User login
N.Y. governor declares state disaster emergency to boost polio vaccination
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
Roflumilast foam effectively eases seborrheic dermatitis
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
.
More than half experienced clearance of their symptoms, and three out of five achieved a significant improvement in pruritus, it was revealed during a late-breaking session at the annual congress of the European Academy of Dermatology and Venereology.
Common condition led to rapid recruitment
“Seborrheic dermatitis is a disease that’s very common, yet in my opinion, undertreated in dermatology,” said Andrew Blauvelt, MD, MBA, who presented the findings.
“It’s so common that when we did this trial, I was very surprised to see how easy it was to recruit,” said Dr. Blauvelt, a dermatologist who is president of the Oregon Medical Research Center, Portland. “Patients came in rapidly, out of the woodwork – they were desperate.”
While there are several tried and tested treatments for the condition, such as topical steroids and antifungal agents, he noted that they have their limitations: “Sometimes efficacy, sometimes the ability to be used on hair-bearing areas.”
Roflumilast is a phosphodiesterase 4 (PDE4) inhibitor that is available for topical use in a 0.3% cream formulation (Zoryve). This formulation gained FDA approval for plaque psoriasis for patients ages 12 and older this summer and is also under investigation as a treatment for atopic dermatitis.
It’s the same product in both preparations, Dr. Blauvelt said during the discussion period. “The only major difference between the cream and the foam is the propellant used to make it into a foam. Otherwise, they have the exact same list of ingredients.”
Dr. Blauvelt reported that just over 450 patients had been recruited at 53 U.S. centers into the 8-week, double-blind, placebo-controlled trial.
For inclusion, patients had to have moderate seborrheic dermatitis, defined as an Investigator’s Global Assessment (IGA) score of three or more. Dr. Blauvelt noted that patients as young as 9 years old could be recruited, and there was no upper age limit. The average age of participating patients, however, was around 42 years.
Multiple improvements seen in ‘happy trial’
The primary endpoint was an IGA score of 0 or 1 with at least a 2-grade improvement (IGA success) after 8 weeks of treatment. This was achieved by 80% of patients who were treated with roflumilast 0.3% foam, compared with 60% of those who were treated with the vehicle (P less than .0001).
Dr. Blauvelt pointed out that significant improvements had also been seen after 2 weeks (about 42% vs. about 26%; P = .0003) and 4 weeks (about 72% vs. about 49%; P less than .0001) of treatment.
“Now if we raise the bar a little higher” and ask how many patients were completely clear of their seborrheic dermatitis, Dr. Blauvelt said, it was 50% at 8 weeks, more than a third at 4 weeks, over 15% at 2 weeks with the foam, and significantly lower at just under 30%, 15%, and 7% in the vehicle group.
A 4-point or more improvement in the Worst Itch Numeric Rating Scale (WI-NRS) – accepted as the minimally clinically important difference – was achieved by more than 60% of patients treated with the foam at week 8, just under 50% at week 4, and just over 30% at week 2. Corresponding rates in the vehicle group were around 40%, 30%, and 15%.
“Many patients responded in this trial. So much so that when I was doing it, I called it the ‘happy trial.’ Every time I saw patients in this trial, they seemed to be happy,” Dr. Blauvelt said anecdotally.
“In terms of adverse events, the drug turned out to be very safe, and there didn’t seem to be any issues with any things that we see with, for example, oral phosphodiesterase inhibitors,” he added.
The tolerability findings suggest that the foam vehicle “was an excellent vehicle to be used for this particular drug,” with no signs of skin irritation, as rated by patients or investigators.
Lesson for practice: Advise patients to moisturize?
“It seems like the vehicle would be a good skincare product for patients,” observed the session’s cochair, Jo Lambert, MD, PhD, professor and academic head of the department of dermatology at Ghent University Hospital, Belgium.
It was “a pretty dramatic vehicle response, right?” Dr. Blauvelt responded. “We normally don’t think of telling seborrheic dermatitis patients to moisturize,” he added.
“I think one of the interesting findings is perhaps we should be telling them to moisturize their scalp or moisturize their face, or it could be something unique to this particular foam.”
The study was funded by Arcutis Biotherapeutics. Dr. Blauvelt disclosed that he was an investigator for the trial and acted as consultant to the company, receiving grants/research funding and/or honoraria. Several of the study’s co-investigators are employees of Arcutis. Dr. Lambert was not involved in the study and cochaired the late-breaking session during which the STRATUM trial findings were reported.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Ustekinumab has comparatively low infection rate in IBD
Patients with inflammatory bowel disease (IBD) taking ustekinumab (Stelara) are less likely to have infections as a side effect than those taking tofacitinib (Xeljanz) or anti–tumor necrosis factor-alpha (anti-TNF), researchers say.
Although the risk of infections is not large for any of these drugs, clinicians should nonetheless take it into account, given their similar efficacy, said Ashwin Ananthakrishnan, MD, associate professor of medicine at Massachusetts General Hospital, Boston.
“These findings may not dramatically change prescribing patterns, but in those who may be particularly vulnerable for infection, they could point toward the safer biologic being Stelara,” he told this news organization.
The study by Dr. Ananthakrishnan and his colleagues was published in Clinical Gastroenterology and Hepatology.
Biologic and small-molecule immunosuppressive therapies have emerged as effective treatments for Crohn’s disease and ulcerative colitis, the two conditions that comprise IBD.
But the recent proliferation of drugs in these classes with different mechanisms of action has made prescribing decisions complicated. And because the drugs suppress the immune system, they can make patients vulnerable to infection.
Randomized controlled trials, even those comparing the drugs head to head, have not been large enough to compare safety outcomes with statistical accuracy, Dr. Ananthakrishnan and his colleagues found.
Accessing a large, real-world cohort
To help fill this gap, they analyzed data on 89,972,617 people enrolled in Aetna, the nationwide U.S. health insurance plan, from 2008-2019.
They identified 19,096 patients whose IBD was treated with anti-TNF agents, 2,420 with ustekinumab, and 305 with tofacitinib. The number of patients taking tofacitinib is small because it is a newer drug, Dr. Ananthakrishnan said.
They found a higher rate of infection and rate of infection-related hospitalization for the patients taking anti-TNF agents (44% and 7%, respectively), compared with those taking ustekinumab (32% and 4%, respectively) over the course of up to a year.
The researchers adjusted for age, sex, IBD duration, prior corticosteroid use, prior immunomodulator use, prior IBD hospitalization, and comorbidities, after which they then compared the risks of infection.
They found that patients taking ustekinumab were 7% less likely to have any infection than patients taking anti-TNF agents (hazard ratio, 0.93), a statistically significant difference (95% confidence interval, 0.86-0.99; P = .041). The reduction in the risk of infection-related hospitalizations was similar but not statistically significant.
The advantage with ustekinumab over anti-TNF agents was larger for patients with comorbidities. There was a 25% reduction in the risk of infections for patients taking ustekinumab who had a Charlson comorbidity index score of at least 2 and who were younger than 65 years (HRm 0.71; 95% CI, 0.58-0.87; P < .001).
For patients taking tofacitinib, there were no significant differences in the rate of infection (HR, 0.97; 95% CI, 0.75-1.24) or infection-related hospitalizations (HR, 0.59; 95% CI, 0.27-1.05), compared with TNF-antagonists.
The respiratory system and the urinary tract were the most common sites of infections. Bacterial, viral, and fungal infections were similarly distributed in the three groups.
Remaining questions
Further research is needed, said Dr. Ananthakrishnan, as “to understand the comparative safety may be particularly important for vulnerable populations, like those who are older or have other underlying comorbidity, where safety is increasingly an important issue.”
The findings are similar to those of other studies comparing infections in association with ustekinumab to anti-TNF medications for related conditions, such as psoriatic arthritis and psoriasis, he said.
Clinicians have seen similar differences among the drugs in clinical practice, said Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at Cleveland Clinic, Ohio, who was not involved in the study.
“It aligns well with what we’ve thought, but it’s nice to see in a publication,” he said in an interview.
