User login
Fine-tuning HR-ARM for constipation diagnoses
Among patients with constipation, reduced rectoanal gradient found during high-resolution anorectal manometry (HR-ARM) is the strongest parameter for predicting how a patient is likely to perform on a rectal balloon expulsion test (BET). Findings of both reduced rectoanal gradient and abnormal BET should be considered diagnostic for a defecatory disorder.
Those are the findings of a study out of the Mayo Clinic in Rochester, Minn., which was published in Gastroenterology. The research could help streamline diagnosis of defecatory disorders: Currently there is a lack of consensus on what tests should be performed and in what order to achieve a reliable diagnosis, according to the authors.
The authors recommend that, in patients with a suggestive history, a prolonged time on BET or reduced rectoanal gradient individually indicate a probable defecatory disorder, while the presence of both could be considered diagnostic even in the absence of defecography.
The research should provide clarity about the findings from HR-ARM, which can be complex, according to Kyle Staller, MD, who was asked to comment on the study. “You get lots of different numbers, you get lots of different parameters [with HR-ARM], and figuring out which ones are really relevant is somewhat difficult. [This study] is really a tour de force that distills down many of these parameters into some that we might want to pay attention to more than others,” said Dr. Staller, who is director of the GI Motility Laboratory at Massachusetts General Hospital and a professor of medicine at Harvard Medical School, both in Boston.
He pointed out that one limitation of the study is it didn’t attempt to answer the question of what parameters predict benefit from biofeedback therapy.
“I think the practice right now is that we tend to put the most weight on the balloon expulsion test. I think what this paper convincingly argues is that maybe someone who has an abnormal rectoanal gradient and a normal balloon expulsion test should also be referred for biofeedback with the caveat that we still don’t know if they’re going to do well on biofeedback or not,” said Dr. Staller.
The study included 658 patients, 474 with constipation and 184 healthy controls. In addition to HR-ARM and BET, 158 underwent defecography. Females made up 89% of constipated patients and 73% of healthy individuals. Healthy individuals were younger than constipated individuals (median age 35 versus 49 years).
Overall, 11% of healthy patients and 32% of constipated patients had prolonged BET time (P < .001) and 11% and 21% had a reduced rectoanal gradient on HR-ARM (P = .003). Among those with a normal BET time, 13% had a reduced rectoanal gradient, compared with 34% of those with a prolonged BET time (P < .001).
The authors report that HR-ARM variables had good specificity but worse sensitivity for predicting prolonged BET. The rectoanal gradient was the best-performing variable, with a specificity of 85% and a sensitivity of 36%.
HR-ARM and BET findings were associated with reduced rectal evacuation found on defecography. The median rectal evacuation was 79% if both BET and rectoanal gradient were normal, 35% if either one was abnormal, and 3% if both were abnormal (P < .001). Having either prolonged BET time or reduced anorectal gradient alone had a 73% sensitivity and specificity of 72% for incomplete evacuation on defecography. If both abnormalities were present, the sensitivity was 31% but the specificity was 95%.
A reduced rectoanal gradient was associated with incomplete rectal evacuation (odds ratio, 4.35; P = .002); prolonged BET time showed a similar association (OR, 4.57; P < .001).
The authors proposed terminology to help differentiate the findings: “probable defecatory disorder,” or “probable DD,” to describe patients with one abnormal result from the three tests and “definite DD” for those with two abnormal test results. “Although a single abnormal finding may be a false-positive result, pursuing a trial of biofeedback therapy for patients with a probable DD may be reasonable, especially when defecography is not feasible. However, this approach should be confirmed by prospective studies that assess the response to anorectal biofeedback therapy in patients with probable and definite DD, as defined above.”
The authors disclosed no conflicts of interest. Dr. Staller has consulted for GI Supply.
Among patients with constipation, reduced rectoanal gradient found during high-resolution anorectal manometry (HR-ARM) is the strongest parameter for predicting how a patient is likely to perform on a rectal balloon expulsion test (BET). Findings of both reduced rectoanal gradient and abnormal BET should be considered diagnostic for a defecatory disorder.
Those are the findings of a study out of the Mayo Clinic in Rochester, Minn., which was published in Gastroenterology. The research could help streamline diagnosis of defecatory disorders: Currently there is a lack of consensus on what tests should be performed and in what order to achieve a reliable diagnosis, according to the authors.
The authors recommend that, in patients with a suggestive history, a prolonged time on BET or reduced rectoanal gradient individually indicate a probable defecatory disorder, while the presence of both could be considered diagnostic even in the absence of defecography.
The research should provide clarity about the findings from HR-ARM, which can be complex, according to Kyle Staller, MD, who was asked to comment on the study. “You get lots of different numbers, you get lots of different parameters [with HR-ARM], and figuring out which ones are really relevant is somewhat difficult. [This study] is really a tour de force that distills down many of these parameters into some that we might want to pay attention to more than others,” said Dr. Staller, who is director of the GI Motility Laboratory at Massachusetts General Hospital and a professor of medicine at Harvard Medical School, both in Boston.
He pointed out that one limitation of the study is it didn’t attempt to answer the question of what parameters predict benefit from biofeedback therapy.
“I think the practice right now is that we tend to put the most weight on the balloon expulsion test. I think what this paper convincingly argues is that maybe someone who has an abnormal rectoanal gradient and a normal balloon expulsion test should also be referred for biofeedback with the caveat that we still don’t know if they’re going to do well on biofeedback or not,” said Dr. Staller.
The study included 658 patients, 474 with constipation and 184 healthy controls. In addition to HR-ARM and BET, 158 underwent defecography. Females made up 89% of constipated patients and 73% of healthy individuals. Healthy individuals were younger than constipated individuals (median age 35 versus 49 years).
Overall, 11% of healthy patients and 32% of constipated patients had prolonged BET time (P < .001) and 11% and 21% had a reduced rectoanal gradient on HR-ARM (P = .003). Among those with a normal BET time, 13% had a reduced rectoanal gradient, compared with 34% of those with a prolonged BET time (P < .001).
The authors report that HR-ARM variables had good specificity but worse sensitivity for predicting prolonged BET. The rectoanal gradient was the best-performing variable, with a specificity of 85% and a sensitivity of 36%.
HR-ARM and BET findings were associated with reduced rectal evacuation found on defecography. The median rectal evacuation was 79% if both BET and rectoanal gradient were normal, 35% if either one was abnormal, and 3% if both were abnormal (P < .001). Having either prolonged BET time or reduced anorectal gradient alone had a 73% sensitivity and specificity of 72% for incomplete evacuation on defecography. If both abnormalities were present, the sensitivity was 31% but the specificity was 95%.
A reduced rectoanal gradient was associated with incomplete rectal evacuation (odds ratio, 4.35; P = .002); prolonged BET time showed a similar association (OR, 4.57; P < .001).
The authors proposed terminology to help differentiate the findings: “probable defecatory disorder,” or “probable DD,” to describe patients with one abnormal result from the three tests and “definite DD” for those with two abnormal test results. “Although a single abnormal finding may be a false-positive result, pursuing a trial of biofeedback therapy for patients with a probable DD may be reasonable, especially when defecography is not feasible. However, this approach should be confirmed by prospective studies that assess the response to anorectal biofeedback therapy in patients with probable and definite DD, as defined above.”
The authors disclosed no conflicts of interest. Dr. Staller has consulted for GI Supply.
