User login
A gruesome murder changes two docs’ lives, and one was the killer
Driving from his home in Asheville, N.C., to his new job at the tiny Cane Creek clinic, Benjamin Gilmer, MD, was eager to start his new life and pay off his medical school debts.
The rural clinic had been forced to close after his predecessor, family physician Vince Gilmer, MD, (no relation) had been convicted of first-degree murder 4 years earlier. He was serving a life sentence in a West Virginia prison without the possibility of parole. He is still behind bars and could not comment on this story.
As the months flew by, Benjamin Gilmer’s patients shared stories about the other Dr. Gilmer that surprised him. They described Vince Gilmer as a caring, generous person who went out of his way to help them. He made house calls, and if a patient couldn’t afford to pay him, he would accept a bushel of corn instead.
Yet there was no doubt about the gruesome murder. Vince Gilmer was convicted of strangling his frail 60-year-old father with a rope in his Toyota truck. He then cut off all his father’s fingers and dumped his father’s body by the side of the road.
“Four years later, his patients were still shocked about what happened and couldn’t reconcile the person they knew with the event that happened,” says Benjamin Gilmer.
Yet, Vince Gilmer had admitted to the killing, and the prosecution had presented evidence at the trial that it was premeditated and that he tried to cover up the crime. The detectives found the “murder” weapons in Vince’s truck: the ropes he strangled his father with and the garden shears that he cut off his fingers with. They also had evidence that he drove to Virginia to dump the body, returned to see patients for several days as if nothing had happened, and then ran away when a detective came to arrest him.
But something kept gnawing away at Benjamin Gilmer.
Little did he know that he would embark on a journey to solve a medical mystery, and then even fight to get the convicted killer out of prison.
Solving a medical mystery
Benjamin Gilmer decided to investigate what might have happened to Vince in the months leading up to the murder. He talked to his friends and found several clues about Vince’s medical history. They recalled that he suffered a concussion in a car accident 6 months before the murder, which suggested he could have had a traumatic brain injury.
Benjamin Gilmer also discovered that Vince’s father was diagnosed with schizophrenia and had been in a residential psychiatric facility in Virginia until he was released that fateful night to Vince’s custody.
Vince had written to friends that “something is wrong with my brain and help me.” He mentioned SSRI discontinuation syndrome because he abruptly stopped taking his medication the week of the murder (which can cause electric shock sensations and mood swings among other symptoms).
Vince had mentioned the SSRI discontinuation syndrome at his trial and that his father had sexually molested him for years and that he tried to molest him again during the ride in his truck. However, the court dismissed that information because Vince represented himself, dismissed his court-appointed attorneys, and lacked expert testimony about his mental state.
The prosecutor portrayed Vince as a lying sociopath who had planned his father’s murder down to the last detail. The judge agreed. Two psychiatrists and a psychologist who later evaluated him in prison concluded that he was faking his symptoms and denied his requests for an SSRI.
Meanwhile, Benjamin Gilmer became increasingly preoccupied with what happened to Vince. “It was hard to erase a memory that had so tainted that community,” he said.
When Sarah Koenig, a journalist and former producer of the radio program This American Life, called Benjamin Gilmer to interview him about the coincidence of taking over Vince Gilmer’s practice and sharing the same last name, he refused. “I was scared and didn’t want to be on his radar, I was afraid of how he might react.”
In spring 2012, he called Koenig and agreed to collaborate on an episode about Vince’s case. Benjamin Gilmer wrote to Vince Gilmer in prison, asking for a meeting. To his surprise, Vince wanted to meet them.
When Vince shuffled into the waiting area at the Wallens Ridge State Prison in West Virginia, Benjamin Gilmer was shocked by his appearance. “He looked like a caged animal, it was very hard for him to string together ideas and express himself, and he was twitching and shaking dramatically. He looked 20 years older than his actual age of 50 and like someone you would imagine in the movie One Flew Over the Cuckoo’s Nest,” said Benjamin Gilmer.
He felt that “there was something clearly wrong with him.” They agreed to a second meeting, and this time Benjamin Gilmer invited a psychiatrist, Steve Buie, MD, to observe Vince. As the visit ended and Vince turned to leave, Dr. Buie watched his shuffling gait. They suspected he may have Huntington’s disease, “which explained why he had delusions and his mind was unraveling,” says Benjamin Gilmer. But they had no way of testing him in prison.
Unexpectedly, an event happened that turned the whole case on its head. Vince was moved to a psychiatric hospital in southern Virginia because he had threatened to commit suicide. The chief psychiatrist, Colin Angliker, MD, was willing to order a genetic test, and the results confirmed the diagnosis: Vince Gilmer had a terminal degenerative brain disease.
Benjamin Gilmer worried how Vince would take the news. To his surprise, Vince was grateful and relieved. He finally knew what was wrong with him.
Vince also improved with the SSRI that Dr. Angliker prescribed — he was less anxious and more mentally alert. “He expressed joy for the first time, despite the death sentence of a diagnosis.”
Still, he was going to spend the rest of his life in prison for the crime he committed.
After the This American Life episode aired in 2013, Benjamin Gilmer felt that he couldn’t just abandon Vince to the prison system, where thousands of inmates with mental illness languish without adequate treatment.
Benjamin Gilmer decided he had a new — although controversial — mission — to get Vince out.
Confronting the politics of a pardon
After nearly a decade of trying, Benjamin Gilmer now admits that he was naive to think he could get him released quickly.
After the episode aired, offers of legal help started to arrive, and a team was assembled who agreed to work on the case pro bono. They wanted justice for Vince but also to prevent anyone else with mental illness from experiencing a similar tragedy.
The goal was to get Vince transferred to a secure hospital, a psychiatric facility dedicated to Huntington’s patients, or a nursing home with a dementia unit.
However, after realizing that Vince may not survive a potentially lengthy court battle, the legal team decided to ask the governor of Virginia to grant a clemency pardon.
They gathered the evidence for Vince’s case and presented their petition to Gov. Terry McAuliffe (D). He rejected it at the end of his term in 2017.
The team tried again with his successor, Gov. Ralph Northam (D), a neurologist. He dashed their hopes when he rejected their petition in late 2021.
That was a huge setback. The team had spent $1 million and had exhausted every contact they could make with the governor’s office, says Gilmer. “We were totally demoralized.”
He dreaded having to tell Vince that yet another governor had rejected their clemency petition. “I went to prison and could see the hopelessness and despair in his reaction. I lost it emotionally,” says Benjamin Gilmer.
Vince surprised him by hugging and comforting him and thanking him for all his efforts. They had developed a strong bond over a decade of visits and calls. Benjamin Gilmer had even brought his wife and children along on special occasions.
“I thought of him as a friend, as a patient, and someone who was really suffering, all those things helped our relationship evolve and kept me engaged with him all these years and continued to inspire me to fight for him. I also liked him because I knew what he was like before the murder from the stories I was hearing from his friends and patients.”
But his continuous advocacy came at a personal cost. “This battle pushed me to my limits emotionally and intellectually. I was busy building my career, trying to be a good doctor, teacher, husband, and father to two young children. I became so distracted that my wife confronted me several times about not being more emotionally present,” says Benjamin Gilmer.
But he knows that without Vince in his life, he would not have written his first book (released earlier this year) about the case and their unlikely friendship.
A pardon is finally granted
He had also given Gov. Northam’s staff advance copies of the book. In a highly unusual move, the governor reversed his previous rejection and granted Vince Gilmer his long-awaited pardon on January 12.
Benjamin Gilmer isn’t ready to celebrate yet. “Despite being a free man, Vince is still living behind bars because we haven’t been able to find him an available bed in a secure treatment facility. There has been a shortage of beds due to COVID.”
He says Vince is looking forward to being safe and being surrounded by people who are committed to caring for him and not punishing him. He can’t wait to be around his family and to give and receive hugs.
“After a while, it was hard not to believe that I was supposed to be in his path and this was just part of my destiny,” says Benjamin Gilmer.
A version of this article first appeared on Medscape.com.
Driving from his home in Asheville, N.C., to his new job at the tiny Cane Creek clinic, Benjamin Gilmer, MD, was eager to start his new life and pay off his medical school debts.
The rural clinic had been forced to close after his predecessor, family physician Vince Gilmer, MD, (no relation) had been convicted of first-degree murder 4 years earlier. He was serving a life sentence in a West Virginia prison without the possibility of parole. He is still behind bars and could not comment on this story.
As the months flew by, Benjamin Gilmer’s patients shared stories about the other Dr. Gilmer that surprised him. They described Vince Gilmer as a caring, generous person who went out of his way to help them. He made house calls, and if a patient couldn’t afford to pay him, he would accept a bushel of corn instead.
Yet there was no doubt about the gruesome murder. Vince Gilmer was convicted of strangling his frail 60-year-old father with a rope in his Toyota truck. He then cut off all his father’s fingers and dumped his father’s body by the side of the road.
“Four years later, his patients were still shocked about what happened and couldn’t reconcile the person they knew with the event that happened,” says Benjamin Gilmer.
Yet, Vince Gilmer had admitted to the killing, and the prosecution had presented evidence at the trial that it was premeditated and that he tried to cover up the crime. The detectives found the “murder” weapons in Vince’s truck: the ropes he strangled his father with and the garden shears that he cut off his fingers with. They also had evidence that he drove to Virginia to dump the body, returned to see patients for several days as if nothing had happened, and then ran away when a detective came to arrest him.
But something kept gnawing away at Benjamin Gilmer.
Little did he know that he would embark on a journey to solve a medical mystery, and then even fight to get the convicted killer out of prison.
Solving a medical mystery
Benjamin Gilmer decided to investigate what might have happened to Vince in the months leading up to the murder. He talked to his friends and found several clues about Vince’s medical history. They recalled that he suffered a concussion in a car accident 6 months before the murder, which suggested he could have had a traumatic brain injury.
Benjamin Gilmer also discovered that Vince’s father was diagnosed with schizophrenia and had been in a residential psychiatric facility in Virginia until he was released that fateful night to Vince’s custody.
Vince had written to friends that “something is wrong with my brain and help me.” He mentioned SSRI discontinuation syndrome because he abruptly stopped taking his medication the week of the murder (which can cause electric shock sensations and mood swings among other symptoms).
Vince had mentioned the SSRI discontinuation syndrome at his trial and that his father had sexually molested him for years and that he tried to molest him again during the ride in his truck. However, the court dismissed that information because Vince represented himself, dismissed his court-appointed attorneys, and lacked expert testimony about his mental state.
The prosecutor portrayed Vince as a lying sociopath who had planned his father’s murder down to the last detail. The judge agreed. Two psychiatrists and a psychologist who later evaluated him in prison concluded that he was faking his symptoms and denied his requests for an SSRI.
Meanwhile, Benjamin Gilmer became increasingly preoccupied with what happened to Vince. “It was hard to erase a memory that had so tainted that community,” he said.
When Sarah Koenig, a journalist and former producer of the radio program This American Life, called Benjamin Gilmer to interview him about the coincidence of taking over Vince Gilmer’s practice and sharing the same last name, he refused. “I was scared and didn’t want to be on his radar, I was afraid of how he might react.”
In spring 2012, he called Koenig and agreed to collaborate on an episode about Vince’s case. Benjamin Gilmer wrote to Vince Gilmer in prison, asking for a meeting. To his surprise, Vince wanted to meet them.
When Vince shuffled into the waiting area at the Wallens Ridge State Prison in West Virginia, Benjamin Gilmer was shocked by his appearance. “He looked like a caged animal, it was very hard for him to string together ideas and express himself, and he was twitching and shaking dramatically. He looked 20 years older than his actual age of 50 and like someone you would imagine in the movie One Flew Over the Cuckoo’s Nest,” said Benjamin Gilmer.
He felt that “there was something clearly wrong with him.” They agreed to a second meeting, and this time Benjamin Gilmer invited a psychiatrist, Steve Buie, MD, to observe Vince. As the visit ended and Vince turned to leave, Dr. Buie watched his shuffling gait. They suspected he may have Huntington’s disease, “which explained why he had delusions and his mind was unraveling,” says Benjamin Gilmer. But they had no way of testing him in prison.
Unexpectedly, an event happened that turned the whole case on its head. Vince was moved to a psychiatric hospital in southern Virginia because he had threatened to commit suicide. The chief psychiatrist, Colin Angliker, MD, was willing to order a genetic test, and the results confirmed the diagnosis: Vince Gilmer had a terminal degenerative brain disease.
Benjamin Gilmer worried how Vince would take the news. To his surprise, Vince was grateful and relieved. He finally knew what was wrong with him.
Vince also improved with the SSRI that Dr. Angliker prescribed — he was less anxious and more mentally alert. “He expressed joy for the first time, despite the death sentence of a diagnosis.”
Still, he was going to spend the rest of his life in prison for the crime he committed.
After the This American Life episode aired in 2013, Benjamin Gilmer felt that he couldn’t just abandon Vince to the prison system, where thousands of inmates with mental illness languish without adequate treatment.
Benjamin Gilmer decided he had a new — although controversial — mission — to get Vince out.
Confronting the politics of a pardon
After nearly a decade of trying, Benjamin Gilmer now admits that he was naive to think he could get him released quickly.
After the episode aired, offers of legal help started to arrive, and a team was assembled who agreed to work on the case pro bono. They wanted justice for Vince but also to prevent anyone else with mental illness from experiencing a similar tragedy.
The goal was to get Vince transferred to a secure hospital, a psychiatric facility dedicated to Huntington’s patients, or a nursing home with a dementia unit.
However, after realizing that Vince may not survive a potentially lengthy court battle, the legal team decided to ask the governor of Virginia to grant a clemency pardon.
They gathered the evidence for Vince’s case and presented their petition to Gov. Terry McAuliffe (D). He rejected it at the end of his term in 2017.
The team tried again with his successor, Gov. Ralph Northam (D), a neurologist. He dashed their hopes when he rejected their petition in late 2021.
That was a huge setback. The team had spent $1 million and had exhausted every contact they could make with the governor’s office, says Gilmer. “We were totally demoralized.”
He dreaded having to tell Vince that yet another governor had rejected their clemency petition. “I went to prison and could see the hopelessness and despair in his reaction. I lost it emotionally,” says Benjamin Gilmer.
Vince surprised him by hugging and comforting him and thanking him for all his efforts. They had developed a strong bond over a decade of visits and calls. Benjamin Gilmer had even brought his wife and children along on special occasions.
“I thought of him as a friend, as a patient, and someone who was really suffering, all those things helped our relationship evolve and kept me engaged with him all these years and continued to inspire me to fight for him. I also liked him because I knew what he was like before the murder from the stories I was hearing from his friends and patients.”
But his continuous advocacy came at a personal cost. “This battle pushed me to my limits emotionally and intellectually. I was busy building my career, trying to be a good doctor, teacher, husband, and father to two young children. I became so distracted that my wife confronted me several times about not being more emotionally present,” says Benjamin Gilmer.
