User login
‘Cool’ way of eradicating fat a promising therapy for many medical conditions
SAN DIEGO – During her third year in the combined Harvard/Massachusetts General Hospital dermatology residency program in 2011, Lilit Garibyan, MD, PhD, attended a lecture presented by R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH. He described the concept of selective cryolipolysis – the method of removing fat by topical cooling that eventually led to the development of the CoolSculpting device.
“He was saying that this is such a great noninvasive technology for fat removal and that patients love it,” Dr. Garibyan recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “But one of the most common side effects after cryolipolysis that is long-lasting, but completely reversible, is hypoesthesia. I was intrigued by this because even as a dermatology resident, I had seen how pain and itch symptoms are present in many dermatologic diseases, and we don’t have great treatments for them. I thought to myself, not the fat.
Following Dr. Anderson’s lecture, Dr. Garibyan asked him if anyone knew the mechanism of action or if anyone was working to find out. He did not, but Dr. Anderson invited her to join his lab to investigate. “I didn’t have a background in lasers or energy devices, but I thought this was such a great opportunity” and addressed an unmet need, she said at the meeting.
Dr. Garibyan then led a clinical trial to characterize the effect of a single cryolipolysis treatment in 11 healthy people and to quantitatively analyze what sensory functions change with treatment over a period of 56 days. Skin biopsies revealed that cryolipolysis mainly decreased myelinated dermal nerve fiber density, which persisted throughout the study.
“The conclusion was that yes, controlled topical cooling does lead to significant and long-lasting but reversible reduction of sensory function, including pain,” said Dr. Garibyan, who is now an assistant professor of dermatology at Harvard Medical School, Boston, and director of the Magic Wand Initiative at the Wellman Center.
Ice slurry injections
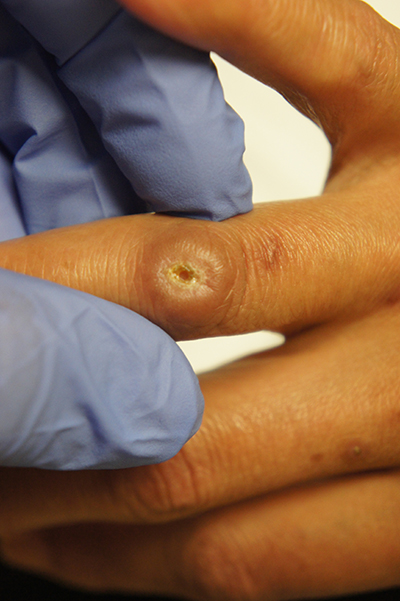
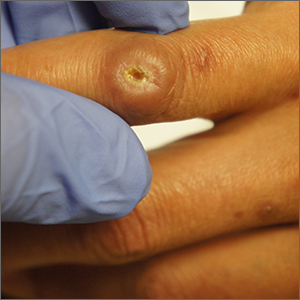
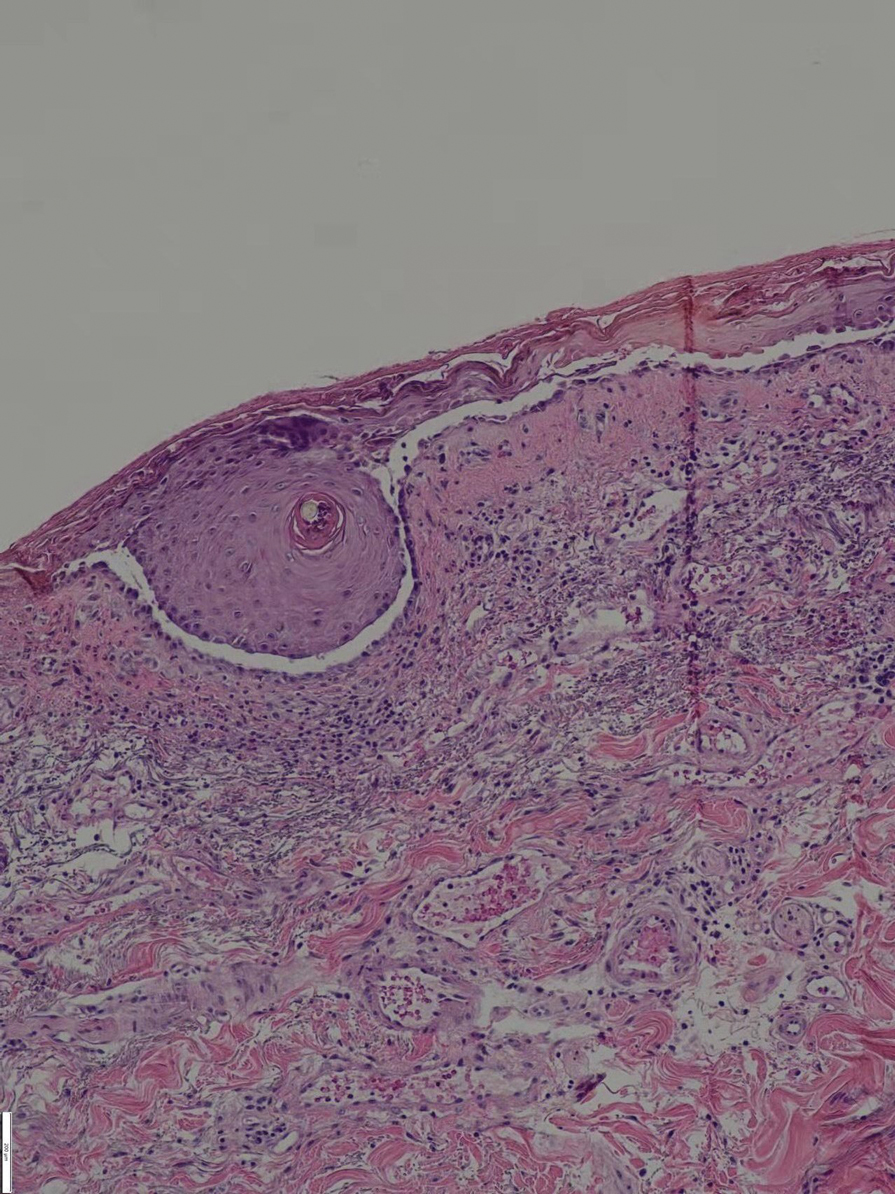
Enter ice slurry, a chilly mix of ice, saline, and glycol that can be directly injected into adipose tissue. In a swine study published online in January 2020, Dr. Garibyan and colleagues at the Wellman Center injected ice slurry into the flanks of swine and followed them for up to 8 weeks, using ultrasound imaging to quantify and show the location of fat loss. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% increase of fat in controls. “On histology, this was very selective,” she said. “Only adipose tissue was affected. There was no damage to the underlying muscle or to the dermis or epidermis.”
In 2021, researchers tested the injection of ice slurry in 12 humans for the first time, injected into tissue, and followed them for 12 weeks. As observed by thermal imaging, ultrasound, and tissue histology, they concluded that ice slurry injection was feasible and safe as a way of inducing cryolipolysis, and was well tolerated by patients.
“This can become a promising treatment for a precise, effective, and customizable way of removing unwanted fat for aesthetic application,” Dr. Garibyan said. However, she added, it is not approved by the Food and Drug Administration and more studies are needed, “but it’s promising and encouraging to see this move forward in patients.”
Potential nonaesthetic uses
The potential applications of injectable ice slurry extend well beyond cosmetic dermatology, she continued, noting that it is being explored as a treatment for many medical conditions including obstructive sleep apnea (OSA). At the University of Pennsylvania, Philadelphia, researchers used MRI to image the tongue fat in a case-control study of 31 obese patients without OSA and 90 obese patients with OSA. They found that patients with OSA had increased deposition of fat at the base of their tongue, which can lead to airway obstruction in this subset of patients with OSA, pointed out Dr. Garibyan, who was not involved with the study. “This also gave us a hint. If we can remove that tongue fat, we could potentially help reduce severity or even cure OSA in this population of patients. This points to tongue fat as a therapeutic target.”
With help from researchers at Uniformed Services University of the Health Sciences, Bethesda, Md., she and her Wellman Center colleagues recently completed a swine study that showed the safety and feasibility of injecting the base of the tongue with ice slurry, targeting adipose tissue. The work has been submitted for publication in a journal, but at the meeting, she said that, 8 weeks after injecting the ice slurry, there were no changes to any tongue tissue other than fat.
“On histology, we only see selective damage to the adipose tissue,” she said. “It is very promising that it’s safe in animal models and we’re hoping to conduct a human trial later this year to test the ability of this injectable ice slurry to remove fat at the base of the tongue with the hope that this will treat OSA.”
Another potential application of this technology is in the cardiology field. Dr. Garibyan is part of a multidisciplinary team at MGH that includes cardiac surgeons, cardiologists, and imaging experts who plan to investigate whether injecting ice slurry into fat around the heart can modify heart disease in humans. “Visceral fat around the heart – pericardial fat and epicardial fat – is involved in cardiovascular disease, arrhythmias, and many other unwanted effects on the heart,” she said. “Imagine if you could inject this around the heart, ablate the fat, and halt cardiovascular disease?”
