids) via the induction of specific gut bacterial taxa,” Dr. Wu and colleagues wrote.
Clinically, the results as a whole suggest that the combination of antibiotics with EEN may be less effective in patients with Crohn’s disease than EEN alone, and can be potentially harmful, they said.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Wu said that, for patients in the ICU on EEN treatment and antibiotics, “we do need to think carefully about microbiota reconstitution because it could have a very significant effect not only on short-chain fatty acid metabolites but on amino acid metabolites that may be good or bad in the setting of disease.”
The scientific rationale for the effectiveness of EEN for IBD is still not well understood, he noted. “All I can say is that EEN works in IBD, but there are aspects about the microbiota and diet and IBD that we don’t understand.”
Dietary impact through the immigration lens
In another presentation, Dan Knights, PhD, of the University of Minnesota, Minneapolis, described his lab’s findings on the association of U.S. immigration with loss of gut microbiome diversity, and the role of diet.
As part of the Immigration Microbiome Project reported several years ago, his team collected stool, dietary recalls, and anthropometrics from 550 Hmong and Karen individuals living in Thailand and the United States, including first- and second-generation immigrants, as well as some U.S.-born European American individuals. They found that the gut microbiome of immigrants changed within months of arriving in the United States, and that immigration status had a stronger effect on the microbiome than obesity status.
“By the time people were in their second generation, their microbiomes were roughly on the same order of diversity as U.S. controls,” said Dr. Knights, associate professor in the Biotechnology Institute and the department of computer science and engineering.
Dietary changes only partly explained microbiome variation, however. “By the second generation, the microbiome tended to be fully Westernized, but the diet was only partly Westernized,” he said. “Diet is only part of the story.”
Other research from his lab, including one study that performed daily fecal shotgun sequencing on 34 people, has found that effects of diet on the microbiome are likely to be observable within days, and that microbial responses to food are highly personalized. Diet appears to explain about 6% of the daily microbiome variation within an individual, and “an average diet explains about 4% of microbiome variation between people,” Dr. Knights said.
The impact of cooking
“The gut microbiota responds to food and to its form,” said Rachel N. Carmody, PhD, of the department of human evolutionary biology at Harvard University, Cambridge, during the plenary session. Her research has shown that, in mice, a plant diet served raw versus cooked quickly reshaped the gut microbiome and disrupted gut microbial physiology. Notably, shifts in gut microbiota modulated host energy status – one of the many areas that begs further research.
The effects of cooking have also been detectable in human pilot studies. “We saw different changes in the microbiome when [study participants] were eating the same plant items either raw or cooked,” she said. “Some microbes were affected only on the raw diet, other were affected only on the cooked diet.”
Other research in animal models and humans has demonstrated a significant amount of plasticity in the microbiome in response to diet. “In mice you get the microbiome signatures to shift within 24 hours by feeding them a new diet,” Dr. Carmody said.
In an interview about the plenary session, Eugene B. Chang, MD, the Martin Boyer Distinguished Professor of Medicine at the University of Chicago, said that he was struck both by the resiliency of individual gut microbiomes overall and by findings that, “in animal models where conditions and diets can be carefully controlled, diet and environment are major drivers of gut microbial membership and function.”
Dr. Chang co-led a separate workshop on “defining a healthy gut microbiome” – a task that he said remains “a challenge [and is not yet] resolved, at least with general consensus.”
Dr. Chang, Dr. Wu, and Dr. Carmody reported no relevant disclosures. Dr. Knights disclosed that he is a paid adviser to Diversigen, a company involved with the commercialization of microbiome analysis.
The 2022 Gut Microbiota for Health World Summit was supported by sponsorships from Danone, Ferring Pharmaceuticals, Aimmune Therapeutics and Seres Therapeutics, Sanofi, and Intrinsic Medicine Inc. with additional support from educational grants provided by Ferring Pharmaceuticals and Salix Pharmaceuticals.
This article was updated 4/5/22.
WASHINGTON – – including amino acid metabolites – that may modify health, said Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia.
During the 2022 Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility, Dr. Wu led a plenary session in which the impact of diet on the microbiome was characterized as important, rapid, personalized, likely modest relative to other contributing ecological factors, influenced by the process of cooking, and exceedingly difficult to tease apart and characterize in human studies.
In a human study published in 2021, Dr. Wu and coinvestigators performed a controlled feeding experiment with 30 healthy volunteers, randomizing them to several weeks of a vegan diet, an omnivore diet (a typical American diet), and an exclusive enteral nutrition diet (EEN) devoid of dietary fiber.
They compared the composition and metabolic function of the gut microbiome during three phases: an initial dietary phase (days 1-5), a purge phase in which antibiotics and polyethylene glycol were administered to transiently reduce bacterial load in the gut (days 6-8), and a recovery phase (days 9-15).
Diversity of the gut microbiota recovered from the purge phase in both vegans and omnivores, but not in those receiving EEN. “The EEN diet was having a profound effect on the [short-term] recovery of microbiota,” said Dr. Wu, the Ferdinand G. Weisbrod Professor in Gastroenterology, in describing the Food And Resulting Microbial Metabolites study.
Using genetic sequencing, microbial culturing, and bioinformatics processing, the researchers also determined that EEN subsequently led to metabolites that were distinct from omnivores and vegans. Unexpectedly, bacterial metabolites of amino acid origin – not only carbohydrate origin – were altered in the EEN group, suggesting a broad impact of dietary fiber on the bacterial metabolome. EEN-induced alterations in the microbiome and metabolome resolved after the study period, he noted.
In other words, “depriving or supplying the gut microbiome with one dietary component (i.e., fiber) can directly impact metabolites of an unrelated portion of the diet (i.e., amino acids) via the induction of specific gut bacterial taxa,” Dr. Wu and colleagues wrote.
Clinically, the results as a whole suggest that the combination of antibiotics with EEN may be less effective in patients with Crohn’s disease than EEN alone, and can be potentially harmful, they said.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Wu said that, for patients in the ICU on EEN treatment and antibiotics, “we do need to think carefully about microbiota reconstitution because it could have a very significant effect not only on short-chain fatty acid metabolites but on amino acid metabolites that may be good or bad in the setting of disease.”
The scientific rationale for the effectiveness of EEN for IBD is still not well understood, he noted. “All I can say is that EEN works in IBD, but there are aspects about the microbiota and diet and IBD that we don’t understand.”
Dietary impact through the immigration lens
In another presentation, Dan Knights, PhD, of the University of Minnesota, Minneapolis, described his lab’s findings on the association of U.S. immigration with loss of gut microbiome diversity, and the role of diet.
As part of the Immigration Microbiome Project reported several years ago, his team collected stool, dietary recalls, and anthropometrics from 550 Hmong and Karen individuals living in Thailand and the United States, including first- and second-generation immigrants, as well as some U.S.-born European American individuals. They found that the gut microbiome of immigrants changed within months of arriving in the United States, and that immigration status had a stronger effect on the microbiome than obesity status.
“By the time people were in their second generation, their microbiomes were roughly on the same order of diversity as U.S. controls,” said Dr. Knights, associate professor in the Biotechnology Institute and the department of computer science and engineering.
Dietary changes only partly explained microbiome variation, however. “By the second generation, the microbiome tended to be fully Westernized, but the diet was only partly Westernized,” he said. “Diet is only part of the story.”
Other research from his lab, including one study that performed daily fecal shotgun sequencing on 34 people, has found that effects of diet on the microbiome are likely to be observable within days, and that microbial responses to food are highly personalized. Diet appears to explain about 6% of the daily microbiome variation within an individual, and “an average diet explains about 4% of microbiome variation between people,” Dr. Knights said.
The impact of cooking
“The gut microbiota responds to food and to its form,” said Rachel N. Carmody, PhD, of the department of human evolutionary biology at Harvard University, Cambridge, during the plenary session. Her research has shown that, in mice, a plant diet served raw versus cooked quickly reshaped the gut microbiome and disrupted gut microbial physiology. Notably, shifts in gut microbiota modulated host energy status – one of the many areas that begs further research.
The effects of cooking have also been detectable in human pilot studies. “We saw different changes in the microbiome when [study participants] were eating the same plant items either raw or cooked,” she said. “Some microbes were affected only on the raw diet, other were affected only on the cooked diet.”
Other research in animal models and humans has demonstrated a significant amount of plasticity in the microbiome in response to diet. “In mice you get the microbiome signatures to shift within 24 hours by feeding them a new diet,” Dr. Carmody said.
In an interview about the plenary session, Eugene B. Chang, MD, the Martin Boyer Distinguished Professor of Medicine at the University of Chicago, said that he was struck both by the resiliency of individual gut microbiomes overall and by findings that, “in animal models where conditions and diets can be carefully controlled, diet and environment are major drivers of gut microbial membership and function.”
Dr. Chang co-led a separate workshop on “defining a healthy gut microbiome” – a task that he said remains “a challenge [and is not yet] resolved, at least with general consensus.”
Dr. Chang, Dr. Wu, and Dr. Carmody reported no relevant disclosures. Dr. Knights disclosed that he is a paid adviser to Diversigen, a company involved with the commercialization of microbiome analysis.
The 2022 Gut Microbiota for Health World Summit was supported by sponsorships from Danone, Ferring Pharmaceuticals, Aimmune Therapeutics and Seres Therapeutics, Sanofi, and Intrinsic Medicine Inc. with additional support from educational grants provided by Ferring Pharmaceuticals and Salix Pharmaceuticals.
This article was updated 4/5/22.
WASHINGTON – – including amino acid metabolites – that may modify health, said Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia.
During the 2022 Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility, Dr. Wu led a plenary session in which the impact of diet on the microbiome was characterized as important, rapid, personalized, likely modest relative to other contributing ecological factors, influenced by the process of cooking, and exceedingly difficult to tease apart and characterize in human studies.
In a human study published in 2021, Dr. Wu and coinvestigators performed a controlled feeding experiment with 30 healthy volunteers, randomizing them to several weeks of a vegan diet, an omnivore diet (a typical American diet), and an exclusive enteral nutrition diet (EEN) devoid of dietary fiber.
They compared the composition and metabolic function of the gut microbiome during three phases: an initial dietary phase (days 1-5), a purge phase in which antibiotics and polyethylene glycol were administered to transiently reduce bacterial load in the gut (days 6-8), and a recovery phase (days 9-15).
Diversity of the gut microbiota recovered from the purge phase in both vegans and omnivores, but not in those receiving EEN. “The EEN diet was having a profound effect on the [short-term] recovery of microbiota,” said Dr. Wu, the Ferdinand G. Weisbrod Professor in Gastroenterology, in describing the Food And Resulting Microbial Metabolites study.
