User login
Nonsurgical options for stress urinary incontinence
In the article, “Nonsurgical treatments for patients with urinary incontinence” (OBG Manag. September 2022;34:36- 42.), the authors, Ashley J. Murillo, MD, and Halina M. Zyczynski, MD, discuss the successful nonsurgical management of urge urinary incontinence, stress urinary incontinence, and mixed urinary incontinence, presenting the case of a 39-year-old woman with urine leakage during exercise. As a follow-up for readers, OBG Management posted a quiz question asking, “Which of the following is a nonsurgical treatment for stress urinary incontinence?”
A total of 129 readers cast their vote:
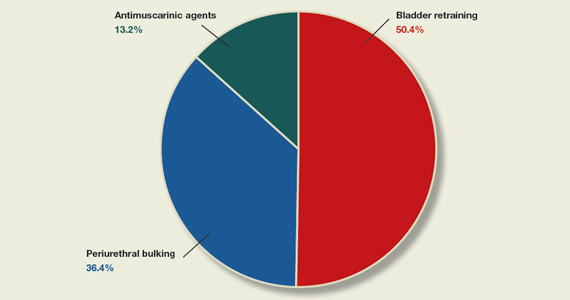
50.4% (65 readers) said bladder retraining
36.4% (47 readers) said periurethral bulking
13.2% (17 readers) said antimuscarinic agents
The correct answer was periurethral bulking, as bladder retraining and antimuscarinic agents, according to TABLE 1 in Murillo and Zyczynski’s article, are appropriate for managing urge urinary incontinence.
In the article, “Nonsurgical treatments for patients with urinary incontinence” (OBG Manag. September 2022;34:36- 42.), the authors, Ashley J. Murillo, MD, and Halina M. Zyczynski, MD, discuss the successful nonsurgical management of urge urinary incontinence, stress urinary incontinence, and mixed urinary incontinence, presenting the case of a 39-year-old woman with urine leakage during exercise. As a follow-up for readers, OBG Management posted a quiz question asking, “Which of the following is a nonsurgical treatment for stress urinary incontinence?”
A total of 129 readers cast their vote:
50.4% (65 readers) said bladder retraining
36.4% (47 readers) said periurethral bulking
13.2% (17 readers) said antimuscarinic agents
The correct answer was periurethral bulking, as bladder retraining and antimuscarinic agents, according to TABLE 1 in Murillo and Zyczynski’s article, are appropriate for managing urge urinary incontinence.

In the article, “Nonsurgical treatments for patients with urinary incontinence” (OBG Manag. September 2022;34:36- 42.), the authors, Ashley J. Murillo, MD, and Halina M. Zyczynski, MD, discuss the successful nonsurgical management of urge urinary incontinence, stress urinary incontinence, and mixed urinary incontinence, presenting the case of a 39-year-old woman with urine leakage during exercise. As a follow-up for readers, OBG Management posted a quiz question asking, “Which of the following is a nonsurgical treatment for stress urinary incontinence?”
A total of 129 readers cast their vote:
50.4% (65 readers) said bladder retraining
36.4% (47 readers) said periurethral bulking
13.2% (17 readers) said antimuscarinic agents
The correct answer was periurethral bulking, as bladder retraining and antimuscarinic agents, according to TABLE 1 in Murillo and Zyczynski’s article, are appropriate for managing urge urinary incontinence.

Dermoscopy, other modalities for improving melanoma diagnoses reviewed
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
AT MELANOMA 2023
No spike in overdose deaths from relaxed buprenorphine regulations
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers say the data add weight to the argument for permanently adopting the pandemic-era prescribing regulations for buprenorphine, a treatment for opioid use disorder.
“We saw no evidence that increased availability of buprenorphine through the loosening of rules around prescribing and dispensing of buprenorphine during the pandemic increased overdose deaths,” investigator Wilson Compton, MD, deputy director of the National Institute on Drug Abuse, told this news organization.
“This is reassuring that, even when we opened up the doors to easier access to buprenorphine, we didn’t see that most serious consequence,” Dr. Compton said.
The findings were published online in JAMA Network Open .
Cause and effect
Federal agencies relaxed prescribing regulations for buprenorphine in March 2020 to make it easier for clinicians to prescribe the drug via telemedicine and for patients to take the medication at home.
The number of buprenorphine prescriptions has increased since that change, with more than 1 million people receiving the medication in 2021 from retail pharmacies in the United States.
However, questions remained about whether increased access would lead to an increase in buprenorphine-involved overdose.
Researchers with NIDA and the Centers for Disease Control and Prevention analyzed data from the State Unintentional Drug Overdose Reporting System, a CDC database that combines medical examiner and coroner reports and postmortem toxicology testing.
The study included information about overdose deaths from July 2019 to June 2021 in 46 states and the District of Columbia.
Between July 2019 and June 2021, there were 1,955 buprenorphine-involved overdose deaths, which accounted for 2.2% of all drug overdose deaths and 2.6% of opioid-involved overdose deaths.
However, researchers went beyond overall numbers and evaluated details from coroner’s and medical examiner reports, something they had not done before.
“For the first time we looked at the characteristics of decedents from buprenorphine because this has not been studied in this type of detail with a near-national sample,” Dr. Compton said.
“That allowed us to look at patterns of use of other substances as well as the circumstances that are recorded at the death scene that are in the data set,” he added.
Important insights
Reports from nearly all buprenorphine-involved deaths included the presence of at least one other drug, compared with opioid overdose deaths that typically involved only one drug.
“This is consistent with the pharmacology of buprenorphine being a partial agonist, so it may not be as fatal all by itself as some of the other opioids,” Dr. Compton said.
Deaths involving buprenorphine were less likely to include illicitly manufactured fentanyls, and other prescription medications were more often found on the scene, such as antidepressants.
Compared with opioid decedents, buprenorphine decedents were more likely to be women, age 35-44, White, and receiving treatment for mental health conditions, including for substance use disorder (SUD).
These kinds of characteristics provide important insights about potential ways to improve safety and clinical outcomes, Dr. Compton noted.
“When we see things like a little higher rate of SUD treatment and this evidence of other prescription drugs on the scene, and some higher rates of antidepressants in these decedents than I might have expected, I’m very curious about their use of other medical services outside of substance use treatment, because that might be a place where some interventions could be implemented,” he said.
A similar study showed pandemic-era policy changes that allowed methadone to be taken at home was followed by a decrease in methadone-related overdose deaths.
The new findings are consistent with those results, Dr. Compton said.
‘Chipping away’ at stigma
Commenting on the study, O. Trent Hall, DO, assistant professor of addiction medicine, Department of Psychiatry and Behavioral Health, Ohio State University Wexner Medical Center, Columbus, said that, although he welcomed the findings, they aren’t unexpected.
“Buprenorphine is well established as a safe and effective medication for opioid use disorder and as a physician who routinely cares for patients in the hospital after opioid overdose, I am not at all surprised by these results,” said Dr. Hall, who was not involved with the research.
“When my patients leave the hospital with a buprenorphine prescription, they are much less likely to return with another overdose or serious opioid-related medical problem,” he added.
U.S. drug overdose deaths topped 100,000 for the first time in 2021, and most were opioid-related. Although the latest data from the CDC shows drug overdose deaths have been declining slowly since early 2022, the numbers remain high.
Buprenorphine is one of only two drugs known to reduce the risk of opioid overdose. While prescriptions have increased since 2020, the medication remains underutilized, despite its known effectiveness in treating opioid use disorder.
Dr. Hall noted that research such as the new study could help increase buprenorphine’s use.
“Studies like this one chip away at the stigma that has been misapplied to buprenorphine,” he said. “I hope this article will encourage more providers to offer buprenorphine to patients with opioid use disorder.”
The study was funded internally by NIDA and the CDC. Dr. Compton reported owning stock in General Electric, 3M, and Pfizer outside the submitted work. Dr. Hall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Can pediatricians’ offices be urgent care centers again?
