User login
Neoadjuvant denosumab ineffective in breast cancer
Results from a new phase IIb, 2x2 randomized, open-label trial (GeparSEPTO) showed no improvement in pathologic complete response (pCR) rates when the RANKL inhibitor denosumab was added to anthracycline/taxane-based neoadjuvant chemotherapy in the treatment of primary breast cancer. The study found that a weekly anthracycline/taxane-based neoadjuvant chemotherapy led to improved pCR compared to an every 3-week schedule both overall and in patients with triple-negative breast cancer (TNBC), though it was associated with greater toxicity.
“Currently, I do not see a place for antiosteolytic agents as part of the neoadjuvant therapy in breast cancer,” said lead author Sibylle Loibl, MD, PhD, who is a breast cancer researcher at Goethe (Germany) University. The researchers can’t exclude the possibility of a long-term benefit, and patients will be followed for disease-free and overall survival.
, prompting optimism that the agents might improve pCR rates and improve survival rates in the neoadjuvant setting.
The failure may be because of the shorter treatment duration, and it’s possible that pCR is not the best endpoint to study for a drug that has long-acting potential, according to Dr. Loibl.
Patients were randomized to 120 mg denosumab every 4 weeks for six cycles or to receive no supplementary treatment. Patients with or without denosumab either received nab-paclitaxel 125 mg/m2 weekly for 12 weeks or on days 1 and 8 every 3 weeks, over four cycles (eight total doses), then epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 and 3 weeks, respectively). Patients with triple-negative breast cancer also received carboplatin. Patients with ERBB2-positive breast cancer received the trastuzumab biosimilar ABP980 plus pertuzumab.
The study included 780 patients (1 male), with a median age of 49.0 years. There was no difference in pCR among denosumab recipients and nonrecipients (41.0% versus 42.8%; P = .58). Weekly nab-paclitaxel led to a higher pCR rate than a schedule of days 1 and 8 every 3 weeks (44.9% versus 39.0%; P = .06, significance level of alpha = .10). Among subgroups, there was only a difference in pCR rates among those with TNBC (60.4% versus 50.0%; P = .06). Grade 3-4 toxic effects were similar regardless of denosumab exposure, but nonhematologic grade 3-4 toxicity was higher with weekly nab-paclitaxel (33.7% versus 24.1%; P = .004).
“The overall pCR difference (with nab-paclitaxel as neoadjuvant chemotherapy) seems small, but looking at the data from the GeparSEPTO study we would expect a transformation into a better invasive disease-free survival. Looking specifically into patients with TNBC, there is clear pCR increase by using the weekly regimen. With just over 60%, this is the highest pCR rate reported so far for a chemotherapy-only regimen. I would prefer to use nab-paclitaxel also in early breast cancer and would use the weekly regimen for women with TNBC who are at high risk,” Dr. Loibl said.
The study was limited by an imbalance of tumor subtypes between the treatment groups. The authors wrote that the results should guide further research, but nab-paclitaxel should not currently be viewed as a standard neoadjuvant treatment of breast cancer.
The study was funded by the German Breast Group, Bristol Myers Squibb, and Amgen. Dr. Loibl has served on the advisory boards or given lectures for AbbVie, Amgen, Bayer, Celgene, EirGenix, GSK, Lilly, Merck, Puma, Seagen, AstraZeneca, Daiichi Sankyo, Novartis, Pierre Fabre, Prime/Medscape, Bristol Myers Squibb, Chugai, Ipsen, Roche, and Samsung. She also holds a related patent.
Results from a new phase IIb, 2x2 randomized, open-label trial (GeparSEPTO) showed no improvement in pathologic complete response (pCR) rates when the RANKL inhibitor denosumab was added to anthracycline/taxane-based neoadjuvant chemotherapy in the treatment of primary breast cancer. The study found that a weekly anthracycline/taxane-based neoadjuvant chemotherapy led to improved pCR compared to an every 3-week schedule both overall and in patients with triple-negative breast cancer (TNBC), though it was associated with greater toxicity.
“Currently, I do not see a place for antiosteolytic agents as part of the neoadjuvant therapy in breast cancer,” said lead author Sibylle Loibl, MD, PhD, who is a breast cancer researcher at Goethe (Germany) University. The researchers can’t exclude the possibility of a long-term benefit, and patients will be followed for disease-free and overall survival.
, prompting optimism that the agents might improve pCR rates and improve survival rates in the neoadjuvant setting.
The failure may be because of the shorter treatment duration, and it’s possible that pCR is not the best endpoint to study for a drug that has long-acting potential, according to Dr. Loibl.
Patients were randomized to 120 mg denosumab every 4 weeks for six cycles or to receive no supplementary treatment. Patients with or without denosumab either received nab-paclitaxel 125 mg/m2 weekly for 12 weeks or on days 1 and 8 every 3 weeks, over four cycles (eight total doses), then epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 and 3 weeks, respectively). Patients with triple-negative breast cancer also received carboplatin. Patients with ERBB2-positive breast cancer received the trastuzumab biosimilar ABP980 plus pertuzumab.
The study included 780 patients (1 male), with a median age of 49.0 years. There was no difference in pCR among denosumab recipients and nonrecipients (41.0% versus 42.8%; P = .58). Weekly nab-paclitaxel led to a higher pCR rate than a schedule of days 1 and 8 every 3 weeks (44.9% versus 39.0%; P = .06, significance level of alpha = .10). Among subgroups, there was only a difference in pCR rates among those with TNBC (60.4% versus 50.0%; P = .06). Grade 3-4 toxic effects were similar regardless of denosumab exposure, but nonhematologic grade 3-4 toxicity was higher with weekly nab-paclitaxel (33.7% versus 24.1%; P = .004).
“The overall pCR difference (with nab-paclitaxel as neoadjuvant chemotherapy) seems small, but looking at the data from the GeparSEPTO study we would expect a transformation into a better invasive disease-free survival. Looking specifically into patients with TNBC, there is clear pCR increase by using the weekly regimen. With just over 60%, this is the highest pCR rate reported so far for a chemotherapy-only regimen. I would prefer to use nab-paclitaxel also in early breast cancer and would use the weekly regimen for women with TNBC who are at high risk,” Dr. Loibl said.
The study was limited by an imbalance of tumor subtypes between the treatment groups. The authors wrote that the results should guide further research, but nab-paclitaxel should not currently be viewed as a standard neoadjuvant treatment of breast cancer.
The study was funded by the German Breast Group, Bristol Myers Squibb, and Amgen. Dr. Loibl has served on the advisory boards or given lectures for AbbVie, Amgen, Bayer, Celgene, EirGenix, GSK, Lilly, Merck, Puma, Seagen, AstraZeneca, Daiichi Sankyo, Novartis, Pierre Fabre, Prime/Medscape, Bristol Myers Squibb, Chugai, Ipsen, Roche, and Samsung. She also holds a related patent.
Results from a new phase IIb, 2x2 randomized, open-label trial (GeparSEPTO) showed no improvement in pathologic complete response (pCR) rates when the RANKL inhibitor denosumab was added to anthracycline/taxane-based neoadjuvant chemotherapy in the treatment of primary breast cancer. The study found that a weekly anthracycline/taxane-based neoadjuvant chemotherapy led to improved pCR compared to an every 3-week schedule both overall and in patients with triple-negative breast cancer (TNBC), though it was associated with greater toxicity.
“Currently, I do not see a place for antiosteolytic agents as part of the neoadjuvant therapy in breast cancer,” said lead author Sibylle Loibl, MD, PhD, who is a breast cancer researcher at Goethe (Germany) University. The researchers can’t exclude the possibility of a long-term benefit, and patients will be followed for disease-free and overall survival.
, prompting optimism that the agents might improve pCR rates and improve survival rates in the neoadjuvant setting.
The failure may be because of the shorter treatment duration, and it’s possible that pCR is not the best endpoint to study for a drug that has long-acting potential, according to Dr. Loibl.
Patients were randomized to 120 mg denosumab every 4 weeks for six cycles or to receive no supplementary treatment. Patients with or without denosumab either received nab-paclitaxel 125 mg/m2 weekly for 12 weeks or on days 1 and 8 every 3 weeks, over four cycles (eight total doses), then epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 and 3 weeks, respectively). Patients with triple-negative breast cancer also received carboplatin. Patients with ERBB2-positive breast cancer received the trastuzumab biosimilar ABP980 plus pertuzumab.
The study included 780 patients (1 male), with a median age of 49.0 years. There was no difference in pCR among denosumab recipients and nonrecipients (41.0% versus 42.8%; P = .58). Weekly nab-paclitaxel led to a higher pCR rate than a schedule of days 1 and 8 every 3 weeks (44.9% versus 39.0%; P = .06, significance level of alpha = .10). Among subgroups, there was only a difference in pCR rates among those with TNBC (60.4% versus 50.0%; P = .06). Grade 3-4 toxic effects were similar regardless of denosumab exposure, but nonhematologic grade 3-4 toxicity was higher with weekly nab-paclitaxel (33.7% versus 24.1%; P = .004).
“The overall pCR difference (with nab-paclitaxel as neoadjuvant chemotherapy) seems small, but looking at the data from the GeparSEPTO study we would expect a transformation into a better invasive disease-free survival. Looking specifically into patients with TNBC, there is clear pCR increase by using the weekly regimen. With just over 60%, this is the highest pCR rate reported so far for a chemotherapy-only regimen. I would prefer to use nab-paclitaxel also in early breast cancer and would use the weekly regimen for women with TNBC who are at high risk,” Dr. Loibl said.
The study was limited by an imbalance of tumor subtypes between the treatment groups. The authors wrote that the results should guide further research, but nab-paclitaxel should not currently be viewed as a standard neoadjuvant treatment of breast cancer.