Using genetic sequencing, microbial culturing, and bioinformatics processing, the researchers also determined that EEN subsequently led to metabolites that were distinct from omnivores and vegans. Unexpectedly, bacterial metabolites of amino acid origin – not only carbohydrate origin – were altered in the EEN group, suggesting a broad impact of dietary fiber on the bacterial metabolome. EEN-induced alterations in the microbiome and metabolome resolved after the study period, he noted.
In other words, “depriving or supplying the gut microbiome with one dietary component (i.e., fiber) can directly impact metabolites of an unrelated portion of the diet (i.e., amino acids) via the induction of specific gut bacterial taxa,” Dr. Wu and colleagues wrote.
Clinically, the results as a whole suggest that the combination of antibiotics with EEN may be less effective in patients with Crohn’s disease than EEN alone, and can be potentially harmful, they said.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Wu said that, for patients in the ICU on EEN treatment and antibiotics, “we do need to think carefully about microbiota reconstitution because it could have a very significant effect not only on short-chain fatty acid metabolites but on amino acid metabolites that may be good or bad in the setting of disease.”
The scientific rationale for the effectiveness of EEN for IBD is still not well understood, he noted. “All I can say is that EEN works in IBD, but there are aspects about the microbiota and diet and IBD that we don’t understand.”
Dietary impact through the immigration lens
In another presentation, Dan Knights, PhD, of the University of Minnesota, Minneapolis, described his lab’s findings on the association of U.S. immigration with loss of gut microbiome diversity, and the role of diet.
As part of the Immigration Microbiome Project reported several years ago, his team collected stool, dietary recalls, and anthropometrics from 550 Hmong and Karen individuals living in Thailand and the United States, including first- and second-generation immigrants, as well as some U.S.-born European American individuals. They found that the gut microbiome of immigrants changed within months of arriving in the United States, and that immigration status had a stronger effect on the microbiome than obesity status.
“By the time people were in their second generation, their microbiomes were roughly on the same order of diversity as U.S. controls,” said Dr. Knights, associate professor in the Biotechnology Institute and the department of computer science and engineering.
Dietary changes only partly explained microbiome variation, however. “By the second generation, the microbiome tended to be fully Westernized, but the diet was only partly Westernized,” he said. “Diet is only part of the story.”
Other research from his lab, including one study that performed daily fecal shotgun sequencing on 34 people, has found that effects of diet on the microbiome are likely to be observable within days, and that microbial responses to food are highly personalized. Diet appears to explain about 6% of the daily microbiome variation within an individual, and “an average diet explains about 4% of microbiome variation between people,” Dr. Knights said.
The impact of cooking
“The gut microbiota responds to food and to its form,” said Rachel N. Carmody, PhD, of the department of human evolutionary biology at Harvard University, Cambridge, during the plenary session. Her research has shown that, in mice, a plant diet served raw versus cooked quickly reshaped the gut microbiome and disrupted gut microbial physiology. Notably, shifts in gut microbiota modulated host energy status – one of the many areas that begs further research.
The effects of cooking have also been detectable in human pilot studies. “We saw different changes in the microbiome when [study participants] were eating the same plant items either raw or cooked,” she said. “Some microbes were affected only on the raw diet, other were affected only on the cooked diet.”
Other research in animal models and humans has demonstrated a significant amount of plasticity in the microbiome in response to diet. “In mice you get the microbiome signatures to shift within 24 hours by feeding them a new diet,” Dr. Carmody said.
In an interview about the plenary session, Eugene B. Chang, MD, the Martin Boyer Distinguished Professor of Medicine at the University of Chicago, said that he was struck both by the resiliency of individual gut microbiomes overall and by findings that, “in animal models where conditions and diets can be carefully controlled, diet and environment are major drivers of gut microbial membership and function.”
Dr. Chang co-led a separate workshop on “defining a healthy gut microbiome” – a task that he said remains “a challenge [and is not yet] resolved, at least with general consensus.”
Dr. Chang, Dr. Wu, and Dr. Carmody reported no relevant disclosures. Dr. Knights disclosed that he is a paid adviser to Diversigen, a company involved with the commercialization of microbiome analysis.
The 2022 Gut Microbiota for Health World Summit was supported by sponsorships from Danone, Ferring Pharmaceuticals, Aimmune Therapeutics and Seres Therapeutics, Sanofi, and Intrinsic Medicine Inc. with additional support from educational grants provided by Ferring Pharmaceuticals and Salix Pharmaceuticals.
This article was updated 4/5/22.
AT GMFH 2022
Science says this is the ‘most boring person in the world’
Apologies up front to anyone who spends their weekends bird-watching or doing math for fun. They are among the people expected to be boring, based on stereotypes about what they do for work or how they spend their spare time, new research reveals.
Researchers surveyed more than 500 people across five related experiments to identify what people perceive as the most boring jobs, traits, and hobbies. They also report how we could all be missing out by spending as little time as possible with our tax consultant, accountant, or financial adviser outside of work.
People who work in banking, finance, accounting, data analytics, and cleaning topped the most boring list in the study, published earlier this month in Personality and Social Psychology Bulletin.
Sleeping, religion, watching television, observing animals, and spending spare time on mathematics were the stereotypical most boring hobbies and activities. The “observing animals” group includes people who bird-watch or study ants.
On the flip side, the top five most exciting jobs, in order, were in the performing arts, science, journalism, health professions, and teaching.
The researchers also looked at the how likely people are to avoid spending time with stereotypical dullards.
“Are people who are stereotyped as being boring avoided, if possible? Our current research shows that this is likely,” says Wijnand A.P. Van Tilburg, PhD, one of the researchers who did the study.
Beyond specific traits and stereotypes, Dr. Van Tilburg and colleagues found that boring people are seen as lacking skills and warmth.
“To our surprise, it appears that they are seen as both unfriendly and incompetent,” says Dr. Van Tilburg, an experimental social psychologist at the University of Essex in the United Kingdom.
What qualities do people most often ascribe to boring people? Besides being “dull,” “dry,” “bland,” and “not interesting,” common stereotypes include thinking someone who is likely boring will have no sense of humor, lack opinions, or complain.
The people surveyed also were more likely to place boring people in towns and small cities rather than large metropolitan areas.
A vicious cycle?
What’s the possible harm of relying on boring stereotypes? If people are stereotyped as being boring solely based on professions and hobbies, “then that suggests that they will incur the negative consequences associated with being a stereotypically boring person -- even when others haven’t actually interacted with them,” Dr. Van Tilburg says.
“Having a stereotypically boring profession or hobby may come with the inability to prove the biased perceivers wrong,” he says.
So making distinctions between stereotypes and realities is important, Dr. Van Tilburg says. “Those who have hobbies or occupations that are stereotypically boring do, of course, not actually have to be boring.”
Mark Leary, PhD, a professor in the department of psychology and neuroscience at Duke University in Durham, N.C., agrees. “The research actually dealt with stereotypes about the kinds of people who hold certain jobs, have certain hobbies, and live in certain places -- and not about boring people per se,” he says.
Dr. Leary points out that few people encounter bankers, tax experts, and others perceived as most boring outside a professional setting.
“When we have interactions with data analysts, accountants, insurance agents, and bankers, for example, those interactions are often boring not because the people are boring, but rather because the context is not interesting.”
To get past the preconceptions, “the best advice might be to get people to try to separate people from their roles when forming impressions of them.”
“We need to recognize that many of our interactions with other people are tied up in particular roles and topics and, thus, don’t reveal much about the other people themselves,” Dr. Leary says. “Maybe my accountant is the life of the party in other contexts.”
Dollars to avoid the dull?
The researchers also found that as the perception of how boring a person is increased, people were more likely to say they would avoid them.
To find a way to measure this avoidance, they asked people in the study how much money they would have to be paid to pal around with a perceived bore for 1 to 7 days. The payments people said they would need varied by perceptions that their boredom would be low, intermediate, or high.
As an example, they would require an average of $50 to spend one day with a highly boring person. That amount would double to $100 to spend almost 4 days in their company, and up to $230 for the week.
Dr. Leary says boredom happens when people try to pay attention to an experience or event. This means boredom goes beyond simple disinterest or trying to pay attention to someone that is not “intrinsically captivating.” When it takes more brain power to pay attention, you’ll perceive the experience as even more boring.
“Perhaps the best way to see if other people are actually boring is to talk about interesting things and see how they respond,” Dr. Leary says. “But, be careful: The topics you think make interesting conversations may be boring to others.”
A version of this article first appeared on WebMD.com.
Apologies up front to anyone who spends their weekends bird-watching or doing math for fun. They are among the people expected to be boring, based on stereotypes about what they do for work or how they spend their spare time, new research reveals.
Researchers surveyed more than 500 people across five related experiments to identify what people perceive as the most boring jobs, traits, and hobbies. They also report how we could all be missing out by spending as little time as possible with our tax consultant, accountant, or financial adviser outside of work.
People who work in banking, finance, accounting, data analytics, and cleaning topped the most boring list in the study, published earlier this month in Personality and Social Psychology Bulletin.
Sleeping, religion, watching television, observing animals, and spending spare time on mathematics were the stereotypical most boring hobbies and activities. The “observing animals” group includes people who bird-watch or study ants.
On the flip side, the top five most exciting jobs, in order, were in the performing arts, science, journalism, health professions, and teaching.
The researchers also looked at the how likely people are to avoid spending time with stereotypical dullards.
“Are people who are stereotyped as being boring avoided, if possible? Our current research shows that this is likely,” says Wijnand A.P. Van Tilburg, PhD, one of the researchers who did the study.
Beyond specific traits and stereotypes, Dr. Van Tilburg and colleagues found that boring people are seen as lacking skills and warmth.
“To our surprise, it appears that they are seen as both unfriendly and incompetent,” says Dr. Van Tilburg, an experimental social psychologist at the University of Essex in the United Kingdom.
What qualities do people most often ascribe to boring people? Besides being “dull,” “dry,” “bland,” and “not interesting,” common stereotypes include thinking someone who is likely boring will have no sense of humor, lack opinions, or complain.
The people surveyed also were more likely to place boring people in towns and small cities rather than large metropolitan areas.
A vicious cycle?
What’s the possible harm of relying on boring stereotypes? If people are stereotyped as being boring solely based on professions and hobbies, “then that suggests that they will incur the negative consequences associated with being a stereotypically boring person -- even when others haven’t actually interacted with them,” Dr. Van Tilburg says.
