User login
Simulation training on management of shoulder dystocia reduces incidence of permanent BPBI
Key clinical point: Weekly 3-hour simulation-based training of midwives and doctors on shoulder dystocia (SD) management significantly reduced the incidence of permanent brachial plexus birth injury (BPBI).
Major finding: Despite an increase in the incidence of SD cases (0.1% vs 0.3%; P < .001) and risk factors in pre-training vs post-training period, the incidence of permanent BPBI decreased significantly (0.05% vs 0.02%; P < .001), with the risk for permanent BPBI among those with SD reducing (43.5% vs 6.0%; P < .001) and the rate of successful posterior arm delivery increasing (11.3% vs 23.4%; P = .04) significantly after the implementation of systematic simulation-based training.
Study details: Findings are from a retrospective observational study including 113,785 vertex deliveries performed by a team of doctors and midwives after receiving the weekly 3-hour simulation-based training.
Disclosures: This study was funded by Helsinki University State Research Funding. No conflicts of interest were declared.
Source: Kaijomaa M et al. Impact of simulation training on the management of shoulder dystocia and incidence of permanent brachial plexus birth injury: An observational study. BJOG. 2022 (Aug 10). Doi: 10.1111/1471-0528.17278
Key clinical point: Weekly 3-hour simulation-based training of midwives and doctors on shoulder dystocia (SD) management significantly reduced the incidence of permanent brachial plexus birth injury (BPBI).
Major finding: Despite an increase in the incidence of SD cases (0.1% vs 0.3%; P < .001) and risk factors in pre-training vs post-training period, the incidence of permanent BPBI decreased significantly (0.05% vs 0.02%; P < .001), with the risk for permanent BPBI among those with SD reducing (43.5% vs 6.0%; P < .001) and the rate of successful posterior arm delivery increasing (11.3% vs 23.4%; P = .04) significantly after the implementation of systematic simulation-based training.
Study details: Findings are from a retrospective observational study including 113,785 vertex deliveries performed by a team of doctors and midwives after receiving the weekly 3-hour simulation-based training.
Disclosures: This study was funded by Helsinki University State Research Funding. No conflicts of interest were declared.
Source: Kaijomaa M et al. Impact of simulation training on the management of shoulder dystocia and incidence of permanent brachial plexus birth injury: An observational study. BJOG. 2022 (Aug 10). Doi: 10.1111/1471-0528.17278
Key clinical point: Weekly 3-hour simulation-based training of midwives and doctors on shoulder dystocia (SD) management significantly reduced the incidence of permanent brachial plexus birth injury (BPBI).
Major finding: Despite an increase in the incidence of SD cases (0.1% vs 0.3%; P < .001) and risk factors in pre-training vs post-training period, the incidence of permanent BPBI decreased significantly (0.05% vs 0.02%; P < .001), with the risk for permanent BPBI among those with SD reducing (43.5% vs 6.0%; P < .001) and the rate of successful posterior arm delivery increasing (11.3% vs 23.4%; P = .04) significantly after the implementation of systematic simulation-based training.
Study details: Findings are from a retrospective observational study including 113,785 vertex deliveries performed by a team of doctors and midwives after receiving the weekly 3-hour simulation-based training.
Disclosures: This study was funded by Helsinki University State Research Funding. No conflicts of interest were declared.
Source: Kaijomaa M et al. Impact of simulation training on the management of shoulder dystocia and incidence of permanent brachial plexus birth injury: An observational study. BJOG. 2022 (Aug 10). Doi: 10.1111/1471-0528.17278
November 2022 - ICYMI
Gastroenterology
August 2022
Johnson-Laghi KA, Mattar MC. Integrating cognitive apprenticeship into gastroenterology clinical training. Gastroenterology. 2022 Aug;163(2):364-7. doi: 10.1053/j.gastro.2022.06.013.
Wood LD et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022 Aug;163(2):386-402.e1. doi: 10.1053/j.gastro.2022.03.056.
Calderwood AH and Robertson DJ. Stopping surveillance in gastrointestinal conditions: Thoughts on the scope of the problem and potential solutions. Gastroenterology. 2022 Aug;163(2):345-9. doi: 10.1053/j.gastro.2022.04.009.
September 2022
Donnangelo LL et al. Disclosure and reflection after an adverse event: Tips for training and practice. Gastroenterology. 2022 Sep;163(3):568-71. doi: 10.1053/j.gastro.2022.07.003.
Chey WD et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022 Sep;163(3):608-19. doi: 10.1053/j.gastro.2022.05.055.
Bushyhead D and Quigley EMM. Small intestinal bacterial overgrowth-pathophysiology and its implications for definition and management. Gastroenterology. 2022 Sep;163(3):593-607. doi: 10.1053/j.gastro.2022.04.002.
Long MT et al. AGA Clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology. 2022 Sep;163(3):764-74.e1. doi: 10.1053/j.gastro.2022.06.023.
CGH
August 2022
Lennon AM and Vege SS. Pancreatic cyst surveillance. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1663-7.e1. doi: 10.1016/j.cgh.2022.03.002.
Crockett SD et al. Large Polyp Study Group Consortium. Clip closure does not reduce risk of bleeding after resection of large serrated polyps: Results from a randomized trial. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1757-17--65.e4. doi: 10.1016/j.cgh.2021.12.036.
Martin P et al. Treatment algorithm for managing chronic hepatitis b virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1766-75. doi: 10.1016/j.cgh.2021.07.036.
September 2022
Pawlak KM et al. How to train the next generation to provide high-quality peer-reviews. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1902-6. doi: 10.1016/j.cgh.2022.05.018.
Choung RS et al. Collagenous gastritis: Characteristics and response to topical budesonide. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1977-85.e1. doi: 10.1016/j.cgh.2021.11.033.
Basnayake C et al. Long-term outcome of multidisciplinary versus standard gastroenterologist care for functional gastrointestinal disorders: A randomized trial. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2102-11.e9. doi: 10.1016/j.cgh.2021.12.005.
Deutsch-Link S et al. Alcohol-associated liver disease mortality increased from 2017 to 2020 and accelerated during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2142-4.e2. doi: 10.1016/j.cgh.2022.03.017.
TIGE
Nakamatsu, Dai et al. Safety of cold snare polypectomy for small colorectal polyps in patients receiving antithrombotic therapy. Tech Innov Gastrointest Endosc. 2022 Apr 8;24[3]:246-53. doi: 10.1016/j.tige.2022.03.008.
Gastro Hep Advances
Brindusa Truta et al. Outcomes of continuation vs. discontinuation of adalimumab therapy during third trimester of pregnancy in inflammatory bowel disease. Gastro Hep Advances. 2022 Jan 1;1[5]:785-91. doi: 10.1016/j.gastha.2022.04.009.
Gastroenterology
August 2022
Johnson-Laghi KA, Mattar MC. Integrating cognitive apprenticeship into gastroenterology clinical training. Gastroenterology. 2022 Aug;163(2):364-7. doi: 10.1053/j.gastro.2022.06.013.
