User login
Intravenous Immunoglobulin in Treating Nonventilated COVID-19 Patients With Moderate-to-Severe Hypoxia: A Pharmacoeconomic Analysis
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.
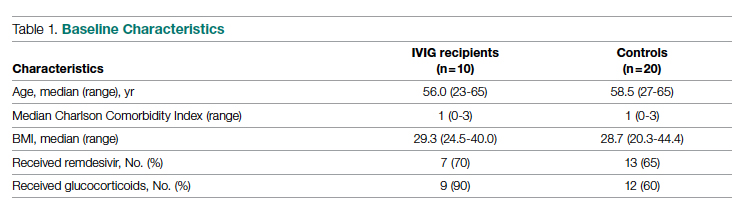
Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).
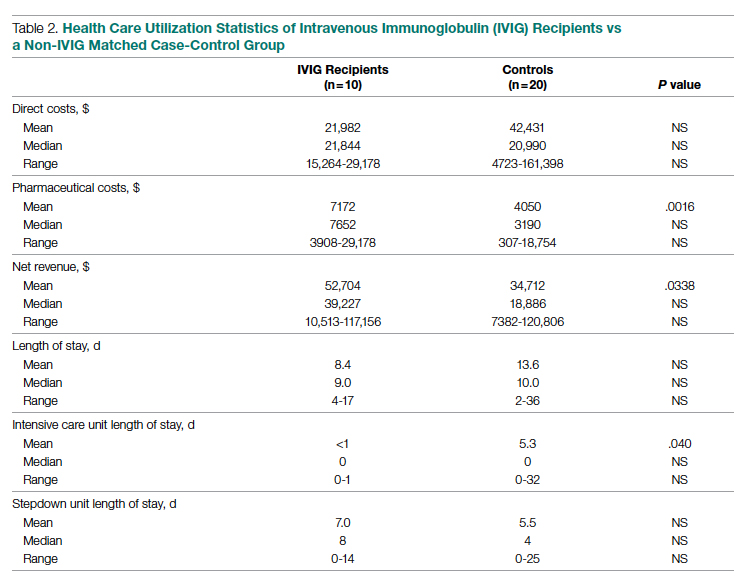
LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.

Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).

LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
From Sharp Memorial Hospital, San Diego, CA (Drs. Poremba, Dehner, Perreiter, Semma, and Mills), Sharp Rees-Stealy Medical Group, San Diego, CA (Dr. Sakoulas), and Collaborative to Halt Antibiotic-Resistant Microbes (CHARM), Department of Pediatrics, University of California San Diego School of Medicine, La Jolla, CA (Dr. Sakoulas).
Abstract
Objective: To compare the costs of hospitalization of patients with moderate-to-severe COVID-19 who received intravenous immunoglobulin (IVIG) with those of patients of similar comorbidity and illness severity who did not.
Design: Analysis 1 was a case-control study of 10 nonventilated, moderately to severely hypoxic patients with COVID-19 who received IVIG (Privigen [CSL Behring]) matched 1:2 with 20 control patients of similar age, body mass index, degree of hypoxemia, and comorbidities. Analysis 2 consisted of patients enrolled in a previously published, randomized, open-label prospective study of 14 patients with COVID-19 receiving standard of care vs 13 patients who received standard of care plus IVIG (Octagam 10% [Octapharma]).
Setting and participants: Patients with COVID-19 with moderate-to-severe hypoxemia hospitalized at a single site located in San Diego, California.
Measurements: Direct cost of hospitalization.
Results: In the first (case-control) population, mean total direct costs, including IVIG, for the treatment group were $21,982 per IVIG-treated case vs $42,431 per case for matched non-IVIG-receiving controls, representing a net cost reduction of $20,449 (48%) per case. For the second (randomized) group, mean total direct costs, including IVIG, for the treatment group were $28,268 per case vs $62,707 per case for untreated controls, representing a net cost reduction of $34,439 (55%) per case. Of the patients who did not receive IVIG, 24% had hospital costs exceeding $80,000; none of the IVIG-treated patients had costs exceeding this amount (P = .016, Fisher exact test).
Conclusion: If allocated early to the appropriate patient type (moderate-to-severe illness without end-organ comorbidities and age <70 years), IVIG can significantly reduce hospital costs in COVID-19 care. More important, in our study it reduced the demand for scarce critical care resources during the COVID-19 pandemic.
Keywords: IVIG, SARS-CoV-2, cost saving, direct hospital costs.
Intravenous immunoglobulin (IVIG) has been available in most hospitals for 4 decades, with broad therapeutic applications in the treatment of Kawasaki disease and a variety of inflammatory, infectious, autoimmune, and viral diseases, via multifactorial mechanisms of immune modulation.1 Reports of COVID-19−associated multisystem inflammatory syndrome in adults and children have supported the use of IVIG in treatment.2,3 Previous studies of IVIG treatment for COVID-19 have produced mixed results. Although retrospective studies have largely been positive,4-8 prospective clinical trials have been mixed, with some favorable results9-11 and another, more recent study showing no benefit.12 However, there is still considerable debate regarding whether some subgroups of patients with COVID-19 may benefit from IVIG; the studies that support this argument, however, have been diluted by broad clinical trials that lack granularity among the heterogeneity of patient characteristics and the timing of IVIG administration.13,14 One study suggests that patients with COVID-19 who may be particularly poised to benefit from IVIG are those who are younger, have fewer comorbidities, and are treated early.8
At our institution, we selectively utilized IVIG to treat patients within 48 hours of rapidly increasing oxygen requirements due to COVID-19, targeting those younger than 70 years, with no previous irreversible end-organ damage, no significant comorbidities (renal failure, heart failure, dementia, active cancer malignancies), and no active treatment for cancer. We analyzed the costs of care of these IVIG (Privigen) recipients and compared them to costs for patients with COVID-19 matched by comorbidities, age, and illness severity who did not receive IVIG. To look for consistency, we examined the cost of care of COVID-19 patients who received IVIG (Octagam) as compared to controls from a previously published pilot trial.10
Methods
Setting and Treatment
All patients in this study were hospitalized at a single site located in San Diego, California. Treatment patients in both cohorts received IVIG 0.5 g/kg adjusted for body weight daily for 3 consecutive days.
Patient Cohort #1: Retrospective Case-Control Trial
Intravenous immunoglobulin (Privigen 10%, CSL Behring) was utilized off-label to treat moderately to severely ill non-intensive care unit (ICU) patients with COVID-19 requiring ≥3 L of oxygen by nasal cannula who were not mechanically ventilated but were considered at high risk for respiratory failure. Preset exclusion criteria for off-label use of IVIG in the treatment of COVID-19 were age >70 years, active malignancy, organ transplant recipient, renal failure, heart failure, or dementia. Controls were obtained from a list of all admitted patients with COVID-19, matched to cases 2:1 on the basis of age (±10 years), body mass index (±1), gender, comorbidities present at admission (eg, hypertension, diabetes mellitus, lung disease, or history of tobacco use), and maximum oxygen requirements within the first 48 hours of admission. In situations where more than 2 potential matched controls were identified for a patient, the 2 controls closest in age to the treatment patient were selected. One IVIG patient was excluded because only 1 matched-age control could be found. Pregnant patients who otherwise fulfilled the criteria for IVIG administration were also excluded from this analysis.
Patient Cohort #2: Prospective, Randomized, Open-Label Trial
Use of IVIG (Octagam 10%, Octapharma) in COVID-19 was studied in a previously published, prospective, open-label randomized trial.10 This pilot trial included 16 IVIG-treated patients and 17 control patients, of which 13 and 14 patients, respectively, had hospital cost data available for analysis.10 Most notably, COVID-19 patients in this study were required to have ≥4 L of oxygen via nasal cannula to maintain arterial oxygen saturationof ≤96%.
Outcomes
Cost data were independently obtained from our finance team, which provided us with the total direct cost and the total pharmaceutical cost associated with each admission. We also compared total length of stay (LOS) and ICU LOS between treatment arms, as these were presumed to be the major drivers of cost difference.
Statistics
Nonparametric comparisons of medians were performed with the Mann-Whitney U test. Comparison of means was done by Student t test. Categorical data were analyzed by Fisher exact test.
This analysis was initiated as an internal quality assessment. It received approval from the Sharp Healthcare Institutional Review Board ([email protected]), and was granted a waiver of subject authorization and consent given the retrospective nature of the study.
Results
Case-Control Analysis
A total of 10 hypoxic patients with COVID-19 received Privigen IVIG outside of clinical trial settings. None of the patients was vaccinated against SARS-CoV-2, as hospitalization occurred prior to vaccine availability. In addition, the original SARS-CoV-2 strain was circulating while these patients were hospitalized, preceding subsequent emerging variants. Oxygen requirements within the first 48 hours ranged from 3 L via nasal cannula to requiring bi-level positive pressure airway therapy with 100% oxygen; median age was 56 years and median Charlson comorbidity index was 1. These 10 patients were each matched to 2 control patients hospitalized during a comparable time period and who, based on oxygen requirements, did not receive IVIG. The 20 control patients had a median age of 58.5 years and a Charlson comorbidity index of 1 (Table 1). Rates of comorbidities, such as hypertension, diabetes mellitus, and obesity, were identical in the 2 groups. None of the patients in either group died during the index hospitalization. Fewer control patients received glucocorticoids, which was reflective of lower illness severity/degree of hypoxia in some controls.

Health care utilization in terms of costs and hospital LOS between the 2 groups are shown in Table 2. The mean total direct hospital cost per case, including IVIG and other drug costs, for the 10 IVIG-treated COVID-19 patients was $21,982 vs $42,431 for the matched controls, a reduction of $20,449 (48%) per case (P = .6187) with IVIG. This difference was heavily driven by 4 control patients (20%) with hospital costs >$80,000, marked by need for ICU transfer, mechanical ventilation during admission, and longer hospital stays. This reduction in progression to mechanical ventilation was consistent with our previously published, open-label, randomized prospective IVIG study, the financial assessment of which is reviewed below. While total direct costs were lower in the treatment arm, the mean drug cost for the treatment arm was $3122 greater than the mean drug cost in the control arm (P = .001622), consistent with the high cost of IVIG therapy (Table 2).

LOS information was obtained, as this was thought to be a primary driver of direct costs. The average LOS in the IVIG arm was 8.4 days, and the average LOS in the control arm was 13.6 days (P = NS). The average ICU LOS in the IVIG arm was 0 days, while the average ICU LOS in the control arm was 5.3 days (P = .04). As with the differences in cost, the differences in LOS were primarily driven by the 4 outlier cases in our control arm, who each had a LOS >25 days, as well as an ICU LOS >20 days.
Randomized, Open-Label, Patient Cohort Analysis
Patient characteristics, LOS, and rates of mechanical ventilation for the IVIG and control patients were previously published and showed a reduction in mechanical ventilation and hospital LOS with IVIG treatment.10 In this group of patients, 1 patient treated with IVIG (6%) and 3 patients not treated with IVIG (18%) died. To determine the consistency of these results from the case-control patients with a set of patients obtained from clinical trial randomization, we examined the health care costs of patients from the prior study.10 As with the case-control group, patients in this portion of the analysis were hospitalized before vaccines were available and prior to any identified variants.
Comparing the hospital cost of the IVIG-treated patients to the control patients from this trial revealed results similar to the matched case-control analysis discussed earlier. Average total direct cost per case, including IVIG, for the IVIG treatment group was $28,268, vs $62,707 per case for non-IVIG controls. This represented a net cost reduction of $34,439 (55%) per case, very similar to that of the prior cohort.
IVIG Reduces Costly Outlier Cases
The case-control and randomized trial groups, yielding a combined 23 IVIG and 34 control patients, showed a median cost per case of $22,578 (range $10,115-$70,929) and $22,645 (range $4723-$279,797) for the IVIG and control groups, respectively. Cases with a cost >$80,000 were 0/23 (0%) vs 8/34 (24%) in the IVIG and control groups, respectively (P = .016, Fisher exact test).
Improving care while simultaneously keeping care costs below reimbursement payment levels received from third-party payers is paramount to the financial survival of health care systems. IVIG appears to do this by reducing the number of patients with COVID-19 who progress to ICU care. We compared the costs of care of our combined case-control and randomized trial cohorts to published data on average reimbursements hospitals receive for COVID-19 care from Medicaid, Medicare, and private insurance (Figure).15 IVIG demonstrated a reduction in cases where costs exceed reimbursement. Indeed, a comparison of net revenue per case of the case-control group showed significantly higher revenue for the IVIG group compared to controls ($52,704 vs $34,712, P = .0338, Table 2).

Discussion
As reflected in at least 1 other study,16 our hospital had been successfully utilizing IVIG in the treatment of viral acute respiratory distress syndrome (ARDS) prior to COVID-19. Therefore, we moved quickly to perform a randomized, open-label pilot study of IVIG (Octagam 10%) in COVID-19, and noted significant clinical benefit that might translate into hospital cost savings.10 Over the course of the pandemic, evidence has accumulated that IVIG may play an important role in COVID-19 therapeutics, as summarized in a recent review.17 However, despite promising but inconsistent results, the relatively high acquisition costs of IVIG raised questions as to its pharmacoeconomic value, particularly with such a high volume of COVID-19 patients with hypoxia, in light of limited clinical data.
COVID-19 therapeutics data can be categorized into either high-quality trials showing marginal benefit for some agents or low-quality trials showing greater benefit for other agents, with IVIG studies falling into the latter category.18 This phenomenon may speak to the pathophysiological heterogeneity of the COVID-19 patient population. High-quality trials enrolling broad patient types lack the granularity to capture and single out relevant patient subsets who would derive maximal therapeutic benefit, with those subsets diluted by other patient types for which no benefit is seen. Meanwhile, the more granular low-quality trials are criticized as underpowered and lacking in translatability to practice.
Positive results from our pilot trial allowed the use of IVIG (Privigen) off-label in hospitalized COVID-19 patients restricted to specific criteria. Patients had to be moderately to severely ill, requiring >3 L of oxygen via nasal cannula; show high risk of clinical deterioration based on respiratory rate and decline in respiratory status; and have underlying comorbidities (such as hypertension, obesity, or diabetes mellitus). However, older patients (>age 70 years) and those with underlying comorbidities marked by organ failure (such as heart failure, renal failure, dementia, or receipt of organ transplant) and active malignancy were excluded, as their clinical outcome in COVID-19 may be considered less modifiable by therapeutics, while simultaneously carrying potentially a higher risk of adverse events from IVIG (volume overload, renal failure). These exclusions are reflected in the overall low Charlson comorbidity index (mean of 1) of the patients in the case-control study arm. As anticipated, we found a net cost reduction: $20,449 (48%) per case among the 10 IVIG-treated patients compared to the 20 matched controls.
We then went back to the patients from the randomized prospective trial and compared costs for the 13 of 16 IVIG patients and 14 of 17 of the control patients for whom data were available. Among untreated controls, we found a net cost reduction of $34,439 (55%) per case. The higher costs seen in the randomized patient cohort compared to the latter case-control group may be due to a combination of the fact that the treated patients had slightly higher comorbidity indices than the case-control group (median Charlson comorbidity index of 2 in both groups) and the fact that they were treated earlier in the pandemic (May/June 2020), as opposed to the case-control group patients, who were treated in November/December 2020.
It was notable that the cost savings across both groups were derived largely from the reduction in the approximately 20% to 25% of control patients who went on to critical illness, including mechanical ventilation, extracorporeal membrane oxygenation (ECMO), and prolonged ICU stays. Indeed, 8 of 34 of the control patients—but none of the 23 IVIG-treated patients—generated hospital costs in excess of $80,000, a difference that was statistically significant even for such a small sample size. Therefore, reducing these very costly outlier events translated into net savings across the board.
In addition to lowering costs, reducing progression to critical illness is extremely important during heavy waves of COVID-19, when the sheer volume of patients results in severe strain due to the relative scarcity of ICU beds, mechanical ventilators, and ECMO. Therefore, reducing the need for these resources would have a vital role that cannot be measured economically.
