User login
Treatment of an Unresectable Cutaneous Squamous Cell Carcinoma With ED&C and 5-FU
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
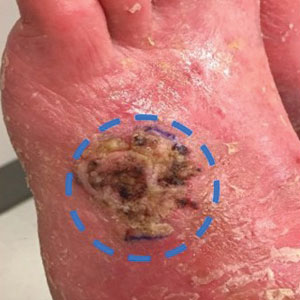
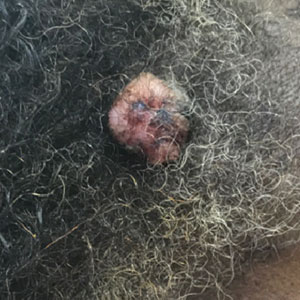
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.
Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
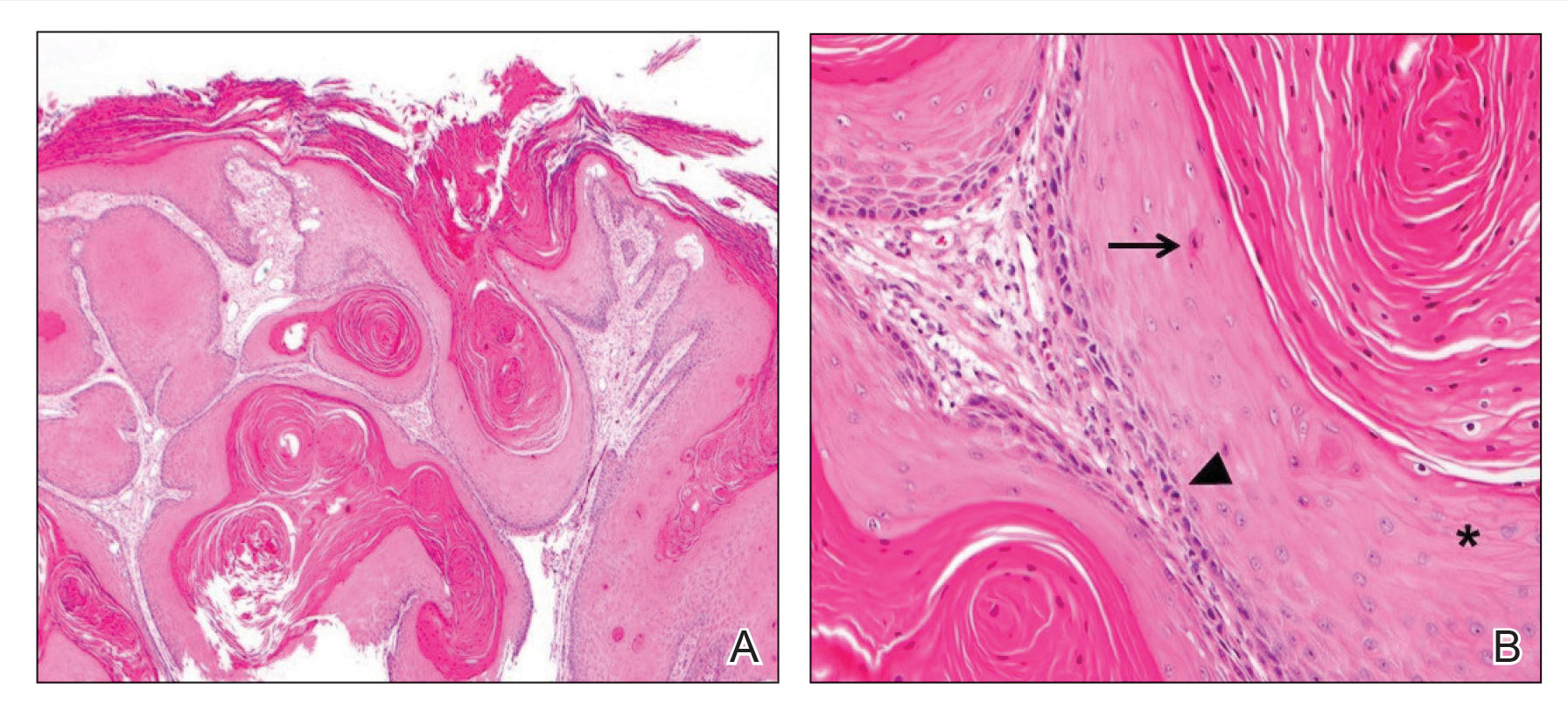
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.
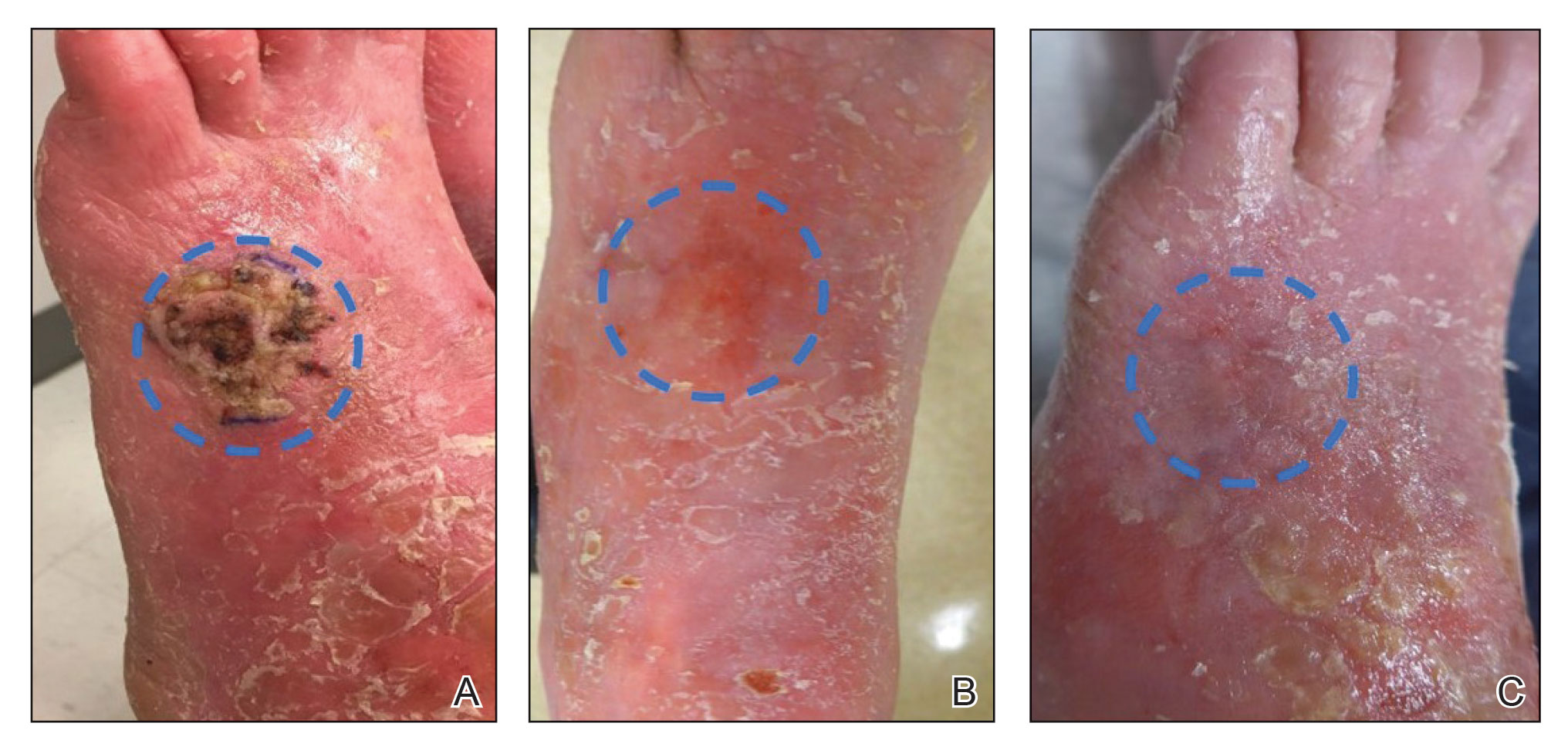
Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
To the Editor:
Most cutaneous squamous cell carcinomas (cSCCs) are successfully treated with standard modalities such as surgical excision; however, a subset of tumors is not amenable to surgical resection.1,2 Patients who are not able to undergo surgical treatment may instead receive radiation therapy, topical 5-fluorouracil (5-FU), imiquimod, cryosurgery, photodynamic therapy, or systemic treatment (eg, immunotherapy) in addition to intralesional approaches for localized disease.1-4 However, the adverse effects associated with these treatments and their modest effect in preventing the recurrence of cutaneous lesions limit their efficacy against unresectable cSCC.4-6 We present a case that demonstrates the efficacy of electrodesiccation and curettage (ED&C) followed by topical 5-FU for an invasive cSCC not amenable to surgical therapy.
A 58-year-old woman presented for evaluation of a 3.5×3.4-cm, incisional biopsy–proven, invasive stage T2a cSCC (Brigham and Women’s Hospital tumor staging system [Boston, Massachusetts]) on the dorsal aspect of the left foot, which had developed over several months (Figure 1A). She had a history of treatment with psoralen plus UV light therapy for erythroderma of unknown cause and peripheral neuropathy. She was not a surgical candidate because of suspected underlying cutaneous sclerosis and a history of poor wound healing on the lower legs.

Prior to presentation to dermatology, the patient had been treated with intralesional methotrexate, intralesional 5-FU, and the antiangiogenic and antiproliferative combination agent OLCAT-0053—consisting of equal parts [by volume] of diclofenac gel 3%, imiquimod cream 5%, hydrocortisone valerate cream 0.2%, calcipotriene cream 0.005%, and tretinoin cream 0.05—which failed, and the patient reported that OLCAT-005 made the pain from the cSCC worse.
Upon growth of the lesion over several months, the patient was referred to the High-Risk Skin Cancer Clinic at Massachusetts General Hospital (Boston, Massachusetts). A repeat biopsy demonstrated an invasive well-differentiated cSCC (Figure 2). The size and invasive features of the lesion on clinical examination prompted a referral to surgical oncology for a wide local excision. However, surgical oncology concluded she was not a surgical candidate.