“Having a stereotypically boring profession or hobby may come with the inability to prove the biased perceivers wrong,” he says.
So making distinctions between stereotypes and realities is important, Dr. Van Tilburg says. “Those who have hobbies or occupations that are stereotypically boring do, of course, not actually have to be boring.”
Mark Leary, PhD, a professor in the department of psychology and neuroscience at Duke University in Durham, N.C., agrees. “The research actually dealt with stereotypes about the kinds of people who hold certain jobs, have certain hobbies, and live in certain places -- and not about boring people per se,” he says.
Dr. Leary points out that few people encounter bankers, tax experts, and others perceived as most boring outside a professional setting.
“When we have interactions with data analysts, accountants, insurance agents, and bankers, for example, those interactions are often boring not because the people are boring, but rather because the context is not interesting.”
To get past the preconceptions, “the best advice might be to get people to try to separate people from their roles when forming impressions of them.”
“We need to recognize that many of our interactions with other people are tied up in particular roles and topics and, thus, don’t reveal much about the other people themselves,” Dr. Leary says. “Maybe my accountant is the life of the party in other contexts.”
Dollars to avoid the dull?
The researchers also found that as the perception of how boring a person is increased, people were more likely to say they would avoid them.
To find a way to measure this avoidance, they asked people in the study how much money they would have to be paid to pal around with a perceived bore for 1 to 7 days. The payments people said they would need varied by perceptions that their boredom would be low, intermediate, or high.
As an example, they would require an average of $50 to spend one day with a highly boring person. That amount would double to $100 to spend almost 4 days in their company, and up to $230 for the week.
Dr. Leary says boredom happens when people try to pay attention to an experience or event. This means boredom goes beyond simple disinterest or trying to pay attention to someone that is not “intrinsically captivating.” When it takes more brain power to pay attention, you’ll perceive the experience as even more boring.
“Perhaps the best way to see if other people are actually boring is to talk about interesting things and see how they respond,” Dr. Leary says. “But, be careful: The topics you think make interesting conversations may be boring to others.”
A version of this article first appeared on WebMD.com.
Apologies up front to anyone who spends their weekends bird-watching or doing math for fun. They are among the people expected to be boring, based on stereotypes about what they do for work or how they spend their spare time, new research reveals.
Researchers surveyed more than 500 people across five related experiments to identify what people perceive as the most boring jobs, traits, and hobbies. They also report how we could all be missing out by spending as little time as possible with our tax consultant, accountant, or financial adviser outside of work.
People who work in banking, finance, accounting, data analytics, and cleaning topped the most boring list in the study, published earlier this month in Personality and Social Psychology Bulletin.
Sleeping, religion, watching television, observing animals, and spending spare time on mathematics were the stereotypical most boring hobbies and activities. The “observing animals” group includes people who bird-watch or study ants.
On the flip side, the top five most exciting jobs, in order, were in the performing arts, science, journalism, health professions, and teaching.
The researchers also looked at the how likely people are to avoid spending time with stereotypical dullards.
“Are people who are stereotyped as being boring avoided, if possible? Our current research shows that this is likely,” says Wijnand A.P. Van Tilburg, PhD, one of the researchers who did the study.
Beyond specific traits and stereotypes, Dr. Van Tilburg and colleagues found that boring people are seen as lacking skills and warmth.
“To our surprise, it appears that they are seen as both unfriendly and incompetent,” says Dr. Van Tilburg, an experimental social psychologist at the University of Essex in the United Kingdom.
What qualities do people most often ascribe to boring people? Besides being “dull,” “dry,” “bland,” and “not interesting,” common stereotypes include thinking someone who is likely boring will have no sense of humor, lack opinions, or complain.
The people surveyed also were more likely to place boring people in towns and small cities rather than large metropolitan areas.
A vicious cycle?
What’s the possible harm of relying on boring stereotypes? If people are stereotyped as being boring solely based on professions and hobbies, “then that suggests that they will incur the negative consequences associated with being a stereotypically boring person -- even when others haven’t actually interacted with them,” Dr. Van Tilburg says.
“Having a stereotypically boring profession or hobby may come with the inability to prove the biased perceivers wrong,” he says.
So making distinctions between stereotypes and realities is important, Dr. Van Tilburg says. “Those who have hobbies or occupations that are stereotypically boring do, of course, not actually have to be boring.”
Mark Leary, PhD, a professor in the department of psychology and neuroscience at Duke University in Durham, N.C., agrees. “The research actually dealt with stereotypes about the kinds of people who hold certain jobs, have certain hobbies, and live in certain places -- and not about boring people per se,” he says.
Dr. Leary points out that few people encounter bankers, tax experts, and others perceived as most boring outside a professional setting.
“When we have interactions with data analysts, accountants, insurance agents, and bankers, for example, those interactions are often boring not because the people are boring, but rather because the context is not interesting.”
To get past the preconceptions, “the best advice might be to get people to try to separate people from their roles when forming impressions of them.”
“We need to recognize that many of our interactions with other people are tied up in particular roles and topics and, thus, don’t reveal much about the other people themselves,” Dr. Leary says. “Maybe my accountant is the life of the party in other contexts.”
Dollars to avoid the dull?
The researchers also found that as the perception of how boring a person is increased, people were more likely to say they would avoid them.
To find a way to measure this avoidance, they asked people in the study how much money they would have to be paid to pal around with a perceived bore for 1 to 7 days. The payments people said they would need varied by perceptions that their boredom would be low, intermediate, or high.
As an example, they would require an average of $50 to spend one day with a highly boring person. That amount would double to $100 to spend almost 4 days in their company, and up to $230 for the week.
Dr. Leary says boredom happens when people try to pay attention to an experience or event. This means boredom goes beyond simple disinterest or trying to pay attention to someone that is not “intrinsically captivating.” When it takes more brain power to pay attention, you’ll perceive the experience as even more boring.
“Perhaps the best way to see if other people are actually boring is to talk about interesting things and see how they respond,” Dr. Leary says. “But, be careful: The topics you think make interesting conversations may be boring to others.”
A version of this article first appeared on WebMD.com.
FROM PERSONALITY AND SOCIAL PSYCHOLOGY BULLETIN
Reporting from: 48th annual scientific meeting of the Society of Gynecologic Surgeons
Wednesday, March 30. Day 3 of SGS.
The final day of #SGS2022 began with the last round of oral and video presentations on topics including: the efficacy and safety of restrictive blood transfusion protocols in gynecologic surgical patients, restricted opioid use following midurethral sling procedures, and the efficacy of trigger point injections for myofascial pelvic pain. Next, the prestigious Distinguished Surgeon Award was presented to Dr. Jeffrey Cornella, professor of Obstetrics and Gynecology at Mayo Clinic College of Medicine, for his contributions to the field of gynecologic surgery.
This was followed by the passing of the presidential gavel from current SGS president Dr. Carl Zimmerman to incoming president Dr. Cheryl Iglesia, Director of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center, Washington DC, and Director of the National Center for Advanced Pelvic Surgery (NCAPS) at Medstar Health. Dr. Iglesia has been internationally and nationally recognized for her work in advancing the field of pelvic surgery and urogynecology through extensive research, clinical excellence, and support of medical education.
Needless to say, #SGS2022 was a huge success! While many of us are sad to leave San Antonio today, we are returning to our respective programs feeling motivated and rejuvenated. There is nothing more inspiring than spending time with such a highly committed group of physicians who strive to improve patient care through their excellent contributions to research and medical education. I am grateful for the new mentors, colleagues, and friends I have met at this meeting.
Thank you to the Society of Gynecologic Surgeons and OBG Management for giving me the opportunity to reflect on my experience at #SGS2022, as well as the companies that support the Fellows Scholar program.
I can’t wait to attend the 49th Annual Scientific Meeting in Tuscon, Arizona, in 2023!
Tuesday, March 29, 2022. Day 2 of SGS.
The second day of #SGS2022 began with several academic roundtables on a variety of topics including hysteroscopy, uterine-preserving prolapse surgeries, how to select patients for vaginal hysterectomy, and the role of minimally invasive surgery in transabdominal cerclage. The general session continued with more outstanding poster and video presentations that were followed by the annual presidential address. SGS president Dr. Carl Zimmerman spoke about the changing surgical landscape and SGS’s commitment to improving surgical education: “The women of America and the world deserve better.” He went on to announce the creation of a presidential task force on surgical training, whose members will include: Dr. Ted Anderson, Dr. Emily Weber LeBrun, and Dr. Mike Moen.
This year’s TeLinde Lecture was given by the executive director of the American College of Surgeons, Dr. Dr. Patricia Turner. Her talk was entitled, “Surgeons: More to Unite Us Than Divide Us.” Dr. Turner began by reminding us of the shared history between general surgery and gynecologic surgery. In fact, the American College of Surgeons was founded by gynecologist Dr. Franklin H. Martin. She went on to thoughtfully discuss the need to rethink surgical training and the way we assess surgical trainees. She highlighted the importance of collaboration between all surgical specialties to improve surgical education, improve outcomes, and advocate for patients. “In order to heal all, we have to have ALL surgeons at the table.”
Today’s general session was concluded with a panel discussion on “Operating Room Safety and Efficiency” in which Dr. Kenneth Catchpole, Dr. Teodor Grantcharov, and Dr. Jason Wright shared some interesting ideas on how we can improve patient care in the operating room. The afternoon continued with a number of social activities, providing an opportunity to enjoy the beautiful landscape of San Antonio, Texas, including: a tour of Eisenhower park, kayaking on the Guadelupe River, and the SGS golf tournament.
The fun continued into the evening at the annual “SGS’ Got Talent” in which participants could be spotted in cowboy hats, bandanas, and boots. The night was filled with food, drinks, laughter, and line dancing! #SGS2022
Monday, March 28, 2022. Day 1 of SGS.
“How do you become brave? How do you become an advocate? How do you make a change?” These are just some of the questions asked during our thought-provoking early morning session entitled, “Healthcare Inequity Awareness—A Conversation to Empower Providers and Enhance the Patient Experience” at this year’s annual scientific meeting of the Society of Gynecologic Surgeons #SGS2022. The panelists, which included Dr. Olivia Cardenas-Trowers, Dr. Maria Florian-Rodriguez, and Dr. Tristi Muir, emphasized the importance of acknowledging our own bias as physicians, as well as the role structural racism plays in the health care access and outcomes of our patients. We were reminded that “Diversity, Equity, Inclusion (aka DEI) is a journey. It is progress over time, not over night.”