Among patients with constipation, reduced rectoanal gradient found during high-resolution anorectal manometry (HR-ARM) is the strongest parameter for predicting how a patient is likely to perform on a rectal balloon expulsion test (BET). Findings of both reduced rectoanal gradient and abnormal BET should be considered diagnostic for a defecatory disorder.
Those are the findings of a study out of the Mayo Clinic in Rochester, Minn., which was published in Gastroenterology. The research could help streamline diagnosis of defecatory disorders: Currently there is a lack of consensus on what tests should be performed and in what order to achieve a reliable diagnosis, according to the authors.
The authors recommend that, in patients with a suggestive history, a prolonged time on BET or reduced rectoanal gradient individually indicate a probable defecatory disorder, while the presence of both could be considered diagnostic even in the absence of defecography.
The research should provide clarity about the findings from HR-ARM, which can be complex, according to Kyle Staller, MD, who was asked to comment on the study. “You get lots of different numbers, you get lots of different parameters [with HR-ARM], and figuring out which ones are really relevant is somewhat difficult. [This study] is really a tour de force that distills down many of these parameters into some that we might want to pay attention to more than others,” said Dr. Staller, who is director of the GI Motility Laboratory at Massachusetts General Hospital and a professor of medicine at Harvard Medical School, both in Boston.
He pointed out that one limitation of the study is it didn’t attempt to answer the question of what parameters predict benefit from biofeedback therapy.
“I think the practice right now is that we tend to put the most weight on the balloon expulsion test. I think what this paper convincingly argues is that maybe someone who has an abnormal rectoanal gradient and a normal balloon expulsion test should also be referred for biofeedback with the caveat that we still don’t know if they’re going to do well on biofeedback or not,” said Dr. Staller.
The study included 658 patients, 474 with constipation and 184 healthy controls. In addition to HR-ARM and BET, 158 underwent defecography. Females made up 89% of constipated patients and 73% of healthy individuals. Healthy individuals were younger than constipated individuals (median age 35 versus 49 years).
Overall, 11% of healthy patients and 32% of constipated patients had prolonged BET time (P < .001) and 11% and 21% had a reduced rectoanal gradient on HR-ARM (P = .003). Among those with a normal BET time, 13% had a reduced rectoanal gradient, compared with 34% of those with a prolonged BET time (P < .001).
The authors report that HR-ARM variables had good specificity but worse sensitivity for predicting prolonged BET. The rectoanal gradient was the best-performing variable, with a specificity of 85% and a sensitivity of 36%.
HR-ARM and BET findings were associated with reduced rectal evacuation found on defecography. The median rectal evacuation was 79% if both BET and rectoanal gradient were normal, 35% if either one was abnormal, and 3% if both were abnormal (P < .001). Having either prolonged BET time or reduced anorectal gradient alone had a 73% sensitivity and specificity of 72% for incomplete evacuation on defecography. If both abnormalities were present, the sensitivity was 31% but the specificity was 95%.
A reduced rectoanal gradient was associated with incomplete rectal evacuation (odds ratio, 4.35; P = .002); prolonged BET time showed a similar association (OR, 4.57; P < .001).
The authors proposed terminology to help differentiate the findings: “probable defecatory disorder,” or “probable DD,” to describe patients with one abnormal result from the three tests and “definite DD” for those with two abnormal test results. “Although a single abnormal finding may be a false-positive result, pursuing a trial of biofeedback therapy for patients with a probable DD may be reasonable, especially when defecography is not feasible. However, this approach should be confirmed by prospective studies that assess the response to anorectal biofeedback therapy in patients with probable and definite DD, as defined above.”
The authors disclosed no conflicts of interest. Dr. Staller has consulted for GI Supply.
FROM GASTROENTEROLOGY
Commentary: Evaluating HCC Treatments, October 2022
Komatsu and colleagues performed a case-matched analysis to evaluate the best first-line treatment for HCC in patients with macroscopic portal vein tumor thrombus (PVTT). Patients had advanced HCC and macroscopic PVTT that invaded an ipsilateral first-order portal branch, main trunk, or contralateral portal vein. The propensity score–matched groups underwent either hepatectomy (n = 36) or received sorafenib (n = 36). To be considered for resection, patients had to have Child-Pugh (CP) grade A or B liver function, an Eastern Cooperative Oncology Group Performance Status score of ≤ 1, life expectancy of > 3 months, and the macroscopic resection of the targeted tumor could be planned with an estimated remnant liver volume ≥ 35%.
Out of 36 patients who underwent surgery, 23 underwent reductive hepatectomy, and 13 underwent complete resection of tumor. Out of 36 patients who received sorafenib, 21 underwent subsequent treatments. The median overall survival (OS) of patients who underwent hepatectomy was 15.1 months, significantly longer than the 4.5 months for patients who were treated with sorafenib. The authors concluded that selected patients who underwent tumor resection lived longer than patients who received systemic therapy with sorafenib first, despite the presence of macroscopic PVTT.
Lenvatinib is an approved treatment for patients with HCC. Because most patients with HCC have underlying cirrhosis, monitoring the underlying liver function is an important facet of patient management. Huynh and colleagues reported on patients in the REFLECT trial whose liver function deteriorated from CP-A to CP-B while receiving systemic therapy. This post hoc analysis included patients whose liver function deteriorated to CP-B or remained CP-A within 8 weeks of randomization to lenvatinib (CP-B: n = 60; CP-A: n = 413) or sorafenib (CP-B: n = 47; CP-A: n = 427). Patients receiving lenvatinib who developed CP-B cirrhosis compared with patients who maintained CP-A cirrhosis had a median progression-free survival (PFS) of 3.7 months (95% CI 1.8-7.4) vs 6.5 months (95% CI 5.6-7.4) and OS of 6.8 months (95% CI 2.6-10.3) vs 13.3 months (95% CI 11.6-16.1). CP-B patients receiving sorafenib had a median PFS and OS of only 0.5 months (95% CI 0.1-3.6) and 4.5 months (95% CI 2.9-6.1), respectively. No new safety signals were reported in CP-B patients. The investigators concluded that deterioration of liver function to CP-B does not require the discontinuation of lenvatinib therapy.
Finally, Brown and colleagues performed a meta-analysis of studies that evaluated transarterial radioembolization (TARE) and transarterial chemoembolization (TACE) in patients with HCC by reviewing 17 studies involving 2465 patients that directly compared TACE and TARE. TARE significantly prolonged the mean time to progression (17.5 vs 9.8 months; 95% CI 1.3-8.3 months) but resulted in comparable OS (absolute difference −0.55 months; 95% CI −1.95 to 3.05 months). Safety profiles appeared to favor TARE. The authors concluded that TACE and TARE should be compared in larger prospective studies to better compare survival, progression, and safety data.
Komatsu and colleagues performed a case-matched analysis to evaluate the best first-line treatment for HCC in patients with macroscopic portal vein tumor thrombus (PVTT). Patients had advanced HCC and macroscopic PVTT that invaded an ipsilateral first-order portal branch, main trunk, or contralateral portal vein. The propensity score–matched groups underwent either hepatectomy (n = 36) or received sorafenib (n = 36). To be considered for resection, patients had to have Child-Pugh (CP) grade A or B liver function, an Eastern Cooperative Oncology Group Performance Status score of ≤ 1, life expectancy of > 3 months, and the macroscopic resection of the targeted tumor could be planned with an estimated remnant liver volume ≥ 35%.