The major limitations of this study include the small sample size and the potential lack of generalizability of these results to all hospital centers and treating providers. Our group has considerable experience in IVIG utilization in COVID-19 and, as a result, has identified a “sweet spot,” where benefits were seen clinically and economically. However, it remains to be determined whether IVIG will benefit patients with greater illness severity, such as those in the ICU, on mechanical ventilation, or ECMO. Furthermore, while a significant morbidity and mortality burden of COVID-19 rests in extremely elderly patients and those with end-organ comorbidities such as renal failure and heart failure, it is uncertain whether their COVID-19 adverse outcomes can be improved with IVIG or other therapies. We believe such patients may limit the pharmacoeconomic value of IVIG due to their generally poorer prognosis, regardless of intervention. On the other hand, COVID-19 patients who are not that severely ill, with minimal to no hypoxia, generally will do well regardless of therapy. Therefore, IVIG intervention may be an unnecessary treatment expense. Evidence for this was suggested in our pilot trial10 and supported in a recent meta-analysis of IVIG therapy in COVID-19.19
Several other therapeutic options with high acquisition costs have seen an increase in use during the COVID-19 pandemic despite relatively lukewarm data. Remdesivir, the first drug found to have a beneficial effect on hospitalized patients with COVID-19, is priced at $3120 for a complete 5-day treatment course in the United States. This was in line with initial pricing models from the Institute for Clinical and Economic Review (ICER) in May 2020, assuming a mortality benefit with remdesivir use. After the SOLIDARITY trial was published, which showed no mortality benefit associated with remdesivir, ICER updated their pricing models in June 2020 and released a statement that the price of remdesivir was too high to align with demonstrated benefits.20,21 More recent data demonstrate that remdesivir may be beneficial, but only if administered to patients with fewer than 6 days of symptoms.22 However, only a minority of patients present to the hospital early enough in their illness for remdesivir to be beneficial.22
Tocilizumab, an interleukin-6 inhibitor, saw an increase in use during the pandemic. An 800-mg treatment course for COVID-19 costs $3584. The efficacy of this treatment option came into question after the COVACTA trial failed to show a difference in clinical status or mortality in COVID-19 patients who received tocilizumab vs placebo.23,24 A more recent study pointed to a survival benefit of tocilizumab in COVID-19, driven by a very large sample size (>4000), yielding statistically significant, but perhaps clinically less significant, effects on survival.25 This latter study points to the extremely large sample sizes required to capture statistically significant benefits of expensive interventions in COVID-19, which our data demonstrate may benefit only a fraction of patients (20%-25% of patients in the case of IVIG). A more granular clinical assessment of these other interventions is needed to be able to capture the patient subtypes where tocilizumab, remdesivir, and other therapies will be cost effective in the treatment of COVID-19 or other virally mediated cases of ARDS.
Conclusion
While IVIG has a high acquisition cost, the drug’s use in hypoxic COVID-19 patients resulted in reduced costs per COVID-19 case of approximately 50% and use of less critical care resources. The difference was consistent between 2 cohorts (randomized trial vs off-label use in prespecified COVID-19 patient types), IVIG products used (Octagam 10% and Privigen), and time period in the pandemic (waves 1 and 2 in May/June 2020 vs wave 3 in November/December 2020), thereby adjusting for potential differences in circulating viral strains. Furthermore, patients from both groups predated SARS-CoV-2 vaccine availability and major circulating viral variants (eg, delta, omicron), thereby eliminating confounding on outcomes posed by these factors. Control patients’ higher costs of care were driven largely by the approximately 25% of patients who required costly hospital critical care resources, a group mitigated by IVIG. When allocated to the appropriate patient type (patients with moderate-to-severe but not critical illness, <age 70 without preexisting comorbidities of end-organ failure or active cancer), IVIG can reduce hospital costs for COVID-19 care. Identification of specific patient populations where IVIG has the most anticipated benefits in viral illness is needed.
Corresponding author: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, 2020 Genesee Avenue, 2nd Floor, San Diego, CA 92123; [email protected]
Disclosures: Dr Sakoulas has worked as a consultant for Abbvie, Paratek, and Octapharma, has served as a speaker for Abbvie and Paratek, and has received research funding from Octapharma. The other authors did not report any disclosures.
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
1. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29(11):491-498. doi:10.1093/intimm/dxx039
2. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771-1778. doi:10.1016/S0140-6736(20)31103-X
3. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429-436. doi:10.1161/CIRCULATIONAHA.120.048360
4. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunology. 2020;9(10):e1192. doi:10.1002/cti2.1192
5. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318-356. doi:10.1016/j.jinf.2020.03.044
6. Zhou ZG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. 2020:2020030065. doi:10.20944/preprints202003.0065.v1
7. Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3):ofaa102. doi:10.1093/ofid/ofaa102
8. Cao W, Liu X, Hong K, et al. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:627844. doi:10.3389/fimmu.2021.627844
9. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi SR, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20(1):786. doi:10.1186/s12879-020-05507-4
10. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi:10.1097/CCE.0000000000000280
11. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538-1543. doi:10.1093/infdis/jiab098
12. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):158-166. doi:10.1016/S2213-2600(21)00440-9
13. Kindgen-Milles D, Feldt T, Jensen BEO, Dimski T, Brandenburger T. Why the application of IVIG might be beneficial in patients with COVID-19. Lancet Respir Med. 2022;10(2):e15. doi:10.1016/S2213-2600(21)00549-X
14. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID-19 ARDS. Lancet Respir Med. 2022;10(2):123-125. doi:10.1016/S2213-2600(21)00450-1
15. Bazell C, Kramer M, Mraz M, Silseth S. How much are hospitals paid for inpatient COVID-19 treatment? June 2020. https://us.milliman.com/-/media/milliman/pdfs/articles/how-much-hospitals-paid-for-inpatient-covid19-treatment.ashx
16. Liu X, Cao W, Li T. High-dose intravenous immunoglobulins in the treatment of severe acute viral pneumonia: the known mechanisms and clinical effects. Front Immunol. 2020;11:1660. doi:10.3389/fimmu.2020.01660
17. Danieli MG, Piga MA, Paladini A, et al. Intravenous immunoglobulin as an important adjunct in prevention and therapy of coronavirus 19 disease. Scand J Immunol. 2021;94(5):e13101. doi:10.1111/sji.13101
18. Starshinova A, Malkova A, Zinchenko U, et al. Efficacy of different types of therapy for COVID-19: a comprehensive review. Life (Basel). 2021;11(8):753. doi:10.3390/life11080753
19. Xiang HR, Cheng X, Li Y, Luo WW, Zhang QZ, Peng WX. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96:107732. doi:10.1016/j.intimp.2021.107732
20. ICER’s second update to pricing models of remdesivir for COVID-19. PharmacoEcon Outcomes News. 2020;867(1):2. doi:10.1007/s40274-020-7299-y
21. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
22. Garcia-Vidal C, Alonso R, Camon AM, et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76(12):3296-3302. doi:10.1093/jac/dkab321
23. Golimumab (Simponi) IV: In combination with methotrexate (MTX) for the treatment of adult patients with moderately to severely active rheumatoid arthritis [Internet]. Canadian Agency for Drugs and Technologies in Health; 2015. Table 1: Cost comparison table for biologic disease-modifying antirheumatic drugs. https://www.ncbi.nlm.nih.gov/books/NBK349397/table/T34/
24. Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi:10.1056/NEJMoa2028700
25. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
Overall Survival Gain With Adding Darolutamide to ADT and Docetaxel in Metastatic, Hormone-Sensitive Prostate Cancer
Study Overview
Objective: To evaluate whether the addition of the potent androgen-receptor inhibitor (ARA) darolutamide to the standard doublet androgen-deprivation therapy (ADT) and docetaxel in metastatic, hormone-sensitive prostate cancer (mHSPC) would increase survival.
Design: A randomized, double-blind, placebo-controlled, multicenter, phase 3 study. The results reported in this publication are from the prespecified interim analysis.
Intervention: Patients with mHSPC were randomly assigned to receive either darolutamide 600 mg twice daily or placebo. All patients received standard ADT with 6 cycles of docetaxel 75 mg/m2 on day 1 every 21 days along with prednisone given within 6 weeks after randomization. Patients receiving luteinizing hormone–releasing hormone (LHRH) agonists as ADT were bridged with at least 4 weeks of first-generation antiandrogen therapy, which was discontinued before randomization. Treatments were continued until symptomatic disease progression, a change in neoplastic therapy, unacceptable toxicity, patient or physician decision, death, or nonadherence.
Setting and participants: Eligible patients included those newly diagnosed with mHSPC with metastases detected on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and bone scan. Patients were excluded if they had regional lymph node–only involvement or if they had received more than 12 weeks of ADT before randomization. Between November 2016 and June 2018, 1306 patients (651 in the darolutamide group and 655 in the placebo group) were randomized in a 1:1 manner to receive darolutamide 600 mg twice daily or placebo in addition to ADT and docetaxel. Randomization was stratified based on the TNM staging system (M1a—nonregional lymph node–only metastasis, M1b—bone metastasis with or without lymph node, or M1c—bone metastases) as well as baseline alkaline phosphatase levels.
Main outcome measures: The primary end point for the study was overall survival. Other meaningful secondary end points included time to castration resistance, time to pain progression, time to first symptomatic skeletal event, symptomatic skeletal event-free survival, time to subsequent systemic antineoplastic therapy, time to worsening of disease-related physical symptoms, initiation of opioid therapy for ≥7 days, and safety.
Results: The baseline and demographic characteristics were well balanced between the 2 groups. Median age was 67 years. Nearly 80% of patients had bone metastasis, and approximately 17% had visceral metastasis. At the data cutoff date for the primary analysis, the median duration of therapy was 41 months for darolutamide compared with 16.7 months in the placebo group; 45.9% in the darolutamide group and 19.1% in the placebo group were receiving the allotted trial therapy at the time of the analysis. Six cycles of docetaxel were completed in approximately 85% of patients in both arms. Median overall survival follow-up was 43.7 months (darolutamide) and 42.4 months (placebo). A significant improvement in overall survival was observed in the darolutamide group. The risk of death was 32.5% lower in the darolutamide cohort than in the placebo cohort (hazard ratio [HR], 0.68; 95% CI, 0.57-0.80; P < .001). The overall survival at 4 years was 62.7% (95% CI, 58.7-66.7) in the darolutamide arm and 50.4% (95% CI, 46.3-54.6) in the placebo arm. The overall survival results remained favorable across most subgroups.
Darolutamide was associated with improvement in all key secondary endpoints. Time to castration-resistance was significantly longer in the darolutamide group (HR, 0.36; 95% CI, 0.30-0.42; P < .001). Time to pain progression was also significantly longer in the darolutamide group (HR, 0.79; 95% CI, 0.66-0.95; P = .01). Time to first symptomatic skeletal events (HR, 0.71; 95% CI, 0.54-0.94; P = .02) and time to initiation of subsequent systemic therapy (HR, 0.39; 95% CI, 0.33-0.46; P < .001) were also found to be longer in the darolutamide group.
Safety: The risk of grade 3 or higher adverse events was similar across the 2 groups. Most common adverse events were known toxic effects of docetaxel therapy and were highest during the initial period when both groups received this therapy. These side effects progressively decreased after the initial period. The most common grade 3 or 4 adverse event was neutropenia, and its frequency was similar between the darolutamide and placebo groups (33.7% and 34.2%, respectively). The most frequently reported adverse events were alopecia, neutropenia, fatigue, and anemia and were similar between the groups. Adverse events of special significance, including fatigue, falls, fractures, and cardiovascular events, were also similar between the 2 groups. Adverse events causing deaths in each arm were low and similar (4.1% in the darolutamide group and 4.0% in the placebo group). The rates of discontinuation of darolutamide or placebo were similar (13.5% and 10.6%, respectively).
Conclusion: Among patients with mHSPC, overall survival was significantly longer among patients who received darolutamide plus ADT and docetaxel than among those who received ADT and docetaxel alone. This was observed despite a high percentage of patients in the placebo group receiving subsequent systemic therapy at the time of progression. The survival benefit of darolutamide was maintained across most subgroups. An improvement was also observed in the darolutamide arm in terms of key secondary end points. The adverse events were similar across the groups and were consistent with known safety profiles of ADT and docetaxel, and no new safety signals were identified in this trial.
Commentary
The results of the current study add to the body of literature supporting multi-agent systemic therapy in newly diagnosed mHSPC. Prior phase 3 trials of combination therapy using androgen-receptor pathway inhibitors, ADT, and docetaxel have shown conflicting results. The results from the previously reported PEACE-1 study showed improved overall survival among patients who received abiraterone with ADT and docetaxel as compared with those who received ADT and docetaxel alone.1 However, as noted by the authors, the subgroup of patients in the ENZAMET trial who received docetaxel, enzalutamide, and ADT did not appear to have a survival advantage compared with those who received ADT and docetaxel alone.2 The results from the current ARASENS trial provide compelling evidence in a population of prospectively randomized patients that combination therapy with darolutamide, docetaxel, and ADT improves overall survival in men with mHSPC. The survival advantage was maintained across subgroups analyzed in this study. Improvements were observed in regards to several key secondary end points with use of darolutamide. This benefit was maintained despite many patients receiving subsequent therapy at the time of progression. Importantly, there did not appear to be a significant increase in toxicity with triplet therapy. However, it is important to note that this cohort of patients appeared largely asymptomatic at the time of enrollment, with 70% of patients having an Eastern Cooperative Oncology Group performance status of 0.
Additionally, the average age in this study was 67 years, with only about 15% of the population being older than 75 years. In the reported subgroup analysis, those older than 75 years appeared to derive a similar benefit in overall survival, however. Whether triplet therapy should be universally adopted in all patients remains unclear. For example, there is a subset of patients with mHSPC with favorable- risk disease (ie, those with recurrent metastatic disease, node-only disease). In this population, the risk-benefit analysis is less clear, and whether these patients should receive this combination is not certain. Nevertheless, the results of this well-designed study are compelling and certainly represent a potential new standard treatment option for men with mHSPC. One of the strengths of this study was its large sample size that allowed for vigorous statistical analysis to evaluate the efficacy of darolutamide in combination with ADT and docetaxel.
Application for Clinical Practice
The ARASENS study provides convincing evidence that in men with mHSPC, the addition of darolutamide to docetaxel and ADT improves overall survival. This combination appeared to be well tolerated, with no evidence of increased toxicity noted. Certainly, this combination represents a potential new standard treatment option in this population; however, further understanding of which subgroups of men benefit from enhanced therapy is needed to aid in proper patient selection.
—Santosh Kagathur, MD, and Daniel Isaac, DO, MS
Michigan State University, East Lansing, MI
1. Fizazi K, Carles Galceran J, Foulon S, et al. LBA5 A phase III trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann Oncol. 2021;32:Suppl 5:S1299. doi:10.1016/j.annonc.2021.08.2099
2. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121-131. doi:10.1056/NEJMoa1903835
Study Overview
Objective: To evaluate whether the addition of the potent androgen-receptor inhibitor (ARA) darolutamide to the standard doublet androgen-deprivation therapy (ADT) and docetaxel in metastatic, hormone-sensitive prostate cancer (mHSPC) would increase survival.
Design: A randomized, double-blind, placebo-controlled, multicenter, phase 3 study. The results reported in this publication are from the prespecified interim analysis.
Intervention: Patients with mHSPC were randomly assigned to receive either darolutamide 600 mg twice daily or placebo. All patients received standard ADT with 6 cycles of docetaxel 75 mg/m2 on day 1 every 21 days along with prednisone given within 6 weeks after randomization. Patients receiving luteinizing hormone–releasing hormone (LHRH) agonists as ADT were bridged with at least 4 weeks of first-generation antiandrogen therapy, which was discontinued before randomization. Treatments were continued until symptomatic disease progression, a change in neoplastic therapy, unacceptable toxicity, patient or physician decision, death, or nonadherence.
Setting and participants: Eligible patients included those newly diagnosed with mHSPC with metastases detected on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and bone scan. Patients were excluded if they had regional lymph node–only involvement or if they had received more than 12 weeks of ADT before randomization. Between November 2016 and June 2018, 1306 patients (651 in the darolutamide group and 655 in the placebo group) were randomized in a 1:1 manner to receive darolutamide 600 mg twice daily or placebo in addition to ADT and docetaxel. Randomization was stratified based on the TNM staging system (M1a—nonregional lymph node–only metastasis, M1b—bone metastasis with or without lymph node, or M1c—bone metastases) as well as baseline alkaline phosphatase levels.
Main outcome measures: The primary end point for the study was overall survival. Other meaningful secondary end points included time to castration resistance, time to pain progression, time to first symptomatic skeletal event, symptomatic skeletal event-free survival, time to subsequent systemic antineoplastic therapy, time to worsening of disease-related physical symptoms, initiation of opioid therapy for ≥7 days, and safety.
Results: The baseline and demographic characteristics were well balanced between the 2 groups. Median age was 67 years. Nearly 80% of patients had bone metastasis, and approximately 17% had visceral metastasis. At the data cutoff date for the primary analysis, the median duration of therapy was 41 months for darolutamide compared with 16.7 months in the placebo group; 45.9% in the darolutamide group and 19.1% in the placebo group were receiving the allotted trial therapy at the time of the analysis. Six cycles of docetaxel were completed in approximately 85% of patients in both arms. Median overall survival follow-up was 43.7 months (darolutamide) and 42.4 months (placebo). A significant improvement in overall survival was observed in the darolutamide group. The risk of death was 32.5% lower in the darolutamide cohort than in the placebo cohort (hazard ratio [HR], 0.68; 95% CI, 0.57-0.80; P < .001). The overall survival at 4 years was 62.7% (95% CI, 58.7-66.7) in the darolutamide arm and 50.4% (95% CI, 46.3-54.6) in the placebo arm. The overall survival results remained favorable across most subgroups.
