User login
Ultraprocessed foods tied to faster rate of cognitive decline
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), which included more than 10,000 people aged 35 and older, showed that higher intake of UPF was significantly associated with a faster rate of decline in executive and global cognitive function.
“These findings show that lifestyle choices, particularly high intake of ultraprocessed foods, can influence our cognitive health many years later,” coinvestigator Natalia Goncalves, PhD, University of São Paulo, Brazil, said in an interview.
The study was published online in JAMA Neurology.
The study’s findings were presented in August at the Alzheimer’s Association International Conference (AAIC) 2022 and were reported by this news organization at that time.
High sugar, salt, fat
The new results align with another recent study linking a diet high in UPFs to an increased risk for dementia.
UPFs are highly manipulated, are packed with added ingredients, including sugar, fat, and salt, and are low in protein and fiber. Examples of UPFs are soft drinks, chips, chocolate, candy, ice cream, sweetened breakfast cereals, packaged soups, chicken nuggets, hot dogs, and fries.
The ELSA-Brasil study comprised 10,775 adults (mean age, 50.6 years at baseline; 55% women; 53% White) who were evaluated in three waves approximately 4 years apart from 2008 to 2017.
Information on diet was obtained via food frequency questionnaires and included details regarding consumption of unprocessed foods, minimally processed foods, and UPFs.
Participants were grouped according to UPF consumption quartiles (lowest to highest). Cognitive performance was evaluated by use of a standardized battery of tests.
During median follow-up of 8 years, people who consumed more than 20% of daily calories from UPFs (quartiles 2-4) experienced a 28% faster rate of decline in global cognition (beta = –0.004; 95% confidence interval [CI], –0.006 to –0.001; P = .003) and a 25% faster rate of decline in executive function (beta = –0.003, 95% CI, –0.005 to 0.000; P = .01) compared to peers in quartile 1 who consumed less than 20% of daily calories from UPFs.
The researchers did not investigate individual groups of UPFs.
However, Dr. Goncalves noted that some studies have linked the consumption of sugar-sweetened beverages with lower cognitive performance, lower brain volume, and poorer memory performance. Another group of ultraprocessed foods, processed meats, has been associated with increased all-cause dementia and Alzheimer’s disease.
Other limitations include the fact that self-reported diet habits were assessed only at baseline using a food frequency questionnaire that was not designed to assess the degree of processing.
While analyses were adjusted for several sociodemographic and clinical confounders, the researchers said they could not exclude the possibility of residual confounding.
Also, since neuroimaging is not available in the ELSA-Brasil study, they were not able to investigate potential mechanisms that could explain the association between higher UPF consumption and cognitive decline.
Despite these limitations, the researchers said their findings suggest that “limiting UPF consumption, particularly in middle-aged adults, may be an efficient form to prevent cognitive decline.”
Weighing the evidence
Several experts weighed in on the results in a statement from the UK nonprofit organization, Science Media Centre.
Kevin McConway, PhD, with Open University, Milton Keynes, England, said it’s important to note that the study suggests “an association, a correlation, and that doesn’t necessarily mean that the cognitive decline was caused by eating more ultra-processed foods.”
He also noted that some types of cognitive decline that are associated with aging occurred in participants in all four quartiles, which were defined by the percentage of their daily energy that came from consuming UPFs.
“That’s hardly surprising – it’s a sad fact of life that pretty well all of us gradually lose some of our cognitive functions as we go through middle and older age,” Dr. McConway said.
“The study doesn’t establish that differences in speed of cognitive decline are caused by ultra-processed food consumption anyway. That’s because it’s an observational study. If the consumption of ultra-processed food causes the differences in rate of cognitive decline, then eating less of it might slow cognitive decline, but if the cause is something else, then that won’t happen,” Dr. McConway added.
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading, England, noted that UPFs have become a “fashionable term to explain associations between diet and ill health, and many studies have attempted to show associations.
“Most studies have been observational and had a key limitation: It is very difficult to determine ultra-processed food intake using methods that are not designed to do so, and so authors need to make a lot of assumptions. Bread and meat products are often classed as ‘ultra-processed,’ even though this is often wrong,” Dr. Kuhnle noted.
“The same applies to this study – the method used to measure ultra-processed food intake was not designed for that task and relied on assumptions. This makes it virtually impossible to draw any conclusions,” Dr. Kuhnle said.
Duane Mellor, PhD, RD, RNutr, registered dietitian and senior teaching fellow, Aston University, Birmingham, England, said the study does not change how we should try to eat to maintain good brain function and cognition.
“We should try to eat less foods which are high in added sugar, salt, and fat, which would include many of the foods classified as being ultra-processed, while eating more in terms of both quantity and variety of vegetables, fruit, nuts, seeds, and pulses, which are known to be beneficial for both our cognitive and overall health,” Dr. Mellor said.
The ELSA-Brasil study was supported by the Brazilian Ministry of Health, the Ministry of Science, Technology and Innovation, and the National Council for Scientific and Technological Development. The authors as well as Dr. McConway, Dr. Mellor, and Dr. Kuhnle have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
‘Meth’ heart failure on the rise, often more severe
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
a literature review indicates.
MethHF is associated with increased severity for HF, longer inpatient stay, and more readmissions, compared with non-MethHF, the data show.
Clinicians “need to consider methamphetamine as a potential etiology for heart failure and include a substance use history when evaluating patients. Treating methamphetamine use disorder improves heart failure outcomes,” first author Veena Manja, MD, PhD, with Stanford (Calif.) University, said in an interview.
The study was published online in the journal Heart.
Poor outcomes, ‘staggering’ costs
This “thoughtful” review is “important and necessary,” Jonathan Davis, MD, director of the heart failure program, Zuckerberg San Francisco General Hospital, wrote in an editorial in the journal.
Dr. Davis noted that patients with Meth HF are at increased risk for poor outcomes and death and the health care costs related to MethHF are “staggering.”
As an example, inpatient data for California show annual charges related to MethHF rose by 840% from 2008 to 2018, from $41.5 million to $390.2 million, compared with 82% for all HF, which rose from $3.5 billion to $6.8 billion.
Illicit use of methamphetamine – also known as “crystal meth,” “ice,” and “speed” – has been linked to hypertension, MI, stroke, aortic dissection, and sudden death. But until now, there was no comprehensive systematic review of published studies on MethHF.
“Our goal was to compile current knowledge on the topic, increase awareness of this condition and identify areas for future research,” Dr. Manja said.
The researchers reviewed 21 observational studies, mostly from the United States (14 from California), between 1997 and 2020. The mean age of adults with MethHF ranged in age from 35 to 60 and more than half were male (57%).
Illicit methamphetamine was inhaled, injected, swallowed, smoked, and snorted. The reported frequency ranged from daily to every other week, and the total monthly dose ranged from 0.35 g to 24.5 g.
The average duration of meth use before HF diagnosis was 5 years. However, 18% of users developed HF within 1 year of starting to use illicit methamphetamine. In some cases, HF was diagnosed after a single use.
The researchers also note that MethHF with preserved left ventricular ejection fraction, seen in up to 44% of cases, is a distinct entity that may progress to reduced LVEF with continued use.
MethHF is also associated with a greater likelihood of other substance abuse, PTSD, depression, and other heart and kidney disease.
Factors associated with improved MethHF outcomes include female sex, meth abstinence, and adherence to guideline-directed HF therapy.
Improvement in MethHF outcomes is possible even if abstinence is not consistent, a finding that lends support to harm reduction principles of “meeting patients where they are instead of insisting on complete abstinence,” the researchers said.
Large gaps in knowledge
They were unable to combine the results into a meta-analysis because of heterogeneity in study design, population, comparator, and outcome assessment. Also, the overall risk of bias is moderate because of the presence of confounders, selection bias and poor matching, and the overall certainty in the evidence is very low,.
No study evaluated the incidence or prevalence of HF among methamphetamine users and inconsistent history taking and testing in patients with HF impeded accurate MethHF prevalence assessment.
Several studies, however, document an increasing incidence of MethHF, particularly over the past decade.
One study from California reported a 585% increase in MethHF hospital admissions between 2008 and 2018. An analysis of the National Inpatient Survey found a 12-fold increase in annual MethHF hospitalizations between 2002 and 2014.
“The results of this systematic review highlight large gaps in our knowledge” of MethHF, Dr. Manja said in an interview.
“We need to understand the epidemiology, prevalence, factors that confer susceptibility to cardiovascular outcomes, and need research into treatment targeted toward this disease,” Dr. Manja added. “We should consider options to integrate substance use treatment in HF/cardiology/primary care clinics and design a multidisciplinary patient-centered approach.”
Dr. Davis agreed. This work “highlights that the standard of care academically and clinically must be a broad team across the care spectrum to simultaneously address methamphetamine use, heart failure, and social determinants of health.”
This research had no specific funding. Dr. Manja and Dr. Davis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HEART
New melting hydrogel bandage could treat burn wounds faster, with less pain
Surgically debriding burn wounds can be tedious for doctors and excruciating for patients. To change that, bioengineers have created a new hydrogel formula that dissolves rapidly from wound sites, melting off in 6 minutes or less.
“The removal of dressings, with the current standard of care, is very hard and time-consuming. It becomes very painful for the patient. People are screaming, or they’re given a lot of opioids,” said senior author O. Berk Usta, PhD, of the Center for Engineering in Medicine and Surgery at Massachusetts General Hospital, Boston. “Those are the things we wanted to minimize: the pain and the time.”
Although beneficial for all patients, a short, painless bandage change would be a particular boon for younger patients. At the pediatric burns care center at Shriners Hospitals for Children (an MGH partner), researchers “observe a lot of children who go through therapy or treatment after burns,” said Dr. Usta. The team at MGH collaborated with scientists at Tufts University, Boston, with those patients in mind, setting out to create a new hydrogel that would transform burn wound care.
A better bandage
Hydrogels provide cooling relief to burn wounds and maintain a moist environment that can speed healing. There are currently hydrogel sheets and hydrogel-infused dressings, as well as gel that is applied directly to burn wounds before being covered with protective material. These dressings must be replaced frequently to prevent infections, but that can be unbearably painful and drawn out, as dressings often stick to wounds.
Mechanical debridement can be especially difficult for second-degree burn patients, whose wounds may still retain nerve endings. Debridement tends to also remove some healthy tissue and can damage newly formed tissue, slowing down healing.
“It can take up to 2, 3 hours, and it requires multiple people working on it,” said Dr. Usta.
The new hydrogel treatment can be applied directly to a wound and it forms a protective barrier around the site in 15 seconds. The hydrogel is then covered by a protective dressing until it needs to be changed.
“After you take off the protective covering, you add another solution, which dissolves the [hydrogel] dressing, so that it can be easily removed from the burn site,” Dr. Usta said.
The solution dissolves the hydrogel in 4-6 minutes.
Hybrid gels
Many hydrogels currently used for burn wounds feature physically cross-linked molecules. This makes them strong and capable of retaining moisture, but also difficult to dissolve. The researchers used a different approach.
“This is not physical cross-linking like the traditional approaches, but rather, softer covalent bonds between the different molecules. And that’s why, when you bring in another solution, the hydrogel dissolves away,” Dr. Usta said.
The new hydrogels rely on a supramolecular assembly: a network of synthetic polymers whose connections can be reversed more easily, meaning they can be dissolved quickly. Another standout feature of the new hydrogels is their hybrid composition, displaying characteristics of both liquids and solids. The polymers are knitted together into a mesh-like network that enables water retention, with the goal of maintaining the moist environment needed for wound healing.
The supramolecular assembly is also greener, Dr. Usta explained; traditional cross-linking approaches produce a lot of toxic by-products that could harm the environment.
And whereas traditional hydrogels can require a dozen chemistry steps to produce, the new hydrogels are ready after mixing two solutions, Dr. Usta explained. This makes them easy to prepare at bedside, ideal for treating large wounds in the ER or even on battlefields.
When tested in vitro, using skin cells, and in vivo, on mice, the new hydrogels were shown to be safe to use on wounds. Additional studies on mice, as well as large animals, will focus on safety and efficacy, and may be followed by human clinical trials, said Dr. Usta.
“The next phase of the project will be to look at whether these dressings will help wound healing by creating a moist environment,” said Dr. Usta.
The researchers are also exploring how to manufacture individual prewrapped hydrogels that could be applied in a clinical setting – or even in people’s homes. The consumer market is “another possibility,” said Dr. Usta, particularly among patients with “smaller, more superficial burns” or patients whose large burn wounds are still healing once they leave the hospital.
