User login
AAP vs. AED on obesity treatment: Is there a middle ground?
While there is little controversy that both obesity and eating disorders represent important public health concerns, each deserving of clinical attention, how best to address one without worsening the other has been the crux of the discussion.
Sparking the dispute was a recent publication from the American Academy of Pediatrics that outlines the scope of the obesity problem and makes specific recommendations for assessment and treatment.1 The ambitious 100-page document, with 801 citations, puts new emphasis on the medical and psychological costs associated with obesity and advocates that pediatric primary care clinicians be more assertive in its treatment. While the guidelines certainly don’t urge the use of medications or surgery options as first-line treatment, the new recommendations do put them on the table as options.
In response, the Academy of Eating Disorders issued a public statement outlining several concerns regarding these guidelines that centered around a lack of a detailed plan to screen and address eating disorders; concerns that pediatricians don’t have the level of training and “skills” to conduct these conversations with patients and families with enough sensitivity; and worries about the premature use of antiobesity medications and surgeries in this population.2
It is fair to say that the critique was sharply worded, invoking physicians’ Hippocratic oath, criticizing their training, and suggesting that the guidelines could be biased by pharmaceutical industry influence (of note, the authors of the guidelines reported no ties to any pharmaceutical company). The AED urged that the guidelines be “revised” after consultation with other groups, including them.
Not unexpectedly, this response, especially coming from a group whose leadership and members are primarily nonphysicians, triggered its own sharp rebukes, including a recent commentary that counter-accused some of the eating disorder clinicians of being more concerned with their pet diets than actual health improvements.3
After everyone takes some deep breaths, it’s worth looking to see if there is some middle ground to explore here. The AAP document, to my reading, shows some important acknowledgments of the stigma associated with being overweight, even coming from pediatricians themselves. One passage reads, “Pediatricians and other PHCPs [primary health care providers] have been – and remain – a source of weight bias. They first need to uncover and address their own attitudes regarding children with obesity. Understanding weight stigma and bias, and learning how to reduce it in the clinical setting, sets the stage for productive discussions and improved relationships between families and pediatricians or other PHCPs.”
The guidelines also include some suggestions for how to talk to youth and families about obesity in less stigmatizing ways and offer a fairly lengthy summary of motivational interviewing techniques as they might apply to obesity discussions and lifestyle change. There is also a section on the interface between obesity and eating disorders with suggestions for further reading on their assessment and management.4
Indeed, research has looked specifically at how to minimize the triggering of eating disorders when addressing weight problems, a concern that has been raised by pediatricians themselves as documented in a qualitative study that also invoked the “do no harm” principle.5 One study asked more than 2,000 teens about how various conversations about weight affected their behavior.6 A main finding from that study was that conversations that focused on healthy eating rather than weight per se were less likely to be associated with unhealthy weight control behaviors. This message was emphasized in a publication that came from the AAP itself; it addresses the interaction between eating disorders and obesity.7 Strangely, however, the suggestion to try to minimize the focus on weight in discussions with patients isn’t well emphasized in the publication.
Overall, though, the AAP guidelines offer a well-informed and balanced approach to helping overweight youth. Pediatricians and other pediatric primary care clinicians are frequently called upon to engage in extremely sensitive and difficult discussions with patients and families on a wide variety of topics and most do so quite skillfully, especially when given the proper time and tools. While it is an area in which many of us, including mental health professionals, could do better, it’s no surprise that the AED’s disparaging of pediatricians’ communication competence came off as insulting. Similarly, productive dialogue would be likely enhanced if both sides avoided unfounded speculation about bias and motive and worked from a good faith perspective that all of us are engaged in this important discussion because of a desire to improve the lives of kids.
From my reading, it is quite a stretch to conclude that this document is urging a hasty and financially driven descent into GLP-1 analogues and bariatric surgery. That said, this wouldn’t be the first time a professional organization issues detailed, thoughtful, and nuanced care guidelines only to have them “condensed” within the practical confines of a busy office practice. Leaders would do well to remember that there remains much work to do to empower clinicians to be able to follow these guidelines as intended.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Hampl SE et al. Pediatrics. 2023;151(2):e2022060640.
2. Academy of Eating Disorders. Jan. 26, 2023. Accessed February 2, 2023. Available at The Academy for Eating Disorders Releases a Statement on the Recent American Academy of Pediatrics Clinical Practice Guideline for Weight-Related Care: First, Do No Harm (newswise.com).
3. Freedhoff Y. MDedge Pediatrics 2023. Available at https://www.mdedge.com/pediatrics/article/260894/obesity/weight-bias-affects-views-kids-obesity-recommendations?channel=52.
4. Hornberger LL, Lane MA et al. Pediatrics. 2021;147(1):e202004027989.
5. Loth KA, Lebow J et al. Global Pediatric Health. 2021;8:1-9.
6. Berge JM et al. JAMA Pediatrics. 2013;167(8):746-53.
7. Golden NH et al. Pediatrics. 2016;138(3):e20161649.
While there is little controversy that both obesity and eating disorders represent important public health concerns, each deserving of clinical attention, how best to address one without worsening the other has been the crux of the discussion.
Sparking the dispute was a recent publication from the American Academy of Pediatrics that outlines the scope of the obesity problem and makes specific recommendations for assessment and treatment.1 The ambitious 100-page document, with 801 citations, puts new emphasis on the medical and psychological costs associated with obesity and advocates that pediatric primary care clinicians be more assertive in its treatment. While the guidelines certainly don’t urge the use of medications or surgery options as first-line treatment, the new recommendations do put them on the table as options.
In response, the Academy of Eating Disorders issued a public statement outlining several concerns regarding these guidelines that centered around a lack of a detailed plan to screen and address eating disorders; concerns that pediatricians don’t have the level of training and “skills” to conduct these conversations with patients and families with enough sensitivity; and worries about the premature use of antiobesity medications and surgeries in this population.2
It is fair to say that the critique was sharply worded, invoking physicians’ Hippocratic oath, criticizing their training, and suggesting that the guidelines could be biased by pharmaceutical industry influence (of note, the authors of the guidelines reported no ties to any pharmaceutical company). The AED urged that the guidelines be “revised” after consultation with other groups, including them.
Not unexpectedly, this response, especially coming from a group whose leadership and members are primarily nonphysicians, triggered its own sharp rebukes, including a recent commentary that counter-accused some of the eating disorder clinicians of being more concerned with their pet diets than actual health improvements.3
After everyone takes some deep breaths, it’s worth looking to see if there is some middle ground to explore here. The AAP document, to my reading, shows some important acknowledgments of the stigma associated with being overweight, even coming from pediatricians themselves. One passage reads, “Pediatricians and other PHCPs [primary health care providers] have been – and remain – a source of weight bias. They first need to uncover and address their own attitudes regarding children with obesity. Understanding weight stigma and bias, and learning how to reduce it in the clinical setting, sets the stage for productive discussions and improved relationships between families and pediatricians or other PHCPs.”
The guidelines also include some suggestions for how to talk to youth and families about obesity in less stigmatizing ways and offer a fairly lengthy summary of motivational interviewing techniques as they might apply to obesity discussions and lifestyle change. There is also a section on the interface between obesity and eating disorders with suggestions for further reading on their assessment and management.4
Indeed, research has looked specifically at how to minimize the triggering of eating disorders when addressing weight problems, a concern that has been raised by pediatricians themselves as documented in a qualitative study that also invoked the “do no harm” principle.5 One study asked more than 2,000 teens about how various conversations about weight affected their behavior.6 A main finding from that study was that conversations that focused on healthy eating rather than weight per se were less likely to be associated with unhealthy weight control behaviors. This message was emphasized in a publication that came from the AAP itself; it addresses the interaction between eating disorders and obesity.7 Strangely, however, the suggestion to try to minimize the focus on weight in discussions with patients isn’t well emphasized in the publication.
Overall, though, the AAP guidelines offer a well-informed and balanced approach to helping overweight youth. Pediatricians and other pediatric primary care clinicians are frequently called upon to engage in extremely sensitive and difficult discussions with patients and families on a wide variety of topics and most do so quite skillfully, especially when given the proper time and tools. While it is an area in which many of us, including mental health professionals, could do better, it’s no surprise that the AED’s disparaging of pediatricians’ communication competence came off as insulting. Similarly, productive dialogue would be likely enhanced if both sides avoided unfounded speculation about bias and motive and worked from a good faith perspective that all of us are engaged in this important discussion because of a desire to improve the lives of kids.
From my reading, it is quite a stretch to conclude that this document is urging a hasty and financially driven descent into GLP-1 analogues and bariatric surgery. That said, this wouldn’t be the first time a professional organization issues detailed, thoughtful, and nuanced care guidelines only to have them “condensed” within the practical confines of a busy office practice. Leaders would do well to remember that there remains much work to do to empower clinicians to be able to follow these guidelines as intended.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Hampl SE et al. Pediatrics. 2023;151(2):e2022060640.
2. Academy of Eating Disorders. Jan. 26, 2023. Accessed February 2, 2023. Available at The Academy for Eating Disorders Releases a Statement on the Recent American Academy of Pediatrics Clinical Practice Guideline for Weight-Related Care: First, Do No Harm (newswise.com).
3. Freedhoff Y. MDedge Pediatrics 2023. Available at https://www.mdedge.com/pediatrics/article/260894/obesity/weight-bias-affects-views-kids-obesity-recommendations?channel=52.
4. Hornberger LL, Lane MA et al. Pediatrics. 2021;147(1):e202004027989.
5. Loth KA, Lebow J et al. Global Pediatric Health. 2021;8:1-9.
6. Berge JM et al. JAMA Pediatrics. 2013;167(8):746-53.
7. Golden NH et al. Pediatrics. 2016;138(3):e20161649.
While there is little controversy that both obesity and eating disorders represent important public health concerns, each deserving of clinical attention, how best to address one without worsening the other has been the crux of the discussion.
Sparking the dispute was a recent publication from the American Academy of Pediatrics that outlines the scope of the obesity problem and makes specific recommendations for assessment and treatment.1 The ambitious 100-page document, with 801 citations, puts new emphasis on the medical and psychological costs associated with obesity and advocates that pediatric primary care clinicians be more assertive in its treatment. While the guidelines certainly don’t urge the use of medications or surgery options as first-line treatment, the new recommendations do put them on the table as options.
In response, the Academy of Eating Disorders issued a public statement outlining several concerns regarding these guidelines that centered around a lack of a detailed plan to screen and address eating disorders; concerns that pediatricians don’t have the level of training and “skills” to conduct these conversations with patients and families with enough sensitivity; and worries about the premature use of antiobesity medications and surgeries in this population.2
It is fair to say that the critique was sharply worded, invoking physicians’ Hippocratic oath, criticizing their training, and suggesting that the guidelines could be biased by pharmaceutical industry influence (of note, the authors of the guidelines reported no ties to any pharmaceutical company). The AED urged that the guidelines be “revised” after consultation with other groups, including them.
Not unexpectedly, this response, especially coming from a group whose leadership and members are primarily nonphysicians, triggered its own sharp rebukes, including a recent commentary that counter-accused some of the eating disorder clinicians of being more concerned with their pet diets than actual health improvements.3
After everyone takes some deep breaths, it’s worth looking to see if there is some middle ground to explore here. The AAP document, to my reading, shows some important acknowledgments of the stigma associated with being overweight, even coming from pediatricians themselves. One passage reads, “Pediatricians and other PHCPs [primary health care providers] have been – and remain – a source of weight bias. They first need to uncover and address their own attitudes regarding children with obesity. Understanding weight stigma and bias, and learning how to reduce it in the clinical setting, sets the stage for productive discussions and improved relationships between families and pediatricians or other PHCPs.”
The guidelines also include some suggestions for how to talk to youth and families about obesity in less stigmatizing ways and offer a fairly lengthy summary of motivational interviewing techniques as they might apply to obesity discussions and lifestyle change. There is also a section on the interface between obesity and eating disorders with suggestions for further reading on their assessment and management.4
Indeed, research has looked specifically at how to minimize the triggering of eating disorders when addressing weight problems, a concern that has been raised by pediatricians themselves as documented in a qualitative study that also invoked the “do no harm” principle.5 One study asked more than 2,000 teens about how various conversations about weight affected their behavior.6 A main finding from that study was that conversations that focused on healthy eating rather than weight per se were less likely to be associated with unhealthy weight control behaviors. This message was emphasized in a publication that came from the AAP itself; it addresses the interaction between eating disorders and obesity.7 Strangely, however, the suggestion to try to minimize the focus on weight in discussions with patients isn’t well emphasized in the publication.
Overall, though, the AAP guidelines offer a well-informed and balanced approach to helping overweight youth. Pediatricians and other pediatric primary care clinicians are frequently called upon to engage in extremely sensitive and difficult discussions with patients and families on a wide variety of topics and most do so quite skillfully, especially when given the proper time and tools. While it is an area in which many of us, including mental health professionals, could do better, it’s no surprise that the AED’s disparaging of pediatricians’ communication competence came off as insulting. Similarly, productive dialogue would be likely enhanced if both sides avoided unfounded speculation about bias and motive and worked from a good faith perspective that all of us are engaged in this important discussion because of a desire to improve the lives of kids.
From my reading, it is quite a stretch to conclude that this document is urging a hasty and financially driven descent into GLP-1 analogues and bariatric surgery. That said, this wouldn’t be the first time a professional organization issues detailed, thoughtful, and nuanced care guidelines only to have them “condensed” within the practical confines of a busy office practice. Leaders would do well to remember that there remains much work to do to empower clinicians to be able to follow these guidelines as intended.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. His latest book is “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood.”
References
1. Hampl SE et al. Pediatrics. 2023;151(2):e2022060640.
2. Academy of Eating Disorders. Jan. 26, 2023. Accessed February 2, 2023. Available at The Academy for Eating Disorders Releases a Statement on the Recent American Academy of Pediatrics Clinical Practice Guideline for Weight-Related Care: First, Do No Harm (newswise.com).
3. Freedhoff Y. MDedge Pediatrics 2023. Available at https://www.mdedge.com/pediatrics/article/260894/obesity/weight-bias-affects-views-kids-obesity-recommendations?channel=52.
4. Hornberger LL, Lane MA et al. Pediatrics. 2021;147(1):e202004027989.
5. Loth KA, Lebow J et al. Global Pediatric Health. 2021;8:1-9.
6. Berge JM et al. JAMA Pediatrics. 2013;167(8):746-53.
7. Golden NH et al. Pediatrics. 2016;138(3):e20161649.
President's Report
Here we are, 1 month into the new year, and it already feels like my time as President of the American College of Chest Physicians will pass too quickly. One of my goals is to share some thoughts on issues important to our profession by contributing quarterly to CHEST Physician. CHEST has always been like an extended family to me, and I look forward to having this regular touchpoint with all of you.
For my first written contribution, I want to focus on the future of medicine through medical education and involvement in professional associations because I am, at heart, a medical educator.
During my address at the CHEST Annual Meeting 2022, I spoke on how CHEST provided me with networking, mentoring, and volunteer opportunities that were critical in advancing my career. Those same opportunities should be extended to everyone in pulmonary, critical care, and sleep medicine – whether a current member or prospective member.
Lighting a fire
Attending my first CHEST Annual Meeting was possible due to my nomination for a leadership development course. The connections I made during the meeting really lit a fire within me. We need to engage with early career clinicians and provide them the same exposure and encouragement that I received.
To instill this fire in the next generation, I encourage each of our established members, years (or decades) into their careers, to pass along their expertise to someone who is just starting out, whether it be a trainee or a junior faculty member. If this applies to you: encourage a new attending who has never been to a CHEST event to attend with you; invite a fellow or resident to submit an abstract or case report to the journal CHEST® with your oversight; or simply volunteer to speak at your medical school or residency program about why you chose PCCM and the career it has given you.
Think back to when you were embarking on your journey toward where you are now – what would it have meant to be able to get career advice or even just a friendly conversation started with someone at your current level?
CHEST offerings and accreditations
Beyond bringing someone to a CHEST Annual Meeting – which you should definitely do – work with your learners at medical schools and residency programs to expose them to CHEST much earlier in their careers. The Trainings and Transitions Committee is an excellent resource to guide newer clinicians and can provide a vital source of encouragement and support. If your institution doesn’t have a simulation learning center or if it has limited offerings, the hands-on learning opportunities offered at CHEST headquarters may be a fit. Accredited by the Society for Simulation in Healthcare (SSH) and the Accreditation Council for Continuing Medical Education (ACCME), CHEST currently offers 24 courses with four new courses planned for 2023 in a wide variety of areas, including courses on ultrasound and bronchoscopy.