Following the early morning panel, the 48th annual scientific meeting officially began with a brief welcome and recognition of new SGS members by current president Dr. Carl Zimmerman and scientific program committee chair Dr. Oz Harmanli. The rest of the morning session was filled with outstanding oral and video presentations on topics ranging from the role of oophorectomy in patients with breast cancer, creating simulation models to enhance medical education, and tips for navigating the altered retroperitoneum.
Next, the Mark D. Walters endowed lecture was given by Dr. Marta A. Crispens, entitled “Restructuring Gynecologic Surgical Education: It’s a Matter of Equity.” In her exceptionally powerful address, Dr. Crispens began by discussing the historical context in which the fields of obstetrics and gynecology were combined and comparing it to a shift in current practice toward a national decrease in number of hysterectomies and an increase in the complexity of surgical cases. She highlighted the well-studied fact that low-volume surgeons have higher complication rates and that many new ObGyn residency graduates perform only 3 to 4 hysterectomies annually during the first few years of practice. Finally, she asserted that, by separating the practices of obstetrics and gynecology, we can improve surgical education and the quality of surgical care for our patients. The audience’s enthusiasm was undeniable, resulting in resounding applause and a standing ovation.
The afternoon was filled with unique opportunities for fellows, including: the Fellow’s Pelvic Research Network (FPRN) meeting, an incredibly informative panel on how to navigate the first year out of fellowship with Dr. Mireille Truong, Dr. Christine Foley, and Dr. Jon Pennycuff, and finally, the Mentorship Mingle.
The first day was concluded with the President’s Award Ceremony in which Dr. John DeLancey was presented with the illustrious President’s Award, followed by the President’s Reception with food, drinks, and lively conversation. Looking forward to day 2 of #SGS2022. @gynsurgery
Wednesday, March 30. Day 3 of SGS.
The final day of #SGS2022 began with the last round of oral and video presentations on topics including: the efficacy and safety of restrictive blood transfusion protocols in gynecologic surgical patients, restricted opioid use following midurethral sling procedures, and the efficacy of trigger point injections for myofascial pelvic pain. Next, the prestigious Distinguished Surgeon Award was presented to Dr. Jeffrey Cornella, professor of Obstetrics and Gynecology at Mayo Clinic College of Medicine, for his contributions to the field of gynecologic surgery.
This was followed by the passing of the presidential gavel from current SGS president Dr. Carl Zimmerman to incoming president Dr. Cheryl Iglesia, Director of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center, Washington DC, and Director of the National Center for Advanced Pelvic Surgery (NCAPS) at Medstar Health. Dr. Iglesia has been internationally and nationally recognized for her work in advancing the field of pelvic surgery and urogynecology through extensive research, clinical excellence, and support of medical education.
Needless to say, #SGS2022 was a huge success! While many of us are sad to leave San Antonio today, we are returning to our respective programs feeling motivated and rejuvenated. There is nothing more inspiring than spending time with such a highly committed group of physicians who strive to improve patient care through their excellent contributions to research and medical education. I am grateful for the new mentors, colleagues, and friends I have met at this meeting.
Thank you to the Society of Gynecologic Surgeons and OBG Management for giving me the opportunity to reflect on my experience at #SGS2022, as well as the companies that support the Fellows Scholar program.
I can’t wait to attend the 49th Annual Scientific Meeting in Tuscon, Arizona, in 2023!
Tuesday, March 29, 2022. Day 2 of SGS.
The second day of #SGS2022 began with several academic roundtables on a variety of topics including hysteroscopy, uterine-preserving prolapse surgeries, how to select patients for vaginal hysterectomy, and the role of minimally invasive surgery in transabdominal cerclage. The general session continued with more outstanding poster and video presentations that were followed by the annual presidential address. SGS president Dr. Carl Zimmerman spoke about the changing surgical landscape and SGS’s commitment to improving surgical education: “The women of America and the world deserve better.” He went on to announce the creation of a presidential task force on surgical training, whose members will include: Dr. Ted Anderson, Dr. Emily Weber LeBrun, and Dr. Mike Moen.
This year’s TeLinde Lecture was given by the executive director of the American College of Surgeons, Dr. Dr. Patricia Turner. Her talk was entitled, “Surgeons: More to Unite Us Than Divide Us.” Dr. Turner began by reminding us of the shared history between general surgery and gynecologic surgery. In fact, the American College of Surgeons was founded by gynecologist Dr. Franklin H. Martin. She went on to thoughtfully discuss the need to rethink surgical training and the way we assess surgical trainees. She highlighted the importance of collaboration between all surgical specialties to improve surgical education, improve outcomes, and advocate for patients. “In order to heal all, we have to have ALL surgeons at the table.”
Today’s general session was concluded with a panel discussion on “Operating Room Safety and Efficiency” in which Dr. Kenneth Catchpole, Dr. Teodor Grantcharov, and Dr. Jason Wright shared some interesting ideas on how we can improve patient care in the operating room. The afternoon continued with a number of social activities, providing an opportunity to enjoy the beautiful landscape of San Antonio, Texas, including: a tour of Eisenhower park, kayaking on the Guadelupe River, and the SGS golf tournament.
The fun continued into the evening at the annual “SGS’ Got Talent” in which participants could be spotted in cowboy hats, bandanas, and boots. The night was filled with food, drinks, laughter, and line dancing! #SGS2022
Monday, March 28, 2022. Day 1 of SGS.
“How do you become brave? How do you become an advocate? How do you make a change?” These are just some of the questions asked during our thought-provoking early morning session entitled, “Healthcare Inequity Awareness—A Conversation to Empower Providers and Enhance the Patient Experience” at this year’s annual scientific meeting of the Society of Gynecologic Surgeons #SGS2022. The panelists, which included Dr. Olivia Cardenas-Trowers, Dr. Maria Florian-Rodriguez, and Dr. Tristi Muir, emphasized the importance of acknowledging our own bias as physicians, as well as the role structural racism plays in the health care access and outcomes of our patients. We were reminded that “Diversity, Equity, Inclusion (aka DEI) is a journey. It is progress over time, not over night.”
Following the early morning panel, the 48th annual scientific meeting officially began with a brief welcome and recognition of new SGS members by current president Dr. Carl Zimmerman and scientific program committee chair Dr. Oz Harmanli. The rest of the morning session was filled with outstanding oral and video presentations on topics ranging from the role of oophorectomy in patients with breast cancer, creating simulation models to enhance medical education, and tips for navigating the altered retroperitoneum.
Next, the Mark D. Walters endowed lecture was given by Dr. Marta A. Crispens, entitled “Restructuring Gynecologic Surgical Education: It’s a Matter of Equity.” In her exceptionally powerful address, Dr. Crispens began by discussing the historical context in which the fields of obstetrics and gynecology were combined and comparing it to a shift in current practice toward a national decrease in number of hysterectomies and an increase in the complexity of surgical cases. She highlighted the well-studied fact that low-volume surgeons have higher complication rates and that many new ObGyn residency graduates perform only 3 to 4 hysterectomies annually during the first few years of practice. Finally, she asserted that, by separating the practices of obstetrics and gynecology, we can improve surgical education and the quality of surgical care for our patients. The audience’s enthusiasm was undeniable, resulting in resounding applause and a standing ovation.
The afternoon was filled with unique opportunities for fellows, including: the Fellow’s Pelvic Research Network (FPRN) meeting, an incredibly informative panel on how to navigate the first year out of fellowship with Dr. Mireille Truong, Dr. Christine Foley, and Dr. Jon Pennycuff, and finally, the Mentorship Mingle.
The first day was concluded with the President’s Award Ceremony in which Dr. John DeLancey was presented with the illustrious President’s Award, followed by the President’s Reception with food, drinks, and lively conversation. Looking forward to day 2 of #SGS2022. @gynsurgery
Wednesday, March 30. Day 3 of SGS.
The final day of #SGS2022 began with the last round of oral and video presentations on topics including: the efficacy and safety of restrictive blood transfusion protocols in gynecologic surgical patients, restricted opioid use following midurethral sling procedures, and the efficacy of trigger point injections for myofascial pelvic pain. Next, the prestigious Distinguished Surgeon Award was presented to Dr. Jeffrey Cornella, professor of Obstetrics and Gynecology at Mayo Clinic College of Medicine, for his contributions to the field of gynecologic surgery.
This was followed by the passing of the presidential gavel from current SGS president Dr. Carl Zimmerman to incoming president Dr. Cheryl Iglesia, Director of Female Pelvic Medicine and Reconstructive Surgery at MedStar Washington Hospital Center, Washington DC, and Director of the National Center for Advanced Pelvic Surgery (NCAPS) at Medstar Health. Dr. Iglesia has been internationally and nationally recognized for her work in advancing the field of pelvic surgery and urogynecology through extensive research, clinical excellence, and support of medical education.
Needless to say, #SGS2022 was a huge success! While many of us are sad to leave San Antonio today, we are returning to our respective programs feeling motivated and rejuvenated. There is nothing more inspiring than spending time with such a highly committed group of physicians who strive to improve patient care through their excellent contributions to research and medical education. I am grateful for the new mentors, colleagues, and friends I have met at this meeting.
Thank you to the Society of Gynecologic Surgeons and OBG Management for giving me the opportunity to reflect on my experience at #SGS2022, as well as the companies that support the Fellows Scholar program.
I can’t wait to attend the 49th Annual Scientific Meeting in Tuscon, Arizona, in 2023!
Tuesday, March 29, 2022. Day 2 of SGS.
The second day of #SGS2022 began with several academic roundtables on a variety of topics including hysteroscopy, uterine-preserving prolapse surgeries, how to select patients for vaginal hysterectomy, and the role of minimally invasive surgery in transabdominal cerclage. The general session continued with more outstanding poster and video presentations that were followed by the annual presidential address. SGS president Dr. Carl Zimmerman spoke about the changing surgical landscape and SGS’s commitment to improving surgical education: “The women of America and the world deserve better.” He went on to announce the creation of a presidential task force on surgical training, whose members will include: Dr. Ted Anderson, Dr. Emily Weber LeBrun, and Dr. Mike Moen.
This year’s TeLinde Lecture was given by the executive director of the American College of Surgeons, Dr. Dr. Patricia Turner. Her talk was entitled, “Surgeons: More to Unite Us Than Divide Us.” Dr. Turner began by reminding us of the shared history between general surgery and gynecologic surgery. In fact, the American College of Surgeons was founded by gynecologist Dr. Franklin H. Martin. She went on to thoughtfully discuss the need to rethink surgical training and the way we assess surgical trainees. She highlighted the importance of collaboration between all surgical specialties to improve surgical education, improve outcomes, and advocate for patients. “In order to heal all, we have to have ALL surgeons at the table.”
