User login
Safety and Efficacy of GLP-1 Receptor Agonists and SGLT2 Inhibitors Among Veterans With Type 2 Diabetes
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
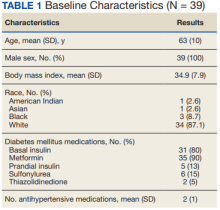
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
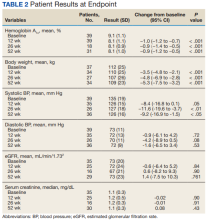
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
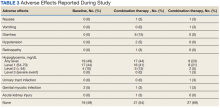
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR). A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
Celiac disease linked to higher risk for rheumatoid arthritis, juvenile idiopathic arthritis
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Sick call
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Higher metal contact allergy rates found in metalworkers
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Optimize HF meds rapidly and fully after hospital discharge: STRONG-HF
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO – Clinicians who prescribe heart failure meds are holding the best hand they’ve ever had, but with so much underuse and suboptimal dosing in actual practice, it seems many may not appreciate the value of their cards. But a major randomized trial that has captured the field’s attention may embolden them to go all in.
Results showed that a strategy of early, rapid up-titration of multiple guideline-directed meds in patients hospitalized with heart failure, compared with a usual-care approach, cut their 6-month risk for death or HF readmission by a steep 34% (P = .002).
The drugs had been started and partly up-titrated in the hospital with the goal of full up-titration within 2 weeks after discharge.
Patients well tolerated the high-intensity approach, researchers said. Their quality-of-life scores improved (P < .0001) compared with the usual-care group, and adverse events were considered few and manageable in the international trial with more than 1,000 patients.
Safety on the high-intensity strategy depended on close patient monitoring at frequently planned clinic visits along with guidance for the up-titrations from clinical signs and natriuretic peptide levels, observed Alexandre Mebazaa, MD, PhD, University of Paris and Public Hospitals of Paris.
Dr. Mebazaa is principal investigator on the trial, called STRONG-HF, which he presented at the American Heart Association scientific sessions, held in Chicago and virtually. He is also lead author on the study’s same-day publication in the Lancet.
The high-intensity strategy’s superiority emerged early in the trial, which was halted early on the data safety monitoring board’s recommendation, with about 90% of follow-ups completed. The board “felt it was unethical to keep patients in usual care,” Dr. Mebazaa said at a press conference.
A dramatic change
The next step, he said, will be to educate the heart failure community on the high-intensity care technique so it can swiftly enter clinical practice. Currently in acute heart failure, “very few patients are monitored after discharge and treated with full doses of heart failure therapies.”
Adoption of the strategy “would be a dramatic change from what’s currently being done,” said Martin B. Leon, MD, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, who moderated the press conference.
Only an estimated 5% of patients with HF in the United States receive full guideline-directed medical therapy, Dr. Leon said, “so the generalizability of this strategy, with careful follow-up that has safety involved in it, is absolutely crucial.”
But the potential impact of this high-intensity approach on resource use is unknown, raising questions about how widely and consistently it could be implemented, said Dr. Leon, who is not connected with STRONG-HF.
The trial called for in-hospital initiation of the three distinct drug classes that, at the time, were the core of guideline-directed HF therapy, with up-titration to 50% of recommended dosage by hospital discharge, and then to 100% within 2 weeks later.
The meds included a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system inhibitor (RASI). The latter could be an ACE inhibitor, angiotensin-receptor blocker (ARB), or angiotensin receptor-neprilysin inhibitor (ARNI).
How about a fourth drug?
Conspicuously absent from the list, for contemporary practice, was an SGLT2 inhibitor, a class that entered the HF guidelines well after STRONG-HF was designed. They would undoubtedly join the other three agents were the high-intensity strategy to enter practice, potentially changing its complexity and safety profile.
But Dr. Mebazaa and other experts don’t see that as a big challenge and would expect a smooth transition to a high-intensity approach that also includes the SGLT2 inhibitors.
STRONG-HF was necessary in part because many clinicians have been “reluctant” to take full advantage of three agents that had been the basis of guideline-directed therapy, he told this news organization.
That reluctance stemmed from concerns that beta-blockers might worsen the heart failure, ACE inhibitors could hurt the kidneys, or MRAs might cause hyperkalemia, Dr. Mebazaa said. The STRONG-HF high-intensity regimen, therefore, demanded multiple clinic visits for close follow-up.
But the SGLT2 inhibitors “are known to be rather safe drugs, at least much safer than the three others,” he said. So, it seems unlikely that their addition to a beta-blocker, RASI, and MRA in patients with HF would worsen the risk of adverse events.
