User login
Compulsively checking social media linked with altered brain patterns in teens
Teens who compulsively checked social media networks showed different development patterns in parts of the brain that involve reward and punishment than did those who didn’t check their platforms as often, new research suggests.
Results were published online in JAMA Pediatrics.
Researchers, led by Maria T. Maza, of the department of psychology and neuroscience at University of North Carolina at Chapel Hill, included 169 6th- and 7th-grade students recruited from three public middle schools in rural North Carolina in a 3-year longitudinal cohort.
Participants reported how frequently they checked Facebook, Instagram, and Snapchat. Answers were grouped into eight score groups depending on their per-day check times: less than 1; 1; 2-3; 4-5; 6-10; 11-15; 16-20; or more than 20 times. Those groups were then broken into three categories: low (nonhabitual); moderate; and high (habitual).
Imaging shows reactions
Researchers used functional magnetic resonance imaging (fMRI) to see how different areas of the brain react when participants looked at a series of indicators, such as happy and angry faces, which mimic social media rewards, punishments, or neutral feedback.
The research team focused on adolescents, for whom social media participation and neural sensitivity to social feedback from peers are high.
They found that participants who frequently checked social media showed distinct brain patterns when anticipating social feedback compared with those who had moderate or low use, “suggesting that habitual social media checking early in adolescence is associated with divergent brain development over time.”
The affected regions of the brain included the networks that respond to motivation and cognitive control.
However, the study was not able to determine whether the differences are a good or bad thing.
“While for some individuals with habitual checking behaviors, an initial hyposensitivity to potential social rewards and punishments followed by hypersensitivity may contribute to checking behaviors on social media becoming compulsive and problematic, for others, this change in sensitivity may reflect an adaptive behavior that allows them to better navigate their increasingly digital environment,” the authors wrote.
Chicken-and-egg questions
David Rettew, MD, a child and adolescent psychiatrist at the Oregon Health & Science University in Portland, who was not part of this research, said in an interview that it’s not clear from this study which came first – different brain development in the teens prior to this study that caused compulsive checking, or checking behaviors that caused different brain development. The authors acknowledge this is a limitation of the study.
“Hopefully, someday researchers will look at some of these brain activation patterns before kids have been exposed to social media to help us sort some of these questions out,” Dr. Rettew said.
“It wasn’t as though the groups looked the same at baseline and then diverged as they used more and more social media,” Dr. Rettew said. “It looked like there were some baseline differences that could be traced back maybe years before the study even started.”
People hear “divergent brain development” associated with social media and naturally get alarmed, he acknowledged.
“I get that, but the study isn’t really equipped to tell us what should be happening in the brain and what changes may have implications for other parts of an adolescent’s life,” Dr. Rettew said, “In the end, what we have is an association between heavy social media use and certain brain activation patterns which is cool to see and measure.”
He agrees with the authors, however, that overuse of social media is concerning and studying its effects is important.
Seventy-eight percent of early adolescents check every hour
According to the paper, 78% of 13- to 17-year-olds report checking their devices at least every hour and 46% check “almost constantly.”
“Regardless of which brain regions light up when looking at various emoji responses to their Instagram post, I think it is valid already to have some concerns about youth who can’t stay off their phone for more than 10 minutes,” Dr. Rettew said. “Technology is here to stay, but how we can learn to use it rather than have it use us is probably the more pressing question at this point.”
One coauthor reports grants from the National Institute on Drug Abuse (NIDA) during the conduct of the study and grants from NIDA and the National Science Foundation outside the submitted work; a coauthor reports grants from the Winston Family Foundation; and a coauthor reports a grant from NIDA and funds from the Winston Family Foundation – both during the conduct of the study. No other disclosures were reported. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Teens who compulsively checked social media networks showed different development patterns in parts of the brain that involve reward and punishment than did those who didn’t check their platforms as often, new research suggests.
Results were published online in JAMA Pediatrics.
Researchers, led by Maria T. Maza, of the department of psychology and neuroscience at University of North Carolina at Chapel Hill, included 169 6th- and 7th-grade students recruited from three public middle schools in rural North Carolina in a 3-year longitudinal cohort.
Participants reported how frequently they checked Facebook, Instagram, and Snapchat. Answers were grouped into eight score groups depending on their per-day check times: less than 1; 1; 2-3; 4-5; 6-10; 11-15; 16-20; or more than 20 times. Those groups were then broken into three categories: low (nonhabitual); moderate; and high (habitual).
Imaging shows reactions
Researchers used functional magnetic resonance imaging (fMRI) to see how different areas of the brain react when participants looked at a series of indicators, such as happy and angry faces, which mimic social media rewards, punishments, or neutral feedback.
The research team focused on adolescents, for whom social media participation and neural sensitivity to social feedback from peers are high.
They found that participants who frequently checked social media showed distinct brain patterns when anticipating social feedback compared with those who had moderate or low use, “suggesting that habitual social media checking early in adolescence is associated with divergent brain development over time.”
The affected regions of the brain included the networks that respond to motivation and cognitive control.
However, the study was not able to determine whether the differences are a good or bad thing.
“While for some individuals with habitual checking behaviors, an initial hyposensitivity to potential social rewards and punishments followed by hypersensitivity may contribute to checking behaviors on social media becoming compulsive and problematic, for others, this change in sensitivity may reflect an adaptive behavior that allows them to better navigate their increasingly digital environment,” the authors wrote.
Chicken-and-egg questions
David Rettew, MD, a child and adolescent psychiatrist at the Oregon Health & Science University in Portland, who was not part of this research, said in an interview that it’s not clear from this study which came first – different brain development in the teens prior to this study that caused compulsive checking, or checking behaviors that caused different brain development. The authors acknowledge this is a limitation of the study.
“Hopefully, someday researchers will look at some of these brain activation patterns before kids have been exposed to social media to help us sort some of these questions out,” Dr. Rettew said.
“It wasn’t as though the groups looked the same at baseline and then diverged as they used more and more social media,” Dr. Rettew said. “It looked like there were some baseline differences that could be traced back maybe years before the study even started.”
People hear “divergent brain development” associated with social media and naturally get alarmed, he acknowledged.
“I get that, but the study isn’t really equipped to tell us what should be happening in the brain and what changes may have implications for other parts of an adolescent’s life,” Dr. Rettew said, “In the end, what we have is an association between heavy social media use and certain brain activation patterns which is cool to see and measure.”
He agrees with the authors, however, that overuse of social media is concerning and studying its effects is important.
Seventy-eight percent of early adolescents check every hour
According to the paper, 78% of 13- to 17-year-olds report checking their devices at least every hour and 46% check “almost constantly.”
“Regardless of which brain regions light up when looking at various emoji responses to their Instagram post, I think it is valid already to have some concerns about youth who can’t stay off their phone for more than 10 minutes,” Dr. Rettew said. “Technology is here to stay, but how we can learn to use it rather than have it use us is probably the more pressing question at this point.”
One coauthor reports grants from the National Institute on Drug Abuse (NIDA) during the conduct of the study and grants from NIDA and the National Science Foundation outside the submitted work; a coauthor reports grants from the Winston Family Foundation; and a coauthor reports a grant from NIDA and funds from the Winston Family Foundation – both during the conduct of the study. No other disclosures were reported. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Teens who compulsively checked social media networks showed different development patterns in parts of the brain that involve reward and punishment than did those who didn’t check their platforms as often, new research suggests.
Results were published online in JAMA Pediatrics.
Researchers, led by Maria T. Maza, of the department of psychology and neuroscience at University of North Carolina at Chapel Hill, included 169 6th- and 7th-grade students recruited from three public middle schools in rural North Carolina in a 3-year longitudinal cohort.
Participants reported how frequently they checked Facebook, Instagram, and Snapchat. Answers were grouped into eight score groups depending on their per-day check times: less than 1; 1; 2-3; 4-5; 6-10; 11-15; 16-20; or more than 20 times. Those groups were then broken into three categories: low (nonhabitual); moderate; and high (habitual).
Imaging shows reactions
Researchers used functional magnetic resonance imaging (fMRI) to see how different areas of the brain react when participants looked at a series of indicators, such as happy and angry faces, which mimic social media rewards, punishments, or neutral feedback.
The research team focused on adolescents, for whom social media participation and neural sensitivity to social feedback from peers are high.
They found that participants who frequently checked social media showed distinct brain patterns when anticipating social feedback compared with those who had moderate or low use, “suggesting that habitual social media checking early in adolescence is associated with divergent brain development over time.”
The affected regions of the brain included the networks that respond to motivation and cognitive control.
However, the study was not able to determine whether the differences are a good or bad thing.
“While for some individuals with habitual checking behaviors, an initial hyposensitivity to potential social rewards and punishments followed by hypersensitivity may contribute to checking behaviors on social media becoming compulsive and problematic, for others, this change in sensitivity may reflect an adaptive behavior that allows them to better navigate their increasingly digital environment,” the authors wrote.
Chicken-and-egg questions
David Rettew, MD, a child and adolescent psychiatrist at the Oregon Health & Science University in Portland, who was not part of this research, said in an interview that it’s not clear from this study which came first – different brain development in the teens prior to this study that caused compulsive checking, or checking behaviors that caused different brain development. The authors acknowledge this is a limitation of the study.
“Hopefully, someday researchers will look at some of these brain activation patterns before kids have been exposed to social media to help us sort some of these questions out,” Dr. Rettew said.
“It wasn’t as though the groups looked the same at baseline and then diverged as they used more and more social media,” Dr. Rettew said. “It looked like there were some baseline differences that could be traced back maybe years before the study even started.”
People hear “divergent brain development” associated with social media and naturally get alarmed, he acknowledged.
“I get that, but the study isn’t really equipped to tell us what should be happening in the brain and what changes may have implications for other parts of an adolescent’s life,” Dr. Rettew said, “In the end, what we have is an association between heavy social media use and certain brain activation patterns which is cool to see and measure.”
He agrees with the authors, however, that overuse of social media is concerning and studying its effects is important.
Seventy-eight percent of early adolescents check every hour
According to the paper, 78% of 13- to 17-year-olds report checking their devices at least every hour and 46% check “almost constantly.”
“Regardless of which brain regions light up when looking at various emoji responses to their Instagram post, I think it is valid already to have some concerns about youth who can’t stay off their phone for more than 10 minutes,” Dr. Rettew said. “Technology is here to stay, but how we can learn to use it rather than have it use us is probably the more pressing question at this point.”
One coauthor reports grants from the National Institute on Drug Abuse (NIDA) during the conduct of the study and grants from NIDA and the National Science Foundation outside the submitted work; a coauthor reports grants from the Winston Family Foundation; and a coauthor reports a grant from NIDA and funds from the Winston Family Foundation – both during the conduct of the study. No other disclosures were reported. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
FROM JAMA PEDIATRICS
The anecdote as antidote: Psychiatric paradigms in Disney films
A common refrain in psychiatry is that the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision, (DSM-5-TR), published in 2022, is the best we can do.
