User login
Endometriosis and infertility – Combining a chronic physical and emotional pain
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
NAMS affirms value of hormone therapy for menopausal women
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
Hormone therapy remains a topic for debate, but a constant in the 2 decades since the Women’s Health Initiative has been the demonstrated effectiveness for relief of vasomotor symptoms and reduction of fracture risk in menopausal women, according to the latest hormone therapy position statement of the North American Menopause Society.
“Healthcare professionals caring for menopausal women should understand the basic concepts of relative risk and absolute risk,” wrote Stephanie S. Faubion, MD, director of the Mayo Clinic Center for Women’s Health and medical director of NAMS, and members of the NAMS 2022 Hormone Therapy Position Statement Advisory Panel in Menopause.
The authors noted that the risks of hormone therapy vary considerably based on type, dose, duration, route of administration, timing of the start of therapy, and whether or not a progestogen is included.
The 2022 statement was commissioned to review new literature and identify the strength of recommendations and quality of evidence since the previous statement in 2017.
The current statement represents not so much a practice-changing update, “but rather that the literature has filled out in some areas,” Dr. Faubion said in an interview. “The recommendations overall haven’t changed,” she said. “The position statement reiterates that hormone therapy, which is significantly underutilized, remains a safe and effective treatment for menopause symptoms, which remain undertreated, with the benefits outweighing the risks for most healthy women who are within 10 years of menopause onset and under the age of 60 years,” she emphasized. “Individualizing therapy is key to maximizing benefits and minimizing risks,” she added.
Overall, the authors confirmed that hormone therapy remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause (GSM), and has been shown to prevent bone loss and fracture. The risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestogen is used.
Risks and benefits should be stratified by age and time since the start of menopause, according to the statement.
For women younger than 60 years or within 10 years of the onset of menopause who have no contraindications, the potential benefits outweigh the risks in most cases for use of hormone therapy to manage vasomotor symptoms and to help prevent bone loss and reduce fracture risk.
For women who begin hormone therapy more than 10 or 20 years from the start of menopause, or who are aged 60 years and older, the risk-benefit ratio may be less favorable because of the increased absolute risk of coronary heart disease, stroke, venous thromboembolism, and dementia. However, strategies such as lower doses and transdermal administration may reduce this risk, according to the statement.
The authors continue to recommend that longer durations of hormone therapy be for documented indications, such as VMS relief, and that patients on longer duration of therapy be reassessed periodically as part of a shared decision-making process. Women with persistent VMS or quality of life issues, or those at risk for osteoporosis, may continue hormone therapy beyond age 60 or 65 years after appropriate evaluation and risk-benefit counseling.
Women with ongoing GSM without indications for systemic therapy whose GSM persists after over-the-counter therapies may try low-dose vaginal estrogen or other nonestrogen therapies regardless of age and for an extended duration if needed, according to the statement.
Challenges, research gaps, and goals
“Barriers to the use of hormone therapy include lack of access to high quality care,” Dr. Faubion said in an interview. The NAMS website, menopause.org, features an option to search for a NAMS-certified provider by ZIP code, she noted.
“Coverage of hormone therapy is highly variable and depends on the insurance company, but most women have access to one form or another with insurance coverage,” she said. “We need to continue to advocate for adequate coverage of menopause symptom treatments, including hormone therapy, so that women’s symptoms – which can significantly affect quality of life – are adequately managed.
“Additional research is needed on the thrombotic risk (venous thromboembolism, pulmonary embolism, and stroke) of oral versus transdermal therapies (including different formulations, doses, and durations of therapy),” Dr. Faubion told this news organization. “More clinical trial data are needed to confirm or refute the potential beneficial effects of hormone therapy on coronary heart disease and all-cause mortality when initiated in perimenopause or early postmenopause,” she said.
Other areas for research include “the breast effects of different estrogen preparations, including the role for selective estrogen receptor modulator (SERM) and tissue selective estrogen complex therapies, optimal progestogen or SERM regimens to prevent endometrial hyperplasia, the relationship between vasomotor symptoms and the risk for heart disease and cognitive changes, and the risks of premature ovarian insufficiency,” Dr. Faubion emphasized.
Looking ahead, “Studies are needed on the effects of longer use of low-dose vaginal estrogen therapy after breast or endometrial cancer, extended use of hormone therapy in women who are early initiators, improved tools to personalize or individualize benefits and risks of hormone therapy, and the role of aging and genetics,” said Dr. Faubion. Other areas for further research include “the long-term benefits and risks on women’s health of lifestyle modification or complementary or nonhormone therapies, if chosen in addition to or over hormone therapy for vasomotor symptoms, bone health, and cardiovascular disease risk reduction,” she added.
The complete statement was published in Menopause: The Journal of the North American Menopause Society.
The position statement received no outside funding. The authors had no financial conflicts to disclose.
FROM MENOPAUSE
Concerns that low LDL-C alters cognitive function challenged in novel analysis
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
PCSK9 inhibitors, which are among the most effective therapies for reducing LDL cholesterol (LDL-C), are associated with a neutral effect on cognitive function, according to a genetics-based Mendelian randomization study intended to sort out through the complexity of confounders.
The same study linked HMG-Co A reductase inhibitors (statins) with the potential for modest adverse neurocognitive effects, although these are likely to be outweighed by cardiovascular benefits, according to a collaborating team of investigators from the U.S. National Institutes of Health and the University of Oxford (England).
For clinicians and patients who continue to harbor concerns that cognitive function is threatened by very low LDL-C, this novel approach to evaluating risk is “reassuring,” according to the authors.
Early in clinical testing of PCSK9 inhibitors, a potential signal for adverse effects on cognitive function was reported but unconfirmed. This signal raised concern that extremely low levels of LDL-C, such as < 25 mg/dL, achieved with PCSK9 inhibitors might pose a risk to neurocognitive function.
Of several factors that provided a basis for concern, the PCSK9 enzyme is known to participate in brain development, according to the authors of this newly published study.
Mendelian randomization addresses complex issue
The objective of this Mendelian randomization analysis was to evaluate the relationship of PCSK9 inhibitors and statins on long-term neurocognitive function. Used previously to address other clinical issues, a drug-effect Mendelian randomization analysis evaluates genetic variants to determine whether there is a causal relationship between a risk, which in this case was lipid-lowering drugs, to a specific outcome, which was cognitive performance.
By looking directly at genetic variants that simulate the pharmacological inhibition of drug gene targets, the bias of confounders of clinical effects, such as baseline cognitive function, are avoided, according to the authors.
The message from this drug-effect Mendelian analysis was simple, according to the senior author of the study, Falk W. Lohoff, MD, chief of the section on clinical genomics and experimental therapeutics, National Institute of Alcohol Abuse and Alcoholism.
“Based on our data, we do not see a significant cognitive risk profile with PCSK9 inhibition associated with low LDL-C,” Dr. Lohoff said in an interview. He cautioned that “future long-term clinical studies are needed to confirm the absence of this effect,” but he and his coauthors noted that these data concur with the clinical studies.
From genome-wide association studies, single-nucleotide polymorphisms in PCSK9 and HMG-Co A reductase were extracted from a sample of more than 700,000 individuals of predominantly European ancestry. In the analysis, the investigators evaluated whether inhibition of PCSK9 or HMG-Co A reductase had an effect on seven clinical outcomes that relate to neurocognitive function, including memory, verbal intelligence, and reaction time, as well as biomarkers of cognitive function, such as cortical surface area.
The genetic effect of PCSK9 inhibition was “null for every cognitive-related outcome evaluated,” the investigators reported. The genetic effect of HMG-Co A reductase inhibition had a statistically significant but modest effect on cognitive performance (P = .03) and cortical surface area (P = .03). While the impact of HMG-Co A reductase inhibition on reaction time was stronger on a statistical basis (P = .0002), the investigators reported that it translated into a decrease of only 0.067 milliseconds per 38.7 mg/dL. They characterized this as a “small impact” unlikely to outweigh clinical benefits.
In an editorial that accompanied publication of this study, Brian A. Ference, MD, MPhil, provided context for the suitability of a Mendelian randomization analysis to address this or other questions regarding the impact of lipid-lowering therapies on clinical outcomes, and he ultimately concurred with the major conclusions
Ultimately, this analysis is consistent with other evidence that PCSK9 inhibition does not pose a risk of impaired cognitive function, he wrote. For statins, he concluded that this study “does not provide compelling evidence” to challenge their current clinical use.
Data do not support low LDL-C as cognitive risk factor
Moreover, this study – as well as other evidence – argues strongly against very low levels of LDL-C, regardless of how they are achieved, as a risk factor for diminished cognitive function, Dr. Ference, director of research in the division of translational therapeutics, University of Cambridge (England), said in an interview.
“There is no evidence from Mendelian randomization studies that lifelong exposure to lower LDL-C increases the risk of cognitive impairment,” he said. “This is true when evaluating lifelong exposure to lower LDL-C due to genetic variants in a wide variety of different genes or the genes that encode the target PCKS9 inhibitors, statins, or other lipid-lowering therapies.”
In other words, this study “adds to the accumulating evidence” that LDL-C lowering by itself does not contribute to an adverse impact on cognitive function despite persistent concern. This should not be surprising. Dr. Ference emphasized that there has never been strong evidence for an association.
“As I point out in the editorial, there is no biologically plausible mechanism by which reducing peripheral LDL-C should impact neurological function in any way, because the therapies do not cross the blood brain barrier, and because the nervous system produces its own cholesterol to maintain the integrity of membranes in nervous system cells,” he explained.
Dr. Lohoff reports no potential conflicts of interest. Dr. Ference has financial relationships with numerous pharmaceutical companies including those that make lipid-lowering therapies.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Is prostasin a clue to diabetes/cancer link?
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
People with elevated levels of protein prostasin seem to have a higher risk of developing diabetes and dying from cancer, according to a large, prospective, population-based study. The finding may provide new insights into why people with diabetes have an increased risk of cancer.
The study claims to be the first to investigate the link between plasma prostasin levels and cancer mortality, the study authors wrote in Diabetologia. The study analyzed plasma prostasin samples from 4,297 older adults (average age, 57.5 years) from the Malmö (Sweden) Diet and Cancer Study Cardiovascular Cohort.
“This study from the general population shows that prostasin, a protein that could be measured in blood, is associated with increased risk of developing diabetes,” senior author Gunnar Engström, MD, PhD, professor of epidemiology at Lund University in Malmö, Sweden, said in a comment. “Furthermore, it was associated with increased risk of death from cancer, especially in individuals with elevated glucose levels in the prediabetic range.
“The relationship between diabetes and cancer is poorly understood,” Dr. Engström said. “To our knowledge, this is the first big population study of prostasin and risk of diabetes.”
He noted previous studies have found a relationship between prostasin and cancer outcomes. “Prostasin could be a possible shared link between the two diseases and the results could help us understand why individuals with diabetes have increased risk of cancer.”
Patients in the study were assigned to quartiles based on prostasin levels. Those in the highest quartile had almost twice the risk of prevalent diabetes than did those in the lowest quartile (adjusted odds ratio, 1.95; 95% confidence interval, 1.39-2.76; P < .0001).
During the follow-up periods of 21.9 years for diabetes and 23.5 years for cancer, on average, 702 participants developed diabetes and 651 died from cancer. Again, the analysis found a significantly higher adjusted hazard ratio for participants in the fourth quartile: about 75% higher for diabetes (HR, 1.76; 95% CI, 1.41-2.19; P < .0001), and, after multivariable analysis, about 40% higher for death from cancer (HR, 1.43; 95% CI, 1.14-1.8; P = .0008).
Potential diabetes-cancer ‘interaction’
The study also identified what it called “a significant interaction” between prostasin and fasting blood glucose for cancer mortality risk (P = .022). In patients with impaired fasting blood glucose levels at baseline, the risk for cancer mortality was about 50% greater with each standard deviation increase in prostasin (HR, 1.52; 95% CI, 1.07-2.16; P = .019). Those with normal fasting blood glucose at baseline had a significantly lower risk with each SD increase in prostasin (HR, 1.11; 95% CI, 1.01-1.21; P = .025).
Further research is needed to validate the potential of prostasin as a biomarker for diabetes and cancer risks, Dr. Engström said. “The results need to be replicated in other studies. A study of cancer mortality in a big cohort of diabetes patients would be of great interest. We also need to examine whether prostasin is causally related to cancer and/or diabetes, or whether prostasin could act as a valuable risk marker in clinical settings. If causal, there could a possible molecular target for treatment.”
He added: “Biomarkers of diabetes and cancer are of great interest in the era of personalized medicine, both for disease prevention and for treatment of those with established disease.”
Li-Mei Chen, MD, PhD, a research associate professor at the University of Central Florida, Orlando, has studied the role of prostasin in epidemiology. She noted that one of the challenges of using prostasin in clinical or research settings is the lack of a standardized assay, which the Malmö study acknowledged. Dr. Engström and colleagues wrote that “prostasin levels were measured in arbitrary units (NPX values), and thus could not be compared directly with absolute values.”
Dr. Chen pointed out that the study reported a lower range of 0.24 pg/mL and an upper range of 7,800 pg/mL.
This means that, “in different groups that measure prostasin, the absolute quantity could have a difference in the thousands or tens of thousands,” she said. “That makes the judgment difficult of whether for this person you have a high level of prostasin in the blood and the other one you don’t if the difference is over a thousandfold.”
The Malmö study used the Proseek Multiplex Oncology I panel to determine plasma prostasin concentration, but Dr. Chen noted that she couldn’t find any data validating the panel for measuring prostasin. “It’s really hard for me to say whether this is of value or not because if the method that generated the data is not verified by another method, you don’t really know what you’re measuring.
“If the data are questionable, it’s really hard to say whether it means whether it’s a marker for cancer or diabetes,” Dr. Chen added. “That’s the biggest question I have, but actually the authors realize that.”
Dr. Engström confirmed that, “if prostasin is used to identify patients with increased risk of diabetes and cancer mortality, we also need to develop standardized assays for clinical use.”
Dr. Engström and coauthors had no disclosures. The study received funding from the Swedish Heart Lung Foundation, the National Natural Science Foundation of China, and the Natural Science Foundation of Jiangsu Province. The Malmö Diet and Cancer study received grants from the Swedish Cancer Society, the Swedish Medical Research Council, AFA Insurance, the Albert Påhlsson and Gunnar Nilsson Foundations, Malmö City Council, and Lund University. Dr. Chen had no relevant disclosures.
FROM DIABETOLOGIA
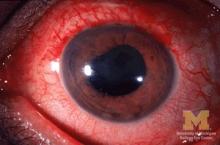
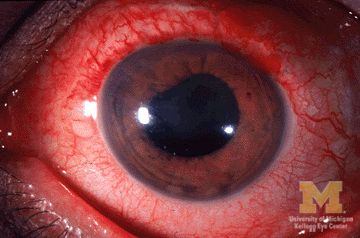
Many patients with acute anterior uveitis may have undiagnosed spondyloarthritis
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
FROM ARTHRITIS & RHEUMATOLOGY
Increasing data link ME/CFS, long COVID, and dysautonomia
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (IACFSME), speakers presented data showing similar pathophysiologic abnormalities in people with systemic symptoms associated with ME/CFS who had a prior SARS-CoV-2 infection and those who did not, including individuals whose illness preceded the COVID-19 pandemic.
Core clinical diagnostic criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for 6 months or longer, postexertional malaise, or a worsening of symptoms following even minor exertion (often described by patients as “crashes”), unrefreshing sleep, and cognitive dysfunction and/or orthostatic intolerance that are frequent and severe.
Long COVID has been defined in several different ways using different terminology. The U.S. Centers for Disease Control and Prevention, for example, defines “post-COVID conditions” as those continuing four or more weeks beyond first symptoms. The World Health Organization’s clinical case definition of “post COVID-19 condition” includes otherwise unexplained symptoms 3 months from COVID-19 onset and lasting longer than 2 months.
Both ME/CFS and long COVID commonly involve numerous symptoms beyond the defining ones, affecting nearly every organ system in the body, including systemic, neurocognitive, endocrine, cardiovascular, pulmonary, musculoskeletal, and gastrointestinal, with wide variation among individuals. Autonomic dysfunction is common to both conditions, particularly postural orthostatic tachycardia syndrome (POTS).
“My way of understanding these illnesses is that they’re not just multisystem illnesses, but all these interactive systems that lean on each other are dysregulated. … I would say that a very common underlying mediator of both ME/CFS and long COVID is autonomic dysfunction, and it presents as POTS,” Nancy Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University, Fort Lauderdale, Fla., told this news organization.
