User login
Clinical Edge Journal Scan Commentary: Migraine April 2022
Neuromodulation is an up-and-coming subtype of treatments for migraine. These treatments vary significantly from transcutaneous electrical nerve stimulation (TENS)–like devices to transcranial magnetic stimulation to remote electrical stimulation of nociceptors in the arm or the vagus nerve. Some of these devices are primarily preventive in nature, whereas others are primarily for the acute treatment of migraine. Transcranial direct-current stimulation (TDCS) has recently been investigated in a number of other neurologic conditions, including multiple sclerosis and stroke, specifically for its ability to reverse manifestations of specific pathologic changes. With migraine, the question remains of whether central sensitization can similarly be reversed.
Prior studies looking at TDCS in the context of episodic migraine were mostly inconclusive. These were looking primarily at acute treatment rather than prevention. In a recent study, Hodai and colleagues took a small group of patients with treatment-refractory chronic migraine and randomly assigned them to TDCS or sham stimulation over a course of 2 months. The stimulations that the patients received were similar to protocols that have been investigated in multiple sclerosis and stroke, specifically anodal TDCS, which is thought to reverse gamma-aminobutyric acid (GABA)-ergic and glutamatergic dysregulations when the right or left cortex was stimulated.
The primary outcome of this study was decrease in baseline migraine attack frequency per month; secondary endpoints were improvement in the Headache Impact Test (HIT-6) and Migraine Disability Assessment (MIDAS) scores, the Short-Form Survey (SF-12) quality of life assessment, the Hospital Anxiety and Depression Scale (HADS) assessment, and a Clinical Global Impression (CGI) scale.
A total of 36 patients were randomly assigned to a sham or TDCS intervention. A larger reduction of migraine days per month was seen by the intervention group. The interventions were also well tolerated, and no serious adverse events were reported. None of the secondary outcomes, however, showed significance. Further analysis of responder rates showed a 50% responder rate of 36% in the intervention group vs. 14% in the sham group.
This is the first sham-controlled study investigating the use of this neuromodulation therapy for the prevention of migraine. TDCS appears to show promise even when selected for some of the most refractory situations. The question will become how this can be more practical for patient use in the future.
Prognosticating treatment effects in chronic migraine is extremely difficult to do. Most specialists have an extensive discussion with their patients that includes the likelihood of improvement in addition to the risks and benefits of the medications they are considering starting. There has been background discussion in the headache community over whether improvement with one calcitonin gene–related peptide (CGRP) antagonist medication is predictive of benefit with other medications in the class or with long-term improvement in migraine. Buse and colleagues present findings from a post hoc analysis of the PROMISE-2 study of eptinezumab for the prevention of chronic migraine.
Eptinezumab is an intravenously administered CGRP monoclonal antibody, given at either 100 mg or 300 mg every 3 months. PROMISE-2 was a randomized controlled trial that led to US Food and Drug Administration approval of eptinezumab for the prevention of chronic migraine. The authors here reviewed the data between the two intervention groups and the placebo group and then regrouped these patients according to response at month 1, defined by whether the patient was in a response group of 25%, 50%, or 75% response after 1 month of treatment. This was then compared with the patient global impact of change (PGIC) score at month 6.
This post hoc analysis did not include patients that had no response at all to either intervention or placebo at month 6. A total of 1072 patients were included in this analysis; the 100-mg, 300-mg, and placebo groups had approximately one third of patients in each.
The majority of patients in the 75% responder group continued to improve; more than half of those patients maintained the 75% response rate at month 6. More than two thirds of the 50% responders remained at a 50% response at 6 months as well. Those who responded at < 25% at month 1 were much less likely to achieve 50% response at month 6; however, the patients in the active groups were more likely to achieve a response compared with those in the placebo group.
The PGIC scores also showed significant improvement when comparing among the groups. Those who were "very much improved" at month 1 were significantly more likely to remain that way at the conclusion of the study.
Although prognosticating among different subtypes of CGRP antagonists is not yet possible, the authors here do show the ability to better inform and educate our patients when considering eptinezumab therapy for chronic migraine.
There is an age-old debate among headache specialists about overused medications: to wean or not to wean. The overuse of acute medications has long been shown to contribute to a higher frequency of migraine attacks over time, initially being called "transformed migraine" and subsequently being understood either as a subtype of chronic migraine or a separate headache disorder completely. Medication overuse headache (MOH) is something screened for by all headache providers when evaluating patients for worsening headaches. The addition of a preventive medication is the mainstay of treatment of any instance of higher frequency migraine; when MOH is a contributing factor, many practitioners will recommend complete discontinuation of the overused medications, whereas others will recommend waiting for the preventive medication to offer benefit first. As yet, there have not been any head-to-head trials investigating discontinuation vs. non-discontinuation of overused medications in this population.
Schwedt and colleagues designed a multisite trial prospectively enrolling patients with an International Classification of Headache Disorders (ICHD-3) diagnosis of both chronic migraine and MOH. Participants were told not to change their preventive medications for 4 weeks prior to enrollment. A total of 720 participants were enrolled through 14 clinics. Any patients already on preventive therapy were optimized to the best dose of that therapy or switched to other medications on the basis of the clinical investigator's judgement; all participants were randomly assigned to either discontinuation of the overused medication and given a novel acute therapy or were told to remain on their current acute therapy. No bridging therapies were recommended when switching or discontinuing acute therapies.
Of the 720 participants enrolled, 42% were already on preventive medicine. The overused medications ranged from simple analgesics for 64% of the study population to triptans, combination analgesics, and even opiates in 4% of the population. Butalbital use was included in the combination analgesic group. The primary outcome was reduction in moderate to severe migraine days, and secondary outcomes were scores for disability, depression, and quality of life (based on questionnaires).
There appeared to be no significant difference between the discontinuation and non-discontinuation groups. The authors describe this as noninferiority between the groups. To answer the age-old question of to wean or not to wean — there probably is not an answer that fits every patient. Patient adherence determines the effectiveness of anything we recommend. When evaluating patients with MOH, we have to consider whether discontinuing a medication that the patient has been depending on for months or longer will make it more or less likely for them to adhere to the other recommendations that we are making. Some patients will be very agreeable to try another acute option and stop overusing altogether. Others will be very apprehensive, and a slower, steadier approach that includes using the overused medication may be necessary. We aim always to individualize our recommendations for patients, and this should be no different.
Neuromodulation is an up-and-coming subtype of treatments for migraine. These treatments vary significantly from transcutaneous electrical nerve stimulation (TENS)–like devices to transcranial magnetic stimulation to remote electrical stimulation of nociceptors in the arm or the vagus nerve. Some of these devices are primarily preventive in nature, whereas others are primarily for the acute treatment of migraine. Transcranial direct-current stimulation (TDCS) has recently been investigated in a number of other neurologic conditions, including multiple sclerosis and stroke, specifically for its ability to reverse manifestations of specific pathologic changes. With migraine, the question remains of whether central sensitization can similarly be reversed.
Prior studies looking at TDCS in the context of episodic migraine were mostly inconclusive. These were looking primarily at acute treatment rather than prevention. In a recent study, Hodai and colleagues took a small group of patients with treatment-refractory chronic migraine and randomly assigned them to TDCS or sham stimulation over a course of 2 months. The stimulations that the patients received were similar to protocols that have been investigated in multiple sclerosis and stroke, specifically anodal TDCS, which is thought to reverse gamma-aminobutyric acid (GABA)-ergic and glutamatergic dysregulations when the right or left cortex was stimulated.
The primary outcome of this study was decrease in baseline migraine attack frequency per month; secondary endpoints were improvement in the Headache Impact Test (HIT-6) and Migraine Disability Assessment (MIDAS) scores, the Short-Form Survey (SF-12) quality of life assessment, the Hospital Anxiety and Depression Scale (HADS) assessment, and a Clinical Global Impression (CGI) scale.
A total of 36 patients were randomly assigned to a sham or TDCS intervention. A larger reduction of migraine days per month was seen by the intervention group. The interventions were also well tolerated, and no serious adverse events were reported. None of the secondary outcomes, however, showed significance. Further analysis of responder rates showed a 50% responder rate of 36% in the intervention group vs. 14% in the sham group.
This is the first sham-controlled study investigating the use of this neuromodulation therapy for the prevention of migraine. TDCS appears to show promise even when selected for some of the most refractory situations. The question will become how this can be more practical for patient use in the future.
Prognosticating treatment effects in chronic migraine is extremely difficult to do. Most specialists have an extensive discussion with their patients that includes the likelihood of improvement in addition to the risks and benefits of the medications they are considering starting. There has been background discussion in the headache community over whether improvement with one calcitonin gene–related peptide (CGRP) antagonist medication is predictive of benefit with other medications in the class or with long-term improvement in migraine. Buse and colleagues present findings from a post hoc analysis of the PROMISE-2 study of eptinezumab for the prevention of chronic migraine.
Eptinezumab is an intravenously administered CGRP monoclonal antibody, given at either 100 mg or 300 mg every 3 months. PROMISE-2 was a randomized controlled trial that led to US Food and Drug Administration approval of eptinezumab for the prevention of chronic migraine. The authors here reviewed the data between the two intervention groups and the placebo group and then regrouped these patients according to response at month 1, defined by whether the patient was in a response group of 25%, 50%, or 75% response after 1 month of treatment. This was then compared with the patient global impact of change (PGIC) score at month 6.
This post hoc analysis did not include patients that had no response at all to either intervention or placebo at month 6. A total of 1072 patients were included in this analysis; the 100-mg, 300-mg, and placebo groups had approximately one third of patients in each.
The majority of patients in the 75% responder group continued to improve; more than half of those patients maintained the 75% response rate at month 6. More than two thirds of the 50% responders remained at a 50% response at 6 months as well. Those who responded at < 25% at month 1 were much less likely to achieve 50% response at month 6; however, the patients in the active groups were more likely to achieve a response compared with those in the placebo group.
The PGIC scores also showed significant improvement when comparing among the groups. Those who were "very much improved" at month 1 were significantly more likely to remain that way at the conclusion of the study.
Although prognosticating among different subtypes of CGRP antagonists is not yet possible, the authors here do show the ability to better inform and educate our patients when considering eptinezumab therapy for chronic migraine.
There is an age-old debate among headache specialists about overused medications: to wean or not to wean. The overuse of acute medications has long been shown to contribute to a higher frequency of migraine attacks over time, initially being called "transformed migraine" and subsequently being understood either as a subtype of chronic migraine or a separate headache disorder completely. Medication overuse headache (MOH) is something screened for by all headache providers when evaluating patients for worsening headaches. The addition of a preventive medication is the mainstay of treatment of any instance of higher frequency migraine; when MOH is a contributing factor, many practitioners will recommend complete discontinuation of the overused medications, whereas others will recommend waiting for the preventive medication to offer benefit first. As yet, there have not been any head-to-head trials investigating discontinuation vs. non-discontinuation of overused medications in this population.
Schwedt and colleagues designed a multisite trial prospectively enrolling patients with an International Classification of Headache Disorders (ICHD-3) diagnosis of both chronic migraine and MOH. Participants were told not to change their preventive medications for 4 weeks prior to enrollment. A total of 720 participants were enrolled through 14 clinics. Any patients already on preventive therapy were optimized to the best dose of that therapy or switched to other medications on the basis of the clinical investigator's judgement; all participants were randomly assigned to either discontinuation of the overused medication and given a novel acute therapy or were told to remain on their current acute therapy. No bridging therapies were recommended when switching or discontinuing acute therapies.
Of the 720 participants enrolled, 42% were already on preventive medicine. The overused medications ranged from simple analgesics for 64% of the study population to triptans, combination analgesics, and even opiates in 4% of the population. Butalbital use was included in the combination analgesic group. The primary outcome was reduction in moderate to severe migraine days, and secondary outcomes were scores for disability, depression, and quality of life (based on questionnaires).