Magnetic resonance imaging showed no deep invasion of the cSCC to the tendons or bones. Electrodesiccation and curettage was performed to debulk the tumor, followed by twice-daily application of topical 5-FU for 4 weeks to improve the odds of tumor clearance (Figure 1B). Fourteen weeks after completion of 5-FU treatment, the cSCC showed complete clinical regression (Figure 1C). No recurrence has been detected clinically more than 3 years following treatment.
Prior to the advent of Mohs micrographic surgery, ED&C commonly was used to treat skin cancer, with a lower cost and a cure rate close to 95%.7,8 We postulate that the mechanism of tumor regression in our patient was ED&C-mediated removal and necrosis of neoplastic tissue combined with 5-FU–induced cancer-cell DNA damage and apoptosis. An antitumor immune response also may have contributed to the complete regression of the cSCC.
Although antiangiogenic and antiproliferative agents are suitable for primary cSCC treatment, it is possible that this patient’s prior therapies alone—in the absence of debulking by ED&C to sufficiently reduce disease burden—did not allow for tumor clearance and were ineffective. Many clinicians are reluctant to apply 5-FU to a wound bed because it can impede wound healing.9 In this case, re-epithelialization likely occurred primarily after completion of 5-FU treatment.
We recommend consideration of ED&C with 5-FU for similar malignant lesions that are not amenable to surgical excision. Nevertheless, Mohs micrographic surgery and wide local excision remain the gold standards for definitive treatment of invasive skin cancer in a patient who is a candidate for surgical treatment.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
- Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med. 2018;379:363-374. doi:10.1056/NEJMra1708701
- de Jong E, Lammerts MUPA, Genders RE, et al. Update of advanced cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2022;36(suppl 1):6-10. doi:10.1111/jdv.17728
- Li VW, Ball RA, Vasan N, et al. Antiangiogenic therapy for squamous cell carcinoma using combinatorial agents [abstract]. J Clin Oncol. 2005;23(16 suppl):3032. doi:10.1200/jco.2005.23.16_suppl.3032
- Lansbury L, Bath-Hextall F, Perkins W, et al. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. doi:10.1136/bmj.f6153
- Behshad R, Garcia‐Zuazaga J, Bordeaux J. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: a review of the literature. Br J Dermatol. 2011;165:1169-1177. doi:10.1111/j.1365-2133.2011.10524.x
- Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976-990. doi:10.1016/0190-9622(92)70144-5
- Knox JM, Lyles TW, Shapiro EM, et al. Curettage and electrodesiccation in the treatment of skin cancer. Arch Dermatol. 1960;82:197-204.
- Chren M-M, Linos E, Torres JS, et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188-1196. doi:10.1038/jid.2012.403
- Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatologic Surgery. 2017;43:S3-S18.
Practice Points
- In a subset of cases of cutaneous squamous cell carcinoma (cSCC), the tumor is not amenable to surgical resection or other standard treatment modalities.
- Electrodesiccation and curettage followed by topical 5-fluorouracil may be an effective option in eliminating unresectable primary cSCCs that do not respond to intralesional treatment.
Porocarcinoma Development in a Prior Trauma Site
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
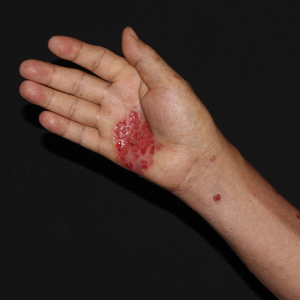
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.
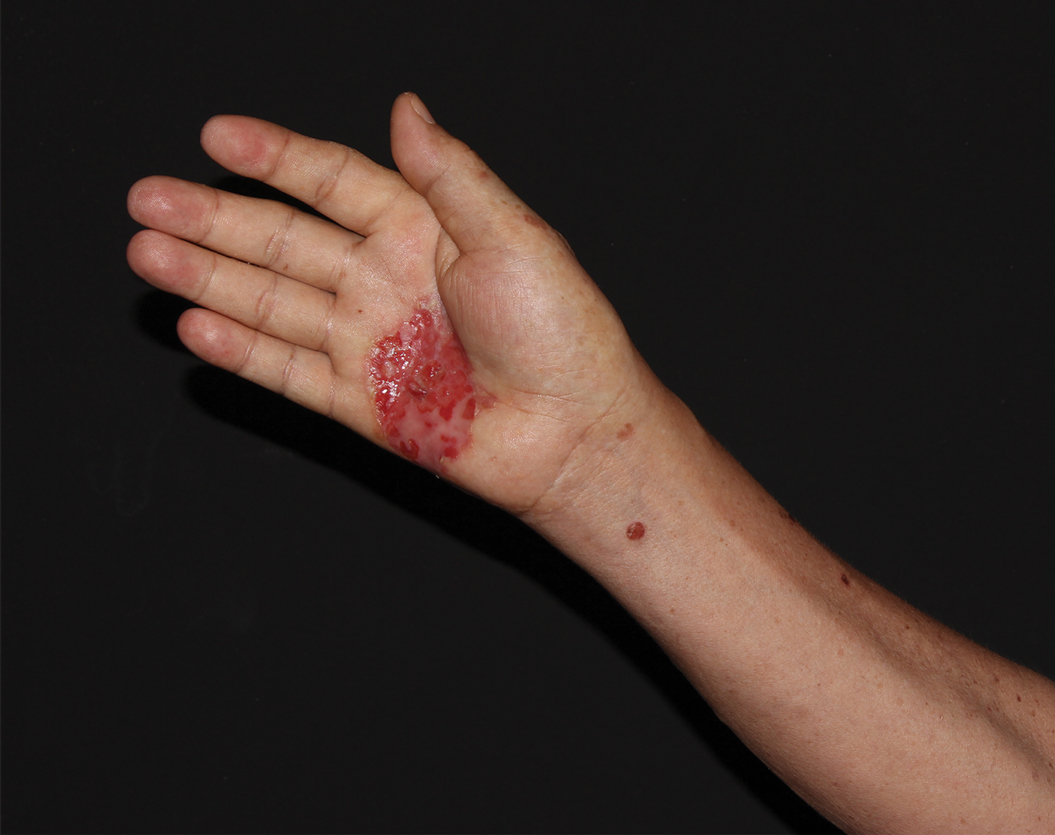
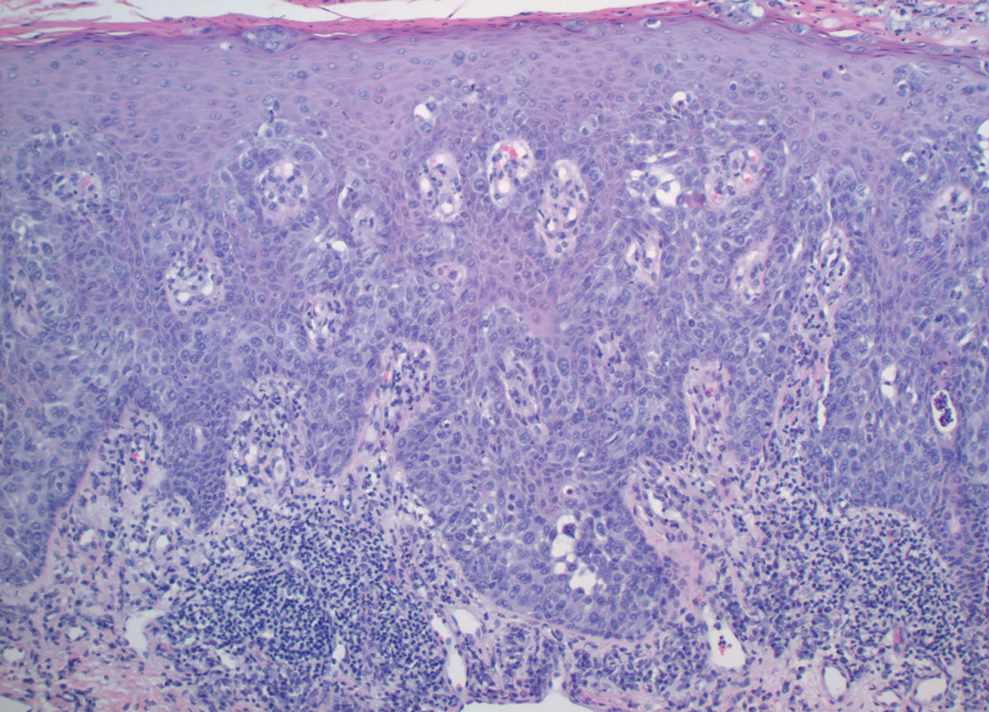
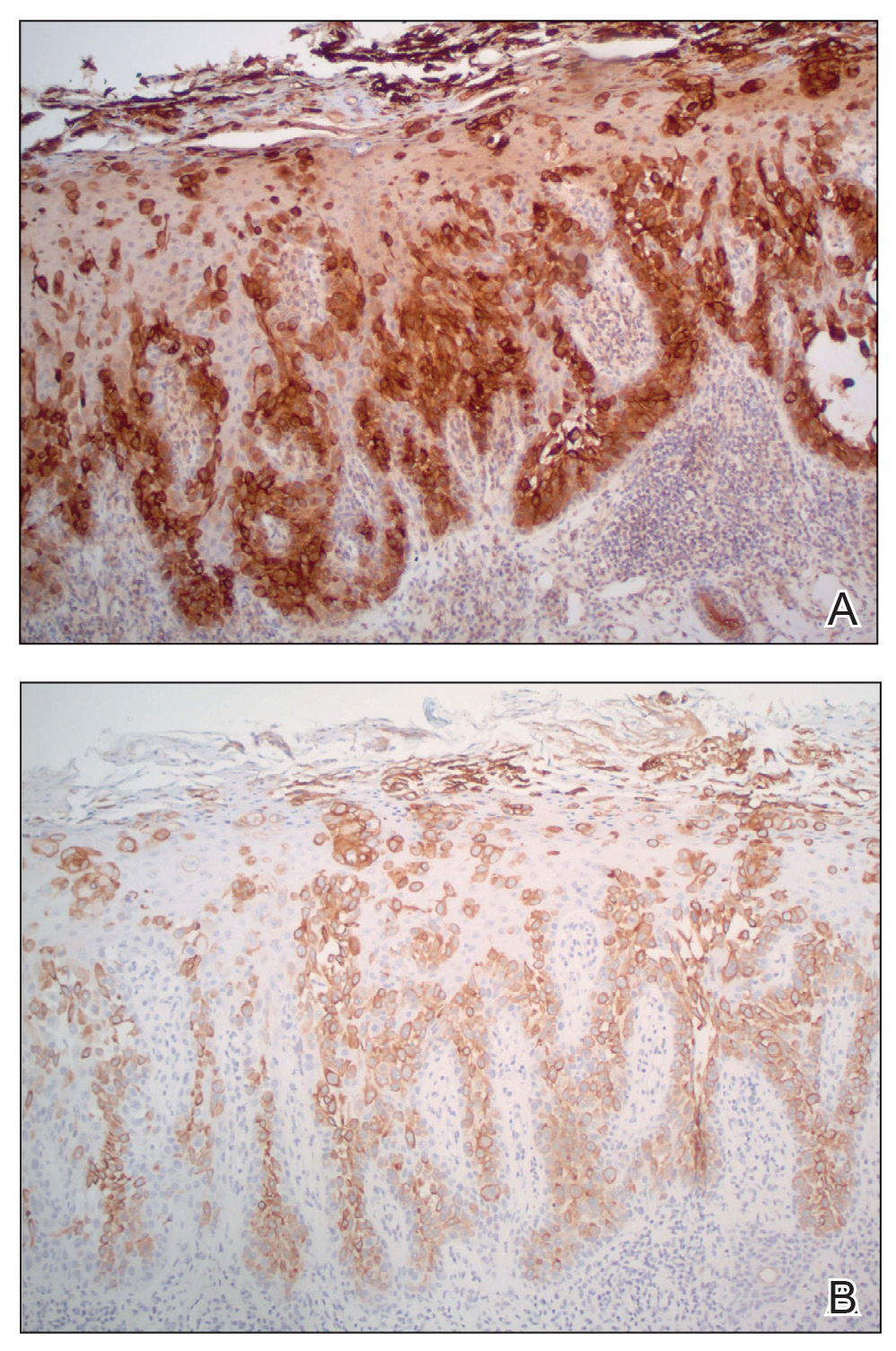
Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.
The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.
Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
Practice Points
- Porocarcinoma is a rare, potentially aggressive, glandular malignancy that should be a clinical consideration in patients presenting with a cutaneous neoplasm.
- Although wide local excision historically has been the treatment of choice for porocarcinoma, Mohs micrographic surgery has demonstrated excellent cure rates.
- Patients with unresectable or metastatic porocarcinomas have a poor prognosis but may respond to combination chemotherapy regimens.
Palliative Care: Utilization Patterns in Inpatient Dermatology
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
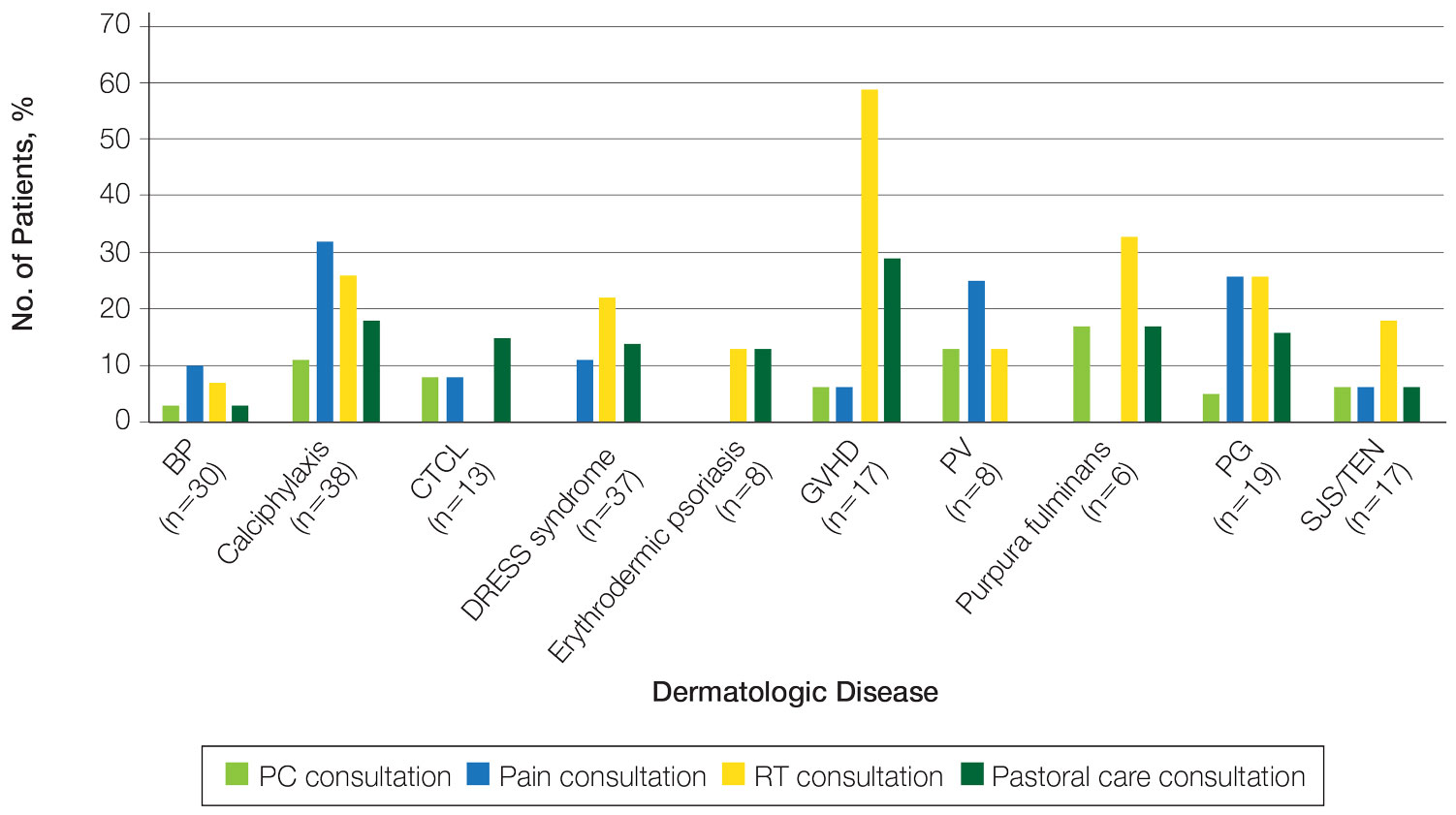
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2
Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Practice Points
- Although severe dermatologic disease negatively impacts patients’ quality of life, palliative care may be underutilized in this population.