Out of 36 patients who underwent surgery, 23 underwent reductive hepatectomy, and 13 underwent complete resection of tumor. Out of 36 patients who received sorafenib, 21 underwent subsequent treatments. The median overall survival (OS) of patients who underwent hepatectomy was 15.1 months, significantly longer than the 4.5 months for patients who were treated with sorafenib. The authors concluded that selected patients who underwent tumor resection lived longer than patients who received systemic therapy with sorafenib first, despite the presence of macroscopic PVTT.
Lenvatinib is an approved treatment for patients with HCC. Because most patients with HCC have underlying cirrhosis, monitoring the underlying liver function is an important facet of patient management. Huynh and colleagues reported on patients in the REFLECT trial whose liver function deteriorated from CP-A to CP-B while receiving systemic therapy. This post hoc analysis included patients whose liver function deteriorated to CP-B or remained CP-A within 8 weeks of randomization to lenvatinib (CP-B: n = 60; CP-A: n = 413) or sorafenib (CP-B: n = 47; CP-A: n = 427). Patients receiving lenvatinib who developed CP-B cirrhosis compared with patients who maintained CP-A cirrhosis had a median progression-free survival (PFS) of 3.7 months (95% CI 1.8-7.4) vs 6.5 months (95% CI 5.6-7.4) and OS of 6.8 months (95% CI 2.6-10.3) vs 13.3 months (95% CI 11.6-16.1). CP-B patients receiving sorafenib had a median PFS and OS of only 0.5 months (95% CI 0.1-3.6) and 4.5 months (95% CI 2.9-6.1), respectively. No new safety signals were reported in CP-B patients. The investigators concluded that deterioration of liver function to CP-B does not require the discontinuation of lenvatinib therapy.
Finally, Brown and colleagues performed a meta-analysis of studies that evaluated transarterial radioembolization (TARE) and transarterial chemoembolization (TACE) in patients with HCC by reviewing 17 studies involving 2465 patients that directly compared TACE and TARE. TARE significantly prolonged the mean time to progression (17.5 vs 9.8 months; 95% CI 1.3-8.3 months) but resulted in comparable OS (absolute difference −0.55 months; 95% CI −1.95 to 3.05 months). Safety profiles appeared to favor TARE. The authors concluded that TACE and TARE should be compared in larger prospective studies to better compare survival, progression, and safety data.
Komatsu and colleagues performed a case-matched analysis to evaluate the best first-line treatment for HCC in patients with macroscopic portal vein tumor thrombus (PVTT). Patients had advanced HCC and macroscopic PVTT that invaded an ipsilateral first-order portal branch, main trunk, or contralateral portal vein. The propensity score–matched groups underwent either hepatectomy (n = 36) or received sorafenib (n = 36). To be considered for resection, patients had to have Child-Pugh (CP) grade A or B liver function, an Eastern Cooperative Oncology Group Performance Status score of ≤ 1, life expectancy of > 3 months, and the macroscopic resection of the targeted tumor could be planned with an estimated remnant liver volume ≥ 35%.
Out of 36 patients who underwent surgery, 23 underwent reductive hepatectomy, and 13 underwent complete resection of tumor. Out of 36 patients who received sorafenib, 21 underwent subsequent treatments. The median overall survival (OS) of patients who underwent hepatectomy was 15.1 months, significantly longer than the 4.5 months for patients who were treated with sorafenib. The authors concluded that selected patients who underwent tumor resection lived longer than patients who received systemic therapy with sorafenib first, despite the presence of macroscopic PVTT.
Lenvatinib is an approved treatment for patients with HCC. Because most patients with HCC have underlying cirrhosis, monitoring the underlying liver function is an important facet of patient management. Huynh and colleagues reported on patients in the REFLECT trial whose liver function deteriorated from CP-A to CP-B while receiving systemic therapy. This post hoc analysis included patients whose liver function deteriorated to CP-B or remained CP-A within 8 weeks of randomization to lenvatinib (CP-B: n = 60; CP-A: n = 413) or sorafenib (CP-B: n = 47; CP-A: n = 427). Patients receiving lenvatinib who developed CP-B cirrhosis compared with patients who maintained CP-A cirrhosis had a median progression-free survival (PFS) of 3.7 months (95% CI 1.8-7.4) vs 6.5 months (95% CI 5.6-7.4) and OS of 6.8 months (95% CI 2.6-10.3) vs 13.3 months (95% CI 11.6-16.1). CP-B patients receiving sorafenib had a median PFS and OS of only 0.5 months (95% CI 0.1-3.6) and 4.5 months (95% CI 2.9-6.1), respectively. No new safety signals were reported in CP-B patients. The investigators concluded that deterioration of liver function to CP-B does not require the discontinuation of lenvatinib therapy.
Finally, Brown and colleagues performed a meta-analysis of studies that evaluated transarterial radioembolization (TARE) and transarterial chemoembolization (TACE) in patients with HCC by reviewing 17 studies involving 2465 patients that directly compared TACE and TARE. TARE significantly prolonged the mean time to progression (17.5 vs 9.8 months; 95% CI 1.3-8.3 months) but resulted in comparable OS (absolute difference −0.55 months; 95% CI −1.95 to 3.05 months). Safety profiles appeared to favor TARE. The authors concluded that TACE and TARE should be compared in larger prospective studies to better compare survival, progression, and safety data.
Commentary: Evaluating HCC Treatments, October 2022
Komatsu and colleagues performed a case-matched analysis to evaluate the best first-line treatment for HCC in patients with macroscopic portal vein tumor thrombus (PVTT). Patients had advanced HCC and macroscopic PVTT that invaded an ipsilateral first-order portal branch, main trunk, or contralateral portal vein. The propensity score–matched groups underwent either hepatectomy (n = 36) or received sorafenib (n = 36). To be considered for resection, patients had to have Child-Pugh (CP) grade A or B liver function, an Eastern Cooperative Oncology Group Performance Status score of ≤ 1, life expectancy of > 3 months, and the macroscopic resection of the targeted tumor could be planned with an estimated remnant liver volume ≥ 35%.
Out of 36 patients who underwent surgery, 23 underwent reductive hepatectomy, and 13 underwent complete resection of tumor. Out of 36 patients who received sorafenib, 21 underwent subsequent treatments. The median overall survival (OS) of patients who underwent hepatectomy was 15.1 months, significantly longer than the 4.5 months for patients who were treated with sorafenib. The authors concluded that selected patients who underwent tumor resection lived longer than patients who received systemic therapy with sorafenib first, despite the presence of macroscopic PVTT.
Lenvatinib is an approved treatment for patients with HCC. Because most patients with HCC have underlying cirrhosis, monitoring the underlying liver function is an important facet of patient management. Huynh and colleagues reported on patients in the REFLECT trial whose liver function deteriorated from CP-A to CP-B while receiving systemic therapy. This post hoc analysis included patients whose liver function deteriorated to CP-B or remained CP-A within 8 weeks of randomization to lenvatinib (CP-B: n = 60; CP-A: n = 413) or sorafenib (CP-B: n = 47; CP-A: n = 427). Patients receiving lenvatinib who developed CP-B cirrhosis compared with patients who maintained CP-A cirrhosis had a median progression-free survival (PFS) of 3.7 months (95% CI 1.8-7.4) vs 6.5 months (95% CI 5.6-7.4) and OS of 6.8 months (95% CI 2.6-10.3) vs 13.3 months (95% CI 11.6-16.1). CP-B patients receiving sorafenib had a median PFS and OS of only 0.5 months (95% CI 0.1-3.6) and 4.5 months (95% CI 2.9-6.1), respectively. No new safety signals were reported in CP-B patients. The investigators concluded that deterioration of liver function to CP-B does not require the discontinuation of lenvatinib therapy.