There appeared to be no significant difference between the discontinuation and non-discontinuation groups. The authors describe this as noninferiority between the groups. To answer the age-old question of to wean or not to wean — there probably is not an answer that fits every patient. Patient adherence determines the effectiveness of anything we recommend. When evaluating patients with MOH, we have to consider whether discontinuing a medication that the patient has been depending on for months or longer will make it more or less likely for them to adhere to the other recommendations that we are making. Some patients will be very agreeable to try another acute option and stop overusing altogether. Others will be very apprehensive, and a slower, steadier approach that includes using the overused medication may be necessary. We aim always to individualize our recommendations for patients, and this should be no different.
Neuromodulation is an up-and-coming subtype of treatments for migraine. These treatments vary significantly from transcutaneous electrical nerve stimulation (TENS)–like devices to transcranial magnetic stimulation to remote electrical stimulation of nociceptors in the arm or the vagus nerve. Some of these devices are primarily preventive in nature, whereas others are primarily for the acute treatment of migraine. Transcranial direct-current stimulation (TDCS) has recently been investigated in a number of other neurologic conditions, including multiple sclerosis and stroke, specifically for its ability to reverse manifestations of specific pathologic changes. With migraine, the question remains of whether central sensitization can similarly be reversed.
Prior studies looking at TDCS in the context of episodic migraine were mostly inconclusive. These were looking primarily at acute treatment rather than prevention. In a recent study, Hodai and colleagues took a small group of patients with treatment-refractory chronic migraine and randomly assigned them to TDCS or sham stimulation over a course of 2 months. The stimulations that the patients received were similar to protocols that have been investigated in multiple sclerosis and stroke, specifically anodal TDCS, which is thought to reverse gamma-aminobutyric acid (GABA)-ergic and glutamatergic dysregulations when the right or left cortex was stimulated.
The primary outcome of this study was decrease in baseline migraine attack frequency per month; secondary endpoints were improvement in the Headache Impact Test (HIT-6) and Migraine Disability Assessment (MIDAS) scores, the Short-Form Survey (SF-12) quality of life assessment, the Hospital Anxiety and Depression Scale (HADS) assessment, and a Clinical Global Impression (CGI) scale.
A total of 36 patients were randomly assigned to a sham or TDCS intervention. A larger reduction of migraine days per month was seen by the intervention group. The interventions were also well tolerated, and no serious adverse events were reported. None of the secondary outcomes, however, showed significance. Further analysis of responder rates showed a 50% responder rate of 36% in the intervention group vs. 14% in the sham group.
This is the first sham-controlled study investigating the use of this neuromodulation therapy for the prevention of migraine. TDCS appears to show promise even when selected for some of the most refractory situations. The question will become how this can be more practical for patient use in the future.
Prognosticating treatment effects in chronic migraine is extremely difficult to do. Most specialists have an extensive discussion with their patients that includes the likelihood of improvement in addition to the risks and benefits of the medications they are considering starting. There has been background discussion in the headache community over whether improvement with one calcitonin gene–related peptide (CGRP) antagonist medication is predictive of benefit with other medications in the class or with long-term improvement in migraine. Buse and colleagues present findings from a post hoc analysis of the PROMISE-2 study of eptinezumab for the prevention of chronic migraine.
Eptinezumab is an intravenously administered CGRP monoclonal antibody, given at either 100 mg or 300 mg every 3 months. PROMISE-2 was a randomized controlled trial that led to US Food and Drug Administration approval of eptinezumab for the prevention of chronic migraine. The authors here reviewed the data between the two intervention groups and the placebo group and then regrouped these patients according to response at month 1, defined by whether the patient was in a response group of 25%, 50%, or 75% response after 1 month of treatment. This was then compared with the patient global impact of change (PGIC) score at month 6.
This post hoc analysis did not include patients that had no response at all to either intervention or placebo at month 6. A total of 1072 patients were included in this analysis; the 100-mg, 300-mg, and placebo groups had approximately one third of patients in each.
The majority of patients in the 75% responder group continued to improve; more than half of those patients maintained the 75% response rate at month 6. More than two thirds of the 50% responders remained at a 50% response at 6 months as well. Those who responded at < 25% at month 1 were much less likely to achieve 50% response at month 6; however, the patients in the active groups were more likely to achieve a response compared with those in the placebo group.
The PGIC scores also showed significant improvement when comparing among the groups. Those who were "very much improved" at month 1 were significantly more likely to remain that way at the conclusion of the study.
Although prognosticating among different subtypes of CGRP antagonists is not yet possible, the authors here do show the ability to better inform and educate our patients when considering eptinezumab therapy for chronic migraine.
There is an age-old debate among headache specialists about overused medications: to wean or not to wean. The overuse of acute medications has long been shown to contribute to a higher frequency of migraine attacks over time, initially being called "transformed migraine" and subsequently being understood either as a subtype of chronic migraine or a separate headache disorder completely. Medication overuse headache (MOH) is something screened for by all headache providers when evaluating patients for worsening headaches. The addition of a preventive medication is the mainstay of treatment of any instance of higher frequency migraine; when MOH is a contributing factor, many practitioners will recommend complete discontinuation of the overused medications, whereas others will recommend waiting for the preventive medication to offer benefit first. As yet, there have not been any head-to-head trials investigating discontinuation vs. non-discontinuation of overused medications in this population.
Schwedt and colleagues designed a multisite trial prospectively enrolling patients with an International Classification of Headache Disorders (ICHD-3) diagnosis of both chronic migraine and MOH. Participants were told not to change their preventive medications for 4 weeks prior to enrollment. A total of 720 participants were enrolled through 14 clinics. Any patients already on preventive therapy were optimized to the best dose of that therapy or switched to other medications on the basis of the clinical investigator's judgement; all participants were randomly assigned to either discontinuation of the overused medication and given a novel acute therapy or were told to remain on their current acute therapy. No bridging therapies were recommended when switching or discontinuing acute therapies.
Of the 720 participants enrolled, 42% were already on preventive medicine. The overused medications ranged from simple analgesics for 64% of the study population to triptans, combination analgesics, and even opiates in 4% of the population. Butalbital use was included in the combination analgesic group. The primary outcome was reduction in moderate to severe migraine days, and secondary outcomes were scores for disability, depression, and quality of life (based on questionnaires).
There appeared to be no significant difference between the discontinuation and non-discontinuation groups. The authors describe this as noninferiority between the groups. To answer the age-old question of to wean or not to wean — there probably is not an answer that fits every patient. Patient adherence determines the effectiveness of anything we recommend. When evaluating patients with MOH, we have to consider whether discontinuing a medication that the patient has been depending on for months or longer will make it more or less likely for them to adhere to the other recommendations that we are making. Some patients will be very agreeable to try another acute option and stop overusing altogether. Others will be very apprehensive, and a slower, steadier approach that includes using the overused medication may be necessary. We aim always to individualize our recommendations for patients, and this should be no different.
Clinical Edge Journal Scan Commentary: PsA April 2022
Treatment of psoriatic arthritis (PsA) was the focus of clinical research papers published this month. Despite the advances made in treating PsA with targeted therapies, in most parts of the world, conventional disease-modifying antirheumatic drugs (DMARDs) are the first line of treatment. Methotrexate (MTX) and leflunomide (LEF) are commonly used, but there are limited data on the effectiveness of combination therapy. To address this issue, Mulder and colleagues enrolled 78 patients with active PsA who have two or more swollen joints and randomly allocated them to either 25 mg oral MTX weekly after 4 weeks of 15 mg weekly plus 20 mg LEF daily (n = 39) or MTX plus placebo (monotherapy; n = 39). At week 16, PsA disease activity score was improved significantly in the MTX + LEF vs. MTX monotherapy group (3.1 vs. 3.7; P = .025). Incidence of mild adverse events, such as nausea/vomiting (44% vs. 28%) and altered bowel habits (26% vs. 8%), was higher with MTX + LEF vs. MTX + placebo. So although less well tolerated, MTX + LEF therapy was superior to MTX monotherapy at improving disease activity in patients with PsA.
Biologics targeting tumor necrosis factor (TNF), interleukin (IL) -12/23, -23, and -17A are efficacious for the management of PsA, but questions remain about comparative effectiveness. Gossec and colleagues reported the results from their prospective observational PsABio study that evaluated real-world treatment persistence and effectiveness at 1 year after initiation of first-line to third-line IL-12/23 inhibitor ustekinumab or a TNF inhibitor (TNFi). Their study followed 893 patients. After 1 year of treatment, ustekinumab and the TNFi showed similar persistence (hazard ratio [HR] for stopping/switching treatment 0.82; 95% CI 0.60-1.13) and a similar proportion of patients achieving (on the Disease Activity Index for PsA) clinical low disease activity (odds ratio [OR] 0.80; 95% CI 0.57-1.10) and remission (OR 0.73; 95% CI 0.49-1.07), along with similar safety profiles. Thus in real-world studies, TNFi and ustekinumab seem to have similar effectiveness and safety.
Drug persistence between patients with psoriasis alone vs. those with PsA is also of interest. In a real-life study including 62 patients with psoriasis and 90 patients with PsA who initiated treatment with secukinumab and were followed up for 24 months or until discontinuation, Ortolan and colleagues demonstrated that the retention rate of secukinumab was higher in psoriasis vs. PsA at 12 (85% vs. 68%) and 24 (61% vs. 57%) months, with the risk for secukinumab discontinuation being higher among patients with PsA in the overall cohort (HR 2.43; P = .035) and in patients with obesity in the PsA cohort (P = .021). Thus, the presence of PsA and obesity lower the secukinumab retention rate.
- Despite the advent of many targeted therapies for PsA, there remain many unmet needs. Deucravacitinib is a novel oral selective inhibitor of tyrosine kinase 2 (TYK2) acting via binding to the TYK2 regulatory domain. In a phase 2 study including 203 patients with active PsA that was intolerant to at least one therapy who were randomly assigned to receive 6 mg deucravacitinib once daily, 12 mg deucravacitinib once daily, or placebo for 16 weeks, Mease and colleagues demonstrated that at week 16, American College of Rheumatology 20 (ACR20) response was significantly higher with 6 mg once-daily deucravacitinib (52.9%, adjusted OR [aOR] 2.4; P = .0134) and 12 mg (62.7%, aOR 3.6; P = .0004) vs. placebo (31.8%), with 12 mg deucravacitinib improving ACR20 response as early as at 8 weeks (P < .05). No serious adverse events were reported. Thus, TYK2 inhibition shows promise in the treatment of PsA and the results from phase 3 trials are awaited.
Treatment of psoriatic arthritis (PsA) was the focus of clinical research papers published this month. Despite the advances made in treating PsA with targeted therapies, in most parts of the world, conventional disease-modifying antirheumatic drugs (DMARDs) are the first line of treatment. Methotrexate (MTX) and leflunomide (LEF) are commonly used, but there are limited data on the effectiveness of combination therapy. To address this issue, Mulder and colleagues enrolled 78 patients with active PsA who have two or more swollen joints and randomly allocated them to either 25 mg oral MTX weekly after 4 weeks of 15 mg weekly plus 20 mg LEF daily (n = 39) or MTX plus placebo (monotherapy; n = 39). At week 16, PsA disease activity score was improved significantly in the MTX + LEF vs. MTX monotherapy group (3.1 vs. 3.7; P = .025). Incidence of mild adverse events, such as nausea/vomiting (44% vs. 28%) and altered bowel habits (26% vs. 8%), was higher with MTX + LEF vs. MTX + placebo. So although less well tolerated, MTX + LEF therapy was superior to MTX monotherapy at improving disease activity in patients with PsA.