She led a study that examined the effect of injecting ice slurry into swine with significant amounts of adipose tissue around their hearts, based on baseline CT scans. She and her coinvestigators observed a significant loss of that fat tissue on follow-up CT scans 8 weeks later. “On average, there was about a 30% reduction of this pericardial adipose tissue after a single injection,” and the procedure “was safe and well tolerated by the animals,” she added.
Ice slurry could also play a role in managing pain by targeting peripheral nerves. Peripheral nerves are composed of 75%-80% lipids, such as the myelin sheaths around the nerves, she noted. “That’s lipid-rich tissue. We think that by targeting that we’re able to block pain.”
She led a study that showed that a single injection of ice slurry around the sciatic nerve in rats served as a sustained anesthetic by blocking mechanical pain sensation for up to 56 days. They imaged the peripheral nerves in the rats and showed that the mechanism involved was loss of the lipid-rich myelin tissue around the nerves, which blocks the signaling of the nerve, she said.
Dr. Garibyan disclosed that she is a member of the advisory board for Brixton Biosciences, Vyome Therapeutics, and Aegle Therapeutics. She is also a consultant for Aegle Therapeutics and Blossom Innovations and holds equity in Brixton Biosciences and EyeCool Therapeutics.
SAN DIEGO – During her third year in the combined Harvard/Massachusetts General Hospital dermatology residency program in 2011, Lilit Garibyan, MD, PhD, attended a lecture presented by R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH. He described the concept of selective cryolipolysis – the method of removing fat by topical cooling that eventually led to the development of the CoolSculpting device.
“He was saying that this is such a great noninvasive technology for fat removal and that patients love it,” Dr. Garibyan recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “But one of the most common side effects after cryolipolysis that is long-lasting, but completely reversible, is hypoesthesia. I was intrigued by this because even as a dermatology resident, I had seen how pain and itch symptoms are present in many dermatologic diseases, and we don’t have great treatments for them. I thought to myself, not the fat.
Following Dr. Anderson’s lecture, Dr. Garibyan asked him if anyone knew the mechanism of action or if anyone was working to find out. He did not, but Dr. Anderson invited her to join his lab to investigate. “I didn’t have a background in lasers or energy devices, but I thought this was such a great opportunity” and addressed an unmet need, she said at the meeting.
Dr. Garibyan then led a clinical trial to characterize the effect of a single cryolipolysis treatment in 11 healthy people and to quantitatively analyze what sensory functions change with treatment over a period of 56 days. Skin biopsies revealed that cryolipolysis mainly decreased myelinated dermal nerve fiber density, which persisted throughout the study.
“The conclusion was that yes, controlled topical cooling does lead to significant and long-lasting but reversible reduction of sensory function, including pain,” said Dr. Garibyan, who is now an assistant professor of dermatology at Harvard Medical School, Boston, and director of the Magic Wand Initiative at the Wellman Center.
Ice slurry injections
Enter ice slurry, a chilly mix of ice, saline, and glycol that can be directly injected into adipose tissue. In a swine study published online in January 2020, Dr. Garibyan and colleagues at the Wellman Center injected ice slurry into the flanks of swine and followed them for up to 8 weeks, using ultrasound imaging to quantify and show the location of fat loss. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% increase of fat in controls. “On histology, this was very selective,” she said. “Only adipose tissue was affected. There was no damage to the underlying muscle or to the dermis or epidermis.”
In 2021, researchers tested the injection of ice slurry in 12 humans for the first time, injected into tissue, and followed them for 12 weeks. As observed by thermal imaging, ultrasound, and tissue histology, they concluded that ice slurry injection was feasible and safe as a way of inducing cryolipolysis, and was well tolerated by patients.
“This can become a promising treatment for a precise, effective, and customizable way of removing unwanted fat for aesthetic application,” Dr. Garibyan said. However, she added, it is not approved by the Food and Drug Administration and more studies are needed, “but it’s promising and encouraging to see this move forward in patients.”
Potential nonaesthetic uses
The potential applications of injectable ice slurry extend well beyond cosmetic dermatology, she continued, noting that it is being explored as a treatment for many medical conditions including obstructive sleep apnea (OSA). At the University of Pennsylvania, Philadelphia, researchers used MRI to image the tongue fat in a case-control study of 31 obese patients without OSA and 90 obese patients with OSA. They found that patients with OSA had increased deposition of fat at the base of their tongue, which can lead to airway obstruction in this subset of patients with OSA, pointed out Dr. Garibyan, who was not involved with the study. “This also gave us a hint. If we can remove that tongue fat, we could potentially help reduce severity or even cure OSA in this population of patients. This points to tongue fat as a therapeutic target.”
With help from researchers at Uniformed Services University of the Health Sciences, Bethesda, Md., she and her Wellman Center colleagues recently completed a swine study that showed the safety and feasibility of injecting the base of the tongue with ice slurry, targeting adipose tissue. The work has been submitted for publication in a journal, but at the meeting, she said that, 8 weeks after injecting the ice slurry, there were no changes to any tongue tissue other than fat.
“On histology, we only see selective damage to the adipose tissue,” she said. “It is very promising that it’s safe in animal models and we’re hoping to conduct a human trial later this year to test the ability of this injectable ice slurry to remove fat at the base of the tongue with the hope that this will treat OSA.”
Another potential application of this technology is in the cardiology field. Dr. Garibyan is part of a multidisciplinary team at MGH that includes cardiac surgeons, cardiologists, and imaging experts who plan to investigate whether injecting ice slurry into fat around the heart can modify heart disease in humans. “Visceral fat around the heart – pericardial fat and epicardial fat – is involved in cardiovascular disease, arrhythmias, and many other unwanted effects on the heart,” she said. “Imagine if you could inject this around the heart, ablate the fat, and halt cardiovascular disease?”
She led a study that examined the effect of injecting ice slurry into swine with significant amounts of adipose tissue around their hearts, based on baseline CT scans. She and her coinvestigators observed a significant loss of that fat tissue on follow-up CT scans 8 weeks later. “On average, there was about a 30% reduction of this pericardial adipose tissue after a single injection,” and the procedure “was safe and well tolerated by the animals,” she added.
Ice slurry could also play a role in managing pain by targeting peripheral nerves. Peripheral nerves are composed of 75%-80% lipids, such as the myelin sheaths around the nerves, she noted. “That’s lipid-rich tissue. We think that by targeting that we’re able to block pain.”
She led a study that showed that a single injection of ice slurry around the sciatic nerve in rats served as a sustained anesthetic by blocking mechanical pain sensation for up to 56 days. They imaged the peripheral nerves in the rats and showed that the mechanism involved was loss of the lipid-rich myelin tissue around the nerves, which blocks the signaling of the nerve, she said.
Dr. Garibyan disclosed that she is a member of the advisory board for Brixton Biosciences, Vyome Therapeutics, and Aegle Therapeutics. She is also a consultant for Aegle Therapeutics and Blossom Innovations and holds equity in Brixton Biosciences and EyeCool Therapeutics.
SAN DIEGO – During her third year in the combined Harvard/Massachusetts General Hospital dermatology residency program in 2011, Lilit Garibyan, MD, PhD, attended a lecture presented by R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at MGH. He described the concept of selective cryolipolysis – the method of removing fat by topical cooling that eventually led to the development of the CoolSculpting device.
“He was saying that this is such a great noninvasive technology for fat removal and that patients love it,” Dr. Garibyan recalled at the annual meeting of the American Society for Laser Medicine and Surgery. “But one of the most common side effects after cryolipolysis that is long-lasting, but completely reversible, is hypoesthesia. I was intrigued by this because even as a dermatology resident, I had seen how pain and itch symptoms are present in many dermatologic diseases, and we don’t have great treatments for them. I thought to myself, not the fat.
Following Dr. Anderson’s lecture, Dr. Garibyan asked him if anyone knew the mechanism of action or if anyone was working to find out. He did not, but Dr. Anderson invited her to join his lab to investigate. “I didn’t have a background in lasers or energy devices, but I thought this was such a great opportunity” and addressed an unmet need, she said at the meeting.
Dr. Garibyan then led a clinical trial to characterize the effect of a single cryolipolysis treatment in 11 healthy people and to quantitatively analyze what sensory functions change with treatment over a period of 56 days. Skin biopsies revealed that cryolipolysis mainly decreased myelinated dermal nerve fiber density, which persisted throughout the study.
“The conclusion was that yes, controlled topical cooling does lead to significant and long-lasting but reversible reduction of sensory function, including pain,” said Dr. Garibyan, who is now an assistant professor of dermatology at Harvard Medical School, Boston, and director of the Magic Wand Initiative at the Wellman Center.
Ice slurry injections
Enter ice slurry, a chilly mix of ice, saline, and glycol that can be directly injected into adipose tissue. In a swine study published online in January 2020, Dr. Garibyan and colleagues at the Wellman Center injected ice slurry into the flanks of swine and followed them for up to 8 weeks, using ultrasound imaging to quantify and show the location of fat loss. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% increase of fat in controls. “On histology, this was very selective,” she said. “Only adipose tissue was affected. There was no damage to the underlying muscle or to the dermis or epidermis.”
In 2021, researchers tested the injection of ice slurry in 12 humans for the first time, injected into tissue, and followed them for 12 weeks. As observed by thermal imaging, ultrasound, and tissue histology, they concluded that ice slurry injection was feasible and safe as a way of inducing cryolipolysis, and was well tolerated by patients.