This research was supported by the National Institutes of Health, National Science Foundation, Massachusetts General Hospital Executive Committee on Research Interim Support Fund, and Shriners Hospitals.
A version of this article first appeared on Medscape.com.
Surgically debriding burn wounds can be tedious for doctors and excruciating for patients. To change that, bioengineers have created a new hydrogel formula that dissolves rapidly from wound sites, melting off in 6 minutes or less.
“The removal of dressings, with the current standard of care, is very hard and time-consuming. It becomes very painful for the patient. People are screaming, or they’re given a lot of opioids,” said senior author O. Berk Usta, PhD, of the Center for Engineering in Medicine and Surgery at Massachusetts General Hospital, Boston. “Those are the things we wanted to minimize: the pain and the time.”
Although beneficial for all patients, a short, painless bandage change would be a particular boon for younger patients. At the pediatric burns care center at Shriners Hospitals for Children (an MGH partner), researchers “observe a lot of children who go through therapy or treatment after burns,” said Dr. Usta. The team at MGH collaborated with scientists at Tufts University, Boston, with those patients in mind, setting out to create a new hydrogel that would transform burn wound care.
A better bandage
Hydrogels provide cooling relief to burn wounds and maintain a moist environment that can speed healing. There are currently hydrogel sheets and hydrogel-infused dressings, as well as gel that is applied directly to burn wounds before being covered with protective material. These dressings must be replaced frequently to prevent infections, but that can be unbearably painful and drawn out, as dressings often stick to wounds.
Mechanical debridement can be especially difficult for second-degree burn patients, whose wounds may still retain nerve endings. Debridement tends to also remove some healthy tissue and can damage newly formed tissue, slowing down healing.
“It can take up to 2, 3 hours, and it requires multiple people working on it,” said Dr. Usta.
The new hydrogel treatment can be applied directly to a wound and it forms a protective barrier around the site in 15 seconds. The hydrogel is then covered by a protective dressing until it needs to be changed.
“After you take off the protective covering, you add another solution, which dissolves the [hydrogel] dressing, so that it can be easily removed from the burn site,” Dr. Usta said.
The solution dissolves the hydrogel in 4-6 minutes.
Hybrid gels
Many hydrogels currently used for burn wounds feature physically cross-linked molecules. This makes them strong and capable of retaining moisture, but also difficult to dissolve. The researchers used a different approach.
“This is not physical cross-linking like the traditional approaches, but rather, softer covalent bonds between the different molecules. And that’s why, when you bring in another solution, the hydrogel dissolves away,” Dr. Usta said.
The new hydrogels rely on a supramolecular assembly: a network of synthetic polymers whose connections can be reversed more easily, meaning they can be dissolved quickly. Another standout feature of the new hydrogels is their hybrid composition, displaying characteristics of both liquids and solids. The polymers are knitted together into a mesh-like network that enables water retention, with the goal of maintaining the moist environment needed for wound healing.
The supramolecular assembly is also greener, Dr. Usta explained; traditional cross-linking approaches produce a lot of toxic by-products that could harm the environment.
And whereas traditional hydrogels can require a dozen chemistry steps to produce, the new hydrogels are ready after mixing two solutions, Dr. Usta explained. This makes them easy to prepare at bedside, ideal for treating large wounds in the ER or even on battlefields.
When tested in vitro, using skin cells, and in vivo, on mice, the new hydrogels were shown to be safe to use on wounds. Additional studies on mice, as well as large animals, will focus on safety and efficacy, and may be followed by human clinical trials, said Dr. Usta.
“The next phase of the project will be to look at whether these dressings will help wound healing by creating a moist environment,” said Dr. Usta.
The researchers are also exploring how to manufacture individual prewrapped hydrogels that could be applied in a clinical setting – or even in people’s homes. The consumer market is “another possibility,” said Dr. Usta, particularly among patients with “smaller, more superficial burns” or patients whose large burn wounds are still healing once they leave the hospital.
This research was supported by the National Institutes of Health, National Science Foundation, Massachusetts General Hospital Executive Committee on Research Interim Support Fund, and Shriners Hospitals.
A version of this article first appeared on Medscape.com.
Surgically debriding burn wounds can be tedious for doctors and excruciating for patients. To change that, bioengineers have created a new hydrogel formula that dissolves rapidly from wound sites, melting off in 6 minutes or less.
“The removal of dressings, with the current standard of care, is very hard and time-consuming. It becomes very painful for the patient. People are screaming, or they’re given a lot of opioids,” said senior author O. Berk Usta, PhD, of the Center for Engineering in Medicine and Surgery at Massachusetts General Hospital, Boston. “Those are the things we wanted to minimize: the pain and the time.”
Although beneficial for all patients, a short, painless bandage change would be a particular boon for younger patients. At the pediatric burns care center at Shriners Hospitals for Children (an MGH partner), researchers “observe a lot of children who go through therapy or treatment after burns,” said Dr. Usta. The team at MGH collaborated with scientists at Tufts University, Boston, with those patients in mind, setting out to create a new hydrogel that would transform burn wound care.
A better bandage
Hydrogels provide cooling relief to burn wounds and maintain a moist environment that can speed healing. There are currently hydrogel sheets and hydrogel-infused dressings, as well as gel that is applied directly to burn wounds before being covered with protective material. These dressings must be replaced frequently to prevent infections, but that can be unbearably painful and drawn out, as dressings often stick to wounds.
Mechanical debridement can be especially difficult for second-degree burn patients, whose wounds may still retain nerve endings. Debridement tends to also remove some healthy tissue and can damage newly formed tissue, slowing down healing.
“It can take up to 2, 3 hours, and it requires multiple people working on it,” said Dr. Usta.
The new hydrogel treatment can be applied directly to a wound and it forms a protective barrier around the site in 15 seconds. The hydrogel is then covered by a protective dressing until it needs to be changed.
“After you take off the protective covering, you add another solution, which dissolves the [hydrogel] dressing, so that it can be easily removed from the burn site,” Dr. Usta said.
The solution dissolves the hydrogel in 4-6 minutes.
Hybrid gels
Many hydrogels currently used for burn wounds feature physically cross-linked molecules. This makes them strong and capable of retaining moisture, but also difficult to dissolve. The researchers used a different approach.
“This is not physical cross-linking like the traditional approaches, but rather, softer covalent bonds between the different molecules. And that’s why, when you bring in another solution, the hydrogel dissolves away,” Dr. Usta said.
The new hydrogels rely on a supramolecular assembly: a network of synthetic polymers whose connections can be reversed more easily, meaning they can be dissolved quickly. Another standout feature of the new hydrogels is their hybrid composition, displaying characteristics of both liquids and solids. The polymers are knitted together into a mesh-like network that enables water retention, with the goal of maintaining the moist environment needed for wound healing.
The supramolecular assembly is also greener, Dr. Usta explained; traditional cross-linking approaches produce a lot of toxic by-products that could harm the environment.
And whereas traditional hydrogels can require a dozen chemistry steps to produce, the new hydrogels are ready after mixing two solutions, Dr. Usta explained. This makes them easy to prepare at bedside, ideal for treating large wounds in the ER or even on battlefields.
When tested in vitro, using skin cells, and in vivo, on mice, the new hydrogels were shown to be safe to use on wounds. Additional studies on mice, as well as large animals, will focus on safety and efficacy, and may be followed by human clinical trials, said Dr. Usta.
“The next phase of the project will be to look at whether these dressings will help wound healing by creating a moist environment,” said Dr. Usta.
The researchers are also exploring how to manufacture individual prewrapped hydrogels that could be applied in a clinical setting – or even in people’s homes. The consumer market is “another possibility,” said Dr. Usta, particularly among patients with “smaller, more superficial burns” or patients whose large burn wounds are still healing once they leave the hospital.
This research was supported by the National Institutes of Health, National Science Foundation, Massachusetts General Hospital Executive Committee on Research Interim Support Fund, and Shriners Hospitals.
A version of this article first appeared on Medscape.com.
FROM BIOACTIVE MATERIALS
Advances in Lupus From ACR 2022
Dr Anca Askanase, director of the Lupus Center at Columbia University Medical Center, highlights the latest research on systemic lupus erythematosus (SLE) and lupus nephritis from the American College of Rheumatology (ACR) 2022.
Dr Askanase first discusses a small study using autologous chimeric antigen receptor T-cell (CAR-T) therapy, which is approved for use in several blood cancers, as an alternative for patients with refractory SLE. Five patients received CAR-T cells, and all achieved sustained, drug-free remission.
Next, Dr Askanase highlights a phase 2B study evaluating the tyrosine kinase 2 inhibitor deucravacitinib in patients with SLE. In early results, patients taking deucravacitinib showed a statistically meaningful response in disease activity compared with placebo.
She then summarizes her presentation on oral cenerimod. The phase 2, 12-week study demonstrated that cenerimod reduced total lymphocyte count compared with placebo in SLE patients.
Next, Dr Askanase details the long-term extension of the TULIP trials. Researchers found that anifrolumab has a favorable benefit-risk profile when compared with placebo and is therefore a possible long-term treatment option for patients with moderate to severe SLE.
Finally, Dr Askanase discusses the positive findings from the phase 3 AURORA 1 and AURORA 2 studies, which sought to determine whether the addition of voclosporin to mycophenolate mofetil and low-dose steroids could maintain the reduction in proteinuria in patients with lupus nephritis.
--
Anca Askanase, MD, MPH, Professor of Medicine, Director, Lupus Center, Department of Rheumatology, Columbia University Medical Center, New York, New York
Anca Askanase, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Dr Anca Askanase, director of the Lupus Center at Columbia University Medical Center, highlights the latest research on systemic lupus erythematosus (SLE) and lupus nephritis from the American College of Rheumatology (ACR) 2022.
Dr Askanase first discusses a small study using autologous chimeric antigen receptor T-cell (CAR-T) therapy, which is approved for use in several blood cancers, as an alternative for patients with refractory SLE. Five patients received CAR-T cells, and all achieved sustained, drug-free remission.
Next, Dr Askanase highlights a phase 2B study evaluating the tyrosine kinase 2 inhibitor deucravacitinib in patients with SLE. In early results, patients taking deucravacitinib showed a statistically meaningful response in disease activity compared with placebo.
She then summarizes her presentation on oral cenerimod. The phase 2, 12-week study demonstrated that cenerimod reduced total lymphocyte count compared with placebo in SLE patients.
Next, Dr Askanase details the long-term extension of the TULIP trials. Researchers found that anifrolumab has a favorable benefit-risk profile when compared with placebo and is therefore a possible long-term treatment option for patients with moderate to severe SLE.
Finally, Dr Askanase discusses the positive findings from the phase 3 AURORA 1 and AURORA 2 studies, which sought to determine whether the addition of voclosporin to mycophenolate mofetil and low-dose steroids could maintain the reduction in proteinuria in patients with lupus nephritis.
--
Anca Askanase, MD, MPH, Professor of Medicine, Director, Lupus Center, Department of Rheumatology, Columbia University Medical Center, New York, New York
Anca Askanase, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Dr Anca Askanase, director of the Lupus Center at Columbia University Medical Center, highlights the latest research on systemic lupus erythematosus (SLE) and lupus nephritis from the American College of Rheumatology (ACR) 2022.
Dr Askanase first discusses a small study using autologous chimeric antigen receptor T-cell (CAR-T) therapy, which is approved for use in several blood cancers, as an alternative for patients with refractory SLE. Five patients received CAR-T cells, and all achieved sustained, drug-free remission.
Next, Dr Askanase highlights a phase 2B study evaluating the tyrosine kinase 2 inhibitor deucravacitinib in patients with SLE. In early results, patients taking deucravacitinib showed a statistically meaningful response in disease activity compared with placebo.
She then summarizes her presentation on oral cenerimod. The phase 2, 12-week study demonstrated that cenerimod reduced total lymphocyte count compared with placebo in SLE patients.
Next, Dr Askanase details the long-term extension of the TULIP trials. Researchers found that anifrolumab has a favorable benefit-risk profile when compared with placebo and is therefore a possible long-term treatment option for patients with moderate to severe SLE.
Finally, Dr Askanase discusses the positive findings from the phase 3 AURORA 1 and AURORA 2 studies, which sought to determine whether the addition of voclosporin to mycophenolate mofetil and low-dose steroids could maintain the reduction in proteinuria in patients with lupus nephritis.
--
Anca Askanase, MD, MPH, Professor of Medicine, Director, Lupus Center, Department of Rheumatology, Columbia University Medical Center, New York, New York
Anca Askanase, MD, MPH, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer
Received income in an amount equal to or greater than $250 from: AstraZeneca; Bristol Myers Squibb; Celgene; Eli Lilly; GSK; Idorsia; Janssen; Pfizer

