User login
Müllerian anomalies: Operative considerations
Telemedicine in cancer care: Not all patients can access
CHICAGO – for patients with cancer, but uptake of telemedicine was plagued by inequities, a retrospective study suggests.
Before March 2020, only a very small percentage of patients with cancer used telemedicine services.
By November 2021, nearly 16% of patients initiating cancer treatment were using this approach.
However, certain groups were less likely to use telemedicine, in particular, patients who were Black, uninsured, did not live in cities, and were less affluent, noted lead author Jenny S. Guadamuz, PhD, a quantitative scientist at Flatiron Health and a postdoctoral research associate at the University of Southern California, Los Angeles.
The results are concerning because they suggest that telemedicine expansion could widen cancer care disparities, Dr. Guadamuz said. Previous studies found racial disparities in care access and outcomes early on in the pandemic.
“These findings are critically important considering recent efforts to make coverage of telemedicine services permanent, instead of tied to the [Health and Human Services] public health emergency declaration,” she said. “There are also efforts to increase reimbursement rates for telemedicine services by Medicare, several Medicaid programs, and private insurers.”
This study was highlighted at a press briefing held in advance of the American Society of Clinical Oncology annual meeting, where it will be presented at a poster session (abstract 6511).
ASCO President Everett E. Vokes, MD, said telemedicine is “an important tool to communicate with patients” but that it is important to consider the “digital divide.”
He also emphasized the need “to expand and learn to use telehealth not in a crisis but as part of our regular care moving forward.” In July 2021, as telemedicine services were expanding, ASCO released practice recommendations specific to telehealth and oncology.
“Telemedicine can improve access to timely cancer care, but, as this study points out, telemedicine must be available equitably, so that every patient can access the care they need and deserve,” he said in a press statement.
Study details
For the study, Dr. Guadamuz and colleagues assessed telemedicine uptake by nearly 27,000 patients in a Flatiron electronic health record–derived deidentified database of patients who initiated treatment for any of 21 common cancers at about 280 community oncology clinics between March 2020 and November 2021.
They found that Black patients were significantly less likely than were White patients to use telemedicine (13.2% vs. 15.6%; odds ratio [OR], 0.82), as were patients without documented insurance, compared with those who were well insured (11.6% vs. 16.4%; OR, 0.68).
Those in rural and suburban areas were less likely than were those in urban areas to use telemedicine (9.7% and 13.0% vs. 17.7%; ORs, 0.50 and 0.69, respectively), and those in less affluent vs. more affluent areas were also less likely to use telemedicine (10.6% vs. 23.6%, OR, 0.39).
Dr. Guadamuz noted that the differences remained statistically significant after adjustment for clinical characteristics and that racial inequities were seen across cancer types and over time.
Future work should assess other potential characteristics associated with telemedicine inequities, evaluate whether health care delivered via telemedicine is of similar quality as in-person services, and determine the types of practice that are providing telemedicine more equitably to their patients, she concluded.
A version of this article first appeared on Medscape.com.
CHICAGO – for patients with cancer, but uptake of telemedicine was plagued by inequities, a retrospective study suggests.
Before March 2020, only a very small percentage of patients with cancer used telemedicine services.
By November 2021, nearly 16% of patients initiating cancer treatment were using this approach.
However, certain groups were less likely to use telemedicine, in particular, patients who were Black, uninsured, did not live in cities, and were less affluent, noted lead author Jenny S. Guadamuz, PhD, a quantitative scientist at Flatiron Health and a postdoctoral research associate at the University of Southern California, Los Angeles.
The results are concerning because they suggest that telemedicine expansion could widen cancer care disparities, Dr. Guadamuz said. Previous studies found racial disparities in care access and outcomes early on in the pandemic.
“These findings are critically important considering recent efforts to make coverage of telemedicine services permanent, instead of tied to the [Health and Human Services] public health emergency declaration,” she said. “There are also efforts to increase reimbursement rates for telemedicine services by Medicare, several Medicaid programs, and private insurers.”
This study was highlighted at a press briefing held in advance of the American Society of Clinical Oncology annual meeting, where it will be presented at a poster session (abstract 6511).
ASCO President Everett E. Vokes, MD, said telemedicine is “an important tool to communicate with patients” but that it is important to consider the “digital divide.”
He also emphasized the need “to expand and learn to use telehealth not in a crisis but as part of our regular care moving forward.” In July 2021, as telemedicine services were expanding, ASCO released practice recommendations specific to telehealth and oncology.
“Telemedicine can improve access to timely cancer care, but, as this study points out, telemedicine must be available equitably, so that every patient can access the care they need and deserve,” he said in a press statement.
Study details
For the study, Dr. Guadamuz and colleagues assessed telemedicine uptake by nearly 27,000 patients in a Flatiron electronic health record–derived deidentified database of patients who initiated treatment for any of 21 common cancers at about 280 community oncology clinics between March 2020 and November 2021.
They found that Black patients were significantly less likely than were White patients to use telemedicine (13.2% vs. 15.6%; odds ratio [OR], 0.82), as were patients without documented insurance, compared with those who were well insured (11.6% vs. 16.4%; OR, 0.68).
Those in rural and suburban areas were less likely than were those in urban areas to use telemedicine (9.7% and 13.0% vs. 17.7%; ORs, 0.50 and 0.69, respectively), and those in less affluent vs. more affluent areas were also less likely to use telemedicine (10.6% vs. 23.6%, OR, 0.39).
Dr. Guadamuz noted that the differences remained statistically significant after adjustment for clinical characteristics and that racial inequities were seen across cancer types and over time.
Future work should assess other potential characteristics associated with telemedicine inequities, evaluate whether health care delivered via telemedicine is of similar quality as in-person services, and determine the types of practice that are providing telemedicine more equitably to their patients, she concluded.
A version of this article first appeared on Medscape.com.
CHICAGO – for patients with cancer, but uptake of telemedicine was plagued by inequities, a retrospective study suggests.
Before March 2020, only a very small percentage of patients with cancer used telemedicine services.
By November 2021, nearly 16% of patients initiating cancer treatment were using this approach.
However, certain groups were less likely to use telemedicine, in particular, patients who were Black, uninsured, did not live in cities, and were less affluent, noted lead author Jenny S. Guadamuz, PhD, a quantitative scientist at Flatiron Health and a postdoctoral research associate at the University of Southern California, Los Angeles.
The results are concerning because they suggest that telemedicine expansion could widen cancer care disparities, Dr. Guadamuz said. Previous studies found racial disparities in care access and outcomes early on in the pandemic.
“These findings are critically important considering recent efforts to make coverage of telemedicine services permanent, instead of tied to the [Health and Human Services] public health emergency declaration,” she said. “There are also efforts to increase reimbursement rates for telemedicine services by Medicare, several Medicaid programs, and private insurers.”
This study was highlighted at a press briefing held in advance of the American Society of Clinical Oncology annual meeting, where it will be presented at a poster session (abstract 6511).
ASCO President Everett E. Vokes, MD, said telemedicine is “an important tool to communicate with patients” but that it is important to consider the “digital divide.”
He also emphasized the need “to expand and learn to use telehealth not in a crisis but as part of our regular care moving forward.” In July 2021, as telemedicine services were expanding, ASCO released practice recommendations specific to telehealth and oncology.
“Telemedicine can improve access to timely cancer care, but, as this study points out, telemedicine must be available equitably, so that every patient can access the care they need and deserve,” he said in a press statement.
Study details
For the study, Dr. Guadamuz and colleagues assessed telemedicine uptake by nearly 27,000 patients in a Flatiron electronic health record–derived deidentified database of patients who initiated treatment for any of 21 common cancers at about 280 community oncology clinics between March 2020 and November 2021.
They found that Black patients were significantly less likely than were White patients to use telemedicine (13.2% vs. 15.6%; odds ratio [OR], 0.82), as were patients without documented insurance, compared with those who were well insured (11.6% vs. 16.4%; OR, 0.68).
Those in rural and suburban areas were less likely than were those in urban areas to use telemedicine (9.7% and 13.0% vs. 17.7%; ORs, 0.50 and 0.69, respectively), and those in less affluent vs. more affluent areas were also less likely to use telemedicine (10.6% vs. 23.6%, OR, 0.39).
Dr. Guadamuz noted that the differences remained statistically significant after adjustment for clinical characteristics and that racial inequities were seen across cancer types and over time.
Future work should assess other potential characteristics associated with telemedicine inequities, evaluate whether health care delivered via telemedicine is of similar quality as in-person services, and determine the types of practice that are providing telemedicine more equitably to their patients, she concluded.
A version of this article first appeared on Medscape.com.
AT ASCO 2022
Increased social services spending ups cancer survival of Blacks
Five-year overall survival increased among non-Hispanic Black patients by 2.02% in conjunction with a 10% increase in spending. In addition, there was a decrease in racial disparities in survival between non-Hispanic Black patients and White patients for many types of cancers.
However, public welfare spending had no real impact on the overall 5-year survival for the entire cohort (0.25 % per 10% increase in spending; P = .78) or for non-Hispanic White patients (0.52% per 10% increase in spending, P = .58).
“We know from prior research that outcomes are worse for minorities,” said lead author Justin Michael Barnes, MD, from the department of radiation oncology at Washington University in St. Louis, Mo. “It’s thought that some of the differences are related to impaired access to health care for minorities, which is related to social determinants of health. This includes socioeconomic factors, educational attainment, place of residence, as well as environmental stressors.
“Our data show that greater state welfare expenditures were associated with greater 5-year survival among Black patients and decreased Black–White disparities,” said Dr. Barnes. “I think these data are thought provoking, but they certainly aren’t the end. I see these data as a proof-of-concept project.”
Dr. Barnes reported the findings at a press conference held in advance of the annual meeting of the American Society of Clinical Oncology, during which the study will be presented (Abstract 6509).
Improved 5-year survival in Black patients
For the study, Dr. Barnes and colleagues evaluated the association of 5-year overall survival and public welfare spending in 2,925,550 individuals aged 18 years and older who were diagnosed with cancer during the period 2007-2016. The cohort was drawn from the Surveillance, Epidemiology, and End Results Program. In addition, annual state spending data were obtained from the U.S. Census Bureau. The team examined survival outcomes by race and ethnicity as well as by cancer site. The investigators accounted for factors such as age, sex, metropolitan residence, state, county-level income and education, insurance status, cancer site, stage at diagnosis, and year of diagnosis.
Much of public welfare spending was related to Medicaid but also included programs that provide subsidy assistance for individuals, such as Supplemental Security Income.
As compared with White patients, the 5-year overall survival rate was 10.8% lower among non-Hispanic Black patients. But there was a 4.46% (P for interaction <.001) narrowing of the 5-year overall survival disparity in non-Hispanic Black patients in comparison with White patients per 10% increase in spending, or a 42% closure of the 10.8% disparity.
Regarding specific cancer types, increased public welfare spending was associated with a narrowing of the 5-year overall survival disparity between Black patients and non-Hispanic White patients for the following cancers: breast (a 6.15% survival increase for Black patients led to a 39% closing of the disparity), cervix (a 11.9% survival increase led to a 46% closing of the disparity), colorectum (a 4.42% survival increase led to a 48% closing of the disparity), head and neck (a 9.41% survival increase led to a 38% closing of the disparity), liver (a 7.02% survival increase led to a 49% closing of the disparity), ovary (an 8.95% survival increase led to a 41% closing of the disparity), bladder (an 8.18% survival increase led to a 44% closing of the disparity), and uterus (a 14.1% survival increase led to a 40% closing of the disparity).