“This can become a promising treatment for a precise, effective, and customizable way of removing unwanted fat for aesthetic application,” Dr. Garibyan said. However, she added, it is not approved by the Food and Drug Administration and more studies are needed, “but it’s promising and encouraging to see this move forward in patients.”
Potential nonaesthetic uses
The potential applications of injectable ice slurry extend well beyond cosmetic dermatology, she continued, noting that it is being explored as a treatment for many medical conditions including obstructive sleep apnea (OSA). At the University of Pennsylvania, Philadelphia, researchers used MRI to image the tongue fat in a case-control study of 31 obese patients without OSA and 90 obese patients with OSA. They found that patients with OSA had increased deposition of fat at the base of their tongue, which can lead to airway obstruction in this subset of patients with OSA, pointed out Dr. Garibyan, who was not involved with the study. “This also gave us a hint. If we can remove that tongue fat, we could potentially help reduce severity or even cure OSA in this population of patients. This points to tongue fat as a therapeutic target.”
With help from researchers at Uniformed Services University of the Health Sciences, Bethesda, Md., she and her Wellman Center colleagues recently completed a swine study that showed the safety and feasibility of injecting the base of the tongue with ice slurry, targeting adipose tissue. The work has been submitted for publication in a journal, but at the meeting, she said that, 8 weeks after injecting the ice slurry, there were no changes to any tongue tissue other than fat.
“On histology, we only see selective damage to the adipose tissue,” she said. “It is very promising that it’s safe in animal models and we’re hoping to conduct a human trial later this year to test the ability of this injectable ice slurry to remove fat at the base of the tongue with the hope that this will treat OSA.”
Another potential application of this technology is in the cardiology field. Dr. Garibyan is part of a multidisciplinary team at MGH that includes cardiac surgeons, cardiologists, and imaging experts who plan to investigate whether injecting ice slurry into fat around the heart can modify heart disease in humans. “Visceral fat around the heart – pericardial fat and epicardial fat – is involved in cardiovascular disease, arrhythmias, and many other unwanted effects on the heart,” she said. “Imagine if you could inject this around the heart, ablate the fat, and halt cardiovascular disease?”
She led a study that examined the effect of injecting ice slurry into swine with significant amounts of adipose tissue around their hearts, based on baseline CT scans. She and her coinvestigators observed a significant loss of that fat tissue on follow-up CT scans 8 weeks later. “On average, there was about a 30% reduction of this pericardial adipose tissue after a single injection,” and the procedure “was safe and well tolerated by the animals,” she added.
Ice slurry could also play a role in managing pain by targeting peripheral nerves. Peripheral nerves are composed of 75%-80% lipids, such as the myelin sheaths around the nerves, she noted. “That’s lipid-rich tissue. We think that by targeting that we’re able to block pain.”
She led a study that showed that a single injection of ice slurry around the sciatic nerve in rats served as a sustained anesthetic by blocking mechanical pain sensation for up to 56 days. They imaged the peripheral nerves in the rats and showed that the mechanism involved was loss of the lipid-rich myelin tissue around the nerves, which blocks the signaling of the nerve, she said.
Dr. Garibyan disclosed that she is a member of the advisory board for Brixton Biosciences, Vyome Therapeutics, and Aegle Therapeutics. She is also a consultant for Aegle Therapeutics and Blossom Innovations and holds equity in Brixton Biosciences and EyeCool Therapeutics.
AT ASLMS 2022
Fidaxomicin favored over vancomycin in real-world C. diff study
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
AT DDW 2022
Gout app improves treat to target, reduces flares
Self-management of gout using a smartphone app to record self-test urate levels and flares, and communicate those results to clinicians, could see more patients reaching target urate levels and even reducing flare frequency, a study has found.
Writing in The Lancet Rheumatology, Philip Riches, PhD, of the rheumatic disease unit at Western General Hospital in Edinburgh, and coauthors presented the findings of their randomized, controlled feasibility study of a new gout self-management approach aimed at helping patients treat to target.
While current rheumatology guidelines stress the importance of keeping urate below target levels to reduce flares and improve clinical outcomes, this isn’t always achieved in clinical practice. A previous trial of a nurse-led treat-to-target intervention did show a reduced incidence of flares and tophaceous disease, but the authors said, despite its cost-effectiveness, this approach has yet to be implemented in the United Kingdom.
Dr. Riches and colleagues developed a self-management strategy in which all 60 patients in the study self-tested their urate levels and were prompted to enter that data into the GoutSMART smartphone app once a month or opportunistically, along with information on disease severity and quality of life. All patients had been recommended for initiation or escalation of urate-lowering therapy, and had a serum urate of 0.36 mmol/L (6 mg/dL) or higher at baseline, and all received a gout management plan at the start of the study.
Patients in the intervention group who recorded a urate level above 0.30 mmol/L (5 mg/dL) via the app during the study were prompted to do a self-test every 2 weeks and given daily reminders in the app. Their urate levels were transmitted securely to the study team who then advised on dose escalation or treatment change. Those in the usual-care group also used the app but it only prompted them to record gout flares, keep quality of life diaries, or message the researchers.
At 24 weeks after the start of the study, 73% of 40 participants in the self-management group had reached the urate target of 0.30 mmol/L or below, compared with 15% of the 20 participants in the usual-care group (P < .0001).
The difference between the two groups was sustained even 1 year after starting the intervention, when 80% of those in the self-management group had reached that target, compared with 45% of those in the usual-care group.
Patients in the intervention group also had fewer flares, experiencing a mean of 2.03 flares in the first 24 weeks, compared with a mean of 3 among the control group, although the study didn’t report any difference in the rates of tophaceous disease.
Those in the self-management group had fewer medical appointments, but were prescribed higher doses of allopurinol at the 24- and 52-week visits.
“Qualitative feedback suggests that the self-monitoring approach was accepted by most participants and was enthusiastically endorsed by many,” the authors wrote. “The approach empowers patients and provides feedback on the effect of medication.”
It will be important to determine if the success of this self-management intervention can be replicated in an even broader patient population, Lisa K. Stamp, MBChB, PhD, of University of Otago, Christchurch, New Zealand, and Angelo L. Gaffo, MD, of University of Alabama at Birmingham, noted in an accompanying editorial. They wrote it was encouraging that only 7% of the 92 people screened for the trial did not have a smartphone and that it the patient sample had a mean age of 53 years. However, the trial did not include people with chronic kidney disease who make up nearly a quarter of all people with gout.
“It remains unknown whether the characteristics of those who did not reach target urate are the same or different as those who did, and a head-to-head comparison of these interventions would be of interest,” Dr. Stamp and Dr. Gaffo wrote. “A key challenge in managing gout is to determine which treatment strategy will be best suited to an individual with gout and to identify those for whom more support might be required.”
This study was supported by the University of Edinburgh and funded by NHS Lothian Health Foundation. No conflicts of interest were declared.
Self-management of gout using a smartphone app to record self-test urate levels and flares, and communicate those results to clinicians, could see more patients reaching target urate levels and even reducing flare frequency, a study has found.
Writing in The Lancet Rheumatology, Philip Riches, PhD, of the rheumatic disease unit at Western General Hospital in Edinburgh, and coauthors presented the findings of their randomized, controlled feasibility study of a new gout self-management approach aimed at helping patients treat to target.
While current rheumatology guidelines stress the importance of keeping urate below target levels to reduce flares and improve clinical outcomes, this isn’t always achieved in clinical practice. A previous trial of a nurse-led treat-to-target intervention did show a reduced incidence of flares and tophaceous disease, but the authors said, despite its cost-effectiveness, this approach has yet to be implemented in the United Kingdom.
Dr. Riches and colleagues developed a self-management strategy in which all 60 patients in the study self-tested their urate levels and were prompted to enter that data into the GoutSMART smartphone app once a month or opportunistically, along with information on disease severity and quality of life. All patients had been recommended for initiation or escalation of urate-lowering therapy, and had a serum urate of 0.36 mmol/L (6 mg/dL) or higher at baseline, and all received a gout management plan at the start of the study.
Patients in the intervention group who recorded a urate level above 0.30 mmol/L (5 mg/dL) via the app during the study were prompted to do a self-test every 2 weeks and given daily reminders in the app. Their urate levels were transmitted securely to the study team who then advised on dose escalation or treatment change. Those in the usual-care group also used the app but it only prompted them to record gout flares, keep quality of life diaries, or message the researchers.
At 24 weeks after the start of the study, 73% of 40 participants in the self-management group had reached the urate target of 0.30 mmol/L or below, compared with 15% of the 20 participants in the usual-care group (P < .0001).
The difference between the two groups was sustained even 1 year after starting the intervention, when 80% of those in the self-management group had reached that target, compared with 45% of those in the usual-care group.
Patients in the intervention group also had fewer flares, experiencing a mean of 2.03 flares in the first 24 weeks, compared with a mean of 3 among the control group, although the study didn’t report any difference in the rates of tophaceous disease.
Those in the self-management group had fewer medical appointments, but were prescribed higher doses of allopurinol at the 24- and 52-week visits.
“Qualitative feedback suggests that the self-monitoring approach was accepted by most participants and was enthusiastically endorsed by many,” the authors wrote. “The approach empowers patients and provides feedback on the effect of medication.”
