User login
Pooled safety data analysis of tralokinumab reported
The most , according to a review published in the British Journal of Dermatology.
These findings underscore the mechanistic elegance of interleukin (IL)-13 inhibition and highlight potential advantages of flexible dosing, according to the study’s lead author, Eric Simpson, MD, MCR. Overall, the pooled analysis of safety data from five phase 2 and 3 trials shows that “blockade of a single cytokine provides excellent short- and long-term safety, which is useful for a severe chronic disease,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University in Portland.
Most patients with AD require years of treatment. “So for clinicians to confidently report to patients the low rates of serious adverse events (AEs) and lack of immune suppression side-effect profile is very encouraging for both the provider and patient,” Dr. Simpson said, noting there were no new signals or concerning short-term AEs.
Tralokinumab (Adbry), an IL-13 antagonist administered subcutaneously, was approved by the Food and Drug Administration for treatment of moderate to severe AD in adults in December 2021.
Minor differences vs. placebo
In the pooled analysis involving 1,605 patients treated for 16 weeks with tralokinumab and 680 who received placebo, frequency of any AE was 65.7% and 67.2%, respectively. Severe AEs occurred in 4.6% and 6.3% of patients, respectively.
The most common AE overall was AD, which occurred less often in tralokinumab-treated patients (15.4%) than those on placebo (26.2%). Other common AEs that occurred more frequently with tralokinumab included viral upper respiratory tract infections (15.7% vs. 12.2%), upper respiratory tract infections (URTI, 5.6% vs. 4.8%), conjunctivitis (5.4% vs. 1.9%), and injection-site reactions (3.5% vs. 0.3%).
AEs that occurred less often with tralokinumab than placebo included skin infections (3.7% vs. 9.2%, respectively) and infected dermatitis (1.6% vs. 6.4%).
Regarding safety areas of special interest, eye disorders classified as conjunctivitis, keratoconjunctivitis, or keratitis occurred more commonly with tralokinumab (7.9%) than placebo (3.4%). Most eye disorders were mild or moderate and resolved during the study. During maintenance treatment up to 52 weeks, AE rates mirrored those in the initial treatment period and did not increase with treatment duration.
In fact, Dr. Simpson said, the low rate of AEs that are known to accompany type 2 blockade, such as conjunctivitis, do not increase but rather appear to drop with longer-term use. The fact that skin infections were reduced vs. placebo and decreased over time suggests that long-term IL-13 blockade with tralokinumab positively impacts skin infections, a well-known comorbidity in uncontrolled AD, he added.
Raj Chovatiya, MD, PhD, who was asked to comment on the study, said, “These findings provide additional data supporting the safety and tolerability of tralokinumab and support my personal real-world experience with tralokinumab as a safe and effective biologic therapy for patients with moderate to severe AD.”
Dr. Chovatiya is assistant professor, director of the Center for Eczema and Itch, and medical director of clinical trials at Northwestern University in Chicago.
Four-week dosing
Consistent with ECZTRA 3, the rates of URTIs and conjunctivitis were lower with maintenance dosing 300 mg every 4 weeks, consideration of which is approved for responders weighing less than 220 pounds, vs. 300 mg every 2 weeks. Specifically, 6.7% of patients on every 4-week dosing schedule experienced URTIs, vs. 9.4% on the every 2-week dosing schedule and 7% of those on the every 2-week dosing schedule plus optional topical corticosteroids. Corresponding figures for conjunctivitis were 3%, 5%, and 5.6%, respectively.
“Four-week dosing is a possibility in your patients with a good clinical response at 16 weeks,” Dr. Simpson said. Advantages include improved convenience for patients, he added, and this analysis shows that dosing every 4 weeks may improve tolerability, with a lower rate of conjunctivitis.
Although it is difficult to directly compare review data to other studies, said Dr. Chovatiya, findings also suggest that tralokinumab may be associated with reduced infections and conjunctivitis compared with other advanced AD therapies. Head-to-head trials and real-world studies are needed to better understand comparative safety, he added.
Some patients will lose a degree of response with the 4-week dosing schedule, Dr. Simpson said. In ECZTRA 1 and 2, 55.9% of patients who achieved investigator global assessment (IGA) scores of 0 or 1 after 16 weeks of dosing every 2 weeks maintained this response level through week 52, vs. 42.4% of responders who switched from dosing every 2 weeks to every 4 weeks after week 16. But according to data that Dr. Simpson recently presented, 95% of patients switched to monthly dosing who relapsed and returned to dosing every 2 weeks regained their original response level within approximately 4 weeks.
In his personal practice, Dr. Simpson has prescribed tralokinumab for patients with AD for up to a year. However, he and fellow investigators have been following much larger populations for more than 2 years and are planning additional publications. “Safety data will continue to accrue” said Dr. Simpson, “but I don’t expect any surprises.”
The clinical trials were sponsored by MedImmune (phase 2b) and LEO Pharma ( ECZTRA phase 3 trials), which also sponsored the review. Dr. Simpson reports grants and personal fees from numerous pharmaceutical companies. Dr. Chovatiya has been an advisory board member, consultant, investigator, and speaker for numerous pharmaceutical companies including LEO Pharma.
A version of this article first appeared on Medscape.com.
The most , according to a review published in the British Journal of Dermatology.
These findings underscore the mechanistic elegance of interleukin (IL)-13 inhibition and highlight potential advantages of flexible dosing, according to the study’s lead author, Eric Simpson, MD, MCR. Overall, the pooled analysis of safety data from five phase 2 and 3 trials shows that “blockade of a single cytokine provides excellent short- and long-term safety, which is useful for a severe chronic disease,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University in Portland.
Most patients with AD require years of treatment. “So for clinicians to confidently report to patients the low rates of serious adverse events (AEs) and lack of immune suppression side-effect profile is very encouraging for both the provider and patient,” Dr. Simpson said, noting there were no new signals or concerning short-term AEs.
Tralokinumab (Adbry), an IL-13 antagonist administered subcutaneously, was approved by the Food and Drug Administration for treatment of moderate to severe AD in adults in December 2021.
Minor differences vs. placebo
In the pooled analysis involving 1,605 patients treated for 16 weeks with tralokinumab and 680 who received placebo, frequency of any AE was 65.7% and 67.2%, respectively. Severe AEs occurred in 4.6% and 6.3% of patients, respectively.
The most common AE overall was AD, which occurred less often in tralokinumab-treated patients (15.4%) than those on placebo (26.2%). Other common AEs that occurred more frequently with tralokinumab included viral upper respiratory tract infections (15.7% vs. 12.2%), upper respiratory tract infections (URTI, 5.6% vs. 4.8%), conjunctivitis (5.4% vs. 1.9%), and injection-site reactions (3.5% vs. 0.3%).
AEs that occurred less often with tralokinumab than placebo included skin infections (3.7% vs. 9.2%, respectively) and infected dermatitis (1.6% vs. 6.4%).
Regarding safety areas of special interest, eye disorders classified as conjunctivitis, keratoconjunctivitis, or keratitis occurred more commonly with tralokinumab (7.9%) than placebo (3.4%). Most eye disorders were mild or moderate and resolved during the study. During maintenance treatment up to 52 weeks, AE rates mirrored those in the initial treatment period and did not increase with treatment duration.
In fact, Dr. Simpson said, the low rate of AEs that are known to accompany type 2 blockade, such as conjunctivitis, do not increase but rather appear to drop with longer-term use. The fact that skin infections were reduced vs. placebo and decreased over time suggests that long-term IL-13 blockade with tralokinumab positively impacts skin infections, a well-known comorbidity in uncontrolled AD, he added.
Raj Chovatiya, MD, PhD, who was asked to comment on the study, said, “These findings provide additional data supporting the safety and tolerability of tralokinumab and support my personal real-world experience with tralokinumab as a safe and effective biologic therapy for patients with moderate to severe AD.”
Dr. Chovatiya is assistant professor, director of the Center for Eczema and Itch, and medical director of clinical trials at Northwestern University in Chicago.