If you live in a suburban or semirural community you have seen at least one urgent care center open up in the last decade. They now number nearly 12,000 nationwide and are growing in number at a 7% rate. Urgent care center patient volume surged during the pandemic and an industry trade group reports it has risen 60% since 2019 (Meyerson N. Why urgent care centers are popping up everywhere. CNN Business. 2023 Jan 28).
According to a report on the CNN Business website, this growth is the result of “convenience, gaps in primary care, high costs of emergency room visits, and increased investment by health systems and equity groups.” Initially, these centers were generally staffed by physicians (70% in 2009) but as of 2022 this number has fallen to 16%. While there are conflicting data to support the claim that urgent care centers are overprescribing, it is pretty clear that their presence in a community encourages fragmented care and weakens established provider-patient relationships. One study has shown that although urgent care centers can prevent a costly emergency room visit ($1,649/visit) this advantage is offset by urgent care cost of more than $6,000.
In the same CNN report, Susan Kressly MD, chair of the AAP’s Private Payer Advocacy Advisory Committee, said: “There’s a need to keep up with society’s demand for quick turnaround, on-demand services that can’t be supported by underfunded primary care.”
Her observation suggests that there is an accelerating demand for timely primary care services. From my perch here in semirural Maine, I don’t see an increasing or unreasonable demand for timeliness by patients and families. Two decades ago, the practice I was in offered evening and weekend morning office hours and call-in times when patientsor parents could speak directly to a physician. These avenues of accessibility have disappeared community wide.
Back in the 1990s “the medical home” was all the buzz. We were encouraged to be the first and primary place to go for a broad range of preventive and responsive care. One-stop shopping at its best. Now it’s “knock, knock ... is anybody home?” Not if it’s getting dark, or it’s the weekend, or you have a minor injury. “Please call the urgent care center.”
I will admit that our dedicated call-in times were unusual and probably not sustainable for most practices. But, most practices back then would see children with acute illness and minor scrapes and trauma on a same-day basis. We dressed burns, splinted joints, and closed minor lacerations. What has changed to create the void that urgent care centers see as an opportunity to make money?
One explanation is the difficulty in finding folks (both providers and support people) who are willing to work a schedule that includes evenings and weekends. One study predicts that there will be a shortfall of 55,000 primary care physicians in the next decade, regardless of their work-life balance preferences. Sometimes it is a lack of creativity and foresight in creating flexible booking schedules that include ample time for patient- and parent-friendly same-day appointments. Minor injuries and skin problems can usually be managed quickly and effectively by an experienced clinician. Unquestionably, one of the big changes has been the shift in the patient mix leaning more toward time-consuming mental health complaints, which make it more difficult to leave open same-day slots. Restoring pediatricians’ offices to their former role as urgent care centers will require training not just more primary care physicians but also mental health consultants and providers.
First, we must decide that we want to become a real medical home that answers to a knock with a receptive response at almost any hour. By failing to accept the challenge of seeing our patients in a timely manner for their minor problems we will continue to fragment their care and threaten to make our relationship with them increasingly irrelevant.
It will mean rethinking how we schedule ourselves and our offices. It may require taking a hard look at how we spend our professional time. For example are annual checkups a must for every child at every age? Are all follow-up visits equally important? Would a phone call be just as effective? Most of all it will require adopting a mindset that we want to be complete physicians for our patients.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If you live in a suburban or semirural community you have seen at least one urgent care center open up in the last decade. They now number nearly 12,000 nationwide and are growing in number at a 7% rate. Urgent care center patient volume surged during the pandemic and an industry trade group reports it has risen 60% since 2019 (Meyerson N. Why urgent care centers are popping up everywhere. CNN Business. 2023 Jan 28).
According to a report on the CNN Business website, this growth is the result of “convenience, gaps in primary care, high costs of emergency room visits, and increased investment by health systems and equity groups.” Initially, these centers were generally staffed by physicians (70% in 2009) but as of 2022 this number has fallen to 16%. While there are conflicting data to support the claim that urgent care centers are overprescribing, it is pretty clear that their presence in a community encourages fragmented care and weakens established provider-patient relationships. One study has shown that although urgent care centers can prevent a costly emergency room visit ($1,649/visit) this advantage is offset by urgent care cost of more than $6,000.
In the same CNN report, Susan Kressly MD, chair of the AAP’s Private Payer Advocacy Advisory Committee, said: “There’s a need to keep up with society’s demand for quick turnaround, on-demand services that can’t be supported by underfunded primary care.”
Her observation suggests that there is an accelerating demand for timely primary care services. From my perch here in semirural Maine, I don’t see an increasing or unreasonable demand for timeliness by patients and families. Two decades ago, the practice I was in offered evening and weekend morning office hours and call-in times when patientsor parents could speak directly to a physician. These avenues of accessibility have disappeared community wide.
Back in the 1990s “the medical home” was all the buzz. We were encouraged to be the first and primary place to go for a broad range of preventive and responsive care. One-stop shopping at its best. Now it’s “knock, knock ... is anybody home?” Not if it’s getting dark, or it’s the weekend, or you have a minor injury. “Please call the urgent care center.”
I will admit that our dedicated call-in times were unusual and probably not sustainable for most practices. But, most practices back then would see children with acute illness and minor scrapes and trauma on a same-day basis. We dressed burns, splinted joints, and closed minor lacerations. What has changed to create the void that urgent care centers see as an opportunity to make money?
One explanation is the difficulty in finding folks (both providers and support people) who are willing to work a schedule that includes evenings and weekends. One study predicts that there will be a shortfall of 55,000 primary care physicians in the next decade, regardless of their work-life balance preferences. Sometimes it is a lack of creativity and foresight in creating flexible booking schedules that include ample time for patient- and parent-friendly same-day appointments. Minor injuries and skin problems can usually be managed quickly and effectively by an experienced clinician. Unquestionably, one of the big changes has been the shift in the patient mix leaning more toward time-consuming mental health complaints, which make it more difficult to leave open same-day slots. Restoring pediatricians’ offices to their former role as urgent care centers will require training not just more primary care physicians but also mental health consultants and providers.
First, we must decide that we want to become a real medical home that answers to a knock with a receptive response at almost any hour. By failing to accept the challenge of seeing our patients in a timely manner for their minor problems we will continue to fragment their care and threaten to make our relationship with them increasingly irrelevant.
It will mean rethinking how we schedule ourselves and our offices. It may require taking a hard look at how we spend our professional time. For example are annual checkups a must for every child at every age? Are all follow-up visits equally important? Would a phone call be just as effective? Most of all it will require adopting a mindset that we want to be complete physicians for our patients.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
If you live in a suburban or semirural community you have seen at least one urgent care center open up in the last decade. They now number nearly 12,000 nationwide and are growing in number at a 7% rate. Urgent care center patient volume surged during the pandemic and an industry trade group reports it has risen 60% since 2019 (Meyerson N. Why urgent care centers are popping up everywhere. CNN Business. 2023 Jan 28).
According to a report on the CNN Business website, this growth is the result of “convenience, gaps in primary care, high costs of emergency room visits, and increased investment by health systems and equity groups.” Initially, these centers were generally staffed by physicians (70% in 2009) but as of 2022 this number has fallen to 16%. While there are conflicting data to support the claim that urgent care centers are overprescribing, it is pretty clear that their presence in a community encourages fragmented care and weakens established provider-patient relationships. One study has shown that although urgent care centers can prevent a costly emergency room visit ($1,649/visit) this advantage is offset by urgent care cost of more than $6,000.
In the same CNN report, Susan Kressly MD, chair of the AAP’s Private Payer Advocacy Advisory Committee, said: “There’s a need to keep up with society’s demand for quick turnaround, on-demand services that can’t be supported by underfunded primary care.”
Her observation suggests that there is an accelerating demand for timely primary care services. From my perch here in semirural Maine, I don’t see an increasing or unreasonable demand for timeliness by patients and families. Two decades ago, the practice I was in offered evening and weekend morning office hours and call-in times when patientsor parents could speak directly to a physician. These avenues of accessibility have disappeared community wide.