It will be important to determine if the success of this self-management intervention can be replicated in an even broader patient population, Lisa K. Stamp, MBChB, PhD, of University of Otago, Christchurch, New Zealand, and Angelo L. Gaffo, MD, of University of Alabama at Birmingham, noted in an accompanying editorial. They wrote it was encouraging that only 7% of the 92 people screened for the trial did not have a smartphone and that it the patient sample had a mean age of 53 years. However, the trial did not include people with chronic kidney disease who make up nearly a quarter of all people with gout.
“It remains unknown whether the characteristics of those who did not reach target urate are the same or different as those who did, and a head-to-head comparison of these interventions would be of interest,” Dr. Stamp and Dr. Gaffo wrote. “A key challenge in managing gout is to determine which treatment strategy will be best suited to an individual with gout and to identify those for whom more support might be required.”
This study was supported by the University of Edinburgh and funded by NHS Lothian Health Foundation. No conflicts of interest were declared.
Self-management of gout using a smartphone app to record self-test urate levels and flares, and communicate those results to clinicians, could see more patients reaching target urate levels and even reducing flare frequency, a study has found.
Writing in The Lancet Rheumatology, Philip Riches, PhD, of the rheumatic disease unit at Western General Hospital in Edinburgh, and coauthors presented the findings of their randomized, controlled feasibility study of a new gout self-management approach aimed at helping patients treat to target.
While current rheumatology guidelines stress the importance of keeping urate below target levels to reduce flares and improve clinical outcomes, this isn’t always achieved in clinical practice. A previous trial of a nurse-led treat-to-target intervention did show a reduced incidence of flares and tophaceous disease, but the authors said, despite its cost-effectiveness, this approach has yet to be implemented in the United Kingdom.
Dr. Riches and colleagues developed a self-management strategy in which all 60 patients in the study self-tested their urate levels and were prompted to enter that data into the GoutSMART smartphone app once a month or opportunistically, along with information on disease severity and quality of life. All patients had been recommended for initiation or escalation of urate-lowering therapy, and had a serum urate of 0.36 mmol/L (6 mg/dL) or higher at baseline, and all received a gout management plan at the start of the study.
Patients in the intervention group who recorded a urate level above 0.30 mmol/L (5 mg/dL) via the app during the study were prompted to do a self-test every 2 weeks and given daily reminders in the app. Their urate levels were transmitted securely to the study team who then advised on dose escalation or treatment change. Those in the usual-care group also used the app but it only prompted them to record gout flares, keep quality of life diaries, or message the researchers.
At 24 weeks after the start of the study, 73% of 40 participants in the self-management group had reached the urate target of 0.30 mmol/L or below, compared with 15% of the 20 participants in the usual-care group (P < .0001).
The difference between the two groups was sustained even 1 year after starting the intervention, when 80% of those in the self-management group had reached that target, compared with 45% of those in the usual-care group.
Patients in the intervention group also had fewer flares, experiencing a mean of 2.03 flares in the first 24 weeks, compared with a mean of 3 among the control group, although the study didn’t report any difference in the rates of tophaceous disease.
Those in the self-management group had fewer medical appointments, but were prescribed higher doses of allopurinol at the 24- and 52-week visits.
“Qualitative feedback suggests that the self-monitoring approach was accepted by most participants and was enthusiastically endorsed by many,” the authors wrote. “The approach empowers patients and provides feedback on the effect of medication.”
It will be important to determine if the success of this self-management intervention can be replicated in an even broader patient population, Lisa K. Stamp, MBChB, PhD, of University of Otago, Christchurch, New Zealand, and Angelo L. Gaffo, MD, of University of Alabama at Birmingham, noted in an accompanying editorial. They wrote it was encouraging that only 7% of the 92 people screened for the trial did not have a smartphone and that it the patient sample had a mean age of 53 years. However, the trial did not include people with chronic kidney disease who make up nearly a quarter of all people with gout.
“It remains unknown whether the characteristics of those who did not reach target urate are the same or different as those who did, and a head-to-head comparison of these interventions would be of interest,” Dr. Stamp and Dr. Gaffo wrote. “A key challenge in managing gout is to determine which treatment strategy will be best suited to an individual with gout and to identify those for whom more support might be required.”
This study was supported by the University of Edinburgh and funded by NHS Lothian Health Foundation. No conflicts of interest were declared.
FROM THE LANCET RHEUMATOLOGY
Crohn’s disease research goes to the dogs
Why it might be better to be a dog person
Here’s that old debate again: Dogs or cats? You probably have your own opinion, but research presented at this year’s Digestive Disease Week may have tipped the scale by showing that children who lived with dogs may be less likely to have Crohn’s disease as adults.
The research was done by having approximately 4,300 people closely related to patients with Crohn’s disease fill out an environmental questionnaire. Using these data, the research team looked into environmental factors such as size of the families, where the home was, how many bathrooms the homes had, and quality of drinking water.
The researchers found that those who had or were exposed to dogs between the ages of 5 and 15 years were more likely to have healthy gut permeability and balanced microbes, which increased their protection against Crohn’s disease.
“Our study seems to add to others that have explored the ‘hygiene hypothesis’ which suggests that the lack of exposure to microbes early in life may lead to lack of immune regulation toward environmental microbes,” senior author Williams Turpin, PhD, said in the written statement.
The researchers aren’t sure why they didn’t get the same findings with cats, but Dr. Turpin theorized that dog owners tend to be outside more with their dogs or live in places with more green space, which are good protectors against Crohn’s disease.
It’s all good for dog owners, but do their pets’ parasites make you more attractive? Just more fuel for the ongoing debate.
Come for the history, stay for the fossilized parasites
Another week, another analysis of old British poop. LOTME really is your one-stop shop for all the important, hard-hitting news about historic parasites. You’re welcome, Internet.
The news this week is from Stonehenge, which is apparently kind of a big deal. Rocks in a circle, celestial calendar, cultural significance, whatever. We’re not here to talk about rocks. We’re here to talk about, uh, rocks. Smaller rocks. Specifically, coprolites, which are essentially poop turned into a rock. (Though now we’re imagining Stonehenge made out of fossilized poop rocks. Would it still be a big tourist destination? We can see both sides of the argument on that one.)
Archaeologists from the University of Cambridge have conducted an analysis of coprolites from Durrington Walls, a Neolithic settlement just a few kilometers from Stonehenge. The town dates to the same time that Stonehenge was constructed, and it’s believed that the residents were responsible for building the landmark. These coprolites, depending on what’s inside, can tell us a lot about how the builders of Stonehenge lived and, more specifically, how they ate.
In this case, the coprolites of one human and three dogs contained capillariid worm eggs. These worms come from cows, and when a human is typically infected, the eggs embed in the liver and do not pass through the body. Finding them in excrement indicates that the people were eating raw cow organs and feeding leftovers to their dogs. This is interesting, because a preponderance of pottery and cooking implements also found at the site indicates that the residents of Durrington Walls were spit-roasting or boiling their beef and pork. So the meat was cooked, but not the organs. That is an interesting dietary decision, ancient British people. Then again, modern British cuisine exists. At least now we know where they got it from.
This new research raises one other very important question: When are we going to get a full-on guided tour of all the important coprolite sites in Britain? They’ve clearly got plenty of them, and the tourist demand for ancient parasites must be sky-high. Come on, capitalism, follow through on this. We’d go.
Everyone lies: Food intake edition
Do you have any patients on special diets? Do you ask them if they are following those diets? Don’t bother, because they’re lying. Everyone lies about the food they eat. Everyone. Obese people lie, and nonobese people lie.
Investigators at the University of Essex in England asked 221 adults to keep food diaries, and then they checked on energy consumption by analyzing radioactive water levels in the participants’ urine over a 10-day period.
Underreporting of food consumption was rampant, even among those who were not obese. The obese subjects did underreport by a greater extent (1,200 calories per day) than did those who were not obese, who were off by only 800 calories, but the obese participants burned about 400 calories more each day than did the nonobese, so the difference was a wash.
Everyone ended up underreporting their calorie consumption by an average of about 900 calories, and the investigators were good enough to provide some food equivalents, tops on the list being three MacDonald’s cheeseburgers.
“Public health recommendations have historically relied heavily on self-reported energy intake values,” senior author Gavin Sandercock, PhD, said in a EurekAlert statement, and “recognising that the measures of energy intake are incorrect might result in the setting of more realistic targets.”
Maybe you can be more realistic with your patients, too. Go ahead and ask Mr. Smith about the burger sticking out of his coat pocket, because there are probably two more you can’t see. We’ve each got 900 calories hiding on us somewhere. Ours is usually pizza.
The art of the gallbladder
Ever thought you would see a portrait of a gallbladder hanging up in a gallery? Not just an artist’s rendition, but an actual photo from an actual patient? Well, you can at the Soloway Gallery in Brooklyn, N.Y., at least until June 12.
The artist? K.C. Joseph, MD, a general surgeon from St. Marie, Pa., who died in 2015. His daughter Melissa is the curator of the show and told ARTnews about the interesting connection her father had with art and surgery.
In 2010, Dr. Joseph gave his daughter a box of photos and said “Make me a famous artist,” she recalled. At first, “I was like, ‘These are weird,’ and then I put them under my bed for 10 years.”