Wood LD et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022 Aug;163(2):386-402.e1. doi: 10.1053/j.gastro.2022.03.056.
Calderwood AH and Robertson DJ. Stopping surveillance in gastrointestinal conditions: Thoughts on the scope of the problem and potential solutions. Gastroenterology. 2022 Aug;163(2):345-9. doi: 10.1053/j.gastro.2022.04.009.
September 2022
Donnangelo LL et al. Disclosure and reflection after an adverse event: Tips for training and practice. Gastroenterology. 2022 Sep;163(3):568-71. doi: 10.1053/j.gastro.2022.07.003.
Chey WD et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022 Sep;163(3):608-19. doi: 10.1053/j.gastro.2022.05.055.
Bushyhead D and Quigley EMM. Small intestinal bacterial overgrowth-pathophysiology and its implications for definition and management. Gastroenterology. 2022 Sep;163(3):593-607. doi: 10.1053/j.gastro.2022.04.002.
Long MT et al. AGA Clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology. 2022 Sep;163(3):764-74.e1. doi: 10.1053/j.gastro.2022.06.023.
CGH
August 2022
Lennon AM and Vege SS. Pancreatic cyst surveillance. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1663-7.e1. doi: 10.1016/j.cgh.2022.03.002.
Crockett SD et al. Large Polyp Study Group Consortium. Clip closure does not reduce risk of bleeding after resection of large serrated polyps: Results from a randomized trial. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1757-17--65.e4. doi: 10.1016/j.cgh.2021.12.036.
Martin P et al. Treatment algorithm for managing chronic hepatitis b virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1766-75. doi: 10.1016/j.cgh.2021.07.036.
September 2022
Pawlak KM et al. How to train the next generation to provide high-quality peer-reviews. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1902-6. doi: 10.1016/j.cgh.2022.05.018.
Choung RS et al. Collagenous gastritis: Characteristics and response to topical budesonide. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1977-85.e1. doi: 10.1016/j.cgh.2021.11.033.
Basnayake C et al. Long-term outcome of multidisciplinary versus standard gastroenterologist care for functional gastrointestinal disorders: A randomized trial. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2102-11.e9. doi: 10.1016/j.cgh.2021.12.005.
Deutsch-Link S et al. Alcohol-associated liver disease mortality increased from 2017 to 2020 and accelerated during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2142-4.e2. doi: 10.1016/j.cgh.2022.03.017.
TIGE
Nakamatsu, Dai et al. Safety of cold snare polypectomy for small colorectal polyps in patients receiving antithrombotic therapy. Tech Innov Gastrointest Endosc. 2022 Apr 8;24[3]:246-53. doi: 10.1016/j.tige.2022.03.008.
Gastro Hep Advances
Brindusa Truta et al. Outcomes of continuation vs. discontinuation of adalimumab therapy during third trimester of pregnancy in inflammatory bowel disease. Gastro Hep Advances. 2022 Jan 1;1[5]:785-91. doi: 10.1016/j.gastha.2022.04.009.
Gastroenterology
August 2022
Johnson-Laghi KA, Mattar MC. Integrating cognitive apprenticeship into gastroenterology clinical training. Gastroenterology. 2022 Aug;163(2):364-7. doi: 10.1053/j.gastro.2022.06.013.
Wood LD et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022 Aug;163(2):386-402.e1. doi: 10.1053/j.gastro.2022.03.056.
Calderwood AH and Robertson DJ. Stopping surveillance in gastrointestinal conditions: Thoughts on the scope of the problem and potential solutions. Gastroenterology. 2022 Aug;163(2):345-9. doi: 10.1053/j.gastro.2022.04.009.
September 2022
Donnangelo LL et al. Disclosure and reflection after an adverse event: Tips for training and practice. Gastroenterology. 2022 Sep;163(3):568-71. doi: 10.1053/j.gastro.2022.07.003.
Chey WD et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022 Sep;163(3):608-19. doi: 10.1053/j.gastro.2022.05.055.
Bushyhead D and Quigley EMM. Small intestinal bacterial overgrowth-pathophysiology and its implications for definition and management. Gastroenterology. 2022 Sep;163(3):593-607. doi: 10.1053/j.gastro.2022.04.002.
Long MT et al. AGA Clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology. 2022 Sep;163(3):764-74.e1. doi: 10.1053/j.gastro.2022.06.023.
CGH
August 2022
Lennon AM and Vege SS. Pancreatic cyst surveillance. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1663-7.e1. doi: 10.1016/j.cgh.2022.03.002.
Crockett SD et al. Large Polyp Study Group Consortium. Clip closure does not reduce risk of bleeding after resection of large serrated polyps: Results from a randomized trial. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1757-17--65.e4. doi: 10.1016/j.cgh.2021.12.036.
Martin P et al. Treatment algorithm for managing chronic hepatitis b virus infection in the United States: 2021 update. Clin Gastroenterol Hepatol. 2022 Aug;20(8):1766-75. doi: 10.1016/j.cgh.2021.07.036.
September 2022
Pawlak KM et al. How to train the next generation to provide high-quality peer-reviews. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1902-6. doi: 10.1016/j.cgh.2022.05.018.
Choung RS et al. Collagenous gastritis: Characteristics and response to topical budesonide. Clin Gastroenterol Hepatol. 2022 Sep;20(9):1977-85.e1. doi: 10.1016/j.cgh.2021.11.033.
Basnayake C et al. Long-term outcome of multidisciplinary versus standard gastroenterologist care for functional gastrointestinal disorders: A randomized trial. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2102-11.e9. doi: 10.1016/j.cgh.2021.12.005.
Deutsch-Link S et al. Alcohol-associated liver disease mortality increased from 2017 to 2020 and accelerated during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2022 Sep;20(9):2142-4.e2. doi: 10.1016/j.cgh.2022.03.017.
TIGE
Nakamatsu, Dai et al. Safety of cold snare polypectomy for small colorectal polyps in patients receiving antithrombotic therapy. Tech Innov Gastrointest Endosc. 2022 Apr 8;24[3]:246-53. doi: 10.1016/j.tige.2022.03.008.
Gastro Hep Advances
Brindusa Truta et al. Outcomes of continuation vs. discontinuation of adalimumab therapy during third trimester of pregnancy in inflammatory bowel disease. Gastro Hep Advances. 2022 Jan 1;1[5]:785-91. doi: 10.1016/j.gastha.2022.04.009.
