User login
Children and COVID: Hospitalizations provide a tale of two sources
New cases of COVID-19 in children largely held steady over the Thanksgiving holiday, but hospital admissions are telling a somewhat different story.
New pediatric COVID cases for the week ending on Thanksgiving (11/18-11/24) were up by 5.3% over the previous week, but in the most recent week (11/25-12/1) new cases dropped by 2.6%, according to state data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
In both weeks, though, the total case count stayed below 30,000 – a streak that has now lasted 8 weeks – so the actual number of weekly cases remained fairly low, the AAP/CHA weekly report indicates.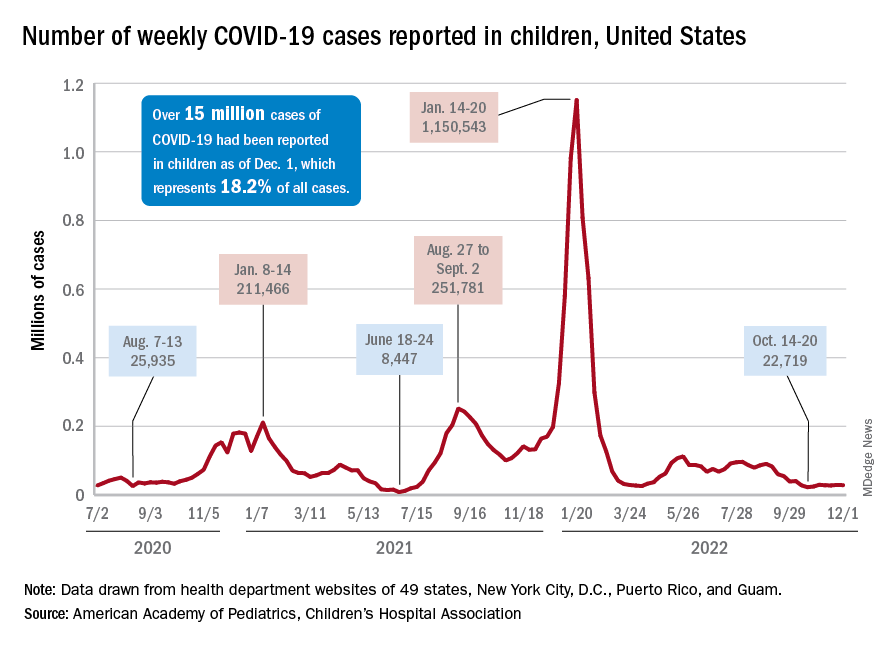
The nation’s emergency departments also experienced a small Thanksgiving bump, as the proportion of visits with diagnosed COVID went from 1.0% of all ED visits for children aged 0-11 years on Nov. 14 to 2.0% on Nov. 27, just 3 days after the official holiday, based on data from the Centers for Disease Control and Prevention. The rate was down to 1.5% on Dec. 1, and similar patterns can be seen for children aged 12-15 and 16-17 years.
New hospital admissions, on the other hand, seem to be following a different path, at least according to the CDC. The hospitalization rate for children aged 0-17 years bottomed out at 0.16 new admissions per 100,000 population back on Oct. 21 and has climbed fairly steadily since then. It was up to 0.20 per 100,000 by Nov. 14, had reached 0.22 per 100,000 on Thanksgiving day (11/24), and then continued to 0.26 per 100,000 by Dec. 2, the latest date for which CDC data are available.
The hospitalization story, however, offers yet another twist. The New York Times, using data from the U.S. Department of Health & Human Services, reports that new COVID-related admissions have held steady at 1.0 per 100,000 since Nov. 18. The rate is much higher than has been reported by the CDC, but no increase can be seen in recent weeks among children, which is not the case for Americans overall, Medscape recently reported.
New cases of COVID-19 in children largely held steady over the Thanksgiving holiday, but hospital admissions are telling a somewhat different story.
New pediatric COVID cases for the week ending on Thanksgiving (11/18-11/24) were up by 5.3% over the previous week, but in the most recent week (11/25-12/1) new cases dropped by 2.6%, according to state data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
In both weeks, though, the total case count stayed below 30,000 – a streak that has now lasted 8 weeks – so the actual number of weekly cases remained fairly low, the AAP/CHA weekly report indicates.
The nation’s emergency departments also experienced a small Thanksgiving bump, as the proportion of visits with diagnosed COVID went from 1.0% of all ED visits for children aged 0-11 years on Nov. 14 to 2.0% on Nov. 27, just 3 days after the official holiday, based on data from the Centers for Disease Control and Prevention. The rate was down to 1.5% on Dec. 1, and similar patterns can be seen for children aged 12-15 and 16-17 years.
New hospital admissions, on the other hand, seem to be following a different path, at least according to the CDC. The hospitalization rate for children aged 0-17 years bottomed out at 0.16 new admissions per 100,000 population back on Oct. 21 and has climbed fairly steadily since then. It was up to 0.20 per 100,000 by Nov. 14, had reached 0.22 per 100,000 on Thanksgiving day (11/24), and then continued to 0.26 per 100,000 by Dec. 2, the latest date for which CDC data are available.
The hospitalization story, however, offers yet another twist. The New York Times, using data from the U.S. Department of Health & Human Services, reports that new COVID-related admissions have held steady at 1.0 per 100,000 since Nov. 18. The rate is much higher than has been reported by the CDC, but no increase can be seen in recent weeks among children, which is not the case for Americans overall, Medscape recently reported.
New cases of COVID-19 in children largely held steady over the Thanksgiving holiday, but hospital admissions are telling a somewhat different story.
New pediatric COVID cases for the week ending on Thanksgiving (11/18-11/24) were up by 5.3% over the previous week, but in the most recent week (11/25-12/1) new cases dropped by 2.6%, according to state data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
In both weeks, though, the total case count stayed below 30,000 – a streak that has now lasted 8 weeks – so the actual number of weekly cases remained fairly low, the AAP/CHA weekly report indicates.
The nation’s emergency departments also experienced a small Thanksgiving bump, as the proportion of visits with diagnosed COVID went from 1.0% of all ED visits for children aged 0-11 years on Nov. 14 to 2.0% on Nov. 27, just 3 days after the official holiday, based on data from the Centers for Disease Control and Prevention. The rate was down to 1.5% on Dec. 1, and similar patterns can be seen for children aged 12-15 and 16-17 years.
New hospital admissions, on the other hand, seem to be following a different path, at least according to the CDC. The hospitalization rate for children aged 0-17 years bottomed out at 0.16 new admissions per 100,000 population back on Oct. 21 and has climbed fairly steadily since then. It was up to 0.20 per 100,000 by Nov. 14, had reached 0.22 per 100,000 on Thanksgiving day (11/24), and then continued to 0.26 per 100,000 by Dec. 2, the latest date for which CDC data are available.
The hospitalization story, however, offers yet another twist. The New York Times, using data from the U.S. Department of Health & Human Services, reports that new COVID-related admissions have held steady at 1.0 per 100,000 since Nov. 18. The rate is much higher than has been reported by the CDC, but no increase can be seen in recent weeks among children, which is not the case for Americans overall, Medscape recently reported.
Diagnosed too late
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
‘Game changer’: Thyroid cancer recurrence no higher with lobectomy
Patients with intermediate-risk papillary thyroid cancer and lymph node metastasis show no significant increase in tumor recurrence when undergoing lobectomy compared with a total thyroidectomy, new research shows.
“Results of this cohort study suggest that patients with ipsilateral clinical lateral neck metastasis (cN1b) papillary thyroid cancer who underwent lobectomy exhibited recurrence-free survival rates similar to those who underwent total thyroidectomy after controlling for major prognostic factors,” the authors conclude in the study published online in JAMA Surgery.
“These findings suggest that cN1b alone should not be an absolute indication for total thyroidectomy,” they note.
The study, involving the largest cohort to date to compare patients with intermediate-risk papillary thyroid cancer treated with lobectomy versus total thyroidectomy, “challenged the current guidelines and pushed the boundary of limited surgical treatment even further,” say Michelle B. Mulder, MD, and Quan-Yang Duh, MD, of the department of surgery, University of California, San Francisco, in an accompanying editorial.
“It can be a game changer if confirmed by future prospective and multicenter studies,” they add.
Guidelines still recommend total thyroidectomy with subsequent RAI
While lower-intensity treatment options, with a lower risk of complications, have gained favor in the treatment of low-risk papillary thyroid cancer, guidelines still recommend the consideration of total thyroidectomy and subsequent radioactive iodine ablation (RAI) for intermediate-risk cancers because of the higher chance of recurrence, particularly among those with clinically positive nodes.
However, data on the superiority of a total thyroidectomy, with or without RAI, versus lobectomy is inconsistent, prompting first author Siyuan Xu, MD, of the department of head and neck surgical oncology, National Cancer Center, Beijing, and colleagues to compare the risk of recurrence with the two approaches.
For the study, patients with intermediate-risk papillary thyroid cancer treated at the Chinese Academy of Medical Sciences Cancer Hospital in Beijing between January 2000 and December 2017, who had a lobectomy or total thyroidectomy, were paired 1:1 in a propensity score matching analysis.
Other than treatment type, the 265 pairs of patients were matched based on all other potential prognostic factors, including age, sex, primary tumor size, minor extrathyroidal extension, multifocality, number of lymph node metastases, and lymph node ratio.
Participants were a mean age of 37 years and 66% were female.
With a median follow-up of 60 months in the lobectomy group and 58 months in the total thyroidectomy group, structural recurrences occurred in 7.9% (21) and 6.4% (17) of patients, respectively, which was not significantly different.
The primary endpoint, 5-year rate of recurrence-free survival, was also not significantly different between the lobectomy (92.3%) and total thyroidectomy groups (93.7%) (adjusted hazard ratio, 1.10; P = .77).
In a further stratified analysis of patients treated with total thyroidectomy along with RAI (n = 75), the lack of a significant difference in recurrence-free survival versus lobectomy remained (aHR, 0.59; P = .46).
The results were similar in unadjusted as well as adjusted analyses, and a power analysis indicated that the study had a 90% power to detect a more than 4.9% difference in recurrence-free survival.
“Given the lower complication rate of lobectomy, a maximal 4.9% recurrence-free survival difference is acceptable, which enhances the reliability of the study results,” the authors say.
They conclude that “our findings call into question whether cN1b alone [ipsilateral clinical lateral neck metastasis papillary thyroid cancer] should be an absolute determinant for deciding the optimal extent of thyroid surgery for papillary thyroid cancer.”
With total thyroidectomy, RAI can be given
An important argument in favor of total thyroidectomy is that with the complete resection of thyroid tissue, RAI ablation can then be used for postoperative detection of residual or metastatic disease, as well as for treatment, the authors note.
Indeed, a study using the Surveillance, Epidemiology, and End Results (SEER) database showed RAI ablation is associated with a 29% reduction in the risk of death in patients with intermediate-risk papillary thyroid cancer, with a hazard risk of 0.71.
However, conflicting data from Memorial Sloan-Kettering Cancer Center, New York, suggests no significant benefit with total thyroidectomy and RAI ablation.
The current study’s analysis of patients treated with RAI, though limited in size, supports the latter study’s findings, the authors note.
“When we performed further stratified analyses in patients treated with total thyroidectomy plus RAI ablation and their counterparts, no significant difference was found, which conformed with [the] result from the whole cohort.”
“Certainly, the stratified comparison did not have enough power to examine the effect of RAI ablation on tumor recurrence subject to the limitation of sample size and case selection [and] further study is needed on this topic,” they write.
Some limitations warrant cautious interpretation
In their editorial, Dr. Mulder and Dr. Duh note that while some previous studies have shown similar outcomes relating to tumor size, thyroid hormone suppression therapy, and multifocality, “few have addressed lateral neck involvement.”
They suggest cautious interpretation, however, due to limitations, acknowledged by the authors, including the single-center nature of the study.
“Appropriate propensity matching may mitigate selection bias but cannot eliminate it entirely and their findings may not be replicated in other institutions by other surgeons,” they note.
Other limitations include that changes in clinical practice and patient selection were likely over the course of the study because of significant changes in American Thyroid Association (ATA) guidelines between 2009 and 2017, and characteristics including molecular genetic testing, which could have influenced final results, were not taken into consideration.
Furthermore, for patients with intermediate-risk cancer, modifications in postoperative follow-up are necessary following lobectomy versus total thyroidectomy; “the role of radioiodine is limited and the levels of thyroglobulin more complicated to interpret,” they note.
The study and editorial authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Patients with intermediate-risk papillary thyroid cancer and lymph node metastasis show no significant increase in tumor recurrence when undergoing lobectomy compared with a total thyroidectomy, new research shows.
“Results of this cohort study suggest that patients with ipsilateral clinical lateral neck metastasis (cN1b) papillary thyroid cancer who underwent lobectomy exhibited recurrence-free survival rates similar to those who underwent total thyroidectomy after controlling for major prognostic factors,” the authors conclude in the study published online in JAMA Surgery.
“These findings suggest that cN1b alone should not be an absolute indication for total thyroidectomy,” they note.
The study, involving the largest cohort to date to compare patients with intermediate-risk papillary thyroid cancer treated with lobectomy versus total thyroidectomy, “challenged the current guidelines and pushed the boundary of limited surgical treatment even further,” say Michelle B. Mulder, MD, and Quan-Yang Duh, MD, of the department of surgery, University of California, San Francisco, in an accompanying editorial.
“It can be a game changer if confirmed by future prospective and multicenter studies,” they add.
Guidelines still recommend total thyroidectomy with subsequent RAI
While lower-intensity treatment options, with a lower risk of complications, have gained favor in the treatment of low-risk papillary thyroid cancer, guidelines still recommend the consideration of total thyroidectomy and subsequent radioactive iodine ablation (RAI) for intermediate-risk cancers because of the higher chance of recurrence, particularly among those with clinically positive nodes.
However, data on the superiority of a total thyroidectomy, with or without RAI, versus lobectomy is inconsistent, prompting first author Siyuan Xu, MD, of the department of head and neck surgical oncology, National Cancer Center, Beijing, and colleagues to compare the risk of recurrence with the two approaches.
For the study, patients with intermediate-risk papillary thyroid cancer treated at the Chinese Academy of Medical Sciences Cancer Hospital in Beijing between January 2000 and December 2017, who had a lobectomy or total thyroidectomy, were paired 1:1 in a propensity score matching analysis.