“Some type of public welfare seems to be helping improve oncologic outcomes for some of our most socioeconomically at-risk patients, but we don’t know the specifics,” Dr. Barnes concluded. “Additional work is needed to identify the most influential public health expenditures. If we can do this, we can more rigorously evaluate state-level policies and their association with cancer outcomes.”
Public welfare improves outcomes
Weighing in on the data, Sarah P. Cate, MD, director, Special Surveillance and Breast Program, Mount Sinai Health System, New York, noted that racial disparities have been identified in many areas of health care and with respect to many diseases. “In the world of oncology, time to diagnosis and treatment significantly impacts overall survival,” she told this news organization. “Many studies are currently underway to investigate why certain ethnic groups have worse cancer outcomes.”
This study is important, she noted, in that it “highlights a discrete source of correcting these disparities in a large group of patients. Obviously there are multiple barriers to care, but increased public welfare spending in oncology should decrease some of these disparities.”
Julie R. Gralow, MD, ASCO’s chief medical officer and executive vice president, commented that it is known that state public welfare spending can mitigate structural racism and at least partially address social determinants of health, such as financial stability, education, place of residence, and insurance status. “This research found that states that increased their public health spending improved overall survival in Black patients with a variety of solid tumors and also resulted in a decrease in racial disparities in survival,” she said. “This important data provides clear support for the benefits of investment in public welfare spending at the state level, including Medicaid expansion.”
The study did not receive funding. Dr. Barnes and Dr. Cate have disclosed no relevant financial relationships. Dr. Gralow has relationships with Genentech, AstraZeneca Hexal, Puma BioTechnology, Roche, Novartis, Seagen, and Genomic Health.
A version of this article first appeared on Medscape.com.
Five-year overall survival increased among non-Hispanic Black patients by 2.02% in conjunction with a 10% increase in spending. In addition, there was a decrease in racial disparities in survival between non-Hispanic Black patients and White patients for many types of cancers.
However, public welfare spending had no real impact on the overall 5-year survival for the entire cohort (0.25 % per 10% increase in spending; P = .78) or for non-Hispanic White patients (0.52% per 10% increase in spending, P = .58).
“We know from prior research that outcomes are worse for minorities,” said lead author Justin Michael Barnes, MD, from the department of radiation oncology at Washington University in St. Louis, Mo. “It’s thought that some of the differences are related to impaired access to health care for minorities, which is related to social determinants of health. This includes socioeconomic factors, educational attainment, place of residence, as well as environmental stressors.
“Our data show that greater state welfare expenditures were associated with greater 5-year survival among Black patients and decreased Black–White disparities,” said Dr. Barnes. “I think these data are thought provoking, but they certainly aren’t the end. I see these data as a proof-of-concept project.”
Dr. Barnes reported the findings at a press conference held in advance of the annual meeting of the American Society of Clinical Oncology, during which the study will be presented (Abstract 6509).
Improved 5-year survival in Black patients
For the study, Dr. Barnes and colleagues evaluated the association of 5-year overall survival and public welfare spending in 2,925,550 individuals aged 18 years and older who were diagnosed with cancer during the period 2007-2016. The cohort was drawn from the Surveillance, Epidemiology, and End Results Program. In addition, annual state spending data were obtained from the U.S. Census Bureau. The team examined survival outcomes by race and ethnicity as well as by cancer site. The investigators accounted for factors such as age, sex, metropolitan residence, state, county-level income and education, insurance status, cancer site, stage at diagnosis, and year of diagnosis.
Much of public welfare spending was related to Medicaid but also included programs that provide subsidy assistance for individuals, such as Supplemental Security Income.
As compared with White patients, the 5-year overall survival rate was 10.8% lower among non-Hispanic Black patients. But there was a 4.46% (P for interaction <.001) narrowing of the 5-year overall survival disparity in non-Hispanic Black patients in comparison with White patients per 10% increase in spending, or a 42% closure of the 10.8% disparity.
Regarding specific cancer types, increased public welfare spending was associated with a narrowing of the 5-year overall survival disparity between Black patients and non-Hispanic White patients for the following cancers: breast (a 6.15% survival increase for Black patients led to a 39% closing of the disparity), cervix (a 11.9% survival increase led to a 46% closing of the disparity), colorectum (a 4.42% survival increase led to a 48% closing of the disparity), head and neck (a 9.41% survival increase led to a 38% closing of the disparity), liver (a 7.02% survival increase led to a 49% closing of the disparity), ovary (an 8.95% survival increase led to a 41% closing of the disparity), bladder (an 8.18% survival increase led to a 44% closing of the disparity), and uterus (a 14.1% survival increase led to a 40% closing of the disparity).
“Some type of public welfare seems to be helping improve oncologic outcomes for some of our most socioeconomically at-risk patients, but we don’t know the specifics,” Dr. Barnes concluded. “Additional work is needed to identify the most influential public health expenditures. If we can do this, we can more rigorously evaluate state-level policies and their association with cancer outcomes.”
Public welfare improves outcomes
Weighing in on the data, Sarah P. Cate, MD, director, Special Surveillance and Breast Program, Mount Sinai Health System, New York, noted that racial disparities have been identified in many areas of health care and with respect to many diseases. “In the world of oncology, time to diagnosis and treatment significantly impacts overall survival,” she told this news organization. “Many studies are currently underway to investigate why certain ethnic groups have worse cancer outcomes.”
This study is important, she noted, in that it “highlights a discrete source of correcting these disparities in a large group of patients. Obviously there are multiple barriers to care, but increased public welfare spending in oncology should decrease some of these disparities.”
Julie R. Gralow, MD, ASCO’s chief medical officer and executive vice president, commented that it is known that state public welfare spending can mitigate structural racism and at least partially address social determinants of health, such as financial stability, education, place of residence, and insurance status. “This research found that states that increased their public health spending improved overall survival in Black patients with a variety of solid tumors and also resulted in a decrease in racial disparities in survival,” she said. “This important data provides clear support for the benefits of investment in public welfare spending at the state level, including Medicaid expansion.”
The study did not receive funding. Dr. Barnes and Dr. Cate have disclosed no relevant financial relationships. Dr. Gralow has relationships with Genentech, AstraZeneca Hexal, Puma BioTechnology, Roche, Novartis, Seagen, and Genomic Health.
A version of this article first appeared on Medscape.com.
Five-year overall survival increased among non-Hispanic Black patients by 2.02% in conjunction with a 10% increase in spending. In addition, there was a decrease in racial disparities in survival between non-Hispanic Black patients and White patients for many types of cancers.
However, public welfare spending had no real impact on the overall 5-year survival for the entire cohort (0.25 % per 10% increase in spending; P = .78) or for non-Hispanic White patients (0.52% per 10% increase in spending, P = .58).
“We know from prior research that outcomes are worse for minorities,” said lead author Justin Michael Barnes, MD, from the department of radiation oncology at Washington University in St. Louis, Mo. “It’s thought that some of the differences are related to impaired access to health care for minorities, which is related to social determinants of health. This includes socioeconomic factors, educational attainment, place of residence, as well as environmental stressors.
“Our data show that greater state welfare expenditures were associated with greater 5-year survival among Black patients and decreased Black–White disparities,” said Dr. Barnes. “I think these data are thought provoking, but they certainly aren’t the end. I see these data as a proof-of-concept project.”
Dr. Barnes reported the findings at a press conference held in advance of the annual meeting of the American Society of Clinical Oncology, during which the study will be presented (Abstract 6509).
Improved 5-year survival in Black patients
For the study, Dr. Barnes and colleagues evaluated the association of 5-year overall survival and public welfare spending in 2,925,550 individuals aged 18 years and older who were diagnosed with cancer during the period 2007-2016. The cohort was drawn from the Surveillance, Epidemiology, and End Results Program. In addition, annual state spending data were obtained from the U.S. Census Bureau. The team examined survival outcomes by race and ethnicity as well as by cancer site. The investigators accounted for factors such as age, sex, metropolitan residence, state, county-level income and education, insurance status, cancer site, stage at diagnosis, and year of diagnosis.
Much of public welfare spending was related to Medicaid but also included programs that provide subsidy assistance for individuals, such as Supplemental Security Income.
As compared with White patients, the 5-year overall survival rate was 10.8% lower among non-Hispanic Black patients. But there was a 4.46% (P for interaction <.001) narrowing of the 5-year overall survival disparity in non-Hispanic Black patients in comparison with White patients per 10% increase in spending, or a 42% closure of the 10.8% disparity.
Regarding specific cancer types, increased public welfare spending was associated with a narrowing of the 5-year overall survival disparity between Black patients and non-Hispanic White patients for the following cancers: breast (a 6.15% survival increase for Black patients led to a 39% closing of the disparity), cervix (a 11.9% survival increase led to a 46% closing of the disparity), colorectum (a 4.42% survival increase led to a 48% closing of the disparity), head and neck (a 9.41% survival increase led to a 38% closing of the disparity), liver (a 7.02% survival increase led to a 49% closing of the disparity), ovary (an 8.95% survival increase led to a 41% closing of the disparity), bladder (an 8.18% survival increase led to a 44% closing of the disparity), and uterus (a 14.1% survival increase led to a 40% closing of the disparity).
“Some type of public welfare seems to be helping improve oncologic outcomes for some of our most socioeconomically at-risk patients, but we don’t know the specifics,” Dr. Barnes concluded. “Additional work is needed to identify the most influential public health expenditures. If we can do this, we can more rigorously evaluate state-level policies and their association with cancer outcomes.”
Public welfare improves outcomes
Weighing in on the data, Sarah P. Cate, MD, director, Special Surveillance and Breast Program, Mount Sinai Health System, New York, noted that racial disparities have been identified in many areas of health care and with respect to many diseases. “In the world of oncology, time to diagnosis and treatment significantly impacts overall survival,” she told this news organization. “Many studies are currently underway to investigate why certain ethnic groups have worse cancer outcomes.”
This study is important, she noted, in that it “highlights a discrete source of correcting these disparities in a large group of patients. Obviously there are multiple barriers to care, but increased public welfare spending in oncology should decrease some of these disparities.”
Julie R. Gralow, MD, ASCO’s chief medical officer and executive vice president, commented that it is known that state public welfare spending can mitigate structural racism and at least partially address social determinants of health, such as financial stability, education, place of residence, and insurance status. “This research found that states that increased their public health spending improved overall survival in Black patients with a variety of solid tumors and also resulted in a decrease in racial disparities in survival,” she said. “This important data provides clear support for the benefits of investment in public welfare spending at the state level, including Medicaid expansion.”
The study did not receive funding. Dr. Barnes and Dr. Cate have disclosed no relevant financial relationships. Dr. Gralow has relationships with Genentech, AstraZeneca Hexal, Puma BioTechnology, Roche, Novartis, Seagen, and Genomic Health.
A version of this article first appeared on Medscape.com.
