User login
TIME: CV events similar with evening or morning dose of BP meds
BARCELONA – Patients with hypertension who took their antihypertensive medication in the evening or in the morning had similar rates of cardiovascular events over the following 5 years, in the much-anticipated TIME trial.
The trial, which contradicts several previous studies suggesting that evening dosing may be better, was presented at the annual congress of the European Society of Cardiology.
“The key message from this study is that taking antihypertensive medication in the evening makes no difference at all from taking it in the morning for the prevention of heart attacks, strokes, and vascular deaths,” concluded TIME lead investigator Tom MacDonald, MBChB, MD, professor of clinical pharmacology & pharmacoepidemiology at the University of Dundee (Scotland).
The hazard ratio was 0.95 for the primary endpoint, a composite of hospitalization for nonfatal myocardial infarction, nonfatal stroke, or vascular death, in the intention-to-treat population.
Similar results, with a hazard ratio around 1, were seen for all the secondary outcomes and in all subgroups.
“There is nothing to see – not a smidge of a difference – in the primary outcome or any of the secondary outcomes,” Dr. MacDonald commented.
The study also showed that evening dosing was not harmful in terms of falls or other adverse effects. Dr. MacDonald explained that taking the medication at night could result in an increase in nocturnal hypotension that may translate into more dizziness and falls if patients get up to use the bathroom during the night. “But, if anything, there were more dizzy turns during the day. The rate of fractures and hospitalization for fractures were identical in the two groups,” he reported.
“Our take-home message is that patients can take their blood pressure tablets at any time they like – whenever is most convenient – as long as they take them. It’s probably best to get into a routine of taking your tablets at the same time every day. That way you are more likely to remember to take them – but it won’t matter if that is in the morning or in the evening,” he said.
Non-dippers
Dr. MacDonald explained that the rationale for the study was that in some patients blood pressure does not drop at night, a group known as “non-dippers,” and nighttime blood pressure is the best predictor of bad outcomes. In addition, previous studies have suggested that evening dosing of antihypertensives reduces nighttime blood pressure more effectively than daytime dosing.
“We and others thought that giving medication in the evening so that its peak effect occurs during the night might be beneficial,” he said. “We did the trial because if it had turned out that taking tablets in the evening was beneficial, it would have been one of the cheapest and most cost-effective interventions known to man. It is a nice hypothesis and most people thought this would turn out with a benefit, but it actually didn’t.”
The study did find some differences in the blood pressure profile between the two dosing schedules.
“Our results show that when antihypertensive medication is taken in the morning, then blood pressure is higher in the morning and lower in the evening. With evening dosing, blood pressure is lower in the morning and higher in the evening. It’s not a huge difference – just 1-2 mm Hg – and this didn’t translate into any difference in outcomes,” Dr. MacDonald said.
“Ideally we need medication that lowers blood pressure effectively over the whole 24-hour period. That is where the push should be,” he added.
The TIME study randomized 21,104 patients with treated hypertension to take their antihypertensive medication in the morning or in the evening. Baseline characteristics show the average age of participants was 65 years, 14% had diabetes, 4% were smokers, 13% had prior cardiovascular disease, and mean blood pressure at entry was 135/79 mmHg.
TIME was a pragmatic study, with participants recruited from primary and secondary care registering on the Internet, and information on hospitalizations and deaths obtained from participants by email and through record linkage to national databases, with further data gathered from family doctors and hospitals and independently adjudicated by a blinded committee.
The median follow-up duration was 5.2 years, but some patients were followed for over 9 years.
The primary endpoint occurred in 362 (3.4%) participants in the evening-dosing group (0.69 events per 100 patient-years) and 390 (3.7%) in the morning-dosing group (0.72 events per 100 patient-years), giving an unadjusted hazard ratio of 0.95 (95% confidence interval, 0.83-1.10; P = .53).
What to recommend in clinical practice?
Outside commentators had mixed opinions on how the TIME results should be applied to clinical practice.
Discussant of the TIME study at the ESC Hotline session, Rhian Touyz, MBBCh, University of Glasgow (Scotland), said the trial asked a “very pertinent” question and the data “are certainly provocative.”
She cited several previous studies suggesting that evening dosing improved nighttime blood pressure and reduced cardiovascular events.
“The finding of no difference in event rate in the TIME study is therefore very intriguing.”
She pointed out that other studies have shown benefit of nighttime dosing in certain patient groups such as those with sleep apnea, non-dippers, and those with nocturnal hypertension.
“With all these previous data, we have to ask why the TIME trial has produced this unexpected result,” she said.
Dr. MacDonald replied that the study was completely neutral. “That is the result, and I believe it is definitive. I’m absolutely confident that we did the study as best we could. All events were adjudicated. Compliance was quite good at 60%. I can’t believe there is anything in our data that invalidates these results,” he said. “If we want to look at specific groups of patients then we have to do larger studies in those particular groups, but for a general population of hypertensive patients we didn’t find any difference at all in morning versus evening dosing.”
The TIME results are in direct contradiction of a previous high-profile study – the Hygia Chronotherapy Trial – published in 2020, which found a large protective effect of nocturnal dosing on cardiovascular events, and attracted much media attention. But this study has subsequently attracted criticism, with an “expression of concern” and a commentary raising several questions.
And a systematic review from the International Society of Hypertension published earlier this month concludes that previous trials of bedtime antihypertensive dosing had “major flaws.”
The review notes that three ongoing, well-designed, prospective, randomized controlled outcome trials are expected to provide high-quality data on the efficacy and safety of evening or bedtime versus morning drug dosing.
“Until that information is available, preferred use of bedtime drug dosing of antihypertensive drugs should not be routinely recommended in clinical practice. Complete 24-h control of BP should be targeted using readily available, long-acting antihypertensive medications as monotherapy or combinations administered in a single morning dose,” it concludes.
On the new TIME results, lead author of the ISH review, George Stergiou, MD, commented: “The benefits of bedtime dosing were not confirmed – as we well expected. So, I think bedtime drug dosing should not be routinely recommended in clinical practice.”
Although the TIME trial did not show any harms with bedtime dose, Dr. Stergiou added, “I am not too happy with their conclusion that patients should do as they wish. The vast majority of well-conducted outcomes studies which we use to guide the treatment of hypertension administered all drugs in the morning.”
One of the authors of the commentary criticizing the Hygia trial, Sverre E. Kjeldsen, MD, University of Oslo, said in an interview that the TIME trial was an important study, far more reliable than the Hygia study, and the results were as expected.
“From a scientific point of view, patients have a choice as to when to take their medication, but we strongly recommend taking blood pressure meds in the morning. Adherence is proven to be worse at bedtime. However, physicians may still consider bedtime dosing in patients proven to have high night-time blood pressure,” Dr. Kjeldsen added.
Lead investigator of the Hygia study, Ramón C. Hermida, PhD, University of Vigo (Spain), told this news organization he and his coauthors are standing by their results.
“The design and conduct of the TIME trial does not comply with the quality requirements listed in the guidelines by the International Society for Chronobiology for conducting chronotherapy trials in hypertension, and the results are not in line with the reported findings of multiple clinical trials on the effects of timed hypertension treatment on blood pressure control and circadian pattern regulation, kidney function, and cardiac pathology,” Dr. Hermida said.
Chair of an ESC press conference on the TIME study, Steen Dalby Kristensen, MD, Aarhus University Hospital, Skejby, Denmark, said he thought the trial was “very well done.”
The TIME results, he said, “are quite clear, whether you take your blood pressure tablets in the morning or the evening it makes no difference for the hard outcomes that we fear in patients with hypertension.
“I think that this solves a question that we’ve had for a long time now,” he commented. “Even though there were some changes in the blood pressure measured in the evening or in the morning it doesn’t seem to matter in terms of clinical events. This means that life might be a bit easier for patients in that they can choose when they take their medication at the time most convenient to them.
“I don’t know why previous studies suggested such a big benefit of evening dosing,” he added. “I would say the TIME trial is a more definitive result. It is a very important trial.”
Dipti Itchhaporia, MD, University of California, Irvine, and immediate past president of the American College of Cardiology, agreed that the TIME study was well conducted.
“On the basis of these results I wouldn’t recommend a specific time,” she said. “That’s kind of a relief, as it can be difficult to always take medications at a set time and this gives patients more flexibility.”
She suggested a possible alternative approach for patients taking more than one drug – taking one in the morning and the other in the evening. “That might give better 24-hour coverage.”
The study was funded by the British Heart Foundation. Dr. MacDonald has reported receiving research funding from Novartis and consulting fees from Novartis and AstraZeneca.
A version of this article first appeared on Medscape.com.
BARCELONA – Patients with hypertension who took their antihypertensive medication in the evening or in the morning had similar rates of cardiovascular events over the following 5 years, in the much-anticipated TIME trial.
The trial, which contradicts several previous studies suggesting that evening dosing may be better, was presented at the annual congress of the European Society of Cardiology.
“The key message from this study is that taking antihypertensive medication in the evening makes no difference at all from taking it in the morning for the prevention of heart attacks, strokes, and vascular deaths,” concluded TIME lead investigator Tom MacDonald, MBChB, MD, professor of clinical pharmacology & pharmacoepidemiology at the University of Dundee (Scotland).
The hazard ratio was 0.95 for the primary endpoint, a composite of hospitalization for nonfatal myocardial infarction, nonfatal stroke, or vascular death, in the intention-to-treat population.
Similar results, with a hazard ratio around 1, were seen for all the secondary outcomes and in all subgroups.
“There is nothing to see – not a smidge of a difference – in the primary outcome or any of the secondary outcomes,” Dr. MacDonald commented.
The study also showed that evening dosing was not harmful in terms of falls or other adverse effects. Dr. MacDonald explained that taking the medication at night could result in an increase in nocturnal hypotension that may translate into more dizziness and falls if patients get up to use the bathroom during the night. “But, if anything, there were more dizzy turns during the day. The rate of fractures and hospitalization for fractures were identical in the two groups,” he reported.
“Our take-home message is that patients can take their blood pressure tablets at any time they like – whenever is most convenient – as long as they take them. It’s probably best to get into a routine of taking your tablets at the same time every day. That way you are more likely to remember to take them – but it won’t matter if that is in the morning or in the evening,” he said.
Non-dippers
Dr. MacDonald explained that the rationale for the study was that in some patients blood pressure does not drop at night, a group known as “non-dippers,” and nighttime blood pressure is the best predictor of bad outcomes. In addition, previous studies have suggested that evening dosing of antihypertensives reduces nighttime blood pressure more effectively than daytime dosing.
“We and others thought that giving medication in the evening so that its peak effect occurs during the night might be beneficial,” he said. “We did the trial because if it had turned out that taking tablets in the evening was beneficial, it would have been one of the cheapest and most cost-effective interventions known to man. It is a nice hypothesis and most people thought this would turn out with a benefit, but it actually didn’t.”
The study did find some differences in the blood pressure profile between the two dosing schedules.
“Our results show that when antihypertensive medication is taken in the morning, then blood pressure is higher in the morning and lower in the evening. With evening dosing, blood pressure is lower in the morning and higher in the evening. It’s not a huge difference – just 1-2 mm Hg – and this didn’t translate into any difference in outcomes,” Dr. MacDonald said.
“Ideally we need medication that lowers blood pressure effectively over the whole 24-hour period. That is where the push should be,” he added.
The TIME study randomized 21,104 patients with treated hypertension to take their antihypertensive medication in the morning or in the evening. Baseline characteristics show the average age of participants was 65 years, 14% had diabetes, 4% were smokers, 13% had prior cardiovascular disease, and mean blood pressure at entry was 135/79 mmHg.
TIME was a pragmatic study, with participants recruited from primary and secondary care registering on the Internet, and information on hospitalizations and deaths obtained from participants by email and through record linkage to national databases, with further data gathered from family doctors and hospitals and independently adjudicated by a blinded committee.
The median follow-up duration was 5.2 years, but some patients were followed for over 9 years.
The primary endpoint occurred in 362 (3.4%) participants in the evening-dosing group (0.69 events per 100 patient-years) and 390 (3.7%) in the morning-dosing group (0.72 events per 100 patient-years), giving an unadjusted hazard ratio of 0.95 (95% confidence interval, 0.83-1.10; P = .53).
What to recommend in clinical practice?
Outside commentators had mixed opinions on how the TIME results should be applied to clinical practice.
Discussant of the TIME study at the ESC Hotline session, Rhian Touyz, MBBCh, University of Glasgow (Scotland), said the trial asked a “very pertinent” question and the data “are certainly provocative.”
She cited several previous studies suggesting that evening dosing improved nighttime blood pressure and reduced cardiovascular events.
“The finding of no difference in event rate in the TIME study is therefore very intriguing.”
She pointed out that other studies have shown benefit of nighttime dosing in certain patient groups such as those with sleep apnea, non-dippers, and those with nocturnal hypertension.
“With all these previous data, we have to ask why the TIME trial has produced this unexpected result,” she said.
Dr. MacDonald replied that the study was completely neutral. “That is the result, and I believe it is definitive. I’m absolutely confident that we did the study as best we could. All events were adjudicated. Compliance was quite good at 60%. I can’t believe there is anything in our data that invalidates these results,” he said. “If we want to look at specific groups of patients then we have to do larger studies in those particular groups, but for a general population of hypertensive patients we didn’t find any difference at all in morning versus evening dosing.”
The TIME results are in direct contradiction of a previous high-profile study – the Hygia Chronotherapy Trial – published in 2020, which found a large protective effect of nocturnal dosing on cardiovascular events, and attracted much media attention. But this study has subsequently attracted criticism, with an “expression of concern” and a commentary raising several questions.
And a systematic review from the International Society of Hypertension published earlier this month concludes that previous trials of bedtime antihypertensive dosing had “major flaws.”
The review notes that three ongoing, well-designed, prospective, randomized controlled outcome trials are expected to provide high-quality data on the efficacy and safety of evening or bedtime versus morning drug dosing.
“Until that information is available, preferred use of bedtime drug dosing of antihypertensive drugs should not be routinely recommended in clinical practice. Complete 24-h control of BP should be targeted using readily available, long-acting antihypertensive medications as monotherapy or combinations administered in a single morning dose,” it concludes.
On the new TIME results, lead author of the ISH review, George Stergiou, MD, commented: “The benefits of bedtime dosing were not confirmed – as we well expected. So, I think bedtime drug dosing should not be routinely recommended in clinical practice.”
Although the TIME trial did not show any harms with bedtime dose, Dr. Stergiou added, “I am not too happy with their conclusion that patients should do as they wish. The vast majority of well-conducted outcomes studies which we use to guide the treatment of hypertension administered all drugs in the morning.”
One of the authors of the commentary criticizing the Hygia trial, Sverre E. Kjeldsen, MD, University of Oslo, said in an interview that the TIME trial was an important study, far more reliable than the Hygia study, and the results were as expected.
“From a scientific point of view, patients have a choice as to when to take their medication, but we strongly recommend taking blood pressure meds in the morning. Adherence is proven to be worse at bedtime. However, physicians may still consider bedtime dosing in patients proven to have high night-time blood pressure,” Dr. Kjeldsen added.
Lead investigator of the Hygia study, Ramón C. Hermida, PhD, University of Vigo (Spain), told this news organization he and his coauthors are standing by their results.
“The design and conduct of the TIME trial does not comply with the quality requirements listed in the guidelines by the International Society for Chronobiology for conducting chronotherapy trials in hypertension, and the results are not in line with the reported findings of multiple clinical trials on the effects of timed hypertension treatment on blood pressure control and circadian pattern regulation, kidney function, and cardiac pathology,” Dr. Hermida said.
Chair of an ESC press conference on the TIME study, Steen Dalby Kristensen, MD, Aarhus University Hospital, Skejby, Denmark, said he thought the trial was “very well done.”
The TIME results, he said, “are quite clear, whether you take your blood pressure tablets in the morning or the evening it makes no difference for the hard outcomes that we fear in patients with hypertension.