Dr. Klimas, who is also director of Clinical Immunology Research at the Miami Veterans Affairs Medical Center, added that “if basic bioenergetics are disrupted and in an oxidative-stress state [then] they have downregulated energy production at the cellular level, which seems to be the case in ME/CFS and now in long COVID.”
New ICD-10 codes better characterize the syndromes
New ICD-10 codes for 2023, being implemented on Oct. 1, will enable clinicians to better document all of these interrelated conditions.
Under the existing G93.3, Postviral and related fatigue syndromes, there will now be:
- G93.31 – Postviral fatigue syndrome.
- G93.32 – Myalgic encephalomyelitis/chronic fatigue syndrome (and the separate terms).
- G93.39 – Other postinfection and related fatigue syndromes.
The old R53.82, “Chronic fatigue, unspecified” code now excludes all of the above conditions.
The additional code U09.9 for “post COVID-19 condition, unspecified,” may also be used if applicable.
In addition, a new code for POTS, G90.A, which wasn’t previously mentioned in ICD-10, may also be used starting Oct. 1.
Lucinda Bateman, MD, founder and director of the Bateman Horne Center, Salt Lake City, advises using all applicable codes for a given patient. “If a patient came into my office with long COVID and met criteria for ME/CFS, we would code both, and also any other syndrome criteria that they may meet, such as POTS or fibromyalgia.
“If people use the codes appropriately, then you can understand the overlap better. It increases the likelihood of reimbursement, creates a more accurate medical record for the patient, and provides them with a better tool should they require disability benefits.”
Dr. Bateman advises in-office orthostatic evaluation for all patients with this symptom constellation, using a passive standing evaluation such as the 10-minute NASA Lean test.
“Clinicians should take the time to do orthostatic testing in these patients because it provides objective markers and will help lead us to potential interventions to help improve people’s function.”
The Bateman Horne center offers clinician resources on management of ME/CFS and related conditions.
How common is ME/CFS after COVID-19?
According to one published meta-analysis, the global prevalence of “post-acute sequelae of SARS-CoV-2,” defined by any symptom, is about 43% of patients overall following infection, and 49% at 120 days. Fatigue was the most commonly reported symptom, followed by memory problems. As of March 22, the World Health Organization estimated that there have been more than 470 million COVID-19 cases, which would give a figure of about 200 million people who are experiencing a wide range of long-COVID symptoms.
On the final day of the IACFSME conference, Luis Nacul, MD, of the University of British Columbia, Vancouver, presented several sets of data from his group and others aiming to determine the proportion of individuals who develop symptoms suggestive of ME/CFS following a COVID-19 infection.
Among a cohort of 88 adults hospitalized with confirmed SARS-CoV-2 infections during the first pandemic wave in 2020 and followed up in the respiratory clinic, rates of reported generalized fatigue were 67% at 3 months and 59.5% at 6 months. Substantial fatigue (that is, present most days and affecting activity levels) were reported by 16% at 3 months and 7% at 6 months. “This should represent in principle the maximum prevalence of cases who would meet the criteria for ME/CFS,” Dr. Nacul said.
Baseline age was indirectly associated with fatigue at 3 and 6 months, while the number of comorbidities a patient had was directly associated. Comorbidities also predicted severe fatigue at 3 months, but the numbers were too small for assessment at 6 months.
Studies involving nonhospitalized patients suggested lower rates. One meta-analysis showed 1-year rates of fatigue in 32% and cognitive impairment in 22%. Another showed very similar rates, reporting fatigue in 28% and memory/concentration difficulties in 18%-19%.
Dr. Nacul cautioned that these figures are likely overestimates since many of the study populations are taken from respiratory or long-COVID clinics. “The evidence on ‘post-COVID fatigue syndrome’ or ME/CFS following COVID is still evolving. There is a huge need for studies looking more closely at cases meeting well-defined ME/CFS criteria. This unfortunately hasn’t been done for most studies.”
Immune system dysfunction appears to underlie many cases
In a keynote address during the conference, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., pointed out that long COVID and ME/CFS are among many unexplained postacute infection syndromes associated with a long list of viral pathogens, including Ebola, the prior SARS viruses, Epstein-Barr virus, and Dengue, as well as nonviral pathogens such as Coxiella burnetii (Q fever syndrome) and Borrelia (posttreatment Lyme disease syndrome).
Dr. Iwasaki cited a recent Nature Medicine review article that she coauthored on this topic with an ME/CFS patient, noting: “We really need to understand why some people are failing to recover from these types of diseases.”
Emerging evidence supports four different hypotheses regarding pathogenesis: viral reservoir/viral pathogen-associated molecular pattern molecules, autoimmunity, dysbiosis/viral reactivation, and tissue damage
“Right now, it’s too early to exclude or make any conclusions about these. We need to have an open mind to dissect these various possibilities,” she said.
Two speakers reported findings of immune dysregulation in both ME/CFS and long COVID. Wakiro Sato, MD, PhD, of the National Center of Neurology and Psychiatry, Tokyo, reported that anti–G-protein coupled receptor antibodies were found in 33 (55%) of 60 patients with long COVID, and more than 40% had peripheral immune cell profile abnormalities. These findings were similar to those found in patients with ME/CFS, published by Sato’s team (Brain Behav Immun. 2021 Mar 29. doi: 10.1016/j.bbi.2021.03.023) and other researchers in Germany.
Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, presented data for an analysis of peripheral blood mononuclear cells from 26 donors with ME/CFS (8 with long COVID) and 24 healthy controls. In both patient groups, they found altered expression of inflammatory markers and decreases in CD8 T-cell number and function. The patients with long COVID showed evidence of sustained activation of both T-cell populations with increased CD38 and HLA-DR, associated with a compensatory increased frequency of activated CD4+CD8+ T cells.
“These results are consistent with immune dysregulation associated with overactivation and exhaustion of CD8 T cells, as observed in chronic viral infections and tumor environments,” Dr. Selin said.
ME/CFS and long COVID ‘frighteningly similar, if not identical’
Data for a different system derangement in long COVID and ME/CFS, the pathophysiology of exercise intolerance, were presented in another keynote talk by David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital cardiopulmonary laboratory, both in Boston. He has conducted invasive cardiopulmonary exercise testing in patients with ME/CFS and patients with long COVID.
Previously, Dr. Systrom and his team found that patients with ME/CFS have distinct defects in both ventricular filling pressure and oxygen extraction from the muscles. Neither of those are features of deconditioning, which is often blamed for exercise intolerance in people with ME/CFS. Rather, the major defect in deconditioning is decreased stroke volume and cardiac output. In ME/CFS patients, he found supranormal pulmonary blood flow, compared with VO2 max, suggesting peripheral left-to-right shunting.
In addition, Dr. Systrom and colleagues found that a large proportion of ME/CFS patients with these peripheral vascular defects also have biopsy-demonstrated small-fiber neuropathy, suggesting that acute exercise intolerance is related to underlying autonomic nervous system dysfunction.
In Dr. Systrom and colleagues’ long COVID study, invasive cardiopulmonary exercise testing in 10 patients who had recovered from COVID-19 at least 6 months prior and did not have cardiopulmonary disease had significantly revealed reduced peak exercise aerobic capacity (VO2 max), compared with 10 age- and sex-matched controls. The reduction in peak VO2 was associated with impaired systemic oxygen extraction, compared with the controls, despite a preserved peak cardiac index.
The long-COVID patients also showed greater ventilatory inefficiency, which “is entirely related to hyperventilation, not intrinsic lung disease,” Dr. Systrom said, adding that while there may be subsets of patients with interstitial lung disease after acute respiratory distress syndrome, these patients didn’t have that. “This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” Dr. Systrom said.
In a third study for which Dr. Systrom was a coauthor, published in Annals of Neurology, multisystem involvement was found in nine patients following mild COVID-19 infection, using standardized autonomic assessments including Valsalva maneuver, sudomotor and tilt tests, and skin biopsies for small-fiber neuropathy. The findings included cerebrovascular dysregulation with persistent cerebral arteriolar vasoconstriction, small-fiber neuropathy and related dysautonomia, respiratory dysregulation, and chronic inflammation.
Dr. Systrom’s conclusion: “Dyspnea and hyperventilation are common in ME/CFS and long COVID and there is significant overlap with POTS.”
Dr. Bateman disclosed that she is conducting research for Terra Biological. Dr. Systrom said he is conducting research for Astellas.
A version of this article first appeared on Medscape.com.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (IACFSME), speakers presented data showing similar pathophysiologic abnormalities in people with systemic symptoms associated with ME/CFS who had a prior SARS-CoV-2 infection and those who did not, including individuals whose illness preceded the COVID-19 pandemic.
Core clinical diagnostic criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for 6 months or longer, postexertional malaise, or a worsening of symptoms following even minor exertion (often described by patients as “crashes”), unrefreshing sleep, and cognitive dysfunction and/or orthostatic intolerance that are frequent and severe.
Long COVID has been defined in several different ways using different terminology. The U.S. Centers for Disease Control and Prevention, for example, defines “post-COVID conditions” as those continuing four or more weeks beyond first symptoms. The World Health Organization’s clinical case definition of “post COVID-19 condition” includes otherwise unexplained symptoms 3 months from COVID-19 onset and lasting longer than 2 months.
Both ME/CFS and long COVID commonly involve numerous symptoms beyond the defining ones, affecting nearly every organ system in the body, including systemic, neurocognitive, endocrine, cardiovascular, pulmonary, musculoskeletal, and gastrointestinal, with wide variation among individuals. Autonomic dysfunction is common to both conditions, particularly postural orthostatic tachycardia syndrome (POTS).
“My way of understanding these illnesses is that they’re not just multisystem illnesses, but all these interactive systems that lean on each other are dysregulated. … I would say that a very common underlying mediator of both ME/CFS and long COVID is autonomic dysfunction, and it presents as POTS,” Nancy Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University, Fort Lauderdale, Fla., told this news organization.
Dr. Klimas, who is also director of Clinical Immunology Research at the Miami Veterans Affairs Medical Center, added that “if basic bioenergetics are disrupted and in an oxidative-stress state [then] they have downregulated energy production at the cellular level, which seems to be the case in ME/CFS and now in long COVID.”
New ICD-10 codes better characterize the syndromes
New ICD-10 codes for 2023, being implemented on Oct. 1, will enable clinicians to better document all of these interrelated conditions.
Under the existing G93.3, Postviral and related fatigue syndromes, there will now be:
- G93.31 – Postviral fatigue syndrome.
- G93.32 – Myalgic encephalomyelitis/chronic fatigue syndrome (and the separate terms).
- G93.39 – Other postinfection and related fatigue syndromes.
The old R53.82, “Chronic fatigue, unspecified” code now excludes all of the above conditions.
The additional code U09.9 for “post COVID-19 condition, unspecified,” may also be used if applicable.
In addition, a new code for POTS, G90.A, which wasn’t previously mentioned in ICD-10, may also be used starting Oct. 1.
Lucinda Bateman, MD, founder and director of the Bateman Horne Center, Salt Lake City, advises using all applicable codes for a given patient. “If a patient came into my office with long COVID and met criteria for ME/CFS, we would code both, and also any other syndrome criteria that they may meet, such as POTS or fibromyalgia.
“If people use the codes appropriately, then you can understand the overlap better. It increases the likelihood of reimbursement, creates a more accurate medical record for the patient, and provides them with a better tool should they require disability benefits.”
Dr. Bateman advises in-office orthostatic evaluation for all patients with this symptom constellation, using a passive standing evaluation such as the 10-minute NASA Lean test.
“Clinicians should take the time to do orthostatic testing in these patients because it provides objective markers and will help lead us to potential interventions to help improve people’s function.”
The Bateman Horne center offers clinician resources on management of ME/CFS and related conditions.
How common is ME/CFS after COVID-19?
According to one published meta-analysis, the global prevalence of “post-acute sequelae of SARS-CoV-2,” defined by any symptom, is about 43% of patients overall following infection, and 49% at 120 days. Fatigue was the most commonly reported symptom, followed by memory problems. As of March 22, the World Health Organization estimated that there have been more than 470 million COVID-19 cases, which would give a figure of about 200 million people who are experiencing a wide range of long-COVID symptoms.
On the final day of the IACFSME conference, Luis Nacul, MD, of the University of British Columbia, Vancouver, presented several sets of data from his group and others aiming to determine the proportion of individuals who develop symptoms suggestive of ME/CFS following a COVID-19 infection.
Among a cohort of 88 adults hospitalized with confirmed SARS-CoV-2 infections during the first pandemic wave in 2020 and followed up in the respiratory clinic, rates of reported generalized fatigue were 67% at 3 months and 59.5% at 6 months. Substantial fatigue (that is, present most days and affecting activity levels) were reported by 16% at 3 months and 7% at 6 months. “This should represent in principle the maximum prevalence of cases who would meet the criteria for ME/CFS,” Dr. Nacul said.
Baseline age was indirectly associated with fatigue at 3 and 6 months, while the number of comorbidities a patient had was directly associated. Comorbidities also predicted severe fatigue at 3 months, but the numbers were too small for assessment at 6 months.
Studies involving nonhospitalized patients suggested lower rates. One meta-analysis showed 1-year rates of fatigue in 32% and cognitive impairment in 22%. Another showed very similar rates, reporting fatigue in 28% and memory/concentration difficulties in 18%-19%.
Dr. Nacul cautioned that these figures are likely overestimates since many of the study populations are taken from respiratory or long-COVID clinics. “The evidence on ‘post-COVID fatigue syndrome’ or ME/CFS following COVID is still evolving. There is a huge need for studies looking more closely at cases meeting well-defined ME/CFS criteria. This unfortunately hasn’t been done for most studies.”
Immune system dysfunction appears to underlie many cases
In a keynote address during the conference, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., pointed out that long COVID and ME/CFS are among many unexplained postacute infection syndromes associated with a long list of viral pathogens, including Ebola, the prior SARS viruses, Epstein-Barr virus, and Dengue, as well as nonviral pathogens such as Coxiella burnetii (Q fever syndrome) and Borrelia (posttreatment Lyme disease syndrome).
Dr. Iwasaki cited a recent Nature Medicine review article that she coauthored on this topic with an ME/CFS patient, noting: “We really need to understand why some people are failing to recover from these types of diseases.”
Emerging evidence supports four different hypotheses regarding pathogenesis: viral reservoir/viral pathogen-associated molecular pattern molecules, autoimmunity, dysbiosis/viral reactivation, and tissue damage
“Right now, it’s too early to exclude or make any conclusions about these. We need to have an open mind to dissect these various possibilities,” she said.
Two speakers reported findings of immune dysregulation in both ME/CFS and long COVID. Wakiro Sato, MD, PhD, of the National Center of Neurology and Psychiatry, Tokyo, reported that anti–G-protein coupled receptor antibodies were found in 33 (55%) of 60 patients with long COVID, and more than 40% had peripheral immune cell profile abnormalities. These findings were similar to those found in patients with ME/CFS, published by Sato’s team (Brain Behav Immun. 2021 Mar 29. doi: 10.1016/j.bbi.2021.03.023) and other researchers in Germany.
Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, presented data for an analysis of peripheral blood mononuclear cells from 26 donors with ME/CFS (8 with long COVID) and 24 healthy controls. In both patient groups, they found altered expression of inflammatory markers and decreases in CD8 T-cell number and function. The patients with long COVID showed evidence of sustained activation of both T-cell populations with increased CD38 and HLA-DR, associated with a compensatory increased frequency of activated CD4+CD8+ T cells.
“These results are consistent with immune dysregulation associated with overactivation and exhaustion of CD8 T cells, as observed in chronic viral infections and tumor environments,” Dr. Selin said.
ME/CFS and long COVID ‘frighteningly similar, if not identical’
Data for a different system derangement in long COVID and ME/CFS, the pathophysiology of exercise intolerance, were presented in another keynote talk by David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital cardiopulmonary laboratory, both in Boston. He has conducted invasive cardiopulmonary exercise testing in patients with ME/CFS and patients with long COVID.
Previously, Dr. Systrom and his team found that patients with ME/CFS have distinct defects in both ventricular filling pressure and oxygen extraction from the muscles. Neither of those are features of deconditioning, which is often blamed for exercise intolerance in people with ME/CFS. Rather, the major defect in deconditioning is decreased stroke volume and cardiac output. In ME/CFS patients, he found supranormal pulmonary blood flow, compared with VO2 max, suggesting peripheral left-to-right shunting.