Apparently he had been making art with his patients’ organs for about 15 years and had a system in which he put each one together. Before a surgery Dr. Joseph would make a note card with the patient’s name handwritten in calligraphy with a couple of pages taken out of the magazine from the waiting room as the backdrop. Afterward, when the patient was in recovery, the removed organ would be placed among the pages and the name card. A photo was taken with the same endoscope that was used for the procedure.
After the show’s debut, people reached out expressing their love for their photos. “I wish, before he died, I had asked him more questions about it,” Ms. Joseph told ARTnews. “I’m regretting it so much now, kicking myself.”
Who gets to take home an artsy photo of their gallbladder after getting it removed? Not us, that’s who. Each collage is a one-of-a-kind piece. They definitely should be framed and shown in an art gallery. Oh, right. Never mind.
Why it might be better to be a dog person
Here’s that old debate again: Dogs or cats? You probably have your own opinion, but research presented at this year’s Digestive Disease Week may have tipped the scale by showing that children who lived with dogs may be less likely to have Crohn’s disease as adults.
The research was done by having approximately 4,300 people closely related to patients with Crohn’s disease fill out an environmental questionnaire. Using these data, the research team looked into environmental factors such as size of the families, where the home was, how many bathrooms the homes had, and quality of drinking water.
The researchers found that those who had or were exposed to dogs between the ages of 5 and 15 years were more likely to have healthy gut permeability and balanced microbes, which increased their protection against Crohn’s disease.
“Our study seems to add to others that have explored the ‘hygiene hypothesis’ which suggests that the lack of exposure to microbes early in life may lead to lack of immune regulation toward environmental microbes,” senior author Williams Turpin, PhD, said in the written statement.
The researchers aren’t sure why they didn’t get the same findings with cats, but Dr. Turpin theorized that dog owners tend to be outside more with their dogs or live in places with more green space, which are good protectors against Crohn’s disease.
It’s all good for dog owners, but do their pets’ parasites make you more attractive? Just more fuel for the ongoing debate.
Come for the history, stay for the fossilized parasites
Another week, another analysis of old British poop. LOTME really is your one-stop shop for all the important, hard-hitting news about historic parasites. You’re welcome, Internet.
The news this week is from Stonehenge, which is apparently kind of a big deal. Rocks in a circle, celestial calendar, cultural significance, whatever. We’re not here to talk about rocks. We’re here to talk about, uh, rocks. Smaller rocks. Specifically, coprolites, which are essentially poop turned into a rock. (Though now we’re imagining Stonehenge made out of fossilized poop rocks. Would it still be a big tourist destination? We can see both sides of the argument on that one.)
Archaeologists from the University of Cambridge have conducted an analysis of coprolites from Durrington Walls, a Neolithic settlement just a few kilometers from Stonehenge. The town dates to the same time that Stonehenge was constructed, and it’s believed that the residents were responsible for building the landmark. These coprolites, depending on what’s inside, can tell us a lot about how the builders of Stonehenge lived and, more specifically, how they ate.
In this case, the coprolites of one human and three dogs contained capillariid worm eggs. These worms come from cows, and when a human is typically infected, the eggs embed in the liver and do not pass through the body. Finding them in excrement indicates that the people were eating raw cow organs and feeding leftovers to their dogs. This is interesting, because a preponderance of pottery and cooking implements also found at the site indicates that the residents of Durrington Walls were spit-roasting or boiling their beef and pork. So the meat was cooked, but not the organs. That is an interesting dietary decision, ancient British people. Then again, modern British cuisine exists. At least now we know where they got it from.
This new research raises one other very important question: When are we going to get a full-on guided tour of all the important coprolite sites in Britain? They’ve clearly got plenty of them, and the tourist demand for ancient parasites must be sky-high. Come on, capitalism, follow through on this. We’d go.
Everyone lies: Food intake edition
Do you have any patients on special diets? Do you ask them if they are following those diets? Don’t bother, because they’re lying. Everyone lies about the food they eat. Everyone. Obese people lie, and nonobese people lie.
Investigators at the University of Essex in England asked 221 adults to keep food diaries, and then they checked on energy consumption by analyzing radioactive water levels in the participants’ urine over a 10-day period.
Underreporting of food consumption was rampant, even among those who were not obese. The obese subjects did underreport by a greater extent (1,200 calories per day) than did those who were not obese, who were off by only 800 calories, but the obese participants burned about 400 calories more each day than did the nonobese, so the difference was a wash.
Everyone ended up underreporting their calorie consumption by an average of about 900 calories, and the investigators were good enough to provide some food equivalents, tops on the list being three MacDonald’s cheeseburgers.
“Public health recommendations have historically relied heavily on self-reported energy intake values,” senior author Gavin Sandercock, PhD, said in a EurekAlert statement, and “recognising that the measures of energy intake are incorrect might result in the setting of more realistic targets.”
Maybe you can be more realistic with your patients, too. Go ahead and ask Mr. Smith about the burger sticking out of his coat pocket, because there are probably two more you can’t see. We’ve each got 900 calories hiding on us somewhere. Ours is usually pizza.
The art of the gallbladder
Ever thought you would see a portrait of a gallbladder hanging up in a gallery? Not just an artist’s rendition, but an actual photo from an actual patient? Well, you can at the Soloway Gallery in Brooklyn, N.Y., at least until June 12.
The artist? K.C. Joseph, MD, a general surgeon from St. Marie, Pa., who died in 2015. His daughter Melissa is the curator of the show and told ARTnews about the interesting connection her father had with art and surgery.
In 2010, Dr. Joseph gave his daughter a box of photos and said “Make me a famous artist,” she recalled. At first, “I was like, ‘These are weird,’ and then I put them under my bed for 10 years.”
Apparently he had been making art with his patients’ organs for about 15 years and had a system in which he put each one together. Before a surgery Dr. Joseph would make a note card with the patient’s name handwritten in calligraphy with a couple of pages taken out of the magazine from the waiting room as the backdrop. Afterward, when the patient was in recovery, the removed organ would be placed among the pages and the name card. A photo was taken with the same endoscope that was used for the procedure.
After the show’s debut, people reached out expressing their love for their photos. “I wish, before he died, I had asked him more questions about it,” Ms. Joseph told ARTnews. “I’m regretting it so much now, kicking myself.”
Who gets to take home an artsy photo of their gallbladder after getting it removed? Not us, that’s who. Each collage is a one-of-a-kind piece. They definitely should be framed and shown in an art gallery. Oh, right. Never mind.
Why it might be better to be a dog person
Here’s that old debate again: Dogs or cats? You probably have your own opinion, but research presented at this year’s Digestive Disease Week may have tipped the scale by showing that children who lived with dogs may be less likely to have Crohn’s disease as adults.
The research was done by having approximately 4,300 people closely related to patients with Crohn’s disease fill out an environmental questionnaire. Using these data, the research team looked into environmental factors such as size of the families, where the home was, how many bathrooms the homes had, and quality of drinking water.
The researchers found that those who had or were exposed to dogs between the ages of 5 and 15 years were more likely to have healthy gut permeability and balanced microbes, which increased their protection against Crohn’s disease.
“Our study seems to add to others that have explored the ‘hygiene hypothesis’ which suggests that the lack of exposure to microbes early in life may lead to lack of immune regulation toward environmental microbes,” senior author Williams Turpin, PhD, said in the written statement.
The researchers aren’t sure why they didn’t get the same findings with cats, but Dr. Turpin theorized that dog owners tend to be outside more with their dogs or live in places with more green space, which are good protectors against Crohn’s disease.
It’s all good for dog owners, but do their pets’ parasites make you more attractive? Just more fuel for the ongoing debate.
Come for the history, stay for the fossilized parasites
Another week, another analysis of old British poop. LOTME really is your one-stop shop for all the important, hard-hitting news about historic parasites. You’re welcome, Internet.
The news this week is from Stonehenge, which is apparently kind of a big deal. Rocks in a circle, celestial calendar, cultural significance, whatever. We’re not here to talk about rocks. We’re here to talk about, uh, rocks. Smaller rocks. Specifically, coprolites, which are essentially poop turned into a rock. (Though now we’re imagining Stonehenge made out of fossilized poop rocks. Would it still be a big tourist destination? We can see both sides of the argument on that one.)
Archaeologists from the University of Cambridge have conducted an analysis of coprolites from Durrington Walls, a Neolithic settlement just a few kilometers from Stonehenge. The town dates to the same time that Stonehenge was constructed, and it’s believed that the residents were responsible for building the landmark. These coprolites, depending on what’s inside, can tell us a lot about how the builders of Stonehenge lived and, more specifically, how they ate.
In this case, the coprolites of one human and three dogs contained capillariid worm eggs. These worms come from cows, and when a human is typically infected, the eggs embed in the liver and do not pass through the body. Finding them in excrement indicates that the people were eating raw cow organs and feeding leftovers to their dogs. This is interesting, because a preponderance of pottery and cooking implements also found at the site indicates that the residents of Durrington Walls were spit-roasting or boiling their beef and pork. So the meat was cooked, but not the organs. That is an interesting dietary decision, ancient British people. Then again, modern British cuisine exists. At least now we know where they got it from.
