User login
Dialysis not always best option in advanced kidney disease
ORLANDO – , new research shows.
“Patients mostly start dialysis because of unpleasant symptoms that cause suffering, including high potassium levels and high levels of uremic toxins in the blood,” senior author Kamyar Kalantar-Zadeh, MD, PhD, MPH, told this news organization.
“Conservative management serves to address and manage these symptoms and levels of toxicities without dialysis, so conservative management is an alternative approach, and patients should always be given a choice between [the two],” stressed Dr. Kalantar-Zadeh, professor of medicine at the University of California, Irvine.
The results were presented during the annual meeting of the American Society of Nephrology.
“There has been growing recognition of the importance of conservative nondialytic management as an alternative patient-centered treatment strategy for advanced kidney disease. However, conservative management remains under-utilized in the United States, which may in part be due to uncertainties regarding which patients will most benefit from dialysis versus nondialytic treatment,” said first author Connie Rhee, MD, also of the University of California, Irvine.
“We hope that these findings and further research can help inform treatment options for patients, care partners, and providers in the shared decision-making process of conservative management versus dialysis,” added Dr. Rhee, in a press release from the American Society of Nephrology.
Asked for comment, Sarah Davison, MD, noted that part of the Society’s strategy is, in fact, to promote conservative kidney management (CKM) as a key component of integrated care for patients with kidney failure. Dr. Davison is professor of medicine and chair of the International Society Working Group for Kidney Supportive Care and Conservative Kidney Management.
“We’ve recognized for a long time that there are many patients for whom dialysis provides neither a survival advantage nor a quality of life advantage,” she told this news organization.
“These patients tend to be those who have multiple morbidities, who are more frail, and who tend to be older, and in fact, the patients can live as long, if not longer, with better symptom management and better quality of life by not being on dialysis,” she stressed.
Study details
In the study, using data from the Optum Labs Data Warehouse, patients with advanced CKD were categorized according to whether or not they received conservative management, defined as those who did not receive dialysis within 2 years of the index eGFR (first eGFR < 25 mL/min/1.73m2) versus receipt of dialysis parsed as late versus early dialysis transition (eGFR < 15 vs. ≥ 15 mL/min/1.73m2 at dialysis initiation).
Hospitalization rates were compared between those treated with conservative management, compared with late or early dialysis.
“Among 309,188 advanced CKD patients who met eligibility [criteria], 55% of patients had greater than or equal to 1 hospitalization(s) within 2 years of the index eGFR,” the authors report. The most common causes of hospitalization among all patients were congestive heart failure, respiratory symptoms, or hypertension.
In most racial groups (non-Hispanic White, non-Hispanic Black, and Hispanic patients), patients on dialysis had higher hospitalization rates than those who received conservative management, and patients who started dialysis early (transitioned to dialysis at higher levels of kidney function) demonstrated the highest rates across all age groups, compared with those who started dialysis late (transitioned to dialysis at lower levels of kidney function) or were treated with conservative management.
Among Asian patients, those on dialysis also had higher hospitalization rates than those receiving conservative management, but patients who started dialysis late had higher rates than those on early dialysis, especially in older age groups, possibly because they were sicker, Dr. Kalantar-Zadeh suggested.
Conservative care has pros and cons, but Canada has embraced it
As Dr. Kalantar-Zadeh explained, conservative management has its pros and cons, compared with dialysis. “Conservative management requires that patients work with the multidisciplinary team including nephrologists, nutritionists, and others to try to manage CKD without dialysis, so it requires patient participation.”
On the other hand, dialysis is both easier and more lucrative than conservative management, at least for nephrologists, as they are well-trained in dialysis care, and it can be systematically applied. As to which patients with CKD might be optimal candidates for conservative management, Dr. Kalantar-Zadeh agreed this requires further study.
But he acknowledged that most nephrologists are not hugely supportive of conservative management because they are less well-trained in it, and it is more time-consuming. The one promising change is a new model introduced in 2022, a value-based kidney care model, that, if implemented, will be more incentivizing for nephrologists to offer conservative care more widely.
Dr. Davison meanwhile believes the “vast majority” of nephrologists based in Canada – as she is – are “highly supportive” of CKM as an important modality.
“The challenge, however, is that many nephrologists remain unsure as to how to best deliver or optimize all aspects of CKM, whether that is symptom management, advanced care planning, or how they must manage symptoms to align with a patient’s goals,” Dr. Davison explained.
“But it’s not that they do not believe in the value of CKM.”
Indeed, in her province, Alberta, nephrologists have been offering CKM for decades, and while they are currently standardizing care to make it easier to deliver, there is no financial incentive to offer dialysis over CKM.
“We are now seeing those elements of kidney supportive care as part of core competencies to manage any person with chronic illness, including CKD,” Dr. Davison said.
“So it’s absolutely doable, and contrary to one of the myths about CKM, it is not more time-consuming than dialysis – not when you know how to do it. You are just shifting your focus,” she emphasized.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Kalantar-Zadeh has reported receiving honoraria and medical directorship fees from Fresenius and DaVita. Dr. Davison has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – , new research shows.
“Patients mostly start dialysis because of unpleasant symptoms that cause suffering, including high potassium levels and high levels of uremic toxins in the blood,” senior author Kamyar Kalantar-Zadeh, MD, PhD, MPH, told this news organization.
“Conservative management serves to address and manage these symptoms and levels of toxicities without dialysis, so conservative management is an alternative approach, and patients should always be given a choice between [the two],” stressed Dr. Kalantar-Zadeh, professor of medicine at the University of California, Irvine.
The results were presented during the annual meeting of the American Society of Nephrology.
“There has been growing recognition of the importance of conservative nondialytic management as an alternative patient-centered treatment strategy for advanced kidney disease. However, conservative management remains under-utilized in the United States, which may in part be due to uncertainties regarding which patients will most benefit from dialysis versus nondialytic treatment,” said first author Connie Rhee, MD, also of the University of California, Irvine.
“We hope that these findings and further research can help inform treatment options for patients, care partners, and providers in the shared decision-making process of conservative management versus dialysis,” added Dr. Rhee, in a press release from the American Society of Nephrology.
Asked for comment, Sarah Davison, MD, noted that part of the Society’s strategy is, in fact, to promote conservative kidney management (CKM) as a key component of integrated care for patients with kidney failure. Dr. Davison is professor of medicine and chair of the International Society Working Group for Kidney Supportive Care and Conservative Kidney Management.
“We’ve recognized for a long time that there are many patients for whom dialysis provides neither a survival advantage nor a quality of life advantage,” she told this news organization.
“These patients tend to be those who have multiple morbidities, who are more frail, and who tend to be older, and in fact, the patients can live as long, if not longer, with better symptom management and better quality of life by not being on dialysis,” she stressed.
Study details
In the study, using data from the Optum Labs Data Warehouse, patients with advanced CKD were categorized according to whether or not they received conservative management, defined as those who did not receive dialysis within 2 years of the index eGFR (first eGFR < 25 mL/min/1.73m2) versus receipt of dialysis parsed as late versus early dialysis transition (eGFR < 15 vs. ≥ 15 mL/min/1.73m2 at dialysis initiation).
Hospitalization rates were compared between those treated with conservative management, compared with late or early dialysis.
“Among 309,188 advanced CKD patients who met eligibility [criteria], 55% of patients had greater than or equal to 1 hospitalization(s) within 2 years of the index eGFR,” the authors report. The most common causes of hospitalization among all patients were congestive heart failure, respiratory symptoms, or hypertension.
In most racial groups (non-Hispanic White, non-Hispanic Black, and Hispanic patients), patients on dialysis had higher hospitalization rates than those who received conservative management, and patients who started dialysis early (transitioned to dialysis at higher levels of kidney function) demonstrated the highest rates across all age groups, compared with those who started dialysis late (transitioned to dialysis at lower levels of kidney function) or were treated with conservative management.
Among Asian patients, those on dialysis also had higher hospitalization rates than those receiving conservative management, but patients who started dialysis late had higher rates than those on early dialysis, especially in older age groups, possibly because they were sicker, Dr. Kalantar-Zadeh suggested.
Conservative care has pros and cons, but Canada has embraced it
As Dr. Kalantar-Zadeh explained, conservative management has its pros and cons, compared with dialysis. “Conservative management requires that patients work with the multidisciplinary team including nephrologists, nutritionists, and others to try to manage CKD without dialysis, so it requires patient participation.”
On the other hand, dialysis is both easier and more lucrative than conservative management, at least for nephrologists, as they are well-trained in dialysis care, and it can be systematically applied. As to which patients with CKD might be optimal candidates for conservative management, Dr. Kalantar-Zadeh agreed this requires further study.
But he acknowledged that most nephrologists are not hugely supportive of conservative management because they are less well-trained in it, and it is more time-consuming. The one promising change is a new model introduced in 2022, a value-based kidney care model, that, if implemented, will be more incentivizing for nephrologists to offer conservative care more widely.
Dr. Davison meanwhile believes the “vast majority” of nephrologists based in Canada – as she is – are “highly supportive” of CKM as an important modality.
“The challenge, however, is that many nephrologists remain unsure as to how to best deliver or optimize all aspects of CKM, whether that is symptom management, advanced care planning, or how they must manage symptoms to align with a patient’s goals,” Dr. Davison explained.
“But it’s not that they do not believe in the value of CKM.”
Indeed, in her province, Alberta, nephrologists have been offering CKM for decades, and while they are currently standardizing care to make it easier to deliver, there is no financial incentive to offer dialysis over CKM.
“We are now seeing those elements of kidney supportive care as part of core competencies to manage any person with chronic illness, including CKD,” Dr. Davison said.
“So it’s absolutely doable, and contrary to one of the myths about CKM, it is not more time-consuming than dialysis – not when you know how to do it. You are just shifting your focus,” she emphasized.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Kalantar-Zadeh has reported receiving honoraria and medical directorship fees from Fresenius and DaVita. Dr. Davison has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – , new research shows.
“Patients mostly start dialysis because of unpleasant symptoms that cause suffering, including high potassium levels and high levels of uremic toxins in the blood,” senior author Kamyar Kalantar-Zadeh, MD, PhD, MPH, told this news organization.
“Conservative management serves to address and manage these symptoms and levels of toxicities without dialysis, so conservative management is an alternative approach, and patients should always be given a choice between [the two],” stressed Dr. Kalantar-Zadeh, professor of medicine at the University of California, Irvine.
The results were presented during the annual meeting of the American Society of Nephrology.
“There has been growing recognition of the importance of conservative nondialytic management as an alternative patient-centered treatment strategy for advanced kidney disease. However, conservative management remains under-utilized in the United States, which may in part be due to uncertainties regarding which patients will most benefit from dialysis versus nondialytic treatment,” said first author Connie Rhee, MD, also of the University of California, Irvine.
“We hope that these findings and further research can help inform treatment options for patients, care partners, and providers in the shared decision-making process of conservative management versus dialysis,” added Dr. Rhee, in a press release from the American Society of Nephrology.
Asked for comment, Sarah Davison, MD, noted that part of the Society’s strategy is, in fact, to promote conservative kidney management (CKM) as a key component of integrated care for patients with kidney failure. Dr. Davison is professor of medicine and chair of the International Society Working Group for Kidney Supportive Care and Conservative Kidney Management.
“We’ve recognized for a long time that there are many patients for whom dialysis provides neither a survival advantage nor a quality of life advantage,” she told this news organization.
“These patients tend to be those who have multiple morbidities, who are more frail, and who tend to be older, and in fact, the patients can live as long, if not longer, with better symptom management and better quality of life by not being on dialysis,” she stressed.
Study details
In the study, using data from the Optum Labs Data Warehouse, patients with advanced CKD were categorized according to whether or not they received conservative management, defined as those who did not receive dialysis within 2 years of the index eGFR (first eGFR < 25 mL/min/1.73m2) versus receipt of dialysis parsed as late versus early dialysis transition (eGFR < 15 vs. ≥ 15 mL/min/1.73m2 at dialysis initiation).
Hospitalization rates were compared between those treated with conservative management, compared with late or early dialysis.
“Among 309,188 advanced CKD patients who met eligibility [criteria], 55% of patients had greater than or equal to 1 hospitalization(s) within 2 years of the index eGFR,” the authors report. The most common causes of hospitalization among all patients were congestive heart failure, respiratory symptoms, or hypertension.
In most racial groups (non-Hispanic White, non-Hispanic Black, and Hispanic patients), patients on dialysis had higher hospitalization rates than those who received conservative management, and patients who started dialysis early (transitioned to dialysis at higher levels of kidney function) demonstrated the highest rates across all age groups, compared with those who started dialysis late (transitioned to dialysis at lower levels of kidney function) or were treated with conservative management.
Among Asian patients, those on dialysis also had higher hospitalization rates than those receiving conservative management, but patients who started dialysis late had higher rates than those on early dialysis, especially in older age groups, possibly because they were sicker, Dr. Kalantar-Zadeh suggested.
Conservative care has pros and cons, but Canada has embraced it
As Dr. Kalantar-Zadeh explained, conservative management has its pros and cons, compared with dialysis. “Conservative management requires that patients work with the multidisciplinary team including nephrologists, nutritionists, and others to try to manage CKD without dialysis, so it requires patient participation.”
On the other hand, dialysis is both easier and more lucrative than conservative management, at least for nephrologists, as they are well-trained in dialysis care, and it can be systematically applied. As to which patients with CKD might be optimal candidates for conservative management, Dr. Kalantar-Zadeh agreed this requires further study.
But he acknowledged that most nephrologists are not hugely supportive of conservative management because they are less well-trained in it, and it is more time-consuming. The one promising change is a new model introduced in 2022, a value-based kidney care model, that, if implemented, will be more incentivizing for nephrologists to offer conservative care more widely.
Dr. Davison meanwhile believes the “vast majority” of nephrologists based in Canada – as she is – are “highly supportive” of CKM as an important modality.
“The challenge, however, is that many nephrologists remain unsure as to how to best deliver or optimize all aspects of CKM, whether that is symptom management, advanced care planning, or how they must manage symptoms to align with a patient’s goals,” Dr. Davison explained.
“But it’s not that they do not believe in the value of CKM.”
Indeed, in her province, Alberta, nephrologists have been offering CKM for decades, and while they are currently standardizing care to make it easier to deliver, there is no financial incentive to offer dialysis over CKM.
“We are now seeing those elements of kidney supportive care as part of core competencies to manage any person with chronic illness, including CKD,” Dr. Davison said.
“So it’s absolutely doable, and contrary to one of the myths about CKM, it is not more time-consuming than dialysis – not when you know how to do it. You are just shifting your focus,” she emphasized.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Kalantar-Zadeh has reported receiving honoraria and medical directorship fees from Fresenius and DaVita. Dr. Davison has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT KIDNEY WEEK 2022
Patients complain some obesity care startups offer pills, and not much else
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Many Americans turn to the latest big idea to lose weight – fad diets, fitness crazes, dodgy herbs and pills, bariatric surgery, just to name a few. They’re rarely the magic solution people dream of.
Now a wave of startups offer access to a new category of drugs coupled with intensive behavioral coaching online. But already concerns are emerging.
