User login
Clinical factors drive hospitalization after self-harm
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
Clinicians who assess suicidal patients in the emergency department setting face the challenge of whether to admit the patient to inpatient or outpatient care, and data on predictors of compulsory admission are limited, wrote Laurent Michaud, MD, of the University of Lausanne, Switzerland, and colleagues.
To better identify predictors of hospitalization after self-harm, the researchers reviewed data from 1,832 patients aged 18 years and older admitted to four emergency departments in Switzerland between December 2016 and November 2019 .
Self-harm (SH) was defined in this study as “all nonfatal intentional acts of self-poisoning or self-injury, irrespective of degree of suicidal intent or other types of motivation,” the researchers noted. The study included 2,142 episodes of self-harm.
The researchers conducted two analyses. They compared episodes followed by any hospitalization and those with outpatient follow-up (1,083 episodes vs. 1,059 episodes) and episodes followed by compulsory hospitalization (357 episodes) with all other episodes followed by either outpatient care or voluntary hospitalization (1,785 episodes).
Overall, women were significantly more likely to be referred to outpatient follow-up compared with men (61.8% vs. 38.1%), and hospitalized patients were significantly older than outpatients (mean age of 41 years vs. 36 years, P < .001 for both).
“Not surprisingly, major psychopathological conditions such as depression, mania, dementia, and schizophrenia were predictive of hospitalization,” the researchers noted.
Other sociodemographic factors associated with hospitalization included living alone, no children, problematic socioeconomic status, and unemployment. Clinical factors associated with hospitalization included physical pain, more lethal suicide attempt method, and clear intent to die.
In a multivariate analysis, independent predictors of any hospitalization included male gender, older age, assessment in the Neuchatel location vs. Lausanne, depression vs. personality disorders, substance use, or anxiety disorder, difficult socioeconomic status, a clear vs. unclear intent to die, and a serious suicide attempt vs. less serious.
Differences in hospitalization based on hospital setting was a striking finding, the researchers wrote in their discussion. These differences may be largely explained by the organization of local mental health services and specific institutional cultures; the workload of staff and availability of beds also may have played a role in decisions to hospitalize, they said.
The findings were limited by several factors including the lack of data on the realization level of a self-harm episode and significant events such as a breakup, the researchers explained. Other limitations included missing data, multiple analyses that could increase the risk of false positives, the reliance on clinical diagnosis rather than formal instruments, and the cross-sectional study design, they said.
However, the results have clinical implications, as the clinical factors identified could be used to target subgroups of suicidal populations and refine treatment strategies, they concluded.
The study was supported by institutional funding and the Swiss Federal Office of Public Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRIC RESEARCH
Stroke management: A 30-year retrospective
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
FDA tweaks Impella indications on basis of postapproval study
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
GOLD Report 2023: Important updates and revisions
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report is revised annually and is used widely throughout the world as a tool for implementing effective management.
Among the updates in the 2023 GOLD Report, the section on diagnostic criteria added a proposed new category “PRISm,” denoting “preserved ratio impaired spirometry,” encompassing individuals who present with structural lung lesions (for example, emphysema) and/or other physiological abnormalities such as low-normal forced expiratory volume in 1 second (FEV1), gas trapping, hyperinflation, reduced lung diffusing capacity and/or rapid FEV1 decline, but without airflow obstruction (FEV1/FEV ≥ 0.7 post bronchodilation). Some of these “pre-COPD” (chronic obstructive pulmonary disease) individuals, who have a normal ratio but abnormal spirometry are at risk over time of developing airflow obstruction. The best treatment for them, beyond smoking cessation, needs to be determined through research, the report states.
Clinical updates
The GOLD 2023 Report also offers proposed clinical guidance, in the absence of high-quality clinical trial evidence, on initial pharmacologic management of COPD. The proposal is based on individual assessment of symptoms and exacerbation risk following use of the ABE Assessment Tool, a revised version of the ABCD Assessment Tool that recognizes the clinical relevance of exacerbations independent of symptom level.
These updates to information and figures pertaining to initial pharmacological treatment and follow-up pharmacological treatment revise the positioning of LABA (long-acting beta2 agonists) plus LAMA (long-acting muscarinic agonists) and LABA/ICS (inhaled corticosteroids). Among GOLD group A patients with 0 or 1 moderate exacerbations that do not lead to hospital admission, a bronchodilator is recommended.
The recommendation for group B patients is LABA/LAMA with the caveat that single inhaler therapy may be more convenient and effective than multiple inhalers. For group E patients with two or more moderate exacerbations or one or more leading to hospitalization, LABA/LAMA is recommended (with the same inhaler therapy caveat). With blood eosinophil levels at 300 or higher, LABA/LAMA/ICS may be considered.
Commenting on the combination recommendations in a press release, Antonio Anzueto, MD, professor of medicine, pulmonary critical care, University of Texas Health, San Antonio, stated: “From a physician’s perspective, we are always grateful to receive well-vetted and informed recommendations on how we can best utilize available treatment options to provide the most benefit to our patients. The new 2023 GOLD recommendations represent a meaningful change for the treatment of COPD by prioritizing the utilization of a fixed LAMA/LABA combination.”
More interventions
In a section on therapeutic interventions to reduce COPD mortality, the report lists studies showing mortality benefits for fixed-dose inhaled triple combinations (LABA + LAMA + ICS) versus dual inhaled long-acting bronchodilations, and for smoking cessation and pulmonary rehabilitation.
Also new is a strong emphasis on inhaler choice, education, and technique training with assessment of inhaler technique and adherence urged as a prerequisite to judging whether current therapy as insufficient. The report summarizes principles guiding inhaler type selection.
The report also added a section on chronic bronchitis, defining it as a common but variable condition in COPD patients with cough and expectorated sputum on a regular basis over a defined period in the absence of other conditions plausibly causing symptoms.
The fact that chronic bronchitis is sometimes found in never-smokers suggests the involvement of other factors such as exposure to inhaled dusts, biomass fuels, chemical fumes, or domestic heating and cooking fuels, according to the report. Gastroesophageal reflux may also be associated with chronic bronchitis.