“I think that this solves a question that we’ve had for a long time now,” he commented. “Even though there were some changes in the blood pressure measured in the evening or in the morning it doesn’t seem to matter in terms of clinical events. This means that life might be a bit easier for patients in that they can choose when they take their medication at the time most convenient to them.
“I don’t know why previous studies suggested such a big benefit of evening dosing,” he added. “I would say the TIME trial is a more definitive result. It is a very important trial.”
Dipti Itchhaporia, MD, University of California, Irvine, and immediate past president of the American College of Cardiology, agreed that the TIME study was well conducted.
“On the basis of these results I wouldn’t recommend a specific time,” she said. “That’s kind of a relief, as it can be difficult to always take medications at a set time and this gives patients more flexibility.”
She suggested a possible alternative approach for patients taking more than one drug – taking one in the morning and the other in the evening. “That might give better 24-hour coverage.”
The study was funded by the British Heart Foundation. Dr. MacDonald has reported receiving research funding from Novartis and consulting fees from Novartis and AstraZeneca.
A version of this article first appeared on Medscape.com.
BARCELONA – Patients with hypertension who took their antihypertensive medication in the evening or in the morning had similar rates of cardiovascular events over the following 5 years, in the much-anticipated TIME trial.
The trial, which contradicts several previous studies suggesting that evening dosing may be better, was presented at the annual congress of the European Society of Cardiology.
“The key message from this study is that taking antihypertensive medication in the evening makes no difference at all from taking it in the morning for the prevention of heart attacks, strokes, and vascular deaths,” concluded TIME lead investigator Tom MacDonald, MBChB, MD, professor of clinical pharmacology & pharmacoepidemiology at the University of Dundee (Scotland).
The hazard ratio was 0.95 for the primary endpoint, a composite of hospitalization for nonfatal myocardial infarction, nonfatal stroke, or vascular death, in the intention-to-treat population.
Similar results, with a hazard ratio around 1, were seen for all the secondary outcomes and in all subgroups.
“There is nothing to see – not a smidge of a difference – in the primary outcome or any of the secondary outcomes,” Dr. MacDonald commented.
The study also showed that evening dosing was not harmful in terms of falls or other adverse effects. Dr. MacDonald explained that taking the medication at night could result in an increase in nocturnal hypotension that may translate into more dizziness and falls if patients get up to use the bathroom during the night. “But, if anything, there were more dizzy turns during the day. The rate of fractures and hospitalization for fractures were identical in the two groups,” he reported.
“Our take-home message is that patients can take their blood pressure tablets at any time they like – whenever is most convenient – as long as they take them. It’s probably best to get into a routine of taking your tablets at the same time every day. That way you are more likely to remember to take them – but it won’t matter if that is in the morning or in the evening,” he said.
Non-dippers
Dr. MacDonald explained that the rationale for the study was that in some patients blood pressure does not drop at night, a group known as “non-dippers,” and nighttime blood pressure is the best predictor of bad outcomes. In addition, previous studies have suggested that evening dosing of antihypertensives reduces nighttime blood pressure more effectively than daytime dosing.
“We and others thought that giving medication in the evening so that its peak effect occurs during the night might be beneficial,” he said. “We did the trial because if it had turned out that taking tablets in the evening was beneficial, it would have been one of the cheapest and most cost-effective interventions known to man. It is a nice hypothesis and most people thought this would turn out with a benefit, but it actually didn’t.”
The study did find some differences in the blood pressure profile between the two dosing schedules.
“Our results show that when antihypertensive medication is taken in the morning, then blood pressure is higher in the morning and lower in the evening. With evening dosing, blood pressure is lower in the morning and higher in the evening. It’s not a huge difference – just 1-2 mm Hg – and this didn’t translate into any difference in outcomes,” Dr. MacDonald said.
“Ideally we need medication that lowers blood pressure effectively over the whole 24-hour period. That is where the push should be,” he added.
The TIME study randomized 21,104 patients with treated hypertension to take their antihypertensive medication in the morning or in the evening. Baseline characteristics show the average age of participants was 65 years, 14% had diabetes, 4% were smokers, 13% had prior cardiovascular disease, and mean blood pressure at entry was 135/79 mmHg.
TIME was a pragmatic study, with participants recruited from primary and secondary care registering on the Internet, and information on hospitalizations and deaths obtained from participants by email and through record linkage to national databases, with further data gathered from family doctors and hospitals and independently adjudicated by a blinded committee.
The median follow-up duration was 5.2 years, but some patients were followed for over 9 years.
The primary endpoint occurred in 362 (3.4%) participants in the evening-dosing group (0.69 events per 100 patient-years) and 390 (3.7%) in the morning-dosing group (0.72 events per 100 patient-years), giving an unadjusted hazard ratio of 0.95 (95% confidence interval, 0.83-1.10; P = .53).
What to recommend in clinical practice?
Outside commentators had mixed opinions on how the TIME results should be applied to clinical practice.
Discussant of the TIME study at the ESC Hotline session, Rhian Touyz, MBBCh, University of Glasgow (Scotland), said the trial asked a “very pertinent” question and the data “are certainly provocative.”
She cited several previous studies suggesting that evening dosing improved nighttime blood pressure and reduced cardiovascular events.
“The finding of no difference in event rate in the TIME study is therefore very intriguing.”
She pointed out that other studies have shown benefit of nighttime dosing in certain patient groups such as those with sleep apnea, non-dippers, and those with nocturnal hypertension.
“With all these previous data, we have to ask why the TIME trial has produced this unexpected result,” she said.
Dr. MacDonald replied that the study was completely neutral. “That is the result, and I believe it is definitive. I’m absolutely confident that we did the study as best we could. All events were adjudicated. Compliance was quite good at 60%. I can’t believe there is anything in our data that invalidates these results,” he said. “If we want to look at specific groups of patients then we have to do larger studies in those particular groups, but for a general population of hypertensive patients we didn’t find any difference at all in morning versus evening dosing.”
The TIME results are in direct contradiction of a previous high-profile study – the Hygia Chronotherapy Trial – published in 2020, which found a large protective effect of nocturnal dosing on cardiovascular events, and attracted much media attention. But this study has subsequently attracted criticism, with an “expression of concern” and a commentary raising several questions.
And a systematic review from the International Society of Hypertension published earlier this month concludes that previous trials of bedtime antihypertensive dosing had “major flaws.”
The review notes that three ongoing, well-designed, prospective, randomized controlled outcome trials are expected to provide high-quality data on the efficacy and safety of evening or bedtime versus morning drug dosing.
“Until that information is available, preferred use of bedtime drug dosing of antihypertensive drugs should not be routinely recommended in clinical practice. Complete 24-h control of BP should be targeted using readily available, long-acting antihypertensive medications as monotherapy or combinations administered in a single morning dose,” it concludes.
On the new TIME results, lead author of the ISH review, George Stergiou, MD, commented: “The benefits of bedtime dosing were not confirmed – as we well expected. So, I think bedtime drug dosing should not be routinely recommended in clinical practice.”
Although the TIME trial did not show any harms with bedtime dose, Dr. Stergiou added, “I am not too happy with their conclusion that patients should do as they wish. The vast majority of well-conducted outcomes studies which we use to guide the treatment of hypertension administered all drugs in the morning.”
One of the authors of the commentary criticizing the Hygia trial, Sverre E. Kjeldsen, MD, University of Oslo, said in an interview that the TIME trial was an important study, far more reliable than the Hygia study, and the results were as expected.
“From a scientific point of view, patients have a choice as to when to take their medication, but we strongly recommend taking blood pressure meds in the morning. Adherence is proven to be worse at bedtime. However, physicians may still consider bedtime dosing in patients proven to have high night-time blood pressure,” Dr. Kjeldsen added.
Lead investigator of the Hygia study, Ramón C. Hermida, PhD, University of Vigo (Spain), told this news organization he and his coauthors are standing by their results.
“The design and conduct of the TIME trial does not comply with the quality requirements listed in the guidelines by the International Society for Chronobiology for conducting chronotherapy trials in hypertension, and the results are not in line with the reported findings of multiple clinical trials on the effects of timed hypertension treatment on blood pressure control and circadian pattern regulation, kidney function, and cardiac pathology,” Dr. Hermida said.
Chair of an ESC press conference on the TIME study, Steen Dalby Kristensen, MD, Aarhus University Hospital, Skejby, Denmark, said he thought the trial was “very well done.”
The TIME results, he said, “are quite clear, whether you take your blood pressure tablets in the morning or the evening it makes no difference for the hard outcomes that we fear in patients with hypertension.
“I think that this solves a question that we’ve had for a long time now,” he commented. “Even though there were some changes in the blood pressure measured in the evening or in the morning it doesn’t seem to matter in terms of clinical events. This means that life might be a bit easier for patients in that they can choose when they take their medication at the time most convenient to them.
“I don’t know why previous studies suggested such a big benefit of evening dosing,” he added. “I would say the TIME trial is a more definitive result. It is a very important trial.”
Dipti Itchhaporia, MD, University of California, Irvine, and immediate past president of the American College of Cardiology, agreed that the TIME study was well conducted.
“On the basis of these results I wouldn’t recommend a specific time,” she said. “That’s kind of a relief, as it can be difficult to always take medications at a set time and this gives patients more flexibility.”
She suggested a possible alternative approach for patients taking more than one drug – taking one in the morning and the other in the evening. “That might give better 24-hour coverage.”
The study was funded by the British Heart Foundation. Dr. MacDonald has reported receiving research funding from Novartis and consulting fees from Novartis and AstraZeneca.
A version of this article first appeared on Medscape.com.
AT ESC CONGRESS 2022
Body contouring tops list of cosmetic procedures with adverse event reports
of data from the Manufacturer and User Facility Device Experience (MAUDE).
The number of noninvasive body-contouring procedures performed in the United States increased by fivefold from 2011 to 2019, attributed in part to a combination of improved technology and new medical devices, as well as a “cosmetically savvy consumer base heavily influenced by social media,” wrote Young Lim, MD, PhD, of the department of dermatology, Massachusetts General Hospital, Boston, and coauthors.
However, premarket evaluations of many new medical devices fail to capture rare or delayed onset complications, and consumers and providers may not be fully aware of potential adverse events, they said. The MAUDE database was created by the Food and Drug Administration in 1991 to collect information on device-related deaths, serious injuries, or malfunctions based on reports from manufacturers, patients, and health care providers.
The researchers used the MAUDE database to identify and highlight adverse events associated with noninvasive body contouring technology in order to improve patient safety and satisfaction.
In their report, published in Lasers in Surgery and Medicine, they analyzed 723 medical device reports (MDRs) reported between 2015 and 2021: 660 for noninvasive body contouring, 55 for cellulite treatments, and 8 for muscle stimulation.
“Notably, of the 723 total MDRs between 2015 and 2021, 515 (71.2%) were reported in 2021, with the next highest reported being 64 in 2019 (8.8%),” the researchers wrote.
Overall, paradoxical hyperplasia (PAH) accounted for the majority of adverse reactions in the noninvasive body-contouring category (73.2%). In PAH, patients develop additional adipose tissue in areas treated with cryolipolysis. In this study, all reports of PAH as well as all 47 reported cases of abdominal hernias were attributed to the CoolSculpting device.
For cellulite treatments, the most common MDRs – 11 of 55 – were scars and keloids (20%). The Cellfina subcision technique accounted for 47% (26 of 55) of the MDRs in this category, including 9 of the scar and keloid cases.
Only eight of the MDRs analyzed were in the muscle stimulation category; of these, burns were the most common adverse event and accounted for three of the reports. The other reported AEs were two cases of pain and one report each of electrical shock, urticaria, and arrhythmia.
Patients are increasingly opting for noninvasive cosmetic procedures, but adverse events may be underreported despite the existence of databases such as MAUDE, the researchers wrote in their discussion.
“PAH, first reported in 2014 as an adverse sequelae of cryolipolysis, remains without known pathophysiology, though it proportionately affects men more than women,” they noted. The incidence of PAH varies widely, and the current treatment of choice is power-assisted liposuction, they said, although surgical abdominoplasty may be needed in severe cases.
The findings were limited by several factors including the reliance of the quality of submissions, the selection biases of the MAUDE database, and the potential for underreporting, the researchers noted.
However, “by cataloging the AEs of the growing noninvasive cosmetics market, the MAUDE can educate providers and inform patients to maximize safety and efficacy,” they said.
The size of the database and volume of reports provides a picture that likely reflects overall trends occurring in clinical practice, but in order to be effective, such databases require diligence on the part of manufacturers and clinicians to provide accurate, up-to-date information, the researchers concluded.
More procedures mean more complications
“As the market for minimally and noninvasive cosmetic procedures continues to expand, clinicians will likely encounter a greater number of patients with complications from these procedures,” said Jacqueline Watchmaker, MD, a general and cosmetic dermatologist in Scottsdale, Ariz., in an interview.
“Now more than ever, it is important for providers to understand potential side effects of procedures so that they can adequately counsel patients and optimize patient safety,” and therefore the current study is important at this time, she commented.
Dr. Watchmaker, who was not involved in the study, said that, overall, she was not surprised by the findings. “The adverse events analyzed from the Manufacturer and User Facility Device Experience parallel what is seen in clinical practice,” she said. “I did find it slightly surprising that an overwhelming majority of the medical device reports (515 of 723) were from 2021.” As the authors discuss, the reasons for this increase may include such factors as more flexible pandemic work schedules, pandemic weight gain, and the rise in MedSpas in recent years, she added.
“Some patients mistakenly think that ‘noninvasive’ or ‘minimally invasive’ procedures are risk free,” said Dr. Watchmaker. “However, as this review clearly demonstrates, complications can and do occur with these procedures. It is our job as clinicians to educate our patients on potential adverse events prior to treatment,” she emphasized. Also, she added, it is important for clinicians to report all adverse events to the MAUDE database so the true risks of noninvasive procedures can be more accurately assessed.
As for additional research, “It would be interesting to repeat the same study but to look at other minimally and noninvasive cosmetic devices such as radiofrequency and ultrasound devices,” Dr. Watchmaker noted.
The study received no outside funding. Dr. Lim and his coauthors, Adam Wulkan, MD, of the Lahey Clinic, Burlington, Mass., and Mathew Avram, MD, JD, of Massachusetts General Hospital, had no financial conflicts to disclose. Dr. Watchmaker had no financial conflicts to disclose.
Medical device–related adverse events can be reported to the FDA’s MAUDE database here .
of data from the Manufacturer and User Facility Device Experience (MAUDE).
The number of noninvasive body-contouring procedures performed in the United States increased by fivefold from 2011 to 2019, attributed in part to a combination of improved technology and new medical devices, as well as a “cosmetically savvy consumer base heavily influenced by social media,” wrote Young Lim, MD, PhD, of the department of dermatology, Massachusetts General Hospital, Boston, and coauthors.
However, premarket evaluations of many new medical devices fail to capture rare or delayed onset complications, and consumers and providers may not be fully aware of potential adverse events, they said. The MAUDE database was created by the Food and Drug Administration in 1991 to collect information on device-related deaths, serious injuries, or malfunctions based on reports from manufacturers, patients, and health care providers.
The researchers used the MAUDE database to identify and highlight adverse events associated with noninvasive body contouring technology in order to improve patient safety and satisfaction.
In their report, published in Lasers in Surgery and Medicine, they analyzed 723 medical device reports (MDRs) reported between 2015 and 2021: 660 for noninvasive body contouring, 55 for cellulite treatments, and 8 for muscle stimulation.
“Notably, of the 723 total MDRs between 2015 and 2021, 515 (71.2%) were reported in 2021, with the next highest reported being 64 in 2019 (8.8%),” the researchers wrote.
Overall, paradoxical hyperplasia (PAH) accounted for the majority of adverse reactions in the noninvasive body-contouring category (73.2%). In PAH, patients develop additional adipose tissue in areas treated with cryolipolysis. In this study, all reports of PAH as well as all 47 reported cases of abdominal hernias were attributed to the CoolSculpting device.