John G.F. Cleland, MD, PhD, agrees. With addition of the fourth agent, “You may need to be a little bit more careful with renal function, just in that first couple of weeks,” he told this news organization. “But I think it would be easy to add an SGLT2 inhibitor into this regimen. And in general, there’s no titration with an SGLT2 inhibitor, so they’ll all be on full dose predischarge.”
Given the drugs’ diuretic-like action, moreover, some patients might be able to pull back on their loop diuretics, speculated Dr. Cleland, from the University of Glasgow’s School of Health and Wellbeing.
The prospect of a high-intensity strategy’s wide implementation in practice presents both “challenges and opportunities,” Amanda R. Vest, MBBS, MPH, Tufts University, Boston, told this news organization.
“There may be additional challenges in terms of ensuring we avoid hypotension or acute kidney injury in the up-titration phase,” said Dr. Vest, who is medical director of her center’s cardiac transplantation program but not connected with STRONG-HF.
“But it also gives us opportunities,” she added, “because there are some patients, especially in that vulnerable postdischarge phase, who are actually much more able to tolerate introduction of an SGLT2 inhibitor than, for example, an ACE inhibitor, ARB, or ARNI – or maybe a beta-blocker if they’ve been in a low cardiac-output state.” Effective dosing would depend on “the personalization and skill of the clinician in optimizing the medications in their correct sequence,” Dr. Vest said.
“It’s challenging to think that we would ever get to 100% up-titration,” she added, “and even in this excellent study, they didn’t get to 100%.” But as clinicians gain experience with the high-intensity strategy, especially as the SGLT2 inhibitors are included, “I think we can reasonably expect more progress to be made in these up-titration skills.”
No restrictions on LVEF
The researchers entered 1,078 patients hospitalized with acute HF in 14 countries across Africa, Europe, the Middle East, and South America, and randomly assigned them to the high-intensity management strategy or usual care.
About 60% of the patients were male and 77% were White. There were no entry restrictions based on left ventricular ejection fraction (LVEF), which exceeded 40% in almost a third of cases.
In the high-intensity care group’s 542 patients, the three agents were up-titrated to 50% of the maximum guideline-recommended dosage prior to hospital discharge, and to 100% within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were monitored closely at four planned clinical visits over the following 6 weeks.
The 536 patients assigned to usual care were discharged and managed according to local standards, with their meds handled by their own primary care doctors or cardiologists, the published report notes. They were reevaluated by STRONG-HF clinicians 90 days after discharge.
The number of clinic visits in the first 90 postdischarge days averaged 4.8 in the high-intensity care group and 1.0 for those receiving usual care. Full up-titration was far more likely in the high-intensity care group: 55% vs. 2% for RASI agents, 49% vs. 4% for beta-blockers, and 84% vs. 46% for MRAs.
They also fared significantly better on all measured parameters associated with decongestion, including weight, prevalence of peripheral edema, jugular venous pressure, NYHA functional class, and natriuretic peptide levels, the researchers said.
The primary endpoint of 180-day death from any cause or HF readmission was met by 15.2% of the high-intensity care group and 23.3% of usual-care patients, for an adjusted risk ratio (RR) of 0.66 (95% CI, 0.50-0.86; P = .0021).
Subgroup analyses saw no significant interactions by age, sex, race, geography, or baseline blood pressure, renal function, or LVEF. Patients with higher vs. lower baseline natriuretic peptide levels trend toward better responses to high-intensity care (P = .08)
The COVID effect
The group performed a sensitivity analysis that excluded deaths attributed to COVID-19 in STRONG-HF, which launched prior to the pandemic. The high-intensity strategy’s benefit for the primary endpoint grew, with an adjusted RR of 0.61 (95% CI, 0.46-0.82; P = .0005). There was no corresponding effect on death from any cause (P = .15).
Treatment-related adverse effects in the overall trial were seen in 41.1% of the high-intensity care group and in 29.5% of those assigned to usual care.
The higher rate in the high-intensity care arm “may be related to their higher number of [clinic] visits compared to usual care,” Dr. Mebazaa said. “However, serious adverse events and fatal adverse events were similar in both arms.”
Cardiac failure was the most common adverse event, developing in about 15% in both groups. It was followed by hypotension, hyperkalemia, and renal impairment, according to the published report.
Dr. Cleland cautioned that the risk of adverse events would potentially be higher should the high-intensity strategy become common clinical practice. The median age in STRONG-HF was 63, which is “10-15 years younger, on average, than the population with recently admitted heart failure that we see. There’s no doubt that older people have more multimorbidity.”
STRONG-HF was funded by Roche Diagnostics. Dr. Mebazaa discloses receiving grants from Roche Diagnostics, Abbott Laboratories, 4TEEN4, and Windtree Therapeutics; honoraria for lectures from Roche Diagnostics, Bayer, and Merck, Sharp & Dohme; and consulting for Corteria Pharmaceuticals, S-form Pharma, FIRE-1, Implicity, 4TEEN4, and Adrenomed; and to being a co-inventor on a patent involving combination therapy for patients having acute or persistent dyspnea.