Finally, Brown and colleagues performed a meta-analysis of studies that evaluated transarterial radioembolization (TARE) and transarterial chemoembolization (TACE) in patients with HCC by reviewing 17 studies involving 2465 patients that directly compared TACE and TARE. TARE significantly prolonged the mean time to progression (17.5 vs 9.8 months; 95% CI 1.3-8.3 months) but resulted in comparable OS (absolute difference −0.55 months; 95% CI −1.95 to 3.05 months). Safety profiles appeared to favor TARE. The authors concluded that TACE and TARE should be compared in larger prospective studies to better compare survival, progression, and safety data.
Komatsu and colleagues performed a case-matched analysis to evaluate the best first-line treatment for HCC in patients with macroscopic portal vein tumor thrombus (PVTT). Patients had advanced HCC and macroscopic PVTT that invaded an ipsilateral first-order portal branch, main trunk, or contralateral portal vein. The propensity score–matched groups underwent either hepatectomy (n = 36) or received sorafenib (n = 36). To be considered for resection, patients had to have Child-Pugh (CP) grade A or B liver function, an Eastern Cooperative Oncology Group Performance Status score of ≤ 1, life expectancy of > 3 months, and the macroscopic resection of the targeted tumor could be planned with an estimated remnant liver volume ≥ 35%.
Out of 36 patients who underwent surgery, 23 underwent reductive hepatectomy, and 13 underwent complete resection of tumor. Out of 36 patients who received sorafenib, 21 underwent subsequent treatments. The median overall survival (OS) of patients who underwent hepatectomy was 15.1 months, significantly longer than the 4.5 months for patients who were treated with sorafenib. The authors concluded that selected patients who underwent tumor resection lived longer than patients who received systemic therapy with sorafenib first, despite the presence of macroscopic PVTT.
Lenvatinib is an approved treatment for patients with HCC. Because most patients with HCC have underlying cirrhosis, monitoring the underlying liver function is an important facet of patient management. Huynh and colleagues reported on patients in the REFLECT trial whose liver function deteriorated from CP-A to CP-B while receiving systemic therapy. This post hoc analysis included patients whose liver function deteriorated to CP-B or remained CP-A within 8 weeks of randomization to lenvatinib (CP-B: n = 60; CP-A: n = 413) or sorafenib (CP-B: n = 47; CP-A: n = 427). Patients receiving lenvatinib who developed CP-B cirrhosis compared with patients who maintained CP-A cirrhosis had a median progression-free survival (PFS) of 3.7 months (95% CI 1.8-7.4) vs 6.5 months (95% CI 5.6-7.4) and OS of 6.8 months (95% CI 2.6-10.3) vs 13.3 months (95% CI 11.6-16.1). CP-B patients receiving sorafenib had a median PFS and OS of only 0.5 months (95% CI 0.1-3.6) and 4.5 months (95% CI 2.9-6.1), respectively. No new safety signals were reported in CP-B patients. The investigators concluded that deterioration of liver function to CP-B does not require the discontinuation of lenvatinib therapy.
Finally, Brown and colleagues performed a meta-analysis of studies that evaluated transarterial radioembolization (TARE) and transarterial chemoembolization (TACE) in patients with HCC by reviewing 17 studies involving 2465 patients that directly compared TACE and TARE. TARE significantly prolonged the mean time to progression (17.5 vs 9.8 months; 95% CI 1.3-8.3 months) but resulted in comparable OS (absolute difference −0.55 months; 95% CI −1.95 to 3.05 months). Safety profiles appeared to favor TARE. The authors concluded that TACE and TARE should be compared in larger prospective studies to better compare survival, progression, and safety data.
Komatsu and colleagues performed a case-matched analysis to evaluate the best first-line treatment for HCC in patients with macroscopic portal vein tumor thrombus (PVTT). Patients had advanced HCC and macroscopic PVTT that invaded an ipsilateral first-order portal branch, main trunk, or contralateral portal vein. The propensity score–matched groups underwent either hepatectomy (n = 36) or received sorafenib (n = 36). To be considered for resection, patients had to have Child-Pugh (CP) grade A or B liver function, an Eastern Cooperative Oncology Group Performance Status score of ≤ 1, life expectancy of > 3 months, and the macroscopic resection of the targeted tumor could be planned with an estimated remnant liver volume ≥ 35%.
Out of 36 patients who underwent surgery, 23 underwent reductive hepatectomy, and 13 underwent complete resection of tumor. Out of 36 patients who received sorafenib, 21 underwent subsequent treatments. The median overall survival (OS) of patients who underwent hepatectomy was 15.1 months, significantly longer than the 4.5 months for patients who were treated with sorafenib. The authors concluded that selected patients who underwent tumor resection lived longer than patients who received systemic therapy with sorafenib first, despite the presence of macroscopic PVTT.
Lenvatinib is an approved treatment for patients with HCC. Because most patients with HCC have underlying cirrhosis, monitoring the underlying liver function is an important facet of patient management. Huynh and colleagues reported on patients in the REFLECT trial whose liver function deteriorated from CP-A to CP-B while receiving systemic therapy. This post hoc analysis included patients whose liver function deteriorated to CP-B or remained CP-A within 8 weeks of randomization to lenvatinib (CP-B: n = 60; CP-A: n = 413) or sorafenib (CP-B: n = 47; CP-A: n = 427). Patients receiving lenvatinib who developed CP-B cirrhosis compared with patients who maintained CP-A cirrhosis had a median progression-free survival (PFS) of 3.7 months (95% CI 1.8-7.4) vs 6.5 months (95% CI 5.6-7.4) and OS of 6.8 months (95% CI 2.6-10.3) vs 13.3 months (95% CI 11.6-16.1). CP-B patients receiving sorafenib had a median PFS and OS of only 0.5 months (95% CI 0.1-3.6) and 4.5 months (95% CI 2.9-6.1), respectively. No new safety signals were reported in CP-B patients. The investigators concluded that deterioration of liver function to CP-B does not require the discontinuation of lenvatinib therapy.
Finally, Brown and colleagues performed a meta-analysis of studies that evaluated transarterial radioembolization (TARE) and transarterial chemoembolization (TACE) in patients with HCC by reviewing 17 studies involving 2465 patients that directly compared TACE and TARE. TARE significantly prolonged the mean time to progression (17.5 vs 9.8 months; 95% CI 1.3-8.3 months) but resulted in comparable OS (absolute difference −0.55 months; 95% CI −1.95 to 3.05 months). Safety profiles appeared to favor TARE. The authors concluded that TACE and TARE should be compared in larger prospective studies to better compare survival, progression, and safety data.
Consider the mnemonic ‘CLEAR’ when counseling acne patients
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
What we know about long COVID so far
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Long COVID: The name says it all. It’s an illness that, for many people, has not yet stopped.
