User login
Pound of flesh buys less prison time
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Maternal COVID-19 vaccine curbs infant infection
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
a new study shows.
Previous research has confirmed that COVID-19 neutralizing antibodies following maternal vaccination or maternal COVID-19 infection are present in umbilical cord blood, breast milk, and infant serum specimens, wrote Sarah C.J. Jorgensen, PharmD, MPH, of the University of Toronto, and colleagues in their article published in The BMJ.
In the study, the researchers identified maternal and newborn pairs using administrative databases from Canada. The study population included 8,809 infants aged younger than 6 months who were born between May 7, 2021, and March 31, 2022, and who underwent testing for COVID-19 between May 7, 2021, and September 5, 2022.
Maternal vaccination with the primary COVID-19 mRNA monovalent vaccine series was defined as two vaccine doses administered up to 14 days before delivery, with at least one of the doses after the conception date.
Maternal vaccination with the primary series plus one booster was defined as three doses administered up to 14 days before delivery, with at least one of these doses after the conception date.
The primary outcome was the presence of delta or omicron COVID-19 infection or hospital admission of the infants.
The study population included 99 COVID-19 cases with the delta variant (with 4,365 controls) and 1,501 cases with the omicron variant (with 4,847 controls).
Overall, the vaccine effectiveness of maternal doses was 95% against delta infection and 45% against omicron.
The effectiveness against hospital admission in cases of delta and omicron variants were 97% and 53%, respectively.
The effectiveness of three doses was 73% against omicron infant infection and 80% against omicron-related infant hospitalization. Data were not available for the effectiveness of three doses against the delta variant.
The effectiveness of two doses of vaccine against infant omicron infection was highest when mothers received the second dose during the third trimester of pregnancy, compared with during the first trimester or second trimester (53% vs. 47% and 53% vs. 37%, respectively).
Vaccine effectiveness with two doses against infant infection from omicron was highest in the first 8 weeks of life (57%), then decreased to 40% among infants after 16 weeks of age.
Although the study was not designed to assess the mechanism of action of the impact of maternal vaccination on infants, the current study results were consistent with other recent studies showing a reduction in infections and hospitalizations among infants whose mothers received COVID-19 vaccines during pregnancy, the researchers wrote in their discussion.
The findings were limited by several factors including the potential unmeasured confounders not available in databases, such as whether infants were breastfed, the researchers noted. Other limitations included a lack of data on home test results and the inability to assess the waning impact of the vaccine effectiveness against the delta variant because of the small number of delta cases, they said. However, the results suggest that the mRNA COVID-19 vaccine during pregnancy was moderately to highly effective for protection against omicron and delta infection and infection-related hospitalization – especially during the first 8 weeks of life.
Effectiveness is encouraging, but updates are needed
The effectiveness of maternal vaccination to prevent COVID-19 infection and related hospitalizations in infants is promising, especially since those younger than 6 months have no other source of vaccine protection against COVID-19 infection, wrote Dana Danino, MD, of Soroka University Medical Center, Israel, and Ilan Youngster, MD, of Shamir Medical Center, Israel, in an accompanying editorial also published in The BMJ.
They also noted that maternal vaccination during pregnancy is an established method of protecting infants from infections such as influenza and pertussis.
Data from previous studies show that most infants whose mothers were vaccinated against COVID-19 during pregnancy retained maternal antibodies at 6 months, “but evidence for protection against neonatal COVID-19 infection has been deficient,” they said.
The current study findings support the value of vaccination during pregnancy, and the findings were strengthened by the large study population, the editorialists wrote. However, whether the same effectiveness holds for other COVID-19 strains such as BQ.1, BQ.1.1, BF.7, XBB, and XBB.1 remains unknown, they said.
Other areas in need of exploration include the optimal timing of vaccination during pregnancy, the protective effects of a bivalent mRNA vaccine (vs. the primary monovalent vaccine in the current study), and the potential benefits of additional boosters, they added.
“Although Jorgenson and colleagues’ study reinforces the value of maternal vaccination against COVID-19 during pregnancy, more studies are needed to better inform vaccination recommendations in an evolving landscape of new SARS-CoV-2 strains and novel vaccines,” the editorialists concluded.