Back in the 1990s “the medical home” was all the buzz. We were encouraged to be the first and primary place to go for a broad range of preventive and responsive care. One-stop shopping at its best. Now it’s “knock, knock ... is anybody home?” Not if it’s getting dark, or it’s the weekend, or you have a minor injury. “Please call the urgent care center.”
I will admit that our dedicated call-in times were unusual and probably not sustainable for most practices. But, most practices back then would see children with acute illness and minor scrapes and trauma on a same-day basis. We dressed burns, splinted joints, and closed minor lacerations. What has changed to create the void that urgent care centers see as an opportunity to make money?
One explanation is the difficulty in finding folks (both providers and support people) who are willing to work a schedule that includes evenings and weekends. One study predicts that there will be a shortfall of 55,000 primary care physicians in the next decade, regardless of their work-life balance preferences. Sometimes it is a lack of creativity and foresight in creating flexible booking schedules that include ample time for patient- and parent-friendly same-day appointments. Minor injuries and skin problems can usually be managed quickly and effectively by an experienced clinician. Unquestionably, one of the big changes has been the shift in the patient mix leaning more toward time-consuming mental health complaints, which make it more difficult to leave open same-day slots. Restoring pediatricians’ offices to their former role as urgent care centers will require training not just more primary care physicians but also mental health consultants and providers.
First, we must decide that we want to become a real medical home that answers to a knock with a receptive response at almost any hour. By failing to accept the challenge of seeing our patients in a timely manner for their minor problems we will continue to fragment their care and threaten to make our relationship with them increasingly irrelevant.
It will mean rethinking how we schedule ourselves and our offices. It may require taking a hard look at how we spend our professional time. For example are annual checkups a must for every child at every age? Are all follow-up visits equally important? Would a phone call be just as effective? Most of all it will require adopting a mindset that we want to be complete physicians for our patients.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Keeping physician stress in check
Fahri Saatcioglu, PhD, and colleagues, whose report was published in the Journal of Clinical Oncology, described it as a “dire situation” with resolutions needed “urgently” to “mitigate the negative consequences of physician burnout.” Both individual and whole-system approaches are needed, wrote Dr. Saatcioglu, a researcher with Oslo University Hospital in Norway who reviewed well-being interventions designed to mitigate physician stress.
When burnout sets in it is marked by emotional exhaustion, depersonalization, and a lack of confidence in one’s ability to do his or her job effectively (often because of lack of support or organizational constraints). It can lead to reduced work efficacy, medical errors, job dissatisfaction, and turnover, Fay J. Hlubocky, PhD, and colleagues, wrote in a report published in the Journal of Clinical Oncology.
During the COVID-19 pandemic, patients postponed doctor visits and procedures. Telemedicine was adopted in place of in-person visits, surgeries were delayed, and oral chemotherapy was prescribed over intravenous therapies, wrote Dr. Hlubocky and colleagues, who addressed the heightened sense of burnout oncologists experienced during the COVID-19 pandemic.
But before the pandemic, oncologists were already overburdened by a system unable to meet the demand for services. And now, because patients delayed doctor visits, more patients are being diagnosed with advanced malignancies.
According to the American Society of Clinical Oncology, the demand for cancer-related services is expected to grow by 40% over the next 6 years. And, by 2025, there will be a shortage of more than 2,200 oncologists in the United States.
Addressing physician burnout can affect the bottom line. According to a report published in Annals of Internal Medicine, physician turnover and reduced clinical hours due to burnout costs the United States $4.6 billion each year.
“It is estimated that 30%-50% of physicians either have burnout symptoms or they experience burnout. A recent study on oncologists in Canada found that symptoms of burnout may reach 73%,” wrote Dr. Saatcioglu and colleagues. “It is clear, for example, that an appropriate workload, resource sufficiency, positive work culture and values, and sufficient social and community support are all very critical for a sustainable and successful health care organization. All of these are also required for the professional satisfaction and well-being of physicians.”
Physician stress has become so serious, that Dr. Saatcioglu and colleagues recommend that hospital administrators “firmly establish the culture of wellness at the workplace” by including physician wellness under the institutional initiatives umbrella. Hospital leadership, they wrote, should strive to mitigate burnout at all levels by addressing issues and adopting strategies for physicians as a workforce and as individuals.
“There is a distinct need to approach the personal needs of the physician as an individual who is experiencing chronic stress that can trigger psychologic symptoms, which further affects not only their own health, family life, etc., but also their clinical performance, quality of the resulting health care, patient satisfaction, and finally the health economy,” the authors wrote.
Some health care organizations have adopted programs and made institutional changes designed to reduce burnout for health care workers. These include online wellness programs both free and paid, but there is little data on the efficacy of these programs.
The review by Dr. Saatcioglu and coauthors included the Online Breath and Meditation Program, a Sudarshan Kriya Yoga (SKY) program of three 90-minute sessions on yoga, effective breathing techniques, and cognitive coping and stressor evaluation strategies that have been effective in helping war veterans, prisoners, patients, and students. The ultimate goal would be to have participants adopt a daily yoga routine. Among 803 health care workers who participated in the program and completed a survey, 85% said they benefited from the program and 94% reported experiencing less stress. And, 81% felt the program would help improve their job performance.
“In the future, we believe that the best place for the individual approaches to physician wellness would be to have them as an integral part of the organizational measures, and ideally, implemented as part of the daily work routine of the physician where the organizational and individual responsibilities would merge,” the authors wrote.
Freelance writer Lorraine L. Janeczko, MPH, contributed to this article.
Fahri Saatcioglu, PhD, and colleagues, whose report was published in the Journal of Clinical Oncology, described it as a “dire situation” with resolutions needed “urgently” to “mitigate the negative consequences of physician burnout.” Both individual and whole-system approaches are needed, wrote Dr. Saatcioglu, a researcher with Oslo University Hospital in Norway who reviewed well-being interventions designed to mitigate physician stress.
When burnout sets in it is marked by emotional exhaustion, depersonalization, and a lack of confidence in one’s ability to do his or her job effectively (often because of lack of support or organizational constraints). It can lead to reduced work efficacy, medical errors, job dissatisfaction, and turnover, Fay J. Hlubocky, PhD, and colleagues, wrote in a report published in the Journal of Clinical Oncology.
During the COVID-19 pandemic, patients postponed doctor visits and procedures. Telemedicine was adopted in place of in-person visits, surgeries were delayed, and oral chemotherapy was prescribed over intravenous therapies, wrote Dr. Hlubocky and colleagues, who addressed the heightened sense of burnout oncologists experienced during the COVID-19 pandemic.
But before the pandemic, oncologists were already overburdened by a system unable to meet the demand for services. And now, because patients delayed doctor visits, more patients are being diagnosed with advanced malignancies.
According to the American Society of Clinical Oncology, the demand for cancer-related services is expected to grow by 40% over the next 6 years. And, by 2025, there will be a shortage of more than 2,200 oncologists in the United States.
Addressing physician burnout can affect the bottom line. According to a report published in Annals of Internal Medicine, physician turnover and reduced clinical hours due to burnout costs the United States $4.6 billion each year.
“It is estimated that 30%-50% of physicians either have burnout symptoms or they experience burnout. A recent study on oncologists in Canada found that symptoms of burnout may reach 73%,” wrote Dr. Saatcioglu and colleagues. “It is clear, for example, that an appropriate workload, resource sufficiency, positive work culture and values, and sufficient social and community support are all very critical for a sustainable and successful health care organization. All of these are also required for the professional satisfaction and well-being of physicians.”
Physician stress has become so serious, that Dr. Saatcioglu and colleagues recommend that hospital administrators “firmly establish the culture of wellness at the workplace” by including physician wellness under the institutional initiatives umbrella. Hospital leadership, they wrote, should strive to mitigate burnout at all levels by addressing issues and adopting strategies for physicians as a workforce and as individuals.
“There is a distinct need to approach the personal needs of the physician as an individual who is experiencing chronic stress that can trigger psychologic symptoms, which further affects not only their own health, family life, etc., but also their clinical performance, quality of the resulting health care, patient satisfaction, and finally the health economy,” the authors wrote.