Other than treatment type, the 265 pairs of patients were matched based on all other potential prognostic factors, including age, sex, primary tumor size, minor extrathyroidal extension, multifocality, number of lymph node metastases, and lymph node ratio.
Participants were a mean age of 37 years and 66% were female.
With a median follow-up of 60 months in the lobectomy group and 58 months in the total thyroidectomy group, structural recurrences occurred in 7.9% (21) and 6.4% (17) of patients, respectively, which was not significantly different.
The primary endpoint, 5-year rate of recurrence-free survival, was also not significantly different between the lobectomy (92.3%) and total thyroidectomy groups (93.7%) (adjusted hazard ratio, 1.10; P = .77).
In a further stratified analysis of patients treated with total thyroidectomy along with RAI (n = 75), the lack of a significant difference in recurrence-free survival versus lobectomy remained (aHR, 0.59; P = .46).
The results were similar in unadjusted as well as adjusted analyses, and a power analysis indicated that the study had a 90% power to detect a more than 4.9% difference in recurrence-free survival.
“Given the lower complication rate of lobectomy, a maximal 4.9% recurrence-free survival difference is acceptable, which enhances the reliability of the study results,” the authors say.
They conclude that “our findings call into question whether cN1b alone [ipsilateral clinical lateral neck metastasis papillary thyroid cancer] should be an absolute determinant for deciding the optimal extent of thyroid surgery for papillary thyroid cancer.”
With total thyroidectomy, RAI can be given
An important argument in favor of total thyroidectomy is that with the complete resection of thyroid tissue, RAI ablation can then be used for postoperative detection of residual or metastatic disease, as well as for treatment, the authors note.
Indeed, a study using the Surveillance, Epidemiology, and End Results (SEER) database showed RAI ablation is associated with a 29% reduction in the risk of death in patients with intermediate-risk papillary thyroid cancer, with a hazard risk of 0.71.
However, conflicting data from Memorial Sloan-Kettering Cancer Center, New York, suggests no significant benefit with total thyroidectomy and RAI ablation.
The current study’s analysis of patients treated with RAI, though limited in size, supports the latter study’s findings, the authors note.
“When we performed further stratified analyses in patients treated with total thyroidectomy plus RAI ablation and their counterparts, no significant difference was found, which conformed with [the] result from the whole cohort.”
“Certainly, the stratified comparison did not have enough power to examine the effect of RAI ablation on tumor recurrence subject to the limitation of sample size and case selection [and] further study is needed on this topic,” they write.
Some limitations warrant cautious interpretation
In their editorial, Dr. Mulder and Dr. Duh note that while some previous studies have shown similar outcomes relating to tumor size, thyroid hormone suppression therapy, and multifocality, “few have addressed lateral neck involvement.”
They suggest cautious interpretation, however, due to limitations, acknowledged by the authors, including the single-center nature of the study.
“Appropriate propensity matching may mitigate selection bias but cannot eliminate it entirely and their findings may not be replicated in other institutions by other surgeons,” they note.
Other limitations include that changes in clinical practice and patient selection were likely over the course of the study because of significant changes in American Thyroid Association (ATA) guidelines between 2009 and 2017, and characteristics including molecular genetic testing, which could have influenced final results, were not taken into consideration.
Furthermore, for patients with intermediate-risk cancer, modifications in postoperative follow-up are necessary following lobectomy versus total thyroidectomy; “the role of radioiodine is limited and the levels of thyroglobulin more complicated to interpret,” they note.
The study and editorial authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
Patients with intermediate-risk papillary thyroid cancer and lymph node metastasis show no significant increase in tumor recurrence when undergoing lobectomy compared with a total thyroidectomy, new research shows.
“Results of this cohort study suggest that patients with ipsilateral clinical lateral neck metastasis (cN1b) papillary thyroid cancer who underwent lobectomy exhibited recurrence-free survival rates similar to those who underwent total thyroidectomy after controlling for major prognostic factors,” the authors conclude in the study published online in JAMA Surgery.
“These findings suggest that cN1b alone should not be an absolute indication for total thyroidectomy,” they note.
The study, involving the largest cohort to date to compare patients with intermediate-risk papillary thyroid cancer treated with lobectomy versus total thyroidectomy, “challenged the current guidelines and pushed the boundary of limited surgical treatment even further,” say Michelle B. Mulder, MD, and Quan-Yang Duh, MD, of the department of surgery, University of California, San Francisco, in an accompanying editorial.
“It can be a game changer if confirmed by future prospective and multicenter studies,” they add.
Guidelines still recommend total thyroidectomy with subsequent RAI
While lower-intensity treatment options, with a lower risk of complications, have gained favor in the treatment of low-risk papillary thyroid cancer, guidelines still recommend the consideration of total thyroidectomy and subsequent radioactive iodine ablation (RAI) for intermediate-risk cancers because of the higher chance of recurrence, particularly among those with clinically positive nodes.
However, data on the superiority of a total thyroidectomy, with or without RAI, versus lobectomy is inconsistent, prompting first author Siyuan Xu, MD, of the department of head and neck surgical oncology, National Cancer Center, Beijing, and colleagues to compare the risk of recurrence with the two approaches.
For the study, patients with intermediate-risk papillary thyroid cancer treated at the Chinese Academy of Medical Sciences Cancer Hospital in Beijing between January 2000 and December 2017, who had a lobectomy or total thyroidectomy, were paired 1:1 in a propensity score matching analysis.
Other than treatment type, the 265 pairs of patients were matched based on all other potential prognostic factors, including age, sex, primary tumor size, minor extrathyroidal extension, multifocality, number of lymph node metastases, and lymph node ratio.
Participants were a mean age of 37 years and 66% were female.
With a median follow-up of 60 months in the lobectomy group and 58 months in the total thyroidectomy group, structural recurrences occurred in 7.9% (21) and 6.4% (17) of patients, respectively, which was not significantly different.
The primary endpoint, 5-year rate of recurrence-free survival, was also not significantly different between the lobectomy (92.3%) and total thyroidectomy groups (93.7%) (adjusted hazard ratio, 1.10; P = .77).
In a further stratified analysis of patients treated with total thyroidectomy along with RAI (n = 75), the lack of a significant difference in recurrence-free survival versus lobectomy remained (aHR, 0.59; P = .46).
The results were similar in unadjusted as well as adjusted analyses, and a power analysis indicated that the study had a 90% power to detect a more than 4.9% difference in recurrence-free survival.
“Given the lower complication rate of lobectomy, a maximal 4.9% recurrence-free survival difference is acceptable, which enhances the reliability of the study results,” the authors say.
They conclude that “our findings call into question whether cN1b alone [ipsilateral clinical lateral neck metastasis papillary thyroid cancer] should be an absolute determinant for deciding the optimal extent of thyroid surgery for papillary thyroid cancer.”
With total thyroidectomy, RAI can be given
An important argument in favor of total thyroidectomy is that with the complete resection of thyroid tissue, RAI ablation can then be used for postoperative detection of residual or metastatic disease, as well as for treatment, the authors note.
Indeed, a study using the Surveillance, Epidemiology, and End Results (SEER) database showed RAI ablation is associated with a 29% reduction in the risk of death in patients with intermediate-risk papillary thyroid cancer, with a hazard risk of 0.71.
However, conflicting data from Memorial Sloan-Kettering Cancer Center, New York, suggests no significant benefit with total thyroidectomy and RAI ablation.
The current study’s analysis of patients treated with RAI, though limited in size, supports the latter study’s findings, the authors note.
“When we performed further stratified analyses in patients treated with total thyroidectomy plus RAI ablation and their counterparts, no significant difference was found, which conformed with [the] result from the whole cohort.”
“Certainly, the stratified comparison did not have enough power to examine the effect of RAI ablation on tumor recurrence subject to the limitation of sample size and case selection [and] further study is needed on this topic,” they write.
Some limitations warrant cautious interpretation
In their editorial, Dr. Mulder and Dr. Duh note that while some previous studies have shown similar outcomes relating to tumor size, thyroid hormone suppression therapy, and multifocality, “few have addressed lateral neck involvement.”
They suggest cautious interpretation, however, due to limitations, acknowledged by the authors, including the single-center nature of the study.
“Appropriate propensity matching may mitigate selection bias but cannot eliminate it entirely and their findings may not be replicated in other institutions by other surgeons,” they note.
Other limitations include that changes in clinical practice and patient selection were likely over the course of the study because of significant changes in American Thyroid Association (ATA) guidelines between 2009 and 2017, and characteristics including molecular genetic testing, which could have influenced final results, were not taken into consideration.
Furthermore, for patients with intermediate-risk cancer, modifications in postoperative follow-up are necessary following lobectomy versus total thyroidectomy; “the role of radioiodine is limited and the levels of thyroglobulin more complicated to interpret,” they note.
The study and editorial authors had no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Youths have strong opinions on language about body weight
With youth obesity on the rise – an estimated 1 in 5 youths are impacted by obesity, according to the Centers for Disease Control and Prevention – conversations about healthy weight are becoming more commonplace, not only in the pediatrician’s office, but at home, too. But the language we use around this sensitive topic is important, as youths are acutely aware that words have a direct impact on their mental health.
Sixteen-year-old Avery DiCocco of Northbrook, Ill., knows how vulnerable teenagers feel.
“I think it definitely matters the way that parents and doctors address weight,” she said. “You never know who may be insecure, and using negative words could go a lot further than they think with impacting self-esteem.”
A new study published in Pediatrics brings light to the words that parents (and providers) use when speaking to youths (ages 10-17) about their weight.
Researchers from the University of Connecticut Rudd Center for Food Policy & Health, Hartford, led an online survey of youths and their parents. Those who took part were asked about 27 terms related to body weight. Parents were asked to comment on their use of these words, while youths commented on the emotional response. The researchers said 1,936 parents and 2,032 adolescents were surveyed between September and December 2021.
Although results skewed toward the use of more positive words, such as “healthy weight,” over terms like “obese,” “fat” or “large,” there was variation across ethnicity, sexual orientation, and weight status. For example, it was noted in the study, funded by WW International, that preference for the word “curvy” was higher among Hispanic/Latino youths, sexual minority youths, and those with a body mass index in the 95th percentile, compared with their White, heterosexual, and lower-weight peers.
Words matter
In 2017, the American Academy of Pediatrics came out with a policy statement on weight stigma and the need for doctors to use more neutral language and less stigmatizing terms in practice when discussing weight among youths.
But one of the reasons this new study is important, said Gregory Germain, MD, associate chief of pediatrics at Yale New Haven (Conn.) Children’s Hospital, is that this study focuses on parents who interact with their kids much more often than a pediatrician who sees them a few times a year.
“Parental motivation, discussion, interaction on a consistent basis – that dialogue is so critical in kids with obesity,” said Germain, who stresses that all adults, coaches, and educators should consider this study as well.
“When we think about those detrimental impacts on mental health when more stigmatizing language is used, just us being more mindful in how we are talking to youth can make such a profound impact,” said Rebecca Kamody, PhD, a clinical psychologist at Yale University, with a research and clinical focus on eating and weight disorders.
“In essence, this is a low-hanging fruit intervention,” she said.
Dr. Kamody recommends we take a lesson from “cultural humility” in psychology to understand how to approach this with kids, calling for “the humbleness as parents or providers in asking someone what they want used to make it the safest place for a discussion with our youth.”
One piece of the puzzle
Dr. Germain and Dr. Kamody agreed that language in discussing this topic is important but that we need to recognize that this topic in general is extremely tricky.
For one, “there are these very real metabolic complications of having high weight at a young age,” said Kamody, who stresses the need for balance to make real change.
Dr. Germain agreed. “Finding a fine line between discussing obesity and not kicking your kid into disordered eating is important.”
The researchers also recognize the limits of an online study, where self-reporting parents may not want to admit using negative weight terminology, but certainly believe it’s a start in identifying some of the undesirable patterns that may be occurring when it comes to weight.
“The overarching message is a positive one, that with our preteens and teenage kids, we need to watch our language, to create a nonjudgmental and safe environment to discuss weight and any issue involved with taking care of themselves,” said Dr. Germain.
A version of this article first appeared on Medscape.com.
With youth obesity on the rise – an estimated 1 in 5 youths are impacted by obesity, according to the Centers for Disease Control and Prevention – conversations about healthy weight are becoming more commonplace, not only in the pediatrician’s office, but at home, too. But the language we use around this sensitive topic is important, as youths are acutely aware that words have a direct impact on their mental health.
Sixteen-year-old Avery DiCocco of Northbrook, Ill., knows how vulnerable teenagers feel.
“I think it definitely matters the way that parents and doctors address weight,” she said. “You never know who may be insecure, and using negative words could go a lot further than they think with impacting self-esteem.”
A new study published in Pediatrics brings light to the words that parents (and providers) use when speaking to youths (ages 10-17) about their weight.
Researchers from the University of Connecticut Rudd Center for Food Policy & Health, Hartford, led an online survey of youths and their parents. Those who took part were asked about 27 terms related to body weight. Parents were asked to comment on their use of these words, while youths commented on the emotional response. The researchers said 1,936 parents and 2,032 adolescents were surveyed between September and December 2021.
Although results skewed toward the use of more positive words, such as “healthy weight,” over terms like “obese,” “fat” or “large,” there was variation across ethnicity, sexual orientation, and weight status. For example, it was noted in the study, funded by WW International, that preference for the word “curvy” was higher among Hispanic/Latino youths, sexual minority youths, and those with a body mass index in the 95th percentile, compared with their White, heterosexual, and lower-weight peers.
Words matter
In 2017, the American Academy of Pediatrics came out with a policy statement on weight stigma and the need for doctors to use more neutral language and less stigmatizing terms in practice when discussing weight among youths.
But one of the reasons this new study is important, said Gregory Germain, MD, associate chief of pediatrics at Yale New Haven (Conn.) Children’s Hospital, is that this study focuses on parents who interact with their kids much more often than a pediatrician who sees them a few times a year.
“Parental motivation, discussion, interaction on a consistent basis – that dialogue is so critical in kids with obesity,” said Germain, who stresses that all adults, coaches, and educators should consider this study as well.
“When we think about those detrimental impacts on mental health when more stigmatizing language is used, just us being more mindful in how we are talking to youth can make such a profound impact,” said Rebecca Kamody, PhD, a clinical psychologist at Yale University, with a research and clinical focus on eating and weight disorders.
“In essence, this is a low-hanging fruit intervention,” she said.