Janus Kinase Inhibitors in the Treatment of Atopic Dermatitis: Military Considerations
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
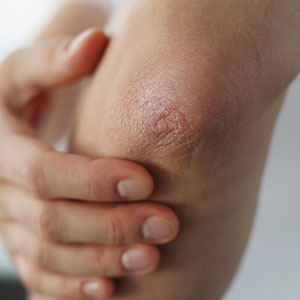
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
Practice Points
- Oral Janus kinase (JAK) inhibitors are novel therapies available for the treatment of atopic dermatitis (AD), with multiple recently approved agents within the class.
- Recommended laboratory monitoring during treatment with oral JAK inhibitors may limit the use of these medications in the active-duty military population or in those with special-duty assignments.
- The oral and topical bioavailability of these medications makes them a more feasible option for deploying service members or for those requiring flexible dosing.
- The rapid improvement in AD seen in multiple trials of oral JAK inhibitors suggests these agents could prove useful in management of acute AD flares, especially in military environments, where injectable agents are either unavailable or unsupported.
Advocacy Update: Ringing in 2023
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.
Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2
Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5
The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.
In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
Practice Points
- New digital pathology codes proposed by the American Medical Association can be used starting January 1, 2023.
- A proposed 2023 fee schedule negatively impacting dermatology practices was published by the Centers for Medicare & Medicaid Services in July 2022.
- Advocacy involvement provides a collaborative collective voice for our specialty to help our patients improve their care.
Nurturing a Satisfying Career in Dermatology
The residents of our program asked me to serve as their commencement speaker in June. Since I was retiring from my position as department chair, this touching honor seemed a fitting capstone for my career. It gave me the opportunity to reflect on the enormity of the changes that have occurred between my graduation from residency in 1983 and the current time, which is marked by disruption from the digital revolution and the COVID-19 pandemic. Throughout this 40-year period, there were times of external global turmoil, economic instability, significant changes in the business of medicine, stressful changes in documentation of competency and certification, and the difficult transition to electronic medical records. Another epidemic—AIDS—changed surgical practices. During my residency, we did biopsies without wearing gloves or masks. Gloves were added to protect the person doing the procedure as well as to prevent spread of disease to other patients, not to reduce the infection rate for the patient undergoing the procedure. Of course, change in the last 40 years also occurred outside of work and included various familial stresses. The irritations of daily life easily mounted up to being overwhelming. However, I had gone to work every day for 40 years, seeking to do my best for my patients and my colleagues and the staff with whom I worked, sometimes feeling successful and sometimes feeling incompetent. Some days went smoothly, and some days were filled with challenges that I could not begin to imagine how I would solve. I have a habit of seeing problems rather than successes, which creates its own difficulties. I did, however, grab opportunities that continually improved my practice of medicine and allowed me to serve in several professional positions as well as in leadership positions of multiple professional societies. As I prepared the commencement address, I realized that the totality of my career was very satisfying.
The Merriam-Webster dictionary definition of satisfying is “producing pleasure or contentment by providing what is needed or wanted.”1 My use of the word means that my career over the long term has pleased me—maybe not some of the people I reported to, but rather me.
My approach to my career can be summarized in 3 words: purpose, serendipity, and curiosity.
The first element is purpose. Job satisfaction generally is associated with work being aligned with values, an appreciation that you are accomplishing the purpose with which you set out on your journey. It is not associated with every day being wonderful and problem free or every task being completed without setbacks or complications. The reality of working is not that every moment brings pure happiness or that every task fulfills a passion. How does a person ensure that the days add up to be satisfying? Start with values. Why did you decide to pursue medical school? Some may have chosen it for economic security, but there are many ways to achieve economic security. Maybe being a physician feeds into the family lore, but families generally have broad ranges of acceptable careers. Maybe it appealed scientifically, but a PhD in biology also fulfills that interest. Maybe it is that you noticed respect for physicians in the community when you were growing up, but that is changing and does not represent an internal value anyway. Consider your values carefully, write them down, and keep them at the forefront of the day. Go back to them consciously any time you have a rough day and understand why you are doing what you are doing. When you are 55 years old and going through your umpteenth change in reimbursement process, go back to the day you decided on medicine as a career. Focus on your values as the grounding for your purpose. Also note that purpose is different than goals. Some goals will be reached, and some will not. Goals change with external realities and/ or internal factors. Purpose and values remain the same if we have thoughtfully identified them.
The second element is serendipity. Serendipity often is thought of as luck, as karma, as being in the right place at the right time. It feels random, and at first glance it appears that purpose and serendipity are complete opposites and do not intersect. Serendipity is, however, not just luck. It is an ability to distinguish events and observations in meaningful ways. It is a close relative of creativity and benefits from sloppiness, playfulness, tinkering, and discussion. It cannot exist in a vacuum. History is replete with serendipitous discoveries. It is thought that James Watson and Francis Crick would never have been able to elucidate the nature of DNA without sharing offices with people with whom they argued daily. In fact, figuring out the DNA structure was not even the main focus of their laboratories. It was just a side angle that several people loved to think about. Appreciating serendipity by being truly open to opportunities that are out on the wings brings experiences that are deeply rewarding even if not planned. I had no idea at all, no plan, no goal of serving as president of the American Academy of Dermatology or as Department Chair, and yet these happened. These experiences have allowed me to work on my purpose as I have defined it. How can you harness serendipity in your own life? My philosophy may be somewhat simple, but I think if you show up every day doing the best job you can at the tasks on hand, doors will appear, at odd intervals and in odd directions. You must be open enough and in tune with your purpose to an extent that you can sense the direction in which to turn and what doorways through which to walk.
The third element is curiosity. One definition is that curiosity is the motivation to learn new information. Another definition is that curiosity is a special form of information seeking distinguished by the fact that it is internally motivated. We are all familiar with intellectual curiosity. For example, a patient has a basal cell carcinoma on the upper back. What does the literature say about the cure rates of various treatments for that particular tumor? In addition, we can be curious about other things as well. Is it a really small tumor? How was it found and why is the patient anxious? Why does it make me irritated that the patient is worried about such a small, easily treated tumor? Or is it a large neglected tumor? Why was it not treated before? Why does it make me sad that it is so large? Why does it annoy me that I have a difficult situation to manage? Being able to define an emotional reaction by being curious about its presence helps us manage destructive responses and promote more positive outcomes. This curiosity is related to emotional intelligence and is mindfully harnessed by effective leaders. Curiosity will get you through tough days when your office team is stressed and the tough years that are complicated by professional and personal challenges.
Curiosity also will help you identify your purpose and harness serendipity, and so we come full circle with our 3 elements: purpose, serendipity, and curiosity.
My wish for all of you is that when you are at the tail end of your career, you will look back and say, “This has been a great ride.” I am very grateful that I can acknowledge this for myself. I have been so fortunate to have found dermatology, where I can go to work every day making a difference for patients in a stimulating environment with good colleagues. One of my values is to try and make life better in some way for everyone around me, even if it is just a smile at the start of the workday. As I look back, this value has allowed me to meet interesting people, hear fascinating stories, make good friends, and have enduring relationships. I have held onto fellow travelers, and we have supported each other through tough times as well as celebrated together the good times.
Nurturing a satisfying career includes these essential fundamentals. First, accept the reality of constant change. Second, develop productive relationships with fellow travelers. And third and most importantly, go forth with purpose, serendipity, and curiosity.
- Merriam-Webster. Satisfying. Merriam-Webster.com Dictionary. Accessed November 18, 2022. https://www.merriam-webster.com/dictionary/satisfying
The residents of our program asked me to serve as their commencement speaker in June. Since I was retiring from my position as department chair, this touching honor seemed a fitting capstone for my career. It gave me the opportunity to reflect on the enormity of the changes that have occurred between my graduation from residency in 1983 and the current time, which is marked by disruption from the digital revolution and the COVID-19 pandemic. Throughout this 40-year period, there were times of external global turmoil, economic instability, significant changes in the business of medicine, stressful changes in documentation of competency and certification, and the difficult transition to electronic medical records. Another epidemic—AIDS—changed surgical practices. During my residency, we did biopsies without wearing gloves or masks. Gloves were added to protect the person doing the procedure as well as to prevent spread of disease to other patients, not to reduce the infection rate for the patient undergoing the procedure. Of course, change in the last 40 years also occurred outside of work and included various familial stresses. The irritations of daily life easily mounted up to being overwhelming. However, I had gone to work every day for 40 years, seeking to do my best for my patients and my colleagues and the staff with whom I worked, sometimes feeling successful and sometimes feeling incompetent. Some days went smoothly, and some days were filled with challenges that I could not begin to imagine how I would solve. I have a habit of seeing problems rather than successes, which creates its own difficulties. I did, however, grab opportunities that continually improved my practice of medicine and allowed me to serve in several professional positions as well as in leadership positions of multiple professional societies. As I prepared the commencement address, I realized that the totality of my career was very satisfying.
The Merriam-Webster dictionary definition of satisfying is “producing pleasure or contentment by providing what is needed or wanted.”1 My use of the word means that my career over the long term has pleased me—maybe not some of the people I reported to, but rather me.
My approach to my career can be summarized in 3 words: purpose, serendipity, and curiosity.
The first element is purpose. Job satisfaction generally is associated with work being aligned with values, an appreciation that you are accomplishing the purpose with which you set out on your journey. It is not associated with every day being wonderful and problem free or every task being completed without setbacks or complications. The reality of working is not that every moment brings pure happiness or that every task fulfills a passion. How does a person ensure that the days add up to be satisfying? Start with values. Why did you decide to pursue medical school? Some may have chosen it for economic security, but there are many ways to achieve economic security. Maybe being a physician feeds into the family lore, but families generally have broad ranges of acceptable careers. Maybe it appealed scientifically, but a PhD in biology also fulfills that interest. Maybe it is that you noticed respect for physicians in the community when you were growing up, but that is changing and does not represent an internal value anyway. Consider your values carefully, write them down, and keep them at the forefront of the day. Go back to them consciously any time you have a rough day and understand why you are doing what you are doing. When you are 55 years old and going through your umpteenth change in reimbursement process, go back to the day you decided on medicine as a career. Focus on your values as the grounding for your purpose. Also note that purpose is different than goals. Some goals will be reached, and some will not. Goals change with external realities and/ or internal factors. Purpose and values remain the same if we have thoughtfully identified them.
The second element is serendipity. Serendipity often is thought of as luck, as karma, as being in the right place at the right time. It feels random, and at first glance it appears that purpose and serendipity are complete opposites and do not intersect. Serendipity is, however, not just luck. It is an ability to distinguish events and observations in meaningful ways. It is a close relative of creativity and benefits from sloppiness, playfulness, tinkering, and discussion. It cannot exist in a vacuum. History is replete with serendipitous discoveries. It is thought that James Watson and Francis Crick would never have been able to elucidate the nature of DNA without sharing offices with people with whom they argued daily. In fact, figuring out the DNA structure was not even the main focus of their laboratories. It was just a side angle that several people loved to think about. Appreciating serendipity by being truly open to opportunities that are out on the wings brings experiences that are deeply rewarding even if not planned. I had no idea at all, no plan, no goal of serving as president of the American Academy of Dermatology or as Department Chair, and yet these happened. These experiences have allowed me to work on my purpose as I have defined it. How can you harness serendipity in your own life? My philosophy may be somewhat simple, but I think if you show up every day doing the best job you can at the tasks on hand, doors will appear, at odd intervals and in odd directions. You must be open enough and in tune with your purpose to an extent that you can sense the direction in which to turn and what doorways through which to walk.
The third element is curiosity. One definition is that curiosity is the motivation to learn new information. Another definition is that curiosity is a special form of information seeking distinguished by the fact that it is internally motivated. We are all familiar with intellectual curiosity. For example, a patient has a basal cell carcinoma on the upper back. What does the literature say about the cure rates of various treatments for that particular tumor? In addition, we can be curious about other things as well. Is it a really small tumor? How was it found and why is the patient anxious? Why does it make me irritated that the patient is worried about such a small, easily treated tumor? Or is it a large neglected tumor? Why was it not treated before? Why does it make me sad that it is so large? Why does it annoy me that I have a difficult situation to manage? Being able to define an emotional reaction by being curious about its presence helps us manage destructive responses and promote more positive outcomes. This curiosity is related to emotional intelligence and is mindfully harnessed by effective leaders. Curiosity will get you through tough days when your office team is stressed and the tough years that are complicated by professional and personal challenges.
Curiosity also will help you identify your purpose and harness serendipity, and so we come full circle with our 3 elements: purpose, serendipity, and curiosity.
My wish for all of you is that when you are at the tail end of your career, you will look back and say, “This has been a great ride.” I am very grateful that I can acknowledge this for myself. I have been so fortunate to have found dermatology, where I can go to work every day making a difference for patients in a stimulating environment with good colleagues. One of my values is to try and make life better in some way for everyone around me, even if it is just a smile at the start of the workday. As I look back, this value has allowed me to meet interesting people, hear fascinating stories, make good friends, and have enduring relationships. I have held onto fellow travelers, and we have supported each other through tough times as well as celebrated together the good times.
Nurturing a satisfying career includes these essential fundamentals. First, accept the reality of constant change. Second, develop productive relationships with fellow travelers. And third and most importantly, go forth with purpose, serendipity, and curiosity.
The residents of our program asked me to serve as their commencement speaker in June. Since I was retiring from my position as department chair, this touching honor seemed a fitting capstone for my career. It gave me the opportunity to reflect on the enormity of the changes that have occurred between my graduation from residency in 1983 and the current time, which is marked by disruption from the digital revolution and the COVID-19 pandemic. Throughout this 40-year period, there were times of external global turmoil, economic instability, significant changes in the business of medicine, stressful changes in documentation of competency and certification, and the difficult transition to electronic medical records. Another epidemic—AIDS—changed surgical practices. During my residency, we did biopsies without wearing gloves or masks. Gloves were added to protect the person doing the procedure as well as to prevent spread of disease to other patients, not to reduce the infection rate for the patient undergoing the procedure. Of course, change in the last 40 years also occurred outside of work and included various familial stresses. The irritations of daily life easily mounted up to being overwhelming. However, I had gone to work every day for 40 years, seeking to do my best for my patients and my colleagues and the staff with whom I worked, sometimes feeling successful and sometimes feeling incompetent. Some days went smoothly, and some days were filled with challenges that I could not begin to imagine how I would solve. I have a habit of seeing problems rather than successes, which creates its own difficulties. I did, however, grab opportunities that continually improved my practice of medicine and allowed me to serve in several professional positions as well as in leadership positions of multiple professional societies. As I prepared the commencement address, I realized that the totality of my career was very satisfying.
The Merriam-Webster dictionary definition of satisfying is “producing pleasure or contentment by providing what is needed or wanted.”1 My use of the word means that my career over the long term has pleased me—maybe not some of the people I reported to, but rather me.
My approach to my career can be summarized in 3 words: purpose, serendipity, and curiosity.
The first element is purpose. Job satisfaction generally is associated with work being aligned with values, an appreciation that you are accomplishing the purpose with which you set out on your journey. It is not associated with every day being wonderful and problem free or every task being completed without setbacks or complications. The reality of working is not that every moment brings pure happiness or that every task fulfills a passion. How does a person ensure that the days add up to be satisfying? Start with values. Why did you decide to pursue medical school? Some may have chosen it for economic security, but there are many ways to achieve economic security. Maybe being a physician feeds into the family lore, but families generally have broad ranges of acceptable careers. Maybe it appealed scientifically, but a PhD in biology also fulfills that interest. Maybe it is that you noticed respect for physicians in the community when you were growing up, but that is changing and does not represent an internal value anyway. Consider your values carefully, write them down, and keep them at the forefront of the day. Go back to them consciously any time you have a rough day and understand why you are doing what you are doing. When you are 55 years old and going through your umpteenth change in reimbursement process, go back to the day you decided on medicine as a career. Focus on your values as the grounding for your purpose. Also note that purpose is different than goals. Some goals will be reached, and some will not. Goals change with external realities and/ or internal factors. Purpose and values remain the same if we have thoughtfully identified them.
The second element is serendipity. Serendipity often is thought of as luck, as karma, as being in the right place at the right time. It feels random, and at first glance it appears that purpose and serendipity are complete opposites and do not intersect. Serendipity is, however, not just luck. It is an ability to distinguish events and observations in meaningful ways. It is a close relative of creativity and benefits from sloppiness, playfulness, tinkering, and discussion. It cannot exist in a vacuum. History is replete with serendipitous discoveries. It is thought that James Watson and Francis Crick would never have been able to elucidate the nature of DNA without sharing offices with people with whom they argued daily. In fact, figuring out the DNA structure was not even the main focus of their laboratories. It was just a side angle that several people loved to think about. Appreciating serendipity by being truly open to opportunities that are out on the wings brings experiences that are deeply rewarding even if not planned. I had no idea at all, no plan, no goal of serving as president of the American Academy of Dermatology or as Department Chair, and yet these happened. These experiences have allowed me to work on my purpose as I have defined it. How can you harness serendipity in your own life? My philosophy may be somewhat simple, but I think if you show up every day doing the best job you can at the tasks on hand, doors will appear, at odd intervals and in odd directions. You must be open enough and in tune with your purpose to an extent that you can sense the direction in which to turn and what doorways through which to walk.
The third element is curiosity. One definition is that curiosity is the motivation to learn new information. Another definition is that curiosity is a special form of information seeking distinguished by the fact that it is internally motivated. We are all familiar with intellectual curiosity. For example, a patient has a basal cell carcinoma on the upper back. What does the literature say about the cure rates of various treatments for that particular tumor? In addition, we can be curious about other things as well. Is it a really small tumor? How was it found and why is the patient anxious? Why does it make me irritated that the patient is worried about such a small, easily treated tumor? Or is it a large neglected tumor? Why was it not treated before? Why does it make me sad that it is so large? Why does it annoy me that I have a difficult situation to manage? Being able to define an emotional reaction by being curious about its presence helps us manage destructive responses and promote more positive outcomes. This curiosity is related to emotional intelligence and is mindfully harnessed by effective leaders. Curiosity will get you through tough days when your office team is stressed and the tough years that are complicated by professional and personal challenges.
Curiosity also will help you identify your purpose and harness serendipity, and so we come full circle with our 3 elements: purpose, serendipity, and curiosity.
My wish for all of you is that when you are at the tail end of your career, you will look back and say, “This has been a great ride.” I am very grateful that I can acknowledge this for myself. I have been so fortunate to have found dermatology, where I can go to work every day making a difference for patients in a stimulating environment with good colleagues. One of my values is to try and make life better in some way for everyone around me, even if it is just a smile at the start of the workday. As I look back, this value has allowed me to meet interesting people, hear fascinating stories, make good friends, and have enduring relationships. I have held onto fellow travelers, and we have supported each other through tough times as well as celebrated together the good times.
Nurturing a satisfying career includes these essential fundamentals. First, accept the reality of constant change. Second, develop productive relationships with fellow travelers. And third and most importantly, go forth with purpose, serendipity, and curiosity.
- Merriam-Webster. Satisfying. Merriam-Webster.com Dictionary. Accessed November 18, 2022. https://www.merriam-webster.com/dictionary/satisfying
- Merriam-Webster. Satisfying. Merriam-Webster.com Dictionary. Accessed November 18, 2022. https://www.merriam-webster.com/dictionary/satisfying
The Universal Dermatology Bandage Kit: A Succinct Collection of Supplies
Practice Gap
Biopsies, excisions, and other invasive cutaneous procedures are performed regularly in dermatology clinics and require placement of a bandage after the procedure. Postprocedural bandaging varies by the type of procedure performed, anatomic site, and the physician’s preference of materials. Dermatologists can be left with an overwhelming choice of supplies and little practical education, as bandaging methods are not routinely addressed in residency curricula. To address this concern, we provide a succinct list of basic materials that are versatile and easily adapted to encompass all bandaging needs for dermatology procedures (Table).
With these few components, one can create an array of distinct bandages to cover wounds as small as a shave biopsy to linear closures and basic flaps or grafts. Even traditionally difficult-to-bandage areas are easily addressed. Simple modifications of the basic materials are required for each bandage adaptation, as outlined below.
The Techniques
Shave and Punch Biopsy Sites—Layer (from bottom to top) the emollient of choice, a cut 4×4-inch gauze pad, and flexible polyester tape cut to the appropriate size (Figure 1). This simple bandage conforms well to any anatomic site and can replace an adhesive bandage, if desired.
Cutaneous Surgery Sites—Pressure bandages are recommended on cutaneous surgery sites. One of the most common closures performed in dermatology is the layered closure with dissolvable subcutaneous sutures and nondissolvable cutaneous sutures. When this closure is performed on the trunk and proximal extremities, undermining often is required to adequately approximate skin. This technique eliminates tension on the wound but can increase the risk for hematoma.1 A pressure bandage left in place and kept dry for 48 hours after surgery helps eliminate the risk for postoperative bleeding.
To make a pressure bandage, layer (from bottom to top) the emollient of choice, a nonstick pad cut to size, folded 4×4-inch gauze pads, and flexible polyester tape (Figure 2). Our practice routinely utilizes the tape fanning technique2 to impart equal and firm pressure over the wound.
Complex Sites—When making a pressure bandage for an anatomically complex site—the ear, nose, or lip—nonstick pads and 4×4-inch gauze pads can be cut and folded or rolled to match the size and shape of the wound. Flexible polyester tape then conforms to these custom bandage shapes, allowing maintenance of targeted wound pressure (Figure 3).
Dental rolls can be of assistance on these sites. For example, a dental roll placed in the postauricular sulcus prior to bandaging an ear maintains comfortable anatomic positioning. Rolls can be placed in the nose, maintaining its architecture while the wound heals and providing counterpressure for added hemostasis of wounds on the lateral nasal sidewall and ala. We recommend coating dental rolls in petrolatum prior to placement in the nares for ease of removal and patient comfort.
Distal Arms and Legs—Another layer of compression is added to pressure bandages on the distal upper and lower extremities using a fabric and elastic wrap (Figure 4). The extra layer keeps the bandage in place on the upper extremities while the patient continues their daily activities. It also helps prevent edema and pain in the lower extremities.
The degree of postoperative lower extremity swelling varies by patient and procedure performed but largely is inevitable with surgery on the leg, given the potential for superficial lymphatic disruption and the dependent position of the leg when standing. Elevation is always advised, but a well-wrapped, long-stretch elastic bandage provides extra support, especially if the patient has baseline venous insufficiency or needs to be on their feet during the day. The wrap is applied from the distal to the proximal leg with graded compression, overlapping by half with each rotation. The wrap is tightest near the ankle, with gradual and subtle easing of tension as it is placed superiorly.
Healing by Secondary Intention, Full-Thickness and Split-Thickness Skin Grafts, and Partial Wound Closure—These postoperative scenarios require bandages with appropriate pressure; however, dressings need to remain moist against the patient’s skin for comfortable removal, which can be accomplished with petrolatum-impregnated gauze with or without antibacterial properties. The gauze is folded to the appropriate size and placed directly on the wound or sutured in place (Figure 5). A pressure bandage is then applied on top of the gauze.
Practice Implications
The universal bandage kit and instructions for its adaptation to accommodate multiple clinical needs can serve as a helpful resource for dermatologists and their staff.
- Bunick CG, Aasi SZ. Hemorrhagic complications in dermatologic surgery. Dermatol Ther. 2011;24:537-550. doi:10.1111/j.1529-8019.2012.01454.x
- Ardilla C, Tarantino I, Goldberg LH, et al. Improved postoperative bleeding control using the fanning pressure dressing technique [published May 31, 2021]. J Am Acad Dermatol. 2021:S0190-9622(21)01040-9. doi:10.1016/j.jaad.2021.05.045
Practice Gap
Biopsies, excisions, and other invasive cutaneous procedures are performed regularly in dermatology clinics and require placement of a bandage after the procedure. Postprocedural bandaging varies by the type of procedure performed, anatomic site, and the physician’s preference of materials. Dermatologists can be left with an overwhelming choice of supplies and little practical education, as bandaging methods are not routinely addressed in residency curricula. To address this concern, we provide a succinct list of basic materials that are versatile and easily adapted to encompass all bandaging needs for dermatology procedures (Table).