The role of repeat uterine curettage in postmolar gestational trophoblastic neoplasia
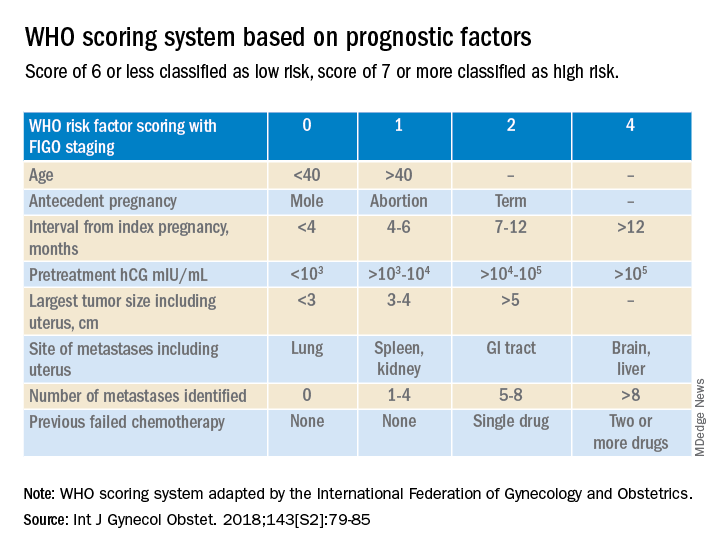
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.
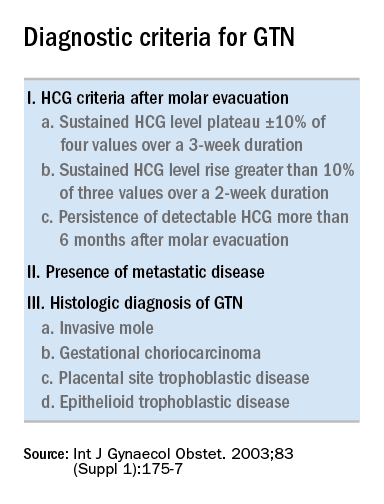
The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Add PCSK9 inhibitor to high-intensity statin at primary PCI, proposes sham-controlled EPIC-STEMI
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
It’s best to have patients on aggressive lipid-lowering therapy before discharge after an acute ST-segment elevation myocardial infarction (STEMI), so why not start it right away – even in the cath lab – using some of the most potent LDL cholesterol–lowering agents available?
That was a main idea behind the randomized, sham-controlled EPIC-STEMI trial, in which STEMI patients were started on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor immediately before direct percutaneous coronary intervention (PCI) and on top of high-intensity statins.
Those in the trial getting the active agent showed a 22% drop in LDL cholesterol levels by 6 weeks, compared with the control group given a sham injection along with high-intensity statins. They were also more likely to meet LDL cholesterol goals specified in some guidelines, including reduction by at least 50%. And those outcomes were achieved regardless of baseline LDL cholesterol levels or prior statin use.
Adoption of the trial’s early, aggressive LDL cholesterolreduction strategy in practice “has the potential to substantially reduce morbidity and mortality” in such cases “by further reducing LDL beyond statins in a much greater number of high-risk patients than are currently being treated with these agents,” suggested principal investigator Shamir R. Mehta, MD, MSc, when presenting the findings at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
Adherence to secondary prevention measures in patients with acute coronary syndromes (ACS) is much better if they are started before hospital discharge, explained Dr. Mehta, senior scientist with Population Health Research Institute and professor of medicine at McMaster University, Hamilton, Ont. But “as soon as the patient has left the hospital, it is much more difficult to get these therapies on board.”
Routine adoption of such aggressive in-hospital, lipid-lowering therapy for the vast population with ACS would likely mean far fewer deaths and cardiovascular events “across a broader patient population.”
EPIC-STEMI is among the first studies to explore the strategy. “I think that’s the point of the trial that we wanted to make, that we don’t yet have data on this. We’re treading very carefully with PCSK9 inhibitors, and it’s just inching forward in populations. And I think we need a bold trial to see whether or not this changes things.”
The PCSK9 inhibitor alirocumab (Praluent) was used in EPIC-STEMI, which was published in EuroIntervention, with Dr. Mehta as lead author, the same day as his presentation. The drug and its sham injection were given on top of either atorvastatin 40-80 mg or rosuvastatin 40 mg.
Early initiation of statins in patients with acute STEMI has become standard, but there’s good evidence from intracoronary imaging studies suggesting that the addition of PCSK9 inhibitors might promote further stabilization of plaques that could potentially cause recurrent ischemic events.
Treatment with the injectable drugs plus statins led to significant coronary lesion regression in the GLAGOV trial of patients with stable coronary disease. And initiation of PCSK9 inhibitors with high-intensity statins soon after PCI for ACS improved atheroma shrinkage in non–infarct-related arteries over 1 year in the recent, placebo-controlled PACMAN-AMI trial.
Dr. Mehta pointed out that LDL reductions on PCSK9 inhibition, compared with the sham control, weren’t necessarily as impressive as might be expected from the major trials of long-term therapy with the drugs.
“You need longer [therapy] in order to see a difference in LDL levels when you use a PCSK9 inhibitor acutely. This is shown also on measures of infarct size.” There was no difference between treatment groups in infarct size as measured by levels of the MB fraction of creatine kinase, he reported.
“What this is telling us is that the acute use of a PCSK9 inhibitor did not modify the size or the severity of the baseline STEMI event.”
And EPIC-STEMI was too small and never intended to assess clinical outcomes; it was more about feasibility and what degree of LDL cholesterol lowering might be expected.
The trial was needed, Dr. Mehta said, because the PCSK9 inhibitors haven’t been extensively adopted into clinical practice and are not getting to the patients who could most benefit. One of the reasons for that is quite clear to him. “We are missing the high-risk patients because we are not treating them acutely,” Dr. Mehta said in an interview.
The strategy “has not yet been evaluated, and there have been barriers,” he observed. “Cost has been a barrier. Access to the drug has been a barrier. But in terms of the science, in terms of reducing cardiovascular events, this is a strategy that has to be tested.”
The aggressive, early LDL cholesterol reduction strategy should be evaluated for its effect on long-term outcomes, “especially knowing that in the first 30 days to 6 months post STEMI there’s a tremendous uptick in ischemic events, including recurrent myocardial infarction,” Roxana Mehran, MD, said at a media briefing on EPIC-STEMI held before Dr. Mehta’s formal presentation.
The “fantastic reduction acutely” with a PCSK9 inhibitor on top of statins, “hopefully reducing inflammation” similarly to what’s been observed in past trials, “absolutely warrants” a STEMI clinical outcomes trial, said Dr. Mehran, Icahn School of Medicine at Mount Sinai, New York, who isn’t connected with EPIC-STEMI.
If better post-discharge medication adherence is one of the acute strategy’s goals, it will be important to consider the potential influence of prescribing a periodically injected drug, proposed Eric A. Cohen, MD, Sunnybrook Health Sciences Center, Toronto, at the press conference.
“Keep in mind that STEMI patients typically come to the hospital on zero medications and leave 2 days later on five medications,” Dr. Cohen observed. “I’m curious whether having one of those as a sub-Q injection every 2 weeks, and reducing the pill burden, will help or deter adherence to therapy. I think it’s worth studying.”
The trial originally included 97 patients undergoing PCI for STEMI who were randomly assigned to receive the PCSK9 inhibitor or a sham injection on top of high-intensity statins, without regard to LDL cholesterol levels. Randomization took place after diagnostic angiography but before PCI.
