User login
Endocrine Mucin-Producing Sweat Gland Carcinoma and Primary Cutaneous Mucinous Carcinoma: A Case Series
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and
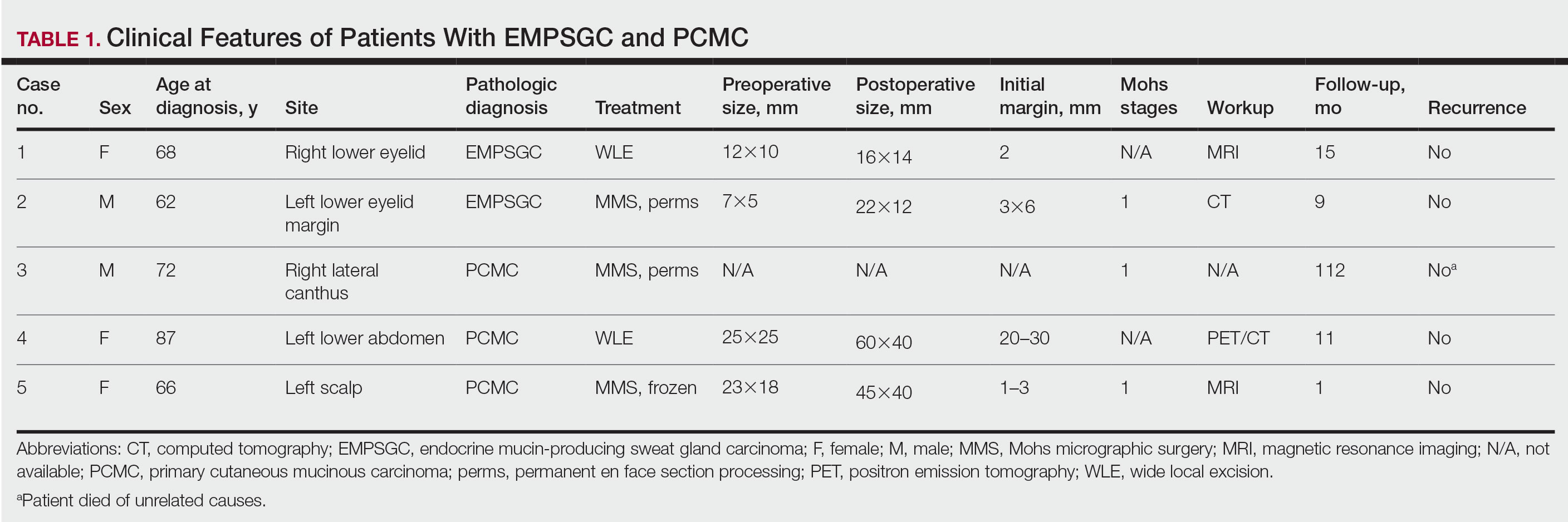
Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.
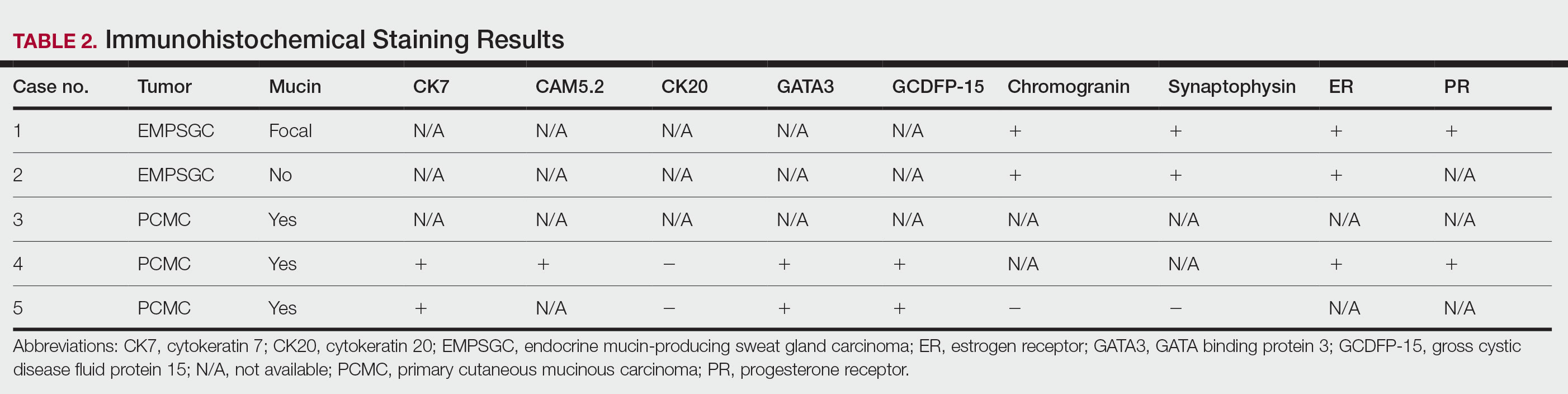
Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.
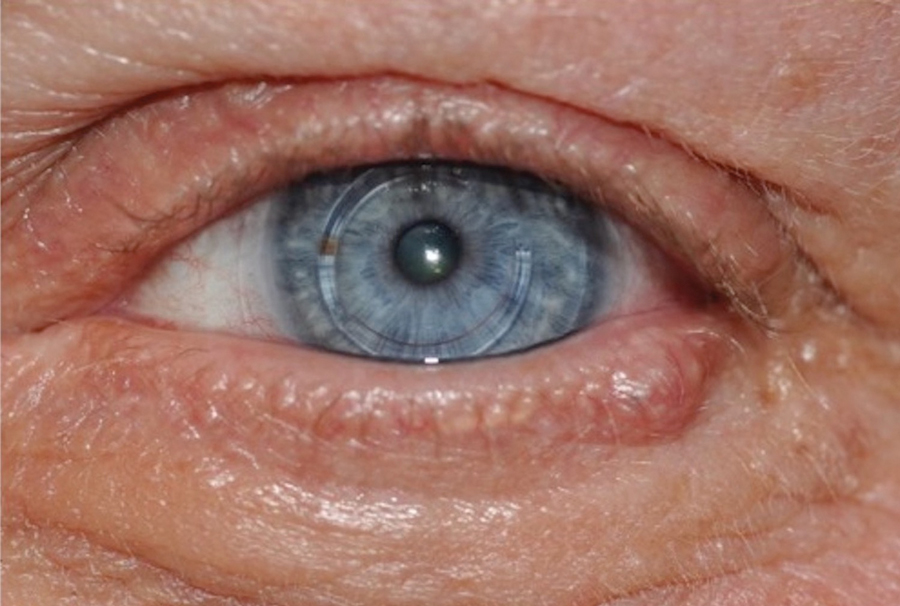
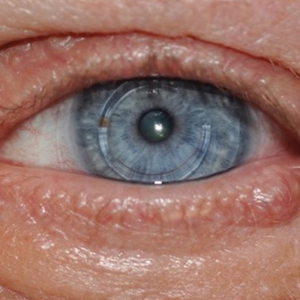
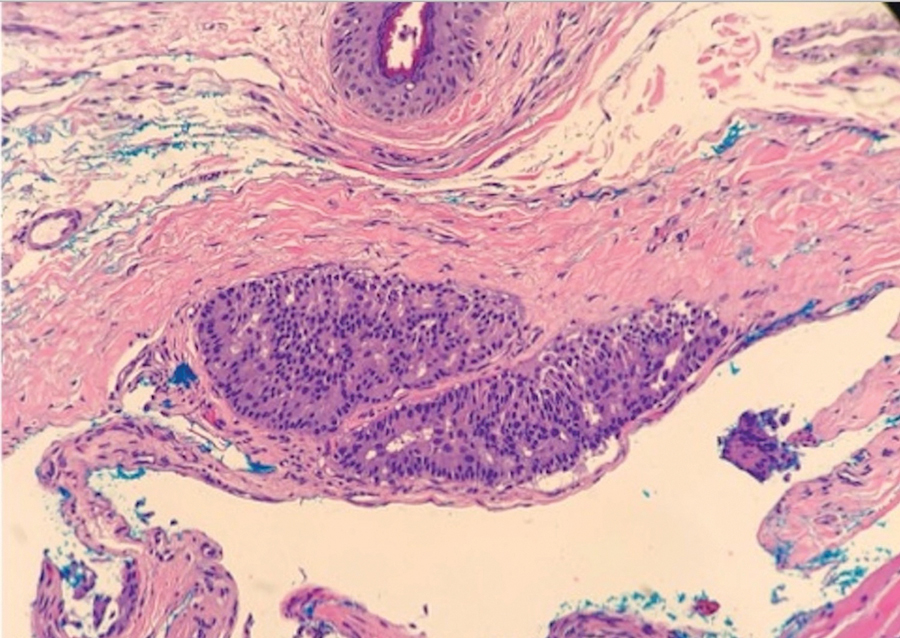
Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.
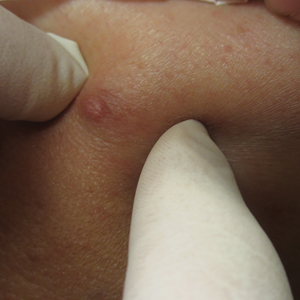
Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.
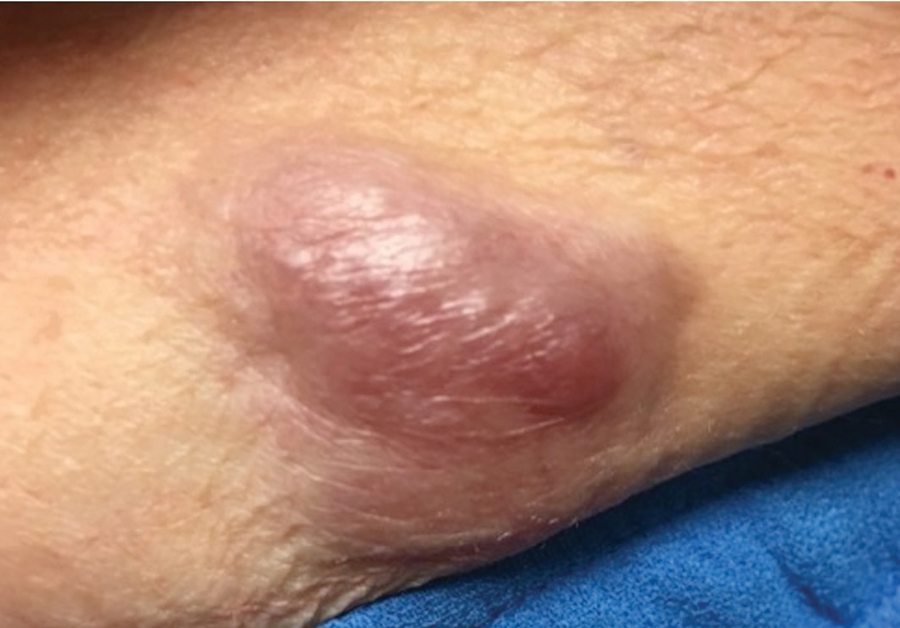
Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.