This new research raises one other very important question: When are we going to get a full-on guided tour of all the important coprolite sites in Britain? They’ve clearly got plenty of them, and the tourist demand for ancient parasites must be sky-high. Come on, capitalism, follow through on this. We’d go.
Everyone lies: Food intake edition
Do you have any patients on special diets? Do you ask them if they are following those diets? Don’t bother, because they’re lying. Everyone lies about the food they eat. Everyone. Obese people lie, and nonobese people lie.
Investigators at the University of Essex in England asked 221 adults to keep food diaries, and then they checked on energy consumption by analyzing radioactive water levels in the participants’ urine over a 10-day period.
Underreporting of food consumption was rampant, even among those who were not obese. The obese subjects did underreport by a greater extent (1,200 calories per day) than did those who were not obese, who were off by only 800 calories, but the obese participants burned about 400 calories more each day than did the nonobese, so the difference was a wash.
Everyone ended up underreporting their calorie consumption by an average of about 900 calories, and the investigators were good enough to provide some food equivalents, tops on the list being three MacDonald’s cheeseburgers.
“Public health recommendations have historically relied heavily on self-reported energy intake values,” senior author Gavin Sandercock, PhD, said in a EurekAlert statement, and “recognising that the measures of energy intake are incorrect might result in the setting of more realistic targets.”
Maybe you can be more realistic with your patients, too. Go ahead and ask Mr. Smith about the burger sticking out of his coat pocket, because there are probably two more you can’t see. We’ve each got 900 calories hiding on us somewhere. Ours is usually pizza.
The art of the gallbladder
Ever thought you would see a portrait of a gallbladder hanging up in a gallery? Not just an artist’s rendition, but an actual photo from an actual patient? Well, you can at the Soloway Gallery in Brooklyn, N.Y., at least until June 12.
The artist? K.C. Joseph, MD, a general surgeon from St. Marie, Pa., who died in 2015. His daughter Melissa is the curator of the show and told ARTnews about the interesting connection her father had with art and surgery.
In 2010, Dr. Joseph gave his daughter a box of photos and said “Make me a famous artist,” she recalled. At first, “I was like, ‘These are weird,’ and then I put them under my bed for 10 years.”
Apparently he had been making art with his patients’ organs for about 15 years and had a system in which he put each one together. Before a surgery Dr. Joseph would make a note card with the patient’s name handwritten in calligraphy with a couple of pages taken out of the magazine from the waiting room as the backdrop. Afterward, when the patient was in recovery, the removed organ would be placed among the pages and the name card. A photo was taken with the same endoscope that was used for the procedure.
After the show’s debut, people reached out expressing their love for their photos. “I wish, before he died, I had asked him more questions about it,” Ms. Joseph told ARTnews. “I’m regretting it so much now, kicking myself.”
Who gets to take home an artsy photo of their gallbladder after getting it removed? Not us, that’s who. Each collage is a one-of-a-kind piece. They definitely should be framed and shown in an art gallery. Oh, right. Never mind.
Depressed patients respond faster to IV ketamine than intranasal ketamine
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
NEW ORLEANS – New research reveals that patients with treatment-resistant depression who were treated with repeated intravenous ketamine show no significant differences in achieving response or remission, compared with those receiving the intranasal formulation of the drug, esketamine – although fewer treatments appear necessary with the intravenous formulation.
“ although at the end, the responses are similar,” said first author Balwinder Singh, MD, of the department of psychiatry and psychology, Mayo Clinic, in Rochester, Minn.
The findings were presented at the annual meeting of the American Psychiatric Association.
Commenting on the study, Roger S. McIntyre, MD, underscored that “this is an important study that addresses the priority questions that everyone wants to know – not only for clinical reasons, but economic reasons.” Dr. McIntyre, a professor of psychiatry and pharmacology at the University of Toronto, and head of the university’s mood disorders psychopharmacology unit, said that “there are implications not only for clinical outcomes and cost, but also implementation because IV is obviously more demanding and complicated.”
As intravenous ketamine increasingly gained interest as a rapid-acting treatment for patients with severe, treatment-resistant depression, the introduction of a more convenient intranasal formulation was seen as a welcome improvement and received approval from the Food and Drug Administration in 2019. However, while the approval ushered in more coverage by insurance companies, the treatment can still be expensive. Intravenous ketamine does not have FDA approval.
With a lack of studies in the real-world setting comparing efficacy of the two formulations, Dr. Singh and his colleagues conducted the observational study, evaluating the responses of 62 adults with treatment-resistant depression who had received either up to six IV ketamine infusions of 0.5 mg/kg, infused over 40 minutes, or up to eight intranasal esketamine treatments of 56/84 mg, as approved by the FDA, at the Mayo Clinic Depression Center.
Of the patients, who had a mean age of 47 years, 59 had major depression and 3 had bipolar depression. Among them, 76% (47) received intravenous ketamine and 24% (15) received esketamine, which Dr. Singh noted reflected the higher number of patients included before esketamine received FDA approval. The patients had similar comorbidity profiles, with the intravenous ketamine group having a higher body mass index at baseline.
Overall, the patients all had significant improvement in their depression at the end of the acute phase of 4 weeks, with a mean change in on the 16-Item Quick Inventory of Depressive Symptomatology (QIDS-SR) scale of –8.6 from baseline (P < .001).
The overall remission rate was 38.7% and overall response rate was 58.1%. Those receiving intravenous ketamine had response and remission rates of 57.4% and 42.6%, versus response and remission rates of 60.0% and 26.7% among the esketamine group, which Dr. Singh said were not significant differences (P > .05).
However, the mean number of treatments necessary to achieve response in the intravenous ketamine group was just 2.3 versus 4.6 with esketamine, and the mean number of treatments to achieve remission were 2.5 versus 6.3, respectively (P = .008).
After a multivariate adjustment, the time to response was determined to be faster with intravenous ketamine versus esketamine (hazard ratio, 2.61; P = .05) and the time to remission was also faster (HR, 5.0; P = .02).
“What this means is you would need fewer treatments to achieve a response or remission with IV ketamine, so there could be an acceleration of patients’ antidepressant response,” Dr. Singh explained.
There were no significant differences between the groups in terms of side effects, and most patients tolerated the treatments well.
Dr. Singh noted the limitation of the study is that it was observational and included a small sample size. Nevertheless, when asked which he would choose if starting treatment when insurance was not an issue, Dr. Singh replied: “I would take patient preference into account, but certainly IV seems to have an advantage.”
Dr. McIntyre noted that, though small, the study’s setting in a real world clinical environment is important.
“Obviously this is observational and not controlled, but the strength is that this involved a real-world cohort of patients and real world applications,” he said. “It’s difficult to have a true comparator head-to-head trial, so that makes this all the more important because it takes into consideration all of the complexities of real world patients.”
Dr. McIntyre emphasized that the study is not “the last word on the story because we need to see a larger sample and replication. But certainly they make an argument that IV ketamine may have an advantage over the speed of onset with intranasal ketamine, which will need to be either replicated or refuted, but it’s a great starting point in the conversation.”
Navigating patient preference
Robert Meisner, MD, founding medical director of the McLean Ketamine Service, Division of Psychiatric Neurotherapeutics, McLean Hospital, Harvard Medical School, in Boston, noted that wide-ranging factors may influence patient as well as clinician decisions about which ketamine treatment approach to use.
“When a patient appears to be equally well-suited for both interventions, I continue to be surprised by why one patient will indicate a preference for intranasal esketamine, while another will lean toward IV racemic ketamine,” he said in an interview.
“Some patients find esketamine’s clear and consistent protocol optimal for scheduling and navigating the logistics of daily life; others value the flexibility offered by certain evidence-based, racemic (IV) protocols,” he said. “Predicting who will prefer each treatment, even with the apparent temporal advantage with IV ketamine, is extremely difficult.”
Likewise, in terms of clinician preference, Dr. Meisner notes that key concerns may sway decisions.
“If I’m concerned with labile pressures or hypertension, for example, or if I have a patient with, say, Erlos Danlos Syndrome without a clear subtype, and hence, some risk of undiscovered aneurysmal vascular disease, I may lean toward racemic IV ketamine.”
On the other hand, “some patients find the simplicity and predictability of the maintenance esketamine protocol comforting and psychologically stabilizing,” he added. “Yet others find that their work or family’s erratic demands on their time make one of the evidence-based racemic regimens preferable – inasmuch as it integrates more flexibility and allows them to remain more fully engaged in the basic activities or work and family.”
Dr. Meisner noted the caveat that efforts to decide which method to use are often complicated by substantial misinformation.
“I can’t emphasize how much misinformation continues to abound regarding appropriate (evidence-based) and safe use of ketamine and esketamine,” he said. “Especially on the IV racemic side, there simply is no substantive evidence base for many of the claims that some providers are preaching.”
The confusion, driven in part by social media, “has diffused into sectors of the field and industry that one might assume are relatively immune (i.e., allied physicians, sophisticated payers, etc),” he added.
“In short, two mantra continue to apply,” Dr. Meisner said. “One – if it sounds too good to be true, it probably is; and two – in pharmacology and interventional psychiatry, we see remarkable progress and potential, but there simply is no such thing as a magic bullet.”