Darolutamide was associated with improvement in all key secondary endpoints. Time to castration-resistance was significantly longer in the darolutamide group (HR, 0.36; 95% CI, 0.30-0.42; P < .001). Time to pain progression was also significantly longer in the darolutamide group (HR, 0.79; 95% CI, 0.66-0.95; P = .01). Time to first symptomatic skeletal events (HR, 0.71; 95% CI, 0.54-0.94; P = .02) and time to initiation of subsequent systemic therapy (HR, 0.39; 95% CI, 0.33-0.46; P < .001) were also found to be longer in the darolutamide group.
Safety: The risk of grade 3 or higher adverse events was similar across the 2 groups. Most common adverse events were known toxic effects of docetaxel therapy and were highest during the initial period when both groups received this therapy. These side effects progressively decreased after the initial period. The most common grade 3 or 4 adverse event was neutropenia, and its frequency was similar between the darolutamide and placebo groups (33.7% and 34.2%, respectively). The most frequently reported adverse events were alopecia, neutropenia, fatigue, and anemia and were similar between the groups. Adverse events of special significance, including fatigue, falls, fractures, and cardiovascular events, were also similar between the 2 groups. Adverse events causing deaths in each arm were low and similar (4.1% in the darolutamide group and 4.0% in the placebo group). The rates of discontinuation of darolutamide or placebo were similar (13.5% and 10.6%, respectively).
Conclusion: Among patients with mHSPC, overall survival was significantly longer among patients who received darolutamide plus ADT and docetaxel than among those who received ADT and docetaxel alone. This was observed despite a high percentage of patients in the placebo group receiving subsequent systemic therapy at the time of progression. The survival benefit of darolutamide was maintained across most subgroups. An improvement was also observed in the darolutamide arm in terms of key secondary end points. The adverse events were similar across the groups and were consistent with known safety profiles of ADT and docetaxel, and no new safety signals were identified in this trial.
Commentary
The results of the current study add to the body of literature supporting multi-agent systemic therapy in newly diagnosed mHSPC. Prior phase 3 trials of combination therapy using androgen-receptor pathway inhibitors, ADT, and docetaxel have shown conflicting results. The results from the previously reported PEACE-1 study showed improved overall survival among patients who received abiraterone with ADT and docetaxel as compared with those who received ADT and docetaxel alone.1 However, as noted by the authors, the subgroup of patients in the ENZAMET trial who received docetaxel, enzalutamide, and ADT did not appear to have a survival advantage compared with those who received ADT and docetaxel alone.2 The results from the current ARASENS trial provide compelling evidence in a population of prospectively randomized patients that combination therapy with darolutamide, docetaxel, and ADT improves overall survival in men with mHSPC. The survival advantage was maintained across subgroups analyzed in this study. Improvements were observed in regards to several key secondary end points with use of darolutamide. This benefit was maintained despite many patients receiving subsequent therapy at the time of progression. Importantly, there did not appear to be a significant increase in toxicity with triplet therapy. However, it is important to note that this cohort of patients appeared largely asymptomatic at the time of enrollment, with 70% of patients having an Eastern Cooperative Oncology Group performance status of 0.
Additionally, the average age in this study was 67 years, with only about 15% of the population being older than 75 years. In the reported subgroup analysis, those older than 75 years appeared to derive a similar benefit in overall survival, however. Whether triplet therapy should be universally adopted in all patients remains unclear. For example, there is a subset of patients with mHSPC with favorable- risk disease (ie, those with recurrent metastatic disease, node-only disease). In this population, the risk-benefit analysis is less clear, and whether these patients should receive this combination is not certain. Nevertheless, the results of this well-designed study are compelling and certainly represent a potential new standard treatment option for men with mHSPC. One of the strengths of this study was its large sample size that allowed for vigorous statistical analysis to evaluate the efficacy of darolutamide in combination with ADT and docetaxel.
Application for Clinical Practice
The ARASENS study provides convincing evidence that in men with mHSPC, the addition of darolutamide to docetaxel and ADT improves overall survival. This combination appeared to be well tolerated, with no evidence of increased toxicity noted. Certainly, this combination represents a potential new standard treatment option in this population; however, further understanding of which subgroups of men benefit from enhanced therapy is needed to aid in proper patient selection.
—Santosh Kagathur, MD, and Daniel Isaac, DO, MS
Michigan State University, East Lansing, MI
Study Overview
Objective: To evaluate whether the addition of the potent androgen-receptor inhibitor (ARA) darolutamide to the standard doublet androgen-deprivation therapy (ADT) and docetaxel in metastatic, hormone-sensitive prostate cancer (mHSPC) would increase survival.
Design: A randomized, double-blind, placebo-controlled, multicenter, phase 3 study. The results reported in this publication are from the prespecified interim analysis.
Intervention: Patients with mHSPC were randomly assigned to receive either darolutamide 600 mg twice daily or placebo. All patients received standard ADT with 6 cycles of docetaxel 75 mg/m2 on day 1 every 21 days along with prednisone given within 6 weeks after randomization. Patients receiving luteinizing hormone–releasing hormone (LHRH) agonists as ADT were bridged with at least 4 weeks of first-generation antiandrogen therapy, which was discontinued before randomization. Treatments were continued until symptomatic disease progression, a change in neoplastic therapy, unacceptable toxicity, patient or physician decision, death, or nonadherence.
Setting and participants: Eligible patients included those newly diagnosed with mHSPC with metastases detected on contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and bone scan. Patients were excluded if they had regional lymph node–only involvement or if they had received more than 12 weeks of ADT before randomization. Between November 2016 and June 2018, 1306 patients (651 in the darolutamide group and 655 in the placebo group) were randomized in a 1:1 manner to receive darolutamide 600 mg twice daily or placebo in addition to ADT and docetaxel. Randomization was stratified based on the TNM staging system (M1a—nonregional lymph node–only metastasis, M1b—bone metastasis with or without lymph node, or M1c—bone metastases) as well as baseline alkaline phosphatase levels.
Main outcome measures: The primary end point for the study was overall survival. Other meaningful secondary end points included time to castration resistance, time to pain progression, time to first symptomatic skeletal event, symptomatic skeletal event-free survival, time to subsequent systemic antineoplastic therapy, time to worsening of disease-related physical symptoms, initiation of opioid therapy for ≥7 days, and safety.
Results: The baseline and demographic characteristics were well balanced between the 2 groups. Median age was 67 years. Nearly 80% of patients had bone metastasis, and approximately 17% had visceral metastasis. At the data cutoff date for the primary analysis, the median duration of therapy was 41 months for darolutamide compared with 16.7 months in the placebo group; 45.9% in the darolutamide group and 19.1% in the placebo group were receiving the allotted trial therapy at the time of the analysis. Six cycles of docetaxel were completed in approximately 85% of patients in both arms. Median overall survival follow-up was 43.7 months (darolutamide) and 42.4 months (placebo). A significant improvement in overall survival was observed in the darolutamide group. The risk of death was 32.5% lower in the darolutamide cohort than in the placebo cohort (hazard ratio [HR], 0.68; 95% CI, 0.57-0.80; P < .001). The overall survival at 4 years was 62.7% (95% CI, 58.7-66.7) in the darolutamide arm and 50.4% (95% CI, 46.3-54.6) in the placebo arm. The overall survival results remained favorable across most subgroups.
Darolutamide was associated with improvement in all key secondary endpoints. Time to castration-resistance was significantly longer in the darolutamide group (HR, 0.36; 95% CI, 0.30-0.42; P < .001). Time to pain progression was also significantly longer in the darolutamide group (HR, 0.79; 95% CI, 0.66-0.95; P = .01). Time to first symptomatic skeletal events (HR, 0.71; 95% CI, 0.54-0.94; P = .02) and time to initiation of subsequent systemic therapy (HR, 0.39; 95% CI, 0.33-0.46; P < .001) were also found to be longer in the darolutamide group.
Safety: The risk of grade 3 or higher adverse events was similar across the 2 groups. Most common adverse events were known toxic effects of docetaxel therapy and were highest during the initial period when both groups received this therapy. These side effects progressively decreased after the initial period. The most common grade 3 or 4 adverse event was neutropenia, and its frequency was similar between the darolutamide and placebo groups (33.7% and 34.2%, respectively). The most frequently reported adverse events were alopecia, neutropenia, fatigue, and anemia and were similar between the groups. Adverse events of special significance, including fatigue, falls, fractures, and cardiovascular events, were also similar between the 2 groups. Adverse events causing deaths in each arm were low and similar (4.1% in the darolutamide group and 4.0% in the placebo group). The rates of discontinuation of darolutamide or placebo were similar (13.5% and 10.6%, respectively).
Conclusion: Among patients with mHSPC, overall survival was significantly longer among patients who received darolutamide plus ADT and docetaxel than among those who received ADT and docetaxel alone. This was observed despite a high percentage of patients in the placebo group receiving subsequent systemic therapy at the time of progression. The survival benefit of darolutamide was maintained across most subgroups. An improvement was also observed in the darolutamide arm in terms of key secondary end points. The adverse events were similar across the groups and were consistent with known safety profiles of ADT and docetaxel, and no new safety signals were identified in this trial.
Commentary
The results of the current study add to the body of literature supporting multi-agent systemic therapy in newly diagnosed mHSPC. Prior phase 3 trials of combination therapy using androgen-receptor pathway inhibitors, ADT, and docetaxel have shown conflicting results. The results from the previously reported PEACE-1 study showed improved overall survival among patients who received abiraterone with ADT and docetaxel as compared with those who received ADT and docetaxel alone.1 However, as noted by the authors, the subgroup of patients in the ENZAMET trial who received docetaxel, enzalutamide, and ADT did not appear to have a survival advantage compared with those who received ADT and docetaxel alone.2 The results from the current ARASENS trial provide compelling evidence in a population of prospectively randomized patients that combination therapy with darolutamide, docetaxel, and ADT improves overall survival in men with mHSPC. The survival advantage was maintained across subgroups analyzed in this study. Improvements were observed in regards to several key secondary end points with use of darolutamide. This benefit was maintained despite many patients receiving subsequent therapy at the time of progression. Importantly, there did not appear to be a significant increase in toxicity with triplet therapy. However, it is important to note that this cohort of patients appeared largely asymptomatic at the time of enrollment, with 70% of patients having an Eastern Cooperative Oncology Group performance status of 0.
Additionally, the average age in this study was 67 years, with only about 15% of the population being older than 75 years. In the reported subgroup analysis, those older than 75 years appeared to derive a similar benefit in overall survival, however. Whether triplet therapy should be universally adopted in all patients remains unclear. For example, there is a subset of patients with mHSPC with favorable- risk disease (ie, those with recurrent metastatic disease, node-only disease). In this population, the risk-benefit analysis is less clear, and whether these patients should receive this combination is not certain. Nevertheless, the results of this well-designed study are compelling and certainly represent a potential new standard treatment option for men with mHSPC. One of the strengths of this study was its large sample size that allowed for vigorous statistical analysis to evaluate the efficacy of darolutamide in combination with ADT and docetaxel.
Application for Clinical Practice
The ARASENS study provides convincing evidence that in men with mHSPC, the addition of darolutamide to docetaxel and ADT improves overall survival. This combination appeared to be well tolerated, with no evidence of increased toxicity noted. Certainly, this combination represents a potential new standard treatment option in this population; however, further understanding of which subgroups of men benefit from enhanced therapy is needed to aid in proper patient selection.
—Santosh Kagathur, MD, and Daniel Isaac, DO, MS
Michigan State University, East Lansing, MI
1. Fizazi K, Carles Galceran J, Foulon S, et al. LBA5 A phase III trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann Oncol. 2021;32:Suppl 5:S1299. doi:10.1016/j.annonc.2021.08.2099
2. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121-131. doi:10.1056/NEJMoa1903835
1. Fizazi K, Carles Galceran J, Foulon S, et al. LBA5 A phase III trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann Oncol. 2021;32:Suppl 5:S1299. doi:10.1016/j.annonc.2021.08.2099
2. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121-131. doi:10.1056/NEJMoa1903835
Coronary CT Angiography Compared to Coronary Angiography or Standard of Care in Patients With Intermediate-Risk Stable Chest Pain
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
Study 1 Overview (SCOT-HEART Investigators)
Objective: To assess cardiovascular mortality and nonfatal myocardial infarction at 5 years in patients with stable chest pain referred to cardiology clinic for management with either standard care plus computed tomography angiography (CTA) or standard care alone.
Design: Multicenter, randomized, open-label prospective study.
Setting and participants: A total of 4146 patients with stable chest pain were randomized to standard care or standard care plus CTA at 12 centers across Scotland and were followed for 5 years.
Main outcome measures: The primary end point was a composite of death from coronary heart disease or nonfatal myocardial infarction. Main secondary end points were nonfatal myocardial infarction, nonfatal stroke, and frequency of invasive coronary angiography (ICA) and coronary revascularization with percutaneous coronary intervention or coronary artery bypass grafting.
Main results: The primary outcome including the composite of cardiovascular death or nonfatal myocardial infarction was lower in the CTA group than in the standard-care group at 2.3% (48 of 2073 patients) vs 3.9% (81 of 2073 patients), respectively (hazard ratio, 0.59; 95% CI, 0.41-0.84; P = .004). Although there was a higher rate of ICA and coronary revascularization in the CTA group than in the standard-care group in the first few months of follow-up, the overall rates were similar at 5 years, with ICA performed in 491 patients and 502 patients in the CTA vs standard-care groups, respectively (hazard ratio, 1.00; 95% CI, 0.88-1.13). Similarly, coronary revascularization was performed in 279 patients in the CTA group and in 267 patients in the standard-care group (hazard ratio, 1.07; 95% CI, 0.91-1.27). There were, however, more preventive therapies initiated in patients in the CTA group than in the standard-care group (odds ratio, 1.40; 95% CI, 1.19-1.65).
Conclusion: In patients with stable chest pain, the use of CTA in addition to standard care resulted in a significantly lower rate of death from coronary heart disease or nonfatal myocardial infarction at 5 years; the main contributor to this outcome was a reduced nonfatal myocardial infarction rate. There was no difference in the rate of coronary angiography or coronary revascularization between the 2 groups at 5 years.
Study 2 Overview (DISCHARGE Trial Group)
Objective: To compare the effectiveness of computed tomography (CT) with ICA as a diagnostic tool in patients with stable chest pain and intermediate pretest probability of coronary artery disease (CAD).
Design: Multicenter, randomized, assessor-blinded pragmatic prospective study.
Setting and participants: A total of 3667 patients with stable chest pain and intermediate pretest probability of CAD were enrolled at 26 centers and randomized into CT or ICA groups. Only 3561 patients were included in the modified intention-to-treat analysis, with 1808 patients and 1753 patients in the CT and ICA groups, respectively.
Main outcome measures: The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke over 3.5 years. The main secondary outcomes were major procedure-related complications and patient-reported angina pectoris during the last 4 weeks of follow up.
Main results: The primary outcome occurred in 38 of 1808 patients (2.1%) in the CT group and in 52 of 1753 patients (3.0%) in the ICA group (hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). The secondary outcomes showed that major procedure-related complications occurred in 9 patients (0.5%) in the CT group and in 33 patients (1.9%) in the ICA group (hazard ratio, 0.26; 95% CI, 0.13-0.55). Rates of patient-reported angina in the final 4 weeks of follow-up were 8.8% in the CT group and 7.5% in the ICA group (odds ratio, 1.17; 95% CI, 0.92-1.48).
Conclusion: Risk of major adverse cardiovascular events from the primary outcome were similar in both the CT and ICA groups among patients with stable chest pain and intermediate pretest probability of CAD. Patients referred for CT had a lower rate of coronary angiography leading to fewer major procedure-related complications in these patients than in those referred for ICA.
Commentary
Evaluation and treatment of obstructive atherosclerosis is an important part of clinical care in patients presenting with angina symptoms.1 Thus, the initial investigation for patients with suspected obstructive CAD includes ruling out acute coronary syndrome and assessing quality of life.1 The diagnostic test should be tailored to the pretest probability for the diagnosis of obstructive CAD.2
In the United States, stress testing traditionally has been used for the initial assessment in patients with suspected CAD,3 but recently CTA has been utilized more frequently for this purpose. Compared to a stress test, which often helps identify and assess ischemia, CTA can provide anatomical assessment, with higher sensitivity to identify CAD.4 Furthermore, it can distinguish nonobstructive plaques that can be challenging to identify with stress test alone.
Whether CTA is superior to stress testing as the initial assessment for CAD has been debated. The randomized PROMISE trial compared patients with stable angina who underwent functional stress testing or CTA as an initial strategy.5 They reported a similar outcome between the 2 groups at a median follow-up of 2 years. However, in the original SCOT-HEART trial (CT coronary angiography in patients with suspected angina due to coronary heart disease), which was published in the same year as the PROMISE trial, the patients who underwent initial assessment with CTA had a numerically lower composite end point of cardiac death and myocardial infarction at a median follow-up of 1.7 years (1.3% vs 2.0%, P = .053).6
Given this result, the SCOT-HEART investigators extended the follow-up to evaluate the composite end point of death from coronary heart disease or nonfatal myocardial infarction at 5 years.7 This trial enrolled patients who were initially referred to a cardiology clinic for evaluation of chest pain, and they were randomized to standard care plus CTA or standard care alone. At a median duration of 4.8 years, the primary outcome was lower in the CTA group (2.3%, 48 patients) than in the standard-care group (3.9%, 81 patients) (hazard ratio, 0.58; 95% CI, 0.41-0.84; P = .004). Both groups had similar rates of invasive coronary angiography and had similar coronary revascularization rates.