Since the DSM-III was released in 1980, the American Psychiatric Association, which publishes the manual, has espoused the position that we should list symptoms, in a manner that is reminiscent of a checklist. For example, having a depressed mood on most days for a 2-week period, or a loss of interest in pleasurable things, as well as 4 additional symptoms – among them changes in appetite, changes in sleep, changes in psychomotor activity, fatigue, worthlessness, poor concentration, or thoughts of death – can lead to a diagnosis of a major depressive episode as part of a major depressive disorder.
Criticisms of this approach can be apparent. Patients subjected to such checklists, including being repeatedly asked to complete the Patient Health Questionnaire 9 (PHQ-9), which closely follows those criteria, can feel lost and even alienated by their providers. After all, one can ask all those questions and make a diagnosis of depression without even knowing about the patient’s stressors, their history, or their social context.
The DSM permits the diagnosis of psychiatric disorders without an understanding of the narrative of the patient. In its defense, the DSM is not a textbook of psychiatry, it is a guide on how to diagnose individuals. The DSM does not demand that psychiatrists only ask about the symptoms on the checklists; it is the providers who can choose to dismiss asking about the important facets of one’s life.
Yet every time we attend a lecture that starts by enumerating the DSM symptoms of the disorder being discussed, we are left with the dissatisfying impression that a specialist of this disorder should have a more nuanced and interesting description of their disorder of study. This feeling of discontent is compounded when we see a movie that encompasses so much of what is missing in today’s psychiatric parlance, and even more so if that movie is ostensibly made for children. Movies, by design, are particularly adept at encapsulating the narrative of someone’s life in a way that psychiatry can learn from.
Other than the embarrassment of not knowing a patient outside the checklist, the importance of narrative cannot be understated. Dr. Erik Erikson rightfully suggested that the point of life is “the acceptance of one’s one and only life cycle”1 or rather to know it was okay to have been oneself without additions or substitutions. Therefore, one must know what it has meant to be themselves to reconcile this question and achieve Ego Integrity rather than disgust and despair. Narrative is the way in which we understand who we are and what it has meant to be ourselves. An understanding of our personal narrative presents a unique opportunity in expressing what is missing in the DSM. Below, we provide two of our favorite examples in Disney films, among many.
‘Ratatouille’ (2007)
One of the missing features of the DSM is its inability to explain to patients the intrapsychic processes that guide us. One of these processes is how our values can lead us to a deep sense of guilt, shame, and the resulting feelings of alienation. It is extremely common for patients to enter our clinical practice feeling shackled by beliefs that they should accomplish more and be more than they are.
The animated film “Ratatouille” does an excellent job at addressing this feeling. The film follows Remy, the protagonist rat, and his adventures as he explores his passion for cooking. Remy teams up with the inept but good-natured human Alfredo Linguini and guides him through cooking while hiding under his chef’s hat. The primary antagonist, Anton Ego, is a particularly harsh food critic. His presence and appearance are somber. He exudes disdain. His trim physique and scarf suggest a man that will break and react to anything, and his skull-shaped typewriter in his coffin-shaped office informs the viewer that he is out to kill with his cruel words. Anton Ego serves as our projected super-ego. He is not an external judge but the judgment deep inside ourselves, goading us to be better with such severity that we are ultimately left feeling condemned.
Remy is the younger of two siblings. He is less physically adept but more intellectual than his older brother, who does not understand why Remy isn’t content eating scraps from the garbage like the rest of their rat clan. Remy is the creative part within us that wants to challenge the status quo and try something new. Remy also represents our shame and guilt for leaving our home. On one hand, we want to dare greatly, in this case at being an extraordinary chef, but on the other we are shy and cook in secret, hiding within the hat of another person. Remy struggles with the deep feeling that we do not deserve our success, that our family will leave us for being who we are, and that we are better off isolating and segregating from our challenges.
The movie concludes that through talent and hard work, our critics will accept us. Furthermore, once accepted for what we do, we can be further accepted for who we are. The movie ends with Remy cooking the eponymous dish ratatouille. He prepares it so remarkably well, the dish transports Anton Ego back to a sublime experience of eating ratatouille as a child, a touching moment which not only underscores food’s evocative link to memory but gives a glimpse at Anton Ego’s own narrative.
Ego is first won over by the dish, and only afterward learns of Remy’s true identity. Remy’s talent is undeniable though, and even the stuffy Ego must accept the film’s theme that “Anyone can cook,” even a rat – the rat that we all sometimes feel we are deep inside, rotten to the core but trying so hard to be accepted by others, and ultimately by ourselves. In the end, we overcome the disgust inherent in the imagery of a rat in a kitchen and instead embrace our hero’s achievement of ego integrity as he combines his identities as a member of a clan of rats, and one of Paris’s finest chefs.
While modern psychiatry can favor looking at people through the lens of biology rather than narrative, “Ratatouille” can serve as a reminder of the powerful unconscious forces that guide our lives. “Ratatouille” is not a successful movie only because of the compelling narrative, but also because the narrative matches the important psychic paradigms that psychiatry once embraced.
‘Inside Out’ (2015)
Another missing feature of the DSM is its inability to explain how symptoms feel and manifest psychologically. One such feeling is that of control – whether one is in control of one’s life, feelings, and action or rather a victim of external forces. It is extremely common for patients to enter our clinical practice feeling traumatized by the life they’ve lived and powerless to produce any change. Part of our role is to guide them through this journey from the object of their lives to the subject of their lives.
In the animated feature “Inside Out,” Riley, a preteen girl, goes through the tribulation of growing up and learning about herself. This seemingly happy child, content playing hockey with her best friend, Meg, on the picturesque frozen lakes of Minnesota, reaches her inevitable conflict. Her parents uproot her life, moving the family to San Francisco. By doing so, they disconnect her from her school, her friends, and her hobbies. While all this is happening, we spend time inside Riley’s psyche with the personified characters of Riley’s emotions as they affect her decisions and daily actions amidst the backdrop of her core memories and islands of personality.
During the move, her parents seemingly change and ultimately destroy every facet of Riley’s sense of self, which is animated as the collapse of her personality islands. Her best friend engages Riley in a video call just to inform her that she has a new friend who plays hockey equally well. Her parents do not hear Riley’s concerns and are portrayed as distracted by their adult problems. Riley feels ridiculed in her new school and unable to share her feelings with her parents, who ask her to still be their “happy girl” and indirectly ask her to fake pleasure to alleviate their own anxiety.
The climax of the movie is when Riley decides to run away from San Francisco and her parents, to return to her perceived true home, Minnesota. The climax is resolved when Riley realizes that her parents’ love, representing the connection we have to others, transcends her need for control. To some degree, we are all powerless in the face of the tremendous forces of life and share the difficult task of accepting the cards we were dealt, thus making the story of Riley so compelling.
Additionally, the climax is further resolved by another argument that psychiatry (and the DSM) should consider embracing. Emotions are not all symptoms and living without negative emotion is not the goal of life. Riley grows from preteen to teenager, and from object to subject of her life, by realizing that her symptoms/feelings are not just nuisances to avoid and hide, but the key to meaning. Our anger drives us to try hard. Our fear protects us from harm. Our sadness attracts the warmth and care of others. Our disgust protects us physically from noxious material (symbolized as a dreaded broccoli floret for preteen Riley) and socially by encouraging us to share societal norms. Similarly, patients and people in general would benefit by being taught that, while symptoms may permit the better assessment of psychiatric conditions using the DSM, life is much more than that.
It is unfair to blame the DSM for things it was not designed to do. The DSM doesn’t advertise itself as a guidebook of all behaviors, at all times. However, for a variety of reasons, it has become the main way psychiatry describes people. While we commend the APA for its effort and do not know that we could make it any better, we are frequently happily reminded that in about 90 minutes, filmmakers are able to display an empathic understanding of personal narratives that biologic psychiatry can miss.
Dr. Pulido is a psychiatry resident at the University of California, San Diego. She is interested in women’s mental health, medical education, and outpatient psychiatry. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Erikson, EH. Childhood and society (New York: WW Norton, 1950).
A common refrain in psychiatry is that the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision, (DSM-5-TR), published in 2022, is the best we can do.
Since the DSM-III was released in 1980, the American Psychiatric Association, which publishes the manual, has espoused the position that we should list symptoms, in a manner that is reminiscent of a checklist. For example, having a depressed mood on most days for a 2-week period, or a loss of interest in pleasurable things, as well as 4 additional symptoms – among them changes in appetite, changes in sleep, changes in psychomotor activity, fatigue, worthlessness, poor concentration, or thoughts of death – can lead to a diagnosis of a major depressive episode as part of a major depressive disorder.
Criticisms of this approach can be apparent. Patients subjected to such checklists, including being repeatedly asked to complete the Patient Health Questionnaire 9 (PHQ-9), which closely follows those criteria, can feel lost and even alienated by their providers. After all, one can ask all those questions and make a diagnosis of depression without even knowing about the patient’s stressors, their history, or their social context.
The DSM permits the diagnosis of psychiatric disorders without an understanding of the narrative of the patient. In its defense, the DSM is not a textbook of psychiatry, it is a guide on how to diagnose individuals. The DSM does not demand that psychiatrists only ask about the symptoms on the checklists; it is the providers who can choose to dismiss asking about the important facets of one’s life.
Yet every time we attend a lecture that starts by enumerating the DSM symptoms of the disorder being discussed, we are left with the dissatisfying impression that a specialist of this disorder should have a more nuanced and interesting description of their disorder of study. This feeling of discontent is compounded when we see a movie that encompasses so much of what is missing in today’s psychiatric parlance, and even more so if that movie is ostensibly made for children. Movies, by design, are particularly adept at encapsulating the narrative of someone’s life in a way that psychiatry can learn from.
Other than the embarrassment of not knowing a patient outside the checklist, the importance of narrative cannot be understated. Dr. Erik Erikson rightfully suggested that the point of life is “the acceptance of one’s one and only life cycle”1 or rather to know it was okay to have been oneself without additions or substitutions. Therefore, one must know what it has meant to be themselves to reconcile this question and achieve Ego Integrity rather than disgust and despair. Narrative is the way in which we understand who we are and what it has meant to be ourselves. An understanding of our personal narrative presents a unique opportunity in expressing what is missing in the DSM. Below, we provide two of our favorite examples in Disney films, among many.
‘Ratatouille’ (2007)
One of the missing features of the DSM is its inability to explain to patients the intrapsychic processes that guide us. One of these processes is how our values can lead us to a deep sense of guilt, shame, and the resulting feelings of alienation. It is extremely common for patients to enter our clinical practice feeling shackled by beliefs that they should accomplish more and be more than they are.
