User login
CDC says about 20% get long COVID. New models try to define it
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
Acute Alopecia Associated With Albendazole Toxicosis
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
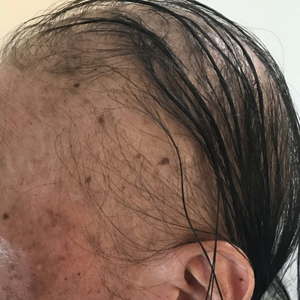
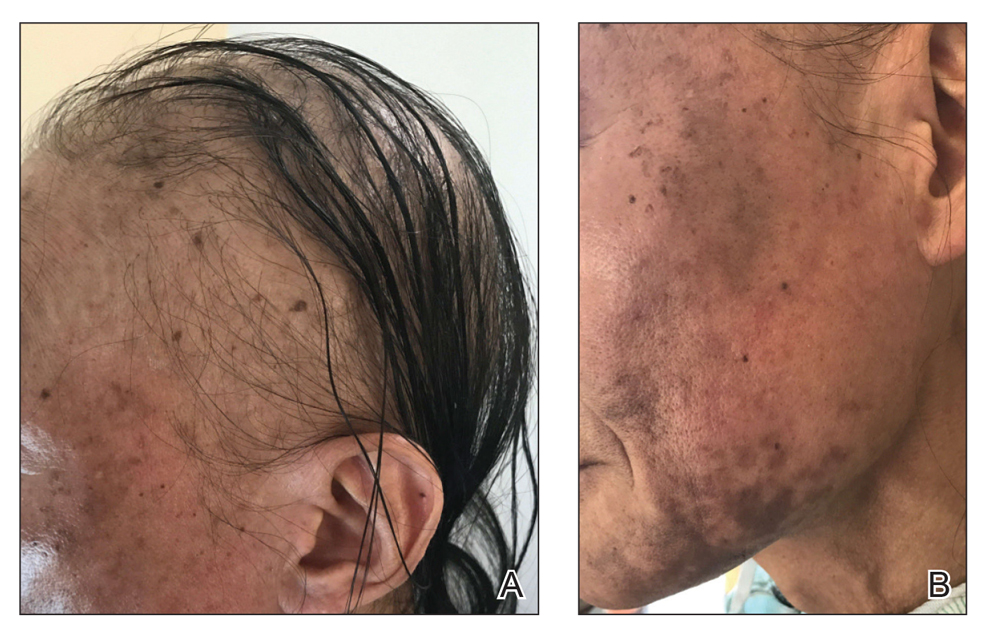
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.
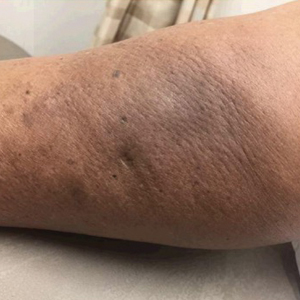
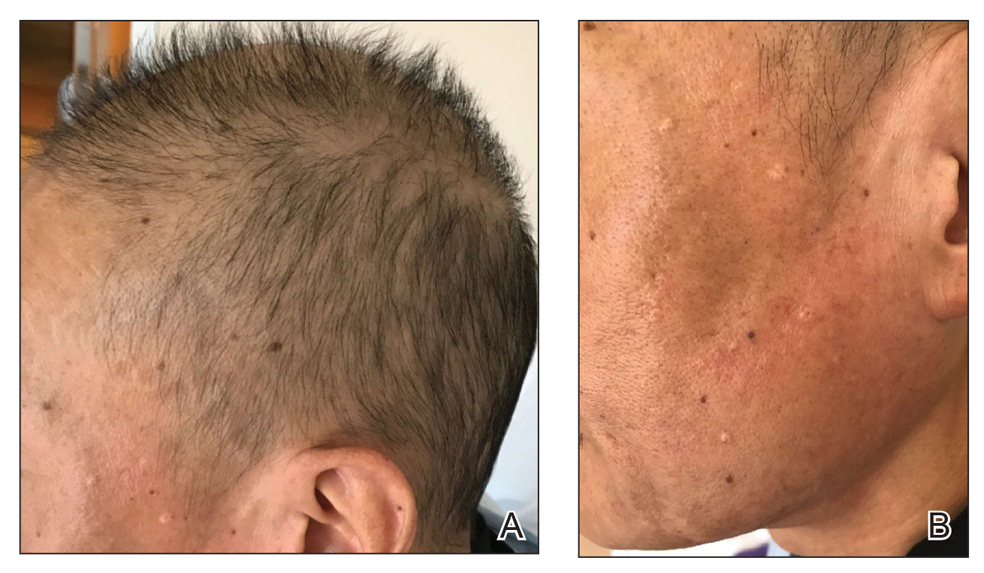
The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).
Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.

The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).

Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.

The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).

Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
PRACTICE POINTS
- Albendazole functions by inhibiting microtubule dynamics and has a remarkably greater binding affinity for helminthic β-tubulin than for its mammalian counterpart.
- An uncommon adverse effect of albendazole at therapeutic dosing is a dose-dependent telogen effluvium in susceptible persons, likely caused by the inherent susceptibility of follicular matrix keratinocytes to antimicrotubule agents.
- Massive albendazole overdose can cause anagen effluvium and myelosuppression similar to the effects of conventional chemotherapy.
Five-year cervical screening interval safe for HPV-negative women
A 5-year cervical screening interval is as safe and effective for women who test negative for human papillomavirus (HPV) as are 3-year intervals, according to a new ‘real life’ study led by King’s College London (KCL) with researchers from the University of Manchester, and the NHS, on behalf of the HPV pilot steering group.
The study, published in The BMJ, used data from the HPV screening pilot to assess rates of detection of high-grade cervical intraepithelial neoplasia (CIN3+) and of cervical cancer following a negative HPV test. It confirmed that 5-yearly screening prevents as many cancers as screening at 3-year intervals, even in women who are not vaccinated against HPV.
Change to primary HPV testing since 2019
Before 2019, the NHS cervical screening program conducted cytology testing first, testing for HPV only if abnormalities were found. In 2019, following reporting of early results of the HPV pilot by the same researchers, the program in England switched to testing for HPV first, on the grounds that since having HPV infection comes before having abnormal cells, HPV testing would detect more women at risk of cervical cancer.
Following the switch to primary HPV testing, the same screening intervals were retained, meaning 3-yearly screening for those aged 24-49 years and testing every 5 years for women aged 50-64 years, or 3 years if they tested positive. However, the National Screening Committee had recommended that invites should be changed from 3 to 5 years for those in the under-50 age group found not to have high-risk HPV at their routine screening test.
For the latest study, funded by Cancer Research UK, the steering group researchers analyzed details for more than 1.3 million women who had attended screening for two rounds of the HPV screening pilot, the first from 2013 to 2016, with a follow-up to the end of 2019. By this time, the data set had doubled in size from the pilot study, and results had been linked with the national cancer registry.
They confirmed that HPV testing was more accurate than a cytology test, irrespective of whether the HPV test assay was DNA- or mRNA-based. With HPV testing, the risk of subsequent cytological changes more than halved overall. Eligible women under 50 who had a negative HPV screen in the first round had a much lower risk of detection of CIN3+ in the second round, with a rate of 1.21 in 1,000, compared with 4.52 in 1,000 after a negative cytology test.
Data support extension of the testing interval
“The study confirms that women in this age group are much less likely to develop clinically relevant cervical lesions and cervical cancer, 3 years after a negative HPV screen, compared with a negative smear test,” the researchers said.
They suggested that most women do not need to be screened as frequently as the current program allows, and that the data support an extension of the screening intervals, regardless of the test assay used, to 5 years after a negative HPV test in women aged 25-49 years, and even longer for women aged 50 years and older.
However, the screening interval for HPV-positive women who have negative HPV tests at early recall should be kept at 3 years, they said.
“These results are very reassuring,” said lead author Matejka Rebolj, PhD, senior epidemiologist at KCL. “They build on previous research that shows that following the introduction of HPV testing for cervical screening, a 5-year interval is at least as safe as the previous 3-year interval. Changing to 5-yearly screening will mean we can prevent just as many cancers as before, while allowing for fewer screens.”
Michelle Mitchell, Cancer Research UK’s chief executive, said: “This large study shows that offering cervical screening using HPV testing effectively prevents cervical cancer, without having to be screened as often. This builds on findings from years of research showing HPV testing is more accurate at predicting who is at risk of developing cervical cancer compared to the previous way of testing. As changes to the screening [programs] are made, they will be monitored to help ensure that cervical screening is as effective as possible for all who take part.”
If HPV is present, testing interval should remain every 3 years
Responding to the study, Theresa Freeman-Wang, MBChB, consultant gynecologist, president of the British Society for Colposcopy and Cervical Pathology, and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “England, Scotland, and Wales and many other countries now use HPV primary screening, which is much better at assessing risk than previous methods. HPV testing is more sensitive and accurate, so changes are picked up earlier.
“Studies have confirmed that if someone is HPV negative (i.e., HPV is not present in the screen test), intervals between tests can very safely be increased from 3 to 5 years.
“If HPV is present, then the program will automatically look for any abnormal cells. If there are no abnormalities, the woman will be advised to have a repeat screen test in a year. If the HPV remains present over 3 successive years or if abnormal cells are detected at any stage, she will be referred for a more detailed screening examination called a colposcopy.
“It’s important that with any change like this, there is clear information available to explain what these changes mean.
“We have an effective cervical screening program in the UK that has significantly reduced the number of cases and deaths from this preventable cancer.
“HPV screening every 5 years is safe and to be fully effective it is vital that women take up the invitation for cervical screening when called.”
A version of this article first appeared on Medscape UK.
A 5-year cervical screening interval is as safe and effective for women who test negative for human papillomavirus (HPV) as are 3-year intervals, according to a new ‘real life’ study led by King’s College London (KCL) with researchers from the University of Manchester, and the NHS, on behalf of the HPV pilot steering group.
The study, published in The BMJ, used data from the HPV screening pilot to assess rates of detection of high-grade cervical intraepithelial neoplasia (CIN3+) and of cervical cancer following a negative HPV test. It confirmed that 5-yearly screening prevents as many cancers as screening at 3-year intervals, even in women who are not vaccinated against HPV.
Change to primary HPV testing since 2019
Before 2019, the NHS cervical screening program conducted cytology testing first, testing for HPV only if abnormalities were found. In 2019, following reporting of early results of the HPV pilot by the same researchers, the program in England switched to testing for HPV first, on the grounds that since having HPV infection comes before having abnormal cells, HPV testing would detect more women at risk of cervical cancer.
Following the switch to primary HPV testing, the same screening intervals were retained, meaning 3-yearly screening for those aged 24-49 years and testing every 5 years for women aged 50-64 years, or 3 years if they tested positive. However, the National Screening Committee had recommended that invites should be changed from 3 to 5 years for those in the under-50 age group found not to have high-risk HPV at their routine screening test.
For the latest study, funded by Cancer Research UK, the steering group researchers analyzed details for more than 1.3 million women who had attended screening for two rounds of the HPV screening pilot, the first from 2013 to 2016, with a follow-up to the end of 2019. By this time, the data set had doubled in size from the pilot study, and results had been linked with the national cancer registry.
They confirmed that HPV testing was more accurate than a cytology test, irrespective of whether the HPV test assay was DNA- or mRNA-based. With HPV testing, the risk of subsequent cytological changes more than halved overall. Eligible women under 50 who had a negative HPV screen in the first round had a much lower risk of detection of CIN3+ in the second round, with a rate of 1.21 in 1,000, compared with 4.52 in 1,000 after a negative cytology test.
Data support extension of the testing interval
“The study confirms that women in this age group are much less likely to develop clinically relevant cervical lesions and cervical cancer, 3 years after a negative HPV screen, compared with a negative smear test,” the researchers said.
They suggested that most women do not need to be screened as frequently as the current program allows, and that the data support an extension of the screening intervals, regardless of the test assay used, to 5 years after a negative HPV test in women aged 25-49 years, and even longer for women aged 50 years and older.
However, the screening interval for HPV-positive women who have negative HPV tests at early recall should be kept at 3 years, they said.
“These results are very reassuring,” said lead author Matejka Rebolj, PhD, senior epidemiologist at KCL. “They build on previous research that shows that following the introduction of HPV testing for cervical screening, a 5-year interval is at least as safe as the previous 3-year interval. Changing to 5-yearly screening will mean we can prevent just as many cancers as before, while allowing for fewer screens.”
Michelle Mitchell, Cancer Research UK’s chief executive, said: “This large study shows that offering cervical screening using HPV testing effectively prevents cervical cancer, without having to be screened as often. This builds on findings from years of research showing HPV testing is more accurate at predicting who is at risk of developing cervical cancer compared to the previous way of testing. As changes to the screening [programs] are made, they will be monitored to help ensure that cervical screening is as effective as possible for all who take part.”
If HPV is present, testing interval should remain every 3 years
Responding to the study, Theresa Freeman-Wang, MBChB, consultant gynecologist, president of the British Society for Colposcopy and Cervical Pathology, and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “England, Scotland, and Wales and many other countries now use HPV primary screening, which is much better at assessing risk than previous methods. HPV testing is more sensitive and accurate, so changes are picked up earlier.
“Studies have confirmed that if someone is HPV negative (i.e., HPV is not present in the screen test), intervals between tests can very safely be increased from 3 to 5 years.
“If HPV is present, then the program will automatically look for any abnormal cells. If there are no abnormalities, the woman will be advised to have a repeat screen test in a year. If the HPV remains present over 3 successive years or if abnormal cells are detected at any stage, she will be referred for a more detailed screening examination called a colposcopy.
“It’s important that with any change like this, there is clear information available to explain what these changes mean.
“We have an effective cervical screening program in the UK that has significantly reduced the number of cases and deaths from this preventable cancer.
“HPV screening every 5 years is safe and to be fully effective it is vital that women take up the invitation for cervical screening when called.”
A version of this article first appeared on Medscape UK.
A 5-year cervical screening interval is as safe and effective for women who test negative for human papillomavirus (HPV) as are 3-year intervals, according to a new ‘real life’ study led by King’s College London (KCL) with researchers from the University of Manchester, and the NHS, on behalf of the HPV pilot steering group.
The study, published in The BMJ, used data from the HPV screening pilot to assess rates of detection of high-grade cervical intraepithelial neoplasia (CIN3+) and of cervical cancer following a negative HPV test. It confirmed that 5-yearly screening prevents as many cancers as screening at 3-year intervals, even in women who are not vaccinated against HPV.
Change to primary HPV testing since 2019
Before 2019, the NHS cervical screening program conducted cytology testing first, testing for HPV only if abnormalities were found. In 2019, following reporting of early results of the HPV pilot by the same researchers, the program in England switched to testing for HPV first, on the grounds that since having HPV infection comes before having abnormal cells, HPV testing would detect more women at risk of cervical cancer.
Following the switch to primary HPV testing, the same screening intervals were retained, meaning 3-yearly screening for those aged 24-49 years and testing every 5 years for women aged 50-64 years, or 3 years if they tested positive. However, the National Screening Committee had recommended that invites should be changed from 3 to 5 years for those in the under-50 age group found not to have high-risk HPV at their routine screening test.
For the latest study, funded by Cancer Research UK, the steering group researchers analyzed details for more than 1.3 million women who had attended screening for two rounds of the HPV screening pilot, the first from 2013 to 2016, with a follow-up to the end of 2019. By this time, the data set had doubled in size from the pilot study, and results had been linked with the national cancer registry.
They confirmed that HPV testing was more accurate than a cytology test, irrespective of whether the HPV test assay was DNA- or mRNA-based. With HPV testing, the risk of subsequent cytological changes more than halved overall. Eligible women under 50 who had a negative HPV screen in the first round had a much lower risk of detection of CIN3+ in the second round, with a rate of 1.21 in 1,000, compared with 4.52 in 1,000 after a negative cytology test.
Data support extension of the testing interval
“The study confirms that women in this age group are much less likely to develop clinically relevant cervical lesions and cervical cancer, 3 years after a negative HPV screen, compared with a negative smear test,” the researchers said.
They suggested that most women do not need to be screened as frequently as the current program allows, and that the data support an extension of the screening intervals, regardless of the test assay used, to 5 years after a negative HPV test in women aged 25-49 years, and even longer for women aged 50 years and older.
However, the screening interval for HPV-positive women who have negative HPV tests at early recall should be kept at 3 years, they said.
“These results are very reassuring,” said lead author Matejka Rebolj, PhD, senior epidemiologist at KCL. “They build on previous research that shows that following the introduction of HPV testing for cervical screening, a 5-year interval is at least as safe as the previous 3-year interval. Changing to 5-yearly screening will mean we can prevent just as many cancers as before, while allowing for fewer screens.”
Michelle Mitchell, Cancer Research UK’s chief executive, said: “This large study shows that offering cervical screening using HPV testing effectively prevents cervical cancer, without having to be screened as often. This builds on findings from years of research showing HPV testing is more accurate at predicting who is at risk of developing cervical cancer compared to the previous way of testing. As changes to the screening [programs] are made, they will be monitored to help ensure that cervical screening is as effective as possible for all who take part.”
If HPV is present, testing interval should remain every 3 years
Responding to the study, Theresa Freeman-Wang, MBChB, consultant gynecologist, president of the British Society for Colposcopy and Cervical Pathology, and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “England, Scotland, and Wales and many other countries now use HPV primary screening, which is much better at assessing risk than previous methods. HPV testing is more sensitive and accurate, so changes are picked up earlier.
“Studies have confirmed that if someone is HPV negative (i.e., HPV is not present in the screen test), intervals between tests can very safely be increased from 3 to 5 years.
“If HPV is present, then the program will automatically look for any abnormal cells. If there are no abnormalities, the woman will be advised to have a repeat screen test in a year. If the HPV remains present over 3 successive years or if abnormal cells are detected at any stage, she will be referred for a more detailed screening examination called a colposcopy.
“It’s important that with any change like this, there is clear information available to explain what these changes mean.
“We have an effective cervical screening program in the UK that has significantly reduced the number of cases and deaths from this preventable cancer.
“HPV screening every 5 years is safe and to be fully effective it is vital that women take up the invitation for cervical screening when called.”
A version of this article first appeared on Medscape UK.
FROM THE BMJ
Sweet Syndrome With Pulmonary Involvement Preceding the Development of Myelodysplastic Syndrome
To the Editor:
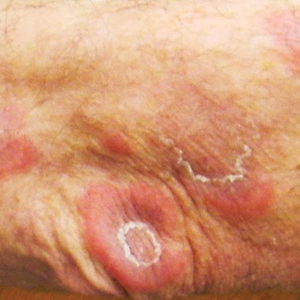
A 59-year-old man was referred to our clinic for a rash, fever, and night sweats following treatment for metastatic seminoma with cisplatin and etoposide. Physical examination revealed indurated erythematous papules and plaques on the trunk and upper and lower extremities, some with annular or arcuate configuration with trailing scale (Figure, A). A skin biopsy demonstrated mild papillary dermal edema with a mixed infiltrate of mononuclear cells, neutrophils, eosinophils, mast cells, lymphocytes, and karyorrhectic debris without evidence of leukocytoclastic vasculitis. The histopathologic differential diagnosis included a histiocytoid variant of Sweet syndrome (SS), and our patient’s rapid clinical response to corticosteroids supported this diagnosis.
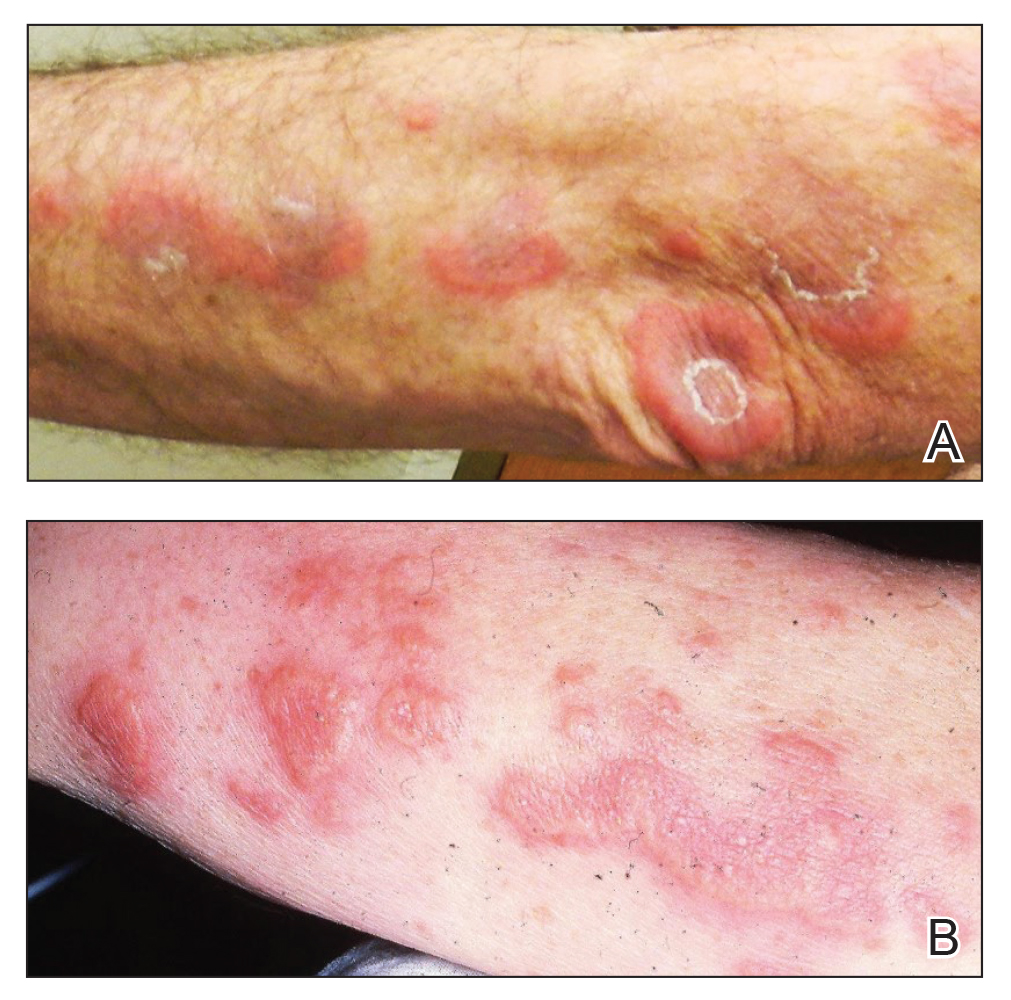
With a relapsing and remitting course over 3 years, the rash eventually evolved into more edematous papules and plaques (Figure, B), and a repeat biopsy 3 years later was consistent with classic SS. Although the patient's condition improved with prednisone, attempts to taper prednisone invariably resulted in relapse. Multiple steroid-sparing agents were trialed over the course of 3 years including dapsone and mycophenolate mofetil, both of which resulted in hypersensitivity drug eruptions. Colchicine and methotrexate were ineffective. Thalidomide strongly was considered but ultimately was avoided due to substantial existing neuropathy associated with his prior chemotherapy for metastatic seminoma.
Four years after the initial diagnosis of SS, our patient presented with dyspnea and weight loss. Computed tomography revealed a nearly confluent miliary pattern of nodularity in the lungs. A wedge biopsy demonstrated pneumonitis with intra-alveolar fibrin and neutrophils with a notable absence of granulomatous inflammation. Fungal and acid-fast bacilli staining as well as tissue cultures were negative. He had a history of Mycobacterium kansasii pulmonary infection treated 18 months prior; however, in this instance, the histopathology, negative microbial cultures, and rapid steroid responsiveness were consistent with pulmonary involvement of SS. Over the ensuing 2 years, the patient developed worsening of his chronic anemia. He was diagnosed with myelodysplastic syndrome (MDS) by bone marrow biopsy, despite having a normal bone marrow biopsy more than 3 years prior to evaluate his anemia. At this time, thalidomide was initiated at 50 mg daily leading to notable improvement in his SS symptoms; however, he developed worsening neuropathy resulting in the discontinuation of this treatment 2 months later. An investigational combination of vosaroxin and azacytidine was used to treat his MDS, resulting in normalization of blood counts and remission from SS.
Sweet syndrome may occur in the setting of undiagnosed cancer or may signal the return of a previously treated malignancy. The first description of SS associated with solid tumors was in a patient with testicular cancer,1 which prompted continuous surveillance for recurrent seminoma in our patient, though none was found. Hematologic malignancies, as well as MDS, often are associated with SS.2 In our patient, multiple atypical features linked the development of SS to the ultimate presentation of MDS. The initial finding of a histiocytoid variant has been described in a case series of 9 patients with chronic relapsing SS who eventually developed MDS with latency of up to 7 years. The histopathology in these cases evolved over time to that of classic neutrophilic SS.3 Pulmonary involvement of SS is another interesting aspect of our case. In one analysis, 18 of 34 (53%) cases with pulmonary involvement featured hematologic pathology, including myelodysplasia and acute leukemia.4
In our patient, SS preceded the clinical manifestation of MDS by 6 years. A similar phenomenon has been described in a patient with SS that preceded myelodysplasia by 30 months and was recalcitrant to numerous steroid-sparing therapies except thalidomide, despite the persistence of myelodysplasia. Tapering thalidomide, however, resulted in recurrence of SS lesions in that patient.5 In another case, resolution of myelodysplasia from azacytidine treatment was associated with remission from SS.6
Our case represents a confluence of atypical features that seem to define myelodysplasia-associated SS, including the initial presentation with a clinically atypical histiocytoid variant, chronic relapsing and remitting course, and extracutaneous involvement of the lungs. These findings should prompt surveillance for hematologic malignancy or myelodysplasia. Serial bone marrow biopsies were required to evaluate persistent anemia before the histopathologic findings of MDS became apparent in our patient. Thalidomide was an effective treatment for the cutaneous manifestations in our patient and should be considered as a steroid-sparing agent in the treatment of recalcitrant SS. Despite the discontinuation of thalidomide therapy, effective control of our patient’s myelodysplasia with chemotherapy has kept him in remission from SS for more than 7 years of follow-up, suggesting a causal relationship between these disorders.
- Shapiro L, Baraf CS, Richheimer LL. Sweet’s syndrome (acute febrile neutrophilic dermatosis): report of a case. Arch Dermatol. 1971;103:81-84.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia. Arch Dermatol. 2006;142:1170-1176.
- Fernandez-Bussy S, Labarca G, Cabello F, et al. Sweet’s syndrome with pulmonary involvement: case report and literature review. Respir Med Case Rep. 2012;6:16-19.
- Browning CE, Dixon DE, Malone JC, et al. Thalidomide in the treatment of recalcitrant Sweet’s syndrome associated with myelodysplasia. J Am Acad Dermatol. 2005;53(2 suppl 1):S135-S138.
- Martinelli S, Rigolin GM, Leo G, et al. Complete remission Sweet’s syndrome after azacytidine treatment for concomitant myelodysplastic syndrome. Int J Hematol. 2014;99:663-667.
To the Editor:
A 59-year-old man was referred to our clinic for a rash, fever, and night sweats following treatment for metastatic seminoma with cisplatin and etoposide. Physical examination revealed indurated erythematous papules and plaques on the trunk and upper and lower extremities, some with annular or arcuate configuration with trailing scale (Figure, A). A skin biopsy demonstrated mild papillary dermal edema with a mixed infiltrate of mononuclear cells, neutrophils, eosinophils, mast cells, lymphocytes, and karyorrhectic debris without evidence of leukocytoclastic vasculitis. The histopathologic differential diagnosis included a histiocytoid variant of Sweet syndrome (SS), and our patient’s rapid clinical response to corticosteroids supported this diagnosis.