The study was funded by the German Breast Group, Bristol Myers Squibb, and Amgen. Dr. Loibl has served on the advisory boards or given lectures for AbbVie, Amgen, Bayer, Celgene, EirGenix, GSK, Lilly, Merck, Puma, Seagen, AstraZeneca, Daiichi Sankyo, Novartis, Pierre Fabre, Prime/Medscape, Bristol Myers Squibb, Chugai, Ipsen, Roche, and Samsung. She also holds a related patent.
REPORTING FROM JAMA ONCOLOGY
The latest on COVID-19 and the heart in children
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
LDL lowering to specific targets may offset risk from high Lp(a)
MILAN – The increased risk for atherosclerotic cardiovascular disease events caused by elevated lipoprotein(a) levels can potentially be precisely offset by lowering LDL cholesterol to specific levels, suggests a novel study that underscores the importance or early intervention.
The results, derived from an analysis of data on Lp(a) and LDL cholesterol levels and associated genetic risk scores in almost 500,000 individuals from the United Kingdom, have been used to develop a series of age-related targets for lowering LDL cholesterol levels to counter the risk associated with lifetime Lp(a) exposure.
Measuring Lp(a) levels can “substantially refine individual estimates of absolute risk of atherosclerotic cardiovascular disease,” said study presenter Brian A. Ference, MD, Centre for Naturally Randomized Trials, University of Cambridge (England).
This can “directly inform treatment decisions about the intensity of LDL lowering or other risk-factor modification needed to overcome the increased risk caused by Lp(a).”
Dr. Ference said this will allow clinicians to personalize the prevention of atherosclerotic cardiovascular disease and identify people “who may benefit from potent Lp(a)-lowering therapies when they become available.”
The research was presented at the European Atherosclerosis Society (EAS) 2022 congress on May 24.
In addition to producing a tabular version of the intensification of LDL-cholesterol reduction needed to overcome the increased cardiovascular risk at different levels of Lp(a), stratified by age, Dr. Ference is working with the EAS to develop an app to further deliver on that personalized prevention.
It will display an individual’s lifetime risk for myocardial infarction or stroke, with and without the inclusion of Lp(a) levels, and determine not only the percentage of increased risk caused by Lp(a), but also the amount by which LDL cholesterol needs to be lowered to overcome that risk.
“The whole rationale for this study was to say, how can we give practical advice on how to use Lp(a) to inform clinical decisions about how to individualize personal risk reduction,” Dr. Ference told this news organization.
“What the app will do is make it very easy for clinicians to, first, understand how much Lp(a) increases risk, but specifically how they can use that information to directly inform their treatment decisions.”
In addition, Dr. Ference said that it will “show patients why it’s important for them” to intensify LDL lowering to overcome their particular level of Lp(a).
Other key takeaways from the results is the importance of intervention as early as possible to minimize the impact of lifetime exposure to increased Lp(a), and that the reduction in LDL cholesterol required to achieve that remains relatively modest.
For Dr. Ference, this means ideally beginning comprehensive health checks at 30 years of age and starting lipid-lowering interventions immediately for those at risk.
“The good thing about LDL and other causes of atherosclerotic cardiovascular disease is it doesn’t really matter how you lower it,” he said, noting that it could be with diet, lifestyle interventions, or medication.
Handy tool
The new app could be a “handy tool to counsel patients,” Florian Kronenberg, MD, Institute of Genetic Epidemiology, Medical University of Innsbruck, Austria, told this news organization.
“We can say, look, you have high Lp(a),” he said. “This is coming from nature, from your genetics, but here we have a point where we can act on your high risk by lowering LDL further. This is important to explain to the patient,” said Dr. Kronenberg, who was not involved in the study.
He emphasized that it is crucial to get across the idea of an individual’s global risk, with not just Lp(a) or cholesterol levels influencing their likelihood of cardiovascular events, but also their age, blood pressure, smoking status, and underlying genetic risk.
Dr. Kronenberg said the current data will be helpful in explaining to clinicians why they should lower LDL-cholesterol levels when a patients had high Lp(a), again centered on the idea of lowering their global risk.
During his presentation, Dr. Ference noted that an increase in Lp(a) levels is associated with a log-linear increase in atherosclerotic cardiovascular disease that is proportional to the absolute, rather than relative, magnitude of Lp(a) increase.
“Unfortunately, unlike other proteins,” he continued, diet and exercise do not affect levels, and there are currently no effective therapies to lower the risks associated with increased Lp(a) concentrations.
“For that reason,” he said, the 2019 ESC/EAS guidelines for the management of dyslipidemias, on which Dr. Ference was a coauthor, “recommend that we intensify life risk-factor modification in persons with elevated risks.”
However, he added, “this guidance is not specific enough to be useful, and that has created a great deal of inertia among clinicians,” with some concluding that they don’t need to measure Lp(a) “because there’s nothing they can do for it.”
Until the development of novel therapies that directly target Lp(a), the authors sought to quantify the amount of LDL lowering needed to “overcome the increased risk caused by Lp(a),” he said.
They studied data on 455,765 individuals from the UK Biobank who did not have a history of cardiovascular events, diabetes, or any cancer before the age of 30. They also had LDL cholesterol levels below 5 mmol/L at the time of enrollment to exclude people with presumed familial hypercholesterolemia.
The researchers used an Lp(a) genetic risk score based on the variants rs10455872 and rs3798220 and an LDL instrumental variable genetic score comprised of 100 variants to randomly categorize individuals with average Lp(a) levels, higher Lp(a) levels, or higher Lp(a) and lower LDL-cholesterol levels.
The data showed that, with elevated absolute levels of measured Lp(a) and with elevated genetic risk scores, there was a progressive increase in the lifetime risk for major coronary events.
When looking at the combination of both increased Lp(a) levels and lower LDL-cholesterol levels, they found that the increase in risk for major coronary events at Lp(a) of 123 nmol/L could be offset by a reduction in LDL-cholesterol levels of 19.5 mg/dL.
For people with an Lp(a) level of 251 nmol/L, the increase in risk for major coronary events was offset by a reduction in LDL-cholesterol levels of 36.1 mg/dL.
Furthermore, the researchers found that the magnitude of intensification of LDL-cholesterol lowering needed to overcome the risk caused by elevated Lp(a) levels varied by age.
For example, in individuals with an Lp(a) level of 220 nmol/L, the reduction in LDL-cholesterol levels needed to offset the risk for major coronary events was calculated to be 0.8 mmol/L if lipid-lowering was started at 30 years of age, rising to 0.9 mmol/L if started at 40 years, 1.2 mmol/L if started at 50 years, and 1.5 mmol/L if started at 60 years.
This, Dr. Ference said, suggests that “diet and lifestyle modification is unlikely to be an effective strategy if started later.”
No funding was declared. Dr. Ference declared relationships with Amgen, Novartis, Merck, Esperion Therapeutics, Pfizer, Regeneron, Sanofi, AstraZeneca, Eli Lilly, Novo Nordisk, The Medicines Company, Mylan, Daiichi Sankyo, Viatris, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, and KrKa Pharmaceuticals. Dr. Kronenberg declared relationships with Amgen, Novartis, and Kaneka.
A version of this article first appeared on Medscape.com.
MILAN – The increased risk for atherosclerotic cardiovascular disease events caused by elevated lipoprotein(a) levels can potentially be precisely offset by lowering LDL cholesterol to specific levels, suggests a novel study that underscores the importance or early intervention.
The results, derived from an analysis of data on Lp(a) and LDL cholesterol levels and associated genetic risk scores in almost 500,000 individuals from the United Kingdom, have been used to develop a series of age-related targets for lowering LDL cholesterol levels to counter the risk associated with lifetime Lp(a) exposure.
Measuring Lp(a) levels can “substantially refine individual estimates of absolute risk of atherosclerotic cardiovascular disease,” said study presenter Brian A. Ference, MD, Centre for Naturally Randomized Trials, University of Cambridge (England).
This can “directly inform treatment decisions about the intensity of LDL lowering or other risk-factor modification needed to overcome the increased risk caused by Lp(a).”
Dr. Ference said this will allow clinicians to personalize the prevention of atherosclerotic cardiovascular disease and identify people “who may benefit from potent Lp(a)-lowering therapies when they become available.”
The research was presented at the European Atherosclerosis Society (EAS) 2022 congress on May 24.
In addition to producing a tabular version of the intensification of LDL-cholesterol reduction needed to overcome the increased cardiovascular risk at different levels of Lp(a), stratified by age, Dr. Ference is working with the EAS to develop an app to further deliver on that personalized prevention.
It will display an individual’s lifetime risk for myocardial infarction or stroke, with and without the inclusion of Lp(a) levels, and determine not only the percentage of increased risk caused by Lp(a), but also the amount by which LDL cholesterol needs to be lowered to overcome that risk.
“The whole rationale for this study was to say, how can we give practical advice on how to use Lp(a) to inform clinical decisions about how to individualize personal risk reduction,” Dr. Ference told this news organization.
“What the app will do is make it very easy for clinicians to, first, understand how much Lp(a) increases risk, but specifically how they can use that information to directly inform their treatment decisions.”
In addition, Dr. Ference said that it will “show patients why it’s important for them” to intensify LDL lowering to overcome their particular level of Lp(a).
Other key takeaways from the results is the importance of intervention as early as possible to minimize the impact of lifetime exposure to increased Lp(a), and that the reduction in LDL cholesterol required to achieve that remains relatively modest.
For Dr. Ference, this means ideally beginning comprehensive health checks at 30 years of age and starting lipid-lowering interventions immediately for those at risk.