The animated film “Ratatouille” does an excellent job at addressing this feeling. The film follows Remy, the protagonist rat, and his adventures as he explores his passion for cooking. Remy teams up with the inept but good-natured human Alfredo Linguini and guides him through cooking while hiding under his chef’s hat. The primary antagonist, Anton Ego, is a particularly harsh food critic. His presence and appearance are somber. He exudes disdain. His trim physique and scarf suggest a man that will break and react to anything, and his skull-shaped typewriter in his coffin-shaped office informs the viewer that he is out to kill with his cruel words. Anton Ego serves as our projected super-ego. He is not an external judge but the judgment deep inside ourselves, goading us to be better with such severity that we are ultimately left feeling condemned.
Remy is the younger of two siblings. He is less physically adept but more intellectual than his older brother, who does not understand why Remy isn’t content eating scraps from the garbage like the rest of their rat clan. Remy is the creative part within us that wants to challenge the status quo and try something new. Remy also represents our shame and guilt for leaving our home. On one hand, we want to dare greatly, in this case at being an extraordinary chef, but on the other we are shy and cook in secret, hiding within the hat of another person. Remy struggles with the deep feeling that we do not deserve our success, that our family will leave us for being who we are, and that we are better off isolating and segregating from our challenges.
The movie concludes that through talent and hard work, our critics will accept us. Furthermore, once accepted for what we do, we can be further accepted for who we are. The movie ends with Remy cooking the eponymous dish ratatouille. He prepares it so remarkably well, the dish transports Anton Ego back to a sublime experience of eating ratatouille as a child, a touching moment which not only underscores food’s evocative link to memory but gives a glimpse at Anton Ego’s own narrative.
Ego is first won over by the dish, and only afterward learns of Remy’s true identity. Remy’s talent is undeniable though, and even the stuffy Ego must accept the film’s theme that “Anyone can cook,” even a rat – the rat that we all sometimes feel we are deep inside, rotten to the core but trying so hard to be accepted by others, and ultimately by ourselves. In the end, we overcome the disgust inherent in the imagery of a rat in a kitchen and instead embrace our hero’s achievement of ego integrity as he combines his identities as a member of a clan of rats, and one of Paris’s finest chefs.
While modern psychiatry can favor looking at people through the lens of biology rather than narrative, “Ratatouille” can serve as a reminder of the powerful unconscious forces that guide our lives. “Ratatouille” is not a successful movie only because of the compelling narrative, but also because the narrative matches the important psychic paradigms that psychiatry once embraced.
‘Inside Out’ (2015)
Another missing feature of the DSM is its inability to explain how symptoms feel and manifest psychologically. One such feeling is that of control – whether one is in control of one’s life, feelings, and action or rather a victim of external forces. It is extremely common for patients to enter our clinical practice feeling traumatized by the life they’ve lived and powerless to produce any change. Part of our role is to guide them through this journey from the object of their lives to the subject of their lives.
In the animated feature “Inside Out,” Riley, a preteen girl, goes through the tribulation of growing up and learning about herself. This seemingly happy child, content playing hockey with her best friend, Meg, on the picturesque frozen lakes of Minnesota, reaches her inevitable conflict. Her parents uproot her life, moving the family to San Francisco. By doing so, they disconnect her from her school, her friends, and her hobbies. While all this is happening, we spend time inside Riley’s psyche with the personified characters of Riley’s emotions as they affect her decisions and daily actions amidst the backdrop of her core memories and islands of personality.
During the move, her parents seemingly change and ultimately destroy every facet of Riley’s sense of self, which is animated as the collapse of her personality islands. Her best friend engages Riley in a video call just to inform her that she has a new friend who plays hockey equally well. Her parents do not hear Riley’s concerns and are portrayed as distracted by their adult problems. Riley feels ridiculed in her new school and unable to share her feelings with her parents, who ask her to still be their “happy girl” and indirectly ask her to fake pleasure to alleviate their own anxiety.
The climax of the movie is when Riley decides to run away from San Francisco and her parents, to return to her perceived true home, Minnesota. The climax is resolved when Riley realizes that her parents’ love, representing the connection we have to others, transcends her need for control. To some degree, we are all powerless in the face of the tremendous forces of life and share the difficult task of accepting the cards we were dealt, thus making the story of Riley so compelling.
Additionally, the climax is further resolved by another argument that psychiatry (and the DSM) should consider embracing. Emotions are not all symptoms and living without negative emotion is not the goal of life. Riley grows from preteen to teenager, and from object to subject of her life, by realizing that her symptoms/feelings are not just nuisances to avoid and hide, but the key to meaning. Our anger drives us to try hard. Our fear protects us from harm. Our sadness attracts the warmth and care of others. Our disgust protects us physically from noxious material (symbolized as a dreaded broccoli floret for preteen Riley) and socially by encouraging us to share societal norms. Similarly, patients and people in general would benefit by being taught that, while symptoms may permit the better assessment of psychiatric conditions using the DSM, life is much more than that.
It is unfair to blame the DSM for things it was not designed to do. The DSM doesn’t advertise itself as a guidebook of all behaviors, at all times. However, for a variety of reasons, it has become the main way psychiatry describes people. While we commend the APA for its effort and do not know that we could make it any better, we are frequently happily reminded that in about 90 minutes, filmmakers are able to display an empathic understanding of personal narratives that biologic psychiatry can miss.
Dr. Pulido is a psychiatry resident at the University of California, San Diego. She is interested in women’s mental health, medical education, and outpatient psychiatry. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Erikson, EH. Childhood and society (New York: WW Norton, 1950).
A common refrain in psychiatry is that the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision, (DSM-5-TR), published in 2022, is the best we can do.
Since the DSM-III was released in 1980, the American Psychiatric Association, which publishes the manual, has espoused the position that we should list symptoms, in a manner that is reminiscent of a checklist. For example, having a depressed mood on most days for a 2-week period, or a loss of interest in pleasurable things, as well as 4 additional symptoms – among them changes in appetite, changes in sleep, changes in psychomotor activity, fatigue, worthlessness, poor concentration, or thoughts of death – can lead to a diagnosis of a major depressive episode as part of a major depressive disorder.
Criticisms of this approach can be apparent. Patients subjected to such checklists, including being repeatedly asked to complete the Patient Health Questionnaire 9 (PHQ-9), which closely follows those criteria, can feel lost and even alienated by their providers. After all, one can ask all those questions and make a diagnosis of depression without even knowing about the patient’s stressors, their history, or their social context.
The DSM permits the diagnosis of psychiatric disorders without an understanding of the narrative of the patient. In its defense, the DSM is not a textbook of psychiatry, it is a guide on how to diagnose individuals. The DSM does not demand that psychiatrists only ask about the symptoms on the checklists; it is the providers who can choose to dismiss asking about the important facets of one’s life.
Yet every time we attend a lecture that starts by enumerating the DSM symptoms of the disorder being discussed, we are left with the dissatisfying impression that a specialist of this disorder should have a more nuanced and interesting description of their disorder of study. This feeling of discontent is compounded when we see a movie that encompasses so much of what is missing in today’s psychiatric parlance, and even more so if that movie is ostensibly made for children. Movies, by design, are particularly adept at encapsulating the narrative of someone’s life in a way that psychiatry can learn from.
Other than the embarrassment of not knowing a patient outside the checklist, the importance of narrative cannot be understated. Dr. Erik Erikson rightfully suggested that the point of life is “the acceptance of one’s one and only life cycle”1 or rather to know it was okay to have been oneself without additions or substitutions. Therefore, one must know what it has meant to be themselves to reconcile this question and achieve Ego Integrity rather than disgust and despair. Narrative is the way in which we understand who we are and what it has meant to be ourselves. An understanding of our personal narrative presents a unique opportunity in expressing what is missing in the DSM. Below, we provide two of our favorite examples in Disney films, among many.
‘Ratatouille’ (2007)
One of the missing features of the DSM is its inability to explain to patients the intrapsychic processes that guide us. One of these processes is how our values can lead us to a deep sense of guilt, shame, and the resulting feelings of alienation. It is extremely common for patients to enter our clinical practice feeling shackled by beliefs that they should accomplish more and be more than they are.
The animated film “Ratatouille” does an excellent job at addressing this feeling. The film follows Remy, the protagonist rat, and his adventures as he explores his passion for cooking. Remy teams up with the inept but good-natured human Alfredo Linguini and guides him through cooking while hiding under his chef’s hat. The primary antagonist, Anton Ego, is a particularly harsh food critic. His presence and appearance are somber. He exudes disdain. His trim physique and scarf suggest a man that will break and react to anything, and his skull-shaped typewriter in his coffin-shaped office informs the viewer that he is out to kill with his cruel words. Anton Ego serves as our projected super-ego. He is not an external judge but the judgment deep inside ourselves, goading us to be better with such severity that we are ultimately left feeling condemned.
Remy is the younger of two siblings. He is less physically adept but more intellectual than his older brother, who does not understand why Remy isn’t content eating scraps from the garbage like the rest of their rat clan. Remy is the creative part within us that wants to challenge the status quo and try something new. Remy also represents our shame and guilt for leaving our home. On one hand, we want to dare greatly, in this case at being an extraordinary chef, but on the other we are shy and cook in secret, hiding within the hat of another person. Remy struggles with the deep feeling that we do not deserve our success, that our family will leave us for being who we are, and that we are better off isolating and segregating from our challenges.
The movie concludes that through talent and hard work, our critics will accept us. Furthermore, once accepted for what we do, we can be further accepted for who we are. The movie ends with Remy cooking the eponymous dish ratatouille. He prepares it so remarkably well, the dish transports Anton Ego back to a sublime experience of eating ratatouille as a child, a touching moment which not only underscores food’s evocative link to memory but gives a glimpse at Anton Ego’s own narrative.
Ego is first won over by the dish, and only afterward learns of Remy’s true identity. Remy’s talent is undeniable though, and even the stuffy Ego must accept the film’s theme that “Anyone can cook,” even a rat – the rat that we all sometimes feel we are deep inside, rotten to the core but trying so hard to be accepted by others, and ultimately by ourselves. In the end, we overcome the disgust inherent in the imagery of a rat in a kitchen and instead embrace our hero’s achievement of ego integrity as he combines his identities as a member of a clan of rats, and one of Paris’s finest chefs.
While modern psychiatry can favor looking at people through the lens of biology rather than narrative, “Ratatouille” can serve as a reminder of the powerful unconscious forces that guide our lives. “Ratatouille” is not a successful movie only because of the compelling narrative, but also because the narrative matches the important psychic paradigms that psychiatry once embraced.
‘Inside Out’ (2015)
Another missing feature of the DSM is its inability to explain how symptoms feel and manifest psychologically. One such feeling is that of control – whether one is in control of one’s life, feelings, and action or rather a victim of external forces. It is extremely common for patients to enter our clinical practice feeling traumatized by the life they’ve lived and powerless to produce any change. Part of our role is to guide them through this journey from the object of their lives to the subject of their lives.
In the animated feature “Inside Out,” Riley, a preteen girl, goes through the tribulation of growing up and learning about herself. This seemingly happy child, content playing hockey with her best friend, Meg, on the picturesque frozen lakes of Minnesota, reaches her inevitable conflict. Her parents uproot her life, moving the family to San Francisco. By doing so, they disconnect her from her school, her friends, and her hobbies. While all this is happening, we spend time inside Riley’s psyche with the personified characters of Riley’s emotions as they affect her decisions and daily actions amidst the backdrop of her core memories and islands of personality.