These startups, spurred by hundreds of millions of dollars in funding from blue-chip venture capital firms, have signed up well over 100,000 patients and could reach millions more. These patients pay hundreds, if not thousands, of dollars to access new drugs, called glucagonlike peptide–1 (GLP-1) agonists, along with online coaching to encourage healthy habits.
The startups initially positioned themselves in lofty terms. “This is the last weight-loss program you’ll try,” said a 2020 marketing analysis by startup Calibrate Health, in messaging designed to reach one of its target demographics, the “working mom.” (Company spokesperson Michelle Wellington said the document does not reflect Calibrate’s current marketing strategy.)
But while doctors and patients are intrigued by the new model, some customers complain online that reality is short of the buildup: They say they got canned advice and unresponsive clinicians – and some report they couldn’t get the newest drugs.
Calibrate Health, a New York City–based startup, reported earlier in 2022 it had served 20,000 people. Another startup, Found, headquartered in San Francisco, has served 135,000 patients since July 2020, CEO Sarah Jones Simmer said in an interview. Calibrate costs patients nearly $1,600 a year, not counting the price of drugs, which can hit nearly $1,500 monthly without insurance, according to drug price savings site GoodRx. (Insurers reimburse for GLP-1agonists in limited circumstances, patients said.) Found offers a 6-month plan for nearly $600, a company spokesperson said. (That price includes generic drugs, but not the newer GLP-1 agonists, like Wegovy.)
The two companies are beneficiaries of over $200 million in combined venture funding, according to tracking by Crunchbase, a repository of venture capital investments. The firms say they’re on the vanguard of weight care, both citing the influence of biology and other scientific factors as key ingredients to their approaches.
There’s potentially a big market for these startups. Just over 4 in 10 Americans are obese, according to the Centers for Disease Control and Prevention, driving up their risk for cardiovascular conditions and type 2 diabetes. Effective medical treatments are elusive and hard to access.
Centers that provide this specialty care “are overwhelmed,” said Fatima Stanford, MD, an obesity medicine specialist at Massachusetts General in Boston, a teaching hospital affiliated with Harvard. Her own clinic has a wait list of 3,000.
Dr. Stanford, who said she has advised several of these telemedicine startups, is bullish on their potential.
Scott Butsch, MD, director of obesity medicine at the Cleveland Clinic, said the startups can offer care with less judgment and stigma than in-person peers. They’re also more convenient.
Dr. Butsch, who learned about the model through consultancies, patients, and colleagues, wonders whether the startups are operating “to strategically find which patients respond to which drug.” He said they should coordinate well with behavioral specialists, as antidepressants or other medications may be driving weight gain. “Obesity is a complex disease and requires treatments that match its complexity. I think programs that do not have a multidisciplinary team are less comprehensive and, in the long term, less effective.”
The startups market a two-pronged product: first, the new class of GLP-1 agonists. While these medications are effective at provoking weight loss, Wegovy, one of two in this class specifically approved for this purpose, is in short supply because of manufacturing difficulties, according to its maker, Novo Nordisk. Others in the category can be prescribed off label. But doctors generally aren’t familiar with the medications, Stanford said. In theory, the startups can bridge some of those gaps: They offer more specialized, knowledgeable clinicians.
Then there’s the other prong: behavioral changes. The companies use televisits and online messaging with nutritionists or coaches to help patients incorporate new diet and exercise habits. The weight loss figures achieved by participants in clinical trials for the new drugs – up to 15% of body mass – were tied to such changes, according to Novo Nordisk.
Social media sites are bursting with these startups’ ads, everywhere from podcasts to Instagram. A search of Meta’s ad library finds 40,000 ads on Facebook and Instagram between the two firms.
The ads complement people’s own postings on social media: Numerous Facebook groups are devoted to the new type of drugs – some even focused on helping patients manage side effects, like changes in their bowel movements. The buzz is quantifiable: On TikTok, mentions of the new GLP-1 agonists tripled from last June to this June, according to an analysis by investment bankers at Morgan Stanley.
There’s now a feverish, expectant appetite for these medications among the startups’ clientele. Patients often complained that their friends had obtained a drug they weren’t offered, recalled Alexandra Coults, a former pharmacist consultant for Found. Ms. Coults said patients may have perceived some sort of bait-and-switch when in reality clinical reasons – like drug contraindications – guide prescribing decisions.
Patient expectations influence care, Ms. Coults said. Customers came in with ideas shaped by the culture of fad diets and New Year’s resolutions. “Quite a few people would sign up for 1 month and not continue.”
In interviews with KHN and in online complaints, patients also questioned the quality of care they received. Some said intake – which began by filling out a form and proceeded to an online visit with a doctor – was perfunctory. Once medication began, they said, requests for counseling about side effects were slow to be answered.
Jess Garrant, a Found patient, recalled that after she was prescribed zonisamide, a generic anticonvulsant that has shown some ability to help with weight loss, she felt “absolutely weird.”
“I was up all night and my thoughts were racing,” she wrote in a blog post. She developed sores in her mouth.
She sought advice and help from Found physicians, but their replies “weren’t quick.” Nonemergency communications are routed through the company’s portal.
It took a week to complete a switch of medications and have a new prescription arrive at her home, she said. Meanwhile, she said, she went to an urgent care clinic for the mouth sores.
Found frequently prescribes generic medications – often off label – rather than just the new GLP-1 agonists, company executives said in an interview. Found said older generics like zonisamide are more accessible than the GLP-1 agonists advertised on social media and their own website. Both Dr. Butsch and Dr. Stanford said they’ve prescribed zonisamide successfully. Dr. Butsch said ramping up dosage rapidly can increase the risk of side effects.
But Kim Boyd, MD, chief medical officer of competitor Calibrate, said the older drugs “just haven’t worked.”
Patients of both companies have critiqued online and in interviews the startups’ behavioral care – which experts across the board maintain is integral to successful weight loss treatment. But some patients felt they simply had canned advice.
Other patients said they had ups and downs with their coaches. Dana Crom, an attorney, said she had gone through many coaches with Calibrate. Some were good, effective cheerleaders; others, not so good. But when kinks in the program arose, she said, the coach wasn’t able to help her navigate them. While the coach can report trouble with medications or the app, it appears those reports are no more effective than messages sent through the portal, Ms. Crom said.
And what about when her yearlong subscription ends? Ms. Crom said she’d consider continuing with Calibrate.
Relationships with coaches, given the need to change behavior, are a critical element of the business models. Patients’ results depend “on how adherent they are to lifestyle changes,” said Found’s chief medical officer, Rehka Kumar, MD.
While the startups offer care to a larger geographic footprint, it’s not clear whether the demographics of their patient populations are different from those of the traditional bricks-and-mortar model. Calibrate’s patients are overwhelmingly White; over 8 in 10 have at least an undergraduate degree; and over 8 in 10 are women, according to the company.
And its earlier marketing strategies reflected that. The September 2020 “segmentation” document laid out three types of customers the company could hope to attract: perimenopausal or menopausal women, with income ranging from $75,000 to $150,000 a year; working mothers, with a similar income; and “men.”
Isabelle Kenyon, Calibrate’s CEO, said the company now hopes to expand its reach to partner with large employers, and that will help diversify its patients.
Patients will need to be convinced that the model – more affordable, more accessible – works for them. For her part, Ms. Garrant, who no longer is using Found, reflected on her experience, writing in her blog post that she was hoping for more follow-up and a more personal approach. “I don’t think it’s a helpful way to lose weight,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Prednisone, colchicine equivalent in efficacy for CPP crystal arthritis
PHILADELPHIA – Prednisone appears to have the edge over colchicine for control of pain in patients with acute calcium pyrophosphate (CPP) crystal arthritis, an intensely painful rheumatic disease primarily affecting older patients.
Among 111 patients with acute CPP crystal arthritis randomized to receive either prednisone or colchicine for control of acute pain in a multicenter study, 2 days of therapy with the oral agents provided equivalent pain relief on the second day, and patients generally tolerated each agent well, reported Tristan Pascart, MD, from the Groupement Hospitalier de l’Institut Catholique de Lille (France).
“Almost three-fourths of patients are considered to be good responders to both drugs on day 3, and, maybe, safety is the key issue distinguishing the two treatments: Colchicine was generally well tolerated, but even with this very short time frame of treatment, one patient out of five had diarrhea, which is more of a concern in this elderly population at risk of dehydration,” he said in an oral abstract session at the annual meeting of the American College of Rheumatology.
In contrast, only about 6% of patients assigned to prednisone had diarrhea, and other adverse events that occurred more frequently with the corticosteroid, including hypertension, hyperglycemia, and insomnia all resolved after the therapy was stopped.
Common and acutely painful
Acute CPP crystal arthritis is a common complication that often occurs during hospitalization for primarily nonrheumatologic causes, Dr. Pascart said, and “in the absence of clinical trials, the management relies on expert opinion, which stems from extrapolated data from gap studies” primarily with prednisone or colchicine, Dr. Pascart said.
To fill in the knowledge gap, Dr. Pascart and colleagues conducted the COLCHICORT study to evaluate whether the two drugs were comparable in efficacy and safety for control of acute pain in a vulnerable population.
The multicenter, open-label trial included patients older than age 65 years with an estimated glomerular filtration rate above 30 mL/min per 1.73 m2 who presented with acute CPP deposition arthritis with symptoms occurring within the previous 36 hours. CPP arthritis was defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on x-rays or ultrasound.
Patients with a history of gout, cognitive decline that could impair pain assessment, or contraindications to either of the study drugs were excluded.
The participants were randomized to receive either colchicine 1.5 mg (1 mg to start, then 0.5 mg one hour later) at baseline and then 1 mg on day 1, or oral prednisone 30 mg at baseline and on day 1. The patients also received 1 g of systemic acetaminophen, and three 50-mg doses of tramadol during the first 24 hours.
Of the 111 patients randomized, 54 were assigned to receive prednisone, and 57 were assigned to receive colchicine. Baseline characteristics were similar between the groups, with a mean age of about 86 years, body mass index of around 25 kg/m2, and blood pressure in the range of 130/69 mm Hg.
For nearly half of all patients in study each arm the most painful joint was the knee, followed by wrists and ankles.
There was no difference between the groups in the primary efficacy outcome of a change at 24 hours over baseline in visual analog scale (VAS) (0-100 mm) scores, either in a per-protocol analysis or modified intention-to-treat analysis. The mean change in VAS at 24 hours in the colchicine group was –36.6 mm, compared with –37.7 mm in the prednisone group. The investigators had previously determined that any difference between the two drugs of less than 13 mm on pain VAS at 24 hours would meet the definition for equivalent efficacy.
In both groups, a majority of patients had either an improvement greater than 50% in pain VAS scores and/or a pain VAS score less than 40 mm at both 24 and 48 hours.
At 7 days of follow-up, 21.8% of patients assigned to colchicine had diarrhea, compared with 5.6% of those assigned to prednisone. Adverse events occurring more frequently with prednisone included hyperglycemia, hypertension, and insomnia.
Patients who received colchicine and were also on statins had a trend toward a higher risk for diarrhea, but the study was not adequately powered to detect an association, and the trend was not statistically significant, Dr. Pascart said.
“Taken together, safety issues suggest that prednisone should be considered as the first-line therapy in acute CPP crystal arthritis. Future research is warranted to determine factors increasing the risk of colchicine-induced diarrhea,” he concluded.
Both drugs are used
Sara K. Tedeschi, MD, from Brigham & Women’s Hospital in Boston, who attended the session where the data were presented, has a special clinical interest in CPP deposition disease. She applauded Dr. Pascart and colleagues for conducting a rare clinical trial in CPP crystal arthritis.
In an interview, she said that the study suggests “we can keep in mind shorter courses of treatment for acute CPP crystal arthritis; I think that’s one big takeaway from this study.”
Asked whether she would change her practice based on the findings, Dr. Tedeschi replied: “I personally am not sure that I would be moved to use prednisone more than colchicine; I actually take away from this that colchicine is equivalent to prednisone for short-term use for CPP arthritis, but I think it’s also really important to note that this is in the context of quite a lot of acetaminophen and quite a lot of tramadol, and frankly I don’t usually use tramadol with my patients, but I might consider doing that, especially as there were no delirium events in this population.”
Dr. Tedeschi was not involved in the study.
Asked the same question, Michael Toprover, MD, from New York University Langone Medical Center, a moderator of the session who was not involved in the study, said: “I usually use a combination of medications. I generally, in someone who is hospitalized in particular and is in such severe pain, use a combination of colchicine and prednisone, unless I’m worried about infection, in which case I’ll start colchicine until we’ve proven that it’s CPPD, and then I’ll add prednisone.”
The study was funded by PHRC-1 GIRCI Nord Ouest, a clinical research program funded by the Ministry of Health in France. Dr. Pascart, Dr. Tedeschi, and Dr. Toprover all reported having no relevant conflicts of interest.
PHILADELPHIA – Prednisone appears to have the edge over colchicine for control of pain in patients with acute calcium pyrophosphate (CPP) crystal arthritis, an intensely painful rheumatic disease primarily affecting older patients.
Among 111 patients with acute CPP crystal arthritis randomized to receive either prednisone or colchicine for control of acute pain in a multicenter study, 2 days of therapy with the oral agents provided equivalent pain relief on the second day, and patients generally tolerated each agent well, reported Tristan Pascart, MD, from the Groupement Hospitalier de l’Institut Catholique de Lille (France).
“Almost three-fourths of patients are considered to be good responders to both drugs on day 3, and, maybe, safety is the key issue distinguishing the two treatments: Colchicine was generally well tolerated, but even with this very short time frame of treatment, one patient out of five had diarrhea, which is more of a concern in this elderly population at risk of dehydration,” he said in an oral abstract session at the annual meeting of the American College of Rheumatology.
In contrast, only about 6% of patients assigned to prednisone had diarrhea, and other adverse events that occurred more frequently with the corticosteroid, including hypertension, hyperglycemia, and insomnia all resolved after the therapy was stopped.
Common and acutely painful
Acute CPP crystal arthritis is a common complication that often occurs during hospitalization for primarily nonrheumatologic causes, Dr. Pascart said, and “in the absence of clinical trials, the management relies on expert opinion, which stems from extrapolated data from gap studies” primarily with prednisone or colchicine, Dr. Pascart said.
To fill in the knowledge gap, Dr. Pascart and colleagues conducted the COLCHICORT study to evaluate whether the two drugs were comparable in efficacy and safety for control of acute pain in a vulnerable population.
The multicenter, open-label trial included patients older than age 65 years with an estimated glomerular filtration rate above 30 mL/min per 1.73 m2 who presented with acute CPP deposition arthritis with symptoms occurring within the previous 36 hours. CPP arthritis was defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on x-rays or ultrasound.
Patients with a history of gout, cognitive decline that could impair pain assessment, or contraindications to either of the study drugs were excluded.
The participants were randomized to receive either colchicine 1.5 mg (1 mg to start, then 0.5 mg one hour later) at baseline and then 1 mg on day 1, or oral prednisone 30 mg at baseline and on day 1. The patients also received 1 g of systemic acetaminophen, and three 50-mg doses of tramadol during the first 24 hours.