There are so many ways to introduce early career clinicians to CHEST, and it can begin with one personal outreach. If you are working on a project for CHEST right now, consider inviting an early career clinician to join you on it – this may be the opportunity that will change their career. It did for me.
As medical professionals, each of us plays an important role in the future of medicine, and the CHEST organization can bring us together to strengthen our impact.
If you are interested in brainstorming ideas for how to engage your medical students, residents, or fellows, please feel free to contact me or anyone at CHEST to help create a plan.
I look forward to the next time we connect.
Doreen J. Addrizzo-Harris, MD, FCCP
CHEST President
Here we are, 1 month into the new year, and it already feels like my time as President of the American College of Chest Physicians will pass too quickly. One of my goals is to share some thoughts on issues important to our profession by contributing quarterly to CHEST Physician. CHEST has always been like an extended family to me, and I look forward to having this regular touchpoint with all of you.
For my first written contribution, I want to focus on the future of medicine through medical education and involvement in professional associations because I am, at heart, a medical educator.
During my address at the CHEST Annual Meeting 2022, I spoke on how CHEST provided me with networking, mentoring, and volunteer opportunities that were critical in advancing my career. Those same opportunities should be extended to everyone in pulmonary, critical care, and sleep medicine – whether a current member or prospective member.
Lighting a fire
Attending my first CHEST Annual Meeting was possible due to my nomination for a leadership development course. The connections I made during the meeting really lit a fire within me. We need to engage with early career clinicians and provide them the same exposure and encouragement that I received.
To instill this fire in the next generation, I encourage each of our established members, years (or decades) into their careers, to pass along their expertise to someone who is just starting out, whether it be a trainee or a junior faculty member. If this applies to you: encourage a new attending who has never been to a CHEST event to attend with you; invite a fellow or resident to submit an abstract or case report to the journal CHEST® with your oversight; or simply volunteer to speak at your medical school or residency program about why you chose PCCM and the career it has given you.
Think back to when you were embarking on your journey toward where you are now – what would it have meant to be able to get career advice or even just a friendly conversation started with someone at your current level?
CHEST offerings and accreditations
Beyond bringing someone to a CHEST Annual Meeting – which you should definitely do – work with your learners at medical schools and residency programs to expose them to CHEST much earlier in their careers. The Trainings and Transitions Committee is an excellent resource to guide newer clinicians and can provide a vital source of encouragement and support. If your institution doesn’t have a simulation learning center or if it has limited offerings, the hands-on learning opportunities offered at CHEST headquarters may be a fit. Accredited by the Society for Simulation in Healthcare (SSH) and the Accreditation Council for Continuing Medical Education (ACCME), CHEST currently offers 24 courses with four new courses planned for 2023 in a wide variety of areas, including courses on ultrasound and bronchoscopy.
There are so many ways to introduce early career clinicians to CHEST, and it can begin with one personal outreach. If you are working on a project for CHEST right now, consider inviting an early career clinician to join you on it – this may be the opportunity that will change their career. It did for me.
As medical professionals, each of us plays an important role in the future of medicine, and the CHEST organization can bring us together to strengthen our impact.
If you are interested in brainstorming ideas for how to engage your medical students, residents, or fellows, please feel free to contact me or anyone at CHEST to help create a plan.
I look forward to the next time we connect.
Doreen J. Addrizzo-Harris, MD, FCCP
CHEST President
Here we are, 1 month into the new year, and it already feels like my time as President of the American College of Chest Physicians will pass too quickly. One of my goals is to share some thoughts on issues important to our profession by contributing quarterly to CHEST Physician. CHEST has always been like an extended family to me, and I look forward to having this regular touchpoint with all of you.
For my first written contribution, I want to focus on the future of medicine through medical education and involvement in professional associations because I am, at heart, a medical educator.
During my address at the CHEST Annual Meeting 2022, I spoke on how CHEST provided me with networking, mentoring, and volunteer opportunities that were critical in advancing my career. Those same opportunities should be extended to everyone in pulmonary, critical care, and sleep medicine – whether a current member or prospective member.
Lighting a fire
Attending my first CHEST Annual Meeting was possible due to my nomination for a leadership development course. The connections I made during the meeting really lit a fire within me. We need to engage with early career clinicians and provide them the same exposure and encouragement that I received.
To instill this fire in the next generation, I encourage each of our established members, years (or decades) into their careers, to pass along their expertise to someone who is just starting out, whether it be a trainee or a junior faculty member. If this applies to you: encourage a new attending who has never been to a CHEST event to attend with you; invite a fellow or resident to submit an abstract or case report to the journal CHEST® with your oversight; or simply volunteer to speak at your medical school or residency program about why you chose PCCM and the career it has given you.
Think back to when you were embarking on your journey toward where you are now – what would it have meant to be able to get career advice or even just a friendly conversation started with someone at your current level?
CHEST offerings and accreditations
Beyond bringing someone to a CHEST Annual Meeting – which you should definitely do – work with your learners at medical schools and residency programs to expose them to CHEST much earlier in their careers. The Trainings and Transitions Committee is an excellent resource to guide newer clinicians and can provide a vital source of encouragement and support. If your institution doesn’t have a simulation learning center or if it has limited offerings, the hands-on learning opportunities offered at CHEST headquarters may be a fit. Accredited by the Society for Simulation in Healthcare (SSH) and the Accreditation Council for Continuing Medical Education (ACCME), CHEST currently offers 24 courses with four new courses planned for 2023 in a wide variety of areas, including courses on ultrasound and bronchoscopy.
There are so many ways to introduce early career clinicians to CHEST, and it can begin with one personal outreach. If you are working on a project for CHEST right now, consider inviting an early career clinician to join you on it – this may be the opportunity that will change their career. It did for me.
As medical professionals, each of us plays an important role in the future of medicine, and the CHEST organization can bring us together to strengthen our impact.
If you are interested in brainstorming ideas for how to engage your medical students, residents, or fellows, please feel free to contact me or anyone at CHEST to help create a plan.
I look forward to the next time we connect.
Doreen J. Addrizzo-Harris, MD, FCCP
CHEST President
Muscle-Related Adverse Events Associated With PCSK9 Inhibitors in a Veteran Population
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
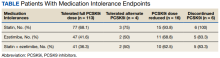
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
HMG-CoA reductase inhibitors (statins) have been shown to effectively reduce low-density lipoprotein cholesterol (LDL-C) as well as morbidity and mortality in patients who have either atherosclerotic cardiovascular disease (ASCVD) or risk factors for ASCVD.1-12 However, research shows that up to 20% of patients are unable to tolerate statin therapy due to muscle-related adverse events (AEs).13 This presents a substantial clinical challenge, as current management strategies for patients with statin-associated muscle symptoms, such as intermittent administration of statins and ezetimibe, seldom achieve the > 50% LDL-C reduction recommended by the 2018 American Heart Association/American College of Cardiology Clinical Practice Guidelines.14 Additionally, statin-intolerant patients who have antihyperlipidemic medication lowered or discontinued are at an increased risk of future cardiovascular events.15 Observational data also show that about 70% of adult patients (primarily with genetic lipid disorders such as heterozygous familial hypercholesterolemia) do not achieve an LDL-C level < 100 mg/dL despite treatment with maximum doses of statins with or without ezetimibe.16,17
PCSK9 inhibitors (PCSK9i) have robust efficacy data to support use in patients who do not meet their LDL-C goal despite maximally tolerated lipid therapy.14 However, long-term safety data for PCSK9i are not as robust as its efficacy data. Specifically, safety data relating to muscle-related AEs, which are the most widely recognized AE associated with statins, have only been reported in a few clinical trials with varying incidence rates, levels of significance, and relatively small study populations. Furthermore, the real-world prevalence of muscle-related PCSK9i AEs is unknown. Clinical guidance for management strategies for muscle-related AEs associated with PCSK9i is largely lacking. For this study, muscle-related AEs were defined as any new or unusual muscle soreness, weakness, cramping, aches, and stiffness that persists, is generally bilateral, and typically affects the large muscles. It is important to note, that muscle-related AEs associated with statins, ezetimibe, and PCSK9i can be attributed to the nocebo effect.
According to the prescribing information for alirocumab and evolocumab, myalgia, muscle spasms, and musculoskeletal pain each occurred in < 5% of the study populations.18,19 From these data, muscle-related PCSK9i AEs are thought to be relatively rare, based on the ODYSSEY-OUTCOME and FOURIER trials, which did not enroll statin-intolerant patients.20,21 However, currently available safety data from 3 small, randomized clinical trials specifically in statin-intolerant patients taking a PCSK9i suggest that muscle-related AEs occur at a rate of 12.2% to 32.5% and discontinuation rates varied from 0% to 15.9%.22-25 As the incidence rates of muscle-related AEs in the prescribing information and clinical trials varied widely, this study will provide quantitative data on the percentage of patients that developed muscle-related PCSK9i AEs in a veteran population to help shed light on a topic that is not well studied.
Methods
This was a single-center, retrospective chart review of patients prescribed a PCSK9i between December 1, 2017, and September 1, 2021, and were managed in a pharmacy-led patient aligned care team (PACT) clinic at the Wilkes-Barre US Department of Veterans Affairs (VA) Medical Center (WBVAMC) in Pennsylvania. This study was approved by the Coatesville VA Medical Center Institutional Review Board, which oversees research conducted at WBVAMC. Veterans aged ≥ 18 years were included in the study. Patients were excluded if they had a history of serious hypersensitivity reaction to a PCSK9i or rhabdomyolysis or did not meet the VA criteria for use.26
The primary outcome was the percentage of patients who developed a muscle-related AE while on a PCSK9i in a PACT clinic. Data were further analyzed based on patients who (1) tolerated a full PCSK9i dose; (2) tolerated alternative PCSK9i following initial intolerance; (3) required a PCSK9i dose reduction, or (4) discontinued PCSK9i. A secondary outcome was the percentage of statin- and/or ezetimibe-intolerant patients in these 4 groups. Another secondary outcome was the management strategies taken for patients who were on a reduced (monthly) dose of PCSK9i who did not reach their LDL-C goal. Management strategies that were assessed included restarting weekly statin, restarting weekly ezetimibe, increasing the dose of the same PCSK9i administered monthly, and switching to an alternative PCSK9i.
Data were collected using the VA Computerized Patient Record System (CPRS) and stored in a secure, locked spreadsheet. Baseline patient demographic characteristics collected included age (at PCSK9i start); sex; race; and PCSK9i name, dose, and frequency. We recorded when a patient switched PCSK9i, whether or not it was due to a muscle-related AE, and the name of the original PCSK9i. Also collected were lipid therapy intolerances prior to PCSK9i initiation (ie, intolerance to statin, ezetimibe, or both).
Patients were considered statin intolerant due to a muscle-related AE in accordance with the VA PCSK9i Criteria for Use, which requires trial of at least 3 statins, one of which was trialed at the lowest dosage approved by the US Food and Drug Administration (FDA) and resulted in intolerable skeletal muscle AEs that worsened during treatment and resolved when the statin was stopped. For our study purposes, patients taking alternative day dosing of statins due to muscle-related AEs (ie, 2- or 3-times weekly dosing) were not considered statin intolerant; however, patients taking once-weekly statin dosing were considered statin intolerant. Patients were considered ezetimibe intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when ezetimibe was stopped. Patients were considered PCSK9i intolerant due to a muscle-related AE if the intolerance was due to skeletal muscle concerns that worsened during treatment and resolved when the PCSK9i was stopped. Patients with non–muscle-related intolerances to statins, ezetimibe, and PCSK9i were not considered statin, ezetimibe, and PCSK9i intolerant.
Alirocumab was initiated at 75 mg subcutaneous (SQ) once every 2 weeks or evolocumab 140 mg SQ once every 2 weeks in our study. The protocol allowed for a dose reduction of alirocumab 75 mg SQ once monthly if a patient experienced AEs, but this dose reduction strategy was not used for any patients on evolocumab in this study. Of note, alirocumab 75 mg SQ once monthly is not an FDA-approved dosing strategy. However, it is similar in concept to the alternative statin dosing (ie, alternate day dosing, once-weekly dosing) and may avoid the need to discontinue PCSK9i therapy altogether.
A review of the CPRS also documented whether a muscle-related AE occurred while the patient was on a PCSK9i (if yes, the specific AE was recorded), the result of PCSK9i therapy (tolerated full dose, required a dose reduction, switched medication, or discontinued), and management strategies taken for patients who did not meet their LDL-C goal while on a reduced (monthly) PCSK9i dose. Prior lipid therapy intolerances, PCSK9i-related AEs, results of PCSK9i therapy, and management strategies for patients who did not meet LDL-C goal while on a reduced PCSK9i dose were obtained by reviewing the PACT pharmacist’s clinic notes and assessment, along with clinic notes and medication history listed within the CPRS.
Statistical Analysis
Descriptive statistics were used for the demographic characteristics of study patients. The primary outcome was calculated as a binary measure (yes/no) of whether the patient developed a muscle-related AE while on a PCSK9i. The secondary outcome of statin, ezetimibe, or statin and ezetimibe intolerances in subgroups also was calculated as a binary measure.
Results
For the study, 156 charts were reviewed and 137 patients were included (Figure).
For the secondary results, 4 patients (2.9%) tolerated an alternate PCSK9i (evolocumab 140 mg SQ every 2 weeks) after initial intolerance to PCSK9i, 16 (11.7%) required a dose reduction, and 6 (4.4%) discontinued PCSK9i due to a muscle-related AE.
Statin intolerance was most common in all groups, followed by ezetimibe intolerance, and intolerance to statins + ezetimibe. Of the 113 patients who tolerated a full dose of PCSK9i, 77 (68.1%) had intolerance to statin, 47 (41.6%) to ezetimibe, and 41 (36.3%) to both statins and ezetimibe. Of the 6 patients who discontinued PCSK9i, all had intolerance to statins, 5 (83.3%) to ezetimibe, and 5 (83.3%) to statins and ezetimibe.
For patients who were on a reduced (monthly) dose of a PCSK9i who did not reach their LDL-C goal, we found that 16 patients (11.7%) required a PCSK9i dose reduction following muscle-related AEs. Of the patients who had their dose of PCSK9i reduced to monthly dosing, 5 (31%) met their LDL-C goal. For the 11 patients who did not meet their LDL-C goal, different management strategies were taken. Lifestyle modifications were made in 6 patients (54%), the monthly PCSK9i dose was increased to alirocumab 150 mg SQ monthly in 4 patients (36%), and 1 patient (9.1%) was switched to an alternative PCSK9i. There were no identified muscle-related AEs recorded in patients whose dose was increased to alirocumab 150 mg SQ monthly.
Discussion
This retrospective study found 17.5% of patients experienced muscle-related PCSK9i AEs. These occurred at a higher rate than reported in the prescribing information (< 5%) and were similar to the incidence rates reported in the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE clinical trials (12.0%-32.5%), which is what we hypothesized.18,19,22-25 It is important to note that the incidence rates of muscle-related AEs reported in the prescribing information for alirocumab and evolocumab were based on trials that did not include statin- and/or ezetimibe-intolerant patients; whereas many patients in our study and patients in the clinical trials were statin and/or ezetimibe intolerant.
Additionally, a new study by Donald and colleagues found an incidence rate of 32% to 36% for muscle-related PCSK9i AEs.27 Collectively, the data from clinical trials and our study indicate that patients with prior intolerances to statin and/or ezetimibe appear to have a higher likelihood of developing a muscle-related PCSK9i intolerance. In our study, 23 of 24 patients who developed a muscle-related PCSK9i AE had a prior history of statin and/or ezetimibe intolerances. This should alert clinicians prescribing PCSK9i in patients with a history of statin and/or ezetimibe intolerance to counsel their patients on the possibility of muscle-related PCSK9i AEs and management strategies. However, it is important to note that there was a substantial number of patients in our study who were statin and/or ezetimibe intolerant due to a prior muscle-related AE who tolerated the full dose of PCSK9i.
To our knowledge, this was the first trial to evaluate muscle-related PCSK9i AEs in a veteran population. Additionally, our study appears to be the first to use 2 PCSK9i dosing strategies that are not FDA approved: Dose reduction for patients who experienced a muscle-related AE on alirocumab 75 mg SQ every 2 weeks and dose escalation for patients who did not meet their LDL-C goal on alirocumab 75 mg SQ monthly following an initial intolerance to 2-week dosing. The dose-reduction strategy allowed patients who experienced a muscle-related AE to alirocumab 75 mg to reduce administration from every 2 weeks to monthly.