But he knows that without Vince in his life, he would not have written his first book (released earlier this year) about the case and their unlikely friendship.
A pardon is finally granted
He had also given Gov. Northam’s staff advance copies of the book. In a highly unusual move, the governor reversed his previous rejection and granted Vince Gilmer his long-awaited pardon on January 12.
Benjamin Gilmer isn’t ready to celebrate yet. “Despite being a free man, Vince is still living behind bars because we haven’t been able to find him an available bed in a secure treatment facility. There has been a shortage of beds due to COVID.”
He says Vince is looking forward to being safe and being surrounded by people who are committed to caring for him and not punishing him. He can’t wait to be around his family and to give and receive hugs.
“After a while, it was hard not to believe that I was supposed to be in his path and this was just part of my destiny,” says Benjamin Gilmer.
A version of this article first appeared on Medscape.com.
Driving from his home in Asheville, N.C., to his new job at the tiny Cane Creek clinic, Benjamin Gilmer, MD, was eager to start his new life and pay off his medical school debts.
The rural clinic had been forced to close after his predecessor, family physician Vince Gilmer, MD, (no relation) had been convicted of first-degree murder 4 years earlier. He was serving a life sentence in a West Virginia prison without the possibility of parole. He is still behind bars and could not comment on this story.
As the months flew by, Benjamin Gilmer’s patients shared stories about the other Dr. Gilmer that surprised him. They described Vince Gilmer as a caring, generous person who went out of his way to help them. He made house calls, and if a patient couldn’t afford to pay him, he would accept a bushel of corn instead.
Yet there was no doubt about the gruesome murder. Vince Gilmer was convicted of strangling his frail 60-year-old father with a rope in his Toyota truck. He then cut off all his father’s fingers and dumped his father’s body by the side of the road.
“Four years later, his patients were still shocked about what happened and couldn’t reconcile the person they knew with the event that happened,” says Benjamin Gilmer.
Yet, Vince Gilmer had admitted to the killing, and the prosecution had presented evidence at the trial that it was premeditated and that he tried to cover up the crime. The detectives found the “murder” weapons in Vince’s truck: the ropes he strangled his father with and the garden shears that he cut off his fingers with. They also had evidence that he drove to Virginia to dump the body, returned to see patients for several days as if nothing had happened, and then ran away when a detective came to arrest him.
But something kept gnawing away at Benjamin Gilmer.
Little did he know that he would embark on a journey to solve a medical mystery, and then even fight to get the convicted killer out of prison.
Solving a medical mystery
Benjamin Gilmer decided to investigate what might have happened to Vince in the months leading up to the murder. He talked to his friends and found several clues about Vince’s medical history. They recalled that he suffered a concussion in a car accident 6 months before the murder, which suggested he could have had a traumatic brain injury.
Benjamin Gilmer also discovered that Vince’s father was diagnosed with schizophrenia and had been in a residential psychiatric facility in Virginia until he was released that fateful night to Vince’s custody.
Vince had written to friends that “something is wrong with my brain and help me.” He mentioned SSRI discontinuation syndrome because he abruptly stopped taking his medication the week of the murder (which can cause electric shock sensations and mood swings among other symptoms).
Vince had mentioned the SSRI discontinuation syndrome at his trial and that his father had sexually molested him for years and that he tried to molest him again during the ride in his truck. However, the court dismissed that information because Vince represented himself, dismissed his court-appointed attorneys, and lacked expert testimony about his mental state.
The prosecutor portrayed Vince as a lying sociopath who had planned his father’s murder down to the last detail. The judge agreed. Two psychiatrists and a psychologist who later evaluated him in prison concluded that he was faking his symptoms and denied his requests for an SSRI.
Meanwhile, Benjamin Gilmer became increasingly preoccupied with what happened to Vince. “It was hard to erase a memory that had so tainted that community,” he said.
When Sarah Koenig, a journalist and former producer of the radio program This American Life, called Benjamin Gilmer to interview him about the coincidence of taking over Vince Gilmer’s practice and sharing the same last name, he refused. “I was scared and didn’t want to be on his radar, I was afraid of how he might react.”
In spring 2012, he called Koenig and agreed to collaborate on an episode about Vince’s case. Benjamin Gilmer wrote to Vince Gilmer in prison, asking for a meeting. To his surprise, Vince wanted to meet them.
When Vince shuffled into the waiting area at the Wallens Ridge State Prison in West Virginia, Benjamin Gilmer was shocked by his appearance. “He looked like a caged animal, it was very hard for him to string together ideas and express himself, and he was twitching and shaking dramatically. He looked 20 years older than his actual age of 50 and like someone you would imagine in the movie One Flew Over the Cuckoo’s Nest,” said Benjamin Gilmer.
He felt that “there was something clearly wrong with him.” They agreed to a second meeting, and this time Benjamin Gilmer invited a psychiatrist, Steve Buie, MD, to observe Vince. As the visit ended and Vince turned to leave, Dr. Buie watched his shuffling gait. They suspected he may have Huntington’s disease, “which explained why he had delusions and his mind was unraveling,” says Benjamin Gilmer. But they had no way of testing him in prison.
Unexpectedly, an event happened that turned the whole case on its head. Vince was moved to a psychiatric hospital in southern Virginia because he had threatened to commit suicide. The chief psychiatrist, Colin Angliker, MD, was willing to order a genetic test, and the results confirmed the diagnosis: Vince Gilmer had a terminal degenerative brain disease.
Benjamin Gilmer worried how Vince would take the news. To his surprise, Vince was grateful and relieved. He finally knew what was wrong with him.
Vince also improved with the SSRI that Dr. Angliker prescribed — he was less anxious and more mentally alert. “He expressed joy for the first time, despite the death sentence of a diagnosis.”
Still, he was going to spend the rest of his life in prison for the crime he committed.
After the This American Life episode aired in 2013, Benjamin Gilmer felt that he couldn’t just abandon Vince to the prison system, where thousands of inmates with mental illness languish without adequate treatment.
Benjamin Gilmer decided he had a new — although controversial — mission — to get Vince out.
Confronting the politics of a pardon
After nearly a decade of trying, Benjamin Gilmer now admits that he was naive to think he could get him released quickly.
After the episode aired, offers of legal help started to arrive, and a team was assembled who agreed to work on the case pro bono. They wanted justice for Vince but also to prevent anyone else with mental illness from experiencing a similar tragedy.
The goal was to get Vince transferred to a secure hospital, a psychiatric facility dedicated to Huntington’s patients, or a nursing home with a dementia unit.
However, after realizing that Vince may not survive a potentially lengthy court battle, the legal team decided to ask the governor of Virginia to grant a clemency pardon.
They gathered the evidence for Vince’s case and presented their petition to Gov. Terry McAuliffe (D). He rejected it at the end of his term in 2017.
The team tried again with his successor, Gov. Ralph Northam (D), a neurologist. He dashed their hopes when he rejected their petition in late 2021.
That was a huge setback. The team had spent $1 million and had exhausted every contact they could make with the governor’s office, says Gilmer. “We were totally demoralized.”
He dreaded having to tell Vince that yet another governor had rejected their clemency petition. “I went to prison and could see the hopelessness and despair in his reaction. I lost it emotionally,” says Benjamin Gilmer.
Vince surprised him by hugging and comforting him and thanking him for all his efforts. They had developed a strong bond over a decade of visits and calls. Benjamin Gilmer had even brought his wife and children along on special occasions.
“I thought of him as a friend, as a patient, and someone who was really suffering, all those things helped our relationship evolve and kept me engaged with him all these years and continued to inspire me to fight for him. I also liked him because I knew what he was like before the murder from the stories I was hearing from his friends and patients.”
But his continuous advocacy came at a personal cost. “This battle pushed me to my limits emotionally and intellectually. I was busy building my career, trying to be a good doctor, teacher, husband, and father to two young children. I became so distracted that my wife confronted me several times about not being more emotionally present,” says Benjamin Gilmer.
But he knows that without Vince in his life, he would not have written his first book (released earlier this year) about the case and their unlikely friendship.
A pardon is finally granted
He had also given Gov. Northam’s staff advance copies of the book. In a highly unusual move, the governor reversed his previous rejection and granted Vince Gilmer his long-awaited pardon on January 12.
Benjamin Gilmer isn’t ready to celebrate yet. “Despite being a free man, Vince is still living behind bars because we haven’t been able to find him an available bed in a secure treatment facility. There has been a shortage of beds due to COVID.”
He says Vince is looking forward to being safe and being surrounded by people who are committed to caring for him and not punishing him. He can’t wait to be around his family and to give and receive hugs.
“After a while, it was hard not to believe that I was supposed to be in his path and this was just part of my destiny,” says Benjamin Gilmer.
A version of this article first appeared on Medscape.com.
Nonhealing boils
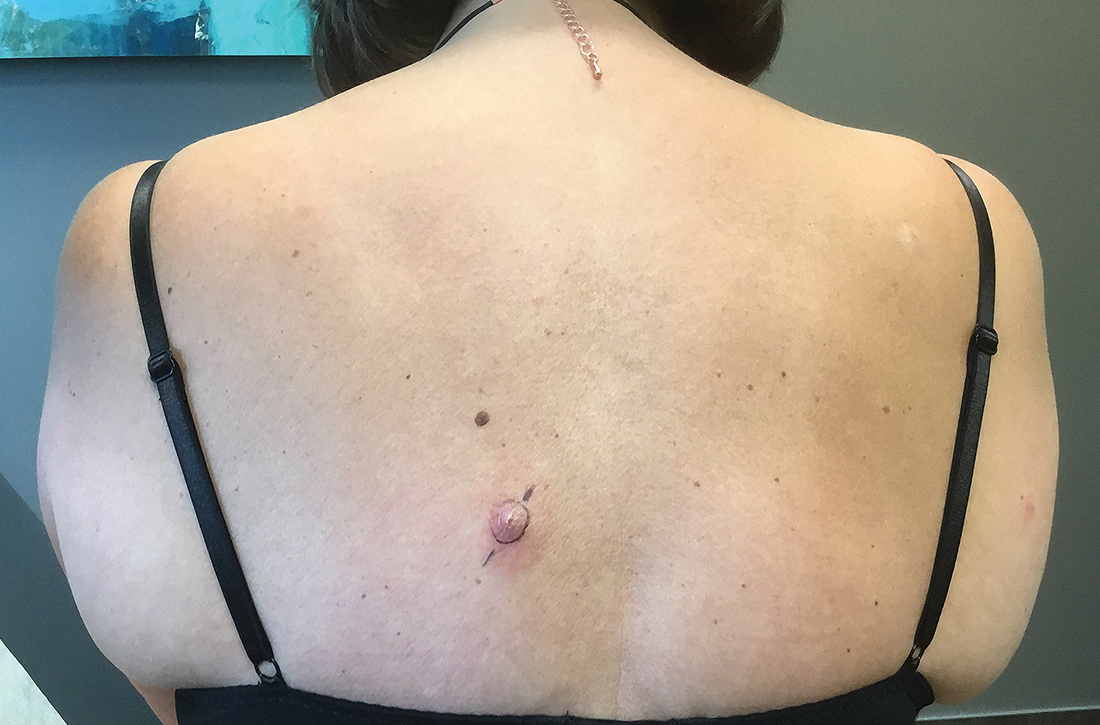
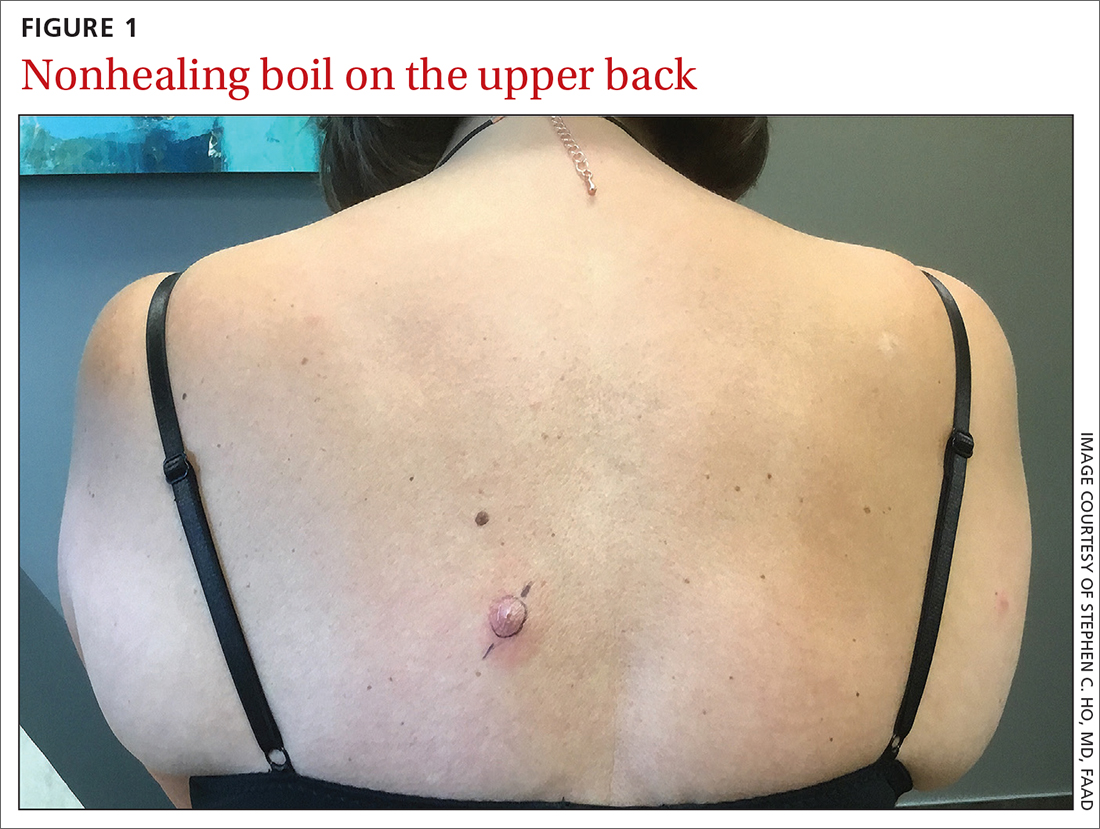
A healthy woman in her 60s presented to the clinic with a 1-month history of red, itchy, and slightly painful nodules on the scalp and back. The patient had travelled to Belize for a vacation in the weeks prior to the onset of the lesions. She was initially given a course of cephalexin for presumed furunculosis at another clinic, without improvement.
Examination revealed inflamed nodules with a central open pore on the left upper back (FIGURE 1) and the occipital scalp. Notably, when the lesions were observed with a dermatoscope, intermittent air bubbles were seen through the skin opening.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Furuncular myiasis
Given the patient’s clinical presentation and travel history, furuncular myiasis infestation was suspected and confirmed by punch biopsy. Pathologic exam revealed botfly larvae in both wounds, consistent with the human botfly, Dermatobia hominis (FIGURE 2). Myiasis is not common in the United States but should be suspected in patients who have recently traveled to tropical or subtropical areas. Furuncular myiasis describes the condition in which fly larvae penetrate healthy skin in a localized fashion, leading to the development of a furuncle-like nodule with 1 or more larvae within it.