FROM ASCO 2022
Long-term schizophrenia treatment may not always be necessary
NEW ORLEANS – Patients with new-onset schizophrenia often ask psychiatrist Stephen R. Marder, MD, whether they’ll need to be on medications forever to treat the disorder. Now, he said, research is showing that the answer isn’t always yes.
In many cases, “it’s an open question” whether lifelong medical treatment is needed, said Dr. Marder, a professor at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles, who spoke in a presentation about schizophrenia treatment at the annual meeting of the American Psychiatric Association.
According to Dr. Marder, research about relapses suggests that there may be a subpopulation of patients who can come off antipsychotics and remain in remission or partial remission. “The problem,” he said, “is that group is very hard to identify.”
Indeed, he highlighted a 2017 study that suggested The study noted choosing the best candidates isn’t simple, as “we do not have clinical measures or biomarkers that allow us to identify them prospectively. Because relapses and delays in the treatment of psychosis have been associated with poorer outcomes, there may be risk associated with withholding or discontinuing medication.”
There are more complications. There’s some evidence that antipsychotic drugs reduce brain volume, Dr. Marder said. But on the other hand, each psychotic episode can itself be harmful. “There is clear evidence that for each psychotic episode, they can take longer to improve, and they need higher doses.”
What to do? “My suggestion for most patients is to keep them on a relatively mild dose of an antipsychotic,” Dr. Marder said, “then to have a gradual decrease in the dose. I’ve done it in many patients.”
Which drug is best over the long term – oral or long-acting injectable antipsychotics? “It’s a hard question to answer because if you rely on randomized clinical trials – with patients who signed consent and are willing to be in a study like that – the subjects are sometimes not the ones who benefit the most from the long-acting drugs. So for many of the randomized clinical trials, the data was incomplete, and it was hard to make the case.”
But if you combine meta-analyses and cohort studies, as a 2021 study did, “you come up with a really clear answer: LAIs [long-acting injectables] are superior. They lead to a superior outcomes when it comes to rehospitalization and psychotic relapse,” Dr. Marder said.
That study reported that “LAIs were more beneficial than oral antipsychotics in 60 [18.3%] of 328 comparisons, not different in 252 [76.8%] comparisons, and less beneficial in 16 [4.9%] comparisons.”
More schizophrenia treatment pearls
People with schizophrenia – including those who aren’t on medication – face three times the risk of developing type 2 diabetes as the general population, “maybe because there’s a shared genetic risk for both disorders,” Dr. Marder said. “Those of you who have a lot of schizophrenia patients, I suspect you’re monitoring if they’re treating their type 2 diabetes and their obesity.”
Which antipsychotics are the best option for these patients? He highlighted a 2020 systematic review and meta-analysis that offers helpful insight into connections between 18 drugs and factors like weight and cholesterol.
Dr. Marder added that “if somebody has an elevation in their triglycerides or [hemoglobin] A1c in one single fasting blood glucose during the first 6 weeks of treatment, even if they haven’t been rated, it suggests that they’re developing insulin resistance.” At that point, he said, it’s a good idea to reconsider the medication choice.
Also, he said, keep in mind that “there’s substantial evidence that metformin is the appropriate treatment for patients who begin to demonstrate insulin resistance. It also works sometimes for weight loss.”
Exercise in people with schizophrenia can pay important dividends. A 2016 meta-analysis suggests that “not only does exercise for people with schizophrenia lead to better cardiovascular health, it’s good for the brain and improves cognitive functioning,” Dr. Marder said. “It’s not easy sometimes to get people with schizophrenia to exercise, but it’s many times worth the effort.”
Dr. Marder reported consulting for Boehringer Ingelheim, Lundbeck, Otsuka, Roche, Neurocrine, Sunovion, Newron, Merck, and Biogen; editor of UptoDate and Schizophrenia Bulletin Open; and research support from Boehringer Ingelheim.
NEW ORLEANS – Patients with new-onset schizophrenia often ask psychiatrist Stephen R. Marder, MD, whether they’ll need to be on medications forever to treat the disorder. Now, he said, research is showing that the answer isn’t always yes.
In many cases, “it’s an open question” whether lifelong medical treatment is needed, said Dr. Marder, a professor at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles, who spoke in a presentation about schizophrenia treatment at the annual meeting of the American Psychiatric Association.
According to Dr. Marder, research about relapses suggests that there may be a subpopulation of patients who can come off antipsychotics and remain in remission or partial remission. “The problem,” he said, “is that group is very hard to identify.”
Indeed, he highlighted a 2017 study that suggested The study noted choosing the best candidates isn’t simple, as “we do not have clinical measures or biomarkers that allow us to identify them prospectively. Because relapses and delays in the treatment of psychosis have been associated with poorer outcomes, there may be risk associated with withholding or discontinuing medication.”
There are more complications. There’s some evidence that antipsychotic drugs reduce brain volume, Dr. Marder said. But on the other hand, each psychotic episode can itself be harmful. “There is clear evidence that for each psychotic episode, they can take longer to improve, and they need higher doses.”
What to do? “My suggestion for most patients is to keep them on a relatively mild dose of an antipsychotic,” Dr. Marder said, “then to have a gradual decrease in the dose. I’ve done it in many patients.”
Which drug is best over the long term – oral or long-acting injectable antipsychotics? “It’s a hard question to answer because if you rely on randomized clinical trials – with patients who signed consent and are willing to be in a study like that – the subjects are sometimes not the ones who benefit the most from the long-acting drugs. So for many of the randomized clinical trials, the data was incomplete, and it was hard to make the case.”
But if you combine meta-analyses and cohort studies, as a 2021 study did, “you come up with a really clear answer: LAIs [long-acting injectables] are superior. They lead to a superior outcomes when it comes to rehospitalization and psychotic relapse,” Dr. Marder said.
That study reported that “LAIs were more beneficial than oral antipsychotics in 60 [18.3%] of 328 comparisons, not different in 252 [76.8%] comparisons, and less beneficial in 16 [4.9%] comparisons.”
More schizophrenia treatment pearls
People with schizophrenia – including those who aren’t on medication – face three times the risk of developing type 2 diabetes as the general population, “maybe because there’s a shared genetic risk for both disorders,” Dr. Marder said. “Those of you who have a lot of schizophrenia patients, I suspect you’re monitoring if they’re treating their type 2 diabetes and their obesity.”
Which antipsychotics are the best option for these patients? He highlighted a 2020 systematic review and meta-analysis that offers helpful insight into connections between 18 drugs and factors like weight and cholesterol.
Dr. Marder added that “if somebody has an elevation in their triglycerides or [hemoglobin] A1c in one single fasting blood glucose during the first 6 weeks of treatment, even if they haven’t been rated, it suggests that they’re developing insulin resistance.” At that point, he said, it’s a good idea to reconsider the medication choice.
Also, he said, keep in mind that “there’s substantial evidence that metformin is the appropriate treatment for patients who begin to demonstrate insulin resistance. It also works sometimes for weight loss.”
Exercise in people with schizophrenia can pay important dividends. A 2016 meta-analysis suggests that “not only does exercise for people with schizophrenia lead to better cardiovascular health, it’s good for the brain and improves cognitive functioning,” Dr. Marder said. “It’s not easy sometimes to get people with schizophrenia to exercise, but it’s many times worth the effort.”
Dr. Marder reported consulting for Boehringer Ingelheim, Lundbeck, Otsuka, Roche, Neurocrine, Sunovion, Newron, Merck, and Biogen; editor of UptoDate and Schizophrenia Bulletin Open; and research support from Boehringer Ingelheim.
NEW ORLEANS – Patients with new-onset schizophrenia often ask psychiatrist Stephen R. Marder, MD, whether they’ll need to be on medications forever to treat the disorder. Now, he said, research is showing that the answer isn’t always yes.
In many cases, “it’s an open question” whether lifelong medical treatment is needed, said Dr. Marder, a professor at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles, who spoke in a presentation about schizophrenia treatment at the annual meeting of the American Psychiatric Association.
According to Dr. Marder, research about relapses suggests that there may be a subpopulation of patients who can come off antipsychotics and remain in remission or partial remission. “The problem,” he said, “is that group is very hard to identify.”
Indeed, he highlighted a 2017 study that suggested The study noted choosing the best candidates isn’t simple, as “we do not have clinical measures or biomarkers that allow us to identify them prospectively. Because relapses and delays in the treatment of psychosis have been associated with poorer outcomes, there may be risk associated with withholding or discontinuing medication.”
There are more complications. There’s some evidence that antipsychotic drugs reduce brain volume, Dr. Marder said. But on the other hand, each psychotic episode can itself be harmful. “There is clear evidence that for each psychotic episode, they can take longer to improve, and they need higher doses.”
What to do? “My suggestion for most patients is to keep them on a relatively mild dose of an antipsychotic,” Dr. Marder said, “then to have a gradual decrease in the dose. I’ve done it in many patients.”
Which drug is best over the long term – oral or long-acting injectable antipsychotics? “It’s a hard question to answer because if you rely on randomized clinical trials – with patients who signed consent and are willing to be in a study like that – the subjects are sometimes not the ones who benefit the most from the long-acting drugs. So for many of the randomized clinical trials, the data was incomplete, and it was hard to make the case.”
But if you combine meta-analyses and cohort studies, as a 2021 study did, “you come up with a really clear answer: LAIs [long-acting injectables] are superior. They lead to a superior outcomes when it comes to rehospitalization and psychotic relapse,” Dr. Marder said.
That study reported that “LAIs were more beneficial than oral antipsychotics in 60 [18.3%] of 328 comparisons, not different in 252 [76.8%] comparisons, and less beneficial in 16 [4.9%] comparisons.”
More schizophrenia treatment pearls
People with schizophrenia – including those who aren’t on medication – face three times the risk of developing type 2 diabetes as the general population, “maybe because there’s a shared genetic risk for both disorders,” Dr. Marder said. “Those of you who have a lot of schizophrenia patients, I suspect you’re monitoring if they’re treating their type 2 diabetes and their obesity.”
Which antipsychotics are the best option for these patients? He highlighted a 2020 systematic review and meta-analysis that offers helpful insight into connections between 18 drugs and factors like weight and cholesterol.
Dr. Marder added that “if somebody has an elevation in their triglycerides or [hemoglobin] A1c in one single fasting blood glucose during the first 6 weeks of treatment, even if they haven’t been rated, it suggests that they’re developing insulin resistance.” At that point, he said, it’s a good idea to reconsider the medication choice.
Also, he said, keep in mind that “there’s substantial evidence that metformin is the appropriate treatment for patients who begin to demonstrate insulin resistance. It also works sometimes for weight loss.”
Exercise in people with schizophrenia can pay important dividends. A 2016 meta-analysis suggests that “not only does exercise for people with schizophrenia lead to better cardiovascular health, it’s good for the brain and improves cognitive functioning,” Dr. Marder said. “It’s not easy sometimes to get people with schizophrenia to exercise, but it’s many times worth the effort.”
Dr. Marder reported consulting for Boehringer Ingelheim, Lundbeck, Otsuka, Roche, Neurocrine, Sunovion, Newron, Merck, and Biogen; editor of UptoDate and Schizophrenia Bulletin Open; and research support from Boehringer Ingelheim.