- Palliative care should be an integral part of caring for patients who are admitted to the hospital with serious dermatologic illnesses.
Use of the Retroauricular Pull-Through Sandwich Flap for Repair of an Extensive Conchal Bowl Defect With Complete Cartilage Loss
Practice Gap
Repair of a conchal defect requires careful consideration to achieve an optimal outcome. Reconstruction should resurface exposed cartilage, restore the natural projection of the auricle, and direct sound into the external auditory meatus. Patients also should be able to wear glasses and a hearing aid.
The reconstructive ladder for most conchal bowl defects includes secondary intention healing, full-thickness skin grafting (FTSG), and either a revolving-door flap or a flip-flop flap. Secondary intention and FTSG are appropriate for superficial defects, in which the loss of cartilage is not substantial.1,2 Revolving-door and flip-flop flaps are single-stage retroauricular approaches used to repair relatively small defects of the conchal bowl.3 However, reconstructive options are limited for a large defect in which there is extensive loss of cartilage; 3-stage retroauricular approaches have been utilized. The anterior pedicled retroauricular flap is a 3-stage repair that can be utilized to reconstruct a through-and-through defect of the central ear:
- Stage 1: an anteriorly based retroauricular pedicle is incised, hinged over, and sutured to the medial aspect of the defect, resurfacing the posterior ear.
- Stage 2: the pedicle is severed and the flap is folded on itself to resurface the anterior ear.
- Stage 3: the folded edge is de-epithelialized and set into the lateral defect.4
The revolving-door flap also uses a 3-stage approach and is utilized for a full-thickness central auricular defect:
- Stage 1: a revolving-door flap is used to resurface the anterior ear.
- Stage 2: a cartilage graft provides structural support.
- Stage 3: division and inset with an FTSG is used to resurface the posterior ear.
The anterior pedicled retroauricular flap and revolving-door flap techniques are useful for defects when there is intact posterior auricular skin but not when there is extensive loss of cartilage. Other downsides to these 3-stage approaches are the time and multiple procedures required.5
We describe the technique of a retroauricular pull-through sandwich flap for repair of a large conchal bowl defect with extensive cartilage loss and intact posterior auricular skin.
Technique
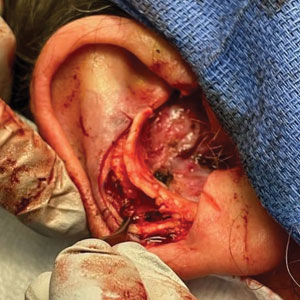
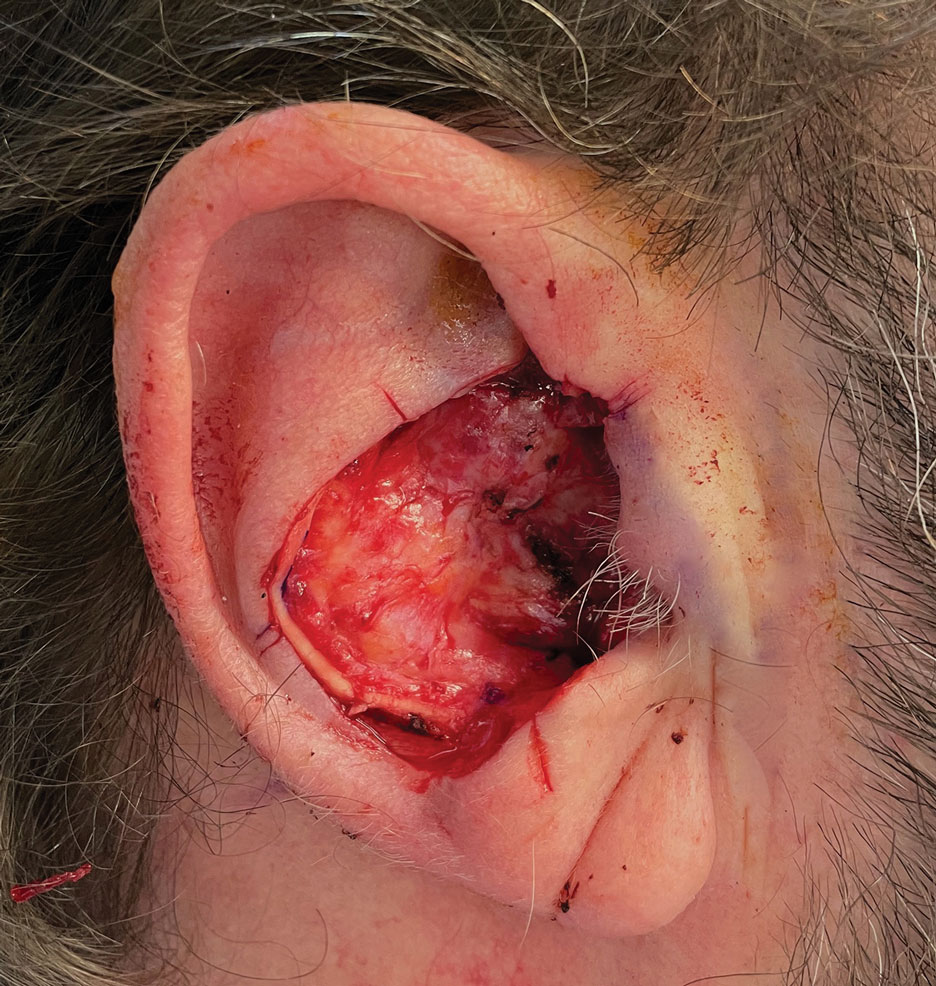
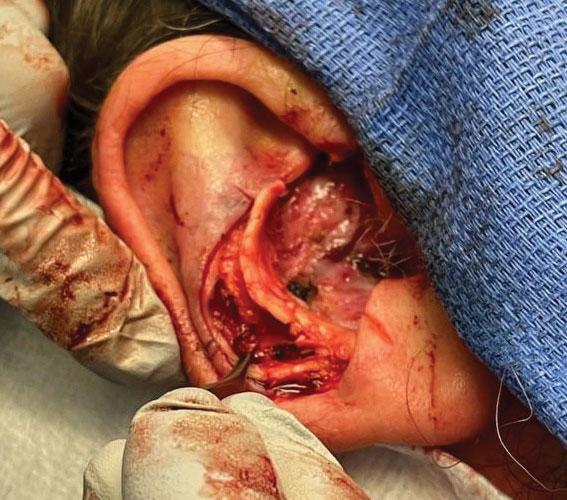
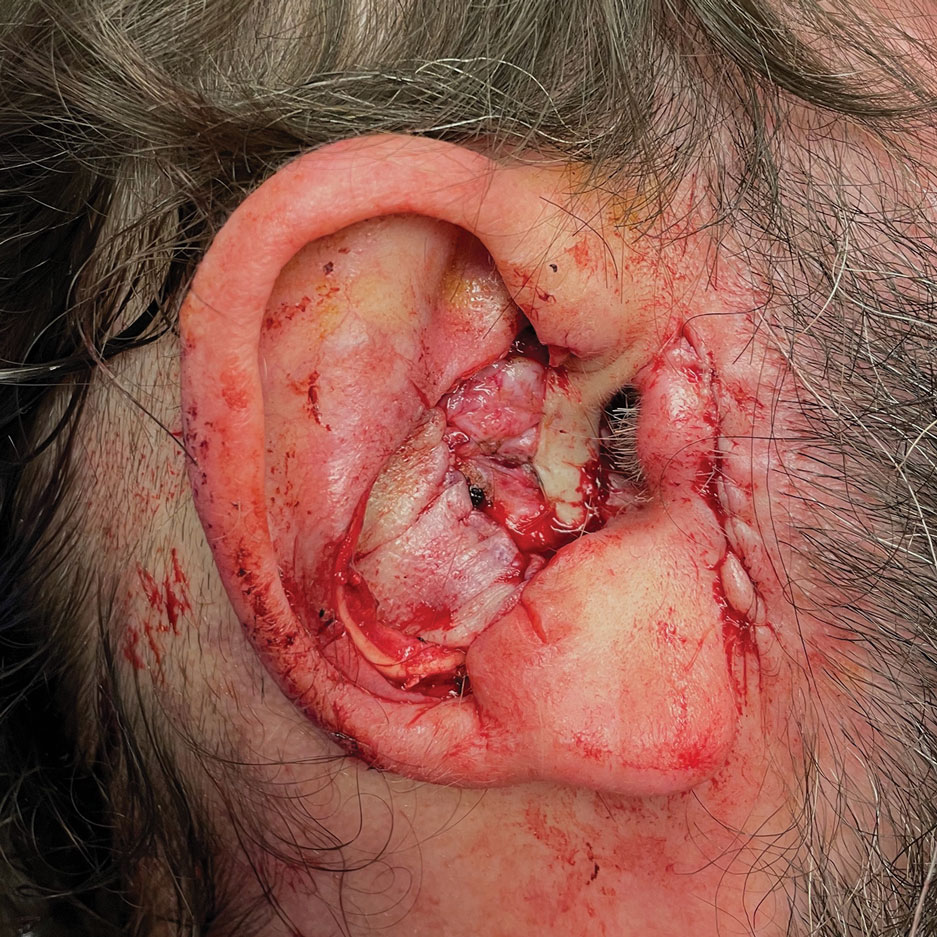
A 62-year-old man presented for treatment of a 2.6×2.4-cm nodular and infiltrative basal cell carcinoma of the right conchal bowl. The tumor was cleared with 3 stages of Mohs micrographic surgery, resulting in a 5.5×4.2-cm defect with complete loss of cartilage throughout the concha, helical crus, and inner rim of the antihelix (Figure 1). A 2-stage repair was performed utilizing a cartilage graft and a pull-through retroauricular interpolation flap.
Stage 1—A cartilage graft was harvested from the left concha and sutured into the central defect for structural support (Figure 2). An incision was then made through the posterior auricular skin, just medial to the residual antihelical cartilage, and a retroauricular interpolation flap was pulled through this incision to resurface the lateral two-thirds of the conchal bowl defect. This created a “sandwich” of tissue, with the following layers (ordered from anterior to posterior): retroauricular interpolation flap, cartilage graft, and intact posterior auricular skin.
A preauricular banner transposition flap was used to repair the medial one-third of the conchal defect. A small area was left to heal by secondary intention (Figure 3).
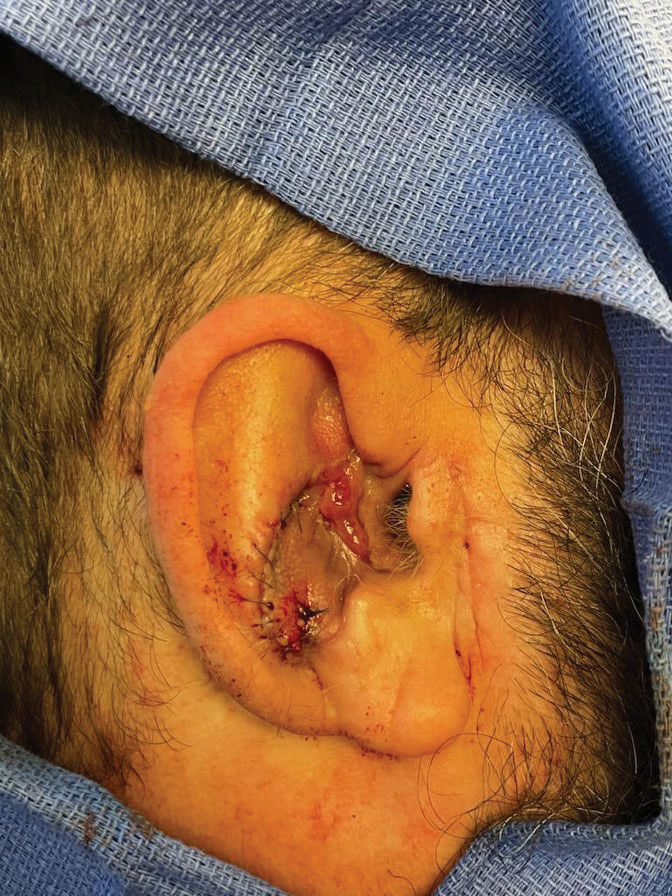
Stage 2—The patient returned 3 weeks later for division and inset of the retroauricular interpolation flap. The pedicle of the flap was severed and its free edge was sutured into the lateral aspect of the defect. The posterior auricular incision that the flap had been pulled through in stage 1 of the repair was closed in a layered fashion, and the secondary defect of the postauricular scalp was left to heal by secondary intention (Figure 4).
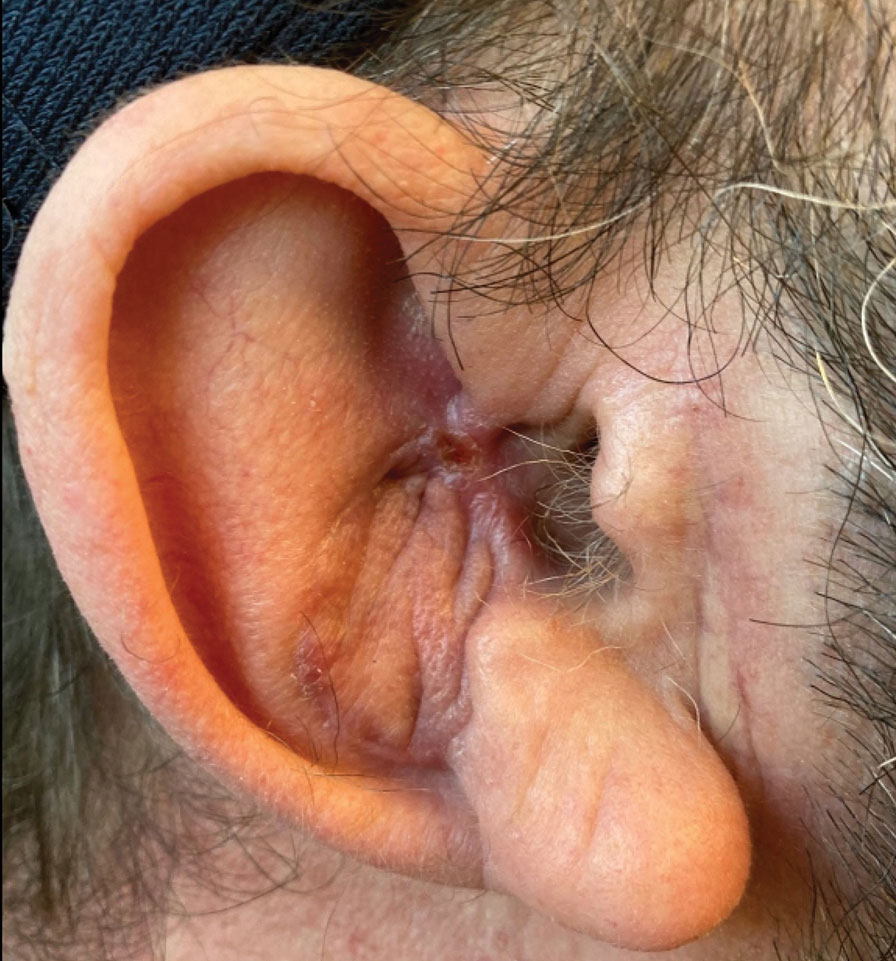
Final Results—At follow-up 1 month later, the patient was noted to have good aesthetic and functional outcomes (Figure 5).
Practice Implications
The retroauricular pull-through sandwich flap combines a cartilage graft and a retroauricular interpolation flap pulled through an incision in the posterior auricular skin to resurface the anterior ear. This repair is most useful for a large conchal bowl defect in which there is extensive missing cartilage but intact posterior auricular skin.