Of the 111 patients randomized, 54 were assigned to receive prednisone, and 57 were assigned to receive colchicine. Baseline characteristics were similar between the groups, with a mean age of about 86 years, body mass index of around 25 kg/m2, and blood pressure in the range of 130/69 mm Hg.
For nearly half of all patients in study each arm the most painful joint was the knee, followed by wrists and ankles.
There was no difference between the groups in the primary efficacy outcome of a change at 24 hours over baseline in visual analog scale (VAS) (0-100 mm) scores, either in a per-protocol analysis or modified intention-to-treat analysis. The mean change in VAS at 24 hours in the colchicine group was –36.6 mm, compared with –37.7 mm in the prednisone group. The investigators had previously determined that any difference between the two drugs of less than 13 mm on pain VAS at 24 hours would meet the definition for equivalent efficacy.
In both groups, a majority of patients had either an improvement greater than 50% in pain VAS scores and/or a pain VAS score less than 40 mm at both 24 and 48 hours.
At 7 days of follow-up, 21.8% of patients assigned to colchicine had diarrhea, compared with 5.6% of those assigned to prednisone. Adverse events occurring more frequently with prednisone included hyperglycemia, hypertension, and insomnia.
Patients who received colchicine and were also on statins had a trend toward a higher risk for diarrhea, but the study was not adequately powered to detect an association, and the trend was not statistically significant, Dr. Pascart said.
“Taken together, safety issues suggest that prednisone should be considered as the first-line therapy in acute CPP crystal arthritis. Future research is warranted to determine factors increasing the risk of colchicine-induced diarrhea,” he concluded.
Both drugs are used
Sara K. Tedeschi, MD, from Brigham & Women’s Hospital in Boston, who attended the session where the data were presented, has a special clinical interest in CPP deposition disease. She applauded Dr. Pascart and colleagues for conducting a rare clinical trial in CPP crystal arthritis.
In an interview, she said that the study suggests “we can keep in mind shorter courses of treatment for acute CPP crystal arthritis; I think that’s one big takeaway from this study.”
Asked whether she would change her practice based on the findings, Dr. Tedeschi replied: “I personally am not sure that I would be moved to use prednisone more than colchicine; I actually take away from this that colchicine is equivalent to prednisone for short-term use for CPP arthritis, but I think it’s also really important to note that this is in the context of quite a lot of acetaminophen and quite a lot of tramadol, and frankly I don’t usually use tramadol with my patients, but I might consider doing that, especially as there were no delirium events in this population.”
Dr. Tedeschi was not involved in the study.
Asked the same question, Michael Toprover, MD, from New York University Langone Medical Center, a moderator of the session who was not involved in the study, said: “I usually use a combination of medications. I generally, in someone who is hospitalized in particular and is in such severe pain, use a combination of colchicine and prednisone, unless I’m worried about infection, in which case I’ll start colchicine until we’ve proven that it’s CPPD, and then I’ll add prednisone.”
The study was funded by PHRC-1 GIRCI Nord Ouest, a clinical research program funded by the Ministry of Health in France. Dr. Pascart, Dr. Tedeschi, and Dr. Toprover all reported having no relevant conflicts of interest.
PHILADELPHIA – Prednisone appears to have the edge over colchicine for control of pain in patients with acute calcium pyrophosphate (CPP) crystal arthritis, an intensely painful rheumatic disease primarily affecting older patients.
Among 111 patients with acute CPP crystal arthritis randomized to receive either prednisone or colchicine for control of acute pain in a multicenter study, 2 days of therapy with the oral agents provided equivalent pain relief on the second day, and patients generally tolerated each agent well, reported Tristan Pascart, MD, from the Groupement Hospitalier de l’Institut Catholique de Lille (France).
“Almost three-fourths of patients are considered to be good responders to both drugs on day 3, and, maybe, safety is the key issue distinguishing the two treatments: Colchicine was generally well tolerated, but even with this very short time frame of treatment, one patient out of five had diarrhea, which is more of a concern in this elderly population at risk of dehydration,” he said in an oral abstract session at the annual meeting of the American College of Rheumatology.
In contrast, only about 6% of patients assigned to prednisone had diarrhea, and other adverse events that occurred more frequently with the corticosteroid, including hypertension, hyperglycemia, and insomnia all resolved after the therapy was stopped.
Common and acutely painful
Acute CPP crystal arthritis is a common complication that often occurs during hospitalization for primarily nonrheumatologic causes, Dr. Pascart said, and “in the absence of clinical trials, the management relies on expert opinion, which stems from extrapolated data from gap studies” primarily with prednisone or colchicine, Dr. Pascart said.
To fill in the knowledge gap, Dr. Pascart and colleagues conducted the COLCHICORT study to evaluate whether the two drugs were comparable in efficacy and safety for control of acute pain in a vulnerable population.
The multicenter, open-label trial included patients older than age 65 years with an estimated glomerular filtration rate above 30 mL/min per 1.73 m2 who presented with acute CPP deposition arthritis with symptoms occurring within the previous 36 hours. CPP arthritis was defined by the identification of CPP crystals on synovial fluid analysis or typical clinical presentation with evidence of chondrocalcinosis on x-rays or ultrasound.
Patients with a history of gout, cognitive decline that could impair pain assessment, or contraindications to either of the study drugs were excluded.
The participants were randomized to receive either colchicine 1.5 mg (1 mg to start, then 0.5 mg one hour later) at baseline and then 1 mg on day 1, or oral prednisone 30 mg at baseline and on day 1. The patients also received 1 g of systemic acetaminophen, and three 50-mg doses of tramadol during the first 24 hours.
Of the 111 patients randomized, 54 were assigned to receive prednisone, and 57 were assigned to receive colchicine. Baseline characteristics were similar between the groups, with a mean age of about 86 years, body mass index of around 25 kg/m2, and blood pressure in the range of 130/69 mm Hg.
For nearly half of all patients in study each arm the most painful joint was the knee, followed by wrists and ankles.
There was no difference between the groups in the primary efficacy outcome of a change at 24 hours over baseline in visual analog scale (VAS) (0-100 mm) scores, either in a per-protocol analysis or modified intention-to-treat analysis. The mean change in VAS at 24 hours in the colchicine group was –36.6 mm, compared with –37.7 mm in the prednisone group. The investigators had previously determined that any difference between the two drugs of less than 13 mm on pain VAS at 24 hours would meet the definition for equivalent efficacy.
In both groups, a majority of patients had either an improvement greater than 50% in pain VAS scores and/or a pain VAS score less than 40 mm at both 24 and 48 hours.
At 7 days of follow-up, 21.8% of patients assigned to colchicine had diarrhea, compared with 5.6% of those assigned to prednisone. Adverse events occurring more frequently with prednisone included hyperglycemia, hypertension, and insomnia.
Patients who received colchicine and were also on statins had a trend toward a higher risk for diarrhea, but the study was not adequately powered to detect an association, and the trend was not statistically significant, Dr. Pascart said.
“Taken together, safety issues suggest that prednisone should be considered as the first-line therapy in acute CPP crystal arthritis. Future research is warranted to determine factors increasing the risk of colchicine-induced diarrhea,” he concluded.
Both drugs are used
Sara K. Tedeschi, MD, from Brigham & Women’s Hospital in Boston, who attended the session where the data were presented, has a special clinical interest in CPP deposition disease. She applauded Dr. Pascart and colleagues for conducting a rare clinical trial in CPP crystal arthritis.
In an interview, she said that the study suggests “we can keep in mind shorter courses of treatment for acute CPP crystal arthritis; I think that’s one big takeaway from this study.”
Asked whether she would change her practice based on the findings, Dr. Tedeschi replied: “I personally am not sure that I would be moved to use prednisone more than colchicine; I actually take away from this that colchicine is equivalent to prednisone for short-term use for CPP arthritis, but I think it’s also really important to note that this is in the context of quite a lot of acetaminophen and quite a lot of tramadol, and frankly I don’t usually use tramadol with my patients, but I might consider doing that, especially as there were no delirium events in this population.”
Dr. Tedeschi was not involved in the study.
Asked the same question, Michael Toprover, MD, from New York University Langone Medical Center, a moderator of the session who was not involved in the study, said: “I usually use a combination of medications. I generally, in someone who is hospitalized in particular and is in such severe pain, use a combination of colchicine and prednisone, unless I’m worried about infection, in which case I’ll start colchicine until we’ve proven that it’s CPPD, and then I’ll add prednisone.”
The study was funded by PHRC-1 GIRCI Nord Ouest, a clinical research program funded by the Ministry of Health in France. Dr. Pascart, Dr. Tedeschi, and Dr. Toprover all reported having no relevant conflicts of interest.
AT ACR 2022
Sham-controlled renal denervation trial for hypertension is a near miss
SPYRAL HTN–ON MED hits headwinds
CHICAGO – Renal denervation, relative to a sham procedure, was linked with statistically significant reductions in blood pressure in the newly completed SPYRAL HTN–ON MED trial, but several factors are likely to have worked in concert to prevent the study from meeting its primary endpoint.
Of these differences, probably none was more important than the substantially higher proportion of patients in the sham group that received additional BP-lowering medications over the course of the study, David E. Kandzari, MD, reported at the American Heart Association scientific sessions.
The SPYRAL HTN–ON MED pivotal trial followed the previously completed SPYRAL HTN–ON MED pilot study, which did show a significant BP-lowering effect on antihypertensive medications followed radiofrequency denervation. In a recent update of the pilot study, the effect was persistent out to 3 years.
In the SPYRAL HTN–ON MED program, patients on their second screening visit were required to have a systolic pressure of between 140 and 170 mm Hg on 24-hour ambulatory BP monitoring (ABPM) while taking up to three antihypertensive medications. Patients who entered the study were randomized to renal denervation or sham control while maintaining their baseline antihypertensive therapies.
The previously reported pilot study comprised 80 patients. The expansion pivotal trial added 257 more patients for a total cohort of 337 patients. The primary efficacy endpoint was based on a Bayesian analysis of change in 24-hour systolic ABPM at 6 months for those in the experimental arm versus those on medications alone. Participants from both the pilot and pivotal trials were included.
The prespecified definition of success for renal denervation was a 97.5% threshold for probability of superiority on the basis of this Bayesian analysis. However, the Bayesian analysis was distorted by differences in the pilot and expansion cohorts, which complicated the superiority calculation. As a result, the analysis only yielded a 51% probability of superiority, a level substantially below the predefined threshold.
Despite differences seen in BP control in favor of renal denervation, several factors were identified that likely contributed to the missed primary endpoint. One stood out.
“Significant differences in medication prescriptions were disproportionate in favor of the sham group,” reported Dr. Kandzari, chief of Piedmont Heart Institute, Atlanta. He said these differences, which were a violation of the protocol mandate, led to a “bias toward the null” for the primary outcome.
The failure to meet the primary outcome was particularly disappointing in the wake of the favorable pilot study and the SPYRAL HTN–OFF MED pivotal trial, which were both positive.
In the pilot study, which did not have a medication imbalance, a 7.3–mm Hg reduction (P = .004) in 24-hour ABPM was seen at 6 months. Relative reductions in office-based systolic pressure reductions for renal denervation versus sham were 6.6 mm Hg (P = .03) and 4.0 mm Hg (P = .03) for the pilot and expansions groups, respectively.
On the basis of a Win ratio derived from a hierarchical analysis of ABMP and medication burden reduction, the 1.50 advantage (P = .005) for the renal denervation arm in the newly completed SPYRAL HTN–ON MED trial was also compelling.
At study entry, the median number of medications was 1.9 in both the renal denervation and sham arms. At the end of 6 months, the median number of medications was unchanged in the experimental arm but rose to 2.1 (P = .01) in the sham group. Similarly, there was little change in the medication burden from the start to the end of the trial in the denervation group (2.8 vs. 3.0), but a statistically significant change in the sham group (2.9 vs. 3.5; P = .04).
Furthermore, the net percentage change of patients receiving medications favoring BP reduction over the course of the study did not differ between the experimental and control arms of the pilot cohort, but was more than 10 times higher among controls in the expansion group (1.9% vs. 21.8%; P < .0001).
Medication changes over the course of the SPYRAL HTN–ON MED trial were even greater in some specific subgroups. Among Black participants, for example, 14.2% of those randomized to renal denervation and 54.6% of those randomized to the sham group increased their antihypertensive therapies over the course of the study.
The COVID-19 epidemic is suspected of playing another role in the negative results, according to Dr. Kandzari. After a brief pause in enrollment, the SPYRAL HTN–ON MED trial was resumed, but approximately 80% of the expansion cohort data were collected during this period. When compared, variances in office and 24-hour ABPM were observed for participants who were or were not evaluated during COVID.
“Significant differences in 24-hour ABPM patterns pre- and during COVID may reflect changes in patient behavior and lifestyle,” Dr. Kandzari speculated.
The data from this study differ from essentially all of the other studies in the SPYRAL HTN program as well as several other sham-controlled studies with renal denervation, according to Dr. Kandzari.
The AHA-invited discussant, Ajay J. Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, largely agreed that several variables appeared to conspire against a positive result in this trial, but he zeroed in on the imbalance of antihypertensive medications.
“Any trial that attempts to show a difference between renal denervation and a sham procedure must insure that antihypertensive medications are the same in the two arms. They cannot be different,” he said.
As an active investigator in the field of renal denervation, Dr. Kirtane thinks the evidence does support a benefit from renal denervation, but he believes data are still needed to determine which patients are candidates.
“Renal denervation is not going to be a replacement for previous established therapies, but it will be an adjunct,” he predicted. The preponderance of evidence supports clinically meaningful reductions in BP with this approach, “but we need to determine who to consider [for this therapy] and to have realistic expectations about the degree of benefit.”
Dr. Kandzari reported financial relationships with Abbott Vascular, Ablative Solutions, Biotronik, Boston Scientific, CSI, Medtronic Cardiovascular, OrbusNeich, and Teleflex. Dr. Kirtane reported financial relationships with Abbott Vascular, Abiomed, Boston Scientific, Cardiovascular Systems, Cathworks, Chiesi, Medtronic, Opens, Philipps, Regeneron, ReCor Medical, Siemens, Spectranetics, and Zoll.
SPYRAL HTN–ON MED hits headwinds
SPYRAL HTN–ON MED hits headwinds
CHICAGO – Renal denervation, relative to a sham procedure, was linked with statistically significant reductions in blood pressure in the newly completed SPYRAL HTN–ON MED trial, but several factors are likely to have worked in concert to prevent the study from meeting its primary endpoint.
Of these differences, probably none was more important than the substantially higher proportion of patients in the sham group that received additional BP-lowering medications over the course of the study, David E. Kandzari, MD, reported at the American Heart Association scientific sessions.
The SPYRAL HTN–ON MED pivotal trial followed the previously completed SPYRAL HTN–ON MED pilot study, which did show a significant BP-lowering effect on antihypertensive medications followed radiofrequency denervation. In a recent update of the pilot study, the effect was persistent out to 3 years.
In the SPYRAL HTN–ON MED program, patients on their second screening visit were required to have a systolic pressure of between 140 and 170 mm Hg on 24-hour ambulatory BP monitoring (ABPM) while taking up to three antihypertensive medications. Patients who entered the study were randomized to renal denervation or sham control while maintaining their baseline antihypertensive therapies.