AT APA 2022
Surgeons, who see it up close, offer ways to stop gun violence
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
Their strategies can work regardless of where you stand on the Second Amendment of the Constitution, said Patricia Turner, MD. “Our proposals are embraced by both gun owners and non–gun owners alike, and we are unique in that regard.”
These “implementable solutions” could prevent the next massacre, Dr. Turner, executive director of the American College of Surgeons, said during a news briefing the group sponsored on June 2.
“Our future – indeed all of our futures – depend on our ability to find durable, actionable steps that we can implement tomorrow to save lives,” she said.
Firsthand perspective
“Sadly I’m here today as a trauma surgeon who has cared for two of the largest mass shootings in modern U.S. history,” said Ronald Stewart, MD, chair of the department of surgery at University Hospital in San Antonio, Texas.
Dr. Stewart treated victims of the 2017 Sutherland Springs First Baptist Church shooting – where 27 people died, including the shooter – and the recent Uvalde school shooting, both in Texas.
“The injuries inflicted by high-velocity weapons used at both of these attacks are horrific. A high-capacity, magazine-fed automatic rifle such as the AR-15 causes extremely destructive tissue wounds,” he said.
One of the group’s proposals is to increase the regulation of high-velocity weapons, including AR-15s.
“These wounds are horribly lethal at close range, and sadly, most victims do not survive long enough to make it to a trauma center,” Dr. Stewart said.
On a positive note, “all of our current [Uvalde] patients are improving, which really brings us joy in this dark time,” he said. “But all of them have a long road to deal with recovery with both the physical and emotional impact of their injuries.”
Jeffrey Kerby, MD, agreed.
“Trauma surgeons see the short-term physical effects of these injuries and watch patients struggle with the long-term impact of these wounds,” said Dr. Kerby, director of trauma and acute care surgery at the University of Alabama at Birmingham.
Surgeons feel ‘profound impact’ of shootings
“Firearm violence has a profound impact on surgeons, and we are the undisputed subject matter experts in treating the tragic results,” said Patrick Bailey, MD, medical director for advocacy at the American College of Surgeons.
“This impacts surgeons as well,” said Dr. Kerby, chair of the Committee on Trauma for the surgeons’ group. “We are human, and we can’t help but share in the grief, the pain, and the suffering that our patients endure.
“As a pediatric surgeon ... I have too often witnessed the impact of firearm violence, and obviously, the devastation extends beyond the victims to their families,” he said. “To put it succinctly, in our culture, parents are not supposed to be put in a position of burying their children.”
A public health crisis
“It’s important to recognize that we’ve been talking about a public health approach,” said Eileen Bulger, MD, acting chief of the trauma division at the University of Washington in Seattle. That strategy is important for engaging both firearm owners and communities that have a higher risk for firearm violence, she said.
A committee of the American College of Surgeons developed specific recommendations in 2018, which are still valid today. The group brought together surgeons from across the U.S. including “passionate firearm owners and experts in firearm safety,” Dr. Bulger said.
The committee, for example, agreed on 10 specific recommendations “that we believe are bipartisan and could have an immediate impact in saving lives.”
“I’m a lifelong gun owner,” Dr. Bailey said, emphasizing that the team’s process included participation and perspective from other surgeons “who, like me, are also gun owners, but gun owners who also seek to reduce the impact of firearm violence in our country.”
The recommendations address these areas:
- Gun ownership
- Firearm registration
- Licensure
- Education and training
- Ownership responsibilities
- Mandatory reporting and risk reduction
- Safety innovation and technology
- Research
- The culture of violence
- Social isolation and mental health
For example, “we currently have certain classes of weapons with significant offensive capability,” Dr. Bulger said, “that are appropriately restricted and regulated under the National Firearms Act as Class 3 weapons.”
This group includes fully automatic machine guns, explosive devices, and short-barrel shotguns.
“We recommend a formal reassessment of the firearms designated within each of these national firearms classifications,” Dr. Bulger said.
For example, high-capacity, magazine-fed semiautomatic rifles, such as the AR-15, should be considered for reclassification as NFA Class 3 firearms, or they should get a new designation with tighter regulation.
The ACS endorses formal firearm safety training for all new gun owners. Also, owners who do not provide reasonably safe firearm storage should be held responsible for events related to the discharge of their firearms, Dr. Bulger said. And people who are deemed an imminent threat to themselves or others through firearm ownership should be temporarily or permanently restricted, with due process.
Research and reporting reforms
The ACS is also calling for research on firearm injuries and firearm injury prevention to be federally funded, Dr. Bulger said. The research should be done in a nonpartisan manner, she said.
“We have concerns that the manner and tone in which information is released to the public may lead to copycat mass killers,” she said. “The ACS recommends that law enforcement officials and the press take steps to eliminate the notoriety of the shooter, for example.”
Dr. Bulger also addressed the mental health angle. “We encourage recognition of mental health warning signs and social isolation by teachers, counselors, peers, and parents.” When identified, immediate referral to professionals is needed.
In addition to these recommendations, another team from the American College of Surgeons has published an overview of ways to address the inequities that contribute to violence. “We advocate for federal funding to support the development of hospital-based and community programs for violence intervention and prevention,” Dr. Bulger said.
Dr. Bailey said that as a gun owner himself, he thinks other gun owners would support these recommendations.
“I do not believe that the steps recommended ... pose undue burden on the rights of individual gun owners,” he said.
The time is now
Most firearm injuries are not from mass shooting events, Dr. Kerby said.
“My own trauma center has seen a 40% increase in the number of firearm injuries just in the last 2 years,” he added, “and these numbers continue to grow.”
A version of this article first appeared on WebMD.com.
NIAID trial to test asthma drug in disadvantaged urban children
The Prevention of Asthma Exacerbations Using Dupilumab in Urban Children and Adolescents (PANDA) trial was launched in order to examine the effects of treatment on children with poorly controlled allergic asthma who live in low-income urban environments in the United States, according to the National Institute of Allergy and Infectious Diseases.
Black and Hispanic children who live in these environments are at particularly high risk for asthma and are prone to attacks. These children and adolescents often have many allergies and are exposed to both high levels of indoor allergens and traffic-related pollution, which can make their asthma even more difficult to control, according to a June 2 NIAID press release.
PANDA is a multicenter, double-blind, placebo-controlled, randomized trial of dupilumab adjunctive therapy for the reduction of asthma exacerbations in urban children and adolescents 6-17 years with T2-high exacerbation-prone asthma. Approximately 240 participants will be randomized 2:1 to one of two study arms: 1) guidelines-based asthma treatment plus dupilumab, or 2) guidelines-based asthma treatment plus placebo. The planned study treatment will continue for 1 year with an additional 3 months of follow-up following completion of study treatment, according to the study details.
In an earlier study, NIAID-supported investigators identified numerous networks of genes that are activated together and are associated with asthma attacks in minority children and adolescents living in low-income urban settings
“We need to find out how well approved asthma drugs work for disadvantaged children of color living in urban areas, and whether biological markers can help predict how the drugs affect their asthma,” NIAID director Anthony S. Fauci, MD, said in the release. “The PANDA trial is an important step toward these goals.”
Participant criteria for the study include children of either sex, ages 6-17 years, who live in prespecified urban areas. Participants must have a diagnosis of asthma and must have had at least two asthma exacerbations in the prior year (defined as a requirement for systemic corticosteroids and/or hospitalization). At the screening visit, participants must have the following requirements for asthma controller medication: For children ages 6-11 years: treatments with at least fluticasone 250 mcg dry powder inhaler (DPI) one puff twice daily or its equivalent; for children ages 12 years and older, treatment with at least fluticasone 250 mcg plus long-acting beta agonist (LABA) DPI one puff twice daily or its equivalent.
ClinicalTrials.gov Identifier: NCT05347771Location: The NIAID-funded Childhood Asthma in Urban Settings (CAUSE) Network is conducting the study at seven medical centers located in Aurora, Colo.; Boston; Chicago; Cincinnati; New York, and Washington, D.C.
Sponsor: NIAID, Regeneron Pharmaceuticals and Sanofi are cofunding the phase 2 trial.
Study start date: April 2022
Expected completion Date: March 31, 2025
The Prevention of Asthma Exacerbations Using Dupilumab in Urban Children and Adolescents (PANDA) trial was launched in order to examine the effects of treatment on children with poorly controlled allergic asthma who live in low-income urban environments in the United States, according to the National Institute of Allergy and Infectious Diseases.
Black and Hispanic children who live in these environments are at particularly high risk for asthma and are prone to attacks. These children and adolescents often have many allergies and are exposed to both high levels of indoor allergens and traffic-related pollution, which can make their asthma even more difficult to control, according to a June 2 NIAID press release.
PANDA is a multicenter, double-blind, placebo-controlled, randomized trial of dupilumab adjunctive therapy for the reduction of asthma exacerbations in urban children and adolescents 6-17 years with T2-high exacerbation-prone asthma. Approximately 240 participants will be randomized 2:1 to one of two study arms: 1) guidelines-based asthma treatment plus dupilumab, or 2) guidelines-based asthma treatment plus placebo. The planned study treatment will continue for 1 year with an additional 3 months of follow-up following completion of study treatment, according to the study details.
In an earlier study, NIAID-supported investigators identified numerous networks of genes that are activated together and are associated with asthma attacks in minority children and adolescents living in low-income urban settings
“We need to find out how well approved asthma drugs work for disadvantaged children of color living in urban areas, and whether biological markers can help predict how the drugs affect their asthma,” NIAID director Anthony S. Fauci, MD, said in the release. “The PANDA trial is an important step toward these goals.”
Participant criteria for the study include children of either sex, ages 6-17 years, who live in prespecified urban areas. Participants must have a diagnosis of asthma and must have had at least two asthma exacerbations in the prior year (defined as a requirement for systemic corticosteroids and/or hospitalization). At the screening visit, participants must have the following requirements for asthma controller medication: For children ages 6-11 years: treatments with at least fluticasone 250 mcg dry powder inhaler (DPI) one puff twice daily or its equivalent; for children ages 12 years and older, treatment with at least fluticasone 250 mcg plus long-acting beta agonist (LABA) DPI one puff twice daily or its equivalent.
ClinicalTrials.gov Identifier: NCT05347771Location: The NIAID-funded Childhood Asthma in Urban Settings (CAUSE) Network is conducting the study at seven medical centers located in Aurora, Colo.; Boston; Chicago; Cincinnati; New York, and Washington, D.C.
Sponsor: NIAID, Regeneron Pharmaceuticals and Sanofi are cofunding the phase 2 trial.
Study start date: April 2022
Expected completion Date: March 31, 2025
The Prevention of Asthma Exacerbations Using Dupilumab in Urban Children and Adolescents (PANDA) trial was launched in order to examine the effects of treatment on children with poorly controlled allergic asthma who live in low-income urban environments in the United States, according to the National Institute of Allergy and Infectious Diseases.
Black and Hispanic children who live in these environments are at particularly high risk for asthma and are prone to attacks. These children and adolescents often have many allergies and are exposed to both high levels of indoor allergens and traffic-related pollution, which can make their asthma even more difficult to control, according to a June 2 NIAID press release.