The retroauricular scalp is a substantial tissue reservoir with robust vasculature; an interpolation flap from this area frequently is used to repair an extensive ear defect. The most common use of an interpolation flap is for a large helical defect; however, the flap also can be pulled through an incision in the posterior auricular skin to the front of the ear in a manner similar to revolving-door and flip-flop flaps, thus allowing for increased flap reach.
A cartilage graft provides structural support, helping to maintain auricular projection. The helical arcades provide a robust vascular supply and maintain viability of the helical rim tissue, despite the large aperture created for the pull-through flap.
We recommend this 2-stage repair for large conchal bowl defects with extensive cartilage loss and intact posterior auricular skin.
- Clark DP, Hanke CW. Neoplasms of the conchal bowl: treatment with Mohs micrographic surgery. J Dermatol Surg Oncol. 1988;14:1223-1228. doi:10.1111/j.1524-4725.1988.tb03479.x
- Dessy LA, Figus A, Fioramonti P, et al. Reconstruction of anterior auricular conchal defect after malignancy excision: revolving-door flap versus full-thickness skin graft. J Plast Reconstr Aesthet Surg. 2010;63:746-752. doi:10.1016/j.bjps.2009.01.073
- Golash A, Bera S, Kanoi AV, et al. The revolving door flap: revisiting an elegant but forgotten flap for ear defect reconstruction. Indian J Plast Surg. 2020;53:64-70. doi:10.1055/s-0040-1709531
- Heinz MB, Hölzle F, Ghassemi A. Repairing a non-marginal full-thickness auricular defect using a reversed flap from the postauricular area. J Oral Maxillofac Surg. 2015;73:764-768. doi:10.1016/j.joms.2014.11.005
- Leitenberger JJ, Golden SK. Reconstruction after full-thickness loss of the antihelix, scapha, and triangular fossa. Dermatol Surg. 2016;42:893-896. doi:10.1097/DSS.0000000000000664
Practice Gap
Repair of a conchal defect requires careful consideration to achieve an optimal outcome. Reconstruction should resurface exposed cartilage, restore the natural projection of the auricle, and direct sound into the external auditory meatus. Patients also should be able to wear glasses and a hearing aid.
The reconstructive ladder for most conchal bowl defects includes secondary intention healing, full-thickness skin grafting (FTSG), and either a revolving-door flap or a flip-flop flap. Secondary intention and FTSG are appropriate for superficial defects, in which the loss of cartilage is not substantial.1,2 Revolving-door and flip-flop flaps are single-stage retroauricular approaches used to repair relatively small defects of the conchal bowl.3 However, reconstructive options are limited for a large defect in which there is extensive loss of cartilage; 3-stage retroauricular approaches have been utilized. The anterior pedicled retroauricular flap is a 3-stage repair that can be utilized to reconstruct a through-and-through defect of the central ear:
- Stage 1: an anteriorly based retroauricular pedicle is incised, hinged over, and sutured to the medial aspect of the defect, resurfacing the posterior ear.
- Stage 2: the pedicle is severed and the flap is folded on itself to resurface the anterior ear.
- Stage 3: the folded edge is de-epithelialized and set into the lateral defect.4
The revolving-door flap also uses a 3-stage approach and is utilized for a full-thickness central auricular defect:
- Stage 1: a revolving-door flap is used to resurface the anterior ear.
- Stage 2: a cartilage graft provides structural support.
- Stage 3: division and inset with an FTSG is used to resurface the posterior ear.
The anterior pedicled retroauricular flap and revolving-door flap techniques are useful for defects when there is intact posterior auricular skin but not when there is extensive loss of cartilage. Other downsides to these 3-stage approaches are the time and multiple procedures required.5
We describe the technique of a retroauricular pull-through sandwich flap for repair of a large conchal bowl defect with extensive cartilage loss and intact posterior auricular skin.
Technique
A 62-year-old man presented for treatment of a 2.6×2.4-cm nodular and infiltrative basal cell carcinoma of the right conchal bowl. The tumor was cleared with 3 stages of Mohs micrographic surgery, resulting in a 5.5×4.2-cm defect with complete loss of cartilage throughout the concha, helical crus, and inner rim of the antihelix (Figure 1). A 2-stage repair was performed utilizing a cartilage graft and a pull-through retroauricular interpolation flap.