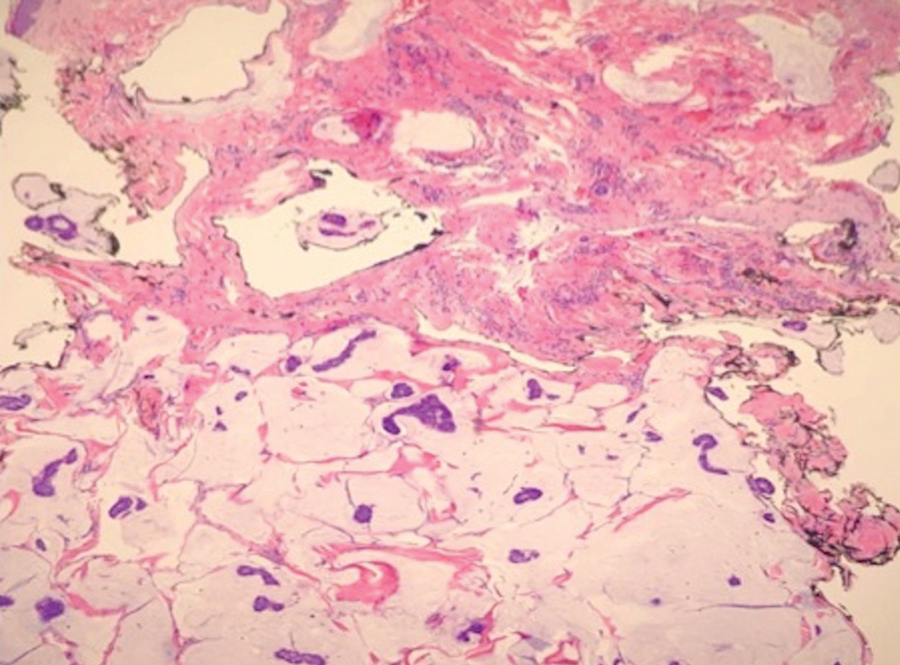
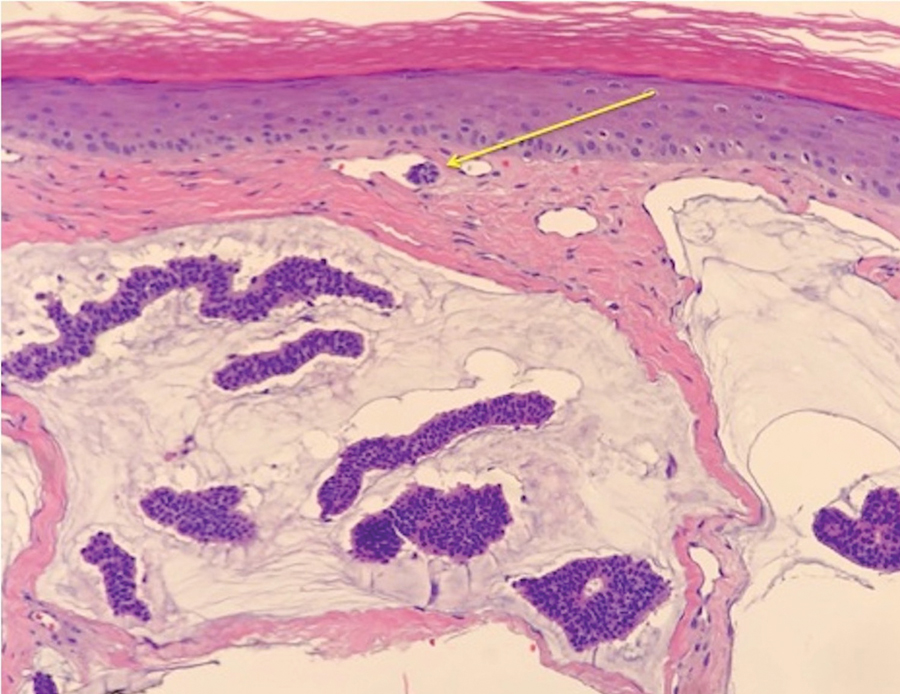
Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.
Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Practice Points
- Endocrine mucin-producing sweat gland carcinoma and primary cutaneous mucinous carcinoma are rare low-grade neoplasms thought to arise from apocrine glands that are morphologically and immunohistochemically analogous to ductal carcinoma in situ and mucinous carcinoma of the breast, respectively.
- Management involves a metastatic workup and either wide local excision with margins greater than 5 mm or Mohs micrographic surgery in anatomically sensitive areas.
High rate of subsequent cancers in MCC
.
In a cohort of 6,146 patients with a first primary MCC, a total of 725 (11.8%) developed subsequent primary cancers. For solid tumors, the risk was highest for cutaneous melanoma and papillary thyroid carcinoma, while for hematologic cancers, the risk was increased for non-Hodgkin lymphoma.
“Our study does confirm that patients with MCC are at higher risk for developing other cancers,” study author Lisa C. Zaba, MD, PhD, associate professor of dermatology and director of the Merkel cell carcinoma multidisciplinary clinic, Stanford (Calif.) Cancer Center, said in an interview. “MCC is a highly malignant cancer with a 40% recurrence risk.”
Because of this high risk, Dr. Zaba noted that patients with MCC get frequent surveillance with both imaging studies (PET-CT and CT) as well as frequent visits in clinic with MCC experts. “Specifically, a patient with MCC is imaged and seen in clinic every 3-6 months for the first 3 years after diagnosis, and every 6-12 months thereafter for up to 5 years,” she said. “Interestingly, this high level of surveillance may be one reason that we find so many cancers in patients who have been diagnosed with MCC, compared to the general population.”
The study was published online in JAMA Dermatology.
With the death of “Margaritaville” singer Jimmy Buffett, who recently died of MCC 4 years after his diagnosis, this rare, aggressive skin cancer has been put in the spotlight. Survival has been increasing, primarily because of the advent of immunotherapy, and the authors note that it is therefore imperative to better understand the risk of subsequent primary tumors to inform screening and treatment recommendations.
In this cohort study, Dr. Zaba and colleagues identified 6,146 patients from 17 registries of the Surveillance, Epidemiology, and End Results (SEER) Program who had been diagnosed with a first primary cutaneous MCC between 2000 and 2018.
Endpoints were the ratio of observed to expected number of cases of subsequent cancer (Standardized incidence ratio, or SIR) and the excess risk.
Overall, there was an elevated risk of developing a subsequent primary cancer after being diagnosed with MCC (SIR, 1.28; excess risk, 57.25 per 10,000 person-years). This included the risk for all solid tumors including liver (SIR, 1.92; excess risk, 2.77 per 10,000 person-years), pancreas (SIR, 1.65; excess risk, 4.55 per 10,000 person-years), cutaneous melanoma (SIR, 2.36; excess risk, 15.27 per 10,000 person-years), and kidney (SIR, 1.64; excess risk, 3.83 per 10,000 person-years).
There was also a higher risk of developing papillary thyroid carcinoma (PTC) (SIR, 5.26; excess risk, 6.16 per 10,000 person-years).
The risk of developing hematological cancers after MCC was also increased, especially for non-Hodgkin lymphoma (SIR, 2.62; excess risk, 15.48 per 10,000 person-years) and myelodysplastic syndrome (SIR, 2.17; excess risk, 2.73 per 10,000 person-years).
The risk for developing subsequent tumors, including melanoma and non-Hodgkin lymphoma, remained significant for up to 10 years, while the risk for developing PTC and kidney cancers remained for up to 5 years.
“After 3-5 years, when a MCC patient’s risk of MCC recurrence drops below 2%, we do not currently have guidelines in place for additional cancer screening,” Dr. Zaba said. “Regarding patient education, patients with MCC are educated to let us know if they experience any symptoms of cancer between visits, including unintentional weight loss, night sweats, headaches that increasingly worsen, or growing lumps or bumps. These symptoms may occur in a multitude of cancers and not just MCC.”
Weighing in on the study, Jeffrey M. Farma, MD, interim chair, department of surgical oncology at Fox Chase Cancer Center, Philadelphia, noted that MCC is considered to be high risk because of its chances of recurring after surgical resection or spreading to lymph nodes or other areas of the body. “There are approximately 3,000 new cases of melanoma a year in the U.S., and it is 40 times rarer than melanoma,” he said. “Patients are usually diagnosed with Merkel cell carcinoma later in life, and the tumors have been associated with sun exposure and immunosuppression and have also been associated with the polyomavirus.”
That said, however, he emphasized that great strides have been made in treatment. “These tumors are very sensitive to radiation, and we generally treat earlier-stage MCC with a combination of surgery and radiation therapy,” said Dr. Farma. “More recently we have had a lot of success with the use of immunotherapy to treat more advanced MCC.”
Dr. Zaba reported receiving grants from the Kuni Foundation outside the submitted work. No other disclosures were reported. Author Eleni Linos, MD, DrPH, MPH, is supported by grant K24AR075060 from the National Institutes of Health. No other outside funding was reported. Dr. Farma had no disclosures.
.
In a cohort of 6,146 patients with a first primary MCC, a total of 725 (11.8%) developed subsequent primary cancers. For solid tumors, the risk was highest for cutaneous melanoma and papillary thyroid carcinoma, while for hematologic cancers, the risk was increased for non-Hodgkin lymphoma.
“Our study does confirm that patients with MCC are at higher risk for developing other cancers,” study author Lisa C. Zaba, MD, PhD, associate professor of dermatology and director of the Merkel cell carcinoma multidisciplinary clinic, Stanford (Calif.) Cancer Center, said in an interview. “MCC is a highly malignant cancer with a 40% recurrence risk.”
Because of this high risk, Dr. Zaba noted that patients with MCC get frequent surveillance with both imaging studies (PET-CT and CT) as well as frequent visits in clinic with MCC experts. “Specifically, a patient with MCC is imaged and seen in clinic every 3-6 months for the first 3 years after diagnosis, and every 6-12 months thereafter for up to 5 years,” she said. “Interestingly, this high level of surveillance may be one reason that we find so many cancers in patients who have been diagnosed with MCC, compared to the general population.”
The study was published online in JAMA Dermatology.
With the death of “Margaritaville” singer Jimmy Buffett, who recently died of MCC 4 years after his diagnosis, this rare, aggressive skin cancer has been put in the spotlight. Survival has been increasing, primarily because of the advent of immunotherapy, and the authors note that it is therefore imperative to better understand the risk of subsequent primary tumors to inform screening and treatment recommendations.
In this cohort study, Dr. Zaba and colleagues identified 6,146 patients from 17 registries of the Surveillance, Epidemiology, and End Results (SEER) Program who had been diagnosed with a first primary cutaneous MCC between 2000 and 2018.
Endpoints were the ratio of observed to expected number of cases of subsequent cancer (Standardized incidence ratio, or SIR) and the excess risk.
Overall, there was an elevated risk of developing a subsequent primary cancer after being diagnosed with MCC (SIR, 1.28; excess risk, 57.25 per 10,000 person-years). This included the risk for all solid tumors including liver (SIR, 1.92; excess risk, 2.77 per 10,000 person-years), pancreas (SIR, 1.65; excess risk, 4.55 per 10,000 person-years), cutaneous melanoma (SIR, 2.36; excess risk, 15.27 per 10,000 person-years), and kidney (SIR, 1.64; excess risk, 3.83 per 10,000 person-years).
There was also a higher risk of developing papillary thyroid carcinoma (PTC) (SIR, 5.26; excess risk, 6.16 per 10,000 person-years).
The risk of developing hematological cancers after MCC was also increased, especially for non-Hodgkin lymphoma (SIR, 2.62; excess risk, 15.48 per 10,000 person-years) and myelodysplastic syndrome (SIR, 2.17; excess risk, 2.73 per 10,000 person-years).
The risk for developing subsequent tumors, including melanoma and non-Hodgkin lymphoma, remained significant for up to 10 years, while the risk for developing PTC and kidney cancers remained for up to 5 years.
“After 3-5 years, when a MCC patient’s risk of MCC recurrence drops below 2%, we do not currently have guidelines in place for additional cancer screening,” Dr. Zaba said. “Regarding patient education, patients with MCC are educated to let us know if they experience any symptoms of cancer between visits, including unintentional weight loss, night sweats, headaches that increasingly worsen, or growing lumps or bumps. These symptoms may occur in a multitude of cancers and not just MCC.”
Weighing in on the study, Jeffrey M. Farma, MD, interim chair, department of surgical oncology at Fox Chase Cancer Center, Philadelphia, noted that MCC is considered to be high risk because of its chances of recurring after surgical resection or spreading to lymph nodes or other areas of the body. “There are approximately 3,000 new cases of melanoma a year in the U.S., and it is 40 times rarer than melanoma,” he said. “Patients are usually diagnosed with Merkel cell carcinoma later in life, and the tumors have been associated with sun exposure and immunosuppression and have also been associated with the polyomavirus.”
That said, however, he emphasized that great strides have been made in treatment. “These tumors are very sensitive to radiation, and we generally treat earlier-stage MCC with a combination of surgery and radiation therapy,” said Dr. Farma. “More recently we have had a lot of success with the use of immunotherapy to treat more advanced MCC.”