PANDA is a multicenter, double-blind, placebo-controlled, randomized trial of dupilumab adjunctive therapy for the reduction of asthma exacerbations in urban children and adolescents 6-17 years with T2-high exacerbation-prone asthma. Approximately 240 participants will be randomized 2:1 to one of two study arms: 1) guidelines-based asthma treatment plus dupilumab, or 2) guidelines-based asthma treatment plus placebo. The planned study treatment will continue for 1 year with an additional 3 months of follow-up following completion of study treatment, according to the study details.
In an earlier study, NIAID-supported investigators identified numerous networks of genes that are activated together and are associated with asthma attacks in minority children and adolescents living in low-income urban settings
“We need to find out how well approved asthma drugs work for disadvantaged children of color living in urban areas, and whether biological markers can help predict how the drugs affect their asthma,” NIAID director Anthony S. Fauci, MD, said in the release. “The PANDA trial is an important step toward these goals.”
Participant criteria for the study include children of either sex, ages 6-17 years, who live in prespecified urban areas. Participants must have a diagnosis of asthma and must have had at least two asthma exacerbations in the prior year (defined as a requirement for systemic corticosteroids and/or hospitalization). At the screening visit, participants must have the following requirements for asthma controller medication: For children ages 6-11 years: treatments with at least fluticasone 250 mcg dry powder inhaler (DPI) one puff twice daily or its equivalent; for children ages 12 years and older, treatment with at least fluticasone 250 mcg plus long-acting beta agonist (LABA) DPI one puff twice daily or its equivalent.
ClinicalTrials.gov Identifier: NCT05347771Location: The NIAID-funded Childhood Asthma in Urban Settings (CAUSE) Network is conducting the study at seven medical centers located in Aurora, Colo.; Boston; Chicago; Cincinnati; New York, and Washington, D.C.
Sponsor: NIAID, Regeneron Pharmaceuticals and Sanofi are cofunding the phase 2 trial.
Study start date: April 2022
Expected completion Date: March 31, 2025
Lupus mutation may unlock targeted drugs for patient subset
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
Scientists have confirmed that a receptor long suspected to be linked to lupus is, in fact, a major driver of the autoimmune disease for at least some subset of patients, according to a study recently published in Nature. Researchers discovered the crucial role of toll-like receptor 7 (TLR7) because of a rare mutation in a pediatric patient with systemic lupus erythematosus (SLE) who had a particularly severe presentation.
“Sometimes it’s valuable to find these very severe cases where there is one mutation that has a strong effect because if we understand how those mutations work, the lessons we learn can generally tell us about disease mechanisms,” explained senior author Carola G. Vinuesa, MD, PhD, of the Centre for Personalised Immunology at Australian National University in Canberra and The Francis Crick Institute in London.

“It’s quite difficult to find one mutation that can alone cause the entire disease,” Dr. Vinuesa added, but what it reveals about how the disease develops may lead to more effective targeted therapies than the immune suppressants most often used to treat lupus currently.
The mutation they found was in the TLR7 gene that encodes the TLR7 protein. TLR7 is a receptor used by immune cells to identify viral RNA so they can fight off viral infections, including COVID-19. But if the body’s own genetic material binds to TLR7 in susceptible individuals, it can lead to an overproduction of type 1 interferons, which are cytokines that trigger or exacerbate the immune reactions that lead to lupus symptoms. The TLR7 gene occurs on the X chromosome, which may explain men’s greater susceptibility to COVID-19 and the greater incidence of lupus in women, who have two X chromosomes instead of the one that men have, Dr. Vinuesa said.
Previous research had shown an association between TLR7 and lupus, but this new study is the first to provide definitive proof that a TLR7 mutation by itself can directly cause human lupus. After discovering the variant in the patient, Dr. Vinuesa’s team used CRISPR to edit the genome of a mouse model and introduce the same mutation the patient had. “And they developed full-blown disease, just with this one single base-pair substitution – 1 letter in the 3 billion letters of the genome,” Dr. Vinuesa said. “It tells us that these receptors are not just there to recognize viral RNA, that in some circumstances, they could be triggered by our own nucleic acids.”
One pathway among many?
The finding does not mean that every lupus patient has this mutation, which remains rare, but suggests that overactivity in this receptor already reported in many lupus patients may be causally related to disease, Dr. Vinuesa said.
Noa Schwartz, MD, an assistant professor of medicine at Albert Einstein College of Medicine, New York, and director of the Montefiore-Einstein Institute for Lupus Care and Research, said in an interview that lupus is thought of as a syndrome, a collection of different but similar diseases that don’t necessarily have a single cause. But finding a single gene mutation that could potentially lead to lupus is an important piece of the puzzle, said Dr. Schwartz, who was not involved in the study. Based on past research in mice models, “we’ve hypothesized that TLR7 is important in humans as well, but this is the last nail in the coffin.”
One of the key questions this finding has prompted is how many patients’ disease results from TLR7 activity. “Because of the evidence from Ignacio Sanz’s group demonstrating TLR7 overactivity in a significant fraction of SLE patients, we believe that it is probably going to be pretty important,” Dr. Vinuesa said. “My feeling is that it is going to be quite a central pathway in lupus pathogenesis, if not the central pathway.”
Dr. Schwartz was more cautious, noting that it is probably important for a subset of patients but may “have a limited effect on the general lupus population.” While it’s not yet clear how large that subset is, it is possible it will include people with cutaneous lupus, those with primarily dermatologic symptoms.
“Hydroxychloroquine works particularly well for cutaneous manifestations of lupus, and one of the ways that works is by inhibiting TLR7 and TLR9, so this [finding] potentially matters for skin disease and lupus, but it’s very early,” Dr. Schwartz said. If it does turn out that TLR7 activity is particularly associated with cutaneous lupus, it may mean therapies with fewer side effects, she said. “Specifically for cutaneous lupus, the concept of suppressing the entire immune system for skin illness sometimes feels, especially to patients, very extreme, so they are [patients] who directed therapy could be so especially relevant for.”
Laura Lewandowski, MD, an assistant clinical investigator and head of the lupus genomics and global health disparities unit at the National Institute of Arthritis and Musculoskeletal and Skin Disease, described this study as particularly remarkable in the way it revealed the mechanism leading to lupus symptoms.
“As whole genome sequencing becomes faster and less expensive, more and more people are employing them in their studies,” most of which report changes in certain genes, Dr. Lewandowski said. “One of the most striking findings about this paper was that they took it to the next step and did a really elegant study on the exact way this gain-of-function TLR7 mutation leads to the autoimmunity that we see in lupus. The detail of mechanism in this paper is really unique.”
A step toward personalized medicine
Dr. Lewandowski is part of a team that recently presented a poster related to genomic sequencing in lupus patients at the annual meeting of the Childhood Arthritis and Rheumatology Research Alliance. Her study reported on the whole genome sequencing of patients with childhood-onset SLE who were already enrolled in the CARRA Lupus Registry. Children with lupus may be more likely than adults to have rare genetic variants, so a registry of childhood-onset SLE patients with fully sequenced genomes provides an opportunity to look for single-gene mutations specifically linked to lupus, said Dr. Lewandowski, who has recently begun a research collaboration with Dr. Vinuesa.
“As we move forward and more and more patients are included in these studies, we will understand a little bit more about the genetic architecture of patients who have rare variations leading to disease, or even common variations,” Dr. Lewandowski said about the intersection between her research and Dr. Vinuesa’s study. The more data they gather, the more they can explore the possible interactions of rare and common variants that play a role in SLE as well as what environmental triggers, such as viral infection or pollution exposure, might tip someone into having an autoimmune disease. “We’re just starting to peek under the hood,” Dr. Lewandowski said.
If further research can reveal the relative contribution of genetics to the disease and what those genetic drivers are, it may allow for greater precision in therapies and “ultimately improve the quality of life for our patients, the ultimate goal of all of these studies,” Dr. Lewandowski said.
Drugs that target TLR7 already exist for other indications, and clinical trials have already begun to see if these TLR7 inhibitors benefit lupus patients.
“If the clinical trials work, this will be quite a nice, targeted therapy with potentially much less side effects than other therapies on the market at the moment,” Dr. Vinuesa said. She is cautiously hopeful, saying it’s likely to make an impact on lupus treatment, but it’s too early to say precisely how much.
“It allows us to understand the disease mechanisms a little bit better and to try and assess what percentage of patients’ disease can be explained by overactivity in this receptor,” Dr. Vinuesa said. She thinks it’s possible that TLR7 over activation may be relevant to other systemic autoimmune diseases as well, such as Sjögren’s syndrome, rheumatoid arthritis, or juvenile dermatomyositis, but it will take more studies to find out.
“Right now, we have medicines that broadly inhibit the immune system and aren’t as targeted, but we have a lot more clinical and scientific work to do before we move this field forward for lupus patients,” Dr. Lewandowski said. “This is one case where they were able to find the exact molecular defect, and it’s not the end of the path of precision medicine — it’s the beginning.”
Dr. Vinuesa, Dr. Schwartz, and Dr. Lewandowski reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
High maternal, fetal morbidity rates in SLE pregnancies
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.
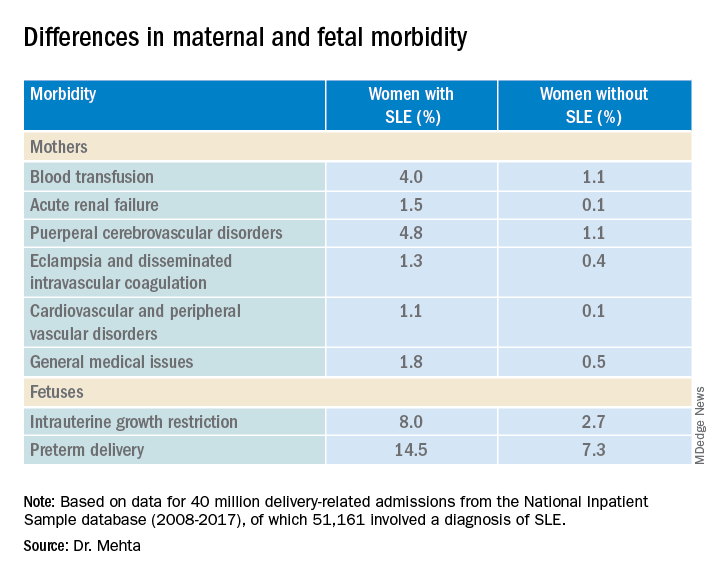
Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.

Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Pregnant women with systemic lupus erythematosus (SLE) are at significantly higher risk of requiring transfusion, developing a cerebrovascular disorder, or developing acute renal failure than pregnant women without SLE, a review of data from an American national sample indicates.
Pregnant women with SLE also have a twofold-higher risk for premature delivery, and a threefold risk of having a fetus with intrauterine growth restriction than their pregnant counterparts without SLE, reported Bella Mehta, MBBS, MS, MD, a rheumatologist at the Hospital for Special Surgery in New York.
“Severe maternal morbidity and fetal morbidity still remain high, but this work can help inform physicians and counsel patients for pregnancy planning and management,” she said at the annual European Congress of Rheumatology.
