User login
Reassessing benzodiazepines: What role should this medication class play in psychiatry?
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Nail Changes Associated With Thyroid Disease
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.
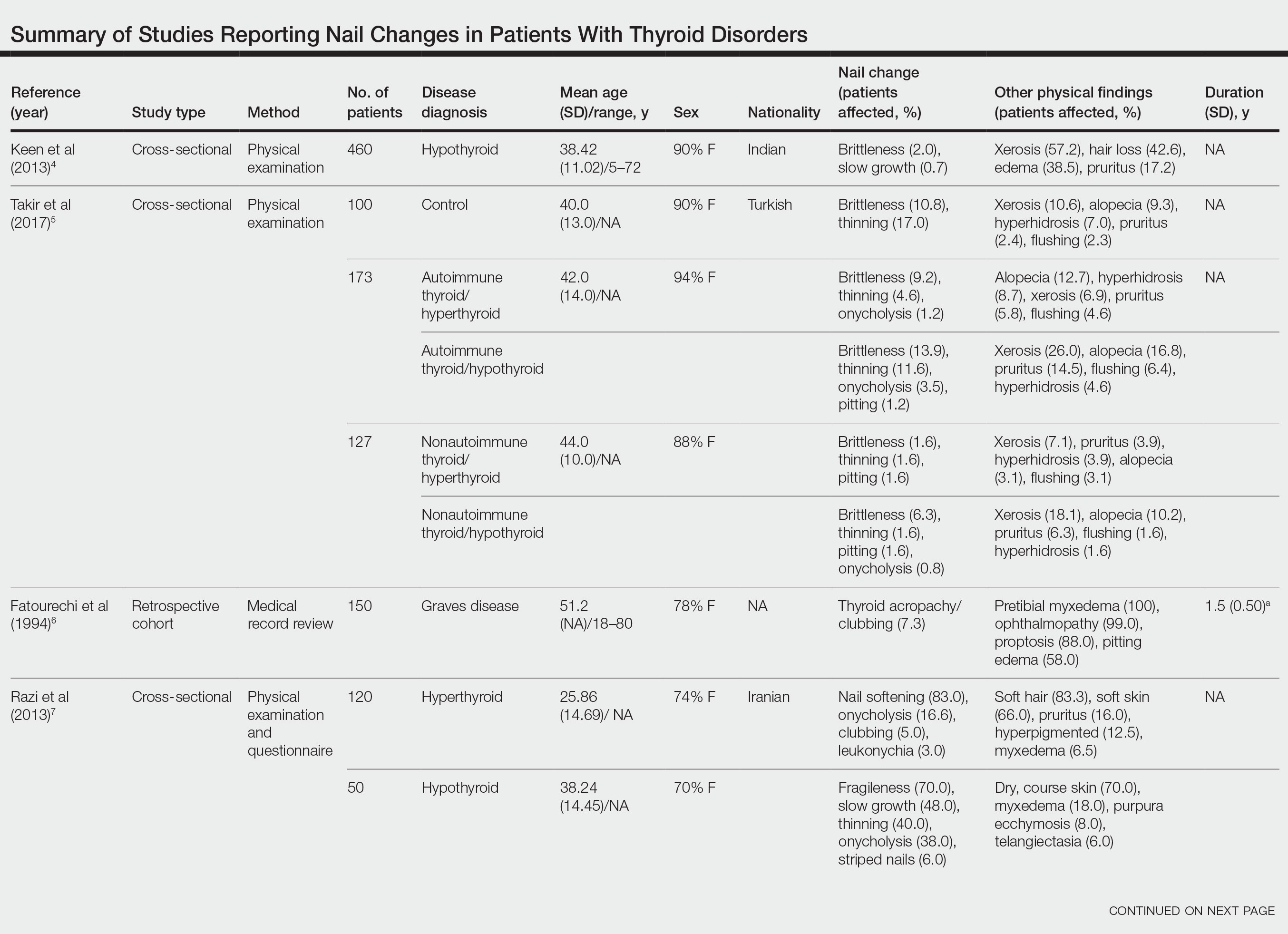
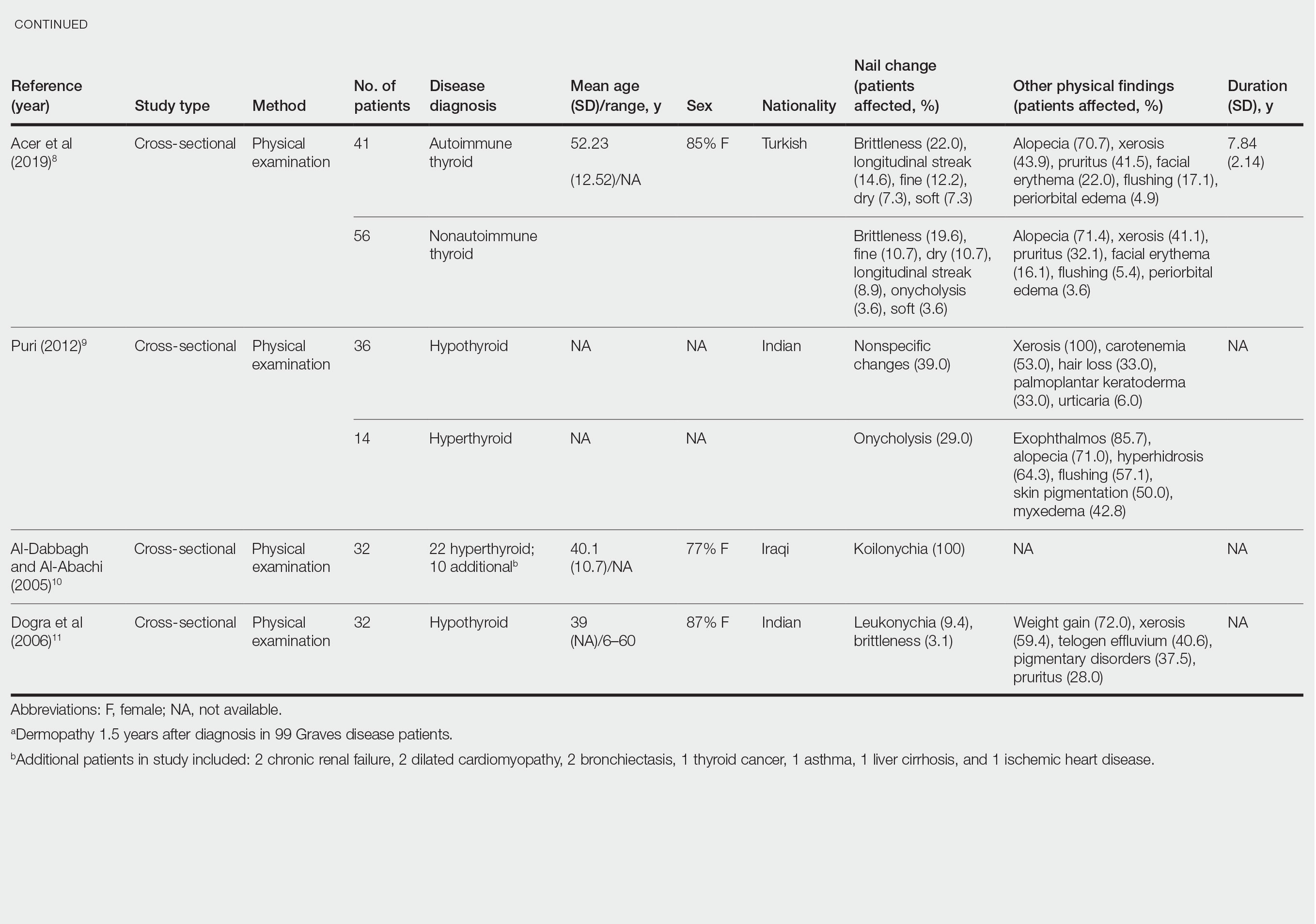
Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
Practice Points
- Koilonychia is associated with hyperthyroidism.