Dr. Zaba reported receiving grants from the Kuni Foundation outside the submitted work. No other disclosures were reported. Author Eleni Linos, MD, DrPH, MPH, is supported by grant K24AR075060 from the National Institutes of Health. No other outside funding was reported. Dr. Farma had no disclosures.
.
In a cohort of 6,146 patients with a first primary MCC, a total of 725 (11.8%) developed subsequent primary cancers. For solid tumors, the risk was highest for cutaneous melanoma and papillary thyroid carcinoma, while for hematologic cancers, the risk was increased for non-Hodgkin lymphoma.
“Our study does confirm that patients with MCC are at higher risk for developing other cancers,” study author Lisa C. Zaba, MD, PhD, associate professor of dermatology and director of the Merkel cell carcinoma multidisciplinary clinic, Stanford (Calif.) Cancer Center, said in an interview. “MCC is a highly malignant cancer with a 40% recurrence risk.”
Because of this high risk, Dr. Zaba noted that patients with MCC get frequent surveillance with both imaging studies (PET-CT and CT) as well as frequent visits in clinic with MCC experts. “Specifically, a patient with MCC is imaged and seen in clinic every 3-6 months for the first 3 years after diagnosis, and every 6-12 months thereafter for up to 5 years,” she said. “Interestingly, this high level of surveillance may be one reason that we find so many cancers in patients who have been diagnosed with MCC, compared to the general population.”
The study was published online in JAMA Dermatology.
With the death of “Margaritaville” singer Jimmy Buffett, who recently died of MCC 4 years after his diagnosis, this rare, aggressive skin cancer has been put in the spotlight. Survival has been increasing, primarily because of the advent of immunotherapy, and the authors note that it is therefore imperative to better understand the risk of subsequent primary tumors to inform screening and treatment recommendations.
In this cohort study, Dr. Zaba and colleagues identified 6,146 patients from 17 registries of the Surveillance, Epidemiology, and End Results (SEER) Program who had been diagnosed with a first primary cutaneous MCC between 2000 and 2018.
Endpoints were the ratio of observed to expected number of cases of subsequent cancer (Standardized incidence ratio, or SIR) and the excess risk.
Overall, there was an elevated risk of developing a subsequent primary cancer after being diagnosed with MCC (SIR, 1.28; excess risk, 57.25 per 10,000 person-years). This included the risk for all solid tumors including liver (SIR, 1.92; excess risk, 2.77 per 10,000 person-years), pancreas (SIR, 1.65; excess risk, 4.55 per 10,000 person-years), cutaneous melanoma (SIR, 2.36; excess risk, 15.27 per 10,000 person-years), and kidney (SIR, 1.64; excess risk, 3.83 per 10,000 person-years).
There was also a higher risk of developing papillary thyroid carcinoma (PTC) (SIR, 5.26; excess risk, 6.16 per 10,000 person-years).
The risk of developing hematological cancers after MCC was also increased, especially for non-Hodgkin lymphoma (SIR, 2.62; excess risk, 15.48 per 10,000 person-years) and myelodysplastic syndrome (SIR, 2.17; excess risk, 2.73 per 10,000 person-years).
The risk for developing subsequent tumors, including melanoma and non-Hodgkin lymphoma, remained significant for up to 10 years, while the risk for developing PTC and kidney cancers remained for up to 5 years.
“After 3-5 years, when a MCC patient’s risk of MCC recurrence drops below 2%, we do not currently have guidelines in place for additional cancer screening,” Dr. Zaba said. “Regarding patient education, patients with MCC are educated to let us know if they experience any symptoms of cancer between visits, including unintentional weight loss, night sweats, headaches that increasingly worsen, or growing lumps or bumps. These symptoms may occur in a multitude of cancers and not just MCC.”
Weighing in on the study, Jeffrey M. Farma, MD, interim chair, department of surgical oncology at Fox Chase Cancer Center, Philadelphia, noted that MCC is considered to be high risk because of its chances of recurring after surgical resection or spreading to lymph nodes or other areas of the body. “There are approximately 3,000 new cases of melanoma a year in the U.S., and it is 40 times rarer than melanoma,” he said. “Patients are usually diagnosed with Merkel cell carcinoma later in life, and the tumors have been associated with sun exposure and immunosuppression and have also been associated with the polyomavirus.”
That said, however, he emphasized that great strides have been made in treatment. “These tumors are very sensitive to radiation, and we generally treat earlier-stage MCC with a combination of surgery and radiation therapy,” said Dr. Farma. “More recently we have had a lot of success with the use of immunotherapy to treat more advanced MCC.”
Dr. Zaba reported receiving grants from the Kuni Foundation outside the submitted work. No other disclosures were reported. Author Eleni Linos, MD, DrPH, MPH, is supported by grant K24AR075060 from the National Institutes of Health. No other outside funding was reported. Dr. Farma had no disclosures.
FROM JAMA DERMATOLOGY
Can skin bleaching lead to cancer?
SINGAPORE –
This question was posed by Ousmane Faye, MD, PhD, director general of Mali’s Bamako Dermatology Hospital, at the World Congress of Dermatology.
Dr. Faye explored the issue during a hot topics session at the meeting, prefacing that it was an important question to ask because “in West Africa, skin bleaching is very common.”
“There are many local names” for skin bleaching, he said. “For example, in Senegal, it’s called xessal; in Mali and Ivory Coast, its name is caco; in South Africa, there are many names, like ukutsheyisa.”
Skin bleaching refers to the cosmetic misuse of topical agents to change one’s natural skin color. It’s a centuries-old practice that people, mainly women, adopt “to increase attractiveness and self-esteem,” explained Dr. Faye.
To demonstrate how pervasive skin bleaching is on the continent, he presented a slide that summarized figures from six studies spanning the past 2 decades. Prevalence ranged from 25% in Mali (based on a 1993 survey of 210 women) to a high of 79.25% in Benin (from a sample size of 511 women in 2019). In other studies of women in Burkina Faso and Togo, the figures were 44.3% and 58.9%, respectively. The most recently conducted study, which involved 2,689 Senegalese women and was published in 2022, found that nearly 6 in 10 (59.2%) respondents used skin-lightening products.
But skin bleaching isn’t just limited to Africa, said session moderator Omar Lupi, MD, PhD, associate professor of dermatology at the Federal University of the State of Rio de Janeiro, when approached for an independent comment. “It’s a traditional practice around the world. Maybe not in the developed countries, but it’s quite common in Africa, in South America, and in Asia.”
His sentiments are echoed in a meta-analysis that was published in the International Journal of Dermatology in 2019. The work examined 68 studies involving more than 67,000 people across Africa, Asia, Europe, the Middle East, and North America. It found that the pooled lifetime prevalence of skin bleaching was 27.7% (95% confidence interval, 19.6-37.5; P < .01).
“This is an important and interesting topic because our world is shrinking,” Dr. Lupi told this news organization. “Even in countries that don’t have bleaching as a common situation, we now have patients who are migrating from one part [of the world] to another, so bleaching is something that can knock on your door and you need to be prepared.”
Misuse leads to complications
The issue is pertinent to dermatologists because skin bleaching is associated with a wide range of complications. Take, for example, topical steroids, which are the most common products used for bleaching, said Dr. Faye in his talk.
“Clobetasol can suppress the hypothalamic-pituitary-adrenal (HPA) function,” he said, referring to the body’s main stress response system. “It can also foster skin infection, including bacterial, fungal, viral, and parasitic infection.”
In addition, topical steroids that are misused as skin lighteners have been reported to cause stretch marks, skin atrophy, inflammatory acne, and even metabolic disorders such as diabetes and hypertension, said Dr. Faye.
To further his point, he cited a 2021 prospective case-control study conducted across five sub-Saharan countries, which found that the use of “voluntary cosmetic depigmentation” significantly increased a person’s risk for necrotizing fasciitis of the lower limbs (odds ratio, 2.29; 95% CI, 1.19-3.73; P = .0226).
Similarly, mercury, another substance found in products commonly used to bleach skin, has been associated with problems ranging from rashes to renal toxicity. And because it’s so incredibly harmful, mercury is also known to cause neurologic abnormalities.
Apart from causing certain conditions, prolonged use of skin-lightening products can change the way existing diseases present themselves as well as their severity, added Dr. Faye.
An increased risk
But what about skin bleaching’s link with cancer? “Skin cancer on Black skin is uncommon, yet it occurs in skin-bleaching women,” said Dr. Faye.
“Since 2000, we have had some cases of skin cancer associated with skin bleaching,” he continued, adding that squamous cell carcinoma (SCC) is the most frequent type of cancer observed.
If you look at what’s been published on the topic so far, you’ll see that “all the cases of skin cancer are located over the neck or some exposed area when skin bleaching products are used for more than 10 years,” said Dr. Faye. “And most of the time, the age of the patient ranges from 30 to 60 years.”
The first known case in Africa was reported in a 58-year-old woman from Ghana, who had been using skin bleaching products for close to 30 years. The patient presented with tumors on her face, neck, and arms.
Dr. Faye then proceeded to share more than 10 such carcinoma cases. “These previous reports strongly suggest a relationship between skin bleaching and skin cancers,” said Dr. Faye.
Indeed, there have been reports and publications in the literature that support his observation, including one last year, which found that use of the tyrosinase inhibitor hydroquinone was associated with approximately a threefold increased risk for skin cancer.
For some, including Brazil’s Dr. Lupi, Dr. Faye’s talk was enlightening: “I didn’t know about this relationship [of bleaching] with skin cancer, it was something new for me.”
But the prevalence of SCC is very low, compared with that of skin bleaching, Dr. Faye acknowledged. Moreover, the cancer observed in the cases reported could have resulted from a number of reasons, including exposure to harmful ultraviolet rays from the sun and genetic predisposition in addition to the use of bleaching products such as hydroquinone. “Other causes of skin cancer are not excluded,” he said.
To further explore the link between skin bleaching and cancer, “we need case-control studies to provide more evidence,” he added. Until then, dermatologists “should keep on promoting messages” to prevent SCC from occurring. This includes encouraging the use of proper sun protection in addition to discouraging the practice of skin bleaching, which still persists despite more than 10 African nations banning the use of toxic skin-lightening products.
Dr. Faye and Dr. Lupi report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE –
This question was posed by Ousmane Faye, MD, PhD, director general of Mali’s Bamako Dermatology Hospital, at the World Congress of Dermatology.
Dr. Faye explored the issue during a hot topics session at the meeting, prefacing that it was an important question to ask because “in West Africa, skin bleaching is very common.”
“There are many local names” for skin bleaching, he said. “For example, in Senegal, it’s called xessal; in Mali and Ivory Coast, its name is caco; in South Africa, there are many names, like ukutsheyisa.”
Skin bleaching refers to the cosmetic misuse of topical agents to change one’s natural skin color. It’s a centuries-old practice that people, mainly women, adopt “to increase attractiveness and self-esteem,” explained Dr. Faye.
To demonstrate how pervasive skin bleaching is on the continent, he presented a slide that summarized figures from six studies spanning the past 2 decades. Prevalence ranged from 25% in Mali (based on a 1993 survey of 210 women) to a high of 79.25% in Benin (from a sample size of 511 women in 2019). In other studies of women in Burkina Faso and Togo, the figures were 44.3% and 58.9%, respectively. The most recently conducted study, which involved 2,689 Senegalese women and was published in 2022, found that nearly 6 in 10 (59.2%) respondents used skin-lightening products.
But skin bleaching isn’t just limited to Africa, said session moderator Omar Lupi, MD, PhD, associate professor of dermatology at the Federal University of the State of Rio de Janeiro, when approached for an independent comment. “It’s a traditional practice around the world. Maybe not in the developed countries, but it’s quite common in Africa, in South America, and in Asia.”
His sentiments are echoed in a meta-analysis that was published in the International Journal of Dermatology in 2019. The work examined 68 studies involving more than 67,000 people across Africa, Asia, Europe, the Middle East, and North America. It found that the pooled lifetime prevalence of skin bleaching was 27.7% (95% confidence interval, 19.6-37.5; P < .01).