This strategy was only performed with alirocumab, the preferred PCSK9i at WBVAMC, but the same dose-reduction strategy can theoretically be used with evolocumab as well. Reduced monthly dosing of alirocumab allowed patients with a prior intolerance to remain on a lower dosage without discontinuation. This is important because as noted by Myers and colleagues, individuals without access to PCSK9i were found to have a significantly higher incidence ratio of cardiovascular events compared with those taking PCSK9i.15 Also of note, > 30% of patients on the reduced monthly dose of alirocumab still met their LDL-C goal. Therefore, using this dose-reduction strategy (instead of patients discontinuing therapy altogether due to a muscle-related intolerance) can lessen the risk of major adverse cardiovascular events (MACE) as well as mitigate muscle-related AEs that occurred while on 2-week PCSK9i dosing regimens. While we acknowledge that this reduced monthly dose of either alirocumab or evolocumab is not FDA approved, it is similar to alternative statin dosing that also is not FDA approved but may minimize the need to discontinue PCSK9i therapy. It would be beneficial if these dosing strategies were investigated by future research.
The dose-escalation strategy for patients who did not meet their LDL-C goal while on the reduced, monthly dose of alirocumab also was unique. Alirocumab was increased from 75 mg SQ once monthly to 150 mg SQ once monthly. Interestingly, we found that through the end of the chart review period, all patients tolerated the increase well, despite having an initial muscle-related AE to alirocumab 75 mg every 2 weeks, which is the same total monthly dosage. This approach is similar to that of once-weekly statin dosing or a drug holiday and may be explained by the long half-life of PCSK9i. Regardless of the mechanism, this finding suggests that an increased monthly dose of PCSK9i is a potential alternative for patients who cannot tolerate the FDA-approved dose. However, the ability for patients to achieve goal LDL-C on the monthly dosage requires future study.
In our study, only 6 patients (4.4%) discontinued PCSK9i therapy. This low discontinuation rate is largely attributable to our unique study design, which allowed for a dose reduction in patients who experienced muscle-related AEs. The earlier ODYSSEY-ALTERNATIVE trial evaluated the safety and efficacy of alirocumab compared with ezetimibe in confirmed statin-intolerant subjects after 24 weeks. This trial did not use a dose-reduction strategy and found 15.9% of patients discontinued alirocumab due to a muscle-related AE.24 This is notably higher than our discontinuation rate of 4.4%. If patients with a muscle-related AE discontinued PCKS9i instead of reducing the dose, they would likely return to their baseline LDL-C, which would increase the risk of MACE.
In general, myalgias due to antihyperlipidemic medications are not completely understood. One possible mechanism for statin-induced myalgias is the depletion of ubiquinone. However, this theory cannot explain muscle-related AEs associated with PCSK9i or ezetimibe, which have not been shown to deplete ubiquinone. We also found that the onset of muscle-related AEs associated with PCSK9i tends to appear later in therapy than what we know about statin therapy. Our study showed that the onset of a muscle-related PCSK9i AEs occurred a mean (SD) 8 (5.3) months after initiation (range, 1-19). Statin muscle-related AEs typically occur within the initial 4 to 8 weeks of treatment, although they can occur at any time.28
Limitations
The results of this study should be considered with the following limitations. First, this was a retrospective chart review performed over a prespecified period. Any muscle-related AEs or LDL-C lowering effects from PCSK9i that occurred outside the review period were not captured. Our study was small and only included 137 patients, though it was similar in size to the GAUSS-2, GAUSS-3, and ODYSSEY-ALTERNATIVE trials.22-24 Additionally, the study was primarily composed of White men and may not be representative of other populations. Some muscle-related PCSK9i AEs may be attributed to the nocebo. Last, our study did not capture patients on a PCSK9i who were not followed in the PACT clinic.
Conclusions
We found that muscle-related PCSK9i AEs occurred at a similar rate as those reported in previous clinical trials and exceeded the incidence rate reported in the prescribing information for alirocumab and evolocumab. It appears that patients who have a prior muscle-related intolerance to a statin and/or ezetimibe had a higher likelihood of developing a muscle-related PCSK9i AE. In our study, only 1 patient developed a muscle-related PCSK9i AE who did not have a prior history of muscle-related intolerance to either a statin or ezetimibe. However, in our study, a substantial percentage of patients with statin and/or ezetimibe intolerances tolerated the full PCSK9i dose well, proving that PCSK9i are still a reasonable alternative for patients with prior intolerances to statins and/or ezetimibe.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the US Department of Veterans Affairs Medical Center, Wilkes-Barre, Pennsylvania.
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389.
2. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001-1009. doi:10.1056/NEJM199610033351401
3. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357. doi:10.1056/NEJM199811053391902.
4. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22. doi:10.1016/S0140-6736(02)09327-3
5. Koren MJ, Hunninghake DB; ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772-1779. doi:10.1016/j.jacc.2004.07.053
6. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622. doi:10.1001/jama.279.20.1615
7. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288(23):2998-3007. doi:10.1001/jama.288.23.2998
8. Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158. doi:10.1016/S0140-6736(03)12948-0
9. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. doi:10.1056/NEJMoa0807646
10. Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155-1163. doi:10.1016/S0140-6736(06)69472-5
11. Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-1630. doi:10.1016/s0140-6736(02)11600-x
12. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301-1307. doi:10.1056/NEJM199511163332001

13. Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
14. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-350. doi:10.1016/j.jacc.2018.11003
15. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
16. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009-2010. J Clin Lipidol. 2015;9(4):525-532. doi:10.1016/j.jacl.2015.05.003
17. Della Badia LA, Elshourbagy NA, Mousa SA. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol Ther. 2016;164:183-194. doi:10.1016/j.pharmthera.2016.04.011
18. Praluent (alirocumab) injection. Prescribing information. Regeneron Pharmaceuticals; 2021.
19. Repatha (evolocumab) injection. Prescribing information. Amgen; 2021.
20. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi:10.1056/NEJMoa1801174
21. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/NEJMoa1615664
22. Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
23. Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
24. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9(6):758-769. doi:10.1016/j.jacl.2015.08.006
25. Mesi O, Lin C, Ahmed H, Cho LS. Statin intolerance and new lipid-lowering treatments. Cleve Clin J Med. 2021;88(7):381-387. Published 2021 Jul 1. doi:10.3949/ccjm.88a.20165
26. US Department of Veterans Affairs. Clinical Guidance - Criteria For Use. September 2016. Accessed January 23, 2023. https://www.pbm.va.gov/clinicalguidance/criteriaforuse.asp
27. Donald DR, Reynolds VW, Hall N, DeClercq J, Choi L. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16(3):315-324. doi:10.1016/j.jacl.2022.03.004
28. Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms: A clinical perspective from the National Lipid Association. J Clin Lipidol. Published online September 10, 2022. doi:10.1016/j.jacl.2022.09.001
Weaponizing Education: The Rise, Fall, and Return of the GI Bill
Growing up I can remember my father telling stories of service members in the medical battalion he commanded in World War II (WWII) who after the war with his encouragement and their GI Bill educational benefits went to school to become doctors, nurses, and dentists. They were among the 2,300,000 veterans who attended US colleges and universities through the Servicemen’s Readjustment Act passed in 1944. The American Legion navigated the bill through the twists and turns of congressional support, and it was one of their leaders who invented the catchy GI Bill shorthand.2
As with most political legislation, there were mixed motives driving passage of the act, and like many policies in America, the primary impetus was economic. While the war was raging overseas, at home the US Department of Labor predicted that by the war’s end, 16 million service members would be jobless. Apprehensive about the prospect of yet another financial depression, in 1943 a White House agency recommended that the federal government fund education and training for the individuals who had served during the war.2
While troops stormed the beaches of Normandy, wartime President Franklin D. Roosevelt (FDR) signed the bill that delivered not only educational and training opportunities for service members and veterans, but also funded home loans and US Department of Veterans Affairs (VA) hospitals. The bill was practical in that it provided not only tuition, but also books, supplies, a living stipend, and counseling for the students. The bill technically expired in 1956, but a series of extensions and expansions has been true to the original intention to offer those who served their nation in the military a better life as citizens.
Articles describing the impact of the GI Bill use terms like life changing and transformative.3,4 Our contemporary culture makes it difficult to imagine how out of reach a college education was for the generation that fought WWII. Universities were primarily for the rich and connected, the powerful and privileged. Were it not for the upward social mobility the GI Bill propelled, the American dream would not have become a reality for many farmers, small town merchants, and factory workers. The GI Bill though could not by itself ensure equity. The systemic racism endemic in the United States and among the elected representatives who debated the bill resulted in many Black service members especially in the South being denied entrance to institutions of higher learning.5 Despite this invidious discrimination, the bill was a profound effort to help many other service members to successfully reintegrate into the society they had preserved and defended.4
“With the signing of this bill, a well-rounded program of special veterans’ benefits is nearly completed,” FDR said, capturing its noble intent: “It gives emphatic notice to the men and women in our armed forces that the American people do not intend to let them down.”6
Regrettably, we have not kept FDR’s pledge. Now unscrupulous businesses are preying on the aspirations of military personnel and veterans for an education and thwarting their ability to seek gainful employment. For more than a decade, respected news media have reported that for-profit universities were exploiting service members trying to improve their lives through obtaining a college education via the GI Bill.7 The sad irony is that what enabled the exploitation to occur was a major expansion of the benefits under the Post-9/11 GI Bill. This version granted educational funding to any individual who had served on active duty for 90 days or more after September 10, 2001.8 Federal law prohibits for-profit educational institutions from receiving more than 90% of their total revenue from federal student aid. A loophole in the law enabled these institutions to categorize GI Bill funding as private not government dollars. Bad old American greed drove these for-profit colleges and universities to aggressively recruit veterans who trusted in the good faith of the academic institutions. Once the GI Bill monies were exhausted, veterans had already invested so much time and energy in a degree or certificate, the schools could persuade them to take out student loans with the promise of job placement assistance that never materialized. They took advantage of the veterans’ hopes to fatten their own bottom line in the face of declining enrolments.9 Journalists, government, think tank reports, and even a documentary described the tragic stories of service members left unemployed with immense debt and degrees that to many of them were now worthless.10
After years of reporters exposing the scam and politically thwarted efforts to stop it, Congress and President Biden closed what was known as the 90/10 loophole. This ended the weaponization of education it had promoted. In October 2022, the US Department of Education announced its final rule to prohibit the widespread educational fraud that had betrayed so many veterans and service members, which Secretary Dennis McDonough described as “abuse.”11Some readers may wonder why I have devoted an editorial to a topic that seems somewhat distant from the health care that is the primary domain of Federal Practitioner. It happens that education is in closer proximity to health for our patients than many of us might have realized. A 2018 Military Medicine study found that veterans who took advantage of the educational opportunities of the GI Bill had better health and reduced smoking, among other benefits.12 This connection between health and education should serve as a source of pride for all of us in federal practice as we are part of organizations that affirm the holistic concept of health that embraces not just medicine but education, housing, and other services essential for comprehensive well-being.
1. Mandela NR. Lighting your way to a better future: speech delivered by Mr. N R Mandela at the launch of Mindset Network. July 16, 2003. Accessed January 23, 2023. http://db.nelsonmandela.org/speeches/pub_view.asp?pg=item&ItemID=NMS909&txtstr=Lighting%20your%20way%20to%20a%20better%20future
2. US National Archives and Records Administration. Milestones Documents: Servicemen’s Readjustment Act (1944). Updated May 3, 2022. Accessed January 23, 2023. https://www.archives.gov/milestone-documents/servicemens-readjustment-act
3. O’Brien C. A brief history of the GI Bill. Army Times. March 10, 2021. Accessed January 23, 2023. https://www.armytimes.com/education-transition/2021/03/10/a-brief-history-of-the-gi-bill
4. US Department of Defense. 75 years of the GI Bill: how transformative it’s been. June 9, 2019. Accessed January 23, 2023. https://www.defense.gov/News/Feature-Stories/story/Article/1727086/75-years-of-the-gi-bill-how-transformative-its-been
5. Thompson J. The GI Bill should’ve been race neutral, politicos made sure it wasn’t. Army Times. November 9, 2019. Accessed January 23, 2023. https://www.armytimes.com/military-honor/salute-veterans/2019/11/10/the-gi-bill-shouldve-been-race-neutral-politicos-made-sure-it-wasnt
6. US Department of Veterans Affairs. Born of controversy: the GI Bill of Rights. Accessed January 23, 2023. https://www.va.gov/opa/publications/celebrate/gi-bill.pdf
7. Lipton E. Profit and scrutiny for colleges courting veterans. New York Times. December 8, 2010. Accessed January 23, 2023. https://www.nytimes.com/2010/12/09/education/09colleges.html
8. Post-9/11 GI Bill. Accessed January 23, 2023. https://www.military.com/education/gi-bill/post-9-11
9. Veterans Education Success. Large for-profit schools remain dependent on recruiting GI Bill students despite overall enrollment declines. Veterans Perspective Brief 2018;4. Accessed January 23, 2023. https://static1.squarespace.com/static/556718b2e4b02e470eb1b186/t/5ae241e588251be6319e24a5/1524777445871/VES+Issue+Brief+%234+Enrollment.FINAL.v2.pdf
10. Hernandez K. Why these veterans regret their for-profit degrees—and debt. PBS Newshour. October 23, 2018. Accessed January 23, 2023. https://www.pbs.org/newshour/education/why-these-veterans-regret-their-for-profit-college-degrees-and-debt
11. US Department of Education. Education Department unveils final rules to protect veterans and service members, improve college access for incarcerated individuals and improve oversight when colleges change owners. Press release. Published October 27, 2022. Accessed January 23, 2023. https://www.ed.gov/news/press-releases/education-department-unveils-final-rules-protect-veterans-and-service-members-improve-college-access-incarcerated-individuals-and-improve-oversight-when-colleges-change-owners
12. Rumery ZR, Patel N, Richard P. The association between the use of the education benefits from the G.I. Bill and veterans’ health. Mil Med. 2018;183(5-6):e241-e248. doi:10.1093/milmed/usx102
Growing up I can remember my father telling stories of service members in the medical battalion he commanded in World War II (WWII) who after the war with his encouragement and their GI Bill educational benefits went to school to become doctors, nurses, and dentists. They were among the 2,300,000 veterans who attended US colleges and universities through the Servicemen’s Readjustment Act passed in 1944. The American Legion navigated the bill through the twists and turns of congressional support, and it was one of their leaders who invented the catchy GI Bill shorthand.2
As with most political legislation, there were mixed motives driving passage of the act, and like many policies in America, the primary impetus was economic. While the war was raging overseas, at home the US Department of Labor predicted that by the war’s end, 16 million service members would be jobless. Apprehensive about the prospect of yet another financial depression, in 1943 a White House agency recommended that the federal government fund education and training for the individuals who had served during the war.2
While troops stormed the beaches of Normandy, wartime President Franklin D. Roosevelt (FDR) signed the bill that delivered not only educational and training opportunities for service members and veterans, but also funded home loans and US Department of Veterans Affairs (VA) hospitals. The bill was practical in that it provided not only tuition, but also books, supplies, a living stipend, and counseling for the students. The bill technically expired in 1956, but a series of extensions and expansions has been true to the original intention to offer those who served their nation in the military a better life as citizens.
Articles describing the impact of the GI Bill use terms like life changing and transformative.3,4 Our contemporary culture makes it difficult to imagine how out of reach a college education was for the generation that fought WWII. Universities were primarily for the rich and connected, the powerful and privileged. Were it not for the upward social mobility the GI Bill propelled, the American dream would not have become a reality for many farmers, small town merchants, and factory workers. The GI Bill though could not by itself ensure equity. The systemic racism endemic in the United States and among the elected representatives who debated the bill resulted in many Black service members especially in the South being denied entrance to institutions of higher learning.5 Despite this invidious discrimination, the bill was a profound effort to help many other service members to successfully reintegrate into the society they had preserved and defended.4
“With the signing of this bill, a well-rounded program of special veterans’ benefits is nearly completed,” FDR said, capturing its noble intent: “It gives emphatic notice to the men and women in our armed forces that the American people do not intend to let them down.”6
Regrettably, we have not kept FDR’s pledge. Now unscrupulous businesses are preying on the aspirations of military personnel and veterans for an education and thwarting their ability to seek gainful employment. For more than a decade, respected news media have reported that for-profit universities were exploiting service members trying to improve their lives through obtaining a college education via the GI Bill.7 The sad irony is that what enabled the exploitation to occur was a major expansion of the benefits under the Post-9/11 GI Bill. This version granted educational funding to any individual who had served on active duty for 90 days or more after September 10, 2001.8 Federal law prohibits for-profit educational institutions from receiving more than 90% of their total revenue from federal student aid. A loophole in the law enabled these institutions to categorize GI Bill funding as private not government dollars. Bad old American greed drove these for-profit colleges and universities to aggressively recruit veterans who trusted in the good faith of the academic institutions. Once the GI Bill monies were exhausted, veterans had already invested so much time and energy in a degree or certificate, the schools could persuade them to take out student loans with the promise of job placement assistance that never materialized. They took advantage of the veterans’ hopes to fatten their own bottom line in the face of declining enrolments.9 Journalists, government, think tank reports, and even a documentary described the tragic stories of service members left unemployed with immense debt and degrees that to many of them were now worthless.10
After years of reporters exposing the scam and politically thwarted efforts to stop it, Congress and President Biden closed what was known as the 90/10 loophole. This ended the weaponization of education it had promoted. In October 2022, the US Department of Education announced its final rule to prohibit the widespread educational fraud that had betrayed so many veterans and service members, which Secretary Dennis McDonough described as “abuse.”11Some readers may wonder why I have devoted an editorial to a topic that seems somewhat distant from the health care that is the primary domain of Federal Practitioner. It happens that education is in closer proximity to health for our patients than many of us might have realized. A 2018 Military Medicine study found that veterans who took advantage of the educational opportunities of the GI Bill had better health and reduced smoking, among other benefits.12 This connection between health and education should serve as a source of pride for all of us in federal practice as we are part of organizations that affirm the holistic concept of health that embraces not just medicine but education, housing, and other services essential for comprehensive well-being.
