User login
Thoracic Oncology & Chest Procedures Network
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
One in eight COVID patients likely to develop long COVID: Large study
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
‘Misleading’ focus on urinary symptoms preventing early prostate cancer diagnoses
Researchers from the University of Cambridge said there was “no evidence of a causal link between prostate cancer and either prostate size or troublesome male urinary symptoms” and called for early prostate cancer to be rebranded “as primarily an asymptomatic disease” to encourage more men to get tested earlier when the condition is more treatable.
The authors of the ‘Opinion’ article, published in the journal BMC Medicine, argued that persistence by health bodies in flagging prostate cancer as a symptomatic disease – frequently presenting with slow urinary flow, frequency, and nocturia – worked against efforts to reduce mortality rates, which had remained largely unaltered in the UK and many other countries over the past decade and largely driven by late detection.
Public advice by the NHS, for instance, acknowledges that prostate cancer may be symptomless for many years but lists ‘an increased need to pee,’ ‘straining while you pee,’ and ‘a feeling that your bladder has not fully emptied’ as the top three signs that should not be ignored.
Messaging gives men ‘a false sense of security’
No wonder, the authors argued, that lower urinary tract symptoms and prostate cancer risk had become “causally associated,” as reflected in a 2003 survey finding that 86% of the public thought that prostate cancer was accompanied by symptoms, while only 1% were aware that it could be asymptomatic.
Lead study author Vincent Gnanapragasam, PhD, professor of urology at the University of Cambridge and an honorary consultant urologist at Addenbrooke’s Hospital, maintained: “We urgently need to recognize that the information currently given to the public risks giving men a false sense of security if they don’t have any urinary symptoms. We need to emphasize that prostate cancer can be a silent or asymptomatic disease, particularly in its curable stages. Waiting out for urinary symptoms may mean missing opportunities to catch the disease when it’s treatable.”
Although prostate enlargement can cause the lower urinary tract problems mentioned in public health messaging, the researchers said this is rarely due to malignant prostate tumors, quoting some research suggesting that “mean prostate volume was lower in men found to have prostate cancer compared to those with benign biopsies.” The Prostate testing for cancer and Treatment (ProtecT) trial in the UK concluded there was “no association or a negative association with more severe symptoms and prostate cancer,” they said.
Screening program
The researchers said they were not advocating introducing an immediate screening program, and they acknowledged that updating advice to focus on the often symptomless nature of the disease could lead to an influx of men requesting a PSA test from their GPs. But concerns this could result in overinvestigation and overtreatment “which previously deterred greater promotion of PSA testing in men with no symptoms” had been lessened by today’s “image-based diagnostics and risk-adapted management strategies,” they said.
The authors hoped for an eventual “intelligent tiered screening program” for prostate cancer to be introduced. In the meantime, Dr. Gnanapragasam said, “We’re calling on organizations such as the NHS, as well as patient charities and the media, to review the current public messaging.”
Amy Rylance, head of improving care at Prostate Cancer UK said: “This study reinforces the fact that men shouldn’t wait for symptoms before they act. Early prostate cancer is often symptomless, which is why we urge men to be aware of their risk instead. This is particularly important for men over 50, Black men, and men with a family history of prostate cancer.
“We know that most people assume that they would have symptoms if they had prostate cancer, and that not having straightforward signs to look out for can cause anxiety or confusion. That’s why our risk checker is designed to help men understand their risk factors and what action they can take, regardless of symptoms.”
A version of this article first appeared on Medscape.co.uk.
Researchers from the University of Cambridge said there was “no evidence of a causal link between prostate cancer and either prostate size or troublesome male urinary symptoms” and called for early prostate cancer to be rebranded “as primarily an asymptomatic disease” to encourage more men to get tested earlier when the condition is more treatable.
The authors of the ‘Opinion’ article, published in the journal BMC Medicine, argued that persistence by health bodies in flagging prostate cancer as a symptomatic disease – frequently presenting with slow urinary flow, frequency, and nocturia – worked against efforts to reduce mortality rates, which had remained largely unaltered in the UK and many other countries over the past decade and largely driven by late detection.
Public advice by the NHS, for instance, acknowledges that prostate cancer may be symptomless for many years but lists ‘an increased need to pee,’ ‘straining while you pee,’ and ‘a feeling that your bladder has not fully emptied’ as the top three signs that should not be ignored.
Messaging gives men ‘a false sense of security’
No wonder, the authors argued, that lower urinary tract symptoms and prostate cancer risk had become “causally associated,” as reflected in a 2003 survey finding that 86% of the public thought that prostate cancer was accompanied by symptoms, while only 1% were aware that it could be asymptomatic.
Lead study author Vincent Gnanapragasam, PhD, professor of urology at the University of Cambridge and an honorary consultant urologist at Addenbrooke’s Hospital, maintained: “We urgently need to recognize that the information currently given to the public risks giving men a false sense of security if they don’t have any urinary symptoms. We need to emphasize that prostate cancer can be a silent or asymptomatic disease, particularly in its curable stages. Waiting out for urinary symptoms may mean missing opportunities to catch the disease when it’s treatable.”
Although prostate enlargement can cause the lower urinary tract problems mentioned in public health messaging, the researchers said this is rarely due to malignant prostate tumors, quoting some research suggesting that “mean prostate volume was lower in men found to have prostate cancer compared to those with benign biopsies.” The Prostate testing for cancer and Treatment (ProtecT) trial in the UK concluded there was “no association or a negative association with more severe symptoms and prostate cancer,” they said.
Screening program
The researchers said they were not advocating introducing an immediate screening program, and they acknowledged that updating advice to focus on the often symptomless nature of the disease could lead to an influx of men requesting a PSA test from their GPs. But concerns this could result in overinvestigation and overtreatment “which previously deterred greater promotion of PSA testing in men with no symptoms” had been lessened by today’s “image-based diagnostics and risk-adapted management strategies,” they said.
The authors hoped for an eventual “intelligent tiered screening program” for prostate cancer to be introduced. In the meantime, Dr. Gnanapragasam said, “We’re calling on organizations such as the NHS, as well as patient charities and the media, to review the current public messaging.”
Amy Rylance, head of improving care at Prostate Cancer UK said: “This study reinforces the fact that men shouldn’t wait for symptoms before they act. Early prostate cancer is often symptomless, which is why we urge men to be aware of their risk instead. This is particularly important for men over 50, Black men, and men with a family history of prostate cancer.
“We know that most people assume that they would have symptoms if they had prostate cancer, and that not having straightforward signs to look out for can cause anxiety or confusion. That’s why our risk checker is designed to help men understand their risk factors and what action they can take, regardless of symptoms.”
A version of this article first appeared on Medscape.co.uk.
Researchers from the University of Cambridge said there was “no evidence of a causal link between prostate cancer and either prostate size or troublesome male urinary symptoms” and called for early prostate cancer to be rebranded “as primarily an asymptomatic disease” to encourage more men to get tested earlier when the condition is more treatable.
The authors of the ‘Opinion’ article, published in the journal BMC Medicine, argued that persistence by health bodies in flagging prostate cancer as a symptomatic disease – frequently presenting with slow urinary flow, frequency, and nocturia – worked against efforts to reduce mortality rates, which had remained largely unaltered in the UK and many other countries over the past decade and largely driven by late detection.
Public advice by the NHS, for instance, acknowledges that prostate cancer may be symptomless for many years but lists ‘an increased need to pee,’ ‘straining while you pee,’ and ‘a feeling that your bladder has not fully emptied’ as the top three signs that should not be ignored.
Messaging gives men ‘a false sense of security’
No wonder, the authors argued, that lower urinary tract symptoms and prostate cancer risk had become “causally associated,” as reflected in a 2003 survey finding that 86% of the public thought that prostate cancer was accompanied by symptoms, while only 1% were aware that it could be asymptomatic.
Lead study author Vincent Gnanapragasam, PhD, professor of urology at the University of Cambridge and an honorary consultant urologist at Addenbrooke’s Hospital, maintained: “We urgently need to recognize that the information currently given to the public risks giving men a false sense of security if they don’t have any urinary symptoms. We need to emphasize that prostate cancer can be a silent or asymptomatic disease, particularly in its curable stages. Waiting out for urinary symptoms may mean missing opportunities to catch the disease when it’s treatable.”
Although prostate enlargement can cause the lower urinary tract problems mentioned in public health messaging, the researchers said this is rarely due to malignant prostate tumors, quoting some research suggesting that “mean prostate volume was lower in men found to have prostate cancer compared to those with benign biopsies.” The Prostate testing for cancer and Treatment (ProtecT) trial in the UK concluded there was “no association or a negative association with more severe symptoms and prostate cancer,” they said.
Screening program
The researchers said they were not advocating introducing an immediate screening program, and they acknowledged that updating advice to focus on the often symptomless nature of the disease could lead to an influx of men requesting a PSA test from their GPs. But concerns this could result in overinvestigation and overtreatment “which previously deterred greater promotion of PSA testing in men with no symptoms” had been lessened by today’s “image-based diagnostics and risk-adapted management strategies,” they said.
The authors hoped for an eventual “intelligent tiered screening program” for prostate cancer to be introduced. In the meantime, Dr. Gnanapragasam said, “We’re calling on organizations such as the NHS, as well as patient charities and the media, to review the current public messaging.”
Amy Rylance, head of improving care at Prostate Cancer UK said: “This study reinforces the fact that men shouldn’t wait for symptoms before they act. Early prostate cancer is often symptomless, which is why we urge men to be aware of their risk instead. This is particularly important for men over 50, Black men, and men with a family history of prostate cancer.
“We know that most people assume that they would have symptoms if they had prostate cancer, and that not having straightforward signs to look out for can cause anxiety or confusion. That’s why our risk checker is designed to help men understand their risk factors and what action they can take, regardless of symptoms.”
A version of this article first appeared on Medscape.co.uk.
Anti-BDCA2 antibody meets primary endpoint in phase 2 cutaneous lupus trial
Treatment with the humanized monoclonal antibody litifilimab improved scores on a validated measure of skin disease activity in an international phase 2 trial of patients with cutaneous lupus erythematosus (CLE).
Improvements in Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores in patients randomly assigned to receive subcutaneous litifilimab were superior to changes in patients randomly assigned to placebo over the trial period of 16 weeks. The double-blind study was published in the New England Journal of Medicine.
“This validated measure is working, and it’s very important to now go into phase 3 using the instrument that worked in phase 2 to measure improvement in the skin,” Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and lead author of the study, said in an interview.
Research on lupus erythematosus has focused on systemic lupus erythematosus (SLE), with few randomized controlled trials addressing CLE, she said, and no Food and Drug Administration–approved treatments for CLE in the last 50 years.