Biologics targeting tumor necrosis factor (TNF), interleukin (IL) -12/23, -23, and -17A are efficacious for the management of PsA, but questions remain about comparative effectiveness. Gossec and colleagues reported the results from their prospective observational PsABio study that evaluated real-world treatment persistence and effectiveness at 1 year after initiation of first-line to third-line IL-12/23 inhibitor ustekinumab or a TNF inhibitor (TNFi). Their study followed 893 patients. After 1 year of treatment, ustekinumab and the TNFi showed similar persistence (hazard ratio [HR] for stopping/switching treatment 0.82; 95% CI 0.60-1.13) and a similar proportion of patients achieving (on the Disease Activity Index for PsA) clinical low disease activity (odds ratio [OR] 0.80; 95% CI 0.57-1.10) and remission (OR 0.73; 95% CI 0.49-1.07), along with similar safety profiles. Thus in real-world studies, TNFi and ustekinumab seem to have similar effectiveness and safety.
Drug persistence between patients with psoriasis alone vs. those with PsA is also of interest. In a real-life study including 62 patients with psoriasis and 90 patients with PsA who initiated treatment with secukinumab and were followed up for 24 months or until discontinuation, Ortolan and colleagues demonstrated that the retention rate of secukinumab was higher in psoriasis vs. PsA at 12 (85% vs. 68%) and 24 (61% vs. 57%) months, with the risk for secukinumab discontinuation being higher among patients with PsA in the overall cohort (HR 2.43; P = .035) and in patients with obesity in the PsA cohort (P = .021). Thus, the presence of PsA and obesity lower the secukinumab retention rate.
- Despite the advent of many targeted therapies for PsA, there remain many unmet needs. Deucravacitinib is a novel oral selective inhibitor of tyrosine kinase 2 (TYK2) acting via binding to the TYK2 regulatory domain. In a phase 2 study including 203 patients with active PsA that was intolerant to at least one therapy who were randomly assigned to receive 6 mg deucravacitinib once daily, 12 mg deucravacitinib once daily, or placebo for 16 weeks, Mease and colleagues demonstrated that at week 16, American College of Rheumatology 20 (ACR20) response was significantly higher with 6 mg once-daily deucravacitinib (52.9%, adjusted OR [aOR] 2.4; P = .0134) and 12 mg (62.7%, aOR 3.6; P = .0004) vs. placebo (31.8%), with 12 mg deucravacitinib improving ACR20 response as early as at 8 weeks (P < .05). No serious adverse events were reported. Thus, TYK2 inhibition shows promise in the treatment of PsA and the results from phase 3 trials are awaited.
Treatment of psoriatic arthritis (PsA) was the focus of clinical research papers published this month. Despite the advances made in treating PsA with targeted therapies, in most parts of the world, conventional disease-modifying antirheumatic drugs (DMARDs) are the first line of treatment. Methotrexate (MTX) and leflunomide (LEF) are commonly used, but there are limited data on the effectiveness of combination therapy. To address this issue, Mulder and colleagues enrolled 78 patients with active PsA who have two or more swollen joints and randomly allocated them to either 25 mg oral MTX weekly after 4 weeks of 15 mg weekly plus 20 mg LEF daily (n = 39) or MTX plus placebo (monotherapy; n = 39). At week 16, PsA disease activity score was improved significantly in the MTX + LEF vs. MTX monotherapy group (3.1 vs. 3.7; P = .025). Incidence of mild adverse events, such as nausea/vomiting (44% vs. 28%) and altered bowel habits (26% vs. 8%), was higher with MTX + LEF vs. MTX + placebo. So although less well tolerated, MTX + LEF therapy was superior to MTX monotherapy at improving disease activity in patients with PsA.
Biologics targeting tumor necrosis factor (TNF), interleukin (IL) -12/23, -23, and -17A are efficacious for the management of PsA, but questions remain about comparative effectiveness. Gossec and colleagues reported the results from their prospective observational PsABio study that evaluated real-world treatment persistence and effectiveness at 1 year after initiation of first-line to third-line IL-12/23 inhibitor ustekinumab or a TNF inhibitor (TNFi). Their study followed 893 patients. After 1 year of treatment, ustekinumab and the TNFi showed similar persistence (hazard ratio [HR] for stopping/switching treatment 0.82; 95% CI 0.60-1.13) and a similar proportion of patients achieving (on the Disease Activity Index for PsA) clinical low disease activity (odds ratio [OR] 0.80; 95% CI 0.57-1.10) and remission (OR 0.73; 95% CI 0.49-1.07), along with similar safety profiles. Thus in real-world studies, TNFi and ustekinumab seem to have similar effectiveness and safety.
Drug persistence between patients with psoriasis alone vs. those with PsA is also of interest. In a real-life study including 62 patients with psoriasis and 90 patients with PsA who initiated treatment with secukinumab and were followed up for 24 months or until discontinuation, Ortolan and colleagues demonstrated that the retention rate of secukinumab was higher in psoriasis vs. PsA at 12 (85% vs. 68%) and 24 (61% vs. 57%) months, with the risk for secukinumab discontinuation being higher among patients with PsA in the overall cohort (HR 2.43; P = .035) and in patients with obesity in the PsA cohort (P = .021). Thus, the presence of PsA and obesity lower the secukinumab retention rate.
- Despite the advent of many targeted therapies for PsA, there remain many unmet needs. Deucravacitinib is a novel oral selective inhibitor of tyrosine kinase 2 (TYK2) acting via binding to the TYK2 regulatory domain. In a phase 2 study including 203 patients with active PsA that was intolerant to at least one therapy who were randomly assigned to receive 6 mg deucravacitinib once daily, 12 mg deucravacitinib once daily, or placebo for 16 weeks, Mease and colleagues demonstrated that at week 16, American College of Rheumatology 20 (ACR20) response was significantly higher with 6 mg once-daily deucravacitinib (52.9%, adjusted OR [aOR] 2.4; P = .0134) and 12 mg (62.7%, aOR 3.6; P = .0004) vs. placebo (31.8%), with 12 mg deucravacitinib improving ACR20 response as early as at 8 weeks (P < .05). No serious adverse events were reported. Thus, TYK2 inhibition shows promise in the treatment of PsA and the results from phase 3 trials are awaited.
Anaphylaxis risk with IV iron low, but varies with formulation
The results of the new retrospective cohort study were published online March 29 in Annals of Internal Medicine (doi: 10.7326/M21-4009).
“The rates of anaphylaxis were very low with all IV iron products but were three- to eightfold greater for iron dextran and ferumoxytol than for iron sucrose,” wrote Chintan V. Dave, PharmD, PhD, of Rutgers University, New Brunswick, N.J., and colleagues.
Using data from Medicare insurance claims, the researchers evaluated the incidence of anaphylaxis among patients 65 years or older receiving their first dose of one of five different IV iron formulations for the treatment of iron deficiency anemia. Patients were treated between July 2013 and December 2018 and the iron formulations were ferric carboxymaltose, ferumoxytol, ferric gluconate, iron dextran, or iron sucrose.
Overall, 167,925 patients were included and categorized based on the iron supplement they received. Dr. Dave and colleagues found that the adjusted incidence rates (IRs) for anaphylaxis per 10,000 first administrations were 9.8 cases for iron dextran (95% confidence interval [CI], 6.2 to 15.3 cases), 4.0 cases for ferumoxytol (95% CI, 2.5 to 6.6 cases), 1.5 cases for ferric gluconate (95% CI, 0.3 to 6.6 cases), 1.2 cases for iron sucrose (95% CI, 0.6 to 2.5 cases), and 0.8 cases for ferric carboxymaltose (95% CI, 0.3 to 2.6 cases).
Only those patients receiving iron dextran or ferumoxytol had anaphylactic reactions requiring hospitalization.
Using iron sucrose as the referent category, the researchers found that the odds ratios (ORs) for anaphylaxis were 8.3 for iron dextran (95% CI, 3.5-19.8) and 3.4 for ferumoxytol (95% CI, 1.4-8.3).
“Anaphylaxis is just one of many factors one should consider when deciding on the choice of IV iron therapy,” Dr. Dave noted in an interview, when asked whether he feels that these findings will change the use of parenteral iron in practice.
Acknowledging that anaphylaxis is a severe but rare complication, Dr. Dave stated that other factors such as “clinical indication, setting, dose, the number and duration of administrations required to replenish iron reserves, risk of other adverse reactions, and costs,” should also be considered when designing treatment plans using intravenous iron.
In the study, anaphylaxis was defined as reactions that occurred within 24 hours of IV iron administration and was restricted to the following:
- Anaphylaxis resulting in hospitalization.
- An outpatient or emergency department visit due to anaphylactic shock accompanied by codes relating to the administration of cardiopulmonary resuscitation or epinephrine or the occurrence of hypotension.
- Two separate encounters for anaphylactic shock within the same day representing different encounter types, that is, inpatient, outpatient, or emergency department visit.
Dr. Dave and colleagues acknowledged study limitations, such as the fact the anaphylaxis criteria included only the most severe cases and could therefore have missed milder cases of anaphylaxis secondary to IV iron. Further, they noted that these findings may not be applicable to a younger patient population.
Patients were excluded from the study if they had received IV iron between January 2007 and July 2013, had a diagnosis of HIV or end-stage renal disease, had a recent blood transfusion, or had a history of anaphylactic reactions.
The study authors disclosed no relevant financial relationships.
The results of the new retrospective cohort study were published online March 29 in Annals of Internal Medicine (doi: 10.7326/M21-4009).
“The rates of anaphylaxis were very low with all IV iron products but were three- to eightfold greater for iron dextran and ferumoxytol than for iron sucrose,” wrote Chintan V. Dave, PharmD, PhD, of Rutgers University, New Brunswick, N.J., and colleagues.
Using data from Medicare insurance claims, the researchers evaluated the incidence of anaphylaxis among patients 65 years or older receiving their first dose of one of five different IV iron formulations for the treatment of iron deficiency anemia. Patients were treated between July 2013 and December 2018 and the iron formulations were ferric carboxymaltose, ferumoxytol, ferric gluconate, iron dextran, or iron sucrose.
Overall, 167,925 patients were included and categorized based on the iron supplement they received. Dr. Dave and colleagues found that the adjusted incidence rates (IRs) for anaphylaxis per 10,000 first administrations were 9.8 cases for iron dextran (95% confidence interval [CI], 6.2 to 15.3 cases), 4.0 cases for ferumoxytol (95% CI, 2.5 to 6.6 cases), 1.5 cases for ferric gluconate (95% CI, 0.3 to 6.6 cases), 1.2 cases for iron sucrose (95% CI, 0.6 to 2.5 cases), and 0.8 cases for ferric carboxymaltose (95% CI, 0.3 to 2.6 cases).
Only those patients receiving iron dextran or ferumoxytol had anaphylactic reactions requiring hospitalization.
Using iron sucrose as the referent category, the researchers found that the odds ratios (ORs) for anaphylaxis were 8.3 for iron dextran (95% CI, 3.5-19.8) and 3.4 for ferumoxytol (95% CI, 1.4-8.3).
“Anaphylaxis is just one of many factors one should consider when deciding on the choice of IV iron therapy,” Dr. Dave noted in an interview, when asked whether he feels that these findings will change the use of parenteral iron in practice.
Acknowledging that anaphylaxis is a severe but rare complication, Dr. Dave stated that other factors such as “clinical indication, setting, dose, the number and duration of administrations required to replenish iron reserves, risk of other adverse reactions, and costs,” should also be considered when designing treatment plans using intravenous iron.
In the study, anaphylaxis was defined as reactions that occurred within 24 hours of IV iron administration and was restricted to the following:
- Anaphylaxis resulting in hospitalization.