“This is an important and interesting topic because our world is shrinking,” Dr. Lupi told this news organization. “Even in countries that don’t have bleaching as a common situation, we now have patients who are migrating from one part [of the world] to another, so bleaching is something that can knock on your door and you need to be prepared.”
Misuse leads to complications
The issue is pertinent to dermatologists because skin bleaching is associated with a wide range of complications. Take, for example, topical steroids, which are the most common products used for bleaching, said Dr. Faye in his talk.
“Clobetasol can suppress the hypothalamic-pituitary-adrenal (HPA) function,” he said, referring to the body’s main stress response system. “It can also foster skin infection, including bacterial, fungal, viral, and parasitic infection.”
In addition, topical steroids that are misused as skin lighteners have been reported to cause stretch marks, skin atrophy, inflammatory acne, and even metabolic disorders such as diabetes and hypertension, said Dr. Faye.
To further his point, he cited a 2021 prospective case-control study conducted across five sub-Saharan countries, which found that the use of “voluntary cosmetic depigmentation” significantly increased a person’s risk for necrotizing fasciitis of the lower limbs (odds ratio, 2.29; 95% CI, 1.19-3.73; P = .0226).
Similarly, mercury, another substance found in products commonly used to bleach skin, has been associated with problems ranging from rashes to renal toxicity. And because it’s so incredibly harmful, mercury is also known to cause neurologic abnormalities.
Apart from causing certain conditions, prolonged use of skin-lightening products can change the way existing diseases present themselves as well as their severity, added Dr. Faye.
An increased risk
But what about skin bleaching’s link with cancer? “Skin cancer on Black skin is uncommon, yet it occurs in skin-bleaching women,” said Dr. Faye.
“Since 2000, we have had some cases of skin cancer associated with skin bleaching,” he continued, adding that squamous cell carcinoma (SCC) is the most frequent type of cancer observed.
If you look at what’s been published on the topic so far, you’ll see that “all the cases of skin cancer are located over the neck or some exposed area when skin bleaching products are used for more than 10 years,” said Dr. Faye. “And most of the time, the age of the patient ranges from 30 to 60 years.”
The first known case in Africa was reported in a 58-year-old woman from Ghana, who had been using skin bleaching products for close to 30 years. The patient presented with tumors on her face, neck, and arms.
Dr. Faye then proceeded to share more than 10 such carcinoma cases. “These previous reports strongly suggest a relationship between skin bleaching and skin cancers,” said Dr. Faye.
Indeed, there have been reports and publications in the literature that support his observation, including one last year, which found that use of the tyrosinase inhibitor hydroquinone was associated with approximately a threefold increased risk for skin cancer.
For some, including Brazil’s Dr. Lupi, Dr. Faye’s talk was enlightening: “I didn’t know about this relationship [of bleaching] with skin cancer, it was something new for me.”
But the prevalence of SCC is very low, compared with that of skin bleaching, Dr. Faye acknowledged. Moreover, the cancer observed in the cases reported could have resulted from a number of reasons, including exposure to harmful ultraviolet rays from the sun and genetic predisposition in addition to the use of bleaching products such as hydroquinone. “Other causes of skin cancer are not excluded,” he said.
To further explore the link between skin bleaching and cancer, “we need case-control studies to provide more evidence,” he added. Until then, dermatologists “should keep on promoting messages” to prevent SCC from occurring. This includes encouraging the use of proper sun protection in addition to discouraging the practice of skin bleaching, which still persists despite more than 10 African nations banning the use of toxic skin-lightening products.
Dr. Faye and Dr. Lupi report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SINGAPORE –
This question was posed by Ousmane Faye, MD, PhD, director general of Mali’s Bamako Dermatology Hospital, at the World Congress of Dermatology.
Dr. Faye explored the issue during a hot topics session at the meeting, prefacing that it was an important question to ask because “in West Africa, skin bleaching is very common.”
“There are many local names” for skin bleaching, he said. “For example, in Senegal, it’s called xessal; in Mali and Ivory Coast, its name is caco; in South Africa, there are many names, like ukutsheyisa.”
Skin bleaching refers to the cosmetic misuse of topical agents to change one’s natural skin color. It’s a centuries-old practice that people, mainly women, adopt “to increase attractiveness and self-esteem,” explained Dr. Faye.
To demonstrate how pervasive skin bleaching is on the continent, he presented a slide that summarized figures from six studies spanning the past 2 decades. Prevalence ranged from 25% in Mali (based on a 1993 survey of 210 women) to a high of 79.25% in Benin (from a sample size of 511 women in 2019). In other studies of women in Burkina Faso and Togo, the figures were 44.3% and 58.9%, respectively. The most recently conducted study, which involved 2,689 Senegalese women and was published in 2022, found that nearly 6 in 10 (59.2%) respondents used skin-lightening products.
But skin bleaching isn’t just limited to Africa, said session moderator Omar Lupi, MD, PhD, associate professor of dermatology at the Federal University of the State of Rio de Janeiro, when approached for an independent comment. “It’s a traditional practice around the world. Maybe not in the developed countries, but it’s quite common in Africa, in South America, and in Asia.”
His sentiments are echoed in a meta-analysis that was published in the International Journal of Dermatology in 2019. The work examined 68 studies involving more than 67,000 people across Africa, Asia, Europe, the Middle East, and North America. It found that the pooled lifetime prevalence of skin bleaching was 27.7% (95% confidence interval, 19.6-37.5; P < .01).
“This is an important and interesting topic because our world is shrinking,” Dr. Lupi told this news organization. “Even in countries that don’t have bleaching as a common situation, we now have patients who are migrating from one part [of the world] to another, so bleaching is something that can knock on your door and you need to be prepared.”
Misuse leads to complications
The issue is pertinent to dermatologists because skin bleaching is associated with a wide range of complications. Take, for example, topical steroids, which are the most common products used for bleaching, said Dr. Faye in his talk.
“Clobetasol can suppress the hypothalamic-pituitary-adrenal (HPA) function,” he said, referring to the body’s main stress response system. “It can also foster skin infection, including bacterial, fungal, viral, and parasitic infection.”
In addition, topical steroids that are misused as skin lighteners have been reported to cause stretch marks, skin atrophy, inflammatory acne, and even metabolic disorders such as diabetes and hypertension, said Dr. Faye.
To further his point, he cited a 2021 prospective case-control study conducted across five sub-Saharan countries, which found that the use of “voluntary cosmetic depigmentation” significantly increased a person’s risk for necrotizing fasciitis of the lower limbs (odds ratio, 2.29; 95% CI, 1.19-3.73; P = .0226).
Similarly, mercury, another substance found in products commonly used to bleach skin, has been associated with problems ranging from rashes to renal toxicity. And because it’s so incredibly harmful, mercury is also known to cause neurologic abnormalities.
Apart from causing certain conditions, prolonged use of skin-lightening products can change the way existing diseases present themselves as well as their severity, added Dr. Faye.
An increased risk
But what about skin bleaching’s link with cancer? “Skin cancer on Black skin is uncommon, yet it occurs in skin-bleaching women,” said Dr. Faye.
“Since 2000, we have had some cases of skin cancer associated with skin bleaching,” he continued, adding that squamous cell carcinoma (SCC) is the most frequent type of cancer observed.
If you look at what’s been published on the topic so far, you’ll see that “all the cases of skin cancer are located over the neck or some exposed area when skin bleaching products are used for more than 10 years,” said Dr. Faye. “And most of the time, the age of the patient ranges from 30 to 60 years.”
The first known case in Africa was reported in a 58-year-old woman from Ghana, who had been using skin bleaching products for close to 30 years. The patient presented with tumors on her face, neck, and arms.
Dr. Faye then proceeded to share more than 10 such carcinoma cases. “These previous reports strongly suggest a relationship between skin bleaching and skin cancers,” said Dr. Faye.
Indeed, there have been reports and publications in the literature that support his observation, including one last year, which found that use of the tyrosinase inhibitor hydroquinone was associated with approximately a threefold increased risk for skin cancer.
For some, including Brazil’s Dr. Lupi, Dr. Faye’s talk was enlightening: “I didn’t know about this relationship [of bleaching] with skin cancer, it was something new for me.”
But the prevalence of SCC is very low, compared with that of skin bleaching, Dr. Faye acknowledged. Moreover, the cancer observed in the cases reported could have resulted from a number of reasons, including exposure to harmful ultraviolet rays from the sun and genetic predisposition in addition to the use of bleaching products such as hydroquinone. “Other causes of skin cancer are not excluded,” he said.
To further explore the link between skin bleaching and cancer, “we need case-control studies to provide more evidence,” he added. Until then, dermatologists “should keep on promoting messages” to prevent SCC from occurring. This includes encouraging the use of proper sun protection in addition to discouraging the practice of skin bleaching, which still persists despite more than 10 African nations banning the use of toxic skin-lightening products.
Dr. Faye and Dr. Lupi report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT WCD 2023
Cadaveric Split-Thickness Skin Graft With Partial Guiding Closure for Scalp Defects Extending to the Periosteum
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

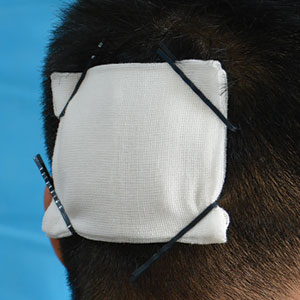
Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Mohs found to confer survival benefit in localized Merkel cell carcinoma
results from a national retrospective cohort study suggest.
The study found that, in patients with pathologically confirmed, localized T1/T2 MCC, “treatment with MMS was associated with an approximately 40% reduction in hazard of death compared with WLE,” reported John A. Carucci, MD, PhD, and colleagues in the department of dermatology at NYU Langone Health, New York. The results provide “preliminary data suggesting that treatment of localized, early-stage MCC with MMS may result in the most optimal patient survival outcomes for this aggressive form of skin cancer,” they added. The study was published online in JAMA Dermatology.
“Although data for keratinocytic nonmelanoma skin cancers have been definitive in demonstrating the advantage of peripheral and deep en face margin assessment over conventional WLE or NME [narrow-margin excision], the data for MCC, likely because of the disease’s rarity and limitations of available data sets, have been mixed,” they wrote.
Results from national studies published in the Journal of the National Cancer Institute and the Journal of the American Academy of Dermatology found no difference in survival among patients with localized MCC treated with WLE versus MMS. “However, these studies did not have confirmed pathologic node status, a substantial limitation considering that clinically node-negative cases of localized MCC have sentinel lymph node positivity rates ranging from 25% to 40%,” the authors noted.
To evaluate the association of the surgical excision modality and patient survival for pathologically confirmed localized T1/T2 MCC, Dr. Carucci and coauthors examined a cohort of 2,313 patients from the National Cancer Database with T1/T2 MCC diagnosed between Jan. 1, 2004, and Dec. 31, 2018, with pathologically confirmed, negative regional lymph nodes and treated with surgery. Their mean age was 71 years and 57.9% were male. Of the 2,313 patients, 1,452 underwent WLE, 104 underwent MMS, and 757 underwent NME.
The unadjusted analysis revealed that, compared with WLE, excision with MMS had the best unadjusted mean survival rates: 87.4% versus 86.1%, respectively, at 3 years, 84.5% versus 76.9% at 5 years, and 81.8% versus 60.9% at 10 years. Patients treated with NME had similar mean survival rates as those treated with WLE: 84.8% at 3 years, 78.3% at 5 years, and 60.8% at 10 years.
Multivariable survival analysis demonstrated that treatment with MMS was associated with significantly improved survival, compared with WLE (hazard ratio, 0.59; 95% CI, 0.36-0.97; P = .04).
“These data suggest that MMS may provide a survival benefit in the treatment of localized MCC, although further prospective work studying this issue is required,” the authors concluded. “Future directions may also focus on elucidating the benefit of adjuvant radiotherapy in localized cases treated with MMS.”