The study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care; the study also received funding from the Canadian Immunization Research Network and the Public Health Agency of Canada. Dr. Jorgensen and the editorialists had no financial conflicts to disclose.
*This article was updated on 3/2/2023.
FROM THE BMJ
Service-Related Toxin Exposure and Acute Myeloid Leukemia in Veterans
Members of the United States military who served in Vietnam between 1961 and 1971 risked exposure to the weaponized chemical defoliant known as Agent Orange. Among the components of Agent Orange, benzene and tetrachlorodibenzo-p-dioxin (TCDD) are known carcinogens linked to several cancers. They include multiple myeloma, Hodgkin and non-Hodgkin lymphoma, as well as bladder, prostate, and lung cancer.
In this ReCAP, Dr Timothy O'Brien, section chief of hematology at the Louis Stokes Cleveland VA Medical Center, examines the evidence that suggests a link between service-related Agent Orange exposure and acute myeloid leukemia (AML). He discusses preclinical models that show a relationship between benzene and TCDD exposure and the development of AML.
Dr O'Brien also explains the factors that have limited researchers' ability to positively connect Agent Orange and AML. For example, there is a dwindling cohort of affected patients to study because dioxins can lie latent in fat cells for more than a decade, delaying the development of AML. During that time, many veterans will have died from unrelated causes.
More research is needed for veterans to receive service-connected benefits for AML diagnoses. However, as Dr O'Brien notes, the PACT Act provides coverage for veterans who developed AML after exposure to benzene-contaminated water at Camp Lejeune.
--
Timothy O'Brien, MD, Associate Professor, Case Western Reserve University School of Medicine; Chief of Hematology, Louis Stokes Cleveland VA Medical Center, Cleveland, Ohio
Timothy O'Brien, MD, has disclosed no relevant financial relationships.
Members of the United States military who served in Vietnam between 1961 and 1971 risked exposure to the weaponized chemical defoliant known as Agent Orange. Among the components of Agent Orange, benzene and tetrachlorodibenzo-p-dioxin (TCDD) are known carcinogens linked to several cancers. They include multiple myeloma, Hodgkin and non-Hodgkin lymphoma, as well as bladder, prostate, and lung cancer.
In this ReCAP, Dr Timothy O'Brien, section chief of hematology at the Louis Stokes Cleveland VA Medical Center, examines the evidence that suggests a link between service-related Agent Orange exposure and acute myeloid leukemia (AML). He discusses preclinical models that show a relationship between benzene and TCDD exposure and the development of AML.
Dr O'Brien also explains the factors that have limited researchers' ability to positively connect Agent Orange and AML. For example, there is a dwindling cohort of affected patients to study because dioxins can lie latent in fat cells for more than a decade, delaying the development of AML. During that time, many veterans will have died from unrelated causes.
More research is needed for veterans to receive service-connected benefits for AML diagnoses. However, as Dr O'Brien notes, the PACT Act provides coverage for veterans who developed AML after exposure to benzene-contaminated water at Camp Lejeune.
--
Timothy O'Brien, MD, Associate Professor, Case Western Reserve University School of Medicine; Chief of Hematology, Louis Stokes Cleveland VA Medical Center, Cleveland, Ohio
Timothy O'Brien, MD, has disclosed no relevant financial relationships.
Members of the United States military who served in Vietnam between 1961 and 1971 risked exposure to the weaponized chemical defoliant known as Agent Orange. Among the components of Agent Orange, benzene and tetrachlorodibenzo-p-dioxin (TCDD) are known carcinogens linked to several cancers. They include multiple myeloma, Hodgkin and non-Hodgkin lymphoma, as well as bladder, prostate, and lung cancer.
In this ReCAP, Dr Timothy O'Brien, section chief of hematology at the Louis Stokes Cleveland VA Medical Center, examines the evidence that suggests a link between service-related Agent Orange exposure and acute myeloid leukemia (AML). He discusses preclinical models that show a relationship between benzene and TCDD exposure and the development of AML.
Dr O'Brien also explains the factors that have limited researchers' ability to positively connect Agent Orange and AML. For example, there is a dwindling cohort of affected patients to study because dioxins can lie latent in fat cells for more than a decade, delaying the development of AML. During that time, many veterans will have died from unrelated causes.