Dr. Vest reports modest relationships with Boehringer Ingelheim, Corvia, and CareDx; and receiving research grants from the American Heart Association and the National Institutes of Health. Dr. Cleland discloses receiving honoraria from Idorsia; and research grants from Vifor Pharma, Medtronic, Bayer, and Bristol-Myers Squibb. Dr. Leon had no disclosures.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Electrolyte disturbances a harbinger of eating disorders?
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Flu vaccination associated with reduced stroke risk
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
The risk of stroke was about 23% lower in the 6 months following a flu shot, regardless of the patient’s age, sex, or underlying health conditions.
“There is an established link between upper respiratory infection and both heart attack and stroke. This has been very salient in the past few years throughout the COVID-19 pandemic,” study author Jessalyn Holodinsky, PhD, a stroke epidemiologist and postdoctoral fellow in clinical neurosciences at the University of Calgary (Alta.) told this news organization.
“It is also known that the flu shot can reduce risk of heart attack and hospitalization for those with heart disease,” she said. “Given both of these [observations], we thought it prudent to study whether there is a link between vaccination for influenza and stroke.”
The study was published in the Lancet Public Health.
Large effect size
The investigators analyzed administrative data from 2009 through 2018 from the Alberta Health Care Insurance Plan, which covers all residents of Alberta. The province provides free seasonal influenza vaccines to residents under the insurance plan.
The research team looked for stroke events such as acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and transient ischemic attack. They then analyzed the risk of stroke events among those with or without a flu shot in the previous 6 months. They accounted for multiple factors, including age, sex, income, location, and factors related to stroke risk, such as anticoagulant use, atrial fibrillation, chronic obstructive pulmonary disease, diabetes, and hypertension.
Among the 4.1 million adults included in the researchers’ analysis, about 1.8 million (43%) received at least one vaccination during the study period. Nearly 97,000 people received a flu vaccine in each year they were in the study, including 29,288 who received a shot in all 10 flu seasons included in the study.
About 38,000 stroke events were recorded, including about 34,000 (90%) first stroke events. Among the 10% of strokes that were recurrent events, the maximum number of stroke events in one person was nine.
Overall, patients who received at least one influenza vaccine were more likely to be older, be women, and have higher rates of comorbidities. The vaccinated group had a slightly higher proportion of people who lived in urban areas, but the income levels were similar between the vaccinated and unvaccinated groups.
The crude incidence of stroke was higher among people who had ever received an influenza vaccination, at 1.25%, compared with 0.52% among those who hadn’t been vaccinated. However, after adjusting for age, sex, underlying conditions, and socioeconomic status, recent flu vaccination (that is, in the previous 6 months) was associated with a 23% reduced risk of stroke.
The significant reduction in risk applied to all stroke types, particularly acute ischemic stroke and intracerebral hemorrhage. In addition, influenza vaccination was associated with a reduced risk across all ages and risk profiles, except patients without hypertension.
“What we were most surprised by was the sheer magnitude of the effect and that it existed across different adult age groups, for both sexes, and for those with and without risk factors for stroke,” said Dr. Holodinsky.
Vaccination was associated with a larger reduction in stroke risk in men than in women, perhaps because unvaccinated men had a significantly higher baseline risk for stroke than unvaccinated women, the study authors write.
Promoting cardiovascular health
In addition, vaccination was associated with a greater relative reduction in stroke risk in younger age groups, lower income groups, and those with diabetes, chronic obstructive pulmonary disease, and anticoagulant use.
Among 2.4 million people observed for the entire study period, vaccination protection increased with the number of vaccines received. People who were vaccinated serially each year had a significantly lower risk of stroke than those who received one shot.
Dr. Holodinsky and colleagues are conducting additional research into influenza vaccination, including stroke risk in children. They’re also investigating whether the reduced risk applies to other vaccinations for respiratory illnesses, such as COVID-19 and pneumonia.
“We hope that this added effect of vaccination encourages more adults to receive the flu shot,” she said. “One day, vaccinations might be considered a key pillar of cardiovascular health, along with diet, exercise, control of hypertension and high cholesterol, and smoking cessation.”
Future research should also investigate the reasons why adults – particularly people at high risk with underlying conditions – don’t receive recommended influenza vaccines, the study authors wrote.
‘Call to action’
Bahar Behrouzi, an MD-PhD candidate focused on clinical epidemiology at the Institute of Health Policy, Management, and Evaluation, University of Toronto, said: “There are a variety of observational studies around the world that show that flu vaccine uptake is low among the general population and high-risk persons. In studying these questions, our hope is that we can continue to build confidence in viral respiratory vaccines like the influenza vaccine by continuing to generate rigorous evidence with the latest data.”