For cellulite treatments, the most common MDRs – 11 of 55 – were scars and keloids (20%). The Cellfina subcision technique accounted for 47% (26 of 55) of the MDRs in this category, including 9 of the scar and keloid cases.
Only eight of the MDRs analyzed were in the muscle stimulation category; of these, burns were the most common adverse event and accounted for three of the reports. The other reported AEs were two cases of pain and one report each of electrical shock, urticaria, and arrhythmia.
Patients are increasingly opting for noninvasive cosmetic procedures, but adverse events may be underreported despite the existence of databases such as MAUDE, the researchers wrote in their discussion.
“PAH, first reported in 2014 as an adverse sequelae of cryolipolysis, remains without known pathophysiology, though it proportionately affects men more than women,” they noted. The incidence of PAH varies widely, and the current treatment of choice is power-assisted liposuction, they said, although surgical abdominoplasty may be needed in severe cases.
The findings were limited by several factors including the reliance of the quality of submissions, the selection biases of the MAUDE database, and the potential for underreporting, the researchers noted.
However, “by cataloging the AEs of the growing noninvasive cosmetics market, the MAUDE can educate providers and inform patients to maximize safety and efficacy,” they said.
The size of the database and volume of reports provides a picture that likely reflects overall trends occurring in clinical practice, but in order to be effective, such databases require diligence on the part of manufacturers and clinicians to provide accurate, up-to-date information, the researchers concluded.
More procedures mean more complications
“As the market for minimally and noninvasive cosmetic procedures continues to expand, clinicians will likely encounter a greater number of patients with complications from these procedures,” said Jacqueline Watchmaker, MD, a general and cosmetic dermatologist in Scottsdale, Ariz., in an interview.
“Now more than ever, it is important for providers to understand potential side effects of procedures so that they can adequately counsel patients and optimize patient safety,” and therefore the current study is important at this time, she commented.
Dr. Watchmaker, who was not involved in the study, said that, overall, she was not surprised by the findings. “The adverse events analyzed from the Manufacturer and User Facility Device Experience parallel what is seen in clinical practice,” she said. “I did find it slightly surprising that an overwhelming majority of the medical device reports (515 of 723) were from 2021.” As the authors discuss, the reasons for this increase may include such factors as more flexible pandemic work schedules, pandemic weight gain, and the rise in MedSpas in recent years, she added.
“Some patients mistakenly think that ‘noninvasive’ or ‘minimally invasive’ procedures are risk free,” said Dr. Watchmaker. “However, as this review clearly demonstrates, complications can and do occur with these procedures. It is our job as clinicians to educate our patients on potential adverse events prior to treatment,” she emphasized. Also, she added, it is important for clinicians to report all adverse events to the MAUDE database so the true risks of noninvasive procedures can be more accurately assessed.
As for additional research, “It would be interesting to repeat the same study but to look at other minimally and noninvasive cosmetic devices such as radiofrequency and ultrasound devices,” Dr. Watchmaker noted.
The study received no outside funding. Dr. Lim and his coauthors, Adam Wulkan, MD, of the Lahey Clinic, Burlington, Mass., and Mathew Avram, MD, JD, of Massachusetts General Hospital, had no financial conflicts to disclose. Dr. Watchmaker had no financial conflicts to disclose.
Medical device–related adverse events can be reported to the FDA’s MAUDE database here .
of data from the Manufacturer and User Facility Device Experience (MAUDE).
The number of noninvasive body-contouring procedures performed in the United States increased by fivefold from 2011 to 2019, attributed in part to a combination of improved technology and new medical devices, as well as a “cosmetically savvy consumer base heavily influenced by social media,” wrote Young Lim, MD, PhD, of the department of dermatology, Massachusetts General Hospital, Boston, and coauthors.
However, premarket evaluations of many new medical devices fail to capture rare or delayed onset complications, and consumers and providers may not be fully aware of potential adverse events, they said. The MAUDE database was created by the Food and Drug Administration in 1991 to collect information on device-related deaths, serious injuries, or malfunctions based on reports from manufacturers, patients, and health care providers.
The researchers used the MAUDE database to identify and highlight adverse events associated with noninvasive body contouring technology in order to improve patient safety and satisfaction.
In their report, published in Lasers in Surgery and Medicine, they analyzed 723 medical device reports (MDRs) reported between 2015 and 2021: 660 for noninvasive body contouring, 55 for cellulite treatments, and 8 for muscle stimulation.
“Notably, of the 723 total MDRs between 2015 and 2021, 515 (71.2%) were reported in 2021, with the next highest reported being 64 in 2019 (8.8%),” the researchers wrote.
Overall, paradoxical hyperplasia (PAH) accounted for the majority of adverse reactions in the noninvasive body-contouring category (73.2%). In PAH, patients develop additional adipose tissue in areas treated with cryolipolysis. In this study, all reports of PAH as well as all 47 reported cases of abdominal hernias were attributed to the CoolSculpting device.
For cellulite treatments, the most common MDRs – 11 of 55 – were scars and keloids (20%). The Cellfina subcision technique accounted for 47% (26 of 55) of the MDRs in this category, including 9 of the scar and keloid cases.
Only eight of the MDRs analyzed were in the muscle stimulation category; of these, burns were the most common adverse event and accounted for three of the reports. The other reported AEs were two cases of pain and one report each of electrical shock, urticaria, and arrhythmia.
Patients are increasingly opting for noninvasive cosmetic procedures, but adverse events may be underreported despite the existence of databases such as MAUDE, the researchers wrote in their discussion.
“PAH, first reported in 2014 as an adverse sequelae of cryolipolysis, remains without known pathophysiology, though it proportionately affects men more than women,” they noted. The incidence of PAH varies widely, and the current treatment of choice is power-assisted liposuction, they said, although surgical abdominoplasty may be needed in severe cases.
The findings were limited by several factors including the reliance of the quality of submissions, the selection biases of the MAUDE database, and the potential for underreporting, the researchers noted.
However, “by cataloging the AEs of the growing noninvasive cosmetics market, the MAUDE can educate providers and inform patients to maximize safety and efficacy,” they said.
The size of the database and volume of reports provides a picture that likely reflects overall trends occurring in clinical practice, but in order to be effective, such databases require diligence on the part of manufacturers and clinicians to provide accurate, up-to-date information, the researchers concluded.
More procedures mean more complications
“As the market for minimally and noninvasive cosmetic procedures continues to expand, clinicians will likely encounter a greater number of patients with complications from these procedures,” said Jacqueline Watchmaker, MD, a general and cosmetic dermatologist in Scottsdale, Ariz., in an interview.
“Now more than ever, it is important for providers to understand potential side effects of procedures so that they can adequately counsel patients and optimize patient safety,” and therefore the current study is important at this time, she commented.
Dr. Watchmaker, who was not involved in the study, said that, overall, she was not surprised by the findings. “The adverse events analyzed from the Manufacturer and User Facility Device Experience parallel what is seen in clinical practice,” she said. “I did find it slightly surprising that an overwhelming majority of the medical device reports (515 of 723) were from 2021.” As the authors discuss, the reasons for this increase may include such factors as more flexible pandemic work schedules, pandemic weight gain, and the rise in MedSpas in recent years, she added.
“Some patients mistakenly think that ‘noninvasive’ or ‘minimally invasive’ procedures are risk free,” said Dr. Watchmaker. “However, as this review clearly demonstrates, complications can and do occur with these procedures. It is our job as clinicians to educate our patients on potential adverse events prior to treatment,” she emphasized. Also, she added, it is important for clinicians to report all adverse events to the MAUDE database so the true risks of noninvasive procedures can be more accurately assessed.
As for additional research, “It would be interesting to repeat the same study but to look at other minimally and noninvasive cosmetic devices such as radiofrequency and ultrasound devices,” Dr. Watchmaker noted.
The study received no outside funding. Dr. Lim and his coauthors, Adam Wulkan, MD, of the Lahey Clinic, Burlington, Mass., and Mathew Avram, MD, JD, of Massachusetts General Hospital, had no financial conflicts to disclose. Dr. Watchmaker had no financial conflicts to disclose.
Medical device–related adverse events can be reported to the FDA’s MAUDE database here .
FROM LASERS IN SURGERY AND MEDICINE
Dermatologists and the Aging Eye: Visual Performance in Physicians
The years start coming and they don’t stop coming.
Smash Mouth, “All Star”
Dermatologists, similar to everyone else, are subject to the inevitable: aging. More than 80% of the US population develops presbyopia, an age-related reduction in visual acuity, in their lifetime. The most common cause of refractive error in adults, presbyopia can contribute to reduced professional productivity, and individuals with uncorrected presbyopia face an estimated 8-fold increase in difficulty performing demanding near-vision tasks.1
As specialists who rely heavily on visual assessment, dermatologists likely are aware of presbyopia, seeking care as appropriate; however, visual correction is not one size fits all, and identifying effective job-specific adjustments may require considerable trial and error. To this end, if visual correction may be needed by a large majority of dermatologists at some point, why do we not have specialized recommendations to guide the corrective process according to the individual’s defect and type of practice within the specialty? Do we need resources for dermatologists concerning ophthalmologic wellness and key warning signs of visual acuity deficits and other ocular complications?
These matters are difficult to address, made more so by the lack of data examining correctable visual impairment (CVI) in dermatology. The basis for discussion is clear; however, visual skills are highly relevant to the practice of dermatology, and age-related visual changes often are inevitable. This article will provide an overview of CVI in related disciplines and the importance of understanding CVI and corrective options in dermatology.
CVI Across Medical Disciplines
Other predominantly visual medical specialties such as pathology, radiology, and surgery have initiated research evaluating the impact of CVI on their respective practices, although consistent data still are limited. Much of the work surrounding CVI in medicine can be identified in surgery and its subspecialties. A 2020 study by Tuna et al2 found that uncorrected myopia with greater than 1.75 diopter, hyperopia regardless of grade, and presbyopia with greater than 1.25 diopter correlated with reduced surgical performance when using the Da Vinci robotic system. A 2002 report by Wanzel et al3 was among the first of many studies to demonstrate the importance of visuospatial ability in surgical success. In radiology, Krupinski et al4 demonstrated reduced accuracy in detecting pulmonary nodules that correlated with increased myopia and decreased accommodation secondary to visual strain.
Most reports examining CVI across medical disciplines are primarily conversational or observational, with some utilizing surveys to assess the prevalence of CVI and the opinions of physicians in the field. For example, in a survey of 93 pathologists in Turkey, 93.5% (87/93) reported at least 1 type of refractive error. Eyeglasses were the most common form of correction (64.5% [60/93]); of those, 33.3% (31/93) reported using eyeglasses during microscopy.5
The importance of visual ability in other highly visual specialties suggests that parallels can be drawn to similar practices in dermatology. Detection of cutaneous lesions might be affected by changes in vision, similar to detection of pulmonary lesions in radiology. Likewise, dermatologic surgeons might experience a similar reduction in surgical performance due to impaired visual acuity or visuospatial ability.
The Importance of Visual Performancein Dermatology
With presbyopia often becoming clinically apparent at approximately 40 years of age,1,6 CVI has the potential to be present for much of a dermatologist’s career. Responsibility falls on the individual practitioner to recognize their visual deficit and seek appropriate optometric or ophthalmologic care. It should be emphasized that there are many effective avenues to correct refractive error, most of which can functionally restore an individual’s vision; however, each option prioritizes different visual attributes (eg, contrast, depth perception, clarity) that have varying degrees of importance in particular areas of dermatologic practice. For example, in addition to visual acuity, dermatologic surgeons might require optimized depth perception, whereas dermatologists performing detailed visual inspection or dermoscopy might instead require optimized contrast sensitivity and acuity. At present, the literature is silent on guiding dermatologists in selecting corrective approaches that enhance the visual characteristics most important for their practice. Lack of research and direction surrounding which visual correction techniques are best suited for individual tasks risks inaccurate and nonspecific conversations with our eye care providers. Focused educated dialogues about visual needs would streamline the process of finding appropriate correction, thereby reducing unnecessary trial and error. As each dermatologic subspecialty might require a unique subset of visual skills, the conceivable benefit of dermatology-specific visual correction resources is evident.
Additionally (although beyond the scope of this commentary), guidance on how a dermatologist should increase their awareness and approach to more serious ophthalmologic conditions—including retinal tear or detachment, age-related macular degeneration, and glaucoma—also would serve as a valuable resource. Overall, prompt identification of visual changes and educated discussions surrounding their correction would allow for optimization based on the required skill set and would improve overall outcomes.
Final Thoughts
Age-related visual changes are a highly prevalent and normal process that carry the potential to impact clinical practice. Fortunately, there are multiple corrective mechanisms that can functionally restore an individual’s eyesight. However, there are no resources to guide dermatologists in seeking specialty-specific correction centered on their daily tasks, which places the responsibility for such correction on the individual. This is a circumstance in which the task at hand is clear, yet we continue to individually reinvent the wheel. We should consider this an opportunity to work together with our optometry and ophthalmology colleagues to create centralized resources that assist dermatologists in navigating age-related visual changes.
Acknowledgments—The authors thank Delaney Stratton, DNP, FNP-BC (Tucson, Arizona); J. Daniel Twelker, OD, PhD (Tucson, Arizona); and Julia Freeman, MD (Pittsburgh, Pennsylvania), for their contributions to the manuscript, as well as Susan M. Swetter, MD (Palo Alto, California) for reviewing and providing feedback.
- Berdahl J, Bala C, Dhariwal M, et al. Patient and economic burden of presbyopia: a systematic literature review. Clin Ophthalmol. 2020;14:3439-3450. doi:10.2147/OPTH.S269597
- Tuna MB, Kilavuzoglu AE, Mourmouris P, et al. Impact of refractive errors on Da Vinci SI robotic system. JSLS. 2020;24:e2020.00031. doi:10.4293/JSLS.2020.00031
- Wanzel KR, Hamstra SJ, Anastakis DJ, et al. Effect of visual-spatial ability on learning of spatially-complex surgical skills. Lancet. 2002;359:230-231. doi:10.1016/S0140-6736(02)07441-X
- Krupinski EA, Berbaum KS, Caldwell RT, et al. Do long radiology workdays affect nodule detection in dynamic CT interpretation? J Am Coll Radiol. 2012;9:191-198. doi:10.1016/j.jacr.2011.11.013
- Akman O, Kösemehmetog˘lu K. Ocular diseases among pathologists and pathologists’ perceptions on ocular diseases: a survey study. Turk Patoloji Derg. 2015;31:194-199. doi:10.5146/tjpath.2015.01326
- Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111-1119. doi:10.1001/archopht.126.8.1111
The years start coming and they don’t stop coming.
Smash Mouth, “All Star”
Dermatologists, similar to everyone else, are subject to the inevitable: aging. More than 80% of the US population develops presbyopia, an age-related reduction in visual acuity, in their lifetime. The most common cause of refractive error in adults, presbyopia can contribute to reduced professional productivity, and individuals with uncorrected presbyopia face an estimated 8-fold increase in difficulty performing demanding near-vision tasks.1
As specialists who rely heavily on visual assessment, dermatologists likely are aware of presbyopia, seeking care as appropriate; however, visual correction is not one size fits all, and identifying effective job-specific adjustments may require considerable trial and error. To this end, if visual correction may be needed by a large majority of dermatologists at some point, why do we not have specialized recommendations to guide the corrective process according to the individual’s defect and type of practice within the specialty? Do we need resources for dermatologists concerning ophthalmologic wellness and key warning signs of visual acuity deficits and other ocular complications?
These matters are difficult to address, made more so by the lack of data examining correctable visual impairment (CVI) in dermatology. The basis for discussion is clear; however, visual skills are highly relevant to the practice of dermatology, and age-related visual changes often are inevitable. This article will provide an overview of CVI in related disciplines and the importance of understanding CVI and corrective options in dermatology.