With these few components, one can create an array of distinct bandages to cover wounds as small as a shave biopsy to linear closures and basic flaps or grafts. Even traditionally difficult-to-bandage areas are easily addressed. Simple modifications of the basic materials are required for each bandage adaptation, as outlined below.
The Techniques
Shave and Punch Biopsy Sites—Layer (from bottom to top) the emollient of choice, a cut 4×4-inch gauze pad, and flexible polyester tape cut to the appropriate size (Figure 1). This simple bandage conforms well to any anatomic site and can replace an adhesive bandage, if desired.

Cutaneous Surgery Sites—Pressure bandages are recommended on cutaneous surgery sites. One of the most common closures performed in dermatology is the layered closure with dissolvable subcutaneous sutures and nondissolvable cutaneous sutures. When this closure is performed on the trunk and proximal extremities, undermining often is required to adequately approximate skin. This technique eliminates tension on the wound but can increase the risk for hematoma.1 A pressure bandage left in place and kept dry for 48 hours after surgery helps eliminate the risk for postoperative bleeding.
To make a pressure bandage, layer (from bottom to top) the emollient of choice, a nonstick pad cut to size, folded 4×4-inch gauze pads, and flexible polyester tape (Figure 2). Our practice routinely utilizes the tape fanning technique2 to impart equal and firm pressure over the wound.