Growing up I can remember my father telling stories of service members in the medical battalion he commanded in World War II (WWII) who after the war with his encouragement and their GI Bill educational benefits went to school to become doctors, nurses, and dentists. They were among the 2,300,000 veterans who attended US colleges and universities through the Servicemen’s Readjustment Act passed in 1944. The American Legion navigated the bill through the twists and turns of congressional support, and it was one of their leaders who invented the catchy GI Bill shorthand.2
As with most political legislation, there were mixed motives driving passage of the act, and like many policies in America, the primary impetus was economic. While the war was raging overseas, at home the US Department of Labor predicted that by the war’s end, 16 million service members would be jobless. Apprehensive about the prospect of yet another financial depression, in 1943 a White House agency recommended that the federal government fund education and training for the individuals who had served during the war.2
While troops stormed the beaches of Normandy, wartime President Franklin D. Roosevelt (FDR) signed the bill that delivered not only educational and training opportunities for service members and veterans, but also funded home loans and US Department of Veterans Affairs (VA) hospitals. The bill was practical in that it provided not only tuition, but also books, supplies, a living stipend, and counseling for the students. The bill technically expired in 1956, but a series of extensions and expansions has been true to the original intention to offer those who served their nation in the military a better life as citizens.
Articles describing the impact of the GI Bill use terms like life changing and transformative.3,4 Our contemporary culture makes it difficult to imagine how out of reach a college education was for the generation that fought WWII. Universities were primarily for the rich and connected, the powerful and privileged. Were it not for the upward social mobility the GI Bill propelled, the American dream would not have become a reality for many farmers, small town merchants, and factory workers. The GI Bill though could not by itself ensure equity. The systemic racism endemic in the United States and among the elected representatives who debated the bill resulted in many Black service members especially in the South being denied entrance to institutions of higher learning.5 Despite this invidious discrimination, the bill was a profound effort to help many other service members to successfully reintegrate into the society they had preserved and defended.4
“With the signing of this bill, a well-rounded program of special veterans’ benefits is nearly completed,” FDR said, capturing its noble intent: “It gives emphatic notice to the men and women in our armed forces that the American people do not intend to let them down.”6
Regrettably, we have not kept FDR’s pledge. Now unscrupulous businesses are preying on the aspirations of military personnel and veterans for an education and thwarting their ability to seek gainful employment. For more than a decade, respected news media have reported that for-profit universities were exploiting service members trying to improve their lives through obtaining a college education via the GI Bill.7 The sad irony is that what enabled the exploitation to occur was a major expansion of the benefits under the Post-9/11 GI Bill. This version granted educational funding to any individual who had served on active duty for 90 days or more after September 10, 2001.8 Federal law prohibits for-profit educational institutions from receiving more than 90% of their total revenue from federal student aid. A loophole in the law enabled these institutions to categorize GI Bill funding as private not government dollars. Bad old American greed drove these for-profit colleges and universities to aggressively recruit veterans who trusted in the good faith of the academic institutions. Once the GI Bill monies were exhausted, veterans had already invested so much time and energy in a degree or certificate, the schools could persuade them to take out student loans with the promise of job placement assistance that never materialized. They took advantage of the veterans’ hopes to fatten their own bottom line in the face of declining enrolments.9 Journalists, government, think tank reports, and even a documentary described the tragic stories of service members left unemployed with immense debt and degrees that to many of them were now worthless.10
After years of reporters exposing the scam and politically thwarted efforts to stop it, Congress and President Biden closed what was known as the 90/10 loophole. This ended the weaponization of education it had promoted. In October 2022, the US Department of Education announced its final rule to prohibit the widespread educational fraud that had betrayed so many veterans and service members, which Secretary Dennis McDonough described as “abuse.”11Some readers may wonder why I have devoted an editorial to a topic that seems somewhat distant from the health care that is the primary domain of Federal Practitioner. It happens that education is in closer proximity to health for our patients than many of us might have realized. A 2018 Military Medicine study found that veterans who took advantage of the educational opportunities of the GI Bill had better health and reduced smoking, among other benefits.12 This connection between health and education should serve as a source of pride for all of us in federal practice as we are part of organizations that affirm the holistic concept of health that embraces not just medicine but education, housing, and other services essential for comprehensive well-being.
1. Mandela NR. Lighting your way to a better future: speech delivered by Mr. N R Mandela at the launch of Mindset Network. July 16, 2003. Accessed January 23, 2023. http://db.nelsonmandela.org/speeches/pub_view.asp?pg=item&ItemID=NMS909&txtstr=Lighting%20your%20way%20to%20a%20better%20future
2. US National Archives and Records Administration. Milestones Documents: Servicemen’s Readjustment Act (1944). Updated May 3, 2022. Accessed January 23, 2023. https://www.archives.gov/milestone-documents/servicemens-readjustment-act
3. O’Brien C. A brief history of the GI Bill. Army Times. March 10, 2021. Accessed January 23, 2023. https://www.armytimes.com/education-transition/2021/03/10/a-brief-history-of-the-gi-bill
4. US Department of Defense. 75 years of the GI Bill: how transformative it’s been. June 9, 2019. Accessed January 23, 2023. https://www.defense.gov/News/Feature-Stories/story/Article/1727086/75-years-of-the-gi-bill-how-transformative-its-been
5. Thompson J. The GI Bill should’ve been race neutral, politicos made sure it wasn’t. Army Times. November 9, 2019. Accessed January 23, 2023. https://www.armytimes.com/military-honor/salute-veterans/2019/11/10/the-gi-bill-shouldve-been-race-neutral-politicos-made-sure-it-wasnt
6. US Department of Veterans Affairs. Born of controversy: the GI Bill of Rights. Accessed January 23, 2023. https://www.va.gov/opa/publications/celebrate/gi-bill.pdf
7. Lipton E. Profit and scrutiny for colleges courting veterans. New York Times. December 8, 2010. Accessed January 23, 2023. https://www.nytimes.com/2010/12/09/education/09colleges.html
8. Post-9/11 GI Bill. Accessed January 23, 2023. https://www.military.com/education/gi-bill/post-9-11
9. Veterans Education Success. Large for-profit schools remain dependent on recruiting GI Bill students despite overall enrollment declines. Veterans Perspective Brief 2018;4. Accessed January 23, 2023. https://static1.squarespace.com/static/556718b2e4b02e470eb1b186/t/5ae241e588251be6319e24a5/1524777445871/VES+Issue+Brief+%234+Enrollment.FINAL.v2.pdf
10. Hernandez K. Why these veterans regret their for-profit degrees—and debt. PBS Newshour. October 23, 2018. Accessed January 23, 2023. https://www.pbs.org/newshour/education/why-these-veterans-regret-their-for-profit-college-degrees-and-debt
11. US Department of Education. Education Department unveils final rules to protect veterans and service members, improve college access for incarcerated individuals and improve oversight when colleges change owners. Press release. Published October 27, 2022. Accessed January 23, 2023. https://www.ed.gov/news/press-releases/education-department-unveils-final-rules-protect-veterans-and-service-members-improve-college-access-incarcerated-individuals-and-improve-oversight-when-colleges-change-owners
12. Rumery ZR, Patel N, Richard P. The association between the use of the education benefits from the G.I. Bill and veterans’ health. Mil Med. 2018;183(5-6):e241-e248. doi:10.1093/milmed/usx102
1. Mandela NR. Lighting your way to a better future: speech delivered by Mr. N R Mandela at the launch of Mindset Network. July 16, 2003. Accessed January 23, 2023. http://db.nelsonmandela.org/speeches/pub_view.asp?pg=item&ItemID=NMS909&txtstr=Lighting%20your%20way%20to%20a%20better%20future
2. US National Archives and Records Administration. Milestones Documents: Servicemen’s Readjustment Act (1944). Updated May 3, 2022. Accessed January 23, 2023. https://www.archives.gov/milestone-documents/servicemens-readjustment-act
3. O’Brien C. A brief history of the GI Bill. Army Times. March 10, 2021. Accessed January 23, 2023. https://www.armytimes.com/education-transition/2021/03/10/a-brief-history-of-the-gi-bill
4. US Department of Defense. 75 years of the GI Bill: how transformative it’s been. June 9, 2019. Accessed January 23, 2023. https://www.defense.gov/News/Feature-Stories/story/Article/1727086/75-years-of-the-gi-bill-how-transformative-its-been
5. Thompson J. The GI Bill should’ve been race neutral, politicos made sure it wasn’t. Army Times. November 9, 2019. Accessed January 23, 2023. https://www.armytimes.com/military-honor/salute-veterans/2019/11/10/the-gi-bill-shouldve-been-race-neutral-politicos-made-sure-it-wasnt
6. US Department of Veterans Affairs. Born of controversy: the GI Bill of Rights. Accessed January 23, 2023. https://www.va.gov/opa/publications/celebrate/gi-bill.pdf
7. Lipton E. Profit and scrutiny for colleges courting veterans. New York Times. December 8, 2010. Accessed January 23, 2023. https://www.nytimes.com/2010/12/09/education/09colleges.html
8. Post-9/11 GI Bill. Accessed January 23, 2023. https://www.military.com/education/gi-bill/post-9-11
9. Veterans Education Success. Large for-profit schools remain dependent on recruiting GI Bill students despite overall enrollment declines. Veterans Perspective Brief 2018;4. Accessed January 23, 2023. https://static1.squarespace.com/static/556718b2e4b02e470eb1b186/t/5ae241e588251be6319e24a5/1524777445871/VES+Issue+Brief+%234+Enrollment.FINAL.v2.pdf
10. Hernandez K. Why these veterans regret their for-profit degrees—and debt. PBS Newshour. October 23, 2018. Accessed January 23, 2023. https://www.pbs.org/newshour/education/why-these-veterans-regret-their-for-profit-college-degrees-and-debt
11. US Department of Education. Education Department unveils final rules to protect veterans and service members, improve college access for incarcerated individuals and improve oversight when colleges change owners. Press release. Published October 27, 2022. Accessed January 23, 2023. https://www.ed.gov/news/press-releases/education-department-unveils-final-rules-protect-veterans-and-service-members-improve-college-access-incarcerated-individuals-and-improve-oversight-when-colleges-change-owners
12. Rumery ZR, Patel N, Richard P. The association between the use of the education benefits from the G.I. Bill and veterans’ health. Mil Med. 2018;183(5-6):e241-e248. doi:10.1093/milmed/usx102
Trauma-Informed Training for Veterans Treatment Court Professionals: Program Development and Initial Feedback
Veterans who interact with the criminal justice system (ie, justice-involved veterans) have heightened rates of mental health and psychosocial needs, including posttraumatic stress disorder (PTSD), substance use disorder, depression, suicidal ideation and attempt, and homelessness.1,2 Alongside these criminogenic risk factors, recidivism is common among justice-involved veterans: About 70% of incarcerated veterans disclosed at least one prior incarceration.3
To address the complex interplay of psychosocial factors, mental health concerns, and justice involvement among veterans, veterans treatment courts (VTCs) emerged as an alternative to incarceration.4 VTC participation often consists of integrated treatment and rehabilitative services (eg, vocational training, health care), ongoing monitoring for substance use, graduated responses to address treatment adherence, and ongoing communication with the judge and legal counsel.4
A primary aim of these courts is to address psychosocial needs believed to underlie criminal behavior, thus reducing risk of recidivism and promoting successful recovery and community integration for eligible veterans. To do so, VTCs collaborate with community-based and/or US Department of Veterans Affairs services, such as the Veterans Justice Outreach program (VJO). VJO specialists identify and refer justice-involved veterans to Veterans Health Administration (VHA) and community care and serve as a liaison between VTC staff and VHA health care professionals (HCPs).5
VTC outcome studies highlight the importance of not only diverting veterans to problem-solving courts, but also ensuring their optimal participation. Successful graduates of VTC programs demonstrate significant improvements in mental health symptoms, life satisfaction, and social support, as well as lower rates of law enforcement interactions.6,7 However, less is known about supporting those veterans who have difficulty engaging in VTCs and either discontinue participation or require lengthier periods of participation to meet court graduation requirements.8 One possibility to improve engagement among these veterans is to enhance court practices to best meet their needs.
In addition to delivering treatment, VHA mental health professionals may serve a critical interdisciplinary role by lending expertise to support VTC practices. For example, equipping court professionals with clinical knowledge and skills related to motivation may strengthen the staff’s interactions with participants, enabling them to address barriers as they arise and to facilitate veterans’ treatment adherence. Additionally, responsiveness to the impact of trauma exposure, which is common among this population, may prove important as related symptoms can affect veterans’ engagement, receptivity, and behavior in court settings. Indeed, prior examinations of justice-involved veterans have found trauma exposure rates ranging from 60% to 90% and PTSD rates ranging from 27% to 40%.1,2 Notably, involvement with the justice system (eg, incarceration) may itself further increase risk of trauma exposure (eg, experiencing a physical or sexual assault in prison) or exacerbate existing PTSD.9 Nonetheless, whereas many drug courts and domestic violence courts have been established, problem-solving courts with a specialized focus on trauma exposure remain rare, suggesting a potential gap in court training.
VHA HCPs have the potential to facilitate justice-involved veterans’ successful court and treatment participation by coordinating with VJO specialists to provide training and consultation to the courts. Supporting efforts to effectively and responsively address criminogenic risk (eg, mental health) in VTC settings may in turn reduce the likelihood of recidivism.10 Given the elevated rates of trauma exposure among justice-involved veterans and the relative lack of trauma-focused VTCs, we developed a trauma-informed training for VTC professionals that centered on related clinical presentations of justice-involved veterans and frequently occurring challenges in the context of court participation.
Program Development
This educational program aimed to (1) provide psychoeducation on trauma exposure, PTSD, and existing evidence-based treatments; (2) present clinical considerations for justice-involved veterans related to trauma exposure and/or PTSD; and (3) introduce skills to facilitate effective communication and trauma-informed care practices among professionals working with veterans in a treatment court.
Prior to piloting the program, we conducted a needs assessment with VTC professionals and identified relevant theoretical constructs and brief interventions for inclusion in the training. Additionally, given the dearth of prior research on mental health education for VTCs, the team consulted with the developers of PTSD 101, a VHA workshop for veterans’ families that promotes psychoeducation, support, and effective communication.11 Doing so informed approaches to delivering education to nonclinical audiences that interact with veterans with histories of trauma exposure. As this was a program development project, it was determined to be exempt from institutional review board review.
Needs Assessment
In the initial stages of development, local VJO specialists identified regional VTCs and facilitated introductions to these courts. Two of the 3 Rocky Mountain region VTCs that were contacted expressed interest in receiving trauma-informed training. Based on preliminary interest, the facilitators conducted a needs assessment with VJO and VTC staff from these 2 courts to capture requests for specific content and past experiences with other mental health trainings.
Guided by the focus group model, the needs assessments took place during three 1-hour meetings with VJO specialists and a 1-hour meeting with VJO specialists, VTC professionals, and community-based clinical partners.12 Additionally, attending a VTC graduation and court session allowed for observations of court practices and interactions with veterans. A total of 13 professionals (judges, court coordinators, case managers, peer mentors, VJO specialists, and clinicians who specialize in substance use disorder and intimate partner violence) participated in the needs assessments.