CVI Across Medical Disciplines
Other predominantly visual medical specialties such as pathology, radiology, and surgery have initiated research evaluating the impact of CVI on their respective practices, although consistent data still are limited. Much of the work surrounding CVI in medicine can be identified in surgery and its subspecialties. A 2020 study by Tuna et al2 found that uncorrected myopia with greater than 1.75 diopter, hyperopia regardless of grade, and presbyopia with greater than 1.25 diopter correlated with reduced surgical performance when using the Da Vinci robotic system. A 2002 report by Wanzel et al3 was among the first of many studies to demonstrate the importance of visuospatial ability in surgical success. In radiology, Krupinski et al4 demonstrated reduced accuracy in detecting pulmonary nodules that correlated with increased myopia and decreased accommodation secondary to visual strain.
Most reports examining CVI across medical disciplines are primarily conversational or observational, with some utilizing surveys to assess the prevalence of CVI and the opinions of physicians in the field. For example, in a survey of 93 pathologists in Turkey, 93.5% (87/93) reported at least 1 type of refractive error. Eyeglasses were the most common form of correction (64.5% [60/93]); of those, 33.3% (31/93) reported using eyeglasses during microscopy.5
The importance of visual ability in other highly visual specialties suggests that parallels can be drawn to similar practices in dermatology. Detection of cutaneous lesions might be affected by changes in vision, similar to detection of pulmonary lesions in radiology. Likewise, dermatologic surgeons might experience a similar reduction in surgical performance due to impaired visual acuity or visuospatial ability.
The Importance of Visual Performancein Dermatology
With presbyopia often becoming clinically apparent at approximately 40 years of age,1,6 CVI has the potential to be present for much of a dermatologist’s career. Responsibility falls on the individual practitioner to recognize their visual deficit and seek appropriate optometric or ophthalmologic care. It should be emphasized that there are many effective avenues to correct refractive error, most of which can functionally restore an individual’s vision; however, each option prioritizes different visual attributes (eg, contrast, depth perception, clarity) that have varying degrees of importance in particular areas of dermatologic practice. For example, in addition to visual acuity, dermatologic surgeons might require optimized depth perception, whereas dermatologists performing detailed visual inspection or dermoscopy might instead require optimized contrast sensitivity and acuity. At present, the literature is silent on guiding dermatologists in selecting corrective approaches that enhance the visual characteristics most important for their practice. Lack of research and direction surrounding which visual correction techniques are best suited for individual tasks risks inaccurate and nonspecific conversations with our eye care providers. Focused educated dialogues about visual needs would streamline the process of finding appropriate correction, thereby reducing unnecessary trial and error. As each dermatologic subspecialty might require a unique subset of visual skills, the conceivable benefit of dermatology-specific visual correction resources is evident.
Additionally (although beyond the scope of this commentary), guidance on how a dermatologist should increase their awareness and approach to more serious ophthalmologic conditions—including retinal tear or detachment, age-related macular degeneration, and glaucoma—also would serve as a valuable resource. Overall, prompt identification of visual changes and educated discussions surrounding their correction would allow for optimization based on the required skill set and would improve overall outcomes.
Final Thoughts
Age-related visual changes are a highly prevalent and normal process that carry the potential to impact clinical practice. Fortunately, there are multiple corrective mechanisms that can functionally restore an individual’s eyesight. However, there are no resources to guide dermatologists in seeking specialty-specific correction centered on their daily tasks, which places the responsibility for such correction on the individual. This is a circumstance in which the task at hand is clear, yet we continue to individually reinvent the wheel. We should consider this an opportunity to work together with our optometry and ophthalmology colleagues to create centralized resources that assist dermatologists in navigating age-related visual changes.
Acknowledgments—The authors thank Delaney Stratton, DNP, FNP-BC (Tucson, Arizona); J. Daniel Twelker, OD, PhD (Tucson, Arizona); and Julia Freeman, MD (Pittsburgh, Pennsylvania), for their contributions to the manuscript, as well as Susan M. Swetter, MD (Palo Alto, California) for reviewing and providing feedback.
The years start coming and they don’t stop coming.
Smash Mouth, “All Star”
Dermatologists, similar to everyone else, are subject to the inevitable: aging. More than 80% of the US population develops presbyopia, an age-related reduction in visual acuity, in their lifetime. The most common cause of refractive error in adults, presbyopia can contribute to reduced professional productivity, and individuals with uncorrected presbyopia face an estimated 8-fold increase in difficulty performing demanding near-vision tasks.1
As specialists who rely heavily on visual assessment, dermatologists likely are aware of presbyopia, seeking care as appropriate; however, visual correction is not one size fits all, and identifying effective job-specific adjustments may require considerable trial and error. To this end, if visual correction may be needed by a large majority of dermatologists at some point, why do we not have specialized recommendations to guide the corrective process according to the individual’s defect and type of practice within the specialty? Do we need resources for dermatologists concerning ophthalmologic wellness and key warning signs of visual acuity deficits and other ocular complications?
These matters are difficult to address, made more so by the lack of data examining correctable visual impairment (CVI) in dermatology. The basis for discussion is clear; however, visual skills are highly relevant to the practice of dermatology, and age-related visual changes often are inevitable. This article will provide an overview of CVI in related disciplines and the importance of understanding CVI and corrective options in dermatology.
CVI Across Medical Disciplines
Other predominantly visual medical specialties such as pathology, radiology, and surgery have initiated research evaluating the impact of CVI on their respective practices, although consistent data still are limited. Much of the work surrounding CVI in medicine can be identified in surgery and its subspecialties. A 2020 study by Tuna et al2 found that uncorrected myopia with greater than 1.75 diopter, hyperopia regardless of grade, and presbyopia with greater than 1.25 diopter correlated with reduced surgical performance when using the Da Vinci robotic system. A 2002 report by Wanzel et al3 was among the first of many studies to demonstrate the importance of visuospatial ability in surgical success. In radiology, Krupinski et al4 demonstrated reduced accuracy in detecting pulmonary nodules that correlated with increased myopia and decreased accommodation secondary to visual strain.
Most reports examining CVI across medical disciplines are primarily conversational or observational, with some utilizing surveys to assess the prevalence of CVI and the opinions of physicians in the field. For example, in a survey of 93 pathologists in Turkey, 93.5% (87/93) reported at least 1 type of refractive error. Eyeglasses were the most common form of correction (64.5% [60/93]); of those, 33.3% (31/93) reported using eyeglasses during microscopy.5
The importance of visual ability in other highly visual specialties suggests that parallels can be drawn to similar practices in dermatology. Detection of cutaneous lesions might be affected by changes in vision, similar to detection of pulmonary lesions in radiology. Likewise, dermatologic surgeons might experience a similar reduction in surgical performance due to impaired visual acuity or visuospatial ability.
The Importance of Visual Performancein Dermatology
With presbyopia often becoming clinically apparent at approximately 40 years of age,1,6 CVI has the potential to be present for much of a dermatologist’s career. Responsibility falls on the individual practitioner to recognize their visual deficit and seek appropriate optometric or ophthalmologic care. It should be emphasized that there are many effective avenues to correct refractive error, most of which can functionally restore an individual’s vision; however, each option prioritizes different visual attributes (eg, contrast, depth perception, clarity) that have varying degrees of importance in particular areas of dermatologic practice. For example, in addition to visual acuity, dermatologic surgeons might require optimized depth perception, whereas dermatologists performing detailed visual inspection or dermoscopy might instead require optimized contrast sensitivity and acuity. At present, the literature is silent on guiding dermatologists in selecting corrective approaches that enhance the visual characteristics most important for their practice. Lack of research and direction surrounding which visual correction techniques are best suited for individual tasks risks inaccurate and nonspecific conversations with our eye care providers. Focused educated dialogues about visual needs would streamline the process of finding appropriate correction, thereby reducing unnecessary trial and error. As each dermatologic subspecialty might require a unique subset of visual skills, the conceivable benefit of dermatology-specific visual correction resources is evident.
Additionally (although beyond the scope of this commentary), guidance on how a dermatologist should increase their awareness and approach to more serious ophthalmologic conditions—including retinal tear or detachment, age-related macular degeneration, and glaucoma—also would serve as a valuable resource. Overall, prompt identification of visual changes and educated discussions surrounding their correction would allow for optimization based on the required skill set and would improve overall outcomes.
Final Thoughts
Age-related visual changes are a highly prevalent and normal process that carry the potential to impact clinical practice. Fortunately, there are multiple corrective mechanisms that can functionally restore an individual’s eyesight. However, there are no resources to guide dermatologists in seeking specialty-specific correction centered on their daily tasks, which places the responsibility for such correction on the individual. This is a circumstance in which the task at hand is clear, yet we continue to individually reinvent the wheel. We should consider this an opportunity to work together with our optometry and ophthalmology colleagues to create centralized resources that assist dermatologists in navigating age-related visual changes.
Acknowledgments—The authors thank Delaney Stratton, DNP, FNP-BC (Tucson, Arizona); J. Daniel Twelker, OD, PhD (Tucson, Arizona); and Julia Freeman, MD (Pittsburgh, Pennsylvania), for their contributions to the manuscript, as well as Susan M. Swetter, MD (Palo Alto, California) for reviewing and providing feedback.
- Berdahl J, Bala C, Dhariwal M, et al. Patient and economic burden of presbyopia: a systematic literature review. Clin Ophthalmol. 2020;14:3439-3450. doi:10.2147/OPTH.S269597
- Tuna MB, Kilavuzoglu AE, Mourmouris P, et al. Impact of refractive errors on Da Vinci SI robotic system. JSLS. 2020;24:e2020.00031. doi:10.4293/JSLS.2020.00031
- Wanzel KR, Hamstra SJ, Anastakis DJ, et al. Effect of visual-spatial ability on learning of spatially-complex surgical skills. Lancet. 2002;359:230-231. doi:10.1016/S0140-6736(02)07441-X
- Krupinski EA, Berbaum KS, Caldwell RT, et al. Do long radiology workdays affect nodule detection in dynamic CT interpretation? J Am Coll Radiol. 2012;9:191-198. doi:10.1016/j.jacr.2011.11.013
- Akman O, Kösemehmetog˘lu K. Ocular diseases among pathologists and pathologists’ perceptions on ocular diseases: a survey study. Turk Patoloji Derg. 2015;31:194-199. doi:10.5146/tjpath.2015.01326
- Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111-1119. doi:10.1001/archopht.126.8.1111
- Berdahl J, Bala C, Dhariwal M, et al. Patient and economic burden of presbyopia: a systematic literature review. Clin Ophthalmol. 2020;14:3439-3450. doi:10.2147/OPTH.S269597
- Tuna MB, Kilavuzoglu AE, Mourmouris P, et al. Impact of refractive errors on Da Vinci SI robotic system. JSLS. 2020;24:e2020.00031. doi:10.4293/JSLS.2020.00031
- Wanzel KR, Hamstra SJ, Anastakis DJ, et al. Effect of visual-spatial ability on learning of spatially-complex surgical skills. Lancet. 2002;359:230-231. doi:10.1016/S0140-6736(02)07441-X
- Krupinski EA, Berbaum KS, Caldwell RT, et al. Do long radiology workdays affect nodule detection in dynamic CT interpretation? J Am Coll Radiol. 2012;9:191-198. doi:10.1016/j.jacr.2011.11.013
- Akman O, Kösemehmetog˘lu K. Ocular diseases among pathologists and pathologists’ perceptions on ocular diseases: a survey study. Turk Patoloji Derg. 2015;31:194-199. doi:10.5146/tjpath.2015.01326
- Vitale S, Ellwein L, Cotch MF, et al. Prevalence of refractive error in the United States, 1999-2004. Arch Ophthalmol. 2008;126:1111-1119. doi:10.1001/archopht.126.8.1111
Practice Points
- With presbyopia becoming clinically apparent starting at 40 years of age, dermatologists should be vigilant for correctable visual impairment.
- Although many corrective options exist, more research is needed to understand whether dermatologic subspecialties are better suited to specific options.
- As a specialty, we should consider standardized visual correction guidance.
Transverse Leukonychia and Beau Lines Following COVID-19 Vaccination
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
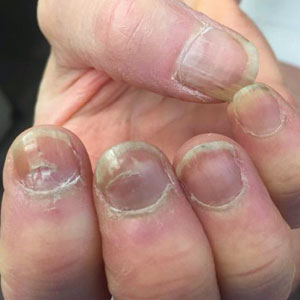
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).
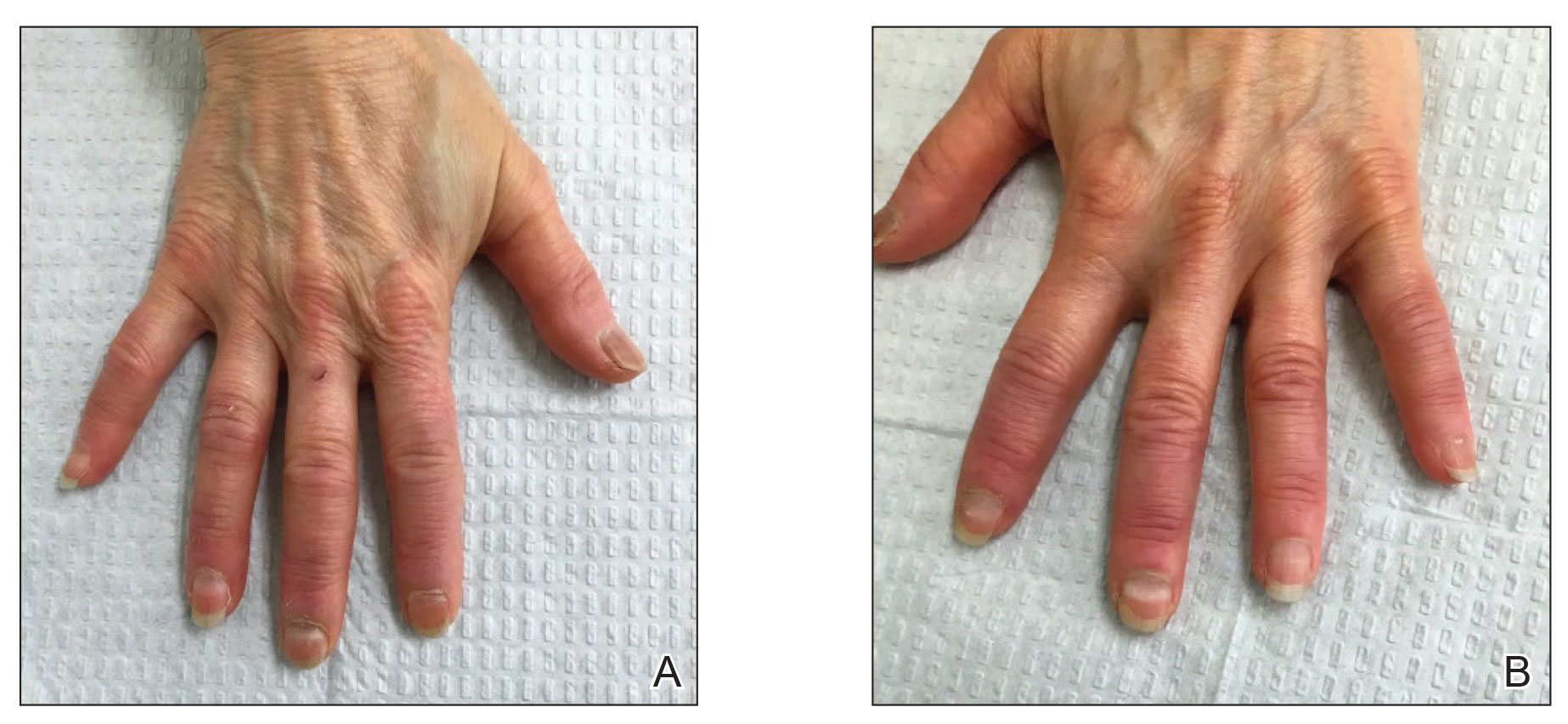
Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.