The previously reported pilot study comprised 80 patients. The expansion pivotal trial added 257 more patients for a total cohort of 337 patients. The primary efficacy endpoint was based on a Bayesian analysis of change in 24-hour systolic ABPM at 6 months for those in the experimental arm versus those on medications alone. Participants from both the pilot and pivotal trials were included.
The prespecified definition of success for renal denervation was a 97.5% threshold for probability of superiority on the basis of this Bayesian analysis. However, the Bayesian analysis was distorted by differences in the pilot and expansion cohorts, which complicated the superiority calculation. As a result, the analysis only yielded a 51% probability of superiority, a level substantially below the predefined threshold.
Despite differences seen in BP control in favor of renal denervation, several factors were identified that likely contributed to the missed primary endpoint. One stood out.
“Significant differences in medication prescriptions were disproportionate in favor of the sham group,” reported Dr. Kandzari, chief of Piedmont Heart Institute, Atlanta. He said these differences, which were a violation of the protocol mandate, led to a “bias toward the null” for the primary outcome.
The failure to meet the primary outcome was particularly disappointing in the wake of the favorable pilot study and the SPYRAL HTN–OFF MED pivotal trial, which were both positive.
In the pilot study, which did not have a medication imbalance, a 7.3–mm Hg reduction (P = .004) in 24-hour ABPM was seen at 6 months. Relative reductions in office-based systolic pressure reductions for renal denervation versus sham were 6.6 mm Hg (P = .03) and 4.0 mm Hg (P = .03) for the pilot and expansions groups, respectively.
On the basis of a Win ratio derived from a hierarchical analysis of ABMP and medication burden reduction, the 1.50 advantage (P = .005) for the renal denervation arm in the newly completed SPYRAL HTN–ON MED trial was also compelling.
At study entry, the median number of medications was 1.9 in both the renal denervation and sham arms. At the end of 6 months, the median number of medications was unchanged in the experimental arm but rose to 2.1 (P = .01) in the sham group. Similarly, there was little change in the medication burden from the start to the end of the trial in the denervation group (2.8 vs. 3.0), but a statistically significant change in the sham group (2.9 vs. 3.5; P = .04).
Furthermore, the net percentage change of patients receiving medications favoring BP reduction over the course of the study did not differ between the experimental and control arms of the pilot cohort, but was more than 10 times higher among controls in the expansion group (1.9% vs. 21.8%; P < .0001).
Medication changes over the course of the SPYRAL HTN–ON MED trial were even greater in some specific subgroups. Among Black participants, for example, 14.2% of those randomized to renal denervation and 54.6% of those randomized to the sham group increased their antihypertensive therapies over the course of the study.
The COVID-19 epidemic is suspected of playing another role in the negative results, according to Dr. Kandzari. After a brief pause in enrollment, the SPYRAL HTN–ON MED trial was resumed, but approximately 80% of the expansion cohort data were collected during this period. When compared, variances in office and 24-hour ABPM were observed for participants who were or were not evaluated during COVID.
“Significant differences in 24-hour ABPM patterns pre- and during COVID may reflect changes in patient behavior and lifestyle,” Dr. Kandzari speculated.
The data from this study differ from essentially all of the other studies in the SPYRAL HTN program as well as several other sham-controlled studies with renal denervation, according to Dr. Kandzari.
The AHA-invited discussant, Ajay J. Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, largely agreed that several variables appeared to conspire against a positive result in this trial, but he zeroed in on the imbalance of antihypertensive medications.
“Any trial that attempts to show a difference between renal denervation and a sham procedure must insure that antihypertensive medications are the same in the two arms. They cannot be different,” he said.
As an active investigator in the field of renal denervation, Dr. Kirtane thinks the evidence does support a benefit from renal denervation, but he believes data are still needed to determine which patients are candidates.
“Renal denervation is not going to be a replacement for previous established therapies, but it will be an adjunct,” he predicted. The preponderance of evidence supports clinically meaningful reductions in BP with this approach, “but we need to determine who to consider [for this therapy] and to have realistic expectations about the degree of benefit.”
Dr. Kandzari reported financial relationships with Abbott Vascular, Ablative Solutions, Biotronik, Boston Scientific, CSI, Medtronic Cardiovascular, OrbusNeich, and Teleflex. Dr. Kirtane reported financial relationships with Abbott Vascular, Abiomed, Boston Scientific, Cardiovascular Systems, Cathworks, Chiesi, Medtronic, Opens, Philipps, Regeneron, ReCor Medical, Siemens, Spectranetics, and Zoll.
CHICAGO – Renal denervation, relative to a sham procedure, was linked with statistically significant reductions in blood pressure in the newly completed SPYRAL HTN–ON MED trial, but several factors are likely to have worked in concert to prevent the study from meeting its primary endpoint.
Of these differences, probably none was more important than the substantially higher proportion of patients in the sham group that received additional BP-lowering medications over the course of the study, David E. Kandzari, MD, reported at the American Heart Association scientific sessions.
The SPYRAL HTN–ON MED pivotal trial followed the previously completed SPYRAL HTN–ON MED pilot study, which did show a significant BP-lowering effect on antihypertensive medications followed radiofrequency denervation. In a recent update of the pilot study, the effect was persistent out to 3 years.
In the SPYRAL HTN–ON MED program, patients on their second screening visit were required to have a systolic pressure of between 140 and 170 mm Hg on 24-hour ambulatory BP monitoring (ABPM) while taking up to three antihypertensive medications. Patients who entered the study were randomized to renal denervation or sham control while maintaining their baseline antihypertensive therapies.
The previously reported pilot study comprised 80 patients. The expansion pivotal trial added 257 more patients for a total cohort of 337 patients. The primary efficacy endpoint was based on a Bayesian analysis of change in 24-hour systolic ABPM at 6 months for those in the experimental arm versus those on medications alone. Participants from both the pilot and pivotal trials were included.
The prespecified definition of success for renal denervation was a 97.5% threshold for probability of superiority on the basis of this Bayesian analysis. However, the Bayesian analysis was distorted by differences in the pilot and expansion cohorts, which complicated the superiority calculation. As a result, the analysis only yielded a 51% probability of superiority, a level substantially below the predefined threshold.
Despite differences seen in BP control in favor of renal denervation, several factors were identified that likely contributed to the missed primary endpoint. One stood out.
“Significant differences in medication prescriptions were disproportionate in favor of the sham group,” reported Dr. Kandzari, chief of Piedmont Heart Institute, Atlanta. He said these differences, which were a violation of the protocol mandate, led to a “bias toward the null” for the primary outcome.
The failure to meet the primary outcome was particularly disappointing in the wake of the favorable pilot study and the SPYRAL HTN–OFF MED pivotal trial, which were both positive.
In the pilot study, which did not have a medication imbalance, a 7.3–mm Hg reduction (P = .004) in 24-hour ABPM was seen at 6 months. Relative reductions in office-based systolic pressure reductions for renal denervation versus sham were 6.6 mm Hg (P = .03) and 4.0 mm Hg (P = .03) for the pilot and expansions groups, respectively.
On the basis of a Win ratio derived from a hierarchical analysis of ABMP and medication burden reduction, the 1.50 advantage (P = .005) for the renal denervation arm in the newly completed SPYRAL HTN–ON MED trial was also compelling.
At study entry, the median number of medications was 1.9 in both the renal denervation and sham arms. At the end of 6 months, the median number of medications was unchanged in the experimental arm but rose to 2.1 (P = .01) in the sham group. Similarly, there was little change in the medication burden from the start to the end of the trial in the denervation group (2.8 vs. 3.0), but a statistically significant change in the sham group (2.9 vs. 3.5; P = .04).
Furthermore, the net percentage change of patients receiving medications favoring BP reduction over the course of the study did not differ between the experimental and control arms of the pilot cohort, but was more than 10 times higher among controls in the expansion group (1.9% vs. 21.8%; P < .0001).
Medication changes over the course of the SPYRAL HTN–ON MED trial were even greater in some specific subgroups. Among Black participants, for example, 14.2% of those randomized to renal denervation and 54.6% of those randomized to the sham group increased their antihypertensive therapies over the course of the study.
The COVID-19 epidemic is suspected of playing another role in the negative results, according to Dr. Kandzari. After a brief pause in enrollment, the SPYRAL HTN–ON MED trial was resumed, but approximately 80% of the expansion cohort data were collected during this period. When compared, variances in office and 24-hour ABPM were observed for participants who were or were not evaluated during COVID.
“Significant differences in 24-hour ABPM patterns pre- and during COVID may reflect changes in patient behavior and lifestyle,” Dr. Kandzari speculated.
The data from this study differ from essentially all of the other studies in the SPYRAL HTN program as well as several other sham-controlled studies with renal denervation, according to Dr. Kandzari.
The AHA-invited discussant, Ajay J. Kirtane, MD, director of the Cardiac Catheterization Laboratories at Columbia University, New York, largely agreed that several variables appeared to conspire against a positive result in this trial, but he zeroed in on the imbalance of antihypertensive medications.
“Any trial that attempts to show a difference between renal denervation and a sham procedure must insure that antihypertensive medications are the same in the two arms. They cannot be different,” he said.
As an active investigator in the field of renal denervation, Dr. Kirtane thinks the evidence does support a benefit from renal denervation, but he believes data are still needed to determine which patients are candidates.
“Renal denervation is not going to be a replacement for previous established therapies, but it will be an adjunct,” he predicted. The preponderance of evidence supports clinically meaningful reductions in BP with this approach, “but we need to determine who to consider [for this therapy] and to have realistic expectations about the degree of benefit.”
Dr. Kandzari reported financial relationships with Abbott Vascular, Ablative Solutions, Biotronik, Boston Scientific, CSI, Medtronic Cardiovascular, OrbusNeich, and Teleflex. Dr. Kirtane reported financial relationships with Abbott Vascular, Abiomed, Boston Scientific, Cardiovascular Systems, Cathworks, Chiesi, Medtronic, Opens, Philipps, Regeneron, ReCor Medical, Siemens, Spectranetics, and Zoll.
AT AHA 2022
Children and COVID: Weekly cases continue to hold fairly steady
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.
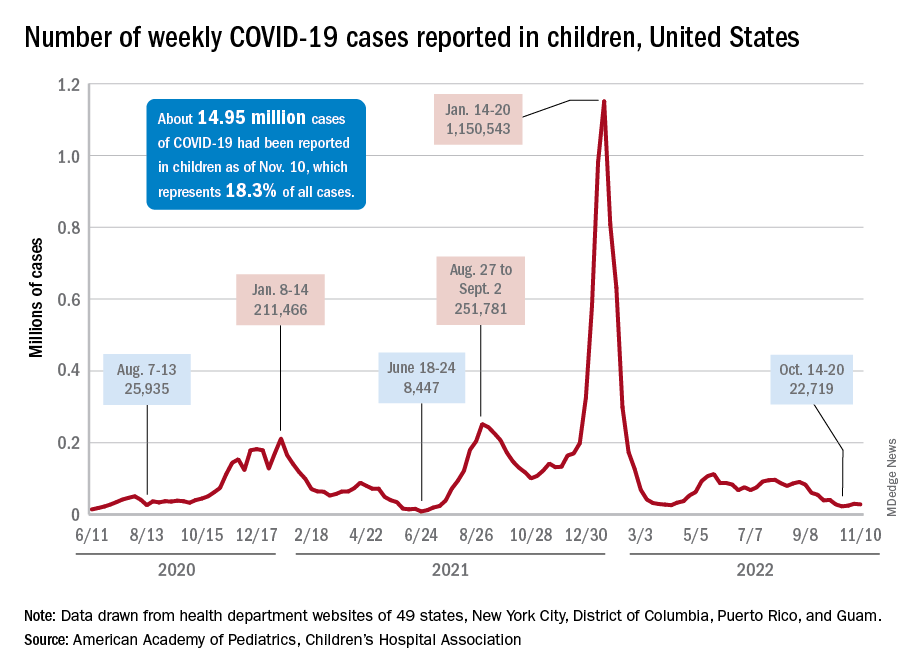
The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
The incidence of new COVID-19 cases in children seems to have stabilized as the national count remained under 30,000 for the fifth consecutive week, but hospitalization data may indicate some possible turbulence.
Just over 28,000 pediatric cases were reported during the week of Nov. 4-10, a drop of 5.4% from the previous week, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report involving data from state and territorial health departments, several of which are no longer updating their websites.

The stability in weekly cases, however, comes in contrast to a very recent and considerable increase in new hospital admissions of children aged 0-17 years with confirmed COVID-19. That rate, which was 0.18 hospitalizations per 100,000 population on Nov. 7 and 0.19 per 100,000 on Nov. 8 and 9, jumped all the way to 0.34 on Nov. 10 and 0.48 on Nov. 11, according to data from the Centers for Disease Control and Prevention. That is the highest rate since the closing days of the Omicron surge in February.
The rate for Nov. 12, the most recent one available, was down slightly to 0.47 admissions per 100,000. There doesn’t seem to be any evidence in the CDC’s data of a similar sudden increase in new hospitalizations among any other age group, and no age group, including children, shows any sign of a recent increase in emergency department visits with diagnosed COVID. (The CDC has not yet responded to our inquiry about this development.)
The two most recent 7-day averages for new admissions in children aged 0-17 show a small increase, but they cover the periods of Oct. 15 to Oct. 31, when there were 126 admissions per day, and Nov. 1 to Nov. 7, when the average went up to 133 per day, the CDC said on its COVID Data Tracker.
The CDC does not publish a weekly count of new COVID cases, but its latest data on the rate of incident cases seem to agree with the AAP/CHA figures: A gradual decline in all age groups, including children, since the beginning of September.
Vaccinations, on the other hand, bucked their recent trend and increased in the last week. About 43,000 children under age 5 years received their initial dose of COVID vaccine during Nov. 3-9, compared with 30,000 and 33,000 the 2 previous weeks, while 5- to 11-year-olds hit their highest weekly mark (31,000) since late August and 12- to 17-year-olds had their biggest week (27,000) since mid-August, the AAP reported based on CDC data.
Meditation for children
Meditation has become a popular practice in the United States over the last decade. It is not limited to adults, but can be learned and practiced by children and teenagers also. Variants are being used in many schools as parts of a social and emotional learning curriculum, and different kinds of mindfulness practices are common parts of psychological treatments. In this month’s column, we will review the evidence that supports the efficacy of a meditation practice to treat the mental health problems that are common in children and adolescents, and review how it might be a useful adjunct to the screening, education, and treatments that you offer your young patients.
There are many different types of meditation practices, but the unifying feature is known as mindfulness. Most broadly, mindfulness refers to a state of nonjudgmental awareness of one’s thoughts, feelings, or sensations. A mindfulness meditation practice involves physical stillness and focused attention, typically on the physical sensations of one’s breath. When thoughts, feelings, or physical sensations intrude on the stillness, one learns to cultivate a nonjudgmental awareness of those experiences without disrupting the state of quiet concentration. It could be said that meditation is easy to learn and difficult to master, and that is why it should be practiced regularly. Part of its growing popularity has undoubtedly been served by the ease with which people can access a variety of guided meditations (through apps, YouTube, and beyond) that make it relatively easy to access a variety of methods to learn how to practice mindfulness meditation.