During the move, her parents seemingly change and ultimately destroy every facet of Riley’s sense of self, which is animated as the collapse of her personality islands. Her best friend engages Riley in a video call just to inform her that she has a new friend who plays hockey equally well. Her parents do not hear Riley’s concerns and are portrayed as distracted by their adult problems. Riley feels ridiculed in her new school and unable to share her feelings with her parents, who ask her to still be their “happy girl” and indirectly ask her to fake pleasure to alleviate their own anxiety.
The climax of the movie is when Riley decides to run away from San Francisco and her parents, to return to her perceived true home, Minnesota. The climax is resolved when Riley realizes that her parents’ love, representing the connection we have to others, transcends her need for control. To some degree, we are all powerless in the face of the tremendous forces of life and share the difficult task of accepting the cards we were dealt, thus making the story of Riley so compelling.
Additionally, the climax is further resolved by another argument that psychiatry (and the DSM) should consider embracing. Emotions are not all symptoms and living without negative emotion is not the goal of life. Riley grows from preteen to teenager, and from object to subject of her life, by realizing that her symptoms/feelings are not just nuisances to avoid and hide, but the key to meaning. Our anger drives us to try hard. Our fear protects us from harm. Our sadness attracts the warmth and care of others. Our disgust protects us physically from noxious material (symbolized as a dreaded broccoli floret for preteen Riley) and socially by encouraging us to share societal norms. Similarly, patients and people in general would benefit by being taught that, while symptoms may permit the better assessment of psychiatric conditions using the DSM, life is much more than that.
It is unfair to blame the DSM for things it was not designed to do. The DSM doesn’t advertise itself as a guidebook of all behaviors, at all times. However, for a variety of reasons, it has become the main way psychiatry describes people. While we commend the APA for its effort and do not know that we could make it any better, we are frequently happily reminded that in about 90 minutes, filmmakers are able to display an empathic understanding of personal narratives that biologic psychiatry can miss.
Dr. Pulido is a psychiatry resident at the University of California, San Diego. She is interested in women’s mental health, medical education, and outpatient psychiatry. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest.
References
1. Erikson, EH. Childhood and society (New York: WW Norton, 1950).
Children and COVID: New cases fell as the old year ended
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.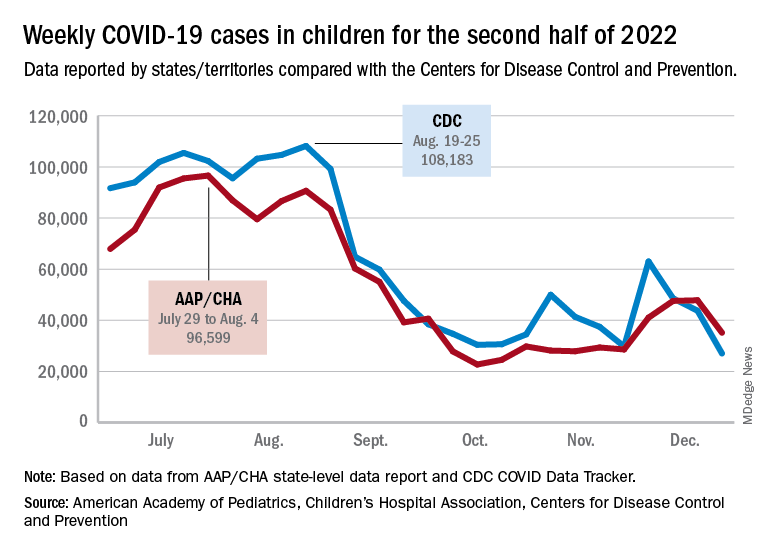
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
Nearly 1,400% rise in young children ingesting cannabis edibles
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
FDA approves Wegovy (semaglutide) for obesity in teens 12 and up
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
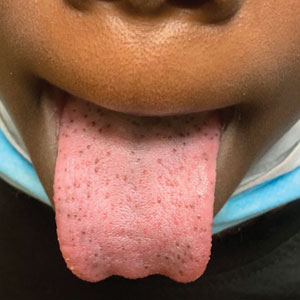
Hyperpigmented Papules on the Tongue of a Child
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
A 9-year-old Black boy presented to the dermatology clinic for evaluation of dark spots on the tongue. The family first noted these spots 5 months prior and reported that they remained stable during that time. The patient’s medical history was notable for autism spectrum disorder and multiple food allergies. His family history was negative for similar oral pigmentation or other pigmentary anomalies. A review of systems was positive only for selective eating and rare nosebleeds. Physical examination revealed numerous dark brown, pinpoint papules across the dorsal aspect of the tongue. No hyperpigmentation of the buccal mucosae, lips, palms, or soles was identified. Several light brown streaks were present on the fingernails and toenails, consistent with longitudinal melanonychia. A prior complete blood cell count was within reference range.
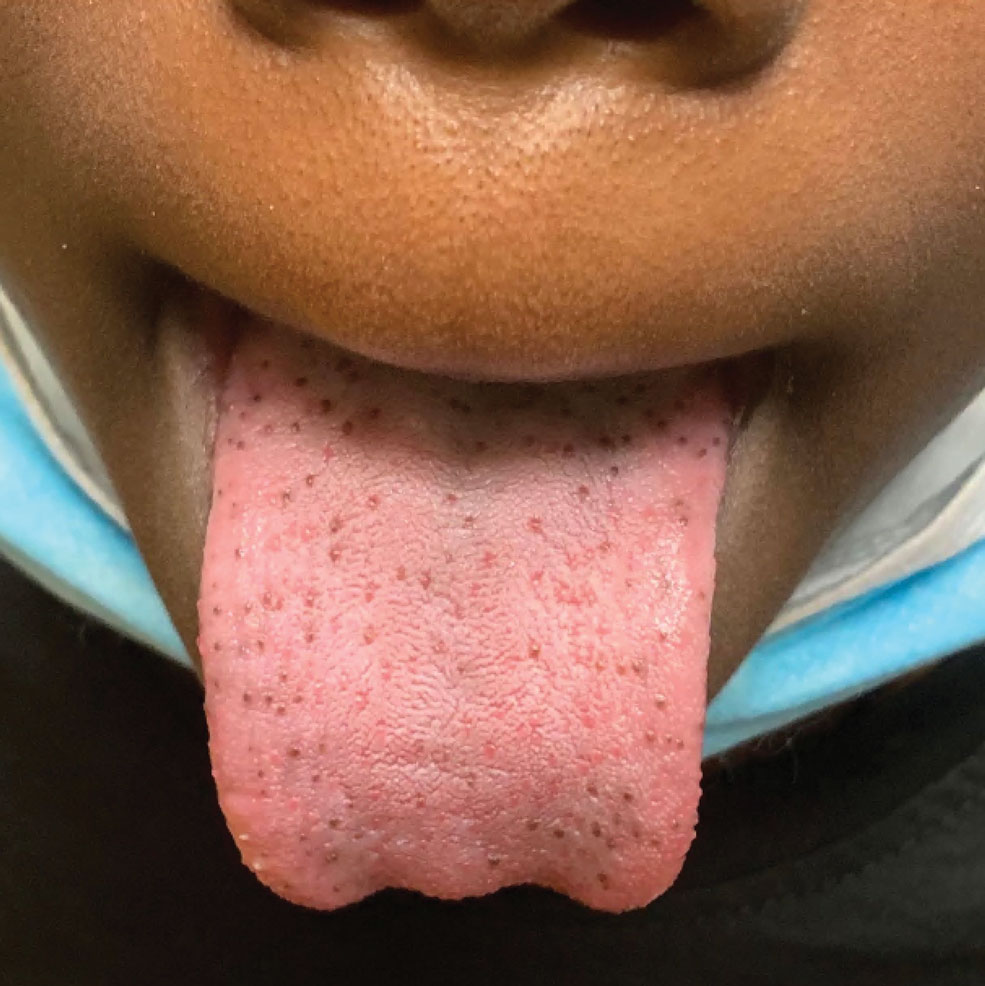
Topical treatment options for acne continue to expand
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
AT MOAS 2022
Double CF combination safe, effective in 1- to 2-year-olds
In children with cystic fibrosis (CF) and homozygous for the F508 mutation, the combination of lumacaftor and ivacaftor appears generally safe, and biomarker data suggest its efficacy, according to results from a new open-label phase 3 trial.
In September, the Food and Drug Administration approved lumacaftor/ivacaftor for patients aged 1 years and above. Waiting in the wings is the triple combination of elexacaftor, tezacaftor, and ivacaftor (ETI), which is available for patients with at least one copy of the F508 mutation aged 6 and over. ETI is also being tested in younger patients.
One driving factor for early treatment is countering the malnutrition that can occur among CF patients because of poor pancreatic insufficiency and chronic inflammation. “We’ve known for many years that (being) at or above average body weight and height predict better lung function. And we’ve known for quite a while that the height-for-age percentile, in preschool years, actually predicts your lung function later, and how long you’re going to live, so nutrition is incredibly important,” said study author Susanna McColley, MD, in an interview. It’s also difficult to use lung function tests in young children, since even adults can find them challenging, she said.
“FEV1 [forced expiratory volume in 1 second] is the strongest predictor of survival, and then nutrition is the highest predictor of FEV1, so that’s kind of the construct. They had similar improvement in the functional measures of their pancreas and in the measures of inflammation in the gut. I think the story here is that starting a modulator early has a likelihood to have positive health effects that go forward. We can’t say that from the data in the paper. It’s a 24-week study, but looking at the pancreatic and intestinal functioning and also the fact that there was a decrease in sweat chloride is important,” said Dr. McColley, who is a professor of pediatrics in pulmonary and sleep medicine at Northwestern University, Chicago.
The study adds more evidence that earlier treatment in CF may lead to better outcomes, but the digestive improvements are an overlooked factor, according to Dr. McColley. “Even with early treatment, and even with pancreatic enzymes and supplements taken to digest food, it’s a huge burden. When they come in and say that digestion seems better, there is less bloating, things like that, these are the things that aren’t captured so much in the clinical trial data, but they’re meaningful to families,” she said.
Single-agent ivacaftor is available for children as young as 4 months, but is limited to patients with the G551D gating mutation. Most young children with CF can only be treated for symptoms.
The lack of new safety signals in the new study is reassuring, and the research presents some hope to young children who are not yet eligible to receive ETI, according to Carlos Milla, MD, who is a pediatric pulmonary physician at Stanford (Calif.) University. “We already know that the next version of this drug is much more efficacious, the triple-combination therapy. It’s a little bit like we’re falling behind when it comes to treating these young kids because we are offering right now what we know is a less effective drug as opposed to the ones that are available now down to age 6, and hopefully sometime soon down to age 2. It’s better [than] to have no treatment at all, so it’s a good start,” said Dr. Milla.