The analysis, however, subsequently excluded 29 patients who could not continue with the study, “mainly because of hospital research clinic closure due to the COVID-19 pandemic,” the published report states.
That left 68 patients who had received at least one dose of PCSK9 inhibitor, alirocumab 150 mg subcutaneously, or the sham injection, and had at least one blood draw for LDL cholesterol response which, Dr. Mehta said, still left adequate statistical power for the LDL cholesterol–based primary endpoint.
By 6 weeks, LDL cholesterol levels had fallen 72.9% in the active-therapy group and by 48.1% in the control group (P < .001). Also, 92.1% and 56.7% of patients, respectively (P = .002), had achieved levels below the 1.4 mmol/L (54 mg/dL) goal in the European guidelines, Dr. Mehta reported.
Levels fell more than 50% compared with baseline in 89.5% of alirocumab patients and 60% (P = .007) of controls, respectively.
There was no significant difference in rates of attaining LDL cholesterol levels below the 70 mg/dL (1.8 mmol/L) threshold specified in U.S. guidelines for very high-risk patients: 94.7% of alirocumab patients and 83.4% of controls (P = .26).
Nor did the groups differ significantly in natriuretic peptide levels, which reflect ventricular remodeling; or in 6-week change in the inflammatory biomarker high-sensitivity C-reactive protein.
An open-label, randomized trial scheduled to launch before the end of 2022 will explore similarly early initiation of a PCSK9 inhibitor, compared with standard lipid management, in an estimated 4,000 patients hospitalized with STEMI or non-STEMI.
The EVOLVE MI trial is looking at the monoclonal antibody evolocumab (Repatha) for its effect on the primary endpoint of myocardial infarction, ischemic stroke, arterial revascularization, or death from any cause over an expected 3-4 years.
EPIC-STEMI was supported in part by Sanofi. Dr. Mehta reported an unrestricted grant from Sanofi to Hamilton Health Sciences for the present study and consulting fees from Amgen, Sanofi, and Novartis. Dr. Cohen disclosed receiving grant support from and holding research contracts with Abbott Vascular; and receiving fees for consulting, honoraria, or serving on a speaker’s bureau for Abbott Vascular, Medtronic, and Baylis. Dr. Mehran disclosed receiving grants or research support from numerous pharmaceutical companies; receiving consultant fee or honoraria or serving on a speaker’s bureau for Novartis, Abbott Vascular, Janssen, Medtronic, Medscape/WebMD, and Cine-Med Research; and holding equity, stock, or stock options with Control Rad, Applied Therapeutics, and Elixir Medical.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
Gender-affirming mastectomy boosts image and quality of life in gender-diverse youth
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
Adolescents and young adults who undergo “top surgery” for gender dysphoria overwhelmingly report being satisfied with the procedure in the near-term, new research shows.
The results of the prospective cohort study, reported recently in JAMA Pediatrics, suggest that the surgery can help facilitate gender congruence and comfort with body image for transmasculine and nonbinary youth. The authors, from Northwestern University, Chicago, said the findings may “help dispel misconceptions that gender-affirming treatment is experimental and support evidence-based practices of top surgery.”
Sumanas Jordan, MD, PhD, assistant professor of plastic surgery at Northwestern University, Chicago, and a coauthor of the study, said the study was the first prospective, matched cohort analysis showing that chest surgery improves outcomes in this age group.
“We focused our study on chest dysphoria, the distress due to the presence of breasts, and gender congruence, the feeling of alignment between identity and physical characteristics,” Dr. Jordan said. “We will continue to study the effect of surgery in other areas of health, such as physical functioning and quality of life, and follow our patients longer term.”
As many as 9% of adolescents and young adults identify as transgender or nonbinary - a group underrepresented in the pediatric literature, Dr. Jordan’s group said. Chest dysphoria often is associated with psychosocial issues such as depression and anxiety.
“Dysphoria can lead to a range of negative physical and emotional consequences, such as avoidance of exercise and sports, harmful chest-binding practices, functional limitations, and suicidal ideation, said M. Brett Cooper, MD, MEd, assistant professor of pediatrics, and adolescent and young adult medicine, at UT Southwestern Medical Center/Children’s Health, Dallas. “These young people often bind for several hours a day to reduce the presence of their chest.”
The study
The Northwestern team recruited 81 patients with a mean age of 18.6 years whose sex at birth was assigned female. Patients were overwhelmingly White (89%), and the majority (59%) were transgender male, the remaining patients nonbinary.
The population sample included patients aged 13-24 who underwent top surgery from December 2019 to April 2021 and a matched control group of those who did not have surgery.
Outcomes measures were assessed preoperatively and 3 months after surgery.
Thirty-six surgical patients and 34 of those in the control arm completed the outcomes measures. Surgical complications were minimal. Propensity analyses suggested an association between surgery and substantial improvements in scores on the following study endpoints:
- Chest dysphoria measure (–25.58 points, 95% confidence interval [CI], –29.18 to –21.98).
- Transgender congruence scale (7.78 points, 95%: CI, 6.06-9.50)
- Body image scale (–7.20 points, 95% CI, –11.68 to –2.72).
The patients who underwent top surgery reported significant improvements in scores of chest dysphoria, transgender congruence, and body image. The results for patients younger than age 18 paralleled those for older participants in the study.
While the results corroborate other studies showing that gender-affirming therapy improves mental health and quality of life among these young people, the researchers cautioned that some insurers require testosterone therapy for 1 year before their plans will cover the costs of gender-affirming surgery.
This may negatively affect those nonbinary patients who do not undergo hormone therapy,” the researchers wrote. They are currently collecting 1-year follow-up data to determine the long-term effects of top surgery on chest dysphoria, gender congruence, and body image.
As surgical patients progress through adult life, does the risk of regret increase? “We did not address regret in this short-term study,” Dr. Jordan said. “However, previous studies have shown very low levels of regret.”
An accompanying editorial concurred that top surgery is effective and medically necessary in this population of young people.
Calling the study “an important milestone in gender affirmation research,” Kishan M. Thadikonda, MD, and Katherine M. Gast, MD, MS, of the school of medicine and public health at the University of Wisconsin in Madison, said it will be important to follow this young cohort to prove these benefits will endure as patients age.
They cautioned, however, that nonbinary patients represented just 13% of the patient total and only 8% of the surgical cohort. Nonbinary patients are not well understood as a patient population when it comes to gender-affirmation surgery and are often included in studies with transgender patients despite clear differences, they noted.
Current setbacks
According to Dr. Cooper, politics is already affecting care in Texas. “Due to the sociopolitical climate in my state in regard to gender-affirming care, I have also seen a few young people have their surgeries either canceled or postponed by their parents,” he said. “This has led to a worsening of mental health in these patients.”
Dr. Cooper stressed the need for more research on the perspective of non-White and socioeconomically disadvantaged youth.