Some health care organizations have adopted programs and made institutional changes designed to reduce burnout for health care workers. These include online wellness programs both free and paid, but there is little data on the efficacy of these programs.
The review by Dr. Saatcioglu and coauthors included the Online Breath and Meditation Program, a Sudarshan Kriya Yoga (SKY) program of three 90-minute sessions on yoga, effective breathing techniques, and cognitive coping and stressor evaluation strategies that have been effective in helping war veterans, prisoners, patients, and students. The ultimate goal would be to have participants adopt a daily yoga routine. Among 803 health care workers who participated in the program and completed a survey, 85% said they benefited from the program and 94% reported experiencing less stress. And, 81% felt the program would help improve their job performance.
“In the future, we believe that the best place for the individual approaches to physician wellness would be to have them as an integral part of the organizational measures, and ideally, implemented as part of the daily work routine of the physician where the organizational and individual responsibilities would merge,” the authors wrote.
Freelance writer Lorraine L. Janeczko, MPH, contributed to this article.
Fahri Saatcioglu, PhD, and colleagues, whose report was published in the Journal of Clinical Oncology, described it as a “dire situation” with resolutions needed “urgently” to “mitigate the negative consequences of physician burnout.” Both individual and whole-system approaches are needed, wrote Dr. Saatcioglu, a researcher with Oslo University Hospital in Norway who reviewed well-being interventions designed to mitigate physician stress.
When burnout sets in it is marked by emotional exhaustion, depersonalization, and a lack of confidence in one’s ability to do his or her job effectively (often because of lack of support or organizational constraints). It can lead to reduced work efficacy, medical errors, job dissatisfaction, and turnover, Fay J. Hlubocky, PhD, and colleagues, wrote in a report published in the Journal of Clinical Oncology.
During the COVID-19 pandemic, patients postponed doctor visits and procedures. Telemedicine was adopted in place of in-person visits, surgeries were delayed, and oral chemotherapy was prescribed over intravenous therapies, wrote Dr. Hlubocky and colleagues, who addressed the heightened sense of burnout oncologists experienced during the COVID-19 pandemic.
But before the pandemic, oncologists were already overburdened by a system unable to meet the demand for services. And now, because patients delayed doctor visits, more patients are being diagnosed with advanced malignancies.
According to the American Society of Clinical Oncology, the demand for cancer-related services is expected to grow by 40% over the next 6 years. And, by 2025, there will be a shortage of more than 2,200 oncologists in the United States.
Addressing physician burnout can affect the bottom line. According to a report published in Annals of Internal Medicine, physician turnover and reduced clinical hours due to burnout costs the United States $4.6 billion each year.
“It is estimated that 30%-50% of physicians either have burnout symptoms or they experience burnout. A recent study on oncologists in Canada found that symptoms of burnout may reach 73%,” wrote Dr. Saatcioglu and colleagues. “It is clear, for example, that an appropriate workload, resource sufficiency, positive work culture and values, and sufficient social and community support are all very critical for a sustainable and successful health care organization. All of these are also required for the professional satisfaction and well-being of physicians.”
Physician stress has become so serious, that Dr. Saatcioglu and colleagues recommend that hospital administrators “firmly establish the culture of wellness at the workplace” by including physician wellness under the institutional initiatives umbrella. Hospital leadership, they wrote, should strive to mitigate burnout at all levels by addressing issues and adopting strategies for physicians as a workforce and as individuals.
“There is a distinct need to approach the personal needs of the physician as an individual who is experiencing chronic stress that can trigger psychologic symptoms, which further affects not only their own health, family life, etc., but also their clinical performance, quality of the resulting health care, patient satisfaction, and finally the health economy,” the authors wrote.
Some health care organizations have adopted programs and made institutional changes designed to reduce burnout for health care workers. These include online wellness programs both free and paid, but there is little data on the efficacy of these programs.
The review by Dr. Saatcioglu and coauthors included the Online Breath and Meditation Program, a Sudarshan Kriya Yoga (SKY) program of three 90-minute sessions on yoga, effective breathing techniques, and cognitive coping and stressor evaluation strategies that have been effective in helping war veterans, prisoners, patients, and students. The ultimate goal would be to have participants adopt a daily yoga routine. Among 803 health care workers who participated in the program and completed a survey, 85% said they benefited from the program and 94% reported experiencing less stress. And, 81% felt the program would help improve their job performance.
“In the future, we believe that the best place for the individual approaches to physician wellness would be to have them as an integral part of the organizational measures, and ideally, implemented as part of the daily work routine of the physician where the organizational and individual responsibilities would merge,” the authors wrote.
Freelance writer Lorraine L. Janeczko, MPH, contributed to this article.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Spectrum of dermatologic adverse events associated with amivantamab use
associated with EGFR inhibitors and atypical presentations. Toxic effects, however, were mitigated by dose interruptions, dAE management, and amivantamab dose reductions, allowing for cancer therapy continuation in all cases. Amivantamab doses were reduced in 5 out of 6 cases, according to a research letter published in JAMA Dermatology.
The EGFR exon 20 insertion–mutation portends insensitivity to EGFR tyrosine kinase inhibitors and poor prognosis. Amivantamab, a bispecific monoclonal antibody targeting EGFR and mesenchymal epithelial transition factor (MET) is Food and Drug Administration approved for this population. Acneiform eruptions and pruritus are the most common dAEs associated with EGFR inhibitors, with xerosis, fissures, and nail and hair changes occurring additionally. While no FDA-approved monoclonal antibody targets MET exclusively, capmatinib and tepotinib (both tyrosine kinase inhibitors) inhibit MET. They have been associated with photosensitivity, acneiform rash, paronychia, xerosis, pruritus, and mucositis.
The Belzer et al. letter reviewed six consecutive cases (mean age, 58) of dAEs associated with amivantamab at two academic health centers (treated June 2021 to August 2022) in order to describe dAEs associated with amivantamab use. “I suspect the rate of dAEs with amivantamab is similar to the rate of dAEs associated with first- and second-generation EGFR inhibitors, where the majority of patients, actually 75%-90%, develop cutaneous toxicity,” said Jonathan Leventhal, MD, Yale University, New Haven, Conn., corresponding author for the Belzer et al. letter.
Time from treatment initiation with amivantamab to dAE ranged from less than 1 month to 4 months. All dAEs were grade 2 or 3 and all included acneiform eruptions. These were widespread in four cases and in another case complicated by impetiginization (culture results positive for methicillin-susceptible Staphylococcus aureus), and a further case was limited to the scalp, face, upper back, and upper chest. Others with widespread acneiform eruption included the face with hyperkeratotic crust of the scalp and dermatitis of the posterior neck. Fissuring of the palms and soles was noted in two cases with widespread acneiform eruptions. Paronychia with pyogenic granulomas was reported in four cases. Another case included onycholysis with suppurative paronychia.
In five cases amivantamab was stopped but successfully reinitiated at 67%-75% of the original dose. In one case amivantamab was continued at the original dose.
Doxycycline at 100 mg twice daily was included among all of the treatments for cutaneous dAEs. Silver nitrate cautery was applied for pyogenic granulomas in clinic. The case of grade 3 acneiform eruption of the scalp and face was treated with hydrogen peroxide soaks with debridement in clinic, doxycycline, aluminum acetate soaks, and triamcinolone ointment. All dermatologic cases resolved fully without scarring.
“It is very likely that this series highlights the more severe and unusual presentations of dAEs which were referred to oncodermatology. I suspect milder presentations were likely managed by oncologists,” Dr. Leventhal said in the interview.
“It is important for dermatologists and oncologists to be aware of the more severe and atypical dAEs associated with this novel FDA-approved targeted therapy.” Dr. Belzer said. “As amivantamab use increases, oncologists and dermatologists need to collaborate to ensure swift diagnosis and management of dAEs.”
One trial, the authors stated, revealed more than half of patients receiving EGFR inhibitors taking preemptive treatment with moisturizers, sunscreen, topical corticosteroids, and an oral tetracycline to have more than a 50% reduction in grade 2 or higher dAEs. Belzer et al. concluded that prophylactic treatment, including sun protection, should be considered before initiating treatment with amivantamab.