The most critical need identified by court professionals was a focus on how to apply knowledge about trauma and PTSD to interactions with justice-involved veterans. This was reportedly absent from prior training sessions the courts had received. Both Rocky Mountain region VTCs expressed a strong interest in and openness to adapting practices based on research and practice recommendations. Additional requests that emerged included a refresher on psychoeducation related to trauma and how to address the personal impact of working with this population (eg, compassion fatigue).
Training Components
Based on the needs identified by VTC professionals and informed by consultation with the developers of PTSD 101,
Psychoeducation. The initial portion of the training consisted of psychoeducation to increase VTC staff familiarity with the distinctions between trauma exposure and a formal diagnosis of PTSD, mechanisms underlying PTSD, and evidence-based treatment. To deepen conceptual understanding of trauma and PTSD beyond an overview of criteria set forth in The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), psychoeducation centered on the drivers of avoidance (eg, short-term benefit vs long-term consequences), behaviors that often facilitate avoidance (eg, substance use), functions underlying these behaviors (eg, distress reduction), and structure and mechanisms of change in evidence-based treatments for PTSD, including cognitive processing therapy and prolonged exposure.13,14
Fostering court familiarity with cognitive processing therapy and prolonged exposure may bolster veteran engagement in treatment through regular reinforcement of skills and concepts introduced in therapy. This may prove particularly salient given the limited engagement with mental health treatment and elevated dropout rates from PTSD treatment among the general veteran population.15,16
Exercises and metaphors were used to illustrate concepts in multiple ways. For example, training attendees engaged in a “stop, drop, and roll” thought exercise in which they were asked to brainstorm behavioral reactions to catching on fire. This exercise illustrated the tendency for individuals to revert to common yet unhelpful attempts at problem solving (eg, running due to panic, which would exacerbate the fire), particularly in crisis and without prior education regarding adaptive ways to respond. Attendee-generated examples, such as running, were used to demonstrate the importance of practicing and reinforcing skill development prior to a crisis, to ensure proficiency and optimal response. Additionally, in prompting consideration of one’s response tendencies, this exercise may engender empathy and understanding for veterans.
Skills training. Efforts to promote veteran engagement in court, facilitate motivation and readiness for change, and address barriers that arise (eg, distress associated with court appearances) may support successful and timely graduation. As such, skills training constituted the largest component of the training and drew from observations of court practices and the VTCs’ identified challenges. Consistent with the project’s aims and reported needs of the court, skills that target common presentations following trauma exposure (eg, avoidance, hypervigilance) were prioritized for this pilot training. Strategies included brief interventions from dialectical behavior therapy, acceptance and commitment therapy, and motivational interviewing to strengthen the support provided by staff to veterans and address their needs (Table 2).
Training attendees also participated in exercises to reiterate skills. For example, attendees completed an ambivalence matrix using an audience-identified common behavior that is difficult to change (eg, heavy alcohol use as a coping mechanism for distress).
Attendees engaged in an exercise that involved identifying unhelpful thoughts and behaviors, targets for validation, and veteran strengths from a hypothetical case vignette. This vignette involved a VTC participant who initially engaged effectively but began to demonstrate difficulty appropriately engaging in court and mental health treatment as well as challenging interactions with VTC staff (eg, raised voice during court sessions, not respecting communication boundaries).
Pilot Test
Based on scheduling parameters communicated by court coordinators, the pilot training was designed as a presentation during times reserved for court staffing meetings. To accommodate court preferences due to the COVID-19 pandemic, one 90-minute training was conducted virtually in March 2022, and the other training was conducted in person in April 2022 for 2 hours. The trainings were facilitated by 2 VHA clinical psychologists and included the judge, court coordinator, VJO specialist, peer mentors, case managers, probation/parole officers, and community-based HCPs who partner with the court (eg, social workers, psychologists). About 12 to 15 professionals attended each training session.
Feedback
Feedback was solicited from attendees via an anonymous online survey. Seven participants completed the survey; the response rate of about 20% was consistent with those observed for other surveys of court professionals.20 Many attendees also provided feedback directly to the facilitators. Feedback highlighted that the skills-based components not only were perceived as most helpful but also notably distinguished this training. “What set this training apart from other training events was the practical applications,” one attendee noted. “It was not just information or education, both instructors did an incredible job of explaining exactly how we could apply the knowledge they were sharing. They did this in such a way that it was easy to understand and apply.”
Specific skills were consistently identified as helpful, including managing intense emotions, addressing ambivalence, and approaching sanctions and rewards. Additionally, employing a less formal approach to the training, with relatable overviews of concepts and immediate responsiveness to requests for expansion on a topic, was perceived as a unique benefit: Another attendee appreciated that “It was beneficial to sit around a table with a less formal presentation and be able to ask questions.” This approach seemed particularly well suited for the program’s cross-disciplinary audience. Attendees reported that they valued the relatively limited focus on DSM-5 criteria. Attendees emphasized that education specific to veterans on evidence-based PTSD treatments, psychoeducation, and avoidance was very helpful. Respondents also recommended that the training be lengthened to a daylong workshop to accommodate greater opportunity to practice skills and consultation.
The consultation portion of the training provided insight into additional areas of importance to incorporate into future iterations. Identified needs included appropriate and realistic boundary setting (eg, addressing disruptions in the courtroom), suggestions for improving and expanding homework assigned by the court, and ways to address concerns about PTSD treatment shared by veterans in court (eg, attributing substance use relapses to the intensiveness of trauma-focused treatment vs lack of familiarity with alternate coping skills). Additionally, the VTC professionals’ desire to support mental health professionals’ work with veterans was clearly evident, highlighting the bidirectional value of interdisciplinary collaboration between VHA mental health professionals and VTC professionals.
Discussion
A trauma-informed training was developed and delivered to 2 VTCs in the Rocky Mountain region with the goal of providing relevant psychoeducation and introducing skills to bolster court practices that address veteran needs. Psychoeducational components of the training that were particularly well received and prompted significant participant engagement included discussions and examples of avoidance, levels of validation, language to facilitate motivation and address barriers, mechanisms underlying treatment, and potential functions underlying limited veteran treatment engagement. Distress tolerance, approaches to sanctions and rewards, and use of ambivalence matrices to guide motivation were identified as particularly helpful skills.
The pilot phase of this trauma-informed training provided valuable insights into developing mental health trainings for VTCs. Specifically, VTCs may benefit from the expertise of VHA HCPs and are particularly interested in learning brief skills to improve their practices. The usefulness of such trainings may be bolstered by efforts to form relationships with the court to identify their perceived needs and employing an iterative process that is responsive to feedback both during and after the training. Last, each stage of this project was strengthened by collaboration with VJO specialists, highlighting the importance of future collaboration between VJO and VHA mental health clinics to further support justice-involved veterans. For example, VJO specialists were instrumental in identifying training needs related to veterans’ clinical presentations in court, facilitating introductions to local VTCs, and helping to address barriers to piloting, like scheduling.
Modifications and Future Directions
The insights gained through the process of training design, delivery, and feedback inform future development of this training. Based on the feedback received, subsequent versions of the training may be expanded into a half- or full-day workshop to allow for adequate time for skills training and feedback, as well as consultation. Doing so will enable facilitators to further foster attendees’ familiarity with and confidence in their ability to use these skills. Furthermore, the consultation portion of this training revealed areas that may benefit from greater attention, including how to address challenging interactions in court (eg, addressing gender dynamics between court professionals and participants) and better support veterans who are having difficulty engaging in mental health treatment (eg, courts’ observation of high rates of dropout around the third or fourth session of evidence-based treatment for PTSD). Last, all attendees who responded to the survey expressed interest in a brief resource guide based on the training, emphasizing the need for ready access to key skills and concepts to support the use of strategies learned.
An additional future aim of this project is to conduct a more thorough evaluation of the needs and outcomes related to this trauma-informed training for VTC professionals. With the rapid growth of VTCs nationwide, relatively little examination of court processes and practices has occurred, and there is a lack of research on the development or effectiveness of mental health trainings provided to VTCs.21 Therefore, we intend to conduct larger scale qualitative interviews with court personnel and VJO specialists to obtain a clearer understanding of the needs related to skills-based training and gaps in psychoeducation. These comprehensive needs assessments may also capture common comorbidities that were not incorporated into the pilot training (eg, substance use disorders) but may be important training targets for court professionals. This information will be used to inform subsequent expansion and adaptation of the training into a longer workshop. Program evaluation will be conducted via survey-based feedback on perceived usefulness of the workshop and self-report of confidence in and use of strategies to improve court practices. Furthermore, efforts to obtain veteran outcome data, such as treatment engagement and successful participation in VTC, may be pursued.
Limitations
This training development and pilot project provided valuable foundational information regarding a largely unexamined component of treatment courts—the benefit of skills-based trainings to facilitate court practices related to justice-involved veterans. However, it is worth noting that survey responses were limited; thus, the feedback received may not reflect all attendees’ perceptions. Additionally, because both training sessions were conducted solely with 2 courts in the Rocky Mountain area, feedback may be limited to the needs of this geographic region.
Conclusions
A trauma-informed training was developed for VTCs to facilitate relevant understanding of justice-involved veterans’ needs and presentations in court, introduce skills to address challenges that arise (eg, motivation, emotional dysregulation), and provide interdisciplinary support to court professionals. This training was an important step toward fostering strong collaborations between VHA HCPs and community-based veterans courts, and feedback received during development and following implementation highlighted the perceived need for a skills-based approach to such trainings. Further program development and evaluation can strengthen this training and provide a foundation for dissemination to a broader scope of VTCs, with the goal of reducing recidivism risk among justice-involved veterans by promoting effective engagement in problem-solving court.
1. Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justice-involved veterans. Epidemiol Rev. 2015;37(1):163-176. doi:10.1093/epirev/mxu003
2. Saxon AJ, Davis TM, Sloan KL, McKnight KM, McFall ME, Kivlahan DR. Trauma, symptoms of posttraumatic stress disorder, and associated problems among incarcerated veterans. Psychiatr Serv. 2001;52(7):959-964. doi:10.1176/appi.ps.52.7.959
3. Bronson J, Carson AC, Noonan M. Veterans in prison and jail, 2011-12. December 2015. Accessed January 11, 2023. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
4. Cartwright T. “To care for him who shall have borne the battle”: the recent development of veterans treatment courts in America. Stanford Law Rev. 2011;22(1):295-316.
5. Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs Veterans Justice Outreach Program: connecting justice-involved veterans with mental health and substance use disorder Treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
6. Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
7. Montgomery LM, Olson JN. Veterans treatment court impact on veteran mental health and life satisfaction. J Psychol Behav Sci. 2018;6(1):1-4. doi:10.15640/jpbs.v6n1a1
8. Tsai J, Finlay A, Flatley B, Kasprow WJ, Clark S. A national study of veterans treatment court participants: who benefits and who recidivates. Adm Policy Ment Health. 2018;45(2):236-244. doi:10.1007/s10488-017-0816-z
9. Wolff NL, Shi J. Trauma and incarcerated persons. In: Scott CL, ed. Handbook of Correctional Mental Health. American Psychiatric Publishing, Inc.; 2010:277-320.
10. Bonta J, Andrews DA. Risk-need-responsivity model for offender assessment and rehabilitation. Rehabilitation. 2007;6:1-22. https://www.publicsafety.gc.ca/cnt/rsrcs/pblctns/rsk-nd-rspnsvty/index-en.aspx
11. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention, Family Services Section; Caska-Wallace CM, Campbell SB, Glynn SM. PTSD 101 for family and friends: a support and education workshop. 2020.
12. Tipping J. Focus groups: a method of needs assessment. J Contin Educ Health Prof. 1998;18(3):150-154. doi:10.1002/chp.1340180304
13. Resick PA, Monson CM, Chard KM. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. The Guilford Press; 2017.
14. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. Oxford University Press; 2007. doi:10.1093/med:psych/9780195308501.001.0001
15. Seal KH, Maguen S, Cohen B, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23(1):5-16. doi:10.1002/jts.20493
16. Edwards-Stewart A, Smolenski DJ, Bush NE, et al. Posttraumatic stress disorder treatment dropout among military and veteran populations: a systematic review and meta-analysis. J Trauma Stress. 2021;34(4):808-818. doi:10.1002/jts.22653
17. Linehan MM. Dialectical Behavior Therapy Skills Training Manual. 2nd ed. Guildford Press; 2015.
18. Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change. 2nd ed. Guildford Press; 2016.
19. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. The Guildford Press; 2002.
20. National Center for State Courts. A survey of members of major national court organizations. October 2010. Accessed January 11, 2023. https://www.ncsc.org/__data/assets/pdf_file/0015/16350/survey-summary-10-26.pdf
21. Baldwin JM, Brooke EJ. Pausing in the wake of rapid adoption: a call to critically examine the veterans treatment court concept. J Offender Rehabil. 2019;58(1):1-29. doi:10.1080/10509674.2018.1549181
Veterans who interact with the criminal justice system (ie, justice-involved veterans) have heightened rates of mental health and psychosocial needs, including posttraumatic stress disorder (PTSD), substance use disorder, depression, suicidal ideation and attempt, and homelessness.1,2 Alongside these criminogenic risk factors, recidivism is common among justice-involved veterans: About 70% of incarcerated veterans disclosed at least one prior incarceration.3
To address the complex interplay of psychosocial factors, mental health concerns, and justice involvement among veterans, veterans treatment courts (VTCs) emerged as an alternative to incarceration.4 VTC participation often consists of integrated treatment and rehabilitative services (eg, vocational training, health care), ongoing monitoring for substance use, graduated responses to address treatment adherence, and ongoing communication with the judge and legal counsel.4
A primary aim of these courts is to address psychosocial needs believed to underlie criminal behavior, thus reducing risk of recidivism and promoting successful recovery and community integration for eligible veterans. To do so, VTCs collaborate with community-based and/or US Department of Veterans Affairs services, such as the Veterans Justice Outreach program (VJO). VJO specialists identify and refer justice-involved veterans to Veterans Health Administration (VHA) and community care and serve as a liaison between VTC staff and VHA health care professionals (HCPs).5
VTC outcome studies highlight the importance of not only diverting veterans to problem-solving courts, but also ensuring their optimal participation. Successful graduates of VTC programs demonstrate significant improvements in mental health symptoms, life satisfaction, and social support, as well as lower rates of law enforcement interactions.6,7 However, less is known about supporting those veterans who have difficulty engaging in VTCs and either discontinue participation or require lengthier periods of participation to meet court graduation requirements.8 One possibility to improve engagement among these veterans is to enhance court practices to best meet their needs.
In addition to delivering treatment, VHA mental health professionals may serve a critical interdisciplinary role by lending expertise to support VTC practices. For example, equipping court professionals with clinical knowledge and skills related to motivation may strengthen the staff’s interactions with participants, enabling them to address barriers as they arise and to facilitate veterans’ treatment adherence. Additionally, responsiveness to the impact of trauma exposure, which is common among this population, may prove important as related symptoms can affect veterans’ engagement, receptivity, and behavior in court settings. Indeed, prior examinations of justice-involved veterans have found trauma exposure rates ranging from 60% to 90% and PTSD rates ranging from 27% to 40%.1,2 Notably, involvement with the justice system (eg, incarceration) may itself further increase risk of trauma exposure (eg, experiencing a physical or sexual assault in prison) or exacerbate existing PTSD.9 Nonetheless, whereas many drug courts and domestic violence courts have been established, problem-solving courts with a specialized focus on trauma exposure remain rare, suggesting a potential gap in court training.
VHA HCPs have the potential to facilitate justice-involved veterans’ successful court and treatment participation by coordinating with VJO specialists to provide training and consultation to the courts. Supporting efforts to effectively and responsively address criminogenic risk (eg, mental health) in VTC settings may in turn reduce the likelihood of recidivism.10 Given the elevated rates of trauma exposure among justice-involved veterans and the relative lack of trauma-focused VTCs, we developed a trauma-informed training for VTC professionals that centered on related clinical presentations of justice-involved veterans and frequently occurring challenges in the context of court participation.
Program Development
This educational program aimed to (1) provide psychoeducation on trauma exposure, PTSD, and existing evidence-based treatments; (2) present clinical considerations for justice-involved veterans related to trauma exposure and/or PTSD; and (3) introduce skills to facilitate effective communication and trauma-informed care practices among professionals working with veterans in a treatment court.
Prior to piloting the program, we conducted a needs assessment with VTC professionals and identified relevant theoretical constructs and brief interventions for inclusion in the training. Additionally, given the dearth of prior research on mental health education for VTCs, the team consulted with the developers of PTSD 101, a VHA workshop for veterans’ families that promotes psychoeducation, support, and effective communication.11 Doing so informed approaches to delivering education to nonclinical audiences that interact with veterans with histories of trauma exposure. As this was a program development project, it was determined to be exempt from institutional review board review.