Asked to comment on the results, Alisa Femia, MD, associate professor and director of autoimmune connective tissue disease in the department of dermatology at New York University, who was not involved in the research, said it is “exciting to have a trial that specifically investigates the effect of a drug on cutaneous lupus, as well-designed investigations into this potentially disfiguring disease are relatively sparse and novel treatment pathways are needed.”
The investigational drug targets blood dendritic cell antigen 2 (BDCA2) – a receptor expressed solely on the surface of plasmacytoid dendritic cells (pDCs) – and inhibits the production of type 1 interferon and other inflammatory cytokines and chemokines believed to play a major role in the pathogenesis of cutaneous and systemic lupus, the investigators said.
Rheumatologist Edward Vital, MD, who leads a lupus research group at the University of Leeds (England), said he’s most interested in how the therapy works. The “idea [has been] that pDCs are the main source of type 1 interferon. But there’s a lot of data emerging at present that suggests there are many other sources of interferons, and the drug may work in other ways,” Dr. Vital, an associate professor at the university, said in an interview. He was not involved with the study.
“Maybe pDCs have other important roles. Or maybe other cells are targeted by the therapy, too,” he said. “Understanding this will help us understand the pathogenesis of lupus and which patients will benefit the most.”
Improvements in CLASI-A scores
Across 54 centers, the study enrolled 132 patients with primarily moderate to severe active subacute CLE or chronic CLE (including discoid lupus erythematosus), or both subacute and chronic CLE with or without systemic manifestations. Active CLE was defined as a score of at least 8 on CLASI-A, which measures erythema and scaling or hypertrophy in 13 skin regions.
Patients were randomly assigned to receive placebo or litifilimab at doses of 50 mg, 150 mg, or 450 mg subcutaneously at weeks 0, 2, 4, 8, and 12. Mean CLASI-A scores at baseline for placebo and each of the dosage groups were 16.5, 15.2, 18.4, and 16.5, respectively.
The investigators used a test of dose-response to assess response across the four groups on the basis of the percent change in CLASI-A scores from baseline to 16 weeks, the primary endpoint. The percent changes in CLASI-A score were –38.8 ± 7.5 in the 50-mg group; –47.9 ± 7.5 in the 150-mg group; –42.5 ± 5.5 in the 450-mg group; and –14.5 ± 6.4 in the placebo group. (Negative value indicates improvement from baseline.)
When compared with placebo, the change in CLASI-A scores in each of the litifilimab groups was –24.3 percentage points for the 50-mg dose (95% confidence interval, –43.7 to –4.9); –33.4 percentage points for the 150-mg dose (95% CI, –52.7 to –14.1); and –28.0 percentage points for the 450-mg dose (95% CI, –44.6 to –11.4).
“All three dosages caused a similar skin response,” said Dr. Werth. “And importantly, the placebo response is fairly low, much lower than in SLE trials, possibly because the background therapies tend to be less overall [including with slightly lower doses of prednisone]. So we can really see the broad effect of the drug.”
Just under half of participants – 42%-48% of patients receiving litifilimab and 42% of those in the placebo group – had concomitant SLE with low to moderate disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index 2000. Patients could meet SLE criteria based on previous findings, and “didn’t have to have active SLE,” Dr. Werth noted.
The trial allowed background therapy as long as treatment had begun at least 12 weeks before randomization, with a stable dose starting at least 4 weeks before randomization and maintained throughout the trial period.
Most patients had moderate to severe CLE at baseline “despite approximately 90% having received concomitant background therapy and 80% of those participants having received antimalarial drugs, either alone or with other agents,” Dr. Werth and coinvestigators wrote.
CLASI-A has been shown to correlate to patients’ quality of life, Dr. Werth emphasized in the interview.
Most of the reported side effects in the phase 2 CLE trial were mild or moderate. The treatment was associated with three cases of hypersensitivity, three cases of oral herpes infection, and one case of herpes zoster infection. One case of herpes zoster meningitis occurred 4 months after the last dose of litifilimab.
Approximately 10% of study participants who reported race and ethnicity were Black or African American.
Phase 3 trials
The trial was one part of a two-part phase 2 study of litifilimab, named the LILAC trial, sponsored by Biogen. The other part, which will be published separately, involved patients who had SLE with active joint and skin manifestations.
Biogen is currently enrolling patients in phase 3 studies – the TOPAZ-1 and TOPAZ-2 studies – to evaluate the efficacy and safety of the drug in patients with active SLE. As secondary endpoints, both trials will measure the percentage of participants with a CLASI-A score of at least 10 at baseline who achieve improvement in the score, including a 50% improvement from baseline to week 16, Nathalie Franchimont, MD, PhD, of Biogen, a coauthor of the NEJM study, said in an email.
Biogen also has “plans to initiate a pivotal study in CLE this year,” she said.
With respect to the newly published phase 2 study, Dr. Femia said that, while “conclusions about the magnitude of efficacy are difficult to extrapolate in this trial design, there’s reason for cautious optimism.” There is “good theoretical basis to be optimistic about a drug such as litifilimab, that ultimately reduces type 1 interferon response,” she added.
Anifrolumab, a type 1 interferon receptor monoclonal antibody marketed as Saphnelo, was approved by the FDA for SLE in July 2021, but CLE subtypes were not characterized in trials and CLE was not studied independently of SLE, the authors pointed out in their NEJM article.
The study was supported by Biogen. In addition to working with Biogen, Dr. Werth serves as a consultant to Gilead Sciences and other pharmaceutical companies. Dr. Vital has research grants and has received honoraria from AstraZeneca. Dr. Femia disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab improved scores on a validated measure of skin disease activity in an international phase 2 trial of patients with cutaneous lupus erythematosus (CLE).
Improvements in Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores in patients randomly assigned to receive subcutaneous litifilimab were superior to changes in patients randomly assigned to placebo over the trial period of 16 weeks. The double-blind study was published in the New England Journal of Medicine.
“This validated measure is working, and it’s very important to now go into phase 3 using the instrument that worked in phase 2 to measure improvement in the skin,” Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and lead author of the study, said in an interview.
Research on lupus erythematosus has focused on systemic lupus erythematosus (SLE), with few randomized controlled trials addressing CLE, she said, and no Food and Drug Administration–approved treatments for CLE in the last 50 years.
Asked to comment on the results, Alisa Femia, MD, associate professor and director of autoimmune connective tissue disease in the department of dermatology at New York University, who was not involved in the research, said it is “exciting to have a trial that specifically investigates the effect of a drug on cutaneous lupus, as well-designed investigations into this potentially disfiguring disease are relatively sparse and novel treatment pathways are needed.”
The investigational drug targets blood dendritic cell antigen 2 (BDCA2) – a receptor expressed solely on the surface of plasmacytoid dendritic cells (pDCs) – and inhibits the production of type 1 interferon and other inflammatory cytokines and chemokines believed to play a major role in the pathogenesis of cutaneous and systemic lupus, the investigators said.
Rheumatologist Edward Vital, MD, who leads a lupus research group at the University of Leeds (England), said he’s most interested in how the therapy works. The “idea [has been] that pDCs are the main source of type 1 interferon. But there’s a lot of data emerging at present that suggests there are many other sources of interferons, and the drug may work in other ways,” Dr. Vital, an associate professor at the university, said in an interview. He was not involved with the study.
“Maybe pDCs have other important roles. Or maybe other cells are targeted by the therapy, too,” he said. “Understanding this will help us understand the pathogenesis of lupus and which patients will benefit the most.”
Improvements in CLASI-A scores
Across 54 centers, the study enrolled 132 patients with primarily moderate to severe active subacute CLE or chronic CLE (including discoid lupus erythematosus), or both subacute and chronic CLE with or without systemic manifestations. Active CLE was defined as a score of at least 8 on CLASI-A, which measures erythema and scaling or hypertrophy in 13 skin regions.
Patients were randomly assigned to receive placebo or litifilimab at doses of 50 mg, 150 mg, or 450 mg subcutaneously at weeks 0, 2, 4, 8, and 12. Mean CLASI-A scores at baseline for placebo and each of the dosage groups were 16.5, 15.2, 18.4, and 16.5, respectively.
The investigators used a test of dose-response to assess response across the four groups on the basis of the percent change in CLASI-A scores from baseline to 16 weeks, the primary endpoint. The percent changes in CLASI-A score were –38.8 ± 7.5 in the 50-mg group; –47.9 ± 7.5 in the 150-mg group; –42.5 ± 5.5 in the 450-mg group; and –14.5 ± 6.4 in the placebo group. (Negative value indicates improvement from baseline.)
When compared with placebo, the change in CLASI-A scores in each of the litifilimab groups was –24.3 percentage points for the 50-mg dose (95% confidence interval, –43.7 to –4.9); –33.4 percentage points for the 150-mg dose (95% CI, –52.7 to –14.1); and –28.0 percentage points for the 450-mg dose (95% CI, –44.6 to –11.4).
“All three dosages caused a similar skin response,” said Dr. Werth. “And importantly, the placebo response is fairly low, much lower than in SLE trials, possibly because the background therapies tend to be less overall [including with slightly lower doses of prednisone]. So we can really see the broad effect of the drug.”
Just under half of participants – 42%-48% of patients receiving litifilimab and 42% of those in the placebo group – had concomitant SLE with low to moderate disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index 2000. Patients could meet SLE criteria based on previous findings, and “didn’t have to have active SLE,” Dr. Werth noted.
The trial allowed background therapy as long as treatment had begun at least 12 weeks before randomization, with a stable dose starting at least 4 weeks before randomization and maintained throughout the trial period.
Most patients had moderate to severe CLE at baseline “despite approximately 90% having received concomitant background therapy and 80% of those participants having received antimalarial drugs, either alone or with other agents,” Dr. Werth and coinvestigators wrote.
CLASI-A has been shown to correlate to patients’ quality of life, Dr. Werth emphasized in the interview.
Most of the reported side effects in the phase 2 CLE trial were mild or moderate. The treatment was associated with three cases of hypersensitivity, three cases of oral herpes infection, and one case of herpes zoster infection. One case of herpes zoster meningitis occurred 4 months after the last dose of litifilimab.
Approximately 10% of study participants who reported race and ethnicity were Black or African American.
Phase 3 trials
The trial was one part of a two-part phase 2 study of litifilimab, named the LILAC trial, sponsored by Biogen. The other part, which will be published separately, involved patients who had SLE with active joint and skin manifestations.
Biogen is currently enrolling patients in phase 3 studies – the TOPAZ-1 and TOPAZ-2 studies – to evaluate the efficacy and safety of the drug in patients with active SLE. As secondary endpoints, both trials will measure the percentage of participants with a CLASI-A score of at least 10 at baseline who achieve improvement in the score, including a 50% improvement from baseline to week 16, Nathalie Franchimont, MD, PhD, of Biogen, a coauthor of the NEJM study, said in an email.
Biogen also has “plans to initiate a pivotal study in CLE this year,” she said.