Dr. Kamody recommends we take a lesson from “cultural humility” in psychology to understand how to approach this with kids, calling for “the humbleness as parents or providers in asking someone what they want used to make it the safest place for a discussion with our youth.”
One piece of the puzzle
Dr. Germain and Dr. Kamody agreed that language in discussing this topic is important but that we need to recognize that this topic in general is extremely tricky.
For one, “there are these very real metabolic complications of having high weight at a young age,” said Kamody, who stresses the need for balance to make real change.
Dr. Germain agreed. “Finding a fine line between discussing obesity and not kicking your kid into disordered eating is important.”
The researchers also recognize the limits of an online study, where self-reporting parents may not want to admit using negative weight terminology, but certainly believe it’s a start in identifying some of the undesirable patterns that may be occurring when it comes to weight.
“The overarching message is a positive one, that with our preteens and teenage kids, we need to watch our language, to create a nonjudgmental and safe environment to discuss weight and any issue involved with taking care of themselves,” said Dr. Germain.
A version of this article first appeared on Medscape.com.
With youth obesity on the rise – an estimated 1 in 5 youths are impacted by obesity, according to the Centers for Disease Control and Prevention – conversations about healthy weight are becoming more commonplace, not only in the pediatrician’s office, but at home, too. But the language we use around this sensitive topic is important, as youths are acutely aware that words have a direct impact on their mental health.
Sixteen-year-old Avery DiCocco of Northbrook, Ill., knows how vulnerable teenagers feel.
“I think it definitely matters the way that parents and doctors address weight,” she said. “You never know who may be insecure, and using negative words could go a lot further than they think with impacting self-esteem.”
A new study published in Pediatrics brings light to the words that parents (and providers) use when speaking to youths (ages 10-17) about their weight.
Researchers from the University of Connecticut Rudd Center for Food Policy & Health, Hartford, led an online survey of youths and their parents. Those who took part were asked about 27 terms related to body weight. Parents were asked to comment on their use of these words, while youths commented on the emotional response. The researchers said 1,936 parents and 2,032 adolescents were surveyed between September and December 2021.
Although results skewed toward the use of more positive words, such as “healthy weight,” over terms like “obese,” “fat” or “large,” there was variation across ethnicity, sexual orientation, and weight status. For example, it was noted in the study, funded by WW International, that preference for the word “curvy” was higher among Hispanic/Latino youths, sexual minority youths, and those with a body mass index in the 95th percentile, compared with their White, heterosexual, and lower-weight peers.
Words matter
In 2017, the American Academy of Pediatrics came out with a policy statement on weight stigma and the need for doctors to use more neutral language and less stigmatizing terms in practice when discussing weight among youths.
But one of the reasons this new study is important, said Gregory Germain, MD, associate chief of pediatrics at Yale New Haven (Conn.) Children’s Hospital, is that this study focuses on parents who interact with their kids much more often than a pediatrician who sees them a few times a year.
“Parental motivation, discussion, interaction on a consistent basis – that dialogue is so critical in kids with obesity,” said Germain, who stresses that all adults, coaches, and educators should consider this study as well.
“When we think about those detrimental impacts on mental health when more stigmatizing language is used, just us being more mindful in how we are talking to youth can make such a profound impact,” said Rebecca Kamody, PhD, a clinical psychologist at Yale University, with a research and clinical focus on eating and weight disorders.
“In essence, this is a low-hanging fruit intervention,” she said.
Dr. Kamody recommends we take a lesson from “cultural humility” in psychology to understand how to approach this with kids, calling for “the humbleness as parents or providers in asking someone what they want used to make it the safest place for a discussion with our youth.”
One piece of the puzzle
Dr. Germain and Dr. Kamody agreed that language in discussing this topic is important but that we need to recognize that this topic in general is extremely tricky.
For one, “there are these very real metabolic complications of having high weight at a young age,” said Kamody, who stresses the need for balance to make real change.
Dr. Germain agreed. “Finding a fine line between discussing obesity and not kicking your kid into disordered eating is important.”
The researchers also recognize the limits of an online study, where self-reporting parents may not want to admit using negative weight terminology, but certainly believe it’s a start in identifying some of the undesirable patterns that may be occurring when it comes to weight.
“The overarching message is a positive one, that with our preteens and teenage kids, we need to watch our language, to create a nonjudgmental and safe environment to discuss weight and any issue involved with taking care of themselves,” said Dr. Germain.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
FDA expands list of Getinge IABP system and component shortages
The U.S. Food and Drug Administration issued a letter to health care providers describing a current shortage of Getinge intra-aortic balloon pump (IABP) catheters and other components.
Earlier, the agency announced shortages of the company’s Maquet/Datascope IAB catheters, new Cardiosave IABP devices, and Cardiosave IABP parts. The new notification adds Getinge Maquet/Datascope IABP systems to the list.
The company’s letter explains that “ongoing supply chain issues have significantly impacted our ability to build intra-aortic balloon pumps, intra-aortic balloon catheters, and spare parts due to raw material shortages.”
It also offers guidance on maintaining Cardiosave Safety Disks and lithium-ion batteries in the face of the shortages. “In the event that you need a replacement pump while your IABP is undergoing service, please contact your local sales representative who may be able to assist with a temporary IABP.”
Providers are instructed to inform the company through its sales representatives “if you have any underutilized Maquet/Datascope IAB catheters or IABPs and are willing to share them with hospitals in need.”
The shortages are expected to continue into 2023, the FDA states in its letter.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration issued a letter to health care providers describing a current shortage of Getinge intra-aortic balloon pump (IABP) catheters and other components.
Earlier, the agency announced shortages of the company’s Maquet/Datascope IAB catheters, new Cardiosave IABP devices, and Cardiosave IABP parts. The new notification adds Getinge Maquet/Datascope IABP systems to the list.
The company’s letter explains that “ongoing supply chain issues have significantly impacted our ability to build intra-aortic balloon pumps, intra-aortic balloon catheters, and spare parts due to raw material shortages.”
It also offers guidance on maintaining Cardiosave Safety Disks and lithium-ion batteries in the face of the shortages. “In the event that you need a replacement pump while your IABP is undergoing service, please contact your local sales representative who may be able to assist with a temporary IABP.”
Providers are instructed to inform the company through its sales representatives “if you have any underutilized Maquet/Datascope IAB catheters or IABPs and are willing to share them with hospitals in need.”
The shortages are expected to continue into 2023, the FDA states in its letter.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration issued a letter to health care providers describing a current shortage of Getinge intra-aortic balloon pump (IABP) catheters and other components.
Earlier, the agency announced shortages of the company’s Maquet/Datascope IAB catheters, new Cardiosave IABP devices, and Cardiosave IABP parts. The new notification adds Getinge Maquet/Datascope IABP systems to the list.
The company’s letter explains that “ongoing supply chain issues have significantly impacted our ability to build intra-aortic balloon pumps, intra-aortic balloon catheters, and spare parts due to raw material shortages.”
It also offers guidance on maintaining Cardiosave Safety Disks and lithium-ion batteries in the face of the shortages. “In the event that you need a replacement pump while your IABP is undergoing service, please contact your local sales representative who may be able to assist with a temporary IABP.”
Providers are instructed to inform the company through its sales representatives “if you have any underutilized Maquet/Datascope IAB catheters or IABPs and are willing to share them with hospitals in need.”
The shortages are expected to continue into 2023, the FDA states in its letter.
A version of this article first appeared on Medscape.com.
Medically speaking, 2022 was the best year yet for children
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Employers use patient assistance programs to offset their own costs
Anna Sutton was shocked when she received a letter from her husband’s job-based health plan stating that Humira, an expensive drug used to treat her daughter’s juvenile arthritis, was now on a long list of medications considered “nonessential benefits.”
The July 2021 letter said the family could either participate in a new effort overseen by a company called SaveOnSP and get the drug free of charge or be saddled with a monthly copayment that could top $1,000.
“It really gave us no choice,” said Mrs. Sutton, of Woodinville, Wash. She added that “every single [Food and Drug Administration]–approved medication for juvenile arthritis” was on the list of nonessential benefits.
Mrs. Sutton had unwittingly become part of a strategy that employers are using to deal with the high cost of drugs prescribed to treat conditions such as arthritis, psoriasis, cancer, and hemophilia.
Those employers are tapping into dollars provided through programs they have previously criticized: patient financial assistance initiatives set up by drugmakers, which some benefit managers have complained encourage patients to stay on expensive brand-name drugs when less expensive options might be available.
Now, though, employers, or the vendors and insurers they hire specifically to oversee such efforts, are seeking that money to offset their own costs. Drugmakers object, saying the money was intended primarily for patients. But some benefit brokers and companies like SaveOnSP say they can help trim employers’ spending on insurance – which, they say, could be the difference between an employer offering coverage to workers or not.
It’s the latest twist in a long-running dispute between the drug industry and insurers over which group is more to blame for rising costs to patients. And patients are, again, caught in the middle.
Patient advocates say the term “nonessential” stresses patients out even though it doesn’t mean the drugs – often called “specialty” drugs because of their high prices or the way they are made – are unnecessary.
Some advocates fear the new strategies could be “a way to weed out those with costly health care needs,” said Rachel Klein, deputy executive director of the AIDS Institute, a nonprofit advocacy group. Workers who rely on the drugs may feel pressured to change insurers or jobs.
Two versions of the new strategy are in play. Both are used mainly by self-insured employers that hire vendors, like SaveOnSP, which then work with the employers’ pharmacy benefit managers, such as Express Scripts/Cigna, to implement the strategy. There are also smaller vendors, like SHARx and Payer Matrix, some of which work directly with employers.
In one approach, insurers or employers continue to cover the drugs but designate them as “nonessential,” which allows the health plans to bypass annual limits set by the Affordable Care Act on how much patients can pay in out-of-pocket costs for drugs. The employer or hired vendor then raises the copay required of the worker, often sharply, but offers to substantially cut or eliminate that copay if the patient participates in the new effort. Workers who agree enroll in drugmaker financial assistance programs meant to cover the drug copays, and the vendor monitoring the effort aims to capture the maximum amount the drugmaker provides annually, according to a lawsuit filed in May by drugmaker Johnson & Johnson against SaveOnSP, which is based in Elma, N.Y.
The employer must still cover part of the cost of the drug, but the amount is reduced by the amount of copay assistance that is accessed. That assistance can vary widely and be as much as $20,000 a year for some drugs.
In the other approach, employers don’t bother naming drugs nonessential; they simply drop coverage for specific drugs or classes of drugs. Then, the outside vendor helps patients provide the financial and other information needed to apply for free medication from drugmakers through charity programs intended for uninsured patients.
“We’re seeing it in every state at this point,” said Becky Burns, chief operating officer and chief financial officer at the Bleeding and Clotting Disorders Institute in Peoria, Ill., a federally funded hemophilia treatment center.
The strategies are mostly being used in self-insured employer health plans, which are governed by federal laws that give broad flexibility to employers in designing health benefits.
Still, some patient advocates say these programs can lead to delays for patients in accessing medications while applications are processed – and sometimes unexpected bills for consumers.
“We have patients get billed after they max out their assistance,” said Kollet Koulianos, vice president of payer relations at the National Hemophilia Foundation. Once she gets involved, vendors often claim the bills were sent in error.
Even though only about 2% of the workforce needs the drugs, which can cost thousands of dollars a dose, they can lead to a hefty financial liability for self-insured employers, said Drew Mann, a benefits consultant in Knoxville, Tenn., whose clientele includes employers that use variations of these programs.
Before employer health plans took advantage of such assistance, patients often signed up for these programs on their own, receiving coupons that covered their share of the drug’s cost. In that circumstance, drugmakers often paid less than they do under the new employer schemes because a patient’s out-of-pocket costs were capped at lower amounts.
Brokers and the CEOs of firms offering the new programs say that in most cases patients continue to get their drugs, often with little or no out-of-pocket costs.
If workers do not qualify for charity because their income is too high, or for another reason, the employer might make an exception and pay the claim or look for an alternative solution, Mr. Mann said. Patient groups noted that some specialty drugs may not have any alternatives.
How this practice will play out in the long run remains uncertain. Drugmakers offer both copay assistance and charity care in part because they know many patients, even those with insurance, cannot afford their products. The programs are also good public relations and a tax write-off. But the new emphasis by some employers on maximizing the amount they or their insurers can collect from the programs could cause some drugmakers to take issue with the new strategies or even reconsider their programs.
“Even though our client, like most manufacturers, provides billions in discounts and rebates to health insurers as part of their negotiations, the insurers also want this additional pool of funds, which is meant to help people who can’t meet the copay,” said Harry Sandick, a lawyer representing J&J.
J&J’s lawsuit, filed in U.S. District Court in New Jersey, alleges that patients are “coerced” into participating in copay assistance programs after their drugs are deemed “nonessential” and therefore are “no longer subject to the ACA’s annual out-of-pocket maximum.”
Once patients enroll, the money from the drugmaker goes to the insurer or employer plan, with SaveOnSP retaining 25%, according to the lawsuit. It claims J&J has lost $100 million to these efforts.
None of that money counts toward patients’ deductibles or out-of-pocket maximums for the year.
In addition to the lawsuit over the copay assistance program efforts, there has been other reaction to the new employer strategies. In an October letter to physicians, the Johnson & Johnson Patient Assistance Foundation, a separate entity, said it will no longer offer free medications to patients with insurance starting in January, citing the rise of such “alternative funding programs.”
Still, J&J spokesperson L.D. Platt said the drugmaker has plans, also in January, to roll out other assistance to patients who may be “underinsured” so they won’t be affected by the foundation’s decision.
In a statement, SaveOnSP said that employers object to drug companies’ “using their employees’ ongoing need for these drugs as an excuse to keep hiking the drugs’ prices” and that the firm simply “advises these employers on how to fight back against rising prices while getting employees the drugs they need at no cost to the employees.”
In a court filing, SaveOnSP said drugmakers have another option if they don’t like efforts by insurers and employers to max out what they can get from the programs: reduce the amount of assistance available. J&J, the filing said, did just that when it recently cut its allotted amount of copay assistance for psoriasis drugs Stelara and Tremfya from $20,000 to $6,000 per participant annually. The filing noted that SaveOnSP participants would still have no copay for those drugs.
For Mrs. Sutton’s part, her family did participate in the program offered through her husband’s work-based insurance plan, agreeing to have SaveOnSP monitor their enrollment and payments from the drugmaker.
So far, her 15-year-old daughter has continued to get Humira, and she has not been billed a copay.
