User login
Chemotherapy meets its match against aggressive ER+/HER2– breast cancers
SAN ANTONIO – Results of a study being hailed as practice changing showed that, for pre- or perimenopausal women with aggressive hormone receptor-positive, HER2-negative (HR+/HER2–) untreated breast cancers,
That’s according to investigators of the phase 2 RIGHT Choice study who found that first-line ribociclib, combined with either letrozole or anastrozole plus goserelin, was associated with a doubling of progression-free survival (PFS), compared with the investigator’s choice of combination chemotherapy, reported Yen-Shen Lu, MD, from National Taiwan University Hospital in Taipei, at the San Antonio Breast Cancer Symposium.
“These results from RIGHT Choice have now shown that first-line ribociclib plus endocrine therapy should be considered the preferred treatment option for this patient population,” he said.
Chemo loses its luster
“This is not time first time that we’ve looked at a CDK4/6 inhibitor compared to chemotherapy, but this is the first time that we’ve seen it compared to a combination chemotherapy,” commented Virginia Kaklamani, MD, from University of Texas Health, San Antonio, who moderated a media briefing held prior to Dr. Lu’s presentation of the data in an oral abstract session.
“I think with this study we’re finding that chemotherapy, at least in the early stages of [estrogen receptor]–positive breast cancer, is probably not appropriate for our patients,” she said.
Chemotherapy is the current standard of care for patients with advanced breast cancers with aggressive disease features that can include rapidly progressive disease, high symptom burden, and/or life-threatening visceral crises requiring rapid control of disease, Dr. Lu said.
Compared with single-agent chemotherapy, combination chemotherapy, for those who can tolerate it, is associated with higher overall response rates and longer PFS.
Although ribociclib plus endocrine therapy has been shown to offer significant PFS and overall survival (OS) benefits, compared with endocrine therapy alone, there have not been any head-to-head studies pitting these agents against combination chemotherapy.
Study details
To rectify this, Dr. Lu and colleagues enrolled 222 pre- or perimenopausal women with HR+/HER2– advanced breast cancers with aggressive features who had not yet received systemic therapy for advanced breast cancer.
After stratification for the presence or absence of liver metastases and by length of disease-free interval (time from complete resection of a primary tumor to documented recurrence), the patients were randomly assigned to receive either ribociclib 600 mg 3 weeks on, 1 week off) plus letrozole or anastrozole and goserelin, or the investigators choice of either docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine.
After a median follow-up of 24.1 months at the time of data cutoff in April 2022, median PFS, the primary endpoint, was 24 months in the ribociclib plus endocrine therapy arm versus 12.3 months in the chemotherapy arm.
This translated into a hazard ratio for progression on ribociclib plus endocrine therapy of 0.54 (P = .0007).
The benefit for the ribociclib combination, compared with combination chemotherapy, was consistent across most patient subgroups, Dr. Lu said.
The median time to treatment failure was also longer with ribociclib, at 18.6 months versus 8.5 months, respectively, translating into a HR of 0.45 favoring ribociclib, with a statistically significant confidence interval.
Overall response rates were similar between the groups, at 65.2% with ribociclib versus 60% with chemotherapy. The respective clinical benefit rates (including complete and partial responses plus stable disease) were 80.4% versus 72.7%.
The time to response was similar between the treatment arms, an important consideration for patients with rapidly progressive disease, Dr. Lu noted.
Adverse events that occurred more frequently with ribociclib were neutropenia and leukopenia. Events more common with chemotherapy included anemia, liver enzyme elevations, nausea, vomiting, diarrhea, alopecia, fatigue, and palmar-plantar erythrodysesthesia.
Confirmation
“These data are just confirming what we’ve already known, and that is that with ER-positive, HER2-negative breast cancer where you have metastatic disease and more aggressive characteristics, treating with a CDK4/6 inhibitor and endocrine therapy leads to high response rates,” breast cancer specialist Matthew P. Goetz, MD, from the Mayo Clinic in Rochester, Minn., said in an interview. Dr. Goetz was not involved in the study.
“What was surprising to me was the fact that the response rates with chemotherapy were not higher,” he said. “We sometime think that the more chemotherapy, the higher the response rates. It was nice to see a direct comparison with chemotherapy, and really to see that giving a target therapy actually led to very, very good results. That tells us that there should be very few situations where we would be prescribing chemotherapy over CDK4/6 inhibitor–based therapies.”
The study was funded by Novartis Pharma. Dr. Lu disclosed personal funding from Novartis and others. Dr. Goetz disclosed grants and other supports for work with the development of abemaciclib and palbociclib, and consulting for Pfizer and others. Dr. Kaklamani disclosed speakers bureau activity for Novartis and others, research support from Eisai, and consulting for other companies.
SAN ANTONIO – Results of a study being hailed as practice changing showed that, for pre- or perimenopausal women with aggressive hormone receptor-positive, HER2-negative (HR+/HER2–) untreated breast cancers,
That’s according to investigators of the phase 2 RIGHT Choice study who found that first-line ribociclib, combined with either letrozole or anastrozole plus goserelin, was associated with a doubling of progression-free survival (PFS), compared with the investigator’s choice of combination chemotherapy, reported Yen-Shen Lu, MD, from National Taiwan University Hospital in Taipei, at the San Antonio Breast Cancer Symposium.
“These results from RIGHT Choice have now shown that first-line ribociclib plus endocrine therapy should be considered the preferred treatment option for this patient population,” he said.
Chemo loses its luster
“This is not time first time that we’ve looked at a CDK4/6 inhibitor compared to chemotherapy, but this is the first time that we’ve seen it compared to a combination chemotherapy,” commented Virginia Kaklamani, MD, from University of Texas Health, San Antonio, who moderated a media briefing held prior to Dr. Lu’s presentation of the data in an oral abstract session.
“I think with this study we’re finding that chemotherapy, at least in the early stages of [estrogen receptor]–positive breast cancer, is probably not appropriate for our patients,” she said.
Chemotherapy is the current standard of care for patients with advanced breast cancers with aggressive disease features that can include rapidly progressive disease, high symptom burden, and/or life-threatening visceral crises requiring rapid control of disease, Dr. Lu said.
Compared with single-agent chemotherapy, combination chemotherapy, for those who can tolerate it, is associated with higher overall response rates and longer PFS.
Although ribociclib plus endocrine therapy has been shown to offer significant PFS and overall survival (OS) benefits, compared with endocrine therapy alone, there have not been any head-to-head studies pitting these agents against combination chemotherapy.
Study details
To rectify this, Dr. Lu and colleagues enrolled 222 pre- or perimenopausal women with HR+/HER2– advanced breast cancers with aggressive features who had not yet received systemic therapy for advanced breast cancer.
After stratification for the presence or absence of liver metastases and by length of disease-free interval (time from complete resection of a primary tumor to documented recurrence), the patients were randomly assigned to receive either ribociclib 600 mg 3 weeks on, 1 week off) plus letrozole or anastrozole and goserelin, or the investigators choice of either docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine.
After a median follow-up of 24.1 months at the time of data cutoff in April 2022, median PFS, the primary endpoint, was 24 months in the ribociclib plus endocrine therapy arm versus 12.3 months in the chemotherapy arm.
This translated into a hazard ratio for progression on ribociclib plus endocrine therapy of 0.54 (P = .0007).
The benefit for the ribociclib combination, compared with combination chemotherapy, was consistent across most patient subgroups, Dr. Lu said.
The median time to treatment failure was also longer with ribociclib, at 18.6 months versus 8.5 months, respectively, translating into a HR of 0.45 favoring ribociclib, with a statistically significant confidence interval.
Overall response rates were similar between the groups, at 65.2% with ribociclib versus 60% with chemotherapy. The respective clinical benefit rates (including complete and partial responses plus stable disease) were 80.4% versus 72.7%.
The time to response was similar between the treatment arms, an important consideration for patients with rapidly progressive disease, Dr. Lu noted.
Adverse events that occurred more frequently with ribociclib were neutropenia and leukopenia. Events more common with chemotherapy included anemia, liver enzyme elevations, nausea, vomiting, diarrhea, alopecia, fatigue, and palmar-plantar erythrodysesthesia.
Confirmation
“These data are just confirming what we’ve already known, and that is that with ER-positive, HER2-negative breast cancer where you have metastatic disease and more aggressive characteristics, treating with a CDK4/6 inhibitor and endocrine therapy leads to high response rates,” breast cancer specialist Matthew P. Goetz, MD, from the Mayo Clinic in Rochester, Minn., said in an interview. Dr. Goetz was not involved in the study.
“What was surprising to me was the fact that the response rates with chemotherapy were not higher,” he said. “We sometime think that the more chemotherapy, the higher the response rates. It was nice to see a direct comparison with chemotherapy, and really to see that giving a target therapy actually led to very, very good results. That tells us that there should be very few situations where we would be prescribing chemotherapy over CDK4/6 inhibitor–based therapies.”
The study was funded by Novartis Pharma. Dr. Lu disclosed personal funding from Novartis and others. Dr. Goetz disclosed grants and other supports for work with the development of abemaciclib and palbociclib, and consulting for Pfizer and others. Dr. Kaklamani disclosed speakers bureau activity for Novartis and others, research support from Eisai, and consulting for other companies.
SAN ANTONIO – Results of a study being hailed as practice changing showed that, for pre- or perimenopausal women with aggressive hormone receptor-positive, HER2-negative (HR+/HER2–) untreated breast cancers,
That’s according to investigators of the phase 2 RIGHT Choice study who found that first-line ribociclib, combined with either letrozole or anastrozole plus goserelin, was associated with a doubling of progression-free survival (PFS), compared with the investigator’s choice of combination chemotherapy, reported Yen-Shen Lu, MD, from National Taiwan University Hospital in Taipei, at the San Antonio Breast Cancer Symposium.
“These results from RIGHT Choice have now shown that first-line ribociclib plus endocrine therapy should be considered the preferred treatment option for this patient population,” he said.
Chemo loses its luster
“This is not time first time that we’ve looked at a CDK4/6 inhibitor compared to chemotherapy, but this is the first time that we’ve seen it compared to a combination chemotherapy,” commented Virginia Kaklamani, MD, from University of Texas Health, San Antonio, who moderated a media briefing held prior to Dr. Lu’s presentation of the data in an oral abstract session.
“I think with this study we’re finding that chemotherapy, at least in the early stages of [estrogen receptor]–positive breast cancer, is probably not appropriate for our patients,” she said.
Chemotherapy is the current standard of care for patients with advanced breast cancers with aggressive disease features that can include rapidly progressive disease, high symptom burden, and/or life-threatening visceral crises requiring rapid control of disease, Dr. Lu said.
Compared with single-agent chemotherapy, combination chemotherapy, for those who can tolerate it, is associated with higher overall response rates and longer PFS.
Although ribociclib plus endocrine therapy has been shown to offer significant PFS and overall survival (OS) benefits, compared with endocrine therapy alone, there have not been any head-to-head studies pitting these agents against combination chemotherapy.
Study details
To rectify this, Dr. Lu and colleagues enrolled 222 pre- or perimenopausal women with HR+/HER2– advanced breast cancers with aggressive features who had not yet received systemic therapy for advanced breast cancer.
After stratification for the presence or absence of liver metastases and by length of disease-free interval (time from complete resection of a primary tumor to documented recurrence), the patients were randomly assigned to receive either ribociclib 600 mg 3 weeks on, 1 week off) plus letrozole or anastrozole and goserelin, or the investigators choice of either docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine.
After a median follow-up of 24.1 months at the time of data cutoff in April 2022, median PFS, the primary endpoint, was 24 months in the ribociclib plus endocrine therapy arm versus 12.3 months in the chemotherapy arm.
This translated into a hazard ratio for progression on ribociclib plus endocrine therapy of 0.54 (P = .0007).
The benefit for the ribociclib combination, compared with combination chemotherapy, was consistent across most patient subgroups, Dr. Lu said.
The median time to treatment failure was also longer with ribociclib, at 18.6 months versus 8.5 months, respectively, translating into a HR of 0.45 favoring ribociclib, with a statistically significant confidence interval.
Overall response rates were similar between the groups, at 65.2% with ribociclib versus 60% with chemotherapy. The respective clinical benefit rates (including complete and partial responses plus stable disease) were 80.4% versus 72.7%.
The time to response was similar between the treatment arms, an important consideration for patients with rapidly progressive disease, Dr. Lu noted.
Adverse events that occurred more frequently with ribociclib were neutropenia and leukopenia. Events more common with chemotherapy included anemia, liver enzyme elevations, nausea, vomiting, diarrhea, alopecia, fatigue, and palmar-plantar erythrodysesthesia.
Confirmation
“These data are just confirming what we’ve already known, and that is that with ER-positive, HER2-negative breast cancer where you have metastatic disease and more aggressive characteristics, treating with a CDK4/6 inhibitor and endocrine therapy leads to high response rates,” breast cancer specialist Matthew P. Goetz, MD, from the Mayo Clinic in Rochester, Minn., said in an interview. Dr. Goetz was not involved in the study.
“What was surprising to me was the fact that the response rates with chemotherapy were not higher,” he said. “We sometime think that the more chemotherapy, the higher the response rates. It was nice to see a direct comparison with chemotherapy, and really to see that giving a target therapy actually led to very, very good results. That tells us that there should be very few situations where we would be prescribing chemotherapy over CDK4/6 inhibitor–based therapies.”
The study was funded by Novartis Pharma. Dr. Lu disclosed personal funding from Novartis and others. Dr. Goetz disclosed grants and other supports for work with the development of abemaciclib and palbociclib, and consulting for Pfizer and others. Dr. Kaklamani disclosed speakers bureau activity for Novartis and others, research support from Eisai, and consulting for other companies.
AT SABCS 2022
Potential cause of worse outcomes among Black breast cancer patients found
SAN ANTONIO – compared with White women, a discovery that may at least partially explain racial differences in breast cancer outcomes, investigators say.
The finding, which comes from a retrospective study comparing differences in tumor microenvironment of metastasis (TMEM) “doorways” between Black and White women suggest that tumors in Black women may have a stronger prometastatic response to neoadjuvant chemotherapy than tumors in White women, reported Maja H. Oktay, MD, PhD, of Montefiore Einstein Cancer Center, Albert Einstein College of Medicine, New York, at the San Antonio Breast Cancer Symposium.
“Looking forward ... we propose to use TMEM doorway density as a prognostic marker for distant recurrence-free survival as a marker of dissemination, and also as a predictive marker of response to drugs that can block TMEM doorways,” she said at a briefing held prior to the presentation of data in an oral abstract session.
Entry points
As their name implies, TMEM doorways are transient entry points or portals that allow cancer cells to disseminate to distant sites. TMEM doorways are composed of tumor cells, macrophages, and endothelial cells that come into direct contact and together create temporary vascular openings that allow tumor cells to cross cell walls into circulation, where they can then hitch a ride and travel to distant organ sites.
Previous studies have shown that TMEM doorway density is a prognostic marker of metastasis in breast cancer patients treated with adjuvant chemotherapy. And as Dr. Oktay and colleagues showed in the current study, TMEM doorway density, as measured by a TMEM doorway score, is a prognostic marker for distant metastatic recurrence of ER+/HER2– breast cancer following neoadjuvant chemotherapy.
They also showed that neoadjuvant chemotherapy may increase the TMEM doorway score and lead to a pro–metastatic tumor microenvironment in some women.
Doorway scores
The investigators measured TMEM doorway scores from residual breast cancers in women who had undergone standard neoadjuvant chemotherapy. The cohort consisted of 96 Black women, 43 of whom had ER+/HER2– breast cancer and 37 of whom had triple-negative breast cancer (TNBC), and 87 White women, 50 with ER+/HER2– cancer and 22 with TNBC. The remaining patients had other breast cancer subtypes.
They found that TNBCs had higher TMEM doorway density score and higher macrophage density scores, which may explain why patients with TNBC often have early recurrence of disease.
They also found that, compared with White patients, Black patients with ER+/HER2– tumors, but not TNBC tumors, had higher TMEM doorway density scores. Similarly, Black patients with ER+/HER– cancers, but not TNBC, had higher macrophage levels than White women, a finding that may explain racial disparity in ER+/HER2– disease, Dr. Oktay said.
For the entire cohort, patients with high TMEM doorway density scores had significantly worse distant recurrence–free survival than patients with intermediate or low scores (P = .008), and there was a trend toward worse DRFS among all patients with ER+/HER2– who were in the highest third of scores, but this did not quite reach statistical significance.
High versus low TMEM doorway density score was also an independent prognostic factor for worse outcomes among the entire cohort (P = .01).
There was no significant difference in TMEM density scores among patients with TNBC.
Neither high macrophage counts nor microvascular density alone were significantly associated with inferior DRFS. TMEM doorway score was the only factor significantly prognostic for worse outcomes among patients in the entire cohort.
Hypothesis needs further testing
Invited discussant Lori Pierce, MD, a radiation oncologist with Michigan Medicine, University of Michigan, Ann Arbor, said it’s unclear whether TMEM doorway density changed following neoadjuvant chemotherapy as there were no prechemotherapy scores available in this study.
“But I think the key part is that, if we think neoadjuvant chemotherapy promotes metastasis, then there should be an inferior outcome compared to adjuvant chemotherapy, but that’s not what we see. Well-powered randomized trials show equivalent outcomes with neoadjuvant chemotherapy as well as adjuvant,” she said.
She noted that a 2018 meta-analysis of individual patient data from 10 randomized trials comparing neoadjuvant with adjuvant chemotherapy in early breast cancer showed no differences in long-term distant recurrences, breast cancer–specific mortality, or all-cause mortality between the two modalities.
“While I think these data are very provocative, I certainly wouldn’t want Black women or any women who need neoadjuvant therapy to be discouraged because of these data. We need these data to be tested rigorously, so I look forward to the clinical trials that will test this question and can really give us more information about this very interesting hypothesis,” Dr. Pierce said.
The study was funded by the National Institutes of Health, New York State Department of Health Peter T. Rowley Breast Cancer Scientific Research Projects, Helen & Irving Spatz Family Foundation, Evelyn Gruss Lipper Charitable Foundation, and the Gruss-Lipper Biophotonics Center and the integrated imaging program at the Albert Einstein College of Medicine. Dr. Oktay reported no conflicts of interests.
SAN ANTONIO – compared with White women, a discovery that may at least partially explain racial differences in breast cancer outcomes, investigators say.
The finding, which comes from a retrospective study comparing differences in tumor microenvironment of metastasis (TMEM) “doorways” between Black and White women suggest that tumors in Black women may have a stronger prometastatic response to neoadjuvant chemotherapy than tumors in White women, reported Maja H. Oktay, MD, PhD, of Montefiore Einstein Cancer Center, Albert Einstein College of Medicine, New York, at the San Antonio Breast Cancer Symposium.
“Looking forward ... we propose to use TMEM doorway density as a prognostic marker for distant recurrence-free survival as a marker of dissemination, and also as a predictive marker of response to drugs that can block TMEM doorways,” she said at a briefing held prior to the presentation of data in an oral abstract session.
Entry points
As their name implies, TMEM doorways are transient entry points or portals that allow cancer cells to disseminate to distant sites. TMEM doorways are composed of tumor cells, macrophages, and endothelial cells that come into direct contact and together create temporary vascular openings that allow tumor cells to cross cell walls into circulation, where they can then hitch a ride and travel to distant organ sites.
Previous studies have shown that TMEM doorway density is a prognostic marker of metastasis in breast cancer patients treated with adjuvant chemotherapy. And as Dr. Oktay and colleagues showed in the current study, TMEM doorway density, as measured by a TMEM doorway score, is a prognostic marker for distant metastatic recurrence of ER+/HER2– breast cancer following neoadjuvant chemotherapy.
They also showed that neoadjuvant chemotherapy may increase the TMEM doorway score and lead to a pro–metastatic tumor microenvironment in some women.
Doorway scores
The investigators measured TMEM doorway scores from residual breast cancers in women who had undergone standard neoadjuvant chemotherapy. The cohort consisted of 96 Black women, 43 of whom had ER+/HER2– breast cancer and 37 of whom had triple-negative breast cancer (TNBC), and 87 White women, 50 with ER+/HER2– cancer and 22 with TNBC. The remaining patients had other breast cancer subtypes.
They found that TNBCs had higher TMEM doorway density score and higher macrophage density scores, which may explain why patients with TNBC often have early recurrence of disease.
They also found that, compared with White patients, Black patients with ER+/HER2– tumors, but not TNBC tumors, had higher TMEM doorway density scores. Similarly, Black patients with ER+/HER– cancers, but not TNBC, had higher macrophage levels than White women, a finding that may explain racial disparity in ER+/HER2– disease, Dr. Oktay said.
For the entire cohort, patients with high TMEM doorway density scores had significantly worse distant recurrence–free survival than patients with intermediate or low scores (P = .008), and there was a trend toward worse DRFS among all patients with ER+/HER2– who were in the highest third of scores, but this did not quite reach statistical significance.
High versus low TMEM doorway density score was also an independent prognostic factor for worse outcomes among the entire cohort (P = .01).
There was no significant difference in TMEM density scores among patients with TNBC.
Neither high macrophage counts nor microvascular density alone were significantly associated with inferior DRFS. TMEM doorway score was the only factor significantly prognostic for worse outcomes among patients in the entire cohort.
Hypothesis needs further testing
Invited discussant Lori Pierce, MD, a radiation oncologist with Michigan Medicine, University of Michigan, Ann Arbor, said it’s unclear whether TMEM doorway density changed following neoadjuvant chemotherapy as there were no prechemotherapy scores available in this study.
“But I think the key part is that, if we think neoadjuvant chemotherapy promotes metastasis, then there should be an inferior outcome compared to adjuvant chemotherapy, but that’s not what we see. Well-powered randomized trials show equivalent outcomes with neoadjuvant chemotherapy as well as adjuvant,” she said.
She noted that a 2018 meta-analysis of individual patient data from 10 randomized trials comparing neoadjuvant with adjuvant chemotherapy in early breast cancer showed no differences in long-term distant recurrences, breast cancer–specific mortality, or all-cause mortality between the two modalities.
“While I think these data are very provocative, I certainly wouldn’t want Black women or any women who need neoadjuvant therapy to be discouraged because of these data. We need these data to be tested rigorously, so I look forward to the clinical trials that will test this question and can really give us more information about this very interesting hypothesis,” Dr. Pierce said.
The study was funded by the National Institutes of Health, New York State Department of Health Peter T. Rowley Breast Cancer Scientific Research Projects, Helen & Irving Spatz Family Foundation, Evelyn Gruss Lipper Charitable Foundation, and the Gruss-Lipper Biophotonics Center and the integrated imaging program at the Albert Einstein College of Medicine. Dr. Oktay reported no conflicts of interests.
SAN ANTONIO – compared with White women, a discovery that may at least partially explain racial differences in breast cancer outcomes, investigators say.
The finding, which comes from a retrospective study comparing differences in tumor microenvironment of metastasis (TMEM) “doorways” between Black and White women suggest that tumors in Black women may have a stronger prometastatic response to neoadjuvant chemotherapy than tumors in White women, reported Maja H. Oktay, MD, PhD, of Montefiore Einstein Cancer Center, Albert Einstein College of Medicine, New York, at the San Antonio Breast Cancer Symposium.