With respect to the newly published phase 2 study, Dr. Femia said that, while “conclusions about the magnitude of efficacy are difficult to extrapolate in this trial design, there’s reason for cautious optimism.” There is “good theoretical basis to be optimistic about a drug such as litifilimab, that ultimately reduces type 1 interferon response,” she added.
Anifrolumab, a type 1 interferon receptor monoclonal antibody marketed as Saphnelo, was approved by the FDA for SLE in July 2021, but CLE subtypes were not characterized in trials and CLE was not studied independently of SLE, the authors pointed out in their NEJM article.
The study was supported by Biogen. In addition to working with Biogen, Dr. Werth serves as a consultant to Gilead Sciences and other pharmaceutical companies. Dr. Vital has research grants and has received honoraria from AstraZeneca. Dr. Femia disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab improved scores on a validated measure of skin disease activity in an international phase 2 trial of patients with cutaneous lupus erythematosus (CLE).
Improvements in Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores in patients randomly assigned to receive subcutaneous litifilimab were superior to changes in patients randomly assigned to placebo over the trial period of 16 weeks. The double-blind study was published in the New England Journal of Medicine.
“This validated measure is working, and it’s very important to now go into phase 3 using the instrument that worked in phase 2 to measure improvement in the skin,” Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and lead author of the study, said in an interview.
Research on lupus erythematosus has focused on systemic lupus erythematosus (SLE), with few randomized controlled trials addressing CLE, she said, and no Food and Drug Administration–approved treatments for CLE in the last 50 years.
Asked to comment on the results, Alisa Femia, MD, associate professor and director of autoimmune connective tissue disease in the department of dermatology at New York University, who was not involved in the research, said it is “exciting to have a trial that specifically investigates the effect of a drug on cutaneous lupus, as well-designed investigations into this potentially disfiguring disease are relatively sparse and novel treatment pathways are needed.”
The investigational drug targets blood dendritic cell antigen 2 (BDCA2) – a receptor expressed solely on the surface of plasmacytoid dendritic cells (pDCs) – and inhibits the production of type 1 interferon and other inflammatory cytokines and chemokines believed to play a major role in the pathogenesis of cutaneous and systemic lupus, the investigators said.
Rheumatologist Edward Vital, MD, who leads a lupus research group at the University of Leeds (England), said he’s most interested in how the therapy works. The “idea [has been] that pDCs are the main source of type 1 interferon. But there’s a lot of data emerging at present that suggests there are many other sources of interferons, and the drug may work in other ways,” Dr. Vital, an associate professor at the university, said in an interview. He was not involved with the study.
“Maybe pDCs have other important roles. Or maybe other cells are targeted by the therapy, too,” he said. “Understanding this will help us understand the pathogenesis of lupus and which patients will benefit the most.”
Improvements in CLASI-A scores
Across 54 centers, the study enrolled 132 patients with primarily moderate to severe active subacute CLE or chronic CLE (including discoid lupus erythematosus), or both subacute and chronic CLE with or without systemic manifestations. Active CLE was defined as a score of at least 8 on CLASI-A, which measures erythema and scaling or hypertrophy in 13 skin regions.
Patients were randomly assigned to receive placebo or litifilimab at doses of 50 mg, 150 mg, or 450 mg subcutaneously at weeks 0, 2, 4, 8, and 12. Mean CLASI-A scores at baseline for placebo and each of the dosage groups were 16.5, 15.2, 18.4, and 16.5, respectively.
The investigators used a test of dose-response to assess response across the four groups on the basis of the percent change in CLASI-A scores from baseline to 16 weeks, the primary endpoint. The percent changes in CLASI-A score were –38.8 ± 7.5 in the 50-mg group; –47.9 ± 7.5 in the 150-mg group; –42.5 ± 5.5 in the 450-mg group; and –14.5 ± 6.4 in the placebo group. (Negative value indicates improvement from baseline.)
When compared with placebo, the change in CLASI-A scores in each of the litifilimab groups was –24.3 percentage points for the 50-mg dose (95% confidence interval, –43.7 to –4.9); –33.4 percentage points for the 150-mg dose (95% CI, –52.7 to –14.1); and –28.0 percentage points for the 450-mg dose (95% CI, –44.6 to –11.4).
“All three dosages caused a similar skin response,” said Dr. Werth. “And importantly, the placebo response is fairly low, much lower than in SLE trials, possibly because the background therapies tend to be less overall [including with slightly lower doses of prednisone]. So we can really see the broad effect of the drug.”
Just under half of participants – 42%-48% of patients receiving litifilimab and 42% of those in the placebo group – had concomitant SLE with low to moderate disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index 2000. Patients could meet SLE criteria based on previous findings, and “didn’t have to have active SLE,” Dr. Werth noted.
The trial allowed background therapy as long as treatment had begun at least 12 weeks before randomization, with a stable dose starting at least 4 weeks before randomization and maintained throughout the trial period.
Most patients had moderate to severe CLE at baseline “despite approximately 90% having received concomitant background therapy and 80% of those participants having received antimalarial drugs, either alone or with other agents,” Dr. Werth and coinvestigators wrote.
CLASI-A has been shown to correlate to patients’ quality of life, Dr. Werth emphasized in the interview.
Most of the reported side effects in the phase 2 CLE trial were mild or moderate. The treatment was associated with three cases of hypersensitivity, three cases of oral herpes infection, and one case of herpes zoster infection. One case of herpes zoster meningitis occurred 4 months after the last dose of litifilimab.
Approximately 10% of study participants who reported race and ethnicity were Black or African American.
Phase 3 trials
The trial was one part of a two-part phase 2 study of litifilimab, named the LILAC trial, sponsored by Biogen. The other part, which will be published separately, involved patients who had SLE with active joint and skin manifestations.
Biogen is currently enrolling patients in phase 3 studies – the TOPAZ-1 and TOPAZ-2 studies – to evaluate the efficacy and safety of the drug in patients with active SLE. As secondary endpoints, both trials will measure the percentage of participants with a CLASI-A score of at least 10 at baseline who achieve improvement in the score, including a 50% improvement from baseline to week 16, Nathalie Franchimont, MD, PhD, of Biogen, a coauthor of the NEJM study, said in an email.
Biogen also has “plans to initiate a pivotal study in CLE this year,” she said.
With respect to the newly published phase 2 study, Dr. Femia said that, while “conclusions about the magnitude of efficacy are difficult to extrapolate in this trial design, there’s reason for cautious optimism.” There is “good theoretical basis to be optimistic about a drug such as litifilimab, that ultimately reduces type 1 interferon response,” she added.
Anifrolumab, a type 1 interferon receptor monoclonal antibody marketed as Saphnelo, was approved by the FDA for SLE in July 2021, but CLE subtypes were not characterized in trials and CLE was not studied independently of SLE, the authors pointed out in their NEJM article.
The study was supported by Biogen. In addition to working with Biogen, Dr. Werth serves as a consultant to Gilead Sciences and other pharmaceutical companies. Dr. Vital has research grants and has received honoraria from AstraZeneca. Dr. Femia disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Doctor faces apparent retaliation after alleging data manipulation in published trial
A rheumatologist was suspended from a professional society and his license to practice medicine was threatened after he raised concerns about data manipulation in a published study for which he recruited patients, according to documents seen by Retraction Watch.
The study, “Added Value of Anti-CD74 Autoantibodies in Axial SpondyloArthritis in a Population With Low HLA-B27 Prevalence,” was published in Frontiers in Immunology in 2019 and has been cited 13 times, according to Clarivate’s Web of Science. In its acknowledgments, it listed Fouad Fayad, PhD, a rheumatologist at the University of Saint Joseph and Hotel-Dieu de France University Medical Center in Beirut, as one of the researchers who recruited patients for the trial.
Dr. Fayad alleged that the researchers tested patient samples multiple times and used a mix of old and new values in their analysis. After he reported his concerns to the journal and then the university, which both concluded that they could not confirm or refute his allegations, he has faced apparent retaliation, including the suspension of his membership in the Lebanese Society of Rheumatology.
In comments to Retraction Watch, the corresponding author for the study noted that the two investigations did not find data manipulation, and said the issue was “based on a background of personal and professional conflicts.”
In an April video recorded with Nassim Nicholas Taleb, PhD, a former quant trader and retired distinguished professor of finance and risk engineering at New York University’s Tandon School of Engineering, Dr. Fayad explained that he was originally an author on the paper, but after expressing concerns about the methodology to the other authors, they didn’t respond to him and his name was dropped from the author list without warning or explanation.
Dr. Taleb also detailed the issues with the study, showing graphs that indicate “very poor correlation” between the old and new test results from participant samples.
In October 2019, Dr. Fayad contacted Frontiers in Immunology with his concerns. But the journal’s investigation was inconclusive, and a staffer on the research integrity team told him in July 2020 to contact his institution to investigate, according to emails seen by Retraction Watch.
Dr. Fayad did so, but the University of Saint Joseph “rushed an incomplete investigation,” he said. It began in September of 2021 and concluded 2 months later that the investigation committee could not confirm or disprove Dr. Fayad’s allegations of data manipulation, according to a copy of the report seen by Retraction Watch. He said that their statistical reviewer did not receive all of the relevant documents, although he had provided them to the university.
A university official sent the findings from the investigation to the Lebanese Order of Physicians – Beirut, which decided to suspend Dr. Fayad’s membership in the Lebanese Society of Rheumatology. It’s “needless to explain the damage resulting from this suspension,” Dr. Fayad said.
The Beirut organization wrote to the Lebanese Order of Physicians – Tripoli, the body with which Dr. Fayad’s license is registered, informing them of the decision. In a copy of the letter seen by Retraction Watch, the Beirut organization cited the university investigation finding Dr. Fayad’s allegations to be invalid, as well as a letter in which he alleged mismanagement of the rheumatology society, as reasons for the decision, and referred the matter to the Tripoli organization for further investigation.
Dr. Fayad told us that the letter asking the Tripoli organization to investigate him could have led to the suspension of his license to practice medicine:
“My license is registered with the Lebanese Order of Physicians – Tripoli. So legally speaking, it is only Tripoli organization that can suspend my license/permit to practice. Beirut Organization has tried to summon me to their investigation committee, but my license (being registered in Tripoli Organization) does not fall under Beirut’s jurisdiction; in other words Beirut Organization violated the law; they can not approach me directly, they have to go through the Tripoli Organization.
“As such, and since Beirut organization could not suspend my license (as they did for my membership in the Lebanese Society of Rheumatology) they sent the letter to Tripoli organization asking them to investigate the matter and take necessary disciplinary action. This was a threat to suspending my license to practice medicine. Should Tripoli Organization have used the [University of Saint Joseph] letter and investigation report without conducting their own international investigation, my permit to practice would have been suspended.”