The report discusses various taxonomic terms for different types of COPD, such as COPD-G for genetically determined COPD, COPD-D for those with abnormal lung development, and COPD-C for COPD associated with cigarette smoking, etc.
Change in exacerbations
The report also revises the definition of a COPD exacerbation as “an event characterized by increased dyspnea and/or cough and sputum that worsens in less than 14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and system inflammation caused by infection, pollution, or other insult to the airways.” To overcome limitations conferred by the current grading of COPD exacerbations, the 2023 report proposes a four-step point-of-contact diagnosis and assessment tool.
Telemedicine
Given the constraints brought on by COVID-19 on top of the generally sparse availability of programs and facilities for delivering well-proven pulmonary rehabilitation methods, tele-rehabilitation has been proposed as an alternative to traditional approaches. While the evidence base is still evolving and best practices have not yet been established, the GOLD Report calls for better understanding of barriers to tele-rehabilitation success.
Comorbidities update
The GOLD Report chapter on COPD and comorbidities was also updated, and lists cardiovascular disease, lung cancer, osteoporosis, depression/anxiety, and gastroesophageal reflux disease as common comorbid conditions which may affect prognosis and, in the case of cancer, mortality. The report urges simplicity of treatment to minimize polypharmacy. While annual low-dose CT is recommended for COPD caused by smoking, it is not recommended for COPD caused by smoking; data are insufficient to establish benefit over harm.
While the GOLD Report “COVID-19 and COPD” chapter summarizes current evidence stating that individuals with COPD do not seem to be at substantially greater risk of infection with SARS-CoV-2, it underscores that they are at higher risk of hospitalization for COVID-19 and may be at higher risk for developing severe disease and death.
Many other topics are included in the updated report, among them screening, imaging, vaccinations, adherence to therapy, and surgical and bronchoscopic interventions. In its closing section, the GOLD Report 2023 reiterates its mission, stating: “The GOLD initiative will continue to work with National Leaders and other interested health care professionals to bring COPD to the attention of governments, public health officials, health care workers, and the general public, to raise awareness of the burden of COPD and to develop programs for early detection, prevention and approaches to management.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report is revised annually and is used widely throughout the world as a tool for implementing effective management.
Among the updates in the 2023 GOLD Report, the section on diagnostic criteria added a proposed new category “PRISm,” denoting “preserved ratio impaired spirometry,” encompassing individuals who present with structural lung lesions (for example, emphysema) and/or other physiological abnormalities such as low-normal forced expiratory volume in 1 second (FEV1), gas trapping, hyperinflation, reduced lung diffusing capacity and/or rapid FEV1 decline, but without airflow obstruction (FEV1/FEV ≥ 0.7 post bronchodilation). Some of these “pre-COPD” (chronic obstructive pulmonary disease) individuals, who have a normal ratio but abnormal spirometry are at risk over time of developing airflow obstruction. The best treatment for them, beyond smoking cessation, needs to be determined through research, the report states.
Clinical updates
The GOLD 2023 Report also offers proposed clinical guidance, in the absence of high-quality clinical trial evidence, on initial pharmacologic management of COPD. The proposal is based on individual assessment of symptoms and exacerbation risk following use of the ABE Assessment Tool, a revised version of the ABCD Assessment Tool that recognizes the clinical relevance of exacerbations independent of symptom level.
These updates to information and figures pertaining to initial pharmacological treatment and follow-up pharmacological treatment revise the positioning of LABA (long-acting beta2 agonists) plus LAMA (long-acting muscarinic agonists) and LABA/ICS (inhaled corticosteroids). Among GOLD group A patients with 0 or 1 moderate exacerbations that do not lead to hospital admission, a bronchodilator is recommended.
The recommendation for group B patients is LABA/LAMA with the caveat that single inhaler therapy may be more convenient and effective than multiple inhalers. For group E patients with two or more moderate exacerbations or one or more leading to hospitalization, LABA/LAMA is recommended (with the same inhaler therapy caveat). With blood eosinophil levels at 300 or higher, LABA/LAMA/ICS may be considered.
Commenting on the combination recommendations in a press release, Antonio Anzueto, MD, professor of medicine, pulmonary critical care, University of Texas Health, San Antonio, stated: “From a physician’s perspective, we are always grateful to receive well-vetted and informed recommendations on how we can best utilize available treatment options to provide the most benefit to our patients. The new 2023 GOLD recommendations represent a meaningful change for the treatment of COPD by prioritizing the utilization of a fixed LAMA/LABA combination.”
More interventions
In a section on therapeutic interventions to reduce COPD mortality, the report lists studies showing mortality benefits for fixed-dose inhaled triple combinations (LABA + LAMA + ICS) versus dual inhaled long-acting bronchodilations, and for smoking cessation and pulmonary rehabilitation.
Also new is a strong emphasis on inhaler choice, education, and technique training with assessment of inhaler technique and adherence urged as a prerequisite to judging whether current therapy as insufficient. The report summarizes principles guiding inhaler type selection.
The report also added a section on chronic bronchitis, defining it as a common but variable condition in COPD patients with cough and expectorated sputum on a regular basis over a defined period in the absence of other conditions plausibly causing symptoms.
The fact that chronic bronchitis is sometimes found in never-smokers suggests the involvement of other factors such as exposure to inhaled dusts, biomass fuels, chemical fumes, or domestic heating and cooking fuels, according to the report. Gastroesophageal reflux may also be associated with chronic bronchitis.
The report discusses various taxonomic terms for different types of COPD, such as COPD-G for genetically determined COPD, COPD-D for those with abnormal lung development, and COPD-C for COPD associated with cigarette smoking, etc.
Change in exacerbations
The report also revises the definition of a COPD exacerbation as “an event characterized by increased dyspnea and/or cough and sputum that worsens in less than 14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and system inflammation caused by infection, pollution, or other insult to the airways.” To overcome limitations conferred by the current grading of COPD exacerbations, the 2023 report proposes a four-step point-of-contact diagnosis and assessment tool.
Telemedicine
Given the constraints brought on by COVID-19 on top of the generally sparse availability of programs and facilities for delivering well-proven pulmonary rehabilitation methods, tele-rehabilitation has been proposed as an alternative to traditional approaches. While the evidence base is still evolving and best practices have not yet been established, the GOLD Report calls for better understanding of barriers to tele-rehabilitation success.