- An outpatient or emergency department visit due to anaphylactic shock accompanied by codes relating to the administration of cardiopulmonary resuscitation or epinephrine or the occurrence of hypotension.
- Two separate encounters for anaphylactic shock within the same day representing different encounter types, that is, inpatient, outpatient, or emergency department visit.
Dr. Dave and colleagues acknowledged study limitations, such as the fact the anaphylaxis criteria included only the most severe cases and could therefore have missed milder cases of anaphylaxis secondary to IV iron. Further, they noted that these findings may not be applicable to a younger patient population.
Patients were excluded from the study if they had received IV iron between January 2007 and July 2013, had a diagnosis of HIV or end-stage renal disease, had a recent blood transfusion, or had a history of anaphylactic reactions.
The study authors disclosed no relevant financial relationships.
The results of the new retrospective cohort study were published online March 29 in Annals of Internal Medicine (doi: 10.7326/M21-4009).
“The rates of anaphylaxis were very low with all IV iron products but were three- to eightfold greater for iron dextran and ferumoxytol than for iron sucrose,” wrote Chintan V. Dave, PharmD, PhD, of Rutgers University, New Brunswick, N.J., and colleagues.
Using data from Medicare insurance claims, the researchers evaluated the incidence of anaphylaxis among patients 65 years or older receiving their first dose of one of five different IV iron formulations for the treatment of iron deficiency anemia. Patients were treated between July 2013 and December 2018 and the iron formulations were ferric carboxymaltose, ferumoxytol, ferric gluconate, iron dextran, or iron sucrose.
Overall, 167,925 patients were included and categorized based on the iron supplement they received. Dr. Dave and colleagues found that the adjusted incidence rates (IRs) for anaphylaxis per 10,000 first administrations were 9.8 cases for iron dextran (95% confidence interval [CI], 6.2 to 15.3 cases), 4.0 cases for ferumoxytol (95% CI, 2.5 to 6.6 cases), 1.5 cases for ferric gluconate (95% CI, 0.3 to 6.6 cases), 1.2 cases for iron sucrose (95% CI, 0.6 to 2.5 cases), and 0.8 cases for ferric carboxymaltose (95% CI, 0.3 to 2.6 cases).
Only those patients receiving iron dextran or ferumoxytol had anaphylactic reactions requiring hospitalization.
Using iron sucrose as the referent category, the researchers found that the odds ratios (ORs) for anaphylaxis were 8.3 for iron dextran (95% CI, 3.5-19.8) and 3.4 for ferumoxytol (95% CI, 1.4-8.3).
“Anaphylaxis is just one of many factors one should consider when deciding on the choice of IV iron therapy,” Dr. Dave noted in an interview, when asked whether he feels that these findings will change the use of parenteral iron in practice.
Acknowledging that anaphylaxis is a severe but rare complication, Dr. Dave stated that other factors such as “clinical indication, setting, dose, the number and duration of administrations required to replenish iron reserves, risk of other adverse reactions, and costs,” should also be considered when designing treatment plans using intravenous iron.
In the study, anaphylaxis was defined as reactions that occurred within 24 hours of IV iron administration and was restricted to the following:
- Anaphylaxis resulting in hospitalization.
- An outpatient or emergency department visit due to anaphylactic shock accompanied by codes relating to the administration of cardiopulmonary resuscitation or epinephrine or the occurrence of hypotension.
- Two separate encounters for anaphylactic shock within the same day representing different encounter types, that is, inpatient, outpatient, or emergency department visit.
Dr. Dave and colleagues acknowledged study limitations, such as the fact the anaphylaxis criteria included only the most severe cases and could therefore have missed milder cases of anaphylaxis secondary to IV iron. Further, they noted that these findings may not be applicable to a younger patient population.
Patients were excluded from the study if they had received IV iron between January 2007 and July 2013, had a diagnosis of HIV or end-stage renal disease, had a recent blood transfusion, or had a history of anaphylactic reactions.
The study authors disclosed no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Clinical clarity grows about toenail disorder, experts report
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
BOSTON – The main commonly leading to the wrong therapy and no resolution to the problem, according to an expert update at the annual meeting of the American Academy of Dermatology.
Misinterpretation of the yellow discoloration, a common feature of retronychia, means “many patients are maintained on antifungal therapy for years and years with no change in their condition,” reported Phoebe Rich, MD, director of the Nail Disorders Clinic, Oregon Health & Science University, Portland.
Infection is not commonly involved in retronychia, but importantly, antifungals and antibiotics “have no role in treating the underlying disorder,” Dr. Rich said.
The term retronychia and its description is only about 20 years old, according to Dr. Rich, who cited work by David A. de Berker, MBBS, PhD, a consultant dermatologist at University Hospitals in Bristol, England. His publication on this disorder appeared in 1999, with a more detailed description published about 10 years later.
Recently, the body of literature on this disorder has been growing, contributing to an increasing consensus about etiology, diagnosis, and treatments to consider in the context of causes and severity, Dr. Rich said.
Some but not all patients have abnormal formation of the nail bed, increasing susceptibility to retronychia, but trauma or microtrauma typically serve as a trigger in most cases. Dancing, high heels, steel-toed shoes, and other sources of trauma to the toes are implicated.
Whether or not patients have an inherent susceptibility, injury separates the existing nail from the matrix and nail bed so that newly forming nail begins to grow under the nail rather continuing to push out the old nail.
Susceptibility is increased substantially in individuals with a shortened nail bed, according to Dr. Rich. In severe cases, when there is simply inadequate nail bed for the nail growth to attach, recurrence is common or even inevitable. Even when the nail is removed and regrowth appears normal at the end of a year, those patients with very short nail beds cannot count on a cure.
“Due to the slow growth of nails, it might take 2 or 3 years for the problem to recur,” Dr. Rich cautioned. For this reason, cure rates reported for the various interventions at 1 year might not predict longer-term benefit.
Retronychia is usually a clinical diagnosis based on the presence of the increased bulk of the toenail when overlapping nails cannot be seen. This is not necessarily a single overgrowth. In some cases, multiple layers of nails are stacked one on top of the other. Xanthonychia (yellow nail) is usually present.
“The layering might not be visible without removing the nail,” said Dr. Rich, explaining one reason that the diagnosis is sometimes missed. Ultrasound is a noninvasive means to confirm the problem, although Rich warned that imaging is not necessarily reimbursed.
“There is no diagnosis by histopathology, so it cannot be confirmed with biopsy,” Dr. Rich said.
Treatments range from conservative strategies, particularly topical or intralesional steroids in mild cases, to more invasive procedures such as clipping of the nail plate or surgical avulsion. All can be effective when used appropriately, according to Dr. Rich.
“The more invasive procedures are the more effective, but the caveat is they are also associated with more complications,” said Dr. Rich, citing, for example, the risk of nail dystrophies. Because of the increasing number of studies, the relative benefits and risks of retronychia treatment have now been summarized in a recent review. Dr. Rich suggested the review is one of the most recent and detailed evaluations of the topic that “I encourage everyone to read.”
Despite progress in describing retronychia, Dr. Rich said that there might be more to learn about risk. In particular, she cited the work of Dana W. Stern, MD, a specialist in nail disorders who is in private practice in New York. Dr. Stern is pursuing a hypothesis that at least some cases are caused by potentially targetable biomechanical issues.
“I have observed that many of the younger patients in my practice with retronychia seem to have atypical foot anatomy,” Dr. Stern said in an interview. “I am collecting cases and hoping to explore this issue in more depth.”
She said that foot anatomy in relationship to retronychia has not been adequately evaluated.
“In my review of the literature, I could not find a single study that showed imagery of the feet,” she said. She is considering a collaboration with others, including Rich, to explore this as a factor in retronychia.
Asked about risk of misdiagnosis, Dr. Stern reiterated some of the points made by Dr. Rich. In particular, she agreed that discolored nails alone should not be a reason to initiate antimycotic therapy without considering the possibility of retronychia.
“So many providers are not familiar with the diagnosis, and only 50% of yellow thickened nails are in fact onychomycosis,” she said. “We end up seeing a plethora of patients [with retronychia] who are unfortunately misdiagnosed for years.”
Dr. Rich reported financial relationships with numerous pharmaceutical companies. Dr. Stern reported a financial relationship with Rare Beauty Brands. Neither Dr. Rich nor Dr. Stern said they had any disclosures related to this topic.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Sustained jawline definition from hyaluronic gel, study reports
BOSTON – After several promising early phase studies, from what study authors characterized as a “pivotal” randomized multicenter trial. The results were presented during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The primary outcome, assessed at 6 months, was at least a 1-point improvement in a photonumeric scale used to grade jawline sagging, reported Jeremy Green, MD, Skin Associates of South Florida, Coral Gables.
When those randomized to the hyaluronic filler gel VYC-25L (Vycross, Juvéderm) were compared with untreated controls, 68.5% versus 38.4% met the criterion for benefit at 6 months. Importantly, the effect in treated patients was sustained when reevaluated at 12 months. Green reported that the response is generally sustained at the maximum follow-up, now out to 17 months.
Most enrolled patients are severely affected
In this study, 208 patients with severe (74%) or moderate loss of jawline definition were randomized in a 3:1 ratio to receive the filler or serve as controls. The initially untreated controls received the gel after the primary outcome analysis at 6 months.
The hyaluronic gel was injected at five sites along the jawline. The mean age of participants was 58 years. The majority were women, and most were White.
Dermatologists blinded to treatment compared photos at 6 months with those taken at baseline using the photonumeric grading system of 1-5. Change in patient satisfaction at 6 months and again at 12 months relative to baseline was also evaluated.
From baseline, when 28.9% of participants reported satisfaction on the Global Aesthetic Improvement Scale (GAIS), rates rose to 89.0% at month 6. There was a decline at month 12, but 79.9% remained satisfied after this period of follow-up.
Most patients experienced injection site reactions that were mainly mild to moderate and all resolved within several days of treatment. Pain with mastication was initially reported by 1.9%, but again this complaint was also mild and transient. All complaints had largely resolved by day 3.
The results are consistent with several previous clinical studies of VYC-25L for the same indication. In a similarly designed trial conducted in Europe that also used a 3:1 randomization scheme, the primary outcome assessed at 3 months was change in facial angle. Relative to controls, the angle improved by 2.51 degrees (P < .0001).
Patient satisfaction supports filler benefit
In the similar European trial, the clinical significance of the objective primary outcome also was supported by patient satisfaction assessed with several instruments, including the GAIS. Some degree of swelling or tenderness was experienced by almost all patients after injection, but none were serious, and all resolved.
In another trial, 202 patients with chin retrusion were randomized in a 3:1 ratio to VYC-25L or a control group. In that study, the primary outcome was at least a 1-point improvement in the Allergan Chin Retrusion Scale at 6 months. This advantage for treatment (56.3% vs. 27.5%) was again supported by several instruments for evaluating patient satisfaction, including GAIS.
As in the other studies, most patients had injection site reactions. Although all resolved within days of treatment, one patient left the study after experiencing cellulitis and injection-site inflammation.
Dissatisfaction with jawline definition is a relatively common complaint in Dr. Green’s experience, who said that there is a need for more effective and well-tolerated treatments. Given the efficacy, tolerability, and safety of VYC-25L in this controlled study, he suggested this product has potential utility.
In the field of cosmetic dermatology, there appears to be incremental progress in fillers with favorable clinical characteristics, according to Sandy U. Tsao, MD, a dermatologic surgeon at Massachusetts General Hospital, Boston.
“We are seeing filler lasting longer and longer,” she said, commenting specifically about the results presented by Dr. Green. She called sustained aesthetic improvement at 12 months for the filler in this study “really exciting.”