The Lebanese Order of Physicians – Tripoli conducted its own investigation and confirmed “the existence of manipulation in the study data and failure to respect the data integrity,” according to an official translation of the investigation report seen by Retraction Watch. The Lebanese Order of Physicians – Tripoli decided after its investigation that Dr. Fayad’s suspension from the rheumatology society was invalid.
The lead author of the study in question, Nelly R. Ziade of Saint Joseph University and Hotel-Dieu de France Hospital in Beirut, told Retraction Watch that the investigation by the Lebanese Order of Physicians – Tripoli “cannot be considered as final or official” and that she was “never approached, interviewed, or asked to provide any documents related to this complaint.”
She continued: “I will always be available to give any scientific clarification requested by the Order of Physicians in Beirut where a serious investigation giving equal voice to both parties is currently conducted.
“Kindly note that the concerned journal has already conducted an internal investigation where both parties provided all documents and it was concluded that there was no scientific foundation for the accusations.
“Again, a similar investigation was conducted by the Saint-Joseph University in Beirut (where myself and the other party work). Both parties presented study documents to a committee including the president of the IRB, the vice president of the University, the medical director of the University Hospital, experts in musculoskeletal system and biostatistics. In brief, the case against the authors was dismissed, no data manipulation was found and the colleague from Tripoli also was submitted to University sanctions. The report of the University can be shared with you should you need it.
“I’m afraid that this issue is based on a background of personal and professional conflicts.”
Dr. Fayad added: “The beauty of science is that the truth will always prevail and cannot be obscured for long time.”
A version of this article first appeared on RetractionWatch.com.
A rheumatologist was suspended from a professional society and his license to practice medicine was threatened after he raised concerns about data manipulation in a published study for which he recruited patients, according to documents seen by Retraction Watch.
The study, “Added Value of Anti-CD74 Autoantibodies in Axial SpondyloArthritis in a Population With Low HLA-B27 Prevalence,” was published in Frontiers in Immunology in 2019 and has been cited 13 times, according to Clarivate’s Web of Science. In its acknowledgments, it listed Fouad Fayad, PhD, a rheumatologist at the University of Saint Joseph and Hotel-Dieu de France University Medical Center in Beirut, as one of the researchers who recruited patients for the trial.
Dr. Fayad alleged that the researchers tested patient samples multiple times and used a mix of old and new values in their analysis. After he reported his concerns to the journal and then the university, which both concluded that they could not confirm or refute his allegations, he has faced apparent retaliation, including the suspension of his membership in the Lebanese Society of Rheumatology.
In comments to Retraction Watch, the corresponding author for the study noted that the two investigations did not find data manipulation, and said the issue was “based on a background of personal and professional conflicts.”
In an April video recorded with Nassim Nicholas Taleb, PhD, a former quant trader and retired distinguished professor of finance and risk engineering at New York University’s Tandon School of Engineering, Dr. Fayad explained that he was originally an author on the paper, but after expressing concerns about the methodology to the other authors, they didn’t respond to him and his name was dropped from the author list without warning or explanation.
Dr. Taleb also detailed the issues with the study, showing graphs that indicate “very poor correlation” between the old and new test results from participant samples.
In October 2019, Dr. Fayad contacted Frontiers in Immunology with his concerns. But the journal’s investigation was inconclusive, and a staffer on the research integrity team told him in July 2020 to contact his institution to investigate, according to emails seen by Retraction Watch.
Dr. Fayad did so, but the University of Saint Joseph “rushed an incomplete investigation,” he said. It began in September of 2021 and concluded 2 months later that the investigation committee could not confirm or disprove Dr. Fayad’s allegations of data manipulation, according to a copy of the report seen by Retraction Watch. He said that their statistical reviewer did not receive all of the relevant documents, although he had provided them to the university.
A university official sent the findings from the investigation to the Lebanese Order of Physicians – Beirut, which decided to suspend Dr. Fayad’s membership in the Lebanese Society of Rheumatology. It’s “needless to explain the damage resulting from this suspension,” Dr. Fayad said.
The Beirut organization wrote to the Lebanese Order of Physicians – Tripoli, the body with which Dr. Fayad’s license is registered, informing them of the decision. In a copy of the letter seen by Retraction Watch, the Beirut organization cited the university investigation finding Dr. Fayad’s allegations to be invalid, as well as a letter in which he alleged mismanagement of the rheumatology society, as reasons for the decision, and referred the matter to the Tripoli organization for further investigation.
Dr. Fayad told us that the letter asking the Tripoli organization to investigate him could have led to the suspension of his license to practice medicine:
“My license is registered with the Lebanese Order of Physicians – Tripoli. So legally speaking, it is only Tripoli organization that can suspend my license/permit to practice. Beirut Organization has tried to summon me to their investigation committee, but my license (being registered in Tripoli Organization) does not fall under Beirut’s jurisdiction; in other words Beirut Organization violated the law; they can not approach me directly, they have to go through the Tripoli Organization.
“As such, and since Beirut organization could not suspend my license (as they did for my membership in the Lebanese Society of Rheumatology) they sent the letter to Tripoli organization asking them to investigate the matter and take necessary disciplinary action. This was a threat to suspending my license to practice medicine. Should Tripoli Organization have used the [University of Saint Joseph] letter and investigation report without conducting their own international investigation, my permit to practice would have been suspended.”
The Lebanese Order of Physicians – Tripoli conducted its own investigation and confirmed “the existence of manipulation in the study data and failure to respect the data integrity,” according to an official translation of the investigation report seen by Retraction Watch. The Lebanese Order of Physicians – Tripoli decided after its investigation that Dr. Fayad’s suspension from the rheumatology society was invalid.
The lead author of the study in question, Nelly R. Ziade of Saint Joseph University and Hotel-Dieu de France Hospital in Beirut, told Retraction Watch that the investigation by the Lebanese Order of Physicians – Tripoli “cannot be considered as final or official” and that she was “never approached, interviewed, or asked to provide any documents related to this complaint.”
She continued: “I will always be available to give any scientific clarification requested by the Order of Physicians in Beirut where a serious investigation giving equal voice to both parties is currently conducted.
“Kindly note that the concerned journal has already conducted an internal investigation where both parties provided all documents and it was concluded that there was no scientific foundation for the accusations.
“Again, a similar investigation was conducted by the Saint-Joseph University in Beirut (where myself and the other party work). Both parties presented study documents to a committee including the president of the IRB, the vice president of the University, the medical director of the University Hospital, experts in musculoskeletal system and biostatistics. In brief, the case against the authors was dismissed, no data manipulation was found and the colleague from Tripoli also was submitted to University sanctions. The report of the University can be shared with you should you need it.
“I’m afraid that this issue is based on a background of personal and professional conflicts.”
Dr. Fayad added: “The beauty of science is that the truth will always prevail and cannot be obscured for long time.”
A version of this article first appeared on RetractionWatch.com.
A rheumatologist was suspended from a professional society and his license to practice medicine was threatened after he raised concerns about data manipulation in a published study for which he recruited patients, according to documents seen by Retraction Watch.
The study, “Added Value of Anti-CD74 Autoantibodies in Axial SpondyloArthritis in a Population With Low HLA-B27 Prevalence,” was published in Frontiers in Immunology in 2019 and has been cited 13 times, according to Clarivate’s Web of Science. In its acknowledgments, it listed Fouad Fayad, PhD, a rheumatologist at the University of Saint Joseph and Hotel-Dieu de France University Medical Center in Beirut, as one of the researchers who recruited patients for the trial.
Dr. Fayad alleged that the researchers tested patient samples multiple times and used a mix of old and new values in their analysis. After he reported his concerns to the journal and then the university, which both concluded that they could not confirm or refute his allegations, he has faced apparent retaliation, including the suspension of his membership in the Lebanese Society of Rheumatology.
In comments to Retraction Watch, the corresponding author for the study noted that the two investigations did not find data manipulation, and said the issue was “based on a background of personal and professional conflicts.”
In an April video recorded with Nassim Nicholas Taleb, PhD, a former quant trader and retired distinguished professor of finance and risk engineering at New York University’s Tandon School of Engineering, Dr. Fayad explained that he was originally an author on the paper, but after expressing concerns about the methodology to the other authors, they didn’t respond to him and his name was dropped from the author list without warning or explanation.
Dr. Taleb also detailed the issues with the study, showing graphs that indicate “very poor correlation” between the old and new test results from participant samples.
In October 2019, Dr. Fayad contacted Frontiers in Immunology with his concerns. But the journal’s investigation was inconclusive, and a staffer on the research integrity team told him in July 2020 to contact his institution to investigate, according to emails seen by Retraction Watch.
Dr. Fayad did so, but the University of Saint Joseph “rushed an incomplete investigation,” he said. It began in September of 2021 and concluded 2 months later that the investigation committee could not confirm or disprove Dr. Fayad’s allegations of data manipulation, according to a copy of the report seen by Retraction Watch. He said that their statistical reviewer did not receive all of the relevant documents, although he had provided them to the university.
A university official sent the findings from the investigation to the Lebanese Order of Physicians – Beirut, which decided to suspend Dr. Fayad’s membership in the Lebanese Society of Rheumatology. It’s “needless to explain the damage resulting from this suspension,” Dr. Fayad said.
The Beirut organization wrote to the Lebanese Order of Physicians – Tripoli, the body with which Dr. Fayad’s license is registered, informing them of the decision. In a copy of the letter seen by Retraction Watch, the Beirut organization cited the university investigation finding Dr. Fayad’s allegations to be invalid, as well as a letter in which he alleged mismanagement of the rheumatology society, as reasons for the decision, and referred the matter to the Tripoli organization for further investigation.
Dr. Fayad told us that the letter asking the Tripoli organization to investigate him could have led to the suspension of his license to practice medicine:
“My license is registered with the Lebanese Order of Physicians – Tripoli. So legally speaking, it is only Tripoli organization that can suspend my license/permit to practice. Beirut Organization has tried to summon me to their investigation committee, but my license (being registered in Tripoli Organization) does not fall under Beirut’s jurisdiction; in other words Beirut Organization violated the law; they can not approach me directly, they have to go through the Tripoli Organization.
“As such, and since Beirut organization could not suspend my license (as they did for my membership in the Lebanese Society of Rheumatology) they sent the letter to Tripoli organization asking them to investigate the matter and take necessary disciplinary action. This was a threat to suspending my license to practice medicine. Should Tripoli Organization have used the [University of Saint Joseph] letter and investigation report without conducting their own international investigation, my permit to practice would have been suspended.”
The Lebanese Order of Physicians – Tripoli conducted its own investigation and confirmed “the existence of manipulation in the study data and failure to respect the data integrity,” according to an official translation of the investigation report seen by Retraction Watch. The Lebanese Order of Physicians – Tripoli decided after its investigation that Dr. Fayad’s suspension from the rheumatology society was invalid.