“The good thing about LDL and other causes of atherosclerotic cardiovascular disease is it doesn’t really matter how you lower it,” he said, noting that it could be with diet, lifestyle interventions, or medication.
Handy tool
The new app could be a “handy tool to counsel patients,” Florian Kronenberg, MD, Institute of Genetic Epidemiology, Medical University of Innsbruck, Austria, told this news organization.
“We can say, look, you have high Lp(a),” he said. “This is coming from nature, from your genetics, but here we have a point where we can act on your high risk by lowering LDL further. This is important to explain to the patient,” said Dr. Kronenberg, who was not involved in the study.
He emphasized that it is crucial to get across the idea of an individual’s global risk, with not just Lp(a) or cholesterol levels influencing their likelihood of cardiovascular events, but also their age, blood pressure, smoking status, and underlying genetic risk.
Dr. Kronenberg said the current data will be helpful in explaining to clinicians why they should lower LDL-cholesterol levels when a patients had high Lp(a), again centered on the idea of lowering their global risk.
During his presentation, Dr. Ference noted that an increase in Lp(a) levels is associated with a log-linear increase in atherosclerotic cardiovascular disease that is proportional to the absolute, rather than relative, magnitude of Lp(a) increase.
“Unfortunately, unlike other proteins,” he continued, diet and exercise do not affect levels, and there are currently no effective therapies to lower the risks associated with increased Lp(a) concentrations.
“For that reason,” he said, the 2019 ESC/EAS guidelines for the management of dyslipidemias, on which Dr. Ference was a coauthor, “recommend that we intensify life risk-factor modification in persons with elevated risks.”
However, he added, “this guidance is not specific enough to be useful, and that has created a great deal of inertia among clinicians,” with some concluding that they don’t need to measure Lp(a) “because there’s nothing they can do for it.”
Until the development of novel therapies that directly target Lp(a), the authors sought to quantify the amount of LDL lowering needed to “overcome the increased risk caused by Lp(a),” he said.
They studied data on 455,765 individuals from the UK Biobank who did not have a history of cardiovascular events, diabetes, or any cancer before the age of 30. They also had LDL cholesterol levels below 5 mmol/L at the time of enrollment to exclude people with presumed familial hypercholesterolemia.
The researchers used an Lp(a) genetic risk score based on the variants rs10455872 and rs3798220 and an LDL instrumental variable genetic score comprised of 100 variants to randomly categorize individuals with average Lp(a) levels, higher Lp(a) levels, or higher Lp(a) and lower LDL-cholesterol levels.
The data showed that, with elevated absolute levels of measured Lp(a) and with elevated genetic risk scores, there was a progressive increase in the lifetime risk for major coronary events.
When looking at the combination of both increased Lp(a) levels and lower LDL-cholesterol levels, they found that the increase in risk for major coronary events at Lp(a) of 123 nmol/L could be offset by a reduction in LDL-cholesterol levels of 19.5 mg/dL.
For people with an Lp(a) level of 251 nmol/L, the increase in risk for major coronary events was offset by a reduction in LDL-cholesterol levels of 36.1 mg/dL.
Furthermore, the researchers found that the magnitude of intensification of LDL-cholesterol lowering needed to overcome the risk caused by elevated Lp(a) levels varied by age.
For example, in individuals with an Lp(a) level of 220 nmol/L, the reduction in LDL-cholesterol levels needed to offset the risk for major coronary events was calculated to be 0.8 mmol/L if lipid-lowering was started at 30 years of age, rising to 0.9 mmol/L if started at 40 years, 1.2 mmol/L if started at 50 years, and 1.5 mmol/L if started at 60 years.
This, Dr. Ference said, suggests that “diet and lifestyle modification is unlikely to be an effective strategy if started later.”
No funding was declared. Dr. Ference declared relationships with Amgen, Novartis, Merck, Esperion Therapeutics, Pfizer, Regeneron, Sanofi, AstraZeneca, Eli Lilly, Novo Nordisk, The Medicines Company, Mylan, Daiichi Sankyo, Viatris, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, and KrKa Pharmaceuticals. Dr. Kronenberg declared relationships with Amgen, Novartis, and Kaneka.
A version of this article first appeared on Medscape.com.
MILAN – The increased risk for atherosclerotic cardiovascular disease events caused by elevated lipoprotein(a) levels can potentially be precisely offset by lowering LDL cholesterol to specific levels, suggests a novel study that underscores the importance or early intervention.
The results, derived from an analysis of data on Lp(a) and LDL cholesterol levels and associated genetic risk scores in almost 500,000 individuals from the United Kingdom, have been used to develop a series of age-related targets for lowering LDL cholesterol levels to counter the risk associated with lifetime Lp(a) exposure.
Measuring Lp(a) levels can “substantially refine individual estimates of absolute risk of atherosclerotic cardiovascular disease,” said study presenter Brian A. Ference, MD, Centre for Naturally Randomized Trials, University of Cambridge (England).
This can “directly inform treatment decisions about the intensity of LDL lowering or other risk-factor modification needed to overcome the increased risk caused by Lp(a).”
Dr. Ference said this will allow clinicians to personalize the prevention of atherosclerotic cardiovascular disease and identify people “who may benefit from potent Lp(a)-lowering therapies when they become available.”
The research was presented at the European Atherosclerosis Society (EAS) 2022 congress on May 24.
In addition to producing a tabular version of the intensification of LDL-cholesterol reduction needed to overcome the increased cardiovascular risk at different levels of Lp(a), stratified by age, Dr. Ference is working with the EAS to develop an app to further deliver on that personalized prevention.
It will display an individual’s lifetime risk for myocardial infarction or stroke, with and without the inclusion of Lp(a) levels, and determine not only the percentage of increased risk caused by Lp(a), but also the amount by which LDL cholesterol needs to be lowered to overcome that risk.
“The whole rationale for this study was to say, how can we give practical advice on how to use Lp(a) to inform clinical decisions about how to individualize personal risk reduction,” Dr. Ference told this news organization.
“What the app will do is make it very easy for clinicians to, first, understand how much Lp(a) increases risk, but specifically how they can use that information to directly inform their treatment decisions.”
In addition, Dr. Ference said that it will “show patients why it’s important for them” to intensify LDL lowering to overcome their particular level of Lp(a).
Other key takeaways from the results is the importance of intervention as early as possible to minimize the impact of lifetime exposure to increased Lp(a), and that the reduction in LDL cholesterol required to achieve that remains relatively modest.
For Dr. Ference, this means ideally beginning comprehensive health checks at 30 years of age and starting lipid-lowering interventions immediately for those at risk.
“The good thing about LDL and other causes of atherosclerotic cardiovascular disease is it doesn’t really matter how you lower it,” he said, noting that it could be with diet, lifestyle interventions, or medication.
Handy tool
The new app could be a “handy tool to counsel patients,” Florian Kronenberg, MD, Institute of Genetic Epidemiology, Medical University of Innsbruck, Austria, told this news organization.
“We can say, look, you have high Lp(a),” he said. “This is coming from nature, from your genetics, but here we have a point where we can act on your high risk by lowering LDL further. This is important to explain to the patient,” said Dr. Kronenberg, who was not involved in the study.
He emphasized that it is crucial to get across the idea of an individual’s global risk, with not just Lp(a) or cholesterol levels influencing their likelihood of cardiovascular events, but also their age, blood pressure, smoking status, and underlying genetic risk.
Dr. Kronenberg said the current data will be helpful in explaining to clinicians why they should lower LDL-cholesterol levels when a patients had high Lp(a), again centered on the idea of lowering their global risk.
During his presentation, Dr. Ference noted that an increase in Lp(a) levels is associated with a log-linear increase in atherosclerotic cardiovascular disease that is proportional to the absolute, rather than relative, magnitude of Lp(a) increase.
“Unfortunately, unlike other proteins,” he continued, diet and exercise do not affect levels, and there are currently no effective therapies to lower the risks associated with increased Lp(a) concentrations.
“For that reason,” he said, the 2019 ESC/EAS guidelines for the management of dyslipidemias, on which Dr. Ference was a coauthor, “recommend that we intensify life risk-factor modification in persons with elevated risks.”
However, he added, “this guidance is not specific enough to be useful, and that has created a great deal of inertia among clinicians,” with some concluding that they don’t need to measure Lp(a) “because there’s nothing they can do for it.”
Until the development of novel therapies that directly target Lp(a), the authors sought to quantify the amount of LDL lowering needed to “overcome the increased risk caused by Lp(a),” he said.
They studied data on 455,765 individuals from the UK Biobank who did not have a history of cardiovascular events, diabetes, or any cancer before the age of 30. They also had LDL cholesterol levels below 5 mmol/L at the time of enrollment to exclude people with presumed familial hypercholesterolemia.
The researchers used an Lp(a) genetic risk score based on the variants rs10455872 and rs3798220 and an LDL instrumental variable genetic score comprised of 100 variants to randomly categorize individuals with average Lp(a) levels, higher Lp(a) levels, or higher Lp(a) and lower LDL-cholesterol levels.
The data showed that, with elevated absolute levels of measured Lp(a) and with elevated genetic risk scores, there was a progressive increase in the lifetime risk for major coronary events.
When looking at the combination of both increased Lp(a) levels and lower LDL-cholesterol levels, they found that the increase in risk for major coronary events at Lp(a) of 123 nmol/L could be offset by a reduction in LDL-cholesterol levels of 19.5 mg/dL.
For people with an Lp(a) level of 251 nmol/L, the increase in risk for major coronary events was offset by a reduction in LDL-cholesterol levels of 36.1 mg/dL.
Furthermore, the researchers found that the magnitude of intensification of LDL-cholesterol lowering needed to overcome the risk caused by elevated Lp(a) levels varied by age.