Dr. Green has reported financial relationships with numerous pharmaceutical companies. Dr. Tsao has reported financial relationships with Epiphany Dermatology, Lazarus AI, and UpToDate.
A version of this article first appeared on Medscape.com.
BOSTON – After several promising early phase studies, from what study authors characterized as a “pivotal” randomized multicenter trial. The results were presented during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The primary outcome, assessed at 6 months, was at least a 1-point improvement in a photonumeric scale used to grade jawline sagging, reported Jeremy Green, MD, Skin Associates of South Florida, Coral Gables.
When those randomized to the hyaluronic filler gel VYC-25L (Vycross, Juvéderm) were compared with untreated controls, 68.5% versus 38.4% met the criterion for benefit at 6 months. Importantly, the effect in treated patients was sustained when reevaluated at 12 months. Green reported that the response is generally sustained at the maximum follow-up, now out to 17 months.
Most enrolled patients are severely affected
In this study, 208 patients with severe (74%) or moderate loss of jawline definition were randomized in a 3:1 ratio to receive the filler or serve as controls. The initially untreated controls received the gel after the primary outcome analysis at 6 months.
The hyaluronic gel was injected at five sites along the jawline. The mean age of participants was 58 years. The majority were women, and most were White.
Dermatologists blinded to treatment compared photos at 6 months with those taken at baseline using the photonumeric grading system of 1-5. Change in patient satisfaction at 6 months and again at 12 months relative to baseline was also evaluated.
From baseline, when 28.9% of participants reported satisfaction on the Global Aesthetic Improvement Scale (GAIS), rates rose to 89.0% at month 6. There was a decline at month 12, but 79.9% remained satisfied after this period of follow-up.
Most patients experienced injection site reactions that were mainly mild to moderate and all resolved within several days of treatment. Pain with mastication was initially reported by 1.9%, but again this complaint was also mild and transient. All complaints had largely resolved by day 3.
The results are consistent with several previous clinical studies of VYC-25L for the same indication. In a similarly designed trial conducted in Europe that also used a 3:1 randomization scheme, the primary outcome assessed at 3 months was change in facial angle. Relative to controls, the angle improved by 2.51 degrees (P < .0001).
Patient satisfaction supports filler benefit
In the similar European trial, the clinical significance of the objective primary outcome also was supported by patient satisfaction assessed with several instruments, including the GAIS. Some degree of swelling or tenderness was experienced by almost all patients after injection, but none were serious, and all resolved.
In another trial, 202 patients with chin retrusion were randomized in a 3:1 ratio to VYC-25L or a control group. In that study, the primary outcome was at least a 1-point improvement in the Allergan Chin Retrusion Scale at 6 months. This advantage for treatment (56.3% vs. 27.5%) was again supported by several instruments for evaluating patient satisfaction, including GAIS.
As in the other studies, most patients had injection site reactions. Although all resolved within days of treatment, one patient left the study after experiencing cellulitis and injection-site inflammation.
Dissatisfaction with jawline definition is a relatively common complaint in Dr. Green’s experience, who said that there is a need for more effective and well-tolerated treatments. Given the efficacy, tolerability, and safety of VYC-25L in this controlled study, he suggested this product has potential utility.
In the field of cosmetic dermatology, there appears to be incremental progress in fillers with favorable clinical characteristics, according to Sandy U. Tsao, MD, a dermatologic surgeon at Massachusetts General Hospital, Boston.
“We are seeing filler lasting longer and longer,” she said, commenting specifically about the results presented by Dr. Green. She called sustained aesthetic improvement at 12 months for the filler in this study “really exciting.”
Dr. Green has reported financial relationships with numerous pharmaceutical companies. Dr. Tsao has reported financial relationships with Epiphany Dermatology, Lazarus AI, and UpToDate.
A version of this article first appeared on Medscape.com.
BOSTON – After several promising early phase studies, from what study authors characterized as a “pivotal” randomized multicenter trial. The results were presented during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
The primary outcome, assessed at 6 months, was at least a 1-point improvement in a photonumeric scale used to grade jawline sagging, reported Jeremy Green, MD, Skin Associates of South Florida, Coral Gables.
When those randomized to the hyaluronic filler gel VYC-25L (Vycross, Juvéderm) were compared with untreated controls, 68.5% versus 38.4% met the criterion for benefit at 6 months. Importantly, the effect in treated patients was sustained when reevaluated at 12 months. Green reported that the response is generally sustained at the maximum follow-up, now out to 17 months.
Most enrolled patients are severely affected
In this study, 208 patients with severe (74%) or moderate loss of jawline definition were randomized in a 3:1 ratio to receive the filler or serve as controls. The initially untreated controls received the gel after the primary outcome analysis at 6 months.
The hyaluronic gel was injected at five sites along the jawline. The mean age of participants was 58 years. The majority were women, and most were White.
Dermatologists blinded to treatment compared photos at 6 months with those taken at baseline using the photonumeric grading system of 1-5. Change in patient satisfaction at 6 months and again at 12 months relative to baseline was also evaluated.
From baseline, when 28.9% of participants reported satisfaction on the Global Aesthetic Improvement Scale (GAIS), rates rose to 89.0% at month 6. There was a decline at month 12, but 79.9% remained satisfied after this period of follow-up.
Most patients experienced injection site reactions that were mainly mild to moderate and all resolved within several days of treatment. Pain with mastication was initially reported by 1.9%, but again this complaint was also mild and transient. All complaints had largely resolved by day 3.
The results are consistent with several previous clinical studies of VYC-25L for the same indication. In a similarly designed trial conducted in Europe that also used a 3:1 randomization scheme, the primary outcome assessed at 3 months was change in facial angle. Relative to controls, the angle improved by 2.51 degrees (P < .0001).
Patient satisfaction supports filler benefit
In the similar European trial, the clinical significance of the objective primary outcome also was supported by patient satisfaction assessed with several instruments, including the GAIS. Some degree of swelling or tenderness was experienced by almost all patients after injection, but none were serious, and all resolved.
In another trial, 202 patients with chin retrusion were randomized in a 3:1 ratio to VYC-25L or a control group. In that study, the primary outcome was at least a 1-point improvement in the Allergan Chin Retrusion Scale at 6 months. This advantage for treatment (56.3% vs. 27.5%) was again supported by several instruments for evaluating patient satisfaction, including GAIS.
As in the other studies, most patients had injection site reactions. Although all resolved within days of treatment, one patient left the study after experiencing cellulitis and injection-site inflammation.
Dissatisfaction with jawline definition is a relatively common complaint in Dr. Green’s experience, who said that there is a need for more effective and well-tolerated treatments. Given the efficacy, tolerability, and safety of VYC-25L in this controlled study, he suggested this product has potential utility.
In the field of cosmetic dermatology, there appears to be incremental progress in fillers with favorable clinical characteristics, according to Sandy U. Tsao, MD, a dermatologic surgeon at Massachusetts General Hospital, Boston.
“We are seeing filler lasting longer and longer,” she said, commenting specifically about the results presented by Dr. Green. She called sustained aesthetic improvement at 12 months for the filler in this study “really exciting.”
Dr. Green has reported financial relationships with numerous pharmaceutical companies. Dr. Tsao has reported financial relationships with Epiphany Dermatology, Lazarus AI, and UpToDate.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Angioimmunoblastic T-cell Lymphoma Mimicking DRESS Syndrome
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
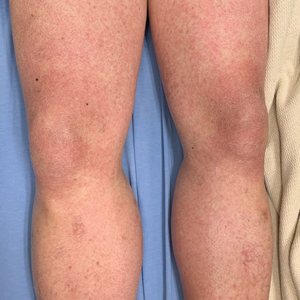
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
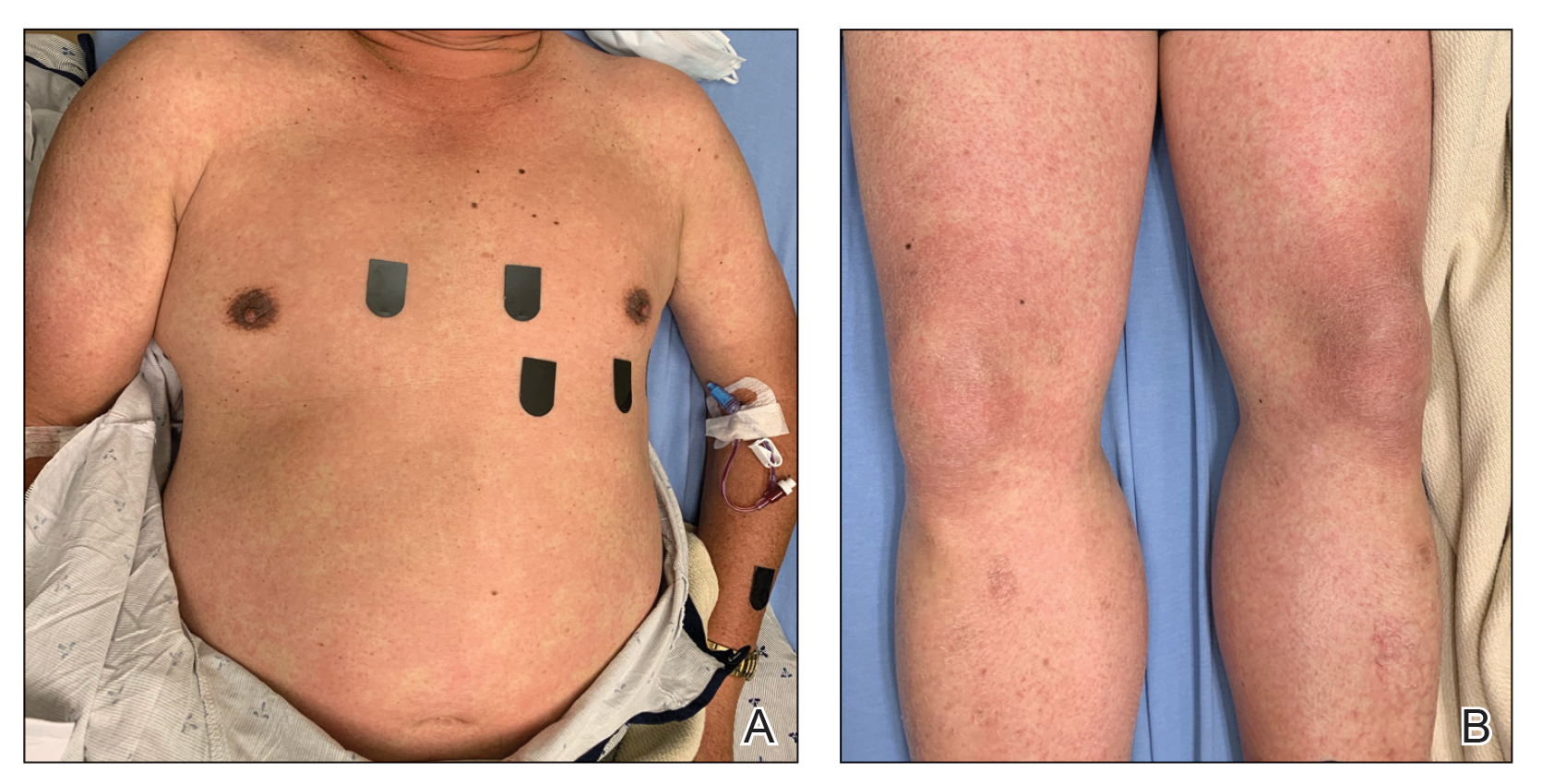
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Practice Points
- It is important to maintain a high index of suspicion for angioimmunoblastic T-cell lymphoma in older patients with a longstanding rash and no clear culprit for drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
- Consider performing a lymph node biopsy early in the course of disease in patients with presumed DRESS syndrome who do not improve with drug withdrawal and steroid therapy.