The implications of the study are limited, because it was not a prospective randomized trial and because the number of patients taking tofacitinib was so small, Dr. Regueiro added.
Another limitation is that the patients who were admitted to the hospital in this database were not necessarily admitted because of their infections, said Stephen Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who also was not involved in the study.
Dr. Hanauer told this news organization that comparisons with other agents would be helpful.
“They didn’t look at vedolizumab (Entyvio) in this database,” he said. “Entyvio is generally considered to be safer than TNF inhibitors or tofacitinib with fairly comparable safety to ustekinumab.”
Dr. Ananthakrishnan reported financial relationships with Gilead, Ikena Therapeutics, and Sun Pharma. Dr. Regueiro reported financial relationships with AbbVie, BMS, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, UCB, Miraca Labs, Amgen, Celgene, Seres, Allergan, Salix, Prometheus, Lilly, TARGET Pharma Solutions, Alfasigigma, and BMS. Dr. Hanauer reported financial relationships with Janssen Pharmaceuticals, AbbVie, Takeda Pharmaceutical, and Pfizer.
A version of this article first appeared on Medscape.com.
With our growing armamentarium of effective medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC) come increasing decisions for patients and providers for treatments based on comparative effectiveness and safety. One of the most frequent concerns by both patients and providers is risk of infection. While we have partial data on safety from clinical trials, trials are underpowered to compare safety outcomes and also typically compare placebo rather than other active treatments.
This study by Cheng et al. provides further reassurance that ustekinumab and tofacitinib are at least as safe from an infection standpoint as TNF inhibitors, with UST having a small, but statistically significant lower risk of infection overall compared to TNF inhibitors. While there have been signals that ustekinumab may have lower infection risk compared to TNF inhibitors from clinical trials and real-world analyses of ustekinumab in other disease states, this study is remarkable in that it studies CD and UC specifically. Ustekinumab dosing for CD and UC are higher than that used for other indications, so it’s highly relevant to study ustekinumab in CD and UC, specifically for safety. This study is also notable in that no statistically significant difference in infection, particularly herpes zoster, was observed in tofacitinib vs. TNF inhibitors.
This study has limitations as a retrospective administrative dataset, including its inability to determine indication of prescription for CD or UC vs. rheumatologic or dermatologic condition, lack of adjustment for concomitant immunomodulator use, and inability to determine primary indication for hospitalization. However, this study should allow providers to discuss with patients with greater confidence that infection risks of ustekinumab and tofacitinib were overall low and that ustekinumab has lower risks of infections than TNF inhibitors.
Jason K. Hou, MD, MS, is an investigator in the clinical epidemiology and outcomes program in the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center and an associate professor at the Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
With our growing armamentarium of effective medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC) come increasing decisions for patients and providers for treatments based on comparative effectiveness and safety. One of the most frequent concerns by both patients and providers is risk of infection. While we have partial data on safety from clinical trials, trials are underpowered to compare safety outcomes and also typically compare placebo rather than other active treatments.
This study by Cheng et al. provides further reassurance that ustekinumab and tofacitinib are at least as safe from an infection standpoint as TNF inhibitors, with UST having a small, but statistically significant lower risk of infection overall compared to TNF inhibitors. While there have been signals that ustekinumab may have lower infection risk compared to TNF inhibitors from clinical trials and real-world analyses of ustekinumab in other disease states, this study is remarkable in that it studies CD and UC specifically. Ustekinumab dosing for CD and UC are higher than that used for other indications, so it’s highly relevant to study ustekinumab in CD and UC, specifically for safety. This study is also notable in that no statistically significant difference in infection, particularly herpes zoster, was observed in tofacitinib vs. TNF inhibitors.
This study has limitations as a retrospective administrative dataset, including its inability to determine indication of prescription for CD or UC vs. rheumatologic or dermatologic condition, lack of adjustment for concomitant immunomodulator use, and inability to determine primary indication for hospitalization. However, this study should allow providers to discuss with patients with greater confidence that infection risks of ustekinumab and tofacitinib were overall low and that ustekinumab has lower risks of infections than TNF inhibitors.
Jason K. Hou, MD, MS, is an investigator in the clinical epidemiology and outcomes program in the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center and an associate professor at the Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
With our growing armamentarium of effective medical therapies for Crohn’s disease (CD) and ulcerative colitis (UC) come increasing decisions for patients and providers for treatments based on comparative effectiveness and safety. One of the most frequent concerns by both patients and providers is risk of infection. While we have partial data on safety from clinical trials, trials are underpowered to compare safety outcomes and also typically compare placebo rather than other active treatments.
This study by Cheng et al. provides further reassurance that ustekinumab and tofacitinib are at least as safe from an infection standpoint as TNF inhibitors, with UST having a small, but statistically significant lower risk of infection overall compared to TNF inhibitors. While there have been signals that ustekinumab may have lower infection risk compared to TNF inhibitors from clinical trials and real-world analyses of ustekinumab in other disease states, this study is remarkable in that it studies CD and UC specifically. Ustekinumab dosing for CD and UC are higher than that used for other indications, so it’s highly relevant to study ustekinumab in CD and UC, specifically for safety. This study is also notable in that no statistically significant difference in infection, particularly herpes zoster, was observed in tofacitinib vs. TNF inhibitors.
This study has limitations as a retrospective administrative dataset, including its inability to determine indication of prescription for CD or UC vs. rheumatologic or dermatologic condition, lack of adjustment for concomitant immunomodulator use, and inability to determine primary indication for hospitalization. However, this study should allow providers to discuss with patients with greater confidence that infection risks of ustekinumab and tofacitinib were overall low and that ustekinumab has lower risks of infections than TNF inhibitors.
Jason K. Hou, MD, MS, is an investigator in the clinical epidemiology and outcomes program in the Center for Innovations in Quality, Effectiveness and Safety at the Michael E. DeBakey VA Medical Center and an associate professor at the Baylor College of Medicine, both in Houston. He has no relevant conflicts of interest.
Patients with inflammatory bowel disease (IBD) taking ustekinumab (Stelara) are less likely to have infections as a side effect than those taking tofacitinib (Xeljanz) or anti–tumor necrosis factor-alpha (anti-TNF), researchers say.
Although the risk of infections is not large for any of these drugs, clinicians should nonetheless take it into account, given their similar efficacy, said Ashwin Ananthakrishnan, MD, associate professor of medicine at Massachusetts General Hospital, Boston.
“These findings may not dramatically change prescribing patterns, but in those who may be particularly vulnerable for infection, they could point toward the safer biologic being Stelara,” he told this news organization.
The study by Dr. Ananthakrishnan and his colleagues was published in Clinical Gastroenterology and Hepatology.
Biologic and small-molecule immunosuppressive therapies have emerged as effective treatments for Crohn’s disease and ulcerative colitis, the two conditions that comprise IBD.
But the recent proliferation of drugs in these classes with different mechanisms of action has made prescribing decisions complicated. And because the drugs suppress the immune system, they can make patients vulnerable to infection.
Randomized controlled trials, even those comparing the drugs head to head, have not been large enough to compare safety outcomes with statistical accuracy, Dr. Ananthakrishnan and his colleagues found.
Accessing a large, real-world cohort
To help fill this gap, they analyzed data on 89,972,617 people enrolled in Aetna, the nationwide U.S. health insurance plan, from 2008-2019.
They identified 19,096 patients whose IBD was treated with anti-TNF agents, 2,420 with ustekinumab, and 305 with tofacitinib. The number of patients taking tofacitinib is small because it is a newer drug, Dr. Ananthakrishnan said.
They found a higher rate of infection and rate of infection-related hospitalization for the patients taking anti-TNF agents (44% and 7%, respectively), compared with those taking ustekinumab (32% and 4%, respectively) over the course of up to a year.
The researchers adjusted for age, sex, IBD duration, prior corticosteroid use, prior immunomodulator use, prior IBD hospitalization, and comorbidities, after which they then compared the risks of infection.
They found that patients taking ustekinumab were 7% less likely to have any infection than patients taking anti-TNF agents (hazard ratio, 0.93), a statistically significant difference (95% confidence interval, 0.86-0.99; P = .041). The reduction in the risk of infection-related hospitalizations was similar but not statistically significant.