For example, in individuals with an Lp(a) level of 220 nmol/L, the reduction in LDL-cholesterol levels needed to offset the risk for major coronary events was calculated to be 0.8 mmol/L if lipid-lowering was started at 30 years of age, rising to 0.9 mmol/L if started at 40 years, 1.2 mmol/L if started at 50 years, and 1.5 mmol/L if started at 60 years.
This, Dr. Ference said, suggests that “diet and lifestyle modification is unlikely to be an effective strategy if started later.”
No funding was declared. Dr. Ference declared relationships with Amgen, Novartis, Merck, Esperion Therapeutics, Pfizer, Regeneron, Sanofi, AstraZeneca, Eli Lilly, Novo Nordisk, The Medicines Company, Mylan, Daiichi Sankyo, Viatris, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, and KrKa Pharmaceuticals. Dr. Kronenberg declared relationships with Amgen, Novartis, and Kaneka.
A version of this article first appeared on Medscape.com.
AT EAS 2022
Meet the JCOM Author with Dr. Barkoudah: IVIG in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia
The AGA Research Foundation awards $2.56 million in funding
AGA is proud to announce the 61 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“Our award recipients demonstrate an undeniable determination to improve the care of digestive health patients,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We are investing in talented early-career investigators knowing that their work will ultimately benefit patients with critical needs.”

“In the past year, we expanded our awards program and elevated the importance of engaging underrepresented groups into the field of GI research,” Dr. Sandler said. “We are encouraged by the range of candidates who applied for funding and look forward to the results of their research.”
The AGA Research Foundation Awards Program is made possible thanks to generous donors and funders.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Kathleen Curtius, PhD, MS, University of California, San Diego, La Jolla
Trisha Satya Pasricha, MD, MPH, Massachusetts General Hospital, Boston
Bomi Lee, PhD, MS, Stanford University, Palo Alto, Calif.
Christine E. Eyler, MD, PhD, Duke University, Durham, N.C.
Joel Gabre, MD, Columbia University Irving Medical Center, New York
AGA–Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Srinivas Gaddam, MD, MPH, Cedars-Sinai Medical Center, Los Angeles
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
Claire L. Jansson-Knodell, MD, Cleveland Clinic Foundation, Cleveland
Specialty Awards
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Eunyoung Choi, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Sarah Palmer Short, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
Pilot Awards
AGA–Medtronic Pilot Research Award in Artificial Intelligence
Dennis Shung, MD, MHS, Yale School of Medicine, New Haven, Conn.
AGA–Merck Pilot Research Award in Colorectal Cancer Health Disparities
Sonia Kupfer, MD, The University of Chicago, Chicago
AGA–Bristol Myers Squibb Pilot Research Award in Inflammatory Bowel Disease Health Disparities
Chung Sang Tse, MD, University of California, San Diego
AGA Pilot Research Award in Health Disparities (funded by Janssen Biotech)
Jennifer Flemming, MD, MAS, Queen’s University, Kingston, Ont.
AGA Pilot Research Award in Digestive Disease Health Disparities
Young-Rock Hong, PhD, MPH, University of Florida, Gainesville, Fla.
AGA–Amgen Pilot Research Award in Digestive Disease Health Disparities
Zachary Reichenbach, MD, PhD, Lewis Katz School of Medicine, Temple University, Philadelphia
AGA–Pfizer Pilot Research Award in Inflammatory Bowel Disease
Melinda Engevik, PhD, MS, Medical University of South Carolina, Charleston
Andre Paes Batista da Silva, PhD, MSC, DDS, Case Western Reserve University, Cleveland
Karen Edelblum, PhD, Rutgers New Jersey Medical School, Newark, N.J.
Undergraduate Research Awards
AGA–Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gabriela Ortiz, Washington University School of Medicine, St. Louis
Daniella Montalvo, University of Miami Miller School of Medicine, Miami
Subear Hussein, Children’s Hospital, Boston
Hussein Herz, University of Iowa Carver College of Medicine, Iowa City
Kaleb Tesfai, University of California, San Diego
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor, Mich.
Abstract Awards
AGA Fellow Abstract of the Year Award
Masaru Sasaki, MD, PhD, The Children’s Hospital of Philadelphia
AGA Student Abstract of the Year Award
Anitha Vijay, MS, Penn State University, State College, Pa.
Maafi Rizwana Islam, PhD, Marshall University, Huntington, W.V.
Fellow Abstract Awards
Nicolette Rodriguez, MD, MPH, Brigham and Women’s Hospital, Boston
Hyunseok Kim, MD, PhD, MPH, Baylor College of Medicine, Houston
Margaret Zhou, MD, Stanford University, Palo Alto, Calif.
Steven Steinway, MD, PhD, Johns Hopkins University, Baltimore
Su-Hyung Lee, PhD, DVM, Vanderbilt University Medical Center, Nashville, Tenn.
Ian Greenberg, MD, Dallas Methodist Hospital, Dallas
Jonathan Xia, MD, PhD, Northwestern Memorial Hospital, Chicago
Donevan Westerveld, MD, NewYork-Presbyterian Weill Cornell Medicine, New York
Haley Zylberberg, MD, Columbia University Irving Medical Center, New York
Maria Jesus Villanueva Millan, PhD, Cedars-Sinai Medical Center, Los Angeles
Duke Geem, MD, PhD. Children s Healthcare of Atlanta/Emory University, Atlanta
Fauzi Feris Jassir, MD, Mayo Clinic, Rochester, Minn.
Melissa Musser, MD, PhD, Boston Children’s Hospital, Boston
Student Abstract Awards
Kushal Saha, MS, BS, Penn State College of Medicine, Hershey, Pa.
Winston Liu, BS. Duke University, Durham, N.C.
Yoojin Sohn, BS, Vanderbilt University Medical Center, Nashville, Tenn.
Jamie Yang, BS, David Geffen School of Medicine at University of California, Los Angeles
Rachel Hopton, BS, University of Oregon, Eugene
Alina Li, BS, Columbia University, New York
Eleazar Montalvan Sanchez, MD, Indiana University School of Medicine, Indianapolis
Christina Lin, MD, BA, BS, Kaiser Permanente Northern California, Santa Clara, Calif.
Conrad Fernandes, MD, BA, Hospital of the University of Pennsylvania, Philadelphia
Hajar Hazime, MS, BS, University of Miami
Blaine Prichard, BS, Pennsylvania State University College of Medicine, Hershey, Pa.
Georgetta Skinner, MS, BS, A.T. Still University, Kirksville, Mo.
AGA Abstract Award for Health Disparities Research
Kai Wang, PhD (Fellow), Harvard T.H. Chan School of Public Health, Boston
Alan De La Rosa, MD (Fellow), Mayo Clinic, Rochester, Minn.
Timothy Andrew Zaki, MD, BS (Student), UT Southwestern Medical Center, Dallas
Megan McLeod, MD, MS, BA, University of California, Los Angeles (student)
AGA–APFED Abstract Award in Eosinophilic GI Diseases
Takeo Hara, MD, PhD, Children’s Hospital of Philadelphia
Michael Wang, BS, Duke University School of Medicine, Durham, N.C.
Melissa Nelson, MD, Baylor University Medical Center, Dallas
AGA–Moti L. & Kamla Rustgi International Travel Award
Joost Algera, MD, University of Gothenburg (Sweden)
Ashkan Rezazadeh Ardabili, MD, MS, BS, Maastricht (Netherlands) University Medical Center+
AGA research awards cycle now open
This year the AGA Research Foundation is awarding more than $2.5 million dollars to investigators who are passionate about improving digestive health. Get your piece of the research funding pie with one of our awards!
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level. View our awards portfolio by career stage below, then mark your calendar for upcoming application deadlines. View additional information about each award.
AGA is proud to announce the 61 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“Our award recipients demonstrate an undeniable determination to improve the care of digestive health patients,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We are investing in talented early-career investigators knowing that their work will ultimately benefit patients with critical needs.”

“In the past year, we expanded our awards program and elevated the importance of engaging underrepresented groups into the field of GI research,” Dr. Sandler said. “We are encouraged by the range of candidates who applied for funding and look forward to the results of their research.”
The AGA Research Foundation Awards Program is made possible thanks to generous donors and funders.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Kathleen Curtius, PhD, MS, University of California, San Diego, La Jolla
Trisha Satya Pasricha, MD, MPH, Massachusetts General Hospital, Boston
Bomi Lee, PhD, MS, Stanford University, Palo Alto, Calif.
Christine E. Eyler, MD, PhD, Duke University, Durham, N.C.
Joel Gabre, MD, Columbia University Irving Medical Center, New York
AGA–Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Srinivas Gaddam, MD, MPH, Cedars-Sinai Medical Center, Los Angeles
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
Claire L. Jansson-Knodell, MD, Cleveland Clinic Foundation, Cleveland
Specialty Awards
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Eunyoung Choi, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Sarah Palmer Short, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
Pilot Awards
AGA–Medtronic Pilot Research Award in Artificial Intelligence
Dennis Shung, MD, MHS, Yale School of Medicine, New Haven, Conn.
AGA–Merck Pilot Research Award in Colorectal Cancer Health Disparities
Sonia Kupfer, MD, The University of Chicago, Chicago
AGA–Bristol Myers Squibb Pilot Research Award in Inflammatory Bowel Disease Health Disparities
Chung Sang Tse, MD, University of California, San Diego
AGA Pilot Research Award in Health Disparities (funded by Janssen Biotech)
Jennifer Flemming, MD, MAS, Queen’s University, Kingston, Ont.
AGA Pilot Research Award in Digestive Disease Health Disparities
Young-Rock Hong, PhD, MPH, University of Florida, Gainesville, Fla.