With a relapsing and remitting course over 3 years, the rash eventually evolved into more edematous papules and plaques (Figure, B), and a repeat biopsy 3 years later was consistent with classic SS. Although the patient's condition improved with prednisone, attempts to taper prednisone invariably resulted in relapse. Multiple steroid-sparing agents were trialed over the course of 3 years including dapsone and mycophenolate mofetil, both of which resulted in hypersensitivity drug eruptions. Colchicine and methotrexate were ineffective. Thalidomide strongly was considered but ultimately was avoided due to substantial existing neuropathy associated with his prior chemotherapy for metastatic seminoma.
Four years after the initial diagnosis of SS, our patient presented with dyspnea and weight loss. Computed tomography revealed a nearly confluent miliary pattern of nodularity in the lungs. A wedge biopsy demonstrated pneumonitis with intra-alveolar fibrin and neutrophils with a notable absence of granulomatous inflammation. Fungal and acid-fast bacilli staining as well as tissue cultures were negative. He had a history of Mycobacterium kansasii pulmonary infection treated 18 months prior; however, in this instance, the histopathology, negative microbial cultures, and rapid steroid responsiveness were consistent with pulmonary involvement of SS. Over the ensuing 2 years, the patient developed worsening of his chronic anemia. He was diagnosed with myelodysplastic syndrome (MDS) by bone marrow biopsy, despite having a normal bone marrow biopsy more than 3 years prior to evaluate his anemia. At this time, thalidomide was initiated at 50 mg daily leading to notable improvement in his SS symptoms; however, he developed worsening neuropathy resulting in the discontinuation of this treatment 2 months later. An investigational combination of vosaroxin and azacytidine was used to treat his MDS, resulting in normalization of blood counts and remission from SS.
Sweet syndrome may occur in the setting of undiagnosed cancer or may signal the return of a previously treated malignancy. The first description of SS associated with solid tumors was in a patient with testicular cancer,1 which prompted continuous surveillance for recurrent seminoma in our patient, though none was found. Hematologic malignancies, as well as MDS, often are associated with SS.2 In our patient, multiple atypical features linked the development of SS to the ultimate presentation of MDS. The initial finding of a histiocytoid variant has been described in a case series of 9 patients with chronic relapsing SS who eventually developed MDS with latency of up to 7 years. The histopathology in these cases evolved over time to that of classic neutrophilic SS.3 Pulmonary involvement of SS is another interesting aspect of our case. In one analysis, 18 of 34 (53%) cases with pulmonary involvement featured hematologic pathology, including myelodysplasia and acute leukemia.4
In our patient, SS preceded the clinical manifestation of MDS by 6 years. A similar phenomenon has been described in a patient with SS that preceded myelodysplasia by 30 months and was recalcitrant to numerous steroid-sparing therapies except thalidomide, despite the persistence of myelodysplasia. Tapering thalidomide, however, resulted in recurrence of SS lesions in that patient.5 In another case, resolution of myelodysplasia from azacytidine treatment was associated with remission from SS.6
Our case represents a confluence of atypical features that seem to define myelodysplasia-associated SS, including the initial presentation with a clinically atypical histiocytoid variant, chronic relapsing and remitting course, and extracutaneous involvement of the lungs. These findings should prompt surveillance for hematologic malignancy or myelodysplasia. Serial bone marrow biopsies were required to evaluate persistent anemia before the histopathologic findings of MDS became apparent in our patient. Thalidomide was an effective treatment for the cutaneous manifestations in our patient and should be considered as a steroid-sparing agent in the treatment of recalcitrant SS. Despite the discontinuation of thalidomide therapy, effective control of our patient’s myelodysplasia with chemotherapy has kept him in remission from SS for more than 7 years of follow-up, suggesting a causal relationship between these disorders.
To the Editor:
A 59-year-old man was referred to our clinic for a rash, fever, and night sweats following treatment for metastatic seminoma with cisplatin and etoposide. Physical examination revealed indurated erythematous papules and plaques on the trunk and upper and lower extremities, some with annular or arcuate configuration with trailing scale (Figure, A). A skin biopsy demonstrated mild papillary dermal edema with a mixed infiltrate of mononuclear cells, neutrophils, eosinophils, mast cells, lymphocytes, and karyorrhectic debris without evidence of leukocytoclastic vasculitis. The histopathologic differential diagnosis included a histiocytoid variant of Sweet syndrome (SS), and our patient’s rapid clinical response to corticosteroids supported this diagnosis.