The advantage with ustekinumab over anti-TNF agents was larger for patients with comorbidities. There was a 25% reduction in the risk of infections for patients taking ustekinumab who had a Charlson comorbidity index score of at least 2 and who were younger than 65 years (HRm 0.71; 95% CI, 0.58-0.87; P < .001).
For patients taking tofacitinib, there were no significant differences in the rate of infection (HR, 0.97; 95% CI, 0.75-1.24) or infection-related hospitalizations (HR, 0.59; 95% CI, 0.27-1.05), compared with TNF-antagonists.
The respiratory system and the urinary tract were the most common sites of infections. Bacterial, viral, and fungal infections were similarly distributed in the three groups.
Remaining questions
Further research is needed, said Dr. Ananthakrishnan, as “to understand the comparative safety may be particularly important for vulnerable populations, like those who are older or have other underlying comorbidity, where safety is increasingly an important issue.”
The findings are similar to those of other studies comparing infections in association with ustekinumab to anti-TNF medications for related conditions, such as psoriatic arthritis and psoriasis, he said.
Clinicians have seen similar differences among the drugs in clinical practice, said Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at Cleveland Clinic, Ohio, who was not involved in the study.
“It aligns well with what we’ve thought, but it’s nice to see in a publication,” he said in an interview.
The implications of the study are limited, because it was not a prospective randomized trial and because the number of patients taking tofacitinib was so small, Dr. Regueiro added.
Another limitation is that the patients who were admitted to the hospital in this database were not necessarily admitted because of their infections, said Stephen Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who also was not involved in the study.
Dr. Hanauer told this news organization that comparisons with other agents would be helpful.
“They didn’t look at vedolizumab (Entyvio) in this database,” he said. “Entyvio is generally considered to be safer than TNF inhibitors or tofacitinib with fairly comparable safety to ustekinumab.”
Dr. Ananthakrishnan reported financial relationships with Gilead, Ikena Therapeutics, and Sun Pharma. Dr. Regueiro reported financial relationships with AbbVie, BMS, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, UCB, Miraca Labs, Amgen, Celgene, Seres, Allergan, Salix, Prometheus, Lilly, TARGET Pharma Solutions, Alfasigigma, and BMS. Dr. Hanauer reported financial relationships with Janssen Pharmaceuticals, AbbVie, Takeda Pharmaceutical, and Pfizer.
A version of this article first appeared on Medscape.com.
Patients with inflammatory bowel disease (IBD) taking ustekinumab (Stelara) are less likely to have infections as a side effect than those taking tofacitinib (Xeljanz) or anti–tumor necrosis factor-alpha (anti-TNF), researchers say.
Although the risk of infections is not large for any of these drugs, clinicians should nonetheless take it into account, given their similar efficacy, said Ashwin Ananthakrishnan, MD, associate professor of medicine at Massachusetts General Hospital, Boston.
“These findings may not dramatically change prescribing patterns, but in those who may be particularly vulnerable for infection, they could point toward the safer biologic being Stelara,” he told this news organization.
The study by Dr. Ananthakrishnan and his colleagues was published in Clinical Gastroenterology and Hepatology.
Biologic and small-molecule immunosuppressive therapies have emerged as effective treatments for Crohn’s disease and ulcerative colitis, the two conditions that comprise IBD.
But the recent proliferation of drugs in these classes with different mechanisms of action has made prescribing decisions complicated. And because the drugs suppress the immune system, they can make patients vulnerable to infection.
Randomized controlled trials, even those comparing the drugs head to head, have not been large enough to compare safety outcomes with statistical accuracy, Dr. Ananthakrishnan and his colleagues found.
Accessing a large, real-world cohort
To help fill this gap, they analyzed data on 89,972,617 people enrolled in Aetna, the nationwide U.S. health insurance plan, from 2008-2019.
They identified 19,096 patients whose IBD was treated with anti-TNF agents, 2,420 with ustekinumab, and 305 with tofacitinib. The number of patients taking tofacitinib is small because it is a newer drug, Dr. Ananthakrishnan said.
They found a higher rate of infection and rate of infection-related hospitalization for the patients taking anti-TNF agents (44% and 7%, respectively), compared with those taking ustekinumab (32% and 4%, respectively) over the course of up to a year.
The researchers adjusted for age, sex, IBD duration, prior corticosteroid use, prior immunomodulator use, prior IBD hospitalization, and comorbidities, after which they then compared the risks of infection.
They found that patients taking ustekinumab were 7% less likely to have any infection than patients taking anti-TNF agents (hazard ratio, 0.93), a statistically significant difference (95% confidence interval, 0.86-0.99; P = .041). The reduction in the risk of infection-related hospitalizations was similar but not statistically significant.
The advantage with ustekinumab over anti-TNF agents was larger for patients with comorbidities. There was a 25% reduction in the risk of infections for patients taking ustekinumab who had a Charlson comorbidity index score of at least 2 and who were younger than 65 years (HRm 0.71; 95% CI, 0.58-0.87; P < .001).
For patients taking tofacitinib, there were no significant differences in the rate of infection (HR, 0.97; 95% CI, 0.75-1.24) or infection-related hospitalizations (HR, 0.59; 95% CI, 0.27-1.05), compared with TNF-antagonists.
The respiratory system and the urinary tract were the most common sites of infections. Bacterial, viral, and fungal infections were similarly distributed in the three groups.
Remaining questions
Further research is needed, said Dr. Ananthakrishnan, as “to understand the comparative safety may be particularly important for vulnerable populations, like those who are older or have other underlying comorbidity, where safety is increasingly an important issue.”
The findings are similar to those of other studies comparing infections in association with ustekinumab to anti-TNF medications for related conditions, such as psoriatic arthritis and psoriasis, he said.
Clinicians have seen similar differences among the drugs in clinical practice, said Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at Cleveland Clinic, Ohio, who was not involved in the study.
“It aligns well with what we’ve thought, but it’s nice to see in a publication,” he said in an interview.
The implications of the study are limited, because it was not a prospective randomized trial and because the number of patients taking tofacitinib was so small, Dr. Regueiro added.
Another limitation is that the patients who were admitted to the hospital in this database were not necessarily admitted because of their infections, said Stephen Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who also was not involved in the study.
Dr. Hanauer told this news organization that comparisons with other agents would be helpful.
“They didn’t look at vedolizumab (Entyvio) in this database,” he said. “Entyvio is generally considered to be safer than TNF inhibitors or tofacitinib with fairly comparable safety to ustekinumab.”
Dr. Ananthakrishnan reported financial relationships with Gilead, Ikena Therapeutics, and Sun Pharma. Dr. Regueiro reported financial relationships with AbbVie, BMS, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, UCB, Miraca Labs, Amgen, Celgene, Seres, Allergan, Salix, Prometheus, Lilly, TARGET Pharma Solutions, Alfasigigma, and BMS. Dr. Hanauer reported financial relationships with Janssen Pharmaceuticals, AbbVie, Takeda Pharmaceutical, and Pfizer.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Sex is still a taboo subject for patients with breast cancer
and 20% noted a negative impact on their sex life. And while meeting with a specialist in psycho-oncology was universally viewed as an acceptable option, only one out of four patients considered consulting a sexologist. All these women should be encouraged to face and address issues related to sexuality so that they can truly regain a good quality of life, the study suggests.
The study, which was conducted at the breast unit of Santa Maria Goretti Hospital in Latina, Italy, enrolled 141 patients who had undergone breast cancer surgery. Participants were asked to complete a questionnaire that included questions regarding self-image, sexual activity, and sexual satisfaction, and it analyzed these aspects before and after treatment. The participants were then asked whether they felt that they needed to see a sexologist or a specialist in psycho-oncology.
The findings clearly showed a worsening in terms of body image perception. When the women were asked about the relationship they had with their body, femininity, and beauty prior to being diagnosed, 37.4% characterized it as very good and 58.9% as “normal,” with ups and downs but nothing that they would term “conflictual.” After diagnosis, 48.9% noted that the disease had an impact on their body image with a partial conditioning about their femininity and beauty. However, 7.2% had difficulty when it came to recognizing their own body, and their relationship with femininity also became difficult.