A limitation of the study, Belzer et al. acknowledged, was the small sample size.
Dr. Leventhal reported receiving personal fees from the advisory boards of Sanofi, Regeneron, and La Roche-Posay as well as clinical trial funding from Azitra and OnQuality Pharmaceuticals outside the submitted work.
associated with EGFR inhibitors and atypical presentations. Toxic effects, however, were mitigated by dose interruptions, dAE management, and amivantamab dose reductions, allowing for cancer therapy continuation in all cases. Amivantamab doses were reduced in 5 out of 6 cases, according to a research letter published in JAMA Dermatology.
The EGFR exon 20 insertion–mutation portends insensitivity to EGFR tyrosine kinase inhibitors and poor prognosis. Amivantamab, a bispecific monoclonal antibody targeting EGFR and mesenchymal epithelial transition factor (MET) is Food and Drug Administration approved for this population. Acneiform eruptions and pruritus are the most common dAEs associated with EGFR inhibitors, with xerosis, fissures, and nail and hair changes occurring additionally. While no FDA-approved monoclonal antibody targets MET exclusively, capmatinib and tepotinib (both tyrosine kinase inhibitors) inhibit MET. They have been associated with photosensitivity, acneiform rash, paronychia, xerosis, pruritus, and mucositis.
The Belzer et al. letter reviewed six consecutive cases (mean age, 58) of dAEs associated with amivantamab at two academic health centers (treated June 2021 to August 2022) in order to describe dAEs associated with amivantamab use. “I suspect the rate of dAEs with amivantamab is similar to the rate of dAEs associated with first- and second-generation EGFR inhibitors, where the majority of patients, actually 75%-90%, develop cutaneous toxicity,” said Jonathan Leventhal, MD, Yale University, New Haven, Conn., corresponding author for the Belzer et al. letter.
Time from treatment initiation with amivantamab to dAE ranged from less than 1 month to 4 months. All dAEs were grade 2 or 3 and all included acneiform eruptions. These were widespread in four cases and in another case complicated by impetiginization (culture results positive for methicillin-susceptible Staphylococcus aureus), and a further case was limited to the scalp, face, upper back, and upper chest. Others with widespread acneiform eruption included the face with hyperkeratotic crust of the scalp and dermatitis of the posterior neck. Fissuring of the palms and soles was noted in two cases with widespread acneiform eruptions. Paronychia with pyogenic granulomas was reported in four cases. Another case included onycholysis with suppurative paronychia.
In five cases amivantamab was stopped but successfully reinitiated at 67%-75% of the original dose. In one case amivantamab was continued at the original dose.
Doxycycline at 100 mg twice daily was included among all of the treatments for cutaneous dAEs. Silver nitrate cautery was applied for pyogenic granulomas in clinic. The case of grade 3 acneiform eruption of the scalp and face was treated with hydrogen peroxide soaks with debridement in clinic, doxycycline, aluminum acetate soaks, and triamcinolone ointment. All dermatologic cases resolved fully without scarring.
“It is very likely that this series highlights the more severe and unusual presentations of dAEs which were referred to oncodermatology. I suspect milder presentations were likely managed by oncologists,” Dr. Leventhal said in the interview.
“It is important for dermatologists and oncologists to be aware of the more severe and atypical dAEs associated with this novel FDA-approved targeted therapy.” Dr. Belzer said. “As amivantamab use increases, oncologists and dermatologists need to collaborate to ensure swift diagnosis and management of dAEs.”
One trial, the authors stated, revealed more than half of patients receiving EGFR inhibitors taking preemptive treatment with moisturizers, sunscreen, topical corticosteroids, and an oral tetracycline to have more than a 50% reduction in grade 2 or higher dAEs. Belzer et al. concluded that prophylactic treatment, including sun protection, should be considered before initiating treatment with amivantamab.
A limitation of the study, Belzer et al. acknowledged, was the small sample size.
Dr. Leventhal reported receiving personal fees from the advisory boards of Sanofi, Regeneron, and La Roche-Posay as well as clinical trial funding from Azitra and OnQuality Pharmaceuticals outside the submitted work.
associated with EGFR inhibitors and atypical presentations. Toxic effects, however, were mitigated by dose interruptions, dAE management, and amivantamab dose reductions, allowing for cancer therapy continuation in all cases. Amivantamab doses were reduced in 5 out of 6 cases, according to a research letter published in JAMA Dermatology.
The EGFR exon 20 insertion–mutation portends insensitivity to EGFR tyrosine kinase inhibitors and poor prognosis. Amivantamab, a bispecific monoclonal antibody targeting EGFR and mesenchymal epithelial transition factor (MET) is Food and Drug Administration approved for this population. Acneiform eruptions and pruritus are the most common dAEs associated with EGFR inhibitors, with xerosis, fissures, and nail and hair changes occurring additionally. While no FDA-approved monoclonal antibody targets MET exclusively, capmatinib and tepotinib (both tyrosine kinase inhibitors) inhibit MET. They have been associated with photosensitivity, acneiform rash, paronychia, xerosis, pruritus, and mucositis.
The Belzer et al. letter reviewed six consecutive cases (mean age, 58) of dAEs associated with amivantamab at two academic health centers (treated June 2021 to August 2022) in order to describe dAEs associated with amivantamab use. “I suspect the rate of dAEs with amivantamab is similar to the rate of dAEs associated with first- and second-generation EGFR inhibitors, where the majority of patients, actually 75%-90%, develop cutaneous toxicity,” said Jonathan Leventhal, MD, Yale University, New Haven, Conn., corresponding author for the Belzer et al. letter.
Time from treatment initiation with amivantamab to dAE ranged from less than 1 month to 4 months. All dAEs were grade 2 or 3 and all included acneiform eruptions. These were widespread in four cases and in another case complicated by impetiginization (culture results positive for methicillin-susceptible Staphylococcus aureus), and a further case was limited to the scalp, face, upper back, and upper chest. Others with widespread acneiform eruption included the face with hyperkeratotic crust of the scalp and dermatitis of the posterior neck. Fissuring of the palms and soles was noted in two cases with widespread acneiform eruptions. Paronychia with pyogenic granulomas was reported in four cases. Another case included onycholysis with suppurative paronychia.
In five cases amivantamab was stopped but successfully reinitiated at 67%-75% of the original dose. In one case amivantamab was continued at the original dose.
Doxycycline at 100 mg twice daily was included among all of the treatments for cutaneous dAEs. Silver nitrate cautery was applied for pyogenic granulomas in clinic. The case of grade 3 acneiform eruption of the scalp and face was treated with hydrogen peroxide soaks with debridement in clinic, doxycycline, aluminum acetate soaks, and triamcinolone ointment. All dermatologic cases resolved fully without scarring.
“It is very likely that this series highlights the more severe and unusual presentations of dAEs which were referred to oncodermatology. I suspect milder presentations were likely managed by oncologists,” Dr. Leventhal said in the interview.
“It is important for dermatologists and oncologists to be aware of the more severe and atypical dAEs associated with this novel FDA-approved targeted therapy.” Dr. Belzer said. “As amivantamab use increases, oncologists and dermatologists need to collaborate to ensure swift diagnosis and management of dAEs.”
One trial, the authors stated, revealed more than half of patients receiving EGFR inhibitors taking preemptive treatment with moisturizers, sunscreen, topical corticosteroids, and an oral tetracycline to have more than a 50% reduction in grade 2 or higher dAEs. Belzer et al. concluded that prophylactic treatment, including sun protection, should be considered before initiating treatment with amivantamab.
A limitation of the study, Belzer et al. acknowledged, was the small sample size.
Dr. Leventhal reported receiving personal fees from the advisory boards of Sanofi, Regeneron, and La Roche-Posay as well as clinical trial funding from Azitra and OnQuality Pharmaceuticals outside the submitted work.