Ms. Behrouzi, who wasn’t involved with this study, has researched influenza vaccination and cardiovascular risk. She and her colleagues have found that flu vaccines were associated with a 34% lower risk of major adverse cardiovascular events, including a 45% reduced risk among patients with recent acute coronary syndrome.
“The broader public health message is for people to advocate for themselves and get the seasonal flu vaccine, especially if they are part of an at-risk group,” she said. “In our studies, we have positioned this message as a call to action not only for the public, but also for health care professionals – particularly specialists such as cardiologists or neurologists – to encourage or remind them to engage in conversation about the broad benefits of vaccination beyond just preventing or reducing the severity of flu infection.”
The study was conducted without outside funding. Dr. Holodinsky and Ms. Behrouzi have reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM LANCET PUBLIC HEALTH
Give bacterial diversity a chance: The antibiotic dichotomy
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
Don’t let amoxicillin shortage go to waste, antibiotic stewards say
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Some experts are encouraging clinicians to see the amoxicillin shortage through pink-colored glasses.
The ongoing shortage, which was first reported in October and was prompted by a surge in demand linked in part to influenza and respiratory syncytial virus (RSV), could be an opportunity for clinicians to refine their prescribing practices and avoid unnecessary and potentially harmful orders for the medication, they say.
Antibiotics are often prescribed to patients who do not need them. In many cases, patients’ symptoms are caused by viral infections, not bacteria, so antibiotics do not help.
Even when symptoms resolve after a patient takes an antibiotic, the drug may have had nothing to do with their improvement.
Time to double-down on assessment; use antibiotics only when needed,” Jason Gallagher, PharmD, of Temple University School of Pharmacy in Philadelphia, posted on Twitter.
When antibiotics are not helping, they still may cause harm. Treatment with antibiotics entails risks for antibiotic resistance, infection with Clostridioides difficile, and side effects, such as rashes and – as Dr. Gallagher noted – diarrhea.
“They say ‘never let a good shortage go to waste,’ ” Michael Cosimini, MD, a pediatrician at Oregon Health & Science University, Portland, tweeted about the lack of amoxicillin in October.
Dr. Cosimini offered his thoughts about “improving our amoxicillin prescribing patterns” in pediatrics and encouraged colleagues to do so.
For example, he highlighted guidelines that state that antimicrobial therapy is not routinely required for preschool-aged children with community-acquired pneumonia (CAP) because most cases are caused by viral pathogens.
And trials show that when antibiotics are used for CAP, a shorter treatment duration, such as 5 days, rather than the standard 7-10 days, can be sufficient.
“As physicians, a shortage like this is an opportunity to do our best in the short term, as well as reflect on our current practice and make changes for the better in the long run,” Dr. Cosimini told this news organization.
Amoxicillin is the most commonly prescribed antibiotic in the outpatient setting and is the first choice among antimicrobial agents for common infections, such as otitis media, strep throat, and pneumonia, he said. “We use it frequently, so even small changes could go a long way to improve our prescribing practice,” Dr. Cosimini said.
Inappropriate antibiotic prescribing may be common
A 2021 statement on antibiotic stewardship from the American Academy of Pediatrics (AAP) declared that while antibiotics have saved countless lives, they can also cause harm and are frequently used inappropriately.
“One in five pediatric ambulatory visits result in an antibiotic prescription, accounting for nearly 50 million antibiotic prescriptions annually in the United States, at least half of which are considered inappropriate. [Acute respiratory tract infections] account for more than two-thirds of antibiotic prescriptions for children, at least one-third of which are unnecessary,” according to the society.
Outpatient antibiotic stewardship efforts could focus on clinical encounters in which the medications could be avoided altogether, the AAP suggested.
“Examples include antibiotic prescribing for nonspecific upper respiratory infection, bronchiolitis, acute bronchitis, asthma exacerbation, or conjunctivitis,” the group said.
Given the epidemiology of bacterial infections seen in ambulatory care settings that warrant antibiotic therapy, researchers conservatively estimate “that antibiotic prescribing could be safely reduced by 30%,” the statement noted.
That said, treatment decisions are not always clear cut.
“Certain infections in children, such as ear infections and lung infections, can be caused by viruses, bacteria, or both at the same time,” Dr. Cosimini said. “As such, it is very difficult to know which children benefit from which antibiotics.”
Watching, waiting, vaccinating
Pediatricians know that many children with ear infections will get better without antibiotics. “Parents should know that their doctor may suggest watching an ear infection without antibiotics, as is the recommendation from the AAP,” Dr. Cosimini said.