They acknowledged certain limitations of the study, including the fewer numbers of patients receiving MMS surgery, lack of randomization, and potential for selection bias.
In an interview, Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the study, said that the field of MCC “has undergone rapid and robust transformation over the past 20 years. These changes encompass advancements in diagnosing the condition, identifying linked viruses, and developing systemic treatments.”
The study findings “imply that comprehensive assessment of histologic margins might offer advantages beyond minimizing scars, minimizing functional impact, and reducing the likelihood of local recurrence,” he said.
“It’s beyond doubt,” he added, that the study “furnishes us with yet another set of real-world insights that will undoubtedly influence patient outcomes. These insights serve to bring clarity to the ways in which we can deliver precisely targeted surgical treatment with durable outcomes for localized MCC.”
Patricia M. Richey, MD, director of Mohs surgery at Boston University, who was also asked to comment on the study, added that, because of the nature of the National Cancer Database, “the authors of this study were unfortunately unable to report disease-specific survival or immunosuppression status. That being said, the preliminary data presented are convincing and should result in us further exploring this topic, as well as readdressing and questioning related issues such as whether or not adjuvant radiotherapy is truly beneficial in cases with histologic clearance via Mohs.”
Dr. Carucci reported receiving grant funding from Regeneron for investigator-initiated basic research. No other author disclosures were reported. Neither Dr. Blalock nor Dr. Richey had relevant disclosures.
results from a national retrospective cohort study suggest.
The study found that, in patients with pathologically confirmed, localized T1/T2 MCC, “treatment with MMS was associated with an approximately 40% reduction in hazard of death compared with WLE,” reported John A. Carucci, MD, PhD, and colleagues in the department of dermatology at NYU Langone Health, New York. The results provide “preliminary data suggesting that treatment of localized, early-stage MCC with MMS may result in the most optimal patient survival outcomes for this aggressive form of skin cancer,” they added. The study was published online in JAMA Dermatology.
“Although data for keratinocytic nonmelanoma skin cancers have been definitive in demonstrating the advantage of peripheral and deep en face margin assessment over conventional WLE or NME [narrow-margin excision], the data for MCC, likely because of the disease’s rarity and limitations of available data sets, have been mixed,” they wrote.
Results from national studies published in the Journal of the National Cancer Institute and the Journal of the American Academy of Dermatology found no difference in survival among patients with localized MCC treated with WLE versus MMS. “However, these studies did not have confirmed pathologic node status, a substantial limitation considering that clinically node-negative cases of localized MCC have sentinel lymph node positivity rates ranging from 25% to 40%,” the authors noted.
To evaluate the association of the surgical excision modality and patient survival for pathologically confirmed localized T1/T2 MCC, Dr. Carucci and coauthors examined a cohort of 2,313 patients from the National Cancer Database with T1/T2 MCC diagnosed between Jan. 1, 2004, and Dec. 31, 2018, with pathologically confirmed, negative regional lymph nodes and treated with surgery. Their mean age was 71 years and 57.9% were male. Of the 2,313 patients, 1,452 underwent WLE, 104 underwent MMS, and 757 underwent NME.
The unadjusted analysis revealed that, compared with WLE, excision with MMS had the best unadjusted mean survival rates: 87.4% versus 86.1%, respectively, at 3 years, 84.5% versus 76.9% at 5 years, and 81.8% versus 60.9% at 10 years. Patients treated with NME had similar mean survival rates as those treated with WLE: 84.8% at 3 years, 78.3% at 5 years, and 60.8% at 10 years.
Multivariable survival analysis demonstrated that treatment with MMS was associated with significantly improved survival, compared with WLE (hazard ratio, 0.59; 95% CI, 0.36-0.97; P = .04).
“These data suggest that MMS may provide a survival benefit in the treatment of localized MCC, although further prospective work studying this issue is required,” the authors concluded. “Future directions may also focus on elucidating the benefit of adjuvant radiotherapy in localized cases treated with MMS.”
They acknowledged certain limitations of the study, including the fewer numbers of patients receiving MMS surgery, lack of randomization, and potential for selection bias.
In an interview, Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the study, said that the field of MCC “has undergone rapid and robust transformation over the past 20 years. These changes encompass advancements in diagnosing the condition, identifying linked viruses, and developing systemic treatments.”
The study findings “imply that comprehensive assessment of histologic margins might offer advantages beyond minimizing scars, minimizing functional impact, and reducing the likelihood of local recurrence,” he said.
“It’s beyond doubt,” he added, that the study “furnishes us with yet another set of real-world insights that will undoubtedly influence patient outcomes. These insights serve to bring clarity to the ways in which we can deliver precisely targeted surgical treatment with durable outcomes for localized MCC.”
Patricia M. Richey, MD, director of Mohs surgery at Boston University, who was also asked to comment on the study, added that, because of the nature of the National Cancer Database, “the authors of this study were unfortunately unable to report disease-specific survival or immunosuppression status. That being said, the preliminary data presented are convincing and should result in us further exploring this topic, as well as readdressing and questioning related issues such as whether or not adjuvant radiotherapy is truly beneficial in cases with histologic clearance via Mohs.”
Dr. Carucci reported receiving grant funding from Regeneron for investigator-initiated basic research. No other author disclosures were reported. Neither Dr. Blalock nor Dr. Richey had relevant disclosures.
results from a national retrospective cohort study suggest.
The study found that, in patients with pathologically confirmed, localized T1/T2 MCC, “treatment with MMS was associated with an approximately 40% reduction in hazard of death compared with WLE,” reported John A. Carucci, MD, PhD, and colleagues in the department of dermatology at NYU Langone Health, New York. The results provide “preliminary data suggesting that treatment of localized, early-stage MCC with MMS may result in the most optimal patient survival outcomes for this aggressive form of skin cancer,” they added. The study was published online in JAMA Dermatology.
“Although data for keratinocytic nonmelanoma skin cancers have been definitive in demonstrating the advantage of peripheral and deep en face margin assessment over conventional WLE or NME [narrow-margin excision], the data for MCC, likely because of the disease’s rarity and limitations of available data sets, have been mixed,” they wrote.
Results from national studies published in the Journal of the National Cancer Institute and the Journal of the American Academy of Dermatology found no difference in survival among patients with localized MCC treated with WLE versus MMS. “However, these studies did not have confirmed pathologic node status, a substantial limitation considering that clinically node-negative cases of localized MCC have sentinel lymph node positivity rates ranging from 25% to 40%,” the authors noted.
To evaluate the association of the surgical excision modality and patient survival for pathologically confirmed localized T1/T2 MCC, Dr. Carucci and coauthors examined a cohort of 2,313 patients from the National Cancer Database with T1/T2 MCC diagnosed between Jan. 1, 2004, and Dec. 31, 2018, with pathologically confirmed, negative regional lymph nodes and treated with surgery. Their mean age was 71 years and 57.9% were male. Of the 2,313 patients, 1,452 underwent WLE, 104 underwent MMS, and 757 underwent NME.
The unadjusted analysis revealed that, compared with WLE, excision with MMS had the best unadjusted mean survival rates: 87.4% versus 86.1%, respectively, at 3 years, 84.5% versus 76.9% at 5 years, and 81.8% versus 60.9% at 10 years. Patients treated with NME had similar mean survival rates as those treated with WLE: 84.8% at 3 years, 78.3% at 5 years, and 60.8% at 10 years.
Multivariable survival analysis demonstrated that treatment with MMS was associated with significantly improved survival, compared with WLE (hazard ratio, 0.59; 95% CI, 0.36-0.97; P = .04).
“These data suggest that MMS may provide a survival benefit in the treatment of localized MCC, although further prospective work studying this issue is required,” the authors concluded. “Future directions may also focus on elucidating the benefit of adjuvant radiotherapy in localized cases treated with MMS.”
They acknowledged certain limitations of the study, including the fewer numbers of patients receiving MMS surgery, lack of randomization, and potential for selection bias.
In an interview, Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, who was asked to comment on the study, said that the field of MCC “has undergone rapid and robust transformation over the past 20 years. These changes encompass advancements in diagnosing the condition, identifying linked viruses, and developing systemic treatments.”
The study findings “imply that comprehensive assessment of histologic margins might offer advantages beyond minimizing scars, minimizing functional impact, and reducing the likelihood of local recurrence,” he said.
“It’s beyond doubt,” he added, that the study “furnishes us with yet another set of real-world insights that will undoubtedly influence patient outcomes. These insights serve to bring clarity to the ways in which we can deliver precisely targeted surgical treatment with durable outcomes for localized MCC.”
Patricia M. Richey, MD, director of Mohs surgery at Boston University, who was also asked to comment on the study, added that, because of the nature of the National Cancer Database, “the authors of this study were unfortunately unable to report disease-specific survival or immunosuppression status. That being said, the preliminary data presented are convincing and should result in us further exploring this topic, as well as readdressing and questioning related issues such as whether or not adjuvant radiotherapy is truly beneficial in cases with histologic clearance via Mohs.”
Dr. Carucci reported receiving grant funding from Regeneron for investigator-initiated basic research. No other author disclosures were reported. Neither Dr. Blalock nor Dr. Richey had relevant disclosures.
FROM JAMA DERMATOLOGY
Cystic Presentation of High-Grade Ductal Carcinoma In Situ in an Inframammary Accessory Nipple
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.
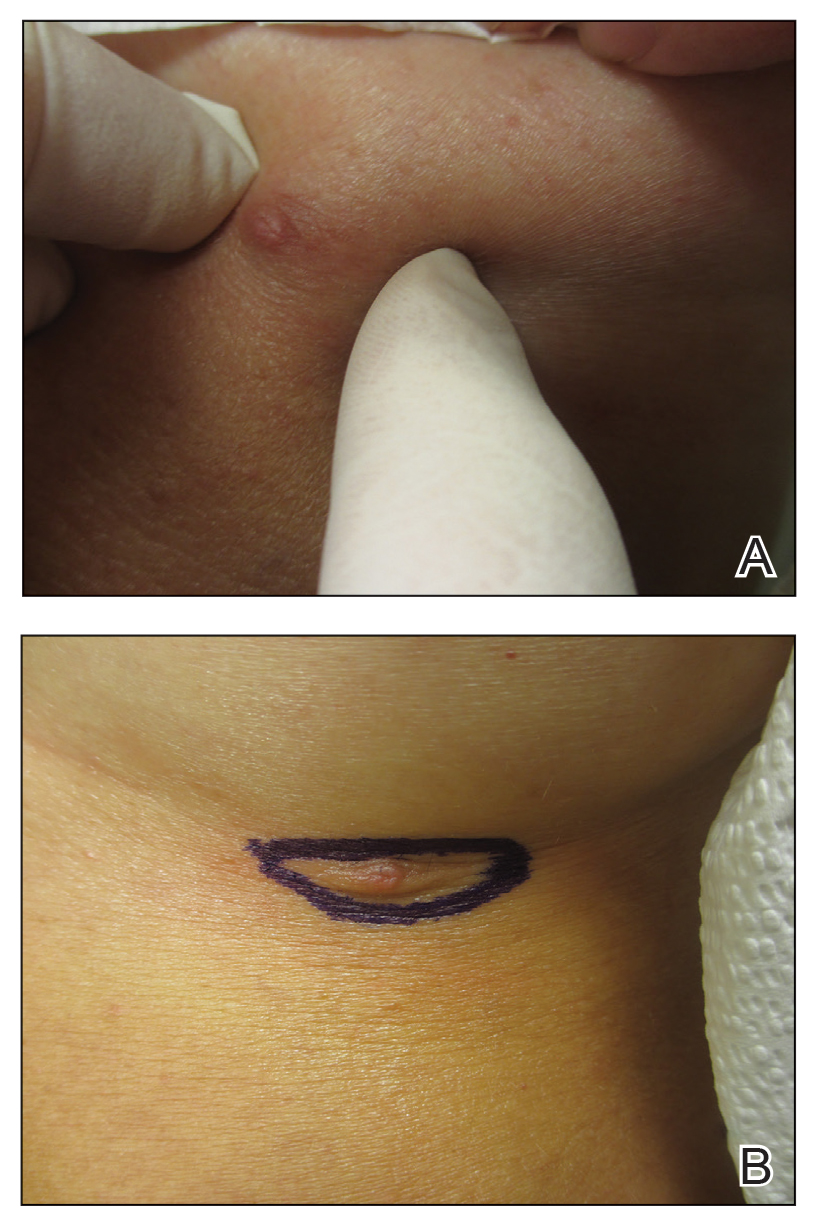
A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).