FROM JAMA DERMATOLOGY
Acute cardiac events common during COVID hospitalization
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
particularly among those with underlying heart disease, and are associated with more severe disease outcomes, a new study suggests.
“We expected to see acute cardiac events occurring among adults hospitalized with COVID-19 but were surprised by how frequently they occurred,” Rebecca C. Woodruff, PhD, MPH, of the U.S. Centers for Disease Control and Prevention, Atlanta, told this news organization.
Overall, she said, “about 1 in 10 adults experienced an acute cardiac event – including heart attacks and acute heart failure – while hospitalized with COVID-19, and this included people with no preexisting heart disease.”
However, she added, “about a quarter of those with underlying heart disease had an acute cardiac event. These patients tended to experience more severe disease outcomes relative to patients hospitalized with COVID-19 who did not experience an acute cardiac event.”
The findings might be relevant to hospitalizations for other viral diseases, “though we can’t say for sure,” she noted. “This study was modeled off a previous study conducted before the COVID-19 pandemic among adults hospitalized with influenza. About 11.7% of [those] adults experienced an acute cardiac event, which was a similar percentage as what we found among patients hospitalized with COVID-19.”
The study was published online in the Journal of the American College of Cardiology.
Underlying cardiac disease key
Dr. Woodruff and colleagues analyzed medical records on a probability sample of 8,460 adults hospitalized with SARS-CoV-2 infection identified from 99 U.S. counties in 14 U.S. states (about 10% of the United States population) from January to November 2021.
Among participants, 11.4% had an acute cardiac event during their hospitalization. The median age was 69 years; 56.5% were men; 48.7%, non-Hispanic White; 33.6%, non-Hispanic Black; 7.4%, Hispanic; and 7.1%, non-Hispanic Asian or Pacific Islander.
As indicated, the prevalence was higher among those with underlying cardiac disease (23.4%), compared with those without (6.2%).
Acute ischemic heart disease (5.5%) and acute heart failure (5.4%) were the most prevalent events; 0.3% of participants had acute myocarditis or pericarditis.
Risk factors varied, depending on underlying cardiac disease status. Those who experienced one or more acute cardiac events had a greater risk for intensive care unit admission (adjusted risk ratio,1.9) and in-hospital death (aRR, 1.7) versus those who did not.
In multivariable analyses, the risk of experiencing acute heart failure was significantly greater among men (aRR, 1.5) and among those with a history of congestive heart failure (aRR, 13.5), atrial fibrillation (aRR, 1.6) or hypertension (aRR,1.3).
Among patients who experienced one or more acute cardiac events, 39.2% required an intensive care unit stay for a median of 5 days. Approximately 22.4% required invasive mechanical ventilation or extracorporeal membrane oxygenation, and 21.1% died while hospitalized.
“Persons at greater risk for experiencing acute cardiac events during COVID-19–associated hospitalizations might benefit from more intensive clinical evaluation and monitoring during hospitalization,” the authors conclude.
The team currently is taking a closer look at acute myocarditis among patients hospitalized with COVID-19, Dr. Woodruff said. Preliminary results were presented at the 2022 annual scientific sessions of the American Heart Association and a paper is forthcoming.
Contemporary data needed
James A. de Lemos, MD, co-chair of the American Heart Association’s COVID-19 CVD Registry Steering Committee and professor of medicine at the University of Texas Southwestern Medical Center, Dallas, said the findings mirror his team’s clinical experience in 2020 and 2021 and echo what was seen in the AHA COVID registry: that is, a 0.3% rate of myocarditis.
“The major caveat is that [the findings] may not be generalizable to contemporary COVID infection, both due to changing viral variants and higher levels of immunity in the population,” he said.
“Rates of COVID hospitalization are markedly lower with the current dominant variants, and we would expect the cardiac risk to be lower as well. I would like to see more contemporary data with current variants, particularly focused on higher risk patients with cardiovascular disease,” Dr. de Lemos added.
In a related editorial, George A. Mensa, MD, of the National Heart, Lung, and Blood Institute in Bethesda, Md., and colleagues suggest that the broader impact of the COVID-19 pandemic on human health remains “incompletely examined.”
“The impact of COVID-19 on cardiovascular mortality, in particular, appears to have varied widely, with no large increases seen in a number of the most developed countries but marked increases in hypertensive heart disease mortality seen in the United States in 2021,” they conclude. “The potential contribution of COVID-19 to these deaths, either directly or indirectly, remains to be determined.”
No commercial funding or relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Increased dietary fiber intake might protect from migraine
Key clinical point: Increased intake of dietary fiber seemed protective against migraine or severe headache in a large population of US adults.
Major finding: Risk for migraine or severe headache was 26% lower among patients in the highest (22.10-95.50 g/day) vs lowest (0.0-7.79 g/day) quintile of dietary fiber intake (adjusted odds ratio 0.74; P = .0029).
Study details: This cross-sectional study included 12,710 participants from the US National Health and Nutrition Examination Survey, of which 2527 experienced migraine or severe headache.
Disclosures: This study did not declare the source of funding. The authors declared no conflicts of interest.
Source: Huang H and He K. The association between dietary fiber intake and severe headaches or migraine in US adults. Front Nutr. 2023;9:1044066 (Jan 4). Doi: 10.3389/fnut.2022.1044066
Key clinical point: Increased intake of dietary fiber seemed protective against migraine or severe headache in a large population of US adults.
Major finding: Risk for migraine or severe headache was 26% lower among patients in the highest (22.10-95.50 g/day) vs lowest (0.0-7.79 g/day) quintile of dietary fiber intake (adjusted odds ratio 0.74; P = .0029).
Study details: This cross-sectional study included 12,710 participants from the US National Health and Nutrition Examination Survey, of which 2527 experienced migraine or severe headache.
Disclosures: This study did not declare the source of funding. The authors declared no conflicts of interest.
Source: Huang H and He K. The association between dietary fiber intake and severe headaches or migraine in US adults. Front Nutr. 2023;9:1044066 (Jan 4). Doi: 10.3389/fnut.2022.1044066
Key clinical point: Increased intake of dietary fiber seemed protective against migraine or severe headache in a large population of US adults.
Major finding: Risk for migraine or severe headache was 26% lower among patients in the highest (22.10-95.50 g/day) vs lowest (0.0-7.79 g/day) quintile of dietary fiber intake (adjusted odds ratio 0.74; P = .0029).
Study details: This cross-sectional study included 12,710 participants from the US National Health and Nutrition Examination Survey, of which 2527 experienced migraine or severe headache.
Disclosures: This study did not declare the source of funding. The authors declared no conflicts of interest.
Source: Huang H and He K. The association between dietary fiber intake and severe headaches or migraine in US adults. Front Nutr. 2023;9:1044066 (Jan 4). Doi: 10.3389/fnut.2022.1044066
What is the psychological cost of performing CPR?
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
One year ago, as the sun was setting on a late fall day, Andrés Snitcofsky, a 40-year-old designer from Buenos Aires, Argentina, heard harrowing cries for help. It was the niece and the wife of one of his neighbors: a man in his 60s who the women had found “passed out” in the bedroom. “I did CPR for 5 minutes straight until a friend of the victim came in and asked me to stop, telling me that the man had probably been dead for 2 or 3 hours already. But I had no idea because I’d never seen a dead body before,” Mr. Snitcofsky told this news organization. A few minutes later, the ambulance arrived. The doctor confirmed that there was nothing more that could be done.
Mr. Snitcofsky went home. Nobody had asked for his name or address or phone number. … And it wasn’t because they already knew who he was. In fact, there wasn’t any sort of relationship there. Mr. Snitcofsky had only known his neighbors by sight. His actions that day, however, “did not come without a cost. It took me weeks – months, actually – to put myself together again,” he said. The things he saw, the things he heard, everything about that night played over and over in his head. “I had trouble sleeping. I would play out different scenarios in my head. I questioned myself. I second-guessed myself, criticized myself. It’s like some taboo subject. There’s no one to share the experience with, no one who gets it. But with time, I was able to process the event.