AGA–Amgen Pilot Research Award in Digestive Disease Health Disparities
Zachary Reichenbach, MD, PhD, Lewis Katz School of Medicine, Temple University, Philadelphia
AGA–Pfizer Pilot Research Award in Inflammatory Bowel Disease
Melinda Engevik, PhD, MS, Medical University of South Carolina, Charleston
Andre Paes Batista da Silva, PhD, MSC, DDS, Case Western Reserve University, Cleveland
Karen Edelblum, PhD, Rutgers New Jersey Medical School, Newark, N.J.
Undergraduate Research Awards
AGA–Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gabriela Ortiz, Washington University School of Medicine, St. Louis
Daniella Montalvo, University of Miami Miller School of Medicine, Miami
Subear Hussein, Children’s Hospital, Boston
Hussein Herz, University of Iowa Carver College of Medicine, Iowa City
Kaleb Tesfai, University of California, San Diego
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor, Mich.
Abstract Awards
AGA Fellow Abstract of the Year Award
Masaru Sasaki, MD, PhD, The Children’s Hospital of Philadelphia
AGA Student Abstract of the Year Award
Anitha Vijay, MS, Penn State University, State College, Pa.
Maafi Rizwana Islam, PhD, Marshall University, Huntington, W.V.
Fellow Abstract Awards
Nicolette Rodriguez, MD, MPH, Brigham and Women’s Hospital, Boston
Hyunseok Kim, MD, PhD, MPH, Baylor College of Medicine, Houston
Margaret Zhou, MD, Stanford University, Palo Alto, Calif.
Steven Steinway, MD, PhD, Johns Hopkins University, Baltimore
Su-Hyung Lee, PhD, DVM, Vanderbilt University Medical Center, Nashville, Tenn.
Ian Greenberg, MD, Dallas Methodist Hospital, Dallas
Jonathan Xia, MD, PhD, Northwestern Memorial Hospital, Chicago
Donevan Westerveld, MD, NewYork-Presbyterian Weill Cornell Medicine, New York
Haley Zylberberg, MD, Columbia University Irving Medical Center, New York
Maria Jesus Villanueva Millan, PhD, Cedars-Sinai Medical Center, Los Angeles
Duke Geem, MD, PhD. Children s Healthcare of Atlanta/Emory University, Atlanta
Fauzi Feris Jassir, MD, Mayo Clinic, Rochester, Minn.
Melissa Musser, MD, PhD, Boston Children’s Hospital, Boston
Student Abstract Awards
Kushal Saha, MS, BS, Penn State College of Medicine, Hershey, Pa.
Winston Liu, BS. Duke University, Durham, N.C.
Yoojin Sohn, BS, Vanderbilt University Medical Center, Nashville, Tenn.
Jamie Yang, BS, David Geffen School of Medicine at University of California, Los Angeles
Rachel Hopton, BS, University of Oregon, Eugene
Alina Li, BS, Columbia University, New York
Eleazar Montalvan Sanchez, MD, Indiana University School of Medicine, Indianapolis
Christina Lin, MD, BA, BS, Kaiser Permanente Northern California, Santa Clara, Calif.
Conrad Fernandes, MD, BA, Hospital of the University of Pennsylvania, Philadelphia
Hajar Hazime, MS, BS, University of Miami
Blaine Prichard, BS, Pennsylvania State University College of Medicine, Hershey, Pa.
Georgetta Skinner, MS, BS, A.T. Still University, Kirksville, Mo.
AGA Abstract Award for Health Disparities Research
Kai Wang, PhD (Fellow), Harvard T.H. Chan School of Public Health, Boston
Alan De La Rosa, MD (Fellow), Mayo Clinic, Rochester, Minn.
Timothy Andrew Zaki, MD, BS (Student), UT Southwestern Medical Center, Dallas
Megan McLeod, MD, MS, BA, University of California, Los Angeles (student)
AGA–APFED Abstract Award in Eosinophilic GI Diseases
Takeo Hara, MD, PhD, Children’s Hospital of Philadelphia
Michael Wang, BS, Duke University School of Medicine, Durham, N.C.
Melissa Nelson, MD, Baylor University Medical Center, Dallas
AGA–Moti L. & Kamla Rustgi International Travel Award
Joost Algera, MD, University of Gothenburg (Sweden)
Ashkan Rezazadeh Ardabili, MD, MS, BS, Maastricht (Netherlands) University Medical Center+
AGA research awards cycle now open
This year the AGA Research Foundation is awarding more than $2.5 million dollars to investigators who are passionate about improving digestive health. Get your piece of the research funding pie with one of our awards!
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level. View our awards portfolio by career stage below, then mark your calendar for upcoming application deadlines. View additional information about each award.
AGA is proud to announce the 61 recipients selected to receive research funding through its annual AGA Research Foundation Awards Program. The program serves as a catalyst for discovery and career growth among the most promising researchers in gastroenterology and hepatology.
“Our award recipients demonstrate an undeniable determination to improve the care of digestive health patients,” said Robert S. Sandler, MD, MPH, AGAF, chair of the AGA Research Foundation. “We are investing in talented early-career investigators knowing that their work will ultimately benefit patients with critical needs.”

“In the past year, we expanded our awards program and elevated the importance of engaging underrepresented groups into the field of GI research,” Dr. Sandler said. “We are encouraged by the range of candidates who applied for funding and look forward to the results of their research.”
The AGA Research Foundation Awards Program is made possible thanks to generous donors and funders.
Here are this year’s award recipients:
Research Scholar Awards
AGA Research Scholar Award
Kathleen Curtius, PhD, MS, University of California, San Diego, La Jolla
Trisha Satya Pasricha, MD, MPH, Massachusetts General Hospital, Boston
Bomi Lee, PhD, MS, Stanford University, Palo Alto, Calif.
Christine E. Eyler, MD, PhD, Duke University, Durham, N.C.
Joel Gabre, MD, Columbia University Irving Medical Center, New York
AGA–Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Srinivas Gaddam, MD, MPH, Cedars-Sinai Medical Center, Los Angeles
AGA–Takeda Pharmaceuticals Research Scholar Award in Celiac Disease
Claire L. Jansson-Knodell, MD, Cleveland Clinic Foundation, Cleveland
Specialty Awards
AGA–R. Robert & Sally Funderburg Research Award in Gastric Cancer
Eunyoung Choi, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
AGA–Caroline Craig Augustyn & Damian Augustyn Award in Digestive Cancer
Sarah Palmer Short, PhD, Vanderbilt University Medical Center, Nashville, Tenn.
Pilot Awards
AGA–Medtronic Pilot Research Award in Artificial Intelligence
Dennis Shung, MD, MHS, Yale School of Medicine, New Haven, Conn.
AGA–Merck Pilot Research Award in Colorectal Cancer Health Disparities
Sonia Kupfer, MD, The University of Chicago, Chicago
AGA–Bristol Myers Squibb Pilot Research Award in Inflammatory Bowel Disease Health Disparities
Chung Sang Tse, MD, University of California, San Diego
AGA Pilot Research Award in Health Disparities (funded by Janssen Biotech)
Jennifer Flemming, MD, MAS, Queen’s University, Kingston, Ont.
AGA Pilot Research Award in Digestive Disease Health Disparities
Young-Rock Hong, PhD, MPH, University of Florida, Gainesville, Fla.
AGA–Amgen Pilot Research Award in Digestive Disease Health Disparities
Zachary Reichenbach, MD, PhD, Lewis Katz School of Medicine, Temple University, Philadelphia
AGA–Pfizer Pilot Research Award in Inflammatory Bowel Disease
Melinda Engevik, PhD, MS, Medical University of South Carolina, Charleston
Andre Paes Batista da Silva, PhD, MSC, DDS, Case Western Reserve University, Cleveland
Karen Edelblum, PhD, Rutgers New Jersey Medical School, Newark, N.J.
Undergraduate Research Awards
AGA–Aman Armaan Ahmed Family Summer Undergraduate Research Award
Gabriela Ortiz, Washington University School of Medicine, St. Louis
Daniella Montalvo, University of Miami Miller School of Medicine, Miami
Subear Hussein, Children’s Hospital, Boston
Hussein Herz, University of Iowa Carver College of Medicine, Iowa City
Kaleb Tesfai, University of California, San Diego
Varun Ponnusamy, University of Michigan Medical School, Ann Arbor, Mich.
Abstract Awards
AGA Fellow Abstract of the Year Award
Masaru Sasaki, MD, PhD, The Children’s Hospital of Philadelphia
AGA Student Abstract of the Year Award
Anitha Vijay, MS, Penn State University, State College, Pa.
Maafi Rizwana Islam, PhD, Marshall University, Huntington, W.V.
Fellow Abstract Awards
Nicolette Rodriguez, MD, MPH, Brigham and Women’s Hospital, Boston
Hyunseok Kim, MD, PhD, MPH, Baylor College of Medicine, Houston
Margaret Zhou, MD, Stanford University, Palo Alto, Calif.
Steven Steinway, MD, PhD, Johns Hopkins University, Baltimore
Su-Hyung Lee, PhD, DVM, Vanderbilt University Medical Center, Nashville, Tenn.
Ian Greenberg, MD, Dallas Methodist Hospital, Dallas
Jonathan Xia, MD, PhD, Northwestern Memorial Hospital, Chicago
Donevan Westerveld, MD, NewYork-Presbyterian Weill Cornell Medicine, New York
Haley Zylberberg, MD, Columbia University Irving Medical Center, New York
Maria Jesus Villanueva Millan, PhD, Cedars-Sinai Medical Center, Los Angeles
Duke Geem, MD, PhD. Children s Healthcare of Atlanta/Emory University, Atlanta
Fauzi Feris Jassir, MD, Mayo Clinic, Rochester, Minn.