Dermatobia hominis is the most common causative organism for furuncular myiasis in the regions of the Americas. Patients typically present with 1 lesion on an exposed part of the body (eg, scalp, face, extremities). The lesions typically contain a central pore with purulent or serosanguinous exudate.1 Upon dermatoscopic inspection, one can often see the posterior part of the larva or the respiratory spiracles, which look like tiny black dots on the surface of the wound. The organism may also be indirectly viewed through respiratory bubble formation within the exudate.1,2 Diagnosis is confirmed by extracting the larvae from the wound and having the species identified by an experienced pathologist or parasitologist.
Mode of transmission
Phoresis is the name of the process by which Dermatobia hominis invades the skin.3 The female fly lays her eggs onto captured mosquitos using a quick-drying adhesive. The eggs are then transferred to the host by a mosquito bite. The host’s body heat induces egg hatching, and the larvae burrow into follicular openings or skin perforations. This leads to the development of a small erythematous papule, which can further lead to a furuncle-like nodule with a central pore that allows the organism to respirate. When ready to pupate, the larvae work their way to the skin surface and drop to the soil, where they can further develop. After pupation, the fly hatches and develops into an adult, and the cycle repeats.
Common infectious and inflammatory lesions are in the differential Dx
The differential diagnosis includes bacterial abscess, exaggerated arthropod reaction, and ruptured epidermal cyst. With our patient, the travel history and unique exam findings led to the suspicion of myiasis.
Bacterial abscess is likely to have more notable purulence with response to appropriate oral antibiotics and no bubbling phenomenon on exam. It is also unlikely to be present for 1 month without progressive worsening.
Continue to: Exaggerated arthropod reaction
Exaggerated arthropod reaction usually manifests as a smooth, red, papular lesion without bubble formation from a central pore.
Ruptured epidermal cyst would be considered if there was a known preexisting cyst that recently changed. No bubble formation would be observed from the central pore.
Extraction is the treatment of choice
Treatment of furuncular myiasis involves removing the larvae or forcing them out of the lesion. Wounds can be covered with a substance, such as petrolatum, nail polish, beeswax, paraffin, or mineral oil, to block respiration.3 Occlusion may be needed for 24 hours to create adequate localized hypoxia to force the larvae to migrate from the wound and allow for easier manual extraction. Surgical removal of the larvae is also effective. A cruciate incision can be made adjacent to the central pore to avoid damaging the organisms.3 A topical, broad-based antiparasitic, such as a 10% ivermectin solution, has also been successfully used to treat furuncular myiasis. This approach works by either inducing larval migration outward or simply killing the larvae.3
Our patient recovered well after we performed a punch biopsy to make a larger wound opening and remove the intact larvae.
ACKNOWLEDGMENT
We thank Richard Pollack, PhD, at IdentifyUS, LLC, for providing the botfly larvae photo.
1. Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi: 10.1128/cmr.00010-11
2. Diaz JH. The epidemiology, diagnosis, management, and prevention of ectoparasitic diseases in travelers. J Travel Med. 2006;13:100-111. doi: 10.1111/j.1708-8305.2006.00021.x
3. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926. doi: 10.1016/j.jaad.2008.03.014
A healthy woman in her 60s presented to the clinic with a 1-month history of red, itchy, and slightly painful nodules on the scalp and back. The patient had travelled to Belize for a vacation in the weeks prior to the onset of the lesions. She was initially given a course of cephalexin for presumed furunculosis at another clinic, without improvement.
Examination revealed inflamed nodules with a central open pore on the left upper back (FIGURE 1) and the occipital scalp. Notably, when the lesions were observed with a dermatoscope, intermittent air bubbles were seen through the skin opening.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Furuncular myiasis
Given the patient’s clinical presentation and travel history, furuncular myiasis infestation was suspected and confirmed by punch biopsy. Pathologic exam revealed botfly larvae in both wounds, consistent with the human botfly, Dermatobia hominis (FIGURE 2). Myiasis is not common in the United States but should be suspected in patients who have recently traveled to tropical or subtropical areas. Furuncular myiasis describes the condition in which fly larvae penetrate healthy skin in a localized fashion, leading to the development of a furuncle-like nodule with 1 or more larvae within it.

Dermatobia hominis is the most common causative organism for furuncular myiasis in the regions of the Americas. Patients typically present with 1 lesion on an exposed part of the body (eg, scalp, face, extremities). The lesions typically contain a central pore with purulent or serosanguinous exudate.1 Upon dermatoscopic inspection, one can often see the posterior part of the larva or the respiratory spiracles, which look like tiny black dots on the surface of the wound. The organism may also be indirectly viewed through respiratory bubble formation within the exudate.1,2 Diagnosis is confirmed by extracting the larvae from the wound and having the species identified by an experienced pathologist or parasitologist.
Mode of transmission
Phoresis is the name of the process by which Dermatobia hominis invades the skin.3 The female fly lays her eggs onto captured mosquitos using a quick-drying adhesive. The eggs are then transferred to the host by a mosquito bite. The host’s body heat induces egg hatching, and the larvae burrow into follicular openings or skin perforations. This leads to the development of a small erythematous papule, which can further lead to a furuncle-like nodule with a central pore that allows the organism to respirate. When ready to pupate, the larvae work their way to the skin surface and drop to the soil, where they can further develop. After pupation, the fly hatches and develops into an adult, and the cycle repeats.
Common infectious and inflammatory lesions are in the differential Dx
The differential diagnosis includes bacterial abscess, exaggerated arthropod reaction, and ruptured epidermal cyst. With our patient, the travel history and unique exam findings led to the suspicion of myiasis.
Bacterial abscess is likely to have more notable purulence with response to appropriate oral antibiotics and no bubbling phenomenon on exam. It is also unlikely to be present for 1 month without progressive worsening.
Continue to: Exaggerated arthropod reaction
Exaggerated arthropod reaction usually manifests as a smooth, red, papular lesion without bubble formation from a central pore.
Ruptured epidermal cyst would be considered if there was a known preexisting cyst that recently changed. No bubble formation would be observed from the central pore.
Extraction is the treatment of choice
Treatment of furuncular myiasis involves removing the larvae or forcing them out of the lesion. Wounds can be covered with a substance, such as petrolatum, nail polish, beeswax, paraffin, or mineral oil, to block respiration.3 Occlusion may be needed for 24 hours to create adequate localized hypoxia to force the larvae to migrate from the wound and allow for easier manual extraction. Surgical removal of the larvae is also effective. A cruciate incision can be made adjacent to the central pore to avoid damaging the organisms.3 A topical, broad-based antiparasitic, such as a 10% ivermectin solution, has also been successfully used to treat furuncular myiasis. This approach works by either inducing larval migration outward or simply killing the larvae.3
Our patient recovered well after we performed a punch biopsy to make a larger wound opening and remove the intact larvae.
ACKNOWLEDGMENT
We thank Richard Pollack, PhD, at IdentifyUS, LLC, for providing the botfly larvae photo.
A healthy woman in her 60s presented to the clinic with a 1-month history of red, itchy, and slightly painful nodules on the scalp and back. The patient had travelled to Belize for a vacation in the weeks prior to the onset of the lesions. She was initially given a course of cephalexin for presumed furunculosis at another clinic, without improvement.
Examination revealed inflamed nodules with a central open pore on the left upper back (FIGURE 1) and the occipital scalp. Notably, when the lesions were observed with a dermatoscope, intermittent air bubbles were seen through the skin opening.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Furuncular myiasis
Given the patient’s clinical presentation and travel history, furuncular myiasis infestation was suspected and confirmed by punch biopsy. Pathologic exam revealed botfly larvae in both wounds, consistent with the human botfly, Dermatobia hominis (FIGURE 2). Myiasis is not common in the United States but should be suspected in patients who have recently traveled to tropical or subtropical areas. Furuncular myiasis describes the condition in which fly larvae penetrate healthy skin in a localized fashion, leading to the development of a furuncle-like nodule with 1 or more larvae within it.

Dermatobia hominis is the most common causative organism for furuncular myiasis in the regions of the Americas. Patients typically present with 1 lesion on an exposed part of the body (eg, scalp, face, extremities). The lesions typically contain a central pore with purulent or serosanguinous exudate.1 Upon dermatoscopic inspection, one can often see the posterior part of the larva or the respiratory spiracles, which look like tiny black dots on the surface of the wound. The organism may also be indirectly viewed through respiratory bubble formation within the exudate.1,2 Diagnosis is confirmed by extracting the larvae from the wound and having the species identified by an experienced pathologist or parasitologist.
Mode of transmission
Phoresis is the name of the process by which Dermatobia hominis invades the skin.3 The female fly lays her eggs onto captured mosquitos using a quick-drying adhesive. The eggs are then transferred to the host by a mosquito bite. The host’s body heat induces egg hatching, and the larvae burrow into follicular openings or skin perforations. This leads to the development of a small erythematous papule, which can further lead to a furuncle-like nodule with a central pore that allows the organism to respirate. When ready to pupate, the larvae work their way to the skin surface and drop to the soil, where they can further develop. After pupation, the fly hatches and develops into an adult, and the cycle repeats.
Common infectious and inflammatory lesions are in the differential Dx
The differential diagnosis includes bacterial abscess, exaggerated arthropod reaction, and ruptured epidermal cyst. With our patient, the travel history and unique exam findings led to the suspicion of myiasis.
Bacterial abscess is likely to have more notable purulence with response to appropriate oral antibiotics and no bubbling phenomenon on exam. It is also unlikely to be present for 1 month without progressive worsening.
Continue to: Exaggerated arthropod reaction
Exaggerated arthropod reaction usually manifests as a smooth, red, papular lesion without bubble formation from a central pore.
Ruptured epidermal cyst would be considered if there was a known preexisting cyst that recently changed. No bubble formation would be observed from the central pore.
Extraction is the treatment of choice
Treatment of furuncular myiasis involves removing the larvae or forcing them out of the lesion. Wounds can be covered with a substance, such as petrolatum, nail polish, beeswax, paraffin, or mineral oil, to block respiration.3 Occlusion may be needed for 24 hours to create adequate localized hypoxia to force the larvae to migrate from the wound and allow for easier manual extraction. Surgical removal of the larvae is also effective. A cruciate incision can be made adjacent to the central pore to avoid damaging the organisms.3 A topical, broad-based antiparasitic, such as a 10% ivermectin solution, has also been successfully used to treat furuncular myiasis. This approach works by either inducing larval migration outward or simply killing the larvae.3
Our patient recovered well after we performed a punch biopsy to make a larger wound opening and remove the intact larvae.
ACKNOWLEDGMENT
We thank Richard Pollack, PhD, at IdentifyUS, LLC, for providing the botfly larvae photo.
1. Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi: 10.1128/cmr.00010-11
2. Diaz JH. The epidemiology, diagnosis, management, and prevention of ectoparasitic diseases in travelers. J Travel Med. 2006;13:100-111. doi: 10.1111/j.1708-8305.2006.00021.x
3. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926. doi: 10.1016/j.jaad.2008.03.014
1. Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79-105. doi: 10.1128/cmr.00010-11
2. Diaz JH. The epidemiology, diagnosis, management, and prevention of ectoparasitic diseases in travelers. J Travel Med. 2006;13:100-111. doi: 10.1111/j.1708-8305.2006.00021.x
3. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926. doi: 10.1016/j.jaad.2008.03.014
Steroids counter ataxia telangiectasia
SEATTLE –
The disease is an autosomal recessive disorder caused by mutations in the ATM gene, which is critical to the response to cellular insults such as DNA breaks, oxidative damage, and other forms of stress. The result is clinical manifestations that range from a suppressed immune system to organ damage and neurological symptoms that typically lead patients to be wheelchair bound by their teenage years.
“It’s really multisystem and a very, very difficult disease for people to live with,” Howard M. Lederman, MD, PhD, said in an interview. Dr. Lederman is a coauthor of the study, which was presented by Stefan Zielen, PhD, professor at the University of Goethe, at the 2022 annual meeting of the American Academy of Neurology.
Various therapies have been developed to improve immunodeficiency, lung disease, and some of the other clinical aspects of the condition, but there is no treatment for its neurological effects. “There’s not really been a good animal model, which has been a big problem in trying to test drugs and design treatment trials,” said Dr. Lederman, professor of pediatrics and medicine at Johns Hopkins University, Baltimore.
The new results may change that. “In the children under the age of 9, there was really a very clear slowdown in the neurodegeneration, and specifically the time that it took for them to lose the ability to ambulate. It’s very exciting, because it’s the first time that anybody has really shown in a double-blind, placebo-controlled, large phase 3 study that any drug has been able to do this. And there were really no steroid side effects, which is the other really remarkable thing about this study,” said Dr. Lederman.
The therapy grew out of a study by researchers in Italy who treated pediatric ataxia telangiectasia patients with corticosteroids and found some transitory improvements in gross motor function, but concerns about long-term exposure to steroids limited its application. EryDel, which specializes in encapsulating therapeutics in red blood cells, became interested and developed a formulation using the patient’s own red blood cells infused with DSP. Reinfused to the patients, the red blood cells slowly release the steroid.
It isn’t clear how dexamethasone works. There are data suggesting that it might lead to transcription of small pieces of the ATM protein, “but that has really not been nailed down in any way at this point. Corticosteroids act on all kinds of cells in all kinds of ways, and so there might be a little bit of this so-called mini-ATM that’s produced, but that may or may not be related to the way in which corticosteroids have a beneficial effect on the rate of neurodegeneration,” said Dr. Lederman.
The treatment process is not easy. Children must have 50-60 cc of blood removed. Red blood cells treated to become porous are exposed to DSP, and then resealed. Then the cells are reinfused. “The whole process takes from beginning to end probably about 3 hours, with a really experienced team of people doing it. And it’s limiting because it’s not easy to put in an IV and take 50 or 60 cc of blood out of children much younger than 5 or 6. The process is now being modified to see whether we could do it with 20 to 30 cc instead,” said Dr. Lederman.
A ‘promising and impressive’ study
The study is promising, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “They were able to show a slower rate of neurological degeneration or duration on both the lower and higher dose compared with the placebo. This is promising and impressive, in the sense that it’s a really large (trial) for a rare condition,” Dr. Johnson, vice chair of research at Virginia Commonwealth University, Richmond, said in an interview.
The study included 164 patients Europe, Australia, Israel, Tunisia, India, and the United States, who received 5-10 mg dexamethasone, 14-22 mg DSP, or placebo. Mean ages in each group ranged from 9.6 to 10.4 years.
