User login
The Charlie Brown tree
I put a Christmas tree up early in November.
It’s not like it’s a real tree, or even a fancy one. For that matter, I’m Jewish.
Growing up in the 1970s one thing that could be relied on every year was the Charlie Brown Christmas special. It never changed. By age 5 you knew most of the lines, and loved the highlight when Charlie Brown brings home the saddest-looking tree ever, which collapses when he puts a single bauble on it.
Years ago, my kids gave me a Charlie Brown tree as a gift. It even plays the late Vince Guaraldi’s immortal Peanuts theme when you push a button. I forgot about it for a few years, then discovered it, and immediately brought it to my office.
I’m not a fan of holiday creep, where they move up earlier in the year, so I used to put it up after Thanksgiving. But we close the office 2-3 weeks later for the rest of the year. I like the tree, my staff likes the tree, and my patients like the tree, so I just started putting it up in early November so we can enjoy it for a month.
It’s whimsical and brings back memories of innocence, childhood, and (of course) Peanuts. It sets a cheerful tone when you see it there. Very few of my patients can resist pressing the button and playing the music as they go by.
The start of a new year is a relatively arbitrary date, chosen long ago. But its approach is always a reminder that life goes on. We continue our trips around the sun. Good times and bad times come and go, but time never stops.
In bad years the tree reminds me that it’s coming to an end, and to look toward the next. In good years it reminds me that it’s time to be ready for the surprises of the coming one.
In mid-December, after the patients are done for the last day of the year, I quietly put it away. It’s a vaguely somber moment, but at the same time I’m glad to know I now have 2-3 weeks of home time. It mostly involves working at my desk and returning phone calls, but there’s also time to relax with my kids, do jigsaw puzzles, and enjoy the Phoenix winter weather as a break before the next round starts.
To those who disagree with my choice of decoration or its timing, I simply respond: “Good grief!”
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I put a Christmas tree up early in November.
It’s not like it’s a real tree, or even a fancy one. For that matter, I’m Jewish.
Growing up in the 1970s one thing that could be relied on every year was the Charlie Brown Christmas special. It never changed. By age 5 you knew most of the lines, and loved the highlight when Charlie Brown brings home the saddest-looking tree ever, which collapses when he puts a single bauble on it.
Years ago, my kids gave me a Charlie Brown tree as a gift. It even plays the late Vince Guaraldi’s immortal Peanuts theme when you push a button. I forgot about it for a few years, then discovered it, and immediately brought it to my office.
I’m not a fan of holiday creep, where they move up earlier in the year, so I used to put it up after Thanksgiving. But we close the office 2-3 weeks later for the rest of the year. I like the tree, my staff likes the tree, and my patients like the tree, so I just started putting it up in early November so we can enjoy it for a month.
It’s whimsical and brings back memories of innocence, childhood, and (of course) Peanuts. It sets a cheerful tone when you see it there. Very few of my patients can resist pressing the button and playing the music as they go by.
The start of a new year is a relatively arbitrary date, chosen long ago. But its approach is always a reminder that life goes on. We continue our trips around the sun. Good times and bad times come and go, but time never stops.
In bad years the tree reminds me that it’s coming to an end, and to look toward the next. In good years it reminds me that it’s time to be ready for the surprises of the coming one.
In mid-December, after the patients are done for the last day of the year, I quietly put it away. It’s a vaguely somber moment, but at the same time I’m glad to know I now have 2-3 weeks of home time. It mostly involves working at my desk and returning phone calls, but there’s also time to relax with my kids, do jigsaw puzzles, and enjoy the Phoenix winter weather as a break before the next round starts.
To those who disagree with my choice of decoration or its timing, I simply respond: “Good grief!”
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I put a Christmas tree up early in November.
It’s not like it’s a real tree, or even a fancy one. For that matter, I’m Jewish.
Growing up in the 1970s one thing that could be relied on every year was the Charlie Brown Christmas special. It never changed. By age 5 you knew most of the lines, and loved the highlight when Charlie Brown brings home the saddest-looking tree ever, which collapses when he puts a single bauble on it.
Years ago, my kids gave me a Charlie Brown tree as a gift. It even plays the late Vince Guaraldi’s immortal Peanuts theme when you push a button. I forgot about it for a few years, then discovered it, and immediately brought it to my office.
I’m not a fan of holiday creep, where they move up earlier in the year, so I used to put it up after Thanksgiving. But we close the office 2-3 weeks later for the rest of the year. I like the tree, my staff likes the tree, and my patients like the tree, so I just started putting it up in early November so we can enjoy it for a month.
It’s whimsical and brings back memories of innocence, childhood, and (of course) Peanuts. It sets a cheerful tone when you see it there. Very few of my patients can resist pressing the button and playing the music as they go by.
The start of a new year is a relatively arbitrary date, chosen long ago. But its approach is always a reminder that life goes on. We continue our trips around the sun. Good times and bad times come and go, but time never stops.
In bad years the tree reminds me that it’s coming to an end, and to look toward the next. In good years it reminds me that it’s time to be ready for the surprises of the coming one.
In mid-December, after the patients are done for the last day of the year, I quietly put it away. It’s a vaguely somber moment, but at the same time I’m glad to know I now have 2-3 weeks of home time. It mostly involves working at my desk and returning phone calls, but there’s also time to relax with my kids, do jigsaw puzzles, and enjoy the Phoenix winter weather as a break before the next round starts.
To those who disagree with my choice of decoration or its timing, I simply respond: “Good grief!”
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Watching violent TV in preschool linked with emotional, behavioral issues at age 12
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
Preschoolers who watch violent television are more likely to have emotional and behavioral issues at the age of 12, according to investigators.
These findings align with previous studies that have shown the negative effects of watching violent content, reinforcing the importance of restricting childhood screen time, lead author Linda S. Pagani, PhD, of Université de Montréal and colleagues reported.
Past research measured the immediate or short-term effects of seeing violent media. This study examined how TV violence could be leading to issues almost a decade later, the investigators wrote in the Journal of Developmental & Behavioral Pediatrics.
Their study looked at 1,976 children from the Quebec Longitudinal Study of Child Development, a random representative cohort of boys and girls followed since their births in 1997 and 1998.
At the cohort study follow-ups at ages 3.5 and 4.5 years, the parents of these children reported if their kids watched violent TV, showing that about half of them were exposed. At age 12, the same children were scored by their teachers on a range of psychosocial outcomes, including emotional distress, inattentive behavior, disorderly behavior, social withdrawal, classroom engagement, and overall academic achievement. At this second time point, the children also scored themselves on their own academic motivation and confidence in writing.
To adjust for other factors that could be playing a role, the investigators accounted for participant characteristics at various ages between 5 months and 12 years, as well as differences in parenting styles, home environment, and socioeconomic status.
Dr. Pagani noted that these were not “garden-variety” statistical techniques.
“We did them in such a way that we set ourselves up for not finding results,” Dr. Pagani said in an interview. “That’s why this is really interesting.”
She and her colleagues found that watching TV violence during preschool was significantly associated with multiple negative outcomes at age 12.
For girls, negative outcomes included greater emotional distress, less classroom engagement, lower academic achievement, and less academic motivation. Boys showed greater emotional distress, decreased attention, disorderly behavior, social withdrawal, less classroom engagement, lower academic achievement, and less academic motivation.
“As expected, early screen violence exposure seems to come at a cost,” the investigators wrote.
Seeing TV through a child’s eyes
According to Dr. Pagani, many parents think that TV shows watched by preschoolers – like cartoons – are harmless, but these parents need to understand that the brains of children are not yet fully developed.
“The kid has an interpretation that’s very concrete,” Dr. Pagani said. “They don’t have abstract thinking.”
Because of this, kids who see “good guys” beating up “bad guys” don’t understand that the violence is comical and justified; they just see violence being used to address social disagreement, Dr. Pagani said. This leads children to believe that violence is an acceptable way to solve problems in daily life. Children are also more likely to see hostility in others when it isn’t present, leading to conflict.
Although the natural response to these findings is to restrict childhood exposure to violent content, this may be easier said than done, the investigators noted, particularly because TV is no longer the only screen in the home, as it was when this study began. Nowadays, parents need to monitor multiple devices, including smartphones, tablets, and computers, all of which may negatively impact normal brain development.
“People think this technology is innocuous,” Dr. Pagani said. “We are asleep at the wheel.”
She advised parents to wake up and follow the World Health Organization guidelines for sedentary screen time. The guidelines call for no screen time at all until a child is at least 2 years old, and then less than 1 hour per day until age 5.
“It’s the parents who should be in charge,” she said. “They’re the ones who have the cognitive ability to make decisions for their children.”
Choosing quality time over screen time
Loredana Marchica, PhD, of Montreal Children’s Hospital and McGill University, also in Montreal, expressed confidence in the study findings, because the results line up with past research, and because the investigators accounted for other explanations.
There is a “very strong probability” that watching violent TV in preschool leads to psychological issues down the line, Dr. Marchica said.
If a child is exposed to violent content, then parents should help children understand the difference between what happens in TV shows and real life, she added, as this can reduce negative effects on behavior.
“Parents need to explain that it’s a TV show,” Dr. Marchica said. “It’s not real, and if [that violent act] happened in real life, it would actually hurt a person.”
In addition to limiting screen time and explaining any violent content, she encouraged parents to spend quality time with their children, especially during the preschool years.
“Those are the years to fortify the attachment you have with that child,” Dr. Marchica said. “Even 15 minutes a day of quality, interactive play time can make such a difference in their development, their imagination, and their social engagement and abilities.”
Parents should also try to have conversations with their young children, she said, noting that it’s okay to share personal feelings, as this teaches kids how to manage their own emotions.
“Not everything is wonderful in life, and we’re allowed to talk about that,” Dr. Marchica said. “[Parents can say,] ‘Mommy had a bad day today. This bad thing happened. But here’s what I did to make myself feel better.’ ”
Dr. Pagani and coauthors termed their findings “robust,” but also cautioned that, in their correlational study, TV violence cannot be interpreted as causal. In other limitations, they noted that the study relies on a single parent-reported item that yielded a low rate of reported exposure. Or the findings could result from other things, such as family chaos or parenting style or something else.
The longitudinal study was supported by Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, and others. The investigators and Dr. Marchica reported no relevant conflicts of interest.
FROM THE JOURNAL OF DEVELOPMENTAL & BEHAVIORAL PEDIATRICS
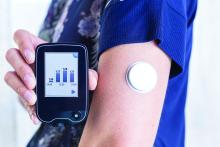
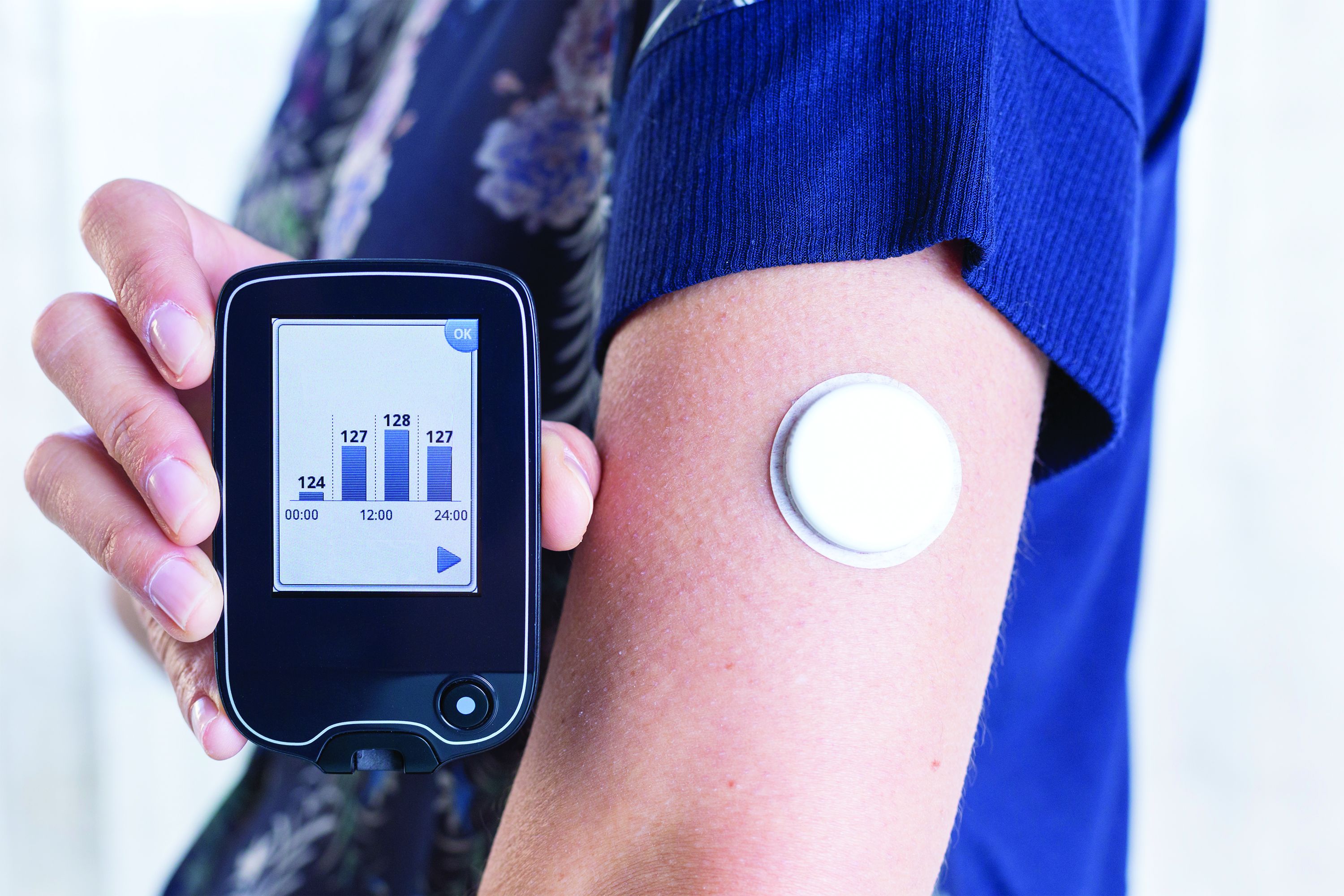
Has the time come for glucose monitors for people without diabetes?