“Looking forward ... we propose to use TMEM doorway density as a prognostic marker for distant recurrence-free survival as a marker of dissemination, and also as a predictive marker of response to drugs that can block TMEM doorways,” she said at a briefing held prior to the presentation of data in an oral abstract session.
Entry points
As their name implies, TMEM doorways are transient entry points or portals that allow cancer cells to disseminate to distant sites. TMEM doorways are composed of tumor cells, macrophages, and endothelial cells that come into direct contact and together create temporary vascular openings that allow tumor cells to cross cell walls into circulation, where they can then hitch a ride and travel to distant organ sites.
Previous studies have shown that TMEM doorway density is a prognostic marker of metastasis in breast cancer patients treated with adjuvant chemotherapy. And as Dr. Oktay and colleagues showed in the current study, TMEM doorway density, as measured by a TMEM doorway score, is a prognostic marker for distant metastatic recurrence of ER+/HER2– breast cancer following neoadjuvant chemotherapy.
They also showed that neoadjuvant chemotherapy may increase the TMEM doorway score and lead to a pro–metastatic tumor microenvironment in some women.
Doorway scores
The investigators measured TMEM doorway scores from residual breast cancers in women who had undergone standard neoadjuvant chemotherapy. The cohort consisted of 96 Black women, 43 of whom had ER+/HER2– breast cancer and 37 of whom had triple-negative breast cancer (TNBC), and 87 White women, 50 with ER+/HER2– cancer and 22 with TNBC. The remaining patients had other breast cancer subtypes.
They found that TNBCs had higher TMEM doorway density score and higher macrophage density scores, which may explain why patients with TNBC often have early recurrence of disease.
They also found that, compared with White patients, Black patients with ER+/HER2– tumors, but not TNBC tumors, had higher TMEM doorway density scores. Similarly, Black patients with ER+/HER– cancers, but not TNBC, had higher macrophage levels than White women, a finding that may explain racial disparity in ER+/HER2– disease, Dr. Oktay said.
For the entire cohort, patients with high TMEM doorway density scores had significantly worse distant recurrence–free survival than patients with intermediate or low scores (P = .008), and there was a trend toward worse DRFS among all patients with ER+/HER2– who were in the highest third of scores, but this did not quite reach statistical significance.
High versus low TMEM doorway density score was also an independent prognostic factor for worse outcomes among the entire cohort (P = .01).
There was no significant difference in TMEM density scores among patients with TNBC.
Neither high macrophage counts nor microvascular density alone were significantly associated with inferior DRFS. TMEM doorway score was the only factor significantly prognostic for worse outcomes among patients in the entire cohort.
Hypothesis needs further testing
Invited discussant Lori Pierce, MD, a radiation oncologist with Michigan Medicine, University of Michigan, Ann Arbor, said it’s unclear whether TMEM doorway density changed following neoadjuvant chemotherapy as there were no prechemotherapy scores available in this study.
“But I think the key part is that, if we think neoadjuvant chemotherapy promotes metastasis, then there should be an inferior outcome compared to adjuvant chemotherapy, but that’s not what we see. Well-powered randomized trials show equivalent outcomes with neoadjuvant chemotherapy as well as adjuvant,” she said.
She noted that a 2018 meta-analysis of individual patient data from 10 randomized trials comparing neoadjuvant with adjuvant chemotherapy in early breast cancer showed no differences in long-term distant recurrences, breast cancer–specific mortality, or all-cause mortality between the two modalities.
“While I think these data are very provocative, I certainly wouldn’t want Black women or any women who need neoadjuvant therapy to be discouraged because of these data. We need these data to be tested rigorously, so I look forward to the clinical trials that will test this question and can really give us more information about this very interesting hypothesis,” Dr. Pierce said.
The study was funded by the National Institutes of Health, New York State Department of Health Peter T. Rowley Breast Cancer Scientific Research Projects, Helen & Irving Spatz Family Foundation, Evelyn Gruss Lipper Charitable Foundation, and the Gruss-Lipper Biophotonics Center and the integrated imaging program at the Albert Einstein College of Medicine. Dr. Oktay reported no conflicts of interests.
AT SABCS 2022
Subset of patients with melanoma have very low mortality risk
Although melanoma is the most serious skin cancer, most patients do have high chances of survival. New research has now identified a subset of patients with early disease who have a very low risk of dying from the disease.
In a cohort of almost 11,600 patients, the overall 7-year rate of death from melanoma was 2.5%, but the risk in a subset of 25% of patients was below 1%. Conversely, the study authors were also able to identify a small subset of high‐risk patients with a greater than 20% risk for death.
and may help to begin to address the problem of overdiagnosis, they note.
“While the topic of very low-risk melanomas has been presented at national and international meetings, there have been no formal discussions to define the classification of ‘melanocytic neoplasms of low malignant potential’ at this time,” first author Megan M. Eguchi, MPH, of the department of medicine, University of California, Los Angeles, said in an interview. “Criteria would need to be established using study designs beyond those available using SEER data.”
She emphasized that currently, they do not propose any change to treatment of these lesions, just a change to the terminology. “A diagnosis of ‘MNLMP’ rather than ‘melanoma’ may potentially alleviate people’s concerns related to prognosis and begin to address the problem of overdiagnosis,” said Ms. Eguchi. The study was recently published online in Cancer.
Even though melanoma is considered to be the most common potentially lethal tumor of the skin, prognosis is often very good for those with T1 tumors, the lowest risk category. Prognostic modeling has been used to predict survival in patients with melanoma and identify prognostic variables, the authors note, with the most prominent attributes being Breslow thickness and ulceration of the primary tumor, which form the basis of the current American Joint Committee on Cancer (AJCC) staging system.
There is evidence that the increasing incidence of melanoma is partly due to overdiagnosis, meaning the diagnosis of lesions that will not lead to symptoms or death. The authors write that they were interested in identifying lesions that are currently diagnosed as melanoma but might lack the capacity for metastasis, cases that could potentially be part of the phenomenon of overdiagnosis.
Subsets with low and high risk for death
In the study, Ms. Eguchi and colleagues analyzed information from the United States Surveillance, Epidemiology, and End Results (SEER) database and identified 11,594 patients who were diagnosed in 2010 and 2011 with stage 1 melanoma that was less than or equal to 1.0 mm in thickness and had not spread to the lymph nodes. Prognostic models for risk for death from melanoma in patients with low-risk melanomas were developed, then the ability of the models to identify very‐low risk subsets of patients with melanoma‐specific survival surpassing that of T1 overall was evaluated.
The median age of the patients was 58 years, the median Breslow thickness was 0.45 mm (interquartile range, 0.30-0.65 mm), and 71% were assigned stage IA. Ulceration was present in 4% of cases, 27% were mitogenic, and 45% were Clark level II, and within this cohort, 292 (2.5%) patients died of melanoma within 7 years. In the training data set, 177 of 7,652 (2.3%) patients died of melanoma within 7 years, and numbers were similar in the testing set (115 of 3,942; 2.9%).
Overall, the investigators identified three large subsets of patients who were in the AJCC seventh edition classification for stage I (“thin”) melanoma, who had a risk for death of approximately less than 1%. This was a marked improvement from the rate of the overall sample. In the simplest model (Model 1A), patients who were younger than 70 years at diagnosis with Clark level II invasion were deemed as very low risk.
In Model 1B, the same initial classification was used, but it was further refined and limited to patients who were either age 43 years or younger or 44-69 years with Breslow thickness less than 0.40 mm. At 10 years postdiagnosis, this subset also showed a less than 1% risk for death from melanoma. The logistic regression model (Model 2) was similar, as it identified about 25% of patients with a predicted risk for death of less than 0.5%, incorporating patient age, sex, mitogenicity, Clark level, and ulceration. Model 2 was also able to further identify a small subset of patients with no deaths.
The logistic regression model was also able to identify a very small subset (0.7% and 0.8%) of patients who had a risk for death that exceeded 20%, which was markedly higher, compared with most patients with T1b tumors.
This study was supported by the National Cancer Institute. Ms. Eguchi had no disclosures to report.
A version of this article first appeared on Medscape.com.
Although melanoma is the most serious skin cancer, most patients do have high chances of survival. New research has now identified a subset of patients with early disease who have a very low risk of dying from the disease.
In a cohort of almost 11,600 patients, the overall 7-year rate of death from melanoma was 2.5%, but the risk in a subset of 25% of patients was below 1%. Conversely, the study authors were also able to identify a small subset of high‐risk patients with a greater than 20% risk for death.
and may help to begin to address the problem of overdiagnosis, they note.
“While the topic of very low-risk melanomas has been presented at national and international meetings, there have been no formal discussions to define the classification of ‘melanocytic neoplasms of low malignant potential’ at this time,” first author Megan M. Eguchi, MPH, of the department of medicine, University of California, Los Angeles, said in an interview. “Criteria would need to be established using study designs beyond those available using SEER data.”
She emphasized that currently, they do not propose any change to treatment of these lesions, just a change to the terminology. “A diagnosis of ‘MNLMP’ rather than ‘melanoma’ may potentially alleviate people’s concerns related to prognosis and begin to address the problem of overdiagnosis,” said Ms. Eguchi. The study was recently published online in Cancer.
Even though melanoma is considered to be the most common potentially lethal tumor of the skin, prognosis is often very good for those with T1 tumors, the lowest risk category. Prognostic modeling has been used to predict survival in patients with melanoma and identify prognostic variables, the authors note, with the most prominent attributes being Breslow thickness and ulceration of the primary tumor, which form the basis of the current American Joint Committee on Cancer (AJCC) staging system.
There is evidence that the increasing incidence of melanoma is partly due to overdiagnosis, meaning the diagnosis of lesions that will not lead to symptoms or death. The authors write that they were interested in identifying lesions that are currently diagnosed as melanoma but might lack the capacity for metastasis, cases that could potentially be part of the phenomenon of overdiagnosis.
Subsets with low and high risk for death
In the study, Ms. Eguchi and colleagues analyzed information from the United States Surveillance, Epidemiology, and End Results (SEER) database and identified 11,594 patients who were diagnosed in 2010 and 2011 with stage 1 melanoma that was less than or equal to 1.0 mm in thickness and had not spread to the lymph nodes. Prognostic models for risk for death from melanoma in patients with low-risk melanomas were developed, then the ability of the models to identify very‐low risk subsets of patients with melanoma‐specific survival surpassing that of T1 overall was evaluated.
The median age of the patients was 58 years, the median Breslow thickness was 0.45 mm (interquartile range, 0.30-0.65 mm), and 71% were assigned stage IA. Ulceration was present in 4% of cases, 27% were mitogenic, and 45% were Clark level II, and within this cohort, 292 (2.5%) patients died of melanoma within 7 years. In the training data set, 177 of 7,652 (2.3%) patients died of melanoma within 7 years, and numbers were similar in the testing set (115 of 3,942; 2.9%).
Overall, the investigators identified three large subsets of patients who were in the AJCC seventh edition classification for stage I (“thin”) melanoma, who had a risk for death of approximately less than 1%. This was a marked improvement from the rate of the overall sample. In the simplest model (Model 1A), patients who were younger than 70 years at diagnosis with Clark level II invasion were deemed as very low risk.
In Model 1B, the same initial classification was used, but it was further refined and limited to patients who were either age 43 years or younger or 44-69 years with Breslow thickness less than 0.40 mm. At 10 years postdiagnosis, this subset also showed a less than 1% risk for death from melanoma. The logistic regression model (Model 2) was similar, as it identified about 25% of patients with a predicted risk for death of less than 0.5%, incorporating patient age, sex, mitogenicity, Clark level, and ulceration. Model 2 was also able to further identify a small subset of patients with no deaths.
The logistic regression model was also able to identify a very small subset (0.7% and 0.8%) of patients who had a risk for death that exceeded 20%, which was markedly higher, compared with most patients with T1b tumors.
This study was supported by the National Cancer Institute. Ms. Eguchi had no disclosures to report.
A version of this article first appeared on Medscape.com.
Although melanoma is the most serious skin cancer, most patients do have high chances of survival. New research has now identified a subset of patients with early disease who have a very low risk of dying from the disease.
In a cohort of almost 11,600 patients, the overall 7-year rate of death from melanoma was 2.5%, but the risk in a subset of 25% of patients was below 1%. Conversely, the study authors were also able to identify a small subset of high‐risk patients with a greater than 20% risk for death.
and may help to begin to address the problem of overdiagnosis, they note.
“While the topic of very low-risk melanomas has been presented at national and international meetings, there have been no formal discussions to define the classification of ‘melanocytic neoplasms of low malignant potential’ at this time,” first author Megan M. Eguchi, MPH, of the department of medicine, University of California, Los Angeles, said in an interview. “Criteria would need to be established using study designs beyond those available using SEER data.”
She emphasized that currently, they do not propose any change to treatment of these lesions, just a change to the terminology. “A diagnosis of ‘MNLMP’ rather than ‘melanoma’ may potentially alleviate people’s concerns related to prognosis and begin to address the problem of overdiagnosis,” said Ms. Eguchi. The study was recently published online in Cancer.
Even though melanoma is considered to be the most common potentially lethal tumor of the skin, prognosis is often very good for those with T1 tumors, the lowest risk category. Prognostic modeling has been used to predict survival in patients with melanoma and identify prognostic variables, the authors note, with the most prominent attributes being Breslow thickness and ulceration of the primary tumor, which form the basis of the current American Joint Committee on Cancer (AJCC) staging system.
There is evidence that the increasing incidence of melanoma is partly due to overdiagnosis, meaning the diagnosis of lesions that will not lead to symptoms or death. The authors write that they were interested in identifying lesions that are currently diagnosed as melanoma but might lack the capacity for metastasis, cases that could potentially be part of the phenomenon of overdiagnosis.
Subsets with low and high risk for death
In the study, Ms. Eguchi and colleagues analyzed information from the United States Surveillance, Epidemiology, and End Results (SEER) database and identified 11,594 patients who were diagnosed in 2010 and 2011 with stage 1 melanoma that was less than or equal to 1.0 mm in thickness and had not spread to the lymph nodes. Prognostic models for risk for death from melanoma in patients with low-risk melanomas were developed, then the ability of the models to identify very‐low risk subsets of patients with melanoma‐specific survival surpassing that of T1 overall was evaluated.
The median age of the patients was 58 years, the median Breslow thickness was 0.45 mm (interquartile range, 0.30-0.65 mm), and 71% were assigned stage IA. Ulceration was present in 4% of cases, 27% were mitogenic, and 45% were Clark level II, and within this cohort, 292 (2.5%) patients died of melanoma within 7 years. In the training data set, 177 of 7,652 (2.3%) patients died of melanoma within 7 years, and numbers were similar in the testing set (115 of 3,942; 2.9%).
Overall, the investigators identified three large subsets of patients who were in the AJCC seventh edition classification for stage I (“thin”) melanoma, who had a risk for death of approximately less than 1%. This was a marked improvement from the rate of the overall sample. In the simplest model (Model 1A), patients who were younger than 70 years at diagnosis with Clark level II invasion were deemed as very low risk.
In Model 1B, the same initial classification was used, but it was further refined and limited to patients who were either age 43 years or younger or 44-69 years with Breslow thickness less than 0.40 mm. At 10 years postdiagnosis, this subset also showed a less than 1% risk for death from melanoma. The logistic regression model (Model 2) was similar, as it identified about 25% of patients with a predicted risk for death of less than 0.5%, incorporating patient age, sex, mitogenicity, Clark level, and ulceration. Model 2 was also able to further identify a small subset of patients with no deaths.
The logistic regression model was also able to identify a very small subset (0.7% and 0.8%) of patients who had a risk for death that exceeded 20%, which was markedly higher, compared with most patients with T1b tumors.
This study was supported by the National Cancer Institute. Ms. Eguchi had no disclosures to report.
A version of this article first appeared on Medscape.com.
FROM CANCER
Chromosomal test may ID risk for sudden infant deaths
Researchers have identified pathogenic gene variations in 12% of cases of sudden unexplained death in children.
The new study, which involved 116 cases of sudden infant death syndrome or sudden unexplained deaths in children (SUDC), suggests that available methods of chromosome testing could be used to help screen for the conditions, which together account for roughly 1,800 fatalities a year in the United States.
“Even though the Back to Sleep campaign has been incredibly effective and safe sleep practices have been promoted for years, sudden unexplained death in pediatrics remains a leading cause of death for infants and children,” said Catherine Brownstein, MPH, PhD, of Boston Children’s Hospital, lead author of the new study.
The findings suggest that chromosomal microarray analysis (CMA), the method that the researchers used in the study, “should be considered in the genetic evaluation of SUDC,” Dr. Brownstein said. The approach is the first-line method of identifying conditions such as autism spectrum disorder, developmental disabilities, multiple congenital anomalies, and epilepsy, she noted.
In the study, published in Advanced Genetics, Dr. Brownstein and her colleagues used CMA to test genes from 116 deceased infants and toddlers up to age 28 months whose deaths were classified as SIDS or SUDC (the latter term applies to children older than 1 year).
The average age at the time of death was 5.7 months; 59% of the patients were boys. In 14 of the children (12%), the CMA test identified genetic variations in the form of deletions or duplications that were pathogenic (five cases) or uncertain but “favoring pathogenicity” (nine cases). Such deletions or duplications are known as copy number variants (CNVs).
CNVs are present in most people and are not necessarily associated with disease, according to the researchers. However, certain CNVs have been linked to ASD, attention-deficit/hyperactivity disorder, schizophrenia, Crohn’s disease, epilepsy, and various congenital abnormalities.
Dr. Brownstein’s group also compared pathogenicity in the SUDC group with that of a cohort of children with ASD and with healthy control persons. They found no significant difference in pathogenicity between SUDC and autism with regard to duplications. However, children in the SUDC group were significantly more likely to have higher pathogenicity scores for deletions, compared with control persons. Some of the CMVs did not appear connected to SIDS or SUDC; two cases in boys were undiagnosed cases of Klinefelter syndrome.
The study findings were limited by several factors, including the small sample size and the inability to conduct CMA analyses on parents or obtain family history, the researchers note. Other limitations were that phenotypic data were available only from autopsy material and medical records and that the study focused on younger children, the researchers add. They did not speculate about the causes of deaths in the other cases they examined.
In the current study, the other 88% of cases could involve nongenetic factors or genetic factors that aren’t measured by next-generation sequencing or chromosomal microarray, Dr. Brownstein said. “Undiagnosed disease programs looking for genetic causes for diseases in living patients identify a cause in about 1 in 4 cases,” she said. “While 12% is a modest percentage, the CNVs identified provide additional information. In the future, the goal would be to capture the full range of potential genetic changes.”
Previous research by Dr. Brownstein’s group at Robert’s Program, a clinical service for SUDC families at Boston Children’s Hospital, found genetic variants that might cause sudden death in children.
“We began this study with the simple question of whether, as a population, these children carry more copy number variation, which they do,” she said. “However, none of the CMA findings we identified are currently associated with SUDC, so much more investigation is necessary to find out if they are coincidental, risk factors, or causative.”
Looking ahead, she said, “Ideally, we would want every family affected by sudden unexplained death in pediatrics to have genetic testing, including a chromosomal microarray. Once we have more families enrolled and tested, we will be able to understand the risk factors for SIDS and SUDC much better.”
Benjamin Solomon, MD, clinical director at the National Human Genome Research Institute, Bethesda, Md., said the new research “may bring answers for individual situations as well as enable research to understand the overall biological underpinnings and causes of disease.”
The findings “help reinforce the heterogeneous nature of SUDC and related conditions,” Dr. Solomon said. “The results also highlight some of the challenges regarding how to interpret the possible clinical effects of genetic changes. That is, every person has genetic changes, and interpreting how certain genetic changes may or may not contribute to a disease or health care outcome can be challenging.”
Research is needed to understand not only the overall causes of SUDC but also how the different causes interact, Dr. Solomon said. “Eventually, better understanding of the causes could lead to knowledge that would enable interventions that could help prevent or reduce these devastating outcomes.”
The study was supported by the Robert’s Program on Sudden Unexplained Death in Pediatrics, the Jude Zayac Foundation, multiple grants from the Eunice Kennedy Shriver National Institutes of Health/National Institute of Child Health and Human Development, the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center Molecular Genetics Core Facility (supported by the NIH/NICHD), and by the NIH National Institute of Mental Health. The researchers and Dr. Solomon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have identified pathogenic gene variations in 12% of cases of sudden unexplained death in children.
The new study, which involved 116 cases of sudden infant death syndrome or sudden unexplained deaths in children (SUDC), suggests that available methods of chromosome testing could be used to help screen for the conditions, which together account for roughly 1,800 fatalities a year in the United States.
“Even though the Back to Sleep campaign has been incredibly effective and safe sleep practices have been promoted for years, sudden unexplained death in pediatrics remains a leading cause of death for infants and children,” said Catherine Brownstein, MPH, PhD, of Boston Children’s Hospital, lead author of the new study.
The findings suggest that chromosomal microarray analysis (CMA), the method that the researchers used in the study, “should be considered in the genetic evaluation of SUDC,” Dr. Brownstein said. The approach is the first-line method of identifying conditions such as autism spectrum disorder, developmental disabilities, multiple congenital anomalies, and epilepsy, she noted.
In the study, published in Advanced Genetics, Dr. Brownstein and her colleagues used CMA to test genes from 116 deceased infants and toddlers up to age 28 months whose deaths were classified as SIDS or SUDC (the latter term applies to children older than 1 year).
The average age at the time of death was 5.7 months; 59% of the patients were boys. In 14 of the children (12%), the CMA test identified genetic variations in the form of deletions or duplications that were pathogenic (five cases) or uncertain but “favoring pathogenicity” (nine cases). Such deletions or duplications are known as copy number variants (CNVs).
CNVs are present in most people and are not necessarily associated with disease, according to the researchers. However, certain CNVs have been linked to ASD, attention-deficit/hyperactivity disorder, schizophrenia, Crohn’s disease, epilepsy, and various congenital abnormalities.
Dr. Brownstein’s group also compared pathogenicity in the SUDC group with that of a cohort of children with ASD and with healthy control persons. They found no significant difference in pathogenicity between SUDC and autism with regard to duplications. However, children in the SUDC group were significantly more likely to have higher pathogenicity scores for deletions, compared with control persons. Some of the CMVs did not appear connected to SIDS or SUDC; two cases in boys were undiagnosed cases of Klinefelter syndrome.
The study findings were limited by several factors, including the small sample size and the inability to conduct CMA analyses on parents or obtain family history, the researchers note. Other limitations were that phenotypic data were available only from autopsy material and medical records and that the study focused on younger children, the researchers add. They did not speculate about the causes of deaths in the other cases they examined.
In the current study, the other 88% of cases could involve nongenetic factors or genetic factors that aren’t measured by next-generation sequencing or chromosomal microarray, Dr. Brownstein said. “Undiagnosed disease programs looking for genetic causes for diseases in living patients identify a cause in about 1 in 4 cases,” she said. “While 12% is a modest percentage, the CNVs identified provide additional information. In the future, the goal would be to capture the full range of potential genetic changes.”
Previous research by Dr. Brownstein’s group at Robert’s Program, a clinical service for SUDC families at Boston Children’s Hospital, found genetic variants that might cause sudden death in children.