In an intention-to-treat analysis, modified International Cooperative Ataxia Rating Scale (mICARS) scores trended toward improvement in the low-dose (–1.37; P = .0847) and high-dose groups (–1.40; P = .0765) when determined by central raters during the COVID-19 pandemic. There was also a trend toward improvement when determined by local raters in the low dose group (–1.73; P = .0720) and a statistically significant change in the high dose group (–2.11; P = .0277). The researchers noted some inconsistency between local and central raters, due to inconsistency of videography and language challenges for central raters.
An intention-to-treat analysis of a subgroup of 89 patients age 6-9, who were compared with natural history data from 245 patients, found a deterioration of mICARS of 3.7 per year, compared with 0.92 in the high-dose group, for a reduction of 75% (P = .020). In the high-dose group, 51.7% had a minimal or significant improvement compared with baseline according to the Clinical Global Impression of Change, as did 29.0% on low dose, and 27.6% in the placebo group.
SEATTLE –
The disease is an autosomal recessive disorder caused by mutations in the ATM gene, which is critical to the response to cellular insults such as DNA breaks, oxidative damage, and other forms of stress. The result is clinical manifestations that range from a suppressed immune system to organ damage and neurological symptoms that typically lead patients to be wheelchair bound by their teenage years.
“It’s really multisystem and a very, very difficult disease for people to live with,” Howard M. Lederman, MD, PhD, said in an interview. Dr. Lederman is a coauthor of the study, which was presented by Stefan Zielen, PhD, professor at the University of Goethe, at the 2022 annual meeting of the American Academy of Neurology.
Various therapies have been developed to improve immunodeficiency, lung disease, and some of the other clinical aspects of the condition, but there is no treatment for its neurological effects. “There’s not really been a good animal model, which has been a big problem in trying to test drugs and design treatment trials,” said Dr. Lederman, professor of pediatrics and medicine at Johns Hopkins University, Baltimore.
The new results may change that. “In the children under the age of 9, there was really a very clear slowdown in the neurodegeneration, and specifically the time that it took for them to lose the ability to ambulate. It’s very exciting, because it’s the first time that anybody has really shown in a double-blind, placebo-controlled, large phase 3 study that any drug has been able to do this. And there were really no steroid side effects, which is the other really remarkable thing about this study,” said Dr. Lederman.
The therapy grew out of a study by researchers in Italy who treated pediatric ataxia telangiectasia patients with corticosteroids and found some transitory improvements in gross motor function, but concerns about long-term exposure to steroids limited its application. EryDel, which specializes in encapsulating therapeutics in red blood cells, became interested and developed a formulation using the patient’s own red blood cells infused with DSP. Reinfused to the patients, the red blood cells slowly release the steroid.
It isn’t clear how dexamethasone works. There are data suggesting that it might lead to transcription of small pieces of the ATM protein, “but that has really not been nailed down in any way at this point. Corticosteroids act on all kinds of cells in all kinds of ways, and so there might be a little bit of this so-called mini-ATM that’s produced, but that may or may not be related to the way in which corticosteroids have a beneficial effect on the rate of neurodegeneration,” said Dr. Lederman.
The treatment process is not easy. Children must have 50-60 cc of blood removed. Red blood cells treated to become porous are exposed to DSP, and then resealed. Then the cells are reinfused. “The whole process takes from beginning to end probably about 3 hours, with a really experienced team of people doing it. And it’s limiting because it’s not easy to put in an IV and take 50 or 60 cc of blood out of children much younger than 5 or 6. The process is now being modified to see whether we could do it with 20 to 30 cc instead,” said Dr. Lederman.
A ‘promising and impressive’ study
The study is promising, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “They were able to show a slower rate of neurological degeneration or duration on both the lower and higher dose compared with the placebo. This is promising and impressive, in the sense that it’s a really large (trial) for a rare condition,” Dr. Johnson, vice chair of research at Virginia Commonwealth University, Richmond, said in an interview.
The study included 164 patients Europe, Australia, Israel, Tunisia, India, and the United States, who received 5-10 mg dexamethasone, 14-22 mg DSP, or placebo. Mean ages in each group ranged from 9.6 to 10.4 years.
In an intention-to-treat analysis, modified International Cooperative Ataxia Rating Scale (mICARS) scores trended toward improvement in the low-dose (–1.37; P = .0847) and high-dose groups (–1.40; P = .0765) when determined by central raters during the COVID-19 pandemic. There was also a trend toward improvement when determined by local raters in the low dose group (–1.73; P = .0720) and a statistically significant change in the high dose group (–2.11; P = .0277). The researchers noted some inconsistency between local and central raters, due to inconsistency of videography and language challenges for central raters.
An intention-to-treat analysis of a subgroup of 89 patients age 6-9, who were compared with natural history data from 245 patients, found a deterioration of mICARS of 3.7 per year, compared with 0.92 in the high-dose group, for a reduction of 75% (P = .020). In the high-dose group, 51.7% had a minimal or significant improvement compared with baseline according to the Clinical Global Impression of Change, as did 29.0% on low dose, and 27.6% in the placebo group.
SEATTLE –
The disease is an autosomal recessive disorder caused by mutations in the ATM gene, which is critical to the response to cellular insults such as DNA breaks, oxidative damage, and other forms of stress. The result is clinical manifestations that range from a suppressed immune system to organ damage and neurological symptoms that typically lead patients to be wheelchair bound by their teenage years.
“It’s really multisystem and a very, very difficult disease for people to live with,” Howard M. Lederman, MD, PhD, said in an interview. Dr. Lederman is a coauthor of the study, which was presented by Stefan Zielen, PhD, professor at the University of Goethe, at the 2022 annual meeting of the American Academy of Neurology.
Various therapies have been developed to improve immunodeficiency, lung disease, and some of the other clinical aspects of the condition, but there is no treatment for its neurological effects. “There’s not really been a good animal model, which has been a big problem in trying to test drugs and design treatment trials,” said Dr. Lederman, professor of pediatrics and medicine at Johns Hopkins University, Baltimore.
The new results may change that. “In the children under the age of 9, there was really a very clear slowdown in the neurodegeneration, and specifically the time that it took for them to lose the ability to ambulate. It’s very exciting, because it’s the first time that anybody has really shown in a double-blind, placebo-controlled, large phase 3 study that any drug has been able to do this. And there were really no steroid side effects, which is the other really remarkable thing about this study,” said Dr. Lederman.
The therapy grew out of a study by researchers in Italy who treated pediatric ataxia telangiectasia patients with corticosteroids and found some transitory improvements in gross motor function, but concerns about long-term exposure to steroids limited its application. EryDel, which specializes in encapsulating therapeutics in red blood cells, became interested and developed a formulation using the patient’s own red blood cells infused with DSP. Reinfused to the patients, the red blood cells slowly release the steroid.
It isn’t clear how dexamethasone works. There are data suggesting that it might lead to transcription of small pieces of the ATM protein, “but that has really not been nailed down in any way at this point. Corticosteroids act on all kinds of cells in all kinds of ways, and so there might be a little bit of this so-called mini-ATM that’s produced, but that may or may not be related to the way in which corticosteroids have a beneficial effect on the rate of neurodegeneration,” said Dr. Lederman.
The treatment process is not easy. Children must have 50-60 cc of blood removed. Red blood cells treated to become porous are exposed to DSP, and then resealed. Then the cells are reinfused. “The whole process takes from beginning to end probably about 3 hours, with a really experienced team of people doing it. And it’s limiting because it’s not easy to put in an IV and take 50 or 60 cc of blood out of children much younger than 5 or 6. The process is now being modified to see whether we could do it with 20 to 30 cc instead,” said Dr. Lederman.
A ‘promising and impressive’ study
The study is promising, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “They were able to show a slower rate of neurological degeneration or duration on both the lower and higher dose compared with the placebo. This is promising and impressive, in the sense that it’s a really large (trial) for a rare condition,” Dr. Johnson, vice chair of research at Virginia Commonwealth University, Richmond, said in an interview.
The study included 164 patients Europe, Australia, Israel, Tunisia, India, and the United States, who received 5-10 mg dexamethasone, 14-22 mg DSP, or placebo. Mean ages in each group ranged from 9.6 to 10.4 years.
In an intention-to-treat analysis, modified International Cooperative Ataxia Rating Scale (mICARS) scores trended toward improvement in the low-dose (–1.37; P = .0847) and high-dose groups (–1.40; P = .0765) when determined by central raters during the COVID-19 pandemic. There was also a trend toward improvement when determined by local raters in the low dose group (–1.73; P = .0720) and a statistically significant change in the high dose group (–2.11; P = .0277). The researchers noted some inconsistency between local and central raters, due to inconsistency of videography and language challenges for central raters.
An intention-to-treat analysis of a subgroup of 89 patients age 6-9, who were compared with natural history data from 245 patients, found a deterioration of mICARS of 3.7 per year, compared with 0.92 in the high-dose group, for a reduction of 75% (P = .020). In the high-dose group, 51.7% had a minimal or significant improvement compared with baseline according to the Clinical Global Impression of Change, as did 29.0% on low dose, and 27.6% in the placebo group.
AT AAN 2022
Restless legs syndrome occurs often in X-linked adrenoleukodystrophy
Patients with X-linked adrenoleukodystrophy (ALD), a neurodegenerative disease, often experience gait and balance problems, as well as leg discomfort, sleep disturbances, and pain, wrote John W. Winkelman, MD, of Massachusetts General Hospital, Boston, and colleagues. Restless legs syndrome (RLS) has been associated with neurological conditions including Parkinson’s disease, but the prevalence of RLS in ALD patients has not been examined, they said.
In a pilot study published in Sleep Medicine, the researchers identified 21 women and 11 men with ALD who were treated at a single center. The median age of the patients was 45.9 years. Twenty-seven patients had symptoms of myelopathy, with a median age of onset of 34 years.
The researchers assessed RLS severity using questionnaires and the Hopkins Telephone Diagnostic Interview (HTDI), a validated RLS assessment tool. They also reviewed patients’ charts for data on neurological examinations, functional gait measures, and laboratory assessments. Functional gait assessments included the 25-Foot Walk test (25-FW), the Timed Up and Go test (TUG), and Six Minute Walk test (6MW).
Thirteen patients (10 women and 3 men) met criteria for RLS based on the HTDI. The median age of RLS onset was 35 years. Six RLS patients (46.2%) reported using medication to relieve symptoms, and eight RLS patients had a history of antidepressant use.
In addition, six patients with RLS reported a history of anemia or iron deficiency. Ferritin levels were available for 14 patients: 8 women with RLS and 4 women and 2 men without RLS; the mean ferritin levels were 74.0 mcg/L in RLS patients and 99.5 mcg/L in those without RLS.
Of the seven ALD patients with brain lesions, all were men, only two were diagnosed with RLS, and all seven cases were mild, the researchers noted.
Overall, patients with RLS had more neurological signs and symptoms than those without RLS; the most significant were pain and gait difficulty. However, patients with RLS also were more likely than were those without RLS to report spasticity, muscle weakness, impaired coordination, hyperreflexia, impaired sensation, and paraesthesia, as well as bladder, bowel, and erectile dysfunction.
The 40.6% prevalence of RLS in patients with ALD is notably higher than that of the general population, in which the prevalence of RLS is 5%-10%, the researchers wrote in their discussion.
“Consistent with patterns observed in the general population, risk factors for RLS in this cohort of adults with ALD included female gender, increased age, lower iron indices, and use of serotonergic antidepressants,” they said.
The study findings were limited by several factors including the small size and the possible contribution of antidepressant use to the high rate of RLS, the researchers noted.
“Awareness of RLS in patients with ALD would allow for its effective treatment, which may improve the functional impairments as well as quality of life, mood, and anxiety issues in those with ALD,” they concluded.
The study received no outside funding.
Dr. Winkelman disclosed ties with Advance Medical, Avadel, Disc Medicine, Eisai, Emalex, Idorsia, Noctrix, UpToDate, and Merck Pharmaceuticals, as well as research support from the National Institute on Drug Abuse and the Baszucki Brain Research Foundation. The study also was supported by grants from the National Institute of Neurological Disorders and Stroke, the European Leukodystrophy Association, the Arrivederci Foundation, the Leblang Foundation, and the Hammer Family Fund Journal Preproof for ALD Research and Therapies for Women.
Patients with X-linked adrenoleukodystrophy (ALD), a neurodegenerative disease, often experience gait and balance problems, as well as leg discomfort, sleep disturbances, and pain, wrote John W. Winkelman, MD, of Massachusetts General Hospital, Boston, and colleagues. Restless legs syndrome (RLS) has been associated with neurological conditions including Parkinson’s disease, but the prevalence of RLS in ALD patients has not been examined, they said.
In a pilot study published in Sleep Medicine, the researchers identified 21 women and 11 men with ALD who were treated at a single center. The median age of the patients was 45.9 years. Twenty-seven patients had symptoms of myelopathy, with a median age of onset of 34 years.
The researchers assessed RLS severity using questionnaires and the Hopkins Telephone Diagnostic Interview (HTDI), a validated RLS assessment tool. They also reviewed patients’ charts for data on neurological examinations, functional gait measures, and laboratory assessments. Functional gait assessments included the 25-Foot Walk test (25-FW), the Timed Up and Go test (TUG), and Six Minute Walk test (6MW).
Thirteen patients (10 women and 3 men) met criteria for RLS based on the HTDI. The median age of RLS onset was 35 years. Six RLS patients (46.2%) reported using medication to relieve symptoms, and eight RLS patients had a history of antidepressant use.
In addition, six patients with RLS reported a history of anemia or iron deficiency. Ferritin levels were available for 14 patients: 8 women with RLS and 4 women and 2 men without RLS; the mean ferritin levels were 74.0 mcg/L in RLS patients and 99.5 mcg/L in those without RLS.
Of the seven ALD patients with brain lesions, all were men, only two were diagnosed with RLS, and all seven cases were mild, the researchers noted.
Overall, patients with RLS had more neurological signs and symptoms than those without RLS; the most significant were pain and gait difficulty. However, patients with RLS also were more likely than were those without RLS to report spasticity, muscle weakness, impaired coordination, hyperreflexia, impaired sensation, and paraesthesia, as well as bladder, bowel, and erectile dysfunction.
The 40.6% prevalence of RLS in patients with ALD is notably higher than that of the general population, in which the prevalence of RLS is 5%-10%, the researchers wrote in their discussion.
“Consistent with patterns observed in the general population, risk factors for RLS in this cohort of adults with ALD included female gender, increased age, lower iron indices, and use of serotonergic antidepressants,” they said.
The study findings were limited by several factors including the small size and the possible contribution of antidepressant use to the high rate of RLS, the researchers noted.
“Awareness of RLS in patients with ALD would allow for its effective treatment, which may improve the functional impairments as well as quality of life, mood, and anxiety issues in those with ALD,” they concluded.
The study received no outside funding.