More research is needed for veterans to receive service-connected benefits for AML diagnoses. However, as Dr O'Brien notes, the PACT Act provides coverage for veterans who developed AML after exposure to benzene-contaminated water at Camp Lejeune.
--
Timothy O'Brien, MD, Associate Professor, Case Western Reserve University School of Medicine; Chief of Hematology, Louis Stokes Cleveland VA Medical Center, Cleveland, Ohio
Timothy O'Brien, MD, has disclosed no relevant financial relationships.

Infiltrating Wound Vacuum-Assisted Closure With Topical Amphotericin for Mucormycosis Infection of the Achilles Tendon
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
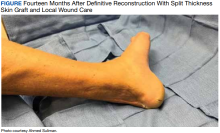
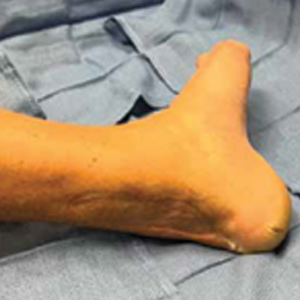
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
Drinking tea can keep your heart healthy as you age
according to the Heart Foundation and researchers from Edith Cowan University, Perth, Australia.
What to know
- Elderly women who drank black tea on a regular basis or consumed a high level of flavonoids in their diet were found to be far less likely to develop extensive AAC.
- AAC is calcification of the large artery that supplies oxygenated blood from the heart to the abdominal organs and lower limbs. It is associated with cardiovascular disorders, such as heart attack and stroke, as well as late-life dementia.
- Flavonoids are naturally occurring substances that regulate cellular activity. They are found in many common foods and beverages, such as black tea, green tea, apples, nuts, citrus fruit, berries, red wine, dark chocolate, and others.
- Study participants who had a higher intake of total flavonoids, flavan-3-ols, and flavonols were almost 40% less likely to have extensive AAC, while those who drank two to six cups of black tea per day had up to 42% less chance of experiencing extensive AAC.
- People who do not drink tea can still benefit by including foods rich in flavonoids in their diet, which protects against extensive calcification of the arteries.
This is a summary of the article, “Higher Habitual Dietary Flavonoid Intake Associates With Less Extensive Abdominal Aortic Calcification in a Cohort of Older Women,” published in Arteriosclerosis, Thrombosis, and Vascular Biology on Nov. 2, 2022. The full article can be found on ahajournals.org. A version of this article originally appeared on Medscape.com.
according to the Heart Foundation and researchers from Edith Cowan University, Perth, Australia.
What to know
- Elderly women who drank black tea on a regular basis or consumed a high level of flavonoids in their diet were found to be far less likely to develop extensive AAC.
- AAC is calcification of the large artery that supplies oxygenated blood from the heart to the abdominal organs and lower limbs. It is associated with cardiovascular disorders, such as heart attack and stroke, as well as late-life dementia.
- Flavonoids are naturally occurring substances that regulate cellular activity. They are found in many common foods and beverages, such as black tea, green tea, apples, nuts, citrus fruit, berries, red wine, dark chocolate, and others.
- Study participants who had a higher intake of total flavonoids, flavan-3-ols, and flavonols were almost 40% less likely to have extensive AAC, while those who drank two to six cups of black tea per day had up to 42% less chance of experiencing extensive AAC.
- People who do not drink tea can still benefit by including foods rich in flavonoids in their diet, which protects against extensive calcification of the arteries.
This is a summary of the article, “Higher Habitual Dietary Flavonoid Intake Associates With Less Extensive Abdominal Aortic Calcification in a Cohort of Older Women,” published in Arteriosclerosis, Thrombosis, and Vascular Biology on Nov. 2, 2022. The full article can be found on ahajournals.org. A version of this article originally appeared on Medscape.com.
according to the Heart Foundation and researchers from Edith Cowan University, Perth, Australia.
What to know
- Elderly women who drank black tea on a regular basis or consumed a high level of flavonoids in their diet were found to be far less likely to develop extensive AAC.
- AAC is calcification of the large artery that supplies oxygenated blood from the heart to the abdominal organs and lower limbs. It is associated with cardiovascular disorders, such as heart attack and stroke, as well as late-life dementia.