Today’s general session was concluded with a panel discussion on “Operating Room Safety and Efficiency” in which Dr. Kenneth Catchpole, Dr. Teodor Grantcharov, and Dr. Jason Wright shared some interesting ideas on how we can improve patient care in the operating room. The afternoon continued with a number of social activities, providing an opportunity to enjoy the beautiful landscape of San Antonio, Texas, including: a tour of Eisenhower park, kayaking on the Guadelupe River, and the SGS golf tournament.
The fun continued into the evening at the annual “SGS’ Got Talent” in which participants could be spotted in cowboy hats, bandanas, and boots. The night was filled with food, drinks, laughter, and line dancing! #SGS2022
Monday, March 28, 2022. Day 1 of SGS.
“How do you become brave? How do you become an advocate? How do you make a change?” These are just some of the questions asked during our thought-provoking early morning session entitled, “Healthcare Inequity Awareness—A Conversation to Empower Providers and Enhance the Patient Experience” at this year’s annual scientific meeting of the Society of Gynecologic Surgeons #SGS2022. The panelists, which included Dr. Olivia Cardenas-Trowers, Dr. Maria Florian-Rodriguez, and Dr. Tristi Muir, emphasized the importance of acknowledging our own bias as physicians, as well as the role structural racism plays in the health care access and outcomes of our patients. We were reminded that “Diversity, Equity, Inclusion (aka DEI) is a journey. It is progress over time, not over night.”
Following the early morning panel, the 48th annual scientific meeting officially began with a brief welcome and recognition of new SGS members by current president Dr. Carl Zimmerman and scientific program committee chair Dr. Oz Harmanli. The rest of the morning session was filled with outstanding oral and video presentations on topics ranging from the role of oophorectomy in patients with breast cancer, creating simulation models to enhance medical education, and tips for navigating the altered retroperitoneum.
Next, the Mark D. Walters endowed lecture was given by Dr. Marta A. Crispens, entitled “Restructuring Gynecologic Surgical Education: It’s a Matter of Equity.” In her exceptionally powerful address, Dr. Crispens began by discussing the historical context in which the fields of obstetrics and gynecology were combined and comparing it to a shift in current practice toward a national decrease in number of hysterectomies and an increase in the complexity of surgical cases. She highlighted the well-studied fact that low-volume surgeons have higher complication rates and that many new ObGyn residency graduates perform only 3 to 4 hysterectomies annually during the first few years of practice. Finally, she asserted that, by separating the practices of obstetrics and gynecology, we can improve surgical education and the quality of surgical care for our patients. The audience’s enthusiasm was undeniable, resulting in resounding applause and a standing ovation.
The afternoon was filled with unique opportunities for fellows, including: the Fellow’s Pelvic Research Network (FPRN) meeting, an incredibly informative panel on how to navigate the first year out of fellowship with Dr. Mireille Truong, Dr. Christine Foley, and Dr. Jon Pennycuff, and finally, the Mentorship Mingle.
The first day was concluded with the President’s Award Ceremony in which Dr. John DeLancey was presented with the illustrious President’s Award, followed by the President’s Reception with food, drinks, and lively conversation. Looking forward to day 2 of #SGS2022. @gynsurgery
TikTok trends: Sleepy lettuce water, cyst smacking, and prostaglandin pain
Spring is the time for new beginnings, cleaning out your dusty musty basement, battling seasonal allergies, and, of course, discovering the latest TikTok trends.
With potentially permanent daylight savings on the table, that extra time spent luxuriating in the sunshine could mean more time to scroll (and scroll and scroll) through the latest health fads on the platform – for better or for worse.
The good: Doctor explains menstrual pain in an unexpected place
For a long time, menstrual cycles were considered taboo, and discussing them is still seen as inappropriate in many parts of the world. Organizations and online resources such as Clue or Flo are seeking to normalize period-talk. Pixar has jumped on board with its latest film, Turning Red, which depicts a 13-year-old’s experience getting her first period. With a 95% rating on Rotten Tomatoes, it’s clear that the public is willing to talk about periods.
Many people feel shame around discussing their periods, but this is something that social media has actually handled positively (for once).
Karan Raj, MD, a National Health Service surgeon and educator at Imperial College London, uses his TikTok account to educate his followers and explain health concerns and issues. Dr. Raj stitched this video from someone else that was captioned, “When you’re on your [period] and feel the stab in your booty.”
In his video, Dr. Raj uses anatomical diagrams to explain exactly what’s going on with this pain in the butt. The pain, which Dr. Raj says is called proctalgia fugax, is caused by a type of hormone released during menstruation called prostaglandins. Prostaglandins tell the uterus to contract and shed its lining, but the uterus isn’t the only part of the body receiving that message from the prostaglandins.
“The prostaglandins also cause contraction of the rectum, pelvic floor muscles, and muscles around the anal canal,” Dr. Raj tells viewers. “These intense contractions can cause muscle spasms and anal cramps.”
The bad: Lettuce water sleep aid
This video from Elliott Norris (@callmebelly) shows him preparing an unusual sleep aid. It’s one of many videos of people trying the same trend, usually with the tag #lettucewater attached.
As Mr. Elliott explains, the rumor is that boiling romaine lettuce and then drinking the water is a way to help one fall asleep faster, or sleep better at night. At the end of the video, Mr. Elliott even said that it worked for him. So where does this come from, and is it just a placebo?
Videos on TikTok recreating this trend cite a 2017 study titled, “Sleep-inducing effect of lettuce (Lactuca sativa) varieties on pentobarbital-induced sleep” (there’s even a New York Times article about it). The star of the study is lactucarium, a milky white substance that›s found in lettuce, usually visible if you squeeze it or break the stalks. The study suggests that lactucarium has “sedative-hypnotic” properties after lettuce extract was used in tandem with pentobarbital on mice, and it was found that the mice›s sleep latency then decreased.
In an article from Parade discussing the trend, Rachel Salas, MD, MEd, weighs in. Dr. Salas is the assistant medical director at Johns Hopkins Center for Sleep and Wellness in Baltimore, so you could say she knows a thing or two about sleep. Her response to the mice study was that its limitations made the results not entirely reliable.
According to Dr. Salas, there was no control group, the extract the mice were given is much more concentrated than what’s found in lettuce, and the mice were given a sedative that was going to make them sleepy anyway: “The mice were drugged to put the animals to sleep soon after they took the lettuce water.”
So while Dr. Salas thinks it’s good that people are open to talking about sleeping solutions, “lettuce tea” just doesn’t have any evidence to back up what people claim it does.
The ugly: Using a book to pop ganglion cysts
Everyone knows how popular pimple popping, blackhead squeezing, and cyst squashing are on social media. Dermatologist Sandra Lee, better known as Dr. Pimple Popper, used her YouTube platform of the same name – which boasts over 7.42 million subscribers – to cinch a reality television show on TLC. Viewers on TikTok are no different and love the satisfying (and often gross) relief of clearing out a nasty pimple or two.
In this stitched TikTok from emergency medicine physician Fayez Ajib, DO, aka @lifeofadoctor, Dr. Ajib reacts to an original video of someone popping a ganglion cyst with a textbook.
There are plenty of other videos on TikTok of people using books to smack ganglion cysts, which develop on the wrist. People have looked for remedies for ganglion cysts since the 1700s, at which point many strange options arose, as discussed in BBC Future. The one that still holds up, however, is smacking the cyst with a heavy book, like a Bible (hence the ganglion cyst’s nickname, “Bible bump”).
In his video, Dr. Ajib explains why smacking the cyst is a bad idea, even if it appears to temporarily resolve the issue.
“Not only have people broken the delicate bones in their wrist from getting hit,” Dr. Ajib says, “but they actually have a high chance of recurrence. A doctor will actually remove the sac itself rather than just draining it from being hit.”
The lessons we glean from TikTok remain the same: Leave it up to the professionals.
A version of this article first appeared on Medscape.com.
Spring is the time for new beginnings, cleaning out your dusty musty basement, battling seasonal allergies, and, of course, discovering the latest TikTok trends.
With potentially permanent daylight savings on the table, that extra time spent luxuriating in the sunshine could mean more time to scroll (and scroll and scroll) through the latest health fads on the platform – for better or for worse.
The good: Doctor explains menstrual pain in an unexpected place
For a long time, menstrual cycles were considered taboo, and discussing them is still seen as inappropriate in many parts of the world. Organizations and online resources such as Clue or Flo are seeking to normalize period-talk. Pixar has jumped on board with its latest film, Turning Red, which depicts a 13-year-old’s experience getting her first period. With a 95% rating on Rotten Tomatoes, it’s clear that the public is willing to talk about periods.
Many people feel shame around discussing their periods, but this is something that social media has actually handled positively (for once).
Karan Raj, MD, a National Health Service surgeon and educator at Imperial College London, uses his TikTok account to educate his followers and explain health concerns and issues. Dr. Raj stitched this video from someone else that was captioned, “When you’re on your [period] and feel the stab in your booty.”
In his video, Dr. Raj uses anatomical diagrams to explain exactly what’s going on with this pain in the butt. The pain, which Dr. Raj says is called proctalgia fugax, is caused by a type of hormone released during menstruation called prostaglandins. Prostaglandins tell the uterus to contract and shed its lining, but the uterus isn’t the only part of the body receiving that message from the prostaglandins.
“The prostaglandins also cause contraction of the rectum, pelvic floor muscles, and muscles around the anal canal,” Dr. Raj tells viewers. “These intense contractions can cause muscle spasms and anal cramps.”
The bad: Lettuce water sleep aid
This video from Elliott Norris (@callmebelly) shows him preparing an unusual sleep aid. It’s one of many videos of people trying the same trend, usually with the tag #lettucewater attached.
As Mr. Elliott explains, the rumor is that boiling romaine lettuce and then drinking the water is a way to help one fall asleep faster, or sleep better at night. At the end of the video, Mr. Elliott even said that it worked for him. So where does this come from, and is it just a placebo?
Videos on TikTok recreating this trend cite a 2017 study titled, “Sleep-inducing effect of lettuce (Lactuca sativa) varieties on pentobarbital-induced sleep” (there’s even a New York Times article about it). The star of the study is lactucarium, a milky white substance that›s found in lettuce, usually visible if you squeeze it or break the stalks. The study suggests that lactucarium has “sedative-hypnotic” properties after lettuce extract was used in tandem with pentobarbital on mice, and it was found that the mice›s sleep latency then decreased.