The lead author of the study in question, Nelly R. Ziade of Saint Joseph University and Hotel-Dieu de France Hospital in Beirut, told Retraction Watch that the investigation by the Lebanese Order of Physicians – Tripoli “cannot be considered as final or official” and that she was “never approached, interviewed, or asked to provide any documents related to this complaint.”
She continued: “I will always be available to give any scientific clarification requested by the Order of Physicians in Beirut where a serious investigation giving equal voice to both parties is currently conducted.
“Kindly note that the concerned journal has already conducted an internal investigation where both parties provided all documents and it was concluded that there was no scientific foundation for the accusations.
“Again, a similar investigation was conducted by the Saint-Joseph University in Beirut (where myself and the other party work). Both parties presented study documents to a committee including the president of the IRB, the vice president of the University, the medical director of the University Hospital, experts in musculoskeletal system and biostatistics. In brief, the case against the authors was dismissed, no data manipulation was found and the colleague from Tripoli also was submitted to University sanctions. The report of the University can be shared with you should you need it.
“I’m afraid that this issue is based on a background of personal and professional conflicts.”
Dr. Fayad added: “The beauty of science is that the truth will always prevail and cannot be obscured for long time.”
A version of this article first appeared on RetractionWatch.com.
Sleep Medicine Network
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Airways Disorders Network
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.
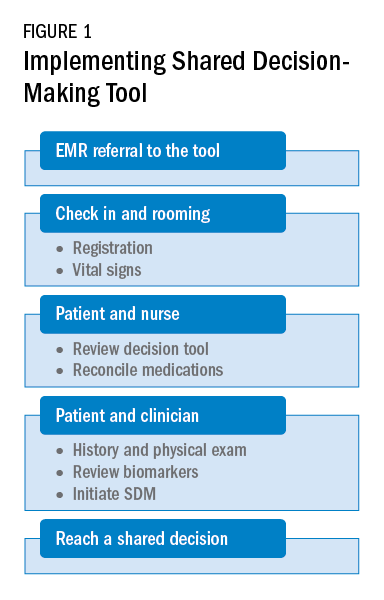
Farrukh Abbas, MBBS
Fellow-in-Training
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.

Farrukh Abbas, MBBS
Fellow-in-Training
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.

Farrukh Abbas, MBBS
Fellow-in-Training
Long COVID doubles risk of some serious outcomes in children, teens
Researchers from the Centers for Disease Control and Prevention report that
Heart inflammation; a blood clot in the lung; or a blood clot in the lower leg, thigh, or pelvis were the most common bad outcomes in a new study. Even though the risk was higher for these and some other serious events, the overall numbers were small.
“Many of these conditions were rare or uncommon among children in this analysis, but even a small increase in these conditions is notable,” a CDC new release stated.
The investigators said their findings stress the importance of COVID-19 vaccination in Americans under the age of 18.
The study was published online in the CDC’s Morbidity and Mortality Weekly Report.
Less is known about long COVID in children
Lyudmyla Kompaniyets, PhD, and colleagues noted that most research on long COVID to date has been done in adults, so little information is available about the risks to Americans ages 17 and younger.
To learn more, they compared post–COVID-19 symptoms and conditions between 781,419 children and teenagers with confirmed COVID-19 to another 2,344,257 without COVID-19. They looked at medical claims and laboratory data for these children and teenagers from March 1, 2020, through Jan. 31, 2022, to see who got any of 15 specific outcomes linked to long COVID-19.
Long COVID was defined as a condition where symptoms that last for or begin at least 4 weeks after a COVID-19 diagnosis.
Compared to children with no history of a COVID-19 diagnosis, the long COVID-19 group was 101% more likely to have an acute pulmonary embolism, 99% more likely to have myocarditis or cardiomyopathy, 87% more likely to have a venous thromboembolic event, 32% more likely to have acute and unspecified renal failure, and 23% more likely to have type 1 diabetes.
“This report points to the fact that the risks of COVID infection itself, both in terms of the acute effects, MIS-C [multisystem inflammatory syndrome in children], as well as the long-term effects, are real, are concerning, and are potentially very serious,” said Stuart Berger, MD, chair of the American Academy of Pediatrics Section on Cardiology and Cardiac Surgery.
“The message that we should take away from this is that we should be very keen on all the methods of prevention for COVID, especially the vaccine,” said Dr. Berger, chief of cardiology in the department of pediatrics at Northwestern University in Chicago.
A ‘wake-up call’
The study findings are “sobering” and are “a reminder of the seriousness of COVID infection,” says Gregory Poland, MD, an infectious disease expert at the Mayo Clinic in Rochester, Minn.
“When you look in particular at the more serious complications from COVID in this young age group, those are life-altering complications that will have consequences and ramifications throughout their lives,” he said.
“I would take this as a serious wake-up call to parents [at a time when] the immunization rates in younger children are so pitifully low,” Dr. Poland said.
Still early days
The study is suggestive but not definitive, said Peter Katona, MD, professor of medicine and infectious diseases expert at the UCLA Fielding School of Public Health.
It’s still too early to draw conclusions about long COVID, including in children, because many questions remain, he said: Should long COVID be defined as symptoms at 1 month or 3 months after infection? How do you define brain fog?
Dr. Katona and colleagues are studying long COVID intervention among students at UCLA to answer some of these questions, including the incidence and effect of early intervention.
The study had “at least seven limitations,” the researchers noted. Among them was the use of medical claims data that noted long COVID outcomes but not how severe they were; some people in the no COVID group might have had the illness but not been diagnosed; and the researchers did not adjust for vaccination status.
Dr. Poland noted that the study was done during surges in COVID variants including Delta and Omicron. In other words, any long COVID effects linked to more recent variants such as BA.5 or BA.2.75 are unknown.
A version of this article first appeared on WebMD.com.
Researchers from the Centers for Disease Control and Prevention report that
Heart inflammation; a blood clot in the lung; or a blood clot in the lower leg, thigh, or pelvis were the most common bad outcomes in a new study. Even though the risk was higher for these and some other serious events, the overall numbers were small.
“Many of these conditions were rare or uncommon among children in this analysis, but even a small increase in these conditions is notable,” a CDC new release stated.
The investigators said their findings stress the importance of COVID-19 vaccination in Americans under the age of 18.
The study was published online in the CDC’s Morbidity and Mortality Weekly Report.
Less is known about long COVID in children
Lyudmyla Kompaniyets, PhD, and colleagues noted that most research on long COVID to date has been done in adults, so little information is available about the risks to Americans ages 17 and younger.
To learn more, they compared post–COVID-19 symptoms and conditions between 781,419 children and teenagers with confirmed COVID-19 to another 2,344,257 without COVID-19. They looked at medical claims and laboratory data for these children and teenagers from March 1, 2020, through Jan. 31, 2022, to see who got any of 15 specific outcomes linked to long COVID-19.
Long COVID was defined as a condition where symptoms that last for or begin at least 4 weeks after a COVID-19 diagnosis.
Compared to children with no history of a COVID-19 diagnosis, the long COVID-19 group was 101% more likely to have an acute pulmonary embolism, 99% more likely to have myocarditis or cardiomyopathy, 87% more likely to have a venous thromboembolic event, 32% more likely to have acute and unspecified renal failure, and 23% more likely to have type 1 diabetes.
“This report points to the fact that the risks of COVID infection itself, both in terms of the acute effects, MIS-C [multisystem inflammatory syndrome in children], as well as the long-term effects, are real, are concerning, and are potentially very serious,” said Stuart Berger, MD, chair of the American Academy of Pediatrics Section on Cardiology and Cardiac Surgery.
“The message that we should take away from this is that we should be very keen on all the methods of prevention for COVID, especially the vaccine,” said Dr. Berger, chief of cardiology in the department of pediatrics at Northwestern University in Chicago.
A ‘wake-up call’
The study findings are “sobering” and are “a reminder of the seriousness of COVID infection,” says Gregory Poland, MD, an infectious disease expert at the Mayo Clinic in Rochester, Minn.
“When you look in particular at the more serious complications from COVID in this young age group, those are life-altering complications that will have consequences and ramifications throughout their lives,” he said.
“I would take this as a serious wake-up call to parents [at a time when] the immunization rates in younger children are so pitifully low,” Dr. Poland said.
Still early days
The study is suggestive but not definitive, said Peter Katona, MD, professor of medicine and infectious diseases expert at the UCLA Fielding School of Public Health.
It’s still too early to draw conclusions about long COVID, including in children, because many questions remain, he said: Should long COVID be defined as symptoms at 1 month or 3 months after infection? How do you define brain fog?
Dr. Katona and colleagues are studying long COVID intervention among students at UCLA to answer some of these questions, including the incidence and effect of early intervention.
The study had “at least seven limitations,” the researchers noted. Among them was the use of medical claims data that noted long COVID outcomes but not how severe they were; some people in the no COVID group might have had the illness but not been diagnosed; and the researchers did not adjust for vaccination status.
Dr. Poland noted that the study was done during surges in COVID variants including Delta and Omicron. In other words, any long COVID effects linked to more recent variants such as BA.5 or BA.2.75 are unknown.
A version of this article first appeared on WebMD.com.
Researchers from the Centers for Disease Control and Prevention report that
Heart inflammation; a blood clot in the lung; or a blood clot in the lower leg, thigh, or pelvis were the most common bad outcomes in a new study. Even though the risk was higher for these and some other serious events, the overall numbers were small.
“Many of these conditions were rare or uncommon among children in this analysis, but even a small increase in these conditions is notable,” a CDC new release stated.
The investigators said their findings stress the importance of COVID-19 vaccination in Americans under the age of 18.
The study was published online in the CDC’s Morbidity and Mortality Weekly Report.
Less is known about long COVID in children
Lyudmyla Kompaniyets, PhD, and colleagues noted that most research on long COVID to date has been done in adults, so little information is available about the risks to Americans ages 17 and younger.
To learn more, they compared post–COVID-19 symptoms and conditions between 781,419 children and teenagers with confirmed COVID-19 to another 2,344,257 without COVID-19. They looked at medical claims and laboratory data for these children and teenagers from March 1, 2020, through Jan. 31, 2022, to see who got any of 15 specific outcomes linked to long COVID-19.
Long COVID was defined as a condition where symptoms that last for or begin at least 4 weeks after a COVID-19 diagnosis.
Compared to children with no history of a COVID-19 diagnosis, the long COVID-19 group was 101% more likely to have an acute pulmonary embolism, 99% more likely to have myocarditis or cardiomyopathy, 87% more likely to have a venous thromboembolic event, 32% more likely to have acute and unspecified renal failure, and 23% more likely to have type 1 diabetes.
“This report points to the fact that the risks of COVID infection itself, both in terms of the acute effects, MIS-C [multisystem inflammatory syndrome in children], as well as the long-term effects, are real, are concerning, and are potentially very serious,” said Stuart Berger, MD, chair of the American Academy of Pediatrics Section on Cardiology and Cardiac Surgery.