On the topic of sexuality, 71.2% of patients were completely satisfied with their sex life before they were diagnosed with breast cancer, 23.7% were partially satisfied, and 5.0% were unsatisfied. As for their sex life after diagnosis and surgery, 20.1% stated that it continued to be fulfilling and 55.4% said that it had gotten worse; 18.8% reported significant sexual dissatisfaction.
The participants were asked whether consulting a professional would be warranted, and whether that would provide useful support for overcoming the difficulties and challenges arising from the disease and the related treatments. In response, 97.1% said they would go to a specialist in psycho-oncology, but only 27.3% would seek help from a sexologist.
“Despite the negative impact on body image and on sexuality, few patients would seek the help of a sexologist; nearly all of the patients, however, would seek the help of a specialist in psycho-oncology. This was very surprising to us,” write the authors. They went on to note that they are carrying out another project to understand the reason for this disparity.
In addition, they advised clinicians to encourage communication about sexuality – a topic that is regularly overlooked and not included in discussions with patients, mostly because of cultural barriers. Often, physicians aren’t comfortable talking about sexuality, as they don’t feel they have the proper training to do so. Patients who are experiencing issues related to sexuality also often have difficulty asking for help. And so, in their conclusion, the authors point out that “collaborating together in the right direction is the basis of change and good communication.”
This article was translated from Univadis Italy and appeared on Medscape.com.
and 20% noted a negative impact on their sex life. And while meeting with a specialist in psycho-oncology was universally viewed as an acceptable option, only one out of four patients considered consulting a sexologist. All these women should be encouraged to face and address issues related to sexuality so that they can truly regain a good quality of life, the study suggests.
The study, which was conducted at the breast unit of Santa Maria Goretti Hospital in Latina, Italy, enrolled 141 patients who had undergone breast cancer surgery. Participants were asked to complete a questionnaire that included questions regarding self-image, sexual activity, and sexual satisfaction, and it analyzed these aspects before and after treatment. The participants were then asked whether they felt that they needed to see a sexologist or a specialist in psycho-oncology.
The findings clearly showed a worsening in terms of body image perception. When the women were asked about the relationship they had with their body, femininity, and beauty prior to being diagnosed, 37.4% characterized it as very good and 58.9% as “normal,” with ups and downs but nothing that they would term “conflictual.” After diagnosis, 48.9% noted that the disease had an impact on their body image with a partial conditioning about their femininity and beauty. However, 7.2% had difficulty when it came to recognizing their own body, and their relationship with femininity also became difficult.
On the topic of sexuality, 71.2% of patients were completely satisfied with their sex life before they were diagnosed with breast cancer, 23.7% were partially satisfied, and 5.0% were unsatisfied. As for their sex life after diagnosis and surgery, 20.1% stated that it continued to be fulfilling and 55.4% said that it had gotten worse; 18.8% reported significant sexual dissatisfaction.
The participants were asked whether consulting a professional would be warranted, and whether that would provide useful support for overcoming the difficulties and challenges arising from the disease and the related treatments. In response, 97.1% said they would go to a specialist in psycho-oncology, but only 27.3% would seek help from a sexologist.
“Despite the negative impact on body image and on sexuality, few patients would seek the help of a sexologist; nearly all of the patients, however, would seek the help of a specialist in psycho-oncology. This was very surprising to us,” write the authors. They went on to note that they are carrying out another project to understand the reason for this disparity.
In addition, they advised clinicians to encourage communication about sexuality – a topic that is regularly overlooked and not included in discussions with patients, mostly because of cultural barriers. Often, physicians aren’t comfortable talking about sexuality, as they don’t feel they have the proper training to do so. Patients who are experiencing issues related to sexuality also often have difficulty asking for help. And so, in their conclusion, the authors point out that “collaborating together in the right direction is the basis of change and good communication.”
This article was translated from Univadis Italy and appeared on Medscape.com.
and 20% noted a negative impact on their sex life. And while meeting with a specialist in psycho-oncology was universally viewed as an acceptable option, only one out of four patients considered consulting a sexologist. All these women should be encouraged to face and address issues related to sexuality so that they can truly regain a good quality of life, the study suggests.
The study, which was conducted at the breast unit of Santa Maria Goretti Hospital in Latina, Italy, enrolled 141 patients who had undergone breast cancer surgery. Participants were asked to complete a questionnaire that included questions regarding self-image, sexual activity, and sexual satisfaction, and it analyzed these aspects before and after treatment. The participants were then asked whether they felt that they needed to see a sexologist or a specialist in psycho-oncology.
The findings clearly showed a worsening in terms of body image perception. When the women were asked about the relationship they had with their body, femininity, and beauty prior to being diagnosed, 37.4% characterized it as very good and 58.9% as “normal,” with ups and downs but nothing that they would term “conflictual.” After diagnosis, 48.9% noted that the disease had an impact on their body image with a partial conditioning about their femininity and beauty. However, 7.2% had difficulty when it came to recognizing their own body, and their relationship with femininity also became difficult.
On the topic of sexuality, 71.2% of patients were completely satisfied with their sex life before they were diagnosed with breast cancer, 23.7% were partially satisfied, and 5.0% were unsatisfied. As for their sex life after diagnosis and surgery, 20.1% stated that it continued to be fulfilling and 55.4% said that it had gotten worse; 18.8% reported significant sexual dissatisfaction.
The participants were asked whether consulting a professional would be warranted, and whether that would provide useful support for overcoming the difficulties and challenges arising from the disease and the related treatments. In response, 97.1% said they would go to a specialist in psycho-oncology, but only 27.3% would seek help from a sexologist.
“Despite the negative impact on body image and on sexuality, few patients would seek the help of a sexologist; nearly all of the patients, however, would seek the help of a specialist in psycho-oncology. This was very surprising to us,” write the authors. They went on to note that they are carrying out another project to understand the reason for this disparity.
In addition, they advised clinicians to encourage communication about sexuality – a topic that is regularly overlooked and not included in discussions with patients, mostly because of cultural barriers. Often, physicians aren’t comfortable talking about sexuality, as they don’t feel they have the proper training to do so. Patients who are experiencing issues related to sexuality also often have difficulty asking for help. And so, in their conclusion, the authors point out that “collaborating together in the right direction is the basis of change and good communication.”
This article was translated from Univadis Italy and appeared on Medscape.com.