Data indicate that doctors are not following this practice as often as they could be, he said.
When antibiotic treatment is needed during the shortage, agents other than amoxicillin suspension can be used.
“Even though amoxicillin suspension is our go-to antibiotic for many infections, there are effective alternative options,” Dr. Cosimini said. “Children’s Hospital of Philadelphia has a good list for doctors looking for alternatives.”
Another approach to reducing the use of antibiotics in the future involves preventing infections through vaccination.
Research shows that routine childhood vaccines may have averted millions of respiratory and ear infections. And because bacterial infections can follow viral infections, the annual flu vaccine and COVID-19 vaccines “are also great tools to reduce antibiotic use,” Dr. Cosimini said.
A turn to more toxic options?
The shortage of amoxicillin oral powder for suspension was reported by the Food and Drug Administration and the American Society of Health-System Pharmacists (ASHP) in October.
On Nov. 4, the Society of Infectious Diseases Pharmacists (SIDP) issued a statement on the amoxicillin shortage, noting that increased demand for the drug coincided with a surge in respiratory viral infections, including RSV and influenza, among children.
“Though supportive care is the mainstay of treatment for viral infections, antibiotics may be indicated for the treatment of superimposed bacterial infections, including pneumonia and acute otitis media,” the SIDP statement said. “While alternative antibiotics may be available depending on the indication, many have a broader spectrum of activity, increased toxicity, and excess cost relative to amoxicillin. Furthermore, it is anticipated alternatives may soon become in short supply as well, given increased usage.”
SIDP “encourages the judicious use of antibiotics” and supports watch-and-wait strategies and the use of the shortest effective duration of therapy when appropriate.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, monitors around 250 drug shortages at any given time.
The amoxicillin shortage, while not “overly worrisome,” stands out because of how widely the drug is used and the fact that the shortage appears to have been sparked by an increase in demand rather than supply chain or manufacturing quality problems that more typically lead to shortages, he said.
Unlike some other shortages, the amoxicillin shortfall largely does not involve disrupting a medication regimen that someone was already receiving, and substitutions should be available.
“That said, it’s very, very disruptive to parents or a caregiver when you have a sick child who needs an antibiotic and it’s not available,” Dr. Ganio said.
Can a poster change practice?
In an unrelated move, the U.S. Agency for Healthcare Research and Quality published new resources and strategies to reduce inappropriate antibiotic use in ambulatory care settings.
One of the tools is a poster that doctors can print and hang in their offices. It states: “We commit to only prescribing antibiotics when they will help you. Taking antibiotics when you do not need them will NOT make you better. You will still feel sick, and the antibiotic may give you a skin rash, diarrhea, or a yeast infection.”
Jeffrey A. Linder, MD, MPH, a general internist and researcher at Northwestern University in Chicago, helped develop some of the approaches to improve prescribing practices in primary care.
Dr. Linder explained on a recent episode of the Freakonomics, M.D. podcast that the poster can be key.
One reason clinicians may prescribe antibiotics inappropriately is because they assume – perhaps erroneously – that patients want and expect them. By addressing the issue up front by displaying the poster, they may be able to “short-circuit” that type of thinking.
A minority of patients do expect antibiotics. “But the vast majority of patients are thinking, ‘I don’t feel well, I want to know what’s going on, and I want to know how to feel better and what’s going to happen.’ ”
For their part, patients can tell their doctors that they want an antibiotic only if they really need it, Dr. Linder said.
A version of this article first appeared on Medscape.com.
Postpartum posttraumatic stress disorder: An underestimated reality?
PAU, FRANCE – Postpartum posttraumatic stress disorder tends to get worse over the months following the birth of a child. Therefore, it’s necessary to screen for it as early on as possible and to ensure that women who are affected are given the proper treatment. This was the message delivered during the Infogyn 2022 conference by Ludivine Franchitto, MD, a child psychiatrist at Toulouse University Hospital, France. Because postpartum PTSD is still not fully recognized, treatment remains inadequate and poorly documented.
Impact on the caregivers as well
“The situation is the same as what we saw with postpartum depression. The debate went on for 20 years before its existence was formally declared,” Dr. Franchitto noted. But for her, the important thing is not knowing whether a traumatic stress state may be experienced by the mother who had complications during pregnancy or delivery. Instead, it’s about focusing on the repercussions for the child.
During her presentation, Dr. Franchitto also pointed out that it’s necessary to recognize that caregivers who work in maternity wards may also be negatively impacted, as they routinely see the complications that women have during pregnancy and delivery. These workers may also develop a PTSD state, requiring support so that they can properly carry out their duties.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), posttraumatic stress disorder arises after exposure to actual (or threatened) death, serious injury, or sexual violence. Individuals who have witnessed a traumatic event in person or who have experienced repeated (or extreme) exposure to aversive details of traumatic events may also develop PTSD.