“We began this study with the simple question of whether, as a population, these children carry more copy number variation, which they do,” she said. “However, none of the CMA findings we identified are currently associated with SUDC, so much more investigation is necessary to find out if they are coincidental, risk factors, or causative.”
Looking ahead, she said, “Ideally, we would want every family affected by sudden unexplained death in pediatrics to have genetic testing, including a chromosomal microarray. Once we have more families enrolled and tested, we will be able to understand the risk factors for SIDS and SUDC much better.”
Benjamin Solomon, MD, clinical director at the National Human Genome Research Institute, Bethesda, Md., said the new research “may bring answers for individual situations as well as enable research to understand the overall biological underpinnings and causes of disease.”
The findings “help reinforce the heterogeneous nature of SUDC and related conditions,” Dr. Solomon said. “The results also highlight some of the challenges regarding how to interpret the possible clinical effects of genetic changes. That is, every person has genetic changes, and interpreting how certain genetic changes may or may not contribute to a disease or health care outcome can be challenging.”
Research is needed to understand not only the overall causes of SUDC but also how the different causes interact, Dr. Solomon said. “Eventually, better understanding of the causes could lead to knowledge that would enable interventions that could help prevent or reduce these devastating outcomes.”
The study was supported by the Robert’s Program on Sudden Unexplained Death in Pediatrics, the Jude Zayac Foundation, multiple grants from the Eunice Kennedy Shriver National Institutes of Health/National Institute of Child Health and Human Development, the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center Molecular Genetics Core Facility (supported by the NIH/NICHD), and by the NIH National Institute of Mental Health. The researchers and Dr. Solomon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have identified pathogenic gene variations in 12% of cases of sudden unexplained death in children.
The new study, which involved 116 cases of sudden infant death syndrome or sudden unexplained deaths in children (SUDC), suggests that available methods of chromosome testing could be used to help screen for the conditions, which together account for roughly 1,800 fatalities a year in the United States.
“Even though the Back to Sleep campaign has been incredibly effective and safe sleep practices have been promoted for years, sudden unexplained death in pediatrics remains a leading cause of death for infants and children,” said Catherine Brownstein, MPH, PhD, of Boston Children’s Hospital, lead author of the new study.
The findings suggest that chromosomal microarray analysis (CMA), the method that the researchers used in the study, “should be considered in the genetic evaluation of SUDC,” Dr. Brownstein said. The approach is the first-line method of identifying conditions such as autism spectrum disorder, developmental disabilities, multiple congenital anomalies, and epilepsy, she noted.
In the study, published in Advanced Genetics, Dr. Brownstein and her colleagues used CMA to test genes from 116 deceased infants and toddlers up to age 28 months whose deaths were classified as SIDS or SUDC (the latter term applies to children older than 1 year).
The average age at the time of death was 5.7 months; 59% of the patients were boys. In 14 of the children (12%), the CMA test identified genetic variations in the form of deletions or duplications that were pathogenic (five cases) or uncertain but “favoring pathogenicity” (nine cases). Such deletions or duplications are known as copy number variants (CNVs).
CNVs are present in most people and are not necessarily associated with disease, according to the researchers. However, certain CNVs have been linked to ASD, attention-deficit/hyperactivity disorder, schizophrenia, Crohn’s disease, epilepsy, and various congenital abnormalities.
Dr. Brownstein’s group also compared pathogenicity in the SUDC group with that of a cohort of children with ASD and with healthy control persons. They found no significant difference in pathogenicity between SUDC and autism with regard to duplications. However, children in the SUDC group were significantly more likely to have higher pathogenicity scores for deletions, compared with control persons. Some of the CMVs did not appear connected to SIDS or SUDC; two cases in boys were undiagnosed cases of Klinefelter syndrome.
The study findings were limited by several factors, including the small sample size and the inability to conduct CMA analyses on parents or obtain family history, the researchers note. Other limitations were that phenotypic data were available only from autopsy material and medical records and that the study focused on younger children, the researchers add. They did not speculate about the causes of deaths in the other cases they examined.
In the current study, the other 88% of cases could involve nongenetic factors or genetic factors that aren’t measured by next-generation sequencing or chromosomal microarray, Dr. Brownstein said. “Undiagnosed disease programs looking for genetic causes for diseases in living patients identify a cause in about 1 in 4 cases,” she said. “While 12% is a modest percentage, the CNVs identified provide additional information. In the future, the goal would be to capture the full range of potential genetic changes.”
Previous research by Dr. Brownstein’s group at Robert’s Program, a clinical service for SUDC families at Boston Children’s Hospital, found genetic variants that might cause sudden death in children.
“We began this study with the simple question of whether, as a population, these children carry more copy number variation, which they do,” she said. “However, none of the CMA findings we identified are currently associated with SUDC, so much more investigation is necessary to find out if they are coincidental, risk factors, or causative.”
Looking ahead, she said, “Ideally, we would want every family affected by sudden unexplained death in pediatrics to have genetic testing, including a chromosomal microarray. Once we have more families enrolled and tested, we will be able to understand the risk factors for SIDS and SUDC much better.”
Benjamin Solomon, MD, clinical director at the National Human Genome Research Institute, Bethesda, Md., said the new research “may bring answers for individual situations as well as enable research to understand the overall biological underpinnings and causes of disease.”
The findings “help reinforce the heterogeneous nature of SUDC and related conditions,” Dr. Solomon said. “The results also highlight some of the challenges regarding how to interpret the possible clinical effects of genetic changes. That is, every person has genetic changes, and interpreting how certain genetic changes may or may not contribute to a disease or health care outcome can be challenging.”
Research is needed to understand not only the overall causes of SUDC but also how the different causes interact, Dr. Solomon said. “Eventually, better understanding of the causes could lead to knowledge that would enable interventions that could help prevent or reduce these devastating outcomes.”
The study was supported by the Robert’s Program on Sudden Unexplained Death in Pediatrics, the Jude Zayac Foundation, multiple grants from the Eunice Kennedy Shriver National Institutes of Health/National Institute of Child Health and Human Development, the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center Molecular Genetics Core Facility (supported by the NIH/NICHD), and by the NIH National Institute of Mental Health. The researchers and Dr. Solomon have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ADVANCED GENETICS
They trusted their prenatal test. They didn’t know the industry is an unregulated ‘Wild West.’
Amanda wanted to warn someone. In June 2021, her daughter – the one she and her husband had tried for 3 years to conceive – had died after only 28 hours. With an underdeveloped nose, she had battled for every breath.
Nobody knew why. Later, an autopsy report revealed their daughter had an extra 13th chromosome. The condition is nearly always fatal.
“But didn’t we test for that?” Amanda recalled asking herself. “That was kind of where the light bulb clicked.”
Through her doctor, Amanda had gotten a popular prenatal screening from a lab company. It had come back “negative.”
For three major conditions, including the one her baby had, the report gave the impression of near certainty. The likelihood that she would be born without them was “greater than 99%.”
As she recovered from a cesarean section, Amanda found herself facing a long maternity leave without a child. She shut the door to the empty nursery and began spending what seemed like endless hours of that hazy summer learning about the test.
It’s a simple blood draw designed to check for an array of genetic anomalies. But Amanda, a science researcher, read academic articles showing there was a higher risk of inaccurate results than she had realized. (She asked to be identified by only her first name to protect her privacy.)
On Reddit, she found other women reporting problems with the tests, too. She thought Labcorp, the company that made her test, would want to know about the screening that failed her. Maybe by alerting them, she could help other families. Maybe it would help her understand what happened.
“I was trying to gain answers,” said Amanda, now 32. She tried calling Labcorp’s customer service line, but she said she was passed along from one person to another. “It was just a circle,” she remembered.
She phoned Labcorp a second time. The call ended when an employee hung up on her.
Amanda was baffled. Why didn’t the company seem interested in her experience? Why, she wondered, wouldn’t it want to collect this data? Why wasn’t there someone who could answer her questions about how often this happens, and why?
If she had taken any number of other common commercial tests – including certain tests for COVID-19 or, say, pregnancy – the company would have been required to inform the U.S. Food and Drug Administration about reports of so-called adverse events.
But the test Amanda had falls into a regulatory void. No federal agency checks to make sure these prenatal screenings work the way they claim before they’re sold to health care providers. The FDA doesn’t ensure that marketing claims are backed up by evidence before screenings reach patients. And companies aren’t required to publicly report instances of when the tests get it wrong – sometimes catastrophically.
The broader lab testing industry and its lobbyists have successfully fought for years to keep it this way, cowing regulators into staying on the sidelines.
Worried about a growing variety of tests escaping scrutiny, the FDA was on the cusp of stepping in 6 years ago. But then it backed down.
Peter Lurie, then a top agency official, was at the meetings where the FDA tabled its plans. Not pushing harder, he told ProPublica, “remains one of my greatest regrets.”
The risk of false positives from prenatal screenings, in particular, has been known for years.
In 2014, the New England Center for Investigative Reporting detailed how some companies gave a misleading impression of the precision of the prenatal screenings. Women often didn’t understand they needed diagnostic testing to confirm the results. Some had gotten abortions based on false positive results, the story said. Earlier this year, the New York Times reported how companies sell optional extra screenings that are “usually wrong” when they predict a disorder.
Despite these stories and calls for reform by patient advocates, the government has done little to improve oversight of prenatal screenings. ProPublica set out to examine the forces that led to this inertia and left patients like Amanda feeling misled. Interviews with more than three dozen women revealed ongoing confusion about the screenings – and anger when their reliability proved to be overblown.
“This is a Wild West scenario where everybody is on their own,” said Lawrence Gostin, a Georgetown University, Washington, law professor specializing in bioethics.
The stakes for families are increasing. Upward of half of all pregnant people now receive one of these prenatal screenings. And with many states banning abortions or limiting them to early in pregnancies, the need for fast, accurate information has become more urgent.
The FDA itself acknowledges the problem. In correspondence with ProPublica, a spokesperson cited an “outdated policy” regarding the lack of vetting of many lab tests that the agency has “spent the better part of the last 2 decades trying to address.”
The screening industry, meanwhile, continues to expand, proving lucrative for those who lead it. The chief executive of Natera, which claims about 40% of the market share of prenatal screenings, received a $23 million compensation package last year, the highest of any executive at a publicly traded lab company.
Testing companies told ProPublica that, even without the FDA, there is significant oversight. Labs must abide by state regulations, and another federal agency, the Centers for Medicare and Medicaid Services, is charged with monitoring quality standards. It does not, however, check whether the tests the labs perform are clinically valid.
Companies also said the screenings offer important guidance to expectant families. Echoing others in the field, Labcorp said in a statement that the screenings, when used properly, “provide vital information about the presence of increased risk, but do not provide a definitive diagnosis.” (It declined to discuss the specifics of Amanda’s experience.)
Natera pointed out that its materials tell patients that “this test does not make a final diagnosis.” It reports results as “high-risk” or “low-risk,” not positive or negative.
Companies have stressed that, ultimately, it’s the responsibility of health care providers, who order the tests, to inform patients about the limits of screenings.
For all that, the statistical nuances of the test aren’t easy to parse for patients and even some doctors and nurses. For example, the test for trisomy 13, which doomed Amanda’s baby, is actually less likely to correctly predict the condition than other tests in the standard bundle of screenings offered to every patient.
When ProPublica asked readers to share their experiences with noninvasive prenatal screening tests, often referred to as NIPTs or NIPS, more than a thousand responded. Many said the tests had given them peace of mind. Some said they had provided an early warning about problems.
But others had more questions than answers. None more so than Amanda.
“What are these tests?” she wondered. “And how did mine end up in the margin of error?”
‘They started using it on humans, and then they went back and said: “Was our test accurate?” ’
Scientists have long tried to find ways to help parents and doctors understand what’s happening inside the womb. Amniocentesis was first used to reveal genetic anomalies in the late 1960s. But it didn’t become more popular until it began to be paired with ultrasound to precisely guide the procedure.
In the 1980s, doctors started using chorionic villus sampling, or CVS, an analysis of placental tissue that offers a diagnosis earlier in pregnancy. But, like amniocentesis, it is an invasive test that involves some risk to the fetus, though experts say it’s exceptionally low.
A breakthrough came in the late 1990s, when a scientist recognized that free-floating placental DNA could be detected in the mother’s blood. This meant that the fetus’s chromosomes could be examined by collecting a blood sample as soon as 9 weeks into pregnancy. This also provides an early opportunity to learn the likely fetal sex – a particularly popular feature.
Champions of the new science celebrated the arrival of a simple technique for patients that was particularly precise, at least for some conditions. Many favored it over other noninvasive options. But the industry that developed around NIPT has been marred by controversy from the beginning.
Dr. Ronald Wapner, director of reproductive genetics at Columbia University, described that time as “very chaotic.”
The tests had not been appropriately evaluated in clinical practice, said Dr. Wapner, whose research has sometimes been funded by testing companies. Because of this, he said, the industry “had very incomplete data on how well it worked.”
That didn’t stop the excitement. The chief executive of Sequenom, a biotechnology company that planned to release the first NIPT for Down syndrome, championed the company as the “Google of Molecular Diagnostics.” Its stock price soared.
Then, about 2 months before an expected launch in 2009, Sequenom killed the plan. The company’s research director, it turned out, had manipulated testing data and made misleading claims about how well the screening worked.
The U.S. Securities and Exchange Commission and Federal Bureau of Investigation opened investigations. Top executives were fired, and the research director pleaded guilty to conspiracy to commit securities fraud. Sequenom still managed to commercialize the test in 2011. (Labcorp, which later acquired Sequenom, said it uses a different kind of test.)
Other companies soon debuted their own tests. Still, there was little data on their clinical performance, researchers said.
As Megan Allyse, a bioethicist at the Mayo Clinic, put it, the companies “launched the test, they started using it on humans, and then they went back and said, ‘Was our test accurate?’ ” She also questioned the lack of attention to the ethics of how tests are presented to patients.
Despite missteps by the industry, the FDA didn’t scrutinize the screenings because they were considered lab-developed tests, which means they are created by the same laboratory that conducts them.
In 1976, Congress revamped oversight over medical devices. Since then, the FDA has effectively exempted such “home-brew” tests from key regulatory requirements. The idea was that when, say, a hospital lab wanted to create a simple test for its own patients, it was spared the time, money, and hassle of getting approval from Washington bureaucrats.
Today, lab-developed tests are vastly more numerous and complex. Because they aren’t registered with the federal government, nobody knows how many exist.
The distinction between tests the FDA actively regulates and those they don’t can seem nonsensical. It isn’t based on the complexity of the tests, or how people use them. It’s simply a matter of where the test is made.
The prenatal genetic screening industry took off almost immediately, powered by an army of aggressive sales representatives.
“At the very beginning, obstetricians in practice were being just completely inundated with visits from the sales reps,” said Dr. John Williams, director of reproductive health at Cedars-Sinai Medical Center in Los Angeles. The push left many ob.gyns. and patients thinking the screenings were accurate enough to substitute for diagnostic tests, such as amniocentesis or CVS.
In some cases, sales tactics escalated into lawbreaking.
Former Sequenom executives who exited during the fraud scandal created a new company that became Progenity, which also offered prenatal screening. Shortly after the company went public in 2020, it finalized a $49 million settlement with federal and state governments, where it admitted to falsifying insurance claims and giving kickbacks to physicians and their staff. According to a legal filing, one sales rep spent $65,658 on meals and alcohol for physicians in 1 year.
Now called Biora Therapeutics, the company said in a statement it no longer does any laboratory testing, including prenatal screenings.
Industry revenue continues to grow, but some testing companies are still fighting to make a profit, and competition to survive is fierce. “There’s a multibillion-dollar market, and they all want a piece of it,” said a former Progenity sales rep who quit in disgust after 5 months in 2016.
The rep, who requested anonymity because she continues to work in the field, said she still sees competitors from NIPT companies visiting medical practices “every week, buying breakfast or dinner, or taking them out for happy hour.”
Over time, companies pointed to new peer-reviewed studies, research the industry itself funded, to earn the confidence of doctors and other stakeholders. They showed that two tests – for Down syndrome and trisomy 18 – often performed better than other screening methods.
This research was valid, said Dr. Mary Norton, a perinatologist and clinical geneticist at the University of California, San Francisco, Medical Center’s prenatal diagnostic center. Considered a leading researcher in the field, she was an author of many of these key industry-funded studies.
But, she said, when research findings were presented publicly, the companies sometimes downplayed “inconvenient truths,” such as the exclusion of inconclusive results from accuracy estimates. Crucial caveats were also glossed over by some companies when they translated research into promotional copy aimed at health care providers and patients. Those materials didn’t always mention the many factors that can limit the performance of the screenings, including high body weight, the rarity of the condition tested, and younger maternal age.
Testing companies said they try to help patients understand the screenings through online resources and other materials. Some offer genetic counseling services.
The younger a person is, the lower the test’s positive predictive value – that is, the probability that a positive screening result will turn out to be correct – will be for some conditions. For instance, because Down syndrome is less prevalent in younger people’s pregnancies, a positive screening test is more likely to be a false positive for them.
Kristina was 30 years old in 2016, when her Progenity test came back positive for Down syndrome. She and her husband, who asked not to be fully named to protect their privacy, said they didn’t plan to carry a pregnancy with this condition to term.
But waiting to get an amniocentesis, and then waiting for the results, took 5 agonizing weeks, she said. It showed her son did not have Down syndrome.
Kristina, who lives in Texas, is still troubled by what she describes as a traumatic experience.
“I researched both late-term abortion providers and cemeteries,” she said. They even picked out a burial place, near their house.
She bought a blue baby blanket she intended to bury the baby’s tiny body in. She still has it. Her son, now 5, sleeps with it every night.
‘I can’t believe I didn’t say more’
As lab-developed tests became a bigger business, moving well past their home-brew origins, regulators looked for a way to assert oversight. In 2014, after years of study and debate, the time seemed right.
The FDA released plans proposing to regulate the tests, prioritizing those used to make major medical decisions. The agency has pointed to NIPTs as 1 of 20 concerning tests.
But, over the next 2 years, a coalition of power players urged the FDA to back off. Professional associations issued statements and hosted webinars devoted to the issue. Some created polished websites featuring sample letters to send to Washington.
Academic medical centers and pathology departments joined the fight, too. Scientists from 23 of them put it bluntly in a letter to the Office of Management and Budget: “FDA regulation of LDTs would be contrary to the public health,” it said, using a common acronym for the tests.
“Critical testing would be unavailable in the ‘lag time’ between development of new tests and FDA authorizing them,” the authors of the letter wrote, “and subsequent improvements on existing tests would slow significantly under the rigid, inflexible, and duplicative FDA regulatory scheme.”
This could delay essential care for patients. What’s more, opponents argued, existing lab reviews by the Centers for Medicare and Medicaid Services are sufficiently rigorous. Some have suggested modernizing the CMS review process to improve oversight.
An FDA spokesperson told ProPublica that the agency encountered “continued, negative feedback,” including a 25-page paper written by two legal heavyweights hired by the American Clinical Laboratory Association: Paul Clement, President George W. Bush’s former solicitor general, and Laurence Tribe, law professor at Harvard University.
Mr. Clement has reportedly commanded rates of $1,350 per hour. He and Mr. Tribe did not respond to ProPublica’s queries about their work.
Their brief argued that the FDA “lacked legal authority” to regulate lab-developed tests because they are properly seen as the practice of medicine: a service, rather than a product.
However, as lawyers representing the American Association of Bioanalysts countered, the FDA would vet tests before they reach the market, not control how doctors use them. The government proposal, they wrote, is “similar to imposing requirements to screen blood or label drugs.”
After the election of President Donald Trump, but before he took office, a handful of FDA officials discussed their battered proposal. It had represented a breakthrough in the decades of excruciating back-and-forth with industry. But now, with an incoming administration bent on deregulation, their efforts seemed futile.
The regulators feared anything they enacted would be undone by Congress – and, under the Congressional Review Act, they might not be able to reissue anything “substantially similar” in the future. So the FDA published a white paper instead, summarizing the issue “for further public discussion.”
After the meeting where officials made this call, Mr. Lurie, then the FDA’s associate commissioner, recalled a colleague approaching him: “I can’t believe you didn’t say more.”
“And I was like, ‘Yeah, actually, I can’t believe I didn’t say more either,’ ” Mr. Lurie later told ProPublica. (After leaving the agency, Mr. Lurie went on to lead the Center for Science in the Public Interest, a consumer advocacy nonprofit, which has pushed the FDA to finally assert oversight over lab-developed tests.)
Nancy Stade, an attorney and senior policy official who left the FDA in 2015, said the agency often moves slowly as it seeks to get buy-in from industry and professional groups. In her work on regulatory policy, she saw it happen with lab-developed tests.
The agency is “always testing the waters,” she said, “and always coming out with something a little bit softer.”
In 2020, the influential American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine, representing doctors who handle pregnancies, gave the screening industry another huge boost.
In a bulletin updating their advice on the tests, the two groups described growing research on the performance of some of the standard tests and said people have the right to information about their pregnancies, so the tests should be offered to all patients. Previously, they recommended this only for those facing higher risk of genetic anomalies.
The bulletin said the coauthors had disclosed no conflicts of interest. But two of the four coauthors, including Mary Norton, had disclosed in prior publications that test-makers had provided funding for their research. A company had provided a third coauthor with laboratory services needed to run tests, according to that researcher, a connection she also disclosed in past papers.
ACOG, in a statement to ProPublica, said the organization “identified no conflicts because research funding is provided to academic institutions with institutional review boards, not to individual investigators.” Two of the three researchers responded to questions from ProPublica and said they maintained independence over their work.
One test-maker, Illumina, celebrated the ACOG guidance in a tweet, saying it “recognizes the superior performance of #NIPT and the benefit it provides expectant families.” Natera’s share prices doubled in 5 months. UnitedHealthcare, the nation’s largest private insurer and long a target of industry lobbying, told ProPublica it changed its stance to cover screenings for all patients, regardless of risk, because of the recommendation.
In a recent shareholder report, Natera stated that prenatal genetic and carrier screenings “represent the significant majority of our revenues,” which totaled $625.5 million in 2021. The company expects more growth to come.
“The NIPT market is still very underpenetrated, compared to the 4 to 5 million pregnancies in the U.S.,” Natera’s chief executive said on a 2021 earnings call, “so there’s a long way to go.”
But even Dr. Norton, who coauthored the ACOG recommendation and favors NIPTs for patients 40 and over, has concerns about screenings becoming widespread among those who are younger. In most cases, she prefers other screening methods that catch the nongenetic problems younger moms are more likely to face. Negative results from an NIPT, she said, can be “falsely reassuring.”
In the years after the FDA set aside its regulatory proposal, the agency has assisted members of Congress on a proposed legislative solution. That effort, dubbed the VALID Act, aims to end any debate over the agency’s authority over lab-developed tests. An FDA press officer said the legislation would ensure the prenatal screening tests and others are “accurate and reliable.”
But, as in the past, intense lobbying followed the proposal. The VALID Act was a rider to a funding reauthorization bill, but in September the House and Senate agreed to remove it. Advocates now hope to attach it to proposed end-of-year legislation.
Meanwhile, earlier this year, 4 months after the New York Times story on the usefulness of some screenings, the FDA took a step toward more public awareness about prenatal genetic screening. It issued its first safety communication on them, noting the potential for false results.
It cautioned patients about making “critical health care decisions based on results from these screening tests alone.”