“I think this is a great bridge for babies while they’re waiting to grow up to be old enough to get [triple combination therapy] and will prevent some of the complications until they can get the even more highly effective therapy in the future,” said Jennifer Taylor-Cousar, MD, who was asked to comment on the study. Dr. Taylor-Cousar is codirector of the Adult CF Program at National Jewish Health, Denver.
She also noted that the therapy could rapidly become more important. Since the approval of elexacaftor/tezacaftor/ivacaftor in 2019, pregnancies in women with CF have increased markedly. There were 310 such pregnancies in 2019, and 675 in 2020 after the combination became generally available in November of 2019. Many of the resulting babies had false-negative CF diagnoses because the mother was taking the triple combination and the medication crossed the placenta and prevented disease progression. The drugs are present in breast milk, but when breastfeeding isn’t possible, newborns are left without a therapeutic option. “There was no approval for babies who had two copies of F508. This helps tremendously with that albeit small population, although I suspect it may grow larger over the upcoming years as we continue to see so many pregnancies in women with CF because they are so much healthier,” said Dr. Taylor-Cousar.
The study was a phase 3, open-label trial with a cohort aged 18-24 months (cohort 1, n = 14) and another aged 12-18 months (cohort 2, n = 46). Participants received a 15-day treatment with a dose based on weight at screening. Participants then underwent a 24-week treatment period with a dose determined by pharmacokinetic data collected during the initial treatment, the authors wrote in the American Journal of Respiratory and Critical Care Medicine.
A total of 95.7% of children experienced adverse events during the 24-week treatment period; 52.2% of events were mild, and 39.1% were moderate. The most frequent adverse events were cough (34.8%), infective exacerbation of CF (21.7%), pyrexia (21.7%), and vomiting (17.4%); 10.9% had elevations of alanine aminotransferase and/or aspartate aminotransferase higher than three times the upper limit of normal, and one (2.2%) had concentrations of both high enough that the study drug was discontinued.
There were significant reductions in sweat chloride concentration at week 24, suggesting strong efficacy (–29.1 mmol/L; 95% confidence interval, –34.8 to –23.4 mmol/L). Body mass, weight, and length remained normal during the 24-week treatment period, and there were trends towards improvement in biomarkers of pancreatic function and intestinal inflammation, including fecal elastase-1 (+73.1mcg/g; 95% CI, 29.40-116.80 mcg/g), serum immunoreactive trypsinogen (–295.50 mcg/g; 95% CI, –416.60 to –174.50 mcg/g), and fecal calprotectin (–106.63 mg/kg; 95% CI, –180.60 to –32.66 mg/kg)
Dr. McColley, Dr. Taylor-Cousar, and Dr. Milla have no relevant financial disclosures. The study was funded by Merck.
In children with cystic fibrosis (CF) and homozygous for the F508 mutation, the combination of lumacaftor and ivacaftor appears generally safe, and biomarker data suggest its efficacy, according to results from a new open-label phase 3 trial.
In September, the Food and Drug Administration approved lumacaftor/ivacaftor for patients aged 1 years and above. Waiting in the wings is the triple combination of elexacaftor, tezacaftor, and ivacaftor (ETI), which is available for patients with at least one copy of the F508 mutation aged 6 and over. ETI is also being tested in younger patients.
One driving factor for early treatment is countering the malnutrition that can occur among CF patients because of poor pancreatic insufficiency and chronic inflammation. “We’ve known for many years that (being) at or above average body weight and height predict better lung function. And we’ve known for quite a while that the height-for-age percentile, in preschool years, actually predicts your lung function later, and how long you’re going to live, so nutrition is incredibly important,” said study author Susanna McColley, MD, in an interview. It’s also difficult to use lung function tests in young children, since even adults can find them challenging, she said.
“FEV1 [forced expiratory volume in 1 second] is the strongest predictor of survival, and then nutrition is the highest predictor of FEV1, so that’s kind of the construct. They had similar improvement in the functional measures of their pancreas and in the measures of inflammation in the gut. I think the story here is that starting a modulator early has a likelihood to have positive health effects that go forward. We can’t say that from the data in the paper. It’s a 24-week study, but looking at the pancreatic and intestinal functioning and also the fact that there was a decrease in sweat chloride is important,” said Dr. McColley, who is a professor of pediatrics in pulmonary and sleep medicine at Northwestern University, Chicago.
The study adds more evidence that earlier treatment in CF may lead to better outcomes, but the digestive improvements are an overlooked factor, according to Dr. McColley. “Even with early treatment, and even with pancreatic enzymes and supplements taken to digest food, it’s a huge burden. When they come in and say that digestion seems better, there is less bloating, things like that, these are the things that aren’t captured so much in the clinical trial data, but they’re meaningful to families,” she said.
Single-agent ivacaftor is available for children as young as 4 months, but is limited to patients with the G551D gating mutation. Most young children with CF can only be treated for symptoms.
The lack of new safety signals in the new study is reassuring, and the research presents some hope to young children who are not yet eligible to receive ETI, according to Carlos Milla, MD, who is a pediatric pulmonary physician at Stanford (Calif.) University. “We already know that the next version of this drug is much more efficacious, the triple-combination therapy. It’s a little bit like we’re falling behind when it comes to treating these young kids because we are offering right now what we know is a less effective drug as opposed to the ones that are available now down to age 6, and hopefully sometime soon down to age 2. It’s better [than] to have no treatment at all, so it’s a good start,” said Dr. Milla.
“I think this is a great bridge for babies while they’re waiting to grow up to be old enough to get [triple combination therapy] and will prevent some of the complications until they can get the even more highly effective therapy in the future,” said Jennifer Taylor-Cousar, MD, who was asked to comment on the study. Dr. Taylor-Cousar is codirector of the Adult CF Program at National Jewish Health, Denver.
She also noted that the therapy could rapidly become more important. Since the approval of elexacaftor/tezacaftor/ivacaftor in 2019, pregnancies in women with CF have increased markedly. There were 310 such pregnancies in 2019, and 675 in 2020 after the combination became generally available in November of 2019. Many of the resulting babies had false-negative CF diagnoses because the mother was taking the triple combination and the medication crossed the placenta and prevented disease progression. The drugs are present in breast milk, but when breastfeeding isn’t possible, newborns are left without a therapeutic option. “There was no approval for babies who had two copies of F508. This helps tremendously with that albeit small population, although I suspect it may grow larger over the upcoming years as we continue to see so many pregnancies in women with CF because they are so much healthier,” said Dr. Taylor-Cousar.
The study was a phase 3, open-label trial with a cohort aged 18-24 months (cohort 1, n = 14) and another aged 12-18 months (cohort 2, n = 46). Participants received a 15-day treatment with a dose based on weight at screening. Participants then underwent a 24-week treatment period with a dose determined by pharmacokinetic data collected during the initial treatment, the authors wrote in the American Journal of Respiratory and Critical Care Medicine.
A total of 95.7% of children experienced adverse events during the 24-week treatment period; 52.2% of events were mild, and 39.1% were moderate. The most frequent adverse events were cough (34.8%), infective exacerbation of CF (21.7%), pyrexia (21.7%), and vomiting (17.4%); 10.9% had elevations of alanine aminotransferase and/or aspartate aminotransferase higher than three times the upper limit of normal, and one (2.2%) had concentrations of both high enough that the study drug was discontinued.
There were significant reductions in sweat chloride concentration at week 24, suggesting strong efficacy (–29.1 mmol/L; 95% confidence interval, –34.8 to –23.4 mmol/L). Body mass, weight, and length remained normal during the 24-week treatment period, and there were trends towards improvement in biomarkers of pancreatic function and intestinal inflammation, including fecal elastase-1 (+73.1mcg/g; 95% CI, 29.40-116.80 mcg/g), serum immunoreactive trypsinogen (–295.50 mcg/g; 95% CI, –416.60 to –174.50 mcg/g), and fecal calprotectin (–106.63 mg/kg; 95% CI, –180.60 to –32.66 mg/kg)
Dr. McColley, Dr. Taylor-Cousar, and Dr. Milla have no relevant financial disclosures. The study was funded by Merck.
In children with cystic fibrosis (CF) and homozygous for the F508 mutation, the combination of lumacaftor and ivacaftor appears generally safe, and biomarker data suggest its efficacy, according to results from a new open-label phase 3 trial.
In September, the Food and Drug Administration approved lumacaftor/ivacaftor for patients aged 1 years and above. Waiting in the wings is the triple combination of elexacaftor, tezacaftor, and ivacaftor (ETI), which is available for patients with at least one copy of the F508 mutation aged 6 and over. ETI is also being tested in younger patients.
One driving factor for early treatment is countering the malnutrition that can occur among CF patients because of poor pancreatic insufficiency and chronic inflammation. “We’ve known for many years that (being) at or above average body weight and height predict better lung function. And we’ve known for quite a while that the height-for-age percentile, in preschool years, actually predicts your lung function later, and how long you’re going to live, so nutrition is incredibly important,” said study author Susanna McColley, MD, in an interview. It’s also difficult to use lung function tests in young children, since even adults can find them challenging, she said.
“FEV1 [forced expiratory volume in 1 second] is the strongest predictor of survival, and then nutrition is the highest predictor of FEV1, so that’s kind of the construct. They had similar improvement in the functional measures of their pancreas and in the measures of inflammation in the gut. I think the story here is that starting a modulator early has a likelihood to have positive health effects that go forward. We can’t say that from the data in the paper. It’s a 24-week study, but looking at the pancreatic and intestinal functioning and also the fact that there was a decrease in sweat chloride is important,” said Dr. McColley, who is a professor of pediatrics in pulmonary and sleep medicine at Northwestern University, Chicago.
The study adds more evidence that earlier treatment in CF may lead to better outcomes, but the digestive improvements are an overlooked factor, according to Dr. McColley. “Even with early treatment, and even with pancreatic enzymes and supplements taken to digest food, it’s a huge burden. When they come in and say that digestion seems better, there is less bloating, things like that, these are the things that aren’t captured so much in the clinical trial data, but they’re meaningful to families,” she said.
Single-agent ivacaftor is available for children as young as 4 months, but is limited to patients with the G551D gating mutation. Most young children with CF can only be treated for symptoms.
The lack of new safety signals in the new study is reassuring, and the research presents some hope to young children who are not yet eligible to receive ETI, according to Carlos Milla, MD, who is a pediatric pulmonary physician at Stanford (Calif.) University. “We already know that the next version of this drug is much more efficacious, the triple-combination therapy. It’s a little bit like we’re falling behind when it comes to treating these young kids because we are offering right now what we know is a less effective drug as opposed to the ones that are available now down to age 6, and hopefully sometime soon down to age 2. It’s better [than] to have no treatment at all, so it’s a good start,” said Dr. Milla.