In an article from Parade discussing the trend, Rachel Salas, MD, MEd, weighs in. Dr. Salas is the assistant medical director at Johns Hopkins Center for Sleep and Wellness in Baltimore, so you could say she knows a thing or two about sleep. Her response to the mice study was that its limitations made the results not entirely reliable.
According to Dr. Salas, there was no control group, the extract the mice were given is much more concentrated than what’s found in lettuce, and the mice were given a sedative that was going to make them sleepy anyway: “The mice were drugged to put the animals to sleep soon after they took the lettuce water.”
So while Dr. Salas thinks it’s good that people are open to talking about sleeping solutions, “lettuce tea” just doesn’t have any evidence to back up what people claim it does.
The ugly: Using a book to pop ganglion cysts
Everyone knows how popular pimple popping, blackhead squeezing, and cyst squashing are on social media. Dermatologist Sandra Lee, better known as Dr. Pimple Popper, used her YouTube platform of the same name – which boasts over 7.42 million subscribers – to cinch a reality television show on TLC. Viewers on TikTok are no different and love the satisfying (and often gross) relief of clearing out a nasty pimple or two.
In this stitched TikTok from emergency medicine physician Fayez Ajib, DO, aka @lifeofadoctor, Dr. Ajib reacts to an original video of someone popping a ganglion cyst with a textbook.
There are plenty of other videos on TikTok of people using books to smack ganglion cysts, which develop on the wrist. People have looked for remedies for ganglion cysts since the 1700s, at which point many strange options arose, as discussed in BBC Future. The one that still holds up, however, is smacking the cyst with a heavy book, like a Bible (hence the ganglion cyst’s nickname, “Bible bump”).
In his video, Dr. Ajib explains why smacking the cyst is a bad idea, even if it appears to temporarily resolve the issue.
“Not only have people broken the delicate bones in their wrist from getting hit,” Dr. Ajib says, “but they actually have a high chance of recurrence. A doctor will actually remove the sac itself rather than just draining it from being hit.”
The lessons we glean from TikTok remain the same: Leave it up to the professionals.
A version of this article first appeared on Medscape.com.
Spring is the time for new beginnings, cleaning out your dusty musty basement, battling seasonal allergies, and, of course, discovering the latest TikTok trends.
With potentially permanent daylight savings on the table, that extra time spent luxuriating in the sunshine could mean more time to scroll (and scroll and scroll) through the latest health fads on the platform – for better or for worse.
The good: Doctor explains menstrual pain in an unexpected place
For a long time, menstrual cycles were considered taboo, and discussing them is still seen as inappropriate in many parts of the world. Organizations and online resources such as Clue or Flo are seeking to normalize period-talk. Pixar has jumped on board with its latest film, Turning Red, which depicts a 13-year-old’s experience getting her first period. With a 95% rating on Rotten Tomatoes, it’s clear that the public is willing to talk about periods.
Many people feel shame around discussing their periods, but this is something that social media has actually handled positively (for once).
Karan Raj, MD, a National Health Service surgeon and educator at Imperial College London, uses his TikTok account to educate his followers and explain health concerns and issues. Dr. Raj stitched this video from someone else that was captioned, “When you’re on your [period] and feel the stab in your booty.”
In his video, Dr. Raj uses anatomical diagrams to explain exactly what’s going on with this pain in the butt. The pain, which Dr. Raj says is called proctalgia fugax, is caused by a type of hormone released during menstruation called prostaglandins. Prostaglandins tell the uterus to contract and shed its lining, but the uterus isn’t the only part of the body receiving that message from the prostaglandins.
“The prostaglandins also cause contraction of the rectum, pelvic floor muscles, and muscles around the anal canal,” Dr. Raj tells viewers. “These intense contractions can cause muscle spasms and anal cramps.”
The bad: Lettuce water sleep aid
This video from Elliott Norris (@callmebelly) shows him preparing an unusual sleep aid. It’s one of many videos of people trying the same trend, usually with the tag #lettucewater attached.
As Mr. Elliott explains, the rumor is that boiling romaine lettuce and then drinking the water is a way to help one fall asleep faster, or sleep better at night. At the end of the video, Mr. Elliott even said that it worked for him. So where does this come from, and is it just a placebo?
Videos on TikTok recreating this trend cite a 2017 study titled, “Sleep-inducing effect of lettuce (Lactuca sativa) varieties on pentobarbital-induced sleep” (there’s even a New York Times article about it). The star of the study is lactucarium, a milky white substance that›s found in lettuce, usually visible if you squeeze it or break the stalks. The study suggests that lactucarium has “sedative-hypnotic” properties after lettuce extract was used in tandem with pentobarbital on mice, and it was found that the mice›s sleep latency then decreased.
In an article from Parade discussing the trend, Rachel Salas, MD, MEd, weighs in. Dr. Salas is the assistant medical director at Johns Hopkins Center for Sleep and Wellness in Baltimore, so you could say she knows a thing or two about sleep. Her response to the mice study was that its limitations made the results not entirely reliable.
According to Dr. Salas, there was no control group, the extract the mice were given is much more concentrated than what’s found in lettuce, and the mice were given a sedative that was going to make them sleepy anyway: “The mice were drugged to put the animals to sleep soon after they took the lettuce water.”
So while Dr. Salas thinks it’s good that people are open to talking about sleeping solutions, “lettuce tea” just doesn’t have any evidence to back up what people claim it does.
The ugly: Using a book to pop ganglion cysts
Everyone knows how popular pimple popping, blackhead squeezing, and cyst squashing are on social media. Dermatologist Sandra Lee, better known as Dr. Pimple Popper, used her YouTube platform of the same name – which boasts over 7.42 million subscribers – to cinch a reality television show on TLC. Viewers on TikTok are no different and love the satisfying (and often gross) relief of clearing out a nasty pimple or two.
In this stitched TikTok from emergency medicine physician Fayez Ajib, DO, aka @lifeofadoctor, Dr. Ajib reacts to an original video of someone popping a ganglion cyst with a textbook.
There are plenty of other videos on TikTok of people using books to smack ganglion cysts, which develop on the wrist. People have looked for remedies for ganglion cysts since the 1700s, at which point many strange options arose, as discussed in BBC Future. The one that still holds up, however, is smacking the cyst with a heavy book, like a Bible (hence the ganglion cyst’s nickname, “Bible bump”).
In his video, Dr. Ajib explains why smacking the cyst is a bad idea, even if it appears to temporarily resolve the issue.
“Not only have people broken the delicate bones in their wrist from getting hit,” Dr. Ajib says, “but they actually have a high chance of recurrence. A doctor will actually remove the sac itself rather than just draining it from being hit.”
The lessons we glean from TikTok remain the same: Leave it up to the professionals.
A version of this article first appeared on Medscape.com.
Avocados linked to lower cardiovascular risk
A prospective study that followed more than 110,000 men and women for more than 30 years suggests that .
Researchers also found that replacing half a serving of butter, cheese, bacon, or other animal product with an equivalent amount of avocado was associated with up to 22% lower risk for CVD events.
The findings add to evidence from other studies that has shown that avocados – which contain multiple nutrients, including fiber and unsaturated, healthy fats – have a positive impact on cardiovascular risk factors, first author Lorena S. Pacheco, PhD, a postdoctoral research fellow at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
“This research complements and expands on the current literature that we have on unsaturated fats and reduced risk of cardiovascular disease and also underscores how bad saturated fats, like butter, cheese, and processed meats are for the heart,” Dr. Pacheco said.
“For the most part, we have known that avocados are healthy, but I think this study, because of its numbers and duration, adds a little more substance to that knowledge now,” Dr. Pacheco said.
The findings were published online March 30 in the Journal of the American Heart Association.
Avocados are dense with nutrients. They are high in fat, but in monounsaturated fats (MUFAs) and polyunsaturated fats (PUFAs), which are viewed as good.
A medium-sized (136 g) Haas avocado, which is the most commonly consumed avocado in the United States, contains roughly 13 g of oleic acid. Avocados also contain dietary fiber, potassium, magnesium, phytonutrients, and bioactive compounds.
To see the effect avocados can have on cardiovascular health, Dr. Pacheco and her team turned to two large, long-running cohort studies: the Nurses’ Health Study (NHS), which began in the early 1970s with 68,786 women 30-55 years of age; and the Health Professionals Follow-up Study (HPFS), which ran from 1986 to 2016 and followed 41,701 men 40-75 years of age.
All were free of cancer, coronary heart disease, and stroke at study entry.
Participants completed a validated food frequency questionnaire at baseline and every 4 years thereafter. The questionnaire asked about the amount and frequency of avocado consumed. One serving equaled half an avocado, or half a cup.
In the early days of the NHS, very few participants said they ate avocados, but that began to change over the years, as the popularity of avocados grew.
“The NHS cohort was recruited back in the late ‘70s, and the health professionals cohort did not start until the mid 1980s, when avocado consumption was really low,” Dr. Pacheco said.
“What is beautiful about these cohorts is we are able to ask participants questions and then save the answers that they give us throughout the years to answer questions that might arise whenever the question is right. So it just depends on when you accrue enough data to ask those questions about potential cardiovascular benefit with avocados,” she said.
There were 9,185 coronary heart disease events and 5,290 strokes documented over 30 years of follow-up.
After adjustment for lifestyle and other dietary factors, those with a higher avocado intake – at least two servings per week – had a 16% lower risk for CVD (pooled hazard ratio, 0.84; 95% CI, 0.75-0.95) and a 21% lower risk for coronary heart disease (pooled HR, 0.79; 95% CI, 0.68-0.91).
No significant associations were seen for stroke, but this is because the study did not have sufficient numbers, Dr. Pacheco explained.
A statistical model also determined that replacing half a serving daily of margarine, butter, egg, yogurt, cheese, or processed meats, such as bacon, with the same amount of avocado was associated with a 16%-22% lower risk for CVD events.
“I want to emphasize that the study is an epidemiological observational study and cannot prove cause and effect,” Dr. Pacheco said.
“It’s not a clinical trial – it’s based on observational epidemiology – but we saw patterns in the model: Avocado consumption and substituting avocado for other unhealthy fats reduced the risk of having a cardiovascular event or coronary heart disease,” she said.
The findings are significant “because a healthy dietary pattern is the cornerstone for cardiovascular health; however, it can be difficult for many Americans to achieve and adhere to healthy eating patterns,” Cheryl Anderson, PhD, professor and dean of the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, who is chair of the AHA’s Council on Epidemiology and Prevention, said in a statement.