Melissa Musser, MD, PhD, Boston Children’s Hospital, Boston
Student Abstract Awards
Kushal Saha, MS, BS, Penn State College of Medicine, Hershey, Pa.
Winston Liu, BS. Duke University, Durham, N.C.
Yoojin Sohn, BS, Vanderbilt University Medical Center, Nashville, Tenn.
Jamie Yang, BS, David Geffen School of Medicine at University of California, Los Angeles
Rachel Hopton, BS, University of Oregon, Eugene
Alina Li, BS, Columbia University, New York
Eleazar Montalvan Sanchez, MD, Indiana University School of Medicine, Indianapolis
Christina Lin, MD, BA, BS, Kaiser Permanente Northern California, Santa Clara, Calif.
Conrad Fernandes, MD, BA, Hospital of the University of Pennsylvania, Philadelphia
Hajar Hazime, MS, BS, University of Miami
Blaine Prichard, BS, Pennsylvania State University College of Medicine, Hershey, Pa.
Georgetta Skinner, MS, BS, A.T. Still University, Kirksville, Mo.
AGA Abstract Award for Health Disparities Research
Kai Wang, PhD (Fellow), Harvard T.H. Chan School of Public Health, Boston
Alan De La Rosa, MD (Fellow), Mayo Clinic, Rochester, Minn.
Timothy Andrew Zaki, MD, BS (Student), UT Southwestern Medical Center, Dallas
Megan McLeod, MD, MS, BA, University of California, Los Angeles (student)
AGA–APFED Abstract Award in Eosinophilic GI Diseases
Takeo Hara, MD, PhD, Children’s Hospital of Philadelphia
Michael Wang, BS, Duke University School of Medicine, Durham, N.C.
Melissa Nelson, MD, Baylor University Medical Center, Dallas
AGA–Moti L. & Kamla Rustgi International Travel Award
Joost Algera, MD, University of Gothenburg (Sweden)
Ashkan Rezazadeh Ardabili, MD, MS, BS, Maastricht (Netherlands) University Medical Center+
AGA research awards cycle now open
This year the AGA Research Foundation is awarding more than $2.5 million dollars to investigators who are passionate about improving digestive health. Get your piece of the research funding pie with one of our awards!
The AGA Research Foundation Awards Program recruits, retains, and supports the most promising researchers in gastroenterology and hepatology. With funding from the foundation, recipients have protected time to take their research to the next level. View our awards portfolio by career stage below, then mark your calendar for upcoming application deadlines. View additional information about each award.
Children & COVID: Rise in new cases slows
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.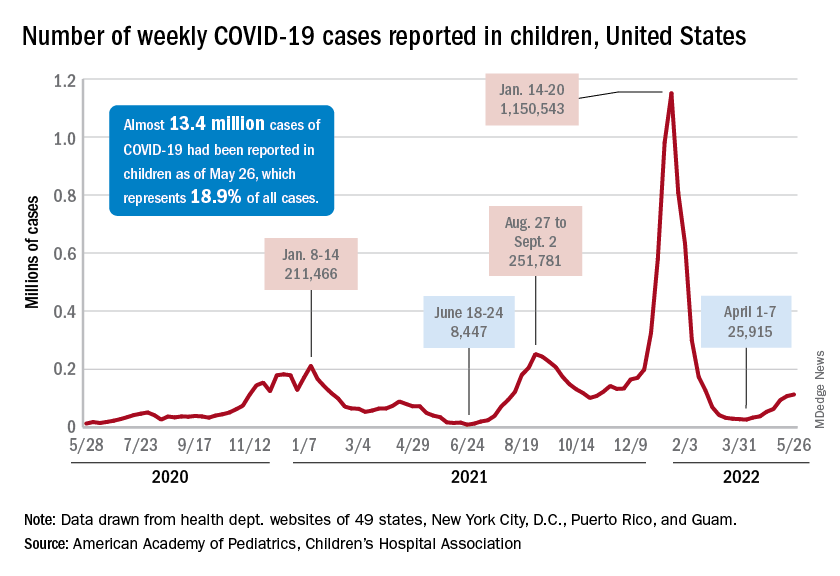
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
Then and Now: Demographics of the AGA
The demographics of the American Gastroenterological Association have changed markedly since the first issue of GI and Hepatology News (GIHN) was published in 2007. GIHN’s first editorial team published news that was reliable and informative.
The first GIHN editor in chief, Dr. Charles J. Lightdale, described GIHN as “irresistible reading for all those seeking comprehensive, current, authoritative information in the field.” Today, GIHN continues the tradition of publishing news that is “irresistible,” “comprehensive,” and “authoritative.” However, GIHN’s board of editors looks quite different in 2022 than it did in 2007. The current board comprises people from both academic and private practice settings; we are from diverse ethnic and racial backgrounds. Finally, Dr. Megan A. Adams is the first woman to serve as editor in chief of the publication.
The last 15 years have seen an increase in the number of women and underrepresented minorities in gastroenterology. This, in turn, has changed the demographics of the AGA, and more women and underrepresented minorities are assuming leadership roles within the organization. The AGA values diversity and inclusion in its membership, but more importantly in its leadership.
On April 2, 2022, I had the privilege of participating in the AGA Future Leaders Program as a mentor representing Private Practice. The program also included participants of AGA’s FORWARD Program. The meeting started with the usual welcome and introductions. During the morning session, Tom Serena, CEO, spoke about the early history of the AGA. The AGA was an “elite” club 125 years ago, established as a research society with a membership limited to those with investigative achievements.
Next, Dr. John M. Carethers spoke about the importance of diversity and leadership. That afternoon, I sat on a panel with Dr. Anna S. Lok and Dr. Guadalupe Garcia-Tsao. The panel was asked to speak about mentoring across practice settings. As I sat there, I became acutely aware of the diversity represented on the panel and in the audience. The panelists were all women, and women of color. The future leaders of the AGA are from diverse backgrounds.
The physicians participating in that meeting came from academia and private practice – young men and women leaders from various racial and ethnic backgrounds. It was so uplifting to witness how the AGA is evolving. I am proud to be a member of an organization that values having different voices at the table. This diversity will make our organization stronger as we face the challenges in our profession today and in the future.
The traditional 15th-year anniversary gift is crystal, which symbolizes clarity and durability. On GIHN’s 15th-year anniversary, the path before us looks bright. The changing demographics of GI and of our organization brings together unfamiliar faces, fresh perspectives and new ideas that will help the organization build a clear and resilient path forward. Our future is bright!
Kimberly M. Persley, MD, AGAF, is a partner with Texas Digestive Disease Consultants/GI Alliance in Dallas, is on the medical staff of Texas Health Presbyterian Hospital, and is an associate editor of GI & Hepatology News. She has no relevant conflicts of interest.
The demographics of the American Gastroenterological Association have changed markedly since the first issue of GI and Hepatology News (GIHN) was published in 2007. GIHN’s first editorial team published news that was reliable and informative.
The first GIHN editor in chief, Dr. Charles J. Lightdale, described GIHN as “irresistible reading for all those seeking comprehensive, current, authoritative information in the field.” Today, GIHN continues the tradition of publishing news that is “irresistible,” “comprehensive,” and “authoritative.” However, GIHN’s board of editors looks quite different in 2022 than it did in 2007. The current board comprises people from both academic and private practice settings; we are from diverse ethnic and racial backgrounds. Finally, Dr. Megan A. Adams is the first woman to serve as editor in chief of the publication.
The last 15 years have seen an increase in the number of women and underrepresented minorities in gastroenterology. This, in turn, has changed the demographics of the AGA, and more women and underrepresented minorities are assuming leadership roles within the organization. The AGA values diversity and inclusion in its membership, but more importantly in its leadership.
On April 2, 2022, I had the privilege of participating in the AGA Future Leaders Program as a mentor representing Private Practice. The program also included participants of AGA’s FORWARD Program. The meeting started with the usual welcome and introductions. During the morning session, Tom Serena, CEO, spoke about the early history of the AGA. The AGA was an “elite” club 125 years ago, established as a research society with a membership limited to those with investigative achievements.
Next, Dr. John M. Carethers spoke about the importance of diversity and leadership. That afternoon, I sat on a panel with Dr. Anna S. Lok and Dr. Guadalupe Garcia-Tsao. The panel was asked to speak about mentoring across practice settings. As I sat there, I became acutely aware of the diversity represented on the panel and in the audience. The panelists were all women, and women of color. The future leaders of the AGA are from diverse backgrounds.
The physicians participating in that meeting came from academia and private practice – young men and women leaders from various racial and ethnic backgrounds. It was so uplifting to witness how the AGA is evolving. I am proud to be a member of an organization that values having different voices at the table. This diversity will make our organization stronger as we face the challenges in our profession today and in the future.
The traditional 15th-year anniversary gift is crystal, which symbolizes clarity and durability. On GIHN’s 15th-year anniversary, the path before us looks bright. The changing demographics of GI and of our organization brings together unfamiliar faces, fresh perspectives and new ideas that will help the organization build a clear and resilient path forward. Our future is bright!
Kimberly M. Persley, MD, AGAF, is a partner with Texas Digestive Disease Consultants/GI Alliance in Dallas, is on the medical staff of Texas Health Presbyterian Hospital, and is an associate editor of GI & Hepatology News. She has no relevant conflicts of interest.
The demographics of the American Gastroenterological Association have changed markedly since the first issue of GI and Hepatology News (GIHN) was published in 2007. GIHN’s first editorial team published news that was reliable and informative.