Comorbidities update
The GOLD Report chapter on COPD and comorbidities was also updated, and lists cardiovascular disease, lung cancer, osteoporosis, depression/anxiety, and gastroesophageal reflux disease as common comorbid conditions which may affect prognosis and, in the case of cancer, mortality. The report urges simplicity of treatment to minimize polypharmacy. While annual low-dose CT is recommended for COPD caused by smoking, it is not recommended for COPD caused by smoking; data are insufficient to establish benefit over harm.
While the GOLD Report “COVID-19 and COPD” chapter summarizes current evidence stating that individuals with COPD do not seem to be at substantially greater risk of infection with SARS-CoV-2, it underscores that they are at higher risk of hospitalization for COVID-19 and may be at higher risk for developing severe disease and death.
Many other topics are included in the updated report, among them screening, imaging, vaccinations, adherence to therapy, and surgical and bronchoscopic interventions. In its closing section, the GOLD Report 2023 reiterates its mission, stating: “The GOLD initiative will continue to work with National Leaders and other interested health care professionals to bring COPD to the attention of governments, public health officials, health care workers, and the general public, to raise awareness of the burden of COPD and to develop programs for early detection, prevention and approaches to management.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report is revised annually and is used widely throughout the world as a tool for implementing effective management.
Among the updates in the 2023 GOLD Report, the section on diagnostic criteria added a proposed new category “PRISm,” denoting “preserved ratio impaired spirometry,” encompassing individuals who present with structural lung lesions (for example, emphysema) and/or other physiological abnormalities such as low-normal forced expiratory volume in 1 second (FEV1), gas trapping, hyperinflation, reduced lung diffusing capacity and/or rapid FEV1 decline, but without airflow obstruction (FEV1/FEV ≥ 0.7 post bronchodilation). Some of these “pre-COPD” (chronic obstructive pulmonary disease) individuals, who have a normal ratio but abnormal spirometry are at risk over time of developing airflow obstruction. The best treatment for them, beyond smoking cessation, needs to be determined through research, the report states.
Clinical updates
The GOLD 2023 Report also offers proposed clinical guidance, in the absence of high-quality clinical trial evidence, on initial pharmacologic management of COPD. The proposal is based on individual assessment of symptoms and exacerbation risk following use of the ABE Assessment Tool, a revised version of the ABCD Assessment Tool that recognizes the clinical relevance of exacerbations independent of symptom level.
These updates to information and figures pertaining to initial pharmacological treatment and follow-up pharmacological treatment revise the positioning of LABA (long-acting beta2 agonists) plus LAMA (long-acting muscarinic agonists) and LABA/ICS (inhaled corticosteroids). Among GOLD group A patients with 0 or 1 moderate exacerbations that do not lead to hospital admission, a bronchodilator is recommended.
The recommendation for group B patients is LABA/LAMA with the caveat that single inhaler therapy may be more convenient and effective than multiple inhalers. For group E patients with two or more moderate exacerbations or one or more leading to hospitalization, LABA/LAMA is recommended (with the same inhaler therapy caveat). With blood eosinophil levels at 300 or higher, LABA/LAMA/ICS may be considered.
Commenting on the combination recommendations in a press release, Antonio Anzueto, MD, professor of medicine, pulmonary critical care, University of Texas Health, San Antonio, stated: “From a physician’s perspective, we are always grateful to receive well-vetted and informed recommendations on how we can best utilize available treatment options to provide the most benefit to our patients. The new 2023 GOLD recommendations represent a meaningful change for the treatment of COPD by prioritizing the utilization of a fixed LAMA/LABA combination.”
More interventions
In a section on therapeutic interventions to reduce COPD mortality, the report lists studies showing mortality benefits for fixed-dose inhaled triple combinations (LABA + LAMA + ICS) versus dual inhaled long-acting bronchodilations, and for smoking cessation and pulmonary rehabilitation.
Also new is a strong emphasis on inhaler choice, education, and technique training with assessment of inhaler technique and adherence urged as a prerequisite to judging whether current therapy as insufficient. The report summarizes principles guiding inhaler type selection.
The report also added a section on chronic bronchitis, defining it as a common but variable condition in COPD patients with cough and expectorated sputum on a regular basis over a defined period in the absence of other conditions plausibly causing symptoms.
The fact that chronic bronchitis is sometimes found in never-smokers suggests the involvement of other factors such as exposure to inhaled dusts, biomass fuels, chemical fumes, or domestic heating and cooking fuels, according to the report. Gastroesophageal reflux may also be associated with chronic bronchitis.
The report discusses various taxonomic terms for different types of COPD, such as COPD-G for genetically determined COPD, COPD-D for those with abnormal lung development, and COPD-C for COPD associated with cigarette smoking, etc.
Change in exacerbations
The report also revises the definition of a COPD exacerbation as “an event characterized by increased dyspnea and/or cough and sputum that worsens in less than 14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and system inflammation caused by infection, pollution, or other insult to the airways.” To overcome limitations conferred by the current grading of COPD exacerbations, the 2023 report proposes a four-step point-of-contact diagnosis and assessment tool.
Telemedicine
Given the constraints brought on by COVID-19 on top of the generally sparse availability of programs and facilities for delivering well-proven pulmonary rehabilitation methods, tele-rehabilitation has been proposed as an alternative to traditional approaches. While the evidence base is still evolving and best practices have not yet been established, the GOLD Report calls for better understanding of barriers to tele-rehabilitation success.
Comorbidities update
The GOLD Report chapter on COPD and comorbidities was also updated, and lists cardiovascular disease, lung cancer, osteoporosis, depression/anxiety, and gastroesophageal reflux disease as common comorbid conditions which may affect prognosis and, in the case of cancer, mortality. The report urges simplicity of treatment to minimize polypharmacy. While annual low-dose CT is recommended for COPD caused by smoking, it is not recommended for COPD caused by smoking; data are insufficient to establish benefit over harm.