In addition, Dr. Systrom and colleagues found that a large proportion of ME/CFS patients with these peripheral vascular defects also have biopsy-demonstrated small-fiber neuropathy, suggesting that acute exercise intolerance is related to underlying autonomic nervous system dysfunction.
In Dr. Systrom and colleagues’ long COVID study, invasive cardiopulmonary exercise testing in 10 patients who had recovered from COVID-19 at least 6 months prior and did not have cardiopulmonary disease had significantly revealed reduced peak exercise aerobic capacity (VO2 max), compared with 10 age- and sex-matched controls. The reduction in peak VO2 was associated with impaired systemic oxygen extraction, compared with the controls, despite a preserved peak cardiac index.
The long-COVID patients also showed greater ventilatory inefficiency, which “is entirely related to hyperventilation, not intrinsic lung disease,” Dr. Systrom said, adding that while there may be subsets of patients with interstitial lung disease after acute respiratory distress syndrome, these patients didn’t have that. “This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” Dr. Systrom said.
In a third study for which Dr. Systrom was a coauthor, published in Annals of Neurology, multisystem involvement was found in nine patients following mild COVID-19 infection, using standardized autonomic assessments including Valsalva maneuver, sudomotor and tilt tests, and skin biopsies for small-fiber neuropathy. The findings included cerebrovascular dysregulation with persistent cerebral arteriolar vasoconstriction, small-fiber neuropathy and related dysautonomia, respiratory dysregulation, and chronic inflammation.
Dr. Systrom’s conclusion: “Dyspnea and hyperventilation are common in ME/CFS and long COVID and there is significant overlap with POTS.”
Dr. Bateman disclosed that she is conducting research for Terra Biological. Dr. Systrom said he is conducting research for Astellas.
A version of this article first appeared on Medscape.com.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (IACFSME), speakers presented data showing similar pathophysiologic abnormalities in people with systemic symptoms associated with ME/CFS who had a prior SARS-CoV-2 infection and those who did not, including individuals whose illness preceded the COVID-19 pandemic.
Core clinical diagnostic criteria for ME/CFS established by the Institute of Medicine in 2015 include substantial decrement in functioning for 6 months or longer, postexertional malaise, or a worsening of symptoms following even minor exertion (often described by patients as “crashes”), unrefreshing sleep, and cognitive dysfunction and/or orthostatic intolerance that are frequent and severe.
Long COVID has been defined in several different ways using different terminology. The U.S. Centers for Disease Control and Prevention, for example, defines “post-COVID conditions” as those continuing four or more weeks beyond first symptoms. The World Health Organization’s clinical case definition of “post COVID-19 condition” includes otherwise unexplained symptoms 3 months from COVID-19 onset and lasting longer than 2 months.
Both ME/CFS and long COVID commonly involve numerous symptoms beyond the defining ones, affecting nearly every organ system in the body, including systemic, neurocognitive, endocrine, cardiovascular, pulmonary, musculoskeletal, and gastrointestinal, with wide variation among individuals. Autonomic dysfunction is common to both conditions, particularly postural orthostatic tachycardia syndrome (POTS).
“My way of understanding these illnesses is that they’re not just multisystem illnesses, but all these interactive systems that lean on each other are dysregulated. … I would say that a very common underlying mediator of both ME/CFS and long COVID is autonomic dysfunction, and it presents as POTS,” Nancy Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University, Fort Lauderdale, Fla., told this news organization.
Dr. Klimas, who is also director of Clinical Immunology Research at the Miami Veterans Affairs Medical Center, added that “if basic bioenergetics are disrupted and in an oxidative-stress state [then] they have downregulated energy production at the cellular level, which seems to be the case in ME/CFS and now in long COVID.”
New ICD-10 codes better characterize the syndromes
New ICD-10 codes for 2023, being implemented on Oct. 1, will enable clinicians to better document all of these interrelated conditions.
Under the existing G93.3, Postviral and related fatigue syndromes, there will now be:
- G93.31 – Postviral fatigue syndrome.
- G93.32 – Myalgic encephalomyelitis/chronic fatigue syndrome (and the separate terms).
- G93.39 – Other postinfection and related fatigue syndromes.
The old R53.82, “Chronic fatigue, unspecified” code now excludes all of the above conditions.
The additional code U09.9 for “post COVID-19 condition, unspecified,” may also be used if applicable.
In addition, a new code for POTS, G90.A, which wasn’t previously mentioned in ICD-10, may also be used starting Oct. 1.
Lucinda Bateman, MD, founder and director of the Bateman Horne Center, Salt Lake City, advises using all applicable codes for a given patient. “If a patient came into my office with long COVID and met criteria for ME/CFS, we would code both, and also any other syndrome criteria that they may meet, such as POTS or fibromyalgia.
“If people use the codes appropriately, then you can understand the overlap better. It increases the likelihood of reimbursement, creates a more accurate medical record for the patient, and provides them with a better tool should they require disability benefits.”
Dr. Bateman advises in-office orthostatic evaluation for all patients with this symptom constellation, using a passive standing evaluation such as the 10-minute NASA Lean test.
“Clinicians should take the time to do orthostatic testing in these patients because it provides objective markers and will help lead us to potential interventions to help improve people’s function.”
The Bateman Horne center offers clinician resources on management of ME/CFS and related conditions.
How common is ME/CFS after COVID-19?
According to one published meta-analysis, the global prevalence of “post-acute sequelae of SARS-CoV-2,” defined by any symptom, is about 43% of patients overall following infection, and 49% at 120 days. Fatigue was the most commonly reported symptom, followed by memory problems. As of March 22, the World Health Organization estimated that there have been more than 470 million COVID-19 cases, which would give a figure of about 200 million people who are experiencing a wide range of long-COVID symptoms.
On the final day of the IACFSME conference, Luis Nacul, MD, of the University of British Columbia, Vancouver, presented several sets of data from his group and others aiming to determine the proportion of individuals who develop symptoms suggestive of ME/CFS following a COVID-19 infection.
Among a cohort of 88 adults hospitalized with confirmed SARS-CoV-2 infections during the first pandemic wave in 2020 and followed up in the respiratory clinic, rates of reported generalized fatigue were 67% at 3 months and 59.5% at 6 months. Substantial fatigue (that is, present most days and affecting activity levels) were reported by 16% at 3 months and 7% at 6 months. “This should represent in principle the maximum prevalence of cases who would meet the criteria for ME/CFS,” Dr. Nacul said.
Baseline age was indirectly associated with fatigue at 3 and 6 months, while the number of comorbidities a patient had was directly associated. Comorbidities also predicted severe fatigue at 3 months, but the numbers were too small for assessment at 6 months.
Studies involving nonhospitalized patients suggested lower rates. One meta-analysis showed 1-year rates of fatigue in 32% and cognitive impairment in 22%. Another showed very similar rates, reporting fatigue in 28% and memory/concentration difficulties in 18%-19%.
Dr. Nacul cautioned that these figures are likely overestimates since many of the study populations are taken from respiratory or long-COVID clinics. “The evidence on ‘post-COVID fatigue syndrome’ or ME/CFS following COVID is still evolving. There is a huge need for studies looking more closely at cases meeting well-defined ME/CFS criteria. This unfortunately hasn’t been done for most studies.”
Immune system dysfunction appears to underlie many cases
In a keynote address during the conference, Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., pointed out that long COVID and ME/CFS are among many unexplained postacute infection syndromes associated with a long list of viral pathogens, including Ebola, the prior SARS viruses, Epstein-Barr virus, and Dengue, as well as nonviral pathogens such as Coxiella burnetii (Q fever syndrome) and Borrelia (posttreatment Lyme disease syndrome).
Dr. Iwasaki cited a recent Nature Medicine review article that she coauthored on this topic with an ME/CFS patient, noting: “We really need to understand why some people are failing to recover from these types of diseases.”
Emerging evidence supports four different hypotheses regarding pathogenesis: viral reservoir/viral pathogen-associated molecular pattern molecules, autoimmunity, dysbiosis/viral reactivation, and tissue damage
“Right now, it’s too early to exclude or make any conclusions about these. We need to have an open mind to dissect these various possibilities,” she said.
Two speakers reported findings of immune dysregulation in both ME/CFS and long COVID. Wakiro Sato, MD, PhD, of the National Center of Neurology and Psychiatry, Tokyo, reported that anti–G-protein coupled receptor antibodies were found in 33 (55%) of 60 patients with long COVID, and more than 40% had peripheral immune cell profile abnormalities. These findings were similar to those found in patients with ME/CFS, published by Sato’s team (Brain Behav Immun. 2021 Mar 29. doi: 10.1016/j.bbi.2021.03.023) and other researchers in Germany.
Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, presented data for an analysis of peripheral blood mononuclear cells from 26 donors with ME/CFS (8 with long COVID) and 24 healthy controls. In both patient groups, they found altered expression of inflammatory markers and decreases in CD8 T-cell number and function. The patients with long COVID showed evidence of sustained activation of both T-cell populations with increased CD38 and HLA-DR, associated with a compensatory increased frequency of activated CD4+CD8+ T cells.
“These results are consistent with immune dysregulation associated with overactivation and exhaustion of CD8 T cells, as observed in chronic viral infections and tumor environments,” Dr. Selin said.
ME/CFS and long COVID ‘frighteningly similar, if not identical’
Data for a different system derangement in long COVID and ME/CFS, the pathophysiology of exercise intolerance, were presented in another keynote talk by David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital cardiopulmonary laboratory, both in Boston. He has conducted invasive cardiopulmonary exercise testing in patients with ME/CFS and patients with long COVID.
Previously, Dr. Systrom and his team found that patients with ME/CFS have distinct defects in both ventricular filling pressure and oxygen extraction from the muscles. Neither of those are features of deconditioning, which is often blamed for exercise intolerance in people with ME/CFS. Rather, the major defect in deconditioning is decreased stroke volume and cardiac output. In ME/CFS patients, he found supranormal pulmonary blood flow, compared with VO2 max, suggesting peripheral left-to-right shunting.
In addition, Dr. Systrom and colleagues found that a large proportion of ME/CFS patients with these peripheral vascular defects also have biopsy-demonstrated small-fiber neuropathy, suggesting that acute exercise intolerance is related to underlying autonomic nervous system dysfunction.
In Dr. Systrom and colleagues’ long COVID study, invasive cardiopulmonary exercise testing in 10 patients who had recovered from COVID-19 at least 6 months prior and did not have cardiopulmonary disease had significantly revealed reduced peak exercise aerobic capacity (VO2 max), compared with 10 age- and sex-matched controls. The reduction in peak VO2 was associated with impaired systemic oxygen extraction, compared with the controls, despite a preserved peak cardiac index.
The long-COVID patients also showed greater ventilatory inefficiency, which “is entirely related to hyperventilation, not intrinsic lung disease,” Dr. Systrom said, adding that while there may be subsets of patients with interstitial lung disease after acute respiratory distress syndrome, these patients didn’t have that. “This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” Dr. Systrom said.
In a third study for which Dr. Systrom was a coauthor, published in Annals of Neurology, multisystem involvement was found in nine patients following mild COVID-19 infection, using standardized autonomic assessments including Valsalva maneuver, sudomotor and tilt tests, and skin biopsies for small-fiber neuropathy. The findings included cerebrovascular dysregulation with persistent cerebral arteriolar vasoconstriction, small-fiber neuropathy and related dysautonomia, respiratory dysregulation, and chronic inflammation.
Dr. Systrom’s conclusion: “Dyspnea and hyperventilation are common in ME/CFS and long COVID and there is significant overlap with POTS.”
Dr. Bateman disclosed that she is conducting research for Terra Biological. Dr. Systrom said he is conducting research for Astellas.
A version of this article first appeared on Medscape.com.
FROM IACFSME 2022
Commentary: Conditions Associated with AD, August 2022
In a cross-sectional observational study of 502 Finnish patients with AD, Salava and colleagues found that severe AD was associated with older age, male sex, early age of disease onset, higher body mass index, history of smoking, concomitant asthma, palmar hyperlinearity, hand dermatitis, history of contact allergy, and history of elevated immunoglobulin E levels. Some of these findings are correlated with each other. For example, palmar hyperlinearity was previously found to be a sign associated with early-onset AD in conjunction with Filaggrin loss-of-function mutations and atopic comorbidities.1,2 The association of AD with increased body mass index is consistent with previous studies that found associations of AD with overweight and obesity.3 In some instances, more severe AD may precede or lead to the association, eg, asthma and hand dermatitis. These results highlight the heterogeneity and complexity of AD, especially in moderate-to-severe disease.
AD is also associated with heterogeneous triggers. In clinical practice, we commonly see patients who consider food a potential trigger for AD. To better understand the role of food-triggered AD, Li and colleagues performed a retrospective study of 372 pediatric patients with AD. They found that more than half of the children with mild, moderate, and severe AD had an immunoglobulin E–mediated food allergy. Nevertheless, food-triggered AD occurred in only 3% of patients with AD. These results are doubly important because they indicate that clinicians should address food allergies to holistically improve the health of patients with AD. On the other hand, food is rarely a reproducible trigger of AD and appropriate treatment should generally not be withheld in favor of testing for food triggers of AD.
That said, it is important to address cutaneous and extra-cutaneous infections that occur in patients with AD to prevent worsening of AD and serious sequelae of infection. Indeed, Han and colleagues examined data from the Korean National Health Insurance Service, a nationwide population-based registry including 70,205 patients with AD and an unspecified number of control patients without AD. They found that AD was associated with significantly higher odds of molluscum contagiosum, impetigo, chickenpox, otitis media, eczema herpeticum, viral warts, and viral conjunctivitis. These results are consistent with previous studies from my research group showing higher rates of these and other infections.4-8 Anecdotally, I have seen all of these occur commonly in patients with AD, and in many instances these conditions worsen the underlying AD, eg, impetigo and eczema herpeticum.
The above-mentioned studies highlight the heterogeneity and complexity of AD, especially moderate-to-severe disease. Elsawi and colleagues conducted a survey-based study of 1065 adults with AD and found that moderate-to-severe AD was associated with increased patient burden, increased time spent managing AD symptoms, and comorbid depression. In addition, time spent managing AD symptoms was in and of itself a predictor of increased patient burden. These results underscore the many unmet needs that remain in the management of AD, with substantial patient burden from inadequate treatment as well as the inherent burden from the treatments themselves.
Additional References
1. Meng L, Wang L, Tang H, et al. Filaggrin gene mutation c.3321delA is associated with various clinical features of atopic dermatitis in the Chinese Han population. PloS One. 2014;9:e98235. Doi: 10.1371/journal.pone.0098235
2. Weidinger S, Illig T, Baurecht H, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214-219. Doi: 10.1016/j.jaci.2006.05.004
3. Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606-616.e4. Doi: 10.1016/j.jaad.2014.12.013
4. Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468. Doi: 10.1111/bjd.16482
5. Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Anb Allergy Asthma Immunol. 2018;120:66-72e11. Doi: 10.1016/j.anai.2017.10.019
6. Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164. Doi: 10.1097/DER.0000000000000526
7. Serrano L, Patel KR, Silverberg JI. Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: a systematic review and meta-analysis. J Acad Am Acad Dermatol. 2019;80:904-912. Doi: 10.1016/j.jaad.2018.11.028
8. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047. Doi: 10.1016/j.jaci.2013.08.012
In a cross-sectional observational study of 502 Finnish patients with AD, Salava and colleagues found that severe AD was associated with older age, male sex, early age of disease onset, higher body mass index, history of smoking, concomitant asthma, palmar hyperlinearity, hand dermatitis, history of contact allergy, and history of elevated immunoglobulin E levels. Some of these findings are correlated with each other. For example, palmar hyperlinearity was previously found to be a sign associated with early-onset AD in conjunction with Filaggrin loss-of-function mutations and atopic comorbidities.1,2 The association of AD with increased body mass index is consistent with previous studies that found associations of AD with overweight and obesity.3 In some instances, more severe AD may precede or lead to the association, eg, asthma and hand dermatitis. These results highlight the heterogeneity and complexity of AD, especially in moderate-to-severe disease.
AD is also associated with heterogeneous triggers. In clinical practice, we commonly see patients who consider food a potential trigger for AD. To better understand the role of food-triggered AD, Li and colleagues performed a retrospective study of 372 pediatric patients with AD. They found that more than half of the children with mild, moderate, and severe AD had an immunoglobulin E–mediated food allergy. Nevertheless, food-triggered AD occurred in only 3% of patients with AD. These results are doubly important because they indicate that clinicians should address food allergies to holistically improve the health of patients with AD. On the other hand, food is rarely a reproducible trigger of AD and appropriate treatment should generally not be withheld in favor of testing for food triggers of AD.