- Clubbing is a manifestation of thyroid acropachy in Graves disease and also affects other patients with hyperthyroidism.
- Onycholysis improves in patients with hypothyroidism treated with thyroid hormone replacement therapy.
Should patients undergoing surgical treatment for cervical lesions also receive an HPV vaccination?
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Human papillomavirus (HPV) vaccine given around the time women have surgery for precancerous cervical lesions might lead to a reduction in the risk of lesions returning, as well as other HPV-related diseases, but the effects of this remain unclear.
The authors of the new study, published in The BMJ, explained that women who have been treated for high-grade cervical intra-epithelial neoplasia (CIN) have a “lifelong residual high risk of cervical cancer and other malignancies related to HPV infection,” and some research suggests that giving a preventive HPV vaccine alongside treatment for CIN might help to “reduce the risk in these women.”
HPV vaccination is highly effective at preventing the development of precancerous cervical lesions, CIN, and in the U.K., HPV vaccination is offered to girls and boys around the age of 12 or 13.
Eluned Hughes, head of information and engagement at Jo’s Cervical Cancer Trust, said: “Recent evidence has found that cases of cervical cancer have fallen 87% since the introduction of the HPV vaccine program in U.K. schools in 2008.”
“However, women over the age of 27, for whom the vaccine was not available, remain at increased risk of cervical cancer,” she highlighted.
Significant risk of bias and scarcity of data
In the study, researchers set out to explore the efficacy of HPV vaccination on the risk of HPV infection and recurrent diseases related to HPV infection in individuals undergoing local surgical treatment of preinvasive genital disease.
The systematic review and meta-analysis, led by researchers at Imperial College London, screened data from PubMed (Medline), Scopus, Cochrane, Web of Science, and ClinicalTrials.gov from inception to March 31, 2021.
The researchers analyzed the results of 18 studies – two randomized controlled trials (RCTs), 12 observational studies, and four post-hoc analyses of RCTs.
The authors said that the two RCTs were classified as low risk of bias, while in the observational studies and post-hoc analyses, risk of bias was moderate for seven, serious for seven, and critical for two. Average length of follow-up was 36 months.
There was a reduction of 57% in the risk of recurrence of high-grade pre-invasive disease (CIN2+) in individuals who were vaccinated, compared with those who were not vaccinated. “The effect estimate was “even more pronounced” – a relative 74% reduction – when the risk of recurrence of CIN2+ was assessed for disease related to the two high-risk HPV types – HPV16 and HPV18,” explained the authors.
However, the researchers noted that these effects are unclear because of the “scarcity of data” and the “moderate to high overall risk of bias” of the available studies.
Quality of evidence inconclusive – more trials needed
With regards to CIN3, the risk of recurrence of was also reduced in patients who were vaccinated, but there was a high level of uncertainty about the quality of this evidence, cautioned the authors.
Evidence was also lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, and anal lesions, as well as genital warts.
Analysis of the post-hoc studies from randomized controlled trial data with historic vaccination at randomization before the development of the disease reported inconsistent results, the authors said.
Several study limitations were acknowledged by the authors, including that most of the studies were observational, of low to moderate quality, and with relatively short follow-up times, which they pointed out prevented assessment of long-term effects. In addition, the average age of participants was not provided in most studies, and factors such as smoking – associated with a higher risk of recurrence – were not controlled for in many studies.
“HPV vaccination might reduce the risk of recurrence of CIN, in particular when related to HPV16 or HPV18, in women treated with local excision,” they concluded. However, they cautioned that “quality of evidence indicated that the data were inconclusive.”
“Large, appropriately powered, randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN,” they recommended.
“Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the United Kingdom, absolute risks and a cost effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment,” the authors said.
Ms. Hughes said that the charity was pleased to see emerging research into the value of using the HPV vaccine to prevent the recurrence of cervical cell changes. She said that the charity looks forward to seeing “further large-scale studies into the effectiveness of this method.”
In the meantime, the charity encourages all women and other people with a cervix to attend their cervical screening and for young people to have the HPV vaccination when invited, as “these are the best tools we currently have to prevent cervical cancer,” she said.
A version of this article first appeared on Medscape UK.
Regular exercise appears to slow cognitive decline in MCI
(MCI), new research from the largest study of its kind suggests. Topline results from the EXERT trial showed patients with MCI who participated regularly in either aerobic exercise or stretching/balance/range-of-motion exercises maintained stable global cognitive function over 12 months of follow-up – with no differences between the two types of exercise.
“We’re excited about these findings, because these types of exercises that we’re seeing can protect against cognitive decline are accessible to everyone and therefore scalable to the public,” study investigator Laura Baker, PhD, Wake Forest University School of Medicine, Winston-Salem, N.C., said at a press briefing.
The topline results were presented at the 2022 Alzheimer’s Association International Conference.
No decline
The 18-month EXERT trial was designed to be the definitive study to answer the question about whether exercise can slow cognitive decline in older adults with amnestic MCI, Dr. Baker reported. Investigators enrolled 296 sedentary men and women with MCI (mean age, about 75 years). All were randomly allocated to either an aerobic exercise group (maintaining a heart rate at about 70%-85%) or a stretching and balance group (maintaining heart rate less than 35%).
Both groups exercised four times per week for about 30-40 minutes. In the first 12 months they were supervised by a trainer at the YMCA and then they exercised independently for the final 6 months.
Participants were assessed at baseline and every 6 months. The primary endpoint was change from baseline on the ADAS-Cog-Exec, a validated measure of global cognitive function, at the end of the 12 months of supervised exercise.
During the first 12 months, participants completed over 31,000 sessions of exercise, which is “quite impressive,” Dr. Baker said.
Over the first 12 months, neither the aerobic group nor the stretch/balance group showed a decline on the ADAS-Cog-Exec.
“We saw no group differences, and importantly, no decline after 12 months,” Dr. Baker reported.
Supported exercise is ‘crucial’
To help “make sense” of these findings, Dr. Baker noted that 12-month changes in the ADAS-Cog-Exec for the EXERT intervention groups were also compared with a “usual care” cohort of adults matched for age, sex, education, baseline cognitive status, and APOE4 genotype.
In this “apples-to-apples” comparison, the usual care cohort showed the expected decline or worsening of cognitive function over 12 months on the ADAS-Cog-Exec, but the EXERT exercise groups did not.
Dr. Baker noted that both exercise groups received equal amounts of weekly socialization, which may have contributed to the apparent protective effects on the brain.