Four-week dosing
Consistent with ECZTRA 3, the rates of URTIs and conjunctivitis were lower with maintenance dosing 300 mg every 4 weeks, consideration of which is approved for responders weighing less than 220 pounds, vs. 300 mg every 2 weeks. Specifically, 6.7% of patients on every 4-week dosing schedule experienced URTIs, vs. 9.4% on the every 2-week dosing schedule and 7% of those on the every 2-week dosing schedule plus optional topical corticosteroids. Corresponding figures for conjunctivitis were 3%, 5%, and 5.6%, respectively.
“Four-week dosing is a possibility in your patients with a good clinical response at 16 weeks,” Dr. Simpson said. Advantages include improved convenience for patients, he added, and this analysis shows that dosing every 4 weeks may improve tolerability, with a lower rate of conjunctivitis.
Although it is difficult to directly compare review data to other studies, said Dr. Chovatiya, findings also suggest that tralokinumab may be associated with reduced infections and conjunctivitis compared with other advanced AD therapies. Head-to-head trials and real-world studies are needed to better understand comparative safety, he added.
Some patients will lose a degree of response with the 4-week dosing schedule, Dr. Simpson said. In ECZTRA 1 and 2, 55.9% of patients who achieved investigator global assessment (IGA) scores of 0 or 1 after 16 weeks of dosing every 2 weeks maintained this response level through week 52, vs. 42.4% of responders who switched from dosing every 2 weeks to every 4 weeks after week 16. But according to data that Dr. Simpson recently presented, 95% of patients switched to monthly dosing who relapsed and returned to dosing every 2 weeks regained their original response level within approximately 4 weeks.
In his personal practice, Dr. Simpson has prescribed tralokinumab for patients with AD for up to a year. However, he and fellow investigators have been following much larger populations for more than 2 years and are planning additional publications. “Safety data will continue to accrue” said Dr. Simpson, “but I don’t expect any surprises.”
The clinical trials were sponsored by MedImmune (phase 2b) and LEO Pharma ( ECZTRA phase 3 trials), which also sponsored the review. Dr. Simpson reports grants and personal fees from numerous pharmaceutical companies. Dr. Chovatiya has been an advisory board member, consultant, investigator, and speaker for numerous pharmaceutical companies including LEO Pharma.
A version of this article first appeared on Medscape.com.
The most , according to a review published in the British Journal of Dermatology.
These findings underscore the mechanistic elegance of interleukin (IL)-13 inhibition and highlight potential advantages of flexible dosing, according to the study’s lead author, Eric Simpson, MD, MCR. Overall, the pooled analysis of safety data from five phase 2 and 3 trials shows that “blockade of a single cytokine provides excellent short- and long-term safety, which is useful for a severe chronic disease,” said Dr. Simpson, professor of dermatology at Oregon Health & Science University in Portland.
Most patients with AD require years of treatment. “So for clinicians to confidently report to patients the low rates of serious adverse events (AEs) and lack of immune suppression side-effect profile is very encouraging for both the provider and patient,” Dr. Simpson said, noting there were no new signals or concerning short-term AEs.
Tralokinumab (Adbry), an IL-13 antagonist administered subcutaneously, was approved by the Food and Drug Administration for treatment of moderate to severe AD in adults in December 2021.
Minor differences vs. placebo
In the pooled analysis involving 1,605 patients treated for 16 weeks with tralokinumab and 680 who received placebo, frequency of any AE was 65.7% and 67.2%, respectively. Severe AEs occurred in 4.6% and 6.3% of patients, respectively.
The most common AE overall was AD, which occurred less often in tralokinumab-treated patients (15.4%) than those on placebo (26.2%). Other common AEs that occurred more frequently with tralokinumab included viral upper respiratory tract infections (15.7% vs. 12.2%), upper respiratory tract infections (URTI, 5.6% vs. 4.8%), conjunctivitis (5.4% vs. 1.9%), and injection-site reactions (3.5% vs. 0.3%).
AEs that occurred less often with tralokinumab than placebo included skin infections (3.7% vs. 9.2%, respectively) and infected dermatitis (1.6% vs. 6.4%).
Regarding safety areas of special interest, eye disorders classified as conjunctivitis, keratoconjunctivitis, or keratitis occurred more commonly with tralokinumab (7.9%) than placebo (3.4%). Most eye disorders were mild or moderate and resolved during the study. During maintenance treatment up to 52 weeks, AE rates mirrored those in the initial treatment period and did not increase with treatment duration.
In fact, Dr. Simpson said, the low rate of AEs that are known to accompany type 2 blockade, such as conjunctivitis, do not increase but rather appear to drop with longer-term use. The fact that skin infections were reduced vs. placebo and decreased over time suggests that long-term IL-13 blockade with tralokinumab positively impacts skin infections, a well-known comorbidity in uncontrolled AD, he added.
Raj Chovatiya, MD, PhD, who was asked to comment on the study, said, “These findings provide additional data supporting the safety and tolerability of tralokinumab and support my personal real-world experience with tralokinumab as a safe and effective biologic therapy for patients with moderate to severe AD.”
Dr. Chovatiya is assistant professor, director of the Center for Eczema and Itch, and medical director of clinical trials at Northwestern University in Chicago.
Four-week dosing
Consistent with ECZTRA 3, the rates of URTIs and conjunctivitis were lower with maintenance dosing 300 mg every 4 weeks, consideration of which is approved for responders weighing less than 220 pounds, vs. 300 mg every 2 weeks. Specifically, 6.7% of patients on every 4-week dosing schedule experienced URTIs, vs. 9.4% on the every 2-week dosing schedule and 7% of those on the every 2-week dosing schedule plus optional topical corticosteroids. Corresponding figures for conjunctivitis were 3%, 5%, and 5.6%, respectively.
“Four-week dosing is a possibility in your patients with a good clinical response at 16 weeks,” Dr. Simpson said. Advantages include improved convenience for patients, he added, and this analysis shows that dosing every 4 weeks may improve tolerability, with a lower rate of conjunctivitis.
Although it is difficult to directly compare review data to other studies, said Dr. Chovatiya, findings also suggest that tralokinumab may be associated with reduced infections and conjunctivitis compared with other advanced AD therapies. Head-to-head trials and real-world studies are needed to better understand comparative safety, he added.
Some patients will lose a degree of response with the 4-week dosing schedule, Dr. Simpson said. In ECZTRA 1 and 2, 55.9% of patients who achieved investigator global assessment (IGA) scores of 0 or 1 after 16 weeks of dosing every 2 weeks maintained this response level through week 52, vs. 42.4% of responders who switched from dosing every 2 weeks to every 4 weeks after week 16. But according to data that Dr. Simpson recently presented, 95% of patients switched to monthly dosing who relapsed and returned to dosing every 2 weeks regained their original response level within approximately 4 weeks.
In his personal practice, Dr. Simpson has prescribed tralokinumab for patients with AD for up to a year. However, he and fellow investigators have been following much larger populations for more than 2 years and are planning additional publications. “Safety data will continue to accrue” said Dr. Simpson, “but I don’t expect any surprises.”
The clinical trials were sponsored by MedImmune (phase 2b) and LEO Pharma ( ECZTRA phase 3 trials), which also sponsored the review. Dr. Simpson reports grants and personal fees from numerous pharmaceutical companies. Dr. Chovatiya has been an advisory board member, consultant, investigator, and speaker for numerous pharmaceutical companies including LEO Pharma.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Know the right resuscitation for right-sided heart failure
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Does dopamine dysregulation cause schizophrenia?
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
Investigators identified a mechanism on the dopamine receptor, known as the autoreceptor, which regulates how much dopamine is released from the presynaptic neuron. Impairment of this autoreceptor leads to poorly controlled dopamine release and excessive dopamine flow.
The researchers found decreased expression of this autoreceptor accounts for the genetic evidence of schizophrenia risk, and, using a suite of statistical routines, they showed that this relationship is probably causative.