With a relapsing and remitting course over 3 years, the rash eventually evolved into more edematous papules and plaques (Figure, B), and a repeat biopsy 3 years later was consistent with classic SS. Although the patient's condition improved with prednisone, attempts to taper prednisone invariably resulted in relapse. Multiple steroid-sparing agents were trialed over the course of 3 years including dapsone and mycophenolate mofetil, both of which resulted in hypersensitivity drug eruptions. Colchicine and methotrexate were ineffective. Thalidomide strongly was considered but ultimately was avoided due to substantial existing neuropathy associated with his prior chemotherapy for metastatic seminoma.
Four years after the initial diagnosis of SS, our patient presented with dyspnea and weight loss. Computed tomography revealed a nearly confluent miliary pattern of nodularity in the lungs. A wedge biopsy demonstrated pneumonitis with intra-alveolar fibrin and neutrophils with a notable absence of granulomatous inflammation. Fungal and acid-fast bacilli staining as well as tissue cultures were negative. He had a history of Mycobacterium kansasii pulmonary infection treated 18 months prior; however, in this instance, the histopathology, negative microbial cultures, and rapid steroid responsiveness were consistent with pulmonary involvement of SS. Over the ensuing 2 years, the patient developed worsening of his chronic anemia. He was diagnosed with myelodysplastic syndrome (MDS) by bone marrow biopsy, despite having a normal bone marrow biopsy more than 3 years prior to evaluate his anemia. At this time, thalidomide was initiated at 50 mg daily leading to notable improvement in his SS symptoms; however, he developed worsening neuropathy resulting in the discontinuation of this treatment 2 months later. An investigational combination of vosaroxin and azacytidine was used to treat his MDS, resulting in normalization of blood counts and remission from SS.
Sweet syndrome may occur in the setting of undiagnosed cancer or may signal the return of a previously treated malignancy. The first description of SS associated with solid tumors was in a patient with testicular cancer,1 which prompted continuous surveillance for recurrent seminoma in our patient, though none was found. Hematologic malignancies, as well as MDS, often are associated with SS.2 In our patient, multiple atypical features linked the development of SS to the ultimate presentation of MDS. The initial finding of a histiocytoid variant has been described in a case series of 9 patients with chronic relapsing SS who eventually developed MDS with latency of up to 7 years. The histopathology in these cases evolved over time to that of classic neutrophilic SS.3 Pulmonary involvement of SS is another interesting aspect of our case. In one analysis, 18 of 34 (53%) cases with pulmonary involvement featured hematologic pathology, including myelodysplasia and acute leukemia.4
In our patient, SS preceded the clinical manifestation of MDS by 6 years. A similar phenomenon has been described in a patient with SS that preceded myelodysplasia by 30 months and was recalcitrant to numerous steroid-sparing therapies except thalidomide, despite the persistence of myelodysplasia. Tapering thalidomide, however, resulted in recurrence of SS lesions in that patient.5 In another case, resolution of myelodysplasia from azacytidine treatment was associated with remission from SS.6
Our case represents a confluence of atypical features that seem to define myelodysplasia-associated SS, including the initial presentation with a clinically atypical histiocytoid variant, chronic relapsing and remitting course, and extracutaneous involvement of the lungs. These findings should prompt surveillance for hematologic malignancy or myelodysplasia. Serial bone marrow biopsies were required to evaluate persistent anemia before the histopathologic findings of MDS became apparent in our patient. Thalidomide was an effective treatment for the cutaneous manifestations in our patient and should be considered as a steroid-sparing agent in the treatment of recalcitrant SS. Despite the discontinuation of thalidomide therapy, effective control of our patient’s myelodysplasia with chemotherapy has kept him in remission from SS for more than 7 years of follow-up, suggesting a causal relationship between these disorders.
- Shapiro L, Baraf CS, Richheimer LL. Sweet’s syndrome (acute febrile neutrophilic dermatosis): report of a case. Arch Dermatol. 1971;103:81-84.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia. Arch Dermatol. 2006;142:1170-1176.
- Fernandez-Bussy S, Labarca G, Cabello F, et al. Sweet’s syndrome with pulmonary involvement: case report and literature review. Respir Med Case Rep. 2012;6:16-19.
- Browning CE, Dixon DE, Malone JC, et al. Thalidomide in the treatment of recalcitrant Sweet’s syndrome associated with myelodysplasia. J Am Acad Dermatol. 2005;53(2 suppl 1):S135-S138.
- Martinelli S, Rigolin GM, Leo G, et al. Complete remission Sweet’s syndrome after azacytidine treatment for concomitant myelodysplastic syndrome. Int J Hematol. 2014;99:663-667.
- Shapiro L, Baraf CS, Richheimer LL. Sweet’s syndrome (acute febrile neutrophilic dermatosis): report of a case. Arch Dermatol. 1971;103:81-84.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia. Arch Dermatol. 2006;142:1170-1176.
- Fernandez-Bussy S, Labarca G, Cabello F, et al. Sweet’s syndrome with pulmonary involvement: case report and literature review. Respir Med Case Rep. 2012;6:16-19.
- Browning CE, Dixon DE, Malone JC, et al. Thalidomide in the treatment of recalcitrant Sweet’s syndrome associated with myelodysplasia. J Am Acad Dermatol. 2005;53(2 suppl 1):S135-S138.
- Martinelli S, Rigolin GM, Leo G, et al. Complete remission Sweet’s syndrome after azacytidine treatment for concomitant myelodysplastic syndrome. Int J Hematol. 2014;99:663-667.
Practice Points
- Sweet syndrome is characterized by the clinical constellation of fever, a skin eruption of tender erythematous papules or plaques, and response to corticosteroids.
- Skin biopsy characteristically demonstrates marked papillary dermal edema with a dense infiltrate of mature neutrophils without leukocytoclasia.
- Sweet syndrome often is idiopathic, though it has been associated with infection, autoimmunity, medication, and malignancy.
Hospital medicine gains popularity among newly minted physicians
In a new study, published in Annals of Internal Medicine, researchers from ABIM reviewed certification data from 67,902 general internists, accounting for 80% of all general internists certified in the United States from 1990 to 2017.
The researchers also used data from Medicare fee-for-service claims from 2008-2018 to measure and categorize practice setting types. The claims were from patients aged 65 years or older with at least 20 evaluation and management visits each year. Practice settings were categorized as hospitalist, outpatient, or mixed.
“ABIM is always working to understand the real-life experience of physicians, and this project grew out of that sort of analysis,” lead author Bradley M. Gray, PhD, a health services researcher at ABIM in Philadelphia, said in an interview. “We wanted to better understand practice setting, because that relates to the kinds of questions that we ask on our certifying exams. When we did this, we noticed a trend toward hospital medicine.”
Overall, the percentages of general internists in hospitalist practice and outpatient-only practice increased during the study period, from 25% to 40% and from 23% to 38%, respectively. By contrast, the percentage of general internists in a mixed-practice setting decreased from 52% to 23%, a 56% decline. Most of the physicians who left the mixed practice setting switched to outpatient-only practices.
Among the internists certified in 2017, 71% practiced as hospitalists, compared with 8% practicing as outpatient-only physicians. Most physicians remained in their original choice of practice setting. For physicians certified in 1999 and 2012, 86% and 85%, respectively, of those who chose hospitalist medicine remained in the hospital setting 5 years later, as did 95% of outpatient physicians, but only 57% of mixed-practice physicians.
The shift to outpatient practice among senior physicians offset the potential decline in outpatient primary care resulting from the increased choice of hospitalist medicine by new internists, the researchers noted.
The study findings were limited by several factors, including the reliance on Medicare fee-for-service claims, the researchers noted.
“We were surprised by both the dramatic shift toward hospital medicine by new physicians and the shift to outpatient only (an extreme category) for more senior physicians,” Dr. Gray said in an interview.
The shift toward outpatient practice among older physicians may be driven by convenience, said Dr. Gray. “I suspect that it is more efficient to specialize in terms of practice setting. Only seeing patients in the outpatient setting means that you don’t have to travel to the hospital, which can be time consuming.
“Also, with fewer new physicians going into primary care, older physicians need to focus on outpatient visits. This could be problematic in the future as more senior physicians retire and are replaced by new physicians who focus on hospital care,” which could lead to more shortages in primary care physicians, he explained.
The trend toward hospital medicine as a career has been going on since before the pandemic, said Dr. Gray. “I don’t think the pandemic will ultimately impact this trend. That said, at least in the short run, there may have been a decreased demand for primary care, but that is just my speculation. As more data flow in we will be able to answer this question more directly.”
Next steps for research included digging deeper into the data to understand the nature of conditions facing hospitalists, Dr. Gray said.
Implications for primary care
“This study provides an updated snapshot of the popularity of hospital medicine,” said Bradley A. Sharpe, MD, of the division of hospital medicine at the University of California, San Francisco. “It is also important to conduct this study now as health systems think about the challenge of providing high-quality primary care with a rapidly decreasing number of internists choosing to practice outpatient medicine.” Dr. Sharpe was not involved in the study.
“The most surprising finding to me was not the increase in general internists focusing on hospital medicine, but the amount of the increase; it is remarkable that nearly three quarters of general internists are choosing to practice as hospitalists,” Dr. Sharpe noted.
“I think there are a number of key factors at play,” he said. “First, as hospital medicine as a field is now more than 25 years old, hospitals and health systems have evolved to create hospital medicine jobs that are interesting, engaging, rewarding (financially and otherwise), doable, and sustainable. Second, being an outpatient internist is incredibly challenging; multiple studies have shown that it is essentially impossible to complete the evidence-based preventive care for a panel of patients on top of everything else. We know burnout rates are often higher among primary care and family medicine providers. On top of that, the expansion of electronic health records and patient access has led to a massive increase in messages to providers; this has been shown to be associated with burnout.”
The potential impact of the pandemic on physicians’ choices and the trend toward hospital medicine is an interested question, Dr. Sharpe said. The current study showed only trends through 2017.
“To be honest, I think it is difficult to predict,” he said. “Hospitalists shouldered much of the burden of COVID care nationally and burnout rates are high. One could imagine the extra work (as well as concern for personal safety) could lead to fewer providers choosing hospital medicine.
“At the same time, the pandemic has driven many of us to reflect on life and our values and what is important and, through that lens, providers might choose hospital medicine as a more sustainable, do-able, rewarding, and enjoyable career choice,” Dr. Sharpe emphasized.
“Additional research could explore the drivers of this clear trend toward hospital medicine. Determining what is motivating this trend could help hospitals and health systems ensure they have the right workforce for the future and, in particular, how to create outpatient positions that are attractive and rewarding,” he said.
The study received no outside funding. The researchers and Dr. Sharpe disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a new study, published in Annals of Internal Medicine, researchers from ABIM reviewed certification data from 67,902 general internists, accounting for 80% of all general internists certified in the United States from 1990 to 2017.
The researchers also used data from Medicare fee-for-service claims from 2008-2018 to measure and categorize practice setting types. The claims were from patients aged 65 years or older with at least 20 evaluation and management visits each year. Practice settings were categorized as hospitalist, outpatient, or mixed.
“ABIM is always working to understand the real-life experience of physicians, and this project grew out of that sort of analysis,” lead author Bradley M. Gray, PhD, a health services researcher at ABIM in Philadelphia, said in an interview. “We wanted to better understand practice setting, because that relates to the kinds of questions that we ask on our certifying exams. When we did this, we noticed a trend toward hospital medicine.”
Overall, the percentages of general internists in hospitalist practice and outpatient-only practice increased during the study period, from 25% to 40% and from 23% to 38%, respectively. By contrast, the percentage of general internists in a mixed-practice setting decreased from 52% to 23%, a 56% decline. Most of the physicians who left the mixed practice setting switched to outpatient-only practices.
Among the internists certified in 2017, 71% practiced as hospitalists, compared with 8% practicing as outpatient-only physicians. Most physicians remained in their original choice of practice setting. For physicians certified in 1999 and 2012, 86% and 85%, respectively, of those who chose hospitalist medicine remained in the hospital setting 5 years later, as did 95% of outpatient physicians, but only 57% of mixed-practice physicians.
The shift to outpatient practice among senior physicians offset the potential decline in outpatient primary care resulting from the increased choice of hospitalist medicine by new internists, the researchers noted.
The study findings were limited by several factors, including the reliance on Medicare fee-for-service claims, the researchers noted.
“We were surprised by both the dramatic shift toward hospital medicine by new physicians and the shift to outpatient only (an extreme category) for more senior physicians,” Dr. Gray said in an interview.
The shift toward outpatient practice among older physicians may be driven by convenience, said Dr. Gray. “I suspect that it is more efficient to specialize in terms of practice setting. Only seeing patients in the outpatient setting means that you don’t have to travel to the hospital, which can be time consuming.
“Also, with fewer new physicians going into primary care, older physicians need to focus on outpatient visits. This could be problematic in the future as more senior physicians retire and are replaced by new physicians who focus on hospital care,” which could lead to more shortages in primary care physicians, he explained.
The trend toward hospital medicine as a career has been going on since before the pandemic, said Dr. Gray. “I don’t think the pandemic will ultimately impact this trend. That said, at least in the short run, there may have been a decreased demand for primary care, but that is just my speculation. As more data flow in we will be able to answer this question more directly.”
Next steps for research included digging deeper into the data to understand the nature of conditions facing hospitalists, Dr. Gray said.
Implications for primary care
“This study provides an updated snapshot of the popularity of hospital medicine,” said Bradley A. Sharpe, MD, of the division of hospital medicine at the University of California, San Francisco. “It is also important to conduct this study now as health systems think about the challenge of providing high-quality primary care with a rapidly decreasing number of internists choosing to practice outpatient medicine.” Dr. Sharpe was not involved in the study.
“The most surprising finding to me was not the increase in general internists focusing on hospital medicine, but the amount of the increase; it is remarkable that nearly three quarters of general internists are choosing to practice as hospitalists,” Dr. Sharpe noted.
“I think there are a number of key factors at play,” he said. “First, as hospital medicine as a field is now more than 25 years old, hospitals and health systems have evolved to create hospital medicine jobs that are interesting, engaging, rewarding (financially and otherwise), doable, and sustainable. Second, being an outpatient internist is incredibly challenging; multiple studies have shown that it is essentially impossible to complete the evidence-based preventive care for a panel of patients on top of everything else. We know burnout rates are often higher among primary care and family medicine providers. On top of that, the expansion of electronic health records and patient access has led to a massive increase in messages to providers; this has been shown to be associated with burnout.”
The potential impact of the pandemic on physicians’ choices and the trend toward hospital medicine is an interested question, Dr. Sharpe said. The current study showed only trends through 2017.
“To be honest, I think it is difficult to predict,” he said. “Hospitalists shouldered much of the burden of COVID care nationally and burnout rates are high. One could imagine the extra work (as well as concern for personal safety) could lead to fewer providers choosing hospital medicine.
“At the same time, the pandemic has driven many of us to reflect on life and our values and what is important and, through that lens, providers might choose hospital medicine as a more sustainable, do-able, rewarding, and enjoyable career choice,” Dr. Sharpe emphasized.
“Additional research could explore the drivers of this clear trend toward hospital medicine. Determining what is motivating this trend could help hospitals and health systems ensure they have the right workforce for the future and, in particular, how to create outpatient positions that are attractive and rewarding,” he said.
The study received no outside funding. The researchers and Dr. Sharpe disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a new study, published in Annals of Internal Medicine, researchers from ABIM reviewed certification data from 67,902 general internists, accounting for 80% of all general internists certified in the United States from 1990 to 2017.
The researchers also used data from Medicare fee-for-service claims from 2008-2018 to measure and categorize practice setting types. The claims were from patients aged 65 years or older with at least 20 evaluation and management visits each year. Practice settings were categorized as hospitalist, outpatient, or mixed.