“I think this is a great bridge for babies while they’re waiting to grow up to be old enough to get [triple combination therapy] and will prevent some of the complications until they can get the even more highly effective therapy in the future,” said Jennifer Taylor-Cousar, MD, who was asked to comment on the study. Dr. Taylor-Cousar is codirector of the Adult CF Program at National Jewish Health, Denver.
She also noted that the therapy could rapidly become more important. Since the approval of elexacaftor/tezacaftor/ivacaftor in 2019, pregnancies in women with CF have increased markedly. There were 310 such pregnancies in 2019, and 675 in 2020 after the combination became generally available in November of 2019. Many of the resulting babies had false-negative CF diagnoses because the mother was taking the triple combination and the medication crossed the placenta and prevented disease progression. The drugs are present in breast milk, but when breastfeeding isn’t possible, newborns are left without a therapeutic option. “There was no approval for babies who had two copies of F508. This helps tremendously with that albeit small population, although I suspect it may grow larger over the upcoming years as we continue to see so many pregnancies in women with CF because they are so much healthier,” said Dr. Taylor-Cousar.
The study was a phase 3, open-label trial with a cohort aged 18-24 months (cohort 1, n = 14) and another aged 12-18 months (cohort 2, n = 46). Participants received a 15-day treatment with a dose based on weight at screening. Participants then underwent a 24-week treatment period with a dose determined by pharmacokinetic data collected during the initial treatment, the authors wrote in the American Journal of Respiratory and Critical Care Medicine.
A total of 95.7% of children experienced adverse events during the 24-week treatment period; 52.2% of events were mild, and 39.1% were moderate. The most frequent adverse events were cough (34.8%), infective exacerbation of CF (21.7%), pyrexia (21.7%), and vomiting (17.4%); 10.9% had elevations of alanine aminotransferase and/or aspartate aminotransferase higher than three times the upper limit of normal, and one (2.2%) had concentrations of both high enough that the study drug was discontinued.
There were significant reductions in sweat chloride concentration at week 24, suggesting strong efficacy (–29.1 mmol/L; 95% confidence interval, –34.8 to –23.4 mmol/L). Body mass, weight, and length remained normal during the 24-week treatment period, and there were trends towards improvement in biomarkers of pancreatic function and intestinal inflammation, including fecal elastase-1 (+73.1mcg/g; 95% CI, 29.40-116.80 mcg/g), serum immunoreactive trypsinogen (–295.50 mcg/g; 95% CI, –416.60 to –174.50 mcg/g), and fecal calprotectin (–106.63 mg/kg; 95% CI, –180.60 to –32.66 mg/kg)
Dr. McColley, Dr. Taylor-Cousar, and Dr. Milla have no relevant financial disclosures. The study was funded by Merck.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
MD-researcher keeps her eyes on the prize
As a toddler undergoing treatment at McMaster Children’s Hospital in Hamilton, Ont., Caroline Diorio, MD, couldn’t grasp what the nice doctors scurrying in and out of her room were doing. She just knew they were taking care of her.
Dr. Diorio had pediatric immune thrombocytopenia (ITP), a type of platelet disorder in which the immune system attacks blood platelets for usually unknown reasons.
“I remember very much how worried my parents were,” recalled Dr. Diorio, now a hematologist-oncologist at Children’s Hospital of Philadelphia. “And I remember how the tone of the doctor’s voice and the way the doctors communicated provided so much reassurance to my parents.”
Dr. Diorio’s ITP resolved within a few years, but her experience left a lasting impression.
“From that moment on, I don’t remember a time that I didn’t want to be a doctor,” she said. “I had these really formative experiences with doctors who were so lovely, and I thought, ‘I want to do that.’ ”
Though she considered other specialties in medical school at the University of Toronto, Dr. Diorio kept feeling drawn back to pediatric oncology and hematology.
“I have always loved the commitment that parents have to their kids and the team approach that exists,” she said. “Hematology/oncology allowed me to take care of really sick kids but also have this long-term relationship with them and their parents, which I really value and love.”
Dr. Diorio even completed her residency at McMaster alongside one of the same physicians who had cared for her as a child, Ronald Duncan Barr, MD. “It sort of all came full circle,” she said.
For B-cell ALL and several other blood cancers, an effective option is CAR T-cell therapy, in which physicians collect T-cells from the patient, re-engineer the T cells in the lab so they recognize the proteins expressed on the surface of cancerous cells – called blasts – and then introduce the modified T-cells back into the patient. Once infused, the re-engineered T-cells attack the blasts with the tell-tale proteins.
But with T-ALL, T-cells themselves are infected with cancer, so autologous CAR T-cell therapy is not currently an option, and no allogeneic CAR T-cell therapies have been approved. Dr. Diorio is part of a cutting-edge research team led by David T. Teachey, MD, striving for breakthroughs. “She’s a brilliant clinician, extremely smart and hard-working, exceptional work ethic, great interaction with patients and families with a great bedside manner,” Dr. Teachey said of Dr. Diorio. “She’s just a superstar all around.”
Dr. Teachey first piqued Dr. Diorio’s interest in researching innovative T-ALL therapies when she arrived at CHOP as a hematology/oncology fellow in 2018 and pursued a master of science degree in translational research under his tutelage at the University of Pennsylvania. Then, for a time, the COVID-19 pandemic shut down most research.
“Caroline pivoted and was at the front line, collecting samples and helping with research on SARS-CoV-2 very early in the pandemic,” Dr. Teachey said. “She was able to then pivot back, taking the skills she learned from that work in the pandemic and applying it to what she was doing in the CAR T-cell space and T-ALL.”
Extraordinary gains in pediatric cancer over the past several decades mean that more than 80% of children diagnosed with cancer today will become long-term survivors. “The 20% of the time that we don’t get the result we want is obviously devastating,” Dr. Diorio said. “However, that’s incredibly motivating to try to make better treatments.”
Her current focus is finding a way to use CAR T-cell therapy in children with T-ALL. About 85% of children with T-ALL do well with standard first-line treatments of chemotherapy, but the 15% who relapse or have chemo-refractory disease have a far lower survival rate – less than 30%, Dr. Diorio said.
The problem with autologous CAR T-cell therapy in T-ALL is twofold: It’s difficult to sort out healthy T cells from the cancerous T cells, and the target current re-engineered T-cells go after is on healthy cells, too.
“What happens is a problem called fratricide – basically the CAR T-cells are killing their brothers,” she said. So Dr. Diorio and her colleagues are trying to modify CAR T-cell strategies to target different markers. One target they’re investigating is CD7, but using CRISPR to gene-edit out CD7 from healthy cells requires making two cuts in the DNA.
“Any time you break DNA, you have to repair it, and any time you repair it, there’s a chance of making a mistake,” Dr. Diorio said. So she used a different technique, cytosine-based editing, which requires only one cut. “You put in what you want, and it’s much more precise and less error-prone.” Cytosine-based editing also preserves T cells’ vitality; too many cuts impair T-cell growth, but that doesn’t happen with cytosine-based editing. In August of 2022, Dr. Diorio published a study demonstrating this technique while the team has continued looking for other targets that show up on cancer cells but not on healthy T-cells.
“I’m not invested in one particular strategy,” Dr. Diorio said. “I’m invested in finding a strategy that works for the maximum number of patients.”
That pragmatic approach may be why Dr. Teachey describes her as an out-of-the-box thinker.
“She brings novel ideas to the table, and not everybody who’s a physician-scientist has that ability to really think about taking things in the bench to the bedside and then back again,” Dr. Teachey said. “It’s knowing what questions are important to ask for our patients and how to study those and the research base, so that you can improve treatments for kids with leukemia.”
Their research looks promising so far. Clinical trials are in development for the CD7-targeted CAR T, and they’re collaborating with others on clinical trials for CAR-T targeting another protein, CD38. In the midst of it all, Dr. Diorio remains focused on her patients.
“It’s really a privilege to see the incredible grace people have in these very difficult circumstances,” Dr. Diorio said. “I find it really motivating to try to make things easier for people, and I try to spend every day looking for better treatments so people don’t have to go through that.”
Dr. Diorio has no disclosures. Dr. Teachey has served on the advisory boards of BEAM, Jazz, Janssen, and Sobi and has received research funding from BEAM, Jazz, Servier, and Neoimmune Tech. He has multiple patents pending on CAR-T therapy.
As a toddler undergoing treatment at McMaster Children’s Hospital in Hamilton, Ont., Caroline Diorio, MD, couldn’t grasp what the nice doctors scurrying in and out of her room were doing. She just knew they were taking care of her.
Dr. Diorio had pediatric immune thrombocytopenia (ITP), a type of platelet disorder in which the immune system attacks blood platelets for usually unknown reasons.
“I remember very much how worried my parents were,” recalled Dr. Diorio, now a hematologist-oncologist at Children’s Hospital of Philadelphia. “And I remember how the tone of the doctor’s voice and the way the doctors communicated provided so much reassurance to my parents.”
Dr. Diorio’s ITP resolved within a few years, but her experience left a lasting impression.
“From that moment on, I don’t remember a time that I didn’t want to be a doctor,” she said. “I had these really formative experiences with doctors who were so lovely, and I thought, ‘I want to do that.’ ”
Though she considered other specialties in medical school at the University of Toronto, Dr. Diorio kept feeling drawn back to pediatric oncology and hematology.
“I have always loved the commitment that parents have to their kids and the team approach that exists,” she said. “Hematology/oncology allowed me to take care of really sick kids but also have this long-term relationship with them and their parents, which I really value and love.”
Dr. Diorio even completed her residency at McMaster alongside one of the same physicians who had cared for her as a child, Ronald Duncan Barr, MD. “It sort of all came full circle,” she said.
For B-cell ALL and several other blood cancers, an effective option is CAR T-cell therapy, in which physicians collect T-cells from the patient, re-engineer the T cells in the lab so they recognize the proteins expressed on the surface of cancerous cells – called blasts – and then introduce the modified T-cells back into the patient. Once infused, the re-engineered T-cells attack the blasts with the tell-tale proteins.
But with T-ALL, T-cells themselves are infected with cancer, so autologous CAR T-cell therapy is not currently an option, and no allogeneic CAR T-cell therapies have been approved. Dr. Diorio is part of a cutting-edge research team led by David T. Teachey, MD, striving for breakthroughs. “She’s a brilliant clinician, extremely smart and hard-working, exceptional work ethic, great interaction with patients and families with a great bedside manner,” Dr. Teachey said of Dr. Diorio. “She’s just a superstar all around.”
Dr. Teachey first piqued Dr. Diorio’s interest in researching innovative T-ALL therapies when she arrived at CHOP as a hematology/oncology fellow in 2018 and pursued a master of science degree in translational research under his tutelage at the University of Pennsylvania. Then, for a time, the COVID-19 pandemic shut down most research.
“Caroline pivoted and was at the front line, collecting samples and helping with research on SARS-CoV-2 very early in the pandemic,” Dr. Teachey said. “She was able to then pivot back, taking the skills she learned from that work in the pandemic and applying it to what she was doing in the CAR T-cell space and T-ALL.”