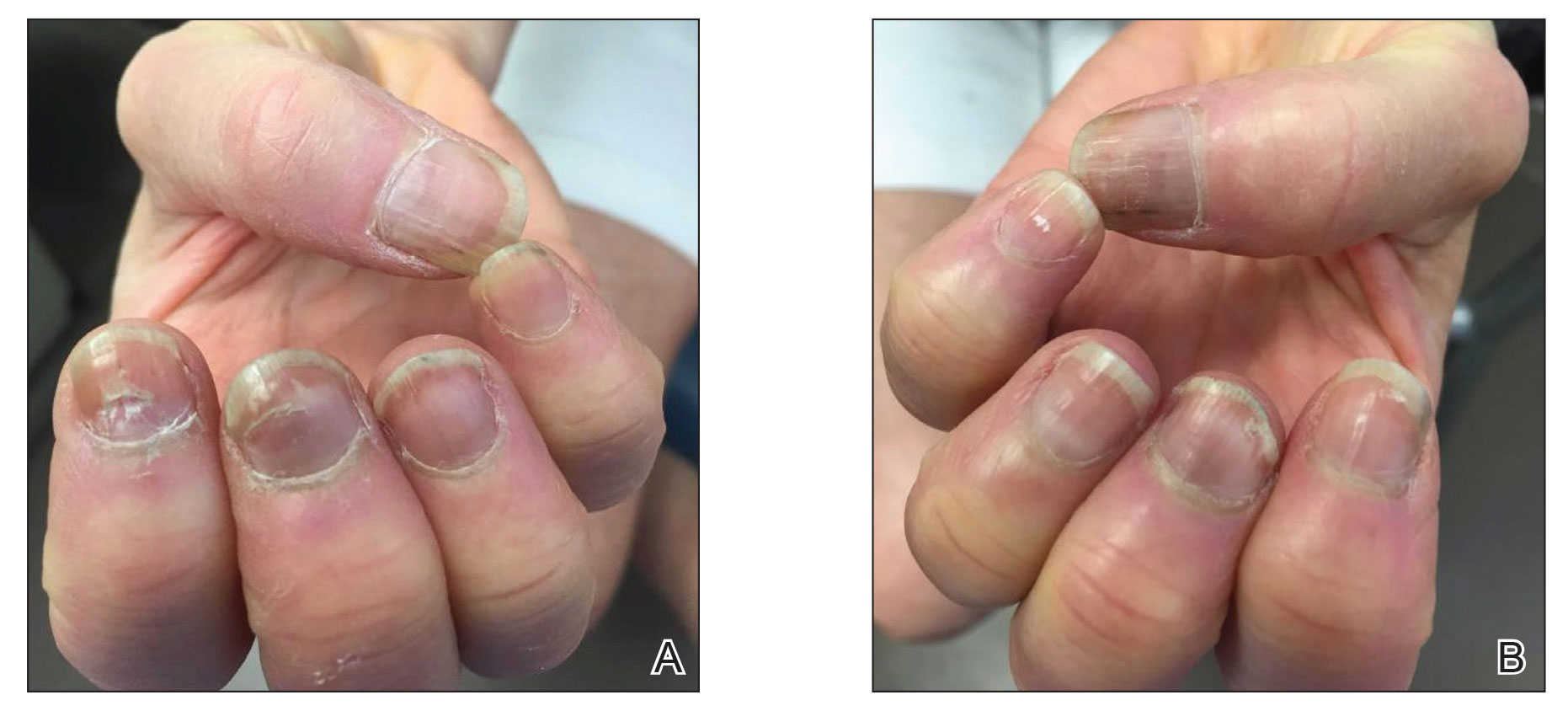
She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
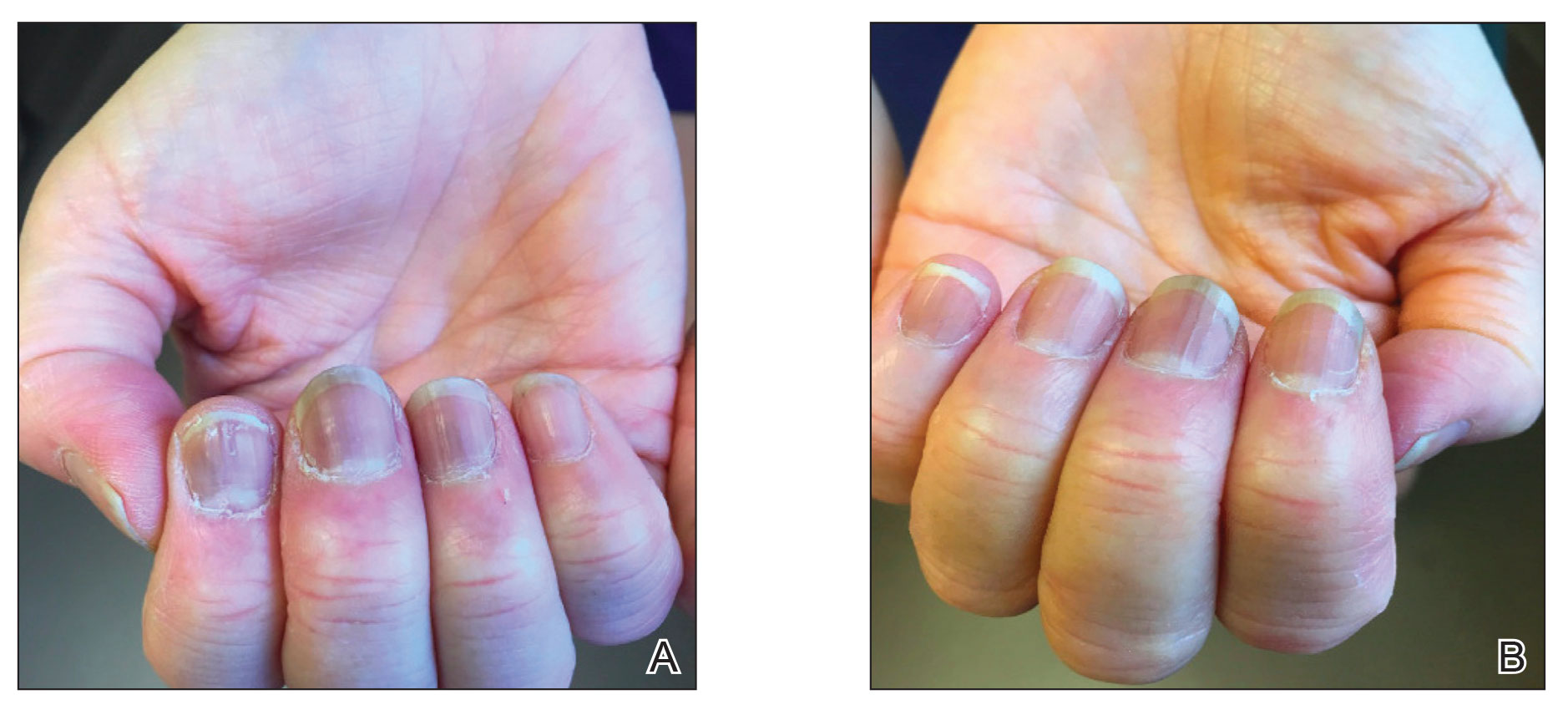
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.
Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
Practice Points
- Given accelerated global vaccination efforts to control the COVID-19 pandemic, cases of nail changes associated with COVID-19 vaccines are expected.
- Nail abnormalities are a potential general, temporary, and self-limiting adverse effect of COVID-19 vaccines that should not discourage patients from getting vaccinated.
Sacubitril/valsartan shows cognitive safety in heart failure: PERSPECTIVE
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
AT ESC CONGRESS 2022
High-dose loop diuretic can raise post–cardiac surgery mortality
The study covered in this summary was published on ResearchSquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
- High-dose furosemide after cardiac surgery is associated with increased mortality and other adverse outcomes.
Why this matters
- The influence of furosemide on prognosis after cardiac surgery is not fully understood.
- The current findings suggest that high-dose furosemide after cardiac surgery is associated with increased risk for death and other adverse events and therefore should be used cautiously in that setting.
Study design
- The retrospective cohort of 6,752 cardiac surgery patients was divided into two groups according to average daily furosemide dosage after cardiac surgery: less than 20 mg (low-dose group, n = 6,033) and at least 20 mg (high-dose group, n = 719).
- The group were compared for total furosemide dose, total furosemide dose of at least 200 mg, total dose of furosemide by patient weight, and average daily furosemide dose of at least 20 mg.
- The primary outcomes were in-hospital mortality and mortality at 1 year after cardiac surgery. Secondary outcomes were length of hospital stay of at least 14 days, length of ICU stay of at least 3 days, and mechanical ventilation for at least 48 hours.
- The study excluded patients aged younger than 18 whose weight data was missing or who had more than 5% of their data missing.
Key results
- Patients in the high-dose furosemide group tended to be older and have a higher body mass index (BMI) and higher rates of diabetes, chronic pulmonary diseases, heart failure, renal failure, blood transfusion, vasopressor use, and valvular surgery.
- They also tended have higher white cell counts and higher levels of blood urea nitrogen, creatinine, glucose, and lactate.
- Those in the high-dose group also were on vasopressors and ventilatory support longer.
- In adjusted multivariate analysis, increased in-hospital mortality was associated with average daily furosemide dose, average daily dose of at least 20 mg/d, and total dose of at least 200 mg.
- Increased mortality at 1 year was associated with total furosemide dose and average daily furosemide dose.
- Significant multivariate predictors of hospital stay of at least 14 days, length of ICU stay of at least 3 days, and mechanical ventilation for at least 48 hours after cardiac surgery included total furosemide dose, total dose by weight, average daily furosemide dose of at least 20 mg/d, and total dose of at least 200 mg.
- In subgroup analyses, average daily furosemide dose of at least 20 mg/d significantly increased risk for in-hospital mortality among patients younger than 60 years or with BMI of at least 28 who received vasopressors or blood transfusions, those with renal failure, and those with heart failure not involving congestion.
Limitations
- No limitations were discussed.
Disclosures
- The study was supported by grants from the National Natural Science Foundation of China, China Postdoctoral Science Foundation, and Jiangsu Postdoctoral Science Foundation.
- The authors declared that they have no competing interests.
This is a summary of a preprint research study, “Association between furosemide administration and outcomes in patients undergoing cardiac surgery,” from Jinghang Li, First Affiliated Hospital of Nanjing (China) Medical University, and colleagues on published on ResearchSquare.com. This study has not yet been peer reviewed. The full text of the study can be found on ResearchSquare.com. A version of this article first appeared on Medscape.com.
The study covered in this summary was published on ResearchSquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
- High-dose furosemide after cardiac surgery is associated with increased mortality and other adverse outcomes.
Why this matters
- The influence of furosemide on prognosis after cardiac surgery is not fully understood.
- The current findings suggest that high-dose furosemide after cardiac surgery is associated with increased risk for death and other adverse events and therefore should be used cautiously in that setting.
Study design
- The retrospective cohort of 6,752 cardiac surgery patients was divided into two groups according to average daily furosemide dosage after cardiac surgery: less than 20 mg (low-dose group, n = 6,033) and at least 20 mg (high-dose group, n = 719).
- The group were compared for total furosemide dose, total furosemide dose of at least 200 mg, total dose of furosemide by patient weight, and average daily furosemide dose of at least 20 mg.
- The primary outcomes were in-hospital mortality and mortality at 1 year after cardiac surgery. Secondary outcomes were length of hospital stay of at least 14 days, length of ICU stay of at least 3 days, and mechanical ventilation for at least 48 hours.
- The study excluded patients aged younger than 18 whose weight data was missing or who had more than 5% of their data missing.
Key results
- Patients in the high-dose furosemide group tended to be older and have a higher body mass index (BMI) and higher rates of diabetes, chronic pulmonary diseases, heart failure, renal failure, blood transfusion, vasopressor use, and valvular surgery.
- They also tended have higher white cell counts and higher levels of blood urea nitrogen, creatinine, glucose, and lactate.
- Those in the high-dose group also were on vasopressors and ventilatory support longer.
- In adjusted multivariate analysis, increased in-hospital mortality was associated with average daily furosemide dose, average daily dose of at least 20 mg/d, and total dose of at least 200 mg.
- Increased mortality at 1 year was associated with total furosemide dose and average daily furosemide dose.
- Significant multivariate predictors of hospital stay of at least 14 days, length of ICU stay of at least 3 days, and mechanical ventilation for at least 48 hours after cardiac surgery included total furosemide dose, total dose by weight, average daily furosemide dose of at least 20 mg/d, and total dose of at least 200 mg.
- In subgroup analyses, average daily furosemide dose of at least 20 mg/d significantly increased risk for in-hospital mortality among patients younger than 60 years or with BMI of at least 28 who received vasopressors or blood transfusions, those with renal failure, and those with heart failure not involving congestion.
Limitations
- No limitations were discussed.
Disclosures
- The study was supported by grants from the National Natural Science Foundation of China, China Postdoctoral Science Foundation, and Jiangsu Postdoctoral Science Foundation.
- The authors declared that they have no competing interests.
This is a summary of a preprint research study, “Association between furosemide administration and outcomes in patients undergoing cardiac surgery,” from Jinghang Li, First Affiliated Hospital of Nanjing (China) Medical University, and colleagues on published on ResearchSquare.com. This study has not yet been peer reviewed. The full text of the study can be found on ResearchSquare.com. A version of this article first appeared on Medscape.com.
The study covered in this summary was published on ResearchSquare.com as a preprint and has not yet been peer reviewed.
Key takeaway
- High-dose furosemide after cardiac surgery is associated with increased mortality and other adverse outcomes.
Why this matters
- The influence of furosemide on prognosis after cardiac surgery is not fully understood.
- The current findings suggest that high-dose furosemide after cardiac surgery is associated with increased risk for death and other adverse events and therefore should be used cautiously in that setting.
Study design
- The retrospective cohort of 6,752 cardiac surgery patients was divided into two groups according to average daily furosemide dosage after cardiac surgery: less than 20 mg (low-dose group, n = 6,033) and at least 20 mg (high-dose group, n = 719).
- The group were compared for total furosemide dose, total furosemide dose of at least 200 mg, total dose of furosemide by patient weight, and average daily furosemide dose of at least 20 mg.
- The primary outcomes were in-hospital mortality and mortality at 1 year after cardiac surgery. Secondary outcomes were length of hospital stay of at least 14 days, length of ICU stay of at least 3 days, and mechanical ventilation for at least 48 hours.
- The study excluded patients aged younger than 18 whose weight data was missing or who had more than 5% of their data missing.
Key results
- Patients in the high-dose furosemide group tended to be older and have a higher body mass index (BMI) and higher rates of diabetes, chronic pulmonary diseases, heart failure, renal failure, blood transfusion, vasopressor use, and valvular surgery.
- They also tended have higher white cell counts and higher levels of blood urea nitrogen, creatinine, glucose, and lactate.
- Those in the high-dose group also were on vasopressors and ventilatory support longer.
- In adjusted multivariate analysis, increased in-hospital mortality was associated with average daily furosemide dose, average daily dose of at least 20 mg/d, and total dose of at least 200 mg.
- Increased mortality at 1 year was associated with total furosemide dose and average daily furosemide dose.
- Significant multivariate predictors of hospital stay of at least 14 days, length of ICU stay of at least 3 days, and mechanical ventilation for at least 48 hours after cardiac surgery included total furosemide dose, total dose by weight, average daily furosemide dose of at least 20 mg/d, and total dose of at least 200 mg.
- In subgroup analyses, average daily furosemide dose of at least 20 mg/d significantly increased risk for in-hospital mortality among patients younger than 60 years or with BMI of at least 28 who received vasopressors or blood transfusions, those with renal failure, and those with heart failure not involving congestion.
Limitations
- No limitations were discussed.
Disclosures
- The study was supported by grants from the National Natural Science Foundation of China, China Postdoctoral Science Foundation, and Jiangsu Postdoctoral Science Foundation.
- The authors declared that they have no competing interests.
This is a summary of a preprint research study, “Association between furosemide administration and outcomes in patients undergoing cardiac surgery,” from Jinghang Li, First Affiliated Hospital of Nanjing (China) Medical University, and colleagues on published on ResearchSquare.com. This study has not yet been peer reviewed. The full text of the study can be found on ResearchSquare.com. A version of this article first appeared on Medscape.com.