Dr. Singh and Dr. Meisner had no disclosures to report. Dr. McIntyre has received research grant support from Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/National Natural Science Foundation of China, and speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
AT APA 2022
Ulcer on knuckle
Since the papules were worrisome for vasculitis, 2 punch biopsies were performed on smaller, younger lesions on the hand and 1 was submitted for direct immunofluorescence. Findings revealed a leukocytoclastic vasculitis (LCV) with prominent immunoglobulin A (IgA) deposits around the vessel wall. The clinical and pathologic findings were consistent with a rare fibrosing LCV called erythema elevatum diutinum (EED). The practice of sampling nonblanching purpura or papules for both standard pathology (hematoxylin and eosin) and direct immunofluorescence can facilitate the diagnosis of unusual conditions that may occur only a few times in one’s career.
EED is rare, chronic, and may be associated with IgA gammopathy, IgA antineutrophil cytoplasmic antibodies, recent streptococcal or HIV infections, or myelodysplastic syndrome. A robust work-up to identify whether any of these factors are at work is critical.1 Distinct from other vasculitides, EED forms granulation tissue that becomes reinjured.
This patient was found to have an IgA monoclonal gammopathy that was monitored by Hematology. After checking her glucose-6-phosphate dehydrogenase activity, she was treated with oral dapsone 25 mg/d. Dapsone was titrated up to 100 mg/d, which improved her symptoms considerably and the ulcerated papule was surgically revised and closed. EED can last for years and ultimately clear or can persist indefinitely.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Sandhu JK, Albrecht J, Agnihotri G, et al. Erythema elevatum et diutinum as a systemic disease. Clin Dermatol. 2019;37:679-683. doi: 10.1016/j.clindermatol.2019.07.028
Since the papules were worrisome for vasculitis, 2 punch biopsies were performed on smaller, younger lesions on the hand and 1 was submitted for direct immunofluorescence. Findings revealed a leukocytoclastic vasculitis (LCV) with prominent immunoglobulin A (IgA) deposits around the vessel wall. The clinical and pathologic findings were consistent with a rare fibrosing LCV called erythema elevatum diutinum (EED). The practice of sampling nonblanching purpura or papules for both standard pathology (hematoxylin and eosin) and direct immunofluorescence can facilitate the diagnosis of unusual conditions that may occur only a few times in one’s career.
EED is rare, chronic, and may be associated with IgA gammopathy, IgA antineutrophil cytoplasmic antibodies, recent streptococcal or HIV infections, or myelodysplastic syndrome. A robust work-up to identify whether any of these factors are at work is critical.1 Distinct from other vasculitides, EED forms granulation tissue that becomes reinjured.
This patient was found to have an IgA monoclonal gammopathy that was monitored by Hematology. After checking her glucose-6-phosphate dehydrogenase activity, she was treated with oral dapsone 25 mg/d. Dapsone was titrated up to 100 mg/d, which improved her symptoms considerably and the ulcerated papule was surgically revised and closed. EED can last for years and ultimately clear or can persist indefinitely.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
Since the papules were worrisome for vasculitis, 2 punch biopsies were performed on smaller, younger lesions on the hand and 1 was submitted for direct immunofluorescence. Findings revealed a leukocytoclastic vasculitis (LCV) with prominent immunoglobulin A (IgA) deposits around the vessel wall. The clinical and pathologic findings were consistent with a rare fibrosing LCV called erythema elevatum diutinum (EED). The practice of sampling nonblanching purpura or papules for both standard pathology (hematoxylin and eosin) and direct immunofluorescence can facilitate the diagnosis of unusual conditions that may occur only a few times in one’s career.
EED is rare, chronic, and may be associated with IgA gammopathy, IgA antineutrophil cytoplasmic antibodies, recent streptococcal or HIV infections, or myelodysplastic syndrome. A robust work-up to identify whether any of these factors are at work is critical.1 Distinct from other vasculitides, EED forms granulation tissue that becomes reinjured.
This patient was found to have an IgA monoclonal gammopathy that was monitored by Hematology. After checking her glucose-6-phosphate dehydrogenase activity, she was treated with oral dapsone 25 mg/d. Dapsone was titrated up to 100 mg/d, which improved her symptoms considerably and the ulcerated papule was surgically revised and closed. EED can last for years and ultimately clear or can persist indefinitely.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Sandhu JK, Albrecht J, Agnihotri G, et al. Erythema elevatum et diutinum as a systemic disease. Clin Dermatol. 2019;37:679-683. doi: 10.1016/j.clindermatol.2019.07.028
1. Sandhu JK, Albrecht J, Agnihotri G, et al. Erythema elevatum et diutinum as a systemic disease. Clin Dermatol. 2019;37:679-683. doi: 10.1016/j.clindermatol.2019.07.028
Manufacturer announces FDA approval for molluscum treatment delayed
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Pharmaceuticals, which is developing the product.
VP-102 is a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
According to a press release from Verrica, the only deficiency listed in the FDA’s complete response letter stemmed from a general reinspection of Sterling Pharmaceuticals Services, which manufactures Verrica’s bulk solution drug product. Although none of the issues identified by the FDA during the reinspection were specific to the manufacturing of VP-102, FDA policy prevents approval of a new drug application when a contract manufacturing organization has an unresolved classification status or is placed on “official action indicated” status.
According to the press release, Verrica will “continue to work collaboratively” with the FDA to bring VP-102 to the market as soon as possible. The company has completed phase 2 studies of VP-102 for the treatment of common warts and for the treatment of external genital warts, the release said.
A version of this article first appeared on Medscape.com.
Experts endorse plant-based diet for type 2 diabetes remission
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Many adults can achieve remission of type 2 diabetes with a primary intervention consisting of a diet that emphasizes whole, plant-based foods, according to a new publication from the American College of Lifestyle Medicine (ACLM).
The document was developed to assist clinicians treating adults with type 2 diabetes, with the goal of remission using diet as a primary intervention. A panel of 15 experts from seven societies reached consensus on 69 statements.
“A healthy diet is a foundational component of current lifestyle guidelines for treatment of type 2 diabetes, but it is often overlooked because of the lack of physician training and patient awareness,” Felice A. Caldarella, MD, president of the American Association of Clinical Endocrinology (AACE), said in a press release from ACLM.
“The consensus statements produced by this panel of experts are invaluable in bringing awareness to the value of diet for diabetes remission in addition to management,” he summarized.
The initiative was cosponsored by the Endocrine Society, endorsed by AACE, and supported by the Academy of Nutrition and Dietetics. The expert panel also included representatives from the American College of Cardiology, the American Heart Association, and the American Academy of Family Physicians. It was published in the American Journal of Lifestyle Medicine.
“I think many patients would do the challenging work of making lifestyle modifications if it meant remission of [type 2 diabetes] and sparing them the burden and cost of medications or surgery,” said Amy E. Rothberg, MD, PhD, who represented the Endocrine Society on the panel.
“By changing the course of the disease, i.e., if in remission, they are unlikely to get the complications related to [type 2 diabetes],” Dr. Rothberg, professor of nutritional sciences at the University of Michigan, Ann Arbor, told this news organization.
Consensus on 69 statements
The panel members used a modified Delphi process to develop the consensus statement. They identified 49 articles from the literature regarding dietary interventions in adults with type 2 diabetes. They reached consensus on 69 statements that cover seven topics: definitions and basic concepts; diet and remission of type 2 diabetes; dietary specifics and types of diets; adjuvant and alternative interventions; support, monitoring, and adherence to therapy; weight loss; and payment and policy.
Dr. Rothberg identified six key areas:
- Definition of remission: Type 2 diabetes remission is defined as A1c < 6.5% for at least 3 months with no surgery, devices, or active pharmacologic therapy for lowering blood glucose, consistent with the diabetes remission timeline published in 2021 by the American Diabetes Association. Remission does not exclude the possibility of recurrence. Remission is a realistic and achievable goal for some adults with type 2 diabetes.
- High-intensity diet, short duration of diabetes: Patients are more likely to attain remission with a high-intensity diet (e.g., high level of restrictions plus frequent patient contact or counseling) accompanied by physical activity and if the patient has had diabetes for 4 years or less. A high-fiber diet is essential.
- Fewer calories, focus on plant-based foods: Calorie reduction could be achieved by reducing food volume, portion sizes, or energy density, or by using liquid meal replacements, or by a combination of these approaches. It should mainly include whole, plant-based foods (whole grains, vegetables, legumes, fruits, nuts, and seeds) and avoid or minimize meat and other animal products, refined foods, ultra-processed foods, and foods with added fats.
- A very low energy diet as initial intervention is optional: There was consensus that this approach can achieve remission, but there was not agreement that low calorie content was essential for achieving remission, Dr. Rothberg noted.
- Beyond type 2 remission: Diet as a primary intervention can also lower the risk of cardiovascular disease and improve lipoprotein profile.
- Self-management, support, and monitoring: The group recognizes the importance of patient education and support. “This can play a vital role and should be part of any comprehensive lifestyle treatment,” said Dr. Rothberg. The diet and lifestyle strategies should be acceptable to most patients, easy to adhere to, accommodate patient preferences and values, and be culturally sensitive.
Intensive lifestyle change can equate to bariatric surgery
Also invited to comment, Yehuda Handelsman, MD, who coauthored a 2020 type 2 diabetes management algorithm by AACE and the American College of Endocrinology, and was not involved with the current initiative, agrees with the importance of lifestyle in the management of type 2 diabetes but takes issue with a few points.