“The message that we should take away from this is that we should be very keen on all the methods of prevention for COVID, especially the vaccine,” said Dr. Berger, chief of cardiology in the department of pediatrics at Northwestern University in Chicago.
A ‘wake-up call’
The study findings are “sobering” and are “a reminder of the seriousness of COVID infection,” says Gregory Poland, MD, an infectious disease expert at the Mayo Clinic in Rochester, Minn.
“When you look in particular at the more serious complications from COVID in this young age group, those are life-altering complications that will have consequences and ramifications throughout their lives,” he said.
“I would take this as a serious wake-up call to parents [at a time when] the immunization rates in younger children are so pitifully low,” Dr. Poland said.
Still early days
The study is suggestive but not definitive, said Peter Katona, MD, professor of medicine and infectious diseases expert at the UCLA Fielding School of Public Health.
It’s still too early to draw conclusions about long COVID, including in children, because many questions remain, he said: Should long COVID be defined as symptoms at 1 month or 3 months after infection? How do you define brain fog?
Dr. Katona and colleagues are studying long COVID intervention among students at UCLA to answer some of these questions, including the incidence and effect of early intervention.
The study had “at least seven limitations,” the researchers noted. Among them was the use of medical claims data that noted long COVID outcomes but not how severe they were; some people in the no COVID group might have had the illness but not been diagnosed; and the researchers did not adjust for vaccination status.
Dr. Poland noted that the study was done during surges in COVID variants including Delta and Omicron. In other words, any long COVID effects linked to more recent variants such as BA.5 or BA.2.75 are unknown.
A version of this article first appeared on WebMD.com.
FROM THE MMWR
‘Children are not little adults’ and need special protection during heat waves
After more than a week of record-breaking temperatures across much of the country, public health experts are cautioning that children are more susceptible to heat illness than adults are – even more so when they’re on the athletic field, living without air conditioning, or waiting in a parked car.
Cases of heat-related illness are rising with average air temperatures, and experts say almost half of those getting sick are children. The reason is twofold: Children’s bodies have more trouble regulating temperature than do those of adults, and they rely on adults to help protect them from overheating.
Parents, coaches, and other caretakers, who can experience the same heat very differently from the way children do, may struggle to identify a dangerous situation or catch the early symptoms of heat-related illness in children.
“Children are not little adults,” said Dr. Aaron Bernstein, a pediatric hospitalist at Boston Children’s Hospital.
Jan Null, a meteorologist in California, recalled being surprised at the effect of heat in a car. It was 86 degrees on a July afternoon more than 2 decades ago when an infant in San Jose was forgotten in a parked car and died of heatstroke.
Mr. Null said a reporter asked him after the death, “How hot could it have gotten in that car?”
Mr. Null’s research with two emergency doctors at Stanford University eventually produced a startling answer. Within an hour, the temperature in that car could have exceeded 120 degrees Fahrenheit. Their work revealed that a quick errand can be dangerous for a child left behind in the car – even for less than 15 minutes, even with the windows cracked, and even on a mild day.
As record heat becomes more frequent, posing serious risks even to healthy adults, the number of cases of heat-related illnesses has gone up, including among children. Those most at risk are young children in parked vehicles and adolescents returning to school and participating in sports during the hottest days of the year.
More than 9,000 high school athletes are treated for heat-related illnesses every year.
Heat-related illnesses occur when exposure to high temperatures and humidity, which can be intensified by physical exertion, overwhelms the body’s ability to cool itself. Cases range from mild, like benign heat rashes in infants, to more serious, when the body’s core temperature increases. That can lead to life-threatening instances of heatstroke, diagnosed once the body temperature rises above 104 degrees, potentially causing organ failure.
Prevention is key. Experts emphasize that drinking plenty of water, avoiding the outdoors during the hot midday and afternoon hours, and taking it slow when adjusting to exercise are the most effective ways to avoid getting sick.
Children’s bodies take longer to increase sweat production and otherwise acclimatize in a warm environment than adults’ do, research shows. Young children are more susceptible to dehydration because a larger percentage of their body weight is water.
Infants and younger children have more trouble regulating their body temperature, in part because they often don’t recognize when they should drink more water or remove clothing to cool down. A 1995 study showed that young children who spent 30 minutes in a 95-degree room saw their core temperatures rise significantly higher and faster than their mothers’ – even though they sweat more than adults do relative to their size.
Pediatricians advise caretakers to monitor how much water children consume and encourage them to drink before they ask for it. Thirst indicates the body is already dehydrated.
They should dress children in light-colored, lightweight clothes; limit outdoor time during the hottest hours; and look for ways to cool down, such as by visiting an air-conditioned place like a library, taking a cool bath, or going for a swim.
To address the risks to student athletes, the National Athletic Trainers’ Association recommends that high school athletes acclimatize by gradually building their activity over the course of 2 weeks when returning to their sport for a new season – including by slowly stepping up the amount of any protective equipment they wear.
“You’re gradually increasing that intensity over a week to 2 weeks so your body can get used to the heat,” said Kathy Dieringer, president of NATA.
Warning signs and solutions
Experts note a flushed face, fatigue, muscle cramps, headache, dizziness, vomiting, and a lot of sweating are among the symptoms of heat exhaustion, which can develop into heatstroke if untreated. A doctor should be notified if symptoms worsen, such as if the child seems disoriented or cannot drink.
Taking immediate steps to cool a child experiencing heat exhaustion or heatstroke is critical. The child should be taken to a shaded or cool area; be given cool fluids with salt, like sports drinks; and have any sweaty or heavy garments removed.
For adolescents, being submerged in an ice bath is the most effective way to cool the body, while younger children can be wrapped in cold, wet towels or misted with lukewarm water and placed in front of a fan.
Although children’s deaths in parked cars have been well documented, the tragic incidents continue to occur. According to federal statistics, 23 children died of vehicular heatstroke in 2021. Mr. Null, who collects his own data, said 13 children have died so far this year.
Caretakers should never leave children alone in a parked car, Mr. Null said. Take steps to prevent young children from entering the car themselves and becoming trapped, including locking the car while it’s parked at home.
More than half of cases of vehicular pediatric heatstroke occur because a caretaker accidentally left a child behind, he said. While in-car technology reminding adults to check their back seats has become more common, only a fraction of vehicles have it, requiring parents to come up with their own methods, like leaving a stuffed animal in the front seat.
The good news, Mr. Null said, is that simple behavioral changes can protect youngsters. “This is preventable in 100% of the cases,” he said.
A lopsided risk
People living in low-income areas fare worse when temperatures climb. Access to air conditioning, which includes the ability to afford the electricity bill, is a serious health concern.
A study of heat in urban areas released last year showed that low-income neighborhoods and communities of color experience much higher temperatures than those of wealthier, White residents. In more impoverished areas during the summer, temperatures can be as much as 7 degrees Fahrenheit warmer.
The study’s authors said their findings in the United States reflect that “the legacy of redlining looms large,” referring to a federal housing policy that refused to insure mortgages in or near predominantly Black neighborhoods.
“These areas have less tree canopy, more streets, and higher building densities, meaning that in addition to their other racist outcomes, redlining policies directly codified into law existing disparity in urban land use and reinforced urban design choices that magnify urban heating into the present,” they concluded.
Dr. Bernstein, who leads Harvard’s Center for Climate, Health, and the Global Environment, coauthored a commentary in JAMA arguing that advancing health equity is critical to action on climate change.
The center works with front-line health clinics to help their predominantly low-income patients respond to the health impacts of climate change. Federally backed clinics alone provide care to about 30 million Americans, including many children, he said.
Dr. Bernstein also recently led a nationwide study that found that from May through September, days with higher temperatures are associated with more visits to children’s hospital emergency rooms. Many visits were more directly linked to heat, although the study also pointed to how high temperatures can exacerbate existing health conditions such as neurological disorders.
“Children are more vulnerable to climate change through how these climate shocks reshape the world in which they grow up,” Dr. Bernstein said.
Helping people better understand the health risks of extreme heat and how to protect themselves and their families are among the public health system’s major challenges, experts said.
The National Weather Service’s heat alert system is mainly based on the heat index, a measure of how hot it feels when relative humidity is factored in with air temperature.
But the alerts are not related to effects on health, said Kathy Baughman McLeod, director of the Adrienne Arsht-Rockefeller Foundation Resilience Center. By the time temperatures rise to the level that a weather alert is issued, many vulnerable people – like children, pregnant women, and the elderly – may already be experiencing heat exhaustion or heatstroke.
The center developed a new heat alert system, which is being tested in Seville, Spain, historically one of the hottest cities in Europe.
The system marries metrics such as air temperature and humidity with public health data to categorize heat waves and, when they are serious enough, give them names – making it easier for people to understand heat as an environmental threat that requires prevention measures.
The categories are determined through a metric known as excess deaths, which compares how many people died on a day with the forecast temperature versus an average day. That may help health officials understand how severe a heat wave is expected to be and make informed recommendations to the public based on risk factors such as age or medical history.
The health-based alert system would also allow officials to target caretakers of children and seniors through school systems, preschools, and senior centers, Ms. Baughman McLeod said.
Giving people better ways to conceptualize heat is critical, she said.
“It’s not dramatic. It doesn’t rip the roof off of your house,” Ms. Baughman McLeod said. “It’s silent and invisible.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
After more than a week of record-breaking temperatures across much of the country, public health experts are cautioning that children are more susceptible to heat illness than adults are – even more so when they’re on the athletic field, living without air conditioning, or waiting in a parked car.
Cases of heat-related illness are rising with average air temperatures, and experts say almost half of those getting sick are children. The reason is twofold: Children’s bodies have more trouble regulating temperature than do those of adults, and they rely on adults to help protect them from overheating.
Parents, coaches, and other caretakers, who can experience the same heat very differently from the way children do, may struggle to identify a dangerous situation or catch the early symptoms of heat-related illness in children.
“Children are not little adults,” said Dr. Aaron Bernstein, a pediatric hospitalist at Boston Children’s Hospital.
Jan Null, a meteorologist in California, recalled being surprised at the effect of heat in a car. It was 86 degrees on a July afternoon more than 2 decades ago when an infant in San Jose was forgotten in a parked car and died of heatstroke.
Mr. Null said a reporter asked him after the death, “How hot could it have gotten in that car?”
Mr. Null’s research with two emergency doctors at Stanford University eventually produced a startling answer. Within an hour, the temperature in that car could have exceeded 120 degrees Fahrenheit. Their work revealed that a quick errand can be dangerous for a child left behind in the car – even for less than 15 minutes, even with the windows cracked, and even on a mild day.
As record heat becomes more frequent, posing serious risks even to healthy adults, the number of cases of heat-related illnesses has gone up, including among children. Those most at risk are young children in parked vehicles and adolescents returning to school and participating in sports during the hottest days of the year.