Complex Sites—When making a pressure bandage for an anatomically complex site—the ear, nose, or lip—nonstick pads and 4×4-inch gauze pads can be cut and folded or rolled to match the size and shape of the wound. Flexible polyester tape then conforms to these custom bandage shapes, allowing maintenance of targeted wound pressure (Figure 3).

Dental rolls can be of assistance on these sites. For example, a dental roll placed in the postauricular sulcus prior to bandaging an ear maintains comfortable anatomic positioning. Rolls can be placed in the nose, maintaining its architecture while the wound heals and providing counterpressure for added hemostasis of wounds on the lateral nasal sidewall and ala. We recommend coating dental rolls in petrolatum prior to placement in the nares for ease of removal and patient comfort.
Distal Arms and Legs—Another layer of compression is added to pressure bandages on the distal upper and lower extremities using a fabric and elastic wrap (Figure 4). The extra layer keeps the bandage in place on the upper extremities while the patient continues their daily activities. It also helps prevent edema and pain in the lower extremities.

The degree of postoperative lower extremity swelling varies by patient and procedure performed but largely is inevitable with surgery on the leg, given the potential for superficial lymphatic disruption and the dependent position of the leg when standing. Elevation is always advised, but a well-wrapped, long-stretch elastic bandage provides extra support, especially if the patient has baseline venous insufficiency or needs to be on their feet during the day. The wrap is applied from the distal to the proximal leg with graded compression, overlapping by half with each rotation. The wrap is tightest near the ankle, with gradual and subtle easing of tension as it is placed superiorly.
Healing by Secondary Intention, Full-Thickness and Split-Thickness Skin Grafts, and Partial Wound Closure—These postoperative scenarios require bandages with appropriate pressure; however, dressings need to remain moist against the patient’s skin for comfortable removal, which can be accomplished with petrolatum-impregnated gauze with or without antibacterial properties. The gauze is folded to the appropriate size and placed directly on the wound or sutured in place (Figure 5). A pressure bandage is then applied on top of the gauze.

Practice Implications
The universal bandage kit and instructions for its adaptation to accommodate multiple clinical needs can serve as a helpful resource for dermatologists and their staff.
Practice Gap
Biopsies, excisions, and other invasive cutaneous procedures are performed regularly in dermatology clinics and require placement of a bandage after the procedure. Postprocedural bandaging varies by the type of procedure performed, anatomic site, and the physician’s preference of materials. Dermatologists can be left with an overwhelming choice of supplies and little practical education, as bandaging methods are not routinely addressed in residency curricula. To address this concern, we provide a succinct list of basic materials that are versatile and easily adapted to encompass all bandaging needs for dermatology procedures (Table).

With these few components, one can create an array of distinct bandages to cover wounds as small as a shave biopsy to linear closures and basic flaps or grafts. Even traditionally difficult-to-bandage areas are easily addressed. Simple modifications of the basic materials are required for each bandage adaptation, as outlined below.
The Techniques
Shave and Punch Biopsy Sites—Layer (from bottom to top) the emollient of choice, a cut 4×4-inch gauze pad, and flexible polyester tape cut to the appropriate size (Figure 1). This simple bandage conforms well to any anatomic site and can replace an adhesive bandage, if desired.

Cutaneous Surgery Sites—Pressure bandages are recommended on cutaneous surgery sites. One of the most common closures performed in dermatology is the layered closure with dissolvable subcutaneous sutures and nondissolvable cutaneous sutures. When this closure is performed on the trunk and proximal extremities, undermining often is required to adequately approximate skin. This technique eliminates tension on the wound but can increase the risk for hematoma.1 A pressure bandage left in place and kept dry for 48 hours after surgery helps eliminate the risk for postoperative bleeding.
To make a pressure bandage, layer (from bottom to top) the emollient of choice, a nonstick pad cut to size, folded 4×4-inch gauze pads, and flexible polyester tape (Figure 2). Our practice routinely utilizes the tape fanning technique2 to impart equal and firm pressure over the wound.

Complex Sites—When making a pressure bandage for an anatomically complex site—the ear, nose, or lip—nonstick pads and 4×4-inch gauze pads can be cut and folded or rolled to match the size and shape of the wound. Flexible polyester tape then conforms to these custom bandage shapes, allowing maintenance of targeted wound pressure (Figure 3).

Dental rolls can be of assistance on these sites. For example, a dental roll placed in the postauricular sulcus prior to bandaging an ear maintains comfortable anatomic positioning. Rolls can be placed in the nose, maintaining its architecture while the wound heals and providing counterpressure for added hemostasis of wounds on the lateral nasal sidewall and ala. We recommend coating dental rolls in petrolatum prior to placement in the nares for ease of removal and patient comfort.
Distal Arms and Legs—Another layer of compression is added to pressure bandages on the distal upper and lower extremities using a fabric and elastic wrap (Figure 4). The extra layer keeps the bandage in place on the upper extremities while the patient continues their daily activities. It also helps prevent edema and pain in the lower extremities.

The degree of postoperative lower extremity swelling varies by patient and procedure performed but largely is inevitable with surgery on the leg, given the potential for superficial lymphatic disruption and the dependent position of the leg when standing. Elevation is always advised, but a well-wrapped, long-stretch elastic bandage provides extra support, especially if the patient has baseline venous insufficiency or needs to be on their feet during the day. The wrap is applied from the distal to the proximal leg with graded compression, overlapping by half with each rotation. The wrap is tightest near the ankle, with gradual and subtle easing of tension as it is placed superiorly.
Healing by Secondary Intention, Full-Thickness and Split-Thickness Skin Grafts, and Partial Wound Closure—These postoperative scenarios require bandages with appropriate pressure; however, dressings need to remain moist against the patient’s skin for comfortable removal, which can be accomplished with petrolatum-impregnated gauze with or without antibacterial properties. The gauze is folded to the appropriate size and placed directly on the wound or sutured in place (Figure 5). A pressure bandage is then applied on top of the gauze.

Practice Implications
The universal bandage kit and instructions for its adaptation to accommodate multiple clinical needs can serve as a helpful resource for dermatologists and their staff.
- Bunick CG, Aasi SZ. Hemorrhagic complications in dermatologic surgery. Dermatol Ther. 2011;24:537-550. doi:10.1111/j.1529-8019.2012.01454.x
- Ardilla C, Tarantino I, Goldberg LH, et al. Improved postoperative bleeding control using the fanning pressure dressing technique [published May 31, 2021]. J Am Acad Dermatol. 2021:S0190-9622(21)01040-9. doi:10.1016/j.jaad.2021.05.045
- Bunick CG, Aasi SZ. Hemorrhagic complications in dermatologic surgery. Dermatol Ther. 2011;24:537-550. doi:10.1111/j.1529-8019.2012.01454.x
- Ardilla C, Tarantino I, Goldberg LH, et al. Improved postoperative bleeding control using the fanning pressure dressing technique [published May 31, 2021]. J Am Acad Dermatol. 2021:S0190-9622(21)01040-9. doi:10.1016/j.jaad.2021.05.045
New Razor Technology Improves Appearance and Quality of Life in Men With Pseudofolliculitis Barbae
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.
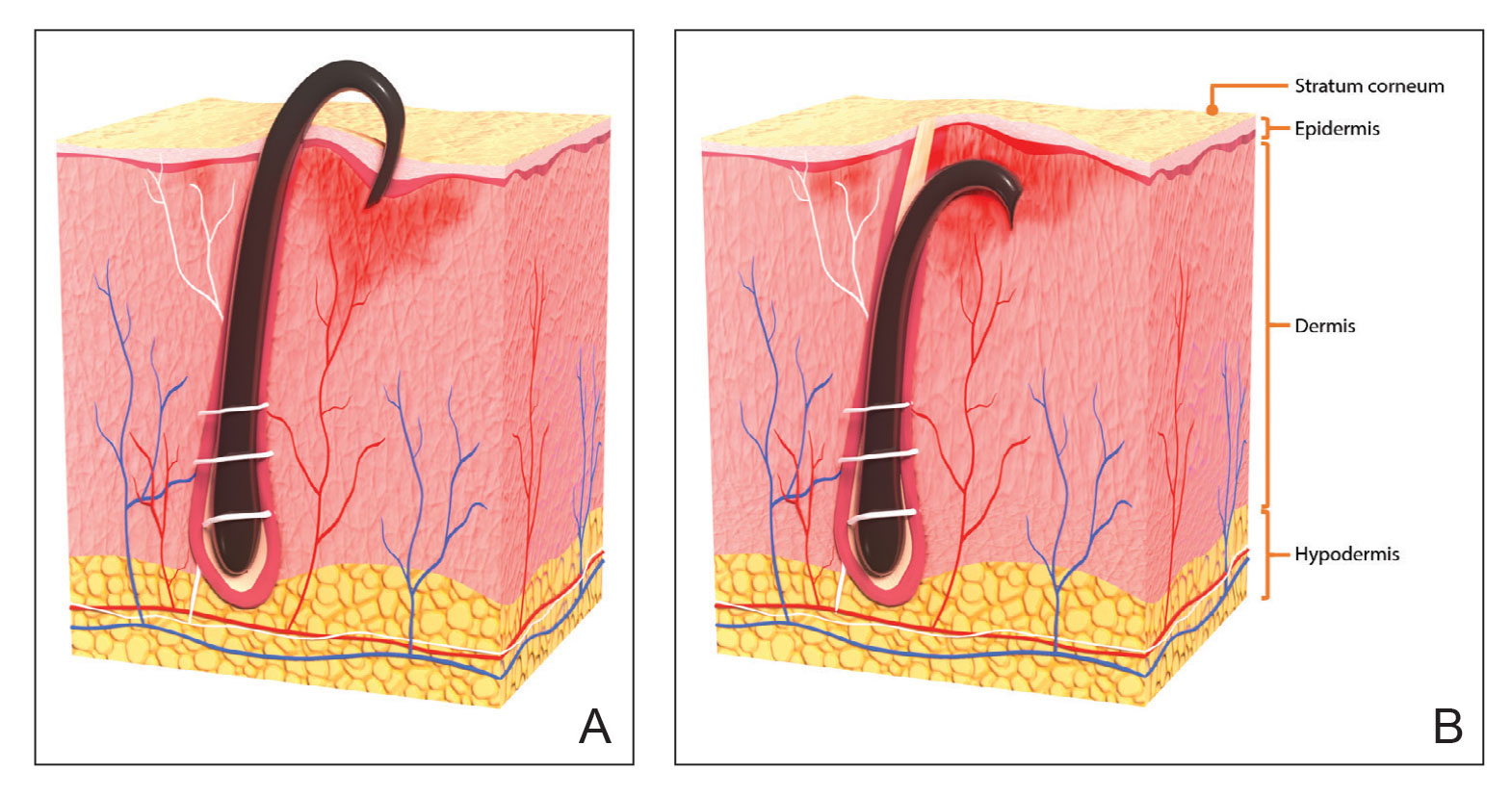
With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.
The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.
Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.
Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.
Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.