Extraordinary gains in pediatric cancer over the past several decades mean that more than 80% of children diagnosed with cancer today will become long-term survivors. “The 20% of the time that we don’t get the result we want is obviously devastating,” Dr. Diorio said. “However, that’s incredibly motivating to try to make better treatments.”
Her current focus is finding a way to use CAR T-cell therapy in children with T-ALL. About 85% of children with T-ALL do well with standard first-line treatments of chemotherapy, but the 15% who relapse or have chemo-refractory disease have a far lower survival rate – less than 30%, Dr. Diorio said.
The problem with autologous CAR T-cell therapy in T-ALL is twofold: It’s difficult to sort out healthy T cells from the cancerous T cells, and the target current re-engineered T-cells go after is on healthy cells, too.
“What happens is a problem called fratricide – basically the CAR T-cells are killing their brothers,” she said. So Dr. Diorio and her colleagues are trying to modify CAR T-cell strategies to target different markers. One target they’re investigating is CD7, but using CRISPR to gene-edit out CD7 from healthy cells requires making two cuts in the DNA.
“Any time you break DNA, you have to repair it, and any time you repair it, there’s a chance of making a mistake,” Dr. Diorio said. So she used a different technique, cytosine-based editing, which requires only one cut. “You put in what you want, and it’s much more precise and less error-prone.” Cytosine-based editing also preserves T cells’ vitality; too many cuts impair T-cell growth, but that doesn’t happen with cytosine-based editing. In August of 2022, Dr. Diorio published a study demonstrating this technique while the team has continued looking for other targets that show up on cancer cells but not on healthy T-cells.
“I’m not invested in one particular strategy,” Dr. Diorio said. “I’m invested in finding a strategy that works for the maximum number of patients.”
That pragmatic approach may be why Dr. Teachey describes her as an out-of-the-box thinker.
“She brings novel ideas to the table, and not everybody who’s a physician-scientist has that ability to really think about taking things in the bench to the bedside and then back again,” Dr. Teachey said. “It’s knowing what questions are important to ask for our patients and how to study those and the research base, so that you can improve treatments for kids with leukemia.”
Their research looks promising so far. Clinical trials are in development for the CD7-targeted CAR T, and they’re collaborating with others on clinical trials for CAR-T targeting another protein, CD38. In the midst of it all, Dr. Diorio remains focused on her patients.
“It’s really a privilege to see the incredible grace people have in these very difficult circumstances,” Dr. Diorio said. “I find it really motivating to try to make things easier for people, and I try to spend every day looking for better treatments so people don’t have to go through that.”
Dr. Diorio has no disclosures. Dr. Teachey has served on the advisory boards of BEAM, Jazz, Janssen, and Sobi and has received research funding from BEAM, Jazz, Servier, and Neoimmune Tech. He has multiple patents pending on CAR-T therapy.
As a toddler undergoing treatment at McMaster Children’s Hospital in Hamilton, Ont., Caroline Diorio, MD, couldn’t grasp what the nice doctors scurrying in and out of her room were doing. She just knew they were taking care of her.
Dr. Diorio had pediatric immune thrombocytopenia (ITP), a type of platelet disorder in which the immune system attacks blood platelets for usually unknown reasons.
“I remember very much how worried my parents were,” recalled Dr. Diorio, now a hematologist-oncologist at Children’s Hospital of Philadelphia. “And I remember how the tone of the doctor’s voice and the way the doctors communicated provided so much reassurance to my parents.”
Dr. Diorio’s ITP resolved within a few years, but her experience left a lasting impression.
“From that moment on, I don’t remember a time that I didn’t want to be a doctor,” she said. “I had these really formative experiences with doctors who were so lovely, and I thought, ‘I want to do that.’ ”
Though she considered other specialties in medical school at the University of Toronto, Dr. Diorio kept feeling drawn back to pediatric oncology and hematology.
“I have always loved the commitment that parents have to their kids and the team approach that exists,” she said. “Hematology/oncology allowed me to take care of really sick kids but also have this long-term relationship with them and their parents, which I really value and love.”
Dr. Diorio even completed her residency at McMaster alongside one of the same physicians who had cared for her as a child, Ronald Duncan Barr, MD. “It sort of all came full circle,” she said.
For B-cell ALL and several other blood cancers, an effective option is CAR T-cell therapy, in which physicians collect T-cells from the patient, re-engineer the T cells in the lab so they recognize the proteins expressed on the surface of cancerous cells – called blasts – and then introduce the modified T-cells back into the patient. Once infused, the re-engineered T-cells attack the blasts with the tell-tale proteins.
But with T-ALL, T-cells themselves are infected with cancer, so autologous CAR T-cell therapy is not currently an option, and no allogeneic CAR T-cell therapies have been approved. Dr. Diorio is part of a cutting-edge research team led by David T. Teachey, MD, striving for breakthroughs. “She’s a brilliant clinician, extremely smart and hard-working, exceptional work ethic, great interaction with patients and families with a great bedside manner,” Dr. Teachey said of Dr. Diorio. “She’s just a superstar all around.”
Dr. Teachey first piqued Dr. Diorio’s interest in researching innovative T-ALL therapies when she arrived at CHOP as a hematology/oncology fellow in 2018 and pursued a master of science degree in translational research under his tutelage at the University of Pennsylvania. Then, for a time, the COVID-19 pandemic shut down most research.
“Caroline pivoted and was at the front line, collecting samples and helping with research on SARS-CoV-2 very early in the pandemic,” Dr. Teachey said. “She was able to then pivot back, taking the skills she learned from that work in the pandemic and applying it to what she was doing in the CAR T-cell space and T-ALL.”
Extraordinary gains in pediatric cancer over the past several decades mean that more than 80% of children diagnosed with cancer today will become long-term survivors. “The 20% of the time that we don’t get the result we want is obviously devastating,” Dr. Diorio said. “However, that’s incredibly motivating to try to make better treatments.”
Her current focus is finding a way to use CAR T-cell therapy in children with T-ALL. About 85% of children with T-ALL do well with standard first-line treatments of chemotherapy, but the 15% who relapse or have chemo-refractory disease have a far lower survival rate – less than 30%, Dr. Diorio said.
The problem with autologous CAR T-cell therapy in T-ALL is twofold: It’s difficult to sort out healthy T cells from the cancerous T cells, and the target current re-engineered T-cells go after is on healthy cells, too.
“What happens is a problem called fratricide – basically the CAR T-cells are killing their brothers,” she said. So Dr. Diorio and her colleagues are trying to modify CAR T-cell strategies to target different markers. One target they’re investigating is CD7, but using CRISPR to gene-edit out CD7 from healthy cells requires making two cuts in the DNA.
“Any time you break DNA, you have to repair it, and any time you repair it, there’s a chance of making a mistake,” Dr. Diorio said. So she used a different technique, cytosine-based editing, which requires only one cut. “You put in what you want, and it’s much more precise and less error-prone.” Cytosine-based editing also preserves T cells’ vitality; too many cuts impair T-cell growth, but that doesn’t happen with cytosine-based editing. In August of 2022, Dr. Diorio published a study demonstrating this technique while the team has continued looking for other targets that show up on cancer cells but not on healthy T-cells.
“I’m not invested in one particular strategy,” Dr. Diorio said. “I’m invested in finding a strategy that works for the maximum number of patients.”
That pragmatic approach may be why Dr. Teachey describes her as an out-of-the-box thinker.
“She brings novel ideas to the table, and not everybody who’s a physician-scientist has that ability to really think about taking things in the bench to the bedside and then back again,” Dr. Teachey said. “It’s knowing what questions are important to ask for our patients and how to study those and the research base, so that you can improve treatments for kids with leukemia.”
Their research looks promising so far. Clinical trials are in development for the CD7-targeted CAR T, and they’re collaborating with others on clinical trials for CAR-T targeting another protein, CD38. In the midst of it all, Dr. Diorio remains focused on her patients.
“It’s really a privilege to see the incredible grace people have in these very difficult circumstances,” Dr. Diorio said. “I find it really motivating to try to make things easier for people, and I try to spend every day looking for better treatments so people don’t have to go through that.”
Dr. Diorio has no disclosures. Dr. Teachey has served on the advisory boards of BEAM, Jazz, Janssen, and Sobi and has received research funding from BEAM, Jazz, Servier, and Neoimmune Tech. He has multiple patents pending on CAR-T therapy.
Cochrane Review bolsters case that emollients don’t prevent AD
associated with early use of emollients.
The document, published in November 2022, updates a February 2021 version, said Robert Boyle, MD, PhD, senior author of the Cochrane Review and a pediatric allergist at Imperial College London. “The differences were slight,” he told this news organization. “Mainly, we had a little more data about food allergy outcomes, which slightly strengthened the concern about a possible increase in food allergy with emollients; and we had some new genetic information, which allowed us to add some further interaction analyses and confirm that chromosome 11 intergenic variant rs2212434 doesn’t seem to impact the effect – or lack of effect – of emollient on eczema development.”
The updated Cochrane Review concludes that, “based on low‐ to moderate-certainty evidence, skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema; may increase risk of food allergy; and probably increase risk of skin infection.”
The latest publication should strengthen clinicians’ confidence in not recommending emollient use for preventing AD in at-risk infants – however, that message is being diluted by a stream of contradictory conclusions from poor-quality systematic reviews, say Dr. Boyle and two coauthors. “It’s a systematic problem of people churning out endless systematic reviews without much rigor,” explained the lead author Maeve Kelleher, MD, from Children’s Health Ireland, Crumlin. There have been “misleading systematic reviews published, often in high-ranking journals,” agreed Dr. Boyle.
“I have been an advocate of systematic reviews for the last 20 years, but they have gone completely out of control,” added Hywel Williams, MD, PhD, another of the Cochrane Review coauthors, who is professor of dermato-epidemiology and codirector of the Centre of Evidence Based Dermatology, at Nottingham (England) University Hospitals NHS Trust. In an editorial, published last year, Dr. Williams even posed the question: “Are Dermatology Systematic Reviews Spinning Out of Control?” in which he blamed “the misrepresentation of study results” – which he calls “the sin of spin” – for degrading the quality of science in dermatology.
“The field has become a ‘sausage machine’ industry that undermines the value of systematic reviews in providing a summary of the best evidence to inform patient care,” he wrote. “Fewer systematic reviews are needed in dermatology,” but “better ones” are needed, he continued, calling for all systematic reviews to be registered prospectively, and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Earlier this year, in a letter to the editor, Dr. Kelleher, Dr. Boyle, Dr. Williams, and several others outlined their concerns after a systemic review and meta-analysis was published, “which came to very different conclusions” than their Cochrane Review.
“It is quite common to see non-Cochrane reviews published in leading specialty journals, which interpret data in a more positive light than Cochrane reviews, which have assessed a similar dataset/topic,” Dr. Boyle said in the interview.