The benefits of meditation in adults are well-established, including lower blood pressure, lower rates of heart disease, lower markers of inflammation, better sleep, and self-described levels of well-being. Meditation appears to be especially effective at mitigating the cardiovascular, metabolic, autoimmune, and inflammatory consequences of high-stress or unhealthy lifestyles in adults. Children and adolescents typically do not suffer from these diseases, but there is growing evidence that mindfulness practices can improve self-reported stress management skills, well-being, and sleep in young people; skills that can protect their physical and mental health. In addition, there is some evidence that mindfulness can be effective as a treatment for the common psychiatric illnesses of youth.
Anxiety
There is robust evidence for the efficacy of mindfulness-based interventions (including a regular mindfulness meditation practice) in the treatment of anxiety disorders in youth. Multiple studies and meta-analyses have demonstrated significant and sustained improvement in anxiety symptoms in these young patients. This makes sense when one considers that most psychotherapy treatments for anxiety include the cultivation of self-awareness and the ability to recognize the feelings of anxiety. This is critical as youth with anxiety disorders often mistake these feelings for facts. The treatment then shifts toward practice tolerating these feelings to help children develop an appreciation that they can face and manage anxiety and that it does not need to be avoided. Part of tolerating these feelings includes building skills to facilitate calm and physical relaxation in the face of these anxious feelings.
This is the core of exposure-based psychotherapies. Mindfulness practices echo the cultivation of self-awareness with focus and physical calm. Studies have shown that mindfulness-based interventions have significant and lasting effects on the symptoms of anxiety disorders in youth, including those youth with comorbid ADHD and learning disabilities. It is important to be aware that, for youth who have experienced trauma, mindfulness meditation can trigger a flood of re-experiencing phenomena, and it is important that those youth also are receiving treatment for PTSD.
Depression
There is evidence that some of the symptoms that occur as part of depression in adolescents improve with mindfulness-based interventions. In particular, symptoms of anger, irritability, disruptive behaviors, suicidality, and even impulsive self-injury improve with mindfulness-based interventions. Dialectical behavioral therapy (DBT) and acceptance and commitment therapy (ACT) have the nonjudgmental self-awareness of mindfulness built in as a component of the therapy. But mindfulness practices without explicit cognitive and behavioral components of psychotherapy for depression are not effective as stand-alone treatment of major depressive disorder in youth.
Multiple meta-analyses have demonstrated that stimulant treatment is more effective than behavioral or environmental interventions in the treatment of ADHD in children and adolescents, and combined treatments have not shown substantial additional improvement over medications alone in randomized controlled studies. But there is a lot of interest in finding effective treatments beyond medications that will help children with ADHD build important cognitive and behavioral skills that may lag developmentally.
Now there is an emerging body of evidence indicating that mindfulness skills in children with ADHD are quite effective for improving their sustained attention, social skills, behavioral control, and even hyperactivity. Additionally, methods to teach mindfulness skills to children who struggle with stillness and focused attention have been developed for these studies (“mindful martial arts”). Again, this intervention has not yet shown the same level of efficacy as medication treatments for ADHD symptoms, but it has demonstrated promise in early trials. Interestingly, it has also shown promise as a component of parenting interventions for youth with ADHD.
You do not need to wait for decisive evidence from randomized controlled trials to recommend mindfulness training for your patients with anxiety, ADHD, or even depression. Indeed, this practice alone may be adequate as a treatment for mild to moderate anxiety disorders. But you can also recommend it as an empowering and effective adjunctive treatment for almost every psychiatric illness and subclinical syndrome, and one that is affordable and easy for families to access. It would be valuable for you to recommend that your patients and their parents both try a mindfulness practice alongside your recommendations about healthy sleep, exercise, and nutrition. There are free apps such as Smiling Mind, Sound Mind, and Thrive Global that families can try together. Some children may need to move physically to be able to practice mindfulness, so yoga or walking meditations can be a better practice for them. When parents can try mindfulness practice alongside their children, it will facilitate their child’s efforts to develop these skills, and the improved sleep, focus, and stress management skills in parents can make a significant difference in the health and well-being of the whole family.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Meditation has become a popular practice in the United States over the last decade. It is not limited to adults, but can be learned and practiced by children and teenagers also. Variants are being used in many schools as parts of a social and emotional learning curriculum, and different kinds of mindfulness practices are common parts of psychological treatments. In this month’s column, we will review the evidence that supports the efficacy of a meditation practice to treat the mental health problems that are common in children and adolescents, and review how it might be a useful adjunct to the screening, education, and treatments that you offer your young patients.
There are many different types of meditation practices, but the unifying feature is known as mindfulness. Most broadly, mindfulness refers to a state of nonjudgmental awareness of one’s thoughts, feelings, or sensations. A mindfulness meditation practice involves physical stillness and focused attention, typically on the physical sensations of one’s breath. When thoughts, feelings, or physical sensations intrude on the stillness, one learns to cultivate a nonjudgmental awareness of those experiences without disrupting the state of quiet concentration. It could be said that meditation is easy to learn and difficult to master, and that is why it should be practiced regularly. Part of its growing popularity has undoubtedly been served by the ease with which people can access a variety of guided meditations (through apps, YouTube, and beyond) that make it relatively easy to access a variety of methods to learn how to practice mindfulness meditation.
The benefits of meditation in adults are well-established, including lower blood pressure, lower rates of heart disease, lower markers of inflammation, better sleep, and self-described levels of well-being. Meditation appears to be especially effective at mitigating the cardiovascular, metabolic, autoimmune, and inflammatory consequences of high-stress or unhealthy lifestyles in adults. Children and adolescents typically do not suffer from these diseases, but there is growing evidence that mindfulness practices can improve self-reported stress management skills, well-being, and sleep in young people; skills that can protect their physical and mental health. In addition, there is some evidence that mindfulness can be effective as a treatment for the common psychiatric illnesses of youth.
Anxiety
There is robust evidence for the efficacy of mindfulness-based interventions (including a regular mindfulness meditation practice) in the treatment of anxiety disorders in youth. Multiple studies and meta-analyses have demonstrated significant and sustained improvement in anxiety symptoms in these young patients. This makes sense when one considers that most psychotherapy treatments for anxiety include the cultivation of self-awareness and the ability to recognize the feelings of anxiety. This is critical as youth with anxiety disorders often mistake these feelings for facts. The treatment then shifts toward practice tolerating these feelings to help children develop an appreciation that they can face and manage anxiety and that it does not need to be avoided. Part of tolerating these feelings includes building skills to facilitate calm and physical relaxation in the face of these anxious feelings.
This is the core of exposure-based psychotherapies. Mindfulness practices echo the cultivation of self-awareness with focus and physical calm. Studies have shown that mindfulness-based interventions have significant and lasting effects on the symptoms of anxiety disorders in youth, including those youth with comorbid ADHD and learning disabilities. It is important to be aware that, for youth who have experienced trauma, mindfulness meditation can trigger a flood of re-experiencing phenomena, and it is important that those youth also are receiving treatment for PTSD.
Depression
There is evidence that some of the symptoms that occur as part of depression in adolescents improve with mindfulness-based interventions. In particular, symptoms of anger, irritability, disruptive behaviors, suicidality, and even impulsive self-injury improve with mindfulness-based interventions. Dialectical behavioral therapy (DBT) and acceptance and commitment therapy (ACT) have the nonjudgmental self-awareness of mindfulness built in as a component of the therapy. But mindfulness practices without explicit cognitive and behavioral components of psychotherapy for depression are not effective as stand-alone treatment of major depressive disorder in youth.
Multiple meta-analyses have demonstrated that stimulant treatment is more effective than behavioral or environmental interventions in the treatment of ADHD in children and adolescents, and combined treatments have not shown substantial additional improvement over medications alone in randomized controlled studies. But there is a lot of interest in finding effective treatments beyond medications that will help children with ADHD build important cognitive and behavioral skills that may lag developmentally.
Now there is an emerging body of evidence indicating that mindfulness skills in children with ADHD are quite effective for improving their sustained attention, social skills, behavioral control, and even hyperactivity. Additionally, methods to teach mindfulness skills to children who struggle with stillness and focused attention have been developed for these studies (“mindful martial arts”). Again, this intervention has not yet shown the same level of efficacy as medication treatments for ADHD symptoms, but it has demonstrated promise in early trials. Interestingly, it has also shown promise as a component of parenting interventions for youth with ADHD.
You do not need to wait for decisive evidence from randomized controlled trials to recommend mindfulness training for your patients with anxiety, ADHD, or even depression. Indeed, this practice alone may be adequate as a treatment for mild to moderate anxiety disorders. But you can also recommend it as an empowering and effective adjunctive treatment for almost every psychiatric illness and subclinical syndrome, and one that is affordable and easy for families to access. It would be valuable for you to recommend that your patients and their parents both try a mindfulness practice alongside your recommendations about healthy sleep, exercise, and nutrition. There are free apps such as Smiling Mind, Sound Mind, and Thrive Global that families can try together. Some children may need to move physically to be able to practice mindfulness, so yoga or walking meditations can be a better practice for them. When parents can try mindfulness practice alongside their children, it will facilitate their child’s efforts to develop these skills, and the improved sleep, focus, and stress management skills in parents can make a significant difference in the health and well-being of the whole family.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Meditation has become a popular practice in the United States over the last decade. It is not limited to adults, but can be learned and practiced by children and teenagers also. Variants are being used in many schools as parts of a social and emotional learning curriculum, and different kinds of mindfulness practices are common parts of psychological treatments. In this month’s column, we will review the evidence that supports the efficacy of a meditation practice to treat the mental health problems that are common in children and adolescents, and review how it might be a useful adjunct to the screening, education, and treatments that you offer your young patients.
There are many different types of meditation practices, but the unifying feature is known as mindfulness. Most broadly, mindfulness refers to a state of nonjudgmental awareness of one’s thoughts, feelings, or sensations. A mindfulness meditation practice involves physical stillness and focused attention, typically on the physical sensations of one’s breath. When thoughts, feelings, or physical sensations intrude on the stillness, one learns to cultivate a nonjudgmental awareness of those experiences without disrupting the state of quiet concentration. It could be said that meditation is easy to learn and difficult to master, and that is why it should be practiced regularly. Part of its growing popularity has undoubtedly been served by the ease with which people can access a variety of guided meditations (through apps, YouTube, and beyond) that make it relatively easy to access a variety of methods to learn how to practice mindfulness meditation.
The benefits of meditation in adults are well-established, including lower blood pressure, lower rates of heart disease, lower markers of inflammation, better sleep, and self-described levels of well-being. Meditation appears to be especially effective at mitigating the cardiovascular, metabolic, autoimmune, and inflammatory consequences of high-stress or unhealthy lifestyles in adults. Children and adolescents typically do not suffer from these diseases, but there is growing evidence that mindfulness practices can improve self-reported stress management skills, well-being, and sleep in young people; skills that can protect their physical and mental health. In addition, there is some evidence that mindfulness can be effective as a treatment for the common psychiatric illnesses of youth.
Anxiety
There is robust evidence for the efficacy of mindfulness-based interventions (including a regular mindfulness meditation practice) in the treatment of anxiety disorders in youth. Multiple studies and meta-analyses have demonstrated significant and sustained improvement in anxiety symptoms in these young patients. This makes sense when one considers that most psychotherapy treatments for anxiety include the cultivation of self-awareness and the ability to recognize the feelings of anxiety. This is critical as youth with anxiety disorders often mistake these feelings for facts. The treatment then shifts toward practice tolerating these feelings to help children develop an appreciation that they can face and manage anxiety and that it does not need to be avoided. Part of tolerating these feelings includes building skills to facilitate calm and physical relaxation in the face of these anxious feelings.
This is the core of exposure-based psychotherapies. Mindfulness practices echo the cultivation of self-awareness with focus and physical calm. Studies have shown that mindfulness-based interventions have significant and lasting effects on the symptoms of anxiety disorders in youth, including those youth with comorbid ADHD and learning disabilities. It is important to be aware that, for youth who have experienced trauma, mindfulness meditation can trigger a flood of re-experiencing phenomena, and it is important that those youth also are receiving treatment for PTSD.
Depression
There is evidence that some of the symptoms that occur as part of depression in adolescents improve with mindfulness-based interventions. In particular, symptoms of anger, irritability, disruptive behaviors, suicidality, and even impulsive self-injury improve with mindfulness-based interventions. Dialectical behavioral therapy (DBT) and acceptance and commitment therapy (ACT) have the nonjudgmental self-awareness of mindfulness built in as a component of the therapy. But mindfulness practices without explicit cognitive and behavioral components of psychotherapy for depression are not effective as stand-alone treatment of major depressive disorder in youth.
Multiple meta-analyses have demonstrated that stimulant treatment is more effective than behavioral or environmental interventions in the treatment of ADHD in children and adolescents, and combined treatments have not shown substantial additional improvement over medications alone in randomized controlled studies. But there is a lot of interest in finding effective treatments beyond medications that will help children with ADHD build important cognitive and behavioral skills that may lag developmentally.
Now there is an emerging body of evidence indicating that mindfulness skills in children with ADHD are quite effective for improving their sustained attention, social skills, behavioral control, and even hyperactivity. Additionally, methods to teach mindfulness skills to children who struggle with stillness and focused attention have been developed for these studies (“mindful martial arts”). Again, this intervention has not yet shown the same level of efficacy as medication treatments for ADHD symptoms, but it has demonstrated promise in early trials. Interestingly, it has also shown promise as a component of parenting interventions for youth with ADHD.