“This study also highlights the disparity between patients who have commercial insurance versus those who are on Medicaid,” he said. “Medicaid plans often do not cover this, so those patients usually have to continue to suffer or pay for this surgery out of their own pocket.”
This study was supported by the Northwestern University Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. Funding also came from the Plastic Surgery Foundation and American Association of Pediatric Plastic Surgery. Dr. Jordan received grants from the Plastic Surgery Foundation during the study. One coauthor reported consultant fees from CVS Caremark for consulting outside the submitted work, and another reported grants from the National Institutes of Health outside the submitted work. Dr. Cooper disclosed no competing interests relevant to his comments. The editorial commentators disclosed no conflicts of interest.
FROM JAMA PEDIATRICS
Presence of community health workers linked with better results in patients with T2D
The researchers, led by Robert L. Ferrer, MD, MPH, with the department of family and community medicine at the University of Texas Health Science Center, San Antonio, enrolled 986 people in a Latino, inner-city cohort in primary care in San Antonio. Patients had uncontrolled type 2 diabetes and psychosocial risk factors. The study was published in Annals of Family Medicine.
The primary outcome measured was whether patients progressed through three stages of self-care: outreach (meeting face to face with a community health care worker), stabilization (collaborating with community health care workers to address life circumstances), and a third stage the researchers called “self-care generativity” (being able to manage blood sugar levels at home). The intervention lasted up to 12 weeks and had a 4-year follow-up.
Of participating patients, the researchers reported, 27% remained in outreach, 41% progressed to stabilization, 32% achieved self-care generativity status.
Coauthor Carlos Roberto Jaén, MD, PhD, also from the UT Health Science Center at San Antonio, said in an interview, “I don’t know any other intervention for diabetes that has 32% of participants having this kind of effect 4 years later.”
Dr. Jaén added that the study is unusual in that it had a 4-year follow-up and showed positive effects throughout that period, as most CHW studies have followed patients only up to one year.
The positive results over the 4 years after a short intervention “is a testimony of the power of intervention,” he said.
A1c drops with more progress in the intervention
The secondary outcome was change in hemoglobin A1c and need for urgent care or emergency department or hospital care.
Study participants who worked with a CHW – regardless of which group they were in at the end of the intervention – collectively saw a 2% drop in blood sugar.
Over a similar time period to when the study was conducted, the researchers analyzed 27,000 A1c measurements of patients with type 2 diabetes in a comparator group. For these patients, who did not receive the study intervention but were part of the same practice as those who received the intervention, the researchers observed a reduction in A1c levels of 0.05%.
Among the study participants, for those who remained in outreach, hospital visits were 6% higher than for those who advanced to the level of self-care generativity, but this difference was not statistically significant. Hospital visits were 90% higher for those who achieved stabilization versus those who remained in outreach (P = .014) The average count of emergency department visits was 74% higher for those who achieved stabilization versus those who achieved self-care generativity, and 31% higher in the group remaining at outreach versus those who reached the highest level of self-care.
Advantages of community workers
In San Antonio, the authors noted, type 2 diabetes prevalence is high: 15.5% of its 1.6 million residents have been diagnosed with the disease.
The CHWs built trust with patients and helped them set goals, navigate the health system and connect to community resources. They worked with behavioral health clinicians, nurse care managers, and medical assistants toward population management.
“Community health workers’ detailed understanding of patients’ circumstances help to tailor their care rather than apply fixed interventions,” the authors wrote.
Ricardo Correa, MD, director of the endocrinology, diabetes, and metabolism fellowship program in the University of Arizona, Phoenix, who was not involved with the study, said in an interview he was not surprised by the positive results.
He described the difference when CHWs get involved with type 2 diabetes care, particularly in the Latino community.
“They understand the culture, not just the language,” he said. “They have the trust of the community.”
It’s different when a provider not from the community tells a person with type 2 diabetes he or she needs to eat healthier or exercise more, he said.
The CHW can understand, for instance, that the nearest fresh market may be two towns away and open only on Saturdays and the parks are not safe for exercise outside at certain times of the day. Then they can help the patient find a sustainable solution.
“Community workers also won’t be looking at your immigration status,” something important to many in the Latino community, he explained.
Though this study looked at type 2 diabetes management, community health workers are also effective in other areas, he explained, such as increasing COVID-19 vaccinations, also do them being trustworthy and understanding.
Other study strengths
The group of people with type 2 diabetes they studied has the highest rates of poverty – “the poorest of the poor” – and the highest rates of diabetes-related amputations in San Antonio, Dr. Jaén said.
The intervention “is more focused on what people want to do, less so on the disease,” he explained. People are asked what goals they want to achieve and how the care team can help.
“It becomes an alliance between the community health worker and the patient,” he continued.
Others interested in implementing a program should know that building that relationship takes time and takes a broad multidisciplinary team working together, he said. “We would not necessarily see these effects in 6 months. You have to use a larger perspective.”
The researchers include with this study under the first-page tab “more online” access to tools, including resources for training, for others who want to implement such a program.
The study authors and Dr. Correa reported no relevant financial relationships.
The researchers, led by Robert L. Ferrer, MD, MPH, with the department of family and community medicine at the University of Texas Health Science Center, San Antonio, enrolled 986 people in a Latino, inner-city cohort in primary care in San Antonio. Patients had uncontrolled type 2 diabetes and psychosocial risk factors. The study was published in Annals of Family Medicine.
The primary outcome measured was whether patients progressed through three stages of self-care: outreach (meeting face to face with a community health care worker), stabilization (collaborating with community health care workers to address life circumstances), and a third stage the researchers called “self-care generativity” (being able to manage blood sugar levels at home). The intervention lasted up to 12 weeks and had a 4-year follow-up.
Of participating patients, the researchers reported, 27% remained in outreach, 41% progressed to stabilization, 32% achieved self-care generativity status.
Coauthor Carlos Roberto Jaén, MD, PhD, also from the UT Health Science Center at San Antonio, said in an interview, “I don’t know any other intervention for diabetes that has 32% of participants having this kind of effect 4 years later.”
Dr. Jaén added that the study is unusual in that it had a 4-year follow-up and showed positive effects throughout that period, as most CHW studies have followed patients only up to one year.
The positive results over the 4 years after a short intervention “is a testimony of the power of intervention,” he said.
A1c drops with more progress in the intervention
The secondary outcome was change in hemoglobin A1c and need for urgent care or emergency department or hospital care.
Study participants who worked with a CHW – regardless of which group they were in at the end of the intervention – collectively saw a 2% drop in blood sugar.
Over a similar time period to when the study was conducted, the researchers analyzed 27,000 A1c measurements of patients with type 2 diabetes in a comparator group. For these patients, who did not receive the study intervention but were part of the same practice as those who received the intervention, the researchers observed a reduction in A1c levels of 0.05%.