The first GIHN editor in chief, Dr. Charles J. Lightdale, described GIHN as “irresistible reading for all those seeking comprehensive, current, authoritative information in the field.” Today, GIHN continues the tradition of publishing news that is “irresistible,” “comprehensive,” and “authoritative.” However, GIHN’s board of editors looks quite different in 2022 than it did in 2007. The current board comprises people from both academic and private practice settings; we are from diverse ethnic and racial backgrounds. Finally, Dr. Megan A. Adams is the first woman to serve as editor in chief of the publication.
The last 15 years have seen an increase in the number of women and underrepresented minorities in gastroenterology. This, in turn, has changed the demographics of the AGA, and more women and underrepresented minorities are assuming leadership roles within the organization. The AGA values diversity and inclusion in its membership, but more importantly in its leadership.
On April 2, 2022, I had the privilege of participating in the AGA Future Leaders Program as a mentor representing Private Practice. The program also included participants of AGA’s FORWARD Program. The meeting started with the usual welcome and introductions. During the morning session, Tom Serena, CEO, spoke about the early history of the AGA. The AGA was an “elite” club 125 years ago, established as a research society with a membership limited to those with investigative achievements.
Next, Dr. John M. Carethers spoke about the importance of diversity and leadership. That afternoon, I sat on a panel with Dr. Anna S. Lok and Dr. Guadalupe Garcia-Tsao. The panel was asked to speak about mentoring across practice settings. As I sat there, I became acutely aware of the diversity represented on the panel and in the audience. The panelists were all women, and women of color. The future leaders of the AGA are from diverse backgrounds.
The physicians participating in that meeting came from academia and private practice – young men and women leaders from various racial and ethnic backgrounds. It was so uplifting to witness how the AGA is evolving. I am proud to be a member of an organization that values having different voices at the table. This diversity will make our organization stronger as we face the challenges in our profession today and in the future.
The traditional 15th-year anniversary gift is crystal, which symbolizes clarity and durability. On GIHN’s 15th-year anniversary, the path before us looks bright. The changing demographics of GI and of our organization brings together unfamiliar faces, fresh perspectives and new ideas that will help the organization build a clear and resilient path forward. Our future is bright!
Kimberly M. Persley, MD, AGAF, is a partner with Texas Digestive Disease Consultants/GI Alliance in Dallas, is on the medical staff of Texas Health Presbyterian Hospital, and is an associate editor of GI & Hepatology News. She has no relevant conflicts of interest.
Commentary: Effects on Colorectal Cancer Treatment, June 2022
Next, a retrospective analysis compared adjuvant capecitabine or capecitabine + oxaliplatin (CapeOX) for resected stage II-III colorectal cancer in 606 patients. Fifty-four of these patients were taking a proton pump inhibitor (PPI) as well. The authors found that concomitant use of a PPI with capecitabine monotherapy led to shorter relapse-free survival (adjusted hazard ratio, 2.48; P = .013) compared with those not taking a PPI. Interestingly, the effect on RFS was not observed in patients receiving CapeOX. A proposed mechanism for this finding is that the increased pH in PPI-treated stomachs decreases dissolution of the capecitabine tablet. Certainly, direct observation would be required to prove this, but these data alone may be enough for oncologists to think twice before prescribing capecitabine to patients who must remain on a PPI.
Lastly, a well-done analysis from the Nurses' Health Study found that higher intake of sugar-sweetened beverages and total fructose was associated with increased incidence of and mortality from proximal colon cancer, but interestingly not distal colon or rectal cancers. The hazard ratios for both the incremental incidence of proximal colon cancer for intake of one serving of sugar-sweetened beverage per day and for 25 g/day of fructose were 1.18 (Ptrend = .02), and the hazard ratios for mortality were 1.39 (Ptrend = .002) and 1.42 (Ptrend = .003), respectively. I am often asked by my patients what, if any, utility there might be in limiting sugar intake when undergoing cancer treatment. This study provides the basis for an answer that is more than just hand-waving.
Next, a retrospective analysis compared adjuvant capecitabine or capecitabine + oxaliplatin (CapeOX) for resected stage II-III colorectal cancer in 606 patients. Fifty-four of these patients were taking a proton pump inhibitor (PPI) as well. The authors found that concomitant use of a PPI with capecitabine monotherapy led to shorter relapse-free survival (adjusted hazard ratio, 2.48; P = .013) compared with those not taking a PPI. Interestingly, the effect on RFS was not observed in patients receiving CapeOX. A proposed mechanism for this finding is that the increased pH in PPI-treated stomachs decreases dissolution of the capecitabine tablet. Certainly, direct observation would be required to prove this, but these data alone may be enough for oncologists to think twice before prescribing capecitabine to patients who must remain on a PPI.
Lastly, a well-done analysis from the Nurses' Health Study found that higher intake of sugar-sweetened beverages and total fructose was associated with increased incidence of and mortality from proximal colon cancer, but interestingly not distal colon or rectal cancers. The hazard ratios for both the incremental incidence of proximal colon cancer for intake of one serving of sugar-sweetened beverage per day and for 25 g/day of fructose were 1.18 (Ptrend = .02), and the hazard ratios for mortality were 1.39 (Ptrend = .002) and 1.42 (Ptrend = .003), respectively. I am often asked by my patients what, if any, utility there might be in limiting sugar intake when undergoing cancer treatment. This study provides the basis for an answer that is more than just hand-waving.
Next, a retrospective analysis compared adjuvant capecitabine or capecitabine + oxaliplatin (CapeOX) for resected stage II-III colorectal cancer in 606 patients. Fifty-four of these patients were taking a proton pump inhibitor (PPI) as well. The authors found that concomitant use of a PPI with capecitabine monotherapy led to shorter relapse-free survival (adjusted hazard ratio, 2.48; P = .013) compared with those not taking a PPI. Interestingly, the effect on RFS was not observed in patients receiving CapeOX. A proposed mechanism for this finding is that the increased pH in PPI-treated stomachs decreases dissolution of the capecitabine tablet. Certainly, direct observation would be required to prove this, but these data alone may be enough for oncologists to think twice before prescribing capecitabine to patients who must remain on a PPI.
Lastly, a well-done analysis from the Nurses' Health Study found that higher intake of sugar-sweetened beverages and total fructose was associated with increased incidence of and mortality from proximal colon cancer, but interestingly not distal colon or rectal cancers. The hazard ratios for both the incremental incidence of proximal colon cancer for intake of one serving of sugar-sweetened beverage per day and for 25 g/day of fructose were 1.18 (Ptrend = .02), and the hazard ratios for mortality were 1.39 (Ptrend = .002) and 1.42 (Ptrend = .003), respectively. I am often asked by my patients what, if any, utility there might be in limiting sugar intake when undergoing cancer treatment. This study provides the basis for an answer that is more than just hand-waving.
FDA withdraws lymphoma drug approval after investigation
Umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
But safety concerns began to emerge in the phase 3 UNITY-CLL trial, which evaluated the drug in a related cancer type: chronic lymphocytic leukemia.
Last February, the FDA said it was investigating a possible increased risk of death associated with umbralisib.
Five months later, the results are in.
“Updated findings from the UNITY-CLL clinical trial continued to show a possible increased risk of death in patients receiving Ukoniq. As a result, we determined the risks of treatment with Ukoniq outweigh its benefits,” the FDA wrote in a drug safety communication published June 1.
In April, the drug manufacturer, TG Therapeutics, announced it was voluntarily withdrawing umbralisib from the market for its approved uses in marginal zone lymphoma and follicular lymphoma.
The FDA’s safety notice includes instructions for physicians and patients. The FDA urges health care professionals to “stop prescribing Ukoniq and switch patients to alternative treatments” and to “inform patients currently taking Ukoniq of the increased risk of death seen in the clinical trial and advise them to stop taking the medicine.”
In special instances in which a patient may be benefiting from the drug, the company plans to make umbralisib available under expanded access.
The FDA also recommends that patients who discontinue taking the drug dispose of unused umbralisib using a drug take-back location, such as a pharmacy, or throwing it away in the household trash after placing it in a sealed bag mixed with dirt or cat litter and removing personal identification information.
A version of this article first appeared on Medscape.com.
Umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
But safety concerns began to emerge in the phase 3 UNITY-CLL trial, which evaluated the drug in a related cancer type: chronic lymphocytic leukemia.
Last February, the FDA said it was investigating a possible increased risk of death associated with umbralisib.
Five months later, the results are in.
“Updated findings from the UNITY-CLL clinical trial continued to show a possible increased risk of death in patients receiving Ukoniq. As a result, we determined the risks of treatment with Ukoniq outweigh its benefits,” the FDA wrote in a drug safety communication published June 1.
In April, the drug manufacturer, TG Therapeutics, announced it was voluntarily withdrawing umbralisib from the market for its approved uses in marginal zone lymphoma and follicular lymphoma.
The FDA’s safety notice includes instructions for physicians and patients. The FDA urges health care professionals to “stop prescribing Ukoniq and switch patients to alternative treatments” and to “inform patients currently taking Ukoniq of the increased risk of death seen in the clinical trial and advise them to stop taking the medicine.”
In special instances in which a patient may be benefiting from the drug, the company plans to make umbralisib available under expanded access.
The FDA also recommends that patients who discontinue taking the drug dispose of unused umbralisib using a drug take-back location, such as a pharmacy, or throwing it away in the household trash after placing it in a sealed bag mixed with dirt or cat litter and removing personal identification information.
A version of this article first appeared on Medscape.com.
Umbralisib had received accelerated approval in February 2021 to treat adults with relapsed or refractory marginal zone lymphoma following at least one prior therapy and those with relapsed or refractory follicular lymphoma who had received at least three prior therapies.