A greater volume of exercise in EXERT, compared with other trials, may also be a factor. Each individual participant in EXERT completed more than 100 hours of exercise.
“The take-home message is that an increased amount of either low-intensity or high-intensity exercise for 120-150 minutes per week for 12 months may slow cognitive decline in sedentary older adults with MCI,” Dr. Baker said.
“What’s critical is that this regular exercise must be supported in these older [patients] with MCI. It must be supervised. There has to be some social component,” she added.
In her view, 120 minutes of regular supported exercise for sedentary individuals with MCI “needs to be part of the recommendation for risk reduction.”
Important study
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that several studies over the years have suggested that different types of exercise can have benefits on the brain.
“What’s important about this study is that it’s in a population of people that have MCI and are already experiencing memory changes,” Dr. Snyder said.
“The results suggest that engaging in both of these types of exercise may be beneficial for our brain. And given that this is the largest study of its kind in a population of people with MCI, it suggests it’s ‘never too late’ to start exercising,” she added.
Dr. Snyder noted the importance of continuing this work and to continue following these individuals “over time as well.”
The study was funded by the National Institutes of Health, National Institute on Aging. Dr. Baker and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(MCI), new research from the largest study of its kind suggests. Topline results from the EXERT trial showed patients with MCI who participated regularly in either aerobic exercise or stretching/balance/range-of-motion exercises maintained stable global cognitive function over 12 months of follow-up – with no differences between the two types of exercise.
“We’re excited about these findings, because these types of exercises that we’re seeing can protect against cognitive decline are accessible to everyone and therefore scalable to the public,” study investigator Laura Baker, PhD, Wake Forest University School of Medicine, Winston-Salem, N.C., said at a press briefing.
The topline results were presented at the 2022 Alzheimer’s Association International Conference.
No decline
The 18-month EXERT trial was designed to be the definitive study to answer the question about whether exercise can slow cognitive decline in older adults with amnestic MCI, Dr. Baker reported. Investigators enrolled 296 sedentary men and women with MCI (mean age, about 75 years). All were randomly allocated to either an aerobic exercise group (maintaining a heart rate at about 70%-85%) or a stretching and balance group (maintaining heart rate less than 35%).
Both groups exercised four times per week for about 30-40 minutes. In the first 12 months they were supervised by a trainer at the YMCA and then they exercised independently for the final 6 months.
Participants were assessed at baseline and every 6 months. The primary endpoint was change from baseline on the ADAS-Cog-Exec, a validated measure of global cognitive function, at the end of the 12 months of supervised exercise.
During the first 12 months, participants completed over 31,000 sessions of exercise, which is “quite impressive,” Dr. Baker said.
Over the first 12 months, neither the aerobic group nor the stretch/balance group showed a decline on the ADAS-Cog-Exec.
“We saw no group differences, and importantly, no decline after 12 months,” Dr. Baker reported.
Supported exercise is ‘crucial’
To help “make sense” of these findings, Dr. Baker noted that 12-month changes in the ADAS-Cog-Exec for the EXERT intervention groups were also compared with a “usual care” cohort of adults matched for age, sex, education, baseline cognitive status, and APOE4 genotype.
In this “apples-to-apples” comparison, the usual care cohort showed the expected decline or worsening of cognitive function over 12 months on the ADAS-Cog-Exec, but the EXERT exercise groups did not.
Dr. Baker noted that both exercise groups received equal amounts of weekly socialization, which may have contributed to the apparent protective effects on the brain.
A greater volume of exercise in EXERT, compared with other trials, may also be a factor. Each individual participant in EXERT completed more than 100 hours of exercise.
“The take-home message is that an increased amount of either low-intensity or high-intensity exercise for 120-150 minutes per week for 12 months may slow cognitive decline in sedentary older adults with MCI,” Dr. Baker said.
“What’s critical is that this regular exercise must be supported in these older [patients] with MCI. It must be supervised. There has to be some social component,” she added.
In her view, 120 minutes of regular supported exercise for sedentary individuals with MCI “needs to be part of the recommendation for risk reduction.”
Important study
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that several studies over the years have suggested that different types of exercise can have benefits on the brain.
“What’s important about this study is that it’s in a population of people that have MCI and are already experiencing memory changes,” Dr. Snyder said.
“The results suggest that engaging in both of these types of exercise may be beneficial for our brain. And given that this is the largest study of its kind in a population of people with MCI, it suggests it’s ‘never too late’ to start exercising,” she added.
Dr. Snyder noted the importance of continuing this work and to continue following these individuals “over time as well.”
The study was funded by the National Institutes of Health, National Institute on Aging. Dr. Baker and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(MCI), new research from the largest study of its kind suggests. Topline results from the EXERT trial showed patients with MCI who participated regularly in either aerobic exercise or stretching/balance/range-of-motion exercises maintained stable global cognitive function over 12 months of follow-up – with no differences between the two types of exercise.
“We’re excited about these findings, because these types of exercises that we’re seeing can protect against cognitive decline are accessible to everyone and therefore scalable to the public,” study investigator Laura Baker, PhD, Wake Forest University School of Medicine, Winston-Salem, N.C., said at a press briefing.
The topline results were presented at the 2022 Alzheimer’s Association International Conference.
No decline
The 18-month EXERT trial was designed to be the definitive study to answer the question about whether exercise can slow cognitive decline in older adults with amnestic MCI, Dr. Baker reported. Investigators enrolled 296 sedentary men and women with MCI (mean age, about 75 years). All were randomly allocated to either an aerobic exercise group (maintaining a heart rate at about 70%-85%) or a stretching and balance group (maintaining heart rate less than 35%).
Both groups exercised four times per week for about 30-40 minutes. In the first 12 months they were supervised by a trainer at the YMCA and then they exercised independently for the final 6 months.
Participants were assessed at baseline and every 6 months. The primary endpoint was change from baseline on the ADAS-Cog-Exec, a validated measure of global cognitive function, at the end of the 12 months of supervised exercise.
During the first 12 months, participants completed over 31,000 sessions of exercise, which is “quite impressive,” Dr. Baker said.
Over the first 12 months, neither the aerobic group nor the stretch/balance group showed a decline on the ADAS-Cog-Exec.
“We saw no group differences, and importantly, no decline after 12 months,” Dr. Baker reported.
Supported exercise is ‘crucial’
To help “make sense” of these findings, Dr. Baker noted that 12-month changes in the ADAS-Cog-Exec for the EXERT intervention groups were also compared with a “usual care” cohort of adults matched for age, sex, education, baseline cognitive status, and APOE4 genotype.
In this “apples-to-apples” comparison, the usual care cohort showed the expected decline or worsening of cognitive function over 12 months on the ADAS-Cog-Exec, but the EXERT exercise groups did not.
Dr. Baker noted that both exercise groups received equal amounts of weekly socialization, which may have contributed to the apparent protective effects on the brain.
A greater volume of exercise in EXERT, compared with other trials, may also be a factor. Each individual participant in EXERT completed more than 100 hours of exercise.
“The take-home message is that an increased amount of either low-intensity or high-intensity exercise for 120-150 minutes per week for 12 months may slow cognitive decline in sedentary older adults with MCI,” Dr. Baker said.