While the GOLD Report “COVID-19 and COPD” chapter summarizes current evidence stating that individuals with COPD do not seem to be at substantially greater risk of infection with SARS-CoV-2, it underscores that they are at higher risk of hospitalization for COVID-19 and may be at higher risk for developing severe disease and death.
Many other topics are included in the updated report, among them screening, imaging, vaccinations, adherence to therapy, and surgical and bronchoscopic interventions. In its closing section, the GOLD Report 2023 reiterates its mission, stating: “The GOLD initiative will continue to work with National Leaders and other interested health care professionals to bring COPD to the attention of governments, public health officials, health care workers, and the general public, to raise awareness of the burden of COPD and to develop programs for early detection, prevention and approaches to management.
Eliminating the language of blame in lung cancer
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Ask knee OA patients about stair climbing difficulty
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
FROM ACR 2022
Commentary: Prevention in AD, December 2022
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
We are in the golden age of atopic dermatitis (AD) drug development. We are fortunate to have numerous topicals, oral systemics, and biologics recently approved or in late-stage clinical development. Yet, we are still lacking effective strategies for primary prevention of incident AD and secondary prevention of AD exacerbations.
Kottner and colleagues published the results from the ADAPI study of 150 infants who were at an enhanced risk for AD. The children were randomly assigned to receive either a skincare regimen that was standardized or unstandardized skincare preferred by parents. They found that in the first year of life, the overall cumulative incidence rate of AD was similar between standardized skincare and skincare preferred by parents (P = .999).
Bradshaw and colleagues also published results from the BEEP study (a 5-year prospective study) of 1394 infants who were at high risk for AD. The children were randomly assigned to receive either emollient for the first year plus standard skincare or standard skincare alone. They found a similar proportion of children were clinically diagnosed with AD between 12 and 60 months in the emollient plus skincare group vs skincare alone group (31% vs 28%; adjusted relative risk 1.10; 95% CI 0.93-1.30). Unfortunately, the results from both studies are consistent with earlier results from BEEP, as well as other studies, and did not show that early application of emollients successfully prevent AD.
The use of applying emollients for primary prevention is unclear. However, proactive application of topical corticosteroids (TCS) and other topical nonsteroidal agents is well accepted in AD treatment guidelines for secondary prevention of AD exacerbations.1 Although, a recent study from Kamiya and colleagues suggested that proactive application of topical corticosteroids may not work as well as we think. They conducted an open-label, active-controlled, parallel-group study of 49 pediatric patients with moderate to severe AD who achieved remission with potent TCS. The children were then randomly assigned to receive proactive therapy with or discontinuation of TCS. The authors found no significant decrease in relapse rates with proactive vs no proactive treatment groups (8.33% vs 20.0%; P = .0859). I don't think these results will change our guidelines. But I do think these results raise important questions about the myriad aspects of proactive therapy that require appropriate counseling, including frequency of application per week (1-3 times), choice of therapies (corticosteroid or nonsteroidal agent), additional emollient use, bathing practice, etc. I personally would strongly recommend use of proactive therapy in clinical practice, but these results highlight that it is not a magic bullet for all patients either.
Additional Reference
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10-22.e2. Doi: 10.1016/j.anai.2017.10.039
Vaccination cuts long COVID risk for rheumatic disease patients
Patients with rheumatic disease are at least half as likely to develop long COVID after a SARS-CoV-2 infection if they have been fully vaccinated against COVID-19, according to research published in Annals of the Rheumatic Diseases (2022 Nov 28. doi: 10.1136/ard-2022-223439).
“Moreover, those who were vaccinated prior to getting COVID-19 had less pain and fatigue after their infection,” Zachary S. Wallace, MD, MSc, an assistant professor of medicine at Harvard Medical School, Boston, and a study author, said in an interview. “These findings reinforce the importance of vaccination in this population.”
Messaging around the value of COVID vaccination has been confusing for some with rheumatic disease “because our concern regarding a blunted response to vaccination has led many patients to think that they do not provide much benefit if they are on immunosuppression,” Dr. Wallace said. “In our cohort, which included many patients on immunosuppression of varying degrees, being vaccinated was quite beneficial.”
Leonard H. Calabrese, DO, director of the R.J. Fasenmyer Center for Clinical Immunology and a professor of medicine at the Cleveland Clinic, said in an interview that the study is an “extremely important contribution to our understanding of COVID-19 and its pattern of recovery in patients with immune-mediated inflammatory diseases [IMIDs].” Remaining unanswered questions are “whether patients with IMIDs develop more frequent PASC [post–acute sequelae of COVID-19] from COVID-19 and, if so, is it milder or more severe, and does it differ in its clinical phenotype?”
Long COVID risk assessed at 4 weeks and 3 months after infection
The researchers prospectively tracked 280 adult patients in the Mass General Brigham health care system in the greater Boston area who had systemic autoimmune rheumatic diseases and had an acute COVID-19 infection between March 2020 and July 2022. Patients were an average 53 years old, and most were White (82%) and female (80%). More than half (59%) had inflammatory arthritis, a quarter (24%) had connective tissue disease, and most others had a vasculitis condition or multiple conditions.
A total of 11% of patients were unvaccinated, 28% were partially vaccinated with one mRNA COVID-19 vaccine dose, and 41% were fully vaccinated with two mRNA vaccine doses or one Johnson & Johnson dose. The 116 fully vaccinated patients were considered to have a breakthrough infection while the other 164 were considered to have a nonbreakthrough infection. The breakthrough and nonbreakthrough groups were similar in terms of age, sex, race, ethnicity, smoking status, and type of rheumatic disease. Comorbidities were also similar, except obesity, which was more common in the non–breakthrough infection group (25%) than the breakthrough infection group (10%).
The researchers queried patients on their COVID-19 symptoms, how long symptoms lasted, treatments they received, and hospitalization details. COVID-19 symptoms assessed included fever, sore throat, new cough, nasal congestion/rhinorrhea, dyspnea, chest pain, rash, myalgia, fatigue/malaise, headache, nausea/vomiting, diarrhea, anosmia, dysgeusia, and joint pain.
Patients completed surveys about symptoms at 4 weeks and 3 months after infection. Long COVID, or PASC, was defined as any persistent symptom at the times assessed.