Needs Assessment
In the initial stages of development, local VJO specialists identified regional VTCs and facilitated introductions to these courts. Two of the 3 Rocky Mountain region VTCs that were contacted expressed interest in receiving trauma-informed training. Based on preliminary interest, the facilitators conducted a needs assessment with VJO and VTC staff from these 2 courts to capture requests for specific content and past experiences with other mental health trainings.
Guided by the focus group model, the needs assessments took place during three 1-hour meetings with VJO specialists and a 1-hour meeting with VJO specialists, VTC professionals, and community-based clinical partners.12 Additionally, attending a VTC graduation and court session allowed for observations of court practices and interactions with veterans. A total of 13 professionals (judges, court coordinators, case managers, peer mentors, VJO specialists, and clinicians who specialize in substance use disorder and intimate partner violence) participated in the needs assessments.
The most critical need identified by court professionals was a focus on how to apply knowledge about trauma and PTSD to interactions with justice-involved veterans. This was reportedly absent from prior training sessions the courts had received. Both Rocky Mountain region VTCs expressed a strong interest in and openness to adapting practices based on research and practice recommendations. Additional requests that emerged included a refresher on psychoeducation related to trauma and how to address the personal impact of working with this population (eg, compassion fatigue).
Training Components
Based on the needs identified by VTC professionals and informed by consultation with the developers of PTSD 101,
Psychoeducation. The initial portion of the training consisted of psychoeducation to increase VTC staff familiarity with the distinctions between trauma exposure and a formal diagnosis of PTSD, mechanisms underlying PTSD, and evidence-based treatment. To deepen conceptual understanding of trauma and PTSD beyond an overview of criteria set forth in The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), psychoeducation centered on the drivers of avoidance (eg, short-term benefit vs long-term consequences), behaviors that often facilitate avoidance (eg, substance use), functions underlying these behaviors (eg, distress reduction), and structure and mechanisms of change in evidence-based treatments for PTSD, including cognitive processing therapy and prolonged exposure.13,14
Fostering court familiarity with cognitive processing therapy and prolonged exposure may bolster veteran engagement in treatment through regular reinforcement of skills and concepts introduced in therapy. This may prove particularly salient given the limited engagement with mental health treatment and elevated dropout rates from PTSD treatment among the general veteran population.15,16
Exercises and metaphors were used to illustrate concepts in multiple ways. For example, training attendees engaged in a “stop, drop, and roll” thought exercise in which they were asked to brainstorm behavioral reactions to catching on fire. This exercise illustrated the tendency for individuals to revert to common yet unhelpful attempts at problem solving (eg, running due to panic, which would exacerbate the fire), particularly in crisis and without prior education regarding adaptive ways to respond. Attendee-generated examples, such as running, were used to demonstrate the importance of practicing and reinforcing skill development prior to a crisis, to ensure proficiency and optimal response. Additionally, in prompting consideration of one’s response tendencies, this exercise may engender empathy and understanding for veterans.
Skills training. Efforts to promote veteran engagement in court, facilitate motivation and readiness for change, and address barriers that arise (eg, distress associated with court appearances) may support successful and timely graduation. As such, skills training constituted the largest component of the training and drew from observations of court practices and the VTCs’ identified challenges. Consistent with the project’s aims and reported needs of the court, skills that target common presentations following trauma exposure (eg, avoidance, hypervigilance) were prioritized for this pilot training. Strategies included brief interventions from dialectical behavior therapy, acceptance and commitment therapy, and motivational interviewing to strengthen the support provided by staff to veterans and address their needs (Table 2).
Training attendees also participated in exercises to reiterate skills. For example, attendees completed an ambivalence matrix using an audience-identified common behavior that is difficult to change (eg, heavy alcohol use as a coping mechanism for distress).
Attendees engaged in an exercise that involved identifying unhelpful thoughts and behaviors, targets for validation, and veteran strengths from a hypothetical case vignette. This vignette involved a VTC participant who initially engaged effectively but began to demonstrate difficulty appropriately engaging in court and mental health treatment as well as challenging interactions with VTC staff (eg, raised voice during court sessions, not respecting communication boundaries).
Pilot Test
Based on scheduling parameters communicated by court coordinators, the pilot training was designed as a presentation during times reserved for court staffing meetings. To accommodate court preferences due to the COVID-19 pandemic, one 90-minute training was conducted virtually in March 2022, and the other training was conducted in person in April 2022 for 2 hours. The trainings were facilitated by 2 VHA clinical psychologists and included the judge, court coordinator, VJO specialist, peer mentors, case managers, probation/parole officers, and community-based HCPs who partner with the court (eg, social workers, psychologists). About 12 to 15 professionals attended each training session.
Feedback
Feedback was solicited from attendees via an anonymous online survey. Seven participants completed the survey; the response rate of about 20% was consistent with those observed for other surveys of court professionals.20 Many attendees also provided feedback directly to the facilitators. Feedback highlighted that the skills-based components not only were perceived as most helpful but also notably distinguished this training. “What set this training apart from other training events was the practical applications,” one attendee noted. “It was not just information or education, both instructors did an incredible job of explaining exactly how we could apply the knowledge they were sharing. They did this in such a way that it was easy to understand and apply.”
Specific skills were consistently identified as helpful, including managing intense emotions, addressing ambivalence, and approaching sanctions and rewards. Additionally, employing a less formal approach to the training, with relatable overviews of concepts and immediate responsiveness to requests for expansion on a topic, was perceived as a unique benefit: Another attendee appreciated that “It was beneficial to sit around a table with a less formal presentation and be able to ask questions.” This approach seemed particularly well suited for the program’s cross-disciplinary audience. Attendees reported that they valued the relatively limited focus on DSM-5 criteria. Attendees emphasized that education specific to veterans on evidence-based PTSD treatments, psychoeducation, and avoidance was very helpful. Respondents also recommended that the training be lengthened to a daylong workshop to accommodate greater opportunity to practice skills and consultation.
The consultation portion of the training provided insight into additional areas of importance to incorporate into future iterations. Identified needs included appropriate and realistic boundary setting (eg, addressing disruptions in the courtroom), suggestions for improving and expanding homework assigned by the court, and ways to address concerns about PTSD treatment shared by veterans in court (eg, attributing substance use relapses to the intensiveness of trauma-focused treatment vs lack of familiarity with alternate coping skills). Additionally, the VTC professionals’ desire to support mental health professionals’ work with veterans was clearly evident, highlighting the bidirectional value of interdisciplinary collaboration between VHA mental health professionals and VTC professionals.
Discussion
A trauma-informed training was developed and delivered to 2 VTCs in the Rocky Mountain region with the goal of providing relevant psychoeducation and introducing skills to bolster court practices that address veteran needs. Psychoeducational components of the training that were particularly well received and prompted significant participant engagement included discussions and examples of avoidance, levels of validation, language to facilitate motivation and address barriers, mechanisms underlying treatment, and potential functions underlying limited veteran treatment engagement. Distress tolerance, approaches to sanctions and rewards, and use of ambivalence matrices to guide motivation were identified as particularly helpful skills.
The pilot phase of this trauma-informed training provided valuable insights into developing mental health trainings for VTCs. Specifically, VTCs may benefit from the expertise of VHA HCPs and are particularly interested in learning brief skills to improve their practices. The usefulness of such trainings may be bolstered by efforts to form relationships with the court to identify their perceived needs and employing an iterative process that is responsive to feedback both during and after the training. Last, each stage of this project was strengthened by collaboration with VJO specialists, highlighting the importance of future collaboration between VJO and VHA mental health clinics to further support justice-involved veterans. For example, VJO specialists were instrumental in identifying training needs related to veterans’ clinical presentations in court, facilitating introductions to local VTCs, and helping to address barriers to piloting, like scheduling.
Modifications and Future Directions
The insights gained through the process of training design, delivery, and feedback inform future development of this training. Based on the feedback received, subsequent versions of the training may be expanded into a half- or full-day workshop to allow for adequate time for skills training and feedback, as well as consultation. Doing so will enable facilitators to further foster attendees’ familiarity with and confidence in their ability to use these skills. Furthermore, the consultation portion of this training revealed areas that may benefit from greater attention, including how to address challenging interactions in court (eg, addressing gender dynamics between court professionals and participants) and better support veterans who are having difficulty engaging in mental health treatment (eg, courts’ observation of high rates of dropout around the third or fourth session of evidence-based treatment for PTSD). Last, all attendees who responded to the survey expressed interest in a brief resource guide based on the training, emphasizing the need for ready access to key skills and concepts to support the use of strategies learned.
An additional future aim of this project is to conduct a more thorough evaluation of the needs and outcomes related to this trauma-informed training for VTC professionals. With the rapid growth of VTCs nationwide, relatively little examination of court processes and practices has occurred, and there is a lack of research on the development or effectiveness of mental health trainings provided to VTCs.21 Therefore, we intend to conduct larger scale qualitative interviews with court personnel and VJO specialists to obtain a clearer understanding of the needs related to skills-based training and gaps in psychoeducation. These comprehensive needs assessments may also capture common comorbidities that were not incorporated into the pilot training (eg, substance use disorders) but may be important training targets for court professionals. This information will be used to inform subsequent expansion and adaptation of the training into a longer workshop. Program evaluation will be conducted via survey-based feedback on perceived usefulness of the workshop and self-report of confidence in and use of strategies to improve court practices. Furthermore, efforts to obtain veteran outcome data, such as treatment engagement and successful participation in VTC, may be pursued.
Limitations
This training development and pilot project provided valuable foundational information regarding a largely unexamined component of treatment courts—the benefit of skills-based trainings to facilitate court practices related to justice-involved veterans. However, it is worth noting that survey responses were limited; thus, the feedback received may not reflect all attendees’ perceptions. Additionally, because both training sessions were conducted solely with 2 courts in the Rocky Mountain area, feedback may be limited to the needs of this geographic region.
Conclusions
A trauma-informed training was developed for VTCs to facilitate relevant understanding of justice-involved veterans’ needs and presentations in court, introduce skills to address challenges that arise (eg, motivation, emotional dysregulation), and provide interdisciplinary support to court professionals. This training was an important step toward fostering strong collaborations between VHA HCPs and community-based veterans courts, and feedback received during development and following implementation highlighted the perceived need for a skills-based approach to such trainings. Further program development and evaluation can strengthen this training and provide a foundation for dissemination to a broader scope of VTCs, with the goal of reducing recidivism risk among justice-involved veterans by promoting effective engagement in problem-solving court.
Veterans who interact with the criminal justice system (ie, justice-involved veterans) have heightened rates of mental health and psychosocial needs, including posttraumatic stress disorder (PTSD), substance use disorder, depression, suicidal ideation and attempt, and homelessness.1,2 Alongside these criminogenic risk factors, recidivism is common among justice-involved veterans: About 70% of incarcerated veterans disclosed at least one prior incarceration.3
To address the complex interplay of psychosocial factors, mental health concerns, and justice involvement among veterans, veterans treatment courts (VTCs) emerged as an alternative to incarceration.4 VTC participation often consists of integrated treatment and rehabilitative services (eg, vocational training, health care), ongoing monitoring for substance use, graduated responses to address treatment adherence, and ongoing communication with the judge and legal counsel.4
A primary aim of these courts is to address psychosocial needs believed to underlie criminal behavior, thus reducing risk of recidivism and promoting successful recovery and community integration for eligible veterans. To do so, VTCs collaborate with community-based and/or US Department of Veterans Affairs services, such as the Veterans Justice Outreach program (VJO). VJO specialists identify and refer justice-involved veterans to Veterans Health Administration (VHA) and community care and serve as a liaison between VTC staff and VHA health care professionals (HCPs).5
VTC outcome studies highlight the importance of not only diverting veterans to problem-solving courts, but also ensuring their optimal participation. Successful graduates of VTC programs demonstrate significant improvements in mental health symptoms, life satisfaction, and social support, as well as lower rates of law enforcement interactions.6,7 However, less is known about supporting those veterans who have difficulty engaging in VTCs and either discontinue participation or require lengthier periods of participation to meet court graduation requirements.8 One possibility to improve engagement among these veterans is to enhance court practices to best meet their needs.
In addition to delivering treatment, VHA mental health professionals may serve a critical interdisciplinary role by lending expertise to support VTC practices. For example, equipping court professionals with clinical knowledge and skills related to motivation may strengthen the staff’s interactions with participants, enabling them to address barriers as they arise and to facilitate veterans’ treatment adherence. Additionally, responsiveness to the impact of trauma exposure, which is common among this population, may prove important as related symptoms can affect veterans’ engagement, receptivity, and behavior in court settings. Indeed, prior examinations of justice-involved veterans have found trauma exposure rates ranging from 60% to 90% and PTSD rates ranging from 27% to 40%.1,2 Notably, involvement with the justice system (eg, incarceration) may itself further increase risk of trauma exposure (eg, experiencing a physical or sexual assault in prison) or exacerbate existing PTSD.9 Nonetheless, whereas many drug courts and domestic violence courts have been established, problem-solving courts with a specialized focus on trauma exposure remain rare, suggesting a potential gap in court training.
VHA HCPs have the potential to facilitate justice-involved veterans’ successful court and treatment participation by coordinating with VJO specialists to provide training and consultation to the courts. Supporting efforts to effectively and responsively address criminogenic risk (eg, mental health) in VTC settings may in turn reduce the likelihood of recidivism.10 Given the elevated rates of trauma exposure among justice-involved veterans and the relative lack of trauma-focused VTCs, we developed a trauma-informed training for VTC professionals that centered on related clinical presentations of justice-involved veterans and frequently occurring challenges in the context of court participation.
Program Development
This educational program aimed to (1) provide psychoeducation on trauma exposure, PTSD, and existing evidence-based treatments; (2) present clinical considerations for justice-involved veterans related to trauma exposure and/or PTSD; and (3) introduce skills to facilitate effective communication and trauma-informed care practices among professionals working with veterans in a treatment court.
Prior to piloting the program, we conducted a needs assessment with VTC professionals and identified relevant theoretical constructs and brief interventions for inclusion in the training. Additionally, given the dearth of prior research on mental health education for VTCs, the team consulted with the developers of PTSD 101, a VHA workshop for veterans’ families that promotes psychoeducation, support, and effective communication.11 Doing so informed approaches to delivering education to nonclinical audiences that interact with veterans with histories of trauma exposure. As this was a program development project, it was determined to be exempt from institutional review board review.
Needs Assessment
In the initial stages of development, local VJO specialists identified regional VTCs and facilitated introductions to these courts. Two of the 3 Rocky Mountain region VTCs that were contacted expressed interest in receiving trauma-informed training. Based on preliminary interest, the facilitators conducted a needs assessment with VJO and VTC staff from these 2 courts to capture requests for specific content and past experiences with other mental health trainings.
Guided by the focus group model, the needs assessments took place during three 1-hour meetings with VJO specialists and a 1-hour meeting with VJO specialists, VTC professionals, and community-based clinical partners.12 Additionally, attending a VTC graduation and court session allowed for observations of court practices and interactions with veterans. A total of 13 professionals (judges, court coordinators, case managers, peer mentors, VJO specialists, and clinicians who specialize in substance use disorder and intimate partner violence) participated in the needs assessments.
The most critical need identified by court professionals was a focus on how to apply knowledge about trauma and PTSD to interactions with justice-involved veterans. This was reportedly absent from prior training sessions the courts had received. Both Rocky Mountain region VTCs expressed a strong interest in and openness to adapting practices based on research and practice recommendations. Additional requests that emerged included a refresher on psychoeducation related to trauma and how to address the personal impact of working with this population (eg, compassion fatigue).
Training Components
Based on the needs identified by VTC professionals and informed by consultation with the developers of PTSD 101,
Psychoeducation. The initial portion of the training consisted of psychoeducation to increase VTC staff familiarity with the distinctions between trauma exposure and a formal diagnosis of PTSD, mechanisms underlying PTSD, and evidence-based treatment. To deepen conceptual understanding of trauma and PTSD beyond an overview of criteria set forth in The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), psychoeducation centered on the drivers of avoidance (eg, short-term benefit vs long-term consequences), behaviors that often facilitate avoidance (eg, substance use), functions underlying these behaviors (eg, distress reduction), and structure and mechanisms of change in evidence-based treatments for PTSD, including cognitive processing therapy and prolonged exposure.13,14
Fostering court familiarity with cognitive processing therapy and prolonged exposure may bolster veteran engagement in treatment through regular reinforcement of skills and concepts introduced in therapy. This may prove particularly salient given the limited engagement with mental health treatment and elevated dropout rates from PTSD treatment among the general veteran population.15,16
Exercises and metaphors were used to illustrate concepts in multiple ways. For example, training attendees engaged in a “stop, drop, and roll” thought exercise in which they were asked to brainstorm behavioral reactions to catching on fire. This exercise illustrated the tendency for individuals to revert to common yet unhelpful attempts at problem solving (eg, running due to panic, which would exacerbate the fire), particularly in crisis and without prior education regarding adaptive ways to respond. Attendee-generated examples, such as running, were used to demonstrate the importance of practicing and reinforcing skill development prior to a crisis, to ensure proficiency and optimal response. Additionally, in prompting consideration of one’s response tendencies, this exercise may engender empathy and understanding for veterans.