“ABIM is always working to understand the real-life experience of physicians, and this project grew out of that sort of analysis,” lead author Bradley M. Gray, PhD, a health services researcher at ABIM in Philadelphia, said in an interview. “We wanted to better understand practice setting, because that relates to the kinds of questions that we ask on our certifying exams. When we did this, we noticed a trend toward hospital medicine.”
Overall, the percentages of general internists in hospitalist practice and outpatient-only practice increased during the study period, from 25% to 40% and from 23% to 38%, respectively. By contrast, the percentage of general internists in a mixed-practice setting decreased from 52% to 23%, a 56% decline. Most of the physicians who left the mixed practice setting switched to outpatient-only practices.
Among the internists certified in 2017, 71% practiced as hospitalists, compared with 8% practicing as outpatient-only physicians. Most physicians remained in their original choice of practice setting. For physicians certified in 1999 and 2012, 86% and 85%, respectively, of those who chose hospitalist medicine remained in the hospital setting 5 years later, as did 95% of outpatient physicians, but only 57% of mixed-practice physicians.
The shift to outpatient practice among senior physicians offset the potential decline in outpatient primary care resulting from the increased choice of hospitalist medicine by new internists, the researchers noted.
The study findings were limited by several factors, including the reliance on Medicare fee-for-service claims, the researchers noted.
“We were surprised by both the dramatic shift toward hospital medicine by new physicians and the shift to outpatient only (an extreme category) for more senior physicians,” Dr. Gray said in an interview.
The shift toward outpatient practice among older physicians may be driven by convenience, said Dr. Gray. “I suspect that it is more efficient to specialize in terms of practice setting. Only seeing patients in the outpatient setting means that you don’t have to travel to the hospital, which can be time consuming.
“Also, with fewer new physicians going into primary care, older physicians need to focus on outpatient visits. This could be problematic in the future as more senior physicians retire and are replaced by new physicians who focus on hospital care,” which could lead to more shortages in primary care physicians, he explained.
The trend toward hospital medicine as a career has been going on since before the pandemic, said Dr. Gray. “I don’t think the pandemic will ultimately impact this trend. That said, at least in the short run, there may have been a decreased demand for primary care, but that is just my speculation. As more data flow in we will be able to answer this question more directly.”
Next steps for research included digging deeper into the data to understand the nature of conditions facing hospitalists, Dr. Gray said.
Implications for primary care
“This study provides an updated snapshot of the popularity of hospital medicine,” said Bradley A. Sharpe, MD, of the division of hospital medicine at the University of California, San Francisco. “It is also important to conduct this study now as health systems think about the challenge of providing high-quality primary care with a rapidly decreasing number of internists choosing to practice outpatient medicine.” Dr. Sharpe was not involved in the study.
“The most surprising finding to me was not the increase in general internists focusing on hospital medicine, but the amount of the increase; it is remarkable that nearly three quarters of general internists are choosing to practice as hospitalists,” Dr. Sharpe noted.
“I think there are a number of key factors at play,” he said. “First, as hospital medicine as a field is now more than 25 years old, hospitals and health systems have evolved to create hospital medicine jobs that are interesting, engaging, rewarding (financially and otherwise), doable, and sustainable. Second, being an outpatient internist is incredibly challenging; multiple studies have shown that it is essentially impossible to complete the evidence-based preventive care for a panel of patients on top of everything else. We know burnout rates are often higher among primary care and family medicine providers. On top of that, the expansion of electronic health records and patient access has led to a massive increase in messages to providers; this has been shown to be associated with burnout.”
The potential impact of the pandemic on physicians’ choices and the trend toward hospital medicine is an interested question, Dr. Sharpe said. The current study showed only trends through 2017.
“To be honest, I think it is difficult to predict,” he said. “Hospitalists shouldered much of the burden of COVID care nationally and burnout rates are high. One could imagine the extra work (as well as concern for personal safety) could lead to fewer providers choosing hospital medicine.
“At the same time, the pandemic has driven many of us to reflect on life and our values and what is important and, through that lens, providers might choose hospital medicine as a more sustainable, do-able, rewarding, and enjoyable career choice,” Dr. Sharpe emphasized.
“Additional research could explore the drivers of this clear trend toward hospital medicine. Determining what is motivating this trend could help hospitals and health systems ensure they have the right workforce for the future and, in particular, how to create outpatient positions that are attractive and rewarding,” he said.
The study received no outside funding. The researchers and Dr. Sharpe disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ADA 2022 preview: Tirzepatide and much more
The full results on Lilly’s tirzepatide for obesity will likely dominate the headlines from the annual scientific sessions of the American Diabetes Association, but the conference program is jam-packed with new findings – and new paradigms – in both type 1 and type 2 diabetes management and prevention.
Taking place June 3-7 both in person – for the first time in 3 years – in New Orleans, and virtually, the “hybrid” meeting is mandating COVID-19 vaccination and mask wearing for all on-site attendees.
A major topic will be new findings and thinking in the treatment of type 2 diabetes, including the new twincretin tirzepatide, as well as discussions about the role of weight loss and the concept of “remission.” In type 1 diabetes, sessions will examine intervention trials to prevent progression, progress in islet transplantation, and the latest findings in diabetes technology.
Other key conference themes include the often interrelated topics of disparities, mental health, and COVID-19.
“I think that the scientific planning committee has put together a really outstanding program this year, covering the entire spectrum of diabetes care and research and translation for both type 1 and type 2 diabetes,” Scientific Planning Committee Chair Dana Dabelea, MD, PhD, professor of epidemiology and pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, told this news organization.
Tirzepatide: The next big thing?
The presentation likely to generate the most buzz will take place Saturday morning, with the full detailed results from Lilly’s phase 3 SURMOUNT-1 trial of its dual-incretin tirzepatide for weight loss in people with obesity or overweight with at least one comorbidity but not diabetes.
Top-line results released by Lilly in April 2022 showed that the drug induced weight loss of up to 22%. Tirzepatide was approved May 13 by the Food and Drug Administration for type 2 diabetes under the brand name Mounjaro. It is not approved for weight loss.
“Certainly the general public will latch on to this idea that there is a drug they can lose 22% of their weight on,” Robert A. Gabbay, MD, PhD, ADA chief science and medical officer, told this news organization. “It’s hard to comment on a press release, so that’s why this presentation is going to be key.”
Another tirzepatide analysis, this one comparing its use to insulin glargine on kidney outcomes in participants with diabetes in the pivotal SURPASS-4 study, will be presented as an ADA Presidents’ Select Abstract on Friday afternoon.
“I think tirzepatide could be the great new thing, but I think we need to know a little bit more. Weight loss seems to be better than with glucagon-like peptide-1 (GLP-1) receptor agonists. Renal outcomes are important. Next will be to see if it has cardiovascular benefit. It makes one think about its use versus GLP-1 agonists,” Dr. Gabbay said.
Managing type 2 diabetes: Shifting paradigms
With the emergence of tirzepatide and other pharmacologic agents with benefits beyond glucose lowering, there has been much discussion in recent years about alternatives to the current metformin monotherapy first, stepwise approach to managing type 2 diabetes.
As has been done previously, on Monday afternoon, there will be a joint ADA/European Association for the Study of Diabetes (EASD) session during which a draft of the latest update will be presented on the management of hyperglycemia in type 2 diabetes. The final version will be presented at the EASD meeting in September.
While it won’t include tirzepatide, as the drug is not yet approved in Europe, there will be discussion about the role of weight loss goals in type 2 diabetes management, Dr. Gabbay said.
The concept of a 15% weight loss as a primary treatment goal of type 2 diabetes management is a new focus, initiated at the EASD 2021 annual meeting and published in The Lancet.
“With tirzepatide becoming available, there’s the opportunity for more significant weight loss. So, there’s been this debate, starting with the somewhat controversial opinion piece in Lancet ... Maybe it was stating things a bit too far but it certainly got everyone in the field thinking. You’ll see that come up in lots of places at this meeting,” Dr. Gabbay said.
Indeed, those sessions include a Sunday morning symposium titled: “Obesity Management as a Primary Treatment Goal for Type 2 Diabetes – It’s Time for a Paradigm Shift,” in which speakers will address both lifestyle and pharmacologic intervention. On Saturday afternoon, two speakers will debate the question: “Weighing the Evidence – Should Obesity Be the Primary Target of Treatment in Type 2 Diabetes?” Yet another session on Sunday afternoon, will cover “Incorporating Weight Management Strategies for Obesity Into Type 2 Diabetes Care – Medical Management and Surgery.”
From weight loss to type 2 diabetes ‘remission’?
Related to the issue of weight loss as first-line therapy is the concept of type 2 diabetes “remission.” “There is a school of thought that says early in the course of disease we probably want to be a lot more aggressive because there’s a greater chance of putting someone into remission,” Dr. Gabbay noted. “The opportunities for remission after someone has had diabetes for a number of years are relatively low.”
In September 2021, ADA, along with EASD, the Endocrine Society, and Diabetes UK, published a joint consensus statement aiming to standardize use of the term “remission” in type 2 diabetes.
At the ADA meeting, a symposium on Monday afternoon, titled, “Definition and Interpretation of Remission in Type 2 Diabetes,” will cover lifestyle, pharmacotherapy, and metabolic surgery approaches. One noteworthy talk in that session will address the question: “Can Type 2 Diabetes Remission Be Diagnosed While Glucose-Lowering Drugs Are Being Used?”
Asked how all of this – tirzepatide, weight loss, and “remission” – might play out clinically, Dr. Dabelea replied: “We are still debating the strategy. That’s why we’re having the scientific talks.
“I think they will be very interesting and very well-attended, but there isn’t a strategy yet ... The important thing is we have these ‘miracle drugs,’ if you want, and once we’ve learned all we need to know about how they act and who we should target, perhaps next year we can talk about a strategy.”
Type 1 diabetes: Progress in preventing, treating, and ... curing?
Type 1 diabetes also will be well represented at the conference, with topics covering prevention, treatment, and progress toward a cure. On Saturday afternoon, a symposium will cover data from a trial of low-dose IL-2 in people with recently diagnosed type 1 diabetes, while a Friday afternoon symposium will address “Emerging Approaches to Beta Cell Replacement.”
On Saturday afternoon, a symposium will provide an update on islet cell transplantation, including immune tolerance strategies, while an oral abstract session will cover “Clinical Outcomes in Islet and Pancreas Transplantation.” And on Monday afternoon, yet another symposium will examine “Emerging Data on Therapies to Treat the Underlying Autoimmunity in Type 1 Diabetes.”
As usual, there will also be numerous presentations on the latest in diabetes technology. Particularly noteworthy among these will be an oral abstract presentation on Monday afternoon, “The CREATE Trial: Randomized Clinical Trial Comparing Open-Source Automated Insulin Delivery With Sensor Augmented Pump Therapy in Type 1 Diabetes,” and results from the insulin-only “bionic pancreas” pivotal randomized clinical trial on Friday afternoon.
“I’m happy to see a plethora of studies in type 1 diabetes. Dr. Dabelea said. “As with tirzepatide in type 2 diabetes, we are witnessing discoveries and we need to have some time to really understand the results, understand who are they targeting, who is going to benefit, and then move into a strategy.”
However, she added, in both type 1 and type 2 diabetes, “we’re seeing these disparities [where] these novel technologies and therapeutics are not getting to the people who need them most,” which brings up another major meeting theme, health disparities.
Overlapping themes: Disparities, mental health, and COVID-19
The topics of health disparities in diabetes prevention, management, and care and promoting health equity, as well as the impact of COVID-19, are “certainly timely this year,” said Dr. Dabelea.
At least eight meeting sessions will address various aspects of disparity, including a Friday afternoon symposium, “Race, Racism, and Diabetes Research,” a Saturday morning oral presentation on “Mitigating Disparities in the Screening and Diagnosis of Diabetes,” and on Monday morning, the symposium “Disparities in the Use of Diabetes Medications and Technologies.”
A related topic, insulin access, will be addressed in a Friday morning “mini-symposium” that will cover the issue from U.S. and international perspectives, including humanitarian crisis situations. Related to that, on Sunday afternoon a panel will discuss the Ukraine situation specifically.
Regarding mental health, one noteworthy session is a symposium on Saturday afternoon: “Suicide and Self-Injury – Unveiling and Addressing the Hidden Nightmare in Diabetes.”
“It’s an underrecognized problem and we’ve devoted a symposium to really drill into it. I think that’s going to be an important story for all of us to think about,” Dr. Gabbay said.
Another mental health session on Saturday afternoon will examine “Stigma in Diabetes Care – Evidence and Solutions.” Dr. Dabelea noted, “Mental health is a rising concern in the United States, especially in people with chronic diseases in the wake of the pandemic ... Of course there’s overlap in mechanisms in type 1 and type 2, but I think there are also distinct pathways.”
COVID-19 will be somewhat less of a focus than in the past 2 years, but there will certainly still be plenty about it. A Friday morning mini-symposium will cover new findings in pathophysiology, another session on Monday afternoon will look at the impact of the pandemic on hypoglycemia risk, and COVID-19 will be the subject of several late-breaking posters on Sunday afternoon. One in particular will report a review of diabetes as a risk factor for long COVID.
Celebrating in person in the Big Easy
But unlike the past 2 years, COVID-19 has not kept ADA from meeting in person in 2022. “I think it’s going to be amazing ... We’re so excited to be in person and interacting,” Dr. Gabbay said.
He observed that virtual meetings – as ADA and most other medical societies have been forced into for the past 2 years during the pandemic – fail to capture “how science is advanced by the casual conversations in the hallway and collaborations and new ideas. It’s really this incredible incubator. For me, that’s the most exciting part.”
The location, New Orleans, also factors into his excitement: “What a great place to do this. It’s conducive to celebrating. It’s been a long couple of years.”
A version of this article first appeared on Medscape.com.
The full results on Lilly’s tirzepatide for obesity will likely dominate the headlines from the annual scientific sessions of the American Diabetes Association, but the conference program is jam-packed with new findings – and new paradigms – in both type 1 and type 2 diabetes management and prevention.
Taking place June 3-7 both in person – for the first time in 3 years – in New Orleans, and virtually, the “hybrid” meeting is mandating COVID-19 vaccination and mask wearing for all on-site attendees.
A major topic will be new findings and thinking in the treatment of type 2 diabetes, including the new twincretin tirzepatide, as well as discussions about the role of weight loss and the concept of “remission.” In type 1 diabetes, sessions will examine intervention trials to prevent progression, progress in islet transplantation, and the latest findings in diabetes technology.
Other key conference themes include the often interrelated topics of disparities, mental health, and COVID-19.
“I think that the scientific planning committee has put together a really outstanding program this year, covering the entire spectrum of diabetes care and research and translation for both type 1 and type 2 diabetes,” Scientific Planning Committee Chair Dana Dabelea, MD, PhD, professor of epidemiology and pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, told this news organization.
Tirzepatide: The next big thing?
The presentation likely to generate the most buzz will take place Saturday morning, with the full detailed results from Lilly’s phase 3 SURMOUNT-1 trial of its dual-incretin tirzepatide for weight loss in people with obesity or overweight with at least one comorbidity but not diabetes.
Top-line results released by Lilly in April 2022 showed that the drug induced weight loss of up to 22%. Tirzepatide was approved May 13 by the Food and Drug Administration for type 2 diabetes under the brand name Mounjaro. It is not approved for weight loss.
“Certainly the general public will latch on to this idea that there is a drug they can lose 22% of their weight on,” Robert A. Gabbay, MD, PhD, ADA chief science and medical officer, told this news organization. “It’s hard to comment on a press release, so that’s why this presentation is going to be key.”
Another tirzepatide analysis, this one comparing its use to insulin glargine on kidney outcomes in participants with diabetes in the pivotal SURPASS-4 study, will be presented as an ADA Presidents’ Select Abstract on Friday afternoon.
“I think tirzepatide could be the great new thing, but I think we need to know a little bit more. Weight loss seems to be better than with glucagon-like peptide-1 (GLP-1) receptor agonists. Renal outcomes are important. Next will be to see if it has cardiovascular benefit. It makes one think about its use versus GLP-1 agonists,” Dr. Gabbay said.