That said, it is important to address cutaneous and extra-cutaneous infections that occur in patients with AD to prevent worsening of AD and serious sequelae of infection. Indeed, Han and colleagues examined data from the Korean National Health Insurance Service, a nationwide population-based registry including 70,205 patients with AD and an unspecified number of control patients without AD. They found that AD was associated with significantly higher odds of molluscum contagiosum, impetigo, chickenpox, otitis media, eczema herpeticum, viral warts, and viral conjunctivitis. These results are consistent with previous studies from my research group showing higher rates of these and other infections.4-8 Anecdotally, I have seen all of these occur commonly in patients with AD, and in many instances these conditions worsen the underlying AD, eg, impetigo and eczema herpeticum.
The above-mentioned studies highlight the heterogeneity and complexity of AD, especially moderate-to-severe disease. Elsawi and colleagues conducted a survey-based study of 1065 adults with AD and found that moderate-to-severe AD was associated with increased patient burden, increased time spent managing AD symptoms, and comorbid depression. In addition, time spent managing AD symptoms was in and of itself a predictor of increased patient burden. These results underscore the many unmet needs that remain in the management of AD, with substantial patient burden from inadequate treatment as well as the inherent burden from the treatments themselves.
Additional References
1. Meng L, Wang L, Tang H, et al. Filaggrin gene mutation c.3321delA is associated with various clinical features of atopic dermatitis in the Chinese Han population. PloS One. 2014;9:e98235. Doi: 10.1371/journal.pone.0098235
2. Weidinger S, Illig T, Baurecht H, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214-219. Doi: 10.1016/j.jaci.2006.05.004
3. Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606-616.e4. Doi: 10.1016/j.jaad.2014.12.013
4. Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468. Doi: 10.1111/bjd.16482
5. Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Anb Allergy Asthma Immunol. 2018;120:66-72e11. Doi: 10.1016/j.anai.2017.10.019
6. Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164. Doi: 10.1097/DER.0000000000000526
7. Serrano L, Patel KR, Silverberg JI. Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: a systematic review and meta-analysis. J Acad Am Acad Dermatol. 2019;80:904-912. Doi: 10.1016/j.jaad.2018.11.028
8. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047. Doi: 10.1016/j.jaci.2013.08.012
In a cross-sectional observational study of 502 Finnish patients with AD, Salava and colleagues found that severe AD was associated with older age, male sex, early age of disease onset, higher body mass index, history of smoking, concomitant asthma, palmar hyperlinearity, hand dermatitis, history of contact allergy, and history of elevated immunoglobulin E levels. Some of these findings are correlated with each other. For example, palmar hyperlinearity was previously found to be a sign associated with early-onset AD in conjunction with Filaggrin loss-of-function mutations and atopic comorbidities.1,2 The association of AD with increased body mass index is consistent with previous studies that found associations of AD with overweight and obesity.3 In some instances, more severe AD may precede or lead to the association, eg, asthma and hand dermatitis. These results highlight the heterogeneity and complexity of AD, especially in moderate-to-severe disease.
AD is also associated with heterogeneous triggers. In clinical practice, we commonly see patients who consider food a potential trigger for AD. To better understand the role of food-triggered AD, Li and colleagues performed a retrospective study of 372 pediatric patients with AD. They found that more than half of the children with mild, moderate, and severe AD had an immunoglobulin E–mediated food allergy. Nevertheless, food-triggered AD occurred in only 3% of patients with AD. These results are doubly important because they indicate that clinicians should address food allergies to holistically improve the health of patients with AD. On the other hand, food is rarely a reproducible trigger of AD and appropriate treatment should generally not be withheld in favor of testing for food triggers of AD.
That said, it is important to address cutaneous and extra-cutaneous infections that occur in patients with AD to prevent worsening of AD and serious sequelae of infection. Indeed, Han and colleagues examined data from the Korean National Health Insurance Service, a nationwide population-based registry including 70,205 patients with AD and an unspecified number of control patients without AD. They found that AD was associated with significantly higher odds of molluscum contagiosum, impetigo, chickenpox, otitis media, eczema herpeticum, viral warts, and viral conjunctivitis. These results are consistent with previous studies from my research group showing higher rates of these and other infections.4-8 Anecdotally, I have seen all of these occur commonly in patients with AD, and in many instances these conditions worsen the underlying AD, eg, impetigo and eczema herpeticum.
The above-mentioned studies highlight the heterogeneity and complexity of AD, especially moderate-to-severe disease. Elsawi and colleagues conducted a survey-based study of 1065 adults with AD and found that moderate-to-severe AD was associated with increased patient burden, increased time spent managing AD symptoms, and comorbid depression. In addition, time spent managing AD symptoms was in and of itself a predictor of increased patient burden. These results underscore the many unmet needs that remain in the management of AD, with substantial patient burden from inadequate treatment as well as the inherent burden from the treatments themselves.
Additional References
1. Meng L, Wang L, Tang H, et al. Filaggrin gene mutation c.3321delA is associated with various clinical features of atopic dermatitis in the Chinese Han population. PloS One. 2014;9:e98235. Doi: 10.1371/journal.pone.0098235
2. Weidinger S, Illig T, Baurecht H, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214-219. Doi: 10.1016/j.jaci.2006.05.004
3. Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606-616.e4. Doi: 10.1016/j.jaad.2014.12.013
4. Narla S, Silverberg JI. Association between childhood atopic dermatitis and cutaneous, extracutaneous and systemic infections. Br J Dermatol. 2018;178:1467-1468. Doi: 10.1111/bjd.16482
5. Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Anb Allergy Asthma Immunol. 2018;120:66-72e11. Doi: 10.1016/j.anai.2017.10.019
6. Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164. Doi: 10.1097/DER.0000000000000526
7. Serrano L, Patel KR, Silverberg JI. Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: a systematic review and meta-analysis. J Acad Am Acad Dermatol. 2019;80:904-912. Doi: 10.1016/j.jaad.2018.11.028
8. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047. Doi: 10.1016/j.jaci.2013.08.012
Treatments explored to ease postviral symptoms of ME/CFS and long COVID
A variety of treatments, most already commercially available, are under investigation for treating the constellation of overlapping symptoms associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), “long COVID,” and dysautonomia.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, speakers presented data for a variety of approaches to ease symptoms common across postviral conditions, such as extreme fatigue, postexertional malaise (“crash”), cognitive dysfunction (“brain fog”), orthostatic intolerance including postural orthostatic tachycardia syndrome (POTS), and chronic pain. Most of the modalities are already commercially available for other indications, although some are costly and not covered by payers for these conditions.
“ ... In the past, patients were told ‘you have chronic fatigue syndrome but there’s nothing we can do for it.’ That certainly is not the case. There aren’t cures, but there are many management techniques to improve symptoms,” Charles W. Lapp, MD, medical director of the Hunter-Hopkins Center, Charlotte, N.C., said in an interview.
A current mainstay of treatment for ME/CFS – including that triggered by COVID-19 – is activity pacing, in which patients learn to stay within their “energy envelopes” in order to avoid postexertional malaise, a worsening of all symptoms with exertion. The use of “graded exercise” is no longer recommended, per U.K. and U.S. guidelines.
Data for the following approaches were presented at the IACFS/ME conference:
Pyridostigmine (mestinon, others)
Pyridostigmine, an acetylcholinesterase inhibitor, is approved for the treatment of muscle weakness resulting from myasthenia gravis and is available in generic form. It has previously been shown to produce significant improvement in both symptom burden and heart rate response in POTS.
At the IACFS/ME conference, David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital Cardiopulmonary laboratory, both in Boston, summarized his group’s study in patients with ME/CFS using pyridostigmine as both a potential treatment for improving exercise capacity and a proof-of-concept that neurovascular dysregulation underlies exertional intolerance in the condition.
A total of 45 patients were randomized to 60 mg oral pyridostigmine or placebo after an invasive cardiopulmonary exercise test, and a second test performed 50 minutes later. Peak VO2 increased after pyridostigmine but decreased after placebo (+13.3 mL/min vs. –40.2 mL/min, P < .05). Cardiac output and right atrial pressure were also significantly improved with pyridostigmine and worse with placebo.
“We suggest that treatable neurovascular dysregulation underlies acute exercise intolerance in ME/CFS. ... Pyridostigmine may be a useful repurposed off-label treatment [for] a subset of patients with exercise intolerance,” Dr. Systrom said.
Asked to comment, Dr. Lapp said: “We’ve used Mestinon for years because it helps with POTS and also with neurally mediated hypotension. Systrom is taking it to a new level because he’s shown that it increases preload to the heart.” However, he noted that it’s unclear whether the drug will help patients who don’t have POTS specifically. On the other hand, patients rarely experience side effects from the drug.
Since the generic tablets come only in 60-mg doses, and the starting dose is 30 mg three times a day, he advised cutting the tablets in half during titration up to 60 mg three times a day.
Oxaloacetate (benaGene)
David Lyons Kaufman, MD, of the Center for Complex Diseases, Mountain View, Calif., summarized data from his group’s recently published open-label, nonrandomized, “proof-of-concept” study on use of the commercially available nutritional supplement anhydrous enol-oxaloacetate for treating mental and physical fatigue in 76 patients with longstanding ME/CFS and 43 with long-COVID fatigue.
Oxaloacetate is a major step in the Krebs cycle within the mitochondria that are depleted in patients with ME/CFS. It is also an energy metabolite that has multiple effects in cells and mitochondria, Dr. Kaufman explained.
Doses ranging from 500 mg twice daily up to 1,000 mg three times a day were given for 6 weeks. Up to 33% of the patients with ME/CFS and up to 46.8% of the long-COVID group achieved clinical efficacy as measured by physical and mental fatigue scores, compared with just 5.9% of historical ME/CFS controls. All doses showed highly significant improvements.
The only adverse effects were occasional dyspepsia, which was avoided by taking the supplement with food, and insomnia, resolved by having them dose at breakfast and lunch, Dr. Kaufman said.
Following those preliminary data, there is now an ongoing 90-day, randomized, placebo-controlled clinical trial of 80 patients with ME/CFS using 2,000 mg anhydrous enol-oxaloacetate per day. Endpoints include multiple objective measures.
“We have a health care crisis with long COVID, and we’ve had this smoldering crisis with ME/CFS for decades that’s never been addressed. ME/CFS and long COVID, if not identical, are certainly overlapping. ... We have to pursue these translational medicine pilot studies as rapidly as possible,” Dr. Kaufman remarked.
Dr. Lapp told this news organization that it makes sense to use constituents of the Krebs cycle to improve mitochondrial function, but the problem with oxaloacetate is its cost. Dr. Kaufman mentioned that based on the preliminary trial, the therapeutic “sweet spot” appeared to be 1,000 mg twice daily. The manufacturer’s website lists the price for a single bottle of 30 250-mg capsules at $49, or $42 if purchased via a monthly subscription.
“It’s a benign drug, and it’s over the counter. I would give it to any patient who’s got a big wallet,” Dr. Lapp quipped, adding: “If they’ve got the money, they can order it tonight.”
Inspiritol
Inspiritol is an investigational “nebulized, inhaled, multimechanism medication designed to treat the major symptoms of respiratory distress with antioxidant, anti-inflammatory, and broad-spectrum antiviral and antibacterial properties. Inspiritol is composed of both endogenously produced and naturally occurring, well-tolerated biochemicals,” according to the company website.
The hypothesis, Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, said at the meeting, is that “ME/CFS and long COVID-19 result from an aberrant response to an immunological trigger like infection, which results in a permanently dysregulated immune system as a result of overactivation of CD8 T cells and subsequent exhaustion.”
Inspiritol, containing five antioxidants, acts as an immune modulator to reverse the CD8 T cell exhaustion and improve symptoms. Administration by inhaler delivers it directly to the brain from the lung. It was originally designed for use in chronic obstructive pulmonary disease and asthma and has shown efficacy for acute COVID-19, Dr. Selin said.
In a preliminary study, four patients with ME/CFS and five with long COVID have been treated with Inspiritol for 2-15 months, and all have self-reported improved symptoms. Cough has been the only reported side effect.
The company is pursuing an Investigational New Drug Application for the product with the Food and Drug Administration and has several patents pending. Dr. Lapp called Inspiritol “very interesting,” and said that reversal of CD8 “exhaustion” also would appear to be a promising approach. However, he noted, “the problem is that we don’t know what’s in it.”
Stellate ganglion block
Injection of local anesthetic near the stellate ganglion to block activity of the entire cervical sympathetic chain has been used for nearly a century to treat a variety of sympathetically mediated conditions, including complex regional pain syndrome (CRPS), shingles, and phantom-limb pain. More recently, it has been used in a variety of other conditions, including PTSD, Raynaud’s disease, menopausal hot flashes, and hyperhidrosis.
Insurance companies typically cover it for CRPS, neuropathic upper-extremity pain, hyperhidrosis, and Raynaud’s, said Luke Liu, MD, an anesthesiologist who is founder and chief executive officer of Alaska-based pain management company Neuroversion.
Deborah Duricka, PhD, also with Neuroversion, presented results from a now-published case series of 11 patients with long COVID who underwent stellate ganglion block by a board-certified anesthesiologist, first on one side at the level of C6, then on the contralateral side the following day.
Clinically meaningful benefits were seen in at least five of the patients in fatigue, memory problems, problems concentrating, rapid heartbeat, orthostatic intolerance, sleep problems, postexertional malaise, anxiety, and depression.
The hypothetical mechanism, she said, is that “sympathetic block prevents sympathetically driven vasoconstriction in carotid and vertebral arteries.”
Dr. Liu presented another case series of five patients with ME/CFS who underwent the procedure with ultrasound guidance, again on one side and the other side the next day. All had upper-limb autonomic issues such as Raynaud’s and/or neuropathic pain that had been refractory to more conventional treatments.
All five patients reported improvements in symptoms of ME/CFS, including energy level, cognition, pain, and postexertional malaise. One patient reported “feeling well for the first time in decades.” However, that patient relapsed after a mild viral illness 3.5 months after treatment. Some of the patients have required further treatments.
Dr. Lapp commented that, although the procedure is generally safe when performed by an experienced clinician, “Any time you do an injection like that, there’s a high risk that you could nick an artery or a vein or hit an essential nerve in the neck. That’s why it has to be done under fluoroscopy or ultrasound.”
He said he’s had a few patients undergo the procedure, mostly for CRPS, and they seem to have benefited from it. “It might increase cerebral blood flow and preload to the heart, so it might decrease ME/CFS symptoms and help with POTS as well.”
Nonetheless, Dr. Lapp said he wouldn’t consider stellate ganglion block as first-line treatment for ME/CFS or long COVID. “I think it would be for the treatment-resistant patient, when you’ve gone through all the treatments that we know and addressed all the comorbidities and they’re still not getting better.”
But, he added, it is a standard procedure. “Any pain clinic can do a stellate block.”
Transcutaneous auricular vagus nerve stimulation
Nicola Clague-Baker, PhD, a physiotherapist at the University of Liverpool (England), presented findings from an international survey of people with ME/CFS regarding their experience with transcutaneous auricular vagus nerve stimulation (taVNS) to manage their autonomic symptoms. The technique involves stimulation of the autonomic nervous system via the vagus nerve using electrodes applied to part of the ear. The theory is that the technique stimulates the parasympathetic nervous system and improves autonomic balance.
Two small previous trials showing benefit of vagus nerve stimulation for people with ME/CFS used more invasive and less comfortable methods of applying the stimulation rather than to the ear, Dr. Clague-Baker and colleagues noted in a poster. It has also been used successfully in treating POTS, another conference speaker noted.
A total of 131 people with ME/CFS (called simply “ME” in the United Kingdom) responded to a survey advertised on social media and websites. The majority (60%) were from the United Kingdom while the rest were from Europe, Australia, and North America. Most were female, and slightly more than half had lived with ME for 10 or more years.
The majority (72%) were still using taVNS, while 28% had stopped using it. Only 9% had used the modality for longer than a year. Respondents identified more than 30 benefits in symptoms and activities, with improvements in postexertional malaise (39%) and brain fog (37%) being the most common. One reported significant reduction in constipation.
However, respondents also mentioned more than 20 short- and long-term negatives, including headaches (15%) and long-term irritation at the site (9%). One participant reported a “big improvement in neuropathic pain, but not so much for muscles and joints.”