Although in-hospital maternal and fetal mortality rates for women with SLE have declined over the past 2 decades, the same cannot be said for morbidities, prompting the investigators to conduct a study to determine the proportion of fetal and maternal morbidity in SLE deliveries, compared with non-SLE deliveries over a decade.
Inpatient Sample
Dr. Mehta and colleagues studied retrospective data on 40 million delivery-related admissions from the National Inpatient Sample database. Of these patients, 51,161 had a diagnosis of SLE.
They identified all delivery-related hospital admissions for patients with and without SLE from 2008 through 2017 using diagnostic codes.
The researchers looked at fetal morbidity indicators, including preterm delivery and intrauterine growth restriction, and used the Centers for Disease Control and Prevention standard definition of severe maternal morbidity as “unexpected outcomes of labor and delivery that result in significant short- or long- term consequences to a woman’s health.”
They identified 21 severe maternal morbidity outcomes, including blood transfusion requirements, acute renal failure, eclampsia and disseminated intravascular coagulation, cardiovascular and peripheral vascular disorders, and general medical issues (hysterectomy, shock, sepsis, adult respiratory distress syndrome, severe anesthesia complications, temporary tracheostomy, and ventilation).
Study results
Women with SLE were slightly older at the time of delivery (mean age, 30.05 vs. 29.19 years) and had more comorbidities, according to the Elixhauser Comorbidity Scale, with 97.84% of women in this group having one to four comorbidities, compared with 19.4% of women without SLE.

Dr. Mehta acknowledged that the study was limited by the inability to capture outpatient deliveries, although she noted that only about 1.3% of deliveries in the United States occur outside the inpatient setting.
In addition, she noted that the database does not include information on lupus disease activity, Apgar scores, SLE flares, the presence of nephritis, antiphospholipid or anti-Ro/SSA antibodies, or medication use.
A rheumatologist who was not involved in the study said in an interview that the data from this study are in line with those in other recently published studies.
“The problem is that these data were not corrected for further disease activity or drugs,” said Frauke Förger, MD, professor of rheumatology and immunology at the University of Bern (Switzerland), who comoderated the oral abstract session where the data were presented.
She said prospective studies that adjusted for factors such as SLE disease activity and medication use will be required to give clinicians a better understanding of how to manage pregnancies in women with SLE.
The study was supported by an award from Weill Cornell Medicine. Dr. Mehta and Dr. Förger reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS
mTOR inhibitor shows early promise in endometrial cancer
In an open-label, phase 1/2, randomized clinical trial (VICTORIA) performed at 12 cancer centers in France, with good tolerability.
The study was published in JAMA Oncology.
Treatment of endometrial cancer involves a combination of surgery, radiation, and chemotherapy, but about 20% of patients relapse, usually within 5 years. HR+ endometrial cancer represents about 65% of endometrial cancers. It is usually endometrioid, and about 80% have phosphatase and tensin homologue (PTEN) mutations, while 36-52% have a mutation in the phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.
Endocrine therapy alone elicits a response rate of 15%-30% in HR+ endometrial cancer, generally in low-grade endometrioid subtypes. Most responses are short in duration. Aromatase inhibitors like anastrozole are used for more often than progestogens because they are better tolerated and they have a lower thromboembolic risk in this patient population. Previously, the phase 2 PARAGON trial showed a response rate of just 7% with anastrozole monotherapy, but 44% in women with recurrent HR+ endometrial cancer gained a clinical benefit.
Deregulation in the PI3K/AKT/mTOR pathway can also lead to hormone resistance, suggesting that combination of an mTOR inhibitor with endocrine therapy might have a synergistic effect.
mTOR inhibition alone or in combination with endocrine treatment has been investigated in some single-arm studies, with some encouraging progression-free survival results, but no clear objective response rate or overall survival benefit.
The new study included just 73 patients with a median age of 69.5 years: 49 received 125 mg vistusertib 2 days per week and 1 mg anastrozole daily and 24 received anastrozole only. The 8-week progression-free rate was 67.3% (unilateral 95% confidence interval, 54.7%) in the combination arm versus 39.1% (unilateral 95% CI, 22.2%) in the anastrozole-only arm.
Among 6 patients in the safety run-in period of the combination arm, there were no serious adverse events. Overall response rate was 24.5% (95% CI, 13.3-38.9%) in the combination arm and 17.4% (95% CI, 5.0-38.8%) in the anastrozole-only arm.
Over a median follow-up of 27.7 months, progression-free survival was 5.2 months in the combination arm (95% CI, 3.4-8.9 months) and 1.9 months (95% CI, 1.6-8.9) In the anastrozole-only arm. Common grade 2 or higher side effects linked to vistusertib included fatigue, lymphopenia, hyperglycemia, and diarrhea.
Although low tumor grade, endometrioid subtype, and HR+ status are associated with response to endocrine therapy, the low overall response and progression-free survival in the anastrozole arm suggest that better patient selection is needed. “The choice of treatment according to the histologic characteristics is not sufficient, and highly selected molecular criteria are necessary,” the authors wrote.
The study is limited by its small size and a lack of data on expression level of hormone receptors.
The study was funded by the National Cancer Institute of France.
In an open-label, phase 1/2, randomized clinical trial (VICTORIA) performed at 12 cancer centers in France, with good tolerability.
The study was published in JAMA Oncology.
Treatment of endometrial cancer involves a combination of surgery, radiation, and chemotherapy, but about 20% of patients relapse, usually within 5 years. HR+ endometrial cancer represents about 65% of endometrial cancers. It is usually endometrioid, and about 80% have phosphatase and tensin homologue (PTEN) mutations, while 36-52% have a mutation in the phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.
Endocrine therapy alone elicits a response rate of 15%-30% in HR+ endometrial cancer, generally in low-grade endometrioid subtypes. Most responses are short in duration. Aromatase inhibitors like anastrozole are used for more often than progestogens because they are better tolerated and they have a lower thromboembolic risk in this patient population. Previously, the phase 2 PARAGON trial showed a response rate of just 7% with anastrozole monotherapy, but 44% in women with recurrent HR+ endometrial cancer gained a clinical benefit.
Deregulation in the PI3K/AKT/mTOR pathway can also lead to hormone resistance, suggesting that combination of an mTOR inhibitor with endocrine therapy might have a synergistic effect.
mTOR inhibition alone or in combination with endocrine treatment has been investigated in some single-arm studies, with some encouraging progression-free survival results, but no clear objective response rate or overall survival benefit.
The new study included just 73 patients with a median age of 69.5 years: 49 received 125 mg vistusertib 2 days per week and 1 mg anastrozole daily and 24 received anastrozole only. The 8-week progression-free rate was 67.3% (unilateral 95% confidence interval, 54.7%) in the combination arm versus 39.1% (unilateral 95% CI, 22.2%) in the anastrozole-only arm.
Among 6 patients in the safety run-in period of the combination arm, there were no serious adverse events. Overall response rate was 24.5% (95% CI, 13.3-38.9%) in the combination arm and 17.4% (95% CI, 5.0-38.8%) in the anastrozole-only arm.
Over a median follow-up of 27.7 months, progression-free survival was 5.2 months in the combination arm (95% CI, 3.4-8.9 months) and 1.9 months (95% CI, 1.6-8.9) In the anastrozole-only arm. Common grade 2 or higher side effects linked to vistusertib included fatigue, lymphopenia, hyperglycemia, and diarrhea.
Although low tumor grade, endometrioid subtype, and HR+ status are associated with response to endocrine therapy, the low overall response and progression-free survival in the anastrozole arm suggest that better patient selection is needed. “The choice of treatment according to the histologic characteristics is not sufficient, and highly selected molecular criteria are necessary,” the authors wrote.
The study is limited by its small size and a lack of data on expression level of hormone receptors.
The study was funded by the National Cancer Institute of France.
In an open-label, phase 1/2, randomized clinical trial (VICTORIA) performed at 12 cancer centers in France, with good tolerability.
The study was published in JAMA Oncology.
Treatment of endometrial cancer involves a combination of surgery, radiation, and chemotherapy, but about 20% of patients relapse, usually within 5 years. HR+ endometrial cancer represents about 65% of endometrial cancers. It is usually endometrioid, and about 80% have phosphatase and tensin homologue (PTEN) mutations, while 36-52% have a mutation in the phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.
Endocrine therapy alone elicits a response rate of 15%-30% in HR+ endometrial cancer, generally in low-grade endometrioid subtypes. Most responses are short in duration. Aromatase inhibitors like anastrozole are used for more often than progestogens because they are better tolerated and they have a lower thromboembolic risk in this patient population. Previously, the phase 2 PARAGON trial showed a response rate of just 7% with anastrozole monotherapy, but 44% in women with recurrent HR+ endometrial cancer gained a clinical benefit.
Deregulation in the PI3K/AKT/mTOR pathway can also lead to hormone resistance, suggesting that combination of an mTOR inhibitor with endocrine therapy might have a synergistic effect.
mTOR inhibition alone or in combination with endocrine treatment has been investigated in some single-arm studies, with some encouraging progression-free survival results, but no clear objective response rate or overall survival benefit.
The new study included just 73 patients with a median age of 69.5 years: 49 received 125 mg vistusertib 2 days per week and 1 mg anastrozole daily and 24 received anastrozole only. The 8-week progression-free rate was 67.3% (unilateral 95% confidence interval, 54.7%) in the combination arm versus 39.1% (unilateral 95% CI, 22.2%) in the anastrozole-only arm.
Among 6 patients in the safety run-in period of the combination arm, there were no serious adverse events. Overall response rate was 24.5% (95% CI, 13.3-38.9%) in the combination arm and 17.4% (95% CI, 5.0-38.8%) in the anastrozole-only arm.
Over a median follow-up of 27.7 months, progression-free survival was 5.2 months in the combination arm (95% CI, 3.4-8.9 months) and 1.9 months (95% CI, 1.6-8.9) In the anastrozole-only arm. Common grade 2 or higher side effects linked to vistusertib included fatigue, lymphopenia, hyperglycemia, and diarrhea.
Although low tumor grade, endometrioid subtype, and HR+ status are associated with response to endocrine therapy, the low overall response and progression-free survival in the anastrozole arm suggest that better patient selection is needed. “The choice of treatment according to the histologic characteristics is not sufficient, and highly selected molecular criteria are necessary,” the authors wrote.
The study is limited by its small size and a lack of data on expression level of hormone receptors.
The study was funded by the National Cancer Institute of France.
FROM JAMA ONCOLOGY
EULAR recommends starting methotrexate and glucocorticoids in RA management
COPENHAGEN – New recommendations for the management of rheumatoid arthritis from the European Alliance of Associations for Rheumatology suggest starting short-term methotrexate and glucocorticoids when starting or changing conventional synthetic disease-modifying antirheumatic drugs (DMARDs), although rapid glucocorticoid dose reduction and discontinuation is also emphasized.
“In this respect we are at odds with the American College of Rheumatology guideline,” said Josef S. Smolen, MD, professor of internal medicine at the Medical University of Vienna, who presented the update at the annual European Congress of Rheumatology.
More evidence supports the recommendation to start methotrexate plus glucocorticoids since this is not surpassed by several biologic DMARDs (bDMARDs) plus methotrexate, said Dr. Smolen, who spoke on behalf of his coauthors, Robert Landewe, MD, PhD, from Amsterdam Rheumatology and Clinical Immunology Center, and the rest of the Global Task Force for the 2022 Update of the EULAR RA-Management Recommendations.