Eric Roach became ill with COVID-19 in November 2020, and he’s still sick. “I have brain fog, memory loss,” says the 67-year-old Navy veteran from Spearfish, S.D. “The fatigue has just been insane.”
Long COVID, more formally known as post-acute sequelae of COVID (PASC), is the lay term to describe when people start to recover, or seem to recover, from a bout of COVID-19 but then continue to suffer from symptoms. For some, it’s gone on for 2 years or longer. While the governments of the United Statesand several other countries formally recognize the existence of long COVID, the National Institutes of Health (NIH) has yet to formally define it. There’s no approved treatment, and the causes are not understood.
Here’s what is known: and it is affecting enough people to cause concern for employers, health insurers, and governments.
First, the many symptoms
According to the Centers for Disease Control and Prvention, long COVID symptoms may include:
- Tiredness or fatigue that interferes with daily life.
- Symptoms that get worse after physical or mental effort.
- Fever.
- Difficulty breathing or shortness of breath.
- Cough.
- Chest pain.
- Heart palpitations.
- Difficulty thinking or concentrating (sometimes referred to as “brain fog”).
- Headache.
- Sleep problems.
- Dizziness when standing.
- Pins-and-needles feelings.
- Change in smell or taste.
- Depression or anxiety.
- Diarrhea.
- Stomach pain.
- Joint or muscle pain.
- Rash.
- Changes in menstrual cycles.
“People with post-COVID conditions may develop or continue to have symptoms that are hard to explain and manage,” the CDC says on its website. “Clinical evaluations and results of routine blood tests, chest x-rays, and electrocardiograms may be normal. The symptoms are similar to those reported by people with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and other poorly understood chronic illnesses that may occur after other infections.”
Doctors may not fully appreciate the subtle nature of some of the symptoms.
“People with these unexplained symptoms may be misunderstood by their health care providers, which can result in a long time for them to get a diagnosis and receive appropriate care or treatment,” the CDC says.
Health professionals should recognize that long COVID can be disabling, the U.S. Department of Health and Human Services says. “Long COVID can substantially limit a major life activity,” HHS says in civil rights guidance. One possible example: “A person with long COVID who has lung damage that causes shortness of breath, fatigue, and related effects is substantially limited in respiratory function, among other major life activities,” the HHS notes.
How many people are affected?
This has been difficult to judge because not everyone who has had COVID-19 gets tested for it and there are no formal diagnostic criteria yet for long COVID. The CDC estimates that 19% of patients in the United States who have ever had COVID-19 have long COVID symptoms.
Some estimates go higher. A University of Oxford study in September 2021 found more than a third of patients had symptoms of long COVID between 3 months and 6 months after a COVID-19 diagnosis. As many as 55% of COVID-19 patients in one Chinese study had one or more lingering symptoms 2 years later, Lixue Huang, MD, of the China-Japan Friendship Hospital in Beijing, and colleagues reported in the journal Lancet Respiratory Medicine in May.
According to the CDC, age is a factor. “Older adults are less likely to have long COVID than younger adults. Nearly three times as many adults ages 50-59 currently have long COVID than those age 80 and older,” the CDC says. Women and racial and ethnic minorities are more likely to be affected.
Many people are experiencing neurological effects, such as the so-called brain fog, according to Ziyad Al-Aly, MD, of Washington University and the VA St. Louis Health Care System, and colleagues, whose report was published in Nature Medicine in September. They estimated that 6.6 million Americans have brain impairments associated with COVID infection.
“Some of the neurologic disorders reported here are serious chronic conditions that will impact some people for a lifetime,” they wrote. “Given the colossal scale of the pandemic, and even though the absolute numbers reported in this work are small, these may translate into a large number of affected individuals around the world – and this will likely contribute to a rise in the burden of neurologic diseases.”
Causes
It’s not clear what the underlying causes are, but most research points to a combination of factors. Suspects include ongoing inflammation, tiny blood clots, and reactivation of latent viruses. In May, Brent Palmer, PhD, of the University of Colorado, Denver, and colleagues found people with long COVID had persistent activation of T-cells that were specific for SARS-CoV-2.
COVID-19 itself can damage organs, and long COVID might be caused by ongoing damage. In August, Alexandros Rovas, MD, of University Hospital Munster in Germany, and colleagues found patients with long COVID had evidence of damage to their capillaries. “Whether, to what extent, and when the observed damage might be reversible remains unclear,” they wrote in the journal Angiogenesis.
People with long COVID have immune responses to other viruses, such as Epstein-Barr – evidence that COVID-19 might reactivate latent viruses. “Our data suggest the involvement of persistent antigen, reactivation of latent herpesviruses, and chronic inflammation,” immunobiologist Akiko Iwasaki, PhD, of Yale University, New Haven, Conn., and colleagues wrote in a study posted in August that had not yet been peer-reviewed for publication.
This might be causing an autoimmune response. “The infection may cause the immune system to start making autoantibodies that attack a person’s own organs and tissues,” the NIH says.
There could be other factors. A study by Harvard researchers found that people who felt stressed, depressed, or lonely before catching COVID-19 were more likely to develop long COVID afterward. “Distress was more strongly associated with developing long COVID than physical health risk factors such as obesity, asthma, and hypertension,” Siwen Wang, MD, a research fellow with Harvard University’s T.H. Chan School of Public Health, Boston, said in a statement. Plus, nearly 44% of those in the study developed COVID-19 infections after having been assessed for stress, Dr. Wang and colleagues reported in the journal JAMA Psychiatry.
Vaccine protection
There’s evidence that vaccination protects against long COVID, both by preventing infection in the first place, but also even for people who have breakthrough infections.
A meta-analysis covering studies involving 17 million people found evidence vaccination might reduce the severity of COVID-19 or might help the body clear any lingering virus after an infection.
“Overall, vaccination was associated with reduced risks or odds of long COVID, with preliminary evidence suggesting that two doses are more effective than one dose,” wrote Cesar Fernandez de las Penas, PhD, of King Juan Carlos University in Madrid, and colleagues. Their report is in The Lancet’s eClinicalMedicine.
A team in Milan found that unvaccinated people in their study were nearly three times as likely to have serious symptoms for longer than 4 weeks compared to vaccinated volunteers. According to their report in JAMA, Elena Azzolini, MD, PhD, assistant professor at Humanitas Research Hospital, and colleagues found two or three doses of vaccine reduced the risk of hospitalization from COVID to 16% or 17% compared to 42% for the unvaccinated.
Treatments
With no diagnostic criteria and no understanding of the causes, it’s hard for doctors to determine treatments.
Most experts dealing with long COVID, even those at the specialty centers that have been set up at hospitals and health systems in the United States, recommend that patients start with their primary care doctors before moving on to specialists.
“The mainstay of management is supportive, holistic care, symptom control, and detection of treatable complications,” Trish Greenhalgh, MD, professor of primary care health sciences at the University of Oxford, England, and colleagues wrote in the journal The BMJ in September. “Patients with long COVID greatly value input from their primary care clinician. Generalist clinicians can help patients considerably by hearing the patient’s story and validating their experience … (and) making the diagnosis of long COVID (which does not have to be by exclusion) and excluding alternative diagnoses.”
Evidence is building that long COVID closely resembles other postviral conditions – something that can provide clues for treatment. For example, several studies indicate that exercise doesn’t help most patients.