Vaccinated patients fared better across outcomes
At 4 weeks after infection, 41% of fully vaccinated patients had at least one persistent symptom, compared with 54% of unvaccinated or partially vaccinated patients (P = .04). At 3 months after infection, 21% of fully vaccinated patients had at least one persistent symptom, compared with 41% of unvaccinated or partially vaccinated patients (P < .0001).
Vaccinated patients were half as likely to have long COVID at 4 weeks after infection (adjusted odds ratio, 0.49) and 90% less likely to have long COVID 3 months after infection (aOR, 0.1), after adjustment for age, sex, race, comorbidities, and use of any of four immune-suppressing medications (anti-CD20 monoclonal antibodies, methotrexate, mycophenolate, or glucocorticoids).
Fully vaccinated patients with breakthrough infections had an average 21 additional days without symptoms during follow-up, compared with unvaccinated and partially vaccinated patients (P = .04).
Reduced risk of long COVID did not change for vaccinated patients after sensitivity analyses for those who did not receive nirmatrelvir/ritonavir (Paxlovid) or monoclonal antibodies, those who didn’t receive any COVID-19-related treatment, those who completed their questionnaires within 6 months after infection, and those who were not hospitalized.
“One important message is that among those who did get PASC, the severity appears similar among those with and without a breakthrough infection,” Dr. Wallace said. “This highlights the need for ongoing research to improve recognition, diagnosis, and treatment of PASC.”
Many more breakthrough infections (72%) than nonbreakthrough infections (2%) occurred during Omicron. The authors acknowledged that different variants might play a role in different long COVID risks but said such potential confounding is unlikely to fully explain the results.
“Even with data suggesting that the Omicron variants may be intrinsically less severe, vaccination still has an impact on severity of infection, rates of hospitalization, and other outcomes and thus may play a role in the risk of PASC,” lead author Naomi Patel, MD, an instructor at Harvard Medical School and a rheumatologist at Massachusetts General Hospital, said in an interview. “A study evaluating the proportions with PASC by vaccination status during the time in which a single variant is predominant, such as the early Omicron era, could help to better assess the more isolated impact of vaccination on PASC.”
Dr. Calabrese said he is convinced that Omicron infections are less likely to result in more severe forms of acute COVID than pre-Omicron infections, and he suspects Omicron infections are also less likely to result in long COVID, although less evidence currently supports this hypothesis.
Hospitalization was more common in unvaccinated/partly vaccinated patients than in vaccinated patients (27% vs. 5%; P = .001). Although pain and fatigue were lower in those with breakthrough infections, functional scores and health-related quality of life were similar in both groups.
Some symptoms significantly differed between vaccinated and unvaccinated/partly vaccinated groups, possibly caused partly by different variants. Nasal congestion was more common (73%) in those with breakthrough infections than in those with nonbreakthrough infections (46%; P < .0001). Those who were unvaccinated/partly vaccinated were significantly more likely to have loss of smell (46% vs. 22%) or taste (45% vs. 28%) or to have joint pain (11% vs. 4%).
Treatment with nirmatrelvir/ritonavir was also more common in vaccinated patients (12%) than in unvaccinated/partly vaccinated patients (1%; P < .0001), as was treatment with monoclonal antibodies (34% vs. 8%; P < .0001).
The study was limited by its low diversity and being at a single health care system, the authors said. Study coauthor Jeffrey A. Sparks, MD, MMSc, an assistant professor of medicine at Brigham and Women’s Hospital and Harvard Medical School, said in an interview that the group is planning additional studies as their cohort grows, including “investigating the relationships between COVID-19 and specific rheumatic diseases and immunomodulating medications, expansion of autoimmunity and systemic inflammation, and lung damage among specific patient populations.”
Dr. Calabrese said it will be important for follow-up study of the symptomatic patients to “determine how many of these patients will fit the clinical picture of long COVID or long-haul phenotypes over the months and years ahead, including documenting exertional malaise and quality of life.
This study only assessed patients who received zero, one, or two doses of a vaccine, but many patients with rheumatic disease today will likely have received booster doses. However, Dr. Calabrese said it would be difficult to quantify whether a third, fourth, or fifth dose offers additional protection from long-term COVID complications after full vaccination or hybrid vaccination.
The research was funded by the Rheumatology Research Foundation, the National Institutes of Health, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Wallace has received research support from Bristol-Myers Squibb and Principia/Sanofi and consulting fees from Zenas BioPharma, Horizon, Sanofi, Shionogi, Viela Bio, and Medpace. Dr. Sparks has received research support from Bristol-Myers Squibb and consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. Dr. Patel has received consulting fees from FVC Health. Calabrese has consulted for Genentech, Sanofi-Regeneron, AstraZeneca, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Patients with rheumatic disease are at least half as likely to develop long COVID after a SARS-CoV-2 infection if they have been fully vaccinated against COVID-19, according to research published in Annals of the Rheumatic Diseases (2022 Nov 28. doi: 10.1136/ard-2022-223439).
“Moreover, those who were vaccinated prior to getting COVID-19 had less pain and fatigue after their infection,” Zachary S. Wallace, MD, MSc, an assistant professor of medicine at Harvard Medical School, Boston, and a study author, said in an interview. “These findings reinforce the importance of vaccination in this population.”
Messaging around the value of COVID vaccination has been confusing for some with rheumatic disease “because our concern regarding a blunted response to vaccination has led many patients to think that they do not provide much benefit if they are on immunosuppression,” Dr. Wallace said. “In our cohort, which included many patients on immunosuppression of varying degrees, being vaccinated was quite beneficial.”
Leonard H. Calabrese, DO, director of the R.J. Fasenmyer Center for Clinical Immunology and a professor of medicine at the Cleveland Clinic, said in an interview that the study is an “extremely important contribution to our understanding of COVID-19 and its pattern of recovery in patients with immune-mediated inflammatory diseases [IMIDs].” Remaining unanswered questions are “whether patients with IMIDs develop more frequent PASC [post–acute sequelae of COVID-19] from COVID-19 and, if so, is it milder or more severe, and does it differ in its clinical phenotype?”