Overall, 80% reported that they would continue using taVNS and 67% said they would recommend it to others with ME, and 56% said that the system was mildly to very beneficial.
Dr. Lapp noted that several types of transcutaneous electrical nerve stimulation units with ear clips are sold online, and he’s seen them work well for migraine treatment. However, he cautioned that some patients have had side effects from the treatment, such as headaches and dizziness. “It’s putting an electrical current through your brain. In my mind, it’s another last-ditch measure.”
Dr. Lapp reported no financial disclosures.
A version of this article first appeared on Medscape.com.
A variety of treatments, most already commercially available, are under investigation for treating the constellation of overlapping symptoms associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), “long COVID,” and dysautonomia.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, speakers presented data for a variety of approaches to ease symptoms common across postviral conditions, such as extreme fatigue, postexertional malaise (“crash”), cognitive dysfunction (“brain fog”), orthostatic intolerance including postural orthostatic tachycardia syndrome (POTS), and chronic pain. Most of the modalities are already commercially available for other indications, although some are costly and not covered by payers for these conditions.
“ ... In the past, patients were told ‘you have chronic fatigue syndrome but there’s nothing we can do for it.’ That certainly is not the case. There aren’t cures, but there are many management techniques to improve symptoms,” Charles W. Lapp, MD, medical director of the Hunter-Hopkins Center, Charlotte, N.C., said in an interview.
A current mainstay of treatment for ME/CFS – including that triggered by COVID-19 – is activity pacing, in which patients learn to stay within their “energy envelopes” in order to avoid postexertional malaise, a worsening of all symptoms with exertion. The use of “graded exercise” is no longer recommended, per U.K. and U.S. guidelines.
Data for the following approaches were presented at the IACFS/ME conference:
Pyridostigmine (mestinon, others)
Pyridostigmine, an acetylcholinesterase inhibitor, is approved for the treatment of muscle weakness resulting from myasthenia gravis and is available in generic form. It has previously been shown to produce significant improvement in both symptom burden and heart rate response in POTS.
At the IACFS/ME conference, David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital Cardiopulmonary laboratory, both in Boston, summarized his group’s study in patients with ME/CFS using pyridostigmine as both a potential treatment for improving exercise capacity and a proof-of-concept that neurovascular dysregulation underlies exertional intolerance in the condition.
A total of 45 patients were randomized to 60 mg oral pyridostigmine or placebo after an invasive cardiopulmonary exercise test, and a second test performed 50 minutes later. Peak VO2 increased after pyridostigmine but decreased after placebo (+13.3 mL/min vs. –40.2 mL/min, P < .05). Cardiac output and right atrial pressure were also significantly improved with pyridostigmine and worse with placebo.
“We suggest that treatable neurovascular dysregulation underlies acute exercise intolerance in ME/CFS. ... Pyridostigmine may be a useful repurposed off-label treatment [for] a subset of patients with exercise intolerance,” Dr. Systrom said.
Asked to comment, Dr. Lapp said: “We’ve used Mestinon for years because it helps with POTS and also with neurally mediated hypotension. Systrom is taking it to a new level because he’s shown that it increases preload to the heart.” However, he noted that it’s unclear whether the drug will help patients who don’t have POTS specifically. On the other hand, patients rarely experience side effects from the drug.
Since the generic tablets come only in 60-mg doses, and the starting dose is 30 mg three times a day, he advised cutting the tablets in half during titration up to 60 mg three times a day.
Oxaloacetate (benaGene)
David Lyons Kaufman, MD, of the Center for Complex Diseases, Mountain View, Calif., summarized data from his group’s recently published open-label, nonrandomized, “proof-of-concept” study on use of the commercially available nutritional supplement anhydrous enol-oxaloacetate for treating mental and physical fatigue in 76 patients with longstanding ME/CFS and 43 with long-COVID fatigue.
Oxaloacetate is a major step in the Krebs cycle within the mitochondria that are depleted in patients with ME/CFS. It is also an energy metabolite that has multiple effects in cells and mitochondria, Dr. Kaufman explained.
Doses ranging from 500 mg twice daily up to 1,000 mg three times a day were given for 6 weeks. Up to 33% of the patients with ME/CFS and up to 46.8% of the long-COVID group achieved clinical efficacy as measured by physical and mental fatigue scores, compared with just 5.9% of historical ME/CFS controls. All doses showed highly significant improvements.
The only adverse effects were occasional dyspepsia, which was avoided by taking the supplement with food, and insomnia, resolved by having them dose at breakfast and lunch, Dr. Kaufman said.
Following those preliminary data, there is now an ongoing 90-day, randomized, placebo-controlled clinical trial of 80 patients with ME/CFS using 2,000 mg anhydrous enol-oxaloacetate per day. Endpoints include multiple objective measures.
“We have a health care crisis with long COVID, and we’ve had this smoldering crisis with ME/CFS for decades that’s never been addressed. ME/CFS and long COVID, if not identical, are certainly overlapping. ... We have to pursue these translational medicine pilot studies as rapidly as possible,” Dr. Kaufman remarked.
Dr. Lapp told this news organization that it makes sense to use constituents of the Krebs cycle to improve mitochondrial function, but the problem with oxaloacetate is its cost. Dr. Kaufman mentioned that based on the preliminary trial, the therapeutic “sweet spot” appeared to be 1,000 mg twice daily. The manufacturer’s website lists the price for a single bottle of 30 250-mg capsules at $49, or $42 if purchased via a monthly subscription.
“It’s a benign drug, and it’s over the counter. I would give it to any patient who’s got a big wallet,” Dr. Lapp quipped, adding: “If they’ve got the money, they can order it tonight.”
Inspiritol
Inspiritol is an investigational “nebulized, inhaled, multimechanism medication designed to treat the major symptoms of respiratory distress with antioxidant, anti-inflammatory, and broad-spectrum antiviral and antibacterial properties. Inspiritol is composed of both endogenously produced and naturally occurring, well-tolerated biochemicals,” according to the company website.
The hypothesis, Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, said at the meeting, is that “ME/CFS and long COVID-19 result from an aberrant response to an immunological trigger like infection, which results in a permanently dysregulated immune system as a result of overactivation of CD8 T cells and subsequent exhaustion.”
Inspiritol, containing five antioxidants, acts as an immune modulator to reverse the CD8 T cell exhaustion and improve symptoms. Administration by inhaler delivers it directly to the brain from the lung. It was originally designed for use in chronic obstructive pulmonary disease and asthma and has shown efficacy for acute COVID-19, Dr. Selin said.
In a preliminary study, four patients with ME/CFS and five with long COVID have been treated with Inspiritol for 2-15 months, and all have self-reported improved symptoms. Cough has been the only reported side effect.
The company is pursuing an Investigational New Drug Application for the product with the Food and Drug Administration and has several patents pending. Dr. Lapp called Inspiritol “very interesting,” and said that reversal of CD8 “exhaustion” also would appear to be a promising approach. However, he noted, “the problem is that we don’t know what’s in it.”
Stellate ganglion block
Injection of local anesthetic near the stellate ganglion to block activity of the entire cervical sympathetic chain has been used for nearly a century to treat a variety of sympathetically mediated conditions, including complex regional pain syndrome (CRPS), shingles, and phantom-limb pain. More recently, it has been used in a variety of other conditions, including PTSD, Raynaud’s disease, menopausal hot flashes, and hyperhidrosis.
Insurance companies typically cover it for CRPS, neuropathic upper-extremity pain, hyperhidrosis, and Raynaud’s, said Luke Liu, MD, an anesthesiologist who is founder and chief executive officer of Alaska-based pain management company Neuroversion.
Deborah Duricka, PhD, also with Neuroversion, presented results from a now-published case series of 11 patients with long COVID who underwent stellate ganglion block by a board-certified anesthesiologist, first on one side at the level of C6, then on the contralateral side the following day.
Clinically meaningful benefits were seen in at least five of the patients in fatigue, memory problems, problems concentrating, rapid heartbeat, orthostatic intolerance, sleep problems, postexertional malaise, anxiety, and depression.
The hypothetical mechanism, she said, is that “sympathetic block prevents sympathetically driven vasoconstriction in carotid and vertebral arteries.”
Dr. Liu presented another case series of five patients with ME/CFS who underwent the procedure with ultrasound guidance, again on one side and the other side the next day. All had upper-limb autonomic issues such as Raynaud’s and/or neuropathic pain that had been refractory to more conventional treatments.
All five patients reported improvements in symptoms of ME/CFS, including energy level, cognition, pain, and postexertional malaise. One patient reported “feeling well for the first time in decades.” However, that patient relapsed after a mild viral illness 3.5 months after treatment. Some of the patients have required further treatments.
Dr. Lapp commented that, although the procedure is generally safe when performed by an experienced clinician, “Any time you do an injection like that, there’s a high risk that you could nick an artery or a vein or hit an essential nerve in the neck. That’s why it has to be done under fluoroscopy or ultrasound.”
He said he’s had a few patients undergo the procedure, mostly for CRPS, and they seem to have benefited from it. “It might increase cerebral blood flow and preload to the heart, so it might decrease ME/CFS symptoms and help with POTS as well.”
Nonetheless, Dr. Lapp said he wouldn’t consider stellate ganglion block as first-line treatment for ME/CFS or long COVID. “I think it would be for the treatment-resistant patient, when you’ve gone through all the treatments that we know and addressed all the comorbidities and they’re still not getting better.”
But, he added, it is a standard procedure. “Any pain clinic can do a stellate block.”
Transcutaneous auricular vagus nerve stimulation
Nicola Clague-Baker, PhD, a physiotherapist at the University of Liverpool (England), presented findings from an international survey of people with ME/CFS regarding their experience with transcutaneous auricular vagus nerve stimulation (taVNS) to manage their autonomic symptoms. The technique involves stimulation of the autonomic nervous system via the vagus nerve using electrodes applied to part of the ear. The theory is that the technique stimulates the parasympathetic nervous system and improves autonomic balance.
Two small previous trials showing benefit of vagus nerve stimulation for people with ME/CFS used more invasive and less comfortable methods of applying the stimulation rather than to the ear, Dr. Clague-Baker and colleagues noted in a poster. It has also been used successfully in treating POTS, another conference speaker noted.
A total of 131 people with ME/CFS (called simply “ME” in the United Kingdom) responded to a survey advertised on social media and websites. The majority (60%) were from the United Kingdom while the rest were from Europe, Australia, and North America. Most were female, and slightly more than half had lived with ME for 10 or more years.
The majority (72%) were still using taVNS, while 28% had stopped using it. Only 9% had used the modality for longer than a year. Respondents identified more than 30 benefits in symptoms and activities, with improvements in postexertional malaise (39%) and brain fog (37%) being the most common. One reported significant reduction in constipation.
However, respondents also mentioned more than 20 short- and long-term negatives, including headaches (15%) and long-term irritation at the site (9%). One participant reported a “big improvement in neuropathic pain, but not so much for muscles and joints.”
Overall, 80% reported that they would continue using taVNS and 67% said they would recommend it to others with ME, and 56% said that the system was mildly to very beneficial.
Dr. Lapp noted that several types of transcutaneous electrical nerve stimulation units with ear clips are sold online, and he’s seen them work well for migraine treatment. However, he cautioned that some patients have had side effects from the treatment, such as headaches and dizziness. “It’s putting an electrical current through your brain. In my mind, it’s another last-ditch measure.”
Dr. Lapp reported no financial disclosures.
A version of this article first appeared on Medscape.com.
A variety of treatments, most already commercially available, are under investigation for treating the constellation of overlapping symptoms associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), “long COVID,” and dysautonomia.
At the virtual annual meeting of the International Association for Chronic Fatigue Syndrome/Myalgic Encephalomyelitis, speakers presented data for a variety of approaches to ease symptoms common across postviral conditions, such as extreme fatigue, postexertional malaise (“crash”), cognitive dysfunction (“brain fog”), orthostatic intolerance including postural orthostatic tachycardia syndrome (POTS), and chronic pain. Most of the modalities are already commercially available for other indications, although some are costly and not covered by payers for these conditions.
“ ... In the past, patients were told ‘you have chronic fatigue syndrome but there’s nothing we can do for it.’ That certainly is not the case. There aren’t cures, but there are many management techniques to improve symptoms,” Charles W. Lapp, MD, medical director of the Hunter-Hopkins Center, Charlotte, N.C., said in an interview.
A current mainstay of treatment for ME/CFS – including that triggered by COVID-19 – is activity pacing, in which patients learn to stay within their “energy envelopes” in order to avoid postexertional malaise, a worsening of all symptoms with exertion. The use of “graded exercise” is no longer recommended, per U.K. and U.S. guidelines.
Data for the following approaches were presented at the IACFS/ME conference:
Pyridostigmine (mestinon, others)
Pyridostigmine, an acetylcholinesterase inhibitor, is approved for the treatment of muscle weakness resulting from myasthenia gravis and is available in generic form. It has previously been shown to produce significant improvement in both symptom burden and heart rate response in POTS.
At the IACFS/ME conference, David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital and director of the Massachusetts General Hospital Cardiopulmonary laboratory, both in Boston, summarized his group’s study in patients with ME/CFS using pyridostigmine as both a potential treatment for improving exercise capacity and a proof-of-concept that neurovascular dysregulation underlies exertional intolerance in the condition.
A total of 45 patients were randomized to 60 mg oral pyridostigmine or placebo after an invasive cardiopulmonary exercise test, and a second test performed 50 minutes later. Peak VO2 increased after pyridostigmine but decreased after placebo (+13.3 mL/min vs. –40.2 mL/min, P < .05). Cardiac output and right atrial pressure were also significantly improved with pyridostigmine and worse with placebo.
“We suggest that treatable neurovascular dysregulation underlies acute exercise intolerance in ME/CFS. ... Pyridostigmine may be a useful repurposed off-label treatment [for] a subset of patients with exercise intolerance,” Dr. Systrom said.
Asked to comment, Dr. Lapp said: “We’ve used Mestinon for years because it helps with POTS and also with neurally mediated hypotension. Systrom is taking it to a new level because he’s shown that it increases preload to the heart.” However, he noted that it’s unclear whether the drug will help patients who don’t have POTS specifically. On the other hand, patients rarely experience side effects from the drug.
Since the generic tablets come only in 60-mg doses, and the starting dose is 30 mg three times a day, he advised cutting the tablets in half during titration up to 60 mg three times a day.
Oxaloacetate (benaGene)
David Lyons Kaufman, MD, of the Center for Complex Diseases, Mountain View, Calif., summarized data from his group’s recently published open-label, nonrandomized, “proof-of-concept” study on use of the commercially available nutritional supplement anhydrous enol-oxaloacetate for treating mental and physical fatigue in 76 patients with longstanding ME/CFS and 43 with long-COVID fatigue.
Oxaloacetate is a major step in the Krebs cycle within the mitochondria that are depleted in patients with ME/CFS. It is also an energy metabolite that has multiple effects in cells and mitochondria, Dr. Kaufman explained.
Doses ranging from 500 mg twice daily up to 1,000 mg three times a day were given for 6 weeks. Up to 33% of the patients with ME/CFS and up to 46.8% of the long-COVID group achieved clinical efficacy as measured by physical and mental fatigue scores, compared with just 5.9% of historical ME/CFS controls. All doses showed highly significant improvements.
The only adverse effects were occasional dyspepsia, which was avoided by taking the supplement with food, and insomnia, resolved by having them dose at breakfast and lunch, Dr. Kaufman said.
Following those preliminary data, there is now an ongoing 90-day, randomized, placebo-controlled clinical trial of 80 patients with ME/CFS using 2,000 mg anhydrous enol-oxaloacetate per day. Endpoints include multiple objective measures.
“We have a health care crisis with long COVID, and we’ve had this smoldering crisis with ME/CFS for decades that’s never been addressed. ME/CFS and long COVID, if not identical, are certainly overlapping. ... We have to pursue these translational medicine pilot studies as rapidly as possible,” Dr. Kaufman remarked.
Dr. Lapp told this news organization that it makes sense to use constituents of the Krebs cycle to improve mitochondrial function, but the problem with oxaloacetate is its cost. Dr. Kaufman mentioned that based on the preliminary trial, the therapeutic “sweet spot” appeared to be 1,000 mg twice daily. The manufacturer’s website lists the price for a single bottle of 30 250-mg capsules at $49, or $42 if purchased via a monthly subscription.