“We desperately need strategies to improve intake of AHA-recommended healthy diets, such as the Mediterranean diet, that are rich in vegetables and fruits. Although no one food is the solution to routinely eating a healthy diet, this study is evidence that avocados have possible health benefits. This is promising because it is a food item that is popular, accessible, desirable, and easy to include in meals eaten by many Americans at home and in restaurants,” said Dr. Anderson, who was not part of the study.
Dr. Pacheco and Dr. Anderson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A prospective study that followed more than 110,000 men and women for more than 30 years suggests that .
Researchers also found that replacing half a serving of butter, cheese, bacon, or other animal product with an equivalent amount of avocado was associated with up to 22% lower risk for CVD events.
The findings add to evidence from other studies that has shown that avocados – which contain multiple nutrients, including fiber and unsaturated, healthy fats – have a positive impact on cardiovascular risk factors, first author Lorena S. Pacheco, PhD, a postdoctoral research fellow at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
“This research complements and expands on the current literature that we have on unsaturated fats and reduced risk of cardiovascular disease and also underscores how bad saturated fats, like butter, cheese, and processed meats are for the heart,” Dr. Pacheco said.
“For the most part, we have known that avocados are healthy, but I think this study, because of its numbers and duration, adds a little more substance to that knowledge now,” Dr. Pacheco said.
The findings were published online March 30 in the Journal of the American Heart Association.
Avocados are dense with nutrients. They are high in fat, but in monounsaturated fats (MUFAs) and polyunsaturated fats (PUFAs), which are viewed as good.
A medium-sized (136 g) Haas avocado, which is the most commonly consumed avocado in the United States, contains roughly 13 g of oleic acid. Avocados also contain dietary fiber, potassium, magnesium, phytonutrients, and bioactive compounds.
To see the effect avocados can have on cardiovascular health, Dr. Pacheco and her team turned to two large, long-running cohort studies: the Nurses’ Health Study (NHS), which began in the early 1970s with 68,786 women 30-55 years of age; and the Health Professionals Follow-up Study (HPFS), which ran from 1986 to 2016 and followed 41,701 men 40-75 years of age.
All were free of cancer, coronary heart disease, and stroke at study entry.
Participants completed a validated food frequency questionnaire at baseline and every 4 years thereafter. The questionnaire asked about the amount and frequency of avocado consumed. One serving equaled half an avocado, or half a cup.
In the early days of the NHS, very few participants said they ate avocados, but that began to change over the years, as the popularity of avocados grew.
“The NHS cohort was recruited back in the late ‘70s, and the health professionals cohort did not start until the mid 1980s, when avocado consumption was really low,” Dr. Pacheco said.
“What is beautiful about these cohorts is we are able to ask participants questions and then save the answers that they give us throughout the years to answer questions that might arise whenever the question is right. So it just depends on when you accrue enough data to ask those questions about potential cardiovascular benefit with avocados,” she said.
There were 9,185 coronary heart disease events and 5,290 strokes documented over 30 years of follow-up.
After adjustment for lifestyle and other dietary factors, those with a higher avocado intake – at least two servings per week – had a 16% lower risk for CVD (pooled hazard ratio, 0.84; 95% CI, 0.75-0.95) and a 21% lower risk for coronary heart disease (pooled HR, 0.79; 95% CI, 0.68-0.91).
No significant associations were seen for stroke, but this is because the study did not have sufficient numbers, Dr. Pacheco explained.
A statistical model also determined that replacing half a serving daily of margarine, butter, egg, yogurt, cheese, or processed meats, such as bacon, with the same amount of avocado was associated with a 16%-22% lower risk for CVD events.
“I want to emphasize that the study is an epidemiological observational study and cannot prove cause and effect,” Dr. Pacheco said.
“It’s not a clinical trial – it’s based on observational epidemiology – but we saw patterns in the model: Avocado consumption and substituting avocado for other unhealthy fats reduced the risk of having a cardiovascular event or coronary heart disease,” she said.
The findings are significant “because a healthy dietary pattern is the cornerstone for cardiovascular health; however, it can be difficult for many Americans to achieve and adhere to healthy eating patterns,” Cheryl Anderson, PhD, professor and dean of the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, who is chair of the AHA’s Council on Epidemiology and Prevention, said in a statement.
“We desperately need strategies to improve intake of AHA-recommended healthy diets, such as the Mediterranean diet, that are rich in vegetables and fruits. Although no one food is the solution to routinely eating a healthy diet, this study is evidence that avocados have possible health benefits. This is promising because it is a food item that is popular, accessible, desirable, and easy to include in meals eaten by many Americans at home and in restaurants,” said Dr. Anderson, who was not part of the study.
Dr. Pacheco and Dr. Anderson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A prospective study that followed more than 110,000 men and women for more than 30 years suggests that .
Researchers also found that replacing half a serving of butter, cheese, bacon, or other animal product with an equivalent amount of avocado was associated with up to 22% lower risk for CVD events.
The findings add to evidence from other studies that has shown that avocados – which contain multiple nutrients, including fiber and unsaturated, healthy fats – have a positive impact on cardiovascular risk factors, first author Lorena S. Pacheco, PhD, a postdoctoral research fellow at the Harvard T.H. Chan School of Public Health, Boston, said in an interview.
“This research complements and expands on the current literature that we have on unsaturated fats and reduced risk of cardiovascular disease and also underscores how bad saturated fats, like butter, cheese, and processed meats are for the heart,” Dr. Pacheco said.
“For the most part, we have known that avocados are healthy, but I think this study, because of its numbers and duration, adds a little more substance to that knowledge now,” Dr. Pacheco said.
The findings were published online March 30 in the Journal of the American Heart Association.
Avocados are dense with nutrients. They are high in fat, but in monounsaturated fats (MUFAs) and polyunsaturated fats (PUFAs), which are viewed as good.
A medium-sized (136 g) Haas avocado, which is the most commonly consumed avocado in the United States, contains roughly 13 g of oleic acid. Avocados also contain dietary fiber, potassium, magnesium, phytonutrients, and bioactive compounds.
To see the effect avocados can have on cardiovascular health, Dr. Pacheco and her team turned to two large, long-running cohort studies: the Nurses’ Health Study (NHS), which began in the early 1970s with 68,786 women 30-55 years of age; and the Health Professionals Follow-up Study (HPFS), which ran from 1986 to 2016 and followed 41,701 men 40-75 years of age.
All were free of cancer, coronary heart disease, and stroke at study entry.
Participants completed a validated food frequency questionnaire at baseline and every 4 years thereafter. The questionnaire asked about the amount and frequency of avocado consumed. One serving equaled half an avocado, or half a cup.
In the early days of the NHS, very few participants said they ate avocados, but that began to change over the years, as the popularity of avocados grew.
“The NHS cohort was recruited back in the late ‘70s, and the health professionals cohort did not start until the mid 1980s, when avocado consumption was really low,” Dr. Pacheco said.
“What is beautiful about these cohorts is we are able to ask participants questions and then save the answers that they give us throughout the years to answer questions that might arise whenever the question is right. So it just depends on when you accrue enough data to ask those questions about potential cardiovascular benefit with avocados,” she said.
There were 9,185 coronary heart disease events and 5,290 strokes documented over 30 years of follow-up.
After adjustment for lifestyle and other dietary factors, those with a higher avocado intake – at least two servings per week – had a 16% lower risk for CVD (pooled hazard ratio, 0.84; 95% CI, 0.75-0.95) and a 21% lower risk for coronary heart disease (pooled HR, 0.79; 95% CI, 0.68-0.91).
No significant associations were seen for stroke, but this is because the study did not have sufficient numbers, Dr. Pacheco explained.
A statistical model also determined that replacing half a serving daily of margarine, butter, egg, yogurt, cheese, or processed meats, such as bacon, with the same amount of avocado was associated with a 16%-22% lower risk for CVD events.
“I want to emphasize that the study is an epidemiological observational study and cannot prove cause and effect,” Dr. Pacheco said.
“It’s not a clinical trial – it’s based on observational epidemiology – but we saw patterns in the model: Avocado consumption and substituting avocado for other unhealthy fats reduced the risk of having a cardiovascular event or coronary heart disease,” she said.
The findings are significant “because a healthy dietary pattern is the cornerstone for cardiovascular health; however, it can be difficult for many Americans to achieve and adhere to healthy eating patterns,” Cheryl Anderson, PhD, professor and dean of the Herbert Wertheim School of Public Health and Human Longevity Science at the University of California, San Diego, who is chair of the AHA’s Council on Epidemiology and Prevention, said in a statement.
“We desperately need strategies to improve intake of AHA-recommended healthy diets, such as the Mediterranean diet, that are rich in vegetables and fruits. Although no one food is the solution to routinely eating a healthy diet, this study is evidence that avocados have possible health benefits. This is promising because it is a food item that is popular, accessible, desirable, and easy to include in meals eaten by many Americans at home and in restaurants,” said Dr. Anderson, who was not part of the study.
Dr. Pacheco and Dr. Anderson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Below the belt: sexual dysfunction overlooked in women with diabetes
Among patients with diabetes, women are just as likely as men to suffer from sexual dysfunction, but their issues are overlooked, with the narrative focusing mainly on the impact of this issue on men, say experts.
Women with diabetes can experience reduced sexual desire, painful sex, reduced lubrication, and sexual distress, increasing the risk of depression, and such issues often go unnoticed despite treatments being available, said Kirsty Winkley, PhD, diabetes nurse and health psychologist, King’s College London.
There is also the “embarrassment factor” on the side of both the health care professional and the patient, she said in a session she chaired at the Diabetes UK Professional Conference 2022. Many women with diabetes “wouldn’t necessarily know” that their sexual dysfunction “is related to their diabetes,” she told this news organization.
For women, sexual health conversations are “often about contraception and pregnancy,” as well as menstrual disorders, genital infections, and hormone replacement therapy. “As health care professionals, you’re trained to focus on those things, and you’re not really considering there might be sexual dysfunction. If women aren’t aware that it’s related to diabetes, you’ve got the perfect situation where it goes under the radar.”
However, cochair Debbie Cooke, PhD, health psychologist at the University of Surrey in Guildford, explained that having psychotherapy embedded within the diabetes team and “integrated throughout the whole service” means that the problem can be identified and treatment offered.
The issue is that such integration is “very uncommon” and access needs to be improved, Dr. Cooke said in an interview.
Sexual dysfunction major predictor of depression in women
Jacqueline Fosbury, psychotherapy lead at Diabetes Care for You, Sussex Community NHS Foundation Trust, said that “intimate activity is clearly beneficial for emotional and physical health,” as it is associated with increased oxytocin release, the burning of calories, better immunity, and improved sleep.