Dr. Winkelman disclosed ties with Advance Medical, Avadel, Disc Medicine, Eisai, Emalex, Idorsia, Noctrix, UpToDate, and Merck Pharmaceuticals, as well as research support from the National Institute on Drug Abuse and the Baszucki Brain Research Foundation. The study also was supported by grants from the National Institute of Neurological Disorders and Stroke, the European Leukodystrophy Association, the Arrivederci Foundation, the Leblang Foundation, and the Hammer Family Fund Journal Preproof for ALD Research and Therapies for Women.
Patients with X-linked adrenoleukodystrophy (ALD), a neurodegenerative disease, often experience gait and balance problems, as well as leg discomfort, sleep disturbances, and pain, wrote John W. Winkelman, MD, of Massachusetts General Hospital, Boston, and colleagues. Restless legs syndrome (RLS) has been associated with neurological conditions including Parkinson’s disease, but the prevalence of RLS in ALD patients has not been examined, they said.
In a pilot study published in Sleep Medicine, the researchers identified 21 women and 11 men with ALD who were treated at a single center. The median age of the patients was 45.9 years. Twenty-seven patients had symptoms of myelopathy, with a median age of onset of 34 years.
The researchers assessed RLS severity using questionnaires and the Hopkins Telephone Diagnostic Interview (HTDI), a validated RLS assessment tool. They also reviewed patients’ charts for data on neurological examinations, functional gait measures, and laboratory assessments. Functional gait assessments included the 25-Foot Walk test (25-FW), the Timed Up and Go test (TUG), and Six Minute Walk test (6MW).
Thirteen patients (10 women and 3 men) met criteria for RLS based on the HTDI. The median age of RLS onset was 35 years. Six RLS patients (46.2%) reported using medication to relieve symptoms, and eight RLS patients had a history of antidepressant use.
In addition, six patients with RLS reported a history of anemia or iron deficiency. Ferritin levels were available for 14 patients: 8 women with RLS and 4 women and 2 men without RLS; the mean ferritin levels were 74.0 mcg/L in RLS patients and 99.5 mcg/L in those without RLS.
Of the seven ALD patients with brain lesions, all were men, only two were diagnosed with RLS, and all seven cases were mild, the researchers noted.
Overall, patients with RLS had more neurological signs and symptoms than those without RLS; the most significant were pain and gait difficulty. However, patients with RLS also were more likely than were those without RLS to report spasticity, muscle weakness, impaired coordination, hyperreflexia, impaired sensation, and paraesthesia, as well as bladder, bowel, and erectile dysfunction.
The 40.6% prevalence of RLS in patients with ALD is notably higher than that of the general population, in which the prevalence of RLS is 5%-10%, the researchers wrote in their discussion.
“Consistent with patterns observed in the general population, risk factors for RLS in this cohort of adults with ALD included female gender, increased age, lower iron indices, and use of serotonergic antidepressants,” they said.
The study findings were limited by several factors including the small size and the possible contribution of antidepressant use to the high rate of RLS, the researchers noted.
“Awareness of RLS in patients with ALD would allow for its effective treatment, which may improve the functional impairments as well as quality of life, mood, and anxiety issues in those with ALD,” they concluded.
The study received no outside funding.
Dr. Winkelman disclosed ties with Advance Medical, Avadel, Disc Medicine, Eisai, Emalex, Idorsia, Noctrix, UpToDate, and Merck Pharmaceuticals, as well as research support from the National Institute on Drug Abuse and the Baszucki Brain Research Foundation. The study also was supported by grants from the National Institute of Neurological Disorders and Stroke, the European Leukodystrophy Association, the Arrivederci Foundation, the Leblang Foundation, and the Hammer Family Fund Journal Preproof for ALD Research and Therapies for Women.
FROM SLEEP MEDICINE
JIA disease activity, disability linked to social factors
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For children with polyarticular juvenile idiopathic arthritis (pJIA), functional disability lasts longer and disease activity is increased among those who belong to a racial/ethnic minority or come from homes with low household income or low family education, according to a study published online in Pediatric Rheumatology. The findings also initially revealed a higher likelihood of functional disability among those living in a poorer community, but that association lost statistical significance after adjustment for confounders.
“We chose community poverty level as the primary predictor for outcomes in pJIA because the socioeconomic context of communities and neighborhoods affects the characteristics of the social, service, and physical environments to which all residents are exposed regardless of their own socioeconomic position and may have a greater negative impact on those with fewer individual resources,” the authors write. “While community poverty level was not associated with an increase in odds of moderate-to-severe disease activity, those with high community poverty level did have higher disease activity scores (0.33 points greater on average than those with low community poverty level, in adjusted analysis).”
Nayimisha Balmuri, MD, an assistant professor of pediatrics at Johns Hopkins Medicine and study coauthor, told this news organization that anecdotal experience from everyday practice has shown that “patients with myriad social determinants of health stacked against them present sicker, take longer to present, and require far more aggressive therapies and follow-up,” which wreaks havoc in terms of disease activity. “It’s really difficult, then, to play catch-up to other cohorts of patients,” Dr. Balmuri added.
Disparities in outcomes persist
A key clinical take-home message from these findings is that the differences in clinical outcomes are relevant throughout the entire year of therapy, Dr. Balmuri said. “Patients get better; however, they don’t get better the same,” she said, and this is because of a variety of reasons. “Getting in the door is one of [those reasons] but then continuing to follow-up care is another.” For general practitioners, it’s especially important to refer patients who complain of joint pains to a specialist and to then follow up to be sure they’re improving and they’re getting the care they need.
For pediatric rheumatologists and subspecialists, “it’s important for us to realize that the disparity doesn’t end when patients come into your door to begin with,” Dr. Balmuri said. “It continues over the short term and far past that into adulthood.”
Candace Feldman, MD, MPH, ScD, an assistant professor of medicine in the Division of Rheumatology, Inflammation, and Immunity at Brigham and Women’s Hospital, Boston, told this news organization that the research “provides an important foundation to the study of the impact of social determinants of health on disease activity and disability among children with JIA. Individuals with rheumatic conditions should be screened for social determinants of health–related needs, and infrastructure should exist within the rheumatology clinic to help address the needs uncovered.” Dr. Feldman was not involved in the study.
In addition to the results’ clinical significance, Dr. Feldman also noted the policy implications of these findings. “Physicians should advocate for efforts to dismantle structural racism, to address income inequality, and to mitigate the effects of climate change, which also disproportionately affect historically marginalized populations,” Dr. Feldman said. Although this study focused predominantly on poverty, she noted that financial insecurity, food insecurity, homelessness, or housing instability were other social determinants of health to consider in future research.
Dr. Balmuri and William Daniel Soulsby, MD, a clinical fellow in pediatric rheumatology at the University of California, San Francisco, who is the study’s lead author, said they focused on poverty in this study not only because it’s so understudied in patients with pJIA but also because research in adults with lupus has found that leaving poverty was associated with a reversal of accrued disease damage.
Interactions of social determinants
The authors analyzed retrospective data from 1,684 pediatric patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry covering the period of April 2015 to February 2020. All study participants had been diagnosed with pJIA. Symptom onset occurred before age 16, and at least five joints were involved. The authors excluded patients who had been diagnosed with other systemic inflammatory or autoimmune diseases.
The authors defined exposure to a high level of community poverty as living in a ZIP code where at least 20% of residents lived at or below the federal poverty level. The authors also collected data on household income, although these data were missing for more than a quarter of participants (27%) and were therefore included only in sensitivity analyses. They used the clinical Juvenile Arthritis Disease Activity Score–10 (cJADAS-10) and the Child Health Assessment Questionnaire (CHAQ) to assess disease activity and disability at baseline and 6 and 12 months later. A cutoff of 2.5 on the cJADAS-10 distinguished mild disease activity from moderate to high disease activity, and a CHAQ score of 0.25 was the cutoff for having functional disability.
Among those who reported household income, just over half the cohort had an income of at least $50,000. The study population was 74% White, and more non-White patients lived in high-poverty communities (36.4%) than did White patients (21.3%). Patients whose families had no more than a high school education (23.1% vs. 13.7%) and those with public insurance (43.0% vs. 21.5%) were also over-represented in poorer communities.
The median cJADAS-10 scores declined overall during patients’ first year of therapy. However, those with public insurance, a lower family education level, or residency in poorer communities made up the greatest proportion of patients who continued to have moderate to severe disease activity a year after diagnosis.
The unadjusted calculations showed that children living in high community poverty had 1.8 times greater odds of functional disability (odds ratio, 1.82; P < .001). However, after adjustment for age, sex, race/ethnicity, insurance status, family education, rheumatoid factor, and cyclic citrullinated peptide antibody, the association lost statistical significance (P = .3). Community poverty level was not associated with disease activity before or after adjustment.
“Race was adjusted for as a confounder; however, the association between race/ethnicity and social determinants of health is likely more complex,” Dr. Feldman said. “Interactions, for example, between individual race and area-level poverty could be investigated.”
Odds of persistent function disability were 1.5 times greater for children with public insurance (adjusted OR, 1.56; P = .023) and 1.9 times greater for those whose families had a lower education level (aOR, 1.89; P = .013). Children whose race/ethnicity was indicated as being other than White had more than double the odds of higher disease activity (aOR, 2.48; P = .002) and were nearly twice as likely to have persistent functional disability (aOR, 1.91; P = .031).
Future directions
Dr. Soulsby was struck by the difference in statistical significance between individual-level poverty, as measured by household income, and community-level poverty. “It’s interesting because it may suggest that both of these forms of poverty are different and have different impacts on disease,” he said. Dr. Balmuri elaborated on the nuances and interactions that exist with social determinants of health and how objective outcomes, such as disease activity as measured by clinical tools, can differ from subjective outcomes, such as patients’ reports of pain, daily disability, and social experiences.
“The human condition is far more complicated, unfortunately, than any dataset could have on their own collected,” Dr. Balmuri said. She said she plans to expand her pJIA research into other social determinants of health. “It’s first about getting people’s eyes and minds open to something we see every day that, for some reason, sometimes people are blinded to, [using] the data that we do have, and then our hope is to build upon that.”
Dr. Feldman noted that ZIP codes, which were used as a proxy for community poverty, may not provide the best perspective regarding a patient’s neighborhood, because significant variation may exist within a single ZIP code, which is something the authors noted as well. The investigators were limited in the data available from the registry, and Dr. Balmuri and Dr. Soulsby suggested that 9-digit ZIP codes or census tracts might better capture neighborhood deprivation.
The research was funded by the Arthritis Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Feldman has received research support from Pfizer and the Bristol-Myers Squibb Foundation. Dr. Soulsby and Dr. Balmuri have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC RHEUMATOLOGY
Nanoparticle shows promise for ALS
SEATTLE – , which was the change in the summated motor unit index (MUNIX) from baseline to week 36.
The drug, CNM-Au8, is being developed by Clene, and would represent a novel mechanism of action. “This is a brand-new approach. We used it complementary with riluzole and it was well tolerated, so I see this as an add-on therapy. I think if we can show some more positivity and longer-term results, it’s going to be a game changer for ALS,” Matthew Kiernan, MBBS, PhD, said in an interview. Dr. Kiernan presented the results at the 2022 annual meeting of the American Academy of Neurology.
Riluzole (Rilutek), which received Food and Drug Administration approval in 1995, inhibits glutamate release to counter excitotoxicity, which is believed to play a role in ALS, Huntington’s disease, ischemia, and other acute and chronic neurodegenerative diseases. The other FDA-approved agent for ALS is the neuroprotective agent and free-radical scavenger edaravone (Radicava), approved in 2017.
CNM-Au8 is made up of catalytically active gold nanocrystals that cross the blood-brain barrier, but lacks the toxicity associated with other synthetic gold compounds, according to the company. The formulation is also being investigated for the treatment of Parkinson’s disease and multiple sclerosis. Basic research has shown that it stabilizes mitochondria and reduces accumulation of the TDP-43 protein, which is linked to spread of ALS through the brain, Dr. Kiernan said during his presentation.
The treatment is well tolerated. “Normally in an ALS trial, we see about a 25% dropout rate. There were no dropouts on the active compound in the clinical trial. There are less deaths, so improved survival,” said Dr. Kiernan, the Bushell chair of neurology at the University of Sydney and codirector of the Brain and Mind Center in Sydney.
Good safety signal
The fact that the trial missed its primary endpoint isn’t too concerning, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “ALS clinical trials are incredibly difficult to conduct, especially a phase 2 learning-phase clinical trial. At this phase, I’m much more buoyed by the fact that they have a good safety signal, and that they’re willing to move forward to that phase 3 clinical trial,” Dr. Johnson said in an interview. He is vice chair of research at Virginia Commonwealth University, Richmond.
A phase 3 clinical trial is in development in the United States and Europe. The drug also is included as part of the HEALEY ALS Platform Trial, which is testing multiple ALS therapies simultaneously. “The results from that should be available by the second half of this year and it will also inform us as to what the approach should be,” said Dr. Kiernan.
Dr. Johnson also was enthusiastic. “I’m excited to see the results in terms of the primary endpoints for that next phase 3 clinical trial,” he said.
Ongoing research
In September 2021, Clene announced a second expanded access program for people with ALS.
The study included a 36-week double-blind treatment period followed by long-term, open-label follow-up. Twenty-three patients received 30 mg CNM-Au8, and 22 received placebo. In the first 36 weeks, the treatment group was more likely to have no disease progression, defined as death, tracheostomy, noninvasive ventilation, or a gastronomy tube (P = .0125). The researchers compared the probability of experiencing a less than 6-point decline in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale. At 12 weeks, it was about 85% in both groups. At 24 weeks, it was about 60% to 50% in favor of the CNM-Au8 group, and at 36 weeks it was about 50% to 20% (P = .0350).
At 36 weeks, quality of life as measured by the ALS Specific Quality of Life–Short Form was better in the treatment group at 36 weeks (mean change, 0.9; P = .0177).
Survival was better in the treatment group at 96 weeks than the mortality derived from a European Network for the Cure of ALS prediction model (hazard ratio [HR], 0.2974; P = .0068). This benefit also was experienced by patients who received drug throughout the study (HR, 0.36; 95% confidence interval [CI], 0.12-1.1) and those who started out on placebo and converted to active drug during the open-label period (HR, 0.24; 95% CI, 0.064-0.88).
The study was funded by Clene and FightMND. Dr. Kiernan and Dr. Johnson have no relevant financial disclosures.
SEATTLE – , which was the change in the summated motor unit index (MUNIX) from baseline to week 36.
The drug, CNM-Au8, is being developed by Clene, and would represent a novel mechanism of action. “This is a brand-new approach. We used it complementary with riluzole and it was well tolerated, so I see this as an add-on therapy. I think if we can show some more positivity and longer-term results, it’s going to be a game changer for ALS,” Matthew Kiernan, MBBS, PhD, said in an interview. Dr. Kiernan presented the results at the 2022 annual meeting of the American Academy of Neurology.