But there are approaches that can work. Treatments may include pulmonary rehabilitation; autonomic conditioning therapy, which includes breathing therapy; and cognitive rehabilitation to relieve brain fog. Doctors are also trying the antidepressant amitriptyline to help with sleep disturbances and headaches; the antiseizure medication gabapentin to help with pain, numbness, and other neurological symptoms; and drugs to relieve low blood pressure in patients experiencing postural orthostatic tachycardia syndrome (POTS).
The NIH is sponsoring studies that have recruited just over 8,200 adults. And more than two dozen researchers from Harvard; Stanford; the University of California, San Francisco; the J. Craig Venter Institute; Johns Hopkins University; the University of Pennsylvania; Mount Sinai Hospitals; Cardiff University; and Yale announced in September they were forming the Long COVID Research Initiative to speed up studies.
The group, with funding from private enterprise, plans to conduct tissue biopsy, imaging studies, and autopsies and will search for potential biomarkers in the blood of patients.
A version of this article first appeared on WebMD.com.
Children and COVID: September slowdown continues
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.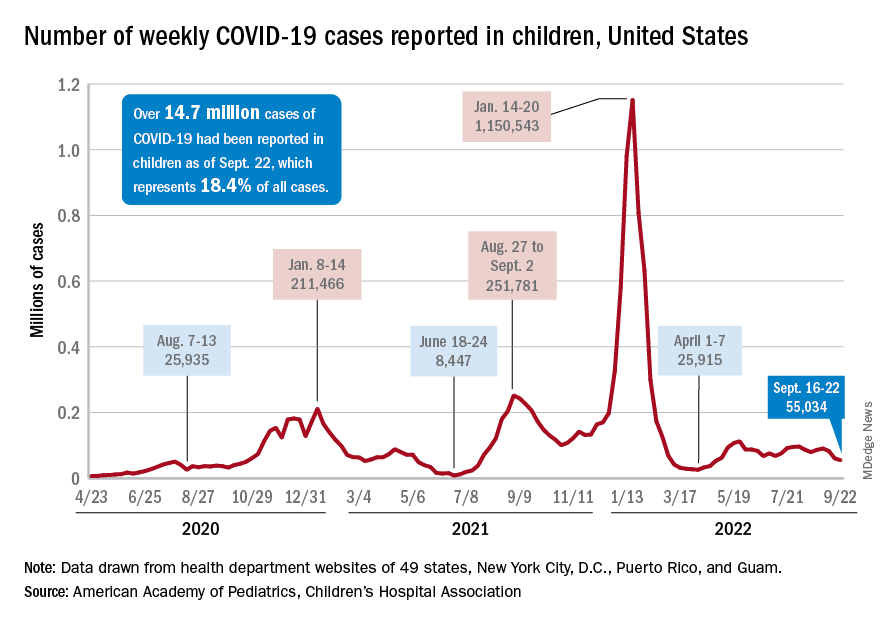
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
Many factors linked with higher, lower risk for hand eczema
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Unconventional wisdom: Major depression tied to childhood trauma is treatable
Despite a higher symptom burden, patients with major depressive disorder (MDD) and a history of childhood trauma (CT) can achieve significant recovery following treatment with a combination of pharmacotherapy and psychotherapy, new research suggests.
Results from a meta-analysis of 29 studies from 1966 to 2019, which included almost 7,000 adults with MDD, showed that more than 60% reported a history of CT. But despite having more severe depression at baseline, those with CT benefited from active treatment. Effect sizes were comparable, and dropout rates were similar to those of their counterparts without CT.
“Evidence-based psychotherapy and pharmacotherapy should be offered to depressed patients, regardless of their childhood trauma status,” lead author Erika Kuzminskaite, MSc, a PhD candidate at Amsterdam UMC department of psychiatry, the Netherlands, told this news organization.
“Screening for childhood trauma is important to identify individuals at risk for more severe course of the disorder and post-treatment residual symptoms,” she added.
The study was published online in the Lancet Psychiatry.
Common and potent risk factor
The researchers note that CT is common and is a potent risk factor for depression. Previous studies have “consistently indicated significantly higher severity and persistence of depressive symptoms in adult patients with depression and a history of childhood trauma.”
Previous individual and meta-analytic studies “indicated poorer response to first-line depression treatments in patients with childhood trauma, compared to those without trauma, suggesting the need for new personalized treatments for depressed patients with childhood trauma history,” Ms. Kuzminskaite said.
“However, the evidence on poorer treatment outcomes has not been definitive, and a comprehensive meta-analysis of available findings has been lacking,” she added.
The previous meta-analyses showed high between-study heterogeneity, and some primary studies reported similar or even superior improvement for patients with CT, compared with those without such history, following treatment with evidence-based psychotherapy or pharmacotherapy.
Previous studies also did not investigate the “relative contribution of different childhood trauma types.”
To address this gap, investigators in the Childhood Trauma Meta-Analysis Study Group conducted the “largest and most comprehensive study of available evidence examining the effects of childhood trauma on the efficacy and effectiveness of first-line treatments for adults with MDD.”
To be included, a study had to focus on adults over 18 years old who had received a primary diagnosis of depression. The study had to have included an available assessment of childhood trauma, and patients were required to have undergone psychotherapy and/or pharmacotherapy for depression alone or in combination with other guideline-recommended treatments. Studies were also required to have a comparator group, when applicable, and to have reported depression severity before and after the acute treatment phase.
Of 10,505 publications, 54 trials met inclusion criteria; of these, 29 (20 randomized controlled trials and 9 open trials), encompassing 6,830 participants aged 18-85 years, included data that had been made available by authors of the various studies and were included in the current analysis.
Most studies focused on MDD; 11 trials focused on patients with chronic or treatment-resistant depression.
The primary outcome was “depression severity change from baseline to the end of the acute treatment phase” (expressed as standardized effect size – Hedges’ g).
Greater treatment motivation?
Of the included patients, 62% reported a history of CT. They were found to have more severe depression at baseline, compared with those without CT (g = .202; 95% confidence interval, 0.145-0.258; I² = 0%).
The benefits from active treatment obtained by these patients with CT were similar to the benefits obtained by their counterparts without CT (between-group treatment effect difference: g = .016; 95% CI, –0.094-0.125; I² = 44.3%).
No significant difference in active treatment effects (in comparison with control condition) was found between individuals with and those without CT (g = .605; 95% CI, 0.294-0.916; I² = 58.0%; and g = .178; 95% CI, –0.195-0.552; I² = 67.5%, respectively; between-group difference P = .051).
Dropout rates were similar for the participants with and those without CT (risk ratio, 1.063; 95% CI, 0.945-1.195; I² = 0%).
“Findings did not significantly differ by childhood trauma type, study design, depression diagnosis, assessment method of childhood trauma, study quality, year, or treatment type or length,” the authors report.
The findings did, however, differ by country, with North American studies showing larger treatment effects for patients with CT, compared with studies conducted in Asian-Pacific countries (g = 0.150; 95% CI, 0.030-0.269; vs. g = 0.255; 95% CI, –0.508- –0.002, respectively; corrected false discovery rate, 0.0080). “However, because of limited power, these findings should be interpreted with caution,” the authors warn.
“It could be a chance finding and is certainly not causal,” Ms. Kuzminskaite suggested.
Most studies (21 of the 29) had a “moderate to high risk of bias.” But when the researchers conducted a sensitivity analysis in the low-bias studies, they found that results were similar to those of the primary analysis that included all the studies.