Dr. Franchitto mentioned some of the criteria needed to make the diagnosis. “Intrusive distressing memories of the event, recurrent distressing dreams related to the event, persistent avoidance of stimuli associated with the traumatic event, or negative alterations in cognitions and mood associated with the traumatic event. And the duration of the disturbance is more than 1 month.” There may also be marked alterations in arousal and reactivity associated with the traumatic event (for example, irritable behavior, loss of awareness of present surroundings).
Prevalent in 18% of women in high-risk groups
According to the studies, there is a wide variability of PTSD rates. If referring only to traumatic symptoms (for example, depressive syndrome, suicidal ideation, hyperreactivity, and persistent avoidance), the rate could reach up to 40%. A 2016 meta-analysis of 59 studies found that the prevalence of childbirth-related PTSD was 5.9%.
The authors distinguished two groups of women: those without complications during pregnancy or during delivery and those with severe complications related to the pregnancy, a fear of giving birth, a difficult delivery, an emergency C-section, a baby born prematurely with birth defects, etc. Their analysis showed PTSD rates of 4% and 18.5%, respectively.
Surprisingly, the major risk factor for PTSD turned out to be uncontrollable vomiting during pregnancy (seen in 40% of postpartum PTSD cases). The birth of a baby with birth defects was the second risk factor (35%), and the third, a history of violence in the mother’s childhood (34%). Women who experienced depression during the delivery were also at higher risk.
Other risk factors identified were lack of communication with the health care team, lack of consent, lack of support from the medical staff, and a long labor. Conversely, a sense of control and the support of a partner play a protective role.
Early screening
“If the symptoms of posttraumatic stress disorder aren’t treated after delivery, they tend to get worse over the period of 1 to 6 months following the child’s birth,” Dr. Franchitto indicated. This is why it’s necessary to screen for it as early as possible – in particular, by having the women fill out the City Birth Trauma Scale questionnaire – and provide proper treatment accordingly. When seeking to limit the effects of stress, early intervention by a psychologist may be beneficial.
Psychotherapy is the recommended first-line treatment for PTSD, especially cognitive behavioral therapy and Eye Movement Desensitization and Reprocessing therapy. This approach aims to limit the mental and behavioral avoidance that prevents the traumatic memory from being integrated and processed as a regular memory.
The consequences that the mother’s PTSD state has on the child are well documented. “Children whose mothers had PTSD during pregnancy have a lower birth weight and a shorter breast-feeding duration,” Dr. Franchitto reported. With respect to the quality of the mother-child relationship and the long-term development of the child, “the studies have highly conflicting findings.”
At the end of the presentation, Professor Israël Nisand, MD, an ob.gyn. at the American Hospital of Paris and the former president of the National College of French Gynecologists and Obstetricians, made the following comment: “I often think that we underestimate the consequences that the mother’s posttraumatic stress has on the child postpartum.” He added, “Postpartum posttraumatic stress disorder is a reality. Yet it isn’t screened for, let alone treated, even though it has serious consequences for the child.”
Dr. Franchitto also brought up the impact on members of the health care staff, the “second victims” of the traumatic events that occur while caring for the women in the maternity ward. “The estimated prevalence of PTSD symptoms among midwives is 22.9%,” which could lead to “a loss of confidence and a desire to leave the profession.”
Providing psychoeducation to health care staff
Dr. Franchitto believes that it’s essential to also protect caregivers who work in maternity wards. “It’s important to have the support of colleagues” – in particular, of team leaders – “and to share one’s experiences,” as long as one knows how to recognize the symptoms of posttraumatic stress through one’s emotions and is able to verbalize them.
She went on to say that providing psychoeducation to health care staff is therefore to be encouraged, as is “simulation-based training, for learning how to manage problematic situations.”
This content was originally published on Medscape French edition. A translated version appeared on Medscape.com.
PAU, FRANCE – Postpartum posttraumatic stress disorder tends to get worse over the months following the birth of a child. Therefore, it’s necessary to screen for it as early on as possible and to ensure that women who are affected are given the proper treatment. This was the message delivered during the Infogyn 2022 conference by Ludivine Franchitto, MD, a child psychiatrist at Toulouse University Hospital, France. Because postpartum PTSD is still not fully recognized, treatment remains inadequate and poorly documented.
Impact on the caregivers as well
“The situation is the same as what we saw with postpartum depression. The debate went on for 20 years before its existence was formally declared,” Dr. Franchitto noted. But for her, the important thing is not knowing whether a traumatic stress state may be experienced by the mother who had complications during pregnancy or delivery. Instead, it’s about focusing on the repercussions for the child.