It is hypothesized that this lower rate of nonfatal myocardial infarction in patients with CTA plus standard care is associated with a higher rate of preventive therapies initiated in patients in the CTA-plus-standard-care group compared to standard care alone. However, the difference in the standard-care group should be noted when compared to the PROMISE trial. In the PROMISE trial, the comparator group had predominantly stress imaging (either nuclear stress test or echocardiography), while in the SCOT-HEART trial, the group had predominantly stress electrocardiogram (ECG), and only 10% of the patients underwent stress imaging. It is possible the difference seen in the rate of nonfatal myocardial infarction was due to suboptimal diagnosis of CAD with stress ECG, which has lower sensitivity compared to stress imaging.
The DISCHARGE trial investigated the effectiveness of CTA vs ICA as the initial diagnostic test in the management of patients with stable chest pain and an intermediate pretest probability of obstructive CAD.8 At 3.5 years of follow-up, the primary composite of cardiovascular death, myocardial infarction, or stroke was similar in both groups (2.1% vs 3.0; hazard ratio, 0.70; 95% CI, 0.46-1.07; P = .10). Importantly, as fewer patients underwent ICA, the risk of procedure-related complication was lower in the CTA group than in the ICA group. However, it is important to note that only 25% of the patients diagnosed with obstructive CAD had greater than 50% vessel stenosis, which raises the question of whether an initial invasive strategy is appropriate for this population.
The strengths of these 2 studies include the large number of patients enrolled along with adequate follow-up, 5 years in the SCOT-HEART trial and 3.5 years in the DISCHARGE trial. The 2 studies overall suggest the usefulness of CTA for assessment of CAD. However, the control groups were very different in these 2 trials. In the SCOT-HEART study, the comparator group was primarily assessed by stress ECG, while in the DISCHARGE study, the comparator group was primary assessed by ICA. In the PROMISE trial, the composite end point of death, myocardial infarction, hospitalization for unstable angina, or major procedural complication was similar when the strategy of initial CTA was compared to functional testing with imaging (exercise ECG, nuclear stress testing, or echocardiography).5 Thus, clinical assessment is still needed when clinicians are selecting the appropriate diagnostic test for patients with suspected CAD. The most recent guidelines give similar recommendations for CTA compared to stress imaging.9 Whether further improvement in CTA acquisition or the addition of CT fractional flow reserve can further improve outcomes requires additional study.
Applications for Clinical Practice and System Implementation
In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful in diagnosis compared to stress ECG and in reducing utilization of low-yield ICA. Whether CTA is more useful compared to the other noninvasive stress imaging modalities in this population requires further study.
Practice Points
- In patients with stable chest pain and intermediate pretest probability of CAD, CTA is useful compared to stress ECG.
- Use of CTA can potentially reduce the use of low-yield coronary angiography.
–Thai Nguyen, MD, Albert Chan, MD, Taishi Hirai, MD
University of Missouri, Columbia, MO
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
1. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407-477. doi:10.1093/eurheartj/ehz425
2. Nakano S, Kohsaka S, Chikamori T et al. JCS 2022 guideline focused update on diagnosis and treatment in patients with stable coronary artery disease. Circ J. 2022;86(5):882-915. doi:10.1253/circj.CJ-21-1041.
3. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44-e164. doi:10.1016/j.jacc.2012.07.013
4. Arbab-Zadeh A, Di Carli MF, Cerci R, et al. Accuracy of computed tomographic angiography and single-photon emission computed tomography-acquired myocardial perfusion imaging for the diagnosis of coronary artery disease. Circ Cardiovasc Imaging. 2015;8(10):e003533. doi:10.1161/CIRCIMAGING
5. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291-300. doi:10.1056/NEJMoa1415516
6. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383-2391. doi:10.1016/S0140-6736(15)60291-4
7. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018;379(10):924-933. doi:10.1056/NEJMoa1805971
8. DISCHARGE Trial Group, Maurovich-Horvat P, Bosserdt M, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med. 2022;386(17):1591-1602. doi:10.1056/NEJMoa2200963
9. Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
Fall Injury Among Community-Dwelling Older Adults: Effect of a Multifactorial Intervention and a Home Hazard Removal Program
Study 1 Overview (Bhasin et al)
Objective: To examine the effect of a multifactorial intervention for fall prevention on fall injury in community-dwelling older adults.
Design: This was a pragmatic, cluster randomized trial conducted in 86 primary care practices across 10 health care systems.
Setting and participants: The primary care sites were selected based on the prespecified criteria of size, ability to implement the intervention, proximity to other practices, accessibility to electronic health records, and access to community-based exercise programs. The primary care practices were randomly assigned to intervention or control.
Eligibility criteria for participants at those practices included age 70 years or older, dwelling in the community, and having an increased risk of falls, as determined by a history of fall-related injury in the past year, 2 or more falls in the past year, or being afraid of falling because of problems with balance or walking. Exclusion criteria were inability to provide consent or lack of proxy consent for participants who were determined to have cognitive impairment based on screening, and inability to speak English or Spanish. A total of 2802 participants were enrolled in the intervention group, and 2649 participants were enrolled in the control group.
Intervention: The intervention contained 5 components: a standardized assessment of 7 modifiable risk factors for fall injuries; standardized protocol-driven recommendations for management of risk factors; an individualized care plan focused on 1 to 3 risk factors; implementation of care plans, including referrals to community-based programs; and follow-up care conducted by telephone or in person. The modifiable risk factors included impairment of strength, gait, or balance; use of medications related to falls; postural hypotension; problems with feet or footwear; visual impairment; osteoporosis or vitamin D deficiency; and home safety hazards. The intervention was delivered by nurses who had completed online training modules and face-to-face training sessions focused on the intervention and motivational interviewing along with continuing education, in partnership with participants and their primary care providers. In the control group, participants received enhanced usual care, including an informational pamphlet, and were encouraged to discuss fall prevention with their primary care provider, including the results of their screening evaluation.
Main outcome measures: The primary outcome of the study was the first serious fall injury in a time-to-event analysis, defined as a fall resulting in a fracture (other than thoracic or lumbar vertebral fracture), joint dislocation, cut requiring closure, head injury requiring hospitalization, sprain or strain, bruising or swelling, or other serious injury. The secondary outcome was first patient-reported fall injury, also in a time-to-event analysis, ascertained by telephone interviews conducted every 4 months. Other outcomes included hospital admissions, emergency department visits, and other health care utilization. Adjudication of fall events and injuries was conducted by a team blinded to treatment assignment and verified using administrative claims data, encounter data, or electronic health record review.
Main results: The intervention and control groups were similar in terms of sex and age: 62.5% vs 61.5% of participants were women, and mean (SD) age was 79.9 (5.7) years and 79.5 (5.8) years, respectively. Other demographic characteristics were similar between groups. For the primary outcome, the rate of first serious injury was 4.9 per 100 person-years in the intervention group and 5.3 per 100 person-years in the control group, with a hazard ratio of 0.92 (95% CI, 0.80-1.06; P = .25). For the secondary outcome of patient-reported fall injury, there were 25.6 events per 100 person-years in the intervention group and 28.6 in the control group, with a hazard ratio of 0.90 (95% CI, 0.83-0.99; P =0.004). Rates of hospitalization and other secondary outcomes were similar between groups.
Conclusion: The multifactorial STRIDE intervention did not reduce the rate of serious fall injury when compared to enhanced usual care. The intervention did result in lower rates of fall injury by patient report, but no other significant outcomes were seen.
Study 2 Overview (Stark et al)
Objective: To examine the effect of a behavioral home hazard removal intervention for fall prevention on risk of fall in community-dwelling older adults.
Design: This randomized clinical trial was conducted at a single site in St. Louis, Missouri. Participants were community-dwelling older adults who received services from the Area Agency on Aging (AAA). Inclusion criteria included age 65 years and older, having 1 or more falls in the previous 12 months or being worried about falling by self report, and currently receiving services from an AAA. Exclusion criteria included living in an institution or being severely cognitively impaired and unable to follow directions or report falls. Participants who met the criteria were contacted by phone and invited to participate. A total of 310 participants were enrolled in the study, with an equal number of participants assigned to the intervention and control groups.
Intervention: The intervention included hazard identification and removal after a comprehensive assessment of participants, their behaviors, and the environment; this assessment took place during the first visit, which lasted approximately 80 minutes. A home hazard removal plan was developed, and in the second session, which lasted approximately 40 minutes, remediation of hazards was carried out. A third session for home modification that lasted approximately 30 minutes was conducted, if needed. At 6 months after the intervention, a booster session to identify and remediate any new home hazards and address issues was conducted. Specific interventions, as identified by the assessment, included minor home repair such as grab bars, adaptive equipment, task modification, and education. Shared decision making that enabled older adults to control changes in their homes, self-management strategies to improve awareness, and motivational enhancement strategies to improve acceptance were employed. Scripted algorithms and checklists were used to deliver the intervention. For usual care, an annual assessment and referrals to community services, if needed, were conducted in the AAA.
Main outcome measures: The primary outcome of the study was the number of days to first fall in 12 months. Falls were defined as unintentional movements to the floor, ground, or object below knee level, and falls were recorded through a daily journal for 12 months. Participants were contacted by phone if they did not return the journal or reported a fall. Participants were interviewed to verify falls and determine whether a fall was injurious. Secondary outcomes included rate of falls per person per 12 months; daily activity performance measured using the Older Americans Resources and Services Activities of Daily Living scale; falls self-efficacy, which measures confidence performing daily activities without falling; and quality of life using the SF-36 at 12 months.
Main results: Most of the study participants were women (74%), and mean (SD) age was 75 (7.4) years. Study retention was similar between the intervention and control groups, with 82% completing the study in the intervention group compared with 81% in the control group. Fidelity to the intervention, as measured by a checklist by the interventionist, was 99%, and adherence to home modification, as measured by number of home modifications in use by self report, was high at 92% at 6 months and 91% at 12 months. For the primary outcome, fall hazard was not different between the intervention and control groups (hazard ratio, 0.9; 95% CI, 0.66-1.27). For the secondary outcomes, the rate of falling was lower in the intervention group compared with the control group, with a relative risk of 0.62 (95% CI, 0.40-0.95). There was no difference in other secondary outcomes of daily activity performance, falls self-efficacy, or quality of life.
Conclusion: Despite high adherence to home modifications and fidelity to the intervention, this home hazard removal program did not reduce the risk of falling when compared to usual care. It did reduce the rate of falls, although no other effects were observed.
Commentary
Observational studies have identified factors that contribute to falls,1 and over the past 30 years a number of intervention trials designed to reduce the risk of falling have been conducted. A recent Cochrane review, published prior to the Bhasin et al and Stark et al trials, looked at the effect of multifactorial interventions for fall prevention across 62 trials that included 19,935 older adults living in the community. The review concluded that multifactorial interventions may reduce the rate of falls, but this conclusion was based on low-quality evidence and there was significant heterogeneity across the studies.2
The STRIDE randomized trial represents the latest effort to address the evidence gap around fall prevention, with the STRIDE investigators hoping this would be the definitive trial that leads to practice change in fall prevention. Smaller trials that have demonstrated effectiveness were brought to scale in this large randomized trial that included 86 practices and more than 5000 participants. The investigators used risk of injurious falls as the primary outcome, as this outcome is considered the most clinically meaningful for the study population. The results, however, were disappointing: the multifactorial intervention in STRIDE did not result in a reduction of risk of injurious falls. Challenges in the implementation of this large trial may have contributed to its results; falls care managers, key to this multifactorial intervention, reported difficulties in navigating complex relationships with patients, families, study staff, and primary care practices during the study. Barriers reported included clinical space limitations, variable buy-in from providers, and turnover of practice staff and providers.3 Such implementation factors may have resulted in the divergent results between smaller clinical trials and this large-scale trial conducted across multiple settings.
The second study, by Stark et al, examined a home modification program and its effect on risk of falls. A prior Cochrane review examining the effect of home safety assessment and modification indicates that these strategies are effective in reducing the rate of falls as well as the risk of falling.4 The results of the current trial showed a reduction in the rate of falls but not in the risk of falling; however, this study did not examine outcomes of serious injurious falls, which may be more clinically meaningful. The Stark et al study adds to the existing literature showing that home modification may have an impact on fall rates. One noteworthy aspect of the Stark et al trial is the high adherence rate to home modification in a community-based approach; perhaps the investigators’ approach can be translated to real-world use.
Applications for Clinical Practice and System Implementation
The role of exercise programs in reducing fall rates is well established,5 but neither of these studies focused on exercise interventions. STRIDE offered community-based exercise program referral, but there is variability in such programs and study staff reported challenges in matching participants with appropriate exercise programs.3 Further studies that examine combinations of multifactorial falls risk reduction, exercise, and home safety, with careful consideration of implementation challenges to assure fidelity and adherence to the intervention, are needed to ascertain the best strategy for fall prevention for older adults at risk.
Given the results of these trials, it is difficult to recommend one falls prevention intervention over another. Clinicians should continue to identify falls risk factors using standardized assessments and determine which factors are modifiable.
Practice Points
- Incorporating assessments of falls risk in primary care is feasible, and such assessments can identify important risk factors.
- Clinicians and health systems should identify avenues, such as developing programmatic approaches, to providing home safety assessment and intervention, exercise options, medication review, and modification of other risk factors.
- Ensuring delivery of these elements reliably through programmatic approaches with adequate follow-up is key to preventing falls in this population.
—William W. Hung, MD, MPH
1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988; 319:1701-1707. doi:10.1056/NEJM198812293192604
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:0.1002/14651858.CD012221.pub2
3. Reckrey JM, Gazarian P, Reuben DB, et al. Barriers to implementation of STRIDE, a national study to prevent fall-related injuries. J Am Geriatr Soc. 2021;69(5):1334-1342. doi:10.1111/jgs.17056
4. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. doi:10.1002/14651858.CD007146.pub3
5. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1(1):CD012424. doi:10.1002/14651858.CD012424.pub2
Study 1 Overview (Bhasin et al)
Objective: To examine the effect of a multifactorial intervention for fall prevention on fall injury in community-dwelling older adults.
Design: This was a pragmatic, cluster randomized trial conducted in 86 primary care practices across 10 health care systems.
Setting and participants: The primary care sites were selected based on the prespecified criteria of size, ability to implement the intervention, proximity to other practices, accessibility to electronic health records, and access to community-based exercise programs. The primary care practices were randomly assigned to intervention or control.
Eligibility criteria for participants at those practices included age 70 years or older, dwelling in the community, and having an increased risk of falls, as determined by a history of fall-related injury in the past year, 2 or more falls in the past year, or being afraid of falling because of problems with balance or walking. Exclusion criteria were inability to provide consent or lack of proxy consent for participants who were determined to have cognitive impairment based on screening, and inability to speak English or Spanish. A total of 2802 participants were enrolled in the intervention group, and 2649 participants were enrolled in the control group.
Intervention: The intervention contained 5 components: a standardized assessment of 7 modifiable risk factors for fall injuries; standardized protocol-driven recommendations for management of risk factors; an individualized care plan focused on 1 to 3 risk factors; implementation of care plans, including referrals to community-based programs; and follow-up care conducted by telephone or in person. The modifiable risk factors included impairment of strength, gait, or balance; use of medications related to falls; postural hypotension; problems with feet or footwear; visual impairment; osteoporosis or vitamin D deficiency; and home safety hazards. The intervention was delivered by nurses who had completed online training modules and face-to-face training sessions focused on the intervention and motivational interviewing along with continuing education, in partnership with participants and their primary care providers. In the control group, participants received enhanced usual care, including an informational pamphlet, and were encouraged to discuss fall prevention with their primary care provider, including the results of their screening evaluation.
Main outcome measures: The primary outcome of the study was the first serious fall injury in a time-to-event analysis, defined as a fall resulting in a fracture (other than thoracic or lumbar vertebral fracture), joint dislocation, cut requiring closure, head injury requiring hospitalization, sprain or strain, bruising or swelling, or other serious injury. The secondary outcome was first patient-reported fall injury, also in a time-to-event analysis, ascertained by telephone interviews conducted every 4 months. Other outcomes included hospital admissions, emergency department visits, and other health care utilization. Adjudication of fall events and injuries was conducted by a team blinded to treatment assignment and verified using administrative claims data, encounter data, or electronic health record review.