Multibiomarker risk score predicts complex revascularization
A multibiomarker risk score helps predict increased risk for future cardiovascular (CV) events as well as high-risk anatomy at revascularization in stable patients with atherosclerotic cardiovascular disease (ASCVD), a FOURIER trial analysis suggests.
The risk score incorporates high-sensitivity C-reactive protein (hsCRP), N-terminal pro B-type natriuretic peptide (NT-proBNP), high-sensitivity troponin I (hsTnI), and growth differentiation factor 15 (GDF-15).
These routine biomarkers of inflammation and fibrosis, ventricular strain, and myocardial injury are individually associated with incident CV in stable ASCVD and were shown in earlier work to be a multimarker score to predict CV events in patients stabilized after an acute coronary syndrome in the IMPROVE-IT trial.
Validating the score, however, wasn’t really the intent here, explained senior author Brian Bergmark, MD, with the TIMI Study Group, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston.
“We know broadly speaking people with high troponin, BNP, et cetera, are going to have broadly defined clinical events like MIs [myocardial infarctions], death. And we also know on a granular level at a single time point that people who, for example, get a coronary CT scan and have a contemporary troponin level tend to have a little bit more coronary disease,” he said.
“But that leaves this broad swath of, what if we follow people over time? Can biomarkers in some form actually predict specific coronary anatomical characteristics and revascularization procedures in conjunction with clinical events?” Dr. Bergmark continued. “That’s sort of an untouched link or translational step between some of the granular data and these clinical events.”
As published in the Journal of the American College of Cardiology, the post hoc study analyzed baseline blood samples from 21,644 FOURIER participants and adapted the previously studied multimarker score to use hsTnI in place of high-sensitivity troponin T (hsTnT). One point was assigned for each elevated biomarker: hsCRP ≥ 2 mg/L, NT-proBNP ≥ 450 pg/mL, hsTnI ≥ 6 ng/L, and GDF-15 ≥ 1,800 pg/mL.
A total of 6,444 patients had a low score (0 points), 12,439 an intermediate score (1-2 points), and 2,761 a high score (3-4 points). Patients with higher biomarker scores were older and were more likely to have hypertension, diabetes, multiple prior MIs, heart failure, prior coronary artery bypass grafting (CABG), and peripheral artery disease but were less likely to have prior percutaneous coronary intervention (PCI).
Results showed a stepwise increase in 3-year risk for major coronary events (coronary death, MI, or coronary revascularization) from 7.3% with a low score to 11.3% with an intermediate score and 21.0% with a high score. A near tripling of risk remained in those with a high score after adjustment (hazard ratio, 2.90).
Individuals with a high score had twice the risk for any coronary revascularization (HR, 2.10) and complex revascularization (HR, 2.07), as well as increased risks for complex PCI (HR, 1.80), CABG (HR, 2.57), and in-stent restenosis (ISR) revascularization (HR, 1.78).
The study is the first to show an association of these biomarkers with future ISR revascularization in a broad cohort of patients with stable ASCVD, the investigators observe.
It could be a random signal, but “it’s one piece of data as people start to look at other datasets, as we start to understand who’s at risk for ISR, as we understand this disease entity that’s really a pandemic at this point,” Dr. Bergmark said, “I think this is one piece of the puzzle that’s novel.”
Compared with those with a low score, patients with a high biomarker score had significantly higher risks for left main disease greater than 50% (HR, 2.22; P = .003), multivessel disease (HR, 1.99; P < .001), and chronic total occlusion (HR, 2.50; P < .001) at the time of revascularization.
There was no significant interaction between the biomarker score and the effect of evolocumab used in the trial; however, the assessment had limited statistical power, the authors note.
Dr. Bergmark said that the results can inform trial design to select a population at risk for specific types of events and when trying to risk adjust in a population for reimbursement purposes to understand quality metrics, for example, for people coming back with ISR.
“I think refining risk estimates has broad applicability clinically and academically,” he added. “This is one step, with one dataset, pushing these typically broad clinical endpoints to be more specific.”
In an related editorial, Giles Montalescot, MD, PhD, Pitié-Salpêtrière Hospital, Paris, and colleagues write, “Not only does this study validate the multibiomarker score in a new cohort of patients and with new coronary-focused outcomes, but it also opens novel and interesting avenues, on a global approach of cardiovascular risk.”
Possibilities include using this or another multibiomarker risk score to streamline enrichment or selection criteria for a trial or as a surrogate endpoint in proof-of-concept trials to test a new drug aimed at reducing CV risk.
“Beyond clinical research, we could imagine in the future to base our therapeutic decisions on such a score, just like we decide anticoagulation in patients with atrial fibrillation according to the CHA₂DS₂-VASc score,” the editorialists say.
This being said, Dr. Montalescot and colleagues point out that the current multibiomarker risk score assigned equal prognostic value to each of the components, whereas IMPROVE-IT and FOURIER both showed that elevated hsTnT and NT-proBNP were associated with much higher hazard ratios than hsCRP and GDF-15.
Other limitations, they say, are that the categorical nature of the variables, albeit user friendly, prevent any subtle analysis; the score does not include biological risk factors; and questions remain about the impact of the lipid-lowering intervention across risk categories.
FOURIER was funded by Amgen. The TIMI Study Group has received institutional grant support through Brigham and Women’s Hospital from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, The Medicines Company, and Zora Biosciences. Dr. Bergmark reports grant support from Pfizer, Ionis, AstraZeneca, and Abbott Vascular; and consulting fees from Philips, Abbott Vascular, Servier, Daiichi-Sankyo, Janssen, and Quark Pharmaceuticals. Dr. Montalescot reports research grants to his institution or consulting/lecture fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cell Prothera, CSL Behring, Europa, Idorsia, IRIS-Servier, Medtronic, MSD, Novartis, Pfizer, Quantum Genomics, and Sanofi-Aventis.
A version of this article first appeared on Medscape.com.
A multibiomarker risk score helps predict increased risk for future cardiovascular (CV) events as well as high-risk anatomy at revascularization in stable patients with atherosclerotic cardiovascular disease (ASCVD), a FOURIER trial analysis suggests.
The risk score incorporates high-sensitivity C-reactive protein (hsCRP), N-terminal pro B-type natriuretic peptide (NT-proBNP), high-sensitivity troponin I (hsTnI), and growth differentiation factor 15 (GDF-15).
These routine biomarkers of inflammation and fibrosis, ventricular strain, and myocardial injury are individually associated with incident CV in stable ASCVD and were shown in earlier work to be a multimarker score to predict CV events in patients stabilized after an acute coronary syndrome in the IMPROVE-IT trial.
Validating the score, however, wasn’t really the intent here, explained senior author Brian Bergmark, MD, with the TIMI Study Group, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston.
“We know broadly speaking people with high troponin, BNP, et cetera, are going to have broadly defined clinical events like MIs [myocardial infarctions], death. And we also know on a granular level at a single time point that people who, for example, get a coronary CT scan and have a contemporary troponin level tend to have a little bit more coronary disease,” he said.
“But that leaves this broad swath of, what if we follow people over time? Can biomarkers in some form actually predict specific coronary anatomical characteristics and revascularization procedures in conjunction with clinical events?” Dr. Bergmark continued. “That’s sort of an untouched link or translational step between some of the granular data and these clinical events.”
As published in the Journal of the American College of Cardiology, the post hoc study analyzed baseline blood samples from 21,644 FOURIER participants and adapted the previously studied multimarker score to use hsTnI in place of high-sensitivity troponin T (hsTnT). One point was assigned for each elevated biomarker: hsCRP ≥ 2 mg/L, NT-proBNP ≥ 450 pg/mL, hsTnI ≥ 6 ng/L, and GDF-15 ≥ 1,800 pg/mL.
A total of 6,444 patients had a low score (0 points), 12,439 an intermediate score (1-2 points), and 2,761 a high score (3-4 points). Patients with higher biomarker scores were older and were more likely to have hypertension, diabetes, multiple prior MIs, heart failure, prior coronary artery bypass grafting (CABG), and peripheral artery disease but were less likely to have prior percutaneous coronary intervention (PCI).
Results showed a stepwise increase in 3-year risk for major coronary events (coronary death, MI, or coronary revascularization) from 7.3% with a low score to 11.3% with an intermediate score and 21.0% with a high score. A near tripling of risk remained in those with a high score after adjustment (hazard ratio, 2.90).
Individuals with a high score had twice the risk for any coronary revascularization (HR, 2.10) and complex revascularization (HR, 2.07), as well as increased risks for complex PCI (HR, 1.80), CABG (HR, 2.57), and in-stent restenosis (ISR) revascularization (HR, 1.78).
The study is the first to show an association of these biomarkers with future ISR revascularization in a broad cohort of patients with stable ASCVD, the investigators observe.
It could be a random signal, but “it’s one piece of data as people start to look at other datasets, as we start to understand who’s at risk for ISR, as we understand this disease entity that’s really a pandemic at this point,” Dr. Bergmark said, “I think this is one piece of the puzzle that’s novel.”
Compared with those with a low score, patients with a high biomarker score had significantly higher risks for left main disease greater than 50% (HR, 2.22; P = .003), multivessel disease (HR, 1.99; P < .001), and chronic total occlusion (HR, 2.50; P < .001) at the time of revascularization.
There was no significant interaction between the biomarker score and the effect of evolocumab used in the trial; however, the assessment had limited statistical power, the authors note.
Dr. Bergmark said that the results can inform trial design to select a population at risk for specific types of events and when trying to risk adjust in a population for reimbursement purposes to understand quality metrics, for example, for people coming back with ISR.
“I think refining risk estimates has broad applicability clinically and academically,” he added. “This is one step, with one dataset, pushing these typically broad clinical endpoints to be more specific.”
In an related editorial, Giles Montalescot, MD, PhD, Pitié-Salpêtrière Hospital, Paris, and colleagues write, “Not only does this study validate the multibiomarker score in a new cohort of patients and with new coronary-focused outcomes, but it also opens novel and interesting avenues, on a global approach of cardiovascular risk.”
Possibilities include using this or another multibiomarker risk score to streamline enrichment or selection criteria for a trial or as a surrogate endpoint in proof-of-concept trials to test a new drug aimed at reducing CV risk.
“Beyond clinical research, we could imagine in the future to base our therapeutic decisions on such a score, just like we decide anticoagulation in patients with atrial fibrillation according to the CHA₂DS₂-VASc score,” the editorialists say.
This being said, Dr. Montalescot and colleagues point out that the current multibiomarker risk score assigned equal prognostic value to each of the components, whereas IMPROVE-IT and FOURIER both showed that elevated hsTnT and NT-proBNP were associated with much higher hazard ratios than hsCRP and GDF-15.
Other limitations, they say, are that the categorical nature of the variables, albeit user friendly, prevent any subtle analysis; the score does not include biological risk factors; and questions remain about the impact of the lipid-lowering intervention across risk categories.
FOURIER was funded by Amgen. The TIMI Study Group has received institutional grant support through Brigham and Women’s Hospital from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, The Medicines Company, and Zora Biosciences. Dr. Bergmark reports grant support from Pfizer, Ionis, AstraZeneca, and Abbott Vascular; and consulting fees from Philips, Abbott Vascular, Servier, Daiichi-Sankyo, Janssen, and Quark Pharmaceuticals. Dr. Montalescot reports research grants to his institution or consulting/lecture fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cell Prothera, CSL Behring, Europa, Idorsia, IRIS-Servier, Medtronic, MSD, Novartis, Pfizer, Quantum Genomics, and Sanofi-Aventis.
A version of this article first appeared on Medscape.com.
A multibiomarker risk score helps predict increased risk for future cardiovascular (CV) events as well as high-risk anatomy at revascularization in stable patients with atherosclerotic cardiovascular disease (ASCVD), a FOURIER trial analysis suggests.
The risk score incorporates high-sensitivity C-reactive protein (hsCRP), N-terminal pro B-type natriuretic peptide (NT-proBNP), high-sensitivity troponin I (hsTnI), and growth differentiation factor 15 (GDF-15).
These routine biomarkers of inflammation and fibrosis, ventricular strain, and myocardial injury are individually associated with incident CV in stable ASCVD and were shown in earlier work to be a multimarker score to predict CV events in patients stabilized after an acute coronary syndrome in the IMPROVE-IT trial.
Validating the score, however, wasn’t really the intent here, explained senior author Brian Bergmark, MD, with the TIMI Study Group, Brigham and Women’s Hospital, and Harvard Medical School, both in Boston.
“We know broadly speaking people with high troponin, BNP, et cetera, are going to have broadly defined clinical events like MIs [myocardial infarctions], death. And we also know on a granular level at a single time point that people who, for example, get a coronary CT scan and have a contemporary troponin level tend to have a little bit more coronary disease,” he said.
“But that leaves this broad swath of, what if we follow people over time? Can biomarkers in some form actually predict specific coronary anatomical characteristics and revascularization procedures in conjunction with clinical events?” Dr. Bergmark continued. “That’s sort of an untouched link or translational step between some of the granular data and these clinical events.”
As published in the Journal of the American College of Cardiology, the post hoc study analyzed baseline blood samples from 21,644 FOURIER participants and adapted the previously studied multimarker score to use hsTnI in place of high-sensitivity troponin T (hsTnT). One point was assigned for each elevated biomarker: hsCRP ≥ 2 mg/L, NT-proBNP ≥ 450 pg/mL, hsTnI ≥ 6 ng/L, and GDF-15 ≥ 1,800 pg/mL.
A total of 6,444 patients had a low score (0 points), 12,439 an intermediate score (1-2 points), and 2,761 a high score (3-4 points). Patients with higher biomarker scores were older and were more likely to have hypertension, diabetes, multiple prior MIs, heart failure, prior coronary artery bypass grafting (CABG), and peripheral artery disease but were less likely to have prior percutaneous coronary intervention (PCI).
Results showed a stepwise increase in 3-year risk for major coronary events (coronary death, MI, or coronary revascularization) from 7.3% with a low score to 11.3% with an intermediate score and 21.0% with a high score. A near tripling of risk remained in those with a high score after adjustment (hazard ratio, 2.90).
Individuals with a high score had twice the risk for any coronary revascularization (HR, 2.10) and complex revascularization (HR, 2.07), as well as increased risks for complex PCI (HR, 1.80), CABG (HR, 2.57), and in-stent restenosis (ISR) revascularization (HR, 1.78).
The study is the first to show an association of these biomarkers with future ISR revascularization in a broad cohort of patients with stable ASCVD, the investigators observe.
It could be a random signal, but “it’s one piece of data as people start to look at other datasets, as we start to understand who’s at risk for ISR, as we understand this disease entity that’s really a pandemic at this point,” Dr. Bergmark said, “I think this is one piece of the puzzle that’s novel.”
Compared with those with a low score, patients with a high biomarker score had significantly higher risks for left main disease greater than 50% (HR, 2.22; P = .003), multivessel disease (HR, 1.99; P < .001), and chronic total occlusion (HR, 2.50; P < .001) at the time of revascularization.
There was no significant interaction between the biomarker score and the effect of evolocumab used in the trial; however, the assessment had limited statistical power, the authors note.
Dr. Bergmark said that the results can inform trial design to select a population at risk for specific types of events and when trying to risk adjust in a population for reimbursement purposes to understand quality metrics, for example, for people coming back with ISR.
“I think refining risk estimates has broad applicability clinically and academically,” he added. “This is one step, with one dataset, pushing these typically broad clinical endpoints to be more specific.”
In an related editorial, Giles Montalescot, MD, PhD, Pitié-Salpêtrière Hospital, Paris, and colleagues write, “Not only does this study validate the multibiomarker score in a new cohort of patients and with new coronary-focused outcomes, but it also opens novel and interesting avenues, on a global approach of cardiovascular risk.”
Possibilities include using this or another multibiomarker risk score to streamline enrichment or selection criteria for a trial or as a surrogate endpoint in proof-of-concept trials to test a new drug aimed at reducing CV risk.