“What’s critical is that this regular exercise must be supported in these older [patients] with MCI. It must be supervised. There has to be some social component,” she added.
In her view, 120 minutes of regular supported exercise for sedentary individuals with MCI “needs to be part of the recommendation for risk reduction.”
Important study
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, noted that several studies over the years have suggested that different types of exercise can have benefits on the brain.
“What’s important about this study is that it’s in a population of people that have MCI and are already experiencing memory changes,” Dr. Snyder said.
“The results suggest that engaging in both of these types of exercise may be beneficial for our brain. And given that this is the largest study of its kind in a population of people with MCI, it suggests it’s ‘never too late’ to start exercising,” she added.
Dr. Snyder noted the importance of continuing this work and to continue following these individuals “over time as well.”
The study was funded by the National Institutes of Health, National Institute on Aging. Dr. Baker and Dr. Snyder have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAIC 2022
Fecal microbiota transplants: Finding new microbial markers for donor efficacy in UC
Donor microbiota stability and species evenness, along with presence of certain microbial species, may predict donor efficacy in fecal microbiota transplantation (FMT) for the treatment of patients with ulcerative colitis (UC), a new study suggests.
The authors noted that these markers of donor efficacy could be used to optimize selection of donors to treat patients with UC and improve outcomes.
The investigators hypothesized that “there are features beyond microbial richness, individual bacterial species, and specific metabolites that may aid in successful identification of effective donors.” They published these findings in Gut.
The LOTUS clinical trial explored the efficacy of lyophilized FMT in patients with UC, but was cut short because of the COVID-19 pandemic. The study investigators analyzed fecal samples from the two donors enrolled in the trial to identify functional and taxonomic differences within the donors’ microbiota that have clinical relevance to their efficacy in active UC. Donor 1’s samples showed 100% efficacy among patients with UC, while donor 2’s samples showed 36% efficacy.
In donor 1, the researchers observed “robust stability in species richness” during the sampling periods, whereas donor 2 exhibited larger fluctuations. Although the species richness was significantly greater in the donor 2, the researchers reported that donor 1 exhibited significantly greater diversity at the higher taxonomic level of phylum. According to the investigators, this was reflected by the detection of Euryarchaeota, Synergistetes, and Verrucomicrobia in donor 1 but not in the second donor.
Despite a higher species richness in donor 2, the researchers found that a higher rate of uniquely classified metagenome-assembled genomes was produced per sample in the donor 1, which indicates greater species evenness, the second marker of efficacy according to investigators.
Blautia wexlerae was a highly prevalent metagenome-assembled genome that was enriched in donor 1 compared with donor 2, and the researchers reported that “a taxon with high similarity (OTU215) showed evidence of engraftment in patients receiving donor 1.” In addition, B. wexlerae demonstrated a trend toward enrichment in donor 2 samples that were associated with positive outcomes in patients and demonstrated evidence of engraftment in patients who received donor 2.
Ninety bacterial species as well as one archaeon were differentially abundant between donors, including 44 donor samples which were greater than 0.1% in relative abundance. According to the researchers, 17 out of the 44 species were enriched in the effective donor, with 11 (64.7%) assembled into high-quality genomes highly prevalent in that donor and 6 that demonstrated evidence of engraftment in patients.
Lastly, the investigators sought to validate the observed associations between certain microbial taxa and donor clinical efficacy in an independent cohort. In this analysis, the investigators evaluated shotgun metagenomics data of donors used to treat patients with UC and examined relative abundances against patient outcomes. Species associated with treatment success included Ruminococcus bromii, B. wexlerae, Eubacterium hallii, Coprococcus catus, Fusicatenibacter saccharivorans, and Parabacteroides merdae.
“We identified microbiota stability and species evenness as markers of donor efficacy, as well as specific microbial species that could be employed to improve donor selection and build artificial microbial consortia to treat UC,” the investigators concluded.
Given that the study enrolled only two donors, the generalizability of the findings may be limited. The researchers wrote that another limitation of the data analysis was “the bias towards more relatively abundant taxa due to the inability to assemble genomes from low-abundance species.” The lack of prospective validation studies on the novel metrics is another limitation of the study.
Some investigators disclosed relationships with biomedical companies, such as Takeda and Janssen.
Donor microbiota stability and species evenness, along with presence of certain microbial species, may predict donor efficacy in fecal microbiota transplantation (FMT) for the treatment of patients with ulcerative colitis (UC), a new study suggests.
The authors noted that these markers of donor efficacy could be used to optimize selection of donors to treat patients with UC and improve outcomes.
The investigators hypothesized that “there are features beyond microbial richness, individual bacterial species, and specific metabolites that may aid in successful identification of effective donors.” They published these findings in Gut.
The LOTUS clinical trial explored the efficacy of lyophilized FMT in patients with UC, but was cut short because of the COVID-19 pandemic. The study investigators analyzed fecal samples from the two donors enrolled in the trial to identify functional and taxonomic differences within the donors’ microbiota that have clinical relevance to their efficacy in active UC. Donor 1’s samples showed 100% efficacy among patients with UC, while donor 2’s samples showed 36% efficacy.
In donor 1, the researchers observed “robust stability in species richness” during the sampling periods, whereas donor 2 exhibited larger fluctuations. Although the species richness was significantly greater in the donor 2, the researchers reported that donor 1 exhibited significantly greater diversity at the higher taxonomic level of phylum. According to the investigators, this was reflected by the detection of Euryarchaeota, Synergistetes, and Verrucomicrobia in donor 1 but not in the second donor.
Despite a higher species richness in donor 2, the researchers found that a higher rate of uniquely classified metagenome-assembled genomes was produced per sample in the donor 1, which indicates greater species evenness, the second marker of efficacy according to investigators.
Blautia wexlerae was a highly prevalent metagenome-assembled genome that was enriched in donor 1 compared with donor 2, and the researchers reported that “a taxon with high similarity (OTU215) showed evidence of engraftment in patients receiving donor 1.” In addition, B. wexlerae demonstrated a trend toward enrichment in donor 2 samples that were associated with positive outcomes in patients and demonstrated evidence of engraftment in patients who received donor 2.
Ninety bacterial species as well as one archaeon were differentially abundant between donors, including 44 donor samples which were greater than 0.1% in relative abundance. According to the researchers, 17 out of the 44 species were enriched in the effective donor, with 11 (64.7%) assembled into high-quality genomes highly prevalent in that donor and 6 that demonstrated evidence of engraftment in patients.
Lastly, the investigators sought to validate the observed associations between certain microbial taxa and donor clinical efficacy in an independent cohort. In this analysis, the investigators evaluated shotgun metagenomics data of donors used to treat patients with UC and examined relative abundances against patient outcomes. Species associated with treatment success included Ruminococcus bromii, B. wexlerae, Eubacterium hallii, Coprococcus catus, Fusicatenibacter saccharivorans, and Parabacteroides merdae.
“We identified microbiota stability and species evenness as markers of donor efficacy, as well as specific microbial species that could be employed to improve donor selection and build artificial microbial consortia to treat UC,” the investigators concluded.