- Flavonoids are naturally occurring substances that regulate cellular activity. They are found in many common foods and beverages, such as black tea, green tea, apples, nuts, citrus fruit, berries, red wine, dark chocolate, and others.
- Study participants who had a higher intake of total flavonoids, flavan-3-ols, and flavonols were almost 40% less likely to have extensive AAC, while those who drank two to six cups of black tea per day had up to 42% less chance of experiencing extensive AAC.
- People who do not drink tea can still benefit by including foods rich in flavonoids in their diet, which protects against extensive calcification of the arteries.
This is a summary of the article, “Higher Habitual Dietary Flavonoid Intake Associates With Less Extensive Abdominal Aortic Calcification in a Cohort of Older Women,” published in Arteriosclerosis, Thrombosis, and Vascular Biology on Nov. 2, 2022. The full article can be found on ahajournals.org. A version of this article originally appeared on Medscape.com.
Longer life after bariatric surgery, but suicide risk in young
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM OBESITY
Renewed calls for fallopian tube removal to avoid ovarian cancer
All women, regardless of their risk profile, should consider prophylactic removal of the fallopian tubes at the same time as other pelvic surgery once they are finished having children, the Ovarian Cancer Research Alliance has advised.
The recommendation, announced Feb. 1, replaces the decades-old focus on symptom awareness and early detection and follows “sobering and deeply disappointing” results from a large U.K. study published 2 years ago, the organization said.
That was the UK Collaborative Trial of Ovarian Cancer Screening published in The Lancet in 2021, which followed more than 200,000 women for a median 16 years. It showed that screening average-risk women with a CA-125 blood test and ultrasound does not reduce deaths from the disease, as reported at the time by this news organization.
“We all hoped that the trial would show that early detection was effective in changing mortality rates. When the results came out, it was very hard to accept,” Audra Moran, OCRA president and CEO, said in an interview.
“We have an obligation to let people know that symptom awareness and early detection will not save lives” but considering opportunistic salpingectomy “absolutely will,” said Ms. Moran. Hence the renewed call for women to consider having their fallopian tubes removed.
What sounds new about this call is that the group is directing fallopian tube removal to all women “who are undergoing pelvic surgeries for benign conditions,” irrespective of what perceived risk they have of developing ovarian cancer (for example, based on family history).
But this advice has been in place for years for women who are known to be at higher risk for the disease.
For instance, women at high risk for ovarian cancer based on Hereditary Breast and Ovarian Cancer Syndrome (HBOC) have long been recommended to undergo surgery to remove ovaries and fallopian tubes (risk-reducing bilateral salpingo-oophorectomy or RRBSO) once there is no longer a desire for pregnancy.
Approached for comment about the new messaging, Stephanie V. Blank, MD, president of the Society of Gynecologic Oncology, says that the new recommendation – that all women who are finished childbearing consider opportunistic salpingectomy at the time of other pelvic surgery for benign conditions – is “not aggressive.”
“It’s reasonable and makes sense,” Dr. Blank said in an interview.
And she pointed out that it’s actually not “new”; it is, however, getting “new attention” based on the disappointing U.K. screening study, said Dr. Blank, director of gynecologic oncology for the Mount Sinai Health System in New York and professor of gynecologic oncology at Icahn School of Medicine at Mount Sinai.
She noted that the procedure of opportunistic salpingectomy has been endorsed by SGO since 2013 and by the American College of Obstetricians and Gynecologists since 2015.
There is increasing evidence that most high-grade serous ovarian cancers arise from cells in the fallopian tubes, William Dahut, MD, chief scientific officer for the American Cancer Society, told this news organization.
“Indirect evidence suggests a fairly strong degree of risk reduction associated with opportunistic salpingectomy for the most prevalent type of ovarian cancer (serous), and some risk reduction of epithelial ovarian cancer. At this time, these discussions seem warranted,” Dr. Dahut said.
At this point, however, the fact that leading organizations advise “consideration” means that the evidence base has “not been judged to be sufficiently strong (in terms of what we can say about benefits and harms) to advise a direct recommendation for opportunistic salpingectomy,” Dr. Dahut added.