Skills training. Efforts to promote veteran engagement in court, facilitate motivation and readiness for change, and address barriers that arise (eg, distress associated with court appearances) may support successful and timely graduation. As such, skills training constituted the largest component of the training and drew from observations of court practices and the VTCs’ identified challenges. Consistent with the project’s aims and reported needs of the court, skills that target common presentations following trauma exposure (eg, avoidance, hypervigilance) were prioritized for this pilot training. Strategies included brief interventions from dialectical behavior therapy, acceptance and commitment therapy, and motivational interviewing to strengthen the support provided by staff to veterans and address their needs (Table 2).
Training attendees also participated in exercises to reiterate skills. For example, attendees completed an ambivalence matrix using an audience-identified common behavior that is difficult to change (eg, heavy alcohol use as a coping mechanism for distress).
Attendees engaged in an exercise that involved identifying unhelpful thoughts and behaviors, targets for validation, and veteran strengths from a hypothetical case vignette. This vignette involved a VTC participant who initially engaged effectively but began to demonstrate difficulty appropriately engaging in court and mental health treatment as well as challenging interactions with VTC staff (eg, raised voice during court sessions, not respecting communication boundaries).
Pilot Test
Based on scheduling parameters communicated by court coordinators, the pilot training was designed as a presentation during times reserved for court staffing meetings. To accommodate court preferences due to the COVID-19 pandemic, one 90-minute training was conducted virtually in March 2022, and the other training was conducted in person in April 2022 for 2 hours. The trainings were facilitated by 2 VHA clinical psychologists and included the judge, court coordinator, VJO specialist, peer mentors, case managers, probation/parole officers, and community-based HCPs who partner with the court (eg, social workers, psychologists). About 12 to 15 professionals attended each training session.
Feedback
Feedback was solicited from attendees via an anonymous online survey. Seven participants completed the survey; the response rate of about 20% was consistent with those observed for other surveys of court professionals.20 Many attendees also provided feedback directly to the facilitators. Feedback highlighted that the skills-based components not only were perceived as most helpful but also notably distinguished this training. “What set this training apart from other training events was the practical applications,” one attendee noted. “It was not just information or education, both instructors did an incredible job of explaining exactly how we could apply the knowledge they were sharing. They did this in such a way that it was easy to understand and apply.”
Specific skills were consistently identified as helpful, including managing intense emotions, addressing ambivalence, and approaching sanctions and rewards. Additionally, employing a less formal approach to the training, with relatable overviews of concepts and immediate responsiveness to requests for expansion on a topic, was perceived as a unique benefit: Another attendee appreciated that “It was beneficial to sit around a table with a less formal presentation and be able to ask questions.” This approach seemed particularly well suited for the program’s cross-disciplinary audience. Attendees reported that they valued the relatively limited focus on DSM-5 criteria. Attendees emphasized that education specific to veterans on evidence-based PTSD treatments, psychoeducation, and avoidance was very helpful. Respondents also recommended that the training be lengthened to a daylong workshop to accommodate greater opportunity to practice skills and consultation.
The consultation portion of the training provided insight into additional areas of importance to incorporate into future iterations. Identified needs included appropriate and realistic boundary setting (eg, addressing disruptions in the courtroom), suggestions for improving and expanding homework assigned by the court, and ways to address concerns about PTSD treatment shared by veterans in court (eg, attributing substance use relapses to the intensiveness of trauma-focused treatment vs lack of familiarity with alternate coping skills). Additionally, the VTC professionals’ desire to support mental health professionals’ work with veterans was clearly evident, highlighting the bidirectional value of interdisciplinary collaboration between VHA mental health professionals and VTC professionals.
Discussion
A trauma-informed training was developed and delivered to 2 VTCs in the Rocky Mountain region with the goal of providing relevant psychoeducation and introducing skills to bolster court practices that address veteran needs. Psychoeducational components of the training that were particularly well received and prompted significant participant engagement included discussions and examples of avoidance, levels of validation, language to facilitate motivation and address barriers, mechanisms underlying treatment, and potential functions underlying limited veteran treatment engagement. Distress tolerance, approaches to sanctions and rewards, and use of ambivalence matrices to guide motivation were identified as particularly helpful skills.
The pilot phase of this trauma-informed training provided valuable insights into developing mental health trainings for VTCs. Specifically, VTCs may benefit from the expertise of VHA HCPs and are particularly interested in learning brief skills to improve their practices. The usefulness of such trainings may be bolstered by efforts to form relationships with the court to identify their perceived needs and employing an iterative process that is responsive to feedback both during and after the training. Last, each stage of this project was strengthened by collaboration with VJO specialists, highlighting the importance of future collaboration between VJO and VHA mental health clinics to further support justice-involved veterans. For example, VJO specialists were instrumental in identifying training needs related to veterans’ clinical presentations in court, facilitating introductions to local VTCs, and helping to address barriers to piloting, like scheduling.
Modifications and Future Directions
The insights gained through the process of training design, delivery, and feedback inform future development of this training. Based on the feedback received, subsequent versions of the training may be expanded into a half- or full-day workshop to allow for adequate time for skills training and feedback, as well as consultation. Doing so will enable facilitators to further foster attendees’ familiarity with and confidence in their ability to use these skills. Furthermore, the consultation portion of this training revealed areas that may benefit from greater attention, including how to address challenging interactions in court (eg, addressing gender dynamics between court professionals and participants) and better support veterans who are having difficulty engaging in mental health treatment (eg, courts’ observation of high rates of dropout around the third or fourth session of evidence-based treatment for PTSD). Last, all attendees who responded to the survey expressed interest in a brief resource guide based on the training, emphasizing the need for ready access to key skills and concepts to support the use of strategies learned.
An additional future aim of this project is to conduct a more thorough evaluation of the needs and outcomes related to this trauma-informed training for VTC professionals. With the rapid growth of VTCs nationwide, relatively little examination of court processes and practices has occurred, and there is a lack of research on the development or effectiveness of mental health trainings provided to VTCs.21 Therefore, we intend to conduct larger scale qualitative interviews with court personnel and VJO specialists to obtain a clearer understanding of the needs related to skills-based training and gaps in psychoeducation. These comprehensive needs assessments may also capture common comorbidities that were not incorporated into the pilot training (eg, substance use disorders) but may be important training targets for court professionals. This information will be used to inform subsequent expansion and adaptation of the training into a longer workshop. Program evaluation will be conducted via survey-based feedback on perceived usefulness of the workshop and self-report of confidence in and use of strategies to improve court practices. Furthermore, efforts to obtain veteran outcome data, such as treatment engagement and successful participation in VTC, may be pursued.
Limitations
This training development and pilot project provided valuable foundational information regarding a largely unexamined component of treatment courts—the benefit of skills-based trainings to facilitate court practices related to justice-involved veterans. However, it is worth noting that survey responses were limited; thus, the feedback received may not reflect all attendees’ perceptions. Additionally, because both training sessions were conducted solely with 2 courts in the Rocky Mountain area, feedback may be limited to the needs of this geographic region.
Conclusions
A trauma-informed training was developed for VTCs to facilitate relevant understanding of justice-involved veterans’ needs and presentations in court, introduce skills to address challenges that arise (eg, motivation, emotional dysregulation), and provide interdisciplinary support to court professionals. This training was an important step toward fostering strong collaborations between VHA HCPs and community-based veterans courts, and feedback received during development and following implementation highlighted the perceived need for a skills-based approach to such trainings. Further program development and evaluation can strengthen this training and provide a foundation for dissemination to a broader scope of VTCs, with the goal of reducing recidivism risk among justice-involved veterans by promoting effective engagement in problem-solving court.
1. Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justice-involved veterans. Epidemiol Rev. 2015;37(1):163-176. doi:10.1093/epirev/mxu003
2. Saxon AJ, Davis TM, Sloan KL, McKnight KM, McFall ME, Kivlahan DR. Trauma, symptoms of posttraumatic stress disorder, and associated problems among incarcerated veterans. Psychiatr Serv. 2001;52(7):959-964. doi:10.1176/appi.ps.52.7.959
3. Bronson J, Carson AC, Noonan M. Veterans in prison and jail, 2011-12. December 2015. Accessed January 11, 2023. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
4. Cartwright T. “To care for him who shall have borne the battle”: the recent development of veterans treatment courts in America. Stanford Law Rev. 2011;22(1):295-316.
5. Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs Veterans Justice Outreach Program: connecting justice-involved veterans with mental health and substance use disorder Treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
6. Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
7. Montgomery LM, Olson JN. Veterans treatment court impact on veteran mental health and life satisfaction. J Psychol Behav Sci. 2018;6(1):1-4. doi:10.15640/jpbs.v6n1a1
8. Tsai J, Finlay A, Flatley B, Kasprow WJ, Clark S. A national study of veterans treatment court participants: who benefits and who recidivates. Adm Policy Ment Health. 2018;45(2):236-244. doi:10.1007/s10488-017-0816-z
9. Wolff NL, Shi J. Trauma and incarcerated persons. In: Scott CL, ed. Handbook of Correctional Mental Health. American Psychiatric Publishing, Inc.; 2010:277-320.
10. Bonta J, Andrews DA. Risk-need-responsivity model for offender assessment and rehabilitation. Rehabilitation. 2007;6:1-22. https://www.publicsafety.gc.ca/cnt/rsrcs/pblctns/rsk-nd-rspnsvty/index-en.aspx
11. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention, Family Services Section; Caska-Wallace CM, Campbell SB, Glynn SM. PTSD 101 for family and friends: a support and education workshop. 2020.
12. Tipping J. Focus groups: a method of needs assessment. J Contin Educ Health Prof. 1998;18(3):150-154. doi:10.1002/chp.1340180304
13. Resick PA, Monson CM, Chard KM. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. The Guilford Press; 2017.
14. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. Oxford University Press; 2007. doi:10.1093/med:psych/9780195308501.001.0001
15. Seal KH, Maguen S, Cohen B, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23(1):5-16. doi:10.1002/jts.20493
16. Edwards-Stewart A, Smolenski DJ, Bush NE, et al. Posttraumatic stress disorder treatment dropout among military and veteran populations: a systematic review and meta-analysis. J Trauma Stress. 2021;34(4):808-818. doi:10.1002/jts.22653
17. Linehan MM. Dialectical Behavior Therapy Skills Training Manual. 2nd ed. Guildford Press; 2015.
18. Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change. 2nd ed. Guildford Press; 2016.
19. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. The Guildford Press; 2002.
20. National Center for State Courts. A survey of members of major national court organizations. October 2010. Accessed January 11, 2023. https://www.ncsc.org/__data/assets/pdf_file/0015/16350/survey-summary-10-26.pdf
21. Baldwin JM, Brooke EJ. Pausing in the wake of rapid adoption: a call to critically examine the veterans treatment court concept. J Offender Rehabil. 2019;58(1):1-29. doi:10.1080/10509674.2018.1549181
1. Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justice-involved veterans. Epidemiol Rev. 2015;37(1):163-176. doi:10.1093/epirev/mxu003
2. Saxon AJ, Davis TM, Sloan KL, McKnight KM, McFall ME, Kivlahan DR. Trauma, symptoms of posttraumatic stress disorder, and associated problems among incarcerated veterans. Psychiatr Serv. 2001;52(7):959-964. doi:10.1176/appi.ps.52.7.959
3. Bronson J, Carson AC, Noonan M. Veterans in prison and jail, 2011-12. December 2015. Accessed January 11, 2023. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
4. Cartwright T. “To care for him who shall have borne the battle”: the recent development of veterans treatment courts in America. Stanford Law Rev. 2011;22(1):295-316.
5. Finlay AK, Smelson D, Sawh L, et al. U.S. Department of Veterans Affairs Veterans Justice Outreach Program: connecting justice-involved veterans with mental health and substance use disorder Treatment. Crim Justice Policy Rev. 2016;27(2):10.1177/0887403414562601. doi:10.1177/0887403414562601
6. Knudsen KJ, Wingenfeld S. A specialized treatment court for veterans with trauma exposure: implications for the field. Community Ment Health J. 2016;52(2):127-135. doi:10.1007/s10597-015-9845-9
7. Montgomery LM, Olson JN. Veterans treatment court impact on veteran mental health and life satisfaction. J Psychol Behav Sci. 2018;6(1):1-4. doi:10.15640/jpbs.v6n1a1
8. Tsai J, Finlay A, Flatley B, Kasprow WJ, Clark S. A national study of veterans treatment court participants: who benefits and who recidivates. Adm Policy Ment Health. 2018;45(2):236-244. doi:10.1007/s10488-017-0816-z
9. Wolff NL, Shi J. Trauma and incarcerated persons. In: Scott CL, ed. Handbook of Correctional Mental Health. American Psychiatric Publishing, Inc.; 2010:277-320.
10. Bonta J, Andrews DA. Risk-need-responsivity model for offender assessment and rehabilitation. Rehabilitation. 2007;6:1-22. https://www.publicsafety.gc.ca/cnt/rsrcs/pblctns/rsk-nd-rspnsvty/index-en.aspx
11. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention, Family Services Section; Caska-Wallace CM, Campbell SB, Glynn SM. PTSD 101 for family and friends: a support and education workshop. 2020.
12. Tipping J. Focus groups: a method of needs assessment. J Contin Educ Health Prof. 1998;18(3):150-154. doi:10.1002/chp.1340180304
13. Resick PA, Monson CM, Chard KM. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. The Guilford Press; 2017.
14. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. Oxford University Press; 2007. doi:10.1093/med:psych/9780195308501.001.0001
15. Seal KH, Maguen S, Cohen B, et al. VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress. 2010;23(1):5-16. doi:10.1002/jts.20493
16. Edwards-Stewart A, Smolenski DJ, Bush NE, et al. Posttraumatic stress disorder treatment dropout among military and veteran populations: a systematic review and meta-analysis. J Trauma Stress. 2021;34(4):808-818. doi:10.1002/jts.22653
17. Linehan MM. Dialectical Behavior Therapy Skills Training Manual. 2nd ed. Guildford Press; 2015.
18. Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change. 2nd ed. Guildford Press; 2016.
19. Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. The Guildford Press; 2002.
20. National Center for State Courts. A survey of members of major national court organizations. October 2010. Accessed January 11, 2023. https://www.ncsc.org/__data/assets/pdf_file/0015/16350/survey-summary-10-26.pdf
21. Baldwin JM, Brooke EJ. Pausing in the wake of rapid adoption: a call to critically examine the veterans treatment court concept. J Offender Rehabil. 2019;58(1):1-29. doi:10.1080/10509674.2018.1549181
Evaluation of the Appropriateness of Aspirin Therapy in a Veteran Population
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
Aspirin is an antiplatelet agent that binds irreversibly to COX-1 and COX-2 enzymes, which results in decreased prostaglandin and thromboxane A2 production and inhibition of platelet aggregation. Aspirin often is used for its antipyretic, analgesic, and antiplatelet properties. Its use in cardiovascular disease (CVD) has been studied extensively over the past few decades, and recent data are changing the framework for aspirin use in primary prevention of atherosclerotic cardiovascular disease (ASCVD). Primary prevention refers to efforts to prevent the incidence of cardiovascular events, whereas secondary prevention refers to efforts to prevent a cardiovascular event after one has occurred.1 This differentiation is important as it guides the course of treatment.
Three trials published in 2018 evaluated aspirin use in primary prevention of ASCVD. The ASCEND trial evaluated aspirin use for primary prevention of ASCVD in patients with diabetes mellitus (DM). This study concluded that although aspirin prevented serious vascular events in patients with DM, the benefit observed was largely counteracted by the bleeding hazard.2 The ARRIVE trial evaluated aspirin use for primary prevention in patients with a moderate CVD risk. The study concluded that aspirin use in patients at moderate risk of CVD could not be assessed due to the low incidence rate of CVD; however, the study concluded that aspirin did not reduce the incidence of cardiovascular events for patients at low CVD risk and that aspirin caused more mild gastrointestinal bleeds compared with placebo.3 The ASPREE trial evaluated aspirin use for primary prevention in patients aged > 70 years to determine whether its use prolonged a healthy lifespan. This trial concluded that patients who received daily aspirin were at a higher risk of major hemorrhage and that aspirin did not diminish CVD risk compared with placebo.4
These studies led to a paradigm shift in therapy to reevaluate aspirin use for primary prevention. Current indications for aspirin include secondary prevention of ASCVD (ie, myocardial infarction [MI], coronary artery bypass graft, transient ischemic attack [TIA], and stroke), venous thromboembolism prophylaxis in the setting of orthopedic surgery, or valvular disease with replacement and analgesia. It is important to note that certain clinical circumstances may warrant aspirin use for primary prevention of ASCVD on a patient-specific basis, and this decision should be made using a risk/benefit analysis with the patient.