“For 2 months, I talked to my psychologist about it all,” he continued. “That really helped me a lot. In addition to therapy, I reached out to a couple I know – they’re both physicians – and to a firefighter who teaches CPR. Their insight and guidance allowed me to get to a point where I was able to understand that what I did was a good thing and that what I did was all that could have been done. But anyone who finds themselves in the position of having to do CPR – they’re going to be affected in many, many ways. It goes beyond the euphoria of seeing a person come back to life. Of that, I’m quite certain.”
We’ve all seen campaigns encouraging people to learn CPR and to be prepared if the need arises. But in training the public (and even health care professionals), not much, if anything, is said about the “collateral damage”: the psychological and emotional consequences of carrying out the procedure. These especially come into play when you don’t know whether the person survived, when your efforts weren’t able to reverse the sudden cardiac arrest, or when the person you gave CPR to was a loved one – a case that may entail immediate therapeutic interventions to minimize or prevent the risk of suffering long-lasting trauma.
In May 2020, popular American activist and educator Kristin Flanary saw someone suffering cardiac arrest. She stepped in and started doing CPR. And she continued doing CPR … for 10 long minutes. The person she was trying to save was her 34-year-old husband, ophthalmologist and comedian Will Flanary. On Twitter, where she’s known as Lady Glaucomflecken, Ms. Flanary recently shared the following message, putting the topic of CPR and automated external defibrillator training front and center.
“Yes, everyone should learn #CPRandAED. But if we are going to ask people to perform such a brutal task, it’s imperative that we also provide them with the info and resources they need to process it mentally and emotionally. It’s traumatic and life changing. It’s irresponsible and unethical to ask people to help in such a brutal and traumatic way and then neglect to help them in return.” In less than a month, the tweet has racked up over 200,000 views.
Doing one’s duty
There are many people who work to promote CPR and strengthen the other links in the chain of survival for out-of-hospital sudden cardiac arrest, such as prompt access to and delivery of early defibrillation. According to them, any negative psychological impact of intervening is temporary and, when compared with the satisfaction of having done one’s duty, quite insignificant – even if the efforts to save a person’s life are not successful.
“In 99.9% of cases, people who have performed CPR feel a sense of satisfaction, even happiness, knowing that they’ve helped. The individuals I’ve spoken with, I’ve never heard any of them say that they felt worse after the event or that they needed to see a psychologist,” said Mario Fitz Maurice, MD, director of the Arrhythmia Council of the Argentine Society of Cardiology and head of Electrophysiology at Rivadavia Hospital in Buenos Aires. He went on to tell this news organization, “Of course, some degree of fear, sadness, or melancholy can remain afterward. But it seems to me, and there are reports saying as much, that, in the end, what stands out in the person’s mind is the fact that they tried to save a life. And for them, there’s joy in knowing this.”
Dr. Fitz Maurice, who is also the director of the National Arrhythmia Institute in Buenos Aires, pointed out that the kind of person who takes CPR classes “has a profile that’s going to allow them to be psychologically involved; they’re the caring person, the one who’s ready and willing to help people.” And he added that, at his hospital, if they can identify the individuals or first responders who have done CPR on a patient, the protocol is to always contact them to offer psychological care and assistance. “But in 99% of cases, they don’t even understand why we’re calling them, they’re extremely happy to have taken part.”
Some studies, though, paint a much different picture, one that shows that providing CPR can be emotionally challenging and have consequences in terms of one’s family and work life. A qualitative study published in 2016 looked into the experiences of 20 lay rescuers in Norway – five were health educated – who had provided CPR to 18 out-of-hospital cardiac arrest (OHCA) victims, 66% of whom survived. The time from experiencing the OHCA incident to participating in the interview ranged from 6 days to 13 years (median 5.5 years). Several participants reported the OHCA incident as a “shocking and terrifying” experience. Tiredness, exhaustion, confusion, and feeling alone about the OHCA experience were individual reactions that could vary in time from days to months. Anxiety and insomnia were also experienced following the incident.
Some lay rescuers described the influence on work and family life, and a few of them described deep sorrow, even several years after the incident. Overall, they reported repetitive self-criticism regarding whether they could have carried out anything else to achieve a better outcome for the cardiac arrest victim. All of them wanted to be informed about the outcome. And four of the lay rescuers needed professional counseling to process the OHCA experience.
In 2020, another qualitative study was conducted, this time in Taiwan. There were nine participants, none of whom were health professionals. Each had provided initial CPR and defibrillation with AED in public locations. Event-to-interview duration was within 1 year and 1-2 years. The major findings from the study were the following:
- The lay rescuers possessed helping traits and high motivation.
- The lay rescuers reported certain aspects of rescue reality that differed much from prior training and expectations, including difficulty in the depth of chest compression, and uncertainties in real emergency situations.
- The lay rescuers gained positive personal fulfillment in sharing their experience and receiving positive feedback from others, and were willing to help next time, although they experienced a short-term negative psychological impact from the event. “Measures should be taken to increase [a] layperson’s confidence and situation awareness, to reduce training-reality discrepancy, and to build up a support system to avoid negative psychological effects.” This was the conclusion of the study team, which was led by Matthew Huei-Ming Ma, MD, PhD. A professor in the department of emergency medicine at National Taiwan University in Taipei, he is also on the board of directors of the Resuscitation Council of Asia.
Potential trauma
In recalling his experience, Mr. Snitcofsky said, “The hardest part of it all was the moment that I stopped giving CPR, that moment of letting go. This became the image that kept coming back to me, the traumatic moment I hadn’t thought about.”
Psychiatrist Daniel Mosca, MD, is the founder and former president of the Argentine Society of Trauma Psychology. He is also the coordinator of the human factors team at the City of Buenos Aires Emergency Medical Care System. “Any event has the potential to be traumatic, all the more so when it’s an event where you come face to face with death and uncertainty. But how a rescuer reacts will depend on their psychological makeup.” Of the individuals who were held for months or years in the jungle as hostages of the Revolutionary Armed Forces of Colombia, “only” half developed symptoms of posttraumatic stress disorder.
Dr. Mosca believes that a comment by Frank Ochberg, MD, speaks to this finding. “In many cases, peritraumatic symptoms are a normal person’s normal response to an abnormal situation.” For a lot of people who have found themselves having to perform CPR, the symptoms associated with the initial acute stress reaction will resolve on their own in 30-90 days. “But if this doesn’t happen, and those symptoms persist, psychotherapeutic or pharmacological intervention will be necessary,” he noted.
“In CPR classes, it would be good for the instructors to talk about the warning signs that people should look out for in themselves and their fellow rescuers. So, for example, insomnia, anxiety, a heightened state of alertness, feeling disconnected from reality,” Dr. Mosca told this news organization.
“Another thing that can help rescuers is letting them know what happened to the person they gave CPR to. This way, they can get closure,” suggested Manlio Márquez Murillo, MD, a cardiologist and electrophysiologist in Mexico. He is also the coordinator of the Alliance Against Sudden Cardiac Death at the Interamerican Society of Cardiology.
“Medical and nursing societies would have to develop a brief protocol or performance standard. The goal would be to ensure that rescuers are asked for their contact information and that someone gets in touch to debrief them and to offer them care. Next would come the treatment part, to resolve any remaining aftereffects,” said in an interview.
For example, a three-stage Lay Responder Support Model (LRSM) was developed and implemented as part of a lay responder support program established in 2014 by the Peel Regional Council in Ontario. The LRSM identifies and engages individuals who witnessed or participated directly or indirectly in an OHCA, inviting them to participate in a debriefing session facilitated by a trained practitioner. Held 24-48 hours post event, the debriefing allows lay responders to contextualize their reaction to the event. The conversation also serves as an opportunity for them to fully articulate their concerns, questions, and thoughts. The facilitator can communicate stress reduction techniques and address psychological first aid needs as they emerge. Approximately 1 week post event, a secondary follow-up occurs. If the lay responder communicates a continuing struggle with symptoms impacting and interfering with everyday life, the facilitator offers a coordinated or facilitated referral for mental health support.