In addition, “JAK [Janus kinase] inhibitors are now only recommended for patients who do not have risk factors for cardiovascular or malignant diseases, but otherwise they remain on the same level [phase 2] as bDMARDS,” he said.
“Registries hitherto do not observe what is reported in the ORAL Surveillance randomized controlled trial [RCT],” but, he added, “RCTs are the decisive studies and we await the baricitinib data on a similar population at risk.”
Dr. Smolen also noted that the ENTRACTE trial comparing tocilizumab with tumor necrosis factor (TNF)–alpha inhibitors did not report similar data as ORAL Surveillance.
“Tapering b/ts [biologic/targeted synthetic] and cs [conventional synthetic] DMARDs in sustained remission have been brought together with the need to discontinue glucocorticoids before other drugs are tapered has been more strongly emphasized,” he explained.
Most of the recommendations from the 2019 update remain unchanged, including all five overarching principles and 6 of the 12 individual items.
Rheumatologist Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, joined the meeting remotely and commented on the working draft of the treatment recommendations. “While much was retained from the previous version, there were several important updates,” he said. “Regarding the use of steroids, it is recommended that when they are used, they should be stopped as soon as possible. Regarding jakinibs, which EULAR considers as a class, they recommended consideration of risk factors for MACE events prior to their utilization,” he said.
Methotrexate plus glucocorticoids (Recommendation 6)
In recent years, many recommendations have suggested combining methotrexate with glucocorticoids as a first-treatment strategy upon diagnosis of RA, said Dr. Smolen, and initially “guidance from the ACR was in agreement.”
In 2021, however, “the ACR published a paper, albeit with a very low level of evidence, that one should not primarily use a combination of methotrexate plus glucocorticoids,” he added, with an emphasis on the “very low level of evidence.”
“Some people on the task force even interpreted it as being in favor of using expensive drugs,” he explained. “This needed to be addressed in the 2022 update.”
The global task force wanted to look further at the benefit-to-risk ratio, despite it being discussed in the 2019 recommendations. “We wanted to check that short-term use of glucocorticoids was not associated with major risks,” said Dr. Smolen. “Glucocorticoids are not used for a long time if used as a bridging therapy. We felt we had to more clearly define what we meant by short term.”
A systematic review of around 7,000 papers, led to consideration of 10 unique studies. “One study published a few years ago in PLOS One, did not find any evidence of increased cardiovascular risk,” Dr. Smolen reported, “however, use of over 1,000 mg of glucocorticoid was associated with a trend for high cardiovascular risk.”
“This trend was confirmed by data from the CorEvitas registry, which shows that up to 1,100 mg of cumulative dose was associated with no increased risk, but above this with increasing dose there was an increased and significant risk,” he added.
When the task force looked at trials that mandated and prespecified a reduction and stopping of glucocorticoids, they found less than 10% persistence of glucocorticoids at 12 months in all trials, some even reduced use to zero.
Dr. Smolen and colleagues also looked at data from the NORD-STAR trial, that compared methotrexate and glucocorticoids with methotrexate and three bDMARDs, namely an anti-TNF inhibitor, certolizumab pegol; anti–co-stimulation, abatacept; and an anti–interleukin-6 receptor, tocilizumab.
“These data prove the validity of the EULAR RA management recommendations regarding the unsurpassed benefit of methotrexate plus glucocorticoids in early RA,” Dr. Smolen said.
“This is confirmation of efficacy and that if you induce tapering and stopping it is not dangerous,” he added. “The level of evidence was very high, and it is the highest level of agreement we have had for any glucocorticoid recommendation over recent years.”
As such, Recommendation 6 says that shortening glucocorticoids should be considered when initiating or changing csDMARDS, in different dose regimens and routes of administration, but should be tapered and discontinued as rapidly as clinically feasible.
JAK inhibitor placed relative to DMARDs (Recommendation 10)
A paper published in the New England Journal of Medicine suggested cardiovascular risks and malignancy risks were higher with the JAK inhibitor, tofacitinib, compared with TNF-alpha inhibitors.
The task force therefore felt the need to evaluate the place of JAK inhibitors next to biologic DMARDs “once phase one with methotrexate plus glucocorticoids has failed,” Dr. Smolen said.
After a systematic literature review of around 4,500 papers, the researchers evaluated 88 safety papers including the ORAL Surveillance study. “This very clearly showed that tocilizumab was not noninferior according to the noninferiority criteria with an upper limit of 1.8 [hazard ratio] and this was independent of dose, compared with TNF-alpha inhibitor,” said Dr. Smolen. “The major adverse cardiovascular events [MACE] were not different, nor were malignancies and overall mortality.”
Dr. Smolen also referred to the ENTRACTE trial that compared etanercept with tocilizumab, and again, there was no evidence of an increased risk of MACE nor mortality for tocilizumab compared with a TNF-alpha inhibitor.
“The increased MACE risk in the ORAL Surveillance trial is unlikely due to inhibition of IL-6 and must be due to some other effects than IL-6 signaling,” he said.
As such, the agreed-on recommendation was that, “if the treatment target is not achieved with the first csDMARD strategy, when poor prognostic factors are present, a bDMARD should be added; JAK inhibitors may be considered but pertinent risk factors must be taken into account [aged over 65 years, history of current or past smoking, either cardiovascular or malignancy risk factors, and risk factors for thromboembolic events].”
There was a high level of agreement by the group for this recommendation.
Switching DMARDs
The task force considered their recommendations on switching DMARDs based on a systematic literature review of 47 papers.
EULAR previously strongly recommended a combination of csDMARDs with bDMARDs (including JAK inhibitors), and this recommendation remains the same except for a note added about risks of tsDMARDs.
Recommendation 10 relates to failure of phase 2 treatment and what to do when the first bDMARD or a tsDMARD has failed (including as per new recommendations, a JAK inhibitor), and if one TNF or IL-6 receptor inhibitor therapy has failed. In this case, patients may receive an agent with another mode of action or a second TNF/IL-6 receptor inhibitor, said Dr. Smolen.
Recommendation 11 has been combined with recommendation 12, he added. “If a patient is in persistent remission after having tapered glucocorticoids, one can consider tapering bDMARDs, or tsDMARDs especially if this treatment is combined with a csDMARD.
“We decided to put more emphasis on the stopping of glucocorticoids, namely not saying ‘tapering’ but ‘discontinued,’ and if the patient is in sustained remission, then consider reduction of DMARDs [biologic, targeted synthetic or conventional synthetic DMARDs],” he explained. “This is left to the discretion of the patient and the physician as to which one should be tapered first. We don’t recommend to taper everything because the patient might be affected by flares but this needs further discussion.”
Dr. Smolen ended his presentation by looking ahead to the next set of recommendations: “With the current rate of evidence development, we expect an update of the recommendations to be necessary in about 3-4 years.”
This article was updated on 6/9/2022.
COPENHAGEN – New recommendations for the management of rheumatoid arthritis from the European Alliance of Associations for Rheumatology suggest starting short-term methotrexate and glucocorticoids when starting or changing conventional synthetic disease-modifying antirheumatic drugs (DMARDs), although rapid glucocorticoid dose reduction and discontinuation is also emphasized.
“In this respect we are at odds with the American College of Rheumatology guideline,” said Josef S. Smolen, MD, professor of internal medicine at the Medical University of Vienna, who presented the update at the annual European Congress of Rheumatology.
More evidence supports the recommendation to start methotrexate plus glucocorticoids since this is not surpassed by several biologic DMARDs (bDMARDs) plus methotrexate, said Dr. Smolen, who spoke on behalf of his coauthors, Robert Landewe, MD, PhD, from Amsterdam Rheumatology and Clinical Immunology Center, and the rest of the Global Task Force for the 2022 Update of the EULAR RA-Management Recommendations.
In addition, “JAK [Janus kinase] inhibitors are now only recommended for patients who do not have risk factors for cardiovascular or malignant diseases, but otherwise they remain on the same level [phase 2] as bDMARDS,” he said.
“Registries hitherto do not observe what is reported in the ORAL Surveillance randomized controlled trial [RCT],” but, he added, “RCTs are the decisive studies and we await the baricitinib data on a similar population at risk.”
Dr. Smolen also noted that the ENTRACTE trial comparing tocilizumab with tumor necrosis factor (TNF)–alpha inhibitors did not report similar data as ORAL Surveillance.
“Tapering b/ts [biologic/targeted synthetic] and cs [conventional synthetic] DMARDs in sustained remission have been brought together with the need to discontinue glucocorticoids before other drugs are tapered has been more strongly emphasized,” he explained.
Most of the recommendations from the 2019 update remain unchanged, including all five overarching principles and 6 of the 12 individual items.
Rheumatologist Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, joined the meeting remotely and commented on the working draft of the treatment recommendations. “While much was retained from the previous version, there were several important updates,” he said. “Regarding the use of steroids, it is recommended that when they are used, they should be stopped as soon as possible. Regarding jakinibs, which EULAR considers as a class, they recommended consideration of risk factors for MACE events prior to their utilization,” he said.
Methotrexate plus glucocorticoids (Recommendation 6)
In recent years, many recommendations have suggested combining methotrexate with glucocorticoids as a first-treatment strategy upon diagnosis of RA, said Dr. Smolen, and initially “guidance from the ACR was in agreement.”
In 2021, however, “the ACR published a paper, albeit with a very low level of evidence, that one should not primarily use a combination of methotrexate plus glucocorticoids,” he added, with an emphasis on the “very low level of evidence.”
“Some people on the task force even interpreted it as being in favor of using expensive drugs,” he explained. “This needed to be addressed in the 2022 update.”
The global task force wanted to look further at the benefit-to-risk ratio, despite it being discussed in the 2019 recommendations. “We wanted to check that short-term use of glucocorticoids was not associated with major risks,” said Dr. Smolen. “Glucocorticoids are not used for a long time if used as a bridging therapy. We felt we had to more clearly define what we meant by short term.”
A systematic review of around 7,000 papers, led to consideration of 10 unique studies. “One study published a few years ago in PLOS One, did not find any evidence of increased cardiovascular risk,” Dr. Smolen reported, “however, use of over 1,000 mg of glucocorticoid was associated with a trend for high cardiovascular risk.”
“This trend was confirmed by data from the CorEvitas registry, which shows that up to 1,100 mg of cumulative dose was associated with no increased risk, but above this with increasing dose there was an increased and significant risk,” he added.
When the task force looked at trials that mandated and prespecified a reduction and stopping of glucocorticoids, they found less than 10% persistence of glucocorticoids at 12 months in all trials, some even reduced use to zero.
Dr. Smolen and colleagues also looked at data from the NORD-STAR trial, that compared methotrexate and glucocorticoids with methotrexate and three bDMARDs, namely an anti-TNF inhibitor, certolizumab pegol; anti–co-stimulation, abatacept; and an anti–interleukin-6 receptor, tocilizumab.
“These data prove the validity of the EULAR RA management recommendations regarding the unsurpassed benefit of methotrexate plus glucocorticoids in early RA,” Dr. Smolen said.