There is no current recommendation to have fallopian tubes removed as a stand-alone procedure, he pointed out. However, he commented that “the occasion of scheduled gynecologic surgery presents an opportunity to possibly reduce the risk of ovarian cancer without known adverse effects in women who have completed childbearing. Having the discussion seems to be justified by the current evidence,” Dr. Dahut said.
Deanna Gerber, MD, a gynecologic oncologist at NYU Langone Perlmutter Cancer Center-Long Island, agrees. “In women who are scheduled to have a gynecologic or pelvic procedure, clinicians should discuss the possibility of removing the fallopian tubes at that time. A salpingectomy is a relatively low-risk procedure and adds little time to the surgery,” Dr. Gerber said in an interview.
“Women should understand that there is still ongoing research on this topic, but this low-risk procedure may reduce their risk of developing an ovarian or fallopian tube cancer,” Dr. Gerber said.
OCRA also encourages all women (or anyone born with ovaries) to know their risk for ovarian cancer. To that end, the organization has launched a pilot program offering free, at-home genetic testing kits to people with a personal or family history of breast, ovarian, uterine, or colorectal cancer.
Ms. Moran, Dr. Blank, Dr. Dahut, and Dr. Gerber report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
All women, regardless of their risk profile, should consider prophylactic removal of the fallopian tubes at the same time as other pelvic surgery once they are finished having children, the Ovarian Cancer Research Alliance has advised.
The recommendation, announced Feb. 1, replaces the decades-old focus on symptom awareness and early detection and follows “sobering and deeply disappointing” results from a large U.K. study published 2 years ago, the organization said.
That was the UK Collaborative Trial of Ovarian Cancer Screening published in The Lancet in 2021, which followed more than 200,000 women for a median 16 years. It showed that screening average-risk women with a CA-125 blood test and ultrasound does not reduce deaths from the disease, as reported at the time by this news organization.
“We all hoped that the trial would show that early detection was effective in changing mortality rates. When the results came out, it was very hard to accept,” Audra Moran, OCRA president and CEO, said in an interview.
“We have an obligation to let people know that symptom awareness and early detection will not save lives” but considering opportunistic salpingectomy “absolutely will,” said Ms. Moran. Hence the renewed call for women to consider having their fallopian tubes removed.
What sounds new about this call is that the group is directing fallopian tube removal to all women “who are undergoing pelvic surgeries for benign conditions,” irrespective of what perceived risk they have of developing ovarian cancer (for example, based on family history).
But this advice has been in place for years for women who are known to be at higher risk for the disease.
For instance, women at high risk for ovarian cancer based on Hereditary Breast and Ovarian Cancer Syndrome (HBOC) have long been recommended to undergo surgery to remove ovaries and fallopian tubes (risk-reducing bilateral salpingo-oophorectomy or RRBSO) once there is no longer a desire for pregnancy.
Approached for comment about the new messaging, Stephanie V. Blank, MD, president of the Society of Gynecologic Oncology, says that the new recommendation – that all women who are finished childbearing consider opportunistic salpingectomy at the time of other pelvic surgery for benign conditions – is “not aggressive.”
“It’s reasonable and makes sense,” Dr. Blank said in an interview.
And she pointed out that it’s actually not “new”; it is, however, getting “new attention” based on the disappointing U.K. screening study, said Dr. Blank, director of gynecologic oncology for the Mount Sinai Health System in New York and professor of gynecologic oncology at Icahn School of Medicine at Mount Sinai.
She noted that the procedure of opportunistic salpingectomy has been endorsed by SGO since 2013 and by the American College of Obstetricians and Gynecologists since 2015.
There is increasing evidence that most high-grade serous ovarian cancers arise from cells in the fallopian tubes, William Dahut, MD, chief scientific officer for the American Cancer Society, told this news organization.
“Indirect evidence suggests a fairly strong degree of risk reduction associated with opportunistic salpingectomy for the most prevalent type of ovarian cancer (serous), and some risk reduction of epithelial ovarian cancer. At this time, these discussions seem warranted,” Dr. Dahut said.
At this point, however, the fact that leading organizations advise “consideration” means that the evidence base has “not been judged to be sufficiently strong (in terms of what we can say about benefits and harms) to advise a direct recommendation for opportunistic salpingectomy,” Dr. Dahut added.