Cara Tenenbaum, a former FDA policy advisor, was pleased to see the statement. Still, she said, it was long overdue.
“This has been known – known, or should have been known – for 10 years,” she said.
‘It had me so messed up’
With the demise of Roe v. Wade, restrictive and ever-changing abortion laws can pressure people to act quickly with limited information, heightening the stakes of prenatal screening.
Julia, a mom from Mississippi’s Gulf Coast, knows what it’s like to face harrowing consequences while navigating state-imposed time limits – and doing so with little guidance. Last fall, she was pregnant with her fourth child when, she said, a nurse practitioner suggested prenatal genetic screening.
At 33, Julia had no risk factors. Her previous pregnancies hadn’t been screened with an NIPT. But with three sons and 18 nephews, she and her husband were curious about the baby’s sex. And the screening seemed like it had no downside.
Julia figured it would only be offered if it was reliable, so her nurse practitioner ordered her both the basic bundle of screenings and the extra tests. (The medical practice didn’t respond to interview requests. Julia is a family nickname that’s used here to protect her privacy.)
The screenings showed the baby was a girl – but the extra tests also detected trisomy 16, a condition caused by an extra chromosome that is so rare, the nurse didn’t know what it was, Julia recalled.
The nurse borrowed Julia’s phone, using it to search online and read aloud what she found. Julia was stunned to hear trisomy 16 was incompatible with life.
“I was utterly devastated,” she said. “I made it out of my doctor’s office but completely broke down in the car.”
But ACOG does not recommend the trisomy 16 screening, saying “its accuracy with regard to detection and the false-positive rate is not established.” Julia wasn’t informed of this, she said, and she’s not sure if her health care providers knew it either.
The lab report recommended diagnostic testing to confirm the results, but time was short. She had her amniocentesis at 17 weeks. It could take up to 4 more weeks to receive results.
That would be too late for a legal abortion in Mississippi. So she made an appointment for one in Florida, where the cutoff was 24 weeks. (It’s now 15 weeks in Florida, while Mississippi went from 15 weeks for legal procedures to a ban on nearly all abortions.)
The wait was excruciating. Julia was driving twice a week to New Orleans for specialized care. With work and child care, it was too hard. She quit the teaching job she loved.
One winter night, she felt the fetus move for the first time – ordinarily a milestone, but now, facing a fatal prognosis, she didn’t want to get attached. “It had me so messed up,” she said.
On the way to the amniocentesis, Julia and her husband chose a name. Drawing from a language conjured by J.R.R. Tolkien in the fantasy novels they love, it means “hope.”
More than halfway through her pregnancy, the amnio results arrived. The prenatal screening had given a false positive. The baby would be fine. In May, Julia gave birth to a healthy daughter.
Julia and her husband are upset about the needless anguish brought on by the screening. “They like to have it both ways,” said Julia’s husband. “They say they are 99% accurate, but when there’s a false positive, they say, ‘Well, we’re not diagnostic.’ ”
Believing the prenatal screening was likely accurate, they had seriously considered canceling the amniocentesis, saving their limited funds for an abortion in Florida, hundreds of miles away.
Their dilemma points to a longtime concern: ending pregnancies based on false positives. The FDA cited it as a risk as far back as 2015. Now, those with positive results are facing an even tighter time crunch. They must consider whether waiting for a definitive test, and possibly traveling to another state for an abortion later in pregnancy, is worth it.
In their promotional material, some companies not only sidestep the variability of the standard tests, they fail to distinguish them from the least reliable ones – those for exceptionally rare conditions. They tout the extra screenings as “premium,” “plus,” or “advanced” options.
“Going to greater lengths for the answers that matter most,” says a brochure aimed at health care providers from test-maker Illumina. Elsewhere it states that the “expanded” panel of tests provides “confident results” and “the additional insights you need.”
But the companies themselves know the accuracy of some of their tests has yet to be established in the research. Natera acknowledged in a recent shareholder report that many insurers won’t pay for screenings for missing chromosomal fragments, known as microdeletions, in part because there isn’t enough published data behind them.
The company, responding to ProPublica, stressed the quality of the data over the quantity, saying the research so far has been favorable. “Natera’s microdeletion testing was thoroughly validated with results published in peer-reviewed publications,” it said in a statement.
Natera pointed to a recent study that looked at DiGeorge syndrome, one of several chromosomal anomalies it checks for with its microdeletion screenings. Researchers found the positive predictive value (PPV) of the test to be 52.6%, meaning that nearly half of positive results are false positives. (For many patients, PPVs for more common conditions can exceed 90%.)
Natera said the performance of the diGeorge syndrome test “is excellent and not considered a low PPV,” because of the condition being extremely rare.
Companies also play up the danger of diagnostic tests like amnio. They “can cause miscarriages,” warns the marketing from Labcorp, which made Amanda’s screening, while its test “does not cause miscarriages.” But medical experts emphasize that diagnostic tests, such as amniocentesis, are more accurate and, in fact, carry little risk to the pregnancy.
Labcorp, in a statement, said the company “acknowledges the well-documented risk associated with amniocentesis and CVS in our literature. It is the patient’s prerogative to decide which risks they are willing or unwilling to take.”
Marketing claims also sometimes skate over the nuances in the guidance from the leading professional societies. On a webpage targeting health care providers, for example, a Labcorp chart said groups such as ACOG “endorse and/or recognize” prenatal screenings as an option for all pregnancies. But the chart listed screenings ACOG does not recommend, including trisomy 16.
When asked about it, Labcorp said in a statement that ACOG “endorses NIPS for all pregnancies.” In fact, the guidance is not so sweeping. It says only that the basic bundle of tests should be offered to all, alongside other screening options. It explicitly advises providers to not offer patients the extra tests.
Soon after ProPublica’s query, the Labcorp webpage was updated to remove any mention of the professional societies.
Patients say they often don’t know where to turn for informed and unbiased information. That’s why the r/NIPT Reddit page became such a robust community. Facing difficult news, Julia turned to it for counsel from other prospective parents. Kristina in Texas found the same community. Amanda, too.
‘The margin of error is a human life’
On a warm and cloudy day this past June, on what would have been their daughter’s first birthday, Amanda and her husband visited her grave. They brought a unicorn balloon and vanilla cake, which they ate nearby on the grass. Her husband read a poem.
To them, their baby had been perfect. She had fingers and toes. A thatch of dark hair. While in intensive care, peering up at her parents, she grabbed for her mother’s hand.
Had her condition been known, they would’ve spared her futile medical interventions, as doctors tried to save her life. Their family priest would have been able to baptize her. As it was, they never got to hold their child while she was alive.
These days, when Amanda and her husband say grace before dinner, they give thanks for the 28 hours of their daughter’s life.
They’re also thinking about making comfort boxes the hospital could give to other parents who lose a child. It might include books on grief. Softer tissues. Something that says, as Amanda puts it, “This is to help you get through.”
Amid their grief, they had a prayer answered: Amanda is pregnant again.
It’s frightening to go through this again. She barely sleeps the night before visiting the doctor. It feels like she never stopped being pregnant. It will feel that way, she said, until she brings a baby home – one who lives past the first 2 nights.
Amanda planned to get another genetic screening test. At first she couldn’t bear it, wasn’t sure she could trust it. “The margin of error is a human life,” Amanda said.
The 10-week appointment passed. Then the 12-week appointment. After her 13th week, she took the plunge. The test she was given was from Labcorp.
Around this time, more than a year after Amanda had desperately tried to alert the company about what had happened to her and her first baby, she finally heard back. Labcorp’s vice president of genetic counseling and services reached out – after ProPublica contacted the company and shared Amanda’s story.
The executive would only speak to Amanda without a reporter present.
Amanda said that during the call, the executive told her that prenatal genetic tests are evolving, and doctors should be clear about what the screenings can and cannot do. By the end of the conversation, the executive offered Amanda her cell number.
Amanda said she appreciated the call. “I feel better. I feel like I got something.”
The same day, her screening results came back. They were negative.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Amanda wanted to warn someone. In June 2021, her daughter – the one she and her husband had tried for 3 years to conceive – had died after only 28 hours. With an underdeveloped nose, she had battled for every breath.
Nobody knew why. Later, an autopsy report revealed their daughter had an extra 13th chromosome. The condition is nearly always fatal.
“But didn’t we test for that?” Amanda recalled asking herself. “That was kind of where the light bulb clicked.”
Through her doctor, Amanda had gotten a popular prenatal screening from a lab company. It had come back “negative.”
For three major conditions, including the one her baby had, the report gave the impression of near certainty. The likelihood that she would be born without them was “greater than 99%.”
As she recovered from a cesarean section, Amanda found herself facing a long maternity leave without a child. She shut the door to the empty nursery and began spending what seemed like endless hours of that hazy summer learning about the test.
It’s a simple blood draw designed to check for an array of genetic anomalies. But Amanda, a science researcher, read academic articles showing there was a higher risk of inaccurate results than she had realized. (She asked to be identified by only her first name to protect her privacy.)
On Reddit, she found other women reporting problems with the tests, too. She thought Labcorp, the company that made her test, would want to know about the screening that failed her. Maybe by alerting them, she could help other families. Maybe it would help her understand what happened.
“I was trying to gain answers,” said Amanda, now 32. She tried calling Labcorp’s customer service line, but she said she was passed along from one person to another. “It was just a circle,” she remembered.
She phoned Labcorp a second time. The call ended when an employee hung up on her.
Amanda was baffled. Why didn’t the company seem interested in her experience? Why, she wondered, wouldn’t it want to collect this data? Why wasn’t there someone who could answer her questions about how often this happens, and why?
If she had taken any number of other common commercial tests – including certain tests for COVID-19 or, say, pregnancy – the company would have been required to inform the U.S. Food and Drug Administration about reports of so-called adverse events.
But the test Amanda had falls into a regulatory void. No federal agency checks to make sure these prenatal screenings work the way they claim before they’re sold to health care providers. The FDA doesn’t ensure that marketing claims are backed up by evidence before screenings reach patients. And companies aren’t required to publicly report instances of when the tests get it wrong – sometimes catastrophically.
The broader lab testing industry and its lobbyists have successfully fought for years to keep it this way, cowing regulators into staying on the sidelines.
Worried about a growing variety of tests escaping scrutiny, the FDA was on the cusp of stepping in 6 years ago. But then it backed down.
Peter Lurie, then a top agency official, was at the meetings where the FDA tabled its plans. Not pushing harder, he told ProPublica, “remains one of my greatest regrets.”
The risk of false positives from prenatal screenings, in particular, has been known for years.
In 2014, the New England Center for Investigative Reporting detailed how some companies gave a misleading impression of the precision of the prenatal screenings. Women often didn’t understand they needed diagnostic testing to confirm the results. Some had gotten abortions based on false positive results, the story said. Earlier this year, the New York Times reported how companies sell optional extra screenings that are “usually wrong” when they predict a disorder.
Despite these stories and calls for reform by patient advocates, the government has done little to improve oversight of prenatal screenings. ProPublica set out to examine the forces that led to this inertia and left patients like Amanda feeling misled. Interviews with more than three dozen women revealed ongoing confusion about the screenings – and anger when their reliability proved to be overblown.
“This is a Wild West scenario where everybody is on their own,” said Lawrence Gostin, a Georgetown University, Washington, law professor specializing in bioethics.
The stakes for families are increasing. Upward of half of all pregnant people now receive one of these prenatal screenings. And with many states banning abortions or limiting them to early in pregnancies, the need for fast, accurate information has become more urgent.
The FDA itself acknowledges the problem. In correspondence with ProPublica, a spokesperson cited an “outdated policy” regarding the lack of vetting of many lab tests that the agency has “spent the better part of the last 2 decades trying to address.”
The screening industry, meanwhile, continues to expand, proving lucrative for those who lead it. The chief executive of Natera, which claims about 40% of the market share of prenatal screenings, received a $23 million compensation package last year, the highest of any executive at a publicly traded lab company.
Testing companies told ProPublica that, even without the FDA, there is significant oversight. Labs must abide by state regulations, and another federal agency, the Centers for Medicare and Medicaid Services, is charged with monitoring quality standards. It does not, however, check whether the tests the labs perform are clinically valid.
Companies also said the screenings offer important guidance to expectant families. Echoing others in the field, Labcorp said in a statement that the screenings, when used properly, “provide vital information about the presence of increased risk, but do not provide a definitive diagnosis.” (It declined to discuss the specifics of Amanda’s experience.)
Natera pointed out that its materials tell patients that “this test does not make a final diagnosis.” It reports results as “high-risk” or “low-risk,” not positive or negative.
Companies have stressed that, ultimately, it’s the responsibility of health care providers, who order the tests, to inform patients about the limits of screenings.
For all that, the statistical nuances of the test aren’t easy to parse for patients and even some doctors and nurses. For example, the test for trisomy 13, which doomed Amanda’s baby, is actually less likely to correctly predict the condition than other tests in the standard bundle of screenings offered to every patient.
When ProPublica asked readers to share their experiences with noninvasive prenatal screening tests, often referred to as NIPTs or NIPS, more than a thousand responded. Many said the tests had given them peace of mind. Some said they had provided an early warning about problems.
But others had more questions than answers. None more so than Amanda.
“What are these tests?” she wondered. “And how did mine end up in the margin of error?”
‘They started using it on humans, and then they went back and said: “Was our test accurate?” ’
Scientists have long tried to find ways to help parents and doctors understand what’s happening inside the womb. Amniocentesis was first used to reveal genetic anomalies in the late 1960s. But it didn’t become more popular until it began to be paired with ultrasound to precisely guide the procedure.
In the 1980s, doctors started using chorionic villus sampling, or CVS, an analysis of placental tissue that offers a diagnosis earlier in pregnancy. But, like amniocentesis, it is an invasive test that involves some risk to the fetus, though experts say it’s exceptionally low.
A breakthrough came in the late 1990s, when a scientist recognized that free-floating placental DNA could be detected in the mother’s blood. This meant that the fetus’s chromosomes could be examined by collecting a blood sample as soon as 9 weeks into pregnancy. This also provides an early opportunity to learn the likely fetal sex – a particularly popular feature.
Champions of the new science celebrated the arrival of a simple technique for patients that was particularly precise, at least for some conditions. Many favored it over other noninvasive options. But the industry that developed around NIPT has been marred by controversy from the beginning.
Dr. Ronald Wapner, director of reproductive genetics at Columbia University, described that time as “very chaotic.”
The tests had not been appropriately evaluated in clinical practice, said Dr. Wapner, whose research has sometimes been funded by testing companies. Because of this, he said, the industry “had very incomplete data on how well it worked.”
That didn’t stop the excitement. The chief executive of Sequenom, a biotechnology company that planned to release the first NIPT for Down syndrome, championed the company as the “Google of Molecular Diagnostics.” Its stock price soared.
Then, about 2 months before an expected launch in 2009, Sequenom killed the plan. The company’s research director, it turned out, had manipulated testing data and made misleading claims about how well the screening worked.
The U.S. Securities and Exchange Commission and Federal Bureau of Investigation opened investigations. Top executives were fired, and the research director pleaded guilty to conspiracy to commit securities fraud. Sequenom still managed to commercialize the test in 2011. (Labcorp, which later acquired Sequenom, said it uses a different kind of test.)
Other companies soon debuted their own tests. Still, there was little data on their clinical performance, researchers said.
As Megan Allyse, a bioethicist at the Mayo Clinic, put it, the companies “launched the test, they started using it on humans, and then they went back and said, ‘Was our test accurate?’ ” She also questioned the lack of attention to the ethics of how tests are presented to patients.
Despite missteps by the industry, the FDA didn’t scrutinize the screenings because they were considered lab-developed tests, which means they are created by the same laboratory that conducts them.
In 1976, Congress revamped oversight over medical devices. Since then, the FDA has effectively exempted such “home-brew” tests from key regulatory requirements. The idea was that when, say, a hospital lab wanted to create a simple test for its own patients, it was spared the time, money, and hassle of getting approval from Washington bureaucrats.
Today, lab-developed tests are vastly more numerous and complex. Because they aren’t registered with the federal government, nobody knows how many exist.
The distinction between tests the FDA actively regulates and those they don’t can seem nonsensical. It isn’t based on the complexity of the tests, or how people use them. It’s simply a matter of where the test is made.
The prenatal genetic screening industry took off almost immediately, powered by an army of aggressive sales representatives.
“At the very beginning, obstetricians in practice were being just completely inundated with visits from the sales reps,” said Dr. John Williams, director of reproductive health at Cedars-Sinai Medical Center in Los Angeles. The push left many ob.gyns. and patients thinking the screenings were accurate enough to substitute for diagnostic tests, such as amniocentesis or CVS.
In some cases, sales tactics escalated into lawbreaking.
Former Sequenom executives who exited during the fraud scandal created a new company that became Progenity, which also offered prenatal screening. Shortly after the company went public in 2020, it finalized a $49 million settlement with federal and state governments, where it admitted to falsifying insurance claims and giving kickbacks to physicians and their staff. According to a legal filing, one sales rep spent $65,658 on meals and alcohol for physicians in 1 year.
Now called Biora Therapeutics, the company said in a statement it no longer does any laboratory testing, including prenatal screenings.
Industry revenue continues to grow, but some testing companies are still fighting to make a profit, and competition to survive is fierce. “There’s a multibillion-dollar market, and they all want a piece of it,” said a former Progenity sales rep who quit in disgust after 5 months in 2016.
The rep, who requested anonymity because she continues to work in the field, said she still sees competitors from NIPT companies visiting medical practices “every week, buying breakfast or dinner, or taking them out for happy hour.”
Over time, companies pointed to new peer-reviewed studies, research the industry itself funded, to earn the confidence of doctors and other stakeholders. They showed that two tests – for Down syndrome and trisomy 18 – often performed better than other screening methods.
This research was valid, said Dr. Mary Norton, a perinatologist and clinical geneticist at the University of California, San Francisco, Medical Center’s prenatal diagnostic center. Considered a leading researcher in the field, she was an author of many of these key industry-funded studies.
But, she said, when research findings were presented publicly, the companies sometimes downplayed “inconvenient truths,” such as the exclusion of inconclusive results from accuracy estimates. Crucial caveats were also glossed over by some companies when they translated research into promotional copy aimed at health care providers and patients. Those materials didn’t always mention the many factors that can limit the performance of the screenings, including high body weight, the rarity of the condition tested, and younger maternal age.
Testing companies said they try to help patients understand the screenings through online resources and other materials. Some offer genetic counseling services.
The younger a person is, the lower the test’s positive predictive value – that is, the probability that a positive screening result will turn out to be correct – will be for some conditions. For instance, because Down syndrome is less prevalent in younger people’s pregnancies, a positive screening test is more likely to be a false positive for them.
Kristina was 30 years old in 2016, when her Progenity test came back positive for Down syndrome. She and her husband, who asked not to be fully named to protect their privacy, said they didn’t plan to carry a pregnancy with this condition to term.
But waiting to get an amniocentesis, and then waiting for the results, took 5 agonizing weeks, she said. It showed her son did not have Down syndrome.
Kristina, who lives in Texas, is still troubled by what she describes as a traumatic experience.
“I researched both late-term abortion providers and cemeteries,” she said. They even picked out a burial place, near their house.
She bought a blue baby blanket she intended to bury the baby’s tiny body in. She still has it. Her son, now 5, sleeps with it every night.
‘I can’t believe I didn’t say more’
As lab-developed tests became a bigger business, moving well past their home-brew origins, regulators looked for a way to assert oversight. In 2014, after years of study and debate, the time seemed right.
The FDA released plans proposing to regulate the tests, prioritizing those used to make major medical decisions. The agency has pointed to NIPTs as 1 of 20 concerning tests.
But, over the next 2 years, a coalition of power players urged the FDA to back off. Professional associations issued statements and hosted webinars devoted to the issue. Some created polished websites featuring sample letters to send to Washington.
Academic medical centers and pathology departments joined the fight, too. Scientists from 23 of them put it bluntly in a letter to the Office of Management and Budget: “FDA regulation of LDTs would be contrary to the public health,” it said, using a common acronym for the tests.
“Critical testing would be unavailable in the ‘lag time’ between development of new tests and FDA authorizing them,” the authors of the letter wrote, “and subsequent improvements on existing tests would slow significantly under the rigid, inflexible, and duplicative FDA regulatory scheme.”
This could delay essential care for patients. What’s more, opponents argued, existing lab reviews by the Centers for Medicare and Medicaid Services are sufficiently rigorous. Some have suggested modernizing the CMS review process to improve oversight.
An FDA spokesperson told ProPublica that the agency encountered “continued, negative feedback,” including a 25-page paper written by two legal heavyweights hired by the American Clinical Laboratory Association: Paul Clement, President George W. Bush’s former solicitor general, and Laurence Tribe, law professor at Harvard University.
Mr. Clement has reportedly commanded rates of $1,350 per hour. He and Mr. Tribe did not respond to ProPublica’s queries about their work.
Their brief argued that the FDA “lacked legal authority” to regulate lab-developed tests because they are properly seen as the practice of medicine: a service, rather than a product.
However, as lawyers representing the American Association of Bioanalysts countered, the FDA would vet tests before they reach the market, not control how doctors use them. The government proposal, they wrote, is “similar to imposing requirements to screen blood or label drugs.”
After the election of President Donald Trump, but before he took office, a handful of FDA officials discussed their battered proposal. It had represented a breakthrough in the decades of excruciating back-and-forth with industry. But now, with an incoming administration bent on deregulation, their efforts seemed futile.
The regulators feared anything they enacted would be undone by Congress – and, under the Congressional Review Act, they might not be able to reissue anything “substantially similar” in the future. So the FDA published a white paper instead, summarizing the issue “for further public discussion.”
After the meeting where officials made this call, Mr. Lurie, then the FDA’s associate commissioner, recalled a colleague approaching him: “I can’t believe you didn’t say more.”
“And I was like, ‘Yeah, actually, I can’t believe I didn’t say more either,’ ” Mr. Lurie later told ProPublica. (After leaving the agency, Mr. Lurie went on to lead the Center for Science in the Public Interest, a consumer advocacy nonprofit, which has pushed the FDA to finally assert oversight over lab-developed tests.)
Nancy Stade, an attorney and senior policy official who left the FDA in 2015, said the agency often moves slowly as it seeks to get buy-in from industry and professional groups. In her work on regulatory policy, she saw it happen with lab-developed tests.
The agency is “always testing the waters,” she said, “and always coming out with something a little bit softer.”
In 2020, the influential American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine, representing doctors who handle pregnancies, gave the screening industry another huge boost.
In a bulletin updating their advice on the tests, the two groups described growing research on the performance of some of the standard tests and said people have the right to information about their pregnancies, so the tests should be offered to all patients. Previously, they recommended this only for those facing higher risk of genetic anomalies.
The bulletin said the coauthors had disclosed no conflicts of interest. But two of the four coauthors, including Mary Norton, had disclosed in prior publications that test-makers had provided funding for their research. A company had provided a third coauthor with laboratory services needed to run tests, according to that researcher, a connection she also disclosed in past papers.
ACOG, in a statement to ProPublica, said the organization “identified no conflicts because research funding is provided to academic institutions with institutional review boards, not to individual investigators.” Two of the three researchers responded to questions from ProPublica and said they maintained independence over their work.