Riluzole (Rilutek), which received Food and Drug Administration approval in 1995, inhibits glutamate release to counter excitotoxicity, which is believed to play a role in ALS, Huntington’s disease, ischemia, and other acute and chronic neurodegenerative diseases. The other FDA-approved agent for ALS is the neuroprotective agent and free-radical scavenger edaravone (Radicava), approved in 2017.
CNM-Au8 is made up of catalytically active gold nanocrystals that cross the blood-brain barrier, but lacks the toxicity associated with other synthetic gold compounds, according to the company. The formulation is also being investigated for the treatment of Parkinson’s disease and multiple sclerosis. Basic research has shown that it stabilizes mitochondria and reduces accumulation of the TDP-43 protein, which is linked to spread of ALS through the brain, Dr. Kiernan said during his presentation.
The treatment is well tolerated. “Normally in an ALS trial, we see about a 25% dropout rate. There were no dropouts on the active compound in the clinical trial. There are less deaths, so improved survival,” said Dr. Kiernan, the Bushell chair of neurology at the University of Sydney and codirector of the Brain and Mind Center in Sydney.
Good safety signal
The fact that the trial missed its primary endpoint isn’t too concerning, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “ALS clinical trials are incredibly difficult to conduct, especially a phase 2 learning-phase clinical trial. At this phase, I’m much more buoyed by the fact that they have a good safety signal, and that they’re willing to move forward to that phase 3 clinical trial,” Dr. Johnson said in an interview. He is vice chair of research at Virginia Commonwealth University, Richmond.
A phase 3 clinical trial is in development in the United States and Europe. The drug also is included as part of the HEALEY ALS Platform Trial, which is testing multiple ALS therapies simultaneously. “The results from that should be available by the second half of this year and it will also inform us as to what the approach should be,” said Dr. Kiernan.
Dr. Johnson also was enthusiastic. “I’m excited to see the results in terms of the primary endpoints for that next phase 3 clinical trial,” he said.
Ongoing research
In September 2021, Clene announced a second expanded access program for people with ALS.
The study included a 36-week double-blind treatment period followed by long-term, open-label follow-up. Twenty-three patients received 30 mg CNM-Au8, and 22 received placebo. In the first 36 weeks, the treatment group was more likely to have no disease progression, defined as death, tracheostomy, noninvasive ventilation, or a gastronomy tube (P = .0125). The researchers compared the probability of experiencing a less than 6-point decline in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale. At 12 weeks, it was about 85% in both groups. At 24 weeks, it was about 60% to 50% in favor of the CNM-Au8 group, and at 36 weeks it was about 50% to 20% (P = .0350).
At 36 weeks, quality of life as measured by the ALS Specific Quality of Life–Short Form was better in the treatment group at 36 weeks (mean change, 0.9; P = .0177).
Survival was better in the treatment group at 96 weeks than the mortality derived from a European Network for the Cure of ALS prediction model (hazard ratio [HR], 0.2974; P = .0068). This benefit also was experienced by patients who received drug throughout the study (HR, 0.36; 95% confidence interval [CI], 0.12-1.1) and those who started out on placebo and converted to active drug during the open-label period (HR, 0.24; 95% CI, 0.064-0.88).
The study was funded by Clene and FightMND. Dr. Kiernan and Dr. Johnson have no relevant financial disclosures.
SEATTLE – , which was the change in the summated motor unit index (MUNIX) from baseline to week 36.
The drug, CNM-Au8, is being developed by Clene, and would represent a novel mechanism of action. “This is a brand-new approach. We used it complementary with riluzole and it was well tolerated, so I see this as an add-on therapy. I think if we can show some more positivity and longer-term results, it’s going to be a game changer for ALS,” Matthew Kiernan, MBBS, PhD, said in an interview. Dr. Kiernan presented the results at the 2022 annual meeting of the American Academy of Neurology.
Riluzole (Rilutek), which received Food and Drug Administration approval in 1995, inhibits glutamate release to counter excitotoxicity, which is believed to play a role in ALS, Huntington’s disease, ischemia, and other acute and chronic neurodegenerative diseases. The other FDA-approved agent for ALS is the neuroprotective agent and free-radical scavenger edaravone (Radicava), approved in 2017.
CNM-Au8 is made up of catalytically active gold nanocrystals that cross the blood-brain barrier, but lacks the toxicity associated with other synthetic gold compounds, according to the company. The formulation is also being investigated for the treatment of Parkinson’s disease and multiple sclerosis. Basic research has shown that it stabilizes mitochondria and reduces accumulation of the TDP-43 protein, which is linked to spread of ALS through the brain, Dr. Kiernan said during his presentation.
The treatment is well tolerated. “Normally in an ALS trial, we see about a 25% dropout rate. There were no dropouts on the active compound in the clinical trial. There are less deaths, so improved survival,” said Dr. Kiernan, the Bushell chair of neurology at the University of Sydney and codirector of the Brain and Mind Center in Sydney.
Good safety signal
The fact that the trial missed its primary endpoint isn’t too concerning, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “ALS clinical trials are incredibly difficult to conduct, especially a phase 2 learning-phase clinical trial. At this phase, I’m much more buoyed by the fact that they have a good safety signal, and that they’re willing to move forward to that phase 3 clinical trial,” Dr. Johnson said in an interview. He is vice chair of research at Virginia Commonwealth University, Richmond.
A phase 3 clinical trial is in development in the United States and Europe. The drug also is included as part of the HEALEY ALS Platform Trial, which is testing multiple ALS therapies simultaneously. “The results from that should be available by the second half of this year and it will also inform us as to what the approach should be,” said Dr. Kiernan.
Dr. Johnson also was enthusiastic. “I’m excited to see the results in terms of the primary endpoints for that next phase 3 clinical trial,” he said.
Ongoing research
In September 2021, Clene announced a second expanded access program for people with ALS.
The study included a 36-week double-blind treatment period followed by long-term, open-label follow-up. Twenty-three patients received 30 mg CNM-Au8, and 22 received placebo. In the first 36 weeks, the treatment group was more likely to have no disease progression, defined as death, tracheostomy, noninvasive ventilation, or a gastronomy tube (P = .0125). The researchers compared the probability of experiencing a less than 6-point decline in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale. At 12 weeks, it was about 85% in both groups. At 24 weeks, it was about 60% to 50% in favor of the CNM-Au8 group, and at 36 weeks it was about 50% to 20% (P = .0350).
At 36 weeks, quality of life as measured by the ALS Specific Quality of Life–Short Form was better in the treatment group at 36 weeks (mean change, 0.9; P = .0177).
Survival was better in the treatment group at 96 weeks than the mortality derived from a European Network for the Cure of ALS prediction model (hazard ratio [HR], 0.2974; P = .0068). This benefit also was experienced by patients who received drug throughout the study (HR, 0.36; 95% confidence interval [CI], 0.12-1.1) and those who started out on placebo and converted to active drug during the open-label period (HR, 0.24; 95% CI, 0.064-0.88).
The study was funded by Clene and FightMND. Dr. Kiernan and Dr. Johnson have no relevant financial disclosures.
AT AAN 2022
Brain implant is a potential life-changer for paralyzed patients
, results of a small, first-in-human study show.
A potential life changer for patients with amyotrophic lateral sclerosis (ALS), the minimally invasive device enables patients to carry out important activities of daily living.
“Our participants are able to use the device to perform tasks like sending email, texting loved ones and caregivers, browsing the web, and doing personal finances such as online banking,” study investigator Douglas J. Weber, PhD, professor of mechanical engineering and neuroscience, Carnegie Mellon University, Pittsburgh, told a press briefing.
The technology allowed one patient to write a book (due out later this year) and another patient to maintain communication despite losing his ability to speak, said the study’s lead investigator, Bruce Campbell, MBBS, PhD, professor of neurology, Royal Melbourne Hospital, University of Melbourne.
“In addition to providing patients with communicative capabilities not possible as a result of their disease, it is our goal to enable patients to be more independently involved in their care going forward, by enabling effective and faster communication directly with their caregiver and physician,” said Dr. Campbell.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Minimally invasive
ALS, also known as Lou Gehrig’s disease, is a progressive neurodegenerative disease that affects nerve cells in the brain and spinal cord. Patients with ALS eventually lose the ability to control muscle movement, often leading to total paralysis.
“Extending the period in which patients are able to communicate with loved ones and caregivers could provide a very meaningful benefit to patients with ALS,” said Dr. Weber.
Brain-computer interfaces measure and translate brain signals, with some functioning as motor neuro-prostheses. These devices provide direct communication between the brain and an external device by recording and decoding signals from the precentral gyrus as the result of movement intention.
“The technology has potential to empower the more than five million people in the U.S. who are severely paralyzed to once again perform important activities of daily living independently,” said Dr. Weber.
Until now, motor neuro-prostheses required surgery to remove a portion of the skull and place electrodes on to the brain. However, the new minimally invasive motor neuro-prostheses reach the brain by vascular access, dispensing with the need for a craniotomy.
“The brain-computer interface device used in our study is unique in that it does not require invasive open surgery to implant,” said Dr. Weber. “Instead this is an endovascular brain-computer interface.”
Using a catheter, surgeons feed the BCI through one of two jugular veins in the neck. They position an array of 16 sensors or electrodes on a stent-like scaffold that deploys against the walls of the superior sagittal sinus.
No adverse events
Describing the device, Dr. Weber said the electrodes or sensing elements are tiny and the body of the stent, which serves as a scaffold to support the electrodes, resembles a standard endovascular stent.
“It’s very small at the time of delivery because it’s held within the body of a catheter, but then when deployed it expands to contact the wall of the vein.”
The device transmits brain signals from the motor cortex to an electronics unit, located in a subcutaneous pocket that decodes movement signals. The machine-learning decoder is programmed as follows: When a trainer asked participants to attempt certain movements, like tapping their foot or extending their knee, the decoder analyzes nerve cell signals from those movement attempts. The decoder is able to translate movement signals into computer navigation.
The study included four patients with ALS who were paralyzed because of the disease and were trained to use the device.
A key safety endpoint was device-related serious adverse events resulting in death or increased disability during the post-implant evaluation period. Results showed all four participants successfully completed the 12-month follow-up with no serious adverse events.
Researchers also assessed target vessel patency and incidence of device migration at 3 and 12 months. Postoperative imaging showed that in all participants, the blood vessel that held the implanted device remained open and stayed in place.
Addressing the potential for blood clots, Dr. Weber said that so far there has been no sign of clotting or vascular occlusion.
“The device itself integrates well into the walls of the blood vessel over time,” he said. “Within the acute period after implantation, there’s time where the device is exposed to the blood stream, but once it becomes encapsulated and fully integrated into the blood vessel wall, the risks of thrombosis diminish.”
Greater independence
Researchers also recorded signal fidelity and stability over 12 months and use of the brain-computer interface to perform routine tasks. All participants learned to use the motor neuro-prostheses with eye tracking for computer use. Eye tracking technology helps a computer determine what a person is looking at.
Using the system, patients were able to complete tasks without help. These included text messaging and managing finances. “Since the device is fully implanted and easy for patients to use, they can use the technology independently and in their own home,” said Dr. Weber.
Although the study started with patients with ALS, those paralyzed from other causes, such as an upper spinal cord injury or brain-stem stroke could also benefit from this technology, Dr. Weber said. In addition, the technology could be expanded to broaden brain communication capabilities potentially to include robotic limbs, he said.
There’s even the potential to use this minimally invasive brain interface technology to deliver therapies like deep brain stimulation, which Dr. Weber noted is a growing field. “It’s [the] early days, but it’s a very exciting new direction for brain interface technology,” he said.
Researchers are now recruiting patients for the first U.S.-based feasibility trial of the device that will be funded by the NIH, said Dr. Weber. A limitation of the research was the study’s small size.
Advancing the field
Reached for a comment, Kevin C. Davis, an MD and PhD student in the department of biomedical engineering, University of Miami Miller School of Medicine, said this new work moves the field forward in an important way.
Dr. Davis and colleagues have shown the effectiveness of another technology used to overcome paralysis – a small portable system that facilitates hand grasp of a patient with a spinal cord injury. He reported on this DBS-based BCI system at the American Association of Neurological Surgeons (AANS) 2021 Annual Meeting.
Developing effective brain-computer interfaces, and motor neural prosthetics that avoid surgery, as the team did in this new study, is “worth exploring,” said Dr. Davis.
However, although the device used in this new study avoids cranial surgery, “sole vascular access may limit the device’s ability to reach other areas of the brain more suitable for upper-limb motor prosthetics,” he said.
“Determining how much function such a device could provide to individuals with locked-in syndrome or paralysis will be important in determining its viability as an eventual clinical tool for patients.”
The study was supported by Synchron, the maker of the device, the U.S. Defense Advanced Research Projects Agency, the Office of Naval Research, the National Health and Medical Research Council of Australia, the Australian Federal Government Foundation, and the Motor Neuron Disease Research Institute of Australia.
A version of this article first appeared on Medscape.com.
, results of a small, first-in-human study show.
A potential life changer for patients with amyotrophic lateral sclerosis (ALS), the minimally invasive device enables patients to carry out important activities of daily living.
“Our participants are able to use the device to perform tasks like sending email, texting loved ones and caregivers, browsing the web, and doing personal finances such as online banking,” study investigator Douglas J. Weber, PhD, professor of mechanical engineering and neuroscience, Carnegie Mellon University, Pittsburgh, told a press briefing.
The technology allowed one patient to write a book (due out later this year) and another patient to maintain communication despite losing his ability to speak, said the study’s lead investigator, Bruce Campbell, MBBS, PhD, professor of neurology, Royal Melbourne Hospital, University of Melbourne.
“In addition to providing patients with communicative capabilities not possible as a result of their disease, it is our goal to enable patients to be more independently involved in their care going forward, by enabling effective and faster communication directly with their caregiver and physician,” said Dr. Campbell.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Minimally invasive
ALS, also known as Lou Gehrig’s disease, is a progressive neurodegenerative disease that affects nerve cells in the brain and spinal cord. Patients with ALS eventually lose the ability to control muscle movement, often leading to total paralysis.
“Extending the period in which patients are able to communicate with loved ones and caregivers could provide a very meaningful benefit to patients with ALS,” said Dr. Weber.
Brain-computer interfaces measure and translate brain signals, with some functioning as motor neuro-prostheses. These devices provide direct communication between the brain and an external device by recording and decoding signals from the precentral gyrus as the result of movement intention.
“The technology has potential to empower the more than five million people in the U.S. who are severely paralyzed to once again perform important activities of daily living independently,” said Dr. Weber.
Until now, motor neuro-prostheses required surgery to remove a portion of the skull and place electrodes on to the brain. However, the new minimally invasive motor neuro-prostheses reach the brain by vascular access, dispensing with the need for a craniotomy.
“The brain-computer interface device used in our study is unique in that it does not require invasive open surgery to implant,” said Dr. Weber. “Instead this is an endovascular brain-computer interface.”