With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.

The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.

Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.

Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.

Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
Pseudofolliculitis barbae (PFB)(also known as razor bumps or shaving bumps)1 is a skin condition that consists of papules resulting from ingrown hairs.2 In more severe cases, papules become pustules, then abscesses, which can cause scarring.1,2 The condition can be distressing for patients, with considerable negative impact on their daily lives.3 The condition also is associated with shaving-related stinging, burning, pruritus, and cuts on the skin.4
Pseudofolliculitis barbae is most common in men of African descent due to the curved nature of the hair follicle,2,5,6 with an estimated prevalence in this population of 45% to 83%,1,6 but it can affect men of other ethnicities.7 A genetic polymorphism in a gene encoding a keratin specific to the hair follicle also has been found to predispose some individuals to PFB.5 When hair from a curved or destabilized hair follicle is cut to form a sharp tip, it is susceptible to extrafollicular and/or transfollicular penetration,5,6,8 as illustrated in Figure 1.

With extrafollicular or transfollicular penetration, the hair shaft re-enters or retracts into the dermis, triggering an inflammatory response that may be exacerbated by subsequent shaving.2 Few studies have been published that aim to identify potential shaving solutions for individuals with PFB who elect to or need to continue shaving.
A new razor technology comprising 2 blades separated by a bridge feature has been designed specifically for men with razor bumps (SkinGuard [Procter & Gamble]). The SkinGuard razor redistributes shaving pressure so that there is less force from the blades on the skin and inflamed lesions than without the bridge, as seen in Figure 2. The razor has been designed to protect the skin from the blades, thereby minimizing the occurrence of new lesions and allowing existing lesions to heal.
![Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company. Test razor bridge feature (SkinGuard [Procter & Gamble]) minimizes the force of the razor blades on the skin. Copyright 2022 The Procter & Gamble Company.](https://cdn.mdedge.com/files/s3fs-public/Moran_2.jpg)
The primary purpose of this study was to assess the appearance of males with razor bumps and shaving irritation when using the new razor technology in a regular shaving routine. The secondary objective was to measure satisfaction of the shaving experience when using the new razor by means of assessing itching, burning, and stinging using the participant global severity assessment (PGSA) and the impact on quality of life (QOL) measures.
Methods
Participants—Eligible participants were male, aged 20 to 60 years, and had clinically diagnosed PFB as well as symptoms of skin irritation from shaving. Participants were recruited from a dermatology clinic and via institutional review board–approved advertising.
Those eligible for inclusion in the study had a shaving routine that comprised shaving at least 3 times a week using a wet-shave, blade-razor technique accompanied by only a shave gel or foam. In addition, eligible participants had mild to moderate symptoms of skin irritation (a minimum of 10 razor bumps) from shaving based on investigator global severity assessment (IGSA) rating scales and were willing to shave at least 5 times a week during the study period. Participants could continue certain topical and systemic interventions for their skin.
Participants were excluded from the study if they had an underlying inflammatory disease that could manifest with a skin rash or were using any of these medications: topical benzoyl peroxide, topical clindamycin, topical retinoids, or oral antibiotics.
Study Design—A prospective, open-label study was conducted over a period of 12 weeks at a single site in the United States. Investigators instructed participants to shave 5 or more times per week with the test razor and to keep a daily shaving journal to track the number of shaves and compliance.
Participants were evaluated at the baseline screening visit, then at 4, 8, and 12 weeks. Evaluations included an investigator lesion count, the IGSA, and the PGSA. The PGSA was used to evaluate subjective clinical measurements (ie, indicate how much postshave burning/itching/stinging the participant was experiencing). The impact of shaving on daily life was evaluated at the baseline screening visit and at 12 weeks with the Participant Quality of Life Questionnaire comprised of 22 QOL statements. eTable 1 summarizes the investigator assessments used in the study, and eTable 2 summarizes the participant self-assessments. Both tables include the scale details and results interpretation for each assessment.

The study was approved by the local institutional review board, and all participants provided written informed consent in accordance with Title 21 of the Code of Federal Regulations, Part 50.

Study Visits—At the baseline screening visit, participants provided written informed consent and completed a prestudy shave questionnaire concerning shaving preparations, techniques, and opinions. Participants also provided a medical history, including prior and concomitant medications, and were evaluated using the inclusion/exclusion criteria. Investigators explained adverse event reporting to the participants. Participants were provided with an adequate supply of test razors for the 12-week period.
Data Analysis—Means and SDs were calculated for the study measures assessed at each visit. Analyses were performed evaluating change from baseline in repeated-measures analysis of variance models. These models were adjusted for baseline levels of the outcome measure and visit number. The magnitude of change from baseline was evaluated against a null hypothesis of 0% change. This longitudinal model adjusted for any potential differing baseline levels among participants. Statistical significance was defined as P<.05. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
In total, 21 individuals were enrolled, and 20 completed the study. Participants who completed the study were non-Hispanic Black (n=10); non-Hispanic White (n=8); Asian (n=1); or White, American Indian (n=1). All participants adhered to the protocol and reported shaving at least 5 times a week for 12 weeks using the test razor. One participant was removed after he was found to have a history of sarcoidosis, making him ineligible for the study. No study-related adverse events were reported.
Papules and Pustules—Over the course of the 12-week study, the papule count decreased significantly from baseline. Results from the investigator lesion count (see eTable 1 for key) indicated that by week 12—adjusted for number of papules at baseline—the mean percentage reduction was estimated to be 59.6% (P<.0001). A significant decrease in papule count also was observed between the baseline visit and week 8 (57.2%; P<.0001). A nonsignificant decrease was observed at week 4 (18.9%; P=.17). Only 3 participants presented with pustules at baseline, and the pustule count remained low over the course of the study. No significant change was noted at week 12 vs baseline (P=.98). Notably, there was no increase in pustule count at the end of the study compared with baseline (Table 1).
Skin Appearance—An improvement in the skin’s appearance over the course of the study from baseline was consistent with an improvement in the IGSA. The IGSA score significantly improved from a mean (SD) measurement of 2.5 (0.6) (indicating mild to moderate inflammation) at baseline to 1.4 (0.8) at week 8 (P<.0001) and 1.2 (1.1) (indicating mild inflammation to almost clear) at week 12 (P<.0001). The observed decrease in severity of skin condition and skin inflammation is shown in Figure 3.

Significant improvements were observed in every category of the PGSA at week 12 vs baseline (P≤.0007)(Table 2). At week 12, there was a significant (P≤.05) increase from baseline in participant agreement for all 22 QOL metrics describing positive shave experience, achieving results, skin feel, self-confidence, and social interactions (Figure 4), which supports the positive impact of adopting a shaving regimen with the test razor. Notably, after using the test razor for 12 weeks, men reported that they were more likely to agree with the statements “my skin felt smooth,” “my skin felt good to touch,” and “I was able to achieve a consistently good shave.” Other meaningful increases occurred in “shaving was something I looked forward to doing,” “others thought I looked clean cut,” “I looked my best for my family/others/work,” and “I felt comfortable/confident getting closer to others.” All QOL statements are shown in Figure 4.