You do not need to wait for decisive evidence from randomized controlled trials to recommend mindfulness training for your patients with anxiety, ADHD, or even depression. Indeed, this practice alone may be adequate as a treatment for mild to moderate anxiety disorders. But you can also recommend it as an empowering and effective adjunctive treatment for almost every psychiatric illness and subclinical syndrome, and one that is affordable and easy for families to access. It would be valuable for you to recommend that your patients and their parents both try a mindfulness practice alongside your recommendations about healthy sleep, exercise, and nutrition. There are free apps such as Smiling Mind, Sound Mind, and Thrive Global that families can try together. Some children may need to move physically to be able to practice mindfulness, so yoga or walking meditations can be a better practice for them. When parents can try mindfulness practice alongside their children, it will facilitate their child’s efforts to develop these skills, and the improved sleep, focus, and stress management skills in parents can make a significant difference in the health and well-being of the whole family.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
How Low Is Too Low? A Retrospective Analysis of Very Low LDL-C Levels in Veterans
According to the Centers for Disease Control and Prevention (CDC), approximately 795,000 strokes occur in the United States yearly and are the fifth leading cause of death.1 The CDC also states that about 43 million Americans who could benefit from cholesterol medication are currently taking them.2 As of 2019, West Virginia, Ohio, and Kentucky are 3 states with the highest rates of heart disease mortality.3
Low-density lipoprotein cholesterol (LDL-C) accumulates on the walls of blood vessels, which can lead to coronary heart disease. However, some LDL-C is necessary to maintain proper brain function. Guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) recommend LDL-C goal levels < 70 mg/dL.4 Yet, there is no consensus on how low LDL-C levels should be. According to clinical practice guidelines for dyslipidemia, developed by the US Department of Veterans Affairs (VA) and US Department of Defense, statin medications are first-line agents for lowering LDL-C. The intensity of the statin medication is based on primary or secondary prevention, atherosclerotic cardiovascular disease (ASCVD) risk, and current LDL-C levels prior to treatment.5
Statin medications are used for primary and secondary prevention of ASCVD. In addition, statin medications decrease total cholesterol, LDL-C, and triglycerides while causing a mild increase in high-density lipoprotein cholesterol. Although statin medications are first-line therapy for LDL-C lowering, other medications can be used to assist in decreasing LDL-C. Ezetimibe, fenofibrates, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can also be used.5 Statin medications do pose a risk of severe adverse drug reactions (ADRs), such as rhabdomyolysis and myopathy.6
One prospective cohort study looked at 27,937 women and analyzed total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglycerides, and strokes. The study noted a mean 19.3-year follow-up and within that follow-up, 137 hemorrhagic strokes occurred. Based on the study’s results, LDL-C levels < 70 mg/dL had 2.17 times the risk of experiencing a hemorrhagic stroke.7 A meta-analysis of prospective studies analyzed 476,173 patients and 7487 hemorrhagic stroke cases. This review concluded that a 10 mg/dL increase in LDL-C was associated with a 3% lower risk of hemorrhagic stroke.8
An observational study conducted in Asia of Chinese adults found that 22% of all strokes were hemorrhagic. The incidence of the hemorrhagic strokes was higher for patients who had an LDL-C < 1.8 mmol/L than those who had an LDL-C between 1.8 and 2.6 mmol/L. This study also showed that if hypertension was inadequately treated, the risk of hemorrhagic stroke increased. This study concluded that the benefit of reducing ASCVD outweighs the small risk of hemorrhagic strokes.9
Another prospective cohort study included 96,043 stroke-free participants and analyzed LDL-C concentrations and incidence of intracranial hemorrhage. The average LDL-C concentrations were calculated from data collected in 4 separate reporting years, and incidence of intracranial hemorrhage was confirmed through review of medication records. Over a 9-year follow-up period, the study concluded that participants with an LDL-C level of < 70 mg/dL had a significantly higher risk of developing intracranial hemorrhage than participants with LDL-C levels 70 to 99 mg/dL.10
The safety and effects of prolonged very low LDL-C levels are currently unknown. The current study sought to gather information to determine the risks of very low LDL-C levels in a veteran population.
Methods
A retrospective chart review was conducted on patients aged 18 to 90 years receiving care at the Hershel “Woody” Williams Veterans Affairs Medical Center (HWW VAMC) in Huntington, West Virginia, between January 1, 2010, and September 1, 2020. Approval of the current study was obtained through the Marshall University Institutional Review Board, HWW VAMC Research and Development Committee, and Veterans Health Administration (VHA) DATA Access Request Tracker (DART)/VA Informatic and Computing Infrastructure (VINCI). Data were obtained via the VHA Corporate Data Warehouse (CDW) for the HWW VAMC using Microsoft Structured Query Language (SQL) server available in VINCI. Analysis of the data was conducted using STATA v. 15.
Patients were included if they had a diagnosis of hyperlipidemia/dyslipidemia, received treatment with HMG-CoA reductase inhibitors or PCSK9 medications, and had an LDL-C level ≤ 40 mg/dL. The primary outcome was the rate of intracranial hemorrhage that could be caused by very low LDL-C levels. The secondary outcomes included actions taken by clinicians to address LDL-C level < 40 mg/dL, ADRs, duration of therapy, and medication adherence. Patients were excluded if they were aged < 18 or > 90 years, were pregnant during the study period, had hypothyroidism, received chronic anticoagulation medications, or had a triglyceride level > 300 mg/dL.
Results
The study included 3027 patients. Of those patients, 78 patients were female while 2949 were male, and the mean (SD) age was 68.3 (9.4) years. A subsample of 32 patients was analyzed to determine whether an ADR was noted or low LDL-C level was addressed in the chart. The subsample size was determined through chart review and included patients who had a documented intracranial hemorrhage. None of the 32 patients had an ADR documented, and 6 (19%) had the low LDL-C level addressed in the chart by monitoring levels, reducing statin doses, or discontinuing the medication. Of the total population analyzed, 8 patients (0.3%) had a documented intracranial hemorrhage within 1 year following the low LDL-C level.
We also analyzed the intensity of statin related to the low LDL-C level (Table 1).
The most common ADRs were muscle, joint, and leg pain, rash, and cramps (Table 2).
Adherence to the medications and duration of therapy was also analyzed and was found to be similar among the various medications. Lovastatin had the highest percent adherence with 91.2% while atorvastatin had the lowest with 85.5%. It can be noted that lovastatin had a lower documented percentage of ADRs while atorvastatin had a higher documented percentage of ADRs, which can be clinically meaningful when prescribing these medications; however, these similar adherence rates are not influencing the primary outcome of the rate of intracranial hemorrhage due to LDL-C level < 40 mg/dL. Mean duration of therapy lasted between 1 year and > 4 years with 1.1 years for alirocumab and 4.2 for simvastatin. The duration of therapy could be influenced by formulary restrictions during the study time. Nonetheless, patients, regardless of formulary restrictions, have taken these medications for a duration long enough to affect LDL-C levels.
Eight patients of the total sample analyzed had an intracranial hemorrhage within 1 year of having a recorded LDL-C level < 40 mg/dL. Secondarily, 32 patients had clinicians address an LDL-C level < 40 mg/dL through documentation or modifying the medication therapy. The most common ADRs among all medications analyzed were leg and joint pain, rash, and cramps. Of all medications included in this study, the mean duration of therapy was > 1 year, which would allow them to affect LDL-C levels and have those levels monitored and recorded in patients’ charts.
Discussion
When comparing our primary outcome of risk of intracranial hemorrhage with previous literature, the results are consistent with previous outcomes. Previous literature had a smaller sample size but analyzed LDL-C levels < 50 mg/dL and had an outcome of 48 patients experiencing an intracranial hemorrhage within 1 year of an LDL-C level < 50 mg/dL. Due to this study having stricter parameters of LDL-C levels < 40 mg/dL, there were fewer patients with documented intracranial hemorrhages. With there being a risk of intracranial hemorrhage with low LDL-C levels, the results demonstrate the need to monitor and address LDL-C levels.
Limitations
There were several notable limitations to this study. The retrospective, single-center nature coupled with the predominately male study population may affect the generalizability of the study results to patients outside of the facility in which the study was performed. Additionally, the study only included statin medications and PCSK9 inhibitors. With future studies, all lipid-lowering medications could be analyzed. The study was largely reliant on the proper documentation of International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes exclusive to the HWW VAMC, which may exclude patients who first present to outside facilities. Due to time restraints, the incidence of hemorrhage was only analyzed 1 year following an LDL-C level < 40 mg/dL. For considerations for future investigation, the length of time to analyze incidence of hemorrhage could be expanded to be similar to previous studies, and the study could be expanded across the local Veterans Integrated Service Network or VA system. Additionally, the study could have analyzed the percentage of time a patient had an LDL-C level < 40 mg/dL in their lifetime.
Conclusions
These results show there is a risk that patients with an LDL-C level < 40 mg/dL may experience an intracranial hemorrhage. As seen by the results, there is a clinical need for practitioners to routinely monitor and address LDL-C levels. With various guidelines that recommend starting statin medication to reduce risk of ASCVD, it is necessary that practitioners routinely monitor cholesterol levels and adjust the medications according to laboratory results.11
Within 1 year of an LDL-C level < 40 mg/dL, 0.3% of patients had an intracranial hemorrhage. There was no statistical significance between the rate of ADRs among the medications analyzed. High-intensity statin medications were statistically significant in resulting in an LDL-C level < 40 mg/dL compared with moderate- and low-intensity statin medications. Of the 32 subsample of patients, LDL-C levels < 40 mg/mL are not routinely being addressed in the chart by the clinician.
1. Centers for Disease Control and Prevention. Stroke facts. Updated April 5, 2022. Accessed September 21, 2022. https://www.cdc.gov/stroke/facts.htm
2. Centers for Disease Control and Prevention. High cholesterol facts. Updated July 12, 2022. Accessed September 21, 2022. https://www.cdc.gov/cholesterol/facts.htm
3. Centers for Disease Control and Prevention. Heart disease mortality by state. Updated February 25, 2022. Accessed September 21, 2022. https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. US Department of Veterans Affairs. June 2020. Accessed September 21, 2022. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
6. Tomaszewski M, Ste¸pien´ KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63(4):859-66. doi:10.1016/s1734-1140(11)70601-6
7. Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92(19):e2286-e2294. doi:10.1212/WNL.0000000000007454
8. Ma C, Na M, Neumann S, Gao X. Low-density lipoprotein cholesterol and risk of hemorrhagic stroke: a systematic review and dose-response meta-analysis of prospective studies. Curr Atheroscler Rep. 2019;21(12):52. Published 2019 Nov 20. doi:10.1007/s11883-019-0815-5
9. Lui DT, Tan KC. Low-density lipoprotein cholesterol and stroke: How low should we go? J Diabetes Investig. 2020;11(6):1379-1381. doi:10.1111/jdi.13310
10. Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93(5):e445-e457. doi:10.1212/WNL.0000000000007853
11. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S144–S174. doi:10.2337/dc22-S010
According to the Centers for Disease Control and Prevention (CDC), approximately 795,000 strokes occur in the United States yearly and are the fifth leading cause of death.1 The CDC also states that about 43 million Americans who could benefit from cholesterol medication are currently taking them.2 As of 2019, West Virginia, Ohio, and Kentucky are 3 states with the highest rates of heart disease mortality.3
Low-density lipoprotein cholesterol (LDL-C) accumulates on the walls of blood vessels, which can lead to coronary heart disease. However, some LDL-C is necessary to maintain proper brain function. Guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) recommend LDL-C goal levels < 70 mg/dL.4 Yet, there is no consensus on how low LDL-C levels should be. According to clinical practice guidelines for dyslipidemia, developed by the US Department of Veterans Affairs (VA) and US Department of Defense, statin medications are first-line agents for lowering LDL-C. The intensity of the statin medication is based on primary or secondary prevention, atherosclerotic cardiovascular disease (ASCVD) risk, and current LDL-C levels prior to treatment.5
Statin medications are used for primary and secondary prevention of ASCVD. In addition, statin medications decrease total cholesterol, LDL-C, and triglycerides while causing a mild increase in high-density lipoprotein cholesterol. Although statin medications are first-line therapy for LDL-C lowering, other medications can be used to assist in decreasing LDL-C. Ezetimibe, fenofibrates, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can also be used.5 Statin medications do pose a risk of severe adverse drug reactions (ADRs), such as rhabdomyolysis and myopathy.6
One prospective cohort study looked at 27,937 women and analyzed total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglycerides, and strokes. The study noted a mean 19.3-year follow-up and within that follow-up, 137 hemorrhagic strokes occurred. Based on the study’s results, LDL-C levels < 70 mg/dL had 2.17 times the risk of experiencing a hemorrhagic stroke.7 A meta-analysis of prospective studies analyzed 476,173 patients and 7487 hemorrhagic stroke cases. This review concluded that a 10 mg/dL increase in LDL-C was associated with a 3% lower risk of hemorrhagic stroke.8
An observational study conducted in Asia of Chinese adults found that 22% of all strokes were hemorrhagic. The incidence of the hemorrhagic strokes was higher for patients who had an LDL-C < 1.8 mmol/L than those who had an LDL-C between 1.8 and 2.6 mmol/L. This study also showed that if hypertension was inadequately treated, the risk of hemorrhagic stroke increased. This study concluded that the benefit of reducing ASCVD outweighs the small risk of hemorrhagic strokes.9
Another prospective cohort study included 96,043 stroke-free participants and analyzed LDL-C concentrations and incidence of intracranial hemorrhage. The average LDL-C concentrations were calculated from data collected in 4 separate reporting years, and incidence of intracranial hemorrhage was confirmed through review of medication records. Over a 9-year follow-up period, the study concluded that participants with an LDL-C level of < 70 mg/dL had a significantly higher risk of developing intracranial hemorrhage than participants with LDL-C levels 70 to 99 mg/dL.10
The safety and effects of prolonged very low LDL-C levels are currently unknown. The current study sought to gather information to determine the risks of very low LDL-C levels in a veteran population.
Methods
A retrospective chart review was conducted on patients aged 18 to 90 years receiving care at the Hershel “Woody” Williams Veterans Affairs Medical Center (HWW VAMC) in Huntington, West Virginia, between January 1, 2010, and September 1, 2020. Approval of the current study was obtained through the Marshall University Institutional Review Board, HWW VAMC Research and Development Committee, and Veterans Health Administration (VHA) DATA Access Request Tracker (DART)/VA Informatic and Computing Infrastructure (VINCI). Data were obtained via the VHA Corporate Data Warehouse (CDW) for the HWW VAMC using Microsoft Structured Query Language (SQL) server available in VINCI. Analysis of the data was conducted using STATA v. 15.
Patients were included if they had a diagnosis of hyperlipidemia/dyslipidemia, received treatment with HMG-CoA reductase inhibitors or PCSK9 medications, and had an LDL-C level ≤ 40 mg/dL. The primary outcome was the rate of intracranial hemorrhage that could be caused by very low LDL-C levels. The secondary outcomes included actions taken by clinicians to address LDL-C level < 40 mg/dL, ADRs, duration of therapy, and medication adherence. Patients were excluded if they were aged < 18 or > 90 years, were pregnant during the study period, had hypothyroidism, received chronic anticoagulation medications, or had a triglyceride level > 300 mg/dL.
Results
The study included 3027 patients. Of those patients, 78 patients were female while 2949 were male, and the mean (SD) age was 68.3 (9.4) years. A subsample of 32 patients was analyzed to determine whether an ADR was noted or low LDL-C level was addressed in the chart. The subsample size was determined through chart review and included patients who had a documented intracranial hemorrhage. None of the 32 patients had an ADR documented, and 6 (19%) had the low LDL-C level addressed in the chart by monitoring levels, reducing statin doses, or discontinuing the medication. Of the total population analyzed, 8 patients (0.3%) had a documented intracranial hemorrhage within 1 year following the low LDL-C level.
We also analyzed the intensity of statin related to the low LDL-C level (Table 1).
The most common ADRs were muscle, joint, and leg pain, rash, and cramps (Table 2).
Adherence to the medications and duration of therapy was also analyzed and was found to be similar among the various medications. Lovastatin had the highest percent adherence with 91.2% while atorvastatin had the lowest with 85.5%. It can be noted that lovastatin had a lower documented percentage of ADRs while atorvastatin had a higher documented percentage of ADRs, which can be clinically meaningful when prescribing these medications; however, these similar adherence rates are not influencing the primary outcome of the rate of intracranial hemorrhage due to LDL-C level < 40 mg/dL. Mean duration of therapy lasted between 1 year and > 4 years with 1.1 years for alirocumab and 4.2 for simvastatin. The duration of therapy could be influenced by formulary restrictions during the study time. Nonetheless, patients, regardless of formulary restrictions, have taken these medications for a duration long enough to affect LDL-C levels.