Long COVID risk assessed at 4 weeks and 3 months after infection
The researchers prospectively tracked 280 adult patients in the Mass General Brigham health care system in the greater Boston area who had systemic autoimmune rheumatic diseases and had an acute COVID-19 infection between March 2020 and July 2022. Patients were an average 53 years old, and most were White (82%) and female (80%). More than half (59%) had inflammatory arthritis, a quarter (24%) had connective tissue disease, and most others had a vasculitis condition or multiple conditions.
A total of 11% of patients were unvaccinated, 28% were partially vaccinated with one mRNA COVID-19 vaccine dose, and 41% were fully vaccinated with two mRNA vaccine doses or one Johnson & Johnson dose. The 116 fully vaccinated patients were considered to have a breakthrough infection while the other 164 were considered to have a nonbreakthrough infection. The breakthrough and nonbreakthrough groups were similar in terms of age, sex, race, ethnicity, smoking status, and type of rheumatic disease. Comorbidities were also similar, except obesity, which was more common in the non–breakthrough infection group (25%) than the breakthrough infection group (10%).
The researchers queried patients on their COVID-19 symptoms, how long symptoms lasted, treatments they received, and hospitalization details. COVID-19 symptoms assessed included fever, sore throat, new cough, nasal congestion/rhinorrhea, dyspnea, chest pain, rash, myalgia, fatigue/malaise, headache, nausea/vomiting, diarrhea, anosmia, dysgeusia, and joint pain.
Patients completed surveys about symptoms at 4 weeks and 3 months after infection. Long COVID, or PASC, was defined as any persistent symptom at the times assessed.
Vaccinated patients fared better across outcomes
At 4 weeks after infection, 41% of fully vaccinated patients had at least one persistent symptom, compared with 54% of unvaccinated or partially vaccinated patients (P = .04). At 3 months after infection, 21% of fully vaccinated patients had at least one persistent symptom, compared with 41% of unvaccinated or partially vaccinated patients (P < .0001).
Vaccinated patients were half as likely to have long COVID at 4 weeks after infection (adjusted odds ratio, 0.49) and 90% less likely to have long COVID 3 months after infection (aOR, 0.1), after adjustment for age, sex, race, comorbidities, and use of any of four immune-suppressing medications (anti-CD20 monoclonal antibodies, methotrexate, mycophenolate, or glucocorticoids).
Fully vaccinated patients with breakthrough infections had an average 21 additional days without symptoms during follow-up, compared with unvaccinated and partially vaccinated patients (P = .04).
Reduced risk of long COVID did not change for vaccinated patients after sensitivity analyses for those who did not receive nirmatrelvir/ritonavir (Paxlovid) or monoclonal antibodies, those who didn’t receive any COVID-19-related treatment, those who completed their questionnaires within 6 months after infection, and those who were not hospitalized.
“One important message is that among those who did get PASC, the severity appears similar among those with and without a breakthrough infection,” Dr. Wallace said. “This highlights the need for ongoing research to improve recognition, diagnosis, and treatment of PASC.”
Many more breakthrough infections (72%) than nonbreakthrough infections (2%) occurred during Omicron. The authors acknowledged that different variants might play a role in different long COVID risks but said such potential confounding is unlikely to fully explain the results.
“Even with data suggesting that the Omicron variants may be intrinsically less severe, vaccination still has an impact on severity of infection, rates of hospitalization, and other outcomes and thus may play a role in the risk of PASC,” lead author Naomi Patel, MD, an instructor at Harvard Medical School and a rheumatologist at Massachusetts General Hospital, said in an interview. “A study evaluating the proportions with PASC by vaccination status during the time in which a single variant is predominant, such as the early Omicron era, could help to better assess the more isolated impact of vaccination on PASC.”
Dr. Calabrese said he is convinced that Omicron infections are less likely to result in more severe forms of acute COVID than pre-Omicron infections, and he suspects Omicron infections are also less likely to result in long COVID, although less evidence currently supports this hypothesis.
Hospitalization was more common in unvaccinated/partly vaccinated patients than in vaccinated patients (27% vs. 5%; P = .001). Although pain and fatigue were lower in those with breakthrough infections, functional scores and health-related quality of life were similar in both groups.
Some symptoms significantly differed between vaccinated and unvaccinated/partly vaccinated groups, possibly caused partly by different variants. Nasal congestion was more common (73%) in those with breakthrough infections than in those with nonbreakthrough infections (46%; P < .0001). Those who were unvaccinated/partly vaccinated were significantly more likely to have loss of smell (46% vs. 22%) or taste (45% vs. 28%) or to have joint pain (11% vs. 4%).
Treatment with nirmatrelvir/ritonavir was also more common in vaccinated patients (12%) than in unvaccinated/partly vaccinated patients (1%; P < .0001), as was treatment with monoclonal antibodies (34% vs. 8%; P < .0001).
The study was limited by its low diversity and being at a single health care system, the authors said. Study coauthor Jeffrey A. Sparks, MD, MMSc, an assistant professor of medicine at Brigham and Women’s Hospital and Harvard Medical School, said in an interview that the group is planning additional studies as their cohort grows, including “investigating the relationships between COVID-19 and specific rheumatic diseases and immunomodulating medications, expansion of autoimmunity and systemic inflammation, and lung damage among specific patient populations.”
Dr. Calabrese said it will be important for follow-up study of the symptomatic patients to “determine how many of these patients will fit the clinical picture of long COVID or long-haul phenotypes over the months and years ahead, including documenting exertional malaise and quality of life.
This study only assessed patients who received zero, one, or two doses of a vaccine, but many patients with rheumatic disease today will likely have received booster doses. However, Dr. Calabrese said it would be difficult to quantify whether a third, fourth, or fifth dose offers additional protection from long-term COVID complications after full vaccination or hybrid vaccination.
The research was funded by the Rheumatology Research Foundation, the National Institutes of Health, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Wallace has received research support from Bristol-Myers Squibb and Principia/Sanofi and consulting fees from Zenas BioPharma, Horizon, Sanofi, Shionogi, Viela Bio, and Medpace. Dr. Sparks has received research support from Bristol-Myers Squibb and consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. Dr. Patel has received consulting fees from FVC Health. Calabrese has consulted for Genentech, Sanofi-Regeneron, AstraZeneca, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
Patients with rheumatic disease are at least half as likely to develop long COVID after a SARS-CoV-2 infection if they have been fully vaccinated against COVID-19, according to research published in Annals of the Rheumatic Diseases (2022 Nov 28. doi: 10.1136/ard-2022-223439).