Among the study participants, for those who remained in outreach, hospital visits were 6% higher than for those who advanced to the level of self-care generativity, but this difference was not statistically significant. Hospital visits were 90% higher for those who achieved stabilization versus those who remained in outreach (P = .014) The average count of emergency department visits was 74% higher for those who achieved stabilization versus those who achieved self-care generativity, and 31% higher in the group remaining at outreach versus those who reached the highest level of self-care.
Advantages of community workers
In San Antonio, the authors noted, type 2 diabetes prevalence is high: 15.5% of its 1.6 million residents have been diagnosed with the disease.
The CHWs built trust with patients and helped them set goals, navigate the health system and connect to community resources. They worked with behavioral health clinicians, nurse care managers, and medical assistants toward population management.
“Community health workers’ detailed understanding of patients’ circumstances help to tailor their care rather than apply fixed interventions,” the authors wrote.
Ricardo Correa, MD, director of the endocrinology, diabetes, and metabolism fellowship program in the University of Arizona, Phoenix, who was not involved with the study, said in an interview he was not surprised by the positive results.
He described the difference when CHWs get involved with type 2 diabetes care, particularly in the Latino community.
“They understand the culture, not just the language,” he said. “They have the trust of the community.”
It’s different when a provider not from the community tells a person with type 2 diabetes he or she needs to eat healthier or exercise more, he said.
The CHW can understand, for instance, that the nearest fresh market may be two towns away and open only on Saturdays and the parks are not safe for exercise outside at certain times of the day. Then they can help the patient find a sustainable solution.
“Community workers also won’t be looking at your immigration status,” something important to many in the Latino community, he explained.
Though this study looked at type 2 diabetes management, community health workers are also effective in other areas, he explained, such as increasing COVID-19 vaccinations, also do them being trustworthy and understanding.
Other study strengths
The group of people with type 2 diabetes they studied has the highest rates of poverty – “the poorest of the poor” – and the highest rates of diabetes-related amputations in San Antonio, Dr. Jaén said.
The intervention “is more focused on what people want to do, less so on the disease,” he explained. People are asked what goals they want to achieve and how the care team can help.
“It becomes an alliance between the community health worker and the patient,” he continued.
Others interested in implementing a program should know that building that relationship takes time and takes a broad multidisciplinary team working together, he said. “We would not necessarily see these effects in 6 months. You have to use a larger perspective.”
The researchers include with this study under the first-page tab “more online” access to tools, including resources for training, for others who want to implement such a program.
The study authors and Dr. Correa reported no relevant financial relationships.
The researchers, led by Robert L. Ferrer, MD, MPH, with the department of family and community medicine at the University of Texas Health Science Center, San Antonio, enrolled 986 people in a Latino, inner-city cohort in primary care in San Antonio. Patients had uncontrolled type 2 diabetes and psychosocial risk factors. The study was published in Annals of Family Medicine.
The primary outcome measured was whether patients progressed through three stages of self-care: outreach (meeting face to face with a community health care worker), stabilization (collaborating with community health care workers to address life circumstances), and a third stage the researchers called “self-care generativity” (being able to manage blood sugar levels at home). The intervention lasted up to 12 weeks and had a 4-year follow-up.
Of participating patients, the researchers reported, 27% remained in outreach, 41% progressed to stabilization, 32% achieved self-care generativity status.
Coauthor Carlos Roberto Jaén, MD, PhD, also from the UT Health Science Center at San Antonio, said in an interview, “I don’t know any other intervention for diabetes that has 32% of participants having this kind of effect 4 years later.”
Dr. Jaén added that the study is unusual in that it had a 4-year follow-up and showed positive effects throughout that period, as most CHW studies have followed patients only up to one year.
The positive results over the 4 years after a short intervention “is a testimony of the power of intervention,” he said.
A1c drops with more progress in the intervention
The secondary outcome was change in hemoglobin A1c and need for urgent care or emergency department or hospital care.
Study participants who worked with a CHW – regardless of which group they were in at the end of the intervention – collectively saw a 2% drop in blood sugar.
Over a similar time period to when the study was conducted, the researchers analyzed 27,000 A1c measurements of patients with type 2 diabetes in a comparator group. For these patients, who did not receive the study intervention but were part of the same practice as those who received the intervention, the researchers observed a reduction in A1c levels of 0.05%.
Among the study participants, for those who remained in outreach, hospital visits were 6% higher than for those who advanced to the level of self-care generativity, but this difference was not statistically significant. Hospital visits were 90% higher for those who achieved stabilization versus those who remained in outreach (P = .014) The average count of emergency department visits was 74% higher for those who achieved stabilization versus those who achieved self-care generativity, and 31% higher in the group remaining at outreach versus those who reached the highest level of self-care.
Advantages of community workers
In San Antonio, the authors noted, type 2 diabetes prevalence is high: 15.5% of its 1.6 million residents have been diagnosed with the disease.
The CHWs built trust with patients and helped them set goals, navigate the health system and connect to community resources. They worked with behavioral health clinicians, nurse care managers, and medical assistants toward population management.
“Community health workers’ detailed understanding of patients’ circumstances help to tailor their care rather than apply fixed interventions,” the authors wrote.
Ricardo Correa, MD, director of the endocrinology, diabetes, and metabolism fellowship program in the University of Arizona, Phoenix, who was not involved with the study, said in an interview he was not surprised by the positive results.
He described the difference when CHWs get involved with type 2 diabetes care, particularly in the Latino community.
“They understand the culture, not just the language,” he said. “They have the trust of the community.”
It’s different when a provider not from the community tells a person with type 2 diabetes he or she needs to eat healthier or exercise more, he said.
The CHW can understand, for instance, that the nearest fresh market may be two towns away and open only on Saturdays and the parks are not safe for exercise outside at certain times of the day. Then they can help the patient find a sustainable solution.
“Community workers also won’t be looking at your immigration status,” something important to many in the Latino community, he explained.
Though this study looked at type 2 diabetes management, community health workers are also effective in other areas, he explained, such as increasing COVID-19 vaccinations, also do them being trustworthy and understanding.
Other study strengths
The group of people with type 2 diabetes they studied has the highest rates of poverty – “the poorest of the poor” – and the highest rates of diabetes-related amputations in San Antonio, Dr. Jaén said.
The intervention “is more focused on what people want to do, less so on the disease,” he explained. People are asked what goals they want to achieve and how the care team can help.
“It becomes an alliance between the community health worker and the patient,” he continued.
Others interested in implementing a program should know that building that relationship takes time and takes a broad multidisciplinary team working together, he said. “We would not necessarily see these effects in 6 months. You have to use a larger perspective.”
The researchers include with this study under the first-page tab “more online” access to tools, including resources for training, for others who want to implement such a program.
The study authors and Dr. Correa reported no relevant financial relationships.