“It’s a benign drug, and it’s over the counter. I would give it to any patient who’s got a big wallet,” Dr. Lapp quipped, adding: “If they’ve got the money, they can order it tonight.”
Inspiritol
Inspiritol is an investigational “nebulized, inhaled, multimechanism medication designed to treat the major symptoms of respiratory distress with antioxidant, anti-inflammatory, and broad-spectrum antiviral and antibacterial properties. Inspiritol is composed of both endogenously produced and naturally occurring, well-tolerated biochemicals,” according to the company website.
The hypothesis, Liisa K. Selin, MD, PhD, professor of pathology at the University of Massachusetts, Worcester, said at the meeting, is that “ME/CFS and long COVID-19 result from an aberrant response to an immunological trigger like infection, which results in a permanently dysregulated immune system as a result of overactivation of CD8 T cells and subsequent exhaustion.”
Inspiritol, containing five antioxidants, acts as an immune modulator to reverse the CD8 T cell exhaustion and improve symptoms. Administration by inhaler delivers it directly to the brain from the lung. It was originally designed for use in chronic obstructive pulmonary disease and asthma and has shown efficacy for acute COVID-19, Dr. Selin said.
In a preliminary study, four patients with ME/CFS and five with long COVID have been treated with Inspiritol for 2-15 months, and all have self-reported improved symptoms. Cough has been the only reported side effect.
The company is pursuing an Investigational New Drug Application for the product with the Food and Drug Administration and has several patents pending. Dr. Lapp called Inspiritol “very interesting,” and said that reversal of CD8 “exhaustion” also would appear to be a promising approach. However, he noted, “the problem is that we don’t know what’s in it.”
Stellate ganglion block
Injection of local anesthetic near the stellate ganglion to block activity of the entire cervical sympathetic chain has been used for nearly a century to treat a variety of sympathetically mediated conditions, including complex regional pain syndrome (CRPS), shingles, and phantom-limb pain. More recently, it has been used in a variety of other conditions, including PTSD, Raynaud’s disease, menopausal hot flashes, and hyperhidrosis.
Insurance companies typically cover it for CRPS, neuropathic upper-extremity pain, hyperhidrosis, and Raynaud’s, said Luke Liu, MD, an anesthesiologist who is founder and chief executive officer of Alaska-based pain management company Neuroversion.
Deborah Duricka, PhD, also with Neuroversion, presented results from a now-published case series of 11 patients with long COVID who underwent stellate ganglion block by a board-certified anesthesiologist, first on one side at the level of C6, then on the contralateral side the following day.
Clinically meaningful benefits were seen in at least five of the patients in fatigue, memory problems, problems concentrating, rapid heartbeat, orthostatic intolerance, sleep problems, postexertional malaise, anxiety, and depression.
The hypothetical mechanism, she said, is that “sympathetic block prevents sympathetically driven vasoconstriction in carotid and vertebral arteries.”
Dr. Liu presented another case series of five patients with ME/CFS who underwent the procedure with ultrasound guidance, again on one side and the other side the next day. All had upper-limb autonomic issues such as Raynaud’s and/or neuropathic pain that had been refractory to more conventional treatments.
All five patients reported improvements in symptoms of ME/CFS, including energy level, cognition, pain, and postexertional malaise. One patient reported “feeling well for the first time in decades.” However, that patient relapsed after a mild viral illness 3.5 months after treatment. Some of the patients have required further treatments.
Dr. Lapp commented that, although the procedure is generally safe when performed by an experienced clinician, “Any time you do an injection like that, there’s a high risk that you could nick an artery or a vein or hit an essential nerve in the neck. That’s why it has to be done under fluoroscopy or ultrasound.”
He said he’s had a few patients undergo the procedure, mostly for CRPS, and they seem to have benefited from it. “It might increase cerebral blood flow and preload to the heart, so it might decrease ME/CFS symptoms and help with POTS as well.”
Nonetheless, Dr. Lapp said he wouldn’t consider stellate ganglion block as first-line treatment for ME/CFS or long COVID. “I think it would be for the treatment-resistant patient, when you’ve gone through all the treatments that we know and addressed all the comorbidities and they’re still not getting better.”
But, he added, it is a standard procedure. “Any pain clinic can do a stellate block.”
Transcutaneous auricular vagus nerve stimulation
Nicola Clague-Baker, PhD, a physiotherapist at the University of Liverpool (England), presented findings from an international survey of people with ME/CFS regarding their experience with transcutaneous auricular vagus nerve stimulation (taVNS) to manage their autonomic symptoms. The technique involves stimulation of the autonomic nervous system via the vagus nerve using electrodes applied to part of the ear. The theory is that the technique stimulates the parasympathetic nervous system and improves autonomic balance.
Two small previous trials showing benefit of vagus nerve stimulation for people with ME/CFS used more invasive and less comfortable methods of applying the stimulation rather than to the ear, Dr. Clague-Baker and colleagues noted in a poster. It has also been used successfully in treating POTS, another conference speaker noted.
A total of 131 people with ME/CFS (called simply “ME” in the United Kingdom) responded to a survey advertised on social media and websites. The majority (60%) were from the United Kingdom while the rest were from Europe, Australia, and North America. Most were female, and slightly more than half had lived with ME for 10 or more years.
The majority (72%) were still using taVNS, while 28% had stopped using it. Only 9% had used the modality for longer than a year. Respondents identified more than 30 benefits in symptoms and activities, with improvements in postexertional malaise (39%) and brain fog (37%) being the most common. One reported significant reduction in constipation.
However, respondents also mentioned more than 20 short- and long-term negatives, including headaches (15%) and long-term irritation at the site (9%). One participant reported a “big improvement in neuropathic pain, but not so much for muscles and joints.”
Overall, 80% reported that they would continue using taVNS and 67% said they would recommend it to others with ME, and 56% said that the system was mildly to very beneficial.
Dr. Lapp noted that several types of transcutaneous electrical nerve stimulation units with ear clips are sold online, and he’s seen them work well for migraine treatment. However, he cautioned that some patients have had side effects from the treatment, such as headaches and dizziness. “It’s putting an electrical current through your brain. In my mind, it’s another last-ditch measure.”
Dr. Lapp reported no financial disclosures.
A version of this article first appeared on Medscape.com.
FROM IACFSME 2022
Some GIs receive more industry money than others
Industry payments to U.S. gastroenterologists and hepatologists increased from 2014 to 2016 before beginning to steadily decrease after 2016, but they're largely concentrated among a small few, according to new research published in Gastroenterology.
The study aimed to identify trends in these specialties in the years after the Sunshine Act, enacted in 2010, and the federal program Open Payments, established in 2013.
“Although Open Payments launched in September of 2014, all the joinpoints in our study occurred more than a year later in 2016, suggesting a delay in observable changes in behavior on industry physician relationships,” wrote Xiaohan Ying, MD, of Weill Cornell Medicine in New York, and colleagues. “Since 2016, we have seen a sustained reduction in general industry payments to physicians while research payments remained stable, which is likely the desired outcome of this program.”
That’s also the conclusion of Lawrence Kosinski, MD, MBA, a spokesperson for the American Gastroenterological Association, who was not involved in the study.
“Most all of us are aware of the Sunshine Act and have reacted accordingly, so I am not surprised that reimbursement per physician has declined over the time period,” Dr. Kosinski told this news organization. “Many physicians are very sensitive to their reporting and have decreased their exposures,” said Dr. Kosinski, founder of SonarMD and a member of the Health & Human Services Advisory Committee on Value-Based Payment. “What does surprise me is the marked disparity in payments with a very small number of physicians receiving tremendous reimbursement from speaking engagements and promotions.”
The researchers retrospectively analyzed industry payments to 26,981 practicing pediatric and adults gastroenterologists and hepatologists using the National Plan and Provider Enumeration System and data from Open Payments between January 2014 and December 2020. The researchers excluded education payments and focused on general payments, which “include charitable contribution, speaker fees, consulting fees, ownership and investments, education, entertainment, food and beverages, gift, honoraria, royalty and license, and travel and lodging,” they reported.
Who gets paid, and how much?
While $27.5 million was going to research and grants, most of the payments ($403.3 million) were general payments; out of the total payments to specialists, $30 million went to hepatology, and $400.8 million went to gastroenterology. Nearly all of the general payments ($398.1 million) were for noneducation purposes; 90.5% of general payments went to men and 9.5% went to women, at an average of $17,167 per person. Nearly half the payments (43.8%) were for speaker fees, totaling $174.3 million, followed by 18.4% going to consulting ($73.1 million) and 12.9% going to food and beverages ($51.5 million).
Most of the physicians accepting payments (86.6%) received less than $10,000, but this made up only 8.3% of all payments. Meanwhile, 74% of all the payments, $294.6 million, went to just 3.1% of the physicians, all of whom received more than $100,000.
That breakdown is what most caught Dr. Kosinki’s attention.
“It’s one thing for a speaker to declare that they are receiving funds from pharma, but they never let us know how much,” Dr. Kosinski said. “Some of these speakers are realizing a very significant payment, which could change the opinions of those listening to their presentations.”
The authors reported that a group of 50 top earners (0.2%) received more than $1 million between 2014 and 2020. Their payments totaled $94.8 million and accounted for nearly a quarter (23.8%) of all the payments. All but one of these physicians were men, and one physician has received more than $1 million every year since 2014.
Payments for guideline authors explored
The authors examined payments to practicing U.S. gastroenterologists and hepatologists who helped write clinical guidelines for the following organizations:
- American Gastroenterological Association (AGA).
- American College of Gastroenterology (ACG).
- American Association for the Study of Liver Disease (AASLD).
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN).
- American Society for Gastrointestinal Endoscopy (ASGE).
The 186 guidelines published between 2014 and 2020 had 632 physician authors, 415 of whom were practicing gastroenterologists and hepatologists in the United States. Most of these physicians (85.8%) received at least one industry payment, with payments to guideline authors totaling $43.6 million.
Similar to the lopsided breakdown for total payments across all physicians, the majority of the payments (87.4%, or $38.1 million) went to one-quarter of the authors, who each received more than $100,000 per person. Meanwhile, 38.2% of the guideline authors received less than $10,000.
“However, these numbers are likely to decrease in the future as professional societies, such as AASLD, require a majority of the guideline authors to be free of conflict of interest relevant to the subject matter,” the authors wrote. They added that members selected as part of the AGA’s guideline development group (GDG) must report all conflicts of interest, including indirect and intellectual ones, and are recused or excluded when appropriate. These guideline development group participants must also forgo speaking and consulting arrangements until one year after the guideline’s publication.
Trends have been shifting
Total industry payments initially grew at a rate of 11.4% a year between 2014 and 2016 before decreasing at a rate of 5.8% per year after 2016 (P = .03). Though a similar trend occurred at the individual level, it did not reach significance.
However, the trend differed slightly between men and women: Payments to men increased 10.4% annually until 2016 then decreased 6.8% per year thereafter, but women’s payments increased 11.3% per year until 2019. Between 2014 and 2019, the amount per person payment dropped 3.5% annually to physicians overall, but payments to women initially increased 35.4% a year between 2014 and 2016 before decreasing.
Although not statistically significant, trends for types of payments showed that speaker and food/beverage fees have been declining since 2016 while consulting fees have been declining since 2014.
“The reduction in industry payments could be due to the Hawthorne effect, where physicians alter their behavior after becoming aware that their payments were being monitored,” the authors wrote. “Although many physicians see themselves as less vulnerable to be biased by industry compensation, studies have shown that even small payments can affect behavior such as prescription pattern. Additionally, studies have found that patients are less likely to trust physicians who have received industry payments.”
The authors acknowledged the role of industry payments in funding clinical trials but noted that pharmaceutical companies themselves have been taking on more design and execution of trials in recent decades. Further, only 6% of all payments went to research and grant funding, a little more than half the payments for food and beverages.
“While industry research funding is undeniably crucial, it simply plays a very small role in total industry compensation for physicians,” the authors wrote. “While speaker events could be beneficial and educational for physicians and other audiences, these events could also be utilized as means to promote specific products. While it is beneficial to seek input from experienced gastroenterologists for novel therapies and devices, actions should be taken to place limitations on industry payments to physicians, especially for the top earners.”
One author reported speaker fees from W.L. Gore & Associates and Cook Medical. The other two others had no disclosures. No external funding was noted. Dr. Kosinski reported having no relevant disclosures.
This article was updated Aug. 9, 2022.
Industry payments to U.S. gastroenterologists and hepatologists increased from 2014 to 2016 before beginning to steadily decrease after 2016, but they're largely concentrated among a small few, according to new research published in Gastroenterology.
The study aimed to identify trends in these specialties in the years after the Sunshine Act, enacted in 2010, and the federal program Open Payments, established in 2013.
“Although Open Payments launched in September of 2014, all the joinpoints in our study occurred more than a year later in 2016, suggesting a delay in observable changes in behavior on industry physician relationships,” wrote Xiaohan Ying, MD, of Weill Cornell Medicine in New York, and colleagues. “Since 2016, we have seen a sustained reduction in general industry payments to physicians while research payments remained stable, which is likely the desired outcome of this program.”
That’s also the conclusion of Lawrence Kosinski, MD, MBA, a spokesperson for the American Gastroenterological Association, who was not involved in the study.
“Most all of us are aware of the Sunshine Act and have reacted accordingly, so I am not surprised that reimbursement per physician has declined over the time period,” Dr. Kosinski told this news organization. “Many physicians are very sensitive to their reporting and have decreased their exposures,” said Dr. Kosinski, founder of SonarMD and a member of the Health & Human Services Advisory Committee on Value-Based Payment. “What does surprise me is the marked disparity in payments with a very small number of physicians receiving tremendous reimbursement from speaking engagements and promotions.”
The researchers retrospectively analyzed industry payments to 26,981 practicing pediatric and adults gastroenterologists and hepatologists using the National Plan and Provider Enumeration System and data from Open Payments between January 2014 and December 2020. The researchers excluded education payments and focused on general payments, which “include charitable contribution, speaker fees, consulting fees, ownership and investments, education, entertainment, food and beverages, gift, honoraria, royalty and license, and travel and lodging,” they reported.
Who gets paid, and how much?
While $27.5 million was going to research and grants, most of the payments ($403.3 million) were general payments; out of the total payments to specialists, $30 million went to hepatology, and $400.8 million went to gastroenterology. Nearly all of the general payments ($398.1 million) were for noneducation purposes; 90.5% of general payments went to men and 9.5% went to women, at an average of $17,167 per person. Nearly half the payments (43.8%) were for speaker fees, totaling $174.3 million, followed by 18.4% going to consulting ($73.1 million) and 12.9% going to food and beverages ($51.5 million).
Most of the physicians accepting payments (86.6%) received less than $10,000, but this made up only 8.3% of all payments. Meanwhile, 74% of all the payments, $294.6 million, went to just 3.1% of the physicians, all of whom received more than $100,000.
That breakdown is what most caught Dr. Kosinki’s attention.
“It’s one thing for a speaker to declare that they are receiving funds from pharma, but they never let us know how much,” Dr. Kosinski said. “Some of these speakers are realizing a very significant payment, which could change the opinions of those listening to their presentations.”
The authors reported that a group of 50 top earners (0.2%) received more than $1 million between 2014 and 2020. Their payments totaled $94.8 million and accounted for nearly a quarter (23.8%) of all the payments. All but one of these physicians were men, and one physician has received more than $1 million every year since 2014.
Payments for guideline authors explored
The authors examined payments to practicing U.S. gastroenterologists and hepatologists who helped write clinical guidelines for the following organizations:
- American Gastroenterological Association (AGA).
- American College of Gastroenterology (ACG).
- American Association for the Study of Liver Disease (AASLD).
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN).
- American Society for Gastrointestinal Endoscopy (ASGE).
The 186 guidelines published between 2014 and 2020 had 632 physician authors, 415 of whom were practicing gastroenterologists and hepatologists in the United States. Most of these physicians (85.8%) received at least one industry payment, with payments to guideline authors totaling $43.6 million.
Similar to the lopsided breakdown for total payments across all physicians, the majority of the payments (87.4%, or $38.1 million) went to one-quarter of the authors, who each received more than $100,000 per person. Meanwhile, 38.2% of the guideline authors received less than $10,000.
“However, these numbers are likely to decrease in the future as professional societies, such as AASLD, require a majority of the guideline authors to be free of conflict of interest relevant to the subject matter,” the authors wrote. They added that members selected as part of the AGA’s guideline development group (GDG) must report all conflicts of interest, including indirect and intellectual ones, and are recused or excluded when appropriate. These guideline development group participants must also forgo speaking and consulting arrangements until one year after the guideline’s publication.