An FP’s guide to exercise counseling for older adults
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
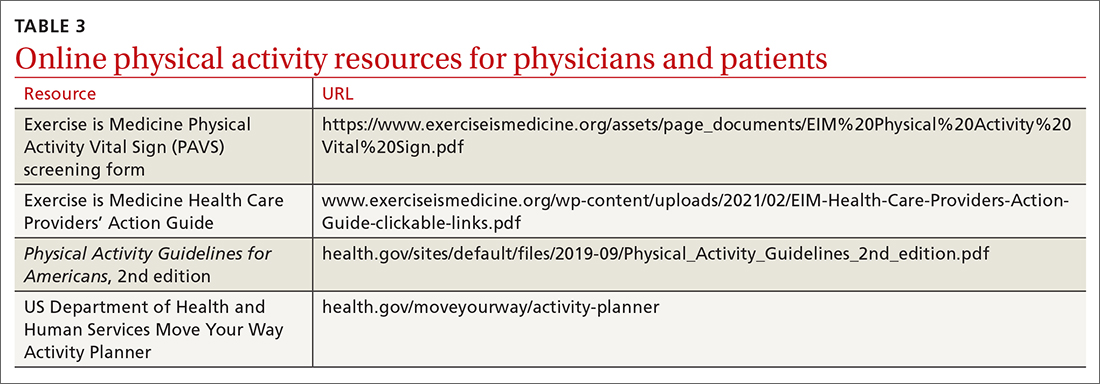
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)
Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2
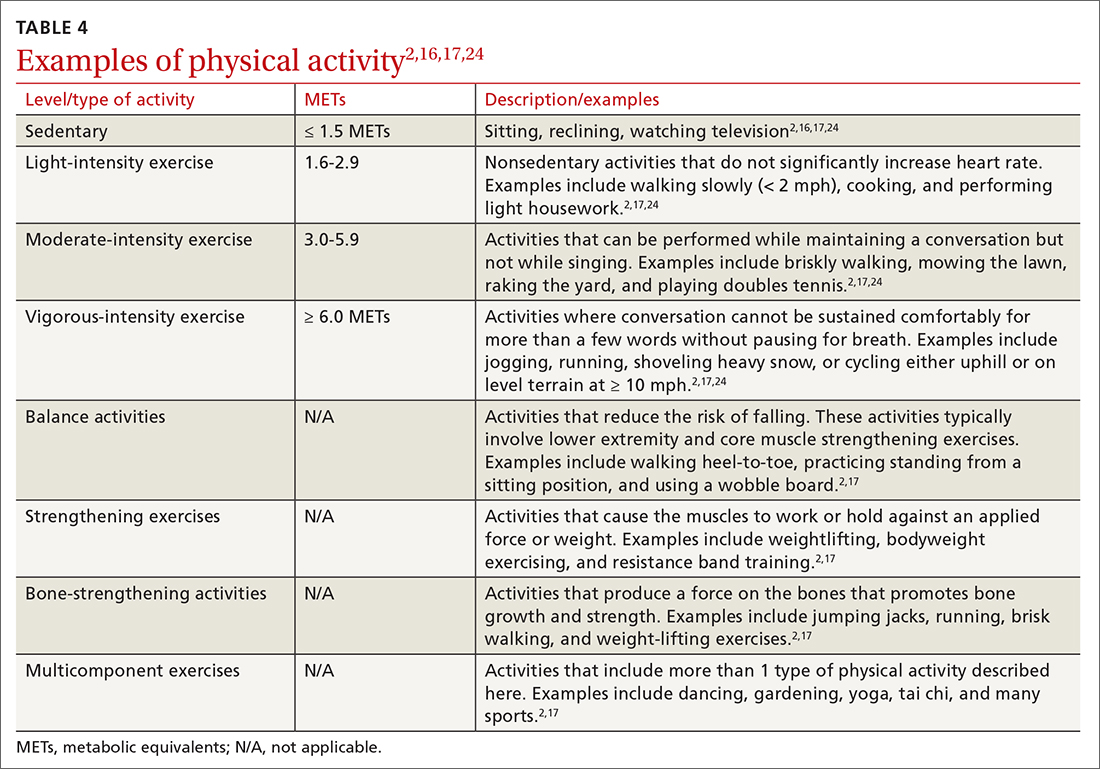
The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
PRACTICE RECOMMENDATIONS
› Encourage older adults to engage in at least 150 minutes of moderate-intensity aerobic physical activity throughout the week, OR at least 75 minutes of vigorous-intensity aerobic physical activity throughout the week, OR an equivalent combination of moderate- and vigorous-intensity activity. A
› Recommend older adults perform muscle-strengthening activities involving major muscle groups on 2 or more days per week. A
› Encourage older adults to be as physically active as possible, even when their health conditions and abilities prevent them from reaching their minimum levels of physical activity. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Congenital cytomegalovirus declined in wake of COVID-19
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM JAMA NETWORK OPEN
Dupilumab offers ‘clinically meaningful’ improvements in prurigo nodularis
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
(Dupixent), indicate results from the phase 2 LIBERTY-PN PRIME trial.
The research was presented at the annual Congress of the European Academy of Dermatology and Venereology.
More than 150 patients with severe PN whose quality of life was impaired were randomly assigned to receive dupilumab (Dupixent) or placebo for 24 weeks. Use of the monoclonal antibody was associated with significant improvements in itch scores.
The researchers also found that the percentage of patients who had no or few PN lesions increased substantially with use of dupilumab, and there were no new safety signals, confirming results from previous studies. Dupilumab, an interleukin-4 receptor alpha antagonist administered by injection, was initially approved by the U.S. Food and Drug Administration for treating atopic dermatitis in 2022.
Study presenter Gil Yosipovitch, MD, professor of dermatology at the University of Miami, emphasized that the improvements in itch and skin lesions seen in these patients were “clinically meaningful.”
In the discussion after the presentation, Dr. Yosipovitch was asked whether the presence or absence of atopy had any bearing on the results.
He replied that although there were too few patients with atopy in the current study to answer that question, other data indicate that there is no overall difference between patients with atopy and those without atopy.
Asked whether dupilumab should be used for only 24 weeks, Dr. Yosipovitch said his that “impression” is that there can be a “honeymoon period” during which the medication is stopped and the treating clinician sees “what happens.”
“It would be interesting in the future” to find out, he added, but he noted that whatever the result, patients would need treatment “for the rest of their life.”
Dr. Yosipovitch, director of the Miami Itch Center and the study’s principal investigator, began his presentation by noting that currently, no systemic therapies have been approved by the FDA or the European Medicines Agency for PN.
Although treatments such as topical medications, ultraviolet light therapy, immunosuppressive agents, and systemic neuromodulators are used off label, for many patients with moderate to severe PN, disease control is inadequate, and the patients are “miserable.”
Recently, the phase 3 LIBERTY-PN PRIME2 trial showed that dupilumab significantly reduced itch and skin lesions for patients with PN, and the safety profile was consistent with that seen in approved indications for the drug.
Dr. Yosipovitch explained that LIBERTY-PN PRIME was a phase 2 study in which, after a screening period, patients with PN were randomly assigned in a 1:1 ratio to receive dupilumab as a 600-mg loading dose followed by 300 mg twice weekly or a matched placebo. Treatment was given for 24 weeks, after which there was a post treatment 12-week follow-up period.
Participants were aged 18-80 years and had been diagnosed with PN for a period of at least 3 months. To be included in the trial, patients had to have an average Worst Itch Numerical Rating Scale (WI-NRS) score of at least 7 and at least 20 lesions, among other criteria. (Patients were allowed to continue treatment with mid- to low-potency topical steroids or topical calcineurin inhibitors if they had been taking them at baseline.)
Among 151 patients in the study, the mean age was 50.1 years, and 66.2% were women. The majority (53.0%) were White; 7.3% were Black; and 35.8% were Asian; 40.4% of patients had a history of atopy. The mean WI-NRS was 8.5, and the mean skin pain score on a 10-point scale was 7.2.
The Investigator’s Global Assessment for PN stage of disease (IGA PN-S) was also employed in the trial. That measure uses a 5-point scale to assess disease severity, with 0 indicating no lesions and 4 indicating more than 100 lesions. At baseline, 28.7% of patients had a score of 4, and the remainder had a score of 3, indicating the presence of 20-100 PN lesions.
Dr. Yosipovitch said that quality of life for these patients was “low” and that scores on the Hospital Anxiety and Depression scale indicated that the participants, many of whom had previously received topical and systemic medications for their PN, indicated they were depressed.
He showed that at week 24, the proportion of patients who had experienced an improvement in the WI-NRS score of greater than or equal to 4 (the study’s primary endpoint) was significantly greater with dupilumab, at 60.0% versus 18.4% among patients given placebo (P < .0001).
Moreover, the proportion of patients at week 24 with an IGA PN-S score of 0 or 1 (the secondary endpoint) was 48.0% in the active treatment group, versus 18.4% with placebo (P =.0004).
With regard to safety, rates of any treatment-emergent adverse events were similar between the groups, at 70.7% for dupilumab and 62.7% for placebo, as were rates for severe treatment-emergent adverse events, at 6.7% and 10.7%, respectively.
Rates of treatment-emergent adverse events of interest, such as skin infections, conjunctivitis, herpes viral infections, and injection site reactions, also suggested that there was no increased risk with active treatment.
Dupilumab is currently under review at the FDA and in Europe for the treatment of PN, according to dupilumab manufacturers Regeneron and Sanofi.
The study was sponsored by Sanofi in collaboration with Regeneron Pharmaceuticals. Dr. Yosipovitch has relationships with Arcutis Biotherapeutics, Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Dermatoses often occur in people who wear face masks
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
Risk factors linked to post–COVID vaccination death identified
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
The researchers have identified factors that put a person at greater risk of COVID-related death after they have completed both doses of the primary COVID vaccination schedule and a booster dose.