Stage 1—A cartilage graft was harvested from the left concha and sutured into the central defect for structural support (Figure 2). An incision was then made through the posterior auricular skin, just medial to the residual antihelical cartilage, and a retroauricular interpolation flap was pulled through this incision to resurface the lateral two-thirds of the conchal bowl defect. This created a “sandwich” of tissue, with the following layers (ordered from anterior to posterior): retroauricular interpolation flap, cartilage graft, and intact posterior auricular skin.

A preauricular banner transposition flap was used to repair the medial one-third of the conchal defect. A small area was left to heal by secondary intention (Figure 3).

Stage 2—The patient returned 3 weeks later for division and inset of the retroauricular interpolation flap. The pedicle of the flap was severed and its free edge was sutured into the lateral aspect of the defect. The posterior auricular incision that the flap had been pulled through in stage 1 of the repair was closed in a layered fashion, and the secondary defect of the postauricular scalp was left to heal by secondary intention (Figure 4).

Final Results—At follow-up 1 month later, the patient was noted to have good aesthetic and functional outcomes (Figure 5).

Practice Implications
The retroauricular pull-through sandwich flap combines a cartilage graft and a retroauricular interpolation flap pulled through an incision in the posterior auricular skin to resurface the anterior ear. This repair is most useful for a large conchal bowl defect in which there is extensive missing cartilage but intact posterior auricular skin.
The retroauricular scalp is a substantial tissue reservoir with robust vasculature; an interpolation flap from this area frequently is used to repair an extensive ear defect. The most common use of an interpolation flap is for a large helical defect; however, the flap also can be pulled through an incision in the posterior auricular skin to the front of the ear in a manner similar to revolving-door and flip-flop flaps, thus allowing for increased flap reach.
A cartilage graft provides structural support, helping to maintain auricular projection. The helical arcades provide a robust vascular supply and maintain viability of the helical rim tissue, despite the large aperture created for the pull-through flap.
We recommend this 2-stage repair for large conchal bowl defects with extensive cartilage loss and intact posterior auricular skin.
Practice Gap
Repair of a conchal defect requires careful consideration to achieve an optimal outcome. Reconstruction should resurface exposed cartilage, restore the natural projection of the auricle, and direct sound into the external auditory meatus. Patients also should be able to wear glasses and a hearing aid.
The reconstructive ladder for most conchal bowl defects includes secondary intention healing, full-thickness skin grafting (FTSG), and either a revolving-door flap or a flip-flop flap. Secondary intention and FTSG are appropriate for superficial defects, in which the loss of cartilage is not substantial.1,2 Revolving-door and flip-flop flaps are single-stage retroauricular approaches used to repair relatively small defects of the conchal bowl.3 However, reconstructive options are limited for a large defect in which there is extensive loss of cartilage; 3-stage retroauricular approaches have been utilized. The anterior pedicled retroauricular flap is a 3-stage repair that can be utilized to reconstruct a through-and-through defect of the central ear:
- Stage 1: an anteriorly based retroauricular pedicle is incised, hinged over, and sutured to the medial aspect of the defect, resurfacing the posterior ear.
- Stage 2: the pedicle is severed and the flap is folded on itself to resurface the anterior ear.
- Stage 3: the folded edge is de-epithelialized and set into the lateral defect.4
The revolving-door flap also uses a 3-stage approach and is utilized for a full-thickness central auricular defect:
- Stage 1: a revolving-door flap is used to resurface the anterior ear.
- Stage 2: a cartilage graft provides structural support.
- Stage 3: division and inset with an FTSG is used to resurface the posterior ear.
The anterior pedicled retroauricular flap and revolving-door flap techniques are useful for defects when there is intact posterior auricular skin but not when there is extensive loss of cartilage. Other downsides to these 3-stage approaches are the time and multiple procedures required.5
We describe the technique of a retroauricular pull-through sandwich flap for repair of a large conchal bowl defect with extensive cartilage loss and intact posterior auricular skin.
Technique
A 62-year-old man presented for treatment of a 2.6×2.4-cm nodular and infiltrative basal cell carcinoma of the right conchal bowl. The tumor was cleared with 3 stages of Mohs micrographic surgery, resulting in a 5.5×4.2-cm defect with complete loss of cartilage throughout the concha, helical crus, and inner rim of the antihelix (Figure 1). A 2-stage repair was performed utilizing a cartilage graft and a pull-through retroauricular interpolation flap.

Stage 1—A cartilage graft was harvested from the left concha and sutured into the central defect for structural support (Figure 2). An incision was then made through the posterior auricular skin, just medial to the residual antihelical cartilage, and a retroauricular interpolation flap was pulled through this incision to resurface the lateral two-thirds of the conchal bowl defect. This created a “sandwich” of tissue, with the following layers (ordered from anterior to posterior): retroauricular interpolation flap, cartilage graft, and intact posterior auricular skin.

A preauricular banner transposition flap was used to repair the medial one-third of the conchal defect. A small area was left to heal by secondary intention (Figure 3).

Stage 2—The patient returned 3 weeks later for division and inset of the retroauricular interpolation flap. The pedicle of the flap was severed and its free edge was sutured into the lateral aspect of the defect. The posterior auricular incision that the flap had been pulled through in stage 1 of the repair was closed in a layered fashion, and the secondary defect of the postauricular scalp was left to heal by secondary intention (Figure 4).

Final Results—At follow-up 1 month later, the patient was noted to have good aesthetic and functional outcomes (Figure 5).