Take action: Turn up the heat on prior auth
In our recent member survey, 99% of respondents expressed that prior authorization has a negative impact on patients’ access to clinically appropriate treatments. We need to continue to put pressure on legislators to eliminate prior authorization burdens.
AGA endorses the Improving Seniors Timely Access to Care Act, which would streamline the prior authorization process in Medicare Advantage by approving in real-time commonly approved services and implementing a standardized electronic prior authorization process.
Despite large bipartisan support, we need your help getting this bill across the finish line! Please take five minutes to ask your Representative to cosponsor this necessary bill by participating in our campaign.
Go to the AGA action center to contact your lawmakers!
In our recent member survey, 99% of respondents expressed that prior authorization has a negative impact on patients’ access to clinically appropriate treatments. We need to continue to put pressure on legislators to eliminate prior authorization burdens.
AGA endorses the Improving Seniors Timely Access to Care Act, which would streamline the prior authorization process in Medicare Advantage by approving in real-time commonly approved services and implementing a standardized electronic prior authorization process.
Despite large bipartisan support, we need your help getting this bill across the finish line! Please take five minutes to ask your Representative to cosponsor this necessary bill by participating in our campaign.
Go to the AGA action center to contact your lawmakers!
In our recent member survey, 99% of respondents expressed that prior authorization has a negative impact on patients’ access to clinically appropriate treatments. We need to continue to put pressure on legislators to eliminate prior authorization burdens.
AGA endorses the Improving Seniors Timely Access to Care Act, which would streamline the prior authorization process in Medicare Advantage by approving in real-time commonly approved services and implementing a standardized electronic prior authorization process.
Despite large bipartisan support, we need your help getting this bill across the finish line! Please take five minutes to ask your Representative to cosponsor this necessary bill by participating in our campaign.
Go to the AGA action center to contact your lawmakers!
2022 AGA recognition prize award recipients

“AGA is proud to officially announce the exceptional individuals selected for 2022 AGA Recognition Prizes. I wish to thank all the nominators and those who provided nomination letters, and the selection committees for the tough task they had to select among the many superb nominees,” said Bishr Omary, MD, PhD, AGAF, chair of the AGA. “Please join us in congratulating this year’s distinguished awardees and applauding their contributions to the field of gastroenterology that advance our profession and the patients we serve.”
AGA looks forward to celebrating the recipients during Digestive Disease Week® 2022, May 21-24, in San Diego, Calif.
Meet and learn more about our award recipients here.

“AGA is proud to officially announce the exceptional individuals selected for 2022 AGA Recognition Prizes. I wish to thank all the nominators and those who provided nomination letters, and the selection committees for the tough task they had to select among the many superb nominees,” said Bishr Omary, MD, PhD, AGAF, chair of the AGA. “Please join us in congratulating this year’s distinguished awardees and applauding their contributions to the field of gastroenterology that advance our profession and the patients we serve.”
AGA looks forward to celebrating the recipients during Digestive Disease Week® 2022, May 21-24, in San Diego, Calif.
Meet and learn more about our award recipients here.

“AGA is proud to officially announce the exceptional individuals selected for 2022 AGA Recognition Prizes. I wish to thank all the nominators and those who provided nomination letters, and the selection committees for the tough task they had to select among the many superb nominees,” said Bishr Omary, MD, PhD, AGAF, chair of the AGA. “Please join us in congratulating this year’s distinguished awardees and applauding their contributions to the field of gastroenterology that advance our profession and the patients we serve.”
AGA looks forward to celebrating the recipients during Digestive Disease Week® 2022, May 21-24, in San Diego, Calif.
Meet and learn more about our award recipients here.
New governing board members
M. Bishr Omary, MD, PhD, AGAF, chair of the AGA Nominating Committee, is pleased to announce that Maria T. Abreu, MD, AGAF, joins the presidential line-up for AGA.
Vice President
Maria T. Abreu, MD, AGAF
Director, Crohn’s and Colitis Center
University of Miami
Maria T. Abreu, MD, AGAF, has more than 20 years of leadership experience in basic, translational, and clinical research and mentoring. She is AGA’s current councillor at-large, past chair of the AGA Institute Council, and an AGA Institute Council Section Research Mentor Award recipient (2020) for the IMIBD section. Dr. Abreu is also a recipient of the 2019 Sherman Prize by The Bruce and Cynthia Sherman Charitable Foundation that recognizes outstanding achievements in intestinal bowel disease.
Read her bio from the University of Miami.
The nominating committee also appointed the following slate of councillors which is subject to membership vote.
At-Large Councillor
Kim Barrett, PhD, AGAF
Vice dean for research
University of California, Davis
Kim Barrett, PhD, AGAF, is the current chair of the AGA Publications Committee, former chair of the AGA Ethics And Audit Committees, and served twice as director of the Academic Skills Workshop. She was recognized with AGA’s top research award, the AGA Distinguished Achievement Award in Basic Science (2021).
Her research interests have centered on the physiology and pathophysiology of the intestinal epithelium and their relevance to inflammatory bowel diseases and diarrheal diseases and have resulted in more than 300 publications.
Read her bio from UC Davis.
Councillor For Development And Growth
Lawrence Kosinski, MD, MBA, AGAF
Chief medical officer
SonarMD
A serial entrepreneur and thought leader in the world of value-based payment, Larry Kosinski, MD, MBA, AGAF, currently serves as chief medical officer of SonarMD, the leading value-based care coordination solution for complex chronic diseases. He founded SonarMD in 2014 to make it easier for specialists and patients to work together to manage symptomatic chronic illness and prevent clinical deterioration, improving health outcomes, and lowering the cost of care.
In 2021, Dr. Kosinski was selected for his expertise in value-based payment to serve on the Centers for Medicare & Medicaid Services’ Physician-Focused Payment Model Technical Advisory Committee and help develop bold, new Medicare payment models.
Read his bio from the SonarMD website.
Education & Training Councillor
Sheryl Pfeil, MD, AGAF
Medical director and professor of clinical medicine, Clinical Skills Education and Assessment Center
The Ohio State University Wexner Medical Center
Sheryl Pfeil, MD, AGAF, has been an AGA member for 30 years, serving on the Education And Training Committee, as past chair of the Academy of Educators, as cochair of the AGA future leaders program, and on the editorial board for Gastro Hep Advances. Dr. Pfeil has 30 years of experience in medical education, leading medical students, residents, and fellow education.
Her educational research interests include professional development, training and assessment methods, and virtual education.
Read her bio from The Ohio State University.
Pending approval by the voting membership, all board members begin their terms after DDW 2022. The voting membership will be sent a ballot to approve the slate of councillors on or before March 28, 2022, with a response date of no later than April 29, 2022. Results will be announced at the AGA Annual Business Meeting on June 1, 2022.
M. Bishr Omary, MD, PhD, AGAF, chair of the AGA Nominating Committee, is pleased to announce that Maria T. Abreu, MD, AGAF, joins the presidential line-up for AGA.
Vice President
Maria T. Abreu, MD, AGAF
Director, Crohn’s and Colitis Center
University of Miami
Maria T. Abreu, MD, AGAF, has more than 20 years of leadership experience in basic, translational, and clinical research and mentoring. She is AGA’s current councillor at-large, past chair of the AGA Institute Council, and an AGA Institute Council Section Research Mentor Award recipient (2020) for the IMIBD section. Dr. Abreu is also a recipient of the 2019 Sherman Prize by The Bruce and Cynthia Sherman Charitable Foundation that recognizes outstanding achievements in intestinal bowel disease.
Read her bio from the University of Miami.
The nominating committee also appointed the following slate of councillors which is subject to membership vote.
At-Large Councillor
Kim Barrett, PhD, AGAF
Vice dean for research
University of California, Davis
Kim Barrett, PhD, AGAF, is the current chair of the AGA Publications Committee, former chair of the AGA Ethics And Audit Committees, and served twice as director of the Academic Skills Workshop. She was recognized with AGA’s top research award, the AGA Distinguished Achievement Award in Basic Science (2021).
Her research interests have centered on the physiology and pathophysiology of the intestinal epithelium and their relevance to inflammatory bowel diseases and diarrheal diseases and have resulted in more than 300 publications.
Read her bio from UC Davis.
Councillor For Development And Growth
Lawrence Kosinski, MD, MBA, AGAF
Chief medical officer
SonarMD
A serial entrepreneur and thought leader in the world of value-based payment, Larry Kosinski, MD, MBA, AGAF, currently serves as chief medical officer of SonarMD, the leading value-based care coordination solution for complex chronic diseases. He founded SonarMD in 2014 to make it easier for specialists and patients to work together to manage symptomatic chronic illness and prevent clinical deterioration, improving health outcomes, and lowering the cost of care.
In 2021, Dr. Kosinski was selected for his expertise in value-based payment to serve on the Centers for Medicare & Medicaid Services’ Physician-Focused Payment Model Technical Advisory Committee and help develop bold, new Medicare payment models.
Read his bio from the SonarMD website.
Education & Training Councillor
Sheryl Pfeil, MD, AGAF
Medical director and professor of clinical medicine, Clinical Skills Education and Assessment Center
The Ohio State University Wexner Medical Center
Sheryl Pfeil, MD, AGAF, has been an AGA member for 30 years, serving on the Education And Training Committee, as past chair of the Academy of Educators, as cochair of the AGA future leaders program, and on the editorial board for Gastro Hep Advances. Dr. Pfeil has 30 years of experience in medical education, leading medical students, residents, and fellow education.
Her educational research interests include professional development, training and assessment methods, and virtual education.
Read her bio from The Ohio State University.
Pending approval by the voting membership, all board members begin their terms after DDW 2022. The voting membership will be sent a ballot to approve the slate of councillors on or before March 28, 2022, with a response date of no later than April 29, 2022. Results will be announced at the AGA Annual Business Meeting on June 1, 2022.
M. Bishr Omary, MD, PhD, AGAF, chair of the AGA Nominating Committee, is pleased to announce that Maria T. Abreu, MD, AGAF, joins the presidential line-up for AGA.
Vice President
Maria T. Abreu, MD, AGAF
Director, Crohn’s and Colitis Center
University of Miami
Maria T. Abreu, MD, AGAF, has more than 20 years of leadership experience in basic, translational, and clinical research and mentoring. She is AGA’s current councillor at-large, past chair of the AGA Institute Council, and an AGA Institute Council Section Research Mentor Award recipient (2020) for the IMIBD section. Dr. Abreu is also a recipient of the 2019 Sherman Prize by The Bruce and Cynthia Sherman Charitable Foundation that recognizes outstanding achievements in intestinal bowel disease.
Read her bio from the University of Miami.
The nominating committee also appointed the following slate of councillors which is subject to membership vote.
At-Large Councillor
Kim Barrett, PhD, AGAF
Vice dean for research
University of California, Davis
Kim Barrett, PhD, AGAF, is the current chair of the AGA Publications Committee, former chair of the AGA Ethics And Audit Committees, and served twice as director of the Academic Skills Workshop. She was recognized with AGA’s top research award, the AGA Distinguished Achievement Award in Basic Science (2021).
Her research interests have centered on the physiology and pathophysiology of the intestinal epithelium and their relevance to inflammatory bowel diseases and diarrheal diseases and have resulted in more than 300 publications.
Read her bio from UC Davis.
Councillor For Development And Growth
Lawrence Kosinski, MD, MBA, AGAF
Chief medical officer
SonarMD
A serial entrepreneur and thought leader in the world of value-based payment, Larry Kosinski, MD, MBA, AGAF, currently serves as chief medical officer of SonarMD, the leading value-based care coordination solution for complex chronic diseases. He founded SonarMD in 2014 to make it easier for specialists and patients to work together to manage symptomatic chronic illness and prevent clinical deterioration, improving health outcomes, and lowering the cost of care.