“Treatments were similarly effective for patients with and without childhood trauma, with slightly larger active treatment (vs. control condition – placebo, wait list, care-as-usual) effects for patients with childhood trauma history,” Ms. Kuzminskaite said.
“Some evidence suggests that patients with childhood trauma are characterized by greater treatment motivation,” she noted. Moreover, “they are also more severely depressed prior to treatment [and] thus have more room for improvement.”
‘Hopeful message’
Commenting for this news organization, Yvette Sheline, MD, McLure professor of psychiatry, radiology, and neurology and director of the center for neuromodulation in depression and Stress, University of Pennsylvania, Philadelphia, called it a “well-executed” and “straightforward” study “with clear-cut findings.”
Dr. Sheline, the director of the section on mood, anxiety, and trauma, who was not involved with the study, agrees with the authors’ conclusions – “to use evidence-based treatments for depression in all patients,” with or without a history of CT.
In an accompanying editorial, Antoine Yrondi, MD, PhD, of Université de Toulouse (France), called the findings “important and encouraging” but cautioned that CT could be associated with conditions other than depression, which could make MDD “more difficult to treat.”
Nevertheless, the meta-analysis “delivers a hopeful message to patients with childhood trauma that evidence-based psychotherapy and pharmacotherapy could improve depressive symptoms,” Dr. Yrondi said.
Dr. Yrondi encouraged physicians not to neglect CT in patients with MDD. “For this, it is important that physicians are trained to evaluate childhood trauma and to take it into account in their daily practice.”
No source of funding for the study was listed. The authors and Dr. Sheline have disclosed no relevant financial relationships. Dr. Yrondi has received speaker’s honoraria from AstraZeneca, Janssen, Lundbeck, Otsuka, and Jazz and has carried out clinical studies in relation to the development of a medicine for Janssen and Lundbeck that are unrelated to this work.
A version of this article first appeared on Medscape.com.
Despite a higher symptom burden, patients with major depressive disorder (MDD) and a history of childhood trauma (CT) can achieve significant recovery following treatment with a combination of pharmacotherapy and psychotherapy, new research suggests.
Results from a meta-analysis of 29 studies from 1966 to 2019, which included almost 7,000 adults with MDD, showed that more than 60% reported a history of CT. But despite having more severe depression at baseline, those with CT benefited from active treatment. Effect sizes were comparable, and dropout rates were similar to those of their counterparts without CT.
“Evidence-based psychotherapy and pharmacotherapy should be offered to depressed patients, regardless of their childhood trauma status,” lead author Erika Kuzminskaite, MSc, a PhD candidate at Amsterdam UMC department of psychiatry, the Netherlands, told this news organization.
“Screening for childhood trauma is important to identify individuals at risk for more severe course of the disorder and post-treatment residual symptoms,” she added.
The study was published online in the Lancet Psychiatry.
Common and potent risk factor
The researchers note that CT is common and is a potent risk factor for depression. Previous studies have “consistently indicated significantly higher severity and persistence of depressive symptoms in adult patients with depression and a history of childhood trauma.”
Previous individual and meta-analytic studies “indicated poorer response to first-line depression treatments in patients with childhood trauma, compared to those without trauma, suggesting the need for new personalized treatments for depressed patients with childhood trauma history,” Ms. Kuzminskaite said.
“However, the evidence on poorer treatment outcomes has not been definitive, and a comprehensive meta-analysis of available findings has been lacking,” she added.
The previous meta-analyses showed high between-study heterogeneity, and some primary studies reported similar or even superior improvement for patients with CT, compared with those without such history, following treatment with evidence-based psychotherapy or pharmacotherapy.
Previous studies also did not investigate the “relative contribution of different childhood trauma types.”
To address this gap, investigators in the Childhood Trauma Meta-Analysis Study Group conducted the “largest and most comprehensive study of available evidence examining the effects of childhood trauma on the efficacy and effectiveness of first-line treatments for adults with MDD.”
To be included, a study had to focus on adults over 18 years old who had received a primary diagnosis of depression. The study had to have included an available assessment of childhood trauma, and patients were required to have undergone psychotherapy and/or pharmacotherapy for depression alone or in combination with other guideline-recommended treatments. Studies were also required to have a comparator group, when applicable, and to have reported depression severity before and after the acute treatment phase.
Of 10,505 publications, 54 trials met inclusion criteria; of these, 29 (20 randomized controlled trials and 9 open trials), encompassing 6,830 participants aged 18-85 years, included data that had been made available by authors of the various studies and were included in the current analysis.
Most studies focused on MDD; 11 trials focused on patients with chronic or treatment-resistant depression.
The primary outcome was “depression severity change from baseline to the end of the acute treatment phase” (expressed as standardized effect size – Hedges’ g).
Greater treatment motivation?
Of the included patients, 62% reported a history of CT. They were found to have more severe depression at baseline, compared with those without CT (g = .202; 95% confidence interval, 0.145-0.258; I² = 0%).
The benefits from active treatment obtained by these patients with CT were similar to the benefits obtained by their counterparts without CT (between-group treatment effect difference: g = .016; 95% CI, –0.094-0.125; I² = 44.3%).
No significant difference in active treatment effects (in comparison with control condition) was found between individuals with and those without CT (g = .605; 95% CI, 0.294-0.916; I² = 58.0%; and g = .178; 95% CI, –0.195-0.552; I² = 67.5%, respectively; between-group difference P = .051).
Dropout rates were similar for the participants with and those without CT (risk ratio, 1.063; 95% CI, 0.945-1.195; I² = 0%).
“Findings did not significantly differ by childhood trauma type, study design, depression diagnosis, assessment method of childhood trauma, study quality, year, or treatment type or length,” the authors report.
The findings did, however, differ by country, with North American studies showing larger treatment effects for patients with CT, compared with studies conducted in Asian-Pacific countries (g = 0.150; 95% CI, 0.030-0.269; vs. g = 0.255; 95% CI, –0.508- –0.002, respectively; corrected false discovery rate, 0.0080). “However, because of limited power, these findings should be interpreted with caution,” the authors warn.
“It could be a chance finding and is certainly not causal,” Ms. Kuzminskaite suggested.
Most studies (21 of the 29) had a “moderate to high risk of bias.” But when the researchers conducted a sensitivity analysis in the low-bias studies, they found that results were similar to those of the primary analysis that included all the studies.
“Treatments were similarly effective for patients with and without childhood trauma, with slightly larger active treatment (vs. control condition – placebo, wait list, care-as-usual) effects for patients with childhood trauma history,” Ms. Kuzminskaite said.
“Some evidence suggests that patients with childhood trauma are characterized by greater treatment motivation,” she noted. Moreover, “they are also more severely depressed prior to treatment [and] thus have more room for improvement.”
‘Hopeful message’
Commenting for this news organization, Yvette Sheline, MD, McLure professor of psychiatry, radiology, and neurology and director of the center for neuromodulation in depression and Stress, University of Pennsylvania, Philadelphia, called it a “well-executed” and “straightforward” study “with clear-cut findings.”
Dr. Sheline, the director of the section on mood, anxiety, and trauma, who was not involved with the study, agrees with the authors’ conclusions – “to use evidence-based treatments for depression in all patients,” with or without a history of CT.
In an accompanying editorial, Antoine Yrondi, MD, PhD, of Université de Toulouse (France), called the findings “important and encouraging” but cautioned that CT could be associated with conditions other than depression, which could make MDD “more difficult to treat.”