“Moreover, those who were vaccinated prior to getting COVID-19 had less pain and fatigue after their infection,” Zachary S. Wallace, MD, MSc, an assistant professor of medicine at Harvard Medical School, Boston, and a study author, said in an interview. “These findings reinforce the importance of vaccination in this population.”
Messaging around the value of COVID vaccination has been confusing for some with rheumatic disease “because our concern regarding a blunted response to vaccination has led many patients to think that they do not provide much benefit if they are on immunosuppression,” Dr. Wallace said. “In our cohort, which included many patients on immunosuppression of varying degrees, being vaccinated was quite beneficial.”
Leonard H. Calabrese, DO, director of the R.J. Fasenmyer Center for Clinical Immunology and a professor of medicine at the Cleveland Clinic, said in an interview that the study is an “extremely important contribution to our understanding of COVID-19 and its pattern of recovery in patients with immune-mediated inflammatory diseases [IMIDs].” Remaining unanswered questions are “whether patients with IMIDs develop more frequent PASC [post–acute sequelae of COVID-19] from COVID-19 and, if so, is it milder or more severe, and does it differ in its clinical phenotype?”
Long COVID risk assessed at 4 weeks and 3 months after infection
The researchers prospectively tracked 280 adult patients in the Mass General Brigham health care system in the greater Boston area who had systemic autoimmune rheumatic diseases and had an acute COVID-19 infection between March 2020 and July 2022. Patients were an average 53 years old, and most were White (82%) and female (80%). More than half (59%) had inflammatory arthritis, a quarter (24%) had connective tissue disease, and most others had a vasculitis condition or multiple conditions.
A total of 11% of patients were unvaccinated, 28% were partially vaccinated with one mRNA COVID-19 vaccine dose, and 41% were fully vaccinated with two mRNA vaccine doses or one Johnson & Johnson dose. The 116 fully vaccinated patients were considered to have a breakthrough infection while the other 164 were considered to have a nonbreakthrough infection. The breakthrough and nonbreakthrough groups were similar in terms of age, sex, race, ethnicity, smoking status, and type of rheumatic disease. Comorbidities were also similar, except obesity, which was more common in the non–breakthrough infection group (25%) than the breakthrough infection group (10%).
The researchers queried patients on their COVID-19 symptoms, how long symptoms lasted, treatments they received, and hospitalization details. COVID-19 symptoms assessed included fever, sore throat, new cough, nasal congestion/rhinorrhea, dyspnea, chest pain, rash, myalgia, fatigue/malaise, headache, nausea/vomiting, diarrhea, anosmia, dysgeusia, and joint pain.
Patients completed surveys about symptoms at 4 weeks and 3 months after infection. Long COVID, or PASC, was defined as any persistent symptom at the times assessed.
Vaccinated patients fared better across outcomes
At 4 weeks after infection, 41% of fully vaccinated patients had at least one persistent symptom, compared with 54% of unvaccinated or partially vaccinated patients (P = .04). At 3 months after infection, 21% of fully vaccinated patients had at least one persistent symptom, compared with 41% of unvaccinated or partially vaccinated patients (P < .0001).
Vaccinated patients were half as likely to have long COVID at 4 weeks after infection (adjusted odds ratio, 0.49) and 90% less likely to have long COVID 3 months after infection (aOR, 0.1), after adjustment for age, sex, race, comorbidities, and use of any of four immune-suppressing medications (anti-CD20 monoclonal antibodies, methotrexate, mycophenolate, or glucocorticoids).
Fully vaccinated patients with breakthrough infections had an average 21 additional days without symptoms during follow-up, compared with unvaccinated and partially vaccinated patients (P = .04).
Reduced risk of long COVID did not change for vaccinated patients after sensitivity analyses for those who did not receive nirmatrelvir/ritonavir (Paxlovid) or monoclonal antibodies, those who didn’t receive any COVID-19-related treatment, those who completed their questionnaires within 6 months after infection, and those who were not hospitalized.
“One important message is that among those who did get PASC, the severity appears similar among those with and without a breakthrough infection,” Dr. Wallace said. “This highlights the need for ongoing research to improve recognition, diagnosis, and treatment of PASC.”
Many more breakthrough infections (72%) than nonbreakthrough infections (2%) occurred during Omicron. The authors acknowledged that different variants might play a role in different long COVID risks but said such potential confounding is unlikely to fully explain the results.
“Even with data suggesting that the Omicron variants may be intrinsically less severe, vaccination still has an impact on severity of infection, rates of hospitalization, and other outcomes and thus may play a role in the risk of PASC,” lead author Naomi Patel, MD, an instructor at Harvard Medical School and a rheumatologist at Massachusetts General Hospital, said in an interview. “A study evaluating the proportions with PASC by vaccination status during the time in which a single variant is predominant, such as the early Omicron era, could help to better assess the more isolated impact of vaccination on PASC.”
Dr. Calabrese said he is convinced that Omicron infections are less likely to result in more severe forms of acute COVID than pre-Omicron infections, and he suspects Omicron infections are also less likely to result in long COVID, although less evidence currently supports this hypothesis.
Hospitalization was more common in unvaccinated/partly vaccinated patients than in vaccinated patients (27% vs. 5%; P = .001). Although pain and fatigue were lower in those with breakthrough infections, functional scores and health-related quality of life were similar in both groups.
Some symptoms significantly differed between vaccinated and unvaccinated/partly vaccinated groups, possibly caused partly by different variants. Nasal congestion was more common (73%) in those with breakthrough infections than in those with nonbreakthrough infections (46%; P < .0001). Those who were unvaccinated/partly vaccinated were significantly more likely to have loss of smell (46% vs. 22%) or taste (45% vs. 28%) or to have joint pain (11% vs. 4%).
Treatment with nirmatrelvir/ritonavir was also more common in vaccinated patients (12%) than in unvaccinated/partly vaccinated patients (1%; P < .0001), as was treatment with monoclonal antibodies (34% vs. 8%; P < .0001).