Main results: The intervention and control groups were similar in terms of sex and age: 62.5% vs 61.5% of participants were women, and mean (SD) age was 79.9 (5.7) years and 79.5 (5.8) years, respectively. Other demographic characteristics were similar between groups. For the primary outcome, the rate of first serious injury was 4.9 per 100 person-years in the intervention group and 5.3 per 100 person-years in the control group, with a hazard ratio of 0.92 (95% CI, 0.80-1.06; P = .25). For the secondary outcome of patient-reported fall injury, there were 25.6 events per 100 person-years in the intervention group and 28.6 in the control group, with a hazard ratio of 0.90 (95% CI, 0.83-0.99; P =0.004). Rates of hospitalization and other secondary outcomes were similar between groups.
Conclusion: The multifactorial STRIDE intervention did not reduce the rate of serious fall injury when compared to enhanced usual care. The intervention did result in lower rates of fall injury by patient report, but no other significant outcomes were seen.
Study 2 Overview (Stark et al)
Objective: To examine the effect of a behavioral home hazard removal intervention for fall prevention on risk of fall in community-dwelling older adults.
Design: This randomized clinical trial was conducted at a single site in St. Louis, Missouri. Participants were community-dwelling older adults who received services from the Area Agency on Aging (AAA). Inclusion criteria included age 65 years and older, having 1 or more falls in the previous 12 months or being worried about falling by self report, and currently receiving services from an AAA. Exclusion criteria included living in an institution or being severely cognitively impaired and unable to follow directions or report falls. Participants who met the criteria were contacted by phone and invited to participate. A total of 310 participants were enrolled in the study, with an equal number of participants assigned to the intervention and control groups.
Intervention: The intervention included hazard identification and removal after a comprehensive assessment of participants, their behaviors, and the environment; this assessment took place during the first visit, which lasted approximately 80 minutes. A home hazard removal plan was developed, and in the second session, which lasted approximately 40 minutes, remediation of hazards was carried out. A third session for home modification that lasted approximately 30 minutes was conducted, if needed. At 6 months after the intervention, a booster session to identify and remediate any new home hazards and address issues was conducted. Specific interventions, as identified by the assessment, included minor home repair such as grab bars, adaptive equipment, task modification, and education. Shared decision making that enabled older adults to control changes in their homes, self-management strategies to improve awareness, and motivational enhancement strategies to improve acceptance were employed. Scripted algorithms and checklists were used to deliver the intervention. For usual care, an annual assessment and referrals to community services, if needed, were conducted in the AAA.
Main outcome measures: The primary outcome of the study was the number of days to first fall in 12 months. Falls were defined as unintentional movements to the floor, ground, or object below knee level, and falls were recorded through a daily journal for 12 months. Participants were contacted by phone if they did not return the journal or reported a fall. Participants were interviewed to verify falls and determine whether a fall was injurious. Secondary outcomes included rate of falls per person per 12 months; daily activity performance measured using the Older Americans Resources and Services Activities of Daily Living scale; falls self-efficacy, which measures confidence performing daily activities without falling; and quality of life using the SF-36 at 12 months.
Main results: Most of the study participants were women (74%), and mean (SD) age was 75 (7.4) years. Study retention was similar between the intervention and control groups, with 82% completing the study in the intervention group compared with 81% in the control group. Fidelity to the intervention, as measured by a checklist by the interventionist, was 99%, and adherence to home modification, as measured by number of home modifications in use by self report, was high at 92% at 6 months and 91% at 12 months. For the primary outcome, fall hazard was not different between the intervention and control groups (hazard ratio, 0.9; 95% CI, 0.66-1.27). For the secondary outcomes, the rate of falling was lower in the intervention group compared with the control group, with a relative risk of 0.62 (95% CI, 0.40-0.95). There was no difference in other secondary outcomes of daily activity performance, falls self-efficacy, or quality of life.
Conclusion: Despite high adherence to home modifications and fidelity to the intervention, this home hazard removal program did not reduce the risk of falling when compared to usual care. It did reduce the rate of falls, although no other effects were observed.
Commentary
Observational studies have identified factors that contribute to falls,1 and over the past 30 years a number of intervention trials designed to reduce the risk of falling have been conducted. A recent Cochrane review, published prior to the Bhasin et al and Stark et al trials, looked at the effect of multifactorial interventions for fall prevention across 62 trials that included 19,935 older adults living in the community. The review concluded that multifactorial interventions may reduce the rate of falls, but this conclusion was based on low-quality evidence and there was significant heterogeneity across the studies.2
The STRIDE randomized trial represents the latest effort to address the evidence gap around fall prevention, with the STRIDE investigators hoping this would be the definitive trial that leads to practice change in fall prevention. Smaller trials that have demonstrated effectiveness were brought to scale in this large randomized trial that included 86 practices and more than 5000 participants. The investigators used risk of injurious falls as the primary outcome, as this outcome is considered the most clinically meaningful for the study population. The results, however, were disappointing: the multifactorial intervention in STRIDE did not result in a reduction of risk of injurious falls. Challenges in the implementation of this large trial may have contributed to its results; falls care managers, key to this multifactorial intervention, reported difficulties in navigating complex relationships with patients, families, study staff, and primary care practices during the study. Barriers reported included clinical space limitations, variable buy-in from providers, and turnover of practice staff and providers.3 Such implementation factors may have resulted in the divergent results between smaller clinical trials and this large-scale trial conducted across multiple settings.
The second study, by Stark et al, examined a home modification program and its effect on risk of falls. A prior Cochrane review examining the effect of home safety assessment and modification indicates that these strategies are effective in reducing the rate of falls as well as the risk of falling.4 The results of the current trial showed a reduction in the rate of falls but not in the risk of falling; however, this study did not examine outcomes of serious injurious falls, which may be more clinically meaningful. The Stark et al study adds to the existing literature showing that home modification may have an impact on fall rates. One noteworthy aspect of the Stark et al trial is the high adherence rate to home modification in a community-based approach; perhaps the investigators’ approach can be translated to real-world use.
Applications for Clinical Practice and System Implementation
The role of exercise programs in reducing fall rates is well established,5 but neither of these studies focused on exercise interventions. STRIDE offered community-based exercise program referral, but there is variability in such programs and study staff reported challenges in matching participants with appropriate exercise programs.3 Further studies that examine combinations of multifactorial falls risk reduction, exercise, and home safety, with careful consideration of implementation challenges to assure fidelity and adherence to the intervention, are needed to ascertain the best strategy for fall prevention for older adults at risk.
Given the results of these trials, it is difficult to recommend one falls prevention intervention over another. Clinicians should continue to identify falls risk factors using standardized assessments and determine which factors are modifiable.
Practice Points
- Incorporating assessments of falls risk in primary care is feasible, and such assessments can identify important risk factors.
- Clinicians and health systems should identify avenues, such as developing programmatic approaches, to providing home safety assessment and intervention, exercise options, medication review, and modification of other risk factors.
- Ensuring delivery of these elements reliably through programmatic approaches with adequate follow-up is key to preventing falls in this population.
—William W. Hung, MD, MPH
Study 1 Overview (Bhasin et al)
Objective: To examine the effect of a multifactorial intervention for fall prevention on fall injury in community-dwelling older adults.
Design: This was a pragmatic, cluster randomized trial conducted in 86 primary care practices across 10 health care systems.
Setting and participants: The primary care sites were selected based on the prespecified criteria of size, ability to implement the intervention, proximity to other practices, accessibility to electronic health records, and access to community-based exercise programs. The primary care practices were randomly assigned to intervention or control.
Eligibility criteria for participants at those practices included age 70 years or older, dwelling in the community, and having an increased risk of falls, as determined by a history of fall-related injury in the past year, 2 or more falls in the past year, or being afraid of falling because of problems with balance or walking. Exclusion criteria were inability to provide consent or lack of proxy consent for participants who were determined to have cognitive impairment based on screening, and inability to speak English or Spanish. A total of 2802 participants were enrolled in the intervention group, and 2649 participants were enrolled in the control group.
Intervention: The intervention contained 5 components: a standardized assessment of 7 modifiable risk factors for fall injuries; standardized protocol-driven recommendations for management of risk factors; an individualized care plan focused on 1 to 3 risk factors; implementation of care plans, including referrals to community-based programs; and follow-up care conducted by telephone or in person. The modifiable risk factors included impairment of strength, gait, or balance; use of medications related to falls; postural hypotension; problems with feet or footwear; visual impairment; osteoporosis or vitamin D deficiency; and home safety hazards. The intervention was delivered by nurses who had completed online training modules and face-to-face training sessions focused on the intervention and motivational interviewing along with continuing education, in partnership with participants and their primary care providers. In the control group, participants received enhanced usual care, including an informational pamphlet, and were encouraged to discuss fall prevention with their primary care provider, including the results of their screening evaluation.
Main outcome measures: The primary outcome of the study was the first serious fall injury in a time-to-event analysis, defined as a fall resulting in a fracture (other than thoracic or lumbar vertebral fracture), joint dislocation, cut requiring closure, head injury requiring hospitalization, sprain or strain, bruising or swelling, or other serious injury. The secondary outcome was first patient-reported fall injury, also in a time-to-event analysis, ascertained by telephone interviews conducted every 4 months. Other outcomes included hospital admissions, emergency department visits, and other health care utilization. Adjudication of fall events and injuries was conducted by a team blinded to treatment assignment and verified using administrative claims data, encounter data, or electronic health record review.
Main results: The intervention and control groups were similar in terms of sex and age: 62.5% vs 61.5% of participants were women, and mean (SD) age was 79.9 (5.7) years and 79.5 (5.8) years, respectively. Other demographic characteristics were similar between groups. For the primary outcome, the rate of first serious injury was 4.9 per 100 person-years in the intervention group and 5.3 per 100 person-years in the control group, with a hazard ratio of 0.92 (95% CI, 0.80-1.06; P = .25). For the secondary outcome of patient-reported fall injury, there were 25.6 events per 100 person-years in the intervention group and 28.6 in the control group, with a hazard ratio of 0.90 (95% CI, 0.83-0.99; P =0.004). Rates of hospitalization and other secondary outcomes were similar between groups.
Conclusion: The multifactorial STRIDE intervention did not reduce the rate of serious fall injury when compared to enhanced usual care. The intervention did result in lower rates of fall injury by patient report, but no other significant outcomes were seen.
Study 2 Overview (Stark et al)
Objective: To examine the effect of a behavioral home hazard removal intervention for fall prevention on risk of fall in community-dwelling older adults.
Design: This randomized clinical trial was conducted at a single site in St. Louis, Missouri. Participants were community-dwelling older adults who received services from the Area Agency on Aging (AAA). Inclusion criteria included age 65 years and older, having 1 or more falls in the previous 12 months or being worried about falling by self report, and currently receiving services from an AAA. Exclusion criteria included living in an institution or being severely cognitively impaired and unable to follow directions or report falls. Participants who met the criteria were contacted by phone and invited to participate. A total of 310 participants were enrolled in the study, with an equal number of participants assigned to the intervention and control groups.
Intervention: The intervention included hazard identification and removal after a comprehensive assessment of participants, their behaviors, and the environment; this assessment took place during the first visit, which lasted approximately 80 minutes. A home hazard removal plan was developed, and in the second session, which lasted approximately 40 minutes, remediation of hazards was carried out. A third session for home modification that lasted approximately 30 minutes was conducted, if needed. At 6 months after the intervention, a booster session to identify and remediate any new home hazards and address issues was conducted. Specific interventions, as identified by the assessment, included minor home repair such as grab bars, adaptive equipment, task modification, and education. Shared decision making that enabled older adults to control changes in their homes, self-management strategies to improve awareness, and motivational enhancement strategies to improve acceptance were employed. Scripted algorithms and checklists were used to deliver the intervention. For usual care, an annual assessment and referrals to community services, if needed, were conducted in the AAA.
Main outcome measures: The primary outcome of the study was the number of days to first fall in 12 months. Falls were defined as unintentional movements to the floor, ground, or object below knee level, and falls were recorded through a daily journal for 12 months. Participants were contacted by phone if they did not return the journal or reported a fall. Participants were interviewed to verify falls and determine whether a fall was injurious. Secondary outcomes included rate of falls per person per 12 months; daily activity performance measured using the Older Americans Resources and Services Activities of Daily Living scale; falls self-efficacy, which measures confidence performing daily activities without falling; and quality of life using the SF-36 at 12 months.
Main results: Most of the study participants were women (74%), and mean (SD) age was 75 (7.4) years. Study retention was similar between the intervention and control groups, with 82% completing the study in the intervention group compared with 81% in the control group. Fidelity to the intervention, as measured by a checklist by the interventionist, was 99%, and adherence to home modification, as measured by number of home modifications in use by self report, was high at 92% at 6 months and 91% at 12 months. For the primary outcome, fall hazard was not different between the intervention and control groups (hazard ratio, 0.9; 95% CI, 0.66-1.27). For the secondary outcomes, the rate of falling was lower in the intervention group compared with the control group, with a relative risk of 0.62 (95% CI, 0.40-0.95). There was no difference in other secondary outcomes of daily activity performance, falls self-efficacy, or quality of life.
Conclusion: Despite high adherence to home modifications and fidelity to the intervention, this home hazard removal program did not reduce the risk of falling when compared to usual care. It did reduce the rate of falls, although no other effects were observed.
Commentary
Observational studies have identified factors that contribute to falls,1 and over the past 30 years a number of intervention trials designed to reduce the risk of falling have been conducted. A recent Cochrane review, published prior to the Bhasin et al and Stark et al trials, looked at the effect of multifactorial interventions for fall prevention across 62 trials that included 19,935 older adults living in the community. The review concluded that multifactorial interventions may reduce the rate of falls, but this conclusion was based on low-quality evidence and there was significant heterogeneity across the studies.2
The STRIDE randomized trial represents the latest effort to address the evidence gap around fall prevention, with the STRIDE investigators hoping this would be the definitive trial that leads to practice change in fall prevention. Smaller trials that have demonstrated effectiveness were brought to scale in this large randomized trial that included 86 practices and more than 5000 participants. The investigators used risk of injurious falls as the primary outcome, as this outcome is considered the most clinically meaningful for the study population. The results, however, were disappointing: the multifactorial intervention in STRIDE did not result in a reduction of risk of injurious falls. Challenges in the implementation of this large trial may have contributed to its results; falls care managers, key to this multifactorial intervention, reported difficulties in navigating complex relationships with patients, families, study staff, and primary care practices during the study. Barriers reported included clinical space limitations, variable buy-in from providers, and turnover of practice staff and providers.3 Such implementation factors may have resulted in the divergent results between smaller clinical trials and this large-scale trial conducted across multiple settings.
The second study, by Stark et al, examined a home modification program and its effect on risk of falls. A prior Cochrane review examining the effect of home safety assessment and modification indicates that these strategies are effective in reducing the rate of falls as well as the risk of falling.4 The results of the current trial showed a reduction in the rate of falls but not in the risk of falling; however, this study did not examine outcomes of serious injurious falls, which may be more clinically meaningful. The Stark et al study adds to the existing literature showing that home modification may have an impact on fall rates. One noteworthy aspect of the Stark et al trial is the high adherence rate to home modification in a community-based approach; perhaps the investigators’ approach can be translated to real-world use.
Applications for Clinical Practice and System Implementation
The role of exercise programs in reducing fall rates is well established,5 but neither of these studies focused on exercise interventions. STRIDE offered community-based exercise program referral, but there is variability in such programs and study staff reported challenges in matching participants with appropriate exercise programs.3 Further studies that examine combinations of multifactorial falls risk reduction, exercise, and home safety, with careful consideration of implementation challenges to assure fidelity and adherence to the intervention, are needed to ascertain the best strategy for fall prevention for older adults at risk.
Given the results of these trials, it is difficult to recommend one falls prevention intervention over another. Clinicians should continue to identify falls risk factors using standardized assessments and determine which factors are modifiable.
Practice Points
- Incorporating assessments of falls risk in primary care is feasible, and such assessments can identify important risk factors.
- Clinicians and health systems should identify avenues, such as developing programmatic approaches, to providing home safety assessment and intervention, exercise options, medication review, and modification of other risk factors.
- Ensuring delivery of these elements reliably through programmatic approaches with adequate follow-up is key to preventing falls in this population.
—William W. Hung, MD, MPH
1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988; 319:1701-1707. doi:10.1056/NEJM198812293192604
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:0.1002/14651858.CD012221.pub2
3. Reckrey JM, Gazarian P, Reuben DB, et al. Barriers to implementation of STRIDE, a national study to prevent fall-related injuries. J Am Geriatr Soc. 2021;69(5):1334-1342. doi:10.1111/jgs.17056
4. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. doi:10.1002/14651858.CD007146.pub3
5. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1(1):CD012424. doi:10.1002/14651858.CD012424.pub2
1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988; 319:1701-1707. doi:10.1056/NEJM198812293192604
2. Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7(7):CD012221. doi:0.1002/14651858.CD012221.pub2
3. Reckrey JM, Gazarian P, Reuben DB, et al. Barriers to implementation of STRIDE, a national study to prevent fall-related injuries. J Am Geriatr Soc. 2021;69(5):1334-1342. doi:10.1111/jgs.17056
4. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;2012(9):CD007146. doi:10.1002/14651858.CD007146.pub3
5. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1(1):CD012424. doi:10.1002/14651858.CD012424.pub2
Index cholecystectomy reduces readmissions after acute cholangitis
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
AT DDW 2022
‘Double-edged’ impact of sparring on the brains of MMA fighters
, early research suggests.