“Our research confirms the scientific hypothesis that too much dopamine plays a likely causative role in psychosis and precisely how this is based on genetic factors,” study investigator Daniel Weinberger, MD, director and CEO of the Lieber Institute for Brain Development, Baltimore, told this news organization.
“Drugs that treat psychosis symptoms by simply blocking dopamine receptors have harsh side effects. ... Theoretically, scientists could now develop therapies that target these malfunctioning autoreceptors to treat this devastating condition with fewer side effects,” he said.
The study was published online in Nature Neuroscience.
‘Privileged spot’
“Large international genetic studies known as genomewide association studies have identified hundreds of regions of the human genome housing potential risk genes for schizophrenia,” Dr. Weinberger said.
“However, these regions are still poorly resolved in terms of specific genes, and treatments and diagnostic techniques are far from what they should be.” Moreover, “treatments for schizophrenia address the symptoms of psychosis but not the cause,” he said.
“For more than 70 years, neuroscientists have suspected that dopamine plays a key role in schizophrenia, but what kind of role, exactly, has remained a mystery,” Dr. Weinberger noted. “It occupied a privileged spot in the principal hypothesis about schizophrenia for over 60 years – the so-called ‘dopamine hypothesis.’ ”
Antipsychotic drugs that reduce dopamine “are the principal medical treatments but they cause serious side effects, including an inability to experience pleasure and joy – a sad reality for patients and their families,” he continued.
The current study “set out to understand how dopamine acts in schizophrenia” using “analysis of the genetic and transcriptional landscape” of the postmortem caudate nucleus from 443 donors (245 neurotypical, 154 with schizophrenia, and 44 with bipolar disorder).
Brain samples were from individuals of diverse ancestry (210 were of African ancestry and 2,233 were of European ancestry).
New treatment target?
The researchers performed an analysis of transancestry expression quantitative trait loci, genetic variants that explain variations in gene expression levels, which express in the caudate, annotating “hundreds of caudate-specific cis-eQTLs.”
Then they integrated this analysis with gene expression that emerged from the latest genomewide association study and transcriptome-wide association study, identifying hundreds of genes that “showed a potential causal association with schizophrenia risk in the caudate nucleus,” including a specific isoform of the dopamine D2 receptor, which is upregulated in the caudate nucleus of those with schizophrenia.
“If autoreceptors don’t function properly the flow of dopamine in the brain is poorly controlled and too much dopamine flows for too long,” said Dr. Weinberger.
In particular, they observed “extensive differential gene expression” for schizophrenia in 2,701 genes in those with schizophrenia, compared with those without: glial cell–derived neurotrophic factor antisense RNA was a top-up gene and tyrosine hydroxylase, which is a rate-limiting enzyme in dopamine synthesis, was a down-regulated gene. Dopamine receptors DRD2 and DRD3 were differentially expressed.
Having done this, they looked at the effects of antipsychotic medications that target D2 regions on gene expression in the caudate by testing for differences between individuals with schizophrenia who were taking antipsychotics at the time of death, those not taking antipsychotics at the time of death (n = 104 and 49, respectively), and neurotypical individuals (n = 239).
There were 2,692 differentially expressed genes between individuals taking antipsychotics versus neurotypical individuals (false discovery rate < 0.05). By contrast, there were only 665 differentially expressed genes (FDR < .05) between those not taking antipsychotics and neurotypical individuals.
“We found that antipsychotic medication has an extensive influence on caudate gene expression,” the investigators noted.
They then developed a new approach to “infer gene networks from expression data.” This method is based on deep neural networks, obtaining a “low-dimensional representation of each gene’s expression across individuals.” The representation is then used to build a “gene neighborhood graph and assign genes to modules.”
This method identified “several modules enriched for genes associated with schizophrenia risk.” The expression representations captured in this approach placed genes in “biologically meaningful neighborhoods, which can provide insight into potential interactions if these genes are targeted for therapeutic intervention,” the authors summarized.
“Now that our new research has identified the specific mechanism by which dopamine plays a causative role in schizophrenia, we hope we have opened the door for more targeted drugs or diagnostic tests that could make life better for patients and their families,” Dr. Weinberger said.
No causal link?
Commenting on the study, Rifaat El-Mallakh, MD, director of the mood disorders research program, department of psychiatry and behavioral sciences, University of Louisville (Ky.), called it an “excellent study performed by an excellent research group” that “fills an important lacuna in our research database.”
However, Dr. El-Mallakh, who was not involved in the research, disagreed that the findings show causality. “The data that can be gleaned from this study is limited and the design has significant limitations. As with all genetic studies, this is an association study. It tells us nothing about the cause-effect relationship between the genes and the illness.
“We do not know why genes are associated with the illness. Genetic overrepresentation can have multiple causes, and more so when the data is a convenience sample. As noted by the authors, much of what they observed was probably related to medication effect. I don’t think this study specifically tells us anything clinically,” he added.
The study was supported by the LIBD, the BrainSeq Consortium, an National Institutes of Health fellowship to two of the authors, and a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to one of the authors. Dr. Weinberger has reported no relevant financial relationships. Dr. El-Mallakh declared no specific financial relationships relevant to the study but has reported being a speaker for several companies that manufacture antipsychotics.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
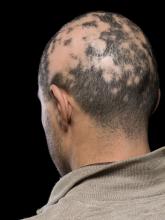
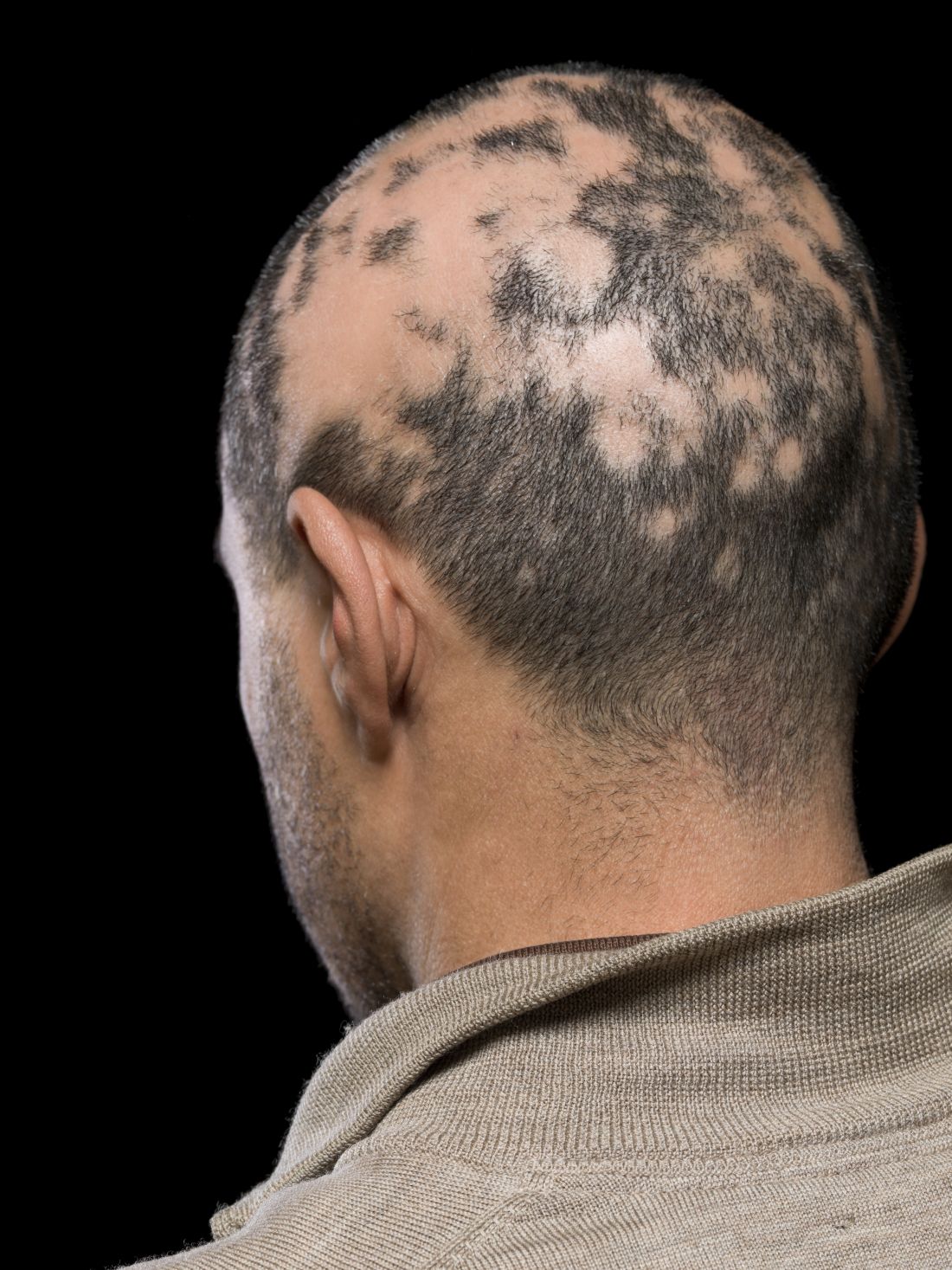
Current alopecia areata options include old and new therapies
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Mind the geriatrician gap
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
These should be the best of times for geriatric medicine.