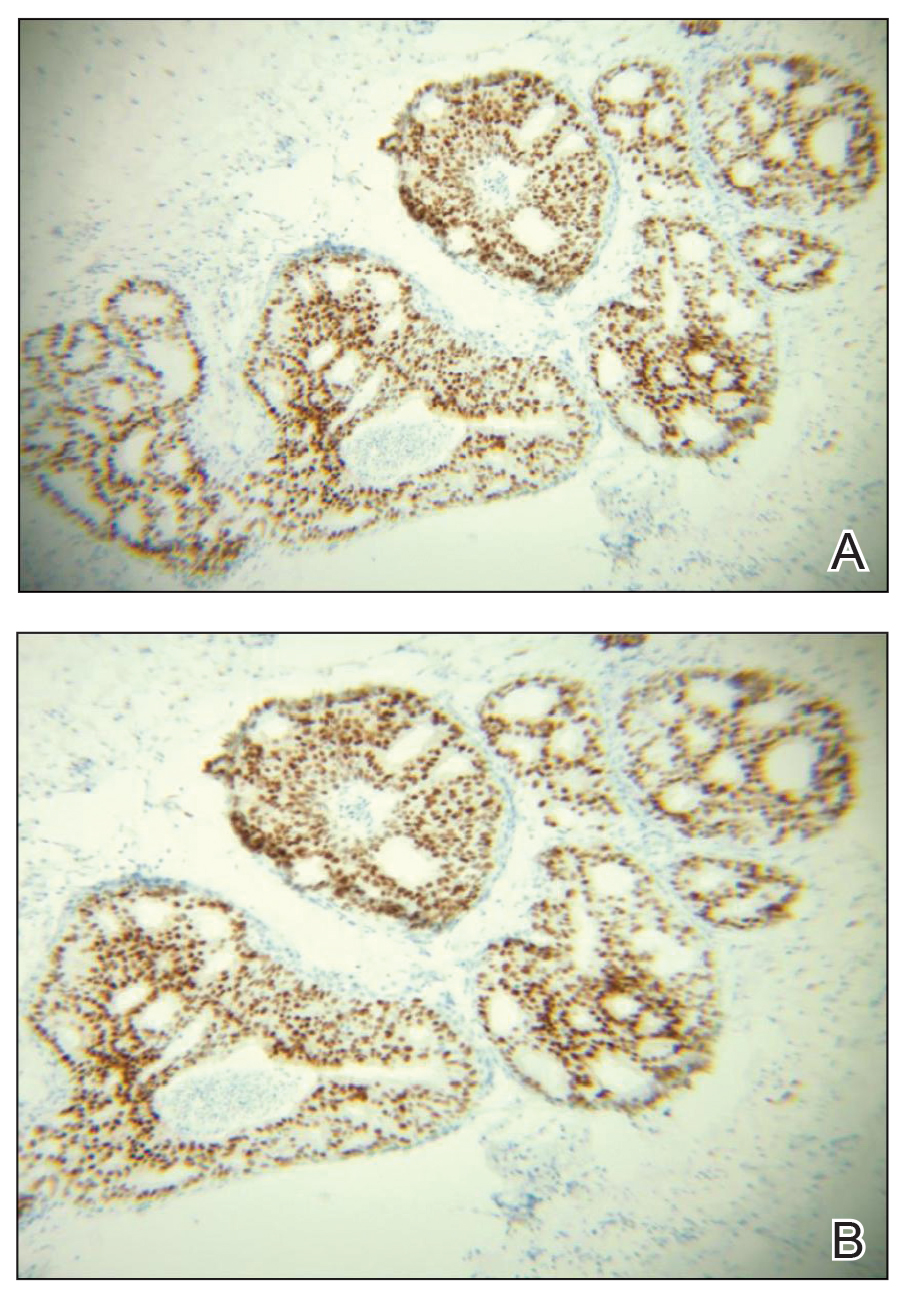
Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11
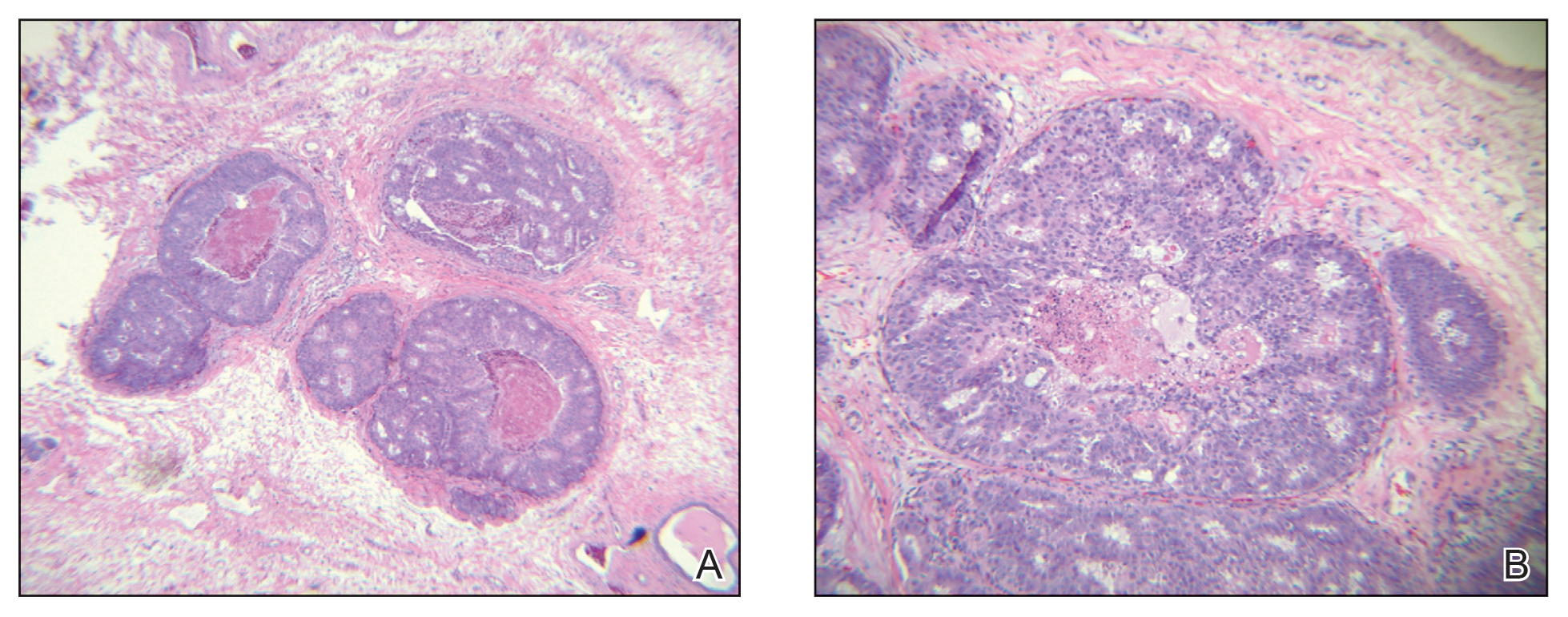
There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
Practice Points
- Accessory breasts (also referred to as ectopic breast tissue) develop when breast tissue is retained along the mammary ridge outside of the usual pectoral regions.
- Because accessory breasts may contain the same structures as anatomically correct breasts, they can be subject to the same benign or malignant changes.
- Clinical and pathologic correlation is prudent when interpreting ectopic mammary tissue, as various benign or malignant neoplasms may arise in this setting, especially if there are underlying genetic aberrancies or genodermatoses.
Affixing a Scalp Dressing With Hairpins
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).
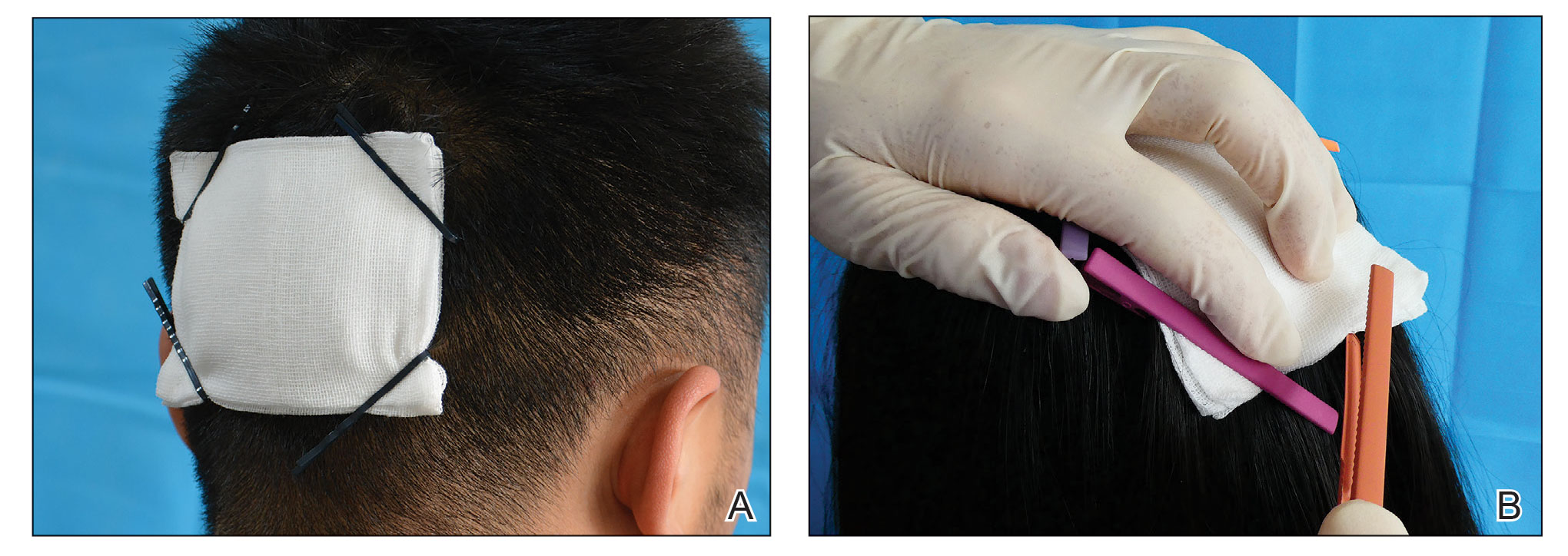
Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).

Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).

Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
Cancer Screening for Dermatomyositis: A Survey of Indirect Costs, Burden, and Patient Willingness to Pay
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18
The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).