Eight patients of the total sample analyzed had an intracranial hemorrhage within 1 year of having a recorded LDL-C level < 40 mg/dL. Secondarily, 32 patients had clinicians address an LDL-C level < 40 mg/dL through documentation or modifying the medication therapy. The most common ADRs among all medications analyzed were leg and joint pain, rash, and cramps. Of all medications included in this study, the mean duration of therapy was > 1 year, which would allow them to affect LDL-C levels and have those levels monitored and recorded in patients’ charts.
Discussion
When comparing our primary outcome of risk of intracranial hemorrhage with previous literature, the results are consistent with previous outcomes. Previous literature had a smaller sample size but analyzed LDL-C levels < 50 mg/dL and had an outcome of 48 patients experiencing an intracranial hemorrhage within 1 year of an LDL-C level < 50 mg/dL. Due to this study having stricter parameters of LDL-C levels < 40 mg/dL, there were fewer patients with documented intracranial hemorrhages. With there being a risk of intracranial hemorrhage with low LDL-C levels, the results demonstrate the need to monitor and address LDL-C levels.
Limitations
There were several notable limitations to this study. The retrospective, single-center nature coupled with the predominately male study population may affect the generalizability of the study results to patients outside of the facility in which the study was performed. Additionally, the study only included statin medications and PCSK9 inhibitors. With future studies, all lipid-lowering medications could be analyzed. The study was largely reliant on the proper documentation of International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes exclusive to the HWW VAMC, which may exclude patients who first present to outside facilities. Due to time restraints, the incidence of hemorrhage was only analyzed 1 year following an LDL-C level < 40 mg/dL. For considerations for future investigation, the length of time to analyze incidence of hemorrhage could be expanded to be similar to previous studies, and the study could be expanded across the local Veterans Integrated Service Network or VA system. Additionally, the study could have analyzed the percentage of time a patient had an LDL-C level < 40 mg/dL in their lifetime.
Conclusions
These results show there is a risk that patients with an LDL-C level < 40 mg/dL may experience an intracranial hemorrhage. As seen by the results, there is a clinical need for practitioners to routinely monitor and address LDL-C levels. With various guidelines that recommend starting statin medication to reduce risk of ASCVD, it is necessary that practitioners routinely monitor cholesterol levels and adjust the medications according to laboratory results.11
Within 1 year of an LDL-C level < 40 mg/dL, 0.3% of patients had an intracranial hemorrhage. There was no statistical significance between the rate of ADRs among the medications analyzed. High-intensity statin medications were statistically significant in resulting in an LDL-C level < 40 mg/dL compared with moderate- and low-intensity statin medications. Of the 32 subsample of patients, LDL-C levels < 40 mg/mL are not routinely being addressed in the chart by the clinician.
According to the Centers for Disease Control and Prevention (CDC), approximately 795,000 strokes occur in the United States yearly and are the fifth leading cause of death.1 The CDC also states that about 43 million Americans who could benefit from cholesterol medication are currently taking them.2 As of 2019, West Virginia, Ohio, and Kentucky are 3 states with the highest rates of heart disease mortality.3
Low-density lipoprotein cholesterol (LDL-C) accumulates on the walls of blood vessels, which can lead to coronary heart disease. However, some LDL-C is necessary to maintain proper brain function. Guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA) recommend LDL-C goal levels < 70 mg/dL.4 Yet, there is no consensus on how low LDL-C levels should be. According to clinical practice guidelines for dyslipidemia, developed by the US Department of Veterans Affairs (VA) and US Department of Defense, statin medications are first-line agents for lowering LDL-C. The intensity of the statin medication is based on primary or secondary prevention, atherosclerotic cardiovascular disease (ASCVD) risk, and current LDL-C levels prior to treatment.5
Statin medications are used for primary and secondary prevention of ASCVD. In addition, statin medications decrease total cholesterol, LDL-C, and triglycerides while causing a mild increase in high-density lipoprotein cholesterol. Although statin medications are first-line therapy for LDL-C lowering, other medications can be used to assist in decreasing LDL-C. Ezetimibe, fenofibrates, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can also be used.5 Statin medications do pose a risk of severe adverse drug reactions (ADRs), such as rhabdomyolysis and myopathy.6
One prospective cohort study looked at 27,937 women and analyzed total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglycerides, and strokes. The study noted a mean 19.3-year follow-up and within that follow-up, 137 hemorrhagic strokes occurred. Based on the study’s results, LDL-C levels < 70 mg/dL had 2.17 times the risk of experiencing a hemorrhagic stroke.7 A meta-analysis of prospective studies analyzed 476,173 patients and 7487 hemorrhagic stroke cases. This review concluded that a 10 mg/dL increase in LDL-C was associated with a 3% lower risk of hemorrhagic stroke.8
An observational study conducted in Asia of Chinese adults found that 22% of all strokes were hemorrhagic. The incidence of the hemorrhagic strokes was higher for patients who had an LDL-C < 1.8 mmol/L than those who had an LDL-C between 1.8 and 2.6 mmol/L. This study also showed that if hypertension was inadequately treated, the risk of hemorrhagic stroke increased. This study concluded that the benefit of reducing ASCVD outweighs the small risk of hemorrhagic strokes.9
Another prospective cohort study included 96,043 stroke-free participants and analyzed LDL-C concentrations and incidence of intracranial hemorrhage. The average LDL-C concentrations were calculated from data collected in 4 separate reporting years, and incidence of intracranial hemorrhage was confirmed through review of medication records. Over a 9-year follow-up period, the study concluded that participants with an LDL-C level of < 70 mg/dL had a significantly higher risk of developing intracranial hemorrhage than participants with LDL-C levels 70 to 99 mg/dL.10
The safety and effects of prolonged very low LDL-C levels are currently unknown. The current study sought to gather information to determine the risks of very low LDL-C levels in a veteran population.
Methods
A retrospective chart review was conducted on patients aged 18 to 90 years receiving care at the Hershel “Woody” Williams Veterans Affairs Medical Center (HWW VAMC) in Huntington, West Virginia, between January 1, 2010, and September 1, 2020. Approval of the current study was obtained through the Marshall University Institutional Review Board, HWW VAMC Research and Development Committee, and Veterans Health Administration (VHA) DATA Access Request Tracker (DART)/VA Informatic and Computing Infrastructure (VINCI). Data were obtained via the VHA Corporate Data Warehouse (CDW) for the HWW VAMC using Microsoft Structured Query Language (SQL) server available in VINCI. Analysis of the data was conducted using STATA v. 15.
Patients were included if they had a diagnosis of hyperlipidemia/dyslipidemia, received treatment with HMG-CoA reductase inhibitors or PCSK9 medications, and had an LDL-C level ≤ 40 mg/dL. The primary outcome was the rate of intracranial hemorrhage that could be caused by very low LDL-C levels. The secondary outcomes included actions taken by clinicians to address LDL-C level < 40 mg/dL, ADRs, duration of therapy, and medication adherence. Patients were excluded if they were aged < 18 or > 90 years, were pregnant during the study period, had hypothyroidism, received chronic anticoagulation medications, or had a triglyceride level > 300 mg/dL.
Results
The study included 3027 patients. Of those patients, 78 patients were female while 2949 were male, and the mean (SD) age was 68.3 (9.4) years. A subsample of 32 patients was analyzed to determine whether an ADR was noted or low LDL-C level was addressed in the chart. The subsample size was determined through chart review and included patients who had a documented intracranial hemorrhage. None of the 32 patients had an ADR documented, and 6 (19%) had the low LDL-C level addressed in the chart by monitoring levels, reducing statin doses, or discontinuing the medication. Of the total population analyzed, 8 patients (0.3%) had a documented intracranial hemorrhage within 1 year following the low LDL-C level.
We also analyzed the intensity of statin related to the low LDL-C level (Table 1).
The most common ADRs were muscle, joint, and leg pain, rash, and cramps (Table 2).
Adherence to the medications and duration of therapy was also analyzed and was found to be similar among the various medications. Lovastatin had the highest percent adherence with 91.2% while atorvastatin had the lowest with 85.5%. It can be noted that lovastatin had a lower documented percentage of ADRs while atorvastatin had a higher documented percentage of ADRs, which can be clinically meaningful when prescribing these medications; however, these similar adherence rates are not influencing the primary outcome of the rate of intracranial hemorrhage due to LDL-C level < 40 mg/dL. Mean duration of therapy lasted between 1 year and > 4 years with 1.1 years for alirocumab and 4.2 for simvastatin. The duration of therapy could be influenced by formulary restrictions during the study time. Nonetheless, patients, regardless of formulary restrictions, have taken these medications for a duration long enough to affect LDL-C levels.
Eight patients of the total sample analyzed had an intracranial hemorrhage within 1 year of having a recorded LDL-C level < 40 mg/dL. Secondarily, 32 patients had clinicians address an LDL-C level < 40 mg/dL through documentation or modifying the medication therapy. The most common ADRs among all medications analyzed were leg and joint pain, rash, and cramps. Of all medications included in this study, the mean duration of therapy was > 1 year, which would allow them to affect LDL-C levels and have those levels monitored and recorded in patients’ charts.
Discussion
When comparing our primary outcome of risk of intracranial hemorrhage with previous literature, the results are consistent with previous outcomes. Previous literature had a smaller sample size but analyzed LDL-C levels < 50 mg/dL and had an outcome of 48 patients experiencing an intracranial hemorrhage within 1 year of an LDL-C level < 50 mg/dL. Due to this study having stricter parameters of LDL-C levels < 40 mg/dL, there were fewer patients with documented intracranial hemorrhages. With there being a risk of intracranial hemorrhage with low LDL-C levels, the results demonstrate the need to monitor and address LDL-C levels.
Limitations
There were several notable limitations to this study. The retrospective, single-center nature coupled with the predominately male study population may affect the generalizability of the study results to patients outside of the facility in which the study was performed. Additionally, the study only included statin medications and PCSK9 inhibitors. With future studies, all lipid-lowering medications could be analyzed. The study was largely reliant on the proper documentation of International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes exclusive to the HWW VAMC, which may exclude patients who first present to outside facilities. Due to time restraints, the incidence of hemorrhage was only analyzed 1 year following an LDL-C level < 40 mg/dL. For considerations for future investigation, the length of time to analyze incidence of hemorrhage could be expanded to be similar to previous studies, and the study could be expanded across the local Veterans Integrated Service Network or VA system. Additionally, the study could have analyzed the percentage of time a patient had an LDL-C level < 40 mg/dL in their lifetime.
Conclusions
These results show there is a risk that patients with an LDL-C level < 40 mg/dL may experience an intracranial hemorrhage. As seen by the results, there is a clinical need for practitioners to routinely monitor and address LDL-C levels. With various guidelines that recommend starting statin medication to reduce risk of ASCVD, it is necessary that practitioners routinely monitor cholesterol levels and adjust the medications according to laboratory results.11
Within 1 year of an LDL-C level < 40 mg/dL, 0.3% of patients had an intracranial hemorrhage. There was no statistical significance between the rate of ADRs among the medications analyzed. High-intensity statin medications were statistically significant in resulting in an LDL-C level < 40 mg/dL compared with moderate- and low-intensity statin medications. Of the 32 subsample of patients, LDL-C levels < 40 mg/mL are not routinely being addressed in the chart by the clinician.
1. Centers for Disease Control and Prevention. Stroke facts. Updated April 5, 2022. Accessed September 21, 2022. https://www.cdc.gov/stroke/facts.htm
2. Centers for Disease Control and Prevention. High cholesterol facts. Updated July 12, 2022. Accessed September 21, 2022. https://www.cdc.gov/cholesterol/facts.htm
3. Centers for Disease Control and Prevention. Heart disease mortality by state. Updated February 25, 2022. Accessed September 21, 2022. https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. US Department of Veterans Affairs. June 2020. Accessed September 21, 2022. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
6. Tomaszewski M, Ste¸pien´ KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63(4):859-66. doi:10.1016/s1734-1140(11)70601-6
7. Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92(19):e2286-e2294. doi:10.1212/WNL.0000000000007454
8. Ma C, Na M, Neumann S, Gao X. Low-density lipoprotein cholesterol and risk of hemorrhagic stroke: a systematic review and dose-response meta-analysis of prospective studies. Curr Atheroscler Rep. 2019;21(12):52. Published 2019 Nov 20. doi:10.1007/s11883-019-0815-5
9. Lui DT, Tan KC. Low-density lipoprotein cholesterol and stroke: How low should we go? J Diabetes Investig. 2020;11(6):1379-1381. doi:10.1111/jdi.13310
10. Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93(5):e445-e457. doi:10.1212/WNL.0000000000007853
11. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S144–S174. doi:10.2337/dc22-S010
1. Centers for Disease Control and Prevention. Stroke facts. Updated April 5, 2022. Accessed September 21, 2022. https://www.cdc.gov/stroke/facts.htm
2. Centers for Disease Control and Prevention. High cholesterol facts. Updated July 12, 2022. Accessed September 21, 2022. https://www.cdc.gov/cholesterol/facts.htm
3. Centers for Disease Control and Prevention. Heart disease mortality by state. Updated February 25, 2022. Accessed September 21, 2022. https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm
4. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi:10.1161/CIR.0000000000000625
5. US Department of Veterans Affairs, US Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction. Version 4.0. US Department of Veterans Affairs. June 2020. Accessed September 21, 2022. https://www.healthquality.va.gov/guidelines/CD/lipids/VADoDDyslipidemiaCPG5087212020.pdf
6. Tomaszewski M, Ste¸pien´ KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63(4):859-66. doi:10.1016/s1734-1140(11)70601-6
7. Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92(19):e2286-e2294. doi:10.1212/WNL.0000000000007454
8. Ma C, Na M, Neumann S, Gao X. Low-density lipoprotein cholesterol and risk of hemorrhagic stroke: a systematic review and dose-response meta-analysis of prospective studies. Curr Atheroscler Rep. 2019;21(12):52. Published 2019 Nov 20. doi:10.1007/s11883-019-0815-5
9. Lui DT, Tan KC. Low-density lipoprotein cholesterol and stroke: How low should we go? J Diabetes Investig. 2020;11(6):1379-1381. doi:10.1111/jdi.13310
10. Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93(5):e445-e457. doi:10.1212/WNL.0000000000007853
11. American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S144–S174. doi:10.2337/dc22-S010
Evaluation of a Pharmacist-Driven Ambulatory Aspirin Deprescribing Protocol
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
Assessment of Glucagon-like Peptide-1 Receptor Agonists in Veterans Taking Basal/Bolus Insulin Regimens
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
In 2019, diabetes mellitus (DM) was the seventh leading cause of death in the United States, and currently, about 11% of the American population has a DM diagnosis.1 Most have a diagnosis of type 2 diabetes (T2DM), which has a strong genetic predisposition, and the risk of developing T2DM increases with age, obesity, and lack of physical activity.1,2 Nearly one-quarter of veterans have a diagnosis of DM, and DM is the leading cause of comorbidities, such as blindness, end-stage renal disease, and amputation for patients receiving care from the Veterans Health Administration (VHA).2 The elevated incidence of DM in the veteran population is attributed to a variety of factors, including exposure to herbicides, such as Agent Orange, advanced age, increased risk of obesity, and limited access to high-quality food.3
After diagnosis, both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) emphasize the appropriate use of lifestyle management and pharmacologic therapy for DM care. The use of pharmacologic agents (oral medications, insulin, or noninsulin injectables) is often determined by efficacy, cost, potential adverse effects (AEs), and patient factors and comorbidities.4,5
The initial recommendation for pharmacologic treatment for T2DM differs slightly between expert guidelines. The ADA and AACE/ACE recommend any of the following as initial monotherapy, listed in order to represent a hierarchy of usage: metformin, glucagon-like peptide-1 receptor agonists (GLP-1 RAs), sodium-glucose cotransporter 2 (SGLT-2) inhibitors, or dipeptidyl peptidase-4 (DPP-4) inhibitors, with the first 3 agents carrying the strongest recommendations.4,5 For patients with established atherosclerotic cardiovascular disease (CVD), chronic kidney disease, or heart failure, it is recommended to start a long-acting GLP-1 RA or SGLT-2 inhibitor. For patients with T2DM and hemoglobin A1c (HbA1c) between 7.5% and 9.0% at diagnosis, the AACE/ACE recommend initiation of dual therapy using metformin alongside another first-line agent and recommend the addition of another antidiabetic agent if glycemic goals are not met after regular follow-up. AACE/ACE recommend the consideration of insulin therapy in symptomatic patients with HbA1c > 9.0%.5 In contrast, the ADA recommends metformin as first-line therapy for all patients with T2DM and recommends dual therapy using metformin and another preferred agent (selection based on comorbidities) when HbA1c is 1.5% to 2% above target. The ADA recommends the consideration of insulin with HbA1c > 10% or with evidence of ongoing catabolism or symptoms of hyperglycemia.4 There are several reasons why insulin may be initiated prior to GLP-1 RAs, including profound hyperglycemia at time of diagnosis or implementation of insulin agents prior to commercial availability of GLP-1 RA.