The baby boom has become a senior surge, bringing in a rapidly growing pool of aging patients for geriatricians to treat. According to the U.S. Census Bureau, more than 56 million adults aged 65 and older live in the United States. They account for about 17% of the nation’s population. That number is expected to hit 73 million by 2030 and 86 million by 2050.
The American Geriatrics Society estimates that 30% of older people require the attention of geriatricians. These clinicians excel in managing complex cases – patients with multiple comorbidities, such as coronary artery disease, dementia, and osteoporosis, who are taking a half dozen, and often more, medications.
. In the 2010s, geriatricians called for “25,000 [such specialists] by 2025.” As of 2021, 7123 certified geriatricians were practicing in the United States, according to the American Board of Medical Specialties.
The Health Resources and Services Administration, a federal agency that addresses medical workforce shortages, estimates that there will be 6,230 geriatricians by 2025, or approximately 1 for every 3,000 older adults requiring geriatric care. HRSA projects a shortage of 27,000 geriatricians by 2025.
The specialty has faced an uphill battle to attract fellows. This year, only 43% of the nation’s 177 geriatrics fellowship slots were filled, according to November’s National Resident Match Program report. Family medicine–based geriatrics achieved only a 32% fill rate, while internal medicine–based programs saw a rate of 45%.
“Our numbers are shrinking so we need another approach to make sure older adults get the care they need and deserve,” said G. Michael Harper, MD, president of the 6,000-member AGS.
But Dr. Harper, who practices at the University of California, San Francisco, and the San Francisco VA Medical Center, added a positive note: “We may be struggling to increase the number of board-certified geriatricians, but the field itself has made a lot of progress in terms of improving clinical care through advancements in science and in the ways we deliver care.”
Dr. Harper cited the Hospital Elder Life Program, a hospital model developed at the Harvard-affiliated Marcus Institute for Aging Research, which uses an interprofessional team and trained volunteers to prevent delirium and functional decline. HELP has been adopted by more than 200 hospitals worldwide and has been successful at returning older adults to their homes or previous living situations with maintained or improved ability to function, he said.
Mark Supiano, MD, professor and chief of geriatrics at the University of Utah, Salt Lake City, said the specialty has been in shortage mode since ABMS recognized it in 1988. He was in the initial cohort of fellowship-trained geriatricians, sitting for the first certifying exam in geriatrics offered that year.
“Back then, the demographic imperative of the aging of our society was on the horizon. We’re living it now. I knew enough to recognize it was coming and saw an opportunity,” Dr. Supiano said in an interview. “There was so much then that we didn’t know about how to understand aging or how to care for older adults that there really was such a knowledge gap.”
Dr. Supiano is an associate editor of Hazzard’s Geriatric Medicine and Gerontology (McGraw-Hill Education), which has more than doubled in pages and word count during his career.
Unfavorable finances
Katherine Thompson, MD, director of the geriatrics fellowship program at the University of Chicago and codirector of UChicago’s Successful Aging and Frailty Evaluation Clinic, said money is a major reason for the struggle. “I think probably the biggest driver is financial,” she said. “A lot of people are graduating medical school with really astronomical amounts of medical school loans.”
Geriatricians, like other doctors, carry a large debt – $200,000, on average, not counting undergraduate debt, according to the Association of American Medical Colleges.
But the typical geriatrician earns less than an internist or family medicine doctor who doesn’t undergo the additional year of training, Dr. Thompson said. “There’s not a lot of financial motivation to do this fellowship,” she said.
The jobs website Zippia reports that geriatricians earned roughly $165,000 per year on average in 2022. The average annual incomes in 2022 were $191,000 for pediatricians, $215,000 for family physicians, and $223,000 for internists, according to the site.
In other words, Dr. Harper said, “geriatrics is one of the few professions where you can actually do additional training and make less money.”
The reason for the pay issue is simple: Geriatricians treat patients covered by Medicare, whose reimbursement schedules lag behind those of commercial insurers. The Kaiser Family Foundation reported in 2020 that private insurance paid 143% of Medicare rates on average for physician services.
Dr. Harper said overall compensation for geriatricians has “not gained a lot of traction,” but they can earn comfortable livings.
Still, representation of the specialty on the American Medical Association’s Relative Value Scale Update Committee has led to approval by the Centers for Medicare & Medicaid Services of billing codes that pay geriatricians “for what they do. Examples include chronic care management, advance care planning, and dementia evaluation,” he said.
But the geriatrician gap goes beyond money.
Ageism, too, may play a role in residents not choosing geriatrics.
“Our culture is ageist. It definitely focuses on youth and looks at aging as being loss rather than just a change in what works well and what doesn’t work well,” said Mary Tinetti, MD, a geriatrician and researcher at Yale University, New Haven, Conn. “Ageism happens among physicians, just because they’re part of the broader society.”
Time for a new goal?
Dr. Tinetti said she’s optimistic that new ideas about geriatricians teaching other primary care clinicians about the tenets of geriatric medicine, which offer a wholistic approach to comorbidities, such as diabetes, atrial fibrillation, dementia, hypertension, hyperlipidemia, and polypharmacy problems faced by this population, especially those 85 and older.
She has called on her profession to abandon the goal of increasing the numbers of board-certified geriatricians – whom she refers to as big “G” geriatricians. She instead wants to develop a “small, elite workforce” that discovers and tests geriatrics principles through research, teaches these principles to all healthcare professions and to the public, and disseminates and implements the policies.
“We need a cadre of geriatricians who train all other clinicians in the care of older adults,” Dr. Tinetti said. “The goal is not more geriatricians but rather the preparation of all clinicians in the care of older adults.”
Dr. Thompson said geriatricians are teaching primary care specialists, nurses, social workers, and other health care providers the principles of age-friendly care. AGS has for the past 20 years led a program called the Geriatrics for Specialists Initiative to increase geriatrics knowledge and expertise of surgical and medical specialists.
Some specialties have taken the cue and have added geriatrics-related hyphens through additional training: geriatric-emergency, geriatric-general surgery, geriatric-hospitalists, and more.
HRSA runs programs to encourage physicians to train as geriatricians and geriatrics faculty, and it encourages the geriatrics interdisciplinary team approach.
Richard Olague, director of public affairs for HRSA, said his agency has invested over $160 million over the past 4 years in the education and training of geriatricians and other health care professionals who care for the elderly through its Geriatrics Workforce Enhancement Program and Geriatrics Academic Career Awards Program. In the academic year 2020-2021, the two programs trained 109 geriatricians; 456 other geriatric/gerontology providers and students; 44,450 other healthcare workforce professionals and students; and served 17,666 patients and 5,409 caregivers.
Dr. Harper, like his fellow geriatricians, tells young doctors that geriatrics is a fulfilling specialty.