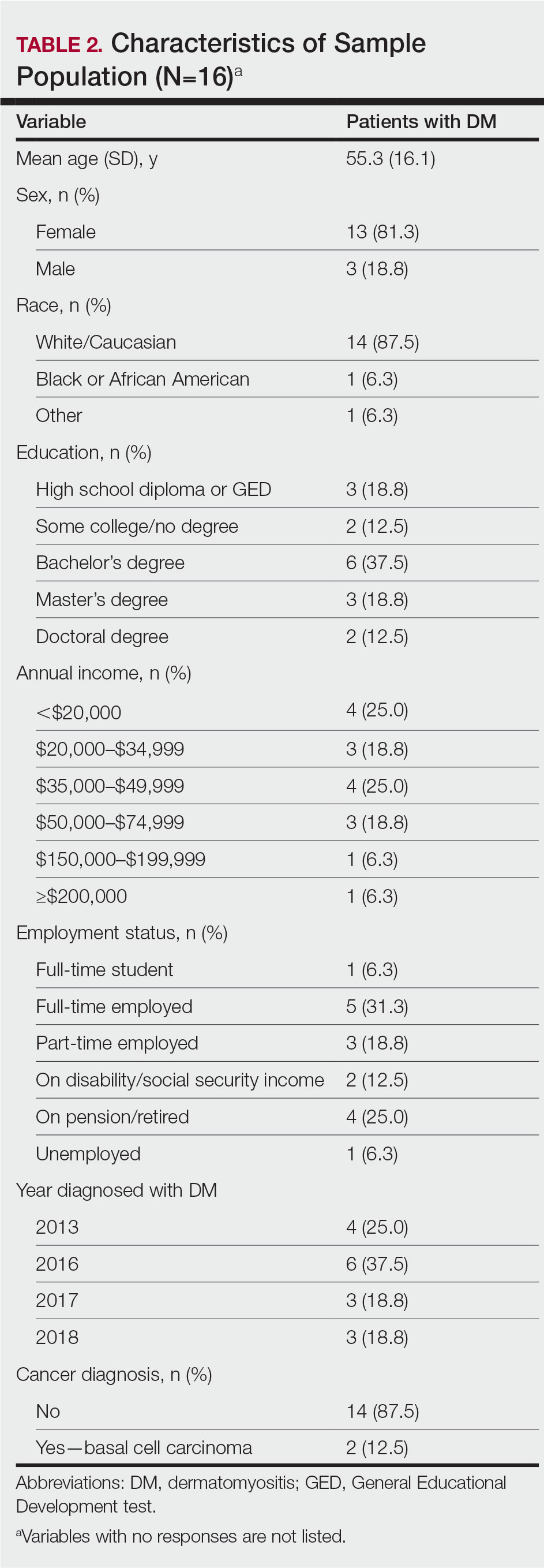
Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.
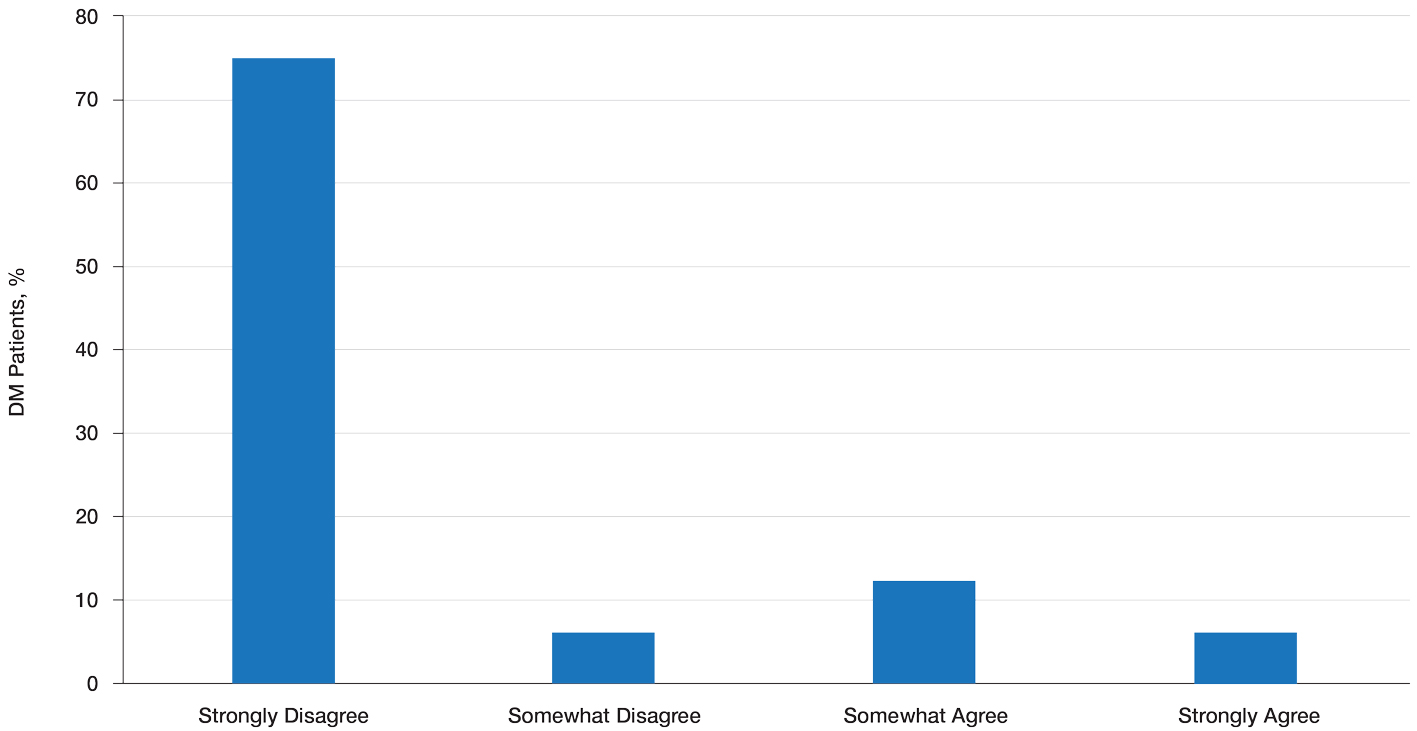
Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.
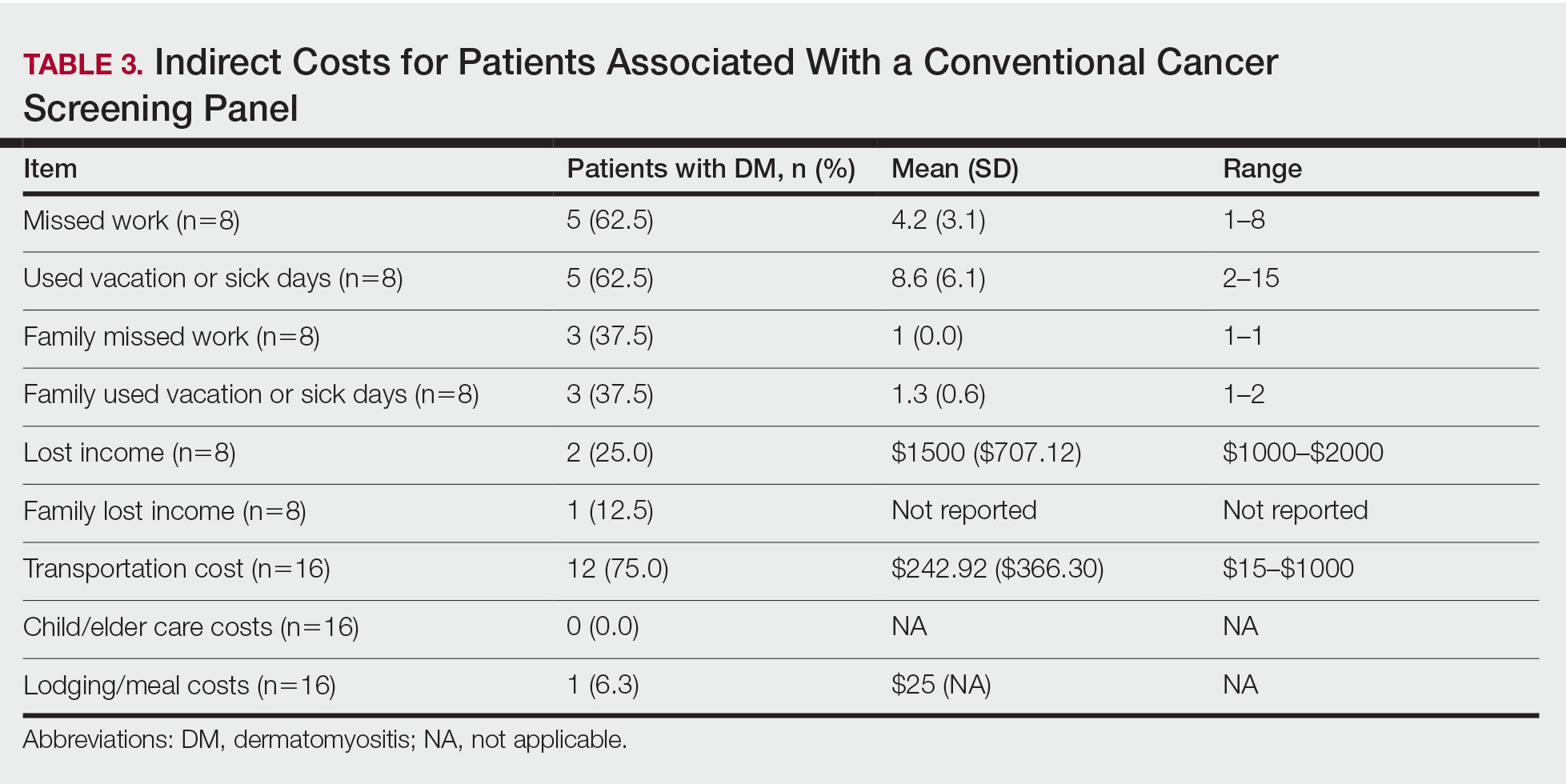
Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17
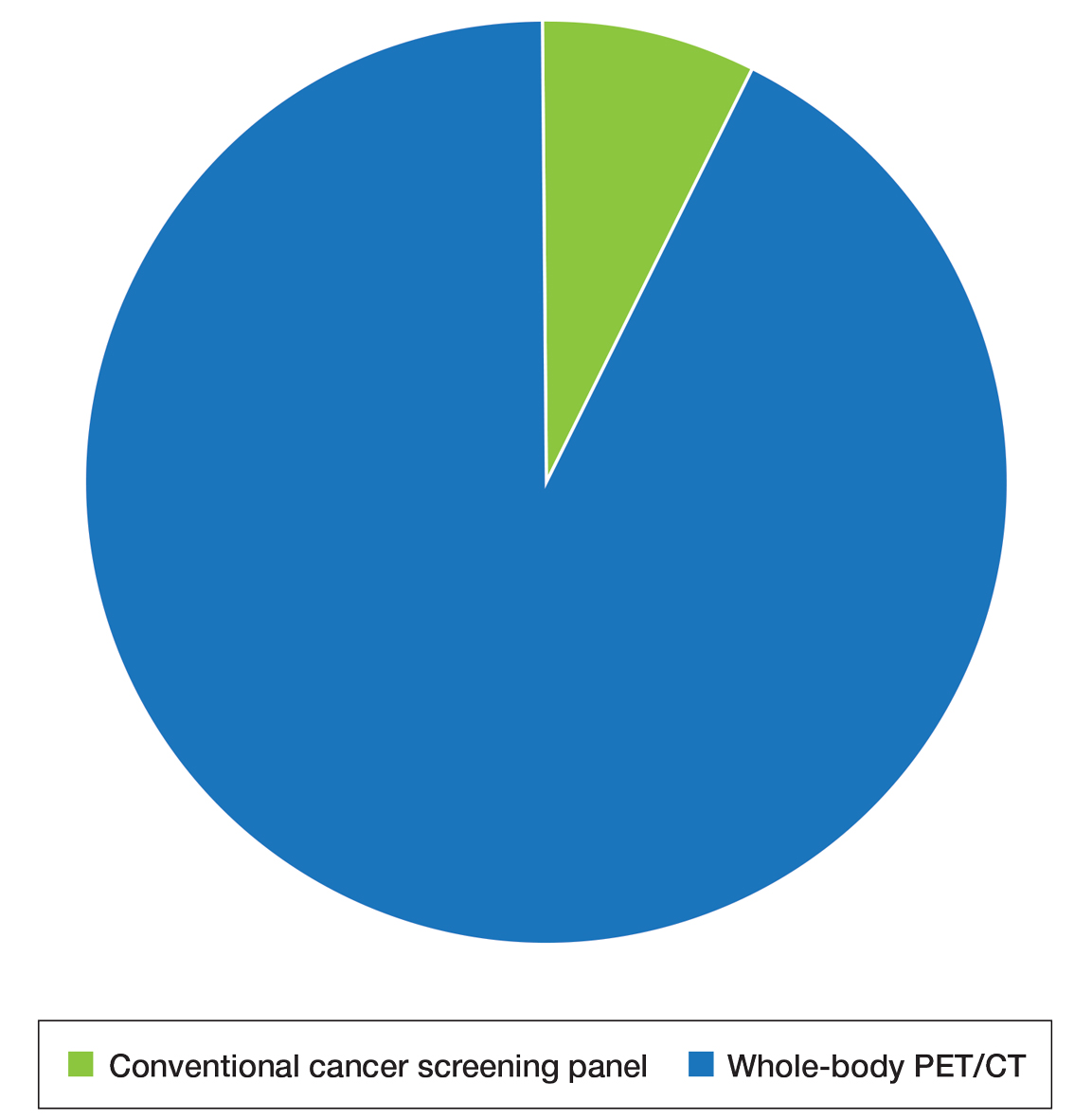
The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)
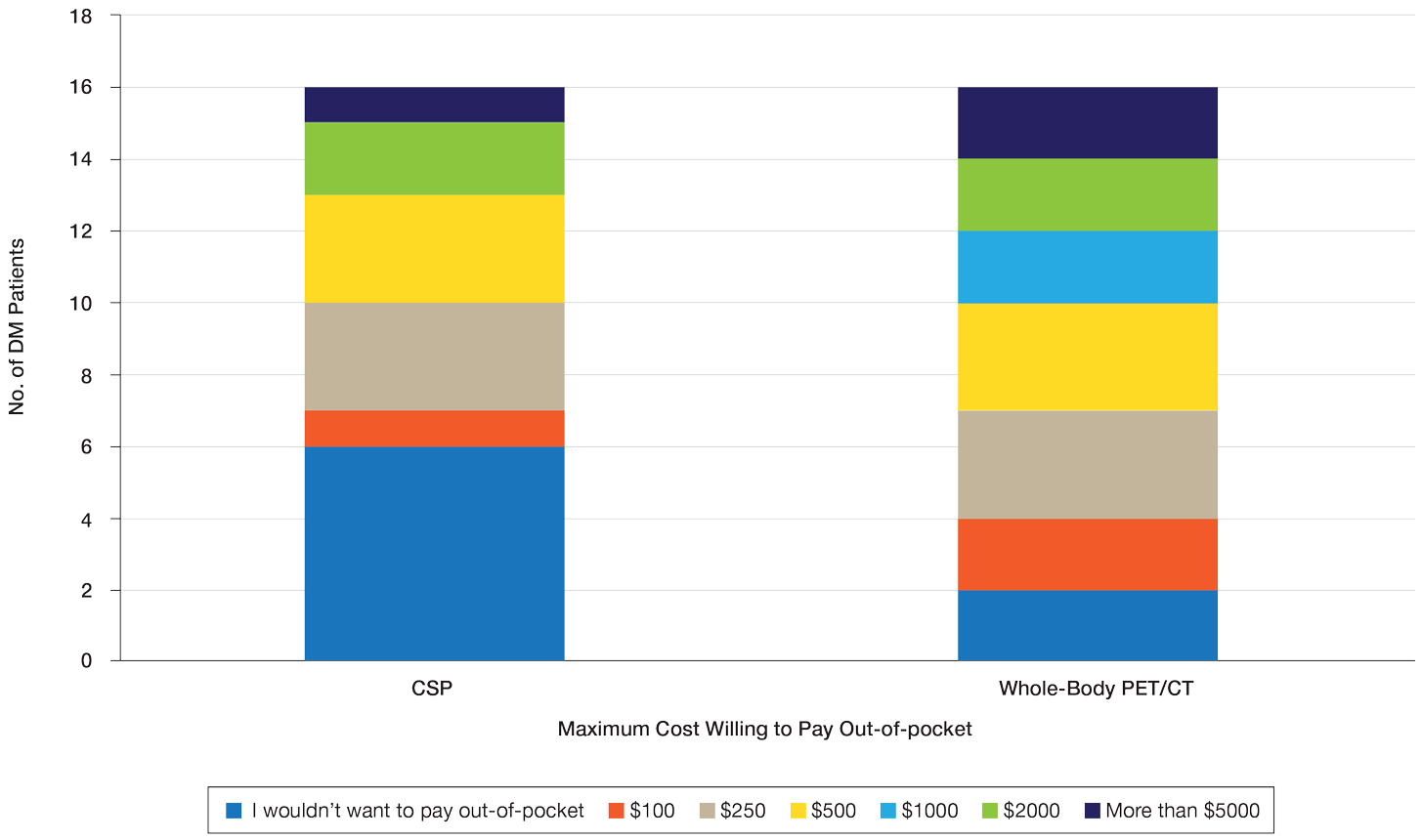
“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Practice Points
- Dermatomyositis (DM) is associated with an increased risk for malignancy. Patient perspective needs to be considered in developing cancer screening guidelines for patients with DM, particularly given the similar efficacy of available screening modalities.
- Current modalities for cancer screening in DM include whole-body positron emission tomography/computed tomography (PET/CT) and a conventional cancer screening panel (CSP), which includes a battery of tests typically requiring multiple visits. Patients may find the simplicity of PET/CT more preferrable than the more complex CSP.
- Indirect costs of cancer screening include missed work, travel and childcare expenses, and lost wages. Conventional cancer screening has greater indirect costs than PET/CT.
Are AI-powered skin-check tools on the horizon for dermatologists, PCPs?
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
What makes teens choose to use sunscreen?
a cornerstone of skin cancer prevention, according to results from a systematic review.
“We know that skin cancer is one of the most common malignancies in the world, and sun protection methods such as sunscreen make it highly preventable,” first author Carly R. Stevens, a student at Tulane University, New Orleans, said in an interview. “This study demonstrates the adolescent populations that are most vulnerable to sun damage and how we can help mitigate their risk of developing skin cancer through education methods, such as Sun Protection Outreach Teaching by Students.”
Ms. Stevens and coauthors presented the findings during a poster session at the annual meeting of the Society for Pediatric Dermatology.
To investigate predictors of sunscreen use among high school students, they searched PubMed, Embase, and Web of Science using the terms (“sunscreen” or “SPF” or “sun protection”) and (“high school” or “teen” or “teenager” or “adolescent”) and limited the analysis to English studies reporting data on sunscreen use in U.S. high school students up to November 2021.
A total of 20 studies were included in the final review. The study populations ranged in number from 208 to 24,645. Of 11 studies that examined gender, all showed increased sunscreen use in females compared with males. Of five studies that examined age, all showed increased sunscreen use in younger adolescents, compared with their older counterparts.
Of four studies that examined the role of ethnicity on sunscreen use, White students were more likely to use sunscreen, compared with their peers of other ethnicities. “This may be due to perceived sun sensitivity, as [these four studies] also showed increased sunscreen use in populations that believed were more susceptible to sun damage,” the researchers wrote in their abstract.
In other findings, two studies that examined perceived self-efficacy concluded that higher levels of sunscreen use correlated with higher self-efficacy, while four studies concluded that high school students were more likely to use sunscreen if their parents encouraged them the wear it or if the parent used it themselves.
“With 40%-50% of ultraviolet damage being done before the age of 20, it’s crucial that we find ways to educate adolescents on the importance of sunscreen use and target those populations who were found to rarely use sunscreen in our study,” Ms. Stevens said.
In one outreach program, Sun Protection Outreach Teaching by Students (SPOTS), medical students visit middle and high schools to educate them about the importance of practicing sun protection. The program began as a collaboration between Saint Louis University and Washington University in St. Louis, but has expanded nationwide. Ms. Stevens described SPOTS as “a great way for medical students to present the information to middle and high school students in a way that is engaging and interactive.”
The researchers reported having no disclosures.
a cornerstone of skin cancer prevention, according to results from a systematic review.
“We know that skin cancer is one of the most common malignancies in the world, and sun protection methods such as sunscreen make it highly preventable,” first author Carly R. Stevens, a student at Tulane University, New Orleans, said in an interview. “This study demonstrates the adolescent populations that are most vulnerable to sun damage and how we can help mitigate their risk of developing skin cancer through education methods, such as Sun Protection Outreach Teaching by Students.”
Ms. Stevens and coauthors presented the findings during a poster session at the annual meeting of the Society for Pediatric Dermatology.