Using a catheter, surgeons feed the BCI through one of two jugular veins in the neck. They position an array of 16 sensors or electrodes on a stent-like scaffold that deploys against the walls of the superior sagittal sinus.
No adverse events
Describing the device, Dr. Weber said the electrodes or sensing elements are tiny and the body of the stent, which serves as a scaffold to support the electrodes, resembles a standard endovascular stent.
“It’s very small at the time of delivery because it’s held within the body of a catheter, but then when deployed it expands to contact the wall of the vein.”
The device transmits brain signals from the motor cortex to an electronics unit, located in a subcutaneous pocket that decodes movement signals. The machine-learning decoder is programmed as follows: When a trainer asked participants to attempt certain movements, like tapping their foot or extending their knee, the decoder analyzes nerve cell signals from those movement attempts. The decoder is able to translate movement signals into computer navigation.
The study included four patients with ALS who were paralyzed because of the disease and were trained to use the device.
A key safety endpoint was device-related serious adverse events resulting in death or increased disability during the post-implant evaluation period. Results showed all four participants successfully completed the 12-month follow-up with no serious adverse events.
Researchers also assessed target vessel patency and incidence of device migration at 3 and 12 months. Postoperative imaging showed that in all participants, the blood vessel that held the implanted device remained open and stayed in place.
Addressing the potential for blood clots, Dr. Weber said that so far there has been no sign of clotting or vascular occlusion.
“The device itself integrates well into the walls of the blood vessel over time,” he said. “Within the acute period after implantation, there’s time where the device is exposed to the blood stream, but once it becomes encapsulated and fully integrated into the blood vessel wall, the risks of thrombosis diminish.”
Greater independence
Researchers also recorded signal fidelity and stability over 12 months and use of the brain-computer interface to perform routine tasks. All participants learned to use the motor neuro-prostheses with eye tracking for computer use. Eye tracking technology helps a computer determine what a person is looking at.
Using the system, patients were able to complete tasks without help. These included text messaging and managing finances. “Since the device is fully implanted and easy for patients to use, they can use the technology independently and in their own home,” said Dr. Weber.
Although the study started with patients with ALS, those paralyzed from other causes, such as an upper spinal cord injury or brain-stem stroke could also benefit from this technology, Dr. Weber said. In addition, the technology could be expanded to broaden brain communication capabilities potentially to include robotic limbs, he said.
There’s even the potential to use this minimally invasive brain interface technology to deliver therapies like deep brain stimulation, which Dr. Weber noted is a growing field. “It’s [the] early days, but it’s a very exciting new direction for brain interface technology,” he said.
Researchers are now recruiting patients for the first U.S.-based feasibility trial of the device that will be funded by the NIH, said Dr. Weber. A limitation of the research was the study’s small size.
Advancing the field
Reached for a comment, Kevin C. Davis, an MD and PhD student in the department of biomedical engineering, University of Miami Miller School of Medicine, said this new work moves the field forward in an important way.
Dr. Davis and colleagues have shown the effectiveness of another technology used to overcome paralysis – a small portable system that facilitates hand grasp of a patient with a spinal cord injury. He reported on this DBS-based BCI system at the American Association of Neurological Surgeons (AANS) 2021 Annual Meeting.
Developing effective brain-computer interfaces, and motor neural prosthetics that avoid surgery, as the team did in this new study, is “worth exploring,” said Dr. Davis.
However, although the device used in this new study avoids cranial surgery, “sole vascular access may limit the device’s ability to reach other areas of the brain more suitable for upper-limb motor prosthetics,” he said.
“Determining how much function such a device could provide to individuals with locked-in syndrome or paralysis will be important in determining its viability as an eventual clinical tool for patients.”
The study was supported by Synchron, the maker of the device, the U.S. Defense Advanced Research Projects Agency, the Office of Naval Research, the National Health and Medical Research Council of Australia, the Australian Federal Government Foundation, and the Motor Neuron Disease Research Institute of Australia.
A version of this article first appeared on Medscape.com.
, results of a small, first-in-human study show.
A potential life changer for patients with amyotrophic lateral sclerosis (ALS), the minimally invasive device enables patients to carry out important activities of daily living.
“Our participants are able to use the device to perform tasks like sending email, texting loved ones and caregivers, browsing the web, and doing personal finances such as online banking,” study investigator Douglas J. Weber, PhD, professor of mechanical engineering and neuroscience, Carnegie Mellon University, Pittsburgh, told a press briefing.
The technology allowed one patient to write a book (due out later this year) and another patient to maintain communication despite losing his ability to speak, said the study’s lead investigator, Bruce Campbell, MBBS, PhD, professor of neurology, Royal Melbourne Hospital, University of Melbourne.
“In addition to providing patients with communicative capabilities not possible as a result of their disease, it is our goal to enable patients to be more independently involved in their care going forward, by enabling effective and faster communication directly with their caregiver and physician,” said Dr. Campbell.
The findings were presented at the 2022 annual meeting of the American Academy of Neurology.
Minimally invasive
ALS, also known as Lou Gehrig’s disease, is a progressive neurodegenerative disease that affects nerve cells in the brain and spinal cord. Patients with ALS eventually lose the ability to control muscle movement, often leading to total paralysis.
“Extending the period in which patients are able to communicate with loved ones and caregivers could provide a very meaningful benefit to patients with ALS,” said Dr. Weber.
Brain-computer interfaces measure and translate brain signals, with some functioning as motor neuro-prostheses. These devices provide direct communication between the brain and an external device by recording and decoding signals from the precentral gyrus as the result of movement intention.
“The technology has potential to empower the more than five million people in the U.S. who are severely paralyzed to once again perform important activities of daily living independently,” said Dr. Weber.
Until now, motor neuro-prostheses required surgery to remove a portion of the skull and place electrodes on to the brain. However, the new minimally invasive motor neuro-prostheses reach the brain by vascular access, dispensing with the need for a craniotomy.
“The brain-computer interface device used in our study is unique in that it does not require invasive open surgery to implant,” said Dr. Weber. “Instead this is an endovascular brain-computer interface.”
Using a catheter, surgeons feed the BCI through one of two jugular veins in the neck. They position an array of 16 sensors or electrodes on a stent-like scaffold that deploys against the walls of the superior sagittal sinus.
No adverse events
Describing the device, Dr. Weber said the electrodes or sensing elements are tiny and the body of the stent, which serves as a scaffold to support the electrodes, resembles a standard endovascular stent.
“It’s very small at the time of delivery because it’s held within the body of a catheter, but then when deployed it expands to contact the wall of the vein.”
The device transmits brain signals from the motor cortex to an electronics unit, located in a subcutaneous pocket that decodes movement signals. The machine-learning decoder is programmed as follows: When a trainer asked participants to attempt certain movements, like tapping their foot or extending their knee, the decoder analyzes nerve cell signals from those movement attempts. The decoder is able to translate movement signals into computer navigation.
The study included four patients with ALS who were paralyzed because of the disease and were trained to use the device.
A key safety endpoint was device-related serious adverse events resulting in death or increased disability during the post-implant evaluation period. Results showed all four participants successfully completed the 12-month follow-up with no serious adverse events.
Researchers also assessed target vessel patency and incidence of device migration at 3 and 12 months. Postoperative imaging showed that in all participants, the blood vessel that held the implanted device remained open and stayed in place.
Addressing the potential for blood clots, Dr. Weber said that so far there has been no sign of clotting or vascular occlusion.
“The device itself integrates well into the walls of the blood vessel over time,” he said. “Within the acute period after implantation, there’s time where the device is exposed to the blood stream, but once it becomes encapsulated and fully integrated into the blood vessel wall, the risks of thrombosis diminish.”
Greater independence
Researchers also recorded signal fidelity and stability over 12 months and use of the brain-computer interface to perform routine tasks. All participants learned to use the motor neuro-prostheses with eye tracking for computer use. Eye tracking technology helps a computer determine what a person is looking at.
Using the system, patients were able to complete tasks without help. These included text messaging and managing finances. “Since the device is fully implanted and easy for patients to use, they can use the technology independently and in their own home,” said Dr. Weber.
Although the study started with patients with ALS, those paralyzed from other causes, such as an upper spinal cord injury or brain-stem stroke could also benefit from this technology, Dr. Weber said. In addition, the technology could be expanded to broaden brain communication capabilities potentially to include robotic limbs, he said.
There’s even the potential to use this minimally invasive brain interface technology to deliver therapies like deep brain stimulation, which Dr. Weber noted is a growing field. “It’s [the] early days, but it’s a very exciting new direction for brain interface technology,” he said.
Researchers are now recruiting patients for the first U.S.-based feasibility trial of the device that will be funded by the NIH, said Dr. Weber. A limitation of the research was the study’s small size.
Advancing the field
Reached for a comment, Kevin C. Davis, an MD and PhD student in the department of biomedical engineering, University of Miami Miller School of Medicine, said this new work moves the field forward in an important way.
Dr. Davis and colleagues have shown the effectiveness of another technology used to overcome paralysis – a small portable system that facilitates hand grasp of a patient with a spinal cord injury. He reported on this DBS-based BCI system at the American Association of Neurological Surgeons (AANS) 2021 Annual Meeting.
Developing effective brain-computer interfaces, and motor neural prosthetics that avoid surgery, as the team did in this new study, is “worth exploring,” said Dr. Davis.
However, although the device used in this new study avoids cranial surgery, “sole vascular access may limit the device’s ability to reach other areas of the brain more suitable for upper-limb motor prosthetics,” he said.
“Determining how much function such a device could provide to individuals with locked-in syndrome or paralysis will be important in determining its viability as an eventual clinical tool for patients.”
The study was supported by Synchron, the maker of the device, the U.S. Defense Advanced Research Projects Agency, the Office of Naval Research, the National Health and Medical Research Council of Australia, the Australian Federal Government Foundation, and the Motor Neuron Disease Research Institute of Australia.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Novel drug significantly reduces tics in Tourette syndrome – without side effects
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Early puberty cases among girls surged during pandemic
Overwhelming numbers of early puberty cases among girls have been reported during the pandemic, according a report copublished by the Washington Post and The Fuller Project.
Early puberty is uncommon, affecting about 1 in every 5,000 to 10,000 children, with cases about 10 times higher in girls than boys. But since the pandemic started, doctors and parents around the world have noted a substantial surge in early puberty.
In some cases, girls as young as 5 have begun developing breasts and girls younger than 8 have started menstruation.
“I noticed that quite a few of my [girl patients] got their period after a lockdown,” Adiaha Spinks-Franklin, MD, a pediatrician at Texas Children’s Hospital, Houston, told the news outlets.
The condition, also called precocious puberty, is defined as puberty-related changes earlier than normal or expected, which starts around age 8 for girls and age 9 for boys. It can sometimes be caused by genetic syndromes, central nervous system issues, or tumors on the ovaries, adrenal glands, pituitary gland, or brain.
Pediatricians across the world have reported more precocious puberty cases, the news outlets reported, including in the United States, India, Italy, and Turkey.
A recent study found that more than 300 girls were referred to five pediatric endocrinology centers in Italy between March and September 2020, as opposed to 140 referrals during the same time period in 2019.
In another study, a Turkish pediatric endocrinology clinic reported 58 cases during the first year of the pandemic, as compared with 66 total cases during the 3 previous years.
Early puberty tends to mean there are other mental and physical issues, though in most cases, an exact cause can’t be found. Doctors have tied the current uptick to the stress of the pandemic and lockdowns, including reduced physical activity and increased consumption of unhealthy food, which are things linked to a higher risk of early puberty.
“I think it’s directly related to the amount of stress that the children have gone through,” Vaishakhi Rustagi, MD, a pediatric endocrinologist in Delhi, India, told the news outlets.
In a typical year, Dr. Rustagi sees about 20 patients with early puberty. Since mid-2020, she’s seen more than 300 girls with the condition. Imaging scans and ultrasounds haven’t found tumors, and the cause has been mostly unidentifiable, though Dr. Rustagi attributed it to stress and grief.
“These children have lost family members,” she said.
Early puberty is known to increase depression, eating disorders, substance abuse, and antisocial behavior, the news outlets reported.
The main treatment for the condition, a form of hormone therapy known as gonadotropin-releasing hormone analogue therapy, is known to work very well. But some patients and families may not seek treatment because of a lack of awareness or stigmas that come with menstruation.
A version of this article first appeared on WebMD.com.
Overwhelming numbers of early puberty cases among girls have been reported during the pandemic, according a report copublished by the Washington Post and The Fuller Project.
Early puberty is uncommon, affecting about 1 in every 5,000 to 10,000 children, with cases about 10 times higher in girls than boys. But since the pandemic started, doctors and parents around the world have noted a substantial surge in early puberty.
In some cases, girls as young as 5 have begun developing breasts and girls younger than 8 have started menstruation.
“I noticed that quite a few of my [girl patients] got their period after a lockdown,” Adiaha Spinks-Franklin, MD, a pediatrician at Texas Children’s Hospital, Houston, told the news outlets.
The condition, also called precocious puberty, is defined as puberty-related changes earlier than normal or expected, which starts around age 8 for girls and age 9 for boys. It can sometimes be caused by genetic syndromes, central nervous system issues, or tumors on the ovaries, adrenal glands, pituitary gland, or brain.
Pediatricians across the world have reported more precocious puberty cases, the news outlets reported, including in the United States, India, Italy, and Turkey.
A recent study found that more than 300 girls were referred to five pediatric endocrinology centers in Italy between March and September 2020, as opposed to 140 referrals during the same time period in 2019.
In another study, a Turkish pediatric endocrinology clinic reported 58 cases during the first year of the pandemic, as compared with 66 total cases during the 3 previous years.
Early puberty tends to mean there are other mental and physical issues, though in most cases, an exact cause can’t be found. Doctors have tied the current uptick to the stress of the pandemic and lockdowns, including reduced physical activity and increased consumption of unhealthy food, which are things linked to a higher risk of early puberty.
“I think it’s directly related to the amount of stress that the children have gone through,” Vaishakhi Rustagi, MD, a pediatric endocrinologist in Delhi, India, told the news outlets.
In a typical year, Dr. Rustagi sees about 20 patients with early puberty. Since mid-2020, she’s seen more than 300 girls with the condition. Imaging scans and ultrasounds haven’t found tumors, and the cause has been mostly unidentifiable, though Dr. Rustagi attributed it to stress and grief.
“These children have lost family members,” she said.
Early puberty is known to increase depression, eating disorders, substance abuse, and antisocial behavior, the news outlets reported.
The main treatment for the condition, a form of hormone therapy known as gonadotropin-releasing hormone analogue therapy, is known to work very well. But some patients and families may not seek treatment because of a lack of awareness or stigmas that come with menstruation.
A version of this article first appeared on WebMD.com.
Overwhelming numbers of early puberty cases among girls have been reported during the pandemic, according a report copublished by the Washington Post and The Fuller Project.