Most clinicians and experts do not believe that diabetes can be reversed, as such, only controlled, noted Dr. Handelsman, medical director of the Metabolic Institute of America, Tarzana, Calif.
“We always have approached type 2 diabetes treatment with lifestyle – diet, exercise, and (as of late) sleep – as the mainstay of therapy,” he said.
However, most patients do not adhere to diet modifications by 6 months and especially by 1 year, which has led to universal recommendations to add medication to lifestyle from inception, he continued.
Most clinicians have not been trained in lifestyle modalities. And many patients with type 2 diabetes are not adherent to medications, which “led to the relative success of bariatric surgery leading to remission (at least for 3-5 years).”
“Remission, which in broad terms implies the disappearance of signs and symptoms, should be a top priority for individuals with type 2 diabetes,” the consensus statement authors wrote.
“While [bariatric surgery] can induce remission in 25% to 80% of targeted patients, it carries risk and its effectiveness wanes as subjects regain lost weight,” and “more dramatic and intensive [lifestyle] change produces remission rates equivalent to bariatric surgery,” they noted.
Need for more randomized trials
Dr. Handelsman also stressed that remission may be temporary. “Three months or 6 months cannot be a measure of success. We must have at least 1 year,” he added. “In fact, there are data to show that remission requires 3 years.”
Nevertheless, the consensus statement does highlight the importance of lifestyle in remission of diabetes, he agreed.
The expert panel also noted that patients can benefit from a healthy lifestyle, even if they do not attain remission, Dr. Rothberg pointed out.
Moving forward, the statement concludes that “there is ... an ongoing need for additional randomized controlled trials to assess sustainable plant-based dietary interventions with whole or minimally processed foods, as a primary means of treating [type 2 diabetes] with the goal of remission, as well as factors that lead to successful patient adherence and effective dissemination and implementation of such interventions.”
This study was supported by the Lisa Wendel Memorial Foundation. Dr. Rothberg has disclosed being the medical director of Rewind, a virtual platform created for weight control with the goal to “defeat” type 2 diabetes, and a consultant for a study for which Nestle provides product. Dr. Handelsman has disclosed receiving research grants and consultant and speaker honoraria from Amarin, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Corcept, Esperion, Ionis, Mankind, Merck, Merck-Pfizer, Novartis, Novo Nordisk, Regor, Sanofi, and Vertis.
A version of this article first appeared on Medscape.com.
Pemphigus Vulgaris Aggravated: Rifampicin Found at the Scene of the Crime
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
Case Report
A 60-year-old man presented with eroded areas in the mouth and blistering eruptions on the scalp, face, trunk, arms, and legs. He initially presented to an outside hospital 4 years prior and was treated with oral prednisone 50 mg daily, to which the eruptions responded rapidly; however, following a nearly 5-mg reduction of the dose per week by the patient and irregular oral administration, he experienced several episodes of recurrence, but he could not remember the exact dosage of prednisone he had taken during that period. Subsequently, he was admitted to our hospital because of large areas of erythema and erosions on the scalp, trunk, arms, and legs.
Since starting the prednisone regimen 4 years prior, the patient had experienced onset of hypertension, diabetes, glaucoma, cataracts, optic nerve atrophy, aseptic necrosis of the femoral head, and osteoporosis. Biopsy of a new skin lesion
The patient initially was started again prednisone 50 mg daily, to which the skin eruptions responded, and 2 weeks later, the disease was considered controlled. The prednisone dosage was tapered to 20 mg daily 3 months later with no new blister formation. However, 2 weeks later, the patient was diagnosed by a tuberculosis specialist with pulmonary tuberculosis, and a daily regimen of isoniazid, rifampicin, ethambutol, and levofloxacin was instituted.
Ten days after starting antituberculosis therapy, the patient developed new erythematous blisters that could not be controlled and self-adjusted the prednisone dose to 50 mg daily. Two months later, blister formation continued.
Six months after the initial presentation, the patient returned to our hospital because of uncontrollable rashes (Figure 2). On admission, he had a Pemphigus Disease Area Index (PDAI) score of 32 with disease involving 30% of the body surface area. Laboratory testing showed a desmoglein 1 level of 233 U/mL and desmoglein 3 level of 228 U/mL. A tuberculosis specialist from an outside hospital was consulted to evaluate the patient’s condition and assist in treatment. Based on findings from a pulmonary computed tomography scan, which showed the inflammation was considerably absorbed, treatment was adjusted to stop using ethambutol and levofloxacin and continue rifampicin and isoniazid. For the PV, prednisone was titrated upward to 75 mg daily, mycophenolate mofetil (MMF) 1 g twice daily was added, and IVIG 400 mg/kg daily was administered for 7 days. After 3 weeks, the rash still expanded.
In considering possible interactions between the drugs, we consulted the literature and found reports1-3 that rifampicin accelerated glucocorticoid metabolism, of which the tuberculosis specialist that we consulted was not aware. Therefore, rifampicin was stopped, and the antituberculosis therapy was adjusted to levofloxacin and isoniazid. Meanwhile, the steroid was changed to methylprednisolone 120 mg daily for 3 days, then to 80 mg daily for 2 days.
After 5 days, the rash was controlled with no new development and the patient was discharged. He continued on prednisone 80 mg daily and MMF 1 g twice daily.
At 2-month follow-up, no new rash had developed. The patient had already self-discontinued the MMF for 1 month because it was difficult to obtain at local hospitals. The prednisone was reduced to 40 mg daily. Pulmonary computed tomography showed no signs of reactivation of tuberculosis.
Comment
Drugs that depend on these enzymes for their metabolism are prone to
Rifampicin causes a marked reduction in dose-corrected mycophenolic acid exposure when administered simultaneously with MMF through induction of glucuronidation activity and inhibition of enterohepatic recirculation.5,10In in vitro studies, rifampin and other cytochrome P450 inducers have been identified as potentially useful for increasing the rate of cyclophosphamide and ifosfamide (an isomeric analogue of cyclophosphamide) 4-hydroxylation in the human liver in a manner that could have a favorable impact on the clinical pharmacokinetics of these anticancer prodrugs.11 However, clinical analysis of 16 patients indicated that co-administration of ifosfamide with rifampin did not result in changes in the pharmacokinetics of the parent drug or its metabolites.12
The steroids and
Conclusion
In our patient, the use of rifapentine resulted in a recurrence of previously controlled PV and resistance to treatment. The patient’s disease was quickly controlled after discontinuation of rifampicin and with a short-term course of high-dose methylprednisolone and remained stable when the dosages of MMF and prednisone were reduced.
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
- Miyagawa S, Yamashina Y, Okuchi T, et al. Exacerbation of pemphigus by rifampicin. Br J Dermatol. 1986;114:729-732. doi:10.1111/j.1365-2133.1986.tb04882.x
- Gange RW, Rhodes EL, Edwards CO, et al. Pemphigus induced by rifampicin. Br J Dermatol. 1976;95:445-448. doi:10.1111/j.1365-2133.1976.tb00849.x
- Bergrem H, Refvem OK. Altered prednisolone pharmacokinetics in patients treated with rifampicin. Acta Med Scand. 1983;213:339-343. doi:10.1111/j.0954-6820.1983.tb03748.x
- McAllister WA, Thompson PJ, Al-Habet SM, et al. Rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed). 1983;286:923-925. doi:10.1136/bmj.286.6369.923
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106. doi:10.1007/s00403-017-1790-8
- Barman H, Dass R, Duwarah SG. Use of high-dose prednisolone to overcome rifampicin-induced corticosteroid non-responsiveness in childhood nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016;27:157-160. doi:10.4103/1319-2442.174198
- Okey AB, Roberts EA, Harper PA, et al. Induction of drug-metabolizing enzymes: mechanisms and consequences. Clin Biochem. 1986;19:132-141. doi:10.1016/s0009-9120(86)80060-1
- Venkatesan K. Pharmacokinetic interactions with rifampicin. Clin Pharmacokinet. 1992;22:47-65. doi:10.2165/00003088-199222010-00005
- Naesens M, Kuypers DRJ, Streit F, et al. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin Pharmacol Ther. 2006;80:509-521. doi:10.1016/j.clpt.2006.08.002
- Kuypers DRJ, Verleden G, Naesens M, et al. Drug interaction between mycophenolate mofetil and rifampin: possible induction of uridine diphosphate–glucuronosyltransferase. Clin Pharmacol Ther. 2005;78:81-88. doi:10.1016/j.clpt.2005.03.004
- Chenhsu RY, Loong CC, Chou MH, et al. Renal allograft dysfunction associated with rifampin–tacrolimus interaction. Ann Pharmacother. 2000;34:27-31. doi:10.1345/aph.19069
- Douglas JG, McLeod MJ. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin Pharmacokinet. 1999;37:127-146. doi:10.2165/00003088-199937020-00003
Practice Points
- Long-term use of immunosuppressants requires constant attention for infections, especially latent infections in the body.
- Clinicians should carefully inquire with patients about concomitant diseases and medications used, and be vigilant about drug interactions.
Fidaxomicin favored over vancomycin in real-world C. diff study
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT DDW 2022