Trends have been shifting
Total industry payments initially grew at a rate of 11.4% a year between 2014 and 2016 before decreasing at a rate of 5.8% per year after 2016 (P = .03). Though a similar trend occurred at the individual level, it did not reach significance.
However, the trend differed slightly between men and women: Payments to men increased 10.4% annually until 2016 then decreased 6.8% per year thereafter, but women’s payments increased 11.3% per year until 2019. Between 2014 and 2019, the amount per person payment dropped 3.5% annually to physicians overall, but payments to women initially increased 35.4% a year between 2014 and 2016 before decreasing.
Although not statistically significant, trends for types of payments showed that speaker and food/beverage fees have been declining since 2016 while consulting fees have been declining since 2014.
“The reduction in industry payments could be due to the Hawthorne effect, where physicians alter their behavior after becoming aware that their payments were being monitored,” the authors wrote. “Although many physicians see themselves as less vulnerable to be biased by industry compensation, studies have shown that even small payments can affect behavior such as prescription pattern. Additionally, studies have found that patients are less likely to trust physicians who have received industry payments.”
The authors acknowledged the role of industry payments in funding clinical trials but noted that pharmaceutical companies themselves have been taking on more design and execution of trials in recent decades. Further, only 6% of all payments went to research and grant funding, a little more than half the payments for food and beverages.
“While industry research funding is undeniably crucial, it simply plays a very small role in total industry compensation for physicians,” the authors wrote. “While speaker events could be beneficial and educational for physicians and other audiences, these events could also be utilized as means to promote specific products. While it is beneficial to seek input from experienced gastroenterologists for novel therapies and devices, actions should be taken to place limitations on industry payments to physicians, especially for the top earners.”
One author reported speaker fees from W.L. Gore & Associates and Cook Medical. The other two others had no disclosures. No external funding was noted. Dr. Kosinski reported having no relevant disclosures.
This article was updated Aug. 9, 2022.
Industry payments to U.S. gastroenterologists and hepatologists increased from 2014 to 2016 before beginning to steadily decrease after 2016, but they're largely concentrated among a small few, according to new research published in Gastroenterology.
The study aimed to identify trends in these specialties in the years after the Sunshine Act, enacted in 2010, and the federal program Open Payments, established in 2013.
“Although Open Payments launched in September of 2014, all the joinpoints in our study occurred more than a year later in 2016, suggesting a delay in observable changes in behavior on industry physician relationships,” wrote Xiaohan Ying, MD, of Weill Cornell Medicine in New York, and colleagues. “Since 2016, we have seen a sustained reduction in general industry payments to physicians while research payments remained stable, which is likely the desired outcome of this program.”
That’s also the conclusion of Lawrence Kosinski, MD, MBA, a spokesperson for the American Gastroenterological Association, who was not involved in the study.
“Most all of us are aware of the Sunshine Act and have reacted accordingly, so I am not surprised that reimbursement per physician has declined over the time period,” Dr. Kosinski told this news organization. “Many physicians are very sensitive to their reporting and have decreased their exposures,” said Dr. Kosinski, founder of SonarMD and a member of the Health & Human Services Advisory Committee on Value-Based Payment. “What does surprise me is the marked disparity in payments with a very small number of physicians receiving tremendous reimbursement from speaking engagements and promotions.”
The researchers retrospectively analyzed industry payments to 26,981 practicing pediatric and adults gastroenterologists and hepatologists using the National Plan and Provider Enumeration System and data from Open Payments between January 2014 and December 2020. The researchers excluded education payments and focused on general payments, which “include charitable contribution, speaker fees, consulting fees, ownership and investments, education, entertainment, food and beverages, gift, honoraria, royalty and license, and travel and lodging,” they reported.
Who gets paid, and how much?
While $27.5 million was going to research and grants, most of the payments ($403.3 million) were general payments; out of the total payments to specialists, $30 million went to hepatology, and $400.8 million went to gastroenterology. Nearly all of the general payments ($398.1 million) were for noneducation purposes; 90.5% of general payments went to men and 9.5% went to women, at an average of $17,167 per person. Nearly half the payments (43.8%) were for speaker fees, totaling $174.3 million, followed by 18.4% going to consulting ($73.1 million) and 12.9% going to food and beverages ($51.5 million).
Most of the physicians accepting payments (86.6%) received less than $10,000, but this made up only 8.3% of all payments. Meanwhile, 74% of all the payments, $294.6 million, went to just 3.1% of the physicians, all of whom received more than $100,000.
That breakdown is what most caught Dr. Kosinki’s attention.
“It’s one thing for a speaker to declare that they are receiving funds from pharma, but they never let us know how much,” Dr. Kosinski said. “Some of these speakers are realizing a very significant payment, which could change the opinions of those listening to their presentations.”
The authors reported that a group of 50 top earners (0.2%) received more than $1 million between 2014 and 2020. Their payments totaled $94.8 million and accounted for nearly a quarter (23.8%) of all the payments. All but one of these physicians were men, and one physician has received more than $1 million every year since 2014.
Payments for guideline authors explored
The authors examined payments to practicing U.S. gastroenterologists and hepatologists who helped write clinical guidelines for the following organizations:
- American Gastroenterological Association (AGA).
- American College of Gastroenterology (ACG).
- American Association for the Study of Liver Disease (AASLD).
- North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN).
- American Society for Gastrointestinal Endoscopy (ASGE).
The 186 guidelines published between 2014 and 2020 had 632 physician authors, 415 of whom were practicing gastroenterologists and hepatologists in the United States. Most of these physicians (85.8%) received at least one industry payment, with payments to guideline authors totaling $43.6 million.
Similar to the lopsided breakdown for total payments across all physicians, the majority of the payments (87.4%, or $38.1 million) went to one-quarter of the authors, who each received more than $100,000 per person. Meanwhile, 38.2% of the guideline authors received less than $10,000.
“However, these numbers are likely to decrease in the future as professional societies, such as AASLD, require a majority of the guideline authors to be free of conflict of interest relevant to the subject matter,” the authors wrote. They added that members selected as part of the AGA’s guideline development group (GDG) must report all conflicts of interest, including indirect and intellectual ones, and are recused or excluded when appropriate. These guideline development group participants must also forgo speaking and consulting arrangements until one year after the guideline’s publication.
Trends have been shifting
Total industry payments initially grew at a rate of 11.4% a year between 2014 and 2016 before decreasing at a rate of 5.8% per year after 2016 (P = .03). Though a similar trend occurred at the individual level, it did not reach significance.
However, the trend differed slightly between men and women: Payments to men increased 10.4% annually until 2016 then decreased 6.8% per year thereafter, but women’s payments increased 11.3% per year until 2019. Between 2014 and 2019, the amount per person payment dropped 3.5% annually to physicians overall, but payments to women initially increased 35.4% a year between 2014 and 2016 before decreasing.
Although not statistically significant, trends for types of payments showed that speaker and food/beverage fees have been declining since 2016 while consulting fees have been declining since 2014.
“The reduction in industry payments could be due to the Hawthorne effect, where physicians alter their behavior after becoming aware that their payments were being monitored,” the authors wrote. “Although many physicians see themselves as less vulnerable to be biased by industry compensation, studies have shown that even small payments can affect behavior such as prescription pattern. Additionally, studies have found that patients are less likely to trust physicians who have received industry payments.”
The authors acknowledged the role of industry payments in funding clinical trials but noted that pharmaceutical companies themselves have been taking on more design and execution of trials in recent decades. Further, only 6% of all payments went to research and grant funding, a little more than half the payments for food and beverages.
“While industry research funding is undeniably crucial, it simply plays a very small role in total industry compensation for physicians,” the authors wrote. “While speaker events could be beneficial and educational for physicians and other audiences, these events could also be utilized as means to promote specific products. While it is beneficial to seek input from experienced gastroenterologists for novel therapies and devices, actions should be taken to place limitations on industry payments to physicians, especially for the top earners.”
One author reported speaker fees from W.L. Gore & Associates and Cook Medical. The other two others had no disclosures. No external funding was noted. Dr. Kosinski reported having no relevant disclosures.
This article was updated Aug. 9, 2022.
FROM GASTROENTEROLOGY
My patient planned to murder me
San Diego internist David B. Bittleman, MD, was finishing an appointment with a patient when the man’s caregiver slipped Dr. Bittleman a note as the patient walked out of the room.
“Call me tomorrow,” the mysterious message read.
Dr. Bittleman phoned the caregiver, who was the patient’s ex-wife, the next day. He assumed she wanted to discuss a routine issue, such as the patient’s treatment. But the reason she wanted to talk privately was far more ominous.
“He wants to kill you,” she said.
Dr. Bittleman was shocked. The caregiver told Dr. Bittleman she believed her ex-husband was serious.
“The ex-wife and two adult sons were very alarmed by his erratic behavior,” Dr. Bittleman recalled. “She made it very clear that he said he planned to kill me. I feared for my life because I took his threat at face value.”
Patient sends alarming message, makes threats
When he went into medicine, Dr. Bittleman never imagined that he’d have to worry about being attacked or killed by a patient.
After spending 20 years in private practice, Dr. Bittleman was excited to accept a position at the Veterans Affairs San Diego health system. His extended family lived in the area, and he looked forward to helping veterans and to working with students, he said.
Dr. Bittleman had practiced primary care at the VA for about 5 years when he encountered the threatening patient, a veteran in his 60’s. The man was suffering from musculoskeletal pain and mental illness.
The patient had taken opioids on and off for many years. Dr. Bittleman felt that to continue the medication would not be safe, considering the man’s lifestyle.
“He had been maintained on oxycodone for chronic pain by previous providers, but I thought that was dangerous, given that he was mixing it with alcohol and marijuana,” he said. “I met with him and a substance use disorder physician for a conference call, and we explained we would need to taper the medication and eventually stop the opioids.”
Dr. Bittleman pleaded with the patient to enter drug rehab, and he offered him inpatient care for treatment of withdrawal. The man refused.
A few weeks later, Dr. Bittleman was checking the health center’s electronic messaging system. He found a disturbing message from the patient.
“You better learn jiu jitsu and hand-to-hand combat if you ever take my opioids away,” the message read. “You better learn how to defend yourself!”
Dr. Bittleman contacted the VA police and reported the message. The patient was interviewed by mental health professionals, but they did not believe he was dangerous, according to Dr. Bittleman.
“They are pretty limited to what they can do,” he said. “At a private practice, the patient might be fired or no longer allowed to come into the building, but the VA is a safety net institution. I’m not sure if he was even reprimanded.”
Two months later, the patient’s ex-wife shared the alarming news that the patient wanted to kill the doctor.
Dr. Bittleman went back to the police. They suggested he file a restraining order, which he sought that afternoon. By the end of the day, the judge had issued the restraining order, according to Dr. Bittleman and court records. The patient could not come within 100 yards of the physician, his clinic, car, or home.
But there was one frightening caveat. The order was temporary. It would last for only 2 weeks. To make the order permanent, Dr. Bittleman would have to go before the judge and argue why it was needed.
He wouldn’t be alone at the hearing. Someone else would be just paces away – the patient who wanted to murder him.
Doctor and patient face off before judge
As the hearing neared, Dr. Bittleman felt anxious, outraged, and fearful. He wondered whether the patient might make good on his threat.
Some colleagues suggested that Bittleman buy a gun, while others recommended he carry pepper spray. Dr. Bittleman had no interest in learning how to use a gun, he said. He took comfort in the fact that there were armed guards and metal detectors in his building, and there was a panic button under his desk.
“I was not sure I wanted to take care of patients anymore, especially chronic pain patients,” he said. “However, I went for some counseling with the Employee Assistance Program, and the therapist was helpful in normalizing my anxiety and acknowledging my fear.”
On the day of the hearing, Dr. Bittleman sat in the back of the courtroom. The patient, who sat near the front, glanced at Dr. Bittleman with a slight smile.
When his case was called, the judge explained that as the plaintiff, the burden was on Dr. Bittleman to prove the patient was a threat to his safety. He provided the judge a copy of the threatening message and a copy of the ex-wife’s note.
After reading the documents, the judge asked the patient to explain his side. The patient complained that the VA had denied him certain benefits and that he was forced to receive mental health treatment rehab that he “didn’t need.” The judge eventually interrupted the man to ask if he had threatened to kill Dr. Bittleman.
“Oh yes, your honor, I did say that, but I was only joking,” he told the judge.
The admission was enough. The judge issued a restraining order against the patient that would last 1 year. He could not have firearms, and if he violated the order, he would be arrested.
The terrifying saga was finally over.
“I never heard from the patient again,” Dr. Bittleman said. “His [care] location was changed, and police were required to come to all his visits with his new provider. I was relieved that if he ever came near me, he was going to jail.”
To raise awareness about such ordeals and the hassles that can follow, Dr. Bittleman wrote an article about his experience, which was published in the Annals of Family Medicine. He continues to treat patients at the VA, including those with chronic pain, but the memory of the menacing patient resurfaces from time to time.
“I do still think about it,” he said. “I know how to use my panic button, and I test it every 90 days. If there is a patient who concerns me, I will have the VA police wait nearby. I am very aware and upset by violence. When I hear about a doctor getting killed, I feel a clutch in my chest. How could I not relate? Here is a doctor who worked hard, who dedicated their life to help patients, and it comes to this? It’s so revolting. It makes me sick.”
Can you identify a violent patient?
Concern over threatening patients has grown across the country after recent violent attacks against physicians in Oklahoma and California. Two physicians were shot to death in June 2022 when a patient opened fire inside a Tulsa medical building. The primary target of the shooting was a surgeon who had performed surgery on the patient. Also in June, two nurses and an emergency physician were stabbed by a patient inside the Encino Hospital Medical Center. They survived.
The attacks raise questions about how to identify potentially violent patients and how to mitigate possible violence.
Threats and violence against health care professionals are nothing new, but they’re finally getting the attention they deserve, says Derek Schaller, MD, an emergency physician and assistant professor of emergency medicine at Central Michigan University in Mount Pleasant.
“Violence against personnel in medicine has been an issue for a long time; it’s just finally making headlines,” he said. “Way back when, it almost seemed like it was part of the job, part of the gig. But it shouldn’t be part of the gig. It’s not something we should be dealing with.”
It’s common for health care professionals and health centers to take a reactive approach to violent patients, but Dr. Schaller encourages a more proactive strategy. Central Michigan University Health, for example, recently studied its past violent encounters and analyzed the characteristics of violent patients. The analysis came after an increase in violent patient episodes at the health center in the past year, Dr. Schaller said.
The study yielded some interesting results, including that a large percentage of patients who became violent in the emergency department did so within the first hour they were in the hospital, he said.
“You would have thought it’s the patients who have been there and have been stuck in the emergency department for a while and who became disgruntled, but that was not the case,” Dr. Schaller said.
He recommends that physicians, medical practices, and hospitals carry out similar assessments of their patient populations and of past violent encounters to determine trends. His institution will be implementing a screening tool in triage to identify patients more likely to become violent so that health care professionals can intervene earlier, he said.
Such a screening tool is already demonstrating success in a variety of medical settings.
About 10 years ago, a research team led by Son Chae Kim, PhD, RN, found that the 10-item Aggressive Behavior Risk Assessment Tool (ABRAT) was able to identify potentially violent patients with reasonable sensitivity and specificity in hospital medical-surgical units.
Subsequently, the tool was modified for long-term care facilities, and again, researchers found that ABRAT was able to identify potentially violent residents with reasonable sensitivity and specificity, said Dr. Kim, ABRAT developer and a professor at Point Loma Nazarene University, San Diego.
In 2021, researchers embedded the checklist into an electronic health record (EHR) system and tested ABRAT in emergency departments.
“Currently, we are working with computer programmers to build an app that would make the ABRAT very easy to use in conjunction with EHR,” Dr. Kim said. “Instead of a nurse searching the EHR to find out if the patient has history of mental illness or aggressive behavior in the past, the app would automatically search the EHR and combine the nurse’s quick observation whether the patient is confused, agitated, staring, or threatening, to automatically calculate the violence risk.”
Dr. Kim and her team also developed a tool called VEST (Violent Event Severity Tool), a standardized objective workplace violence severity assessment. They are working with programmers to incorporate VEST into the app as well.
Dr. Kim’s hope is that the ABRAT tool can be modified for use in a range of health care settings.
A version of this article first appeared on Medscape.com.