One test-maker, Illumina, celebrated the ACOG guidance in a tweet, saying it “recognizes the superior performance of #NIPT and the benefit it provides expectant families.” Natera’s share prices doubled in 5 months. UnitedHealthcare, the nation’s largest private insurer and long a target of industry lobbying, told ProPublica it changed its stance to cover screenings for all patients, regardless of risk, because of the recommendation.
In a recent shareholder report, Natera stated that prenatal genetic and carrier screenings “represent the significant majority of our revenues,” which totaled $625.5 million in 2021. The company expects more growth to come.
“The NIPT market is still very underpenetrated, compared to the 4 to 5 million pregnancies in the U.S.,” Natera’s chief executive said on a 2021 earnings call, “so there’s a long way to go.”
But even Dr. Norton, who coauthored the ACOG recommendation and favors NIPTs for patients 40 and over, has concerns about screenings becoming widespread among those who are younger. In most cases, she prefers other screening methods that catch the nongenetic problems younger moms are more likely to face. Negative results from an NIPT, she said, can be “falsely reassuring.”
In the years after the FDA set aside its regulatory proposal, the agency has assisted members of Congress on a proposed legislative solution. That effort, dubbed the VALID Act, aims to end any debate over the agency’s authority over lab-developed tests. An FDA press officer said the legislation would ensure the prenatal screening tests and others are “accurate and reliable.”
But, as in the past, intense lobbying followed the proposal. The VALID Act was a rider to a funding reauthorization bill, but in September the House and Senate agreed to remove it. Advocates now hope to attach it to proposed end-of-year legislation.
Meanwhile, earlier this year, 4 months after the New York Times story on the usefulness of some screenings, the FDA took a step toward more public awareness about prenatal genetic screening. It issued its first safety communication on them, noting the potential for false results.
It cautioned patients about making “critical health care decisions based on results from these screening tests alone.”
Cara Tenenbaum, a former FDA policy advisor, was pleased to see the statement. Still, she said, it was long overdue.
“This has been known – known, or should have been known – for 10 years,” she said.
‘It had me so messed up’
With the demise of Roe v. Wade, restrictive and ever-changing abortion laws can pressure people to act quickly with limited information, heightening the stakes of prenatal screening.
Julia, a mom from Mississippi’s Gulf Coast, knows what it’s like to face harrowing consequences while navigating state-imposed time limits – and doing so with little guidance. Last fall, she was pregnant with her fourth child when, she said, a nurse practitioner suggested prenatal genetic screening.
At 33, Julia had no risk factors. Her previous pregnancies hadn’t been screened with an NIPT. But with three sons and 18 nephews, she and her husband were curious about the baby’s sex. And the screening seemed like it had no downside.
Julia figured it would only be offered if it was reliable, so her nurse practitioner ordered her both the basic bundle of screenings and the extra tests. (The medical practice didn’t respond to interview requests. Julia is a family nickname that’s used here to protect her privacy.)
The screenings showed the baby was a girl – but the extra tests also detected trisomy 16, a condition caused by an extra chromosome that is so rare, the nurse didn’t know what it was, Julia recalled.
The nurse borrowed Julia’s phone, using it to search online and read aloud what she found. Julia was stunned to hear trisomy 16 was incompatible with life.
“I was utterly devastated,” she said. “I made it out of my doctor’s office but completely broke down in the car.”
But ACOG does not recommend the trisomy 16 screening, saying “its accuracy with regard to detection and the false-positive rate is not established.” Julia wasn’t informed of this, she said, and she’s not sure if her health care providers knew it either.
The lab report recommended diagnostic testing to confirm the results, but time was short. She had her amniocentesis at 17 weeks. It could take up to 4 more weeks to receive results.
That would be too late for a legal abortion in Mississippi. So she made an appointment for one in Florida, where the cutoff was 24 weeks. (It’s now 15 weeks in Florida, while Mississippi went from 15 weeks for legal procedures to a ban on nearly all abortions.)
The wait was excruciating. Julia was driving twice a week to New Orleans for specialized care. With work and child care, it was too hard. She quit the teaching job she loved.
One winter night, she felt the fetus move for the first time – ordinarily a milestone, but now, facing a fatal prognosis, she didn’t want to get attached. “It had me so messed up,” she said.
On the way to the amniocentesis, Julia and her husband chose a name. Drawing from a language conjured by J.R.R. Tolkien in the fantasy novels they love, it means “hope.”
More than halfway through her pregnancy, the amnio results arrived. The prenatal screening had given a false positive. The baby would be fine. In May, Julia gave birth to a healthy daughter.
Julia and her husband are upset about the needless anguish brought on by the screening. “They like to have it both ways,” said Julia’s husband. “They say they are 99% accurate, but when there’s a false positive, they say, ‘Well, we’re not diagnostic.’ ”
Believing the prenatal screening was likely accurate, they had seriously considered canceling the amniocentesis, saving their limited funds for an abortion in Florida, hundreds of miles away.
Their dilemma points to a longtime concern: ending pregnancies based on false positives. The FDA cited it as a risk as far back as 2015. Now, those with positive results are facing an even tighter time crunch. They must consider whether waiting for a definitive test, and possibly traveling to another state for an abortion later in pregnancy, is worth it.
In their promotional material, some companies not only sidestep the variability of the standard tests, they fail to distinguish them from the least reliable ones – those for exceptionally rare conditions. They tout the extra screenings as “premium,” “plus,” or “advanced” options.
“Going to greater lengths for the answers that matter most,” says a brochure aimed at health care providers from test-maker Illumina. Elsewhere it states that the “expanded” panel of tests provides “confident results” and “the additional insights you need.”
But the companies themselves know the accuracy of some of their tests has yet to be established in the research. Natera acknowledged in a recent shareholder report that many insurers won’t pay for screenings for missing chromosomal fragments, known as microdeletions, in part because there isn’t enough published data behind them.
The company, responding to ProPublica, stressed the quality of the data over the quantity, saying the research so far has been favorable. “Natera’s microdeletion testing was thoroughly validated with results published in peer-reviewed publications,” it said in a statement.
Natera pointed to a recent study that looked at DiGeorge syndrome, one of several chromosomal anomalies it checks for with its microdeletion screenings. Researchers found the positive predictive value (PPV) of the test to be 52.6%, meaning that nearly half of positive results are false positives. (For many patients, PPVs for more common conditions can exceed 90%.)
Natera said the performance of the diGeorge syndrome test “is excellent and not considered a low PPV,” because of the condition being extremely rare.
Companies also play up the danger of diagnostic tests like amnio. They “can cause miscarriages,” warns the marketing from Labcorp, which made Amanda’s screening, while its test “does not cause miscarriages.” But medical experts emphasize that diagnostic tests, such as amniocentesis, are more accurate and, in fact, carry little risk to the pregnancy.
Labcorp, in a statement, said the company “acknowledges the well-documented risk associated with amniocentesis and CVS in our literature. It is the patient’s prerogative to decide which risks they are willing or unwilling to take.”
Marketing claims also sometimes skate over the nuances in the guidance from the leading professional societies. On a webpage targeting health care providers, for example, a Labcorp chart said groups such as ACOG “endorse and/or recognize” prenatal screenings as an option for all pregnancies. But the chart listed screenings ACOG does not recommend, including trisomy 16.
When asked about it, Labcorp said in a statement that ACOG “endorses NIPS for all pregnancies.” In fact, the guidance is not so sweeping. It says only that the basic bundle of tests should be offered to all, alongside other screening options. It explicitly advises providers to not offer patients the extra tests.
Soon after ProPublica’s query, the Labcorp webpage was updated to remove any mention of the professional societies.
Patients say they often don’t know where to turn for informed and unbiased information. That’s why the r/NIPT Reddit page became such a robust community. Facing difficult news, Julia turned to it for counsel from other prospective parents. Kristina in Texas found the same community. Amanda, too.
‘The margin of error is a human life’
On a warm and cloudy day this past June, on what would have been their daughter’s first birthday, Amanda and her husband visited her grave. They brought a unicorn balloon and vanilla cake, which they ate nearby on the grass. Her husband read a poem.
To them, their baby had been perfect. She had fingers and toes. A thatch of dark hair. While in intensive care, peering up at her parents, she grabbed for her mother’s hand.
Had her condition been known, they would’ve spared her futile medical interventions, as doctors tried to save her life. Their family priest would have been able to baptize her. As it was, they never got to hold their child while she was alive.
These days, when Amanda and her husband say grace before dinner, they give thanks for the 28 hours of their daughter’s life.
They’re also thinking about making comfort boxes the hospital could give to other parents who lose a child. It might include books on grief. Softer tissues. Something that says, as Amanda puts it, “This is to help you get through.”
Amid their grief, they had a prayer answered: Amanda is pregnant again.
It’s frightening to go through this again. She barely sleeps the night before visiting the doctor. It feels like she never stopped being pregnant. It will feel that way, she said, until she brings a baby home – one who lives past the first 2 nights.
Amanda planned to get another genetic screening test. At first she couldn’t bear it, wasn’t sure she could trust it. “The margin of error is a human life,” Amanda said.
The 10-week appointment passed. Then the 12-week appointment. After her 13th week, she took the plunge. The test she was given was from Labcorp.
Around this time, more than a year after Amanda had desperately tried to alert the company about what had happened to her and her first baby, she finally heard back. Labcorp’s vice president of genetic counseling and services reached out – after ProPublica contacted the company and shared Amanda’s story.
The executive would only speak to Amanda without a reporter present.
Amanda said that during the call, the executive told her that prenatal genetic tests are evolving, and doctors should be clear about what the screenings can and cannot do. By the end of the conversation, the executive offered Amanda her cell number.
Amanda said she appreciated the call. “I feel better. I feel like I got something.”
The same day, her screening results came back. They were negative.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Amanda wanted to warn someone. In June 2021, her daughter – the one she and her husband had tried for 3 years to conceive – had died after only 28 hours. With an underdeveloped nose, she had battled for every breath.
Nobody knew why. Later, an autopsy report revealed their daughter had an extra 13th chromosome. The condition is nearly always fatal.
“But didn’t we test for that?” Amanda recalled asking herself. “That was kind of where the light bulb clicked.”
Through her doctor, Amanda had gotten a popular prenatal screening from a lab company. It had come back “negative.”
For three major conditions, including the one her baby had, the report gave the impression of near certainty. The likelihood that she would be born without them was “greater than 99%.”
As she recovered from a cesarean section, Amanda found herself facing a long maternity leave without a child. She shut the door to the empty nursery and began spending what seemed like endless hours of that hazy summer learning about the test.
It’s a simple blood draw designed to check for an array of genetic anomalies. But Amanda, a science researcher, read academic articles showing there was a higher risk of inaccurate results than she had realized. (She asked to be identified by only her first name to protect her privacy.)
On Reddit, she found other women reporting problems with the tests, too. She thought Labcorp, the company that made her test, would want to know about the screening that failed her. Maybe by alerting them, she could help other families. Maybe it would help her understand what happened.
“I was trying to gain answers,” said Amanda, now 32. She tried calling Labcorp’s customer service line, but she said she was passed along from one person to another. “It was just a circle,” she remembered.
She phoned Labcorp a second time. The call ended when an employee hung up on her.
Amanda was baffled. Why didn’t the company seem interested in her experience? Why, she wondered, wouldn’t it want to collect this data? Why wasn’t there someone who could answer her questions about how often this happens, and why?
If she had taken any number of other common commercial tests – including certain tests for COVID-19 or, say, pregnancy – the company would have been required to inform the U.S. Food and Drug Administration about reports of so-called adverse events.
But the test Amanda had falls into a regulatory void. No federal agency checks to make sure these prenatal screenings work the way they claim before they’re sold to health care providers. The FDA doesn’t ensure that marketing claims are backed up by evidence before screenings reach patients. And companies aren’t required to publicly report instances of when the tests get it wrong – sometimes catastrophically.
The broader lab testing industry and its lobbyists have successfully fought for years to keep it this way, cowing regulators into staying on the sidelines.
Worried about a growing variety of tests escaping scrutiny, the FDA was on the cusp of stepping in 6 years ago. But then it backed down.
Peter Lurie, then a top agency official, was at the meetings where the FDA tabled its plans. Not pushing harder, he told ProPublica, “remains one of my greatest regrets.”
The risk of false positives from prenatal screenings, in particular, has been known for years.
In 2014, the New England Center for Investigative Reporting detailed how some companies gave a misleading impression of the precision of the prenatal screenings. Women often didn’t understand they needed diagnostic testing to confirm the results. Some had gotten abortions based on false positive results, the story said. Earlier this year, the New York Times reported how companies sell optional extra screenings that are “usually wrong” when they predict a disorder.
Despite these stories and calls for reform by patient advocates, the government has done little to improve oversight of prenatal screenings. ProPublica set out to examine the forces that led to this inertia and left patients like Amanda feeling misled. Interviews with more than three dozen women revealed ongoing confusion about the screenings – and anger when their reliability proved to be overblown.
“This is a Wild West scenario where everybody is on their own,” said Lawrence Gostin, a Georgetown University, Washington, law professor specializing in bioethics.
The stakes for families are increasing. Upward of half of all pregnant people now receive one of these prenatal screenings. And with many states banning abortions or limiting them to early in pregnancies, the need for fast, accurate information has become more urgent.
The FDA itself acknowledges the problem. In correspondence with ProPublica, a spokesperson cited an “outdated policy” regarding the lack of vetting of many lab tests that the agency has “spent the better part of the last 2 decades trying to address.”
The screening industry, meanwhile, continues to expand, proving lucrative for those who lead it. The chief executive of Natera, which claims about 40% of the market share of prenatal screenings, received a $23 million compensation package last year, the highest of any executive at a publicly traded lab company.
Testing companies told ProPublica that, even without the FDA, there is significant oversight. Labs must abide by state regulations, and another federal agency, the Centers for Medicare and Medicaid Services, is charged with monitoring quality standards. It does not, however, check whether the tests the labs perform are clinically valid.
Companies also said the screenings offer important guidance to expectant families. Echoing others in the field, Labcorp said in a statement that the screenings, when used properly, “provide vital information about the presence of increased risk, but do not provide a definitive diagnosis.” (It declined to discuss the specifics of Amanda’s experience.)
Natera pointed out that its materials tell patients that “this test does not make a final diagnosis.” It reports results as “high-risk” or “low-risk,” not positive or negative.
Companies have stressed that, ultimately, it’s the responsibility of health care providers, who order the tests, to inform patients about the limits of screenings.
For all that, the statistical nuances of the test aren’t easy to parse for patients and even some doctors and nurses. For example, the test for trisomy 13, which doomed Amanda’s baby, is actually less likely to correctly predict the condition than other tests in the standard bundle of screenings offered to every patient.
When ProPublica asked readers to share their experiences with noninvasive prenatal screening tests, often referred to as NIPTs or NIPS, more than a thousand responded. Many said the tests had given them peace of mind. Some said they had provided an early warning about problems.
But others had more questions than answers. None more so than Amanda.
“What are these tests?” she wondered. “And how did mine end up in the margin of error?”
‘They started using it on humans, and then they went back and said: “Was our test accurate?” ’
Scientists have long tried to find ways to help parents and doctors understand what’s happening inside the womb. Amniocentesis was first used to reveal genetic anomalies in the late 1960s. But it didn’t become more popular until it began to be paired with ultrasound to precisely guide the procedure.
In the 1980s, doctors started using chorionic villus sampling, or CVS, an analysis of placental tissue that offers a diagnosis earlier in pregnancy. But, like amniocentesis, it is an invasive test that involves some risk to the fetus, though experts say it’s exceptionally low.
A breakthrough came in the late 1990s, when a scientist recognized that free-floating placental DNA could be detected in the mother’s blood. This meant that the fetus’s chromosomes could be examined by collecting a blood sample as soon as 9 weeks into pregnancy. This also provides an early opportunity to learn the likely fetal sex – a particularly popular feature.
Champions of the new science celebrated the arrival of a simple technique for patients that was particularly precise, at least for some conditions. Many favored it over other noninvasive options. But the industry that developed around NIPT has been marred by controversy from the beginning.
Dr. Ronald Wapner, director of reproductive genetics at Columbia University, described that time as “very chaotic.”
The tests had not been appropriately evaluated in clinical practice, said Dr. Wapner, whose research has sometimes been funded by testing companies. Because of this, he said, the industry “had very incomplete data on how well it worked.”
That didn’t stop the excitement. The chief executive of Sequenom, a biotechnology company that planned to release the first NIPT for Down syndrome, championed the company as the “Google of Molecular Diagnostics.” Its stock price soared.
Then, about 2 months before an expected launch in 2009, Sequenom killed the plan. The company’s research director, it turned out, had manipulated testing data and made misleading claims about how well the screening worked.
The U.S. Securities and Exchange Commission and Federal Bureau of Investigation opened investigations. Top executives were fired, and the research director pleaded guilty to conspiracy to commit securities fraud. Sequenom still managed to commercialize the test in 2011. (Labcorp, which later acquired Sequenom, said it uses a different kind of test.)
Other companies soon debuted their own tests. Still, there was little data on their clinical performance, researchers said.
As Megan Allyse, a bioethicist at the Mayo Clinic, put it, the companies “launched the test, they started using it on humans, and then they went back and said, ‘Was our test accurate?’ ” She also questioned the lack of attention to the ethics of how tests are presented to patients.
Despite missteps by the industry, the FDA didn’t scrutinize the screenings because they were considered lab-developed tests, which means they are created by the same laboratory that conducts them.
In 1976, Congress revamped oversight over medical devices. Since then, the FDA has effectively exempted such “home-brew” tests from key regulatory requirements. The idea was that when, say, a hospital lab wanted to create a simple test for its own patients, it was spared the time, money, and hassle of getting approval from Washington bureaucrats.
Today, lab-developed tests are vastly more numerous and complex. Because they aren’t registered with the federal government, nobody knows how many exist.
The distinction between tests the FDA actively regulates and those they don’t can seem nonsensical. It isn’t based on the complexity of the tests, or how people use them. It’s simply a matter of where the test is made.
The prenatal genetic screening industry took off almost immediately, powered by an army of aggressive sales representatives.
“At the very beginning, obstetricians in practice were being just completely inundated with visits from the sales reps,” said Dr. John Williams, director of reproductive health at Cedars-Sinai Medical Center in Los Angeles. The push left many ob.gyns. and patients thinking the screenings were accurate enough to substitute for diagnostic tests, such as amniocentesis or CVS.
In some cases, sales tactics escalated into lawbreaking.
Former Sequenom executives who exited during the fraud scandal created a new company that became Progenity, which also offered prenatal screening. Shortly after the company went public in 2020, it finalized a $49 million settlement with federal and state governments, where it admitted to falsifying insurance claims and giving kickbacks to physicians and their staff. According to a legal filing, one sales rep spent $65,658 on meals and alcohol for physicians in 1 year.
Now called Biora Therapeutics, the company said in a statement it no longer does any laboratory testing, including prenatal screenings.
Industry revenue continues to grow, but some testing companies are still fighting to make a profit, and competition to survive is fierce. “There’s a multibillion-dollar market, and they all want a piece of it,” said a former Progenity sales rep who quit in disgust after 5 months in 2016.
The rep, who requested anonymity because she continues to work in the field, said she still sees competitors from NIPT companies visiting medical practices “every week, buying breakfast or dinner, or taking them out for happy hour.”
Over time, companies pointed to new peer-reviewed studies, research the industry itself funded, to earn the confidence of doctors and other stakeholders. They showed that two tests – for Down syndrome and trisomy 18 – often performed better than other screening methods.
This research was valid, said Dr. Mary Norton, a perinatologist and clinical geneticist at the University of California, San Francisco, Medical Center’s prenatal diagnostic center. Considered a leading researcher in the field, she was an author of many of these key industry-funded studies.
But, she said, when research findings were presented publicly, the companies sometimes downplayed “inconvenient truths,” such as the exclusion of inconclusive results from accuracy estimates. Crucial caveats were also glossed over by some companies when they translated research into promotional copy aimed at health care providers and patients. Those materials didn’t always mention the many factors that can limit the performance of the screenings, including high body weight, the rarity of the condition tested, and younger maternal age.
Testing companies said they try to help patients understand the screenings through online resources and other materials. Some offer genetic counseling services.
The younger a person is, the lower the test’s positive predictive value – that is, the probability that a positive screening result will turn out to be correct – will be for some conditions. For instance, because Down syndrome is less prevalent in younger people’s pregnancies, a positive screening test is more likely to be a false positive for them.
Kristina was 30 years old in 2016, when her Progenity test came back positive for Down syndrome. She and her husband, who asked not to be fully named to protect their privacy, said they didn’t plan to carry a pregnancy with this condition to term.
But waiting to get an amniocentesis, and then waiting for the results, took 5 agonizing weeks, she said. It showed her son did not have Down syndrome.
Kristina, who lives in Texas, is still troubled by what she describes as a traumatic experience.
“I researched both late-term abortion providers and cemeteries,” she said. They even picked out a burial place, near their house.
She bought a blue baby blanket she intended to bury the baby’s tiny body in. She still has it. Her son, now 5, sleeps with it every night.
‘I can’t believe I didn’t say more’
As lab-developed tests became a bigger business, moving well past their home-brew origins, regulators looked for a way to assert oversight. In 2014, after years of study and debate, the time seemed right.
The FDA released plans proposing to regulate the tests, prioritizing those used to make major medical decisions. The agency has pointed to NIPTs as 1 of 20 concerning tests.
But, over the next 2 years, a coalition of power players urged the FDA to back off. Professional associations issued statements and hosted webinars devoted to the issue. Some created polished websites featuring sample letters to send to Washington.
Academic medical centers and pathology departments joined the fight, too. Scientists from 23 of them put it bluntly in a letter to the Office of Management and Budget: “FDA regulation of LDTs would be contrary to the public health,” it said, using a common acronym for the tests.
“Critical testing would be unavailable in the ‘lag time’ between development of new tests and FDA authorizing them,” the authors of the letter wrote, “and subsequent improvements on existing tests would slow significantly under the rigid, inflexible, and duplicative FDA regulatory scheme.”
This could delay essential care for patients. What’s more, opponents argued, existing lab reviews by the Centers for Medicare and Medicaid Services are sufficiently rigorous. Some have suggested modernizing the CMS review process to improve oversight.
An FDA spokesperson told ProPublica that the agency encountered “continued, negative feedback,” including a 25-page paper written by two legal heavyweights hired by the American Clinical Laboratory Association: Paul Clement, President George W. Bush’s former solicitor general, and Laurence Tribe, law professor at Harvard University.
Mr. Clement has reportedly commanded rates of $1,350 per hour. He and Mr. Tribe did not respond to ProPublica’s queries about their work.
Their brief argued that the FDA “lacked legal authority” to regulate lab-developed tests because they are properly seen as the practice of medicine: a service, rather than a product.
However, as lawyers representing the American Association of Bioanalysts countered, the FDA would vet tests before they reach the market, not control how doctors use them. The government proposal, they wrote, is “similar to imposing requirements to screen blood or label drugs.”
After the election of President Donald Trump, but before he took office, a handful of FDA officials discussed their battered proposal. It had represented a breakthrough in the decades of excruciating back-and-forth with industry. But now, with an incoming administration bent on deregulation, their efforts seemed futile.
The regulators feared anything they enacted would be undone by Congress – and, under the Congressional Review Act, they might not be able to reissue anything “substantially similar” in the future. So the FDA published a white paper instead, summarizing the issue “for further public discussion.”