More than 9,000 high school athletes are treated for heat-related illnesses every year.
Heat-related illnesses occur when exposure to high temperatures and humidity, which can be intensified by physical exertion, overwhelms the body’s ability to cool itself. Cases range from mild, like benign heat rashes in infants, to more serious, when the body’s core temperature increases. That can lead to life-threatening instances of heatstroke, diagnosed once the body temperature rises above 104 degrees, potentially causing organ failure.
Prevention is key. Experts emphasize that drinking plenty of water, avoiding the outdoors during the hot midday and afternoon hours, and taking it slow when adjusting to exercise are the most effective ways to avoid getting sick.
Children’s bodies take longer to increase sweat production and otherwise acclimatize in a warm environment than adults’ do, research shows. Young children are more susceptible to dehydration because a larger percentage of their body weight is water.
Infants and younger children have more trouble regulating their body temperature, in part because they often don’t recognize when they should drink more water or remove clothing to cool down. A 1995 study showed that young children who spent 30 minutes in a 95-degree room saw their core temperatures rise significantly higher and faster than their mothers’ – even though they sweat more than adults do relative to their size.
Pediatricians advise caretakers to monitor how much water children consume and encourage them to drink before they ask for it. Thirst indicates the body is already dehydrated.
They should dress children in light-colored, lightweight clothes; limit outdoor time during the hottest hours; and look for ways to cool down, such as by visiting an air-conditioned place like a library, taking a cool bath, or going for a swim.
To address the risks to student athletes, the National Athletic Trainers’ Association recommends that high school athletes acclimatize by gradually building their activity over the course of 2 weeks when returning to their sport for a new season – including by slowly stepping up the amount of any protective equipment they wear.
“You’re gradually increasing that intensity over a week to 2 weeks so your body can get used to the heat,” said Kathy Dieringer, president of NATA.
Warning signs and solutions
Experts note a flushed face, fatigue, muscle cramps, headache, dizziness, vomiting, and a lot of sweating are among the symptoms of heat exhaustion, which can develop into heatstroke if untreated. A doctor should be notified if symptoms worsen, such as if the child seems disoriented or cannot drink.
Taking immediate steps to cool a child experiencing heat exhaustion or heatstroke is critical. The child should be taken to a shaded or cool area; be given cool fluids with salt, like sports drinks; and have any sweaty or heavy garments removed.
For adolescents, being submerged in an ice bath is the most effective way to cool the body, while younger children can be wrapped in cold, wet towels or misted with lukewarm water and placed in front of a fan.
Although children’s deaths in parked cars have been well documented, the tragic incidents continue to occur. According to federal statistics, 23 children died of vehicular heatstroke in 2021. Mr. Null, who collects his own data, said 13 children have died so far this year.
Caretakers should never leave children alone in a parked car, Mr. Null said. Take steps to prevent young children from entering the car themselves and becoming trapped, including locking the car while it’s parked at home.
More than half of cases of vehicular pediatric heatstroke occur because a caretaker accidentally left a child behind, he said. While in-car technology reminding adults to check their back seats has become more common, only a fraction of vehicles have it, requiring parents to come up with their own methods, like leaving a stuffed animal in the front seat.
The good news, Mr. Null said, is that simple behavioral changes can protect youngsters. “This is preventable in 100% of the cases,” he said.
A lopsided risk
People living in low-income areas fare worse when temperatures climb. Access to air conditioning, which includes the ability to afford the electricity bill, is a serious health concern.
A study of heat in urban areas released last year showed that low-income neighborhoods and communities of color experience much higher temperatures than those of wealthier, White residents. In more impoverished areas during the summer, temperatures can be as much as 7 degrees Fahrenheit warmer.
The study’s authors said their findings in the United States reflect that “the legacy of redlining looms large,” referring to a federal housing policy that refused to insure mortgages in or near predominantly Black neighborhoods.
“These areas have less tree canopy, more streets, and higher building densities, meaning that in addition to their other racist outcomes, redlining policies directly codified into law existing disparity in urban land use and reinforced urban design choices that magnify urban heating into the present,” they concluded.
Dr. Bernstein, who leads Harvard’s Center for Climate, Health, and the Global Environment, coauthored a commentary in JAMA arguing that advancing health equity is critical to action on climate change.
The center works with front-line health clinics to help their predominantly low-income patients respond to the health impacts of climate change. Federally backed clinics alone provide care to about 30 million Americans, including many children, he said.
Dr. Bernstein also recently led a nationwide study that found that from May through September, days with higher temperatures are associated with more visits to children’s hospital emergency rooms. Many visits were more directly linked to heat, although the study also pointed to how high temperatures can exacerbate existing health conditions such as neurological disorders.
“Children are more vulnerable to climate change through how these climate shocks reshape the world in which they grow up,” Dr. Bernstein said.
Helping people better understand the health risks of extreme heat and how to protect themselves and their families are among the public health system’s major challenges, experts said.
The National Weather Service’s heat alert system is mainly based on the heat index, a measure of how hot it feels when relative humidity is factored in with air temperature.
But the alerts are not related to effects on health, said Kathy Baughman McLeod, director of the Adrienne Arsht-Rockefeller Foundation Resilience Center. By the time temperatures rise to the level that a weather alert is issued, many vulnerable people – like children, pregnant women, and the elderly – may already be experiencing heat exhaustion or heatstroke.
The center developed a new heat alert system, which is being tested in Seville, Spain, historically one of the hottest cities in Europe.
The system marries metrics such as air temperature and humidity with public health data to categorize heat waves and, when they are serious enough, give them names – making it easier for people to understand heat as an environmental threat that requires prevention measures.
The categories are determined through a metric known as excess deaths, which compares how many people died on a day with the forecast temperature versus an average day. That may help health officials understand how severe a heat wave is expected to be and make informed recommendations to the public based on risk factors such as age or medical history.
The health-based alert system would also allow officials to target caretakers of children and seniors through school systems, preschools, and senior centers, Ms. Baughman McLeod said.
Giving people better ways to conceptualize heat is critical, she said.
“It’s not dramatic. It doesn’t rip the roof off of your house,” Ms. Baughman McLeod said. “It’s silent and invisible.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
After more than a week of record-breaking temperatures across much of the country, public health experts are cautioning that children are more susceptible to heat illness than adults are – even more so when they’re on the athletic field, living without air conditioning, or waiting in a parked car.
Cases of heat-related illness are rising with average air temperatures, and experts say almost half of those getting sick are children. The reason is twofold: Children’s bodies have more trouble regulating temperature than do those of adults, and they rely on adults to help protect them from overheating.
Parents, coaches, and other caretakers, who can experience the same heat very differently from the way children do, may struggle to identify a dangerous situation or catch the early symptoms of heat-related illness in children.
“Children are not little adults,” said Dr. Aaron Bernstein, a pediatric hospitalist at Boston Children’s Hospital.
Jan Null, a meteorologist in California, recalled being surprised at the effect of heat in a car. It was 86 degrees on a July afternoon more than 2 decades ago when an infant in San Jose was forgotten in a parked car and died of heatstroke.
Mr. Null said a reporter asked him after the death, “How hot could it have gotten in that car?”
Mr. Null’s research with two emergency doctors at Stanford University eventually produced a startling answer. Within an hour, the temperature in that car could have exceeded 120 degrees Fahrenheit. Their work revealed that a quick errand can be dangerous for a child left behind in the car – even for less than 15 minutes, even with the windows cracked, and even on a mild day.
As record heat becomes more frequent, posing serious risks even to healthy adults, the number of cases of heat-related illnesses has gone up, including among children. Those most at risk are young children in parked vehicles and adolescents returning to school and participating in sports during the hottest days of the year.
More than 9,000 high school athletes are treated for heat-related illnesses every year.
Heat-related illnesses occur when exposure to high temperatures and humidity, which can be intensified by physical exertion, overwhelms the body’s ability to cool itself. Cases range from mild, like benign heat rashes in infants, to more serious, when the body’s core temperature increases. That can lead to life-threatening instances of heatstroke, diagnosed once the body temperature rises above 104 degrees, potentially causing organ failure.
Prevention is key. Experts emphasize that drinking plenty of water, avoiding the outdoors during the hot midday and afternoon hours, and taking it slow when adjusting to exercise are the most effective ways to avoid getting sick.
Children’s bodies take longer to increase sweat production and otherwise acclimatize in a warm environment than adults’ do, research shows. Young children are more susceptible to dehydration because a larger percentage of their body weight is water.
Infants and younger children have more trouble regulating their body temperature, in part because they often don’t recognize when they should drink more water or remove clothing to cool down. A 1995 study showed that young children who spent 30 minutes in a 95-degree room saw their core temperatures rise significantly higher and faster than their mothers’ – even though they sweat more than adults do relative to their size.
Pediatricians advise caretakers to monitor how much water children consume and encourage them to drink before they ask for it. Thirst indicates the body is already dehydrated.
They should dress children in light-colored, lightweight clothes; limit outdoor time during the hottest hours; and look for ways to cool down, such as by visiting an air-conditioned place like a library, taking a cool bath, or going for a swim.
To address the risks to student athletes, the National Athletic Trainers’ Association recommends that high school athletes acclimatize by gradually building their activity over the course of 2 weeks when returning to their sport for a new season – including by slowly stepping up the amount of any protective equipment they wear.
“You’re gradually increasing that intensity over a week to 2 weeks so your body can get used to the heat,” said Kathy Dieringer, president of NATA.
Warning signs and solutions
Experts note a flushed face, fatigue, muscle cramps, headache, dizziness, vomiting, and a lot of sweating are among the symptoms of heat exhaustion, which can develop into heatstroke if untreated. A doctor should be notified if symptoms worsen, such as if the child seems disoriented or cannot drink.
Taking immediate steps to cool a child experiencing heat exhaustion or heatstroke is critical. The child should be taken to a shaded or cool area; be given cool fluids with salt, like sports drinks; and have any sweaty or heavy garments removed.
For adolescents, being submerged in an ice bath is the most effective way to cool the body, while younger children can be wrapped in cold, wet towels or misted with lukewarm water and placed in front of a fan.
Although children’s deaths in parked cars have been well documented, the tragic incidents continue to occur. According to federal statistics, 23 children died of vehicular heatstroke in 2021. Mr. Null, who collects his own data, said 13 children have died so far this year.
Caretakers should never leave children alone in a parked car, Mr. Null said. Take steps to prevent young children from entering the car themselves and becoming trapped, including locking the car while it’s parked at home.
More than half of cases of vehicular pediatric heatstroke occur because a caretaker accidentally left a child behind, he said. While in-car technology reminding adults to check their back seats has become more common, only a fraction of vehicles have it, requiring parents to come up with their own methods, like leaving a stuffed animal in the front seat.
The good news, Mr. Null said, is that simple behavioral changes can protect youngsters. “This is preventable in 100% of the cases,” he said.