Practice Implications
The retroauricular pull-through sandwich flap combines a cartilage graft and a retroauricular interpolation flap pulled through an incision in the posterior auricular skin to resurface the anterior ear. This repair is most useful for a large conchal bowl defect in which there is extensive missing cartilage but intact posterior auricular skin.
The retroauricular scalp is a substantial tissue reservoir with robust vasculature; an interpolation flap from this area frequently is used to repair an extensive ear defect. The most common use of an interpolation flap is for a large helical defect; however, the flap also can be pulled through an incision in the posterior auricular skin to the front of the ear in a manner similar to revolving-door and flip-flop flaps, thus allowing for increased flap reach.
A cartilage graft provides structural support, helping to maintain auricular projection. The helical arcades provide a robust vascular supply and maintain viability of the helical rim tissue, despite the large aperture created for the pull-through flap.
We recommend this 2-stage repair for large conchal bowl defects with extensive cartilage loss and intact posterior auricular skin.
- Clark DP, Hanke CW. Neoplasms of the conchal bowl: treatment with Mohs micrographic surgery. J Dermatol Surg Oncol. 1988;14:1223-1228. doi:10.1111/j.1524-4725.1988.tb03479.x
- Dessy LA, Figus A, Fioramonti P, et al. Reconstruction of anterior auricular conchal defect after malignancy excision: revolving-door flap versus full-thickness skin graft. J Plast Reconstr Aesthet Surg. 2010;63:746-752. doi:10.1016/j.bjps.2009.01.073
- Golash A, Bera S, Kanoi AV, et al. The revolving door flap: revisiting an elegant but forgotten flap for ear defect reconstruction. Indian J Plast Surg. 2020;53:64-70. doi:10.1055/s-0040-1709531
- Heinz MB, Hölzle F, Ghassemi A. Repairing a non-marginal full-thickness auricular defect using a reversed flap from the postauricular area. J Oral Maxillofac Surg. 2015;73:764-768. doi:10.1016/j.joms.2014.11.005
- Leitenberger JJ, Golden SK. Reconstruction after full-thickness loss of the antihelix, scapha, and triangular fossa. Dermatol Surg. 2016;42:893-896. doi:10.1097/DSS.0000000000000664
- Clark DP, Hanke CW. Neoplasms of the conchal bowl: treatment with Mohs micrographic surgery. J Dermatol Surg Oncol. 1988;14:1223-1228. doi:10.1111/j.1524-4725.1988.tb03479.x
- Dessy LA, Figus A, Fioramonti P, et al. Reconstruction of anterior auricular conchal defect after malignancy excision: revolving-door flap versus full-thickness skin graft. J Plast Reconstr Aesthet Surg. 2010;63:746-752. doi:10.1016/j.bjps.2009.01.073
- Golash A, Bera S, Kanoi AV, et al. The revolving door flap: revisiting an elegant but forgotten flap for ear defect reconstruction. Indian J Plast Surg. 2020;53:64-70. doi:10.1055/s-0040-1709531
- Heinz MB, Hölzle F, Ghassemi A. Repairing a non-marginal full-thickness auricular defect using a reversed flap from the postauricular area. J Oral Maxillofac Surg. 2015;73:764-768. doi:10.1016/j.joms.2014.11.005
- Leitenberger JJ, Golden SK. Reconstruction after full-thickness loss of the antihelix, scapha, and triangular fossa. Dermatol Surg. 2016;42:893-896. doi:10.1097/DSS.0000000000000664
Ulcerated Nodule on the Lip
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
A 79-year-old man with a medical history of type 2 diabetes mellitus, hypothyroidism, and atrial fibrillation presented with an enlarging lesion on the right side of the upper cutaneous lip of 5 weeks’ duration. He had no personal history of skin cancer or other malignancy and was up to date on all routine cancer screenings. He reported associated lip and oral cavity tenderness, weakness, and a 13.6-kg (30-lb) unintentional weight loss over the last 6 months. He had used over-the-counter bacitracin ointment on the lesion without relief. A full-body skin examination revealed a firm, mobile, flesh-colored, nondraining nodule in the right axillary vault.
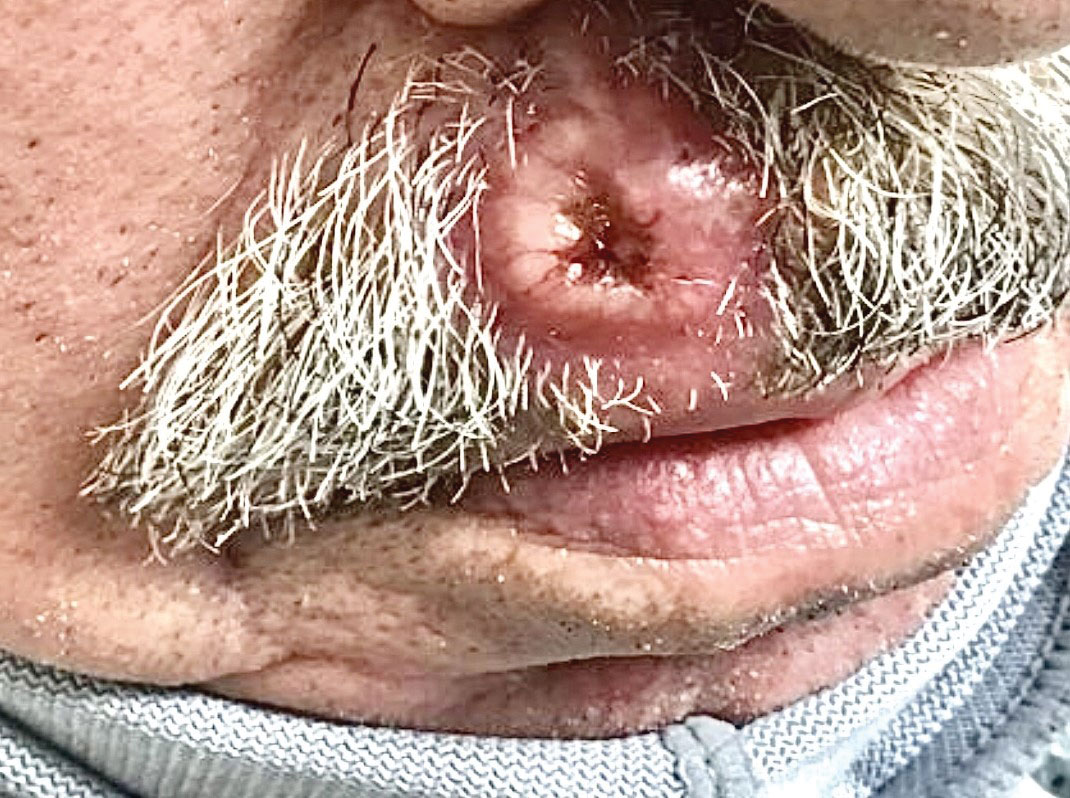
Eccrine Porocarcinoma in 2 Patients
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.
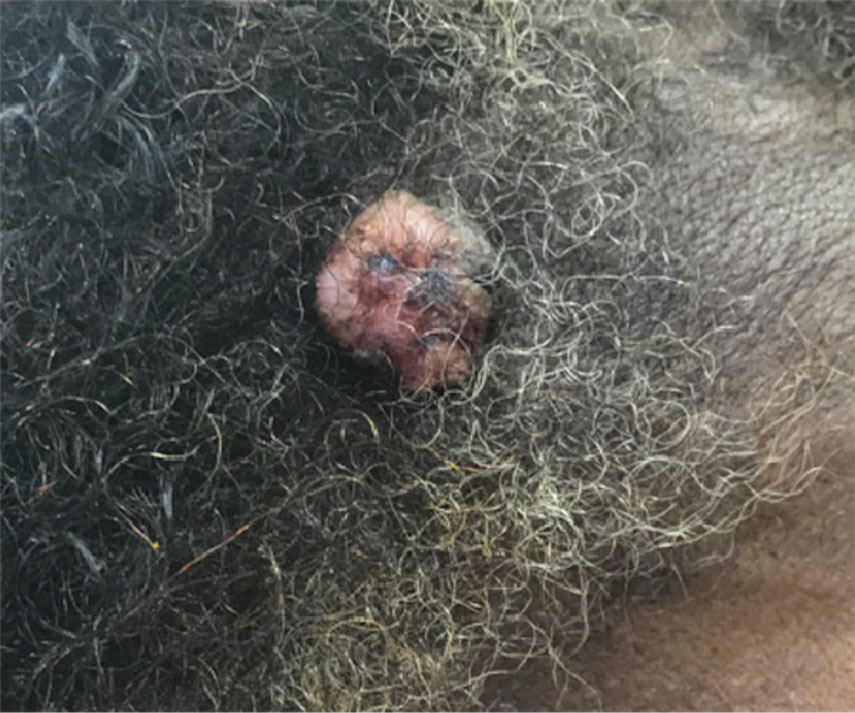
A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.
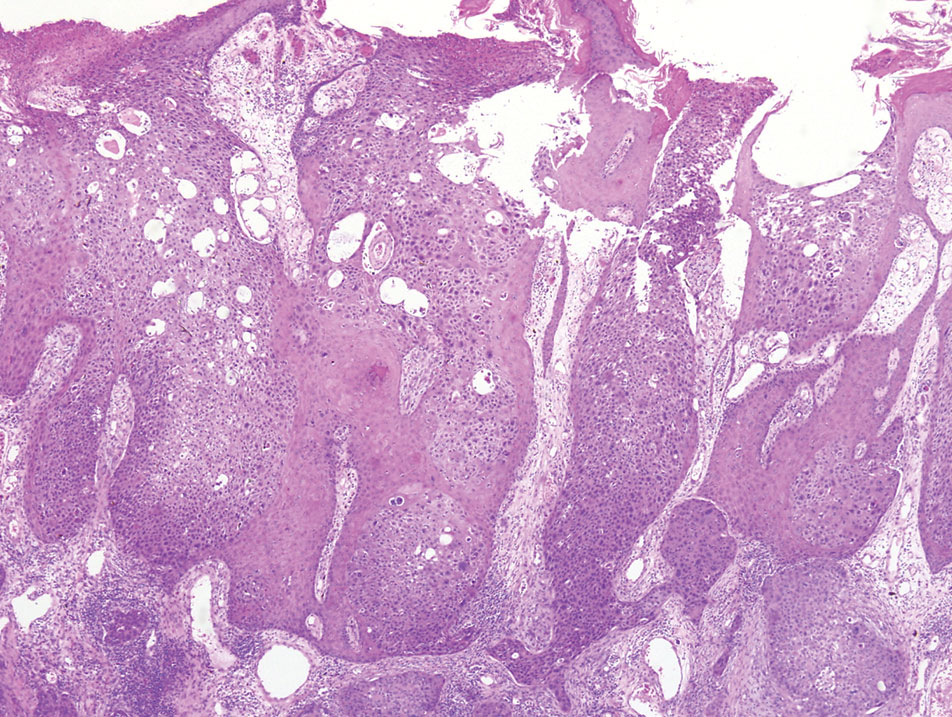
A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.
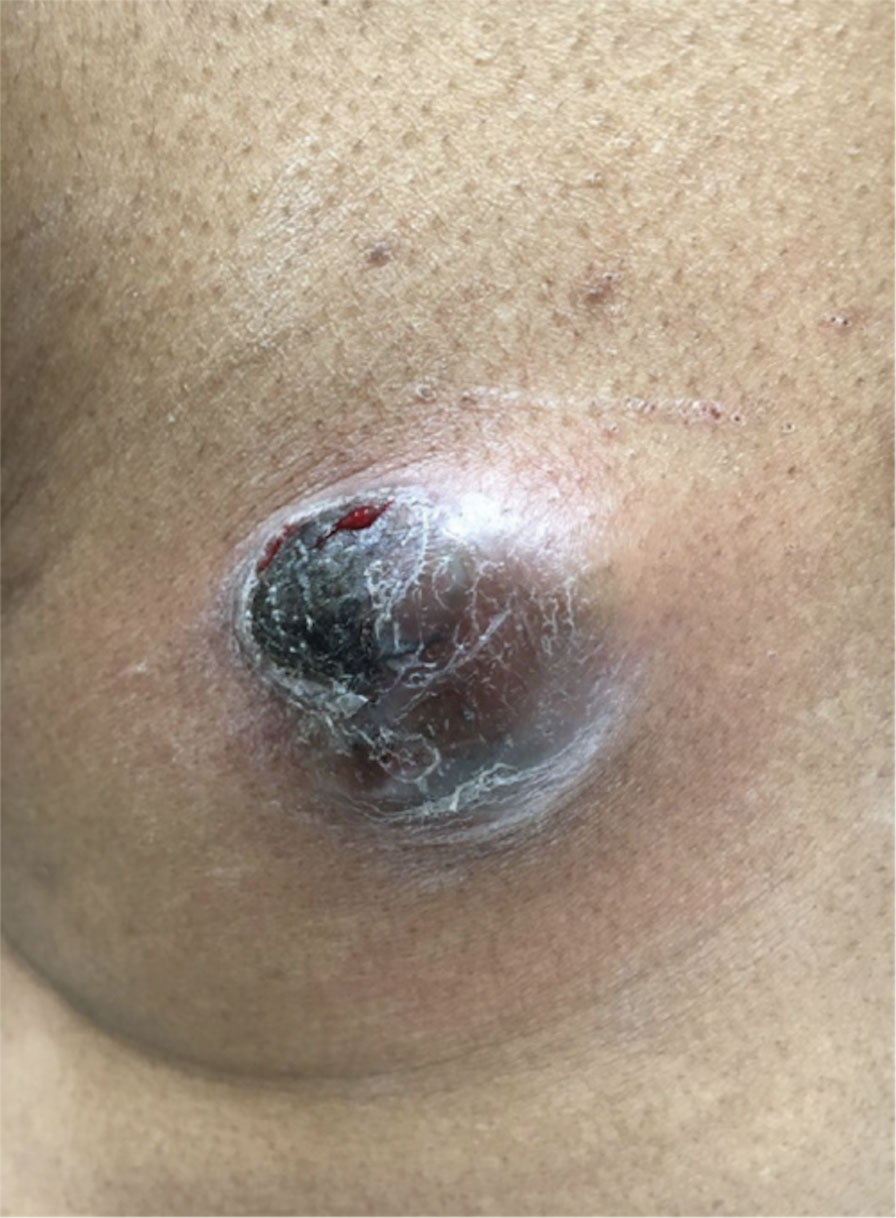
Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.
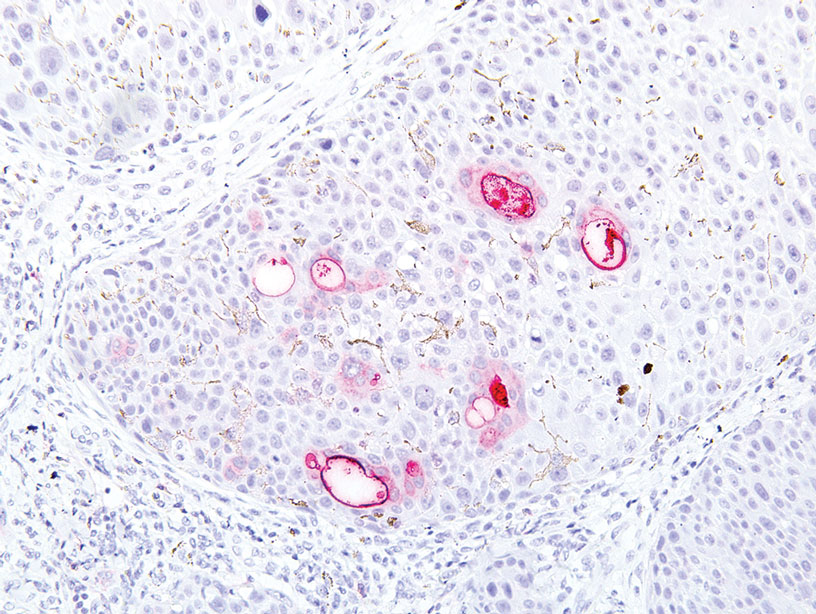
Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.

A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.

A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.

Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.

Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
To the Editor:
Porocarcinoma is a rare malignancy of the eccrine sweat glands and is commonly misdiagnosed clinically. We present 2 cases of porocarcinoma and highlight key features of this uncommon disease.

A 65-year-old man presented to the emergency department with a chief concern of a bump on the head of 8 months' duration that gradually enlarged. The lesion recently became painful and contributed to frequent headaches. He reported a history of smoking 1 pack per day and denied trauma to the area or history of immunosuppression. He had no personal or family history of skin cancer. Physical examination revealed a 1.4-cm, heterochromic, pedunculated, keratotic tumor with crusting on the right temporal scalp (Figure 1). No lymphadenopathy was appreciated. The clinical differential diagnosis included irritated seborrheic keratosis, pyogenic granuloma, polypoid malignant melanoma, and nonmelanoma skin cancer. A biopsy of the lesion demonstrated a proliferation of cuboidal cells with focal ductular differentiation arranged in interanastamosing strands arising from the epidermis (Figure 2). Scattered mitotic figures, including atypical forms, cytologic atypia, and foci of necrosis, also were present. The findings were consistent with features of porocarcinoma. Contrast computed tomography of the neck showed no evidence of metastatic disease within the neck. A wide local excision was performed and yielded a tumor measuring 1.8×1.6×0.7 cm with a depth of 0.3 cm and uninvolved margins. No lymphovascular or perineural invasion was identified. At 4-month follow-up, the patient had a well-healed scar on the right scalp without evidence of recurrence or lymphadenopathy.

A 32-year-old woman presented to dermatology with a chief concern of a mass on the back of 2 years’ duration that rapidly enlarged and became painful following irritation from her bra strap 2 months earlier. She had no relevant medical history. Physical examination revealed a firm, tender, heterochromic nodule measuring 3.0×2.8 cm on the left mid back inferior to the left scapula (Figure 3). The lesion expressed serosanguineous discharge. No lymphadenopathy was appreciated on examination. The clinical differential diagnosis included an inflamed cyst, nodular melanoma, cutaneous metastasis, and nonmelanoma skin cancer. The patient underwent an excisional biopsy, which demonstrated porocarcinoma with positive margins, microsatellitosis, and evidence of lymphovascular invasion. Carcinoembryonic antigen immunohistochemistry highlighted ducts within the tumor (Figure 4). The patient underwent re-excision with 2-cm margins, and no residual tumor was appreciated on pathology.

Positron emission tomography and computed tomography revealed a hypermetabolic left axillary lymph node. Ultrasound-guided fine-needle aspiration was positive for malignant cells consistent with metastatic carcinoma. Dissection of left axillary lymph nodes yielded metastatic porocarcinoma in 2 of 13 nodes. The largest tumor deposit measured 0.9 cm, and no extracapsular extension was identified. The patient continues to be monitored with semiannual full-body skin examinations as well as positron emission tomography and computed tomography scans, with no evidence of recurrence 2 years later.

Porocarcinoma is a rare malignancy of the skin arising from the eccrine sweat glands1 with an incidence rate of 0.4 cases per 1 million person-years in the United States. These tumors represent 0.005% to 0.01% of all skin cancers.2 The mean age of onset is approximately 65 years with no predilection for sex. The mean time from initial presentation to treatment is 5.6 to 8.5 years.3-5
Eccrine sweat glands consist of a straight intradermal duct (syrinx); coiled intradermal duct; and spiral intraepidermal duct (acrosyringium), which opens onto the skin. Both eccrine poromas (solitary benign eccrine gland tumors) and eccrine porocarcinomas develop from the acrosyringium. Eccrine poromas most commonly are found in sites containing the highest density of eccrine glands such as the palms, soles, axillae, and forehead, whereas porocarcinomas most commonly are found on the head, neck, arms, and legs.1,3,4,6,7 A solitary painless nodule that may ulcerate or bleed is the most common presentation.1,3-5,7
The etiology of eccrine porocarcinoma is poorly understood, but it has been found to arise de novo or to develop from pre-existing poromas or even from nevus sebaceus of Jadassohn. Chronic sunlight exposure, irradiation, lymphedema, trauma, and immunosuppression (eg, Hodgkin disease, chronic lymphocytic leukemia, HIV) have been reported as potential predisposing factors.3,4,6,8,9
Eccrine porocarcinoma often is clinically misdiagnosed as nonmelanoma skin cancer, pyogenic granuloma, amelanotic melanoma, fibroma, verruca vulgaris, or metastatic carcinoma. Appropriate classification is essential, as metastasis is present in 25% to 31% of cases, and local recurrence occurs in 20% to 25% of cases.1,3-5,7
Microscopically, porocarcinomas are comprised of atypical basaloid epithelial cells with focal ductular differentiation. Typically, there is an extensive intraepidermal component that invades into the dermis in anastomosing ribbons and cords. The degree of nuclear atypia, mitotic activity, and invasive growth pattern, as well as the presence of necrosis, are useful histologic features to differentiate porocarcinoma from poroma, which may be present in the background. Given the sometimes-extensive squamous differentiation, porocarcinoma can be confused with squamous cell carcinoma. In these cases, immunohistochemical stains such as epithelial membrane antigen or carcinoembryonic antigen can be used to highlight the ductal differentiation.1,5,8,10
Poor histologic prognostic indicators include a high mitotic index (>14 mitoses per field), a tumor depth greater than 7 mm, and evidence of lymphovascular invasion. Positive lymph node involvement is associated with a 65% to 67% mortality rate.1,8
Because of its propensity to metastasize via the lymphatic system and the high mortality rate associated with such metastases, early identification and treatment are essential. Treatment is accomplished via Mohs micrographic surgery or wide local excision with negative margins. Lymphadenectomy is indicated if regional lymph nodes are involved. Radiation and chemotherapy have been used in patients with metastatic and recurrent disease with mixed results.1,3-5,7 There is no adequate standardized chemotherapy or drug regimen established for porocarcinoma.5 Tsunoda et al11 proposed that sentinel lymph node biopsy should be considered first-line management of eccrine porocarcinoma; however, this remains unproven on the basis of a limited case series. Others conclude that sentinel lymph node biopsy should be recommended for cases with poor histologic prognostic features.1,5
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
- Marone U, Caraco C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
- Blake PW, Bradford PT, Devesa SS, et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010;146:625-632.
- Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg (Lond). 2017;20:74-79.
- Ritter AM, Graham RS, Amaker B, et al. Intracranial extension of an eccrine porocarcinoma. case report and review of the literature. J Neurosurg. 1999;90:138-140.
- Khaja M, Ashraf U, Mehershahi S, et al. Recurrent metastatic eccrine porocarcinoma: a case report and review of the literature. Am J Case Rep. 2019;20:179-183.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Lloyd MS, El-Muttardi N, Robson A. Eccrine porocarcinoma: a case report and review of the literature. Can J Plast Surg. 2003;11:153-156.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Tarkhan II, Domingo J. Metastasizing eccrine porocarcinoma developing in a sebaceous nevus of Jadassohn. report of a case. Arch Dermatol. 1985;121:413‐415.
- Prieto VG, Shea CR, Celebi JK, et al. Adnexal tumors. In: Busam KJ. Dermatopathology: A Volume in the Foundations in Diagnostic Pathology Series. 2nd ed. Elsevier; 2016:388-446.
- Tsunoda K, Onishi M, Maeda F, et al. Evaluation of sentinel lymph node biopsy for eccrine porocarcinoma. Acta Derm Venereol. 2019;99:691-692.
Practice Points
- Eccrine porocarcinoma is a rare malignancy that clinically mimics other cutaneous malignancies.
- Early histologic diagnosis is essential, as lymphatic metastasis is common and carries a 65% to 67% mortality rate.
Imaging techniques will revolutionize cancer detection, expert predicts
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
AT ASLMS 2023
Study supports new NCCN classification for cutaneous squamous cell carcinoma
, according to new findings.
In addition, regardless of the NCCN risk group, the study found that Mohs surgery or peripheral and deep en face margin assessment (PDEMA) conferred a lower risk of developing LR, DM, and disease-related death.
“Although the NCCN included this new high-risk group in the last iteration of the guidelines, there were no studies that identified whether the high-risk group achieved the goal of identifying riskier tumors,” said senior author Emily Ruiz, MD, MPH, associate physician at the Mohs and Dermatologic Surgery Center at Brigham and Women’s Faulkner Hospital, Boston. “Based on the data in our study, the risk groups did risk stratify tumors and so clinicians can utilize the high-risk group risk factors to identify which tumors may require additional surveillance or treatment.”
The study was published online in JAMA Dermatology.
Most patients with CSCC are successfully treated with Mohs micrographic surgery or wide local excision (WLE) alone, but a subset will experience more severe and aggressive disease. While useful for prognostication, current staging systems do not incorporate patient factors or other high-risk tumor features that influence outcomes, which led to the NCCN reclassifying CSCC into low-, high-, and very high-risk groups. The NCCN guidelines also made a new recommendation that Mohs or PDEMA be the preferred method for tissue processing for high- and very-high-risk tumors, based on this new stratification.
However, these changes to the NCCN guidelines have not been validated. The goal of this study was to compare outcomes in very-high-, high-, and low-risk NCCN groups as well as comparing outcomes of CSCCs stratified by Mohs and WLE.
Dr. Ruiz and colleagues conducted a retrospective cohort study using patient data from two tertiary care academic medical centers. Their analysis included 10,196 tumors from 8,727 patients that were then stratified into low-risk (3,054 tumors [30.0%]), high-risk (6,269 tumors [61.5%]), and very-high-risk (873 tumors [8.6%]) groups.
Tumors in the very-high-risk group were more likely to have high-risk tumor and histologic features, such as large-caliber perineural invasion, large diameter, invasion beyond the subcutaneous fat or bone, poor differentiation, and lymphovascular invasion.
The authors found that, compared with the low-risk group, the high- and very-high-risk groups demonstrated a greater risk of LR (high-risk subhazard ratio, 1.99; P = .007; very-high-risk SHR, 12.66; P < .001); NM (high-risk SHR, 4.26; P = .02; very-high-risk SHR, 62.98; P < .001); DM (high-risk SHR, 2.2 × 107; P < .001; very-high-risk SHR, 6.3 × 108; P < .001); and DSD (high-risk SHR, 4.02; P = .03; very-high-risk SHR, 93.87; P < .001).
Adjusted 5-year cumulative incidence was also significantly higher in very-high- vs. high- and low-risk groups for all endpoints.
They next compared the procedures used to treat the tumors. Compared with WLE, patients treated with Mohs or PDEMA had a lower risk of LR (SHR, 0.65; P = .009), DM (SHR, 0.38; P = .02), and DSD (SHR, 0.55; P = .006).
Mohs and PDEMA have already became preferred surgical modalities for high- and very-high-risk tumors, and Dr. Ruiz pointed out that their analysis was for the entire cohort.
“We did not stratify this by risk group,” she said. “So our results do not change anything clinically at this time, but support prior studies that have found Mohs/PDEMA to have improved outcomes, compared to WLE. Further studies are needed evaluating surgical approach by risk-group.”
However, she emphasized, “our studies further validate prior evidence showing Mohs/PDEMA to have the lowest rates of recurrence and in this study, even disease-related death.”
Approached for an independent comment, Jeffrey M. Farma, MD, codirector of the melanoma and skin cancer program, and interim chair, department of surgical oncology, Fox Chase Cancer Center, Philadelphia, noted that this study supports the new reclassification of CSCC tumors by the NCCN, and confirms that the high-risk and very-high-risk tumors surely have a higher propensity for worse outcomes overall.
“That being said, the notion for type of resection and margin assessment is still an area of controversy in the dermatology, surgical oncology, and pathology community,” said Dr. Farma, who is also on the NCCN panel. “I believe we need further studies to truly understand the role of the type of resection and the pathologic evaluation play in this disease process.”
He also pointed out that it is unclear in this dataset if patients initially had any imaging to evaluate for local or regional metastatic disease. “It would be helpful to have a further understanding of which type of provider was performing the excisions, the type of excision decided upon, and if there was a standardized approach to [decide] which patients had MOHS or PDEMA and what was the surveillance for these patients both with imaging and physical examinations,” said Dr. Farma. “This data also evaluated patients over a long time period where practice patterns have evolved.”
Finally, he noted that the number of local and metastatic events subjectively seems low in this cohort. “We also do not know any information about the initial workup of the patients, patterns of recurrence, and adjuvant or palliative treatment after recurrence,” he added. “It is unclear from this manuscript how the type of resection or pathologic evaluation of margins leads to improved outcomes and further prospective studies are warranted.”
Dr. Ruiz reports reported serving as a coinvestigator and principal investigator for Regeneron Pharmaceuticals and as a coinvestigator for Merck and consulting for Checkpoint Therapeutics, BDO, and Genentech outside the submitted work. Dr. Farma has no disclosures other than the NCCN panel. The study was supported by Harvard Catalyst and the Harvard University Clinical and Translational Science Center and by Harvard University and its affiliated academic health care centers and partially supported by the Melvin Markey Discovery Fund at Cleveland Clinic Foundation.
, according to new findings.
In addition, regardless of the NCCN risk group, the study found that Mohs surgery or peripheral and deep en face margin assessment (PDEMA) conferred a lower risk of developing LR, DM, and disease-related death.
“Although the NCCN included this new high-risk group in the last iteration of the guidelines, there were no studies that identified whether the high-risk group achieved the goal of identifying riskier tumors,” said senior author Emily Ruiz, MD, MPH, associate physician at the Mohs and Dermatologic Surgery Center at Brigham and Women’s Faulkner Hospital, Boston. “Based on the data in our study, the risk groups did risk stratify tumors and so clinicians can utilize the high-risk group risk factors to identify which tumors may require additional surveillance or treatment.”
The study was published online in JAMA Dermatology.
Most patients with CSCC are successfully treated with Mohs micrographic surgery or wide local excision (WLE) alone, but a subset will experience more severe and aggressive disease. While useful for prognostication, current staging systems do not incorporate patient factors or other high-risk tumor features that influence outcomes, which led to the NCCN reclassifying CSCC into low-, high-, and very high-risk groups. The NCCN guidelines also made a new recommendation that Mohs or PDEMA be the preferred method for tissue processing for high- and very-high-risk tumors, based on this new stratification.
However, these changes to the NCCN guidelines have not been validated. The goal of this study was to compare outcomes in very-high-, high-, and low-risk NCCN groups as well as comparing outcomes of CSCCs stratified by Mohs and WLE.
Dr. Ruiz and colleagues conducted a retrospective cohort study using patient data from two tertiary care academic medical centers. Their analysis included 10,196 tumors from 8,727 patients that were then stratified into low-risk (3,054 tumors [30.0%]), high-risk (6,269 tumors [61.5%]), and very-high-risk (873 tumors [8.6%]) groups.
Tumors in the very-high-risk group were more likely to have high-risk tumor and histologic features, such as large-caliber perineural invasion, large diameter, invasion beyond the subcutaneous fat or bone, poor differentiation, and lymphovascular invasion.
The authors found that, compared with the low-risk group, the high- and very-high-risk groups demonstrated a greater risk of LR (high-risk subhazard ratio, 1.99; P = .007; very-high-risk SHR, 12.66; P < .001); NM (high-risk SHR, 4.26; P = .02; very-high-risk SHR, 62.98; P < .001); DM (high-risk SHR, 2.2 × 107; P < .001; very-high-risk SHR, 6.3 × 108; P < .001); and DSD (high-risk SHR, 4.02; P = .03; very-high-risk SHR, 93.87; P < .001).
Adjusted 5-year cumulative incidence was also significantly higher in very-high- vs. high- and low-risk groups for all endpoints.
They next compared the procedures used to treat the tumors. Compared with WLE, patients treated with Mohs or PDEMA had a lower risk of LR (SHR, 0.65; P = .009), DM (SHR, 0.38; P = .02), and DSD (SHR, 0.55; P = .006).
Mohs and PDEMA have already became preferred surgical modalities for high- and very-high-risk tumors, and Dr. Ruiz pointed out that their analysis was for the entire cohort.
“We did not stratify this by risk group,” she said. “So our results do not change anything clinically at this time, but support prior studies that have found Mohs/PDEMA to have improved outcomes, compared to WLE. Further studies are needed evaluating surgical approach by risk-group.”
However, she emphasized, “our studies further validate prior evidence showing Mohs/PDEMA to have the lowest rates of recurrence and in this study, even disease-related death.”
Approached for an independent comment, Jeffrey M. Farma, MD, codirector of the melanoma and skin cancer program, and interim chair, department of surgical oncology, Fox Chase Cancer Center, Philadelphia, noted that this study supports the new reclassification of CSCC tumors by the NCCN, and confirms that the high-risk and very-high-risk tumors surely have a higher propensity for worse outcomes overall.
“That being said, the notion for type of resection and margin assessment is still an area of controversy in the dermatology, surgical oncology, and pathology community,” said Dr. Farma, who is also on the NCCN panel. “I believe we need further studies to truly understand the role of the type of resection and the pathologic evaluation play in this disease process.”
He also pointed out that it is unclear in this dataset if patients initially had any imaging to evaluate for local or regional metastatic disease. “It would be helpful to have a further understanding of which type of provider was performing the excisions, the type of excision decided upon, and if there was a standardized approach to [decide] which patients had MOHS or PDEMA and what was the surveillance for these patients both with imaging and physical examinations,” said Dr. Farma. “This data also evaluated patients over a long time period where practice patterns have evolved.”
Finally, he noted that the number of local and metastatic events subjectively seems low in this cohort. “We also do not know any information about the initial workup of the patients, patterns of recurrence, and adjuvant or palliative treatment after recurrence,” he added. “It is unclear from this manuscript how the type of resection or pathologic evaluation of margins leads to improved outcomes and further prospective studies are warranted.”
Dr. Ruiz reports reported serving as a coinvestigator and principal investigator for Regeneron Pharmaceuticals and as a coinvestigator for Merck and consulting for Checkpoint Therapeutics, BDO, and Genentech outside the submitted work. Dr. Farma has no disclosures other than the NCCN panel. The study was supported by Harvard Catalyst and the Harvard University Clinical and Translational Science Center and by Harvard University and its affiliated academic health care centers and partially supported by the Melvin Markey Discovery Fund at Cleveland Clinic Foundation.
, according to new findings.
In addition, regardless of the NCCN risk group, the study found that Mohs surgery or peripheral and deep en face margin assessment (PDEMA) conferred a lower risk of developing LR, DM, and disease-related death.
“Although the NCCN included this new high-risk group in the last iteration of the guidelines, there were no studies that identified whether the high-risk group achieved the goal of identifying riskier tumors,” said senior author Emily Ruiz, MD, MPH, associate physician at the Mohs and Dermatologic Surgery Center at Brigham and Women’s Faulkner Hospital, Boston. “Based on the data in our study, the risk groups did risk stratify tumors and so clinicians can utilize the high-risk group risk factors to identify which tumors may require additional surveillance or treatment.”
The study was published online in JAMA Dermatology.
Most patients with CSCC are successfully treated with Mohs micrographic surgery or wide local excision (WLE) alone, but a subset will experience more severe and aggressive disease. While useful for prognostication, current staging systems do not incorporate patient factors or other high-risk tumor features that influence outcomes, which led to the NCCN reclassifying CSCC into low-, high-, and very high-risk groups. The NCCN guidelines also made a new recommendation that Mohs or PDEMA be the preferred method for tissue processing for high- and very-high-risk tumors, based on this new stratification.
However, these changes to the NCCN guidelines have not been validated. The goal of this study was to compare outcomes in very-high-, high-, and low-risk NCCN groups as well as comparing outcomes of CSCCs stratified by Mohs and WLE.
Dr. Ruiz and colleagues conducted a retrospective cohort study using patient data from two tertiary care academic medical centers. Their analysis included 10,196 tumors from 8,727 patients that were then stratified into low-risk (3,054 tumors [30.0%]), high-risk (6,269 tumors [61.5%]), and very-high-risk (873 tumors [8.6%]) groups.
Tumors in the very-high-risk group were more likely to have high-risk tumor and histologic features, such as large-caliber perineural invasion, large diameter, invasion beyond the subcutaneous fat or bone, poor differentiation, and lymphovascular invasion.
The authors found that, compared with the low-risk group, the high- and very-high-risk groups demonstrated a greater risk of LR (high-risk subhazard ratio, 1.99; P = .007; very-high-risk SHR, 12.66; P < .001); NM (high-risk SHR, 4.26; P = .02; very-high-risk SHR, 62.98; P < .001); DM (high-risk SHR, 2.2 × 107; P < .001; very-high-risk SHR, 6.3 × 108; P < .001); and DSD (high-risk SHR, 4.02; P = .03; very-high-risk SHR, 93.87; P < .001).
Adjusted 5-year cumulative incidence was also significantly higher in very-high- vs. high- and low-risk groups for all endpoints.
They next compared the procedures used to treat the tumors. Compared with WLE, patients treated with Mohs or PDEMA had a lower risk of LR (SHR, 0.65; P = .009), DM (SHR, 0.38; P = .02), and DSD (SHR, 0.55; P = .006).
Mohs and PDEMA have already became preferred surgical modalities for high- and very-high-risk tumors, and Dr. Ruiz pointed out that their analysis was for the entire cohort.
“We did not stratify this by risk group,” she said. “So our results do not change anything clinically at this time, but support prior studies that have found Mohs/PDEMA to have improved outcomes, compared to WLE. Further studies are needed evaluating surgical approach by risk-group.”
However, she emphasized, “our studies further validate prior evidence showing Mohs/PDEMA to have the lowest rates of recurrence and in this study, even disease-related death.”
Approached for an independent comment, Jeffrey M. Farma, MD, codirector of the melanoma and skin cancer program, and interim chair, department of surgical oncology, Fox Chase Cancer Center, Philadelphia, noted that this study supports the new reclassification of CSCC tumors by the NCCN, and confirms that the high-risk and very-high-risk tumors surely have a higher propensity for worse outcomes overall.
“That being said, the notion for type of resection and margin assessment is still an area of controversy in the dermatology, surgical oncology, and pathology community,” said Dr. Farma, who is also on the NCCN panel. “I believe we need further studies to truly understand the role of the type of resection and the pathologic evaluation play in this disease process.”
He also pointed out that it is unclear in this dataset if patients initially had any imaging to evaluate for local or regional metastatic disease. “It would be helpful to have a further understanding of which type of provider was performing the excisions, the type of excision decided upon, and if there was a standardized approach to [decide] which patients had MOHS or PDEMA and what was the surveillance for these patients both with imaging and physical examinations,” said Dr. Farma. “This data also evaluated patients over a long time period where practice patterns have evolved.”
Finally, he noted that the number of local and metastatic events subjectively seems low in this cohort. “We also do not know any information about the initial workup of the patients, patterns of recurrence, and adjuvant or palliative treatment after recurrence,” he added. “It is unclear from this manuscript how the type of resection or pathologic evaluation of margins leads to improved outcomes and further prospective studies are warranted.”
Dr. Ruiz reports reported serving as a coinvestigator and principal investigator for Regeneron Pharmaceuticals and as a coinvestigator for Merck and consulting for Checkpoint Therapeutics, BDO, and Genentech outside the submitted work. Dr. Farma has no disclosures other than the NCCN panel. The study was supported by Harvard Catalyst and the Harvard University Clinical and Translational Science Center and by Harvard University and its affiliated academic health care centers and partially supported by the Melvin Markey Discovery Fund at Cleveland Clinic Foundation.
FROM JAMA DERMATOLOGY
Multiprong strategy makes clinical trials less White
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023
CBSM phone app eases anxiety, depression in cancer patients
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023