To investigate predictors of sunscreen use among high school students, they searched PubMed, Embase, and Web of Science using the terms (“sunscreen” or “SPF” or “sun protection”) and (“high school” or “teen” or “teenager” or “adolescent”) and limited the analysis to English studies reporting data on sunscreen use in U.S. high school students up to November 2021.
A total of 20 studies were included in the final review. The study populations ranged in number from 208 to 24,645. Of 11 studies that examined gender, all showed increased sunscreen use in females compared with males. Of five studies that examined age, all showed increased sunscreen use in younger adolescents, compared with their older counterparts.
Of four studies that examined the role of ethnicity on sunscreen use, White students were more likely to use sunscreen, compared with their peers of other ethnicities. “This may be due to perceived sun sensitivity, as [these four studies] also showed increased sunscreen use in populations that believed were more susceptible to sun damage,” the researchers wrote in their abstract.
In other findings, two studies that examined perceived self-efficacy concluded that higher levels of sunscreen use correlated with higher self-efficacy, while four studies concluded that high school students were more likely to use sunscreen if their parents encouraged them the wear it or if the parent used it themselves.
“With 40%-50% of ultraviolet damage being done before the age of 20, it’s crucial that we find ways to educate adolescents on the importance of sunscreen use and target those populations who were found to rarely use sunscreen in our study,” Ms. Stevens said.
In one outreach program, Sun Protection Outreach Teaching by Students (SPOTS), medical students visit middle and high schools to educate them about the importance of practicing sun protection. The program began as a collaboration between Saint Louis University and Washington University in St. Louis, but has expanded nationwide. Ms. Stevens described SPOTS as “a great way for medical students to present the information to middle and high school students in a way that is engaging and interactive.”
The researchers reported having no disclosures.
a cornerstone of skin cancer prevention, according to results from a systematic review.
“We know that skin cancer is one of the most common malignancies in the world, and sun protection methods such as sunscreen make it highly preventable,” first author Carly R. Stevens, a student at Tulane University, New Orleans, said in an interview. “This study demonstrates the adolescent populations that are most vulnerable to sun damage and how we can help mitigate their risk of developing skin cancer through education methods, such as Sun Protection Outreach Teaching by Students.”
Ms. Stevens and coauthors presented the findings during a poster session at the annual meeting of the Society for Pediatric Dermatology.
To investigate predictors of sunscreen use among high school students, they searched PubMed, Embase, and Web of Science using the terms (“sunscreen” or “SPF” or “sun protection”) and (“high school” or “teen” or “teenager” or “adolescent”) and limited the analysis to English studies reporting data on sunscreen use in U.S. high school students up to November 2021.
A total of 20 studies were included in the final review. The study populations ranged in number from 208 to 24,645. Of 11 studies that examined gender, all showed increased sunscreen use in females compared with males. Of five studies that examined age, all showed increased sunscreen use in younger adolescents, compared with their older counterparts.
Of four studies that examined the role of ethnicity on sunscreen use, White students were more likely to use sunscreen, compared with their peers of other ethnicities. “This may be due to perceived sun sensitivity, as [these four studies] also showed increased sunscreen use in populations that believed were more susceptible to sun damage,” the researchers wrote in their abstract.
In other findings, two studies that examined perceived self-efficacy concluded that higher levels of sunscreen use correlated with higher self-efficacy, while four studies concluded that high school students were more likely to use sunscreen if their parents encouraged them the wear it or if the parent used it themselves.
“With 40%-50% of ultraviolet damage being done before the age of 20, it’s crucial that we find ways to educate adolescents on the importance of sunscreen use and target those populations who were found to rarely use sunscreen in our study,” Ms. Stevens said.
In one outreach program, Sun Protection Outreach Teaching by Students (SPOTS), medical students visit middle and high schools to educate them about the importance of practicing sun protection. The program began as a collaboration between Saint Louis University and Washington University in St. Louis, but has expanded nationwide. Ms. Stevens described SPOTS as “a great way for medical students to present the information to middle and high school students in a way that is engaging and interactive.”
The researchers reported having no disclosures.
FROM SPD 2023