Nevertheless, the meta-analysis “delivers a hopeful message to patients with childhood trauma that evidence-based psychotherapy and pharmacotherapy could improve depressive symptoms,” Dr. Yrondi said.
Dr. Yrondi encouraged physicians not to neglect CT in patients with MDD. “For this, it is important that physicians are trained to evaluate childhood trauma and to take it into account in their daily practice.”
No source of funding for the study was listed. The authors and Dr. Sheline have disclosed no relevant financial relationships. Dr. Yrondi has received speaker’s honoraria from AstraZeneca, Janssen, Lundbeck, Otsuka, and Jazz and has carried out clinical studies in relation to the development of a medicine for Janssen and Lundbeck that are unrelated to this work.
A version of this article first appeared on Medscape.com.
Despite a higher symptom burden, patients with major depressive disorder (MDD) and a history of childhood trauma (CT) can achieve significant recovery following treatment with a combination of pharmacotherapy and psychotherapy, new research suggests.
Results from a meta-analysis of 29 studies from 1966 to 2019, which included almost 7,000 adults with MDD, showed that more than 60% reported a history of CT. But despite having more severe depression at baseline, those with CT benefited from active treatment. Effect sizes were comparable, and dropout rates were similar to those of their counterparts without CT.
“Evidence-based psychotherapy and pharmacotherapy should be offered to depressed patients, regardless of their childhood trauma status,” lead author Erika Kuzminskaite, MSc, a PhD candidate at Amsterdam UMC department of psychiatry, the Netherlands, told this news organization.
“Screening for childhood trauma is important to identify individuals at risk for more severe course of the disorder and post-treatment residual symptoms,” she added.
The study was published online in the Lancet Psychiatry.
Common and potent risk factor
The researchers note that CT is common and is a potent risk factor for depression. Previous studies have “consistently indicated significantly higher severity and persistence of depressive symptoms in adult patients with depression and a history of childhood trauma.”
Previous individual and meta-analytic studies “indicated poorer response to first-line depression treatments in patients with childhood trauma, compared to those without trauma, suggesting the need for new personalized treatments for depressed patients with childhood trauma history,” Ms. Kuzminskaite said.
“However, the evidence on poorer treatment outcomes has not been definitive, and a comprehensive meta-analysis of available findings has been lacking,” she added.
The previous meta-analyses showed high between-study heterogeneity, and some primary studies reported similar or even superior improvement for patients with CT, compared with those without such history, following treatment with evidence-based psychotherapy or pharmacotherapy.
Previous studies also did not investigate the “relative contribution of different childhood trauma types.”
To address this gap, investigators in the Childhood Trauma Meta-Analysis Study Group conducted the “largest and most comprehensive study of available evidence examining the effects of childhood trauma on the efficacy and effectiveness of first-line treatments for adults with MDD.”
To be included, a study had to focus on adults over 18 years old who had received a primary diagnosis of depression. The study had to have included an available assessment of childhood trauma, and patients were required to have undergone psychotherapy and/or pharmacotherapy for depression alone or in combination with other guideline-recommended treatments. Studies were also required to have a comparator group, when applicable, and to have reported depression severity before and after the acute treatment phase.
Of 10,505 publications, 54 trials met inclusion criteria; of these, 29 (20 randomized controlled trials and 9 open trials), encompassing 6,830 participants aged 18-85 years, included data that had been made available by authors of the various studies and were included in the current analysis.
Most studies focused on MDD; 11 trials focused on patients with chronic or treatment-resistant depression.
The primary outcome was “depression severity change from baseline to the end of the acute treatment phase” (expressed as standardized effect size – Hedges’ g).
Greater treatment motivation?
Of the included patients, 62% reported a history of CT. They were found to have more severe depression at baseline, compared with those without CT (g = .202; 95% confidence interval, 0.145-0.258; I² = 0%).
The benefits from active treatment obtained by these patients with CT were similar to the benefits obtained by their counterparts without CT (between-group treatment effect difference: g = .016; 95% CI, –0.094-0.125; I² = 44.3%).
No significant difference in active treatment effects (in comparison with control condition) was found between individuals with and those without CT (g = .605; 95% CI, 0.294-0.916; I² = 58.0%; and g = .178; 95% CI, –0.195-0.552; I² = 67.5%, respectively; between-group difference P = .051).
Dropout rates were similar for the participants with and those without CT (risk ratio, 1.063; 95% CI, 0.945-1.195; I² = 0%).
“Findings did not significantly differ by childhood trauma type, study design, depression diagnosis, assessment method of childhood trauma, study quality, year, or treatment type or length,” the authors report.
The findings did, however, differ by country, with North American studies showing larger treatment effects for patients with CT, compared with studies conducted in Asian-Pacific countries (g = 0.150; 95% CI, 0.030-0.269; vs. g = 0.255; 95% CI, –0.508- –0.002, respectively; corrected false discovery rate, 0.0080). “However, because of limited power, these findings should be interpreted with caution,” the authors warn.
“It could be a chance finding and is certainly not causal,” Ms. Kuzminskaite suggested.
Most studies (21 of the 29) had a “moderate to high risk of bias.” But when the researchers conducted a sensitivity analysis in the low-bias studies, they found that results were similar to those of the primary analysis that included all the studies.
“Treatments were similarly effective for patients with and without childhood trauma, with slightly larger active treatment (vs. control condition – placebo, wait list, care-as-usual) effects for patients with childhood trauma history,” Ms. Kuzminskaite said.
“Some evidence suggests that patients with childhood trauma are characterized by greater treatment motivation,” she noted. Moreover, “they are also more severely depressed prior to treatment [and] thus have more room for improvement.”
‘Hopeful message’
Commenting for this news organization, Yvette Sheline, MD, McLure professor of psychiatry, radiology, and neurology and director of the center for neuromodulation in depression and Stress, University of Pennsylvania, Philadelphia, called it a “well-executed” and “straightforward” study “with clear-cut findings.”
Dr. Sheline, the director of the section on mood, anxiety, and trauma, who was not involved with the study, agrees with the authors’ conclusions – “to use evidence-based treatments for depression in all patients,” with or without a history of CT.
In an accompanying editorial, Antoine Yrondi, MD, PhD, of Université de Toulouse (France), called the findings “important and encouraging” but cautioned that CT could be associated with conditions other than depression, which could make MDD “more difficult to treat.”
Nevertheless, the meta-analysis “delivers a hopeful message to patients with childhood trauma that evidence-based psychotherapy and pharmacotherapy could improve depressive symptoms,” Dr. Yrondi said.
Dr. Yrondi encouraged physicians not to neglect CT in patients with MDD. “For this, it is important that physicians are trained to evaluate childhood trauma and to take it into account in their daily practice.”
No source of funding for the study was listed. The authors and Dr. Sheline have disclosed no relevant financial relationships. Dr. Yrondi has received speaker’s honoraria from AstraZeneca, Janssen, Lundbeck, Otsuka, and Jazz and has carried out clinical studies in relation to the development of a medicine for Janssen and Lundbeck that are unrelated to this work.
A version of this article first appeared on Medscape.com.
FROM LANCET PSYCHIATRY
Apremilast may have some cardiometabolic benefits in patients with psoriasis
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Early emollient use reduces dermatitis in at-risk infants
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY




