User login
Children and COVID: New cases increase; hospital admissions could follow
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.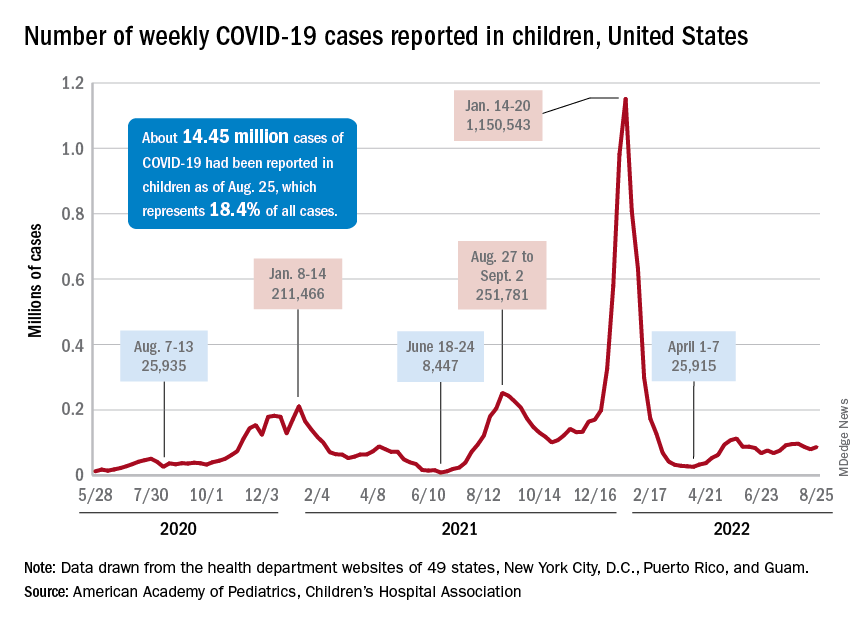
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.
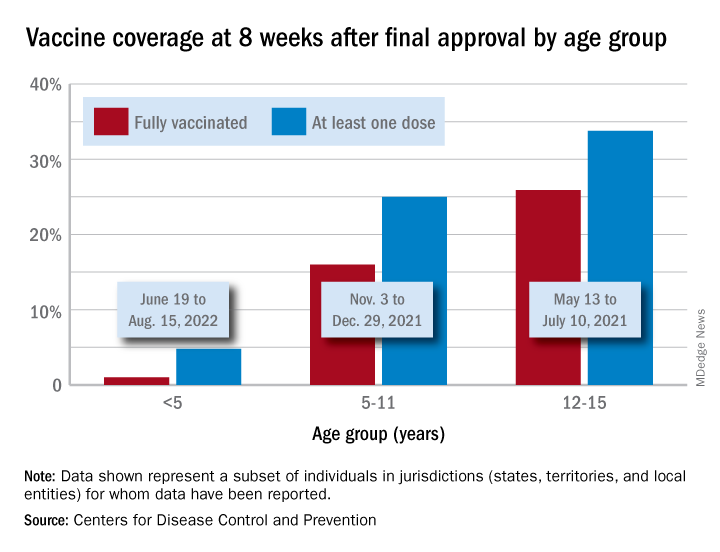
Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
OMERACT continues to set standards on research outcomes, enhancing the patient voice
Clinical research in rheumatology was suffering from an identity crisis of sorts 40 years ago. A lack of consensus across continents resulted in differing views about clinical outcome measures and judgments about treatments.
Patients were not allowed to be the generating source of a clinical outcome, according to Peter Tugwell, MSc, MD. “The only outcomes that were acceptable were clinician assessments, blood tests, and imaging,” said Dr. Tugwell, professor of medicine, epidemiology, and public health at the University of Ottawa (Ont.) and a practicing rheumatologist at Ottawa Hospital.
Clinicians were coming to different conclusions about patient responses to treatment when managing rheumatoid arthritis in clinical practice.
OMERACT sought to address this lack of uniformity. This international group, formed in 1992, leverages stakeholder groups to improve outcome measurement in rheumatology endpoints through a consensus-building, data-driven format.
It was originally known as “Outcome Measures in Rheumatoid Arthritis Clinical Trials,” but its leaders have since broadened its scope to “Outcome Measures in Rheumatology.” Over the years, it has evolved into an international network that assesses measurement across a wide variety of intervention studies. Now 30 years old, the network spans 40 active working groups and has influenced work in patient outcomes across 500 peer-reviewed publications.
The network meets every 2 years to address what is always a challenging agenda, said Dr. Tugwell, one of its founding members and chair. “There’s lots of strong opinions.” Participating in the discussions are individuals from all stages of seniority in rheumatology and clinical epidemiology, patient research partners, industry, approval agencies, and many countries who are committed to the spirit of OMERACT.
“The secret to our success has been getting world leaders to come together and have those discussions, work them through, and identify common ground in such a way that the approval agencies accept these outcome measures in clinical trials,” he added.
“My impression was the founders perceived a problem in the early 1990s and devised a consensus method in an attempt to quantify clinical parameters to define disease activity in rheumatoid arthritis – an important first step to do clinical trials and allow comparisons between them,” said Patricia Woo, CBE, FMedSci, FRCP, emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. At that time, even disease definitions varied between the United States and Europe and other parts of the world, said Dr. Woo, who is not a part of OMERACT. “This was especially true for pediatric rheumatology.”
Fusing the continental divide
OMERACT arose from a need to streamline clinical outcome measures in rheumatology. Research papers during the 1980s demonstrated a lack of coherence in managing patients with rheumatoid arthritis in routine practice. In addition, the measures used to define clinical endpoints in clinical trials operated in silos – they were either too specific to a certain trial, overlapped with other concepts, or didn’t reflect changes in treatment.
Approval agencies in Europe and North America were approving only outcomes measures developed by their respective researchers. This was also true of patients they tested on. “This seemed crazy,” Dr. Tugwell said.
Dr. Tugwell was involved in the Cochrane collaboration, which conducts systematic reviews of best evidence across the world that assesses the magnitude of benefits versus harms.
To achieve this goal, “you need to pull studies from around the world,” he said. Maarten Boers, MD, PhD, a rheumatologist (and later professor of clinical epidemiology at Amsterdam University Medical Center) from the Netherlands, spent a year in Ontario, Canada, to train as a clinical epidemiologist. Together, Dr. Tugwell and Dr. Boers began discussing options to develop more streamlined outcome measures.
They initiated the first OMERACT conference in Maastricht, the Netherlands, in 1992. The Food and Drug Administration and European Medicines Agency participated, along with leaders of outcomes measurement in Europe and in North America.
Discussions centered on methods to develop outcomes in a meaningful fashion. During the first meeting, North American and European approval agencies agreed to accept each other’s studies and endpoints and patient reported outcomes.
Agreement was achieved on a preliminary set of outcome domains and measures that later became known as the WHO-ILAR (World Health Organization–International League of Associations for Rheumatology) core set. The set included seven outcome domains: tender joints, swollen joints, pain, physician global assessment, patient global assessment, physical disability, and acute phase reactants, and one additional outcome domain for studies lasting 1 year or more: radiographs of the joints.
“A proactive program was planned to test not only the validity of these endpoints, but also the methods for their measurement. This was the start of a continuing process,” OMERACT members said in a joint statement for this article. Meetings have since taken place every 2 years.

OMERACT accomplishments
OMERACT now requires buy-in from four continents: Asia, Australia, Europe, and North America.
Its leaders have developed an explicit process for gaining endorsement of core outcome domains and instrument measurement sets. To fully capture the possibilities of “what to measure,” i.e., “measurable aspects of health conditions,” OMERACT has developed a framework of concepts, core areas, and outcome domains. The key concepts are pathophysiology (with a core area termed “manifestations/abnormalities”) and impact (with core areas of “death/lifespan,” and “life impact,” and the optional area of “societal/resource use”). An outcome domain defines an element of a core area to measure the effects of a treatment, such as blood markers, pain intensity, physical function, or emotional well-being.
A core outcome domain set is developed by agreeing to at least one outcome domain within one of the three core areas. Subsequently, a core outcome measurement set is developed by agreeing to at least one applicable measurement instrument for each core outcome domain. This requires documentation of validity, summarized under three metrics: truth, discrimination, and feasibility.
OMERACT’s handbook provides tutelage on establishing and implementing core outcomes, and several workbooks offer guidance on developing core outcome domain sets, selecting instruments for core outcome measurement sets, and OMERACT methodology.
All this work has led to widespread adoption.
Approval agencies have accepted OMERACT’s filter and methods advances, which have been adopted by many research groups in rheumatology and among nonrheumatology research groups. Organizations such as the U.S. National Institutes of Health’s National Institute of Neurological Disorders and Stroke have sought its advice.
Its core outcomes have been adopted and used for approval in the great majority of studies on rheumatoid arthritis, Dr. Tugwell said.
Several BMJ articles underscore the influence and uptake of OMERACT’s core outcome set. One 2017 paper, which analyzed 273 randomized trials of rheumatoid arthritis drug treatments on ClinicalTrials.gov, found that the WHO-ILAR arthritis core outcome set was reported in 81% of the studies. “The adoption of a core outcome set has the potential to increase consistency in outcomes measured across trials and ensure that trials are more likely to measure appropriate outcomes,” the authors concluded.
Since the initial 1992 meeting, OMERACT has broadened its focus from rheumatoid arthritis to 25 other musculoskeletal conditions.
For example, other OMERACT conferences have led to consensus on core sets of measures for osteoarthritis and osteoporosis, psoriasis/psoriatic arthritis, psychosocial measures, and a core set of data for cost-effectiveness evaluations.
‘Speed is a limitation’
OMERACT is a bottom-up volunteer organization. It doesn’t represent any official organization of any clinical society. “We’ve not asked to be adopted by the American College of Rheumatology, EULAR [European Alliance of Associations for Rheumatology], or other international organizations,” Dr. Tugwell said. It offers a chance for patients, users, and doers of research to work together to agree on rigorous criteria accepted by the approval agencies and take the necessary time to work things through.
This is not a fast process, usually taking 4-6 years to initiate and establish an outcome domain set, he emphasized. “It would be beneficial to do it faster if we had the resources to meet every year. The fact is we’re a volunteer organization that meets every 2 years.”
Speed is a limitation, he acknowledged, but it’s an acceptable trade-off for doing things correctly.
The group has faced other challenges during the COVID-19 pandemic, pivoting to a virtual format that had benefits and limitations.
In one respect, moving to a virtual meeting increased uptake in participation and voting, Dr. Tugwell said. Patient participants with severe rheumatoid arthritis no longer faced the challenges of travel. “On the other hand, we didn’t have the same opportunity to achieve common ground virtually,” he said. “Where there are strong disagreements, I’m a great believer that people need to know one another. There needs to be relationship building.”
OMERACT’s emerging leader program has been a cornerstone of its in-person meetings, engaging young rheumatologists to interact with some of the leaders of outcome measurement. The virtual format dampened this process somewhat, eliminating those important “café chats” between the stakeholders.
The hope is to bring people face-to-face once more at the next meeting in May 2023. The agenda will focus on relationship building, identifying controversial areas, and bringing younger people to develop relationships, Dr. Tugwell said. OMERACT will retain a virtual option for the worldwide voting, “which will allow for more buy-in from so many more people,” he added.
A consensus on pain
The onus of developing outcome measures that move with the times is sometimes too great for one group to manage. In 2018, OMERACT became a part of the Red Hat Group (RHG), an organization conceived at the COMET (Core Outcome Measures in Effectiveness Trials) VII meeting in Amsterdam.
RHG aims to improve the choice of outcomes in health research. It includes eight groups: COMET; OMERACT; the Cochrane Skin Core Outcome Set Initiative; Grading of Recommendations, Assessment, Development and Evaluations; Center for Medical Technology Policy; COnsensus-based Standards for the selection of health Measurement Instruments; Clinical Data Interchange Standards Consortium; and Standardized Outcomes in Nephrology.
The collaboration between groups offers a “very interesting interface between consensus building as well as hard evidence,” Dr. Tugwell said. The focus goes beyond rheumatology to other clinical areas of common interest, exploring how one classifies outcome domains in terms of symptoms, life impact, or death.
Pain is an important common denominator that the RHG has evaluated.
“We believe it’s too general. We’re trying to define pain across all Red Hat Groups because it’s clear that the research community has all these different scales for defining pain severity,” Dr. Tugwell said. “We have to find a way to make ruthless decisions and rules for doing it. And of course, it has to be transparent.”
Looking ahead
As part of its ongoing work, OMERACT is evaluating the robustness of instruments that rheumatologists use as outcome measures in clinical trials, which can be a laborious process. The OMERACT Filter 2.0, part of the latest iteration of the handbook, offers strong guidance for researchers but needs a long-term strategy and key methodological support. “To that end, we set up a technical advisory group to help people in the instrument selection work and that remains an ongoing process,” OMERACT leaders said in their joint statement.
OMERACT is looking at opportunities to create benchmark processes for developing core sets outside of rheumatology and a methodology around outcome measures such as contextual factors, composites, and surrogates.
It will also be taking a step back to solicit opinions from the approval agencies represented by the OMERACT membership on the OMERACT handbook.
The goal is to make sure the handbook aligns with everyone else’s approval and labeling requirements.
OMERACT’s patient participants bring important perspectives
OMERACT over the years has sought to become a more patient-centered group. Patients have been involved in OMERACT activities since its sixth meeting, forming an independent, yet integrated, group within the network. They have their own steering committee and produced and helped to update a glossary for OMERACT patients and professionals.
Catherine (McGowan) Hofstetter, who was diagnosed with rheumatoid arthritis 30 years ago, chairs OMERACT’s Patient Research Partners Support Team. In a Q&A, she discussed the importance of patient voices and OMERACT’s plans to further educate and include patients in the dialogue on outcomes.
Question: Have patients always been a part of OMERACT meetings?
Answer: Patients have been involved with OMERACT since 2002. The patient voice adds relevance to all the work that OMERACT does. You can’t begin to talk about outcomes unless there is a patient at the table with lived experience.
Q: Can you cite a few examples of how the patient voice enriches the conversation on outcomes research?
A: Outcomes and priorities that are important to patients are often completely different than those of the clinician. For instance, a work outcome is important to someone who doesn’t have any medical insurance or disability insurance, so that you can ensure that there is food on the table and a roof over your head. Or it may be important to someone because the employment provides medical and disability insurance to provide security for them and their family. These are two different perspectives on work and therefore work priorities and outcomes.
Q: What have been some of the challenges of getting patients to participate?
A: Training patients is one challenge. OMERACT’s work has a very steep learning curve, and while the basics are the same between the groups in terms of looking at what we measure and how we measure it, the nuances of different working groups require a lot of time and energy to be comfortable enough with the work, and then be confident enough to bring your perspective and lived experience to the table. It’s also a very accomplished group, which can be quite intimidating. Self-disclosure is a very personal and intimate undertaking that requires patience, compassion, and respect.
Q: Are there any plans to enhance patient engagement?
A: When we had OMERACT 2020 it was a virtual conference that took place over about 6 months. We had far more patient research partners [PRPs] participate than we have ever had at any OMERACT face-to-face meeting. There is a desire and passion on the part of patients to lend their voices to the work. The working groups meet virtually throughout the year to advance their agendas, and PRPs are a part of each of the working groups.
Hopefully, we can start working toward including more voices at the conferences by enabling a hybrid model. The PRP Support Team will begin engaging patients this fall with education, mentoring, and team-building exercises so by the time we meet in person in May 2023, they will have enough background knowledge and information to give them the confidence that will enhance their experience at the face-to-face meeting.
We also need to ensure that those patients who want to stay engaged can. This means that the education and training should continue long after the face-to-face meeting is over. We need to build capacity in the PRP group and look to succession planning and be a resource to working groups struggling to find PRPs to work with them on a longer-term basis.
Clinical research in rheumatology was suffering from an identity crisis of sorts 40 years ago. A lack of consensus across continents resulted in differing views about clinical outcome measures and judgments about treatments.
Patients were not allowed to be the generating source of a clinical outcome, according to Peter Tugwell, MSc, MD. “The only outcomes that were acceptable were clinician assessments, blood tests, and imaging,” said Dr. Tugwell, professor of medicine, epidemiology, and public health at the University of Ottawa (Ont.) and a practicing rheumatologist at Ottawa Hospital.
Clinicians were coming to different conclusions about patient responses to treatment when managing rheumatoid arthritis in clinical practice.
OMERACT sought to address this lack of uniformity. This international group, formed in 1992, leverages stakeholder groups to improve outcome measurement in rheumatology endpoints through a consensus-building, data-driven format.
It was originally known as “Outcome Measures in Rheumatoid Arthritis Clinical Trials,” but its leaders have since broadened its scope to “Outcome Measures in Rheumatology.” Over the years, it has evolved into an international network that assesses measurement across a wide variety of intervention studies. Now 30 years old, the network spans 40 active working groups and has influenced work in patient outcomes across 500 peer-reviewed publications.
The network meets every 2 years to address what is always a challenging agenda, said Dr. Tugwell, one of its founding members and chair. “There’s lots of strong opinions.” Participating in the discussions are individuals from all stages of seniority in rheumatology and clinical epidemiology, patient research partners, industry, approval agencies, and many countries who are committed to the spirit of OMERACT.
“The secret to our success has been getting world leaders to come together and have those discussions, work them through, and identify common ground in such a way that the approval agencies accept these outcome measures in clinical trials,” he added.
“My impression was the founders perceived a problem in the early 1990s and devised a consensus method in an attempt to quantify clinical parameters to define disease activity in rheumatoid arthritis – an important first step to do clinical trials and allow comparisons between them,” said Patricia Woo, CBE, FMedSci, FRCP, emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. At that time, even disease definitions varied between the United States and Europe and other parts of the world, said Dr. Woo, who is not a part of OMERACT. “This was especially true for pediatric rheumatology.”
Fusing the continental divide
OMERACT arose from a need to streamline clinical outcome measures in rheumatology. Research papers during the 1980s demonstrated a lack of coherence in managing patients with rheumatoid arthritis in routine practice. In addition, the measures used to define clinical endpoints in clinical trials operated in silos – they were either too specific to a certain trial, overlapped with other concepts, or didn’t reflect changes in treatment.
Approval agencies in Europe and North America were approving only outcomes measures developed by their respective researchers. This was also true of patients they tested on. “This seemed crazy,” Dr. Tugwell said.
Dr. Tugwell was involved in the Cochrane collaboration, which conducts systematic reviews of best evidence across the world that assesses the magnitude of benefits versus harms.
To achieve this goal, “you need to pull studies from around the world,” he said. Maarten Boers, MD, PhD, a rheumatologist (and later professor of clinical epidemiology at Amsterdam University Medical Center) from the Netherlands, spent a year in Ontario, Canada, to train as a clinical epidemiologist. Together, Dr. Tugwell and Dr. Boers began discussing options to develop more streamlined outcome measures.
They initiated the first OMERACT conference in Maastricht, the Netherlands, in 1992. The Food and Drug Administration and European Medicines Agency participated, along with leaders of outcomes measurement in Europe and in North America.
Discussions centered on methods to develop outcomes in a meaningful fashion. During the first meeting, North American and European approval agencies agreed to accept each other’s studies and endpoints and patient reported outcomes.
Agreement was achieved on a preliminary set of outcome domains and measures that later became known as the WHO-ILAR (World Health Organization–International League of Associations for Rheumatology) core set. The set included seven outcome domains: tender joints, swollen joints, pain, physician global assessment, patient global assessment, physical disability, and acute phase reactants, and one additional outcome domain for studies lasting 1 year or more: radiographs of the joints.
“A proactive program was planned to test not only the validity of these endpoints, but also the methods for their measurement. This was the start of a continuing process,” OMERACT members said in a joint statement for this article. Meetings have since taken place every 2 years.

OMERACT accomplishments
OMERACT now requires buy-in from four continents: Asia, Australia, Europe, and North America.
Its leaders have developed an explicit process for gaining endorsement of core outcome domains and instrument measurement sets. To fully capture the possibilities of “what to measure,” i.e., “measurable aspects of health conditions,” OMERACT has developed a framework of concepts, core areas, and outcome domains. The key concepts are pathophysiology (with a core area termed “manifestations/abnormalities”) and impact (with core areas of “death/lifespan,” and “life impact,” and the optional area of “societal/resource use”). An outcome domain defines an element of a core area to measure the effects of a treatment, such as blood markers, pain intensity, physical function, or emotional well-being.
A core outcome domain set is developed by agreeing to at least one outcome domain within one of the three core areas. Subsequently, a core outcome measurement set is developed by agreeing to at least one applicable measurement instrument for each core outcome domain. This requires documentation of validity, summarized under three metrics: truth, discrimination, and feasibility.
OMERACT’s handbook provides tutelage on establishing and implementing core outcomes, and several workbooks offer guidance on developing core outcome domain sets, selecting instruments for core outcome measurement sets, and OMERACT methodology.
All this work has led to widespread adoption.
Approval agencies have accepted OMERACT’s filter and methods advances, which have been adopted by many research groups in rheumatology and among nonrheumatology research groups. Organizations such as the U.S. National Institutes of Health’s National Institute of Neurological Disorders and Stroke have sought its advice.
Its core outcomes have been adopted and used for approval in the great majority of studies on rheumatoid arthritis, Dr. Tugwell said.
Several BMJ articles underscore the influence and uptake of OMERACT’s core outcome set. One 2017 paper, which analyzed 273 randomized trials of rheumatoid arthritis drug treatments on ClinicalTrials.gov, found that the WHO-ILAR arthritis core outcome set was reported in 81% of the studies. “The adoption of a core outcome set has the potential to increase consistency in outcomes measured across trials and ensure that trials are more likely to measure appropriate outcomes,” the authors concluded.
Since the initial 1992 meeting, OMERACT has broadened its focus from rheumatoid arthritis to 25 other musculoskeletal conditions.
For example, other OMERACT conferences have led to consensus on core sets of measures for osteoarthritis and osteoporosis, psoriasis/psoriatic arthritis, psychosocial measures, and a core set of data for cost-effectiveness evaluations.
‘Speed is a limitation’
OMERACT is a bottom-up volunteer organization. It doesn’t represent any official organization of any clinical society. “We’ve not asked to be adopted by the American College of Rheumatology, EULAR [European Alliance of Associations for Rheumatology], or other international organizations,” Dr. Tugwell said. It offers a chance for patients, users, and doers of research to work together to agree on rigorous criteria accepted by the approval agencies and take the necessary time to work things through.
This is not a fast process, usually taking 4-6 years to initiate and establish an outcome domain set, he emphasized. “It would be beneficial to do it faster if we had the resources to meet every year. The fact is we’re a volunteer organization that meets every 2 years.”
Speed is a limitation, he acknowledged, but it’s an acceptable trade-off for doing things correctly.
The group has faced other challenges during the COVID-19 pandemic, pivoting to a virtual format that had benefits and limitations.
In one respect, moving to a virtual meeting increased uptake in participation and voting, Dr. Tugwell said. Patient participants with severe rheumatoid arthritis no longer faced the challenges of travel. “On the other hand, we didn’t have the same opportunity to achieve common ground virtually,” he said. “Where there are strong disagreements, I’m a great believer that people need to know one another. There needs to be relationship building.”
OMERACT’s emerging leader program has been a cornerstone of its in-person meetings, engaging young rheumatologists to interact with some of the leaders of outcome measurement. The virtual format dampened this process somewhat, eliminating those important “café chats” between the stakeholders.
The hope is to bring people face-to-face once more at the next meeting in May 2023. The agenda will focus on relationship building, identifying controversial areas, and bringing younger people to develop relationships, Dr. Tugwell said. OMERACT will retain a virtual option for the worldwide voting, “which will allow for more buy-in from so many more people,” he added.
A consensus on pain
The onus of developing outcome measures that move with the times is sometimes too great for one group to manage. In 2018, OMERACT became a part of the Red Hat Group (RHG), an organization conceived at the COMET (Core Outcome Measures in Effectiveness Trials) VII meeting in Amsterdam.
RHG aims to improve the choice of outcomes in health research. It includes eight groups: COMET; OMERACT; the Cochrane Skin Core Outcome Set Initiative; Grading of Recommendations, Assessment, Development and Evaluations; Center for Medical Technology Policy; COnsensus-based Standards for the selection of health Measurement Instruments; Clinical Data Interchange Standards Consortium; and Standardized Outcomes in Nephrology.
The collaboration between groups offers a “very interesting interface between consensus building as well as hard evidence,” Dr. Tugwell said. The focus goes beyond rheumatology to other clinical areas of common interest, exploring how one classifies outcome domains in terms of symptoms, life impact, or death.
Pain is an important common denominator that the RHG has evaluated.
“We believe it’s too general. We’re trying to define pain across all Red Hat Groups because it’s clear that the research community has all these different scales for defining pain severity,” Dr. Tugwell said. “We have to find a way to make ruthless decisions and rules for doing it. And of course, it has to be transparent.”
Looking ahead
As part of its ongoing work, OMERACT is evaluating the robustness of instruments that rheumatologists use as outcome measures in clinical trials, which can be a laborious process. The OMERACT Filter 2.0, part of the latest iteration of the handbook, offers strong guidance for researchers but needs a long-term strategy and key methodological support. “To that end, we set up a technical advisory group to help people in the instrument selection work and that remains an ongoing process,” OMERACT leaders said in their joint statement.
OMERACT is looking at opportunities to create benchmark processes for developing core sets outside of rheumatology and a methodology around outcome measures such as contextual factors, composites, and surrogates.
It will also be taking a step back to solicit opinions from the approval agencies represented by the OMERACT membership on the OMERACT handbook.
The goal is to make sure the handbook aligns with everyone else’s approval and labeling requirements.
OMERACT’s patient participants bring important perspectives
OMERACT over the years has sought to become a more patient-centered group. Patients have been involved in OMERACT activities since its sixth meeting, forming an independent, yet integrated, group within the network. They have their own steering committee and produced and helped to update a glossary for OMERACT patients and professionals.
Catherine (McGowan) Hofstetter, who was diagnosed with rheumatoid arthritis 30 years ago, chairs OMERACT’s Patient Research Partners Support Team. In a Q&A, she discussed the importance of patient voices and OMERACT’s plans to further educate and include patients in the dialogue on outcomes.
Question: Have patients always been a part of OMERACT meetings?
Answer: Patients have been involved with OMERACT since 2002. The patient voice adds relevance to all the work that OMERACT does. You can’t begin to talk about outcomes unless there is a patient at the table with lived experience.
Q: Can you cite a few examples of how the patient voice enriches the conversation on outcomes research?
A: Outcomes and priorities that are important to patients are often completely different than those of the clinician. For instance, a work outcome is important to someone who doesn’t have any medical insurance or disability insurance, so that you can ensure that there is food on the table and a roof over your head. Or it may be important to someone because the employment provides medical and disability insurance to provide security for them and their family. These are two different perspectives on work and therefore work priorities and outcomes.
Q: What have been some of the challenges of getting patients to participate?
A: Training patients is one challenge. OMERACT’s work has a very steep learning curve, and while the basics are the same between the groups in terms of looking at what we measure and how we measure it, the nuances of different working groups require a lot of time and energy to be comfortable enough with the work, and then be confident enough to bring your perspective and lived experience to the table. It’s also a very accomplished group, which can be quite intimidating. Self-disclosure is a very personal and intimate undertaking that requires patience, compassion, and respect.
Q: Are there any plans to enhance patient engagement?
A: When we had OMERACT 2020 it was a virtual conference that took place over about 6 months. We had far more patient research partners [PRPs] participate than we have ever had at any OMERACT face-to-face meeting. There is a desire and passion on the part of patients to lend their voices to the work. The working groups meet virtually throughout the year to advance their agendas, and PRPs are a part of each of the working groups.
Hopefully, we can start working toward including more voices at the conferences by enabling a hybrid model. The PRP Support Team will begin engaging patients this fall with education, mentoring, and team-building exercises so by the time we meet in person in May 2023, they will have enough background knowledge and information to give them the confidence that will enhance their experience at the face-to-face meeting.
We also need to ensure that those patients who want to stay engaged can. This means that the education and training should continue long after the face-to-face meeting is over. We need to build capacity in the PRP group and look to succession planning and be a resource to working groups struggling to find PRPs to work with them on a longer-term basis.
Clinical research in rheumatology was suffering from an identity crisis of sorts 40 years ago. A lack of consensus across continents resulted in differing views about clinical outcome measures and judgments about treatments.
Patients were not allowed to be the generating source of a clinical outcome, according to Peter Tugwell, MSc, MD. “The only outcomes that were acceptable were clinician assessments, blood tests, and imaging,” said Dr. Tugwell, professor of medicine, epidemiology, and public health at the University of Ottawa (Ont.) and a practicing rheumatologist at Ottawa Hospital.
Clinicians were coming to different conclusions about patient responses to treatment when managing rheumatoid arthritis in clinical practice.
OMERACT sought to address this lack of uniformity. This international group, formed in 1992, leverages stakeholder groups to improve outcome measurement in rheumatology endpoints through a consensus-building, data-driven format.
It was originally known as “Outcome Measures in Rheumatoid Arthritis Clinical Trials,” but its leaders have since broadened its scope to “Outcome Measures in Rheumatology.” Over the years, it has evolved into an international network that assesses measurement across a wide variety of intervention studies. Now 30 years old, the network spans 40 active working groups and has influenced work in patient outcomes across 500 peer-reviewed publications.
The network meets every 2 years to address what is always a challenging agenda, said Dr. Tugwell, one of its founding members and chair. “There’s lots of strong opinions.” Participating in the discussions are individuals from all stages of seniority in rheumatology and clinical epidemiology, patient research partners, industry, approval agencies, and many countries who are committed to the spirit of OMERACT.
“The secret to our success has been getting world leaders to come together and have those discussions, work them through, and identify common ground in such a way that the approval agencies accept these outcome measures in clinical trials,” he added.
“My impression was the founders perceived a problem in the early 1990s and devised a consensus method in an attempt to quantify clinical parameters to define disease activity in rheumatoid arthritis – an important first step to do clinical trials and allow comparisons between them,” said Patricia Woo, CBE, FMedSci, FRCP, emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. At that time, even disease definitions varied between the United States and Europe and other parts of the world, said Dr. Woo, who is not a part of OMERACT. “This was especially true for pediatric rheumatology.”
Fusing the continental divide
OMERACT arose from a need to streamline clinical outcome measures in rheumatology. Research papers during the 1980s demonstrated a lack of coherence in managing patients with rheumatoid arthritis in routine practice. In addition, the measures used to define clinical endpoints in clinical trials operated in silos – they were either too specific to a certain trial, overlapped with other concepts, or didn’t reflect changes in treatment.
Approval agencies in Europe and North America were approving only outcomes measures developed by their respective researchers. This was also true of patients they tested on. “This seemed crazy,” Dr. Tugwell said.
Dr. Tugwell was involved in the Cochrane collaboration, which conducts systematic reviews of best evidence across the world that assesses the magnitude of benefits versus harms.
To achieve this goal, “you need to pull studies from around the world,” he said. Maarten Boers, MD, PhD, a rheumatologist (and later professor of clinical epidemiology at Amsterdam University Medical Center) from the Netherlands, spent a year in Ontario, Canada, to train as a clinical epidemiologist. Together, Dr. Tugwell and Dr. Boers began discussing options to develop more streamlined outcome measures.
They initiated the first OMERACT conference in Maastricht, the Netherlands, in 1992. The Food and Drug Administration and European Medicines Agency participated, along with leaders of outcomes measurement in Europe and in North America.
Discussions centered on methods to develop outcomes in a meaningful fashion. During the first meeting, North American and European approval agencies agreed to accept each other’s studies and endpoints and patient reported outcomes.
Agreement was achieved on a preliminary set of outcome domains and measures that later became known as the WHO-ILAR (World Health Organization–International League of Associations for Rheumatology) core set. The set included seven outcome domains: tender joints, swollen joints, pain, physician global assessment, patient global assessment, physical disability, and acute phase reactants, and one additional outcome domain for studies lasting 1 year or more: radiographs of the joints.
“A proactive program was planned to test not only the validity of these endpoints, but also the methods for their measurement. This was the start of a continuing process,” OMERACT members said in a joint statement for this article. Meetings have since taken place every 2 years.

OMERACT accomplishments
OMERACT now requires buy-in from four continents: Asia, Australia, Europe, and North America.
Its leaders have developed an explicit process for gaining endorsement of core outcome domains and instrument measurement sets. To fully capture the possibilities of “what to measure,” i.e., “measurable aspects of health conditions,” OMERACT has developed a framework of concepts, core areas, and outcome domains. The key concepts are pathophysiology (with a core area termed “manifestations/abnormalities”) and impact (with core areas of “death/lifespan,” and “life impact,” and the optional area of “societal/resource use”). An outcome domain defines an element of a core area to measure the effects of a treatment, such as blood markers, pain intensity, physical function, or emotional well-being.
A core outcome domain set is developed by agreeing to at least one outcome domain within one of the three core areas. Subsequently, a core outcome measurement set is developed by agreeing to at least one applicable measurement instrument for each core outcome domain. This requires documentation of validity, summarized under three metrics: truth, discrimination, and feasibility.
OMERACT’s handbook provides tutelage on establishing and implementing core outcomes, and several workbooks offer guidance on developing core outcome domain sets, selecting instruments for core outcome measurement sets, and OMERACT methodology.
All this work has led to widespread adoption.
Approval agencies have accepted OMERACT’s filter and methods advances, which have been adopted by many research groups in rheumatology and among nonrheumatology research groups. Organizations such as the U.S. National Institutes of Health’s National Institute of Neurological Disorders and Stroke have sought its advice.
Its core outcomes have been adopted and used for approval in the great majority of studies on rheumatoid arthritis, Dr. Tugwell said.
Several BMJ articles underscore the influence and uptake of OMERACT’s core outcome set. One 2017 paper, which analyzed 273 randomized trials of rheumatoid arthritis drug treatments on ClinicalTrials.gov, found that the WHO-ILAR arthritis core outcome set was reported in 81% of the studies. “The adoption of a core outcome set has the potential to increase consistency in outcomes measured across trials and ensure that trials are more likely to measure appropriate outcomes,” the authors concluded.
Since the initial 1992 meeting, OMERACT has broadened its focus from rheumatoid arthritis to 25 other musculoskeletal conditions.
For example, other OMERACT conferences have led to consensus on core sets of measures for osteoarthritis and osteoporosis, psoriasis/psoriatic arthritis, psychosocial measures, and a core set of data for cost-effectiveness evaluations.
‘Speed is a limitation’
OMERACT is a bottom-up volunteer organization. It doesn’t represent any official organization of any clinical society. “We’ve not asked to be adopted by the American College of Rheumatology, EULAR [European Alliance of Associations for Rheumatology], or other international organizations,” Dr. Tugwell said. It offers a chance for patients, users, and doers of research to work together to agree on rigorous criteria accepted by the approval agencies and take the necessary time to work things through.
This is not a fast process, usually taking 4-6 years to initiate and establish an outcome domain set, he emphasized. “It would be beneficial to do it faster if we had the resources to meet every year. The fact is we’re a volunteer organization that meets every 2 years.”
Speed is a limitation, he acknowledged, but it’s an acceptable trade-off for doing things correctly.
The group has faced other challenges during the COVID-19 pandemic, pivoting to a virtual format that had benefits and limitations.
In one respect, moving to a virtual meeting increased uptake in participation and voting, Dr. Tugwell said. Patient participants with severe rheumatoid arthritis no longer faced the challenges of travel. “On the other hand, we didn’t have the same opportunity to achieve common ground virtually,” he said. “Where there are strong disagreements, I’m a great believer that people need to know one another. There needs to be relationship building.”
OMERACT’s emerging leader program has been a cornerstone of its in-person meetings, engaging young rheumatologists to interact with some of the leaders of outcome measurement. The virtual format dampened this process somewhat, eliminating those important “café chats” between the stakeholders.
The hope is to bring people face-to-face once more at the next meeting in May 2023. The agenda will focus on relationship building, identifying controversial areas, and bringing younger people to develop relationships, Dr. Tugwell said. OMERACT will retain a virtual option for the worldwide voting, “which will allow for more buy-in from so many more people,” he added.
A consensus on pain
The onus of developing outcome measures that move with the times is sometimes too great for one group to manage. In 2018, OMERACT became a part of the Red Hat Group (RHG), an organization conceived at the COMET (Core Outcome Measures in Effectiveness Trials) VII meeting in Amsterdam.
RHG aims to improve the choice of outcomes in health research. It includes eight groups: COMET; OMERACT; the Cochrane Skin Core Outcome Set Initiative; Grading of Recommendations, Assessment, Development and Evaluations; Center for Medical Technology Policy; COnsensus-based Standards for the selection of health Measurement Instruments; Clinical Data Interchange Standards Consortium; and Standardized Outcomes in Nephrology.
The collaboration between groups offers a “very interesting interface between consensus building as well as hard evidence,” Dr. Tugwell said. The focus goes beyond rheumatology to other clinical areas of common interest, exploring how one classifies outcome domains in terms of symptoms, life impact, or death.
Pain is an important common denominator that the RHG has evaluated.
“We believe it’s too general. We’re trying to define pain across all Red Hat Groups because it’s clear that the research community has all these different scales for defining pain severity,” Dr. Tugwell said. “We have to find a way to make ruthless decisions and rules for doing it. And of course, it has to be transparent.”
Looking ahead
As part of its ongoing work, OMERACT is evaluating the robustness of instruments that rheumatologists use as outcome measures in clinical trials, which can be a laborious process. The OMERACT Filter 2.0, part of the latest iteration of the handbook, offers strong guidance for researchers but needs a long-term strategy and key methodological support. “To that end, we set up a technical advisory group to help people in the instrument selection work and that remains an ongoing process,” OMERACT leaders said in their joint statement.
OMERACT is looking at opportunities to create benchmark processes for developing core sets outside of rheumatology and a methodology around outcome measures such as contextual factors, composites, and surrogates.
It will also be taking a step back to solicit opinions from the approval agencies represented by the OMERACT membership on the OMERACT handbook.
The goal is to make sure the handbook aligns with everyone else’s approval and labeling requirements.
OMERACT’s patient participants bring important perspectives
OMERACT over the years has sought to become a more patient-centered group. Patients have been involved in OMERACT activities since its sixth meeting, forming an independent, yet integrated, group within the network. They have their own steering committee and produced and helped to update a glossary for OMERACT patients and professionals.
Catherine (McGowan) Hofstetter, who was diagnosed with rheumatoid arthritis 30 years ago, chairs OMERACT’s Patient Research Partners Support Team. In a Q&A, she discussed the importance of patient voices and OMERACT’s plans to further educate and include patients in the dialogue on outcomes.
Question: Have patients always been a part of OMERACT meetings?
Answer: Patients have been involved with OMERACT since 2002. The patient voice adds relevance to all the work that OMERACT does. You can’t begin to talk about outcomes unless there is a patient at the table with lived experience.
Q: Can you cite a few examples of how the patient voice enriches the conversation on outcomes research?
A: Outcomes and priorities that are important to patients are often completely different than those of the clinician. For instance, a work outcome is important to someone who doesn’t have any medical insurance or disability insurance, so that you can ensure that there is food on the table and a roof over your head. Or it may be important to someone because the employment provides medical and disability insurance to provide security for them and their family. These are two different perspectives on work and therefore work priorities and outcomes.
Q: What have been some of the challenges of getting patients to participate?
A: Training patients is one challenge. OMERACT’s work has a very steep learning curve, and while the basics are the same between the groups in terms of looking at what we measure and how we measure it, the nuances of different working groups require a lot of time and energy to be comfortable enough with the work, and then be confident enough to bring your perspective and lived experience to the table. It’s also a very accomplished group, which can be quite intimidating. Self-disclosure is a very personal and intimate undertaking that requires patience, compassion, and respect.
Q: Are there any plans to enhance patient engagement?
A: When we had OMERACT 2020 it was a virtual conference that took place over about 6 months. We had far more patient research partners [PRPs] participate than we have ever had at any OMERACT face-to-face meeting. There is a desire and passion on the part of patients to lend their voices to the work. The working groups meet virtually throughout the year to advance their agendas, and PRPs are a part of each of the working groups.
Hopefully, we can start working toward including more voices at the conferences by enabling a hybrid model. The PRP Support Team will begin engaging patients this fall with education, mentoring, and team-building exercises so by the time we meet in person in May 2023, they will have enough background knowledge and information to give them the confidence that will enhance their experience at the face-to-face meeting.
We also need to ensure that those patients who want to stay engaged can. This means that the education and training should continue long after the face-to-face meeting is over. We need to build capacity in the PRP group and look to succession planning and be a resource to working groups struggling to find PRPs to work with them on a longer-term basis.
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022
VTE risk not elevated in AD patients on JAK inhibitors: Study
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
FROM JAMA DERMATOLOGY
How docs in firearm-friendly states talk gun safety
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Vaccine hope now for leading cause of U.S. infant hospitalizations: RSV
Respiratory syncytial virus (RSV) is the leading cause of U.S. infant hospitalizations overall and across population subgroups, new data published in the Journal of Infectious Diseases confirm.
Acute bronchiolitis caused by RSV accounted for 9.6% (95% confidence interval, 9.4%-9.9%) and 9.3% (95% CI, 9.0%-9.6%) of total infant hospitalizations from January 2009 to September 2015 and October 2015 to December 2019, respectively.
Journal issue includes 14 RSV studies
The latest issue of the journal includes a special section with results from 14 studies related to the widespread, easy-to-catch virus, highlighting the urgency of finding a solution for all infants.
In one study, authors led by Mina Suh, MPH, with EpidStrategies, a division of ToxStrategies in Rockville, Md., reported that, in children under the age of 5 years in the United States, RSV caused 58,000 annual hospitalizations and from 100 to 500 annual deaths from 2009 to 2019 (the latest year data were available).
Globally, in 2015, among infants younger than 6 months, an estimated 1.4 million hospital admissions and 27,300 in-hospital deaths were attributed to RSV lower respiratory tract infection (LRTI).
The researchers used the largest publicly available, all-payer database in the United States – the National (Nationwide) Inpatient Sample – to describe the leading causes of infant hospitalizations.
The authors noted that, because clinicians don’t routinely perform lab tests for RSV, the true health care burden is likely higher and its public health impact greater than these numbers show.
Immunization candidates advance
There are no preventative options currently available to substantially cut RSV infections in all infants, though immunization candidates are advancing, showing safety and efficacy in clinical trials.
Palivizumab is currently the only available option in the United States to prevent RSV and is recommended only for a small group of infants with particular forms of heart or lung disease and those born prematurely at 29 weeks’ gestational age. Further, palivizumab has to be given monthly throughout the RSV season.
Another of the studies in the journal supplement concluded that a universal immunization strategy with one of the candidates, nirsevimab (Sanofi, AstraZeneca), an investigational long-acting monoclonal antibody, could substantially reduce the health burden and economic burden for U.S. infants in their first RSV season.
The researchers, led by Alexia Kieffer, MSc, MPH, with Sanofi, used static decision-analytic modeling for the estimates. Modeled RSV-related outcomes included primary care and ED visits, hospitalizations, including ICU admission and mechanical ventilations, and RSV-related deaths.
“The results of this model suggested that the use of nirsevimab in all infants could reduce health events by 55% and the overall costs to the payer by 49%,” the authors of the study wrote.
According to the study, universal immunization of all infants with nirsevimab is expected to reduce 290,174 RSV-related medically attended LRTI (MALRTI), 24,986 hospitalizations, and cut $612 million in costs to the health care system.
The authors wrote: “While this reduction would be driven by term infants, who account for most of the RSV-MALRTI burden; all infants, including palivizumab-eligible and preterm infants who suffer from significantly higher rates of disease, would benefit from this immunization strategy.”
Excitement for another option
Jörn-Hendrik Weitkamp, MD, professor of pediatrics and director for patient-oriented research at Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said in an interview there is much excitement in the field for nirsevimab as it has significant advantages over palivizumab.
RSV “is a huge burden to the children, the families, the hospitals, and the medical system,” he said.
Ideally there would be a vaccine to offer the best protection, he noted.
“People have spent their lives, their careers trying to develop a vaccine for RSV,” he said, but that has been elusive for more than 60 years. Therefore, passive immunization is the best of the current options, he says, and nirsevimab “seems to be very effective.”
What’s not clear, Dr. Weitkamp said, is how much nirsevimab will cost as it is not yet approved by the Food and Drug Administration. However, it has the great advantage of being given only once before the season starts instead of monthly (as required for palivizumab) through the season, “which is painful, inconvenient, and traumatizing. We limit that one to the children at highest risk.”
Rolling out an infant nirsevimab program would likely vary by geographic region, Ms. Kieffer and colleagues said, to help ensure infants are protected during the peak of their region’s RSV season.
The journal’s RSV supplement was supported by Sanofi and AstraZeneca. The studies by Ms. Suh and colleagues and Ms. Kieffer and colleagues were supported by AstraZeneca and Sanofi. Ms. Suh and several coauthors are employees of EpidStrategies. One coauthor is an employee of Sanofi and may hold shares and/or stock options in the company. Ms. Kieffer and several coauthors are employees of Sanofi and may hold shares and/or stock options in the company. Dr. Weitkamp reported no relevant financial relationships.
Respiratory syncytial virus (RSV) is the leading cause of U.S. infant hospitalizations overall and across population subgroups, new data published in the Journal of Infectious Diseases confirm.
Acute bronchiolitis caused by RSV accounted for 9.6% (95% confidence interval, 9.4%-9.9%) and 9.3% (95% CI, 9.0%-9.6%) of total infant hospitalizations from January 2009 to September 2015 and October 2015 to December 2019, respectively.
Journal issue includes 14 RSV studies
The latest issue of the journal includes a special section with results from 14 studies related to the widespread, easy-to-catch virus, highlighting the urgency of finding a solution for all infants.
In one study, authors led by Mina Suh, MPH, with EpidStrategies, a division of ToxStrategies in Rockville, Md., reported that, in children under the age of 5 years in the United States, RSV caused 58,000 annual hospitalizations and from 100 to 500 annual deaths from 2009 to 2019 (the latest year data were available).
Globally, in 2015, among infants younger than 6 months, an estimated 1.4 million hospital admissions and 27,300 in-hospital deaths were attributed to RSV lower respiratory tract infection (LRTI).
The researchers used the largest publicly available, all-payer database in the United States – the National (Nationwide) Inpatient Sample – to describe the leading causes of infant hospitalizations.
The authors noted that, because clinicians don’t routinely perform lab tests for RSV, the true health care burden is likely higher and its public health impact greater than these numbers show.
Immunization candidates advance
There are no preventative options currently available to substantially cut RSV infections in all infants, though immunization candidates are advancing, showing safety and efficacy in clinical trials.
Palivizumab is currently the only available option in the United States to prevent RSV and is recommended only for a small group of infants with particular forms of heart or lung disease and those born prematurely at 29 weeks’ gestational age. Further, palivizumab has to be given monthly throughout the RSV season.
Another of the studies in the journal supplement concluded that a universal immunization strategy with one of the candidates, nirsevimab (Sanofi, AstraZeneca), an investigational long-acting monoclonal antibody, could substantially reduce the health burden and economic burden for U.S. infants in their first RSV season.
The researchers, led by Alexia Kieffer, MSc, MPH, with Sanofi, used static decision-analytic modeling for the estimates. Modeled RSV-related outcomes included primary care and ED visits, hospitalizations, including ICU admission and mechanical ventilations, and RSV-related deaths.
“The results of this model suggested that the use of nirsevimab in all infants could reduce health events by 55% and the overall costs to the payer by 49%,” the authors of the study wrote.
According to the study, universal immunization of all infants with nirsevimab is expected to reduce 290,174 RSV-related medically attended LRTI (MALRTI), 24,986 hospitalizations, and cut $612 million in costs to the health care system.
The authors wrote: “While this reduction would be driven by term infants, who account for most of the RSV-MALRTI burden; all infants, including palivizumab-eligible and preterm infants who suffer from significantly higher rates of disease, would benefit from this immunization strategy.”
Excitement for another option
Jörn-Hendrik Weitkamp, MD, professor of pediatrics and director for patient-oriented research at Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said in an interview there is much excitement in the field for nirsevimab as it has significant advantages over palivizumab.
RSV “is a huge burden to the children, the families, the hospitals, and the medical system,” he said.
Ideally there would be a vaccine to offer the best protection, he noted.
“People have spent their lives, their careers trying to develop a vaccine for RSV,” he said, but that has been elusive for more than 60 years. Therefore, passive immunization is the best of the current options, he says, and nirsevimab “seems to be very effective.”
What’s not clear, Dr. Weitkamp said, is how much nirsevimab will cost as it is not yet approved by the Food and Drug Administration. However, it has the great advantage of being given only once before the season starts instead of monthly (as required for palivizumab) through the season, “which is painful, inconvenient, and traumatizing. We limit that one to the children at highest risk.”
Rolling out an infant nirsevimab program would likely vary by geographic region, Ms. Kieffer and colleagues said, to help ensure infants are protected during the peak of their region’s RSV season.