Even so, “the whole process seems kind of slimy to me,” she said. “The patients are caught in the middle between the drug industry and the insurance industry, each trying to get as much money as possible out of the other.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anna Sutton was shocked when she received a letter from her husband’s job-based health plan stating that Humira, an expensive drug used to treat her daughter’s juvenile arthritis, was now on a long list of medications considered “nonessential benefits.”
The July 2021 letter said the family could either participate in a new effort overseen by a company called SaveOnSP and get the drug free of charge or be saddled with a monthly copayment that could top $1,000.
“It really gave us no choice,” said Mrs. Sutton, of Woodinville, Wash. She added that “every single [Food and Drug Administration]–approved medication for juvenile arthritis” was on the list of nonessential benefits.
Mrs. Sutton had unwittingly become part of a strategy that employers are using to deal with the high cost of drugs prescribed to treat conditions such as arthritis, psoriasis, cancer, and hemophilia.
Those employers are tapping into dollars provided through programs they have previously criticized: patient financial assistance initiatives set up by drugmakers, which some benefit managers have complained encourage patients to stay on expensive brand-name drugs when less expensive options might be available.
Now, though, employers, or the vendors and insurers they hire specifically to oversee such efforts, are seeking that money to offset their own costs. Drugmakers object, saying the money was intended primarily for patients. But some benefit brokers and companies like SaveOnSP say they can help trim employers’ spending on insurance – which, they say, could be the difference between an employer offering coverage to workers or not.
It’s the latest twist in a long-running dispute between the drug industry and insurers over which group is more to blame for rising costs to patients. And patients are, again, caught in the middle.
Patient advocates say the term “nonessential” stresses patients out even though it doesn’t mean the drugs – often called “specialty” drugs because of their high prices or the way they are made – are unnecessary.
Some advocates fear the new strategies could be “a way to weed out those with costly health care needs,” said Rachel Klein, deputy executive director of the AIDS Institute, a nonprofit advocacy group. Workers who rely on the drugs may feel pressured to change insurers or jobs.
Two versions of the new strategy are in play. Both are used mainly by self-insured employers that hire vendors, like SaveOnSP, which then work with the employers’ pharmacy benefit managers, such as Express Scripts/Cigna, to implement the strategy. There are also smaller vendors, like SHARx and Payer Matrix, some of which work directly with employers.
In one approach, insurers or employers continue to cover the drugs but designate them as “nonessential,” which allows the health plans to bypass annual limits set by the Affordable Care Act on how much patients can pay in out-of-pocket costs for drugs. The employer or hired vendor then raises the copay required of the worker, often sharply, but offers to substantially cut or eliminate that copay if the patient participates in the new effort. Workers who agree enroll in drugmaker financial assistance programs meant to cover the drug copays, and the vendor monitoring the effort aims to capture the maximum amount the drugmaker provides annually, according to a lawsuit filed in May by drugmaker Johnson & Johnson against SaveOnSP, which is based in Elma, N.Y.
The employer must still cover part of the cost of the drug, but the amount is reduced by the amount of copay assistance that is accessed. That assistance can vary widely and be as much as $20,000 a year for some drugs.
In the other approach, employers don’t bother naming drugs nonessential; they simply drop coverage for specific drugs or classes of drugs. Then, the outside vendor helps patients provide the financial and other information needed to apply for free medication from drugmakers through charity programs intended for uninsured patients.
“We’re seeing it in every state at this point,” said Becky Burns, chief operating officer and chief financial officer at the Bleeding and Clotting Disorders Institute in Peoria, Ill., a federally funded hemophilia treatment center.
The strategies are mostly being used in self-insured employer health plans, which are governed by federal laws that give broad flexibility to employers in designing health benefits.
Still, some patient advocates say these programs can lead to delays for patients in accessing medications while applications are processed – and sometimes unexpected bills for consumers.
“We have patients get billed after they max out their assistance,” said Kollet Koulianos, vice president of payer relations at the National Hemophilia Foundation. Once she gets involved, vendors often claim the bills were sent in error.
Even though only about 2% of the workforce needs the drugs, which can cost thousands of dollars a dose, they can lead to a hefty financial liability for self-insured employers, said Drew Mann, a benefits consultant in Knoxville, Tenn., whose clientele includes employers that use variations of these programs.
Before employer health plans took advantage of such assistance, patients often signed up for these programs on their own, receiving coupons that covered their share of the drug’s cost. In that circumstance, drugmakers often paid less than they do under the new employer schemes because a patient’s out-of-pocket costs were capped at lower amounts.
Brokers and the CEOs of firms offering the new programs say that in most cases patients continue to get their drugs, often with little or no out-of-pocket costs.
If workers do not qualify for charity because their income is too high, or for another reason, the employer might make an exception and pay the claim or look for an alternative solution, Mr. Mann said. Patient groups noted that some specialty drugs may not have any alternatives.
How this practice will play out in the long run remains uncertain. Drugmakers offer both copay assistance and charity care in part because they know many patients, even those with insurance, cannot afford their products. The programs are also good public relations and a tax write-off. But the new emphasis by some employers on maximizing the amount they or their insurers can collect from the programs could cause some drugmakers to take issue with the new strategies or even reconsider their programs.
“Even though our client, like most manufacturers, provides billions in discounts and rebates to health insurers as part of their negotiations, the insurers also want this additional pool of funds, which is meant to help people who can’t meet the copay,” said Harry Sandick, a lawyer representing J&J.
J&J’s lawsuit, filed in U.S. District Court in New Jersey, alleges that patients are “coerced” into participating in copay assistance programs after their drugs are deemed “nonessential” and therefore are “no longer subject to the ACA’s annual out-of-pocket maximum.”
Once patients enroll, the money from the drugmaker goes to the insurer or employer plan, with SaveOnSP retaining 25%, according to the lawsuit. It claims J&J has lost $100 million to these efforts.
None of that money counts toward patients’ deductibles or out-of-pocket maximums for the year.
In addition to the lawsuit over the copay assistance program efforts, there has been other reaction to the new employer strategies. In an October letter to physicians, the Johnson & Johnson Patient Assistance Foundation, a separate entity, said it will no longer offer free medications to patients with insurance starting in January, citing the rise of such “alternative funding programs.”
Still, J&J spokesperson L.D. Platt said the drugmaker has plans, also in January, to roll out other assistance to patients who may be “underinsured” so they won’t be affected by the foundation’s decision.
In a statement, SaveOnSP said that employers object to drug companies’ “using their employees’ ongoing need for these drugs as an excuse to keep hiking the drugs’ prices” and that the firm simply “advises these employers on how to fight back against rising prices while getting employees the drugs they need at no cost to the employees.”
In a court filing, SaveOnSP said drugmakers have another option if they don’t like efforts by insurers and employers to max out what they can get from the programs: reduce the amount of assistance available. J&J, the filing said, did just that when it recently cut its allotted amount of copay assistance for psoriasis drugs Stelara and Tremfya from $20,000 to $6,000 per participant annually. The filing noted that SaveOnSP participants would still have no copay for those drugs.
For Mrs. Sutton’s part, her family did participate in the program offered through her husband’s work-based insurance plan, agreeing to have SaveOnSP monitor their enrollment and payments from the drugmaker.
So far, her 15-year-old daughter has continued to get Humira, and she has not been billed a copay.
Even so, “the whole process seems kind of slimy to me,” she said. “The patients are caught in the middle between the drug industry and the insurance industry, each trying to get as much money as possible out of the other.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anna Sutton was shocked when she received a letter from her husband’s job-based health plan stating that Humira, an expensive drug used to treat her daughter’s juvenile arthritis, was now on a long list of medications considered “nonessential benefits.”
The July 2021 letter said the family could either participate in a new effort overseen by a company called SaveOnSP and get the drug free of charge or be saddled with a monthly copayment that could top $1,000.
“It really gave us no choice,” said Mrs. Sutton, of Woodinville, Wash. She added that “every single [Food and Drug Administration]–approved medication for juvenile arthritis” was on the list of nonessential benefits.
Mrs. Sutton had unwittingly become part of a strategy that employers are using to deal with the high cost of drugs prescribed to treat conditions such as arthritis, psoriasis, cancer, and hemophilia.
Those employers are tapping into dollars provided through programs they have previously criticized: patient financial assistance initiatives set up by drugmakers, which some benefit managers have complained encourage patients to stay on expensive brand-name drugs when less expensive options might be available.
Now, though, employers, or the vendors and insurers they hire specifically to oversee such efforts, are seeking that money to offset their own costs. Drugmakers object, saying the money was intended primarily for patients. But some benefit brokers and companies like SaveOnSP say they can help trim employers’ spending on insurance – which, they say, could be the difference between an employer offering coverage to workers or not.
It’s the latest twist in a long-running dispute between the drug industry and insurers over which group is more to blame for rising costs to patients. And patients are, again, caught in the middle.
Patient advocates say the term “nonessential” stresses patients out even though it doesn’t mean the drugs – often called “specialty” drugs because of their high prices or the way they are made – are unnecessary.
Some advocates fear the new strategies could be “a way to weed out those with costly health care needs,” said Rachel Klein, deputy executive director of the AIDS Institute, a nonprofit advocacy group. Workers who rely on the drugs may feel pressured to change insurers or jobs.
Two versions of the new strategy are in play. Both are used mainly by self-insured employers that hire vendors, like SaveOnSP, which then work with the employers’ pharmacy benefit managers, such as Express Scripts/Cigna, to implement the strategy. There are also smaller vendors, like SHARx and Payer Matrix, some of which work directly with employers.
In one approach, insurers or employers continue to cover the drugs but designate them as “nonessential,” which allows the health plans to bypass annual limits set by the Affordable Care Act on how much patients can pay in out-of-pocket costs for drugs. The employer or hired vendor then raises the copay required of the worker, often sharply, but offers to substantially cut or eliminate that copay if the patient participates in the new effort. Workers who agree enroll in drugmaker financial assistance programs meant to cover the drug copays, and the vendor monitoring the effort aims to capture the maximum amount the drugmaker provides annually, according to a lawsuit filed in May by drugmaker Johnson & Johnson against SaveOnSP, which is based in Elma, N.Y.
The employer must still cover part of the cost of the drug, but the amount is reduced by the amount of copay assistance that is accessed. That assistance can vary widely and be as much as $20,000 a year for some drugs.
In the other approach, employers don’t bother naming drugs nonessential; they simply drop coverage for specific drugs or classes of drugs. Then, the outside vendor helps patients provide the financial and other information needed to apply for free medication from drugmakers through charity programs intended for uninsured patients.
“We’re seeing it in every state at this point,” said Becky Burns, chief operating officer and chief financial officer at the Bleeding and Clotting Disorders Institute in Peoria, Ill., a federally funded hemophilia treatment center.
The strategies are mostly being used in self-insured employer health plans, which are governed by federal laws that give broad flexibility to employers in designing health benefits.
Still, some patient advocates say these programs can lead to delays for patients in accessing medications while applications are processed – and sometimes unexpected bills for consumers.
“We have patients get billed after they max out their assistance,” said Kollet Koulianos, vice president of payer relations at the National Hemophilia Foundation. Once she gets involved, vendors often claim the bills were sent in error.
Even though only about 2% of the workforce needs the drugs, which can cost thousands of dollars a dose, they can lead to a hefty financial liability for self-insured employers, said Drew Mann, a benefits consultant in Knoxville, Tenn., whose clientele includes employers that use variations of these programs.
Before employer health plans took advantage of such assistance, patients often signed up for these programs on their own, receiving coupons that covered their share of the drug’s cost. In that circumstance, drugmakers often paid less than they do under the new employer schemes because a patient’s out-of-pocket costs were capped at lower amounts.
Brokers and the CEOs of firms offering the new programs say that in most cases patients continue to get their drugs, often with little or no out-of-pocket costs.
If workers do not qualify for charity because their income is too high, or for another reason, the employer might make an exception and pay the claim or look for an alternative solution, Mr. Mann said. Patient groups noted that some specialty drugs may not have any alternatives.
How this practice will play out in the long run remains uncertain. Drugmakers offer both copay assistance and charity care in part because they know many patients, even those with insurance, cannot afford their products. The programs are also good public relations and a tax write-off. But the new emphasis by some employers on maximizing the amount they or their insurers can collect from the programs could cause some drugmakers to take issue with the new strategies or even reconsider their programs.
“Even though our client, like most manufacturers, provides billions in discounts and rebates to health insurers as part of their negotiations, the insurers also want this additional pool of funds, which is meant to help people who can’t meet the copay,” said Harry Sandick, a lawyer representing J&J.
J&J’s lawsuit, filed in U.S. District Court in New Jersey, alleges that patients are “coerced” into participating in copay assistance programs after their drugs are deemed “nonessential” and therefore are “no longer subject to the ACA’s annual out-of-pocket maximum.”
Once patients enroll, the money from the drugmaker goes to the insurer or employer plan, with SaveOnSP retaining 25%, according to the lawsuit. It claims J&J has lost $100 million to these efforts.
None of that money counts toward patients’ deductibles or out-of-pocket maximums for the year.
In addition to the lawsuit over the copay assistance program efforts, there has been other reaction to the new employer strategies. In an October letter to physicians, the Johnson & Johnson Patient Assistance Foundation, a separate entity, said it will no longer offer free medications to patients with insurance starting in January, citing the rise of such “alternative funding programs.”
Still, J&J spokesperson L.D. Platt said the drugmaker has plans, also in January, to roll out other assistance to patients who may be “underinsured” so they won’t be affected by the foundation’s decision.
In a statement, SaveOnSP said that employers object to drug companies’ “using their employees’ ongoing need for these drugs as an excuse to keep hiking the drugs’ prices” and that the firm simply “advises these employers on how to fight back against rising prices while getting employees the drugs they need at no cost to the employees.”
In a court filing, SaveOnSP said drugmakers have another option if they don’t like efforts by insurers and employers to max out what they can get from the programs: reduce the amount of assistance available. J&J, the filing said, did just that when it recently cut its allotted amount of copay assistance for psoriasis drugs Stelara and Tremfya from $20,000 to $6,000 per participant annually. The filing noted that SaveOnSP participants would still have no copay for those drugs.
For Mrs. Sutton’s part, her family did participate in the program offered through her husband’s work-based insurance plan, agreeing to have SaveOnSP monitor their enrollment and payments from the drugmaker.
So far, her 15-year-old daughter has continued to get Humira, and she has not been billed a copay.