The study was limited by its low diversity and being at a single health care system, the authors said. Study coauthor Jeffrey A. Sparks, MD, MMSc, an assistant professor of medicine at Brigham and Women’s Hospital and Harvard Medical School, said in an interview that the group is planning additional studies as their cohort grows, including “investigating the relationships between COVID-19 and specific rheumatic diseases and immunomodulating medications, expansion of autoimmunity and systemic inflammation, and lung damage among specific patient populations.”
Dr. Calabrese said it will be important for follow-up study of the symptomatic patients to “determine how many of these patients will fit the clinical picture of long COVID or long-haul phenotypes over the months and years ahead, including documenting exertional malaise and quality of life.
This study only assessed patients who received zero, one, or two doses of a vaccine, but many patients with rheumatic disease today will likely have received booster doses. However, Dr. Calabrese said it would be difficult to quantify whether a third, fourth, or fifth dose offers additional protection from long-term COVID complications after full vaccination or hybrid vaccination.
The research was funded by the Rheumatology Research Foundation, the National Institutes of Health, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. Dr. Wallace has received research support from Bristol-Myers Squibb and Principia/Sanofi and consulting fees from Zenas BioPharma, Horizon, Sanofi, Shionogi, Viela Bio, and Medpace. Dr. Sparks has received research support from Bristol-Myers Squibb and consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer. Dr. Patel has received consulting fees from FVC Health. Calabrese has consulted for Genentech, Sanofi-Regeneron, AstraZeneca, and GlaxoSmithKline.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Immunity debt and the tripledemic
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
What is the diagnosis?
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
A 48-year-old man with HIV infection (PCR undetectable CD4 483) who used cocaine and was a heavy user of alcohol presented with jaundice, fever, and acute-onset left-upper quadrant abdominal pain. The pain was exacerbated by breathing. He had associated intermittent fevers and weight loss starting 3 weeks before presentation. He denied chest pain, nausea, vomiting, or a change in bowel habits. Home medications included dolutegravir, emtricitabine, tenofovir disoproxil fumarate, and recent intake of tamoxifen, clomiphene, and chorionic gonadotropin to counteract the effects of anabolic steroids that were used 4 months before presentation.
On examination, his temperature was 39°C, he was jaundiced, and he had icteric sclera. The abdomen was soft and nondistended with minimal left-upper quadrant tenderness. Blood work showed a white blood cell count of 7,800/mcL, hemoglobin of 12.2 g/dL, platelets of 378,000/mcL, alanine aminotransferase 236 IU/L (upper limit of normal [ULN], 65 IU/L), aspartate aminotransferase 166 IU/L (ULN, 50 IU/L), total bilirubin 3.4 mg/dL (ULN, 1.2 mg/dL), direct bilirubin 2.6 mg/dL (ULN, 0.3 mg/dL), alkaline phosphatase 1,064 IU/L (ULN, 120 IU/L), gamma-glutamyl transferase (GGT) of 655 (ULN, 50 IU/L), protein of 73 g/L, and albumin of 34 g/L. Lipase, lactate dehydrogenase, and international normalized ratio were normal. Blood smear was unrevealing. A contrasted computed tomography scan showed multiple subcentimetric mesenteric and multiple retroperitoneal lymph nodes, the largest of which was 1.3 cm in the aortocaval area. All medications were discontinued. Hepatitis A, B, and C serologies were negative, including hepatitis B and C PCR. Epstein-Barr virus IgM was negative and cytomegalovirus IgM was equivocal.
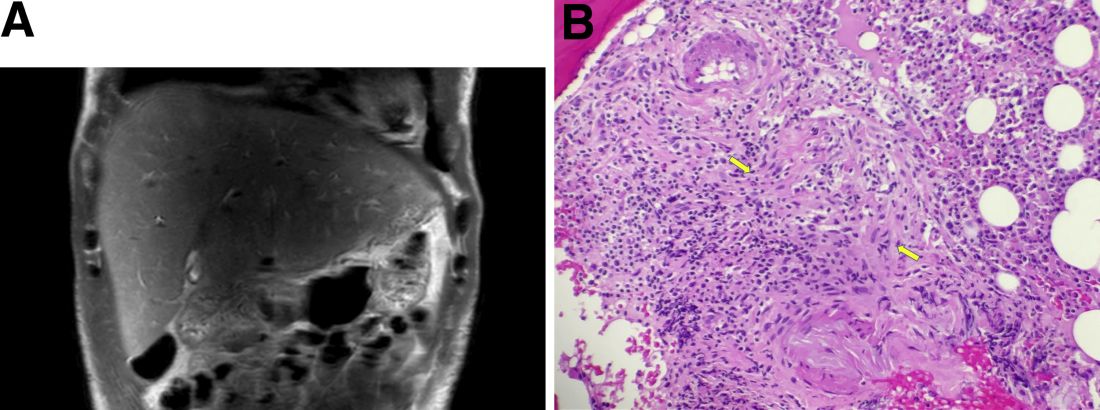
During this hospitalization, his cholestatic liver enzymes continued to rise, reaching a maximum value of total bilirubin of 7.8 mg/dL, direct bilirubin of 6.5 mg/dL, and 3 days later, alkaline phosphatase of 1,637 IU/L and GGT of 1,171 IU/L. Alanine aminotransferase and aspartate aminotransferase slowly downtrended during the hospitalization. Magnetic resonance cholangiopancreatography showed an edematous enlarged liver with minimal peripheral intrahepatic dilatation without an obstructing mass or extrahepatic biliary ductal dilatation (Figure A). Comprehensive autoimmune hepatic serology, iron studies, ceruloplasmin, and alpha-1 antitrypsin labs were negative. The patient remained febrile, so a positron emission tomography computed tomography scan was done and it showed active and enlarged (2.8-cm) portocaval and porta hepatis lymph nodes. Bone marrow biopsy showed no lymphoproliferative disorder, but there was a small poorly formed granuloma (Figure B, between the arrows).
What other testing would you obtain to evaluate this patient's fever and abnormal liver enzymes?
Previously published in Gastroenterology