Comment
Improvement With Novel Razor Technology—For the first time, frequent use of a novel razor technology designed specifically for men with PFB was found to significantly improve skin appearance, shave satisfaction, and QOL after 12 weeks vs baseline in participants clinically diagnosed with PFB. In men with shave-related skin irritation and razor bumps who typically wet-shaved with a razor at least 3 times a week, use of the test razor with their regular shaving preparation product 5 or more times per week for 12 weeks was associated with significant improvements from baseline in investigator lesion count, IGSA, PGSA, and Participant Quality of Life Questionnaire measurements.
Study strengths included the quantification of the change in the number of lesions and the degree of severity by a trained investigator in a prospective clinical study along with an assessment of the impact on participant QOL. A lack of a control arm could be considered a limitation of the study; however, study end points were evaluated compared with baseline, with each participant serving as their own control. Spontaneous resolution of the condition with their standard routine was considered highly unlikely in these participants; therefore, in the absence of any other changes, improvements were attributed to regular use of the test product over the course of the study. The results presented here provide strong support for the effectiveness of the new razor technology in improving the appearance of men with razor bumps and shaving irritation.
Hair Removal Tools for the Management of PFB—Although various tools and techniques have been proposed in the past for men with PFB, the current test razor technology provided unique benefits, including improvements in appearance and severity of the condition as well as a positive impact on QOL. In 1979, Conte and Lawrence9 evaluated the effect of using an electric hair clipper and twice-daily use of a skin-cleansing pad on the occurrence of PFB. Participants (n=96) allowed their beards to grow out for 1 month, after which they started shaving with an electric clipper with a triple O head. The authors reported a favorable response in 95% (91/96) of cases. However, the electric clippers left 1 mm of beard at the skin level,9 which may not be acceptable for those who prefer a clean-shaven appearance.6
A prospective survey of 22 men of African descent with PFB found use of a safety razor was preferred over an electric razor.10 The single-arm study evaluated use of a foil-guarded shaver (single-razor blade) in the management of PFB based on investigator lesion counts and a participant questionnaire. Participants were asked to shave at least every other day and use a specially designed preshave brush. A mean reduction in lesion counts was observed at 2 weeks (29.6%), 4 weeks (38.1%), and 6 weeks (47.1%); statistical significance was not reported. At 6 weeks, 77.3% (17/22) of participants judged the foil-guarded shaver to be superior to other shaving devices in controlling their razor bumps, and 90.9% (20/22) indicated they would recommend the shaver to others with PFB. The authors hypothesized that the guard buffered the skin from the blade, which might otherwise facilitate the penetration of ingrowing hairs and cause trauma to existing lesions.
The mean reduction in lesion count from baseline observed at week 4 was greater in the study with the foil-guarded shaver and preshave brush (38% reduction)10 than in our study (19% reduction in papule count). Different methodologies, use of a preshave brush in the earlier study, and a difference in lesion severity at baseline may have contributed to this difference. The study with the foil-guarded shaver concluded after 6 weeks, and there was a 47.1% reduction in lesion counts vs baseline.10 In contrast, the current study continued for 12 weeks, and a 59.6% reduction in lesion counts was reported. Participants from both studies reported an improved shaving experience compared with their usual practice,10 though only the current study explored the positive impact of the new razor technology on participant QOL.
Preventing Hairs From Being Cut Too Close—The closeness of the shave is believed to be a contributory factor in the development and persistence of PFB6,8,11 based on a tendency for the distal portion of tightly curled hair shafts to re-enter the skin after shaving via transfollicular penetration.12 Inclusion of a buffer in the razor between the sharp blades and the skin has been proposed to prevent hairs from being cut too close and causing transfollicular penetration.12
In the test razor used in the current study, the bridge technology acted as the buffer to prevent hairs from being cut too close to the skin and to reduce blade contact with the skin (Figure 2). Having only 2 blades also reduced the closeness of the shave compared with 5-bladed technologies,13 as each hair can only be pulled and cut up to a maximum of 2 times per shaving stroke. Notably, this did not impact the participants’ QOL scores related to achieving a close shave or skin feeling smooth; both attributes were significantly improved at 12 weeks vs baseline (Figure 4).
By reducing blade contact with the skin, the bridge technology in the test razor was designed to prevent excessive force from being applied to the skin through the blades. Reduced blade loading minimizes contact with and impact on sensitive skin.14 Additional design features of the test razor to minimize the impact of shaving on the skin include treatment of the 2 blades with low-friction coatings, which allows the blades to cut through the beard hair with minimal force, helping to reduce the tug-and-pull effect that may otherwise result in irritation and inflammation.13,15 Lubrication strips before and after the blades in the test razor reduce friction between the blades and the skin to further protect the skin from the blades.15
Shaving With Multiblade Razors Does Not Exacerbate PFB—In a 1-week, split-faced, randomized study of 45 Black men, shaving with a manual 3-bladed razor was compared with use of 3 different chemical depilatory formulations.16 Shaving every other day for 1 week with the manual razor resulted in more papule formation but less irritation than use of the depilatories. The authors concluded that a study with longer duration was needed to explore the impact of shaving on papule formation in participants with a history of PFB.16
In 2013, an investigator-blinded study of 90 African American men with PFB compared the impact of different shaving regimens on the signs and symptoms of PFB over a 12-week period.4 Participants were randomized to 1 of 3 arms: (1) shaving 2 to 3 times per week with a triple-blade razor and standard products (control group); (2) shaving daily with a 5-bladed razor and standard products; and (3) shaving daily with a 5-bladed razor and “advanced” specific pre- and postshave products. The researchers found that the mean papule measurement significantly decreased from baseline in the advanced (P=.01) and control (P=.016) groups. Between-group comparison revealed no significant differences for papule or pustule count among each arm. For the investigator-graded severity, the change from baseline was significant for all 3 groups (P≤.04); however, the differences among groups were not significant. Importantly, these data demonstrated that PFB was not exacerbated by multiblade razors used as part of a daily shaving regimen.4
The findings of the current study were consistent with those of Daniel et al4 in that there was no exacerbation of the signs and symptoms of PFB associated with daily shaving. However, rather than requiring participants to change their entire shaving regimen, the present study only required a change of razor type. Moreover, the use of the new razor technology significantly decreased papule counts at week 12 vs the baseline measurement (P<.0001) and was associated with an improvement in subjective skin severity measurements. The participants in the present study reported significantly less burning, stinging, and itching after using the test product for 12 weeks (P<.0001).
Impact of Treatment on QOL—The current study further expanded on prior findings by combining these clinical end points with the QOL results to assess the test razor’s impact on participants’ lives. Results showed that over the course of 12 weeks, the new razor technology significantly improved the participants’ QOL in all questions related to shaving experience, achieving results, skin feel, self-confidence, and social interactions. The significant improvement in QOL included statements such as “shaving was a pleasant experience,” “I was able to achieve a consistently good shave,” and “my skin felt smooth.” Participants also reported improvements in meaningful categories such as “my shave made me feel attractive” and “I felt comfortable/confident getting closer to others.” As the current study showed, a shave regimen has the potential to change participants’ overall assessment of their QOL, a variable that must not be overlooked.
Conclusion
In men with clinically diagnosed PFB, regular shaving with a razor designed to protect the skin was found to significantly decrease lesion counts, increase shave satisfaction, and improve QOL after 12 weeks compared with their usual shaving practice (baseline measures). This razor technology provides another option to help manage PFB for men who wish to or need to continue shaving.
Acknowledgments—The clinical study was funded by the Procter & Gamble Company. Editorial writing assistance, supported financially by the Procter & Gamble Company, was provided by Gill McFeat, PhD, of McFeat Science Ltd (Devon, United Kingdom).
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
- Alexander AM, Delph WI. Pseudofolliculitis barbae in the military. a medical, administrative and social problem. J Natl Med Assoc. 1974;66:459-464, 479.
- Kligman AM, Strauss JS. Pseudofolliculitis of the beard. AMA Arch Derm. 1956;74:533-542.
- Banta J, Bowen C, Wong E, et al. Perceptions of shaving profiles and their potential impacts on career progression in the United States Air Force. Mil Med. 2021;186:187-189.
- Daniel A, Gustafson CJ, Zupkosky PJ, et al. Shave frequency and regimen variation effects on the management of pseudofolliculitis barbae. J Drugs Dermatol. 2013;12:410-418.
- Winter H, Schissel D, Parry DA, et al. An unusual Ala12Thr polymorphism in the 1A alpha-helical segment of the companion layer-specific keratin K6hf: evidence for a risk factor in the etiology of the common hair disorder pseudofolliculitis barbae. J Invest Dermatol. 2004;122:652-657.
- Perry PK, Cook-Bolden FE, Rahman Z, et al. Defining pseudofolliculitis barbae in 2001: a review of the literature and current trends. J Am Acad Dermatol. 2002;46(2 suppl understanding):S113-S119.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21:629-644.
- Ribera M, Fernández-Chico N, Casals M. Pseudofolliculitis barbae [in Spanish]. Actas Dermosifiliogr. 2010;101:749-757.
- Conte MS, Lawrence JE. Pseudofolliculitis barbae. no ‘pseudoproblem.’ JAMA. 1979;241:53-54.
- Alexander AM. Evaluation of a foil-guarded shaver in the management of pseudofolliculitis barbae. Cutis. 1981;27:534-537, 540-542.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328;331-333.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Cowley K, Vanoosthuyze K, Ertel K, et al. Blade shaving. In: Draelos ZD, ed. Cosmetic Dermatology: Products and Procedures. 2nd ed. John Wiley & Sons; 2015:166-173.
- Cowley K, Vanoosthuyze K. Insights into shaving and its impact on skin. Br J Dermatol. 2012;166(suppl 1):6-12.
- Cowley K, Vanoosthuyze K. The biomechanics of blade shaving. Int J Cosmet Sci. 2016;38(suppl 1):17-23.
- Kindred C, Oresajo CO, Yatskayer M, et al. Comparative evaluation of men’s depilatory composition versus razor in black men. Cutis. 2011;88:98-103.
Practice Points
- Pseudofolliculitis barbae (PFB) is a common follicular inflammatory disorder associated with shaving, most commonly seen in men of African ancestry. It can be distressing and cause a substantial impact on quality of life (QOL).
- Frequent use of a novel razor technology designed specifically for men with PFB was found to improve skin appearance and QOL after 12 weeks vs baseline.
- This razor technology provides an alternative approach to help manage PFB for men who wish to or need to continue shaving.
High rates of inappropriate PPI use in hospitalized patients
The research was published online in the journal Digestive and Liver Disease.
First author Orly Sneh-Arbib, MD, division of gastroenterology and liver disease, Clalit Health Services, Talpiot, Jerusalem, said in an interview that she and her co-authors were “very surprised” that the rates of inappropriate prescribing remained so high, especially as they had discussed the adverse effects of the drugs numerous times during departmental meetings.
She believes that, for many clinicians, handing out a prescription for PPIs has become a habit, with the thought being: “Just take this; it’s like a vitamin.”
In the paper, the researchers write, “Undoubtedly, more action is needed to raise physicians’ knowledge and attention to the subject while providing automated and standardized technology-based tools to reduce inappropriate PPI use using an acceptable algorithm.”
For the study, Dr. Sneh-Arbib and her colleagues developed an algorithm to assess inappropriate PPI prescribing in almost 4,000 internal medicine patients. To try to limit future overprescribing, their algorithm is now included in the clinical records system.
Consequently, every time a clinician tries to prescribe a PPI, they have to confirm that there is a valid indication for doing so, with the aim of adding an “extra step in the process,” Dr. Sneh-Arbib said.
Clear but complex PPI indications
There are a small, well-defined number of indications for long-term PPI use, the authors write. The indications include prior upper gastrointestinal bleeding, maintenance treatment after healing of erosive esophagitis, Barrett’s esophagus, the use of nonsteroidal anti-inflammatory drugs or antiplatelet agents in patients with increased bleeding risk, and maintenance therapy for symptom control in patients with gastroesophageal reflux disease.
The authors also note that the “appropriateness of PPIs is complex,” with multiple factors to consider for long-term PPI use, including complex drug combinations and medical history.
But having seen patients in their 30s prescribed PPIs “just because of taking steroids or aspirin,” Dr. Sneh-Arbib said she wanted to investigate further.
To examine the rate of inappropriate long-term PPI prescription on discharge from internal medicine departments, the team conducted a retrospective analysis of adults admitted to their institution for the first 6 months of 2014 and the first 6 months of 2017.
They included all patients prescribed PPIs on discharge with a recommendation for long-term use and excluded those who were transferred between departments or who had a hospital stay longer than 3 months.
Information on age, gender, ethnicity, socioeconomic status, current and past diagnoses including all relevant gastrointestinal diagnoses, Charlson comorbidity index, and medications on admission and discharge were recorded.
Guidelines for use of PPIs
To assess inappropriate PPI prescribing, the team searched for published recommendations on long-term use, Dr. Sneh-Arbib said. They developed an algorithm based on the U.K. National Institute for Health and Care Excellence (NICE) clinical guideline, “Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management.”
The guideline is “not perfect,” Dr. Sneh-Arbib said, but it was suitable. The team then randomly chose 100 patients from their records to validate the resulting algorithm, finding an accuracy of 96%.