“I get to care for the whole person and sometimes their families, too, and in the process form rich and meaningful relationships. And while I’m rarely in the position to cure, I always have the ability to care,” he said. “Sometimes that can mean being an advocate trying to make sure my patients receive the care they need, and other times it might mean protecting them from burdensome care that is unlikely to lead to any meaningful benefit. There is great reward in all of that.”
Dr. Supiano said geriatric patients are being helped by the Age-Friendly Health System initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement in partnership with the American Hospital Association and the Catholic Health Association of the United States. This is sort of a seal of approval for facilities committed to age-friendly care.
“When you go to your hospital, if they don’t have this age-friendly health system banner on the front door ... you either ask why that is not there, or you vote with your feet and go to another health system that is age friendly,” he said. “Geriatricians are eternal optimists.”
A version of this article first appeared on Medscape.com.
Nurses questions answered: Could you face repercussions for your actions?
Nurses are the most trusted profession for one reason. They care.
Nurses are passionate about patient interactions, quality, and giving optimal support, often to the detriment of self-care. Many do not hesitate to voice concerns in an atmosphere that produces anxiety, whether it be regarding supplies, documentation, or staffing. As a result,
In October, three nurses at Ascension Saint Joseph in Joliet, Ill., were escorted off the premises of a hospital emergency room when they began an understaffed shift. They were removed from work by hospital security and then suspended for 1 week. It was a decision that was incomprehensible, because the emergency room faced an overwhelming influx of patients – 46 that evening alone – and only four nurses instead of the more than 10 approved staffing were on duty. Why were they suspended?
Hospital officials have been quiet in responding to their alarmed community as well as in answering the Illinois Nurses Association, who criticized the hospital’s response. It has been suggested that the nurses had been intensely vocal about staffing for several weeks and the hospital might have wanted to silence their voices.
In my opinion, this could be considered a professional repercussion of the post-pandemic work environment. Though the nurses were reinstated after the week expired, nursing organizations believed that the actions of their employer were too harsh.
There was a similar response by employers after a string of large strikes of California nurses earlier this year. For example, a walkout by nurses at Stanford Health Care and Lucile Packard Children’s Hospital resulted in the hospitals withholding wages during the strike period and stating they might withhold health coverage from striking workers.
“Our sincere hope is that an agreement can be reached promptly so that nurses don’t lose additional pay, don’t risk losing the subsidy for employer-paid health benefits, and can return to patient care,” the hospital newsletter StanfordPackardVoice.com reported before the strike began in April. “Nurses who choose to go out on strike will not paid for missed shifts and cannot use PTO, ESL, or Education Hours.”
Nurse: Could a similar repercussion be in my work future?
Nurses may take to picket lines or contact administrators (for example, Human Resources) for multiple reasons, but the most common issues are related to staffing, scheduling, mandatory overtime, or equipment required to do their job: safety or lifting equipment and broken or missing tools for monitoring patients. The lack of hospital security services to assist with violent or threatening patients has also become a concern.
Goodman: The inability to provide safe care is a common fear of all nurses, one that was exacerbated by health care workers leaving during the pandemic, primarily from nursing homes. Although overall safety has improved, that may not be the case in smaller, rural institutions. Staffing for all shifts may also be erratic as the country faces an uphill winter battle with influenza, respiratory syncytial virus, and newer COVID variants.
Report to your supervisor: First, be familiar with your institution’s policy regarding chain of command. Know where to take a complaint when staffing seems unsafe. Contact your immediate supervisor as soon as the situation has been assessed. They might be able to shift resources to your area or find coverage to help. In addition, keep accurate notes related to your actions.
I covered a night shift where I was directly responsible for the care of 13 subacute medical-surgical patients (new admissions and postoperative patients). Patients kept arriving with no regard for the load that was present. One of the patients was completely unhappy with her pain regimen and kept calling for assistance, as is often the case.
While I was doing my best to assess arrivals, another nurse contacted a supervisor. The next thing that happened was an on-site visit by hospital administrators (unusual!) who asked to see my assignment sheet. I had been hesitant to share the list, fearing recrimination from intermediate leadership (this was not my home unit). But it led to an immediate change in staffing. The ordeal ended amicably, but not all do. Thereafter, no nurse was expected to care for more than eight patients on the night shift.
Notify proper authorities: Nurses may believe contacting the Occupational Safety and Health Administration (OSHA) might be helpful; however, OSHA may not have jurisdiction over the hospital, as the Saint Joseph nurses discovered. Working without safety equipment or with reduced supplies (for example, automatic blood pressure cuffs, oxygen saturation monitors, isolation gear) may appear to be a federal complaint, but it depends where the nurse is employed. The hospital in Joliet was covered by the Illinois Department of Public Health.
Federal law entitles you to work in a safe place. Contacting OSHA for direction should not lead to recrimination for nurses. Although OSHA has been overwhelmed with complaints since the onset of the pandemic, their website directs nurses. For example, a whistleblower complaint can be filed up to 30 days after an incident of worker retaliation.
If you are a member of a nursing union, follow union guidelines related to your actions. Thousands of nurses went on strike in the past 2 years. Most remained employed and returned to work with negotiations complete. As far as the nurses in Massachusetts, the state does not have mandatory staffing ratios – most do not – which complicated contract negotiations. At this time, California is the only state that has mandatory nurse-patient ratios written into law.
It is also important to know state law and to be cognizant of nursing organizations within your geographic area. Staying connected means staying informed and having nursing resources.
Respond rationally: An additional reminder for nurses is not to react to a tense situation impulsively. Leaving an assignment unfinished or walking off the job is never a good idea. (A scheduled strike organized by union leaders is different). Leaving work is viewed by institutions as job abandonment and can be grounds for dismissal. Most states are currently “at will” employers, meaning hospitals can terminate nurses without due process.
Above all, know that nurses worry about providing safe practice and avoiding recrimination. Only one of these should be in your future.
Ms. Goodman has no disclosures.
A version of this article first appeared on Medscape.com.
Nurses are the most trusted profession for one reason. They care.
Nurses are passionate about patient interactions, quality, and giving optimal support, often to the detriment of self-care. Many do not hesitate to voice concerns in an atmosphere that produces anxiety, whether it be regarding supplies, documentation, or staffing. As a result,
In October, three nurses at Ascension Saint Joseph in Joliet, Ill., were escorted off the premises of a hospital emergency room when they began an understaffed shift. They were removed from work by hospital security and then suspended for 1 week. It was a decision that was incomprehensible, because the emergency room faced an overwhelming influx of patients – 46 that evening alone – and only four nurses instead of the more than 10 approved staffing were on duty. Why were they suspended?
Hospital officials have been quiet in responding to their alarmed community as well as in answering the Illinois Nurses Association, who criticized the hospital’s response. It has been suggested that the nurses had been intensely vocal about staffing for several weeks and the hospital might have wanted to silence their voices.
In my opinion, this could be considered a professional repercussion of the post-pandemic work environment. Though the nurses were reinstated after the week expired, nursing organizations believed that the actions of their employer were too harsh.
There was a similar response by employers after a string of large strikes of California nurses earlier this year. For example, a walkout by nurses at Stanford Health Care and Lucile Packard Children’s Hospital resulted in the hospitals withholding wages during the strike period and stating they might withhold health coverage from striking workers.
“Our sincere hope is that an agreement can be reached promptly so that nurses don’t lose additional pay, don’t risk losing the subsidy for employer-paid health benefits, and can return to patient care,” the hospital newsletter StanfordPackardVoice.com reported before the strike began in April. “Nurses who choose to go out on strike will not paid for missed shifts and cannot use PTO, ESL, or Education Hours.”
Nurse: Could a similar repercussion be in my work future?
Nurses may take to picket lines or contact administrators (for example, Human Resources) for multiple reasons, but the most common issues are related to staffing, scheduling, mandatory overtime, or equipment required to do their job: safety or lifting equipment and broken or missing tools for monitoring patients. The lack of hospital security services to assist with violent or threatening patients has also become a concern.