Given that the study enrolled only two donors, the generalizability of the findings may be limited. The researchers wrote that another limitation of the data analysis was “the bias towards more relatively abundant taxa due to the inability to assemble genomes from low-abundance species.” The lack of prospective validation studies on the novel metrics is another limitation of the study.
Some investigators disclosed relationships with biomedical companies, such as Takeda and Janssen.
Donor microbiota stability and species evenness, along with presence of certain microbial species, may predict donor efficacy in fecal microbiota transplantation (FMT) for the treatment of patients with ulcerative colitis (UC), a new study suggests.
The authors noted that these markers of donor efficacy could be used to optimize selection of donors to treat patients with UC and improve outcomes.
The investigators hypothesized that “there are features beyond microbial richness, individual bacterial species, and specific metabolites that may aid in successful identification of effective donors.” They published these findings in Gut.
The LOTUS clinical trial explored the efficacy of lyophilized FMT in patients with UC, but was cut short because of the COVID-19 pandemic. The study investigators analyzed fecal samples from the two donors enrolled in the trial to identify functional and taxonomic differences within the donors’ microbiota that have clinical relevance to their efficacy in active UC. Donor 1’s samples showed 100% efficacy among patients with UC, while donor 2’s samples showed 36% efficacy.
In donor 1, the researchers observed “robust stability in species richness” during the sampling periods, whereas donor 2 exhibited larger fluctuations. Although the species richness was significantly greater in the donor 2, the researchers reported that donor 1 exhibited significantly greater diversity at the higher taxonomic level of phylum. According to the investigators, this was reflected by the detection of Euryarchaeota, Synergistetes, and Verrucomicrobia in donor 1 but not in the second donor.
Despite a higher species richness in donor 2, the researchers found that a higher rate of uniquely classified metagenome-assembled genomes was produced per sample in the donor 1, which indicates greater species evenness, the second marker of efficacy according to investigators.
Blautia wexlerae was a highly prevalent metagenome-assembled genome that was enriched in donor 1 compared with donor 2, and the researchers reported that “a taxon with high similarity (OTU215) showed evidence of engraftment in patients receiving donor 1.” In addition, B. wexlerae demonstrated a trend toward enrichment in donor 2 samples that were associated with positive outcomes in patients and demonstrated evidence of engraftment in patients who received donor 2.
Ninety bacterial species as well as one archaeon were differentially abundant between donors, including 44 donor samples which were greater than 0.1% in relative abundance. According to the researchers, 17 out of the 44 species were enriched in the effective donor, with 11 (64.7%) assembled into high-quality genomes highly prevalent in that donor and 6 that demonstrated evidence of engraftment in patients.
Lastly, the investigators sought to validate the observed associations between certain microbial taxa and donor clinical efficacy in an independent cohort. In this analysis, the investigators evaluated shotgun metagenomics data of donors used to treat patients with UC and examined relative abundances against patient outcomes. Species associated with treatment success included Ruminococcus bromii, B. wexlerae, Eubacterium hallii, Coprococcus catus, Fusicatenibacter saccharivorans, and Parabacteroides merdae.
“We identified microbiota stability and species evenness as markers of donor efficacy, as well as specific microbial species that could be employed to improve donor selection and build artificial microbial consortia to treat UC,” the investigators concluded.
Given that the study enrolled only two donors, the generalizability of the findings may be limited. The researchers wrote that another limitation of the data analysis was “the bias towards more relatively abundant taxa due to the inability to assemble genomes from low-abundance species.” The lack of prospective validation studies on the novel metrics is another limitation of the study.
Some investigators disclosed relationships with biomedical companies, such as Takeda and Janssen.
FROM GUT
Applications for the CUTIS 2023 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2023 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2023.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 28. The residents who are selected to write the column for the upcoming year will be notified by November 4.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2023 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2023.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 28. The residents who are selected to write the column for the upcoming year will be notified by November 4.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2023 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2023.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 28. The residents who are selected to write the column for the upcoming year will be notified by November 4.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Why exercise doesn’t help people with long COVID
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
Waking up at night could be your brain boosting your memory
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
FROM NATURE NEUROSCIENCE
Chronically low wages linked to subsequent memory decline
, new research suggests. In a new analysis of more than 3,000 participants in the Health and Retirement Study, those who sustained low wages in midlife showed significantly faster memory decline than their peers who never earned low wages.
The findings could have implications for future public policy and research initiatives, the investigators noted.
“Our findings, which suggest a pattern of sustained low-wage earning is harmful for cognitive health, [are] broadly applicable to researchers across numerous health disciplines,” said co-investigator Katrina Kezios, PhD, postdoctoral researcher, department of epidemiology, Mailman School of Public Health, Columbia University, New York.
The findings were presented at the 2022 Alzheimer’s Association International Conference.
Growing number of low-wage workers
Low-wage workers make up a growing share of the U.S. labor market. Yet little research has examined the long-term relationship between earning low wages and memory decline.
The current investigators assessed 1992-2016 data from the Health and Retirement Study, a longitudinal survey of nationally representative samples of Americans aged 50 years and older. Study participants are interviewed every 2 years and provide, among other things, information on work-related factors, including hourly wages.
Memory function was measured at each visit from 2004 to 2016 using a memory composite score. The score included immediate and delayed word recall memory assessments. For those who became too impaired to complete cognitive assessment, memory tests by proxy informants were utilized.
On average, participants completed 4.8 memory assessments over the course of the study.
Researchers defined “low wage” as an hourly wage lower than two-thirds of the federal median wage for the corresponding year. They categorized low-wage exposure history as “never” or “intermittent” or “sustained” on the basis of wages earned from 1992 to 2004.
The current analysis included 3,803 participants, 1,913 of whom were men. All participants were born from 1936 to 1941. In 2004, the average age was 65 years, and the mean memory score was 1.15 standard units.
The investigators adjusted for factors that could confound the relationship between wages and cognition, including the participant’s education, parental education, household wealth, and marital status. Later, whether the participants’ occupation type was of low skill or not was also included.
Cognitive harm
The confounder-adjusted annual rate of memory decline among workers who never earned low wages was –0.12 standard units (95% confidence interval, –0.14 to –0.10).
Compared with these workers, memory decline was significantly faster among participants with sustained low wage–earning during midlife (beta for interaction between time and exposure group, –0.012; 95% CI, –0.02 to 0.01), corresponding to an annual rate of –0.13 standard units.
Put another way, the cognitive aging experienced by workers earning low wages over a 10-year period was equivalent to what workers who never earned low wages would experience over 11 years.
Although similar associations were found for men and women, it was stronger in magnitude for men – a finding Dr. Kezios said was somewhat surprising. She noted that women are commonly more at risk for dementia than men.
However, she advises caution in interpreting this finding, as there were so few men in the sustained low-wage group. “Women disproportionately make up the group of workers earning low wages,” she said.
The negative low coefficient found for those who persistently earned low wages was also observed for those who intermittently earned low wages, but this was not statistically significant.
“We can speculate or hypothesize the cumulative effect of earning low wages at each exposure interval produces more cognitive harm than maybe earning low wages at some time points over that exposure period,” said Dr. Kezios.
A sensitivity analysis that examined wage earning at the same ages but in two different birth cohorts showed similar results for the two groups. When researchers removed self-employed workers from the study sample, the same association between sustained low wages and memory decline was found.