The full analysis included 3,982 patients, of whom 74% were aged 65 years or older, and 50.8% were women. PPIs were first prescribed before hospital admission in 92.4% of cases.
The researchers found that the overall rate of inappropriate PPI prescriptions was 44.3%, a figure that was stable between the two the study periods. In 2014, the rate of inappropriate use was 43.2%, and it was 45.6% in 2017, a nonsignificant difference.
Inappropriate PPIs were higher (68.1%) in patients younger than 65 years, compared with those aged 65 years and older (36%).
The researchers analyzed factors associated with inappropriate PPIs, first excluding 448 patients with clear gastrointestinal indications.
In the majority of cases, the inappropriate PPI prescriptions occurred in patients who were not taking dual antiplatelet treatment (89.4%), in younger patients who were taking aspirin only (8.6%), and in patients receiving a single antiplatelet agent other than aspirin (1.9%), according to multivariate analysis.
Overall, 42.4% of patients classified as inappropriate PPI use were not using aspirin, nonsteroidal anti-inflammatory drugs, antiplatelets, antiaggregants, anticoagulants, or steroids, the researchers found.
They also note that most patients in the study (92.3%) had received PPIs before admission, prescribed by their general practitioner or during a previous hospitalization. This raises concerns about unneeded continuation of the drugs and lack of review by clinicians, they write.
PPI deprescribing
Approached for comment, Adrienna Jirik, MD, a gastroenterologist at the Cleveland Clinic, told this news organization that “PPI overprescription is a common problem worldwide, with the United States being no exception.”
They are “one of the most prescribed medications in the world, with several formulations readily available as an over-the-counter medication,” she added.
Dr. Jirik, who was not involved with the study, said that the algorithm used is “on par with the United States clinical practice guidelines for PPI use” and a “great start to initiate an encounter with a patient on PPIs in the outpatient setting to review the indications and to de-escalate and deprescribe therapy.”
Indeed, the American Gastroenterological Association recently published a clinical practice update on deprescribing PPIs.
“It may be useful to incorporate a version of this algorithm as a ‘hard stop’ on some outpatient EMR [electronic medical record] templates to remind providers to address this issue prior to closing an encounter,” Dr. Jirik added.
She noted, however, that tackling any medication reconciliation is a very important but difficult and time-consuming task.
“Education is definitely key for both primary and subspecialty providers,” Dr. Jirik said. “If patients have been on a long-term PPI, the inpatient provider can suggest a plan for de-escalation based on practice guidelines and arrange proper outpatient follow-up for eventual deprescribing.”
No funding was declared. The authors and Dr. Jirik report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The research was published online in the journal Digestive and Liver Disease.
First author Orly Sneh-Arbib, MD, division of gastroenterology and liver disease, Clalit Health Services, Talpiot, Jerusalem, said in an interview that she and her co-authors were “very surprised” that the rates of inappropriate prescribing remained so high, especially as they had discussed the adverse effects of the drugs numerous times during departmental meetings.
She believes that, for many clinicians, handing out a prescription for PPIs has become a habit, with the thought being: “Just take this; it’s like a vitamin.”
In the paper, the researchers write, “Undoubtedly, more action is needed to raise physicians’ knowledge and attention to the subject while providing automated and standardized technology-based tools to reduce inappropriate PPI use using an acceptable algorithm.”
For the study, Dr. Sneh-Arbib and her colleagues developed an algorithm to assess inappropriate PPI prescribing in almost 4,000 internal medicine patients. To try to limit future overprescribing, their algorithm is now included in the clinical records system.
Consequently, every time a clinician tries to prescribe a PPI, they have to confirm that there is a valid indication for doing so, with the aim of adding an “extra step in the process,” Dr. Sneh-Arbib said.
Clear but complex PPI indications
There are a small, well-defined number of indications for long-term PPI use, the authors write. The indications include prior upper gastrointestinal bleeding, maintenance treatment after healing of erosive esophagitis, Barrett’s esophagus, the use of nonsteroidal anti-inflammatory drugs or antiplatelet agents in patients with increased bleeding risk, and maintenance therapy for symptom control in patients with gastroesophageal reflux disease.
The authors also note that the “appropriateness of PPIs is complex,” with multiple factors to consider for long-term PPI use, including complex drug combinations and medical history.
But having seen patients in their 30s prescribed PPIs “just because of taking steroids or aspirin,” Dr. Sneh-Arbib said she wanted to investigate further.
To examine the rate of inappropriate long-term PPI prescription on discharge from internal medicine departments, the team conducted a retrospective analysis of adults admitted to their institution for the first 6 months of 2014 and the first 6 months of 2017.
They included all patients prescribed PPIs on discharge with a recommendation for long-term use and excluded those who were transferred between departments or who had a hospital stay longer than 3 months.
Information on age, gender, ethnicity, socioeconomic status, current and past diagnoses including all relevant gastrointestinal diagnoses, Charlson comorbidity index, and medications on admission and discharge were recorded.
Guidelines for use of PPIs
To assess inappropriate PPI prescribing, the team searched for published recommendations on long-term use, Dr. Sneh-Arbib said. They developed an algorithm based on the U.K. National Institute for Health and Care Excellence (NICE) clinical guideline, “Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management.”
The guideline is “not perfect,” Dr. Sneh-Arbib said, but it was suitable. The team then randomly chose 100 patients from their records to validate the resulting algorithm, finding an accuracy of 96%.
The full analysis included 3,982 patients, of whom 74% were aged 65 years or older, and 50.8% were women. PPIs were first prescribed before hospital admission in 92.4% of cases.
The researchers found that the overall rate of inappropriate PPI prescriptions was 44.3%, a figure that was stable between the two the study periods. In 2014, the rate of inappropriate use was 43.2%, and it was 45.6% in 2017, a nonsignificant difference.
Inappropriate PPIs were higher (68.1%) in patients younger than 65 years, compared with those aged 65 years and older (36%).
The researchers analyzed factors associated with inappropriate PPIs, first excluding 448 patients with clear gastrointestinal indications.
In the majority of cases, the inappropriate PPI prescriptions occurred in patients who were not taking dual antiplatelet treatment (89.4%), in younger patients who were taking aspirin only (8.6%), and in patients receiving a single antiplatelet agent other than aspirin (1.9%), according to multivariate analysis.
Overall, 42.4% of patients classified as inappropriate PPI use were not using aspirin, nonsteroidal anti-inflammatory drugs, antiplatelets, antiaggregants, anticoagulants, or steroids, the researchers found.
They also note that most patients in the study (92.3%) had received PPIs before admission, prescribed by their general practitioner or during a previous hospitalization. This raises concerns about unneeded continuation of the drugs and lack of review by clinicians, they write.
PPI deprescribing
Approached for comment, Adrienna Jirik, MD, a gastroenterologist at the Cleveland Clinic, told this news organization that “PPI overprescription is a common problem worldwide, with the United States being no exception.”
They are “one of the most prescribed medications in the world, with several formulations readily available as an over-the-counter medication,” she added.
Dr. Jirik, who was not involved with the study, said that the algorithm used is “on par with the United States clinical practice guidelines for PPI use” and a “great start to initiate an encounter with a patient on PPIs in the outpatient setting to review the indications and to de-escalate and deprescribe therapy.”
Indeed, the American Gastroenterological Association recently published a clinical practice update on deprescribing PPIs.
“It may be useful to incorporate a version of this algorithm as a ‘hard stop’ on some outpatient EMR [electronic medical record] templates to remind providers to address this issue prior to closing an encounter,” Dr. Jirik added.
She noted, however, that tackling any medication reconciliation is a very important but difficult and time-consuming task.
“Education is definitely key for both primary and subspecialty providers,” Dr. Jirik said. “If patients have been on a long-term PPI, the inpatient provider can suggest a plan for de-escalation based on practice guidelines and arrange proper outpatient follow-up for eventual deprescribing.”
No funding was declared. The authors and Dr. Jirik report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The research was published online in the journal Digestive and Liver Disease.
First author Orly Sneh-Arbib, MD, division of gastroenterology and liver disease, Clalit Health Services, Talpiot, Jerusalem, said in an interview that she and her co-authors were “very surprised” that the rates of inappropriate prescribing remained so high, especially as they had discussed the adverse effects of the drugs numerous times during departmental meetings.
She believes that, for many clinicians, handing out a prescription for PPIs has become a habit, with the thought being: “Just take this; it’s like a vitamin.”
In the paper, the researchers write, “Undoubtedly, more action is needed to raise physicians’ knowledge and attention to the subject while providing automated and standardized technology-based tools to reduce inappropriate PPI use using an acceptable algorithm.”
For the study, Dr. Sneh-Arbib and her colleagues developed an algorithm to assess inappropriate PPI prescribing in almost 4,000 internal medicine patients. To try to limit future overprescribing, their algorithm is now included in the clinical records system.
Consequently, every time a clinician tries to prescribe a PPI, they have to confirm that there is a valid indication for doing so, with the aim of adding an “extra step in the process,” Dr. Sneh-Arbib said.
Clear but complex PPI indications
There are a small, well-defined number of indications for long-term PPI use, the authors write. The indications include prior upper gastrointestinal bleeding, maintenance treatment after healing of erosive esophagitis, Barrett’s esophagus, the use of nonsteroidal anti-inflammatory drugs or antiplatelet agents in patients with increased bleeding risk, and maintenance therapy for symptom control in patients with gastroesophageal reflux disease.
The authors also note that the “appropriateness of PPIs is complex,” with multiple factors to consider for long-term PPI use, including complex drug combinations and medical history.
But having seen patients in their 30s prescribed PPIs “just because of taking steroids or aspirin,” Dr. Sneh-Arbib said she wanted to investigate further.
To examine the rate of inappropriate long-term PPI prescription on discharge from internal medicine departments, the team conducted a retrospective analysis of adults admitted to their institution for the first 6 months of 2014 and the first 6 months of 2017.
They included all patients prescribed PPIs on discharge with a recommendation for long-term use and excluded those who were transferred between departments or who had a hospital stay longer than 3 months.
Information on age, gender, ethnicity, socioeconomic status, current and past diagnoses including all relevant gastrointestinal diagnoses, Charlson comorbidity index, and medications on admission and discharge were recorded.
Guidelines for use of PPIs
To assess inappropriate PPI prescribing, the team searched for published recommendations on long-term use, Dr. Sneh-Arbib said. They developed an algorithm based on the U.K. National Institute for Health and Care Excellence (NICE) clinical guideline, “Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management.”
The guideline is “not perfect,” Dr. Sneh-Arbib said, but it was suitable. The team then randomly chose 100 patients from their records to validate the resulting algorithm, finding an accuracy of 96%.
The full analysis included 3,982 patients, of whom 74% were aged 65 years or older, and 50.8% were women. PPIs were first prescribed before hospital admission in 92.4% of cases.
The researchers found that the overall rate of inappropriate PPI prescriptions was 44.3%, a figure that was stable between the two the study periods. In 2014, the rate of inappropriate use was 43.2%, and it was 45.6% in 2017, a nonsignificant difference.
Inappropriate PPIs were higher (68.1%) in patients younger than 65 years, compared with those aged 65 years and older (36%).
The researchers analyzed factors associated with inappropriate PPIs, first excluding 448 patients with clear gastrointestinal indications.
In the majority of cases, the inappropriate PPI prescriptions occurred in patients who were not taking dual antiplatelet treatment (89.4%), in younger patients who were taking aspirin only (8.6%), and in patients receiving a single antiplatelet agent other than aspirin (1.9%), according to multivariate analysis.
Overall, 42.4% of patients classified as inappropriate PPI use were not using aspirin, nonsteroidal anti-inflammatory drugs, antiplatelets, antiaggregants, anticoagulants, or steroids, the researchers found.
They also note that most patients in the study (92.3%) had received PPIs before admission, prescribed by their general practitioner or during a previous hospitalization. This raises concerns about unneeded continuation of the drugs and lack of review by clinicians, they write.
PPI deprescribing
Approached for comment, Adrienna Jirik, MD, a gastroenterologist at the Cleveland Clinic, told this news organization that “PPI overprescription is a common problem worldwide, with the United States being no exception.”
They are “one of the most prescribed medications in the world, with several formulations readily available as an over-the-counter medication,” she added.
Dr. Jirik, who was not involved with the study, said that the algorithm used is “on par with the United States clinical practice guidelines for PPI use” and a “great start to initiate an encounter with a patient on PPIs in the outpatient setting to review the indications and to de-escalate and deprescribe therapy.”
Indeed, the American Gastroenterological Association recently published a clinical practice update on deprescribing PPIs.
“It may be useful to incorporate a version of this algorithm as a ‘hard stop’ on some outpatient EMR [electronic medical record] templates to remind providers to address this issue prior to closing an encounter,” Dr. Jirik added.
She noted, however, that tackling any medication reconciliation is a very important but difficult and time-consuming task.
“Education is definitely key for both primary and subspecialty providers,” Dr. Jirik said. “If patients have been on a long-term PPI, the inpatient provider can suggest a plan for de-escalation based on practice guidelines and arrange proper outpatient follow-up for eventual deprescribing.”
No funding was declared. The authors and Dr. Jirik report no relevant financial relationships.
A version of this article first appeared on Medscape.com.