In April 2022, the US Preventive Services Task Force (USPSTF) recommended against using low-dose aspirin for primary prevention of ASCVD in individuals aged ≥ 60 years. The USPSTF noted that for patients who have a ≥ 10%, 10-year CVD risk, the decision to initiate aspirin should be based on a risk/benefit discussion and may be beneficial in certain patient populations.5A 2019 National Heart, Lung, and Blood Institute survey found that 29 million Americans used aspirin for primary prevention of ASCVD, and 6.6 million of these Americans used aspirin for primary prevention without the recommendation of a health care professional (HCP). Almost half of these individuals were aged > 70 years and, therefore, at an increased risk for bleeding.6 With the recent studies and changes in guidelines highlighting a higher risk rather than benefit with the use of aspirin for primary prevention, the current use of aspirin for primary prevention in the United States needs to be readdressed.
HCPs should assess the appropriateness of aspirin use in their patients to ensure that the risks of aspirin do not outweigh the benefits. Pharmacists can play a vital role in the assessment of aspirin for primary prevention during patient visits and make recommendations to primary care practitioners to deprescribe aspirin when appropriate.
Methods
The objective of this study was to evaluate the appropriateness of aspirin therapy in patient aligned care team (PACT) clinics at the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois. The PACT clinics are a category of clinics that include all the primary care clinics at FHCC.
The primary outcome of this study was to determine the percentage of patients inappropriately on aspirin therapy. To assess the inappropriate use of aspirin, relevant history of ASCVD was collected. Patients were divided into 3 groups: those with a history of ASCVD, those with no risk factors or history of ASCVD, and those with risk factors and no history of ASCVD. Patients were then categorized for their indication for aspirin use, which included either primary or secondary prevention of ASCVD. Patients were categorized into the primary prevention group if they had no history of ASCVD, whereas patients with a history of ASCVD were placed into the secondary prevention group.
ASCVD was defined as patients with acute coronary syndrome (ACS), history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral artery disease (PAD), including aortic aneurysm (all with an atherosclerotic origin). Possible ASCVD risk was defined as patients with DM with a major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, chronic kidney disease [CKD]/albuminuria) or patients diagnosed with coronary artery disease without an event. The percentage of patients followed by a PACT pharmacist, the number of pharmacist follow-up visits during the study period, and the date of the first 81-mg aspirin pharmacy order that was filled at FHCC were also collected.
The secondary outcome of this study focused on patients who were using aspirin for primary prevention and assessed potential reasons that may warrant deprescribing aspirin therapy. One reason for deprescribing is that aspirin may not be indicated for some patients, including those with DM without cardiovascular complications, patients aged > 70 years, and/or patients with CKD (defined as estimated glomerular filtration rate < 60 mL/min). Another reason for deprescribing is contraindication, which included patients with coagulopathy, thrombocytopenia (defined as platelet count < 150,000 mL), a history of gastrointestinal bleeding, peptic ulcer disease or other major bleeds, and/or consistent use of medications that increase bleeding risk (such as nonsteroidal anti-inflammatory agents, steroids, or anticoagulants) for > 14 days.
The safety outcome of this study assessed bleeding events while on aspirin therapy. All patients were categorized depending on if they had a major, minor, or no bleeding event while on aspirin therapy. Hemorrhagic stroke, symptomatic intracranial bleeding, bleeds located in other critical sites or organs (intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial), bleeds causing hemodynamic instability requiring vasopressors, bleeds causing a > 2 g/dL hemoglobin drop since initiation of aspirin therapy, severe extracranial bleeding requiring transfusion or hospitalization, fatal bleeding, or bleeds requiring > 2 units of red blood cell transfusion were considered major bleeding events. Minor bleeding events were any events that did not meet the criteria for major bleeding, including bruising, bleeding gums, epistaxis, hemorrhoidal bleeds, and bleeding that did not require intervention or treatment.7
Patients were included if they were aged > 18 years, had an active prescription for 81-mg aspirin tablet on September 30, 2021, and were seen in FHCC PACT clinics or at affiliated community-based outpatient centers. Other doses of aspirin were excluded as the 81-mg dose is the standard dose for primary prevention of ASCVD in the United States. US Department of Defense patients, home-based primary care patients, and community living center patients were excluded in this study. Patients with an aspirin prescription from a non–US Department of Veterans Affairs (VA) facility and patients on aspirin for reasons other than cardiovascular protection (such as pain, fever, etc) also were excluded from this study.
Data were collected from the FHCC electronic health record. A list was generated to include all active prescriptions for aspirin filled at FHCC as of September 30, 2021. Data were reviewed before this date to capture primary and secondary outcomes. No information was gathered from the chart after that date. This project was approved by the Edward Hines, Jr. VA Hospital Institutional Review Board. The primary and secondary outcomes were reported using descriptive statistics.
Results
This study reviewed 140 patient records and 105 patients met inclusion criteria.
For the primary endpoint, 53 patients (50%) were on aspirin for secondary prevention and 52 (50%) were on aspirin for primary prevention. Of the 105 patients included in the study, 31 (30%) had a possible ASCVD risk and were taking aspirin for primary prevention, while 21 (20%) had no ASCVD and were taking aspirin for primary prevention. Of the 52 patients on aspirin for primary prevention, 31 patients (60%) had a possible risk for ASCVD. Of the 52 patients in the primary prevention group, 15 (29%) were followed by a pharmacist, and the average number of follow-up appointments was 4.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. Of the 52 patients on aspirin for primary prevention, 25 patients were aged > 70 years, 15 patients were concurrently taking medications that may increase bleeding risk,
For the entire study group, 6 patients (6%) experienced a major bleeding event while on aspirin, 46 (44%) experienced a minor bleeding event while on aspirin, and 53 (50%) experienced no bleeding events while on aspirin. Of the 6 patients who experienced major bleeding events, 4 were on aspirin for secondary prevention, and 2 were on aspirin for primary prevention with ASCVD risk factors. The major bleeding events included 4 gastrointestinal bleeds, 1 intracranial hemorrhage, and 1 hemorrhagic stroke. Of the 46 who experienced minor bleeding events, 20 patients were on aspirin for primary prevention; 11 of those patients had possible ASCVD risk factors and 9 had no documented ASCVD. The minor bleeding events included hematuria, epistaxis, bleeding scabs, and dental bleeding.
Discussion
The majority of patients in this study were on aspirin appropriately. Indications deemed appropriate for aspirin therapy include secondary prevention and primary prevention with a possible ASCVD risk. About 20% of the total patient population in this study was taking aspirin for primary prevention with no ASCVD risk. For these patients, the risk of bleeding likely outweighs the benefits of aspirin therapy as patients are at low risk for ASCVD; therefore, aspirin therapy is likely inappropriate in this patient population. These patients may be unnecessarily at an increased risk for bleeding and may benefit from deprescribing aspirin. For the safety of patients, HCPs should be continuously assessing the appropriateness of aspirin for primary prevention and deprescribing when necessary.
About one-third of the patients using aspirin for primary prevention were followed by a pharmacist. Pharmacists can play a key role in deprescribing aspirin for primary prevention when aspirin use is deemed inappropriate. About 30% of the total patient population in this study was on aspirin for primary prevention with possible ASCVD risk. This patient population may benefit from aspirin therapy as they are at a higher risk for ASCVD. For these patients, a risk/benefit discussion is necessary to determine the appropriateness of aspirin for primary prevention. This risk/benefit discussion should be a continuous conversation between patients and HCPs as different factors such as age and changes in comorbid conditions and medications may increase bleeding risk.
The secondary endpoint focused on patients taking aspirin for primary prevention and the factors that may warrant deprescribing aspirin. The most common factors seen in this study included patients who were aged > 70 years, patients who were concurrently taking medications that may increase bleeding risk, and patients with CKD. All of these factors increase bleeding risk, making the risks potentially outweigh the benefits of aspirin for primary prevention. These factors should be the primary focus when assessing patients on aspirin for primary prevention to promote deprescribing aspirin if deemed appropriate as they were the most prevalent in this study.
The safety endpoints focused on bleeding events as a whole as well as the bleeding events seen in the primary prevention group. There were 2 major bleeding events and 20 minor bleeding events in the primary prevention group. The number of bleeding events both major and minor further shows the need for a continuous risk/benefit discussion between patients and HCPs on continued aspirin use for primary prevention. The bleeding risk with aspirin is prevalent. HCPs should continue to assess for factors that increase the bleeding risk that may warrant deprescribing aspirin to prevent future bleeding events in this patient population.
Strengths and Limitations
As there have been recent updates to guidelines on the use of aspirin for primary prevention, a strength of this study is that it evaluates a topic that is relevant in health care. Another strength of this study is that it focuses on specific patient factors that HCPs can assess when determining whether aspirin for primary prevention is appropriate in their patients. These specific patient factors can also be used as a guide to help HCPs deprescribe aspirin for primary prevention when appropriate.
One of the limitations of this study is that bleeding events that occurred outside of the FHCC were unable to be assessed unless the HCP specifically commented on the bleeding event in the chart. This could potentially underestimate the bleeding events seen in this study. Another limitation is that the bleeding risk for patients who were not on aspirin was not assessed. There was no comparison group to ascertain whether the bleeding risk was higher in the aspirin group compared with a no aspirin group. However, many of the major clinical trials saw an increased risk of bleeding in the aspirin group compared with placebo.
Conclusions
Aspirin therapy for secondary prevention remains an important part of treatment. Aspirin therapy for primary prevention may be appropriate for patients with a possible ASCVD risk. The therapy may be inappropriate in cases where patients have an increased bleeding risk and low or no ASCVD risk. It is important to continuously assess the need for aspirin therapy for patients in the setting of primary prevention. Common factors seen in this study to warrant deprescribing aspirin for primary prevention include patients aged > 70 years, concurrent use of medications that increase bleeding risk, and patients with CKD. By assessing ASCVD risk as well as bleeding risk and having a risk/benefit discussion between the HCP and patient, aspirin used for primary prevention can be appropriately deprescribed when the risks of bleeding outweigh the benefits.
Acknowledgments
The authors thank the Captain James A. Lovell Federal Health Care Center research committee (Hong-Yen Vi, PharmD, BCPS; Shaiza Khan, PharmD, BCPS; Yinka Alaka, BPharm, PharmD; Jennifer Kwon, PharmD, BCOP) and coinvestigator Aeman Choudhury, PharmD, BCPS, BCACP.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
1. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619-633. doi:10.1111/j.1365-2125.2011.03943.x
2. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
3. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
4. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(16):1577-1584. doi:10.1001/jama.2022.4983
6. Murphy E, McEvoy JW. Does stopping aspirin differ fundamentally from not starting aspirin in the primary prevention of cardiovascular disease among older adults? Ann Intern Med. 2022;175(5):757-758. doi:10.7326/M22-0550
7. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. doi:10.1111/j.1538-7836.2005.01204.
Guidance for PCI without on-site surgical backup updated
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
FROM THE JOURNAL OF SOCIETY FOR CARDIOVASCULAR ANGIOGRAPHY AND INTERVENTIONS
A doctor intervenes in a fiery car crash
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes physicians find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a Medscape series telling these stories.
I was coming off a 48-hour shift plus a day of doing outpatient sedation at Sparrow Hospital in Lansing. It was December and Michigan-cold.
I drove on the side of the road where I wasn’t really supposed to and got closer. An SUV had crashed into one of the big concrete structures under the bridge. I saw people running around but wasn’t able to spot EMS or any health care workers. From where I was, I could identify four kids who had already been extricated and one adult still in the driver’s seat. I estimated the kids’ ages were around 7, 5, 3, and an infant who was a few months old. I left my car and went to help.
I was able to peg the ages correctly because I’m a pediatric critical care physician. As a specialty, we’re not commonly known. We oversee patient care in intensive care units, except the patients are children. Part of the job is that we’re experts at triaging. We recognize what’s life-threatening and less so.
The kids were with some adults who kept them warm with blankets. I examined each of them. The infant was asleep but arousable and acting like a normal baby. The 3-year-old boy was vomiting and appeared very fatigued. The 5-year-old boy had a forehead laceration and was in and out of consciousness. The 7-year-old girl was screaming because of different injuries.
While all of the children were concerning to me, I identified one in particular: the 5-year-old boy. It was obvious he needed serious medical attention and fast. So, I kept that little guy in mind. The others had sustained significant injuries, but my best guess was they could get to a hospital and be stabilized.
That said, I’m a trauma instructor, and one of the things I always tell trainees is: Trauma is a black box. On the outside, it may seem like a patient doesn’t have a lot of injuries. But underneath, there might be something worse, like a brain injury. Or the chest might have taken a blunt impact affecting the heart. There may be internal bleeding somewhere in the belly. It’s really hard to tease out what exactly is going on without equipment and testing.
I didn’t even have a pulse oximeter or heart rate monitor. I pretty much just went by the appearance of the child: pulse, heart rate, awareness, things like that.
After the kids, I moved to look at the man in the car. The front end had already caught fire. I could see the driver – the kids’ father, I guessed – unconscious and hunched over. I was wondering, Why hasn’t this guy been extricated?
I approached the car on the front passenger side. And then I just had this feeling. I knew I needed to step back. Immediately.
I did. And a few seconds later, the whole car exploded in flames.
I believe God is in control of everything. I tried to get to that man. But the scene was unsafe. Later I learned that several people, including a young nurse at the scene, had tried to get to him as well.
When EMS came, I identified myself. Obviously, these people do very, very important work. But they may be more used to the 60-year-old heart attack, the 25-year-old gunshot wound, the occasional ill child. I thought that four kids – each with possible critical poly-traumatic injuries – posed a challenge to anyone.
I told them, “This is what I do on a daily basis, and this is the kid I’m worried about the most. The other kids are definitely worrisome, but I would prioritize getting this kid to the hospital first. Can I ride with you?” They agreed.
We got that boy and his older sister into the first ambulance (she was in a lot of pain, the result of a femur fracture). The two other kids rode in the second ambulance. The hospital where I had just left was 10 minutes away. I called the other pediatric critical care doctor there, my partner. He thought I was calling for a routine issue – no such luck. I said, “I’m with four kids who are level-1 traumas in two ambulances and I’m heading to the hospital right now, ETA 10 minutes.”
En route, I thought the little boy might lose consciousness at any moment. He needed a breathing tube, and I debated whether it should be done in the ambulance vs. waiting until we got to the emergency room. Based on my judgment and his vital signs, I elected to wait to have it done it in a more controlled environment. Had I felt like he was in immediate need of an airway, I would’ve attempted it. But those are the tough calls that you must make.
My partner had alerted the trauma and emergency medicine teams at the hospital. By the time we arrived, my partner was down in the ER with the trauma team and ER staff. Everyone was ready. Then it was like divide and conquer. He attended to one of the kids. The ER team and I were with the little guy I was really worried about. We had his breathing tube in within minutes. The trauma team attended to the other two.
All the kids were stabilized and then admitted to the pediatric intensive care unit. I’m happy to say that all of them did well in the end. Even the little guy I was worried about the most.
I must say this incident gave me perspective on what EMS goes through. The field medicine we do in the United States is still in its infancy in a lot of ways. One of the things I would love to see in the future is a mobile ICU. After a critical illness hits, sometimes you only have seconds, minutes, maybe hours if you’re lucky. The earlier you can get patients the treatment they need, the better the outcomes.
I like taking care of critically ill children and their families. It fits my personality. And it’s a wonderful cause. But you have to be ready for tragic cases like this one. Yes, the children came out alive, but the accident claimed a life in a horrible way. And there was nothing I could do about it.
Critical care takes an emotional, psychological, and physical toll. It’s a roller coaster: Some kids do well; some kids don’t do well. All I can do is hold myself accountable. I keep my emotions in check, whether the outcome is positive or negative. And I do my best.
Mohamed Hani Farhat, MD, is a pediatric critical care physician at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor and Sparrow Hospital in Lansing, Mich. Are you a physician with a dramatic medical story outside the clinic? Medscape would love to consider your story for Is There a Doctor in the House? Please email your contact information and a short summary of your story to [email protected] . A version of this article appeared on Medscape.com.