Even so, “the whole process seems kind of slimy to me,” she said. “The patients are caught in the middle between the drug industry and the insurance industry, each trying to get as much money as possible out of the other.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Joint replacements: Should there be BMI cutoffs?
For patients with severe arthritis, joint replacement is considered when more conservative treatments have failed. Because patients with obesity have a higher risk of complications during and after surgery, some surgeons, hospitals, and insurance companies have adopted body mass index cutoffs as a basis for deciding whether to offer patients these elective surgeries. But some experts argue that these cutoffs are arbitrary, exclude patients who can still benefit from the surgery, and can increase disparities in care.
“By enforcing cutoffs in general, you’re losing the ability for each surgeon to determine who they want to operate on,” said Daniel Wiznia, MD, assistant professor of orthopedic surgery at Yale University, New Haven, Conn. He is on the leadership committee of the Movement Is Life Caucus, a nonprofit group focused on eliminating disparities in musculoskeletal health. “For every surgeon, it’s up to them to decide if they feel comfortable doing the surgery,” he noted in an interview. “My guidance for that would be, don’t just say no because of the number – look at the patient’s entire medical profile.”
According to the Centers for Disease Control and Prevention, nearly 42% of adults in the United States have a BMI over 30, and 9.2% of adults have a BMI over 40. This excess weight puts additional stress on joints: When a person is walking, experts estimate that the force on the knees can be two to three times someone’s body weight. Over time, this pressure can wear down the cartilage on joints.
As a result, people who are overweight or obese are more likely to develop osteoarthritis and to need joint replacements. According to a Canadian study, patients with a BMI of 30-35 are 3.4 times as likely to require a hip replacement and are 8.5 times as likely to require a knee replacement compared to individuals with a BMI in the “healthy weight” range. With a BMI above 40, individuals were 8.5 times more likely to need a hip replacement and were 32.7 times as likely to need a knee replacement.
More complications, greater expense
While there are no universally recommended BMI cutoffs for joint replacement surgery, it is not uncommon for institutions to require that patients have a BMI below a certain value (usually 35-40) to proceed with surgery. A 2013 survey of physicians from the American Association of Hip and Knee Surgeons found that 52% of surgeons required a BMI below 40 to qualify for surgery.
One of the main reasons for these cutoffs is the elevated risk of complications during and after surgery. Research suggests that obesity is associated with higher rates of wound dehiscence, prosthetic joint infection (PJI), and revision total joint arthroplasty. One 2016 study suggests that patients with a BMI of 35-39.9 are twice as likely to experience PJI compared to patients with a BMI below 35. For patients with a BMI of 40 or higher, PJI is four times as likely.
Another study found that patients whose BMI is 35-40 and who undergo total joint arthroplasty have a 6.4-fold greater risk of deep incision infection. For those with a BMI over 40, that rises to a 12.9-fold increased risk compared to patients with a BMI of 18.5-25. Patients with obesity tend to have other comorbidities that can increase the risk of complications during surgery, such as type 2 diabetes, coronary artery disease, and chronic kidney disease.
Because of the increased risk of complications, health care costs tend to be higher for patients with obesity. The growing popularity of bundled health payments can discourage operating on patients who are more likely to experience complications, such as patients with high BMIs, noted Dr. Wiznia.
Research suggests that minorities and people with lower socioeconomic status are disproportionately affected by these cutoffs. According to the CDC, among non-Hispanic Black Americans and Hispanic Americans, rates of obesity are higher than among their White counterparts, and these patients are less likely to undergo joint replacement. Strictly enforcing this eligibility criterion can worsen those disparities. A study involving 21,294 adults over age 50 from the National Health and Nutrition Examination Survey (NHANES) found that requiring a BMI of under 35 for total joint arthroplasty resulted in Black patients being 39% less likely to be eligible for surgery than White patients. And individuals with an annual household income under $45,000 were 19% less likely to qualify for surgery than those with a household income above $45,000.
BMI no better than other risk factors
Although high BMI is independently associated with a higher risk of complications, the increased risk of complications conferred by a BMI at or above 40 is similar to or lower than those of other comorbidities that surgeons generally accept, said Nicholas Giori, MD, PhD, professor of orthopedic surgery at Stanford (Calif.) University, and chief of orthopedic surgery at the VA Palo Alto Health Care System. These other comorbidities include age older than 75, hypertension that requires medication, and insulin-controlled diabetes. “The independent risk of just having the diagnosis of insulin-dependent diabetes is actually comparable to the independent risk of having obesity by itself,” he told this news organization, “and all of us operate on [patients with] diabetes.”
Also, there is no BMI at which the risk of complications suddenly increases, according to the American Academy of Orthopaedic Surgeons. “It’s a rising complication rate as you go into higher BMIs,” Dr. Giori said. “If you operate on someone with a BMI of 39 vs. 41, you’re not going to find that much of a difference [in risk].” But if a medical system enforced a hard BMI cutoff of 40, one patient would qualify for surgery while the other would be barred.
Weight not as “modifiable” as previously thought
Weight is often considered a “modifiable factor” for a person considering undergoing total joint arthroplasty, but research suggests that the issue is more complicated. “Obesity is tricky, because some people are successful [in weight loss],” said Dr. Giori. Those tend to be the more memorable stories. “But a large majority have a really hard time losing substantial weight – enough to make a difference in risk,” he continued.
A study conducted in North Carolina found that restricting patients with a BMI over 40 from having elective total joint arthroplasty procedures until their weight was optimized did not result in successful weight loss. Only 20% of patients who originally presented with a BMI above this limit eventually underwent surgery after 2 years, and fewer than half of these patients had achieved a BMI of less than 40 at the time of their surgery. A third of all patients in the study did not return to the orthopedic office after their first visit.
“To hold a hard cutoff when it’s very, very hard to modify ... is essentially telling people that they are not going to ever have surgery,” Dr. Giori said; “I think that can be unfair to some patients.”
Bariatric surgery is often suggested for patients with obesity who have not experienced successful weight loss with diet and lifestyle changes alone, but bariatric surgery comes with its own complications. Research on outcomes from total joint arthroplasty among patients with who have lost weight with bariatric surgery has yielded mixed results. “I rarely push anyone hard to go that route but present it as an option for certain patients,” said Benjamin M. Stronach, MD, an orthopedic surgeon at the University of Arkansas for Medical Sciences, in Little Rock. He usually brings up bariatric surgery with patients with a BMI in the high 40s or higher to gauge their interest. If patients are already considering weight loss surgery, his office provides referrals.
But even bariatric surgery does not result in successful long-term weight loss for every patient, Dr. Stronach said. He’s seeing more and more patients who come for consultations after having undergone bariatric surgery 10 to 15 years ago. These patients lost a significant amount of weight, but then gained the weight back. He noted that bariatric surgery can be very successful for some patients who adhere to their postbariatric regimen. “We typically see fairly impressive results in the short term,” he said.
Patients with obesity benefit from joint replacement
Although patients with obesity are at higher risk for complications from joint replacement surgery, research suggests that these patients can still benefit greatly from these surgeries and that these surgeries remain cost-effective. Some studies have found that patients with obesity tend to have worse outcomes after surgery than patients who are not obese, but often, patients with high BMIs are starting from a lower point, with greater joint pain and limited mobility, Dr. Giori said. But the improvements – that is, net change in measured outcomes – can be greater for obese patients.
“Several studies have shown equal or greater improvements in validated outcome scores, function, and satisfaction compared with nonobese patients after surgery,” authors wrote in a recent review article in which they discuss how to optimize joint replacement surgery for patients with obesity. The article, published in the November 2022 issue of the Journal of the American Academy of Orthopaedic Surgeon (JAAOS), is part of a collection of review articles by the Movement Is Life Caucus.
Encourage weight loss, but look beyond the number
Rather than adhering to strict BMI cutoffs, some experts urge surgeons to consider the patient as a whole and to evaluate each individual’s overall health and potential risk. Dr. Giori generally considers high BMI as just another comorbidity when assessing a patient’s overall risk. “For a person who only has a high BMI but is otherwise healthy, I see no reason not to go ahead and schedule that person for surgery, because reducing the patient’s BMI will not substantially reduce the patient’s complication risk, and a delay in surgery may adversely affect the patient’s quality of life and ability to earn a living,” he said.
“If someone is between a BMI of 40 and 45, we are definitely going to have a discussion about weight,” Dr. Stronach said. He generally counsels against surgery for any patient with a BMI at 45 or above. He wants patients to have a BMI below 40 before surgery but considers individual cases for exceptions. “We will still move forward at times with someone with a BMI of 41, as an example, who is otherwise healthy,” he said. Similarly, if a patient has lost a significant amount of weight (e.g., the patient’s BMI was reduced from 50 to 41), the patient is actively engaged in improving their health, and surgeons believe the patient has significantly reduced their risk, “a lot of time, we’re not going to draw a line in the sand right at [a BMI of] 40,” he said.
While using a BMI of under 35 or 40 as a guideline when starting to work with patients is reasonable, working toward a weight loss of 5%-10% of total body weight is another goal to consider, authors advise in the JAAOS obesity review article. Research suggests that even a 5% reduction in overall body weight can reduce surgical complications and can improve a patient’s glucose and lipid levels and cardiac profile. Referrals to dietitians and weight loss programs, as well as behavioral counseling, can also be useful in initiating weight loss and keeping patients engaged in the process, the authors wrote.
Consider a patient’s comorbidities
Many patients with obesity have comorbidities, such as type 2 diabetes and hypertension, that can also be optimized for surgery so as to lower a patient’s overall risk profile. For patients with diabetes, achieving an A1c of 8% or lower can be a reasonable goal and can reduce risk. “We’ve found that an HbA1c level of 8% or less is something that virtually all diabetics (though not everybody) can reach, and it’s something that can be reached in a reasonable amount of time,” Dr. Giori said. Preoperative use of beta-blockers, continued use of ACE inhibitors or angiotensin receptor blockers, and behavioral modifications can improve a patient’s cardiac health before surgery.
Malnutrition can be a correctable problem for patients, regardless of BMI. In the Movement Is Life collection of optimization articles, experts recommend that orthopedists screen for malnutrition with blood tests for albumin, vitamin D, transferrin, and total lymphocyte count. Patients with malnutrition should be screened for food insecurity, experts advise, and surgical candidates with deficiencies can be given supplements of omega-3 fatty acids, arginine, and protein shakes.
Surgeon comfort and shared decision-making
Dr. Wiznia emphasized that the patient and surgeon need to discuss the risks of surgery, concerns about potential complications, and how a complication could affect the patient’s life moving forward. “Ultimately, the surgeon needs to make the decision [of whether or not to proceed [with surgery] with the patient,” he said, “but not every surgeon is going to feel comfortable operating on these patients, and not every medical institution is going to have the equipment and the investments to support surgeons doing it.”
Dr. Giori agreed that surgeons should proceed only with surgical cases they feel comfortable with. Certain surgeons may decide not to operate on individuals with higher BMIs because of the potential complications and can refer these patients to more specialized care centers. Operating on larger patients is more difficult and requires surgical skills and expertise that the surgeon may not have, he noted. “What I do object to is a system-wide BMI cutoff – for example, if an insurance company won’t pay for you to have a joint replacement, regardless of where you go or who your surgeon is,” Dr. Giori added. “I think that’s wrong, because it’s not patient centered and it’s basically excluding people from having a life-altering operation.”
Dr. Giori and Dr. Wiznia report no relevant financial relationships. Dr. Stronach is a consultant for DJ Orthopaedics, Johnson & Johnson, and MiCare Path.
A version of this article first appeared on Medscape.com.
For patients with severe arthritis, joint replacement is considered when more conservative treatments have failed. Because patients with obesity have a higher risk of complications during and after surgery, some surgeons, hospitals, and insurance companies have adopted body mass index cutoffs as a basis for deciding whether to offer patients these elective surgeries. But some experts argue that these cutoffs are arbitrary, exclude patients who can still benefit from the surgery, and can increase disparities in care.
“By enforcing cutoffs in general, you’re losing the ability for each surgeon to determine who they want to operate on,” said Daniel Wiznia, MD, assistant professor of orthopedic surgery at Yale University, New Haven, Conn. He is on the leadership committee of the Movement Is Life Caucus, a nonprofit group focused on eliminating disparities in musculoskeletal health. “For every surgeon, it’s up to them to decide if they feel comfortable doing the surgery,” he noted in an interview. “My guidance for that would be, don’t just say no because of the number – look at the patient’s entire medical profile.”
According to the Centers for Disease Control and Prevention, nearly 42% of adults in the United States have a BMI over 30, and 9.2% of adults have a BMI over 40. This excess weight puts additional stress on joints: When a person is walking, experts estimate that the force on the knees can be two to three times someone’s body weight. Over time, this pressure can wear down the cartilage on joints.
As a result, people who are overweight or obese are more likely to develop osteoarthritis and to need joint replacements. According to a Canadian study, patients with a BMI of 30-35 are 3.4 times as likely to require a hip replacement and are 8.5 times as likely to require a knee replacement compared to individuals with a BMI in the “healthy weight” range. With a BMI above 40, individuals were 8.5 times more likely to need a hip replacement and were 32.7 times as likely to need a knee replacement.
More complications, greater expense
While there are no universally recommended BMI cutoffs for joint replacement surgery, it is not uncommon for institutions to require that patients have a BMI below a certain value (usually 35-40) to proceed with surgery. A 2013 survey of physicians from the American Association of Hip and Knee Surgeons found that 52% of surgeons required a BMI below 40 to qualify for surgery.
One of the main reasons for these cutoffs is the elevated risk of complications during and after surgery. Research suggests that obesity is associated with higher rates of wound dehiscence, prosthetic joint infection (PJI), and revision total joint arthroplasty. One 2016 study suggests that patients with a BMI of 35-39.9 are twice as likely to experience PJI compared to patients with a BMI below 35. For patients with a BMI of 40 or higher, PJI is four times as likely.
Another study found that patients whose BMI is 35-40 and who undergo total joint arthroplasty have a 6.4-fold greater risk of deep incision infection. For those with a BMI over 40, that rises to a 12.9-fold increased risk compared to patients with a BMI of 18.5-25. Patients with obesity tend to have other comorbidities that can increase the risk of complications during surgery, such as type 2 diabetes, coronary artery disease, and chronic kidney disease.
Because of the increased risk of complications, health care costs tend to be higher for patients with obesity. The growing popularity of bundled health payments can discourage operating on patients who are more likely to experience complications, such as patients with high BMIs, noted Dr. Wiznia.