After the meeting where officials made this call, Mr. Lurie, then the FDA’s associate commissioner, recalled a colleague approaching him: “I can’t believe you didn’t say more.”
“And I was like, ‘Yeah, actually, I can’t believe I didn’t say more either,’ ” Mr. Lurie later told ProPublica. (After leaving the agency, Mr. Lurie went on to lead the Center for Science in the Public Interest, a consumer advocacy nonprofit, which has pushed the FDA to finally assert oversight over lab-developed tests.)
Nancy Stade, an attorney and senior policy official who left the FDA in 2015, said the agency often moves slowly as it seeks to get buy-in from industry and professional groups. In her work on regulatory policy, she saw it happen with lab-developed tests.
The agency is “always testing the waters,” she said, “and always coming out with something a little bit softer.”
In 2020, the influential American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine, representing doctors who handle pregnancies, gave the screening industry another huge boost.
In a bulletin updating their advice on the tests, the two groups described growing research on the performance of some of the standard tests and said people have the right to information about their pregnancies, so the tests should be offered to all patients. Previously, they recommended this only for those facing higher risk of genetic anomalies.
The bulletin said the coauthors had disclosed no conflicts of interest. But two of the four coauthors, including Mary Norton, had disclosed in prior publications that test-makers had provided funding for their research. A company had provided a third coauthor with laboratory services needed to run tests, according to that researcher, a connection she also disclosed in past papers.
ACOG, in a statement to ProPublica, said the organization “identified no conflicts because research funding is provided to academic institutions with institutional review boards, not to individual investigators.” Two of the three researchers responded to questions from ProPublica and said they maintained independence over their work.
One test-maker, Illumina, celebrated the ACOG guidance in a tweet, saying it “recognizes the superior performance of #NIPT and the benefit it provides expectant families.” Natera’s share prices doubled in 5 months. UnitedHealthcare, the nation’s largest private insurer and long a target of industry lobbying, told ProPublica it changed its stance to cover screenings for all patients, regardless of risk, because of the recommendation.
In a recent shareholder report, Natera stated that prenatal genetic and carrier screenings “represent the significant majority of our revenues,” which totaled $625.5 million in 2021. The company expects more growth to come.
“The NIPT market is still very underpenetrated, compared to the 4 to 5 million pregnancies in the U.S.,” Natera’s chief executive said on a 2021 earnings call, “so there’s a long way to go.”
But even Dr. Norton, who coauthored the ACOG recommendation and favors NIPTs for patients 40 and over, has concerns about screenings becoming widespread among those who are younger. In most cases, she prefers other screening methods that catch the nongenetic problems younger moms are more likely to face. Negative results from an NIPT, she said, can be “falsely reassuring.”
In the years after the FDA set aside its regulatory proposal, the agency has assisted members of Congress on a proposed legislative solution. That effort, dubbed the VALID Act, aims to end any debate over the agency’s authority over lab-developed tests. An FDA press officer said the legislation would ensure the prenatal screening tests and others are “accurate and reliable.”
But, as in the past, intense lobbying followed the proposal. The VALID Act was a rider to a funding reauthorization bill, but in September the House and Senate agreed to remove it. Advocates now hope to attach it to proposed end-of-year legislation.
Meanwhile, earlier this year, 4 months after the New York Times story on the usefulness of some screenings, the FDA took a step toward more public awareness about prenatal genetic screening. It issued its first safety communication on them, noting the potential for false results.
It cautioned patients about making “critical health care decisions based on results from these screening tests alone.”
Cara Tenenbaum, a former FDA policy advisor, was pleased to see the statement. Still, she said, it was long overdue.
“This has been known – known, or should have been known – for 10 years,” she said.
‘It had me so messed up’
With the demise of Roe v. Wade, restrictive and ever-changing abortion laws can pressure people to act quickly with limited information, heightening the stakes of prenatal screening.
Julia, a mom from Mississippi’s Gulf Coast, knows what it’s like to face harrowing consequences while navigating state-imposed time limits – and doing so with little guidance. Last fall, she was pregnant with her fourth child when, she said, a nurse practitioner suggested prenatal genetic screening.
At 33, Julia had no risk factors. Her previous pregnancies hadn’t been screened with an NIPT. But with three sons and 18 nephews, she and her husband were curious about the baby’s sex. And the screening seemed like it had no downside.
Julia figured it would only be offered if it was reliable, so her nurse practitioner ordered her both the basic bundle of screenings and the extra tests. (The medical practice didn’t respond to interview requests. Julia is a family nickname that’s used here to protect her privacy.)
The screenings showed the baby was a girl – but the extra tests also detected trisomy 16, a condition caused by an extra chromosome that is so rare, the nurse didn’t know what it was, Julia recalled.
The nurse borrowed Julia’s phone, using it to search online and read aloud what she found. Julia was stunned to hear trisomy 16 was incompatible with life.
“I was utterly devastated,” she said. “I made it out of my doctor’s office but completely broke down in the car.”
But ACOG does not recommend the trisomy 16 screening, saying “its accuracy with regard to detection and the false-positive rate is not established.” Julia wasn’t informed of this, she said, and she’s not sure if her health care providers knew it either.
The lab report recommended diagnostic testing to confirm the results, but time was short. She had her amniocentesis at 17 weeks. It could take up to 4 more weeks to receive results.
That would be too late for a legal abortion in Mississippi. So she made an appointment for one in Florida, where the cutoff was 24 weeks. (It’s now 15 weeks in Florida, while Mississippi went from 15 weeks for legal procedures to a ban on nearly all abortions.)
The wait was excruciating. Julia was driving twice a week to New Orleans for specialized care. With work and child care, it was too hard. She quit the teaching job she loved.
One winter night, she felt the fetus move for the first time – ordinarily a milestone, but now, facing a fatal prognosis, she didn’t want to get attached. “It had me so messed up,” she said.
On the way to the amniocentesis, Julia and her husband chose a name. Drawing from a language conjured by J.R.R. Tolkien in the fantasy novels they love, it means “hope.”
More than halfway through her pregnancy, the amnio results arrived. The prenatal screening had given a false positive. The baby would be fine. In May, Julia gave birth to a healthy daughter.
Julia and her husband are upset about the needless anguish brought on by the screening. “They like to have it both ways,” said Julia’s husband. “They say they are 99% accurate, but when there’s a false positive, they say, ‘Well, we’re not diagnostic.’ ”
Believing the prenatal screening was likely accurate, they had seriously considered canceling the amniocentesis, saving their limited funds for an abortion in Florida, hundreds of miles away.
Their dilemma points to a longtime concern: ending pregnancies based on false positives. The FDA cited it as a risk as far back as 2015. Now, those with positive results are facing an even tighter time crunch. They must consider whether waiting for a definitive test, and possibly traveling to another state for an abortion later in pregnancy, is worth it.
In their promotional material, some companies not only sidestep the variability of the standard tests, they fail to distinguish them from the least reliable ones – those for exceptionally rare conditions. They tout the extra screenings as “premium,” “plus,” or “advanced” options.
“Going to greater lengths for the answers that matter most,” says a brochure aimed at health care providers from test-maker Illumina. Elsewhere it states that the “expanded” panel of tests provides “confident results” and “the additional insights you need.”
But the companies themselves know the accuracy of some of their tests has yet to be established in the research. Natera acknowledged in a recent shareholder report that many insurers won’t pay for screenings for missing chromosomal fragments, known as microdeletions, in part because there isn’t enough published data behind them.
The company, responding to ProPublica, stressed the quality of the data over the quantity, saying the research so far has been favorable. “Natera’s microdeletion testing was thoroughly validated with results published in peer-reviewed publications,” it said in a statement.
Natera pointed to a recent study that looked at DiGeorge syndrome, one of several chromosomal anomalies it checks for with its microdeletion screenings. Researchers found the positive predictive value (PPV) of the test to be 52.6%, meaning that nearly half of positive results are false positives. (For many patients, PPVs for more common conditions can exceed 90%.)
Natera said the performance of the diGeorge syndrome test “is excellent and not considered a low PPV,” because of the condition being extremely rare.
Companies also play up the danger of diagnostic tests like amnio. They “can cause miscarriages,” warns the marketing from Labcorp, which made Amanda’s screening, while its test “does not cause miscarriages.” But medical experts emphasize that diagnostic tests, such as amniocentesis, are more accurate and, in fact, carry little risk to the pregnancy.
Labcorp, in a statement, said the company “acknowledges the well-documented risk associated with amniocentesis and CVS in our literature. It is the patient’s prerogative to decide which risks they are willing or unwilling to take.”
Marketing claims also sometimes skate over the nuances in the guidance from the leading professional societies. On a webpage targeting health care providers, for example, a Labcorp chart said groups such as ACOG “endorse and/or recognize” prenatal screenings as an option for all pregnancies. But the chart listed screenings ACOG does not recommend, including trisomy 16.
When asked about it, Labcorp said in a statement that ACOG “endorses NIPS for all pregnancies.” In fact, the guidance is not so sweeping. It says only that the basic bundle of tests should be offered to all, alongside other screening options. It explicitly advises providers to not offer patients the extra tests.
Soon after ProPublica’s query, the Labcorp webpage was updated to remove any mention of the professional societies.
Patients say they often don’t know where to turn for informed and unbiased information. That’s why the r/NIPT Reddit page became such a robust community. Facing difficult news, Julia turned to it for counsel from other prospective parents. Kristina in Texas found the same community. Amanda, too.
‘The margin of error is a human life’
On a warm and cloudy day this past June, on what would have been their daughter’s first birthday, Amanda and her husband visited her grave. They brought a unicorn balloon and vanilla cake, which they ate nearby on the grass. Her husband read a poem.
To them, their baby had been perfect. She had fingers and toes. A thatch of dark hair. While in intensive care, peering up at her parents, she grabbed for her mother’s hand.
Had her condition been known, they would’ve spared her futile medical interventions, as doctors tried to save her life. Their family priest would have been able to baptize her. As it was, they never got to hold their child while she was alive.
These days, when Amanda and her husband say grace before dinner, they give thanks for the 28 hours of their daughter’s life.
They’re also thinking about making comfort boxes the hospital could give to other parents who lose a child. It might include books on grief. Softer tissues. Something that says, as Amanda puts it, “This is to help you get through.”
Amid their grief, they had a prayer answered: Amanda is pregnant again.
It’s frightening to go through this again. She barely sleeps the night before visiting the doctor. It feels like she never stopped being pregnant. It will feel that way, she said, until she brings a baby home – one who lives past the first 2 nights.
Amanda planned to get another genetic screening test. At first she couldn’t bear it, wasn’t sure she could trust it. “The margin of error is a human life,” Amanda said.
The 10-week appointment passed. Then the 12-week appointment. After her 13th week, she took the plunge. The test she was given was from Labcorp.
Around this time, more than a year after Amanda had desperately tried to alert the company about what had happened to her and her first baby, she finally heard back. Labcorp’s vice president of genetic counseling and services reached out – after ProPublica contacted the company and shared Amanda’s story.
The executive would only speak to Amanda without a reporter present.
Amanda said that during the call, the executive told her that prenatal genetic tests are evolving, and doctors should be clear about what the screenings can and cannot do. By the end of the conversation, the executive offered Amanda her cell number.
Amanda said she appreciated the call. “I feel better. I feel like I got something.”
The same day, her screening results came back. They were negative.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
More evidence in utero exposure to antiseizure meds safe for children’s cognition
NASHVILLE, TENN. – There is no negative impact of in utero exposure to antiseizure medications on children’s creativity, new research shows.
The results of this study, along with other research, suggest the risk for cognitive problems “is fairly low” overall for children of women with epilepsy taking lamotrigine or levetiracetam, study investigator, Kimford J. Meador, MD, professor, department of neurology & neurological sciences, Stanford (Calif.) University School of Medicine, told this news organization.
“This is another encouraging piece that’s showing these new drugs are safe with regard to cognition.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Capturing creativity
Fetal exposure to antiseizure medications can produce adverse neurodevelopmental effects. These are typically assessed using measures such as general intelligence, verbal/nonverbal abilities, or additional educational needs.
However, these measures don’t capture creativity, which “is related to intelligence but not completely,” said Dr. Meador. “I have seen wonderful examples of creativity in people who have a lot of cognitive impairment.”
He referred to one of his patients with epilepsy who is “spectacularly good” at painting with watercolors, even though she has significant cognitive impairment.
The new analysis is part of the MONEAD study, a prospective, observational multicenter study examining pregnancy outcomes for both mother and child. It included pregnant women who were enrolled at under 20 weeks’ gestational age.
The women with epilepsy in the study were primarily on monotherapy (73%), and of these, 82% were on lamotrigine or levetiracetam. About 22% were on polytherapy, of which 42% were on dual therapy with lamotrigine and levetiracetam.
Fluency, originality
Researchers assessed the children of these women at age 4½ years using the Torrance Test of Creative Thinking-Figural (TTCT-F). This is a standardized assessment of creative thinking with index scores measuring such things as fluency, originality, abstractness, and elaboration.
Dr. Meador noted the research team used a shorter version of the test battery “so as to not wear out the families and kids.”
During the test, children were given lines of different shapes and asked to draw a picture using these lines. Dr. Meador pointed out the drawings ranged from quite basic to more intricate.
One child cleverly turned a few squiggly lines into a car. “I can look at this and say this kid’s going to do very well,” said Dr. Meador.
Investigators compared scores between 241 children of women with epilepsy (WWE) and 65 children of healthy women (HW). They adjusted for the mother’s IQ, education level, age at enrollment, gestation age at enrollment, post-birth average anxiety score, and the child’s ethnicity and sex.
Investigators found the mean TTCT-F scores did not differ significantly between the two groups: adjusted least squares mean of 89.5 (95% confidence interval, 86.7-92.3) for children of WWE, compared with adjusted least square mean of 92.0 (95% CI, 86.4-97.6) for children of HW.
Balancing act
The researchers haven’t looked at a dose effect in this current study, but Dr. Meador said it’s always “a balancing act” between giving enough of the drug to keep mothers from seizing, which affect both the mother and fetus, and giving as low a dose as possible to protect the fetus.
In addition, as medication levels change during pregnancy, he said he recommends that drug levels are monitored monthly so that medication can be adjusted as necessary.
Looking at what factors might predict creativity scores, researchers found children did less well creatively if their mother didn’t have a college degree (estimate –9.5; 95% CI, –17.9 to –1.2; P = .025).
“It looks like being in a home where the mother has had more education is going to have an impact on the kid’s thinking and creativity,” said Dr. Meador.
These new findings are consistent with a lack of differences in other cognitive abilities that Dr. Meador and his team found when the children were younger.
“At age 3, we did not find an overall difference in cognitive and verbal abilities and intelligence between the children of mothers with epilepsy and those of healthy women,” he said.
The researchers aim to assess cognitive and behavioral outcomes in these children when they are 6 years old.
Helpful information
Commenting on the findings, Stéphane Auvin, MD, PhD, chair of the department of pediatric neurology at the University of Paris, who co-moderated a platform session featuring the research, said the study “is an interesting measure of the impact of being exposed to antiseizure medications.”
Creativity is “complex,” he said. “It’s not only cognition; it could be things like behavior and impulsivity.”
The new information is “very helpful.” Focusing on something broader than just IQ “gives you a better picture of what’s going on.”
The study received funding from NIH, NINDS, and NICH. Dr. Meador has received grants from NIH/NINDS, NIH/NICHD, Veterans Administration, and Eisai. He has been a consultant for Epilepsy Consortium, Novartis, Supernus, Upsher Smith Labs, and UCB Pharma. Dr. Auvin reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – There is no negative impact of in utero exposure to antiseizure medications on children’s creativity, new research shows.
The results of this study, along with other research, suggest the risk for cognitive problems “is fairly low” overall for children of women with epilepsy taking lamotrigine or levetiracetam, study investigator, Kimford J. Meador, MD, professor, department of neurology & neurological sciences, Stanford (Calif.) University School of Medicine, told this news organization.
“This is another encouraging piece that’s showing these new drugs are safe with regard to cognition.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Capturing creativity
Fetal exposure to antiseizure medications can produce adverse neurodevelopmental effects. These are typically assessed using measures such as general intelligence, verbal/nonverbal abilities, or additional educational needs.
However, these measures don’t capture creativity, which “is related to intelligence but not completely,” said Dr. Meador. “I have seen wonderful examples of creativity in people who have a lot of cognitive impairment.”
He referred to one of his patients with epilepsy who is “spectacularly good” at painting with watercolors, even though she has significant cognitive impairment.
The new analysis is part of the MONEAD study, a prospective, observational multicenter study examining pregnancy outcomes for both mother and child. It included pregnant women who were enrolled at under 20 weeks’ gestational age.
The women with epilepsy in the study were primarily on monotherapy (73%), and of these, 82% were on lamotrigine or levetiracetam. About 22% were on polytherapy, of which 42% were on dual therapy with lamotrigine and levetiracetam.
Fluency, originality
Researchers assessed the children of these women at age 4½ years using the Torrance Test of Creative Thinking-Figural (TTCT-F). This is a standardized assessment of creative thinking with index scores measuring such things as fluency, originality, abstractness, and elaboration.
Dr. Meador noted the research team used a shorter version of the test battery “so as to not wear out the families and kids.”
During the test, children were given lines of different shapes and asked to draw a picture using these lines. Dr. Meador pointed out the drawings ranged from quite basic to more intricate.
One child cleverly turned a few squiggly lines into a car. “I can look at this and say this kid’s going to do very well,” said Dr. Meador.
Investigators compared scores between 241 children of women with epilepsy (WWE) and 65 children of healthy women (HW). They adjusted for the mother’s IQ, education level, age at enrollment, gestation age at enrollment, post-birth average anxiety score, and the child’s ethnicity and sex.
Investigators found the mean TTCT-F scores did not differ significantly between the two groups: adjusted least squares mean of 89.5 (95% confidence interval, 86.7-92.3) for children of WWE, compared with adjusted least square mean of 92.0 (95% CI, 86.4-97.6) for children of HW.
Balancing act
The researchers haven’t looked at a dose effect in this current study, but Dr. Meador said it’s always “a balancing act” between giving enough of the drug to keep mothers from seizing, which affect both the mother and fetus, and giving as low a dose as possible to protect the fetus.
In addition, as medication levels change during pregnancy, he said he recommends that drug levels are monitored monthly so that medication can be adjusted as necessary.
Looking at what factors might predict creativity scores, researchers found children did less well creatively if their mother didn’t have a college degree (estimate –9.5; 95% CI, –17.9 to –1.2; P = .025).
“It looks like being in a home where the mother has had more education is going to have an impact on the kid’s thinking and creativity,” said Dr. Meador.
These new findings are consistent with a lack of differences in other cognitive abilities that Dr. Meador and his team found when the children were younger.
“At age 3, we did not find an overall difference in cognitive and verbal abilities and intelligence between the children of mothers with epilepsy and those of healthy women,” he said.
The researchers aim to assess cognitive and behavioral outcomes in these children when they are 6 years old.
Helpful information
Commenting on the findings, Stéphane Auvin, MD, PhD, chair of the department of pediatric neurology at the University of Paris, who co-moderated a platform session featuring the research, said the study “is an interesting measure of the impact of being exposed to antiseizure medications.”
Creativity is “complex,” he said. “It’s not only cognition; it could be things like behavior and impulsivity.”
The new information is “very helpful.” Focusing on something broader than just IQ “gives you a better picture of what’s going on.”
The study received funding from NIH, NINDS, and NICH. Dr. Meador has received grants from NIH/NINDS, NIH/NICHD, Veterans Administration, and Eisai. He has been a consultant for Epilepsy Consortium, Novartis, Supernus, Upsher Smith Labs, and UCB Pharma. Dr. Auvin reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – There is no negative impact of in utero exposure to antiseizure medications on children’s creativity, new research shows.
The results of this study, along with other research, suggest the risk for cognitive problems “is fairly low” overall for children of women with epilepsy taking lamotrigine or levetiracetam, study investigator, Kimford J. Meador, MD, professor, department of neurology & neurological sciences, Stanford (Calif.) University School of Medicine, told this news organization.
“This is another encouraging piece that’s showing these new drugs are safe with regard to cognition.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Capturing creativity
Fetal exposure to antiseizure medications can produce adverse neurodevelopmental effects. These are typically assessed using measures such as general intelligence, verbal/nonverbal abilities, or additional educational needs.
However, these measures don’t capture creativity, which “is related to intelligence but not completely,” said Dr. Meador. “I have seen wonderful examples of creativity in people who have a lot of cognitive impairment.”
He referred to one of his patients with epilepsy who is “spectacularly good” at painting with watercolors, even though she has significant cognitive impairment.
The new analysis is part of the MONEAD study, a prospective, observational multicenter study examining pregnancy outcomes for both mother and child. It included pregnant women who were enrolled at under 20 weeks’ gestational age.
The women with epilepsy in the study were primarily on monotherapy (73%), and of these, 82% were on lamotrigine or levetiracetam. About 22% were on polytherapy, of which 42% were on dual therapy with lamotrigine and levetiracetam.
Fluency, originality
Researchers assessed the children of these women at age 4½ years using the Torrance Test of Creative Thinking-Figural (TTCT-F). This is a standardized assessment of creative thinking with index scores measuring such things as fluency, originality, abstractness, and elaboration.
Dr. Meador noted the research team used a shorter version of the test battery “so as to not wear out the families and kids.”
During the test, children were given lines of different shapes and asked to draw a picture using these lines. Dr. Meador pointed out the drawings ranged from quite basic to more intricate.
One child cleverly turned a few squiggly lines into a car. “I can look at this and say this kid’s going to do very well,” said Dr. Meador.
Investigators compared scores between 241 children of women with epilepsy (WWE) and 65 children of healthy women (HW). They adjusted for the mother’s IQ, education level, age at enrollment, gestation age at enrollment, post-birth average anxiety score, and the child’s ethnicity and sex.
Investigators found the mean TTCT-F scores did not differ significantly between the two groups: adjusted least squares mean of 89.5 (95% confidence interval, 86.7-92.3) for children of WWE, compared with adjusted least square mean of 92.0 (95% CI, 86.4-97.6) for children of HW.
Balancing act
The researchers haven’t looked at a dose effect in this current study, but Dr. Meador said it’s always “a balancing act” between giving enough of the drug to keep mothers from seizing, which affect both the mother and fetus, and giving as low a dose as possible to protect the fetus.
In addition, as medication levels change during pregnancy, he said he recommends that drug levels are monitored monthly so that medication can be adjusted as necessary.
Looking at what factors might predict creativity scores, researchers found children did less well creatively if their mother didn’t have a college degree (estimate –9.5; 95% CI, –17.9 to –1.2; P = .025).
“It looks like being in a home where the mother has had more education is going to have an impact on the kid’s thinking and creativity,” said Dr. Meador.
These new findings are consistent with a lack of differences in other cognitive abilities that Dr. Meador and his team found when the children were younger.
“At age 3, we did not find an overall difference in cognitive and verbal abilities and intelligence between the children of mothers with epilepsy and those of healthy women,” he said.
The researchers aim to assess cognitive and behavioral outcomes in these children when they are 6 years old.