“Our findings held up, which gave us a little more reassurance that what we were seeing is at least signaling there might be something there,” said Dr. Kezios.
She described the study as a “first pass” for documenting the harmful cognitive effects of consistently earning low wages.
It would be interesting, she said, to now determine whether there’s a “dose effect” for having a low salary. However, other studies with different designs would be needed to determine at what income level cognitive health starts to be protected and the impact of raising the minimum wage, she added.
Unique study
Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said the study was unique. “I don’t think we have seen anything like this before,” said Dr. Snyder.
The study, which links sustained low-wage earning in midlife to later memory decline, “is looking beyond some of the other measures we’ve seen when we looked at socioeconomic status,” she noted.
The results “beg the question” of whether people who earn low wages have less access to health care, she added.
“We should think about how to ensure access and equity around health care and around potential ways that may address components of risk individuals have during their life course,” Dr. Snyder said.
She noted that the study provides a “start” at considering potential policies to address the impact of sustained low wages on overall health, particularly cognitive health, throughout life.
The study had no outside funding. Dr. Kezios has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In a new analysis of more than 3,000 participants in the Health and Retirement Study, those who sustained low wages in midlife showed significantly faster memory decline than their peers who never earned low wages.
The findings could have implications for future public policy and research initiatives, the investigators noted.
“Our findings, which suggest a pattern of sustained low-wage earning is harmful for cognitive health, [are] broadly applicable to researchers across numerous health disciplines,” said co-investigator Katrina Kezios, PhD, postdoctoral researcher, department of epidemiology, Mailman School of Public Health, Columbia University, New York.
The findings were presented at the 2022 Alzheimer’s Association International Conference.
Growing number of low-wage workers
Low-wage workers make up a growing share of the U.S. labor market. Yet little research has examined the long-term relationship between earning low wages and memory decline.
The current investigators assessed 1992-2016 data from the Health and Retirement Study, a longitudinal survey of nationally representative samples of Americans aged 50 years and older. Study participants are interviewed every 2 years and provide, among other things, information on work-related factors, including hourly wages.
Memory function was measured at each visit from 2004 to 2016 using a memory composite score. The score included immediate and delayed word recall memory assessments. For those who became too impaired to complete cognitive assessment, memory tests by proxy informants were utilized.
On average, participants completed 4.8 memory assessments over the course of the study.
Researchers defined “low wage” as an hourly wage lower than two-thirds of the federal median wage for the corresponding year. They categorized low-wage exposure history as “never” or “intermittent” or “sustained” on the basis of wages earned from 1992 to 2004.
The current analysis included 3,803 participants, 1,913 of whom were men. All participants were born from 1936 to 1941. In 2004, the average age was 65 years, and the mean memory score was 1.15 standard units.
The investigators adjusted for factors that could confound the relationship between wages and cognition, including the participant’s education, parental education, household wealth, and marital status. Later, whether the participants’ occupation type was of low skill or not was also included.
Cognitive harm
The confounder-adjusted annual rate of memory decline among workers who never earned low wages was –0.12 standard units (95% confidence interval, –0.14 to –0.10).
Compared with these workers, memory decline was significantly faster among participants with sustained low wage–earning during midlife (beta for interaction between time and exposure group, –0.012; 95% CI, –0.02 to 0.01), corresponding to an annual rate of –0.13 standard units.
Put another way, the cognitive aging experienced by workers earning low wages over a 10-year period was equivalent to what workers who never earned low wages would experience over 11 years.
Although similar associations were found for men and women, it was stronger in magnitude for men – a finding Dr. Kezios said was somewhat surprising. She noted that women are commonly more at risk for dementia than men.
However, she advises caution in interpreting this finding, as there were so few men in the sustained low-wage group. “Women disproportionately make up the group of workers earning low wages,” she said.
The negative low coefficient found for those who persistently earned low wages was also observed for those who intermittently earned low wages, but this was not statistically significant.
“We can speculate or hypothesize the cumulative effect of earning low wages at each exposure interval produces more cognitive harm than maybe earning low wages at some time points over that exposure period,” said Dr. Kezios.
A sensitivity analysis that examined wage earning at the same ages but in two different birth cohorts showed similar results for the two groups. When researchers removed self-employed workers from the study sample, the same association between sustained low wages and memory decline was found.
“Our findings held up, which gave us a little more reassurance that what we were seeing is at least signaling there might be something there,” said Dr. Kezios.
She described the study as a “first pass” for documenting the harmful cognitive effects of consistently earning low wages.
It would be interesting, she said, to now determine whether there’s a “dose effect” for having a low salary. However, other studies with different designs would be needed to determine at what income level cognitive health starts to be protected and the impact of raising the minimum wage, she added.
Unique study
Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said the study was unique. “I don’t think we have seen anything like this before,” said Dr. Snyder.
The study, which links sustained low-wage earning in midlife to later memory decline, “is looking beyond some of the other measures we’ve seen when we looked at socioeconomic status,” she noted.
The results “beg the question” of whether people who earn low wages have less access to health care, she added.
“We should think about how to ensure access and equity around health care and around potential ways that may address components of risk individuals have during their life course,” Dr. Snyder said.
She noted that the study provides a “start” at considering potential policies to address the impact of sustained low wages on overall health, particularly cognitive health, throughout life.
The study had no outside funding. Dr. Kezios has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In a new analysis of more than 3,000 participants in the Health and Retirement Study, those who sustained low wages in midlife showed significantly faster memory decline than their peers who never earned low wages.
The findings could have implications for future public policy and research initiatives, the investigators noted.
“Our findings, which suggest a pattern of sustained low-wage earning is harmful for cognitive health, [are] broadly applicable to researchers across numerous health disciplines,” said co-investigator Katrina Kezios, PhD, postdoctoral researcher, department of epidemiology, Mailman School of Public Health, Columbia University, New York.
The findings were presented at the 2022 Alzheimer’s Association International Conference.
Growing number of low-wage workers
Low-wage workers make up a growing share of the U.S. labor market. Yet little research has examined the long-term relationship between earning low wages and memory decline.
The current investigators assessed 1992-2016 data from the Health and Retirement Study, a longitudinal survey of nationally representative samples of Americans aged 50 years and older. Study participants are interviewed every 2 years and provide, among other things, information on work-related factors, including hourly wages.
Memory function was measured at each visit from 2004 to 2016 using a memory composite score. The score included immediate and delayed word recall memory assessments. For those who became too impaired to complete cognitive assessment, memory tests by proxy informants were utilized.
On average, participants completed 4.8 memory assessments over the course of the study.
Researchers defined “low wage” as an hourly wage lower than two-thirds of the federal median wage for the corresponding year. They categorized low-wage exposure history as “never” or “intermittent” or “sustained” on the basis of wages earned from 1992 to 2004.
The current analysis included 3,803 participants, 1,913 of whom were men. All participants were born from 1936 to 1941. In 2004, the average age was 65 years, and the mean memory score was 1.15 standard units.
The investigators adjusted for factors that could confound the relationship between wages and cognition, including the participant’s education, parental education, household wealth, and marital status. Later, whether the participants’ occupation type was of low skill or not was also included.