During her presentation, Dr. Franchitto also pointed out that it’s necessary to recognize that caregivers who work in maternity wards may also be negatively impacted, as they routinely see the complications that women have during pregnancy and delivery. These workers may also develop a PTSD state, requiring support so that they can properly carry out their duties.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), posttraumatic stress disorder arises after exposure to actual (or threatened) death, serious injury, or sexual violence. Individuals who have witnessed a traumatic event in person or who have experienced repeated (or extreme) exposure to aversive details of traumatic events may also develop PTSD.
Dr. Franchitto mentioned some of the criteria needed to make the diagnosis. “Intrusive distressing memories of the event, recurrent distressing dreams related to the event, persistent avoidance of stimuli associated with the traumatic event, or negative alterations in cognitions and mood associated with the traumatic event. And the duration of the disturbance is more than 1 month.” There may also be marked alterations in arousal and reactivity associated with the traumatic event (for example, irritable behavior, loss of awareness of present surroundings).
Prevalent in 18% of women in high-risk groups
According to the studies, there is a wide variability of PTSD rates. If referring only to traumatic symptoms (for example, depressive syndrome, suicidal ideation, hyperreactivity, and persistent avoidance), the rate could reach up to 40%. A 2016 meta-analysis of 59 studies found that the prevalence of childbirth-related PTSD was 5.9%.
The authors distinguished two groups of women: those without complications during pregnancy or during delivery and those with severe complications related to the pregnancy, a fear of giving birth, a difficult delivery, an emergency C-section, a baby born prematurely with birth defects, etc. Their analysis showed PTSD rates of 4% and 18.5%, respectively.
Surprisingly, the major risk factor for PTSD turned out to be uncontrollable vomiting during pregnancy (seen in 40% of postpartum PTSD cases). The birth of a baby with birth defects was the second risk factor (35%), and the third, a history of violence in the mother’s childhood (34%). Women who experienced depression during the delivery were also at higher risk.
Other risk factors identified were lack of communication with the health care team, lack of consent, lack of support from the medical staff, and a long labor. Conversely, a sense of control and the support of a partner play a protective role.
Early screening
“If the symptoms of posttraumatic stress disorder aren’t treated after delivery, they tend to get worse over the period of 1 to 6 months following the child’s birth,” Dr. Franchitto indicated. This is why it’s necessary to screen for it as early as possible – in particular, by having the women fill out the City Birth Trauma Scale questionnaire – and provide proper treatment accordingly. When seeking to limit the effects of stress, early intervention by a psychologist may be beneficial.
Psychotherapy is the recommended first-line treatment for PTSD, especially cognitive behavioral therapy and Eye Movement Desensitization and Reprocessing therapy. This approach aims to limit the mental and behavioral avoidance that prevents the traumatic memory from being integrated and processed as a regular memory.
The consequences that the mother’s PTSD state has on the child are well documented. “Children whose mothers had PTSD during pregnancy have a lower birth weight and a shorter breast-feeding duration,” Dr. Franchitto reported. With respect to the quality of the mother-child relationship and the long-term development of the child, “the studies have highly conflicting findings.”
At the end of the presentation, Professor Israël Nisand, MD, an ob.gyn. at the American Hospital of Paris and the former president of the National College of French Gynecologists and Obstetricians, made the following comment: “I often think that we underestimate the consequences that the mother’s posttraumatic stress has on the child postpartum.” He added, “Postpartum posttraumatic stress disorder is a reality. Yet it isn’t screened for, let alone treated, even though it has serious consequences for the child.”
Dr. Franchitto also brought up the impact on members of the health care staff, the “second victims” of the traumatic events that occur while caring for the women in the maternity ward. “The estimated prevalence of PTSD symptoms among midwives is 22.9%,” which could lead to “a loss of confidence and a desire to leave the profession.”
Providing psychoeducation to health care staff
Dr. Franchitto believes that it’s essential to also protect caregivers who work in maternity wards. “It’s important to have the support of colleagues” – in particular, of team leaders – “and to share one’s experiences,” as long as one knows how to recognize the symptoms of posttraumatic stress through one’s emotions and is able to verbalize them.
She went on to say that providing psychoeducation to health care staff is therefore to be encouraged, as is “simulation-based training, for learning how to manage problematic situations.”
This content was originally published on Medscape French edition. A translated version appeared on Medscape.com.
PAU, FRANCE – Postpartum posttraumatic stress disorder tends to get worse over the months following the birth of a child. Therefore, it’s necessary to screen for it as early on as possible and to ensure that women who are affected are given the proper treatment. This was the message delivered during the Infogyn 2022 conference by Ludivine Franchitto, MD, a child psychiatrist at Toulouse University Hospital, France. Because postpartum PTSD is still not fully recognized, treatment remains inadequate and poorly documented.