Investigators found sparring, defined as strategically hitting opponents with kicks, punches, and other strikes during practice sessions, is linked to increased white matter hyperintensities in the brain, pointing to possible vascular damage from repeated head trauma. However, the study results also show sparring was associated with a larger bilateral caudate which, in theory, is neuroprotective.
“From our preliminary study, sparring practice in MMA fighters may have a ‘double-edged sword’ effect on the brain,” study investigator Aaron Esagoff, a second-year medical student at Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“The combination of complex movements along with constant strategy and anticipation of your opponent’s next move may provide a neuroprotective effect on the caudate,” Mr. Esagoff said. However, he added, more research is needed into understanding this particular finding.
The study results were presented at the American Psychiatric Association (APA) 2022 Annual Meeting.
Growing popularity
MMA is a full-contact combat sport that has become increasingly popular over the past 15 years. It combines techniques from boxing, wrestling, karate, judo, and jujitsu.
To prepare for fights, MMA practitioners incorporate sparring and grappling, which use techniques such as chokes and locks to submit an opponent. Head protection is sometimes incorporated during practice, but is not the norm during a fight, said Mr. Esagoff.
The study investigated sparring during practice rather than fights because, he said, MMA competitors only fight a few times a year but spend hundreds of hours training. “So the health effects of training are going to be really important,” he said.
As with other combat sports, MMA involves hits to the head. Previous research has shown repetitive head trauma can lead to neurodegenerative diseases, including chronic traumatic encephalopathy (CTE) and Alzheimer’s disease, Mr. Esagoff noted.
Previous studies have also linked more professional fights and years of fighting to a decrease in brain volume among MMA fighters, he added.
The new analysis was conducted as part of the Professional Fighters Brain Health Study, a longitudinal cohort study of MMA professional fighters. It included 92 fighters with data available on MRI and habits regarding practicing. The mean age of the participants was 30 years, 62% were White, and 85% were men.
The study examined sparring but did not include grappling because of “several challenges” with the current data analysis, Mr. Esagoff said. Researchers adjusted for age, sex, education, race, number of fights, total intracranial volume, and type of MRI scanner used.
A ‘highly strategic’ sport
Results showed a strong association between the number of sparring rounds per week and increased white matter hyperintensity volume (mcL) on MRI (P = .039).
This suggests white matter damage, possibly a result of direct neuronal injury, vascular damage, or immune modulation, said Mr. Esagoff. However, another mechanism may be involved, he added.
There was also a significant association between sparring and increased size of the caudate nucleus, an area of the brain involved in movement, learning, and memory (P = .014 for right caudate volume, P = .012 for left caudate volume).
There are some theories that might explain this finding, said Mr. Esagoff. For example, individuals who spar more may get better at avoiding impacts and injuries during a fight, which might in turn affect the size of the caudate.
The controlled movements and techniques used during sparring could also affect the caudate. “Some research has shown that behavior, learning, and/or exercise may increase the size of certain brain regions,” Mr. Esagoff said.
He noted the “highly strategic” nature of combat sports – and used the example of Brazilian jiu-jitsu. That sport “is known as human chess because it takes a thoughtful approach to defeat a larger opponent with base, leverage, and technique,” he said.
However, Mr. Esagoff stressed that while it is possible movements involved in MMA increase caudate size, this is just a theory at this point.
A study limitation was that fighters volunteered to participate and may not represent all fighters. As well, the study was cross-sectional and looked at only one point in time, so it cannot infer causation.
Overall, the new findings should help inform fighters, governing bodies, and the public about the potential risks and benefits of different styles of MMA fighting and practice, although more research is needed, said Mr. Esagoff.
He and his team now plan to conduct a longer-term study and investigate effects of grappling on brain structure and function in addition to sparring.
Jury still out
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry and incoming chair of the APA’s Council on Communications, said the jury “is clearly still out” when it comes to the investigation of brain impacts.
“We don’t know quite what these changes fully correlate to,” said Dr. Liu, who moderated a press briefing highlighting the study.
He underlined the importance of protecting athletes vulnerable to head trauma, be they professionals or those involved at the youth sports level.
Dr. Liu also noted the “extreme popularity” and rapid growth of MMA around the world, which he said provides an opportunity for researchers to study these professional fighters.
“This is a unique population that signed up in the midst of hundreds of hours of sparring to advance neuroscience, and that’s quite amazing,” he said.
A version of this article first appeared on Medscape.com.
, early research suggests.
Investigators found sparring, defined as strategically hitting opponents with kicks, punches, and other strikes during practice sessions, is linked to increased white matter hyperintensities in the brain, pointing to possible vascular damage from repeated head trauma. However, the study results also show sparring was associated with a larger bilateral caudate which, in theory, is neuroprotective.
“From our preliminary study, sparring practice in MMA fighters may have a ‘double-edged sword’ effect on the brain,” study investigator Aaron Esagoff, a second-year medical student at Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“The combination of complex movements along with constant strategy and anticipation of your opponent’s next move may provide a neuroprotective effect on the caudate,” Mr. Esagoff said. However, he added, more research is needed into understanding this particular finding.
The study results were presented at the American Psychiatric Association (APA) 2022 Annual Meeting.
Growing popularity
MMA is a full-contact combat sport that has become increasingly popular over the past 15 years. It combines techniques from boxing, wrestling, karate, judo, and jujitsu.
To prepare for fights, MMA practitioners incorporate sparring and grappling, which use techniques such as chokes and locks to submit an opponent. Head protection is sometimes incorporated during practice, but is not the norm during a fight, said Mr. Esagoff.
The study investigated sparring during practice rather than fights because, he said, MMA competitors only fight a few times a year but spend hundreds of hours training. “So the health effects of training are going to be really important,” he said.
As with other combat sports, MMA involves hits to the head. Previous research has shown repetitive head trauma can lead to neurodegenerative diseases, including chronic traumatic encephalopathy (CTE) and Alzheimer’s disease, Mr. Esagoff noted.
Previous studies have also linked more professional fights and years of fighting to a decrease in brain volume among MMA fighters, he added.
The new analysis was conducted as part of the Professional Fighters Brain Health Study, a longitudinal cohort study of MMA professional fighters. It included 92 fighters with data available on MRI and habits regarding practicing. The mean age of the participants was 30 years, 62% were White, and 85% were men.
The study examined sparring but did not include grappling because of “several challenges” with the current data analysis, Mr. Esagoff said. Researchers adjusted for age, sex, education, race, number of fights, total intracranial volume, and type of MRI scanner used.
A ‘highly strategic’ sport
Results showed a strong association between the number of sparring rounds per week and increased white matter hyperintensity volume (mcL) on MRI (P = .039).
This suggests white matter damage, possibly a result of direct neuronal injury, vascular damage, or immune modulation, said Mr. Esagoff. However, another mechanism may be involved, he added.
There was also a significant association between sparring and increased size of the caudate nucleus, an area of the brain involved in movement, learning, and memory (P = .014 for right caudate volume, P = .012 for left caudate volume).
There are some theories that might explain this finding, said Mr. Esagoff. For example, individuals who spar more may get better at avoiding impacts and injuries during a fight, which might in turn affect the size of the caudate.
The controlled movements and techniques used during sparring could also affect the caudate. “Some research has shown that behavior, learning, and/or exercise may increase the size of certain brain regions,” Mr. Esagoff said.
He noted the “highly strategic” nature of combat sports – and used the example of Brazilian jiu-jitsu. That sport “is known as human chess because it takes a thoughtful approach to defeat a larger opponent with base, leverage, and technique,” he said.
However, Mr. Esagoff stressed that while it is possible movements involved in MMA increase caudate size, this is just a theory at this point.
A study limitation was that fighters volunteered to participate and may not represent all fighters. As well, the study was cross-sectional and looked at only one point in time, so it cannot infer causation.
Overall, the new findings should help inform fighters, governing bodies, and the public about the potential risks and benefits of different styles of MMA fighting and practice, although more research is needed, said Mr. Esagoff.
He and his team now plan to conduct a longer-term study and investigate effects of grappling on brain structure and function in addition to sparring.
Jury still out
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry and incoming chair of the APA’s Council on Communications, said the jury “is clearly still out” when it comes to the investigation of brain impacts.
“We don’t know quite what these changes fully correlate to,” said Dr. Liu, who moderated a press briefing highlighting the study.
He underlined the importance of protecting athletes vulnerable to head trauma, be they professionals or those involved at the youth sports level.
Dr. Liu also noted the “extreme popularity” and rapid growth of MMA around the world, which he said provides an opportunity for researchers to study these professional fighters.
“This is a unique population that signed up in the midst of hundreds of hours of sparring to advance neuroscience, and that’s quite amazing,” he said.
A version of this article first appeared on Medscape.com.
, early research suggests.
Investigators found sparring, defined as strategically hitting opponents with kicks, punches, and other strikes during practice sessions, is linked to increased white matter hyperintensities in the brain, pointing to possible vascular damage from repeated head trauma. However, the study results also show sparring was associated with a larger bilateral caudate which, in theory, is neuroprotective.
“From our preliminary study, sparring practice in MMA fighters may have a ‘double-edged sword’ effect on the brain,” study investigator Aaron Esagoff, a second-year medical student at Johns Hopkins University School of Medicine, Baltimore, told this news organization.
“The combination of complex movements along with constant strategy and anticipation of your opponent’s next move may provide a neuroprotective effect on the caudate,” Mr. Esagoff said. However, he added, more research is needed into understanding this particular finding.
The study results were presented at the American Psychiatric Association (APA) 2022 Annual Meeting.
Growing popularity
MMA is a full-contact combat sport that has become increasingly popular over the past 15 years. It combines techniques from boxing, wrestling, karate, judo, and jujitsu.
To prepare for fights, MMA practitioners incorporate sparring and grappling, which use techniques such as chokes and locks to submit an opponent. Head protection is sometimes incorporated during practice, but is not the norm during a fight, said Mr. Esagoff.
The study investigated sparring during practice rather than fights because, he said, MMA competitors only fight a few times a year but spend hundreds of hours training. “So the health effects of training are going to be really important,” he said.
As with other combat sports, MMA involves hits to the head. Previous research has shown repetitive head trauma can lead to neurodegenerative diseases, including chronic traumatic encephalopathy (CTE) and Alzheimer’s disease, Mr. Esagoff noted.
Previous studies have also linked more professional fights and years of fighting to a decrease in brain volume among MMA fighters, he added.
The new analysis was conducted as part of the Professional Fighters Brain Health Study, a longitudinal cohort study of MMA professional fighters. It included 92 fighters with data available on MRI and habits regarding practicing. The mean age of the participants was 30 years, 62% were White, and 85% were men.
The study examined sparring but did not include grappling because of “several challenges” with the current data analysis, Mr. Esagoff said. Researchers adjusted for age, sex, education, race, number of fights, total intracranial volume, and type of MRI scanner used.
A ‘highly strategic’ sport
Results showed a strong association between the number of sparring rounds per week and increased white matter hyperintensity volume (mcL) on MRI (P = .039).
This suggests white matter damage, possibly a result of direct neuronal injury, vascular damage, or immune modulation, said Mr. Esagoff. However, another mechanism may be involved, he added.
There was also a significant association between sparring and increased size of the caudate nucleus, an area of the brain involved in movement, learning, and memory (P = .014 for right caudate volume, P = .012 for left caudate volume).
There are some theories that might explain this finding, said Mr. Esagoff. For example, individuals who spar more may get better at avoiding impacts and injuries during a fight, which might in turn affect the size of the caudate.
The controlled movements and techniques used during sparring could also affect the caudate. “Some research has shown that behavior, learning, and/or exercise may increase the size of certain brain regions,” Mr. Esagoff said.
He noted the “highly strategic” nature of combat sports – and used the example of Brazilian jiu-jitsu. That sport “is known as human chess because it takes a thoughtful approach to defeat a larger opponent with base, leverage, and technique,” he said.
However, Mr. Esagoff stressed that while it is possible movements involved in MMA increase caudate size, this is just a theory at this point.
A study limitation was that fighters volunteered to participate and may not represent all fighters. As well, the study was cross-sectional and looked at only one point in time, so it cannot infer causation.
Overall, the new findings should help inform fighters, governing bodies, and the public about the potential risks and benefits of different styles of MMA fighting and practice, although more research is needed, said Mr. Esagoff.
He and his team now plan to conduct a longer-term study and investigate effects of grappling on brain structure and function in addition to sparring.
Jury still out
Commenting on the study, Howard Liu, MD, chair of the University of Nebraska Medical Center department of psychiatry and incoming chair of the APA’s Council on Communications, said the jury “is clearly still out” when it comes to the investigation of brain impacts.
“We don’t know quite what these changes fully correlate to,” said Dr. Liu, who moderated a press briefing highlighting the study.
He underlined the importance of protecting athletes vulnerable to head trauma, be they professionals or those involved at the youth sports level.
Dr. Liu also noted the “extreme popularity” and rapid growth of MMA around the world, which he said provides an opportunity for researchers to study these professional fighters.
“This is a unique population that signed up in the midst of hundreds of hours of sparring to advance neuroscience, and that’s quite amazing,” he said.
A version of this article first appeared on Medscape.com.
FROM APA 2022
FDA allows import of 2 million cans of baby formula from U.K.
The U.S. Food and Drug Administration is easing rules to allow infant formula imports from the United Kingdom, which would bring about 2 million cans to the U.S. in coming weeks.
Kendal Nutricare will be able to offer certain infant formula products under the Kendamil brand to ease the nationwide formula shortage.
“Importantly, we anticipate additional infant formula products may be safely and quickly imported in the U.S. in the near-term, based on ongoing discussions with manufacturers and suppliers worldwide,” Robert Califf, MD, the FDA commissioner, said in a statement.
Kendal Nutricare has more than 40,000 cans in stock for immediate dispatch, the FDA said, and the U.S. Department of Health and Human Services is talking to the company about the best ways to get the products to the U.S. as quickly as possible.
Kendamil has set up a website for consumers to receive updates and find products once they arrive in the U.S.
After an evaluation, the FDA said it had no safety or nutrition concerns about the products. The evaluation reviewed the company’s microbiological testing, labeling, and information about facility production and inspection history.
On May 24, the FDA announced that Abbott Nutrition will release about 300,000 cans of its EleCare specialty amino acid-based formula to families that need urgent, life-sustaining supplies. The products had more tests for microbes before release.
Although some EleCare products were included in Abbott’s infant formula recall earlier this year, the cans that will be released were in different lots, have never been released, and have been maintained in storage, the FDA said.
“These EleCare product lots were not part of the recall but have been on hold due to concerns that they were produced under unsanitary conditions observed at Abbott Nutrition’s Sturgis, Michigan, facility,” the FDA wrote.
The FDA encourages parents and caregivers to talk with their health care providers to weigh the potential risk of bacterial infection with the critical need for the product, based on its special dietary formulation for infants with severe food allergies or gut disorders.
The FDA also said that Abbott confirmed the EleCare products will be the first formula produced at the Sturgis facility when it restarts production soon. Other specialty metabolic formulas will follow.
Abbott plans to restart production at the Sturgis facility on June 4, the company said in a statement, noting that the early batches of EleCare would be available to consumers around June 20.
The products being released now are EleCare (for infants under 1 year) and EleCare Jr. (for ages 1 and older). Those who want to request products should contact their health care providers or call Abbott directly at 800-881-0876.
A version of this article first appeared on WebMD.com.
The U.S. Food and Drug Administration is easing rules to allow infant formula imports from the United Kingdom, which would bring about 2 million cans to the U.S. in coming weeks.
Kendal Nutricare will be able to offer certain infant formula products under the Kendamil brand to ease the nationwide formula shortage.
“Importantly, we anticipate additional infant formula products may be safely and quickly imported in the U.S. in the near-term, based on ongoing discussions with manufacturers and suppliers worldwide,” Robert Califf, MD, the FDA commissioner, said in a statement.
Kendal Nutricare has more than 40,000 cans in stock for immediate dispatch, the FDA said, and the U.S. Department of Health and Human Services is talking to the company about the best ways to get the products to the U.S. as quickly as possible.
Kendamil has set up a website for consumers to receive updates and find products once they arrive in the U.S.
After an evaluation, the FDA said it had no safety or nutrition concerns about the products. The evaluation reviewed the company’s microbiological testing, labeling, and information about facility production and inspection history.
On May 24, the FDA announced that Abbott Nutrition will release about 300,000 cans of its EleCare specialty amino acid-based formula to families that need urgent, life-sustaining supplies. The products had more tests for microbes before release.
Although some EleCare products were included in Abbott’s infant formula recall earlier this year, the cans that will be released were in different lots, have never been released, and have been maintained in storage, the FDA said.
“These EleCare product lots were not part of the recall but have been on hold due to concerns that they were produced under unsanitary conditions observed at Abbott Nutrition’s Sturgis, Michigan, facility,” the FDA wrote.