In an article published in the Journal of Cardiac Failure. Ms. Flanary speaks about the three kinds of language that anyone who was either forced to or inspired to perform CPR can use to help process their trauma: words that explain what happened, words that name (eg, “forgotten patients”), and words that validate the experience and allow the person to articulate their feelings. The tools and technologies that organizations and health care professionals provide can help the healing process. Empathy and compassion, too, have a place.
But there are virtually no standardized and proactive initiatives of this kind in much of the world, including Latin America. So, most people who just happened to be in the right place at the right time find that they have to navigate the “after” part all on their own.
Other obstacles
Dr. Márquez Murillo finds it unfortunate that countries in the region have yet to enact “Good Samaritan” laws. If individuals render aid to someone suffering cardiac arrest, then these laws would ensure that they will not be held liable in any way. This is the case in Argentina and Uruguay. So, the fear of things turning into a legal matter may be holding people back from taking action; that fear could also create additional stress for those who end up stepping in to help.
Even with the legal safeguards, exceptional circumstances may arise where rescuers find themselves facing unexpected emotional challenges. In Argentina, Virginia Pérez Antonelli, the 17-year-old who tried in vain to save the life of Fernando Báez Sosa, had to testify at the trial of the eight defendants accused of brutally beating him in January 2020. The press, the public – the attention of an entire country – was focused on her. She had to respond to the defense attorneys who were able to ask whether she was sure that she performed the CPR maneuvers correctly. And a few weeks ago, a medical examiner hired by the defense suggested that “the CPR may have made the situation worse” for the victim. An indignant Dr. Fitz Maurice responded on Twitter: “CPR SAVES LIVES!! Let’s not let a CHEAP AND BASELESS argument destroy all the work that’s been done…!”
Of course, there are consequences that are beyond our control and others that can, in fact, be anticipated and planned for. Dr. Fitz Maurice brought up a preventive approach: Make CPR second nature, teach it in schools, help people overcome their fears. “Cardiac deaths are 200 times more frequent than deaths resulting from fires – and we practice fire drills a lot more than we practice CPR,” he told this news organization. In a society where there is widespread training on the procedure, where people regularly practice the technique, those who have had the experience of giving someone CPR will feel less alone, will be better understood by others.
“On the other hand, beyond the initial impact and the lack of a formal support system, the medium- and long-term outcome for those who acted is also psychologically and emotionally favorable,” said Jorge Bombau, MD, an obstetrician/gynecologist in Buenos Aires. After Dr. Bombau’s 14-year-old son Beltrán suddenly died during a school sports tournament, Dr. Bombau became a prominent advocate spreading the word about CPR.
“I don’t know anyone who regrets doing CPR,” he told this news organization. “There may be a brief period when the person feels distressed or depressed, when they have trouble sleeping. But it’s been proven that doing a good deed improves one’s mood. And what better deed is there than trying to save someone’s life? Whether their efforts were successful or in vain, that person has, at the end of the day, done something meaningful and worthwhile.”
Mr. Snitcofsky shares this sentiment. For several months now, he’s been feeling he’s “in a good place.” And he’s been actively promoting CPR on social media. As he recently posted on Twitter, “I’m here to retweet everything that has to do with getting us all to become familiar with how to do CPR and working up the courage to do it. The training takes no more than a few hours.
“I want to know that, if I ever have an out-of-hospital sudden cardiac arrest, there will be neighbors, friends, or family members around who know how to do CPR. Every person who knows how to do CPR can persuade others, and those of us who’ve had to do CPR in real life are even better candidates for persuading others. And if one day a person ends up needing CPR, I want to step in again and make up for lost time. Here’s hoping it’ll do the job,” he concluded.
It’s the same for Matías Alonso, a journalist in Buenos Aires. On New Year’s Eve 15 years ago, he was at a family dinner when, a few minutes before midnight, he found himself giving CPR to his stepmother’s father. “Unfortunately, he passed away, but I continued doing CPR on him until the ambulance arrived. For some time, I felt a little guilty for not taking charge of the situation from the beginning, and because I had this idea in my head that more people pulled through and recovered. But afterwards, they really thanked me a lot. And that helped me realize that I’d done something. I didn’t stand still when faced with the inevitability of death. I understood that it was good to have tried,” Mr. Alonso told this news organization. “And next time … hopefully there won’t be a next time … but I’m more prepared, and I now know how I can do better.”
Mr. Alonso, Mr. Snitcofsky, Dr. Fitz Maurice, Dr. Mosca, Dr. Bombau, and Dr. Márquez Murillo disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from Medscape Spanish.
Index vein diagnoses migraine aura with excellent accuracy in emergency setting
Key clinical point: Index vein served as a good biomarker for migraine aura with a high diagnostic specificity and sensitivity in the emergency setting in patients with acute neurological deficit.
Major finding: Prevalence of index vein was more frequent in patients with migraine aura (17%) vs those with stroke (2%)/epileptic seizure (4%) or control participants (1%; P < .001). Index vein was highly specific to migraine aura (specificity 97%; 95% CI 95%-99%), with an ability to diagnose migraine aura with 94% sensitivity (95% CI 87.4%-97.8%) and 73.5% specificity (95% CI 66.8%-79.5%) at a cut-off of 4 points.
Study details: This retrospective case-control study included 400 patients who presented to the emergency department with an acute neurological deficit, underwent brain magnetic resonance imaging, and were discharged with a diagnosis of migraine aura/ischemic stroke/epileptic seizure or none of these (control participants).
Disclosures: This study did not receive any specific funding. Two authors declared serving as part-time employees at Zynnon or as a consultant, speaker, or advisory board member for various sources.
Source: Scutelnic A et al. The “index vein” as a sign for migraine aura in the emergency setting. Cephalalgia. 2023 (Jan 9). Doi: 10.1177/033310242211320
Key clinical point: Index vein served as a good biomarker for migraine aura with a high diagnostic specificity and sensitivity in the emergency setting in patients with acute neurological deficit.
Major finding: Prevalence of index vein was more frequent in patients with migraine aura (17%) vs those with stroke (2%)/epileptic seizure (4%) or control participants (1%; P < .001). Index vein was highly specific to migraine aura (specificity 97%; 95% CI 95%-99%), with an ability to diagnose migraine aura with 94% sensitivity (95% CI 87.4%-97.8%) and 73.5% specificity (95% CI 66.8%-79.5%) at a cut-off of 4 points.
Study details: This retrospective case-control study included 400 patients who presented to the emergency department with an acute neurological deficit, underwent brain magnetic resonance imaging, and were discharged with a diagnosis of migraine aura/ischemic stroke/epileptic seizure or none of these (control participants).
Disclosures: This study did not receive any specific funding. Two authors declared serving as part-time employees at Zynnon or as a consultant, speaker, or advisory board member for various sources.
Source: Scutelnic A et al. The “index vein” as a sign for migraine aura in the emergency setting. Cephalalgia. 2023 (Jan 9). Doi: 10.1177/033310242211320
Key clinical point: Index vein served as a good biomarker for migraine aura with a high diagnostic specificity and sensitivity in the emergency setting in patients with acute neurological deficit.
Major finding: Prevalence of index vein was more frequent in patients with migraine aura (17%) vs those with stroke (2%)/epileptic seizure (4%) or control participants (1%; P < .001). Index vein was highly specific to migraine aura (specificity 97%; 95% CI 95%-99%), with an ability to diagnose migraine aura with 94% sensitivity (95% CI 87.4%-97.8%) and 73.5% specificity (95% CI 66.8%-79.5%) at a cut-off of 4 points.
Study details: This retrospective case-control study included 400 patients who presented to the emergency department with an acute neurological deficit, underwent brain magnetic resonance imaging, and were discharged with a diagnosis of migraine aura/ischemic stroke/epileptic seizure or none of these (control participants).
Disclosures: This study did not receive any specific funding. Two authors declared serving as part-time employees at Zynnon or as a consultant, speaker, or advisory board member for various sources.
Source: Scutelnic A et al. The “index vein” as a sign for migraine aura in the emergency setting. Cephalalgia. 2023 (Jan 9). Doi: 10.1177/033310242211320