There is no current recommendation to have fallopian tubes removed as a stand-alone procedure, he pointed out. However, he commented that “the occasion of scheduled gynecologic surgery presents an opportunity to possibly reduce the risk of ovarian cancer without known adverse effects in women who have completed childbearing. Having the discussion seems to be justified by the current evidence,” Dr. Dahut said.
Deanna Gerber, MD, a gynecologic oncologist at NYU Langone Perlmutter Cancer Center-Long Island, agrees. “In women who are scheduled to have a gynecologic or pelvic procedure, clinicians should discuss the possibility of removing the fallopian tubes at that time. A salpingectomy is a relatively low-risk procedure and adds little time to the surgery,” Dr. Gerber said in an interview.
“Women should understand that there is still ongoing research on this topic, but this low-risk procedure may reduce their risk of developing an ovarian or fallopian tube cancer,” Dr. Gerber said.
OCRA also encourages all women (or anyone born with ovaries) to know their risk for ovarian cancer. To that end, the organization has launched a pilot program offering free, at-home genetic testing kits to people with a personal or family history of breast, ovarian, uterine, or colorectal cancer.
Ms. Moran, Dr. Blank, Dr. Dahut, and Dr. Gerber report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
All women, regardless of their risk profile, should consider prophylactic removal of the fallopian tubes at the same time as other pelvic surgery once they are finished having children, the Ovarian Cancer Research Alliance has advised.
The recommendation, announced Feb. 1, replaces the decades-old focus on symptom awareness and early detection and follows “sobering and deeply disappointing” results from a large U.K. study published 2 years ago, the organization said.
That was the UK Collaborative Trial of Ovarian Cancer Screening published in The Lancet in 2021, which followed more than 200,000 women for a median 16 years. It showed that screening average-risk women with a CA-125 blood test and ultrasound does not reduce deaths from the disease, as reported at the time by this news organization.
“We all hoped that the trial would show that early detection was effective in changing mortality rates. When the results came out, it was very hard to accept,” Audra Moran, OCRA president and CEO, said in an interview.
“We have an obligation to let people know that symptom awareness and early detection will not save lives” but considering opportunistic salpingectomy “absolutely will,” said Ms. Moran. Hence the renewed call for women to consider having their fallopian tubes removed.
What sounds new about this call is that the group is directing fallopian tube removal to all women “who are undergoing pelvic surgeries for benign conditions,” irrespective of what perceived risk they have of developing ovarian cancer (for example, based on family history).
But this advice has been in place for years for women who are known to be at higher risk for the disease.
For instance, women at high risk for ovarian cancer based on Hereditary Breast and Ovarian Cancer Syndrome (HBOC) have long been recommended to undergo surgery to remove ovaries and fallopian tubes (risk-reducing bilateral salpingo-oophorectomy or RRBSO) once there is no longer a desire for pregnancy.
Approached for comment about the new messaging, Stephanie V. Blank, MD, president of the Society of Gynecologic Oncology, says that the new recommendation – that all women who are finished childbearing consider opportunistic salpingectomy at the time of other pelvic surgery for benign conditions – is “not aggressive.”
“It’s reasonable and makes sense,” Dr. Blank said in an interview.
And she pointed out that it’s actually not “new”; it is, however, getting “new attention” based on the disappointing U.K. screening study, said Dr. Blank, director of gynecologic oncology for the Mount Sinai Health System in New York and professor of gynecologic oncology at Icahn School of Medicine at Mount Sinai.
She noted that the procedure of opportunistic salpingectomy has been endorsed by SGO since 2013 and by the American College of Obstetricians and Gynecologists since 2015.
There is increasing evidence that most high-grade serous ovarian cancers arise from cells in the fallopian tubes, William Dahut, MD, chief scientific officer for the American Cancer Society, told this news organization.
“Indirect evidence suggests a fairly strong degree of risk reduction associated with opportunistic salpingectomy for the most prevalent type of ovarian cancer (serous), and some risk reduction of epithelial ovarian cancer. At this time, these discussions seem warranted,” Dr. Dahut said.
At this point, however, the fact that leading organizations advise “consideration” means that the evidence base has “not been judged to be sufficiently strong (in terms of what we can say about benefits and harms) to advise a direct recommendation for opportunistic salpingectomy,” Dr. Dahut added.