In 2021, Dr. Kosinski was selected for his expertise in value-based payment to serve on the Centers for Medicare & Medicaid Services’ Physician-Focused Payment Model Technical Advisory Committee and help develop bold, new Medicare payment models.
Read his bio from the SonarMD website.
Education & Training Councillor
Sheryl Pfeil, MD, AGAF
Medical director and professor of clinical medicine, Clinical Skills Education and Assessment Center
The Ohio State University Wexner Medical Center
Sheryl Pfeil, MD, AGAF, has been an AGA member for 30 years, serving on the Education And Training Committee, as past chair of the Academy of Educators, as cochair of the AGA future leaders program, and on the editorial board for Gastro Hep Advances. Dr. Pfeil has 30 years of experience in medical education, leading medical students, residents, and fellow education.
Her educational research interests include professional development, training and assessment methods, and virtual education.
Read her bio from The Ohio State University.
Pending approval by the voting membership, all board members begin their terms after DDW 2022. The voting membership will be sent a ballot to approve the slate of councillors on or before March 28, 2022, with a response date of no later than April 29, 2022. Results will be announced at the AGA Annual Business Meeting on June 1, 2022.
Fertility after tubal ligation – It’s a matter of ‘AGE’
Despite the original intent of permanent contraception, tubal sterilization regret is experienced by 2%-26% of women as demonstrated by the United States Collaborative Review of Sterilization “CREST” 14-year study (Obstet Gynecol. 1999 Jun;93[6]:889-95). Regret appears to be higher in the United States than Europe and in resource-limited countries and is more common in women who are less than age 30, African-American, and unmarried. Nevertheless, requests for tubal reversal are estimated to be between 1% and 4% (Contraception. 1981 Jun;23[6]:579-89). The alternative option for fertility is in vitro fertilization (IVF) and this month’s column considers the pros and cons of both methods.
The procedure of tubal reanastomosis involves removing abnormal tissue and reapproximating the healthy tubal segments with attention to minimize adhesion formation through continued gentle irrigation. The surgery involves microsuturing using 6-0 to 10-0 sutures. Tubal patency can be confirmed during the procedure and with a subsequent hysterosalpingogram. While time from sterilization and the type of sterilization technique are factors that may influence the success rate of tubal reanastomosis, the age of the woman is the most predictive for pregnancy outcome.
In the original CREST study, the risk of ectopic pregnancy following tubal reanastomosis was contingent on the method of sterilization: Bipolar electrosurgery resulted in the highest probability of ectopic pregnancy (17.1 per 1,000 procedures at 10 years after permanent contraception), while postpartum partial salpingectomy resulted in the lowest (1.5 per 1,000 procedures at 10 years after permanent contraception) (N Engl J Med. 1997;336[11]:762). Comparatively, the ectopic pregnancy rate during an IVF cycle was 1.9% for pregnancies from transfers of fresh cleavage embryo, followed by transfers of frozen cleavage embryo (1.7%), transfers of fresh blastocyst (1.3%), and transfers of frozen blastocyst (0.8%) (Hum Reprod. 2015;30[9]:2048-54).
Reports vary regarding pregnancy rates from tubal reanastomosis. Prior use of rings and clips for sterilization appear to yield the highest outcomes as opposed to the use of electrocautery. In one large Canadian cohort study of over 300,000 women, those aged 15-30 years, 30-33 years, and 34-49 years had a conception rate of 73%, 64%, and 46%, respectively (Obstet Gynecol. 2003;101[4]:677-84). Most pregnancies were within 2 years after reversal and 48% of women achieved a delivery. Of interest, 23% of patients subsequently underwent another sterilization.
An Australian study of nearly 2,000 women found an overall cumulative live-delivery rate of 20% within the first year after reversal, 40% at 2 years, 51% at 5 years, and 52% at 10 years. As expected, the 5-year cumulative live-delivery rate was significantly lower in women who were aged 40-44 years (26%), compared with younger women. For all women below age 40 years, the live-delivery rate was approximately 50% within 5 years after tubal reanastomosis, while the rate halves after the age of 40 (Fertil Steril. 2015 Oct;104[4]:921-6).
To compare tubal reanastomosis with IVF, a retrospective cohort study of 163 patients demonstrated the cumulative delivery rate over 72 months was comparable for IVF vs. sterilization reversal (52% vs. 60%). The only significant difference was in a subset of patients aged <37 years (52% after IVF and 72% after reversal) and the lower cost of surgery. The authors advocated laparoscopic sterilization reversal in women younger than 37 years who have ≥4 cm of residual tube with IVF as the better alternative for all other women (Hum Reprod. 2007;22[10]:2660).
Indeed, tubal length is another important factor in successful reversal. The pregnancy rate after tubal anastomosis is 75% in women with tubal length of 4 cm or more, but only 19% in those with shorter tubes (Fertil Steril. 1987;48[1]:13-7). The literature does suggest equivalent pregnancy rates after laparoscopic tubal anastomosis and conventional microsurgical anastomosis. Although the laparoscopic approach may be more economical, it is more demanding technically than an open microsurgical procedure.
Tubal reanastomosis can also be performed using robot-assisted laparoscopy. In preliminary studies, robotic surgery appears to have a similar success rate and a shorter recovery time, but longer operative times and higher costs (Obstet Gynecol. 2007;109[6]:1375; Fertil Steril. 2008;90[4]:1175).
To educate women on the success of IVF based on individual characteristics, a valuable tool to approximate the cumulative outcome for a live birth following one cycle of IVF is offered by the Society for Assisted Reproductive Technology. To clarify, a cycle of IVF consists of one egg retrieval and the ultimate transfer of all embryos produced, i.e., fresh and frozen. The website also includes estimations of success following a second and third IVF cycle.
The woman’s age is a significant predictor of IVF success. Ovarian aging is currently best measured by combining chronologic age, antral follicle count (AFC) by transvaginal pelvic ultrasound, and serum anti-Müllerian hormone (AMH). Natural fecundity begins to decline, on average, above age 32-33 years. An AFC less than 11 reflects diminished ovarian reserve (DOR) and less than 6 is severe. AMH levels below 1.6 ng/mL have been shown to reduce the number of eggs retrieved with IVF, while levels below 0.4 ng/mL are very low. Very low AMH levels negatively affect the outcome of IVF cycles as demonstrated in the SART data study from a population of women with a mean age of 39.4 years: Cycle cancellation was 54%; of all retrieval attempts, no oocytes were obtained in 5.4%, and no embryo transfer occurred in 25.1% of cycles; the live birth rate per embryo transfer was 20.5% (9.5% per cycle start and 16.3% per retrieval) from a mean age of 36.8 years (Fertil Steril. 2016 Feb;105[2]:385-93.e3). The predictive ability of AMH on the live birth rate from IVF cycles was also shown in a study of over 85,000 women (Fertil Steril. 2018;109:258-65).
While low AMH has been shown to lessen a successful outcome from IVF, there appears to be no difference in natural pregnancy rates in women aged 30-44 years irrespective of AMH levels (JAMA. 2017;318[14]:1367-76). Of importance, the use of AMH in a population at low risk for DOR will yield a larger number of false-positive results (i.e., characterizing a woman as DOR when in fact she has normal ovarian reserve). Further, users of hormonal contraceptives have a 25.2% lower mean AMH level than nonusers.
When a patient is considering tubal reanastomosis vs. IVF, a useful acronym to remember is to check “AGE” – the A is for AMH because severely diminished ovarian reserve will reduce success with IVF as shown by the SART calculator; the G represents guy, i.e., ensuring a reasonably normal sperm analysis; and E stands for eggs representing ovulation function. In a woman who is anovulatory and who will require fertility medication, it would be reasonable to consider IVF given the need for ovarian stimulation. As in females, advanced paternal age has demonstrated a decline in fertility and sperm analysis parameters. Men above age 45 take approximately five times as long to achieve a pregnancy, compared with men less than 25 years of age. Further, there is evidence for advanced paternal age increasing risk of miscarriage, preterm birth, and birth defects. Men older than 40-45 years have twice the risk of an autistic child and five times the risk of having a child with schizophrenia (Transl Psychiatry 2017;7: e1019; Am J Psychiatry. 2002;159:1528-33).
To conclude, the data support consideration for sterilization reversal in women less than age 37 years with more than 4 cm of residual functional fallopian tube and the prior use of rings or clip sterilization. In other women, IVF may be the better option, particularly when ovulation dysfunction and/or male factor is present. IVF also offers the advantage of maintaining contraception and gender determination. However, given that AMH does not appear to reduce natural fertility, unlike during its effect during an IVF cycle, the option of tubal reversal may be more favorable in women with severe DOR.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Despite the original intent of permanent contraception, tubal sterilization regret is experienced by 2%-26% of women as demonstrated by the United States Collaborative Review of Sterilization “CREST” 14-year study (Obstet Gynecol. 1999 Jun;93[6]:889-95). Regret appears to be higher in the United States than Europe and in resource-limited countries and is more common in women who are less than age 30, African-American, and unmarried. Nevertheless, requests for tubal reversal are estimated to be between 1% and 4% (Contraception. 1981 Jun;23[6]:579-89). The alternative option for fertility is in vitro fertilization (IVF) and this month’s column considers the pros and cons of both methods.
The procedure of tubal reanastomosis involves removing abnormal tissue and reapproximating the healthy tubal segments with attention to minimize adhesion formation through continued gentle irrigation. The surgery involves microsuturing using 6-0 to 10-0 sutures. Tubal patency can be confirmed during the procedure and with a subsequent hysterosalpingogram. While time from sterilization and the type of sterilization technique are factors that may influence the success rate of tubal reanastomosis, the age of the woman is the most predictive for pregnancy outcome.
In the original CREST study, the risk of ectopic pregnancy following tubal reanastomosis was contingent on the method of sterilization: Bipolar electrosurgery resulted in the highest probability of ectopic pregnancy (17.1 per 1,000 procedures at 10 years after permanent contraception), while postpartum partial salpingectomy resulted in the lowest (1.5 per 1,000 procedures at 10 years after permanent contraception) (N Engl J Med. 1997;336[11]:762). Comparatively, the ectopic pregnancy rate during an IVF cycle was 1.9% for pregnancies from transfers of fresh cleavage embryo, followed by transfers of frozen cleavage embryo (1.7%), transfers of fresh blastocyst (1.3%), and transfers of frozen blastocyst (0.8%) (Hum Reprod. 2015;30[9]:2048-54).
Reports vary regarding pregnancy rates from tubal reanastomosis. Prior use of rings and clips for sterilization appear to yield the highest outcomes as opposed to the use of electrocautery. In one large Canadian cohort study of over 300,000 women, those aged 15-30 years, 30-33 years, and 34-49 years had a conception rate of 73%, 64%, and 46%, respectively (Obstet Gynecol. 2003;101[4]:677-84). Most pregnancies were within 2 years after reversal and 48% of women achieved a delivery. Of interest, 23% of patients subsequently underwent another sterilization.
An Australian study of nearly 2,000 women found an overall cumulative live-delivery rate of 20% within the first year after reversal, 40% at 2 years, 51% at 5 years, and 52% at 10 years. As expected, the 5-year cumulative live-delivery rate was significantly lower in women who were aged 40-44 years (26%), compared with younger women. For all women below age 40 years, the live-delivery rate was approximately 50% within 5 years after tubal reanastomosis, while the rate halves after the age of 40 (Fertil Steril. 2015 Oct;104[4]:921-6).