Cognitive harm
The confounder-adjusted annual rate of memory decline among workers who never earned low wages was –0.12 standard units (95% confidence interval, –0.14 to –0.10).
Compared with these workers, memory decline was significantly faster among participants with sustained low wage–earning during midlife (beta for interaction between time and exposure group, –0.012; 95% CI, –0.02 to 0.01), corresponding to an annual rate of –0.13 standard units.
Put another way, the cognitive aging experienced by workers earning low wages over a 10-year period was equivalent to what workers who never earned low wages would experience over 11 years.
Although similar associations were found for men and women, it was stronger in magnitude for men – a finding Dr. Kezios said was somewhat surprising. She noted that women are commonly more at risk for dementia than men.
However, she advises caution in interpreting this finding, as there were so few men in the sustained low-wage group. “Women disproportionately make up the group of workers earning low wages,” she said.
The negative low coefficient found for those who persistently earned low wages was also observed for those who intermittently earned low wages, but this was not statistically significant.
“We can speculate or hypothesize the cumulative effect of earning low wages at each exposure interval produces more cognitive harm than maybe earning low wages at some time points over that exposure period,” said Dr. Kezios.
A sensitivity analysis that examined wage earning at the same ages but in two different birth cohorts showed similar results for the two groups. When researchers removed self-employed workers from the study sample, the same association between sustained low wages and memory decline was found.
“Our findings held up, which gave us a little more reassurance that what we were seeing is at least signaling there might be something there,” said Dr. Kezios.
She described the study as a “first pass” for documenting the harmful cognitive effects of consistently earning low wages.
It would be interesting, she said, to now determine whether there’s a “dose effect” for having a low salary. However, other studies with different designs would be needed to determine at what income level cognitive health starts to be protected and the impact of raising the minimum wage, she added.
Unique study
Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, said the study was unique. “I don’t think we have seen anything like this before,” said Dr. Snyder.
The study, which links sustained low-wage earning in midlife to later memory decline, “is looking beyond some of the other measures we’ve seen when we looked at socioeconomic status,” she noted.
The results “beg the question” of whether people who earn low wages have less access to health care, she added.
“We should think about how to ensure access and equity around health care and around potential ways that may address components of risk individuals have during their life course,” Dr. Snyder said.
She noted that the study provides a “start” at considering potential policies to address the impact of sustained low wages on overall health, particularly cognitive health, throughout life.
The study had no outside funding. Dr. Kezios has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From AAIC 2022
Individualized sensory care for older patients with dementia
Everyone gets by using environmental cues: For example, if you have to go to use the toilet in public, a bathroom sign prompts an immediate response. However, patients with dementia often struggle with environmental cues, which can complicate the already difficult task faced by their caregivers.
Individuals with dementia can lose awareness of such signs, and even colors, making it harder for them to interpret environmental cues.
The study, presented at the Alzheimer’s Association International Conference, recruited 30 pairs of patients and their caregivers. The approach is based on the Dunn model of sensory processing, which focuses on altering environments to maximize chances of success. It “explains that sensory processing is the information coming in, and then our ability to regulate and habituate to those sensations (creates) behavior,” Elizabeth Rhodus, MD, PhD, said during her talk. Dr. Rhodus is assistant professor of medicine at the University of Kentucky, Lexington.
Sensory-based interventions are not uncommon, but most are applied to pediatric populations and tend to focus on sensory processing disorders and autism spectrum disorder. The few programs that do focus on adults have varying methods and produce mixed results. Dr. Rhodus thinks that the key to success is individualization of therapy. “You’re going to like a certain sensation, and I might not like it. You can’t put us in the same room and expect the same results. We have to identify the preferences of how people interact with their environment, and what their brain does at a neuroscience level with that information,” she said.
Caregiving hacks
The program employs telehealth to work with caregivers so they can also create sensory environments within the home, using environment to trigger behavior.
For example, although individuals with dementia may have reduced response to color, the color red is unique. “Red is a cortical trigger. Red always stands out to people, so in our package that we send out as part of this intervention, we send out a roll of red duct tape,” said Dr. Rhodus.
An example of the use of red was a patient with dementia who had stopped drinking on his own, causing his caregiver daughter to be concerned that he would soon have to enter a nursing home. Examining the room, the occupational therapist realized that the water was kept out of sight, and suggested that the water glass be placed within the patient’s view, atop a square created with the red duct tape.
“These are just some of the simple concepts. They kind of seem easy. Some of my participants call them caregiving hacks, but it’s things that are grounded in neuroscience – how the brain processes the environment, and then how can we plug in supports and cues in whatever area is missing,” said Dr. Rhodus.
In the program, the caregiver fills out several online surveys, and an occupational therapist conducts an interview to identify specific challenges, such as bathing, or using the toilet, or going to church. Then an adult sensory profile reveals how the patient perceives his or her environment. “It’s taking those individual pieces, and then boiling it down to these mechanisms at the behavioral and neuroscience level,” said Dr. Rhodus. She said the entire setup process takes about an hour.
Impactful care
The individualized approach of the HARMONY (Helping Older Adults Create and Manage Occupations Successfully) method is promising, according to Monika Gross, executive director of the Poise Project, which uses the Alexander Technique to help people with chronic conditions such as Parkinson’s disease.
“Although it’s always a very simple idea that human beings need sensory processing aspects in their lives, from the time they’re infants through to the end of life, we don’t really focus on the end of life in a way that can bring meaning between the care partner and the person living with dementia. The other thing that was impressive is that this is in a rural community, where there often aren’t a lot of resources available, (such as) classes that the care partner can take their loved one to. So having something where the care partner has some confidence that they can really make an impact in that person that they are seeing decline, that they can see their behavior change [is good],” said Ms. Gross.
Empowering caregivers
The study included 30 pairs of patients and caregivers who were randomized to the individualized care (I), standardized care, or a control group. Adherence to weekly visits was high (I, 88%; S, 100%; C, 60%; P = .061). Retention was strong (I, 80%; S, 60%; C, 50%).
“It was feasible ... and at the end, we found a significant improvement in care partner satisfaction. We actually empowered these people to care for their loved ones, and in doing that, and helping them set up environmental cues, it allowed that person to perform at a more independent level,” said Dr. Rhodus.
The trial was only a proof of concept, so although the researchers saw signs of efficacy, it wasn’t powered to show that. They are currently enrolling additional patients and caregivers for larger studies to further test the approach.
Dr. Rhodus and Ms. Gross have no relevant financial disclosures.
Everyone gets by using environmental cues: For example, if you have to go to use the toilet in public, a bathroom sign prompts an immediate response. However, patients with dementia often struggle with environmental cues, which can complicate the already difficult task faced by their caregivers.
Individuals with dementia can lose awareness of such signs, and even colors, making it harder for them to interpret environmental cues.
The study, presented at the Alzheimer’s Association International Conference, recruited 30 pairs of patients and their caregivers. The approach is based on the Dunn model of sensory processing, which focuses on altering environments to maximize chances of success. It “explains that sensory processing is the information coming in, and then our ability to regulate and habituate to those sensations (creates) behavior,” Elizabeth Rhodus, MD, PhD, said during her talk. Dr. Rhodus is assistant professor of medicine at the University of Kentucky, Lexington.
Sensory-based interventions are not uncommon, but most are applied to pediatric populations and tend to focus on sensory processing disorders and autism spectrum disorder. The few programs that do focus on adults have varying methods and produce mixed results. Dr. Rhodus thinks that the key to success is individualization of therapy. “You’re going to like a certain sensation, and I might not like it. You can’t put us in the same room and expect the same results. We have to identify the preferences of how people interact with their environment, and what their brain does at a neuroscience level with that information,” she said.
Caregiving hacks
The program employs telehealth to work with caregivers so they can also create sensory environments within the home, using environment to trigger behavior.
For example, although individuals with dementia may have reduced response to color, the color red is unique. “Red is a cortical trigger. Red always stands out to people, so in our package that we send out as part of this intervention, we send out a roll of red duct tape,” said Dr. Rhodus.
An example of the use of red was a patient with dementia who had stopped drinking on his own, causing his caregiver daughter to be concerned that he would soon have to enter a nursing home. Examining the room, the occupational therapist realized that the water was kept out of sight, and suggested that the water glass be placed within the patient’s view, atop a square created with the red duct tape.
“These are just some of the simple concepts. They kind of seem easy. Some of my participants call them caregiving hacks, but it’s things that are grounded in neuroscience – how the brain processes the environment, and then how can we plug in supports and cues in whatever area is missing,” said Dr. Rhodus.
In the program, the caregiver fills out several online surveys, and an occupational therapist conducts an interview to identify specific challenges, such as bathing, or using the toilet, or going to church. Then an adult sensory profile reveals how the patient perceives his or her environment. “It’s taking those individual pieces, and then boiling it down to these mechanisms at the behavioral and neuroscience level,” said Dr. Rhodus. She said the entire setup process takes about an hour.
Impactful care
The individualized approach of the HARMONY (Helping Older Adults Create and Manage Occupations Successfully) method is promising, according to Monika Gross, executive director of the Poise Project, which uses the Alexander Technique to help people with chronic conditions such as Parkinson’s disease.
“Although it’s always a very simple idea that human beings need sensory processing aspects in their lives, from the time they’re infants through to the end of life, we don’t really focus on the end of life in a way that can bring meaning between the care partner and the person living with dementia. The other thing that was impressive is that this is in a rural community, where there often aren’t a lot of resources available, (such as) classes that the care partner can take their loved one to. So having something where the care partner has some confidence that they can really make an impact in that person that they are seeing decline, that they can see their behavior change [is good],” said Ms. Gross.
Empowering caregivers
The study included 30 pairs of patients and caregivers who were randomized to the individualized care (I), standardized care, or a control group. Adherence to weekly visits was high (I, 88%; S, 100%; C, 60%; P = .061). Retention was strong (I, 80%; S, 60%; C, 50%).
“It was feasible ... and at the end, we found a significant improvement in care partner satisfaction. We actually empowered these people to care for their loved ones, and in doing that, and helping them set up environmental cues, it allowed that person to perform at a more independent level,” said Dr. Rhodus.
The trial was only a proof of concept, so although the researchers saw signs of efficacy, it wasn’t powered to show that. They are currently enrolling additional patients and caregivers for larger studies to further test the approach.
Dr. Rhodus and Ms. Gross have no relevant financial disclosures.
Everyone gets by using environmental cues: For example, if you have to go to use the toilet in public, a bathroom sign prompts an immediate response. However, patients with dementia often struggle with environmental cues, which can complicate the already difficult task faced by their caregivers.
Individuals with dementia can lose awareness of such signs, and even colors, making it harder for them to interpret environmental cues.
The study, presented at the Alzheimer’s Association International Conference, recruited 30 pairs of patients and their caregivers. The approach is based on the Dunn model of sensory processing, which focuses on altering environments to maximize chances of success. It “explains that sensory processing is the information coming in, and then our ability to regulate and habituate to those sensations (creates) behavior,” Elizabeth Rhodus, MD, PhD, said during her talk. Dr. Rhodus is assistant professor of medicine at the University of Kentucky, Lexington.
Sensory-based interventions are not uncommon, but most are applied to pediatric populations and tend to focus on sensory processing disorders and autism spectrum disorder. The few programs that do focus on adults have varying methods and produce mixed results. Dr. Rhodus thinks that the key to success is individualization of therapy. “You’re going to like a certain sensation, and I might not like it. You can’t put us in the same room and expect the same results. We have to identify the preferences of how people interact with their environment, and what their brain does at a neuroscience level with that information,” she said.
Caregiving hacks
The program employs telehealth to work with caregivers so they can also create sensory environments within the home, using environment to trigger behavior.
For example, although individuals with dementia may have reduced response to color, the color red is unique. “Red is a cortical trigger. Red always stands out to people, so in our package that we send out as part of this intervention, we send out a roll of red duct tape,” said Dr. Rhodus.
An example of the use of red was a patient with dementia who had stopped drinking on his own, causing his caregiver daughter to be concerned that he would soon have to enter a nursing home. Examining the room, the occupational therapist realized that the water was kept out of sight, and suggested that the water glass be placed within the patient’s view, atop a square created with the red duct tape.
“These are just some of the simple concepts. They kind of seem easy. Some of my participants call them caregiving hacks, but it’s things that are grounded in neuroscience – how the brain processes the environment, and then how can we plug in supports and cues in whatever area is missing,” said Dr. Rhodus.
In the program, the caregiver fills out several online surveys, and an occupational therapist conducts an interview to identify specific challenges, such as bathing, or using the toilet, or going to church. Then an adult sensory profile reveals how the patient perceives his or her environment. “It’s taking those individual pieces, and then boiling it down to these mechanisms at the behavioral and neuroscience level,” said Dr. Rhodus. She said the entire setup process takes about an hour.
Impactful care
The individualized approach of the HARMONY (Helping Older Adults Create and Manage Occupations Successfully) method is promising, according to Monika Gross, executive director of the Poise Project, which uses the Alexander Technique to help people with chronic conditions such as Parkinson’s disease.
“Although it’s always a very simple idea that human beings need sensory processing aspects in their lives, from the time they’re infants through to the end of life, we don’t really focus on the end of life in a way that can bring meaning between the care partner and the person living with dementia. The other thing that was impressive is that this is in a rural community, where there often aren’t a lot of resources available, (such as) classes that the care partner can take their loved one to. So having something where the care partner has some confidence that they can really make an impact in that person that they are seeing decline, that they can see their behavior change [is good],” said Ms. Gross.
Empowering caregivers
The study included 30 pairs of patients and caregivers who were randomized to the individualized care (I), standardized care, or a control group. Adherence to weekly visits was high (I, 88%; S, 100%; C, 60%; P = .061). Retention was strong (I, 80%; S, 60%; C, 50%).
“It was feasible ... and at the end, we found a significant improvement in care partner satisfaction. We actually empowered these people to care for their loved ones, and in doing that, and helping them set up environmental cues, it allowed that person to perform at a more independent level,” said Dr. Rhodus.
The trial was only a proof of concept, so although the researchers saw signs of efficacy, it wasn’t powered to show that. They are currently enrolling additional patients and caregivers for larger studies to further test the approach.
Dr. Rhodus and Ms. Gross have no relevant financial disclosures.
FROM AAIC 2022