Helpful information
Commenting on the findings, Stéphane Auvin, MD, PhD, chair of the department of pediatric neurology at the University of Paris, who co-moderated a platform session featuring the research, said the study “is an interesting measure of the impact of being exposed to antiseizure medications.”
Creativity is “complex,” he said. “It’s not only cognition; it could be things like behavior and impulsivity.”
The new information is “very helpful.” Focusing on something broader than just IQ “gives you a better picture of what’s going on.”
The study received funding from NIH, NINDS, and NICH. Dr. Meador has received grants from NIH/NINDS, NIH/NICHD, Veterans Administration, and Eisai. He has been a consultant for Epilepsy Consortium, Novartis, Supernus, Upsher Smith Labs, and UCB Pharma. Dr. Auvin reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AES 2022
Blame IBS on gravity intolerance?
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Study eyes sunscreens marketed to individuals with skin of color
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Telemedicine increases access to care and optimizes practice revenue
The first time I considered telehealth as a viable option for care delivery was in February 2020. I had just heard that one of my patients had been diagnosed with COVID-19 and admitted to Evergreen Health, a hospital our practice covered just outside of Seattle. The news was jarring. Suddenly, it became crystal clear that patient access to care and the economic survival of our business would require another approach. Seemingly overnight, we built a telehealth program and began seeing patients virtually from the comfort and safety of home.
We certainly weren’t alone. From January to March 2020, the Centers for Disease Control and Prevention showed a 154% increase in telehealth visits.1 Even as the postpandemic era settles in, the use of telehealth today is 38 times greater than the pre-COVID baseline, creating a market valued at $250 billion per year.2 What value might gastroenterologists gain from the use of telehealth going forward? 3 For today’s overburdened GI practices, telehealth can improve patient access to care, alleviate the clinician shortage with work-from-home options for practitioners, and present innovative methods of increasing revenue streams – all while improving quality of care.
As GI demand outpaces supply, it’s time to consider alternative channels of care
The prevalence of gastrointestinal illness, the size of the market, and the growing difficulty in gaining access to care makes it natural to consider whether virtual care may benefit patients and GI practices alike. Approximately 70 million Americans, or 1 in 5, live with chronic GI symptoms.4 On an annual basis, more than 50 million primary care visits and 15 million ER visits in the United States have a primary diagnostic code for GI disease.5 Annual expenditures to address GI conditions, valued at $136 billion, outpace those of other high-cost conditions such as heart disease or mental health.6 And with the recent addition of 21 million patients between 45 and 49 years of age who now require colon cancer screening, plus the expected postpandemic increase in GI illness, those numbers are likely to grow.7
Compounding matters is a shortage of clinicians. Between early physician retirements and a limited number of GI fellowships, gastroenterology was recently identified by a Merritt Hawkins survey as the “most in-demand” specialty.8 Patients are already waiting months, and even up to a year in some parts of the country, to see a gastroenterologist. GI physicians, likewise, are running ragged trying to keep up and are burning out in the process.
The case for virtual GI care
Until the pandemic, many of us would not have seriously considered a significant role for virtual care in GI. When necessity demanded it, however, we used this channel effectively with both patients and providers reporting high rates of satisfaction with telehealth for GI clinic visits.9
In a recent published study with a sizable cohort of GI patients across a wide spectrum of conditions, only 17% required a physical exam following a telehealth visit. Over 50% said they were very likely or likely to continue using telehealth in the future. Interestingly, it was not only a young or tech-savvy population that ranked telehealth highly. In fact, Net Promoter Scores (a proven measure of customer experience) were consistently high for employed patients aged 60 or younger.10
Recent research also has demonstrated that telehealth visits meet quality standards and do so efficiently. A Mayo Clinic study demonstrated that telehealth visits in GI were delivered with a similar level of quality based on diagnostic concordance,11 and a recent study by Tang et al. found that 98% of visits for routine GI issues were completed within 20 minutes.12
Finally, establishing a virtual channel allows a clinic to increase its staffing radius by using geographically dispersed GI providers, including appropriately licensed physicians or advanced practice providers who may reside in other states. The use of remote providers opens up the possibility for “time zone arbitrage” to allow for more flexible staffing that’s similar to urgent care with wraparound and weekend hours – all without adding office space or overhead.
Financial implications
Given the long tail of demand in GI, increasing capacity will increase revenue. Telehealth increases capacity by allowing for the efficient use of resources and expanding the reach of practices in engaging potential providers.
The majority of telehealth visits are reimbursable. Since 1995, 40 states and the District of Columbia have enacted mandatory telehealth coverage laws, and 20 states require that telehealth visits be paid on par with in-person visits.13 With the pandemic Medicare waivers, parity was extended through government programs and is expected by many insiders to continue in some form going forward. By an overwhelming bipartisan majority, the House of Representatives recently passed the Advancing Telehealth Beyond COVID-19 Act, which would extend most temporary telemedicine policies through 2024. This legislation would affect only Medicare reimbursement, but changes in Medicare policy often influence the policies of commercial payers.14
While reimbursement for clinic visits is important, the larger financial implication for extending clinics virtually is in the endoscopy suite. Most revenue (70%-80%) in community GI practices is generated from endoscopic services and related ancillary streams. For an endoscopist, spending time in the clinic is effectively a loss leader. Adding capacity with a virtual clinic and geographically dispersed providers can open up GI physicians to spend more time in the endoscopy suite, thereby generating additional revenue.
Given the rapid consolidation of the GI space, income repair post private equity transaction is top of mind for both established physicians and young physicians entering the labor market. Having a virtual ancillary differentiates practices and may prove useful for recruitment. Increasing access by using remote providers during evenings and weekends may “unclog the pipes,” improve the patient and provider experience, and increase revenue.
Overcoming obstacles
Creating a telehealth platform – particularly one that crosses state lines – requires an understanding of a complex and evolving regulatory environment. Licensing is one example. When telehealth is used, it is considered to be rendered at the location of the patient. A provider typically has to be licensed in the state where the patient is located at the time of the clinical encounter. So, if providers cross jurisdictional boundaries to provide care, multiple state licenses may be required.
In addition, medical malpractice and cyber insurance for telemedicine providers are niche products. And as with the use of any technology, risks of a data breach or other unauthorized disclosure of protected health information make it vital to ensure data are fully encrypted, networks are secure, and all safeguards are followed according to the Health Information and Portability and Accountability Act (HIPAA).
Perhaps most challenging are payers, both commercial and governmental. The location of a distant site provider can affect network participation for some but not all payers. Understanding payer reimbursement policies is time-intensive, and building relationships within these organizations is crucial in today’s rapidly changing environment.
The ultimate aim: Better patient outcomes
Of course, the main goal is to take care of patients well and in a timely fashion. Better access will lead to an improved patient experience and a greater emphasis on the important cognitive aspects of GI care. Moreover, efficient use of physician time will also improve clinician satisfaction while increasing revenue and downstream value. Most importantly, increased access via a virtual channel may positively impact patient outcomes. For instance, data show that distance from an endoscopy center is negatively associated with the stage of colon cancer diagnosis.15 Providing a virtual channel to reach these distant patients will likely increase the opportunity for high-impact procedures like colonoscopy.
Change can be hard, but it will come
The old saying is that change comes slowly, then all at once. Access is a chronic pain point for GI practices that has now reached a critical level.
The GI market is enormous and rapidly evolving; it will continue to attract disruptive interest and several early-stage digital first GI companies have entered the ecosystem. There is a risk for disintermediation as well as opportunities for collaboration. The next few years will be interesting.
As we transition to a postpandemic environment, telehealth can continue to improve patient access and present new revenue streams for GI practices – all while improving quality of care. Seeing around the corner likely means expanding the reach of your clinic and offering multiple channels of care. There is likely a significant opportunity for those who choose to adapt.
Dr. Arjal is cofounder, chief medical officer, and president of Telebelly Health and is a board-certified gastroenterologist who previously served as vice president of Puget Sound Gastroenterology and a vice president of clinical affairs for GastroHealth. He currently serves on the American Gastroenterological Association (AGA) Practice Management and Economics Committee. He has no conflicts. He is on LinkedIn and Twitter (@RussArjalMD).
References
1. Koonin LM et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic – United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020. Oct 30;69(43):1595-9.
2. “Telehealth: A quarter-trillion-dollar post-COVID-19 reality?” McKinsey & Company, July 9, 2021.
3. The telehealth era is just beginning, Robert Pearl and Brian Wayling, Harvard Business Review, May-June, 2022.
4. Peery et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019. Jan;156(1):254-72.
5. See id.
6. See id.
7. Sieh, K. Post-COVID-19 functional gastrointestinal disorders: Prepare for a GI aftershock. J Gastroenterol Hepatol. 2022 March;37(3):413-4.
8. Newitt, P. Gastroenterology’s biggest threats. Becker’s, GI & Endoscopy, 2021 Oct 8, and Physician Compensation Report, 2022. Physicians Thrive (projecting a shortage of over 1,600 Gastroenterologists by 2025).
9. Dobrusin et al. Gastroenterologists and patients report high satisfaction rates with Telehealth services during the novel coronavirus 2019 pandemic. Clin Gastroenterol Hepatol. 2020;8(11):2393-7.
10. Dobrusin et al. Patients with gastrointestinal conditions consider telehealth equivalent to in-person care. Gastroenterology. 2022 Oct 4. doi: 10.1053/j.gastro.2022.09.035.
11. Demaerschalk et al. Assessment of clinician diagnostic concordance with video telemedicine in the integrated multispecialty practice at Mayo Clinic during the beginning of COVID-19 pandemic from March to June, 2020. JAMA Netw Open. 2022 Sep;5(9):e2229958.
12. Tang et al. A model for the pandemic and beyond: Telemedicine for all gastroenterology referrals reduces unnecessary clinic visits. J Telemed Telecare. 2022 Sep 28(8):577-82.
13. Dills A. Policy brief: Telehealth payment parity laws at the state level. Mercatus Center, George Mason University.
14. H.R.4040 – Advancing Telehealth Beyond COVID-19 Act of 2021. Congress.gov.
15. Brand et al. Association of distance, region, and insurance with advanced colon cancer at initial diagnosis. JAMA Netw Open. 2022 Sep 1;5(9):e2229954.
The first time I considered telehealth as a viable option for care delivery was in February 2020. I had just heard that one of my patients had been diagnosed with COVID-19 and admitted to Evergreen Health, a hospital our practice covered just outside of Seattle. The news was jarring. Suddenly, it became crystal clear that patient access to care and the economic survival of our business would require another approach. Seemingly overnight, we built a telehealth program and began seeing patients virtually from the comfort and safety of home.
We certainly weren’t alone. From January to March 2020, the Centers for Disease Control and Prevention showed a 154% increase in telehealth visits.1 Even as the postpandemic era settles in, the use of telehealth today is 38 times greater than the pre-COVID baseline, creating a market valued at $250 billion per year.2 What value might gastroenterologists gain from the use of telehealth going forward? 3 For today’s overburdened GI practices, telehealth can improve patient access to care, alleviate the clinician shortage with work-from-home options for practitioners, and present innovative methods of increasing revenue streams – all while improving quality of care.
As GI demand outpaces supply, it’s time to consider alternative channels of care
The prevalence of gastrointestinal illness, the size of the market, and the growing difficulty in gaining access to care makes it natural to consider whether virtual care may benefit patients and GI practices alike. Approximately 70 million Americans, or 1 in 5, live with chronic GI symptoms.4 On an annual basis, more than 50 million primary care visits and 15 million ER visits in the United States have a primary diagnostic code for GI disease.5 Annual expenditures to address GI conditions, valued at $136 billion, outpace those of other high-cost conditions such as heart disease or mental health.6 And with the recent addition of 21 million patients between 45 and 49 years of age who now require colon cancer screening, plus the expected postpandemic increase in GI illness, those numbers are likely to grow.7
Compounding matters is a shortage of clinicians. Between early physician retirements and a limited number of GI fellowships, gastroenterology was recently identified by a Merritt Hawkins survey as the “most in-demand” specialty.8 Patients are already waiting months, and even up to a year in some parts of the country, to see a gastroenterologist. GI physicians, likewise, are running ragged trying to keep up and are burning out in the process.
The case for virtual GI care
Until the pandemic, many of us would not have seriously considered a significant role for virtual care in GI. When necessity demanded it, however, we used this channel effectively with both patients and providers reporting high rates of satisfaction with telehealth for GI clinic visits.9
In a recent published study with a sizable cohort of GI patients across a wide spectrum of conditions, only 17% required a physical exam following a telehealth visit. Over 50% said they were very likely or likely to continue using telehealth in the future. Interestingly, it was not only a young or tech-savvy population that ranked telehealth highly. In fact, Net Promoter Scores (a proven measure of customer experience) were consistently high for employed patients aged 60 or younger.10
Recent research also has demonstrated that telehealth visits meet quality standards and do so efficiently. A Mayo Clinic study demonstrated that telehealth visits in GI were delivered with a similar level of quality based on diagnostic concordance,11 and a recent study by Tang et al. found that 98% of visits for routine GI issues were completed within 20 minutes.12
Finally, establishing a virtual channel allows a clinic to increase its staffing radius by using geographically dispersed GI providers, including appropriately licensed physicians or advanced practice providers who may reside in other states. The use of remote providers opens up the possibility for “time zone arbitrage” to allow for more flexible staffing that’s similar to urgent care with wraparound and weekend hours – all without adding office space or overhead.
Financial implications
Given the long tail of demand in GI, increasing capacity will increase revenue. Telehealth increases capacity by allowing for the efficient use of resources and expanding the reach of practices in engaging potential providers.
The majority of telehealth visits are reimbursable. Since 1995, 40 states and the District of Columbia have enacted mandatory telehealth coverage laws, and 20 states require that telehealth visits be paid on par with in-person visits.13 With the pandemic Medicare waivers, parity was extended through government programs and is expected by many insiders to continue in some form going forward. By an overwhelming bipartisan majority, the House of Representatives recently passed the Advancing Telehealth Beyond COVID-19 Act, which would extend most temporary telemedicine policies through 2024. This legislation would affect only Medicare reimbursement, but changes in Medicare policy often influence the policies of commercial payers.14
While reimbursement for clinic visits is important, the larger financial implication for extending clinics virtually is in the endoscopy suite. Most revenue (70%-80%) in community GI practices is generated from endoscopic services and related ancillary streams. For an endoscopist, spending time in the clinic is effectively a loss leader. Adding capacity with a virtual clinic and geographically dispersed providers can open up GI physicians to spend more time in the endoscopy suite, thereby generating additional revenue.
Given the rapid consolidation of the GI space, income repair post private equity transaction is top of mind for both established physicians and young physicians entering the labor market. Having a virtual ancillary differentiates practices and may prove useful for recruitment. Increasing access by using remote providers during evenings and weekends may “unclog the pipes,” improve the patient and provider experience, and increase revenue.
Overcoming obstacles
Creating a telehealth platform – particularly one that crosses state lines – requires an understanding of a complex and evolving regulatory environment. Licensing is one example. When telehealth is used, it is considered to be rendered at the location of the patient. A provider typically has to be licensed in the state where the patient is located at the time of the clinical encounter. So, if providers cross jurisdictional boundaries to provide care, multiple state licenses may be required.
In addition, medical malpractice and cyber insurance for telemedicine providers are niche products. And as with the use of any technology, risks of a data breach or other unauthorized disclosure of protected health information make it vital to ensure data are fully encrypted, networks are secure, and all safeguards are followed according to the Health Information and Portability and Accountability Act (HIPAA).
Perhaps most challenging are payers, both commercial and governmental. The location of a distant site provider can affect network participation for some but not all payers. Understanding payer reimbursement policies is time-intensive, and building relationships within these organizations is crucial in today’s rapidly changing environment.
The ultimate aim: Better patient outcomes
Of course, the main goal is to take care of patients well and in a timely fashion. Better access will lead to an improved patient experience and a greater emphasis on the important cognitive aspects of GI care. Moreover, efficient use of physician time will also improve clinician satisfaction while increasing revenue and downstream value. Most importantly, increased access via a virtual channel may positively impact patient outcomes. For instance, data show that distance from an endoscopy center is negatively associated with the stage of colon cancer diagnosis.15 Providing a virtual channel to reach these distant patients will likely increase the opportunity for high-impact procedures like colonoscopy.
Change can be hard, but it will come
The old saying is that change comes slowly, then all at once. Access is a chronic pain point for GI practices that has now reached a critical level.
The GI market is enormous and rapidly evolving; it will continue to attract disruptive interest and several early-stage digital first GI companies have entered the ecosystem. There is a risk for disintermediation as well as opportunities for collaboration. The next few years will be interesting.
As we transition to a postpandemic environment, telehealth can continue to improve patient access and present new revenue streams for GI practices – all while improving quality of care. Seeing around the corner likely means expanding the reach of your clinic and offering multiple channels of care. There is likely a significant opportunity for those who choose to adapt.
Dr. Arjal is cofounder, chief medical officer, and president of Telebelly Health and is a board-certified gastroenterologist who previously served as vice president of Puget Sound Gastroenterology and a vice president of clinical affairs for GastroHealth. He currently serves on the American Gastroenterological Association (AGA) Practice Management and Economics Committee. He has no conflicts. He is on LinkedIn and Twitter (@RussArjalMD).
References
1. Koonin LM et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic – United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020. Oct 30;69(43):1595-9.
2. “Telehealth: A quarter-trillion-dollar post-COVID-19 reality?” McKinsey & Company, July 9, 2021.
3. The telehealth era is just beginning, Robert Pearl and Brian Wayling, Harvard Business Review, May-June, 2022.
4. Peery et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019. Jan;156(1):254-72.
5. See id.
6. See id.
7. Sieh, K. Post-COVID-19 functional gastrointestinal disorders: Prepare for a GI aftershock. J Gastroenterol Hepatol. 2022 March;37(3):413-4.
8. Newitt, P. Gastroenterology’s biggest threats. Becker’s, GI & Endoscopy, 2021 Oct 8, and Physician Compensation Report, 2022. Physicians Thrive (projecting a shortage of over 1,600 Gastroenterologists by 2025).
9. Dobrusin et al. Gastroenterologists and patients report high satisfaction rates with Telehealth services during the novel coronavirus 2019 pandemic. Clin Gastroenterol Hepatol. 2020;8(11):2393-7.
10. Dobrusin et al. Patients with gastrointestinal conditions consider telehealth equivalent to in-person care. Gastroenterology. 2022 Oct 4. doi: 10.1053/j.gastro.2022.09.035.
11. Demaerschalk et al. Assessment of clinician diagnostic concordance with video telemedicine in the integrated multispecialty practice at Mayo Clinic during the beginning of COVID-19 pandemic from March to June, 2020. JAMA Netw Open. 2022 Sep;5(9):e2229958.
12. Tang et al. A model for the pandemic and beyond: Telemedicine for all gastroenterology referrals reduces unnecessary clinic visits. J Telemed Telecare. 2022 Sep 28(8):577-82.
13. Dills A. Policy brief: Telehealth payment parity laws at the state level. Mercatus Center, George Mason University.
14. H.R.4040 – Advancing Telehealth Beyond COVID-19 Act of 2021. Congress.gov.
15. Brand et al. Association of distance, region, and insurance with advanced colon cancer at initial diagnosis. JAMA Netw Open. 2022 Sep 1;5(9):e2229954.
The first time I considered telehealth as a viable option for care delivery was in February 2020. I had just heard that one of my patients had been diagnosed with COVID-19 and admitted to Evergreen Health, a hospital our practice covered just outside of Seattle. The news was jarring. Suddenly, it became crystal clear that patient access to care and the economic survival of our business would require another approach. Seemingly overnight, we built a telehealth program and began seeing patients virtually from the comfort and safety of home.
We certainly weren’t alone. From January to March 2020, the Centers for Disease Control and Prevention showed a 154% increase in telehealth visits.1 Even as the postpandemic era settles in, the use of telehealth today is 38 times greater than the pre-COVID baseline, creating a market valued at $250 billion per year.2 What value might gastroenterologists gain from the use of telehealth going forward? 3 For today’s overburdened GI practices, telehealth can improve patient access to care, alleviate the clinician shortage with work-from-home options for practitioners, and present innovative methods of increasing revenue streams – all while improving quality of care.
As GI demand outpaces supply, it’s time to consider alternative channels of care
The prevalence of gastrointestinal illness, the size of the market, and the growing difficulty in gaining access to care makes it natural to consider whether virtual care may benefit patients and GI practices alike. Approximately 70 million Americans, or 1 in 5, live with chronic GI symptoms.4 On an annual basis, more than 50 million primary care visits and 15 million ER visits in the United States have a primary diagnostic code for GI disease.5 Annual expenditures to address GI conditions, valued at $136 billion, outpace those of other high-cost conditions such as heart disease or mental health.6 And with the recent addition of 21 million patients between 45 and 49 years of age who now require colon cancer screening, plus the expected postpandemic increase in GI illness, those numbers are likely to grow.7
Compounding matters is a shortage of clinicians. Between early physician retirements and a limited number of GI fellowships, gastroenterology was recently identified by a Merritt Hawkins survey as the “most in-demand” specialty.8 Patients are already waiting months, and even up to a year in some parts of the country, to see a gastroenterologist. GI physicians, likewise, are running ragged trying to keep up and are burning out in the process.
The case for virtual GI care
Until the pandemic, many of us would not have seriously considered a significant role for virtual care in GI. When necessity demanded it, however, we used this channel effectively with both patients and providers reporting high rates of satisfaction with telehealth for GI clinic visits.9
In a recent published study with a sizable cohort of GI patients across a wide spectrum of conditions, only 17% required a physical exam following a telehealth visit. Over 50% said they were very likely or likely to continue using telehealth in the future. Interestingly, it was not only a young or tech-savvy population that ranked telehealth highly. In fact, Net Promoter Scores (a proven measure of customer experience) were consistently high for employed patients aged 60 or younger.10
Recent research also has demonstrated that telehealth visits meet quality standards and do so efficiently. A Mayo Clinic study demonstrated that telehealth visits in GI were delivered with a similar level of quality based on diagnostic concordance,11 and a recent study by Tang et al. found that 98% of visits for routine GI issues were completed within 20 minutes.12
Finally, establishing a virtual channel allows a clinic to increase its staffing radius by using geographically dispersed GI providers, including appropriately licensed physicians or advanced practice providers who may reside in other states. The use of remote providers opens up the possibility for “time zone arbitrage” to allow for more flexible staffing that’s similar to urgent care with wraparound and weekend hours – all without adding office space or overhead.
Financial implications
Given the long tail of demand in GI, increasing capacity will increase revenue. Telehealth increases capacity by allowing for the efficient use of resources and expanding the reach of practices in engaging potential providers.
The majority of telehealth visits are reimbursable. Since 1995, 40 states and the District of Columbia have enacted mandatory telehealth coverage laws, and 20 states require that telehealth visits be paid on par with in-person visits.13 With the pandemic Medicare waivers, parity was extended through government programs and is expected by many insiders to continue in some form going forward. By an overwhelming bipartisan majority, the House of Representatives recently passed the Advancing Telehealth Beyond COVID-19 Act, which would extend most temporary telemedicine policies through 2024. This legislation would affect only Medicare reimbursement, but changes in Medicare policy often influence the policies of commercial payers.14
While reimbursement for clinic visits is important, the larger financial implication for extending clinics virtually is in the endoscopy suite. Most revenue (70%-80%) in community GI practices is generated from endoscopic services and related ancillary streams. For an endoscopist, spending time in the clinic is effectively a loss leader. Adding capacity with a virtual clinic and geographically dispersed providers can open up GI physicians to spend more time in the endoscopy suite, thereby generating additional revenue.