For their research, published in JAMA Network Open, researchers from the Office for National Statistics (ONS); Public Health Scotland; the University of Strathclyde, Glasgow; and the University of Edinburgh used data from the ONS Public linked data set combining the 2011 Census of England and covering 80% of the population of England. The study population included 19,473,570 individuals aged 18-100 years (mean age 60.8 years, 45.2% men, 92.0% White individuals) living in England who had completed both doses of their primary vaccination schedule and had received their mRNA booster 14 days or more prior to Dec. 31, 2021. The outcome of interest was time to death involving COVID-19 occurring between Jan. 1 and March 16, 2022.
Prioritization of booster doses and COVID-19 treatments
The authors highlighted how it had become “critical” to identify risk factors associated with COVID-19 death in those who had been vaccinated and pointed out that existing evidence was “based on people who have received one or two doses of a COVID-19 vaccine and were infected by the Alpha or Delta variant”. They emphasized that establishing which groups are at increased risk of COVID-19 death after receiving a booster is crucial for the “prioritization of further booster doses and access to COVID-19 therapeutics.”
During the study period the authors found that there were 4,781 (0.02%) deaths involving COVID-19 and 58,020 (0.3%) deaths from other causes. Of those who died of coronavirus, the mean age was 83.3 years, and the authors highlighted how “age was the most important characteristic” associated with the risk of postbooster COVID-19 death. They added that, compared with a 50-year-old, the HR for an 80-year-old individual was 31.3 (95% confidence interval, 26.1-37.6).
They found that women were at lower risk than men with an HR of 0.52 (95% CI, 0.49-0.55). An increased risk of COVID-19 death was also associated with living in a care home or in a socioeconomically deprived area.
Of note, they said that “there was no association between the risk of COVID-19 death and ethnicity, except for those of Indian background”, who they explained were at slightly elevated risk, compared with White individuals. However, they explained how the association with ethnicity was “unclear and differed from previous studies”, with their findings likely to be due “largely to the pronounced differences in vaccination uptake” between ethnic groups in previous studies.
Dementia concern
With regard to existing health conditions the authors commented that “most of the QCovid risk groups were associated with an increased HR of postbooster breakthrough death, except for of congenital heart disease, asthma, and prior fracture.”
Risk was particularly elevated, they said, for people with severe combined immunodeficiency (HR, 6.2; 95% CI, 3.3-11.5), and they also identified several conditions associated with HRs of greater than 3, including dementia.
In July, Alzheimer’s Research UK urged the Government to boost the development and deployment of new dementia treatments having found that a significant proportion of people who died of COVID-19 in 2020 and 2021 were living with the condition. At the time, data published by the ONS of deaths caused by coronavirus in England and Wales in 2021 showed dementia to be the second-most common pre-existing condition.
David Thomas, head of policy at Alzheimer’s Research UK, said: “We’ve known for some time that people with dementia have been hit disproportionately hard during the pandemic, but this new data serves as a stark reminder of the growing challenge we face in tackling the condition, and the urgent need to address it.”
The authors of the new research acknowledged the study’s limitations, notably that only data for the population living in England who were enumerated in the 2011 Census of England and Wales was included.
However, subpopulations “remain at increased risk of COVID-19 fatality” after receiving a booster vaccine during the Omicron wave, they pointed out.
“The subpopulations with the highest risk should be considered a priority for COVID-19 therapeutics and further booster doses,” they urged.
A version of this article first appeared on Medscape UK.
FROM JAMA NETWORK OPEN
‘Spectacular’ polypill results also puzzle docs
But results from the SECURE trial, published in the New England Journal of Medicine, also raise questions.
How do the polypills reduce cardiovascular problems? And will they ever be available in the United States?
Questions about how they work center on a mystery in the trial data: the polypill – containing aspirin, an angiotensin-converting enzyme (ACE) inhibitor, and a statin – apparently conferred substantial cardiovascular protection while producing average blood pressure and lipid levels that were virtually the same as with usual care.
As to when polypills will be available, the answer may hinge on whether companies, government agencies, or philanthropic foundations come to see making and paying for such treatments – combinations of typically inexpensive generic drugs in a single pill for the sake of convenience and greater adherence – as financially worthwhile.
A matter of adherence?
In the SECURE trial, presented late August at the annual congress of the European Society of Cardiology, Barcelona, investigators randomly assigned 2,499 patients with an MI in the previous 6 months to receive usual care or a polypill.
Patients in the usual-care group typically received the same types of treatments included the polypill, only taken separately. Different versions of the polypill were available to allow for titration to tolerated doses of the component medications: aspirin (100 mg), ramipril (2.5, 5, or 10 mg), and atorvastatin (20 mg or 40 mg).
Researchers used the Morisky Medication Adherence Scale to gauge participants’ adherence to their medication regimen and found the polypill group was more adherent. Patients who received the polypill were more likely to have a high level of adherence at 6 months (70.6% vs. 62.7%) and 24 months (74.1% vs. 63.2%), they reported. (The Morisky tool is the subject of some controversy because of aggressive licensing tactics of its creator.)
The primary endpoint of cardiovascular death, MI, stroke, or urgent revascularization was significantly less likely in the polypill group during a median of 3 years of follow-up (hazard ratio, 0.76; P = .02).
“A primary-outcome event occurred in 118 of 1,237 patients (9.5%) in the polypill group and in 156 of 1,229 (12.7%) in the usual-care group,” the researchers report.
“Probably, adherence is the most important reason of how this works,” Valentin Fuster, MD, physician-in-chief at Mount Sinai Hospital, New York, who led the study, said at ESC 2022.
Still, some clinicians were left scratching their heads by the lack of difference between treatment groups in average blood pressure and levels of low-density lipoprotein (LDL) cholesterol.
In the group that received the polypill, average systolic and diastolic blood pressure at 24 months were 135.2 mmHg and 74.8 mmHg, respectively. In the group that received usual care, those values were 135.5 mmHg and 74.9 mmHg, respectively.
Likewise, “no substantial differences were found in LDL-cholesterol levels over time between the groups, with a mean value at 24 months of 67.7 mg/dL in the polypill group and 67.2 mg/dL in the usual-care group,” according to the researchers.
One explanation for the findings is that greater adherence led to beneficial effects that were not reflected in lipid and blood pressure measurements, the investigators said. Alternatively, the open-label trial design could have led to different health behaviors between groups, they suggested.
Martha Gulati, MD, director of preventive cardiology at Cedars-Sinai Medical Center, Los Angeles, said she loves the idea of polypills. But she wonders about the lack of difference in blood pressure and lipids in SECURE.
Dr. Gulati said she sees in practice how medication adherence and measurements of blood pressure and lipids typically go hand in hand.
When a patient initially responds to a medication, but then their LDL cholesterol goes up later, “my first question is, ‘Are you still taking your medication or how frequently are you taking it?’” Dr. Gulati said in an interview. “And I get all kinds of answers.”
“If you are more adherent, why wouldn’t your LDL actually be lower, and why wouldn’t your blood pressure be lower?” she asked.
Can the results be replicated?
Ethan J. Weiss, MD, a cardiologist and volunteer associate clinical professor of medicine at the University of California, San Francisco, said the SECURE results are “spectacular,” but the seeming disconnect with the biomarker measurements “doesn’t make for a clean story.”
“It just seems like if you are making an argument that this is a way to improve compliance ... you would see some evidence of improved compliance objectively” in the biomarker readings, Dr. Weiss said.
Trying to understand how the polypill worked requires more imagination. “Or it makes you just say, ‘Who cares what the mechanism is?’ These people did a lot better, full stop, and that’s all that matters,” he said.
Dr. Weiss said he expects some degree of replication of the results may be needed before practice changes.
To Steven E. Nissen, MD, chief academic officer of the Heart and Vascular Institute at Cleveland Clinic, the results “don’t make any sense.”