Managing type 2 diabetes: Shifting paradigms
With the emergence of tirzepatide and other pharmacologic agents with benefits beyond glucose lowering, there has been much discussion in recent years about alternatives to the current metformin monotherapy first, stepwise approach to managing type 2 diabetes.
As has been done previously, on Monday afternoon, there will be a joint ADA/European Association for the Study of Diabetes (EASD) session during which a draft of the latest update will be presented on the management of hyperglycemia in type 2 diabetes. The final version will be presented at the EASD meeting in September.
While it won’t include tirzepatide, as the drug is not yet approved in Europe, there will be discussion about the role of weight loss goals in type 2 diabetes management, Dr. Gabbay said.
The concept of a 15% weight loss as a primary treatment goal of type 2 diabetes management is a new focus, initiated at the EASD 2021 annual meeting and published in The Lancet.
“With tirzepatide becoming available, there’s the opportunity for more significant weight loss. So, there’s been this debate, starting with the somewhat controversial opinion piece in Lancet ... Maybe it was stating things a bit too far but it certainly got everyone in the field thinking. You’ll see that come up in lots of places at this meeting,” Dr. Gabbay said.
Indeed, those sessions include a Sunday morning symposium titled: “Obesity Management as a Primary Treatment Goal for Type 2 Diabetes – It’s Time for a Paradigm Shift,” in which speakers will address both lifestyle and pharmacologic intervention. On Saturday afternoon, two speakers will debate the question: “Weighing the Evidence – Should Obesity Be the Primary Target of Treatment in Type 2 Diabetes?” Yet another session on Sunday afternoon, will cover “Incorporating Weight Management Strategies for Obesity Into Type 2 Diabetes Care – Medical Management and Surgery.”
From weight loss to type 2 diabetes ‘remission’?
Related to the issue of weight loss as first-line therapy is the concept of type 2 diabetes “remission.” “There is a school of thought that says early in the course of disease we probably want to be a lot more aggressive because there’s a greater chance of putting someone into remission,” Dr. Gabbay noted. “The opportunities for remission after someone has had diabetes for a number of years are relatively low.”
In September 2021, ADA, along with EASD, the Endocrine Society, and Diabetes UK, published a joint consensus statement aiming to standardize use of the term “remission” in type 2 diabetes.
At the ADA meeting, a symposium on Monday afternoon, titled, “Definition and Interpretation of Remission in Type 2 Diabetes,” will cover lifestyle, pharmacotherapy, and metabolic surgery approaches. One noteworthy talk in that session will address the question: “Can Type 2 Diabetes Remission Be Diagnosed While Glucose-Lowering Drugs Are Being Used?”
Asked how all of this – tirzepatide, weight loss, and “remission” – might play out clinically, Dr. Dabelea replied: “We are still debating the strategy. That’s why we’re having the scientific talks.
“I think they will be very interesting and very well-attended, but there isn’t a strategy yet ... The important thing is we have these ‘miracle drugs,’ if you want, and once we’ve learned all we need to know about how they act and who we should target, perhaps next year we can talk about a strategy.”
Type 1 diabetes: Progress in preventing, treating, and ... curing?
Type 1 diabetes also will be well represented at the conference, with topics covering prevention, treatment, and progress toward a cure. On Saturday afternoon, a symposium will cover data from a trial of low-dose IL-2 in people with recently diagnosed type 1 diabetes, while a Friday afternoon symposium will address “Emerging Approaches to Beta Cell Replacement.”
On Saturday afternoon, a symposium will provide an update on islet cell transplantation, including immune tolerance strategies, while an oral abstract session will cover “Clinical Outcomes in Islet and Pancreas Transplantation.” And on Monday afternoon, yet another symposium will examine “Emerging Data on Therapies to Treat the Underlying Autoimmunity in Type 1 Diabetes.”
As usual, there will also be numerous presentations on the latest in diabetes technology. Particularly noteworthy among these will be an oral abstract presentation on Monday afternoon, “The CREATE Trial: Randomized Clinical Trial Comparing Open-Source Automated Insulin Delivery With Sensor Augmented Pump Therapy in Type 1 Diabetes,” and results from the insulin-only “bionic pancreas” pivotal randomized clinical trial on Friday afternoon.
“I’m happy to see a plethora of studies in type 1 diabetes. Dr. Dabelea said. “As with tirzepatide in type 2 diabetes, we are witnessing discoveries and we need to have some time to really understand the results, understand who are they targeting, who is going to benefit, and then move into a strategy.”
However, she added, in both type 1 and type 2 diabetes, “we’re seeing these disparities [where] these novel technologies and therapeutics are not getting to the people who need them most,” which brings up another major meeting theme, health disparities.
Overlapping themes: Disparities, mental health, and COVID-19
The topics of health disparities in diabetes prevention, management, and care and promoting health equity, as well as the impact of COVID-19, are “certainly timely this year,” said Dr. Dabelea.
At least eight meeting sessions will address various aspects of disparity, including a Friday afternoon symposium, “Race, Racism, and Diabetes Research,” a Saturday morning oral presentation on “Mitigating Disparities in the Screening and Diagnosis of Diabetes,” and on Monday morning, the symposium “Disparities in the Use of Diabetes Medications and Technologies.”
A related topic, insulin access, will be addressed in a Friday morning “mini-symposium” that will cover the issue from U.S. and international perspectives, including humanitarian crisis situations. Related to that, on Sunday afternoon a panel will discuss the Ukraine situation specifically.
Regarding mental health, one noteworthy session is a symposium on Saturday afternoon: “Suicide and Self-Injury – Unveiling and Addressing the Hidden Nightmare in Diabetes.”
“It’s an underrecognized problem and we’ve devoted a symposium to really drill into it. I think that’s going to be an important story for all of us to think about,” Dr. Gabbay said.
Another mental health session on Saturday afternoon will examine “Stigma in Diabetes Care – Evidence and Solutions.” Dr. Dabelea noted, “Mental health is a rising concern in the United States, especially in people with chronic diseases in the wake of the pandemic ... Of course there’s overlap in mechanisms in type 1 and type 2, but I think there are also distinct pathways.”
COVID-19 will be somewhat less of a focus than in the past 2 years, but there will certainly still be plenty about it. A Friday morning mini-symposium will cover new findings in pathophysiology, another session on Monday afternoon will look at the impact of the pandemic on hypoglycemia risk, and COVID-19 will be the subject of several late-breaking posters on Sunday afternoon. One in particular will report a review of diabetes as a risk factor for long COVID.
Celebrating in person in the Big Easy
But unlike the past 2 years, COVID-19 has not kept ADA from meeting in person in 2022. “I think it’s going to be amazing ... We’re so excited to be in person and interacting,” Dr. Gabbay said.
He observed that virtual meetings – as ADA and most other medical societies have been forced into for the past 2 years during the pandemic – fail to capture “how science is advanced by the casual conversations in the hallway and collaborations and new ideas. It’s really this incredible incubator. For me, that’s the most exciting part.”
The location, New Orleans, also factors into his excitement: “What a great place to do this. It’s conducive to celebrating. It’s been a long couple of years.”
A version of this article first appeared on Medscape.com.
The full results on Lilly’s tirzepatide for obesity will likely dominate the headlines from the annual scientific sessions of the American Diabetes Association, but the conference program is jam-packed with new findings – and new paradigms – in both type 1 and type 2 diabetes management and prevention.
Taking place June 3-7 both in person – for the first time in 3 years – in New Orleans, and virtually, the “hybrid” meeting is mandating COVID-19 vaccination and mask wearing for all on-site attendees.
A major topic will be new findings and thinking in the treatment of type 2 diabetes, including the new twincretin tirzepatide, as well as discussions about the role of weight loss and the concept of “remission.” In type 1 diabetes, sessions will examine intervention trials to prevent progression, progress in islet transplantation, and the latest findings in diabetes technology.
Other key conference themes include the often interrelated topics of disparities, mental health, and COVID-19.
“I think that the scientific planning committee has put together a really outstanding program this year, covering the entire spectrum of diabetes care and research and translation for both type 1 and type 2 diabetes,” Scientific Planning Committee Chair Dana Dabelea, MD, PhD, professor of epidemiology and pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, told this news organization.
Tirzepatide: The next big thing?
The presentation likely to generate the most buzz will take place Saturday morning, with the full detailed results from Lilly’s phase 3 SURMOUNT-1 trial of its dual-incretin tirzepatide for weight loss in people with obesity or overweight with at least one comorbidity but not diabetes.
Top-line results released by Lilly in April 2022 showed that the drug induced weight loss of up to 22%. Tirzepatide was approved May 13 by the Food and Drug Administration for type 2 diabetes under the brand name Mounjaro. It is not approved for weight loss.
“Certainly the general public will latch on to this idea that there is a drug they can lose 22% of their weight on,” Robert A. Gabbay, MD, PhD, ADA chief science and medical officer, told this news organization. “It’s hard to comment on a press release, so that’s why this presentation is going to be key.”
Another tirzepatide analysis, this one comparing its use to insulin glargine on kidney outcomes in participants with diabetes in the pivotal SURPASS-4 study, will be presented as an ADA Presidents’ Select Abstract on Friday afternoon.
“I think tirzepatide could be the great new thing, but I think we need to know a little bit more. Weight loss seems to be better than with glucagon-like peptide-1 (GLP-1) receptor agonists. Renal outcomes are important. Next will be to see if it has cardiovascular benefit. It makes one think about its use versus GLP-1 agonists,” Dr. Gabbay said.
Managing type 2 diabetes: Shifting paradigms
With the emergence of tirzepatide and other pharmacologic agents with benefits beyond glucose lowering, there has been much discussion in recent years about alternatives to the current metformin monotherapy first, stepwise approach to managing type 2 diabetes.
As has been done previously, on Monday afternoon, there will be a joint ADA/European Association for the Study of Diabetes (EASD) session during which a draft of the latest update will be presented on the management of hyperglycemia in type 2 diabetes. The final version will be presented at the EASD meeting in September.
While it won’t include tirzepatide, as the drug is not yet approved in Europe, there will be discussion about the role of weight loss goals in type 2 diabetes management, Dr. Gabbay said.
The concept of a 15% weight loss as a primary treatment goal of type 2 diabetes management is a new focus, initiated at the EASD 2021 annual meeting and published in The Lancet.
“With tirzepatide becoming available, there’s the opportunity for more significant weight loss. So, there’s been this debate, starting with the somewhat controversial opinion piece in Lancet ... Maybe it was stating things a bit too far but it certainly got everyone in the field thinking. You’ll see that come up in lots of places at this meeting,” Dr. Gabbay said.
Indeed, those sessions include a Sunday morning symposium titled: “Obesity Management as a Primary Treatment Goal for Type 2 Diabetes – It’s Time for a Paradigm Shift,” in which speakers will address both lifestyle and pharmacologic intervention. On Saturday afternoon, two speakers will debate the question: “Weighing the Evidence – Should Obesity Be the Primary Target of Treatment in Type 2 Diabetes?” Yet another session on Sunday afternoon, will cover “Incorporating Weight Management Strategies for Obesity Into Type 2 Diabetes Care – Medical Management and Surgery.”
From weight loss to type 2 diabetes ‘remission’?
Related to the issue of weight loss as first-line therapy is the concept of type 2 diabetes “remission.” “There is a school of thought that says early in the course of disease we probably want to be a lot more aggressive because there’s a greater chance of putting someone into remission,” Dr. Gabbay noted. “The opportunities for remission after someone has had diabetes for a number of years are relatively low.”
In September 2021, ADA, along with EASD, the Endocrine Society, and Diabetes UK, published a joint consensus statement aiming to standardize use of the term “remission” in type 2 diabetes.
At the ADA meeting, a symposium on Monday afternoon, titled, “Definition and Interpretation of Remission in Type 2 Diabetes,” will cover lifestyle, pharmacotherapy, and metabolic surgery approaches. One noteworthy talk in that session will address the question: “Can Type 2 Diabetes Remission Be Diagnosed While Glucose-Lowering Drugs Are Being Used?”
Asked how all of this – tirzepatide, weight loss, and “remission” – might play out clinically, Dr. Dabelea replied: “We are still debating the strategy. That’s why we’re having the scientific talks.
“I think they will be very interesting and very well-attended, but there isn’t a strategy yet ... The important thing is we have these ‘miracle drugs,’ if you want, and once we’ve learned all we need to know about how they act and who we should target, perhaps next year we can talk about a strategy.”
Type 1 diabetes: Progress in preventing, treating, and ... curing?
Type 1 diabetes also will be well represented at the conference, with topics covering prevention, treatment, and progress toward a cure. On Saturday afternoon, a symposium will cover data from a trial of low-dose IL-2 in people with recently diagnosed type 1 diabetes, while a Friday afternoon symposium will address “Emerging Approaches to Beta Cell Replacement.”
On Saturday afternoon, a symposium will provide an update on islet cell transplantation, including immune tolerance strategies, while an oral abstract session will cover “Clinical Outcomes in Islet and Pancreas Transplantation.” And on Monday afternoon, yet another symposium will examine “Emerging Data on Therapies to Treat the Underlying Autoimmunity in Type 1 Diabetes.”
As usual, there will also be numerous presentations on the latest in diabetes technology. Particularly noteworthy among these will be an oral abstract presentation on Monday afternoon, “The CREATE Trial: Randomized Clinical Trial Comparing Open-Source Automated Insulin Delivery With Sensor Augmented Pump Therapy in Type 1 Diabetes,” and results from the insulin-only “bionic pancreas” pivotal randomized clinical trial on Friday afternoon.
“I’m happy to see a plethora of studies in type 1 diabetes. Dr. Dabelea said. “As with tirzepatide in type 2 diabetes, we are witnessing discoveries and we need to have some time to really understand the results, understand who are they targeting, who is going to benefit, and then move into a strategy.”
However, she added, in both type 1 and type 2 diabetes, “we’re seeing these disparities [where] these novel technologies and therapeutics are not getting to the people who need them most,” which brings up another major meeting theme, health disparities.
Overlapping themes: Disparities, mental health, and COVID-19
The topics of health disparities in diabetes prevention, management, and care and promoting health equity, as well as the impact of COVID-19, are “certainly timely this year,” said Dr. Dabelea.
At least eight meeting sessions will address various aspects of disparity, including a Friday afternoon symposium, “Race, Racism, and Diabetes Research,” a Saturday morning oral presentation on “Mitigating Disparities in the Screening and Diagnosis of Diabetes,” and on Monday morning, the symposium “Disparities in the Use of Diabetes Medications and Technologies.”
A related topic, insulin access, will be addressed in a Friday morning “mini-symposium” that will cover the issue from U.S. and international perspectives, including humanitarian crisis situations. Related to that, on Sunday afternoon a panel will discuss the Ukraine situation specifically.
Regarding mental health, one noteworthy session is a symposium on Saturday afternoon: “Suicide and Self-Injury – Unveiling and Addressing the Hidden Nightmare in Diabetes.”
“It’s an underrecognized problem and we’ve devoted a symposium to really drill into it. I think that’s going to be an important story for all of us to think about,” Dr. Gabbay said.
Another mental health session on Saturday afternoon will examine “Stigma in Diabetes Care – Evidence and Solutions.” Dr. Dabelea noted, “Mental health is a rising concern in the United States, especially in people with chronic diseases in the wake of the pandemic ... Of course there’s overlap in mechanisms in type 1 and type 2, but I think there are also distinct pathways.”
COVID-19 will be somewhat less of a focus than in the past 2 years, but there will certainly still be plenty about it. A Friday morning mini-symposium will cover new findings in pathophysiology, another session on Monday afternoon will look at the impact of the pandemic on hypoglycemia risk, and COVID-19 will be the subject of several late-breaking posters on Sunday afternoon. One in particular will report a review of diabetes as a risk factor for long COVID.
Celebrating in person in the Big Easy
But unlike the past 2 years, COVID-19 has not kept ADA from meeting in person in 2022. “I think it’s going to be amazing ... We’re so excited to be in person and interacting,” Dr. Gabbay said.
He observed that virtual meetings – as ADA and most other medical societies have been forced into for the past 2 years during the pandemic – fail to capture “how science is advanced by the casual conversations in the hallway and collaborations and new ideas. It’s really this incredible incubator. For me, that’s the most exciting part.”
The location, New Orleans, also factors into his excitement: “What a great place to do this. It’s conducive to celebrating. It’s been a long couple of years.”
A version of this article first appeared on Medscape.com.
Calcified Urachal Remnant in a Young Adult: An Unusual Case
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.
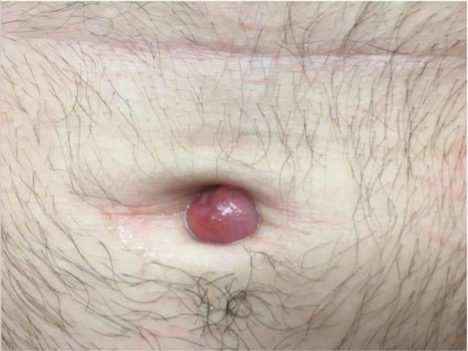
Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.
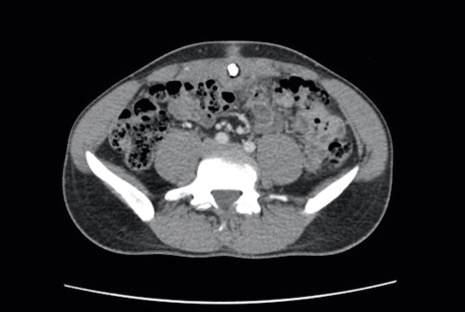
The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.
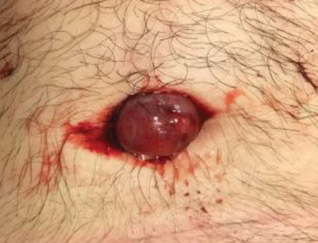
We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.

Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.

The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.

We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.

Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.

The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.

We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
Practice Points
- Visible umbilical nodules occur infrequently; the differential diagnosis is broad and consists of various benign and malignant pathologies.
- Disruption of the involution of the urachus during development can lead to various rare anomalies.
- Urachal anomalies are important to diagnose given the potential for secondary infection or malignancy.
Postirradiation Pseudosclerodermatous Panniculitis: A Rare Complication of Megavoltage External Beam Radiotherapy
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
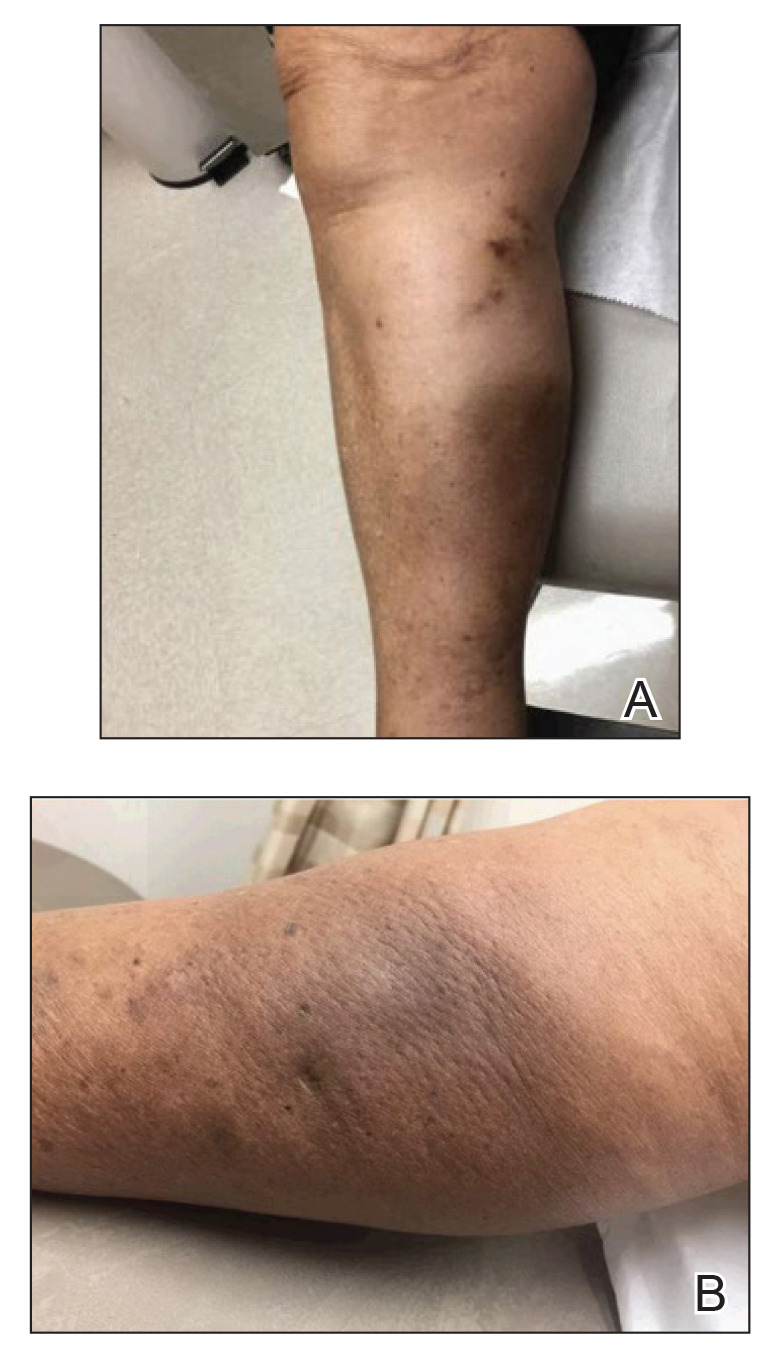
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
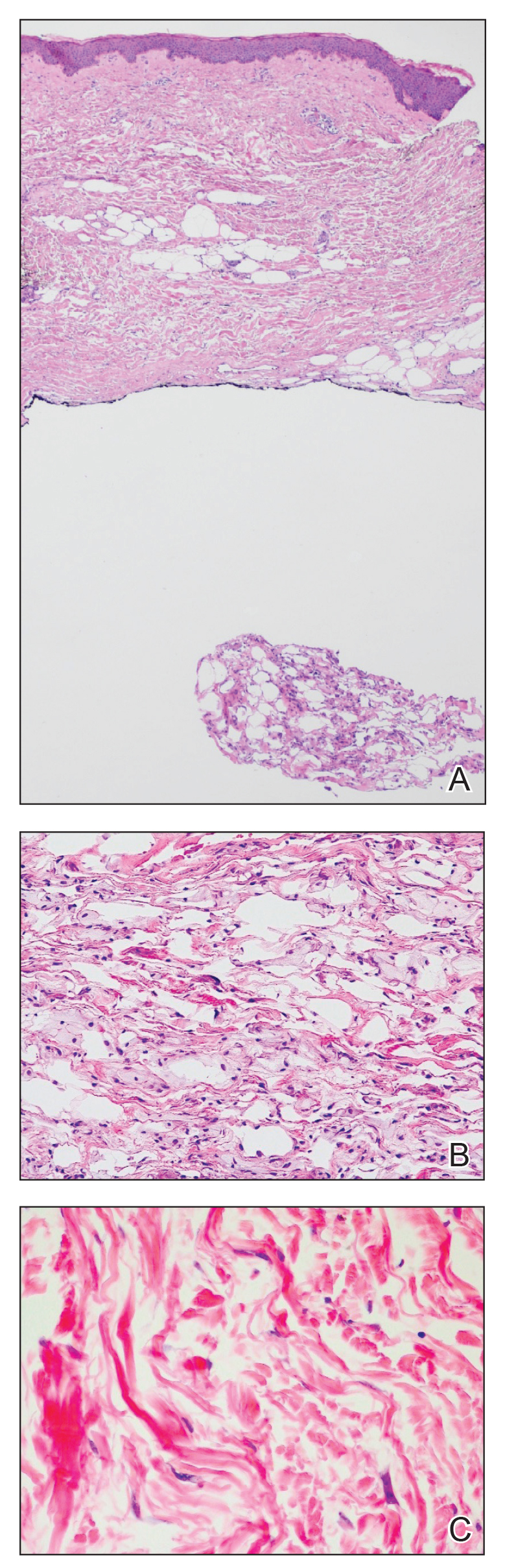
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.

The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.

Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.

The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.

Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
Practice Points
- Postirradiation pseudosclerodermatous panniculitis presents as an erythematous or indurated plaque at a site of prior radiotherapy.
- This rare entity may be underreported and requires biopsy for accurate diagnosis.
Serum brodalumab levels linked with treatment outcomes in patients with psoriasis
In a study of patients with psoriasis who had previously failed treatment with interleukin-17 receptor A inhibitor therapy, “all patients with quantifiable levels of brodalumab after 12 weeks of therapy experienced PASI reductions” and subquantifiable brodalumab levels were associated with a lack of response after 12 weeks, they wrote in JAMA Dermatology.
Lead study author Christian Enevold, PhD, a researcher at the Institute for Inflammation Research at Copenhagen University Hospital, and colleagues monitored patients with plaque psoriasis who had not improved with previous IL-17A inhibitor therapy, to evaluate whether trough levels and antidrug antibodies were associated with clinical response in this group of patients.
The 20 consecutive adult patients were treated at two academic hospital dermatology clinics between 2018 and 2020 and ranged in age from 19 to 66 years; 13 were male. At baseline, their weight ranged from 59 to 182 kg (median, 103 kg), their body mass index (BMI) ranged from 20 to 50 (median, 32), and their Psoriasis Area and Severity Index (PASI) scores ranged from 7 to 26 (median, 13). All had failed treatment with at least one IL-17A inhibitor, and 90% had failed treatment with at least one tumor necrosis factor–alpha or IL-12/-23 inhibitor.
Patients stopped taking systemic psoriasis therapies for 4 weeks before entering the study, then received subcutaneous injections of 210 mg of the IL-17A inhibitor brodalumab (Siliq) at weeks 0, 1, 2, and every 2 weeks thereafter. Patients whose PASI scores did not improve at least 75% from baseline (PASI 75) after 12 weeks of brodalumab discontinued treatment and left the study, while those who maintained PASI 75 were monitored for up to 52 weeks.
The researchers used assays to compare decreases in PASI score with brodalumab levels as well as with antibrodalumab antibodies at 12 weeks, and determined the following:
- Participants with quantifiable brodalumab levels (≥ 0.05 mcg/mL) showed a greater drop in PASI scores (median, 93%; range, 61%-100%) than those without quantifiable brodalumab levels (median, −3; range, −49% to 94%) (P = .006).
- Four of 5 patients (80%) who did not achieve a PASI 75, compared with 3 of 14 PASI 75 responders (21%), had drug levels too low to be measured (< 0.05 mcg/mL).
- The eight patients who did not have obesity (BMI < 30) had PASI reductions of at least 77%, and seven of the eight patients (88%) had quantifiable brodalumab levels.
- Six of the 12 patients with obesity (BMI ≥ 30) had brodalumab levels too low to be measured. Of those, four had increased PASI after 12 weeks of treatment. For all patients with obesity with quantifiable brodalumab levels, PASI scores dropped by at least 61% after 12 weeks.
- Five of the 12 (42%) patients with obesity versus 7 of the 8 (88%) patients without obesity had quantifiable brodalumab levels.
- None of the seven patients (35%) with subquantifiable drug levels after 12 weeks remained PASI responders.
- No antibrodalumab antibodies were detected in any serum samples.
The authors acknowledged that there were limitations of the study, including its retrospective design and restriction to the few available participants with a history of treatment failure.
George Han, MD, PhD, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said in an interview that he found the study interesting. “The authors did an admirable job looking at many factors to try to understand response to treatment in a challenging population of patients who had failed at least one, and in many cases, numerous, biologics from different classes.”
“The most interesting finding is that patients with higher BMIs had much higher rates of low-to-undetectable drug concentration,” said Dr. Han, who was not involved in the study. “This very practical finding could help patient care immediately. While it’s impractical to start performing assays of drug concentration in clinical practice, this finding certainly would guide my conversations with my heavier-set patients who have had multiple failures on previous biologics.
“I’m looking forward to further studies that explore this issue and provide better evidence-based guidance for treating patients who have experienced multi-biologic failure,” he added.
Robert A. Dorschner, MD, assistant professor of dermatology at the UC San Diego Health System, also welcomed the study’s results.
“Current psoriasis treatment is based on trial-and-error application of various biologics targeting different pathways, with initial selection frequently based on insurance preference, not patient characteristics,” he said in an interview.
“Studies like this help clinicians make more informed decisions about whether a patient may benefit from a different dose or may require a different drug, and make those decisions earlier in therapy,” he said. “This can improve patient care and decrease costs associated with prolonged treatments with ineffective drugs.”
But Dr. Dorschner, who also was not involved in the study, cautions clinicians to not draw conclusions about dose adjustments from these results. “These findings need to be verified in a larger cohort,” he advised, “and they should drive future studies with larger cohorts and prospective designs.”
“The last couple of decades have seen an explosion in the availability of biologics targeting different cytokines, with significant benefits to patients,” Dr. Dorschner explained. “However, there is a dearth of information on how to choose the right biologic for a particular patient and how to assess the benefit of dose alteration versus changing the drug target. Medicine needs more studies like this one.”
Several authors of the study report financial relationships with LEO Pharma and other pharmaceutical companies. Most authors, including Dr. Enevold, reported no relevant financial relationships. Dr. Dorschner reported no relevant financial relationships. Dr. Han reported financial relationships with pharmaceutical companies not involved in the study. The study was funded by LEO Pharma and the Danish Biotechnology Program.
In a study of patients with psoriasis who had previously failed treatment with interleukin-17 receptor A inhibitor therapy, “all patients with quantifiable levels of brodalumab after 12 weeks of therapy experienced PASI reductions” and subquantifiable brodalumab levels were associated with a lack of response after 12 weeks, they wrote in JAMA Dermatology.
Lead study author Christian Enevold, PhD, a researcher at the Institute for Inflammation Research at Copenhagen University Hospital, and colleagues monitored patients with plaque psoriasis who had not improved with previous IL-17A inhibitor therapy, to evaluate whether trough levels and antidrug antibodies were associated with clinical response in this group of patients.
The 20 consecutive adult patients were treated at two academic hospital dermatology clinics between 2018 and 2020 and ranged in age from 19 to 66 years; 13 were male. At baseline, their weight ranged from 59 to 182 kg (median, 103 kg), their body mass index (BMI) ranged from 20 to 50 (median, 32), and their Psoriasis Area and Severity Index (PASI) scores ranged from 7 to 26 (median, 13). All had failed treatment with at least one IL-17A inhibitor, and 90% had failed treatment with at least one tumor necrosis factor–alpha or IL-12/-23 inhibitor.
Patients stopped taking systemic psoriasis therapies for 4 weeks before entering the study, then received subcutaneous injections of 210 mg of the IL-17A inhibitor brodalumab (Siliq) at weeks 0, 1, 2, and every 2 weeks thereafter. Patients whose PASI scores did not improve at least 75% from baseline (PASI 75) after 12 weeks of brodalumab discontinued treatment and left the study, while those who maintained PASI 75 were monitored for up to 52 weeks.
The researchers used assays to compare decreases in PASI score with brodalumab levels as well as with antibrodalumab antibodies at 12 weeks, and determined the following:
- Participants with quantifiable brodalumab levels (≥ 0.05 mcg/mL) showed a greater drop in PASI scores (median, 93%; range, 61%-100%) than those without quantifiable brodalumab levels (median, −3; range, −49% to 94%) (P = .006).
- Four of 5 patients (80%) who did not achieve a PASI 75, compared with 3 of 14 PASI 75 responders (21%), had drug levels too low to be measured (< 0.05 mcg/mL).
- The eight patients who did not have obesity (BMI < 30) had PASI reductions of at least 77%, and seven of the eight patients (88%) had quantifiable brodalumab levels.
- Six of the 12 patients with obesity (BMI ≥ 30) had brodalumab levels too low to be measured. Of those, four had increased PASI after 12 weeks of treatment. For all patients with obesity with quantifiable brodalumab levels, PASI scores dropped by at least 61% after 12 weeks.
- Five of the 12 (42%) patients with obesity versus 7 of the 8 (88%) patients without obesity had quantifiable brodalumab levels.
- None of the seven patients (35%) with subquantifiable drug levels after 12 weeks remained PASI responders.
- No antibrodalumab antibodies were detected in any serum samples.
The authors acknowledged that there were limitations of the study, including its retrospective design and restriction to the few available participants with a history of treatment failure.
George Han, MD, PhD, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said in an interview that he found the study interesting. “The authors did an admirable job looking at many factors to try to understand response to treatment in a challenging population of patients who had failed at least one, and in many cases, numerous, biologics from different classes.”
“The most interesting finding is that patients with higher BMIs had much higher rates of low-to-undetectable drug concentration,” said Dr. Han, who was not involved in the study. “This very practical finding could help patient care immediately. While it’s impractical to start performing assays of drug concentration in clinical practice, this finding certainly would guide my conversations with my heavier-set patients who have had multiple failures on previous biologics.
“I’m looking forward to further studies that explore this issue and provide better evidence-based guidance for treating patients who have experienced multi-biologic failure,” he added.
Robert A. Dorschner, MD, assistant professor of dermatology at the UC San Diego Health System, also welcomed the study’s results.
“Current psoriasis treatment is based on trial-and-error application of various biologics targeting different pathways, with initial selection frequently based on insurance preference, not patient characteristics,” he said in an interview.
“Studies like this help clinicians make more informed decisions about whether a patient may benefit from a different dose or may require a different drug, and make those decisions earlier in therapy,” he said. “This can improve patient care and decrease costs associated with prolonged treatments with ineffective drugs.”
But Dr. Dorschner, who also was not involved in the study, cautions clinicians to not draw conclusions about dose adjustments from these results. “These findings need to be verified in a larger cohort,” he advised, “and they should drive future studies with larger cohorts and prospective designs.”
“The last couple of decades have seen an explosion in the availability of biologics targeting different cytokines, with significant benefits to patients,” Dr. Dorschner explained. “However, there is a dearth of information on how to choose the right biologic for a particular patient and how to assess the benefit of dose alteration versus changing the drug target. Medicine needs more studies like this one.”
Several authors of the study report financial relationships with LEO Pharma and other pharmaceutical companies. Most authors, including Dr. Enevold, reported no relevant financial relationships. Dr. Dorschner reported no relevant financial relationships. Dr. Han reported financial relationships with pharmaceutical companies not involved in the study. The study was funded by LEO Pharma and the Danish Biotechnology Program.
In a study of patients with psoriasis who had previously failed treatment with interleukin-17 receptor A inhibitor therapy, “all patients with quantifiable levels of brodalumab after 12 weeks of therapy experienced PASI reductions” and subquantifiable brodalumab levels were associated with a lack of response after 12 weeks, they wrote in JAMA Dermatology.
Lead study author Christian Enevold, PhD, a researcher at the Institute for Inflammation Research at Copenhagen University Hospital, and colleagues monitored patients with plaque psoriasis who had not improved with previous IL-17A inhibitor therapy, to evaluate whether trough levels and antidrug antibodies were associated with clinical response in this group of patients.
The 20 consecutive adult patients were treated at two academic hospital dermatology clinics between 2018 and 2020 and ranged in age from 19 to 66 years; 13 were male. At baseline, their weight ranged from 59 to 182 kg (median, 103 kg), their body mass index (BMI) ranged from 20 to 50 (median, 32), and their Psoriasis Area and Severity Index (PASI) scores ranged from 7 to 26 (median, 13). All had failed treatment with at least one IL-17A inhibitor, and 90% had failed treatment with at least one tumor necrosis factor–alpha or IL-12/-23 inhibitor.
Patients stopped taking systemic psoriasis therapies for 4 weeks before entering the study, then received subcutaneous injections of 210 mg of the IL-17A inhibitor brodalumab (Siliq) at weeks 0, 1, 2, and every 2 weeks thereafter. Patients whose PASI scores did not improve at least 75% from baseline (PASI 75) after 12 weeks of brodalumab discontinued treatment and left the study, while those who maintained PASI 75 were monitored for up to 52 weeks.
The researchers used assays to compare decreases in PASI score with brodalumab levels as well as with antibrodalumab antibodies at 12 weeks, and determined the following:
- Participants with quantifiable brodalumab levels (≥ 0.05 mcg/mL) showed a greater drop in PASI scores (median, 93%; range, 61%-100%) than those without quantifiable brodalumab levels (median, −3; range, −49% to 94%) (P = .006).
- Four of 5 patients (80%) who did not achieve a PASI 75, compared with 3 of 14 PASI 75 responders (21%), had drug levels too low to be measured (< 0.05 mcg/mL).
- The eight patients who did not have obesity (BMI < 30) had PASI reductions of at least 77%, and seven of the eight patients (88%) had quantifiable brodalumab levels.
- Six of the 12 patients with obesity (BMI ≥ 30) had brodalumab levels too low to be measured. Of those, four had increased PASI after 12 weeks of treatment. For all patients with obesity with quantifiable brodalumab levels, PASI scores dropped by at least 61% after 12 weeks.
- Five of the 12 (42%) patients with obesity versus 7 of the 8 (88%) patients without obesity had quantifiable brodalumab levels.
- None of the seven patients (35%) with subquantifiable drug levels after 12 weeks remained PASI responders.
- No antibrodalumab antibodies were detected in any serum samples.
The authors acknowledged that there were limitations of the study, including its retrospective design and restriction to the few available participants with a history of treatment failure.
George Han, MD, PhD, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said in an interview that he found the study interesting. “The authors did an admirable job looking at many factors to try to understand response to treatment in a challenging population of patients who had failed at least one, and in many cases, numerous, biologics from different classes.”
“The most interesting finding is that patients with higher BMIs had much higher rates of low-to-undetectable drug concentration,” said Dr. Han, who was not involved in the study. “This very practical finding could help patient care immediately. While it’s impractical to start performing assays of drug concentration in clinical practice, this finding certainly would guide my conversations with my heavier-set patients who have had multiple failures on previous biologics.
“I’m looking forward to further studies that explore this issue and provide better evidence-based guidance for treating patients who have experienced multi-biologic failure,” he added.
Robert A. Dorschner, MD, assistant professor of dermatology at the UC San Diego Health System, also welcomed the study’s results.
“Current psoriasis treatment is based on trial-and-error application of various biologics targeting different pathways, with initial selection frequently based on insurance preference, not patient characteristics,” he said in an interview.
“Studies like this help clinicians make more informed decisions about whether a patient may benefit from a different dose or may require a different drug, and make those decisions earlier in therapy,” he said. “This can improve patient care and decrease costs associated with prolonged treatments with ineffective drugs.”
But Dr. Dorschner, who also was not involved in the study, cautions clinicians to not draw conclusions about dose adjustments from these results. “These findings need to be verified in a larger cohort,” he advised, “and they should drive future studies with larger cohorts and prospective designs.”
“The last couple of decades have seen an explosion in the availability of biologics targeting different cytokines, with significant benefits to patients,” Dr. Dorschner explained. “However, there is a dearth of information on how to choose the right biologic for a particular patient and how to assess the benefit of dose alteration versus changing the drug target. Medicine needs more studies like this one.”
Several authors of the study report financial relationships with LEO Pharma and other pharmaceutical companies. Most authors, including Dr. Enevold, reported no relevant financial relationships. Dr. Dorschner reported no relevant financial relationships. Dr. Han reported financial relationships with pharmaceutical companies not involved in the study. The study was funded by LEO Pharma and the Danish Biotechnology Program.
FROM JAMA DERMATOLOGY
Today’s medical oxymoron: Healthy overconfidence
Doctor, doctor, gimme the news. I got a bad case of knowing better than you
Stop us if you’ve heard this before. One of your parents (let’s be honest, probably your ornery father) refuses to go to the doctor. You tell him it’s for the best, but in his words, “Doctors don’t know nothin’. I’m fine.” How many TV shows with grumpy fathers feature this exact plot in an episode as the frustrated child attempts increasingly convoluted traps to encourage the stubborn parent to get himself to the doctor?
As is so often the case, wacky sitcoms reflect reality, according to a new study from the Journal of the Economics of Aging. In a massive survey of 80,000 Europeans aged 50 years and older, the researchers found that individuals who were overconfident and rated their health as better than it actually was visited their doctor 17% less often than did those who correctly judge their own health. Fewer medical visits leaves them more vulnerable to chronic disease, since they’re not getting the preventive care they need to catch illnesses early.
Perhaps unsurprisingly, the inverse is also true: People who underestimate their health status visit the doctor 21% more often. On the one hand, regular visits to the doctor are a good thing, as is awareness of how healthy one really is. On the other hand, though, extra visits cost money and time, especially relevant in an aging society with high public health costs.
Nobody likes visiting the doctor, but it is kind of important, especially as we age and our bodies start to let us down. Confidence is fine, but don’t be overly confident. And if you do go, don’t be like a certain former president of the United States. Don’t pay a sycophant to look in your general direction and then declare that you are in very good (great!) condition on Twitter. That’s not how medicine is meant to work.
Your liver stays toddler age
Rapid cell regeneration might seem like something straight out of a sci-fi novel, but it happens to your liver all the time. So much so that the human liver is never a day over 3 years old.
How’s that possible? The liver deals with a lot of toxic substances in its job as the Brita filter of the human body, so it has a unique capacity among organs to regenerate itself after damage.
Dr. Olaf Bergmann and his team at Technical University Dresden’s (Germany) Center for Regenerative Therapies used retrospective radiocarbon birth dating to determine the age of the livers of a group of people who died at the ages of 20-84 years. The results were the same regardless of age.
This information could be a complete game changer for understanding cell regeneration. It’s important in determining cancer cell formation in the liver but also if new heart muscle cells can be generated in people with cardiovascular disease, which the researchers are looking into.
So sure, your liver may be totally capable of filtering those drinks at happy hour, but as old as it is, a juice box might be more appropriate.
To bee, or not to bee? That is the vacation
Sleeping is pretty important for humans, no doubt about that, so anything that improves sleep is worth considering, right? But how far would you go for a good night’s sleep? Would you be willing to travel to Italy to experience the ultimate white-noise generator?
For more on this exciting, yet also sleep-inducing, news story, let’s go to the village of Grottole in southern Italy, where we meet bee keeper and Airbnb host Rocco Filomeno. ”This is the first place in the world where you can sleep immersed in the distinctive sound and aroma of the bees, experiencing ‘bee-therapy’ in the most authentic and natural way,” he said in a written statement for Airbnb.
Mr. Filomeno worked with local NGO Wonder Grottole and a self-build specialist to take the next step in tiny-house evolution. The resulting structure cost just $17,000 – crowdfunded, of course, and built by 25 local bee-lievers (aka volunteers) – and consists of a single room surrounded by nine apiaries, which contain a combined total of 1 million working bees. It is now available to book on Airbnb, and guests “will receive their first lesson on bees and how to live with them,” Airbnb said.
The immersion in bee sound/scent is fully realized through the building’s most prominent interior feature, a screened box in the ceiling with a working hive that allows guests to see the bees and fall asleep to the “gently humming sound,” Airbnb explained. The sound from the hive is said to have a soothing effect that “acts as salve to day-to-day stressors,” according to the BBC.
This is just the start of a trend and we want in on it. Should our tiny house feature the sights/smells/sounds of angry rattlesnakes or a swarm of locusts?
Joysticks can make the world a better place
Someday, it might be possible for surgeons to treat a stroke or aneurysm during the “golden hour,” even if they’re not in the same hospital as the patient. MIT engineers have created a robotic system that can be controlled remotely with a modified joystick, so the patient can go to a closer, smaller hospital and be treated by a surgeon at a larger facility through live imaging.
Endovascular surgery seems difficult enough with the patient and doctor in the same hospital, “but having a robot twist with the same level of sophistication [as a surgeon] is challenging,” Yoonho Kim, lead author of a study in Science Robotics, said in a written statement. “Our system is based on a fundamentally different mechanism.”
It involves “a medical-grade robotic arm with a magnet attached to its wrist. With a joystick and live imaging, an operator can adjust the magnet’s orientation and manipulate the arm to guide a soft and thin magnetic wire through arteries and vessels,” MIT explained in the statement.
The system was tested using life-like models, and it took each surgeon about an hour of training to learn how to use the new joystick and other equipment. Another perk: No exposure to radiation from x-ray imaging.
If someone you know is obsessed with video games, stop thinking “slacker” and start thinking “neurosurgeon.”
Doctor, doctor, gimme the news. I got a bad case of knowing better than you
Stop us if you’ve heard this before. One of your parents (let’s be honest, probably your ornery father) refuses to go to the doctor. You tell him it’s for the best, but in his words, “Doctors don’t know nothin’. I’m fine.” How many TV shows with grumpy fathers feature this exact plot in an episode as the frustrated child attempts increasingly convoluted traps to encourage the stubborn parent to get himself to the doctor?
As is so often the case, wacky sitcoms reflect reality, according to a new study from the Journal of the Economics of Aging. In a massive survey of 80,000 Europeans aged 50 years and older, the researchers found that individuals who were overconfident and rated their health as better than it actually was visited their doctor 17% less often than did those who correctly judge their own health. Fewer medical visits leaves them more vulnerable to chronic disease, since they’re not getting the preventive care they need to catch illnesses early.
Perhaps unsurprisingly, the inverse is also true: People who underestimate their health status visit the doctor 21% more often. On the one hand, regular visits to the doctor are a good thing, as is awareness of how healthy one really is. On the other hand, though, extra visits cost money and time, especially relevant in an aging society with high public health costs.
Nobody likes visiting the doctor, but it is kind of important, especially as we age and our bodies start to let us down. Confidence is fine, but don’t be overly confident. And if you do go, don’t be like a certain former president of the United States. Don’t pay a sycophant to look in your general direction and then declare that you are in very good (great!) condition on Twitter. That’s not how medicine is meant to work.
Your liver stays toddler age
Rapid cell regeneration might seem like something straight out of a sci-fi novel, but it happens to your liver all the time. So much so that the human liver is never a day over 3 years old.
How’s that possible? The liver deals with a lot of toxic substances in its job as the Brita filter of the human body, so it has a unique capacity among organs to regenerate itself after damage.
Dr. Olaf Bergmann and his team at Technical University Dresden’s (Germany) Center for Regenerative Therapies used retrospective radiocarbon birth dating to determine the age of the livers of a group of people who died at the ages of 20-84 years. The results were the same regardless of age.
This information could be a complete game changer for understanding cell regeneration. It’s important in determining cancer cell formation in the liver but also if new heart muscle cells can be generated in people with cardiovascular disease, which the researchers are looking into.
So sure, your liver may be totally capable of filtering those drinks at happy hour, but as old as it is, a juice box might be more appropriate.
To bee, or not to bee? That is the vacation
Sleeping is pretty important for humans, no doubt about that, so anything that improves sleep is worth considering, right? But how far would you go for a good night’s sleep? Would you be willing to travel to Italy to experience the ultimate white-noise generator?
For more on this exciting, yet also sleep-inducing, news story, let’s go to the village of Grottole in southern Italy, where we meet bee keeper and Airbnb host Rocco Filomeno. ”This is the first place in the world where you can sleep immersed in the distinctive sound and aroma of the bees, experiencing ‘bee-therapy’ in the most authentic and natural way,” he said in a written statement for Airbnb.
Mr. Filomeno worked with local NGO Wonder Grottole and a self-build specialist to take the next step in tiny-house evolution. The resulting structure cost just $17,000 – crowdfunded, of course, and built by 25 local bee-lievers (aka volunteers) – and consists of a single room surrounded by nine apiaries, which contain a combined total of 1 million working bees. It is now available to book on Airbnb, and guests “will receive their first lesson on bees and how to live with them,” Airbnb said.
The immersion in bee sound/scent is fully realized through the building’s most prominent interior feature, a screened box in the ceiling with a working hive that allows guests to see the bees and fall asleep to the “gently humming sound,” Airbnb explained. The sound from the hive is said to have a soothing effect that “acts as salve to day-to-day stressors,” according to the BBC.
This is just the start of a trend and we want in on it. Should our tiny house feature the sights/smells/sounds of angry rattlesnakes or a swarm of locusts?
Joysticks can make the world a better place
Someday, it might be possible for surgeons to treat a stroke or aneurysm during the “golden hour,” even if they’re not in the same hospital as the patient. MIT engineers have created a robotic system that can be controlled remotely with a modified joystick, so the patient can go to a closer, smaller hospital and be treated by a surgeon at a larger facility through live imaging.
Endovascular surgery seems difficult enough with the patient and doctor in the same hospital, “but having a robot twist with the same level of sophistication [as a surgeon] is challenging,” Yoonho Kim, lead author of a study in Science Robotics, said in a written statement. “Our system is based on a fundamentally different mechanism.”
It involves “a medical-grade robotic arm with a magnet attached to its wrist. With a joystick and live imaging, an operator can adjust the magnet’s orientation and manipulate the arm to guide a soft and thin magnetic wire through arteries and vessels,” MIT explained in the statement.
The system was tested using life-like models, and it took each surgeon about an hour of training to learn how to use the new joystick and other equipment. Another perk: No exposure to radiation from x-ray imaging.
If someone you know is obsessed with video games, stop thinking “slacker” and start thinking “neurosurgeon.”
Doctor, doctor, gimme the news. I got a bad case of knowing better than you
Stop us if you’ve heard this before. One of your parents (let’s be honest, probably your ornery father) refuses to go to the doctor. You tell him it’s for the best, but in his words, “Doctors don’t know nothin’. I’m fine.” How many TV shows with grumpy fathers feature this exact plot in an episode as the frustrated child attempts increasingly convoluted traps to encourage the stubborn parent to get himself to the doctor?
As is so often the case, wacky sitcoms reflect reality, according to a new study from the Journal of the Economics of Aging. In a massive survey of 80,000 Europeans aged 50 years and older, the researchers found that individuals who were overconfident and rated their health as better than it actually was visited their doctor 17% less often than did those who correctly judge their own health. Fewer medical visits leaves them more vulnerable to chronic disease, since they’re not getting the preventive care they need to catch illnesses early.
Perhaps unsurprisingly, the inverse is also true: People who underestimate their health status visit the doctor 21% more often. On the one hand, regular visits to the doctor are a good thing, as is awareness of how healthy one really is. On the other hand, though, extra visits cost money and time, especially relevant in an aging society with high public health costs.
Nobody likes visiting the doctor, but it is kind of important, especially as we age and our bodies start to let us down. Confidence is fine, but don’t be overly confident. And if you do go, don’t be like a certain former president of the United States. Don’t pay a sycophant to look in your general direction and then declare that you are in very good (great!) condition on Twitter. That’s not how medicine is meant to work.
Your liver stays toddler age
Rapid cell regeneration might seem like something straight out of a sci-fi novel, but it happens to your liver all the time. So much so that the human liver is never a day over 3 years old.
How’s that possible? The liver deals with a lot of toxic substances in its job as the Brita filter of the human body, so it has a unique capacity among organs to regenerate itself after damage.
Dr. Olaf Bergmann and his team at Technical University Dresden’s (Germany) Center for Regenerative Therapies used retrospective radiocarbon birth dating to determine the age of the livers of a group of people who died at the ages of 20-84 years. The results were the same regardless of age.
This information could be a complete game changer for understanding cell regeneration. It’s important in determining cancer cell formation in the liver but also if new heart muscle cells can be generated in people with cardiovascular disease, which the researchers are looking into.
So sure, your liver may be totally capable of filtering those drinks at happy hour, but as old as it is, a juice box might be more appropriate.
To bee, or not to bee? That is the vacation
Sleeping is pretty important for humans, no doubt about that, so anything that improves sleep is worth considering, right? But how far would you go for a good night’s sleep? Would you be willing to travel to Italy to experience the ultimate white-noise generator?
For more on this exciting, yet also sleep-inducing, news story, let’s go to the village of Grottole in southern Italy, where we meet bee keeper and Airbnb host Rocco Filomeno. ”This is the first place in the world where you can sleep immersed in the distinctive sound and aroma of the bees, experiencing ‘bee-therapy’ in the most authentic and natural way,” he said in a written statement for Airbnb.
Mr. Filomeno worked with local NGO Wonder Grottole and a self-build specialist to take the next step in tiny-house evolution. The resulting structure cost just $17,000 – crowdfunded, of course, and built by 25 local bee-lievers (aka volunteers) – and consists of a single room surrounded by nine apiaries, which contain a combined total of 1 million working bees. It is now available to book on Airbnb, and guests “will receive their first lesson on bees and how to live with them,” Airbnb said.
The immersion in bee sound/scent is fully realized through the building’s most prominent interior feature, a screened box in the ceiling with a working hive that allows guests to see the bees and fall asleep to the “gently humming sound,” Airbnb explained. The sound from the hive is said to have a soothing effect that “acts as salve to day-to-day stressors,” according to the BBC.
This is just the start of a trend and we want in on it. Should our tiny house feature the sights/smells/sounds of angry rattlesnakes or a swarm of locusts?
Joysticks can make the world a better place
Someday, it might be possible for surgeons to treat a stroke or aneurysm during the “golden hour,” even if they’re not in the same hospital as the patient. MIT engineers have created a robotic system that can be controlled remotely with a modified joystick, so the patient can go to a closer, smaller hospital and be treated by a surgeon at a larger facility through live imaging.
Endovascular surgery seems difficult enough with the patient and doctor in the same hospital, “but having a robot twist with the same level of sophistication [as a surgeon] is challenging,” Yoonho Kim, lead author of a study in Science Robotics, said in a written statement. “Our system is based on a fundamentally different mechanism.”
It involves “a medical-grade robotic arm with a magnet attached to its wrist. With a joystick and live imaging, an operator can adjust the magnet’s orientation and manipulate the arm to guide a soft and thin magnetic wire through arteries and vessels,” MIT explained in the statement.
The system was tested using life-like models, and it took each surgeon about an hour of training to learn how to use the new joystick and other equipment. Another perk: No exposure to radiation from x-ray imaging.
If someone you know is obsessed with video games, stop thinking “slacker” and start thinking “neurosurgeon.”