Given the rapid consolidation of the GI space, income repair post private equity transaction is top of mind for both established physicians and young physicians entering the labor market. Having a virtual ancillary differentiates practices and may prove useful for recruitment. Increasing access by using remote providers during evenings and weekends may “unclog the pipes,” improve the patient and provider experience, and increase revenue.
Overcoming obstacles
Creating a telehealth platform – particularly one that crosses state lines – requires an understanding of a complex and evolving regulatory environment. Licensing is one example. When telehealth is used, it is considered to be rendered at the location of the patient. A provider typically has to be licensed in the state where the patient is located at the time of the clinical encounter. So, if providers cross jurisdictional boundaries to provide care, multiple state licenses may be required.
In addition, medical malpractice and cyber insurance for telemedicine providers are niche products. And as with the use of any technology, risks of a data breach or other unauthorized disclosure of protected health information make it vital to ensure data are fully encrypted, networks are secure, and all safeguards are followed according to the Health Information and Portability and Accountability Act (HIPAA).
Perhaps most challenging are payers, both commercial and governmental. The location of a distant site provider can affect network participation for some but not all payers. Understanding payer reimbursement policies is time-intensive, and building relationships within these organizations is crucial in today’s rapidly changing environment.
The ultimate aim: Better patient outcomes
Of course, the main goal is to take care of patients well and in a timely fashion. Better access will lead to an improved patient experience and a greater emphasis on the important cognitive aspects of GI care. Moreover, efficient use of physician time will also improve clinician satisfaction while increasing revenue and downstream value. Most importantly, increased access via a virtual channel may positively impact patient outcomes. For instance, data show that distance from an endoscopy center is negatively associated with the stage of colon cancer diagnosis.15 Providing a virtual channel to reach these distant patients will likely increase the opportunity for high-impact procedures like colonoscopy.
Change can be hard, but it will come
The old saying is that change comes slowly, then all at once. Access is a chronic pain point for GI practices that has now reached a critical level.
The GI market is enormous and rapidly evolving; it will continue to attract disruptive interest and several early-stage digital first GI companies have entered the ecosystem. There is a risk for disintermediation as well as opportunities for collaboration. The next few years will be interesting.
As we transition to a postpandemic environment, telehealth can continue to improve patient access and present new revenue streams for GI practices – all while improving quality of care. Seeing around the corner likely means expanding the reach of your clinic and offering multiple channels of care. There is likely a significant opportunity for those who choose to adapt.
Dr. Arjal is cofounder, chief medical officer, and president of Telebelly Health and is a board-certified gastroenterologist who previously served as vice president of Puget Sound Gastroenterology and a vice president of clinical affairs for GastroHealth. He currently serves on the American Gastroenterological Association (AGA) Practice Management and Economics Committee. He has no conflicts. He is on LinkedIn and Twitter (@RussArjalMD).
References
1. Koonin LM et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic – United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020. Oct 30;69(43):1595-9.
2. “Telehealth: A quarter-trillion-dollar post-COVID-19 reality?” McKinsey & Company, July 9, 2021.
3. The telehealth era is just beginning, Robert Pearl and Brian Wayling, Harvard Business Review, May-June, 2022.
4. Peery et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology. 2019. Jan;156(1):254-72.
5. See id.
6. See id.
7. Sieh, K. Post-COVID-19 functional gastrointestinal disorders: Prepare for a GI aftershock. J Gastroenterol Hepatol. 2022 March;37(3):413-4.
8. Newitt, P. Gastroenterology’s biggest threats. Becker’s, GI & Endoscopy, 2021 Oct 8, and Physician Compensation Report, 2022. Physicians Thrive (projecting a shortage of over 1,600 Gastroenterologists by 2025).
9. Dobrusin et al. Gastroenterologists and patients report high satisfaction rates with Telehealth services during the novel coronavirus 2019 pandemic. Clin Gastroenterol Hepatol. 2020;8(11):2393-7.
10. Dobrusin et al. Patients with gastrointestinal conditions consider telehealth equivalent to in-person care. Gastroenterology. 2022 Oct 4. doi: 10.1053/j.gastro.2022.09.035.
11. Demaerschalk et al. Assessment of clinician diagnostic concordance with video telemedicine in the integrated multispecialty practice at Mayo Clinic during the beginning of COVID-19 pandemic from March to June, 2020. JAMA Netw Open. 2022 Sep;5(9):e2229958.
12. Tang et al. A model for the pandemic and beyond: Telemedicine for all gastroenterology referrals reduces unnecessary clinic visits. J Telemed Telecare. 2022 Sep 28(8):577-82.
13. Dills A. Policy brief: Telehealth payment parity laws at the state level. Mercatus Center, George Mason University.
14. H.R.4040 – Advancing Telehealth Beyond COVID-19 Act of 2021. Congress.gov.
15. Brand et al. Association of distance, region, and insurance with advanced colon cancer at initial diagnosis. JAMA Netw Open. 2022 Sep 1;5(9):e2229954.
How a cheap liver drug may be the key to preventing COVID
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.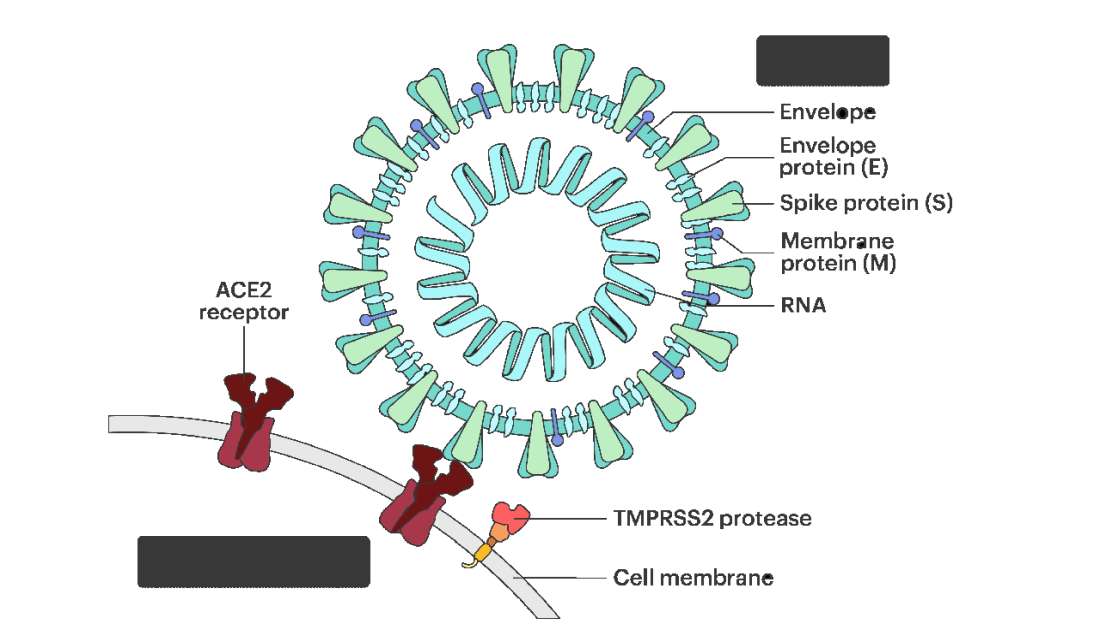
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.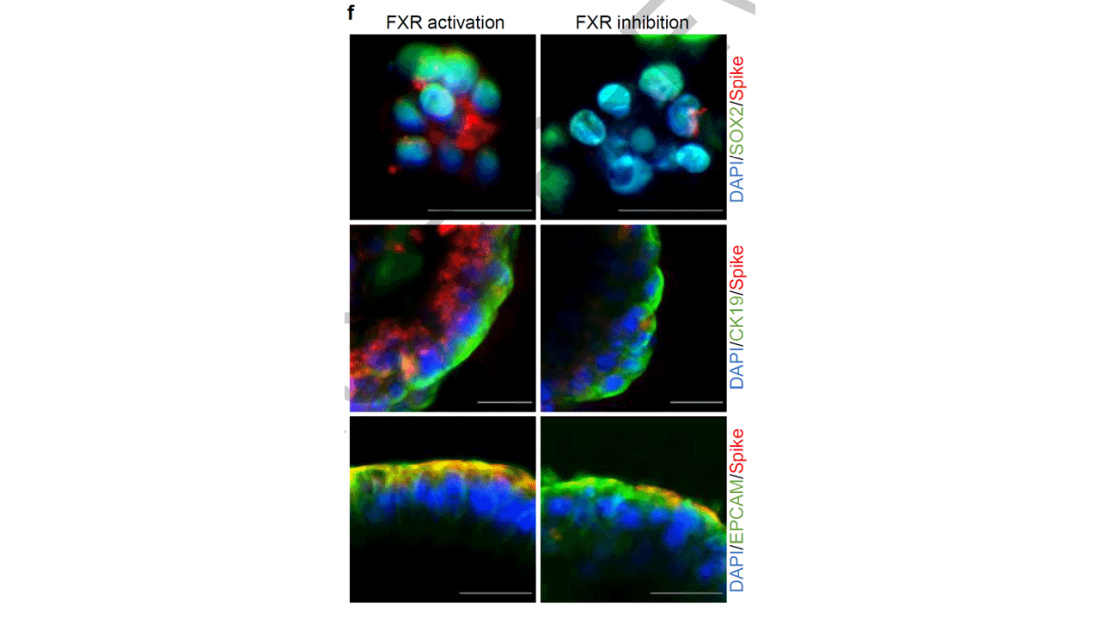
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies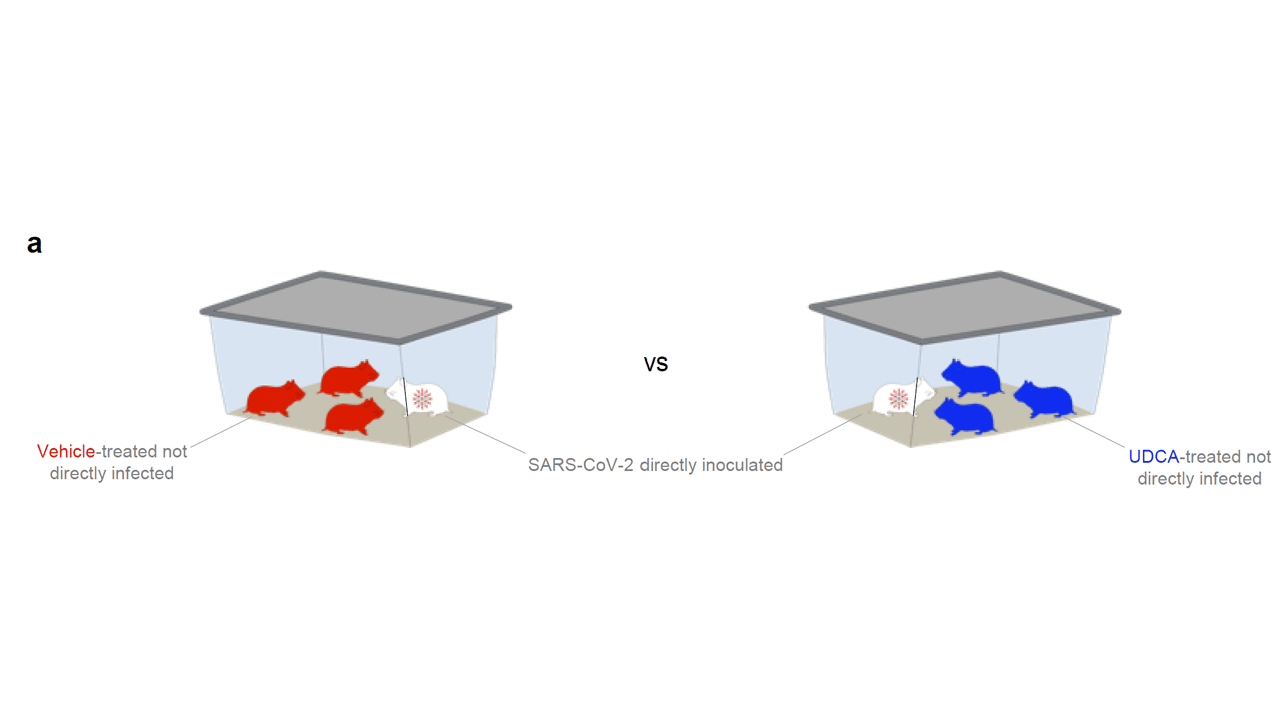
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.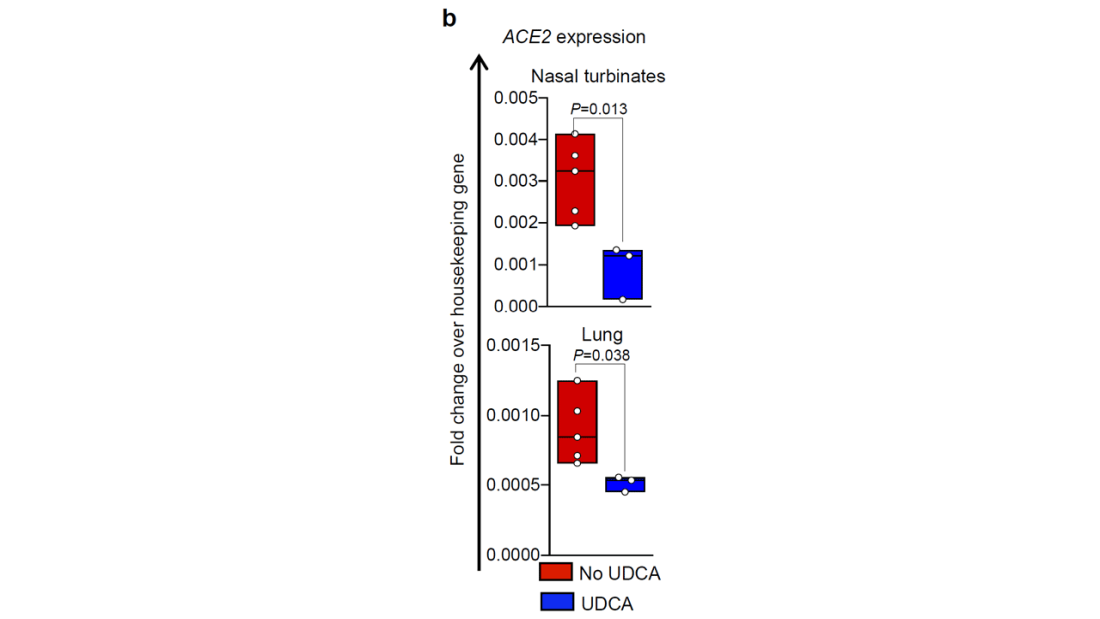
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.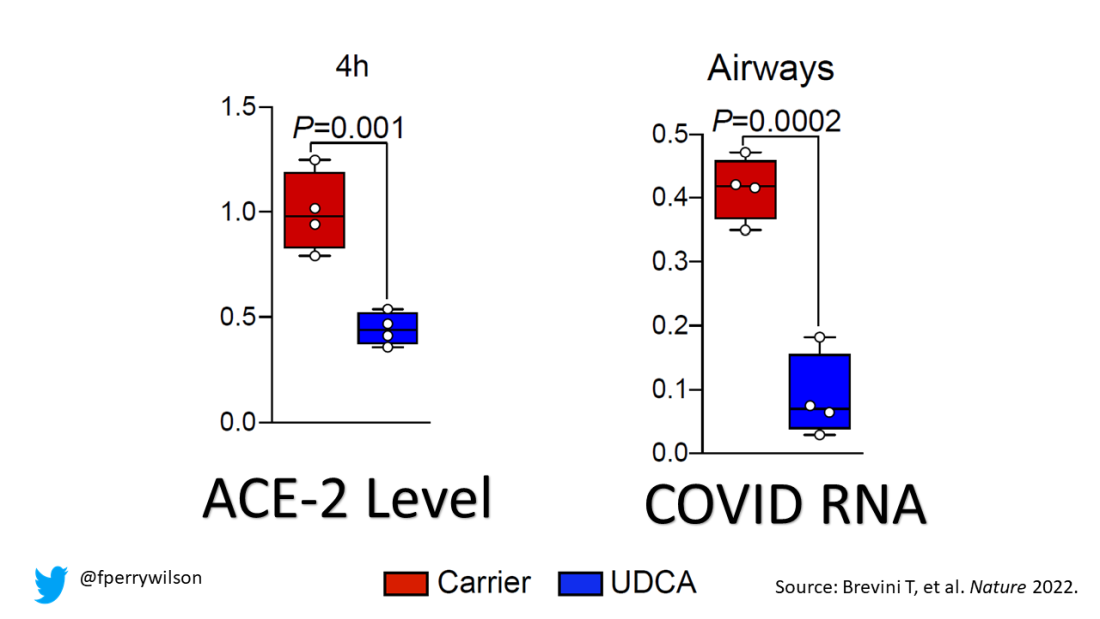
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.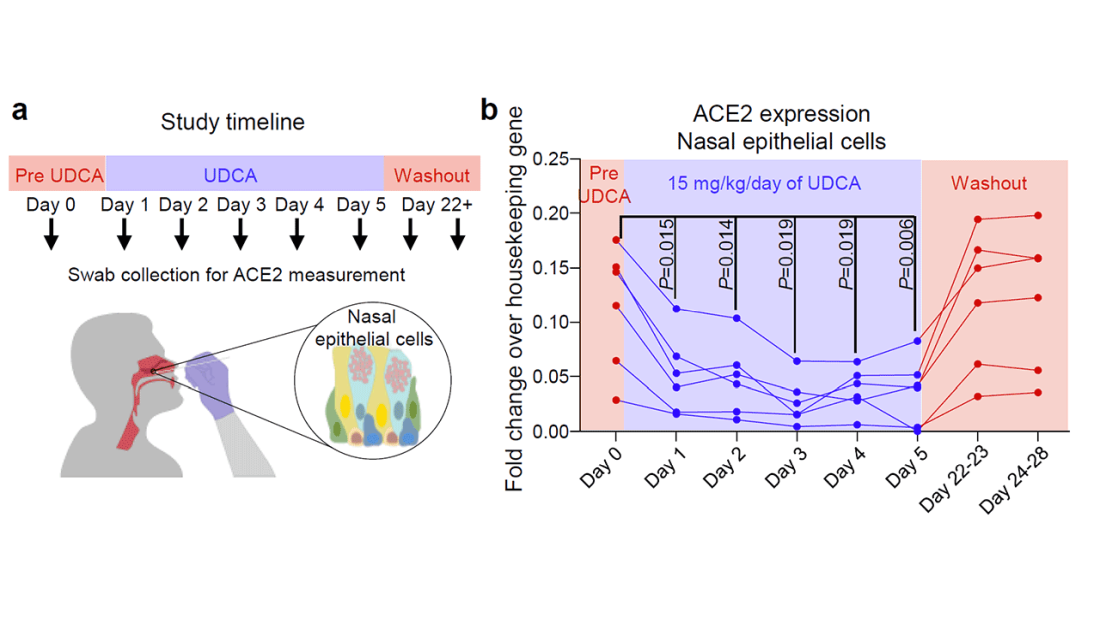
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
As soon as the pandemic started, the search was on for a medication that could stave off infection, or at least the worst consequences of infection.
One that would be cheap to make, safe, easy to distribute, and, ideally, was already available. The search had a quest-like quality, like something from a fairy tale. Society, poisoned by COVID, would find the antidote out there, somewhere, if we looked hard enough.
You know the story. There were some pretty dramatic failures: hydroxychloroquine, ivermectin. There were some successes, like dexamethasone.
I’m not here today to tell you that the antidote has been found – no, it takes large randomized trials to figure that out. But
How do you make a case that an existing drug – UDCA, in this case – might be useful to prevent or treat COVID? In contrast to prior basic-science studies, like the original ivermectin study, which essentially took a bunch of cells and virus in a tube filled with varying concentrations of the antiparasitic agent, the authors of this paper appearing in Nature give us multiple, complementary lines of evidence. Let me walk you through it.
All good science starts with a biologically plausible hypothesis. In this case, the authors recognized that SARS-CoV-2, in all its variants, requires the presence of the ACE2 receptor on the surface of cells to bind.
That is the doorway to infection. Vaccines and antibodies block the key to this door, the spike protein and its receptor binding domain. But what if you could get rid of the doors altogether?
The authors first showed that ACE2 expression is controlled by a certain transcription factor known as the farnesoid X receptor, or FXR. Reducing the binding of FXR should therefore reduce ACE2 expression.
As luck would have it, UDCA – Actigall – reduces the levels of FXR and thus the expression of ACE2 in cells.
Okay. So we have a drug that can reduce ACE2, and we know that ACE2 is necessary for the virus to infect cells. Would UDCA prevent viral infection?
They started with test tubes, showing that cells were less likely to be infected by SARS-CoV-2 in the presence of UDCA at concentrations similar to what humans achieve in their blood after standard dosing. The red staining here is spike protein; you can see that it is markedly lower in the cells exposed to UDCA.
So far, so good. But test tubes aren’t people. So they moved up to mice and Syrian golden hamsters. These cute fellows are quite susceptible to human COVID and have been a model organism in countless studies
Mice and hamsters treated with UDCA in the presence of littermates with COVID infections were less likely to become infected themselves compared with mice not so treated. They also showed that mice and hamsters treated with UDCA had lower levels of ACE2 in their nasal passages.
Of course, mice aren’t humans either. So the researchers didn’t stop there.
To determine the effects of UDCA on human tissue, they utilized perfused human lungs that had been declined for transplantation. The lungs were perfused with a special fluid to keep them viable, and were mechanically ventilated. One lung was exposed to UDCA and the other served as a control. The authors were able to show that ACE2 levels went down in the exposed lung. And, importantly, when samples of tissue from both lungs were exposed to SARS-CoV-2, the lung tissue exposed to UDCA had lower levels of viral infection.
They didn’t stop there.
Eight human volunteers were recruited to take UDCA for 5 days. ACE2 levels in the nasal passages went down over the course of treatment. They confirmed those results from a proteomics dataset with several hundred people who had received UDCA for clinical reasons. Treated individuals had lower ACE2 levels.
Finally, they looked at the epidemiologic effect. They examined a dataset that contained information on over 1,000 patients with liver disease who had contracted COVID-19, 31 of whom had been receiving UDCA. Even after adjustment for baseline differences, those receiving UDCA were less likely to be hospitalized, require an ICU, or die.
Okay, we’ll stop there. Reading this study, all I could think was, Yes! This is how you generate evidence that you have a drug that might work – step by careful step.
But let’s be careful as well. Does this study show that taking Actigall will prevent COVID? Of course not. It doesn’t show that it will treat COVID either. But I bring it up because the rigor of this study stands in contrast to those that generated huge enthusiasm earlier in the pandemic only to let us down in randomized trials. If there has been a drug out there this whole time which will prevent or treat COVID, this is how we’ll find it. The next step? Test it in a randomized trial.
For Medscape, I’m Perry Wilson.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant financial relationships.
A version of this video transcript first appeared on Medscape.com.