Research suggests that minorities and people with lower socioeconomic status are disproportionately affected by these cutoffs. According to the CDC, among non-Hispanic Black Americans and Hispanic Americans, rates of obesity are higher than among their White counterparts, and these patients are less likely to undergo joint replacement. Strictly enforcing this eligibility criterion can worsen those disparities. A study involving 21,294 adults over age 50 from the National Health and Nutrition Examination Survey (NHANES) found that requiring a BMI of under 35 for total joint arthroplasty resulted in Black patients being 39% less likely to be eligible for surgery than White patients. And individuals with an annual household income under $45,000 were 19% less likely to qualify for surgery than those with a household income above $45,000.
BMI no better than other risk factors
Although high BMI is independently associated with a higher risk of complications, the increased risk of complications conferred by a BMI at or above 40 is similar to or lower than those of other comorbidities that surgeons generally accept, said Nicholas Giori, MD, PhD, professor of orthopedic surgery at Stanford (Calif.) University, and chief of orthopedic surgery at the VA Palo Alto Health Care System. These other comorbidities include age older than 75, hypertension that requires medication, and insulin-controlled diabetes. “The independent risk of just having the diagnosis of insulin-dependent diabetes is actually comparable to the independent risk of having obesity by itself,” he told this news organization, “and all of us operate on [patients with] diabetes.”
Also, there is no BMI at which the risk of complications suddenly increases, according to the American Academy of Orthopaedic Surgeons. “It’s a rising complication rate as you go into higher BMIs,” Dr. Giori said. “If you operate on someone with a BMI of 39 vs. 41, you’re not going to find that much of a difference [in risk].” But if a medical system enforced a hard BMI cutoff of 40, one patient would qualify for surgery while the other would be barred.
Weight not as “modifiable” as previously thought
Weight is often considered a “modifiable factor” for a person considering undergoing total joint arthroplasty, but research suggests that the issue is more complicated. “Obesity is tricky, because some people are successful [in weight loss],” said Dr. Giori. Those tend to be the more memorable stories. “But a large majority have a really hard time losing substantial weight – enough to make a difference in risk,” he continued.
A study conducted in North Carolina found that restricting patients with a BMI over 40 from having elective total joint arthroplasty procedures until their weight was optimized did not result in successful weight loss. Only 20% of patients who originally presented with a BMI above this limit eventually underwent surgery after 2 years, and fewer than half of these patients had achieved a BMI of less than 40 at the time of their surgery. A third of all patients in the study did not return to the orthopedic office after their first visit.
“To hold a hard cutoff when it’s very, very hard to modify ... is essentially telling people that they are not going to ever have surgery,” Dr. Giori said; “I think that can be unfair to some patients.”
Bariatric surgery is often suggested for patients with obesity who have not experienced successful weight loss with diet and lifestyle changes alone, but bariatric surgery comes with its own complications. Research on outcomes from total joint arthroplasty among patients with who have lost weight with bariatric surgery has yielded mixed results. “I rarely push anyone hard to go that route but present it as an option for certain patients,” said Benjamin M. Stronach, MD, an orthopedic surgeon at the University of Arkansas for Medical Sciences, in Little Rock. He usually brings up bariatric surgery with patients with a BMI in the high 40s or higher to gauge their interest. If patients are already considering weight loss surgery, his office provides referrals.
But even bariatric surgery does not result in successful long-term weight loss for every patient, Dr. Stronach said. He’s seeing more and more patients who come for consultations after having undergone bariatric surgery 10 to 15 years ago. These patients lost a significant amount of weight, but then gained the weight back. He noted that bariatric surgery can be very successful for some patients who adhere to their postbariatric regimen. “We typically see fairly impressive results in the short term,” he said.
Patients with obesity benefit from joint replacement
Although patients with obesity are at higher risk for complications from joint replacement surgery, research suggests that these patients can still benefit greatly from these surgeries and that these surgeries remain cost-effective. Some studies have found that patients with obesity tend to have worse outcomes after surgery than patients who are not obese, but often, patients with high BMIs are starting from a lower point, with greater joint pain and limited mobility, Dr. Giori said. But the improvements – that is, net change in measured outcomes – can be greater for obese patients.
“Several studies have shown equal or greater improvements in validated outcome scores, function, and satisfaction compared with nonobese patients after surgery,” authors wrote in a recent review article in which they discuss how to optimize joint replacement surgery for patients with obesity. The article, published in the November 2022 issue of the Journal of the American Academy of Orthopaedic Surgeon (JAAOS), is part of a collection of review articles by the Movement Is Life Caucus.
Encourage weight loss, but look beyond the number
Rather than adhering to strict BMI cutoffs, some experts urge surgeons to consider the patient as a whole and to evaluate each individual’s overall health and potential risk. Dr. Giori generally considers high BMI as just another comorbidity when assessing a patient’s overall risk. “For a person who only has a high BMI but is otherwise healthy, I see no reason not to go ahead and schedule that person for surgery, because reducing the patient’s BMI will not substantially reduce the patient’s complication risk, and a delay in surgery may adversely affect the patient’s quality of life and ability to earn a living,” he said.
“If someone is between a BMI of 40 and 45, we are definitely going to have a discussion about weight,” Dr. Stronach said. He generally counsels against surgery for any patient with a BMI at 45 or above. He wants patients to have a BMI below 40 before surgery but considers individual cases for exceptions. “We will still move forward at times with someone with a BMI of 41, as an example, who is otherwise healthy,” he said. Similarly, if a patient has lost a significant amount of weight (e.g., the patient’s BMI was reduced from 50 to 41), the patient is actively engaged in improving their health, and surgeons believe the patient has significantly reduced their risk, “a lot of time, we’re not going to draw a line in the sand right at [a BMI of] 40,” he said.
While using a BMI of under 35 or 40 as a guideline when starting to work with patients is reasonable, working toward a weight loss of 5%-10% of total body weight is another goal to consider, authors advise in the JAAOS obesity review article. Research suggests that even a 5% reduction in overall body weight can reduce surgical complications and can improve a patient’s glucose and lipid levels and cardiac profile. Referrals to dietitians and weight loss programs, as well as behavioral counseling, can also be useful in initiating weight loss and keeping patients engaged in the process, the authors wrote.
Consider a patient’s comorbidities
Many patients with obesity have comorbidities, such as type 2 diabetes and hypertension, that can also be optimized for surgery so as to lower a patient’s overall risk profile. For patients with diabetes, achieving an A1c of 8% or lower can be a reasonable goal and can reduce risk. “We’ve found that an HbA1c level of 8% or less is something that virtually all diabetics (though not everybody) can reach, and it’s something that can be reached in a reasonable amount of time,” Dr. Giori said. Preoperative use of beta-blockers, continued use of ACE inhibitors or angiotensin receptor blockers, and behavioral modifications can improve a patient’s cardiac health before surgery.
Malnutrition can be a correctable problem for patients, regardless of BMI. In the Movement Is Life collection of optimization articles, experts recommend that orthopedists screen for malnutrition with blood tests for albumin, vitamin D, transferrin, and total lymphocyte count. Patients with malnutrition should be screened for food insecurity, experts advise, and surgical candidates with deficiencies can be given supplements of omega-3 fatty acids, arginine, and protein shakes.
Surgeon comfort and shared decision-making
Dr. Wiznia emphasized that the patient and surgeon need to discuss the risks of surgery, concerns about potential complications, and how a complication could affect the patient’s life moving forward. “Ultimately, the surgeon needs to make the decision [of whether or not to proceed [with surgery] with the patient,” he said, “but not every surgeon is going to feel comfortable operating on these patients, and not every medical institution is going to have the equipment and the investments to support surgeons doing it.”
Dr. Giori agreed that surgeons should proceed only with surgical cases they feel comfortable with. Certain surgeons may decide not to operate on individuals with higher BMIs because of the potential complications and can refer these patients to more specialized care centers. Operating on larger patients is more difficult and requires surgical skills and expertise that the surgeon may not have, he noted. “What I do object to is a system-wide BMI cutoff – for example, if an insurance company won’t pay for you to have a joint replacement, regardless of where you go or who your surgeon is,” Dr. Giori added. “I think that’s wrong, because it’s not patient centered and it’s basically excluding people from having a life-altering operation.”
Dr. Giori and Dr. Wiznia report no relevant financial relationships. Dr. Stronach is a consultant for DJ Orthopaedics, Johnson & Johnson, and MiCare Path.
A version of this article first appeared on Medscape.com.
For patients with severe arthritis, joint replacement is considered when more conservative treatments have failed. Because patients with obesity have a higher risk of complications during and after surgery, some surgeons, hospitals, and insurance companies have adopted body mass index cutoffs as a basis for deciding whether to offer patients these elective surgeries. But some experts argue that these cutoffs are arbitrary, exclude patients who can still benefit from the surgery, and can increase disparities in care.
“By enforcing cutoffs in general, you’re losing the ability for each surgeon to determine who they want to operate on,” said Daniel Wiznia, MD, assistant professor of orthopedic surgery at Yale University, New Haven, Conn. He is on the leadership committee of the Movement Is Life Caucus, a nonprofit group focused on eliminating disparities in musculoskeletal health. “For every surgeon, it’s up to them to decide if they feel comfortable doing the surgery,” he noted in an interview. “My guidance for that would be, don’t just say no because of the number – look at the patient’s entire medical profile.”
According to the Centers for Disease Control and Prevention, nearly 42% of adults in the United States have a BMI over 30, and 9.2% of adults have a BMI over 40. This excess weight puts additional stress on joints: When a person is walking, experts estimate that the force on the knees can be two to three times someone’s body weight. Over time, this pressure can wear down the cartilage on joints.
As a result, people who are overweight or obese are more likely to develop osteoarthritis and to need joint replacements. According to a Canadian study, patients with a BMI of 30-35 are 3.4 times as likely to require a hip replacement and are 8.5 times as likely to require a knee replacement compared to individuals with a BMI in the “healthy weight” range. With a BMI above 40, individuals were 8.5 times more likely to need a hip replacement and were 32.7 times as likely to need a knee replacement.
More complications, greater expense
While there are no universally recommended BMI cutoffs for joint replacement surgery, it is not uncommon for institutions to require that patients have a BMI below a certain value (usually 35-40) to proceed with surgery. A 2013 survey of physicians from the American Association of Hip and Knee Surgeons found that 52% of surgeons required a BMI below 40 to qualify for surgery.
One of the main reasons for these cutoffs is the elevated risk of complications during and after surgery. Research suggests that obesity is associated with higher rates of wound dehiscence, prosthetic joint infection (PJI), and revision total joint arthroplasty. One 2016 study suggests that patients with a BMI of 35-39.9 are twice as likely to experience PJI compared to patients with a BMI below 35. For patients with a BMI of 40 or higher, PJI is four times as likely.
Another study found that patients whose BMI is 35-40 and who undergo total joint arthroplasty have a 6.4-fold greater risk of deep incision infection. For those with a BMI over 40, that rises to a 12.9-fold increased risk compared to patients with a BMI of 18.5-25. Patients with obesity tend to have other comorbidities that can increase the risk of complications during surgery, such as type 2 diabetes, coronary artery disease, and chronic kidney disease.
Because of the increased risk of complications, health care costs tend to be higher for patients with obesity. The growing popularity of bundled health payments can discourage operating on patients who are more likely to experience complications, such as patients with high BMIs, noted Dr. Wiznia.
Research suggests that minorities and people with lower socioeconomic status are disproportionately affected by these cutoffs. According to the CDC, among non-Hispanic Black Americans and Hispanic Americans, rates of obesity are higher than among their White counterparts, and these patients are less likely to undergo joint replacement. Strictly enforcing this eligibility criterion can worsen those disparities. A study involving 21,294 adults over age 50 from the National Health and Nutrition Examination Survey (NHANES) found that requiring a BMI of under 35 for total joint arthroplasty resulted in Black patients being 39% less likely to be eligible for surgery than White patients. And individuals with an annual household income under $45,000 were 19% less likely to qualify for surgery than those with a household income above $45,000.
BMI no better than other risk factors
Although high BMI is independently associated with a higher risk of complications, the increased risk of complications conferred by a BMI at or above 40 is similar to or lower than those of other comorbidities that surgeons generally accept, said Nicholas Giori, MD, PhD, professor of orthopedic surgery at Stanford (Calif.) University, and chief of orthopedic surgery at the VA Palo Alto Health Care System. These other comorbidities include age older than 75, hypertension that requires medication, and insulin-controlled diabetes. “The independent risk of just having the diagnosis of insulin-dependent diabetes is actually comparable to the independent risk of having obesity by itself,” he told this news organization, “and all of us operate on [patients with] diabetes.”
Also, there is no BMI at which the risk of complications suddenly increases, according to the American Academy of Orthopaedic Surgeons. “It’s a rising complication rate as you go into higher BMIs,” Dr. Giori said. “If you operate on someone with a BMI of 39 vs. 41, you’re not going to find that much of a difference [in risk].” But if a medical system enforced a hard BMI cutoff of 40, one patient would qualify for surgery while the other would be barred.
Weight not as “modifiable” as previously thought
Weight is often considered a “modifiable factor” for a person considering undergoing total joint arthroplasty, but research suggests that the issue is more complicated. “Obesity is tricky, because some people are successful [in weight loss],” said Dr. Giori. Those tend to be the more memorable stories. “But a large majority have a really hard time losing substantial weight – enough to make a difference in risk,” he continued.
A study conducted in North Carolina found that restricting patients with a BMI over 40 from having elective total joint arthroplasty procedures until their weight was optimized did not result in successful weight loss. Only 20% of patients who originally presented with a BMI above this limit eventually underwent surgery after 2 years, and fewer than half of these patients had achieved a BMI of less than 40 at the time of their surgery. A third of all patients in the study did not return to the orthopedic office after their first visit.
“To hold a hard cutoff when it’s very, very hard to modify ... is essentially telling people that they are not going to ever have surgery,” Dr. Giori said; “I think that can be unfair to some patients.”
Bariatric surgery is often suggested for patients with obesity who have not experienced successful weight loss with diet and lifestyle changes alone, but bariatric surgery comes with its own complications. Research on outcomes from total joint arthroplasty among patients with who have lost weight with bariatric surgery has yielded mixed results. “I rarely push anyone hard to go that route but present it as an option for certain patients,” said Benjamin M. Stronach, MD, an orthopedic surgeon at the University of Arkansas for Medical Sciences, in Little Rock. He usually brings up bariatric surgery with patients with a BMI in the high 40s or higher to gauge their interest. If patients are already considering weight loss surgery, his office provides referrals.