FROM ANNALS OF FAMILY MEDICINE
Meet the JCOM Author with Dr. Barkoudah: Diabetes Population Health Innovations



Assessment of Glucagon-like Peptide-1 Receptor Agonists in Veterans TakingBasal/Bolus Insulin Regimens
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent Hb A1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of T1DM, were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline Hb A1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units. Mean duration of DM was 10 years, and mean use of basal/bolus insulin regimen was 6.1 years. Most participants (91%) used an insulin regimen containing insulin glargine and insulin aspart; the remaining participants used insulin detemir and insulin aspart. Semaglutide and liraglutide were the most commonly used GLP-1 RAs (44% and 39%, respectively) (Table 1).
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up, a different number of patient charts were available for review at each period (Table 2). Glycemic control was significantly improved at all time points when compared with baseline, but over time the benefit declined. The mean change in HbA1c was −1.1% (95% CI, −1.3 to −0.8; P < .001) at 3 months; −1.0% (95% CI, −1.3 to −0.7; P < .001) at 6 months; −0.9% (95% CI, −1.3 to −0.6; P < .001) at 12 months; −0.9% (95% CI −1.4 to −0.3; P = .002) at 18 months; and −0.7% (95% CI, −1.4 to 0.1; P = .07) at 24 months (Figure 1). Mean weight decreased from baseline −2.7 kg (95% CI, −3.7 to −1.6; P < .001); −4.4 kg (95% CI −5.7 to −3.2; P < .001) at 6 months; −3.9 kg (95% CI −6.0 to −1.9; P < .001) at 12 months; −4.7 kg (95% CI −6.7 to −2.6; P < .001) at 18 months; and −2.8 kg (95% CI, −5.9 to 0.3; P = .07) at 24 months (Figure 2). Mean TDD decreased at 3 months −12 units (95% CI, −19 to −5; P < .001); −18 units (95% CI, −27 to −9; P < .001) at 6 months; −14 units (95% CI, −24 to −5; P = .004) at 12 months; −9 units (95% CI, −21 to 3; P = .15) at 18 months; and −18 units (95% CI, −43 to 5 units; P = .12) at 24 months (Figure 3). The most common AEs were hypoglycemia (30%), diarrhea (11%), nausea (4%), and abdominal pain (3%).
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lover than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents being available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older males of White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the length of study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent Hb A1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of T1DM, were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline Hb A1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units. Mean duration of DM was 10 years, and mean use of basal/bolus insulin regimen was 6.1 years. Most participants (91%) used an insulin regimen containing insulin glargine and insulin aspart; the remaining participants used insulin detemir and insulin aspart. Semaglutide and liraglutide were the most commonly used GLP-1 RAs (44% and 39%, respectively) (Table 1).
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up, a different number of patient charts were available for review at each period (Table 2). Glycemic control was significantly improved at all time points when compared with baseline, but over time the benefit declined. The mean change in HbA1c was −1.1% (95% CI, −1.3 to −0.8; P < .001) at 3 months; −1.0% (95% CI, −1.3 to −0.7; P < .001) at 6 months; −0.9% (95% CI, −1.3 to −0.6; P < .001) at 12 months; −0.9% (95% CI −1.4 to −0.3; P = .002) at 18 months; and −0.7% (95% CI, −1.4 to 0.1; P = .07) at 24 months (Figure 1). Mean weight decreased from baseline −2.7 kg (95% CI, −3.7 to −1.6; P < .001); −4.4 kg (95% CI −5.7 to −3.2; P < .001) at 6 months; −3.9 kg (95% CI −6.0 to −1.9; P < .001) at 12 months; −4.7 kg (95% CI −6.7 to −2.6; P < .001) at 18 months; and −2.8 kg (95% CI, −5.9 to 0.3; P = .07) at 24 months (Figure 2). Mean TDD decreased at 3 months −12 units (95% CI, −19 to −5; P < .001); −18 units (95% CI, −27 to −9; P < .001) at 6 months; −14 units (95% CI, −24 to −5; P = .004) at 12 months; −9 units (95% CI, −21 to 3; P = .15) at 18 months; and −18 units (95% CI, −43 to 5 units; P = .12) at 24 months (Figure 3). The most common AEs were hypoglycemia (30%), diarrhea (11%), nausea (4%), and abdominal pain (3%).
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lover than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents being available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older males of White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the length of study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent Hb A1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of T1DM, were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline Hb A1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units. Mean duration of DM was 10 years, and mean use of basal/bolus insulin regimen was 6.1 years. Most participants (91%) used an insulin regimen containing insulin glargine and insulin aspart; the remaining participants used insulin detemir and insulin aspart. Semaglutide and liraglutide were the most commonly used GLP-1 RAs (44% and 39%, respectively) (Table 1).
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up, a different number of patient charts were available for review at each period (Table 2). Glycemic control was significantly improved at all time points when compared with baseline, but over time the benefit declined. The mean change in HbA1c was −1.1% (95% CI, −1.3 to −0.8; P < .001) at 3 months; −1.0% (95% CI, −1.3 to −0.7; P < .001) at 6 months; −0.9% (95% CI, −1.3 to −0.6; P < .001) at 12 months; −0.9% (95% CI −1.4 to −0.3; P = .002) at 18 months; and −0.7% (95% CI, −1.4 to 0.1; P = .07) at 24 months (Figure 1). Mean weight decreased from baseline −2.7 kg (95% CI, −3.7 to −1.6; P < .001); −4.4 kg (95% CI −5.7 to −3.2; P < .001) at 6 months; −3.9 kg (95% CI −6.0 to −1.9; P < .001) at 12 months; −4.7 kg (95% CI −6.7 to −2.6; P < .001) at 18 months; and −2.8 kg (95% CI, −5.9 to 0.3; P = .07) at 24 months (Figure 2). Mean TDD decreased at 3 months −12 units (95% CI, −19 to −5; P < .001); −18 units (95% CI, −27 to −9; P < .001) at 6 months; −14 units (95% CI, −24 to −5; P = .004) at 12 months; −9 units (95% CI, −21 to 3; P = .15) at 18 months; and −18 units (95% CI, −43 to 5 units; P = .12) at 24 months (Figure 3). The most common AEs were hypoglycemia (30%), diarrhea (11%), nausea (4%), and abdominal pain (3%).
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lover than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents being available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older males of White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the length of study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
Yes, we should talk politics and religion with patients
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
From our first days as medical students, we are told that politics and religion are topics to be avoided with patients, but I disagree. Knowing more about our patients allows us to deliver better care.
Politics and religion: New risk factors
The importance of politics and religion in the health of patients was clearly demonstrated during the COVID-19 pandemic. Lives were needlessly lost because of stands taken based on religious beliefs or a political ideology. Families, friends, and the community at large were impacted.
Over my years of practice, I have found that while these are difficult topics to address, they should not be avoided. Studies have shown that open acknowledgement of religious beliefs can affect both clinical outcomes and well-being. Religion and spirituality are as much a part of our patient’s lives as the physical parameters that we measure. To neglect these significant aspects is to miss the very essence of the individual.
I made it a practice to ask patients about their religious beliefs, the extent to which religion shaped their life, and whether they were part of a church community. Knowing this allowed me to separate deep personal belief from stances based on personal freedom, misinformation, conspiracies, and politics.