The journal’s RSV supplement was supported by Sanofi and AstraZeneca. The studies by Ms. Suh and colleagues and Ms. Kieffer and colleagues were supported by AstraZeneca and Sanofi. Ms. Suh and several coauthors are employees of EpidStrategies. One coauthor is an employee of Sanofi and may hold shares and/or stock options in the company. Ms. Kieffer and several coauthors are employees of Sanofi and may hold shares and/or stock options in the company. Dr. Weitkamp reported no relevant financial relationships.
Respiratory syncytial virus (RSV) is the leading cause of U.S. infant hospitalizations overall and across population subgroups, new data published in the Journal of Infectious Diseases confirm.
Acute bronchiolitis caused by RSV accounted for 9.6% (95% confidence interval, 9.4%-9.9%) and 9.3% (95% CI, 9.0%-9.6%) of total infant hospitalizations from January 2009 to September 2015 and October 2015 to December 2019, respectively.
Journal issue includes 14 RSV studies
The latest issue of the journal includes a special section with results from 14 studies related to the widespread, easy-to-catch virus, highlighting the urgency of finding a solution for all infants.
In one study, authors led by Mina Suh, MPH, with EpidStrategies, a division of ToxStrategies in Rockville, Md., reported that, in children under the age of 5 years in the United States, RSV caused 58,000 annual hospitalizations and from 100 to 500 annual deaths from 2009 to 2019 (the latest year data were available).
Globally, in 2015, among infants younger than 6 months, an estimated 1.4 million hospital admissions and 27,300 in-hospital deaths were attributed to RSV lower respiratory tract infection (LRTI).
The researchers used the largest publicly available, all-payer database in the United States – the National (Nationwide) Inpatient Sample – to describe the leading causes of infant hospitalizations.
The authors noted that, because clinicians don’t routinely perform lab tests for RSV, the true health care burden is likely higher and its public health impact greater than these numbers show.
Immunization candidates advance
There are no preventative options currently available to substantially cut RSV infections in all infants, though immunization candidates are advancing, showing safety and efficacy in clinical trials.
Palivizumab is currently the only available option in the United States to prevent RSV and is recommended only for a small group of infants with particular forms of heart or lung disease and those born prematurely at 29 weeks’ gestational age. Further, palivizumab has to be given monthly throughout the RSV season.
Another of the studies in the journal supplement concluded that a universal immunization strategy with one of the candidates, nirsevimab (Sanofi, AstraZeneca), an investigational long-acting monoclonal antibody, could substantially reduce the health burden and economic burden for U.S. infants in their first RSV season.
The researchers, led by Alexia Kieffer, MSc, MPH, with Sanofi, used static decision-analytic modeling for the estimates. Modeled RSV-related outcomes included primary care and ED visits, hospitalizations, including ICU admission and mechanical ventilations, and RSV-related deaths.
“The results of this model suggested that the use of nirsevimab in all infants could reduce health events by 55% and the overall costs to the payer by 49%,” the authors of the study wrote.
According to the study, universal immunization of all infants with nirsevimab is expected to reduce 290,174 RSV-related medically attended LRTI (MALRTI), 24,986 hospitalizations, and cut $612 million in costs to the health care system.
The authors wrote: “While this reduction would be driven by term infants, who account for most of the RSV-MALRTI burden; all infants, including palivizumab-eligible and preterm infants who suffer from significantly higher rates of disease, would benefit from this immunization strategy.”
Excitement for another option
Jörn-Hendrik Weitkamp, MD, professor of pediatrics and director for patient-oriented research at Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said in an interview there is much excitement in the field for nirsevimab as it has significant advantages over palivizumab.
RSV “is a huge burden to the children, the families, the hospitals, and the medical system,” he said.
Ideally there would be a vaccine to offer the best protection, he noted.
“People have spent their lives, their careers trying to develop a vaccine for RSV,” he said, but that has been elusive for more than 60 years. Therefore, passive immunization is the best of the current options, he says, and nirsevimab “seems to be very effective.”
What’s not clear, Dr. Weitkamp said, is how much nirsevimab will cost as it is not yet approved by the Food and Drug Administration. However, it has the great advantage of being given only once before the season starts instead of monthly (as required for palivizumab) through the season, “which is painful, inconvenient, and traumatizing. We limit that one to the children at highest risk.”
Rolling out an infant nirsevimab program would likely vary by geographic region, Ms. Kieffer and colleagues said, to help ensure infants are protected during the peak of their region’s RSV season.
The journal’s RSV supplement was supported by Sanofi and AstraZeneca. The studies by Ms. Suh and colleagues and Ms. Kieffer and colleagues were supported by AstraZeneca and Sanofi. Ms. Suh and several coauthors are employees of EpidStrategies. One coauthor is an employee of Sanofi and may hold shares and/or stock options in the company. Ms. Kieffer and several coauthors are employees of Sanofi and may hold shares and/or stock options in the company. Dr. Weitkamp reported no relevant financial relationships.
FROM THE JOURNAL OF INFECTIOUS DISEASES
TikTok’s impact on adolescent mental health
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
First drug therapy approved for childhood GVHD
Specifically, the indication is for pediatric patients with cGVHD who have already been treated with one or more lines of systemic therapy. The manufacturers have also launched a new oral suspension formulation, in addition to capsules and tablets, which were already available.
Ibrutinib is already approved for use in adults with cGVHD.
The drug is also approved for use in several blood cancers, including chronic lymphocytic leukemia, mantle cell lymphoma, and Waldenström’s macroglobulinemia. All these approvals are for adult patients.
This is the first pediatric indication for the product and is “incredibly meaningful,” said Gauri Sunkersett, DO, associate medical director at AbbVie, which markets the drug together with Jansen. “As a pediatric oncologist, when my patients describe the physical pain they experience from simply hugging their parents, due to their cGVHD, the importance of researching alternative treatment options in this patient population is further validated.”
These children have already been through a lot, having been diagnosed with a leukemia or lymphoma and then undergoing chemotherapy and/or radiotherapy for a stem cell transplant. Just over half (52%-65%) of children who receive allogeneic transplants go on to develop cGVHD, in which the donor bone marrow or stem cells attack the recipient.
“Imagine going through a transplant and then being told you have a moderate to severe chronic disease that can sometimes also be life-threatening,” commented Paul A. Carpenter, MD, attending physician at Seattle Children’s Hospital. “If these children were between 1 and 12 and didn’t respond to steroid treatment, we didn’t have any rigorously studied treatment options – until now.”
The new indication was approved by the U.S. Food and Drug Administration on the basis of results from the iMAGINE trial, for which Dr. Carpenter was a principal investigator.
The phase 1/2 iMAGINE trial was an open-label, multicenter, single-arm trial conducted with 47 patients (mean age, 13 years; range, 1-19 years) with relapsed/refractory cGVHD who had received at least one prior systemic therapy. Ibrutinib was given at a dose of 420 mg orally once daily to patients aged 12 and older and at a dose of 240 mg/m2 orally once daily to patients who were younger than 12 years.
The overall response rate through week 25 was 60% (confidence interval, 95%, 44%-74%). The median duration of response was 5.3 months (95% CI, 2.8-8.8).
The safety profile was consistent with the established profile for ibrutinib. Observed adverse events in pediatric patients were consistent with those observed in adult patients with moderate to severe cGVHD, the companies noted.
The FDA noted that the most common (≥ 20%) adverse reactions, including laboratory abnormalities, were anemia, musculoskeletal pain, pyrexia, diarrhea, pneumonia, abdominal pain, stomatitis, thrombocytopenia, and headache.
Full prescribing information for ibrutinib is available here.
A version of this article first appeared on Medscape.com.
Specifically, the indication is for pediatric patients with cGVHD who have already been treated with one or more lines of systemic therapy. The manufacturers have also launched a new oral suspension formulation, in addition to capsules and tablets, which were already available.
Ibrutinib is already approved for use in adults with cGVHD.
The drug is also approved for use in several blood cancers, including chronic lymphocytic leukemia, mantle cell lymphoma, and Waldenström’s macroglobulinemia. All these approvals are for adult patients.
This is the first pediatric indication for the product and is “incredibly meaningful,” said Gauri Sunkersett, DO, associate medical director at AbbVie, which markets the drug together with Jansen. “As a pediatric oncologist, when my patients describe the physical pain they experience from simply hugging their parents, due to their cGVHD, the importance of researching alternative treatment options in this patient population is further validated.”