“If they got the same results on the biomarkers that the pill was designed to intervene upon, why are the [primary outcome] results different? It’s completely unexplained,” Dr. Nissen said.
In general, Dr. Nissen has not been an advocate of the polypill approach in higher-income countries.
“Medicine is all about customization of therapy,” he said. “Not everybody needs blood pressure lowering. Not everybody needs the same intensity of LDL reduction. We spend much of our lives seeing patients and treating their blood pressure, and if it doesn’t come down adequately, giving them a higher dose or adding another agent.”
Polypills might be reasonable for primary prevention in countries where people have less access to health care resources, he added. In such settings, a low-cost, simple treatment strategy might have benefit.
But Dr. Nissen still doesn’t see a role for a polypill in secondary prevention.
“I think we have to take a step back, take a deep breath, and look very carefully at the science and try to understand whether this, in fact, is sensible,” he said. “We may need another study to see if this can be replicated.”
For Dhruv S. Kazi, MD, the results of the SECURE trial offer an opportunity to rekindle conversations about the use of polypills for cardiovascular protection. These conversations and studies have been taking place for nearly two decades.
Dr. Kazi, associate director of the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center, Boston, has used models to study the expected cost-effectiveness of polypills in various countries.
Although polypills can improve patients’ adherence to their prescribed medications, Dr. Kazi and colleagues have found that treatment gaps are “often at the physician level,” with many patients not prescribed all of the medications from which they could benefit.
Availability of polypills could help address those gaps. At the same time, many patients, even those with higher incomes, may have a strong preference for taking a single pill.
Dr. Kazi’s research also shows that a polypill approach may be more economically attractive as countries develop because successful treatment averts cardiovascular events that are costlier to treat.
“In the United States, in order for this to work, we would need a polypill that is both available widely but also affordable,” Dr. Kazi said. “It is going to require a visionary mover” to make that happen.
That could include philanthropic foundations. But it could also be a business opportunity for a company like Barcelona-based Ferrer, which provided the polypills for the SECURE trial.
The clinical and economic evidence in support of polypills has been compelling, Dr. Kazi said: “We have to get on with the business of implementing something that is effective and has the potential to greatly improve population health at scale.”
The SECURE trial was funded by the European Union Horizon 2020 program and coordinated by the Spanish National Center for Cardiovascular Research (CNIC). Ferrer International provided the polypill that was used in the trial. CNIC receives royalties for sales of the polypill from Ferrer. Dr. Weiss is starting a biotech company unrelated to this area of research.
A version of this article first appeared on Medscape.com.
But results from the SECURE trial, published in the New England Journal of Medicine, also raise questions.
How do the polypills reduce cardiovascular problems? And will they ever be available in the United States?
Questions about how they work center on a mystery in the trial data: the polypill – containing aspirin, an angiotensin-converting enzyme (ACE) inhibitor, and a statin – apparently conferred substantial cardiovascular protection while producing average blood pressure and lipid levels that were virtually the same as with usual care.
As to when polypills will be available, the answer may hinge on whether companies, government agencies, or philanthropic foundations come to see making and paying for such treatments – combinations of typically inexpensive generic drugs in a single pill for the sake of convenience and greater adherence – as financially worthwhile.
A matter of adherence?
In the SECURE trial, presented late August at the annual congress of the European Society of Cardiology, Barcelona, investigators randomly assigned 2,499 patients with an MI in the previous 6 months to receive usual care or a polypill.
Patients in the usual-care group typically received the same types of treatments included the polypill, only taken separately. Different versions of the polypill were available to allow for titration to tolerated doses of the component medications: aspirin (100 mg), ramipril (2.5, 5, or 10 mg), and atorvastatin (20 mg or 40 mg).
Researchers used the Morisky Medication Adherence Scale to gauge participants’ adherence to their medication regimen and found the polypill group was more adherent. Patients who received the polypill were more likely to have a high level of adherence at 6 months (70.6% vs. 62.7%) and 24 months (74.1% vs. 63.2%), they reported. (The Morisky tool is the subject of some controversy because of aggressive licensing tactics of its creator.)
The primary endpoint of cardiovascular death, MI, stroke, or urgent revascularization was significantly less likely in the polypill group during a median of 3 years of follow-up (hazard ratio, 0.76; P = .02).
“A primary-outcome event occurred in 118 of 1,237 patients (9.5%) in the polypill group and in 156 of 1,229 (12.7%) in the usual-care group,” the researchers report.
“Probably, adherence is the most important reason of how this works,” Valentin Fuster, MD, physician-in-chief at Mount Sinai Hospital, New York, who led the study, said at ESC 2022.
Still, some clinicians were left scratching their heads by the lack of difference between treatment groups in average blood pressure and levels of low-density lipoprotein (LDL) cholesterol.
In the group that received the polypill, average systolic and diastolic blood pressure at 24 months were 135.2 mmHg and 74.8 mmHg, respectively. In the group that received usual care, those values were 135.5 mmHg and 74.9 mmHg, respectively.
Likewise, “no substantial differences were found in LDL-cholesterol levels over time between the groups, with a mean value at 24 months of 67.7 mg/dL in the polypill group and 67.2 mg/dL in the usual-care group,” according to the researchers.
One explanation for the findings is that greater adherence led to beneficial effects that were not reflected in lipid and blood pressure measurements, the investigators said. Alternatively, the open-label trial design could have led to different health behaviors between groups, they suggested.
Martha Gulati, MD, director of preventive cardiology at Cedars-Sinai Medical Center, Los Angeles, said she loves the idea of polypills. But she wonders about the lack of difference in blood pressure and lipids in SECURE.
Dr. Gulati said she sees in practice how medication adherence and measurements of blood pressure and lipids typically go hand in hand.
When a patient initially responds to a medication, but then their LDL cholesterol goes up later, “my first question is, ‘Are you still taking your medication or how frequently are you taking it?’” Dr. Gulati said in an interview. “And I get all kinds of answers.”
“If you are more adherent, why wouldn’t your LDL actually be lower, and why wouldn’t your blood pressure be lower?” she asked.
Can the results be replicated?
Ethan J. Weiss, MD, a cardiologist and volunteer associate clinical professor of medicine at the University of California, San Francisco, said the SECURE results are “spectacular,” but the seeming disconnect with the biomarker measurements “doesn’t make for a clean story.”
“It just seems like if you are making an argument that this is a way to improve compliance ... you would see some evidence of improved compliance objectively” in the biomarker readings, Dr. Weiss said.
Trying to understand how the polypill worked requires more imagination. “Or it makes you just say, ‘Who cares what the mechanism is?’ These people did a lot better, full stop, and that’s all that matters,” he said.
Dr. Weiss said he expects some degree of replication of the results may be needed before practice changes.
To Steven E. Nissen, MD, chief academic officer of the Heart and Vascular Institute at Cleveland Clinic, the results “don’t make any sense.”
“If they got the same results on the biomarkers that the pill was designed to intervene upon, why are the [primary outcome] results different? It’s completely unexplained,” Dr. Nissen said.
In general, Dr. Nissen has not been an advocate of the polypill approach in higher-income countries.
“Medicine is all about customization of therapy,” he said. “Not everybody needs blood pressure lowering. Not everybody needs the same intensity of LDL reduction. We spend much of our lives seeing patients and treating their blood pressure, and if it doesn’t come down adequately, giving them a higher dose or adding another agent.”
Polypills might be reasonable for primary prevention in countries where people have less access to health care resources, he added. In such settings, a low-cost, simple treatment strategy might have benefit.
But Dr. Nissen still doesn’t see a role for a polypill in secondary prevention.
“I think we have to take a step back, take a deep breath, and look very carefully at the science and try to understand whether this, in fact, is sensible,” he said. “We may need another study to see if this can be replicated.”
For Dhruv S. Kazi, MD, the results of the SECURE trial offer an opportunity to rekindle conversations about the use of polypills for cardiovascular protection. These conversations and studies have been taking place for nearly two decades.
Dr. Kazi, associate director of the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center, Boston, has used models to study the expected cost-effectiveness of polypills in various countries.
Although polypills can improve patients’ adherence to their prescribed medications, Dr. Kazi and colleagues have found that treatment gaps are “often at the physician level,” with many patients not prescribed all of the medications from which they could benefit.
Availability of polypills could help address those gaps. At the same time, many patients, even those with higher incomes, may have a strong preference for taking a single pill.
Dr. Kazi’s research also shows that a polypill approach may be more economically attractive as countries develop because successful treatment averts cardiovascular events that are costlier to treat.
“In the United States, in order for this to work, we would need a polypill that is both available widely but also affordable,” Dr. Kazi said. “It is going to require a visionary mover” to make that happen.
That could include philanthropic foundations. But it could also be a business opportunity for a company like Barcelona-based Ferrer, which provided the polypills for the SECURE trial.
The clinical and economic evidence in support of polypills has been compelling, Dr. Kazi said: “We have to get on with the business of implementing something that is effective and has the potential to greatly improve population health at scale.”
The SECURE trial was funded by the European Union Horizon 2020 program and coordinated by the Spanish National Center for Cardiovascular Research (CNIC). Ferrer International provided the polypill that was used in the trial. CNIC receives royalties for sales of the polypill from Ferrer. Dr. Weiss is starting a biotech company unrelated to this area of research.
A version of this article first appeared on Medscape.com.
But results from the SECURE trial, published in the New England Journal of Medicine, also raise questions.
How do the polypills reduce cardiovascular problems? And will they ever be available in the United States?
Questions about how they work center on a mystery in the trial data: the polypill – containing aspirin, an angiotensin-converting enzyme (ACE) inhibitor, and a statin – apparently conferred substantial cardiovascular protection while producing average blood pressure and lipid levels that were virtually the same as with usual care.
As to when polypills will be available, the answer may hinge on whether companies, government agencies, or philanthropic foundations come to see making and paying for such treatments – combinations of typically inexpensive generic drugs in a single pill for the sake of convenience and greater adherence – as financially worthwhile.
A matter of adherence?
In the SECURE trial, presented late August at the annual congress of the European Society of Cardiology, Barcelona, investigators randomly assigned 2,499 patients with an MI in the previous 6 months to receive usual care or a polypill.
Patients in the usual-care group typically received the same types of treatments included the polypill, only taken separately. Different versions of the polypill were available to allow for titration to tolerated doses of the component medications: aspirin (100 mg), ramipril (2.5, 5, or 10 mg), and atorvastatin (20 mg or 40 mg).
Researchers used the Morisky Medication Adherence Scale to gauge participants’ adherence to their medication regimen and found the polypill group was more adherent. Patients who received the polypill were more likely to have a high level of adherence at 6 months (70.6% vs. 62.7%) and 24 months (74.1% vs. 63.2%), they reported. (The Morisky tool is the subject of some controversy because of aggressive licensing tactics of its creator.)
The primary endpoint of cardiovascular death, MI, stroke, or urgent revascularization was significantly less likely in the polypill group during a median of 3 years of follow-up (hazard ratio, 0.76; P = .02).
“A primary-outcome event occurred in 118 of 1,237 patients (9.5%) in the polypill group and in 156 of 1,229 (12.7%) in the usual-care group,” the researchers report.
“Probably, adherence is the most important reason of how this works,” Valentin Fuster, MD, physician-in-chief at Mount Sinai Hospital, New York, who led the study, said at ESC 2022.
Still, some clinicians were left scratching their heads by the lack of difference between treatment groups in average blood pressure and levels of low-density lipoprotein (LDL) cholesterol.
In the group that received the polypill, average systolic and diastolic blood pressure at 24 months were 135.2 mmHg and 74.8 mmHg, respectively. In the group that received usual care, those values were 135.5 mmHg and 74.9 mmHg, respectively.
Likewise, “no substantial differences were found in LDL-cholesterol levels over time between the groups, with a mean value at 24 months of 67.7 mg/dL in the polypill group and 67.2 mg/dL in the usual-care group,” according to the researchers.
One explanation for the findings is that greater adherence led to beneficial effects that were not reflected in lipid and blood pressure measurements, the investigators said. Alternatively, the open-label trial design could have led to different health behaviors between groups, they suggested.
Martha Gulati, MD, director of preventive cardiology at Cedars-Sinai Medical Center, Los Angeles, said she loves the idea of polypills. But she wonders about the lack of difference in blood pressure and lipids in SECURE.
Dr. Gulati said she sees in practice how medication adherence and measurements of blood pressure and lipids typically go hand in hand.
When a patient initially responds to a medication, but then their LDL cholesterol goes up later, “my first question is, ‘Are you still taking your medication or how frequently are you taking it?’” Dr. Gulati said in an interview. “And I get all kinds of answers.”
“If you are more adherent, why wouldn’t your LDL actually be lower, and why wouldn’t your blood pressure be lower?” she asked.
Can the results be replicated?
Ethan J. Weiss, MD, a cardiologist and volunteer associate clinical professor of medicine at the University of California, San Francisco, said the SECURE results are “spectacular,” but the seeming disconnect with the biomarker measurements “doesn’t make for a clean story.”
“It just seems like if you are making an argument that this is a way to improve compliance ... you would see some evidence of improved compliance objectively” in the biomarker readings, Dr. Weiss said.
Trying to understand how the polypill worked requires more imagination. “Or it makes you just say, ‘Who cares what the mechanism is?’ These people did a lot better, full stop, and that’s all that matters,” he said.
Dr. Weiss said he expects some degree of replication of the results may be needed before practice changes.
To Steven E. Nissen, MD, chief academic officer of the Heart and Vascular Institute at Cleveland Clinic, the results “don’t make any sense.”
“If they got the same results on the biomarkers that the pill was designed to intervene upon, why are the [primary outcome] results different? It’s completely unexplained,” Dr. Nissen said.
In general, Dr. Nissen has not been an advocate of the polypill approach in higher-income countries.
“Medicine is all about customization of therapy,” he said. “Not everybody needs blood pressure lowering. Not everybody needs the same intensity of LDL reduction. We spend much of our lives seeing patients and treating their blood pressure, and if it doesn’t come down adequately, giving them a higher dose or adding another agent.”
Polypills might be reasonable for primary prevention in countries where people have less access to health care resources, he added. In such settings, a low-cost, simple treatment strategy might have benefit.
But Dr. Nissen still doesn’t see a role for a polypill in secondary prevention.
“I think we have to take a step back, take a deep breath, and look very carefully at the science and try to understand whether this, in fact, is sensible,” he said. “We may need another study to see if this can be replicated.”
For Dhruv S. Kazi, MD, the results of the SECURE trial offer an opportunity to rekindle conversations about the use of polypills for cardiovascular protection. These conversations and studies have been taking place for nearly two decades.
Dr. Kazi, associate director of the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center, Boston, has used models to study the expected cost-effectiveness of polypills in various countries.
Although polypills can improve patients’ adherence to their prescribed medications, Dr. Kazi and colleagues have found that treatment gaps are “often at the physician level,” with many patients not prescribed all of the medications from which they could benefit.
Availability of polypills could help address those gaps. At the same time, many patients, even those with higher incomes, may have a strong preference for taking a single pill.
Dr. Kazi’s research also shows that a polypill approach may be more economically attractive as countries develop because successful treatment averts cardiovascular events that are costlier to treat.
“In the United States, in order for this to work, we would need a polypill that is both available widely but also affordable,” Dr. Kazi said. “It is going to require a visionary mover” to make that happen.
That could include philanthropic foundations. But it could also be a business opportunity for a company like Barcelona-based Ferrer, which provided the polypills for the SECURE trial.
The clinical and economic evidence in support of polypills has been compelling, Dr. Kazi said: “We have to get on with the business of implementing something that is effective and has the potential to greatly improve population health at scale.”
The SECURE trial was funded by the European Union Horizon 2020 program and coordinated by the Spanish National Center for Cardiovascular Research (CNIC). Ferrer International provided the polypill that was used in the trial. CNIC receives royalties for sales of the polypill from Ferrer. Dr. Weiss is starting a biotech company unrelated to this area of research.
A version of this article first appeared on Medscape.com.