Goodman: The inability to provide safe care is a common fear of all nurses, one that was exacerbated by health care workers leaving during the pandemic, primarily from nursing homes. Although overall safety has improved, that may not be the case in smaller, rural institutions. Staffing for all shifts may also be erratic as the country faces an uphill winter battle with influenza, respiratory syncytial virus, and newer COVID variants.
Report to your supervisor: First, be familiar with your institution’s policy regarding chain of command. Know where to take a complaint when staffing seems unsafe. Contact your immediate supervisor as soon as the situation has been assessed. They might be able to shift resources to your area or find coverage to help. In addition, keep accurate notes related to your actions.
I covered a night shift where I was directly responsible for the care of 13 subacute medical-surgical patients (new admissions and postoperative patients). Patients kept arriving with no regard for the load that was present. One of the patients was completely unhappy with her pain regimen and kept calling for assistance, as is often the case.
While I was doing my best to assess arrivals, another nurse contacted a supervisor. The next thing that happened was an on-site visit by hospital administrators (unusual!) who asked to see my assignment sheet. I had been hesitant to share the list, fearing recrimination from intermediate leadership (this was not my home unit). But it led to an immediate change in staffing. The ordeal ended amicably, but not all do. Thereafter, no nurse was expected to care for more than eight patients on the night shift.
Notify proper authorities: Nurses may believe contacting the Occupational Safety and Health Administration (OSHA) might be helpful; however, OSHA may not have jurisdiction over the hospital, as the Saint Joseph nurses discovered. Working without safety equipment or with reduced supplies (for example, automatic blood pressure cuffs, oxygen saturation monitors, isolation gear) may appear to be a federal complaint, but it depends where the nurse is employed. The hospital in Joliet was covered by the Illinois Department of Public Health.
Federal law entitles you to work in a safe place. Contacting OSHA for direction should not lead to recrimination for nurses. Although OSHA has been overwhelmed with complaints since the onset of the pandemic, their website directs nurses. For example, a whistleblower complaint can be filed up to 30 days after an incident of worker retaliation.
If you are a member of a nursing union, follow union guidelines related to your actions. Thousands of nurses went on strike in the past 2 years. Most remained employed and returned to work with negotiations complete. As far as the nurses in Massachusetts, the state does not have mandatory staffing ratios – most do not – which complicated contract negotiations. At this time, California is the only state that has mandatory nurse-patient ratios written into law.
It is also important to know state law and to be cognizant of nursing organizations within your geographic area. Staying connected means staying informed and having nursing resources.
Respond rationally: An additional reminder for nurses is not to react to a tense situation impulsively. Leaving an assignment unfinished or walking off the job is never a good idea. (A scheduled strike organized by union leaders is different). Leaving work is viewed by institutions as job abandonment and can be grounds for dismissal. Most states are currently “at will” employers, meaning hospitals can terminate nurses without due process.
Above all, know that nurses worry about providing safe practice and avoiding recrimination. Only one of these should be in your future.
Ms. Goodman has no disclosures.
A version of this article first appeared on Medscape.com.
Nurses are the most trusted profession for one reason. They care.
Nurses are passionate about patient interactions, quality, and giving optimal support, often to the detriment of self-care. Many do not hesitate to voice concerns in an atmosphere that produces anxiety, whether it be regarding supplies, documentation, or staffing. As a result,
In October, three nurses at Ascension Saint Joseph in Joliet, Ill., were escorted off the premises of a hospital emergency room when they began an understaffed shift. They were removed from work by hospital security and then suspended for 1 week. It was a decision that was incomprehensible, because the emergency room faced an overwhelming influx of patients – 46 that evening alone – and only four nurses instead of the more than 10 approved staffing were on duty. Why were they suspended?
Hospital officials have been quiet in responding to their alarmed community as well as in answering the Illinois Nurses Association, who criticized the hospital’s response. It has been suggested that the nurses had been intensely vocal about staffing for several weeks and the hospital might have wanted to silence their voices.
In my opinion, this could be considered a professional repercussion of the post-pandemic work environment. Though the nurses were reinstated after the week expired, nursing organizations believed that the actions of their employer were too harsh.
There was a similar response by employers after a string of large strikes of California nurses earlier this year. For example, a walkout by nurses at Stanford Health Care and Lucile Packard Children’s Hospital resulted in the hospitals withholding wages during the strike period and stating they might withhold health coverage from striking workers.
“Our sincere hope is that an agreement can be reached promptly so that nurses don’t lose additional pay, don’t risk losing the subsidy for employer-paid health benefits, and can return to patient care,” the hospital newsletter StanfordPackardVoice.com reported before the strike began in April. “Nurses who choose to go out on strike will not paid for missed shifts and cannot use PTO, ESL, or Education Hours.”
Nurse: Could a similar repercussion be in my work future?
Nurses may take to picket lines or contact administrators (for example, Human Resources) for multiple reasons, but the most common issues are related to staffing, scheduling, mandatory overtime, or equipment required to do their job: safety or lifting equipment and broken or missing tools for monitoring patients. The lack of hospital security services to assist with violent or threatening patients has also become a concern.
Goodman: The inability to provide safe care is a common fear of all nurses, one that was exacerbated by health care workers leaving during the pandemic, primarily from nursing homes. Although overall safety has improved, that may not be the case in smaller, rural institutions. Staffing for all shifts may also be erratic as the country faces an uphill winter battle with influenza, respiratory syncytial virus, and newer COVID variants.
Report to your supervisor: First, be familiar with your institution’s policy regarding chain of command. Know where to take a complaint when staffing seems unsafe. Contact your immediate supervisor as soon as the situation has been assessed. They might be able to shift resources to your area or find coverage to help. In addition, keep accurate notes related to your actions.
I covered a night shift where I was directly responsible for the care of 13 subacute medical-surgical patients (new admissions and postoperative patients). Patients kept arriving with no regard for the load that was present. One of the patients was completely unhappy with her pain regimen and kept calling for assistance, as is often the case.
While I was doing my best to assess arrivals, another nurse contacted a supervisor. The next thing that happened was an on-site visit by hospital administrators (unusual!) who asked to see my assignment sheet. I had been hesitant to share the list, fearing recrimination from intermediate leadership (this was not my home unit). But it led to an immediate change in staffing. The ordeal ended amicably, but not all do. Thereafter, no nurse was expected to care for more than eight patients on the night shift.
Notify proper authorities: Nurses may believe contacting the Occupational Safety and Health Administration (OSHA) might be helpful; however, OSHA may not have jurisdiction over the hospital, as the Saint Joseph nurses discovered. Working without safety equipment or with reduced supplies (for example, automatic blood pressure cuffs, oxygen saturation monitors, isolation gear) may appear to be a federal complaint, but it depends where the nurse is employed. The hospital in Joliet was covered by the Illinois Department of Public Health.
Federal law entitles you to work in a safe place. Contacting OSHA for direction should not lead to recrimination for nurses. Although OSHA has been overwhelmed with complaints since the onset of the pandemic, their website directs nurses. For example, a whistleblower complaint can be filed up to 30 days after an incident of worker retaliation.
If you are a member of a nursing union, follow union guidelines related to your actions. Thousands of nurses went on strike in the past 2 years. Most remained employed and returned to work with negotiations complete. As far as the nurses in Massachusetts, the state does not have mandatory staffing ratios – most do not – which complicated contract negotiations. At this time, California is the only state that has mandatory nurse-patient ratios written into law.
It is also important to know state law and to be cognizant of nursing organizations within your geographic area. Staying connected means staying informed and having nursing resources.
Respond rationally: An additional reminder for nurses is not to react to a tense situation impulsively. Leaving an assignment unfinished or walking off the job is never a good idea. (A scheduled strike organized by union leaders is different). Leaving work is viewed by institutions as job abandonment and can be grounds for dismissal. Most states are currently “at will” employers, meaning hospitals can terminate nurses without due process.
Above all, know that nurses worry about providing safe practice and avoiding recrimination. Only one of these should be in your future.
Ms. Goodman has no disclosures.
A version of this article first appeared on Medscape.com.
Saururus chinensis
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
NRS grants target rosacea’s underlying mechanisms
Two new , according to an announcement by the NRS.