San Diego internist David B. Bittleman, MD, was finishing an appointment with a patient when the man’s caregiver slipped Dr. Bittleman a note as the patient walked out of the room.
“Call me tomorrow,” the mysterious message read.
Dr. Bittleman phoned the caregiver, who was the patient’s ex-wife, the next day. He assumed she wanted to discuss a routine issue, such as the patient’s treatment. But the reason she wanted to talk privately was far more ominous.
“He wants to kill you,” she said.
Dr. Bittleman was shocked. The caregiver told Dr. Bittleman she believed her ex-husband was serious.
“The ex-wife and two adult sons were very alarmed by his erratic behavior,” Dr. Bittleman recalled. “She made it very clear that he said he planned to kill me. I feared for my life because I took his threat at face value.”
Patient sends alarming message, makes threats
When he went into medicine, Dr. Bittleman never imagined that he’d have to worry about being attacked or killed by a patient.
After spending 20 years in private practice, Dr. Bittleman was excited to accept a position at the Veterans Affairs San Diego health system. His extended family lived in the area, and he looked forward to helping veterans and to working with students, he said.
Dr. Bittleman had practiced primary care at the VA for about 5 years when he encountered the threatening patient, a veteran in his 60’s. The man was suffering from musculoskeletal pain and mental illness.
The patient had taken opioids on and off for many years. Dr. Bittleman felt that to continue the medication would not be safe, considering the man’s lifestyle.
“He had been maintained on oxycodone for chronic pain by previous providers, but I thought that was dangerous, given that he was mixing it with alcohol and marijuana,” he said. “I met with him and a substance use disorder physician for a conference call, and we explained we would need to taper the medication and eventually stop the opioids.”
Dr. Bittleman pleaded with the patient to enter drug rehab, and he offered him inpatient care for treatment of withdrawal. The man refused.
A few weeks later, Dr. Bittleman was checking the health center’s electronic messaging system. He found a disturbing message from the patient.
“You better learn jiu jitsu and hand-to-hand combat if you ever take my opioids away,” the message read. “You better learn how to defend yourself!”
Dr. Bittleman contacted the VA police and reported the message. The patient was interviewed by mental health professionals, but they did not believe he was dangerous, according to Dr. Bittleman.
“They are pretty limited to what they can do,” he said. “At a private practice, the patient might be fired or no longer allowed to come into the building, but the VA is a safety net institution. I’m not sure if he was even reprimanded.”
Two months later, the patient’s ex-wife shared the alarming news that the patient wanted to kill the doctor.
Dr. Bittleman went back to the police. They suggested he file a restraining order, which he sought that afternoon. By the end of the day, the judge had issued the restraining order, according to Dr. Bittleman and court records. The patient could not come within 100 yards of the physician, his clinic, car, or home.
But there was one frightening caveat. The order was temporary. It would last for only 2 weeks. To make the order permanent, Dr. Bittleman would have to go before the judge and argue why it was needed.
He wouldn’t be alone at the hearing. Someone else would be just paces away – the patient who wanted to murder him.
Doctor and patient face off before judge
As the hearing neared, Dr. Bittleman felt anxious, outraged, and fearful. He wondered whether the patient might make good on his threat.
Some colleagues suggested that Bittleman buy a gun, while others recommended he carry pepper spray. Dr. Bittleman had no interest in learning how to use a gun, he said. He took comfort in the fact that there were armed guards and metal detectors in his building, and there was a panic button under his desk.
“I was not sure I wanted to take care of patients anymore, especially chronic pain patients,” he said. “However, I went for some counseling with the Employee Assistance Program, and the therapist was helpful in normalizing my anxiety and acknowledging my fear.”
On the day of the hearing, Dr. Bittleman sat in the back of the courtroom. The patient, who sat near the front, glanced at Dr. Bittleman with a slight smile.
When his case was called, the judge explained that as the plaintiff, the burden was on Dr. Bittleman to prove the patient was a threat to his safety. He provided the judge a copy of the threatening message and a copy of the ex-wife’s note.
After reading the documents, the judge asked the patient to explain his side. The patient complained that the VA had denied him certain benefits and that he was forced to receive mental health treatment rehab that he “didn’t need.” The judge eventually interrupted the man to ask if he had threatened to kill Dr. Bittleman.
“Oh yes, your honor, I did say that, but I was only joking,” he told the judge.
The admission was enough. The judge issued a restraining order against the patient that would last 1 year. He could not have firearms, and if he violated the order, he would be arrested.
The terrifying saga was finally over.
“I never heard from the patient again,” Dr. Bittleman said. “His [care] location was changed, and police were required to come to all his visits with his new provider. I was relieved that if he ever came near me, he was going to jail.”
To raise awareness about such ordeals and the hassles that can follow, Dr. Bittleman wrote an article about his experience, which was published in the Annals of Family Medicine. He continues to treat patients at the VA, including those with chronic pain, but the memory of the menacing patient resurfaces from time to time.
“I do still think about it,” he said. “I know how to use my panic button, and I test it every 90 days. If there is a patient who concerns me, I will have the VA police wait nearby. I am very aware and upset by violence. When I hear about a doctor getting killed, I feel a clutch in my chest. How could I not relate? Here is a doctor who worked hard, who dedicated their life to help patients, and it comes to this? It’s so revolting. It makes me sick.”
Can you identify a violent patient?
Concern over threatening patients has grown across the country after recent violent attacks against physicians in Oklahoma and California. Two physicians were shot to death in June 2022 when a patient opened fire inside a Tulsa medical building. The primary target of the shooting was a surgeon who had performed surgery on the patient. Also in June, two nurses and an emergency physician were stabbed by a patient inside the Encino Hospital Medical Center. They survived.
The attacks raise questions about how to identify potentially violent patients and how to mitigate possible violence.
Threats and violence against health care professionals are nothing new, but they’re finally getting the attention they deserve, says Derek Schaller, MD, an emergency physician and assistant professor of emergency medicine at Central Michigan University in Mount Pleasant.
“Violence against personnel in medicine has been an issue for a long time; it’s just finally making headlines,” he said. “Way back when, it almost seemed like it was part of the job, part of the gig. But it shouldn’t be part of the gig. It’s not something we should be dealing with.”
It’s common for health care professionals and health centers to take a reactive approach to violent patients, but Dr. Schaller encourages a more proactive strategy. Central Michigan University Health, for example, recently studied its past violent encounters and analyzed the characteristics of violent patients. The analysis came after an increase in violent patient episodes at the health center in the past year, Dr. Schaller said.
The study yielded some interesting results, including that a large percentage of patients who became violent in the emergency department did so within the first hour they were in the hospital, he said.
“You would have thought it’s the patients who have been there and have been stuck in the emergency department for a while and who became disgruntled, but that was not the case,” Dr. Schaller said.
He recommends that physicians, medical practices, and hospitals carry out similar assessments of their patient populations and of past violent encounters to determine trends. His institution will be implementing a screening tool in triage to identify patients more likely to become violent so that health care professionals can intervene earlier, he said.
Such a screening tool is already demonstrating success in a variety of medical settings.
About 10 years ago, a research team led by Son Chae Kim, PhD, RN, found that the 10-item Aggressive Behavior Risk Assessment Tool (ABRAT) was able to identify potentially violent patients with reasonable sensitivity and specificity in hospital medical-surgical units.
Subsequently, the tool was modified for long-term care facilities, and again, researchers found that ABRAT was able to identify potentially violent residents with reasonable sensitivity and specificity, said Dr. Kim, ABRAT developer and a professor at Point Loma Nazarene University, San Diego.
In 2021, researchers embedded the checklist into an electronic health record (EHR) system and tested ABRAT in emergency departments.
“Currently, we are working with computer programmers to build an app that would make the ABRAT very easy to use in conjunction with EHR,” Dr. Kim said. “Instead of a nurse searching the EHR to find out if the patient has history of mental illness or aggressive behavior in the past, the app would automatically search the EHR and combine the nurse’s quick observation whether the patient is confused, agitated, staring, or threatening, to automatically calculate the violence risk.”
Dr. Kim and her team also developed a tool called VEST (Violent Event Severity Tool), a standardized objective workplace violence severity assessment. They are working with programmers to incorporate VEST into the app as well.
Dr. Kim’s hope is that the ABRAT tool can be modified for use in a range of health care settings.
A version of this article first appeared on Medscape.com.
San Diego internist David B. Bittleman, MD, was finishing an appointment with a patient when the man’s caregiver slipped Dr. Bittleman a note as the patient walked out of the room.
“Call me tomorrow,” the mysterious message read.
Dr. Bittleman phoned the caregiver, who was the patient’s ex-wife, the next day. He assumed she wanted to discuss a routine issue, such as the patient’s treatment. But the reason she wanted to talk privately was far more ominous.
“He wants to kill you,” she said.
Dr. Bittleman was shocked. The caregiver told Dr. Bittleman she believed her ex-husband was serious.
“The ex-wife and two adult sons were very alarmed by his erratic behavior,” Dr. Bittleman recalled. “She made it very clear that he said he planned to kill me. I feared for my life because I took his threat at face value.”
Patient sends alarming message, makes threats
When he went into medicine, Dr. Bittleman never imagined that he’d have to worry about being attacked or killed by a patient.
After spending 20 years in private practice, Dr. Bittleman was excited to accept a position at the Veterans Affairs San Diego health system. His extended family lived in the area, and he looked forward to helping veterans and to working with students, he said.
Dr. Bittleman had practiced primary care at the VA for about 5 years when he encountered the threatening patient, a veteran in his 60’s. The man was suffering from musculoskeletal pain and mental illness.
The patient had taken opioids on and off for many years. Dr. Bittleman felt that to continue the medication would not be safe, considering the man’s lifestyle.
“He had been maintained on oxycodone for chronic pain by previous providers, but I thought that was dangerous, given that he was mixing it with alcohol and marijuana,” he said. “I met with him and a substance use disorder physician for a conference call, and we explained we would need to taper the medication and eventually stop the opioids.”
Dr. Bittleman pleaded with the patient to enter drug rehab, and he offered him inpatient care for treatment of withdrawal. The man refused.
A few weeks later, Dr. Bittleman was checking the health center’s electronic messaging system. He found a disturbing message from the patient.
“You better learn jiu jitsu and hand-to-hand combat if you ever take my opioids away,” the message read. “You better learn how to defend yourself!”
Dr. Bittleman contacted the VA police and reported the message. The patient was interviewed by mental health professionals, but they did not believe he was dangerous, according to Dr. Bittleman.
“They are pretty limited to what they can do,” he said. “At a private practice, the patient might be fired or no longer allowed to come into the building, but the VA is a safety net institution. I’m not sure if he was even reprimanded.”
Two months later, the patient’s ex-wife shared the alarming news that the patient wanted to kill the doctor.
Dr. Bittleman went back to the police. They suggested he file a restraining order, which he sought that afternoon. By the end of the day, the judge had issued the restraining order, according to Dr. Bittleman and court records. The patient could not come within 100 yards of the physician, his clinic, car, or home.
But there was one frightening caveat. The order was temporary. It would last for only 2 weeks. To make the order permanent, Dr. Bittleman would have to go before the judge and argue why it was needed.
He wouldn’t be alone at the hearing. Someone else would be just paces away – the patient who wanted to murder him.
Doctor and patient face off before judge
As the hearing neared, Dr. Bittleman felt anxious, outraged, and fearful. He wondered whether the patient might make good on his threat.
Some colleagues suggested that Bittleman buy a gun, while others recommended he carry pepper spray. Dr. Bittleman had no interest in learning how to use a gun, he said. He took comfort in the fact that there were armed guards and metal detectors in his building, and there was a panic button under his desk.
“I was not sure I wanted to take care of patients anymore, especially chronic pain patients,” he said. “However, I went for some counseling with the Employee Assistance Program, and the therapist was helpful in normalizing my anxiety and acknowledging my fear.”
On the day of the hearing, Dr. Bittleman sat in the back of the courtroom. The patient, who sat near the front, glanced at Dr. Bittleman with a slight smile.
When his case was called, the judge explained that as the plaintiff, the burden was on Dr. Bittleman to prove the patient was a threat to his safety. He provided the judge a copy of the threatening message and a copy of the ex-wife’s note.
After reading the documents, the judge asked the patient to explain his side. The patient complained that the VA had denied him certain benefits and that he was forced to receive mental health treatment rehab that he “didn’t need.” The judge eventually interrupted the man to ask if he had threatened to kill Dr. Bittleman.
“Oh yes, your honor, I did say that, but I was only joking,” he told the judge.
The admission was enough. The judge issued a restraining order against the patient that would last 1 year. He could not have firearms, and if he violated the order, he would be arrested.
The terrifying saga was finally over.
“I never heard from the patient again,” Dr. Bittleman said. “His [care] location was changed, and police were required to come to all his visits with his new provider. I was relieved that if he ever came near me, he was going to jail.”
To raise awareness about such ordeals and the hassles that can follow, Dr. Bittleman wrote an article about his experience, which was published in the Annals of Family Medicine. He continues to treat patients at the VA, including those with chronic pain, but the memory of the menacing patient resurfaces from time to time.
“I do still think about it,” he said. “I know how to use my panic button, and I test it every 90 days. If there is a patient who concerns me, I will have the VA police wait nearby. I am very aware and upset by violence. When I hear about a doctor getting killed, I feel a clutch in my chest. How could I not relate? Here is a doctor who worked hard, who dedicated their life to help patients, and it comes to this? It’s so revolting. It makes me sick.”
Can you identify a violent patient?
Concern over threatening patients has grown across the country after recent violent attacks against physicians in Oklahoma and California. Two physicians were shot to death in June 2022 when a patient opened fire inside a Tulsa medical building. The primary target of the shooting was a surgeon who had performed surgery on the patient. Also in June, two nurses and an emergency physician were stabbed by a patient inside the Encino Hospital Medical Center. They survived.
The attacks raise questions about how to identify potentially violent patients and how to mitigate possible violence.
Threats and violence against health care professionals are nothing new, but they’re finally getting the attention they deserve, says Derek Schaller, MD, an emergency physician and assistant professor of emergency medicine at Central Michigan University in Mount Pleasant.
“Violence against personnel in medicine has been an issue for a long time; it’s just finally making headlines,” he said. “Way back when, it almost seemed like it was part of the job, part of the gig. But it shouldn’t be part of the gig. It’s not something we should be dealing with.”
It’s common for health care professionals and health centers to take a reactive approach to violent patients, but Dr. Schaller encourages a more proactive strategy. Central Michigan University Health, for example, recently studied its past violent encounters and analyzed the characteristics of violent patients. The analysis came after an increase in violent patient episodes at the health center in the past year, Dr. Schaller said.
The study yielded some interesting results, including that a large percentage of patients who became violent in the emergency department did so within the first hour they were in the hospital, he said.
“You would have thought it’s the patients who have been there and have been stuck in the emergency department for a while and who became disgruntled, but that was not the case,” Dr. Schaller said.
He recommends that physicians, medical practices, and hospitals carry out similar assessments of their patient populations and of past violent encounters to determine trends. His institution will be implementing a screening tool in triage to identify patients more likely to become violent so that health care professionals can intervene earlier, he said.
Such a screening tool is already demonstrating success in a variety of medical settings.
About 10 years ago, a research team led by Son Chae Kim, PhD, RN, found that the 10-item Aggressive Behavior Risk Assessment Tool (ABRAT) was able to identify potentially violent patients with reasonable sensitivity and specificity in hospital medical-surgical units.
Subsequently, the tool was modified for long-term care facilities, and again, researchers found that ABRAT was able to identify potentially violent residents with reasonable sensitivity and specificity, said Dr. Kim, ABRAT developer and a professor at Point Loma Nazarene University, San Diego.
In 2021, researchers embedded the checklist into an electronic health record (EHR) system and tested ABRAT in emergency departments.
“Currently, we are working with computer programmers to build an app that would make the ABRAT very easy to use in conjunction with EHR,” Dr. Kim said. “Instead of a nurse searching the EHR to find out if the patient has history of mental illness or aggressive behavior in the past, the app would automatically search the EHR and combine the nurse’s quick observation whether the patient is confused, agitated, staring, or threatening, to automatically calculate the violence risk.”
Dr. Kim and her team also developed a tool called VEST (Violent Event Severity Tool), a standardized objective workplace violence severity assessment. They are working with programmers to incorporate VEST into the app as well.
Dr. Kim’s hope is that the ABRAT tool can be modified for use in a range of health care settings.
A version of this article first appeared on Medscape.com.