But even bariatric surgery does not result in successful long-term weight loss for every patient, Dr. Stronach said. He’s seeing more and more patients who come for consultations after having undergone bariatric surgery 10 to 15 years ago. These patients lost a significant amount of weight, but then gained the weight back. He noted that bariatric surgery can be very successful for some patients who adhere to their postbariatric regimen. “We typically see fairly impressive results in the short term,” he said.
Patients with obesity benefit from joint replacement
Although patients with obesity are at higher risk for complications from joint replacement surgery, research suggests that these patients can still benefit greatly from these surgeries and that these surgeries remain cost-effective. Some studies have found that patients with obesity tend to have worse outcomes after surgery than patients who are not obese, but often, patients with high BMIs are starting from a lower point, with greater joint pain and limited mobility, Dr. Giori said. But the improvements – that is, net change in measured outcomes – can be greater for obese patients.
“Several studies have shown equal or greater improvements in validated outcome scores, function, and satisfaction compared with nonobese patients after surgery,” authors wrote in a recent review article in which they discuss how to optimize joint replacement surgery for patients with obesity. The article, published in the November 2022 issue of the Journal of the American Academy of Orthopaedic Surgeon (JAAOS), is part of a collection of review articles by the Movement Is Life Caucus.
Encourage weight loss, but look beyond the number
Rather than adhering to strict BMI cutoffs, some experts urge surgeons to consider the patient as a whole and to evaluate each individual’s overall health and potential risk. Dr. Giori generally considers high BMI as just another comorbidity when assessing a patient’s overall risk. “For a person who only has a high BMI but is otherwise healthy, I see no reason not to go ahead and schedule that person for surgery, because reducing the patient’s BMI will not substantially reduce the patient’s complication risk, and a delay in surgery may adversely affect the patient’s quality of life and ability to earn a living,” he said.
“If someone is between a BMI of 40 and 45, we are definitely going to have a discussion about weight,” Dr. Stronach said. He generally counsels against surgery for any patient with a BMI at 45 or above. He wants patients to have a BMI below 40 before surgery but considers individual cases for exceptions. “We will still move forward at times with someone with a BMI of 41, as an example, who is otherwise healthy,” he said. Similarly, if a patient has lost a significant amount of weight (e.g., the patient’s BMI was reduced from 50 to 41), the patient is actively engaged in improving their health, and surgeons believe the patient has significantly reduced their risk, “a lot of time, we’re not going to draw a line in the sand right at [a BMI of] 40,” he said.
While using a BMI of under 35 or 40 as a guideline when starting to work with patients is reasonable, working toward a weight loss of 5%-10% of total body weight is another goal to consider, authors advise in the JAAOS obesity review article. Research suggests that even a 5% reduction in overall body weight can reduce surgical complications and can improve a patient’s glucose and lipid levels and cardiac profile. Referrals to dietitians and weight loss programs, as well as behavioral counseling, can also be useful in initiating weight loss and keeping patients engaged in the process, the authors wrote.
Consider a patient’s comorbidities
Many patients with obesity have comorbidities, such as type 2 diabetes and hypertension, that can also be optimized for surgery so as to lower a patient’s overall risk profile. For patients with diabetes, achieving an A1c of 8% or lower can be a reasonable goal and can reduce risk. “We’ve found that an HbA1c level of 8% or less is something that virtually all diabetics (though not everybody) can reach, and it’s something that can be reached in a reasonable amount of time,” Dr. Giori said. Preoperative use of beta-blockers, continued use of ACE inhibitors or angiotensin receptor blockers, and behavioral modifications can improve a patient’s cardiac health before surgery.
Malnutrition can be a correctable problem for patients, regardless of BMI. In the Movement Is Life collection of optimization articles, experts recommend that orthopedists screen for malnutrition with blood tests for albumin, vitamin D, transferrin, and total lymphocyte count. Patients with malnutrition should be screened for food insecurity, experts advise, and surgical candidates with deficiencies can be given supplements of omega-3 fatty acids, arginine, and protein shakes.
Surgeon comfort and shared decision-making
Dr. Wiznia emphasized that the patient and surgeon need to discuss the risks of surgery, concerns about potential complications, and how a complication could affect the patient’s life moving forward. “Ultimately, the surgeon needs to make the decision [of whether or not to proceed [with surgery] with the patient,” he said, “but not every surgeon is going to feel comfortable operating on these patients, and not every medical institution is going to have the equipment and the investments to support surgeons doing it.”
Dr. Giori agreed that surgeons should proceed only with surgical cases they feel comfortable with. Certain surgeons may decide not to operate on individuals with higher BMIs because of the potential complications and can refer these patients to more specialized care centers. Operating on larger patients is more difficult and requires surgical skills and expertise that the surgeon may not have, he noted. “What I do object to is a system-wide BMI cutoff – for example, if an insurance company won’t pay for you to have a joint replacement, regardless of where you go or who your surgeon is,” Dr. Giori added. “I think that’s wrong, because it’s not patient centered and it’s basically excluding people from having a life-altering operation.”
Dr. Giori and Dr. Wiznia report no relevant financial relationships. Dr. Stronach is a consultant for DJ Orthopaedics, Johnson & Johnson, and MiCare Path.
A version of this article first appeared on Medscape.com.
Less than a third of Americans aware of cancer risk from alcohol
The new findings, from a nationally representative survey that included responses from 3,865 adults, show a low awareness of the cancer risk from alcohol, and also that the risk varies by type of drink. Just under a third (31.2%) of respondents thought that consuming liquor/spirits was associated with a risk of cancer, but this fell to 24.9% for drinking beer and even further, to 20.3%, for drinking wine.
In fact, some respondents though the opposite – that drinking alcohol has health benefits; 10.3% of respondents thought that drinking wine was associated with a decreased cancer risk, while 2.25% thought the same for drinking beer, and 1.7% thought that for drinking liquor.
Most U.S. adults (> 50%) reported not knowing how these beverages affected cancer risk, the authors report.
“This study’s findings underscore the need to develop interventions for educating the public about the cancer risks of alcohol use, particularly in the prevailing context of national dialogue about the purported heart health benefits of wine,” commented senior author William M. P. Klein, PhD, associate director of the National Cancer Institute’s Behavioral Research Program, in a statement.
“All types of alcoholic beverages, including wine, increase cancer risk,” Dr. Klein said.
The findings were published online in Cancer Epidemiology, Biomarkers & Prevention.
The results echo the findings of a previous national survey that also found that the majority of Americans are not aware that alcohol consumption is associated with an increased risk of developing a variety of cancers.
In contrast, within the scientific community, there is long-standing and increasing awareness of alcohol consumption as a leading modifiable risk factor for cancer, and there is a growing movement calling for more public health awareness of the link.
Recently, there has been some public support for adding written warnings about the cancer risk from alcohol. A Citizen Petition was filed in 2021, and in August 2022, The New England Journal of Medicine issued a call for new labeling.
Several cancer organizations are petitioning for warnings to be added to alcoholic beverages. The petition is supported by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations. Proposed labeling would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Dr. Klein and colleagues suggest that public health interventions, including mass media campaigns, cancer warning labels, and patient-provider communications, could help disseminate information about cancer and alcohol. “Educating the public about how alcohol increases cancer risk will not only empower consumers to make more informed decisions but may also prevent and reduce excessive alcohol use, as well as cancer morbidity and mortality,” Dr. Klein said.
The study was supported by the Division of Cancer Control and Population Sciences at the National Cancer Institute. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, from a nationally representative survey that included responses from 3,865 adults, show a low awareness of the cancer risk from alcohol, and also that the risk varies by type of drink. Just under a third (31.2%) of respondents thought that consuming liquor/spirits was associated with a risk of cancer, but this fell to 24.9% for drinking beer and even further, to 20.3%, for drinking wine.
In fact, some respondents though the opposite – that drinking alcohol has health benefits; 10.3% of respondents thought that drinking wine was associated with a decreased cancer risk, while 2.25% thought the same for drinking beer, and 1.7% thought that for drinking liquor.
Most U.S. adults (> 50%) reported not knowing how these beverages affected cancer risk, the authors report.
“This study’s findings underscore the need to develop interventions for educating the public about the cancer risks of alcohol use, particularly in the prevailing context of national dialogue about the purported heart health benefits of wine,” commented senior author William M. P. Klein, PhD, associate director of the National Cancer Institute’s Behavioral Research Program, in a statement.
“All types of alcoholic beverages, including wine, increase cancer risk,” Dr. Klein said.
The findings were published online in Cancer Epidemiology, Biomarkers & Prevention.
The results echo the findings of a previous national survey that also found that the majority of Americans are not aware that alcohol consumption is associated with an increased risk of developing a variety of cancers.
In contrast, within the scientific community, there is long-standing and increasing awareness of alcohol consumption as a leading modifiable risk factor for cancer, and there is a growing movement calling for more public health awareness of the link.
Recently, there has been some public support for adding written warnings about the cancer risk from alcohol. A Citizen Petition was filed in 2021, and in August 2022, The New England Journal of Medicine issued a call for new labeling.
Several cancer organizations are petitioning for warnings to be added to alcoholic beverages. The petition is supported by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations. Proposed labeling would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Dr. Klein and colleagues suggest that public health interventions, including mass media campaigns, cancer warning labels, and patient-provider communications, could help disseminate information about cancer and alcohol. “Educating the public about how alcohol increases cancer risk will not only empower consumers to make more informed decisions but may also prevent and reduce excessive alcohol use, as well as cancer morbidity and mortality,” Dr. Klein said.
The study was supported by the Division of Cancer Control and Population Sciences at the National Cancer Institute. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The new findings, from a nationally representative survey that included responses from 3,865 adults, show a low awareness of the cancer risk from alcohol, and also that the risk varies by type of drink. Just under a third (31.2%) of respondents thought that consuming liquor/spirits was associated with a risk of cancer, but this fell to 24.9% for drinking beer and even further, to 20.3%, for drinking wine.
In fact, some respondents though the opposite – that drinking alcohol has health benefits; 10.3% of respondents thought that drinking wine was associated with a decreased cancer risk, while 2.25% thought the same for drinking beer, and 1.7% thought that for drinking liquor.
Most U.S. adults (> 50%) reported not knowing how these beverages affected cancer risk, the authors report.
“This study’s findings underscore the need to develop interventions for educating the public about the cancer risks of alcohol use, particularly in the prevailing context of national dialogue about the purported heart health benefits of wine,” commented senior author William M. P. Klein, PhD, associate director of the National Cancer Institute’s Behavioral Research Program, in a statement.
“All types of alcoholic beverages, including wine, increase cancer risk,” Dr. Klein said.
The findings were published online in Cancer Epidemiology, Biomarkers & Prevention.
The results echo the findings of a previous national survey that also found that the majority of Americans are not aware that alcohol consumption is associated with an increased risk of developing a variety of cancers.
In contrast, within the scientific community, there is long-standing and increasing awareness of alcohol consumption as a leading modifiable risk factor for cancer, and there is a growing movement calling for more public health awareness of the link.
Recently, there has been some public support for adding written warnings about the cancer risk from alcohol. A Citizen Petition was filed in 2021, and in August 2022, The New England Journal of Medicine issued a call for new labeling.
Several cancer organizations are petitioning for warnings to be added to alcoholic beverages. The petition is supported by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations. Proposed labeling would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Dr. Klein and colleagues suggest that public health interventions, including mass media campaigns, cancer warning labels, and patient-provider communications, could help disseminate information about cancer and alcohol. “Educating the public about how alcohol increases cancer risk will not only empower consumers to make more informed decisions but may also prevent and reduce excessive alcohol use, as well as cancer morbidity and mortality,” Dr. Klein said.
The study was supported by the Division of Cancer Control and Population Sciences at the National Cancer Institute. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Diabetes decision tool yields ‘modest’ benefit in low-resource clinics
a randomized trial in China showed.
The tool required clinicians to enter patient data into a computer in order to generate individualized treatment recommendations, adding to their administrative burdens. It also couldn’t tackle patients’ problems with access and affordability of medications.
Nevertheless, the model could curtail physician burnout and improve the quality of care in primary care clinics with limited resources, the researchers said in a paper published in the Annals of Internal Medicine.
They concluded that the findings support “widespread adoption” of the model in China and other low- or middle-income countries where diabetes is on the rise.
Co–principal investigator Jiang He, MD, PhD, chair of epidemiology at Tulane University, New Orleans, said the findings could apply to federally qualified health care (FQHC) clinics that treat underserved patients in the United States.
“At many FQHC clinics, nurse practitioners have to take care of patients with multiple chronic disease conditions. Team-based care with a computerized clinical decision support system will help them and improve patient care,” Dr. He said.
Small improvements
To conduct the trial, called Diabetes Complication Control in Community Clinics (D4C), Dr. He and colleagues randomly assigned 19 out of the 38 community health centers in Xiamen, China, to have a clinical decision support tool installed on the computers of primary care physicians and health coaches.
Starting in October 2016 the researchers recruited 11,132 patients aged 50 and older with uncontrolled diabetes and at least one comorbid condition, with 5,475 patients receiving team-based care with the CDSS and the remainder receiving team-based care alone.
The CDSS generated individualized risk factor summaries and treatment recommendations, including prescriptions based on Chinese and U.S. clinical guidelines. It incorporated data on patients’ insurance plans and local availability of drugs.
At all centers, primary care physicians received training in managing glycemia, blood pressure, and lipids. Nurses were certified as health coaches after receiving training on nutrition, lifestyle changes, and medication adherence. Patients met with their coaches for half an hour every 3 months, and diabetes specialists visited each clinic monthly for team meetings and consultations.
After 18 months, patients undergoing team-based care alone lowered their hemoglobin A1c by 0.6 percentage points (95% confidence interval, –0.7 to –0.5 percentage points), LDL cholesterol by 12.5 mg/dL (95% CI, –13.6 to –11.3 mg/dL), and systolic blood pressure by 7.5 mm Hg (95% CI, –8.4 to –6.6 mm Hg).
The group whose care teams used the CDSS further reduced A1c by 0.2 percentage points (95% CI, –0.3 to –0.1 percentage points), LDL cholesterol by 6.5 mg/dL (95% CI, –8.3 to -4.6 mg/dL), and blood pressure by 1.5 mm Hg (95% CI, –2.8 to –0.3 mm Hg).