There is no current recommendation to have fallopian tubes removed as a stand-alone procedure, he pointed out. However, he commented that “the occasion of scheduled gynecologic surgery presents an opportunity to possibly reduce the risk of ovarian cancer without known adverse effects in women who have completed childbearing. Having the discussion seems to be justified by the current evidence,” Dr. Dahut said.
Deanna Gerber, MD, a gynecologic oncologist at NYU Langone Perlmutter Cancer Center-Long Island, agrees. “In women who are scheduled to have a gynecologic or pelvic procedure, clinicians should discuss the possibility of removing the fallopian tubes at that time. A salpingectomy is a relatively low-risk procedure and adds little time to the surgery,” Dr. Gerber said in an interview.
“Women should understand that there is still ongoing research on this topic, but this low-risk procedure may reduce their risk of developing an ovarian or fallopian tube cancer,” Dr. Gerber said.
OCRA also encourages all women (or anyone born with ovaries) to know their risk for ovarian cancer. To that end, the organization has launched a pilot program offering free, at-home genetic testing kits to people with a personal or family history of breast, ovarian, uterine, or colorectal cancer.
Ms. Moran, Dr. Blank, Dr. Dahut, and Dr. Gerber report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IVF-conceived children show strong developmental performance
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
In vitro fertilization has been around long enough that researchers can now compare developmental and academic achievements between these children and peers at school age.
Amber Kennedy, MBBS, and colleagues did just that. They found little difference in these milestones between a total of 11,059 IVF-conceived children and 401,654 spontaneously conceived children in a new study.
“Parents considering IVF and health care professionals can be reassured that the school age developmental and educational outcomes of IVF-conceived children are equivalent to their peers,” said Dr. Kennedy, lead author and obstetrician and gynecologist at Mercy Hospital for Women at the University of Melbourne.
The findings were published online in PLOS Medicine.
“Overall, we know that children born through IVF are doing fine in terms of health, but also emotionally and cognitively. So I wasn’t surprised. I live in this world,” said Ariadna Cymet Lanski, PsyD, chair of the American Society for Reproductive Medicine Mental Health Professional Group, who was not affiliated with the study.
Some previous researchers linked conception via IVF to an increased risk of congenital abnormalities, autism spectrum disorder, developmental delay, and intellectual disability.
Asked why the current study did not find increased risks, Dr. Kennedy said, “Our population included a relatively recent birth cohort, which may explain some differences from previous studies as IVF practices have evolved over time.”
An estimated 8 million people worldwide have been conceived through IVF since the first birth in 1978, the researchers said. In Australia, this has grown from 2% of births in the year 2000 to now nearly 5% or 1 in 20 live births, Dr. Kennedy noted. “Consequently, it is important to understand the longer-term outcomes for this population of children.”
Along with senior author Anthea Lindquist, MBBS, Dr. Kennedy and colleagues studied 585,659 single births in Victoria, Australia, between 2005 and 2014. They did not include multiple births such as twins or triplets.
The investigators compared 4,697 children conceived via IVF and 168,503 others conceived spontaneously using a standard developmental measure, the Australian Early Developmental Census (AEDC). They also assessed 8,976 children in the IVF group and 333,335 other children on a standard educational measure, the National Assessment Program–Literacy and Numeracy (NAPLAN).
For example, the developmental census measures developmental vulnerability. Dr. Kennedy and colleagues found a 0.3% difference in favor of IVF-conceived children, which statistically was no different than zero.
Similarly, the researchers reported that IVF conception had essentially no effect on overall the literacy score, with an adjusted average difference of 0.03.
Dr. Lanski said the results should be reassuring for people considering IVF. “I can see the value of the study.” The findings “probably brings a lot of comfort ... if you want to build a family, and medically this is what’s recommended.”
Not all IVF techniques are the same, and the researchers want to take a deeper dive to evaluate any distinctions among them. For example, Dr. Kennedy said, “We plan to investigate the same school-aged outcomes after specific IVF-associated techniques.”
A version of this article first appeared on WebMD.com.
FROM PLOS MEDICINE
In families with gout, obesity and alcohol add to personal risk
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE AND RESEARCH
Study documents link between preadolescent acne and elevated BMI
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
FROM PEDIATRIC DERMATOLOGY