A lopsided risk
People living in low-income areas fare worse when temperatures climb. Access to air conditioning, which includes the ability to afford the electricity bill, is a serious health concern.
A study of heat in urban areas released last year showed that low-income neighborhoods and communities of color experience much higher temperatures than those of wealthier, White residents. In more impoverished areas during the summer, temperatures can be as much as 7 degrees Fahrenheit warmer.
The study’s authors said their findings in the United States reflect that “the legacy of redlining looms large,” referring to a federal housing policy that refused to insure mortgages in or near predominantly Black neighborhoods.
“These areas have less tree canopy, more streets, and higher building densities, meaning that in addition to their other racist outcomes, redlining policies directly codified into law existing disparity in urban land use and reinforced urban design choices that magnify urban heating into the present,” they concluded.
Dr. Bernstein, who leads Harvard’s Center for Climate, Health, and the Global Environment, coauthored a commentary in JAMA arguing that advancing health equity is critical to action on climate change.
The center works with front-line health clinics to help their predominantly low-income patients respond to the health impacts of climate change. Federally backed clinics alone provide care to about 30 million Americans, including many children, he said.
Dr. Bernstein also recently led a nationwide study that found that from May through September, days with higher temperatures are associated with more visits to children’s hospital emergency rooms. Many visits were more directly linked to heat, although the study also pointed to how high temperatures can exacerbate existing health conditions such as neurological disorders.
“Children are more vulnerable to climate change through how these climate shocks reshape the world in which they grow up,” Dr. Bernstein said.
Helping people better understand the health risks of extreme heat and how to protect themselves and their families are among the public health system’s major challenges, experts said.
The National Weather Service’s heat alert system is mainly based on the heat index, a measure of how hot it feels when relative humidity is factored in with air temperature.
But the alerts are not related to effects on health, said Kathy Baughman McLeod, director of the Adrienne Arsht-Rockefeller Foundation Resilience Center. By the time temperatures rise to the level that a weather alert is issued, many vulnerable people – like children, pregnant women, and the elderly – may already be experiencing heat exhaustion or heatstroke.
The center developed a new heat alert system, which is being tested in Seville, Spain, historically one of the hottest cities in Europe.
The system marries metrics such as air temperature and humidity with public health data to categorize heat waves and, when they are serious enough, give them names – making it easier for people to understand heat as an environmental threat that requires prevention measures.
The categories are determined through a metric known as excess deaths, which compares how many people died on a day with the forecast temperature versus an average day. That may help health officials understand how severe a heat wave is expected to be and make informed recommendations to the public based on risk factors such as age or medical history.
The health-based alert system would also allow officials to target caretakers of children and seniors through school systems, preschools, and senior centers, Ms. Baughman McLeod said.
Giving people better ways to conceptualize heat is critical, she said.
“It’s not dramatic. It doesn’t rip the roof off of your house,” Ms. Baughman McLeod said. “It’s silent and invisible.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
‘Go Ask Alice’: A fake view of teen mental health
If you grew up in the 1970s and 1980s, chances are high you’re familiar with “Go Ask Alice.”
What was then said to be the real diary of a 15-year-old promising teen turned drug addict was released in 1971 as a cautionary tale and has since sold over 5 million copies. The diary was harrowing against the backdrop of the war on drugs and soon became both acclaimed and banned from classrooms across the country.
Schools citied “inappropriate” language that “borders on pornography” as grounds to prohibit teenagers from reading Alice’s story. But as much as the book’s vivid writing offended readers, it drew millions in with its profanity and graphic descriptions of sex, drugs, and mental health struggles.
At the time, The New York Times reviewed the book as “a strong, painfully honest, nakedly candid and true story ... a document of horrifying reality,” but the popular diary was later found to be a ploy – a fake story written by a 54-year-old Mormon youth counselor named Beatrice Sparks.
Now, Ms. Sparks, who died in 2012, has been further exposed in radio personality Rick Emerson’s new book, “Unmask Alice: LSD, Satanic Panic, and the Imposter Behind the World’s Most Notorious Diaries.” Mr. Emerson published the exposé in July, years after he had the idea to investigate Ms. Sparks’s work in 2015. The book details Ms. Sparks’s background, her journey in creating Alice, and her quest to be recognized for the teen diary she had published as “Anonymous.”
“After 30 years of trying, Beatrice Sparks had changed the world. And nobody knew it,” Mr. Emerson told the New York Post.In his work, Mr. Emerson also dives into the profound impact of the diary at a time when not as much research existed on teen mental health.
When the teenager whose diary inspired Ms. Sparks’s writing “died in March 1971, the very first true study of adolescent psychology had just barely come out,” Mr. Emerson said to Rolling Stone. “Mental health, especially for young people, was still very much on training wheels.”
According to Mr. Emerson, a lack of insight into mental health issues allowed Ms. Sparks’s description to go relatively unchallenged and for the book’s influence to spread despite its misinformation.
“It’s indisputable that large sections of ‘Go Ask Alice’ are just embellished and/or false,” he told the Post.
Then versus now
This landscape is in stark contrast to today, where thousands of studies on the topic have been done, compared with the mere dozens in the 1970s.
Anxiety and depression in minors have increased over time, a trend worsened by the COVID-19 pandemic, according to the CDC. Studies have shown that reported drug use in teens has decreased over time, proving significant during the pandemic, according to the National Institutes of Health.
While Alice from “Go Ask Alice” has not existed in either, comparing the two periods can offer insight into teen struggles in the 1970s versus today and sheds light on how literature – fiction or even faked nonfiction – can transform a nation.
A version of this article first appeared on WebMD.com.
If you grew up in the 1970s and 1980s, chances are high you’re familiar with “Go Ask Alice.”
What was then said to be the real diary of a 15-year-old promising teen turned drug addict was released in 1971 as a cautionary tale and has since sold over 5 million copies. The diary was harrowing against the backdrop of the war on drugs and soon became both acclaimed and banned from classrooms across the country.
Schools citied “inappropriate” language that “borders on pornography” as grounds to prohibit teenagers from reading Alice’s story. But as much as the book’s vivid writing offended readers, it drew millions in with its profanity and graphic descriptions of sex, drugs, and mental health struggles.
At the time, The New York Times reviewed the book as “a strong, painfully honest, nakedly candid and true story ... a document of horrifying reality,” but the popular diary was later found to be a ploy – a fake story written by a 54-year-old Mormon youth counselor named Beatrice Sparks.
Now, Ms. Sparks, who died in 2012, has been further exposed in radio personality Rick Emerson’s new book, “Unmask Alice: LSD, Satanic Panic, and the Imposter Behind the World’s Most Notorious Diaries.” Mr. Emerson published the exposé in July, years after he had the idea to investigate Ms. Sparks’s work in 2015. The book details Ms. Sparks’s background, her journey in creating Alice, and her quest to be recognized for the teen diary she had published as “Anonymous.”
“After 30 years of trying, Beatrice Sparks had changed the world. And nobody knew it,” Mr. Emerson told the New York Post.In his work, Mr. Emerson also dives into the profound impact of the diary at a time when not as much research existed on teen mental health.
When the teenager whose diary inspired Ms. Sparks’s writing “died in March 1971, the very first true study of adolescent psychology had just barely come out,” Mr. Emerson said to Rolling Stone. “Mental health, especially for young people, was still very much on training wheels.”
According to Mr. Emerson, a lack of insight into mental health issues allowed Ms. Sparks’s description to go relatively unchallenged and for the book’s influence to spread despite its misinformation.
“It’s indisputable that large sections of ‘Go Ask Alice’ are just embellished and/or false,” he told the Post.
Then versus now
This landscape is in stark contrast to today, where thousands of studies on the topic have been done, compared with the mere dozens in the 1970s.
Anxiety and depression in minors have increased over time, a trend worsened by the COVID-19 pandemic, according to the CDC. Studies have shown that reported drug use in teens has decreased over time, proving significant during the pandemic, according to the National Institutes of Health.
While Alice from “Go Ask Alice” has not existed in either, comparing the two periods can offer insight into teen struggles in the 1970s versus today and sheds light on how literature – fiction or even faked nonfiction – can transform a nation.
A version of this article first appeared on WebMD.com.
If you grew up in the 1970s and 1980s, chances are high you’re familiar with “Go Ask Alice.”
What was then said to be the real diary of a 15-year-old promising teen turned drug addict was released in 1971 as a cautionary tale and has since sold over 5 million copies. The diary was harrowing against the backdrop of the war on drugs and soon became both acclaimed and banned from classrooms across the country.
Schools citied “inappropriate” language that “borders on pornography” as grounds to prohibit teenagers from reading Alice’s story. But as much as the book’s vivid writing offended readers, it drew millions in with its profanity and graphic descriptions of sex, drugs, and mental health struggles.
At the time, The New York Times reviewed the book as “a strong, painfully honest, nakedly candid and true story ... a document of horrifying reality,” but the popular diary was later found to be a ploy – a fake story written by a 54-year-old Mormon youth counselor named Beatrice Sparks.
Now, Ms. Sparks, who died in 2012, has been further exposed in radio personality Rick Emerson’s new book, “Unmask Alice: LSD, Satanic Panic, and the Imposter Behind the World’s Most Notorious Diaries.” Mr. Emerson published the exposé in July, years after he had the idea to investigate Ms. Sparks’s work in 2015. The book details Ms. Sparks’s background, her journey in creating Alice, and her quest to be recognized for the teen diary she had published as “Anonymous.”
“After 30 years of trying, Beatrice Sparks had changed the world. And nobody knew it,” Mr. Emerson told the New York Post.In his work, Mr. Emerson also dives into the profound impact of the diary at a time when not as much research existed on teen mental health.
When the teenager whose diary inspired Ms. Sparks’s writing “died in March 1971, the very first true study of adolescent psychology had just barely come out,” Mr. Emerson said to Rolling Stone. “Mental health, especially for young people, was still very much on training wheels.”
According to Mr. Emerson, a lack of insight into mental health issues allowed Ms. Sparks’s description to go relatively unchallenged and for the book’s influence to spread despite its misinformation.
“It’s indisputable that large sections of ‘Go Ask Alice’ are just embellished and/or false,” he told the Post.
Then versus now
This landscape is in stark contrast to today, where thousands of studies on the topic have been done, compared with the mere dozens in the 1970s.
Anxiety and depression in minors have increased over time, a trend worsened by the COVID-19 pandemic, according to the CDC. Studies have shown that reported drug use in teens has decreased over time, proving significant during the pandemic, according to the National Institutes of Health.
While Alice from “Go Ask Alice” has not existed in either, comparing the two periods can offer insight into teen struggles in the 1970s versus today and sheds light on how literature – fiction or even faked nonfiction – can transform a nation.
A version of this article first appeared on WebMD.com.