As part of the NRS research grants program, the organization recently awarded $10,000 to Emanual Maverakis, MD, professor of dermatology, University of California, Davis, and research fellow Samantha Herbert, MSPH. Their project will characterize rosacea pathophysiology using single-cell RNA sequencing. This novel analytical technique provides specific information on the signals expressed by different cell types and will help researchers better understand the role each subtype may play in rosacea, along with how these cells interact with each other, according to the NRS press release. New knowledge in the foregoing areas may fuel development of better therapies, the release added.
The NRS awarded its second new-research grant to Arisa Ortiz, MD, director of laser and cosmetic dermatology and associate professor of dermatology, University of California, San Diego. She was awarded $5,000 to examine whether laser therapy affects the skin microbiome, the complex ecosystem of bacteria and other microorganisms that reside on the skin. Studies have detected significant differences – such as higher levels of Demodex folliculorum and Staphylococcus epidermidis and lower levels of Cutibacterium acnes – in the microbiome of skin with rosacea compared with healthy skin. Dr. Ortiz’s research also will probe how blood vessels, which laser therapy often target, contribute to the rosacea disease process.
The NRS also renewed its support of an ongoing study led by Sezen Karakus, MD, assistant professor of ophthalmology at the Johns Hopkins Wilmer Eye Institute, Baltimore. She is studying the role of the ocular-surface microbiome in rosacea pathogenesis. Because ocular rosacea can lead to vision-threatening corneal complications, Dr. Karakus said in the press release, identifying microorganisms present on the ocular surface may spur development of targeted treatment strategies.
A second ongoing study for which the NRS renewed funding is investigating whether certain intracellular signals recently found to be elevated in rosacea lesions may drive skin inflammation, which may be a root cause of rosacea. Emmanuel Contassot, PhD, project leader in the dermatology department at the University Hospital of Basel, Switzerland, is leading the study.
To date, the NRS research grants program has awarded more than $1.6 million to research designed to further elucidate potential causes and other key aspects of rosacea with the goal of advancing treatment, prevention, or potential cure of rosacea.
For interested researchers, the deadline to submit proposals for next year’s grants is June 16, 2023. Forms and instructions are available through the research grants section of the NRS website or by contacting the NRS at 4619 N. Ravenswood Ave., Suite 103, Chicago, IL 60640; 888-662-5874; or [email protected].
Two new , according to an announcement by the NRS.
As part of the NRS research grants program, the organization recently awarded $10,000 to Emanual Maverakis, MD, professor of dermatology, University of California, Davis, and research fellow Samantha Herbert, MSPH. Their project will characterize rosacea pathophysiology using single-cell RNA sequencing. This novel analytical technique provides specific information on the signals expressed by different cell types and will help researchers better understand the role each subtype may play in rosacea, along with how these cells interact with each other, according to the NRS press release. New knowledge in the foregoing areas may fuel development of better therapies, the release added.
The NRS awarded its second new-research grant to Arisa Ortiz, MD, director of laser and cosmetic dermatology and associate professor of dermatology, University of California, San Diego. She was awarded $5,000 to examine whether laser therapy affects the skin microbiome, the complex ecosystem of bacteria and other microorganisms that reside on the skin. Studies have detected significant differences – such as higher levels of Demodex folliculorum and Staphylococcus epidermidis and lower levels of Cutibacterium acnes – in the microbiome of skin with rosacea compared with healthy skin. Dr. Ortiz’s research also will probe how blood vessels, which laser therapy often target, contribute to the rosacea disease process.
The NRS also renewed its support of an ongoing study led by Sezen Karakus, MD, assistant professor of ophthalmology at the Johns Hopkins Wilmer Eye Institute, Baltimore. She is studying the role of the ocular-surface microbiome in rosacea pathogenesis. Because ocular rosacea can lead to vision-threatening corneal complications, Dr. Karakus said in the press release, identifying microorganisms present on the ocular surface may spur development of targeted treatment strategies.
A second ongoing study for which the NRS renewed funding is investigating whether certain intracellular signals recently found to be elevated in rosacea lesions may drive skin inflammation, which may be a root cause of rosacea. Emmanuel Contassot, PhD, project leader in the dermatology department at the University Hospital of Basel, Switzerland, is leading the study.
To date, the NRS research grants program has awarded more than $1.6 million to research designed to further elucidate potential causes and other key aspects of rosacea with the goal of advancing treatment, prevention, or potential cure of rosacea.
For interested researchers, the deadline to submit proposals for next year’s grants is June 16, 2023. Forms and instructions are available through the research grants section of the NRS website or by contacting the NRS at 4619 N. Ravenswood Ave., Suite 103, Chicago, IL 60640; 888-662-5874; or [email protected].
Two new , according to an announcement by the NRS.
As part of the NRS research grants program, the organization recently awarded $10,000 to Emanual Maverakis, MD, professor of dermatology, University of California, Davis, and research fellow Samantha Herbert, MSPH. Their project will characterize rosacea pathophysiology using single-cell RNA sequencing. This novel analytical technique provides specific information on the signals expressed by different cell types and will help researchers better understand the role each subtype may play in rosacea, along with how these cells interact with each other, according to the NRS press release. New knowledge in the foregoing areas may fuel development of better therapies, the release added.
The NRS awarded its second new-research grant to Arisa Ortiz, MD, director of laser and cosmetic dermatology and associate professor of dermatology, University of California, San Diego. She was awarded $5,000 to examine whether laser therapy affects the skin microbiome, the complex ecosystem of bacteria and other microorganisms that reside on the skin. Studies have detected significant differences – such as higher levels of Demodex folliculorum and Staphylococcus epidermidis and lower levels of Cutibacterium acnes – in the microbiome of skin with rosacea compared with healthy skin. Dr. Ortiz’s research also will probe how blood vessels, which laser therapy often target, contribute to the rosacea disease process.
The NRS also renewed its support of an ongoing study led by Sezen Karakus, MD, assistant professor of ophthalmology at the Johns Hopkins Wilmer Eye Institute, Baltimore. She is studying the role of the ocular-surface microbiome in rosacea pathogenesis. Because ocular rosacea can lead to vision-threatening corneal complications, Dr. Karakus said in the press release, identifying microorganisms present on the ocular surface may spur development of targeted treatment strategies.
A second ongoing study for which the NRS renewed funding is investigating whether certain intracellular signals recently found to be elevated in rosacea lesions may drive skin inflammation, which may be a root cause of rosacea. Emmanuel Contassot, PhD, project leader in the dermatology department at the University Hospital of Basel, Switzerland, is leading the study.
To date, the NRS research grants program has awarded more than $1.6 million to research designed to further elucidate potential causes and other key aspects of rosacea with the goal of advancing treatment, prevention, or potential cure of rosacea.
For interested researchers, the deadline to submit proposals for next year’s grants is June 16, 2023. Forms and instructions are available through the research grants section of the NRS website or by contacting the NRS at 4619 N. Ravenswood Ave., Suite 103, Chicago, IL 60640; 888-662-5874; or [email protected].
New Year’s resolutions
I can’t presume to know what issues need addressing in your practice, but I do know the ones I get asked about most often, so I can offer some suggestions that might provide inspiration:
1. Keep your website up to date. Check it now, then make a note to check it regularly. Most people find their physicians online these days, and you don’t want them finding a year-old presentation with outdated photos, personnel, services, and rates. Keep your site current, or hire someone to do it for you.
2. Be an authoritative presence on social media. Like it or not, you should be on Facebook, Twitter (at least for now), Instagram, TikTok – wherever your patients congregate. Medical topics are popular search categories, and they are searching for expert advice. You are the expert. There is a ton of medical misinformation online, and it needs to be countered with accurate, factual data from bona fide experts.
3. Follow colleagues. No need to reinvent the wheel; many physicians have already developed large online followings. Track some of them down, follow them yourself, and use them as inspiration for your own online contributions. Your specialty society probably maintains a presence on Instagram and other sites as well, and they are a good source of topics and tips.