These children have already been through a lot, having been diagnosed with a leukemia or lymphoma and then undergoing chemotherapy and/or radiotherapy for a stem cell transplant. Just over half (52%-65%) of children who receive allogeneic transplants go on to develop cGVHD, in which the donor bone marrow or stem cells attack the recipient.
“Imagine going through a transplant and then being told you have a moderate to severe chronic disease that can sometimes also be life-threatening,” commented Paul A. Carpenter, MD, attending physician at Seattle Children’s Hospital. “If these children were between 1 and 12 and didn’t respond to steroid treatment, we didn’t have any rigorously studied treatment options – until now.”
The new indication was approved by the U.S. Food and Drug Administration on the basis of results from the iMAGINE trial, for which Dr. Carpenter was a principal investigator.
The phase 1/2 iMAGINE trial was an open-label, multicenter, single-arm trial conducted with 47 patients (mean age, 13 years; range, 1-19 years) with relapsed/refractory cGVHD who had received at least one prior systemic therapy. Ibrutinib was given at a dose of 420 mg orally once daily to patients aged 12 and older and at a dose of 240 mg/m2 orally once daily to patients who were younger than 12 years.
The overall response rate through week 25 was 60% (confidence interval, 95%, 44%-74%). The median duration of response was 5.3 months (95% CI, 2.8-8.8).
The safety profile was consistent with the established profile for ibrutinib. Observed adverse events in pediatric patients were consistent with those observed in adult patients with moderate to severe cGVHD, the companies noted.
The FDA noted that the most common (≥ 20%) adverse reactions, including laboratory abnormalities, were anemia, musculoskeletal pain, pyrexia, diarrhea, pneumonia, abdominal pain, stomatitis, thrombocytopenia, and headache.
Full prescribing information for ibrutinib is available here.
A version of this article first appeared on Medscape.com.
Specifically, the indication is for pediatric patients with cGVHD who have already been treated with one or more lines of systemic therapy. The manufacturers have also launched a new oral suspension formulation, in addition to capsules and tablets, which were already available.
Ibrutinib is already approved for use in adults with cGVHD.
The drug is also approved for use in several blood cancers, including chronic lymphocytic leukemia, mantle cell lymphoma, and Waldenström’s macroglobulinemia. All these approvals are for adult patients.
This is the first pediatric indication for the product and is “incredibly meaningful,” said Gauri Sunkersett, DO, associate medical director at AbbVie, which markets the drug together with Jansen. “As a pediatric oncologist, when my patients describe the physical pain they experience from simply hugging their parents, due to their cGVHD, the importance of researching alternative treatment options in this patient population is further validated.”
These children have already been through a lot, having been diagnosed with a leukemia or lymphoma and then undergoing chemotherapy and/or radiotherapy for a stem cell transplant. Just over half (52%-65%) of children who receive allogeneic transplants go on to develop cGVHD, in which the donor bone marrow or stem cells attack the recipient.
“Imagine going through a transplant and then being told you have a moderate to severe chronic disease that can sometimes also be life-threatening,” commented Paul A. Carpenter, MD, attending physician at Seattle Children’s Hospital. “If these children were between 1 and 12 and didn’t respond to steroid treatment, we didn’t have any rigorously studied treatment options – until now.”
The new indication was approved by the U.S. Food and Drug Administration on the basis of results from the iMAGINE trial, for which Dr. Carpenter was a principal investigator.
The phase 1/2 iMAGINE trial was an open-label, multicenter, single-arm trial conducted with 47 patients (mean age, 13 years; range, 1-19 years) with relapsed/refractory cGVHD who had received at least one prior systemic therapy. Ibrutinib was given at a dose of 420 mg orally once daily to patients aged 12 and older and at a dose of 240 mg/m2 orally once daily to patients who were younger than 12 years.
The overall response rate through week 25 was 60% (confidence interval, 95%, 44%-74%). The median duration of response was 5.3 months (95% CI, 2.8-8.8).
The safety profile was consistent with the established profile for ibrutinib. Observed adverse events in pediatric patients were consistent with those observed in adult patients with moderate to severe cGVHD, the companies noted.
The FDA noted that the most common (≥ 20%) adverse reactions, including laboratory abnormalities, were anemia, musculoskeletal pain, pyrexia, diarrhea, pneumonia, abdominal pain, stomatitis, thrombocytopenia, and headache.
Full prescribing information for ibrutinib is available here.
A version of this article first appeared on Medscape.com.
Monkeypox in children and women remains rare, CDC data show
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Well-child visits rise, but disparities remain
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Adherence to well-child visits in the United States increased overall over a 10-year period, but a gap of up to 20% persisted between the highest and lowest adherence groups, reflecting disparities by race and ethnicity, poverty level, geography, and insurance status.
Well-child visits are recommended to provide children with preventive health and development services, ensure immunizations, and allow parents to discuss health concerns, wrote Salam Abdus, PhD, and Thomas M. Selden, PhD, of the Agency for Healthcare Research and Quality, Rockville, Md.
“We know from prior studies that as of 2008, well-child visits were trending upward, but often fell short of recommendations among key socioeconomic groups,” they wrote.
To examine recent trends in well-child visits, the researchers conducted a cross-sectional study of data from the Medical Expenditure Panel Survey (MEPS) on children aged 0 to 18 years. The findings were published in JAMA Pediatrics.
The study population included 19,018 children in 2006 and 2007 and 17,533 children in 2016 and 2017.
Adherence was defined as the ratio of reported well-child visits divided by the recommended number of visits in a calendar year.
Overall, the mean adherence increased from 47.9% in 2006-2007 to 62.3% in 2016-2017.
However, significant gaps persisted across race and ethnicity. Notably, adherence in the Hispanic population increased by nearly 22% between the study dates, compared to a 15.3% increase among White non-Hispanic children. However, Hispanic children still trailed White children overall in 2016-2017 (58% vs. 67.8%).
The smallest increase in adherence occurred among Black non-Hispanic children (5.6%) which further widened the gap between Black and White non-Hispanic children in 2016-2017 (52.5% vs. 67.8%).
Adherence rates increased similarly for children with public and private insurance (15.5% and 13.9%, respectively), but the adherence rates for uninsured children remained stable. Adherence in 2016-2017 for children with private, public, and no insurance were 66.3%, 58.7%, and 31.1%.
Also, despite overall increases in adherence across regions, a gap of more than 20% separated the region with the highest adherence (Northeast) from the lowest (West) in both the 2006-2007 and 2016-2017 periods (69.3% vs. 38.4%, and 79.3% vs. 55.2%, respectively).
The findings show an increase in well-child visits that spanned a time period of increased recommendations, economic changes, and the impact of the Affordable Care Act, but unaddressed disparities remain, the researchers noted.
Reducing disparities and improving adherence, “will require the combined efforts of researchers, policymakers, and clinicians to improve our understanding of adherence, to implement policies improving access to care, and to increase health care professional engagement with disadvantaged communities,” they concluded.
Overall increases are encouraging, but barriers need attention
“Demographic data are critical to determine which groups of children need the most support for recommended well child care,” Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H., said in an interview. In the current study, “it was encouraging to see how either public or private insurance significantly increased the percentage of children receiving well child care,” she said.
The level of increased adherence to AAP-recommended guidelines for well-child visits was surprising, said Dr. Boulter. The overall increase is likely attributable in part to the increased coverage for well-child visits in the wake of the Affordable Care Act, as the study authors mention, she said.
“The gains experienced by Hispanic families were especially encouraging,” she added.
However, ongoing barriers to well-child care include “lack of adequate provider numbers and mix, transportation difficulties for patients, and lack of child care and time away from work for parents so they can complete the recommended well child visit schedule,” Dr. Boulter noted. “Provider schedules and locations of care should be improved so families would have easier access. Also, social media should have more positive well-child messages to counteract the negative messaging.”
More research is needed to examine the impact of COVID-19 on well-child visits, Dr. Boulter emphasized. “Most likely, the percentages in all groups will have changed since COVID-19 has impacted office practices,” she said. “Anxiety about COVID-19 transmissibility in the pediatric office decreased routine office visits, and skepticism about vaccines, including vaccine refusal, has significantly changed the percentage of children who have received the AAP recommended vaccines,” she explained. Ideally, the study authors will review the MEPS data again to examine changes since the COVID-19 pandemic began, she told this news organization.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS