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
AT ADA 2022
ICI combinations show survival benefit in advanced renal cancer
The combination treatment should be made readily available worldwide to patients with advanced renal cell carcinoma (RCC), the authors said.
Until recently, first-line therapy for RCC has primarily been TKIs that target vascular endothelial growth factor (VEGF) and other receptors, including sunitinib and pazopanib. Explorations of novel therapeutic regimens focused on the use of multiple TKIs in combination with monoclonal antibodies that directly inhibit VEGF and inhibitors of the mammalian target of rapamycin (mTOR), such as everolimus.
Some ICIs have already become the preferred first-line treatment for RCC. VEGF and VEGF receptors inhibitors are believed to have immunomodulatory effects, including boosting immune cell infiltration as a result of their effect on tumor vasculature. That idea has spurred recent clinical trials have examined ICIs in combination with VEGF-directed therapies.
In a review published online in Therapeutic Advances in Medical Oncology, researchers examined six phase 3 clinical trials. Each compared ICI combinations versus sunitinib as first-line therapy for advanced or metastatic RCC. Four of the studies tested TKI/ICI combinations, and 1 each tested an ICI/anti-VEGF antibody and dual ICIs.
After median follow-ups of 20-30 months, there was no benefit to PD-L1 inhibitor combinations (atezolizumab plus bevacizumab or avelumab plus axitinib) compared to sunitinib. Final survival analyses from one of the trials have not been reported yet.
PD-1 inhibitor combinations fared better. Nivolumab plus ipilimumab led to a 32% reduced risk of death in intermediate poor-risk patients compared to sunitinib, but the combination led to more frequent discontinuation due to toxicity (21.8% versus 12.3%). Nivolumab plus cabozantinib produced a 34% reduction in risk of death (P = .003) and a 48% reduction in risk of progression (P < .0001). Rates of discontinuation due to toxicity were similar to sunitinib.
Pembrolizumab combined with TKIs led to a 32% reduced risk of death (P = .003) and a 29% reduced risk of progression (P < .001). Pembrolizumab plus lenvatinib reduced risk of death by 28% (P value not reported) and the risk of progression by 61% (P < .001). Both combinations had a higher frequency of discontinuation due to toxicity (25.9% versus 10.1% and 37.2% versus 14.4%, respectively).
Given that there are no head-to-head comparisons between dual ICI or PD-1/TKI combinations, the researchers suggest that response outcomes may assist in selection between the two approaches. Overall, PD-1/TKI combinations had better overall response rates. The highest was seen in pembrolizumab plus lenvatinib, where frequency of progressive disease ranged from 5.4% to 11.3%. Complete response rate ranged from 8% to 10%.
The authors suggest that upfront treatment with a PD-1 inhibitor and a TKI could be appropriate for patients with a high tumor burden or aggressive disease, in whom stopping tumor growth is urgent and progression could be particularly worrisome.
Safety concerns associated with dual ICI combination therapy were similar to those seen in RCC and other cancers. Dose delays, rapid diagnostic workups, appropriate timing, and the use of glucocorticoids were among strategies used to manage treatment-related adverse events.
The authors noted that five combinations are approved by either the Food and Drug Administration or the European Medicines Agency for first-line treatment of metastatic RCC. Factors to consider for treatment selection include patient and disease characteristics, International Metastatic RCC Database Consortium (IMDC) risk status, treatment history during earlier disease stage, and eligibility for immunotherapy. Nivolumab plus ipilimumab may be a good choice for patients with an intermediate or poor IMDC risk since it provides a strong and durable overall survival benefit. Pembrolizumab plus axitinib, pembrolizumab plus lenvatinib, and nivolumab plus cabozantinib all have good overall response rates and can prolong life, though extended TKI use can lead to chronic toxicity. Nivolumab plus ipilimumab is not approved for those with a favorable IMDC risk in many regions.
The combination treatment should be made readily available worldwide to patients with advanced renal cell carcinoma (RCC), the authors said.
Until recently, first-line therapy for RCC has primarily been TKIs that target vascular endothelial growth factor (VEGF) and other receptors, including sunitinib and pazopanib. Explorations of novel therapeutic regimens focused on the use of multiple TKIs in combination with monoclonal antibodies that directly inhibit VEGF and inhibitors of the mammalian target of rapamycin (mTOR), such as everolimus.
Some ICIs have already become the preferred first-line treatment for RCC. VEGF and VEGF receptors inhibitors are believed to have immunomodulatory effects, including boosting immune cell infiltration as a result of their effect on tumor vasculature. That idea has spurred recent clinical trials have examined ICIs in combination with VEGF-directed therapies.
In a review published online in Therapeutic Advances in Medical Oncology, researchers examined six phase 3 clinical trials. Each compared ICI combinations versus sunitinib as first-line therapy for advanced or metastatic RCC. Four of the studies tested TKI/ICI combinations, and 1 each tested an ICI/anti-VEGF antibody and dual ICIs.
After median follow-ups of 20-30 months, there was no benefit to PD-L1 inhibitor combinations (atezolizumab plus bevacizumab or avelumab plus axitinib) compared to sunitinib. Final survival analyses from one of the trials have not been reported yet.
PD-1 inhibitor combinations fared better. Nivolumab plus ipilimumab led to a 32% reduced risk of death in intermediate poor-risk patients compared to sunitinib, but the combination led to more frequent discontinuation due to toxicity (21.8% versus 12.3%). Nivolumab plus cabozantinib produced a 34% reduction in risk of death (P = .003) and a 48% reduction in risk of progression (P < .0001). Rates of discontinuation due to toxicity were similar to sunitinib.
Pembrolizumab combined with TKIs led to a 32% reduced risk of death (P = .003) and a 29% reduced risk of progression (P < .001). Pembrolizumab plus lenvatinib reduced risk of death by 28% (P value not reported) and the risk of progression by 61% (P < .001). Both combinations had a higher frequency of discontinuation due to toxicity (25.9% versus 10.1% and 37.2% versus 14.4%, respectively).
Given that there are no head-to-head comparisons between dual ICI or PD-1/TKI combinations, the researchers suggest that response outcomes may assist in selection between the two approaches. Overall, PD-1/TKI combinations had better overall response rates. The highest was seen in pembrolizumab plus lenvatinib, where frequency of progressive disease ranged from 5.4% to 11.3%. Complete response rate ranged from 8% to 10%.
The authors suggest that upfront treatment with a PD-1 inhibitor and a TKI could be appropriate for patients with a high tumor burden or aggressive disease, in whom stopping tumor growth is urgent and progression could be particularly worrisome.
Safety concerns associated with dual ICI combination therapy were similar to those seen in RCC and other cancers. Dose delays, rapid diagnostic workups, appropriate timing, and the use of glucocorticoids were among strategies used to manage treatment-related adverse events.
The authors noted that five combinations are approved by either the Food and Drug Administration or the European Medicines Agency for first-line treatment of metastatic RCC. Factors to consider for treatment selection include patient and disease characteristics, International Metastatic RCC Database Consortium (IMDC) risk status, treatment history during earlier disease stage, and eligibility for immunotherapy. Nivolumab plus ipilimumab may be a good choice for patients with an intermediate or poor IMDC risk since it provides a strong and durable overall survival benefit. Pembrolizumab plus axitinib, pembrolizumab plus lenvatinib, and nivolumab plus cabozantinib all have good overall response rates and can prolong life, though extended TKI use can lead to chronic toxicity. Nivolumab plus ipilimumab is not approved for those with a favorable IMDC risk in many regions.
The combination treatment should be made readily available worldwide to patients with advanced renal cell carcinoma (RCC), the authors said.
Until recently, first-line therapy for RCC has primarily been TKIs that target vascular endothelial growth factor (VEGF) and other receptors, including sunitinib and pazopanib. Explorations of novel therapeutic regimens focused on the use of multiple TKIs in combination with monoclonal antibodies that directly inhibit VEGF and inhibitors of the mammalian target of rapamycin (mTOR), such as everolimus.
Some ICIs have already become the preferred first-line treatment for RCC. VEGF and VEGF receptors inhibitors are believed to have immunomodulatory effects, including boosting immune cell infiltration as a result of their effect on tumor vasculature. That idea has spurred recent clinical trials have examined ICIs in combination with VEGF-directed therapies.
In a review published online in Therapeutic Advances in Medical Oncology, researchers examined six phase 3 clinical trials. Each compared ICI combinations versus sunitinib as first-line therapy for advanced or metastatic RCC. Four of the studies tested TKI/ICI combinations, and 1 each tested an ICI/anti-VEGF antibody and dual ICIs.
After median follow-ups of 20-30 months, there was no benefit to PD-L1 inhibitor combinations (atezolizumab plus bevacizumab or avelumab plus axitinib) compared to sunitinib. Final survival analyses from one of the trials have not been reported yet.
PD-1 inhibitor combinations fared better. Nivolumab plus ipilimumab led to a 32% reduced risk of death in intermediate poor-risk patients compared to sunitinib, but the combination led to more frequent discontinuation due to toxicity (21.8% versus 12.3%). Nivolumab plus cabozantinib produced a 34% reduction in risk of death (P = .003) and a 48% reduction in risk of progression (P < .0001). Rates of discontinuation due to toxicity were similar to sunitinib.
Pembrolizumab combined with TKIs led to a 32% reduced risk of death (P = .003) and a 29% reduced risk of progression (P < .001). Pembrolizumab plus lenvatinib reduced risk of death by 28% (P value not reported) and the risk of progression by 61% (P < .001). Both combinations had a higher frequency of discontinuation due to toxicity (25.9% versus 10.1% and 37.2% versus 14.4%, respectively).
Given that there are no head-to-head comparisons between dual ICI or PD-1/TKI combinations, the researchers suggest that response outcomes may assist in selection between the two approaches. Overall, PD-1/TKI combinations had better overall response rates. The highest was seen in pembrolizumab plus lenvatinib, where frequency of progressive disease ranged from 5.4% to 11.3%. Complete response rate ranged from 8% to 10%.
The authors suggest that upfront treatment with a PD-1 inhibitor and a TKI could be appropriate for patients with a high tumor burden or aggressive disease, in whom stopping tumor growth is urgent and progression could be particularly worrisome.
Safety concerns associated with dual ICI combination therapy were similar to those seen in RCC and other cancers. Dose delays, rapid diagnostic workups, appropriate timing, and the use of glucocorticoids were among strategies used to manage treatment-related adverse events.
The authors noted that five combinations are approved by either the Food and Drug Administration or the European Medicines Agency for first-line treatment of metastatic RCC. Factors to consider for treatment selection include patient and disease characteristics, International Metastatic RCC Database Consortium (IMDC) risk status, treatment history during earlier disease stage, and eligibility for immunotherapy. Nivolumab plus ipilimumab may be a good choice for patients with an intermediate or poor IMDC risk since it provides a strong and durable overall survival benefit. Pembrolizumab plus axitinib, pembrolizumab plus lenvatinib, and nivolumab plus cabozantinib all have good overall response rates and can prolong life, though extended TKI use can lead to chronic toxicity. Nivolumab plus ipilimumab is not approved for those with a favorable IMDC risk in many regions.
FROM THERAPEUTIC ADVANCES IN MEDICAL ONCOLOGY
Retention rates high after biosimilar-to-biosimilar switch for inflammatory arthritis
PHILADELPHIA – When patients with inflammatory rheumatic diseases were switched from one biosimilar agent to another, treatment retention rates were high, investigators in Denmark reported.
The findings suggest patient-related factors rather than drug-related factors appear to determine whether patients will stay on the new drug, the researchers said.
One year after a Danish government-mandated switch from one infliximab (Remicade) biosimilar to another equally efficacious but less costly biosimilar, 83% of patients who had started therapy on a biosimilar (so-called “originator-naive” patients) stayed on the newly assigned therapy. And so did 92% of patients who had started on the original infliximab (“originator experienced”) before they were switched to one biosimilar and then another.
“In regards to potential baseline predictors, we found that treatment withdrawal was more frequent among originator-naive switchers and patients with higher baseline disease activity, especially [in] patient-reported outcomes, which may indicate that treatment-related outcomes may be more affected by patient-related rather than drug-related factors,” said lead author Hafsah Nabi, MD from the Danish biosimilar registry DANBIO and a PhD candidate at the Copenhagen Center for Arthritis Research.
Dr. Nabi reported the results in an oral abstract session at the annual meeting of the American College of Rheumatology.
Annual review of biologic agents
In Denmark, health authorities issue annual recommendations for the use of biologic agents. “And since patients receive this treatment free from the hospital, based on the tax system, the switches are made due to these cost considerations,” Dr. Nabi said in an interview.
To get the nod from Danish pharmaceutical regulators, pharmaceutical manufacturers submit drugs that have already been approved by the European Medicines Agency for consideration for treatment of specific indications, explained coauthor Merete Lund Hetland, MD, PhD, DMSc, from Rigshospitalet in Copenhagen.
“Those drugs that are then considered equally safe and effective are invited to this process where they will give their bid, and then the cheapest one will win,” she said.
The winning formulation will be able to capture about 80% of prescriptions for that indication for the coming year.
Awake at the switch
Dr. Nabi, Dr. Hetland, and colleagues studied how one such recent government-mandated switch from one biosimilar to another affected efficacy and patterns of care among patients with rheumatoid arthritis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
To identify prior comorbidities, they drew data from the DANBIO registry, which is linked to patient specific but anonymous data from other comprehensive birth-to-death patient registries in Denmark.
They looked at all patients with RA, PsA, or axSpA who were switched from CT-P13 (Remsira, Inflectra) to GP1111 (Zessly) from April 1, 2019, to Feb. 1, 2020.
They identified a total of 1,605 patients, including 685 with RA, 314 with PsA, and 606 with axSpa. The median disease duration was 9 years, and 37% of all patients were in remission according to Clinical Disease Activity Index or Ankylosing Spondylitis Disease Activity Scale.
Of this group, 1,171 had started therapy on a biosimilar.
As noted above, 83% of patients who had never received original infliximab, and 92% of those who were originator experienced were still on the new biosimilar 1 year after the switch.
In a multivariate analysis controlling for demographic and clinical factors at baseline, the variables significantly associated with treatment withdrawal from the new biosimilar (GP11110) included previous Remicade exposure (hazard ratio, 0.36), methotrexate use (HR, 0.60), and patient-reported global visual analog scale (HR, 1.02).
Among all patients, disease activity was stable 6 months before and after the switch, Dr. Nabi said, although she did not show data to support it.
Patient education benefit
During the session, Jonathan Kay, MD, professor of rheumatology and chair of the division of rheumatology at the University of Massachusetts, Worcester, who was not involved the study, asked Dr. Nabi whether patients were educated about equivalent efficacy and safety of biosimilars prior to the switch. He noted that education prior to switching led to a much lower patient withdrawal rate in a similar switching study conducted in The Netherlands.
“In this study, we haven’t looked more specifically into the education and which strategies have been used prior to switching, and we also conclude in the study that there may be the presence of a nocebo effect, which can be handled by better educating the patients,” she replied.
The nocebo effect refers to the phenomenon in which a patient’s belief that a specific intervention may cause harm actually can lead to negative outcomes – in other words, the opposite of the placebo effect.
In an interview, Dr. Kay said that he is confident about the efficacy, safety, and equivalency of approved biosimilar agents.
“A biosimilar that has been reviewed and approved by a regulatory agency such as the [Food and Drug Administration or the [European Medicines Agency] should be equivalent in efficacy and comparable in safety and immunogenicity. I would be fully confident in switching from the reference product to the biosimilar,” he said.
Dr. Nabi reported that the study was partly funded by a research grant from Sandoz, the maker of GP1111. Dr. Hetland has disclosed grants from various companies, not including Sandoz. Dr. Kay disclosed consulting fees from various companies, not including Sandoz.
PHILADELPHIA – When patients with inflammatory rheumatic diseases were switched from one biosimilar agent to another, treatment retention rates were high, investigators in Denmark reported.
The findings suggest patient-related factors rather than drug-related factors appear to determine whether patients will stay on the new drug, the researchers said.
One year after a Danish government-mandated switch from one infliximab (Remicade) biosimilar to another equally efficacious but less costly biosimilar, 83% of patients who had started therapy on a biosimilar (so-called “originator-naive” patients) stayed on the newly assigned therapy. And so did 92% of patients who had started on the original infliximab (“originator experienced”) before they were switched to one biosimilar and then another.
“In regards to potential baseline predictors, we found that treatment withdrawal was more frequent among originator-naive switchers and patients with higher baseline disease activity, especially [in] patient-reported outcomes, which may indicate that treatment-related outcomes may be more affected by patient-related rather than drug-related factors,” said lead author Hafsah Nabi, MD from the Danish biosimilar registry DANBIO and a PhD candidate at the Copenhagen Center for Arthritis Research.
Dr. Nabi reported the results in an oral abstract session at the annual meeting of the American College of Rheumatology.
Annual review of biologic agents
In Denmark, health authorities issue annual recommendations for the use of biologic agents. “And since patients receive this treatment free from the hospital, based on the tax system, the switches are made due to these cost considerations,” Dr. Nabi said in an interview.
To get the nod from Danish pharmaceutical regulators, pharmaceutical manufacturers submit drugs that have already been approved by the European Medicines Agency for consideration for treatment of specific indications, explained coauthor Merete Lund Hetland, MD, PhD, DMSc, from Rigshospitalet in Copenhagen.
“Those drugs that are then considered equally safe and effective are invited to this process where they will give their bid, and then the cheapest one will win,” she said.
The winning formulation will be able to capture about 80% of prescriptions for that indication for the coming year.
Awake at the switch
Dr. Nabi, Dr. Hetland, and colleagues studied how one such recent government-mandated switch from one biosimilar to another affected efficacy and patterns of care among patients with rheumatoid arthritis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
To identify prior comorbidities, they drew data from the DANBIO registry, which is linked to patient specific but anonymous data from other comprehensive birth-to-death patient registries in Denmark.
They looked at all patients with RA, PsA, or axSpA who were switched from CT-P13 (Remsira, Inflectra) to GP1111 (Zessly) from April 1, 2019, to Feb. 1, 2020.
They identified a total of 1,605 patients, including 685 with RA, 314 with PsA, and 606 with axSpa. The median disease duration was 9 years, and 37% of all patients were in remission according to Clinical Disease Activity Index or Ankylosing Spondylitis Disease Activity Scale.
Of this group, 1,171 had started therapy on a biosimilar.
As noted above, 83% of patients who had never received original infliximab, and 92% of those who were originator experienced were still on the new biosimilar 1 year after the switch.
In a multivariate analysis controlling for demographic and clinical factors at baseline, the variables significantly associated with treatment withdrawal from the new biosimilar (GP11110) included previous Remicade exposure (hazard ratio, 0.36), methotrexate use (HR, 0.60), and patient-reported global visual analog scale (HR, 1.02).
Among all patients, disease activity was stable 6 months before and after the switch, Dr. Nabi said, although she did not show data to support it.
Patient education benefit
During the session, Jonathan Kay, MD, professor of rheumatology and chair of the division of rheumatology at the University of Massachusetts, Worcester, who was not involved the study, asked Dr. Nabi whether patients were educated about equivalent efficacy and safety of biosimilars prior to the switch. He noted that education prior to switching led to a much lower patient withdrawal rate in a similar switching study conducted in The Netherlands.
“In this study, we haven’t looked more specifically into the education and which strategies have been used prior to switching, and we also conclude in the study that there may be the presence of a nocebo effect, which can be handled by better educating the patients,” she replied.
The nocebo effect refers to the phenomenon in which a patient’s belief that a specific intervention may cause harm actually can lead to negative outcomes – in other words, the opposite of the placebo effect.
In an interview, Dr. Kay said that he is confident about the efficacy, safety, and equivalency of approved biosimilar agents.
“A biosimilar that has been reviewed and approved by a regulatory agency such as the [Food and Drug Administration or the [European Medicines Agency] should be equivalent in efficacy and comparable in safety and immunogenicity. I would be fully confident in switching from the reference product to the biosimilar,” he said.
Dr. Nabi reported that the study was partly funded by a research grant from Sandoz, the maker of GP1111. Dr. Hetland has disclosed grants from various companies, not including Sandoz. Dr. Kay disclosed consulting fees from various companies, not including Sandoz.
PHILADELPHIA – When patients with inflammatory rheumatic diseases were switched from one biosimilar agent to another, treatment retention rates were high, investigators in Denmark reported.
The findings suggest patient-related factors rather than drug-related factors appear to determine whether patients will stay on the new drug, the researchers said.
One year after a Danish government-mandated switch from one infliximab (Remicade) biosimilar to another equally efficacious but less costly biosimilar, 83% of patients who had started therapy on a biosimilar (so-called “originator-naive” patients) stayed on the newly assigned therapy. And so did 92% of patients who had started on the original infliximab (“originator experienced”) before they were switched to one biosimilar and then another.
“In regards to potential baseline predictors, we found that treatment withdrawal was more frequent among originator-naive switchers and patients with higher baseline disease activity, especially [in] patient-reported outcomes, which may indicate that treatment-related outcomes may be more affected by patient-related rather than drug-related factors,” said lead author Hafsah Nabi, MD from the Danish biosimilar registry DANBIO and a PhD candidate at the Copenhagen Center for Arthritis Research.
Dr. Nabi reported the results in an oral abstract session at the annual meeting of the American College of Rheumatology.
Annual review of biologic agents
In Denmark, health authorities issue annual recommendations for the use of biologic agents. “And since patients receive this treatment free from the hospital, based on the tax system, the switches are made due to these cost considerations,” Dr. Nabi said in an interview.
To get the nod from Danish pharmaceutical regulators, pharmaceutical manufacturers submit drugs that have already been approved by the European Medicines Agency for consideration for treatment of specific indications, explained coauthor Merete Lund Hetland, MD, PhD, DMSc, from Rigshospitalet in Copenhagen.
“Those drugs that are then considered equally safe and effective are invited to this process where they will give their bid, and then the cheapest one will win,” she said.
The winning formulation will be able to capture about 80% of prescriptions for that indication for the coming year.
Awake at the switch
Dr. Nabi, Dr. Hetland, and colleagues studied how one such recent government-mandated switch from one biosimilar to another affected efficacy and patterns of care among patients with rheumatoid arthritis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
To identify prior comorbidities, they drew data from the DANBIO registry, which is linked to patient specific but anonymous data from other comprehensive birth-to-death patient registries in Denmark.
They looked at all patients with RA, PsA, or axSpA who were switched from CT-P13 (Remsira, Inflectra) to GP1111 (Zessly) from April 1, 2019, to Feb. 1, 2020.
They identified a total of 1,605 patients, including 685 with RA, 314 with PsA, and 606 with axSpa. The median disease duration was 9 years, and 37% of all patients were in remission according to Clinical Disease Activity Index or Ankylosing Spondylitis Disease Activity Scale.
Of this group, 1,171 had started therapy on a biosimilar.
As noted above, 83% of patients who had never received original infliximab, and 92% of those who were originator experienced were still on the new biosimilar 1 year after the switch.
In a multivariate analysis controlling for demographic and clinical factors at baseline, the variables significantly associated with treatment withdrawal from the new biosimilar (GP11110) included previous Remicade exposure (hazard ratio, 0.36), methotrexate use (HR, 0.60), and patient-reported global visual analog scale (HR, 1.02).
Among all patients, disease activity was stable 6 months before and after the switch, Dr. Nabi said, although she did not show data to support it.
Patient education benefit
During the session, Jonathan Kay, MD, professor of rheumatology and chair of the division of rheumatology at the University of Massachusetts, Worcester, who was not involved the study, asked Dr. Nabi whether patients were educated about equivalent efficacy and safety of biosimilars prior to the switch. He noted that education prior to switching led to a much lower patient withdrawal rate in a similar switching study conducted in The Netherlands.
“In this study, we haven’t looked more specifically into the education and which strategies have been used prior to switching, and we also conclude in the study that there may be the presence of a nocebo effect, which can be handled by better educating the patients,” she replied.
The nocebo effect refers to the phenomenon in which a patient’s belief that a specific intervention may cause harm actually can lead to negative outcomes – in other words, the opposite of the placebo effect.
In an interview, Dr. Kay said that he is confident about the efficacy, safety, and equivalency of approved biosimilar agents.
“A biosimilar that has been reviewed and approved by a regulatory agency such as the [Food and Drug Administration or the [European Medicines Agency] should be equivalent in efficacy and comparable in safety and immunogenicity. I would be fully confident in switching from the reference product to the biosimilar,” he said.
Dr. Nabi reported that the study was partly funded by a research grant from Sandoz, the maker of GP1111. Dr. Hetland has disclosed grants from various companies, not including Sandoz. Dr. Kay disclosed consulting fees from various companies, not including Sandoz.
AT ACR 2022
Use 2022’s advocacy successes and frustrations as a catalyst for the new year
As we come to a close on 2022, let’s take a look at the celebrations and frustrations of the past year’s health policies so that they may act as a catalyst, encouraging us to engage with our representatives. Some of these policies include actions by major companies that rule our health care system, as well as the regulations and legislation passed (or not passed) by our governmental entities. And of course, we must consider how profits and politics influence these policies and often rule the roost!
Insurance
Once again, we are facing increased nonmedical switching of stable patients to different medications through ever-increasing formulary exclusions and higher tiering of less profitable drugs. There are some reports of patients being whipsawed back and forth yearly between reference infliximab and various biosimilars, depending on which is the most profitable to the health plan at the time. And now it’s not just the copay accumulator or maximizer programs that are abusing patient assistance programs, there are new “alternative funding companies” that are carving out expensive and specialty drugs from coverage of employers’ funded health plans. These alternative funding companies then obtain medications – sometimes from other countries – and other forms of assistance from manufacturers and foundations. There have been reports that they make the patient assign power of attorney to them and even pretend to be the patient to obtain the drug, assistance, and copay cards and then bill the employer for getting the free drug or assistance. This abuse of the system, along with copay maximizers, are causing drug manufacturers to rethink their assistance policies, with middlemen reaping the advantages of the assistance plans and not the truly needy patient.
Legislation and regulation
Substantive progress continues to be made on access issues in the states. A total of 5 states signed step-therapy legislation into law, 3 states have new copay accumulator program bans, 13 states began to debate the issue of white bagging, and 16 states began to consider the next stage of pharmacy benefit manager (PBM) reform with rebate-pass-through legislation.
At the federal level, the Inflation Reduction Act (IRA; H.R. 5376) was enacted in August and, like all major pieces of legislation, there are pros and cons. On the positive side, the legislation reforms Medicare Part D cost-sharing, including – for the first time – the creation of an annual cap on cost-sharing by beneficiaries. That will especially help patients with high, ongoing prescription drug needs. On the negative side, despite its extensive drug-pricing provisions, the IRA did not include any reform of PBM practices. In fact, Congress has delayed implementation of the so-called “rebate rule” for 10 years. That rule would have essentially ended payments from drug companies to PBMs in exchange for formulary placement by removing safe harbor protection from antikickback law for these payments, allowing patients to benefit from these payments.
Finally, the IRA included extensive provisions applicable to drug manufacturers, including a mechanism for Medicare to set prices directly for medications that have been on the market for a certain number of years but are still without a biosimilar or generic. This will apply fully to selected Part B drugs as of 2028. The key for rheumatologists and our patients in the next few years will be to engage with the Centers for Medicare & Medicaid Services as it implements this provision to ensure that rheumatologists are not underwater financially on the acquisition of medications subject to the new pricing mechanism.
With regard to utilization management reform at the federal level, the Ensuring Seniors’ Timely Access to Care Act (H.R. 3173) would reform prior authorization in Medicare Advantage, but after passing in the House on Sept. 14, the bill has slowed down in the Senate. In some part, that may be because of a surprising score from the Congressional Budget Office, which projected that the bill would cost $16 billion. However, this is not insurmountable: The legislation enjoys broad bipartisan support in the Senate, and its sponsors remain committed to enactment before the end of the year. Additionally, the Safe Step Act (S. 464) would reform step therapy practices in employer-based coverage, but that legislation has not passed either chamber of Congress despite bipartisan support and is unlikely to be enacted before the end of this congressional session.
As noted above, PBMs escaped meaningful scrutiny or reform in the IRA, but the Federal Trade Commission took a different approach when it announced earlier in 2022 that it would conduct an investigation into the business practices of several major PBMs. That study is ongoing and, when finished, will likely result in some additional ideas for meaningful legislative reform.
Finally, there’s the frustration of the egregious Medicare Physician Fee Schedule that has decreased physicians’ reimbursement in a time of accelerated inflation in the cost of running a practice. At the same time, Medicare Advantage plans and everyone else in the government-reimbursed health system are getting at least an inflationary raise. This has created an ire among all physicians that we have not seen in quite a while and which we are leveraging into grassroots outreach.
The problems in the Fee Schedule result from a combination of factors, but one overarching issue is the concept of “budget neutrality,” which essentially requires CMS to make up for any new spending over a certain amount by a commensurate reduction across the whole Fee Schedule. This has the effect of turning the Fee Schedule into a fixed pie: If someone’s slice gets bigger, someone else’s slice must get smaller, but the pie itself never gets bigger. To make matters worse, the Medicare Access and CHIP Reauthorization Act of 2015 has not resulted in advancing value-driven care as the Congress had envisioned when it enacted that legislation. The good news is that there is widespread recognition in Congress that a system built on temporary legislative “patches” to avoid deep payment reductions is unsustainable and must be fixed. The Supporting Medicare Providers Act of 2022 (H.R. 8800) that’s currently pending in the House to offset the looming 2023 Fee Schedule cuts also includes a Sense of the Congress, or nonbinding resolution, establishing the need for administrative and legislative actions for long-term, meaningful reform of Medicare physician payment, along three principles: ensuring financial stability and predictability, promoting and rewarding value-based care innovation, and safeguarding timely access to high-quality care by advancing health equity and reducing disparities.
Turning frustration into action
Much of the frustration for those of us who take care of patients is that many actions and policies are based on profits and politics and not on patient care.
It is unfortunate that money plays such an important role in politics. We are all aware of the power of the well-heeled lobbyist and how money can lead to legislation that is more beneficial to one for-profit company versus another in the health care sector. But then there is the party politics of health care legislation. We see examples of great legislation offered by one party being buried because it might be beneficial to the “other side” in the next election, in spite of the fact that both sides agree on the issue. Here is where we must fight our cynicism and remember our patients. Building and maintaining a relationship with our representatives, whether we agree with them are not, is a very important part of advocacy.
As we come off the recent elections, it is important that we acquaint ourselves with our newly elected representatives and reacquaint ourselves with our re-elected officials. Recently, the Coalition of State Rheumatology Organizations had an advocacy day asking rheumatologists to invite their legislator (city, state, or federal) to their office to witness first-hand the practice of rheumatology. The importance of asking your representative to visit your office cannot be overemphasized. First, you get to know the staffer who arranges these visits. Having a good relationship with your representative’s staff is important in maintaining future communications. Having your legislator tour your office, while you share the daily challenges of getting the right medication for patients, is invaluable to their understanding of how the delay in care that utilization management tools such as prior authorizations and step therapy can cause. It is also helpful for you or your office manager to highlight how independent practices are small businesses that must be run efficiently to ensure they can stay open. Building a relationship with and educating your representatives on issues they may not be familiar with will encourage them to use you as a resource in the future.
CSRO has a legislator invitation template, and we can provide talking points if the invitation is accepted. Many state legislative sessions begin in January, so now is the time to get to know your legislator.
Let’s celebrate the wins of 2022 and not let the frustrations with the system diminish our passion – that’s the hard part! Onward to 2023 as “Rheums for Action!”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As we come to a close on 2022, let’s take a look at the celebrations and frustrations of the past year’s health policies so that they may act as a catalyst, encouraging us to engage with our representatives. Some of these policies include actions by major companies that rule our health care system, as well as the regulations and legislation passed (or not passed) by our governmental entities. And of course, we must consider how profits and politics influence these policies and often rule the roost!
Insurance
Once again, we are facing increased nonmedical switching of stable patients to different medications through ever-increasing formulary exclusions and higher tiering of less profitable drugs. There are some reports of patients being whipsawed back and forth yearly between reference infliximab and various biosimilars, depending on which is the most profitable to the health plan at the time. And now it’s not just the copay accumulator or maximizer programs that are abusing patient assistance programs, there are new “alternative funding companies” that are carving out expensive and specialty drugs from coverage of employers’ funded health plans. These alternative funding companies then obtain medications – sometimes from other countries – and other forms of assistance from manufacturers and foundations. There have been reports that they make the patient assign power of attorney to them and even pretend to be the patient to obtain the drug, assistance, and copay cards and then bill the employer for getting the free drug or assistance. This abuse of the system, along with copay maximizers, are causing drug manufacturers to rethink their assistance policies, with middlemen reaping the advantages of the assistance plans and not the truly needy patient.
Legislation and regulation
Substantive progress continues to be made on access issues in the states. A total of 5 states signed step-therapy legislation into law, 3 states have new copay accumulator program bans, 13 states began to debate the issue of white bagging, and 16 states began to consider the next stage of pharmacy benefit manager (PBM) reform with rebate-pass-through legislation.
At the federal level, the Inflation Reduction Act (IRA; H.R. 5376) was enacted in August and, like all major pieces of legislation, there are pros and cons. On the positive side, the legislation reforms Medicare Part D cost-sharing, including – for the first time – the creation of an annual cap on cost-sharing by beneficiaries. That will especially help patients with high, ongoing prescription drug needs. On the negative side, despite its extensive drug-pricing provisions, the IRA did not include any reform of PBM practices. In fact, Congress has delayed implementation of the so-called “rebate rule” for 10 years. That rule would have essentially ended payments from drug companies to PBMs in exchange for formulary placement by removing safe harbor protection from antikickback law for these payments, allowing patients to benefit from these payments.
Finally, the IRA included extensive provisions applicable to drug manufacturers, including a mechanism for Medicare to set prices directly for medications that have been on the market for a certain number of years but are still without a biosimilar or generic. This will apply fully to selected Part B drugs as of 2028. The key for rheumatologists and our patients in the next few years will be to engage with the Centers for Medicare & Medicaid Services as it implements this provision to ensure that rheumatologists are not underwater financially on the acquisition of medications subject to the new pricing mechanism.
With regard to utilization management reform at the federal level, the Ensuring Seniors’ Timely Access to Care Act (H.R. 3173) would reform prior authorization in Medicare Advantage, but after passing in the House on Sept. 14, the bill has slowed down in the Senate. In some part, that may be because of a surprising score from the Congressional Budget Office, which projected that the bill would cost $16 billion. However, this is not insurmountable: The legislation enjoys broad bipartisan support in the Senate, and its sponsors remain committed to enactment before the end of the year. Additionally, the Safe Step Act (S. 464) would reform step therapy practices in employer-based coverage, but that legislation has not passed either chamber of Congress despite bipartisan support and is unlikely to be enacted before the end of this congressional session.
As noted above, PBMs escaped meaningful scrutiny or reform in the IRA, but the Federal Trade Commission took a different approach when it announced earlier in 2022 that it would conduct an investigation into the business practices of several major PBMs. That study is ongoing and, when finished, will likely result in some additional ideas for meaningful legislative reform.
Finally, there’s the frustration of the egregious Medicare Physician Fee Schedule that has decreased physicians’ reimbursement in a time of accelerated inflation in the cost of running a practice. At the same time, Medicare Advantage plans and everyone else in the government-reimbursed health system are getting at least an inflationary raise. This has created an ire among all physicians that we have not seen in quite a while and which we are leveraging into grassroots outreach.
The problems in the Fee Schedule result from a combination of factors, but one overarching issue is the concept of “budget neutrality,” which essentially requires CMS to make up for any new spending over a certain amount by a commensurate reduction across the whole Fee Schedule. This has the effect of turning the Fee Schedule into a fixed pie: If someone’s slice gets bigger, someone else’s slice must get smaller, but the pie itself never gets bigger. To make matters worse, the Medicare Access and CHIP Reauthorization Act of 2015 has not resulted in advancing value-driven care as the Congress had envisioned when it enacted that legislation. The good news is that there is widespread recognition in Congress that a system built on temporary legislative “patches” to avoid deep payment reductions is unsustainable and must be fixed. The Supporting Medicare Providers Act of 2022 (H.R. 8800) that’s currently pending in the House to offset the looming 2023 Fee Schedule cuts also includes a Sense of the Congress, or nonbinding resolution, establishing the need for administrative and legislative actions for long-term, meaningful reform of Medicare physician payment, along three principles: ensuring financial stability and predictability, promoting and rewarding value-based care innovation, and safeguarding timely access to high-quality care by advancing health equity and reducing disparities.
Turning frustration into action
Much of the frustration for those of us who take care of patients is that many actions and policies are based on profits and politics and not on patient care.
It is unfortunate that money plays such an important role in politics. We are all aware of the power of the well-heeled lobbyist and how money can lead to legislation that is more beneficial to one for-profit company versus another in the health care sector. But then there is the party politics of health care legislation. We see examples of great legislation offered by one party being buried because it might be beneficial to the “other side” in the next election, in spite of the fact that both sides agree on the issue. Here is where we must fight our cynicism and remember our patients. Building and maintaining a relationship with our representatives, whether we agree with them are not, is a very important part of advocacy.
As we come off the recent elections, it is important that we acquaint ourselves with our newly elected representatives and reacquaint ourselves with our re-elected officials. Recently, the Coalition of State Rheumatology Organizations had an advocacy day asking rheumatologists to invite their legislator (city, state, or federal) to their office to witness first-hand the practice of rheumatology. The importance of asking your representative to visit your office cannot be overemphasized. First, you get to know the staffer who arranges these visits. Having a good relationship with your representative’s staff is important in maintaining future communications. Having your legislator tour your office, while you share the daily challenges of getting the right medication for patients, is invaluable to their understanding of how the delay in care that utilization management tools such as prior authorizations and step therapy can cause. It is also helpful for you or your office manager to highlight how independent practices are small businesses that must be run efficiently to ensure they can stay open. Building a relationship with and educating your representatives on issues they may not be familiar with will encourage them to use you as a resource in the future.
CSRO has a legislator invitation template, and we can provide talking points if the invitation is accepted. Many state legislative sessions begin in January, so now is the time to get to know your legislator.
Let’s celebrate the wins of 2022 and not let the frustrations with the system diminish our passion – that’s the hard part! Onward to 2023 as “Rheums for Action!”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
As we come to a close on 2022, let’s take a look at the celebrations and frustrations of the past year’s health policies so that they may act as a catalyst, encouraging us to engage with our representatives. Some of these policies include actions by major companies that rule our health care system, as well as the regulations and legislation passed (or not passed) by our governmental entities. And of course, we must consider how profits and politics influence these policies and often rule the roost!
Insurance
Once again, we are facing increased nonmedical switching of stable patients to different medications through ever-increasing formulary exclusions and higher tiering of less profitable drugs. There are some reports of patients being whipsawed back and forth yearly between reference infliximab and various biosimilars, depending on which is the most profitable to the health plan at the time. And now it’s not just the copay accumulator or maximizer programs that are abusing patient assistance programs, there are new “alternative funding companies” that are carving out expensive and specialty drugs from coverage of employers’ funded health plans. These alternative funding companies then obtain medications – sometimes from other countries – and other forms of assistance from manufacturers and foundations. There have been reports that they make the patient assign power of attorney to them and even pretend to be the patient to obtain the drug, assistance, and copay cards and then bill the employer for getting the free drug or assistance. This abuse of the system, along with copay maximizers, are causing drug manufacturers to rethink their assistance policies, with middlemen reaping the advantages of the assistance plans and not the truly needy patient.
Legislation and regulation
Substantive progress continues to be made on access issues in the states. A total of 5 states signed step-therapy legislation into law, 3 states have new copay accumulator program bans, 13 states began to debate the issue of white bagging, and 16 states began to consider the next stage of pharmacy benefit manager (PBM) reform with rebate-pass-through legislation.
At the federal level, the Inflation Reduction Act (IRA; H.R. 5376) was enacted in August and, like all major pieces of legislation, there are pros and cons. On the positive side, the legislation reforms Medicare Part D cost-sharing, including – for the first time – the creation of an annual cap on cost-sharing by beneficiaries. That will especially help patients with high, ongoing prescription drug needs. On the negative side, despite its extensive drug-pricing provisions, the IRA did not include any reform of PBM practices. In fact, Congress has delayed implementation of the so-called “rebate rule” for 10 years. That rule would have essentially ended payments from drug companies to PBMs in exchange for formulary placement by removing safe harbor protection from antikickback law for these payments, allowing patients to benefit from these payments.
Finally, the IRA included extensive provisions applicable to drug manufacturers, including a mechanism for Medicare to set prices directly for medications that have been on the market for a certain number of years but are still without a biosimilar or generic. This will apply fully to selected Part B drugs as of 2028. The key for rheumatologists and our patients in the next few years will be to engage with the Centers for Medicare & Medicaid Services as it implements this provision to ensure that rheumatologists are not underwater financially on the acquisition of medications subject to the new pricing mechanism.
With regard to utilization management reform at the federal level, the Ensuring Seniors’ Timely Access to Care Act (H.R. 3173) would reform prior authorization in Medicare Advantage, but after passing in the House on Sept. 14, the bill has slowed down in the Senate. In some part, that may be because of a surprising score from the Congressional Budget Office, which projected that the bill would cost $16 billion. However, this is not insurmountable: The legislation enjoys broad bipartisan support in the Senate, and its sponsors remain committed to enactment before the end of the year. Additionally, the Safe Step Act (S. 464) would reform step therapy practices in employer-based coverage, but that legislation has not passed either chamber of Congress despite bipartisan support and is unlikely to be enacted before the end of this congressional session.
As noted above, PBMs escaped meaningful scrutiny or reform in the IRA, but the Federal Trade Commission took a different approach when it announced earlier in 2022 that it would conduct an investigation into the business practices of several major PBMs. That study is ongoing and, when finished, will likely result in some additional ideas for meaningful legislative reform.
Finally, there’s the frustration of the egregious Medicare Physician Fee Schedule that has decreased physicians’ reimbursement in a time of accelerated inflation in the cost of running a practice. At the same time, Medicare Advantage plans and everyone else in the government-reimbursed health system are getting at least an inflationary raise. This has created an ire among all physicians that we have not seen in quite a while and which we are leveraging into grassroots outreach.
The problems in the Fee Schedule result from a combination of factors, but one overarching issue is the concept of “budget neutrality,” which essentially requires CMS to make up for any new spending over a certain amount by a commensurate reduction across the whole Fee Schedule. This has the effect of turning the Fee Schedule into a fixed pie: If someone’s slice gets bigger, someone else’s slice must get smaller, but the pie itself never gets bigger. To make matters worse, the Medicare Access and CHIP Reauthorization Act of 2015 has not resulted in advancing value-driven care as the Congress had envisioned when it enacted that legislation. The good news is that there is widespread recognition in Congress that a system built on temporary legislative “patches” to avoid deep payment reductions is unsustainable and must be fixed. The Supporting Medicare Providers Act of 2022 (H.R. 8800) that’s currently pending in the House to offset the looming 2023 Fee Schedule cuts also includes a Sense of the Congress, or nonbinding resolution, establishing the need for administrative and legislative actions for long-term, meaningful reform of Medicare physician payment, along three principles: ensuring financial stability and predictability, promoting and rewarding value-based care innovation, and safeguarding timely access to high-quality care by advancing health equity and reducing disparities.
Turning frustration into action
Much of the frustration for those of us who take care of patients is that many actions and policies are based on profits and politics and not on patient care.
It is unfortunate that money plays such an important role in politics. We are all aware of the power of the well-heeled lobbyist and how money can lead to legislation that is more beneficial to one for-profit company versus another in the health care sector. But then there is the party politics of health care legislation. We see examples of great legislation offered by one party being buried because it might be beneficial to the “other side” in the next election, in spite of the fact that both sides agree on the issue. Here is where we must fight our cynicism and remember our patients. Building and maintaining a relationship with our representatives, whether we agree with them are not, is a very important part of advocacy.
As we come off the recent elections, it is important that we acquaint ourselves with our newly elected representatives and reacquaint ourselves with our re-elected officials. Recently, the Coalition of State Rheumatology Organizations had an advocacy day asking rheumatologists to invite their legislator (city, state, or federal) to their office to witness first-hand the practice of rheumatology. The importance of asking your representative to visit your office cannot be overemphasized. First, you get to know the staffer who arranges these visits. Having a good relationship with your representative’s staff is important in maintaining future communications. Having your legislator tour your office, while you share the daily challenges of getting the right medication for patients, is invaluable to their understanding of how the delay in care that utilization management tools such as prior authorizations and step therapy can cause. It is also helpful for you or your office manager to highlight how independent practices are small businesses that must be run efficiently to ensure they can stay open. Building a relationship with and educating your representatives on issues they may not be familiar with will encourage them to use you as a resource in the future.
CSRO has a legislator invitation template, and we can provide talking points if the invitation is accepted. Many state legislative sessions begin in January, so now is the time to get to know your legislator.
Let’s celebrate the wins of 2022 and not let the frustrations with the system diminish our passion – that’s the hard part! Onward to 2023 as “Rheums for Action!”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Rituximab ‘a reasonable alternative to cyclophosphamide’ to improve ILD-CTD
PHILADELPHIA – In the first controlled clinical trial to compare the two drugs, rituximab and cyclophosphamide were similarly effective in improving lung function in patients with interstitial lung disease (ILD) associated with idiopathic inflammatory myositis and mixed connective tissue disease (CTD). The findings also revealed some nuanced findings that could help clarify which drug to use in specific patients.
“We feel that rituximab is a reasonable alternative to cyclophosphamide as a treatment in patients with these diseases,” said Toby Maher, MD, of the University of Southern California, Los Angeles, who presented results of an analysis of three disease subgroups from the RECITAL (Rituximab versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease) study at the annual meeting of the American College of Rheumatology.
“We didn’t show it to be better, so I think you can reasonably choose between the two, but rituximab almost certainly has the advantage of being safer and better tolerated than cyclophosphamide,” Dr. Maher said in an interview. The findings were published simultaneously in The Lancet Respiratory Medicine.
Double-blind, double-dummy
RECITAL is a phase 2b, randomized, controlled trial to test the hypothesis that intravenous rituximab would be superior to cyclophosphamide for ILD-associated CTD.
The study included adults with three separate diagnoses: myositis (n = 44), mixed CTD (n = 16), and systemic sclerosis (SSc, n = 37). The study was done in the United Kingdom when Dr. Maher was with Imperial College London.
Patients in the rituximab group received 1,000 mg of IV treatment at baseline and 2 weeks, then placebo treatment every 4 weeks to week 20. Cyclophosphamide patients received 600 mg/m2 of body surface area intravenously every 4 weeks for six doses.
“When we designed this study there was limited evidence for any treatment for any disease associated with ILD,” Dr. Maher said. “But cyclophosphamide brings with it many challenges. It can be poorly tolerated and carries issues like infertility and risk of bladder cancer.”
Improved lung function
While the study failed to meet its primary endpoint – superiority of rituximab versus cyclophosphamide – it did show that both drugs led to improvement in lung function, measured by the rate of change in forced vital capacity (FVC), as well as quality of life measures, Dr. Maher said.
“Overall by week 48, we saw about a 5% improvement in FVC in the cyclophosphamide group and approximately a 4% improvement in FVC from baseline in the rituximab group, suggesting that both drugs almost certainly had a positive benefit in this patient group,” he said.
But secondary outcomes varied somewhat across the different disease groups. Patients with SSc saw a slight deterioration with cyclophosphamide in the modified Rodnan skin score at 24 weeks (1.6 ± 5.7 units) but an improvement with rituximab (–3.4 ± 8.1 units).
“One area where we did see a difference was in the number of adverse events,” Dr. Maher said. “They were fewer in the rituximab arm – namely gastrointestinal disorders [and] nausea, which we saw quite frequently following cyclophosphamide. Also, they had fewer headaches, which we saw quite frequently following cyclophosphamide.”
Rituximab patients also had fewer infusion reactions, but the number of infections was similar between the two treatment groups, he said.
“The patient group that responded best to treatment was the myositis group,” Dr. Maher said in his presentation. “Cyclophosphamide actually appears to be more effective than rituximab in improving their disease. By the end of 48 weeks, the cyclophosphamide group actually gained about 400 mL in FVC, so a close to 20% improvement.”
The rituximab group had “a little bit of a drop-off” in efficacy from weeks 24 to 48, although the trial didn’t repeat dosing at 6 months, “which is what perhaps one might do in clinical practice,” he said.
Oliver Distler, MD, chair of rheumatology at the University Hospital Zürich, raised questions about concurrent corticosteroid use in study patients that may have caused a “spillover” in the study’s efficacy analysis. But Dr. Maher noted that steroid use was balanced in all treatment arms. Patients in the cyclophosphamide arm averaged 42.9 mg of hydrocortisone daily versus 37.6 mg daily in the rituximab arm. That represents a 12.3% reduction in steroid exposure for the latter.
Dr. Distler noted that the myositis population represented the bulk of those study patients on steroids. “So in the myositis subanalysis we do see a combination of high-dose steroid plus cyclophosphamide and rituximab.”
Dr. Maher disclosed relationships with Boehringer Ingelheim, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Trevi, CSL Behring, Pliant and Veracyte. Dr. Distler disclosed relationships with numerous pharmaceutical companies.
PHILADELPHIA – In the first controlled clinical trial to compare the two drugs, rituximab and cyclophosphamide were similarly effective in improving lung function in patients with interstitial lung disease (ILD) associated with idiopathic inflammatory myositis and mixed connective tissue disease (CTD). The findings also revealed some nuanced findings that could help clarify which drug to use in specific patients.
“We feel that rituximab is a reasonable alternative to cyclophosphamide as a treatment in patients with these diseases,” said Toby Maher, MD, of the University of Southern California, Los Angeles, who presented results of an analysis of three disease subgroups from the RECITAL (Rituximab versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease) study at the annual meeting of the American College of Rheumatology.
“We didn’t show it to be better, so I think you can reasonably choose between the two, but rituximab almost certainly has the advantage of being safer and better tolerated than cyclophosphamide,” Dr. Maher said in an interview. The findings were published simultaneously in The Lancet Respiratory Medicine.
Double-blind, double-dummy
RECITAL is a phase 2b, randomized, controlled trial to test the hypothesis that intravenous rituximab would be superior to cyclophosphamide for ILD-associated CTD.
The study included adults with three separate diagnoses: myositis (n = 44), mixed CTD (n = 16), and systemic sclerosis (SSc, n = 37). The study was done in the United Kingdom when Dr. Maher was with Imperial College London.
Patients in the rituximab group received 1,000 mg of IV treatment at baseline and 2 weeks, then placebo treatment every 4 weeks to week 20. Cyclophosphamide patients received 600 mg/m2 of body surface area intravenously every 4 weeks for six doses.
“When we designed this study there was limited evidence for any treatment for any disease associated with ILD,” Dr. Maher said. “But cyclophosphamide brings with it many challenges. It can be poorly tolerated and carries issues like infertility and risk of bladder cancer.”
Improved lung function
While the study failed to meet its primary endpoint – superiority of rituximab versus cyclophosphamide – it did show that both drugs led to improvement in lung function, measured by the rate of change in forced vital capacity (FVC), as well as quality of life measures, Dr. Maher said.
“Overall by week 48, we saw about a 5% improvement in FVC in the cyclophosphamide group and approximately a 4% improvement in FVC from baseline in the rituximab group, suggesting that both drugs almost certainly had a positive benefit in this patient group,” he said.
But secondary outcomes varied somewhat across the different disease groups. Patients with SSc saw a slight deterioration with cyclophosphamide in the modified Rodnan skin score at 24 weeks (1.6 ± 5.7 units) but an improvement with rituximab (–3.4 ± 8.1 units).
“One area where we did see a difference was in the number of adverse events,” Dr. Maher said. “They were fewer in the rituximab arm – namely gastrointestinal disorders [and] nausea, which we saw quite frequently following cyclophosphamide. Also, they had fewer headaches, which we saw quite frequently following cyclophosphamide.”
Rituximab patients also had fewer infusion reactions, but the number of infections was similar between the two treatment groups, he said.
“The patient group that responded best to treatment was the myositis group,” Dr. Maher said in his presentation. “Cyclophosphamide actually appears to be more effective than rituximab in improving their disease. By the end of 48 weeks, the cyclophosphamide group actually gained about 400 mL in FVC, so a close to 20% improvement.”
The rituximab group had “a little bit of a drop-off” in efficacy from weeks 24 to 48, although the trial didn’t repeat dosing at 6 months, “which is what perhaps one might do in clinical practice,” he said.
Oliver Distler, MD, chair of rheumatology at the University Hospital Zürich, raised questions about concurrent corticosteroid use in study patients that may have caused a “spillover” in the study’s efficacy analysis. But Dr. Maher noted that steroid use was balanced in all treatment arms. Patients in the cyclophosphamide arm averaged 42.9 mg of hydrocortisone daily versus 37.6 mg daily in the rituximab arm. That represents a 12.3% reduction in steroid exposure for the latter.
Dr. Distler noted that the myositis population represented the bulk of those study patients on steroids. “So in the myositis subanalysis we do see a combination of high-dose steroid plus cyclophosphamide and rituximab.”
Dr. Maher disclosed relationships with Boehringer Ingelheim, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Trevi, CSL Behring, Pliant and Veracyte. Dr. Distler disclosed relationships with numerous pharmaceutical companies.
PHILADELPHIA – In the first controlled clinical trial to compare the two drugs, rituximab and cyclophosphamide were similarly effective in improving lung function in patients with interstitial lung disease (ILD) associated with idiopathic inflammatory myositis and mixed connective tissue disease (CTD). The findings also revealed some nuanced findings that could help clarify which drug to use in specific patients.
“We feel that rituximab is a reasonable alternative to cyclophosphamide as a treatment in patients with these diseases,” said Toby Maher, MD, of the University of Southern California, Los Angeles, who presented results of an analysis of three disease subgroups from the RECITAL (Rituximab versus Cyclophosphamide for the Treatment of Connective Tissue Disease Associated Interstitial Lung Disease) study at the annual meeting of the American College of Rheumatology.
“We didn’t show it to be better, so I think you can reasonably choose between the two, but rituximab almost certainly has the advantage of being safer and better tolerated than cyclophosphamide,” Dr. Maher said in an interview. The findings were published simultaneously in The Lancet Respiratory Medicine.
Double-blind, double-dummy
RECITAL is a phase 2b, randomized, controlled trial to test the hypothesis that intravenous rituximab would be superior to cyclophosphamide for ILD-associated CTD.
The study included adults with three separate diagnoses: myositis (n = 44), mixed CTD (n = 16), and systemic sclerosis (SSc, n = 37). The study was done in the United Kingdom when Dr. Maher was with Imperial College London.
Patients in the rituximab group received 1,000 mg of IV treatment at baseline and 2 weeks, then placebo treatment every 4 weeks to week 20. Cyclophosphamide patients received 600 mg/m2 of body surface area intravenously every 4 weeks for six doses.
“When we designed this study there was limited evidence for any treatment for any disease associated with ILD,” Dr. Maher said. “But cyclophosphamide brings with it many challenges. It can be poorly tolerated and carries issues like infertility and risk of bladder cancer.”
Improved lung function
While the study failed to meet its primary endpoint – superiority of rituximab versus cyclophosphamide – it did show that both drugs led to improvement in lung function, measured by the rate of change in forced vital capacity (FVC), as well as quality of life measures, Dr. Maher said.
“Overall by week 48, we saw about a 5% improvement in FVC in the cyclophosphamide group and approximately a 4% improvement in FVC from baseline in the rituximab group, suggesting that both drugs almost certainly had a positive benefit in this patient group,” he said.
But secondary outcomes varied somewhat across the different disease groups. Patients with SSc saw a slight deterioration with cyclophosphamide in the modified Rodnan skin score at 24 weeks (1.6 ± 5.7 units) but an improvement with rituximab (–3.4 ± 8.1 units).
“One area where we did see a difference was in the number of adverse events,” Dr. Maher said. “They were fewer in the rituximab arm – namely gastrointestinal disorders [and] nausea, which we saw quite frequently following cyclophosphamide. Also, they had fewer headaches, which we saw quite frequently following cyclophosphamide.”
Rituximab patients also had fewer infusion reactions, but the number of infections was similar between the two treatment groups, he said.
“The patient group that responded best to treatment was the myositis group,” Dr. Maher said in his presentation. “Cyclophosphamide actually appears to be more effective than rituximab in improving their disease. By the end of 48 weeks, the cyclophosphamide group actually gained about 400 mL in FVC, so a close to 20% improvement.”
The rituximab group had “a little bit of a drop-off” in efficacy from weeks 24 to 48, although the trial didn’t repeat dosing at 6 months, “which is what perhaps one might do in clinical practice,” he said.
Oliver Distler, MD, chair of rheumatology at the University Hospital Zürich, raised questions about concurrent corticosteroid use in study patients that may have caused a “spillover” in the study’s efficacy analysis. But Dr. Maher noted that steroid use was balanced in all treatment arms. Patients in the cyclophosphamide arm averaged 42.9 mg of hydrocortisone daily versus 37.6 mg daily in the rituximab arm. That represents a 12.3% reduction in steroid exposure for the latter.
Dr. Distler noted that the myositis population represented the bulk of those study patients on steroids. “So in the myositis subanalysis we do see a combination of high-dose steroid plus cyclophosphamide and rituximab.”
Dr. Maher disclosed relationships with Boehringer Ingelheim, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, AstraZeneca, Trevi, CSL Behring, Pliant and Veracyte. Dr. Distler disclosed relationships with numerous pharmaceutical companies.
AT ACR 2022
How AI is, or will soon be, relevant in radiation oncology
Artificial intelligence (AI) is impacting many aspects of health care, and radiation oncology is no exception. It has the potential to cut costs and streamline work flows ranging from image analysis to treatment plan formulation, but its specific place in clinical practice is still being debated.
In a session at the annual meeting of the American Society for Radiation Oncology, researchers discussed some of the ways that AI is or will soon be relevant to the clinic. The general consensus was that
In his talk, Sanjay Aneja, MD focused on practical applications of AI that are in the clinic or close to being ready. One example is image classification. “There has been recent evidence that suggests in a variety of different kind of scenarios, deep-learning models can be very good at image classification in automated ways,” said Dr. Aneja, who is a professor of radiology at Yale University, New Haven, Conn. He described one study that used AI to classify 14 different pathologies on chest x-ray images.
Dr. Aneja described the open-source nnU-net tool, which automatically configures itself and segments biomedical images for research or clinical purposes, including therapy planning support, intraoperative support, and tumor growth monitoring. The researchers who developed it also created a “recipe” to systematize configuration of nnU-net, making it useful as an out-of-the-box tool for image segmentation.
He predicted that AI will improve radiology oncology by assisting in the determination of disease extent, including microscopic areas of disease. It could also help plan treatment volume and monitor treatment response. “I think that these are the types of things that will be moving toward the clinic in the future; very specific applications and models trained on very specific scenarios that will help us answer a very important clinical question,” Dr. Aneja said.
He expects AI to contribute to auto-segmenting and clinical contouring, “but I will caution everyone that these algorithms have not been proven to be better than physician contours. They very frequently fail in the specific use cases when anatomy is distorted by, I don’t know, say a tumor. And so a lot of times, we don’t actually have the ability to just make it an automated process. I think it’ll be something that physicians will use to help them but not necessarily replace their contouring ability,” Dr. Aneja said.
Another, potentially more useful application, is in adaptive radiation planning. “I think that AI auto-contouring will be very helpful in establishing contours in a situation in which a physician doing them would not be feasible. We need to have nimble and computationally efficient auto segmentation algorithms that will be able to be easily deployed at the linear accelerator,” he said.
AI in pathology and treatment selection
In another talk, Osama Mohamad, MD talked about AI in pathology, and specifically treatment selection. He described research from his group that digitized pathology data from 5,500 patients drawn from five randomized, clinical trials. They used AI on data from four of the clinical trials to identify a prognostic biomarker for distant metastasis, then validated it on data from the remaining clinical trial, which compared radiation versus radiation plus short-term hormone therapy in prostate cancer.
The results suggested that most patients should receive hormone therapy, but the AI suggested a more nuanced answer. “Patients who had AI biomarker negative do not see any benefit from adding 4 months of hormone therapy ... whereas patients who have biomarker positive have significant difference and improvement in distant metastasis at 10 years and 15 years. This means that we can save a significant proportion of patients from getting [androgen deprivation therapy], which is hormonal therapy and has very well-known side effects, because they simply they will not benefit,” said Dr. Mohamad, who is an assistant professor of radiation oncology at University of California, San Francisco.
That study relied on the ArteraAI prostate cancer test, which is available through a Clinical Laboratory Improvement Amendment–certified laboratory in Florida.
Another example of AI used to plan treatment is On-line Real-time Benchmarking Informatics Technology for Radiotherapy (ORBIT-RT), developed at the University of California, San Diego. It focuses on radiotherapy treatment plan quality control, and has two main components: creating clinically validated plan routines and a free radiotherapy plan quality control system.
No matter how impressive the technical advances may be, AI contributions won’t impact clinical practice if radiation oncologists, physicians, and patients don’t accept AI. Dr. Aneja’s group surveyed patients about which health field they would feel more comfortable with AI having an important role. Most said they were extremely uncomfortable when it came to cancer. “Now, does that mean that we can’t use AI in oncology? No, I think it just means that we have to be a little bit more nuanced in our approach and how we develop AI solutions for cancer patients,” Dr. Aneja said.
Physicians also show reluctance, according to Alejandro Berlin, MD, who is an affiliate scientist at Princess Margaret Cancer Centre in Toronto. He discussed some research looking at physician acceptance of machine learning. His group looked at physician acceptance of treatment plans for prostate cancer that were generated by physicians and in parallel by machine learning. In a theoretical phase, physicians generally agreed that the machine learning plans were better, but when it came to a phase of the study in which physicians chose which plan to implement in a real patient, the acceptance of machine learning-generated plans dropped by 20%.
This tendency to trust humans over machines is what Dr. Berlin called “automation bias,” and he called for a more collaborative approach to implement AI. “In some cases, [machine learning] is going to be good and sufficient. And in some cases, you will need the expertise of a human.”
Dr. Aneja, who also moderated the session, expressed a similar sentiment when summing up the day’s talks: “I do feel like it’s a disruptive technology ... but I think there will still be a need for us to have people who are trained in order to evaluate and make sure that these algorithms are working correctly and efficiently.”
Dr. Aneja, Dr. Mohamad, and Dr. Berlin have no relevant financial disclosures.
* This article was updated on Nov. 15, 2022.
Artificial intelligence (AI) is impacting many aspects of health care, and radiation oncology is no exception. It has the potential to cut costs and streamline work flows ranging from image analysis to treatment plan formulation, but its specific place in clinical practice is still being debated.
In a session at the annual meeting of the American Society for Radiation Oncology, researchers discussed some of the ways that AI is or will soon be relevant to the clinic. The general consensus was that
In his talk, Sanjay Aneja, MD focused on practical applications of AI that are in the clinic or close to being ready. One example is image classification. “There has been recent evidence that suggests in a variety of different kind of scenarios, deep-learning models can be very good at image classification in automated ways,” said Dr. Aneja, who is a professor of radiology at Yale University, New Haven, Conn. He described one study that used AI to classify 14 different pathologies on chest x-ray images.
Dr. Aneja described the open-source nnU-net tool, which automatically configures itself and segments biomedical images for research or clinical purposes, including therapy planning support, intraoperative support, and tumor growth monitoring. The researchers who developed it also created a “recipe” to systematize configuration of nnU-net, making it useful as an out-of-the-box tool for image segmentation.
He predicted that AI will improve radiology oncology by assisting in the determination of disease extent, including microscopic areas of disease. It could also help plan treatment volume and monitor treatment response. “I think that these are the types of things that will be moving toward the clinic in the future; very specific applications and models trained on very specific scenarios that will help us answer a very important clinical question,” Dr. Aneja said.
He expects AI to contribute to auto-segmenting and clinical contouring, “but I will caution everyone that these algorithms have not been proven to be better than physician contours. They very frequently fail in the specific use cases when anatomy is distorted by, I don’t know, say a tumor. And so a lot of times, we don’t actually have the ability to just make it an automated process. I think it’ll be something that physicians will use to help them but not necessarily replace their contouring ability,” Dr. Aneja said.
Another, potentially more useful application, is in adaptive radiation planning. “I think that AI auto-contouring will be very helpful in establishing contours in a situation in which a physician doing them would not be feasible. We need to have nimble and computationally efficient auto segmentation algorithms that will be able to be easily deployed at the linear accelerator,” he said.
AI in pathology and treatment selection
In another talk, Osama Mohamad, MD talked about AI in pathology, and specifically treatment selection. He described research from his group that digitized pathology data from 5,500 patients drawn from five randomized, clinical trials. They used AI on data from four of the clinical trials to identify a prognostic biomarker for distant metastasis, then validated it on data from the remaining clinical trial, which compared radiation versus radiation plus short-term hormone therapy in prostate cancer.
The results suggested that most patients should receive hormone therapy, but the AI suggested a more nuanced answer. “Patients who had AI biomarker negative do not see any benefit from adding 4 months of hormone therapy ... whereas patients who have biomarker positive have significant difference and improvement in distant metastasis at 10 years and 15 years. This means that we can save a significant proportion of patients from getting [androgen deprivation therapy], which is hormonal therapy and has very well-known side effects, because they simply they will not benefit,” said Dr. Mohamad, who is an assistant professor of radiation oncology at University of California, San Francisco.
That study relied on the ArteraAI prostate cancer test, which is available through a Clinical Laboratory Improvement Amendment–certified laboratory in Florida.
Another example of AI used to plan treatment is On-line Real-time Benchmarking Informatics Technology for Radiotherapy (ORBIT-RT), developed at the University of California, San Diego. It focuses on radiotherapy treatment plan quality control, and has two main components: creating clinically validated plan routines and a free radiotherapy plan quality control system.
No matter how impressive the technical advances may be, AI contributions won’t impact clinical practice if radiation oncologists, physicians, and patients don’t accept AI. Dr. Aneja’s group surveyed patients about which health field they would feel more comfortable with AI having an important role. Most said they were extremely uncomfortable when it came to cancer. “Now, does that mean that we can’t use AI in oncology? No, I think it just means that we have to be a little bit more nuanced in our approach and how we develop AI solutions for cancer patients,” Dr. Aneja said.
Physicians also show reluctance, according to Alejandro Berlin, MD, who is an affiliate scientist at Princess Margaret Cancer Centre in Toronto. He discussed some research looking at physician acceptance of machine learning. His group looked at physician acceptance of treatment plans for prostate cancer that were generated by physicians and in parallel by machine learning. In a theoretical phase, physicians generally agreed that the machine learning plans were better, but when it came to a phase of the study in which physicians chose which plan to implement in a real patient, the acceptance of machine learning-generated plans dropped by 20%.
This tendency to trust humans over machines is what Dr. Berlin called “automation bias,” and he called for a more collaborative approach to implement AI. “In some cases, [machine learning] is going to be good and sufficient. And in some cases, you will need the expertise of a human.”
Dr. Aneja, who also moderated the session, expressed a similar sentiment when summing up the day’s talks: “I do feel like it’s a disruptive technology ... but I think there will still be a need for us to have people who are trained in order to evaluate and make sure that these algorithms are working correctly and efficiently.”
Dr. Aneja, Dr. Mohamad, and Dr. Berlin have no relevant financial disclosures.
* This article was updated on Nov. 15, 2022.