4. Post frequently. We all have a finite amount of time, but a few brief posts per week on various social media platforms will attract more attention, and garner more followers than an occasional long treatise. Add relevant hashtags to get more reach and engagement.
5. Participate in trends. When a topic is getting thousands of views, it a trending topic. Post on trending topics, and if you know the trend’s original authors, tag them. That will increase your audience, and the compliment might be reciprocated in the future.
6. Google yourself. You might be surprised by what you find. Being aware of what is being said about you online is a necessary exercise to maintain a healthy online reputation. The good reviews are ego builders, but it’s the bad reviews that you can learn from. They will help you identify your negative personality traits and motivate you to eliminate them.
7. Encrypt your mobile devices. The biggest HIPAA vulnerability in many practices is laptops and tablets carrying confidential patient information; losing one could be a disaster. Encryption software is cheap and readily available, and a lost or stolen mobile device will probably not be treated as a HIPAA breach if it is properly encrypted.
8. Back up your data. Now is an excellent time to verify that the information on your office and personal computers is being backed up – locally and online – on a regular schedule. Don’t wait until something crashes.
9. Keep a closer eye on your office finances. Most physicians delegate the bookkeeping, and that’s fine. But ignoring the financial side completely creates an atmosphere that facilitates embezzlement. Set aside a couple of hours each month to review the books personally. And make sure your employees know you’re doing it.
10. Make sure your long-range financial planning is on track. I’ve said this before, but it can’t be repeated too often. Economic conditions change all the time. Once a year, you should sit down with your accountant and lawyer and make sure your investments are well-diversified and all other aspects of your finances – budgets, credit ratings, insurance coverage, tax situations, college savings, estate plans, retirement accounts – are in the best shape possible.
11. Pay down your debt. Another oldie but goodie. Debt can destroy the best laid retirement plans. If you carry significant debt, set up a plan to pay it off as soon as you can.
12. Take more vacations. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.” If you’ve been working too much, this is the year to start spending more time enjoying your life, your friends and family, and the world. As John Lennon said, “Life is what happens to you while you’re busy making other plans.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I can’t presume to know what issues need addressing in your practice, but I do know the ones I get asked about most often, so I can offer some suggestions that might provide inspiration:
1. Keep your website up to date. Check it now, then make a note to check it regularly. Most people find their physicians online these days, and you don’t want them finding a year-old presentation with outdated photos, personnel, services, and rates. Keep your site current, or hire someone to do it for you.
2. Be an authoritative presence on social media. Like it or not, you should be on Facebook, Twitter (at least for now), Instagram, TikTok – wherever your patients congregate. Medical topics are popular search categories, and they are searching for expert advice. You are the expert. There is a ton of medical misinformation online, and it needs to be countered with accurate, factual data from bona fide experts.
3. Follow colleagues. No need to reinvent the wheel; many physicians have already developed large online followings. Track some of them down, follow them yourself, and use them as inspiration for your own online contributions. Your specialty society probably maintains a presence on Instagram and other sites as well, and they are a good source of topics and tips.
4. Post frequently. We all have a finite amount of time, but a few brief posts per week on various social media platforms will attract more attention, and garner more followers than an occasional long treatise. Add relevant hashtags to get more reach and engagement.
5. Participate in trends. When a topic is getting thousands of views, it a trending topic. Post on trending topics, and if you know the trend’s original authors, tag them. That will increase your audience, and the compliment might be reciprocated in the future.
6. Google yourself. You might be surprised by what you find. Being aware of what is being said about you online is a necessary exercise to maintain a healthy online reputation. The good reviews are ego builders, but it’s the bad reviews that you can learn from. They will help you identify your negative personality traits and motivate you to eliminate them.
7. Encrypt your mobile devices. The biggest HIPAA vulnerability in many practices is laptops and tablets carrying confidential patient information; losing one could be a disaster. Encryption software is cheap and readily available, and a lost or stolen mobile device will probably not be treated as a HIPAA breach if it is properly encrypted.
8. Back up your data. Now is an excellent time to verify that the information on your office and personal computers is being backed up – locally and online – on a regular schedule. Don’t wait until something crashes.
9. Keep a closer eye on your office finances. Most physicians delegate the bookkeeping, and that’s fine. But ignoring the financial side completely creates an atmosphere that facilitates embezzlement. Set aside a couple of hours each month to review the books personally. And make sure your employees know you’re doing it.
10. Make sure your long-range financial planning is on track. I’ve said this before, but it can’t be repeated too often. Economic conditions change all the time. Once a year, you should sit down with your accountant and lawyer and make sure your investments are well-diversified and all other aspects of your finances – budgets, credit ratings, insurance coverage, tax situations, college savings, estate plans, retirement accounts – are in the best shape possible.
11. Pay down your debt. Another oldie but goodie. Debt can destroy the best laid retirement plans. If you carry significant debt, set up a plan to pay it off as soon as you can.
12. Take more vacations. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.” If you’ve been working too much, this is the year to start spending more time enjoying your life, your friends and family, and the world. As John Lennon said, “Life is what happens to you while you’re busy making other plans.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I can’t presume to know what issues need addressing in your practice, but I do know the ones I get asked about most often, so I can offer some suggestions that might provide inspiration:
1. Keep your website up to date. Check it now, then make a note to check it regularly. Most people find their physicians online these days, and you don’t want them finding a year-old presentation with outdated photos, personnel, services, and rates. Keep your site current, or hire someone to do it for you.
2. Be an authoritative presence on social media. Like it or not, you should be on Facebook, Twitter (at least for now), Instagram, TikTok – wherever your patients congregate. Medical topics are popular search categories, and they are searching for expert advice. You are the expert. There is a ton of medical misinformation online, and it needs to be countered with accurate, factual data from bona fide experts.
3. Follow colleagues. No need to reinvent the wheel; many physicians have already developed large online followings. Track some of them down, follow them yourself, and use them as inspiration for your own online contributions. Your specialty society probably maintains a presence on Instagram and other sites as well, and they are a good source of topics and tips.
4. Post frequently. We all have a finite amount of time, but a few brief posts per week on various social media platforms will attract more attention, and garner more followers than an occasional long treatise. Add relevant hashtags to get more reach and engagement.
5. Participate in trends. When a topic is getting thousands of views, it a trending topic. Post on trending topics, and if you know the trend’s original authors, tag them. That will increase your audience, and the compliment might be reciprocated in the future.
6. Google yourself. You might be surprised by what you find. Being aware of what is being said about you online is a necessary exercise to maintain a healthy online reputation. The good reviews are ego builders, but it’s the bad reviews that you can learn from. They will help you identify your negative personality traits and motivate you to eliminate them.
7. Encrypt your mobile devices. The biggest HIPAA vulnerability in many practices is laptops and tablets carrying confidential patient information; losing one could be a disaster. Encryption software is cheap and readily available, and a lost or stolen mobile device will probably not be treated as a HIPAA breach if it is properly encrypted.
8. Back up your data. Now is an excellent time to verify that the information on your office and personal computers is being backed up – locally and online – on a regular schedule. Don’t wait until something crashes.
9. Keep a closer eye on your office finances. Most physicians delegate the bookkeeping, and that’s fine. But ignoring the financial side completely creates an atmosphere that facilitates embezzlement. Set aside a couple of hours each month to review the books personally. And make sure your employees know you’re doing it.
10. Make sure your long-range financial planning is on track. I’ve said this before, but it can’t be repeated too often. Economic conditions change all the time. Once a year, you should sit down with your accountant and lawyer and make sure your investments are well-diversified and all other aspects of your finances – budgets, credit ratings, insurance coverage, tax situations, college savings, estate plans, retirement accounts – are in the best shape possible.
11. Pay down your debt. Another oldie but goodie. Debt can destroy the best laid retirement plans. If you carry significant debt, set up a plan to pay it off as soon as you can.
12. Take more vacations. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.” If you’ve been working too much, this is the year to start spending more time enjoying your life, your friends and family, and the world. As John Lennon said, “Life is what happens to you while you’re busy making other plans.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].