GLP-1 RAs are analogs of the hormone incretin, which increases glucose-dependent insulin secretion, decreases postprandial glucagon secretion, increases satiety, and slows gastric emptying.6,7 When used in combination with noninsulin agents, GLP-1 RAs have demonstrated HbA1c reductions of 0.5% to 1.5%.8 The use of GLP-1 RAs with basal insulin also has been studied extensively.6,8-10 When the combination of GLP-1 RAs and basal insulin was compared with basal/bolus insulin regimens, the use of the GLP-1 RAs resulted in lower HbA1c levels and lower incidence of hypoglycemia.6,9 Data have demonstrated the complementary mechanisms of using basal insulin and GLP 1 RAs in decreasing HbA1c levels, insulin requirements, and weight compared with using basal insulin monotherapy and basal/bolus combinations.6,9-13 Moreover, 3 GLP-1 RA medications currently on the market (liraglutide, dulaglutide, and semaglutide) have displayed cardiovascular and renal benefits, further supporting the use of these medications.2,5
Despite these benefits, GLP-1 RAs may have bothersome AEs and are associated with a high cost.6 In addition, some studies have found that as the length of therapy increases, the positive effects of these agents may diminish.9,11 In one study, which looked at the impact of the addition of exenatide to patients taking basal or basal/bolus insulin regimens, mean changes in weight were −2.4 kg at 0 to 6 months, −4.3 kg at 6 to 12 months, −6.2 kg at 12 to 18 months, and −5.5 kg at 18 to 27 months. After 18 months, an increase in weight was observed, but the increase remained lower than baseline.11 Another study, conducted over 12 months, found no significant decrease in weight or total daily dose (TDD) of insulin when exenatide or liraglutide were added to various insulin regimens (basal or basal/bolus).13 To date, minimal published data exist regarding the addition of newer GLP-1 RAs and the long-term use of these agents beyond 12 months in patients taking basal/bolus insulin regimens. The primary goal of this study was to evaluate the effect of adding GLP-1 RAs to basal/bolus insulin regimens over a 24-month period.
Methods
This study was a retrospective, electronic health record review of all patients on basal and bolus insulin regimens who received additional therapy with a GLP-1 RA at Veteran Health Indiana in Indianapolis from September 1, 2015, to June 30, 2019. Patients meeting inclusion criteria served as their own control. The primary outcome was change in HbA1c at 3, 6, 12, 18, and 24 months after initiation of the GLP-1 RA. Secondary outcomes included change in weight and TDD of insulin at 3, 6, 12, 18, and 24 months after the initiation of the GLP-1 RAs and incidence of patient-reported or laboratory-confirmed hypoglycemia and other AEs.
Patients were included if they were aged ≥ 18 years with a diagnosis of T2DM, had concomitant prescriptions for both a basal insulin (glargine, detemir, or NPH) and a bolus insulin (aspart, lispro, or regular) before receiving add-on therapy with a GLP-1 RA (exenatide, liraglutide, albiglutide, lixisenatide, dulaglutide, or semaglutide) from September 1, 2015, to June 30, 2019, and had baseline and subsequent HbA1c measurements available in the electronic health record. Patients were excluded if they had a diagnosis of type 1 DM (T1DM), were followed by an outside clinician for DM care, or if the GLP-1 RA was discontinued before subsequent HbA1c measurement. The study protocol was approved by the Research and Development Office of Veteran Health Indiana, and the project was deemed exempt from review by the Indiana University Institutional Review Board due to the retrospective nature of the study.
Data analysis was performed using Excel. Change from baseline for each interval was computed, and 1 sample t tests (2-tailed) compared change from baseline to no change. Due to the disparity in the number of patients with data available at each of the time intervals, a mean plot was presented for each group of patients within each interval, allowing mean changes in individual groups to be observed over time.
Results
One hundred twenty-three subjects met inclusion criteria; 16 patients were excluded due to GLP-1 RA discontinuation before follow-up measurement of HbA1c; 14 were excluded due to patients being managed by a clinician outside of the facility; 1 patient was excluded for lack of documentation regarding baseline and subsequent insulin doses. Ninety-two patient charts were reviewed. Participants had a mean age of 64 years, 95% were male, and 89% were White. Mean baseline HbA1c was 9.2%, mean body mass index was 38.9, and the mean TDD of insulin was 184 units.
Since some patients switched between GLP-1 RAs throughout the study and there was variation in timing of laboratory and clinic follow-up,
Discussion
Adding a GLP-1 RA to basal/bolus insulin regimens was associated with a statistically significant decrease in HbA1c at each time point through 18 months. The greatest improvement in glycemic control from baseline was seen at 3 months, with improvements in HbA1c diminishing at each subsequent period. The study also demonstrated a significant decrease in weight at each time point through 18 months. The greatest decrease in weight was observed at both 6 and 12 months. Statistically significant decreases in TDD were observed at 3, 6, and 12 months. Insulin changes after 12 months were not found to be statistically significant.
Few studies have previously evaluated the use of GLP-1 RAs in patients with T2DM who are already taking basal/bolus insulin regimens. Gyorffy and colleagues reported significant improvements in glycemic control at 3 and 6 months in a sample of 54 patients taking basal/bolus insulin when liraglutide or exenatide was added, although statistical significance was not found at the final 12-month time point.13 That study also found a significant decrease in weight at 6 months; however there was not a significant reduction in weight at both 3 and 12 months of GLP-1 RA therapy. There was not a significant decrease in TDD at any of the collected time points. Nonetheless, Gyorffy and colleagues concluded that reduction in TDD leveled off after 12 months, which is consistent with this study’s findings. The small size of the study may have limited the ability to detect statistical significance; however, this study was conducted in a population that was racially diverse and included a higher proportion of women, though average age was similar.13
Yoon and colleagues reported weight loss through 18 months, then saw weight increase, though weights did remain lower than baseline. The study also showed no significant change in TDD of insulin after 12 months of concomitant exenatide and insulin therapy.11 Although these results mirror the outcomes observed in this study, Yoon and colleagues did not differentiate results between basal and basal/bolus insulin groups.11 Seino and colleagues observed no significant change in weight after 36 weeks of GLP-1 RA therapy in Japanese patients when used with basal and basal/bolus insulin regimens. Despite the consideration that the population in the study was not overweight (mean body mass index was 25.6), the results of these studies support the idea that effects of GLP-1 RAs on weight and TDD may diminish over time.14
Within the VHA, GLP-1 RAs are nonformulary medications. Patients must meet certain criteria in order to be approved for these agents, which may include diagnosis of CVD, renal disease, or failure to reach glycemic control with the use of oral agents or insulin. Therefore, participants of this study represent a particular subset of VHA patients, many of whom may have been selected for consideration due to long-standing or uncontrolled T2DM and failure of previous therapies. The baseline demographics support this idea, given poor glycemic control at baseline and high insulin requirements. Once approved for GLP-1 RA therapy, semaglutide is currently the preferred agent within the VHA, with other agents available for select considerations. It should be noted that albiglutide, which was the primary agent selected for some of the patients included in this study, was removed from the market in 2017 for economic considerations.15 In the case for these patients, a conversion to a formulary-preferred GLP-1 RA was made.
Most of the patients included in this study (70%) were maintained on metformin from baseline throughout the study period. Fifty-seven percent of patients were taking TDD of insulin > 150 units. Considering the significant cost of concentrated insulins, the addition of GLP-1 RAs to standard insulin may prove to be beneficial from a cost standpoint. Additional research in this area may be warranted to establish more data regarding this potential benefit of GLP-1 RAs as add-on therapy.
Many adverse drug reactions were reported at different periods; however, most of these were associated with the gastrointestinal system, which is consistent with current literature, drug labeling, and the mechanism of action.16 Hypoglycemia occurred in about one-third of the participants; however, it should be noted that alone, GLP-1 RAs are not associated with a high risk of hypoglycemia. Previous studies have found that GLP-1 RA monotherapy is associated with hypoglycemia in 1.6% to 12.6% of patients.17,18 More likely, the combination of basal/bolus insulin and the GLP-1 RA’s effect on increasing insulin sensitivity through weight loss, improving glucose-dependent insulin secretion, or by decreasing appetite and therefore decreasing carbohydrate intake contributed to the hypoglycemia prevalence.
Limitations and Strengths
Limitations of this study include a small patient population and a gradual reduction in available data as time periods progressed, making even smaller sample sizes for subsequent time periods. A majority of participants were older, males and White race. This could have limited the determination of statistical significance and applicability of the results to other patient populations. Another potential limitation was the retrospective nature of the study design, which may have limited reporting of hypoglycemia and other AEs based on the documentation of the clinician.
Strengths included the study duration and the diversity of GLP-1 RAs used by participants, as the impact of many of these agents has not yet been assessed in the literature. In addition, the retrospective nature of the study allows for a more realistic representation of patient adherence, education, and motivation, which are likely different from those of patients included in prospective clinical trials.
There are no clear guidelines dictating the optimal duration of concomitant GLP-1 RA and insulin therapy; however, our study suggests that there may be continued benefits past short-term use. Also our study suggests that patients with T2DM treated with basal/bolus insulin regimens may glean additional benefit from adding GLP-1 RAs; however, further randomized, controlled studies are warranted, particularly in poorly controlled patients requiring even more aggressive treatment regimens, such as concentrated insulins.
Conclusions
In our study, adding GLP-1 RA to basal/bolus insulin was associated with a significant decrease in HbA1c from baseline through 18 months. An overall decrease in weight and TDD of insulin was observed through 24 months, but the change in weight was not significant past 18 months, and the change in insulin requirement was not significant past 12 months. Hypoglycemia was observed in almost one-third of patients, and gastrointestinal symptoms were the most common AE observed as a result of adding GLP-1 RAs. More studies are needed to better evaluate the durability and cost benefit of GLP-1 RAs, especially in patients with high insulin requirements.
Acknowledgments
This material is the result of work supported with resources and facilities at Veteran Health Indiana in Indianapolis. Study data were collected and managed using REDCap electronic data capture tools hosted at Veteran Health Indiana. The authors also acknowledge George Eckert for his assistance with data analysis.
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
1. American Diabetes Association. Statistics about diabetes. Accessed August 9, 2022. http://www.diabetes.org/diabetes-basics/statistics
2. US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development. VA research on: diabetes. Updated January 15, 2021. Accessed August 9, 2022. https://www.research.va.gov/topics/diabetes.cfm
3. Federal Practitioner. Federal Health Care Data Trends 2017, Diabetes mellitus. Accessed August 9, 2022. https://www.fedprac-digital.com/federalpractitioner/data_trends_2017?pg=20#pg20
4. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S125-S143. doi:10.2337/dc22-S009
5. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2019 executive summary. Endocr Pract. 2019;25(1):69-100. doi:10.4158/CS-2018-0535
6. St Onge E, Miller S, Clements E, Celauro L, Barnes K. The role of glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes. J Transl Int Med. 2017;5(2):79-89. Published 2017 Jun 30. doi:10.1515/jtim-2017-0015
7. Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390-402. doi:10.2337/cd19-0100
8. Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905. doi:10.1002/phar.1792
9. Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 Trial Investigators. Diabetes Care. 2016;39(8):1318-1328. doi:10.2337/dc16-0014
10. Levin PA, Mersey JH, Zhou S, Bromberger LA. Clinical outcomes using long-term combination therapy with insulin glargine and exenatide in patients with type 2 diabetes mellitus. Endocr Pract. 2012;18(1):17-25. doi:10.4158/EP11097.OR
11. Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther. 2009;31(7):1511-1523. doi:10.1016/j.clinthera.2009.07.021
12. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475-2484. doi:10.1007/s00125-014-3360-3
13. Gyorffy JB, Keithler AN, Wardian JL, Zarzabal LA, Rittel A, True MW. The impact of GLP-1 receptor agonists on patients with diabetes on insulin therapy. Endocr Pract. 2019;25(9):935-942. doi:10.4158/EP-2019-0023
14. Seino Y, Kaneko S, Fukuda S, et al. Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36-week, randomized, double-blind, parallel-group trial. J Diabetes Investig. 2016;7(4):565-573. doi:10.1111/jdi.12457
15. Optum. Tanzeum (albiglutide)–drug discontinuation. Published 2017. Accessed August 15, 2022. https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/drug-recalls-shortages/drugwithdrawal_tanzeum_2017-0801.pdf
16. Chun JH, Butts A. Long-acting GLP-1RAs: an overview of efficacy, safety, and their role in type 2 diabetes management. JAAPA. 2020;33(8):3-18. doi:10.1097/01.JAA.0000669456.13763.bd
17. Ozempic semaglutide injection. Prescribing information. Novo Nordisk; 2022. Accessed August 9, 2022. https://www.novo-pi.com/ozempic.pdf
18. Victoza liraglutide injection. Prescribing information. Novo Nordisk; 2021. Accessed August 9, 2022. https://www.novo-pi.com/victoza.pdf
Preoperative Insulin Intensification to Improve Day of Surgery Blood Glucose Control
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922