Artificial intelligence (AI) is impacting many aspects of health care, and radiation oncology is no exception. It has the potential to cut costs and streamline work flows ranging from image analysis to treatment plan formulation, but its specific place in clinical practice is still being debated.
In a session at the annual meeting of the American Society for Radiation Oncology, researchers discussed some of the ways that AI is or will soon be relevant to the clinic. The general consensus was that
In his talk, Sanjay Aneja, MD focused on practical applications of AI that are in the clinic or close to being ready. One example is image classification. “There has been recent evidence that suggests in a variety of different kind of scenarios, deep-learning models can be very good at image classification in automated ways,” said Dr. Aneja, who is a professor of radiology at Yale University, New Haven, Conn. He described one study that used AI to classify 14 different pathologies on chest x-ray images.
Dr. Aneja described the open-source nnU-net tool, which automatically configures itself and segments biomedical images for research or clinical purposes, including therapy planning support, intraoperative support, and tumor growth monitoring. The researchers who developed it also created a “recipe” to systematize configuration of nnU-net, making it useful as an out-of-the-box tool for image segmentation.
He predicted that AI will improve radiology oncology by assisting in the determination of disease extent, including microscopic areas of disease. It could also help plan treatment volume and monitor treatment response. “I think that these are the types of things that will be moving toward the clinic in the future; very specific applications and models trained on very specific scenarios that will help us answer a very important clinical question,” Dr. Aneja said.
He expects AI to contribute to auto-segmenting and clinical contouring, “but I will caution everyone that these algorithms have not been proven to be better than physician contours. They very frequently fail in the specific use cases when anatomy is distorted by, I don’t know, say a tumor. And so a lot of times, we don’t actually have the ability to just make it an automated process. I think it’ll be something that physicians will use to help them but not necessarily replace their contouring ability,” Dr. Aneja said.
Another, potentially more useful application, is in adaptive radiation planning. “I think that AI auto-contouring will be very helpful in establishing contours in a situation in which a physician doing them would not be feasible. We need to have nimble and computationally efficient auto segmentation algorithms that will be able to be easily deployed at the linear accelerator,” he said.
AI in pathology and treatment selection
In another talk, Osama Mohamad, MD talked about AI in pathology, and specifically treatment selection. He described research from his group that digitized pathology data from 5,500 patients drawn from five randomized, clinical trials. They used AI on data from four of the clinical trials to identify a prognostic biomarker for distant metastasis, then validated it on data from the remaining clinical trial, which compared radiation versus radiation plus short-term hormone therapy in prostate cancer.
The results suggested that most patients should receive hormone therapy, but the AI suggested a more nuanced answer. “Patients who had AI biomarker negative do not see any benefit from adding 4 months of hormone therapy ... whereas patients who have biomarker positive have significant difference and improvement in distant metastasis at 10 years and 15 years. This means that we can save a significant proportion of patients from getting [androgen deprivation therapy], which is hormonal therapy and has very well-known side effects, because they simply they will not benefit,” said Dr. Mohamad, who is an assistant professor of radiation oncology at University of California, San Francisco.
That study relied on the ArteraAI prostate cancer test, which is available through a Clinical Laboratory Improvement Amendment–certified laboratory in Florida.
Another example of AI used to plan treatment is On-line Real-time Benchmarking Informatics Technology for Radiotherapy (ORBIT-RT), developed at the University of California, San Diego. It focuses on radiotherapy treatment plan quality control, and has two main components: creating clinically validated plan routines and a free radiotherapy plan quality control system.
No matter how impressive the technical advances may be, AI contributions won’t impact clinical practice if radiation oncologists, physicians, and patients don’t accept AI. Dr. Aneja’s group surveyed patients about which health field they would feel more comfortable with AI having an important role. Most said they were extremely uncomfortable when it came to cancer. “Now, does that mean that we can’t use AI in oncology? No, I think it just means that we have to be a little bit more nuanced in our approach and how we develop AI solutions for cancer patients,” Dr. Aneja said.
Physicians also show reluctance, according to Alejandro Berlin, MD, who is an affiliate scientist at Princess Margaret Cancer Centre in Toronto. He discussed some research looking at physician acceptance of machine learning. His group looked at physician acceptance of treatment plans for prostate cancer that were generated by physicians and in parallel by machine learning. In a theoretical phase, physicians generally agreed that the machine learning plans were better, but when it came to a phase of the study in which physicians chose which plan to implement in a real patient, the acceptance of machine learning-generated plans dropped by 20%.
This tendency to trust humans over machines is what Dr. Berlin called “automation bias,” and he called for a more collaborative approach to implement AI. “In some cases, [machine learning] is going to be good and sufficient. And in some cases, you will need the expertise of a human.”
Dr. Aneja, who also moderated the session, expressed a similar sentiment when summing up the day’s talks: “I do feel like it’s a disruptive technology ... but I think there will still be a need for us to have people who are trained in order to evaluate and make sure that these algorithms are working correctly and efficiently.”
Dr. Aneja, Dr. Mohamad, and Dr. Berlin have no relevant financial disclosures.
* This article was updated on Nov. 15, 2022.
FROM ASTRO 2022
Clinical signs differ between children and adults with vasculitis
Researchers have found a link between age of diagnosis and various clinical characteristics and outcomes in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV).
The findings, presented at the annual meeting of the American College of Rheumatology, may have implications for research and treatment, especially in children.
AAV is a group of conditions characterized by the development of autoantibodies to the neutrophil proteins proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA).
The rare autoimmune condition can cause systemic inflammation and damage, sometimes permanent, to small- and medium-sized arteries. Clinical presentations vary and can include several organs, including skin, stomach, intestines, lung, and kidney, as well as airways in ear, nose, and throat.
Data limited on child vs. adult characteristics
AAV can be diagnosed in any decade of life, but clinical characteristics and outcomes often differ between children and adults, and data are limited. Studies often exclude children.
Lead author Jessica Bloom, MD, MSCS, a pediatric rheumatologist and assistant professor of pediatrics at Children’s Hospital Colorado, Aurora, and colleagues performed an analysis of patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) who were enrolled in the Vasculitis Clinical Research Consortium Longitudinal Studies from 2013 to 2021.
Patients with eosinophilic GPA (EGPA) were analyzed separately. Children and young adults with EGPA were combined because of the small sample size (n = 87).
The groups were sorted by the age they were diagnosed: under 18 years old, 18-40, 40-65, and older than 65.
More than 1,000 patients included
Dr. Bloom’s team analyzed data from 1,020 patients: 61 diagnosed as children, 240 as young adults, 560 as middle-aged adults, and 159 diagnosed as older adults. At all ages, about nine out of 10 patients were White.
They found 852 (84%) had GPA and 165 (16%) had MPA. The analysis also showed 893 (92%) of patients with ANCA results were ANCA positive: 637 (65%) with PR3-ANCA, 247 (25%) with MPO-ANCA, and 9 (1%) with both.
Differences between age groups included:
- Children experienced more subglottic stenosis and alveolar hemorrhage than adults with the condition.
- About half of patients diagnosed in childhood received both cyclophosphamide and rituximab. That rate decreased with increasing age of diagnosis to as low as 14% for those diagnosed in older adulthood.
- More females than males in all age groups were diagnosed with AAV, but the difference was most pronounced when diagnosed in childhood, and female predominance declined as age increased.
- Older adults experienced more neurologic disease and less musculoskeletal and sinus involvement.
Additionally, for those diagnosed after age 65, after adjusting for disease length and whether they were taking cyclophosphamide and/or rituximab, the Vasculitis Damage Index (VDI) and ANCA Vasculitis Index of Damage (AVID) scores were higher than for those diagnosed in childhood.
“However, these differences are no longer significant when medication toxicity and comorbidity-related items are excluded. Thus, differences in the VDI and AVID scores are driven by non–disease-specific damage,” Dr. Bloom said.
Bringing children into the clinical discussion
Dr. Bloom said in an interview that
For example, the findings that children have more subglottic stenosis and alveolar hemorrhage than adults “may warrant more aggressive therapy,” she said. Children also have different growth and psychosocial risk factors during their disease course and may live longer with the disease than those in older age groups.
“Our study helps to point out these differences and bring children into the discussion,” Dr. Bloom said. “It also recognizes that damage scores used in studies and care may not adequately assess disease across the lifespan, as they are largely influenced by items not specific to the disease but rather medication toxicity and comorbidities, such as osteoporosis, cataracts, and malignancy.”
Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at Hospital for Special Surgery, New York, said in an interview that the work highlights interesting information about the fact that disease features are skewed differently in children – “in particular the higher likelihood of upper airway [subglottic] disease, and potentially severe lower airway disease [alveolar hemorrhage].”
However, from a practical standpoint, Dr. Spiera said, “I am not sure that this will change our clinical approach to different patients, but the differences in disease features and even the sex differences in terms of who are afflicted with GPA [more often children and more likely to be female] may offer insights into disease pathogenesis.”
Dr. Bloom received funding from the Vasculitis Clinical Research Consortium and Vasculitis Foundation to conduct this work as a VCRC-VF fellow. Several coauthors reported various conflicts of interest with pharmaceutical companies. Dr. Spiera declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have found a link between age of diagnosis and various clinical characteristics and outcomes in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV).
The findings, presented at the annual meeting of the American College of Rheumatology, may have implications for research and treatment, especially in children.
AAV is a group of conditions characterized by the development of autoantibodies to the neutrophil proteins proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA).
The rare autoimmune condition can cause systemic inflammation and damage, sometimes permanent, to small- and medium-sized arteries. Clinical presentations vary and can include several organs, including skin, stomach, intestines, lung, and kidney, as well as airways in ear, nose, and throat.
Data limited on child vs. adult characteristics
AAV can be diagnosed in any decade of life, but clinical characteristics and outcomes often differ between children and adults, and data are limited. Studies often exclude children.
Lead author Jessica Bloom, MD, MSCS, a pediatric rheumatologist and assistant professor of pediatrics at Children’s Hospital Colorado, Aurora, and colleagues performed an analysis of patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) who were enrolled in the Vasculitis Clinical Research Consortium Longitudinal Studies from 2013 to 2021.
Patients with eosinophilic GPA (EGPA) were analyzed separately. Children and young adults with EGPA were combined because of the small sample size (n = 87).
The groups were sorted by the age they were diagnosed: under 18 years old, 18-40, 40-65, and older than 65.
More than 1,000 patients included
Dr. Bloom’s team analyzed data from 1,020 patients: 61 diagnosed as children, 240 as young adults, 560 as middle-aged adults, and 159 diagnosed as older adults. At all ages, about nine out of 10 patients were White.
They found 852 (84%) had GPA and 165 (16%) had MPA. The analysis also showed 893 (92%) of patients with ANCA results were ANCA positive: 637 (65%) with PR3-ANCA, 247 (25%) with MPO-ANCA, and 9 (1%) with both.
Differences between age groups included:
- Children experienced more subglottic stenosis and alveolar hemorrhage than adults with the condition.
- About half of patients diagnosed in childhood received both cyclophosphamide and rituximab. That rate decreased with increasing age of diagnosis to as low as 14% for those diagnosed in older adulthood.
- More females than males in all age groups were diagnosed with AAV, but the difference was most pronounced when diagnosed in childhood, and female predominance declined as age increased.
- Older adults experienced more neurologic disease and less musculoskeletal and sinus involvement.
Additionally, for those diagnosed after age 65, after adjusting for disease length and whether they were taking cyclophosphamide and/or rituximab, the Vasculitis Damage Index (VDI) and ANCA Vasculitis Index of Damage (AVID) scores were higher than for those diagnosed in childhood.
“However, these differences are no longer significant when medication toxicity and comorbidity-related items are excluded. Thus, differences in the VDI and AVID scores are driven by non–disease-specific damage,” Dr. Bloom said.
Bringing children into the clinical discussion
Dr. Bloom said in an interview that
For example, the findings that children have more subglottic stenosis and alveolar hemorrhage than adults “may warrant more aggressive therapy,” she said. Children also have different growth and psychosocial risk factors during their disease course and may live longer with the disease than those in older age groups.
“Our study helps to point out these differences and bring children into the discussion,” Dr. Bloom said. “It also recognizes that damage scores used in studies and care may not adequately assess disease across the lifespan, as they are largely influenced by items not specific to the disease but rather medication toxicity and comorbidities, such as osteoporosis, cataracts, and malignancy.”
Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at Hospital for Special Surgery, New York, said in an interview that the work highlights interesting information about the fact that disease features are skewed differently in children – “in particular the higher likelihood of upper airway [subglottic] disease, and potentially severe lower airway disease [alveolar hemorrhage].”
However, from a practical standpoint, Dr. Spiera said, “I am not sure that this will change our clinical approach to different patients, but the differences in disease features and even the sex differences in terms of who are afflicted with GPA [more often children and more likely to be female] may offer insights into disease pathogenesis.”
Dr. Bloom received funding from the Vasculitis Clinical Research Consortium and Vasculitis Foundation to conduct this work as a VCRC-VF fellow. Several coauthors reported various conflicts of interest with pharmaceutical companies. Dr. Spiera declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have found a link between age of diagnosis and various clinical characteristics and outcomes in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV).
The findings, presented at the annual meeting of the American College of Rheumatology, may have implications for research and treatment, especially in children.
AAV is a group of conditions characterized by the development of autoantibodies to the neutrophil proteins proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA).
The rare autoimmune condition can cause systemic inflammation and damage, sometimes permanent, to small- and medium-sized arteries. Clinical presentations vary and can include several organs, including skin, stomach, intestines, lung, and kidney, as well as airways in ear, nose, and throat.
Data limited on child vs. adult characteristics
AAV can be diagnosed in any decade of life, but clinical characteristics and outcomes often differ between children and adults, and data are limited. Studies often exclude children.