But safety concerns began to emerge in the phase 3 UNITY-CLL trial, which evaluated the drug in a related cancer type: chronic lymphocytic leukemia.
Last February, the FDA said it was investigating a possible increased risk of death associated with umbralisib.
Five months later, the results are in.
“Updated findings from the UNITY-CLL clinical trial continued to show a possible increased risk of death in patients receiving Ukoniq. As a result, we determined the risks of treatment with Ukoniq outweigh its benefits,” the FDA wrote in a drug safety communication published June 1.
In April, the drug manufacturer, TG Therapeutics, announced it was voluntarily withdrawing umbralisib from the market for its approved uses in marginal zone lymphoma and follicular lymphoma.
The FDA’s safety notice includes instructions for physicians and patients. The FDA urges health care professionals to “stop prescribing Ukoniq and switch patients to alternative treatments” and to “inform patients currently taking Ukoniq of the increased risk of death seen in the clinical trial and advise them to stop taking the medicine.”
In special instances in which a patient may be benefiting from the drug, the company plans to make umbralisib available under expanded access.
The FDA also recommends that patients who discontinue taking the drug dispose of unused umbralisib using a drug take-back location, such as a pharmacy, or throwing it away in the household trash after placing it in a sealed bag mixed with dirt or cat litter and removing personal identification information.
A version of this article first appeared on Medscape.com.
Commentary: New Prognostic Markers in Rheumatoid Arthritis, June 2022
Predicting severe disease is of great importance in rheumatoid arthritis (RA), ideally to establish which poor prognostic factors allow for early aggressive and targeted treatment for a subset of patients. In a post hoc analysis of the AGREE study by Durez and colleagues, 509 treatment-naive patients randomized to either methotrexate or methotrexate with abatacept were evaluated for predictors of joint damage and disease activity. Baseline swelling in the knee, temporomandibular joint (TMJ), elbow, and wrist was correlated with severe disease activity as well as tender and swollen joint counts, whereas baseline swelling at the second metacarpophalangeal joint was correlated with erosive disease. Overall, remission rates were better in patients with baseline wrist, TMJ, elbow, and knee swelling treated with combination therapy vs methotrexate alone, suggestive of a better response to more aggressive therapy. Further studies of patients with RA with poor prognostic factors would be helpful.
Laboratory biomarkers can also serve as prognostic indicators for patients with RA. Based in part on the association of obesity and lower rates of remission in people with RA, Baker and colleagues investigated the possible association of adipocytokines and disease activity in a cohort study of over 1200 patients with Disease Activity Score for Rheumatoid Arthritis (DAS28) > 3.2 enrolled in the Veterans Affairs RA registry. Of these, about 800 achieved low disease activity while the remainder did not. Interestingly, obesity was not a baseline characteristic associated with disease activity, though chronic obstructive pulmonary disease, heart failure, and mood disorders were. Baseline adipocytokine levels on average did not differ between the two groups, though higher baseline adiponectin and leptin levels (based on quartile) were associated with a lower likelihood of achieving low disease activity. Interestingly, this change did not increase progressively with higher quartile. Because these are baseline levels and were not tracked prospectively with medication use, it is difficult to assess the importance of this finding. The association may not reflect a causative relationship but may be affected by medications or disease duration. However, it appears worthwhile investigating in therapy-naive patients as well as those being observed with treatment.
Finally, with respect to novel therapeutic regimens, Fleischmann and colleagues report the results of a multicenter randomized clinical trial of a Bruton tyrosine kinase inhibitor, elsubrutinib, alone or in combination with the Janus kinase (JAK) inhibitor upadicitinib in the treatment of 242 patients with RA. At the end of 12 weeks, DAS28 with C-reactive protein scores were not measurably lower in patients treated with elsubrutinib at different doses. In addition, patients receiving the combination therapy of 15 mg upadicitinib with the highest dose of elsubrutinib (60 mg) did not have greater DAS28 improvement than patients treated with upadicitinib alone, suggesting a lack of synergistic effect. Short-term safety data does not suggest significant differences. Though this combination is also being investigated in systemic lupus erythematosus, it is not clear that the combination of two targeted synthetic disease-modifying antirheumatic drugs is feasible in RA, nor that long-term safety concerns would make it advisable.
Predicting severe disease is of great importance in rheumatoid arthritis (RA), ideally to establish which poor prognostic factors allow for early aggressive and targeted treatment for a subset of patients. In a post hoc analysis of the AGREE study by Durez and colleagues, 509 treatment-naive patients randomized to either methotrexate or methotrexate with abatacept were evaluated for predictors of joint damage and disease activity. Baseline swelling in the knee, temporomandibular joint (TMJ), elbow, and wrist was correlated with severe disease activity as well as tender and swollen joint counts, whereas baseline swelling at the second metacarpophalangeal joint was correlated with erosive disease. Overall, remission rates were better in patients with baseline wrist, TMJ, elbow, and knee swelling treated with combination therapy vs methotrexate alone, suggestive of a better response to more aggressive therapy. Further studies of patients with RA with poor prognostic factors would be helpful.
Laboratory biomarkers can also serve as prognostic indicators for patients with RA. Based in part on the association of obesity and lower rates of remission in people with RA, Baker and colleagues investigated the possible association of adipocytokines and disease activity in a cohort study of over 1200 patients with Disease Activity Score for Rheumatoid Arthritis (DAS28) > 3.2 enrolled in the Veterans Affairs RA registry. Of these, about 800 achieved low disease activity while the remainder did not. Interestingly, obesity was not a baseline characteristic associated with disease activity, though chronic obstructive pulmonary disease, heart failure, and mood disorders were. Baseline adipocytokine levels on average did not differ between the two groups, though higher baseline adiponectin and leptin levels (based on quartile) were associated with a lower likelihood of achieving low disease activity. Interestingly, this change did not increase progressively with higher quartile. Because these are baseline levels and were not tracked prospectively with medication use, it is difficult to assess the importance of this finding. The association may not reflect a causative relationship but may be affected by medications or disease duration. However, it appears worthwhile investigating in therapy-naive patients as well as those being observed with treatment.
Finally, with respect to novel therapeutic regimens, Fleischmann and colleagues report the results of a multicenter randomized clinical trial of a Bruton tyrosine kinase inhibitor, elsubrutinib, alone or in combination with the Janus kinase (JAK) inhibitor upadicitinib in the treatment of 242 patients with RA. At the end of 12 weeks, DAS28 with C-reactive protein scores were not measurably lower in patients treated with elsubrutinib at different doses. In addition, patients receiving the combination therapy of 15 mg upadicitinib with the highest dose of elsubrutinib (60 mg) did not have greater DAS28 improvement than patients treated with upadicitinib alone, suggesting a lack of synergistic effect. Short-term safety data does not suggest significant differences. Though this combination is also being investigated in systemic lupus erythematosus, it is not clear that the combination of two targeted synthetic disease-modifying antirheumatic drugs is feasible in RA, nor that long-term safety concerns would make it advisable.
Predicting severe disease is of great importance in rheumatoid arthritis (RA), ideally to establish which poor prognostic factors allow for early aggressive and targeted treatment for a subset of patients. In a post hoc analysis of the AGREE study by Durez and colleagues, 509 treatment-naive patients randomized to either methotrexate or methotrexate with abatacept were evaluated for predictors of joint damage and disease activity. Baseline swelling in the knee, temporomandibular joint (TMJ), elbow, and wrist was correlated with severe disease activity as well as tender and swollen joint counts, whereas baseline swelling at the second metacarpophalangeal joint was correlated with erosive disease. Overall, remission rates were better in patients with baseline wrist, TMJ, elbow, and knee swelling treated with combination therapy vs methotrexate alone, suggestive of a better response to more aggressive therapy. Further studies of patients with RA with poor prognostic factors would be helpful.
Laboratory biomarkers can also serve as prognostic indicators for patients with RA. Based in part on the association of obesity and lower rates of remission in people with RA, Baker and colleagues investigated the possible association of adipocytokines and disease activity in a cohort study of over 1200 patients with Disease Activity Score for Rheumatoid Arthritis (DAS28) > 3.2 enrolled in the Veterans Affairs RA registry. Of these, about 800 achieved low disease activity while the remainder did not. Interestingly, obesity was not a baseline characteristic associated with disease activity, though chronic obstructive pulmonary disease, heart failure, and mood disorders were. Baseline adipocytokine levels on average did not differ between the two groups, though higher baseline adiponectin and leptin levels (based on quartile) were associated with a lower likelihood of achieving low disease activity. Interestingly, this change did not increase progressively with higher quartile. Because these are baseline levels and were not tracked prospectively with medication use, it is difficult to assess the importance of this finding. The association may not reflect a causative relationship but may be affected by medications or disease duration. However, it appears worthwhile investigating in therapy-naive patients as well as those being observed with treatment.
Finally, with respect to novel therapeutic regimens, Fleischmann and colleagues report the results of a multicenter randomized clinical trial of a Bruton tyrosine kinase inhibitor, elsubrutinib, alone or in combination with the Janus kinase (JAK) inhibitor upadicitinib in the treatment of 242 patients with RA. At the end of 12 weeks, DAS28 with C-reactive protein scores were not measurably lower in patients treated with elsubrutinib at different doses. In addition, patients receiving the combination therapy of 15 mg upadicitinib with the highest dose of elsubrutinib (60 mg) did not have greater DAS28 improvement than patients treated with upadicitinib alone, suggesting a lack of synergistic effect. Short-term safety data does not suggest significant differences. Though this combination is also being investigated in systemic lupus erythematosus, it is not clear that the combination of two targeted synthetic disease-modifying antirheumatic drugs is feasible in RA, nor that long-term safety concerns would make it advisable.