I found that information about political leanings flowed naturally in discussions with patients as we trusted and respected each other over time. If I approached politics objectively and nonjudgmentally, it generally led to meaningful conversation. This helped me to understand the patient as an individual and informed my diagnosis and treatment plan.
Politics as stress
For example, on more than one occasion, a patient with atrial fibrillation presented with persistent elevated blood pressure and pulse rate despite adherence to the medical regimen that I had prescribed. After a few minutes of discussion, it was clear that excessive attention to political commentary on TV and social media raised their anxiety and anger level, putting them at greater risk for adverse outcomes. I advised that they refocus their leisure activities rather than change or increase medication.
It is disappointing to see how one of the great scientific advances of our lifetime, vaccination science, has been tarnished because of political or religious ideology and to see the extent to which these beliefs influenced COVID-19 vaccination compliance. As health care providers, we must promote information based on the scientific method. If patients challenge us and point out that recommendations based on science seem to change over time, we must explain that science evolves on the basis of new information objectively gathered. We need to find out what information the patient has gotten from the Internet, TV, or conspiracy theories and counter this with scientific facts. If we do not discuss religion and politics with our patients along with other risk factors, we may compromise our ability to give them the best advice and treatment.
Our patients have a right to their own spiritual and political ideology. If it differs dramatically from our own, this should not influence our commitment to care for them. But we have an obligation to challenge unfounded beliefs about medicine and counter with scientific facts. There are times when individual freedoms must be secondary to public health. Ultimately, it is up to the patient to choose, but they should not be given a “free pass” on the basis of religion or politics. If I know something is true and I would do it myself or recommend it for my family, I have an obligation to provide this recommendation to my patients.
Religious preference is included in medical records. It is not appropriate to add political preference, but the patient benefits if a long-term caregiver knows this information.
During the pandemic, for the first time in my 40+ years of practice, some patients questioned my recommendations and placed equal or greater weight on religion, politics, or conspiracy theories. This continues to be a very real struggle.
Knowing and understanding our patients as individuals is critical to providing optimum care and that means tackling these formally taboo topics. If having a potentially uncomfortable conversation with patients allows us to save one life, it is worth it.
Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no relevant conflict of interest. A version of this article first appeared on Medscape.com.
Under 2% of eligible have gotten newest COVID booster shot
The newest booster became available to the public around Labor Day weekend, and about 4.4 million people have gotten it as of Sept. 21, according to Centers for Disease Control and Prevention data. That figure represents about 1.5% of the people eligible to receive the booster, NBC News reported.
The White House has said the total is probably closer to 5 million people. The CDC totals don’t yet include Texas and Idaho, which use an aggregate vaccination record reporting method for the Pfizer vaccine.
Scott Roberts, MD, a Yale Medicine infectious disease specialist in New Haven, Conn., told NBC News the low numbers are “demoralizing.”
“I would expect a much higher proportion of Americans to have gotten the booster by this point,” he said. “The fact that this booster came out days before Biden said the pandemic is over is a huge mixed message. Now it’s going to be that much harder to convince those at risk who are on the fence to get a booster.”
White House COVID-19 coordinator Ashish Jha, MD, says he thinks demand will pick up in the coming weeks.
“We’ve been thinking and talking about this as an annual vaccine like the flu vaccine. Flu vaccine season picks up in late September and early October. We’re just getting our education campaign going. So we expect to see, despite the fact that this was a strong start, we actually expect this to ramp up stronger,” Dr. Jha said.
The new booster is the third one authorized by the federal government and was redesigned to protect against the currently circulating subvariants BA.4 and BA.5 of the Omicron strain. People who have received a primary vaccine series or a booster at least 2 months before can receive it.
The new Pfizer booster is available for people 12 and up and the Moderna version for people 18 and up. The vaccines can be mixed and matched.
A version of this article first appeared on WebMD.com.
The newest booster became available to the public around Labor Day weekend, and about 4.4 million people have gotten it as of Sept. 21, according to Centers for Disease Control and Prevention data. That figure represents about 1.5% of the people eligible to receive the booster, NBC News reported.
The White House has said the total is probably closer to 5 million people. The CDC totals don’t yet include Texas and Idaho, which use an aggregate vaccination record reporting method for the Pfizer vaccine.
Scott Roberts, MD, a Yale Medicine infectious disease specialist in New Haven, Conn., told NBC News the low numbers are “demoralizing.”
“I would expect a much higher proportion of Americans to have gotten the booster by this point,” he said. “The fact that this booster came out days before Biden said the pandemic is over is a huge mixed message. Now it’s going to be that much harder to convince those at risk who are on the fence to get a booster.”
White House COVID-19 coordinator Ashish Jha, MD, says he thinks demand will pick up in the coming weeks.
“We’ve been thinking and talking about this as an annual vaccine like the flu vaccine. Flu vaccine season picks up in late September and early October. We’re just getting our education campaign going. So we expect to see, despite the fact that this was a strong start, we actually expect this to ramp up stronger,” Dr. Jha said.
The new booster is the third one authorized by the federal government and was redesigned to protect against the currently circulating subvariants BA.4 and BA.5 of the Omicron strain. People who have received a primary vaccine series or a booster at least 2 months before can receive it.
The new Pfizer booster is available for people 12 and up and the Moderna version for people 18 and up. The vaccines can be mixed and matched.
A version of this article first appeared on WebMD.com.
The newest booster became available to the public around Labor Day weekend, and about 4.4 million people have gotten it as of Sept. 21, according to Centers for Disease Control and Prevention data. That figure represents about 1.5% of the people eligible to receive the booster, NBC News reported.
The White House has said the total is probably closer to 5 million people. The CDC totals don’t yet include Texas and Idaho, which use an aggregate vaccination record reporting method for the Pfizer vaccine.
Scott Roberts, MD, a Yale Medicine infectious disease specialist in New Haven, Conn., told NBC News the low numbers are “demoralizing.”
“I would expect a much higher proportion of Americans to have gotten the booster by this point,” he said. “The fact that this booster came out days before Biden said the pandemic is over is a huge mixed message. Now it’s going to be that much harder to convince those at risk who are on the fence to get a booster.”
White House COVID-19 coordinator Ashish Jha, MD, says he thinks demand will pick up in the coming weeks.
“We’ve been thinking and talking about this as an annual vaccine like the flu vaccine. Flu vaccine season picks up in late September and early October. We’re just getting our education campaign going. So we expect to see, despite the fact that this was a strong start, we actually expect this to ramp up stronger,” Dr. Jha said.
The new booster is the third one authorized by the federal government and was redesigned to protect against the currently circulating subvariants BA.4 and BA.5 of the Omicron strain. People who have received a primary vaccine series or a booster at least 2 months before can receive it.
The new Pfizer booster is available for people 12 and up and the Moderna version for people 18 and up. The vaccines can be mixed and matched.
A version of this article first appeared on WebMD.com.