Impact on the caregivers as well
“The situation is the same as what we saw with postpartum depression. The debate went on for 20 years before its existence was formally declared,” Dr. Franchitto noted. But for her, the important thing is not knowing whether a traumatic stress state may be experienced by the mother who had complications during pregnancy or delivery. Instead, it’s about focusing on the repercussions for the child.
During her presentation, Dr. Franchitto also pointed out that it’s necessary to recognize that caregivers who work in maternity wards may also be negatively impacted, as they routinely see the complications that women have during pregnancy and delivery. These workers may also develop a PTSD state, requiring support so that they can properly carry out their duties.
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), posttraumatic stress disorder arises after exposure to actual (or threatened) death, serious injury, or sexual violence. Individuals who have witnessed a traumatic event in person or who have experienced repeated (or extreme) exposure to aversive details of traumatic events may also develop PTSD.
Dr. Franchitto mentioned some of the criteria needed to make the diagnosis. “Intrusive distressing memories of the event, recurrent distressing dreams related to the event, persistent avoidance of stimuli associated with the traumatic event, or negative alterations in cognitions and mood associated with the traumatic event. And the duration of the disturbance is more than 1 month.” There may also be marked alterations in arousal and reactivity associated with the traumatic event (for example, irritable behavior, loss of awareness of present surroundings).
Prevalent in 18% of women in high-risk groups
According to the studies, there is a wide variability of PTSD rates. If referring only to traumatic symptoms (for example, depressive syndrome, suicidal ideation, hyperreactivity, and persistent avoidance), the rate could reach up to 40%. A 2016 meta-analysis of 59 studies found that the prevalence of childbirth-related PTSD was 5.9%.
The authors distinguished two groups of women: those without complications during pregnancy or during delivery and those with severe complications related to the pregnancy, a fear of giving birth, a difficult delivery, an emergency C-section, a baby born prematurely with birth defects, etc. Their analysis showed PTSD rates of 4% and 18.5%, respectively.
Surprisingly, the major risk factor for PTSD turned out to be uncontrollable vomiting during pregnancy (seen in 40% of postpartum PTSD cases). The birth of a baby with birth defects was the second risk factor (35%), and the third, a history of violence in the mother’s childhood (34%). Women who experienced depression during the delivery were also at higher risk.
Other risk factors identified were lack of communication with the health care team, lack of consent, lack of support from the medical staff, and a long labor. Conversely, a sense of control and the support of a partner play a protective role.
Early screening
“If the symptoms of posttraumatic stress disorder aren’t treated after delivery, they tend to get worse over the period of 1 to 6 months following the child’s birth,” Dr. Franchitto indicated. This is why it’s necessary to screen for it as early as possible – in particular, by having the women fill out the City Birth Trauma Scale questionnaire – and provide proper treatment accordingly. When seeking to limit the effects of stress, early intervention by a psychologist may be beneficial.
Psychotherapy is the recommended first-line treatment for PTSD, especially cognitive behavioral therapy and Eye Movement Desensitization and Reprocessing therapy. This approach aims to limit the mental and behavioral avoidance that prevents the traumatic memory from being integrated and processed as a regular memory.
The consequences that the mother’s PTSD state has on the child are well documented. “Children whose mothers had PTSD during pregnancy have a lower birth weight and a shorter breast-feeding duration,” Dr. Franchitto reported. With respect to the quality of the mother-child relationship and the long-term development of the child, “the studies have highly conflicting findings.”
At the end of the presentation, Professor Israël Nisand, MD, an ob.gyn. at the American Hospital of Paris and the former president of the National College of French Gynecologists and Obstetricians, made the following comment: “I often think that we underestimate the consequences that the mother’s posttraumatic stress has on the child postpartum.” He added, “Postpartum posttraumatic stress disorder is a reality. Yet it isn’t screened for, let alone treated, even though it has serious consequences for the child.”
Dr. Franchitto also brought up the impact on members of the health care staff, the “second victims” of the traumatic events that occur while caring for the women in the maternity ward. “The estimated prevalence of PTSD symptoms among midwives is 22.9%,” which could lead to “a loss of confidence and a desire to leave the profession.”
Providing psychoeducation to health care staff
Dr. Franchitto believes that it’s essential to also protect caregivers who work in maternity wards. “It’s important to have the support of colleagues” – in particular, of team leaders – “and to share one’s experiences,” as long as one knows how to recognize the symptoms of posttraumatic stress through one’s emotions and is able to verbalize them.
She went on to say that providing psychoeducation to health care staff is therefore to be encouraged, as is “simulation-based training, for learning how to manage problematic situations.”
This content was originally published on Medscape French edition. A translated version appeared on Medscape.com.