“Beyond clinical research, we could imagine in the future to base our therapeutic decisions on such a score, just like we decide anticoagulation in patients with atrial fibrillation according to the CHA₂DS₂-VASc score,” the editorialists say.
This being said, Dr. Montalescot and colleagues point out that the current multibiomarker risk score assigned equal prognostic value to each of the components, whereas IMPROVE-IT and FOURIER both showed that elevated hsTnT and NT-proBNP were associated with much higher hazard ratios than hsCRP and GDF-15.
Other limitations, they say, are that the categorical nature of the variables, albeit user friendly, prevent any subtle analysis; the score does not include biological risk factors; and questions remain about the impact of the lipid-lowering intervention across risk categories.
FOURIER was funded by Amgen. The TIMI Study Group has received institutional grant support through Brigham and Women’s Hospital from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, The Medicines Company, and Zora Biosciences. Dr. Bergmark reports grant support from Pfizer, Ionis, AstraZeneca, and Abbott Vascular; and consulting fees from Philips, Abbott Vascular, Servier, Daiichi-Sankyo, Janssen, and Quark Pharmaceuticals. Dr. Montalescot reports research grants to his institution or consulting/lecture fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cell Prothera, CSL Behring, Europa, Idorsia, IRIS-Servier, Medtronic, MSD, Novartis, Pfizer, Quantum Genomics, and Sanofi-Aventis.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
State of the science in PCOS: Emerging neuroendocrine involvement driving research
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
In denial: When patients don’t want to believe they have cancer
In June, Rebecca A. Shatsky, MD, a medical oncologist, turned to Twitter for advice: “What do you do/say when a patient won’t believe you that they have #CANCER. As an oncologist this comes up every now and then and proves very difficult, looking to hear how others have dealt and what works best to help patients here.”
About a dozen people weighed in, offering various thoughts on how to approach these thorny situations. One oncologist suggested revisiting the conversation a few days later, after the patient has more time to process; others suggested sharing the pathology report or images with their patient.
Another person simply noted that “if a [patient] doesn’t want to believe they have cancer, no amount of evidence will change that.”
Based on the initial responses, “it appears there is a paucity of answers sadly,” wrote Dr. Shatsky, a breast cancer specialist at University of California, San Diego.
But for Dr. Shatsky, these incidents spoke to another alarming trend: a rampant mistrust of the medical community that is “becoming MORE common instead of less.”
‘Erosion of trust’
Overall, experts say that situations like the one Dr. Shatsky described – patients who don’t believe their cancer diagnosis – occur infrequently.
But denial comes in many forms, and complete disbelief is probably the most extreme.
Like Dr. Shatsky, these experts say they are also seeing a troubling increase in patients who don’t believe their physicians or don’t trust their recommendations.
“I think there’s an erosion of trust in expertise, in general,” said Ronald M. Epstein, MD, professor of family medicine and psychiatry & oncology at the University of Rochester (N.Y.). “People distrust science more than they did maybe 20 or 30 years ago, or at least that seems to be the case.”
Denial and distrust in cancer care are not new. These responses – along with wishful thinking, distraction, and minimization – are long-established responses among oncology patients. In 1972, Avery D. Weisman, MD, a psychiatrist at Harvard Medical School, Boston, wrote his book “On Dying and Denying,” and ever since, denial and similar responses have been explored in the oncology literature.
Much of this research has focused on the latter stages of illness, but denial can be present at diagnosis as well. One study of patients with breast cancer, carried out nearly 30 years ago, suggested that denial of diagnosis generally occurs early in a patient’s course of illness and decreases over time, but may arise again in the terminal phase of cancer. Another analysis, evaluating this phenomenon across 13 studies, found that the prevalence of denial at diagnosis ranged from 4% to as high as 47%.
An oncologist delivers somewhere between 10,000 to 30,000 episodes of bad news over the course of a career, so there’s always a chance that a patient will respond in a way that’s on the “spectrum of disbelief,” said Paul Helft, MD, professor of medicine and recently retired director of the ethics center at Indiana University, Indianapolis.
Diane Meier, MD, said denial and disbelief are natural, protective responses to difficult or frightening news.
When patients exhibit denial, Dr. Meier advises patience and time. Physicians can also ask the patient if there’s a person they trust – a family member or faith leader, for example – who could speak on their behalf about possible next steps.
“The main thing is not to find ourselves in opposition to the patient ... or threaten them with what will happen if they don’t listen to us,” said Dr. Meier, a professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York.
And physicians should be careful when they feel themselves wanting to argue with or lecture a patient.
“The minute we feel that urge coming on, that’s a signal to us to stop and realize that something is going on inside the patient that we don’t understand,” she said. “Forcing information on a person who is signaling in every way that they don’t want it and can’t handle it is not a recipe for trust or a high-quality relationship.”
Refusing expert advice
Jennifer Lycette, MD, has encountered a growing number of patients who don’t believe their disease should be treated the way she or other oncologists recommend. Some patients remain adamant about sticking with alternative medicine or doing nothing, despite growing sicker.
“I’ve even had situations where the tumor might be visible, like growing through the skin, and people still double down that whatever they’re doing is working,” said Dr. Lycette, a hematologist and medical oncologist at the Providence Seaside Cancer Center in Seaside, Ore.
She encourages these patients to get a second opinion and tries to keep an open mind about alternative approaches. If she’s not familiar with something a patient is considering, she’ll research it with them.
But she makes sure to point out any risks associated with these approaches. While some alternative therapies can support patients through standard treatment, she strongly cautions patients against using these therapies in place of standard treatment.
“The bottom line is to keep the lines of communication open,” she said.
Like Dr. Lycette, Dr. Helft has been encountering more patients with alternative health beliefs who rely on people outside of the medical system for elements of their care.
In the past, he used to tell these patients that science is incomplete, and physicians don’t know everything. But he’s changed his tune.
“I’ve taken to just telling them what I believe, which is that the majority of things that they hear and are being sold are almost certainly ineffective and a waste of money,” he said. “I’ve come to accept that people are adults, and they make their own decisions, and sometimes they make decisions that are not the ones that I would make or want them to make.”
Delivering bad news
Dr. Helft often sees patients seeking a second or third opinion on their cancer. These patients may not all be in denial about having cancer, but they typically don’t want to hear bad news, which can make treatment a challenge.
To handle these scenarios, Dr. Helft has developed a system of responses for engaging with patients. He borrows an approach described in 2008 where he acknowledges a patient’s emotional distress and tries to understand why they may not want to know more.
For instance, he might tell a patient: “I have formulated an opinion about your situation, but it sounds as if you have heard many negative descriptions previously. I don’t want to burden you with one more if you don’t feel prepared to talk about it.”
Trying to understand why a patient is resistant to hearing about their condition may also help build trust. “If you could help me understand your thinking about why you would rather not talk about prognosis, it will help me know more about how to discuss other serious issues,” is one approach highlighted in the 2008 guide.
Behind the scenes, Dr. Helft will privately assess how much information about a patient’s prognosis is salient to their decision making, especially if the patient appears to misunderstand their prognosis or if there are various options for treatment over the long-term.
Dr. Helft will also ask patients how much they want to know. Do they want to discuss no options? A few? All and in detail?
This approach implicitly recognizes that the information is highly stressful but avoids being overly blunt, he notes. It can also help steer patients on the right treatment track and minimize poor decision making.
Samantha Winemaker, MD, a palliative care physician in Hamilton, Ont., finds patients often go through an adjustment period after learning about a new diagnosis. The reaction tends to range from needing time to accept the diagnosis as real to jumping in to understand as much as possible.
Dr. Winemaker, who cohosts “The Waiting Room Revolution” podcast that focuses on helping people deal with a serious illness, encourages physicians to be realistic with patients about their prognosis and deliver news with a dose of gentle truth from the start.
“We should invite patients ‘into the know’ as early as possible, while maintaining hope,” she said.
She calls this approach of balancing hope and reality “walking two roads” and said it extends throughout the illness journey. This way, patients are less likely to be surprised if things make a turn for the worse.
“We should never wait until the 11th hour to give someone bad news,” she said.
‘We all want to hope’
Dr. Epstein has listened to hundreds of hours of discussion between doctors and patients as part of his research on communication. He often hears doctors initiate difficult conversations by lecturing a patient.
Many physicians mistakenly believe that, if they say something authoritatively, patients will believe it, he said. But the opposite often happens – patients shut down and instinctively distrust the physician.
Dr. Epstein teaches doctors to establish trust before providing difficult information. Even when a patient expresses outlandish ideas about their illness, treat them with dignity and respect, he advised. “If people don’t feel respected, you don’t have a leg to stand on and there’s no point in trying to convince them.”
Patients and physicians often leave conversations with discordant views of what’s ahead. In one study, two-thirds of patients held wildly different views on their prognosis, compared with their doctors, and most had no idea they were at odds with their physician.
In the past, Dr. Epstein has tried to close the gap between his understanding of a patient’s prognosis and the patient’s. But more recently he has become less convinced of the need to do so.
“What I try to do now is focus more on the uncertainty there,” he said. He uses phrases like: “Given that we don’t know how long you will live, I just need to know what you would want me to do if things took a turn for the worse” or “I’m worried that if you don’t have the surgery, you might experience more pain in the future.”
He urged doctors to pay attention to their word choices. Use care with the phrase “response rate” – patients sometimes mistake this to mean that they are being cured. And, instead of telling patients they “must” do something, he says that he worries about consequences for them if they don’t.
He asks patients what they’re hearing from other people in their lives or online. Sometimes patients say that people close to them are encouraging them to stop medical treatment or pursue alternative therapies. When that happens, Dr. Epstein asks to meet with that person to talk to them about his concerns for their loved one.
He also acknowledges calculated uncertainty often exists in medicine. That, he says, leaves open the potential for exceptional circumstances.
“And we all want to hope,” Dr. Epstein said.
A version of this article first appeared on Medscape.com.
In June, Rebecca A. Shatsky, MD, a medical oncologist, turned to Twitter for advice: “What do you do/say when a patient won’t believe you that they have #CANCER. As an oncologist this comes up every now and then and proves very difficult, looking to hear how others have dealt and what works best to help patients here.”
About a dozen people weighed in, offering various thoughts on how to approach these thorny situations. One oncologist suggested revisiting the conversation a few days later, after the patient has more time to process; others suggested sharing the pathology report or images with their patient.
Another person simply noted that “if a [patient] doesn’t want to believe they have cancer, no amount of evidence will change that.”
Based on the initial responses, “it appears there is a paucity of answers sadly,” wrote Dr. Shatsky, a breast cancer specialist at University of California, San Diego.
But for Dr. Shatsky, these incidents spoke to another alarming trend: a rampant mistrust of the medical community that is “becoming MORE common instead of less.”
‘Erosion of trust’
Overall, experts say that situations like the one Dr. Shatsky described – patients who don’t believe their cancer diagnosis – occur infrequently.
But denial comes in many forms, and complete disbelief is probably the most extreme.
Like Dr. Shatsky, these experts say they are also seeing a troubling increase in patients who don’t believe their physicians or don’t trust their recommendations.
“I think there’s an erosion of trust in expertise, in general,” said Ronald M. Epstein, MD, professor of family medicine and psychiatry & oncology at the University of Rochester (N.Y.). “People distrust science more than they did maybe 20 or 30 years ago, or at least that seems to be the case.”
Denial and distrust in cancer care are not new. These responses – along with wishful thinking, distraction, and minimization – are long-established responses among oncology patients. In 1972, Avery D. Weisman, MD, a psychiatrist at Harvard Medical School, Boston, wrote his book “On Dying and Denying,” and ever since, denial and similar responses have been explored in the oncology literature.
Much of this research has focused on the latter stages of illness, but denial can be present at diagnosis as well. One study of patients with breast cancer, carried out nearly 30 years ago, suggested that denial of diagnosis generally occurs early in a patient’s course of illness and decreases over time, but may arise again in the terminal phase of cancer. Another analysis, evaluating this phenomenon across 13 studies, found that the prevalence of denial at diagnosis ranged from 4% to as high as 47%.
An oncologist delivers somewhere between 10,000 to 30,000 episodes of bad news over the course of a career, so there’s always a chance that a patient will respond in a way that’s on the “spectrum of disbelief,” said Paul Helft, MD, professor of medicine and recently retired director of the ethics center at Indiana University, Indianapolis.
Diane Meier, MD, said denial and disbelief are natural, protective responses to difficult or frightening news.
When patients exhibit denial, Dr. Meier advises patience and time. Physicians can also ask the patient if there’s a person they trust – a family member or faith leader, for example – who could speak on their behalf about possible next steps.
“The main thing is not to find ourselves in opposition to the patient ... or threaten them with what will happen if they don’t listen to us,” said Dr. Meier, a professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York.
And physicians should be careful when they feel themselves wanting to argue with or lecture a patient.
“The minute we feel that urge coming on, that’s a signal to us to stop and realize that something is going on inside the patient that we don’t understand,” she said. “Forcing information on a person who is signaling in every way that they don’t want it and can’t handle it is not a recipe for trust or a high-quality relationship.”
Refusing expert advice
Jennifer Lycette, MD, has encountered a growing number of patients who don’t believe their disease should be treated the way she or other oncologists recommend. Some patients remain adamant about sticking with alternative medicine or doing nothing, despite growing sicker.
“I’ve even had situations where the tumor might be visible, like growing through the skin, and people still double down that whatever they’re doing is working,” said Dr. Lycette, a hematologist and medical oncologist at the Providence Seaside Cancer Center in Seaside, Ore.
She encourages these patients to get a second opinion and tries to keep an open mind about alternative approaches. If she’s not familiar with something a patient is considering, she’ll research it with them.
But she makes sure to point out any risks associated with these approaches. While some alternative therapies can support patients through standard treatment, she strongly cautions patients against using these therapies in place of standard treatment.
“The bottom line is to keep the lines of communication open,” she said.
Like Dr. Lycette, Dr. Helft has been encountering more patients with alternative health beliefs who rely on people outside of the medical system for elements of their care.
In the past, he used to tell these patients that science is incomplete, and physicians don’t know everything. But he’s changed his tune.
“I’ve taken to just telling them what I believe, which is that the majority of things that they hear and are being sold are almost certainly ineffective and a waste of money,” he said. “I’ve come to accept that people are adults, and they make their own decisions, and sometimes they make decisions that are not the ones that I would make or want them to make.”
Delivering bad news
Dr. Helft often sees patients seeking a second or third opinion on their cancer. These patients may not all be in denial about having cancer, but they typically don’t want to hear bad news, which can make treatment a challenge.
To handle these scenarios, Dr. Helft has developed a system of responses for engaging with patients. He borrows an approach described in 2008 where he acknowledges a patient’s emotional distress and tries to understand why they may not want to know more.
For instance, he might tell a patient: “I have formulated an opinion about your situation, but it sounds as if you have heard many negative descriptions previously. I don’t want to burden you with one more if you don’t feel prepared to talk about it.”
Trying to understand why a patient is resistant to hearing about their condition may also help build trust. “If you could help me understand your thinking about why you would rather not talk about prognosis, it will help me know more about how to discuss other serious issues,” is one approach highlighted in the 2008 guide.
Behind the scenes, Dr. Helft will privately assess how much information about a patient’s prognosis is salient to their decision making, especially if the patient appears to misunderstand their prognosis or if there are various options for treatment over the long-term.
Dr. Helft will also ask patients how much they want to know. Do they want to discuss no options? A few? All and in detail?
This approach implicitly recognizes that the information is highly stressful but avoids being overly blunt, he notes. It can also help steer patients on the right treatment track and minimize poor decision making.
Samantha Winemaker, MD, a palliative care physician in Hamilton, Ont., finds patients often go through an adjustment period after learning about a new diagnosis. The reaction tends to range from needing time to accept the diagnosis as real to jumping in to understand as much as possible.
Dr. Winemaker, who cohosts “The Waiting Room Revolution” podcast that focuses on helping people deal with a serious illness, encourages physicians to be realistic with patients about their prognosis and deliver news with a dose of gentle truth from the start.
“We should invite patients ‘into the know’ as early as possible, while maintaining hope,” she said.
She calls this approach of balancing hope and reality “walking two roads” and said it extends throughout the illness journey. This way, patients are less likely to be surprised if things make a turn for the worse.
“We should never wait until the 11th hour to give someone bad news,” she said.
‘We all want to hope’
Dr. Epstein has listened to hundreds of hours of discussion between doctors and patients as part of his research on communication. He often hears doctors initiate difficult conversations by lecturing a patient.
Many physicians mistakenly believe that, if they say something authoritatively, patients will believe it, he said. But the opposite often happens – patients shut down and instinctively distrust the physician.
Dr. Epstein teaches doctors to establish trust before providing difficult information. Even when a patient expresses outlandish ideas about their illness, treat them with dignity and respect, he advised. “If people don’t feel respected, you don’t have a leg to stand on and there’s no point in trying to convince them.”
Patients and physicians often leave conversations with discordant views of what’s ahead. In one study, two-thirds of patients held wildly different views on their prognosis, compared with their doctors, and most had no idea they were at odds with their physician.
In the past, Dr. Epstein has tried to close the gap between his understanding of a patient’s prognosis and the patient’s. But more recently he has become less convinced of the need to do so.
“What I try to do now is focus more on the uncertainty there,” he said. He uses phrases like: “Given that we don’t know how long you will live, I just need to know what you would want me to do if things took a turn for the worse” or “I’m worried that if you don’t have the surgery, you might experience more pain in the future.”
He urged doctors to pay attention to their word choices. Use care with the phrase “response rate” – patients sometimes mistake this to mean that they are being cured. And, instead of telling patients they “must” do something, he says that he worries about consequences for them if they don’t.
He asks patients what they’re hearing from other people in their lives or online. Sometimes patients say that people close to them are encouraging them to stop medical treatment or pursue alternative therapies. When that happens, Dr. Epstein asks to meet with that person to talk to them about his concerns for their loved one.
He also acknowledges calculated uncertainty often exists in medicine. That, he says, leaves open the potential for exceptional circumstances.
“And we all want to hope,” Dr. Epstein said.
A version of this article first appeared on Medscape.com.
In June, Rebecca A. Shatsky, MD, a medical oncologist, turned to Twitter for advice: “What do you do/say when a patient won’t believe you that they have #CANCER. As an oncologist this comes up every now and then and proves very difficult, looking to hear how others have dealt and what works best to help patients here.”
About a dozen people weighed in, offering various thoughts on how to approach these thorny situations. One oncologist suggested revisiting the conversation a few days later, after the patient has more time to process; others suggested sharing the pathology report or images with their patient.
Another person simply noted that “if a [patient] doesn’t want to believe they have cancer, no amount of evidence will change that.”
Based on the initial responses, “it appears there is a paucity of answers sadly,” wrote Dr. Shatsky, a breast cancer specialist at University of California, San Diego.
But for Dr. Shatsky, these incidents spoke to another alarming trend: a rampant mistrust of the medical community that is “becoming MORE common instead of less.”
‘Erosion of trust’
Overall, experts say that situations like the one Dr. Shatsky described – patients who don’t believe their cancer diagnosis – occur infrequently.
But denial comes in many forms, and complete disbelief is probably the most extreme.
Like Dr. Shatsky, these experts say they are also seeing a troubling increase in patients who don’t believe their physicians or don’t trust their recommendations.
“I think there’s an erosion of trust in expertise, in general,” said Ronald M. Epstein, MD, professor of family medicine and psychiatry & oncology at the University of Rochester (N.Y.). “People distrust science more than they did maybe 20 or 30 years ago, or at least that seems to be the case.”
Denial and distrust in cancer care are not new. These responses – along with wishful thinking, distraction, and minimization – are long-established responses among oncology patients. In 1972, Avery D. Weisman, MD, a psychiatrist at Harvard Medical School, Boston, wrote his book “On Dying and Denying,” and ever since, denial and similar responses have been explored in the oncology literature.
Much of this research has focused on the latter stages of illness, but denial can be present at diagnosis as well. One study of patients with breast cancer, carried out nearly 30 years ago, suggested that denial of diagnosis generally occurs early in a patient’s course of illness and decreases over time, but may arise again in the terminal phase of cancer. Another analysis, evaluating this phenomenon across 13 studies, found that the prevalence of denial at diagnosis ranged from 4% to as high as 47%.
An oncologist delivers somewhere between 10,000 to 30,000 episodes of bad news over the course of a career, so there’s always a chance that a patient will respond in a way that’s on the “spectrum of disbelief,” said Paul Helft, MD, professor of medicine and recently retired director of the ethics center at Indiana University, Indianapolis.
Diane Meier, MD, said denial and disbelief are natural, protective responses to difficult or frightening news.
When patients exhibit denial, Dr. Meier advises patience and time. Physicians can also ask the patient if there’s a person they trust – a family member or faith leader, for example – who could speak on their behalf about possible next steps.
“The main thing is not to find ourselves in opposition to the patient ... or threaten them with what will happen if they don’t listen to us,” said Dr. Meier, a professor of geriatrics and palliative medicine at the Icahn School of Medicine at Mount Sinai, New York.
And physicians should be careful when they feel themselves wanting to argue with or lecture a patient.
“The minute we feel that urge coming on, that’s a signal to us to stop and realize that something is going on inside the patient that we don’t understand,” she said. “Forcing information on a person who is signaling in every way that they don’t want it and can’t handle it is not a recipe for trust or a high-quality relationship.”
Refusing expert advice
Jennifer Lycette, MD, has encountered a growing number of patients who don’t believe their disease should be treated the way she or other oncologists recommend. Some patients remain adamant about sticking with alternative medicine or doing nothing, despite growing sicker.
“I’ve even had situations where the tumor might be visible, like growing through the skin, and people still double down that whatever they’re doing is working,” said Dr. Lycette, a hematologist and medical oncologist at the Providence Seaside Cancer Center in Seaside, Ore.
She encourages these patients to get a second opinion and tries to keep an open mind about alternative approaches. If she’s not familiar with something a patient is considering, she’ll research it with them.
But she makes sure to point out any risks associated with these approaches. While some alternative therapies can support patients through standard treatment, she strongly cautions patients against using these therapies in place of standard treatment.
“The bottom line is to keep the lines of communication open,” she said.
Like Dr. Lycette, Dr. Helft has been encountering more patients with alternative health beliefs who rely on people outside of the medical system for elements of their care.
In the past, he used to tell these patients that science is incomplete, and physicians don’t know everything. But he’s changed his tune.
“I’ve taken to just telling them what I believe, which is that the majority of things that they hear and are being sold are almost certainly ineffective and a waste of money,” he said. “I’ve come to accept that people are adults, and they make their own decisions, and sometimes they make decisions that are not the ones that I would make or want them to make.”
Delivering bad news
Dr. Helft often sees patients seeking a second or third opinion on their cancer. These patients may not all be in denial about having cancer, but they typically don’t want to hear bad news, which can make treatment a challenge.
To handle these scenarios, Dr. Helft has developed a system of responses for engaging with patients. He borrows an approach described in 2008 where he acknowledges a patient’s emotional distress and tries to understand why they may not want to know more.
For instance, he might tell a patient: “I have formulated an opinion about your situation, but it sounds as if you have heard many negative descriptions previously. I don’t want to burden you with one more if you don’t feel prepared to talk about it.”
Trying to understand why a patient is resistant to hearing about their condition may also help build trust. “If you could help me understand your thinking about why you would rather not talk about prognosis, it will help me know more about how to discuss other serious issues,” is one approach highlighted in the 2008 guide.
Behind the scenes, Dr. Helft will privately assess how much information about a patient’s prognosis is salient to their decision making, especially if the patient appears to misunderstand their prognosis or if there are various options for treatment over the long-term.
Dr. Helft will also ask patients how much they want to know. Do they want to discuss no options? A few? All and in detail?
This approach implicitly recognizes that the information is highly stressful but avoids being overly blunt, he notes. It can also help steer patients on the right treatment track and minimize poor decision making.
Samantha Winemaker, MD, a palliative care physician in Hamilton, Ont., finds patients often go through an adjustment period after learning about a new diagnosis. The reaction tends to range from needing time to accept the diagnosis as real to jumping in to understand as much as possible.
Dr. Winemaker, who cohosts “The Waiting Room Revolution” podcast that focuses on helping people deal with a serious illness, encourages physicians to be realistic with patients about their prognosis and deliver news with a dose of gentle truth from the start.
“We should invite patients ‘into the know’ as early as possible, while maintaining hope,” she said.
She calls this approach of balancing hope and reality “walking two roads” and said it extends throughout the illness journey. This way, patients are less likely to be surprised if things make a turn for the worse.
“We should never wait until the 11th hour to give someone bad news,” she said.
‘We all want to hope’
Dr. Epstein has listened to hundreds of hours of discussion between doctors and patients as part of his research on communication. He often hears doctors initiate difficult conversations by lecturing a patient.
Many physicians mistakenly believe that, if they say something authoritatively, patients will believe it, he said. But the opposite often happens – patients shut down and instinctively distrust the physician.
Dr. Epstein teaches doctors to establish trust before providing difficult information. Even when a patient expresses outlandish ideas about their illness, treat them with dignity and respect, he advised. “If people don’t feel respected, you don’t have a leg to stand on and there’s no point in trying to convince them.”
Patients and physicians often leave conversations with discordant views of what’s ahead. In one study, two-thirds of patients held wildly different views on their prognosis, compared with their doctors, and most had no idea they were at odds with their physician.
In the past, Dr. Epstein has tried to close the gap between his understanding of a patient’s prognosis and the patient’s. But more recently he has become less convinced of the need to do so.
“What I try to do now is focus more on the uncertainty there,” he said. He uses phrases like: “Given that we don’t know how long you will live, I just need to know what you would want me to do if things took a turn for the worse” or “I’m worried that if you don’t have the surgery, you might experience more pain in the future.”
He urged doctors to pay attention to their word choices. Use care with the phrase “response rate” – patients sometimes mistake this to mean that they are being cured. And, instead of telling patients they “must” do something, he says that he worries about consequences for them if they don’t.
He asks patients what they’re hearing from other people in their lives or online. Sometimes patients say that people close to them are encouraging them to stop medical treatment or pursue alternative therapies. When that happens, Dr. Epstein asks to meet with that person to talk to them about his concerns for their loved one.
He also acknowledges calculated uncertainty often exists in medicine. That, he says, leaves open the potential for exceptional circumstances.
“And we all want to hope,” Dr. Epstein said.
A version of this article first appeared on Medscape.com.
Secondary CV prevention benefit from polypill promises global health benefit
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
Compared with separate medications in patients with a prior myocardial infarction, a single pill containing aspirin, a lipid-lowering agent, and an ACE inhibitor provided progressively greater protection from a second cardiovascular (CV) event over the course of a trial with several years of follow-up, according to results of a multinational trial.
“The curves began to separate at the very beginning of the trial, and they are continuing to separate, so we can begin to project the possibility that the results would be even more striking if we had an even longer follow-up,” said Valentin Fuster, MD, physician in chief, Mount Sinai Hospital, New York, who presented the results at the annual congress of the European Society of Cardiology.
By “striking,” Dr. Fuster was referring to a 24% reduction in the hazard ratio of major adverse CV events (MACE) for a trial in which patients were followed for a median of 3 years. The primary composite endpoint consisted of cardiovascular death, MI, stroke, and urgent revascularization (HR, 0.76; P = .02).
AS for the secondary composite endpoint, confined to CV death, MI, and stroke, use of the polypill linked to an even greater relative advantage over usual care (HR, 0.70; P = .005).
SECURE trial is latest test of polypill concept
A polypill strategy has been pursued for more than 15 years, according to Dr. Fuster. Other polypill studies have also generated positive results, but the latest trial, called SECURE, is the largest prospective randomized trial to evaluate a single pill combining multiple therapies for secondary prevention.
The degree of relative benefit has “huge implications for clinical care,” reported the ESC-invited commentator, Louise Bowman, MBBS, MD, professor of medicine and clinical trials, University of Oxford (England). She called the findings “in line with what was expected,” but she agreed that the results will drive practice change.
The SECURE trial, published online in the New England Journal of Medicine at the time of its presentation at the ESC congress, randomized 2,499 patients over the age of 65 years who had a MI within the previous 6 months and at least one other risk factor, such as diabetes mellitus, kidney dysfunction, or a prior coronary revascularization. They were enrolled at 113 participating study centers in seven European countries.
Multiple polypill versions permit dose titration
The polypill consisted of aspirin in a fixed dose of 100 mg, the HMG CoA reductase inhibitor atorvastatin, and the ACE inhibitor ramipril. For atorvastatin and ramipril, the target doses were 40 mg and 10 mg, respectively, but different versions of the polypill were available to permit titration to a tolerated dose. Usual care was provided by participating investigators according to ESC recommendations.
The average age of those enrolled was 76 years. Nearly one-third (31%) were women. At baseline, most had hypertension (77.9%), and the majority had diabetes (57.4%).
When the events in the primary endpoint were assessed individually, the polypill was associated with a 33% relative reduction in the risk of CV death (HR, 0.67; P = .03). The reductions in the risk of nonfatal MI (HR, 0.71) and stroke (HR, 0.70) were of the same general magnitude although they did not reach statistical significance. There was no meaningful reduction in urgent revascularization (HR, 0.96).
In addition, the reduction in all-cause mortality (HR, 0.97) was not significant.
The rate of adverse events over the course of the study was 32.7% in the polypill group and 31.6% in the usual-care group, which did not differ significantly. There was also no difference in types of adverse events, including bleeding and other adverse events of interest, according to Dr. Fuster.
Adherence, which was monitored at 6 and 24 months using the Morisky Medication Adherence Scale, was characterized as low, medium, or high. More patients in the polypill group reached high adherence at 6 months (70.6% vs. 62.7%) and at 24 months (74.1% vs. 63.2%). Conversely, fewer patients in the polypill group were deemed to have low adherence at both time points.
“Probably, adherence is the most important reason of how this works,” Dr. Fuster said. Although there were no substantial differences in lipid levels or in systolic or diastolic blood pressure between the two groups when compared at 24 months, there are several theories that might explain the lower event rates in the polypill group, including a more sustained anti-inflammatory effect from greater adherence.
One potential limitation was the open-label design, but Dr. Bowman said that this was unavoidable, given the difficulty of blinding and the fact that comparing a single pill with multiple pills was “the point of the study.” She noted that the 14% withdrawal rate over the course of the trial, which was attributed largely to the COVID-19 pandemic, and the lower than planned enrollment (2,500 vs. a projected 3,000 patients) are also limitations, prohibiting “a more robust result,” but she did not dispute the conclusions.
Polypill benefit documented in all subgroups
While acknowledging these limitations, Dr. Fuster emphasized the consistency of these results with prior polypill studies and within the study. Of the 16 predefined subgroups, such as those created with stratifications for age, sex, comorbidities, and country of treatment, all benefited to a similar degree.
“This really validates the importance of the study,” Dr. Fuster said.
In addition to the implications for risk management globally, Dr. Fuster and others, including Dr. Bowman, spoke of the potential of a relatively inexpensive polypill to improve care in resource-limited settings. Despite the move toward greater personalization of medicine, Dr. Fuster called “simplicity the key to global health” initiatives.
Salim Yusuf, MD, DPhil, a leader in international polypill research, agreed. He believes the supportive data for this approach are conclusive.
“There are four positive trials of the polypill now and collectively the data are overwhelmingly clear,” Dr. Yusuf, professor of medicine, McMaster University, Hamilton, Ont., said in an interview. “The polypill should be considered in secondary prevention as well as in primary prevention for high-risk individuals. We have estimated that, if it is used in even 50% of those who should get it, it would avoid 2 million premature deaths from CV disease and 6 million nonfatal events. The next step is to implement the findings.”
Dr. Fuster, Dr. Bowman, and Dr. Yusuf reported no potential conflicts of interest.
FROM ESC CONGRESS 2022