Such concerns also apply to the publication of another systematic review that was recently published. “Overall, early application of emollients is an effective strategy for preventing AD development in high-risk infants,” reported senior author Xiaojing Kang, MD, PhD, from People’s Hospital of Xinjiang Uygur Autonomous Region, Urumchi, China, and coauthors, who could not be reached for comment. In their discussion, the authors cite several criticisms of the Cochrane Review: that it included two meeting abstracts and two “ineligible” studies; did not do subgroup analysis of high-risk infants; did not look at different types of emollients; and did not examine the risk of food sensitization.
“A Cochrane Review can be quite a large and complex document to negotiate for those who are not very familiar with Cochrane’s methodology,” said Dr. Boyle. He dismissed the criticism, saying “we did do subgroup analysis of high risk infants, we did look at different types of emollient, and we did look at food sensitization and food allergy risk. We only included eligible studies. … Certainly we would include abstracts of trials, which are not reported in any other form, in order to capture as complete a picture.”
Ultimately, Dr. Boyle said, the discrepancy in conclusions between such systematic reviews and the Cochrane Review relates to quality of methodology. “Our Cochrane review was an individual participant data (IPD) meta-analysis, meaning that authors of the main trials in this area shared their original datasets with us,” he said in the interview. “This is the ‘gold standard’ in systematic reviews, and allowed us to check data/ query inconsistencies and to apply a single-analysis methodology across all studies. It also allowed us to undertake some analyses, which are just not possible in aggregate data analysis based on published work without IPD.”
The most recently published systematic review had no registered protocol, “so, there is no transparency about the methods used,” he noted. “It is free and simple to register a protocol – multiple websites such as PROSPERO, open science framework, and zenodo allow this,” he said “In the journal I edit, we use availability of a registered protocol as a marker of quality. We find that systematic reviews with no registered protocol are almost universally poor quality.”
Dr. Williams is a founding member and coordinating editor of the Cochrane Skin Group 1998 to 2017. Dr. Boyle was paid by Cochrane for senior editor work, until recently, and had no other relevant disclosures. Dr. Kelleher had no relevant disclosures.
associated with early use of emollients.
The document, published in November 2022, updates a February 2021 version, said Robert Boyle, MD, PhD, senior author of the Cochrane Review and a pediatric allergist at Imperial College London. “The differences were slight,” he told this news organization. “Mainly, we had a little more data about food allergy outcomes, which slightly strengthened the concern about a possible increase in food allergy with emollients; and we had some new genetic information, which allowed us to add some further interaction analyses and confirm that chromosome 11 intergenic variant rs2212434 doesn’t seem to impact the effect – or lack of effect – of emollient on eczema development.”
The updated Cochrane Review concludes that, “based on low‐ to moderate-certainty evidence, skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema; may increase risk of food allergy; and probably increase risk of skin infection.”
The latest publication should strengthen clinicians’ confidence in not recommending emollient use for preventing AD in at-risk infants – however, that message is being diluted by a stream of contradictory conclusions from poor-quality systematic reviews, say Dr. Boyle and two coauthors. “It’s a systematic problem of people churning out endless systematic reviews without much rigor,” explained the lead author Maeve Kelleher, MD, from Children’s Health Ireland, Crumlin. There have been “misleading systematic reviews published, often in high-ranking journals,” agreed Dr. Boyle.
“I have been an advocate of systematic reviews for the last 20 years, but they have gone completely out of control,” added Hywel Williams, MD, PhD, another of the Cochrane Review coauthors, who is professor of dermato-epidemiology and codirector of the Centre of Evidence Based Dermatology, at Nottingham (England) University Hospitals NHS Trust. In an editorial, published last year, Dr. Williams even posed the question: “Are Dermatology Systematic Reviews Spinning Out of Control?” in which he blamed “the misrepresentation of study results” – which he calls “the sin of spin” – for degrading the quality of science in dermatology.
“The field has become a ‘sausage machine’ industry that undermines the value of systematic reviews in providing a summary of the best evidence to inform patient care,” he wrote. “Fewer systematic reviews are needed in dermatology,” but “better ones” are needed, he continued, calling for all systematic reviews to be registered prospectively, and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Earlier this year, in a letter to the editor, Dr. Kelleher, Dr. Boyle, Dr. Williams, and several others outlined their concerns after a systemic review and meta-analysis was published, “which came to very different conclusions” than their Cochrane Review.
“It is quite common to see non-Cochrane reviews published in leading specialty journals, which interpret data in a more positive light than Cochrane reviews, which have assessed a similar dataset/topic,” Dr. Boyle said in the interview.
Such concerns also apply to the publication of another systematic review that was recently published. “Overall, early application of emollients is an effective strategy for preventing AD development in high-risk infants,” reported senior author Xiaojing Kang, MD, PhD, from People’s Hospital of Xinjiang Uygur Autonomous Region, Urumchi, China, and coauthors, who could not be reached for comment. In their discussion, the authors cite several criticisms of the Cochrane Review: that it included two meeting abstracts and two “ineligible” studies; did not do subgroup analysis of high-risk infants; did not look at different types of emollients; and did not examine the risk of food sensitization.
“A Cochrane Review can be quite a large and complex document to negotiate for those who are not very familiar with Cochrane’s methodology,” said Dr. Boyle. He dismissed the criticism, saying “we did do subgroup analysis of high risk infants, we did look at different types of emollient, and we did look at food sensitization and food allergy risk. We only included eligible studies. … Certainly we would include abstracts of trials, which are not reported in any other form, in order to capture as complete a picture.”
Ultimately, Dr. Boyle said, the discrepancy in conclusions between such systematic reviews and the Cochrane Review relates to quality of methodology. “Our Cochrane review was an individual participant data (IPD) meta-analysis, meaning that authors of the main trials in this area shared their original datasets with us,” he said in the interview. “This is the ‘gold standard’ in systematic reviews, and allowed us to check data/ query inconsistencies and to apply a single-analysis methodology across all studies. It also allowed us to undertake some analyses, which are just not possible in aggregate data analysis based on published work without IPD.”
The most recently published systematic review had no registered protocol, “so, there is no transparency about the methods used,” he noted. “It is free and simple to register a protocol – multiple websites such as PROSPERO, open science framework, and zenodo allow this,” he said “In the journal I edit, we use availability of a registered protocol as a marker of quality. We find that systematic reviews with no registered protocol are almost universally poor quality.”
Dr. Williams is a founding member and coordinating editor of the Cochrane Skin Group 1998 to 2017. Dr. Boyle was paid by Cochrane for senior editor work, until recently, and had no other relevant disclosures. Dr. Kelleher had no relevant disclosures.
associated with early use of emollients.
The document, published in November 2022, updates a February 2021 version, said Robert Boyle, MD, PhD, senior author of the Cochrane Review and a pediatric allergist at Imperial College London. “The differences were slight,” he told this news organization. “Mainly, we had a little more data about food allergy outcomes, which slightly strengthened the concern about a possible increase in food allergy with emollients; and we had some new genetic information, which allowed us to add some further interaction analyses and confirm that chromosome 11 intergenic variant rs2212434 doesn’t seem to impact the effect – or lack of effect – of emollient on eczema development.”
The updated Cochrane Review concludes that, “based on low‐ to moderate-certainty evidence, skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema; may increase risk of food allergy; and probably increase risk of skin infection.”
The latest publication should strengthen clinicians’ confidence in not recommending emollient use for preventing AD in at-risk infants – however, that message is being diluted by a stream of contradictory conclusions from poor-quality systematic reviews, say Dr. Boyle and two coauthors. “It’s a systematic problem of people churning out endless systematic reviews without much rigor,” explained the lead author Maeve Kelleher, MD, from Children’s Health Ireland, Crumlin. There have been “misleading systematic reviews published, often in high-ranking journals,” agreed Dr. Boyle.
“I have been an advocate of systematic reviews for the last 20 years, but they have gone completely out of control,” added Hywel Williams, MD, PhD, another of the Cochrane Review coauthors, who is professor of dermato-epidemiology and codirector of the Centre of Evidence Based Dermatology, at Nottingham (England) University Hospitals NHS Trust. In an editorial, published last year, Dr. Williams even posed the question: “Are Dermatology Systematic Reviews Spinning Out of Control?” in which he blamed “the misrepresentation of study results” – which he calls “the sin of spin” – for degrading the quality of science in dermatology.
“The field has become a ‘sausage machine’ industry that undermines the value of systematic reviews in providing a summary of the best evidence to inform patient care,” he wrote. “Fewer systematic reviews are needed in dermatology,” but “better ones” are needed, he continued, calling for all systematic reviews to be registered prospectively, and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Earlier this year, in a letter to the editor, Dr. Kelleher, Dr. Boyle, Dr. Williams, and several others outlined their concerns after a systemic review and meta-analysis was published, “which came to very different conclusions” than their Cochrane Review.
“It is quite common to see non-Cochrane reviews published in leading specialty journals, which interpret data in a more positive light than Cochrane reviews, which have assessed a similar dataset/topic,” Dr. Boyle said in the interview.
Such concerns also apply to the publication of another systematic review that was recently published. “Overall, early application of emollients is an effective strategy for preventing AD development in high-risk infants,” reported senior author Xiaojing Kang, MD, PhD, from People’s Hospital of Xinjiang Uygur Autonomous Region, Urumchi, China, and coauthors, who could not be reached for comment. In their discussion, the authors cite several criticisms of the Cochrane Review: that it included two meeting abstracts and two “ineligible” studies; did not do subgroup analysis of high-risk infants; did not look at different types of emollients; and did not examine the risk of food sensitization.
“A Cochrane Review can be quite a large and complex document to negotiate for those who are not very familiar with Cochrane’s methodology,” said Dr. Boyle. He dismissed the criticism, saying “we did do subgroup analysis of high risk infants, we did look at different types of emollient, and we did look at food sensitization and food allergy risk. We only included eligible studies. … Certainly we would include abstracts of trials, which are not reported in any other form, in order to capture as complete a picture.”
Ultimately, Dr. Boyle said, the discrepancy in conclusions between such systematic reviews and the Cochrane Review relates to quality of methodology. “Our Cochrane review was an individual participant data (IPD) meta-analysis, meaning that authors of the main trials in this area shared their original datasets with us,” he said in the interview. “This is the ‘gold standard’ in systematic reviews, and allowed us to check data/ query inconsistencies and to apply a single-analysis methodology across all studies. It also allowed us to undertake some analyses, which are just not possible in aggregate data analysis based on published work without IPD.”
The most recently published systematic review had no registered protocol, “so, there is no transparency about the methods used,” he noted. “It is free and simple to register a protocol – multiple websites such as PROSPERO, open science framework, and zenodo allow this,” he said “In the journal I edit, we use availability of a registered protocol as a marker of quality. We find that systematic reviews with no registered protocol are almost universally poor quality.”
Dr. Williams is a founding member and coordinating editor of the Cochrane Skin Group 1998 to 2017. Dr. Boyle was paid by Cochrane for senior editor work, until recently, and had no other relevant disclosures. Dr. Kelleher had no relevant disclosures.
FROM THE COCHRANE REVIEW