Early puberty is uncommon, affecting about 1 in every 5,000 to 10,000 children, with cases about 10 times higher in girls than boys. But since the pandemic started, doctors and parents around the world have noted a substantial surge in early puberty.
In some cases, girls as young as 5 have begun developing breasts and girls younger than 8 have started menstruation.
“I noticed that quite a few of my [girl patients] got their period after a lockdown,” Adiaha Spinks-Franklin, MD, a pediatrician at Texas Children’s Hospital, Houston, told the news outlets.
The condition, also called precocious puberty, is defined as puberty-related changes earlier than normal or expected, which starts around age 8 for girls and age 9 for boys. It can sometimes be caused by genetic syndromes, central nervous system issues, or tumors on the ovaries, adrenal glands, pituitary gland, or brain.
Pediatricians across the world have reported more precocious puberty cases, the news outlets reported, including in the United States, India, Italy, and Turkey.
A recent study found that more than 300 girls were referred to five pediatric endocrinology centers in Italy between March and September 2020, as opposed to 140 referrals during the same time period in 2019.
In another study, a Turkish pediatric endocrinology clinic reported 58 cases during the first year of the pandemic, as compared with 66 total cases during the 3 previous years.
Early puberty tends to mean there are other mental and physical issues, though in most cases, an exact cause can’t be found. Doctors have tied the current uptick to the stress of the pandemic and lockdowns, including reduced physical activity and increased consumption of unhealthy food, which are things linked to a higher risk of early puberty.
“I think it’s directly related to the amount of stress that the children have gone through,” Vaishakhi Rustagi, MD, a pediatric endocrinologist in Delhi, India, told the news outlets.
In a typical year, Dr. Rustagi sees about 20 patients with early puberty. Since mid-2020, she’s seen more than 300 girls with the condition. Imaging scans and ultrasounds haven’t found tumors, and the cause has been mostly unidentifiable, though Dr. Rustagi attributed it to stress and grief.
“These children have lost family members,” she said.
Early puberty is known to increase depression, eating disorders, substance abuse, and antisocial behavior, the news outlets reported.
The main treatment for the condition, a form of hormone therapy known as gonadotropin-releasing hormone analogue therapy, is known to work very well. But some patients and families may not seek treatment because of a lack of awareness or stigmas that come with menstruation.
A version of this article first appeared on WebMD.com.
Gene therapy demonstrates modest success in genetic blindness
SEATTLE – The therapy, delivered by intravitreal injection, uses an adeno-associated virus vector to deliver a corrected copy of the mutated ND4 mitochondrial gene.
LHON is a rare, maternally inherited mitochondrial mutation that can cause blindness, most commonly in young men, though it does not happen in all individuals with the mutation. The condition often starts with blindness in one eye, accompanied or followed shortly by blindness in the second eye. Researchers believe that the injected viral vector gets taken up retinal ganglion cells, where the mutated gene interferes with vision. Once synthesized, a mitochondria-targeting sequence facilitates transport of the protein to the mitochondria.
The study protocol called for injection of the therapy into one eye and a placebo into the other, using the patient as his or own placebo control. The results in the treated eye were encouraging, though modest. “This is not hitting it out of the ballpark. But for people whose vision is devastated by this disease, it certainly is a first step,” said Nancy J. Newman, MD, during a press conference held March 29 in advance of the 2022 annual meeting of the American Academy of Neurology.
Dr. Newman also noted a surprise finding: Visual improvement also occurred in the placebo-control eye. This was noted in previous studies, called RESCUE and REVERSE, and follow-up studies in monkeys found viral vector in the unaffected eye 3-6 months after an injection. “This would imply some kind of transport within retrograde up the opposite optic nerve after crossing in the chiasm to the eye, but this is going to take a fair bit of work to know exactly how that happens,” said Dr. Newman
Unfortunately, the phase 3 REFLECT study was designed before that process was understood. “This was not a case-control study by person, it was by eye. And that was a mistake, because it turns out there is a does appear to be second eye effects. We do not have naive controls here that did not receive any injection at all in any eye. That’s something that we will [do going] forward,” said Dr. Newman.
Despite the problem with placebo, the results were encouraging. “Those patients who had both eyes injected with the drug did better than in those who had one eye injected with drug and one eye injected with placebo, suggesting some sort of dose effect. There were no adverse events other than what we would expect from injecting [into] eyes. Those treated with the drug had more ocular inflammation, as would also be expected, but all were easily treated with topical medications,” said Dr. Newman.
What are the long-term effects?
Natalia Rost, MD, who chairs the AAN Science Committee, commented after the presentation: “We’re quite impressed with advances in gene therapy. The question is, are there early indications that this improvement in vision will have a lasting effect?”
Dr. Newman responded that ongoing data from earlier studies are also encouraging regarding the long-term effect of the treatment. At 4 years, there was a difference of 16.5 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters equivalent between treated patients and natural history controls (P < .01), “which [does] suggest that this effect is maintained,” said Dr. Newman, who is a professor of ophthalmology and neurology at Emory University, Atlanta.
Dr. Rost also wondered if it would be possible to capture patients earlier in their disease process, in the hopes of countering degeneration before it becomes severe enough to impact vision. Dr. Newman answered by noting another surprise from the research. Previous studies had shown that intervention while only a single eye is affected had little impact on spread of the condition to the second eye, “which was very disappointing,” said Dr. Newman. When they stratified patients by time since vision loss, they found that those who received the therapy 6 months or later after vision loss had better responses than those who were treated earlier.
The mechanism of this counter-intuitive finding remains uncertain, “but we do know that acutely in this disease when people are just starting to lose this vision, during the first couple of months, they get swelling of the axons from these retinal ganglion cells. Our hypothesis is that swelling may actually act as a barrier for the drug to get into the retinal ganglion cell bodies themselves and be transfected. So it turns out that earlier may not be better,” said Dr. Newman.
The study included patients at 13 sites worldwide; 48 were treated bilaterally and 50 treated unilaterally. Just under 80% were male, the mean age was 31.5 years, and the mean duration of vision loss was 8.30 months.
After 1.5 years, the improvement in best-corrected visual acuity between second-affected eyes was stronger in the treatment eye, equivalent to +3 ETDRS letters. The first-affected eye improved by 19 ETDRS letters, and the second-affected eye improved by 16 (P < .0001). Improvement in placebo eyes was +13 ETDRS letters (P < .0001).
Dr. Rost has served on a scientific advisory board or data monitoring board for Omniox. Dr. Newman has consulted for GenSight, Santhera/Chiesi, and Neurophoenix, and has received research support from GenSight and Santhera/Chiesi.
SEATTLE – The therapy, delivered by intravitreal injection, uses an adeno-associated virus vector to deliver a corrected copy of the mutated ND4 mitochondrial gene.
LHON is a rare, maternally inherited mitochondrial mutation that can cause blindness, most commonly in young men, though it does not happen in all individuals with the mutation. The condition often starts with blindness in one eye, accompanied or followed shortly by blindness in the second eye. Researchers believe that the injected viral vector gets taken up retinal ganglion cells, where the mutated gene interferes with vision. Once synthesized, a mitochondria-targeting sequence facilitates transport of the protein to the mitochondria.
The study protocol called for injection of the therapy into one eye and a placebo into the other, using the patient as his or own placebo control. The results in the treated eye were encouraging, though modest. “This is not hitting it out of the ballpark. But for people whose vision is devastated by this disease, it certainly is a first step,” said Nancy J. Newman, MD, during a press conference held March 29 in advance of the 2022 annual meeting of the American Academy of Neurology.
Dr. Newman also noted a surprise finding: Visual improvement also occurred in the placebo-control eye. This was noted in previous studies, called RESCUE and REVERSE, and follow-up studies in monkeys found viral vector in the unaffected eye 3-6 months after an injection. “This would imply some kind of transport within retrograde up the opposite optic nerve after crossing in the chiasm to the eye, but this is going to take a fair bit of work to know exactly how that happens,” said Dr. Newman
Unfortunately, the phase 3 REFLECT study was designed before that process was understood. “This was not a case-control study by person, it was by eye. And that was a mistake, because it turns out there is a does appear to be second eye effects. We do not have naive controls here that did not receive any injection at all in any eye. That’s something that we will [do going] forward,” said Dr. Newman.
Despite the problem with placebo, the results were encouraging. “Those patients who had both eyes injected with the drug did better than in those who had one eye injected with drug and one eye injected with placebo, suggesting some sort of dose effect. There were no adverse events other than what we would expect from injecting [into] eyes. Those treated with the drug had more ocular inflammation, as would also be expected, but all were easily treated with topical medications,” said Dr. Newman.
What are the long-term effects?
Natalia Rost, MD, who chairs the AAN Science Committee, commented after the presentation: “We’re quite impressed with advances in gene therapy. The question is, are there early indications that this improvement in vision will have a lasting effect?”
Dr. Newman responded that ongoing data from earlier studies are also encouraging regarding the long-term effect of the treatment. At 4 years, there was a difference of 16.5 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters equivalent between treated patients and natural history controls (P < .01), “which [does] suggest that this effect is maintained,” said Dr. Newman, who is a professor of ophthalmology and neurology at Emory University, Atlanta.
Dr. Rost also wondered if it would be possible to capture patients earlier in their disease process, in the hopes of countering degeneration before it becomes severe enough to impact vision. Dr. Newman answered by noting another surprise from the research. Previous studies had shown that intervention while only a single eye is affected had little impact on spread of the condition to the second eye, “which was very disappointing,” said Dr. Newman. When they stratified patients by time since vision loss, they found that those who received the therapy 6 months or later after vision loss had better responses than those who were treated earlier.
The mechanism of this counter-intuitive finding remains uncertain, “but we do know that acutely in this disease when people are just starting to lose this vision, during the first couple of months, they get swelling of the axons from these retinal ganglion cells. Our hypothesis is that swelling may actually act as a barrier for the drug to get into the retinal ganglion cell bodies themselves and be transfected. So it turns out that earlier may not be better,” said Dr. Newman.
The study included patients at 13 sites worldwide; 48 were treated bilaterally and 50 treated unilaterally. Just under 80% were male, the mean age was 31.5 years, and the mean duration of vision loss was 8.30 months.
After 1.5 years, the improvement in best-corrected visual acuity between second-affected eyes was stronger in the treatment eye, equivalent to +3 ETDRS letters. The first-affected eye improved by 19 ETDRS letters, and the second-affected eye improved by 16 (P < .0001). Improvement in placebo eyes was +13 ETDRS letters (P < .0001).
Dr. Rost has served on a scientific advisory board or data monitoring board for Omniox. Dr. Newman has consulted for GenSight, Santhera/Chiesi, and Neurophoenix, and has received research support from GenSight and Santhera/Chiesi.
SEATTLE – The therapy, delivered by intravitreal injection, uses an adeno-associated virus vector to deliver a corrected copy of the mutated ND4 mitochondrial gene.
LHON is a rare, maternally inherited mitochondrial mutation that can cause blindness, most commonly in young men, though it does not happen in all individuals with the mutation. The condition often starts with blindness in one eye, accompanied or followed shortly by blindness in the second eye. Researchers believe that the injected viral vector gets taken up retinal ganglion cells, where the mutated gene interferes with vision. Once synthesized, a mitochondria-targeting sequence facilitates transport of the protein to the mitochondria.
The study protocol called for injection of the therapy into one eye and a placebo into the other, using the patient as his or own placebo control. The results in the treated eye were encouraging, though modest. “This is not hitting it out of the ballpark. But for people whose vision is devastated by this disease, it certainly is a first step,” said Nancy J. Newman, MD, during a press conference held March 29 in advance of the 2022 annual meeting of the American Academy of Neurology.
Dr. Newman also noted a surprise finding: Visual improvement also occurred in the placebo-control eye. This was noted in previous studies, called RESCUE and REVERSE, and follow-up studies in monkeys found viral vector in the unaffected eye 3-6 months after an injection. “This would imply some kind of transport within retrograde up the opposite optic nerve after crossing in the chiasm to the eye, but this is going to take a fair bit of work to know exactly how that happens,” said Dr. Newman
Unfortunately, the phase 3 REFLECT study was designed before that process was understood. “This was not a case-control study by person, it was by eye. And that was a mistake, because it turns out there is a does appear to be second eye effects. We do not have naive controls here that did not receive any injection at all in any eye. That’s something that we will [do going] forward,” said Dr. Newman.
Despite the problem with placebo, the results were encouraging. “Those patients who had both eyes injected with the drug did better than in those who had one eye injected with drug and one eye injected with placebo, suggesting some sort of dose effect. There were no adverse events other than what we would expect from injecting [into] eyes. Those treated with the drug had more ocular inflammation, as would also be expected, but all were easily treated with topical medications,” said Dr. Newman.
What are the long-term effects?
Natalia Rost, MD, who chairs the AAN Science Committee, commented after the presentation: “We’re quite impressed with advances in gene therapy. The question is, are there early indications that this improvement in vision will have a lasting effect?”
Dr. Newman responded that ongoing data from earlier studies are also encouraging regarding the long-term effect of the treatment. At 4 years, there was a difference of 16.5 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters equivalent between treated patients and natural history controls (P < .01), “which [does] suggest that this effect is maintained,” said Dr. Newman, who is a professor of ophthalmology and neurology at Emory University, Atlanta.
Dr. Rost also wondered if it would be possible to capture patients earlier in their disease process, in the hopes of countering degeneration before it becomes severe enough to impact vision. Dr. Newman answered by noting another surprise from the research. Previous studies had shown that intervention while only a single eye is affected had little impact on spread of the condition to the second eye, “which was very disappointing,” said Dr. Newman. When they stratified patients by time since vision loss, they found that those who received the therapy 6 months or later after vision loss had better responses than those who were treated earlier.
The mechanism of this counter-intuitive finding remains uncertain, “but we do know that acutely in this disease when people are just starting to lose this vision, during the first couple of months, they get swelling of the axons from these retinal ganglion cells. Our hypothesis is that swelling may actually act as a barrier for the drug to get into the retinal ganglion cell bodies themselves and be transfected. So it turns out that earlier may not be better,” said Dr. Newman.
The study included patients at 13 sites worldwide; 48 were treated bilaterally and 50 treated unilaterally. Just under 80% were male, the mean age was 31.5 years, and the mean duration of vision loss was 8.30 months.
After 1.5 years, the improvement in best-corrected visual acuity between second-affected eyes was stronger in the treatment eye, equivalent to +3 ETDRS letters. The first-affected eye improved by 19 ETDRS letters, and the second-affected eye improved by 16 (P < .0001). Improvement in placebo eyes was +13 ETDRS letters (P < .0001).
Dr. Rost has served on a scientific advisory board or data monitoring board for Omniox. Dr. Newman has consulted for GenSight, Santhera/Chiesi, and Neurophoenix, and has received research support from GenSight and Santhera/Chiesi.
AT AAN 2022