The FDA encourages parents and caregivers to talk with their health care providers to weigh the potential risk of bacterial infection with the critical need for the product, based on its special dietary formulation for infants with severe food allergies or gut disorders.
The FDA also said that Abbott confirmed the EleCare products will be the first formula produced at the Sturgis facility when it restarts production soon. Other specialty metabolic formulas will follow.
Abbott plans to restart production at the Sturgis facility on June 4, the company said in a statement, noting that the early batches of EleCare would be available to consumers around June 20.
The products being released now are EleCare (for infants under 1 year) and EleCare Jr. (for ages 1 and older). Those who want to request products should contact their health care providers or call Abbott directly at 800-881-0876.
A version of this article first appeared on WebMD.com.
The U.S. Food and Drug Administration is easing rules to allow infant formula imports from the United Kingdom, which would bring about 2 million cans to the U.S. in coming weeks.
Kendal Nutricare will be able to offer certain infant formula products under the Kendamil brand to ease the nationwide formula shortage.
“Importantly, we anticipate additional infant formula products may be safely and quickly imported in the U.S. in the near-term, based on ongoing discussions with manufacturers and suppliers worldwide,” Robert Califf, MD, the FDA commissioner, said in a statement.
Kendal Nutricare has more than 40,000 cans in stock for immediate dispatch, the FDA said, and the U.S. Department of Health and Human Services is talking to the company about the best ways to get the products to the U.S. as quickly as possible.
Kendamil has set up a website for consumers to receive updates and find products once they arrive in the U.S.
After an evaluation, the FDA said it had no safety or nutrition concerns about the products. The evaluation reviewed the company’s microbiological testing, labeling, and information about facility production and inspection history.
On May 24, the FDA announced that Abbott Nutrition will release about 300,000 cans of its EleCare specialty amino acid-based formula to families that need urgent, life-sustaining supplies. The products had more tests for microbes before release.
Although some EleCare products were included in Abbott’s infant formula recall earlier this year, the cans that will be released were in different lots, have never been released, and have been maintained in storage, the FDA said.
“These EleCare product lots were not part of the recall but have been on hold due to concerns that they were produced under unsanitary conditions observed at Abbott Nutrition’s Sturgis, Michigan, facility,” the FDA wrote.
The FDA encourages parents and caregivers to talk with their health care providers to weigh the potential risk of bacterial infection with the critical need for the product, based on its special dietary formulation for infants with severe food allergies or gut disorders.
The FDA also said that Abbott confirmed the EleCare products will be the first formula produced at the Sturgis facility when it restarts production soon. Other specialty metabolic formulas will follow.
Abbott plans to restart production at the Sturgis facility on June 4, the company said in a statement, noting that the early batches of EleCare would be available to consumers around June 20.
The products being released now are EleCare (for infants under 1 year) and EleCare Jr. (for ages 1 and older). Those who want to request products should contact their health care providers or call Abbott directly at 800-881-0876.
A version of this article first appeared on WebMD.com.
Does taking isotretinoin worsen a patient’s baseline IBD symptoms?
A , results from a small retrospective study suggests.
“Early studies of isotretinoin for use in severe acne suggested the drug may serve as a trigger for new-onset inflammatory bowel disease (IBD),” researchers led by Christina G. Lopez, MD, of the Lewis Katz School of Medicine at Temple University, Philadelphia, wrote in an article published online , in the Journal of the American Academy of Dermatology. “While more recent studies have suggested no such causal relationship, little is known about the medication’s effect on patients with a preexisting IBD diagnosis.”
To investigate this topic further, the researchers identified 19 patients who were diagnosed with IBD and treated with isotretinoin between Jan. 1, 2006, and Jan. 1, 2020, at Mass General Brigham Hospitals, Boston. They determined severity of disease and degree of antecedent management of IBD by evaluating flaring two years prior to starting isotretinoin. The patients were considered to have a flare caused by isotretinoin if the IBD flare occurred during or up to 3 months following course completion.
The mean age of the 19 patients was 35 years, 26% were female, and 95% were White. Nearly half of the patients (42%) had ulcerative colitis, 37% had Crohn’s disease, and 21% had both. The researchers found that nine patients had flared two years before starting isotretinoin. Of these, five (56%) flared and four (44%) did not flare during treatment or within three months of completing the course of isotretinoin.
Of the 10 patients who did not flare two years before starting isotretinoin, seven (70%) did not flare during treatment and three (30%) flared during or within three months following completion of isotretinoin use. The researchers found no statistically significant association between isotretinoin use and flaring among patients with IBD (P = .76).
Dr. Lopez and her colleagues also assessed IBD maintenance therapy with respect to IBD flares in the study population. They observed no statistically significant association between the use of maintenance IBD therapy and the likelihood of having flares during isotretinoin treatment (P = .15).
“The results suggest limited association between isotretinoin and the worsening of a patient’s baseline IBD,” the authors concluded. They acknowledged certain limitations of the study, including its small sample size and retrospective design, and they called for larger and prospective studies to assess the relationship of IBD flaring in this population of patients.
Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, who was asked to comment on the results, characterized the trial as “an important study highlighting how we continue to understand the safe use of isotretinoin in the IBD cohort.”
Isotretinoin, she added, “continues to be a highly important treatment for acne and in patients such as these where oral antibiotics are relatively contraindicated due to risk of exacerbating their bowel disease.” Such data are reassuring, “albeit future studies with larger patient pools are desirable,” she added. “Future studies could also help to elucidate if diet, smoking, sleep, exercise, and medication adherence are potential confounding factors along with whether the cumulative isotretinoin dose has any effect on IBD flares in those who are susceptible.”
Neither the researchers nor Dr. Sodha had financial conflicts. The other authors were from Brigham and Women’s Hospital, Harvard University, Boston, and the University of Massachusetts, Worcester.
A , results from a small retrospective study suggests.
“Early studies of isotretinoin for use in severe acne suggested the drug may serve as a trigger for new-onset inflammatory bowel disease (IBD),” researchers led by Christina G. Lopez, MD, of the Lewis Katz School of Medicine at Temple University, Philadelphia, wrote in an article published online , in the Journal of the American Academy of Dermatology. “While more recent studies have suggested no such causal relationship, little is known about the medication’s effect on patients with a preexisting IBD diagnosis.”
To investigate this topic further, the researchers identified 19 patients who were diagnosed with IBD and treated with isotretinoin between Jan. 1, 2006, and Jan. 1, 2020, at Mass General Brigham Hospitals, Boston. They determined severity of disease and degree of antecedent management of IBD by evaluating flaring two years prior to starting isotretinoin. The patients were considered to have a flare caused by isotretinoin if the IBD flare occurred during or up to 3 months following course completion.
The mean age of the 19 patients was 35 years, 26% were female, and 95% were White. Nearly half of the patients (42%) had ulcerative colitis, 37% had Crohn’s disease, and 21% had both. The researchers found that nine patients had flared two years before starting isotretinoin. Of these, five (56%) flared and four (44%) did not flare during treatment or within three months of completing the course of isotretinoin.
Of the 10 patients who did not flare two years before starting isotretinoin, seven (70%) did not flare during treatment and three (30%) flared during or within three months following completion of isotretinoin use. The researchers found no statistically significant association between isotretinoin use and flaring among patients with IBD (P = .76).
Dr. Lopez and her colleagues also assessed IBD maintenance therapy with respect to IBD flares in the study population. They observed no statistically significant association between the use of maintenance IBD therapy and the likelihood of having flares during isotretinoin treatment (P = .15).
“The results suggest limited association between isotretinoin and the worsening of a patient’s baseline IBD,” the authors concluded. They acknowledged certain limitations of the study, including its small sample size and retrospective design, and they called for larger and prospective studies to assess the relationship of IBD flaring in this population of patients.
Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, who was asked to comment on the results, characterized the trial as “an important study highlighting how we continue to understand the safe use of isotretinoin in the IBD cohort.”
Isotretinoin, she added, “continues to be a highly important treatment for acne and in patients such as these where oral antibiotics are relatively contraindicated due to risk of exacerbating their bowel disease.” Such data are reassuring, “albeit future studies with larger patient pools are desirable,” she added. “Future studies could also help to elucidate if diet, smoking, sleep, exercise, and medication adherence are potential confounding factors along with whether the cumulative isotretinoin dose has any effect on IBD flares in those who are susceptible.”
Neither the researchers nor Dr. Sodha had financial conflicts. The other authors were from Brigham and Women’s Hospital, Harvard University, Boston, and the University of Massachusetts, Worcester.
A , results from a small retrospective study suggests.
“Early studies of isotretinoin for use in severe acne suggested the drug may serve as a trigger for new-onset inflammatory bowel disease (IBD),” researchers led by Christina G. Lopez, MD, of the Lewis Katz School of Medicine at Temple University, Philadelphia, wrote in an article published online , in the Journal of the American Academy of Dermatology. “While more recent studies have suggested no such causal relationship, little is known about the medication’s effect on patients with a preexisting IBD diagnosis.”
To investigate this topic further, the researchers identified 19 patients who were diagnosed with IBD and treated with isotretinoin between Jan. 1, 2006, and Jan. 1, 2020, at Mass General Brigham Hospitals, Boston. They determined severity of disease and degree of antecedent management of IBD by evaluating flaring two years prior to starting isotretinoin. The patients were considered to have a flare caused by isotretinoin if the IBD flare occurred during or up to 3 months following course completion.
The mean age of the 19 patients was 35 years, 26% were female, and 95% were White. Nearly half of the patients (42%) had ulcerative colitis, 37% had Crohn’s disease, and 21% had both. The researchers found that nine patients had flared two years before starting isotretinoin. Of these, five (56%) flared and four (44%) did not flare during treatment or within three months of completing the course of isotretinoin.
Of the 10 patients who did not flare two years before starting isotretinoin, seven (70%) did not flare during treatment and three (30%) flared during or within three months following completion of isotretinoin use. The researchers found no statistically significant association between isotretinoin use and flaring among patients with IBD (P = .76).
Dr. Lopez and her colleagues also assessed IBD maintenance therapy with respect to IBD flares in the study population. They observed no statistically significant association between the use of maintenance IBD therapy and the likelihood of having flares during isotretinoin treatment (P = .15).
“The results suggest limited association between isotretinoin and the worsening of a patient’s baseline IBD,” the authors concluded. They acknowledged certain limitations of the study, including its small sample size and retrospective design, and they called for larger and prospective studies to assess the relationship of IBD flaring in this population of patients.
Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, who was asked to comment on the results, characterized the trial as “an important study highlighting how we continue to understand the safe use of isotretinoin in the IBD cohort.”
Isotretinoin, she added, “continues to be a highly important treatment for acne and in patients such as these where oral antibiotics are relatively contraindicated due to risk of exacerbating their bowel disease.” Such data are reassuring, “albeit future studies with larger patient pools are desirable,” she added. “Future studies could also help to elucidate if diet, smoking, sleep, exercise, and medication adherence are potential confounding factors along with whether the cumulative isotretinoin dose has any effect on IBD flares in those who are susceptible.”
Neither the researchers nor Dr. Sodha had financial conflicts. The other authors were from Brigham and Women’s Hospital, Harvard University, Boston, and the University of Massachusetts, Worcester.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Childhood survivors of gun violence: What’s the long-term outlook?
As the parents of the 19 children shot dead Tuesday in Uvalde, Tex., by a teen gunman grapple with unspeakable grief and funeral preparations, the survivors and their families are dealing with their own angst and likely much more.
While the parents understandably feel lucky that their children made it out, what about the long-term effect on their children of witnessing that carnage, of seeing classmates, friends, and teachers die violently as they stood by helpless and fearful?
The outcome over the next few days, months, and years depends on many factors, but how parents address the trauma both immediately and long-term can make a huge difference, experts say.
Posttraumatic growth
Best long-term case scenario? Survivors can experience what experts call posttraumatic growth – reaching out to give back to society, to make the world a better place, and changing who they are and their view of the world.
A prime example of posttraumatic growth: A month after a teen gunman killed 17 students at Marjory Stoneman Douglas High School in Parkland, Fla., on Valentine’s Day 2018, an army of survivors from that day’s bloodbath headed to Washington, D.C., for the now-famous March for Our Lives. The student-led demonstration, with hundreds of thousands of supporters marching, called for gun control legislation and an end to gun violence. It remains a vibrant, nonprofit organization still advocating for universal background checks and increased support of mental health services.
No sign of future violence
While most children and teens who witness school violence won’t become high-profile activists, as survivors of Parkland and the numerous other school shootings have, neither will they become the next active shooter, mental health experts say. They can’t point to a study that follows the gun violence victims that shows who does OK and who doesn’t, but they know immediate support and therapy can go a long way to recovery.
“I can’t tell you how any particular child will do,” says Robin Gurwitch, PhD, psychologist and professor at Duke University Medical Center, Durham, N.C. “I can tell you the majority of kids will be OK.”
However, that doesn’t mean a surviving child won’t have behavior and other issues, she says. Research does suggest the next few days, weeks, or months will be rough.
What parents and other caretakers do in the days after the violence will help predict the long-term outcome. Dr. Gurwitch and other experts say it’s important to first focus on what they call “psychological first aid,” then phase in therapy such as trauma-focused cognitive behavioral therapy, if and when it’s needed.
First, ‘psychological first aid’
“Psychological first aid is designed to minimize the impact down the road,” Dr. Gurwitch says. “Validate that they are feeling scared or worried.”
Some may be angry, another understandable emotion. In the first few days of witnessing violence – or even just hearing about it – parents should expect clinginess, sleep problems, behavior meltdowns, and irritability, she says.
“Those kinds of changes are likely to last a few weeks,” she says.
If day-to-day functioning is very difficult, “don’t wait for those to pass,” Dr. Gurwitch says. “Reach out for help. Resources will be available. Check with your pediatrician or family physician.”
At home, parents can address specific problems related to the experience, Dr. Gurwitch says. If it’s sleep, she says, parents and kids can work together to figure out how to ease sleep, such as listening to their favorite music before bedtime.
While parents may be inclined to baby the kids after the violence, Dr. Gurwitch says it’s important to maintain routines. So it’s not cruel to insist they do their chores.
Expect change
Things won’t be the same.
“Anytime we go through a particular traumatic event, we are changed,” Dr. Gurwitch says. ‘’The question is, what do we do about it? How do we incorporate that change into who we are and have become?”
Also important is figuring out how to make meaning out of what happened.
“I am so impressed by the families at Sandy Hook (the Connecticut elementary school where a gunman killed 26 in 2012),” she says.
They set up foundations and did other advocacy work.
“These types of events are life-changing events,” agrees David Schonfeld, MD, a pediatrician and director of the National Center for Schools Crisis and Bereavement at Children’s Hospital Los Angeles, California. “They will change who children are as people, but it doesn’t mean they are damaged for life. They will remember it as long as they live, and it will also change who they are as a person.”
While people tend to stress the potential negative effects – and there certainly are some – ‘’some individuals actually emerge from these events with a renewed sense of purpose.’’
He tells parents: “Yes, your child has changed, and you can’t go back. But it doesn’t mean they are destined to never be able to cope [with trauma].”
Research
The effects of gun violence on children can be serious and dramatic, research shows.
- Exposure to neighborhood gun violence is linked with an increase in children’s mental health issues, have found. Children living within two or three blocks of gun violence had nearly twice the risk of going to the emergency department with a mental health complaint in the 14 days following the shooting.
- Exposure to gun violence should be classified, along with maltreatment, household dysfunction, and other issues known to impact children negatively, as an adverse childhood experience, other experts
- Direct gun violence exposure, witnessing it, and hearing gunshots are all associated with children being victimized in other ways, another found. And that poly-victimization, as it is called, was strongly associated with having posttraumatic symptoms.
Adverse Childhood Events, as these sorts of experiences are known, can have long-lasting effects on physical and mental health, as well as on even the economic future of a person, says Hansa Bhargava, MD, a pediatrician and chief medical officer of Medscape, WebMD’s sister site for medical professionals.
“Kids who have suffered through violent events can have brain development affected, as well as their immune systems,” she says. “They are more likely to have chronic disease, substance use disorder, sexually transmitted diseases, teen pregnancy, and lifelong depression. A high risk of [posttraumatic stress disorder] is likely for them and their families.”
The impact of family support
The gun violence and deaths are likely to remind children of other losses they have experienced, Dr. Schonfeld says, and that can make coping more difficult.
If the trauma from the Tuesday shootings is ‘’layered” on top of trauma from COVID-19 deaths or other trauma such as domestic violence, those children may have a more difficult time, says Allan Chrisman, MD, professor emeritus of psychiatry and behavioral sciences at Duke University Health System. However, protective factors such as the family response and the community response can build resilience in survivors, he says.
“The way in which parents handle it for themselves will have a huge impact on the kids,” Dr. Chrisman says. “The worst outcomes are linked with [parents saying], ‘We don’t want to talk about it.’ ”
The parents are understandably upset, Dr. Gurwitch says. It’s OK to show sadness, anger, and other emotions, but she tells parents: “It’s not OK to completely decompose.” It’s important for the children to see that parents can pull themselves together.