Lead author Jessica Bloom, MD, MSCS, a pediatric rheumatologist and assistant professor of pediatrics at Children’s Hospital Colorado, Aurora, and colleagues performed an analysis of patients with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) who were enrolled in the Vasculitis Clinical Research Consortium Longitudinal Studies from 2013 to 2021.
Patients with eosinophilic GPA (EGPA) were analyzed separately. Children and young adults with EGPA were combined because of the small sample size (n = 87).
The groups were sorted by the age they were diagnosed: under 18 years old, 18-40, 40-65, and older than 65.
More than 1,000 patients included
Dr. Bloom’s team analyzed data from 1,020 patients: 61 diagnosed as children, 240 as young adults, 560 as middle-aged adults, and 159 diagnosed as older adults. At all ages, about nine out of 10 patients were White.
They found 852 (84%) had GPA and 165 (16%) had MPA. The analysis also showed 893 (92%) of patients with ANCA results were ANCA positive: 637 (65%) with PR3-ANCA, 247 (25%) with MPO-ANCA, and 9 (1%) with both.
Differences between age groups included:
- Children experienced more subglottic stenosis and alveolar hemorrhage than adults with the condition.
- About half of patients diagnosed in childhood received both cyclophosphamide and rituximab. That rate decreased with increasing age of diagnosis to as low as 14% for those diagnosed in older adulthood.
- More females than males in all age groups were diagnosed with AAV, but the difference was most pronounced when diagnosed in childhood, and female predominance declined as age increased.
- Older adults experienced more neurologic disease and less musculoskeletal and sinus involvement.
Additionally, for those diagnosed after age 65, after adjusting for disease length and whether they were taking cyclophosphamide and/or rituximab, the Vasculitis Damage Index (VDI) and ANCA Vasculitis Index of Damage (AVID) scores were higher than for those diagnosed in childhood.
“However, these differences are no longer significant when medication toxicity and comorbidity-related items are excluded. Thus, differences in the VDI and AVID scores are driven by non–disease-specific damage,” Dr. Bloom said.
Bringing children into the clinical discussion
Dr. Bloom said in an interview that
For example, the findings that children have more subglottic stenosis and alveolar hemorrhage than adults “may warrant more aggressive therapy,” she said. Children also have different growth and psychosocial risk factors during their disease course and may live longer with the disease than those in older age groups.
“Our study helps to point out these differences and bring children into the discussion,” Dr. Bloom said. “It also recognizes that damage scores used in studies and care may not adequately assess disease across the lifespan, as they are largely influenced by items not specific to the disease but rather medication toxicity and comorbidities, such as osteoporosis, cataracts, and malignancy.”
Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at Hospital for Special Surgery, New York, said in an interview that the work highlights interesting information about the fact that disease features are skewed differently in children – “in particular the higher likelihood of upper airway [subglottic] disease, and potentially severe lower airway disease [alveolar hemorrhage].”
However, from a practical standpoint, Dr. Spiera said, “I am not sure that this will change our clinical approach to different patients, but the differences in disease features and even the sex differences in terms of who are afflicted with GPA [more often children and more likely to be female] may offer insights into disease pathogenesis.”
Dr. Bloom received funding from the Vasculitis Clinical Research Consortium and Vasculitis Foundation to conduct this work as a VCRC-VF fellow. Several coauthors reported various conflicts of interest with pharmaceutical companies. Dr. Spiera declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2022
Chronic stress, especially race related, may hasten cancer death
The American folk hero John Henry pitted his hammer against a mechanical steam drill, only to die of exhaustion after winning the battle. In the legend, John Henry was African American, and it’s a fitting metaphor, according to Justin Xavier Moore, PhD.
It’s a metaphor for accumulated stress over a lifetime, also known as allostatic load. Though it affects everyone, Black, Indigenous, and people of color experience it in excess. “It serves as a symbolism for the plight of African Americans within the United States, that regardless of all the triumph and trying to overcompensate and work just as hard as your counterpart, it oftentimes leads to this overtaxing or exhaustion because your competitor has an unfair advantage. You have Jim Crow laws in the South. We have the history of slavery. We have individuals of racial subgroups that are exposed daily to microaggressions, racial discrimination, stereotypes, redlining, all of these different issues that basically reduce to systemic racism,” said Dr. Moore, who is an assistant professor of medicine at the Medical College of Georgia, Augusta.
Dr. Moore is also a coauthor of a new study published online in SSM–Population Health, which examined the association between increased allostatic load and cancer outcomes among participants in the National Health and Nutrition Examination Survey (NHANES) and the National Death Index. They found that both non-Hispanic Black and non-Hispanic White adults with high allostatic load had about a doubled risk of cancer death.
To determine allostatic load, the researchers looked at nine factors collected in NHANES: abnormal values of BMI, diastolic blood pressure, glycohemoglobin, systolic blood pressure, total cholesterol, serum triglycerides, serum albumin, serum creatinine, and C-reactive protein. “The fact that we’re looking at cardiovascular, metabolic and immune function, all in one gives us a better risk assessment for morbidity and mortality. Allostatic load has actually been associated with cardiovascular disease. I think we are one of the first studies to actually look at whether allostatic load is associated with cancer mortality,” said Dr. Moore.
Previous research coauthored by Dr. Moore showed 20-year old African Americans have an allostatic load comparable with that seen in 30-year-old non-Hispanic Whites. That can lead to a proinflammatory state that might be causing increased cancer risk. But stress isn’t a simple concept to pin down, Dr. Moore said. “One of the founding fathers of public health research and epidemiology, Paracelsus, [said] ‘the dose makes the poison.’ ”
In this case, it means that not all stress is bad. Exercise is good stress. “Your heart rate goes up, you compete, and then it comes back down. That’s healthy. But then there’s those stressful situations like dealing with a horrible job, and a boss that may just be overdemanding. Deadlines, and not having a work-life balance. Too much stress, in this case, can cause cancer death,” Dr. Moore said.
In the study, both non-Hispanic Black adults and non-Hispanic White adults heightened risk of cancer death when dealing with high allostatic load, even though the cause of stress may be different. “It’s almost like the cause of the stress does not matter as much. There are millions of Americans that live in environments that are not conducive to their health. The fact of the matter is that because of racial discrimination, because all these different biases, African Americans may have higher allostatic load, which they did on an average, but high allostatic load for even White people is associated with dying from cancer,” Dr. Moore said.
After adjustment, the (adjusted subdistributed hazard ratio, 1.14; 95% CI, 1.04-1.26). After stratification by age, high allostatic load was associated with an 80% increased risk of cancer death among adults (SHR, 1.80; 95% CI, 1.35-2.41). Non-Hispanic White adults had a 95% increased risk (SHR, 1.95; 95% CI, 1.22-3.12), non-Hispanic Black adults had a twofold increased risk (SHR, 1.06; 95% CI, 1.27-3.34), and Hispanic adults had a 36% increased risk.
Dr. Moore has no relevant financial disclosures.
The American folk hero John Henry pitted his hammer against a mechanical steam drill, only to die of exhaustion after winning the battle. In the legend, John Henry was African American, and it’s a fitting metaphor, according to Justin Xavier Moore, PhD.
It’s a metaphor for accumulated stress over a lifetime, also known as allostatic load. Though it affects everyone, Black, Indigenous, and people of color experience it in excess. “It serves as a symbolism for the plight of African Americans within the United States, that regardless of all the triumph and trying to overcompensate and work just as hard as your counterpart, it oftentimes leads to this overtaxing or exhaustion because your competitor has an unfair advantage. You have Jim Crow laws in the South. We have the history of slavery. We have individuals of racial subgroups that are exposed daily to microaggressions, racial discrimination, stereotypes, redlining, all of these different issues that basically reduce to systemic racism,” said Dr. Moore, who is an assistant professor of medicine at the Medical College of Georgia, Augusta.
Dr. Moore is also a coauthor of a new study published online in SSM–Population Health, which examined the association between increased allostatic load and cancer outcomes among participants in the National Health and Nutrition Examination Survey (NHANES) and the National Death Index. They found that both non-Hispanic Black and non-Hispanic White adults with high allostatic load had about a doubled risk of cancer death.
To determine allostatic load, the researchers looked at nine factors collected in NHANES: abnormal values of BMI, diastolic blood pressure, glycohemoglobin, systolic blood pressure, total cholesterol, serum triglycerides, serum albumin, serum creatinine, and C-reactive protein. “The fact that we’re looking at cardiovascular, metabolic and immune function, all in one gives us a better risk assessment for morbidity and mortality. Allostatic load has actually been associated with cardiovascular disease. I think we are one of the first studies to actually look at whether allostatic load is associated with cancer mortality,” said Dr. Moore.
Previous research coauthored by Dr. Moore showed 20-year old African Americans have an allostatic load comparable with that seen in 30-year-old non-Hispanic Whites. That can lead to a proinflammatory state that might be causing increased cancer risk. But stress isn’t a simple concept to pin down, Dr. Moore said. “One of the founding fathers of public health research and epidemiology, Paracelsus, [said] ‘the dose makes the poison.’ ”
In this case, it means that not all stress is bad. Exercise is good stress. “Your heart rate goes up, you compete, and then it comes back down. That’s healthy. But then there’s those stressful situations like dealing with a horrible job, and a boss that may just be overdemanding. Deadlines, and not having a work-life balance. Too much stress, in this case, can cause cancer death,” Dr. Moore said.
In the study, both non-Hispanic Black adults and non-Hispanic White adults heightened risk of cancer death when dealing with high allostatic load, even though the cause of stress may be different. “It’s almost like the cause of the stress does not matter as much. There are millions of Americans that live in environments that are not conducive to their health. The fact of the matter is that because of racial discrimination, because all these different biases, African Americans may have higher allostatic load, which they did on an average, but high allostatic load for even White people is associated with dying from cancer,” Dr. Moore said.
After adjustment, the (adjusted subdistributed hazard ratio, 1.14; 95% CI, 1.04-1.26). After stratification by age, high allostatic load was associated with an 80% increased risk of cancer death among adults (SHR, 1.80; 95% CI, 1.35-2.41). Non-Hispanic White adults had a 95% increased risk (SHR, 1.95; 95% CI, 1.22-3.12), non-Hispanic Black adults had a twofold increased risk (SHR, 1.06; 95% CI, 1.27-3.34), and Hispanic adults had a 36% increased risk.
Dr. Moore has no relevant financial disclosures.
The American folk hero John Henry pitted his hammer against a mechanical steam drill, only to die of exhaustion after winning the battle. In the legend, John Henry was African American, and it’s a fitting metaphor, according to Justin Xavier Moore, PhD.
It’s a metaphor for accumulated stress over a lifetime, also known as allostatic load. Though it affects everyone, Black, Indigenous, and people of color experience it in excess. “It serves as a symbolism for the plight of African Americans within the United States, that regardless of all the triumph and trying to overcompensate and work just as hard as your counterpart, it oftentimes leads to this overtaxing or exhaustion because your competitor has an unfair advantage. You have Jim Crow laws in the South. We have the history of slavery. We have individuals of racial subgroups that are exposed daily to microaggressions, racial discrimination, stereotypes, redlining, all of these different issues that basically reduce to systemic racism,” said Dr. Moore, who is an assistant professor of medicine at the Medical College of Georgia, Augusta.
Dr. Moore is also a coauthor of a new study published online in SSM–Population Health, which examined the association between increased allostatic load and cancer outcomes among participants in the National Health and Nutrition Examination Survey (NHANES) and the National Death Index. They found that both non-Hispanic Black and non-Hispanic White adults with high allostatic load had about a doubled risk of cancer death.
To determine allostatic load, the researchers looked at nine factors collected in NHANES: abnormal values of BMI, diastolic blood pressure, glycohemoglobin, systolic blood pressure, total cholesterol, serum triglycerides, serum albumin, serum creatinine, and C-reactive protein. “The fact that we’re looking at cardiovascular, metabolic and immune function, all in one gives us a better risk assessment for morbidity and mortality. Allostatic load has actually been associated with cardiovascular disease. I think we are one of the first studies to actually look at whether allostatic load is associated with cancer mortality,” said Dr. Moore.
Previous research coauthored by Dr. Moore showed 20-year old African Americans have an allostatic load comparable with that seen in 30-year-old non-Hispanic Whites. That can lead to a proinflammatory state that might be causing increased cancer risk. But stress isn’t a simple concept to pin down, Dr. Moore said. “One of the founding fathers of public health research and epidemiology, Paracelsus, [said] ‘the dose makes the poison.’ ”
In this case, it means that not all stress is bad. Exercise is good stress. “Your heart rate goes up, you compete, and then it comes back down. That’s healthy. But then there’s those stressful situations like dealing with a horrible job, and a boss that may just be overdemanding. Deadlines, and not having a work-life balance. Too much stress, in this case, can cause cancer death,” Dr. Moore said.
In the study, both non-Hispanic Black adults and non-Hispanic White adults heightened risk of cancer death when dealing with high allostatic load, even though the cause of stress may be different. “It’s almost like the cause of the stress does not matter as much. There are millions of Americans that live in environments that are not conducive to their health. The fact of the matter is that because of racial discrimination, because all these different biases, African Americans may have higher allostatic load, which they did on an average, but high allostatic load for even White people is associated with dying from cancer,” Dr. Moore said.
After adjustment, the (adjusted subdistributed hazard ratio, 1.14; 95% CI, 1.04-1.26). After stratification by age, high allostatic load was associated with an 80% increased risk of cancer death among adults (SHR, 1.80; 95% CI, 1.35-2.41). Non-Hispanic White adults had a 95% increased risk (SHR, 1.95; 95% CI, 1.22-3.12), non-Hispanic Black adults had a twofold increased risk (SHR, 1.06; 95% CI, 1.27-3.34), and Hispanic adults had a 36% increased risk.
Dr. Moore has no relevant financial disclosures.
FROM SSM–POPULATION HEALTH