“This is confirmation of efficacy and that if you induce tapering and stopping it is not dangerous,” he added. “The level of evidence was very high, and it is the highest level of agreement we have had for any glucocorticoid recommendation over recent years.”
As such, Recommendation 6 says that shortening glucocorticoids should be considered when initiating or changing csDMARDS, in different dose regimens and routes of administration, but should be tapered and discontinued as rapidly as clinically feasible.
JAK inhibitor placed relative to DMARDs (Recommendation 10)
A paper published in the New England Journal of Medicine suggested cardiovascular risks and malignancy risks were higher with the JAK inhibitor, tofacitinib, compared with TNF-alpha inhibitors.
The task force therefore felt the need to evaluate the place of JAK inhibitors next to biologic DMARDs “once phase one with methotrexate plus glucocorticoids has failed,” Dr. Smolen said.
After a systematic literature review of around 4,500 papers, the researchers evaluated 88 safety papers including the ORAL Surveillance study. “This very clearly showed that tocilizumab was not noninferior according to the noninferiority criteria with an upper limit of 1.8 [hazard ratio] and this was independent of dose, compared with TNF-alpha inhibitor,” said Dr. Smolen. “The major adverse cardiovascular events [MACE] were not different, nor were malignancies and overall mortality.”
Dr. Smolen also referred to the ENTRACTE trial that compared etanercept with tocilizumab, and again, there was no evidence of an increased risk of MACE nor mortality for tocilizumab compared with a TNF-alpha inhibitor.
“The increased MACE risk in the ORAL Surveillance trial is unlikely due to inhibition of IL-6 and must be due to some other effects than IL-6 signaling,” he said.
As such, the agreed-on recommendation was that, “if the treatment target is not achieved with the first csDMARD strategy, when poor prognostic factors are present, a bDMARD should be added; JAK inhibitors may be considered but pertinent risk factors must be taken into account [aged over 65 years, history of current or past smoking, either cardiovascular or malignancy risk factors, and risk factors for thromboembolic events].”
There was a high level of agreement by the group for this recommendation.
Switching DMARDs
The task force considered their recommendations on switching DMARDs based on a systematic literature review of 47 papers.
EULAR previously strongly recommended a combination of csDMARDs with bDMARDs (including JAK inhibitors), and this recommendation remains the same except for a note added about risks of tsDMARDs.
Recommendation 10 relates to failure of phase 2 treatment and what to do when the first bDMARD or a tsDMARD has failed (including as per new recommendations, a JAK inhibitor), and if one TNF or IL-6 receptor inhibitor therapy has failed. In this case, patients may receive an agent with another mode of action or a second TNF/IL-6 receptor inhibitor, said Dr. Smolen.
Recommendation 11 has been combined with recommendation 12, he added. “If a patient is in persistent remission after having tapered glucocorticoids, one can consider tapering bDMARDs, or tsDMARDs especially if this treatment is combined with a csDMARD.
“We decided to put more emphasis on the stopping of glucocorticoids, namely not saying ‘tapering’ but ‘discontinued,’ and if the patient is in sustained remission, then consider reduction of DMARDs [biologic, targeted synthetic or conventional synthetic DMARDs],” he explained. “This is left to the discretion of the patient and the physician as to which one should be tapered first. We don’t recommend to taper everything because the patient might be affected by flares but this needs further discussion.”
Dr. Smolen ended his presentation by looking ahead to the next set of recommendations: “With the current rate of evidence development, we expect an update of the recommendations to be necessary in about 3-4 years.”
This article was updated on 6/9/2022.
COPENHAGEN – New recommendations for the management of rheumatoid arthritis from the European Alliance of Associations for Rheumatology suggest starting short-term methotrexate and glucocorticoids when starting or changing conventional synthetic disease-modifying antirheumatic drugs (DMARDs), although rapid glucocorticoid dose reduction and discontinuation is also emphasized.
“In this respect we are at odds with the American College of Rheumatology guideline,” said Josef S. Smolen, MD, professor of internal medicine at the Medical University of Vienna, who presented the update at the annual European Congress of Rheumatology.
More evidence supports the recommendation to start methotrexate plus glucocorticoids since this is not surpassed by several biologic DMARDs (bDMARDs) plus methotrexate, said Dr. Smolen, who spoke on behalf of his coauthors, Robert Landewe, MD, PhD, from Amsterdam Rheumatology and Clinical Immunology Center, and the rest of the Global Task Force for the 2022 Update of the EULAR RA-Management Recommendations.
In addition, “JAK [Janus kinase] inhibitors are now only recommended for patients who do not have risk factors for cardiovascular or malignant diseases, but otherwise they remain on the same level [phase 2] as bDMARDS,” he said.
“Registries hitherto do not observe what is reported in the ORAL Surveillance randomized controlled trial [RCT],” but, he added, “RCTs are the decisive studies and we await the baricitinib data on a similar population at risk.”
Dr. Smolen also noted that the ENTRACTE trial comparing tocilizumab with tumor necrosis factor (TNF)–alpha inhibitors did not report similar data as ORAL Surveillance.
“Tapering b/ts [biologic/targeted synthetic] and cs [conventional synthetic] DMARDs in sustained remission have been brought together with the need to discontinue glucocorticoids before other drugs are tapered has been more strongly emphasized,” he explained.
Most of the recommendations from the 2019 update remain unchanged, including all five overarching principles and 6 of the 12 individual items.
Rheumatologist Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, joined the meeting remotely and commented on the working draft of the treatment recommendations. “While much was retained from the previous version, there were several important updates,” he said. “Regarding the use of steroids, it is recommended that when they are used, they should be stopped as soon as possible. Regarding jakinibs, which EULAR considers as a class, they recommended consideration of risk factors for MACE events prior to their utilization,” he said.
Methotrexate plus glucocorticoids (Recommendation 6)
In recent years, many recommendations have suggested combining methotrexate with glucocorticoids as a first-treatment strategy upon diagnosis of RA, said Dr. Smolen, and initially “guidance from the ACR was in agreement.”
In 2021, however, “the ACR published a paper, albeit with a very low level of evidence, that one should not primarily use a combination of methotrexate plus glucocorticoids,” he added, with an emphasis on the “very low level of evidence.”
“Some people on the task force even interpreted it as being in favor of using expensive drugs,” he explained. “This needed to be addressed in the 2022 update.”
The global task force wanted to look further at the benefit-to-risk ratio, despite it being discussed in the 2019 recommendations. “We wanted to check that short-term use of glucocorticoids was not associated with major risks,” said Dr. Smolen. “Glucocorticoids are not used for a long time if used as a bridging therapy. We felt we had to more clearly define what we meant by short term.”
A systematic review of around 7,000 papers, led to consideration of 10 unique studies. “One study published a few years ago in PLOS One, did not find any evidence of increased cardiovascular risk,” Dr. Smolen reported, “however, use of over 1,000 mg of glucocorticoid was associated with a trend for high cardiovascular risk.”
“This trend was confirmed by data from the CorEvitas registry, which shows that up to 1,100 mg of cumulative dose was associated with no increased risk, but above this with increasing dose there was an increased and significant risk,” he added.
When the task force looked at trials that mandated and prespecified a reduction and stopping of glucocorticoids, they found less than 10% persistence of glucocorticoids at 12 months in all trials, some even reduced use to zero.
Dr. Smolen and colleagues also looked at data from the NORD-STAR trial, that compared methotrexate and glucocorticoids with methotrexate and three bDMARDs, namely an anti-TNF inhibitor, certolizumab pegol; anti–co-stimulation, abatacept; and an anti–interleukin-6 receptor, tocilizumab.
“These data prove the validity of the EULAR RA management recommendations regarding the unsurpassed benefit of methotrexate plus glucocorticoids in early RA,” Dr. Smolen said.
“This is confirmation of efficacy and that if you induce tapering and stopping it is not dangerous,” he added. “The level of evidence was very high, and it is the highest level of agreement we have had for any glucocorticoid recommendation over recent years.”
As such, Recommendation 6 says that shortening glucocorticoids should be considered when initiating or changing csDMARDS, in different dose regimens and routes of administration, but should be tapered and discontinued as rapidly as clinically feasible.
JAK inhibitor placed relative to DMARDs (Recommendation 10)
A paper published in the New England Journal of Medicine suggested cardiovascular risks and malignancy risks were higher with the JAK inhibitor, tofacitinib, compared with TNF-alpha inhibitors.
The task force therefore felt the need to evaluate the place of JAK inhibitors next to biologic DMARDs “once phase one with methotrexate plus glucocorticoids has failed,” Dr. Smolen said.
After a systematic literature review of around 4,500 papers, the researchers evaluated 88 safety papers including the ORAL Surveillance study. “This very clearly showed that tocilizumab was not noninferior according to the noninferiority criteria with an upper limit of 1.8 [hazard ratio] and this was independent of dose, compared with TNF-alpha inhibitor,” said Dr. Smolen. “The major adverse cardiovascular events [MACE] were not different, nor were malignancies and overall mortality.”
Dr. Smolen also referred to the ENTRACTE trial that compared etanercept with tocilizumab, and again, there was no evidence of an increased risk of MACE nor mortality for tocilizumab compared with a TNF-alpha inhibitor.
“The increased MACE risk in the ORAL Surveillance trial is unlikely due to inhibition of IL-6 and must be due to some other effects than IL-6 signaling,” he said.
As such, the agreed-on recommendation was that, “if the treatment target is not achieved with the first csDMARD strategy, when poor prognostic factors are present, a bDMARD should be added; JAK inhibitors may be considered but pertinent risk factors must be taken into account [aged over 65 years, history of current or past smoking, either cardiovascular or malignancy risk factors, and risk factors for thromboembolic events].”
There was a high level of agreement by the group for this recommendation.
Switching DMARDs
The task force considered their recommendations on switching DMARDs based on a systematic literature review of 47 papers.
EULAR previously strongly recommended a combination of csDMARDs with bDMARDs (including JAK inhibitors), and this recommendation remains the same except for a note added about risks of tsDMARDs.
Recommendation 10 relates to failure of phase 2 treatment and what to do when the first bDMARD or a tsDMARD has failed (including as per new recommendations, a JAK inhibitor), and if one TNF or IL-6 receptor inhibitor therapy has failed. In this case, patients may receive an agent with another mode of action or a second TNF/IL-6 receptor inhibitor, said Dr. Smolen.
Recommendation 11 has been combined with recommendation 12, he added. “If a patient is in persistent remission after having tapered glucocorticoids, one can consider tapering bDMARDs, or tsDMARDs especially if this treatment is combined with a csDMARD.
“We decided to put more emphasis on the stopping of glucocorticoids, namely not saying ‘tapering’ but ‘discontinued,’ and if the patient is in sustained remission, then consider reduction of DMARDs [biologic, targeted synthetic or conventional synthetic DMARDs],” he explained. “This is left to the discretion of the patient and the physician as to which one should be tapered first. We don’t recommend to taper everything because the patient might be affected by flares but this needs further discussion.”
Dr. Smolen ended his presentation by looking ahead to the next set of recommendations: “With the current rate of evidence development, we expect an update of the recommendations to be necessary in about 3-4 years.”
This article was updated on 6/9/2022.
AT THE EULAR 2022 CONGRESS