Sexual dysfunction is common in people with diabetes, she noted. Poor glycemic control can “damage” blood vessels and nerves, causing reduced blood flow and loss of sensation in sexual organs.
A recent study led by Belgian researchers found that among more than 750 adults with diabetes, 36% of men and 33% of women reported sexual dysfunction.
Sexual dysfunction was more common in women with type 1 diabetes, at 36%, compared with 26% for those with type 2 diabetes. The most commonly reported issues were decreased sexual desire, lubrication problems, orgasmic dysfunction, and pain. Body image problems and fear of hypoglycemia also affect sexuality and intimacy, leading to “sexual distress.”
Moreover, Ms. Fosbury said female sexual dysfunction has been identified as a “major predictor” of depression, which in turn reduces libido.
Treatments for women can include lubricants, local estrogen, and medications that are prescribed off-label, such as sildenafil. The same is true of testosterone therapy, which can be used to boost libido.
Couples therapy?
Next, Trudy Hannington, a psychosexual therapist with Leger Clinic, Doncaster, U.K., talked about how to use an integrated approach to address sexuality overall in people with diabetes.
She said this should be seen in a biopsychosocial context, with emphasis on the couple, on sensation and communication, and sexual growth, as well as changes in daily routines.
There should be a move away from “penetrative sex,” Ms. Hannington said, with the goal being “enjoyment, not orgasm.” Pleasure should be facilitated and the opportunities for “performance pressure and/or anxiety” reduced.
She discussed the case of Marie, a 27-year-old woman with type 1 diabetes who had been referred with painful sex and vaginal dryness. Marie had “never experienced orgasm,” despite being in a same-sex relationship with Emily.
Marie’s treatment involved a sexual growth program, to which Emily was invited, as well as recommendations to use lubricants, vibrators, and to try sildenafil.
Prioritize women
Ms. Fosbury reiterated that, in men, sexual dysfunction is “readily identified as a complication of diabetes” and is described as “traumatic” and “crucial to well-being.” It is also seen as “easy to treat” with medication, such as that for erectile dysfunction.
It is therefore crucial to talk to women with diabetes about possible sexual dysfunction, and the scene must be set before the appointment to explain that the subject will be broached. In addition, handouts and leaflets should be available for patients in the clinic so they can read about female sexual health and to lower the stigma around discussing it.
“Cultural stereotypes diminish the importance of female sexuality and prevent us from providing equal consideration to the sexual difficulties of our patients,” she concluded.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Among patients with diabetes, women are just as likely as men to suffer from sexual dysfunction, but their issues are overlooked, with the narrative focusing mainly on the impact of this issue on men, say experts.
Women with diabetes can experience reduced sexual desire, painful sex, reduced lubrication, and sexual distress, increasing the risk of depression, and such issues often go unnoticed despite treatments being available, said Kirsty Winkley, PhD, diabetes nurse and health psychologist, King’s College London.
There is also the “embarrassment factor” on the side of both the health care professional and the patient, she said in a session she chaired at the Diabetes UK Professional Conference 2022. Many women with diabetes “wouldn’t necessarily know” that their sexual dysfunction “is related to their diabetes,” she told this news organization.
For women, sexual health conversations are “often about contraception and pregnancy,” as well as menstrual disorders, genital infections, and hormone replacement therapy. “As health care professionals, you’re trained to focus on those things, and you’re not really considering there might be sexual dysfunction. If women aren’t aware that it’s related to diabetes, you’ve got the perfect situation where it goes under the radar.”
However, cochair Debbie Cooke, PhD, health psychologist at the University of Surrey in Guildford, explained that having psychotherapy embedded within the diabetes team and “integrated throughout the whole service” means that the problem can be identified and treatment offered.
The issue is that such integration is “very uncommon” and access needs to be improved, Dr. Cooke said in an interview.
Sexual dysfunction major predictor of depression in women
Jacqueline Fosbury, psychotherapy lead at Diabetes Care for You, Sussex Community NHS Foundation Trust, said that “intimate activity is clearly beneficial for emotional and physical health,” as it is associated with increased oxytocin release, the burning of calories, better immunity, and improved sleep.
Sexual dysfunction is common in people with diabetes, she noted. Poor glycemic control can “damage” blood vessels and nerves, causing reduced blood flow and loss of sensation in sexual organs.
A recent study led by Belgian researchers found that among more than 750 adults with diabetes, 36% of men and 33% of women reported sexual dysfunction.
Sexual dysfunction was more common in women with type 1 diabetes, at 36%, compared with 26% for those with type 2 diabetes. The most commonly reported issues were decreased sexual desire, lubrication problems, orgasmic dysfunction, and pain. Body image problems and fear of hypoglycemia also affect sexuality and intimacy, leading to “sexual distress.”
Moreover, Ms. Fosbury said female sexual dysfunction has been identified as a “major predictor” of depression, which in turn reduces libido.
Treatments for women can include lubricants, local estrogen, and medications that are prescribed off-label, such as sildenafil. The same is true of testosterone therapy, which can be used to boost libido.
Couples therapy?
Next, Trudy Hannington, a psychosexual therapist with Leger Clinic, Doncaster, U.K., talked about how to use an integrated approach to address sexuality overall in people with diabetes.
She said this should be seen in a biopsychosocial context, with emphasis on the couple, on sensation and communication, and sexual growth, as well as changes in daily routines.
There should be a move away from “penetrative sex,” Ms. Hannington said, with the goal being “enjoyment, not orgasm.” Pleasure should be facilitated and the opportunities for “performance pressure and/or anxiety” reduced.
She discussed the case of Marie, a 27-year-old woman with type 1 diabetes who had been referred with painful sex and vaginal dryness. Marie had “never experienced orgasm,” despite being in a same-sex relationship with Emily.
Marie’s treatment involved a sexual growth program, to which Emily was invited, as well as recommendations to use lubricants, vibrators, and to try sildenafil.
Prioritize women
Ms. Fosbury reiterated that, in men, sexual dysfunction is “readily identified as a complication of diabetes” and is described as “traumatic” and “crucial to well-being.” It is also seen as “easy to treat” with medication, such as that for erectile dysfunction.
It is therefore crucial to talk to women with diabetes about possible sexual dysfunction, and the scene must be set before the appointment to explain that the subject will be broached. In addition, handouts and leaflets should be available for patients in the clinic so they can read about female sexual health and to lower the stigma around discussing it.
“Cultural stereotypes diminish the importance of female sexuality and prevent us from providing equal consideration to the sexual difficulties of our patients,” she concluded.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Among patients with diabetes, women are just as likely as men to suffer from sexual dysfunction, but their issues are overlooked, with the narrative focusing mainly on the impact of this issue on men, say experts.
Women with diabetes can experience reduced sexual desire, painful sex, reduced lubrication, and sexual distress, increasing the risk of depression, and such issues often go unnoticed despite treatments being available, said Kirsty Winkley, PhD, diabetes nurse and health psychologist, King’s College London.
There is also the “embarrassment factor” on the side of both the health care professional and the patient, she said in a session she chaired at the Diabetes UK Professional Conference 2022. Many women with diabetes “wouldn’t necessarily know” that their sexual dysfunction “is related to their diabetes,” she told this news organization.
For women, sexual health conversations are “often about contraception and pregnancy,” as well as menstrual disorders, genital infections, and hormone replacement therapy. “As health care professionals, you’re trained to focus on those things, and you’re not really considering there might be sexual dysfunction. If women aren’t aware that it’s related to diabetes, you’ve got the perfect situation where it goes under the radar.”
However, cochair Debbie Cooke, PhD, health psychologist at the University of Surrey in Guildford, explained that having psychotherapy embedded within the diabetes team and “integrated throughout the whole service” means that the problem can be identified and treatment offered.
The issue is that such integration is “very uncommon” and access needs to be improved, Dr. Cooke said in an interview.
Sexual dysfunction major predictor of depression in women
Jacqueline Fosbury, psychotherapy lead at Diabetes Care for You, Sussex Community NHS Foundation Trust, said that “intimate activity is clearly beneficial for emotional and physical health,” as it is associated with increased oxytocin release, the burning of calories, better immunity, and improved sleep.
Sexual dysfunction is common in people with diabetes, she noted. Poor glycemic control can “damage” blood vessels and nerves, causing reduced blood flow and loss of sensation in sexual organs.
A recent study led by Belgian researchers found that among more than 750 adults with diabetes, 36% of men and 33% of women reported sexual dysfunction.
Sexual dysfunction was more common in women with type 1 diabetes, at 36%, compared with 26% for those with type 2 diabetes. The most commonly reported issues were decreased sexual desire, lubrication problems, orgasmic dysfunction, and pain. Body image problems and fear of hypoglycemia also affect sexuality and intimacy, leading to “sexual distress.”
Moreover, Ms. Fosbury said female sexual dysfunction has been identified as a “major predictor” of depression, which in turn reduces libido.
Treatments for women can include lubricants, local estrogen, and medications that are prescribed off-label, such as sildenafil. The same is true of testosterone therapy, which can be used to boost libido.
Couples therapy?
Next, Trudy Hannington, a psychosexual therapist with Leger Clinic, Doncaster, U.K., talked about how to use an integrated approach to address sexuality overall in people with diabetes.
She said this should be seen in a biopsychosocial context, with emphasis on the couple, on sensation and communication, and sexual growth, as well as changes in daily routines.
There should be a move away from “penetrative sex,” Ms. Hannington said, with the goal being “enjoyment, not orgasm.” Pleasure should be facilitated and the opportunities for “performance pressure and/or anxiety” reduced.
She discussed the case of Marie, a 27-year-old woman with type 1 diabetes who had been referred with painful sex and vaginal dryness. Marie had “never experienced orgasm,” despite being in a same-sex relationship with Emily.
Marie’s treatment involved a sexual growth program, to which Emily was invited, as well as recommendations to use lubricants, vibrators, and to try sildenafil.
Prioritize women
Ms. Fosbury reiterated that, in men, sexual dysfunction is “readily identified as a complication of diabetes” and is described as “traumatic” and “crucial to well-being.” It is also seen as “easy to treat” with medication, such as that for erectile dysfunction.
It is therefore crucial to talk to women with diabetes about possible sexual dysfunction, and the scene must be set before the appointment to explain that the subject will be broached. In addition, handouts and leaflets should be available for patients in the clinic so they can read about female sexual health and to lower the stigma around discussing it.
“Cultural stereotypes diminish the importance of female sexuality and prevent us from providing equal consideration to the sexual difficulties of our patients,” she concluded.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.