Longer-term effects
As time goes on, ‘’a very large percentage will have posttraumatic reactions,” Dr. Schonfeld says. “Those reactions tend to improve over time.”
While people talk about PTSD directly after an incident such as a school shooting, it isn’t officially diagnosed as PTSD until the symptoms describing PTSD have persisted for a month, Dr. Schonfeld says. However, ‘’that doesn’t mean you don’t have a problem” that needs attention from a mental health professional.
“As a country we are already struggling with a mental health crisis,” Dr. Bhargava says. “Events such as this serve to exacerbate even more crisis in a group of innocent children whose only crime was to attend school. We must address the ‘epidemic’ of gun violence and school shootings head on. For the sake of our children and their health. For all of us.”
Therapy that works
Cognitive behavioral therapy (CBT) approaches are effective in reducing the trauma, Dr. Gurwitch says.
She often recommends one type of CBT, called trauma-focused cognitive behavioral therapy. This approach involves children and parents and focuses on safety, coping skills, and gradual exposure. It’s a structured and short-term treatment of about eight to 25 sessions.
A version of this article first appeared on Medscape.com.
As the parents of the 19 children shot dead Tuesday in Uvalde, Tex., by a teen gunman grapple with unspeakable grief and funeral preparations, the survivors and their families are dealing with their own angst and likely much more.
While the parents understandably feel lucky that their children made it out, what about the long-term effect on their children of witnessing that carnage, of seeing classmates, friends, and teachers die violently as they stood by helpless and fearful?
The outcome over the next few days, months, and years depends on many factors, but how parents address the trauma both immediately and long-term can make a huge difference, experts say.
Posttraumatic growth
Best long-term case scenario? Survivors can experience what experts call posttraumatic growth – reaching out to give back to society, to make the world a better place, and changing who they are and their view of the world.
A prime example of posttraumatic growth: A month after a teen gunman killed 17 students at Marjory Stoneman Douglas High School in Parkland, Fla., on Valentine’s Day 2018, an army of survivors from that day’s bloodbath headed to Washington, D.C., for the now-famous March for Our Lives. The student-led demonstration, with hundreds of thousands of supporters marching, called for gun control legislation and an end to gun violence. It remains a vibrant, nonprofit organization still advocating for universal background checks and increased support of mental health services.
No sign of future violence
While most children and teens who witness school violence won’t become high-profile activists, as survivors of Parkland and the numerous other school shootings have, neither will they become the next active shooter, mental health experts say. They can’t point to a study that follows the gun violence victims that shows who does OK and who doesn’t, but they know immediate support and therapy can go a long way to recovery.
“I can’t tell you how any particular child will do,” says Robin Gurwitch, PhD, psychologist and professor at Duke University Medical Center, Durham, N.C. “I can tell you the majority of kids will be OK.”
However, that doesn’t mean a surviving child won’t have behavior and other issues, she says. Research does suggest the next few days, weeks, or months will be rough.
What parents and other caretakers do in the days after the violence will help predict the long-term outcome. Dr. Gurwitch and other experts say it’s important to first focus on what they call “psychological first aid,” then phase in therapy such as trauma-focused cognitive behavioral therapy, if and when it’s needed.
First, ‘psychological first aid’
“Psychological first aid is designed to minimize the impact down the road,” Dr. Gurwitch says. “Validate that they are feeling scared or worried.”
Some may be angry, another understandable emotion. In the first few days of witnessing violence – or even just hearing about it – parents should expect clinginess, sleep problems, behavior meltdowns, and irritability, she says.
“Those kinds of changes are likely to last a few weeks,” she says.
If day-to-day functioning is very difficult, “don’t wait for those to pass,” Dr. Gurwitch says. “Reach out for help. Resources will be available. Check with your pediatrician or family physician.”
At home, parents can address specific problems related to the experience, Dr. Gurwitch says. If it’s sleep, she says, parents and kids can work together to figure out how to ease sleep, such as listening to their favorite music before bedtime.
While parents may be inclined to baby the kids after the violence, Dr. Gurwitch says it’s important to maintain routines. So it’s not cruel to insist they do their chores.
Expect change
Things won’t be the same.
“Anytime we go through a particular traumatic event, we are changed,” Dr. Gurwitch says. ‘’The question is, what do we do about it? How do we incorporate that change into who we are and have become?”
Also important is figuring out how to make meaning out of what happened.
“I am so impressed by the families at Sandy Hook (the Connecticut elementary school where a gunman killed 26 in 2012),” she says.
They set up foundations and did other advocacy work.
“These types of events are life-changing events,” agrees David Schonfeld, MD, a pediatrician and director of the National Center for Schools Crisis and Bereavement at Children’s Hospital Los Angeles, California. “They will change who children are as people, but it doesn’t mean they are damaged for life. They will remember it as long as they live, and it will also change who they are as a person.”
While people tend to stress the potential negative effects – and there certainly are some – ‘’some individuals actually emerge from these events with a renewed sense of purpose.’’
He tells parents: “Yes, your child has changed, and you can’t go back. But it doesn’t mean they are destined to never be able to cope [with trauma].”
Research
The effects of gun violence on children can be serious and dramatic, research shows.
- Exposure to neighborhood gun violence is linked with an increase in children’s mental health issues, have found. Children living within two or three blocks of gun violence had nearly twice the risk of going to the emergency department with a mental health complaint in the 14 days following the shooting.
- Exposure to gun violence should be classified, along with maltreatment, household dysfunction, and other issues known to impact children negatively, as an adverse childhood experience, other experts
- Direct gun violence exposure, witnessing it, and hearing gunshots are all associated with children being victimized in other ways, another found. And that poly-victimization, as it is called, was strongly associated with having posttraumatic symptoms.
Adverse Childhood Events, as these sorts of experiences are known, can have long-lasting effects on physical and mental health, as well as on even the economic future of a person, says Hansa Bhargava, MD, a pediatrician and chief medical officer of Medscape, WebMD’s sister site for medical professionals.
“Kids who have suffered through violent events can have brain development affected, as well as their immune systems,” she says. “They are more likely to have chronic disease, substance use disorder, sexually transmitted diseases, teen pregnancy, and lifelong depression. A high risk of [posttraumatic stress disorder] is likely for them and their families.”
The impact of family support
The gun violence and deaths are likely to remind children of other losses they have experienced, Dr. Schonfeld says, and that can make coping more difficult.
If the trauma from the Tuesday shootings is ‘’layered” on top of trauma from COVID-19 deaths or other trauma such as domestic violence, those children may have a more difficult time, says Allan Chrisman, MD, professor emeritus of psychiatry and behavioral sciences at Duke University Health System. However, protective factors such as the family response and the community response can build resilience in survivors, he says.
“The way in which parents handle it for themselves will have a huge impact on the kids,” Dr. Chrisman says. “The worst outcomes are linked with [parents saying], ‘We don’t want to talk about it.’ ”
The parents are understandably upset, Dr. Gurwitch says. It’s OK to show sadness, anger, and other emotions, but she tells parents: “It’s not OK to completely decompose.” It’s important for the children to see that parents can pull themselves together.
Longer-term effects
As time goes on, ‘’a very large percentage will have posttraumatic reactions,” Dr. Schonfeld says. “Those reactions tend to improve over time.”
While people talk about PTSD directly after an incident such as a school shooting, it isn’t officially diagnosed as PTSD until the symptoms describing PTSD have persisted for a month, Dr. Schonfeld says. However, ‘’that doesn’t mean you don’t have a problem” that needs attention from a mental health professional.
“As a country we are already struggling with a mental health crisis,” Dr. Bhargava says. “Events such as this serve to exacerbate even more crisis in a group of innocent children whose only crime was to attend school. We must address the ‘epidemic’ of gun violence and school shootings head on. For the sake of our children and their health. For all of us.”
Therapy that works
Cognitive behavioral therapy (CBT) approaches are effective in reducing the trauma, Dr. Gurwitch says.
She often recommends one type of CBT, called trauma-focused cognitive behavioral therapy. This approach involves children and parents and focuses on safety, coping skills, and gradual exposure. It’s a structured and short-term treatment of about eight to 25 sessions.
A version of this article first appeared on Medscape.com.
As the parents of the 19 children shot dead Tuesday in Uvalde, Tex., by a teen gunman grapple with unspeakable grief and funeral preparations, the survivors and their families are dealing with their own angst and likely much more.
While the parents understandably feel lucky that their children made it out, what about the long-term effect on their children of witnessing that carnage, of seeing classmates, friends, and teachers die violently as they stood by helpless and fearful?
The outcome over the next few days, months, and years depends on many factors, but how parents address the trauma both immediately and long-term can make a huge difference, experts say.
Posttraumatic growth
Best long-term case scenario? Survivors can experience what experts call posttraumatic growth – reaching out to give back to society, to make the world a better place, and changing who they are and their view of the world.
A prime example of posttraumatic growth: A month after a teen gunman killed 17 students at Marjory Stoneman Douglas High School in Parkland, Fla., on Valentine’s Day 2018, an army of survivors from that day’s bloodbath headed to Washington, D.C., for the now-famous March for Our Lives. The student-led demonstration, with hundreds of thousands of supporters marching, called for gun control legislation and an end to gun violence. It remains a vibrant, nonprofit organization still advocating for universal background checks and increased support of mental health services.
No sign of future violence
While most children and teens who witness school violence won’t become high-profile activists, as survivors of Parkland and the numerous other school shootings have, neither will they become the next active shooter, mental health experts say. They can’t point to a study that follows the gun violence victims that shows who does OK and who doesn’t, but they know immediate support and therapy can go a long way to recovery.
“I can’t tell you how any particular child will do,” says Robin Gurwitch, PhD, psychologist and professor at Duke University Medical Center, Durham, N.C. “I can tell you the majority of kids will be OK.”
However, that doesn’t mean a surviving child won’t have behavior and other issues, she says. Research does suggest the next few days, weeks, or months will be rough.
What parents and other caretakers do in the days after the violence will help predict the long-term outcome. Dr. Gurwitch and other experts say it’s important to first focus on what they call “psychological first aid,” then phase in therapy such as trauma-focused cognitive behavioral therapy, if and when it’s needed.
First, ‘psychological first aid’
“Psychological first aid is designed to minimize the impact down the road,” Dr. Gurwitch says. “Validate that they are feeling scared or worried.”
Some may be angry, another understandable emotion. In the first few days of witnessing violence – or even just hearing about it – parents should expect clinginess, sleep problems, behavior meltdowns, and irritability, she says.
“Those kinds of changes are likely to last a few weeks,” she says.
If day-to-day functioning is very difficult, “don’t wait for those to pass,” Dr. Gurwitch says. “Reach out for help. Resources will be available. Check with your pediatrician or family physician.”
At home, parents can address specific problems related to the experience, Dr. Gurwitch says. If it’s sleep, she says, parents and kids can work together to figure out how to ease sleep, such as listening to their favorite music before bedtime.
While parents may be inclined to baby the kids after the violence, Dr. Gurwitch says it’s important to maintain routines. So it’s not cruel to insist they do their chores.
Expect change
Things won’t be the same.
“Anytime we go through a particular traumatic event, we are changed,” Dr. Gurwitch says. ‘’The question is, what do we do about it? How do we incorporate that change into who we are and have become?”
Also important is figuring out how to make meaning out of what happened.
“I am so impressed by the families at Sandy Hook (the Connecticut elementary school where a gunman killed 26 in 2012),” she says.
They set up foundations and did other advocacy work.
“These types of events are life-changing events,” agrees David Schonfeld, MD, a pediatrician and director of the National Center for Schools Crisis and Bereavement at Children’s Hospital Los Angeles, California. “They will change who children are as people, but it doesn’t mean they are damaged for life. They will remember it as long as they live, and it will also change who they are as a person.”
While people tend to stress the potential negative effects – and there certainly are some – ‘’some individuals actually emerge from these events with a renewed sense of purpose.’’
He tells parents: “Yes, your child has changed, and you can’t go back. But it doesn’t mean they are destined to never be able to cope [with trauma].”
Research
The effects of gun violence on children can be serious and dramatic, research shows.
- Exposure to neighborhood gun violence is linked with an increase in children’s mental health issues, have found. Children living within two or three blocks of gun violence had nearly twice the risk of going to the emergency department with a mental health complaint in the 14 days following the shooting.
- Exposure to gun violence should be classified, along with maltreatment, household dysfunction, and other issues known to impact children negatively, as an adverse childhood experience, other experts
- Direct gun violence exposure, witnessing it, and hearing gunshots are all associated with children being victimized in other ways, another found. And that poly-victimization, as it is called, was strongly associated with having posttraumatic symptoms.
Adverse Childhood Events, as these sorts of experiences are known, can have long-lasting effects on physical and mental health, as well as on even the economic future of a person, says Hansa Bhargava, MD, a pediatrician and chief medical officer of Medscape, WebMD’s sister site for medical professionals.
“Kids who have suffered through violent events can have brain development affected, as well as their immune systems,” she says. “They are more likely to have chronic disease, substance use disorder, sexually transmitted diseases, teen pregnancy, and lifelong depression. A high risk of [posttraumatic stress disorder] is likely for them and their families.”
The impact of family support
The gun violence and deaths are likely to remind children of other losses they have experienced, Dr. Schonfeld says, and that can make coping more difficult.
If the trauma from the Tuesday shootings is ‘’layered” on top of trauma from COVID-19 deaths or other trauma such as domestic violence, those children may have a more difficult time, says Allan Chrisman, MD, professor emeritus of psychiatry and behavioral sciences at Duke University Health System. However, protective factors such as the family response and the community response can build resilience in survivors, he says.
“The way in which parents handle it for themselves will have a huge impact on the kids,” Dr. Chrisman says. “The worst outcomes are linked with [parents saying], ‘We don’t want to talk about it.’ ”
The parents are understandably upset, Dr. Gurwitch says. It’s OK to show sadness, anger, and other emotions, but she tells parents: “It’s not OK to completely decompose.” It’s important for the children to see that parents can pull themselves together.
Longer-term effects
As time goes on, ‘’a very large percentage will have posttraumatic reactions,” Dr. Schonfeld says. “Those reactions tend to improve over time.”
While people talk about PTSD directly after an incident such as a school shooting, it isn’t officially diagnosed as PTSD until the symptoms describing PTSD have persisted for a month, Dr. Schonfeld says. However, ‘’that doesn’t mean you don’t have a problem” that needs attention from a mental health professional.
“As a country we are already struggling with a mental health crisis,” Dr. Bhargava says. “Events such as this serve to exacerbate even more crisis in a group of innocent children whose only crime was to attend school. We must address the ‘epidemic’ of gun violence and school shootings head on. For the sake of our children and their health. For all of us.”
Therapy that works
Cognitive behavioral therapy (CBT) approaches are effective in reducing the trauma, Dr. Gurwitch says.
She often recommends one type of CBT, called trauma-focused cognitive behavioral therapy. This approach involves children and parents and focuses on safety, coping skills, and gradual exposure. It’s a structured and short-term treatment of about eight to 25 sessions.
A version of this article first appeared on Medscape.com.
Urinating multiple times per night
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's history and presentation, this is likely a case of adenocarcinoma of the prostate. Although most patients with prostate cancer are diagnosed on screening, when localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. In some cases, these signs and symptoms may well be related to age-associated prostate enlargement or other conditions; benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA (but because this patient does not report pain, nonbacterial prostatitis is unlikely). Symptomatic patients older than 50 years, such as the one in this case, should be screened for prostate cancer. Those with a PSA > 10 ng/mL are more than 50% likely to have prostate cancer.
National Comprehensive Cancer Network guidelines advise that needle biopsy of the prostate is indicated for tissue diagnosis in those with elevated PSA levels, preferably via a transrectal ultrasound. MRI can be used to assess lesions that are concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density and PSA doubling time should be collected as well. The clinician should ask about high-risk germline mutations and estimate life expectancy because course of treatment is largely based on risk assessment.
Standard treatments for clinically localized prostate cancer include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. Active surveillance is often recommended for those who have very-low-risk disease because of the slow growth of certain types of prostate cancer. Radical prostatectomy is a viable option for any patient with localized disease that can be completely excised surgically, provided the patient has a life expectancy of 10 or more years and no serious comorbidities. In some patients, radical prostatectomy may be followed by radiation with or without a short course of hormone treatment, depending on risk factors for recurrence. Radiation therapy is also potentially curative in localized prostate cancer and may be delivered in the form of external-beam radiation therapy or brachytherapy. For asymptomatic patients who are older and/or have other serious underlying conditions, observation may be recommended.
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: CVICO Medical Solutions.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 62-year-old man presents for routine prostate cancer screening. He notes that he has not been sleeping well as a result of getting up to urinate multiple times per night for the past few months. The patient underwent a prostate cancer screening about 26 months ago, and results were normal. On examination, digital rectal examination is normal, but prostate-specific antigen (PSA) levels are elevated at 10.2 ng/mL.