All-cause mortality did not differ between the groups. Serious adverse events occurred in 9.1% of the CDSS group, compared with 10.9% of the group whose care team did not use the CDSS.
Addressing social needs
Experts who were not involved in the trial said the marginal impact of the CDSS was no surprise given the mixed results of such tools in previous studies.
However, the lackluster result “might be a shock to people investing a lot in clinical decision support,” said Elbert Huang, MD, MPH, director of the Center for Chronic Disease Research and Policy at the University of Chicago.
Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said the administrative burden of entering each patient’s data into the system would slow down care and frustrate clinicians. “The system has to be smarter than this.”
On the other hand, the findings of the D4C trial align with other research showing that team-based care strategies are effective for diabetes management.
Dr. Huang noted that there is a “well-established history” of diabetes quality improvement programs, health coaches, buddy programs, and community health worker programs. He added that the new findings “might help to remind everyone of the importance of these programs, which are not always well supported.”
“The bottom line of the paper might be that investing in patient engagement programs might get us 90% of the way to our goal of improving diabetes care,” Dr. Huang said.
Still, Dr. Peters said the portion of patients in the trial who benefited from team-based care seemed “disturbingly low.” Just 16.9% of patients who received team-based care and CDSS and 13% of those who received team-based care alone improved in all three measures. “This system doesn’t get you to where you want to be by a long shot.”
She added that a team-based approach, particularly the use of health coaches, would be a “huge improvement” over fragmented care provided in much of the U.S. safety-net system.
Another team approach
Many systems are striving to improve diabetes management in response to payment incentives, Dr. Huang said.
In a separate retrospective analysis, published in Annals of Family Medicine, researchers at the Mayo Clinic, Rochester, Minn., reported quality improvement gains among primary care practices that adopted a team-based model called Enhanced Primary Care Diabetes (EPCD). The model deployed a range of strategies, such as empowering nurses to engage with patients outside of scheduled office visits and including pharmacists on care teams.
Mayo’s approach did not specifically target underserved populations. Rather, researchers evaluated the model’s impact on about 17,000 patients treated at 32 Mayo internal medicine and family medicine practices of varying sizes, resources, and community settings.
Among staff clinician practices using the EPCD model improved patients’ scores on a composite quality measure called D5, which incorporates glycemic control, blood pressure control, low-density lipoprotein control, tobacco abstinence, and aspirin use.
Following implementation, the portion of patients in those practices meeting the D5 indicator increased from 42.9% to 45.0% (incident rate ratio, 1.005; P = .001).
Meanwhile, the portion of patients meeting the indicator increased from 38.9% to 42.0% (IRR, 1.011; P = .003) at resident physician practices that used the EPCD model and decreased from 36.2% to 35.5% (IRR, 0.994; P < .001) at staff clinician practices that did not use the model.
In contrast to the team-based approach used in China, the EPCD protocol “is very complex, and it will be difficult to implement in low-resource settings,” Dr. He said.
The D4C trial was funded by the Xiamen Municipal Health Commission. The Mayo study was funded by a National Institutes of Diabetes and Digestive and Kidney Diseases grant. Dr. He, Dr. Peters, and Dr. Huang reported no relevant financial interests.
a randomized trial in China showed.
The tool required clinicians to enter patient data into a computer in order to generate individualized treatment recommendations, adding to their administrative burdens. It also couldn’t tackle patients’ problems with access and affordability of medications.
Nevertheless, the model could curtail physician burnout and improve the quality of care in primary care clinics with limited resources, the researchers said in a paper published in the Annals of Internal Medicine.
They concluded that the findings support “widespread adoption” of the model in China and other low- or middle-income countries where diabetes is on the rise.
Co–principal investigator Jiang He, MD, PhD, chair of epidemiology at Tulane University, New Orleans, said the findings could apply to federally qualified health care (FQHC) clinics that treat underserved patients in the United States.
“At many FQHC clinics, nurse practitioners have to take care of patients with multiple chronic disease conditions. Team-based care with a computerized clinical decision support system will help them and improve patient care,” Dr. He said.
Small improvements
To conduct the trial, called Diabetes Complication Control in Community Clinics (D4C), Dr. He and colleagues randomly assigned 19 out of the 38 community health centers in Xiamen, China, to have a clinical decision support tool installed on the computers of primary care physicians and health coaches.
Starting in October 2016 the researchers recruited 11,132 patients aged 50 and older with uncontrolled diabetes and at least one comorbid condition, with 5,475 patients receiving team-based care with the CDSS and the remainder receiving team-based care alone.
The CDSS generated individualized risk factor summaries and treatment recommendations, including prescriptions based on Chinese and U.S. clinical guidelines. It incorporated data on patients’ insurance plans and local availability of drugs.
At all centers, primary care physicians received training in managing glycemia, blood pressure, and lipids. Nurses were certified as health coaches after receiving training on nutrition, lifestyle changes, and medication adherence. Patients met with their coaches for half an hour every 3 months, and diabetes specialists visited each clinic monthly for team meetings and consultations.
After 18 months, patients undergoing team-based care alone lowered their hemoglobin A1c by 0.6 percentage points (95% confidence interval, –0.7 to –0.5 percentage points), LDL cholesterol by 12.5 mg/dL (95% CI, –13.6 to –11.3 mg/dL), and systolic blood pressure by 7.5 mm Hg (95% CI, –8.4 to –6.6 mm Hg).
The group whose care teams used the CDSS further reduced A1c by 0.2 percentage points (95% CI, –0.3 to –0.1 percentage points), LDL cholesterol by 6.5 mg/dL (95% CI, –8.3 to -4.6 mg/dL), and blood pressure by 1.5 mm Hg (95% CI, –2.8 to –0.3 mm Hg).
All-cause mortality did not differ between the groups. Serious adverse events occurred in 9.1% of the CDSS group, compared with 10.9% of the group whose care team did not use the CDSS.
Addressing social needs
Experts who were not involved in the trial said the marginal impact of the CDSS was no surprise given the mixed results of such tools in previous studies.
However, the lackluster result “might be a shock to people investing a lot in clinical decision support,” said Elbert Huang, MD, MPH, director of the Center for Chronic Disease Research and Policy at the University of Chicago.
Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said the administrative burden of entering each patient’s data into the system would slow down care and frustrate clinicians. “The system has to be smarter than this.”
On the other hand, the findings of the D4C trial align with other research showing that team-based care strategies are effective for diabetes management.
Dr. Huang noted that there is a “well-established history” of diabetes quality improvement programs, health coaches, buddy programs, and community health worker programs. He added that the new findings “might help to remind everyone of the importance of these programs, which are not always well supported.”
“The bottom line of the paper might be that investing in patient engagement programs might get us 90% of the way to our goal of improving diabetes care,” Dr. Huang said.
Still, Dr. Peters said the portion of patients in the trial who benefited from team-based care seemed “disturbingly low.” Just 16.9% of patients who received team-based care and CDSS and 13% of those who received team-based care alone improved in all three measures. “This system doesn’t get you to where you want to be by a long shot.”
She added that a team-based approach, particularly the use of health coaches, would be a “huge improvement” over fragmented care provided in much of the U.S. safety-net system.
Another team approach
Many systems are striving to improve diabetes management in response to payment incentives, Dr. Huang said.
In a separate retrospective analysis, published in Annals of Family Medicine, researchers at the Mayo Clinic, Rochester, Minn., reported quality improvement gains among primary care practices that adopted a team-based model called Enhanced Primary Care Diabetes (EPCD). The model deployed a range of strategies, such as empowering nurses to engage with patients outside of scheduled office visits and including pharmacists on care teams.
Mayo’s approach did not specifically target underserved populations. Rather, researchers evaluated the model’s impact on about 17,000 patients treated at 32 Mayo internal medicine and family medicine practices of varying sizes, resources, and community settings.
Among staff clinician practices using the EPCD model improved patients’ scores on a composite quality measure called D5, which incorporates glycemic control, blood pressure control, low-density lipoprotein control, tobacco abstinence, and aspirin use.
Following implementation, the portion of patients in those practices meeting the D5 indicator increased from 42.9% to 45.0% (incident rate ratio, 1.005; P = .001).
Meanwhile, the portion of patients meeting the indicator increased from 38.9% to 42.0% (IRR, 1.011; P = .003) at resident physician practices that used the EPCD model and decreased from 36.2% to 35.5% (IRR, 0.994; P < .001) at staff clinician practices that did not use the model.
In contrast to the team-based approach used in China, the EPCD protocol “is very complex, and it will be difficult to implement in low-resource settings,” Dr. He said.
The D4C trial was funded by the Xiamen Municipal Health Commission. The Mayo study was funded by a National Institutes of Diabetes and Digestive and Kidney Diseases grant. Dr. He, Dr. Peters, and Dr. Huang reported no relevant financial interests.
a randomized trial in China showed.
The tool required clinicians to enter patient data into a computer in order to generate individualized treatment recommendations, adding to their administrative burdens. It also couldn’t tackle patients’ problems with access and affordability of medications.
Nevertheless, the model could curtail physician burnout and improve the quality of care in primary care clinics with limited resources, the researchers said in a paper published in the Annals of Internal Medicine.
They concluded that the findings support “widespread adoption” of the model in China and other low- or middle-income countries where diabetes is on the rise.
Co–principal investigator Jiang He, MD, PhD, chair of epidemiology at Tulane University, New Orleans, said the findings could apply to federally qualified health care (FQHC) clinics that treat underserved patients in the United States.
“At many FQHC clinics, nurse practitioners have to take care of patients with multiple chronic disease conditions. Team-based care with a computerized clinical decision support system will help them and improve patient care,” Dr. He said.
Small improvements
To conduct the trial, called Diabetes Complication Control in Community Clinics (D4C), Dr. He and colleagues randomly assigned 19 out of the 38 community health centers in Xiamen, China, to have a clinical decision support tool installed on the computers of primary care physicians and health coaches.
Starting in October 2016 the researchers recruited 11,132 patients aged 50 and older with uncontrolled diabetes and at least one comorbid condition, with 5,475 patients receiving team-based care with the CDSS and the remainder receiving team-based care alone.
The CDSS generated individualized risk factor summaries and treatment recommendations, including prescriptions based on Chinese and U.S. clinical guidelines. It incorporated data on patients’ insurance plans and local availability of drugs.
At all centers, primary care physicians received training in managing glycemia, blood pressure, and lipids. Nurses were certified as health coaches after receiving training on nutrition, lifestyle changes, and medication adherence. Patients met with their coaches for half an hour every 3 months, and diabetes specialists visited each clinic monthly for team meetings and consultations.
After 18 months, patients undergoing team-based care alone lowered their hemoglobin A1c by 0.6 percentage points (95% confidence interval, –0.7 to –0.5 percentage points), LDL cholesterol by 12.5 mg/dL (95% CI, –13.6 to –11.3 mg/dL), and systolic blood pressure by 7.5 mm Hg (95% CI, –8.4 to –6.6 mm Hg).
The group whose care teams used the CDSS further reduced A1c by 0.2 percentage points (95% CI, –0.3 to –0.1 percentage points), LDL cholesterol by 6.5 mg/dL (95% CI, –8.3 to -4.6 mg/dL), and blood pressure by 1.5 mm Hg (95% CI, –2.8 to –0.3 mm Hg).
All-cause mortality did not differ between the groups. Serious adverse events occurred in 9.1% of the CDSS group, compared with 10.9% of the group whose care team did not use the CDSS.
Addressing social needs
Experts who were not involved in the trial said the marginal impact of the CDSS was no surprise given the mixed results of such tools in previous studies.
However, the lackluster result “might be a shock to people investing a lot in clinical decision support,” said Elbert Huang, MD, MPH, director of the Center for Chronic Disease Research and Policy at the University of Chicago.
Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said the administrative burden of entering each patient’s data into the system would slow down care and frustrate clinicians. “The system has to be smarter than this.”
On the other hand, the findings of the D4C trial align with other research showing that team-based care strategies are effective for diabetes management.
Dr. Huang noted that there is a “well-established history” of diabetes quality improvement programs, health coaches, buddy programs, and community health worker programs. He added that the new findings “might help to remind everyone of the importance of these programs, which are not always well supported.”
“The bottom line of the paper might be that investing in patient engagement programs might get us 90% of the way to our goal of improving diabetes care,” Dr. Huang said.
Still, Dr. Peters said the portion of patients in the trial who benefited from team-based care seemed “disturbingly low.” Just 16.9% of patients who received team-based care and CDSS and 13% of those who received team-based care alone improved in all three measures. “This system doesn’t get you to where you want to be by a long shot.”
She added that a team-based approach, particularly the use of health coaches, would be a “huge improvement” over fragmented care provided in much of the U.S. safety-net system.
Another team approach
Many systems are striving to improve diabetes management in response to payment incentives, Dr. Huang said.
In a separate retrospective analysis, published in Annals of Family Medicine, researchers at the Mayo Clinic, Rochester, Minn., reported quality improvement gains among primary care practices that adopted a team-based model called Enhanced Primary Care Diabetes (EPCD). The model deployed a range of strategies, such as empowering nurses to engage with patients outside of scheduled office visits and including pharmacists on care teams.
Mayo’s approach did not specifically target underserved populations. Rather, researchers evaluated the model’s impact on about 17,000 patients treated at 32 Mayo internal medicine and family medicine practices of varying sizes, resources, and community settings.
Among staff clinician practices using the EPCD model improved patients’ scores on a composite quality measure called D5, which incorporates glycemic control, blood pressure control, low-density lipoprotein control, tobacco abstinence, and aspirin use.
Following implementation, the portion of patients in those practices meeting the D5 indicator increased from 42.9% to 45.0% (incident rate ratio, 1.005; P = .001).
Meanwhile, the portion of patients meeting the indicator increased from 38.9% to 42.0% (IRR, 1.011; P = .003) at resident physician practices that used the EPCD model and decreased from 36.2% to 35.5% (IRR, 0.994; P < .001) at staff clinician practices that did not use the model.
In contrast to the team-based approach used in China, the EPCD protocol “is very complex, and it will be difficult to implement in low-resource settings,” Dr. He said.
The D4C trial was funded by the Xiamen Municipal Health Commission. The Mayo study was funded by a National Institutes of Diabetes and Digestive and Kidney Diseases grant. Dr. He, Dr. Peters, and Dr. Huang reported no relevant financial interests.
FROM ANNALS OF INTERNAL MEDICINE