To compare tubal reanastomosis with IVF, a retrospective cohort study of 163 patients demonstrated the cumulative delivery rate over 72 months was comparable for IVF vs. sterilization reversal (52% vs. 60%). The only significant difference was in a subset of patients aged <37 years (52% after IVF and 72% after reversal) and the lower cost of surgery. The authors advocated laparoscopic sterilization reversal in women younger than 37 years who have ≥4 cm of residual tube with IVF as the better alternative for all other women (Hum Reprod. 2007;22[10]:2660).
Indeed, tubal length is another important factor in successful reversal. The pregnancy rate after tubal anastomosis is 75% in women with tubal length of 4 cm or more, but only 19% in those with shorter tubes (Fertil Steril. 1987;48[1]:13-7). The literature does suggest equivalent pregnancy rates after laparoscopic tubal anastomosis and conventional microsurgical anastomosis. Although the laparoscopic approach may be more economical, it is more demanding technically than an open microsurgical procedure.
Tubal reanastomosis can also be performed using robot-assisted laparoscopy. In preliminary studies, robotic surgery appears to have a similar success rate and a shorter recovery time, but longer operative times and higher costs (Obstet Gynecol. 2007;109[6]:1375; Fertil Steril. 2008;90[4]:1175).
To educate women on the success of IVF based on individual characteristics, a valuable tool to approximate the cumulative outcome for a live birth following one cycle of IVF is offered by the Society for Assisted Reproductive Technology. To clarify, a cycle of IVF consists of one egg retrieval and the ultimate transfer of all embryos produced, i.e., fresh and frozen. The website also includes estimations of success following a second and third IVF cycle.
The woman’s age is a significant predictor of IVF success. Ovarian aging is currently best measured by combining chronologic age, antral follicle count (AFC) by transvaginal pelvic ultrasound, and serum anti-Müllerian hormone (AMH). Natural fecundity begins to decline, on average, above age 32-33 years. An AFC less than 11 reflects diminished ovarian reserve (DOR) and less than 6 is severe. AMH levels below 1.6 ng/mL have been shown to reduce the number of eggs retrieved with IVF, while levels below 0.4 ng/mL are very low. Very low AMH levels negatively affect the outcome of IVF cycles as demonstrated in the SART data study from a population of women with a mean age of 39.4 years: Cycle cancellation was 54%; of all retrieval attempts, no oocytes were obtained in 5.4%, and no embryo transfer occurred in 25.1% of cycles; the live birth rate per embryo transfer was 20.5% (9.5% per cycle start and 16.3% per retrieval) from a mean age of 36.8 years (Fertil Steril. 2016 Feb;105[2]:385-93.e3). The predictive ability of AMH on the live birth rate from IVF cycles was also shown in a study of over 85,000 women (Fertil Steril. 2018;109:258-65).
While low AMH has been shown to lessen a successful outcome from IVF, there appears to be no difference in natural pregnancy rates in women aged 30-44 years irrespective of AMH levels (JAMA. 2017;318[14]:1367-76). Of importance, the use of AMH in a population at low risk for DOR will yield a larger number of false-positive results (i.e., characterizing a woman as DOR when in fact she has normal ovarian reserve). Further, users of hormonal contraceptives have a 25.2% lower mean AMH level than nonusers.
When a patient is considering tubal reanastomosis vs. IVF, a useful acronym to remember is to check “AGE” – the A is for AMH because severely diminished ovarian reserve will reduce success with IVF as shown by the SART calculator; the G represents guy, i.e., ensuring a reasonably normal sperm analysis; and E stands for eggs representing ovulation function. In a woman who is anovulatory and who will require fertility medication, it would be reasonable to consider IVF given the need for ovarian stimulation. As in females, advanced paternal age has demonstrated a decline in fertility and sperm analysis parameters. Men above age 45 take approximately five times as long to achieve a pregnancy, compared with men less than 25 years of age. Further, there is evidence for advanced paternal age increasing risk of miscarriage, preterm birth, and birth defects. Men older than 40-45 years have twice the risk of an autistic child and five times the risk of having a child with schizophrenia (Transl Psychiatry 2017;7: e1019; Am J Psychiatry. 2002;159:1528-33).
To conclude, the data support consideration for sterilization reversal in women less than age 37 years with more than 4 cm of residual functional fallopian tube and the prior use of rings or clip sterilization. In other women, IVF may be the better option, particularly when ovulation dysfunction and/or male factor is present. IVF also offers the advantage of maintaining contraception and gender determination. However, given that AMH does not appear to reduce natural fertility, unlike during its effect during an IVF cycle, the option of tubal reversal may be more favorable in women with severe DOR.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Despite the original intent of permanent contraception, tubal sterilization regret is experienced by 2%-26% of women as demonstrated by the United States Collaborative Review of Sterilization “CREST” 14-year study (Obstet Gynecol. 1999 Jun;93[6]:889-95). Regret appears to be higher in the United States than Europe and in resource-limited countries and is more common in women who are less than age 30, African-American, and unmarried. Nevertheless, requests for tubal reversal are estimated to be between 1% and 4% (Contraception. 1981 Jun;23[6]:579-89). The alternative option for fertility is in vitro fertilization (IVF) and this month’s column considers the pros and cons of both methods.
The procedure of tubal reanastomosis involves removing abnormal tissue and reapproximating the healthy tubal segments with attention to minimize adhesion formation through continued gentle irrigation. The surgery involves microsuturing using 6-0 to 10-0 sutures. Tubal patency can be confirmed during the procedure and with a subsequent hysterosalpingogram. While time from sterilization and the type of sterilization technique are factors that may influence the success rate of tubal reanastomosis, the age of the woman is the most predictive for pregnancy outcome.
In the original CREST study, the risk of ectopic pregnancy following tubal reanastomosis was contingent on the method of sterilization: Bipolar electrosurgery resulted in the highest probability of ectopic pregnancy (17.1 per 1,000 procedures at 10 years after permanent contraception), while postpartum partial salpingectomy resulted in the lowest (1.5 per 1,000 procedures at 10 years after permanent contraception) (N Engl J Med. 1997;336[11]:762). Comparatively, the ectopic pregnancy rate during an IVF cycle was 1.9% for pregnancies from transfers of fresh cleavage embryo, followed by transfers of frozen cleavage embryo (1.7%), transfers of fresh blastocyst (1.3%), and transfers of frozen blastocyst (0.8%) (Hum Reprod. 2015;30[9]:2048-54).
Reports vary regarding pregnancy rates from tubal reanastomosis. Prior use of rings and clips for sterilization appear to yield the highest outcomes as opposed to the use of electrocautery. In one large Canadian cohort study of over 300,000 women, those aged 15-30 years, 30-33 years, and 34-49 years had a conception rate of 73%, 64%, and 46%, respectively (Obstet Gynecol. 2003;101[4]:677-84). Most pregnancies were within 2 years after reversal and 48% of women achieved a delivery. Of interest, 23% of patients subsequently underwent another sterilization.
An Australian study of nearly 2,000 women found an overall cumulative live-delivery rate of 20% within the first year after reversal, 40% at 2 years, 51% at 5 years, and 52% at 10 years. As expected, the 5-year cumulative live-delivery rate was significantly lower in women who were aged 40-44 years (26%), compared with younger women. For all women below age 40 years, the live-delivery rate was approximately 50% within 5 years after tubal reanastomosis, while the rate halves after the age of 40 (Fertil Steril. 2015 Oct;104[4]:921-6).
To compare tubal reanastomosis with IVF, a retrospective cohort study of 163 patients demonstrated the cumulative delivery rate over 72 months was comparable for IVF vs. sterilization reversal (52% vs. 60%). The only significant difference was in a subset of patients aged <37 years (52% after IVF and 72% after reversal) and the lower cost of surgery. The authors advocated laparoscopic sterilization reversal in women younger than 37 years who have ≥4 cm of residual tube with IVF as the better alternative for all other women (Hum Reprod. 2007;22[10]:2660).
Indeed, tubal length is another important factor in successful reversal. The pregnancy rate after tubal anastomosis is 75% in women with tubal length of 4 cm or more, but only 19% in those with shorter tubes (Fertil Steril. 1987;48[1]:13-7). The literature does suggest equivalent pregnancy rates after laparoscopic tubal anastomosis and conventional microsurgical anastomosis. Although the laparoscopic approach may be more economical, it is more demanding technically than an open microsurgical procedure.
Tubal reanastomosis can also be performed using robot-assisted laparoscopy. In preliminary studies, robotic surgery appears to have a similar success rate and a shorter recovery time, but longer operative times and higher costs (Obstet Gynecol. 2007;109[6]:1375; Fertil Steril. 2008;90[4]:1175).
To educate women on the success of IVF based on individual characteristics, a valuable tool to approximate the cumulative outcome for a live birth following one cycle of IVF is offered by the Society for Assisted Reproductive Technology. To clarify, a cycle of IVF consists of one egg retrieval and the ultimate transfer of all embryos produced, i.e., fresh and frozen. The website also includes estimations of success following a second and third IVF cycle.
The woman’s age is a significant predictor of IVF success. Ovarian aging is currently best measured by combining chronologic age, antral follicle count (AFC) by transvaginal pelvic ultrasound, and serum anti-Müllerian hormone (AMH). Natural fecundity begins to decline, on average, above age 32-33 years. An AFC less than 11 reflects diminished ovarian reserve (DOR) and less than 6 is severe. AMH levels below 1.6 ng/mL have been shown to reduce the number of eggs retrieved with IVF, while levels below 0.4 ng/mL are very low. Very low AMH levels negatively affect the outcome of IVF cycles as demonstrated in the SART data study from a population of women with a mean age of 39.4 years: Cycle cancellation was 54%; of all retrieval attempts, no oocytes were obtained in 5.4%, and no embryo transfer occurred in 25.1% of cycles; the live birth rate per embryo transfer was 20.5% (9.5% per cycle start and 16.3% per retrieval) from a mean age of 36.8 years (Fertil Steril. 2016 Feb;105[2]:385-93.e3). The predictive ability of AMH on the live birth rate from IVF cycles was also shown in a study of over 85,000 women (Fertil Steril. 2018;109:258-65).
While low AMH has been shown to lessen a successful outcome from IVF, there appears to be no difference in natural pregnancy rates in women aged 30-44 years irrespective of AMH levels (JAMA. 2017;318[14]:1367-76). Of importance, the use of AMH in a population at low risk for DOR will yield a larger number of false-positive results (i.e., characterizing a woman as DOR when in fact she has normal ovarian reserve). Further, users of hormonal contraceptives have a 25.2% lower mean AMH level than nonusers.
When a patient is considering tubal reanastomosis vs. IVF, a useful acronym to remember is to check “AGE” – the A is for AMH because severely diminished ovarian reserve will reduce success with IVF as shown by the SART calculator; the G represents guy, i.e., ensuring a reasonably normal sperm analysis; and E stands for eggs representing ovulation function. In a woman who is anovulatory and who will require fertility medication, it would be reasonable to consider IVF given the need for ovarian stimulation. As in females, advanced paternal age has demonstrated a decline in fertility and sperm analysis parameters. Men above age 45 take approximately five times as long to achieve a pregnancy, compared with men less than 25 years of age. Further, there is evidence for advanced paternal age increasing risk of miscarriage, preterm birth, and birth defects. Men older than 40-45 years have twice the risk of an autistic child and five times the risk of having a child with schizophrenia (Transl Psychiatry 2017;7: e1019; Am J Psychiatry. 2002;159:1528-33).
To conclude, the data support consideration for sterilization reversal in women less than age 37 years with more than 4 cm of residual functional fallopian tube and the prior use of rings or clip sterilization. In other women, IVF may be the better option, particularly when ovulation dysfunction and/or male factor is present. IVF also offers the advantage of maintaining contraception and gender determination. However, given that AMH does not appear to reduce natural fertility, unlike during its effect during an IVF cycle, the option of tubal reversal may be more favorable in women with severe DOR.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.